1. Introduction
When facing the dangers of oxidative stress,
temperature changes, toxins, hypoxia and osmotic pressure changes,
eukaryotic cells produce a series of comprehensive stress responses
in order to resist the above-mentioned unfavorable conditions and
reduce damage (1). These include
the production of membraneless messenger ribonucleoprotein (mRNP)
particles, such as stress granules (SGs) and P bodies (PBs) in the
cytoplasm (1-4). The aberrant assembly or disassembly
of SGs has pathological implications in cancer (5-7)
and viral infection (8,9). In addition, SGs are associated with
increased rates of aging-related conditions such as cardiovascular
disease (10,11) and neurodegenerative disorders
(12-15). Therefore, a better understanding
of the initiation, assembly and pathological assembly of SGs in
these diseases may help to discover different target molecules and
help to develop precise pharmacological interventions. The present
article provides a review of current findings on the formation,
composition, dynamics, function and involvement of SGs in different
diseases.
2. Formation of SGs and influencing
factors
When faced with a stress stimulus, the four
monitoring kinases including protein kinase R (PKR), PKR-like
endoplasmic reticulum kinase (PERK), heme-regulated kinase
inhibitor and general control nonderepressible 2 (GCN2) in
eukaryotic cells are activated and the Ser51 site of the
translation initiation factor eukaryotic translation initiation
factor 2A (eIF2) is phosphorylated. As a result, the
eIF2-GTP-tRNAiMet ternary complex required for normal translation
initiation is gradually depleted and the ribosomes disintegrate and
stagnant 48S preinitiation complexes (PICs), PICs including
translation initiation factor, 40S ribosomal subunit, untranslated
mRNA and RNA binding protein are generated, which aggregate to form
the 'nucleus' of SGs and further combine with RNA-binding proteins
(RBPs) to form the 'shell' of SGs (16-18). Furthermore, the activated 'shell'
may exchange its components with ribosomes, PBs and other
structures in the cytoplasm (4,18-20), thus regulating the transport,
translation, isolation and degradation of RNA (2,3).
The low-complexity domains (LCDs) contained in RBPs are critical to
the formation of the 'core' and 'shell' of SGs. This region may
mediate specific protein-protein, protein-RNA and/or RNA-RNA
cross-linking and aggregation through liquid-liquid phase
separation (LLPS), thereby condensing into SGs particles in the
cytoplasm (2,3). In addition, the formation and
depolymerization of SGs are an energy-consuming process and
multiple ATP-dependent complexes affect the assembly of SGs. For
instance, the Chaperonin-Containing T complex inhibits the assembly
of SGs, while the mini-chromosome maintenance and RuvB-like
helicase complexes promote the survival of SGs (20,21).
RBPs are regulated by a variety of intracellular
signaling pathways, thereby affecting translation/protein
synthesis. Under unfavorable circumstances, intracellular
DNA-binding proteins and RBPs join SGs, such as TAR DNA-binding
protein 43 (TDP-43), fused in sarcoma (FUS), GTPase-activating
protein SH3 domain-binding protein (G3BP) SG assembly factor 1/2
(G3BP1/2), ubiquitin specific peptidase 10 (USP10), cell cycle
associated protein 1 (Caprin1), T-cell intracytoplasmic antigen 1
(TIA1) cytotoxic granule associated RNA binding protein
(TIA1)/TIA1-related protein (TIAR) and heat shock proteins
(Hsp)40/70 (18,21-24), participating in different
stresses and the pathological process of disease (Table I). G3BP1/2, Caprin1, USP10 and
TIA1/R have important roles in the formation of SGs (23). Among them, G3BP1/2 is regulated
by the phosphorylation site of S149 and may bind to Caprin1 or
USP10; binding to Caprin1 may promote the formation of SGs, while
binding to USP10 may inhibit the formation of SGs (23). G3BP cross-links with 40S
ribosomal subunits through its arginine- and glycin-rich motif RGG,
which is also one of the necessary conditions for G3BP to mediate
the formation of SGs (23). In
addition, TIA-1 or TIAR may also promote the formation of SGs alone
(23,25).
 | Table IRelations between specific stress
granule initiation factors, proteins and different types of
disease. |
Table I
Relations between specific stress
granule initiation factors, proteins and different types of
disease.
| Initiation factors
and RBPs |
Treatment/effectors | Disease, Refs. for
established relations
|
|---|
| Tumor | VI | AD | ALS/FTD | PD |
|---|
| eIF2α | Bortezomib,
cisplatin, etoposide, morusin, NO, IL-19, PRV, EV71, MERS-CoV,
EBOV, GCN2, PKR, PERK, TDP-43 | (27,34,41,42) | (52,56,57,65) | (74-76,102) | (74,76) | |
| eIF3, eIF4 | NO, EV71, EBOV,
PKR, PERK, rapamycin, DJ-1, COI | (27) | (56,57) | (74) | (74,103) | (100) |
| G3BP1 or G3BP2 | Cisplatin,
etoposide, morusin, NO, SASP, SART3, YB-1, PKM2, IL-19, LRP6, RIG-I
C108, CrPV-1A, PV, EBOV, SOD1, COI | (27,34,35,37,41,46,47) | (53,55,57) | (84,103) | (84,103) | |
| G3BP1 and
G3BP2 | Bortezomib,
MERS-CoV, IFITM1, FMDV | (42,45) | (54,65) | | | |
| RACK1 | NO, morusin, 5-Fu,
DJ-1 | (25,27,34) | | | | (101) |
| TIA1 | NO, EV71, MERS-CoV,
CrPV-1A, YB-1, Tau, DJ-1, COI | (27) | (53,56, 65) | (35,82-84) | (35,82-84,103) | (100) |
| TDP-43 | PKR, PERK, RRM,
SINE, tau, COI | | | (90) | (76,78,79,103) | |
| FUS | Hpo, JNK, AchR,
COI | | | | (86,87,103) | |
| hnRNPs | PrLD, DJ-1, MAP
1B-LC1 | (104) | | | (88,89) | (100) |
According to the translation initiation factors and
RBPs contained, SGs may be divided into different subtypes. For
instance, type I SGs are classical SGs. The formation process
includes phosphorylation of eIF2α, which requires G3BP and 48S
PICs. Its components include eIF3, but lack eIF2 and eIF5. The
formation of type II SGs includes eIF4A inactivation and still
requires G3BP. The group of type II SGs includes eIF2, eIF3 and
eIF5. However, type III SGs lack eIF3 (18,26,27), display reduced recruitment of 40S
ribosomal subunit (26) and are
less dynamic (28). The
formation of type III SGs is independent of the phosphorylation of
eIF2α and may be triggered by sodium selenite (26), glucose starvation (27) and nitric oxide (NO) (28). The different functions and
properties of the diverse SG subtypes remain to be fully
elucidated, but different SG subtypes may exert opposite effects on
cellular metabolism and survival.
There is a full interaction between mRNA and RBPs in
SGs. RBPs may act on mRNA to regulate its metabolism and function;
conversely, mRNA may also be combined with RBPs to affect the
subsequent functions of RBPs (2). After the separation between SGs,
fusion may also form new SGs (29). The formation and depolymerization
of SGs are also affected by a variety of post-translational
modification factors. For instance, the phosphorylation of eIF2α at
Ser51 regulates the initiation of SGs; phosphorylation of
tristetraprolin may regulate the interaction between SGs and PBs;
and phosphorylation of growth factor receptor bound protein 7 may
promote heat shock-induced depolymerization of SGs (18). The demethylation of G3BP1 and
ubiquitin-associated protein 2 like (UBAP2L) protein arginine may
promote the production of SGs (30,31). The UBAP2L RGG motif is able to
mediate the enrollment of SG components, including mRNPs, RBPs and
ribosomal subunits (30,31). The protein arginine
methyltransferase 1 asymmetrically dimethylated UBAP2L by targeting
the RGG motif, thus compromising SG assembly (30). Therefore, the RGG motif may serve
as a critical interface for SG assembly. The RGG motif and arginine
methylation/demethylation are involved in the regulation of the
downstream nucleic acid binding, protein-protein interactions and
signal transduction.
3. Functions of SGs
In eukaryotic cells, there is a competitive
relationship between the signaling pathways that govern protein
translation and mRNA degradation, which jointly regulate cell gene
expression under different conditions. Both SGs and PBs are
membraneless mRNP particles produced by LLPS from local high
concentrations of RBPs and RNA in order to adapt to environmental
changes (24,29). Due to their non-membrane
characteristics, SGs and PBs may serve as a stress signal
processing center where various signaling pathways converge and may
exchange components. Therefore, SGs and PBs share a variety of RBPs
and RNA components (4).
When faced with external, unfavorable factors, cells
suspend most of their protein synthesis to conserve energy. The
mRNAs encoding housekeeping proteins (e.g., β-actin, β-tubulin and
GAPDH) in the ribosome are transported to SGs for temporary storage
(24,32). The mRNAs encoding other proteins
are transferred to PBs or other mRNPs for further storage or
degradation (18,24,29,32). However, certain transcripts
involved in the stress response of SGs are allowed to be translated
to maintain cell survival. For instance, activating transcription
factor 4 and HSP70 help reduce misfolding during protein expression
(32,33). In addition, the pro-apoptotic
protein receptor for activated C kinase 1 (RACK1) is 'isolated' in
SGs, so that the MAP three kinase 1 (MTK1)-stress activated protein
kinase (SAPK) signaling pathway that depends on RACK1 is blocked
(34) and the activation of
caspase-3 is reduced (35),
thereby inhibiting the apoptotic signaling cascade response to
promote cell survival.
When oxidative stress, heat shock and hypoxia, and
other harmful stimuli persist, chronic stress may occur. Continuous
cell stress response may induce mutations in RBPs, block autophagy
pathways and cause deposition of disease-related proteins, leading
to the breakdown of the stability/degradation balance of SGs,
continuous accumulation of SGs, as well as degeneration and
deposition and/or formation of abnormal SGs. Therefore, SGs are a
key factor that determines the fate of mRNA when cells face stress
and affect the translation, stabilization, transport and
degradation of mRNA (Fig. 1)
(18,36,37). When the subsequent stress crisis
is relieved, eIF2α is dephosphorylated, which may restore the
normal translation initiation function and the normal protein
translation process of the cell is restored (21,36). Therefore, the main function of
SGs is to help cells reorganize the translation process in protein
synthesis, store housekeeping protein mRNA and solve the survival
crisis first when faced with unfavorable environments. However, the
persistence of SGs and the imbalance of SG production/decomposition
are also the main reasons for the degeneration and hydrolysis of
important cellular mRNAs and may promote the occurrence of numerous
diseases, including cancer, cardiovascular diseases, viral
infections and neurodegenerative diseases (18,21,36).
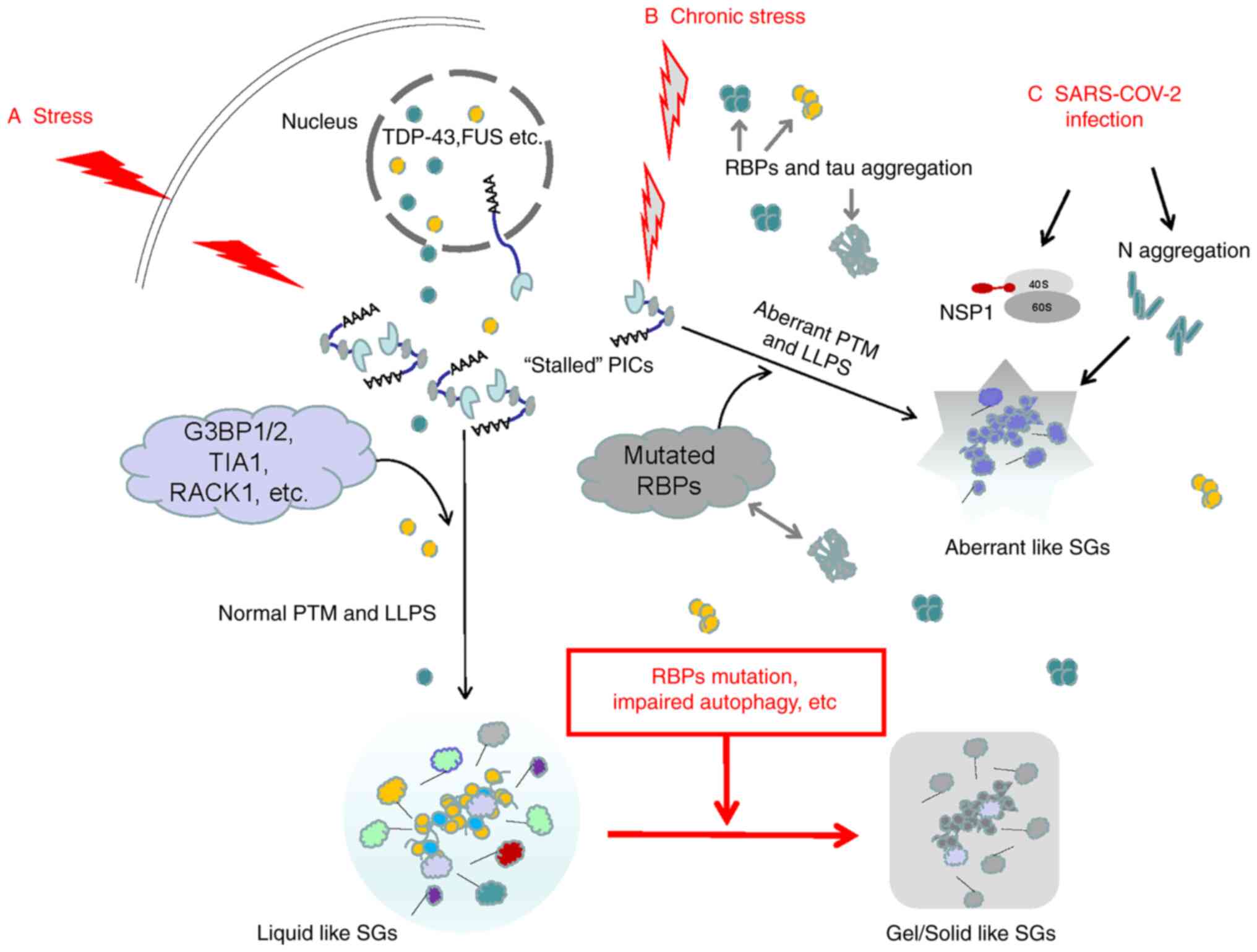 | Figure 1Possible mechanisms of acute and
chronic stress-inducing different types of SGs. (A) When subjected
to acute stresses, the stalled 48S PICs in eukaryotic cells serve
as the 'nucleus' of SGs. The SG 'shell' consists of various dynamic
RBPs. The assembly of SG 'cores' and 'shells' is largely driven by
LLPS. The mRNA in SGs is able to resume translation after the
stress is relieved. (B) Chronic stress may induce mutations and
aggregation of DNA-binding proteins and RBPs, leading to abnormal
LLPS and generating aberrant SGs. The deposited tau may cross-link
with RBPs. As chronic stress persists, RBPs in normal SGs may also
undergo mutations and the autophagy hydrolysis pathway of SGs is
blocked. Normal SGs gradually change from liquid to gel/solid and
persist, eventually inducing various diseases. (C) The typical RBPs
in SGs, such as G3BP1, are the main targets of various viruses. The
N protein in SARS-COV-2 integrates with G3BP1 into SGs, promoting
the degeneration of SGs. In addition, after SARS-COV-2 enters human
host cells, the initially synthesized NSP1 may block the channel of
host mRNA to enter the 40S subunit but promote viral replication.
SG, stress granule; RBP, RNA-binding protein; SARS-COV-2, severe
acute respiratory syndrome-coronavirus disease; LLPS, liquid-liquid
phase separation; G3BP1, G3BP SG assembly factor 1; TIA1, TIA1
cytotoxic granule associated RNA binding protein; RACK1, receptor
for activated C kinase 1; NSP1, non-structural protein 1; PICs,
preinitiation complexes; PTM, post-translational modification. |
4. SGs involved in cell senescence and
tumors
Cell senescence is one of the main pathogenic
factors of cancer. During ageing, cells in the human body gradually
become senescent. Under chronic oxidative stress, senescent cells
inhibit the production of SGs in the cytoplasm, which may be
accompanied by heat shock response and autophagy, which further
promote the decomposition of SGs (38). The key factors, HSP70 and
autophagy related 5, which inhibit the heat-shock response, as well
as the autophagy pathway, may restore the ability of senescent
cells to generate SGs during chronic oxidative stress (38). Aging-related secreted phenotype
(SASP) factors are closely related to the occurrence of tumors
(39,40). A recent study suggested that
G3BP1 is not only the key RBP for cell stress response and SG
generation, but also a necessary factor for activating SASP
factors. G3BP1 may activate the NF-κB and signal transducer and
activator of transcription 3 signaling pathways through cyclic
GMP-AMP synthase (cGAS) to promote the expression of SASP protein;
depleting G3BP1 or inhibiting the cGAS pathway may prevent the
expression of SASP factors and reduce the occurrence and migration
of tumors without affecting the normal senescence of cells
(39).
The survival of tumor cells faces numerous
unfavorable conditions, such as hypoxia, nutrient deficiency,
reactive oxygen species and high osmotic pressure. Tumor cells may
pursue the path of generating SGs to selectively perform mRNA
translation to regulate cell signal transduction pathways,
metabolism and stress response to promote their own survival
(1,41,42). For instance, in liver cancer
cells, PI3K and MAPK/p38 activate the mechanistic target of
rapamycin (mTOR) complex 1-S6 kinase signaling pathway and
phosphorylate eIF2α, eIF3, eIF4B and eIF5 to generate SGs (5-7),
which may promote the development of liver cancer (6). Chemotherapy drugs commonly used in
cancer treatment, such as bortezomib, cisplatin or etoposide, may
stimulate tumor cells to generate SGs that depend on eIF2α
phosphorylation and resist the effects of drugs (43,44). In tumor cells cultured in
vitro, the plant-derived anti-cancer drug Sanxin may activate
PKR and eIF2α phosphorylation in cancer cells and induce the
production of SGs (35). SGs may
also regulate tumor-related signaling pathways and participate in
tumor migration. By inhibiting the phosphorylation of eIF2α and
interfering with the recruitment of SGs or microtubule
polymerization, tumor occurrence and metastasis may be inhibited
(43,45).
NO is one of the key factors in tumorigenesis,
development or suppression, depending on the source and
concentration of NO. The high concentration of NO synthesized by
inducible NO synthase in activated macrophages/lymphocytes may
induce cell cycle arrest and apoptosis and exert anti-tumor
effects. The low and intermediate concentrations of NO synthesized
by tumors and endothelial cells interfere with normal cell DNA
repair and promote the accumulation of mutations, tumor
heterogeneity, angiogenesis, epithelial mesenchyme and tumor
invasion (46). The different
effects of NO in inhibiting or promoting tumorigenesis may be
related to the regulation of different translation initiation
factors by NO, inducing the production of different subtypes of SGs
in cancer cells and promoting the conversion between different
subtypes of SGs. For instance, the lack of eIF3 in type II SGs may
promote tumorigenesis (28).
Various RBPs also have a vital role in the
generation of SGs in tumor cells and the promotion of tumor
development (Table I). The
anti-metabolite drug 5-fluorouracil (5-Fu) is widely used to treat
solid tumors. However, tumors generally develop resistance to 5-Fu
in the later stages of treatment. Kaehler et al (25) indicated that during the process
of 5-Fu inducing SGs in cancer cells, RACK1 was isolated in SGs,
which inhibited MTK1-SAPK and downstream tumor apoptosis signaling
pathways, resulting in a decrease in the effect of 5-Fu
chemotherapy. Similarly, when Sanxin was used to treat tumors,
RACK1 was isolated in SGs, which reduced the activation of
caspase-3 and the therapeutic effect of Sanxin (35). The results of in vitro
experiments indicate that the use of comprehensive therapies such
as hypoxia, arsenite and X-ray therapy may also induce the
production of SGs containing RACK1 in cancer cells, thereby
reducing cancer cell apoptosis and resisting therapeutic
intervention (34).
Compared with the control group, G3BP1 gene knockout
in tumor cells significantly enhanced the sensitivity to Sanxin
treatment (35). G3BP1/2 is able
to regulate the translation of interferon-stimulated genes to
promote tumor development and metastasis (47); G3BP2 was also reported to promote
breast tumors by binding to squamous-cell carcinoma antigens
(48). Studies on SGs of human
sarcoma cells indicated that G3BP1 interacts with Y-box binding
protein (YB-1) to jointly promote the production of SGs; at the
same time, the increased expression of G3BP1 and YB-1 predicts a
poor survival prognosis for tumor patients (49). In the SGs of tumor cells induced
by sodium arsenite, the distributions of G3BP1 and TIA1/R are
highly overlapped and may jointly regulate the stability and
degradation of mRNA in SGs under stress conditions (37). Therefore, by regulating the
phosphorylation of translation initiation factors, regulating the
SGs-promoting effects of various RBPs, such as RACK1, G3BP1/2 and
the downstream signaling pathways of SGs/RBPs, may reduce the
incidence of tumors, improve the efficacy of tumor treatment drugs
and reduce the mortality of patients with cancer.
5. SGs involved in cardiovascular
disease
A recent study indicated that for
monocytes/macrophages isolated from the serum of patients with
myocardial infarction (MI) and coronary artery disease,
hyperglycemia stimulation activated the pyruvate kinase isoenzyme
type M2 (PKM2), followed by SGs marker protein G3BP1 production,
intravascular plaque loosening and shedding; eventually, the risk
of thrombosis was increased (10). A study on atherosclerotic plaques
in low-density lipoprotein (LDL) receptor knockout mice suggested
that increased expression of G3BP1 and poly-A binding protein
(PABP) in macrophages and smooth muscles was significantly
associated with the progression of atherosclerosis (11). In human coronary artery smooth
muscle and macrophages cultured in vitro, application of
oxidized LDL and mitochondrial/oxidative stress mediator may
rapidly induce the production of SGs containing PABP and G3BP;
while application of IL-19 may inhibit eIF2α phosphorylation, PABP
expression and SG production (11).
A molecular genetic study in humans and rodents
indicated that the Wnt and LDL receptor-related protein (LRP)
signaling pathway may be a key step in cardiometabolic diseases.
For cultured mouse aortic smooth muscle cells, under the
stimulation of glycerol phosphate, the expression of G3BP1, a
marker protein of SGs in smooth muscle cells, increased through its
C-terminal arginine methylation domain and mitochondrial antiviral
signal protein retinoic acid-inducible gene I cross-linking to
promote Wnt signaling related to arteriosclerosis and accelerated
arteriosclerosis (50). The
application of G3BP antagonist C108 may reduce G3BP1 methylation
and Wnt signaling (50).
The mutation of human RNA binding motif protein 20
(RBM20) caused by gene mutation is closely related to congenital
dilated cardiomyopathy. A clinical study indicated that mRNP
particles containing RBM20 variants accumulate abnormally in the
sarcoplasm of patients' myocardial cells and promote the fusion of
biomolecules and SGs in the sarcoplasm (51). Therefore, the disorder of the
protein components of mRNP particles, including SGs, is closely
related to cardiovascular diseases such as MI and coronary artery
disease, atherosclerosis and dilated cardiomyopathy. In addition,
the above-mentioned diseases are mostly chronic diseases except for
MI, which may cause intracellular protein translation, RBP
dysregulation and the persistence of SGs, thereby promoting the
development of cardiovascular diseases. For instance, acute
coronary syndrome, otherwise known as type 1 MI, occurs as a result
of vulnerable plaque rupture with following thrombus formation and
arterial spasm and thus coronary occlusion (52). However, the activation of PKM2
may mediate NLR family pyrin domain containing 3 inflammasome and
G3BP1 production, ultimately increasing plaque
vulnerability/rupture and is associated with acute MI (10). Circular RNAs are a subclass of
non-coding RNAs detected within mammalian cells and are generated
from numerous protein-coding genes. It was recently reported that a
circRNA transcript, circFndc3b, is significantly downregulated in
the post-MI mouse and human hearts. Overexpression of circFndc3b
significantly enhanced vascular endothelial growth factor A
expression via reducing the levels of SGs-related protein FUS
(53). These results indicated
that modulation of circRNA expression and/or SGs-related protein
levels may represent a potential strategy to promote cardiac
function and remodeling after MI. In the meantime, meticulous
research is also required to determine the roles of SGs and RBPs in
acute cardiovascular diseases such as MI.
6. SGs involved in viral infection
SGs produced by host cells have an important role in
antiviral innate immunity (54).
During virus attack, viral double-stranded RNA may activate PKR and
PERK in host cells, phosphorylate eIF2α, generate SGs and inhibit
the translation and replication of viral RNA; therefore, SGs have
also become the target of numerous viral attacks (8,9).
Different viruses may affect the various stages of SGs, such as
inhibiting the production of SGs or interfering with the components
of SGs and the function of SGs (8,55). Pseudorabies virus (PRV) may
infect other mammals such as pigs, cattle, sheep, dogs and cats.
The results of in vitro experiments suggest that PRV may
inhibit the process of eIF2α phosphorylation induced by sodium
arsenite, inhibit the production of SGs and promote viral
replication (56). The cricket
paralysis virus (CrPV)-associated protein CrPV-1A may induce the
aggregation of G3BP1 and inhibit the production of SGs (57). Foot-and-mouth disease virus and
equine rhinitis A virus also inhibit the production of SGs by host
cells by lysing G3BP1/2 (58).
Poliomyelitis virus (PV) may induce host cells to
produce SGs at the initial stage of infection, but as time goes by,
PV promotes the cleavage of G3BP1 through protease 3C and inhibits
the production of SGs (59).
Protease 2A in picornavirus, EV71, isolates host mRNA by cleaving
eIF4GI to form atypical SGs, but releases viral mRNA to promote
virus replication (60). Ebola
virus (EBOV) replicates in host cells and infection does not induce
the production of SGs, and may inhibit the process of
arsenite-induced SGs; EBOV virus particles may also inhibit the
production of SGs induced by a variety of drug stresses (61). The effect of EBOV in inhibiting
SGs may lie in the cross-linking between its C-terminal domain and
G3BP1, eIF3 and eEF2 in SGs (61).
Coronavirus disease 2019, which is currently
circulating globally, is a highly contagious disease caused by
severe acute respiratory syndrome coronavirus 2 (SARS-COV-2)
infection. The latest research has indicated that the SARS-COV-2
nucleocapsid (N) protein is cross-linked with G3BP1 in the host
cell SGs through LLPS to isolate G3BP1, thereby inhibiting the
formation of SGs or promoting the degeneration of SGs, and is
beneficial to the virus' self replication (62-64). Prasad et al (65) even observed that 116 human SG
proteins directly interacted with SARS-CoV-2 proteins and they are
involved in 430 different brain disorders.
SARS-COV-2 may also interfere with the initiation of
SGs in the host cell by inhibiting translation initiation; for
instance, after SARS-COV-2 enters the host cell, it first
synthesizes non-structural protein 1 (NSP1), and NSP1 uses its
C-end to bind to the 40S subunit in the host cell ribosome complex,
blocking the passage of host mRNA into the 40S subunit; NSP1 may
also bind to 43S PIC (66-68). In addition, SARS-COV-2 may also
inhibit host cell translation and immune response through NSP1, but
does not restrict the translation of the virus' own protein
(66-68). In the host cell, the aggregated N
protein and SARS-COV-2 RNA polymerase form a high-density complex
through LLPS, which may effectively promote the translation of
viral RNA and viral replication (62). MERS-CoV, similar to SARS-COV-2,
inhibits PKR-mediated phosphorylation of eIF2α, prevents host cells
from producing SGs and promotes viral mRNA translation (69). At present, as is common for
viruses, new coronavirus variants keep on emerging, and certain
strains may be more contagious and harmful. Whether the mutated
SARS-COV-2 has other ways to inhibit host cell translation and
immune response, inhibit the production of SGs in host cells and
promote its own protein translation and virus replication requires
further research.
The diversity of the above-mentioned viruses' attack
methods in host cells highlights the importance of SGs in
anti-viral defense. It is necessary to intervene with specific
proteins according to the interaction between different viruses and
the host cell's SG production process, and determine a treatment
plan. For instance, for SARS-COV-2, it interferes with the LLPS
process in the host cell, inhibits the accumulation of N in large
quantities and interferes with the binding of NSP1 to the 40S
subunit of the host cell's ribosome. However, a latest heavily
mutated Omicron variant (B.1.1.529) puts scientists on alert due to
its increased infectivity and ability to evade infection-blocking
antibodies compared to the Delta and Alpha variants (70). In addition, certain key questions
about this variant remain unanswered, such as whether Omicron is
more transmissible and causes more severe or milder pathologies
than other variants. A question related to the present review,
namely how SGs/RBPs directly and indirectly regulate various
signaling pathways to contribute to Omicron evading the antiviral
immune response is also worthy of in-depth study.
7. SGs involved in neurodegenerative
diseases
Accompanied by chronic encephalopathy caused by
brain aging, ischemia, traumatic brain injury and other factors
such as secondary neuroinflammation, the nervous system of the
elderly is prone to chronic stress and in turn, the occurrence of
chronic stress is closely related to neurodegenerative disease
(19,71). Among the mRNP particles induced
by stress, SGs are most closely related to neurological diseases
(19,71). The above-mentioned risk factors,
such as brain aging, cerebral ischemia, brain injury and
neuroinflammation, may induce the generation of SGs in neurons,
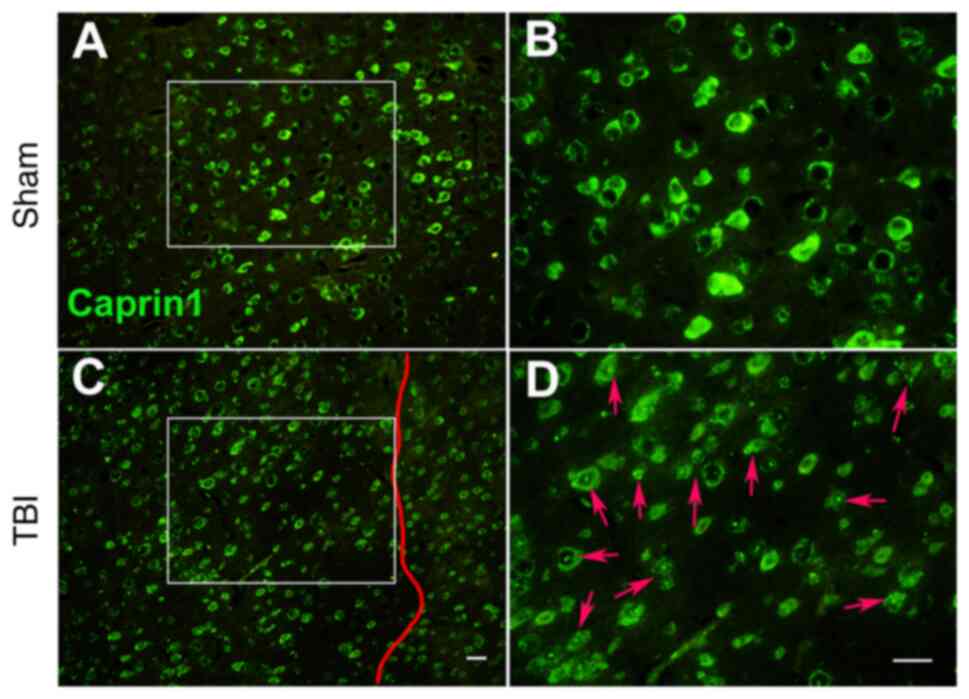
regulate the expression of RBPs (72-75), such as Caprin1 (Fig. 2), and induce irreversible
accumulation of key RBPs and tau (19,76).
In SGs, RBPs and deposited tau may be cross-linked,
hindering the translation of RNA. Eventually, RBP and/or tau
deposits aggregate and transform into an irreversible state of
accumulation (77). The results
of in vitro studies have indicated that under pathological
conditions, SGs transform from liquid to viscous/solid amyloid and
cause tau deposition (12-15), which promotes neurodegenerative
diseases (77). The
phosphorylation of neuron eIF2α and eIF4 is mainly caused by the
activation of signaling pathways such as mTOR, GCN2, PKR and PERK;
therefore, these signaling pathways may also become targets for the
development of neuroprotective drugs (78-80). The disordered LCDs in RBPs may
regulate the liquid-solid transition of mRNPs. Under pathological
conditions, the LCDs of RBPs exhibit a highly ordered structure,
which promotes the aggregation of RBPs and the unidirectional
transformation of mRNPs from liquid to solid, and generates
pathological SGs (13,81). Full-length tau with internal
disorder may also pass through LLPS under pathological conditions
and aggregate (15). Numerous
proteins involved in the composition of SGs, such as TDP-43
(82-85), TIA1 (86,87), G3BP1/2 (88,89), FUS (90,91) and heterogeneous nuclear
ribonucleoproteins (hnRNPs) (92,93), are related to the pathogenesis of
neurodegenerative diseases (Table
I).
Amyotrophic lateral sclerosis (ALS) and
frontotemporal dementia (FTD) have the same pathological and
genetic characteristics, and TDP-43 and FUS are closely related to
ASL/FTD (82-84,90,91). TDP-43 also accumulates in the
brains of patients with Alzheimer's disease (AD) (85,94). In the brains of patients with AD,
in addition to β-amyloid and tau protein, TDP-43 may also
accumulate in the limbic system; AD patients with full-length TDP
accumulation have more obvious FTD symptoms (85). The latest research suggested that
FUS is able to regulate the gene expression of acetylcholine
receptors at the neuromuscular junction and FUS mutations may
directly lead to ALS (91). In
addition, the accumulation of FUS mutations may activate the
protein kinase Hippo and JNK signaling pathways, accelerating
neuronal degeneration and apoptosis (90). RBPs, such as TDP-43 and FUS,
regulate the distribution and metabolism of RNA in cells by binding
to coding and non-coding RNA; these RBPs may interact and
participate in the pathogenesis of ALS/FTD (95,96). Clinical autopsy results suggested
the presence of a large number of TDP-43 accumulations in neurons
of TIA1 mutation carriers; TIA1 mutations may hinder the
decomposition of SGs and promote the accumulation of TDP-43 in
non-dynamic SGs (96).
Under stress, neurons may work with their
intracellular SGs to degrade a large amount of RNA through the
autophagy pathway (18,19). When the crisis is removed, SGs
are also eliminated through autophagy and the normal translation
process is restored (18,19).
However, under continuous stress, the chromosome 9 open reading
frame 72 mutation causes the SG autophagy pathway to be blocked and
promotes the accumulation of TDP-43 in the cytoplasm (97,98), leading to ALS/FTD (99,100). In addition, the methylation of
FUS arginine at R218 by protein arginine methyltransferase 5 is a
prerequisite step for the formation of the above-mentioned complex
and the maintenance of the SG clearance mechanism (99). Mutations in p62 may also reduce
the ability of neurons to clear SGs, resulting in neurotoxicity
(100). Therefore, regulating
the production/disaggregation balance of SGs in stress by arginine
methyltransferase and p62 kinase drugs may help reduce the
occurrence of neurodegenerative diseases. In the pathological
process of AD and ALS/FTD, RBPs and tau in SGs may also interact
with each other (94).
Transgenic animal (87) and
in vitro experiments (86) indicated that TIA1 is able to
promote tau phosphorylation and misfolding, and they then enter
pathological SGs together, affecting the normal metabolism of RNA;
tau may also regulate the distribution of TIA1 (86). In addition to long-term
potentiation (LTP), long-term depression (LTD) is also involved in
regulating the synaptic plasticity of hippocampal neurons and they
jointly affect the formation of different types of memory. The
phosphorylation of eIF2α may reduce LTP, increase mGluR-LTD and
affect learning and memory (101,102).
DJ-1 is a protein/nucleic acid desaccharase encoded
by the gene PARK7 related to familial Parkinson's disease (PD). It
has numerous functions, such as anti-oxidation, transcription
regulation and regulation of protein degradation. The expression of
DJ-1 helps delay the pathological process of neurodegenerative
diseases such as PD and AD (103). During acute stress, DJ-1 is
able to combine with SGs to jointly regulate RNA metabolism and
shunt, and exert a neuroprotective effect (104,105). However, under chronic stress
conditions, DJ-1 mutation promotes the transformation of SGs into
pathological SGs and promotes the occurrence of PD (104). Therefore, eIF2α
phosphorylation, liquid-solid phase transformation of SGs,
incorrect intracellular distribution of RBPs, RNA metabolism
disorders and interactions between RBPs and tau or DJ-1 may all be
targets for the development of drugs for neurodegenerative
diseases.
8. Conclusions
The production of SGs by eukaryotic cells is an
important means of resisting various stress injuries and
maintaining cell survival. SGs contain translation initiation
factors, untranslated mRNA and RBPs. During the stress process,
numerous signaling pathways/proteins affect the distribution,
assembly and degradation of SGs. SGs may also change a variety of
intracellular signaling pathways by isolating various messenger
proteins. Therefore, SGs may be combined with other mRNPs, such as
PBs, to become the 'stress signal processing center' of cells under
stress conditions. However, there are still numerous unknowns about
SGs. For instance, it remains elusive how various intracellular
signaling pathways regulate the initiation factors to affect the
initiation, nucleation and cross-linking process of SGs with RBPs.
Similarly, how various RBPs inside and outside SGs regulate the
storage, retranslation and degradation of specific mRNAs is also
undetermined. In the acute phase of stress, the
assembly/disassembly of SGs is in a dynamic equilibrium. As the
stress continues, SGs may transform into aggregated pathological
SGs. Possibly, varied signaling pathways engage in regulating the
assembly/disassembly of SGs under acute or chronic stress
conditions. The coding and non-coding mRNA components of SGs and
the relationships between different RBP mutations and diseases are
also worthy of in-depth study. Therefore, it is necessary to
clarify the various components of SGs, including mRNA and RBPs, and
the roadmap for the occurrence of various diseases, so as to
provide a theoretical basis for the design and development of drugs
for SG-related diseases.
Supplementary Data
Availability of data and materials
The datasets generated and/or used during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
All authors participated in the design and revision
of the manuscript. JW, YG and WO reviewed the literature and shared
the writing of several drafts of the manuscript. JC and XD provided
the figures and table. WO performed the final review of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
All procedures performed to produce Fig. 2 were performed with the approval
of the Institutional Animal Care and Use Committee (Zhejiang Normal
University, Jinhua, China) and in accordance with the laws and
regulations. The Care and Use Standard for Laboratory Animals
(China Ministry of Health publication) and the National Institute
of Health Guide for the Care and Use of Laboratory Animals (2011
edition) were followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
The authors thank Miss Sophie Ouyang (third-year
grade medical student of UPenn Nursing School, Bachelor of Science
in Nursing Program, Philadelphia, USA) for language editing the
article.
Funding
This work was supported by grants from the Natural Science
Foundation of Zhejiang, China (grant no. LY20H150001), the National
College Student Innovation and Entrepreneurship Training Program
(grant no. 201710345005) and the College Student Innovation and
Entrepreneurship Training Program of Zhejiang, China (grant no.
2019R404011).
References
|
1
|
Ivanov P, Kedersha N and Anderson P:
Stress granules and processing bodies in translational control.
Cold Spring Harb Perspect Biol. 11:a0328132019. View Article : Google Scholar
|
|
2
|
Hentze MW, Castello A, Schwarzl T and
Preiss T: A brave new world of RNA-binding proteins. Nat Rev Mol
Cell Biol. 19:327–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin Y and Brangwynne CP: Liquid phase
condensation in cell physiology and disease. Science.
357:eaaf43822017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riggs CL, Kedersha N, Ivanov P and
Anderson P: Mammalian stress granules and P bodies at a glance. J
Cell Sci. 133:jcs2424872020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heberle AM, Razquin Navas P,
Langelaar-Makkinje M, Kasack K, Sadik A, Faessler E, Hahn U,
Marx-Stoelting P, Opitz CA, Sers C, et al: The PI3K and MAPK/p38
pathways control stress granule assembly in a hierarchical manner.
Life Sci Alliance. 2:e2018002572019. View Article : Google Scholar :
|
|
6
|
Golob-Schwarzl N, Krassnig S, Toeglhofer
AM, Park YN, Gogg-Kamerer M, Vierlinger K, Schröder F, Rhee H,
Schicho R, Fickert P and Haybaeck J: New liver cancer biomarkers:
PI3K/AKT/mTOR pathway members and eukaryotic translation initiation
factors. Eur J Cancer. 83:56–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sfakianos AP, Mellor LE, Pang YF,
Kritsiligkou P, Needs H, Abou-Hamdan H, Désaubry L, Poulin GB, Ashe
MP and Whitmarsh AJ: The mTOR-S6 kinase pathway promotes stress
granule assembly. Cell Death Differ. 25:1766–1780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onomoto K, Yoneyama M, Fung G, Kato H and
Fujita T: Antiviral innate immunity and stress granule responses.
Trends Immunol. 35:420–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCormick C and Khaperskyy DA: Translation
inhibition and stress granules in the antiviral immune response.
Nat Rev Immunol. 17:647–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Leng K, Liu Y, Sun H, Gao J, Ren Q,
Zhou T, Dong J and Xia J: The impact of hyperglycaemia on
PKM2-mediated NLRP3 inflammasome/stress granule signalling in
macrophages and its correlation with plaque vulnerability: An in
vivo and in vitro study. Metabolism. 107:1542312020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herman AB, Silva Afonso M, Kelemen SE, Ray
M, Vrakas CN, Burke AC, Scalia RG, Moore K and Autieri MV:
Regulation of stress granule formation by inflammation, vascular
injury, and atherosclerosis. Arterioscler Thromb Vasc Biol.
39:2014–2027. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel A, Lee HO, Jawerth L, Maharana S,
Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et
al: A Liquid-to-Solid phase transition of the ALS Protein FUS
accelerated by disease mutation. Cell. 162:1066–1077. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramaswami M, Taylor JP and Parker R:
Altered ribostasis: RNA-protein granules in degenerative disorders.
Cell. 154:727–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambadipudi S, Biernat J, Riedel D,
Mandelkow E and Zweckstetter M: Liquid-liquid phase separation of
the microtubule-binding repeats of the Alzheimer-related protein
Tau. Nat Commu. 8:2752017. View Article : Google Scholar
|
|
15
|
Wegmann S, Eftekharzadeh B, Tepper K,
Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D,
Kamath T, Commins C, et al: Tau protein liquid-liquid phase
separation can initiate tau aggregation. EMBO J. 37:e980492018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kedersha N, Chen S, Gilks N, Li W, Miller
IJ, Stahl J and Anderson P: Evidence that ternary complex
(eIF2-GTP-tRNA(i) (Met))-deficient preinitiation complexes are core
constituents of mammalian stress granules. Mol Biol Cell.
13:195–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anderson P and Kedersha N: Visibly
stressed: The role of eIF2, TIA-1, and stress granules in protein
translation. Cell Stress Chaperones. 7:213–221. 2002. View Article : Google Scholar
|
|
18
|
Hofmann S, Kedersha N, Anderson P and
Ivanov P: Molecular mechanisms of stress granule assembly and
disassembly. Biochim Biophys Acta Mol Cell Res. 1868:1188762021.
View Article : Google Scholar
|
|
19
|
Wolozin B and Ivanov P: Stress granules
and neurodegeneration. Na Rev Neurosci. 20:649–666. 2019.
View Article : Google Scholar
|
|
20
|
Jain S, Wheeler JR, Walters RW, Agrawal A,
Barsic A and Parker R: ATPase-modulated stress granules contain a
diverse proteome and substructure. Cell. 164:487–498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Protter DSW and Parker R: Principles and
properties of stress granules. Trends Cell Biol. 26:668–679. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markmiller S, Soltanieh S, Server KL, Mak
R, Jin W, Fang MY, Luo EC, Krach F, Yang D, Sen A, et al:
Context-dependent and disease-specific diversity in protein
interactions within stress granules. Cell. 172:590–604.e13. 2018.
View Article : Google Scholar :
|
|
23
|
Kedersha N, Panas MD, Achorn CA, Lyons S,
Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P
and Anderson P: G3BP-Caprin1-USP10 complexes mediate stress granule
condensation and associate with 40S subunits. J Cell Biol.
212:845–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson P and Kedersha N: Stress
granules: The Tao of RNA triage. Trends Biochem Sci. 33:141–150.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaehler C, Isensee J, Hucho T, Lehrach H
and Krobitsch S: 5-fluorouracil affects assembly of stress granules
based on RNA incorporation. Nucleic Acids Res. 42:6436–6447. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujimura K, Sasaki AT and Anderson P:
Selenite targets eIF4E-binding protein-1 to inhibit translation
initiation and induce the assembly of non-canonical stress
granules. Nucleic Acids Res. 40:8099–8110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohn T, Kedersha N, Hickman T, Tisdale S
and Anderson P: A functional RNAi screen links O-GlcNAc
modification of ribosomal proteins to stress granule and processing
body assembly. Nat Cell Biol. 10:1224–1231. 2008. View Article : Google Scholar
|
|
28
|
Aulas A, Lyons SM, Fay MM, Anderson P and
Ivanov P: Nitric oxide triggers the assembly of 'type II' stress
granules linked to decreased cell viability. Cell Death Dis.
9:11292018. View Article : Google Scholar
|
|
29
|
Anderson P and Kedersha N: RNA granules. J
Cell Biol. 172:803–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang C, Chen Y, Dai H, Zhang H, Xie M,
Zhang H, Chen F, Kang X, Bai X and Chen Z: UBAP2L arginine
methylation by PRMT1 modulates stress granule assembly. Cell Death
Differ. 27:227–241. 2020. View Article : Google Scholar
|
|
31
|
Tsai WC, Gayatri S, Reineke LC, Sbardella
G, Bedford MT and Lloyd RE: Arginine demethylation of G3BP1
promotes stress granule assembly. J Biol Chemistry.
291:22671–22685. 2016. View Article : Google Scholar
|
|
32
|
Kedersha N and Anderson P: Stress
granules: Sites of mRNA triage that regulate mRNA stability and
translatability. Biochem Soc Trans. 30:963–969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mateju D, Eichenberger B, Voigt F,
Eglinger J, Roth G and Chao JA: Single-molecule imaging reveals
translation of mRNAs localized to stress granules. Cell.
183:1801–1812.e13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito
H and Takekawa M: Formation of stress granules inhibits apoptosis
by suppressing stress-responsive MAPK pathways. Nat Cell Biol.
10:1324–1332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park YJ, Choi DW, Cho SW, Han J, Yang S
and Choi CY: Stress granule formation attenuates RACK1-mediated
apoptotic cell death induced by morusin. Int J Mol Sci.
21:53602020. View Article : Google Scholar :
|
|
36
|
Panas MD, Ivanov P and Anderson P:
Mechanistic insights into mammalian stress granule dynamics. J Cell
Biol. 215:313–323. 2016. View Article : Google Scholar :
|
|
37
|
Tourrière H, Chebli K, Zekri L, Courselaud
B, Blanchard JM, Bertrand E and Tazi J: The RasGAP-associated
endoribonuclease G3BP assembles stress granules. J Cell Biol.
160:823–831. 2003. View Article : Google Scholar
|
|
38
|
Omer A, Patel D, Moran JL, Lian XJ, Di
Marco S and Gallouzi IE: Autophagy and heat-shock response impair
stress granule assembly during cellular senescence. Mech Ageing
Dev. 192:1113822020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Omer A, Barrera MC, Moran JL, Lian XJ, Di
Marco S, Beausejour C and Gallouzi IE: G3BP1 controls the
senescence-associated secretome and its impact on cancer
progression. Nat Commun. 11:49792020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anderson P, Kedersha N and Ivanov P:
Stress granules, P-bodies and cancer. Biochim Biophys Acta.
1849:861–870. 2015. View Article : Google Scholar :
|
|
42
|
El-Naggar AM and Sorensen PH:
Translational control of aberrant stress responses as a hallmark of
cancer. J Pathol. 244:650–666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vilas-Boas Fde A, da Silva AM, de Sousa
LP, Lima KM, Vago JP, Bittencourt LF, Dantas AE, Gomes DA, Vilela
MC, Teixeira MM and Barcelos LS: Impairment of stress granule
assembly via inhibition of the eIF2alpha phosphorylation sensitizes
glioma cells to chemotherapeutic agents. J Neurooncol. 127:253–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fournier MJ, Gareau C and Mazroui R: The
chemotherapeutic agent bortezomib induces the formation of stress
granules. Cancer Cell Int. 10:122010. View Article : Google Scholar :
|
|
45
|
Gao X, Jiang L, Gong Y, Chen X, Ying M,
Zhu H, He Q, Yang B and Cao J: Stress granule: A promising target
for cancer treatment. Br J Pharmacol. 176:4421–4433. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khan FH, Dervan E, Bhattacharyya DD,
McAuliffe JD, Miranda KM and Glynn SA: The role of nitric oxide in
cancer: Master regulator or NOt? Int J Mol Sci. 21:93932020.
View Article : Google Scholar :
|
|
47
|
Alam U and Kennedy D: G3BP1 and G3BP2
regulate translation of interferon-stimulated genes: IFITM1, IFITM2
and IFITM3 in the cancer cell line MCF7. Mol Cell Biochem.
459:189–204. 2019. View Article : Google Scholar
|
|
48
|
Gupta N, Badeaux M, Liu Y, Naxerova K,
Sgroi D, Munn LL, Jain RK and Garkavtsev I: Stress
granule-associated protein G3BP2 regulates breast tumor initiation.
Proc Natl Acad Sci USA. 114:1033–1038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Somasekharan SP, El-Naggar A, Leprivier G,
Cheng H, Hajee S, Grunewald TG, Zhang F, Ng T, Delattre O,
Evdokimova V, et al: YB-1 regulates stress granule formation and
tumor progression by translationally activating G3BP1. J Cell Biol.
208:913–929. 2015. View Article : Google Scholar :
|
|
50
|
Ramachandran B, Stabley JN, Cheng SL,
Behrmann AS, Gay A, Li L, Mead M, Kozlitina J, Lemoff A, Mirzaei H,
et al: A GTPase-activating protein-binding protein
(G3BP1)/antiviral protein relay conveys arteriosclerotic Wnt
signals in aortic smooth muscle cells. J Biol Chem. 293:7942–7968.
2018. View Article : Google Scholar :
|
|
51
|
Schneider JW, Oommen S, Qureshi MY,
Goetsch SC, Pease DR, Sundsbak RS, Guo W, Sun M, Sun H, Kuroyanagi
H, et al: Dysregulated ribonucleoprotein granules promote
cardiomyopathy in RBM20 gene-edited pigs. Nat Med. 26:1788–1800.
2020. View Article : Google Scholar
|
|
52
|
Smit M, Coetzee AR and Lochner A: The
pathophysiology of myocardial ischemia and perioperative myocardial
infarction. J Cardiothorac Vasc Anesth. 34:2501–2512. 2020.
View Article : Google Scholar
|
|
53
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mahboubi H and Stochaj U: Cytoplasmic
stress granules: Dynamic modulators of cell signaling and disease.
Biochim Biophys Acta Mol Basis Dis. 1863:884–895. 2017. View Article : Google Scholar
|
|
55
|
Yoneyama M, Jogi M and Onomoto K:
Regulation of antiviral innate immune signaling by stress-induced
RNA granules. J Biochem. 159:279–286. 2016.PubMed/NCBI
|
|
56
|
Xu S, Chen D, Chen D, Hu Q, Zhou L, Ge X,
Han J, Guo X and Yang H: Pseudorabies virus infection inhibits
stress granules formation via dephosphorylating eIF2α. Vet
Microbiol. 247:1087862020. View Article : Google Scholar
|
|
57
|
Khong A, Kerr CH, Yeung CHL, Keatings K,
Nayak A, Allan DW and Jan E: Disruption of stress granule formation
by the multifunctional cricket paralysis virus 1A protein. J Virol.
91:e01779–16. 2017. View Article : Google Scholar :
|
|
58
|
Visser LJ, Medina GN, Rabouw HH, de Groot
RJ, Langereis MA, de Los Santos T and van Kuppeveld FJM:
Foot-and-Mouth disease virus leader protease cleaves G3BP1 and
G3BP2 and inhibits stress granule formation. J Virol. 93:e00922–18.
2019. View Article : Google Scholar :
|
|
59
|
Dougherty JD, Tsai WC and Lloyd RE:
Multiple poliovirus proteins repress cytoplasmic RNA granules.
Viruses. 7:6127–6140. 2015. View Article : Google Scholar
|
|
60
|
Yang X, Hu Z, Fan S, Zhang Q, Zhong Y, Guo
D, Qin Y and Chen M: Picornavirus 2A protease regulates stress
granule formation to facilitate viral translation. PLoS Pathog.
14:e10069012018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Le Sage V, Cinti A, McCarthy S, Amorim R,
Rao S, Daino GL, Tramontano E, Branch DR and Mouland AJ: Ebola
virus VP35 blocks stress granule assembly. Virology. 502:73–83.
2017. View Article : Google Scholar
|
|
62
|
Savastano A, Ibáñez de Opakua A, Rankovic
M and Zweckstetter M: Nucleocapsid protein of SARS-CoV-2 phase
separates into RNA-rich polymerase-containing condensates. Nat
Commun. 11:60412020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang J, Shi C, Xu Q and Yin H: SARS-CoV-2
nucleocapsid protein undergoes liquid-liquid phase separation into
stress granules through its N-terminal intrinsically disordered
region. Cell Discov. 7:52021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lu S, Ye Q, Singh D, Cao Y, Diedrich JK,
Yates JR III, Villa E, Cleveland DW and Corbett KD: The SARS-CoV-2
nucleocapsid phosphoprotein forms mutually exclusive condensates
with RNA and the membrane-associated M protein. Nat Commun.
12:5022021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Prasad K, Alasmari AF, Ali N, Khan R,
Alghamdi A and Kumar V: Insights into the SARS-CoV-2-Mediated
alteration in the stress granule protein regulatory networks in
humans. Pathogens. 10:14592021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thoms M, Buschauer R, Ameismeier M, Koepke
L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T,
Cheng J, et al: Structural basis for translational shutdown and
immune evasion by the Nsp1 protein of SARS-CoV-2. Science.
369:1249–1255. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shi M, Wang L, Fontana P, Vora S, Zhang Y,
Fu TM, Lieberman J and Wu H: SARS-CoV-2 Nsp1 suppresses host but
not viral translation through a bipartite mechanism. bioRxiv.
2020:3029012020.
|
|
68
|
Schubert K, Karousis ED, Jomaa A, Scaiola
A, Echeverria B, Gurzeler LA, Leibundgut M, Thiel V, Mühlemann O
and Ban N: SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to
inhibit translation. Nat Struct Mol Biol. 27:959–966. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nakagawa K, Narayanan K, Wada M and Makino
S: Inhibition of stress granule formation by middle east
respiratory syndrome coronavirus 4a accessory protein facilitates
viral translation, leading to efficient virus replication. J Virol.
92:e00902–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Callaway E: Heavily mutated omicron
variant puts scientists on alert. Nature. 600:212021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dudman J and Qi X: Stress granule
dysregulation in amyotrophic lateral sclerosis. Front Cell
Neurosci. 14:5985172020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Anderson EN, Gochenaur L, Singh A, Grant
R, Patel K, Watkins S, Wu JY and Pandey UB: Traumatic injury
induces stress granule formation and enhances motor dysfunctions in
ALS/FTD models. Hum Mol Genet. 27:1366–1381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ayuso MI, Martínez-Alonso E, Regidor I and
Alcázar A: Stress granule induction after brain ischemia is
independent of eukaryotic translation initiation factor (eIF) 2α
phosphorylation and is correlated with a decrease in eIF4B and
eIF4E proteins. J Biol Chemistry. 291:27252–27264. 2016. View Article : Google Scholar
|
|
74
|
Correia AS, Patel P, Dutta K and Julien
JP: Inflammation induces TDP-43 mislocalization and aggregation.
PLoS One. 10:e01402482015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cao X, Jin X and Liu B: The involvement of
stress granules in aging and aging-associated diseases. Aging Cell.
19:e131362020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cruz A, Verma M and Wolozin B: The
Pathophysiology of tau and stress granules in disease. Adv Exp Med
Biol. 1184:359–372. 2019. View Article : Google Scholar
|
|
77
|
Webber CJ, Lei SE and Wolozin B: The
pathophysiology of neurodegenerative disease: Disturbing the
balance between phase separation and irreversible aggregation. Prog
Mol Biol Transl Sci. 174:187–223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rozpędek-Kamińska W, Siwecka N,
Wawrzynkiewicz A, Wojtczak R, Pytel D, Diehl JA and Majsterek I:
The PERK-dependent molecular mechanisms as a novel therapeutic
target for neurodegenerative diseases. Int J Mol Sci. 21:21082020.
View Article : Google Scholar
|
|
79
|
Ma T, Trinh MA, Wexler AJ, Bourbon C,
Gatti E, Pierre P, Cavener DR and Klann E: Suppression of eIF2α
kinases alleviates Alzheimer's disease-related plasticity and
memory deficits. Nat Neurosci. 16:1299–1305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim HJ, Raphael AR, LaDow ES, McGurk L,
Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD and
Bonini NM: Therapeutic modulation of eIF2α phosphorylation rescues
TDP-43 toxicity in amyotrophic lateral sclerosis disease models.
Nat Genet. 46:152–160. 2014. View Article : Google Scholar
|
|
81
|
Banani SF, Lee HO, Hyman AA and Rosen MK:
Biomolecular condensates: Organizers of cellular biochemistry. Nat
Rev Mol Cell Biol. 18:285–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Flores BN, Li X, Malik AM, Martinez J, Beg
AA and Barmada SJ: An intramolecular salt bridge linking TDP43 RNA
binding, protein stability, and TDP43-dependent neurodegeneration.
Cell Rep. 27:1133–1150.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Archbold HC, Jackson KL, Arora A, Weskamp
K, Tank EM, Li X, Miguez R, Dayton RD, Tamir S, Klein RL and
Barmada SJ: TDP43 nuclear export and neurodegeneration in models of
amyotrophic lateral sclerosis and frontotemporal dementia. Sci Rep.
8:46062018. View Article : Google Scholar :
|
|
84
|
Suk TR and Rousseaux MWC: The role of
TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol
Neurodegener. 15:452020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tomé SO, Vandenberghe R, Ospitalieri S,
Van Schoor E, Tousseyn T, Otto M, von Arnim CAF and Thal DR:
Distinct molecular patterns of TDP-43 pathology in Alzheimer's
disease: Relationship with clinical phenotypes. Acta Neuropathol
Commun. 8:612020. View Article : Google Scholar
|
|
86
|
Vanderweyde T, Apicco DJ, Youmans-Kidder
K, Ash PEA, Cook C, Lummertz da Rocha E, Jansen-West K, Frame AA,
Citro A, Leszyk JD, et al: Interaction of tau with the RNA-binding
Protein TIA1 regulates tau pathophysiology and toxicity. Cell Rep.
15:1455–1466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Apicco DJ, Ash PEA, Maziuk B, LeBlang C,
Medalla M, Al Abdullatif A, Ferragud A, Botelho E, Ballance HI,
Dhawan U, et al: Reducing the RNA binding protein TIA1 protects
against tau-mediated neurodegeneration in vivo. Nat Neurosci.
21:72–80. 2018. View Article : Google Scholar
|
|
88
|
Gal J, Kuang L, Barnett KR, Zhu BZ,
Shissler SC, Korotkov KV, Hayward LJ, Kasarskis EJ and Zhu H: ALS
mutant SOD1 interacts with G3BP1 and affects stress granule
dynamics. Acta Neuropathol. 132:563–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sidibé H, Dubinski A and Vande Velde C:
The multi-functional RNA-binding protein G3BP1 and its potential
implication in neurodegenerative disease. J Neurochem. 157:944–962.
2021. View Article : Google Scholar
|
|
90
|
Gogia N, Sarkar A, Mehta AS, Ramesh N,
Deshpande P, Kango-Singh M, Pandey UB and Singh A: Inactivation of
Hippo and cJun-N-terminal Kinase (JNK) signaling mitigate FUS
mediated neurodegeneration in vivo. Neurobio Dis. 140:1048372020.
View Article : Google Scholar
|
|
91
|
Picchiarelli G, Demestre M, Zuko A, Been
M, Higelin J, Dieterlé S, Goy MA, Mallik M, Sellier C,
Scekic-Zahirovic J, et al: FUS-mediated regulation of acetylcholine
receptor transcription at neuromuscular junctions is compromised in
amyotrophic lateral sclerosis. Nat Neurosci. 22:1793–1805. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fifita JA, Zhang KY, Galper J, Williams
KL, McCann EP, Hogan AL, Saunders N, Bauer D, Tarr IS, Pamphlett R,
et al: Genetic and pathological assessment of hnRNPA1, hnRNPA2/B1,
and hnRNPA3 in familial and sporadic amyotrophic lateral sclerosis.
Neurodegener Dis. 17:304–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim HJ, Kim NC, Wang YD, Scarborough EA,
Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al:
Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause
multi-system proteinopathy and ALS. Nature. 495:467–473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Montalbano M, McAllen S, Cascio FL,
Sengupta U, Garcia S, Bhatt N, Ellsworth A, Heidelman EA, Johnson
OD, Doskocil S and Kayed R: TDP-43 and tau oligomers in Alzheimer's
disease, amyotrophic lateral sclerosis, and frontotemporal
dementia. Neurobiol Dis. 146:1051302020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao M, Kim JR, van Bruggen R and Park J:
RNA-binding proteins in amyotrophic lateral sclerosis. Mol Cells.
41:818–829. 2018.PubMed/NCBI
|
|
96
|
Mackenzie IR, Nicholson AM, Sarkar M,
Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB,
Kurti A, et al: TIA1 mutations in amyotrophic lateral sclerosis and
frontotemporal dementia promote phase separation and alter stress
granule dynamics. Neuron. 95:808–816.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chew J, Gendron TF, Prudencio M, Sasaguri
H, Zhang YJ, Castanedes-Casey M, Lee CW, Jansen-West K, Kurti A,
Murray ME, et al: Neurodegeneration. C9ORF72 repeat expansions in
mice cause TDP-43 pathology, neuronal loss, and behavioral
deficits. Science. 348:1151–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chew J, Cook C, Gendron TF, Jansen-West K,
Del Rosso G, Daughrity LM, Castanedes-Casey M, Kurti A, Stankowski
JN, Disney MD, et al: Aberrant deposition of stress
granule-resident proteins linked to C9orf72-associated TDP-43
proteinopathy. Mol Neurodegener. 14:92019. View Article : Google Scholar :
|
|
99
|
Chitiprolu M, Jagow C, Tremblay V,
Bondy-Chorney E, Paris G, Savard A, Palidwor G, Barry FA, Zinman L,
Keith J, et al: A complex of C9ORF72 and p62 uses arginine
methylation to eliminate stress granules by autophagy. Nat Commun.
9:27942018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Deng Z, Lim J, Wang Q, Purtell K, Wu S,
Palomo GM, Tan H, Manfredi G, Zhao Y, Peng J, et al:
ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy
and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy.
16:917–931. 2020. View Article : Google Scholar :
|
|
101
|
Jiang Z, Belforte JE, Lu Y, Yabe Y, Pickel
J, Smith CB, Je HS, Lu B and Nakazawa K: eIF2alpha
Phosphorylation-dependent translation in CA1 pyramidal cells
impairs hippocampal memory consolidation without affecting general
translation. J Neurosci. 30:2582–2594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Trinh MA, Ma T, Kaphzan H, Bhattacharya A,
Antion MD, Cavener DR, Hoeffer CA and Klann E: The eIF2α kinase
PERK limits the expression of hippocampal metabotropic glutamate
receptor-dependent long-term depression. Learn Mem. 21:298–304.
2014. View Article : Google Scholar :
|
|
103
|
Hijioka M, Inden M, Yanagisawa D and
Kitamura Y: DJ-1/PARK7: A new therapeutic target for
neurodegenerative disorders. Biol Pharm Bull. 40:548–552. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Repici M, Hassanjani M, Maddison DC,
Garção P, Cimini S, Patel B, Szegö ÉM, Straatman KR, Lilley KS,
Borsello T, et al: The Parkinson's disease-linked protein DJ-1
associates with cytoplasmic mRNP granules during stress and
neurodegeneration. Mol Neurobiol. 56:61–77. 2019. View Article : Google Scholar
|
|
105
|
Ma J, Wu R, Zhang Q, Wu JB, Lou J, Zheng
Z, Ding JQ and Yuan Z: DJ-1 interacts with RACK1 and protects
neurons from oxidative-stress-induced apoptosis. Biochem J.
462:489–497. 2014. View Article : Google Scholar
|