Introduction
Asthma is a chronic inflammatory disease
characterized by variable respiratory symptoms and airflow
limitation (1). According to the
Global Initiative for Asthma (GINA), ~300 million individuals
worldwide suffer from asthma and the number is estimated to reach
400 million by the year 2025 (2). As a result, the socio-economic
burden of this disease is increasing. Corticosteroids and
bronchodilators continue to play a major role in the treatment of
asthma (3,4); however, symptom control is poor in
~10% of patients (5).
Furthermore, asthma attacks are common and are associated with an
accelerated and permanent loss of lung function (6). Therefore, effective prevention
measures and novel therapies are required for the more effective
control of symptoms and for the reduction of asthma attacks in
patients with asthma.
As a novel cytokine, interleukin (IL)-27 was
identified as a new member of the IL-6/IL-12 family (7). IL-27 is a heterodimeric molecule
that consists of EBV-induced gene 3 (EBi3) and p28 subunit, and is
primarily produced from activated antigen-presenting cells and
macrophages (7,8). By binding to its receptor [composed
of WSX-1 (T-cell cytokine receptor) and gp130], IL-27 activates
signal transducer and activator of transcription (STAT)1 (8,9)
and thereby functions as a promoter of T-helper 1 (Th1)
differentiation (10,11), and a suppressor of Th2 and Th17
differentiation (12,13). Group 2 innate lymphoid cells
(ILC2) also play a crucial role in allergic inflammation and IL-27
suppresses the proliferation of ILC2 cells in lung tissue (14). Thus, IL-27 may be involved in
allergic inflammatory diseases, including asthma.
Previous studies have found that the levels of IL-27
in serum are altered in patients with acute asthma and are
associated with alterations in lung function (15,16). Jirmo et al (17) reported that IL-27 enhanced the
secretion of IFN-γ, whereas it decreased that of IL-5 and IL-13,
demonstrating its critical role in the control of allergic asthma.
In a previous study by the authors, it was demonstrated that the
preventative intranasal administration of IL-27 alleviated airway
inflammation and remodeling in mouse models of asthma by restoring
both the STAT1 and STAT3 pathways (18). However, the associated mechanisms
have not yet been fully elucidated and required further
investigation. As a distinct subset of T-cells, T regulatory type 1
(Tr1) cells are characterized by the ability to secrete high levels
of IL-10 and the lack of the forkhead box P3 (Foxp3) expression
(19,20). Tr1 cells play a crucial role in
maintaining peripheral tolerance and preventing T-cell-mediated
diseases (21). However, the
effects of IL-27 on Tr1 cells have not yet been investigated in
asthma, at least to the best of our knowledge. Based on the mouse
model of ovalbumin (OVA)-induced airway inflammation, Yoshimoto
et al (22) found that
the intranasal administration of IL-27 reduced OVA-induced airway
hyperresponsiveness (AHR) and inflammation. However, Su et
al (23) reported that IL-27
alleviated airway inflammation and improved the pathological
changes when it was preventatively administered, while IL-27 did
not affect AHR and airway inflammation when delivered
therapeutically. Therefore, whether the therapeutic administration
of IL-27 can suppress airway inflammation, AHR, and airway
remodeling in a mouse model of asthma remains unclear.
In the present study, the effects of the therapeutic
intranasal administration of IL-27 on airway inflammation, AHR and
airway remodeling were investigated in mouse models of OVA-induced
asthma. Additionally, the inflammatory environment in the lungs of
mice was evaluated based on the analysis of bronchoalveolar lavage
(BAL) fluid (BALF) and lung tissue from mice which received IL-27
prophylactically. Finally, the main signaling pathways involved in
the anti-inflammatory effects of IL-27 were examined.
Materials and methods
Animals
A total of 48 healthy female BALB/c mice (6-8 weeks
old, weighing 12-14 g) were purchased from the Experimental Animal
Center of Shandong University (Jinan, China). The animals were
housed in pathogen-free and standard conditions (12-h light/dark
cycle, room temperature of 22°C and a relative humidity of 60%).
The mice were randomly divided into three groups as follows: the
phosphate-buffered saline (PBS) group (n=8), the OVA group (n=8)
and the OVA and IL-27 group (OVA + IL-27, n=8). All animal
procedures were conducted according to the National Institutes of
Health (NIH) Guide for the Care and Use of Laboratory Animals
(24). Moreover, all protocols
were approved by the Ethics Committee for Laboratory Animals Care
and Use in First Affiliated Hospital of Shandong First Medical
University, Jinan, China.
Experimental model of acute asthma
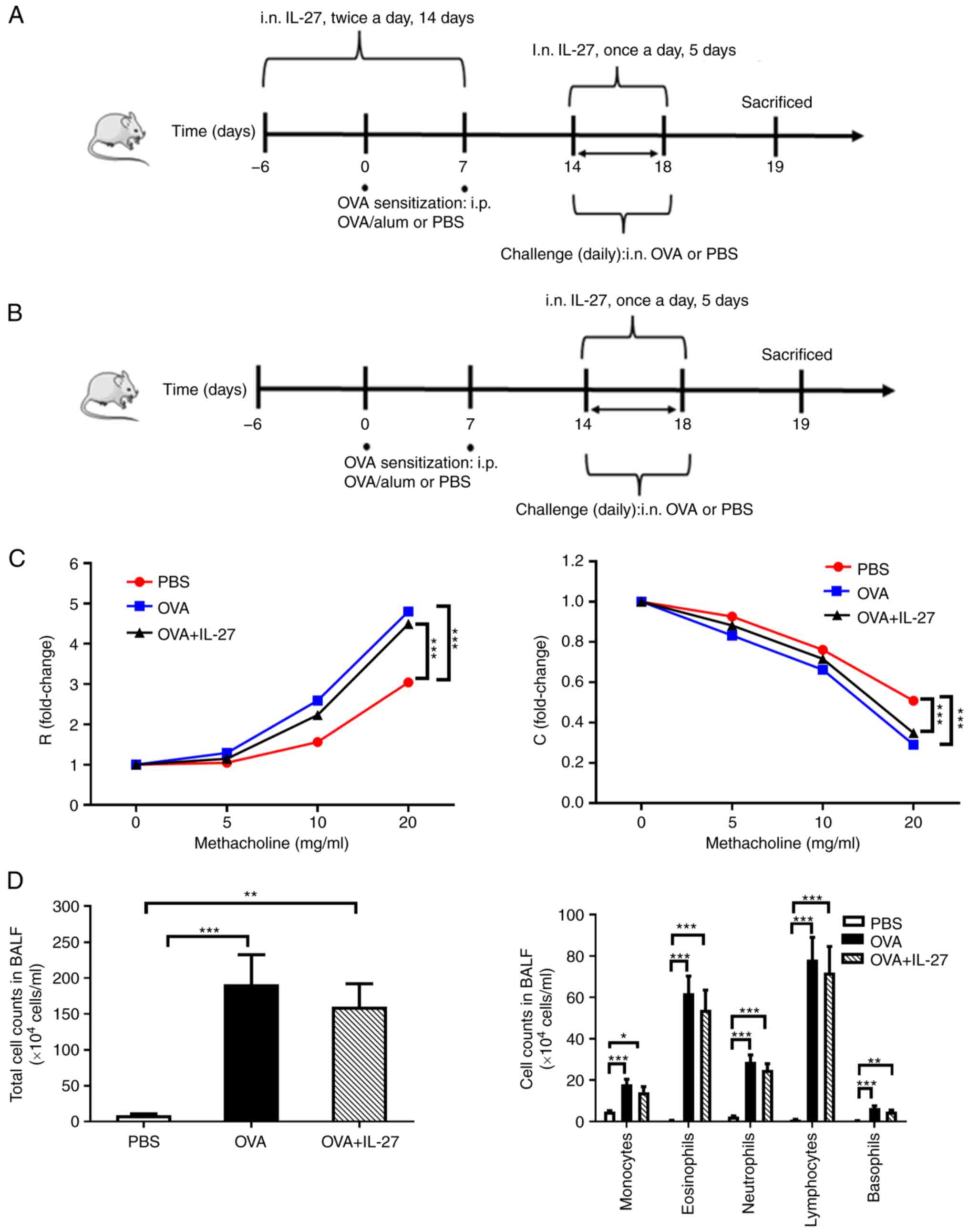
Mice were exposed to OVA as previously described
(25,26) to establish a model of acute
asthma. When IL-27 was administered in a preventative manner to the
mice in the OVA + IL-27 group (Fig.
1A), the mice received 50 µl PBS and 50 ng IL-27
intranasally twice a day from day-6 to day 7. However, the mice
received 50 µl PBS alone on the same days when IL-27 was
delivered in a therapeutic manner in the OVA and PBS groups
(Fig. 1B). The mice were then
sensitized with intraperitoneal (i.p.) injections of 100 µg
OVA (Sigma-Aldrich; Merck KGaA), 2 mg alum (Thermo Fisher
Scientific, Inc.) and 100 µl sterile endotoxin-free PBS
(Invitrogen; Thermo Fisher Scientific, Inc.) on days 0 and 7 in the
OVA and OVA + IL-27 groups. The mice received PBS instead of OVA on
the same days in the PBS group. In the OVA group, the mice were
challenged with 50 µl PBS and 100 µg OVA intranasally
from days 14 to 18 under light isoflurane (3%) anesthesia (27). In the OVA + IL-27 group, the mice
were treated intranasally with 50 µl PBS and 1 µg
IL-27 1 h prior to OVA sensitization and subsequent challenge (50
µl PBS and 100 µg OVA) on days 0, 7 and 14-18. The
mice received PBS alone on the same days in the PBS group. Each
group included 8 mice.
Experimental model of chronic asthma
To establish the phenotype in chronic murine models
of allergic asthma, the mice were treated with OVA as previously
described (28). Briefly, 20
µg OVA/1 mg Alum/200 µl PBS were delivered
subcutaneously (s.c.) to mice in the OVA and OVA + IL-27 groups on
days 0, 7, 14 and 21, while 1 mg alum/200 µl PBS was
administered to the mice in the PBS group. On days 26, 27 and 28,
and on the following 4 weeks (twice a week), the mice were
challenged intranasally with 20 mg of either OVA in 50 µl
PBS (OVA and OVA + IL-27 group) or 50 µl PBS alone (PBS
group) following anesthesia (27). In the OVA + IL-27 group, the mice
received 50 µl PBS and 20 ng IL-27 intranasally 1 h before
the OVA challenge on days 0, 7, 26-28, 35, 38, 42, 49, 52, 56 and
59 (please refer to the protocol scheme illustrated in Fig. 2A). Each group included 8
mice.
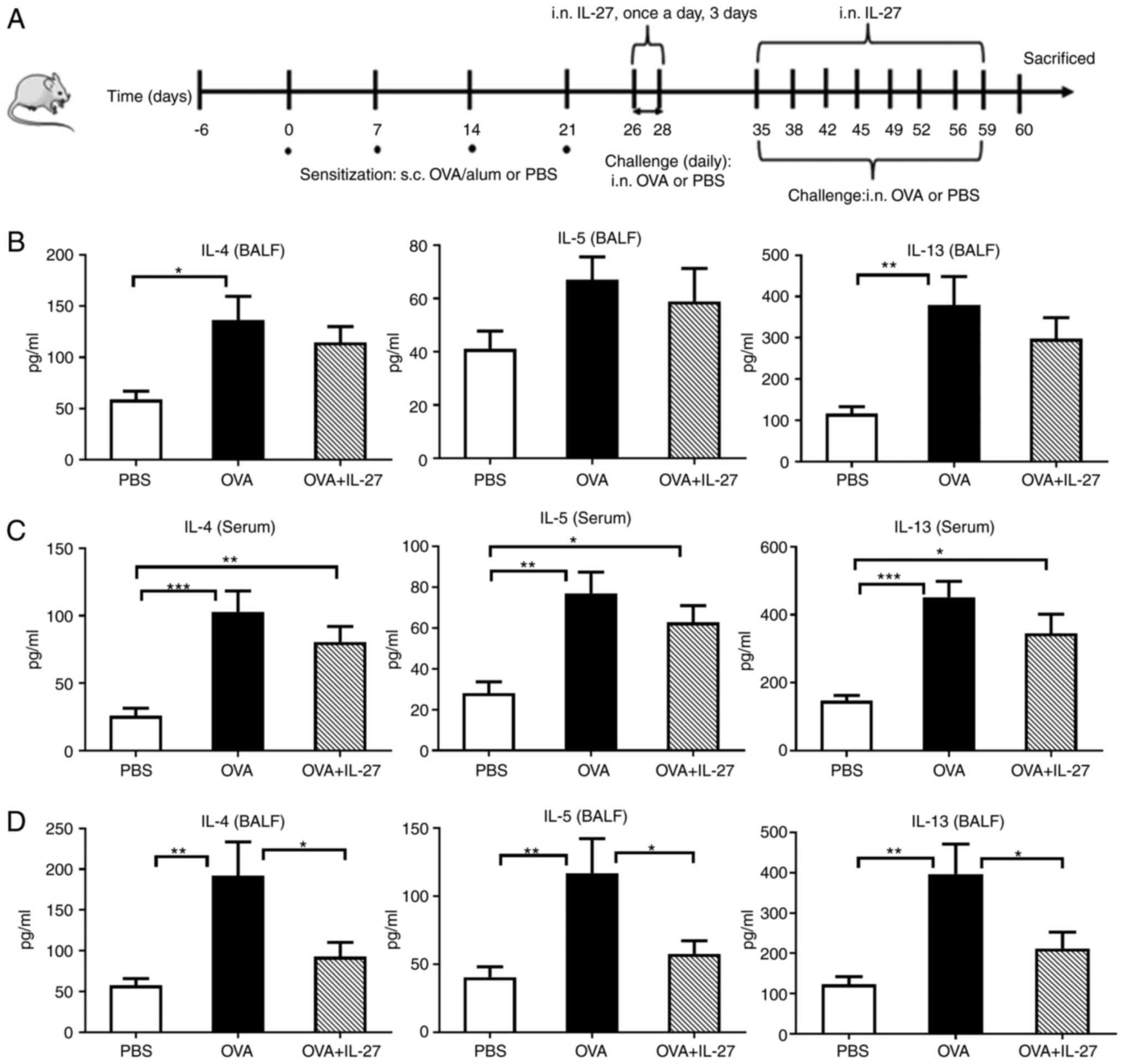 | Figure 2Levels of IL-4, IL-5 and IL-13 in
BALF and serum samples. (A) Protocol of OVA-induced allergic asthma
and administration of IL-27. (B and C) The levels of IL-4, IL-5 and
IL-13 were measured using ELISA when IL-27 was administered in a
therapeutic manner. The concentrations of IL-4, IL-5 and IL-13
exhibited no significant differences in BALF and serum between the
OVA + IL-27 group and OVA group. (D) The levels of IL-4, IL-5 and
IL-13 in the BALF were measured using ELISA when IL-27 was used in
a preventive manner. The levels of IL-4, IL-5 and IL-13 were lower
in the OVA + IL-27 group compared with the OVA group. The data are
expressed as the mean ± SEM of three independent experiments, with
8 animals per group. *P<0.05, **P<0.01,
***P<0.001. BALF, bronchoalveolar lavage fluid;
ELISA, enzyme linked immunosorbent assay. |
Measurement of airway responsiveness
AHR is one of the characteristic features of asthma
(29). In the present study, AHR
was assessed using invasive techniques 24 h following the final OVA
challenge, as previously described (30). In brief, the mice were
anesthetized with pentobarbital sodium (50 mg/kg, i.p. injection)
and their responses to nociceptive stimulation and movement were
assessed in order to determine the depth of anesthesia (27). A tracheostomy was performed and
the mice were connected to the flexiVent system (SCIREQ Scientific
Respiratory Equipment, Inc.). The mice were then mechanically
ventilated according to the following parameters: tidal volume, 5
ml/kg; breathing rate, 150 breaths/min; positive end-expiratory
pressure, 3 cmH2O (31). Subsequently, the mice were
challenged with aerosol saline followed by increasing
concentrations of acetyl-β-methylcholine chloride (methacholine; 0,
5, 10 and 20 mg/ml; Sigma-Aldrich; Merck KGaA) for 10 sec at each
dose. Airway resistance and lung compliance were presented as a
percentage change relative to baseline levels (saline
challenge).
BAL and inflammatory cell analysis in
BALF
At 24 h after the final OVA challenge and
immediately following the AHR assessments, the animals were
euthanized by cervical dislocation and the verification of death
was based on a combination of criteria, including the absence of
breathing, pulse, corneal reflex and response to a firm toe pinch.
After the left main bronchus was clamped, BAL was immediately
performed in the right lung and BALF was subsequently collected and
processed, as previously described (12,32). The BALF was centrifuged (80 × g
for 10 min at 4°C) and the cell pellets were resuspended in 1 ml
PBS-EDTA (Sigma-Aldrich; Merck KGaA). The total number of BALF
cells and BALF differential cell counts were determined on cytospin
slide preparations which were stained with Wright-Giemsa (Beyotime
Institute of Biotechnology) at room temperature for 8 min. A total
of 200 cells per slide were counted per sample under a light
microscope (DP73; Olympus corporation) at ×400 magnification
(33).
Enzyme-linked immunosorbent assay
(ELISA)
IL-4, IL-5 and IL-13 are typical allergic
asthma-associated cytokines which initiate and promote airway
inflammation, mucus overproduction, immunoglobulin E (IgE)
synthesis and fibrosis (34,35). Therefore, in the present study,
ELISA kits (R&D Systems, Inc.) were used to evaluate the levels
of IL-4 (cat. no. M4000B), IL-5 (cat. no. M5000) and IL-13 (cat.
no. dY413) in the serum and in the supernatant of BALF according to
a standard protocol as previously described (36).
Cell isolation for flow cytometry
Cell isolation from the lungs was performed as
previously described (37,38). In brief, the right lung was
excised after the ligation of the left main bronchus. The whole
lungs were minced, homogenized and subsequently incubated with
shaking for 30 min at 37°C with the collagenase type IV solution
(cat. no. C8160; Beijing Solarbio Science & Technology Co.,
Ltd.). The supernatant was removed and then filtered through a
nylon mesh with pore size 48 µm (cat. no. YA0691; Beijing
Solarbio Science & Technology Co., Ltd.), and the resulting
cells were washed in complete medium [CM; RPMI-1640 + 10%
heat-inactivated fetal bovine serum + 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer +
10 mM non-essential amino acids + 10 mM sodium pyruvate + 10 U/ml
penicillin/streptomycin; all from Sigma-Aldrich]. Subsequently, the
isolated cells were enriched by density gradient centrifugation at
800 × g for 30 min at 4°C (Percoll, Sigma-Aldrich; Merck KGaA) and
they were re-suspended in CM for further analysis.
Flow cytometry
The antibodies used in the present study included
the following: fluorescein isothiocyanate (FITC)-labeled anti-mouse
CD4 (1:100; cat. no. 100510), APC-labeled anti-mouse CD49b (1:100;
cat. no. 103516) and PerCP/Cyanine 5.5 anti-mouse CD223 (LAG-3)
(1:100; cat. no. 125212) (all purchased from BioLegend). The cells
were stained in fluorescence-activated cell sorting (FACS) buffer
at 4°C in the dark for 30 min with mixed the antibodies. After
washing, 2% formaldehyde was added to the system at 4°C for 30 min
and the cells were analyzed using a flow cytometer (FACs Verse, BD
Biosciences) and FlowJo software (version 10.4, Tree Star, Inc.)
(39).
Reverse transcription-quantitative PCR
(RT-qPCR)
After the BAL was collected, the left lungs were
immediately excised and total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), as per the manufacturer's protocol. The RNA concentration
was determined using a Nanodrop™ Nd-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). The synthesis of complementary DNA (cDNA)
was conducted using a Superscript III First-Strand Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) at 50°C for 50 min.
qPCR was then performed using TB Green Premix Ex Taq II (Tli RNaseH
Plus; cat. no. RR820A, Takara Bio, Inc.) according to the
manufacturer's instructions. The thermocycling conditions comprised
an initial denaturation at 94°C for 5 min, 40 cycles of 10 sec at
94°C and 20 sec at 60°C, and a final extension of 30 sec at 72°C.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
reference control gene. An ABI 7000 PCR instrument (Thermo Fisher
Scientific, Inc.) was used to quantify the mRNA. The relative
abundance of the mRNA transcripts was determined using the
2−∆∆Cq method (40).
Each sample was analyzed in triplicate and at least three duplicate
wells were used for each group. The sequences of primer and the
expected size of the PCR products are presented in Table SI.
Western blot analysis
Western blot analysis was conducted as previously
described (41). In brief, the
mouse lung tissues were lysed in RIPA buffer (50 mM Tris pH 7.4,
150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate and 0.1% SDS;
Beyotime Institute of Biotechnology) supplemented with protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The bicinchoninic
acid (BCA) protein assay kit (cat. no. 23225; Pierce; Thermo Fisher
Scientific, Inc.) was used to determine protein concentration.
Proteins were separated on 10% acrylamide SDS-PAGE gels. A total of
30 µg protein was loaded per lane. Following
electrophoresis, the isolated proteins were transferred onto PVDF
membranes (EMD Millipore). After blocking with 5% (w/v) dried
skimmed milk at 37°C for 1 h, the membranes were then incubated
overnight at 4°C with primary antibodies (all 1:500) against STAT1
(cat. no. sc-464), phosphorylated (p)-STAT1 (cat. no. sc-8394),
STAT3 (cat. no. sc-482), p-STAT3 (cat. no. sc-8059) and β-tubulin
(cat. no. sc-47778; all from Santa Cruz Biotechnology, Inc.),
separately. Following five washes with PBST (PBS and 0.15%
Tween-20), the membranes were incubated with goat anti-rabbit
IgG-HRP secondary antibody (cat. no. sc-2004; 1:5,000; Santa Cruz
Biotechnology, Inc.) at 37°C for 45 min. β-tubulin was used as an
internal control. The protein lanes were detected using ECL
detection reagents kit (GE Healthcare; Cytiva) as per the
manufacturer's instructions. Densitometry was performed using
ImageJ software (v1.8.0; National Institutes of Health).
Lung histological analysis
The left lungs were excised and were subsequently
prepared for histological analysis, as previously described
(42). The lung tissue samples
were fixed in 4% paraformaldehyde at room temperature for 24 h. The
samples were dehydrated, cleared and embedded in paraffin for
sectioning. The lung sections (5-µm-thick) were processed
and stained with hematoxylin and eosin (H&E), Masson's
trichrome and periodic-acid schiff (PAS) stain following a standard
procedure (43). For H&E
staining, the sections were immersed in 1% hematoxylin (Yuanmu
Biotechnology Co., Ltd.) for 5 min and washed in distilled water. A
1% hydrochloric acid-alcohol solution was used to clear the
residual dye, and 0.5% eosin was then used to stain the sections
(Huihong Reagent Co., Ltd, China) at room temperature for 3 min.
The histology sections were evaluated and scored independently by
two experienced pathologists who were blinded to the identity of
the experimental groups. The H&E-stained lung sections were
used mainly to assess thickening of the basal membrane and
hyperplasia of the airway smooth muscle cells. In total, three
bronchioles that were 150-200 µm in inner diameter were
selected and examined. The perimeter of the basement membrane
(Pbm), the total area of the airway wall (Wat) and the area of
smooth muscle (Wam) were measured by morphometric analysis
(Image-Pro Plus 6.0; Media cybernetics, Inc.) and the ratios of Wat
to Pbm (Wat/Pbm) and Wam to Pbm (Wam/Pbm) were calculated (43).
Masson's trichrome-stained slides were used to
determine subepithelial fibrosis around the airways of the mice.
The sections were stained in Biebrich scarlet-acid fuchsin solution
(Thermo Fisher Scientific, Inc.) at room temperature for 10 min and
washed in distilled water. Subsequently, the sections were
differentiated in phosphomolybdic-phosphotungstic acid solution
(Thermo Fisher Scientific, Inc.) for 10 min and then transferred
directly to aniline blue solution (Thermo Fisher Scientific, Inc.)
and stained at room temperature for 5 min. In total, three sections
in which the epithelial basement membranes of the bronchioles were
1.0-2.5 mm and the diameter of bronchial wall was >250 µm
were selected. The area of collagen fiber (stained in blue) beneath
the basement membrane and Pbm were measured using Image-Pro Plus
6.0 software and the results are expressed as the fibrotic area per
unit length (area of Masson-positive/Pbm) (44).
To evaluate the degree of goblet cell hyperplasia,
the lung sections were stained with PAS. Sections were immersed in
PAS solution (Thermo Fisher Scientific, Inc.) for 10 min and washed
four times with distilled water. They were then immersed in
Schiff's solution (Thermo Fisher Scientific, Inc.) for 15 min and
successively in hematoxylin for 3 min (both at room temperature),
followed by washing with distilled water. The bronchioles with
1.0-2.5 mm long epithelial basement membrane were selected and the
area of goblet cells (PAS-positive stained) was measured using
Image-Pro Plus 6.0 software. Subsequently, the mean score of the
area of PAS-positive staining divided by Pbm was calculated
(45).
To assess the degree of myofibroblast activation and
angiogenesis, the lung sections were heated, blocked with 3%
hydrogen peroxide (Thermo Fisher Scientific, Inc.) for 15 min at
room temperature, and then incubated with primary antibody α-smooth
muscle actin (α-SMA) (1:200; cat. no. sc-15320; Santa Cruz
Biotechnology, Inc.) and CD31 (1:100; cat. no. sc-28188; Santa Cruz
Biotechnology, Inc.) at 4°C for 12 h, respectively. After rinsing
with PBS, the sections were incubated with secondary antibody from
the MaxVision™ HRP-Polymer anti-mouse/rabbit IHC kit (Maixin Group
China Co. Ltd.) at 37°C for 60 min. The peribronchial
a-SMA-positive and CD31-positive areas in the sub-mucosa were
measured using Image-Pro Plus 6.0 software and the results are
expressed as the area of α-SMA and CD31-positive staining per mm of
Pbm of bronchi (46). All the
sections were observed using a BX51 microscopic imaging system
(Olympus Corporation).
Statistical analysis
Data are presented as the mean ± SEM. Statistical
analysis was performed using PRISM version 6 (GraphPad, Inc.).
Comparisons between multiple groups were performed using one-way
ANOVA with the Bonferroni post-test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Therapeutic administration of IL-27 does
not improve airway inflammation, AHR and airway remodeling in a
mouse model of OVA asthma
To investigate whether IL-27 exerts an inhibitory
effect on airway inflammation and AHR, IL-27 was administered to
mice with acute asthma in the final 5 consecutive days of the
experiment (Fig. 1B). As shown
in Fig. 1C, IL-27 was unable to
alleviate AHR, a hallmark of asthma (ROVA + IL-27 vs.
ROVA: 4.49 vs. 4.80, t=2.22, P>0.05; COVA + IL-27 vs.
COVA: 0.31 vs. 0.33, t=2.78, P>0.05). Furthermore,
IL-27 did not decrease the numbers of total cells [OVA + IL-27 vs.
OVA (×104 cells/ml): 191.40 vs. 160.00, t=0.74,
P>0.05] or those of differential cells [OVA + IL-27 vs. OVA
(×104 cells/ml) for monocytes: 14.19 vs. 18.06, t=1.31;
eosinophils: 54.03 vs. 61.95, t=0.77; neutrophils: 24.79 vs. 28.79,
t=1.04; lymphocytes: 71.94 vs. 78.23, t=0.46; basophils: 4.78 vs.
6.48, t=1.53; all P>0.05] in BALF (Fig. 1D). It also did not significantly
decrease the levels of Th2 cytokines in BALF and serum (OVA + IL-27
vs. OVA), such as IL-4 [BALF (pg/ml): 114.60 vs. 136.20, t=0.91;
serum (pg/ml): 80.58 vs. 102.90, t=1.37, both P>0.05], IL-5
[BALF (pg/ml): 58.87 vs. 61.11, t=0.61; serum (pg/ml): 62.80 vs.
72.07, t=1.22, both P>0.05] and IL-13 [BALF (pg/ml): 297.70.6
vs. 380.00, t=1.16; serum (pg/ml): 345.40 vs. 451.90, t=1.75, both
P>0.05] (Fig. 2B and C).
Moreover, the effects of the therapeutic administration of IL-27 on
airway remodeling were assessed in mice with chronic asthma using
different staining methods. As depicted in Fig. 3, no significant differences were
found between the OVA and OVA + IL-27 groups. Taken together, these
results demonstrated that IL-27 did not effectively suppress airway
inflammation, AHR and airway remodeling in mice with OVA-sensitized
allergic asthma when administrated in a therapeutic manner.
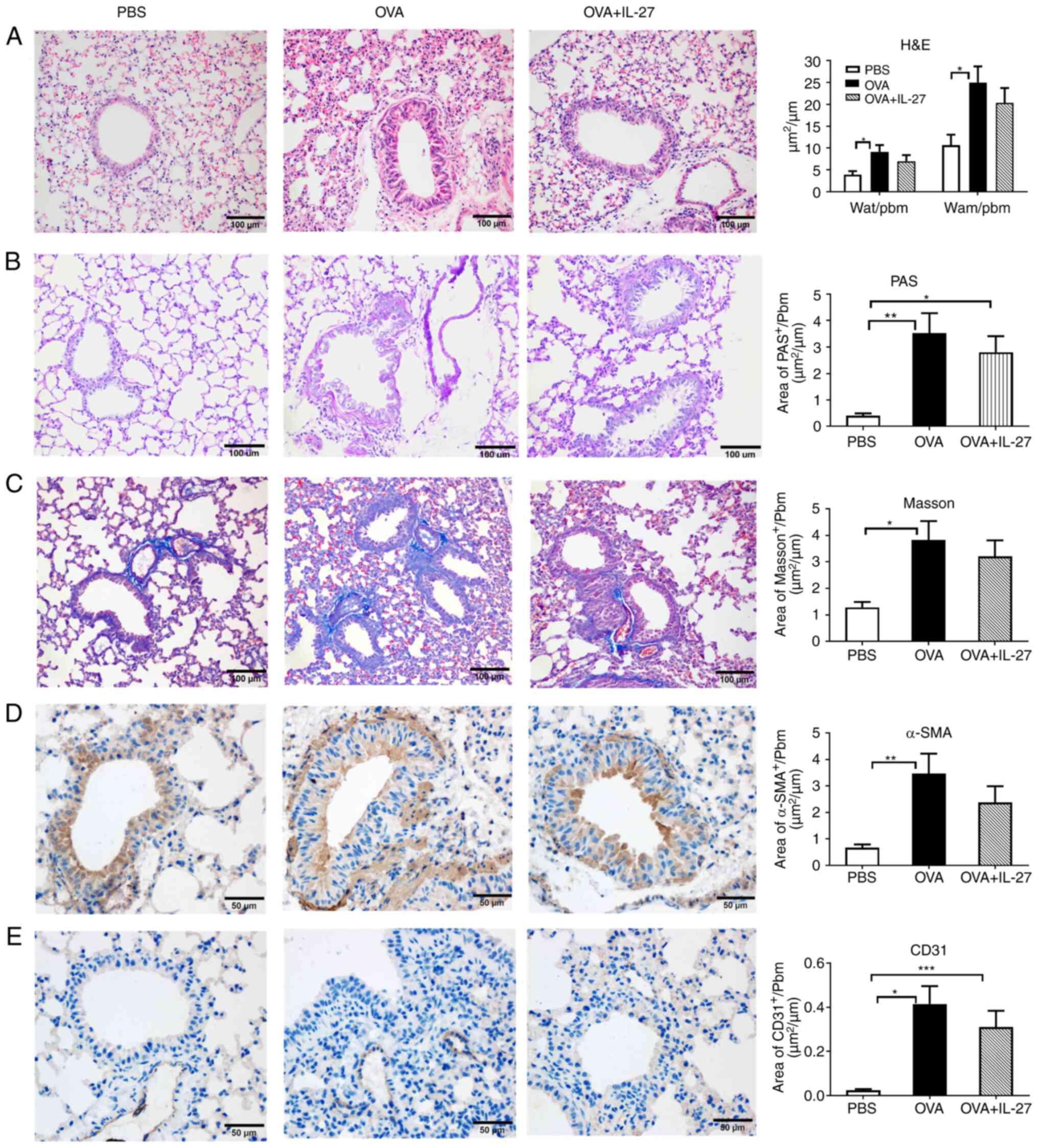 | Figure 3Therapeutic administration of IL-27
does not ameliorate OVA-induced airway remodeling in a chronic
experimental model of asthma. (A) Representative photomicrographs
of H&E-stained lung sections from each group (magnification,
×200). No significant difference was observed in basal membrane
thickening and the hyperplasia of airway smooth muscle cells
between the OVA + IL-27 group and the OVA group. (B) Representative
photomicrographs of PAS-stained lung sections from each group
(magnification, ×200). No significant difference was found in
airway mucus production between the OVA + IL-27 group and the OVA
group. (C) Representative photomicrographs of Masson's
trichrome-stained sections from each group (magnification, ×200).
No obvious difference was found in subepithelial fibrosis between
the OVA + IL-27 group and the OVA group. (D) Representative
photomicrographs of α-SMA-immunostained sections from each group
(magnification, ×400). No significant difference was found in the
peribronchial α-SMA-immunostained area between the OVA + IL-27
group and the OVA group. (E) Representative photomicrographs of
CD31-immunostained sections from each group (magnification, ×400).
There was no significant difference in the peribronchial
CD31-immunostained area between the OVA + IL-27 group and the OVA
group. The data are expressed as mean ± SEM of three independent
experiments with n=8 per group. *P<0.05,
**P<0.01, ***P<0.001. IL-27,
interleukin 27; OVA, ovalbumin; H&E, hematoxylin and eosin;
PAS, periodic-acid Schiff; α-SMA, α-smooth muscle actin; Pbm,
perimeter of basement membrane; Wat, total area of airway wall;
Wam, area of smooth muscle. |
Preventive administration of IL-27
alleviates the lung Th2 inflammatory environment
To examine whether the effects of IL-27 on
inflammation and AHR resulted from the suppression of the
inflammatory environment when IL-27 was prophylactically
administrated, the concentrations of Th2 cytokines (IL-4, IL-5 and
IL-13) were examined in BALF samples obtained from mice. As shown
in Fig. 2D, compared with the
OVA group, the intranasal administration of IL-27 led to a
significant reduction in the levels of the inflammatory cytokines
(OVA + IL-27 vs. OVA, pg/ml) IL-4 (92.81 vs. 191.70, t=2.63,
P<0.05), IL-5 (57.68 vs. 117.00, t=3.64, P<0.05) and IL-13
(212.10 vs. 397.00, t=3.68, P<0.05) in BALF. These results
demonstrated that the preventative administration of IL-27
ameliorated the lung Th2 inflammatory environment which would
result in an improvement of allergic asthma in the experimental
model.
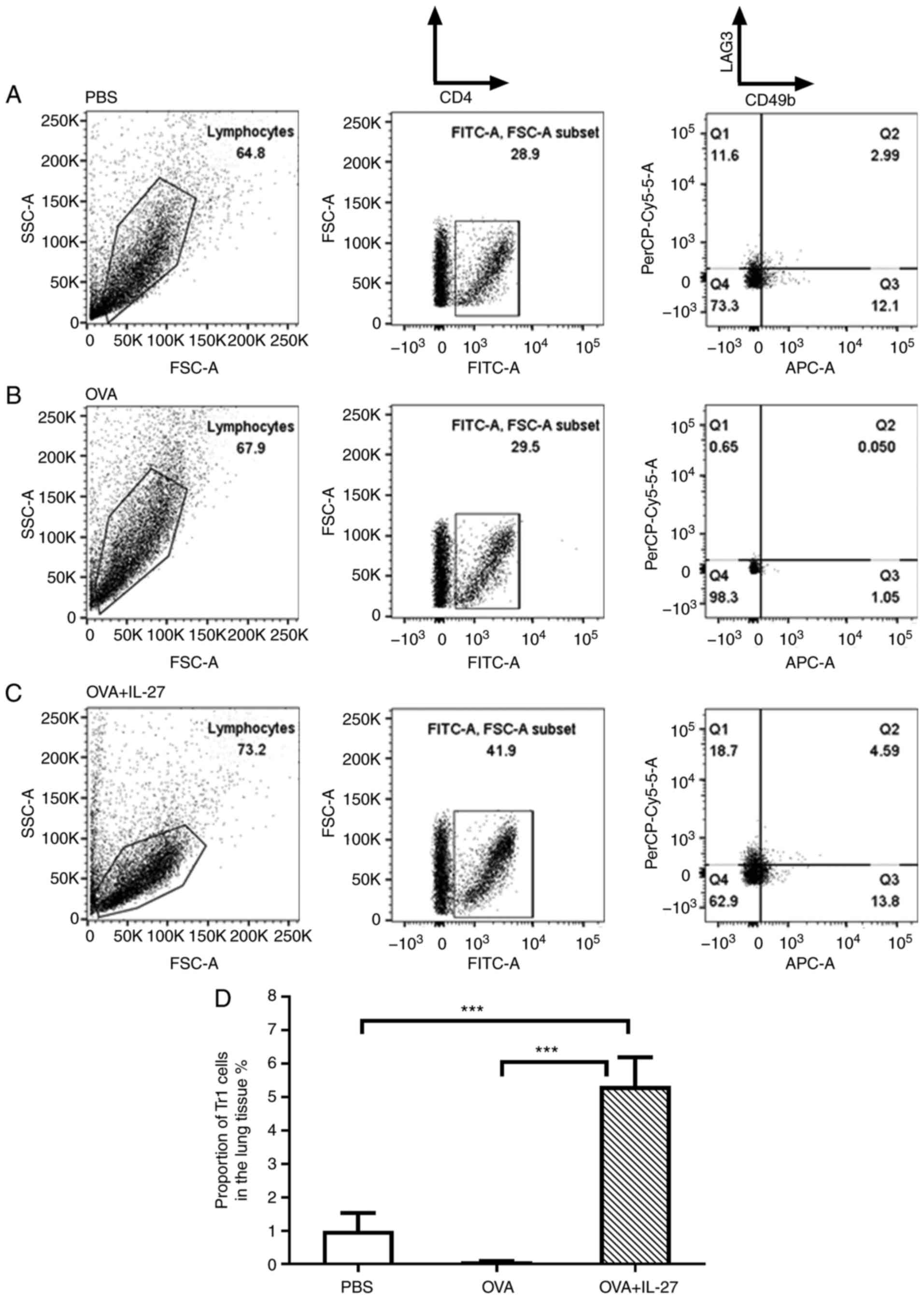
Preventive administration of IL-27
promotes the generation of Tr1 cells in lung tissue
There is emerging evidence to indicate that Tr1
cells play a role in inhibiting the production of the
pro-inflammatory cytokines, IL-5 and IL-13, and are instrumental in
the prevention of tissue inflammation (47,48). Therefore, in the present study,
Tr1 cells in the lung tissue were evaluated by means of FACS
analysis. As shown in the right column of Fig. 4A and B, the proportion of Tr1
cells in the OVA group was lower than that in the PBS group, and
the administration of IL-27 increased the proportion of Tr1 cells
in the OVA + IL-27 group (Fig. 4C
and D). These results suggested that the preventative
administration of IL-27 promoted the generation of Tr1 cells in
mouse lung tissue, which led to the the decreased production of Th2
cytokines.
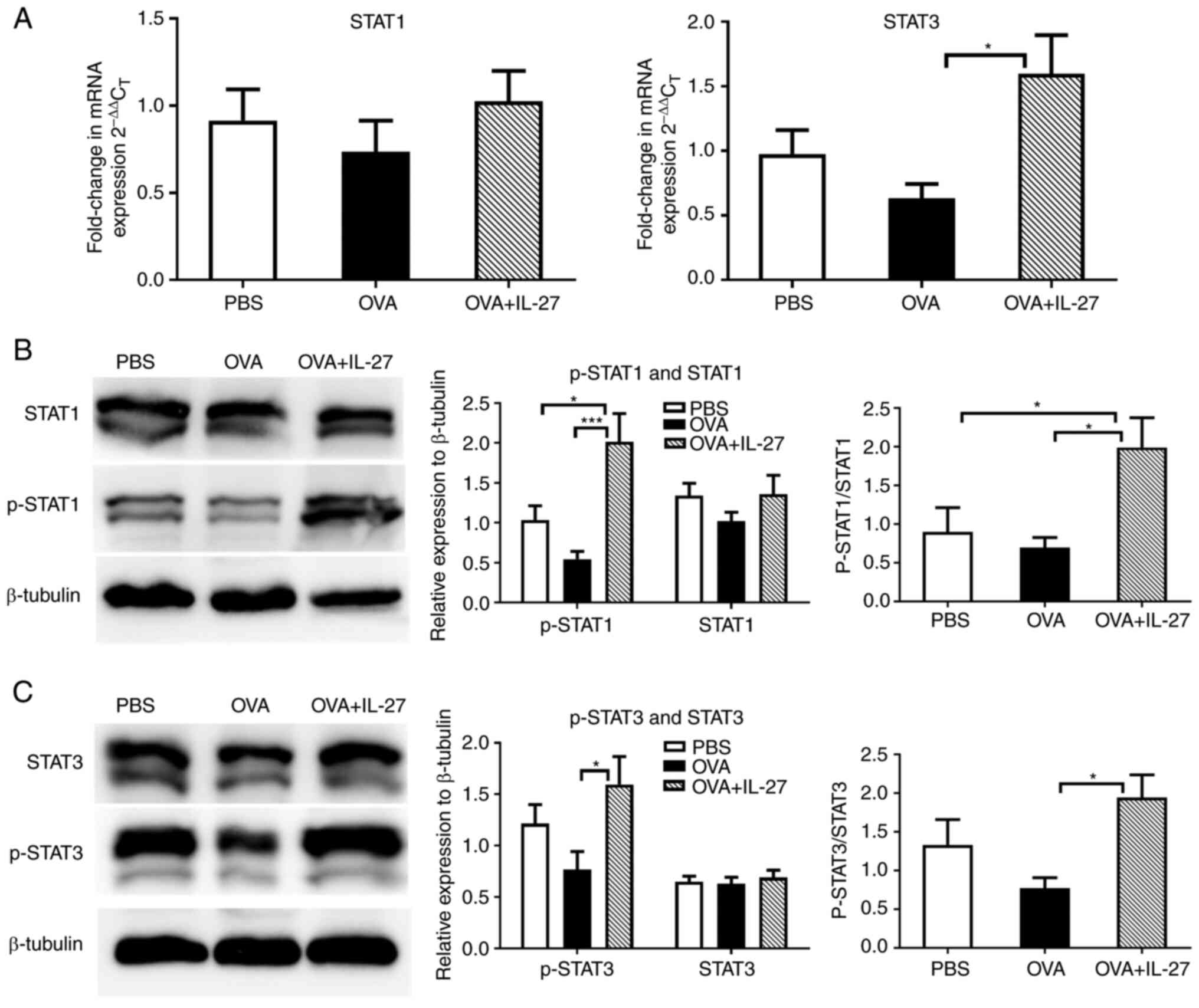
Preventative administration of IL-27
prior to the OVA challenge upregulates both the STAT1 and STAT3
pathways
The possible signaling pathways involved in the
effects of IL-27 were subsequently investigated. The STAT1 pathway
is critical for Th1 differentiation (49,50). Studies have found that the
blockade of IL-6, the activator of STAT3, leads to diminished Th2
responses in mice (51,52). Therefore, whether the
ameliorating effects of IL-27 on the lung Th2 inflammatory
environment are associated with the STAT1 and STAT3 pathways was
further examined. As shown in Fig.
5A, compared with the OVA group, the mRNA expression levels of
STAT1 exhibited no significant changes, while those of STAT3 were
significantly upregulated in the OVA+ IL-27 group. As for the total
levels of STAT1 and STAT3 proteins, no significant differences were
found between the OVA+ IL-27 and the OVA group. However, obvious
differences were observed in the phosphorylation levels of STAT1
and STAT3 between the OVA+ IL-27 and the OVA group. Moreover,
similar trends were observed in the ratios of the p-STAT1/3 and to
STAT1/3 (Fig. 5B and C). These
results indicated that IL-27 inhibited Th2 cell differentiation and
ameliorated the lung Th2 inflammatory environment by reversing the
impairment of the STAT1 and STAT3 pathways.
Discussion
The findings of the present study provide novel
contributions to the current knowledge of the association between
IL-27 and allergic asthma. First, the therapeutic administration of
IL-27 does not attenuate airway inflammation, AHR and airway
remodeling in a mouse model of acute and chronic asthma. Second,
IL-27 increased the proportion of Tr1 cells in the lung tissues of
asthmatic mice, which partially indicated that IL-27 alleviated the
lung Th2 inflammatory environment and sequentially ameliorated
OVA-induced allergic asthma. To the best of our knowledge, this is
first study to evaluate the effects of IL-27 on Tr1 cells in a
model of acute asthma. Finally, IL-27 alleviated airway
inflammation and airway remodeling mainly through the STAT1 and
STAT3 signaling pathways.
The findings that IL-27 exerted no inhibitory effect
on asthmatic models of mice when used following sensitization with
OVA are in accordance with those of a recent study by Su et
al (23), who reported that
the therapeutic administration of IL-27 did not suppress allergic
asthma. However, the results of the present study are contradictory
to those of a previous study by Yoshimoto et al (22), who revealed that IL-27 diminished
AHR and airway inflammation when IL-27 was administered at the time
of the OVA challenge. The reason for these contradictory results is
unclear; however, it may be partly be ascribed to the different
protocols used for the establishment of murine models of allergic
asthma. Mice were challenged with 50 µg of OVA for 3 days in
the study by Yoshimoto et al (22), whereas the mice were stimulated
with 100 µg OVA for 5 days in the present study. In
addition, Yoshimoto et al (22) selected an asthma model of 10
days, whereas the model used herein involved a period of 19 days.
These differences may affect the severity of Th2-mediated airway
inflammation and may result in the differences observed in the
interventional effects of IL-27 on airway inflammation in the mouse
models of allergic asthma. Th2 cells secrete IL-4 and play a
fundamental role in the pathogenesis of allergic asthma. IL-4 from
differentiated Th2 cells can upregulate the mRNA expression of
suppressor of cytokine signaling 3 and impair IL-27-induced STAT1
phosphorylation. Consequently, established Th2 cells are resistant
to reprogramming into Th1 cells (53). Th17 cells can produce IL-17,
which can lead to neutrophilic airway inflammation (54). Airway neutrophilia is associated
with asthma which is refractory to corticosteroids (55). IL-27 suppresses de novo
Th17 development; however, it has a minimal effect on committed
Th17 cells (56). When viewed in
combination, these findings demonstrate that whether IL-27 plays an
anti-inflammatory role in asthma is dependent upon the polarization
of inflammatory cells in the context of an inflammatory
environment.
As type 2 cytokine-producing cells, ILC2 cells are
increasingly recognized as a key controller of type 2 inflammation
and play an essential role in the pathophysiology of asthma
(14,57). ILC2 cell numbers are increased in
patients with allergic rhinitis, chronic rhinosinusitis with nasal
polyps and asthma (58,59). ILC2 cell-derived cytokines, such
as IL-4, IL-5 and IL-13 are associated with the initiation and
amplification of airway inflammation by promoting the activation
and survival of eosinophils, B cells, mast cells, macrophages and
epithelial cells during the Th2 immune response (60). As a negative regulator, IL-27
inhibits the proliferation and type 2 cytokine production of ILC2s
from mice and humans through the activation of STAT1 (60,61). IL-27-deficient mice exhibit an
increased ILC2 cell fraction and an enhanced Th2 immune response
following papain-induced lung inflammation (57). Therefore, IL-27 is a fundamental
environmental cytokine for the regulation of ILC2 cells. Th2
cytokines may be partially produced by ILC2 cells and are involved
in orchestrating the allergic inflammatory responses in the Th2
inflammatory environment.
Tr1 cells, a type of induced regulatory T-cells
(Tregs), are derived from the peripheral lymphoid tissue. They are
negative for Foxp3 and GATA-3, but double positive for LAG-3 and
CD49b (62). By secreting high
levels of the immunosuppressive cytokine, IL-10, and TGF-β, Tr1
cells can suppress immune and autoimmune responses in autoimmune
diseases (63-65). Thus, Tr1 cells play a crucial
role in maintaining the peripheral immune tolerance. Allergic
individuals have lower levels of antigen-specific Tr1 cells in
peripheral blood in comparison to healthy ones (66). The adoptive transfer of Tr1 cells
has been shown to suppress the development of inflammation in a
mouse model of inflammatory bowel disease and asthma (23,67). Allergen immunotherapy (AIT) is
the only etiological therapy and provides a specific and
potentially curative approach for the treatment of allergic asthma.
Tr1 cells downregulate allergen-specific immune responses and play
crucial roles in AIT (68).
Matsuda et al (62)
reported that subcutaneous immunotherapy was associated with an
increased number of Tr1 cells in peripheral blood mononuclear
cells. It has been demonstrated that Tr1 cells are instrumental for
the inhibition of atopic diseases, including allergic asthma
(62).
The mechanisms through which Tr1 cells suppress the
development of allergies may include the following aspects: first,
the production of IL-10 and TGF-β increases in Tr1 cells during
autoimmune responses (63,69). IL-10 is a secreted cytokine that
tempers inflammatory responses by antagonizing the activation of
antigen-presenting cells (APCs) (70,71). TGF-β maintains tolerance by
regulating the development of lymphocytes and the chemotaxis and
survival of lymphocytes and other immune cells (72). Second, Tr1 cells express certain
inhibitory receptors, such as cytotoxic T-lymphocyte antigen 4 and
programmed cell death protein 1, which play inhibitory roles in
T-cell function (19,73). Tr1 cells also express the
ectoenzymes (CD39/CD73) and subsequently produce higher levels of
adenosine and prostaglandin E2, which leads to the suppression of
effector T-cells (74). Third,
Tr1 cells kill myeloid APCs and regulate T-cell responses by a
granzyme B- and perforin-dependent mechanism (19,75). Overall, Tr1 cells directly and
indirectly affect the functions of different immune cells and
molecules in an antagonistic manner to mitigate immune
responses.
The present study found a significantly higher
number of CD4(+)CD49b(+)LAG3(+) T-cells (Tr1) in the lungs of mice
from the IL-27 + OVA group compared with those of mice from the OVA
group, suggesting that IL-27 promoted the generation of Tr1 cells
in the mouse model of asthma (Fig.
4). The present study also found that the levels of IL-4, IL-5
and IL-13 in BALF and serum were lower in mice from the IL-27 + OVA
group compared with those from the OVA group (Fig. 2C), indicating the decreased
production of Th2 cytokines. Studies have revealed that IL-27
promotes the development of Tr1 cells in addition to antagonizing
the differentiation of Th2 and Th17 cells (10,63,76,77). Moreover, Tr1 cell-derived IL-10
can suppress differentiation of Th2 cells and decrease the cytokine
production of eosinophils, basophils and mast cells (63). The balance between Tr1 and Th2
cells plays a critical role in the development of allergic and
normal immune response against allergens (78). In a previous study by the
authors, it was found that when administrated preventatively, IL-27
attenuated airway inflammation and remodeling in mouse models of
asthma (18). Thus, IL-27 helps
to improve allergic asthma by alleviating the Th2 inflammatory
environment.
Some molecular pathways have been involved in the
expansion and differentiation of Tr1 cells by IL-27. Activation
with IL-27 induces the expression of three key factors: the
transcription factor, c-musculoaponeurotic-fibrosarcoma (c-Maf),
the cytokine IL-21, and the receptor for inducible co-stimulator
(ICOS) (79). The
ligand-activated transcription factor aryl hydrocarbon receptor
binds to c-Maf, which leads to the transactivation of the IL-10 and
IL-21 promoters following Tr1 cell induction and IL-10 production
(47). IL-21 is produced by Tr1
cells due to the IL-27-driven c-Maf expression and functions as an
autocrine growth factor for Tr1 cells (79). ICOS further promotes the
differentiation of IL-27-driven Tr1 cells by interacting with ICOS
ligand and subsequently inducing c-Maf expression (80,81). Previous studies have revealed
that the signaling pathways of STAT1 and STAT3 activated by IL-27
are key to the transcriptional output (82,83). Interferon regulatory factor 1
(IRF1) is downstream of STAT1 and basic leucine zipper
transcriptional factor ATF-like (BATF) is dependent on STAT3. It
has been substantiated that IRF1 and BATF play a major role in
preparing the chromatin landscape during IL-27-induced Tr1
development (84). In this
process, IRF1 specifically upregulates the expression of the IL-10
gene and BATF serves as a pioneer factor. Therefore, the two
transcription factors may function as drivers in the earliest steps
during the expansion of Tr1 cells (84).
It has been reported that Janus kinase (JAK)1, JAK2
and tyrosine kinase-2 can be activated by IL-27 in naive
CD4+ T-cells (83).
As downstream signaling pathways of JAKs, STAT1-6 can also be
activated by IL-27 in bronchial epithelial cells and intestinal
epithelial cells (82,85). A previous in vitro study
demonstrated that the activation of STAT1 and STAT3 is required for
the differentiation of Tr1-like cells elicited by IL-27 (86). In addition, this induction is
associated with the expression of IL-10 and IFN-γ (87). Harb et al (88) found that the upregulation of
Notch4 receptor in Tregs in asthmatic lung tissue is indispensable
for allergens to potentiate airway inflammation. Of note, this
upregulation is dependent on STAT3 and IL-6 (88). In the present study, both the
STAT1 and STAT3 pathways were downregulated in the OVA group,
whereas they were upregulated in the OVA + IL-27 group (Fig. 5), which suggested that IL-27
upregulated the phosphorylation of the STAT1 and STAT3 proteins and
restored the STAT1 and STAT3 signaling pathways in the asthmatic
mouse model. When administrated preventatively, IL-27 subverted
naïve CD4+ T-cells into Th2 cells and led to an
improvement in the pathological changes of asthma. Thus, the
ameliorated Th2 inflammatory environment may help to attenuate
allergic asthma by restoring the STAT1 and STAT3 pathways.
In conclusion, the present study demonstrated that
IL-27 did not improve airway inflammation, AHR and airway
remodeling when administrated in a therapeutic manner in a mouse
model of OVA-induced asthma. The preventive administration of IL-27
decreased the production of Th2 cytokines in BALF and increased the
proportion of Tr1 cells in lung tissue. The ameliorated Th2
inflammatory environment is instrumental in attenuating allergic
asthma by STAT1 and STAT3 pathway-dependent mechanisms.
Collectively, these findings may provide insight into the
multifaceted role of IL-27 in allergic asthma. Therapeutic
intervention with IL-27 may be relevant to the improvement of the
inflammatory environment associated with dysregulated type-2
responses in allergic asthma. With further studies, IL-27 is
expected to be used as an immunotherapeutic treatment target for
asthma.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
JL, XJ and DL contributed to the conception and
design of the present study. JL, XJ, LW, YJ, XL, ZG, YJ, CH, HP and
FS performed the experiments and analyzed the data. JL, XJ, HP and
DL wrote and revised the manuscript. DL reviewed the article. JL
and FS confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Ethics committee
for Laboratory Animal care and Use in Shandong Qianfoshan Hospital,
Shandong University (approval no. 2019-S-306). All procedures using
mice were performed in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Xianchen Liu
(Center for Public Health Initiatives, University of Pennsylvania,
Philadelphia, PA, USA) for providing a critical appraisal of the
manuscript.
Funding
The present study was supported by the Collaborative Innovation
Center for Intelligent Molecules with Multi-effects and
Nanomedicine (grant no. 2019-01), Shandong province, China.
References
|
1
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar
|
|
2
|
Adcock IM, Caramori G and Chung KF: New
targets for drug development in asthma. Lancet. 372:1073–1087.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnes CB and Ulrik CS: Asthma and
adherence to inhaled corticosteroids: Current status and future
perspectives. Respir Care. 60:455–468. 2015. View Article : Google Scholar
|
|
4
|
Conner JB and Buck PO: Improving asthma
management: The case for mandatory inclusion of dose counters on
all rescue bronchodilators. J Asthma. 50:658–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campo P, Rodriguez F, Sanchez-Garcia S,
Barranco P, Quirce S, Pérez-Francés C, Gómez-Torrijos E, Cárdenas
R, Olaguibel JM, Delgado J, et al: Phenotypes and endotypes of
uncontrolled severe asthma: New treatments. J Investig Allergol
Clin Immunol. 23:76–88; quiz 71 p follow 88. 2013.PubMed/NCBI
|
|
6
|
Martin MJ, Beasley R and Harrison TW:
Towards a personalised treatment approach for asthma attacks.
Thorax. 75:1119–1129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pflanz S, Timans JC, Cheung J, Rosales R,
Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E,
et al: IL-27, a heterodimeric cytokine composed of EBI3 and p28
protein, induces proliferation of naive CD4+ T cells. Immunity.
16:779–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Owaki T, Asakawa M, Morishima N, Hata K,
Fukai F, Matsui M, Mizuguchi J and Yoshimoto T: A role for IL-27 in
early regulation of Th1 differentiation. J Immunol. 175:2191–2200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunter CA: New IL-12-family members: IL-23
and IL-27, cytokines with divergent functions. Nat Rev Immunol.
5:521–531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meka RR, Venkatesha SH, Dudics S, Acharya
B and Moudgil KD: IL-27-induced modulation of autoimmunity and its
therapeutic potential. Autoimmun Rev. 14:1131–1141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Owaki T, Asakawa M, Fukai F, Mizuguchi J
and Yoshimoto T: IL-27 induces Th1 differentiation via p38
MAPK/T-bet- and intercellular adhesion
molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol.
177:7579–7587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang RX, Yu CR, Mahdi RM and Egwuagu CE:
Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune
uveitis by inhibiting autoreactive Th1/Th17 cells and promoting
expansion of regulatory T cells. J Biol Chem. 287:36012–36021.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Artis D, Villarino A, Silverman M, He W,
Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, et al:
The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive
elements of type 2 immunity. J Immunol. 173:5626–5634. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moro K, Kabata H, Tanabe M, Koga S, Takeno
N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T and Koyasu S:
Interferon and IL-27 antagonize the function of group 2 innate
lymphoid cells and type 2 innate immune responses. Nat Immunol.
17:76–86. 2016. View Article : Google Scholar
|
|
15
|
Qin L, Li Z, Fan Y, Fang X, Zhang C, Yue
J, Xu Y, Wenzel SE and Xie M: Exploration of plasma interleukin-27
levels in asthma patients and the correlation with lung function.
Respir Med. 175:1062082020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Niu C, Ying L, Zhang Q, Long M and
Fu Z: Exploration of the serum interleukin-17 and interleukin-27
expression levels in children with bronchial asthma and their
correlation with indicators of lung function. Genet Test Mol
Biomarkers. 24:10–16. 2020. View Article : Google Scholar
|
|
17
|
Jirmo AC, Daluege K, Happle C, Albrecht M,
Dittrich AM, Busse M, Habener A, Skuljec J and Hansen G: IL-27 is
essential for suppression of experimental allergic asthma by the
TLR7/8 Agonist R848 (Resiquimod). J Immunol. 197:4219–4227. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu D, Lu J, Ji X, Ji Y, Zhang Z, Peng H,
Sun F and Zhang C: IL27 suppresses airway inflammation,
hyperresponsiveness and remodeling via the STAT1 and STAT3 pathways
in mice with allergic asthma. Int J Mol Med. 46:641–652. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roncarolo MG, Gregori S, Bacchetta R and
Battaglia M: Tr1 cells and the counter-regulation of immunity:
Natural mechanisms and therapeutic applications. Curr Top Microbiol
Immunol. 380:39–68. 2014.PubMed/NCBI
|
|
20
|
Song Y, Wang N, Chen L and Fang L: Tr1
cells as a key regulator for maintaining immune homeostasis in
transplantation. Front Immunol. 12:6715792021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Battaglia M, Gregori S, Bacchetta R and
Roncarolo MG: Tr1 cells: From discovery to their clinical
application. Semin Immunol. 18:120–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshimoto T, Yoshimoto T, Yasuda K,
Mizuguchi J and Nakanishi K: IL-27 suppresses Th2 cell development
and Th2 cytokines production from polarized Th2 cells: A novel
therapeutic way for Th2-mediated allergic inflammation. J Immunol.
179:4415–4423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su X, Pan J, Bai F, Yuan H, Dong N, Li D,
Wang X and Chen Z: IL-27 attenuates airway inflammation in a mouse
asthma model via the STAT1 and GADD45Y/p38 MAPK pathways. J Transl
Med. 14:2832016. View Article : Google Scholar
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
25
|
Reddy AT, Lakshmi SP and Reddy RC: Murine
model of allergen induced asthma. J Vis Exp. 63:e37712012.
|
|
26
|
Liu X, Li S, Jin J, Zhu T, Xu K, Liu C,
Zeng Y, Mao R, Wang X and Chen Z: Preventative tracheal
administration of interleukin-27 attenuates allergic asthma by
improving the lung Th1 microenvironment. J Cell Physiol.
234:6642–6653. 2019. View Article : Google Scholar
|
|
27
|
Overmyer KA, Thonusin C, Qi NR, Burant CF
and Evans CR: Impact of anesthesia and euthanasia on metabolomics
of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS One.
10:e01172322015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kirstein F, Nieuwenhuizen NE, Jayakumar J,
Horsnell WGC and Brombacher F: Role of IL-4 receptor α-positive
CD4(+) T cells in chronic airway hyperresponsiveness. J Allergy
Clin Immunol. 137:1852–1862 e9. 2016. View Article : Google Scholar
|
|
29
|
O'Byrne PM and Inman MD: Airway
hyperresponsiveness. Chest. 123(3 Suppl): 411S–416S. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoymann HG: Lung function measurements in
rodents in safety pharmacology studies. Front Pharmacol. 3:1562012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin TR, Gerard NP, Galli SJ and Drazen
JM: Pulmonary responses to bronchoconstrictor agonists in the
mouse. J Appl Physiol (1985). 64:2318–2323. 1988. View Article : Google Scholar
|
|
32
|
Cataldo DD, Tournoy KG, Vermaelen K,
Munaut C, Foidart JM, Louis R, Noël A and Pauwels RA: Matrix
metalloproteinase-9 deficiency impairs cellular infiltration and
bronchial hyperresponsiveness during allergen-induced airway
inflammation. Am J Pathol. 161:491–498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polte T, Behrendt AK and Hansen G: Direct
evidence for a critical role of CD30 in the development of allergic
asthma. J Allergy Clin Immunol. 118:942–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hammad H and Lambrecht BN: The basic
immunology of asthma. Cell. 184:2521–2522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albrecht M, Chen HC, Preston-Hurlburt P,
Ranney P, Hoymann HG, Maxeiner J, Staudt V, Taube C, Bottomly HK
and Dittrich AM: T(H)17 cells mediate pulmonary collateral priming.
J Allergy Clin Immunol. 128:168–177.e8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalidhindi RSR, Ambhore NS and Sathish V:
Cellular and biochemical analysis of bronchoalveolar lavage fluid
from murine lungs. Methods Mol Biol. 2223:201–215. 2021. View Article : Google Scholar :
|
|
38
|
Gregorczyk I and Maslanka T: Blockade of
RANKL/RANK and NF-kB signalling pathways as novel therapeutic
strategies for allergic asthma: A comparative study in a mouse
model of allergic airway inflammation. Eur J Pharmacol.
879:1731292020. View Article : Google Scholar
|
|
39
|
Chauhan PS, Subhashini, Dash D and Singh
R: Intranasal curcumin attenuates airway remodeling in murine model
of chronic asthma. Int Immunopharmacol. 21:63–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
41
|
Loke WS, Freeman A, Garthwaite L,
Prazakova S, Park M, Hsu K, Thomas PS and Herbert C: T-bet and
interleukin-27: Possible TH1 immunomodulators of sarcoidosis.
Inflammopharmacology. 23:283–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kelly-Welch AE, Melo ME, Smith E, Ford AQ,
Haudenschild C, Noben-Trauth N and Keegan AD: Complex role of the
IL-4 receptor alpha in a murine model of airway inflammation:
Expression of the IL-4 receptor alpha on nonlymphoid cells of bone
marrow origin contributes to severity of inflammation. J Immunol.
172:4545–4555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang X, Nian H, Li X, Yang Y, Wang X, Xu
L, Shi H, Yang X and Liu R: Effects of the combined extracts of
Herba Epimedii and Fructus Ligustrilucidi on airway remodeling in
the asthmatic rats with the treatment of budesonide. BMC Complement
Altern Med. 17:3802017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Komai M, Tanaka H, Masuda T, Nagao K,
Ishizaki M, Sawada M and Nagai H: Role of Th2 responses in the
development of allergen-induced airway remodelling in a murine
model of allergic asthma. Br J Pharmacol. 138:912–920. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kohan M, Breuer R and Berkman N:
Osteopontin induces airway remodeling and lung fibroblast
activation in a murine model of asthma. Am J Respir Cell Mol Biol.
41:290–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eifan AO, Orban NT, Jacobson MR and Durham
SR: Severe persistent allergic rhinitis. Inflammation but No
histologic features of structural upper airway remodeling. Am J
Respir Crit Care Med. 192:1431–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Apetoh L, Quintana FJ, Pot C, Joller N,
Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL and Kuchroo VK: The
aryl hydrocarbon receptor interacts with c-Maf to promote the
differentiation of type 1 regulatory T cells induced by IL-27. Nat
Immunol. 11:854–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aron JL and Akbari O: Regulatory T cells
and type 2 innate lymphoid cell-dependent asthma. Allergy.
72:1148–1155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STAT1-induced
regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol.
3:549–557. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lighvani AA, Frucht DM, Jankovic D, Yamane
H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE and
O'Shea JJ: T-bet is rapidly induced by interferon-gamma in lymphoid
and myeloid cells. Proc Natl Acad Sci USA. 98:15137–15142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Doganci A, Eigenbrod T, Krug N, De Sanctis
GT, Hausding M, Erpenbeck VJ, Haddad el-B, Lehr HA, Schmitt E, Bopp
T, et al: The IL-6R alpha chain controls lung CD4+CD25+ Treg
development and function during allergic airway inflammation in
vivo. J Clin Invest. 115:313–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Finotto S, Eigenbrod T, Karwot R, Boross
I, Doganci A, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T,
Rose-John S, et al: Local blockade of IL-6R signaling induces lung
CD4+ T cell apoptosis in a murine model of asthma via regulatory T
cells. Int Immunol. 19:685–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen Z, Wang S, Erekosima N, Li Y, Hong J,
Qi X, Merkel P, Nagabhushanam V, Choo E, Katial R, et al: IL-4
confers resistance to IL-27-mediated suppression on CD4+ T cells by
impairing signal transducer and activator of transcription 1
signaling. J Allergy Clin Immunol. 132:912–921. e1–5. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Boonpiyathad T, Sozener ZC, Satitsuksanoa
P and Akdis CA: Immunologic mechanisms in asthma. Semin Immunol.
46:1013332019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ray A and Kolls JK: Neutrophilic
Inflammation in asthma and association with disease severity.
Trends Immunol. 38:942–954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
El-behi M, Ciric B, Yu S, Zhang GX,
Fitzgerald DC and Rostami A: Differential effect of IL-27 on
developing versus committed Th17 cells. J Immunol. 183:4957–4967.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McHedlidze T, Kindermann M, Neves AT,
Voehringer D, Neurath MF and Wirtz S: IL-27 suppresses type 2
immune responses in vivo via direct effects on group 2 innate
lymphoid cells. Mucosal Immunol. 9:1384–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ho J, Bailey M, Zaunders J, Mrad N, Sacks
R, Sewell W and Harvey RJ: Group 2 innate lymphoid cells (ILC2s)
are increased in chronic rhinosinusitis with nasal polyps or
eosinophilia. Clin Exp Allergy. 45:394–403. 2015. View Article : Google Scholar
|
|
59
|
Kabata H, Moro K, Koyasu S, Fukunaga K,
Asano K and Betsuyaku T: Mechanisms to Suppress ILC2-induced airway
inflammation. Ann Am Thorac Soc. 13(Suppl 1): S952016.PubMed/NCBI
|
|
60
|
Kato A: Group 2 innate lymphoid cells in
airway diseases. Chest. 156:141–149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kabata H, Moro K and Koyasu S: The group 2
innate lymphoid cell (ILC2) regulatory network and its underlying
mechanisms. Immunol Rev. 286:37–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Matsuda M, Doi K, Tsutsumi T, Fujii S,
Kishima M, Nishimura K, Kuroda I, Tanahashi Y, Yuasa R, Kinjo T, et
al: Regulation of allergic airway inflammation by adoptive transfer
of CD4+ T cells preferentially producing IL-10. Eur J
Pharmacol. 812:38–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pot C, Apetoh L and Kuchroo VK: Type 1
regulatory T cells (Tr1) in autoimmunity. Semin Immunol.
23:202–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McGee HS and Agrawal DK: TH2 cells in the
pathogenesis of airway remodeling: Regulatory T cells a plausible
panacea for asthma. Immunol Res. 35:219–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Smith N and Broadley KJ: Optimisation of
the sensitisation conditions for an ovalbumin challenge model of
asthma. Int Immunopharmacol. 7:183–190. 2007. View Article : Google Scholar
|
|
66
|
Akdis M, Verhagen J, Taylor A, Karamloo F,
Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig
H, et al: Immune responses in healthy and allergic individuals are
characterized by a fine balance between allergen-specific T
regulatory 1 and T helper 2 cells. J Exp Med. 199:1567–1575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wirtz S and Neurath MF: Animal models of
intestinal inflammation: New insights into the molecular
pathogenesis and immunotherapy of inflammatory bowel disease. Int J
Colorectal Dis. 15:144–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Akdis CA and Akdis M: Mechanisms of
allergen-specific immunotherapy and immune tolerance to allergens.
World Allergy Organ J. 8:172015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao ST and Wang CZ: Regulatory T cells
and asthma. J Zhejiang Univ Sci B. 19:663–673. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
O'Farrell AM, Liu Y, Moore KW and Mui AL:
IL-10 inhibits macrophage activation and proliferation by distinct
signaling mechanisms: Evidence for Stat3-dependent and -independent
pathways. EMBO J. 17:1006–1018. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stumhofer JS, Silver JS, Laurence A,
Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ and
Hunter CA: Interleukins 27 and 6 induce STAT3-mediated T cell
production of interleukin 10. Nat Immunol. 8:1363–1371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67. 2018.
View Article : Google Scholar
|
|
74
|
Mandapathil M, Szczepanski MJ, Szajnik M,
Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S and Whiteside TL:
Adenosine and prostaglandin E2 cooperate in the suppression of
immune responses mediated by adaptive regulatory T cells. J Biol
Chem. 285:27571–27580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Magnani CF, Alberigo G, Bacchetta R,
Serafini G, Andreani M, Roncarolo MG and Gregori S: Killing of
myeloid APCs via HLA class I, CD2 and CD226 defines a novel
mechanism of suppression by human Tr1 cells. Eur J Immunol.
41:1652–1662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pot C, Apetoh L, Awasthi A and Kuchroo VK:
Molecular pathways in the induction of interleukin-27-driven
regulatory type 1 cells. J Interferon Cytokine Res. 30:381–388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Muallem G, Wagage S, Sun Y, DeLong JH,
Valenzuela A, Christian DA, Harms Pritchard G, Fang Q, Buza EL,
Jain D, et al: IL-27 limits type 2 immunopathology following
parainfluenza virus infection. PLoS Pathog. 13:e10061732017.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Akdis M: T-cell tolerance to inhaled
allergens: Mechanisms and therapeutic approaches. Expert Opin Biol
Ther. 8:769–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pot C, Jin H, Awasthi A, Liu SM, Lai CY,
Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC and Kuchroo VK: Cutting
edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21,
and the costimulatory receptor ICOS that coordinately act together
to promote differentiation of IL-10-producing Tr1 cells. J Immunol.
183:797–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bauquet AT, Jin H, Paterson AM,
Mitsdoerffer M, Ho IC, Sharpe AH and Kuchroo VK: The costimulatory
molecule ICOS regulates the expression of c-Maf and IL-21 in the
development of follicular T helper cells and TH-17 cells. Nat
Immunol. 10:167–175. 2009. View Article : Google Scholar
|
|
81
|
Nurieva RI, Duong J, Kishikawa H, Dianzani
U, Rojo JM, Ho Ic, Flavell RA and Dong C: Transcriptional
regulation of th2 differentiation by inducible costimulator.
Immunity. 18:801–811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Diegelmann J, Olszak T, Goke B, Blumberg
RS and Brand S: A novel role for interleukin-27 (IL-27) as mediator
of intestinal epithelial barrier protection mediated via
differential signal transducer and activator of transcription
(STAT) protein signaling and induction of antibacterial and
anti-inflammatory proteins. J Biol Chem. 287:286–298. 2012.
View Article : Google Scholar
|
|
83
|
Kamiya S, Owaki T, Morishima N, Fukai F,
Mizuguchi J and Yoshimoto T: An indispensable role for STAT1 in
IL-27-induced T-bet expression but not proliferation of naive CD4+
T cells. J Immunol. 173:3871–3877. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Karwacz K, Miraldi ER, Pokrovskii M, Madi
A, Yosef N, Wortman I, Chen X, Watters A, Carriero N, Awasthi A, et
al: Critical role of IRF1 and BATF in forming chromatin landscape
during type 1 regulatory cell differentiation. Nat Immunol.
18:412–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pereira ABM, de Oliveira JR, Teixeira MM,
da Silva PR, Rodrigues Junior V and Rogerio AP: IL-27 regulates
IL-4-induced chemokine production in human bronchial epithelial
cells. Immunobiology. 226:1520292021. View Article : Google Scholar
|
|
86
|
Wang H, Meng R, Li Z, Yang B, Liu Y, Huang
F, Zhang J, Chen H and Wu C: IL-27 induces the differentiation of
Tr1-like cells from human naive CD4+ T cells via the
phosphorylation of STAT1 and STAT3. Immunol Lett. 136:21–28. 2011.
View Article : Google Scholar
|
|
87
|
Pot C, Apetoh L, Awasthi A and Kuchroo VK:
Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by
IL-27. Semin Immunol. 23:438–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Harb H, Stephen-Victor E, Crestani E,
Benamar M, Massoud A, Cui Y, Charbonnier LM, Arbag S, Baris S,
Cunnigham A, et al: A regulatory T cell Notch4-GDF15 axis licenses
tissue inflammation in asthma. Nat Immunol. 21:1359–1370. 2020.
View Article : Google Scholar : PubMed/NCBI
|