Introduction
Cryptococcal meningitis (CM) is associated with
mortality rates of ~15% in patients with acquired immunodeficiency
syndrome (AIDS), accounting for ~181,100 related deaths per year.
China and other developing countries have observed a higher impact
of CM infection in non-HIV AIDS hosts with an unknown risk of
susceptibility (1,2). The lung is the first organ exposed
to fungal spores following inhalation. Colonization of the fungi in
the host lung leads to immune activation in the absence of fungal
clearance during the incubation period, which can be reactivated
when the host becomes immunocompromised, leading to asymptomatic
infection. Common symptoms include pulmonary disease characterized
by pulmonary nodules and inflammation (3,4).
In immunosuppressed individuals, the fungi spread to other organs,
particularly the brain. and cryptococcal cells proliferate
hematogenously to disseminate to the brain through the blood-brain
barrier (BBB) (5).
Ngamskulrungroj et al (6)
previously demonstrated that, unlike Cryptococcus gattii,
Cryptococcus neoformans (C. neoformans) exhibits a
preference for the brain as opposed to the lungs as a site of
infection. C. neoformans can survive under phagocytosis and
can endure the oxidative attack of the innate immune response,
which facilitates dissemination to the central nervous system (CNS)
and causes meningoencephalitis (7).
Microglia are macrophages within the CNS that
participate in the immunosurveillance of C. neoformans. The
efficiency of the immune response dictates the outcome of C.
neoformans infection, producing a disseminated disease or state
of latency. The CNS is immune-privileged and isolated from
peripheral organs due to separation by the BBB. Microglial
activation enhances innate immune response, enhancing the
phagocytosis of invasive bacteria or fungi, thus protecting
neuronal cells (8,9). The assessment of the association
between macrophages, innate immunity and C. neoformans can
enhance the current understanding of the pathogenesis of CM.
MicroRNAs (miRNAs/miRs) are highly conserved small
non-coding RNAs of 18-22 nucleotides (10). miRNAs regulate gene expression by
binding to the 3′ untranslated region (3'UTR) of mRNAs, leading to
a loss of gene expression through mRNA degradation or by preventing
translation. A role for miRNAs in the immune response to fungal
exposure has been documented. miR-21, miR-146, miR-132, miR-155 and
the let-7 family have been shown to regulate inflammatory responses
following exposure to bacterial pathogens, and a range of miRNAs
have been used as therapeutics for cancers and other diseases
(11). The miRNA response to
fungal exposure is comparable to that of inflammatory and allergic
responses (12-17). miR-4792 has been shown to be
downregulated in glioblastoma-infiltrating CD14+ cells,
in contrast to miR-4792 that is upregulated in glioma microvesicles
(18-22). Epidermal growth factor receptor
(EGFR) regulates epithelial tissue development and homeostasis, its
dysregulation being common in lung, breast and glioblastoma tumors.
In previous studies, miRNA-34a, miRNA-101-3p.1 and miRNA-223 were
demonstrated to play important roles in several disease states,
including gastric cancer, chronic obstructive pulmonary disease and
non-small cell lung cancer through their ability to target EGFR
(23-25).
Approximately 50% of patients with cryptococcal
meningitis succumb to the disease within 1 year of infection due to
a lack of successful therapy (26). In the absence of effective
anti-fungal agents, the immune response to fungal infection is
poorly defined. In the present study, the changes in miR-4792
expression following pathogen infection and the regulation of its
target genes during neuroinflammation were investigated, in order
to provide a novel theoretical basis and more effective approaches
for antifungal therapeutics.
Materials and methods
Cells and cell culture
Given the limited availability of primary cultures,
BV2 cells were used as a representative immortalized microglia cell
line due to their known similarities to primary microglia cells
(27,28). BV2 cells were cultured at 37°C in
5% CO2 in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated fetal bovine serum (FBS; Omega Scientific, Inc.)
and 1% penicillin/streptomycin (all from Beyotime Institute of
Biotechnology). Cell densities did not exceed 5×105
cells/cm2. The BV2 and THP-1 cell lines were purchased
from the Stem Cell Bank (The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences).
Induction of BV2 cells
The cells were seeded at a density of
1×107 cells into 6-well plates and incubated at 37°C
with C. neoformans (5×107 of the WM148 strain
obtained from the Centre for Preservation of Medical Mycology,
Shanghai, China) for 6 h at a ratio of 5:1. The cells were
subsequently treated with the EGFR inhibitor (AG1478, 40 µm)
(TargetMol), miR-4792 inhibitor or inhibitor negative controls,
miR-4792 mimics or mimic negative controls (Guangzhou RiboBio Co.,
Ltd.) for 24 h as described below.
Western blot analysis
For western blot analysis, the cells incubated with
WM148 were washed three times in phosphate-buffered saline (PBS)
and lysed in RIPA buffer supplemented with 1 µm
phenylmethylsulfonyl fluoride (PMSF; protease inhibitor). Protein
samples were quantified using a BCA Protein Assay kit (Shanghai
Epizyme Biomedical Technology Co., Ltd.). Equivalent amounts of
protein (15 µg) from each sample were run at 10% SDS-PAGE
and transferred to 0.45 µm polyvinylidene fluoride membranes
(EMD Millipore). After blocking with Protein Free Rapid Blocking
Buffer (1X) (Shanghai Epizyme Biomedical Technology Co., Ltd.) at
room temperature for 10 min, the membranes were first incubated
with the appropriate primary antibodies overnight at 4°C. The
membranes were then washed and labeled with HRP-conjugated
secondary anti-rabbit or anti-mouse antibodies (1:3,000, cat. no.
7074S, or 7076S, Cell Signaling Technology, Inc.) at room
temperature for 1 h. Finally, bands were visualized with enhanced
chemiluminescence (ECL) kits (Beyotime Biotech Inc), and resulting
digital images were analyzed using ImageJ software (version 1.8.0,
National Institute of Health) to obtain the optical densities (OD)
of signals. The primary antibodies used in the study included the
following: Phosphorylated (phosphor/p)-EGFR monoclonal antibodies
(1:200; cat. no. sc-81488, Santa Cruz Biotechnology, Inc.), EGFR
monoclonal antibodies (1:100; cat. no. sc-377229, Santa Cruz
Biotechnology, Inc.), CD11b rabbit monoclonal antibodies (1:1,000;
cat. no. 49420, Cell Signaling Technology, Inc.), major
histocompatibility complex (MHC) class II mouse monoclonal
antibodies (1:1,000; cat. no. 68258, Cell Signaling Technology,
Inc.), phospho-ERK1/2 rabbit monoclonal antibodies (1:2,000; cat.
no. 4370, Cell Signaling Technology, Inc.), phospho-p38 rabbit
monoclonal anti-bodies (1:1,000; cat. no. 4511, Cell Signaling
Technology, Inc.), phospho-JNK rabbit monoclonal antibodies
(1:1,000; cat. no. 4668, Cell Signaling Technology, Inc.), IL-1β
polyclonal antibodies (1:1,000; cat. no. 16806-1-AP, ProteinTech
Group, Inc.) and TNF-α rabbit polyclonal antibodies (1:1,000; cat.
no. 17590-1-AP, ProteinTech Group, Inc.). β-actin (1:5,000; cat.
no. 20536-1-AP, ProteinTech Group, Inc.) was used as an internal
control.
Quantification of cytokine levels
The WM148-infected BV2 cells were treated with
AG1478 (40 µm) for 6 h, or transfected with miR-4792 mimics
and inhibitors for 24 h as described below. Following the
treatments, cell culture supernatants were collected, and IL-1α,
IL-12, eotaxin, granulocyte-macrophage colony stimulating factor
(GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2) were
quantified using Mouse Multi-Analyte kits (cat. no. M60009RDPD,
Bio-Plex Suspension Array System; Bio-Rad Laboratories, Inc.)
according to the manufacturer's instructions. Antibody arrays were
performed by Wayen Biotechnology according to established
protocols. Briefly, 50 µl antibody-conjugated beads were
added to assay plates to which 50 µl tissue lysates,
standards and blank controls were added in the dark at room
temperature with rotational speed of shaker 850 rpm/min for 2 h.
After washing, 50 µl biotinylated antibodies were added in
the dark at room temperature with shaking at 850 rpm/min for 1 h.
The plates were then washed and 50 µl of
streptavidin-phycoerythrin (PE) was added in the dark at room
temperature with shaking at 850 rpm/min for 30 min. The plates were
then washed and read using a Bio-Plex MAGPIX Multiplex Reader
(Bio-Rad Laboratories, Inc.). Bio-Plex Manager™ software (version
6.1, Bio-Rad Laboratories, Inc.) was used for data acquisition and
analysis.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis of miR-4792 and EGFR
The cells (1×105 cells/cm2)
were seeded onto coverslips and treated with 40 µm AG1478
for 24 h following WM148 (5×105 cells/cm2)
induction. RNA was extracted at 0, 3, 6, 9 and 12 h. The expression
levels of miR-4792 and EGFR in CSF were measured using miRNeasy
Serum/Plasma kits (cat. no. 217184, Qiagen, Inc.) according to the
manufacturer's recommendations. Total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
(500 ng) was used for cDNA synthesis with reverse transcriptase
(RR036B, Takara Bio, Inc.). cDNA (1 µl) was used for qPCR
with GoTaq qPCR Master Mix (Promega Corporation). The reaction
conditions were as follows: Hot start at 95°C/5 min (for miRNA) or
95°C/30 sec (for RNA); 40 cycles of 30 sec at 95°C, 30 sec at 60°C
and 30 sec at 72°C, and 10 min at 72°C. Target gene expression was
calculated relative to GAPDH (for RNA) or U6 (for miRNA)and values
were normalized to untreated controls. The 2−ΔΔCq method
was used to quantify the relative levels of gene expression
(29). The primer sequences are
presented in Table I.
 | Table IList of primers used for RT-qPCR and
mimic and inhibitor sequences. |
Table I
List of primers used for RT-qPCR and
mimic and inhibitor sequences.
| Primer/name | Sequence |
|---|
| Human GAPDH-F |
5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Human GAPDH-R |
5′-TGGTGAAGACGCCAGTGGA-3′ |
| Human EGFR-F |
5′-GCCAAGGCACGAGTAACAAGC-3′ |
| Human EGFR -R |
5′-AGGGCAATGAGGACATAACC-3′ |
| Mouse GAPDH-F |
5′-ACCCAGAAGACTGTGGATGG-3′ |
| Mouse GAPDH-R |
5′-TCTAGACGGCAGGTCAGGTC-3′ |
| Mouse EGFR-F |
5′-CTGCCAAAAGTTCCAAGATGAGG-3′ |
| Mouse EGFR-R |
5′-GGGGCACTTCTTCACACAGG-3′ |
| Mouse U6-F |
5′-CTCGCTTCGGCAGCACA-3′ |
| Mouse U6-R |
5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-4792 |
CGGTGAGCGCTCGCTGGC |
| miR-4792
mimics |
5′-CGGUGAGCGCUCGCUGGC -3′ |
|
3′-GCCAGCGAGCGCUCACCG -5′ |
| Mimics negative
control |
5′-UUUGUACUACACAAAAGUACUG -3′ |
|
3′-AAACAUGAUGUGUUUUCAUGAC-5′ |
| miR-4792
inhibitor |
GCCAGCGAGCGCUCACCG |
| Inhibitor negative
control |
5′-CAGUACUUUUGUGUAGUACAAA -3′ |
Transient transfection
The cells were seeded at a density of
2×105 cells/plate and were transfected with miR-4792
mimics (50 nM), mimic controls (50 nM), miR-4792 inhibitor (100 nM)
and inhibitor controls (100 nM) using riboFECT™ CP reagent
(Guangzhou RiboBio Co., Ltd.) as per the manufacturer's
recommendations. The sequences of the mimics and inhibitors are
presented in Table I. Briefly, 5
µl of the miRNA mimic or 10 µl of the miRNA inhibitor
were diluted with 120 µl riboFECT™ CP buffer at 37°C for 10
min. Diluents were mixed with 12 µl of riboFECT™ CP reagent
and incubated for 15 min at 37°C. RiboFECT™ CP-miRNA mixtures were
then added to the cells with 2 ml of DMEM and incubated at 37°C for
24 h.
Luciferase reporter assay
The miR-4792 target gene, EGFR, was searched using
the bioinformatics tool, miRanda (http://www.microrna.org) and TargetScan (http://www.targetscan.org). BV2 cells were exogenously
transfected with either 60 nM miR-controls and 60 nM miR-4792
mimics in combination with wild-type (WT) and mutant type (mut)
3′UTR EGFR using Lipofectamine 3000 (Thermo Fisher Scientific,
Inc.). At 48 h post-transfection, dual-luciferase reporter assays
were performed to monitor Firefly and Renilla luciferase
activity (Promega Corporation).
Flow cytometry
MHC II (FITC anti-mouse I-A/I-E, BioLegend, Inc.)
and CD11b (APC anti-mouse/human CD11b, BioLegend, Inc.) expression
were measured using a BD FACSCalibur™ (version 5.0, BD Biosciences)
using flow cytometer (BD Biosciences).
Patients
CSF samples were obtained by lumbar puncture from 11
patients with CM (7 males and 4 females; age range, 16-62 years;
mean age, 45 years; all HIV(-). CSF was collected before and after
antifungal therapy between January, 2014 and December, 2017 at
Shanghai Changzheng Hospital (patient details are presented in
Table II) (29). The patient inclusion criteria
were as follows: i) Clinical symptoms of CM; ii) CSF ink staining
microscopic examination (+) or CSF fungal culture (+) or CSF latex
agglutination test >1:8; iii) HIV test (-); iv) vital signs were
stable and there are no other serious organ diseases of heart,
liver, kidney, or blood system.
 | Table IIClinical information of the patients
with cryptococcal meningoencephalitis. |
Table II
Clinical information of the patients
with cryptococcal meningoencephalitis.
| Patient no. | Sex | Age (years) | Pre-therapy
| Post-treatment
|
|---|
| No. of
cryptococci | Antibody titer | No. of
cryptococci | Antibody titer |
|---|
| 1 | Male | 51 | 2 | 1:640 | 0 | 1:20 |
| 2 | Male | 16 | 0 | 1:40 | 0 | 1:1 |
| 3 | Male | 62 | 182 | 1:5120 | 0 | 1:20 |
| 4 | Male | 35 | 2 | >1:5120 | 0 | 1:1 |
| 5 | Male | 45 | 10 | 1:5120 | 0 | 1:20 |
| 6 | Male | 56 | 42 | 1:80 | 2 | 1:20 |
| 7 | Male | 18 | 2 | 1:320 | 0 | 1:10 |
| 8 | Female | 40 | 4 | 1:1280 | 0 | 1:10 |
| 9 | Female | 49 | 620 | 1:5120 | 2 | 1:160 |
| 10 | Female | 60 | 0 | 1:80 | 0 | 1:10 |
| 11 | Female | 54 | 2 | 1:160 | 0 | 1:5 |
Treatment included the following: Induction
treatment period: Amphotericin B 0.7-1.0 mg/kg/day intravenously
guttae + flucytosine 100 mg/kg/day p.o. for at least 2 weeks;
Consolidation period: Fluconazole 800 mg/day p.o. for 8-10 weeks;
Maintenance treatment period: Fluconazole 200 mg/day p.o. for 6-12
months. The clinical observation period was 12 months. CSF fungal
culture was negative for at least 3 consecutive times. All patients
fulfilled the criteria of the Infectious Diseases Society of
America (IDSA) (30). All
protocols were approved by the Ethics Committee of Ethics Committee
of Changzheng Hospital and written consent was obtained from all
patients. The study was performed according to the declaration of
Helsinki guidelines.
Statistical analysis
The majority of the experiments were performed
independently at least three times and yielded similar results.
Data are presented in figures as the mean ± SD using GraphPad
Prism8 (GraphPad Software, Inc.). An independent two-tailed
Student's t-test was used to compare two independent groups. For
multiple group comparisons, one-way ANOVA was performed, and a post
hoc analysis was conducted using Tukey's test. Receiver operating
characteristic (ROC) curves were used to analyze the predictive
value of miR-4792 and EGFR. A value P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-4792 and EGFR expression in BV2 cells
following infection with C. neoformans (WM148)
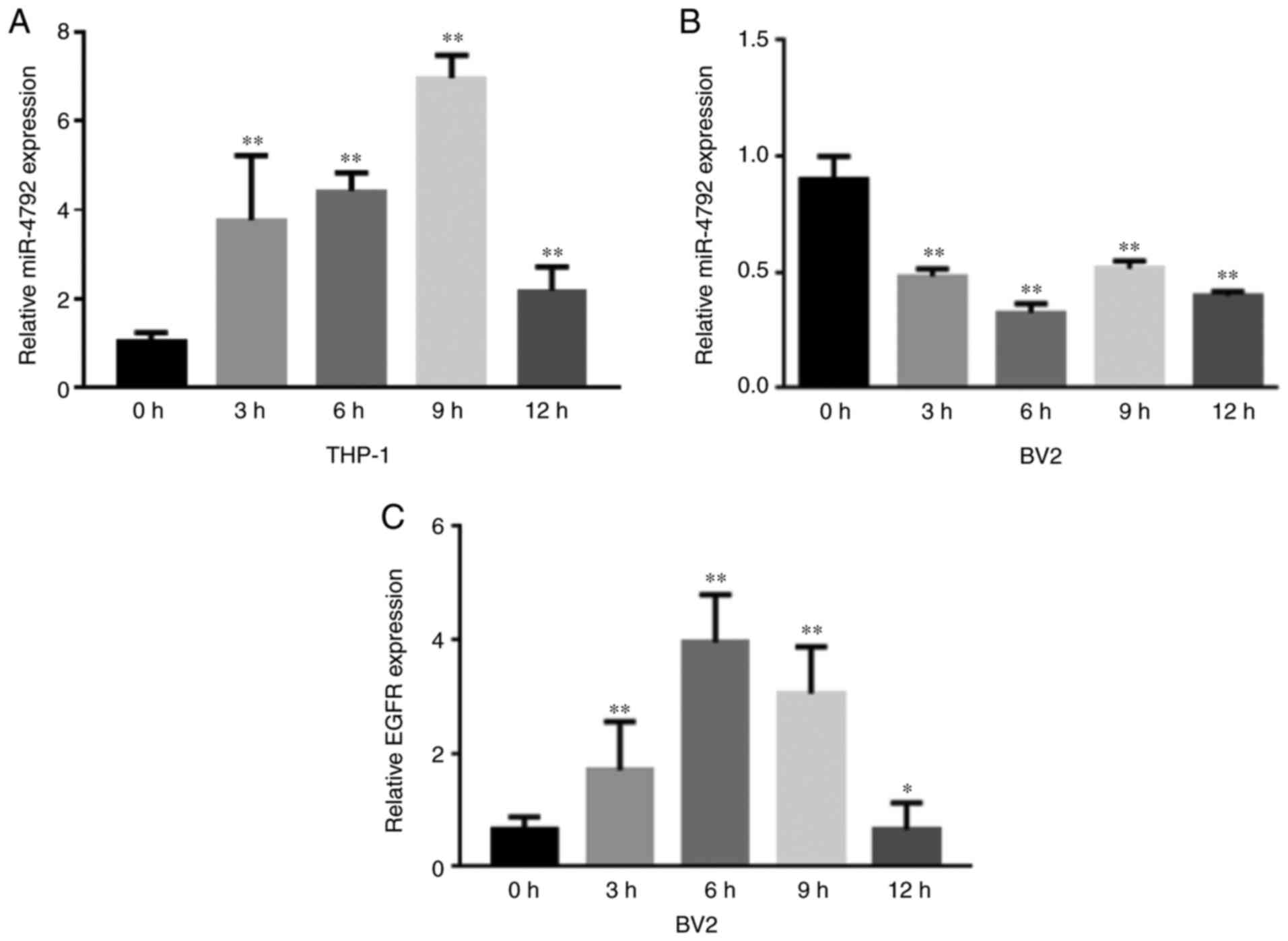
The preliminary data revealed that the expression of
miR-4792 increased in the THP-1 cells infected with C.
neoformans (WM148) (Fig.
1A). To further investigate the expression of miR-4792 in
microglia, BV2 cells were infected with WM148 for 0, 3, 6 and 9 and
12 h and RT-qPCR was performed to detect miR-4792 expression. It
was found that the expression of miR-4792 decreased over time,
reaching its lowest level after 6 h (Fig. 1B), whereas the expression of EGFR
gradually increased, peaking at 6 h (Fig. 1C). In a previous study, the
authors demonstrated that the secretion of the inflammatory
factors, TNF-α and IL-6, by microglia gradually increased over
time, whereas the IL-1β levels exhibit no obvious changes (31).
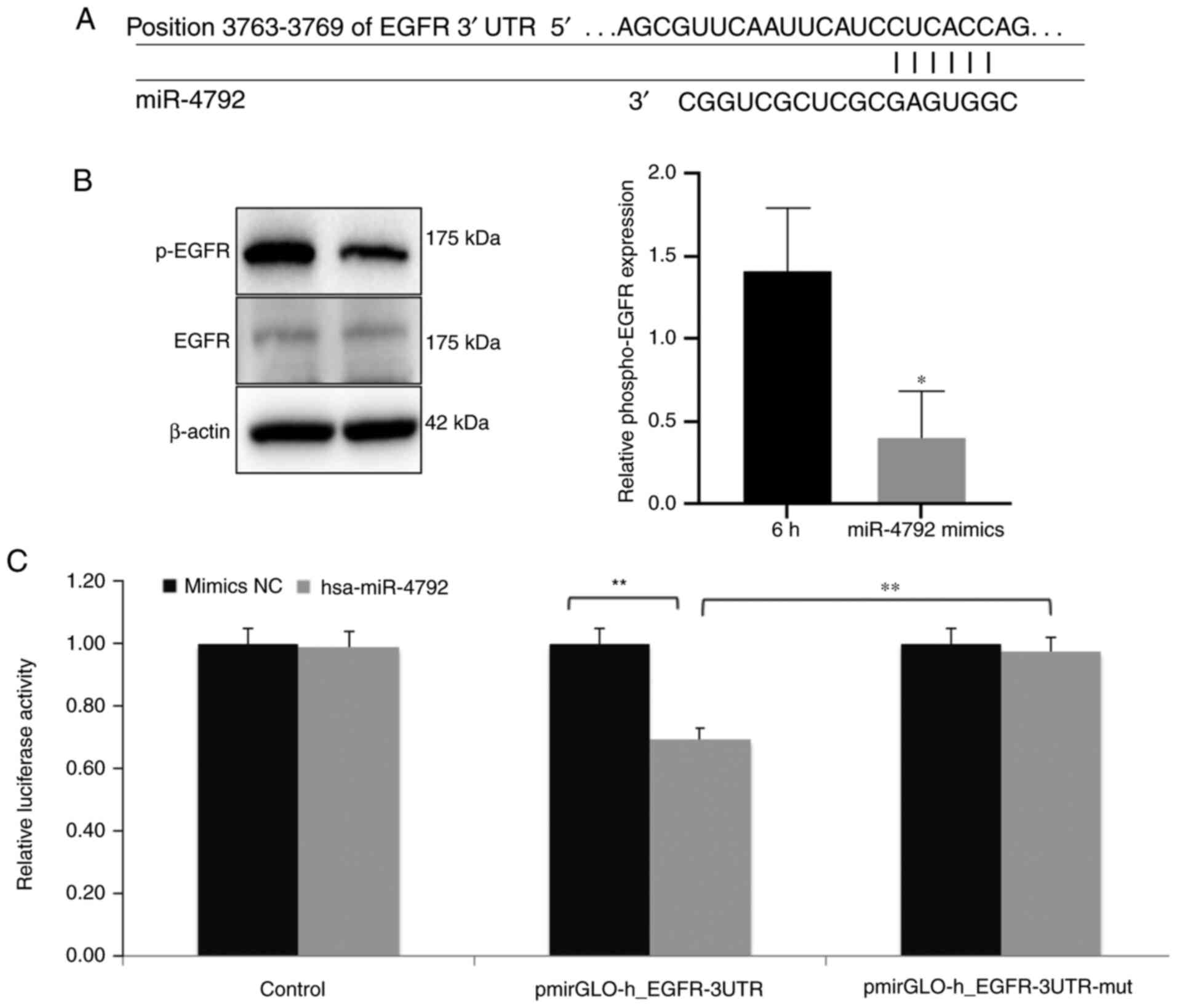
EGFR is targeted by miR-4792
To further define the molecular mechanisms governing
the regulatory effects of miR-4792, the bioinformatics tool,
miRanda (http://www.microrna.org) and TargetScan
(http://www.targetscan.org) were used to
investigate novel miR-4792 targets. These analyses revealed EGFR as
a miRNA target, the sequence of which was assumed to be in the
3′UTR (Fig. 2A). Additionally,
western blot analysis was performed to assess the phosphorylation
status of EGFR (p-EGFR) in the BV2 cells. It was found that
miR-4792 overexpression significantly reduced the p-EGFR levels
compared with the cells infected with WM148 for 6 h (Fig. 2B). Dual luciferase assays also
confirmed EGFR as a direct target of miR-4792. The exogenous
overexpression of miR-4792 significantly inhibited WT EGFR 3′UTR
activity, whereas it exerted minimal effects on the mutant EGFR
3′UTR sequence (Fig. 2C). These
data strongly indicate that EGFR is a direct target of
miR-4792.
EGFR blockade inhibits WM148-induced
microglial activation
Microglia regulate the innate immune responses of
the CNS (32,33). Given that microglia are
responsible for pro-inflammatory cytokine production (34), the present study examined the
effects of WM148 on microglial induction. Western blot analysis and
flow cytometry were performed to assess the cell surface expression
of CD11b and MHC II, considered phenotypic markers of microglial
activation (35). The data
demonstrated that following 6 h of WM148 infection, higher levels
of CD11b, MHC II and p-EGFR were observed in the infected BV2 cells
(Fig. 3A). By contrast, 24 h of
treatment with the EGFR inhibitor, AG1478, led to a loss of CD11b,
MHC II and p-EGFR expression [Fig.
3A (bottom panels) and B] confirmed by western blot analysis
and flow cytometry. Of note, miR-4792 expression was inversely
associated with the EGFR levels, further highlighting EGFR as a
target of miR-4792 (Fig.
3C).
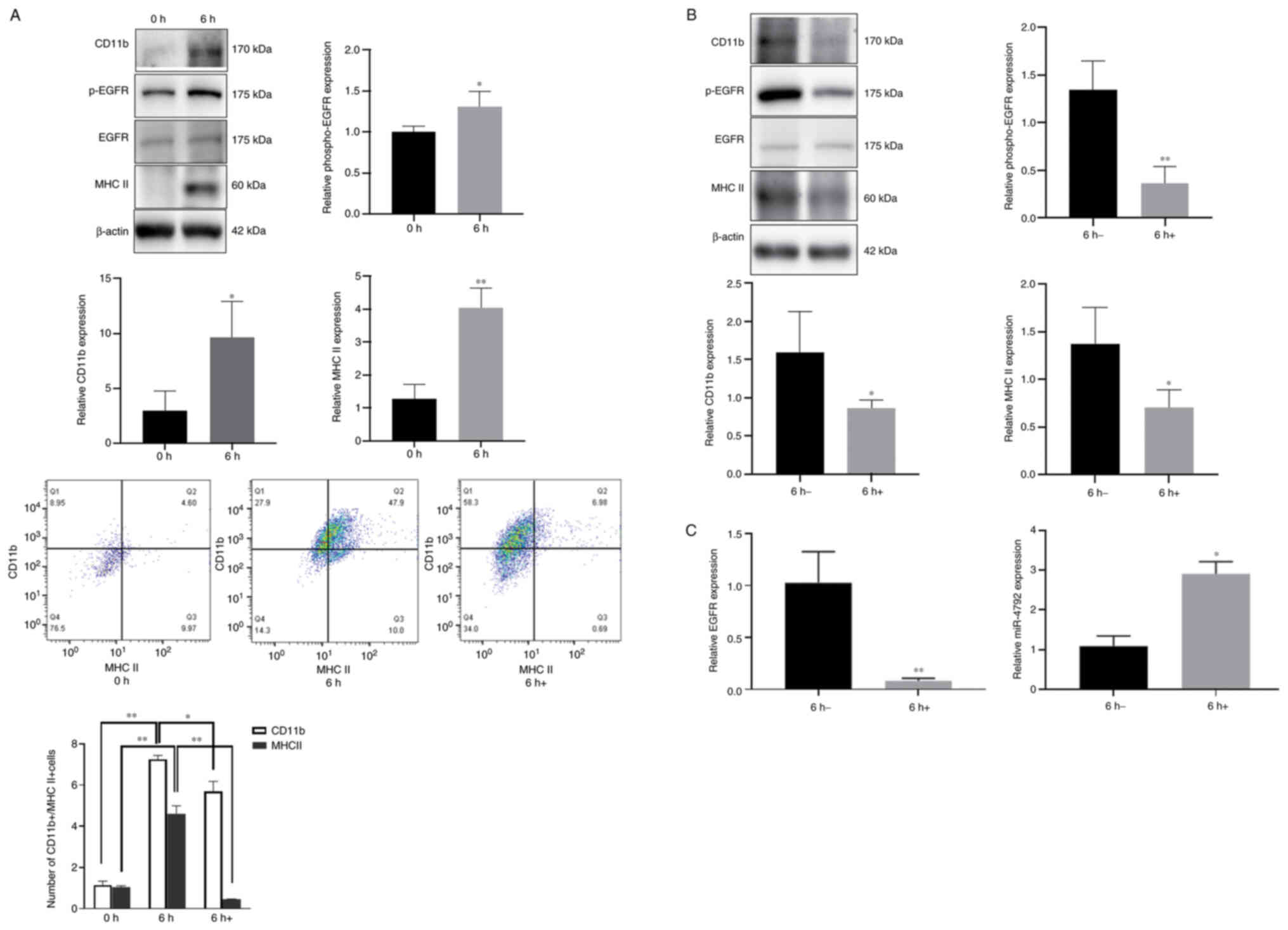 | Figure 3EGFR blockade inhibits WM148-induced
microglial activation, EGFR phosphorylation and increases miR-4792
expression. Purified microglia were infected with WM148 for 6 h
prior to treatment with 40 µm AG1478 for 24 h. (A) Western
blot analysis of BV2 cells revealed that WM148 upregulated CD11b,
MHC II and p-EGFR expression. Flow cytometry revealed that the
number of CD11b+/MHC II+ cells increased in
the presence of WM148, whereas it decreased following AG1478
stimulation. 6 h, represents BV2 cells were treated with WM148 for
6 h; 6 h+, represents BV2 cells were treated with WM148 and for 6 h
and then incubated with 40 µm AG1478 for 24 h. (B) Western
blot analysis showing that AG1478 suppresses CD11b, MHC II and
p-EGFR expression in WM148-infected BV2 cells. (C) RT-qPCR analysis
showing that AG1478 significantly suppressed EGFR expression,
whilst miR-4792 expression increased. 6 h-, represents cells
treated with WM148 for 6 h alone; 6 h+, represents cells treated
with WM148 for 6 h and incubated with 40 µm AG1478 for 24 h.
Data are the mean ± SD from three independent experiments.
*P<0.05; **P<0.01, vs. the 0 h
(control) or 6 h (WM148) group. |
Inhibition of EGFR prevents MAPK-mediated
cytokine production in BV2 cells
Activated microglia stimulate neuronal inflammation
and enhance the levels of pro-inflammatory cytokines, including
IL-1β and TNF-α in the CNS (36). ERK1/2, JNK and p38 MAPK are key
cell signaling cascades. MAPKs are known to enhance the production
and secretion of cytokines (37-39). For example, MAPK signaling
promotes the lipopolysaccharide-induced synthesis of IL-1β and
TNF-α (40). MAPK is also
stimulated following EGFR activation (41,42). Consistent with previous findings,
the present study found that AG1478 treatment inhibited MAPK
phosphorylation, leading to a loss of ERK1/2, p38 MAPK and JNK
activation in WM148-infected BV2 cells. In these cells, p-ERK1/2
and p-p38 MAPK levels were significantly decreased; however, no
significant differences were observed in the p-JNK levels (Fig. 4A). Similarly, AG1478 treatment
inhibited both IL-1β and TNF-α production in the WM148-infected BV2
cells (Fig. 4B). The presents
study then simultaneously assessed the secretion of other
inflammatory factors in WM148-infected BV2 cells. The results
revealed that the IL-1α, IL-12, eotaxin, GM-CSF and MCP-1/CCL2
levels decreased following AG1478 treatment compared with the
control group, amongst which the expression of MCP-1/CCL2
significantly decreased (Fig.
4C).
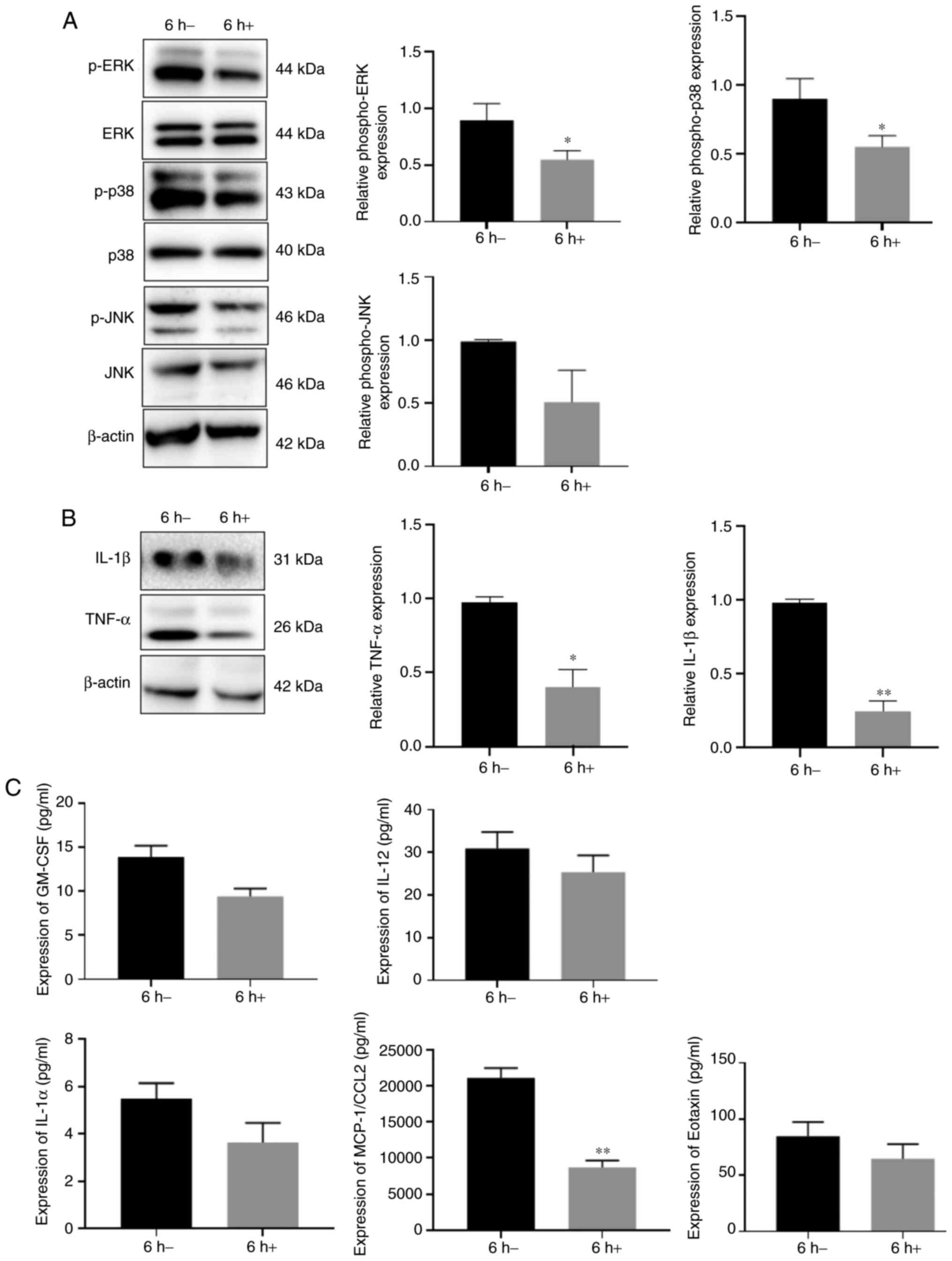 | Figure 4EGFR blockade suppresses EGFR/MAPK
activation and cytokine production. BV2 cells were treated with
WM148 for 6 h, followed by incubation with 40 µm AG1478 for
24 h. Western blot analysis of the phosphorylation status of ERK,
JNK, p38MAPK and cytokine production (IL-1β and TNFα). 6 h-,
represents cells treated with WM148 alone; 6 h+, represents cells
treated with WM148 for 6 h and incubated with 40 µm AG1478
for 24 h. (A) p-ERK and p-p38 levels are significantly decreased by
AG1478, whilst p-JNK levels were not significantly affected (B)
Decreased expression of TNF-α and IL-1β. (C) Expression of IL-1α
(P=0.13), IL-12 (P=0.29), eotaxin (P=0.25), GM-CSF (P=0.055) and
MCP-1/CCL2 (P=0.009) compared to WM148 treatment, amongst which the
expression of MCP-1/CCL2 significantly decreased. Data are the mean
± SD from three independent experiments. *P<0.05;
**P<0.01, vs. the 6 h-group. |
miR-4792 regulates the EGFR/MAPK axis in
BV2 cells
The present study then examined the effects of the
miR-4792-mediated regulation of EGFR on ERK1/2, JNK and p38 MAPK
signaling in BV2 cells infected with WM148. As shown in Figs. 2B and 5A (top and bottom panels), the levels
of active p-EGFR, p-ERK1/2 and p-p38 significantly decreased in the
presence of miR-4792 mimics compared with the WM148 group, whilst
no significant differences in CD11b, MHC II and p-JNK levels were
observed, as shown by western blot analysis. Flow cytometry
revealed that the number of CD11b+/MHC II+
cells decreased in the presence of miR-4792 mimics compared with
the WM148 group, whilst no significant differences were observed in
the CD11b levels (Fig. 5A, top
and middle panels). The levels of the inflammatory cytokines, IL-1β
and TNF-α, also decreased significantly (Fig. 5B). These findings were confirmed
by RT-qPCR analysis, which revealed that the expression of miR-4792
significantly increased, and EGFR expression decreased without
significance following transfection with miR-4792 mimics for 24 h.
Opposite results were observed following transfection with miR-4792
inhibitors (Fig. 5C). A previous
study (12) by the authors
demonstrated that p-EGFR was highly expressed, whilst miR-4792 was
expressed at low levels following WM148 infection (Fig. 1). Furthermore, the secretion of
other inflammatory factors by WM148-infected BV2 cells was
assessed. following transfection with miR-4792 mimics for 24 h, the
levels of pro-inflammatory factors, including IL-1α, IL-12,
eotaxin, GM-CSF and MCP-1/CCL2 decreased, amongst which the
decrease of GM-CSF and MCP-1/CCL2 were significant (Fig. 5D). Taken together, these data
highlight that in BV2 cells, miR-4792 regulates EGFR/MAPK signaling
following WM148 infection and partially alleviates MAPK-mediated
inflammatory responses, thus affecting the production of
pro-inflammatory factors following WM148 infection.
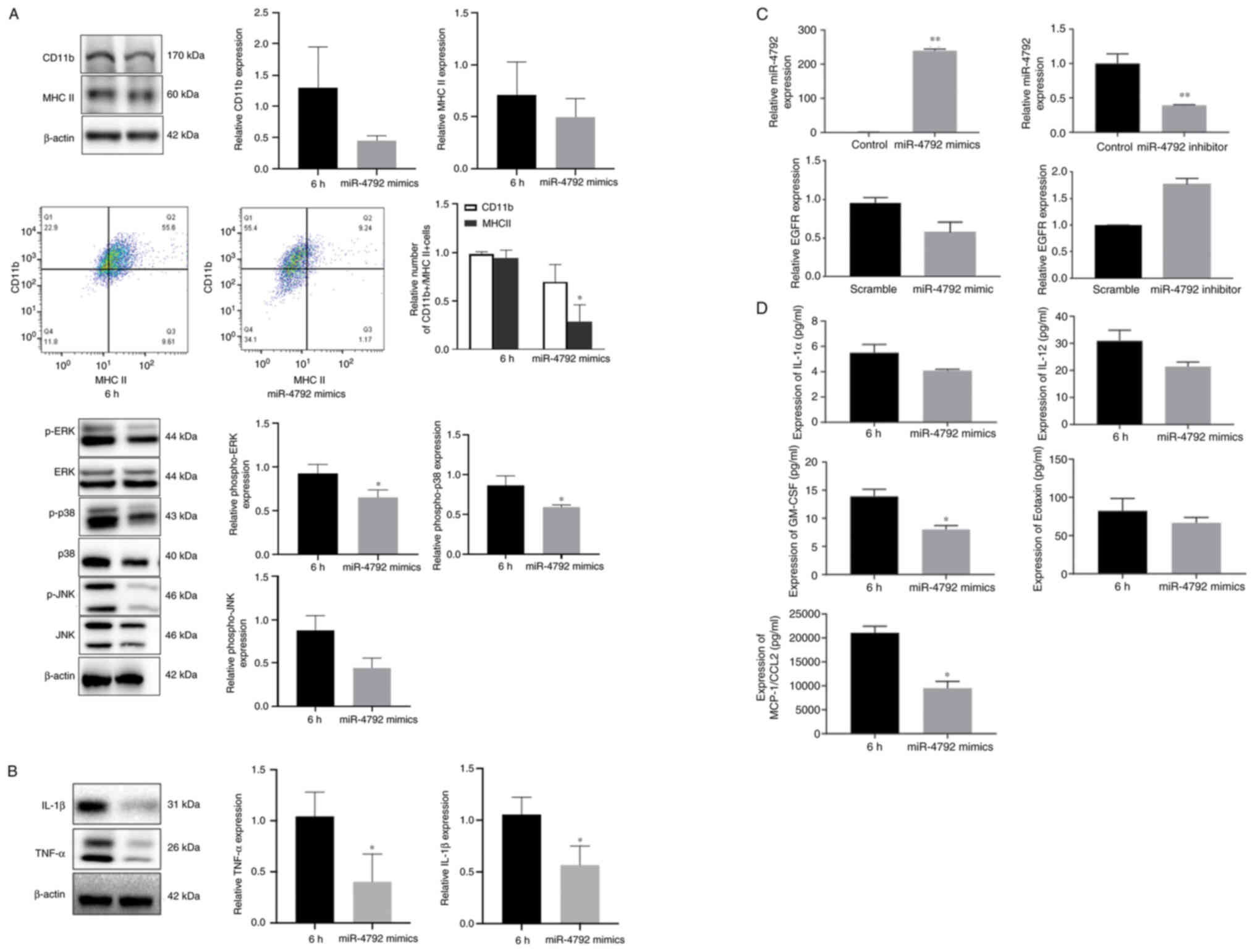 | Figure 5miR-4792 mimics suppress EGFR/MAPK
activation and cytokine production. BV2 cells were treated with
WM148 for 6 h, followed by incubation with 50 nM miR-4792 mimics
for 24 h. Western blot analysis was performed to assess the
phosphorylation status of ERK, JNK and p38 and cytokine production
(IL-1β and TNFα). 6 h, treated with WM148 alone; miR-4792 mimics,
transfected with WM148 for 6 h and 50 nM miR-4792 mimics for 24 h.
(A) Western blot analysis of BV2 cells showing that miR-4792 mimics
suppressed the phosphorylation of ERK and p38 following WM148
induction, whilst the expression of CD11b, MHC II and p-JNK
exhibited no significant differences. Flow cytometry showing that
the number of CD11b+/MHC II+ cells decreases
in the presence of miR-4792 mimics compared with the WM148 group,
whilst no significant differences were observed in the CD11b
levels. (B) Western blot analysis of BV2 cells showing that
miR-4792 mimics suppressed the levels of the inflammatory
cytokines, IL-1β and TNFα. (C) RT-qPCR confirming the effects of
miR-4792 mimics/inhibitors on miR-4792 expression, whilst the
expression of EGFR did not significantly differ from the control
group. (D) Quantification of cytokines showing that miR-4792 mimics
suppressed the expression of IL-1α (P=0.29), IL-12 (P=0.087),
eotaxin (P=0.33), GM-CSF (P=0.028) and MCP-1/CCL2 (P=0.014)
compared with WM148 alone, amongst which the expression of GM-CSF
and MCP-1/CCL2 significantly decreased. Data are the mean ± SD from
three independent experiments. *P<0.05;
**P<0.01, vs. the respective control group. |
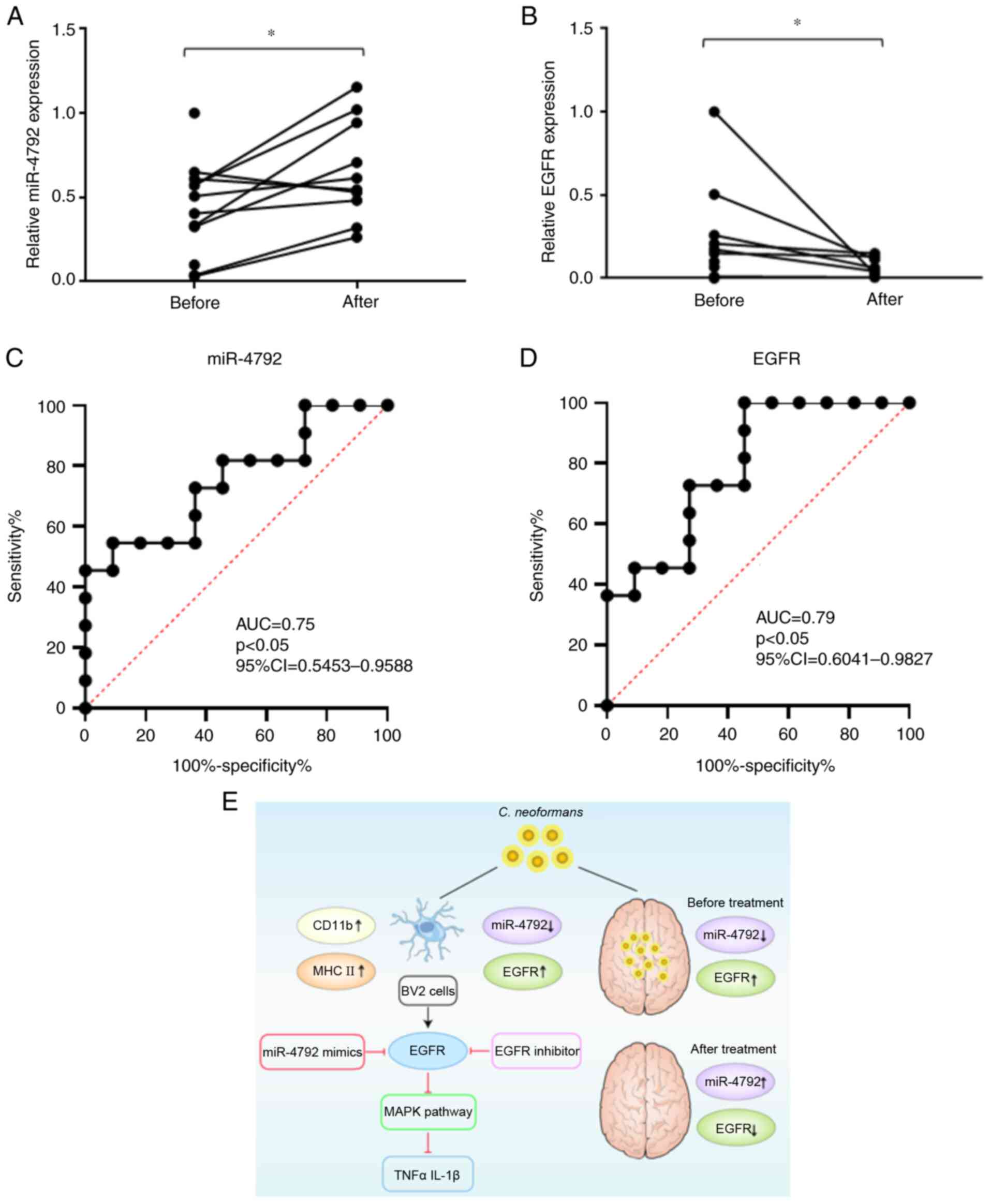
Expression of miR-4792 in patients with
CM before and after treatment
CSF was collected from patients with CM before and
after regular antifungal treatment. The results revealed that the
expression of miR-4792 in the CSF significantly increased following
treatment (Fig. 6A). ROC curve
analysis revealed an area under the curve (AUC) value of 0.75 (95%
CI, 0.54-0.96) (Fig. 6C). The
expression of EGFR in the CSF also significantly decreased
following treatment (Fig. 6B).
ROC curve analysis revealed AUCs of 0.79 (95% CI, 0.6-0.98)
(Fig. 6D). These data confirmed
the reciprocal association between miR-4792 and EGFR in vivo
during fungal infection and demonstrated that the CSF levels of
miR-4792, highlighting EGFR as a useful biomarker when assessing
the treatment efficacy of patients with CM. Fig. 6E showed the proposed model of the
regulatory and functional role for miR-4792 and EGFR in the process
of C. neoformans infecting BV2 cells.
Discussion
Cryptococcosis is a life-threatening fungal
infection, the common clinical manifestation of which is CM. The
time from onset to diagnosis is relatively long, with an average of
172.9 days, and an observed mortality of 57.1% (43). CM diagnostics have rapidly
improved over the past 10 years due to advancements being made in
point-of-care testing that are highly specific, sensitive, accurate
and capable of data production within 10 min of test initiation
(https://www.immy.com/crag; https://www.biosynex.com/flyers/pro/mycologie/en/cryptops.pdf).
Despite these diagnostic advances, the discovery of effective
anti-fungal agents has stalled. The results of the present study
provide a perspective and theoretical basis for the control of CNS
inflammation following cryptococcus infection. miRNAs protect the
host from infection by regulating key genes involved in host immune
defenses amongst different phenotypes of macrophages and immune
responses (44,45). In vitro, macrophage
phagocytosis is directly associated with clinical outcome (46,47). As previously demonstrated,
following C. neoformans exposure in human monocytic THP-1
cells, miR-146a is upregulated and inhibits NF-κB activation and
inflammatory cytokine release (12). miRNAs function in concert to
induce host defenses and are frequently dysregulated in an array of
disease models (23-25,31), including following exposure to
fungal pathogens. As such, miRNAs serve as novel biomarkers for
fungal infections.
In the present study, in BV2 cells, exposure to
WM148 led to a significant upregulation of IL-1β, TNF-α and IL-6
expression levels. Pro-inflammatory cytokines are key mediators of
neuroinflammation in a number of CNS pathologies, highlighting the
ability of C. neoformans to induce a neuroinflammatory
phenotype (32,48-50). BV2 cells are RAF/Myc immortalized
murine neonatal microglia models and act as surrogates for primary
microglia (51). The suitability
of BV2 microglia as an alternative model system has been previously
established (51,52). These in vitro data further
highlight the inflammatory responses induced by C.
neoformans.
Neuroinflammation is the coordinated response of the
CNS to threats posed by a variety of conditions, including
pathogens and trauma. Responses are mediated by the production of
cytokines, chemokines, reactive oxygen species and secondary
messengers. These mediators are produced by resident CNS glia
(microglia and astrocytes), endothelial cells and peripherally
derived immune cells. Initially, inflammation is defined and
determined by the release of pro-inflammatory cytokines, such as
TNF-α, IL-1β and adhesion molecules. IL-1β and TNF-α play an
integral role in pathological inflammation and the acceleration of
disease (49). Microglia are key
effector cells in the host defense to microbial infections and act
as antigen presenting cells that can produce active substances that
promote inflammatory cell death (53,54). Following C. neoformans
exposure, microglia produce TNF-α, IL-1β and IL-6 to upregulate MHC
class II and CD11c expression (55,56). Moreover, microglia can
phagocytose C. neoformans and upregulate inducible nitric
oxide synthase (iNOS) levels with anti-fungal effects that are
dependent on G protein-coupled receptor 43 (GPR43) expression
(57-60). Despite the phagocytic
capabilities of microglia, they are unable to destroy yeast cells
and remain susceptible to latent intracellular infections (60,61). Microglia are also activated in
response to injury in which cell-surface CD11b is a typical
phenotypic marker (35).
Microglia continually survey the microenvironment for noxious
agents and injury, and respond to extracellular signals to clear
debris and toxic substances, and secret trophic factors, providing
neuroprotection. Increasing evidence implicates microglial
activation as a major cause of CNS inflammation, the suppression of
which reduces tissue damage and morphological alterations (62-64). This highlights microglia as
critical for analysis during C. neoformans infection.
In the present study, the role for EGFR/MAPK
signaling in WM148-mediated inflammation in the CNS was confirmed.
EGFR is expressed in astrocytes, neurons oligodendrocytes and
microglia (65,66). The inhibition of EGFR/MAPK
signaling prevents microglial inflammatory responses by attenuating
Ras/Raf/MAPK signaling (65).
MAPK activation is essential for the production of inflammatory
cytokines, including IL-1β, TNF-α and IL-6, and regulates cell
survival, differentiation and proliferation through its effects on
gene expression (37,67). Oral epithelial cells infected
with Candida albicans activate three MAPK subfamilies and
enhance the production of inflammatory mediators (68). Sporothrix schenckii yeast
induces the robust activation of JNK, ERK1/2 and p38 MAPK in
dendritic cells, which is related to IL-6 and TNF-α secretion
(69). Recent study demonstrated
that miR-4792 participates in the apoptotic induction of A549 cells
through RTHF via the MAPK pathway and determines the apoptotic
mechanism (70). In the present
study, it was found that miR-4792 mimics/inhibitors did not
significantly influence EGFR expression at the transcript level,
suggestive of translational regulation. It was found that the
inhibition of EGFR led to the inhibition of downstream MAPK
signaling, preventing microglial activation and inflammatory
cytokine production. Taken together, these data highlight the
importance of EGFR and its effects on MAPK signaling and
pro-inflammatory factors during the microglial inflammatory
response to C. neoformans.
Exposure to dangerous pathogenic fungal infections
poses a risk to human health. Enhancing the current understanding
of the mechanisms governing the host innate immune response to
pathogenic infections can lead to the discovery of novel
anti-fungal agents. Previous studies have solely focused on the
endpoint of the host response to fungal exposure (68,69); however, the specific molecular
mechanisms underlying these responses differ for fungal species.
Although an array of studies has investigated the pulmonary
immunological response following both chronic and acute exposure to
lethal fungal spores (71-73), the miRNAs that mediate these
responses/deficiencies are poorly characterized. In the present
study, using up-to-date models and the assessment of miR-4792 and
EGFR in clinical samples, the role of miR-4792 and EGFR in the host
immune response to fungal exposure was specifically analyzed. The
data provide a theoretical basis for the future development of
anti-fungal immune therapeutic regimens and permit the
identification of at-risk populations, enabling targeted treatments
to those deemed most at risk, providing a novel methodology for the
assessment of the treatment efficacy for patients with CM.
Following effective treatment, the expression of miR-4792 CSF
increases, whilst EGFR expression decreases, which can be used as
an effective index to judge whether patients can be discharged from
hospital. However, the present study had certain limitations. For
example, human primary microglia are difficult to culture in
vitro; thus, BV2 cells were used as an alternative. As miRNAs
are highly conserved, the results may guide future human
assessments. Further studies on the role of miR-4792 in
cryptococcal meningitis animal models are required however, through
its intramedullary injection using liposomes or exosomes.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC, ZW and YJ designed the present study. GY, XW and
YaW performed the experiments. QH, RG and LT analyzed the data and
prepared the figures. GY and YiW drafted the initial manuscript.
YiW, ZW and WL discussed and interpreted the results, commented on
the manuscript and revised the manuscript. GY and JC confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Changzheng Hospital and written consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (NSFC; grant no. 81772158).
References
|
1
|
Pappas PG: Cryptococcal infections in
non-HIV-infected patients. Trans Am Clin Climatol Assoc. 124:61–79.
2013.PubMed/NCBI
|
|
2
|
Rajasingham R, Smith RM, Park BJ, Jarvis
JN, Govender NP, Chiller TM, Denning DW, Loyse A and Boulware DR:
Global burden of disease of HIV-associated cryptococcal meningitis:
An updated analysis. Lancet Infect Dis. 17:873–881. 2017.
View Article : Google Scholar
|
|
3
|
Wu B, Liu H, Huang J, Zhang W and Zhang T:
Pulmonary cryptococcosis in non-AIDS patients. Clin Invest Med.
32:E70–E77. 2009. View Article : Google Scholar
|
|
4
|
Kishi K, Homma S, Kurosaki A, Kohno T,
Motoi N and Yoshimura K: Clinical features and high-resolution CT
findings of pulmonary cryptococcosis in non-AIDS patients. Respir
Med. 100:807–812. 2006. View Article : Google Scholar
|
|
5
|
Chang YC, Stins MF, McCaffery MJ, Miller
GF, Pare DR, Dam T, Paul-Satyaseela M, Kim KS and Kwon-Chung KJ:
Cryptococcal yeast cells invade the central nervous system via
transcellular penetration of the blood-brain barrier. Infect Immun.
72:4985–4995. 2004. View Article : Google Scholar
|
|
6
|
Ngamskulrungroj P, Chang Y, Sionov E and
Kwon-Chung KJ: The primary target organ of Cryptococcus gattii is
different from that of Cryptococcus neoformans in a murine model.
mBio. 3:e00103–e00112. 2012. View Article : Google Scholar
|
|
7
|
Chang YC, Bien CM, Lee H, Espenshade PJ
and Kwon-Chung KJ: Sre1p, a regulator of oxygen sensing and sterol
homeostasis, is required for virulence in Cryptococcus neoformans.
Mol Microbiol. 64:614–629. 2007. View Article : Google Scholar
|
|
8
|
Ribes S, Ebert S, Regen T, Agarwal A,
Tauber SC, Czesnik D, Spreer A, Bunkowski S, Eiffert H, Hanisch UK,
et al: Toll-like receptor stimulation enhances phagocytosis and
intracellular killing of nonencapsulated and encapsulated
Streptococcus pneumoniae by murine microglia. Infect Immun.
78:865–871. 2010. View Article : Google Scholar
|
|
9
|
Redlich S, Ribes S, Schütze S, Eiffert H
and Nau R: Toll-like receptor stimulation increases phagocytosis of
Cryptococcus neoformans by microglial cells. J Neuroinflammation.
10:712013. View Article : Google Scholar
|
|
10
|
Gebert L and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar
|
|
11
|
Rupaimoole R and Frank J: Slack MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar
|
|
12
|
Chen H, Jin Y, Chen H, Liao N, Wang Y and
Chen J: MicroRNA-mediated inflammatory responses induced by
Cryptococcus neoformans are dependent on the NF-κB pathway in human
monocytes. Int J Mol Med. 39:1525–1532. 2017. View Article : Google Scholar
|
|
13
|
De Lacorte Singulani J, De Fátima Da Silva
J, Gullo FP, Costa MC, Fusco-Almeida AM, Enguita FJ and
Mendes-Giannini MJS: Preliminary evaluation of circulating
microRNAs as potential biomarkers in paracoccidioidomycosis. Biomed
Rep. 6:353–357. 2017. View Article : Google Scholar
|
|
14
|
Kumar M, Ahmad T, Sharma A, Mabalirajan U,
Kulshreshtha A, Agrawal A and Ghosh B: Let-7 microRNA-mediated
regulation of IL-13 and allergic airway inflammation. J Allergy
Clin Immunol. 128:1077–1085. 2011. View Article : Google Scholar
|
|
15
|
Essandoh K, Li Y, Huo J and Fan GC:
miRNA-mediated macrophage polarization and its potential role in
the regulation of inflammatory response. Shock. 46:122–131. 2016.
View Article : Google Scholar
|
|
16
|
Nahid MA, Yao B, Dominguez-Gutierrez PR,
Kesavalu L, Satoh M and Chan EK: Regulation of TLR2-mediated
tolerance and cross-tolerance through IRAK4 modulation by miR-132
and miR-212. J Immunol. 190:1250–1263. 2013. View Article : Google Scholar
|
|
17
|
Testa U, Pelosi E, Castelli G and Labbaye
C: miR-146 and miR-155: two key modulators of immune response and
tumor development. Noncoding RNA. 3:22201.
|
|
18
|
Li Y and Chen X: miR-4792 inhibits
epithelial-mesenchymal transition and invasion in nasopharyngeal
carcinoma by targeting FOXC1. Biochem Biophys Res Commun.
468:863–869. 2015. View Article : Google Scholar
|
|
19
|
Georgieva B, Milev I, Minkov I, Dimitrova
I, Bradford AP and Baev V: Characterization of the uterine
leiomyoma microR-NAome by deep sequencing. Genomics. 99:275–281.
2012. View Article : Google Scholar
|
|
20
|
Chickooree D, Zhu K, Ram V, Wu HJ, He ZJ
and Zhang S: A preliminary microarray assay of the miRNA expression
signatures in buccal mucosa of oral submucous fibrosis patients. J
Oral Pathol Med. 45:691–697. 2016. View Article : Google Scholar
|
|
21
|
Gabrusiewicz K, Rodriguez B, Wei J,
Hashimoto Y, Healy LM, Maiti SN, Thomas G, Zhou S, Wang Q, Elakkad
A, et al: Glioblastoma-infiltrated innate immune cells resemble M0
macrophage phenotype. JCI Insight. 1:e858412016. View Article : Google Scholar
|
|
22
|
Li CC, Eaton SA, Young PE, Lee M,
Shuttleworth R, Humphreys DT, Grau GE, Combes V, Bebawy M, Gong J,
et al: Glioma microvesicles carry selectively packaged coding and
non-coding RNAs which alter gene expression in recipient cells. RNA
Biol. 10:1333–1344. 2013. View Article : Google Scholar
|
|
23
|
Liu G, Jiang C, Li D, Wang R and Wang W:
MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in
gastric cancer. Tumor Biol. 35:9801–9806. 2014. View Article : Google Scholar
|
|
24
|
Chen S, Zhang Z, Chen L and Zhang J: miRNA
101 3p.1 as an independent diagnostic biomarker aggravates chronic
obstructive pulmonary disease via activation of the EGFR/PI3K/AKT
signaling pathway. Mol Med Rep. 20:4293–4302. 2019.PubMed/NCBI
|
|
25
|
Ma HP, Kong WX, Li XY, Li W, Zhang Y and
Wu Y: miRNA-223 is an anticancer gene in human non-small cell lung
cancer through the PI3K/AKT pathway by targeting EGFR. Oncol Rep.
41:1549–1559. 2019.PubMed/NCBI
|
|
26
|
Williamson PR: The relentless march of
cryptococcal meningitis. Lancet Infect Dis. 17:790–791. 2017.
View Article : Google Scholar
|
|
27
|
Bocchini V, Mazzolla R, Barluzzi R, Blasi
E, Sick P and Kettenmann H: An immortalized cell line expresses
properties of activated microglial cells. J Neurosci Res.
31:616–621. 1992. View Article : Google Scholar
|
|
28
|
Sheng W, Zong Y, Mohammad A, Ajit D, Cui
J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, et al:
Pro-inflammatory cytokines and lipopolysaccharide induce changes in
cell morphology, and upregulation of ERK1/2, iNOS and sPLA2-IIA
expression in astrocytes and microglia. J Neuroinflammation.
8:1212011. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Saag MS, Graybill RJ, Larsen RA, Pappas
PG, Perfect JR, Powderly WG, Sobel JD and Dismukes WE: Practice
guidelines for the management of cryptococcal disease. Infectious
diseases society of America. Clin Infect Dis. 30:710–718. 2000.
View Article : Google Scholar
|
|
31
|
Jin Y, Yao G, Wang Y, Teng L, Wang Y, Chen
H, Gao R, Lin W, Wang Z and Chen J: MiR-30c-5p mediates
inflammatory responses and promotes microglia survival by targeting
eIF2α during Cryptococcus neoformans infection. Microb Pathog.
141:1039592020. View Article : Google Scholar
|
|
32
|
Wang WY, Tan MS, Yu JT and Tan L: Role of
pro-inflammatory cytokines released from microglia in Alzheimer's
disease. Ann Transl Med. 3:1362015.
|
|
33
|
Jin X and Yamashita T: Microglia in
central nervous system repair after injury. J Biochem. 159:491–496.
2016. View Article : Google Scholar
|
|
34
|
Kraft AD and Harry GJ: Features of
microglia and neuroinflammation relevant to environmental exposure
and neurotoxicity. Int J Environ Res Public Health. 8:2980–3018.
2011. View Article : Google Scholar
|
|
35
|
O'Sullivan JB, Ryan KM, Curtin NM, Harkin
A and Connor TJ: Noradrenaline reuptake inhibitors limit
neuroinflammation in rat cortex following a systemic inflammatory
challenge: Implications for depression and neurodegeneration. Int J
Neuropsychopharmacol. 12:687–699. 2009. View Article : Google Scholar
|
|
36
|
Loane DJ and Byrnes KR: Role of microglia
in neurotrauma. Neurotherapeutics. 7:366–377. 2010. View Article : Google Scholar
|
|
37
|
Bachstetter AD, Xing B, de Almeida L,
Dimayuga ER, Watterson DM and Van Eldik LJ: Microglial p38alpha
MAPK is a key regulator of proinflammatory cytokine up-regulation
induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ). J
Neuroinflammation. 8:792011. View Article : Google Scholar
|
|
38
|
Xu L, Huang Y, Yu X, Yue J, Yang N and Zuo
P: The influence of p38 mitogen-activated protein kinase inhibitor
on synthesis of inflammatory cytokine tumor necrosis factor alpha
in spinal cord of rats with chronic constriction injury. Anesth
Analg. 105:1838–1844. 2007. View Article : Google Scholar
|
|
39
|
Kim SH, Smith CJ and Van Eldik LJ:
Importance of MAPK pathways for microglial pro-inflammatory
cytokine IL-1 beta production. Neurobiol Aging. 25:431–439. 2004.
View Article : Google Scholar
|
|
40
|
Qu WS, Tian DS, Guo ZB, Fang J, Zhang Q,
Yu ZY, Xie MJ, Zhang HQ, Lü JG and Wang W: Inhibition of EGFR/MAPK
signaling reduces microglial inflammatory response and the
associated secondary damage in rats after spinal cord injury. J
Neuroinflammation. 9:1782012. View Article : Google Scholar
|
|
41
|
Yamauchi T, Ueki K, Tobe K, Tamemoto H,
Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, et
al: Growth hormone-induced tyrosine phosphorylation of EGF receptor
as an essential element leading to MAP kinase activation and gene
expression. Endocr J. 45(Suppl): S27–S31. 1998. View Article : Google Scholar
|
|
42
|
Goel S, Hidalgo M and Perez-Soler R: EGFR
inhibitor-mediated apoptosis in solid tumors. J Exp Ther Oncol.
6:305–320. 2007.PubMed/NCBI
|
|
43
|
Pinheiro SB, Sousa ES, Cortez ACA, da
Silva Rocha DF, Menescal LSF, Chagas VS, Gómez ASP, Cruz KS, Santos
LO, Alves MJ, et al: Cryptococcal meningitis in non-HIV patients in
the State of Amazonas, Northern Brazil. J Microbiol. 52:279–288.
2021.
|
|
44
|
Martinez-Nunez RT, Louafi F and
Sanchez-Elsner T: The interleukin 13 (IL-13) pathway in human
macrophages is modulated by microRNA-155 via direct targeting of
interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem.
286:1786–1794. 2011. View Article : Google Scholar
|
|
45
|
Roy S: miRNA in macrophage development and
function. Antioxid Redox Signal. 25:795–804. 2016. View Article : Google Scholar
|
|
46
|
Sabiiti W, Robertson E, Beale MA, Johnston
SA, Brouwer AE, Loyse A, Jarvis JN, Gilbert AS, Fisher MC, Harrison
TS, et al: Efficient phagocytosis and laccase activity affect the
outcome of HIV-associated cryptococcosis. J Clin Invest.
124:2000–2008. 2014. View Article : Google Scholar
|
|
47
|
Alanio A, Desnos-Ollivier M and Dromer F:
Dynamics of Cryptococcus neoformans-macrophage interactions reveal
that fungal background influences outcome during cryptococcal
meningoencephalitis in humans. mBio. 2:e00158–e00111. 2011.
View Article : Google Scholar
|
|
48
|
Ji RR, Nackley A, Huh Y, Terrando N and
Maixner W: Neuroinflammation and central sensitization in chronic
and widespread pain. Anesthesiology. 129:343–366. 2018. View Article : Google Scholar
|
|
49
|
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP
and Ma D: Neuroinflammation: The role and consequences. Neurosci
Res. 79:1–12. 2014. View Article : Google Scholar
|
|
50
|
Kim YK, Na KS, Myint AM and Leonard BE:
The role of pro-inflammatory cytokines in neuroinflammation,
neurogenesis and the neuroendocrine system in major depression.
Prog Neuropsychopharmacol Biol Psychiatry. 64:277–284. 2016.
View Article : Google Scholar
|
|
51
|
Henn A, Lund S, Hedtjärn M, Schrattenholz
A, Pörzgen P and Leist M: The suitability of BV2 cells as
alternative model system for primary microglia cultures or for
animal experiments examining brain inflammation. ALTEX. 26:83–94.
2009. View Article : Google Scholar
|
|
52
|
Stansley B, Post J and Hensley K: A
comparative review of cell culture systems for the study of
microglial biology in Alzheimer's disease. J Neuroinflammation.
9:1152012. View Article : Google Scholar
|
|
53
|
Kofler J and Wiley CA: Microglia: Key
innate immune cells of the brain. Toxicol Pathol. 39:103–114. 2011.
View Article : Google Scholar
|
|
54
|
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran
M, Lokensgard JR and Peterson PK: Role of microglia in central
nervous system infections. Clin Microbiol Rev. 17:942–964. 2004.
View Article : Google Scholar
|
|
55
|
Barluzzi R, Brozzetti A, Delfino D,
Bistoni F and Blasi E: Role of the capsule in microglial
cell-Cryptococcus neoformans interaction: Impairment of antifungal
activity but not of secretory functions. Med Mycol. 36:189–197.
1998.
|
|
56
|
Neal LM, Xing E, Xu J, Kolbe JL,
Osterholzer JJ, Segal BM, Williamson PR and Olszewski MA:
CD4+T cells orchestrate lethal immune pathology despite
fungal clearance during Cryptococcus neoformans
meningoencephalitis. mBio. 11:e01415–e01417. 2017.
|
|
57
|
Adami C, Sorci G, Blasi E, Agneletti AL,
Bistoni F and Donato R: S100B expression in and effects on
microglia. Glia. 33:131–142. 2001. View Article : Google Scholar
|
|
58
|
Song X, Tanaka S, Cox D and Lee SC: Fc
gamma receptor signaling in primary human microglia: Differential
roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and
chemokine induction. J Leukoc Biol. 75:1147–1155. 2004. View Article : Google Scholar
|
|
59
|
Preissler J, Grosche A, Lede V, Le Duc D,
Krügel K, Matyash V, Szulzewsky F, Kallendrusch S, Immig K,
Kettenmann H, et al: Altered microglial phagocytosis in
GPR34-deficient mice. Glia. 63:206–215. 2015. View Article : Google Scholar
|
|
60
|
Lee SC, Kress Y, Dickson DW and Casadevall
A: Human microglia mediate anti-Cryptococcus neoformans activity in
the presence of specific antibody. J Neuroimmunol. 62:43–52. 1995.
View Article : Google Scholar
|
|
61
|
Lee SC, Kress Y, Zhao ML, Dickson DW and
Casadevall A: Cryptococcus neoformans survive and replicate in
human microglia. Lab Invest. 73:871–879. 1995.PubMed/NCBI
|
|
62
|
Popovich PG, Guan Z, Wei P, Huitinga I,
van Rooijen N and Stokes BT: Depletion of hematogenous macrophages
promotes partial hindlimb recovery and neuroanatomical repair after
experimental spinal cord injury. Exp Neurol. 158:351–365. 1999.
View Article : Google Scholar
|
|
63
|
Stirling DP, Khodarahmi K, Liu J, McPhail
LT, McBride CB, Steeves JD, Ramer MS and Tetzlaff W: Minocycline
treatment reduces delayed oligodendrocyte death, attenuates axonal
dieback, and improves functional outcome after spinal cord injury.
J Neurosci. 24:2182–2190. 2004. View Article : Google Scholar
|
|
64
|
Tian DS, Xie MJ, Yu ZY, Zhang Q, Wang YH,
Chen B, Chen C and Wang W: Cell cycle inhibition attenuates
microglia induced inflammatory response and alleviates neuronal
cell death after spinal cord injury in rats. Brain Res.
1135:177–185. 2007. View Article : Google Scholar
|
|
65
|
Gomez-Pinilla F, Knauer DJ and
Nieto-Sampedro M: Epidermal growth factor receptor immunoreactivity
in rat brain. Development and cellular localization. Brain Res.
438:385–390. 1988. View Article : Google Scholar
|
|
66
|
Erschbamer M, Pernold K and Olson L:
Inhibiting epidermal growth factor receptor improves structural,
locomotor, sensory, and bladder recovery from experimental spinal
cord injury. J Neurosci. 27:6428–6435. 2007. View Article : Google Scholar
|
|
67
|
Jung HW, Son HY, Minh CV, Kim YH and Park
YK: Methanol extract of ficus leaf inhibits the production of
nitric oxide and proinflammatory cytokines in LPS-stimulated
microglia via the MAPK pathway. Phytother Res. 22:1064–1069. 2008.
View Article : Google Scholar
|
|
68
|
Correia I, Prieto D, Román E, Wilson D,
Hube B, Alonso-Monge R and Pla J: Cooperative role of MAPK pathways
in the interaction of Candida albicans with the host epithelium.
Microorganisms. 25:482019. View Article : Google Scholar
|
|
69
|
Uenotsuchi T, Takeuchi S, Matsuda T, Urabe
K, Koga T, Uchi H, Nakahara T, Fukagawa S, Kawasaki M, Kajiwara H,
et al: Differential induction of Th1-prone immunity by human
dendritic cells activated with sporothrix schenckii of cutaneous
and visceral origins to determine their different virulence. Int
Immunol. 18:1637–1646. 2006. View Article : Google Scholar
|
|
70
|
Liu P, Pu J, Zhang J, Chen Z, Wei K and
Shi L: Bioinformatic analysis of mir-4792 regulates Radix
Tetrastigma hemsleyani flavone to inhibit proliferation, invasion,
and induce apoptosis of a549 cells. Onco Targets Ther.
12:1401–1412. 2019. View Article : Google Scholar
|
|
71
|
Lindell DM, Ballinger MN, McDonald RA,
Toews GB and Huffnagle GB: Immunologic homeostasis during
infection: coexistence of strong pulmonary cell-mediated immunity
to secondary Cryptococcus neoformans infection while the primary
infection still persists at low levels in the lungs. J Immunol.
177:4652–4661. 2006. View Article : Google Scholar
|
|
72
|
Lindell DM, Ballinger MN, McDonald RA,
Toews GB and Huffnagle GB: Diversity of the T-cell response to
pulmonary Cryptococcus neoformans infection. Infect Immun.
74:4538–4548. 2006. View Article : Google Scholar
|
|
73
|
Eastman AJ, Osterholzer JJ and Olszewski
MA: Role of dendritic cell-pathogen interactions in the immune
response to pulmonary cryptococcal infection. Future Microbiol.
10:1837–1857. 2015. View Article : Google Scholar
|