Introduction
Obesity is a global pandemic demonstrating
increasing international dimensions (1,2).
According to a recent pooled analysis of 2,416 population-based
measurement studies involving 128.9 million subjects, the worldwide
prevalence of overweight adults increased from 21.5 to 38.9% over
the last 40 years (3-5). This complex disease frequently
starts early in childhood and continues through adulthood. It
appears that obesity in both children and adolescents has exhibited
marked increases through the years, with 38 million patients under
the age of five years diagnosed as overweight or obese in 2019
(6-8).
Obesity is unquestionably a disease multifactorial
in origin. Its phenotypic manifestations and metabolic
complications are ascribed to various genetic, environmental and
epigenetic factors (9).
Early-onset severe childhood obesity, a rare disorder caused
predominantly by a variation of a single gene, is described as
monogenic obesity. Single gene variations in the hypothalamic
leptin-melanocortin pathway have been reported to affect the
balance between appetite and energy expenditure from a young age.
Such genes are leptin, leptin receptor, melanocortin-4 receptor
(MC4R), proopiomelanocortin and prohormone convertase 1/3
genes (10-12). Of course, there are other
monogenic syndromic forms of childhood obesity, which are
considered separate entities studied by clinical geneticists. On
the contrary, common obesity is thought to be affected by numerous
genes (polygenic) triggered by behavioral and environmental
factors. Genome-wide association studies (GWAS) have reported
variations in the four above-mentioned major genes of the
hypothalamic leptin-melanocortic pathway or other loci implicated
either in common or monogenic obesity (11,12). In addition, obesity
susceptibility loci associated with alterations in body mass index
(BMI) in both children and adults have been also identified by GWAS
in targeted or population studies (10-12).
Adenylate cyclase 3 (ADCY3) is a candidate
gene gaining increasing attention. ADCY3 loss-of-function
mutations have been associated with early-onset childhood obesity
(13). ADCY3 is located
on chromosome 2p23.3, consisting of 21 exons covering the positions
24,819,169-24,920,237 bp (GRCh38.p13) (14). Its product, an enzyme
characterized by a pseudo-symmetric structure of two transmembrane
and two cytoplasmic domains, is a member of the adenylyl cyclase
family and catalyses the synthesis of 3′,5′-cyclic adenosine
monophosphate (cAMP) from adenosine triphosphate upon G-protein
signaling (14). Research has
been performed in an effort to elucidate its role in metabolic
processes, including energy metabolism, adipogenesis and changes in
BMI (15-18). Its genetic variability has also
been studied in numerous populations of various geographical areas
(19). The present study aimed
to determine the occurrence of variations in the ADCY3 gene
in 33 Greek-Cypriot patients diagnosed with early-onset severe
obesity (as from the age of 3 years).
Materials and methods
Patient inclusion criteria
A total of 33 unrelated patients were included in
the present study, comprising 18 females and 15 males aged 15-20
years. All patients were recruited from the Archbishop Makarios III
Hospital (Nicosia, Cyprus), the Paedi Center for Specialized
Paediatrics (Nicosia, Cyprus) and the Aretaeio Hospital (Nicosia,
Cyprus). All patients were severely obese with BMI > +2.5
standard deviation score (SDS) at the time of genetic testing and
were diagnosed with early-onset obesity defined as BMI > +2 SDS
from the age of 3 years onwards. All patients had a 6-12 month
follow-up in a pediatric endocrinology clinic. According to their
medical records, patients were not diagnosed with any other
underlying medical conditions or complex syndromes. A total of 51
age-matched non-obese individuals (40 females and 11 males) of
Greek-Cypriot origin were included as a control group in the
present study. The project was approved by the Cyprus National
Ethics Committee (Nicosia, Cyprus) and informed consent was given
by patients/parents prior to any genetic testing.
Genomic sequencing and in silico
analysis
Total genomic DNA samples were isolated from
peripheral whole blood using the Gentra Puregene Blood kit (Qiagen
GmbH). DNA sequencing was performed with 100 ng genomic DNA, which
was amplified using primers designed by Primer3 software ver. 0.4.0
(http://frodo.wi.mit.edu/). PCR mixtures were
prepared using the Taq DNA Polymerase kit (Qiagen GmbH); they had a
final volume of 20 µl and contained 2 µl PCR buffer
(10X), 2 µl Q Solution (5X), 2 µl dNTPs (2 mM), 0.3
µl of each primer (10 µM), 0.2 µl Taq
polymerase (5 U/µl) and 100 ng genomic DNA. Amplification
was performed with an initial denaturing temperature at 95°C for 5
min, followed by 30 cycles of denaturation (95°C, 45 sec),
annealing (57°C, 60 sec) and extension (72°C, 45 sec), with a final
extension at 72°C for 5 min. The ADCY3 gene primers covered
all 21 exons (listed in Table
SI). The PCR products were analysed on an Applied Biosystems
3130xl Genetic Analyzer and the results were analysed using
Sequencing Analysis R 5.3 software (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The χ2 test of independence
and Fisher's exact test (for low counts) were used to determine any
association between the presence of variants and sex or BMI SDS.
For the association between the presence of variants and BMI SDS,
patients were separated according to their BMI SDS into three
groups: +2.00-+2.99 SDS, +3.00-+3.99 SDS and ≥ +4.00 SDS. P≤0.05
was considered to indicate statistical significance. In
silico prediction on protein function of the pathogenicity
effect of the novel amino acid substitution was performed using
three different protein functionality prediction tools: PolyPhen-2
(http://genetics.bwh.harvard.edu/pph2/), SIFT
(https://sift.bii.a-star.edu.sg/www/SIFT_seq_submit2.html)
and Mutation Taster (http://www.mutationtaster.org/). The PolyPhen-2 method
was based on sequence homology (values >0.908 were considered
probably damaging, values >0.446 and ≤0.908 were considered
possibly damaging). The SIFT method was based on sequence homology
and the physico-chemical properties of the amino acids (values
<0.05 were considered deleterious whereas values ≥0.05 were
considered tolerated). Mutation Taster was used to determine the
position of a splice site change relative to intron/exon borders. A
confidence score >0.3 was indicative of a gain of a completely
new splice site. Linkage disequilibrium analysis for the variants
was performed in all available populations using the Ensemble
Linkage Disequilibrium Calculator (https://grch37.ensembl.org/Homo_sapiens/Tools/LD?db=core).
Molecular modeling
The homology modelling of ADCY3 was performed using
Molecular Operating Environment (MOE; https://www.chemcomp.com/). The sequence of the human
ADCY3 was used (accession no. 060266 in UniProtKB; https://www.uniprot.org/). The selection of template
crystal structures for homology modelling was based on the primary
sequence identity and the crystal resolution. Therefore, the
selected template for the homology modelling was the structure of a
membrane adenylyl cyclase bound to an activated stimulatory G
protein (protein databank ID, 6R3Q). The primary alignment revealed
a 74% coverage and a marginal 30% sequence identity, which however
allowed for a conventional homology modelling experiment to be
performed. The initial energy minimization for the ADCY3 model was
performed in MOE using the CHARMM27 force-field up to a root mean
square deviation gradient of 0.0001, in an effort to remove the
geometrical strain. The ADCY3 model was subsequently solvated with
simple point charge (SPC) water using the truncated octahedron box
extending to 7Å from the model. Molecular dynamics simulation was
performed at 300 K and 1 atm with a 2 fsecond step size for a total
of 10 nanoseconds, using the NVT ensemble in a canonical
environment (NVT stands for Number of atoms, Volume and Temperature
that remain constant throughout the calculation). The ADCY3 model
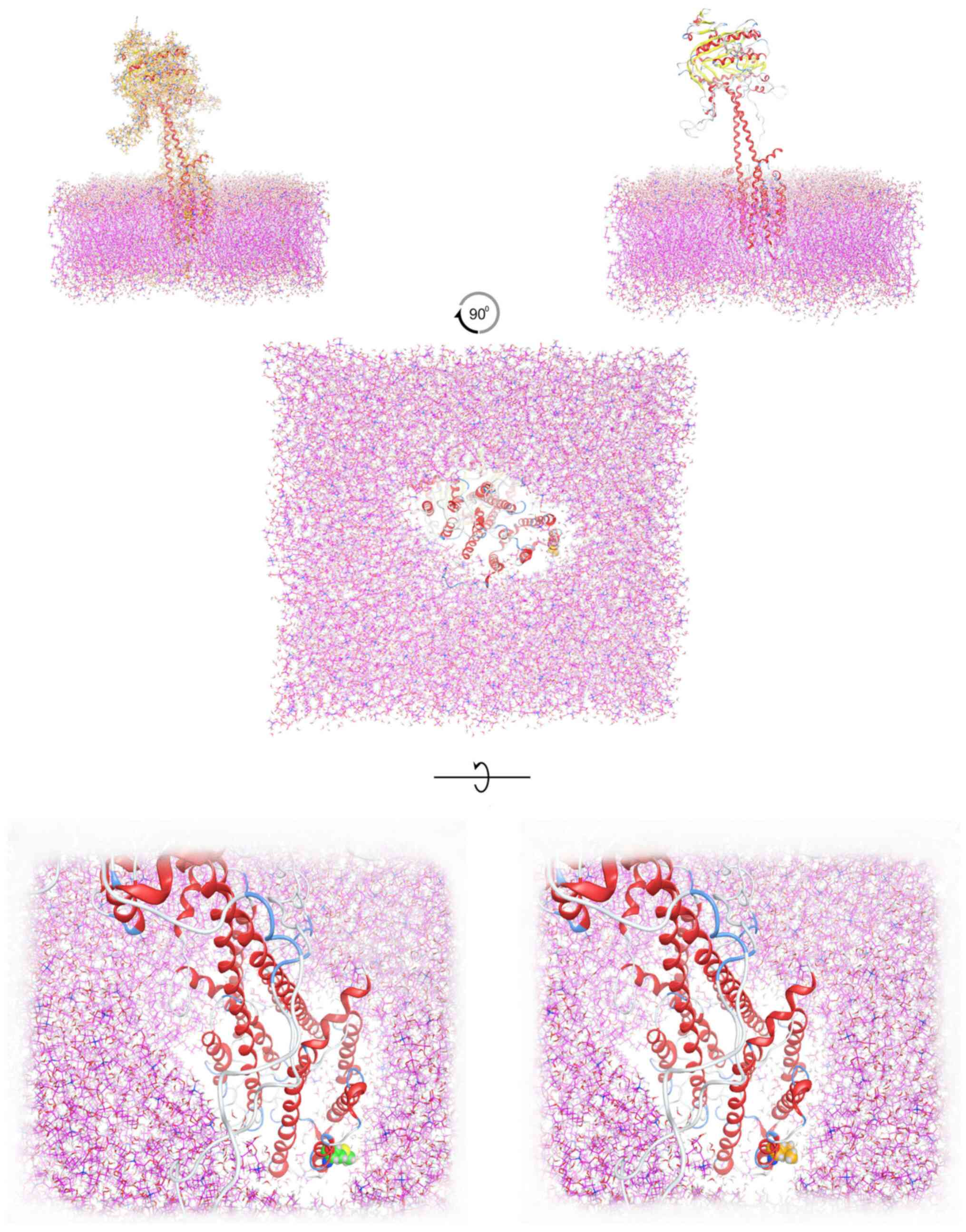
was also modelled in a full explicit phospholipid bilayer membrane
protected by a layer of explicit SPC water molecules in a periodic
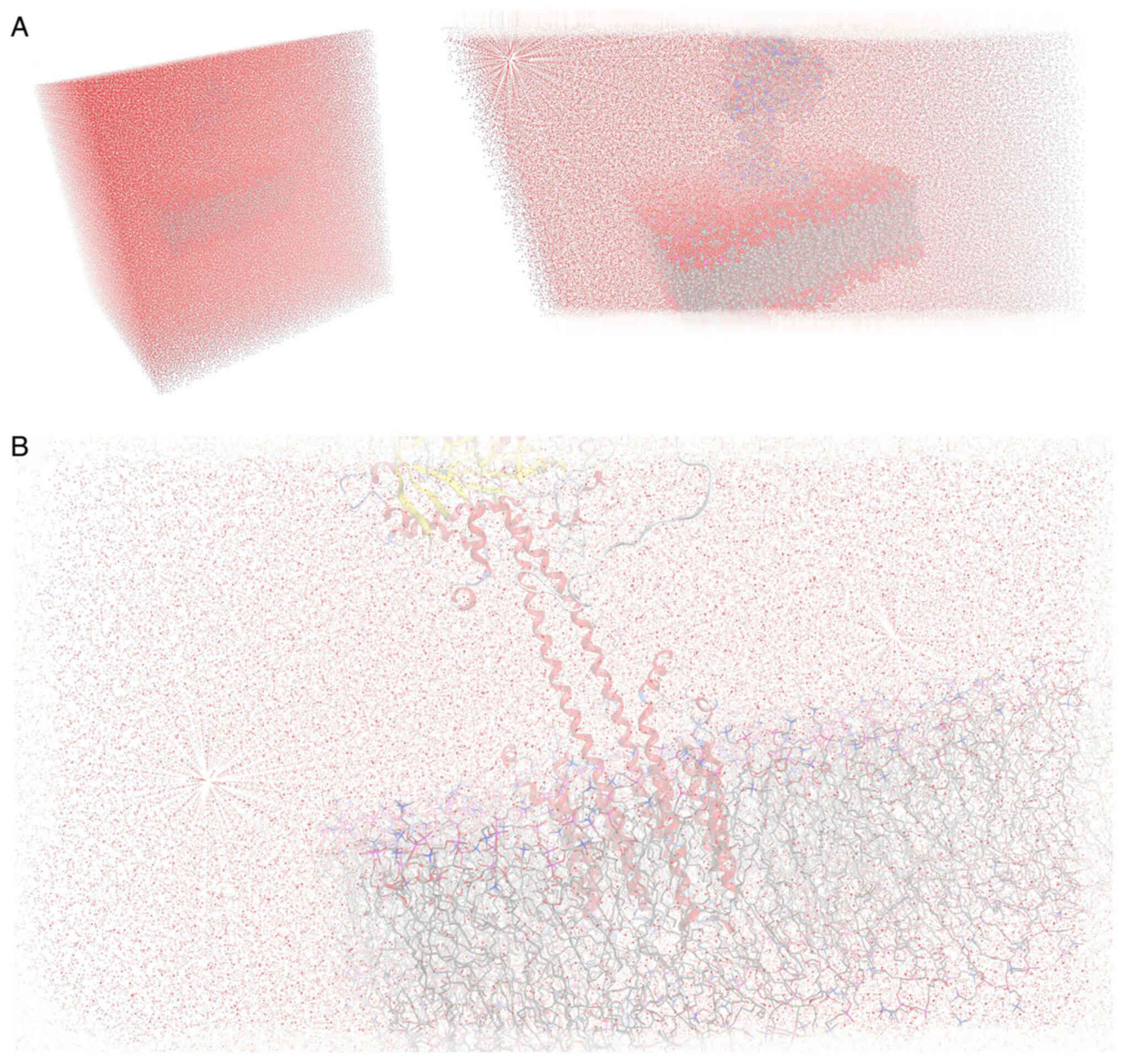
system (Fig. 1). The results of
the molecular dynamics simulation were collected into a database by
MOE for further analysis (Fig.
2).
Results
Genetic findings and prediction analysis
of the novel variation
Sanger sequencing of ADCY3 revealed a total
of five variants in patients (Table
I), four of which were previously reported. A novel variant was
identified in two patients (6%). Clinical and biochemical
parameters for these patients are presented in Table SII. The novel variant involves a
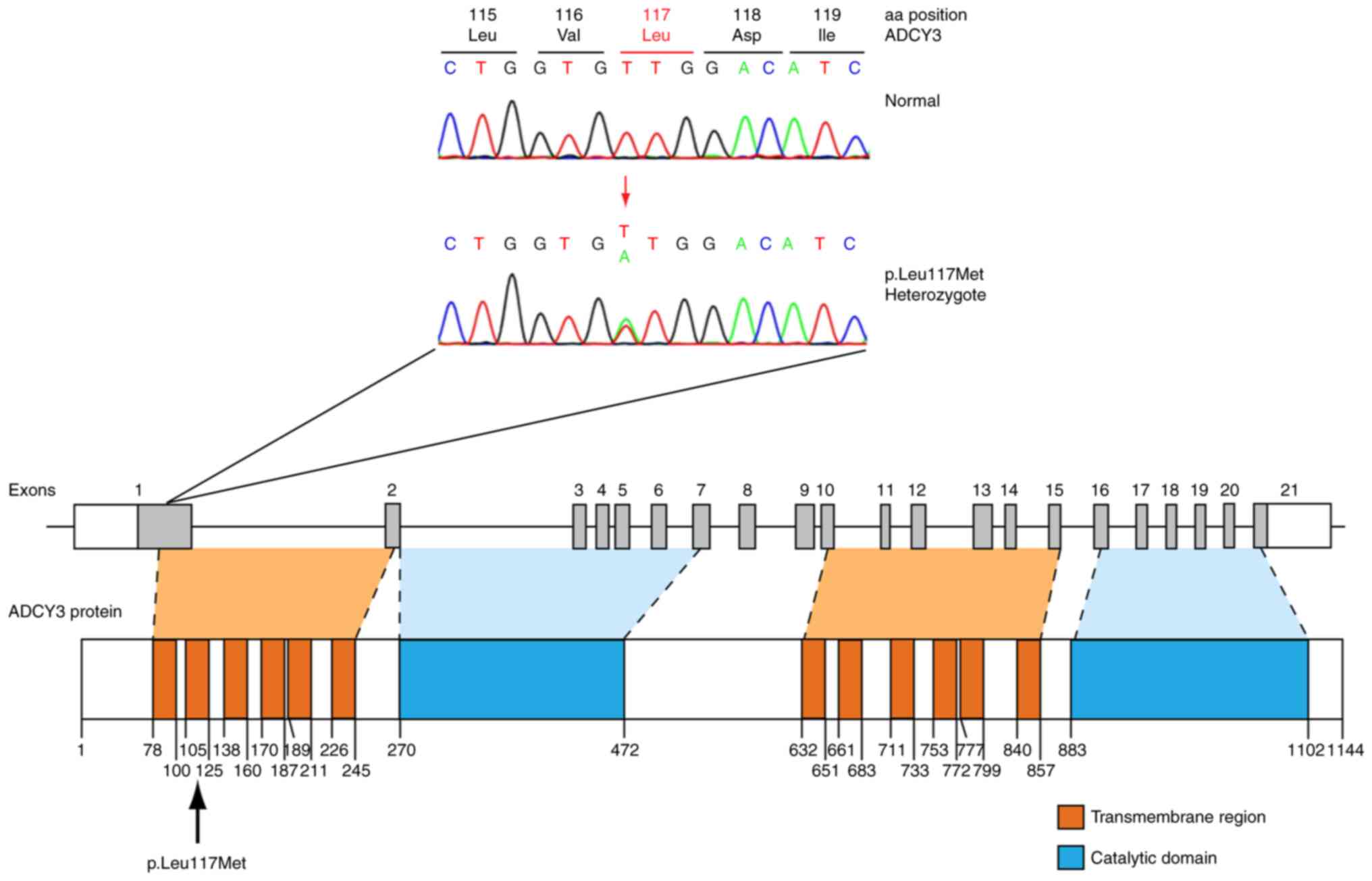
heterozygous c.349T>A change in exon 1 of the gene locus,
leading to a missense p.Leu117Met substitution, as demonstrated in
the sequence electropherogram presented in Fig. 3. Exon 1 codes for an α-helical
transmembrane region of the protein (Fig. 3). A search in the widely used
single nucleotide polymorphism (SNP) repositories such as the
gnomAD V2.1.1 (accessed July 12, 2021; https://gnomad.broadinstitute.org/), 1000Genomes
(accessed July 12, 2021; https://www.internationalgenome.org/) and TOPMED
(accessed July 12, 2021; https://topmed.nhlbi.nih.gov/) verified that the novel
p.Leu117Met variant was not previously reported. In silico
prediction of the pathogenicity effect of the p.Leu117Met
substitution was made using three different protein functionality
prediction tools: PolyPhen-2, SIFT and Mutation Taster. PolyPhen-2
predicted p.Leu117Met to be 'possibly damaging' with a score of
0.646 (sensitivity: 0.80; specificity: 0.84). SIFT predicted
substitution at position 117 from Leu to Met to 'affect protein
function' with a score of 0.03. Finally, Mutation Taster predicted
substitution at position 117 from Leu to Met as a 'polymorphism'
resulting in splice changes with a confidence score of 0.91 for the
predicted splice site. A gain of a completely new splice site was
displayed if the confidence score of the newly created splice site
was >0.3.
 | Table IIdentification of previously reported
variants in association to obesity and the novel adenylate cyclase
3 variant p.Leu117Met. |
Table I
Identification of previously reported
variants in association to obesity and the novel adenylate cyclase
3 variant p.Leu117Met.
| SNP ID no. | Description
nucleotide/amino acid | No. of homozygotes
in patients (sex) | No. of
heterozygotes in patients (sex) | MAF of patients
(n=33) | MAF of controls
(n=51) |
|---|
| Novel |
c.349T>A/p.Leu117Met | 0 | 2 (M:2; F:0) | 0.03 | - |
| rs11676272 |
c.319T>C/p.Ser107Pro | 10 (M:5; F:5) | 13 (M:5; F:8) | 0.50 | - |
| rs2241758 |
c.1167C>G/p.Leu389= | 0 | 3 (M:1; F:2) | 0.05 | 0.06 |
| rs7604576 |
c.2578-3T>C/NA | 9 (M:5; F:4) | 14 (M:6; F:8) | 0.48 | - |
| rs1127568 |
c.2874A>G/p.Ser958= | 21 (M:12; F:9) | 9 (M:2; F:7) | 0.77 | 0.62 |
The four previously reported variants include two
synonymous (rs1127568 and rs2241758), one missense (rs11676272) and
one intronic variant (rs7604576). The control group only revealed
the two synonymous variations rs1127568 and rs2241758, located in
exon 17 and exon 5, respectively. These SNPs have been previously
identified in other populations as well. More specifically,
rs1127568, which has a 0.8493 allele frequency based on gnomAD
v2.1.1, was identified in a Chinese Han population sample in
association to obesity (15),
whereas rs2241758 (0.1068 allele frequency, based on gnomAD v2.1.1)
was reported as a polymorphism in a Swedish population by Nordman
et al (16). Both of the
above-mentioned SNPs are synonymous, thus not causing any change of
the amino acids in the protein structure. The frequency of these
polymorphisms detected in the study population (Table I) was comparable to the results
reported in gnomAD v2.1.1. Intronic variant rs7604576 and the
missense variant rs11676272 were also among the SNPs reported in
the Chinese Han population (15). It should be noted that both of
these SNPs (rs7604576 and rs11676272) did not co-occur with the
novel p.Leu117Met variant in any of the patients.
Linkage disequilibrium analysis for the variants was
performed in all available populations. As expected, only
neighboring variants rs1127568 and rs7604576 demonstrated a
significant association (D=1.0, r2≥0.8).
The χ2 test of independence and Fisher's
exact test were used to determine an association between variants
and sex; however, no association was detected (P>0.05). A
similar analysis revealed no association between the presence of
variants and severity of obesity (measured as BMI SDS; P>0.05).
However, it should be noted that the small sample size in the
present study may have affected these results (Table SIII).
In light of recently reported findings suggesting
the involvement of MC4R and ADCY3 in a common pathway towards
obesity (20), the possible
co-occurrence of MC4R variations in the patients was also
investigated. However, Sanger sequencing of the MC4R locus
in patients and controls revealed no variations within the
MC4R locus (data not shown).
Molecular modeling
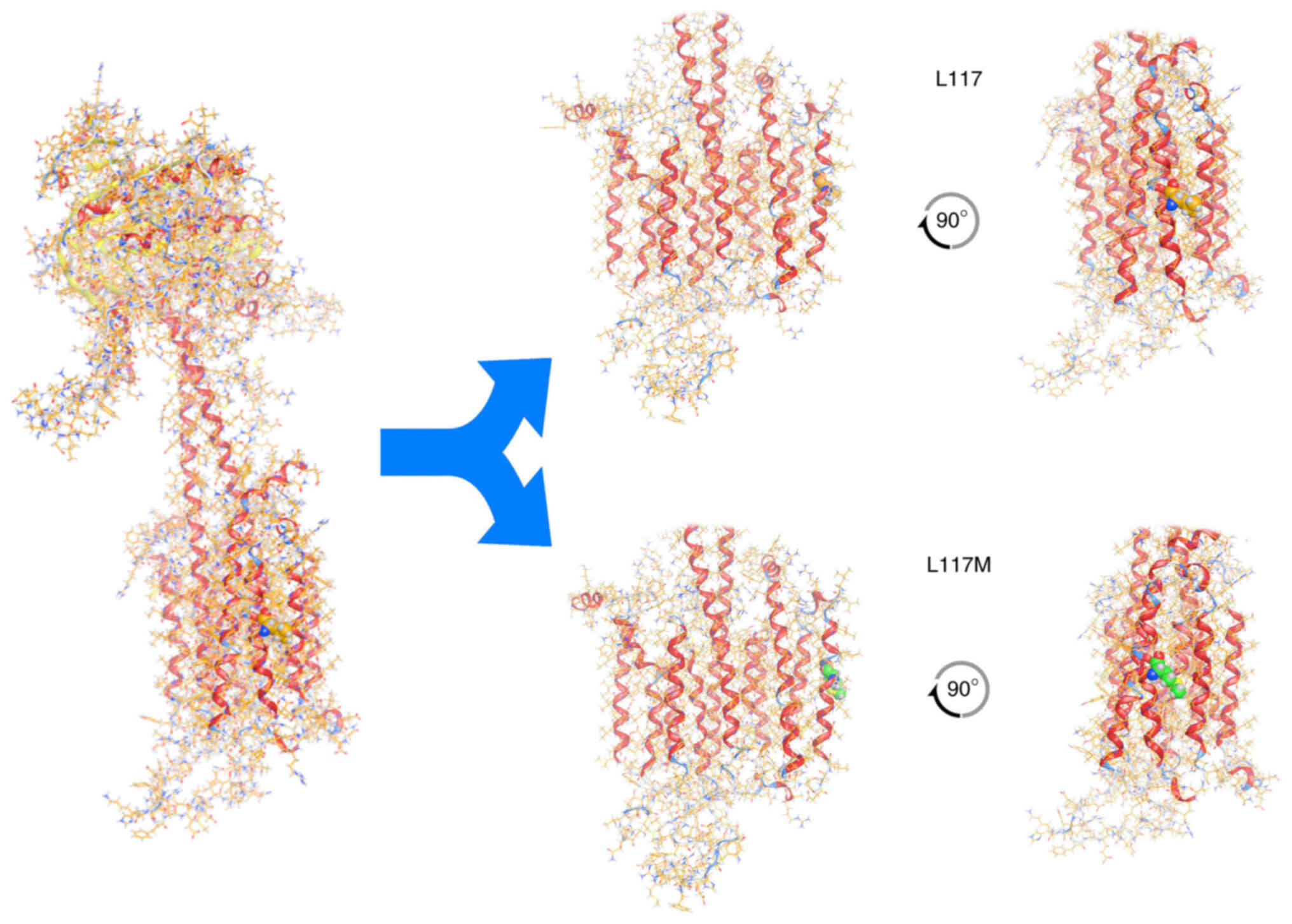
To investigate the possible effects of the novel
p.Leu117Met variation on protein structure and function, the
homology modelling of ADCY3 was performed using MOE. ADCY3 was
modelled initially with the wild-type sequence and a Leu residue at
position 117. The p.Leu117Met mutation was then induced and the
model was energetically re-optimized using molecular dynamics
(Fig. 4). The electrostatic
potential and available conformational space were also calculated
for both wild-type and mutant models. It was indicated, as
expected, that even though Leu and Met have a similar size, the Met
substitution introduces a sulphur atom and is therefore slightly
bulkier than the Leu wild-type residue. More specifically, when
superposing the two models and upon molecular dynamics
optimization, the two interaction plots were deduced for both
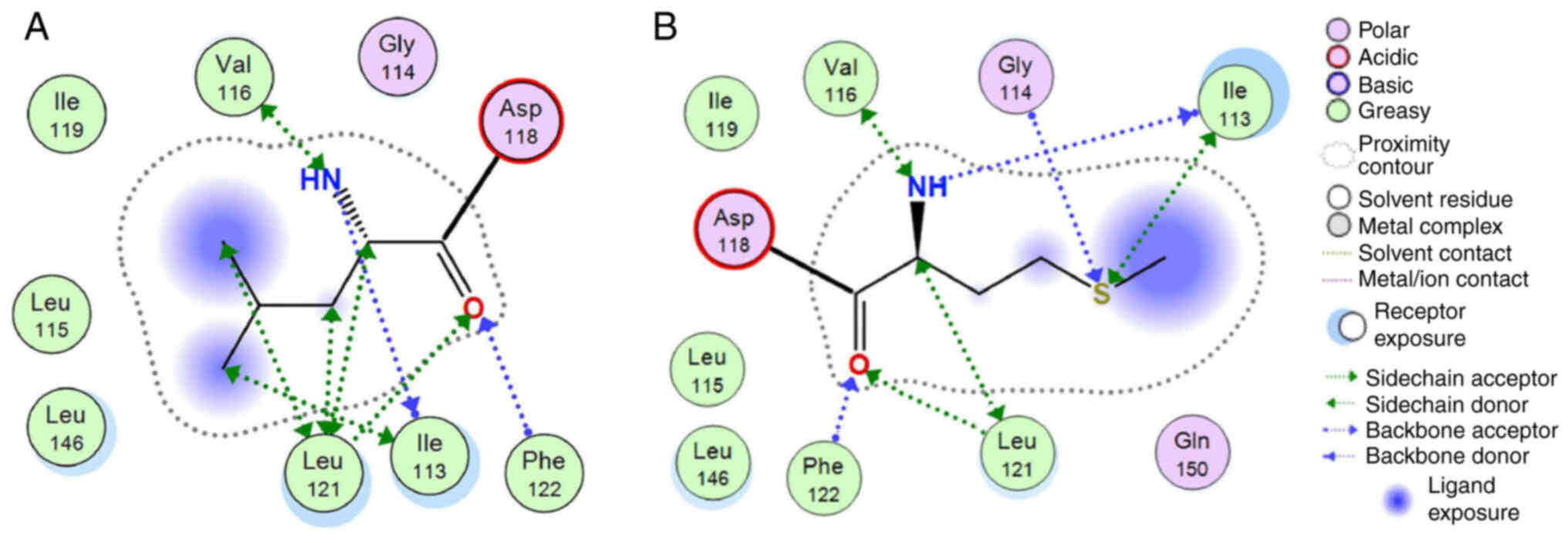
wild-type and mutant ADCY3 models (Fig. 5). Of note, while the wild-type
Leu residue is only capable of establishing mild H-bonding
interactions, the Met mutant substitution is establishing stronger
interactions with adjacent residues. This is functionally profound;
first, the different side chain of the Met residue, and
furthermore, the huge differences in Van der Waals and interaction
energies in the middle of the hydrophobic part of the lipid
bilayer, may functionally and mechanistically explain the
differences in structure that are able to lead to a significant
shift in the functionality of ADCY3 and hence to pathology. The
in silico calculated Van der Waals energy of the Leu to Met
at position 117 was-1.7 and 31.1 respectively, which constitutes a
significant change after the p.Leu117Met mutation (Table II). It is of utmost importance
to focus on the structural and physicochemical significance of the
Leu to Met substitution. Leu is a branched neutral hydrophobic
amino acid, whilst Met contains the sulfur atom that alters its
physicochemical properties and its potential atomic interaction.
Even though the two amino acids exhibit small differences in terms
of volume, charge and polarity, the sulfur of Met is reactive with
electrophilic centers and is prone to oxidation. Leu to Met
substitution decreases the stability of the protein structure with
a higher entropic cost. The difference in their shape also affects
the overall structure and, depending on the side chain's position,
the substitution may cause steric effects. In addition, leucine's
bulky side chain contributes to a greater hydrophobic stabilization
and its substitution by the elongated sulfur-containing Met is able
to destabilize the protein's structure.
 | Table IIIn silico calculated VdW
energy of the L117 wild-type and the L117M mutated biological
system (units are kcal/mol). |
Table II
In silico calculated VdW
energy of the L117 wild-type and the L117M mutated biological
system (units are kcal/mol).
| System | Leucine 117
| Methionine 117
|
|---|
| System energy | VdW | System energy | VdW |
|---|
| Full system | 1263.102 | 115010.1 | 1298.466 | −133304 |
| Interaction | −1.714 | −1.730 | 32.309 | 31.149 |
Discussion
The genetic component of obesity is challenging to
determine and monogenic forms of severe obesity starting at a young
age remain elusive. Mendelian monogenic nonsyndromic obesity is
associated with only 5% of obesity cases in the population
(8). The present study focuses
on the ADCY3 genetic profile of a small group of severely
obese individuals diagnosed with obesity from the age of 3 years
onwards. A small sample size in the present study may be a
limitation and conclusive results may be affected. However, five
ADCY3 gene variants (4 previously reported and one novel
variant) were identified. Of the patients, 2 (6%) were heterozygous
carriers of the novel p.Leu117Met variant in the ADCY3 gene.
Taking into consideration the rarity of monogenic obesity, this is
a noteworthy finding in a targeted group of patients diagnosed with
severe obesity since childhood. Molecular modelling data of the
mutated protein suggest significant changes in structure, as well
as in the interaction with the surrounding lipid bilayer,
indicating the possibility of functional changes.
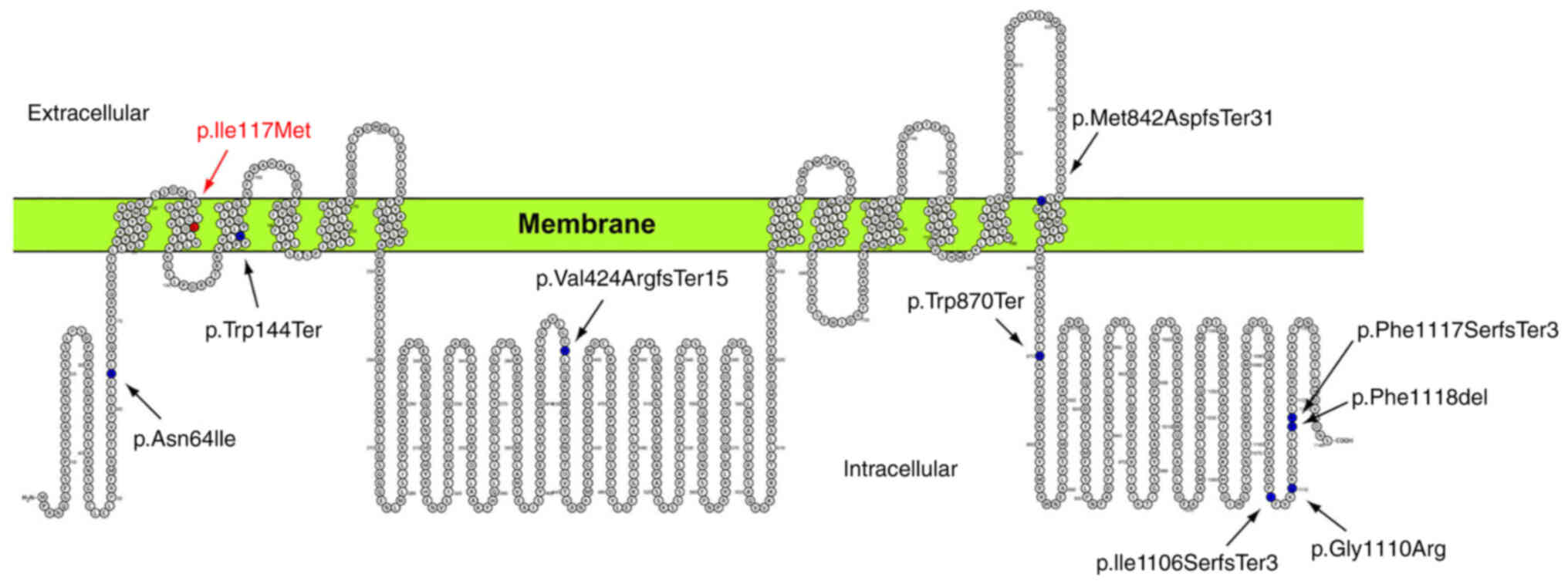
ADCY3 is a 128,960 Da integral protein, which
consists of two transmembrane components of six-transmembrane
helices each. These two transmembrane components alternate with two
cytoplasmic domains, which contain the active site of the molecule
at the N-terminus of the protein (Fig. 6). The amino acid Leu to Met
change at position 117 of the protein is within an α-helical region
embedded in the cellular membrane (105-125aa). Based on the
molecular modelling data of the present study, it is suggested that
the presence of the sulfur-carrying side chain of the Met residue
alters the interaction with the surrounding environment. More
specifically, the Met residue is capable of establishing stronger
interactions with surrounding residues. Furthermore, interaction
energies within the lipid bilayer of the membrane are increased.
Although the enzyme's catalytic centers fall within a different
region, the changes caused by the Leu to Met substitution may
affect the interaction between the transmembrane clusters of the
protein. According to previous studies, the interaction between
these transmembrane regions of the protein appears to significantly
affect the interaction of the catalytic domains (21). Hence, it is possible that the
ADCY3 variant c.349T>A results in a reduction of ADCY3
activity.
According to the literature, ADCY3 has been studied
extensively in a spectrum of processes affecting metabolic
mechanisms and obesity development. There are 10 isoforms of ADCY3
reported and characterized in mammals. A total of 9 isoforms are
membrane-bound proteins found to be expressed in various human
tissues, including adipocyte tissues and the hypothalamus (22). ADCY3 is
Ca2+-calmodulin sensitive and it appears to be linked to
olfactory signal transduction (23,24). A study involving ADCY3
heterozygous null mice demonstrated increased visceral adiposity in
the absence of hyperphagia and impaired insulin sensitivity,
dyslipidemia, as well as increased plasma levels of proinflammatory
cytokines. According to the same study, a high-fat diet decreased
the expression of ADCY3 and genes involved in thermogenesis,
fatty acid oxidation and insulin signaling, whereas it increased
the expression of genes related to adipogenesis (25). In a different study using
diabetic rats, which demonstrated a dysfunctional hypothalamic
melanocortin system and attenuation of the hypothalamic
glucose-sensing pathway, after performing a hypothalamic
transcriptomes analysis, the authors identified ADCY3 among
the genes with a role in hypothalamic regulation. In fact, their
results support a key role of ADCY3 in preventing obesity
(26).
ADCY3 is an enzyme which catalyzes the formation of
cAMP, a second messenger involved in signal transduction processes
in cells, such as the activation of kinases and the control of
metabolic processes of carbohydrates and lipids. More specifically,
cAMP appears to be involved in intracellular signaling of molecules
such as glucagon-like peptide 1, ghrelin, orexins,
α-melanocyte-stimulating hormone and leptin (27,28).
Numerous studies support the involvement of ADCY3 in
metabolism regulation and BMI or obesity risk. For instance, a
study reported on ADCY3-knockout mice demonstrating obesity,
low locomotor activity, hyperphagia and leptin resistance (29). Furthermore, the involvement of
ADCY3 in the regulation of glucose homeostasis was suggested by a
study using Goto-Kakizaki (GK) rats with type 2 diabetes. In that
study, its overexpression was observed in the pancreatic islets as
well as the striatum and hypothalamus regions of the brain in the
GK rats (30). The involvement
of ADCY3 in energy metabolism regulation was reported in another
study, which used mutagenized mice with dominantly inherited
resistance to diet-induced obesity. These mice were genetically
screened and were indicated to contain a p.Met279Leu substitution
in ADCY3 (17).
ADCY3 variants in association with obesity
were identified in various human population studies. All reported
data are presented in Table
III (15,16,31-34). More specifically, ADCY3
variants (rs2033655 and rs1968482) were reported to be associated
with obesity in a population of Swedish males with or without type
2 diabetes in 2008 (16). Two
years later, ADCY3 polymorphisms (rs1127568, rs7604576 and
rs753529) were reported to be associated with obesity in adults but
not in children of a Chinese Han population sample (15). It should be noted that one of
these variants (rs7604576) was also detected in the patients of the
present study (minor allele frequency, 0.48). In 2018, a study
identified a correlation between loss-of-function mutations and
onset obesity and type 2 diabetes in a small Greenlandic population
(rs1331776405). Carriers in this cohort appeared to also have
decreased RNA levels (34). In
the same year, a similar study involving children with severe
obesity of a Pakistani population also revealed ADCY3
variants, including that identified in the Greenlandic study
(13). Most recently,
ADCY3 variant p.Gly1110Arg was detected by targeted exome
sequencing in a Finnish population with early-onset severe obesity
(Table III) (33).
 | Table IIIGenetic variants identified in
ADCY3 gene with association to obesity and BMI. |
Table III
Genetic variants identified in
ADCY3 gene with association to obesity and BMI.
A, Causative
variations
|
|---|
| Nucleotide
change | Amino-acid
change | Genetic location
(relevant to ADCY3 gene) | Type of
variant | dbSNP number | NCBI Refseq | Clinical
characteristic | (Refs.) |
|---|
| c.191A>T | p.Asn64Ile | Exon 1 | Missense | rs541941351 |
NM_004036.5:c.191A>T | Obesity | (13) |
| c.349T>A | p.Leu117Met | Exon 1 | Missense | NA |
NM_004036.5:c.349T>A | Obesity | Present study |
| c.431G>A | p.Trp144Ter | Exon 1 | Stop-gained | rs760195447 |
NM_004036.5:c.431G>A | Obesity/type II
diabetes | (34) |
| c.1072-1G>A | NA | Intron 4 | Splice
acceptor | NA |
NM_004036.5:c.1072-1G>A | Obesity/type II
diabetes | (34) |
| c.1268del |
p.Val424ArgfsTer15 | Exon 6 | Frameshift | rs754914420 |
NM_004036.5:c.1268del | Obesity/type II
diabetes | (13,34) |
| c.1805+2T>C | NA | Exon 10 | Splice donor | rs761428196 |
NM_004036.5:c.1805+2T>C | Obesity/type II
diabetes | (34) |
| c.2433-1G>A | NA | Intron 13 | Splice
acceptor | rs1331776405 |
NM_004036.5:c.2433-1G>A | Obesity | (34) |
| c.2524_2525del |
p.Met842AspfsTer31 | Exon 15 | Frameshift | rs759512660 |
NM_004036.5:c.2524_2525del | Obesity/type II
diabetes | (34) |
| c.2578-1G>A | NA | Intron 15 | Splice
acceptor | rs1553333167 |
NM_004036.5:c.2578-1G>A | Obesity | (13) |
| c.2609G>A | p.Trp870Ter | Exon16 | Stop-gained | rs763802630 |
NM_004036.5:c.2609G>A | Obesity/type II
diabetes | (34) |
| c.3315del |
p.Ile1106SerfsTer3 | Exon 21 | Frameshift | rs1553329804 |
NM_004036.5:c.3315del | Obesity | (13) |
| c.3328G>C | p.Gly1110Arg | Exon 21 | Missense | rs1292057640 |
NM_004036.5:c.3328G>C | Obesity | (33) |
| c.3348AG>A |
p.Phe1117SerfsTer3 | Exon 21 | Frameshift | NA |
NM_004036.5:c.3348AG>A | Obesity/type II
diabetes | (34) |
|
c.3348_3350CTT[2] | p.Phe1118del | Exon 21 | Deletion | rs750852737 |
NM_004036.3:c.3348_3350CTT[2] | Obesity | (13) |
|
B, Associated SNPs
|
| Nucleotide
change | Amino-acid
change | Genetic location
(relevant to ADCY3 gene) | Type of
variant | dbSNP number | NCBI Refseq | Clinical
characteristic | (Refs.) |
|
| c.319T>C | p.Ser107Pro | Exon 1 | SNP | rs11676272 |
NM_004036.5:c.319T>C | Linked to
obesity | (36) |
|
c.675+9866T>C | NA | Intron 1 | SNP | rs6545814 |
NM_004036.5:c.675+9866T>C | Linked to BMI | (35) |
|
c.675+14467G>A | NA | Intron 1 | SNP | rs6545809 |
NM_004036.5:c.675+14467 G>A | Linked to BMI | (38) |
|
c.676-21389G>A | NA | Intron 1 | SNP | rs7586879 |
NM_004036.5:c.676-21389G>A | Linked to BMI | (42) |
|
c.676-5504C>T/G | NA | Intron 1 | SNP | rs2033655 | NM_004036.5:
c.676-5504C>T/G | Linked to
obesity/type II diabetes | (16) |
|
c.825+8581A>G | NA | Intron 2 | SNP | rs1968482 |
NM_004036.5:c.825+8581A>G | Linked to BMI | (16) |
| c.1167C>G | p.Leu389= | Exon 5 | Synonymous
(SNP) | rs2241758 |
NM_004036.5:c.1167C>G | Obesity | (16) |
|
c.2055+270C>T | NA | Intron 11 | SNP | rs753529 |
NM_004036.3:c.2055+270C>T | Linked to
obesity | (15) |
| c.2578-3T>C | NA | Intron 15 | SNP | rs7604576 |
NM_004036.5:c.2578-3T>C | Linked to
obesity | (15) |
| c.2874A>G | p.Ser958= | Exon 17 | Synonymous
(SNP) | rs1127568 |
NM_004036.5:c.2871A>G | Obesity | (15) |
|
g.24927427A>G | NA | Intergenic | SNP | rs10182181 |
NC_000002.12:g.24927427A>G | Linked to
obesity/nutrition | (32) |
|
g.24935139T>C | NA | Intergenic | SNP | rs713586 |
NC_000002.12:g.24935139T>C | Linked to BMI | (43) |
Apart from population genetic studies, GWAS have
also linked ADCY3 genetic polymorphisms to obesity
susceptibility. The ADCY3 locus was indicated to be
associated with BMI in the Genetic Investigation of ANthropometric
Traits consortium meta-analysis (12) and also in a meta-analysis of
East-Asian populations (rs6545814, rs11676272) (35). It should be noted that variant
rs11676272, also detected in the patients of the present study, was
reported in a GWAS of height-adjusted BMI in children. Of note, in
this study, the authors discuss the possible effects on the second
transmembrane-spanning α-helix of the M1 transmembrane cluster of
the protein (same region where the novel p.Leu117Met of the present
study was detected), thus possibly disturbing the interaction of M1
helix and M2 helix (36).
ADCY3 was also identified in a GWAS meta-analysis of BMI
across children aged 1 to 17 years from the Avon Longitudinal Study
of Parents and Children and the Western Australian Pregnancy Cohort
study (37). Similarly, an
association to BMI was concluded in a GWAS concerning a Korean
sample (38).
Adipogenesis is another process linked to ADCY3, as
supported by animal studies performed by Tong et al
(25) and Meng et al
(26). A decrease in
ADCY3 expression in the adipose tissue of obese patients was
also reported in 2011 (18).
However, recent findings concerning MC4R provided strong evidence
regarding its possible relevance to the leptin-melanocortin
pathway. More specifically, it was indicated that MC4R co-localizes
with ADCY3 at the primary cilium of hypothalamic neurons and
obesity-related MC4R mutations impaired ciliary
localization. In addition, inhibition of ADCY3 signaling at the
primary cilia of these neurons increased body weight (20). A previous study by our group
reported a novel MC4R deletion in a pediatric patient with
severe early-onset obesity (39). However, MC4R screening
revealed no mutations in patients and healthy individuals of the
present study.
Finally, epigenetic studies have also suggested a
role of ADCY3 in human obesity, as DNA methylation and
ADCY3 associations were identified in a study involving
obese and healthy individuals (40). Similarly, a genome-wide DNA
methylation quantitative trait locus analysis in human adipose
tissue and genotype-DNA methylation associations were indicated to
involve ADCY3 (41).
In conclusion, according to the literature discussed
above, there appears to be strong evidence to suggest that ADCY3 is
an important mediator of energy homeostasis with a possible role in
the development of obesity. Although the lack of whole-exome
sequencing or whole-genome sequencing may be considered a
limitation of the present study, recent published data suggests
that ADCY3 is considered a strong candidate among numerous
loci previously reported to be associated with obesity (13,20,34). Thus, the ADCY3 locus has
been the focus of the present study. The current findings support
the association of ADCY3 with obesity and provide the first
evidence that ADCY3 variations exist in the genetic spectrum
of the Cypriot population. Further to identifying the novel,
possibly pathogenic ADCY3 variant, causing an amino acid Leu
to Met change at position 117 of the protein in young obese
patients, the present study provided informative modelling data
suggesting a change in the interaction of the two transmembrane
halves of the enzyme, thus causing a disturbance in the
pseudo-twofold symmetry of the transmembrane domain, a symmetry
known to affect catalytic activity (21). Further biological assessment
using cell lines or genetically modified animals may help to
elucidate the role of the novel variant and other previously
reported ADCY3 variants in genetic predisposition to
obesity.
Supplementary Data
Availability of data and materials
The data that support the findings of this study are
openly available from the Zenodo repository at DOI (https://zenodo.org/record/5717389#.YZrIRM_RbX4).
Authors' contributions
MT and NS examined the patients. MT gathered all
clinical information. MP, PF and VN performed the database search
and interpreted the genetic data. PF performed the genomic analysis
experiments and processed genetic data. MP and PF performed data
analysis. MP, MT and DV wrote the draft of the manuscript. DV
worked on the molecular modelling data. LAP and CSM had substantial
contribution to the design of the work and critically reviewed the
manuscript. MP, NS, MT, DV, PF and VN confirm the authenticity of
the raw data. All authors have read and given final approval of the
version to be published.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Written informed consent was obtained from the patients
and the parents of minors that participated in the study. Bioethics
approval was received from the Cyprus National Ethics Committee
(Nicosia, Cyprus).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This research received funding from the AG Leventis Foundation
(grant no. 3317312) and the RCB Bank Ltd. (grant no. 33173151).
References
|
1
|
Lobstein T and Baur L: Obesity in children
and young people: A crisis in public health. Obes Rev. 5(Suppl 1):
S4–S104. 2004. View Article : Google Scholar
|
|
2
|
Nutrition Global Targets 2025. Geneva:
WHO; 2018, http://www.who.int/nutrition/global-target-2025/en/.
Accessed April 2, 2020.
|
|
3
|
NCD Risk Factor Collaboration (NCD-RisC):
Worldwide trends in body-mass index, underweight, overweight, and
obesity from 1975 to 2016: A pooled analysis of 2416
population-based measurement studies in 1289 million children,
adolescents, and adults. Lancet. 390:2627–2642. 2017. View Article : Google Scholar
|
|
4
|
Roberto CA, Swinburn B, Hawkes C, Huang
TT, Costa SA, Ashe M, Zwicker L, Cawley JH and Brownell KD: Patchy
progress on obesity prevention: Emerging examples, entrenched
barriers, and new thinking. Lancet. 385:2400–2409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Templin T, Cravo Oliveira Hashiguchi T,
Thomson B, Dieleman J and Bendavid E: The overweight and obesity
transition from the wealthy to the poor in low- and middle-income
countries: A survey of household data from 103 countries. PLoS Med.
16:e10029682019. View Article : Google Scholar
|
|
6
|
González-Muniesa P, Mártinez-González MA,
Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA and
Martinez JA: Obesity. Nat Rev Dis Primers. 3:170342017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tremmel M, Gerdtham UG, Nilsson PM and
Saha S: Economic burden of obesity: A systematic literature review.
Int J Environ Res Public Health. 14:4352017. View Article : Google Scholar :
|
|
8
|
WHO fact sheet 311. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
Accessed April 2, 2020.
|
|
9
|
Sahoo K, Sahoo B, Choudhury AK, Sofi NY,
Kumar R and Bhadoria AS: Childhood obesity: Causes and
consequences. J Family Med Prim Care. 4:187–192. 2015. View Article : Google Scholar :
|
|
10
|
Frayling TM, Timpson NJ, Weedon MN,
Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H,
Rayner NW, et al: A common variant in the FTO gene is associated
with body mass index and predisposes to childhood and adult
obesity. Science. 316:889–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loos RJ, Lindgren CM, Li S, Wheeler E,
Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann
JS, et al: Common variants near MC4R are associated with fat mass,
weight and risk of obesity. Nat Genet. 40:768–775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Speliotes EK, Willer CJ, Berndt SI, Monda
KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Mägi
R, et al: Association analyses of 249,796 individuals reveal 18 new
loci associated with body mass index. Nat Genet. 42:937–948. 2010.
View Article : Google Scholar
|
|
13
|
Saeed S, Bonnefond A, Tamanini F, Mirza
MU, Manzoor J, Janjua QM, Din SM, Gaitan J, Milochau A, Durand E,
et al: Loss-of-function mutations in ADCY3 cause monogenic severe
obesity. Nat Genet. 50:175–179. 2018. View Article : Google Scholar
|
|
14
|
Ludwig MG and Seuwen K: Characterization
of the human adenylyl cyclase gene family: cDNA, gene structure,
and tissue distribution of the nine isoforms. J Recept Signal
Transduct Res. 22:79–110. 2002. View Article : Google Scholar
|
|
15
|
Wang H, Wu M, Zhu W, Shen J, Shi X, Yang
J, Zhao Q, Ni C, Xu Y, Shen H, et al: Evaluation of the association
between the AC3 genetic polymorphisms and obesity in a Chinese Han
population. PLoS One. 5:e138512010. View Article : Google Scholar :
|
|
16
|
Nordman S, Abulaiti A, Hilding A, Långberg
EC, Humphreys K, Ostenson CG, Efendic S and Gu HF: Genetic
variation of the adenylyl cyclase 3 (AC3) locus and its influence
on type 2 diabetes and obesity susceptibility in Swedish men. Int J
Obes (Lond). 32:407–412. 2008. View Article : Google Scholar
|
|
17
|
Pitman JL, Wheeler MC, Lloyd DJ, Walker
JR, Glynne RJ and Gekakis N: A gain-of-function mutation in
adenylate cyclase 3 protects mice from diet-induced obesity. PLoS
One. 9:e1102262014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurtado del Pozo C, Vesperinas-García G,
Rubio MÁ, Corripio-Sánchez R, Torres-García AJ, Obregon MJ and
Calvo RM: ChREBP expression in the liver, adipose tissue and
differentiated preadipocytes in human obesity. Biochim Biophys
Acta. 1811:1194–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Shen C, Seed Ahmed M, Östenson CG
and Gu HF: Adenylate cyclase 3: A new target for anti-obesity drug
development. Obes Rev. 17:907–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siljee JE, Wang Y, Bernard AA, Ersoy BA,
Zhang S, Marley A, Von Zastrow M, Reiter JF and Vaisse C:
Subcellular localization of MC4R with ADCY3 at neuronal primary
cilia underlies a common pathway for genetic predisposition to
obesity. Nat Genet. 50:180–185. 2018. View Article : Google Scholar :
|
|
21
|
Gu C, Sorkin A and Cooper DM: Persistent
interactions between the two transmembrane clusters dictate the
targeting and functional assembly of adenylyl cyclase. Curr Biol.
11:185–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cooper DM: Regulation and organization of
adenylyl cyclases and cAMP. Biochem J. 375:517–529. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valverde I, Vandermeers A, Anjaneyulu R
and Malaisse WJ: Calmodulin activation of adenylate cyclase in
pancreatic islets. Science. 206:225–227. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong ST, Trinh K, Hacker B, Chan GC, Lowe
G, Gaggar A, Xia Z, Gold GH and Storm DR: Disruption of the type
III adenylyl cyclase gene leads to peripheral and behavioral
anosmia in transgenic mice. Neuron. 27:487–497. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tong T, Shen Y, Lee HW, Yu R and Park T:
Adenylyl cyclase 3 haploinsufficiency confers susceptibility to
diet-induced obesity and insulin resistance in mice. Sci Rep.
6:341792016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng Y, Guan Y, Zhang W, Wu YE, Jia H,
Zhang Y, Zhang X, Du H and Wang X: RNA-seq analysis of the
hypothalamic transcriptome reveals the networks regulating
physiopathological progress in the diabetic GK rat. Sci Rep.
6:341382016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu TR, Yang Y, Ward R, Gao L and Liu Y:
Orexin receptors: Multi-functional therapeutic targets for sleeping
disorders, eating disorders, drug addiction, cancers and other
physiological disorders. Cell Signal. 25:2413–2423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wheeler MB, Lu M, Dillon JS, Leng XH, Chen
C and Boyd AE III: Functional expression of the rat glucagon-like
peptide-I receptor, evidence for coupling to both adenylyl cyclase
and phospholipase-C. Endocrinology. 133:57–62. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Li V, Chan GC, Phan T, Nudelman
AS, Xia Z and Storm DR: Adult type 3 adenylyl cyclase-deficient
mice are obese. PLoS One. 4:e69792009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seed Ahmed M, Kovoor A, Nordman S, Abu
Seman N, Gu T, Efendic S, Brismar K, Östenson CG and Gu HF:
Increased expression of adenylyl cyclase 3 in pancreatic islets and
central nervous system of diabetic Goto-Kakizaki rats: A possible
regulatory role in glucose homeostasis. Islets. 4:343–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andersen MK and Hansen T: Genetics of
metabolic traits in Greenlanders: Lessons from an isolated
population. J Intern Med. 284:464–477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goni L, Riezu-Boj JI, Milagro FI, Corrales
FJ, Ortiz L, Cuervo M and Martínez JA: Interaction between an ADCY3
genetic variant and two weight-lowering diets affecting body
fatness and body composition outcomes depending on macronutrient
distribution: A randomized trial. Nutrients. 10:7892018. View Article : Google Scholar :
|
|
33
|
Loid P, Mustila T, Mäkitie RE, Viljakainen
H, Kämpe A, Tossavainen P, Lipsanen-Nyman M, Pekkinen M and Mäkitie
O: Rare variants in genes linked to appetite control and
hypothalamic development in early-onset severe obesity. Front
Endocrinol (Lausanne). 11:812020. View Article : Google Scholar
|
|
34
|
Grarup N, Moltke I, Andersen MK, Dalby M,
Vitting-Seerup K, Kern T, Mahendran Y, Jørsboe E, Larsen CVL,
Dahl-Petersen IK, et al: Loss-of-function variants in ADCY3
increase risk of obesity and type 2 diabetes. Nat Genet.
50:172–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen W, Cho YS, Zheng W, Dorajoo R, Kato N,
Qi L, Chen CH, Delahanty RJ, Okada Y, Tabara Y, et al:
Meta-analysis identifies common variants associated with body mass
index in east Asians. Nat Genet. 44:307–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stergiakouli E, Gaillard R, Tavaré JM,
Balthasar N, Loos RJ, Taal HR, Evans DM, Rivadeneira F, St Pourcain
B, Uitterlinden AG, et al: Genome-wide association study of
height-adjusted BMI in childhood identifies functional variant in
ADCY3. Obesity (Silver Spring). 22:2252–2259. 2014. View Article : Google Scholar
|
|
37
|
Warrington NM, Howe LD, Paternoster L,
Kaakinen M, Herrala S, Huikari V, Wu YY, Kemp JP, Timpson NJ, St
Pourcain B, et al: A genome-wide association study of body mass
index across early life and childhood. Int J Epidemiol. 44:700–712.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JS, Cheong HS and Shin HD: BMI
prediction within a Korean population. PeerJ. 5:e35102017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neocleous V, Shammas C, Phelan MM, Fanis
P, Pantelidou M, Skordis N, Mantzoros C, Phylactou LA and Toumba M:
A novel MC4R deletion coexisting with FTO and MC1R gene variants,
causes severe early onset obesity. Hormones (Athens). 15:445–452.
2016.
|
|
40
|
Voisin S, Almén MS, Zheleznyakova GY,
Lundberg L, Zarei S, Castillo S, Eriksson FE, Nilsson EK, Blüher M,
Böttcher Y, et al: Many obesity-associated SNPs strongly associate
with DNA methylation changes at proximal promoters and enhancers.
Genome Med. 7:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Volkov P, Olsson AH, Gillberg L, Jørgensen
SW, Brøns C, Eriksson KF, Groop L, Jansson PA, Nilsson E, Rönn T,
et al: A genome-wide mQTL analysis in human adipose tissue
identifies genetic variants associated with DNA methylation, gene
expression and metabolic traits. PLoS One. 11:e01577762016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monda KL, Chen GK, Taylor KC, Palmer C,
Edwards TL, Lange LA, Ng MC, Adeyemo AA, Allison MA, Bielak LF, et
al: A meta-analysis identifies new loci associated with body mass
index in individuals of African ancestry. Nat Genet. 45:690–696.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Locke AE, Kahali B, Berndt SI, Justice AE,
Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et
al: Genetic studies of body mass index yield new insights for
obesity biology. Nature. 518:197–206. 2015. View Article : Google Scholar : PubMed/NCBI
|