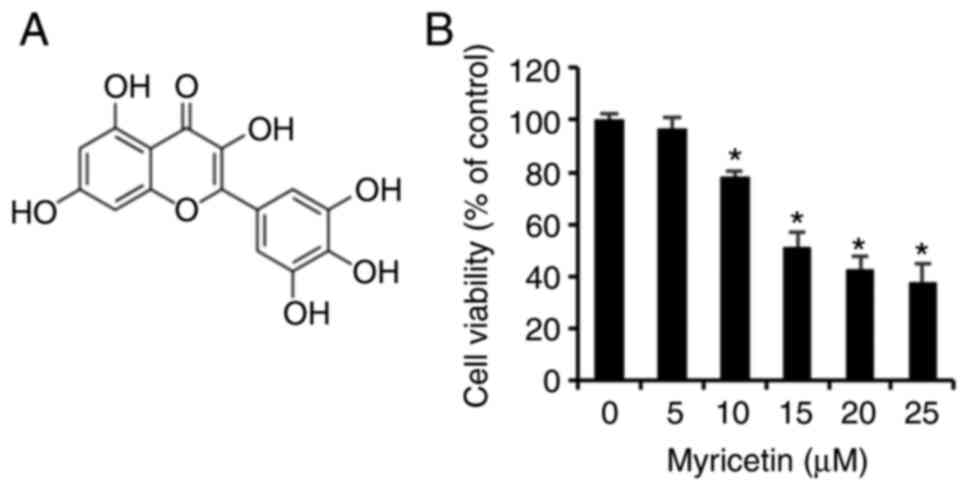
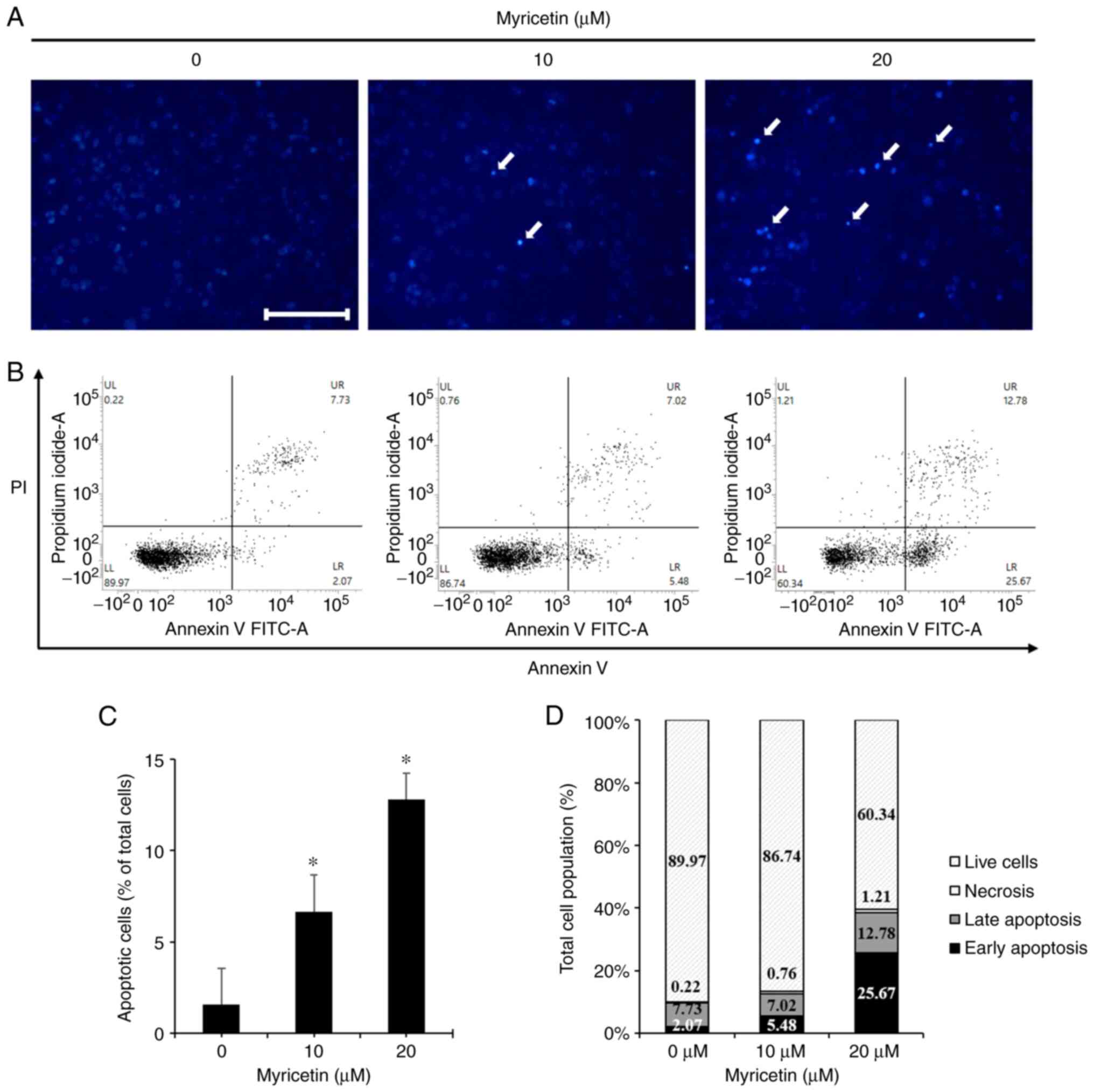
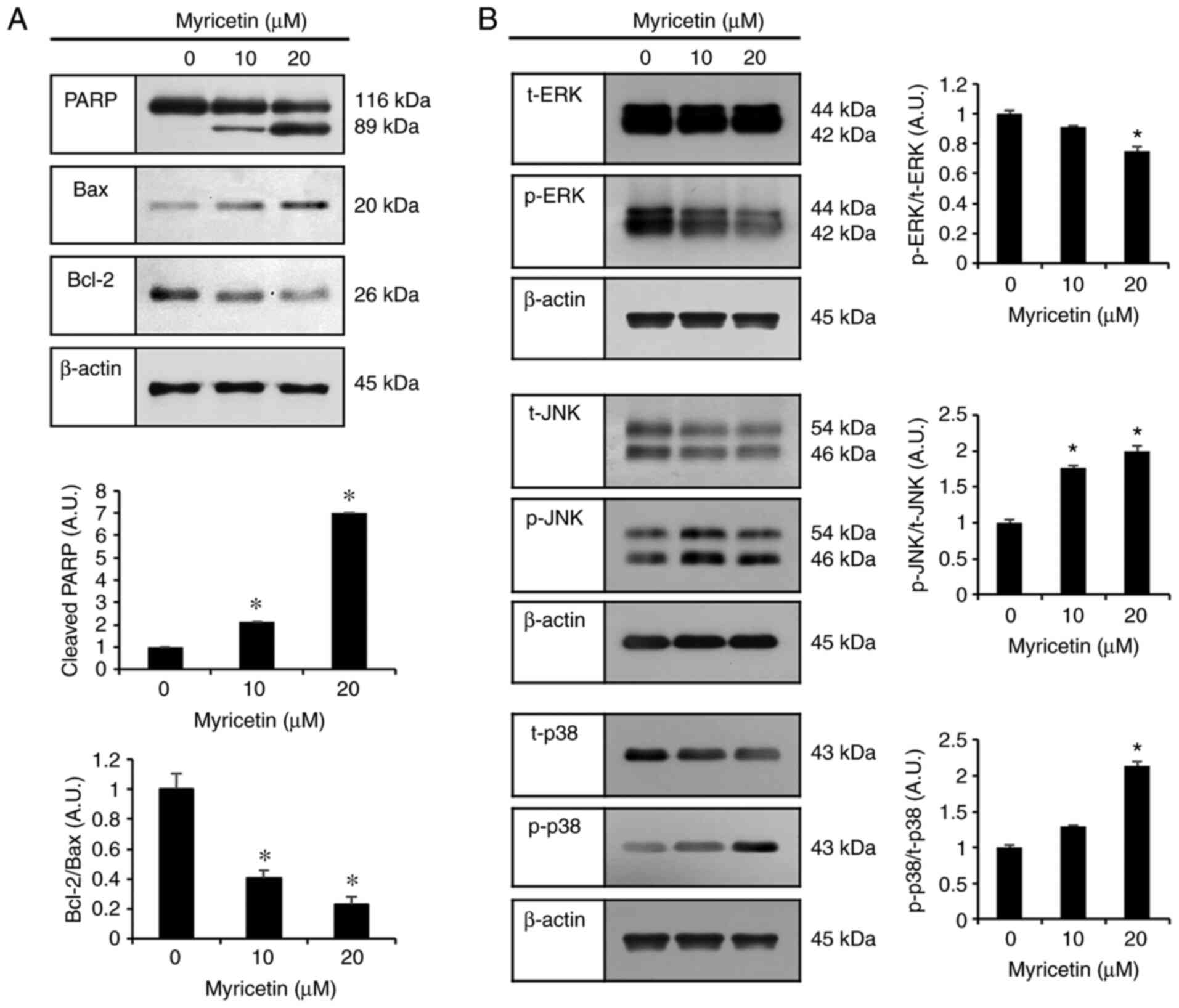
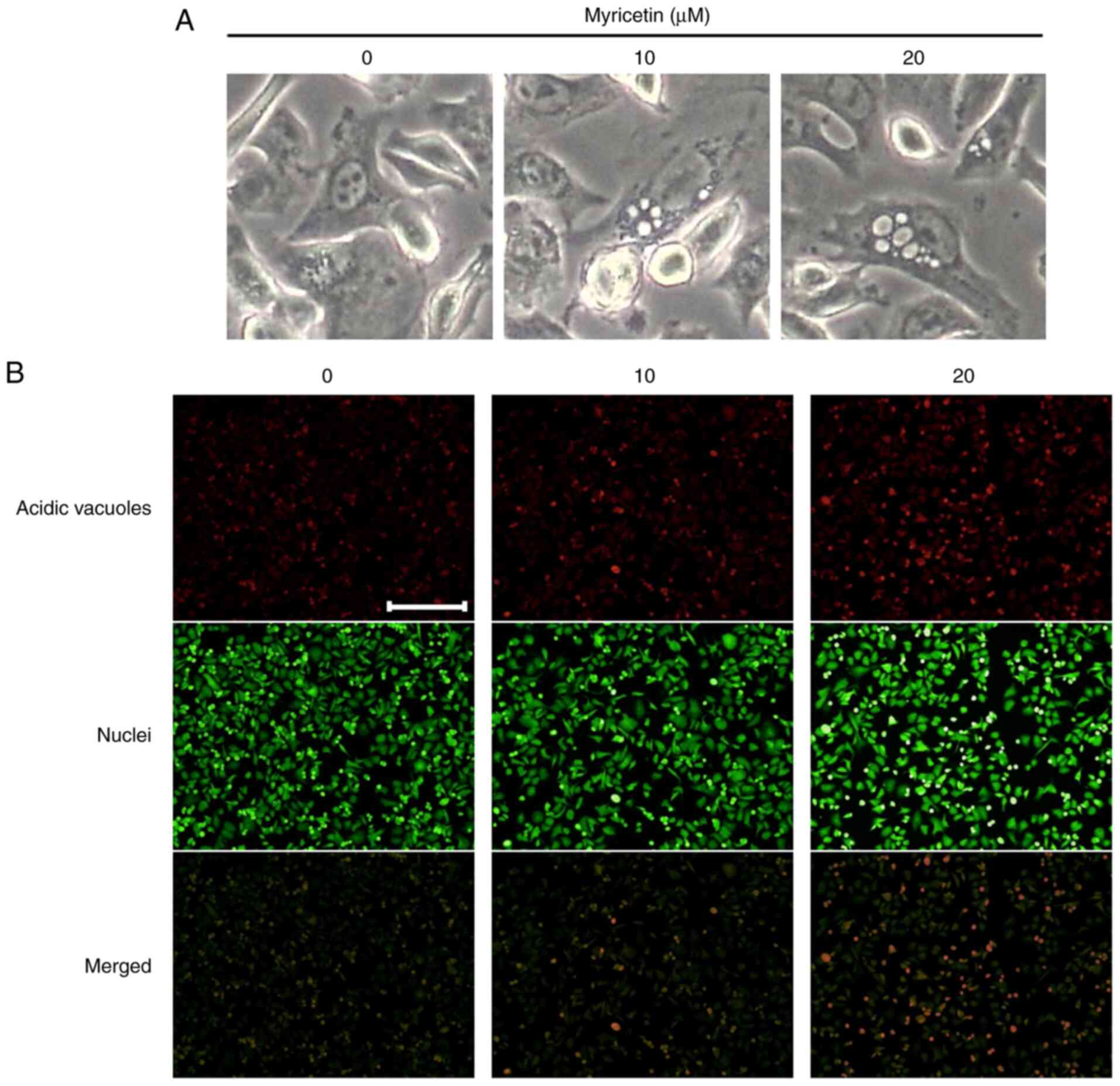
|
1
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar
|
|
3
|
Brown LC, Mutter RW and Halyard MY:
Benefits, risks, and safety of external beam radiation therapy for
breast cancer. Int J Womens Health. 7:449–458. 2015.PubMed/NCBI
|
|
4
|
Seltzer JH, Gintant G, Amiri-Kordestani L,
Singer J, Koplowitz LP, Moslehi JJ, Barac A and Yu AF: Assessing
cardiac safety in oncology drug development. Am Heart J.
214:125–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra S and Dash R: Natural products for
the management and prevention of breast cancer. Evid Based
Complement Alternat Med. 2018:83246962018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar
|
|
7
|
Song X, Tan L, Wang M, Ren C, Guo C, Yang
B, Ren Y, Cao Z, Li Y and Pei J: Myricetin: A review of the most
recent research. Biomed Pharmacother. 134:1110172021. View Article : Google Scholar
|
|
8
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devi KP, Rajavel T, Habtemariam S, Nabavi
SF and Nabavi SM: Molecular mechanisms underlying anticancer
effects of myricetin. Life Sci. 142:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masuda T, Miura Y, Inai M and Masuda A:
Enhancing effect of a cysteinyl thiol on the antioxidant activity
of flavonoids and identification of the antioxidative thiol adducts
of myricetin. Biosci Biotechnol Biochem. 77:1753–1758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng AW, Chen YQ, Zhao LQ and Feng JG:
Myricetin induces apoptosis and enhances chemosensitivity in
ovarian cancer cells. Oncol Lett. 13:4974–4978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ong KC and Khoo HE: Biological effects of
myricetin. Gen Pharmacol. 29:121–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Chen J, Yu X, Xu S, Li D, Zheng Q
and Yin Y: Myricetin suppresses the propagation of hepatocellular
carcinoma via down-regulating expression of YAP. Cells. 8:3582019.
View Article : Google Scholar :
|
|
15
|
Ye C, Zhang C, Huang H, Yang B, Xiao G,
Kong D, Tian Q, Song Q, Song Y, Tan H, et al: The natural compound
myricetin effectively represses the malignant progression of
prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4
interaction. Cell Physiol Biochem. 48:1230–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo S, Ha TK, Han SH, Kim ME, Jung I, Lee
HW, Bae SK and Lee JS: Myricetin induces apoptosis of human
anaplastic thyroid cancer cells via mitochondria dysfunction.
Anticancer Res. 37:1705–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao D and Zhang XD: Myricetin suppresses
p21-activated kinase 1 in human breast cancer MCF-7 cells through
downstream signaling of the β-catenin pathway. Oncol Rep.
36:342–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soleimani M and Sajedi N: Myricetin
apoptotic effects on T47D breast cancer cells is a P53-independent
approach. Asian Pac J Cancer Prev. 21:3697–3704. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knickle A, Fernando W, Greenshields AL,
Rupasinghe HPV and Hoskin DW: Myricetin-induced apoptosis of
triple-negative breast cancer cells is mediated by the
iron-dependent generation of reactive oxygen species from hydrogen
peroxide. Food Chem Toxicol. 118:154–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morales P and Haza AI: Selective apoptotic
effects of piceatannol and myricetin in human cancer cells. J Appl
Toxicol. 32:986–993. 2012. View Article : Google Scholar
|
|
21
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elumalai P, Gunadharini DN, Senthilkumar
K, Banudevi S, Arunkumar R, Benson CS, Sharmila G and Arunakaran J:
Induction of apoptosis in human breast cancer cells by nimbolide
through extrinsic and intrinsic pathway. Toxicol Lett. 215:131–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Naturey. 410:37–40. 2001. View Article : Google Scholar
|
|
26
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He C and Levine B: The beclin 1
interactome. Curr Opin Cell Biol. 22:140–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oberstein A, Jeffrey PD and Shi Y: Crystal
structure of the Bcl-XL-beclin 1 peptide complex: Beclin 1 is a
novel BH3-only protein. J Biol Chem. 282:13123–13132. 2007.
View Article : Google Scholar
|
|
32
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
33
|
Huang H, Chen AY, Ye X, Li B, Rojanasakul
Y, Rankin GO and Chen YC: Myricetin inhibits proliferation of
cisplatin-resistant cancer cells through a p53-dependent apoptotic
pathway. Int J Oncol. 47:1494–1502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cummings BS, Wills LP and Schnellmann RG:
Measurement of cell death in Mammalian cells. Curr Protoc
Pharmacol. 56:12.8.1–12.8.24. 2012. View Article : Google Scholar
|
|
35
|
Yoo GS, Lee JM, Lee CH, Jang JB and Lee
KS: Study of apoptosis by scirpi tuber in Hela cell and MCF-7 cell.
J Korean Obstet Gynecol. 24:1–13. 2011.
|
|
36
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Innajak S, Mahabusrakum W and
Watanapokasin R: Goniothalamin induces apoptosis associated with
autophagy activation through MAPK signaling in SK-BR-3 cells. Oncol
Rep. 35:2851–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Traganos F and Darzynkiewicz Z: Lysosomal
proton pump activity: Supravital cell staining with acridine orange
differentiates leukocyte subpopulations. Methods Cell Biol.
41:185–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu ML, Zhang PM, Jiang M, Yu SW and Wang
L: Myricetin induces apoptosis and autophagy by inhibiting
PI3K/Akt/mTOR signalling in human colon cancer cells. BMC
Complement Med Ther. 20:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu H, Wu CL, Wang X, Ban Q, Quan C, Liu M,
Dong H, Li J, Kim GY, Choi YH, et al: SP600125 enhances C-2-induced
cell death by the switch from autophagy to apoptosis in bladder
cancer cells. J Exp Clin Cancer Res. 38:4482019. View Article : Google Scholar :
|
|
42
|
Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang
Z, Mei N and Guo L: The role of autophagy in usnic acid-induced
toxicity in hepatic cells. Toxicol Sci. 142:33–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin HO, Hong SE, Park JA, Chang YH, Hong
YJ, Park IC and Lee JK: Inhibition of JNK-mediated autophagy
enhances NSCLC cell sensitivity to mTORC1/2 inhibitors. Sci Rep.
6:289452016. View Article : Google Scholar :
|