1. Introduction
Pulmonary fibrosis (PF) is a chronic, aged-related,
progressive, irreversible and life-threatening lung disease caused
by alveolar epithelial cell injury and apoptosis,
epithelial-mesenchymal transition (EMT), fibroblast proliferation,
as well as extracellular matrix (ECM) deposition in the
interstitial tissue. Idiopathic PF (IPF) is the most common type of
PF, and its diagnosis is based on radiographical and/or
histopathological patterns typical of usual interstitial pneumonia
(1). IPF exhibits a high
prevalence among the elderly with a history of smoking, thus
increasing morbidity and mortality; there is also currently a lack
of effective diagnostic and therapeutic measures (1). The incidence of IPF in North
America and Europe (3-9 cases/ 100,000 person-years) is higher than
that in South America and East Asia (<4 cases/100,000
person-years) (2). Although the
pathogenesis and molecular mechanisms of IPF remain unclear, cell
apoptosis and/or senescence, telomere shortening, mitochondrial
dysfunction, endoplasmic reticulum stress, oxidant/antioxidant
imbalance and immunoregulatory dysfunction may be involved
(3-6).
With the US Food and Drug Administration approval of
pirfenidone and nintedanib, the treatment of IPF has reached a new
plateau, and the majority of patients succumb to respiratory
failure within 3-5 years following diagnosis (7,8).
The results of a phase-2, double-blind, randomized,
placebo-controlled clinical study investigating lebrikizumab (an
anti-IL-13 monoclonal antibody) alone or combined with pirfenidone
treatment in patients with IPF indicated that this therapeutic
strategy may not be sufficient to improve pulmonary function
(9). A previous meta-analysis of
the use of pirfenidone, nintedanib and pamrevlumab (monoclonal
antibody targeting connective tissue growth factor, FG-3109) for
the treatment of patients with IPF suggested that pamrevlumab
significantly improved pulmonary function and was effective in
attenuating the decline in forced vital capacity (10). Thus, it may become a potential
candidate for IPF treatment for phase-3 clinical trials. In small
randomized trials, other drugs, such as pentraxin 2 (11) and GLPPG1690 (12), have also resulted in a slower
decline in pulmonary function than the administered placebo
(13). Despite these findings,
to date, there is no effective treatment available for IPF, at
least to the best of our knowledge. All available therapies are
only effective for a small number of patients and have severe
side-effects, together with a low overall survival rate. Therefore,
there is still a need for the development of novel therapies that
may provide additional clinical benefits to patients with IPF.
Extracellular vesicles (EVs) are present in body
fluids, such as serum, plasma, urine, saliva, sputum, uterine
secretions and bronchoalveolar lavage fluid (BALF) (14). EVs, including exosomes, play an
important role in cell-to-cell communications, particularly between
epithelial cells and the pulmonary microenvironment (14). MicroRNAs (miRNAs/miRs), EVs
and/or exosomes may represent potential biomarkers for the
diagnosis and prognosis of IPF, as well as therapeutic targets for
this disease (15). The present
review discusses the current understanding of the role of EVs,
particularly that of exosomes and exosomal miRNAs in the
pathogenesis of PF and/or IPF. The clinical process of IPF
diagnosis and treatment based on exosomes and exosomal miRNAs is
also discussed.
2. Characteristics, classification and
biosynthesis of extracellular vesicles
EVs are structures derived from plasma membranes
with diameters ranging from 30 nm to 5 µm and are composed
of a phospholipid bilayer (14).
EVs were first identified in the late 1960s in cells from mammalian
tissue or fluids (16), although
they were not named as such until 2011 (17). In the 1980s, EVs were found to be
generated either by budding from the plasma membrane or through
intracellular pathways, including incorporating multivesicular
bodies (MVBs) into the plasma membrane (18).
Depending on their biosynthesis, particle size and
biophysical properties, EVs can be classified into four subtypes as
follows: Exosomes, microvesicles (MVs) or micropaticles (19) and apoptotic bodies (20,21), as well as smaller-diameter
subsets of nanoparticle exomeres (22,23). Different subtypes of EVs can be
enriched and broadly separated using different methods, such as
ultracentrifugation, ultrafiltration, polymer precipitation,
immunoaffinity chromatography, flow cytometry, density gradient
centrifugation, microfluidics-based techniques and immunoadsorption
(24). Currently, the gold
standard for EVs isolation is the ultracentrifugation-linked
immunoprecipitation method (25). However, none of these methods can
completely purify a specific subpopulation, resulting in
preparations that are mixed with other EV subtypes. A membrane
affinity spin column can capture nearly 100% mRNA from EVs without
undesired protein-bound extracellular RNA co-precipitate (25). EVs isolation and enrichment is
considered a necessary pre-analytical requirement for biomedical
research and clinical treatment (26,27). A list of different subtypes of
EVs is presented in Table I.
 | Table ISubtypes of EVs. |
Table I
Subtypes of EVs.
| Subtypes | Size | Biomarker | Contents | Biosynthesis
pathway | (Refs.) |
|---|
| Apoptotic bodies
(Large EVs) | 1-5 µm | Annexin, histone
H3, | Nucleoprotein,
Golgi, endoplasmic reticulum, other cellular organelles | Apoptosis
pathway | (20,21,24) |
| Microvesicles
(Medium EVs) | 100-1,000 nm | Integrin, selectin,
annexin A1, CD40L | RNA, cytoplasmic
protein | Budding from the
plasma membranes pathway | (19,20,21,25) |
| Exosomes, (Small
EVs) | 40-120 nm | CD9, CD63, CD81,
HSP70, Alix, TSG101 | DNA, RNA,
cytoplasmic protein, membrane protein | Endocytosis of cell
membrane forming MVBs pathway | (20,21,24,27,32,33) |
| Exomeres
(Nanoparticles EVs) | 35-50 nm | HSP90, HSPA13 | DNA, RNA, protein,
enzymes, lipid | None | (22,23) |
The biosynthesis of exosomes is a progressive
endosome-dependent cytological process (28). This process begins with
endocytosis and is characterized by invagination of the cell
membrane to form an early-sorting endosome; subsequently, the
intranuclear soma re-invaginates and sheds to form small
intraluminal vesicles (ILVs). The formation of ILVs is followed by
the generation of late-sorting endosomes, termed MVBs. When MVBs
coalesce into the cell membrane, ILVs are released into the
extracellular space as exosomes (29).
Exosome biosynthesis and secretion can be triggered
by different mechanisms, such as endosomal sorting
complex-dependent pathways and ceramide-dependent pathways required
for transport (30). The former
are mediated by the endosomal sorting complex required for
transport (ESCRT). ESCRT is a family of proteins that includes
ESCRT-0, -I, -II and -III (31).
These proteins bind to MVB membrane-associated proteins and
regulate the targeted transport of cellular cargo molecules and the
formation of ILVs (32). Also
involved in the formation of MVBs are tumor susceptibility gene 101
and other ESCRT cofactors, such as ALG-2 interacting protein X
(33) and vacuolar protein
sorting-associated protein 4 (34). In addition, some ESCRT-associated
proteins can promote the secretion of exosomes independently of
ubiquitination. The post-translational binding of Sentrin/small
ubiquitin-like modifiers (SUMO) to proteins plays a vital role in
mediating ESCRT-dependent EV biosynthesis (35,36).
The ESCRT-independent mechanism of exosome formation
was first described in the study by Trajkovic et al
(29), reporting that
intracellular ceramide was deposited with the depletion of neutral
sphingomyelinase, causing small craft-based microdomains to fuse
into larger domains to induce exosomes budding. Subsequently, lipid
raft microdomains in exosomal membranes (37), tetraspanins (38), and CD63 (39), CD82 and CD9 (40) were found to be involved in
ESCRT-independent pathways.
When exosomes were first discovered, they were
considered as cellular waste products, and their functions were
unclear. However, several studies have demonstrated that exosomes
are important mediators of intercellular communication that can
transport endogenous proteins (41), lipids (42) and nucleic acids (43) encapsulated in lipid bilayers
through target cell internalization, receptor-ligand interactions
or lipid membrane fusion (44).
The components of exosomes of different cellular origins have a
common protein composition (45).
Exosomes contain various proteins, such as
transmembrane proteins (CD9, CD63, CD81 and CD82), heat shock
proteins involved in signal transduction (such as HSP60, HSP70 and
HSP90), the factor-associated suicide ligand (FasL) regulating the
immune response, cytokines such as TGF-β, interferon-γ and TNF-α,
as well as cytoskeletal proteins (actin and microtubulin) (46). CD9, CD63, CD81 and HSP70 are also
used as biomarkers to identify exosomes (40). The lipid components are mostly
sphingolipids, ceramides, phosphoglycerides, phosphatidylserine and
cholesterol (47). Exosomes also
contain various nucleic acids, including DNA, mRNAs, miRNAs, long
non-coding RNAs (lncRNAs) and fragmented mRNAs (44).
3. Exosomal microRNAs
miRNAs are a class of highly conserved endogenous
small non-coding RNAs widely distributed in plants, animals and DNA
viruses. They are single-stranded RNA molecules consisting of 19-25
nucleotides (average length of 22 nucleotides), which regulate cell
differentiation, proliferation and apoptosis by degrading the
target mRNAs or inhibiting translation to regulate gene expression
(48,49). In 2007, Valadi et al
(50) demonstrated for the first
time that both mouse and human mast cell exosomes contained mRNAs
and miRNAs that could be transferred to and mediate physiological
functions in the recipient cells. Subsequently, other studies
confirmed the existence of several other non-coding RNA species in
exosomes (51,52).
The initial transcription products of miRNAs are
primary transcripts (pri-miRNAs) with a hairpin structure.
Ribonuclease III releases the hairpin-shaped pri-miRNAs to form
precursor miRNAs (pre-miRNAs) with a stem-looped structure
(48). The endonuclease Dicer
removes the pre-miRNA 3′ and 5′ loop structures to form the miRNA
duplex. The miRNA binds to members of the argonaute RISC catalytic
component (Ago) protein family, discard the follower strand, with
the guide strand complementing the miRNA-induced silencing complex
to produce mature miRNA (53,54). Through the exchange of material
between cells, exosomes deliver intronic miRNA molecules to the
cytoplasm of target cells that specifically bind to the 3′
untranslated region of mRNA transcripts to regulate the expression
of target genes.
Accumulating evidence suggests that miRNAs are not
randomly integrated into exosomes. Of all the RNA types, miRNAs are
more prevalent in exosomes than in the cells from which they
originate (55). In a previous
study involving the analysis of exosomes secreted by different cell
lines, some miRNA subgroups, such as miR-150, miR-142-3p and
miR-451, were found to preferentially localize into exosomes
(56). For instance,
miRNA-574-5p has been shown to be only expressed in lung
adenocarcinoma cells, but not in squamous cell carcinoma,
suggesting a cell-specific response to exosomal miRNAs, which may
be due to the unique composition of tetraspanin which strongly
influences EV uptake (57).
Exosomes internalize miRNAs through various mechanisms, and
different RNA subpopulations bind to different exosome-targeted
RNA-binding proteins (RBPs). For example, the miRNA-associated
effector protein, Ago2, has been reported as an exosomal intron in
several cell types. In addition, it has been found that exosomal
miRNAs and Ago2 released from cancer tissue play an essential role
in the metastasis of breast cancer (58). The contents of exosomal miRNAs
depend to some extent on the abundance of exosomal Ago2, and
exosomes are also commonly enriched in miRNAs containing the GGAG
sequence (59). miRNAs are
accompanied by the extensive binding of the RBP heterogeneous
nuclear ribonucleoprotein A2B1 (hnRNPA2B1). These findings identify
hnRNPA2B1 as a vital player in miRNA sorting into exosomes. In
addition, SUMOylated hnRNP effectively controls the integration of
sorting-specific miRNAs into exosomes (59). Another study has suggested that
the RBP synaptotagmin-binding cytoplasmic RNA-interacting protein
(also known as hnRNPQ or NSAP1), directly binds to specific
exosomal miRNA molecules and shares a common extra-seed sequences
(EXO (exosomes) motif), thus mediating miRNA sorting into exosomes
in hepatocytes (60).
4. Role of microRNAs in the pathogenesis of
pulmonary fibrosis
miRNAs in pulmonary diseases
To date, >2,500 miRNA molecules have been
identified, and previous research has examined this RNA type in
pulmonary diseases (61). For
example, in the plasma of patients with chronic obstructive
pulmonary disease (COPD), the expression levels of circulating
hsa-miR-19b-3p and miR-320c were shown to be increased, while those
of hsa-miR-125b-5p were downregulated; these molecules may
represent promising biomarkers for the diagnosis of COPD (62). Through the p38-MAPK-c-Myc
signaling pathway, oxidative stress can induce the expression of
miR-494-3p, which can directly bind to sirtuin (SIRT)3 and reduce
its expression, thereby increasing cell senescence and promoting
the progression of COPD (63).
In asthmatic patients, the expression of let-7a, and
miR-21, -133a, -155, -328 and -1248 in exhaled breath condensates
has been shown to be significantly lower than in healthy
individuals (64). Serum miRNA
expression is age-dependent and is associated with clinical asthma
features and systemic inflammation, suggesting that miRNAs play
differential roles in the pathogenesis of asthma in patients from
different age groups (65).
As previously demonstrated in a systematic review,
in lung cancer, blood-derived miR-20a, -10b, -150 and -223 are
excellent diagnostic biomarkers for non-small cell lung cancer,
whereas miR-205 is specific for squamous cell carcinoma (66). miR-34a, -93, -106b, -181a,
-193a-3p and -375 may serve as theragnostic biomarker candidates to
checkpoint inhibitor treatments (66). Moreover, miR-21, -25, -27b, -19b,
-125b, -146a and -210 can predict responsiveness to platinum-based
treatment (66). The results of
exosomal RNA sequencing in patients with lung cancer suggested that
adenocarcinoma-specific, exosomal miR-181-5p, -30a-3p, -30e-3p and
-361-5p, as well as squamous carcinoma-specific exosomal
miR-10b-5p, -15b-5p and -320b levels were differentially expressed,
and these miRNA molecules may be valid candidates for the
development of highly sensitive and non-invasive biological markers
for early diagnosis (67).
Yang et al (68) analyzed human serum miRNAs from
healthy volunteers and patients with rapidly and slowly progressing
IPF. The results suggested that 47 miRNA molecules were
characteristically expressed in patients with IPF, of which 21 were
upregulated and 26 were downregulated. Moreover, the results from
reverse transcription-quantitative PCR revealed that the expression
of miR-21, miR-199a-5p and miR-200c in patients with IPF was
markedly higher than that in healthy volunteers, while the miR-31,
let-7a and let-7d expression levels were significantly lower. Thus,
miRNA molecules found in serum, BALF, sputum and other body fluids
may have the advantages of disease-specific expression, easy
detection and quantification. They may become useful diagnostic
biomarkers for lung diseases (69).
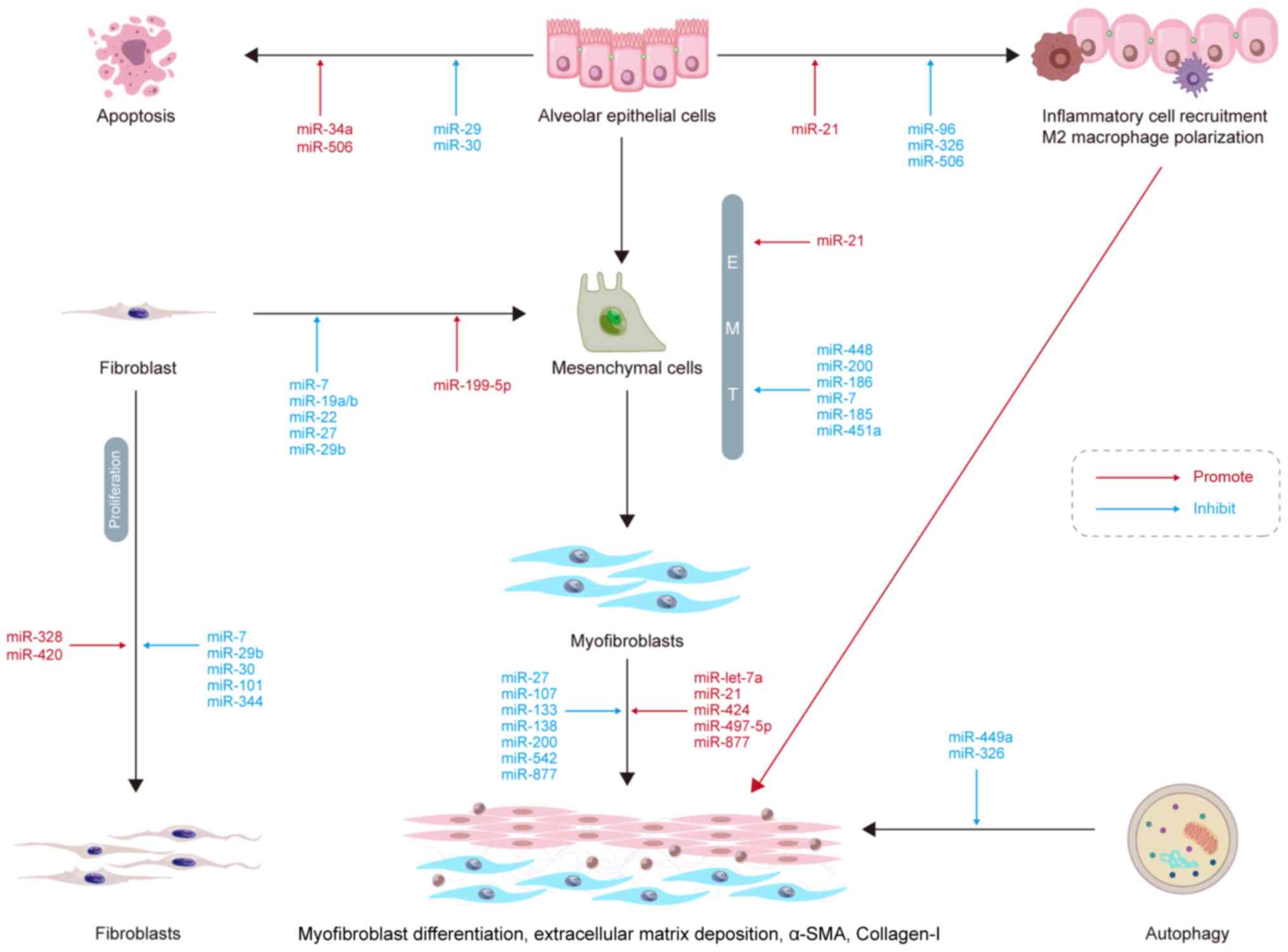
miRNAs and alveolar epithelial cell
apoptosis
When type-I alveolar epithelial cells are injured,
type-II alveolar epithelial cells (AEC-IIs) proliferate to promote
lung tissue repair (70). AEC-II
apoptosis is a key determinant in the initiation and development of
IPF. miR-30a has been shown to be significantly downregulated in a
murine model of bleomycin-induced lung fibrosis (71). Furthermore, miR-30a
overexpression has been shown to inhibit AEC-II apoptosis by
dampening mitochondrial fission through dynamin-related protein-1.
miR-29 is downregulated in IPF lungs, which promotes apoptosis
resistance (72). TGF-β inhibits
the expression of miR-29c and Fas receptors, causing AEC-II
apoptosis and fibrosis. In aged mice, miR-34a upregulation has been
found to accelerate lung epithelial cell senescence, apoptosis and
mitochondrial damage, resulting in epithelial cell dysfunction and
increased lung fibrosis (73).
p53 is an important cellular senescence marker
(74). Shetty et al
(73) suggested that both
miR-34a and p53 acetylation expression were increased in different
factor-induced lung epithelium injury and IPF models, which was
accompanied by the decreased expression of SIRT1, an
NAD+-dependent histone deacetylase that deacetylates the
lysine residues of various proteins. It was also found that the
inhibition of miR-34a upregulated SIRT1, and the overexpression of
SIRT1 reduced the acetylation levels of p53, thus reducing the
apoptosis of lung epithelial cells (73). Therefore, the p53-miR-34a/SIRT1
pathway may be a potential target for the treatment of IPF via the
inhibition of epithelial cell apoptosis (Fig. 1).
miRNAs and EMT
EMT is a dynamic and flexible biological process,
during which epithelial cells lose their cell polarity and basilar
membrane adhesion, change their shape with marked cytoskeletal
alterations and acquire the ability to migrate (75). EMT impairs re-epithelialization,
resulting in abnormal tissue repair, inflammatory cell
infiltration, fibroblast proliferation, ECM deposition and scarring
(76). This process is important
in PF and also serves essential roles in implantation and
gastrulation of the embryo, inflammatory and cancer metastasis
(76). Silica dynamically
downregulates miR-29b expression in RLE-6TN cells, which regulates
mesenchymal-epithelial transition (MET). In vivo, the
administration of miR-29b to mice has been shown to significantly
restrain silica-induced EMT, prevent lung fibrosis and improve lung
function (77). miR-34a
expression is significantly downregulated in silica-induced PF and
TGF-β-treated A549 cells in vitro (78). In addition, miR-34a can inhibit
EMT by suppressing the TGF-β signaling pathway and reducing the
expression of SMAD4 in PF. Odd-skipped related transcription factor
1 (OSR1) is a potential target for miR-451a; indeed, miR-451a
inhibits the expression of OSR1 and impairs EMT in vitro
(79). A previous review article
reported the inhibition of EMT by miR-7, -185, -186, -200, and
-448, while miR-21 promoted EMT in PF (80). Therefore, miRNAs may be a
potential target for the treatment of PF by inhibiting EMT.
miRNAs and myofibroblast
differentiation
IPF progresses primarily through the action of
myofibroblasts in the lungs. TGF-β1 is a profibrogenic cytokine
that induces the differentiation of lung myofibroblasts, and
emerging evidence has demonstrated TGF-β1 can regulate miRNA
expression in IPF (81).
Previous studies have demonstrated that TGF-β1 significantly
upregulates miR-133a expression in a time- and
concentration-dependent manner (82). Moreover, in a previous study,
TGF-β1-induced myofibroblast differentiation was shown to be
inhibited by miR-133a and to be promoted by miR-133a inhibitors
(82). In mice, the transfection
of miR-133a into lung tissue was also found to improve
bleomycin-induced lung fibrosis (82). In another study, lncRNA H19
served as a miRNA sponge for let-7a, which mediated
arsenite-induced M2 polarization of macrophages and c-Myc-induced
myofibroblast differentiation in PF (83). Several other miRNA molecules can
aggravate or attenuate PF by promoting or inhibiting myofibroblast
differentiation, such as miR-21 (84), miR-424 (85), miR-497-5p (86) and miR-877-3p (87).
miRNAs and ECM deposition
Several growth factors, cytokines, chemokines and
matrix metalloproteinases (MMPs) are involved in the development of
IPF. MMPs can disrupt the basilar lamina, allowing lung fibroblasts
to enter the alveolar space where they proliferate and produce
collagen and other ECM components, resulting in abnormal ECM
deposition and further promoting PF development (88). Previous research has suggested
that the lncRNA miR155 host gene (miR155HG) is upregulated in PF
tissue and normal human primary lung fibroblasts (NHLFs) stimulated
with TGF-β1 (89). Moreover,
miR155HG binds directly to miR-627 inhibits miR-627 expression
(89). Thus, the overexpression
of miR-627 reduces TGF-1-induced changes in NHLFs and significantly
reverses the excessive EMC deposition caused by overexpression of
miR155HG. Microvascular pericytes are the source of excess ECM
protein produced by myofibroblasts, contributing to PF (90). miR-107 expression has been shown
to be reduced in clinical or experimental mouse PF tissue samples
and exosomes by PF-derived microvascular endothelial cells
(90). The anti-fibrotic effect
of miR-107 is mediated by the inhibition of the hypoxia inducible
factor 1 α (HIF-1α)/Notch1/platelet-derived growth factor receptor
β (PDGFRβ)/Yes 1-associated transcriptional regulator/Twist1
signaling pathway. Indeed, miR-107 targets HIF-1α mRNA directly,
and HIF-1α directly activates the transcription of Notch1 and
PDGFRβ, thereby inhibiting ECM deposition (90). ECM deposition is also inhibited
by other miRNA molecules, such as miR-133, -138, -200 and -542 and
promoted by miR-497 and -877 (80).
miRNAs and fibroblast proliferation and
differentiation
In PF, fibroblasts differentiate into
myofibroblasts, which may secrete a greater amount ECM and collagen
components than fibroblasts, thus increasing ECM deposition in the
lung interstitium and aggravating PF; on the other hand, the
abnormal activation and proliferation of lung fibroblasts are also
crucial for the onset and progression of PF (91). miRNAs have been shown to play a
role in fibroblast proliferation and differentiation. For instance,
miR-7 expression has been found to be significantly downregulated
in polymyositis-associated interstitial lung diseases, while the
components of neutrophil extracellular trap components,
myeloperoxidase and histone 3, promote the proliferation and
differentiation of lung fibroblasts (92). Another study revealed that miR-7
attenuated the proliferation and differentiation of fibroblasts by
inhibiting SMAD2 expression (92). In vitro, miR-19a, -19b and
-26b have been shown to inhibit connective tissue growth factor
expression in the WI-38 human embryonic lung fibroblast cell line.
In vivo, the expression of miR-19a, -19b and -26b has been
shown to be downregulated in the murine bleomycin-induced PF model,
and these miRNA molecules can ameliorate PF by inhibiting
fibroblast differentiation and activating the MAPK pathway
(93). However, the
Snail/miR-199a-5p axis promotes endothelial-mesenchymal transition
in irradiated endothelial cells, which promotes fibroblast
differentiation into myofibroblasts (94). Other miRNA molecules, such as
miR-30, -101 and -344, can inhibit fibrosis by suppressing
fibroblast proliferation, whereas miR-328 and -420 exert the
opposite effect (80).
Therefore, regulating miRNA expression may represent a novel
therapeutic strategy to prevent or even partially reverse the
development of IPF.
miRNAs and inflammation
Inflammation is related to the onset of PF. However,
the role of the inflammatory response in the development of PF
remains under debate, as anti-inflammatory treatments are
ineffective in the treatment of IPF (95). Nevertheless, there is evidence to
suggest that inflammatory cells play a significant role in
regulating the microenvironment following IPF through innate and
adaptive immune responses (96).
In a previous study, the downregulation of miR-506 was observed
following during lipopolysaccharide-induced PF in rats with acute
respiratory distress syndrome; both in vivo and in
vitro, miR-506 induced apoptosis and reduced inflammation. In
addition, miR-506 was shown to target the 3′-UTR of NF-κB/p65 to
reduce its expression, and p65 inhibition significantly reduced
lung fibrosis and inflammation (97). Thus, miR-506 may regulate
apoptosis and inflammation, as well as lung fibrosis.
It has been shown that there is a positive
correlation between the expression of forkhead transcription factor
O (FOXO) 3a and the activation of NOD-like receptor protein 3
(NLRP3) inflammasome. FOXO3a is one of the targets of miR-96
(98). miR-96 expression is
reduced in carbon black nanoparticle (CBNP)-induced bronchial
epithelial cells, which is accompanied by a significant increase in
the expression of FOXO3a, the NLRP3 inflammasome and α-smooth
muscle actin (α-SMA). These effects were suppressed following
miR-96 transfection, suggesting that miR-96 silencing can lead to
an upregulation of FOXO3a, thereby promoting the activation of
NLRP3 inflammasome and aggravating CBNP-induced PF (98). Another study also found that
miR-21 expression was significantly elevated in a
nano-nickel-induced murine model of lung injury and fibrosis and
that miR-21 silencing inhibited TGF-β1 signaling and alleviated PF
(99).
miRNAs and autophagy
Autophagic cell death, also known as type-II
programmed cell death, is a biological phenomenon that that
eliminates the cellular by-products of eukaryotic cells (100). Autophagy requires the formation
of autophagosomes, which are double-membrane structures containing
isolated cytoplasmic material that eventually fuse with lysosomes
to form autophagic lysosomes for content degradation (101). Autophagy may be involved in
fibrosis and plays a vital role in the degradation of the ECM in
fibrotic diseases (102). Xu
et al (103) suggested
that the expression of miR-326 in the fibrotic lung tissue of mice
treated with silica was downregulated, whereas miR-326 upregulation
could attenuate silica-induced lung fibrosis in vivo. Tumor
necrosis factor superfamily member 14 (TNFSF14) and polypyrimidine
tract binding protein 1 (PTBP1) have been identified as the targets
of miR-326 (103). Thus, the
overexpression of miR-326 can inhibit silica-induced PF by
inhibiting inflammation and promoting autophagy by targeting
TNFSF14 and PTBP1 (103).
miR-499a has also been shown to significantly reduce PF and promote
autophagy in vivo and in vitro (104).
5. Role of exosomal microRNAs in pulmonary
fibrosis
BALF-derived exosomal miRNAs
BALF is the fluid that is collected through a
flexible bronchoscope during bronchoalveolar lavage (105). This fluid is mainly composed of
cells (including resident intrinsic trachea and alveolar cells, as
well as recruited inflammatory cells), but also contains EVs or
exosomes (106). It is an
important diagnostic material that provides information about the
immunological, infectious and inflammatory processes occurring in
the alveoli (105). BALF
contains a large number of EVs. Indeed, a previous study identified
1.8-3.8×108 EV-like particles per milliliter of BALF
using nanoparticle tracking analysis (107). Another study assessed the miRNA
expression patterns in BALF exosomes from elderly patients with
IPF, the high-throughput quantification of miRNA expression using a
microarray revealed that miR-125b, -128, -21, -100, -140-3p and
-374b expression was increased in patients with IPF, whereas that
of let-7d, miR-103, -26 and -30a-5p expression was reduced.
Furthermore, the overexpression of miR-30a-5p, a miRNA that targets
TGF-β activated kinase 1/MAP3K7-binding protein 3 (TAB3), was shown
to inhibit the expression of TAB3, thus delaying the occurrence of
PF (108). miR-145a can
suppress EMT by inhibiting the expression of OSR1 in vitro.
In a PF mouse model, the expression of miR-145a has been shown to
be significantly decreased in BALF-derived EVs, whereas the
expression of OSR1 was increased in PF tissue. The transfection of
miR-451a induces autocrine anti-fibrotic effects by downregulating
OSR1 (79). Zhu et al
(109) found that miR-204-5p
was markedly upregulated in BALF-derived exosomes of rats with PF
and that BALF-derived exosomal miR-204-5p promoted the progression
of the PF by inhibiting autophagy and targeting the AP1S2 gene
in vivo and in vitro. A list of exosomal miRNAs
involved in PF is presented in Table II.
 | Table IIExosomal-miRNAs in pulmonary
fibrosis. |
Table II
Exosomal-miRNAs in pulmonary
fibrosis.
| Origin | Exsomal miRNAs | (Refs.) |
|---|
| Diagnosis | BALF | miR-125b, miR-128,
miR-21, miR-100, miR-140-3p, miR-374b, let 7d, miR-103, miR-26,
miR-30a-5p, miR-145a, miR-204-5p | (79,108,109,118) |
| Sputum | miR-33a-5p,
miR-142-3p, miR-192-5, let-7d-5p, miR-26a-5p, miR-29b-3p,
miR-423-3p, miR-142-3p | (113,125) |
| Blood | miR-141, miR-7,
miR-21-5p, miR-16, miR-21, miR-26a, miR-210, let-7d, miR-18,
miR-142-3p | (116-118,125) |
| Treatment | Blood | miR-22,
miR-16, | (119,120) |
| Macrophages | miR-328,
miR-142-3p | (123,125) |
| BMSCs | miR-29b-3p,
miR-186 | (130,132) |
| EnCs | miR-223,
miR-27b-3p | (135) |
| LSCs | miR-99a-5p,
hsa-miR-100-5p, miR-30a-3p | (136) |
| HBECs | miR-16, miR-26a,
miR-26b, miR-141, miR-148a, miR-200a | (137) |
Differential miRNA expression profiles between
patients with IPF and healthy controls may represent a minimally
invasive biomarker for IPF screening and diagnosis. BALF-derived
exosomal miRNAs may become more practical and reliable biomarkers
than invasive lung tissue biopsies.
Sputum-derived exosomal miRNAs
Although BALF analysis is the most commonly used
technique for the assessment IPF, induced sputum has been regarded
as a safer and less invasive method and has been shown to be
clinically valuable for respiratory diseases (110,111). Previous research has
demonstrated that the expression and production of proteins, such
as insulin-like growth factor binding protein 2, IL-8 and MMP-7 in
the sputum of patients with IPF is significantly increased, which
may be related to the disease (112). Njock et al (113) conducted an miRNA quantitative
PCR array analysis to detect the miRNA expression profile of
sputum-derived exosomes in patients with IPF and healthy subjects.
The results suggested that 21 miRNA molecules were differentially
expressed (seven were unregulated and 14 were downregulated) in
IPF. To assess whether specific abnormally expressed miRNA
molecules were related to the pathophysiology of the disease, they
examined seven abnormally expressed miRNA molecules (three
upregulated miRNA molecules, including miR-33a-5p, -142-3p and
-192-5; four downregulated, including let-7d-5p, miR-26a-5p,
-29b-3p and -423-3p) using the ingenuity pathway analysis tool.
Functional annotation was used to predict their potential role in
the initiation and progression of IPF. They further validated three
miRNA molecules (miR-142-3p, miR-33a-5p and let-7d-5p) in an
independent cohort from 10 patients with IPF and 8 healthy
subjects. Of note, miR-142-3p expression was negatively correlated
with the carbon monoxide/alveolar volume, while that of let-7d-5p
was positively correlated, suggesting that exosomal miRNAs in the
sputum may be associated with the severity of PF (113). Thus, the study of
sputum-derived exosomal miRNAs in patients with IPF may help to
validate miRNAs previously identified from other samples (such as
serum, plasma, BALF and saliva), as well as identify novel miRNAs
with potential functions in IPF progression. This, in turn, this
may provide insight into the role of sputum-derived exosomal miRNA
in the pathogenesis of IPF. In addition, validating sputum exosomal
miRNAs as biomarkers of IPF to predict disease severity may pave
the way for the development of novel therapeutic approaches for
this disease.
Blood-derived exosomal miRNAs
Clinically, EVs can be found and isolated from the
majority of biological fluids, although blood and BALF are the most
well-characterized sources of EVs (114). Increasing evidence has
indicated that exosomes play a cardinal role in intercellular
communication by delivering their shipments to target cells in the
lungs (such as miRNAs). It has been suggested that exosomes and
exosomal miRNAs may eventually be used for the pathogenesis,
diagnosis and treatment of pulmonary diseases (115).
As previously demonstrated, compared with healthy
subjects, the levels of the anti-fibrotic miRNA, miR-141, are
significantly decreased in serum exosomal miRNAs isolated from
patients with IPF, whereas those of the fibrogenic miR-7 are
increased, resulting in ECM deposition (116). In addition, miR-7 upregulation
is associated with the radiologically and physiologically defined
disease burden.
In a previous study, in a murine model of PF,
miR-21-5p expression was found to be upregulated in serum EVs. In
addition, miR-21-5p levels were measured in serum EVs after
accounting for differences in serum EV amounts across patients with
IPF. Kaplan-Meier analysis revealed that individuals with high
blood EV miR-21-5p levels had a poorer survival rate than those
with low EV miR-21-5p levels. During the 30-month follow-up phase,
42% of the patients with elevated serum EV miR-21-5p levels died,
compared with 10% of the individuals with lower levels. Therefore,
serum EV miR-21-5p levels adjusted for EV capacity strongly
predicted disease progression and mortality (117).
Another study analyzed the expression levels of 5
exosomal miRNAs in serum from patients with IPF and found that
miR-16, miR-21, miR-26a, miR-210 and let-7d were downregulated in
serum exosomes from patients with IPF, although only miR-16 and
let-7d had a statistically significant difference, compared with
healthy subjects. Moreover, let-7d was shown to be anti-fibrotic
(118).
A previous study demonstrated that the expression of
miR-22 in the sera of bleomycin-induced PF in mice was increased by
up to 2-fold compared with the control group (119). In vitro experiments
demonstrated that exosomal miR-22 inhibited TGF-β1-induced α-SMA
expression through the ERK1/2 signaling pathway, indicating that
this miRNA could regulate fibroblast-to-myofibroblast
differentiation to ameliorate PF (119). It was also previously
demonstrated that serum exosome miR-18 expression was upregulated
8-fold in bleomycin-induced PF mice, compared with the wild-type
group. In vivo, a miR-16 mimic downregulated the expression
of the rapamycin-insensitive companion of mTOR complex 2 and the
TGFβ-1-induced expression of secreted protein acidic and rich in
cysteine (SPARC) in human lung fibroblasts to reduce PF in lung
tissue. This demonstrated that exosome-derived miR-16 may play a
role in PF through the mTOR-SPARC signaling pathway (120). Blood testing is minimally
invasive, and exosomal miRNAs are stable. Therefore, blood exosomal
miRNAs may serve as reliable markers for the diagnosis and
prognosis of IPF, as well as for the assessment of disease
severity. Exosomes may serve as miRNA delivery platforms for the
targeted treatment of IPF.
Macrophage-derived exosomal miRNAs
Although macrophages are critical cells in the
immune response, they can also promote PF (96). Macrophages differentiate into two
distinct subsets: i) Classically activated or M1 macrophages,
characterized by the secretion of pro-inflammatory cytokines and
chemokines (such as IL-1β, IL-6, IL-12, IL-23, TNF-α and CCL2); or
ii) alternatively activated or M2 macrophages that produce
anti-inflammatory cytokines and chemokines (IL-4, IL-13, TGF-β and
CXCL-8) (121). miRNAs may also
mediate macrophage polarization. A previous study examining the
regulation of M2 macrophage polarization in radiation-induced PF
demonstrated that miR-21 and miR-155 played a fibrogenic role,
whereas let-7i and miR-107, -126, -140, and -511 were anti-fibrotic
(122). In another study, in a
rat model of PF, miR-328 expression was significantly upregulated,
and family with sequence similarity 13 member A (FAM13A) expression
was notably downregulated. In addition, both miR-328 overexpression
and FAM13A silencing promoted the proliferation of lung fibroblasts
and deposition of ECM. By contrast, the overexpression of M2
macrophage-derived exosomal miR-328 contributed to enhanced
fibroblast proliferation and promoted PF through the regulation of
FAM13A in vivo and in vitro (123). Exosomal miRNA profiles derived
from macrophages exposed to silica have been examined using
high-throughput sequencing (124). A total of 298 miRNA molecules
were differentially expressed (155 upregulated and 143
downregulated), compared with unexposed macrophages; additional
research found that plasma exosomal miR-125a-5p expression was
upregulated in patients with silicosis (124). In vitro experiments
revealed that miR-125a-5p may target the ID1 and TGF-β/SMAD1
signaling pathway, promoting fibroblast differentiation to
myofibroblasts and the development of PF (124). In a recent study, the analysis
of exosomal miRNAs demonstrated that miR-142-3p was significantly
elevated in the sputum (8.06-fold) and plasma (1.64-fold) of
patients with IPF. Correlation analysis revealed that exosomal
miR-142-3p correlate positively with the percentage of macrophages
in the sputum of patients with IPF. In addition, macrophage-derived
exosomes inhibit TGF-β receptor 1 by delivering anti-fibrotic
miR-142-3p to AECs and lung fibroblasts, thereby alleviating PF
(125). Therefore, there is
evidence that the effect of exosomal miRNAs derived from
macrophages contributes to the proliferation of fibroblasts and the
progression of PF.
Exosomal miRNAs derived from other
cells
Mesenchymal stem cells (MSCs) have been shown to be
effective in the treatment of IPF (126). Multiple phase-I clinical trials
have been conducted to ensure the safety of MSC treatment (127). In one particular trial, the
2-year survival rate of 14 patients with IPF following the first
administration of adipose-derived stem cells (ADSCs) was 100%, the
median overall progression-free survival time was 26 months, and
overall survival was 32 months (128). In general, MSCs have the
therapeutic ability to repair damaged tissue by releasing EVs that
carry various dynamic cargos. In particular, exosomes extracted
from bone marrow-derived MSCs (BMSCs), contain various proteins,
mRNA transcripts and miRNAs that affect various biological
activities during tissue healing (129). A previous study demonstrated
that the overexpression of miR-29b-3p from BMSC EVs suppressed the
proliferation, migration, invasion and differentiation of lung
interstitial fibroblasts and relieved IPF through Frizzled 6
(130).
Moreover, it has been reported that MSC-derived EVs
can attenuate radiation-induced lung fibrosis by reducing DNA
damage by downregulating the ataxia telangiectasia mutated
(ATM)/P53/P21 signaling pathway; in addition, the downregulation of
ATM has been shown to be regulated by miR-214-3p, which is enriched
in MSC-derived EVs (131).
Another study found that BMSC-EVs inhibit lung fibroblast
activation and postpone the advancement of the IPF in a murine
model. miR-186 is downregulated in IPF but enriched in BMSC-derived
EVs, suggesting that miR-186 transported by BMSC EVs could promote
fibroblast activation and alleviate PF through downregulation of
miR-186 target gene with SRY-related HMG box transcription factor 4
and its downstream gene, Dickkopf-1 (132).
Other biological sources of therapeutic EVs include
differentiated cells, such as lung epithelial cells, lung
fibroblasts and cells of the immune system (133). EV-associated miRNAs, such as
miR-142-3p, miR-144-3p, miR-34b and miR-503-5p, which target
diverse profibrotic pathways, have been found to ameliorate
bleomycin-induced PF by lung-derived epithelial cell EVs collected
from syndecan-1 deficient mice (134). miR-223 and miR-27b-3p in
exosomes derived from vascular endothelial cells and AEC-IIs have
been shown to inhibit regulator of G protein signaling-1 (RGS1)
expression and regulate the frequency of Flt3+,
Tie2+ alveolar macrophages through calcium-dependent
signaling, thereby ameliorating bleomycin-induced acute lung injury
and PF (135). Furthermore, the
inhalation of lung spheroid cell (LSC)-secretome (LSC-Sec) and
exosomes (LSC-Exo) has been examined in various murine models of
lung damage and fibrosis. In LSC-Exo and MSC-Exo specimens, ~600
distinct miRNA molecules were identified. hsa-miR-99a-5p,
hsa-miR-100-5p and miR-30a-3p were among the most upregulated
miRNAs in LSC-Exo, whereas hsa-let-7a-5p and hsa-let-7f-5p were the
most upregulated in the MSC-Exo samples. By re-establishing normal
alveolar structure and decreasing collagen deposition and
myofibroblast proliferation, LSC-Sec and LSC-Exo therapies may
ameliorate and repair bleomycin- and silica-induced PF (136). It has also been demonstrated
that human bronchial epithelial cell (HBEC)-derived EVs prevent
TGF-β-mediated activation of both myofibroblast differentiation and
lung epithelial cellular senescence by inhibiting WNT signaling.
miR-16, miR-26a, miR-26b, miR-141, miR-148a and miR-200a are
mechanistically involved in suppressing WNT5A and WNT10B expression
in lung fibroblasts in vitro and in reducing WNT3A, WNT5A
and WNT10B expression in HBECs, among the enriched miRNAs derived
from HBEC EVs. Further research has revealed that HBEC EVs
treatment may be a viable anti-fibrotic therapy for IPF due to
miRNA-mediated reduction of TGF-β-WNT crosstalk (137).
6. Conclusions and future prospects
As discussed in the present review article, previous
studies have clarified the role of miRNAs and exosomes in the
pathogenesis of IPF, and have suggested that circulating miRNAs and
exosomes may become biomarkers for the diagnosis and prognosis of
IPF and potential therapeutic targets for its treatment. However,
several important issues need to be clarified to determine the
applicability of miRNAs and exosomes in IPF diagnosis and
treatment.
Firstly, different laboratories use different
techniques for the detection, isolation and analysis of exosomes,
which can confound the interpretation of results. Exosomes are
nanoscopic particles that are still difficult to detect and isolate
even using advanced technologies. With exosome isolation and
identification techniques continuously evolving, there is a lack of
standardization; hence, the employment of reproducible and
standardized methodology to quantify specific exosomes in clinical
samples is still a challenge.
In addition, exosomes originate from various body
fluids, such as saliva, sputum, exhaled breath condensates, BALF,
blood, urine, ascites and cerebrospinal fluid (138), and their cargos are organ-,
tissue- and cell-specific. Thus, due to the heterogeneity of
exosomes, their cargos and functions, precise and accurate
characterization methods are necessary. Investigators need to
further explore novel techniques to identify them, such as miRNAs
combined with traditional biomarkers, such as KL6, metalloprotease
and surfactant proteins A and D, to obtain insight into the
pathophysiology of IPF. On the other hand, the EV isolate
technique, and research design are highly heterogeneous (139).
Moreover, specific methods for the delivery of
potentially therapeutic miRNAs in exosomes to individual cells or
organs have not yet been established; therefore, further
investigations into the use of exosomes as miRNA delivery platforms
that can accurately deliver miRNAs to injured lung epithelial cells
are required; these may provide novel therapeutic strategies for
the treatment of IPF.
Furthermore, miRNAs are found in large amounts in
the body, and different miRNA molecules can regulate one target
gene, and conversely, one miRNA can regulate multiple target genes.
This complex association has kept much of the current research in
the exploratory stage. Similarly, in the pathogenesis of PF, one
miRNA can regulate multiple pathological aspects of PF (such as
miR-21), and one pathological pathway may be subjected to
regulation by multiple miRNA molecules, which represents a complex
regulatory network that needs further study.
The epigenetic profile of miRNAs renders them one of
the molecular classes that warrant consideration in future studies.
Moreover, whether they can participate in the signaling pathways
and autonomous biochemical reactions under appropriate
circumstances remains to be clarified (44).
Lastly, large-scale randomized controlled trials are
required to determine the efficacy of particular treatment
strategies and effective preventive measures to reduce the
mortality of patients with IPF.
In conclusion, exosomal miRNAs can mediate
cell-to-cell communications, which has become a topic of extensive
research. Previous studies have indicated that the abnormal
expression of exosomal miRNAs in lung diseases (such as COPD,
asthma, lung cancer and IPF) is essential to their pathogenesis.
The potential for miRNA molecules to be used as biomarkers for the
diagnosis and management of PF, and as therapeutic tools in
clinical practice remains to be investigated. The study of exosomal
miRNAs in IPF is still in its infancy, and further research is
required to investigate the role of exosomal miRNAs in PF
pathogenesis and progression. Future studies on the contribution of
exosomal miRNA to the pathogenesis of PF may clarify the prospects
for their future clinical application in this field.
Availability of data and materials
Data sharing does not apply to this article, as no
datasets were generated or analyzed during the current study.
Authors' contributions
TY performed the literature search and wrote the
manuscript. JZ and YL contributed to the literature search and in
the revision of the manuscript. JW supervised the preparation of
the manuscript and was also involved in the revision of the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Project of The Social
Development of Zhenjiang City of China (grant no. SH2020047).
References
|
1
|
Raghu G, Remy-Jardin M, Myers JL, Richeldi
L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F,
et al: Diagnosis of idiopathic pulmonary fibrosis. An official
ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care
Med. 198:e44–e68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lederer DJ and Martinez FJ: Idiopathic
pulmonary fibrosis. N Engl J Med. 378:1811–1823. 2018. View Article : Google Scholar
|
|
3
|
Schamberger AC, Schiller HB, Fernandez IE,
Sterclova M, Heinzelmann K, Hennen E, Hatz R, Behr J, Vašáková M,
Mann M, et al: Glutathione peroxidase 3 localizes to the epithelial
lining fluid and the extracellular matrix in interstitial lung
disease. Sci Rep. 6:299522016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Wang Y, Pandupuspitasari NS, Wu
G, Xiang X, Gong Q, Xiong W, Wang CY, Yang P and Ren B: Endoplasmic
reticulum stress, a new wrestler, in the pathogenesis of idiopathic
pulmonary fibrosis. Am J Transl Res. 9:722–735. 2017.
|
|
5
|
Kirby T: Living with idiopathic pulmonary
fibrosis. Lancet Respir Med. 9:136–138. 2021. View Article : Google Scholar
|
|
6
|
Shenderov K, Collins SL, Powell JD and
Horton MR: Immune dysregulation as a driver of idiopathic pulmonary
fibrosis. J Clin Invest. 131:e1432262021. View Article : Google Scholar :
|
|
7
|
Guenther A, Krauss E, Tello S, Wagner J,
Paul B, Kuhn S, Maurer O, Heinemann S, Costabel U, Barbero MAN, et
al: The European IPF registry (eurIPFreg): Baseline characteristics
and survival of patients with idiopathic pulmonary fibrosis. Respir
Res. 19:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao J, Kalafatis D, Carlson L, Pesonen
IHA, Li CX, Wheelock Å, Magnusson JM and Sköld CM: Baseline
characteristics and survival of patients of idiopathic pulmonary
fibrosis: A longitudinal analysis of the Swedish IPF Registry.
Respir Res. 22:402021. View Article : Google Scholar :
|
|
9
|
Maher TM, Costabel U, Glassberg MK, Kondoh
Y, Ogura T, Scholand MB, Kardatzke D, Howard M, Olsson J, Neighbors
M, et al: Phase 2 trial to assess lebrikizumab in patients with
idiopathic pulmonary fibrosis. Eur Respir J. 57:19024422021.
View Article : Google Scholar :
|
|
10
|
Di Martino E, Provenzani A, Vitulo P and
Polidori P: Systematic review and meta-analysis of pirfenidone,
nintedanib, and pamrevlumab for the treatment of idiopathic
pulmonary fibrosis. Ann Pharmacother. 55:723–731. 2021. View Article : Google Scholar
|
|
11
|
Raghu G, van den Blink B, Hamblin MJ,
Brown AW, Golden JA, Ho LA, Wijsenbeek MS, Vasakova M, Pesci A,
Antin-Ozerkis DE, et al: Effect of recombinant human pentraxin 2 vs
placebo on change in forced vital capacity in patients with
idiopathic pulmonary fibrosis: A randomized clinical trial. JAMA.
319:2299–2307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maher TM, van der Aar EM, Van de Steen O,
Allamassey L, Desrivot J, Dupont S, Fagard L, Ford P, Fieuw A and
Wuyts W: Safety, tolerability, pharmacokinetics, and
pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat
idiopathic pulmonary fibrosis (FLORA): A phase 2a randomised
placebo-controlled trial. Lancet Respir Med. 6:627–635. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abuserewa ST, Duff R and Becker G:
Treatment of idiopathic pulmonary fibrosis. Cureus.
13:e153602021.PubMed/NCBI
|
|
14
|
Brigstock DR: Extracellular vesicles in
organ fibrosis: Mechanisms, therapies, and diagnostics. Cells.
10:15962021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada M: Extracellular vesicles: Their
emerging roles in the pathogenesis of respiratory diseases. Respir
Investig. 59:302–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anderson HC: Vesicles associated with
calcification in the matrix of epiphyseal cartilage. J Cell Biol.
41:59–72. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
György B, Szabó TG, Pásztói M, Pál Z,
Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al:
Membrane vesicles, current state-of-the-art: Emerging role of
extracellular vesicles. Cell Mol Life Sci. 68:2667–2688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan BT, Teng K, Wu C, Adam M and Johnstone
RM: Electron microscopic evidence for externalization of the
transferrin receptor in vesicular form in sheep reticulocytes. J
Cell Biol. 101:942–948. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menck K, Sivaloganathan S, Bleckmann A and
Binder C: Microvesicles in cancer: Small size, large potential. Int
J Mol Sci. 21:53732020. View Article : Google Scholar :
|
|
20
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cocozza F, Grisard E, Martin-Jaular L,
Mathieu M and Théry C: SnapShot: Extracellular vesicles. Cell.
182:262–262.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Freitas D, Kim HS, Fabijanic K,
Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al:
Identification of distinct nanoparticles and subsets of
extracellular vesicles by asymmetric flow field-flow fractionation.
Nat Cell Biol. 20:332–343. 2018. View Article : Google Scholar :
|
|
23
|
Anand S, Samuel M and Mathivanan S:
Exomeres: A new member of extracellular vesicles family. Subcell
Biochem. 97:89–97. 2021. View Article : Google Scholar
|
|
24
|
Kučuk N, Primožič M, Knez Ž and Leitgeb M:
Exosomes engineering and their roles as therapy delivery tools,
therapeutic targets, and biomarkers. Int J Mol Sci. 22:95432021.
View Article : Google Scholar
|
|
25
|
Kodam SP and Ullah M: Diagnostic and
therapeutic potential of extracellular vesicles. Technol Cancer Res
Treat. 20:153303382110412032021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao H, Im H, Castro CM, Breakefield X,
Weissleder R and Lee H: New technologies for analysis of
extracellular vesicles. Chem Rev. 118:1917–1950. 2018. View Article : Google Scholar :
|
|
27
|
Yang D, Zhang W, Zhang H, Zhang F, Chen L,
Ma L, Larcher LM, Chen S, Liu N, Zhao Q, et al: Progress
opportunity, and perspective on exosome isolation-efforts for
efficient exosome-based theranostics. Theranostics. 10:3684–3707.
2020. View Article : Google Scholar
|
|
28
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trajkovic K, Hsu C, Chiantia S, Rajendran
L, Wenzel D, Wieland F, Schwille P, Brügger B and Simons M:
Ceramide triggers budding of exosome vesicles into multivesicular
endosomes. Science. 319:1244–1247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Niel G, Porto-Carreiro I, Simoes S and
Raposo G: Exosomes: A common pathway for a specialized function. J
Biochem. 140:13–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vietri M, Radulovic M and Stenmark H: The
many functions of ESCRTs. Nat Rev Mol Cell Biol. 21:25–42. 2020.
View Article : Google Scholar
|
|
32
|
Ju Y, Bai H, Ren L and Zhang L: The role
of exosome and the ESCRT pathway on enveloped virus infection. Int
J Mol Sci. 22:90602021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun R, Liu Y, Lu M, Ding Q, Wang P, Zhang
H, Tian X, Lu P, Meng D, Sun N, et al: ALIX increases protein
content and protective function of iPSC-derived exosomes. J Mol Med
(Berl). 97:829–844. 2019. View Article : Google Scholar
|
|
34
|
Han Q, Lv L, Wei J, Lei X, Lin H, Li G,
Cao J, Xie J, Yang W, Wu S, et al: Vps4A mediates the localization
and exosome release of β-catenin to inhibit epithelial-mesenchymal
transition in hepatocellular carcinoma. Cancer Lett. 457:47–59.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kunadt M, Eckermann K, Stuendl A, Gong J,
Russo B, Strauss K, Rai S, Kügler S, Falomir Lockhart L, Schwalbe
M, et al: Extracellular vesicle sorting of α-Synuclein is regulated
by sumoylation. Acta Neuropathol. 129:695–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang HM and Yeh ETH: SUMO: From bench to
bedside. Physiol Rev. 100:1599–1619. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Gassart A, Géminard C, Février B,
Raposo G and Vidal M: Lipid raft-associated protein sorting in
exosomes. Blood. 102:4336–4344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rana S and Zöller M: Exosome target cell
selection and the importance of exosomal tetraspanins: A
hypothesis. Biochem Soc Trans. 39:559–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Niel G, Charrin S, Simoes S, Romao M,
Rochin L, Saftig P, Marks MS, Rubinstein E and Raposo G: The
tetraspanin CD63 regulates ESCRT-Independent and -Dependent
endosomal sorting during melanogenesis. Dev Cell. 21:708–721. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chairoungdua A, Smith DL, Pochard P, Hull
M and Caplan MJ: Exosome release of β-catenin: A novel mechanism
that antagonizes Wnt signaling. J Cell Biol. 190:1079–1091. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar :
|
|
42
|
Dang VD, Jella KK, Ragheb RRT, Denslow ND
and Alli AA: Lipidomic and proteomic analysis. of exosomes from
mouse cortical collecting duct cells. FASEB J. 31:5399–5408. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
O'Brien K, Breyne K, Ughetto S, Laurent LC
and Breakefield XO: RNA delivery by extracellular vesicles in
mammalian cells and its applications. Nat Rev Mol Cell Biol.
21:585–606. 2020. View Article : Google Scholar
|
|
44
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar :
|
|
45
|
Saad MH, Badierah R, Redwan EM and
El-Fakharany EM: A comprehensive insight into the role of exosomes
in viral infection: Dual faces bearing different functions.
Pharmaceutics. 13:14052021. View Article : Google Scholar :
|
|
46
|
Gurunathan S, Kang MH, Qasim M, Khan K and
Kim JH: Biogenesis, membrane trafficking, functions, and next
generation nanotherapeutics medicine of extracellular vesicles. Int
J Nanomedicine. 16:3357–3383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Q, Higginbotham JN, Jeppesen DK,
Yang YP, Li W, McKinley ET, Graves-Deal R, Ping J, Britain CM,
Dorsett KA, et al: Transfer of functional cargo in exomeres. Cell
Rep. 27:940–954.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mohr AM and Mott JL: Overview of MicroRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shao N, Xue L, Wang R, Luo K, Zhi F and
Lan Q: MiR-454-3p is an exosomal biomarker and functions as a tumor
suppressor in glioma. Mol Cancer Ther. 18:459–469. 2019. View Article : Google Scholar
|
|
52
|
Qiu Y, Li P, Zhang Z and Wu M: Insights
into exosomal Non-coding RNAs sorting mechanism and clinical
application. Front Oncol. 11:6649042021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schirle NT, Sheu-Gruttadauria J and MacRae
IJ: Structural basis for microRNA targeting. Science. 346:608–613.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Methods in MicroRNA biogenesis,
identification, function and decay. Methods. 152:1–2. 2019.
View Article : Google Scholar
|
|
55
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guduric-Fuchs J, O'Connor A, Camp B,
O'Neill CL, Medina RJ and Simpson DA: Selective extracellular
vesicle-mediated export of an overlapping set of microRNAs from
multiple cell types. BMC Genomics. 13:3572012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Donzelli J, Proestler E, Riedel A,
Nevermann S, Hertel B, Guenther A, Gattenlöhner S, Savai R, Larsson
K and Saul MJ: Small extracellular vesicle-derived miR-574-5p
regulates PGE2-biosynthesis via TLR7/8 in lung cancer. J Extracell
Vesicles. 10:e121432021. View Article : Google Scholar
|
|
58
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform Cell-Independent MicroRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Villarroya-Beltri C, Gutiérrez-Vázquez C,
Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N,
Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M and
Sánchez-Madrid F: Sumoylated hnRNPA2B1 controls the sorting of
miRNAs into exosomes through binding to specific motifs. Nat
Commun. 4:29802013. View Article : Google Scholar
|
|
60
|
Santangelo L, Giurato G, Cicchini C,
Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A
and Tripodi M: The RNA-Binding Protein SYNCRIP Is a component of
the hepatocyte exosomal machinery controlling MicroRNA sorting.
Cell Rep. 17:799–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Boateng E and Krauss-Etschmann S: miRNAs
in lung development and diseases. Int J Mol Sci. 21:27652020.
View Article : Google Scholar :
|
|
62
|
Bersimbaev R, Aripova A, Bulgakova O,
Kussainova A, Akparova A and Izzotti A: The plasma levels of
hsa-miR-19b-3p hsa-miR-125b-5p and hsamiR-320c in patients with
asthma, COPD and asthma-COPD overlap syndrome (ACOS). MicroRNA.
10:130–138. 2021. View Article : Google Scholar
|
|
63
|
Zeng Q and Zeng J: Inhibition of
miR-494-3p alleviates oxidative stress-induced cell senescence and
inflammation in the primary epithelial cells of COPD patients. Int
Immunopharmacol. 92:1070442021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Weidner J, Bartel S, Kılıç A, Zissler UM,
Renz H, Schwarze J, Schmidt-Weber CB, Maes T, Rebane A,
Krauss-Etschmann S and Rådinger M: Spotlight on microRNAs in
allergy and asthma. Allergy. 76:1661–1678. 2021. View Article : Google Scholar
|
|
65
|
Wardzyńska A, Pawełczyk M, Rywaniak J,
Makowska J, Jamroz-Brzeska J and Kowalski ML: Circulating miRNA
expression in asthmatics is age-related and associated with
clinical asthma parameters, respiratory function and systemic
inflammation. Respir Res. 22:1772021. View Article : Google Scholar
|
|
66
|
Zhong S, Golpon H, Zardo P and Borlak J:
MiRNAs in lung cancer. A systematic review identifies predictive
and prognostic miRNA candidates for precision medicine in lung
cancer. Transl Res. 230:164–196. 2021. View Article : Google Scholar
|
|
67
|
Cainap C, Balacescu O, Cainap SS and Pop
LA: Next generation sequencing technology in lung cancer diagnosis.
Biology (Basel). 10:8642021.
|
|
68
|
Yang G, Yang L, Wang W, Wang J, Wang J and
Xu Z: Discovery and validation of extracellular/circulating
microRNAs during idiopathic pulmonary fibrosis disease progression.
Gene. 562:138–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang H, Song M, Guo J, Ma J, Qiu M and
Yang Z: The function of non-coding RNAs in idiopathic pulmonary
fibrosis. Open Med (Wars). 16:481–490. 2021. View Article : Google Scholar
|
|
70
|
Wang Q, Xie ZL, Wu Q, Jin ZX, Yang C and
Feng J: Role of various imbalances centered on alveolar epithelial
cell/fibroblast apoptosis imbalance in the pathogenesis of
idiopathic pulmonary fibrosis. Chin Med J (Engl). 134:261–274.
2021. View Article : Google Scholar
|
|
71
|
Mao C, Zhang J, Lin S, Jing L, Xiang J,
Wang M, Wang B, Xu P, Liu W, Song X and Lv C: Mi RNA -30a inhibits
AEC s-II apoptosis by blocking mitochondrial fission dependent on
Drp-1. J Cell Mol Med. 18:2404–2416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Matsushima S and Ishiyama J: MicroRNA-29c
regulates apoptosis sensitivity via modulation of the cell-surface
death receptor, Fas, in lung fibroblasts. Am J Physiol Lung Cell
Mol Physiol. 311:L1050–L1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shetty SK, Tiwari N, Marudamuthu AS,
Puthusseri B, Bhandary YP, Fu J, Levin J, Idell S and Shetty S: p53
and miR-34a feedback promotes lung epithelial injury and pulmonary
fibrosis. Am J Pathol. 187:1016–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Milanovic M, Fan DNY, Belenki D, Däbritz
JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa
IA, et al: Senescence-associated reprogramming promotes cancer
stemness. Nature. 553:96–100. 2018. View Article : Google Scholar
|
|
75
|
Wolters PJ, Collard HR and Jones KD:
Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol.
9:157–179. 2014. View Article : Google Scholar :
|
|
76
|
Hussen BM, Shoorei H, Mohaqiq M, Dinger
ME, Hidayat HJ, Taheri M and Ghafouri-Fard S: The impact of
Non-coding RNAs in the epithelial to mesenchymal transition. Front
Mol Biosci. 8:6651992021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sun J, Li Q, Lian X, Zhu Z, Chen X, Pei W,
Li S, Abbas A, Wang Y and Tian L: MicroRNA-29b mediates lung
mesenchymal-epithelial transition and prevents lung fibrosis in the
silicosis model. Mol Ther Nucleic Acids. 14:20–31. 2019. View Article : Google Scholar
|
|
78
|
Qi Y, Zhao A, Yang P, Jin L and Hao C:
MiR-34a-5p Attenuates EMT through targeting SMAD4 in silica-induced
pulmonary fibrosis. J Cell Mol Med. 24:12219–12224. 2020.
View Article : Google Scholar :
|
|
79
|
Jeong MH, Kim HR, Park YJ, Chung KH and
Kim HS: Reprogrammed lung epithelial cells by decrease of miR-451a
in extracellular vesicles contribute to aggravation of pulmonary
fibrosis. Cell Biol Toxicol. Aug 30–2021.Epub ahead of print.
View Article : Google Scholar
|
|
80
|
Liu Y, Nie H, Ding Y, Hou Y, Mao K and Cui
Y: MiRNA, a new treatment strategy for pulmonary fibrosis. Curr
Drug Targets. 22:793–802. 2021. View Article : Google Scholar
|
|
81
|
Rajasekaran S, Rajaguru P and Sudhakar
Gandhi PS: MicroRNAs as potential targets for progressive pulmonary
fibrosis. Front Pharmacol. 6:2542015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wei P, Xie Y, Abel PW, Huang Y, Ma Q, Li
L, Hao J, Wolff DW, Wei T and Tu Y: Transforming growth factor
(TGF)-β1-induced miR-133a inhibits myofibroblast differentiation
and pulmonary fibrosis. Cell Death Dis. 10:6702019. View Article : Google Scholar
|
|
83
|
Xiao T, Zou Z, Xue J, Syed BM, Sun J, Dai
X, Shi M, Li J, Wei S, Tang H, et al: LncRNA H19-mediated M2
polarization of macro-phages promotes myofibroblast differentiation
in pulmonary fibrosis induced by arsenic exposure. Environ Pollut.
268(Pt A): 1158102021. View Article : Google Scholar
|
|
84
|
Wang P, Xiao T, Li J, Wang D, Sun J, Cheng
C, Ma H, Xue J, Li Y, Zhang A and Liu Q: MiR-21 in EVs from
pulmonary epithelial cells promotes myofibroblast differentiation
via glycolysis in arsenic-induced pulmonary fibrosis. Environ
Pollut. 286:1172592021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang Y, Xie Y, Abel PW, Wei P, Plowman J,
Toews ML, Strah H, Siddique A, Bailey KL and Tu Y: TGF-β1-induced
miR-424 promotes pulmonary myofibroblast differentiation by
targeting Slit2 protein expression. Biochem Pharmacol.
180:1141722020. View Article : Google Scholar
|
|
86
|
Chen X, Shi C, Wang C, Liu W, Chu Y, Xiang
Z, Hu K, Dong P and Han X: The role of miR-97-5p in myofibroblast
differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep.
7:409582017. View Article : Google Scholar
|
|
87
|
Wang C, Gu S, Cao H, Li Z, Xiang Z, Hu K
and Han X: MiR-877-3p targets Smad7 and is associated with
myofibroblast differentiation and bleomycin-induced lung fibrosis.
Sci Rep. 6:301222016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Akbari Dilmaghnai N, Shoorei H, Sharifi G,
Mohaqiq M, Majidpoor J, Dinger ME, Taheri M and Ghafouri-Fard S:
Non-coding RNAs modulate function of extracellular matrix proteins.
Biomed Pharmacother. 136:1112402021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li J, Zhang X, Wang T, Li J, Su Q, Zhong
C, Chen Z and Liang Y: The MIR155 host
gene/microRNA-627/HMGB1/NF-κB loop modulates fibroblast
proliferation and extracellular matrix deposition. Life Sci.
269:1190852021. View Article : Google Scholar
|
|
90
|
Wang YC, Xie H, Zhang YC, Meng QH, Xiong
MM, Jia MW, Peng F and Tang DL: Exosomal miR-107 antagonizes
profibrotic phenotypes of pericytes by targeting a pathway
involving HIF-1 α/Notch1/PDGFR β/YAP1/Twist1 axis in vitro. Am J
Physiol-Heart Circ Physiol. 320:H520–H534. 2021. View Article : Google Scholar
|
|
91
|
Phan THG, Paliogiannis P, Nasrallah GK,
Giordo R, Eid AH, Fois AG, Zinellu A, Mangoni AA and Pintus G:
Emerging cellular and molecular determinants of idiopathic
pulmonary fibrosis. Cell Mol Life Sci. 78:2031–2057. 2021.
View Article : Google Scholar
|
|
92
|
Zhang S, Jia X, Zhang Q, Zhang L, Yang J,
Hu C, Shi J, Jiang X, Lu J and Shen H: Neutrophil extracellular
traps activate lung fibroblast to induce polymyositis-related
interstitial lung diseases via TLR9-miR-7-Smad2 pathway. J Cell Mol
Med. 24:1658–1669. 2020. View Article : Google Scholar
|
|
93
|
Chen YC, Chen BC, Yu CC, Lin SH and Lin
CH: MiR-19a, -19b, and -26b Mediate CTGF expression and pulmonary
fibroblast differentiation. J Cell Physiol. 231:2236–2248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yi M, Liu B, Tang Y, Li F, Qin W and Yuan
X: Irradiated human umbilical vein endothelial cells undergo
endothelial-mesenchymal transition via the Snail/miR-199a-5p axis
to promote the differentiation of fibroblasts into myofibroblasts.
Biomed Res Int. 2018:41358062018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Desai O, Winkler J, Minasyan M and Herzog
EL: The role of immune and inflammatory cells in idiopathic
pulmonary fibrosis. Front Med (Lausanne). 5:432018. View Article : Google Scholar
|
|
96
|
Heukels P, Moor CC, von der Thüsen JH,
Wijsenbeek MS and Kool M: Inflammation and immunity in IPF
pathogenesis and treatment. Respir Med. 147:79–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhu M, An Y, Zhang X, Wang Z and Duan H:
Experimental pulmonary fibrosis was suppressed by microRNA-506
through NF-kappa-mediated apoptosis and inflammation. Cell Tissue
Res. 378:255–265. 2019. View Article : Google Scholar
|
|
98
|
Zhou L, Li P, Zhang M, Han B, Chu C, Su X,
Li B, Kang H, Ning J, Zhang B, et al: Carbon black nanoparticles
induce pulmonary fibrosis through NLRP3 inflammasome pathway
modulated by miR-96 targeted FOXO3a. Chemosphere. 241:1250752020.
View Article : Google Scholar
|
|
99
|
Mo Y, Zhang Y, Wan R, Jiang M, Xu Y and
Zhang Q: MiR-21 mediates nickel nanoparticle-induced pulmonary
injury and fibrosis. Nanotoxicology. 14:1175–1197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tan S and Chen S: The mechanism and effect
of autophagy, apoptosis, and pyroptosis on the progression of
silicosis. Int J Mol Sci. 22:81102021. View Article : Google Scholar :
|
|
101
|
Zhao H, Wang Y, Qiu T, Liu W and Yao P:
Autophagy, an important therapeutic target for pulmonary fibrosis
diseases. Clin Chim Acta. 502:139–147. 2020. View Article : Google Scholar
|
|
102
|
Lv X, Li K and Hu Z: Autophagy and
pulmonary fibrosis. Adv Exp Med Biol. 1207:569–579. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xu T, Yan W, Wu Q, Xu Q, Yuan J, Li Y, Li
P, Pan H and Ni C: MiR-326 inhibits inflammation and promotes
autophagy in Silica-Induced pulmonary fibrosis through targeting
TNFSF14 and PTBP1. Chem Res Toxicol. 32:2192–2203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han R, Ji X, Rong R, Li Y, Yao W, Yuan J,
Wu Q, Yang J, Yan W, Han L, et al: MiR-449a regulates autophagy to
inhibit silica-induced. pulmonary fibrosis through targeting Bcl2.
J Mol Med (Berl). 94:1267–1279. 2016. View Article : Google Scholar
|
|
105
|
Zareba L, Szymanski J, Homoncik Z and
Czystowska-Kuzmicz M: EVs from BALF-Mediators of inflammation and
potential biomarkers in lung diseases. Int J Mol Sci. 22:36512021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shaba E, Landi C, Carleo A, Vantaggiato L,
Paccagnini E, Gentile M, Bianchi L, Lupetti P, Bargagli E, Prasse A
and Bini L: Proteome characterization of BALF extracellular
vesicles in idiopathic pulmonary fibrosis: Unveiling undercover
molecular pathways. Int J Mol Sci. 22:56962021. View Article : Google Scholar :
|
|
107
|
Rodríguez M, Silva J, López-Alfonso A,
López-Muñiz MB, Peña C, Domínguez G, García JM, López-Gónzalez A,
Méndez M, Provencio M, et al: Different exosome cargo from
plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes
Chromosomes Cancer. 53:713–724. 2014.PubMed/NCBI
|
|
108
|
Liu B, Jiang T, Hu X, Liu Z, Zhao L, Liu
H, Liu Z and Ma L: Downregulation of microRNA-30a in
bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis
patients. Mol Med Rep. 18:5799–5806. 2018.PubMed/NCBI
|
|
109
|
Zhu L, Chen Y, Chen M and Wang W:
Mechanism of miR-204-5p in exosomes derived from bronchoalveolar
lavage fluid on the progression of pulmonary fibrosis via AP1S2.
Ann Transl Med. 9:10682021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Guiot J, Demarche S, Henket M, Paulus V,
Graff S, Schleich F, Corhay JL, Louis R and Moermans C: Methodology
for Sputum Induction and Laboratory Processing. J Vis Exp.
130:566122017.
|
|
111
|
Pastor L, Vera E, Marin JM and Sanz-Rubio
D: Extracellular Vesicles from Airway Secretions: New insights in
lung diseases. Int J Mol Sci. 22:5832021. View Article : Google Scholar :
|
|
112
|
Guiot J, Henket M, Corhay JL, Moermans C
and Louis R: Sputum biomarkers in IPF: Evidence for raised gene
expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One.
12:e01713442017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Njock MS, Guiot J, Henket MA, Nivelles O,
Thiry M, Dequiedt F, Corhay JL, Louis RE and Struman I: Sputum
exosomes: Promising biomarkers for idiopathic pulmonary fibrosis.
Thorax. 74:309–312. 2019. View Article : Google Scholar
|
|
114
|
Trappe A, Donnelly SC, McNally P and
Coppinger JA: Role of extracellular vesicles in chronic lung
disease. Thorax. 76:1047–1056. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hua Y, Ding Y, Hou Y, Liu Y, Mao K, Cui Y
and Nie H: Exosomal MicroRNA: Diagnostic marker and therapeutic
tool for lung diseases. Curr Pharm Des. 27:2934–2942. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Minnis P, Kane R, Anglin R, Walsh S,
Worrel J, Khan F, Lumsden RV, Whitty S and Keane MP: Serum exosomes
from IPF patients display a fibrotic. miRNA profile that correlates
to clinical measures of disease severity. Eur Respir Rev. 46(Suppl
59): PA38452015.
|
|
117
|
Makiguchi T, Yamada M, Yoshioka Y, Sugiura
H, Koarai A, Chiba S, Fujino N, Tojo Y, Ota C, Kubo H, et al: Serum
extra-cellular vesicular miR-21-5p is a predictor of the prognosis
in idiopathic pulmonary fibrosis. Respir Res. 17:1102016.
View Article : Google Scholar
|
|
118
|
Lacedonia D, Scioscia G, Soccio P, Conese
M, Catucci L, Palladino GP, Simone F, Quarato CMI, Di Gioia S, Rana
R, et al: Downregulation of exosomal let-7d and miR-16 in
idiopathic pulmonary fibrosis. BMC Pulm Med. 21:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kuse N, Kamio K, Azuma A, Matsuda K,
Inomata M, Usuki J, Morinaga A, Tanaka T, Kashiwada T, Atsumi K, et
al: Exosome-derived microRNA-22 ameliorates. pulmonary fibrosis by
regulating fibroblast-to-myofibroblast differentiation in vitro and
in vivo. J Nippon Med Sch. 87:118–128. 2020. View Article : Google Scholar
|
|
120
|
Inomata M, Kamio K, Azuma A, Matsuda K,
Usuki J, Morinaga A, Tanaka T, Kashiwada T, Atsumi K, Hayashi H, et
al: Rictor-targeting exosomal microRNA-16 ameliorates lung fibrosis
by inhibiting the mTORC2-SPARC axis. Exp Cell Res. 398:1124162021.
View Article : Google Scholar
|
|
121
|
Vasse GF, Nizamoglu M, Heijink IH,
Schlepütz M, van Rijn P, Thomas MJ, Burgess JK and Melgert BN:
Macrophage-stroma interactions in fibrosis: Biochemical,
biophysical, and cellular perspectives. J Pathol. 254:344–357.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kishore A and Petrek M: Roles of
macrophage polarization and macrophage-derived. miRNAs in pulmonary
fibrosis. Front Immunol. 12:6784572021. View Article : Google Scholar
|
|
123
|
Yao MY, Zhang WH, Ma WT, Liu QH, Xing LH
and Zhao GF: MicroRNA-328 in. exosomes derived from M2 macrophages
exerts a promotive effect on the progression of pulmonary fibrosis
via FAM13A in a rat model. Exp Mol Med. 51:1–16. 2019. View Article : Google Scholar
|
|
124
|
Wang D, Hao C, Zhang L, Zhang J, Liu S, Li
Y, Qu Y, Zhao Y, Huang R, Wei J and Yao W: Exosomal miR-125a-5p
derived from silica-exposed. macrophages induces fibroblast
transdifferentiation. Ecotoxicol Environ Saf. 192:1102532020.
View Article : Google Scholar
|
|
125
|
Guiot J, Cambier M, Boeckx A, Henket M,
Nivelles O, Gester F, Louis E, Malaise M, Dequiedt F, Louis R, et
al: Macrophage-derived exosomes attenuate fibrosis. in airway
epithelial cells through delivery of antifibrotic miR-142-3p.
Thorax. 75:870–881. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Cruz FF and Rocco PRM: The potential of
mesenchymal stem cell therapy for chronic lung disease. Expert Rev
Respir Med. 14:31–39. 2020. View Article : Google Scholar
|
|
127
|
Yang S, Liu P, Jiang Y, Wang Z, Dai H and
Wang C: Therapeutic applications of. mesenchymal stem cells in
idiopathic pulmonary fibrosis. Front Cell Dev Biol. 9:6396572021.
View Article : Google Scholar
|
|
128
|
Ntolios P, Manoloudi E, Tzouvelekis A,
Bouros E, Steiropoulos P, Anevlavis S, Bouros D and Froudarakis M:
Longitudinal outcomes of patients. Enrolled. in a phase Ib clinical
trial of the adipose-derived stromal cells-stromal vascular
fraction in idiopathic pulmonary fibrosis. Clin Respir J.
12:2084–2089. 2018. View Article : Google Scholar
|
|
129
|
Hade MD, Suire CN and Suo Z: Mesenchymal
stem cell-derived exosomes: Applications in regenerative medicine.
Cells. 10:19592021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wan X, Chen S, Fang Y, Zuo W, Cui J and
Xie S: Mesenchymal stem cell-derived. extracellular vesicles
suppress the fibroblast proliferation by downregulating FZD6
expression in fibroblasts via micrRNA-29b-3p in idiopathic
pulmonary fibrosis. J Cell Physiol. 235:8613–8625. 2020. View Article : Google Scholar
|
|
131
|
Lei X, He N, Zhu L, Zhou M, Zhang K, Wang
C, Huang H, Chen S, Li Y, Liu Q, et al: Mesenchymal stem
cell-derived extracellular vesicles Attenuate radiation-induced
lung injury via miRNA-214-3p. Antioxid Redox Signal. 35:849–862.
2021. View Article : Google Scholar
|
|
132
|
Zhou J, Lin Y, Kang X, Liu Z, Zhang W and
Xu F: MicroRNA-186 in extracellular vesicles. from bone marrow
mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via
interaction with SOX4 and DKK1. Stem Cell Res Ther. 12:962021.
View Article : Google Scholar
|
|
133
|
Ibrahim A, Ibrahim A and Parimon T:
Diagnostic and therapeutic applications of extracellular vesicles
in interstitial lung diseases. Diagnostics (Basel). 11:872021.
View Article : Google Scholar
|
|
134
|
Parimon T, Yao C, Habiel DM, Ge L, Bora
SA, Brauer R, Evans CM, Xie T, Alonso-Valenteen F, Medina-Kauwe LK,
et al: Syndecan-1 promotes lung fibrosis by. regulating epithelial
reprogramming through extracellular vesicles. JCI Insight.
4:e1293592019. View Article : Google Scholar
|
|
135
|
Feng Z, Zhou J, Liu Y, Xia R, Li Q, Yan L,
Chen Q, Chen X, Jiang Y, Chao G, et al: Epithelium- and
endothelium-derived exosomes regulate the. alveolar macrophages by
targeting RGS1 mediated calcium signaling-dependent immune
response. Cell Death Differ. 28:2238–2256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Dinh PC, Paudel D, Brochu H, Popowski KD,
Gracieux MC, Cores J, Huang K, Hensley MT, Harrell E, Vandergriff
AC, et al: Inhalation of lung spheroid cell secretome and exosomes
promotes lung repair in pulmonary fibrosis. Nat Commun.
11:10642020. View Article : Google Scholar :
|
|
137
|
Kadota T, Fujita Y, Araya J, Watanabe N,
Fujimoto S, Kawamoto H, Minagawa S, Hara H, Ohtsuka T, Yamamoto Y,
et al: Human bronchial epithelial cell-derived. Extracellular.
vesicle therapy for pulmonary fibrosis via inhibition of TGF-β-WNT
crosstalk. J Extracell Vesicles. 10:e121242021. View Article : Google Scholar
|
|
138
|
Liu Z, Yan J, Tong L, Liu S and Zhang Y:
The role of exosomes from BALF in lung disease. J Cell Physiol. Aug
13–2021.Epub ahead of print.
|
|
139
|
Tieu A, Hu K, Gnyra C, Montroy J,
Fergusson DA, Allan DS, Stewart DJ, Thébaud B and Lalu MM:
Mesenchymal stromal cell extracellular vesicles as. Therapy. for
acute and chronic respiratory diseases: A meta-analysis. J
Extracell Vesicles. 10:e121412021. View Article : Google Scholar
|