Introduction
The most common orthopedic diseases include
osteoarthritis (OA) and osteoporosis. OA is defined as a joint
disease, characterized by cartilage degeneration, abnormal
remodeling of subchondral bone and joint pain (1), and occurs in aging populations.
However, previous research has demonstrated that subchondral bone
hardens and cartilage degenerates during disease proression.
Anderson-MacKenzie et al (2) reported that the role of subchondral
bone was mainly to reduce impact forces and mitigate cartilage
damage. In addition, subchondral bone density and cartilage
thickness are positively associated in knees without OA, and
negatively associated in knees with OA, in support of the
subchondral bone/chondral functional unit theory. These conclusions
also contribute to the improvement of the understanding of the
pathophysiology of OA (3).
Furthermore, Neve et al (4) studied osteoblasts, which are
mononuclear mesenchymal stem cell-derived cells responsible for
osteogenesis and mineralization in early bone formation and
subsequent bone remodeling, while OA osteoblasts exhibit an
abnormal metabolic behavior. Maruotti et al (5) revealed that osteoblast disorders
play a crucial role in the pathogenesis of OA, stimulating the
osteoblast-induced production of large transcription factor
quantities, growth factors and other proteins involved in OA
pathogenesis. In addition, Di Pompo et al (6) previously reported that osteoblasts
promoted the secretion of osteoclastic cytokines and inflammatory
mediators in an acidic environment (pH 6.8), further exacerbating
OA.
The signaling pathways form a complex network of
molecular interactions in osteoblasts. It has been reported that
the Wnt signaling pathway is involved in OA, in which signaling
molecules and regulatory factors are aberrantly activated or
inhibited (7). The activation of
the classical Wnt/β-catenin signaling pathway increases subchondral
bone remodeling and osteophyte formation, which are two
pathological processes in bone also associated with OA progression
(8). Similarly, decreased levels
of Wnt activity are important for maintaining the viability of
osteocytes (both chondrocytes and osteoblasts); however, excessive
activation has been found to be associated with joint damage
(9).
Osteoblasts and their precursors can control various
Wnt ligands and are involved in the regulation of bone remodeling;
the Wnt signaling may regulate chondrocyte and osteoblast
differentiation and function via classical (β-catenin-dependent) or
non-classical Wnt signaling pathways, as well as cross-linking with
other signaling pathways, affecting cartilage and bone metabolism.
In addition, the excessive activation of the Wnt signaling pathway
may exacerbate cartilage OA-like degeneration (10,11). β-catenin has been reported to be
essential for the differentiation of osteoblasts, and the
overexpression of constitutively active β-catenin forms may also
lead to the development of OA, characterized by increased
chondrocyte hypertrophy (12,13). Thus, the dysregulation of the
Wnt/β-catenin signaling pathway may lead to OA.
The Dickkopf (DKK) family has been reported to
regulate the Wnt signaling pathway by binding to the Wnt receptor
complex (14). Chen et al
(15) demonstrated that the
local injection of DKK1 knockout lentivirus into the rat femur
alleviated the progression of AONFH; Wnt/β-catenin signalling was
activated, the number of empty fat cavities was reduced, the blood
supply was restored and bone formation was significantly increased.
Among DKK family members, Dickkopf-related protein 3 (DKK3) is an
atypical member of the DKK family of Wnt antagonists; human DKK-1,
-2 and -4 are located in the same homology group, whereas DKK3 does
not belong to this group (16).
Furthermore, the mechanisms of action of DKK3 differ from those of
other proteins in the DDK gene family. Lee et al (17) demonstrated that DKK3 attenuated
β-catenin protein expression and its transcriptional activity via
the interaction with β-transducin repeat-containing protein
(β-TrCP) and prevented the translocation of β-catenin to the
nucleus. DKK3 expression levels in the subchondral bone, as well as
its role in osteocyte formation have not yet been fully elucidated.
Therefore, the aim of the present study was to investigate the DKK3
expression levels in osteoblasts in subchondral bone from human
knees with OA, in order to clarify the role of DKK3 in abnormal
bone remodeling in subchondral bone and to determine its potential
association with β-catenin expression level changes.
Materials and methods
Materials
A total of 38 osteotomy specimens (Table I) were collected from the medial
tibial plateau of the knee joint of patients from January, 2019 to
October, 2020 at the Third Department of Orthopedics, General
Hospital of Ningxia Medical University (Yinchuan, China). Tissue
samples from three cases with a normal and five cases with a mild
OA symptomatology were obtained from young patients who underwent
lower limb amputations. Tissue samples from 10 cases of moderate OA
symptomatology were collected from patients diagnosed with OA and
who underwent knee arthroplasty, including single condyle
replacement. Tissue samples from 20 cases with severe OA
symptomatology were also collected, including a case of tibial
plateau osteotomy that was diagnosed with OA in a patient
undergoing total knee replacement. Samples were acquired from 16
male, and 22 female patients, aged 20-80 years, with an average age
of 62 years. The patients included in the present study had no
family history of malignant tumors, hemiplegia, or death from
stroke, and no patients had undergone surgery on the ipsilateral
hip. In total, 23 patients were diagnosed with hypertension (20
patients had grade I and 3 had grade II hypertension), and none
were diagnosed with type 2 diabetes. In addition, 1 patient had a
giant cell tumor of the ipsilateral pelvis (Jaffe grade II). During
tissue collection, all samples were immediately stored in liquid
nitrogen, at −147°C. The sample cohort was divided into two equal
parts, following sample collection. The samples in the first group
were fixed with neutral formalin for 24 h, decalcified with fast
decalcification liquid for 1 week, and then embedded in paraffin,
as described below. Subchondral bone was retained after cartilage
removal in the other sample group, and stored in a refrigerator at
-80°C for later use. The present study was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University
(approval no. KYLL-2020-20; approval date, January 14, 2021), and
written informed consent was obtained from all the enrolled
patients.
 | Table IClinicopathological data of the
patients in the present study. |
Table I
Clinicopathological data of the
patients in the present study.
| Patient no. | Sex | Age (years) | Severity of
symptoms | Left or right
knee |
|---|
| 1 | M | 65 | Moderate | Right |
| 2 | F | 62 | Severe | Right |
| 3 | M | 59 | Severe | Right |
| 4 | F | 40 | Normal | Left |
| 5 | M | 68 | Severe | Left |
| 6 | F | 41 | Mild | Left |
| 7 | M | 80 | Severe | Right |
| 8 | F | 72 | Moderate | Right |
| 9 | M | 32 | Normal | Right |
| 10 | F | 66 | Severe | Right |
| 11 | F | 80 | Moderate | Left |
| 12 | M | 80 | Severe | Left |
| 13 | F | 78 | Severe | Right |
| 14 | F | 59 | Moderate | Left |
| 15 | M | 55 | Moderate | Right |
| 16 | M | 63 | Severe | Left |
| 17 | F | 61 | Moderate | Right |
| 18 | M | 69 | Severe | Right |
| 19 | F | 44 | Mild | Left |
| 20 | M | 64 | Moderate | Left |
| 21 | M | 72 | Moderate | Right |
| 22 | M | 78 | Severe | Right |
| 23 | F | 67 | Severe | Left |
| 24 | M | 20 | Normal | Left |
| 25 | M | 62 | Severe | Left |
| 26 | F | 67 | Severe | Right |
| 27 | F | 62 | Moderate | Right |
| 28 | M | 78 | Severe | Left |
| 29 | F | 58 | Severe | Left |
| 30 | M | 70 | Severe | Left |
| 31 | F | 43 | Mild | Right |
| 32 | M | 64 | Moderate | Left |
| 33 | F | 68 | Severe | Left |
| 34 | M | 45 | Mild | Right |
| 35 | M | 70 | Severe | Right |
| 36 | M | 79 | Severe | Left |
| 37 | M | 76 | Severe | Right |
| 38 | M | 40 | Mild | Right |
Experimental methods
The samples were divided into the normal, mild,
moderate and severe symptom groups, according to the Osteoarthritis
Research Society International (OARSI) (18), and the distribution and sclerosis
of medial osteoblasts were observed following hematoxylin and eosin
(H&E), Safranin O/fast green staining and alkaline phosphatase
(ALP; AZO method) as described below. Along with the increase in
the osteoarthritis pathology degree, alterations in the number of
subchondral osteoblasts were also observed. Changes in DKK3 and
β-catenin expression in the subchondral osteoblasts of the medial
tibial plateau were observed and detected using immunohistochemical
staining, immunofluorescence, western blot analysis and reverse
transcription-quantitative PCR (RT-qPCR) as described below.
Main reagents
Anti-DKK3 antibody (EPR15611; ab186409; rabbit
monoclonal) was purchased from Abcam, and β-catenin antibody
(ab32572; rabbit monoclonal) and GAPDH antibody (ab9485) were
obtained from Abcam. RIPA lysate, 50X cocktail, PMSF (100 mM),
phosphorylated protease inhibitor, BCA protein quantitative
detection kit, 5X protein loading buffer and the SDS-PAGE gel
preparation kit were acquired from Wuhan Google Biotechnology Co.,
Ltd.; the protein marker was acquired from Thermo Fisher
Scientific, Inc. The PVDF membrane was acquired from
MilliporeSigma; and TRIzol Universal and Trizol® Reagent
was purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Histological analysis
All obtained tissues were rinsed with normal saline
three times for 15 min each and fixed in 4% neutral formaldehyde
solution for 24 h. Subsequently, the tissues were incubated in a
rapid decalcification solution (Beijing Zhuoding Biological
Company) for decalcification for 7 days and immersed in PBS
solution for 6 h, with PBS replacement every 1 h. Tissues were
trimmed to a size of ~5×5×3 mm and placed in a plastic embedding
box for subsequent processing. The tissues were sequentially placed
in 50, 70, 80, 95 and 100% ethanol for 10 min each at first, and
subsequently in a 100% ethanol/xylene (2:1) solution, 100%
ethanol/xylene (1:1) solution, 100% ethanol/xylene (1:2) solution,
xylene I, II, and III for 10 min per step. The paraffin wax was
melted at 60°C in an electric heating constant temperature
incubator (constant temperature chamber; Xiaogan Yaguang
Medical-electronic Technology Co., Ltd.), placed afterwards on
tissue paraffin glass for embedding, and xylene was then used to
melt the paraffin wax in order to create a mixed solution. The
tissues were then placed consecutively in xylene/paraffin (2:1)
solution, xylene/paraffin (1:1) solution, and xylene/paraffin (1:2)
solution for 30 min per step. Finally, the tissues were placed in
paraffin I and II in a thermostat at 60°C for 1.5 h,
respectively.
H&E staining
Tissue samples were dewaxed by soaking the sections
in xylene I and II for 10 min, rinsing with distilled water for 30
sec, then soaking consecutively in 100 (I, II), 90, 80 and 70%
alcohol for 5 min, and finally rinsing with tap water for 5 min
three times. The samples were stained with hematoxylin (Beijing
Solarbio Science & Technology Co., Ltd.) for 5 min at room
temperature and rinsed with running water. Samples were
differentiated with 5% acetic acid for 1 min, washed with running
water for 10 min, stained with eosin (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 min at room temperature, and
rinsed three times with running water, for 5 min. The slices were
dehydrated by placing them in 70, 80, 90 and 100% alcohol and
xylene for 1 min each, and were then sealed with neutral glue, and
covered with a cover slip. The tissues were examined using a Leica
microscope (Leica DM2700 M; Leica Microsystems GmbH).
Safranin O/fast green staining
The dewaxing procedure was performed according to
the H&E staining protocol, as described above. The sections
were stained with hematoxylin for 5 min and rinsed with tap water
for 10 min. The sections were dyed with 0.3% solid green for 3 min
and then washed with tap water for 5 min three times. The slices
were placed in 1% acetic acid for 5 sec, then immersed in 1%
safranin staining solution (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 min at room temperature, and then
rinsed three times with distilled water for 5 min. Finally, the
samples were dehydrated and sealed as process as described above
for H&E staining. The samples were examined using a Leica
microscope (Leica Microsystems GmbH).
The final score was based on the Safranin O/fast
green staining results and the OARSI scoring items. The OARIS score
was obtained by multiplying the histological grading of cartilage
degeneration (in 6 grades) by the histological staging of cartilage
degeneration (in five stages) following Safranin O/fast green
staining.
ALP staining (AZO coupling method)
The dewaxing procedure was performed as described
above for the H&E staining protocol. Firstly, the working fluid
was prepared. ALP staining solution B1 (AS-BI staining solution)
and alkaline phosphatase staining solution B2 (FBB staining
solution) (both stains from Beijing Solarbio Science &
Technology Co., Ltd.) were mixed in a volume ratio of 1:1, and the
prepared ALP incubation solution was then dropped onto the cut
sections. The sections were then incubated in a dark incubator for
20 min at 37°C and washed with water for 5 min. The sections were
immersed in nuclear hard red staining solution for counterstaining
for 3 min at room temperature. Finally, samples were dehydrated and
sealed as described above previously described. The samples were
examined using a Leica microscope (Leica Microsystems GmbH).
Immunohistochemical staining
The dewaxing procedure was performed as described
above. A total of 20 µl 0.1% trypsin solution was added to
the tissue, incubated in an incubator at 37°C for 15 min, and
soaked in PBS for 15 min. Endogenous peroxidase blocker (Beijing
Zhongshan Jinqiao Biological Co., Ltd.) was then added (20
µl) to the tissue, incubated at 37°C for 10 min, and soaked
in PBS solution for 15 min, followed by the addition of goat serum
(Beijing Solarbio Science & Technology Co., Ltd.) and
incubation at 37°C for 30 min. Afterwards, the primary antibodies
[DKK3 antibody (goat anti-rabbit, 1:100, ab186409, Abcam) and
β-catenin antibody (goat anti-rabbit, 1:150, ab32572, Abcam)] were
added in a dropwise manner to the tissue and incubated overnight in
a refrigerator at 4°C. The secondary antibody used was enhanced
goat anti-rabbit IgG polymer (1:500, PV-9000, 37°C, 40 min; Beijing
Zhongshan Jinqiao Biological Co., Ltd.). The slides were removed
warmed to 37°C for 40 min the following day. Immunohistochemical
detection reagent 2 (Beijing Zhongshan Jinqiao Biological Co.,
Ltd.) was then added in a dropwise manner to the tissues. The
tissues were incubated in an incubator at 37°C for 30 min and
soaked in PBS solution for 15 min. Immunohistochemical detection
reagent 3 (Beijing Zhongshan Jinqiao Biological Co., Ltd.) was
dropped onto the tissues. The samples were placed in an incubator
at 37°C and incubated for 40 min. Subsequently, the samples were
removed from the incubator and soaked in PBS solution for 15 min at
room temperature. DAB chromogenic solution (Beijing Zhongshan
Jinqiao Biological Co., Ltd.) was then added in a dropwise manner
to the tissues, and they were observed under an optical microscope
(Leica Microsystems GmbH) for 1 min, and soaked in distilled water
for 5 min. The cells were then stained with hematoxylin for 5 min
at room temperture, differentiated with 5% acetic acid for 1 min,
and rinsed with tap water for 10 min. Samples were dehydrated and
sealed as described in the H&E staining section above. Finally,
analysis was performed based on the experimental results. Tissues
were counted in areas of high expression, and with the use of
ImageJ (1.8.0) software (National Institutes of Health) was used to
determine the intensity of protein expression. Afterwards, the
histological score was calculated as the average optical density of
positively stained osteoblasts, which equals to the ratio of the
overall optical density of the positive cells to the positive area.
Positive cells are those with clear brownish-yellow granules in the
cytoplasm or nucleus.
Immunofluorescence
The procedure for the first day of the
immunofluorescence protocol was the same as that used for
immunohistochemistry (described above). The following day, the
slices were warmed to room temperature for 40 min. Under dark
conditions, goat anti-rabbit IgG H&L (Cy3; S0011, Affinity
Biosciences, Ltd.) fluorescent secondary antibody (1:500) was added
to the tissues and incubated for 1 h at 37°C. The samples were then
soaked and washed in PBS for 15 min. DAPI (Beijing Solarbio Science
& Technology Co., Ltd.) was then added in a dropwise manner in
the dark and incubated at room temperature for 5 min at room
temperature. The samples were then soaked in PBS solution for 4
min, followed by anti-fluorescence attenuation sealer (containing
DAPI) drops on the tissue for mounting. The samples were examined
using a Leica microscope (DM4B; Leica Microsystems GmbH). For
immunofluorescence analysis, in a place with high light intensity,
under a 40X lens, the optical density value was analyzed under the
same area and was equal to the ratio of the overall optical density
to the area.
Western blot analysis
All tissues used were removed from −80°C.
Subsequently, the samples were washed with cold PBS 2-3 times for
blood stain removal, cut into small sections (3×3×5 mm) using a
tissue grinder, and placed in a homogenization tube (stored in a
liquid nitrogen tank). Subsequently, a total protein extraction kit
[containing lysate, phosphatase inhibitor (100X), protease
inhibitor (100X), PMSF (100X; Beijing Zhongshan Jinqiao Biological
Co.)] was applied for protein extraction. The homogenization tube
was placed in the homogenizer, the program was selected to
homogenize thoroughly so that the bone texture was 120 sec and was
intermittently cooled during the period (liquid nitrogen was
automatically added intermittently). The homogenized sample tube
was removed, iced for 30 min, and shaken every 5 min to ensure that
the tissue was completely lysed. The supernatant, which is the
total protein solution, was collected and centrifuged at 4°C at
16,000 × g for 10 min. The undenatured protein solution was
determined using the BCA protein concentration determination kit
method and the microplate reader [ESCO (Shanghai) Enterprise
Development Co., Ltd.] was used to measure the protein
concentration (referring to the kit instructions for the specific
method), and the protein concentration was then calculated. The
protein solution was added to 5X protein loading buffer at a ratio
of 4:1 and denatured in a boiling water bath for 15 min.
Subsequently, a 10% SDS-PAGE gel was prepared and 15 µl
protein per sample was loaded in equal volumes of sample (including
markers) into the gel wells. Electrophoresis was performed (80 V
for concentrated gel, 120 V for separation gel, 300 mA for 40 min)
and gel was transferred to a PVDF membrane (MilliporeSigma). The
membrane was washed with TBST three times for 5 min, blocked with
5% skim milk for 1.5 h at room temperature, and incubated with the
primary anti-bodies [anti-DKK3 antibody (ab186409; rabbit
monoclonal, 1:300), anti-GAPDH antibody (ab9485, Abcam, rabbit
monoclonal, 1:300) and anti-β-catenin antibody (ab32572, Abcam,
rabbit monoclonal, 1:200)] at 4°C overnight. On the second day, the
samples were moved to room temperature conditions for 40 min and
the membrane was then washed with TBST three times for 5 min,
incubated with the secondary antibody [anti-horseradish peroxidase
antibody (1:1,000, ab181658, Abcam)] at room temperature for 1.5 h.
Afterwards, the membranes were washed with TBST three times for 5
min. Finally, ECL luminescent agent (Beijing Solarbio Science &
Technology Co., Ltd.) was used for exposure and observation in a
chemical exposure machine. Densitometry was performed using ImageJ
(1.8.0) software (National Institutes of Health). Western blot
analysis utilized statistical analysis based on the gray value
obtained, which is equal to the ratio of the gray value of the
target protein to the gray value of the internal reference.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues using a
high-speed homogenizer for cancellous bone tissue comminution,
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA (1 µg) was then reverse transcribed
into cDNA using the PrimeScript RT kit (Affinity Biosciences, Ltd.)
according to the manufacturer's protocol. The cDNA samples were
then analyzed using LightCycler 480 SYBR-Green I Master (Roche
Diagnostics) for RT-qPCR on a LightCycler 480 system (Roche
Diagnostics). The reaction conditions were as follows: 95°C for 30
sec, followed by 5 sec at 95°C, 20 sec at 60°C and 20 sec at 75°C
40 cycles, and GAPDH expression was used as an internal control.
The data was analyzed for expression changes using the
2−ΔΔCq formula (19).
The primer sequences for β-catenin, DKK3 and GAPDH were as follows:
β-catenin forward, 5′-CAT CTA CAC AGT TTG ATG CTG CT-3′ and
reverse, 5′-GCA GTT TTG TCA GTT CAG GGA-3′; DKK3 forward, 5′-CAA
TGG GAC CAT CTG TGA CAA C-3′ and reverse, 5′-ATC GGT CCA AGG CTC
CAT CA-3′; GAPDH forward, 5′-TGC CAA ATG ATG ACA TCA AGA A-3′ and
reverse, 5′-GGA GTG GGT GTC GCT GTT G-3′.
Statistical analysis
All data were analyzed using SPSS 22.0 (IBM Corp.)
statistical software. Graphs were drawn using GraphPad Prism
software (version 8.0). ImageJ (1.8.0) (National Institutes of
Health) software was used for immunohistochemistry,
immunofluorescence, and western blot analysis. All data were tested
for normality of distribution using the Shapiro-Wilk test. One-way
analysis of variance with Tukey's post hoc test was used to compare
the parametric data between groups. For non-parametric data, the
Kruskal-Wallis test with Dunn's post hoc test were used. A value of
P<0.05 was considered to indicate a statistically significant
difference. Data from all studies were subjected to before
statistical analysis.
Results
Cell count and changes in osteoblast
activation
Histological samples of the medial tibial plateau
were collected and divided into the normal (n=3), mild (n=5),
moderate (n=10) and severe symptom groups (n=20). From the analysis
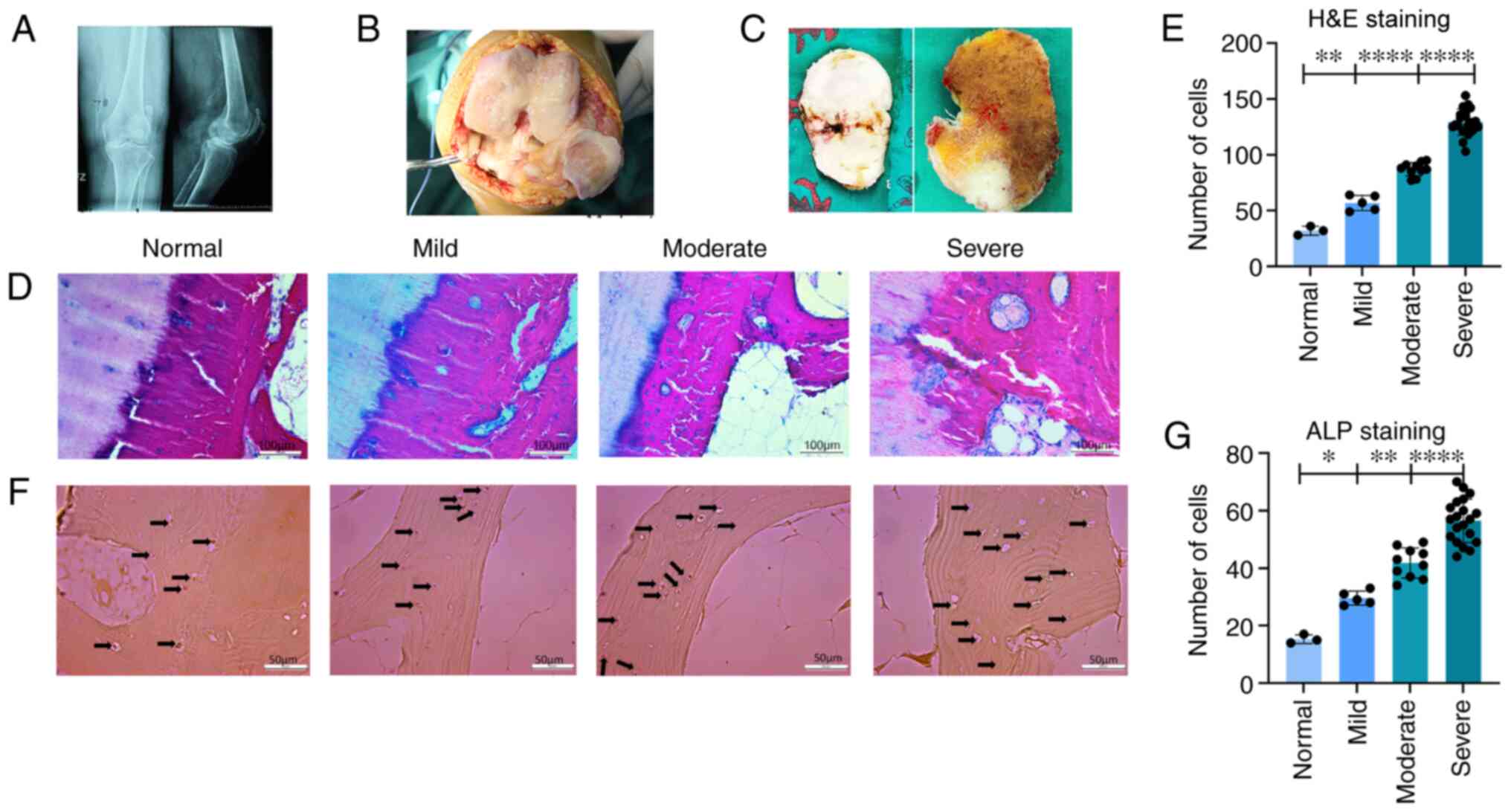
of the pre-operative radiographs from the patients (Fig. 1A), gross pathological images
(Fig. 1B) and intraoperative
cuttings of the bone plate of the tibial plateau, including
cartilage and subchondral bone (Fig.
1C), the medial subchondral bone was found to be markedly
sclerotic and cartilage damage was evident. To detect changes in
the number of bone cells in the subchondral bone, H&E staining
was performed. The results revealed changes in the H&E staining
of the subchondral bone tissue (Fig.
1D), and combined with statistical analysis (Fig. 1E) there was a statistically
significant difference in cell numbers between the normal, mild and
moderate/severe symptom groups. The total number of bone cells
(including osteoblasts and osteoclasts) increased with the
progression of OA. To observe the changes in the number of
osteoblasts in the subchondral bone tissue, ALP staining of the
subchondral bone tissue was performed (Fig. 1F). By observing the results of
ALP staining and combining these with statistical analysis
(Fig. 1G), an increase in the
number of osteoblasts with calcaneal nodules in the subchondral
bone was observed, in parallel with the aggravation of the disease.
There was a statistically significant difference between the mild
and moderate symptom groups and between the moderate and severe
symptom groups. The arrows in Fig.
1F indicate the calcareous nodules within the osteoblasts,
following ALP staining.
OARSI score
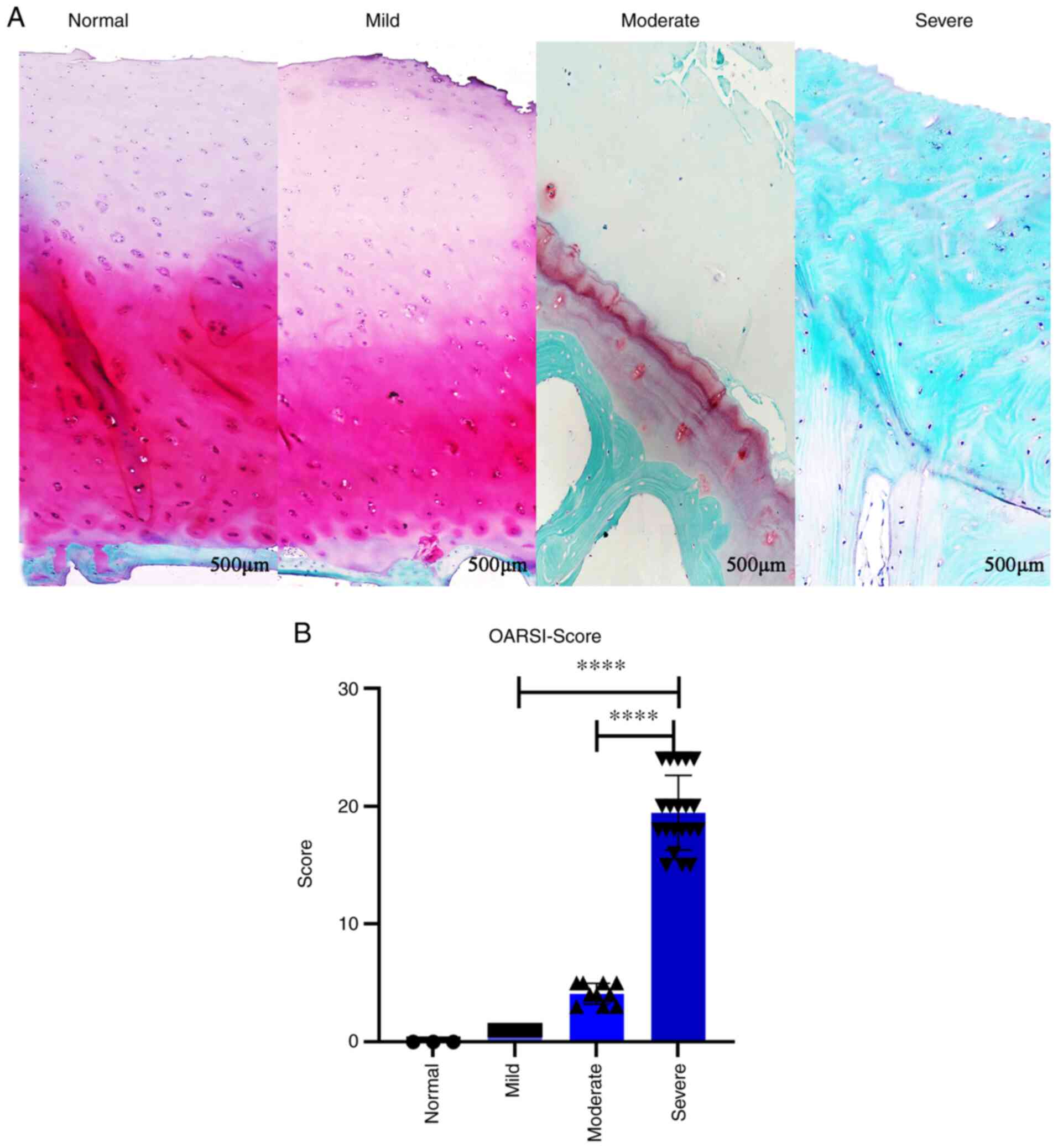
Cartilage damage was investigated by performing
Safranin O/fast green staining (Fig.
2A). The results revealed no notable damage in the mild symptom
group, mild damage in the moderate symptom group and severe damage
in the severe symptom group. In the latter group, some of the
subchondral bone was no longer covered by cartilage and the
subchondral bone was completely exposed. The cartilage was then
scored according to the severity of cartilage damage and in
combination with the OARSI scoring system (OARSI score was equal to
the cartilage degenerative tissue grading multiplied by the stage).
Statistical analysis was then performed, with higher scores
indicating more severe cartilage injury. The differences between
the mild and moderate symptom groups, and the moderate and severe
symptom groups were statistically significant (both P<0.0001;
Fig. 2B).
β-catenin and DKK3 expression evaluation
using immunohistochemistry
To observe the expression patterns of β-catenin and
DKK3 in the osteoblasts in the subchondral bone,
immunohistochemistry was performed, and the results were
statistically analyzed (Fig. 3).
For β-catenin (Fig. 3A), the
differences in cell counts between the normal and mild groups, mild
and moderate groups, and moderate and severe groups were
statistically significant (P<0.05, P<0.01 and P<0.01,
respectively) (Fig. 3C). For
DKK3 (Fig. 3B), there were
statistically significant differences in cell numbers between the
normal and mild, mild and moderate, and moderate and severe symptom
groups (P<0.05, P<0.01 and P<0.05, respectively) (Fig. 3E). In addition, the differences
between the mild and moderate symptom groups were statistically
significant according to the analysis of optical density values
(P<0.001 and P<0.01, respectively; Fig. 3D and F).
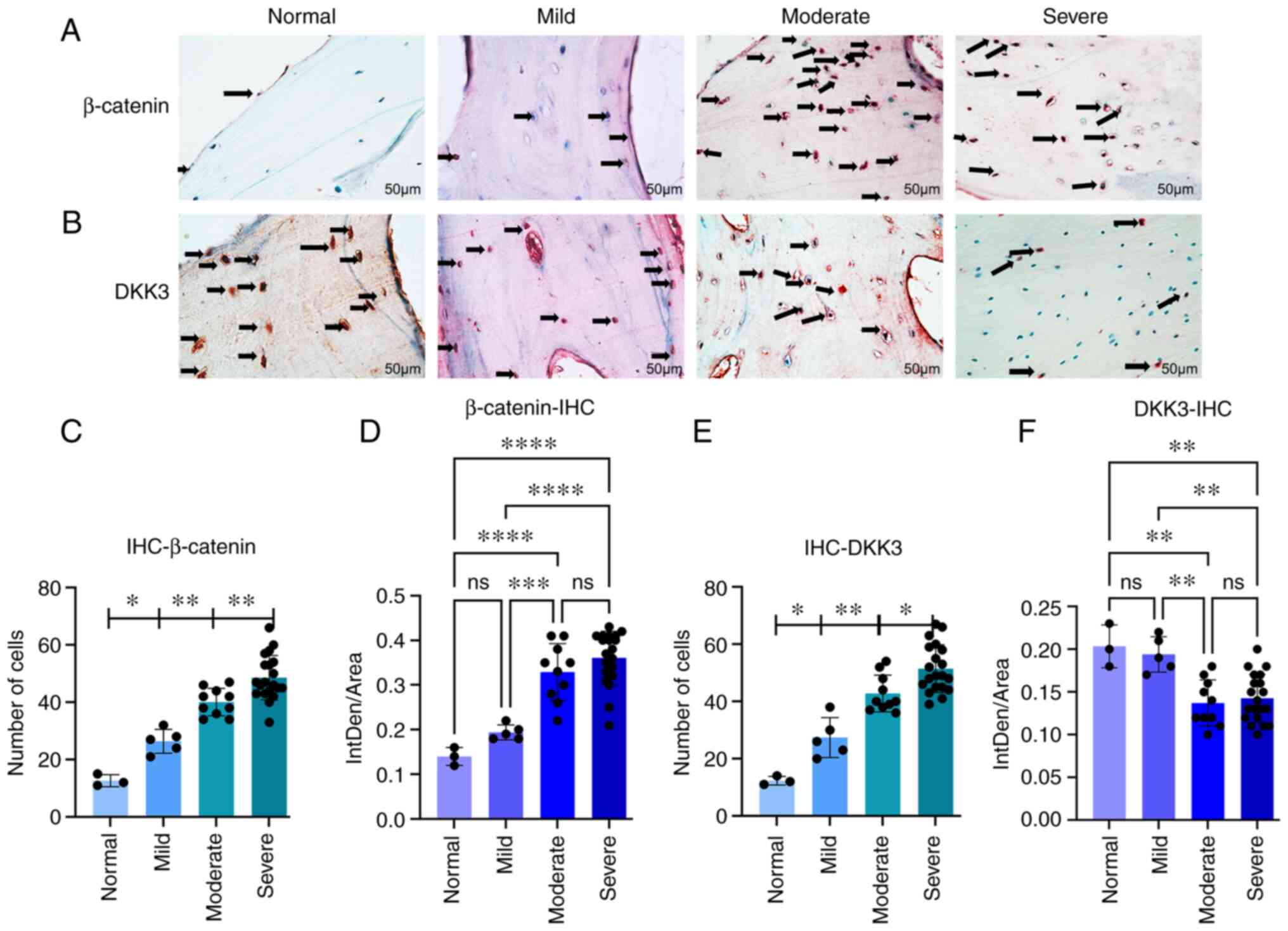 | Figure 3(A and B) Immunohistochemical
staining. Arrows indicate the number of positively stained cells.
(C) In the β-catenin group, the difference in cell number was
statistically significant between the normal and mild, mild and
moderate, and moderate and severe symptom groups
(*P<0.01, **P<0.01 and
**P<0.01 respectively). (D) Statistically significant
differences between the mild and moderate symptom groups according
to the average optical density values (***P<0.001 and
****P<0.0001). (E) In the DKK3 group, cell numbers
were statistically significant between the normal and mild, mild
and moderate, and moderate and severe symptom groups
(*P<0.05, **P<0.01 and
*P<0.05, respectively). (F) Statistically significant
differences between the mild and moderate groups according to the
average optical density values (**P<0.01). (C-F) Data
were analyzed using one-way ANOVA; mean of the 95% confidence
interval: Normal (n=3), mild (n=5), moderate (n=10) and severe
(n=20). DKK3, Dickkopf-related protein 3; ns, not significant. |
Evaluation of β-catenin expression and
DKK3 by immunofluorescence
To further observe β-catenin and DKK3 expression
levels in osteoblasts from the subchondral bone, immunofluorescence
staining was used. The results revealed the optical density changes
in β-catenin and DKK3 expression in the subchondral osteoblasts.
Immunofluorescence staining was performed using Cy3, which is
excited at 532 nm and emits orange light, then images of the
samples were captured using a 40X fluorescence microscope. The
results of β-catenin (Fig. 4A)
and DKK3 expression (Fig. 5A)
were observed and statistically analyzed (Figs. 4B-D, and 5B and C). The cell counts were
statistically significantly different between the normal and mild,
mild and moderate, and moderate and severe symptom groups. The
differences in β-catenin and DKK3 expression were statistically
significant between the mild and moderate symptom groups by
analyzing the mean optical density (P<0.001 and P<0.001,
respectively).
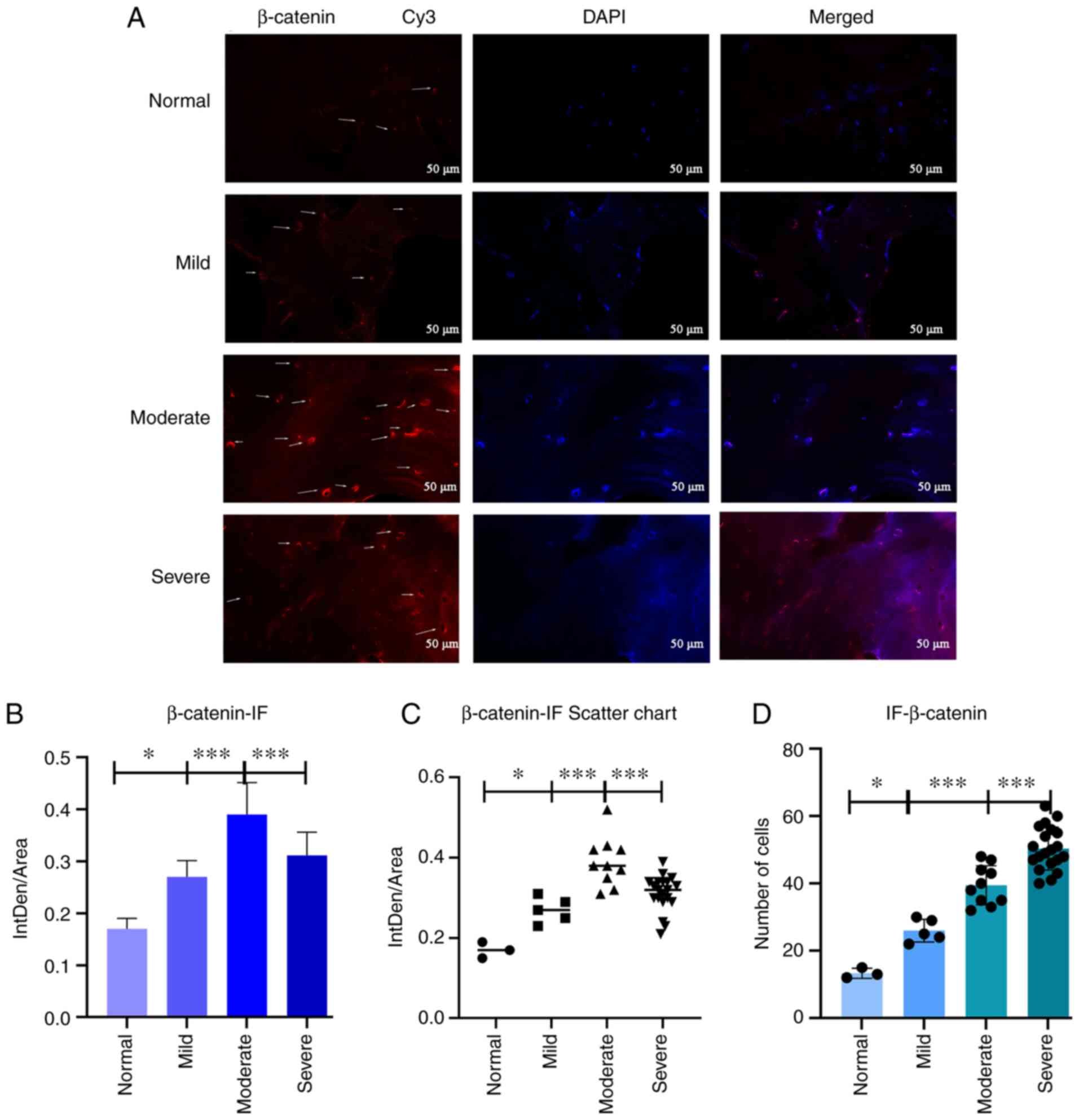 | Figure 4(A) Immunofluorescence staining
(β-catenin). Arrows indicate the number of positively stained
cells. (B and C) Statistical analysis results. Statistical
significant differences in b-catenin expression were revealed
between normal and mild, mild and moderate, and moderate and severe
OA in the β-catenin group (*P<0.05,
***P<0.001 and ***P<0.001,
respectively). (D) Statistical analysis results of cell numbers.
The number of cells was statistically significant between the
normal and mild, mild and moderate, and moderate and severe symptom
groups (*P<0.05, ***P<0.001 and
***P<0.001, respectively). (B-D) Data were analyzed
using one-way ANOVA. Mean 95% confidence interval for average
score: Normal (n=3), mild (n=5), moderate (n=10) and severe (n=20).
OA, osteoarthritis. |
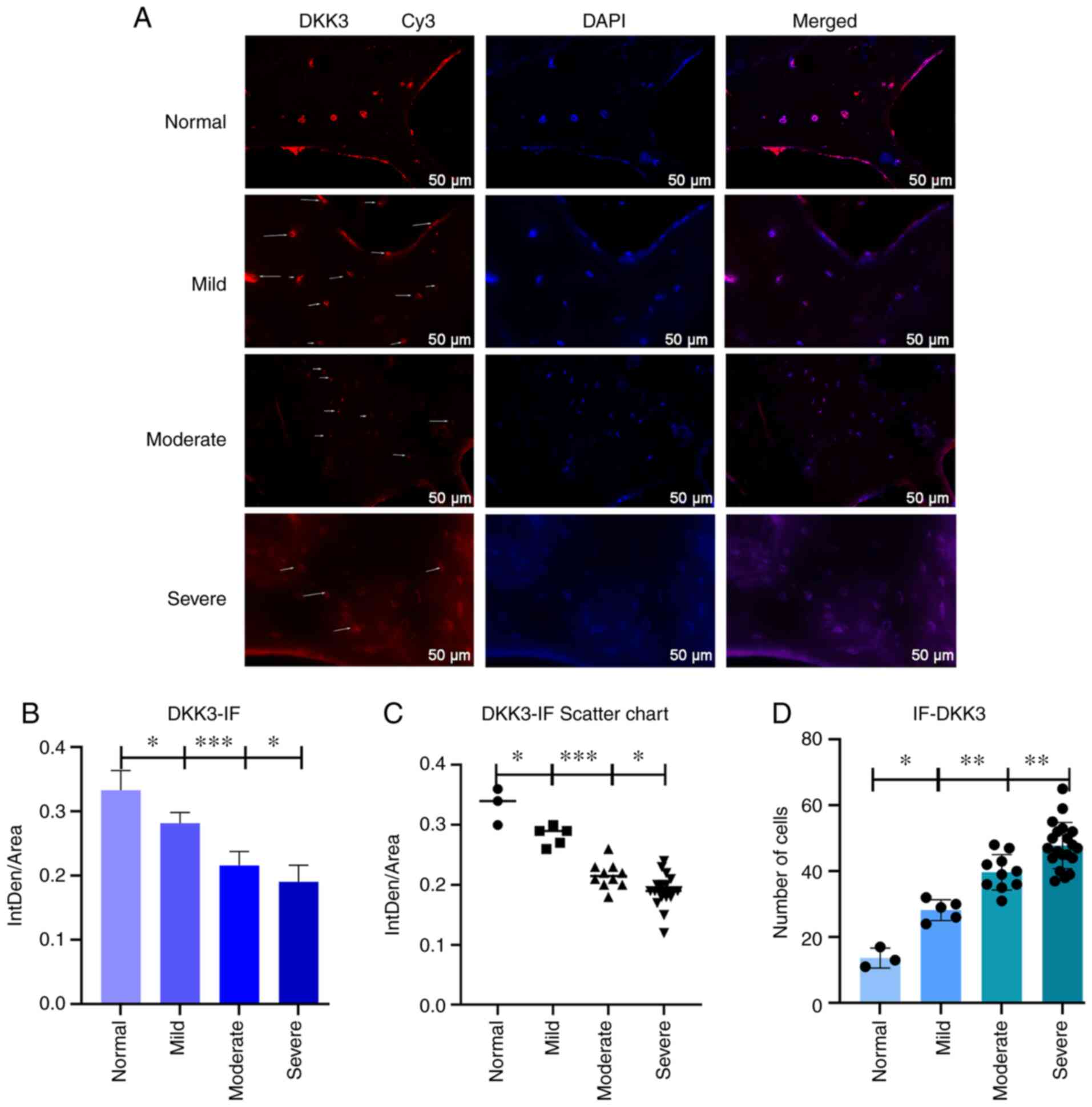 | Figure 5(A) Immunofluorescence staining for
DKK3. Arrows indicate the number of positively stained cells. (B
and C) Statistical analysis results. The difference in DKK3
expression between the normal and mild, mild and moderate, and
moderate and severe groups was statistically significant
(*P<0.05, ***P<0.001 and
*P<0.05, respectively). (D) Cell count statistical
analysis results. The differences in cell numbers was statistically
significant between the normal and mild, mild and moderate, and
moderate and severe symptom groups (*P<0.05,
**P<0.01, and **P<0.01, respectively).
(B-D) Data were analyzed using one-way ANOVA; mean 95% confidence
interval for average score: Normal (n=3), mild (n=5), moderate
(n=10) and severe (n=20). DKK3, Dickkopf-related protein 3. |
Changes in β-catenin and DKK3 protein and
mRNA expression
To detect and quantify β-catenin and DKK3 protein
expression in the subchondral bone tissue, western blot analysis
was performed. By analyzing the results (Fig. 6A-C) using statistical analysis,
significant differences in the protein expression level of
β-catenin were observed between the mild and moderate symptom
groups, and between the moderate and severe symptom groups
(P<0.001 and P<0.01, respectively). The β-catenin expression
levels were more increased in the moderate symptom group. There
were also statistically significant differences in the protein
expression level of DKK3 between the mild and moderate symptom
groups, and between the moderate and severe symptom groups
(P<0.01 and P<0.0001, respectively). Increased DKK3
expression levels were observed in the normal and mild symptom
groups, whereas decreased expression levels were observed in the
moderate and severe symptom groups, exhibiting a trend towards a
decrease in expression levels with the severity of OA. Finally, in
order to detect the gene expression level of β-catenin and DKK3 in
the bone tissue from subchondral bone, RT-qPCR was performed. The
results (Fig. 6D and E)
demonstrated significant differences between the mild and moderate
symptom groups, and between the moderate and severe symptom groups
concerning the β-catenin expression evaluation group (P<0.0001
and P<0.01, respectively). Concerning DKK3 mRNA expression,
there was a statistically significant difference between the mild
and moderate symptom groups and between the moderate and severe
symptom groups (P<0.0001 and P<0.01 respectively),
demonstrating an increased expression in the normal and mild
symptom groups, and a decreased expression in the moderate and
severe symptom groups, exhibiting a trending towards a decreased
expression with the increasing severity of OA.
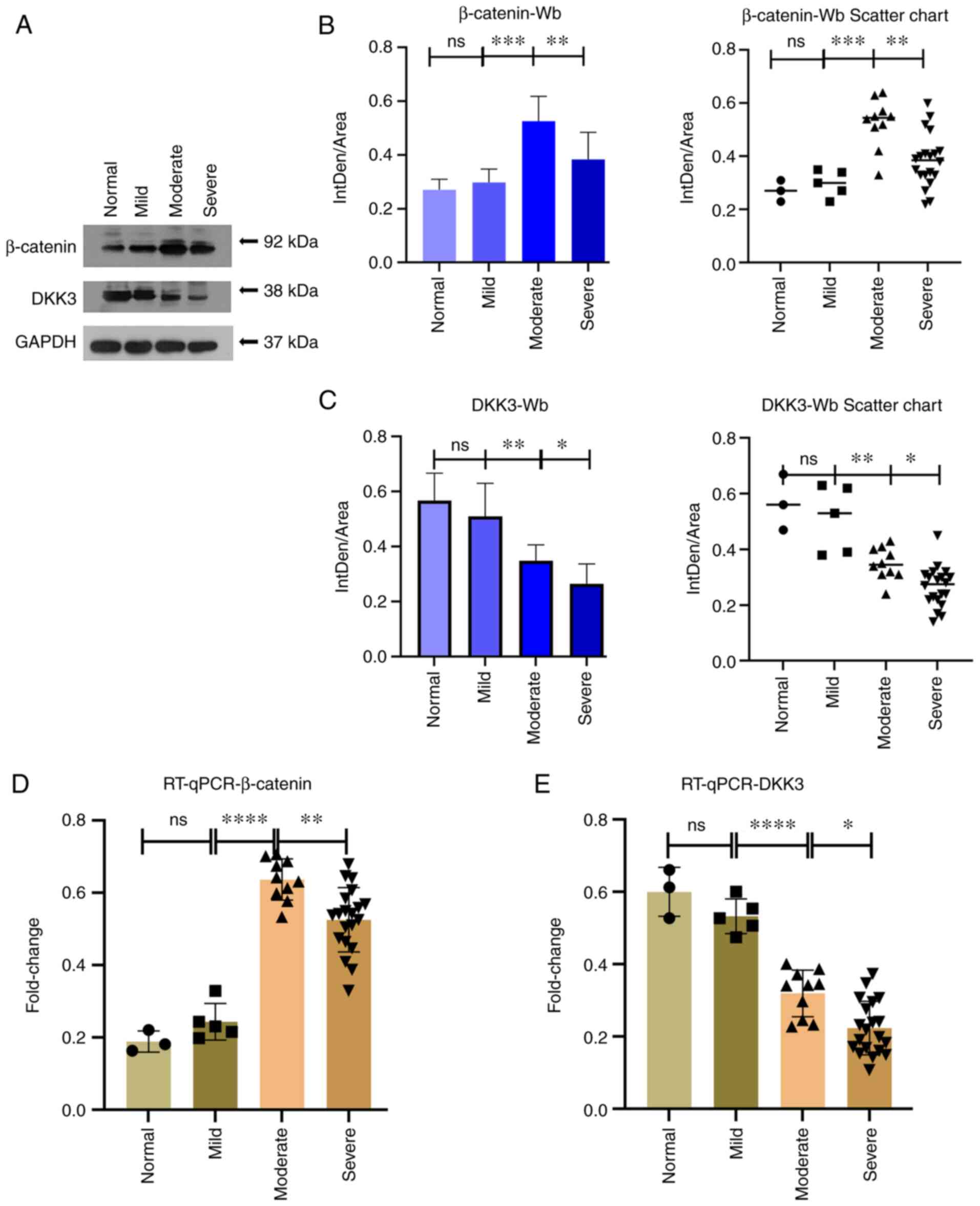 | Figure 6(A-C) The results of western blot
analysis revealed that in the β-catenin group, there was a
statistically significant difference between the mild and moderate
symptom groups and between the moderate and severe symptom groups
(***P<0.001 and **P<0.01,
respectively). In the DKK3 group, there were statistically
significant differences between the mild and moderate symptom
groups, as well as between the moderate and severe symptom groups
(**P<0.01 and *P<0.05, respectively).
(D and E) The results of RT-qPCR revealed significant differences
between the mild and moderate groups as and between the moderate
and severe symptom groups in the β-catenin group
(****P<0.0001, and **P<0.01,
respectively). In the DKK3 group, there was a statistically
significant difference between the mild and moderate symptom groups
and between the moderate and severe symptom groups
(****P<0.0001 and *P<0.01,
respectively). (B-E) Data were analyzed using one-way ANOVA. 95%
confidence interval for average score: Normal (n=3), mild (n=5),
moderate (n=10) and severe (n=20). DKK3, Dickkopf-related protein
3; RT-qPCR, reverse transcription quantitative PCR; ns, not
significant. |
Discussion
Previous research has characterized early-stage OA
with increased subchondral bone remodeling and late OA with
decreased bone resorption and increased bone formation (5). Abnormal subchondral bone remodeling
and osteophyte formation are hallmarks of OA progression (20).
Human β-catenin protein is an 88-kDa cytoskeletal
protein, 781-amino acid long, encoded by the catenin beta-1 gene
(CTNNBI) and located on the 3p21 human chromosome (21). Numerous studies have shown that
β-catenin is a major signaling molecule in the Wnt signaling
pathway (22-24). Wnt signaling pathway activation
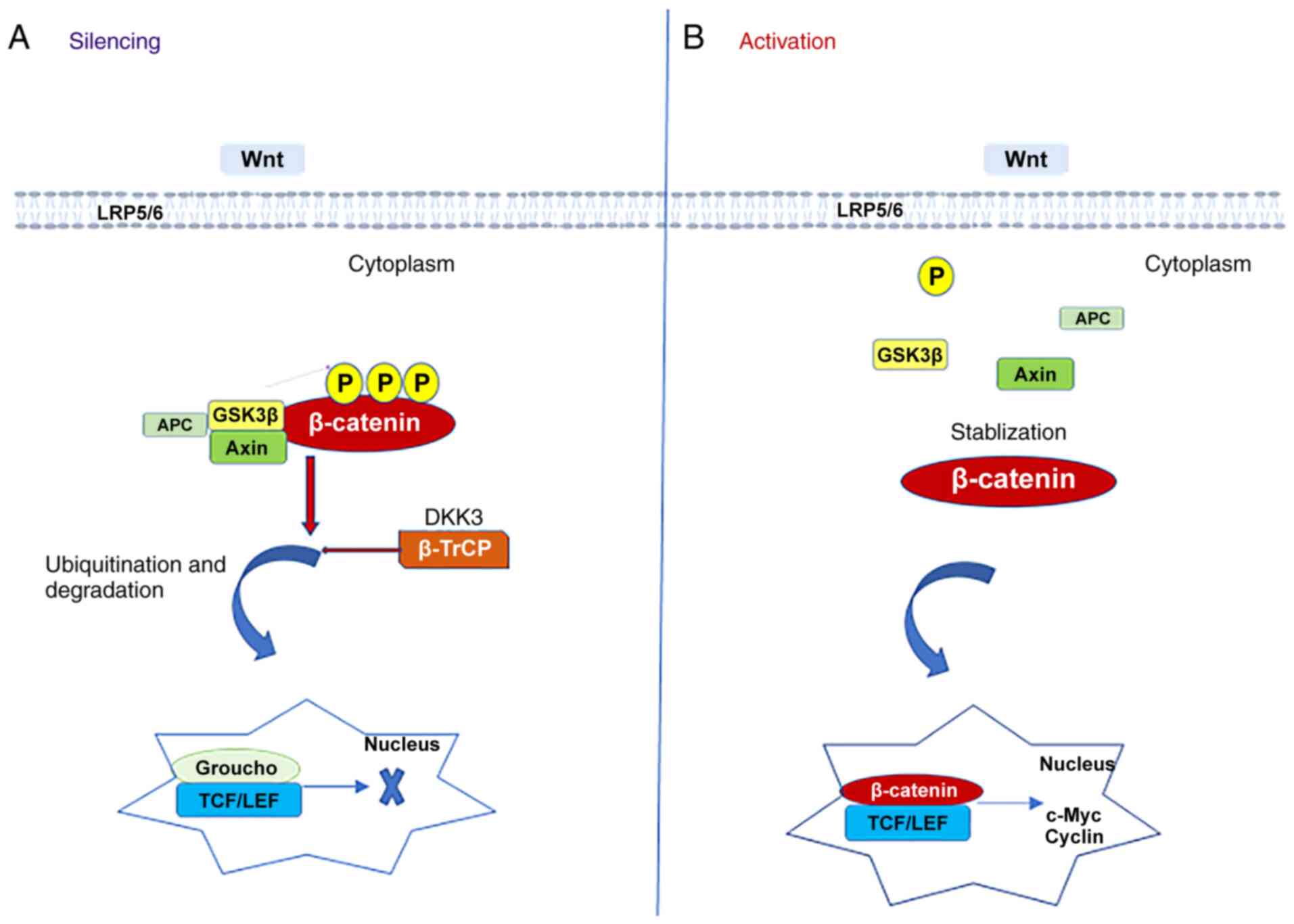
depends on β-catenin expression levels in the cell (Fig. 7). When β-catenin levels are
decreased, the Wnt pathway is not activated; however, when the
levels are increased, the Wnt pathway is activated (25).
DKK3 is a member of the DKK family, among which
DKK-1, -2 and -4 compete for binding to the Wnt protein low-density
lipoprotein 5/6 receptor (26).
DKK3 negatively regulates β-catenin and its downstream signaling
pathways by reducing β-catenin aggregation in the cytoplasm
(27). The graphs presented in
Fig. 1 depict the changing
patterns of β-catenin and DKK3 expression when the Wnt/β-catenin
pathway is inhibited and activated. In addition, DKK3 indirectly
inhibited its role in the Wnt/β-catenin pathway by reducing the
nuclear transcription of β-catenin. Suwa et al (28) reported that various genes in the
Wnt signaling pathway were expressed in the adrenal cortex. Based
on the regional distribution pattern of DKK3 expression, it may be
associated with the Wnt/β-catenin signaling pathway, affecting
regional differentiation or growth. However, it is unclear whether
DKKs are associated with bone formation. Subsequently, Niehrs
(29) reported that, in adults,
DKKs were associated with bone formation in bone disease.
Furthermore, Aslan et al (30) further demonstrated that DKK3
expression affected the osteogenesis process in vivo and
in vitro, suggesting that it may exert an inhibitory effect
on osteogenesis.
As the number of studies on the involvement of the
Wnt signaling pathway in the developmental mechanisms of OA have
increased, it has been reported that the activation of the
classical Wnt/β-catenin pathway accelerates the progression of OA
(31). According to a previously
published study by Funck-Brentano et al (32), it was revealed by using a mouse
model of OA that the classic Wnt pathway was activated to promote
subchondral bone formation osteophytes and inhibition of the
Wnt/β-catenin pathway signaling improved subchondral bone structure
and biomechanical function, thereby alleviating the degeneration of
articular cartilage. This may be attributed to active osteoblasts
in OA altering bone microarchitecture through aberrant bone
remodeling and reduced mineralization. However, this alteration
notably reduced the biomechanical function of the subchondral bone,
depriving it of its cushioning effect against mechanical loads and
its protective function against cartilage, further aggravating
cartilage damage and accelerating the development of OA.
In the present study, a total of 38 subchondral bone
tissue specimens were collected from the tibial plateau in patients
with severe OA. In addition, intraoperative anatomical tissues and
postoperative tibial plateau osteotomy specimens were also
collected, demonstrating severe cartilage damage in the femoral
condyle and tibial plateau, with some areas of cartilage lost and
replaced by sclerotic subchondral bone. H&E and ALP staining of
the subchondral bone tissue revealed that the total number of
osteocytes and osteoblasts in the subchondral bone increased along
with the degree of OA, including various osteoblasts
(pre-osteoblasts, osteoclasts and bone lining cells). Neve et
al (4) reported that the
abnormal differentiation and increased activity of osteoblasts were
observed in OA. In addition, dysfunctional osteoblasts produce in
turn numerous transcription factors, growth factors and other
proteins involved in the pathogenesis of OA (33), further promoting the development
of OA.
It has been found that osteoblast differentiation
requires the classical Wnt/β-catenin signaling pathway (14). It has been reported that some
osteoblasts contain ALP, which can be used as a biochemical marker
to assess bone formation (34);
however, mechanical loading increases ALP activity (35). Thus, in the present study, it was
demonstrated using ALP staining (AZO method) that the number of
ALP-positive cells in the subchondral bone specimens, to be
increased during the progression of OA from mild to moderate; in
addition, in severe OA, there was no significant increase in active
osteoblasts compared with that in moderate OA.
Cartilage changes may be visualized using Safranin
O/fast green staining, revealing that the hyaline cartilage cracks,
thins and eventually disappears, as the degree of OA increases. It
has been reported that articular cartilage is very sensitive to
mechanical loading, subchondral bone provides cartilage support and
cartilage degeneration is a sign of OA aggravation (36). The role of the subchondral bone
in the development of OA remains under investigation; however,
there is evidence to indicate that changes in the subchondral bone
are accompanied by cartilage degeneration (37).
Another study revealed that metabolic modulators
produced by subchondral osteoblasts directly cause chondrocyte
degeneration by affecting the microstructure between the bone and
cartilage, while abnormal expression of osteoblasts can indirectly
expose cartilage to higher stress (38). Thus, this direct and indirect
mode of action of subchondral bone and cartilage accelerates
cartilage degeneration and further aggravates the progression of
OA. Fell et al (39)
reported that the association between the morphology of the
subchondral bone tissue and the viscoelasticity, and thickness of
cartilage may contribute to the development and progression of OA
by altering the ability of cartilage to dissipate energy.
Furthermore, Smieszek et al (40) demonstrated that osteoblasts are
inhibited by high expression of microRNA-21 in OA, through the
reduction of subchondral bone mineralization. Therefore, altering
and alleviating the abnormal remodeling of subchondral bone may
mitigate further cartilage damage and delay the progression of
OA.
Due to this supportive role of subchondral bone on
cartilage, the question on whether DKK3 could reduce subchondral
bone sclerosis via β-catenin inhibition of the Wnt pathway,
preventing further damage to cartilage due to subchondral bone
sclerosis and delaying the progression of OA was raised. Among the
numerous regulators of the classical Wnt pathway, β-catenin is a
key factor, while DKK3 is a downstream inhibitor of the Wnt
pathway. In malignant tumor cells, DKK3 was associated with the
induction of apoptosis and inhibition of invasion by regulating
β-catenin signaling, and c-Jun N-terminal kinase-dependent cellular
pathways, as reported by Lee et al (17). It has been reported that
β-catenin may be a central molecule mediating osteoblast viability
and differentiation (41). In
addition, DKK3 inhibited the Wnt signaling pathway by hydrolyzing
β-catenin/APC/GSK3β, thereby inhibiting the entry of β-catenin into
the nucleus for transcription (17). Therefore, in OA, DKK3 degrades
β-catenin via ubiquitination, thereby degrading the downstream
signaling pathway factors of β-catenin and inhibiting nuclear
transcription of the Wnt pathway (15), delaying abnormal remodeling of
subchondral bone.
According to Huang et al (42) the regulation of the Wnt signaling
pathway reduced the risk of OA. Charlier et al (43) reported that the DKK3 regulation
of the Wnt/β-catenin pathway increased the dedifferentiation of OA
chondrocytes in the human hip and promoted the remission of OA.
Thus, maintaining normal Wnt/β-catenin signaling is critical for
orthogenic bone tissue differentiation and in OA, once this pathway
is activated, it can accelerate OA progression.
In conclusion, the results of the present study
demonstrated that with the increase in the pathological changes of
OA in the knee, the number of osteoblasts may also gradually
increase; however, the number of active osteoblasts was highest in
moderate OA and slightly more increased in severe OA. The
aforementioned association analysis revealed that DKK3 expression
was high in the normal and mild groups, decreased in the moderate
and severe groups, while β-catenin expression increased
significantly in the moderate OA group after activation of the
Wnt/β-catenin pathway. In subchondral bone tissue, DKK3 may play an
opposite role to β-catenin by inhibiting the Wnt/β-catenin
signaling pathway in osteoblasts, reducing subchondral bone
sclerosis, while reducing cartilage damage and delaying OA
progression. In future studies, the authors aim to verify whether
DKK3 can inhibit bone formation in vitro. Furthermore, it
was unexpectedly found that, in the late stages of OA, some
osteoblasts can still be activated and play a role in abnormal bone
remodeling, leading to increased subchondral bone sclerosis and
worsening of pain.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and QJ contributed to the conception and design
of the present study. XL, WJ and XY performed the experiments. XL
performed the statistical analysis and contributed to the
manuscript preparation. QJ and XL confirm the authenticity of all
the raw data. QJ contributed to funding acquisition. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University
(approval no. KYLL-2020-20; approval date, January 14, 2021), and
informed consent was obtained from all the enrolled patients.
Patient consent for publication
The patient whose images are shown in Fig. 1 consents to the publication of
these images.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 8186090221).
References
|
1
|
Qin HJ, Xu T, Wu HT, Yao ZL, Hou YL, Xie
YH, Su JW, Cheng CY, Yang KF, Zhang XR, et al: SDF-1/CXCR4 axis
coordinates crosstalk between subchondral bone and articular
cartilage in osteoarthritis pathogenesis. Bone. 125:140–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson-MacKenzie JM, Quasnichka HL,
Starr RL, Lewis EJ, Billingham ME and Bailey AJ: Fundamental
subchondral bone changes in spontaneous knee osteoarthritis. Int J
Biochem Cell Biol. 37:224–236. 2005. View Article : Google Scholar
|
|
3
|
Omoumi P, Babel H, Jolles BM and Favre J:
Relationships between cartilage thickness and subchondral bone
mineral density in non-osteoarthritic and severely osteoarthritic
knees: In vivo concomitant 3D analysis using CT arthrography.
Osteoarthritis Cartilage. 27:621–629. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neve A, Corrado A and Cantatore FP:
Osteoblast physiology in normal and pathological conditions. Cell
Tissue Res. 343:289–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maruotti N, Corrado A and Cantatore FP:
Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol.
232:2957–2963. 2017. View Article : Google Scholar :
|
|
6
|
Di Pompo G, Errani C, Gillies R, Mercatali
L, Ibrahim T, Tamanti J, Baldini N and Avnet S: Acid-induced
inflammatory cytokines in osteoblasts: A guided path to osteolysis
in bone metastasis. Front Cell Dev Biol. 9:6785322021. View Article : Google Scholar :
|
|
7
|
Wang Y, Fan X, Xing L and Tian F: Wnt
signaling: A promising target for osteoarthritis therapy. Cell
Commun Signal. 17:972019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lories RJ and Monteagudo S: Review
article: Is Wnt signaling an attractive target for the treatment of
osteoarthritis? Rheumatol Ther. 7:259–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cherifi C, Monteagudo S and Lories RJ:
Promising targets for therapy of osteoarthritis: A review on the
Wnt and TGF-β signalling pathways. Ther Adv Musculoskel.
13:1759720X2110069592021.
|
|
10
|
Chen H, Tan XN, Hu S, Liu RQ, Peng LH, Li
YM and Wu P: Molecular mechanisms of chondrocyte proliferation and
differentiation. Front Cell Dev Biol. 9:6641682021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staines KA, Macrae VE and Farquharson C:
Cartilage development and degeneration: A Wnt Wnt situation. Cell
Biochem Funct. 30:633–642. 2012. View
Article : Google Scholar
|
|
12
|
Hill TP, Später D, Taketo MM, Birchmeier W
and Hartmann C: Canonical Wnt/beta-catenin signaling prevents
osteoblasts from differentiating into chondrocytes. Dev Cell.
8:727–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C,
Rosier RN, O'Keefe RJ, Zuscik M and Chen D: Activation of
beta-catenin signaling in articular chondrocytes leads to
osteoarthritis-like phenotype in adult beta-catenin conditional
activation mice. J Bone Miner Res. 24:12–21. 2009. View Article : Google Scholar
|
|
14
|
Fjeld K, Kettunen P, Furmanek T,
Kvinnsland IH and Luukko K: Dynamic expression of Wnt
signaling-related Dickkopf1, -2, and -3 mRNAs in the developing
mouse tooth. Dev Dyn. 233:161–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Yang C, Yang Y, Liang Q, Xie K,
Liu J and Tang Y: Targeting DKK1 prevents development of
alcohol-induced osteonecrosis of the femoral head in rats. Am J
Transl Res. 13:2320–2330. 2021.PubMed/NCBI
|
|
16
|
Baetta R and Banfi C: Dkk (Dickkopf)
proteins. Arterioscler Thromb Vasc Biol. 39:1330–1342. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee EJ, Nguyen QTT and Lee M: Dickkopf-3
in human malignant tumours: A clinical viewpoint. Anticancer Res.
40:5969–5979. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Zhang L and Wen C: Osteocyte dysfunction
in joint homeostasis and osteoarthritis. Int J Mol Sci.
22:65222021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian J, Gao SG, Li YS, Cheng C, Deng ZH,
Luo W and Zhang FJ: The β-catenin/TCF-4 pathway regulates the
expression of OPN in human osteoarthritic chondrocytes. J Orthop
Surg Res. 15:3442020. View Article : Google Scholar
|
|
22
|
Li W, Xiong Y, Chen W and Wu L:
Wnt/β-catenin signaling may induce senescence of chondrocytes in
osteoarthritis. Exp Ther Med. 20:2631–2638. 2020.PubMed/NCBI
|
|
23
|
Xuan F, Yano F, Mori D, Chijimatsu R,
Maenohara Y, Nakamoto H, Mori Y, Makii Y, Oichi T, Taketo MM, et
al: Wnt/β-catenin signaling contributes to articular cartilage
homeostasis through lubricin induction in the superficial zone.
Arthritis Res Ther. 21:2472019. View Article : Google Scholar
|
|
24
|
Yu H, Liu Y, Yang X, He J, Zhang F, Zhong
Q and Guo X: Strontium ranelate promotes chondrogenesis through
inhibition of the Wnt/β-catenin pathway. Stem Cell Res Ther.
12:2962021. View Article : Google Scholar
|
|
25
|
Le NH, Franken P and Fodde R:
Tumour-stroma interactions in colorectal cancer: Converging on
beta-catenin activation and cancer stemness. Brit J Cancer.
98:1886–1893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren C, Gu X, Li H, Lei S, Wang Z, Wang J,
Yin P, Zhang C, Wang F and Liu C: The role of DKK1 in Alzheimer's
disease: A potential intervention point of brain damage prevention?
Pharmacol Res. 144:331–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uribe D, Cardona A, Esposti DD, Cros MP,
Cuenin C, Herceg Z, Camargo M and Cortés-Mancera FM:
Antiproliferative effects of epigenetic modifier drugs through
E-cadherin up-regulation in liver cancer cell lines. Ann Hepatol.
17:444–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suwa T, Chen M, Hawks CL and Hornsby PJ:
Zonal expression of dickkopf-3 and components of the Wnt signalling
pathways in the human adrenal cortex. J Endocrinol. 178:149–158.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aslan H, Ravid-Amir O, Clancy BM,
Rezvankhah S, Pittman D, Pelled G, Turgeman G, Zilberman Y, Gazit
Z, Hoffmann A, et al: Advanced molecular profiling in vivo detects
novel function of dickkopf-3 in the regulation of bone formation. J
Bone Miner Res. 21:1935–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Palma A and Nalesso G: WNT signalling
in osteoarthritis and its pharmacological targeting. Handb Exp
Pharmacol. 269:337–356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Funck-Brentano T, Bouaziz W, Marty C,
Geoffroy V, Hay E and Cohen-Solal M: Dkk-1-mediated inhibition of
Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis
Rheumatol. 66:3028–3039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang A, Xu P, Sun S, Zhao Z, Tan Q, Li W,
Song C and Leng H: Cellular alterations and crosstalk in the
osteochondral joint in osteoarthritis and promising therapeutic
strategies. Connect Tissue Res. 62:709–719. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao F, Hu X and Chen R: The effects of
omarigliptin on promoting osteoblastic differentiation.
Bioengineered. 12:11837–11846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyamoto S, Yoshikawa H and Nakata K:
Axial mechanical loading to ex vivo mouse long bone regulates
endochondral ossification and endosteal mineralization through
activation of the BMP-Smad pathway during postnatal growth. Bone
Rep. 15:1010882021. View Article : Google Scholar
|
|
36
|
Vincent TL and Wann AKT:
Mechanoadaptation: Articular cartilage through thick and thin. J
Physiol. 597:1271–1281. 2019. View Article : Google Scholar :
|
|
37
|
Bhatla JL, Kroker A, Manske SL, Emery CA
and Boyd SK: Differences in subchondral bone plate and cartilage
thickness between women with anterior cruciate ligament
reconstructions and uninjured controls. Osteoarthritis Cartilage.
26:929–939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu W, Chen Y, Dou C and Dong S:
Microenvironment in subchondral bone: Predominant regulator for the
treatment of osteoarthritis. Ann Rheum Dis. 80:413–422. 2020.
View Article : Google Scholar
|
|
39
|
Fell NLA, Lawless BM, Cox SC, Cooke ME,
Eisenstein NM, Shepherd DET and Espino DM: The role of subchondral
bone, and its histomorphology, on the dynamic viscoelasticity of
cartilage, bone and osteochondral cores. Osteoarthritis Cartilage.
27:535–543. 2019. View Article : Google Scholar :
|
|
40
|
Smieszek A, Marcinkowska K, Pielok A,
Sikora M, Valihrach L and Marycz K: The role of miR-21 in
osteoblasts-osteoclasts coupling in vitro. Cells. 9:4792020.
View Article : Google Scholar
|
|
41
|
Chu Y, Gao Y, Yang Y, Liu Y, Guo N, Wang
L, Huang W, Wu L, Sun D and Gu W: β-Catenin mediates
fluoride-induced aberrant osteoblasts activity and osteogenesis.
Environ Pollut. 265:1147342020. View Article : Google Scholar
|
|
42
|
Huang Y, Jiang L, Yang H, Wu L, Xu N, Zhou
X and Li J: Variations of Wnt/β-catenin pathway-related genes in
susceptibility to knee osteoarthritis: A three-centre case-control
study. J Cell Mol Med. 23:8246–8257. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Charlier E, Malaise O, Deroyer C, Zeddou
M, Neuville S, Cobraiville G, Gillet P, Kurth W, de Seny D, Relic B
and Malaise MG: Dickkopf 3 (DKK3) is increased along human hip OA
chondrocytes dedifferentiation and can modulate Wnt/B-catenin and
TGFβ Alk1/Smad1/5 signaling pathways, as well as leptin production.
Osteoarthritis Cartilage. 24(Suppl 1): S1822016. View Article : Google Scholar
|