Oxidative stress generally originates from toxic
by-products resulting from the imbalance between radicals and
antioxidants, which primarily arises from the accumulation of
reactive oxygen species (ROS) (1,2).
The redox balance is maintained by complex cellular biochemical and
genetic mechanisms. Redox imbalance may have profound effects on
physiological and pathophysiological mechanisms (3,4).
ROS disrupt cellular processes by non-specific modifications on
critical amino acid residues in proteins (resulting in protein
oxidation), fatty acids in lipids (to cause lipid peroxidation) and
nucleic acids (inducing DNA damage and strand breaks) (5-8).
ROS mainly includes the superoxide anion
(O2−), hydrogen peroxide
(H2O2), hydroxyl radical (·OH) and singlet
oxygen (1O2) (9). Among these, ·OH is the most
reactive ROS and is able to react with almost any tissue directly,
thereby causing more effective cellular damage than any other ROS
(10). Under pathological
conditions, tumor cells produce elevated levels of ROS compared
with those of normal cells (11-13). Tumor cells always adjust their
metabolism to increase intracellular ROS levels and maintain their
survival and proliferation during tumorigenesis (14,15). However, ROS have a dual role in
cancer development. ROS may lead to epigenetic alterations that
promote the acceleration of tumor progression. By contrast, higher
levels of ROS promote genome instability, inducing activation of
cancer cell death or inhibiting resistance to anticancer treatment
(16-19).
Theoretically, radiotherapy is able to more
precisely target the tumor. The relative toxicity caused by
radiation to the surrounding normal tissues is limited (20). However, several antioxidant
transcription factors may be activated in response to radiotherapy,
resulting in the inhibition of ROS-dependent damaging effects
induced by radiation and in the reduced effectiveness of the
treatment (21). In addition,
the source of ROS is considered to be a double-edged sword, which
has a key initiator role in ionizing radiation (IR)-associated
normal tissue injury (22). The
radioresistance and tumor recurrence following radiotherapy are
significant problems to overcome, which may contribute to treatment
failure and tumor relapse. Specific modifications in the production
of ROS and the concentrations of antioxidants have pivotal roles in
cancer radiotherapy (12,23,24).
Current research demonstrates that targeting oxidative stress may
benefit patients with radiation resistance during radiotherapy
(25). Therefore, the
identification of the mechanisms of oxidative stress has been the
focus of various studies. In the present review article, the
mechanisms underlying the regulation of oxidative stress induced by
radiotherapy were summarized and the benefits of using
radio-protectors or radio-sensitizers were discussed.
The current literature was reviewed and oxidative
stress-related genes were extracted from pertinent papers (Table SI). Finally, 198 gene symbols
were confirmed with the HUGO Gene Nomenclature Committee
Multi-symbol checker tool (https://www.genenames.org/tools/multi-symbol-checker).
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
by R software was used to identify the signaling pathways that were
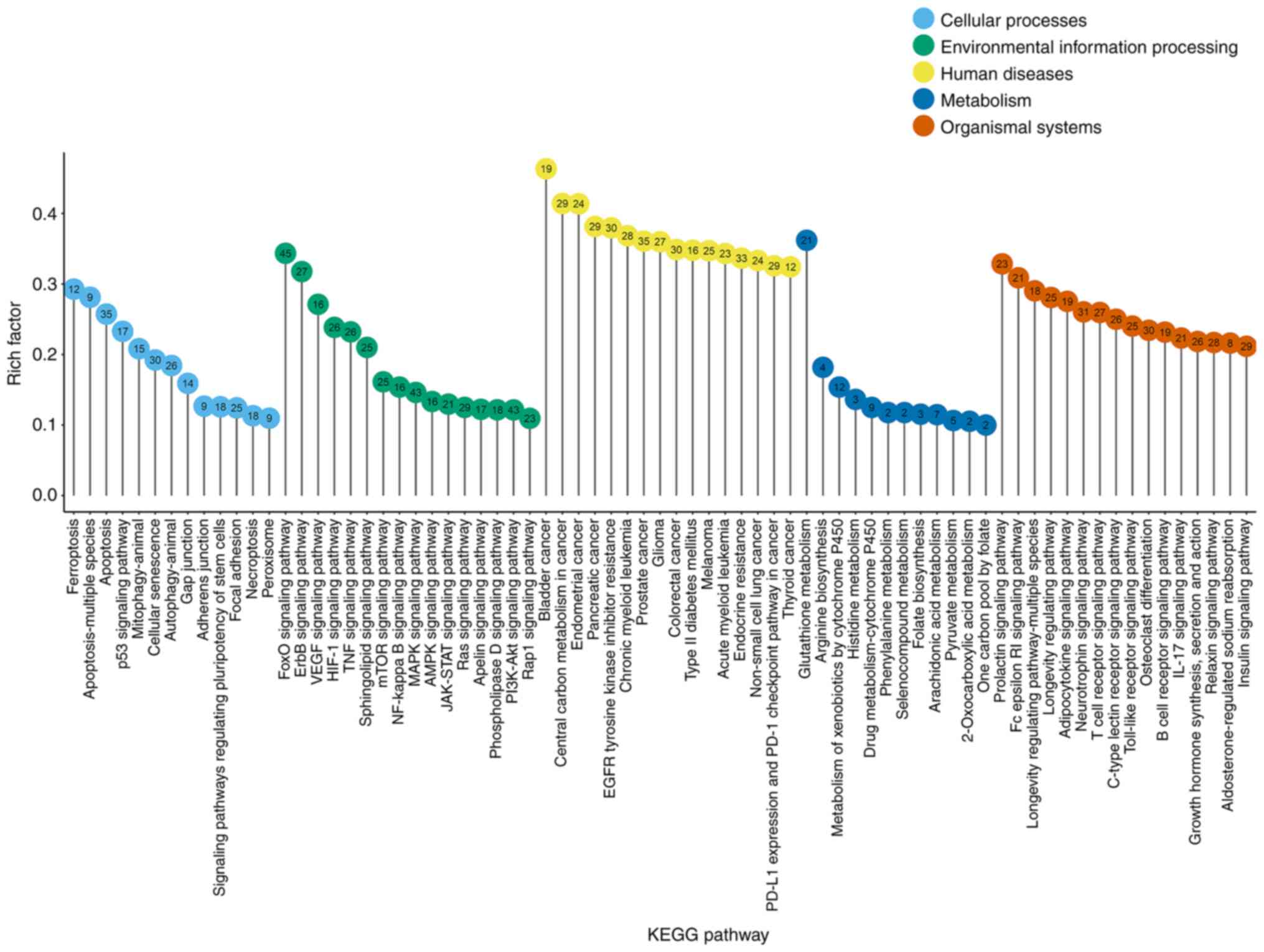
mainly enriched by oxidative stress-related genes. Fig. 1 indicates the significant
pathways identified (rich factor >0.1), which were sub-grouped
by the KEGG main class. The top significant pathways with roles in
cellular processes were as follows: Ferroptosis, apoptosis, p53
signaling pathway, mitophagy, cellular senescence pathway and
autophagy. In addition, the forkhead box protein O (FoxO), Erb-b
receptor tyrosine kinase (ErbB), vascular endothelial growth factor
receptor (VEGF), hypoxia inducible factor-1 (HIF-1), TNF, mTOR,
NF-κB, MAPK, 5′AMP-activated protein kinase, Janus kinase/signal
transducer and activator of transcription, Ras and PI3K/AKT
signaling pathways were the most represented pathways according to
environmental information processing. Glutathione (GSH) metabolism
was dominant in the metabolism category.
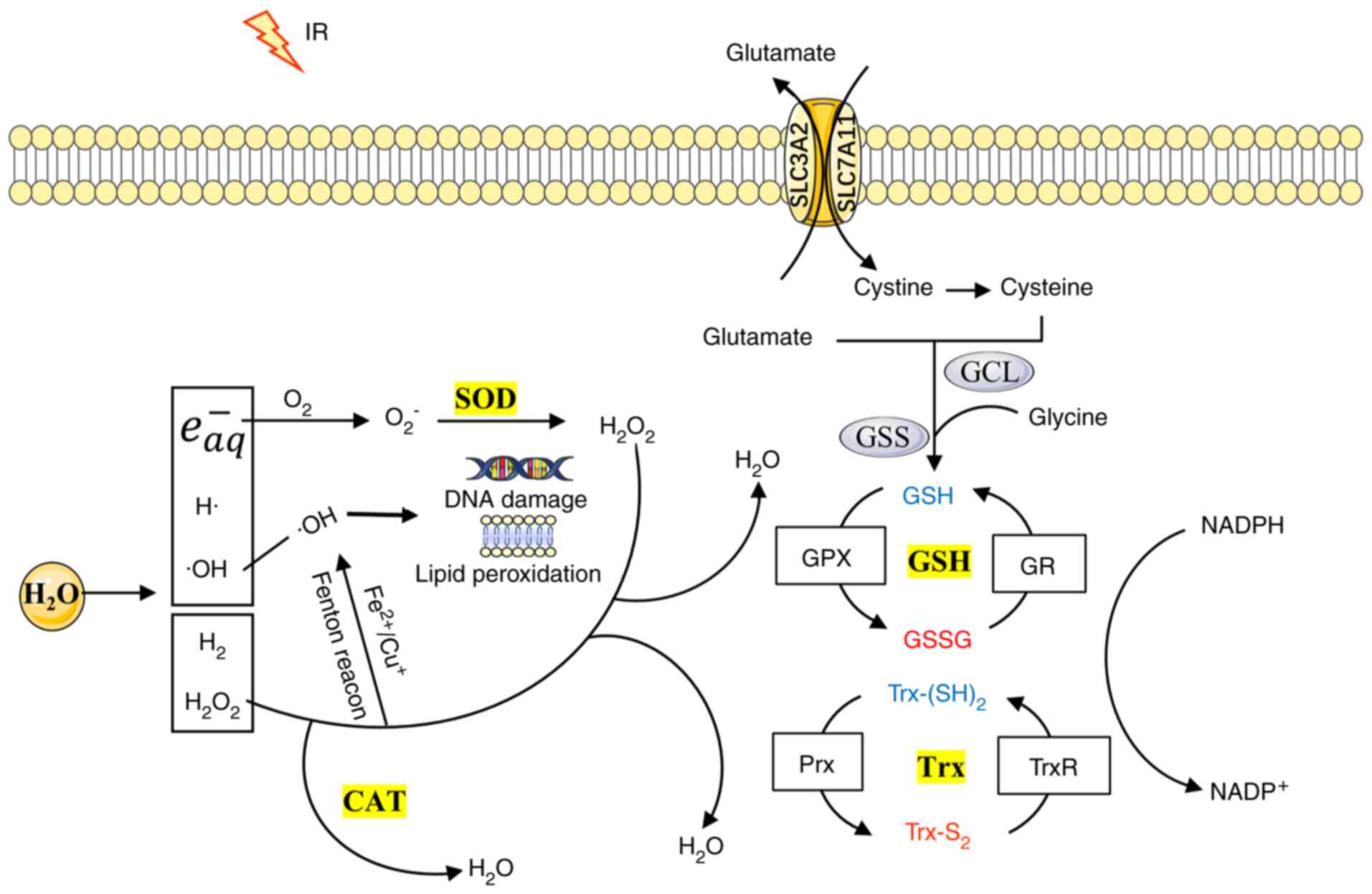
As protectors of cancer cells from the effects of
ROS, the superoxide dismutase (SOD), GSH reductase (GPX),
thioredoxin (Trx) reductase (TrxR) and catalase (CAT) antioxidant
enzymes were investigated, which have a major role in ROS
scavenging (26-28). Fig. 2 indicates the response of the
antioxidant system to radiotherapy. SODs may function in different
cellular compartments to rapidly catalyze O2− into
H2O2 and O2. The other antioxidant
enzymes, including CAT, GPX and TrxR, convert
H2O2 into water (29). In mammalian cells, the following
three types of SOD exist: A copper and zinc SOD termed CuZn-SOD or
SOD1, which is mainly found in the cytosol, a manganese SOD, termed
Mn-SOD or SOD2, which is found in the mitochondrial matrix, and an
extracellular SOD termed EC-SOD or SOD3 (30). CAT is located primarily in the
peroxisomes and is a widespread and highly efficient antioxidant
enzyme present in almost all living organisms, which uses either
iron or manganese as a cofactor (31). The GSH system, which is composed
of glutathione reductase (GR), GSH and NADPH, is the most abundant
cellular thiol antioxidant system and is regulated by its
biosynthesis, redox state and cellular export (32). Its redox cycle is regulated by
GPX and GR (33). At least eight
isoforms of GPX enzymes (GPX1-GPX8) have been found in mammals, of
which GPX4 is the only one that is able to reduce phospholipid
hydroperoxides (34,35). The solute carrier family 7 member
11 (SLC7A11) has a pivotal role in intracellular cysteine balance
and GSH biosynthesis (36).
Similar to the GSH system, Trx is another powerful cellular
disulfide reductase involved in the control of cellular redox
homeostasis, which comprises TrxR, Trx and NADPH (37). The mammalian Trx consists of the
following three isoforms: Trx1 in the cytosol, Trx2 in the
mitochondria and a testis-specific Trx. The following three types
of TrxRs have been characterized: Cytosolic TrxR1, mitochondrial
TrxR2 and testis-specific TrxR3 (38). Trx donates electrons to
peroxyredoxin (Prx) to remove H2O2.
Typically, the Trx and GSH systems are functioning in parallel, and
several types of reciprocal crosstalk have been identified between
these two systems, indicating that the components of one system may
be a backup to those of the other (38).
Radiotherapy has been recognized as one of the
mainstay regimens for various types of cancer treatment (39,40). The changes in the biological
effectiveness of the targeted tissues caused by IR are related to
the energy deposits observed in the encountered molecules of
specific cell signaling pathways (41,42). Oxidative stress has a powerful
function in cancer progression and in the response to radiotherapy.
IR-induced cell damage may originate from direct or indirect
actions. Direct damage to the cell mainly relies on the radiation
that affects the DNA molecules and results in the formation of
either single- or double-strand breaks (43). By contrast, water radiolysis
rapidly produces ROS; the elevated intracellular levels of ROS
cause oxidative stress, which results in indirect damage.
Approximately 80% of the cellular content is composed of water,
which has a leading role in IR-induced biological effects (42,44).
The radiolysis of water leads to the formation of
free radicals, such as hydrated electrons
(e−aq), ·OH, and H·, and certain molecular
products (H2, H2 O2) (45,46). E−aq are
able to indirectly form O−2 with molecular
oxygen (47). In addition,
H2O2 and O2− may be
transformed into the highly reactive ·OH via the Fenton or the
Haber-Weiss reactions in the presence of transition redox metals,
such as iron or copper (48). IR
generates ROS that readily interact with cellular membrane lipids,
proteins and nucleic acids, resulting in the alteration of membrane
permeability, proteolytic degradation, DNA damage and genomic
instability. This eventually induces radiation damage and tumor
cell death (49). Consequently,
radiotherapy may efficiently induce massive cell death by
increasing intracellular ROS levels. Furthermore, the radiation
damage also affects adjacent normal cells via the bystander effect
(50-52). Radiotherapy used in cancer
treatment may cause problems in the heart, as well as in the
hematopoietic, intestinal and nervous systems (53,54).
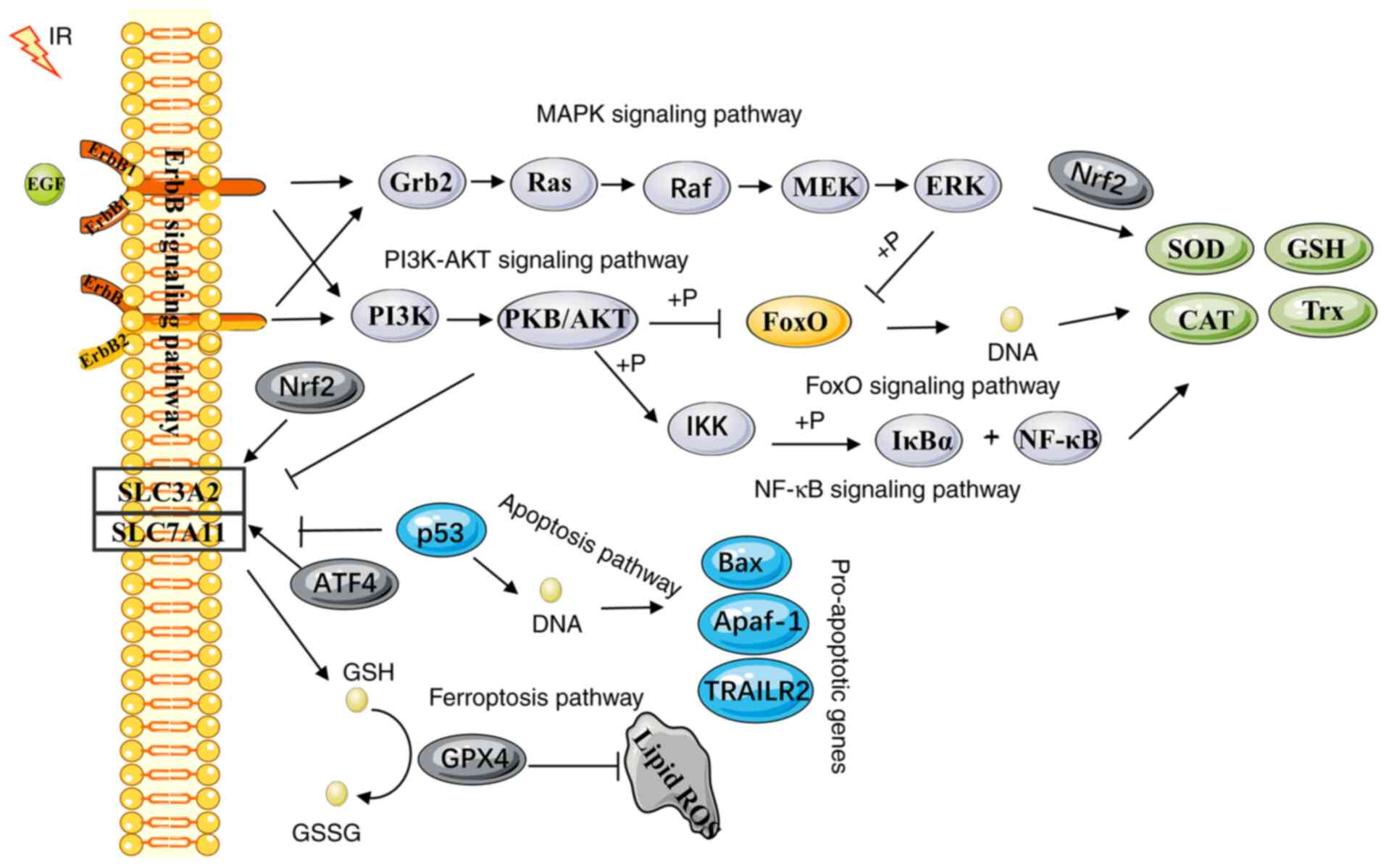
The results of the KEGG pathway analysis revealed
that the dominant pathways that regulate oxidative stress were the
ferroptotic (Fig. S1),
apoptotic (Fig. S2), FoxO
(Fig. S3) and ErbB (Fig. S4) signaling pathways. All of
these pathways may be activated by radiotherapy (55-58). To respond to IR-induced oxidative
stress and the change in redox environmental conditions, multiple
signal transduction pathways crosstalk with each other (Fig. 3). Depending on the IR dose, the
dose rate, the quality and the time period of treatment, these
mechanisms may affect the antioxidant or pro-oxidant effects in a
different manner. To clarify the crosstalk between oxidative stress
and the intracellular IR response, the corresponding molecular
mechanisms were investigated. These molecular events were involved
in the relationship between the major pathways linked to oxidative
stress and the response of the antioxidant defense pathways to
radiotherapy.
Ferroptosis is a recently described form of
regulated cell death, which differs from apoptosis and necrosis and
is characterized by the accumulation of iron-dependent lipid
peroxidation (59,60). The critical role of ferroptosis
in radiotherapy has been established in recent studies (55). The cell membrane is the major
target of IR-induced ROS, since membrane lipids are easily
peroxidized, resulting in structural and functional damage
(61). Glutathione metabolism is
one of the main mechanisms governing ferroptosis. GPX4 and SLC7A11
are key regulators of glutathione metabolism, which have a crucial
role in limiting lipid peroxidation (62).
Radiotherapy results in downregulation of SLC7A11
expression and induces lipid oxidative damage to promote
tumor-associated ferroptosis (63). IR may also cause significant
downregulation in the expression levels of GPX4 (64). However, in certain cases, IR may
induce SLC7A11 or GPX4 expression as an adaptive response to
protect cells from ferroptosis (65). In addition, p53 and nuclear
factor-erythroid factor 2-related factor 2 (Nrf2) may be rapidly
activated by IR, which has an important role in the regulation of
ferroptosis. p53 is able to inhibit the cellular uptake of cystine
by transcriptionally restricting SLC7A11 expression to reduce
antioxidant capacity, resulting in ferroptosis (66,67). The transcription factor Nrf2 is
considered to have a central role in the upregulation of the
expression levels of specific anti-ferroptotic defense biomarkers.
Nrf2 promotes cell survival in irradiated cells via activation of
specific downstream regulator target genes, including SLC7A11.
These genes aim to prevent oxidative damage (68-71). In addition, the Trx system may
also protect cells from lipid peroxidation (72). Nrf2 is able to bind to the TrxR1
and Trx1 promoter antioxidant responsive element (ARE) and improve
its activity (73). In addition,
it has been indicated that Nrf2 is able to bind to the ARE sequence
of various other antioxidant proteins, namely GPX2, Prx1, Prx6 and
glutamate-cysteine ligase catalytic subunit (74-77).
Apoptosis is a form of regulated cell death.
Oxidative stress is considered to be a strong inducer of apoptosis
(78). Apoptosis is triggered by
the following two major signaling pathways: The extrinsic and the
intrinsic pathway. These pathways are independent but interact with
each other (79). It is
suggested that both the intrinsic pathway (activated by
mitochondrial outer membrane permeabilization) and the extrinsic
pathway (initiated by plasma membrane receptors) may be activated
following IR treatment (80).
However, studies have demonstrated that radiotherapy
primarily acts through the intrinsic pathway (80-82). The signatures of several
intrinsic pathway proteins are associated with radiosensitivity,
such as p53, Bcl-2 and Bax (83,84). In response to IR-induced
oxidative stress, p53 has an essential role in the regulation of
the redox state (85,86). The activation of p53 is largely
dependent on the ATM kinase that phosphorylates p53 shortly after
IR (87). A previous research
study suggested that p53 regulated radiotherapy efficacy by
targeting Bcl-2 proteins to release Bax, which in turn promoted
apoptosis or inactivated invasiveness (88). In addition, p53 was also able to
activate the expression levels of SOD2 and GPX1 by binding to their
promoters, which stimulates an antioxidant response (89). It is known that TNFα is a potent
pro-apoptotic molecule, which promotes the expression of several
inflammatory factors. However, TNFα also has a role in cell
survival mechanisms (90-92).
TNFα is able to increase the transcription of GPX4 (93). Activation of the transcription
factor NF-κB has a central role in regulating apoptosis (94). In addition to its apoptotic
activity, NF-κB induces the expression of specific genes, which may
attenuate ROS production and promote survival (95). For instance, the NF-κB pathway
may lead to SOD2 gene activation (96,97). Experimental evidence also
suggests that GPX4 is transcriptionally regulated by NF-κB
(93).
The FoxO family includes several pivotal
transcription factors activated in response to oxidative stress,
such as FoxO1, FoxO3a, FoxO4 and FoxO6. The majority of previously
published studies have focused on the first three members (98,99). The interaction of FoxO and p53
proteins may coordinate tumor suppression via the regulation of
various common target genes, such as p21, growth arrest and DNA
damage, protein phosphatase 1D and sestrin 1 (57). A previous study revealed that JNK
is able to phosphorylate FoxO1, FoxO3a, and FoxO4 to facilitate
nuclear entry of FoxO, leading to the upregulation of the
expression levels of antioxidant genes (98).
FoxO3a is a crucial effector of IR-induced apoptosis
in response to genotoxic stress (100). FOXO3a promotes the cell
survival pathway by directly binding to the SOD2 promoter, causing
increased expression of SOD2. The activation of the latter protects
the cells from oxidative stress-mediated injury (101). By contrast, FoxO3a may
effectively increase cellular antioxidant capacity by enhancing the
levels of CAT and Prx3 to protect against oxidative stress
(102,103). However, the regulation of
oxidative stress by FoxO3a is complex. A previous study indicated
that depletion of FoxO3a expression profoundly reduced kelch-like
ECH associated protein 1 protein levels, thereby activating Nrf2
signaling (104). It was also
indicated that FoxO4 was able to bind to the SOD2 promoter to
upregulate SOD2 expression (105). FoxO1 was able to promote
activating transcription factor 4 expression, which acts as an
important transcription factor for SLC7A11, leading to GSH
synthesis (106,107).
The ErbB family of proteins is also termed the
epithelial growth factor receptor (EGFR) family and consists of the
four following members: EGFR (ErbB1 or Her1), ErbB2 or Her2, ErbB3
or Her3, and ErbB4 or Her4 (108). In response to IR, the ErbB
receptor tyrosine kinase family is rapidly activated, leading to
subsequent activation of multiple downstream pathways (58,109). The activated downstream
pathways mainly include PI3K/AKT, MAPK/ERK1/2, Ras and the mTOR
signaling pathways, leading to alteration in cell proliferation,
apoptosis, autophagy, migration and invasion (110-112). The EGFR transactivation caused
by ROS results in the protection of the cells against oxidative
stress with extensive crosstalk occurring among these pathways
(113).
ErbB receptors, notably EGFR and ErbB2, are closely
associated with the induction of oxidative stress (114). EGFR may stimulate HIF signaling
activity to improve cellular survival (115). A previous research study has
identified a functional transcription start site for GPX3, which is
used for binding with HIF-1 (116). Several mechanisms have also
been reported to explain the increase in Nrf2 transcription by the
PI3K/AKT and Kras signaling pathways (117). The study also indicated that
ErbB2 activated Nrf2 transcriptional activity through direct
protein-protein interactions, which caused the induction of the
expression of antioxidant and detoxification proteins (118). Moreover, Sakurai et al
also reported that overexpression of Nrf2 augmented the TrxR1
promoter activity (119). In
addition, it has been demonstrated that the restriction of ErbB2
receptor contributes to cell death through the production of ROS
(120).
It is important to note that IR-induced ROS leads to
cellular oxidizing stress that has an important role in
radiotherapy. Several proteins are related to the regulation of the
antioxidant systems. These proteins control the expression of
various antioxidant genes and may defend against the induction of
oxidative stress by IR (Table
I). Consequently, the effects of various types of anticancer
treatment may be diminished.
IR-induced oxidative stress is not only involved in
cancer cell death but also in the activation of the damage-repair
and survival signaling to relieve the induction of oxidative
damage. These activations are responsible for radioresistance in
cancer (85). The inhibition of
oxidative stress appears to be the main mechanism, established by
the intracellular antioxidant system, responsible for tumor
radioresistance (121). As
presented in Table II,
increasing evidence has demonstrated that antioxidant system
inhibitors promote radiation sensitization.
Previous studies have suggested that Nrf2 is a key
transcription factor that regulates the expression of various
antioxidant proteins (122,123). Nrf2 inhibitors may be an
effective approach against radioresistance. ML385 is a specific
Nrf2 inhibitor that binds this transcription factor and blocks the
downstream target gene expression, leading to the sensitization of
breast cancer stem cells to IR (124). Brusatol selectively reduces the
protein levels of Nrf2 by enhancing ubiquitination and degradation
of Nrf2 and enhances the radiosensitivity of tumors (125). In addition, IM3829 markedly
enhances the radiosensitivity of human lung cancer cells by
inhibiting the mRNA and protein expression levels of Nrf2 (126). Halofuginone, a less-toxic
febrifugine derivative, is considered to be particularly promising
for cancer treatment. This compound rapidly suppresses the
accumulation of the Nrf2 protein in therapy-resistant cancer cells
(127). Although FoxO3a may be
activated by IR, leading to an increase in the expression levels of
antioxidant markers, FoxO3a-induced apoptosis has received
increasing attention in response to radiation. Butyrate (128) and resveratrol (129) have demonstrated the potential
to overcome the radioresistance effect by enhancing the activation
of FoxO3a transcription. During radioresistance, ferroptosis
inducers also have a key role. A previous study revealed that
sulfasalazine (inhibitor of SLC7A11) and RSL3 (inhibitor of GPX4)
exert significant radiosensitizing effects in vitro
(65). TrxR inhibitors enhance
radiosensitivity by triggering excessive oxidative stress. Specific
examples of these compounds include auranofin (72,130), platinum complexes (20) and selenadiazole (131,132). Since Trx and GSH perform
crosstalk with each other, their dual inhibition has synergetic
antitumor effects in cancer therapy by inducing ROS production
(133). EGFR or ErbB2
inhibitors (e.g. lapatinib) led to increased radiosensitivity in
wild-type K-ras pancreatic cancer (134). The EGFR inhibitor icotinib has
been indicated to increase radiosensitivity by enhancing apoptosis
and downregulating the MAPK-AKT and ERK signaling pathways
(135). In addition,
combination treatment with radiotherapy and an MDM2-p53 inhibitor
(APG-115) made tumors overcome radioresistance and enhance the
antitumor effects (136).
Typically, IR causes the accumulation of endogenous
ROS in irradiated cells, as a consequence of the activation of
intracellular signaling pathways (137-139). These effects result in an
ongoing inflammatory cascade, which may contribute to continuous
damage that surpasses the initial insult and responses noted in
non-irradiated cells, which are neighboring to irradiated cells
(IR-induced bystander effects) (140). The side effects of IR mostly
result from the increased oxidative stress and inflammation
generated during radiotherapy (141). Therefore, it is of particular
importance that the induction of tumor cell death during
radiotherapy occurs without producing extensive damage to
surrounding healthy tissues (142).
To reduce these adverse effects, radioprotectors are
employed to protect against IR damage to healthy tissues. These
compounds act by different mechanisms, which are mainly associated
with the modulation of the antioxidant defense (49). p53 inhibition may reduce damage
to normal tissues and this strategy has been experimentally tested
in mice by using a small-molecule inhibitor of p53 (pifithrin-α)
(143). Isofraxidin may have a
radioprotective effect in human leukemia cells through decreasing
ROS levels in a p53-independent manner (144). Resveratrol has been indicated
to attenuate IR enteritis by inhibiting oxidative stress and
apoptosis through the activation of the Sirtuin 1/FoxO3a and
PI3K/AKT signaling pathways (145). In addition, the endogenous
compounds melatonin and vitamin D are considered to be potent
radioprotectors for the protection against oxidative damage caused
by IR (146). Melatonin has
been reported to possess significant potency in inhibiting the
induction of oxidative stress via regulation of the expression
levels of certain antioxidant genes (including Nrf2) and the
activities of ROS/nitric oxide-producing enzymes (147). In addition, this hormone may
directly scavenge free radicals to alleviate oxidative injury
induced by IR in different cells or organs (147). In previous studies, plant and
plant-derived products, such as herbal medicine, have been
extensively examined for their effectiveness and compatibility in
conferring radioprotection (49). Mn porphyrins are powerful SOD
mimics, which have been indicated to possess radioprotective
effects in different cells, animal models and tissues, including
the lung, the prostate and the brain (148,149). The lead Mn porphyrins, such as
MnTE-2-PyP5+ (BMX-010, AEOL10113),
MnTnBuOE-2-PyP5+ (BMX-001) and
MnTnHex-2-PyP5+ have entered clinical trials for the
assessment of their efficacy in the radioprotection of normal
tissues during cancer radiotherapy (149).
Accumulating evidence suggests that a rational
combination of antioxidants or oxidants with IR is an attractive
approach to improve the tumor treatment response. In the present
review article, the molecular pathways and potential candidate
targets that control the induction of oxidative stress in
radiosensitivity and radioprotection were discussed. Nrf2 was
identified as a key transcriptional target involved in the
resistance of cancer cells to radiotherapy. In addition, Trx and
GSH complement each other. They are parts of powerful antioxidant
mechanisms connected with the protection of cancer cells from
radiation resistance. However, due to the limitations of the
present study, further experiments should be performed to
completely uncover the roles of these antioxidant enzyme systems in
radiotherapy. A deeper understanding of the mechanisms underlying
oxidative stress in cancer radiotherapy may reveal novel
therapeutic opportunities.
All data generated or analysed during this study are
included in this published article.
RL and YB contributed to the preparation,
bioinformatics analyses and drafting of the manuscript. RL, YB, LL
and LCL performed the relevant literature search, assisted in
obtaining data and revised the manuscript. XDL and SMM supervised
the preparation of the manuscript and critically reviewed the
manuscript. All authors have read and approved the final version of
the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81773363, 81872558,
81972969 and 81673092).
|
1
|
Zhang Z, Rong L and Li YP: Flaviviridae
viruses and oxidative stress: Implications for viral pathogenesis.
Oxid Med Cell Longev. 2019:14095822019.
|
|
2
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017:84167632017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beyfuss K and Hood DA: A systematic review
of p53 regulation of oxidative stress in skeletal muscle. Redox
Rep. 23:100–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pisoschi AM, Pop A, Iordache F, Stanca L,
Predoi G and Serban AI: Oxidative stress mitigation by
antioxidants-An overview on their chemistry and influences on
health status. Eur J Med Chem. 209:1128912021. View Article : Google Scholar
|
|
6
|
Zuo L and Wijegunawardana D: Redox role of
ROS and inflammation in pulmonary diseases. Adv Exp Med Biol.
1304:187–204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Czarnocka W and Karpiński S: Friend or
foe? Reactive oxygen species production, scavenging and signaling
in plant response to environmental stresses. Free Radic Biol Med.
122:4–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samavarchi Tehrani S, Mahmoodzadeh
Hosseini H, Yousefi T, Abolghasemi M, Qujeq D, Maniati M and Amani
J: The crosstalk between trace elements with DNA damage response,
repair, and oxidative stress in cancer. J Cell Biochem. Oct
30–2018.Epub ahead of print. PubMed/NCBI
|
|
9
|
Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi
Z, Xiaocheng W, Hong Z and Liang S: Oxidative stress in
radiation-induced cardiotoxicity. Oxid Med Cell Longev.
2020:35791432020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XQ, Wang W, Peng M and Zhang XZ: Free
radicals for cancer theranostics. Biomaterials. 266:1204742021.
View Article : Google Scholar
|
|
11
|
Zhang J, Duan D, Song ZL, Liu T, Hou Y and
Fang J: Small molecules regulating reactive oxygen species
homeostasis for cancer therapy. Med Res Rev. 41:342–394. 2021.
View Article : Google Scholar
|
|
12
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
13
|
Zou Z, Chang H, Li H and Wang S: Induction
of reactive oxygen species: An emerging approach for cancer
therapy. Apoptosis. 22:1321–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodic S and Vincent MD: Reactive oxygen
species (ROS) are a key determinant of Cancer's metabolic
phenotype. Int J Cancer. 142:440–448. 2018. View Article : Google Scholar
|
|
15
|
Wang S, Luo J, Zhang Z, Dong D, Shen Y,
Fang Y, Hu L, Liu M, Dai C, Peng S, et al: Iron and magnetic: New
research direction of the ferroptosis-based cancer therapy. Am J
Cancer Res. 8:1933–1946. 2018.PubMed/NCBI
|
|
16
|
Snezhkina AV, Kudryavtseva AV, Kardymon
OL, Savvateeva MV, Melnikova NV, Krasnov GS and Dmitriev AA: ROS
Generation and antioxidant defense systems in normal and malignant
cells. Oxid Med Cell Longev. 2019:61758042019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helfinger V and Schröder K: Redox control
in cancer development and progression. Mol Aspects Med. 63:88–98.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sánchez-Sánchez AM, Martín V,
García-Santos G, Rodríguez-Blanco J, Casado-Zapico S,
Suarez-Garnacho S, Antolín I and Rodriguez C: Intracellular redox
state as determinant for melatonin antiproliferative vs cytotoxic
effects in cancer cells. Free Radic Res. 45:1333–1341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klaunig JE: Oxidative stress and cancer.
Curr Pharm Des. 24:4771–4778. 2018. View Article : Google Scholar
|
|
20
|
Xie Q, Lan G, Zhou Y, Huang J, Liang Y,
Zheng W, Fu X, Fan C and Chen T: Strategy to enhance the anticancer
efficacy of X-ray radiotherapy in melanoma cells by platinum
complexes, the role of ROS-mediated signaling pathways. Cancer
Lett. 354:58–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galeaz C, Totis C and Bisio A: Radiation
resistance: A matter of transcription factors. Front Oncol.
11:6628402021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sonis ST: Superoxide dismutase as an
intervention for radiation therapy-associated toxicities: Review
and profile of avasopasem manganese as a treatment option for
radiation-induced mucositis. Drug Des Devel Ther. 15:1021–1029.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perillo B, Di Donato M, Pezone A, Di Zazzo
E, Giovannelli P, Galasso G, Castoria G and Migliaccio A: ROS in
cancer therapy: The bright side of the moon. Exp Mol Med.
52:192–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SJ, Kim HS and Seo YR: Understanding
of ROS-inducing strategy in anticancer therapy. Oxid Med Cell
Longev. 2019:53816922019. View Article : Google Scholar
|
|
25
|
Hu J, Li Y, Li H, Shi F, Xie L, Zhao L,
Tang M, Luo X, Jia W, Fan J, et al: Targeting Epstein-Barr virus
oncoprotein LMP1-mediated high oxidative stress suppresses EBV
lytic reactivation and sensitizes tumors to radiation therapy.
Theranostics. 10:11921–11937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poprac P, Jomova K, Simunkova M, Kollar V,
Rhodes CJ and Valko M: Targeting free radicals in oxidative
stress-related human diseases. Trends Pharmacol Sci. 38:592–607.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang H, Wang H and De Ridder M: Targeting
antioxidant enzymes as a radiosensitizing strategy. Cancer Lett.
438:154–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jaganjac M, Milkovic L, Sunjic SB and
Zarkovic N: The NRF2, Thioredoxin, and Glutathione system in
tumorigenesis and anticancer therapies. Antioxidants (Basel).
9:E11512020. View Article : Google Scholar
|
|
29
|
Reczek CR and Chandel NS: The two faces of
reactive oxygen species in cancer. Ann Rev Cancer Biol. 1:79–98.
2017. View Article : Google Scholar
|
|
30
|
Koyama H, Nojiri H, Kawakami S, Sunagawa
T, Shirasawa T and Shimizu T: Antioxidants improve the phenotypes
of dilated cardiomyopathy and muscle fatigue in mitochondrial
superoxide dismutase-deficient mice. Molecules. 18:1383–1393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ighodaro OM and Akinloye OA: First line
defence antioxidantssuperoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alexandria J Med. 54:287–293. 2018.
View Article : Google Scholar
|
|
32
|
Haddad M, Hervé V, Ben Khedher MR, Rabanel
JM and Ramassamy C: Glutathione: An old and small molecule with
great functions and new applications in the brain and in
Alzheimer's disease. Antioxid Redox Signal. 35:270–292. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marrocco I, Altieri F and Peluso I:
Measurement and clinical significance of biomarkers of oxidative
stress in humans. Oxid Med Cell Longev. 2017:65010462017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang ML, Wu HT, Chen WJ, Xu Y, Ye QQ,
Shen JX and Liu J: Involvement of glutathione peroxidases in the
occurrence and development of breast cancers. J Transl Med.
18:2472020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Zhang Y, Zhuang L, Olszewski K and
Gan B: NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated
cystine uptake as a double-edged sword in cellular redox
regulation. Genes Dis. 8:731–745. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Xia X and Huang P: xCT: A critical
molecule that links cancer metabolism to redox signaling. Mol Ther.
28:2358–2366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu J, Chew EH and Holmgren A: Targeting
thioredoxin reductase is a basis for cancer therapy by arsenic
trioxide. Proc Natl Acad Sci USA. 104:12288–12293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren X, Zou L, Zhang X, Branco V, Wang J,
Carvalho C, Holmgren A and Lu J: Redox signaling mediated by
thioredoxin and glutathione systems in the central nervous system.
Antioxid Redox Signal. 27:989–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu TI, Lu TY, Yang YC, Chang SH, Chen HH,
Lu IL, Sabu A and Chiu HC: New combination treatment from
ROS-Induced sensitized radiotherapy with nanophototherapeutics to
fully eradicate orthotopic breast cancer and inhibit metastasis.
Biomaterials. 257:1202292020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shevtsov M, Sato H, Multhoff G and Shibata
A: Novel approaches to improve the efficacy of immuno-Radiotherapy.
Front Oncol. 9:1562019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rabus H: Nanodosimetry-on the 'tracks' of
biological radiation effectiveness. Z Med Phys. 30:91–94. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Jiang H, Van De Gucht M and De
Ridder M: Hypoxic radioresistance: Can ROS be the key to overcome
it? Cancers (Basel). 11:1122019. View Article : Google Scholar
|
|
43
|
Alizadeh E, Orlando TM and Sanche L:
Biomolecular damage induced by ionizing radiation: The direct and
indirect effects of low-energy electrons on DNA. Annu Rev Phys
Chem. 66:379–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baskar R, Dai J, Wenlong N, Yeo R and Yeoh
KW: Biological response of cancer cells to radiation treatment.
Front Mol Biosci. 1:242014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Le Caër S: Water Radiolysis: Influence of
oxide surfaces on H2 production under ionizing radiation. Water.
3:235–253. 2011. View Article : Google Scholar
|
|
46
|
Baldacchino G, Brun E, Denden I, Bouhadoun
S, Roux R, Khodja H and Sicard-Roselli C: Importance of radiolytic
reactions during high-LET irradiation modalities: LET effect, role
of O2 and radiosensitization by nanoparticles. Cancer Nanotechnol.
10:32019. View Article : Google Scholar
|
|
47
|
Gong L, Zhang Y, Liu C, Zhang M and Han S:
Application of radiosensitizers in cancer radiotherapy. Int J
Nanomedicine. 16:1083–1102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Collin F: Chemical basis of reactive
oxygen species reactivity and involvement in neurodegenerative
diseases. Int J Mol Sci. 20:24072019. View Article : Google Scholar :
|
|
49
|
Dayal R, Singh A, Pandey A and Mishra KP:
Reactive oxygen species as mediator of tumor radiosensitivity. J
Cancer Res Ther. 10:811–818. 2014. View Article : Google Scholar
|
|
50
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
51
|
Robinett ZN, Bathla G, Wu A, Clark JJ,
Sibenaller ZA, Wilson T, Kirby P, Allen BG and Hansen MR:
Persistent oxidative stress in vestibular schwannomas after
stereotactic radiation therapy. Otol Neurotol. 39:1184–1190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buonanno M, de Toledo SM, Pain D and Azzam
EI: Long-Term consequences of radiation-induced bystander effects
depend on radiation quality and dose and correlate with oxidative
stress. Radiat Res. 175:405–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang QY, Wang FX, Jia KK and Kong LD:
Natural product interventions for chemotherapy and
radiotherapy-induced side effects. Front Pharmacol. 9:12532018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Musa AE, Shabeeb D and Alhilfi HSQ:
Protective effect of melatonin against radiotherapy-induced small
intestinal oxidative stress: Biochemical evaluation. Medicina
(Kaunas). 55:3082019. View Article : Google Scholar
|
|
55
|
Lei G, Mao C, Yan Y, Zhuang L and Gan B:
Ferroptosis, radiotherapy, and combination therapeutic strategies.
Protein Cell. 12:836–857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu B, Bhatt D, Oltvai ZN, Greenberger JS
and Bahar I: Significance of p53 dynamics in regulating apoptosis
in response to ionizing radiation and polypharmacological
strategies. Sci Rep. 4:62452014. View Article : Google Scholar
|
|
57
|
Zanella F, Link W and Carnero A:
Understanding FOXO, new views on old transcription factors. Curr
Cancer Drug Targets. 10:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schmidt-Ullrich RK, Contessa JN, Lammering
G, Amorino G and Lin PS: ERBB receptor tyrosine kinases and
cellular radiation responses. Oncogene. 22:5855–5865. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of non-apoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu J, Xiong Y, Zhang Y, Wen J, Cai N,
Cheng K, Liang H and Zhang W: The molecular mechanisms of
regulating oxidative stress-induced ferroptosis and therapeutic
strategy in tumors. Oxid Med Cell Longev. 2020:88107852020.
View Article : Google Scholar
|
|
61
|
Barrera G: Oxidative stress and lipid
peroxidation products in cancer progression and therapy. ISRN
Oncol. 2012:1372892012.PubMed/NCBI
|
|
62
|
Stockwell BR, Jiang X and Gu W: Emerging
mechanisms and disease relevance of ferroptosis. Trends Cell Biol.
30:478–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lang X, Green MD, Wang W, Yu J, Choi JE,
Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al: Radiotherapy and
immunotherapy promote tumoral lipid oxidation and ferroptosis via
synergistic repression of SLC7A11. Cancer Discov. 9:1673–1685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li X, Duan L, Yuan S, Zhuang X, Qiao T and
He J: Ferroptosis inhibitor alleviates Radiation-induced lung
fibrosis (RILF) via down-regulation of TGF-β1. J Inflamm (Lond).
16:112019. View Article : Google Scholar
|
|
65
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cameron BD, Sekhar KR, Ofori M and Freeman
ML: The role of Nrf2 in the response to normal tissue radiation
injury. Radiat Res. 190:99–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sekhar KR and Freeman ML: Nrf2 promotes
survival following exposure to ionizing radiation. Free Radic Biol
Med. 88:268–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu
C and Li B: SLC7A11 regulated by NRF2 modulates esophageal squamous
cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl
Med. 19:3672021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lippmann J, Petri K, Fulda S and Liese J:
Redox modulation and induction of ferroptosis as a new therapeutic
strategy in hepatocellular carcinoma. Transl Oncol. 13:1007852020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jakobs P, Serbulea V, Leitinger N, Eckers
A and Haendeler J: Nuclear factor (Erythroid-Derived 2)-Like 2 and
Thioredoxin-1 in atherosclerosis and ischemia/reperfusion injury in
the heart. Antioxid Redox Signal. 26:630–644. 2017. View Article : Google Scholar :
|
|
74
|
Banning A, Deubel S, Kluth D, Zhou Z and
Brigelius-Flohé R: The GI-GPx Gene Is a target for Nrf2. Mol Cell
Biol. 25:4914–4923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y and
Park YM: Human prx1 gene is a target of Nrf2 and is up-regulated by
hypoxia/reoxygenation: Implication to tumor biology. Cancer Res.
67:546–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fisher AB: Peroxiredoxin 6: A Bifunctional
enzyme with glutathione peroxidase and phospholipase A2 activities.
Antioxid Redox Signal. 15:831–844. 2011. View Article : Google Scholar :
|
|
77
|
Yang H, Magilnick N, Lee C, Kalmaz D, Ou
X, Chan JY and Lu SC: Nrf1 and Nrf2 regulate rat glutamate-cysteine
ligase catalytic subunit transcription indirectly via NF-kappaB and
AP-1. Mol Cell Biol. 25:5933–5946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen KW, Demarco B, Heilig R, Shkarina K,
Boettcher A, Farady CJ, Pelczar P and Broz P: Extrinsic and
intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome
assembly. EMBO J. 38:e1016382019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cao X, Wen P, Fu Y, Gao Y, Qi X, Chen B,
Tao Y, Wu L, Xu A, Lu H and Zhao G: Radiation induces apoptosis
primarily through the intrinsic pathway in mammalian cells. Cell
Signal. 62:1093372019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu C, Mann D, Sinha UK and Kokot NC: The
molecular mechanisms of increased radiosensitivity of HPV-positive
oropharyngeal squamous cell carcinoma (OPSCC): An extensive review.
J Otolaryngol Head Neck Surg. 47:592018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mendes F, Sales T, Domingues C, Schugk S,
Abrantes AM, Gonçalves AC, Teixo R, Silva R, Casalta-Lopes J, Rocha
C, et al: Effects of X-radiation on lung cancer cells: the
interplay between oxidative stress and P53 levels. Med Oncol.
32:2662015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mortezaee K, Najafi M, Farhood B, Ahmadi
A, Potes Y, Shabeeb D and Musa AE: Modulation of apoptosis by
melatonin for improving cancer treatment efficiency: An updated
review. Life Sci. 228:228–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qin C, Chen X, Bai Q, Davis MR and Fang Y:
Factors associated with radiosensitivity of cervical cancer.
Anticancer Res. 34:4649–4656. 2014.PubMed/NCBI
|
|
85
|
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E,
Kang J, Seong KM, Youn H and Youn B: Cellular stress responses in
radiotherapy. Cells. 8:11052019. View Article : Google Scholar :
|
|
86
|
Chang HW, Lee M, Lee YS, Kim SH, Lee JC,
Park JJ, Nam HY, Kim MR, Han MW, Kim SW and Kim SY: p53-dependent
glutamine usage determines susceptibility to oxidative stress in
radioresistant head and neck cancer cells. Cell Signal.
77:1098202021. View Article : Google Scholar
|
|
87
|
Maya R, Balass M, Kim ST, Shkedy D, Leal
JF, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, et al:
ATM-dependent phosphorylation of Mdm2 on serine 395: Role in p53
activation by DNA damage. Genes Dev. 15:1067–1077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Budanov AV: The role of tumor suppressor
p53 in the antioxidant defense and metabolism. Subcell Biochem.
85:337–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rastogi S, Rizwani W, Joshi B, Kunigal S
and Chellappan SP: TNF-α response of vascular endothelial and
vascular smooth muscle cells involve differential utilization of
ASK1 kinase and p73. Cell Death Differ. 19:274–283. 2012.
View Article : Google Scholar
|
|
91
|
Feltham R, Jamal K, Tenev T, Liccardi G,
Jaco I, Domingues CM, Morris O, John SW, Annibaldi A, Widya M, et
al: Mind bomb regulates cell death during TNF signaling by
suppressing RIPK1's cytotoxic potential. Cell Rep. 23:470–484.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kokolakis G, Sabat R, Krüger-Krasagakis S
and Eberle J: Ambivalent effects of tumor necrosis factor alpha on
apoptosis of malignant and normal human keratinocytes. Skin
Pharmacol Physiol. 34:94–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stoytcheva ZR and Berry MJ:
Transcriptional regulation of mammalian selenoprotein expression.
Biochim Biophys Acta. 1790:1429–1440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ross MJ, Martinka S, D'Agati VD and
Bruggeman LA: NF-kappaB regulates Fas-mediated apoptosis in
HIV-associated nephropathy. J Am Soc Nephrol. 16:2403–2411. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Morgan MJ and Liu Z: Crosstalk of reactive
oxygen species and NF-κB signaling. Cell Res. 21:103–115. 2011.
View Article : Google Scholar
|
|
96
|
Li Q, Sun Y, Liu B, Li J, Hao X, Ge W,
Zhang X, Bao S, Gong J, Jiang Z, et al: ACT001 modulates the
NF-κB/MnSOD/ROS axis by targeting IKKβ to inhibit glioblastoma cell
growth. J Mol Med (Berl). 98:263–277. 2020. View Article : Google Scholar
|
|
97
|
Kumar S and Clair DS: Radioresistance in
prostate cancer: Focus on the interplay between NF-κB and SOD.
Antioxidants (Basel). 10:19252021. View Article : Google Scholar
|
|
98
|
Soh R, Hardy A and Zur Nieden NI: The FOXO
signaling axis displays conjoined functions in redox homeostasis
and stemness. Free Radic Biol Med. 169:224–237. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ponugoti B, Dong G and Graves DT: Role of
forkhead transcription factors in diabetes-induced oxidative
stress. Exp Diabetes Res. 2012:9397512012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang JY, Xia W and Hu MC: Ionizing
radiation activates expression of FOXO3a, Fas ligand, and Bim, and
induces cell apoptosis. Int J Oncol. 29:643–648. 2006.PubMed/NCBI
|
|
101
|
Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong
SY and Yang CW: Klotho enhances FoxO3-mediated manganese superoxide
dismutase expression by negatively regulating PI3K/AKT pathway
during tacrolimus-induced oxidative stress. Cell Death Dis.
8:e29722017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tseng AH, Wu LH, Shieh SS and Wang DL:
SIRT3 interactions with FOXO3 acetylation, phosphorylation and
ubiquitinylation mediate endothelial cell responses to hypoxia.
Biochem J. 464:157–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang Y, Chen S and Li H: Hydrogen peroxide
stress stimulates phosphorylation of FoxO1 in rat aortic
endothelial cells. Acta Pharmacol Sin. 31:160–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Guan L, Zhang L, Gong Z, Hou X, Xu Y, Feng
X, Wang H and You H: FoxO3 inactivation promotes human
cholangiocarcinoma tumorigenesis and chemoresistance through
Keap1-Nrf2 signaling. Hepatology. 63:1914–1927. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Araujo J, Breuer P, Dieringer S, Krauss S,
Dorn S, Zimmermann K, Pfeifer A, Klockgether T, Wuellner U and
Evert BO: FOXO4-dependent upregulation of superoxide dismutase-2 in
response to oxidative stress is impaired in spinocerebellar ataxia
type 3. Hum Mol Genet. 20:2928–2941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rached MT, Kode A, Xu L, Yoshikawa Y, Paik
JH, DePinho RA and Kousteni S: FoxO1 is a positive regulator of
bone formation by favoring protein synthesis and resistance to
oxidative stress in osteoblasts. Cell Metab. 11:147–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ye P, Mimura J, Okada T, Sato H, Liu T,
Maruyama A, Ohyama C and Itoh K: Nrf2- and ATF4-dependent
upregulation of xCT modulates the sensitivity of T24 bladder
carcinoma cells to proteasome inhibition. Mol Cell Biol.
34:3421–3434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang Z: ErbB receptors and cancer. Methods
Mol Biol. 1652:3–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Valerie K, Yacoub A, Hagan MP, Curiel DT,
Fisher PB, Grant S and Dent P: Radiation-induced cell signaling:
Inside-out and outside-in. Mol Cancer Ther. 6:789–801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar
|
|
112
|
Rezatabar S, Karimian A, Rameshknia V,
Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi
M, Monirialamdari M and Yousefi B: RAS/MAPK signaling functions in
oxidative stress, DNA damage response and cancer progression. J
Cell Physiol. Feb 27–2019.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kim MJ, Choi WG, Ahn KJ, Chae IG, Yu R and
Back SH: Reduced EGFR level in eIF2α PhosphorylationDeficient
hepatocytes is responsible for susceptibility to oxidative stress.
Mol Cells. 43:264–275. 2020.PubMed/NCBI
|
|
114
|
Zhang W, Yang H, Zhu L, Luo Y, Nie L and
Li G: Role of EGFR/ErbB2 and PI3K/AKT/e-NOS in Lycium barbarum
polysaccharides ameliorating endothelial dysfunction induced by
oxidative stress. Am J Chin Med. 47:1523–1539. 2019. View Article : Google Scholar
|
|
115
|
Boeckx C, Van den Bossche J, De Pauw I,
Peeters M, Lardon F, Baay M and Wouters A: The hypoxic tumor
microenvironment and drug resistance against EGFR inhibitors:
Preclinical study in cetuximab-sensitive head and neck squamous
cell carcinoma cell lines. BMC Res Notes. 8:2032015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bierl C, Voetsch B, Jin RC, Handy DE and
Loscalzo J: Determinants of human plasma glutathione peroxidase
(GPx-3) expression. J Biol Chem. 279:26839–26845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tonelli C, Chio IIC and Tuveson DA:
Transcriptional regulation by Nrf2. Antioxid Redox Signal.
29:1727–1745. 2018. View Article : Google Scholar :
|
|
118
|
Kang HJ, Yi YW, Hong YB, Kim HJ, Jang YJ,
Seong YS and Bae I: HER2 confers drug resistance of human breast
cancer cells through activation of NRF2 by direct interaction. Sci
Rep. 4:72012014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sakurai A, Nishimoto M, Himeno S, Imura N,
Tsujimoto M, Kunimoto M and Hara S: Transcriptional regulation of
thioredoxin reductase 1 expression by cadmium in vascular
endothelial cells: Role of NF-E2-related factor-2. J Cell Physiol.
203:529–537. 2005. View Article : Google Scholar
|
|
120
|
Gordon LI, Burke MA, Singh AT, Prachand S,
Lieberman ED, Sun L, Naik TJ, Prasad SV and Ardehali H: Blockade of
the erbB2 receptor induces cardiomyocyte death through
mitochondrial and reactive oxygen species-dependent pathways. J
Biol Chem. 284:2080–2087. 2009. View Article : Google Scholar :
|
|
121
|
Allegra AG, Mannino F, Innao V, Musolino C
and Allegra A: Radioprotective agents and enhancers factors
Preventive and therapeutic strategies for oxidative induced
radiotherapy damages in hematological malignancies. Antioxidants
(Basel). 9:11162020. View Article : Google Scholar
|
|
122
|
Jasek-Gajda E, Jurkowska H, Jasińska M and
Lis GJ: Targeting the MAPK/ERK and PI3K/AKT signaling pathways
affects NRF2, Trx and GSH antioxidant systems in leukemia cells.
Antioxidants (Basel). 9:6332020. View Article : Google Scholar
|
|
123
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
50:77–97. 2018. View Article : Google Scholar :
|
|
124
|
Qin S, He X, Lin H, Schulte BA, Zhao M,
Tew KD and Wang GY: Nrf2 inhibition sensitizes breast cancer stem
cells to ionizing radiation via suppressing DNA repair. Free Radic
Biol Med. 169:238–247. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sun X, Wang Q, Wang Y, Du L, Xu C and Liu
Q: Brusatol enhances the radiosensitivity of A549 cells by
promoting ROS production and enhancing DNA damage. Int J Mol Sci.
17:E9972016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of Human lung Cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tsuchida K, Tsujita T, Hayashi M, Ojima A,
Keleku-Lukwete N, Katsuoka F, Otsuki A, Kikuchi H, Oshima Y, Suzuki
M and Yamamoto M: Halofuginone enhances the chemo-sensitivity of
cancer cells by suppressing NRF2 accumulation. Free Radic Biol Med.
103:236–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Park M, Kwon J, Shin HJ, Moon SM, Kim SB,
Shin US, Han YH and Kim Y: Butyrate enhances the efficacy of
radiotherapy via FOXO3A in colorectal cancer patient-derived
organoids. Int J Oncol. 57:1307–1318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu Z, Li Y, She G, Zheng X, Shao L, Wang
P, Pang M, Xie S and Sun Y: Resveratrol induces cervical cancer
HeLa cell apoptosis through the activation and nuclear
translocation promotion of FOXO3a. Pharmazie. 75:250–254.
2020.PubMed/NCBI
|
|
130
|
Wang H, Bouzakoura S, de Mey S, Jiang H,
Law K, Dufait I, Corbet C, Verovski V, Gevaert T, Feron O, et al:
Auranofin radiosensitizes tumor cells through targeting thioredoxin
reductase and resulting overproduction of reactive oxygen species.
Oncotarget. 8:35728–35742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xie Q, Zhou Y, Lan G, Yang L, Zheng W,
Liang Y and Chen T: Sensitization of cancer cells to radiation by
selenadiazole derivatives by regulation of ROS-mediated DNA damage
and ERK and AKT pathways. Biochem Biophys Res Commun. 449:88–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liang YW, Zheng J, Li X, Zheng W and Chen
T: Selenadiazole derivatives as potent thioredoxin reductase
inhibitors that enhance the radiosensitivity of cancer cells. Eur J
Med Chem. 84:335–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Jia JJ, Geng WS, Wang ZQ, Chen L and Zeng
XS: The role of thioredoxin system in cancer: Strategy for cancer
therapy. Cancer Chemother Pharmacol. 84:453–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kimple RJ, Vaseva AV, Cox AD, Baerman KM,
Calvo BF, Tepper JE, Shields JM and Sartor CI: Radiosensitization
of epidermal growth factor receptor/HER2-positive pancreatic cancer
is mediated by inhibition of Akt independent of ras mutational
status. Clin Cancer Res. 16:912–923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang S, Fu Y, Wang D and Wang J: Icotinib
enhances lung cancer cell radiosensitivity in vitro and in vivo by
inhibiting MAPK/ERK and AKT activation. Clin Exp Pharmacol Physiol.
May 16–2018.Epub ahead of print. View Article : Google Scholar
|
|
136
|
Yi H, Yan X, Luo Q, Yuan L, Li B, Pan W,
Zhang L, Chen H, Wang J, Zhang Y, et al: A novel small molecule
inhibitor of MDM2-p53 (APG-115) enhances radiosensitivity of
gastric adenocarcinoma. J Exp Clin Cancer Res. 37:972018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang J, Wang H and Qian H: Biological
effects of radiation on cancer cells. Mil Med Res.
5:202018.PubMed/NCBI
|
|
138
|
Desouky O, Ding N and Zhou G: Targeted and
non-targeted effects of ionizing radiation. J Radiation Res App
Sci. 8:247–254. 2015.
|
|
139
|
De Ruysscher D, Niedermann G, Burnet NG,
Siva S, Lee AWM and Hegi-Johnson F: Radiotherapy toxicity. Nat Rev
Dis Primers. 5:132019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Mukherjee D, Coates PJ, Lorimore SA and
Wright EG: Responses to ionizing radiation mediated by inflammatory
mechanisms. J Pathol. 232:289–299. 2014. View Article : Google Scholar
|
|
141
|
LeBaron TW, Kura B, Kalocayova B,
Tribulova N and Slezak J: A new approach for the prevention and
treatment of cardiovascular disorders. Molecular Hydrogen
Significantly Reduces the Effects of Oxidative Stress. Molecules.
24:20762019. View Article : Google Scholar :
|
|
142
|
Ebrahimi S, Soltani A and Hashemy SI:
Oxidative stress in cervical cancer pathogenesis and resistance to
therapy. J Cell Biochem. Nov 13–2018.Epub ahead of print.
PubMed/NCBI
|
|
143
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li P, Zhao QL, Wu LH, Jawaid P, Jiao YF,
Kadowaki M and Kondo T: Isofraxidin, a potent reactive oxygen
species (ROS) scavenger, protects human leukemia cells from
radiation-induced apoptosis via ROS/mitochondria pathway in
p53-independent manner. Apoptosis. 19:1043–1053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Qin H, Zhang H, Zhang X, Zhang S, Zhu S
and Wang H: Resveratrol attenuates radiation enteritis through the
SIRT1/FOXO3a and PI3K/AKT signaling pathways. Biochem Biophys Res
Commun. 554:199–205. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Nuszkiewicz J, Woźniak A and
Szewczyk-Golec K: Ionizing radiation as a source of oxidative
stress-the protective role of melatonin and vitamin D. Int J Mol
Sci. 21:58042020. View Article : Google Scholar
|
|
147
|
Farhood B, Goradel NH, Mortezaee K,
Khanlarkhani N, Salehi E, Nashtaei MS, Mirtavoos-Mahyari H,
Motevaseli E, Shabeeb D, Musa AE and Najafi M: Melatonin as an
adjuvant in radiotherapy for radioprotection and
radiosensitization. Clin Transl Oncol. 21:268–279. 2019. View Article : Google Scholar
|
|
148
|
Batinic-Haberle I, Tovmasyan A and
Spasojevic I: Mn Porphyrin-based redox-active drugs: Differential
effects as cancer therapeutics and protectors of normal tissue
against oxidative injury. Antioxid Redox Signal. 29:1691–1724.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Batinic-Haberle I, Tovmasyan A, Huang Z,
Duan W, Du L, Siamakpour-Reihani S, Cao Z, Sheng H, Spasojevic I
and Alvarez Secord A: H2O2-Driven Anticancer
Activity of Mn Porphyrins and the underlying molecular pathways.
Oxid Med Cell Longev. 2021:66537902021. View Article : Google Scholar
|