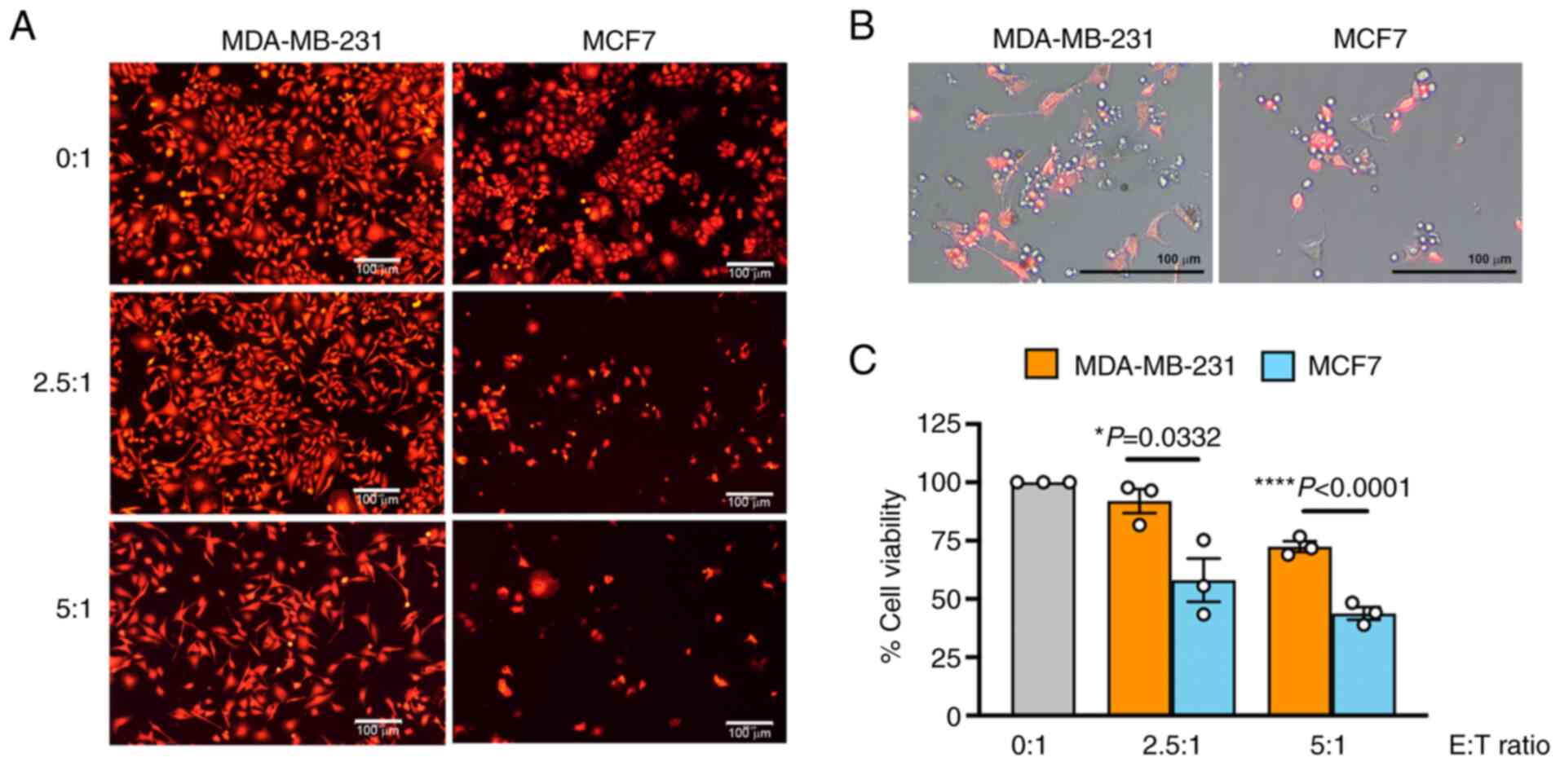
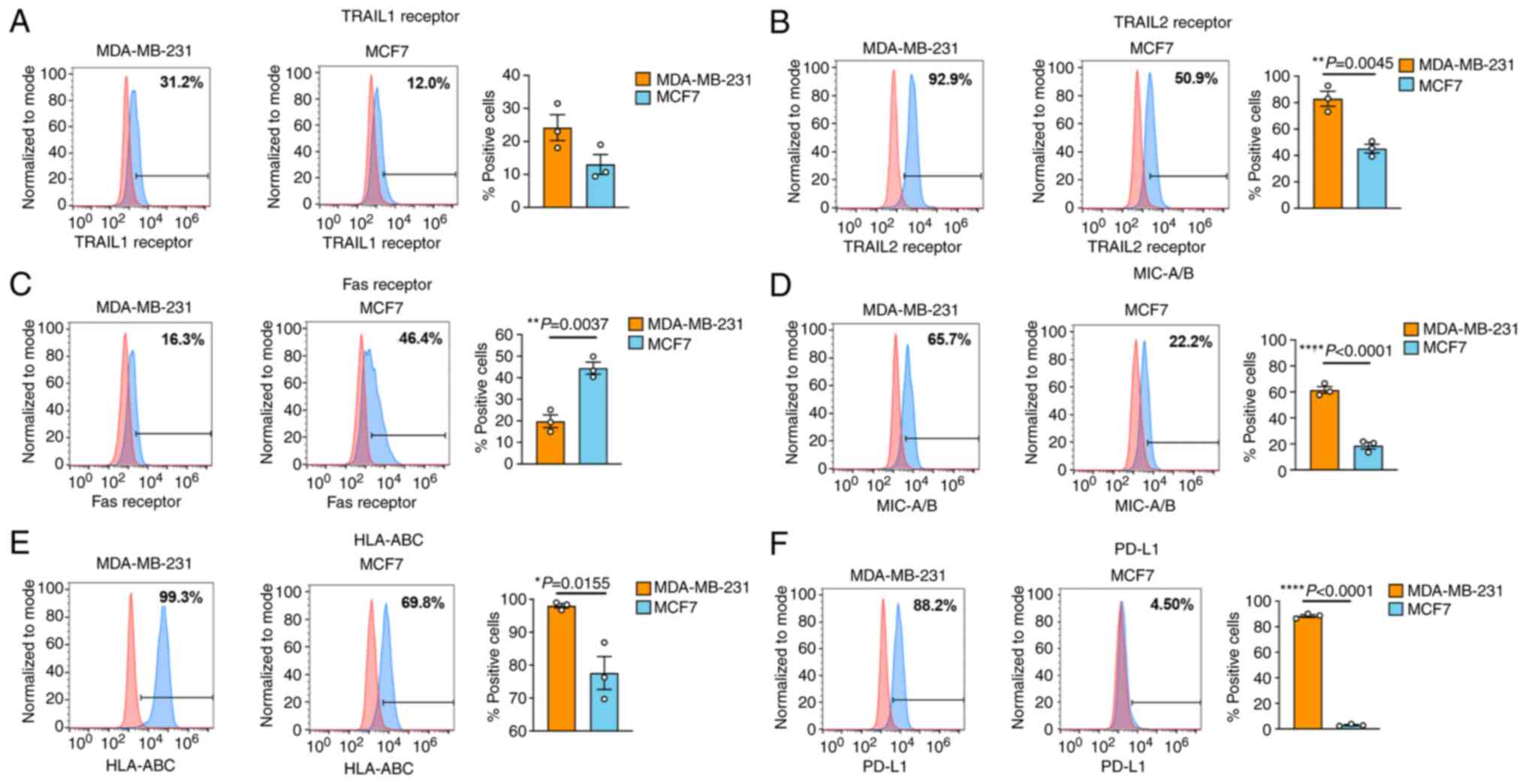
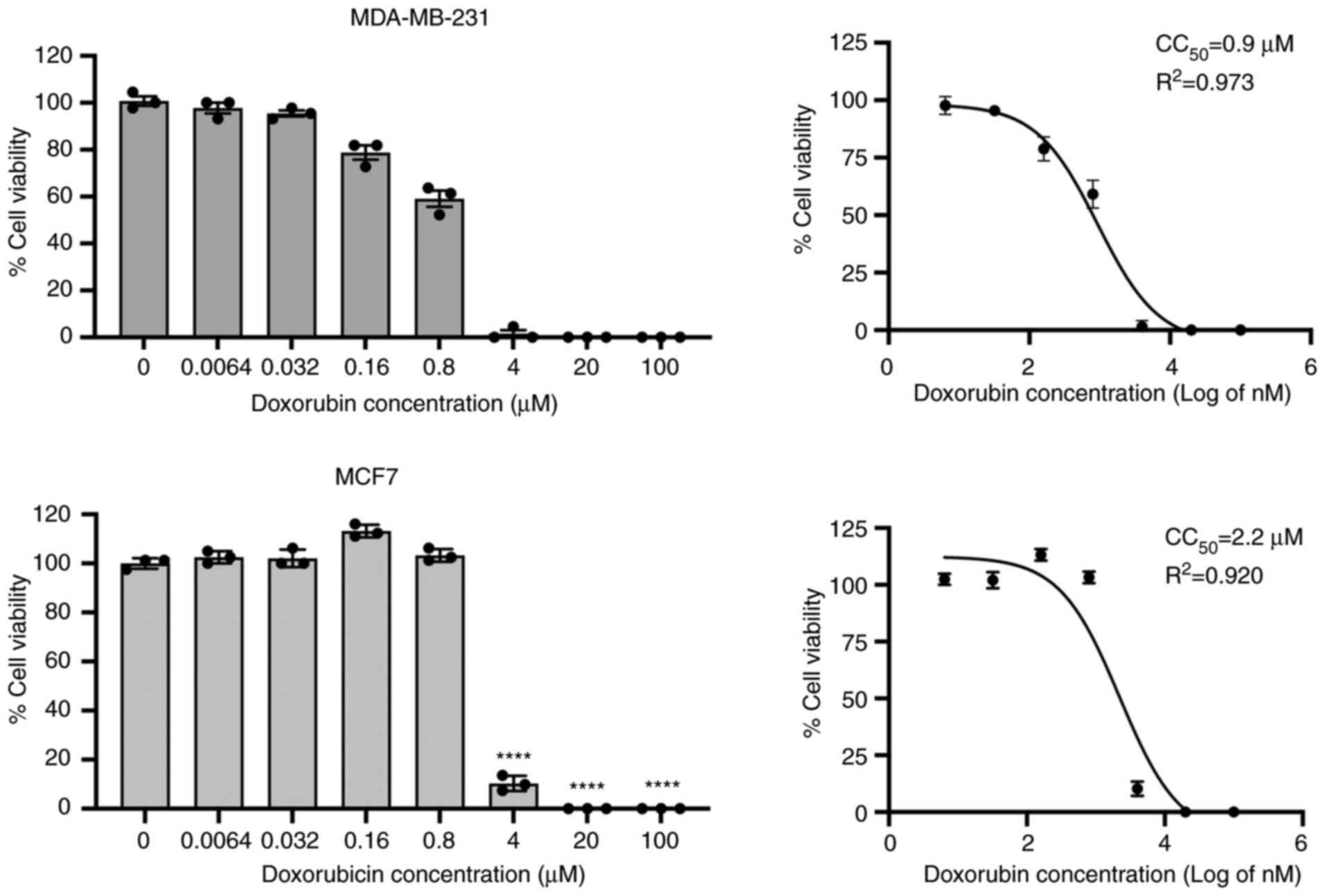
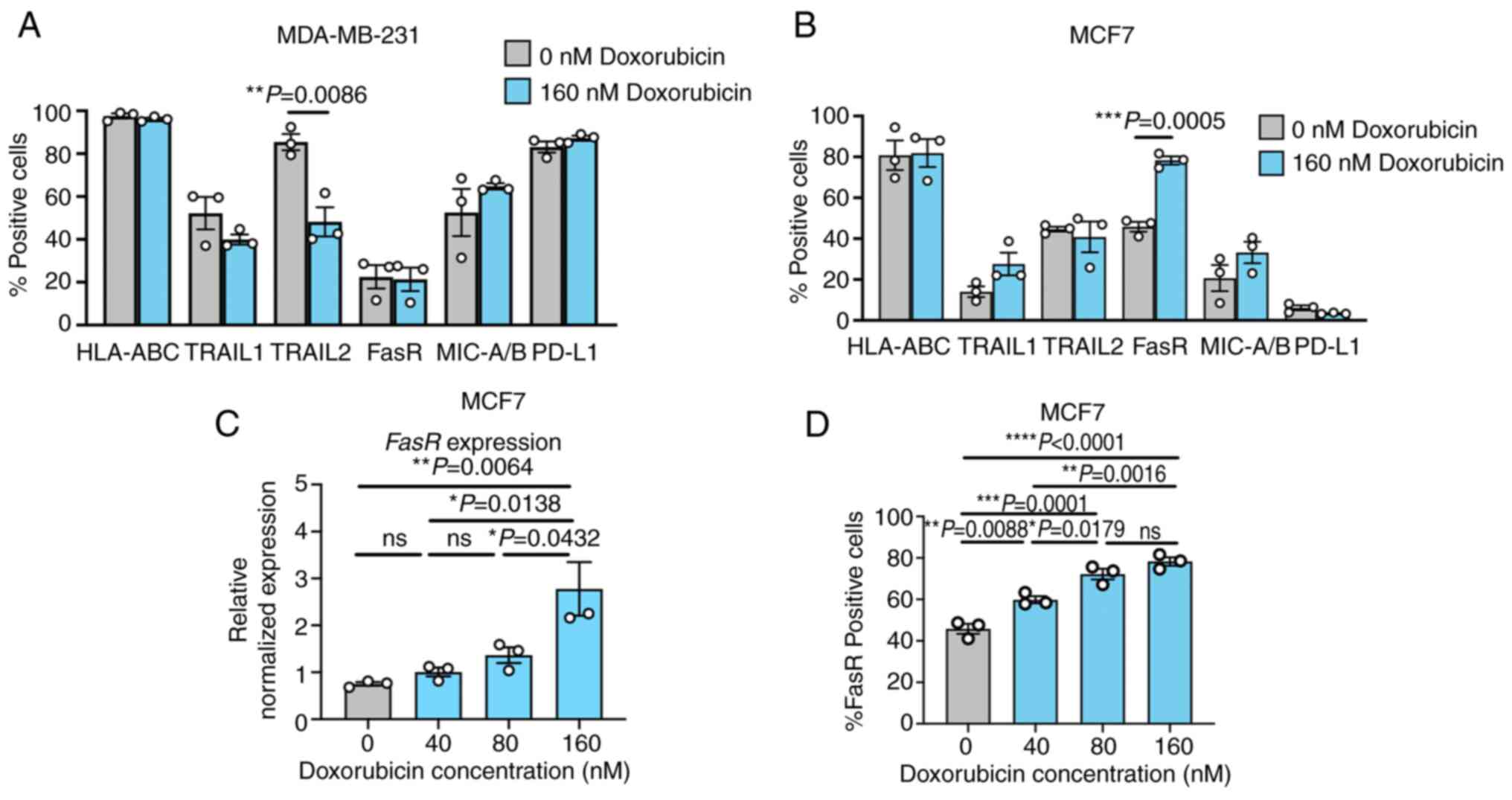
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lakha H, Suriyawongpaisul P, Sangrajrang
S, Leerapan B and Coker R: Breast cancer in Thailand: Policy and
health system challenges to universal healthcare. Health Policy
Plan. 35:1159–1167. 2020. View Article : Google Scholar
|
|
3
|
Treating Breast Cancer. https//:www.cancer.org/cancer/breast-cancer/treatment.html
Accessed October 23, 2021.
|
|
4
|
Saadatmand S, Bretveld R, Siesling S and
Tilanus-Linthorst MM: Influence of tumour stage at breast cancer
detection on survival in modern times: Population based study in
173,797 patients. BMJ. 351:h49012015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donegan WL: Tumor-related prognostic
factors for breast cancer. CA Cancer J Clin. 47:28–51. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robbins GF and Berg J: Curability of
patients with invasive breast carcinoma based on a 30-year study.
World J Surg. 1:284–286. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer. BMC Med.
14:732016. View Article : Google Scholar
|
|
8
|
Kruger S, Ilmer M, Kobold S, Cadilha BL,
Endres S, Ormanns S, Schuebbe G, Renz BW, D'Haese JG, Schloesser H,
et al: Advances in cancer immunotherapy 2019 - latest trends. J Exp
Clin Cancer Res. 38:2682019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaddepally RK, Kharel P, Pandey R and
Garje R: Chandra AB. Review of indications of FDA-approved immune
checkpoint inhibitors per NCCN Guidelines with the level of
evidence. Cancers (Basel). 12:7382020. View Article : Google Scholar
|
|
10
|
Twomey JD and Zhang B: Cancer
immunotherapy update: FDA-approved checkpoint inhibitors and
companion diagnostics. AAPS J. 23:392021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen LT, Saibil SD, Sotov V, Le MX,
Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, et
al: Phase II clinical trial of adoptive cell therapy for patients
with metastatic melanoma with autologous tumor-infiltrating
lymphocytes and low-dose interleukin-2. Cancer Immunol Immunother.
68:773–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Multhoff G, Seier S, Stangl S, Sievert W,
Shevtsov M, Werner C, Pockley AG, Blankenstein C, Hildebrandt M,
Offner R, et al: Targeted natural killer cell-based adoptive
immunotherapy for the treatment of patients with NSCLC after
radiochemotherapy: A randomized phase II clinical trial. Clin
Cancer Res. 26:5368–5379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radosa JC, Stotz L, Muller C, Kaya AC,
Solomayer EF and Radosa MP: Clinical data on immunotherapy in
breast cancer. Breast Care (Basel). 15:450–469. 2020. View Article : Google Scholar
|
|
14
|
Sanchez-Correa B, Valhondo I, Hassouneh F,
Lopez-Sejas N, Pera A, Bergua JM, Arcos MJ, Bañas H, Casas-Avilés
I, Durán E, et al: DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel
immune checkpoints for natural killer cell-based cancer
immunotherapy. Cancers (Basel). 11:8772019. View Article : Google Scholar
|
|
15
|
Xie G, Dong H, Liang Y, Ham JD, Rizwan R
and Chen J: CAR-NK cells: A promising cellular immunotherapy for
cancer. EBioMedicine. 59:1029752020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Galat V, Galat Y, Lee YKA,
Wainwright D and Wu J: NK cell-based cancer immunotherapy: From
basic biology to clinical development. J Hematol Oncol. 14:72021.
View Article : Google Scholar :
|
|
17
|
Krause SW, Gastpar R, Andreesen R, Gross
C, Ullrich H, Thonigs G, Pfister K and Multhoff G: Treatment of
colon and lung cancer patients with ex vivo heat shock protein
70-peptide-activated, autologous natural killer cells: A clinical
phase i trial. Clin Cancer Res. 10:3699–3707. 2004. View Article : Google Scholar
|
|
18
|
Parkhurst MR, Riley JP, Dudley ME and
Rosenberg SA: Adoptive transfer of autologous natural killer cells
leads to high levels of circulating natural killer cells but does
not mediate tumor regression. Clin Cancer Res. 17:6287–6297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruggeri L, Capanni M, Urbani E, Perruccio
K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F,
et al: Effectiveness of donor natural killer cell alloreactivity in
mismatched hematopoietic transplants. Science. 295:2097–2100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geller MA and Miller JS: Use of allogeneic
NK cells for cancer immunotherapy. Immunotherapy. 3:1445–1259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wennerberg E, Sarhan D, Carlsten M,
Kaminskyy VO, D'Arcy P, Zhivotovsky B, Childs R and Lundqvist A:
Doxorubicin sensitizes human tumor cells to NK cell- and
T-cell-mediated killing by augmented TRAIL receptor signaling. Int
J Cancer. 133:1643–1652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–446. 2011. View Article : Google Scholar :
|
|
24
|
Paridaens R, Biganzoli L, Bruning P, Klijn
JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A,
Sylvester R, et al: Paclitaxel versus doxorubicin as first-line
single-agent chemotherapy for metastatic breast cancer: A European
organization for research and treatment of cancer randomized study
with cross-over. J Clin Oncol. 18:724–733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Jinushi M, Takehara T, Tatsumi T, Kanto T,
Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y and
Hayashi N: Expression and role of MICA and MICB in human
hepatocellular carcinomas and their regulation by retinoic acid.
Int J Cancer. 104:354–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jost S and Altfeld M: Control of human
viral infections by natural killer cells. Annu Rev Immunol.
31:163–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quatrini L, Mariotti FR, Munari E, Tumino
N, Vacca P and Moretta L: The immune checkpoint PD-1 in natural
killer cells: Expression, function and targeting in tumour
immunotherapy. Cancers (Basel). 12:32852020. View Article : Google Scholar
|
|
29
|
Oh S, Lee JH and Kwack K: Choi SW. Natural
killer cell therapy: A new treatment paradigm for solid tumors.
Cancers (Basel). 11:15342019. View Article : Google Scholar
|
|
30
|
Xie S, Wu Z, Niu L, Chen J, Ma Y and Zhang
M: Preparation of highly activated natural killer cells for
advanced lung cancer therapy. Onco Targets Ther. 12:5077–5086.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Ostaijen-ten Dam MM, Prins HJ, Boerman
GH, Vervat C, Pende D, Putter H, Lankester A, van Tol MJD, Zwaginga
JJ and Schilham MW: Preparation of cytokine-activated NK cells for
use in adoptive cell therapy in cancer patients: Protocol
optimization and therapeutic potential. J Immunother. 39:90–100.
2016. View Article : Google Scholar
|
|
32
|
Dewan MZ, Terunuma H, Takada M, Tanaka Y,
Abe H, Sata T, Toi M and Yamamoto N: Role of natural killer cells
in hormone-independent rapid tumor formation and spontaneous
metastasis of breast cancer cells in vivo. Breast Cancer Res Treat.
104:267–275. 2007. View Article : Google Scholar
|
|
33
|
Marusyk A and Polyak K: Tumor
heterogeneity: Causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.
|
|
34
|
Larsen SK, Gao Y and Basse PH: NK cells in
the tumor micro-environment. Crit Rev Oncog. 19:91–105. 2014.
View Article : Google Scholar
|
|
35
|
Maki G, Klingemann HG, Martinson JA and
Tam YK: Factors regulating the cytotoxic activity of the human
natural killer cell line, NK-92. J Hematother Stem Cell Res.
10:369–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassin D, Garber OG, Meiraz A,
Schiffenbauer YS and Berke G: Cytotoxic T lymphocyte perforin and
Fas ligand working in concert even when fas ligand lytic action is
still not detectable. Immunology. 133:190–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:885–886. 2015. View Article : Google Scholar :
|
|
38
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun SY, Yue P, Hong WK and Lotan R:
Induction of fas expression and augmentation of fas/fas
ligand-mediated apoptosis by the synthetic retinoid CD437 in human
lung cancer cells. Cancer Res. 60:6537–6543. 2000.PubMed/NCBI
|
|
40
|
Zingoni A, Fionda C, Borrelli C,
Cippitelli M, Santoni A and Soriani A: Natural killer cell response
to chemotherapy-stressed cancer cells: Role in tumor
immunosurveillance. Front Immunol. 8:11942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Soriani A, Zingoni A, Cerboni C, Iannitto
ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C,
Petrucci MT, Guarini A, et al: ATM-ATR-dependent up-regulation of
DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic
agents results in enhanced NK-cell susceptibility and is associated
with a senescent phenotype. Blood. 113:3503–3511. 2009. View Article : Google Scholar
|
|
42
|
Wen J, Ramadevi N, Nguyen D, Perkins C,
Worthington E and Bhalla K: Antileukemic drugs increase death
receptor 5 levels and enhance Apo-2L-induced apoptosis of human
acute leukemia cells. Blood. 96:3900–3906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Müller M, Wilder S, Bannasch D, Israeli D,
Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M
and Krammer PH: P53 activates the CD95 (APO-1/Fas) gene in response
to DNA damage by anticancer drugs. J Exp Med. 188:2033–2045. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Asadzadeh Z, Safarzadeh E, Safaei S,
Baradaran A, Mohammadi A, Hajiasgharzadeh K, Derakhshani A,
Argentiero A and Silvestris N: Baradaran B. Current approaches for
combination therapy of cancer: The role of immunogenic cell death.
Cancers (Basel). 12:10472020. View Article : Google Scholar
|
|
45
|
Kagi D, Vignaux F, Ledermann B, Bürki K,
Depraetere V, Nagata S, Hengartner H and Golstein P: Fas and
perforin pathways as major mechanisms of T cell-mediated
cytotoxicity. Science. 265:528–530. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kojima H, Shinohara N, Hanaoka S,
Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H
and Okumura K: Two distinct pathways of specific killing revealed
by perforin mutant cytotoxic T lymphocytes. Immunity. 1:357–364.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sivaganesh V, Promi N, Maher S and
Peethambaran B: Emerging immunotherapies against novel molecular
targets in breast cancer. Int J Mol Sci. 22:24332021. View Article : Google Scholar :
|
|
48
|
Shenouda MM, Gillgrass A, Nham T, Hogg R,
Lee AJ, Chew MV, Shafaei M, Aarts C, Lee DA, Hassell J, et al: Ex
vivo expanded natural killer cells from breast cancer patients and
healthy donors are highly cytotoxic against breast cancer cell
lines and patient-derived tumours. Breast Cancer Res. 19:762017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao Q, Li L, Zhang C, Sun Y, Liu S and Cui
S: Clinical effects of immunotherapy of DC-CIK combined with
chemotherapy in treating patients with metastatic breast cancer.
Pak J Pharm Sci. 28(Suppl 3): S1055–S1058. 2015.
|
|
50
|
Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y,
Jia Y, Zhou J, Ouyang Y and Zeng Z: Clinical implications of
tumor-infiltrating immune cells in breast cancer. J Cancer.
10:6175–6184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wallstabe L, Gottlich C, Nelke LC,
Kuhnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar
G, Nietzer SL and Hudecek M: ROR1-CAR T cells are effective against
lung and breast cancer in advanced microphysiologic 3D tumor
models. JCI Insight. 4:e1263452019. View Article : Google Scholar :
|
|
52
|
Zhou R, Yazdanifar M, Roy LD, Whilding LM,
Gavrill A, Maher J and Mukherjee P: CAR T cells targeting the tumor
MUC1 glycoprotein reduce triple-negative breast cancer growth.
Front Immunol. 10:11492019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu Z: Tissue factor as a new target for
CAR-NK cell immunotherapy of triple-negative breast cancer. Sci
Rep. 10:28152020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Zhou Y, Huang KH, Fang X, Li Y,
Wang F, An L, Chen Q, Zhang Y, Shi A, et al: Targeting epidermal
growth factor-overexpressing triple-negative breast cancer by
natural killer cells expressing a specific chimeric antigen
receptor. Cell Prolif. 53:e128582020. View Article : Google Scholar
|
|
55
|
Chen X, Han J, Chu J, Zhang L, Zhang J,
Chen C, Chen L, Wang Y, Wang H, Yi L, et al: A combinational
therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1
for breast cancer brain metastases. Oncotarget. 7:27764–27777.
2016. View Article : Google Scholar : PubMed/NCBI
|