Introduction
Osteoclasts are multinucleated bone-resorbing cells
that differentiate from macrophages in bone marrow.
Osteoclastogenesis is primarily governed by two key cytokines,
receptor activator of the nuclear factor-κB (NF-κB) ligand (RANKL)
and macrophage colony-stimulating factor (M-CSF) (1). Osteoprotegerin (OPG), a
glycoprotein mainly synthesized by osteoblasts, functions as a
decoy receptor for RANKL. OPG binds to RANK and blocks its
activity, which results in the inhibition of osteoclast
differentiation and subsequent bone resorption (2). RANK, RANKL and the OPG system can
form a tertiary complex, suggesting that OPG is not only a soluble
decoy receptor for RANKL, but can also be considered as a direct
effector of osteoclast functions (3).
OPG disrupts the attachment structure of osteoclasts
and activates SRC proto-oncogene, non-receptor tyrosine kinase
(SRC) (4). SRC is an adaptor
protein that competes for the reduced amount of phosphorylated
protein tyrosine kinase 2 (PYK2) remaining following OPG short-term
treatment via calcium- and extracellular signal-regulated kinase
(ERK)-dependent signaling pathways (5). In this process, OPG short-term
treatment reduces the intracellular calcium concentration. However,
adenosine triphosphate (ATP) can increase the calcium concentration
and inhibit damage to adhesion structuresinduced by OPG (6). Extracellular ATP is viewed as a
primary messenger as it stimulates purinergic receptor P2X7 (P2X7R)
at the cell surface of autocrine or paracrine cells. Additionally,
P2X7R has been detected in osteoclasts and osteoblasts. Therefore,
P2X7R may play an important role in osteoclast function.
P2X receptors are ATP-gated, non-selective cation
channels, which are divided into seven subtypes. When ATP binds to
a P2X receptor, it causes cytomembrane depolarization and the
subsequent elevation of the intracellular calcium concentration,
either by direct Ca2+ permeation or by the activation of
voltage-gated Ca2+ channels, which triggers a range of
signaling cascades, resulting in both short- and long-term cellular
events (7). P2X receptors are
widely expressed in a number of tissues and cells (8). However, P2X7R differs from the
other P2XRs to a certain degree. First, the brief activation of
P2X7R results in rapid membrane depolarization similar with other
P2XR; however, within seconds, a more profound development of an
additional permeability state occurs, which allows for the
permeation of large cations with a molecular weight of up to 900
kDa. Secondly, 3′-O-(4-Benzoyl)benzoyl ATP (BzATP) is a more potent
activator of P2X7R than ATP, and its activation induces cellular
fusion or apoptosis (9).
However, a recent study indicated that purinergic signaling occurs
in bone metabolism (10);
therefore, the present study focused on P2X7R-mediated signaling in
bone.
P2X7R plays an important role in bone metabolism,
being expressed by both osteoclasts and osteoblasts, in which it
plays a role in modulating differentiation, function and lifespan
(11). It has also been
demonstrated that BALB/c P2x7r−/− miceexhibit
increased bone loss; BALB/c P2x7r−/− derived
precursors have been shown to generate a slightly greater number of
osteoclasts, although with a significant reduction in the amount of
resorption per osteoclast (12).
The nuclear translocation of nuclear factor of activated T-cells 1
(NFATc1) in RANKL- and M-CSF-primed monocytes and in mature,
resorbing osteoclasts has been shown to be dependent on P2X7R
activation (13). Moreover,
abolishing P2X7R activity would interfere with calcium signaling,
thereby influencing osteoclast function by affecting the formation
of the ruffled border and subsequently, bone resorption (14). Therefore, P2X7R plays a critical
role in the regulation of osteoclast fusion and resorption.
However, it is not clear whether P2X7R participates in regulating
osteoclast adhesion structure function induced by OPG.
The present study aimed to examine the regulatory
effects of P2X7R on damage to osteoclast adhesion structures
induced by OPG. It was hypothesized that the effects of P2X7R on
OPG-induced damage to osteoclast function would be mediated via
mitogen-activated protein kinase (MAPK) signaling. Moreover, P2X7R
may be a novel target with OPG in the treatment of
osteoporosis.
Materials and methods
Animals
In the present study, all mice were provided by
Yangzhou University. BALB/c male mice were used (weight, 10-15 g;
age, 4 weeks). All mice were sacrificed by cervical dislocation
method and mouse death was verified by the cessation of respiratory
movements. A total of 40 mice were used for the isolation of
primary cells. No mouse died during the experimental period, which
was 4 weeks. Mouse health and behavior were monitored every day.
All mice were kept in specific pathogen-free (SPF) animal housing
with a temperature of 18-25°C, a humidity of 30-50% anda 12-h
dark/light cycle every day for 1 month. All mice were also provided
with free access to food and water.
Cell culture and osteoclast
induction
Mouse bone marrow-derived macrophages (BMMs) were
used for osteoclast differentiation. BMMs can continually
proliferate under M-CSF stimulation, and can differentiate into
multinuclear cells under RANKL stimulation (4). In the present study, BMMs were
isolated from the femurs and tibiae of BALB/c mice, the marrow was
aspirated from the marrow cavity using a syringe to collect the
cells, seeded and cultured in α-minimal essential medium (MEM)
(Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.) overnight in
humidified atmosphere of 5% CO2 at 37°C. The
experimental procedures were approved by the Ethics Committee of
Yangzhou University [SYXK (Su) 2017-0044]. The suspended cells were
used as osteoclast precursors and further cultured in the presence
of M-CSF (30 ng/ml) and RANKL (60 ng/ml; R&D Systems, Inc.) for
5 days. The medium was changed every 2 days. At the end of the
culture period, mature osteoclasts were visualized using
tartrate-resistant acidic phosphatase (TRAP) staining as described
below. The expression of osteoclast adhesion proteins was examined
by using various concentrations of OPG (0, 20, 40 and 80 ng/ml);
according to the results, the concentration of 80 ng/ml OPG was
used in follow-up experiments.
Cell transfection
A P2X7R short hairpin RNA (shRNA) knockdown
construct was constructed by Hanheng (Shanghai) Corporation Ltd.
Transfection was performed using shRNA-P2X7R and NC-shRNA
adenovirus and the silencing efficiency was determined using
western blot analysis. The cell transfection method was previously
described in the study by Ma et al (14). An adenoviral vector containing
shRNA-P2X7 (MOI, 1-3) was introduced into BMMs in the presence of
M-CSF (30 ng/ml); after 24 h, the transduction efficiency was
observed using fluorescence microscopy, and the silencing and
overexpression efficiency were determined using western blot
analysis. NC-shRNA was used as the non-targeting sequence, and the
sequences of the shRNAs and NC-shRNA were as follows: NC-shRNA: Top
strand, aat tcG TTC TCC GAA CGT GTC ACG TAA TTC AAG AGA TTA CGA CAC
GTT CGC AGA ATT TTT Tg; bottom strand, gat ccA AAA AAT TCT CCG AAC
GTG TCA CGT AAT CTC TTG AAT TAC GTG ACA CGT TCG GAG AAC g. shRNA:
Top strand, AAT TCG ACG AAG TTA GGA CAC AGC ATC TTT Gtt caa gag aCA
AAG ATG CTG TGT CCT AAC TTC GTt ttt ttg; bottom strand, GAT CCA AAA
AAA CGA AGT TAG GAC ACA GCA TCT TTG Tct ctt gaa CAA AGA TGC TGT GTC
CTA ACT TCG TCg GAT CCA AAA AAA CGA AGT TAG GAC ACA GCA TCT TTG Tct
ctt gaa CAA AGA TGC TGT GTC CTA ACT TCG TCg.
TRAP staining
BMMs were cultured in the presence of RANKL and
M-CSF, following BMMs were treated with A438079 (ab120413; Abcam)
for 12 h, after 5 days, the cells were fixed in 10%
paraformaldehyde for 10 min. After washing with PBS, the cells were
stained using a TRAP-Kit 387A (Sigma-Aldrich; MerckKGaA) according
to the manufacturer's instructions. TRAP-positive mature
osteoclasts having more than three nuclei were observed and counted
using a light microscope (LEICA DMI 3000B; Leica Microsystems
GmbH).
Western blot analysis
The cells were washed twice with cold PBS and lysed
in radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute
of Biotechnology) on ice for 30 min, and then centrifuged at 12,000
× g for 10 min to precipitate the cell debrisat 4°C. The protein
concentration was determined using a bicinchoninic acid (BCA)
protein assay kit. The western blot analysis protocol was as
previously described in the study by Fathi et al (15). Equal amounts of protein were
separated using 10-12% SDS-PAGE. The proteins in the gel were
transferred onto polyvinylidene fluoride (PVDF) membranes. After
blocking with 5% skim milk in Tris-buffered saline-Tween-20 (TBST)
for 2 h atroom temperature, the membranes were incubated overnight
with primary antibodies at 4°C. The primary antibodies were as
follows: Anti-phospho (p)-PYK2 (phospho Y402) (1:1,000; ab4800),
anti-PYK2 (1:1,000; ab32571), anti-vinculin (1:1,000; ab129002)
(all from Abcam), anti-p-vinculin (1:1,000; SAB4301470;
Sigma-Aldrich; MerckKGaA), anti-paxillin (1:1,000; ab32084),
anti-p-paxillin (phosphoY31; 1:1,000; ab4832), anti-p-paxillin
(phospho S126; 1:1,000; ab24402), anti-integrin αv (1:1,000;
ab179475), P2X7 (1:1,000; ab259942) (all from Abcam), antiintegrin
β3 (1:500; ET1606-49; HUABIO), anti-SRC (1:1,000; #2109),
anti-p-SRC (Tyr416; 1:1,000; #2101) and anti-p-SRC (Tyr527;
1:1,000; #2105), ERK (1:1,000; #4695), p-ERK (1:1,000; #4370), JNK
(1:1,000; #9252), p-JNK (1:1,000; #4668), p38 (1:1,000; #8690),
p-p38 (1:1,000; #4511), (all from Cell Signaling Technology, Inc.).
The following day, the membranes were washed and incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:5,000; #7076 and #7074; Cell Signaling Technology, Inc.) for 2 h
atroom temperature. The immunoreactive proteins on the membranes
were then visualized using chemiluminescence (cat. no. P2300, New
Cell & Molecular Biotech). Densitometric quantification was
performed using ImageJ software 1.48 (National Institutes of
Health). The protein expression level was calculated according to
the gray value of the bands.
Immunofluorescence staining
The cells were cultured for 5 days following
treatment with various reagents [U0126 (ERK inhibitor), 5
µm; SP600125 (JNK inhibitor), 10 µM; SB202190 (MAPK
inhibitor), 5 µM; MedChemExpress] and 30 µM BzATP
(ab120444; Abcam) for 30 min and OPG (80 ng/ml) for 12 h, and then
fixed using 10% paraformaldehyde for 30 min, permeabilized with
0.3% Triton X-100 for 20 min, and washed with PBS three times. The
cells were first incubated in 5% skim milk in TBST for 30 min, and
then incubated overnight with primary antibodies at 4°C, (the
primary antibodies were the same as those used for western blot
analysis; dilution, 1:200), washed three times in PBS,
Subsequently, the coverslips were washed and incubated for 2 h at
room temperature with Alexa Fluor® 488-conjugated
secondary antibody (cat. no. A0428; 1:200) F-actin was stained with
phalloidin (ab176756; Abcam) for 40 min at room temperature,
followed by washing with PBS. The nuclei were stained using
4′,6-diamidino-2-phenyl-indole (DAPI) for 15 min at room
temperature, and covered using glass coverslips. The
immunofluorescent stained cells were viewed and images captured
using confocal laser scanning microscopy (LSM 880NL0; Carl Zeiss).
The OPG concentration was used acording to the study by Zhao et
al (5). The method used
forimmunofluorescence staining was as described in the study by
Fathi et al (16).
Scanning electron microscopy (SEM)
After the cells were treated with OPG (80 ng/ml) for
12 hand various reagents (U0126, 5 µM; SP600125, 10
µM; SB202190, 5 µM) for 30 min, the cells were fixed
in 2.5% glutaraldehyde solution overnight at 4°C, dehydrated using
increasing concentrations of ethanol from 50-100%, dried and
gold-coated (as the sample does not conduct electricity), using the
carbon coater for gold coating (MC1000; Hitachi, Ltd.). Cell
morphologies were observed using a Hitachi S-4800 Field-Emission
Environmental Scanning Electron Microscope (Hitachi Corporation).
The SEM method was as previously described in the study by Ma et
al (14).
Statistical analysis
Each experiment was repeated at least three times
andno data were excluded. All data were analyzed using SPSS 22.0
software (SPSS, Inc.). The results are expressed as the mean ±
standard deviation. An independent samples t-test (Student's
t-test) was used for two-sample comparisons for data demonstrating
a normal distribution. The t-test used was unpaired in the present
study. Multiple groups were compared using one-way analysis of
variance (ANOVA) followed by Tukey's or Tamhane's test. P<0.05
was considered to indicate statistically significant
differences.
Results
OPG suppresses osteoclast
adhesion-related protein expression
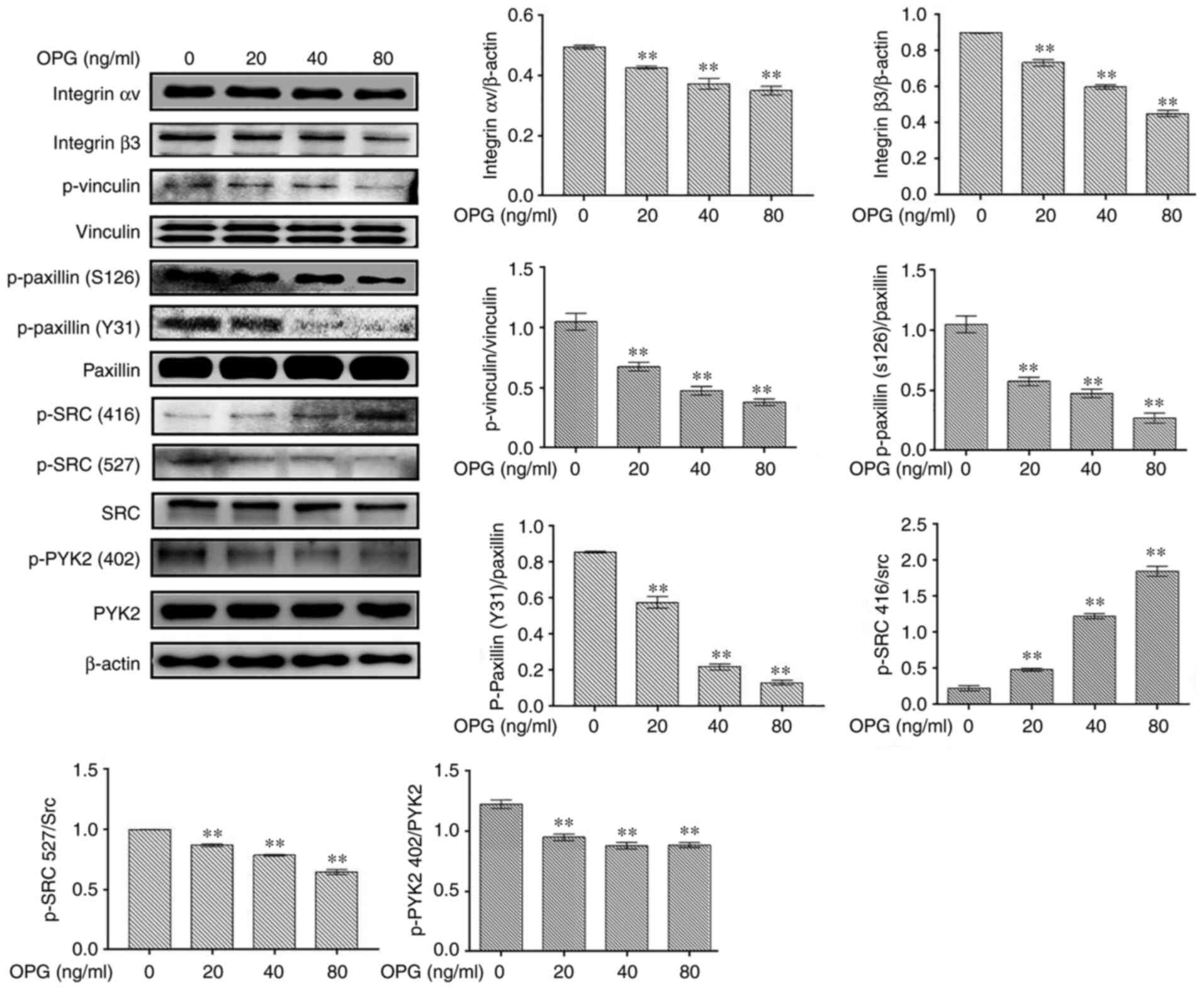
The levels of osteoclast adhesion-related
proteinswere examined using western blot analysis. In response to
OPGstimulation, the levels of osteoclast adhesion proteins,
including integrin αv, integrin β3, p-vinculin, p-paxillinand p-SRC
(527), were significantly decreased in in a concentration-dependent
manner (Fig. 1). However, the
phosphorylation of SRC at amino acid 416 was increased following
treatment with OPG, in a concentration-dependent manner (Fig. 1). Taken together, these data
demonstrated that OPG inhibited the expression of osteoclast
adhesion-related proteins.
OPG damages osteoclast adhesive
structures via the MAPK signaling pathways
Firstly, MAPK signaling path-ways were examined
usingwestern blot analysis. The data demonstrated OPG reduced the
phosphorylation of ERK, JNK and p38 in a concentration-dependent
manner, and the phosphorylation of ERK, JNK and p38 also
significantly decreased AT different time pointS by OPG treatment
(Fig. 2A). These results
suggested that OPG inhibited the MAPK signaling pathways.
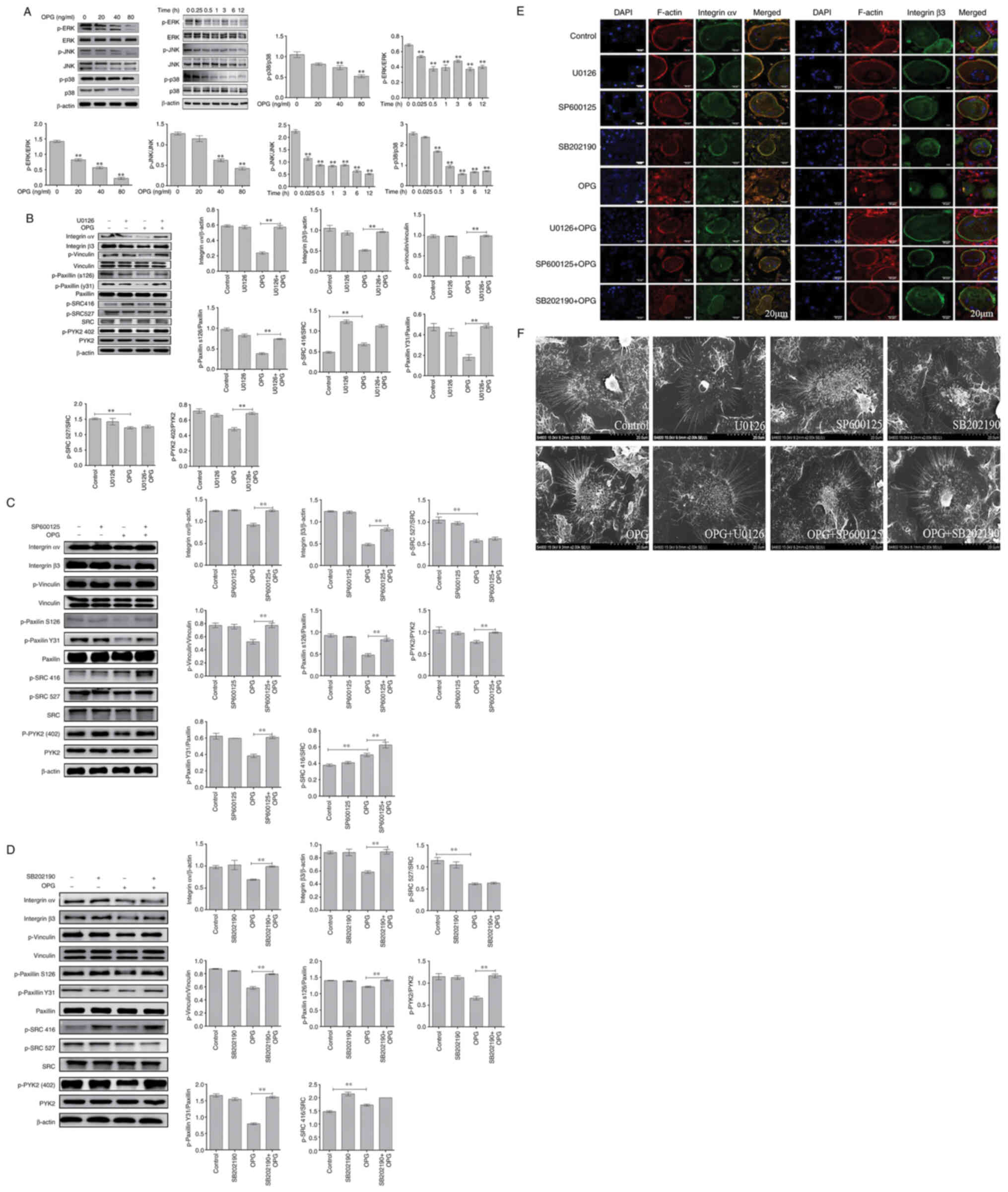 | Figure 2OPG damages osteoclast adhesive
structures via the MAPK signaling pathway. Osteoclasts were
differentiated from mouse bone marrow-derived macrophages in
24-well culture plates, and then cultured in α-MEM in the presence
of receptor activator of the nuclear factor-κB (NF-κB) ligand and
macrophage colony-stimulating factor for 5 days. (A) The activation
of MAPK signaling was determined using western blot analysis.
**P<0.01. (B-D) Effects of U0126, SB202190 and
SP600125 on OPG-mediated osteoclast adhesion structures.
Osteoclasts were pre-treated with 5 µM U0126, 10 µM
SP600125 or 5 µM SB202190 for 30 min followed by 80 ng/ml
OPG for a further 12 h. Western blot analysis was performed for
osteoclast adhesion-related proteins in osteoclasts exposed to OPG,
or OPG and inhibitors. **P<0.01. (E and F)
Osteoclasts were fixed, permeabilized and stained for integrin αv
and β3, actin and nuclei. (E) Colocalization of integrin αv and β3
was analyzed using a confocal fluorescence microscope. Scale bars,
20 µm. (F) Osteoclast adhesion structures were examined
using scanning electron microscopy. Scale bars, 100 µm. Data
are representative of three independent experiments. OPG,
osteoprotegerin; PYK2, phosphorylated protein tyrosine kinase 2;
SRC, SRC proto-oncogene, non-receptor tyrosine kinase |
Subsequently, specific inhibitors of ERK(U0126), JNK
(SP600125) and p38 (SB202190) were used. As shown in Fig. 2B, the decrease in the levels of
osteoclast adhesion proteins was significantly reversed when the
cells were co-treated with OPG and U0126. The results revealed that
when osteoclasts were co-treated with OPG (80 ng/ml) and U0126 (5
µm), the levels of key osteoclast adhesion proteins were
higher than thosein the OPG group, and the phosphorylation levels
of vinculin and paxillinwere mostly recovered compared with those
in the OPG group. In addition, the data also revealed that OPG
altered the phosphorylation levels of SRC at 416 and 527; however,
no recovery of the SRC phosphorylation levels was observed in the
group co-treated with OPG and U0126. Using the same method, the
osteoclasts were cotreated with OPG, SP600125 (10 µm) and
SB202190 (5 µm), respectively. As shown in Fig. 2C and D, co-treatment with OPG and
the inhibitors markedly increased the levels of osteoclast
adhesion-related proteins, which was consistent with the results
obtained with U0126.
Subsequently, the distribution of adhesion-related
proteins was analyzed using immunofluorescence staining, including
integrin αv, integrin β3, vinculin, paxillin, SRC and PYK2. As
shown in Fig. 2E, the
distribution of integrin αv and integrin β3 and F-actin was very
similar, being co-localized in the periphery of the osteoclasts.
This co-localization was not observed in the OPG-treated group. OPG
completely disrupted the distribution of integrin αv and integrin
β3 to the osteoclast periphery. The labeling of integrinwas diffuse
inside the osteoclasts or even disappeared. Of note, the
co-treatment group exhibited a markedly altered integrin αv and
integrin β3 location, with co-localization being observed at the
osteoclast periphery. For other adhesion proteins (Fig. S1), including vinculin and
paxillin, the distribution was consistent compared with that of
integrin, However, SRC and PYK2 remained localized in the cell
center or exhibited a diffuse distribution after treatment with OPG
and inhibitors, which was consistent with the results of western
blot analysis.
Finally, SEM was used to analyze the morphological
changes of the adhesion structures in osteoclasts. The adhesion
structure was distributed as a circular zone near the periphery of
the osteoclast and outside this peripheral circular zone, including
the lamellipodia and filopodia. Following treatment with OPG, as
shown in Fig. 2F, a severe
retraction and reduction of lamellipodia and filopodia-like
structures was observed, some of which were detached from the
substrate. Moreover, the osteoclast adhesion structures in the
control and inhibitor groups exhibited no obvious changes. The OPG
and inhibitor co-treatment groups exhibited no marked retraction
and reduction in osteoclast lamellipodia and filopodia, which
remained attached to the substrate. The aforementioned results
indicated that MAPK was involved in regulating osteoclast adhesion
structures.
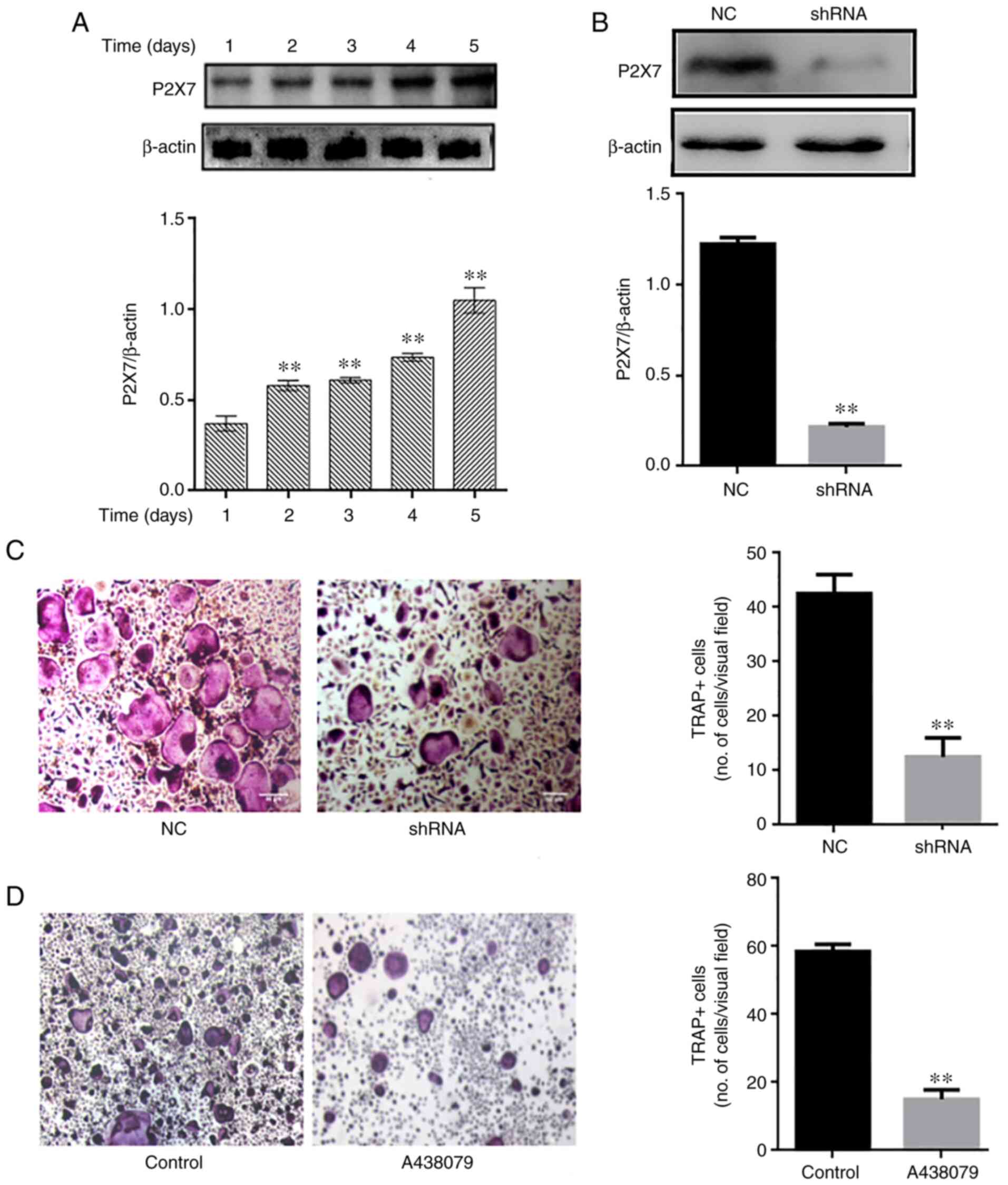
Absence of P2X7R inhibits osteoclast
differentiation
P2X7R expression was analyzed during osteoclast
differentiation. The P2X7R levels were upregulated over time (1-5
days) during osteoclastogenesis (Fig. 3A). Subsequently, mouse BMMs were
transfected with an adenovirus-mediated P2X7R-shRNA, and control
and blank interference adenoviruses, and then cultured in the
presence of RANKL (60 ng/ml) and M-CSF (30 ng/ml). The silencing
efficacy of P2X7R was determined using western blot analysis
(Fig. 3B) The results revealed
that P2X7R deficiency suppressed the formation of TRAP-positive
multinucleated osteoclasts compared with the control and blank
interference adenovirus (Fig.
3C). To further illustrate the effect of P2X7R, the BMMs were
treated with the P2X7R inhibitor, A438079, which does not affect
cell proliferation. After 5 days, A438079 (50 µm/l)
significantly inhibited the formation of multinucleated osteoclasts
(Fig. 3D). These results
suggested that P2X7R plays a crucial role in osteoclast
differentiation.
Effect of MAPK signaling inhibitors on
P2X7R expression
In the present study, osteoclasts treated with OPG
(80 ng/ml) for 12 h were examined for P2X7R expression levels. The
results revealed that OPG reduced the expression of P2X7R in a
concentration-dependent manner, and the expression of P2X7R also
significantly decreased at different time points by OPG treatment
(Fig. 4A). To further illustrate
thatthe OPG-induced reduction in P2X7R levels was related to MAPK
signaling, specific inhibitors of ERK (U0126), JNK (SP600125) and
p38 (SB202190) were used and P2X7R expression was examined. The
results revealed that the OPG-induced reduction in P2X7R levels was
partially or absolutely reversed using the specific inhibitors
(Fig. 4B).
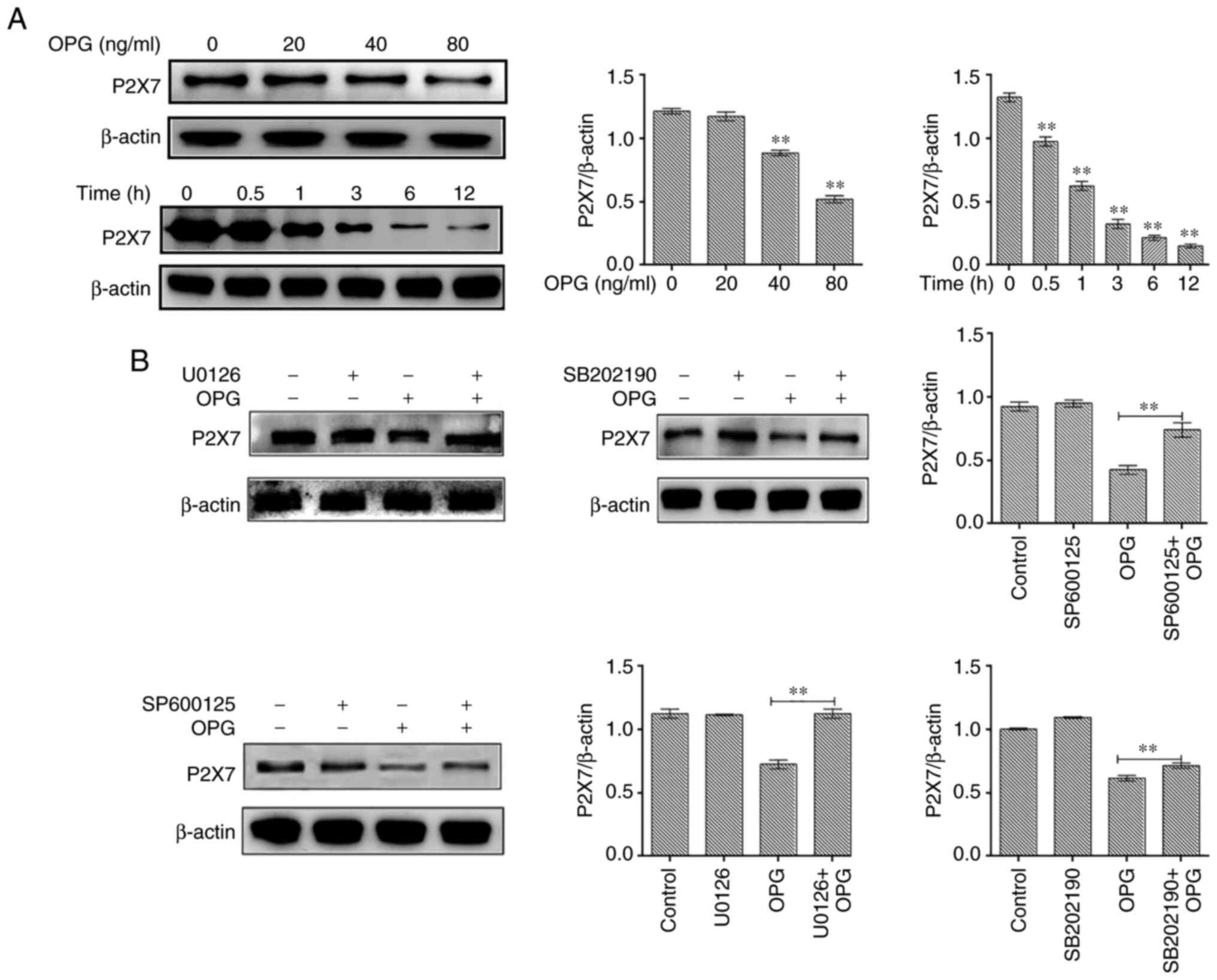 | Figure 4Effect of MAPK signaling inhibitors
on P2X7 expression. (A) Mouse bone marrow-derived macrophages were
cultured in the presence of macro-phage colony-stimulating factor
and receptor activator of the nuclear factor-κB (NF-κB) ligand for
5 days, followed by treatment with OPG (0, 20, 40 and 80 ng/ml) for
a further 12 h, and treatment with OPG (80 ng/ml) for different
time periods (0, 0.5, 1, 3, 6, 12 h). The expression of P2X7R was
examined using western blot analysis. **P<0.01. (B)
Osteoclasts were pre-treated with U0126, SB202190 and SP600125 for
30 min followed by 80 ng/ml OPG for a further 12 h. The expression
of P2X7R was examined using western blot analysis.
**P<0.01 vs. control or as indicated by the lines on
the graphs. Data are representative of three independent
experiments. OPG, osteoprotegerin; PYK2, phosphorylated protein
tyrosine kinase 2. |
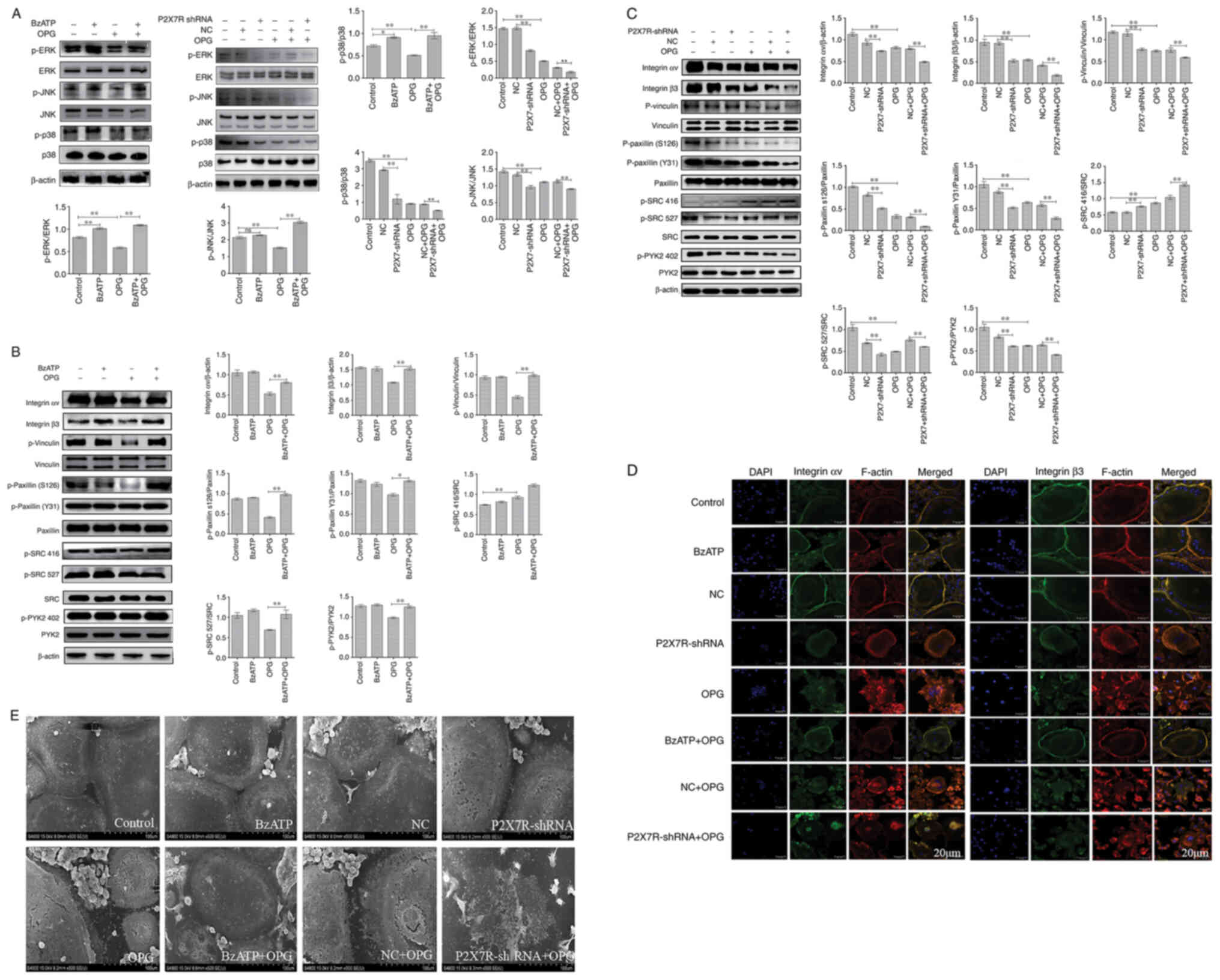
Effect of BzATP/P2X7R-shRNA on
OPG-induced osteoclast adhesion function and MAPK signaling
Osteoclasts were treated with BzATP for 30 min,
followed by OPG treatment for 12 h. In addition, the BMMs were
transfected with adenovirus with P2X7R-shRNA in the presence of
M-CSF and RANKL. As shown in Fig.
5A, compared with the control group, the levels of p-p38 and
p-ERK, but not those of p-JNK, increased significantly following
treatment with BzATP. P2X7R-shRNA decreased the phosphorylation
levels of ERK, JNK and p38 MAPK compared with those in the negative
control group. The results also revealed that OPG markedly
decreased the phosphorylation levels of ERK, JNK and p38. However,
this phenomenon was reversed or aggravated by BzATP or P2X7R-shRNA,
respectively. Therefore, these results indicated that BzATP induced
the activation of P2X7R, which activated ERK and p38. By contrast,
the depletion of P2X7R markedly inhibited MAPK signaling
activation.
Subsequently, the present study examined the effects
of BzATP and P2X7R-shRNA on OPG-induced osteoclast adhesion
function. First, western blot analysis was used to assessthe levels
of adhesion-related protein following treatment with BzATP,
P2X7R-shRNA and OPG. As shown in Fig. 5B and C, the levels of integrin αv
and β3 markedly decreased in the OPG group. BzATP and OPG
co-treatment significantly increased the integrin αv and β3 levels;
however, P2X7R-shRNA and OPG co-treatment further decreased the
levels of integrin αv and β3 compared with those in the negative
control plus OPG group. The results also revealed that OPG reduced
the phosphorylation levels of vinculin and paxillin; however, the
activation of P2X7R with BzATP markedly increased the
phosphorylation levels of vinculin and paxillin. By contrast, the
depletion of P2X7R markedly decreased the phosphorylation levels of
vinculin and paxillin, further disrupting the osteoclast adhesion
ability. The data also demonstrated a significant reduction in PYK2
phosphorylation at position 402 and SRC phosphorylation at position
527 under OPG treatment. BzATP and P2X7R-shRNA treatment increased
and decreased, respectively, the activation of PYK2 and SRC. Of
note, OPG increased the level of SRC phosphorylation at position
416; however, BzATP and OPG co-treatment did not recover the level
of SRC phosphorylation at position 416; P2X7R-shRNA and OPG
co-treatment also did not reverse the level of SRC phosphorylation
at position 416.
As shown in Fig.
5D, OPG treatment resulted in a disrupted distribution of
integrin αv and β3. However, the OPG-induced damage to the
osteoclast adhesion structures was significantly aggravated when
the cells were transfected with P2X7R-shRNA. This was confirmed by
the observation that the OPG-induced disruption of integrin αv and
β3 was markedly attenuated when the cells were pre-treated with
BzATP for 30 min. In addition, the levels of other adhesion
proteins, examined by immunofluorescence staining, were consistent
with those of integrin αv and β3 (Fig. S2). Taken together, these results
further suggested that P2X7R plays a crucial role in regulating the
damage to osteoclast adhesion structures induced by OPG.
SEM was then used to observe osteoclast morphology.
As shown in Fig. 5E, SEM
revealedthat OPG severely damaged osteoclast pseudopodia, including
filopodia breakage or loss offilopodia, the retraction of
lamellipodiaand detachment from the substrate. However, BzATP
pre-treatment for 30 min reversed these OPG-induced osteoclast
morphological changes. By contrast, the depletion of P2X7R did not
recover osteoclast intact morphology, but further aggravated the
OPG-induced adhesive structure damage. The results of the present
study thus suggest that P2X7R-mediated MAPK signaling plays an
essential role inresisting OPG-induced damage to osteoclast
adhesion.
Discussion
The critical role of P2X7R in bone pathogenesis has
been elucidated (11). The
results of the present study demonstrated that P2X7R maybe a
potential target for bone disease therapeutics. The results
indicated that OPG disrupted osteoclast adhesive function by
affecting MAPK signaling. The absence of P2X7R inhibited osteoclast
function, and P2X7R mediated-MAPK signaling was involved in
OPG-induced osteoclast adhesive structure damage.
It has been previously reported that osteoclasts are
multi-nucleated cells generated from monocytes and macrophages,
which adhere to mineralized bone matrix and become polarized,
forming the sealing zone for bone resorption (17). OPG has been observed to inhibit
osteoclast differentiation and bone resorption by blocking the
binding of RANKL to RANK (18).
A previous study (19) also
demonstrated that the cytoskeleton plays an important role in
osteoclast differentiation, maturation and adhesion, Moreover,
filopodia and podosomesundergo drastic and regular variations
during osteoclast differentiation and adhesion. In the present
study, OPG significantly disrupted the filopodia and lamellipodia
in mature osteoclast. In addition, OPG suppressed osteoclastic
adhesion protein expression, including integrin αv, integrin β3,
p-vinculin, p-paxillin and p-SRC at position 527. SRC isan adaptor
protein, and the phosphorylation of SRC at position 416 was
increased by OPG. Previous research has demonstrated that podosomes
and pseudopodium share several components and structural features,
the most important being a multimolecular complex surrounding the
core, which comprises integrin receptors and integrin-associated
proteins that are also found in focal adhesions, such as vinculin
and paxillin (20). Paxillin, an
adaptor protein, is highly phosphorylated on tyrosyl residues and
has been shown to regulate focal adhesion dynamics and cell
migration (21). In the present
study, OPG significantly inhibited the phosphorylation of paxillin
at tyrosine 31 (Y31) and serine (S126). Furthermore, OPG severely
disrupted the distribution of paxillin in mature osteoclasts.
Paxillinplays amain role in regulating osteoclast adhesion and
migration. Additionally, vinculin, as an actinprotein, is closely
related to the maturation of podosomes (22). In the present study, OPG
attenuated the phosphorylation of vinculin and disrupted its
distribution. It has also been found that vinculin-deficient
osteoclasts do not exhibit any actin ring formation or bone
resorption activity (23). In
addition, the cytokine activation of osteoclasts requires
matrix-derived signals transmitted intracellularly via integrin αv
and β3, which induces SRC to stimulate a canonical,
cytoskeleton-organizing complex (24). Herein, the levels of integrinαv
and β3 werereduced following OPG stimulation and exhibited a
scattered distribution in the cell center, which would lead to
osteoclasts losing their adhesion function and to the inhibition of
downstream signaling. Moreover, OPG induced a decrease in PYK2 Tyr
402 and SRC Tyr 527 phosphorylation, where as the phosphorylation
of SRC at Tyr 416 was markedly increased. SRC and PYK2, as part of
the podosome actin cloud, are essential for proper podosome
organization and bone resorption (25). The results of the present study
suggested that OPG disturbed the adhesion structure and the
distribution of podosomes in osteoclasts by causing the
disequilibration of the dormant form of SRC. PYK2-null osteoclasts
have been shown to be unable to form the podosome belt, resulting
in impaired bone resorption (26). This finding is consistent with
the findings of the present study, in which OPG inhibited PYK2
activity, resulting in podosome disassembly and impaired osteoclast
resorption ability.
M-CSF and RANKL playan essential role through MAPK
signaling pathways during osteoclast differentiation and bone
resorption. Of note, in the present study, OPG significantly
suppressed MAPK signaling. A previous study demonstrated that OPG
suppressed osteoclast differentiation by inhibiting the
phosphorylation of ERK1 and ERK2, and directly induced podosome
disassembly in osteoclasts (4).
In the present study, the blocking of ERK signaling using U0126
significantly attenuated the damaging effects of OPG. In addition,
JNK and p38 signaling also plays an important role in the
regulation of apoptosis, and the formation and differentiation of
osteoclasts (27,28). OPG, as an upstream signaling
factor, markedly suppressed JNK and p38 activation, ultimately
impairing osteoclast adhesion structures. Several inflammatory
cytokines that negatively influence osteoclastogenesis via JNK and
p38 inactivation have been identified, including IL-3, IL-4, IL-6
and TNF (29-31). Thus, OPG and inflammatory
cytokines play similar roles in regulating JNK and p38 activity,
which affects osteoclast function. In the present study, the
blocking of JNK and p38 signaling protected the adhesion structure
from OPG induced damage. However, ERK, JNK and p38 inhibitorsdid
not recover SRC Tyr 416 and 527 phosphorylation under OPG
induction. Thus, these findings revealed that MAPK signaling wasnot
involved in modulating SRC activation.
P2X7R is a cation channel and can be activated by
ATP (32). It has been
demonstrated that P2X7R is related to diseasesof the central
nervous system (33), multiple
sclerosis (34) and bone
metabolism (35). P2X7R is
expressed in osteoblasts and osteoclasts (36). In the present study, P2X7R was
highly expressed in mature osteoclasts and OPG reduced the
expression level of P2X7R. Previous studies have also demonstrated
that P2X7R-mediated signaling drives osteoclastic fusion (37) and negatively regulates bone
mineralization (38). In the
present study, the depletion of P2X7R significantly reduced the
number of osteoclasts. Aprevious study also demonstrated that the
blockade of P2X7R inhibited osteoclast formation in vitro
(39), which is consistent with
the present findings. The present study also found that the
depletion of P2X7R markedly suppressed osteoclast adhesion
functions. However, a previous study (40) demonstrated that whenRGC-5 cells
were treated with BzATP (a P2X7R agonist), BzATP reversed the
inhibitory effects of Mongolian compound medicine-Gurigumu-13
(GRGM) on cell apoptosis, oxidative stress and the phosphorylation
of p38. P2X7R knockdown was shown to further enhance the inhibitory
effect of GRGM on pp38 (40). Of
note, BzATP enhanced the phosphorylation of p38 and ERK and
reversed the damaging effects of OPG on osteoclast adhesion in the
present study. In addition, P2X7R silencing significantly inhibited
the MAPK pathway and aggravated the damaging effects of OPG. In
recent years, studies have demonstrated that P2X7R activation can
increase cancer cell invasiveness (41) and migration (42). The present study also revealed
that P2X7R activation restored the normal osteoclast adhesion
structure following OPG-induced damage, which suggested that P2X7R
activation would recover adhesion, invasiveness and migration
functions. The silencing of P2X7R further promoted the OPG-induced
damage to the adhesion structure. In brief, P2X7R-mediated MAPK
signaling plays an essential role in regulating osteoclast adhesion
function, and P2X7R may be a novel target for bone disease
treatment. Furthermore, mesenchymal stem cell therapy may also be
promising in the treatment of a number of diseases (43). In addition, P2X7R, as a targeted
therapeutic strategy, may beworthy of further explorationcompared
with targeted therapeutics for the treatment of acute myeloid
leukemia (44).
In conclusion, the present study demonstrated that
OPG disrupted the osteoclast adhesion structure via P2X7R-mediated
MAPK signaling and revealed the role of P2X7R in mature
osteoclasts. The findings presented herein provide a novel
therapeutic target for bone diseases, such as osteoporosis.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and HZh designed the study. YM and XS performed
the cell transduction and culture. YM, RS and JZ performed the
western blotting and immunofluorescence. YM, XS and HZo analyzed
the data. YM and XS wrote the manuscript. ZL and JZ proofread the
manuscript and confirm the authenticity of all the raw data. All
authors contributed to the article, and read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experimental procedures were approved by
the Ethics Committee of Yangzhou University [SYXK (Su)
2017-0044].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The presentstudy was supported by grants from the National
Nature Science Foundation of China (nos. 31702304, 31872534 and
31502128), the Natural Science Foundation of Jiangsu Province (no.
BK20150447), the Natural Science Foundation Research Grants of
Jiangsu Province (no. BK20181452), the China Postdoctoral Science
Foundation (no. 2017M611932), the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD) and the
Graduate Innovation Project of Jiangsu Province.
Abbreviations:
|
OPG
|
osteoprotegerin
|
|
RANKL
|
receptor activator of the nuclear
factor-κB (NF-κB) ligand
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
P2X7R
|
purinergic receptor P2X7
|
|
PYK2
|
phosphorylated protein tyrosine kinase
2
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
NFATc1
|
nuclear translocation of nuclear
factor of activated T-cells 1
|
References
|
1
|
Xiong J, Piemontese M, Onal M, Campbell J,
Goellner JJ, Dusevich V, Bonewald L, Manolagas SC and O'Brien CA:
Osteocytes, not osteoblasts or lining cells, are the main source of
the RANKL required for osteoclast formation in remodeling bone.
PLoS One. 10:e01381892015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee DW, Kwon JY, Kim HK, Lee HJ, Kim ES,
Kim HJ, Kim HJ and Lee HB: Propofol attenuates osteoclastogenesis
by lowering RANKL/OPG ratio in mouse Osteoblasts. Int J Med Sci.
15:723–729. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Theoleyre S, Wittrant Y, Couillaud S,
Vusio P, Berreur M, Dunstan C, Blanchard F, Rédini F and Heymann D:
Cellular activity and signaling induced by osteoprotegerin in
osteoclasts: involvement of receptor activator of nuclear factor
kappaB ligand and MAPK. Biochim Biophys Acta. 1644:1–7. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao H, Gu J, Dai N, Gao Q, Wang D, Song
R, Liu W, Yuan Y, Bian J, Liu X and Liu Z: Osteoprotegerin exposure
at different stages of osteoclastogenesis differentially affects
osteoclast formation and function. Cytotechnology. 68:1325–1335.
2016. View Article : Google Scholar :
|
|
5
|
Zhao H, Liu X, Zou H, Dai N, Yao L, Zhang
X, Gao Q, Liu W, Gu J, Yuan Y, et al: Osteoprotegerin disrupts
peripheral adhesive structures of osteoclasts by modulating Pyk2
and Src activities. Cell Adh Migr. 10:299–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oshiro T, Shiotani A, Shibasaki Y and
Sasaki T: Osteoclast induction in periodontal tissue during
experimental movement of incisors in osteoprotegerin-deficient
mice. Anat Rec. 266:218–225. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng QZ: Radioligands targeting
purinergic P2X7 receptor. Bioorg Med Chem Lett. 30:1271692020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li LZ, Yue LH, Zhang ZM, Zhao J, Ren LM,
Wang HJ and Li L: Comparison of mRNA Expression of P2X receptor
subtypes in different arterial tissues of rats. Biochem Genet.
58:677–690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miteva A, Gaydukov A and Balezina O:
Interaction between calcium chelators and the activity of P2X7
receptors in mouse motor synapses. Int J Mol Sci. 21:20342020.
View Article : Google Scholar :
|
|
10
|
Dong Y, Chen Y, Zhang L, Tian Z and Dong
S: P2X7 receptor acts as an efficient drug target in regulating
bone metabolism system. Biomed Pharmacother. 125:1100102020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agrawal A and Gartland A: P2X7 receptors:
Role in bone cell formation and function. J Mol Endocrinol.
54:R75–R88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang N, Agrawal A, Jørgensen NR and
Gartland A: P2X7 receptor regulates osteoclast function and bone
loss in a mouse model of osteoporosis. Sci Rep. 8:35072018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agrawal A, Buckley KA, Bowers K, Furber M,
Gallagher JA and Gartland A: The effects of P2X7 receptor
antagonists on the formation and function of human osteoclasts in
vitro. Purinergic Signal. 6:307–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma Y, Zhao H, Chile C, Wang C, Zheng J,
Song R, Zou H, Gu J, YanYuan, Bian J and Liu Z: The effect of
P2X7R-mediated Ca2+ signaling in OPG-induced osteoclasts
adhesive structure damage, Experimental Cell Research. 43:39–98.
2019.
|
|
15
|
Fathi E, Farahzadi R and Valipour B:
Alginate/gelatin encapsulation promotes NK cells differentiation
potential of bone marrow resident C-kit hematopoietic stem cells.
Int J Biol Macromol. 177:317–327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fathi E, Farahzadi R, Vietor I and
Javanmardi S: Cardiac differentiation of bone-marrow-resident
c-kit+ stem cells by L-carnitine increases through
secretion of VEGF, IL-6, IGF-1, and TGF-β as clinical agents in
cardiac regeneration. J Biosci. 45:922020. View Article : Google Scholar
|
|
17
|
Kwon JO, Jin WJ, Kim B, Kim HH and Lee ZH:
Myristoleic acid inhibits osteoclast formation and bone resorption
by suppressing the RANKL activation of Src and Pyk2. Eur J
Pharmacol. 768:189–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong X, Gu J, Song R, Wang D, Sun Z, Sui
C, Zhang C, Liu X, Bian J and Liu Z: Osteoprotegerin inhibit
osteoclast differentiation and bone resorption by enhancing
autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell
Biochem. Sep 6–2018.Epub ahead of print.
|
|
19
|
Song RL, Liu XZ, Zhu JQ, Zhang JM, Gao Q,
Zhao HY, Sheng AZ, Yuan Y, Gu JH, Zou H, et al: New roles of
filopodia and podosomes in the differentiation and fusion process
of osteoclasts. Genetics Mol Res. 13:4776–4787. 2014. View Article : Google Scholar
|
|
20
|
Badowski C, Pawlak G, Grichine A, Chabadel
A, Oddou C, Jurdic P, Pfaff M, Albigès-Rizo C and Block MR:
Paxillin phosphorylation controls invadopodia/podosomes
spatiotemporal organization. Mol Biol Cell. 19:633–645. 2008.
View Article : Google Scholar :
|
|
21
|
Bowden ET, Barth M, Thomas D, Glazer RI
and Mueller SC: An invasion-related complex of cortactin, paxillin
and PKCm associates with invadopodia at sites of extracellular
matrix degradation. Oncogene. 18:4440–4449. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Destaing O, Saltel F, Géminard JC, Jurdic
P and Bard F: Podosomes display actin turnover and dynamic
self-organization in osteoclasts expressing actin-green fluorescent
protein. Mol Biol Cell. 14:407–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukunaga T, Zou W, Warren JT and
Teitelbaum SL: Vinculin regulates osteoclast function. J Biological
Chemistry. 289:13554–13564. 2014. View Article : Google Scholar
|
|
24
|
Zou W, Kitaura H, Reeve J, Long F,
Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP and Teitelbaum SL:
Syk, c-Src, the alphav-beta3 integrin, and ITAM immunoreceptors, in
concert, regulate osteoclastic bone resorption. J Cell Biol.
176:877–888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Novack DV and Faccio R: Osteoclast
motility: Putting the brakes on bone resorption. Ageing Res Rev.
10:54–61. 2011. View Article : Google Scholar
|
|
26
|
Buckbinder L, Crawford DT, Qi H, Ke HZ,
Olson LM, Long KR, Bonnette PC, Baumann AP, Hambor JE, Grasser WA
III, et al: Prolinerich tyrosine kinase 2 regulates osteoprogenitor
cells and bone formation, and offers an anabolic treatment approach
for osteoporosis. Proc Natl Acad Sci USA. 104:10619–10624. 2007.
View Article : Google Scholar
|
|
27
|
Otero JE, Dai S, Foglia D, Alhawagri M,
Vacher J, Pasparakis M and Abu-Amer Y: Defective osteoclastogenesis
by IKKbeta-null precursors is a result of receptor activator of
NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and
impaired differentiation. J BiolChem. 283:24546–24553. 2008.
|
|
28
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khapli SM, Tomar GB, Barhanpurkar AP,
Gupta N, Yogesha SD, Pote ST and Wani MR: Irreversible inhibition
of RANK expression as a possible mechanism for IL-3 inhibition of
RANKL-induced osteoclastogenesis. Biochem Biophys Res Commun.
399:688–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshitake F, Itoh S, Narita H, Ishihara K
and Ebisu S: Interleukin-6 directly inhibits osteoclast
differentiation by suppressing receptor activator of NF-kappaB
signaling pathways. J Biol Chem. 283:11535–11540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takayanagi H, Ogasawara K, Hida S, Chiba
T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al:
T-cell-mediated regulation of osteoclastogenesis by signalling
cross-talk between RANKL and IFN-gamma. Nature. 408:600–605. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greenberg S, Di Virgilio F, Steinberg TH
and Silverstein SC: Extracellular nucleotides mediate Ca2+ fluxes
in J774 macrophages by two distinct mechanisms. J Biol Chem.
263:10337–10343. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McLarnon JG, Ryu JK, Walker DG and Choi
HB: Upregulated expression of purinergic P2X(7) receptor in
Alzheimer disease and amyloid-beta peptide-treated microglia and in
peptide-injected rat hippocampus. J Neuropathol Exp Neurol.
65:1090–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yiangou Y, Facer P, Durrenberger P,
Chessell IP, Naylor A, Bountra C, Banati RR and Anand P: COX-2, CB2
and P2X7-immunoreactivities are increased in activated microglial
cells/macrophages of multiple sclerosis and amyotrophic lateral
sclerosis spinal cord. BMC Neurol. 6:122006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jørgensen N: The purinergic P2X7 ion
channel receptor a 'repair' receptor in bone. CurrOpinImmunol.
52:32–38. 2018.
|
|
36
|
Wesselius M, Bours M, Agrawal A, Gartland
A, Dagnelie P, Schwarz P and Jorgensen N: Role of purinergic
receptor polymorphisms in human bone. Front Biosci (Landmark Ed).
16:2572–2585. 2011. View
Article : Google Scholar
|
|
37
|
Pellegatti P, Falzoni S, Donvito G,
Lemaire I and Di Virgilio F: P2X7 receptor drives osteoclast fusion
by increasing the extra-cellular adenosine concentration. FASEB J.
25:1264–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syberg S, Schwarz P, Petersen S, Steinberg
T, Jensen JE, Teilmann J and Jørgensen NR: Association between P2X7
receptor polymorphisms and bone status in mice. J Osteoporos.
2012:6379862012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gartland A, Buckley KA, Hipskind RA, Perry
MJ, Tobias JH, Buell G, Chessell I, Bowler WB and Gallagher JA:
Multinucleated osteoclast formation in vivo and in vitro by P2X7
receptor-deficient mice. Crit Rev Eukaryot Gene Expr. 13:243–253.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang QL, Wang W, Jiang Y, A-Tuya,
Dongmei, Li LL, Lu ZJ, Chang H and Zhang TZ: GRGM-13 comprising 13
plant and animal products, inhibited oxidative stress induced
apoptosis in retinal ganglion cells by inhibiting P2RX7/p38 MAPK
signaling pathway. Biomed Pharmacother. 101:494–500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jelassi B, Chantôme A, Alcaraz-Pérez F,
Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A and Roger S:
P2X(7) receptor activation enhances SK3 channels- and cystein
cathepsin-dependent cancer cells invasiveness. Oncogene.
30:2108–2122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takai E, Tsukimoto M, Harada H and Kojima
S: Autocrine signaling via release of ATP and activation of P2X7
receptor influences motile activity of human lung cancer cells.
Purinergic Signal. 10:487–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fathi E, Sanaat Z and Farahzadi R:
Mesenchymal stem cells in acute myeloid leukemia: A focus on
mechanisms involved and therapeutic concepts. Blood Res.
54:165–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fathi E, Farahzadi R, Sheervalilou R,
Sanaat Z and Vietor I: A general view of CD33+ leukemic
stem cells and CAR-T cells as interesting targets in acute
myeloblastsic leukemia therapy. Blood Res. 55:10–16. 2020.
View Article : Google Scholar : PubMed/NCBI
|