Introduction
Breast cancer (BC) is the most common cancer
diagnosed in women worldwide (1), and it is the most commonly
diagnosed cancer among women in Thailand (2). The standard treatments for BC
include local treatment (resection and radiotherapy) and systemic
therapy (chemotherapy, endocrine therapy and targeted drug therapy)
(3). The clinical outcome is
associated with the stage of disease and the molecular
characteristics of the cancer diagnosed (4,5).
These standard treatments in the early stage of the disease can
improve survival-free progression and lower the risk of cancer
recurrence and mortality. Patients diagnosed with stage I BC have
prolonged survival (5- to 30-year follow-up) compared with those
diagnosed with stage II BC (6).
However, an alternative therapeutic option is urgently required for
those with advanced stages, and for those who do not respond to the
traditional standard treatments.
Immunotherapy functions by prompting the immune
system to fight against cancer, since host immunity is the key
element for controlling carcinogenesis, progression and metastasis
(7). Components of the immune
system, such as antibodies and immune cells, are used as biological
therapeutics to eliminate and control cancer cells. The success of
immunotherapy in several cancer types greatly expands its
therapeutic utility in modern cancer therapy (7,8).
Several immunotherapy drugs have been approved by the U.S. Food and
Drug Administration (9,10), and a large number of clinical
trials are ongoing (11-13). Natural killer (NK) cells
belonging to the innate immune system are an attractive choice for
adoptive immunotherapy. NK cells directly attack cancer cells
without prior sensitization (12) in a non-major histocompatibility
complex (MHC)-restricted manner. NK cells can lyse cancer cells via
various mechanisms, including antibody-dependent cellular
cytotoxicity, the release of cytolytic granules (perforin and
granzyme) and expression of death receptor-mediated apoptosis
[TNF-related apoptosis-inducing ligand (TRAIL) and Fas signaling
pathway] (14). Adoptive NK cell
immunotherapy is an attractive approach that has been tested in
patients with lung cancer, lymphoma, ovarian cancer and BC
(12,15-18). A previous study using autologous
NK-cell adoptive transfer in patients with metastatic melanoma or
renal cell carcinoma revealed the persistence of adoptive NK cells
in the peripheral circulation for weeks to months after treatment;
however, the adoptive NK cells had low cytotoxic activity and
failed to mediate tumor regression (18). This was due to the inhibitory
signal from self MHC I-expressing tumor cells that prevented the
autologous NK cells from attacking cancer cells. Accordingly,
allogeneic NK cell therapy has gained significant attention. Taking
advantage of the inhibitory ligand mismatch, allogeneic NK cells
demonstrated a markedly improved outcome in myeloid leukemia after
haploidentical hematopoietic cell transplantation (19). However, the therapeutic potential
of adoptive NK cell transfer remains limited by several factors,
one of which is the failure of NK expansion in vivo
(20). Therefore, the in
vivo persistence of NK cells and their rapid actions are likely
the critical factors that influence their antitumor activity. One
strategy to augment the NK killing function is to render tumor
cells more susceptible to NK cytotoxicity, which causes the rapid
killing of tumors in a shorter period.
Previously, the combined effects of chemotherapeutic
drugs and immunotherapy have been reported to enhance the cytotoxic
activity of immunotherapy against cancer cells and promote the
therapeutic effect (21).
Sensitization of cancer cells involves the upregulation of
activating ligand expression, which causes the cancer cells to be
more susceptible to killing by immune cells. Previously, the
treatment with a sublethal dose of doxorubicin was shown to promote
tumor cell susceptibility to NK and T lymphocytes (22). Doxorubicin, which is an
anthracycline antibiotic drug, is the first-line therapy for the
treatment of different cancer types, including leukemia, Hodgkin's
lymphoma, lung cancer, BC and others (23). In BC, doxorubicin treatment
yielded improved progression-free survival and median survival
duration in patients with metastasis compared with paclitaxel
treatment (24); however, the
underlying mechanism of doxorubicin on the sensitization of BC has
not yet been elucidated.
To investigate the sensitization mechanism of
doxorubicin in BC, the killing activity of NK cells in two
different BC cell lines, including MCF7
(ER+/PR+/HER2−) and MDA-MB-231
(ER−/PR−/HER2−), with and without
doxorubicin treatment, was compared in the present study. The
cytotoxicity of doxorubicin and its ability to sensitize MCF7 and
MDA-MB-231 cells to NK-92 cell killing was investigated. It was
demonstrated that the treatment with a sublethal dose of
doxorubicin caused MCF7, but not MDA-MB-231 cells, to become more
sensitive to NK-92 cells and primary NK cells isolated from healthy
donors via upregulation of Fas-receptor (FasR) expression. The
knowledge obtained from the present study supports the use of a
combined chemotherapy-immunotherapy regimen for the treatment of
BC, and suggests that FasR could be used as a marker for predicting
the response to combined doxorubicin-NK cell therapy.
Materials and methods
Cell culture
BC cell lines, MCF7
(ER+/PR+/HER2−), T47D
(ER+/PR+/HER2−) and MDA-MB-231
(ER−/PR−/HER2−) were obtained from
the American Type Culture Collection (ATCC) and maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), and 100 mg/ml of
penicillin/streptomycin (MilliporeSigma). The lymphoblast cell line
K562 was obtained from the ATCC and maintained in Roswell Park
Memorial Institute-1640 (RPMI-1640; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 100 mg/ml
penicillin/streptomycin. An NK cell line, namely NK-92, was
purchased from the ATCC and maintained in Minimum Essential Medium
Eagle-α Modification supplemented with 12.5% horse serum (Gibco;
Thermo Fisher Scientific, Inc.) and 12.5% FBS, 0.2 mM inositol, 0.1
mM 2-mercaptoethanol, 0.02 mM folic acid and 100-200 U/ml
recombinant IL-2 (R&D Systems, Inc.). Mycoplasma testing was
performed for the cell lines used. The cells were cultured at 37°C
in a 5% CO2 humidified incubator.
Construction and generation of
red-fluorescence protein (RFP)-expressing MCF7 and MDA-MB-231
cells
The lentivirus transfer plasmid (pCDH vector) was a
kind gift from Associate Professor Naravat Poungvarin (Faculty of
Medicine Siriraj Hospital, Mahidol University, Thailand). The
RFP-encoding lentivirus, namely pCDH-RFP, was constructed by
cloning the RFP coding sequences to the vector backbone. Using the
2nd generation lentivirus packing system, the construct (500 ng)
was used to transfect into the 293T cells (ATCC; 5×105
cells in a 35-mm dish; maintained in DMEM supplemented with 10% FBS
and 100 mg/ml penicillin/streptomycin), together with 50 ng of
envelope (pMD2.G) and 500 ng of packaging plasmids (psPAX2) to
produce the lentivirus using the Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The cells were incubated for 6 h at 37°C, and the 2 ml of
fresh media was replaced. The lentivirus was harvested at 48 h
after transfection and titrated using qPCR Lentivirus Titer kit
(cat. no. LV900; Applied Biological Materials). The virus was used
to transduce the MCF7 and MDA-MB-231 cells at a multiplicity of
infection of 100 for 24 h. Subsequently, puromycin (Thermo Fisher
Scientific, Inc.) was added at the concentration of 2 µg/ml
for 3 weeks (replacing the fresh media containing puromycin every 3
days thereafter) to select the stable RFP-expressing cells used in
the killing assay.
Isolation and culture of primary NK
cells
Peripheral blood mononuclear cells (PBMCs) were
isolated from two healthy donors using Lymphocyte Separation Medium
(Corning, Inc.) according to the manufacturer's protocol. The
present study was conducted according to the guidelines of the
Declaration of Helsinki and was approved (approval no. COA
286/2021) by the Siriraj Institutional Review Board of the Faculty
of Medicine Siriraj Hospital (Bangkok, Thailand). Written consent
was obtained during April-June 2021 and followed the approved
protocol by which all donors agreed to the use of their samples in
scientific research. The primary NK cells were sorted using CD56
MicroBeads (Miltenyi Biotec GmbH) according to the manufacturer's
protocol. The primary NK cells were maintained in RPMI-1640 medium,
10% FBS, 2 mM GlutaMAX™ (Gibco; Thermo Fisher Scientific, Inc.), 1%
non-essential amino acid and 1% penicillin/streptomycin,
supplemented with 100 U/ml recombinant IL-2. The CD56+
cells were expanded by co-culturing with irradiated membrane-bound
IL-21 expressing K562 cells (artificial antigen-presenting cells)
at a 1:1 ratio in the culture medium supplemented with 100 U/ml
recombinant IL-2. The NK cells were expanded for 6 days, with the
medium being changed on day 3 of co-culture.
Cell viability assay
The cytotoxicity of doxorubicin (MilliporeSigma) was
evaluated in the MCF7 and MDA-MB-231 cells using a cell viability
assay. The cells were plated in a 96-well plate at a density of
7,000 cells/well 1 day before the experiment. Various
concentrations of doxorubicin (0, 0.0064, 0.032, 0.16, 0.8, 4, 20
and 100 µM) were prepared in complete media (10% FBS DMEM)
and added to the cells. Cell viability was determined after 24 h of
incubation at 37°C in a 5% CO2 humidified incubator
using PrestoBLUE™ cell viability reagent (10 µl per well;
Invitrogen; Thermo Fisher Scientific, Inc.) to measure the number
of living cells. The reducing viability of the treated cells
reflected by the color changes of reagent were measured by
monitoring the absorbance at 570 nm and the absorbance at 600 nm (a
reference wavelength) using a Synergy Mx Microplate reader (BioTek
Instruments Inc.). The results are presented as the percentage of
cell viability relative to that of the non-treated cells, which was
set as 100% using the following equation: % cell
viability=[(OD570-OD595)treated cells/
(OD570-OD595)non-treated cells] ×100. The data were used
to calculate the half-maximal cytotoxic concentration
(CC50) using non-linear regression and GraphPad Prism
software version 8 (GraphPad Software, Inc.).
Flow cytometric analysis
Primary NK cells were propagated in vitro and
characterized by flow cytometry. Expanded CD56+ NK cells
were harvested via centrifugation (800 × g) at 4°C for 5 min and
stained with monoclonal antibodies (at 1:50 dilution), including
anti-human CD45-PerCP (cat. no. 368506), anti-human CD56-PE/Cy7
(cat. no. 362510), anti-human CD3-FITC (cat. no. 300406) and
anti-human CD16-BV650 (cat. no. 302041) (all from BioLegend, Inc.),
for 15 min at room temperature. A Zombie Violet™ Fixable Viability
kit (BioLegend, Inc.) was used to exclude dead cells according to
the manufacturer's protocol. Non-specific background signal was
determined using isotype-control antibodies (at 1:50 dilution),
including PerCP mouse IgG1 (cat. no. 400148), PE/Cy7 mouse IgG1
(cat. no. 400125), FITC mouse IgG1 (cat. no. 400108) and BV650
mouse IgG1 (cat. no. 400163) (all from BioLegend, Inc.), for 15 min
at room temperature. The stained cells were analyzed using BD
LSRFortessa™ flow cytometer (BD Biosciences) and FlowJo software
version 10 (BD Biosciences).
The effect of doxorubicin on surface protein
expression was investigated. MCF7 and MDA-MB-231 cells were plated
onto a 24-well plate at a density of 30,000 cells/well 1 day before
the experiment. Doxorubicin was added to the cells at various
concentrations (0, 40, 80 and 160 nM). The cells were cultured for
24 h at 37°C, in a 5% CO2 cell culture incubator, and
harvested for flow cytometric analysis. Briefly, MCF7 and
MDA-MB-231 cells were harvested via centrifugation (800 × g) at 4°C
for 5 min and incubated with monoclonal antibodies (at 1:50
dilution), including anti-TRAIL1-PE (cat. no. 12-6644-42),
anti-TRAIL2-PE (cat. no. 12-9908-42), anti-CD95-APC (cat. no.
17-0959-42), anti-MHC class I polypeptide-related sequence
(MIC)-A/B-FITC (cat. no. 53-5788-42), anti-human leukocyte antigen
(HLA)-ABC-FITC (cat. no. 11-9983-42) (all Thermo Fisher Scientific,
Inc.) and anti-programmed death-ligand 1 (PD-L1)-FITC (cat. no.
393606; BioLegend, Inc.), for 15 min at room temperature. The
respective isotype controls were also obtained from eBioscience;
Thermo Fisher Scientific, Inc. The stained cells were analyzed
using a BD Accuri™ C6 Plus flow cytometer (BD Biosciences) using
FlowJo software version 10.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The BC cell lines, MCF7 and MDA-MB-231, treated with
various concentrations of doxorubicin, were collected for total RNA
extraction using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). RNA was converted into cDNA using a SensiFAST
cDNA Synthesis kit (Bioline; Meridian Bioscience) according to the
manufacturer's protocol. The qPCR analysis was performed using a
iCycler iQ™ real-time PCR detection system (Bio-Rad Laboratories,
Inc.) using the 2X SensiFast™ SYBR® kit (Meridian
Bioscience) and the following primers: FasR forward, 5′-ATG CTG GGC
ATC TGG ACC CT-3′ and reverse, 5′-CAA CAT CAG ATA AAT TTA TTG
CCA-3′; and GAPDH forward, 5′-CGA CCA CTT TGT CAA GCT CA-3′ and
reverse, 5′-AGG GGT CTA CAT GGC AAC TG-3′. The PCR conditions were
as follows: An initial denaturation step at 95°C for 3 min,
followed by 40 cycles of denaturation at 95°C for 30 sec, annealing
at 59°C for 30 sec and extension at 72°C for 45 sec. The final
extension was performed at 72°C for 5 min. The housekeeping gene
GAPDH was used to normalize the expression level of each
gene. The gene expression fold-change was calculated using the
2−∆∆Cq formula (25).
Killing assay
A killing assay was conducted to determine the NK-92
killing activity against BC cells. The MCF7 and MDA-MB-231 cells
were genetically engineered to express RFP. The RFP-expressing MCF7
and MDA-MB-231 cells were used to examine the killing efficiency of
NK-92 cells. The cells were plated in a 96-well plate at a density
of 7,000 cells/well 1 day before the experiment. Co-culture of the
effector NK-92 cells (E) and the RFP-expressing MCF7 or MDA-MB-231
target cells (T) was performed using different E:T ratios,
including 1:1, 2.5:1 and 5:1. At 24 h after co-culture at 37°C in a
5% CO2 humidified incubator, the NK-92 cells and the
dead cells were removed by pipetting and 100 µl PBS was
added to the plate. The living cells (attached cells) were
visualized under a fluorescent microscope. The proportion of living
cells was measured by crystal violet staining at room temperature
for 30 min. The stained cells were solubilized in 50% ethanol and
the absorbance was measured at OD590 using the NS-100 Nano Scan
microplate reader (Hercuvan Lab Systems). The absorbance values of
treated cells were used to calculate the percentage of living cells
relative to the non-treated control (set as 100%). Data were
analyzed from at least three independent experiments. In a combined
treatment of doxorubicin and NK cells, the cancer cells were plated
in the presence of 160 nM of doxorubicin for 24 h before
co-culturing with NK cells at the E:T ratios of 1:1, 2.5:1, and
5:1. The death of the cancer cells was calculated as
aforementioned. The killing ability of primary NK cells was also
determined. The primary NK cells isolated from two healthy donors
were expanded and used for the killing assay, as
aforementioned.
Spheroid formation and killing assay of
spheroids
In addition, the killing assay was conducted using a
3D spheroid model. Briefly, the MCF7 cells (2×103 cells)
were labelled with CellTracker™ Green 5-chloromethylfluorescein
diacetate dye (Thermo Fisher Scientific, Inc.) at 37°C for 10 min
before seeding onto an ultra-low attachment 96-well round-bottomed
plate containing 2.5% Corning Matrigel matrix (both from Corning,
Inc.). The plate was then centrifuged at 1,000 × g at 4°C for 10
min to allow spheroid formation for 24 h at 37°C, in a 5%
CO2 cell culture incubator. Doxorubicin (320 nM) was
added to the spheroids for 24 h before introducing the NK cells in
the culture medium at an E:T ratio of 5:1. The remaining living
cells were analyzed by confocal microscopy (Nikon Corporation).
Images were acquired by Nikon Eclipse Ti confocal microscope and
analyzed using the NIS-Element software, version 4.2. Cell
viability was calculated using the following formula: (mean
fluorescence intensity (MFI) of spheroid with treatment
condition/MFI of spheroid alone) ×100.
Statistical analysis
All experiments were conducted in triplicate.
P<0.05 was used to indicate a statistically significant
difference. Data were analyzed using the unpaired Student's t-test
for two groups, in addition to a one-way ANOVA with Tukey's post
hoc test for multiple comparisons, using GraphPad Prism software
version 8 (GraphPad Software, Inc.).
Results
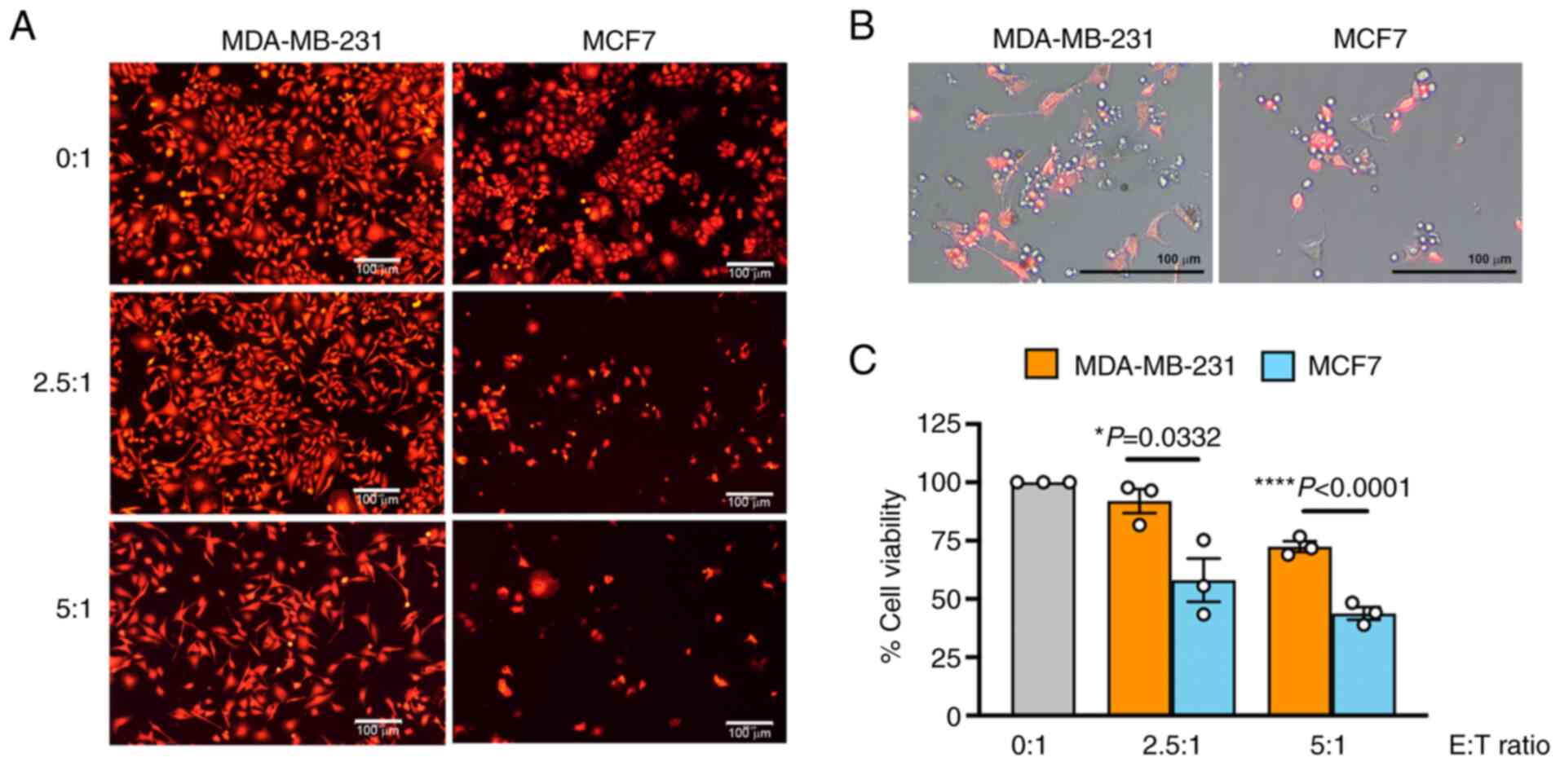
MCF7 is more sensitive to NK cytotoxicity
than MDA-MB-231
The ability of NK cells to eliminate BC cells was
examined in MCF7 cells and the triple-negative BC MDA-MB-231 cells.
The viability of cancer cells after 24 h of co-culturing with NK-92
cells was determined at E:T ratios of 0:1, 2.5:1 and 5:1. The NK-92
cells demonstrated their killing ability in a dose-dependent manner
(Fig. 1A). These results merged
with cell morphology images revealed that the NK-92 cells promptly
attacked the cancer cells, as observed by the NK-92 cells
surrounding the RFP+ cancer cells after co-culturing
(Fig. 1B). The efficiency of
NK-92 cells in killing MCF7 was greater than that in the MDA-MB-231
cells at all tested E:T ratios (Fig.
1C). At the highest tested ratio (E:T, 5:1), NK-92 cells caused
56.19±4.72% of MCF7 cell death (the cell viability decreased to
43.80±4.72%) whereas it was less efficient in MDA-MB-231 cells by
causing 27.55±3.91% of death cell (the cell viability decreased to
72.44±3.91%).
Protein expression profiles of TRAIL
receptors, FasR, MIC-A/B, HLA class I and PD-L1, on MCF7 and
MDA-MB-231 cells
To explain the distinct sensitivity of MCF7 and
MDA-MB-231 cells to NK-92 cytotoxicity, the expression levels of
TRAIL receptors, FasR, MIC-A/B, HLA class I and PD-L1, were
investigated (Fig. 2). Death
receptors, including TRAIL receptors and FasR, are involved in NK
killing activity, whereas MIC-A/B is the ligand for NK cell
activating receptor NKG2D (26).
HLA class I is well established as an inhibitory ligand for
killer-cell immunoglobulin-like receptors (KIRs) (27). PD-L1 plays a critical role in
suppressing NK cell function via binding to the PD1 receptor on NK
cells (28). Flow cytometric
data revealed significant differences in expression between cell
lines among these proteins, except for that in the TRAIL1
receptor.
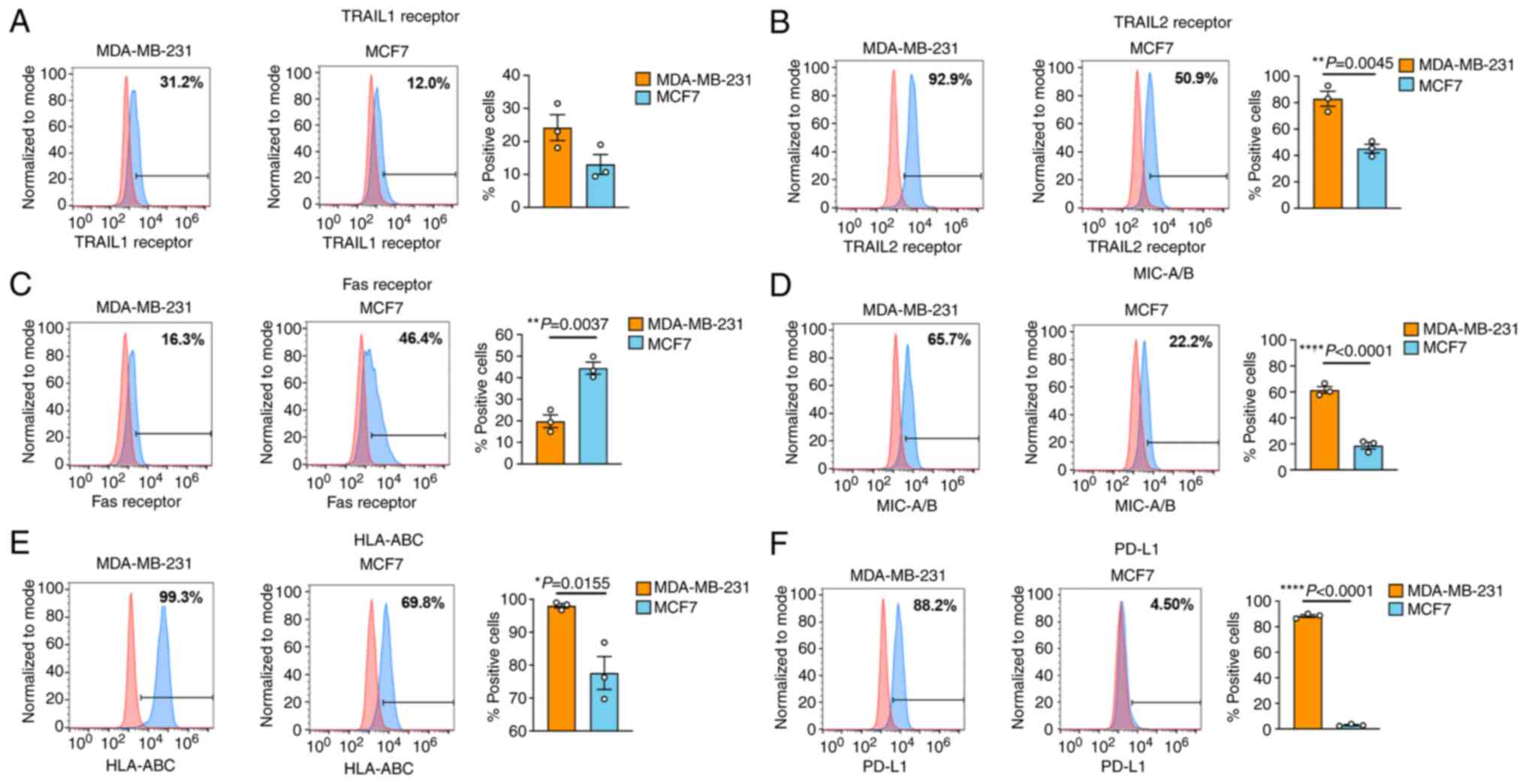 | Figure 2Protein expression profiles of TRAIL
receptors, FasR, MIC-A/B, HLA class I and PD-L1 in BC cell lines.
The expression profiles of (A) TRAIL1 receptor (A and B) TRAIL2
receptor, (C) FasR, (D) MIC-A/B, (E) HLA class I (ABC) and (F)
PD-L1 were compared between two different BC cell lines, MDA-MB-231
(orange bar) and MCF7 (cyan bar), using flow cytometric analysis.
Data**) were obtained from three independent experiments. Data are
presented as the mean ± SEM. TRAIL, TNF-related apoptosis-inducing
ligand; FasR, Fas receptor; HLA, human leukocyte antigen; PD-L1,
programmed death-ligand 1; BC, breast cancer. |
Notably, the death receptors, including TRAIL
receptors and FasR, were expressed in both cancer cell lines, but
in the opposite manner. The MDA-MB-231 cells expressed higher
levels of TRAIL1 and TRAIL2 receptors than those of the MCF7 cells.
In more detail, the mean TRAIL1 receptor expression was 24.18% in
the MDA-MB-231 cells compared with 13.04% in the MCF7 cells and the
mean TRAIL2 receptor expression was 82.98% in the MDA-MB-231 cells
compared with 45.17% in the MCF7 cells. By contrast, the MCF7 cells
expressed significantly higher levels of FasR than that of the
MDA-MB-231 cells (44.47 vs. 19.81%) (Fig. 2A-C). The expression levels of
MIC-A/B, HLA class I and PD-L1 were significantly lower in the MCF7
cells than in those of the MDA-MB-231 cells (18.59 vs. 61.48, 77.62
vs. 98.03, and 2.95 vs. 88.41%, respectively) (Fig. 2D-F).
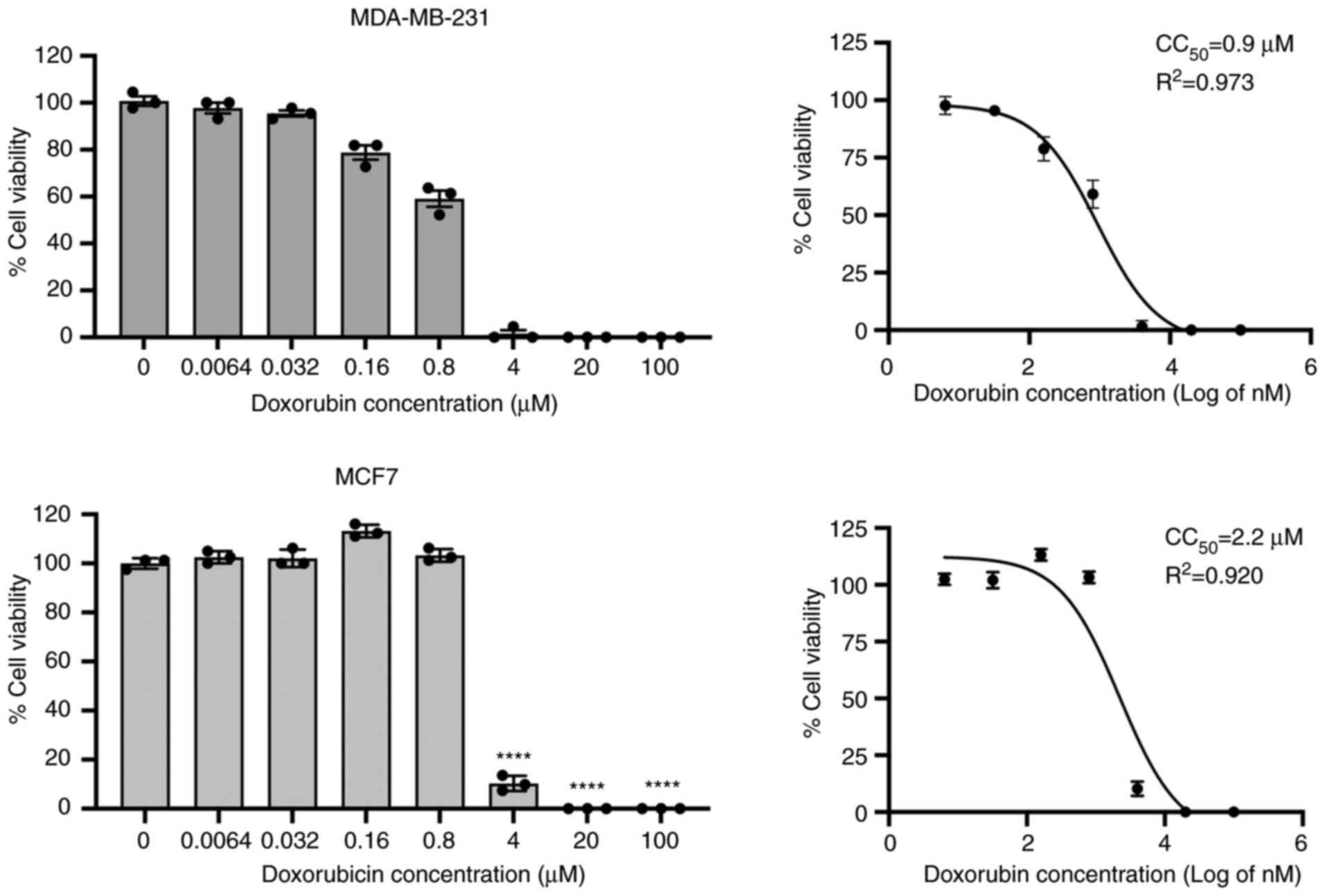
Effects of doxorubicin on modulation of
TRAIL receptor, FasR, MIC-A/B, HLA class I and PD-L1
expression
The immune-modulation activity of doxorubicin has
previously been reported (22);
however, its effect in modulating NK cell-related markers is
under-investigated. We hypothesized that the expression of TRAIL
receptors, FasR, MIC-A/B, HLA class I and PD-L1 may modulate the
sensitivity of BC cells to NK cells. First, the cytotoxicity of
doxorubicin was determined using a cell viability assay, and the
CC50 was determined after a 24-h treatment with
doxorubicin. From this analysis, the MDA-MB-231 cells had a
CC50 at a concentration of 0.9 µM, whereas the
MCF7 cells were more resistant to doxorubicin with a higher
CC50 concentration of 2.2 µM (Fig. 3).
The sublethal dose of doxorubicin (160 nM) was
selected and tested for its activity in modulating TRAIL receptor,
FasR, MIC-A/B, HLA class I and PD-L1 expression (Figs. 4 and S1-S3). Treatment with 160 nM
doxorubicin in the MDA-MB-231 cells caused a significant reduction
in TRAIL2, whereas no significant alteration in other proteins was
observed (Fig. 4A). By contrast,
doxorubicin treatment at the same concentration significantly
increased the expression level of FasR in the MCF7 cells, but it
had only a slight non-significant effect on the expression levels
of the other markers (Fig. 4B).
The dosing effect of doxorubicin in modulating the expression of
FasR in the MCF7 cells at the mRNA and protein levels was further
investigated. qPCR analysis demonstrated that doxorubicin treatment
elevated the mRNA expression of FasR in a dose-dependent
manner. At a concentration of 160 nM, the doxorubicin-treated MCF7
cells reached a 2.77-fold increase in the mRNA expression of
FasR compared with that of the non-treated control (Fig. 4C). Concordantly, the percentages
of FasR-positive cells were significantly increased as doxorubicin
concentrations increased compared with that of the control
(Fig. 4D).
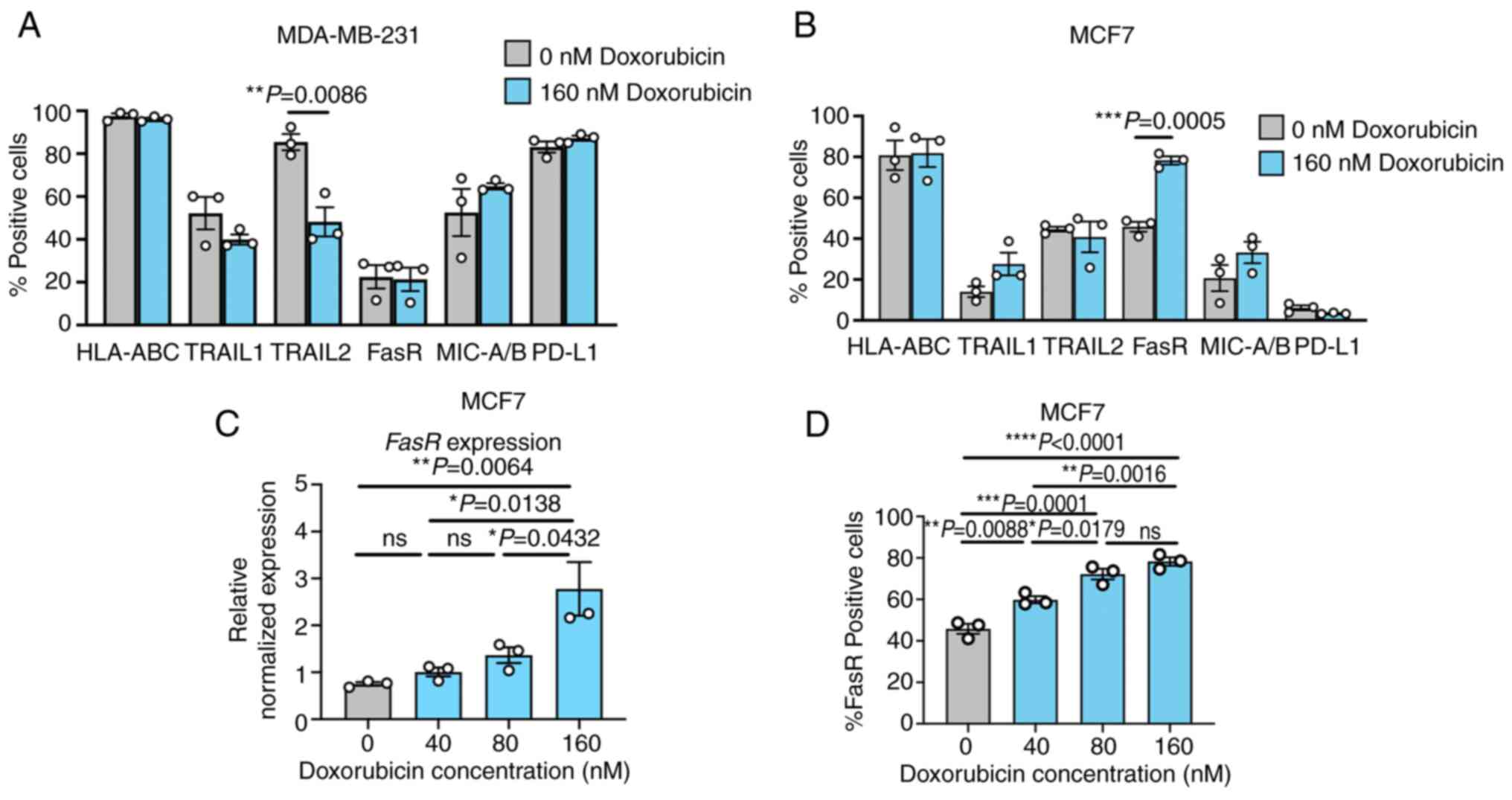 | Figure 4Effects of doxorubicin on the
modulation of TRAIL receptor, FasR, MIC-A/B, HLA class I and PD-L1
expression in breast cancer cell lines. The MDA-MB-231 and MCF7
cells were treated with a sublethal dose of doxorubicin (160 nM).
The percentage of positive cells expressing TRAIL receptors, FasR,
MIC-A/B, HLA class I and PD-L1 protein was monitored after a 24-h
treatment with doxorubicin in (A) the MDA-MB-231 cells and the (B)
MCF7 cells as analyzed by flow cytometry. The dose-dependent
effects of doxorubicin on the expression of (C) FasR mRNA and the
number of (D) FasR-positive cells were also examined. Data were
obtained from three independent experiments. Data are presented as
the mean ± SEM. TRAIL, TNF-related apoptosis-inducing ligand; FasR,
Fas receptor; HLA, human leukocyte antigen; PD-L1, programmed
death-ligand 1; ns, not significant. |
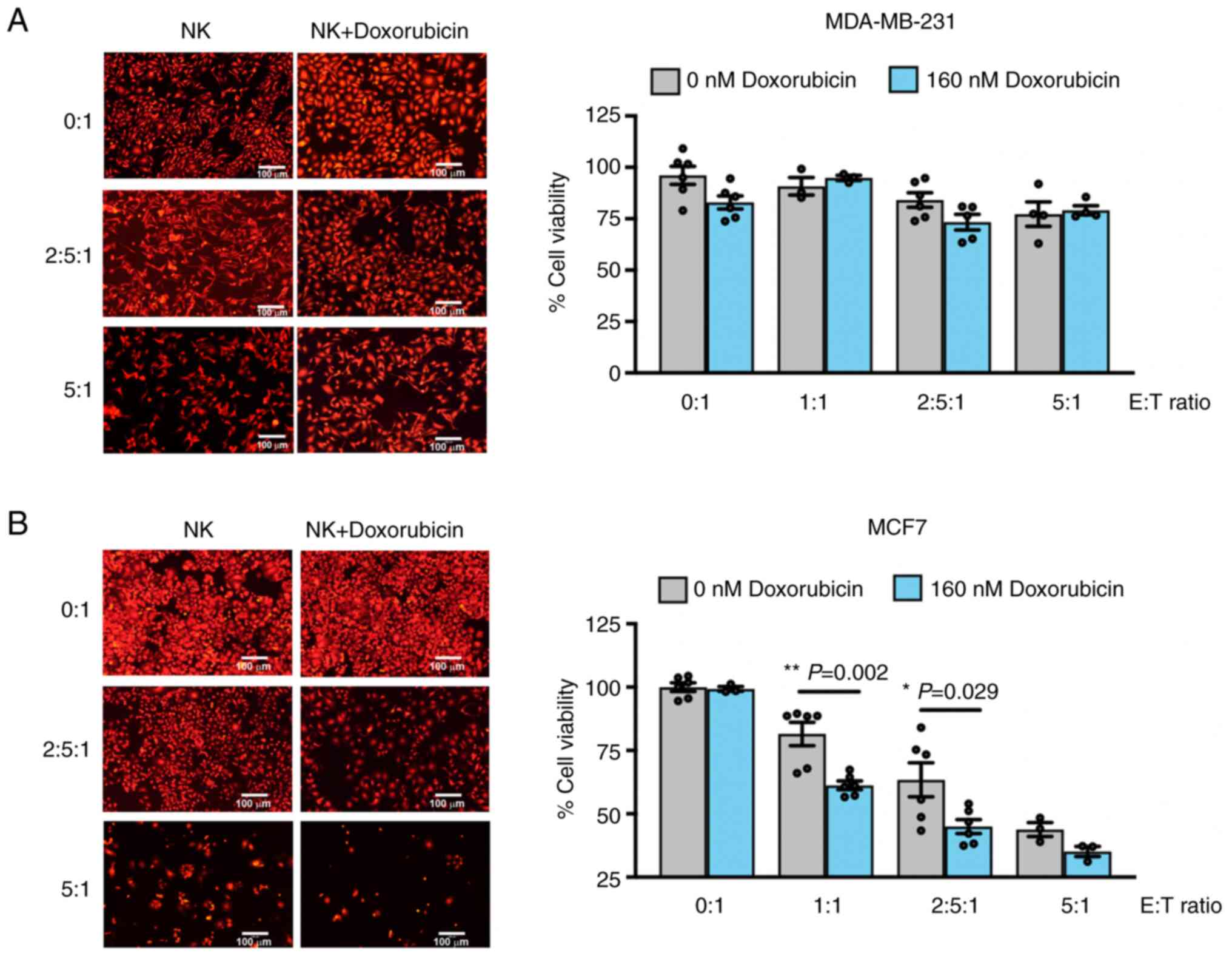
Doxorubicin sensitization increases NK-92
cytotoxicity in MCF7 cells
Based on the flow cytometric results, it was
speculated that the expression profiles of FasR, PD-L1 and HLA
class I may play an important role in MCF7 sensitivity to NK cells.
Additionally, the treatment with doxorubicin resulted in an
increase in FasR expression in the MCF7 cells, but not in the
MDA-MB-231 cells. It was then investigated whether this alteration
in FasR expression sensitized the MCF7 cells and increased the
cytotoxic activity of the NK-92 cells. The percentages of
MDA-MB-231 and MCF7 cell death were compared after co-culturing
with the NK-92 cells in the absence and presence of 160 nM of
doxorubicin. The results demonstrated that the NK-92 cells killed
both types of cells in a dose-dependent manner (Fig. 5). Doxorubicin treatment exerted
no additional killing effect of NK-92 cells in the MDA-MB-231 cells
(Fig. 5A); however, it
significantly increased the NK-92 cytotoxicity in the MCF7 cells
(Fig. 5B). At E:T ratios of 1:1
and 2.5:1, doxorubicin treatment had a greater killing effect, as
observed by a significant reduction in the MCF7 cell viability
compared with the killing effect of the NK-92 cells alone. These
results suggested the possible role of FasR in the modulation of
NK-92 activity.
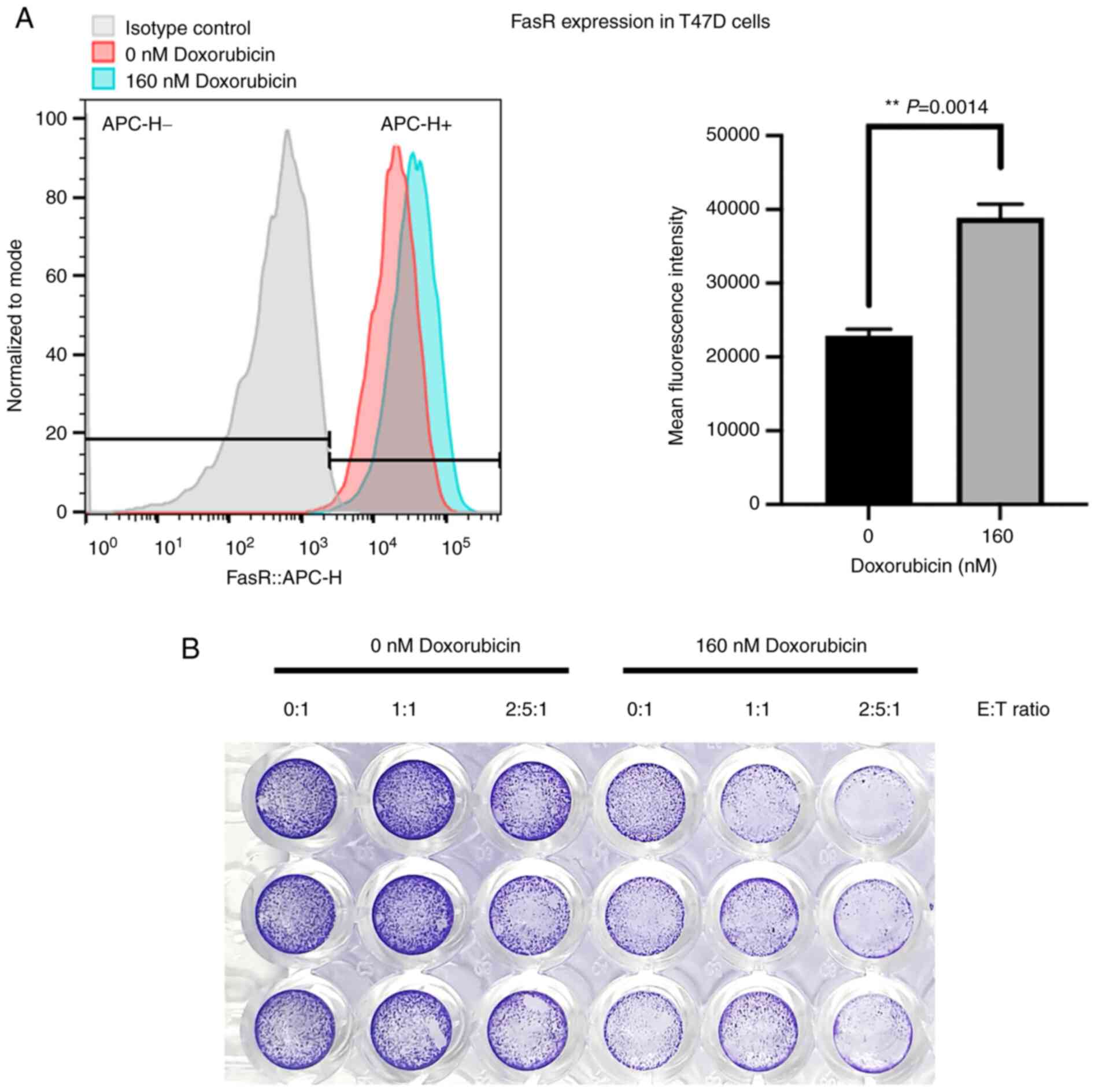
The effect of doxorubicin to increase the FasR
expression and its relationship to enhance the NK killing activity
were further investigated. Another BC cell line, T47D, was used to
demonstrate the doxorubicin sensitization in BC cells. Treatment of
doxorubicin at 160 nM increased the expression of FasR and the
corresponding MFI (Fig. 6A).
Notably, the doxorubicin treatment could sensitize T47D cells to
the NK-92 killing activity, as shown by crystal violet staining to
determine the amount of adherent living cells (Fig. 6B), which emphasized the
contribution of FasR on modulating the NK-92 cytotoxicity.
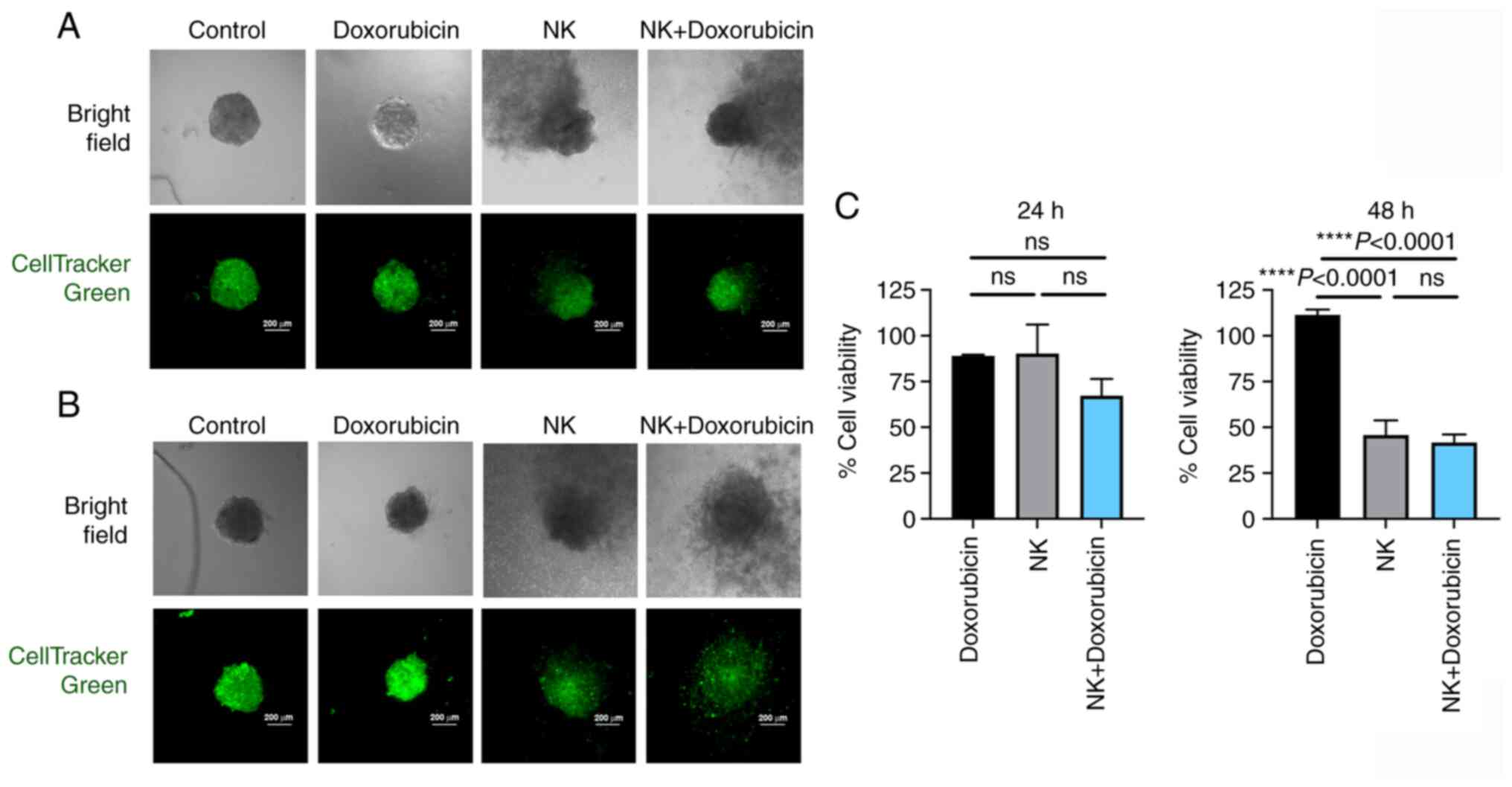
The potential of doxorubicin in combination with the
NK-92 cells was further examined in the present study using
spheroid 3D culture to reflect its effect on controlling tumor
mass. The MCF7 spheroids were generated and treated with
doxorubicin at 320 nM prior to co-culturing with the NK-92 cells.
The number of living cells (green color) was monitored. The cell
viability observed after 24 and 48 h of co-culturing with the NK-92
cells tended to decrease, particularly in the doxorubicin-treated
cells (Fig. 7A and C). Notably,
the NK-92 cells caused apparent destruction of the spheroids after
48 h of co-culturing, resulting in the collapse of the
well-organized structure of spheroids in the doxorubicin-treated
cells (Fig. 7B).
Doxorubicin markedly enhances
cytotoxicity of primary NK cells
To confirm the effect of doxorubicin on cancer cell
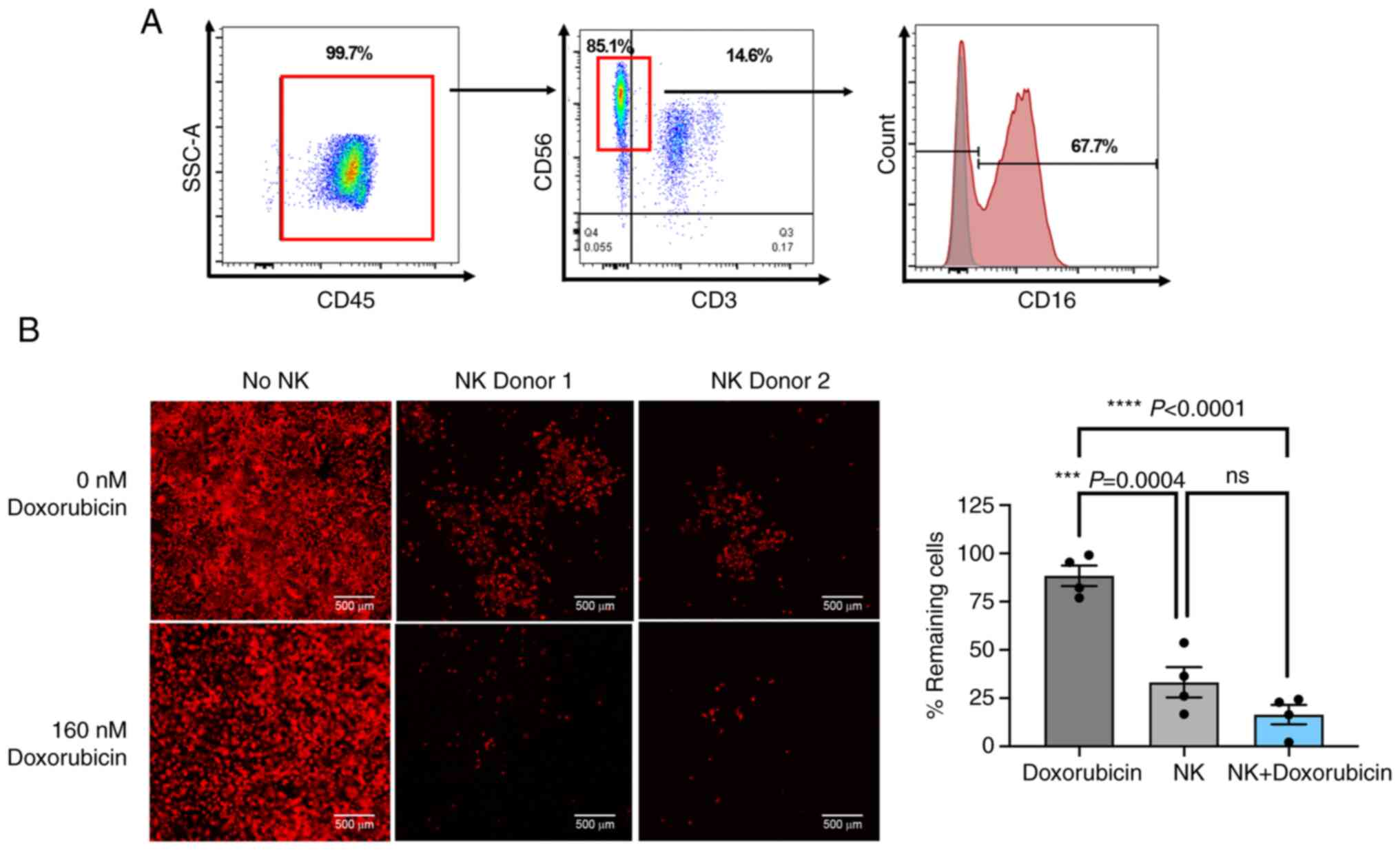
sensitization, the effect of doxorubicin on enhancing primary NK
killing activity was investigated. Primary NK cells were isolated
from the PBMCs of two healthy donors and were expanded ex
vivo. After 6 days of expansion, the cells were analyzed for
CD45 (leukocyte common antigen), CD3 (T cell marker) and CD56 (NK
cell marker) expression. The majority of cells after the expansion
were CD3−/CD56+ NK cells, which also
expressed CD16 at 67.78% (Fig.
8A). The cytotoxic capacity of primary NK cells to eliminate
the MCF7 cells was then examined with and without doxorubicin
treatment. Primary NK cells caused ~66.78% cell death at an E:T
ratio of 1:1 (Fig. 8B). As
expected, this activity was significantly improved in the presence
of 160 nM doxorubicin, which resulted in 83.57% cell death
(Fig. 8B). This finding strongly
suggested the role of doxorubicin in enhancing primary NK killing
activity.
Discussion
The use of immunotherapy to enhance immune response
is currently the front-line strategy for cancer treatment and
control. Among the different types of immune cells used to fight
against cancer cells, NK cells are an attractive cellular
immunotherapy strategy, as reflected by a large number of ongoing
clinical trials (29). Without
MHC restriction recognition and prior antigen exposure requirement,
NK cells could be expanded ex vivo rapidly and transferred
to patients within 5-12 days, depending on the production protocol
(30,31). NK-cell therapy could then be used
as the first line of immunotherapy for cancer treatment. NK cells
have been shown to play a vital role in controlling the formation
and progression of BC. A comparison of BC growth and metastasis
between NOD/SCID/γnull (NSG) mice (without T, B and NK cells) and
SCID mice (with NK-cell, macrophage, complement and dendritic cell
activities) demonstrated the important roles of NK cells for
controlling BC growth and metastasis in SCID mice (32). These data emphasized the
possibility of NK immunotherapy for BC treatment. However, the NK
cell activity after entering the body of the patient has been
problematic, even though the number of NK cells has been found to
be sufficient (18). Thus, the
efficacy of NK cell therapy depends on both the quantity and
quality of NK cells to achieve its therapeutic potential. In the
present study, the combination of NK cells and a chemotherapeutic
drug, doxorubicin, was demonstrated as a strategy for elevating NK
cell activity. In addition, the association between the effect of
doxorubicin treatment on FasR protein (CD95) expression modulation
and NK killing ability was investigated.
Variations in tumor subpopulations caused by genetic
or non-genetic factors, so-called heterogeneity, are known to exert
significant influence on the outcome of cancer treatment (33). To understand the key natural
differences that may have an impact on NK function, the efficiency
of the NK cell line (NK-92) to eliminate well-known candidate BC
cells (ER+/PR+ MCF7 and triple-negative
MDA-MB-231 cells) was first compared in the present study.
Naturally, the proportion of NK cells among peripheral blood
lymphocytes is estimated to be 5-15%; however, the density of NK
cells is relatively higher at the tumor site and is associated with
the tumor progression. Larsen et al (34) described the E:T ratio of
intertumoral NK cells at the tumor site as 1:35, but with pro-NK
cytokine such as IL2, the E:T ratio could reach 1:4 or better than
1:1. In the present experiment, the high E:T ratios of 2.5:1 and
5:1 were used to demonstrate the dose-dependent effect of NK-92
cells comparing between the different breast cancer cell lines. As
expected, the MCF7 cells were more sensitive to the NK-92 cells
than the MDA-MB-231 cells. This result necessitated further
analysis of the biomarkers that manipulate the magnitude of NK
cytotoxicity on these cells. Accordingly, the expression of five
biomarkers that may play a role in NK cytotoxicity was compared,
including TRAIL receptors (TRAIL1/TRAIL2), FasR (CD95), MIC-A/B,
HLA class I and PD-L1. The results demonstrated that the
NK-sensitive MCF7 cells had significantly lower expression of TRAIL
receptors (TRAIL1/TRAIL2), MIC-A/B, HLA class I and PD-L1, whereas
the expression of FasR was significantly higher than that observed
in the MDA-MB-231 cells. Notably, the NK-92 cells express various
activation molecules (NKp30, NKp46, 2B4, NKG2D, NKG2E and CD28),
but only a few inhibitory molecules (NKG2A, NKG2B, low levels of
KIR2DL4 and ILT-2), and they lack most of the killer inhibitory
receptors (KIRs) (35); thus,
the changes of HLA class I expression (as a ligand of KIRs) was
considered as a minor response. In addition, the NK-92 cells
expressed high levels of cytotoxic effector molecules, including
the members of the TNF superfamily (i.e., FasL, TRAIL, TNF-α and
TWEAK) suggesting the involvement of an alternative cytotoxic
mechanism (35). Based on these
findings, we hypothesized that the association of FasR and PD-L1
expression may contribute to the sensitivity of the MCF7 cells to
the NK-92 cells.
FasR (CD95; TNF receptor superfamily member 6) is
constitutionally expressed in human cells, and functions to mediate
the antitumor cytotoxicity of NK cells and lymphocytes (36,37). The specific interaction between
FasR and its ligand (FasL) triggers apoptosis via a cascade of the
extrinsic pathway in which the intracellular protein death-inducing
signaling complex promotes the recruitment and activation of
caspase-8, caspase-3 and caspase-7 cleavage (36). The signaling of FasR/FasL is
considered to be one of the central cell-death mechanisms
underlying the antitumor activity of immune cells. The resistance
of cancer cells to cell death signaling has been reported as one of
the hallmarks of cancer apart from continuous proliferation, growth
suppression escape, effective angiogenesis, tissue invasion and
metastasis (38). The induction
of FasR/FasL expression was reported to augment the apoptotic rate
in cancer. For example, the induction of FasR by CD437 increased
apoptosis in human non-small cell lung cancer (39). These data emphasized the impact
of FasR expression on FasR/FasL signaling, which influences the
modulation of NK cell function. Doxorubicin treatment in the
present study caused the MCF7 and T47D cells to become more
sensitive to NK cells. Notably, the upregulation of FasR expression
was observed in both sensitive cells. This result was consistent
with a previous report demonstrating that the combination of
doxorubicin and immune cells increased cell death in various cancer
cell types (22). In addition,
the present study elucidated the partial involvement of FasR/FasL
signaling in doxorubicin immunomodulation to enhance NK cell
cytotoxicity against BC.
Apart from FasR/FasL signaling, chemotherapy
treatment has been reported to promote sensitization of cancer
cells via other mechanisms, i.e., the stress pathway and
damage-associated molecular pattern (DAMP) (40). A low dose of doxorubicin has been
reported to modulate the MIC-A/B and poliovirus receptor (CD155)
via activation of ataxia telangiectasia mutated (ATM) and ATM- and
Rad3-related protein kinase, resulting in the increase of NK
activity in multiple myeloma models (41). Furthermore, several studies
reported the effect of doxorubicin treatment on the upregulation of
TRAIL receptors in various tumor cells via either p53-dependent or
-independent mechanisms (40,42,43). Increase of immunogenic potency
through the DAMP immunogenic cell death pathway has been reported
as one of the chemotherapy mechanisms. The interaction of DAMPs and
their receptors promoted the recruitment and induction of immune
cells, such as monocytes/dendritic cells, which ultimately modified
the magnitude of immune cell activities (44). These mechanisms likely confer the
sensitization of cancer cells to the immune cells. Since those
potential mechanisms were not explored in the present study, they
could not be excluded from the sensitization activity of
doxorubicin to modulate BC to NK cells. Further investigation is
required to confirm these mechanisms.
BC cell sensitization by doxorubicin could be
applied to adoptive T cell therapy, since both perforin and FasL
individually enhanced the killing ability of T cells and NK cells
(45). A study of a putative
perforin-independent mechanism revealed that the perforin deficient
(−/−) cytotoxic T cells (CD8+) were capable of inducing
antigen-specific target cell lysis compared with T cells lacking
both perforin and FasL, suggesting the additional contribution of
FasR/FasL signaling in T cell killing activity (46). In the present study, primary NK
cells demonstrated the improvement of killing activity in the
doxorubicin-treated MCF7 cells. Notably, based on the results of
flow cytometric analysis, although the major population of the
present samples were the NK cells (~85% of NK cells), there were
other immune cell populations that may contribute to the cancer
cell death, particularly the CD3+ cells (~15% of
population). Considering the contribution of FasL/FasR signaling on
T cell cytotoxicity, the possibility that the induction of cell
death in the doxorubicin-treated MCF7 cells may be the consequences
of both NK cells and T cells could not be excluded.
A broad range of immunotherapeutic approaches has
previously been developed. Adoptive immune cell therapy has been
reported to treat BC (47),
including conventional immune cells (i.e., NK cells) (48), cytokine-induced killer cells
(CIK) (49), tumor-infiltrating
lymphocytes (TILs) (50),
genetically modified immune cells [i.e., chimeric antigen receptor
(CAR)-T lymphocytes] (51,52) and CAR-NK cells (53-55). Doxorubicin could be used in
combination with those cellular immuno-therapies or as an adjuvant
therapy to enhance immune cell function, and to combat rapidly
growing cancer cells and their strong microenvironment. The results
of the present study suggested that FasR/FasL signaling plays an
important role in immune cell function; however, the doxorubicin
response was different among BC cell lines, MCF-7 and MDA-MB-231,
suggesting heterogeneity in drug response.
In conclusion, the results of the present study
demonstrated the role of FasR modulation in the response to
doxorubicin treatment to enhance NK cell killing activity in BC
cells. This finding supports the development and use of combined
chemo-immunotherapy and immuno-modulation for the treatment of
BC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
NS planned the studies, designed and conducted
experimental works, analyzed data and edited the manuscript. MW and
NT conducted the primary NK isolation and culture, and edited the
manuscript. NP conducted the killing assay. CC conducted the
RT-qPCR. CT designed experiments. PTY designed experiments and
edited the manuscript. AP managed the research team, planned the
studies, designed and conducted experiments, analyzed data, and
drafted and edited the manuscript. NS and AP confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved
(approval no. COA 286/2021) by the Siriraj Institutional Review
Board of the Faculty of Medicine Siriraj Hospital, Mahidol
University, (Bangkok, Thailand).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Assistant Professor
Kevin P. Jones (Faculty of Medicine Siriraj Hospital, Mahidol
University, Thailand) for language editing of the original
manuscript.
Funding
The present study was supported by the Center of Excellence on
Medical Biotechnology (CEMB) (grant no. R000018103), the S&T
Postgraduate Education and Research Development Office, the
Commission on Higher Education (Thailand), the Siriraj Research
Fund (grant no. R016034008) Faculty of Medicine Siriraj Hospital,
Mahidol University, and partially supported by the Research Center
in Bioresources for Agriculture, Industry and Medicine, Chiang Mai
University. A Chalermphrakiat Grant also provided by the Faculty of
Medicine Siriraj Hospital, Mahidol University. A Siriraj Graduate
Scholarship from the Faculty of Medicine Siriraj Hospital, Mahidol
University and grant from the National Research Council of Thailand
(grant no. NRCT5-RGJ63012-126) was also provided. A Science
Achievement Scholarship of Thailand also supported the study.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lakha H, Suriyawongpaisul P, Sangrajrang
S, Leerapan B and Coker R: Breast cancer in Thailand: Policy and
health system challenges to universal healthcare. Health Policy
Plan. 35:1159–1167. 2020. View Article : Google Scholar
|
|
3
|
Treating Breast Cancer. https//:www.cancer.org/cancer/breast-cancer/treatment.html
Accessed October 23, 2021.
|
|
4
|
Saadatmand S, Bretveld R, Siesling S and
Tilanus-Linthorst MM: Influence of tumour stage at breast cancer
detection on survival in modern times: Population based study in
173,797 patients. BMJ. 351:h49012015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donegan WL: Tumor-related prognostic
factors for breast cancer. CA Cancer J Clin. 47:28–51. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robbins GF and Berg J: Curability of
patients with invasive breast carcinoma based on a 30-year study.
World J Surg. 1:284–286. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer. BMC Med.
14:732016. View Article : Google Scholar
|
|
8
|
Kruger S, Ilmer M, Kobold S, Cadilha BL,
Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H,
et al: Advances in cancer immunotherapy 2019 - latest trends. J Exp
Clin Cancer Res. 38:2682019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaddepally RK, Kharel P, Pandey R and
Garje R: Chandra AB. Review of indications of FDA-approved immune
checkpoint inhibitors per NCCN Guidelines with the level of
evidence. Cancers (Basel). 12:7382020. View Article : Google Scholar
|
|
10
|
Twomey JD and Zhang B: Cancer
immunotherapy update: FDA-approved checkpoint inhibitors and
companion diagnostics. AAPS J. 23:392021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen LT, Saibil SD, Sotov V, Le MX,
Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, et
al: Phase II clinical trial of adoptive cell therapy for patients
with metastatic melanoma with autologous tumor-infiltrating
lymphocytes and low-dose interleukin-2. Cancer Immunol Immunother.
68:773–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Multhoff G, Seier S, Stangl S, Sievert W,
Shevtsov M, Werner C, Pockley AG, Blankenstein C, Hildebrandt M,
Offner R, et al: Targeted natural killer cell-based adoptive
immunotherapy for the treatment of patients with NSCLC after
radiochemotherapy: A randomized phase II clinical trial. Clin
Cancer Res. 26:5368–5379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radosa JC, Stotz L, Muller C, Kaya AC,
Solomayer EF and Radosa MP: Clinical data on immunotherapy in
breast cancer. Breast Care (Basel). 15:450–469. 2020. View Article : Google Scholar
|
|
14
|
Sanchez-Correa B, Valhondo I, Hassouneh F,
Lopez-Sejas N, Pera A, Bergua JM, Arcos MJ, Bañas H, Casas-Avilés
I, Durán E, et al: DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel
immune checkpoints for natural killer cell-based cancer
immunotherapy. Cancers (Basel). 11:8772019. View Article : Google Scholar
|
|
15
|
Xie G, Dong H, Liang Y, Ham JD, Rizwan R
and Chen J: CAR-NK cells: A promising cellular immunotherapy for
cancer. EBioMedicine. 59:1029752020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Galat V, Galat Y, Lee YKA,
Wainwright D and Wu J: NK cell-based cancer immunotherapy: From
basic biology to clinical development. J Hematol Oncol. 14:72021.
View Article : Google Scholar :
|
|
17
|
Krause SW, Gastpar R, Andreesen R, Gross
C, Ullrich H, Thonigs G, Pfister K and Multhoff G: Treatment of
colon and lung cancer patients with ex vivo heat shock protein
70-peptide-activated, autologous natural killer cells: A clinical
phase i trial. Clin Cancer Res. 10:3699–3707. 2004. View Article : Google Scholar
|
|
18
|
Parkhurst MR, Riley JP, Dudley ME and
Rosenberg SA: Adoptive transfer of autologous natural killer cells
leads to high levels of circulating natural killer cells but does
not mediate tumor regression. Clin Cancer Res. 17:6287–6297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruggeri L, Capanni M, Urbani E, Perruccio
K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F,
et al: Effectiveness of donor natural killer cell alloreactivity in
mismatched hematopoietic transplants. Science. 295:2097–2100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geller MA and Miller JS: Use of allogeneic
NK cells for cancer immunotherapy. Immunotherapy. 3:1445–1259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wennerberg E, Sarhan D, Carlsten M,
Kaminskyy VO, D'Arcy P, Zhivotovsky B, Childs R and Lundqvist A:
Doxorubicin sensitizes human tumor cells to NK cell- and
T-cell-mediated killing by augmented TRAIL receptor signaling. Int
J Cancer. 133:1643–1652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–446. 2011. View Article : Google Scholar :
|
|
24
|
Paridaens R, Biganzoli L, Bruning P, Klijn
JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A,
Sylvester R, et al: Paclitaxel versus doxorubicin as first-line
single-agent chemotherapy for metastatic breast cancer: A European
organization for research and treatment of cancer randomized study
with cross-over. J Clin Oncol. 18:724–733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Jinushi M, Takehara T, Tatsumi T, Kanto T,
Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y and
Hayashi N: Expression and role of MICA and MICB in human
hepatocellular carcinomas and their regulation by retinoic acid.
Int J Cancer. 104:354–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jost S and Altfeld M: Control of human
viral infections by natural killer cells. Annu Rev Immunol.
31:163–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quatrini L, Mariotti FR, Munari E, Tumino
N, Vacca P and Moretta L: The immune checkpoint PD-1 in natural
killer cells: Expression, function and targeting in tumour
immunotherapy. Cancers (Basel). 12:32852020. View Article : Google Scholar
|
|
29
|
Oh S, Lee JH and Kwack K: Choi SW. Natural
killer cell therapy: A new treatment paradigm for solid tumors.
Cancers (Basel). 11:15342019. View Article : Google Scholar
|
|
30
|
Xie S, Wu Z, Niu L, Chen J, Ma Y and Zhang
M: Preparation of highly activated natural killer cells for
advanced lung cancer therapy. Onco Targets Ther. 12:5077–5086.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Ostaijen-ten Dam MM, Prins HJ, Boerman
GH, Vervat C, Pende D, Putter H, Lankester A, van Tol MJD, Zwaginga
JJ and Schilham MW: Preparation of cytokine-activated NK cells for
use in adoptive cell therapy in cancer patients: Protocol
optimization and therapeutic potential. J Immunother. 39:90–100.
2016. View Article : Google Scholar
|
|
32
|
Dewan MZ, Terunuma H, Takada M, Tanaka Y,
Abe H, Sata T, Toi M and Yamamoto N: Role of natural killer cells
in hormone-independent rapid tumor formation and spontaneous
metastasis of breast cancer cells in vivo. Breast Cancer Res Treat.
104:267–275. 2007. View Article : Google Scholar
|
|
33
|
Marusyk A and Polyak K: Tumor
heterogeneity: Causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.
|
|
34
|
Larsen SK, Gao Y and Basse PH: NK cells in
the tumor micro-environment. Crit Rev Oncog. 19:91–105. 2014.
View Article : Google Scholar
|
|
35
|
Maki G, Klingemann HG, Martinson JA and
Tam YK: Factors regulating the cytotoxic activity of the human
natural killer cell line, NK-92. J Hematother Stem Cell Res.
10:369–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassin D, Garber OG, Meiraz A,
Schiffenbauer YS and Berke G: Cytotoxic T lymphocyte perforin and
Fas ligand working in concert even when fas ligand lytic action is
still not detectable. Immunology. 133:190–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:885–886. 2015. View Article : Google Scholar :
|
|
38
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun SY, Yue P, Hong WK and Lotan R:
Induction of fas expression and augmentation of fas/fas
ligand-mediated apoptosis by the synthetic retinoid CD437 in human
lung cancer cells. Cancer Res. 60:6537–6543. 2000.PubMed/NCBI
|
|
40
|
Zingoni A, Fionda C, Borrelli C,
Cippitelli M, Santoni A and Soriani A: Natural killer cell response
to chemotherapy-stressed cancer cells: Role in tumor
immunosurveillance. Front Immunol. 8:11942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Soriani A, Zingoni A, Cerboni C, Iannitto
ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C,
Petrucci MT, Guarini A, et al: ATM-ATR-dependent up-regulation of
DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic
agents results in enhanced NK-cell susceptibility and is associated
with a senescent phenotype. Blood. 113:3503–3511. 2009. View Article : Google Scholar
|
|
42
|
Wen J, Ramadevi N, Nguyen D, Perkins C,
Worthington E and Bhalla K: Antileukemic drugs increase death
receptor 5 levels and enhance Apo-2L-induced apoptosis of human
acute leukemia cells. Blood. 96:3900–3906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Müller M, Wilder S, Bannasch D, Israeli D,
Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M
and Krammer PH: P53 activates the CD95 (APO-1/Fas) gene in response
to DNA damage by anticancer drugs. J Exp Med. 188:2033–2045. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Asadzadeh Z, Safarzadeh E, Safaei S,
Baradaran A, Mohammadi A, Hajiasgharzadeh K, Derakhshani A,
Argentiero A and Silvestris N: Baradaran B. Current approaches for
combination therapy of cancer: The role of immunogenic cell death.
Cancers (Basel). 12:10472020. View Article : Google Scholar
|
|
45
|
Kagi D, Vignaux F, Ledermann B, Bürki K,
Depraetere V, Nagata S, Hengartner H and Golstein P: Fas and
perforin pathways as major mechanisms of T cell-mediated
cytotoxicity. Science. 265:528–530. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kojima H, Shinohara N, Hanaoka S,
Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H
and Okumura K: Two distinct pathways of specific killing revealed
by perforin mutant cytotoxic T lymphocytes. Immunity. 1:357–364.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sivaganesh V, Promi N, Maher S and
Peethambaran B: Emerging immunotherapies against novel molecular
targets in breast cancer. Int J Mol Sci. 22:24332021. View Article : Google Scholar :
|
|
48
|
Shenouda MM, Gillgrass A, Nham T, Hogg R,
Lee AJ, Chew MV, Shafaei M, Aarts C, Lee DA, Hassell J, et al: Ex
vivo expanded natural killer cells from breast cancer patients and
healthy donors are highly cytotoxic against breast cancer cell
lines and patient-derived tumours. Breast Cancer Res. 19:762017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao Q, Li L, Zhang C, Sun Y, Liu S and Cui
S: Clinical effects of immunotherapy of DC-CIK combined with
chemotherapy in treating patients with metastatic breast cancer.
Pak J Pharm Sci. 28(Suppl 3): S1055–S1058. 2015.
|
|
50
|
Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y,
Jia Y, Zhou J, Ouyang Y and Zeng Z: Clinical implications of
tumor-infiltrating immune cells in breast cancer. J Cancer.
10:6175–6184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wallstabe L, Gottlich C, Nelke LC,
Kuhnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar
G, Nietzer SL and Hudecek M: ROR1-CAR T cells are effective against
lung and breast cancer in advanced microphysiologic 3D tumor
models. JCI Insight. 4:e1263452019. View Article : Google Scholar :
|
|
52
|
Zhou R, Yazdanifar M, Roy LD, Whilding LM,
Gavrill A, Maher J and Mukherjee P: CAR T cells targeting the tumor
MUC1 glycoprotein reduce triple-negative breast cancer growth.
Front Immunol. 10:11492019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu Z: Tissue factor as a new target for
CAR-NK cell immunotherapy of triple-negative breast cancer. Sci
Rep. 10:28152020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Zhou Y, Huang KH, Fang X, Li Y,
Wang F, An L, Chen Q, Zhang Y, Shi A, et al: Targeting epidermal
growth factor-overexpressing triple-negative breast cancer by
natural killer cells expressing a specific chimeric antigen
receptor. Cell Prolif. 53:e128582020. View Article : Google Scholar
|
|
55
|
Chen X, Han J, Chu J, Zhang L, Zhang J,
Chen C, Chen L, Wang Y, Wang H, Yi L, et al: A combinational
therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1
for breast cancer brain metastases. Oncotarget. 7:27764–27777.
2016. View Article : Google Scholar : PubMed/NCBI
|