1. Introduction
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) is an enveloped RNA virus that is transmitted mainly
via air droplets, and can cause a viral infectious disease in
humans, namely coronavirus disease 2019 (COVID-19), which was first
identified towards the end of 2019 (1). COVID-19 manifestations range from
mild (asymptomatic or mild respiratory tract infection in the
majority of reported cases) to severe and the disease can be fatal
in high-risk individuals, with a spectrum of respiratory and/or
extra-pulmonary manifestations that may require hospitalization and
potentially mechanical ventilation and intensive care unit support
(1). Of note, SARS-CoV-2 is
highly virulent, has a tendency to mutate and exhibits a cellular
tropism within the human body that appears to be associated with
the expression of various host cell entry mediators, such as the
angiotensin converting enzyme 2 (ACE2) (2-4).
The present review article presents a concise overview of the key
host cell entry mediators which have been identified to play a role
in the infection of human cells by SARS-CoV-2, summarizing the
evidence on the mechanisms through which ACE2 and additional host
receptors/proteins appear to be implicated in the cellular tropism
of SARS-CoV-2 and the pathophysiology of COVID-19. Moreover, the
present review article aims to provide insight into the mechanisms
through which these cell entry mediators may be regulated by
certain microRNAs (miRNAs/miRs).
2. Angiotensin converting enzyme 2, and
transmembrane protease serine 2 and 4
Both SARS-CoV and SARS-CoV-2 have been reported to
use ACE2 as the key receptor for their host cell entry (4-6).
Indeed, an initial step in these viral infections is the binding of
this cellular receptor with the viral spike S glycoprotein (S
protein), which consists of the S1 and S2 subunits (Fig. 1). The S1 subunit contains a
C-terminal receptor-binding domain that recognizes and binds to the
specific host receptor (e.g., ACE2), whilst the S2 subdomain is
responsible for the fusion of the viral membrane with the host cell
membrane via the fusion peptide. Following this binding to ACE2, a
protease cleavage of the S protein follows. This process is
catalysed by transmembrane protease serine (TMPRSS)2, and other
host proteases, such as cathepsin L and furin at the S1-S2 site,
which activate the entry of SARS-CoV-2 (5,7-11).
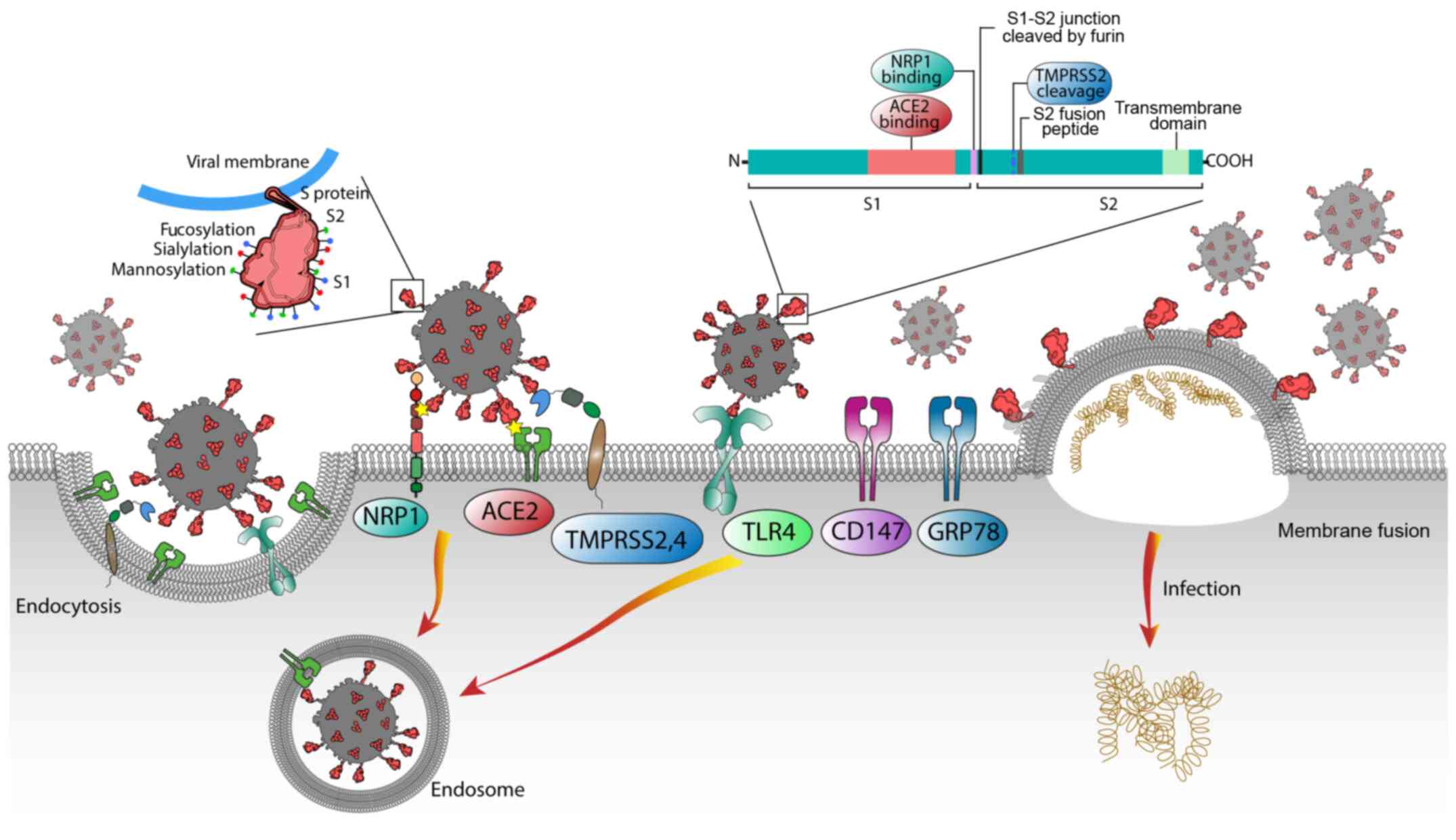 | Figure 1Schematic diagram of the known
pathways facilitating the entry of SARS-CoV-2 into host cells. The
SARS-CoV-2 spike (S) glycoproteins are instrumental for this
process; S1 (being the cell connecting head of the molecule) binds
to the ACE2 receptor, while S2 mediates the viral-cell membrane
fusion by being exposed to TMPRSS2 and TMPRSS4 or other host cell
proteases, such as furin, that is cleaved at the S1-S2 junction and
allows the binding of S1 to NRP1. Additional molecules, including
TLR4, CD147 and GRP78, have also been proposed as potential
SARS-CoV-2 cell entry mediators. SARS-CoV2, severe acute
respiratory syndrome coronavirus 2; ACE2, angiotensin converting
enzyme 2; NPR1, neuropilin-1; TMPRSS2 and 4, transmembrane protease
serine 2 and 4; TLR4, Toll-like receptor 4; GRP78,
glucose-regulated protein 78; CD147, basigin (BSG). |
Based on consistent data, ACE2 is now
well-established as the key mediator that facilitates the entry of
SARS-CoV-2 into human host cells (6,12,13). Notably, ACE2 is highly expressed
in the kidneys, small intestine, prostate and lung alveolar
epithelial type II cells, which explains the documented tropism of
this novel coronavirus and the related pulmonary and extrapulmonary
symptoms that a SARS-CoV-2 infection may cause (14). The analysis of autopsy tissue
from fatal COVID-19 cases has most frequently detected SARS-CoV-2
RNA in the airway epithelium, whilst SARS-CoV-2 RNA has also been
found to be highly co-localised in cells expressing TMPRSS2
(15-18). Of note, it has been suggested
that DNA polymorphisms for ACE2 and/or TMPRSS2 may be associated
with the genetic susceptibility of different populations to severe
COVID-19 infection (9). Along
with TMPRSS2, TMPRSS4 has also been shown to be involved in the
infectivity of SARS-CoV-2. In human small intestinal enterocytes,
the presence of both enzymes drives the cleavage of the spike S
glycoprotein of SARS-CoV-2 and enhances subsequent membrane fusion
(19). Moreover, TMPRSS2 and
TMPRSS4, along with ACE2, are abundantly co-expressed in the small
intestine and colon (20).
Subsequently, the authors have previously demonstrated that TMPRSS4
is overexpressed in 11 types of cancer in the colon mucosa and in
all the brain regions that are associated with the sense of taste
and smell (21).
3. Neuropilin-1
It is noteworthy that the relatively low/moderate
expression of ACE2 in the human respiratory system has triggered
further research focusing on the discovery of potential additional
cell proteins/receptors that may mediate the SARS-CoV-2 infectivity
and contribute to the selective tissue/organ tropism of this new
coronavirus (22,23). In this context, two elegant
studies by Daly et al (24) and Cantuti-Castelvetri (25) et al have demonstrated that
neuropilin (NRP)1 constitutes an additional cellular mediator
implicated in the entry of SARS-CoV-2 into human cells. Indeed,
based on the experimental data of both these studies, it is evident
that, not only ACE2, but also NRP1 can act as a host cell mediator
that enhances the infectivity of SARS-CoV-2 and contributes to its
documented tissue/organ tropism.
Overall, NRPs are classed as non-tyrosine kinase,
single-transmembrane glycoproteins which are greatly conserved in
vertebrates and constitute co-receptors for a number of molecules,
such as semaphorins and vascular endothelial growth factors
(VEGFs), playing a key role in numerous physiological processes
(e.g., neuronal development and axon control, immune function
angiogenesis, cell proliferation and vascular permeability)
(23-26).
Of note, the aforementioned studies by Daly et
al (24) and
Cantuti-Castelvetri et al (25) suggest that, unlike SARS-CoV S
proteins, SARS-CoV-2 S proteins have a polybasic sequence motif
(Arg-Arg-Ala-Arg) at the S1-S2 junction/boundary that conforms to
the 'C-end rule' (CendR motif) [R-XX-R; where R denotes arginine
(Arg), can be replaced by lysine (Lys)] and serves as a cleavage
site for the convertase furin (Fig.
1) (24-26). Thus, SARS-CoV-2 can enter host
cells more easily with the aid of NRP1, boosting its infectivity
and promoting its tropism (24,25). Indeed, with the use of human cell
lines, it has been shown that NRP1 enhances infection by a clinical
SARS-CoV-2 isolate and lentiviral particles pseudotyped with the S
protein of SARS-CoV-2 by binding to this CendR motif of the
furin-cleaved SARS-CoV-2 S1 protein. This NRP1-mediated increase in
SARS-CoV-2 infectivity appears to be due to increased viral entry
into host cells, rather than increased viral binding to the cell
membrane, whilst it is amplified when ACE2 and TMPRSS2 are present.
On the other hand, the rate of NRP1-enhanced SARS-CoV-2 infection
in human cell cultures has been shown to be suppressed by using a
monoclonal blocking antibody against the extracellular NRP1 b1b2
space, or a specific NRP1 mutant which ties the CendR-binding b1
domain (24,26,27). In addition, as previously
demonstrated, mutated variants of SARS-CoV-2 with a modified furin
cleavage site of the S protein (erased poly-basic cleavage site or
impervious to furin-intervened cleavage) were not subject to
NRP1-mediated infectivity, and similarly transformations in the
NRP1 b1 domain likewise restrained the NRP1-S1 interaction
(24,26,27).
Notably, the upregulation of NRP1 gene expression
has also been demonstrated in lung tissue samples and infected
olfactory epithelial cells from patients with COVID-19; further
co-staining revealed the infection of cells that were positive for
oligodendrocyte transcription factor 2, which is primarily
expressed by olfactory neuronal progenitors (27).
Given these data on NRP1 and since extra-pulmonary
symptoms (e.g., gastrointestinal and neurological symptoms) are
frequently identified in relation to COVID-19, extensive additional
research is still required to identify the association between
SARS-CoV-2 tropism and the mechanisms through which host cell
infection mediators, such as NRP1, are dispersed across the various
tissues/organs of the human body. This may further aid in the
development of novel antiviral drugs against COVID-19, which for
example could be targeting NRP1 and its role in promoting the entry
of SARS-CoV-2 into host cells.
4. Emerging SARS-CoV-2 cell entry
mediators
Following the initial findings regarding the role of
host cell receptors/mediators, such as ACE2 and NRP1, data are also
emerging for a number of additional cellular factors which may also
facilitate or inhibit the infectivity of SARS-CoV-2, including
glucose-regulated protein 78 (GRP78), basigin (BSG; CD147), kidney
injury molecule-1 (KIM1), heparan sulfate (HS), ADAM
metallopeptidase domain 17 [also termed tumour necrosis factor-α
convertase (ADAM17)], surfactant protein D (SP-D), metabotropic
glutamate receptor subtype 2 (mGluR2) and Toll-like receptor 4
(TLR4).
In this context, heat shock protein A5 (HSPA5), also
termed GRP78, has been reported to be implicated in a possible
route for SARS-CoV-2 cell attachment and entry. Indeed, it has been
proposed that the recognition site for GRP78 is found in SARS-CoV-2
(28), and that the binding is
more favourable between regions III (C391-C525) and IV (C480-C488)
of the SARS-CoV-2 protein spike model and GRP78 (29). Moreover, CD147 (also known as
EMMPRIN or BSG; a transmembrane glycoprotein that belongs to the
large immunoglobulin superfamily) has also been proposed as another
molecule that can facilitate the entry of SARS-CoV-2 into host
cells by endocytosis. This is supported by the findings of an in
vitro study in which SARS-CoV-2 amplification was inhibited
when CD147 was blocked in Vero E6 and BEAS-2B cell lines (30). However, based on the findings of
a later study, another group argued that there was no evidence for
a direct interaction between the viral spike protein and this
particular molecule (31). Such
contradictory findings highlight the current need for further
research into the entire spectrum of potential host-pathogen
interactions for SARS-CoV-2.
Furthermore, given that kidney-related complications
are common in patients with COVID-19 (32), the involvement of KIM1 has been
recently investigated. Accordingly, through a series of
experiments, it was previously demonstrated that KIM1 is capable of
interacting with the receptor-binding domain of this new
coronavirus, subsequently enabling its attachment to the plasma
membrane (33). These findings
suggest that this molecule may mediate and exacerbate SARS-CoV-2
infection of the kidneys (33).
Moreover, KIM1 may function as a biomarker of acute kidney injury,
which has been associated with a poor prognosis of patients with
COVID-19 (34).
mGluR2 (GRM2), a G protein-coupled receptor which
inhibits adenylyl cyclase and regulates the mechanisms through
which glutamate can control cell excitability, is another potential
cell entry receptor for SARS-CoV-2. Indeed, recent experiments have
demonstrated that mGluR2 interacts directly with the S protein of
SARS-CoV-2, whilst the knockdown of mGluR2, even though it does not
affect binding to the plasma membrane, reduces the
clathrin-mediated endocytosis of this virus (35). Moreover, in vivo studies
corroborated these findings, where viral infectivity in lungs of
mice was significantly reduced, when mGluR2 was knocked out
(35). Of note, angiotensin II
receptor type 2 is another G protein-coupled receptor, which, based
on an in silico simulation data has revealed an interaction
with the S protein of SARS-CoV-2 (36). However, further in vitro
and/or in vivo studies are required to validate these
initial modelling data.
Apart from direct cell entry mediators of SARS-CoV-2
into host cells, there have been molecules which appear to function
as co-factors in this process, such as HS and ADAM17. Of note, HS
is a co-factor for ACE2-mediated viral entry and a potential
therapeutic target (e.g., using mitoxantrone) (37). On the other hand, ADAM17 has been
shown to regulate the shedding of the ACE2 ectodomain (38). This is in line with its role in
releasing ectodomains of a wide repertoire of cytokines, enzymes or
other molecules (38,39). However, due to a potential
redundancy in viral cell entry, it has been demonstrated that
TMPRSS2 can compete with ADAM17 for ACE2 processing (40). Furthermore, SP-D is an innate
immune molecule with a main role in clearing pathogens and
apoptotic/necrotic cells from pulmonary and extra-pulmonary mucosal
sites (41). Given that human
SP-D can recognise the spike glycoprotein of SARS-CoV, a recent
study demonstrated that the treatment of 293T cells overexpressing
ACE2 with a recombinant fragment of human SP-D (rfhSP-D) inhibited
the interaction of the S1 spike proteins with the cell membrane,
raising the possibility of an alternative therapeutic target
(42). Indeed, in another study,
when clinical samples were used, treatment with rfhSP-D inhibited
viral replication, and appeared to be more efficient than the
antiviral drug remdesivir, when Vero cells were treated in
vitro (43). Currently, a
Phase 1 clinical trial (ClinicalTrials.gov identifier: NCT04659122) is
ongoing, entitled: 'Phase 1b Open-label, Single Arm, Cohort Dose
Escalation Study Evaluating the Safety, Tolerability, and
Feasibility of Intervention With AT-100 (rhSP-D) in Intubated
Patients Receiving Invasive Mechanical Ventilation With Severe
COVID-19 Infection'. Finally, in a previous observational study on
39 patients with COVID-19, SP-D levels were found to be
significantly higher in severe compared to mild cases, suggesting
the possibility of its use as a biomarker of severity as well
(44).
Finally, another innate immune molecule that has
gained increasing interest in the context of COVID-19 is TLR4,
which represents a key cell surface receptor that induces the
secretion of pro-inflammatory cytokines and interferons to fight
infection (45). Indeed, there
is accumulating evidence to indicate that SARS-CoV-2 can bind to
TLR4, whilst treatment with resatorvid (a TLR4 specific inhibitor)
has been shown to abolish the secretion of IL1B by SARS-CoV-2 in
THP-1 cells (46,47).
Overall, identifying the underlying mechanisms that
facilitate the entry of SARS-CoV-2 into host cells (Fig. 1) is currently the focus of
intensive research which is expected to provide further valuable
insight into the pathophysiology of COVID-19, identifying the human
tissues/organs that are more vulnerable to a SARS-CoV-2 infection.
Ultimately, this may also aid in the development of more precise
treatments against COVID-19 sequelae. A list of the 'canonical' and
potential cell entry mediators is provided in Table I.
 | Table IGenes related to SARS-CoV-2 entry
into host cells. |
Table I
Genes related to SARS-CoV-2 entry
into host cells.
| Cell entry
mediators | Abbreviation | Proposed
action | (Refs.) |
|---|
| Angiotensin
converting enzyme 2 | ACE2 | Canonical receptor
for the S glycoprotein (S1 domain) | (4,48,49) |
| Transmembrane
protease, serine 2 and serine 4 | TMPRSS2
TMPRSS4 | Mediate fusion of
viral and host cell membranes after proteolytic cleavage at the
S1/S2 region | (7,19,50,51) |
| Neuropilin-1 | NRP1 | Additional
SARS-CoV-2 host cell receptor, increasing its infectivity and
contributing to its tropism | (20,23-25,48) |
| Toll-like receptor
4 | TLR4 | Potential
alternative receptor for SARS-CoV-2 | (46,47,52) |
| Kidney injury
molecule-1 | KIM1 | Potential
interaction with SARS-CoV-2 receptor-binding domain | (33,34) |
| Basigin | BSG/CD147 | Potential
facilitator of the entry of SARS-CoV-2 into host cells by
endocytosis | (30,31) |
| Heat shock protein
A5 or glucose regulating protein 78 | HSPA5/GRP78 | Possible route for
SARS-CoV-2 cell attachment and entry | (28,29) |
| ADAM
metallopeptidase domain 17 or TNF-α convertase enzyme | ADAM17 | Regulates shedding
of the ACE2 ectodomain | (10,38,39) |
5. Identification of miRNAs that can target
and regulate the gene expression of SARS-CoV-2 cell entry
mediators
miRNAs are single-strand, endogenous, non-coding,
short RNAs of 22-25 nucleotides in length, which can regulate gene
expression post-transcriptionally and subsequently alter signalling
pathways (53). A series of
review articles have elegantly summarised the functions of miRNAs
in health and disease (54-57). Of note, miRNAs regulate the
expression of key inflammatory cytokines involved in the massive
recruitment of immune cells to the lungs, such as IL6 and TNFα.
Several studies have demonstrated that changes in the expression of
miRNAs in patients with COVID-19 may be a predictor of tissue
damage and lung inflammation, whilst miRNAs can also be potent
diagnostic biomarkers for COVID-19 (58-60).
Several miRNAs have been either predicted or shown
to target SARS-CoV-2 RNAs (61).
Indeed, a number of miRNAs have been found to target the RNA S
glycoprotein sequence of SARS-CoV-2, which interacts with ACE2 for
viral entry into host cells, including hsa-miR-4661-3p (62), hsa-miR-510-3p, hsa-miR-624-5p,
hsa-miR-497-5p (63),
hsa-miR-622, hsa-miR-761, miR-A3r, hsa-miR-15b-5p, miR-A2r,
hsa-miR-196a-5p (64), and
miR-338-3p, miR-4661-3p, miR-4761-5p, hsa-miR-4464,
hsa-miR-1234-3p, hsa-miR-7107-5p and hsa-miR-885-5p, which have
been shown to bind to the receptor binding domain of the S gene
(62).
As such, a number of studies have identified host
cell miRNAs that target different SARS-CoV-2 proteins. Considering
the aforementioned significance of ACE2 and TMPRSS2 in SARS-CoV-2
infection, in a previous study, a TargetScan analysis revealed
several miRNAs that could directly target these two receptors,
including hsa-mi-200b-3p, hsa-miR-200c-3p and hsa-miR-429 for ACE2,
and hsa-let7a-5p, hsa-let7b-5p, hsa-let7c-5p, hsa-let7d-5p,
hsa-let7e-5p, hsa-let7f-5p, hsa-let7g-5p, hsa-let7i-5p,
hsa-miR-98-5p, has-miR-4458 and hsa-miR-4500 for TMPRSS2; thus,
these miRNAs may be utilised as potent therapeutic molecules to
regulate key proteins that are required for viral entry to the host
airway/lung epithelial cells (59). Moreover, TMPRSS2, which activates
the spike protein of SARS-CoV-2 to promote viral infection, has
been predicted to be regulated by MR147-3p in the gut (62).
Overall, several cell host miRNAs that target the
ACE2 and TMPRSS2 genes can prevent attachment and the entry of
SARS-CoV-2 into different tissues. For example, hsa-miR-98-5p has
been shown to directly target the 3′-UTR of TMPRSS2, both in human
lung microvascular endothelial cells (HMVEC-L) and human umbilical
vein endothelial cells (65).
Furthermore, the hsa-let-7e/hsa-mir-125a and
hsa-mir-141/hsa-miR-200 miRNA families inhibit ACE2 and TMPRSS2
expression (66). It has also
been shown that miR-200c is essential for SARS-CoV-2 binding to the
ACE2 receptor and entry into cardiomyocytes (67), while miR-98-5p can inhibit
TMPRSS2 expression in human endothelial cells (65). In a previous study,
bioinformatics analysis revealed that lysine-specific demethylase
5B can regulate ACE2 and TMPRSS2 via the transcriptional repression
of let-7e/miR-125a and miR-141/miR-200 miRNAs; thus, these miRNAs
are crucial for the function of ACE2 and TMPRSS2 (66).
Notably, a recent study provided novel evidence of
an interplay between host miRNAs and SARS-CoV-2, as well as the
mechanisms through which these interactions can drive pathogenesis
by dysregulating certain immune pathways (68). A recent mini-review, provided an
excellent overview of the role of COVID-19-associated miRNAs as
potential therapeutic targets and biomarkers (69). Currently, there are a number of
clinical trials/studies investigating miRNAs in the context of
COVID-19. For example, in an observational study on the expression
of cytokines, transcriptome and miRNAs in COVID-19 patients is
currently recruiting (ClinicalTrials.gov identifier: NCT04583566).
Similarly, 'The Effect of miRNA and Epigenetic Modifications on
Prognosis in Covid-19 Infection' will also be investigated
(ClinicalTrials.gov Identifier:
NCT04411563), whilst another study aims to assess the mechanisms
through which miRNA levels relate to viral infection (ClinicalTrials.gov Identifier: NCT04346160). In terms
of therapeutics, a phase 2 trial is currently enrolling by
invitation, with miRNA-containing exosomes as an aerosol inhaler
(ClinicalTrials.gov Identifier:
NCT04602442).
Of note, the targeting of PARP-1 by miRNAs has been
suggested to hold therapeutic value for COVID-19-related
pathologies (70). In a recent
in silico study, a number of hypothalamic miRNAs that can
bind and regulate ACE2 and TMPRSS2 were identified (71). The authors of that study
concluded that these miRNAs may be used in the search for novel
therapies for the neurological symptoms in patients with COVID-19.
The present study expanded on these initial observations by
identifying potential miRNA modulators of all remaining SARS-CoV-2
cell entry mediators, using miRDB, an online database for miRNA
target prediction and functional annotations (mirdb.org) (72,73). For the present study, the target
prediction score was set >80 for higher confidence in the
interactions. As such, Table II
provides a list of five potential candidate miRNAs against TMPRRS4;
Table III provides a list of
69 miRNAs for NRP1; Table IV
indicates 36 potential miRNA interactions with ADAM17; Table V presents five interactions with
KIM1; Table VI presents a list
of 27 interactions with GRP78 (HSPA5); Table VII presents only one possible
interaction identified between a miRNA and CD147 (BSG); Table VIII presents 12 interactions
identified between miRNAs and TLR4; and finally, Table IX outlines five possible
interactions of these regulatory RNAs with mGluR2.
 | Table IIList of miRNAs that indicate strong
binding potential against transmembrane serine protease 4. |
Table II
List of miRNAs that indicate strong
binding potential against transmembrane serine protease 4.
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 88 |
hsa-miR-6721-5p |
5′-ugggcaggggcuuauuguaggag-3′ |
| 2 | 85 | hsa-miR-3671 |
5′-aucaaauaaggacuagucugca-3′ |
| 3 | 82 | hsa-miR-496 |
5′-ugaguauuacauggccaaucuc-3′ |
| 4 | 81 |
hsa-miR-551b-3p |
5′-gcgacccauacuugguuucag-3′ |
| 5 | 81 | hsa-miR-551a |
5′-gcgacccacucuugguuucca-3′ |
 | Table IIIList of miRNAs that indicate strong
binding potential against neuropilin-1. |
Table III
List of miRNAs that indicate strong
binding potential against neuropilin-1.
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 98 | hsa-miR-3646 |
5′-aaaaugaaaugagcccagccca-3′ |
| 2 | 97 | hsa-miR-7977 |
5′-uucccagccaacgcacca-3′ |
| 3 | 96 | hsa-miR-6844 |
5′-uucuuuguuuuuaauucacag-3′ |
| 4 | 95 | hsa-miR-124-3p |
5′-uaaggcacgcggugaaugccaa-3′ |
| 5 | 95 | hsa-miR-3133 |
5′-uaaagaacucuuaaaacccaau-3′ |
| 6 | 95 | hsa-miR-5094 |
5′-aaucagugaaugccuugaaccu-3′ |
| 7 | 95 | hsa-miR-506-3p |
5′-uaaggcacccuucugaguaga-3′ |
| 8 | 95 | hsa-miR-153-5p |
5′-ucauuuuugugauguugcagcu-3′ |
| 9 | 94 |
hsa-miR-4755-5p |
5′-uuucccuucagagccuggcuuu-3′ |
| 10 | 94 |
hsa-miR-148a-3p |
5′-ucagugcacuacagaacuuugu-3′ |
| 11 | 94 | hsa-miR-152-3p |
5′-ucagugcaugacagaacuugg-3′ |
| 12 | 94 |
hsa-miR-148b-3p |
5′-ucagugcaucacagaacuuugu-3′ |
| 13 | 94 | hsa-miR-3148 |
5′-uggaaaaaacuggugugugcuu-3′ |
| 14 | 94 |
hsa-miR-5006-3p |
5′-uuucccuuuccauccuggcag-3′ |
| 15 | 93 |
hsa-miR-1285-3p |
5′-ucugggcaacaaagugagaccu-3′ |
| 16 | 93 | hsa-miR-548an |
5′-aaaaggcauugugguuuuug-3′ |
| 17 | 93 | hsa-miR-3686 |
5′-aucuguaagagaaaguaaauga-3′ |
| 18 | 93 | hsa-miR-651-3p |
5′-aaaggaaaguguauccuaaaag-3′ |
| 19 | 93 |
hsa-miR-5189-5p |
5′-ucugggcacaggcggauggacagg-3′ |
| 20 | 92 | hsa-let-7a-3p |
5′-cuauacaaucuacugucuuuc-3′ |
| 21 | 92 | hsa-miR-98-3p |
5′-cuauacaacuuacuacuuuccc-3′ |
| 22 | 92 | hsa-miR-6860 |
5′-acugggcagggcuguggugagu-3′ |
| 23 | 92 | hsa-miR-1-3p |
5′-uggaauguaaagaaguauguau-3′ |
| 24 | 92 | hsa-miR-612 |
5′-gcugggcagggcuucugagcuccuu-3′ |
| 25 | 92 |
hsa-miR-3187-5p |
5′-ccugggcagcguguggcugaagg-3′ |
| 26 | 92 | hsa-let-7b-3p |
5′-cuauacaaccuacugccuuccc-3′ |
| 27 | 92 | hsa-miR-206 |
5′-uggaauguaaggaagugugugg-3′ |
| 28 | 92 |
hsa-miR-5004-5p |
5′-ugaggacagggcaaauucacga-3′ |
| 29 | 92 |
hsa-let-7f-1-3p |
5′-cuauacaaucuauugccuuccc-3′ |
| 30 | 91 | hsa-miR-510-3p |
5′-auugaaaccucuaagagugga-3′ |
| 31 | 90 | hsa-miR-320d |
5′-aaaagcuggguugagagga-3′ |
| 32 | 90 | hsa-miR-613 |
5′-aggaauguuccuucuuugcc-3′ |
| 33 | 90 | hsa-miR-4429 |
5′-aaaagcugggcugagaggcg-3′ |
| 34 | 90 | hsa-miR-320c |
5′-aaaagcuggguugagagggu-3′ |
| 35 | 90 |
hsa-miR-6806-3p |
5′-ugaagcucugacauuccugcag-3′ |
| 36 | 90 |
hsa-miR-3928-5p |
5′-ugaagcucuaagguuccgccugc-3′ |
| 37 | 90 |
hsa-miR-320a-3p |
5′-aaaagcuggguugagagggcga-3′ |
| 38 | 90 | hsa-miR-338-3p |
5′-uccagcaucagugauuuuguug-3′ |
| 39 | 90 | hsa-miR-9-5p |
5′-ucuuugguuaucuagcuguauga-3′ |
| 40 | 90 | hsa-miR-1322 |
5′-gaugaugcugcugaugcug-3′ |
| 41 | 90 | hsa-miR-320b |
5′-aaaagcuggguugagagggcaa-3′ |
| 42 | 89 | hsa-miR-150-3p |
5′-cugguacaggccugggggacag-3′ |
| 43 | 89 | hsa-miR-4261 |
5′-aggaaacagggaccca-3′ |
| 44 | 88 |
hsa-miR-6733-3p |
5′-ucagugucuggauuuccuag-3′ |
| 45 | 88 | hsa-miR-5701 |
5′-uuauugucacguucugauu-3′ |
| 46 | 87 | hsa-miR-3920 |
5′-acugauuaucuuaacucucuga-3′ |
| 47 | 87 |
hsa-miR-147b-5p |
5′-uggaaacauuucugcacaaacu-3′ |
| 48 | 87 |
hsa-miR-4724-5p |
5′-aacugaaccaggagugagcuucg-3′ |
| 49 | 87 | hsa-miR-4251 |
5′-ccugagaaaagggccaa-3′ |
| 50 | 86 | hsa-miR-137-3p |
5′-uuauugcuuaagaauacgcguag-3′ |
| 51 | 86 |
hsa-miR-4789-5p |
5′-guauacaccugauauguguaug-3′ |
| 52 | 86 | hsa-miR-570-3p |
5′-cgaaaacagcaauuaccuuugc-3′ |
| 53 | 85 | hsa-miR-4801 |
5′-uacacaagaaaaccaaggcuca-3′ |
| 54 | 84 | hsa-miR-24-3p |
5′-uggcucaguucagcaggaacag-3′ |
| 55 | 84 | hsa-miR-587 |
5′-uuuccauaggugaugagucac-3′ |
| 56 | 83 | hsa-miR-4324 |
5′-cccugagacccuaaccuuaa-3′ |
| 57 | 83 |
hsa-miR-181a-2-3p |
5′-accacugaccguugacuguacc-3′ |
| 58 | 82 | hsa-miR-186-5p |
5′-caaagaauucuccuuuugggcu-3′ |
| 59 | 82 |
hsa-let-7f-2-3p |
5′-cuauacagucuacugucuuucc-3′ |
| 60 | 82 |
hsa-miR-6843-3p |
5′-auggucuccuguucucugcag-3′ |
| 61 | 82 |
hsa-miR-1185-1-3p |
5′-auauacagggggagacucuuau-3′ |
| 62 | 82 |
hsa-miR-6848-3p |
5′-guggucucuuggcccccag-3′ |
| 63 | 82 |
hsa-miR-6853-3p |
5′-uguucauuggaacccugcgcag-3′ |
| 64 | 82 |
hsa-miR-1185-2-3p |
5′-auauacagggggagacucucau-3′ |
| 65 | 81 |
hsa-miR-6736-3p |
5′-ucagcuccucucuacccacag-3′ |
| 66 | 81 | hsa-miR-4277 |
5′-gcaguucugagcacaguacac-3′ |
| 67 | 81 | hsa-miR-3662 |
5′-gaaaaugaugaguagugacugaug-3′ |
| 68 | 81 | hsa-miR-1289 |
5′-uggaguccaggaaucugcauuuu-3′ |
| 69 | 81 | hsa-miR-1825 |
5′-uccagugcccuccucucc-3′ |
 | Table IVList of miRNAs that indicate strong
binding potential against ADAM metallopeptidase domain 17. |
Table IV
List of miRNAs that indicate strong
binding potential against ADAM metallopeptidase domain 17.
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 99 | hsa-miR-3163 |
5′-uauaaaaugagggcaguaagac-3′ |
| 2 | 97 |
hsa-miR-548ah-3p |
5′-caaaaacugcaguuacuuuugc-3′ |
| 3 | 97 |
hsa-miR-548am-3p |
5′-caaaaacugcaguuacuuuugu-3′ |
| 4 | 97 |
hsa-miR-548x-3p |
5′-uaaaaacugcaauuacuuuc-3′ |
| 5 | 97 | hsa-miR-4465 |
5′-cucaaguagucugaccagggga-3′ |
| 6 | 97 |
hsa-miR-548aj-3p |
5′-uaaaaacugcaauuacuuuua-3′ |
| 7 | 96 |
hsa-miR-548ae-3p |
5′-caaaaacugcaauuacuuuca-3′ |
| 8 | 96 |
hsa-miR-548aq-3p |
5′-caaaaacugcaauuacuuuugc-3′ |
| 9 | 96 | hsa-miR-1297 |
5′-uucaaguaauucaggug-3′ |
| 10 | 96 | hsa-miR-26a-5p |
5′-uucaaguaauccaggauaggcu-3′ |
| 11 | 96 | hsa-miR-26b-5p |
5′-uucaaguaauucaggauaggu-3′ |
| 12 | 96 |
hsa-miR-548j-3p |
5′-caaaaacugcauuacuuuugc-3′ |
| 13 | 89 | hsa-miR-507 |
5′-uuuugcaccuuuuggagugaa-3′ |
| 14 | 88 | hsa-miR-5697 |
5′-ucaaguaguuucaugauaaagg-3′ |
| 15 | 88 |
hsa-miR-4762-5p |
5′-ccaaaucuugaucagaagccu-3′ |
| 16 | 88 | hsa-miR-4719 |
5′-ucacaaaucuauaauaugcagg-3′ |
| 17 | 88 |
hsa-miR-5197-5p |
5′-caauggcacaaacucauucuuga-3′ |
| 18 | 88 | hsa-miR-4686 |
5′-uaucugcugggcuuucugguguu-3′ |
| 19 | 87 | hsa-miR-95-5p |
5′-ucaauaaaugucuguugaauu-3′ |
| 20 | 87 | hsa-miR-8064 |
5′-agcacacugagcgagcggac-3′ |
| 21 | 86 |
hsa-miR-7109-3p |
5′-caagccucuccugcccuuccag-3′ |
| 22 | 86 |
hsa-miR-513a-5p |
5′-uucacagggaggugucau-3′ |
| 23 | 84 |
hsa-miR-5195-3p |
5′-auccaguucucugagggggcu-3′ |
| 24 | 84 | hsa-miR-557 |
5′-guuugcacgggugggccuugucu-3′ |
| 25 | 84 | hsa-miR-145-5p |
5′-guccaguuuucccaggaaucccu-3′ |
| 26 | 84 | hsa-miR-12136 |
5′-gaaaaagucauggaggcc-3′ |
| 27 | 84 | hsa-miR-6126 |
5′-gugaaggcccggcggaga-3′ |
| 28 | 83 |
hsa-miR-4799-5p |
5′-aucuaaaugcagcaugccaguc-3′ |
| 29 | 83 |
hsa-miR-148a-3p |
5′-ucagugcacuacagaacuuugu-3′ |
| 30 | 83 | hsa-miR-152-3p |
5′-ucagugcaugacagaacuugg-3′ |
| 31 | 83 | hsa-miR-4464 |
5′-aagguuuggauagaugcaaua-3′ |
| 32 | 83 | hsa-miR-4748 |
5′-gagguuuggggaggauuugcu-3′ |
| 33 | 83 | hsa-miR-605-3p |
5′-agaaggcacuaugagauuuaga-3′ |
| 34 | 83 |
hsa-miR-148b-3p |
5′-ucagugcaucacagaacuuugu-3′ |
| 35 | 83 | hsa-miR-767-5p |
5′-ugcaccaugguugucugagcaug-3′ |
| 36 | 81 | hsa-miR-548l |
5′-aaaaguauuugcggguuuuguc-3′ |
 | Table VList of miRNAs that indicate strong
binding potential against hepatitis A virus cellular receptor 1
(HAVCR1, KIM1). |
Table V
List of miRNAs that indicate strong
binding potential against hepatitis A virus cellular receptor 1
(HAVCR1, KIM1).
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 90 | hsa-miR-4255 |
5′-caguguucagagaugga-3′ |
| 2 | 87 |
hsa-miR-4738-3p |
5′-ugaaacuggagcgccuggagga-3′ |
| 3 | 86 |
hsa-miR-3117-3p |
5′-auaggacucauauagugccag-3′ |
| 4 | 85 | hsa-miR-3171 |
5′-agauguauggaaucuguauauauc-3′ |
| 5 | 83 | hsa-miR-383-5p |
5′-agaucagaaggugauuguggcu-3′ |
 | Table VIList of miRNAs that indicate strong
binding potential against heat shock protein family A (Hsp70)
member 5 (HSPA5 or GRP78). |
Table VI
List of miRNAs that indicate strong
binding potential against heat shock protein family A (Hsp70)
member 5 (HSPA5 or GRP78).
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 99 |
hsa-miR-3121-3p |
5′-uaaauagaguaggcaaaggaca-3′ |
| 2 | 97 | hsa-miR-5688 |
5′-uaacaaacaccuguaaaacagc-3′ |
| 3 | 96 | hsa-miR-635 |
5′-acuugggcacugaaacaaugucc-3′ |
| 4 | 96 |
hsa-miR-6774-5p |
5′-acuugggcaggagggacccuguaug-3′ |
| 5 | 95 | hsa-miR-495-3p |
5′-aaacaaacauggugcacuucuu-3′ |
| 6 | 93 | hsa-miR-338-5p |
5′-aacaauauccuggugcugagug-3′ |
| 7 | 91 | hsa-miR-7-2-3p |
5′-caacaaaucccagucuaccuaa-3′ |
| 8 | 91 | hsa-miR-7-1-3p |
5′-caacaaaucacagucugccaua-3′ |
| 9 | 90 | hsa-miR-30d-3p |
5′-cuuucagucagauguuugcugc-3′ |
| 10 | 89 |
hsa-miR-199a-5p |
5′-cccaguguucagacuaccuguuc-3′ |
| 11 | 89 |
hsa-miR-199b-5p |
5′-cccaguguuuagacuaucuguuc-3′ |
| 12 | 89 |
hsa-miR-4699-3p |
5′-aauuuacucugcaaucuucucc-3′ |
| 13 | 88 |
hsa-miR-7854-3p |
5′-ugaggugaccgcagaugggaa-3′ |
| 14 | 87 |
hsa-miR-4650-3p |
5′-agguagaaugaggccugacau-3′ |
| 15 | 85 | hsa-miR-222-5p |
5′-cucaguagccaguguagauccu-3′ |
| 16 | 84 |
hsa-miR-16-1-3p |
5′-ccaguauuaacugugcugcuga-3′ |
| 17 | 84 | hsa-miR-30e-3p |
5′-cuuucagucggauguuuacagc-3′ |
| 18 | 84 |
hsa-miR-148a-5p |
5′-aaaguucugagacacuccgacu-3′ |
| 19 | 84 | hsa-miR-30a-3p |
5′-cuuucagucggauguuugcagc-3′ |
| 20 | 84 | hsa-miR-4451 |
5′-ugguagagcugaggaca-3′ |
| 21 | 84 |
hsa-miR-7162-3p |
5′-ucugagguggaacagcagc-3′ |
| 22 | 83 |
hsa-miR-6777-3p |
5′-uccacucuccuggcccccag-3′ |
| 23 | 82 | hsa-miR-770-5p |
5′-uccaguaccacgugucagggcca-3′ |
| 24 | 82 |
hsa-miR-4712-5p |
5′-uccaguacaggucucucauuuc-3′ |
| 25 | 81 |
hsa-miR-3606-3p |
5′-aaaauuucuuucacuacuuag-3′ |
| 26 | 81 |
hsa-miR-513c-3p |
5′-uaaauuucaccuuucugagaaga-3′ |
| 27 | 81 |
hsa-miR-513a-3p |
5′-uaaauuucaccuuucugagaagg-3′ |
 | Table VIIOne miRNA which exhibits strong
binding potential against basigin (BSG, CD147). |
Table VII
One miRNA which exhibits strong
binding potential against basigin (BSG, CD147).
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 93 |
hsa-miR-1252-5p |
5′-agaaggaaauugaauucauuua-3′ |
 | Table VIIIList of miRNAs that indicate strong
binding potential against Toll-like receptor 4. |
Table VIII
List of miRNAs that indicate strong
binding potential against Toll-like receptor 4.
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 93 | hsa-miR-448 |
5′-uugcauauguaggaugucccau-3′ |
| 2 | 91 | hsa-miR-3924 |
5′-auauguauaugugacugcuacu-3′ |
| 3 | 89 | hsa-miR-627-3p |
5′-ucuuuucuuugagacucacu-3′ |
| 4 | 88 | hsa-miR-338-5p |
5′-aacaauauccuggugcugagug-3′ |
| 5 | 88 | hsa-miR-4272 |
5′-cauucaacuagugauugu-3′ |
| 6 | 87 |
hsa-miR-10397-5p |
5′-uccuugaccugaugcuguaggg-3′ |
| 7 | 87 |
hsa-miR-5583-3p |
5′-gaauauggguauauuaguuugg-3′ |
| 8 | 85 |
hsa-miR-4760-3p |
5′-aaauucauguucaaucuaaacc-3′ |
| 9 | 85 |
hsa-miR-4652-3p |
5′-guucuguuaacccauccccuca-3′ |
| 10 | 84 | hsa-miR-1243 |
5′-aacuggaucaauuauaggagug-3′ |
| 11 | 81 | hsa-miR-587 |
5′-uuuccauaggugaugagucac-3′ |
| 12 | 81 | hsa-miR-4678 |
5′-aagguauuguucagacuuauga-3′ |
 | Table IXList of miRNAs that indicate strong
binding potential against metabotropic glutamate receptor subtype
2. |
Table IX
List of miRNAs that indicate strong
binding potential against metabotropic glutamate receptor subtype
2.
| Target rank | Target score | miRNA name | miRNA sequence |
|---|
| 1 | 86 | hsa-miR-4492 |
5′-ggggcugggcgcgcgcc-3′ |
| 2 | 84 | hsa-miR-555 |
5′-aggguaagcugaaccucugau-3′ |
| 3 | 83 |
hsa-miR-4436b-3p |
5′-cagggcaggaagaaguggacaa-3′ |
| 4 | 83 | hsa-miR-497-3p |
5′-caaaccacacugugguguuaga-3′ |
| 5 | 82 | hsa-miR-4498 |
5′-ugggcuggcagggcaagugcug-3′ |
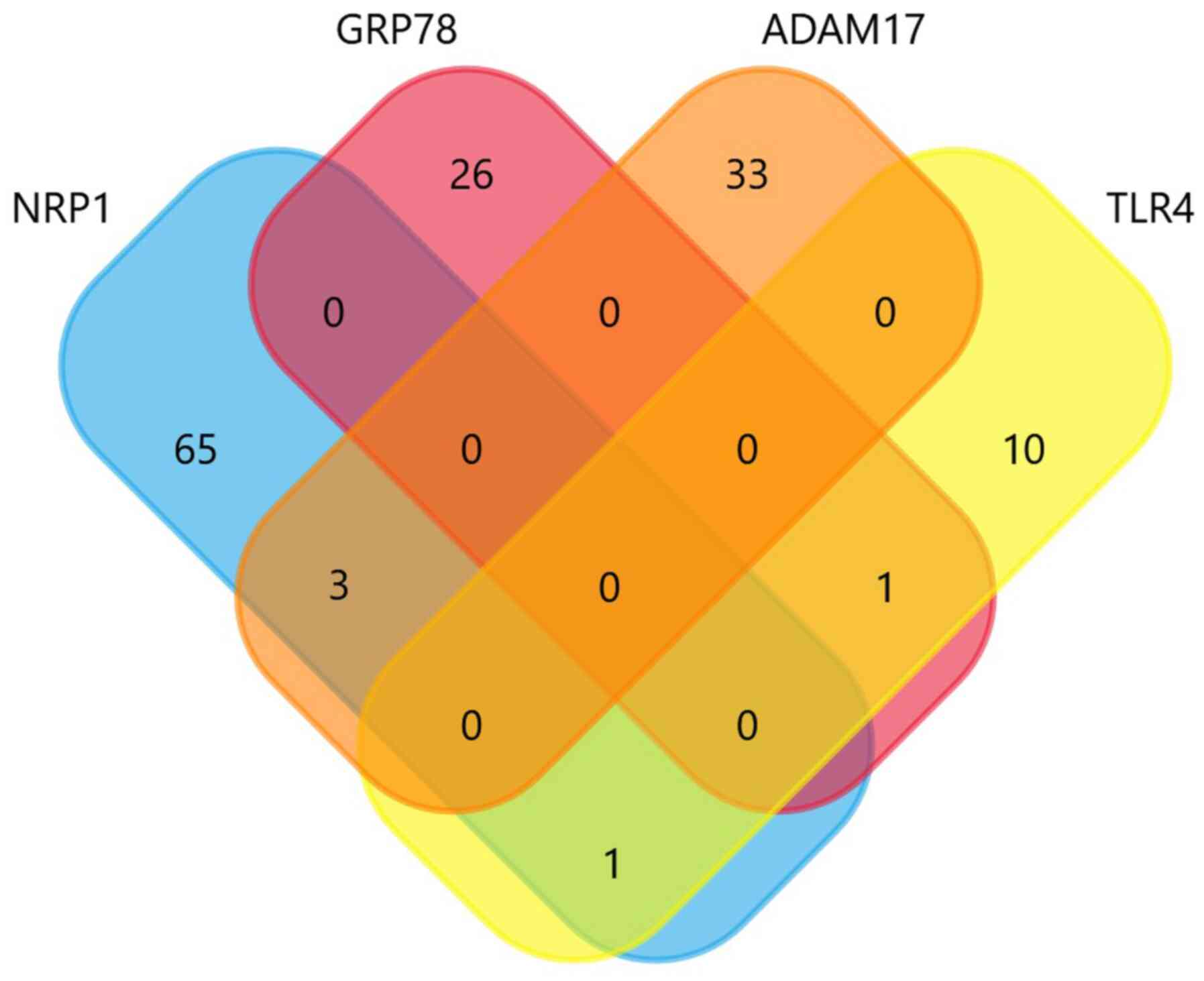
Further analysis using Venn diagrams (Fig. 2), revealed that there were three
common miRNAs amongst NRP1 and ADAM17, namely hsa-miR-148a-3p,
hsa-miR-152-3p and hsa-miR-148b-3p. One common miRNA, i.e.,
hsa-miR-587, was also shared between TLR4 and NRP1, and another
miRNA (hsa-miR-338-5p) between TLR4 and GRP78. Of note,
hsa-miR-148a-3p was also shared with ACE2, as previously reported
(71). Moreover, hsa-miR-587 was
recently identified as a top miRNA regulating ACE2 networks; it was
thus concluded that these miRNAs can be used in the personalized
diagnosis of patients with COVID-19 (74).
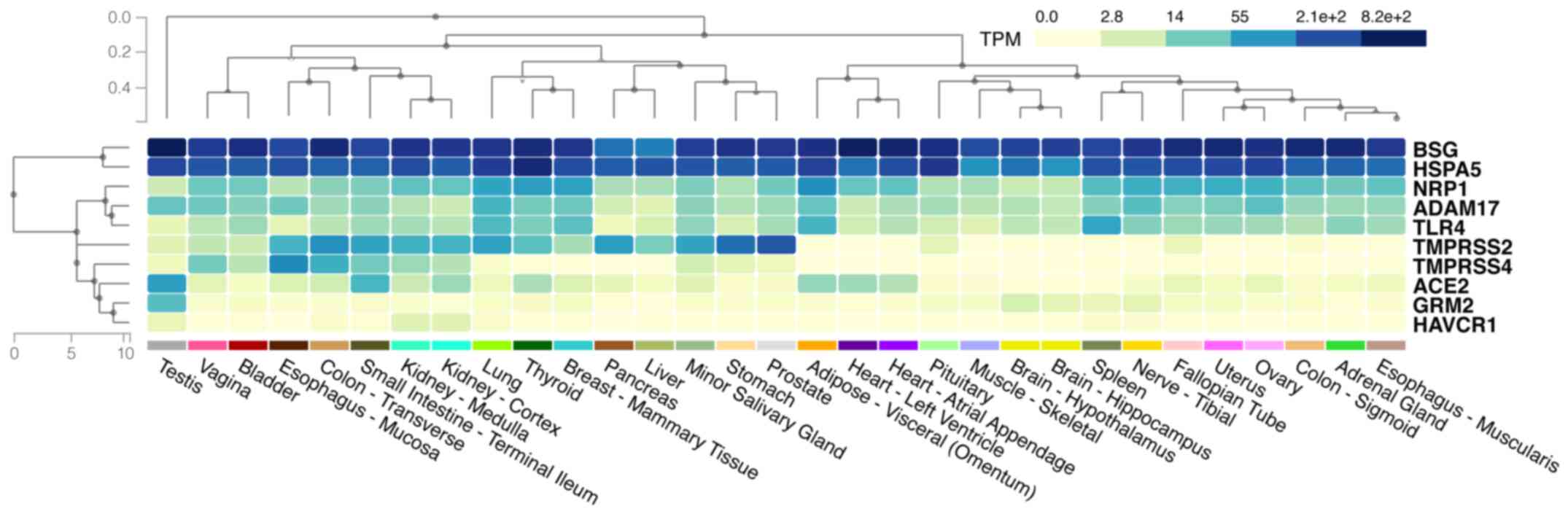
Moreover, making use of the miRNA atlas (ccb-web.
cs.uni-saarland.de/tissueatlas), it was evident that the miRNAs
listed in Tables I-VIII have a wide distribution in the
human body and co-localise with SARS-CoV-2 cell entry mediators
(Fig. 3). For example,
hsa-miR-152-3p is primarily expressed in the epididymis, colon,
artery, oesophagus, thyroid, whereas hsa-miR-338-5p is mostly
present in the kidneys, brain and spleen. This co-localisation
provides a higher order of complexity on the mechanisms through
which these mediators may be regulated by miRNAs. Future studies
are required to evaluate their therapeutic or diagnostic
potential.
6. Conclusion
The pathophysiology of COVID-19 is mainly dependent
on the underlying mechanisms that mediate the entry of SARS-CoV-2
into the host cells of the various human tissues/organs. Based on
the growing body of evidence regarding these mechanisms, it is
evident that there is an array of factors/mediators which play
crucial roles in these processes, often in
synergistic/complimentary ways. The complexity of these viral-host
interactions appears to be further increased when the
co-localization of SARS-CoV-2 host cell entry mediators in
different human tissues/organs is considered, as well as when
considering the potential modulatory effects that several miRNAs
can have on the local expression of these mediators. Accordingly,
intensive research is still warranted in this field in order to
precisely characterise these underlying mechanisms, with the
ultimate objective of aiding in the development of novel
treatments, which will act by blocking the entry of SARS-CoV-2 into
susceptible human host cells.
Availability of data and materials
The data used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PK, EK, SS, HSR and IK contributed to the
conceptualization, methodology, data curation, investigation,
visualization, drafting, editing and reviewing of the manuscript.
HSR, DAS and SS contributed to the literature search, and the
drafting and critical revision of the manuscript. All authors have
read and approved the final manuscript. SS and EK confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Evangelos
Kolettas, Laboratory of Biology, School of Medicine, University of
Ioannina, and Biomedical Research Division, Institute of Molecular
Biology and Biotechnology, Foundation for Research and Technology,
Ioannina, Greece, for critically reading and commenting on the
manuscript.
Funding
No funding was received.
Abbreviations:
|
COVID-19
|
coronavirus disease 2019
|
|
SARS-CoV2
|
severe acute respiratory syndrome
coronavirus 2
|
|
ACE2
|
angiotensin converting enzyme 2
|
|
NPR1
|
neuropilin-1
|
|
TMPRSS2 and 4
|
transmembrane protease serine 2 and
4
|
|
ADAM17
|
ADAM metallopeptidase domain 17 (also
termed tumour necrosis factor-αconvertase)
|
|
KIM1
|
kidney injury molecule-1
|
|
TLR4
|
Toll-like receptor 4
|
|
GRP78
|
glucose-regulated protein 78
|
|
miRNAs
|
microRNAs
|
References
|
1
|
Osuchowski MF, Winkler MS, Skirecki T,
Cajander S, Shankar-Hari M, Lachmann G, Monneret G, Venet F, Bauer
M, Brunkhorst FM, et al: The COVID-19 puzzle: Deciphering
pathophysiology and phenotypes of a new disease entity. Lancet
Respir Med. 9:622–642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh S, Pandey R, Tomar S, Varshney R,
Sharma D and Gangenahalli G: A brief molecular insight of COVID-19:
Epidemiology, clinical manifestation, molecular mechanism, cellular
tropism and immuno-pathogenesis. Mol Cell Biochem. 476:3987–4002.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murgolo N, Therien AG, Howell B, Klein D,
Koeplinger K, Lieberman LA, Adam GC, Flynn J, McKenna P,
Swaminathan G, et al: SARS-CoV-2 tropism, entry, replication, and
propagation: Considerations for drug discovery and development.
PLoS Pathog. 17:e10092252021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao S and Zhang L: ACE2 partially dictates
the host range and tropism of SARS-CoV-2. Comput Struct Biotechnol
J. 18:4040–4047. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai L, Guo X, Cao Y, Ying P, Hong L, Zhang
Y, Yi G and Fu M: Determining available strategies for prevention
and therapy: Exploring COVID-19 from the perspective of ACE2
(Review). Int J Mol Med. 47:432021. View Article : Google Scholar :
|
|
7
|
Glowacka I, Bertram S, Müller MA, Allen P,
Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, et
al: Evidence that TMPRSS2 activates the severe acute respiratory
syndrome coronavirus spike protein for membrane fusion and reduces
viral control by the humoral immune response. J Virol.
85:4122–4134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piva F, Sabanovic B, Cecati M and
Giulietti M: Expression and Co-expression analyses of TMPRSS2, a
key element in COVID-19. Eur J Clin Microbiol Infect Dis.
40:451–455. 2021. View Article : Google Scholar
|
|
9
|
Hou Y, Zhao J, Martin W, Kallianpur A,
Chung MK, Jehi L, Sharifi N, Erzurum S, Eng C and Cheng F: New
insights into genetic susceptibility of COVID-19: An ACE2 and
TMPRSS2 polymorphism analysis. BMC Med. 18:2162020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zipeto D, Palmeira JDF, Argañaraz GA and
Argañaraz ER: ACE2/ADAM17/TMPRSS2 interplay may be the main risk
factor for COVID-19. Front Immunol. 11:5767452020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang J, Wan Y, Luo C, Ye G, Geng Q,
Auerbach A and Li F: Cell entry mechanisms of SARS-CoV-2. Proc Natl
Acad Sci USA. 117:11727–11734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magrone T, Magrone M and Jirillo E: Focus
on receptors for coronaviruses with special reference to
Angiotensin-converting Enzyme 2 as a potential drug Target-A
perspective. Endocr Metab Immune Disord Drug Targets. 20:807–811.
2020. View Article : Google Scholar
|
|
13
|
Jia H, Neptune E and Cui H: Targeting ACE2
for COVID-19 therapy: Opportunities and challenges. Am J Respir
Cell Mol Biol. 64:416–425. 2021. View Article : Google Scholar :
|
|
14
|
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan
B, Yang P, Sarao R, Wada T, Leong-Poi H, et al:
Angiotensin-converting enzyme 2 protects from severe acute lung
failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schuler BA, Habermann AC, Plosa EJ, Taylor
CJ, Jetter C, Negretti NM, Kapp ME, Benjamin JT, Gulleman P,
Nichols DS, et al: Age-determined expression of priming protease
TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin
Invest. 131:e1407662021. View Article : Google Scholar :
|
|
16
|
Musso N, Falzone L, Stracquadanio S,
Bongiorno D, Salerno M, Esposito M, Sessa F, Libra M, Stefani S and
Pomara C: Post-mortem detection of SARS-CoV-2 RNA in Long-buried
lung samples. Diagnostics (Basel). 11:11582021. View Article : Google Scholar
|
|
17
|
Deinhardt-Emmer S, Wittschieber D, Sanft
J, Kleemann S, Elschner S, Haupt KF, Vau V, Häring C, Rödel J,
Henke A, et al: Early postmortem mapping of SARS-CoV-2 RNA in
patients with COVID-19 and the correlation with tissue damage.
Elife. 10:e603612021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang
PP, Cai J, Zhou XD, Jiang DP, Fei XC, et al: A cohort autopsy study
defines COVID-19 systemic pathogenesis. Cell Res. 31:836–846. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zang R, Gomez Castro MF, McCune BT, Zeng
Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB,
et al: TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human
small intestinal enterocytes. Sci Immunol. 5:eabc35822020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kyrou I, Randeva HS, Spandidos DA and
Karteris E: Not only ACE2-the quest for additional host cell
mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel
SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal
Transduct Target Ther. 6:212021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katopodis P, Kerslake R, Davies J, Randeva
HS, Chatha K, Hall M, Spandidos DA, Anikin V, Polychronis A,
Robertus JL, et al: COVID-19 and SARS-CoV-2 host cell entry
mediators: Expression profiling of TMRSS4 in health and disease.
Int J Mol Med. 47:642021. View Article : Google Scholar :
|
|
22
|
Cuervo NZ and Grandvaux N: ACE2: Evidence
of role as entry receptor for SARS-CoV-2 and implications in
comorbidities. Elife. 9:e613902020. View Article : Google Scholar
|
|
23
|
Davies J, Randeva HS, Chatha K, Hall M,
Spandidos DA, Karteris E and Kyrou I: Neuropilin-1 as a new
potential SARS-CoV-2 infection mediator implicated in the
neurologic features and central nervous system involvement of
COVID-19. Mol Med Rep. 22:4221–4226. 2020.PubMed/NCBI
|
|
24
|
Daly JL, Simonetti B, Antón-Plágaro C,
Williamson MK, Shoemark DK, Simón-Gracia L, Klein K, Bauer M,
Hollandi R, Greber UF, et al: Neuropilin-1 is a host factor for
SARS-CoV-2 infection. Science. 370:861–865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cantuti-Castelvetri L, Ojha R, Pedro LD,
Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya
T, Anastasina M, et al: Neuropilin-1 facilitates SARS-CoV-2 cell
entry and infectivity. Science. 370:856–860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kielian M: Enhancing host cell infection
by SARS-CoV-2. Science. 370:765–766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cantuti-Castelvetri L, Ojha R, Pedro LD,
Djannatian M, Franz J, Kuivanen S, Kallio K, Kaya T, Anastasina M,
Smura T, et al: Neuropilin-1 facilitates SARS-CoV-2 cell entry and
provides a possible pathway into the central nervous system.
bioRxiv. Jul 15–2020.Epub ahead of print. View Article : Google Scholar
|
|
28
|
Elfiky AA: SARS-CoV-2 Spike-heat shock
protein A5 (GRP78) recognition may be related to the immersed human
coronaviruses. Front Pharmacol. 11:5774672020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibrahim IM, Abdelmalek DH, Elshahat ME and
Elfiky AA: COVID-19 spike-host cell receptor GRP78 binding site
prediction. J Infect. 80:554–562. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ,
Du P, Wei D, Zhang Y, Sun XX, Gong L, et al: CD147-spike protein is
a novel route for SARS-CoV-2 infection to host cells. Signal
Transduct Target Ther. 5:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shilts J, Crozier TWM, Greenwood EJD,
Lehner PJ and Wright GJ: No evidence for basigin/CD147 as a direct
SARS-CoV-2 spike binding receptor. Sci Rep. 11:4132021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minami T, Iwata Y and Wada T: Renal
complications in coronavirus disease 2019: A systematic review.
Inflamm Regen. 40:312020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang C, Zhang Y, Zeng X, Chen H, Chen Y,
Yang D, Shen Z, Wang X, Liu X, Xiong M, et al: Kidney injury
molecule-1 is a potential receptor for SARS-CoV-2. J Mol Cell Biol.
13:185–196. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan C and Zhang C: Kidney injury
molecule-1: A novel entry factor for SARS-CoV-2. J Mol Cell Biol.
13:159–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bu Z, Wang J, Yang G, Wang X, Wen Z, Shuai
L, Luo J, Wang C, Sun Z, Liu R, et al: Metabotropic glutamate
receptor subtype 2 is a receptor of SARS-CoV-2. Res Sq. April
21–2021.Epub ahead of print. View Article : Google Scholar
|
|
36
|
Cui C, Huang C, Zhou W, Ji X, Zhang F,
Wang L, Zhou Y and Cui Q: AGTR2, one possible novel key gene for
the entry of SARS-CoV-2 into human cells. IEEE/ACM Trans Comput
Biol Bioinforma. 18:1230–1233. 2021. View Article : Google Scholar
|
|
37
|
Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L,
Lee J, Wang AQ, Pradhan M, Hagen N, Chen L, et al: Heparan sulfate
assists SARS-CoV-2 in cell entry and can be targeted by approved
drugs in vitro. Cell Discov. 6:802020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lambert DW, Yarski M, Warner FJ, Thornhill
P, Parkin ET, Smith AI, Hooper NM and Turner AJ: Tumor necrosis
factor-alpha convertase (ADAM17) mediates regulated ectodomain
shedding of the severe-acute respiratory syndrome-coronavirus
(SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol
Chem. 280:30113–30119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Palau V, Riera M and Soler MJ: ADAM17
inhibition may exert a protective effect on COVID-19. Nephrol Dial
Transplant. 35:1071–1072. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heurich A, Hofmann-Winkler H, Gierer S,
Liepold T, Jahn O and Pöhlmann S: TMPRSS2 and ADAM17 cleave ACE2
differentially and only proteolysis by TMPRSS2 augments entry
driven by the severe acute respiratory syndrome coronavirus spike
protein. J Virol. 88:1293–1307. 2014. View Article : Google Scholar :
|
|
41
|
Kumar J, Murugaiah V, Sotiriadis G, Kaur
A, Jeyaneethi J, Sturniolo I, Alhamlan FS, Chatterjee J, Hall M,
Kishore U and Karteris E: Surfactant Protein D as a potential
biomarker and therapeutic target in ovarian cancer. Front Oncol.
9:5422019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh MH, Beirag N, Murugaiah V, Chou YC,
Kuo WS, Kao HF, Madan T, Kishore U and Wang JY: Human surfactant
Protein D binds spike protein and acts as an entry inhibitor of
SARS-CoV-2 pseudotyped viral particles. Front Immunol.
12:6413602021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Madan T, Biswas B, Varghese PM, Subedi R,
Pandit H, Idicula-Thomas S, Kundu I, Rooge S, Agarwal R, Tripathi
DM, et al: A recombinant fragment of human surfactant Protein D
binds spike protein and inhibits infectivity and replication of
SARS-CoV-2 in clinical samples. Am J Respir Cell Mol Biol.
65:41–53. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tong M, Xiong Y, Zhu C, Xu H, Zheng Q,
Jiang Y, Zou L, Xiao X, Chen F, Yan X, et al: Serum surfactant
protein D in COVID-19 is elevated and correlated with disease
severity. BMC Infect Dis. 21:7372021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aboudounya MM and Heads RJ: COVID-19 and
toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4
to increase ACE2 expression, facilitating entry and causing
hyper-inflammation. Mediators Inflamm. 2021:88743392021. View Article : Google Scholar
|
|
46
|
Gadanec LK, McSweeney KR, Qaradakhi T, Ali
B, Zulli A and Apostolopoulos V: Can SARS-CoV-2 virus use multiple
receptors to enter host cells? Int J Mol Sci. 22:9922021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao Y, Kuang M, Li J, Zhu L, Jia Z, Guo
X, Hu Y, Kong J, Yin H, Wang X and You F: SARS-CoV-2 spike protein
interacts with and activates TLR41. Cell Res. 31:818–820. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zamorano Cuervo N and Grandvaux N: ACE2:
Evidence of role as entry receptor for SARS-CoV-2 and implications
in comorbidities. Elife. 9:e613902020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shrimp JH, Kales SC, Sanderson PE,
Simeonov A, Shen M and Hall MD: An enzymatic TMPRSS2 assay for
assessment of clinical candidates and discovery of inhibitors as
potential treatment of COVID-19. ACS Pharmacol Transl Sci.
3:997–1007. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K
and Maitra A: Relative abundance of SARS-CoV-2 entry genes in the
enterocytes of the lower gastrointestinal tract. Genes (Basel).
11:6452020. View Article : Google Scholar :
|
|
52
|
Choudhury A and Mukherjee S: In silico
studies on the comparative characterization of the interactions of
SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and
human TLRs. J Med Virol. 92:2105–2113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dexheimer PJ and Cochella L: MicroRNAs:
From mechanism to organism. Front Cell Dev Biol. 8:4092020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' Action through
miRNA Editing. Int J Mol Sci. 20:62492019. View Article : Google Scholar
|
|
57
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar
|
|
58
|
Liu Z, Wang J, Ge Y, Xu Y, Guo M, Mi K, Xu
R, Pei Y, Zhang Q, Luan X, et al: SARS-CoV-2 encoded microRNAs are
involved in the process of virus infection and host immune
response. J Biomed Res. 35:216–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chauhan N, Jaggi M, Chauhan SC and Yallapu
MM: COVID-19: Fighting the invisible enemy with microRNAs. Expert
Rev Anti Infect Ther. 19:137–145. 2021. View Article : Google Scholar
|
|
60
|
Fani M, Zandi M, Ebrahimi S, Soltani S and
Abbasi S: The role of miRNAs in COVID-19 disease. Future Virol.
16:301–306. 2021. View Article : Google Scholar
|
|
61
|
Bugnon LA, Raad J, Merino GA, Yones C,
Ariel F, Milone DH and Stegmayer G: Deep Learning for the discovery
of new pre-miRNAs: Helping the fight against COVID-19. Mach Learn
with Appl. 6:1001502021. View Article : Google Scholar
|
|
62
|
Abedi F, Rezaee R, Hayes AW, Nasiripour S
and Karimi G: MicroRNAs and SARS-CoV-2 life cycle, pathogenesis,
and mutations: Biomarkers or therapeutic agents? Cell Cycle.
20:143–153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Haddad H and Al-Zyoud W: miRNA target
prediction might explain the reduced transmission of SARS-CoV-2 in
Jordan, Middle East. Noncoding RNA Res. 5:135–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sardar R, Satish D, Birla S and Gupta D:
Integrative analyses of SARS-CoV-2 genomes from different
geographical locations reveal unique features potentially
consequential to host-virus interaction, pathogenesis and clues for
novel therapies. Heliyon. 6:e046582020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Matarese A, Gambardella J, Sardu C and
Santulli G: miR-98 regulates TMPRSS2 expression in human
endothelial cells: Key implications for COVID-19. Biomedicines.
8:4622020. View Article : Google Scholar :
|
|
66
|
Nersisyan S, Shkurnikov M, Turchinovich A,
Knyazev E and Tonevitsky A: Integrative analysis of miRNA and mRNA
sequencing data reveals potential regulatory mechanisms of ACE2 and
TMPRSS2. PLoS One. 15:e02359872020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu D, Chatterjee S, Xiao K, Riedel I, Wang
Y, Foo R, Bär C and Thum T: MicroRNAs targeting the SARS-CoV-2
entry receptor ACE2 in cardiomyocytes. J Mol Cell Cardiol.
148:46–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Khan MA, Sany MRU, Islam MS and Islam
ABMMK: Epigenetic regulator miRNA pattern differences among
SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide isolates delineated
the mystery behind the epic pathogenicity and distinct clinical
characteristics of pandemic COVID-19. Front Genet. 11:7652020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Paul S, Bravo Vázquez LA, Reyes-Pérez PR,
Estrada-Meza C, Aponte Alburquerque RA, Pathak S, Banerjee A,
Bandyopadhyay A, Chakraborty S and Srivastava A: The role of
microRNAs in solving COVID-19 puzzle from infection to
therapeutics: A mini-review. Virus Res. 308:1986312021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dash S, Dash C and Pandhare J: Therapeutic
significance of microRNA-mediated regulation of PARP-1 in
SARS-CoV-2 infection. Noncoding RNA. 7:602021.PubMed/NCBI
|
|
71
|
Mukhopadhyay D and Mussa BM:
Identification of novel hypothalamic MicroRNAs as promising
therapeutics for SARS-CoV-2 by regulating ACE2 and TMPRSS2
expression: An in silico analysis. Brain Sci. 10:6662020.
View Article : Google Scholar :
|
|
72
|
Wang X: miRDB: A microRNA target
prediction and functional annotation database with a wiki
interface. RNA. 14:1012–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:D146–D152. 2015. View Article : Google Scholar :
|
|
74
|
Wicik Z, Eyileten C, Jakubik D, Simões SN,
Martins DC Jr, Pavão R, Siller-Matula JM and Postula M: ACE2
interaction networks in COVID-19: A physiological framework for
prediction of outcome in patients with cardiovascular risk factors.
J Clin Med. 9:37432020. View Article : Google Scholar :
|