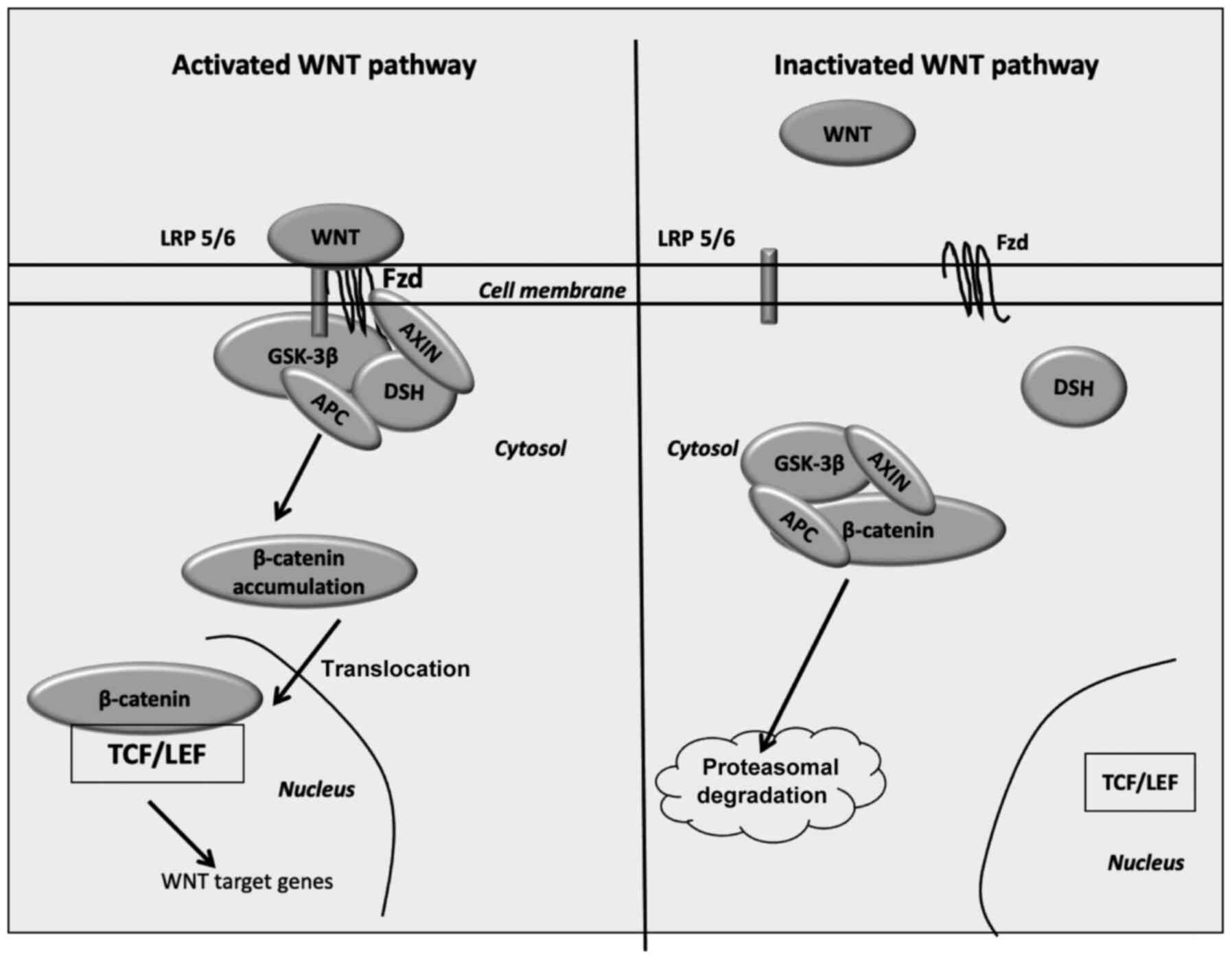
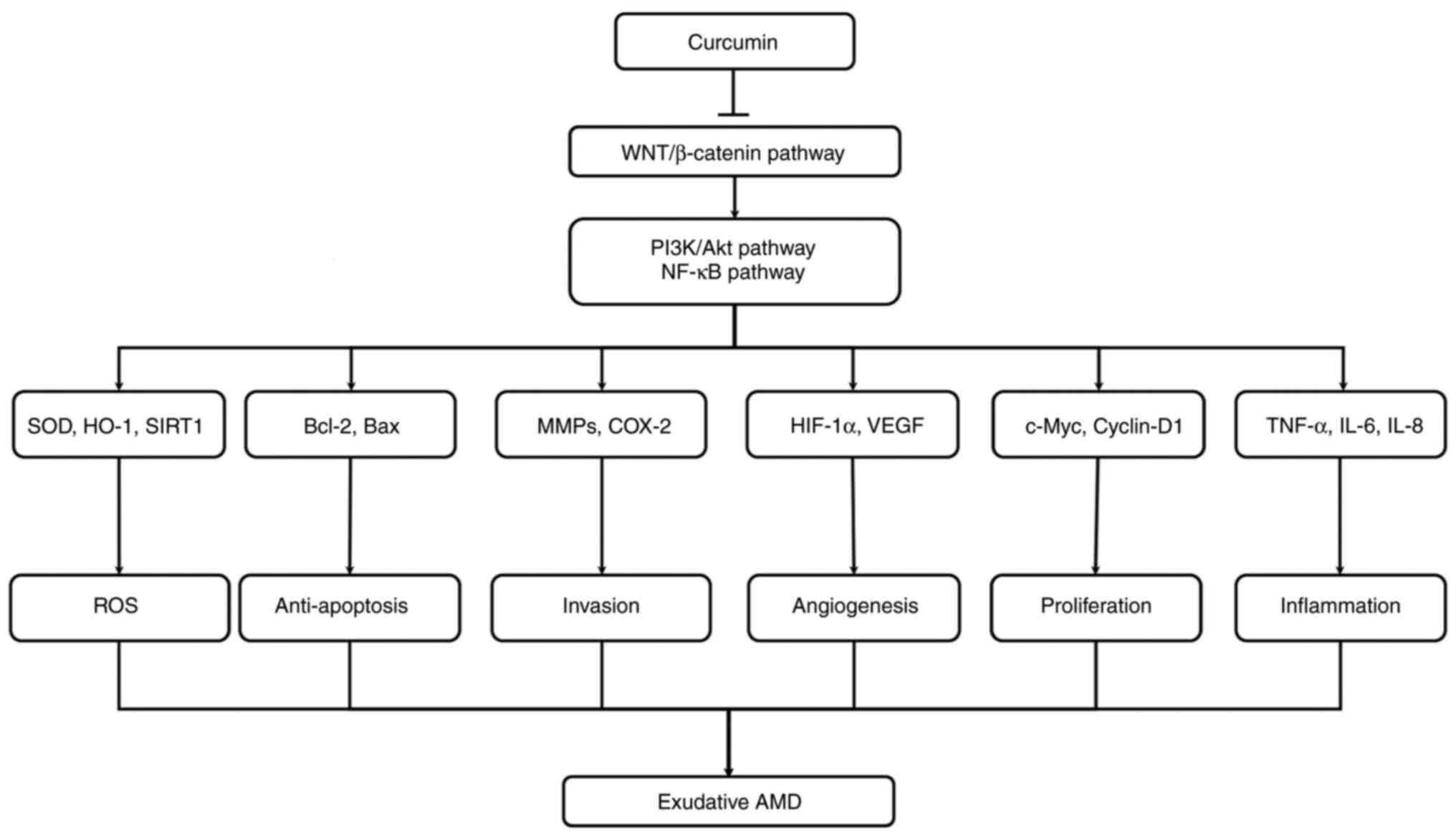
|
1
|
Bird AC: Therapeutic targets in
age-related macular disease. J Clin Invest. 120:3033–3041. 2010.
View Article : Google Scholar :
|
|
2
|
Radomska-Leśniewska DM, Skopiński P, Bałan
BJ, Białoszewska A, Jóźwiak J, Rokicki D, Skopińska-Różewska E,
Borecka A and Hevelke A: Angiomodulatory properties of Rhodiola
spp. and other natural antioxidants. Cent Eur J Immunol.
40:249–262. 2015. View Article : Google Scholar
|
|
3
|
Radomska-Leśniewska DM, Bałan BJ and
Skopiński P: Angiogenesis modulation by exogenous antioxidants.
Cent Eur J Immunol. 42:370–376. 2017. View Article : Google Scholar
|
|
4
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: PPARγ agonists: Potential treatments for exudative
age-related macular degeneration. Life Sci. 188:123–130. 2017.
View Article : Google Scholar
|
|
5
|
Coffe V, Carbajal RC and Salceda R:
Glucose metabolism in rat retinal pigment epithelium. Neurochem
Res. 31:103–108. 2006. View Article : Google Scholar
|
|
6
|
Kaur C, Foulds WS and Ling EA:
Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol.
2:879–889. 2008. View Article : Google Scholar
|
|
7
|
Ferris FL, Fine SL and Hyman L:
Age-related macular degeneration and blindness due to neovascular
maculopathy. Arch Ophthalmol. 102:164016421984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barchitta M and Maugeri A: Association
between vascular endothelial growth factor polymorphisms and
age-related macular degeneration: An updated meta-analysis. Dis
Markers. 2016:84864062016. View Article : Google Scholar
|
|
9
|
Yin F, Boveris A and Cadenas E:
Mitochondrial energy metabolism and redox signaling in brain aging
and neurodegeneration. Antioxid Redox Signal. 20:353–371. 2014.
View Article : Google Scholar
|
|
10
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: opposite interplay between the canonical WNT/β-catenin
pathway and PPAR Gamma: A potential therapeutic target in gliomas.
Neurosci Bull. 34:573–588. 2018. View Article : Google Scholar
|
|
11
|
Vallée A, Lecarpentier Y and Vallée JN:
Curcumin: A therapeutic strategy in cancers by inhibiting the
canonical WNT/β-catenin pathway. J Exp Clin Cancer Res. 38:3232019.
View Article : Google Scholar
|
|
12
|
Yeung AWK, Horbańczuk M, Tzvetkov NT,
Mocan A, Carradori S, Maggi F, Marchewka J, Sut S, Dall'Acqua S,
Gan RY, et al: Curcumin: Total-scale analysis of the scientific
literature. Molecules. 24:13932019. View Article : Google Scholar
|
|
13
|
Kao YW, Hsu SK, Chen JY, Lin IL, Chen KJ,
Lee PY, Ng HS, Chiu CC and Cheng KC: Curcumin metabolite
tetrahydrocurcumin in the treatment of eye diseases. Int J Mol Sci.
22:2122020. View Article : Google Scholar
|
|
14
|
Bhutto I and Lutty G: Understanding
age-related macular degeneration (AMD): Relationships between the
photoreceptor/retinal pigment epithelium/Bruch's
membrane/choriocapillaris complex. Mol Aspects Med. 33:295–317.
2012. View Article : Google Scholar
|
|
15
|
McLeod DS, Grebe R, Bhutto I, Merges C,
Baba T and Lutty GA: Relationship between RPE and choriocapillaris
in age-related macular degeneration. Invest Ophthalmol Vis Sci.
50:4982–4991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takata S, Masuda T, Nakamura S, Kuchimaru
T, Tsuruma K, Shimazawa M, Nagasawa H, Kizaka-Kondoh S and Hara H:
The effect of triamcinolone acetonide on laser-induced choroidal
neovascularization in mice using a hypoxia visualization
bio-imaging probe. Sci Rep. 5:98982015. View Article : Google Scholar
|
|
17
|
Sakurai E, Anand A, Ambati BK, van Rooijen
N and Ambati J: Macrophage depletion inhibits experimental
choroidal neovascularization. Invest Ophthalmol Vis Sci.
44:3578–3585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Indaram M, Ma W, Zhao L, Fariss RN,
Rodriguez IR and Wong WT: 7-Ketocholesterol increases retinal
microglial migration, activation, and angiogenicity: A potential
pathogenic mechanism underlying age-related macular degeneration.
Sci Rep. 5:91442015. View Article : Google Scholar
|
|
19
|
Terasaki H, Kase S, Shirasawa M, Otsuka H,
Hisatomi T, Sonoda S, Ishida S, Ishibashi T and Sakamoto T: TNF-α
decreases VEGF secretion in highly polarized RPE cells but
increases it in non-polarized RPE cells related to crosstalk
between JNK and NF-κB pathways. PLoS One. 8:e699942013. View Article : Google Scholar
|
|
20
|
Hu Y, Chen Y, Lin M, Lee K, Mott RA and Ma
J: Pathogenic role of the Wnt signaling pathway activation in
laser-induced choroidal neovascularization. Invest Ophthalmol Vis
Sci. 54:141–154. 2013. View Article : Google Scholar
|
|
21
|
Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu
F, Qian H, Shen D, Penalva R, Xu H, et al: Wnt signaling in
age-related macular degeneration: Human macular tissue and mouse
model. J Transl Med. 13:3302015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nussenblatt RB and Ferris F: Age-related
macular degeneration and the immune response: Implications for
therapy. Am J Ophthalmol. 144:618–626. 2007. View Article : Google Scholar
|
|
23
|
Radeke MJ, Radeke CM, Shih YH, Hu J, Bok
D, Johnson LV and Coffey PJ: Restoration of mesenchymal retinal
pigmented epithelial cells by TGFβ pathway inhibitors: Implications
for age-related macular degeneration. Genome Med. 7:582015.
View Article : Google Scholar
|
|
24
|
Lin CH, Li CH, Liao PL, Tse LS, Huang WK,
Cheng HW and Cheng YW: Silibinin inhibits VEGF secretion and
age-related macular degeneration in a hypoxia-dependent manner
through the PI-3 kinase/Akt/mTOR pathway. Br J Pharmacol.
168:920–931. 2013. View Article : Google Scholar
|
|
25
|
Ambati J: Age-related macular degeneration
and the other double helix. The Cogan lecture. Invest Ophthalmol
Vis Sci. 52:2165–2169. 2011. View Article : Google Scholar
|
|
26
|
Blasiak J, Petrovski G, Veréb Z, Facskó A
and Kaarniranta K: Oxidative stress, hypoxia, and autophagy in the
neovascular processes of age-related macular degeneration. Biomed
Res Int. 2014:7680262014. View Article : Google Scholar
|
|
27
|
Jaffe GJ, Eliott D, Wells JA, Prenner JL,
Papp A and Patel S: A Phase 1 study of Intravitreous E10030 in
combination with ranibizumab in neovascular age-related macular
degeneration. Ophthalmology. 123:78–85. 2016. View Article : Google Scholar
|
|
28
|
Kwak N, Okamoto N, Wood JM and Campochiaro
PA: VEGF is major stimulator in model of choroidal
neovascularization. Invest Ophthalmol Vis Sci. 41:3158–3164.
2000.
|
|
29
|
Brown DM, Kaiser PK, Michels M, Soubrane
G, Heier JS, Kim RY, Sy JP and Schneider S; ANCHOR Study Group:
Ranibizumab versus verteporfin for neovascular age-related macular
degeneration. N Engl J Med. 355:1432–1444. 2006. View Article : Google Scholar
|
|
30
|
Rosenfeld PJ, Brown DM, Heier JS, Boyer
DS, Kaiser PK, Chung CY and Kim RY; MARINA Study Group: Ranibizumab
for neovascular age-related macular degeneration. N Engl J Med.
355:1419–1431. 2006. View Article : Google Scholar
|
|
31
|
Zhang X, Gaspard JP and Chung DC:
Regulation of vascular endothelial growth factor by the Wnt and
K-ras pathways in colonic neoplasia. Cancer Res. 61:6050–6054.
2001.PubMed/NCBI
|
|
32
|
Katoh Y and Katoh M: Comparative
integromics on VEGF family members. Int J Oncol. 28:1585–1589.
2006.
|
|
33
|
Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B,
Gao G and Ma J: The pathogenic role of the canonical Wnt pathway in
age-related macular degeneration. Invest Ophthalmol Vis Sci.
51:4371–4379. 2010. View Article : Google Scholar :
|
|
34
|
Ma B and Hottiger MO: Crosstalk between
Wnt/β-catenin and NF-κB signaling pathway during inflammation.
Front Immunol. 7:3782016. View Article : Google Scholar
|
|
35
|
Wang H and Hartnett ME: Regulation of
signaling events involved in the pathophysiology of neovascular
AMD. Mol Vis. 22:189–202. 2016.
|
|
36
|
Al-Harthi L: Wnt/β-catenin and its diverse
physiological cell signaling pathways in neurodegenerative and
neuropsychiatric disorders. J Neuroimmune Pharmacol. 7:725–730.
2012. View Article : Google Scholar
|
|
37
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar
|
|
38
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar
|
|
39
|
Fuhrmann S: Wnt signaling in eye
organogenesis. Organogenesis. 4:60–67. 2008. View Article : Google Scholar
|
|
40
|
Fujimura N: WNT/β-catenin signaling in
vertebrate eye development. Front Cell Dev Biol. 4:1382016.
View Article : Google Scholar
|
|
41
|
Machon O, Kreslova J, Ruzickova J, Vacik
T, Klimova L, Fujimura N, Lachova J and Kozmik Z: Lens
morphogenesis is dependent on Pax6-mediated inhibition of the
canonical Wnt/beta-catenin signaling in the lens surface ectoderm.
Genesis. 48:86–95. 2010.
|
|
42
|
Carpenter AC, Smith AN, Wagner H,
Cohen-Tayar Y, Rao S, Wallace V, Ashery-Padan R and Lang RA: Wnt
ligands from the embryonic surface ectoderm regulate 'bimetallic
strip' optic cup morphogenesis in mouse. Development. 142:972–982.
2015. View Article : Google Scholar
|
|
43
|
Hägglund AC, Berghard A and Carlsson L:
Canonical Wnt/β-catenin signalling is essential for optic cup
formation. PLoS One. 8:e811582013. View Article : Google Scholar
|
|
44
|
Birdsey GM, Shah AV, Dufton N, Reynolds
LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana
E, et al: The endothelial transcription factor ERG promotes
vascular stability and growth through Wnt/β-catenin signaling. Dev
Cell. 32:82–96. 2015. View Article : Google Scholar
|
|
45
|
Ye X, Wang Y, Cahill H, Yu M, Badea TC,
Smallwood PM, Peachey NS and Nathans J: Norrin, frizzled-4, and
Lrp5 signaling in endothelial cells controls a genetic program for
retinal vascularization. Cell. 139:285–298. 2009. View Article : Google Scholar
|
|
46
|
Zhou Y, Wang Y, Tischfield M, Williams J,
Smallwood PM, Rattner A, Taketo MM and Nathans J: Canonical WNT
signaling components in vascular development and barrier formation.
J Clin Invest. 124:3825–3846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang W, Li Q, Amiry-Moghaddam M, Hokama
M, Sardi SH, Nagao M, Warman ML and Olsen BR: Critical endothelial
regulation by LRP5 during retinal vascular development. PLoS One.
11:e01528332016. View Article : Google Scholar
|
|
48
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar
|
|
49
|
Nusse R: Wnt signaling. Cold Spring Harb
Perspect Biol. 4:a0111632012. View Article : Google Scholar
|
|
50
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar
|
|
51
|
Sprowl-Tanio S, Habowski AN, Pate KT,
McQuade MM, Wang K, Edwards RA, Grun F, Lyou Y and Waterman ML:
Lactate/pyruvate transporter MCT-1 is a direct Wnt target that
confers sensitivity to 3-bromopyruvate in colon cancer. Cancer
Metab. 4:202016. View Article : Google Scholar :
|
|
52
|
Pate KT, Stringari C, Sprowl-Tanio S, Wang
K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell
MA, et al: Wnt signaling directs a metabolic program of glycolysis
and angiogenesis in colon cancer. EMBO J. 33:1454–1473. 2014.
View Article : Google Scholar :
|
|
53
|
Gao C, Xiao G and Hu J: Regulation of
Wnt/β-catenin signaling by posttranslational modifications. Cell
Biosci. 4:132014. View Article : Google Scholar
|
|
54
|
Cruciat CM and Niehrs C: Secreted and
transmembrane Wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5:a0150812013. View Article : Google Scholar
|
|
55
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: Beta-catenin is a target for the ubiquitin-proteasome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar
|
|
56
|
Wu D and Pan W: GSK3: A multifaceted
kinase in Wnt signaling. Trends Biochem Sci. 35:161–168. 2010.
View Article : Google Scholar :
|
|
57
|
Hur EM and Zhou FQ: GSK3 signalling in
neural development. Nat Rev Neurosci. 11:539–551. 2010. View Article : Google Scholar
|
|
58
|
Ambacher KK, Pitzul KB, Karajgikar M,
Hamilton A, Ferguson SS and Cregan SP: The JNK- and
AKT/GSK3β-signaling pathways converge to regulate puma induction
and neuronal apoptosis induced by trophic factor deprivation. PLoS
One. 7:e468852012. View Article : Google Scholar
|
|
59
|
Yokosako K, Mimura T, Funatsu H, Noma H,
Goto M, Kamei Y, Kondo A and Matsubara M: Glycolysis in patients
with age-related macular degeneration. Open Ophthalmol J. 8:39–47.
2014. View Article : Google Scholar
|
|
60
|
Grossniklaus HE, Kang SJ and Berglin L:
Animal models of choroidal and retinal neovascularization. Prog
Retin Eye Res. 29:500–519. 2010. View Article : Google Scholar
|
|
61
|
Wang Z, Liu CH, Huang S and Chen J: Wnt
Signaling in vascular eye diseases. Prog Retin Eye Res. 70:110–133.
2019. View Article : Google Scholar
|
|
62
|
Wang Z, Cheng R, Lee K, Tyagi P, Ding L,
Kompella UB, Chen J, Xu X and Ma JX: Nanoparticle-mediated
expression of a wnt pathway inhibitor ameliorates ocular
neovascularization. Arterioscler Thromb Vasc Biol. 35:855–864.
2015. View Article : Google Scholar
|
|
63
|
Chen Y, Hu Y, Lu K, Flannery JG and Ma JX:
Very low density lipoprotein receptor, a negative regulator of the
wnt signaling pathway and choroidal neovascularization. J Biol
Chem. 282:34420–34428. 2007. View Article : Google Scholar
|
|
64
|
Lin JB, Sene A, Wiley LA, Santeford A,
Nudleman E, Nakamura R, Lin JB, Moolani HV and Apte RS: WNT7A/B
promote choroidal neovascularization. Exp Eye Res. 174:107–112.
2018. View Article : Google Scholar
|
|
65
|
Park K, Lee K, Zhang B, Zhou T, He X, Gao
G, Murray AR and Ma JX: Identification of a novel inhibitor of the
canonical Wnt pathway. Mol Cell Biol. 31:3038–3051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai Z, Lu L, Yang Z, Mao Y, Lu J, Li C, Qi
W, Chen Y, Yao Y, Li L, et al: Kallikrein-binding protein inhibits
LPS-induced TNF-α by upregulating SOCS3 expression. J Cell Biochem.
114:1020–1028. 2013. View Article : Google Scholar
|
|
67
|
Zhang J, Yang Z, Li P, Bledsoe G, Chao L
and Chao J: Kallistatin antagonizes Wnt/β-catenin signaling and
cancer cell motility via binding to low-density lipoprotein
receptor-related protein 6. Mol Cell Biochem. 379:295–301. 2013.
View Article : Google Scholar
|
|
68
|
Lu SL, Tsai C Y, Luo YH, Kuo C F, Lin WC,
Chang YT, Wu JJ, Chuang WJ, Liu CC, Chao L, et al: Kallistatin
modulates immune cells and confers anti-inflammatory response to
protect mice from Group A streptococcal infection. Antimicrob
Agents Chemother. 57:5366–5372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
McBride JD, Jenkins AJ, Liu X, Zhang B,
Lee K, Berry WL, Janknecht R, Griffin CT, Aston CE, Lyons TJ, et
al: Elevated circulation levels of an antiangiogenic SERPIN in
patients with diabetic microvascular complications impair wound
healing through suppression of Wnt signaling. J Invest Dermatol.
134:1725–1734. 2014. View Article : Google Scholar
|
|
70
|
Bach RR: Initiation of coagulation by
tissue factor. CRC Crit Rev Biochem. 23:339–368. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tuo J, Bojanowski CM, Zhou M, Shen D, Ross
RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, et al:
Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking
human age-related macular degeneration. Invest Ophthalmol Vis Sci.
48:3827–3836. 2007. View Article : Google Scholar
|
|
72
|
Chan CC, Ross RJ, Shen D, Ding X, Majumdar
Z, Bojanowski CM, Zhou M, Salem N Jr, Bonner R and Tuo J:
Ccl2/Cx3cr1-deficient mice: An animal model for age-related macular
degeneration. Ophthalmic Res. 40:124–128. 2008. View Article : Google Scholar
|
|
73
|
Chu XK, Wang Y, Ardeljan D, Tuo J and Chan
CC: Controversial view of a genetically altered mouse model of
focal retinal degeneration. Bioengineered. 4:130–135. 2013.
View Article : Google Scholar :
|
|
74
|
Tuo J, Ross RJ, Herzlich AA, Shen D, Ding
X, Zhou M, Coon SL, Hussein N, Salem N Jr and Chan CC: A high
omega-3 fatty acid diet reduces retinal lesions in a murine model
of macular degeneration. Am J Pathol. 175:799–807. 2009. View Article : Google Scholar
|
|
75
|
Tuo J, Pang JJ, Cao X, Shen D, Zhang J,
Scaria A, Wadsworth SC, Pechan P, Boye SL, Hauswirth WW and Chan
CC: AAV5-mediated sFLT01 gene therapy arrests retinal lesions in
Ccl2(-/-)/Cx3cr1(-/-) mice. Neurobiol Aging. 33. pp. 433.e1–e10.
2012, View Article : Google Scholar
|
|
76
|
Zhang J, Tuo J, Cao X, Shen D, Li W and
Chan CC: Early degeneration of photoreceptor synapse in
Ccl2/Cx3cr1-deficient mice on Crb1(rd8) background. Synapse.
67:515–531. 2013. View Article : Google Scholar
|
|
77
|
Clemons TE, Milton RC, Klein R and Seddon
JM: Risk factors for the incidence of advanced age-related macular
degeneration in the age-related eye disease study (AREDS) AREDS
report no. 19. Ophthalmology. 112:533–539. 2005. View Article : Google Scholar
|
|
78
|
Wang Y, Sang A, Zhu M, Zhang G, Guan H, Ji
M and Chen H: Tissue factor induces VEGF expression via activation
of the Wnt/β-catenin signaling pathway in ARPE-19 cells. Mol Vis.
22:886–897. 2016.
|
|
79
|
Qiu F, Liu Z, Zhou Y, He J, Gong S, Bai X,
Zeng Y, Liu Z and Ma JX: Decreased circulating levels of dickkopf-1
in patients with exudative age-related macular degeneration. Sci
Rep. 7:12632017. View Article : Google Scholar
|
|
80
|
Voorzanger-Rousselot N, Goehrig D, Facon
T, Clézardin P and Garnero P: Platelet is a major contributor to
circulating levels of Dickkopf-1: Clinical implications in patients
with multiple myeloma. Br J Haematol. 145:264–266. 2009. View Article : Google Scholar
|
|
81
|
Esen E, Chen J, Karner CM, Okunade AL,
Patterson BW and Long F: WNT-LRP5 signaling induces Warburg effect
through mTORC2 activation during osteoblast differentiation. Cell
Metab. 17:745–755. 2013. View Article : Google Scholar
|
|
82
|
Pate KT, Stringari C, Sprowl-Tanio S, Wang
K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell
MA, et al: Wnt signaling directs a metabolic program of glycolysis
and angiogenesis in colon cancer. EMBO J. 33:1454–1473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Thompson CB: Wnt meets Warburg: Another
piece in the puzzle? EMBO J. 33:1420–1422. 2014. View Article : Google Scholar
|
|
84
|
Lum JJ, Bui T, Gruber M, Gordan JD,
DeBerardinis RJ, Covello KL, Simon MC and Thompson CB: The
transcription factor HIF-1alpha plays a critical role in the growth
factor-dependent regulation of both aerobic and anaerobic
glycolysis. Genes Dev. 21:1037–1049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Manea A: NADPH oxidase-derived reactive
oxygen species: Involvement in vascular physiology and pathology.
Cell Tissue Res. 342:325–339. 2010. View Article : Google Scholar
|
|
86
|
Radomska-Leśniewska DM, Hevelke A,
Skopiński P, Bałan B, Jóźwiak J, Rokicki D, Skopińska-Różewska E
and Białoszewska A: Reactive oxygen species and synthetic
antioxidants as angiogenesis modulators: Clinical implications.
Pharmacol Rep. 68:462–471. 2016. View Article : Google Scholar
|
|
87
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
88
|
Kim YW, West XZ and Byzova TV:
Inflammation and oxidative stress in angiogenesis and vascular
disease. J Mol Med (Berl). 91:323–328. 2013. View Article : Google Scholar
|
|
89
|
Brambilla D, Mancuso C, Scuderi MR, Bosco
P, Cantarella G, Lempereur L, Di Benedetto G, Pezzino S and
Bernardini R: The role of antioxidant supplement in immune system,
neoplastic, and neurodegenerative disorders: A point of view for an
assessment of the risk/benefit profile. Nutr J. 7:292008.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar
|
|
91
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar :
|
|
92
|
Vallée A, Lecarpentier Y, Vallée R,
Guillevin R and Vallée JN: Circadian rhythms in exudative
age-related macular degeneration: The key role of the canonical
WNT/β-catenin pathway. Int J Mol Sci. 21:8202020. View Article : Google Scholar
|
|
93
|
Brugarolas JB, Vazquez F, Reddy A, Sellers
WR and Kaelin WG: TSC2 regulates VEGF through mTOR-dependent and
-independent pathways. Cancer Cell. 4:147–158. 2003. View Article : Google Scholar
|
|
94
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005. View Article : Google Scholar
|
|
96
|
Land SC and Tee AR: Hypoxia-inducible
factor 1alpha is regulated by the mammalian target of rapamycin
(mTOR) via an mTOR signaling motif. J Biol Chem. 282:20534–20543.
2007. View Article : Google Scholar
|
|
97
|
Toschi A, Lee E, Gadir N, Ohh M and Foster
DA: Differential dependence of hypoxia-inducible factors 1 alpha
and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 283:34495–34499.
2008. View Article : Google Scholar
|
|
98
|
Xu Q, Briggs J, Park S, Niu G, Kortylewski
M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al:
Targeting Stat3 blocks both HIF-1 and VEGF expression induced by
multiple oncogenic growth signaling pathways. Oncogene.
24:5552–5560. 2005. View Article : Google Scholar
|
|
99
|
Kim J, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar
|
|
100
|
Suda T, Takubo K and Semenza GL: Metabolic
regulation of hematopoietic stem cells in the hypoxic niche. Cell
Stem Cell. 9:298–310. 2011. View Article : Google Scholar
|
|
101
|
Firth JD, Ebert BL and Ratcliffe PJ:
Hypoxic regulation of lactate dehydrogenase A. Interaction between
hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem.
270:21021–21027. 1995. View Article : Google Scholar
|
|
102
|
Lewis BC, Shim H, Li Q, Wu CS, Lee LA,
Maity A and Dang CV: Identification of putative c-Myc-responsive
genes: Characterization of rcl, a novel growth-related gene. Mol
Cell Biol. 17:4967–4978. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Semenza GL, Jiang BH, Leung SW, Passantino
R, Concordet JP, Maire P and Giallongo A: Hypoxia response elements
in the Aldolase A, Enolase 1, and lactate dehydrogenase A gene
promoters contain essential binding sites for hypoxia-inducible
factor 1. J Biol Chem. 271:32529–32537. 1996. View Article : Google Scholar
|
|
104
|
Shim H, Dolde C, Lewis BC, Wu CS, Dang G,
Jungmann RA, Dalla-Favera R and Dang CV: c-Myc transactivation of
LDH-A: implications for tumor metabolism and growth. Proc Natl Acad
Sci USA. 94:6658–6663. 1997. View Article : Google Scholar
|
|
105
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar
|
|
106
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Bougioukas G, Didilis V, Gatter KC and Harris AL; Tumour and
Angiogenesis Research Group: Lactate dehydrogenase-5 (LDH-5)
overexpression in non-small-cell lung cancer tissues is linked to
tumour hypoxia, angiogenic factor production and poor prognosis. Br
J Cancer. 89:877–885. 2003. View Article : Google Scholar
|
|
107
|
Vallée A, Lecarpentier Y, Guillevin R and
Vallée JN: Aerobic glycolysis hypothesis through WNT/beta-catenin
pathway in exudative age-related macular degeneration. J Mol
Neurosci. 62:368–379. 2017. View Article : Google Scholar
|
|
108
|
Léveillard T and Sahel JA: Metabolic and
redox signaling in the retina. Cell Mol Life Sci. 74:3649–3665.
2017. View Article : Google Scholar
|
|
109
|
Oguma K, Oshima H and Oshima M:
Inflammation, tumor necrosis factor and Wnt promotion in gastric
cancer development. Future Oncol. 6:515–526. 2010. View Article : Google Scholar
|
|
110
|
Schön S, Flierman I, Ofner A, Stahringer
A, Holdt LM, Kolligs FT and Herbst A: β-catenin regulates NF-κB
activity via TNFRSF19 in colorectal cancer cells. Int J Cancer.
135:1800–1811. 2014. View Article : Google Scholar
|
|
111
|
Oh H, Takagi H, Takagi C, Suzuma K, Otani
A, Ishida K, Matsumura M, Ogura Y and Honda Y: The potential
angiogenic role of macrophages in the formation of choroidal
neovascular membranes. Invest Ophthalmol Vis Sci. 40:1891–1898.
1999.
|
|
112
|
Cousins SW, Espinosa-Heidmann DG and Csaky
KG: Monocyte activation in patients with age-related macular
degeneration: A biomarker of risk for choroidal neovascularization?
Arch Ophthalmol. 122:1013–1018. 2004. View Article : Google Scholar
|
|
113
|
Duguid IG, Boyd AW and Mandel TE: Adhesion
molecules are expressed in the human retina and choroid. Curr Eye
Res. 11(Suppl): S153–S159. 1992. View Article : Google Scholar
|
|
114
|
Elner SG, Elner VM, Pavilack MA, Todd RF
III, Mayo-Bond L, Franklin WA, Strieter RM, Kunkel SL and Huber AR:
Modulation and function of intercellular adhesion molecule-1 (CD54)
on human retinal pigment epithelial cells. Lab Invest. 66:200–211.
1992.
|
|
115
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar
|
|
116
|
Donoso LA, Kim D, Frost A, Callahan A and
Hageman G: The role of inflammation in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 51:137–152.
2006. View Article : Google Scholar
|
|
117
|
Easwaran V, Lee SH, Inge L, Guo L,
Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, et
al: Beta-Catenin regulates vascular endothelial growth factor
expression in colon cancer. Cancer Res. 63:3145–3153. 2003.
|
|
118
|
Ip MS, Scott IU, Brown GC, Brown MM, Ho
AC, Huang SS and Recchia FM; American Academy of Ophthalmology:
Anti-vascular endothelial growth factor pharmacotherapy for
age-related macular degeneration: A report by the American Academy
of Ophthalmology. Ophthalmology. 115:1837–1846. 2008. View Article : Google Scholar
|
|
119
|
Wolf S: Current status of anti-vascular
endothelial growth factor therapy in Europe. Jpn J Ophthalmol.
52:433–439. 2008. View Article : Google Scholar
|
|
120
|
Menon G and Walters G: New paradigms in
the treatment of wet AMD: The impact of anti-VEGF therapy. Eye
(Lond). 23(Suppl 1): S1–S7. 2009. View Article : Google Scholar
|
|
121
|
Grisanti S, Zhu Q, Tatar O, Lueke J, Lueke
M, Tura A and Grisanti S: Differential expression of vascular
endothelial growth factor-a isoforms in neovascular age-related
macular degeneration. Retina. 35:764–772. 2015. View Article : Google Scholar
|
|
122
|
Liu X: Overstimulation can create health
problems due to increases in PI3K/Akt/GSK3 insensitivity and GSK3
activity. Springerplus. 3:3562014. View Article : Google Scholar
|
|
123
|
Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou
J and Du H: Inhibition of VEGF expression by targeting HIF-1 alpha
with small interference RNA in human RPE cells. Ophthalmologica.
221:411–417. 2007. View Article : Google Scholar
|
|
124
|
Zhang P, Zhang X, Hao X, Wang Y, Hui Y,
Wang H, Hu D and Zhou J: Rac1 activates HIF-1 in retinal pigment
epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol.
247:633–639. 2009. View Article : Google Scholar
|
|
125
|
Arjamaa O, Nikinmaa M, Salminen A and
Kaarniranta K: Regulatory role of HIF-1alpha in the pathogenesis of
age-related macular degeneration (AMD). Ageing Res Rev. 8:349–358.
2009. View Article : Google Scholar
|
|
126
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T,
Laurent D, et al: Prognostic and predictive role of lactate
dehydrogenase 5 expression in colorectal cancer patients treated
with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin
Cancer Res. 17:4892–4900. 2011. View Article : Google Scholar
|
|
127
|
Giatromanolaki A, Sivridis E, Gatter KC,
Turley H, Harris AL and Koukourakis MI; Tumour and Angiogenesis
Research Group: Lactate dehydrogenase 5 (LDH-5) expression in
endometrial cancer relates to the activated VEGF/VEGFR2(KDR)
pathway and prognosis. Gynecol Oncol. 103:912–918. 2006. View Article : Google Scholar
|
|
128
|
Kolev Y, Uetake H, Takagi Y and Sugihara
K: Lactate dehydrogenase-5 (LDH-5) expression in human gastric
cancer: Association with hypoxia-inducible factor (HIF-1alpha)
pathway, angiogenic factors production and poor prognosis. Ann Surg
Oncol. 15:2336–2344. 2008. View Article : Google Scholar
|
|
129
|
Dhup S, Dadhich RK, Porporato PE and
Sonveaux P: Multiple biological activities of lactic acid in
cancer: Influences on tumor growth, angiogenesis and metastasis.
Curr Pharm Des. 18:1319–1330. 2012. View Article : Google Scholar
|
|
130
|
Polet F and Feron O: Endothelial cell
metabolism and tumour angiogenesis: Glucose and glutamine as
essential fuels and lactate as the driving force. J Intern Med.
273:156–165. 2013. View Article : Google Scholar
|
|
131
|
San-Millán I and Brooks GA: Reexamining
cancer metabolism: Lactate production for carcinogenesis could be
the purpose and explanation of the Warburg Effect. Carcinogenesis.
38:119–133. 2017.
|
|
132
|
Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun
D and Zhai G: Oral bioavailability of curcumin: problems and
advancements. J Drug Target. 24:694–702. 2016. View Article : Google Scholar
|
|
133
|
Vallée A and Lecarpentier Y: Curcumin and
endometriosis. Int J Mol Sci. 21:24402020. View Article : Google Scholar :
|
|
134
|
Kotha RR and Luthria DL: Curcumin:
Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects.
Molecules. 24:29302019. View Article : Google Scholar
|
|
135
|
Prasad S, Gupta SC, Tyagi AK and Aggarwal
BB: Curcumin, a component of golden spice: From bedside to bench
and back. Biotechnol Adv. 32:1053–1064. 2014. View Article : Google Scholar
|
|
136
|
Priyadarsini KI: The chemistry of
curcumin: From extraction to therapeutic agent. Molecules.
19:20091–20112. 2014. View Article : Google Scholar
|
|
137
|
Zhang L, Zhu W, Yang C, Guo H, Yu A, Ji J,
Gao Y, Sun M and Zhai G: A novel folate-modified
self-microemulsifying drug delivery system of curcumin for colon
targeting. Int J Nanomedicine. 7:151–162. 2012.
|
|
138
|
Shen L, Liu CC, An CY and Ji HF: How does
curcumin work with poor bioavailability? Clues from experimental
and theoretical studies. Sci Rep. 6:208722016. View Article : Google Scholar :
|
|
139
|
Sun M, Su X, Ding B, He X, Liu X, Yu A,
Lou H and Zhai G: Advances in nanotechnology-based delivery systems
for curcumin. Nanomedicine (Lond). 7:1085–1100. 2012. View Article : Google Scholar
|
|
140
|
Naksuriya O, Okonogi S, Schiffelers RM and
Hennink WE: Curcumin nanoformulations: A review of pharmaceutical
properties and preclinical studies and clinical data related to
cancer treatment. Biomaterials. 35:3365–3383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Malam Y, Loizidou M and Seifalian AM:
Liposomes and nanoparticles: Nanosized vehicles for drug delivery
in cancer. Trends Pharmacol Sci. 30:592–599. 2009. View Article : Google Scholar
|
|
142
|
Lee WH, Loo CY, Young PM, Traini D, Mason
RS and Rohanizadeh R: Recent advances in curcumin nanoformulation
for cancer therapy. Expert Opin Drug Deliv. 11:1183–1201. 2014.
View Article : Google Scholar
|
|
143
|
Hatefi A and Amsden B: Biodegradable
injectable in situ forming drug delivery systems. J Control
Release. 80:9–28. 2002. View Article : Google Scholar
|
|
144
|
Yallapu MM, Jaggi M and Chauhan SC:
Beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery
in prostate cancer cells. Colloids Surf B Biointerfaces.
79:113–125. 2010. View Article : Google Scholar
|
|
145
|
Radomska-Leśniewska DM, Osiecka-Iwan A,
Hyc A, Góźdź A, Dąbrowska AM and Skopiński P: Therapeutic potential
of curcumin in eye diseases. Cent Eur J Immunol. 44:181–189. 2019.
View Article : Google Scholar
|
|
146
|
Zhu W, Wu Y, Meng YF, Wang JY, Xu M, Tao
JJ and Lu J: Effect of curcumin on aging retinal pigment epithelial
cells. Drug Des Devel Ther. 9:5337–5344. 2015.
|
|
147
|
Jiang X, Li S, Qiu X, Cong J, Zhou J and
Miu W: Curcumin inhibits cell viability and increases apoptosis of
SW620 human colon adenocarcinoma cells via the caudal type
homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit.
25:7451–7458. 2019. View Article : Google Scholar
|
|
148
|
Howell JC, Chun E, Farrell AN, Hur EY,
Caroti CM, Iuvone PM and Haque R: Global microRNA expression
profiling: Curcumin (diferuloylmethane) alters oxidative
stress-responsive microRNAs in human ARPE-19 cells. Mol Vis.
19:544–560. 2013.
|
|
149
|
Mandal MNA, Patlolla JMR, Zheng L, Agbaga
MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV and
Anderson RE: Curcumin protects retinal cells from light-and oxidant
stress-induced cell death. Free Radic Biol Med. 46:672–679. 2009.
View Article : Google Scholar
|
|
150
|
Muangnoi C, Sharif U, Ratnatilaka Na,
Bhuket P, Rojsitthisak P and Paraoan L: Protective effects of
curcumin ester prodrug, curcumin diethyl disuccinate against
H2O2-Induced oxidative stress in human
retinal pigment epithelial cells: Potential therapeutic avenues for
age-related macular degeneration. Int J Mol Sci. 20:33672019.
View Article : Google Scholar
|
|
151
|
Kim HJ, Park SY, Park OJ and Kim YM:
Curcumin suppresses migration and proliferation of Hep3B
hepatocarcinoma cells through inhibition of the Wnt signaling
pathway. Mol Med Rep. 8:282–286. 2013. View Article : Google Scholar
|
|
152
|
Leow PC, Bahety P, Boon CP, Lee CY, Tan
KL, Yang T and Ee PL: Functionalized curcumin analogs as potent
modulators of the Wnt/β-catenin signaling pathway. Eur J Med Chem.
71:67–80. 2014. View Article : Google Scholar
|
|
153
|
Kolb TM and Davis MA: The tumor promoter
12-O-tetradecanoylphorbol 13-acetate (TPA) provokes a prolonged
morphologic response and ERK activation in Tsc2-null renal tumor
cells. Toxicol Sci. 81:233–242. 2004. View Article : Google Scholar
|
|
154
|
Balasubramanyam K, Varier RA, Altaf M,
Swaminathan V, Siddappa NB, Ranga U and Kundu TK: Curcumin, a novel
p300/CREB-binding protein-specific inhibitor of acetyltransferase,
represses the acetylation of histone/nonhistone proteins and
histone acetyltransferase-dependent chromatin transcription. J Biol
Chem. 279:51163–51171. 2004. View Article : Google Scholar
|
|
155
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK and Tang L: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Menon VP and Sudheer AR: Antioxidant and
anti-inflammatory properties of curcumin. Adv Exp Med Biol.
595:105–125. 2007. View Article : Google Scholar
|
|
157
|
Marchiani A, Rozzo C, Fadda A, Delogu G
and Ruzza P: Curcumin and curcumin-like molecules: From spice to
drugs. Curr Med Chem. 21:204–222. 2014. View Article : Google Scholar
|
|
158
|
Schneider C, Boeglin WE, Yin H, Stec DF
and Voehler M: Convergent oxygenation of arachidonic acid by
5-lipoxygenase and cyclooxygenase-2. J Am Chem Soc. 128:720–721.
2006. View Article : Google Scholar
|
|
159
|
Giménez-Bastida JA, González-Sarrías A,
Laparra-Llopis JM, Schneider C and Espín JC: Targeting mammalian
5-lipoxygenase by dietary phenolics as an anti-inflammatory
mechanism: A systematic review. Int J Mol Sci. 22:79372021.
View Article : Google Scholar :
|
|
160
|
Othman A, Ahmad S, Megyerdi S, Mussell R,
Choksi K, Maddipati KR, Elmarakby A, Rizk N and Al-Shabrawey M:
12/15-Lipoxygenase-derived lipid metabolites induce retinal
endothelial cell barrier dysfunction: Contribution of NADPH
oxidase. PLoS One. 8:e572542013. View Article : Google Scholar
|
|
161
|
Subramanian P, Mendez EF and Becerra SP: A
novel inhibitor of 5-Lipoxygenase (5-LOX) prevents oxidative
stress-induced cell death of retinal pigment epithelium (RPE)
cells. Invest Ophthalmol Vis Sci. 57:4581–4588. 2016. View Article : Google Scholar
|
|
162
|
Yadav UCS and Ramana KV: Regulation of
NF-κB-induced inflammatory signaling by lipid peroxidation-derived
aldehydes. Oxid Med Cell Longev. 2013:6905452013. View Article : Google Scholar
|
|
163
|
Ruan Y, Jiang S and Gericke A: Age-related
macular degeneration: Role of oxidative stress and blood vessels.
Int J Mol Sci. 22:12962021. View Article : Google Scholar :
|
|
164
|
Yabas M, Orhan C, Er B, Tuzcu M, Durmus
AS, Ozercan IH, Sahin N, Bhanuse P, Morde AA, Padigaru M and Sahin
K: A next generation formulation of curcumin ameliorates
experimentally induced osteoarthritis in rats via regulation of
inflammatory mediators. Front Immunol. 12:6096292021. View Article : Google Scholar :
|
|
165
|
Li X, Lu Y, Jin Y, Son JK, Lee SH and
Chang HW: Curcumin inhibits the activation of immunoglobulin
e-mediated mast cells and passive systemic anaphylaxis in mice by
reducing serum eicosanoid and histamine levels. Biomol Ther
(Seoul). 22:27–34. 2014. View Article : Google Scholar
|
|
166
|
Manjunatha H and Srinivasan K: Protective
effect of dietary curcumin and capsaicin on induced oxidation of
low-density lipoprotein, iron-induced hepatotoxicity and
carrageenan-induced inflammation in experimental rats. FEBS J.
273:4528–4537. 2006. View Article : Google Scholar
|
|
167
|
Priyadarsini KI, Maity DK, Naik GH, Kumar
MS, Unnikrishnan MK, Satav JG and Mohan H: Role of phenolic O-H and
methylene hydrogen on the free radical reactions and antioxidant
activity of curcumin. Free Radic Biol Med. 35:475–484. 2003.
View Article : Google Scholar
|
|
168
|
Piwocka K, Jaruga E, Skierski J, Gradzka I
and Sikora E: Effect of glutathione depletion on caspase-3
independent apoptosis pathway induced by curcumin in Jurkat cells.
Free Radic Biol Med. 31:670–678. 2001. View Article : Google Scholar
|
|
169
|
Motterlini R, Foresti R, Bassi R and Green
CJ: Curcumin, an antioxidant and anti-inflammatory agent, induces
heme oxygenase-1 and protects endothelial cells against oxidative
stress. Free Radic Biol Med. 28:1303–1312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Cao K, Dong YT, Xiang J, Xu Y, Hong W,
Song H and Guan ZZ: Reduced expression of SIRT1 and SOD-1 and the
correlation between these levels in various regions of the brains
of patients with Alzheimer's disease. J Clin Pathol. 71:1090–1099.
2018. View Article : Google Scholar
|
|
171
|
Golestaneh N, Chu Y, Cheng SK, Cao H,
Poliakov E and Berinstein DM: Repressed SIRT1/PGC-1α pathway and
mitochondrial disintegration in iPSC-derived RPE disease model of
age-related macular degeneration. J Transl Med. 14:3442016.
View Article : Google Scholar
|
|
172
|
Zuo L, Khan RS, Lee V, Dine K, Wu W and
Shindler KS: SIRT1 promotes RGC survival and delays loss of
function following optic nerve crush. Invest Ophthalmol Vis Sci.
54:5097–5102. 2013. View Article : Google Scholar
|
|
173
|
Li K, Zhai M, Jiang L, Song F, Zhang B, Li
J, Li H, Li B, Xia L, Xu L, et al: Tetrahydrocurcumin ameliorates
diabetic cardiomyopathy by attenuating high glucose-induced
oxidative stress and fibrosis via activating the SIRT1 Pathway.
Oxid Med Cell Longev. 2019:67469072019. View Article : Google Scholar
|
|
174
|
Ghasemi F, Shafiee M, Banikazemi Z,
Pourhanifeh MH, Khanbabaei H, Shamshirian A, Amiri Moghadam S,
ArefNezhad R, Sahebkar A, Avan A and Mirzaei H: Curcumin inhibits
NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol
Res Pract. 215:1525562019. View Article : Google Scholar
|
|
175
|
Olivera A, Moore TW, Hu F, Brown AP, Sun
A, Liotta DC, Snyder JP, Yoon Y, Shim H, Marcus AI, et al:
Inhibition of the NF-κB signaling pathway by the curcumin analog,
3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inf
lammatory and anti-cancer properties. Int Immunopharmacol.
12:368–377. 2012. View Article : Google Scholar
|
|
176
|
da Cruz BO, Cardozo LFM de F, Magliano DC
and Stockler-Pinto MB: Nutritional strategies to modulate
inflammation pathways via regulation of peroxisome
proliferator-activated receptor β/δ. Nutr Rev. 78:207–214.
2020.
|
|
177
|
Lin YG, Kunnumakkara AB, Nair A, Merritt
WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM,
Lutgendorf SK, et al: Curcumin inhibits tumor growth and
angiogenesis in ovarian carcinoma by targeting the nuclear
factor-kappaB pathway. Clin Cancer Res. 13:3423–3430. 2007.
View Article : Google Scholar
|
|
178
|
Zhang ZB, Luo DD, Xie JH, Xian YF, Lai ZQ,
Liu YH, Liu WH, Chen JN, Lai XP, Lin ZX and Su ZR: Curcumin's
metabolites, tetrahydrocurcumin and octahydrocurcumin, possess
superior anti-inflammatory effects in vivo through suppression of
TAK1-NF-κB pathway. Front Pharmacol. 9:11812018. View Article : Google Scholar
|
|
179
|
Chen W, Chen Y and Cui G: Effects of
TNF-alpha and curcumin on the expression of VEGF in Raji and U937
cells and on angiogenesis in ECV304 cells. Chin Med J (Engl).
118:2052–2057. 2005.
|
|
180
|
Yoysungnoen P, Wirachwong P, Bhattarakosol
P, Niimi H and Patumraj S: Effects of curcumin on tumor
angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular
carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc.
34:109–115. 2006.PubMed/NCBI
|
|
181
|
Li L, Braiteh FS and Kurzrock R:
Liposome-encapsulated curcumin: In vitro and in vivo effects on
proliferation, apoptosis, signaling, and angiogenesis. Cancer.
104:1322–1331. 2005. View Article : Google Scholar
|
|
182
|
Arbiser JL, Klauber N, Rohan R, van
Leeuwen R, Huang MT, Fisher C, Flynn E and Byers HR: Curcumin is an
in vivo inhibitor of angiogenesis. Mol Med. 4:376–383. 1998.
View Article : Google Scholar
|
|
183
|
Gururaj AE, Belakavadi M, Venkatesh DA,
Marmé D and Salimath BP: Molecular mechanisms of anti-angiogenic
effect of curcumin. Biochem Biophys Res Commun. 297:934–942. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zahra FT, Sajib MS and Mikelis CM: Role of
bFGF in acquired resistance upon Anti-VEGF therapy in cancer.
Cancers (Basel). 13:14222021. View Article : Google Scholar
|
|
185
|
Compagni A, Wilgenbus P, Impagnatiello MA,
Cotten M and Christofori G: Fibroblast growth factors are required
for efficient tumor angiogenesis. Cancer Res. 60:7163–7169.
2000.
|
|
186
|
Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao
X, Wetterskog D, Funa K, Bråkenhielm E and Cao Y: Angiogenic
factors FGF2 and PDGF-BB synergistically promote murine tumor
neovascularization and metastasis. J Clin Invest. 117:2766–2777.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar
|
|
188
|
Choi HJ, Armaiz Pena GN, Pradeep S, Cho
MS, Coleman RL and Sood AK: Anti-vascular therapies in ovarian
cancer: Moving beyond anti-VEGF approaches. Cancer Metastasis Rev.
34:19–40. 2015. View Article : Google Scholar :
|
|
189
|
Aggarwal BB and Natarajan K: Tumor
necrosis factors: Developments during the last decade. Eur Cytokine
Netw. 7:93–124. 1996.PubMed/NCBI
|
|
190
|
Li H, Soria C, Griscelli F, Opolon P,
Soria J, Yeh P, Legrand C, Vannier JP, Belin D, Perricaudet M and
Lu H: Amino-terminal fragment of urokinase inhibits tumor cell
invasion in vitro and in vivo: Respective contribution of the
urokinase plasminogen activator receptor-dependent or -independent
pathway. Hum Gene Ther. 16:1157–1167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.
|
|
192
|
Wang LL, Sun Y, Huang K and Zheng L:
Curcumin, a potential therapeutic candidate for retinal diseases.
Mol Nutr Food Res. 57:1557–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Pittalà V, Fidilio A, Lazzara F, Platania
CBM, Salerno L, Foresti R, Drago F and Bucolo C: Effects of novel
nitric oxide-releasing molecules against oxidative stress on
retinal pigmented epithelial cells. Oxid Med Cell Longev.
2017:14208922017. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Bucolo C, Drago F, Maisto R, Romano GL,
D'Agata V, Maugeri G and Giunta S: Curcumin prevents high glucose
damage in retinal pigment epithelial cells through ERK1/2-mediated
activation of the Nrf2/HO-1 pathway. J Cell Physiol.
234:17295–17304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Vyas A, Dandawate P, Padhye S, Ahmad A and
Sarkar F: Perspectives on New Synthetic curcumin analogs and their
potential anticancer properties. Curr Pharm Des. 19:2047–2069.
2013.
|
|
196
|
Muangnoi C, Ratnatilaka Na Bhuket P,
Jithavech P, Supasena W, Paraoan L, Patumraj S and Rojsitthisak P:
Curcumin diethyl disuccinate, a prodrug of curcumin, enhances
anti-proliferative effect of curcumin against HepG2 cells via
apoptosis induction. Sci Rep. 9:117182019. View Article : Google Scholar :
|
|
197
|
Ohori H, Yamakoshi H, Tomizawa M, Shibuya
M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C,
et al: Synthesis and biological analysis of new curcumin analogues
bearing an enhanced potential for the medicinal treatment of
cancer. Mol Cancer Ther. 5:2563–2571. 2006. View Article : Google Scholar
|
|
198
|
Shoba G, Joy D, Joseph T, Majeed M,
Rajendran R and Srinivas PS: Influence of piperine on the
pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. 64:353–356. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Sai N, Dong X, Huang P, You L, Yang C, Liu
Y, Wang W, Wu H, Yu Y, Du Y, et al: A novel gel-forming solution
based on PEG-DSPE/Solutol HS 15 Mixed Micelles and Gellan Gum for
ophthalmic delivery of curcumin. Molecules. 25:812019. View Article : Google Scholar
|
|
200
|
Zhang J, Sun H, Zhou N, Zhang B and Ma J:
Preparation and evaluation of biodegradable scleral plug containing
curcumin in rabbit eye. Curr Eye Res. 42:1597–1603. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Mazzolani F, Togni S, Giacomelli L,
Eggenhoffner R and Franceschi F: Oral administration of a
curcumin-phospholipid formulation (Meriva®) for treatment of
chronic diabetic macular edema: A pilot study. Eur Rev Med
Pharmacol Sci. 22:3617–3625. 2018.PubMed/NCBI
|
|
202
|
Allegri P, Mastromarino A and Neri P:
Management of chronic anterior uveitis relapses: Efficacy of oral
phospholipidic curcumin treatment. Long-term follow-up. Clin
Ophthalmol. 4:1201–1206. 2010.
|
|
203
|
Chen J and Smith LEH: Retinopathy of
prematurity. Angiogenesis. 10:133–140. 2007. View Article : Google Scholar
|