Introduction
Aminoglycosides are a group of antibiotics that
includes streptomycin, kanamycin, tobramycin, gentamicin and
neomycin. They are used to treat serious gram-negative bacterial
infections (1). Gentamicin is a
member of the aminoglycoside antibiotics and serves an important
role in the treatment of gram-negative organisms. However, the
clinical utility and dosage of gentamicin are limited by its
well-known side effects, nephrotoxicity, neuropathy and
ototoxicity. The ototoxicity of gentamicin, which is cumulative,
bilateral and irreversible in the inner ear fluid (2). Patients with gentamicin-induced
ototoxicity might suffer from imbalance, dizziness, vertigo,
tinnitus, or hearing loss. After parenteral injection, gentamicin
is transported into cochlear hair cells via endocytosis or by
several aminoglycoside-permeant ion channels (3). Several molecular mechanisms explain
how gentamicin may induce ototoxicity, including increased reactive
oxygen species (ROS), activated c-Jun N-terminal kinase (JNK),
induced caspase signaling cascades and defective mitochondria
metabolism (4,5). Therefore, finding ways to relieve
cell damage can have therapeutic implications in gentamicin-induced
ototoxicity.
The ototoxic effects of gentamicin are mediated by
apoptosis, autophagy and the Akt survival pathway (1,6).
Among these pathways, autophagy serves an important role in
cellular homeostasis under physiological or chemical stress
(7). Autophagy appears both
pro-survival and pro-death mechanisms and the balance between
apoptosis and autophagy determines the fate of injured cells
(8,9). Aberrant autophagy may cause hair
cell loss and influence auditory function (10,11). However, excessive activation of
autophagy might also promote cell death via the apoptosis pathway
and pathological changes (12).
Autophagy induced under severe hypoxia and concomitant to metabolic
stress is often associated with cell death (13).
The mammalian target of rapamycin (mTOR) is a key
regulator in sensing cellular stress that regulates growth,
proliferation, survival and aging (14). The reduction of mTOR may be a
potential strategy to prevent age related hearing loss (15). AMP-activated protein kinase
(AMPK) is an energy-sensing kinase that could activate autophagy
process through the inhibition of mTOR signaling (16,17). Sesn2, a member of the oxidative
stress pathway, is involved in the regulation of mTOR. Sesn2
negatively regulates mTOR via AMPK and recombinant activating genes
(Rag) and thereby attenuates the accumulation of ROS (18). Previous research has shown that
Sesn2 serves a key role in gentamicin-induced hair cell death via
modulation of AMPK/mTOR signaling (19).
A main active compound of the traditional Chinese
herb plant Polygonum multiflorum Thunb is
2,3,4′,5-tetrahydroxy stilbene-2-O-β-D-glucoside (THSG) (20). Pharmacological studies have
demonstrated that THSG exhibits numerous biological functions in
the treatment of atherosclerosis, lipid metabolism, cerebral
ischemia, diabetic complications, hair growth problems and a number
of other conditions (21-24).
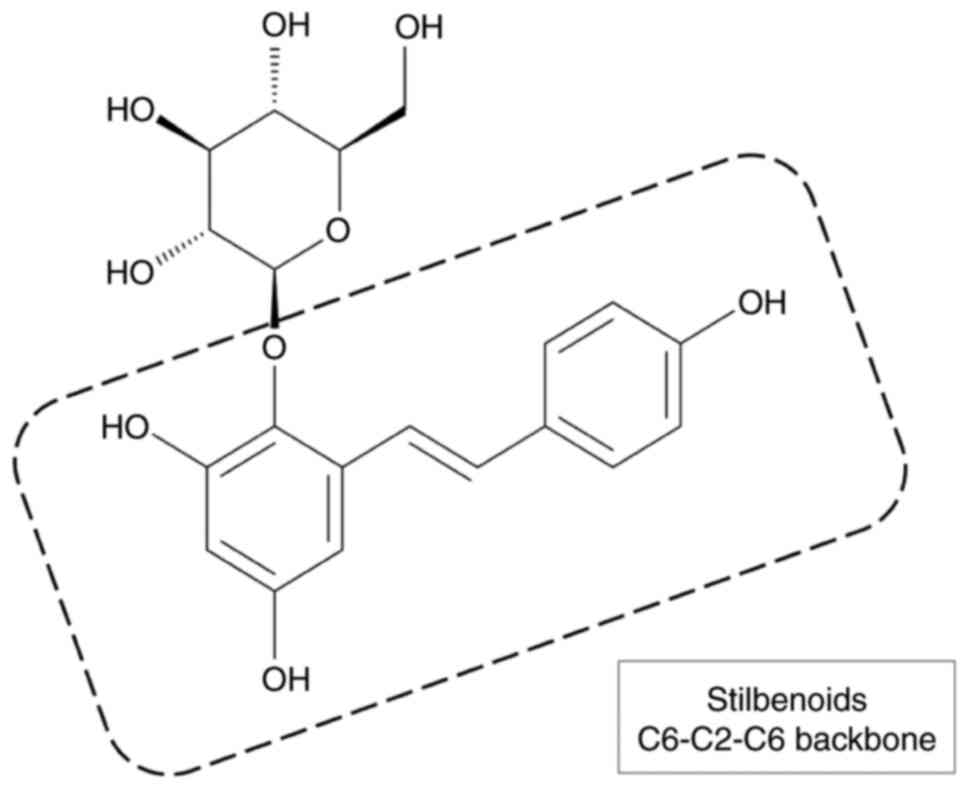
THSG is composed of stilbene and glucoside, which contain a number
of polar hydroxyl groups in chemical structure and it has been
demonstrated to possess strong antioxidant and free radical
scavenging activities (Fig. 1)
(20). THSG can also activate
the AMPK/Nrf2 signaling pathways and increase renal and neural cell
survival (23,24).
Previous studies have shown that THSG may be
effective in gentamicin-induced ototoxicity (6,25). However, very few studies have
attempted to evaluate the functional role of THSG and the molecular
mechanism underlying its effects on gentamicin ototoxicity. The
current study was conducted to determine whether THSG could
attenuate gentamicin-induced autophagy in effort to help develop
new strategies in the protection of patients who are vulnerable to
gentamicin ototoxicity.
Materials and methods
Chemicals and reagents
Gentamicin (cat. no. 2623184) was purchased from
Standard Chemical & Pharmaceutical Co. Ltd. THSG (cat. no.
HY-N0652) was obtained from MedChemExpress and dissolved in
dimethyl sulfoxide (DMSO) to create a 20 mM stock solution.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
cat. no. 298-93-1) was purchased from VWR International, LLC.
Lactate dehydrogenase (LDH) assay kit (cat. no. ENZ-KIT157) and
goat anti-rabbit IgG-HRP antibody (cat. no. ADI-SAB-300) was
purchased from Enzo Life Sciences. Antibodies against Beclin (cat.
no. 3738), autophagy related 5 (ATG5; cat. no. 12994), LC3 (cat.
no. 3868), phosphorylated (p)-mTOR (Ser2448; cat. no. 5536), mTOR
(cat. no. 2983) and β-actin (cat. no. 4967) were obtained from Cell
Signaling Technology. Inc. Antibodies against p-AMPK (Thr172; cat.
no. AP0116), AMPK (cat. no. A1229) and Sesn2 (cat. no. A14220) were
purchased from ABclonal Biotech Co., Ltd. Goat anti-mouse IgG-HRP
antibody (cat. no. NEF822001EA) was purchased from PerkinElmer,
Inc. Bafilomycin A1 (cat. no. B1793), chloroquine (cat. no. C6628)
and monodansylcadaverine (MDC; cat. no. 240141) was purchased from
MilliporeSigma.
Cell culture and drug treatment
The mouse cochlear cell line UB/OC-2 was purchased
from Ximbo. Cells were cultured in minimal essential medium
(MEM)/GlutaMAX (cat. no. 41090036; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; cat. no.
SH30066; Hyclone; Cytiva) and 50 U/ml of IFN-γ (cat. no. 485-MI;
R&D Systems, Inc.) in a humidified incubator at 33°C with 5%
CO2 (26).
Cell viability assay
The MTT method was used to measure cell viability.
The cells were seeded into 24-well plates at a density of
4×104 cells/well and treated with the various
concentrations of gentamicin for 24 h. The cells were then
incubated in culture medium containing 0.2 mg/ml MTT solution for 4
h at 33°C. The formazan crystals were dissolved in DMSO and the
absorbance detected at a wavelength of 570 nm using a microplate
reader (Infinite 200 PRO Series Multimode Reader; Tecan Group,
Ltd.). Cell viability of the control group was considered to be
100% (27).
Lactate dehydrogenase (LDH) release
assay
The LDH method was used to measure cellular
cytotoxicity. The cells were seeded into 96-well plates at a
density of 8×103 cells/well. The cells were pretreated
with THSG (5, 10 and 20 µM) or an autophagy inhibitor (10 nM
bafilomycin A1 or 20 µM chloroquine) for 6 h and then
cotreated with 750 µM gentamicin for 24 h at 33°C. LDH
release was detected using a LDH Cytotoxicity Assay kit according
to the manufacturer's instructions. The supernatants were then
transferred to another 96-well plate and the absorbance measured at
a wavelength of 490 nm using a microplate reader.
Cell morphology
The cells were seeded into 6-well plates at a
density of 4×105 cells/well and treated with 750
µM gentamicin for 6, 12 and 24 h. Cellular morphological
changes were then observed using a light microscope at ×400
magnification (Olympus BX41 microscope; Olympus Corporation) and
then evaluated in 10 randomly selected images per group.
Western blot analysis
Total protein was extracted from the cells and
homogenized using protein extraction reagent (cat. no. 78501;
Thermo Fisher Scientific, Inc.) containing protease (cat. no.
539134) and phosphates inhibitor (cat. no. 524629; MilliporeSigma).
Protein concentration was quantified by BCA reagent (cat. no.
97065; VWR International, LLC). Equal amounts of protein (20
µg/sample) were subjected to 10 or 15% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and the separated
proteins transferred onto polyvinylidene difluoride membranes.
After being blocked with 3% (w/v) bovine serum albumin (BSA;
cat.no. A9418; MilliporeSigma) in Tris-buffered saline (TBS; cat.
no. 75801; VWR International, LLC) for 1 h at room temperature, the
membranes were incubated overnight at 4°C with primary antibodies
diluted 1:1,000. After six washes in TBS containing 0.1% Tween
(TBST), the membranes were incubated for 1 h at room temperature
with the appropriate secondary antibodies diluted 1:5,000. After
six washes in TBST, the target proteins were detected using
enhanced chemiluminescence (Bio-Rad Laboratories, Inc.) and imaged
using a KETA C Chemi Imaging System (Wealtec Corporation) (28).
Transmission electron microscope
(TEM)
The cells were fixed with 2.5% glutaraldehyde in 0.1
M cacodylate buffer overnight at 4°C and then postfixed with 1%
osmium tetroxide in 0.1 M cacodylate buffer at room temperature for
1 h. The cells were stained with 2% uranyl acetate at room
temperature for 30 min, followed by gradient dehydration with
ethanol-acetone at room temperature for 15 min each and finally
embedded in Spurr's resin at 60°C for 12 h. Serial ultrathin
sections (~80 nm) were made with a Ultracut-R ultramicrotome (Leica
Microsystems GmbH) and observed with a Hitachi H-7500 transmission
electron microscope (Hitachi, Ltd.) at an accelerating voltage of
80 kV (29). Images were
captured with AMT XR-16 digital camera system in combination with
AMT Capture Engine, v602.600.51 software (Advanced Microscopy
Techniques Corporation).
Monodansylcadaverine (MDC) staining
The cells were washed twice with PBS and incubated
in 50 µM MDC dye in culture medium for 20 min at 33°C. After
washing twice with PBS, the MDC fluorescence was measured at 335 nm
excitation and 420 nm emission. Images were captured using an
Olympus BX41 fluorescent microscope at ×400 magnification (Olympus
Corporation) (27).
Reverse-transcription-quantitative (RT-q)
PCR
Total RNA was isolated using a RNeasy Mini kit (cat.
no. 74101) and QIAshredder (cat. no. 79654) from Qiagen GmbH
according to the manufacturer's recommended protocol. RT was
performed according to the protocol supplied with the SuperScript
III Reverse Transcriptase kit (cat. no. 18080; Invitrogen; Thermo
Fisher Scientific, Inc.) for qPCR. Gene expression was performed
using a SYBR Green PCR kit (cat. no. 208052) and measured with a
StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The RT conditions were: 50 min at 50°C for and
20 sec at 70°C, followed by preservation at 4°C. The primers used
for Sesn2 were sense 5′-TAG CCT GCA GCC TCA CCT AT-3′ and
antisense 5′-TAT CTG ATG CCA AAG ACG CA-3′; the primers used for
GAPDH were sense 5′-GCC AAA AGG GTC ATC ATC TC-3′ and
antisense 5′-CAC ACC CAT CAC AAA CAT GG-3′. The qPCR was run with
following thermocycling conditions: 10 min at 95°C, followed by 15
sec at 95°C and 1 min at 52°C for 40 cycles. The relative
expression level was calculated according to the 2−ΔΔCq
method normalized with the internal reference gene to GAPDH
(25,30,31).
Statistical analysis
Statistical analysis was performed using SPSS
Version 22.0 software (IBM Corporation) and data were presented as
mean ± the standard deviation (SD) from at least three independent
experiments. The data was used Shapiro-Wilk test to check
normality. Differences were determined using one-way analysis of
variance (ANOVA) followed by Tukey test or Kruskal-Wallis test
followed by Dunn's test for comparing multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Gentamicin decreases viability, increases
cytotoxicity and alters cellular morphology in UB/OC-2 cochlear
cells
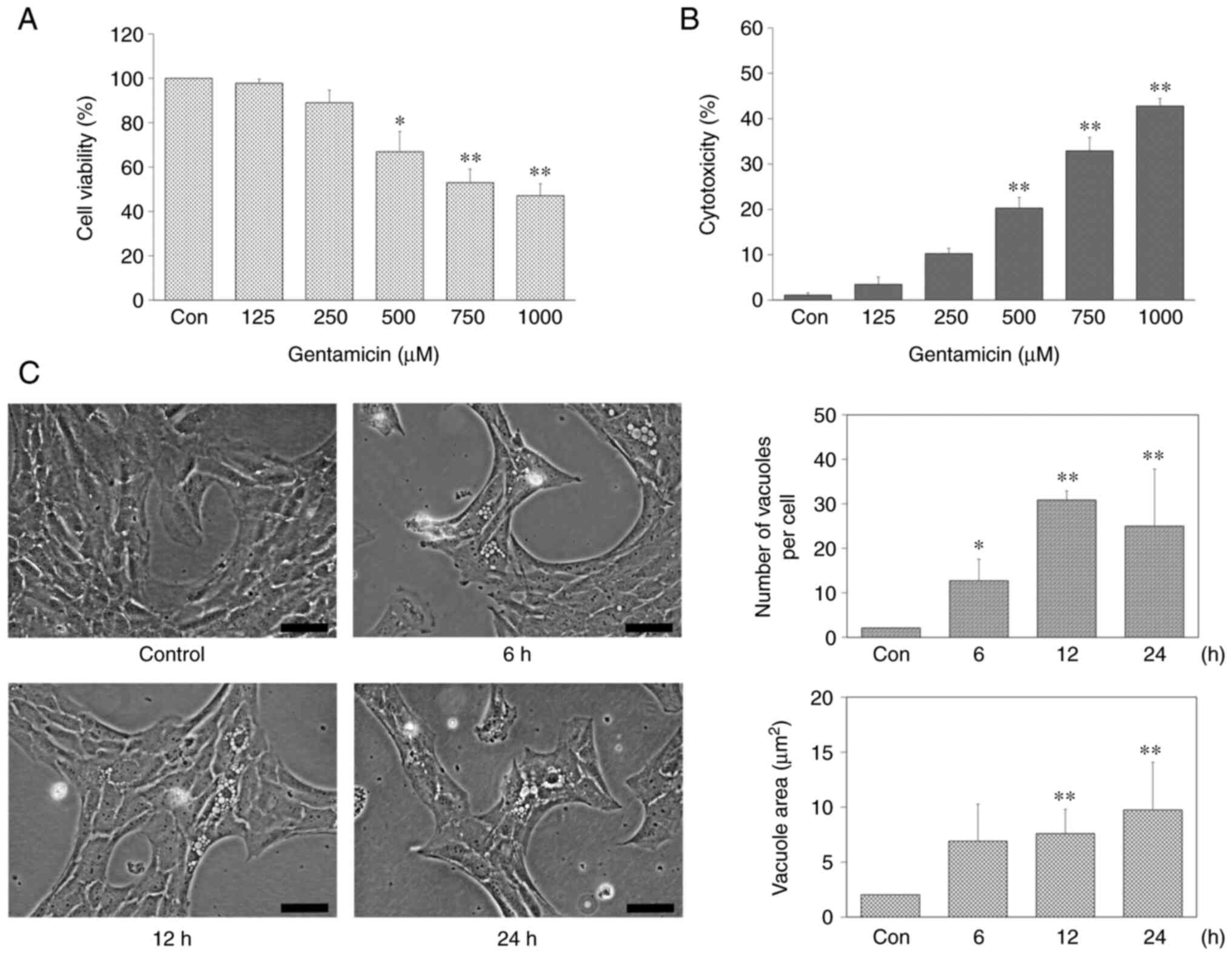
To evaluate the effect of gentamicin on the
viability and cytotoxicity of UB/OC-2 cochlear cells, the cells
were treated with different concentrations of gentamicin (125, 250,
500, 750 and 1,000 µM) for 24 h. The MTT assay results
showed that gentamicin significantly inhibited cell viability in a
concentration-dependent manner and viable cells related to
untreated control were observed 52.95 ± 6.04% at 750 µM
gentamicin (Fig. 2A). Therefore,
750 µM was chosen in the following experiments for UB/OC-2
cells injury but not 1,000 µM gentamicin because the
estimated IC50 was 800 µM.
Subsequently, cellular cytotoxicity was evaluated
using the LDH method. Gentamicin increased cytotoxicity of UB/OC-2
cells in a concentration-dependent manner (Fig. 2B). To investigate the effect of
gentamicin in UB/OC-2 cochlear cells, cell morphology was observed
after treatment with 750 µM gentamicin at various time
points (6, 12 and 24 h). As shown in Fig. 2C, the number and area of vacuoles
in UB/OC-2 cochlear cells increased after 6 h of 750 µM
gentamicin treatment. Overall, these results suggested gentamicin
treatment decreased UB/OC-2 cochlear cell viability, increased
cytotoxicity and altered the cell morphological
characteristics.
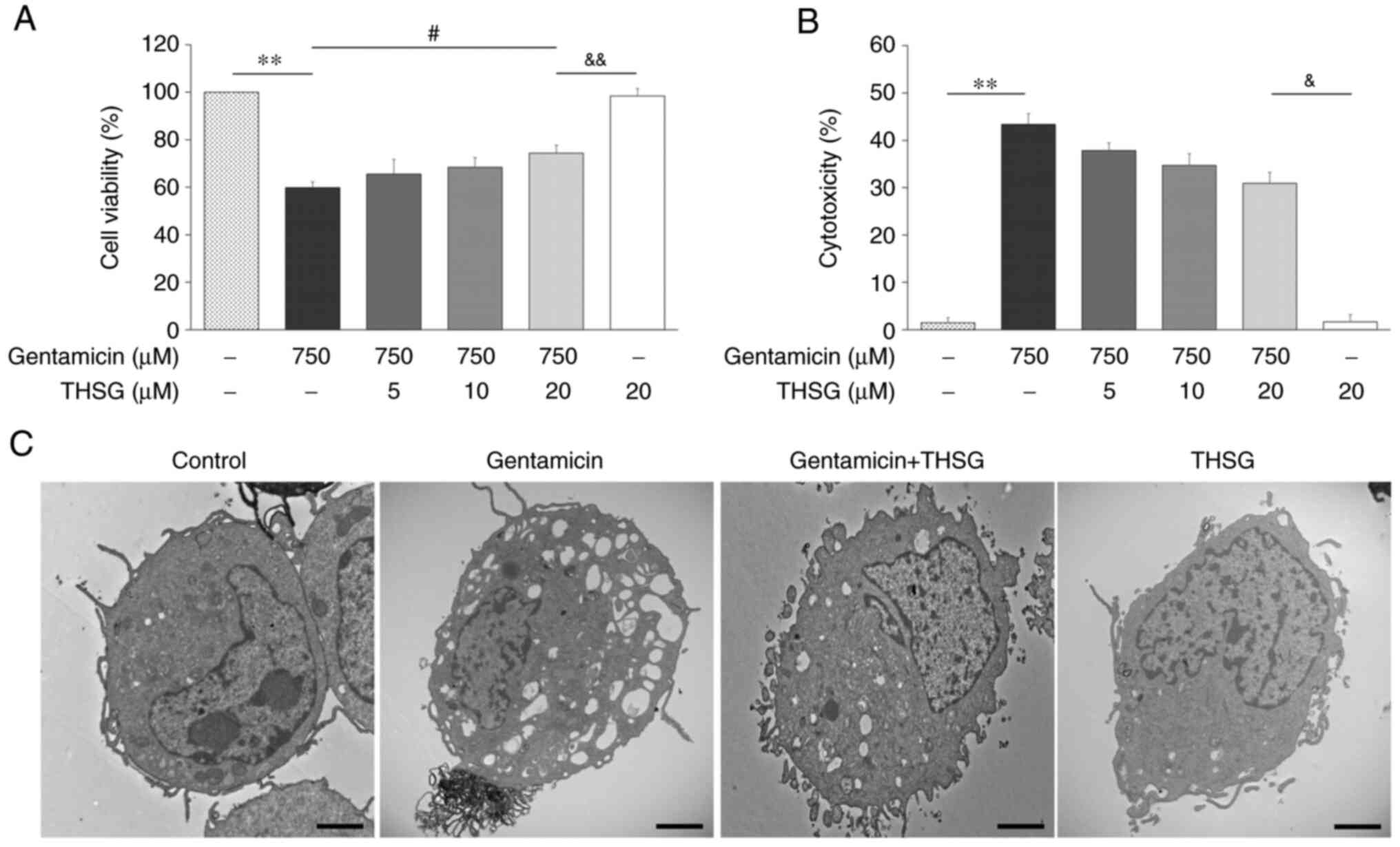
THSG increases the cell viability and
suppresses cytotoxicity under the treatment of gentamicin in
UB/OC-2 cochlear cells
Following a previous study (25), no cellular toxicity was
detectable in THSG-treated UB/OC-2 cochlear cells (≤20 µM).
To confirm that the otoprotective effect of THSG, cell viability
and cytotoxicity were measured after treatment of gentamicin and
THSG for 24 h. The results of MTT assay showed the cell viability
was significantly increased in THSG-treated groups compared to the
gentamicin-only treated group (Fig.
3A). Furthermore, THSG-treated groups were reduced cytotoxicity
compared with the gentamicin-only treated group by measuring LDH
release (Fig. 3B). To check
ultrastructural changes, UB/OC-2 cochlear cells in the control
group, gentamicin group (750 µM), THSG-treated group
(pretreated with 20 µM THSG for 6 h before 750 µM
gentamicin exposure) and THSG-only group (20 µM) were
analyzed by TEM 24 h after treatment. In the THSG-treated group
induced fewer vacuole formation compared with in the gentamicin
group, but no effect was observed on ultrastructural variable in
the THSG-only group (Fig. 3C).
These results indicated that THSG could effectively protect UB/OC-2
cochlear cells against gentamicin-induced ototoxicity.
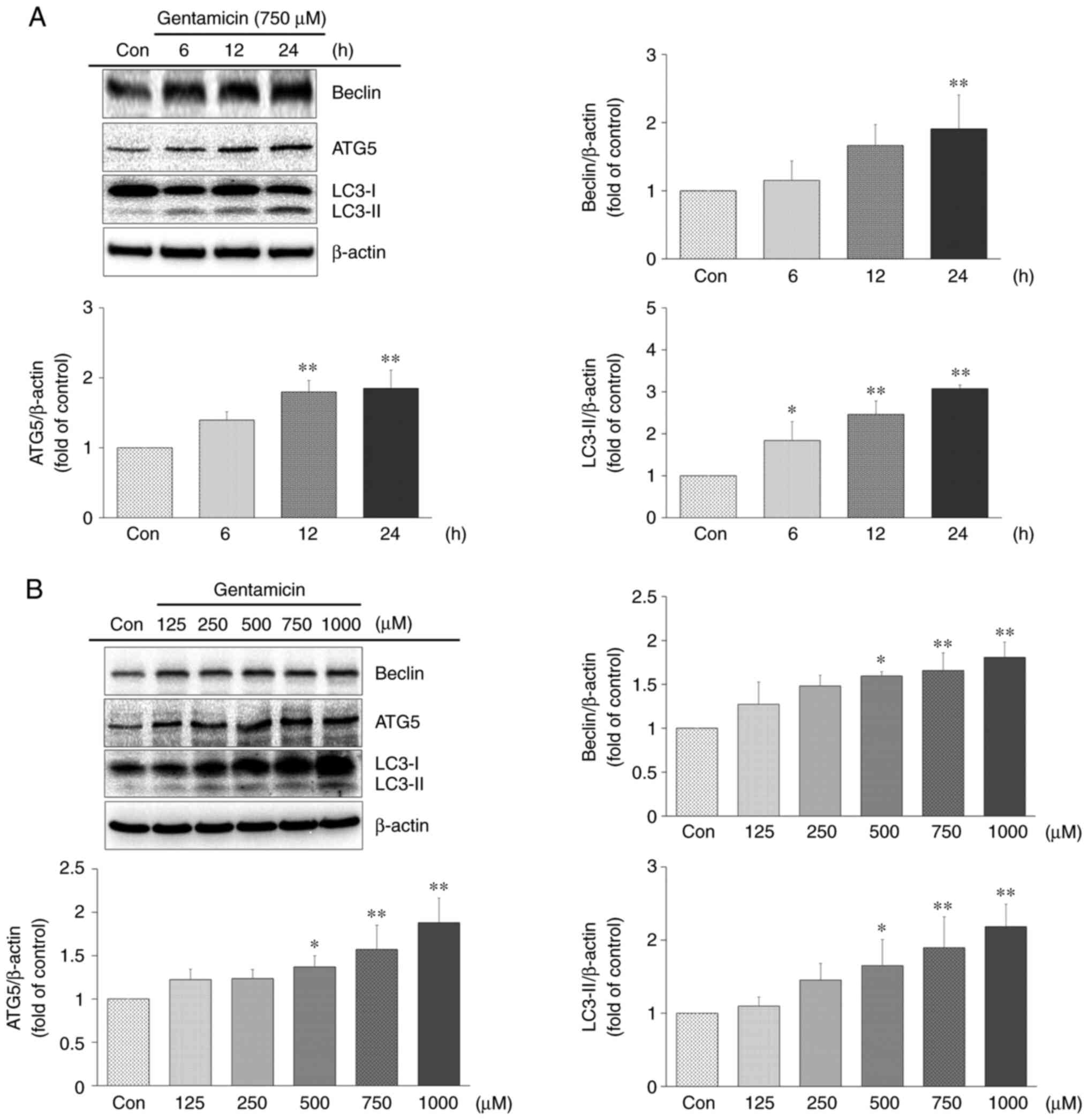
Gentamicin induces autophagy in UB/OC-2
cochlear cells
Based on morphological and ultrastructural
attribute, autophagy may be involved in gentamicin-induced
ototoxicity. Autophagy-related proteins Beclin, ATG5 and LC3-II
were estimated the autophagic levels by western blot analysis. As
shown in Fig. 4A, the protein
expression of Beclin, ATG5 and LC3-II significantly increased in
the gentamicin group compared with the control group. Protein
expression was analyzed after treatment of the cells with 125, 250,
500, 750 and 1,000 µM gentamicin for 24 h (Fig. 4B). The expression of Beclin, ATG5
and LC3-II each increased as gentamicin concentration was
increased. Accordingly, gentamicin might induce autophagy in a
time- and concentration-dependent manner in UB/OC-2 cochlear cells.
Next, to clarify whether the effects were actually due to autophagy
or just proteosomal protein degradation following gentamicin
toxicity, autophagic flux was analyzed in gentamicin-treated
UB/OC-2 cochlear cells by lysosomal degradation inhibitors
(bafilomycin A1 or chloroquine). Bafilomycin A1 is a V-ATPase
inhibitor to prevent lysosome acidification and block
autophagosome-lysosome fusion (32). Chloroquine is a lysosomotropic
agent that can inhibit autophagic degradation in the lysosomes by
altering the lysosomal pH (32).
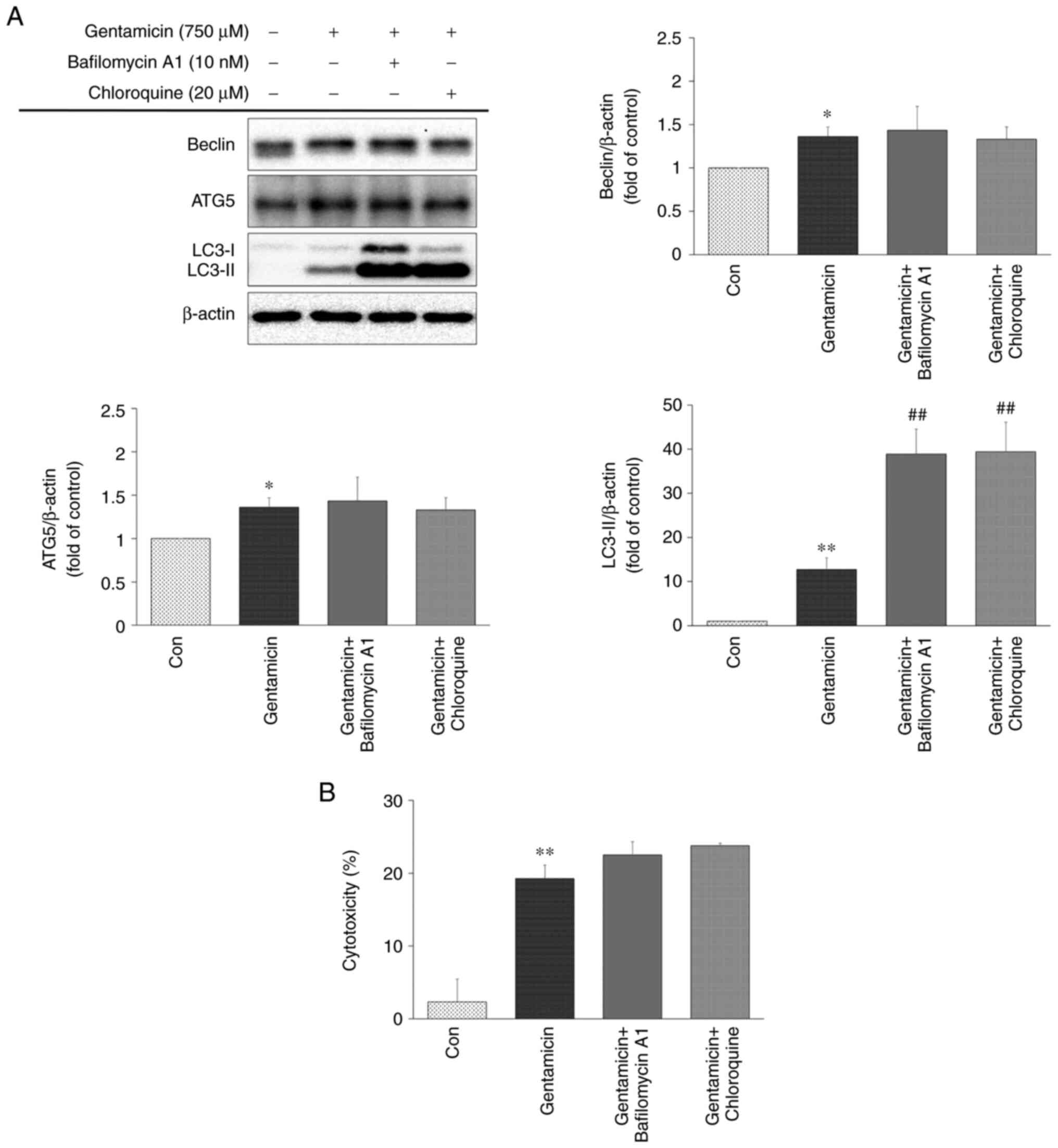
As shown in Fig. 5A, a marked
increase in LC3-II accumulation was found in gentamicin-treated
UB/OC-2 cochlear cells with 10 nM bafilomycin A1 or 20 µM
chloroquine; however, the protein levels of Beclin and ATG5 were
not significantly altered. Impaired autophagic degradation led to
increased LDH release (Fig. 5B).
Disruption of autophagic flux by bafilomycin A1 or chloroquine may
slightly enhance gentamicin-induced cytotoxicity in UB/OC-2
cochlear cells.
THSG decreases gentamicin-induced
autophagy in UB/OC-2 cochlear cells
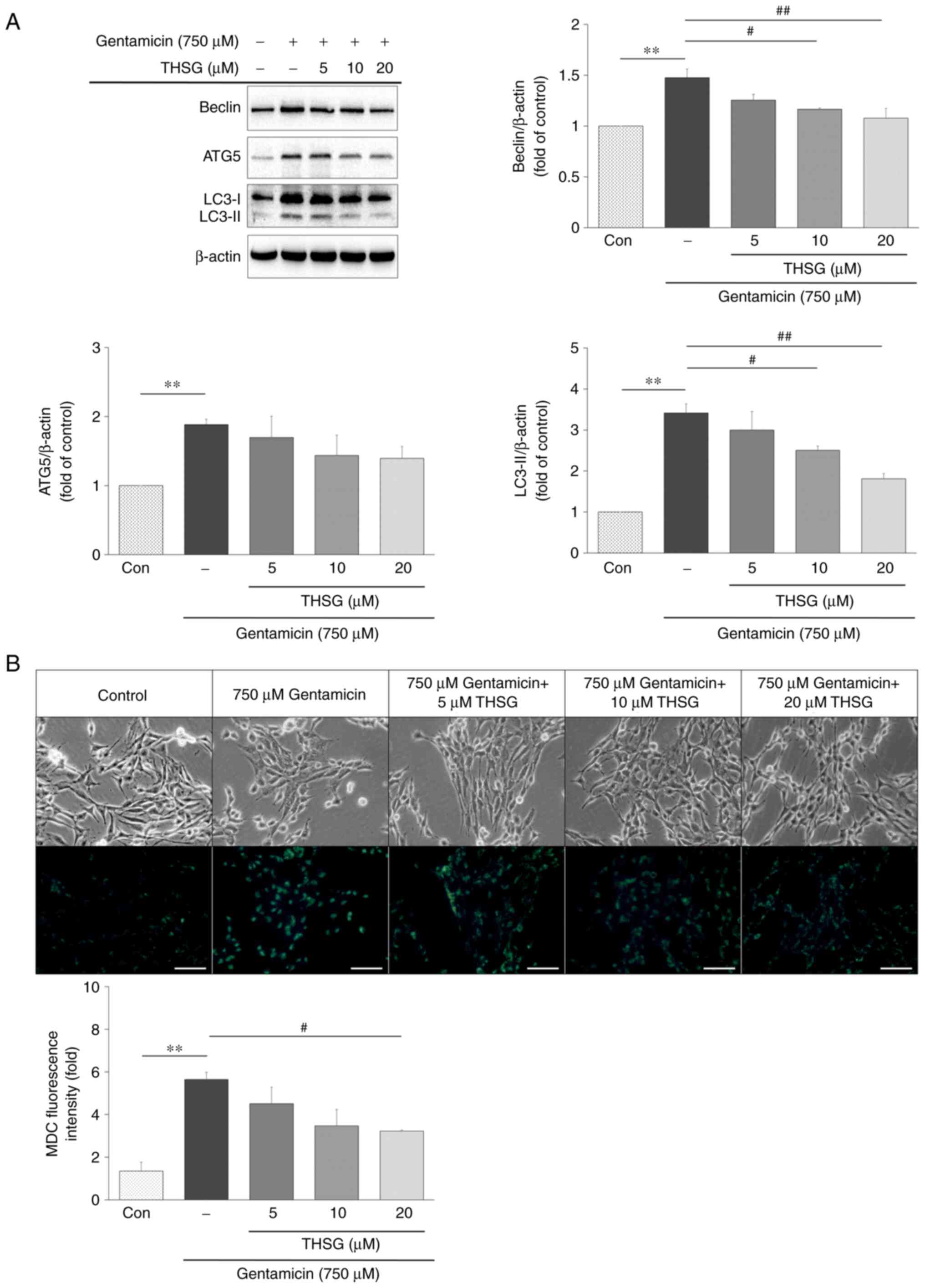
The effect of THSG on gentamicin-induced autophagy
was explored through the determination of autophagy related protein
expression. Protein expression of Beclin, ATG5 and LC3-II were
evaluated by western blot analysis in cells pretreated with 5, 10
and 20 µM THSG for 6 h and then cotreated with 750 µM
gentamicin for 24 h. Levels of all three proteins were decreased in
the THSG-treated groups compared to that in the gentamicin-only
treated group, especially in the 20 µM THSG-treated group
(Fig. 6A). To analyze the effect
of THSG on gentamicin-induced autophagy, the cells were stained
with MDC dye to detect autophagic vacuoles. In addition, the number
of MDC-labeled vacuoles was reduced in the THSG-treated groups
compared with the gentamicin-only treated group (Fig. 6B). Taken together, these results
showed that THSG decreased gentamicin-induced autophagy in UB/OC-2
cochlear cells.
THSG decreases gentamicin-induced
autophagy via modulation of Sesn2/AMPK/mTOR signaling
Sesn2 serves a major role in suppression of
oxidative stress and the regulation of AMPK/mTOR signaling, which
is crucial for autophagy induction (19). In the current study, UB/OC-2
cochlear cells were pretreated with 5, 10 and 20 µM THSG for
6 h and then cotreated with 750 µM gentamicin for 16 h. The
mRNA levels of Sesn2 in the cells decreased as the
concentration of THSG was increased (Fig. 7A). In addition, the protein level
of Sesn2 was measured after pretreated with 5, 10 and 20 µM
THSG for 6 h and then cotreated with 750 µM gentamicin for
24 h. The protein expression level of Sesn2 was diminished in the
THSG-treated groups compared to the gentamicin-only group (Fig. 7B). The results showed that
pretreatment with THSG produced a significant inhibition of this
gentamicin-induced effect on the mRNA and protein levels of
Sesn2.
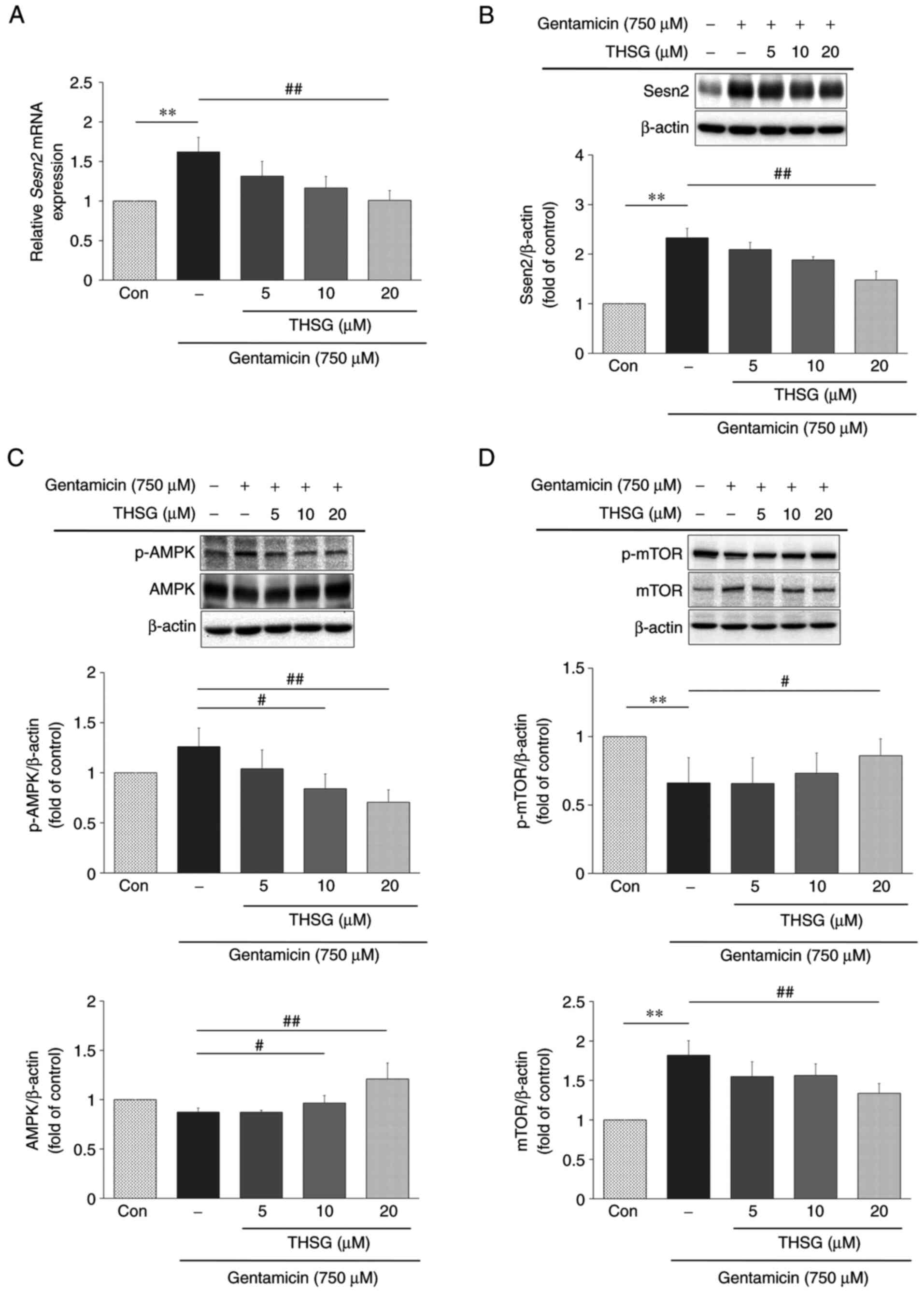 | Figure 7Effects of THSG on Sesn2, AMPK and
mTOR levels in UB/OC-2 cochlear cells following gentamicin
treatment. (A) UB/OC-2 cochlear cells were pretreated with 5, 10
and 20 µM THSG for 6 h and then cotreated with 750 µM
gentamicin for 16 h. The expression levels of Sesn2 mRNA
were evaluated using reverse-transcription-quantitative PCR.
GAPDH was used as an internal control. n=3 per group.
UB/OC-2 cochlear cells were pretreated with 5, 10 and 20 µM
THSG for 6 h and then cotreated with 750 µM gentamicin for
24 h. Protein expression of Sesn2 (B), p-AMPK and AMPK (C), p-mTOR
and mTOR (D) were evaluated by western blotting. β-actin was used
as a loading control. n=3 per group. Quantitative data are
expressed as mean ± SD. **P<0.01 compared with the
control group; #P<0.05, ##P<0.01 vs.
the gentamicin group. THSG,
2,3,4′,5-tetrahydroxystilbene-2-O-β-D-glucoside; AMPK,
AMP-activated protein kinase; p-, phosphorylated. |
The cells were pretreated with 5, 10 and 20
µM THSG for 6 h and then cotreated with 750 µM
gentamicin for 24 h. The following experiments were conducted to
show whether THSG regulates Sesn2 downstream effectors, AMPK and
downstream mTOR. Although AMPK levels in UB/OC-2 cochlear cells
increased as THSG concentrations were increased, levels of the
active form of the enzyme, p-AMPK, inversely decreased (Fig. 7C). On the other hand, mTOR levels
decreased relative to increased THSG concentrations, but again
levels of the active form, p-mTOR, inversely increased (Fig. 7D). Taken together, the results
suggested that THSG could decrease Sesn2 expression at both the
mRNA and protein level and thereby reduce autophagy in the UB/OC-2
cochlear cells in regulating AMPK/mTOR signaling response.
Discussion
Cochleototoxicity is generally observed with the use
of amikacin, kanamycin and neomycin, whereas the use of
streptomycin and gentamicin are associated with vestibulotoxicity
(1). Some patients are more
vulnerable to aminoglycoside ototoxicity, including the elderly,
those with renal insufficiency, diuretic users and those with gene
polymorphisms (33). Gentamicin
ototoxicity is cumulative and dose dependent (4,34). Consistent with previous reports,
data in the current study showed that UB/OC-2 cochlear cells
exhibit reduced viability and increased cytotoxicity as gentamicin
concentrations increase (Fig.
2).
The ototoxicity of aminoglycosides is attributed to
the production of excessive ROS (1). Stilbenoids, a group of phenolic
compounds containing C6-C2-C6 backbone, have antioxidant activities
by reacting with ROS, inducing antioxidant enzymes (such as
catalase, glutathione peroxidase, heme oxygenase and superoxide
dismutase) and activating Nrf2-antioxidant response element system
(Fig. 1) (35,36). Previous studies have reported
that stilbene-glycosides possess biological activities underlying
the antioxidant, anti-inflammatory and anti-apoptotic effects,
which is due to partial deglycosylation by the intestine and/or
liver (37,38). In a previous study, THSG appears
to be a good antioxidant with its free radical scavenging activity
being comparable to ascorbic acid (25). Furthermore, THSG is able to
promote several antioxidant pathways, such as Nrf2-Keap1 and
AMPK/Nrf2 (23,24). THSG also inhibits apoptosis in
gentamicin-induced cell damage (6). Data in the present study showed
that THSG significantly protected against gentamicin-induced
ototoxicity by the MTT assay and LDH method (Fig. 3A and B).
Autophagy serves a protective role in a number of
situations of cochlear hair cell stress or injury, such as
drug-induced ototoxicity, noise-induced hair cell injury and aging
(39-41). However, excessive activation of
autophagy may cause cell damage or death (8,9).
Meclofenamic acid may inhibit excessive autophagy and protect hair
HEI-OC1 cells from cisplatin-induced cell death (12). In the current study, gentamicin
induced vacuole formation in UB/OC-2 cochlear cells, which were
comparable to autophagosomes. The data showed that if the exposure
time or concentration of gentamicin was increased, the level of
autophagy proteins Beclin, ATG5 and LC3-II increased (Fig. 4). Disruption of autophagic flux
by bafilomycin A1 or chloroquine augmented the level of LC3-II and
slightly promoted gentamicin-induced cytotoxicity (Fig. 5). This suggested that autophagy
did not serve a major role in cell survival in the present study.
No further experiments on the synergy between THSG and the
autophagy inhibitors were performed and this was the limitation of
the present study. Furthermore, THSG reduced the expression of
gentamicin-induced autophagy related proteins and autophagic
vacuoles (Fig. 6). In view of
the above, the protection effects of THSG might be initiated in
front of autophagy process.
Autophagy is especially regulated by the AMPK/mTOR
pathway (17). Sesn2 is a
conserved antioxidant protein that reduces ROS (42). In the current study, when UB/OC-2
cochlear cells were cotreated with gentamicin and THSG, the
expression of Sesn2 decreased at both the protein and mRNA levels
and thus decreased autophagy. It is possible that THSG reduced ROS
in the gentamicin-treated cells and Sesn2 expression decreased
consequently. Unlike in the cochlear of mice where the expression
of Sesn2 is unchanged and ultimately downregulated in
gentamicin-treated explants (19), the data showed that Sesn2 levels
increased both on the mRNA and protein level in gentamicin-treated
UB/OC-2 cochlear cells (Fig. 7A and
B). However, there are still some mechanisms of interaction
between Sesn2 and gentamicin that should be further evaluated. When
cells are in a stress situation or when ROS increases, AMPK is
activated to its phosphorylated form, which then suppresses mTOR, a
suppresser of autophagy and autophagy consequently increases in the
cells. Sesn2 activates the AMPK/mTOR pathway and enhances autophagy
(19,42). When mTOR activity is inhibited by
rapamycin, hair cell survival increases following gentamicin
exposure (19). Gentamicin
increased the phosphorylation of AMPK, which then reduced
phosphorylation of mTOR. However, THSG was able to reverse the
effect of gentamicin by decreasing phosphorylation of AMPK and
increasing phosphorylation of mTOR, thereby reducing autophagy
(Fig. 7C and D). The
Sesn2/AMPK/mTOR pathway might have acted as a possible
stress-relieving mechanism and is involved autophagy induced by
gentamicin.
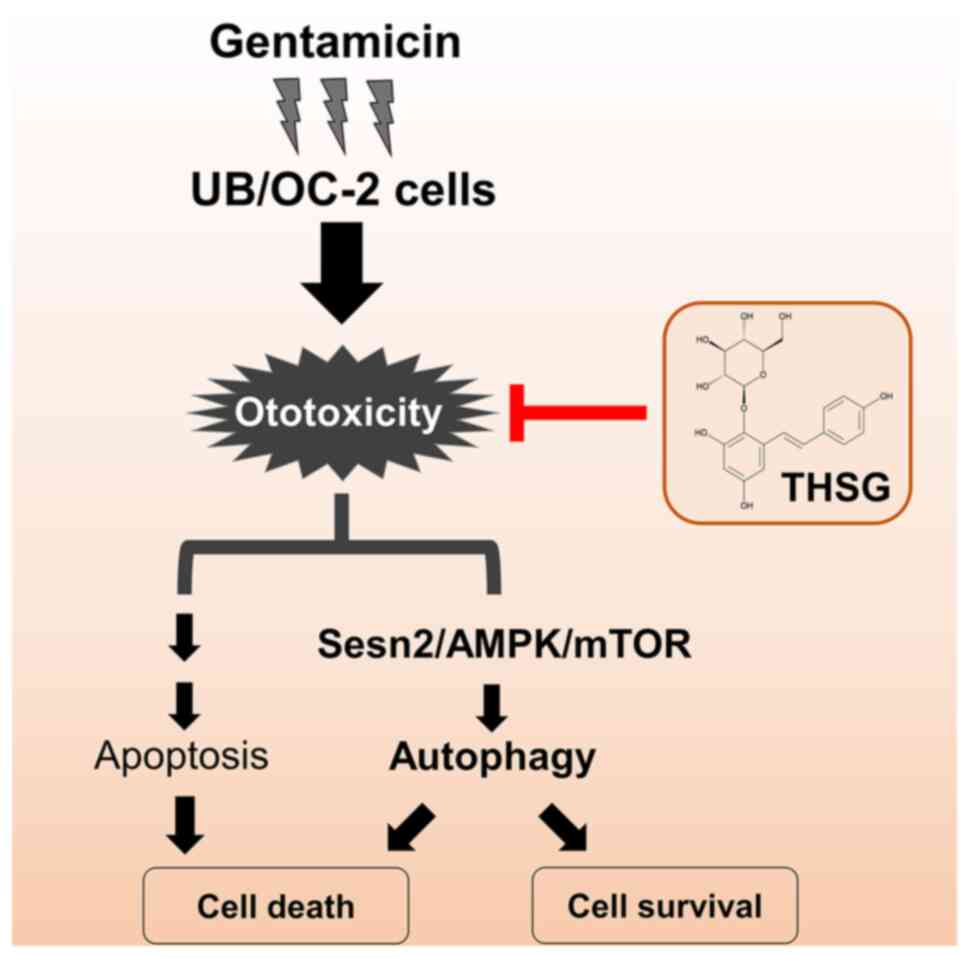
According to the results of the present study
combined with a previous study (6), gentamicin could induce cell
toxicity followed by the programmed cell death pathways, autophagy
and apoptosis. Evidence showed THSG decreased not only
gentamicin-induced apoptosis but also stress-inducible protein
Sesn2 and thereby reduced gentamicin-induced autophagy by
regulating AMPK/mTOR signaling. Under gentamicin treatment, THSG
might modulate the UB/OC-2 cochlear cells toward autophagic
survival but not autophagic cell death or apoptosis (Fig. 8).
Gentamicin can not only induce ototoxicity, but also
damage other organs. Further studies about protection effects of
THSG in other organs such as kidney or nerves will be conducted. In
addition, THSG has idiosyncratic hepatotoxicity that involves the
effect of THSG on cytochrome P450 (CYP) enzyme activity (43). There were only few studies on CYP
and cochlear cells. Maybe studies about the effect of THSG in
cochlear cell CYP activity should be inducted in the future.
In summary, the results of the present study showed
that THSG significantly suppressed gentamicin-induced ototoxicity
and thus modulated autophagy via the Sesn2/AMPK/mTOR signaling
pathway in UB/OC-2 cochlear cells. Therefore, THSG may serve as a
protective agent against gentamicin-induced ototoxicity. The
pharmacological effects of THSG in vivo in animal model
should be explored in future research.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHW and HYL were responsible for the study design
and implementation, data analysis and manuscript preparation. YHW,
HYL and JNL confirm the authenticity of all the raw data. JNL and
CCL interpreted the data and wrote the manuscript. YHW, HYL and JNL
were responsible for data collection and statistical analysis. GFT,
CFH, CJH and HPW served an important role in study design and
guidance and were responsible for the revision of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors wish to thank the Electron Microscopy
Laboratory of Tzu Chi University for helping with the TEM
evaluation.
Funding
This research was supported by grants from the Buddhist Tzu Chi
Medical Foundation (grant nos. TCMF-MP 108-01-01, TCMF-CP 111-09
and TCMF-A 108-03) and the Taichung Tzu Chi Hospital, Buddhist Tzu
Chi Medical Foundation (grant nos. TTCRD 110-03 and TTCRD
110-24).
References
|
1
|
Jiang M, Karasawa T and Steyger PS:
Aminoglycoside-induced cochleotoxicity: A review. Front Cell
Neurosci. 11:3082017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen KS, Bach A, Shoup A and Winick NJ:
Hearing loss and vestibular dysfunction among children with cancer
after receiving aminoglycosides. Pediatr Blood Cancer.
60:1772–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J,
Chai R, Ricci AJ and Cheng AG: Integrity and regeneration of
mechanotransduction machinery regulate aminoglycoside entry and
sensory cell death. PLoS One. 8:e547942013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ylikoski J, Xing-Qun L, Virkkala J and
Pirvola U: Blockade of c-Jun N-terminal kinase pathway attenuates
gentamicin-induced cochlear and vestibular hair cell death. Hear
Res. 166:33–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jensen-Smith HC, Hallworth R and Nichols
MG: Gentamicin rapidly inhibits mitochondrial metabolism in
high-frequency cochlear outer hair cells. PLoS One. 7:e384712012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wen YH, Lin JN, Wu RS, Yu SH, Hsu CJ,
Tseng GF and Wu HP: Otoprotective Effect of
2,3,4′,5-Tetrahydroxystilbene-2-O-β-d-glucoside on
gentamicin-induced apoptosis in mouse cochlear UB/OC-2 cells.
Molecules. 25:30702020. View Article : Google Scholar
|
|
7
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
8
|
Denton D and Kumar S: Autophagy-dependent
cell death. Cell Death Differ. 26:605–616. 2019. View Article : Google Scholar :
|
|
9
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou H, Qian X, Xu N, Zhang S, Zhu G,
Zhang Y, Liu D, Cheng C, Zhu X, Liu Y, et al: Disruption of
Atg7-dependent autophagy causes electromotility disturbances, outer
hair cell loss, and deafness in mice. Cell Death Dis. 11:9132020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto C, Iwasaki S, Urata S, Morishita
H, Sakamaki Y, Fujioka M, Kondo K, Mizushima N and Yamasoba T:
Autophagy is essential for hearing in mice. Cell Death Dis.
8:e27802017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Song Y, He Z, Chen X, Wu X, Li X,
Bai X, Liu W, Li B, Wang S, et al: Meclofenamic acid reduces
reactive oxygen species accumulation and apoptosis, inhibits
excessive autophagy, and protects hair cell-like HEI-OC1 cells from
cisplatin-induced damage. Front Cell Neurosci. 12:1392018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazure NM and Pouyssegur J:
Hypoxia-induced autophagy: Cell death or cell survival? Curr Opin
Cell Biol. 22:177–180. 2010. View Article : Google Scholar
|
|
14
|
Antikainen H, Driscoll M, Haspel G and
Dobrowolski R: TOR-mediated regulation of metabolism in aging.
Aging Cell. 16:1219–1233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu X, Sun X, Zhang L, Jin Y, Chai R, Yang
L, Zhang A, Liu X, Bai X, Li J, et al: Tuberous sclerosis
complex-mediated mTORC1 overactivation promotes age-related hearing
loss. J Clin Invest. 128:4938–4955. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vilimanovich U and Jevremovic SA:
Dihydroquercetin: A novel potent flavonoid antioxidant as an
adjuvant for effective cancer treatment. EC Nutr. 14:660–674.
2019.
|
|
17
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rhee SG and Bae SH: The antioxidant
function of sestrins is mediated by promotion of autophagic
degradation of Keap1 and Nrf2 activation and by inhibition of
mTORC1. Free Radic Biol Med. 88:205–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ebnoether E, Ramseier A, Cortada M, Bodmer
D and Levano-Huaman S: Sesn2 gene ablation enhances susceptibility
to gentamicin-induced hair cell death via modulation of AMPK/mTOR
signaling. Cell Death Discov. 3:170242017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin L, Ni B, Lin H, Zhang M, Li X, Yin X,
Qu C and Ni J: Traditional usages, botany, phytochemistry,
pharmacology and toxicology of Polygonum multiflorum Thunb: A
review. J Ethnopharmacol. 159:158–183. 2015. View Article : Google Scholar
|
|
21
|
Ling S and Xu JW: Biological activities of
2,3,5,4′-Tetrahydrox ystilbene-2-O-β-D-Glucoside in antiaging and
antiaging-related disease treatments. Oxid Med Cell Longev.
2016:49732392016. View Article : Google Scholar
|
|
22
|
Xu S, Liu J, Shi J, Wang Z and Ji L:
2,3,4′,5-tetrahydroxystilbene-2-O-β-D-glucoside exacerbates
acetaminophen-induced hepatotoxicity by inducing hepatic expression
of CYP2E1, CYP3A4 and CYP1A2. Sci Rep. 7:165112017. View Article : Google Scholar
|
|
23
|
Park SY, Jin ML, Wang Z, Park G and Choi
YW: 2,3,4′,5-tetrahyd roxystilbene-2-O-β-d-glucoside exerts
anti-inflammatory effects on lipopolysaccharide-stimulated
microglia by inhibiting NF-κB and activating AMPK/Nrf2 pathways.
Food Chem Toxicol. 97:159–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin EY, Bayarsengee U, Wang CC, Chiang YH
and Cheng CW: The natural compound
2,3,5,4′-tetrahydroxystilbene-2-O-β-d glucoside protects against
adriamycin-induced nephropathy through activating the Nrf2-Keap1
antioxidant pathway. Environ Toxicol. 33:72–82. 2018. View Article : Google Scholar
|
|
25
|
Wu TY, Lin JN, Luo ZY, Hsu CJ, Wang JS and
Wu HP: 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG)
activates the Nrf2 antioxidant pathway and attenuates oxidative
stress-induced cell death in mouse cochlear UB/OC-2 cells.
Biomolecules. 10:4652020. View Article : Google Scholar
|
|
26
|
Rivolta MN, Grix N, Lawlor P, Ashmore JF,
Jagger DJ and Holley MC: Auditory hair cell precursors immortalized
from the mammalian inner ear. Proc Biol Sci. 265:1595–1603. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin HY, Lin JN, Ma JW, Yang NS, Ho CT, Kuo
SC and Way TD: Demethoxycurcumin induces autophagic and apoptotic
responses on breast cancer cells in photodynamic therapy. J Funct
Foods. 12:439–449. 2015. View Article : Google Scholar
|
|
28
|
Lin JN, Lin VC, Rau KM, Shieh PC, Kuo DH,
Shieh JC, Chen WJ, Tsai SC and Way TD: Resveratrol modulates tumor
cell proliferation and protein translation via SIRT1-dependent AMPK
activation. J Agric Food Chem. 58:1584–1592. 2010. View Article : Google Scholar
|
|
29
|
Graham L and Orenstein JM: Processing
tissue and cells for transmission electron microscopy in diagnostic
pathology and research. Nat Protoc. 2:2439–2450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trierweiler C, Hockenjos B, Zatloukal K,
Thimme R, Blum HE, Wagner EF and Hasselblatt P: The transcription
factor c-JUN/AP-1 promotes HBV-related liver tumorigenesis in mice.
Cell Death Differ. 23:576–582. 2016. View Article : Google Scholar :
|
|
31
|
Delbridge GJ and Khachigian LM:
FGF-1-induced platelet-derived growth factor-A chain gene
expression in endothelial cells involves transcriptional activation
by early growth response factor-1. Circ Res. 81:282–288. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY
and Hwang TI: Cisplatin induces protective autophagy through
activation of BECN1 in human bladder cancer cells. Drug Des Devel
Ther. 11:1517–1533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischel-Ghodsian N: Genetic factors in
aminoglycoside toxicity. Pharmacogenomics. 6:27–36. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsui JI, Gale JE and Warchol ME:
Critical signaling events during the aminoglycoside-induced death
of sensory hair cells in vitro. J Neurobiol. 61:250–266. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akinwumi BC, Bordun KM and Anderson HD:
Biological activities of stilbenoids. Int J Mol Sci. 19:7922018.
View Article : Google Scholar :
|
|
36
|
Treml J, Leláková V, Šmejkal K, Paulíčková
T, Labuda Š, Granica S, Havlík J, Jankovská D, Padrtová T and Hošek
J: Antioxidant activity of selected stilbenoid derivatives in a
cellular model system. biomolecules. Biomolecules. 9:4682019.
View Article : Google Scholar
|
|
37
|
Storniolo CE, Quifer-Rada P,
Lamuela-Raventos RM and Moreno JJ: Piceid presents
antiproliferative effects in intestinal epithelial Caco-2 cells,
effects unrelated to resveratrol release. Food Funct. 5:2137–2144.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Storniolo CE and Moreno JJ: Resveratrol
metabolites have an antiproliferative effect on intestinal
epithelial cancer cells. Food Chem. 134:1385–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang B and Xiao H: Rapamycin alleviates
cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun.
448:443–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Iriarte Rodríguez R, Pulido S,
Rodríguez-de la Rosa L, Magariños M and Varela-Nieto I:
Age-regulated function of autophagy in the mouse inner ear. Hear
Res. 330:39–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan H, Wang X, Hill K, Chen J, Lemasters
J, Yang SM and Sha SH: Autophagy attenuates noise-induced hearing
loss by reducing oxidative stress. Antioxid Redox Signal.
22:1308–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei JL, Fang M, Fu ZX, Zhang SR, Guo JB,
Wang R, Lv ZB and Xiong YF: Sestrin 2 suppresses cells
proliferation through AMPK/mTORC1 pathway activation in colorectal
cancer. Oncotarget. 8:49318–49328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li DK, Chen J, Ge ZZ and Sun ZX:
Hepatotoxicity in rats induced by aqueous extract of polygoni
multiflori radix, root of Polygonum multiflorum related to the
activity inhibition of CYP1A2 or CYP2E1. Evid Based Complement
Alternat Med. 2017:94567852017.
|