Ischemic stroke is a common cause of disability,
normally manifested as long-term neurological impairment, and even
death (1,2). The pathogenesis of ischemic stroke
is mainly caused by atherothrombosis at large cervical or
intracranial arteries or by occlusion of cardio-embolus (3), which results in insufficient oxygen
and glucose delivery to meet the requirement of cellular
respiration (4). To date, the
approved therapies of ischemic stroke are intravenous thrombolysis
or thrombectomy (5), which can
only be applied to a very small fraction of patients due to the
narrow time window and strict indication criteria (6). As such, there is an urgent need to
develop novel and alternative treatment strategies to treat
ischemic stroke.
Post-ischemic neuroinflammation is an important
pathological hallmark that affects both the development and
prognosis of ischemic stroke (7,8).
Once the inflammatory cascade is turned on, it aggravates neuron
dysfunction and induces breakdown of blood brain barrier, brain
edema and ultimately neuron death (9). Inflammasomes are intracellular
multiprotein complexes that drive the activation of inflammatory
responses. Among all types of inflammasomes, such as NLR family
pyrin domain containing 1 (NLRP1), NLRP3, NLR family CARD
domain-containing protein 4 (NLRC4), and absent in melanoma 2
(AIM2), NLRP3 is the most studied one, particularly in the central
neural system (10). It was
shown that NLRP3 inflammasome activation regulates inflammatory
response and accelerates neuron damage (11), and it acts as a key intermediate
of neuroinflammation during the progress of ischemic stroke
(12). Thus, inhibition of NLRP3
inflammasome activation may be applied as a novel treatment
strategy for ischemic stroke (13).
Alternatively, mitochondrion is an organelle that
plays roles in energy conversion and metabolism, and its
dysfunction is the crucial pathophysiological change in ischemic
stroke due to oxygen-glucose deprivation (14). It was found that a few studies
have confirmed that NLRC4 (15),
NLRP1 (16) and AIM2 (17) can promote cerebral ischemic
injury, but there is no evidence that mitochondria can affect the
pathological process of cerebral ischemia through them. Previous
studies revealed that mitochondrial dysfunction is a vital event
during the NLPR3 inflammasome activation (18-20). However, the role of NLRP3
inflammasome in sensing mitochondrial damage and how mitochondria
trigger NLRP3 activation in ischemic stroke is to be elucidated
(21). The present review
focused on the role of mitochondria in NLRP3 inflammasome
activation under ischemic stroke and described the currently used
drugs and potential treatment targets for ischemic stroke.
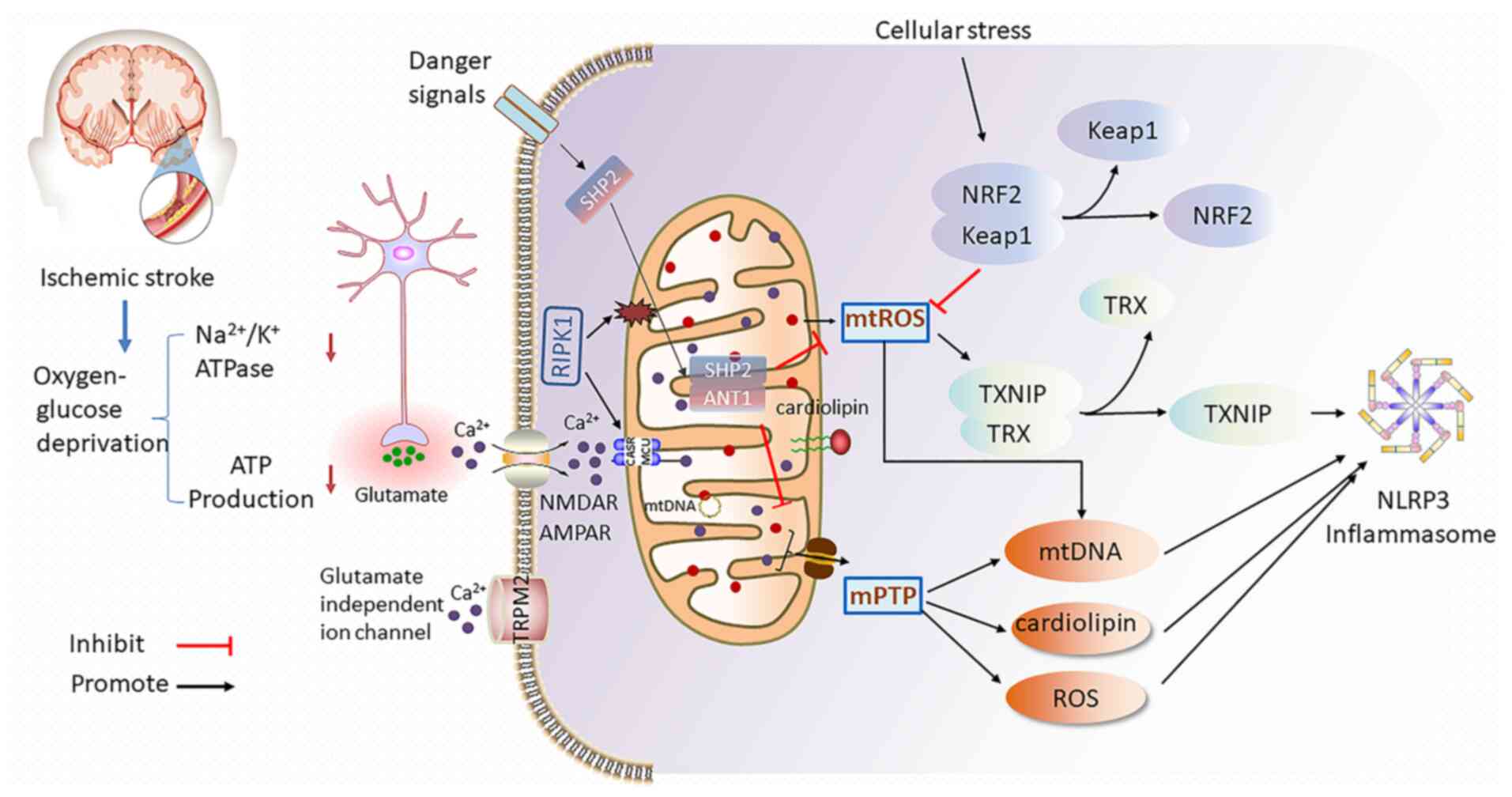
During ischemic stroke, ischemia and hypoxia reduce
ATP production and lead to dysfunction of the
Na+/K+ ATPase pump (35). Within min following the onset of
cerebral ischemia, ATP depletion deactivates the sodium-potassium
pump and then excessive glutamate is released into extracellular
fluid (36). Glutamate, as the
main excitatory neurotransmitter in the central nervous system, is
essential to multiple functions of neurons by binding to different
types of receptors including N-methyl-d-aspartate receptor and
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor
(37). Under ischemia-hypoxia
condition, the high level of extracellular glutamate induces
massive calcium influx, which further aggravates mitochondrial
calcium overload (38). Elevated
mitochondrial calcium launches a series of events ranging from mPTP
opening and dissipation of ΔΨm to excessive ROS production, leading
to neuroinflammation and eventually neuronal death (Fig. 1) (28). While Inhibition of the heat shock
75-kDa glucose-regulated protein was able to effectively ameliorate
mitochondrial calcium overload and alleviate the ischemic stroke
(39).
Besides energy production, mitochondria also
generate a small amount of ROS, which induces oxidative damage
(46). Increased ROS was
generated during cerebral ischemia, particularly during reperfusion
by disrupting mitochondrial electron transport (47,48). The broken mitochondria produce
large amounts of ROS, which in turn affect the function of adjacent
mitochondria. Since mitochondria are both the sources of ROS
production and the targets of ROS, oxidant-induced mitochondrial
dysfunction forms a 'vicious cycle' in patients with ischemic
stroke (49,50). Furthermore, the ROS release
results in oxidation and mutation of mtDNA, release of
mitochondrial proteins and impaired mitophagy as shown in rat model
of middle cerebral artery occlusion (MCAO). Moreover, the
accumulation of ROS promotes neuroinflammation and neuron apoptosis
after oxygen-glucose deprivation/reoxygenation (OGD/R) (51).
In addition to mitochondrial function, changes in
mitochondrial structure play an important role in the progress of
ischemic stroke. Mitochondria are highly dynamic organelles to
regulate their size, form and mtDNA integrity via continuous
fission, fusion and mitophagy (52). When neurons undergo ischemia,
mitochondrial fission and fusion are transitory to maintain its
structure integrity and function. However, under the circumstance
of large amount of ROS production (53) and mtDNA damage (54), mitochondria are divided
excessively, resulting in mitochondrial dysfunction (55). Thus, mitochondrial fission occurs
as an upstream and early event in brain cell death following
ischemia (56,57).
Furthermore, mutation mtDNA genes is correlated
mitochondrial dysfunction with genomic instability. mtDNA is
markedly more prone to mutations than nuclear DNA (58). Particularly, the frequency of
mtDNA mutations was found to be significantly higher in the brain
of patients with ischemic stroke, and numerous of these mutations
resulted in an alteration of amino acid therefore structure of
protein (34). The vulnerability
of mtDNA to mutation is due to its lacking of high-fidelity repair
processes, the high-copy-number of mtDNA within each cell, and the
close proximity to ROS-generating machinery (59). In a word, mutation of
gene-encoded subunits in mtDNA results in more ROS generation,
which turns mtDNA more susceptible to mutation.
Upon stroke attack, the interruption and reperfusion
of blood flow in the brain tissue trigger the infiltration of
inflammatory cells to induce neuronal death (60). Neuroinflammation is a primary
pathological event involved in the process of ischemic injury and
repair (61). In response to
injury in the brain, neuroinflammation occurs in various cells,
including neurons, microglia and astrocytes (62). In the acute phase, the damaged
neurons and resident immune cells secrete inflammatory mediators
and activate microglia, which are the first line of defense in the
nervous system (63) that
produce more inflammatory factors (62,64). Additionally, astrocytes that are
activated by ischemia mediate inflammatory response to aggravate
ischemic injury. However, they also limit the spread of
inflammation by inhibiting excitotoxicity and secreting
neurotrophic factors (65,66). Interestingly, NLRP3 inflammasome
was found to play a key role in driving neuroinflammation in these
cells during acute ischemic stroke, and early blockade of NLRP3
protects neurons from ischemia-reperfusion injury by mitigating
inflammation (67).
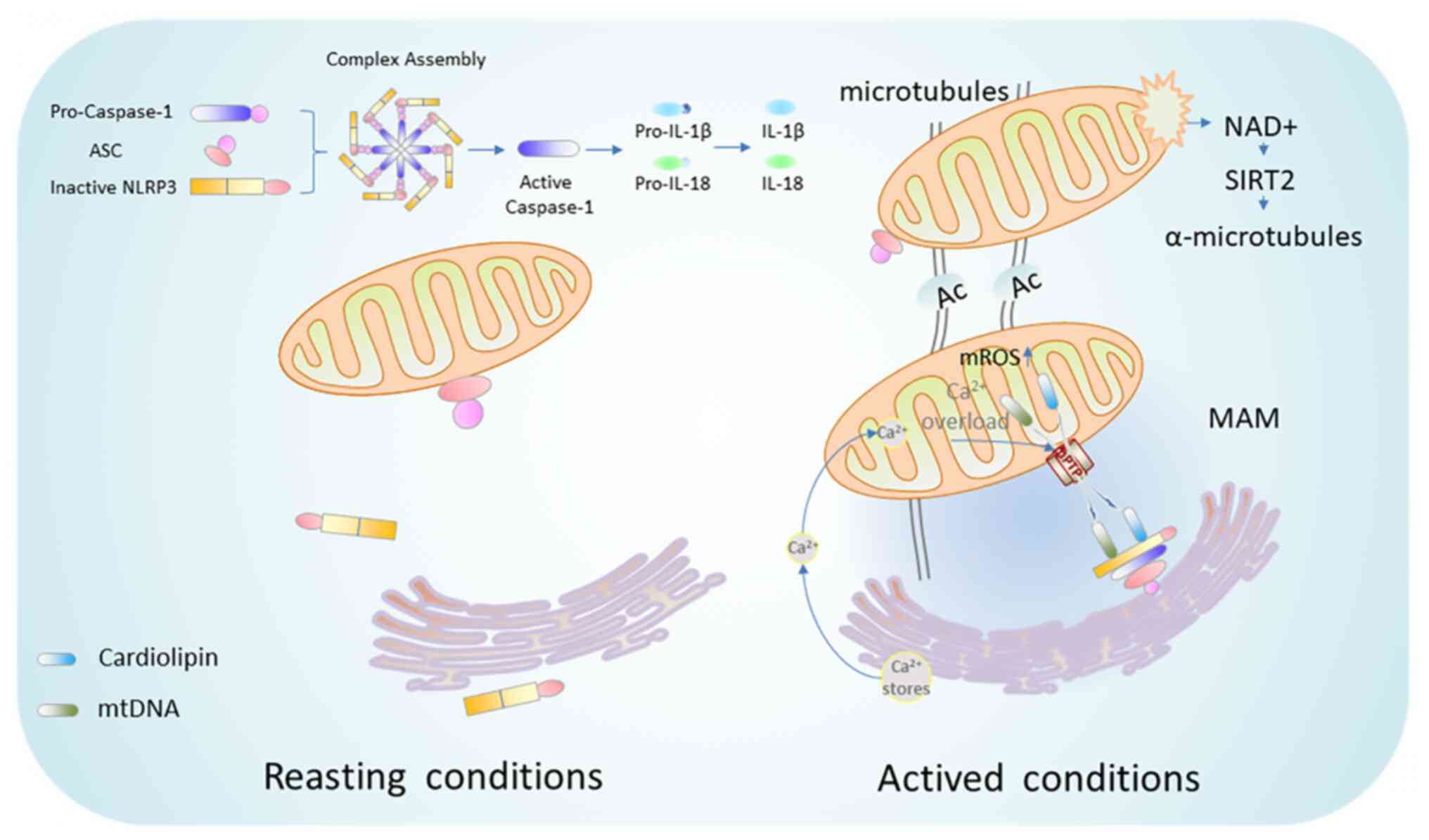
NLRP3 inflammasome is the most widely described
inflammatory complex, which is composed of NLRP3 as its receptor,
apoptosis-associated speck-like protein containing a CARD (ASC) as
its adaptor, and caspase-1 as its effector. Activation of NLRP3
inflammasome includes two steps, namely, priming and activation
(68). The priming step is the
recognition of pathogen-associated molecular pattern (PAMP) or
damage-associated molecular pattern (DAMP) via pattern recognition
receptors (PRRs). Transcription and post-translational modification
(PTM) of NLRP3 inflammasome promote the transcription of NLRP3 and
the precursor of caspase-1, interleukin (IL)-1β and IL-18 (69). The priming of NLRP3 inflammasome
not only provides material for NLRP3 inflammasome activation, but
also allows NLRP3 and ASC to form the inflammasome assembly
(70) or to protect them from
degradation (71) through
various PTM including ubiquitylation, deubiquitylation and
phosphorylation. Then, the activation step is required to initiate
the NLRP3 inflammasome assembly and subsequent activation.
Generally, NLRP3 is oligomerized via its NACHT domains once
activated (72). NLRP3
trimerization rather than dimerization is necessary for the
inflammasome activation (73).
Subsequently, activated NLRP3 interacts and recruits the adaptor
molecule ASC via PYD-PYD interaction (74). The polymerized ASC further
recruits the enzyme caspase-1 through CARD-CARD interactions to
initiate autocatalytic cleavage of caspase-1 (75). Notably, ASC oligomerization is a
key step in caspase-1 activation and caspase-1-dependent
pyrophosphorylation upon NLRP3 stimulation (76). Once activated, caspase-1 cleaves
the inactive pro-IL-1β and pro-IL-18 to release their active form
of cytokines IL-1β and IL-18 to induce neuroinflammation and
pyroptosis (77).
NLRP3 inflammasome is generally expressed in immune
organs and cells, and is also expressed in the central nervous
system (78,79). Liu et al (80) found that NLRP3 was activated in
the microglia of ischemia-reperfusion injury rat model (81). Moreover, recent study suggested
that NLRP3 was also expressed in the endothelial cells, neurons,
and astrocytes of ischemic brain (82). It was demonstrated that NLRP3
inflammasome was firstly activated in microglia and then expressed
in microvascular endothelial cells and neurons of transient MCAO
(tMCAO) rat model (83).
Elevated levels of enlarged infarct size,
neurological function and brain infarct volume were observed in
MCAO rats with activation of NLRP3 inflammasome compared with sham
rats (84). Furthermore, IL-1β
and IL-18 levels were increased in the ipsilateral brain of
ischemia-reperfusion mice model as well as in the postmortem
ipsilateral brain of patients with stroke (79). The NLRP3 inhibitor MCC95
significantly reduced the infarct volume in a dose-dependent
manner, the expression of different pro-inflammatory cytokines and
NLRP3 inflammasome components, indicating its neuroprotective
effect in the MCAO mice (85).
Furthermore, elevated expression of NLRP3, caspase-1, ASC and IL-1β
were present in a murine model of cerebral ischemia, while
caspase-1 inhibition by VX765 prevented these changes and neuronal
death. In summary, NLRP3 inflammasome exerts essential functions in
the pathogenesis of ischemic stroke.
Mitochondria regulate NLRP3 inflammasome activation
under cerebral ischemia in bidirectional mode. Mitochondria are
involved in the initiation and activation of NLRP3 inflammasome,
leading to neuroinflammation and pyroptotic cell death (86). On one hand, the opening of mPTPs
in damaged mitochondria release DAMPs, such as ATP, mtROS,
cardiolipin and mtDNA, which are common causes for NLRP3
inflammasome activation (87).
The localization of mitochondrial and its membrane-associated
proteins also takes positive participation in activation of NLRP3
inflammasome during cerebral ischemic damage. On the other hand,
active mitophagy and fractional mitochondrial-related proteins such
as Src homology 2 domain-containing tyrosine phosphatase 2 (SHP2),
mitofusin 2 (Mfn2) and nuclear factor E2-related factor-2 (Nrf2)
negatively regulate the NLRP3 inflammasome expression to protect
brain from ischemic injury (88)
(Fig. 1).
Dysfunctional mitochondria produce large amounts of
mtROS by transferring electrons from the MRC to molecular oxygen to
form mtROS (77). MtROS are
important in NLRP3 priming and activation. It was revealed that
mtROS regulates NLRP3 initiation earlier than activation by
upregulating its transcription (89). As a non-transcriptional priming
signal, deubiquitination of NLRP3 depends on mtROS generation
(90). Elimination of mtROS
inhibits NLRP3 deubiquitination, in response to lipopolysaccharide
stimulation (91,92). By contrast, mtROS induces
NLRP3-dependent lysosomal damage and inflammasome activation, and
promotes macrophages pyroptosis by inducing Gasdermin D oxidation
(93), which can be reversed by
scavenging mtROS (94).
Next, high concentrations of mtROS results in NLRP3
activation and IL-1β production (92). Furthermore, increased mtROS
induced by ischemia-reperfusion injury leads to release IL-1β,
IL-18 and caspase-1 by cleaving their precursors (95). Conversely, inhibiting mtROS
formation or eliminating mtROS by antioxidants strongly impairs the
activation of NLRP3 inflammasome and IL-1β release (96,97). It was reported that mtROS-NLRP3
signaling is activated in BV2 cells after OGD/R for 24 h.
Inhibition of mtROS release suppresses NLRP3 activation and
alleviates NLRP3-mediating damage in microglia of
ischemia-reperfusion rats (33).
Apart from mtROS, the opening of mPTPs releases
other mitochondria-related DAMPs like mtDNA and mitochondrial
lipids during ischemic stroke (98). Among the different mitochondrial
lipids, cardiolipin is an anionic phospholipid that localizes at
the inner mitochondrial membrane and facilitates OXPHOS in
mitochondria (99). Cardiolipin
oxidation and hydrolysis are a key mechanism of
ischemia-reperfusion-induced brain injury (100). Nowadays, cardiolipin is
reported to be effective for triggering the activation of NLRP3
inflammasome (101) after acute
ischemia (102). On one hand,
cardiolipin directly interacts with both the N-terminal
leucine-rich repeat (LRR) of NLRP3 and full-length of caspase-1 of
NLRP3 inflammasome (86,103). On the other hand, NLRP3 is
tethered to mitochondria by cardiolipin in an mTROs-dependent
manner and is thereby activated (104). Either interference with
cardiolipin synthesis or knockdown of cardiolipin specifically
inhibits NLRP3 inflammasome activation (78).
mtDNA was recognized as one of the endogenous DAMPs,
which is released from damaged mitochondria into the cytoplasm to
activate NLRP3 inflammasome and induce pyroptosis. It was shown
that mtDNA was indispensable for NLRP3 activation by mtROS after
ischemia-reperfusion (105,106). In response to various NLRP3
activators, mtDNA is rapidly released into cytoplasm to be oxidized
to oxidized mtDNA (ox-mtDNA). Then, ox-mtDNA specifically localizes
to NLRP3 (106), and directly
binds to NLRP3 to trigger NLRP3 inflammasome activation (107). It was identified that NLRP3
inflammasome is over-activated in aged individuals, due to
increased production of mtROS and/or mtDNA (108). High level of mtDNA induces the
interaction of ASC with NLRP3 and pro-caspase-1 and promotes
neuronal pyroptosis in the hippocampus of rats with incomplete
ischemia-reperfusion injury (109). Correspondingly, repairing mtDNA
oxidative damage inhibits NLRP3 activation and reduces
reperfusion-associated ischemic brain injury (110). These results imply that mtDNA
interacts with NLRP3 inflammasome to form positive feedback during
the development of ischemic stroke.
ER-mitochondrial contact is mediated by a specific
membrane structure, known as MAM, which plays key roles in material
transfer and signal transduction, including Ca2+
signaling (111,112). Notably, increased MAM
aggravates mitochondrial dysfunction and enhances ROS production
(113). In addition, MAM has
overarching roles in innate immune system. When NLRP3 localizes at
ER membrane, it is in the resting state. By contrast, when it
relocates to MAM, it switches to activated state and functions in
detecting ROS production from damaged mitochondria (114). Recruitment of NLRP3 to
mitochondria enhances the ability of mtROS to activate NLRP3
inflammasome. ASC predominantly localized to the mitochondria, is
transferred to MAM under the stimulation of NLRP3 activators.
Furthermore, colocalization of ASC with MAM requires the presence
of NLRP3 and is Ca2+ dependent (115). Notably, mitochondrial damage by
NLRP3 inflammasome activators leads to the accumulation of NLRP3
and ASC in MAM (116).
Ischemia-reperfusion injury increases the expression of MAM-related
proteins, which accelerated signal communication with mitochondria
through MAM in the male C57BL/6 mice (117). Silencing MAM-related protein
p66Shc protects the integrity of blood-brain barrier, reduces
infarct area, relieves the neurological deficit, and improves the
survival rate of mice after MCAO (118). In a word, MAM is an ideal site
for NLRP3 inflammasome activation and assembly which accelerates
the development of ischemic stroke (119).
Generally, the movement of mitochondria in neurons
is considered to be mediated by microtubules or microfilaments.
Microtubules, which are formed by polymerization of α and β
tubulins, are cell cytoskeleton to support intracellular transport
between various organelles, particularly those involved in
mitochondrial transport (120).
Microtubules undergo various PTMs to take part in the
transportation between mitochondria and ER, as well as in the
subcellular localization of NLRP3 and ASC. Correspondingly, NLRP3
inflammasome activators induce mitochondrial transport to ER
through the microtubule system, thereby facilitating the transfer
of ASC from mitochondria to ER to combine with NLRP3 (121). A previous study found that the
acetylation of microtubule α-tubulin is a necessary step for NLRP3
inflammasome activation (41)
(Fig. 2). Moreover,
NAD+ is an endogenous small molecule that regulates the
microtubules (122).
NAD+ caused by reduced ATP production moves mitochondria
through microtubules, therefore promotes the assembly of NLRP3
inflammasome (41). Nicotinamide
partially increases cellular NAD+ levels and effectively
protects neurons from ischemic damage (40).
TXNIP is an endogenous inhibitor of the thioredoxin
(TRX) system and is expressed in nearly all cytoplasm and
mitochondria (123). MtROS was
revealed to promote the combination of NLRP3 with TXNIP which is a
critical step that links oxidative stress to neuroinflammation
(124,125). In response to mtROS release,
TXNIP dissociates from TRX and translocates to MAM, to bind with
NLRP3 and induces NLRP3 inflammasome activation (126). Inhibition of TRX expression
interrupts the interaction between TRX and TXNIP, thus promoting
the binding of TXNIP to NLRP3 and triggers the assembly and
oligomerization of the NLRP3 inflammasome (127). In addition, TXNIP expression is
upregulated in patients with stroke and rat model of cerebral
ischemia (123,128). The inactivation of TXNIP
relieves neurological deficits, cerebral infarction and edema from
ischemic damage (123).
Finally, TRX inhibitor downregulates the expression of TXNIP and
suppresses NLRP3 inflammasome activation in astrocytes with OGD/R
(80).
RIPK1 is considered an essential regulator of
apoptosis, necroptosis and inflammatory response. Under cerebral
ischemia-hypoxia condition, RIPK1 induces necrotic apoptosis and
neuroinflammation by destroying plasma membrane of endotheliocyte
and microglia (136).
Subsequently, released DAMPs from brain cells cause secondary
inflammatory response, which aggravates ischemic damage (137,138). Upon being transported to out
membrane of mitochondria, RIPK1 interacts with MCU to upregulate
mitochondrial Ca2+ uptake, which leads to mtROS
generation and NLRP3 activation (139). RIPK1 is upregulated in rat
brain upon MCAO and localized to the microglia. Furthermore, RIPK1
triggers activation of NLRP3 inflammasome by disrupting the
mitochondrial membrane integrity and by promoting mtROS release in
ischemic microglia (140). As
the first selective inhibitor, necrostatin-1 (Nec-1) significantly
decreased RIPK1 phosphorylation in rat brain following ischemic
stroke. Consistently, Nec-1 hindered IL-1β maturation in ischemic
brains of rats (141).
Mitochondrial dynamics are regulated by specific
proteins through mitochondrial fission and fusion (142) which play an important role in
NLRP3 inflammasome assembly and activation (143). Mitochondrial fission is
primarily contributed to Drp1 activation (144), which promotes mitochondrial
Ca2+ uptake (145)
to trigger NLRP3 inflammasome activation. Consistently, inhibition
of Drp1-dependent mitochondrial fission protects neurons from
ischemic stroke by preserving the activity of respiratory chain,
reducing superoxide production and delaying Ca2+
dysregulation (146). Moreover,
inhibition of Drp1 withholds NLRP3 inflammasome activation and
protects mitochondrial integrity to exert its neuroprotective
effects (147). A recent study
showed that neuronal death was prevented in MCAO rat model by
lowering Drp1 expression and NLRP3 signal transduction (148).
Following ischemia-reperfusion injury, the damaged
mitochondria are removed by autophagy-related mechanism, which is
known as mitophagy (149).
Mitophagy is an important mechanism of mitochondrial renewal and
metabolism which downregulates the number and controls quality of
mitochondria, induces mitochondria to maintain dynamic homeostasis
(150) and consequently reduces
mitochondria-dependent neuronal cell death (151). In previous studies, it was
identified that induction of mitophagy protects against cerebral
ischemia-reperfusion injury by inhibiting NLRP3 inflammasome
activation (149,152). Following ischemia-reperfusion,
mitophagy increased locally reduces the neuroinflammatory response
induced by NLRP3 inflammasome to relieve neurological deficits
(153). Blockage of mitophagy
enhances the activation of the NLRP3 inflammasome (154). The damaged and dysfunctional
mitochondria enhance NLRP3 inflammasome activation upon treatment
with mitophagy inhibitors (116).
Astrocytes (AS) are the most abundant glial cells in
the central nervous system (155). When ischemic stroke occurs, AS
provide energy support for injured neurons through the changes of
its own bioenergy and mitochondrial dynamics (156). In addition, AS sense stress,
transfer mitochondria as a 'help me' signal to adjacent injured
neurons (157) and rescue
damaged neurons from mitochondrial dysfunction to deal with stress
(158). Concurrently, neurons
also release damaged mitochondria and transfer them to as for
endocytosis and degradation (159), so as to realize the recycling
of mitochondria, making mitochondria crosstalk between healthy
cells and damaged cells.
Mfn2, a mitochondrial outer membrane protein, plays
a pivotal part in mitochondrial fusion. It was reported that Mfn2
is downregulated while NLRP3 inflammasome and pyroptosis are
activated in microglia and astrocytes of rats upon
ischemia-reperfusion injury (162). Mfn2 overexpression attenuates
free fatty acids induced mitochondrial damage, decreases mtROS
production and inhibits NLRP3 inflammasome activation (163). Additionally, Mfn2 improves
hypoxia induced neuronal apoptosis and prolongs the treatment time
window of ischemic stroke by mitochondrial pathway (164).
Nrf2 is a well-known transcription factor that
dissociates from Keap1 then translocates into the nucleus to
initiate gene transcription via binding to antioxidant response
element (ARE) during cellular stress conditions (165,166). The knockout of Nrf2 aggravates
oxidative stress and inflammation (167). Nrf2 abates NLRP3 inflammasome
activation by inhibiting the priming step to limit the assembly of
NLRP3 inflammasome (96,168). Following cerebral
ischemia-reperfusion, the activated Nrf2/ARE pathway inhibits
ROS-induced NLRP3 inflammasome activation in BV2 microglia
(169). Nrf2 knockout mice
showed larger infarct size following ischemia-reperfusion, compared
with that of control counterparts (170). Moreover, Nrf2 siRNA increased
expressions of TXNIP, NLRP3, caspase-1 and IL-1β in brain of MCAO
rats (171). Consequently, Nrf2
protects against NLRP3 inflammasome activation by regulating the
TRX/TXNIP complex during cerebral ischemia-reperfusion injury
(172).
Tissue plasminogen activator (tPA) and tPA
recombinant protein were both used to treat ischemic stroke.
However, these treatments have dangerous complications regarding
reperfusion-injury (173).
Reperfusion-injury generally causes oxidative stress, calcium
overload and excitatory toxicity (174). At present, edaravone,
butylphenol and other drugs are often used in the clinical
treatment of stroke to alleviate the brain injury caused by calcium
overload and excess ROS. In addition, mitochondrial dysfunction is
deemed as a marker of ischemic stroke (175). Therefore, drugs that target
mitochondria and directly or indirectly affect NLRP3 inflammasome
to alleviate brain injury from the aspects of ROS and calcium
overload are summarized in the present review (Table I).
As a sulfonylurea-containing compound, MCC950 was
first identified as an IL-1β inhibitor (198). Then, MCC950 was also used as a
specific inhibitor of NLRP3 inflammasome (199,200). The OGD/R-induced BV-2 cells and
MCAO rats showed high expression of Drp1 and mitochondrial fission,
as well as NLRP3 inflammasome activation, which were abolished by
MCC950 treatments (191).
Oxidative stress, mainly caused by mitochondria, was reported to be
a crucial mechanism for brain damage following ischemic stroke. And
NLRP3 inflammasome perpetuates oxidative stress. MCC950 application
inhibits the effect of NLRP3 on brain oxidative stress in the
animal model of transient global cerebral ischemia (201). In addition, MCC950
administration attenuated brain edema, reduced NADPH oxidase and
infarct area and improved the nervous system in a MCAO rat model
with reperfusion induced by hyperglycemia (202). Moreover, glibenclamide is a
potent NLRP3 inflammasome inhibitor (203) that belongs to a class of
medications known as sulfonylureas. Its neuroprotective role is due
to its effect in reducing inflammatory response and endothelial
cell death (204).
Idebenone was originally used as a drug to treat
dementia. It was regarded as a potent antioxidant (205) which is used as a protective
agent for mitochondria (206).
Upon OGD/R, mtDNA and mtROS were released, resulting in
accumulation of oxidized mtDNA in the cytoplasm which binds to and
activates NLRP3 to initiate inflammation. Furthermore, idebenone
treatment inhibited this process. NLRP3 was activated in microglia
of ischemia-reperfusion rats in vivo. Inhibition of NLRP3
was observed in idebenone treatment which attenuated neurological
deficit and reduced infarct volume (33).
Melatonin, an endogenous regulator, is a metabolite
of tryptophan released from the pineal gland (213). It is involved in numerous
physiological and pathophysiological processes including
antioxidant (214),
anti-apoptotic, and anti-inflammatory effects (215). Melatonin is of neuroprotective
effect, which reduces infarct volume, lowers brain edema, and
increases neurological scores. In addition, melatonin preserves
mitochondrial membrane potential and mitochondrial complex I
activity (216), and inhibits
the opening of MPTPs and the abnormal release of cytochrome c
(217), which is critical in
reducing ischemia-reperfusion injury. Furthermore, melatonin is a
relatively nontoxic molecule, which is safe to use in clinical
trials. Previously, melatonin prevented IL-1β overexpression in the
MCAO rat model (218).
Additionally, Wang et al (219) showed that melatonin inhibited
cell death, loss of mPTP, the release of mitochondrial factors,
pro-IL-1β processing, and activation of caspase-1 induced by OGD.
Furthermore, it decreased infarct size and improved neurological
scores after MCAO in mice (219,220). Consequently, melatonin exerts
neuroprotective and anti-inflammatory effects by modulating
multiple targets in the NLRP3 inflammation (220).
At present, thrombolytic therapy is the only
approved treatment for acute ischemic stroke (229), but only a minority of all
patients with stroke are eligible for this treatment. At present,
some drugs that target mitochondria and NLRP3 inflammasome are
being tested in preclinical research. Idebenone, melatonin,
minocycline and 3-n-butylphthalide were examined in clinical trials
(Table II). It was demonstrated
that no adverse events were reported as to the clinic usage of
3-n-butylphthalide (230).
3-n-butylphthalide showed favorable results and safety in the
treatment of patients with moderate acute ischemic stroke
(ClinicalTrials.gov Identifier:
NCT02149875). Minocycline was reported to be safe and well
tolerated with half-life of ~24 h. It may be an ideal drug to treat
ischemic stroke when used together with tPA (ClinicalTrials.gov Identifier: NCT00630396) (231). In addition, the North Shore
University Hospital is recruiting patients to study the effect of
intra-arterial neuroprotective agents (minocycline) for
recanalization of ischemic stroke (ClinicalTrials.gov Identifier: NCT05032781).
Furthermore, a clinical trial is comparing the efficacy and safety
of low-dose rivastigmine with ezetimibe and high-dose rivastigmine
in the treatment of ischemic stroke (ClinicalTrials.gov Identifier: NCT03993236). In
conclusion, drugs targeting mitochondria and NLRP3 inflammasome
show promising therapeutic effects.
Mitochondria are involved in various processes
essential for cell survival, including energy production and
physiological cell death mechanisms. Emerging knowledge about this
organelle has shed light on its implication in inflammation. It is
well accepted that post-ischemic neuroinflammation is one of the
important mechanisms of ischemic brain injury. NLRP3 inflammasome
has been found to play a key role in driving neuroinflammation in
brain cells, such as cerebral microvascular endothelial cells,
neurons and microglia during acute ischemic stroke. NLRP3
inflammasome was firstly activated in microglia and then expressed
in microvascular endothelial cells and neurons of rat brains after
ischemia-reperfusion injury. NLRP3 inflammasome can be activated by
several factors, including the release of mitochondrial components,
such as mtROS, cardiolipin, and mtDNA and mitochondrial related
proteins, such as TXNIP and RIPK1 and some proteins that regulate
the location of mitochondria. Through the present review, the close
relationship between mitochondria and NLRP3 inflammasome and how
mitochondrial damage contributes to ischemic damage by targeting
neuroinflammation were discussed. Thus, maintaining mitochondrial
homeostasis is important to ischemic stroke. Although the efficacy
of tPA for acute ischemic stroke is well established, there are
still serious side effects and limits. New therapeutic targets
focusing on mitochondria, such as potential antioxidant or
anti-inflammatory medicines, are promising therapeutic approaches
in ischemic stroke. In addition, for ischemic stroke, some
aforementioned drugs shall be administered in clinical trials
currently recruiting patients, or are used in ongoing clinical
trials or have been used in completed clinical trials. Thus,
understanding the biology and regulation of
inflammasome-mitochondria connections is required to treat ischemic
stroke.
Not applicable.
XLZ designed the review, prepared the tables and
figures, and wrote the manuscript. WYZ were involved in the
conception and design of the study. YZ and QY searched the
literature. MZ, JLG and WLZ provided helpful comments. HHL were
involved in the conception and design of the study and revised the
manuscript. XJJ designed the study and revised the manuscript. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82074211 and 82160828) and the
Research and Innovation Project of Graduate (grant nos.
2020YJSS208, YJSKC-20201021 and ZXYCXLX201902).
|
1
|
Wang H, Wang Z, Wu Q, Yuan Y, Cao W and
Zhang X: Regulatory T cells in ischemic stroke. CNS Neurosci Ther.
27:643–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu M, Guo J, Wu B, Zhou Y, Wu M, Farzaneh
M and Khoshnam SE: Mesenchymal stem cell-mediated mitochondrial
transfer: A therapeutic approach for ischemic stroke. Transl Stroke
Res. 12:212–229. 2021. View Article : Google Scholar
|
|
3
|
Feng L, Han CX, Cao SY, Zhang HM and Wu
GY: Deficits in motor and cognitive functions in an adult mouse
model of hypoxia-ischemia induced stroke. Sci Rep. 10:206462020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrington J, Lemarchand E and Allan SM: A
brain in flame; do inflammasomes and pyroptosis influence stroke
pathology? Brain Pathol. 27:205–212. 2017. View Article : Google Scholar
|
|
5
|
Lambertsen KL, Finsen B and Clausen BH:
Post-stroke inflammation-target or tool for therapy? Acta
Neuropathol. 137:693–714. 2019. View Article : Google Scholar
|
|
6
|
Andrabi SS, Parvez S and Tabassum H:
Ischemic stroke and mitochondria: Mechanisms and targets.
Protoplasma. 257:335–343. 2020. View Article : Google Scholar
|
|
7
|
Li X, Huang Z, Liu S, Zeng X, Xie J, Liu
C, Xiao H, Liu R, Li L and Zeng J: 3′-Daidzein sulfonate sodium
provides neuroprotection by promoting the expression of the alpha7
nicotinic acetylcholine receptor and suppressing inflammatory
responses in a rat model of focal cerebral ischemia. Am J Transl
Res. 10:3455–3464. 2018.
|
|
8
|
Mo Y, Sun YY and Liu KY: Autophagy and
inflammation in ischemic stroke. Neural Regen Res. 15:1388–1396.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflammation. 16:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vats K, Sarmah D, Kaur H, Wanve M, Kalia
K, Borah A, Dave KR, Yavagal DR and Bhattacharya P: Inflammasomes
in stroke: A triggering role for acid-sensing ion channels. Ann N Y
Acad Sci. 1431:14–24. 2018. View Article : Google Scholar
|
|
11
|
Forn-Cuni G, Meijer AH and Varela M:
Zebrafish in inflammasome research. Cells. 8:9012019. View Article : Google Scholar :
|
|
12
|
Ma C, Liu S, Zhang S, Xu T, Yu X, Gao Y,
Zhai C, Li C, Lei C, Fan S, et al: Evidence and perspective for the
role of the NLRP3 inflammasome signaling pathway in ischemic stroke
and its therapeutic potential (Review). Int J Mol Med.
42:2979–2990. 2018.
|
|
13
|
Xu Q, Zhao B, Ye Y, Li Y, Zhang Y, Xiong X
and Gu L: Relevant mediators involved in and therapies targeting
the inflammatory response induced by activation of the NLRP3
inflammasome in ischemic stroke. J Neuroinflammation. 18:1232021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Y, Lyu Y, Jiang M, Tang B, Nie T and
Lu S: Human urinary kallidinogenase or edaravone combined with
butylphthalide in the treatment of acute ischemic stroke. Brain
Behav. 9:e014382019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poh L, Kang SW, Baik SH, Ng GYQ, She DT,
Balaganapathy P, Dheen ST, Magnus T, Gelderblom M, Sobey CG, et al:
Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic
microglial death following ischemic stroke. Brain Behav Immun.
75:34–47. 2019. View Article : Google Scholar
|
|
16
|
Cao Y, Zhang H, Lu X, Wang J, Zhang X, Sun
S, Bao Z, Tian W, Ning S, Wang L and Cui L: Overexpression of
MicroRNA-9a-5p Ameliorates NLRP1 inflammasome-mediated ischemic
injury in rats following ischemic stroke. Neuroscience.
444:106–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu SY, Bian HJ, Shu S, Xia SN, Gu Y, Zhang
MJ, Xu Y and Cao X: AIM2 deletion enhances blood-brain barrier
integrity in experimental ischemic stroke. CNS Neurosci Ther.
27:1224–1237. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu JW and Lee MS: Mitochondria and the
NLRP3 inflammasome: Physiological and pathological relevance. Arch
Pharm Res. 39:1503–1518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He Y, Hara H and Nunez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YL, Wu HR, Zhang SS, Xiao HL, Yu J,
Ma YY, Zhang YD and Liu Q: Catalpol ameliorates depressive-like
behaviors in CUMS mice via oxidative stress-mediated NLRP3
inflammasome and neuroinflammation. Transl Psychiatry. 11:3532021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elliott EI and Sutterwala FS: Initiation
and perpetuation of NLRP3 inflammasome activation and assembly.
Immunol Rev. 265:35–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savyuk M, Krivonosov M, Mishchenko T,
Gazaryan I, Ivanchenko M, Khristichenko A, Poloznikov A, Hushpulian
D, Nikulin S, Tonevitsky E, et al: Neuroprotective Effect of HIF
prolyl hydroxylase inhibition in an in vitro hypoxia model.
Antioxidants (Basel). 9:6622020. View Article : Google Scholar
|
|
23
|
Huber W, Zanner R, Schneider G, Schmid R
and Lahmer T: Assessment of regional perfusion and organ function:
Less and non-invasive techniques. Front Med (Lausanne). 6:502019.
View Article : Google Scholar
|
|
24
|
Wang W, Zhao F, Ma X, Perry G and Zhu X:
Mitochondria dysfunction in the pathogenesis of Alzheimer's
disease: Recent advances. Mol Neurodegener. 15:302020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Kui L, Demetrios T, Gong X and Tang
M: A Glimmer of hope: Maintain mitochondrial homeostasis to
mitigate Alzheimer's disease. Aging Dis. 11:1260–1275. 2020.
View Article : Google Scholar :
|
|
26
|
Ham PR and Raju R: Mitochondrial function
in hypoxic ischemic injury and influence of aging. Prog Neurobiol.
157:92–116. 2017. View Article : Google Scholar
|
|
27
|
Liu Y, Lin J, Wu X, Guo X, Sun H, Yu B,
Shen J, Bai J, Chen Z, Yang H, et al: Aspirin-mediated attenuation
of intervertebral disc degeneration by ameliorating reactive oxygen
species in vivo and in vitro. Oxid Med Cell Longev.
2019:71898542019. View Article : Google Scholar
|
|
28
|
Anzell AR, Maizy R, Przyklenk K and
Sanderson TH: Mitochondrial quality control and disease: Insights
into ischemia-reperfusion injury. Mol Neurobiol. 55:2547–2564.
2018. View Article : Google Scholar
|
|
29
|
Babenko VA, Silachev DN, Popkov VA, Zorova
LD, Pevzner IB, Plotnikov EY, Sukhikh GT and Zorov DB: Miro1
enhances mitochondria transfer from multipotent mesenchymal stem
cells (MMSC) to neural cells and improves the efficacy of cell
recovery. Molecules. 23:6872018. View Article : Google Scholar :
|
|
30
|
Mondal NK, Behera J, Kelly KE, George AK,
Tyagi PK and Tyagi N: Tetrahydrocurcumin epigenetically mitigates
mitochondrial dysfunction in brain vasculature during ischemic
stroke. Neurochem Int. 122:120–138. 2019. View Article : Google Scholar :
|
|
31
|
Andrabi SS, Ali M, Tabassum H, Parveen S
and Parvez S: Pramipexole prevents ischemic cell death via
mitochondrial pathways in ischemic stroke. Dis Model Mech.
12:dmm0338602019. View Article : Google Scholar :
|
|
32
|
Chen N, Zhou Z, Li J, Li B, Feng J, He D,
Luo Y, Zheng X, Luo J and Zhang J: 3-n-butylphthalide exerts
neuroprotective effects by enhancing anti-oxidation and attenuating
mitochondrial dysfunction in an in vitro model of ischemic stroke.
Drug Des Devel Ther. 12:4261–4271. 2018. View Article : Google Scholar :
|
|
33
|
Peng J, Wang H, Gong Z, Li X, He L, Shen
Q, Pan J and Peng Y: Idebenone attenuates cerebral inflammatory
injury in ischemia and reperfusion via dampening NLRP3 inflammasome
activity. Mol Immunol. 123:74–87. 2020. View Article : Google Scholar
|
|
34
|
Luan Y, Yang D, Zhang Z, Bie X, Zhao H,
Wang Y, Liu Y, Yang S, Zhou B, Xu Y, et al: Association study
between genetic variation in whole mitochondrial genome and
ischemic stroke. J Mol Neurosci. 71:2152–2162. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarmah D, Datta A, Raut S, Sarkar A, Shah
B, Bohra M, Singh U, Jagtap P, Baidya F, Kalia K, et al: The role
of inflammasomes in atherosclerosis and stroke pathogenesis. Curr
Pharm Des. 26:4234–4245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martynov MY and Gusev EI: Current
knowledge on the neuroprotective and neuroregenerative properties
of citicoline in acute ischemic stroke. J Exp Pharmacol. 7:17–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bissen D, Foss F and Acker-Palmer A: AMPA
receptors and their minions: Auxiliary proteins in AMPA receptor
trafficking. Cell Mol Life Sci. 76:2133–2169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Qin C, Huang J, Tang X, Liu C,
Huang K, Xu J, Guo G, Tong A and Zhou L: The role of astrocytes in
oxidative stress of central nervous system: A mixed blessing. Cell
Prolif. 53:e127812020. View Article : Google Scholar :
|
|
39
|
Wen B, Xu K, Huang R, Jiang T, Wang J,
Chen J, Chen J and He B: Preserving mitochondrial function by
inhibiting GRP75 ameliorates neuron injury under ischemic stroke.
Mol Med Rep. 25:1652022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu D, Gharavi R, Pitta M, Gleichmann M
and Mattson MP: Nicotinamide prevents NAD+ depletion and protects
neurons against excitotoxicity and cerebral ischemia: NAD+
consumption by SIRT1 may endanger energetically compromised
neurons. Neuromolecular Med. 11:28–42. 2009. View Article : Google Scholar :
|
|
41
|
Misawa T, Takahama M, Kozaki T, Lee H, Zou
J, Saitoh T and Akira S: Microtubule-driven spatial arrangement of
mitochondria promotes activation of the NLRP3 inflammasome. Nat
Immunol. 14:454–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Wang T, Zhai L, Ge K, Zhao J, Cong W
and Guo Y: Picroside II exerts a neuroprotective effect by
inhibiting mPTP permeability and EndoG release after cerebral
ischemia/reperfusion injury in rats. J Mol Neurosci. 64:144–155.
2018. View Article : Google Scholar
|
|
43
|
Zheng W, Talley WL, Holstein DM, Wewer J
and Lechleiter JD: P2Y1R-initiated, IP3R-dependent stimulation of
astrocyte mitochondrial metabolism reduces and partially reverses
ischemic neuronal damage in mouse. J Cereb Blood Flow Metab.
33:600–711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bonora M, Bononi A, De Marchi E, Giorgi C,
Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM,
Wojtala A, et al: Role of the c subunit of the FO ATP synthase in
mitochondrial permeability transition. Cell Cycle. 12:674–683.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu
P, Ma Q, Tian F and Chen Y: Mff-Dependent mitochondrial fission
contributes to the pathogenesis of cardiac microvasculature
ischemia/reperfusion injury via induction of mROS-mediated
cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP
opening. J Am Heart Assoc. 6:e0053282017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jin X, Zhang J, An T, Zhao H, Fu W, Li D,
Liu S, Cao X and Liu B: A Genome-wide screen in saccharomyces
cerevisiae reveals a critical role for oxidative phosphorylation in
cellular tolerance to lithium hexafluorophosphate. Cells.
10:8882021. View Article : Google Scholar :
|
|
47
|
He J, Liu J, Huang Y, Zhuo Y, Chen W, Duan
D, Tang X, Lu M and Hu Z: Olfactory mucosa mesenchymal stem cells
alleviate cerebral ischemia/reperfusion injury via Golgi apparatus
secretory pathway Ca2+-ATPase isoform1. Front Cell Dev
Biol. 8:5865412020. View Article : Google Scholar
|
|
48
|
Chen M, Wang M, Yang Q, Wang M, Wang Z,
Zhu Y, Zhang Y, Wang C, Jia Y, Li Y and Wen A: Antioxidant effects
of hydroxysafflor yellow A and acetyl-11-keto-β-boswellic acid in
combination on isoproterenol-induced myocardial injury in rats. Int
J Mol Med. 37:1501–1510. 2016. View Article : Google Scholar :
|
|
49
|
Wang C, Hao J, Liu X, Li C, Yuan X, Lee
RJ, Bai T and Wang D: Isoforsythiaside attenuates Alzheimer's
disease via regulating mitochondrial function through the PI3K/AKT
pathway. Int J Mol Sci. 21:56872020. View Article : Google Scholar
|
|
50
|
Wang T, Wang F, Yu L and Li Z: Nobiletin
alleviates cerebral ischemic-reperfusion injury via MAPK signaling
pathway. Am J Transl Res. 11:5967–5977. 2019.PubMed/NCBI
|
|
51
|
Zhao Q, Zhang C, Wang X, Chen L, Ji H and
Zhang Y: (S)-ZJM-289, a nitric oxide-releasing derivative of
3-n-butylphthalide, protects against ischemic neuronal injury by
attenuating mitochondrial dysfunction and associated cell death.
Neurochem Int. 60:134–144. 2012. View Article : Google Scholar
|
|
52
|
Tan YQ, Zhang X, Zhang S, Zhu T, Garg M,
Lobie PE and Pandey V: Mitochondria: The metabolic switch of
cellular oncogenic transformation. Biochim Biophys Acta Rev Cancer.
1876:1885342021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jahani-Asl A, Cheung EC, Neuspiel M,
MacLaurin JG, Fortin A, Park DS, McBride HM and Slack RS: Mitofusin
2 protects cerebellar granule neurons against injury-induced cell
death. J Biol Chem. 282:23788–23798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McGahan L, Hakim AM and Robertson GS:
Hippocampal Myc and p53 expression following transient global
ischemia. Brain Res Mol Brain Res. 56:133–145. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y and Liu X: Novel insights into the
role of mitochondrial fusion and fission in cardiomyocyte apoptosis
induced by ischemia/reperfusion. J Cell Physiol. 233:5589–5597.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grohm J, Kim SW, Mamrak U, Tobaben S,
Cassidy-Stone A, Nunnari J, Plesnila N and Culmsee C: Inhibition of
Drp1 provides neuroprotection in vitro and in vivo. Cell Death
Differ. 19:1446–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao YX, Cui M, Chen SF, Dong Q and Liu
XY: Amelioration of ischemic mitochondrial injury and Bax-dependent
outer membrane permeabilization by Mdivi-1. CNS Neurosci Ther.
20:528–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang J, Xiong S, Xie C, Markesbery WR and
Lovell MA: Increased oxidative damage in nuclear and mitochondrial
DNA in Alzheimer's disease. J Neurochem. 93:953–962. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
West AP and Shadel GS: Mitochondrial DNA
in innate immune responses and inflammatory pathology. Nat Rev
Immunol. 17:363–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo S, Geng X, Lee H and Ding Y:
Phenothiazine inhibits neuroinflammation and inflammasome
activation independent of hypothermia after ischemic stroke. Mol
Neurobiol. 58:6136–6152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lian L, Zhang Y, Liu L, Yang L, Cai Y,
Zhang J and Xu S: Neuroinflammation in ischemic stroke: Focus on
MicroRNA-mediated polarization of microglia. Front Mol Neurosci.
13:6124392020. View Article : Google Scholar
|
|
62
|
Shaheryar ZA, Khan MA, Adnan CS, Zaidi AA,
Hanggi D and Muhammad S: Neuroinflammatory triangle presenting
novel pharmacological targets for ischemic brain injury. Front
Immunol. 12:7486632021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xue Y, Nie D, Wang LJ, Qiu HC, Ma L, Dong
MX, Tu WJ and Zhao J: Microglial polarization: Novel therapeutic
strategy against ischemic stroke. Aging Dis. 12:466–479. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang L, Yu CC, Liu XY, Deng XN, Tian Q and
Du YJ: Epigenetic modulation of microglia function and phenotypes
in neurodegenerative diseases. Neural Plast. 2021:99126862021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ponsaerts L, Alders L, Schepers M, de
Oliveira RMW, Prickaerts J, Vanmierlo T and Bronckaers A:
Neuroinflammation in ischemic stroke: Inhibition of cAMP-Specific
phosphodiesterases (PDEs) to the rescue. Biomedicines. 9:7032021.
View Article : Google Scholar :
|
|
66
|
Guan X, Zhang Y, Gareev I, Beylerli O, Li
X, Lu X, Lv L and Hai X: MiR-499a prevents astrocytes mediated
inflammation in ischemic stroke by targeting PTEN. Noncoding RNA
Res. 6:146–152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Franke M, Bieber M, Kraft P, Weber A,
Stoll G and Schuhmann MK: The NLRP3 inflammasome drives
inflammation in ischemia/reperfusion injury after transient middle
cerebral artery occlusion in mice. Brain Behav Immun. 92:223–233.
2021. View Article : Google Scholar
|
|
68
|
Gritsenko A, Green JP, Brough D and
Lopez-Castejon G: Mechanisms of NLRP3 priming in inflammaging and
age related diseases. Cytokine Growth Factor Rev. 55:15–25. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu S, Li X, Liu Y, Xia Y, Chang R and
Zhang C: Inflammasome inhibitors: Promising therapeutic approaches
against cancer. J Hematol Oncol. 12:642019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shim DW and Lee KH: Posttranslational
regulation of the NLR family pyrin domain-containing 3
inflammasome. Front Immunol. 9:10542018. View Article : Google Scholar :
|
|
71
|
Han S, Lear TB, Jerome JA, Rajbhandari S,
Snavely CA, Gulick DL, Gibson KF, Zou C, Chen BB and Mallampalli
RK: Lipopolysaccharide primes the NALP3 inflammasome by inhibiting
its ubiquitination and degradation mediated by the SCFFBXL2 E3
ligase. J Biol Chem. 290:18124–18133. 2015. View Article : Google Scholar :
|
|
72
|
Swanson KV, Deng M and Ting JP: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Susjan P, Roskar S and Hafner-Bratkovic I:
The mechanism of NLRP3 inflammasome initiation: Trimerization but
not dimerization of the NLRP3 pyrin domain induces robust
activation of IL-1beta. Biochem Biophys Res Commun. 483:823–828.
2017. View Article : Google Scholar
|
|
74
|
Lu A, Magupalli VG, Ruan J, Yin Q,
Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H and Egelman
EH: Unified polymerization mechanism for the assembly of
ASC-dependent inflammasomes. Cell. 156:1193–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Boucher D, Monteleone M, Coll RC, Chen KW,
Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, et
al: Caspase-1 self-cleavage is an intrinsic mechanism to terminate
inflammasome activity. J Exp Med. 215:827–840. 2018. View Article : Google Scholar :
|
|
76
|
Dick MS, Sborgi L, Ruhl S, Hiller S and
Broz P: ASC filament formation serves as a signal amplification
mechanism for inflammasomes. Nat Commun. 7:119292016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu L, Lu Q, Chen W, Li J, Li C and Zheng
Z: Vitamin D3 protects against diabetic retinopathy by inhibiting
high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome
pathway. J Diabetes Res. 2018:81935232018. View Article : Google Scholar :
|
|
78
|
Ratajczak MZ, Bujko K, Cymer M, Thapa A,
Adamiak M, Ratajczak J, Abdel-Latif AK and Kucia M: The Nlrp3
inflammasome as a 'rising star' in studies of normal and malignant
hematopoiesis. Leukemia. 34:1512–1523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gao L, Dong Q, Song Z, Shen F, Shi J and
Li Y: NLRP3 inflammasome: A promising target in ischemic stroke.
Inflamm Res. 66:17–24. 2017. View Article : Google Scholar
|
|
80
|
Liu H, Wu X, Luo J, Zhao L, Li X, Guo H,
Bai H, Cui W, Guo W, Feng D and Qu Y: Adiponectin peptide
alleviates oxidative stress and NLRP3 inflammasome activation after
cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3beta.
Exp Neurol. 329:1133022020. View Article : Google Scholar
|
|
81
|
Zhao J, Piao X, Wu Y, Liang S, Han F,
Liang Q, Shao S and Zhao D: Cepharanthine attenuates cerebral
ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced
inflammation and oxidative stress via inhibiting 12/15-LOX
signaling. Biomed Pharmacother. 127:1101512020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang F, Wang Z, Wei X, Han H, Meng X,
Zhang Y, Shi W, Li F, Xin T, Pang Q and Yi F: NLRP3 deficiency
ameliorates neurovascular damage in experimental ischemic stroke. J
Cereb Blood Flow Metab. 34:660–667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Feng YS, Tan ZX, Wang MM, Xing Y, Dong F
and Zhang F: Inhibition of NLRP3 inflammasome: A prospective target
for the treatment of ischemic stroke. Front Cell Neurosci.
14:1552020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shi M, Chen J, Liu T, Dai W, Zhou Z, Chen
L and Xie Y: Protective effects of remimazolam on cerebral
ischemia/reperfusion injury in rats by inhibiting of NLRP3
inflammasome-dependent pyroptosis. Drug Des Devel Ther. 16:413–423.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ye Y, Jin T, Zhang X, Zeng Z, Ye B, Wang
J, Zhong Y, Xiong X and Gu L: Meisoindigo protects against focal
cerebral ischemia-reperfusion injury by inhibiting NLRP3
inflammasome activation and regulating microglia/macrophage
polarization via TLR4/NF-κB signaling pathway. Front Cell Neurosci.
13:5532019. View Article : Google Scholar
|
|
86
|
He Z, Ning N, Zhou Q, Khoshnam SE and
Farzaneh M: Mitochondria as a therapeutic target for ischemic
stroke. Free Radic Biol Med. 146:45–58. 2020. View Article : Google Scholar
|
|
87
|
Chen Y, Zhou Z and Min W: Mitochondria,
oxidative stress and innate immunity. Front Physiol. 9:14872018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Meyers AK and Zhu X: The NLRP3
inflammasome: Metabolic regulation and contribution to
inflammaging. Cells. 9:18082020. View Article : Google Scholar :
|
|
89
|
Bauernfeind F, Bartok E, Rieger A, Franchi
L, Nunez G and Hornung V: Cutting edge: Reactive oxygen species
inhibitors block priming, but not activation, of the NLRP3
inflammasome. J Immunol. 187:613–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Paik S, Kim JK, Silwal P, Sasakawa C and
Jo EK: An update on the regulatory mechanisms of NLRP3 inflammasome
activation. Cell Mol Immunol. 18:1141–1160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ren GM, Li J, Zhang XC, Wang Y, Xiao Y,
Zhang XY, Liu X, Zhang W, Ma WB, Zhang J, et al: Pharmacological
targeting of NLRP3 deubiquitination for treatment of
NLRP3-associated inflammatory diseases. Sci Immunol.
6:eabe29332021. View Article : Google Scholar
|
|
92
|
Ren JD, Wu XB, Jiang R, Hao DP and Liu Y:
Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3
inflammasome activation in macrophages by targeting the
mitochondrial reactive oxygen species. Biochim Biophys Acta.
1863:50–55. 2016. View Article : Google Scholar
|
|
93
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D,
Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage
pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol.
11:1069–1082. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Heid ME, Keyel PA, Kamga C, Shiva S,
Watkins SC and Salter RD: Mitochondrial reactive oxygen species
induces NLRP3-dependent lysosomal damage and inflammasome
activation. J Immunol. 191:5230–5238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-Dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019:81518362019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu
P, Wang Y and Hu R: Nuclear factor E2-Related Factor-2 negatively
regulates NLRP3 inflammasome activity by inhibiting reactive oxygen
species-induced NLRP3 priming. Antioxid Redox Signal. 26:28–43.
2017. View Article : Google Scholar :
|
|
97
|
Juliana C, Fernandes-Alnemri T, Kang S,
Farias A, Qin F and Alnemri ES: Non-transcriptional priming and
deubiquitination regulate NLRP3 inflammasome activation. J Biol
Chem. 287:36617–36622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Krysko DV, Agostinis P, Krysko O, Garg AD,
Bachert C, Lambrecht BN and Vandenabeele P: Emerging role of
damage-associated molecular patterns derived from mitochondria in
inflammation. Trends Immunol. 32:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Arias-Cartin R, Grimaldi S, Arnoux P,
Guigliarelli B and Magalon A: Cardiolipin binding in bacterial
respiratory complexes: Structural and functional implications.
Biochim Biophys Acta. 1817:1937–1949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ji J, Baart S, Vikulina AS, Clark RS,
Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J,
et al: Deciphering of mitochondrial cardiolipin oxidative signaling
in cerebral ischemia-reperfusion. J Cereb Blood Flow Metab.
35:319–328. 2015. View Article : Google Scholar :
|
|
101
|
Liu J, Wang T, He K, Xu M and Gong JP:
Cardiolipin inhibitor ameliorates the non-alcoholic steatohepatitis
through suppressing NLRP3 inflammasome activation. Eur Rev Med
Pharmacol Sci. 23:8158–8167. 2019.PubMed/NCBI
|
|
102
|
Szeto HH, Liu S, Soong Y, Seshan SV,
Cohen-Gould L, Manichev V, Feldman LC and Gustafsson T:
Mitochondria protection after acute ischemia prevents prolonged
upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol.
28:1437–1449. 2017. View Article : Google Scholar
|
|
103
|
Carinci M, Vezzani B, Patergnani S,
Ludewig P, Lessmann K, Magnus T, Casetta I, Pugliatti M, Pinton P
and Giorgi C: Different roles of mitochondria in cell death and
inflammation: Focusing on mitochondrial quality control in ischemic
stroke and reperfusion. Biomedicines. 9:1692021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yabal M, Calleja DJ, Simpson DS and Lawlor
KE: Stressing out the mitochondria: Mechanistic insights into NLRP3
inflammasome activation. J Leukoc Biol. 105:377–399. 2019.
View Article : Google Scholar
|
|
105
|
Englander EW, Greeley GJ, Wang G,
Perez-Polo JR and Lee HM: Hypoxia-induced mitochondrial and nuclear
DNA damage in the rat brain. J Neurosci Res. 58:262–269. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nakahira K, Haspel JA, Rathinam VA, Lee
SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim
HP, et al: Autophagy proteins regulate innate immune responses by
inhibiting the release of mitochondrial DNA mediated by the NALP3
inflammasome. Nat Immunol. 12:222–2230. 2011. View Article : Google Scholar
|
|
108
|
Lara PC, Macias-Verde D and Burgos-Burgos
J: Age-induced NLRP3 inflammasome over-activation increases
lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis.
11:756–762. 2020. View Article : Google Scholar :
|
|
109
|
Fu L, Zhang DX, Zhang LM, Song YC, Liu FH,
Li Y, Wang XP, Zheng WC, Wang XD, Gui CX, et al: Exogenous carbon
monoxide protects against mitochondrial DNAinduced hippocampal
pyroptosis in a model of hemorrhagic shock and resuscitation. Int J
Mol Med. 45:1176–1186. 2020.
|
|
110
|
Simon R, Meller R, Yang T, Pearson A and
Wilson G: Enhancing base excision repair of mitochondrial DNA to
reduce ischemic injury following reperfusion. Transl Stroke Res.
10:664–671. 2019. View Article : Google Scholar :
|
|
111
|
Gomez-Suaga P, Bravo-San PJ, Gonzalez-Polo
RA, Fuentes JM and Niso-Santano M: ER-mitochondria signaling in
Parkinson's disease. Cell Death Dis. 9:3372018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tubbs E, Theurey P, Vial G, Bendridi N,
Bravard A, Chauvin MA, Ji-Cao J, Zoulim F, Bartosch B, Ovize M, et
al: Mitochondria-associated endoplasmic reticulum membrane (MAM)
integrity is required for insulin signaling and is implicated in
hepatic insulin resistance. Diabetes. 63:3279–3294. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bravo R, Vicencio JM, Parra V, Troncoso R,
Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J,
et al: Increased ER-mitochondrial coupling promotes mitochondrial
respiration and bioenergetics during early phases of ER stress. J
Cell Sci. 24:2143–2152. 2011. View Article : Google Scholar
|
|
114
|
Elliott EI, Miller AN, Banoth B, Iyer SS,
Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS and Cassel SL:
Cutting Edge: Mitochondrial assembly of the NLRP3 inflammasome
complex is initiated at priming. J Immunol. 200:3047–3052. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hamilton C and Anand PK: Right place,
right time: Localisation and assembly of the NLRP3 inflammasome.
F1000Res. 8:F1000 Faculty Rev-676. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar
|
|
117
|
Gu J, Zhang T, Guo J, Chen K, Li H and
Wang J: PINK1 activation and translocation to
mitochondria-associated membranes mediates mitophagy and protects
against hepatic ischemia/reperfusion injury. Shock. 54:783–793.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Spescha RD, Klohs J, Semerano A, Giacalone
G, Derungs RS, Reiner MF, Rodriguez Gutierrez D, Mendez-Carmona N,
Glanzmann M, Savarese G, et al: Post-ischaemic silencing of p66Shc
reduces ischaemia/reperfusion brain injury and its expression
correlates to clinical outcome in stroke. Eur Heart J.
36:1590–1600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Thoudam T, Jeon JH, Ha CM and Lee IK: Role
of Mitochondria-Associated endoplasmic reticulum membrane in
inflammation-mediated metabolic diseases. Mediators Inflamm.
2016:18514202016. View Article : Google Scholar
|
|
120
|
Fu MM and Holzbaur EL: Integrated
regulation of motor-driven organelle transport by scaffolding
proteins. Trends Cell Biol. 24:564–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Place DE and Kanneganti TD: Recent
advances in inflammasome biology. Curr Opin Immunol. 50:32–38.
2018. View Article : Google Scholar :
|
|
122
|
Harkcom WT, Ghosh AK, Sung MS, Matov A,
Brown KD, Giannakakou P and Jaffrey SR: NAD+ and SIRT3 control
microtubule dynamics and reduce susceptibility to antimicrotubule
agents. Proc Natl Acad Sci USA. 111:E2443–E2452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nasoohi S, Ismael S and Ishrat T:
Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and
Neurodegenerative Diseases: Regulation and Implication. Mol
Neurobiol. 55:7900–7920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nagaraj K, Lapkina-Gendler L, Sarfstein R,
Gurwitz D, Pasmanik-Chor M, Laron Z, Yakar S and Werner H:
Identification of thioredoxin-interacting protein (TXNIP) as a
downstream target for IGF1 action. Proc Natl Acad Sci USA.
115:1045–1050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar
|
|
126
|
Fann DY, Lee SY, Manzanero S, Chunduri P,
Sobey CG and Arumugam TV: Pathogenesis of acute stroke and the role
of inflammasomes. Ageing Res Rev. 12:941–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Han Y, Xu X, Tang C, Gao P, Chen X, Xiong
X, Yang M, Yang S, Zhu X, Yuan S, et al: Reactive oxygen species
promote tubular injury in diabetic nephropathy: The role of the
mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol.
16:32–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang BF and Yoshioka J: The Emerging role
of thioredoxin-interacting protein in myocardial
ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther.
22:219–229. 2017. View Article : Google Scholar :
|
|
129
|
Schafer MK, Pfeiffer A, Jaeckel M, Pouya
A, Dolga AM and Methner A: Regulators of mitochondrial Ca(2+)
homeostasis in cerebral ischemia. Cell Tissue Res. 357:395–405.
2014. View Article : Google Scholar
|
|
130
|
Missiroli S, Patergnani S, Caroccia N,
Pedriali G, Perrone M, Previati M, Wieckowski MR and Giorgi C:
Mitochondria-associated membranes (MAMs) and inflammation. Cell
Death Dis. 9:3292018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lee GS, Subramanian N, Kim AI,
Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner
DL and Chae JJ: The calcium-sensing receptor regulates the NLRP3
inflammasome through Ca2+ and cAMP. Nature. 492:123–127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang C, Jia Q, Sun C and Jing C: Calcium
sensing receptor contribute to early brain injury through the
CaMKII/NLRP3 pathway after subarachnoid hemorrhage in mice. Biochem
Biophys Res Commun. 530:651–657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Triantafilou K, Hughes TR, Triantafilou M
and Morgan BP: The complement membrane attack complex triggers
intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation.
J Cell Sci. 126:2903–2913. 2013.PubMed/NCBI
|
|
134
|
Murakami T, Ockinger J, Yu J, Byles V,
McColl A, Hofer AM and Horng T: Critical role for calcium
mobilization in activation of the NLRP3 inflammasome. Proc Natl
Acad Sci USA. 109:11282–11287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Pan T, Zhu QJ, Xu LX, Ding X, Li JQ, Sun
B, Hua J and Feng X: Knocking down TRPM2 expression reduces cell
injury and NLRP3 inflammasome activation in PC12 cells subjected to
oxygen-glucose deprivation. Neural Regen Res. 15:2154–2161. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF,
Mao L, Xia YP, Jin HJ, Li YN, You MF, et al: Microglia-derived
TNF-α mediates endothelial necroptosis aggravating blood
brain-barrier disruption after ischemic stroke. Cell Death Dis.
10:4872019. View Article : Google Scholar
|
|
137
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
He S and Wang X: RIP kinases as modulators
of inflammation and immunity. Nat Immunol. 19:912–922. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X,
Ma D, Yuan Y, Li Z, Hou N, et al: RIPK1 Binds MCU to mediate
induction of mitochondrial Ca2+ uptake and promotes
colorectal oncogenesis. Cancer Res. 78:2876–2885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jiao Y, Wang J, Zhang H, Cao Y, Qu Y,
Huang S, Kong X, Song C, Li J, Li Q, et al: Inhibition of
microglial receptor-interacting protein kinase 1 ameliorates
neuroinflammation following cerebral ischaemic stroke. J Cell Mol
Med. 24:12585–12598. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Deng XX, Li SS and Sun FY: Necrostatin-1
prevents necroptosis in brains after ischemic stroke via inhibition
of RIPK1-Mediated RIPK3/MLKL signaling. Aging Dis. 10:807–817.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Park S, Won JH, Hwang I, Hong S, Lee HK
and Yu JW: Defective mitochondrial fission augments NLRP3
inflammasome activation. Sci Rep. 5:154892015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xie JH, Li YY and Jin J: The essential
functions of mitochondrial dynamics in immune cells. Cell Mol
Immunol. 17:712–721. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ren L, Chen X, Chen X, Li J, Cheng B and
Xia J: Mitochondrial dynamics: Fission and fusion in fate
determination of mesenchymal stem cells. Front Cell Dev Biol.
8:5800702020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Szabadkai G, Simoni AM, Chami M,
Wieckowski MR, Youle RJ and Rizzuto R: Drp-1-dependent division of
the mitochondrial network blocks intraorganellar Ca2+ waves and
protects against Ca2+-mediated apoptosis. Mol Cell. 16:59–68. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Flippo KH, Gnanasekaran A, Perkins GA,
Ajmal A, Merrill RA, Dickey AS, Taylor SS, McKnight GS, Chauhan AK,
Usachev YM and Strack S: AKAP1 protects from cerebral ischemic
stroke by inhibiting Drp1-dependent mitochondrial fission. J
Neurosci. 38:8233–8242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Guo M, Wang X, Zhao Y, Yang Q, Ding H,
Dong Q, Chen X and Cui M: Ketogenic diet improves brain ischemic
tolerance and inhibits NLRP3 inflammasome activation by preventing
Drp1-Mediated mitochondrial fission and endoplasmic reticulum
stress. Front Mol Neurosci. 11:862018. View Article : Google Scholar :
|
|
148
|
He J and Zhang X: miR-668 inhibitor
attenuates mitochondrial membrane potential and protects against
neuronal apoptosis in cerebral ischemic stroke. Folia Neuropathol.
58:22–29. 2020. View Article : Google Scholar
|
|
149
|
Zhang X, Yan H, Yuan Y, Gao J, Shen Z,
Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al: Cerebral
ischemia-reperfusion-induced autophagy protects against neuronal
injury by mitochondrial clearance. Autophagy. 9:1321–1333. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wang J, Yu S, Li J, Li H, Jiang H, Xiao P,
Pan Y, Zheng J, Yu L and Jiang J: Protective role of
N-acetyl-l-tryptophan against hepatic ischemia-reperfusion injury
via the RIP2/caspase-1/IL-1beta signaling pathway. Pharm Biol.
57:385–391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang Y, Tian J, Qiao X, Su X, Mi Y, Zhang
R and Li R: Intermedin protects against renal ischemia-reperfusion
injury by inhibiting endoplasmic reticulum stress. BMC Nephrol.
16:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
He Q, Li Z, Meng C, Wu J, Zhao Y and Zhao
J: Parkin-dependent mitophagy is required for the inhibition of
ATF4 on NLRP3 inflammasome activation in cerebral
ischemia-reperfusion injury in rats. Cells. 8:8972019. View Article : Google Scholar :
|
|
153
|
Yang J, Chen Y and Pang Y: Occurrence of
mitochondrial autophagy and nlrp3 inflammatory bodies in cerebral
ischemia-reperfusion injury and its correlation with
neuroinflammatory response. Acta Medica Mediterranea. 37:1033–1037.
2021.
|
|
154
|
Su SH, Wu YF, Lin Q, Wang DP and Hai J:
URB597 protects against NLRP3 inflammasome activation by inhibiting
autophagy dysfunction in a rat model of chronic cerebral
hypoperfusion. J Neuroinflammation. 16:2602019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Fan Y, Zhu S, Wang J, Zhao Y and Wang X:
Propofol protects against oxygen/glucose deprivationinduced cell
injury via gap junction inhibition in astrocytes. Mol Med Rep.
22:2896–2904. 2020.PubMed/NCBI
|
|
156
|
Cai Y, Guo H, Fan Z, Zhang X, Wu D, Tang
W, Gu T, Wang S, Yin A, Tao L, et al: Glycogenolysis is crucial for
astrocytic glycogen accumulation and brain damage after reperfusion
in ischemic stroke. iScience. 23:1011362020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gao L, Liu F, Hou PP, Manaenko A, Xiao ZP,
Wang F, Xu TL and Hu Q: Neurons release injured mitochondria as
'Help-Me' signaling after ischemic stroke. Front Aging Neurosci.
14:7857612022. View Article : Google Scholar
|
|
158
|
Jiang D, Gao F, Zhang Y, Wong DS, Li Q,
Tse HF, Xu G, Yu Z and Lian Q: Mitochondrial transfer of
mesenchymal stem cells effectively protects corneal epithelial
cells from mitochondrial damage. Cell Death Dis. 7:e24672016.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Hasan-Olive MM, Enger R, Hansson HA,
Nagelhus EA and Eide PK: Pathological mitochondria in neurons and
perivascular astrocytic endfeet of idiopathic normal pressure
hydrocephalus patients. Fluids Barriers CNS. 16:392019. View Article : Google Scholar :
|
|
160
|
Guo W, Liu W, Chen Z, Gu Y, Peng S, Shen
L, Shen Y, Wang X, Feng GS, Sun Y and Xu Q: Tyrosine phosphatase
SHP2 negatively regulates NLRP3 inflammasome activation via
ANT1-dependent mitochondrial homeostasis. Nat Commun. 8:21682017.
View Article : Google Scholar :
|
|
161
|
Aoki Y, Huang Z, Thomas SS, Bhide PG,
Huang I, Moskowitz MA and Reeves SA: Increased susceptibility to
ischemia-induced brain damage in transgenic mice overexpressing a
dominant negative form of SHP2. FASEB J. 14:1965–1973. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zou X, Xie L, Wang W, Zhao G, Tian X and
Chen M: FK866 alleviates cerebral pyroptosis and inflammation
mediated by Drp1 in a rat cardiopulmonary resuscitation model. Int
Immunopharmacol. 89:1070322020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Dong J, Bobe G, Guan Y, Li G, Zuo R, Shu
X, Wang Y, Sun X, Chen X and Li X: Mitochondrial membrane protein
mitofusin 2 as a potential therapeutic target for treating free
fatty acid-induced hepatic inflammation in dairy cows during early
lactation. J Dairy Sci. 103:5561–5574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Peng C, Rao W, Zhang L, Wang K, Hui H,
Wang L, Su N, Luo P, Hao YL, Tu Y, et al: Mitofusin 2 ameliorates
hypoxia-induced apoptosis via mitochondrial function and signaling
pathways. Int J Biochem Cell Biol. 69:29–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wang X, Chen T, Ma X, Huang W, Huang Q,
Liu K and Liang H: Progress on nuclear factor-E2 related factor 2
transcription factors in sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi
Xue. 30:810–814. 2018.In Chinese. PubMed/NCBI
|
|
166
|
Zhao C, Gillette DD, Li X, Zhang Z and Wen
H: Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3
and AIM2 inflammasome activation. J Biol Chem. 289:17020–17029.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Anandhan A, Nguyen N, Syal A, Dreher LA,
Dodson M, Zhang DD and Madhavan L: NRF2 loss accentuates
Parkinsonian pathology and behavioral dysfunction in human
α-synuclein overexpressing mice. Aging Dis. 12:964–982. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu
S and Kong AN: Activation of Nrf2-antioxidant signaling attenuates
NFkappaB-inflammatory response and elicits apoptosis. Biochem
Pharmacol. 76:1485–1489. 2008. View Article : Google Scholar
|
|
169
|
Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G
and Yu M: Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome
activation in BV2 cells after cerebral ischemia reperfusion.
Inflamm Res. 67:57–65. 2018. View Article : Google Scholar
|
|
170
|
Xu B, Zhang J, Strom J, Lee S and Chen QM:
Myocardial ischemic reperfusion induces de novo Nrf2 protein
translation. Biochim Biophys Acta. 1842:1638–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Yu J, Wang WN, Matei N, Li X, Pang JW, Mo
J, Chen SP, Tang JP, Yan M and Zhang JH: Ezetimibe attenuates
oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP
Pathway after MCAO in Rats. Oxid Med Cell Longev. 2020:47172582020.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y
and Zhao J: Nrf2 inhibits NLRP3 inflammasome activation through
regulating Trx1/TXNIP complex in cerebral ischemia reperfusion
injury. Behav Brain Res. 336:32–39. 2018. View Article : Google Scholar
|
|
173
|
Zhang C, He M, Ni L, He K, Su K, Deng Y,
Li Y and Xia H: The role of arachidonic acid metabolism in
myocardial ischemia-reperfusion injury. Cell Biochem Biophys.
78:255–265. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Shi Y, Peng XH, Li X, Luo GP and Wu MF:
Neuroprotective role of dexmedetomidine pretreatment in cerebral
ischemia injury via ADRA2A-mediated phosphorylation of ERK1/2 in
adult rats. Exp Ther Med. 16:5201–5209. 2018.PubMed/NCBI
|
|
175
|
Liu F, Lu J, Manaenko A, Tang J and Hu Q:
Mitochondria in Ischemic Stroke: New Insight and Implications.
Aging Dis. 9:924–937. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang X, Li R, Wang X, Fu Q and Ma S:
Umbelliferone ameliorates cerebral ischemia-reperfusion injury via
upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3
inflammasome. Neurosci Lett. 600:182–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Li Y, Li J, Li S, Li Y, Wang X, Liu B, Fu
Q and Ma S: Curcumin attenuates glutamate neurotoxicity in the
hippocampus by suppression of ER stress-associated TXNIP/NLRP3
inflammasome activation in a manner dependent on AMPK. Toxicol Appl
Pharmacol. 286:53–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B and Kou J: Ruscogenin attenuates
cerebral ischemia-induced blood-brain barrier dysfunction by
suppressing TXNIP/NLRP3 inflammasome activation and the MAPK
pathway. Int J Mol Sci. 17:14182016. View Article : Google Scholar :
|
|
179
|
Ishrat T, Mohamed IN, Pillai B, Soliman S,
Fouda AY, Ergul A, El-Remessy AB and Fagan SC:
Thioredoxin-interacting protein: A novel target for neuroprotection
in experimental thromboembolic stroke in mice. Mol Neurobiol.
51:766–778. 2015. View Article : Google Scholar
|
|
180
|
Ismael S, Nasoohi S, Yoo A, Ahmed HA and
Ishrat T: Tissue plasminogen activator promotes TXNIP-NLRP3
inflammasome activation after hyperglycemic stroke in mice. Mol
Neurobiol. 57:2495–2508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu T, Wang W, Liu M, Ma Y, Mu F, Feng X,
Zhang Y, Guo C, Ding Y and Wen A: Z-Guggulsterone alleviated
oxidative stress and inflammation through inhibiting the
TXNIP/NLRP3 axis in ischemic stroke. Int Immunopharmacol.
89:1070942020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Yang W, Chen X, Pan J, Ge H, Yin K, Wu Z,
Li X, Sha D and Xu Y: Malibatol A protects against brain injury
through reversing mitochondrial dysfunction in experimental stroke.
Neurochem Int. 80:33–40. 2015. View Article : Google Scholar
|
|
183
|
Gao XJ, Xie GN, Liu L, Fu ZJ, Zhang ZW and
Teng LZ: Sesamol attenuates oxidative stress, apoptosis and
inflammation in focal cerebral ischemia/reperfusion injury. Exp
Ther Med. 14:841–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Lu Y, Xiao G and Luo W: Minocycline
suppresses NLRP3 inflammasome activation in experimental ischemic
stroke. Neuroimmunomodulation. 23:230–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Qiu J, Wang M, Zhang J, Cai Q, Lu D, Li Y,
Dong Y, Zhao T and Chen H: The neuroprotection of Sinomenine
against ischemic stroke in mice by suppressing NLRP3 inflammasome
via AMPK signaling. Int Immunopharmacol. 40:492–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Peng J, Deng X, Huang W, Yu JH, Wang JX,
Wang JP, Yang SB, Liu X, Wang L, Zhang Y, et al: Irisin protects
against neuronal injury induced by oxygen-glucose deprivation in
part depends on the inhibition of ROS-NLRP3 inflammatory signaling
pathway. Mol Immunol. 91:185–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Qin YY, Li M, Feng X, Wang J, Cao L, Shen
XK, Chen J, Sun M, Sheng R, Han F and Qin ZH: Combined NADPH and
the NOX inhibitor apocynin provides greater anti-inflammatory and
neuroprotective effects in a mouse model of stroke. Free Radic Biol
Med. 104:333–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Safakheil M and Safakheil H: The effect of
exosomes derived from bone marrow stem cells in combination with
rosuvastatin on functional recovery and neuroprotection in rats
after ischemic stroke. J Mol Neurosci. 70:724–737. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Barakat W, Fahmy A, Askar M and
El-Kannishy S: Effectiveness of arginase inhibitors against
experimentally induced stroke. Naunyn Schmiedebergs Arch Pharmacol.
391:603–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wang Y, Guan X, Gao CL, Ruan W, Zhao S,
Kai G, Li F and Pang T: Medioresinol as a novel PGC-1α activator
prevents pyroptosis of endothelial cells in ischemic stroke through
PPARα-GOT1 axis. Pharmacol Res. 169:1056402021. View Article : Google Scholar
|
|
191
|
Hu J, Zeng C, Wei J, Duan F, Liu S, Zhao Y
and Tan H: The combination of Panax ginseng and Angelica sinensis
alleviates ischemia brain injury by suppressing NLRP3 inflammasome
activation and microglial pyroptosis. Phytomedicine. 76:1532512020.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Yao Z, Liu N, Zhu X, Wang L, Zhao Y, Liu
Q, Gao C and Li J: Subanesthetic isoflurane abates ROS-activated
MAPK/NF-κB signaling to repress ischemia-induced microglia
inflammation and brain injury. Aging (Albany NY). 12:26121–26139.
2020. View Article : Google Scholar
|
|
193
|
Lin KC, Chen KH, Wallace CG, Chen YL, Ko
SF, Lee MS and Yip HK: Combined therapy with hyperbaric oxygen and
melatonin effectively reduce brain infarct volume and preserve
neurological function after acute ischemic infarct in rat. J
Neuropathol Exp Neurol. 78:949–960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yang M, Lv Y, Tian X, Lou J, An R, Zhang
Q, Li M, Xu L and Dong Z: Neuroprotective effect of β-caryophyllene
on cerebral ischemia-reperfusion injury via regulation of
necroptotic neuronal death and inflammation: In vivo and in vitro.
Front Neurosci. 11:5832017. View Article : Google Scholar
|
|
195
|
Zhong KL, Lu MY, Liu F, Mei Y, Zhang XJ,
Zhang H, Zan J, Sun XO and Tan W: Isosteviol sodium protects neural
cells against hypoxia-induced apoptosis through inhibiting MAPK and
NF-κB pathways. J Stroke Cerebrovasc Dis. 28:175–184. 2019.
View Article : Google Scholar
|
|
196
|
Turovskaya MV, Gaidin SG, Mal'Tseva VN,
Zinchenko VP and Turovsky EA: Taxifolin protects neurons against
ischemic injury in vitro via the activation of antioxidant systems
and signal transduction pathways of GABAergic neurons. Mol Cell
Neurosci. 96:10–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Zhao P, Chang RY, Liu N, Wang J, Zhou R,
Qi X, Liu Y, Ma L, Niu Y, Sun T, et al: Neuroprotective effect of
oxysophocarpine by modulation of MAPK pathway in rat hippocampal
neurons subject to oxygen-glucose deprivation and reperfusion. Cell
Mol Neurobiol. 38:529–540. 2018. View Article : Google Scholar
|
|
198
|
Wu D, Chen Y, Sun Y, Gao Q, Li H, Yang Z,
Wang Y, Jiang X and Yu B: Target of MCC950 in Inhibition of NLRP3
inflammasome activation: A literature review. Inflammation.
43:17–23. 2020. View Article : Google Scholar
|
|
199
|
Coll RC, Hill JR, Day CJ, Zamoshnikova A,
Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson
AAB and Schroder K: MCC950 directly targets the NLRP3
ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol.
15:556–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Jiao J, Zhao G, Wang Y, Ren P and Wu M:
MCC950, a selective inhibitor of NLRP3 inflammasome, reduces the
inflammatory response and improves neurological outcomes in mice
model of spinal cord injury. Front Mol Biosci. 7:372020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Joaquim LS, Danielski LG, Bonfante S,
Biehl E, Mathias K, Denicol T, Bagio E, Lanzzarin EV, Machado RS,
Bernades GC, et al: NLRP3 inflammasome activation increases brain
oxidative stress after transient global cerebral ischemia in rats.
Int J Neurosci. 1–14. 2021.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Chen H, Guan B, Chen S, Yang D and Shen J:
Peroxynitrite activates NLRP3 inflammasome and contributes to
hemorrhagic transformation and poor outcome in ischemic stroke with
hyperglycemia. Free Radic Biol Med. 165:171–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Dwivedi DK and Jena GB: NLRP3 inhibitor
glibenclamide attenuates high-fat diet and streptozotocin-induced
non-alcoholic fatty liver disease in rat: Studies on oxidative
stress, inflammation, DNA damage and insulin signalling pathway.
Naunyn Schmiedebergs Arch Pharmacol. 393:705–716. 2020. View Article : Google Scholar
|
|
204
|
Zhu S, Gao X, Huang K, Gu Y, Hu Y, Wu Y,
Ji Z, Wang Q and Pan S: Glibenclamide enhances the therapeutic
benefits of early hypothermia after severe stroke in rats. Aging
Dis. 9:685–695. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Gueven N, Ravishankar P, Eri R and Rybalka
E: Idebenone: When an antioxidant is not an antioxidant. Redox
Biol. 38:1018122021. View Article : Google Scholar
|
|
206
|
Jiang W, Geng H, Lv X, Ma J, Liu F, Lin P
and Yan C: Idebenone Protects against atherosclerosis in
apolipoprotein E-Deficient mice via activation of the
SIRT3-SOD2-mtROS pathway. Cardiovasc Drugs Ther. 35:1129–1145.
2021. View Article : Google Scholar
|
|
207
|
Akopova O, Kolchinskaya L, Nosar V,
Mankovska I and Sagach V: Diazoxide affects mitochondrial
bioenergetics by the opening of mKATP channel on submicromolar
scale. BMC Mol Cell Biol. 21:312020. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Liu D, Lu C, Wan R, Auyeung WW and Mattson
MP: Activation of mitochondrial ATP-dependent potassium channels
protects neurons against ischemia-induced death by a mechanism
involving suppression of Bax translocation and cytochrome c
release. J Cereb Blood Flow Metab. 22:431–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Lei X, Lei L, Zhang Z and Cheng Y:
Diazoxide inhibits of ER stressmediated apoptosis during
oxygenglucose deprivation in vitro and cerebral ischemiareperfusion
in vivo. Mol Med Rep. 17:8039–8046. 2018.PubMed/NCBI
|
|
210
|
Mishra SR, Mahapatra KK, Behera BP, Patra
S, Bhol CS, Panigrahi DP, Praharaj PP, Singh A, Patil S, Dhiman R
and Bhutia SK: Mitochondrial dysfunction as a driver of NLRP3
inflammasome activation and its modulation through mitophagy for
potential therapeutics. Int J Biochem Cell Biol. 136:1060132021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Nógrádi B, Nyúl-Tóth Á, Kozma M, Molnár K,
Patai R, Siklós L, Wilhelm I and Krizbai IA: Upregulation of
nucleotide-binding oligomerization Domain-, LRR- and pyrin
domain-containing protein 3 in motoneurons following peripheral
nerve injury in mice. Front Pharmacol. 11:5841842020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gong Z, Pan J, Shen Q, Li M and Peng Y:
Mitochondrial dysfunction induces NLRP3 inflammasome activation
during cerebral ischemia/reperfusion injury. J Neuroinflammation.
15:2422018. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Kondoh T, Uneyama H, Nishino H and Torii
K: Melatonin reduces cerebral edema formation caused by transient
forebrain ischemia in rats. Life Sci. 72:583–590. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Kilic E, Caglayan B and Caglar BM:
Physiological and pharmacological roles of melatonin in the
pathophysiological components of cellular injury after ischemic
stroke. Turk J Med Sci. 50:1655–1664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Fan W, He Y, Guan X, Gu W, Wu Z, Zhu X,
Huang F and He H: Involvement of the nitric oxide in
melatonin-mediated protection against injury. Life Sci.
200:142–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Yang Y, Jiang S, Dong Y, Fan C, Zhao L,
Yang X, Li J, Di S, Yue L, Liang G, et al: Melatonin prevents cell
death and mitochondrial dysfunction via a SIRT1-dependent mechanism
during ischemic-stroke in mice. J Pineal Res. 58:61–70. 2015.
View Article : Google Scholar
|
|
217
|
Ramos E, Patino P, Reiter RJ, Gil-Martín
E, Marco-Contelles J, Parada E, de Los Rios C, Romero A and Egea J:
Ischemic brain injury: New insights on the protective role of
melatonin. Free Radic Biol Med. 104:32–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Paredes SD, Rancan L, Kireev R, González
A, Louzao P, González P, Rodríguez-Bobada C, García C, Vara E and
Tresguerres JA: Melatonin counteracts at a transcriptional level
the inflammatory and apoptotic response secondary to ischemic brain
injury induced by middle cerebral artery blockade in aging rats.
Biores Open Access. 4:407–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Wang X, Figueroa BE, Stavrovskaya IG,
Zhang Y, Sirianni AC, Zhu S, Day AL, Kristal BS and Friedlander RM:
Methazolamide and melatonin inhibit mitochondrial cytochrome C
release and are neuroprotective in experimental models of ischemic
injury. Stroke. 40:1877–1885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Gilani GS, Nimal RW, Mueller R and Mazza
G: Effects of source of protein and supplementary extracted
isoflavones and anthocyanins on longevity of Stroke-prone
Spontaneously Hypertensive (SHRSP) rats. J Toxicol Sci. 34:335–341.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Song F, Zhu Y, Shi Z, Tian J, Deng X, Ren
J, Andrews MC, Ni H, Ling W and Yang Y: Plant food anthocyanins
inhibit platelet granule secretion in hypercholesterolaemia:
Involving the signalling pathway of PI3K-Akt. Thromb Haemost.
112:981–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Feng R, Ni HM, Wang SY, Tourkova IL,
Shurin MR, Harada H and Yin XM: Cyanidin-3-rutinoside, a natural
polyphenol antioxidant, selectively kills leukemic cells by
induction of oxidative stress. J Biol Chem. 282:13468–13476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Dreiseitel A, Schreier P, Oehme A, Locher
S, Rogler G, Piberger H, Hajak G and Sand PG: Inhibition of
proteasome activity by anthocyanins and anthocyanidins. Biochem
Biophys Res Commun. 372:57–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Cai Y, Li X, Pan Z, Zhu Y, Tuo J, Meng Q,
Dai G, Yang G and Pan Y: Anthocyanin ameliorates hypoxia and
ischemia induced inflammation and apoptosis by increasing
autophagic flux in SH-SY5Y cells. Eur J Pharmacol. 883:1733602020.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Pan Z, Cui M, Dai G, Yuan T, Li Y, Ji T
and Pan Y: Protective effect of anthocyanin on neurovascular unit
in cerebral ischemia/reperfusion injury in rats. Front Neurosci.
12:9472018. View Article : Google Scholar
|
|
226
|
Liobikas J, Skemiene K, Trumbeckaite S and
Borutaite V: Anthocyanins in cardioprotection: A path through
mitochondria. Pharmacol Res. 113:808–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Min J, Yu SW, Baek SH, Nair KM, Bae ON,
Bhatt A, Kassab M, Nair MG and Majid A: Neuroprotective effect of
cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral
ischemia. Neurosci Lett. 500:157–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Cui HX, Chen JH, Li JW, Cheng FR and Yuan
K: Protection of anthocyanin from myrica rubra against cerebral
ischemia-reperfusion injury via modulation of the TLR4/NF-κB and
NLRP3 pathways. Moleculs. 23:17882018. View Article : Google Scholar
|
|
229
|
Gutierrez-Vargas JA, Munera A and
Cardona-Gomez GP: CDK5 knockdown prevents hippocampal degeneration
and cognitive dysfunction produced by cerebral ischemia. J Cereb
Blood Flow Metab. 35:1937–1949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Xue LX, Zhang T, Zhao YW, Geng Z, Chen JJ
and Chen H: Efficacy and safety comparison of DL-3-n-butylphthalide
and Cerebrolysin: Effects on neurological and behavioral outcomes
in acute ischemic stroke. Exp Ther Med. 11:2015–2020. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Fagan SC, Waller JL, Nichols FT, Edwards
DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A and Hess
DC: Minocycline to improve neurologic outcome in stroke (MINOS): A
dose-finding study. Stroke. 41:2283–2287. 2010. View Article : Google Scholar : PubMed/NCBI
|