Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide. Statistical data from 2018 indicated that
there were >1.8 million new cases of CXRC worldwide, and
>880,000 patients succumbed to the disease. This accounts for
10.2 and 9.2% of new cancer patients and cancer-related deaths,
respectively (1). Chemotherapy
is one of the main treatments for CRC. However, it is associated
with several side-effects, such as nephrotoxicity, cardiotoxicity,
gastrointestinal reactions, neurotoxicity and bone marrow
suppression. Long-term multiple chemotherapy often leads to the
accumulation of toxic side-effects and to an increase in drug
resistance, which is the main reason for the failure of
chemotherapy (2). At present,
there is still a lack of effective chemotherapeutic drugs with low
toxicity. As the source of numerous anticancer agents, the extracts
of natural plants exert minimal side-effects and can prevent
resistance to chemotherapeutic drugs to a certain extent (3).
Ailanthus altissima is a plant of the genus
Ailanthus in the family Simaroubaceae, commonly known as
tree of heaven (4). It can be
found worldwide, essentially in countries with a temperate climate.
As a type of traditional Chinese medicine, it has a long history of
use in China. It can be used in the treatment of diseases, such as
inflammation, malaria, allergy, tuberculosis, ulcers, amoebiasis,
viral infections, HIV and cancer (5). The medicinal ingredient is
considered to be Ailanthone (AIL), a water-soluble quassinoid, and
is mainly extracted from the bark of this plant (chemical structure
shown in Fig. 1A). AIL was first
isolated in 1955 (6) and its
structure was clarified in the early 1980s (7). A number of in vitro studies
have demonstrated that AIL exerts anticancer effects on a variety
of cancer cells, such as lung cancer (8), melanoma (9), bladder (10), stomach (11), prostate (12) and liver cancer (13), etc. In these tumor cells, AIL
plays an anticancer role mainly through the inhibition of
proliferation, the induction of apoptosis and autophagy, and by
mediating a variety of molecular targets and signaling pathways to
exert anticancer effects.
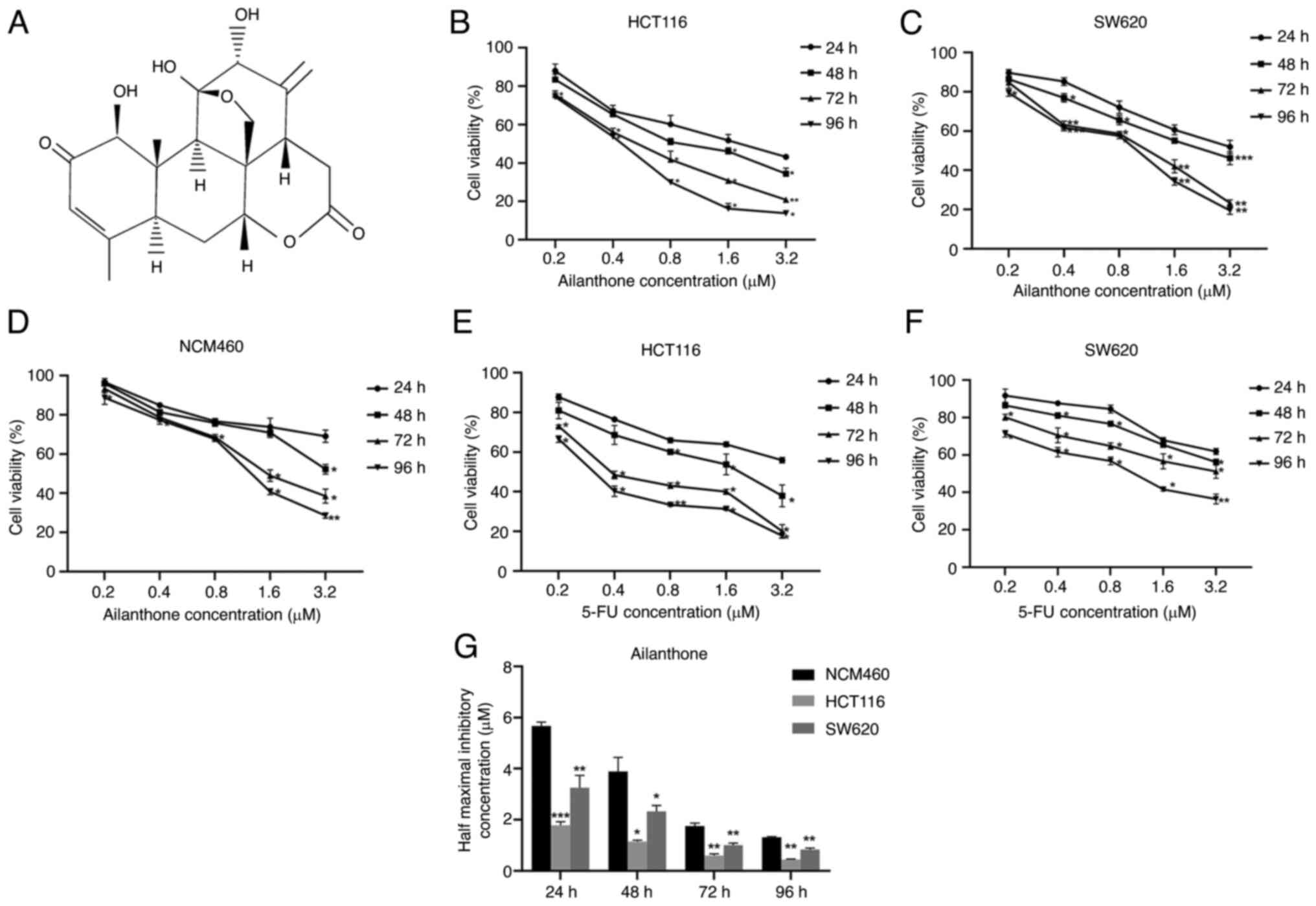 | Figure 1Growth-inhibitory effects of AIL and
5-FU on the HCT116 and SW620 cells, and the growth-inhibitory
effects of AIL on the NCM460 cells. (A) Molecular structure of AIL.
(B-D) AIL exerted inhibitory effects on the growth of HCT116, SW620
and NCM460 cells in a concentration- and time-dependent manner. (E
and F) Effects of 5-FU induced on HCT116 and SW620 cell growth. (G)
Comparison of the IC50 values of AIL in the three cell lines
(HCT116, SW620 and NCM460) following treatment with AIL for
different periods of time (24, 48, 72 and 96 h). Data are presented
as the mean ± standard deviation, n=3. *P<0.05,
**P<0.01 and ***P<0.001, vs. respective
control. AIL, Ailanthone; 5-FU, 5-fluorouracil. |
To date, at least to the best of our knowledge,
there is no relevant research available the effects of AIL on CRC.
Therefore, the present study used the HCT116 and SW620 CRC cells to
observe the effects of AIL on the proliferation, apoptosis and cell
cycle distribution of AIL these cells. Moreover, in order to
explore the specific molecular mechanisms of AIL, the changes in
the expression of cell cycle-related proteins and apoptosis
regulatory proteins were examined. It was also found that AIL
inhibit the activation of the Janus kinase (JAK)/signal transducer
and activator of transcription (STAT)3 signaling pathway. These
findings clarify the molecular mechanisms of AIL against CRC cells
to a certain extent, and provide the experimental and theoretical
basis for the application of AIL in the treatment of CRC.
Materials and methods
Materials, antibodies and reagents
AIL was extracted and isolated from Ailanthus
altissima. The AIL sample (purity ≥98%) was provided by
Shanghai Yiyan Biotechnology Co., Ltd. 5-Fluorouracil (5-FU) was
obtained from MedChemExpress. The Cell Mitochondria Isolation kit
(cat no. C3601) was purchased from the Beyotime Institute of
Biotechnology. All the antibodies used in the present study were as
follows: Antibodies for mouse polyclonal β-actin (cat no.
bsm-33036M), mouse monoclonal B-cell lymphoma-2 (Bcl-2; cat no.
bsm-33411M), rabbit poly-clonal Bcl-2-associated X protein (Bax;
cat no. bs-0127R), rabbit polyclonal cyclin-dependent protein
kinase 1 (CDK1; cat no. bs-1341R), rabbit polyclonal Cyclin B1 (cat
no. bs-0572R), rabbit polyclonal STAT3 (cat no. bs-55208R), rabbit
polyclonal E-cadherin (cat no. bs-10009R), rabbit polyclonal
N-cadherin (cat no. bs-1172R), rabbit polyclonal Vimentin (cat no.
bs-8533R), rabbit polyclonal cytochrome c (cat no.
bs-0013R), rabbit polyclonal voltage-dependent anion-selective
channel (VDAC; cat no. bs-7647R) were purchased from BIOSS. The
antibodies for rabbit monoclonal caspase-3 (cat no. 14220), mouse
monoclonal caspase-9 (cat no. 9508), rabbit monoclonal PARP (cat
no. 9532), horseradish peroxidase (HRP)-conjugated goat anti-mouse
(cat no. 91196) immunoglobulin (Ig)G were obtained from Cell
Signaling Technology, Inc., and goat anti-rabbit (cat no. AS014)
immunoglobulin (Ig)G was purchased from ABclonal Biotech Co.,
Ltd.
Cells and cell culture
The human CRC cell lines, HCT116 and SW620 (cat. no.
TCHu 99 and TCHu101), were obtained from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. The human
normal colonic epithelial cell line, NCM460, was also obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The HCT116 and NCM460 cells were cultured in RPMI-1640
medium supplemented with 10% FBS and 1% PS in a humidified
incubator containing 5% CO2 and 95% air at 37°C for cell
subculture and all the experiments. Under the same conditions, the
SW620 cells were cultured in L-15 medium supplemented with 10% FBS
and 1% PS. Stock solutions of AIL were prepared in DMSO, and stored
at -20°C. Prior to use, stock solutions were immediately diluted to
the required concentration with complete medium; the terminal
concentration of DMSO in the culture medium was ≤0.1%.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay obtained from
MedChemExpress (cat no. HY-K0301) was used to measure cell
viability. 5-FU was used in the positive control. The NCM460,
HCT116, SW620 cells in the exponential growth phase
(5×103 cells/well) were seeded and cultured in 96-well
plates for 24 h, and were then treated with AIL (0.2, 0.4, 0.8, 1.6
and 3.2 µM) or 5-FU (0.2, 0.4, 0.8, 1.6 and 3.2 µM)
for 24, 48, 72 and 96 h at 37°C; each group was analyzed 4 times.
Subsequently, 10 µl CCK-8 solution were added to each well.
Following incubation for 3 h at 37°C, the optical density was
measured at a wavelength of 450 nm using a microplate reader
(SpectraMax M5; Molecular Devices, LLC). The relative cell
viability was calculated based on the optical density value. Prism
9.2 (GraphPad Software, Inc.) was then used to calculate the IC50
value based on the relative cell viability.
Transwell assay
A 0.4 µm Transwell assay (Costar; Corning,
Inc.) was used to assess cell migration. In brief, 6×104
CRC cells (HCT116 and SW620) were seeded in the upper side of the
chamber with non-serum culture medium. The lower chamber was filled
with 500 µl complete culture medium. The chamber was then
placed in a humidified incubator at 37°C for 24 h. The CRC cells
were treated with 0.4 µM AIL for 48 h, and the traversed
cells (the cells in the lower chamber) were then stained with
crystal violet (Sinopharm Group Co., Ltd.) at room temperature for
3 h and counted microscopically using a binocular microscope
(Olympus CX23). Five fields were randomly selected for calculation
of relative migration.
Cell apoptosis analysis
The Annexin-V-FITC Apoptosis Detection kit [Hangzhou
Multi Sciences (Lianke) Biotech Co.] was used to analyze cell
apoptosis. Following treatment with AIL (0.2 and 0.4 µM) for
48 h, 1×105 SW620 and HCT116 cells were collected from
each sample. The cells were suspended in a binding buffer
containing 10 µl Annexin-V-FITC, and then incubated at room
temperature in the dark for 30 min. Subsequently, 5 µl PI
and 200 ml ice-cold PBS were added, and the samples were
immediately analyzed using a FACSscan flow cytometer (CytoFLEX;
Beckman Coulter, Inc.). FITC+ and PI− cells
were considered apoptotic.
JC-1 staining
The HCT116 and SW620 cells were seeded in a 6-well
plate at a density of 1×105 cells/well for 12 h, and
treated with various concentrations of AIL (0, 0.2 and 0.4
µM) for 48 h. The cells were then collected and resuspended
in JC-1 (Solebold) for 30 min at 37°C in the dark. JC-1 is a type
of dye that targets the mitochondrion and is used as a reporter for
membrane potential. It has two states: Monomer and multimer. In
normal cells, JC-1 enters the mitochondria and forms red
fluorescent multimers due to the increase in the concentration. In
apoptotic cells, the mitochondrial membrane potential is lost, JC-1
is released from the mitochondria, the concentration decreases, and
it turns into a green fluorescent monomer form. Therefore, the
changes in mitochondrial membrane potential were quantified by
detecting green and red fluorescence using a flow cytometer
(CytoFLEX; Beckman Coulter, Inc.).
Cell cycle distribution analysis
The cells were seeded in a 6-well plate at a density
of 1×106/ml and treated with AIL (0.2 and 0.4 µM)
for 48 h. Following treatment, the cells were collected and
resuspended in 0.5 ml PBS, fixed with 70% cold ethanol at -20°C
overnight, and then mixed with 1 ml DNA staining solution and
incubated at 0°C for 30 min. The cell cycle distribution was then
analyzed using a flow cytometer (CytoFLEX; Beckman Coulter,
Inc.).
Isolation of mitochondria from cells
The cells were collected and washed with PBS, and
were pelleted by centrifugation at 600 × g for 5 min at 4°C. After
the supernatant was discarded, 1.5 ml mitochondrial separation
reagent (cat no. SM0020; Beijing Solarbio Science & Technology
Co., Ltd.) was added to resuscitate the cells, and the cells were
then placed in an ice bath for 15 min. The cells were homogenized
15 times using a homogenizer (cat no. YA0856; Beijing Solarbio
Science & Technology Co., Ltd.), and the cell homogenate was
then centrifuged at 800 × g for 10 min at 4°C. As a final step, the
supernatant was transferred to another tube and centrifuged again
at 11,000 × g for 10 min at 4°C. Finally, the precipitate obtained
was the separated mitochondria.
Western blot analysis
Following treatment with AIL (0.2, 0.4, 0.8, 1.6 and
3.2 µM) for 48 h at 37°C, the HCT116 and SW620 cells were
washed twice with ice-cold PBS and suspended in lysis buffer (cat
no. R0020; Solarbio Biotech Co., Ltd.) on ice for 30 min. The
lysates were then cleared by centrifugation at 12,000 × g for 15
min at 4°C. Subsequently, the Bradford protein assay kit (cat no.
P0006; Beyotime Biotech Co., Ltd.) was used to measure the total
protein concentration of each sample. A total of 50 µg
protein samples from each group were separated by 12% SDS-PAGE and
were then transferred onto polyvinylidene fluoride membranes
(MilliporeSigma). The membranes were then blocked with 5% non-fat
dry milk dissolved in TBS containing 0.05% Tween-20 (TBST) at room
temperature for 1 h, and were then washed three times with TBST.
Subsequently, the membranes were incubated with the primary
antibodies (1:1,500) overnight at 4°C. After washing three times
with TBST, the membranes were incubated with HRP-conjugated goat
anti-mouse (1:2,000) or anti-rabbit (1:2,000) IgG secondary
antibodies at room temperature for 1 h. The immunoreactive bands
were visualized with chemiluminescent substrates (cat no.
K-12045-D50; Advansta Scientific, Inc.) using an X-ray film
processor (Clinx ChemiScope 3000Mini; Clinx Science Instruments
Co., Ltd.). β-actin and VDAC were used as loading controls. The
experiment was independently repeated three times, and ImageJ
software (version 1.8.0; National Institutes of Health, USA) was
used for densitometric analysis of the protein bands.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
To detect the relative expression levels of JAK and
STAT3, RT-qPCR was performed. Briefly, the HCT116 and SW620 cells
were treated with various concentrations (0, 0.2 and 0.4 µM)
of AIL for 48 h. Total RNA from was then extracted from the CRC
cell lines using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) as per the manufacturer's instructions.
The extracted RNA was then converted into cDNA using the GoScript
Reverse Transcription System (Promega Corporation) using following
the steps: i) Denaturation of the template into single strands; ii)
annealing (25°C, 5 min) of primers to each original strand for new
strand synthesis; and iii) extension (43°C, 55 min) of the new DNA
strands from the primers. Subsequently, qPCR was performed using
GoTaq® 2-Step RT-qPCR System (Promega Corporation) by
applying a Stratagene MX3005P qPCR System (Agilent Technologies,
Inc.) using 5 µl cDNA. The primer sequences used were as
follows: JAK forward, 5′-TCT GGG GAG TAT GTT GCA GAA -3′ and
reverse, 5′-AGA CAT GGT TGG GTG GAT ACC -3′; STAT3 forward, 5′-GAG
AAG GAC ATC AGC GGT AAG -3′ and reverse, 5′-CAG TGG AGA CAC CAG GAT
ATT G-3′; and human GAPDH (used as an internal control) forward,
5′-AAG GTG AAG GTC GGA GTC AA-3′ and reverse, 5′-AAT GAA GGG GTC
ATT GAT GG-3′. ΔΔCq values were calculated to reflect the
expression of JAK and STAT3 (14).
Statistical analysis
All data were analyzed using SPSS software (version
18.0; SPSS, Inc.). All statistical graphs are constructed using
Prism 9.2 (GraphPad Software, Inc.). All the experiments were
conducted in triplicate, and all data are expressed as the mean ±
SD. An unpaired Student's t-test was used to compare differences
between two groups. The statistical significance of the differences
among more than two groups were analyzed using one-way ANOVA and a
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
AIL inhibits CRC cell proliferation
CCK-8 assay was used to detect the effects of AIL on
the growth of the HCT116, SW620 and NCM460 cells treated with AIL
(0-3.2 µM) or 5-FU (0-3.2 µM) for 24, 48, 72 and 96 h
at 37°C. AIL inhibited the viability of these three cells in a
dose- and time-dependent manner (Fig. 1B-D). The half maximal inhibitory
concentration (IC50) values of AIL in the HCT116 cells at 24, 48 72
and 96 h were 1.79±0.139, 1.147±0.056, 0.603±0.067 and 0.449±0.021
µM, respectively. The half maximal inhibitory concentration
(IC50) values of AIL in the SW620 cells at 24, 48 72 and 96 h were
3.255±0.479, 2.333±0.23, 1.01±0.079 and 0.834±0.066 µM,
respectively. Cells treated with 5-FU were considered as the
positive control group; 5-FU also inhibited the growth of these two
CRC cells in a dose- and time-dependent manner (Fig. 1E and F). The IC50 values of 5-FU
in the HCT116 cells at 24, 48 72 and 96 h were 4.511±0.572,
1.691±0.355, 0.576±0.045 and 0.358±0.011 µM, respectively.
The IC50 value of 5-FU in SW620 cells at 24, 48, 72 and 96 h were
5.666±0.259, 4.96±0.61, 3.304±1.059 and 1.065±0.144 µM,
respectively. When comparing the IC50 values of these two drugs, it
was found that the antitumor effect of AIL on the CRC cells was
similar to that of 5-FU.
Furthermore, the cytotoxicity of AIL on normal
intestinal epithelial cells NCM460 was also evaluated. The IC50
values at 24, 48, 72 and 96 h were 5.67±0.155, 3.89±0.553,
1.759±0.119 and 1.314±0.027 µM, respectively (Fig. 1D). It can be seen that in the
experimental group (0.2 and 0.4 µM), AIL exerted more
significant cytotoxic effects on the tumor cells than on the normal
intestinal epithelial cells (Fig.
1G).
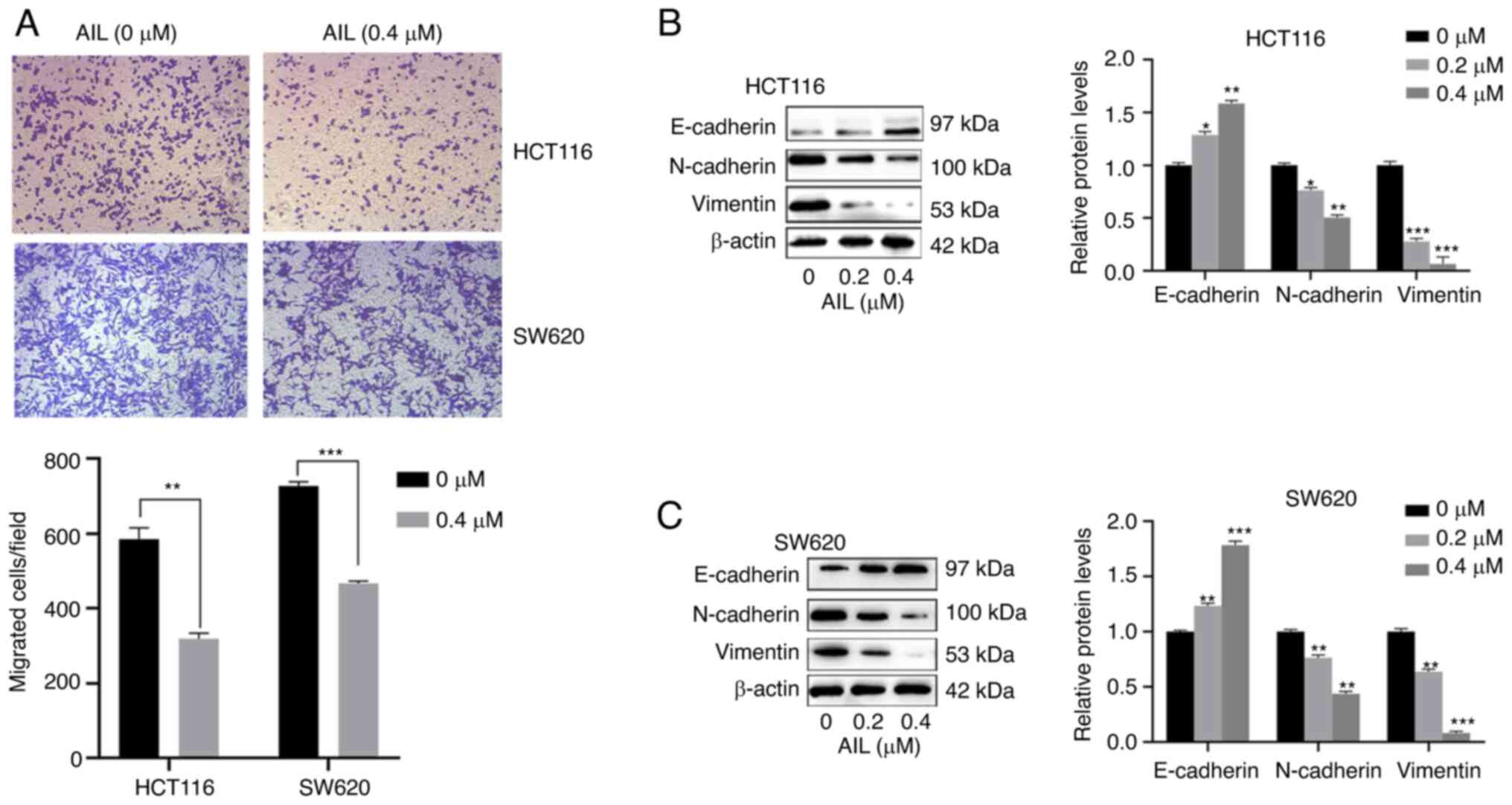
AIL inhibits colorectal cancer cell
migration
Transwell assay was conducted to assess the
migratory capacity of the CRC cells. As shown in Fig. 2A, the relative migration rate was
significantly decreased in the AIL group compared with the control
group (P<0.01 or P<0.001). By performing western blot
analysis, it was found that the protein levels of N-cadherin and
Vimentin were both significantly downregulated in the AIL group, as
compared with the control group, while E-cadherin expression was
significantly upregulated (P<0.05, P<0.01, P<0.001 or
P<0.0001 Fig. 2B and C).
These results demonstrated that AIL was able to suppress the
migratory capacities of the CRC cells.
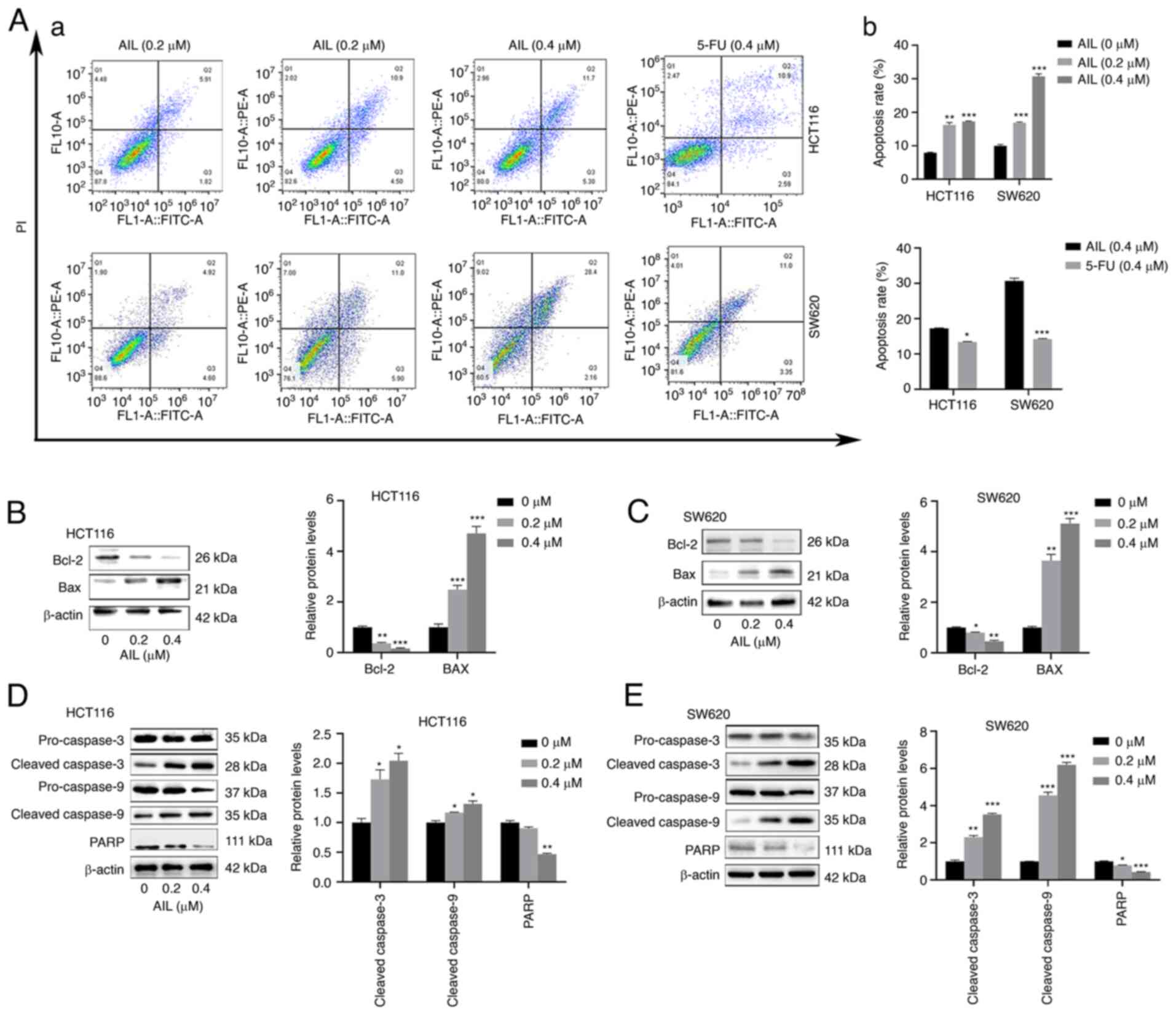
AIL induces caspase-dependent
apoptosis
To confirm the occurrence of apoptosis, an Annexin
V-FITC/PI double-staining assay was performed. As shown in Fig. 3A, the percentage of apoptotic
HCT116 cells (including early and late apoptotic cells)
significantly increased with the increasing AIL concentration, from
7.90±0.18 to 17.25±0.15%, while the apoptotic rate of the SW620
cells increased from 9.97±0.40 to 30.7±0.77%. It was also found
that at the same concentration (0.4 µM), the
apoptosis-promoting effects of AIL on the HCT116 cells
(17.22±0.19%) were more prominent than those of 5-FU (13.41±0.92%).
Similar results were obtained for the SW620 cells. The apoptosis
induction rates of the two drugs for the SW620 cells were
30.70±0.77 and 14.22±0.15%, respectively.
In addition, compared with the control group,
following 48 h of treatment with AIL (0-0.4 µM) at 37°C, the
expression level of the inhibitor of apoptosis protein, Bcl-2, in
the two CRC cells was decreased, while the expression level of the
apoptosis-promoting protein, Bax, was increased (Fig. 3B and C). Since caspase activation
is considered a hallmark of apoptosis, western blot analysis was
performed to examine caspase activation. The levels of cleaved
caspase-9 and cleaved caspase-3 were significantly increased in the
AIL-treated cells. The expression of the caspase cleaved substrate
PARP was significantly decreased, which also indicated caspase
activation (Fig. 3D and E).
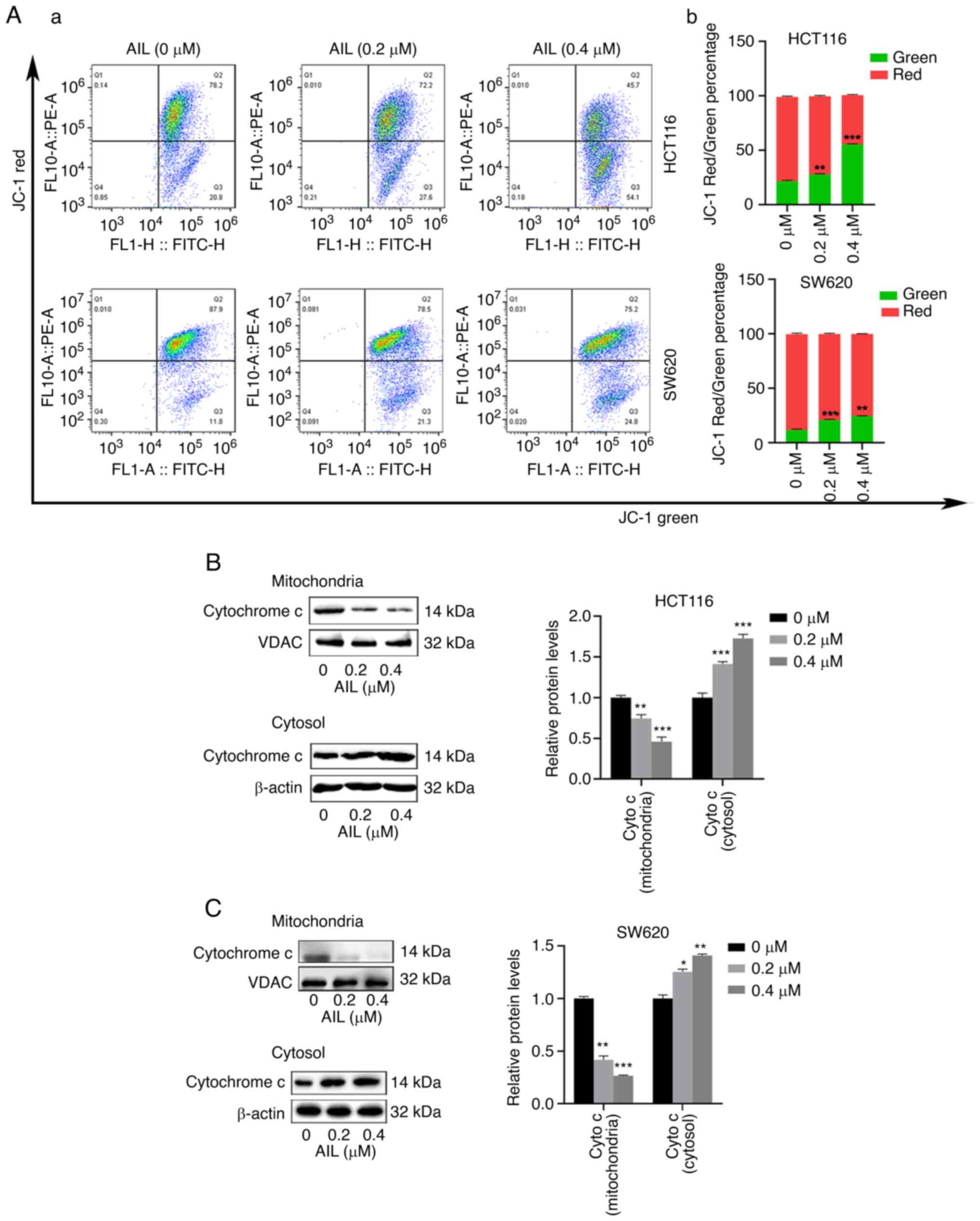
AIL induces apoptosis through
mitochondrial pathways
Subsequently, mitochondrial membrane potential was
analyzed using JC-1 staining. As shown in Fig. 4A, the JC-1 red/green percentage
in the two CRC cell lines decreased following AIL treatment.
Mitochondrial dysfunction, can subsequently lead to
the release of cytochrome c from the mitochondria to the
cytosol. The increased distribution of cytochrome c in the
cytosol is considered to be related to the apoptosis induced by the
caspase cascade pathway (15).
Therefore, to further confirm this, the effects of AIL on
cytochrome c were examined using western blot analysis. The
results revealed that AIL increased the level of cytochrome
c in the cytoplasm and decreased its expression level in the
mitochondria (Fig. 4B and C).
These results indicate that AIL promoted the mitochondrial-mediated
apoptosis of CRC cells.
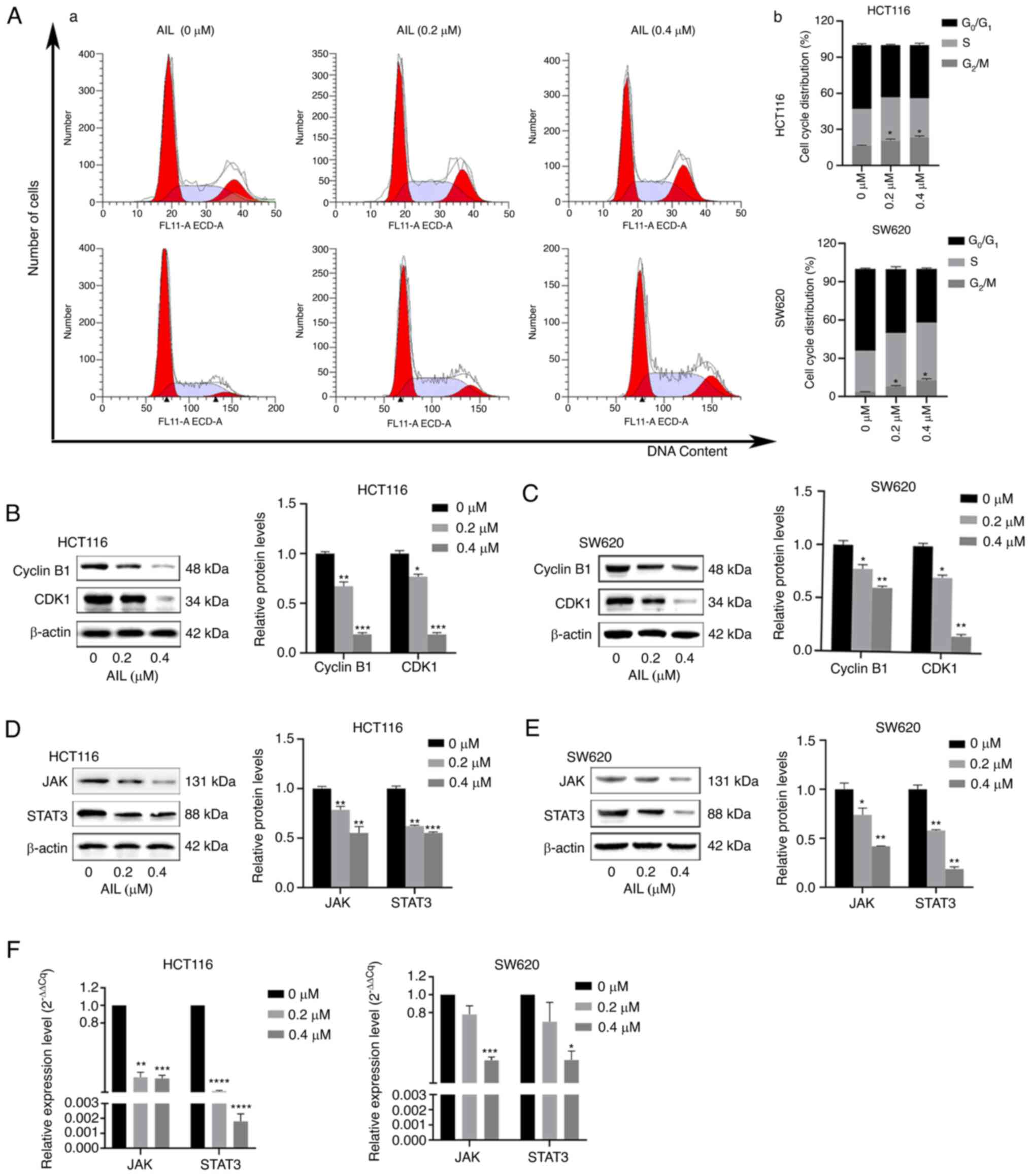
AIL induces cell cycle arrest by
regulating cell cycle regulatory proteins
To investigate whether the anti-proliferative
effects of AIL on CRC cells were triggered by cell cycle arrest,
the cell cycle phase ratio was measured using flow cytometry with
DNA staining solution. As illustrated in Fig. 5A, compared with the control, AIL
treatment induced the significant accumulation of cells in the
G2/M phase. The percentage of HCT116 cells in the
G2/M phase significantly increased with the increasing
AIL concentration, from 16.2±0.65 to 23.43±1.22%, and that of SW620
cells increased from 3.0±0.34 to 13.41±0.92%. To investigate the
molecular mechanisms through which AIL inhibited the
G2/M transition in the tumor cells, the cells were
treated with AIL and the expression of proteins involved in cell
cycle regulation was then analyzed. It was found that AIL treatment
decreased cyclin B1 and CDK1 expression (Fig. 5B and C).
AIL inhibits JAK/STAT3 pathway activation
in CRC cells
JAK/STAT3 is an important signaling pathway related
to the occurrence and development of cancer. This pathway can be
abnormally activated by a variety of upstream signals in cells, can
regulate EMT-related genes, and is closely related to tumor
proliferation, invasion, metastasis and angiogenesis (16,17). It has been confirmed that the
activation of this pathway is directly related to the development
of CRC (18). The present study
found that AIL treatment inhibited JAK3 and STAT3 expression in the
two CRC cell lines (Fig. 5D and
E). Furthermore, the changes in JAK and STAT3 gene levels were
detected using RT-qPCR. The results revealed that AIL inhibited the
expression of the JAK and STAT3 genes in the HCT116 and SW620 cells
(Fig. 5F).
Discussion
Medicinal plants have long been used in cancer
treatment. Countries, such as China, Japan and Thailand have used
traditional medicinal plants in the treatment of cancer for
thousands of years (19).
Several antitumor drugs that have been clinically used are derived
from plants and have significantly prolonged the survival time of
patients. For example, vincristine can be used in the treatment of
leukemia (20), lymphoma
(21), breast cancer (22), lung cancer (23) and pediatric solid tumors
(24); paclitaxel has also been
used in the treatment of ovarian, breast, lung, bladder cancer and
head and neck tumors (25);
docetaxel has been used in the treatment of breast (26) and lung cancer (27); in addition, irinotecan has been
used in the treatment of CRC and lung cancer (28). Ailanthus altissima has a
long history in China as a medicinal plant. AIL, one of the primary
active quassinoids in Ailanthus altissima, has also been
reported to possess certain anticancer properties in numerous in
vitro studies (8,9,11,29,30). However, the antitumor activity of
AIL against human |CRC and its mechanisms of action have not yet
been elucidated. Therefore, the aim of the present study was to
examine the effects of AIL on human CRC and to elucidate the
underlying molecular mechanisms. To the best of our knowledge, the
present study demonstrates for the first time that AIL inhibits the
proliferation of CRC cells in vitro. The present study also
revealed the molecular mechanisms through which AIL affects CRC
cells and further evaluated the toxicity of AIL to normal
intestinal epithelial cells (NCM460); 5-FU was used as a positive
control to reflect the advantages of AIL.
The findings of the present study demonstrated that
AIL suppressed the growth of HCT116 and SW620 cells in a
concentration- and time-dependent manner. In addition, the
inhibitory effects of AIL on the proliferation of NCM460 cells, as
well as its cytotoxic effects were less prominent than those on the
HCT116 and SW620, indicating that AIL exhibited a greater
sensitivity to tumor cells. Moreover, when comparing the IC50
values of AIL and 5-FU, it was found that AIL and 5-FU had a
similar cytotoxicity.
The majority of the cells in the body of healthy
individuals are in a quiescent phase, and cells will only re-enter
the cycle when and where they are needed. The dysregulation of cell
cycle progression is also considered a common characteristic of
cancer, which may lead to excessive or uncontrolled cell
proliferation (31). Therefore,
targeting the regulatory components of the cell cycle machinery is
an important strategy for the treatment of human malignancies. As
previously demonstrated, AIL induced the
G0/G1 and G2/M phase arrest of B16
and A375 melanoma cells by downregulating cyclin E and cyclin B
expression (9). In
hepatocellular carcinoma, AIL has been shown to induce the
G0/G1 phase arrest of Huh7 cells by
downregulating the expression of cyclin D, cyclin E, and CDK2, CDK4
and CDK6 (13). The present
study indicated that AIL induced the G2/M cycle arrest
of HCT116 and SW620 CRC cells by downregulating the levels of
positive regulators of the G2/M phase (cyclin B and
CDK1), and inhibiting cell proliferation.
Apoptosis, also known as programmed cell death, is
an important terminal pathway of multicellular biological cells. In
the majority of eukaryotic cells, there are two major apoptotic
pathways: The death receptor pathway and the mitochondrial pathway.
The process is highly regulated, which can eliminate damaged cells
(such as DNA-damaged cells) and senescent cells in an orderly and
effective manner to maintain the stability of the internal
environment (32). The evasion
of cell death is one of the important characteristics of the
malignant transformation of normal cells to tumor cells (33). It has been found that a variety
of novel drugs, such as Bcl-2 inhibitors (ABT-263, ABT-737,
GX15-070 and fenretinide) (34),
caspase activators (35),
caspase inducers (apoptin) (36), etc. can induce tumor cell
apoptosis. In the present study, it was found that AIL induced cell
apoptosis through the mitochondrial pathway. The Bcl-2 protein
family is considered to be a switch that controls the mitochondrial
apoptotic pathway by affecting the permeability of the
mitochondrial membrane (37). To
further investigate the underlying molecular mechanisms of
AIL-induced apoptosis, the protein expression levels of Bcl-2, Bax
were detected in CRC cells treated with AIL. The results of western
blot analysis revealed that AIL increased the Bax and decreased
Bcl-2 expression, which altered the permeability of the
mitochondrial membrane, resulting in an increase in the amount of
cytochrome c in the cytosol, finally inducing caspase-3 and
-9 activation, as well as apoptosis.
STAT family members are usually divided into two
subgroups. The first group is involved in T-cell development and
IFN-γ signal transduction and mainly includes STAT2, STAT4 and
STAT6. The second group is related to embryogenesis, breast
development and tumor occurrence, and mainly includes STAT1, STAT3
and STAT5 (38). Among them,
STAT3 is considered to be highly related to tumor development. It
can regulate cell proliferation-related genes (c-Myc and cyclin
D1), anti-apoptotic genes (Mcl1, Bcl-xL, Bcl-2 and survivin), and
angiogenesis-related genes (BFGF, HIF-1α, VEGF and HGF) and
metastasis and EMT process-related genes [Snail, Slug, Twist1,
Vimentin, matrix metalloproteinase (MMP)2, MMP9 and HMGB1]
(16,39). The STAT3 pathway is closely
related to the occurrence and development of CRC, and the
activation of this pathway can affect the progression of CRC via
several mechanisms (40,41). The activation of STAT3 signaling
can drive Th17 cells in CRC to secrete cytokines (IL-17A, IL-17F,
IL-21 and IL-22) to promote tumor angiogenesis and tumorigenesis
(42). c-Myc is an important
transcription factor that can affect a variety of cell biological
functions, such as proliferation, differentiation, growth and
apoptosis. The high expression of c-Myc is common in CRC, which is
often associated with a poor prognosis (43). The continuous activation of STAT3
activates c-Myc to promote tumor progression. Furthermore, the
STAT3 signaling pathway can upregulate the expression of the
positive cell cycle regulatory protein, cyclin D1 (44). STAT3 can induce the expression of
the anti-apoptotic proteins, Bcl-2, Mcl-1, survivin and Bcl-xL, to
inhibit apoptosis and promote proliferation (45,46). The activation of the STAT3
signaling pathway can also induce the expression of MMPs and EMT
regulatory factors (Snail and Twist1), and can downregulate
E-cadherin and upregulate vimentin expression, thereby enhancing
the invasiveness and metastatic ability of CRC (47,48). Therefore, targeting the STAT3
signaling pathway may be a feasible and effective treatment
strategy. A number of clinical studies using STAT3
pathway-targeting drugs have been performed, such as IL-6
inhibitors (siltuximab) (49),
JAK inhibitors (ruxolitinib and itacitinib) (50,51) and STAT3 inhibitors (OPB-31121,
GRIM19, AZD9150 and TTI-101) (52-55). In addition, certain naturally
derived compounds (piperine, matrine, luteolin, curcumin, etc.)
have been confirmed to target the STAT3 signaling pathway and to
exert anticancer effects (56-59). In the present study, it was found
that AIL significantly inhibited the expression of JAK and STAT3
proteins in HCT116 and SW620CRC cells. Furthermore, specific
primers for JAK and STAT3 were designed. In addition, the changes
in the mRNA levels of JAK and STAT3 were examined following
treatment with AIL using RT-qPCR, and found that AIL inhibited the
expression of the two genes. It was thus demonstrated that AIL
inhibited the activation of the JAK/STAT3 signaling pathway in the
HCT116 and SW620 CRC cell lines. In addition, compared with the
control group, the mRNA levels of JAK1 and STAT3 were significantly
decreased. All these results indicate that AIL inhibits the
activation of the JAK/STAT3 signaling pathway in the HCT116 and
SW620 CRC cell lines.
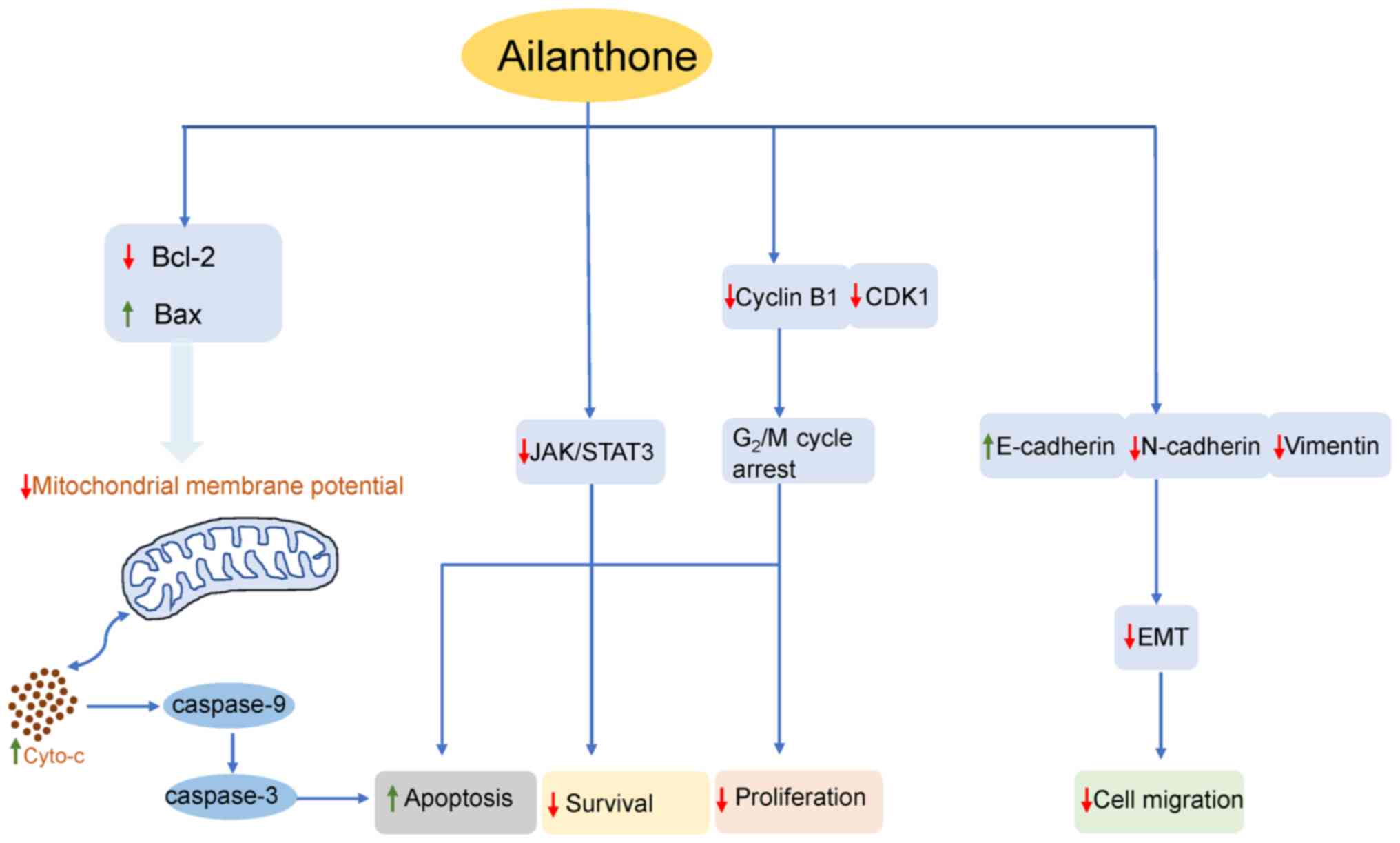
In conclusion, the findings of the present study
indicated that AIL inhibited the proliferation and migration of
HCT116 and SW620 CRC cells in vitro, promoted apoptosis and
exerted inhibitory effects on tumor-related signaling pathways
(Fig. 6). These results indicate
that AIL may have potential for use in the treatment of CRC; thus,
it may be worthy of further investigation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and ZY conceived and supervised the whole study.
HD and XY performed the experiments and the data analysis. HD
drafted the manuscript. XY and ZY revised the manuscript. HD and XY
confirm the authenticity of all the raw data. All authors agree to
be accountable for all aspects of the work ensuring integrity and
accuracy, and all authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Traditional Chinese Medicine
Science and Technology Project of Zhejiang Province (grant no.
2018ZA109), the Medical and Health Science and Technology Project
of Zhejiang Province (grant no. 2018ZH026), the Natural Science
Foundation of Ningbo (grant nos. 2016A610157 and 2018A610371) and
the Science and Technology Projects of Zhejiang Province (grant no.
LGF19H030007).
Abbreviations:
|
AIL
|
Ailanthone
|
|
CRC
|
colorectal cancer
|
|
JAK
|
Janus kinase
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CDKs
|
cyclin-dependent protein kinases
|
|
CKIs
|
CDK inhibitors
|
|
CCK-8
|
Cell Counting Kit-8
|
|
Bcl-2
|
B cell lymphoma-2
|
|
Bax
|
Bcl-2-associated X
|
|
MMPs
|
matrix metalloproteinases
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nikolaou M, Pavlopoulou A, Georgakilas AG
and Kyrodimos E: The challenge of drug resistance in cancer
treatment: a current overview. Clin Exp Metastasis. 35:309–318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang P, Yang HL, Yang YJ, Wang L and Lee
SC: Overcome cancer cell drug resistance using natural products.
Evid Based Complement Alternat Med. 2015:7671362015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alves IA, Miranda HM, Soares LA and Randau
KP: Simaroubaceae family: Botany, chemical composition and
biological activities. Rev Bras Farmacogn. 24:481–501. 2014.
View Article : Google Scholar
|
|
5
|
Ding H, Yu X, Hang C, Gao K, Lao X, Jia Y
and Yan Z: Ailanthone: A novel potential drug for treating human
cancer. Oncol Lett. 20:1489–1503. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casinovi CG, Ceccherelli P, Grandolini G
and Bellavita V: On the structure of Ailanthone. Tetrahedron Lett.
5:3991–3997. 1964. View Article : Google Scholar
|
|
7
|
Ishibashi M, Tsuyuki T, Murae T, Hirota H,
Takahashi T, Itai A and Iitaka Y: Constituents of the root bark of
Ailanthus altissima swingle. Isolation and X-ray crystal structures
of shinjudilactone and shinjulactone C and conversion of ailanthone
into shinjudilactone. Bull Chem Sok Jpn. 56:3683–3693. 1983.
View Article : Google Scholar
|
|
8
|
Hou S, Cheng Z, Wang W, Wang X and Wu Y:
Ailanthone exerts an antitumor function on the development of human
lung cancer by upregulating microRNA-195. J Cell Biochem.
120:10444–10451. 2019. View Article : Google Scholar
|
|
9
|
Liu W, Liu X, Pan Z, Wang D, Li M, Chen X,
Zhou L, Xu M, Li D and Zheng Q: Ailanthone induces cell cycle
arrest and apoptosis in melanoma B16 and A375 cells. Biomolecules.
9:2752019. View Article : Google Scholar :
|
|
10
|
Daga M, Pizzimenti S, Dianzani C, Cucci
MA, Cavalli R, Grattarola M, Ferrara B, Scariot V, Trotta F and
Barrera G: Ailanthone inhibits cell growth and migration of
cisplatin resistant bladder cancer cells through down-regulation of
Nrf2, YAP, and c-Myc expression. Phytomedicine. 56:156–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Zhu L, Yang X, Wei C, Chen C, He Y
and Ji Z: Ailanthone induces G2/M cell cycle arrest and apoptosis
of SGC-7901 human gastric cancer cells. Mol Med Rep. 16:6821–6827.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Peng S, Wang J, Chen H, Cong X, Chen
A, Hu M, Qin M, Wu H, Gao S, et al: Ailanthone targets p23 to
overcome MDV3100 resistance in castration-resistant prostate
cancer. Nat Commun. 7:131222016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng
L, Yao N, Peng Q, Chen Z, Ye W and Zhang D: Ailanthone inhibits
Huh7 cancer cell growth via cell cycle arrest and apoptosis in
vitro and in vivo. Sci Rep. 5:161852015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu
Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang W, Yu LF, Zhong J, Wu W, Zhu JY,
Jiang FX and Wu YL: Stat3 is involved in angiotensin II-induced
expression of MMP2 in gastric cancer cells. Dig Dis Sci.
54:2056–2062. 2009. View Article : Google Scholar
|
|
18
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bezwoda WR, MacDonald DF, Gear JS, Derman
DP, Bothwell TH, Sqi S, Hurwitz S and Lewis D: Combination
chemotherapy including bleomycin in the treatment of advanced
Hodgkin's disease. S Afr Med J. 53:369–373. 1978.PubMed/NCBI
|
|
21
|
Durant JR, Gams RA, Bartolucci AA and
Dorfman RF: BCNU with and without cyclophosphamide, vincristine,
and prednisone (COP) and cycle-active therapy in non-Hodgkin's
lymphoma. Cancer Treat Rep. 61:1085–1096. 1977.PubMed/NCBI
|
|
22
|
Wong MY and Chiu GN: Liposome formulation
of co-encapsulated vincristine and quercetin enhanced antitumor
activity in a trastuzumab-insensitive breast tumor xenograft model.
Nanomedicine. 7:834–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Munker S, Vogelhuber M, Bornschein J,
Stroszczynski C, Evert M, Schlitt H, Herr W and Teufel A: EpiCO
(epirubicin, cyclophosphamide and vincristine) as treatment for
extrapulmonary high-grade neuroendocrine neoplasms. Z
Gastroenterol. 58:133–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Büyükkapu Bay S, Kebudi R, Görgün O,
Zülfikar B, Darendeliler E and Çakır FB: Vincristine, irinotecan,
and temozolomide treatment for refractory/relapsed pediatric solid
tumors: A single center experience. J Oncol Pharm Pract.
25:1343–1348. 2019. View Article : Google Scholar
|
|
25
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caparica R, Bruzzone M, Poggio F, Ceppi M,
de Azambuja E and Lambertini M: Anthracycline and taxane-based
chemotherapy versus docetaxel and cyclophosphamide in the adjuvant
treatment of HER2-negative breast cancer patients: A systematic
review and meta-analysis of randomized controlled trials. Breast
Cancer Res Treat. 174:27–37. 2019. View Article : Google Scholar
|
|
27
|
Reck M, Brahmer J, Bennett B, Taylor F,
Penrod JR, DeRosa M, Dastani H, Spigel DR and Gralla RJ: Evaluation
of health-related quality of life and symptoms in patients with
advanced non-squamous non-small cell lung cancer treated with
nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 102:23–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhang C and Min D: Ailanthone
up-regulates miR-449a to restrain acute myeloid leukemia cells
growth, migration and invasion. Exp Mol Pathol. 108:114–120. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni Z, Yao C, Zhu X, Gong C, Xu Z, Wang L,
Li S, Zou C and Zhu S: Ailanthone inhibits non-small cell lung
cancer cell growth through repressing DNA replication via
downregulating RPA1. Br J Cancer. 117:1621–1630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Philchenkov A, Zavelevich M, Kroczak TJ
and Los M: Caspases and cancer: Mechanisms of inactivation and new
treatment modalities. Exp Oncol. 26:82–97. 2004.PubMed/NCBI
|
|
36
|
Rohn JL and Noteborn MH: The viral death
effector apoptin reveals tumor-specific processes. Apoptosis.
9:315–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terrano DT, Upreti M and Chambers TC:
Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation
acts as a functional link coupling mitotic arrest and apoptosis.
Mol Cell Biol. 30:640–656. 2010. View Article : Google Scholar :
|
|
38
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
39
|
Lee H, Jeong AJ and Ye SK: Highlighted
STAT3 as a potential drug target for cancer therapy. BMB Rep.
52:415–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng J, Tang ZH, Liu S and Guo SS:
Clinicopathological significance of overexpression of interleukin-6
in colorectal cancer. World J Gastroenterol. 23:1780–1786. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waldner MJ and Neurath MF: Master
regulator of intestinal disease: IL-6 in chronic inflammation and
cancer development. Semin Immunol. 26:75–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Velikova TV, Miteva L, Stanilov N,
Spassova Z and Stanilova SA: Interleukin-6 compared to the other
Th17/Treg related cytokines in inflammatory bowel disease and
colorectal cancer. World J Gastroenterol. 26:1912–1925. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee KS, Kwak Y, Nam KH, Kim DW, Kang SB,
Choe G, Kim WH and Lee HS: c-MYC copy-number gain is an independent
prognostic factor in patients with colorectal cancer. PLoS One.
10:e01397272015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leslie K, Lang C, Devgan G, Azare J,
Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, et al:
Cyclin D1 is transcriptionally regulated by and required for
transformation by activated signal transducer and activator of
transcription 3. Cancer Res. 66:2544–2552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee DH, Sung KS, Bartlett DL, Kwon YT and
Lee YJ: HSP90 inhibitor NVP-AUY922 enhances TRAIL-induced apoptosis
by suppressing the JAK2-STAT3-Mcl-1 signal transduction pathway in
colorectal cancer cells. Cell Signal. 27:293–305. 2015. View Article : Google Scholar
|
|
46
|
Tian Y, Ye Y, Gao W, Chen H, Song T, Wang
D, Mao X and Ren C: Aspirin promotes apoptosis in a murine model of
colorectal cancer by mechanisms involving downregulation of
IL-6-STAT3 signaling pathway. Int J Colorectal Dis. 26:13–22. 2011.
View Article : Google Scholar
|
|
47
|
Liu H, Ren G, Wang T, Chen Y, Gong C, Bai
Y, Wang B, Qi H, Shen J, Zhu L, et al: Aberrantly expressed Fra-1
by IL-6/STAT3 transactivation promotes colorectal cancer
aggressiveness through epithelial-mesenchymal transition.
Carcinogenesis. 36:459–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Han C, Sun B, Zhao X, Zhang Y, Gu Q, Liu
F, Zhao N and Wu L: Phosphorylation of STAT3 promotes vasculogenic
mimicry by inducing epithelial-to-mesenchymal transition in
colorectal cancer. Technol Cancer Res Treat. 16:1209–1219. 2017.
View Article : Google Scholar
|
|
49
|
Angevin E, Tabernero J, Elez E, Cohen SJ,
Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S,
Joly F, et al: A phase I/II, multiple-dose, dose-escalation study
of siltuximab, an anti-interleukin-6 monoclonal antibody, in
patients with advanced solid tumors. Clin Cancer Res. 20:2192–2204.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fogelman D, Cubillo A, García-Alfonso P,
Mirón MLL, Nemunaitis J, Flora D, Borg C, Mineur L, Vieitez JM,
Cohn A, et al: Randomized, double-blind, phase two study of
ruxolitinib plus regorafenib in patients with relapsed/refractory
metastatic colorectal cancer. Cancer Med. 7:5382–5393. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beatty GL, Shahda S, Beck T, Uppal N,
Cohen SJ, Donehower R, Gabayan AE, Assad A, Switzky J, Zhen H and
Von Hoff DD: A phase Ib/II study of the JAK1 inhibitor, itacitinib,
plus nab-paclitaxel and gemcitabine in advanced solid tumors.
Oncologist. 24:14–e10. 2019. View Article : Google Scholar
|
|
52
|
Oh DY, Lee SH, Han SW, Kim MJ, Kim TM, Kim
TY, Heo DS, Yuasa M, Yanagihara Y and Bang YJ: Phase I study of
OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid
tumors. Cancer Res Treat. 47:607–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Okamoto T, Inozume T, Mitsui H, Kanzaki M,
Harada K, Shibagaki N and Shimada S: Overexpression of GRIM-19 in
cancer cells suppresses STAT3-mediated signal transduction and
cancer growth. Mol Cancer Ther. 9:2333–2343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hong D, Kurzrock R, Kim Y, Woessner R,
Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M, et al:
AZD9150, a next-generation antisense oligonucleotide inhibitor of
STAT3 with early evidence of clinical activity in lymphoma and lung
cancer. Sci Transl Med. 7:314ra1852015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bharadwaj U, Kasembeli MM, Robinson P and
Tweardy DJ: Targeting janus kinases and signal transducer and
activator of transcription 3 to treat inflammation, fibrosis, and
cancer: Rationale, progress and caution. Pharmacol Rev. 72:486–526.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song L, Wang Y, Zhen Y, Li D, He X, Yang
H, Zhang H and Liu Q: Piperine inhibits colorectal cancer migration
and invasion by regulating STAT3/Snail-mediated
epithelial-mesenchymal transition. Biotechnol Lett. 42:2049–2058.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou H, Chen S, Yang Y, Yang C, Chen D,
Yao Z and Sun B: Matrine enhances the efficacy of adriamycin
chemotherapy in osteosarcoma cells by the STAT3 pathway. Anticancer
Drugs. 30:1006–1012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cummins CB, Wang X, Nunez Lopez O, Graham
G, Tie HY, Zhou J and Radhakrishnan RS: Luteolin-mediated
inhibition of hepatic stellate cell activation via suppression of
the STAT3 pathway. Int J Mol Sci. 19:15672018. View Article : Google Scholar :
|
|
59
|
Hayakawa T, Yaguchi T and Kawakami Y:
Enhanced anti-tumor effects of the PD-1 blockade combined with a
highly absorptive form of curcumin targeting STAT3. Cancer Sci.
111:4326–4335. 2020. View Article : Google Scholar : PubMed/NCBI
|