Introduction
Angiotensin II (Ang II) is known to be a major
effector peptide of the renin-angiotensin system (RAS), and serves
an important role in regulating blood pressure and humoral
homeostasis (1). Epidemiological
and experimental data have suggested that RAS activation serves an
important role in increasing the risk of cardiovascular events
(2). Substantial evidence has
suggested that Ang II, as a RAS effector peptide, may be involved
in the development of cardiovascular diseases, such as
atherosclerosis (3). In humans,
associative evidence has indicated that cardiovascular events
increase alongside increases in renin activity (3). In mice, injections of Ang II have
been reported to promote cardiovascular disease, such as
atherosclerosis, abdominal aortic aneurysms and cardiac hypertrophy
(4-6). Several mechanisms may underlie how
Ang II promotes cardiac damage. Firstly, Ang II may indirectly
influence cardiovascular disease development by increasing arterial
blood pressure (7). Secondly,
Ang II has been reported to possess numerous properties directly
affecting cardiovascular disease, including pro-inflammatory
(8), fibrosis-enhancing
(9) and oxidative
stress-promoting effects (10).
Thymoquinone (TQ) is the most abundant constituent
of the volatile oil of Nigella sativa seeds and the majority
of the properties of this plant are primarily attributed to TQ
(11). The molecular formula of
TQ is C10H12O2. TQ has been
reported to exhibit anti-inflammatory, anti-oxidative stress,
anti-hypertensive, anti-apoptotic and free radical-scavenging
properties (12-14). There has been a growing interest
in the use of TQ as an alternative treatment for several
conditions, including cardiovascular diseases. Xiao et al
(15) reported that TQ
effectively improved cardiac function in rats with
ischemia/reperfusion injury, inhibited oxidative stress, decreased
myocardial enzyme activity and reduced myocardial infarct size. In
addition, other studies have revealed that TQ could attenuate
cisplatin-induced cardiac damage and morphine-induced cardiac cell
apoptosis in rats (16,17). However, the effects of TQ on Ang
II-induced cardiac damage remain unclear. H9c2 cells are often used
to study the mechanisms associated with cardiac damage; therefore,
the present study established an Ang II-induced cardiac damage
model to examine the role of TQ and its potential mechanism in H9c2
cells. Furthermore, apolipoprotein E-deficient (ApoE−/−)
mice are commonly used as a model of Ang II-induced aortic aneurysm
(18,19). Since Ang II has an important role
in the development of atherosclerosis, the present study conducted
a series of experiments using this model and monitored organ damage
in ApoE−/− mice. The kidney, arteries and heart were
described in other studies; however, the present study focused on
cardiac damage.
Materials and methods
Animal maintenance
ApoE−/− male mice (n=30; weight,
24.30±1.04 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. All mice were housed in a room at a
constant temperature of 23-25°C and 40-60% humidity under a 12-h
light/dark cycle. The mice had free access to water and food. At 8
weeks of age, the mice were equally and randomly divided into four
groups as follows: Control group (n=7), TQ group (n=7), Ang II
group (n=8) and TQ + Ang II group (n=8). TQ (50 mg/kg/d; cat. no.
490-91-5; MilliporeSigma) was administered to the TQ and TQ + Ang
II groups by gavage (20).
Osmotic minipumps (Model 2004; ALZET® Osmotic Pumps;
Durect Corporation), filled with either saline vehicle or Ang II
solution (1,000 ng/kg/min; cat. no. 4474-91-3; MilliporeSigma),
were implanted subcutaneously in mice for up to 4 weeks (21). Each group of mice was subjected
to their respective treatment for 4 weeks. Blood pressure was
measured using photoplethysmography using a computerized tail-cuff
system (BP-2000 Blood Pressure Analysis System; Visitech Systems)
in conscious animals, as previously described (22). After 4 weeks, the mice were
weighed and then sacrificed with a high dose of pentobarbital (100
mg/kg, intraperitoneal administration), and a lack of respiration
and heartbeat was used as an indicator of mouse death. Blood
samples were obtained from the abdominal cava, collected in serum
tubes and stored at -80°C until further use. In addition, the
hearts were weighed and coronal sections of heart tissues were
fixed in 10% formalin for 30 min, dehydrated in 75% ethanol
overnight and finally embedded in paraffin for histological
evaluation at room temperature (24-26°C). The remaining heart
tissues were stored at −80°C and later used to perform reverse
transcription-quantitative PCR (RT-qPCR) or western blot analysis.
All experimental procedures in the present study were approved by
the ethical committee of Xi'an No. 3 Hospital (Xi'an, China).
Biochemical analysis
Serum was obtained from the abdominal aorta of mice
and preserved in tubes. The blood samples were immediately
centrifuged at 1,006 × g for 10 min at 4°C after collection and the
serum was subsequently stored at -80°C. Serum high-sensitivity
C-reactive protein (hs-CRP) was measured using an ELISA kit (cat.
no. SEKM-0059; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's protocol. Total cholesterol (TC;
cat. no. A111-1-1), triglyceride (TG; cat. no. A110-1-1) and
low-density lipoprotein cholesterol (LDL-c; cat. no. A113-1-1)
levels were examined using commercial reagent kits (Nanjing
Jiancheng Bioengineering Institute).
Cardiac histological analysis
Cardiac tissues were fixed in 10% formalin for 30
min, dehydrated in 75% ethanol overnight and finally embedded in
paraffin for histological evaluation at room temperature (24-26°C).
According to manufacturer's protocol, serial sections (4 µm)
were subjected to staining with a hematoxylin and eosin (H&E)
staining kit (cat. no. G1120; Beijing Solarbio Science &
Technology Co., Ltd.) and Masson's trichrome stain kit (cat. no.
G1340; Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature (24-26°C) and according to the manufacturer's protocol
to assess pathological changes. Blue staining indicated collagen
accumulation in Masson's trichrome staining. Images of the sections
were analyzed using ImageJ software v1.8.0 (National Institutes of
Health). The mean cross-sectional area and fibrosis of
cardiomyocytes were assessed by computerized planimetry.
Cell culture and treatment
Rat cardiac H9c2 cells were purchased from the
National Collection of Authenticated Cell Cultures (cat. no. GNR 5,
http://www.nccc.com/) and were cultured in
Dulbecco's modified Eagle's medium (cat. no. D0819; MilliporeSigma)
containing 10% fetal calf serum (cat. no. 10099141; Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin-streptomycin (cat.
no. V900929; MilliporeSigma). H9c2 cells were maintained at 37°C in
a humidified atmosphere containing 5% CO2 and were
treated with different doses of Ang II (0, 150 and 300 nmol/l) for
24 h. Cells underwent RNA and protein extraction, and the
expression levels of pro-inflammatory cytokines were assessed using
RT-qPCR and p-ERK expression levels were detected using western
blotting. In addition, cells were pre-incubated with an ERK
inhibitor (20 µmol PD98059; cat. no. HY-12028;
MedChemExpress) for 30 min [PD98059 was dissolved in DMSO (0.4
µl/ml; cat. no. D8371; Beijing Solarbio Science &
Technology Co., Ltd.)], followed by treatment with Ang II (300
nmol/l) for 48 h at 37°C. For TQ treatment, cells were grown to 80%
confluence and were then incubated with TQ at the indicated
concentration (20 µmol/l) for 24 h when treated with Ang II
at the same time. TQ treatment was used to detect the effects of TQ
on p-ERK. For PD + Ang II + TQ group, the cells were pre-incubated
with PD and then treatment with Ang II and TQ as previously
mentioned. DMSO treatment (final concentration, 0.1%) was used as a
sham control for all cell groups. The treated cells were harvested
and washed with PBS for the subsequent analyses.
RNA isolation and RT-qPCR
The primers were designed by Invitrogen; Thermo
Fisher Scientific, Inc. Total RNA was isolated from cardiac tissue
and cells using the TransZol Up Plus RNA kit (cat. no. ER501-01;
Transgen Biotech Co., Ltd.) and cDNA was synthesized using
TransScript® One-Step gDNA Removal and cDNA Synthesis
SuperMix (cat. no. AT311-02; Transgen Biotech Co., Ltd.) according
to the manufacturer's protocols. Gene expression was analyzed
quantitatively by qPCR using the TransStart® Top Green
qPCR SuperMix kit (cat. no. AQ131-01; Transgen Biotech Co., Ltd.).
β-actin cDNA was amplified and quantified in each cDNA preparation
to normalize the relative amounts of the target genes. The cDNA
amplification was performed as follows: The first cycle was
maintained at 95c for 30 sec, followed by 38 cycles consisting of
denaturation (95°C for 10 sec), annealing (60°C for 20 sec), and
extension (72°C for 15 sec). The IL-1β, TNF-α and IL-6 were then
processed using the 2−ΔΔCq method (23), during which a single calibrated
sample was compared against the gene expression of every unknown
sample. Primer sequences are listed in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence |
|---|
| Mouse TNF-α | F:
5′-TCTCATGCACCACCATCAAGGACT-3′ |
| R:
5′-ACCACTCTCCCTTTGCAGAACTCA-3′ |
| Mouse IL-6 | F:
5′-TACCAGTTGCCTTCTTGGGACTGA-3′ |
| R:
5′-TAAGCCTCCGACTTGTGAAGTGGT-3′ |
| Mouse IL-1β | F:
5′-TGCCACCTTTTGACAGTGAT-3′ |
| R:
5′-TGTGCTGCTGCGAGATTTGA-3′ |
| Mouse β-actin | F:
5′-CGATGCCCTGAGGGTCTTT-3′ |
| R:
5′-TGGATGCCACAGGATTCCAT-3′ |
| Rat TNF-α | F:
5′-CACCACGCTCTTCTGTCTACTG-3′ |
| R:
5′-GCTACGGGCTTGTCACTCG-3′ |
| Rat IL-6 | F:
5′-CTTCCATCCAGTTGCCTTCTTG-3′ |
| R:
5′-AATTAAGCCTCCGACTTGTGAAG-3′ |
| Rat IL-1β | F:
5′-GTGGCAGCTACCTATGTCTTGC-3′ |
| R:
5′-CCACTTGTTGGCTTATGTTCTGT-3′ |
| Rat β-actin | F:
5′-CCTGTGGCATCCATGAAACTAC-3′ |
| R:
5′-CCAGGGCAGTAATCTCCTTCTG-3′ |
Immunohistochemistry
Coronal sections of heart tissues were fixed in 10%
formalin for 30 min, dehydrated in 75% ethanol overnight and
finally embedded in paraffin for histological evaluation at room
temperature (24-26°C). For immunohistochemical staining, the heart
sections were deparaffinized with xylene (two times, 10 min each)
and rehydrated in a descending alcohol series (100, 90, 80 and 70%
alcohol; 5 min each). Subsequently, the sections were blocked with
3% H2O2 in methanol for 15 min at room
temperature to inactivate endogenous peroxidases, and incubated
overnight at 4°C with the following primary antibodies: Rabbit
anti-collagen I antibody (cat. no. 14695-1-AP; 1:200; Wuhan Sanying
Biotechnology), rabbit anti-collagen III antibody (cat. no.
22734-1-AP; 1:200; Wuhan Sanying Biotechnology), rabbit anti-Nox4
antibody (cat. no. 14347-1-AP; 1:200; Wuhan Sanying Biotechnology)
and rabbit anti-p53 antibody (cat. no. 10442-1-AP; 1:200; Wuhan
Sanying Biotechnology). The sections were washed three times in
water containing PBS (5 min/wash) and subsequently incubated with a
goat anti-rabbit HRP-conjugated secondary antibody (Histofine
Simple Stain kit; cat. no. 414321; Nichirei Biosciences, Inc.) for
30 min at room temperature. All sections were examined under an
Olympus B 40X upright light microscope (Olympus Corporation).
Western blot analysis
Proteins were extracted from cardiac tissues and
cells using radioimmunoprecipitation assay buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology). Cardiac tissue total protein
concentrations were determined using a BCA Protein assay reagent
kit (cat. no. DQ111-01; Beijing Transgen Biotech Co., Ltd.). The
protein samples (20 µg per lane) were then separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10%
gels and transferred to polyvinylidene fluoride membranes (cat. no.
IPFL00010; MilliporeSigma). The membranes were blocked with 5% skim
milk in TBS containing 0.1% Tween-20 at room temperature for 1 h
and then incubated with the primary antibodies at 4°C overnight.
Primary rabbit antibodies against phosphorylated (p)-extracellular
signal-regulated kinase (ERK) (cat. no. 9102; 1:1,000; Cell
Signaling Technology, Inc.), collagen I (cat. no. 14695-1-AP;
1:1,000; Wuhan Sanying Biotechnology), collagen III (cat. no.
22734-1-AP; 1:1,000; Wuhan Sanying Biotechnology), Nox4 (cat. no.
14347-1-AP; 1:1,000; Wuhan Sanying Biotechnology), p53 (cat. no.
10442-1-AP; 1:1,000; Wuhan Sanying Biotechnology), total (t)-ERK
(cat. no. 11257-1-AP; 1:1,000; Wuhan Sanying Biotechnology) and
β-actin (cat. no. 4970; 1:1,000; Cell Signaling Technology, Inc.)
were used. After the membranes were washed, they were incubated
with the appropriate anti-rabbit IgG secondary antibody (cat. no.
7074; 1:2,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature (24-26°C). Enhanced chemiluminescence reagent (cat. no.
32106; Thermo Fisher Scientific, Inc.) was used to visualize bands.
Signals were imaged using a Bio-Rad imaging system (Bio-Rad
Laboratories, Inc.) with a Chemi 410 HR camera (Analytik; Jena AG)
and analyzed using Gel-Pro Analyzer version 4.0 (Media Cybernetics,
Inc.). The analysis was performed independently three times. The
blotted proteins were semi-quantified using ImageJ software version
1.8.0 (National Institutes of Health). β-actin was used as an
internal control and protein expression levels were expressed as
protein/β-actin ratios, but this was not the case for p-ERK; t-ERK
was used as an internal control for p-ERK.
Statistical analysis
Data are expressed as the mean ± SEM and were
analyzed using SPSS 23.0 statistical software (IBM Corporation).
Intergroup statistical significance of multiple groups was
determined using one-way ANOVA, followed by Tukey's test.
Differences between two groups was determined using one-way ANOVA
without a post hoc test. There were three experimental repeats.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Metabolic characterization
The metabolic characteristics of the mice in the
four groups are shown in Fig. 1.
No differences in body and heart/body weight were detected among
the groups. Notably, a significant increase was observed in the
serum levels of hs-CRP, TC, TG and LDL-c in the Ang II group
compared with those in the control group, which was significantly
decreased upon TQ treatment. Furthermore, an increase in blood
pressure was detected in the Ang II group compared with that in the
control group. Notably, TQ suppressed this increase in blood
pressure in mice in the Ang II + TQ group.
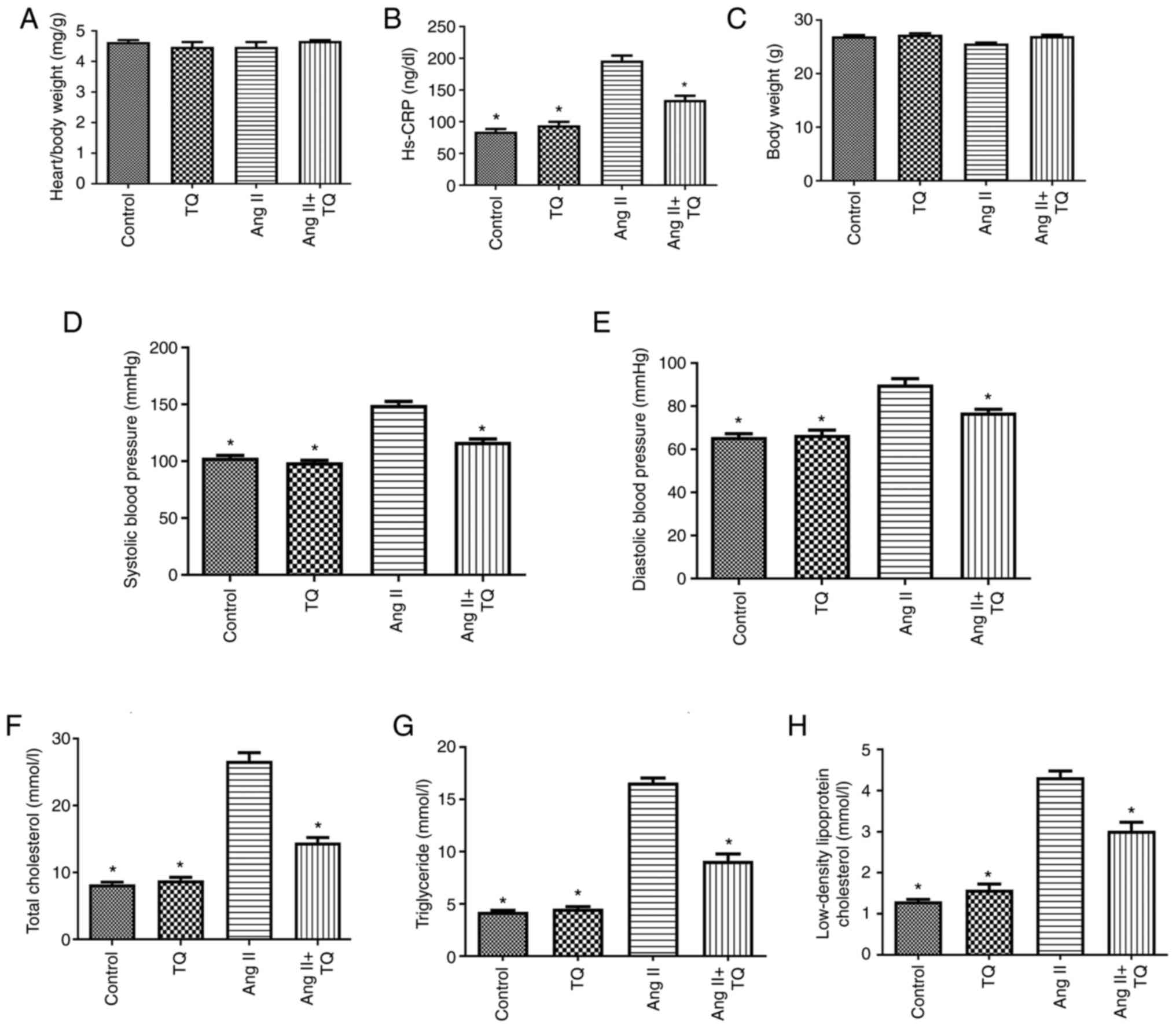 | Figure 1Metabolic data showing the (A)
heart/body weight, (B) hs-CRP, (C) body weight, (D) systolic blood
pressure, (E) diastolic blood pressure, (F) total cholesterol, (G)
triglyceride and (H) low density lipoprotein cholesterol of the
mice in the four groups after different treatments. Data are
presented as the mean ± SEM; n=6-8/group. *P<0.05 vs.
the Ang II group. Hs-CRP, high-sensitivity C-reactive protein; TQ,
thymoquinone; Ang II, angiotensin II. TC, TG and LDL-c. |
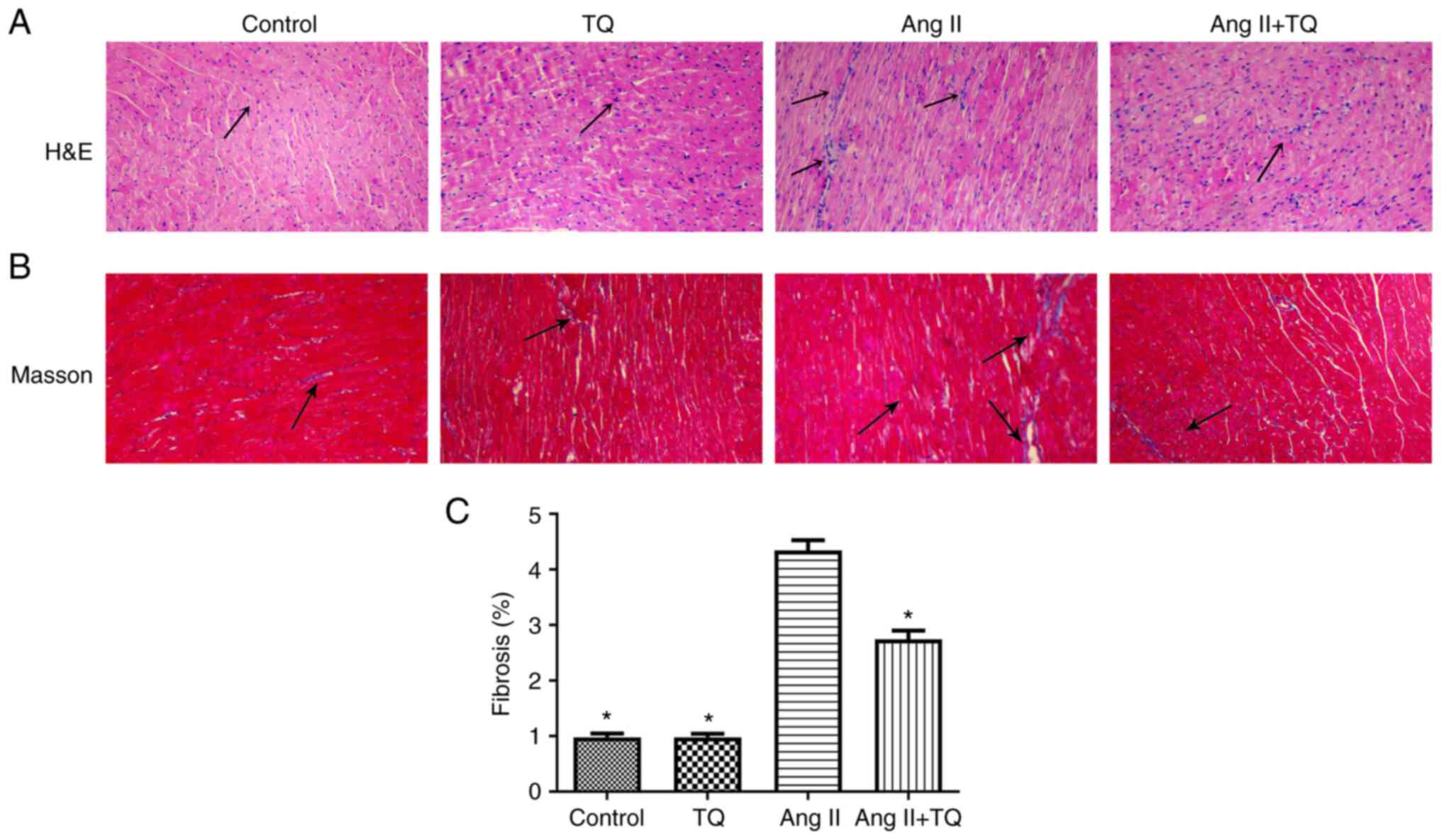
TQ reduces the histopathological changes
in the heart tissues of mice in the Ang II group
To evaluate histopathological changes in heart
tissues, H&E and Masson staining were performed (Fig. 2). Heart tissues from the control
group appeared normal; however, histopathological changes,
including inflammatory cell infiltration and collagen deposition,
were observed in the heart tissues of Ang II-treated mice. Notably,
this damage was suppressed in Ang II + TQ mice. The area of cardiac
fibrosis from Masson trichrome-stained sections were clearly
increased in Ang II group, while TQ treatment suppressed this
increased.
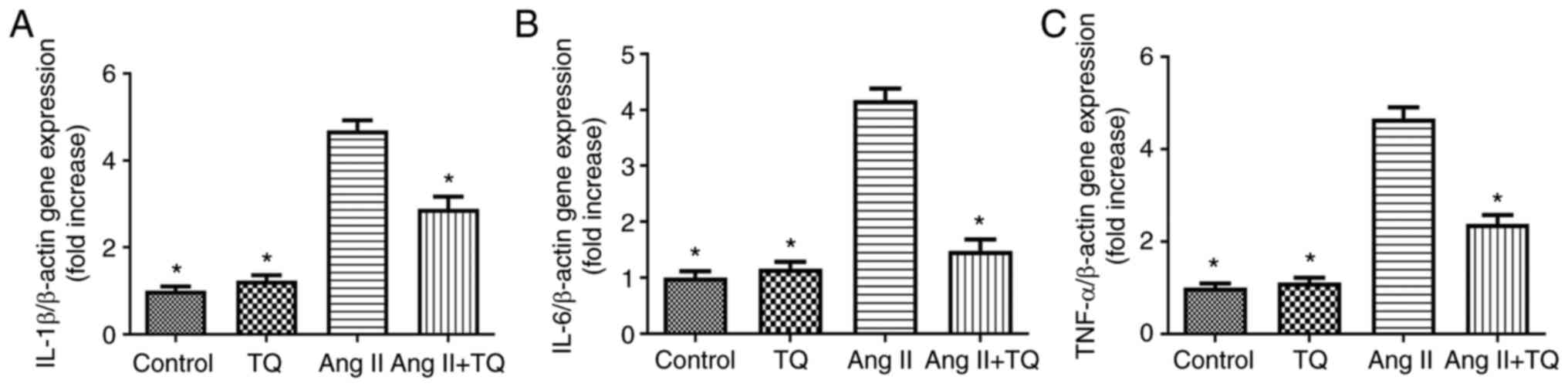
TQ reduces pro-inflammatory cytokine
expression in the heart tissues of mice in the Ang II group
To evaluate the involvement of pro-inflammatory
cytokines in mouse heart tissue, the mRNA expression levels of
IL-1β, IL-6 and TNF-α were measured using RT-qPCR in the different
mouse groups (Fig. 3). The
expression levels of these three genes were increased in Ang
II-treated mice compared with those in the control group; however,
this increase was attenuated in the Ang II + TQ group.
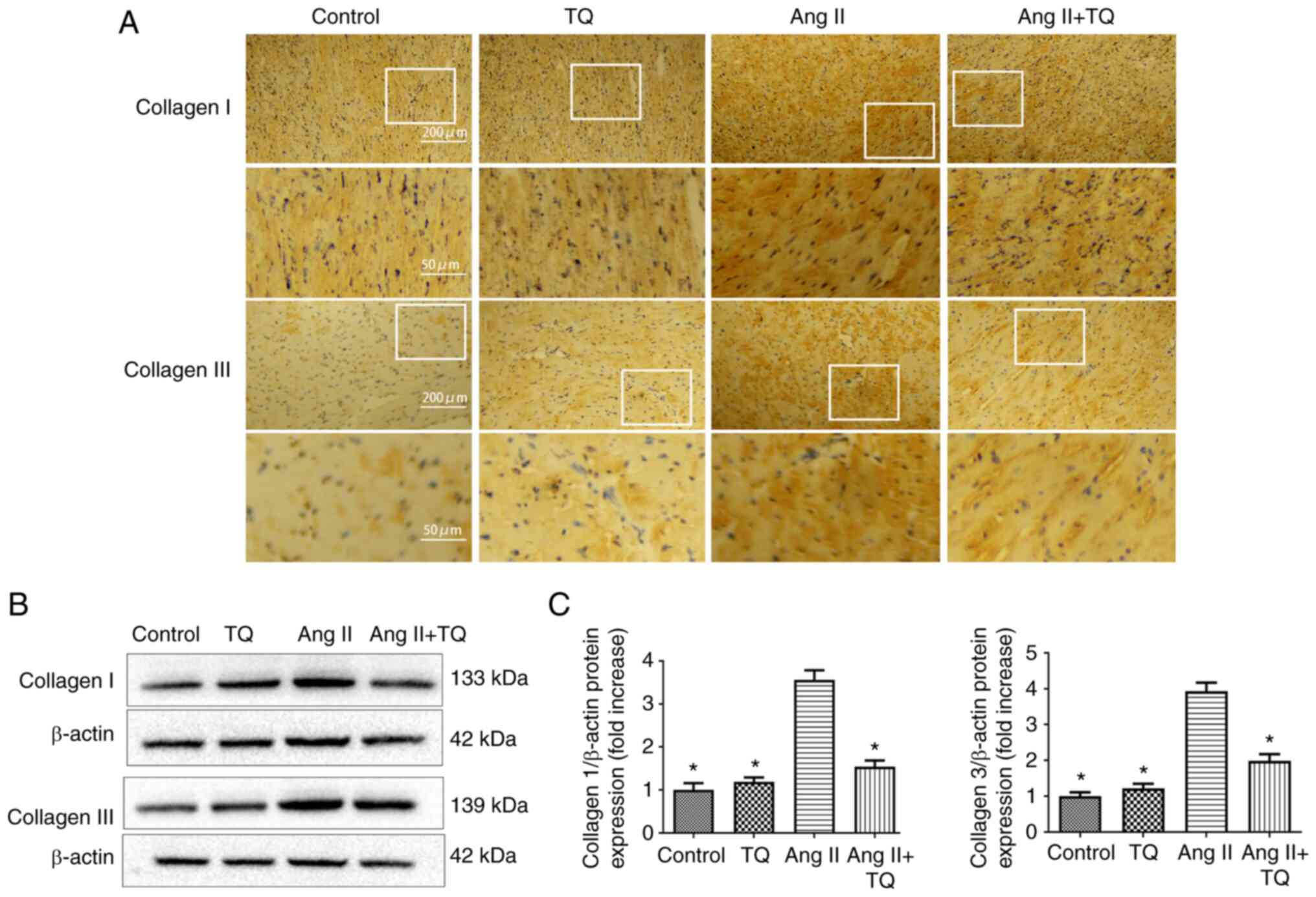
TQ reduces fibrosis-related protein
expression in the heart tissues of mice in the Ang II group
To investigate the mechanism underlying fibrosis in
heart damage, the expression levels of collagen I and III were
examined using immunohistochemistry (Fig. 4A) and western blotting (Fig. 4B and C). The expression collagen
I and III were increased in Ang II group compared with the control
group. However, compared with in the Ang II group, mice in the Ang
II + TQ group exhibited markedly reduced collagen I and III
expression levels. These findings indicated that TQ reduced
collagen I and III expression in Ang II-treated mice.
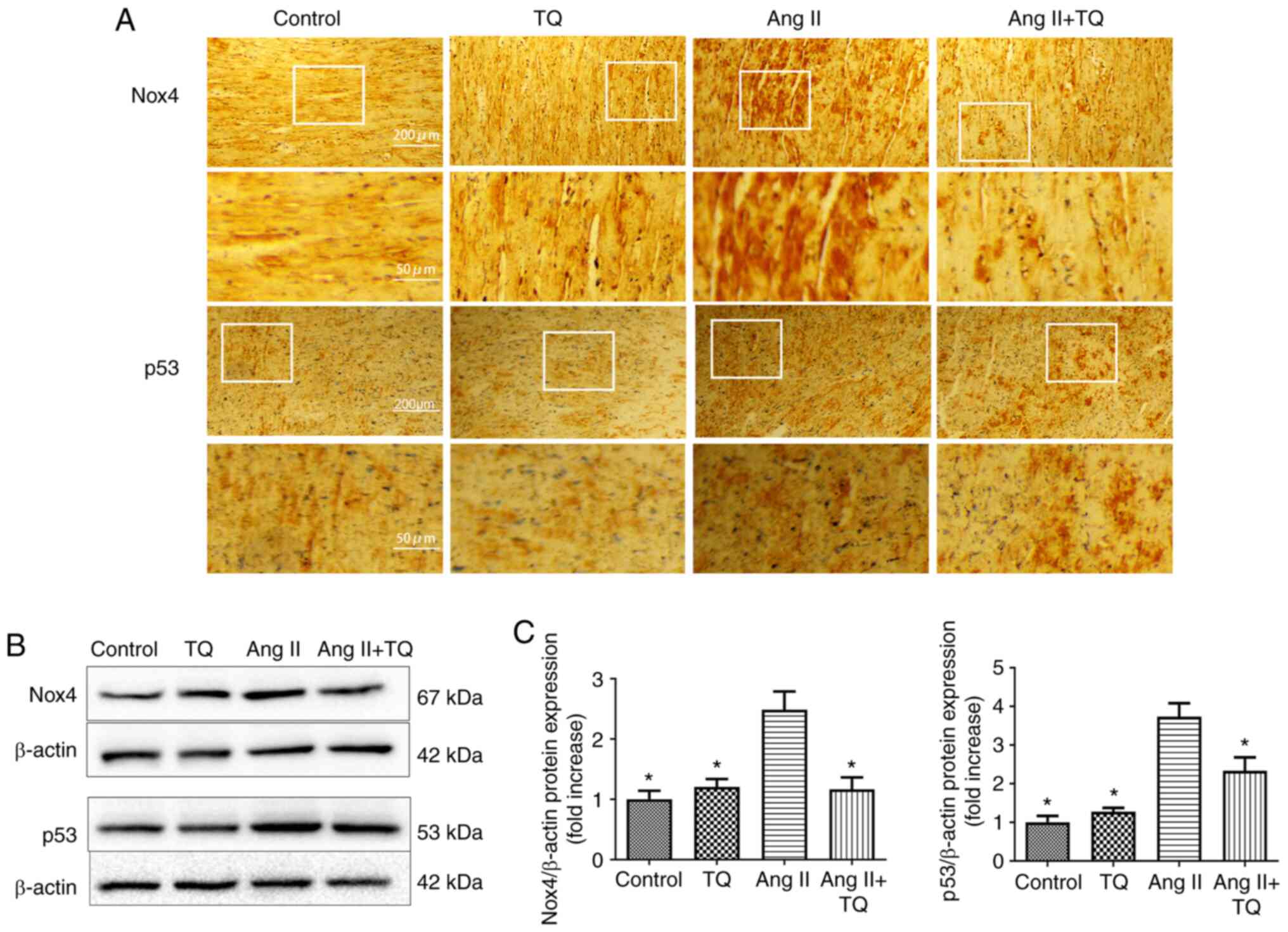
TQ reduces Nox4 and p53 expression in the
heart tissues of mice in the Ang II group
Immunohistochemistry was used to analyze the protein
expression levels of Nox4 and p53 (Fig. 5A). The expression Nox4 and p53
were increased in Ang II group compared with the control group. In
the Ang II + TQ group this increase was markedly reduced in heart
tissue compared with the Ang II group. A similar result was
obtained by western blotting; the Ang II-induced increase in the
expression levels of these proteins was attenuated by TQ treatment
(Fig. 5B and C). These findings
suggested that TQ may reduce metabolic energy-related protein
expression in the heart tissue of Ang II-treated mice.
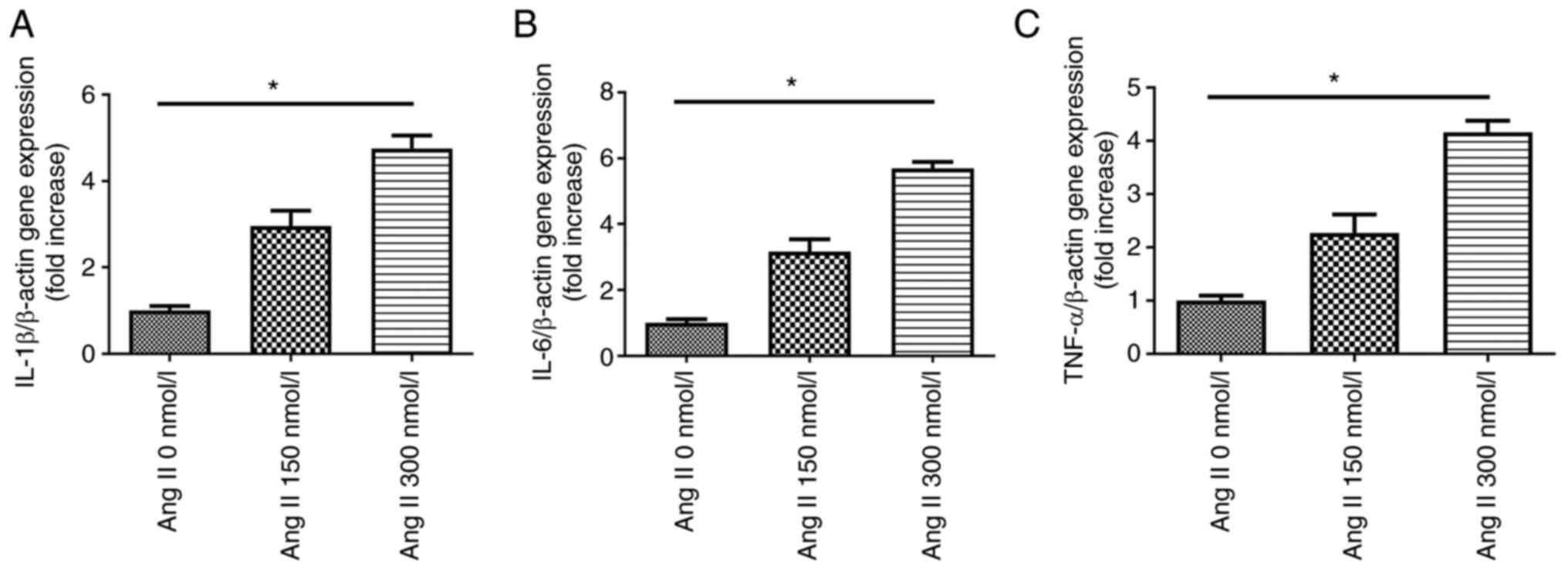
Ang II upregulates the expression levels
of pro-inflammatory cytokines in H9c2 cells
RT-PCR was performed to evaluate the expression
levels of pro-inflammatory cytokines in H9c2 cells exposed to
different concentrations of Ang II. Cultured H9c2 cells were
incubated with Ang II for 24 h. A dose-dependent upregulation of
pro-inflammatory cytokine expression was observed with Ang II
treatment (Fig. 6); the
strongest upregulation was caused by Ang II at a concentration of
300 nmol/l.
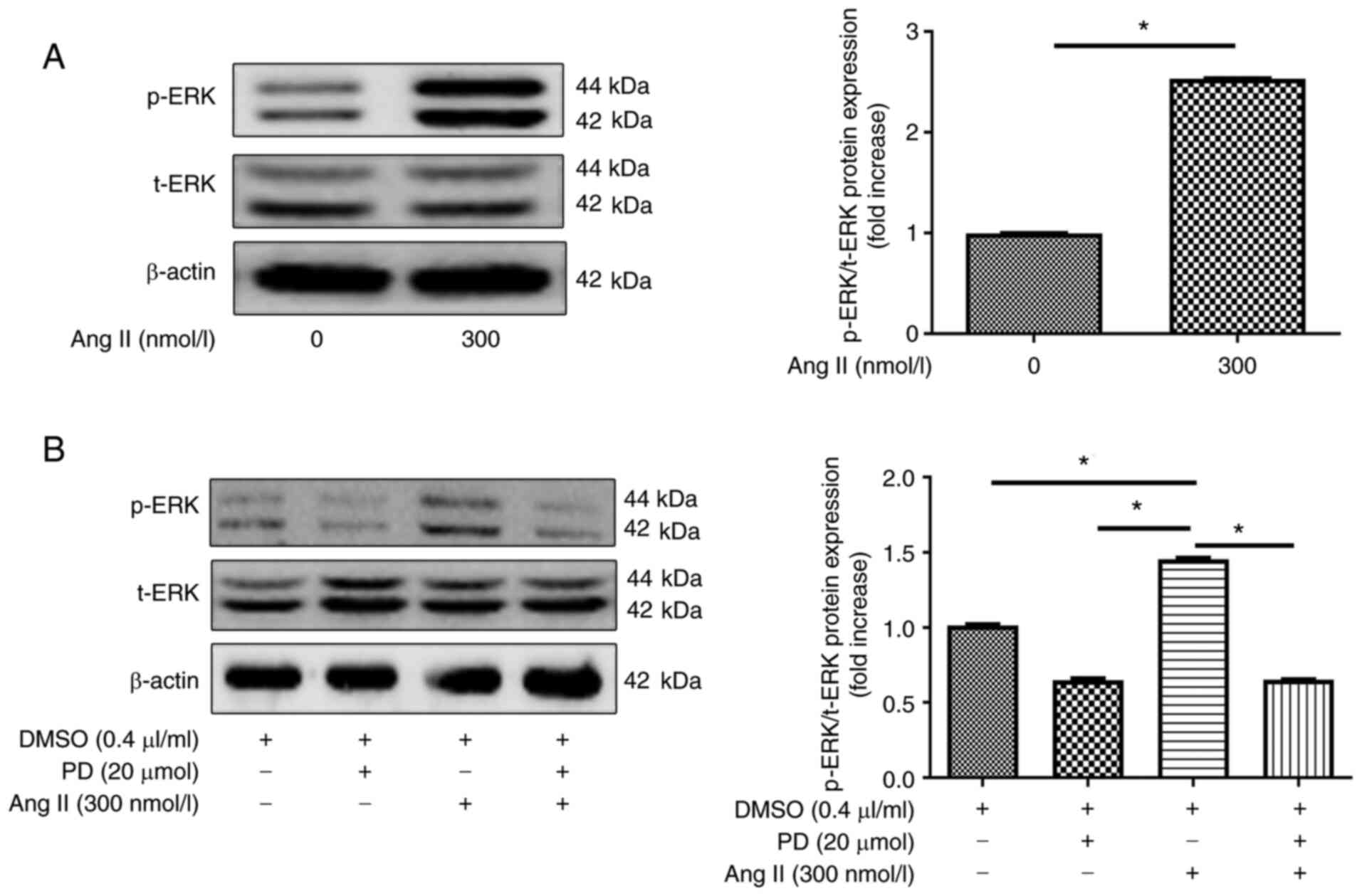
ERK expression in H9c2 cells
The present study examined the involvement of the
ERK pathway in H9c2 cells. ERK phosphorylation was revealed to be
induced by treating H9c2 cells with 300 nmol/l Ang II. p-ERK levels
in cells increased when 300 nmol/l of Ang II was used compared with
treated with 0 nmol/l Ang II (Fig.
7A). Furthermore, pre-incubation with an ERK inhibitor (20
µM PD98059) for 30 min, followed by treatment with Ang II
for 48 h, reduced ERK phosphorylation (Fig. 7B) compared with treatment without
ERK inhibitor. These findings indicated that p-ERK expression
levels were increased when H9c2 cells were incubated with 300
nmol/l Ang II, but were markedly decreased by PD98059
treatment.
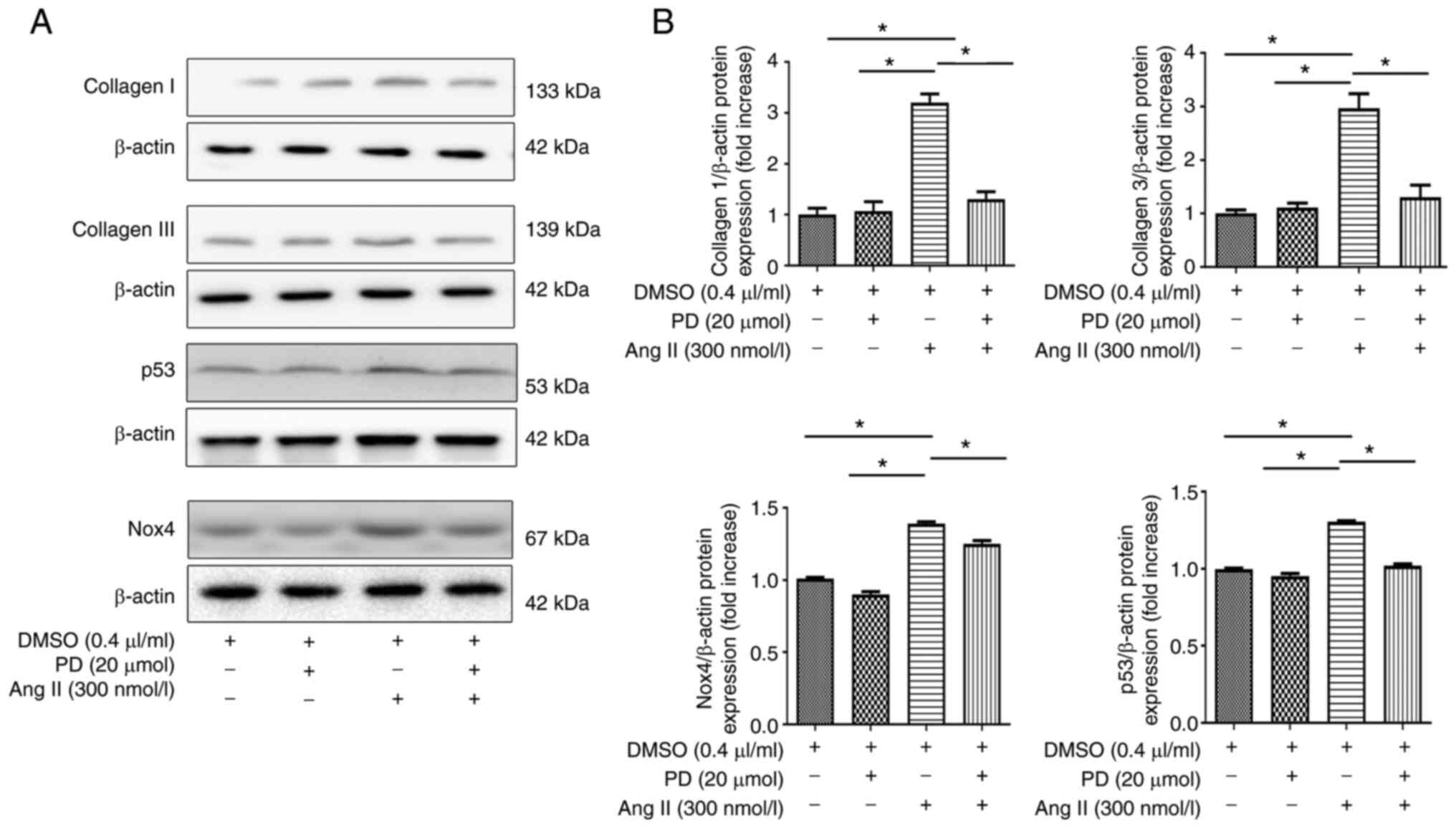
Fibrosis-related protein expression, and
Nox4 and p53 expression in H9c2 cells
The present study examined the expression levels of
fibrosis-related proteins, and Nox4 and p53 in H9c2 cells (Fig. 8). The expression levels of
collagen I and III, Nox4 and p53 were increased when H9c2 cells
were incubated with 300 nmol/l Ang II, but were markedly decreased
with PD98059 treatment.
TQ reduces p-ERK expression in vivo and
vitro
The present study examined the expression levels of
p-ERK in heart tissue and H9c2 cells (Fig. 9). p-ERK expression was clearly
increased in Ang II group compared with the control group. The Ang
II + TQ group exhibited significantly reduced p-ERK expression in
heart tissues compared with that in the Ang II group (Fig. 9A and B). The expression levels of
p-ERK were increased when H9c2 cells were incubated with 300 nmol/l
Ang II, but were decreased with TQ or PD98059 treatment
respectively (Fig. 9C and D).
Notably, p-ERK expression was markedly decreased by TQ and PD98059
treatment. TQ increased the inhibitory effect of the ERK
inhibitor.
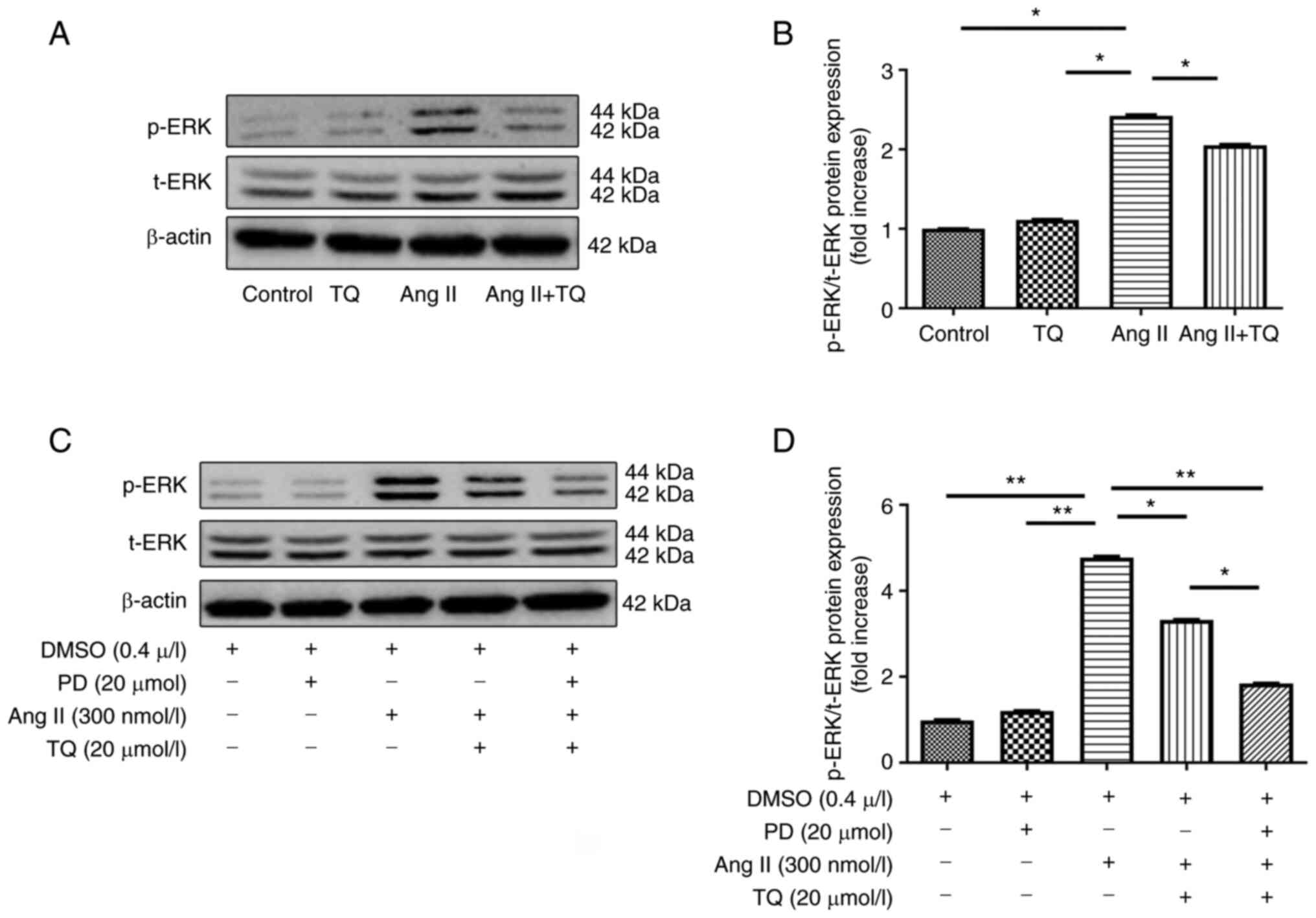 | Figure 9(A) Western blot analysis of p-ERK in
heart tissue. (B) Bar graph shows the semi-quantification of the
expression levels of p-ERK in heart tissue. (C) Western blot
analysis of p-ERK following pre-treatment of cells with an ERK
inhibitor and TQ. (D) Bar graph shows semi-quantification of the
expression levels of collagen I and III, Nox4 and p53 in H9c2
cells. H9c2 cells were cultured and pre-incubated with an ERK
inhibitor (PD; 20 µmol/l) for 30 min followed by treatment
with Ang II for 48 h. For TQ treatment, cells were grown to 80%
confluence and then incubated with TQ at the indicated
concentration (20 µmol/l) for 24 h. For PD + Ang II + TQ
group, the cells were pre-incubated with PD and then treatment with
Ang II and TQ as previously mentioned. DMSO treatment (final
concentration, 0.1%) was used as a sham control. Data are presented
as the mean ± SEM; n=3/group. *P<0.05,
**P<0.01. ERK, extracellular signal-regulated kinase;
p, phosphorylated; PD, PD98059; t, total; TQ, thymoquinone. |
Discussion
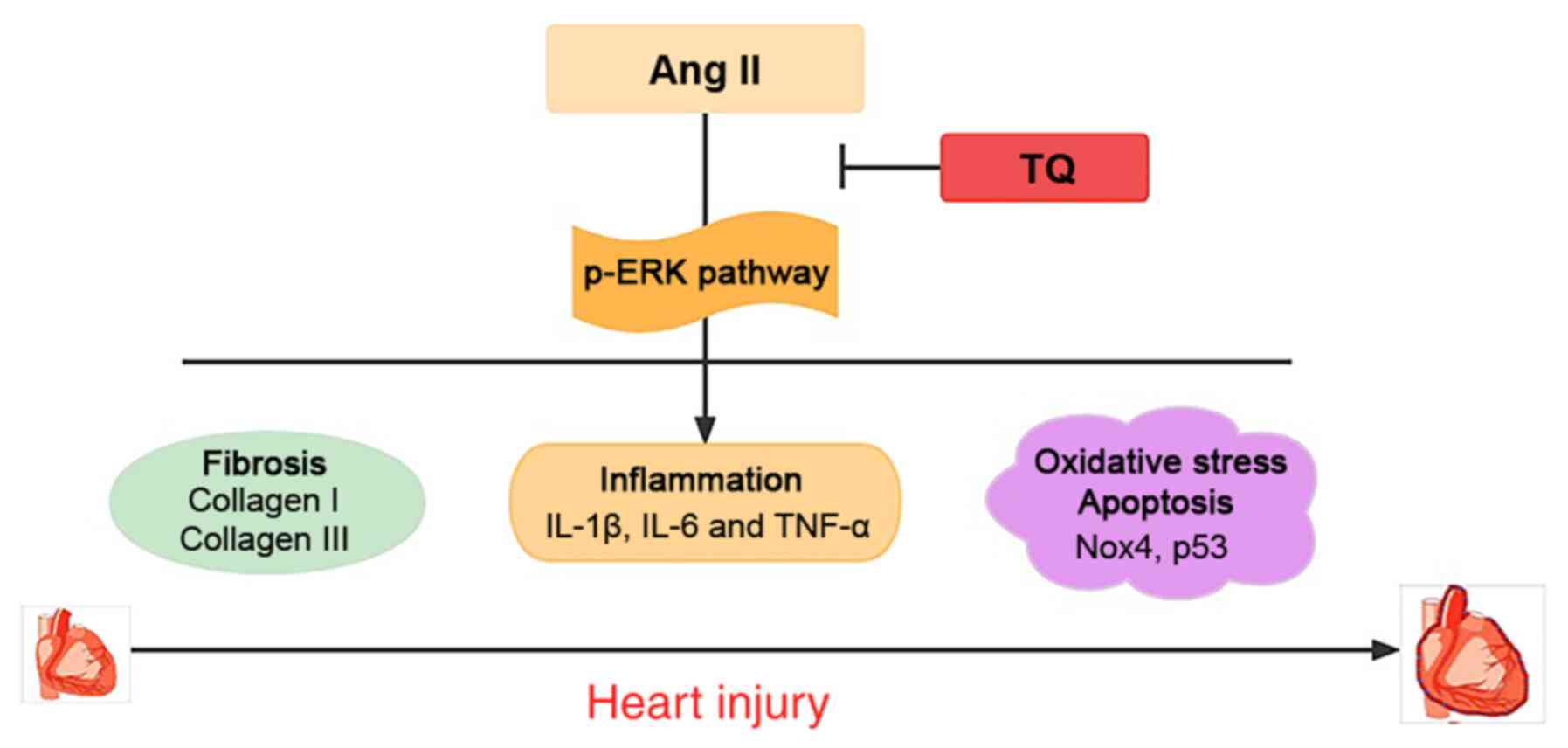
The present study demonstrated that TQ had a
protective effect against cardiac damage via anti-inflammatory,
anti-fibrotic, anti-oxidative stress and anti-apoptotic effects (as
suggested by its effects on proteins associated with oxidative
stress and apoptosis; Fig. 10).
A cardiac damage model was established by implanting osmotic
minipumps filled with Ang II solutions in mice to investigate the
effects of TQ. Body weight did not exhibit obvious changes in Ang
II-infused mice, this may be because there was insufficient time to
detect changes in body weight. In addition, the observation time
has not been long enough in previous studies to show body weight
changes (24,25). With regard to metabolic
characteristics, the serum hs-CRP level has been used to indicate
the instability of atherosclerotic lesions in several clinical
studies and as a risk predictor of cardiovascular events (26-28). In the present study, the Ang II
group exhibited significantly higher indices of cardiac injury
(hs-CRP) compared with in the control group; however, treatment
with TQ significantly reduced the hs-CRP levels, alleviating the
cardiac damage induced by Ang II. Chobanian and Alexander (7) reported that Ang II may influence
cardiovascular disease development by increasing arterial blood
pressure, which is in agreement with the findings of the present
study. Hypertension is a major cause of cardiac damage. Elevated
blood pressure can cause cardiac fibrosis, which in turn leads to
remodeling of the heart structure; uncontrolled cardiac remodeling
can lead to heart failure (8,29). The present study observed an
increase in blood pressure in the Ang II group compared with that
in the control group, which was attenuated by TQ treatment. These
findings suggested that TQ may attenuate cardiac fibrosis by
suppressing the increase in blood pressure early, which was
confirmed by histological analyses.
RAS serves a key role in the development of
cardiovascular diseases. Ang II is a core effector of RAS and an
increase in its concentration can cause inflammation (29). The mechanism underlying the
pro-inflammatory effect of Ang II on cardiovascular diseases
includes triggering of vascular damage and recruitment of
inflammatory cells (8). In the
present study, H&E staining revealed higher inflammatory cell
infiltration in the Ang II group than in the control group.
Notably, TQ reduced inflammatory cell infiltration in the Ang II +
TQ group. Furthermore, it reduced the Ang II-induced increases in
the expression levels of inflammatory molecules (IL-1β, IL-6 and
TNF-α). Ojha et al (30)
reported that TQ may exhibit cardioprotective effects by inhibiting
the expression of pro-inflammatory cytokines (31).
Collagen I and III are derived from cardiac
fibroblasts, and have an important role in cardiac function.
Collectively, these extracellular matrix (ECM) proteins define the
fibrous meshwork of the heart (31,32). The excess accumulation of ECM can
cause cardiac fibrosis, thereby limiting cardiomyocyte contraction
and relaxation (33,34). Ang II acts primarily by binding
to its type I receptor, thus stimulating cardiac fibroblasts to
produce ECM proteins and causing reactive fibrosis in the heart
(35-37). In the present study, Masson
staining was used to evaluate collagen deposition in the cardiac
tissue following Ang II-induced cardiac damage. Masson staining
revealed higher collagen deposition in the Ang II group than in the
control group. Notably, TQ reduced collagen deposition in the Ang
II + TQ group. In addition, immunohistochemistry and western
blotting were used to examine the expression levels of collagen I
and III. Compared with in the Ang II group mice, the Ang II + TQ
group mice exhibited significantly reduced collagen I and III
expression levels, indicating that TQ reduced fibrosis. This is
consistent with our suggestion that TQ may attenuate cardiac
fibrosis by suppressing the increase in blood pressure early.
Several studies have shown that oxidative stress is
an important mechanism underlying cardiovascular disease
development (38,39). Furthermore, inhibition of
oxidative stress has been reported to alleviate the endothelial
dysfunction caused by Ang II in mice (40). Nox4, which induces oxidative
stress, has critical roles in the pathogenesis of cardiovascular
diseases (38,41). Heymes et al (41) reported that Nox4 may have an
important role in cardiomyocyte injury. In addition, Nox4 has been
shown to mediate cardiac hypertrophy by inducing apoptosis
(42). p53 is recognized as a
key molecule in the adaptation to harmful stimuli, including
oxidative stress, and has a pivotal role in myocardial apoptosis
(43,44). In the present study,
immunohistochemistry and western blotting were performed to analyze
the expression levels of Nox4 and p53. The Ang II group exhibited
significantly increased Nox4 and p53 expression in the heart tissue
compared with that in the control group, which indicated that Ang
II induced cardiac damage by increasing Nox4 and p53 levels; this
is in agreement with the findings of Tian et al (45). TQ has been reported to attenuate
cardiomyopathy in streptozotocin-treated diabetic rats via
anti-oxidative stress (20).
Similarly, the present study revealed that TQ reduced Nox4 and p53
expression in the heart tissue of Ang II-treated mice. Therefore,
TQ may have a protective function against cardiac damage caused by
Ang II via anti-oxidative stress and anti-apoptotic effects.
RAS is a central component of the physiological and
pathological responses of the cardiovascular system. Ang II, as a
primary effector hormone, serves a significant role in cardiac
function (1,2). Its effect is achieved through
complex intracellular signal transduction pathways. ERK is a member
of the mitogen-activated protein kinase (MAPK) family and is
closely related to cardiac damage (46,47). MAPKs are potential mediators of
inflammatory responses and atherosclerosis (48,49). Furthermore, it has been reported
that the ERK signaling pathway is related to liver fibrosis
(50,51). In the present study, RT-qPCR was
performed to evaluate the expression levels of pro-inflammatory
cytokines in H9c2 cells exposed to Ang II at different
concentrations. The results demonstrated that the inflammation
caused by Ang II at a concentration of 300 nmol/l was the most
severe. Activated MAP3K phosphorylates MEK 1/2 and catalyzes the
phosphorylation of ERK1/2 at Tyr204/187 and then Thr202/185, and
activated ERK1/2 (p-ERK) promotes the release of its downstream
target genes (52). In the
present study, the expression levels of p-ERK were increased when
H9c2 cells were incubated with 300 nmol/l Ang II; however,
treatment with an inhibitor of ERK (PD98059) decreased the levels
of p-ERK. This revealed that Ang II-induced cardiac inflammation
may be mediated via the p-ERK pathway, which increased the
expression of pro-inflammatory cytokines. A previous study revealed
that urantide can alleviate atherosclerotic myocardial injury by
regulating the MAPK signaling pathway and inhibiting the expression
of p-ERK (53). In addition, the
present study detected the protein expression levels of Nox4, p53,
collagen I and III when cells were stimulated with Ang II and an
ERK inhibitor. The results revealed that the expression levels of
these factors were inhibited by the ERK inhibitor, which suggested
that Ang II could increase the expression of collagen I, collagen
III, Nox4 and p53 by activating the ERK signaling pathway, thus
causing cardiac damage. Previous studies have reported that the
p-ERK pathway is related to oxidative stress, inflammatory response
and fibrosis (54-57), which is consistent with the
present findings. Furthermore, it was revealed that TQ presented a
synergistic action with the ERK inhibitor, whereby TQ increased the
inhibitory effect of the ERK inhibitor. These findings indicated
that TQ may act against Ang II-induced cardiac damage via the p-ERK
signaling pathway.
Notably, there is a limitation of the present study;
an ultrasonographic examination was not performed to determine if
there were changes in cardiac hypertrophy. We plan to investigate
this in future studies.
In conclusion, the present study established that TQ
has a protective effect against Ang II-induced cardiac damage, as
shown by the reduction in hs-CRP levels, and the inhibition of
inflammatory cell infiltration, pro-inflammatory cytokine
expression, fibrosis, oxidative stress and apoptosis associated
proteins via suppressing activation of the p-ERK signaling pathway.
These findings provide novel insights into cardiac damage caused by
Ang II and present the possibility for a new therapeutic
intervention for the treatment of cardiovascular diseases.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD designed the study. LZ, ZP and YW provided their
assistance in the execution of the experiments. LZ and YW confirmed
the authenticity of all raw data. HZ and JM analyzed the data and
interpreted the results. YW prepared the figures. LZ drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures in this study were
approved by the ethical committee of Xi'an No. 3 Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Xi'an Medical
Science Research Program of China (grant no. XA2020-YXYJ-0413).
Abbreviations:
|
TQ
|
thymoquinone
|
|
Ang II
|
angiotensin II
|
|
ApoE−/−
|
apolipoprotein E-deficient
|
|
hs-CRP
|
high-sensitivity C-reactive
protein
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
IL
|
interleukin
|
|
TNF-α
|
tumor necrosis factor α
|
|
ECM
|
extracellular matrix
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Kim JA, Berliner JA and Nadler JL:
Angiotensin II increases monocyte binding to endothelial cells.
Biochem Biophys Res Commun. 226:862–868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lonn EM, Yusuf S, Jha P, Montague TJ, Teo
KK, Benedict CR and Pitt B: Emerging role of angiotensin-converting
enzyme inhibitors in cardiac and vascular protection. Circulation.
90:2056–2069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alderman MH, Madhavan S, Ooi WL, Cohen H,
Sealey JE and Laragh JH: Association of the renin-sodium profile
with the risk of myocardial infarction in patients with
hypertension. N Engl J Med. 324:1098–1104. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daugherty A, Manning MW and Cassis LA:
Angiotensin II promotes atherosclerotic lesions and aneurysms in
apolipoprotein E-deficient mice. J Clin Invest. 105:1605–162. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cassis LA, Gupte M, Thayer S, Zhang X,
Charnigo R, Howatt DA, Rateri DL and Daugherty A: ANG II infusion
promotes abdominal aortic aneurysms independent of increased blood
pressure in hypercholesterolemic mice. Am J Physiol Heart Circ
Physiol. 296:H1660–H1665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guan XH, Hong X, Zhao N, Liu XH, Xiao YF,
Chen TT, Deng LB, Wang XL, Wang JB, Ji GJ, et al: CD38 promotes
angiotensin II-induced cardiac hypertrophy. J Cell Mol Med.
21:1492–1502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chobanian AV and Alexander RW:
Exacerbation of atherosclerosis by hypertension. Potential
mechanisms and clinical implications. Arch Intern Med.
156:1952–1956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L, Li Y, Xiao C and Du J: Angiotensin
II induces inflammation leading to cardiac remodeling. Front Biosci
(Landmark Ed). 17:221–231. 2012. View
Article : Google Scholar
|
|
9
|
She G, Ren YJ, Wang Y, Hou MC, Wang HF,
Gou W, Lai BC, Lei T, Du XJ and Deng XL: KCa 3.1
channels promote cardiac fibrosis through mediating inflammation
and differentiation of monocytes into myofibroblasts in angiotensin
II-treated rats. J Am Heart Assoc. 8:e0104182019. View Article : Google Scholar
|
|
10
|
Touyz RM, Anagnostopoulou A, Camargo LL,
Rios FJ and Montezano AC: Vascular biology of superoxide-generating
NADPH oxidase 5-implications in hypertension and cardiovascular
disease. Antioxid Redox Signal. 30:1027–1040. 2019. View Article : Google Scholar :
|
|
11
|
Gali-Muhtasib H, Roessner A and
Schneider-Stock R: Thymoquinone: A promising anti-cancer drug from
natural sources. Int J Biochem Cell Biol. 38:1249–1253. 2006.
View Article : Google Scholar
|
|
12
|
el Tahir KE, Ashour MM and al-Harbi MM:
The cardiovascular actions of the volatile oil of the black seed
(Nigella sativa) in rats: Elucidation of the mechanism of action.
Gen Pharmacol. 24:1123–1131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar
|
|
14
|
Hosseinzadeh H, Taiari S and Nassiri-Asl
M: Effect of thymoquinone, a constituent of Nigella sativa L., on
ischemia-reperfusion in rat skeletal muscle. Naunyn Schmiedebergs
Arch Pharmacol. 385:503–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao J, Ke ZP, Shi Y, Zeng Q and Cao Z:
The cardioprotective effect of thymoquinone on ischemia-reperfusion
injury in isolated rat heart via regulation of apoptosis and
autophagy. J Cell Biochem. 119:7212–7217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adalı F, Gonul Y, Kocak A, Yuksel Y,
Ozkececi G, Ozdemir C, Tunay K, Bozkurt MF and Sen OG: Effects of
thymoquinone against cisplatin-induced cardiac injury in rats. Acta
Cir Bras. 31:271–277. 2016. View Article : Google Scholar
|
|
17
|
Jalili C, Sohrabi M, Jalili F and
Salahshoor MR: Assessment of thymoquinone effects on apoptotic and
oxidative damage induced by morphine in mice heart. Cell Mol Biol
(Noisy-le-grand). 64:33–38. 2018. View Article : Google Scholar
|
|
18
|
Ortega R, Collado A, Selles F,
Gonzalez-Navarro H, Sanz MJ, Real JT and Piqueras L: SGLT-2
(Sodium-Glucose Cotransporter 2) Inhibition reduces Ang II
(Angiotensin II)-induced dissecting abdominal aortic aneurysm in
ApoE (Apolipoprotein E) knockout mice. Arterioscler Thromb Vasc
Biol. 39:1614–1628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stegbauer J, Thatcher SE, Yang G,
Bottermann K, Rump LC, Daugherty A and Cassis LA: Mas receptor
deficiency augments angiotensin II-induced atherosclerosis and
aortic aneurysm ruptures in hypercholesterolemic male mice. J Vasc
Surg. 70:1658–68.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Atta MS, El-Far AH, Farrag FA, Abdel-Daim
MM, Al Jaouni SK and Mousa SA: Thymoquinone attenuates
cardiomyopathy in streptozotocin-treated diabetic rats. Oxid Med
Cell Longev. 2018:78456812018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satoh K, Nigro P, Zeidan A, Soe NN, Jaffré
F, Oikawa M, O'Dell MR, Cui Z, Menon P, Lu Y, et al: Cyclophilin A
promotes cardiac hypertrophy in apolipoprotein E-deficient mice.
Arterioscler Thromb Vasc Biol. 31:1116–1123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koutnikova H, Laakso M, Lu L, Combe R,
Paananen J, Kuulasmaa T, Kuusisto J, Häring HU, Hansen T, Pedersen
O, et al: Identification of the UBP1 locus as a critical blood
pressure determinant using a combination of mouse and human
genetics. PLoS Genet. 5:e10005912009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chen P, Yang F, Wang W, Li X, Liu D, Zhang
Y, Yin G, Lv F, Guo Z, Mehta JL and Wang X: Liraglutide attenuates
myocardial fibrosis via inhibition of AT1R-mediated ROS production
in hypertensive mice. J Cardiovasc Pharmacol Ther. 26:179–188.
2021. View Article : Google Scholar
|
|
25
|
Madeddu P, Emanueli C, Maestri R, Salis
MB, Minasi A, Capogrossi MC and Olivetti G: Angiotensin II type 1
receptor blockade prevents cardiac remodeling in bradykinin B(2)
receptor knockout mice. Hypertension. 35:391–396. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ridker PM, Hennekens CH, Buring JE and
Rifai N: C-reactive protein and other markers of inflammation in
the prediction of cardiovascular disease in women. N Engl J Med.
342:836–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsushita K, Yatsuya H, Tamakoshi K, Yang
PO, Otsuka R, Wada K, Mitsuhashi H, Hotta Y, Kondo T, Murohara T
and Toyoshima H: High-sensitivity C-reactive protein is quite low
in Japanese men at high coronary risk. Circ J. 71:820–825. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada K, Fujita M, Tanaka A, Yoshida K,
Jisso S, Tanaka H, Yoshikawa J, Kohro T, Hayashi D, Okada Y, et al:
Elevated serum C-reactive protein levels predict cardiovascular
events in the Japanese coronary artery disease (JCAD) study. Circ
J. 73:78–85. 2009. View Article : Google Scholar
|
|
29
|
Touyz RM: Intracellular mechanisms
involved in vascular remodelling of resistance arteries in
hypertension: Role of angiotensin II. Exp Physiol. 90:449–455.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ojha S, Azimullah S, Mohanraj R, Sharma C,
Yasin J, Arya DS and Adem A: Thymoquinone protects against
myocardial ischemic injury by mitigating oxidative stress and
inflammation. Evid Based Complement Alternat Med. 2015:1436292015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ninh VK, El Hajj EC, Ronis MJ and Gardner
JD: N-Acetylcysteine prevents the decreases in cardiac collagen
I/III ratio and systolic function in neonatal mice with prenatal
alcohol exposure. Toxicol Lett. 315:87–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Husse B, Briest W, Homagk L, Isenberg G
and Gekle M: Cyclical mechanical stretch modulates expression of
collagen I and III by PKC and tyrosine kinase in cardiac
fibroblasts. Am J Physiol Regul Integr Comp Physiol.
293:R1898–R1907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cowling RT, Kupsky D, Kahn AM, Daniels LB
and Greenberg BH: Mechanisms of cardiac collagen deposition in
experimental models and human disease. Transl Res. 209:138–155.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villarreal FJ, Kim NN, Ungab GD, Printz MP
and Dillmann WH: Identification of functional angiotensin II
receptors on rat cardiac fibroblasts. Circulation. 88:2849–2861.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schnee JM and Hsueh WA: Angiotensin II,
adhesion, and cardiac fibrosis. Cardiovasc Res. 46:264–268. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leask A: Getting to the heart of the
matter: New insights into cardiac fibrosis. Circ Res.
116:1269–1276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brandes RP, Weissmann N and Schröder K:
NADPH oxidases in cardiovascular disease. Free Radic Biol Med.
49:687–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nabeebaccus A, Zhang M and Shah AM: NADPH
oxidases and cardiac remodelling. Heart Fail Rev. 16:5–12. 2011.
View Article : Google Scholar
|
|
40
|
Li DX, Chen W, Jiang YL, Ni JQ and Lu L:
Antioxidant protein peroxiredoxin 6 suppresses the vascular
inflammation, oxidative stress and endothelial dysfunction in
angiotensin II-induced endotheliocyte. Gen Physiol Biophys.
39:545–555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heymes C, Bendall JK, Ratajczak P, Cave
AC, Samuel JL, Hasenfuss G and Shah AM: Increased myocardial NADPH
oxidase activity in human heart failure. J Am Coll Cardiol.
41:2164–2171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schröder K, Zhang M, Benkhoff S, Mieth A,
Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann
N, et al: Nox4 is a protective reactive oxygen species generating
vascular NADPH oxidase. Circ Res. 110:1217–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Farina F, Sancini G, Mantecca P,
Gallinotti D, Camatini M and Palestini P: The acute toxic effects
of particulate matter in mouse lung are related to size and season
of collection. Toxicol Lett. 202:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qin F, Patel R, Yan C and Liu W: NADPH
oxidase is involved in angiotensin II-induced apoptosis in H9C2
cardiac muscle cells: Effects of apocynin. Free Radic Biol Med.
40:236–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian HP, Sun YH, He L, Yi YF, Gao X and Xu
DL: Single-stranded DNA-binding protein 1 abrogates cardiac
fibroblast proliferation and collagen expression induced by
angiotensin II. Int Heart J. 59:1398–1408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Purcell NH, Wilkins BJ, York A,
Saba-El-Leil MK, Meloche S, Robbins J and Molkentin JD: Genetic
inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and
heart failure but has no effect on hypertrophy in vivo. Proc Natl
Acad Sci USA. 104:14074–14079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cipolletta E, Rusciano MR, Maione AS,
Santulli G, Sorriento D, Del Giudice C, Ciccarelli M, Franco A,
Crola C, Campiglia P, et al: Targeting the CaMKII/ERK interaction
in the heart prevents cardiac hypertrophy. PLoS One.
10:e01304772015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jagavelu K, Tietge UJ, Gaestel M, Drexler
H, Schieffer B and Bavendiek U: Systemic deficiency of the MAP
kinase-activated protein kinase 2 reduces atherosclerosis in
hypercholesterolemic mice. Circ Res. 101:1104–1112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsuzawa A and Ichijo H: Molecular
mechanisms of the decision between life and death: Regulation of
apoptosis by apoptosis signal-regulating kinase 1. J Biochem.
130:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Song J, Bian H, Bo J, Lv S, Pan W
and Lv X: Apelin promotes hepatic fibrosis through ERK signaling in
LX-2 cells. Mol Cell Biochem. 460:205–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ning ZW, Luo XY, Wang GZ, Li Y, Pan MX,
Yang RQ, Ling XG, Huang S, Ma XX, Jin SY, et al: MicroRNA-21
mediates angiotensin II-induced liver fibrosis by activating NLRP3
inflammasome/IL-1β axis via targeting Smad7 and Spry1. Antioxid
Redox Signal. 27:1–20. 2017. View Article : Google Scholar :
|
|
52
|
Xu Z, Sun J, Tong Q, Lin Q, Qian L, Park Y
and Zheng Y: The role of ERK1/2 in the development of diabetic
cardiomyopathy. Int J Mol Sci. 17:20012016. View Article : Google Scholar
|
|
53
|
Zhao J, Miao G, Wang T, Li J and Xie L:
Urantide attenuates myocardial damage in atherosclerotic rats by
regulating the MAPK signalling pathway. Life Sci. 262:1185512020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang
L and Zhang C: Celastrol-induced suppression of the miR-21/ERK
signalling pathway attenuates cardiac fibrosis and dysfunction.
Cell Physiol Biochem. 38:1928–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li P, Chen XR, Xu F, Liu C, Li C, Liu H,
Wang H, Sun W, Sheng YH and Kong XQ: Alamandine attenuates
sepsis-associated cardiac dysfunction via inhibiting MAPKs
signaling pathways. Life Sci. 206:106–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu M, Qin J, Hao Y, Liu M, Luo J, Luo T
and Wei L: Astragalus polysaccharide suppresses skeletal muscle
myostatin expression in diabetes: Involvement of ROS-ERK and NF-κB
pathways. Oxid Med Cell Longev. 2013:7824972013. View Article : Google Scholar
|
|
57
|
Rao RK and Clayton LW: Regulation of
protein phosphatase 2A by hydrogen peroxide and glutathionylation.
Biochem Biophys Res Commun. 293:610–616. 2002. View Article : Google Scholar : PubMed/NCBI
|