1. Introduction
Ischemic injury is caused by insufficient blood
perfusion in cells, tissues and organs due to a limited blood
supply. Given that blood is the carrier of oxygen, the main cause
of ischemia is hypoxia. However, ischemia also involves an
insufficient supply of nutrients and clearance of metabolic wastes,
which leads to inflammation, acidosis and an electrolyte imbalance.
Ischemic injuries to the brain, heart, kidneys, lungs and other
organs can cause severe damage (1). For instance, stroke is an acute
brain injury that involves brain damage and neurological
dysfunction, and is associated with a high mortality or disability
rate globally. Acute renal ischemia can cause acute renal failure,
while heart ischemia can cause myocardial infarction (2-4).
Long non-coding RNAs (lncRNAs) refer to the
transcripts of non-coding RNAs that are >200 nucleotides in
length (5). Previous research
defined lncRNAs as by-products of transcription; however, recent
research has found that they regulate various physiological and
pathophysiological processes (6). lncRNAs regulate gene expression at
the epigenetic, transcriptional, post-transcriptional and chromatin
remodeling levels (6). They also
activate or inhibit the expression of target genes by directly
binding them or recruiting transcription factors (5).
Recently, research on lncRNAs has become a hot
topic, with several studies suggesting that lncRNAs are involved in
ischemic injuries (7,8). For instance, experimental models
(9) have found that lncRNA
expression differs between stroke patients and healthy individuals,
and that genetic variations of lncRNA loci are associated with an
increased risk of suffering from a stroke (2). lncRNAs are highly specific in the
central nervous system and stroke is known to induce changes in
several lncRNAs in the brain. Therefore, lncRNAs play a crucial
role in the complex pathological processes associated with stroke,
and may regulate the development of the central nervous system and
disease progression (10).
During the tra/rensplantation of lungs, kidneys and other organs,
ischemia/reperfusion (I/R) is a critical factor affecting the
success rate of organ transplantation (11,12). The present review article
discusses the functional roles of lncRNAs in ischemic injuries in
different organs.
2. Synthesis of lncRNAs
RNA-producing genes can be divided into two main
types: Protein-coding genes and non-protein-coding genes. Of the
180,000 transcripts in human cells, ~20,000 are protein-coding and
the remaining 160,000 are non-coding transcripts (6). Non-coding RNAs are divided into two
groups based on their size: Short non-coding RNAs (<200 nt),
e.g., microRNAs (miRNAs/miRs) and long non-coding RNAs (≥200 bp),
such as lncRNAS (13). Recent
estimates suggest there are >100,000 lncRNAs (14,15). In most cases, mRNA and lncRNA are
similar in several aspects of their biogenesis, although there are
differences between them (5,16). Similar to mRNAs, the synthesis of
lncRNAs requires one of the two strands of DNA as a template. The
majority of lncRNAs are transcribed by RNA polymerase II (pol II),
m7G capped at 5′-end and polyadenylated at 3′-end
(17). In contrast to mRNAs,
IncRNAs are not well conserved in evolution, consist of fewer exons
and are expressed at relatively low levels; large proportions of
IncRNAS are also retained in the nucleus (18).
Increasing evidence has indicated that lncRNAs play
a crucial role in regulating gene expression, such as gene
silencing or gene activation. Furthermore, the regulation of
lncRNAs can affect the transcription, splicing, translation,
output, import and stability of mRNAs (19). The role of lncRNAs in the process
of ischemic injury will be discussed in detail in the following
sections.
3. Role of lncRNAs in cerebral ischemic
injury
Cerebral ischemic injuries, including ischemic
stroke and cerebral ischemia-reperfusion injuries, can lead to
severe brain dysfunction, disability and a high mortality. lncRNAs
are highly expressed in the brain, indicating that they may be
involved in the physiological and pathological processes of the
brain, such as cerebral ischemic injury, neurodegeneration,
neurodevelopment and plasticity (20,21). A number of studies have
demonstrated that lncRNAs play a central role in ischemic brain
injuries (Table I).
 | Table IRole of lncRNAs in cerebral ischemic
injuries. |
Table I
Role of lncRNAs in cerebral ischemic
injuries.
| lncRNA | Effect on miRNAs
and signaling pathways (Refs.) | Effect on
inflammation | Effect on
apoptosis | Effect on
autophagy | Effect on other
aspects | Effect on cerebral
ischemic injury |
|---|
| KCNQ1OT1 | miR-200a/FOXO3/ATG7
(51) | | | ↑ | | |
| MALAT1 |
miR-30a/Beclin1/autophagy (27) | | | ↑ | | |
| miR-145/AQP42
(28) | | | | | ↑ |
| miR-211-5p
(52) | ↑ | | | | ↑ |
| miR-142-3p/SIRT1
(53) | | | | Acts as ceRNA for
miR-142-3p | ↓ |
| miR-375/PDE4D
(54) | ↑ | | | | ↑ |
| miR-181c-5p/HMGB1
(29) | ↑ | | | Acts as ceRNA for
miR-181c-5p | |
| miR-195a-5p/HMGA1
(55) | | | | Sponging
miR-195a-5p to upregulate HMGA1 | ↑ |
| miR-182-5p/TLR4
(56) | | | | | ↑ |
| miR-375/PDE4D
(54) | | | | | ↑ |
| Bim and E-selectin
(25) | ↓ | ↓ | | | |
| ERK/MAPK (57) | | | | | ↑ |
| MyD88/IRAK1/TRAF6
(58) | ↑ | | | | |
| AQP4 (59) | | | | | ↑ |
| Gm11974 | miR-766-3p/NR3C2
(46) | | | | | ↓ |
| miR-122-5P/SEMA3A
(60) | | | | | ↓ |
| MEG3 | miR-485/AIM2
(35) | | | | | ↑ |
| miR-378/GRB2
(61) | | | | Protect
neurons | |
| miR-181c-5p/ATG7
(62) | | | ↑ | | ↑ |
| NF-κB (63) | ↑ | | | | ↑ |
| p53/GPX4 (64) | | | | Mediate ferroptosis
of brain microvascular | |
| | | | endothelial
cells | |
| H19 | miR-29b/Akt3/mTOR
(40) | | | ↓ | Acts as a miR-29b
sponge | ↓ |
| miR-138-5p/p65
(65) | ↑ | | | | |
|
miRNA-29b/SIRT1/PGC-1α (66) | ↑ | ↑ | | | |
| miR-107 (41) | | ↓ | | | ↓ |
| miR-1306-5p/BCL2L13
(67) | | | | Acts as a
miR-1306-5p sponge | ↑ |
| miR-19a-3p/PTEN
(68) | | | | | ↑ |
| DUSP5-ERK1/2
(69) | | | ↓ | | |
| IGF1/mTOR (42) | | | | Inhibits axon
sprouting and functional recovery | |
| ROR | miR-135a-5p/ROCK1/2
(70) | | | | | ↑ |
| Nespas | Modulates
TNFα-induced apoptosis (71) | | | | | ↓ |
| SNHG12 | miR-199a/SIRT1/AMPK
(72) | | | | | ↓ |
| Inhibits
TRIM8-related K63-linked | ↓ | ↓ | | | |
| polyubiquitination
of TAK1 (73) | | | | | |
| Acts as an
autophagy inducer (74) | | | | | ↓ |
| SIRT1/FOXO3a
(75) | | | | | ↓ |
| SNHG14 | miR-136-5p/ROCK1
(76) | ↑ | | | | |
| miR-182-5p/BINP3
(77) | | | | | ↑ |
| miR-181c-5p/BMF
(78) | | | | | ↑ |
| miR-199b/AQP4
(79) | | | | | ↑ |
| MIAT | miR-204-5p/HMGB1
(80) | | | | | ↑ |
| miR-211/GDNF
(48) | | ↓ | | | |
| REDD1 (81) | | ↑ | ↑ | | |
| TUG1 | miR-145a-5p
(82) | ↑ | | | | ↑ |
| miR-204-5p/COX2
(83) | ↑ | | | Sponging
miR-204-5p | ↑ |
| miR-145/AQP4
(84) | | ↑ | | Sponging
miR-145 | |
| miR-200a-3p/NLRP3
(45) | ↑ | | | | |
| miR-142-3p
(85) | | | | | ↑ |
| miR-493-3p or
miR-410-3p (44) | | | | Sponging
miR-493-3p/miR-410-3p | ↑ |
| RMST | miR-150 (86) | ↑ | | | Inhibits cell
proliferation | |
| Protects against
MCAO-induced | | | | | |
| ischemic stroke
(87) | | | | | |
| N1LR | p53 (86) | | | | | ↓ |
| CHRF | miR-126/SOX6
(47) | | | | | ↑ |
| Inhibits p53
phosphorylation (88) | | | | | ↓ |
| Oprm1 | miR-155/GATA3
(49) | | ↓ | | | |
Metastatic-associated lung adenocarcinoma
transcript 1 (MALAT1)
Cerebral ischemic stroke is a main cause of
mortality and long-term disability globally (5) and is one of the most common
cerebral diseases, particularly among the elderly. MALAT1 is
closely associated with abnormal cellular signaling, as well as
with the occurrence, development and response to the treatment of
human diseases. It has been shown that MALAT1 plays a role in the
pathophysiology of a number of diseases and may be a therapeutic or
prognostic biological target (22,23). A previous study demonstrated that
MALAT1 was strongly induced in anoxic mouse organs, particularly in
the spleen, kidneys, testicles, lungs and brain (24).
In 2017, Zhang et al (25) explored the relevant mechanisms of
MALAT1 in regulating cerebral vascular lesions in ischemic stroke.
These authors established a mouse model of transient focal cerebral
ischemia through intracavitary middle cerebral artery occlusion
(MCAO) and then evaluated the infarct volume, neurological deficits
and sensorimotor function. Additionally, they simulated ischemic
injury in vitro by exposing rat brain microvascular
endothelial cells (BMECs) to oxygen-glucose deprivation (OGD) for
16 h. RNA sequencing revealed that the levels of MALAT1 in the
OGD-exposed BMEC cells and in ischemic cerebral microvessels were
significantly increased, suggesting that the upregulation of MALAT1
may play a crucial role in cerebrovascular pathologies induced by
ischemic stroke (25).
Furthermore, fluorescein-labeled MALAT1-Gapmer was introduced into
mouse BMECs in in vitro culture to achieve targeted MALAT1
degradation, resulting in a significant reduction in the changes of
OGD-induced MALAT1 levels, accompanied by increased cerebrovascular
endothelial cell death and programmed death-activating factor
caspase-3 activity. In addition, MALAT1 knockout mice and litter
control mice were briefly treated with MCAO for 1 h and perfused
for 24 h. The results revealed that MALAT1 knockout mice displayed
a larger infarct volume and significantly more severe neurological
deficits. These results suggest that the loss of MALAT1 exacerbates
ischemic brain injury. In subsequent experiments, the researchers
found that MALAT1 bound directly to Bim (a pro-apoptotic regulator)
and e-selectin. The silencing of MALAT1 increased the expression of
Bim and pro-inflammatory cytokines. Cerebrovascular endothelial
inflammation and subsequent endothelial function damage are
associated with ischemic brain injuries (21).
Similarly, in 2018, Zhang et al (26) also explored the mechanisms of
MALAT1 in ischemic stroke. The mouse dual-2-min homolog (MDM2) gene
encodes the E3 ubiquitin ligase of p53 in mitotic cells, which is
an oncoprotein that blocks the transcriptional activation mediated
by p53 tumor suppressors. MALAT1 was found to be highly expressed
in ischemic stroke samples, including human brain microvascular
endothelial cells (HBMECs). MALAT1 significantly promoted the
occurrence of an ischemic stroke by regulating MDM2 expression and
activating the p53 signaling pathway. Subsequently, Zhang et
al (26) found that MALAT1
and MDM2 were highly expressed in HBMECs and that both MALAT1 and
MDM2 were localized in the nucleus following OGD/reoxygenation
(OGD/R). The expression of the p53 signaling pathway-related
proteins was highly increased in HBMECs exposed to OGD/R.
Furthermore, the knockdown of MALAT1 effectively reduced the
expression of MDM2, while the knockdown of MDM2 had no effect on
the expression of MALAT1. These results revealed that MALAT1
regulated and promoted the expression of MDM2 following OGD/R.
OGD/R also promoted apoptosis through the p53 signaling pathway,
while the silencing of MALAT1 and MDM2 inhibited this effect.
MCAO/reperfusion (MCAO/R) in mouse brains also caused a similar
effect on expression of related proteins and lncRNAs (26).
Additionally, several studies have indicated that
MALAT1 plays a crucial role in the progression of ischemic brain
injury. In 2017, Guo et al (27) found that MALAT1, the
autophagy-related microtubule-related proteins light chain 3-I
(LC3-I)/LC3-II and Beclin1 were upregulated in the cortical neurons
of mice following MCAO/R. Furthermore, neuronal cell death was
significantly increased following OGD compared with the sham group;
the downregulation of MALAT1 significantly reversed this effect.
Additionally, the downregulation of MALAT1 significantly inhibited
the increase in LC3-I/LC3-II and Beclin1 levels induced by OGD
(27). This indicates that the
downregulation of MALAT1 can inhibit the occurrence of autophagy
and ischemic injury. It was also demonstrated that the
downregulation of MALAT1 alleviated neuronal cell death by
regulating the Beclin1-dependent autophagy of miR-30a during an
ischemic stroke (27).
In 2020, Wang et al (28) established the OGD/R an in
vitro astrocyte model and an MCAO/R in vivo mouse model.
In both models, the level of MALAT1 was significantly upregulated
and the expression of aquaporin 4 (AQP4) was significantly
increased. Following intervention with MALAT1 siRNA, the cell
survival rate was increased and apoptosis was reduced. Further
experiments revealed that MALAT1 siRNA could not reduce the
aggravation of the injury induced by a miR-145 inhibitor. This
indicates that MALAT1 silencing protects the cerebral
ischemia-reperfusion injury through miR-145. These results suggest
that MALAT1 positively regulates AQP4 expression through miR-145
and promotes cerebral ischemia-reperfusion injury (28). Cao et al (29) also found that the depletion of
MALAT1 suppressed inflammatory injury in the ischemic brain and
that the overexpression of MALAT1 had an adverse effect; in fact,
MALAT1 positively regulates the high-mobility Group Box 1 (HMGB1)
to promote inflammation by acting as a competing endogenous RNA
(ceRNA) to inhibit the function of miR-181C-5p (29). Another study demonstrated that
MALAT1 also inhibited PI3K/AKT signaling by sponging miR-126 to
promote the OGD-induced apoptosis of cerebral microvascular
endothelial cells (30). Thus,
MALAT1 may be a novel target for the treatment of cerebral ischemic
stroke.
Nevertheless, a few studies have reported
inconsistent results concerning the effects of MALAT1 in ischemic
brain injuries. For example, polydatin (a natural product)
upregulated MALAT1 expression and activated the
C/EBP/MALAT1/CREB/PGC-1/PPAR pathway to protect the brain
microvascular integrity and decrease the damage caused by cerebral
stroke (31). MALAT1 can protect
cerebral micro-vascular endothelial cells from OGD injuries by
sponging miR-26b and upregulating the expression of ULK2 (12,32).
Maternal expression of gene 3 (MEG3)
MEG3 plays an antitumor role in various types of
cancer by regulating the major tumor suppressor genes, p53 and Rb,
inhibiting angiogenesis-related factors and controlling miRNAs
(33). Recent experiments have
also explored the mechanisms of MEG3 involved in cerebral ischemic
injuries.
In 2019, You and You (34) found that MEG3 was upregulated in
rat models of MCAO. The rats with MACO exhibited evident nervous
system injuries, infarction areas, an increase in the blood-brain
barrier (BBB) permeability, water content, neuronal apoptosis and
necrosis. An increase was also observed in the levels of the
Wnt/β-catenin signaling pathway key proteins, including
brain-derived neurotrophic factor (BDNF), the nerve growth factor
(NGF) and basic fibroblast growth factor (bFGF). The depletion of
MEG3 by siRNA (si-MEG3) resulted in alleviated neurological damage,
reduced infarct area, water content, BBB permeability, neuronal
apoptosis and necrosis, and significantly increased neurogenesis,
whereas the overexpression of MEG3 exerted opposite effects.
Following treatment with the classic Wnt pathway inhibitor, DKK1,
the effect of si-MEG3 was reversed. The effect of MEG3 was also
reversed following treatment with LiCl, a classical Wnt pathway
activator. These experimental results suggest that the
downregulation of the lncRNA MEG3 activates the Wnt/β-catenin
signaling pathway, which further promotes nerve cell growth in rats
following cerebral I/R injury, and alleviates nervous tissue
injuries (34).
In 2020, Liang et al (35) reported that MEG3 was
significantly upregulated and miR-485 was significantly
downregulated in brain tissues following cerebral I/R, suggesting
that MEG3 and miR-485 may be involved in the regulation of brain
I/R. The pyroptosis in the brains of rats wit h MCAO was
significantly higher than that in the sham-operated group, while
the expression of absent in melanoma 2 (AIM2), an inflammatory
factor closely related to pyroptosis, was also significantly
increased in the I/R group. The protein levels of several key
components of the AIM2 inflammatory signal pathway, including AIM2,
lysed caspase-1 (c-caspase-1), IL-1β and IL-18 were significantly
increased in the brains of rats in the I/R group, suggesting that
I/R in the brain activates AIM2 inflammasome signaling, and
subsequently induces pyroptosis and the secretion of inflammatory
cytokines (35). Further data
indicated that MEG3 negatively regulated the expression of miR-485,
which can directly bind the 3′-UTR of AIM2 to inhibit its
expression. Furthermore, the knockdown of MEG3 inhibited
OGD/R-induced pyroptosis by directly increasing the level of
miR-485 (35). Other studies
have reported similar observations. For example, several
researchers have found that MEG3 exacerbrates oxygen-deficient
damage in PC12 cells by targeting miR-147 (36). MEG3 silencing also protects PC12
cells from hypoxic damage by sponging miR-21 and regulating both
the PI3K/AKT and NF-κB pathways (37). MEG3 silencing can also enhance
the cerebral protective effect of dexmedetomidine against
hypoxic-ischemic brain injuries in newborn mice via the
upregulation of miR-129-5p, leading to decreased neuronal cell
death and cerebral atrophy (38).
These studies also demonstrate that the regulation
of brain neurogenesis by targeting miRNAs is a common action form
of MEG3. The data suggest that MEG3 inhibits AIM2 expression by
sponging miR-485 during the I/R process in the brain, leading to
pyroptosis and inflammation (31), thus providing a novel direction
for the targeted therapy of ischemic stroke.
lncRNA H19
lncRNA H19 was previously misidentified as
'transcriptional noise' (39).
Existing studies have found that H19 plays a critical role in the
pathogenesis of various central nervous system diseases, such as
Parkinson's disease, Alzheimer's disease, cerebral ischemia,
cerebral hemorrhage and neuroblastoma (39). A number of studies have been
conducted to investigate the mechanisms of the role of H19 in the
process of ischemic brain injury.
In a study in 2020, the researchers established a
neonatal rat model of hypoxic-ischemic encephalopathy (HIE), which
exhibited an increased area of cerebral infarction, apoptosis and
impaired neurological function in neonatal rats with HIE (40). That study confirmed that the
overexpression of H19 functioned as a sponge for miR-29b and
attenuated neural damage and reduced autophagy in brain tissue in
neonatal rats with HIE by upregulating the Akt3/mTOR pathway. The
effect produced by the overexpression of H19 could also be
partially reversed by autophagy activators, which suggests that H19
affects autophagy (40).
Furthermore, research on hypoxic-ischemic brain injury has
confirmed that H19 overexpression is able to decrease miR-107
expression and increase VEGF expression, which results in the
inhibition of neuronal apoptosis and the alleviation of cognitive
dysfunction (41).
The two aforementioned studies demonstrate the
positive role of H19 overexpression in cerebral I/R; other studies
have demonstrated that H19 knockdown is capable of ischemic brain
injury-induced inflammation and neurological damage. For example,
the knockdown of lncRNA H19 has been shown to promotes axon
sprouting and functional recovery following cerebral ischemic
stroke (42). Another study
established an in vivo model of I/R using rats with MCAO and
an in vitro model of cells exposed to OGD/R and revealed
that the knockdown of H19 promoted cell proliferation, decreased
the rate of apoptosis, and alleviated the inflammatory status
following OGD/R (39). That
study also revealed that H19 was involved in this process by
regulating the miR-138-5p/p65 axis (39). Similarly, another study
established in vitro and in vivo models of cerebral
I/R (40). The results of that
study confirmed that lncRNA H19 knockdown attenuated the
OGD-mediated downregulation of miR-29b, SIRT1 and PGC-1α expression
levels, which in turn ameliorated the OGD-induced increase in
apoptosis and the concentration of inflammatory cytokines (40). Another study also demonstrated
that H19 functioned as a sponge for miR-1306-5p and triggered
cerebral I/R-induced injury, and that the knockdown of H19
alleviated injury (41).
Blocking the lncRNA H19-miR-19a-Id2 axis has also been found to
attenuate hypoxia/ischemia-induced neuronal injuries (43). Other pathways in which H19 is
involved are listed in Table I,
which also fully illustrates the close association of H19 with
cerebral I/R.
The existing studies suggest that H19 plays a
crucial role in cerebral I/R and is involved in various mechanisms,
such as autophagy, apoptosis and inflammation.
lncRNA taurine upregulated gene 1
(TUG1)
lncRNA TUG1 is one of the first lncRNAs identified
to be associated with human diseases, and is known to be involved
in and to regulate the biological processes of a number of human
diseases. Several studies have been conducted to investigate its
mechanisms of action in cerebral ischemic injury.
Several studies have described that TUG1 functions
by sponging miRNAs. For example, a study published in 2022
demonstrated that TUG1 sponged miR-204-5p and induced the
downregulation of miR-204-5p in the blood of patients with ischemic
stroke and in the brains of rats with MCAO/R (43). By contrast, the intracerebral
injection of miR-204-5p in rats significantly decreased COX2,
IL-1β, TNFα and PGE2 expression, increased IL-10 expression and
ameliorated brain injury, inhibited apoptosis, and significantly
decreased brain infarcts in rats with MCAO/R. That study confirmed
that the TUG1/miR-204-5p/COX2 axis is one of the pathways involved
in cerebral ischemic injury associated with TUG1 (43).
A study published in 2020 also reported that TUG1
promoted inflammation and apoptosis by sponging miR-145 and thereby
upregulating AQP4 expression in an in vitro cellular model
of OGD/R and a rat model of MCAO (44). TUG1 has also been shown to
activate the JNK and p38 MAPK signaling pathways by sponging
miR-493-3p or miR-410-3p (44).
In addition, TUG1 can also promote inflammation in brain ischemic
injury through other miRNA (miR-145a-5p or miR-200a-3p)-mediated
signaling pathways (45).
All the aforementioned studies have demonstrated
that TUG1 negatively affects ischemic injury, increases the
expression of inflammatory factors and the occurrence of apoptosis,
and that these pathways are dependent on many targeted miRNAs.
Other lncRNAs
Additionally, a large number of studies have
examined the involvement of other lncRNAs in cerebral ischemic
injuries. Similarly, the majority of these lncRNAs aggravate or
protect ischemic brain injuries by directly or indirectly acting on
miRNAs and associated signaling pathways. For example, the
knockdown of lncRNA Gm11974 has been shown to provide protection
against cerebral I/R through the miR-766-3p/NR3C2 axis (46). The lncRNA SNHG12 is a potent
autophagy inducer that exerts neuroprotective effects against
cerebral I/R injuries (46). The
lncRNA CHRF modulates the progression of cerebral I/R injuries via
the miR-126/SOX6 signaling pathway (47).
Both the upregulation and downregulation of several
lncRNAs can influence ischemic brain injuries. For example, the
overexpression of lncRNA MIAT has been found to reduce neuronal
apoptosis in a neonatal rat model of hypoxic-ischemic injury
through the miR-211/GDNF pathway (48). The overexpression of the lncRNA
Oprm1 and Rian attenuate cell apoptosis induced by cerebral
ischemia-reperfusion injuries through the Oprm1/miR-155/GATA3 and
Rian/miR-144-3p/GATA3 axes, respectively (49,50).
On the whole, a number of lncRNAs are involved in
cerebral ischemic injuries and some of these are listed in Table I (25,27-29,35,40-42,44-49,51-88).
4. Role of lncRNAs in heart ischemic
injury
Ischemic heart disease mainly refers to myocardial
ischemia induced by coronary artery obstruction or stenosis. It
often leads to myocardial infarction, left ventricular aneurysm,
ventricular septal defect and mitral insufficiency, which is common
among middle-aged and older individuals. Myocardial infarction is
caused by vascular stenosis, blocked circulation blood flow and
insufficient myocardial blood supply due to atherosclerosis in the
coronary artery wall. The high incidence of myocardial infarction
is a major human health concern. Therefore, it is of utmost
clinical significance to comprehensively understand the relevant
mechanisms underlying ischemic heart diseases.
lncRNA AK139328 and lncRNA CAREL
In recent years, IncRNAs have been reported to be
associated with ischemic heart diseases. Similar to ischemic brain
injuries, lncRNAs can promote or prevent the occurrence of ischemic
heart injuries via acting on various miRNAs. It has been shown that
patients with diabetes display a higher risk of ischemic heart
disease and myocardial I/R injury (89). Yu et al (90) investigated the effect of the
lncRNA AK139328 on myocardial I/R injury in diabetic mice. First,
the researchers established I/R models in normal mice and diabetic
mice. Compared to other RNAs, AK139328 was the most notably
upregulated in DM and the expression levels of phosphocreatine
kinase (CK), creatine kinase myocardial band (CK-MB) and lactate
dehydrogenase (LDH) (enzymes associated with myocardial injury)
were higher following I/R in the diabetic mice compared with the
normal mice. The downregulation of AK139328 significantly inhibited
the expression of CK, CK-MB and LDH. While I/R induced the
apoptosis of myocardial cells of both normal and diabetic mice, the
rate of apoptosis was significantly higher in the myocardial cells
of diabetic mice. The downregulation of AK139328 significantly
inhibited the activity of caspase-3 in DM and inhibited the
apoptosis of cardiomyocytes. Myocardial I/R injuries promoted the
expression of the autophagy-related proteins, Atg7, Atg5 and
LC3-II/LC3-I, and decreased p62 expression; downregulation of
AK139328 reversed the changes in the expression of these proteins
and inhibited autophagy. In fact, the knockdown of AK139328
significantly alleviated I/R injury in diabetic mice by regulating
miR-204-3p expression (86).
Another study explored the influence of the lncRNA
CAREL on heart regeneration, demonstrating that the overexpression
of CAREL in cardiomyocytes decreased the division and proliferation
of cardiomyocytes, and attenuated heart regeneration after injury.
By contrast, the silencing of CAREL significantly promoted cardiac
regeneration and improved cardiac function following myocardial
infarction in both neonatal and adult mice. In fact, CAREL
functioned as the ceRNA for miR-296 which suppressed the expression
of its target genes, Trp53inp1 and Itm2a. Furthermore, the
overexpression of miR-296 significantly increased the replication
of myocardial cells and heart regeneration following injury
(91).
lncRNA nuclear enriched transcript 1
(NEAT1)
In recent studies, the function of lncRNA NEAT1 in
myocardial I/R injuries has been described. For example, a study in
published 2020 demonstrated that NEAT1 was highly expressed in the
blood of patients with myocardial infarction and in mouse
cardiomyocytes (92). lncRNA
NEAT1 inhibited the expression of miR-378a-3p, which in turn
inhibited the expression of Atg12 and affected autophagy in
cardiomyocytes. It was also found that lncRNA NEAT1 significantly
promoted the proliferation and migration of cardiomyocytes and had
a protective effect against myocardial injury (92).
In addition to its functions in autophagy, NEAT1
affects cardiac functions, infarct size and myocardial apoptosis.
The knockdown of NEAT1 has been shown to attenuate myocardial I/R
injury through the miR-140/RhoA axis (93). This demonstrates a negative
effect of NEAT1 on myocardial injury, an observation that was
confirmed by another study, wherein the inhibition of NEAT1 reduced
apoptosis and also attenuated the hypoxia-induced inhibition of
proliferation (94). In another
study, it was demonstrated that in lipopolysaccharide-induced
myocardial injury, the knockdown of NEAT1 significantly ameliorated
I/R-induced cardiac insufficiency, oxidative stress and
inflammatory response, and also alleviated
lipopolysaccharide-induced myocardial injury by inhibiting the
TLR2/NF-κB signaling pathway (91).
The existing studies indicate that NEAT1 is involved
in various pathways, such as apoptosis, autophagy, inflammatory
response and oxidative stress. NEAT1 plays a double-sided role in
ischemic injury of the heart, and may thus provide novel strategies
for the clinical targeting of myocardial injury.
Other lncRNAs
There are other lncRNAs that have also been
implicated in ischemic heart injuries (Table II) (92-121). Several studies found that
lncRNAs play a key role in alleviating myocardial infarction
injuries and myocardial ischemia-reperfusion injuries. These
findings provide new insight into the pathophysiology of acute
myocardial I/R injuries, and may lead to the identification of
novel biomarkers and therapeutic targets for the detection and
treatment of acute myocardial infarction. The pharmacological and
genetic manipulation of lncRNAs has the therapeutic potential to
improve the clinical outcomes of patients with acute myocardial
infarction (122).
 | Table IIRole of lncRNAs in ischemic heart
injury. |
Table II
Role of lncRNAs in ischemic heart
injury.
| lncRNA | Effect on miRNAs
and signaling pathways (Refs.) | Effect on
inflammation | Effect on
apoptosis | Effect on
autophagy | Effect on ischemic
heart injury | Effect on other
aspects |
|---|
| NEAT1 | miR-27b/PINK1
(95) | | | | ↑ | |
| miR-129-5p
(94) | | ↑ | | | Suppresses
proliferation |
| miR-378a-3p/Atg12
(92) | | | | ↑ | |
| miR-140/RhoA
(93) | | ↑ | | ↑ | |
| TLR2/NF-κB
signaling pathway (96) | | | | ↑ | |
| Decreases pri-miRNA
processing (97) | | | | ↑ | |
| H19 | miR-22-3P (98) | | | | ↓ | |
| miR-22-3p/KDM3A
(99) | ↓ | ↓ | | ↓ | |
| miR-675/PPARα
(100) | ↑ | ↑ | | ↑ | |
| miR-29b-3p
(101) | | ↓ | | ↓ | |
| YB-1 protein
(102) | | | | ↑ | Results in cardiac
dilatation, and cardiac fibrosis |
| Increases the
stability of nucleolin protein (103) | | | | ↓ | Is involved in
myocardial ischemic preconditioning (IP) |
| TUG1 | miR-132-3p/HDAC3
(104) | | | | ↑ | |
| miR-142-3p
(105) | | | | ↓ | |
| miR-9a-5p/KLF5
(106) | | ↑ | | | |
| miR-29a-3p
(107) | | | | ↑ | |
| miR-532-5p/Sox8
(108) | | | | ↑ | |
| MALAT1 | miR-20b (109) | | | | ↑ | |
| miR-133 (110) | | ↑ | | | |
| miR-320/Pten
(111) | | | | ↑ | |
| β-catenin (112) | | | | ↑ | |
| ANRIL | miR-7-5p/SIRT1
(113) | | | | ↓ | |
| HOTAIR | miR-125/mmp2
(114) | | | | ↓ | |
| GAS5 | miR-142-5p
(115) | | | | ↑ | |
| CAIF | p53 (116) | | | ↓ | ↓ | |
| XIST | miR-133a/SOCS2
(117) | | | | ↓ | |
| HIF1A-AS1 | miRNA-204/SOCS2
(118) | | | | ↓ | Promotes
ventricular remodeling |
| LET | miR-138 (118) | | | | ↓ | |
| Gm2691 | PI3K/AKT (119) | ↓ | ↓ | | | |
| MIAT | NF-κB (120) | | ↓ | | | |
| RMRP | miR-206/ATG3
(121) | | | | ↑ | |
5. Role of lncRNAs in renal ischemic
injury
An ischemic renal injury refers to damage caused by
severe renal ischemia resulting from severe stenosis or obstruction
of the main renal artery or its branches. Acute renal ischemia and
renal I/R injuries are the main causes of acute renal failure.
Chronic ischemia often leads to chronic ischemic nephropathy in
middle-aged and elderly patients (123). Several studies have
demonstrated that various lncRNAs play a potential role in renal
I/R injuries (124,125).
lncRNA MALAT1
The findings of recent studies analyzing the role of
MALAT1 in renal ischemic injuries have been inconsistent. For
example, one study demonstrated that expression of MALAT1 was
significantly increased in mouse kidneys following I/R injury and
in human proximal renal tubular epithelial cells (HK2) under
hypoxic conditions (111). The
knockdown of MALAT1 significantly increased both the expression of
NF-κB and HIF-1α, and the secretion of IL-6 and TNFα in hypoxic HK2
cells. This suggests that the knockdown of MALAT1 expression can
promote hypoxia-induced inflammation in HK2 cells (111).
Nevertheless, other studies have suggested that
MALAT1 may have no effect on renal I/R injury. For instance,
Kölling et al (126)
found that the level of MALAT1 was increased in kidney biopsies and
plasma from patients with acute kidney injury; MALAT1 expression
was also increased in hypoxic mouse kidney tissues, endothelial
cells and tubular epithelial cells. However, MALAT1 knockout mice
exhibited the same degree of extramedullary injury, capillary
thinning, fibrosis, inflammatory cell infiltration, inflammatory
gene expression and proliferation as that in wild-type mice under
unilateral renal I/R injuries. MALAT1 knockout mice and wild-type
mice also exhibited similar renal dysfunction in bilateral kidney
I/R injuries (126). However, a
review of the role of MALAT1 in vascular and psychosomatic diseases
stated that most experimental studies have shown that the
downregulation of MALAT1 leads to more pronounced atherosclerotic
lesions and aggravates renal and other organ damage after ischemia
(127).
I/R injury is not only a main cause of acute kidney
injury, but also a critical risk factor for delayed or
non-functional graft function in renal transplantation. In 2019, Su
et al (11), using
microarray data, found that MALAT1 was upregulated in I/R injury;
MALAT1 expression was increased 3.79-fold in the kidneys of mice
with I/R injury when compared with sham-operated mice. The
researchers also detected a 40.4% decrease in the expression of
miR-139-5p in the kidneys of the mice with I/R injury compared with
the sham-operated mice, which is closely related to cell
proliferation (11). The
afore-mentioned data indicate that both MALAT1 and miR-139-5p are
involved in renal I/R injury, although the specific association
between them has not yet been fully elucidated.
Other lncRNAs
A number of studies have demonstrated that other
lncRNAs play a crucial role in renal ischemic injuries. Several
studies have analyzed the miRNA axis under renal ischemic injuries.
For example, lncRNA NEAT1 has been shown to promote hypoxia-induced
renal tubular epithelial apoptosis through the downregulation of
miR-27a-3p (128). The lncRNA
GAS5 has also been found to promote apoptosis by competing with
endogenous RNA for miR-21 via thrombospondin 1 in acute ischemic
kidney injury (129).
Furthermore, another study demonstrated that the downregulation of
lncRNA TUG1 attenuated inflammation and apoptosis in renal tubular
epithelial cells induced by ischemia-reperfusion by sponging
miR-449b-5p via HMGB1 and MMP2 (130).
Other studies have confirmed that lncRNAs can
regulate renal ischemic injuries by activating other signal
pathways. For example, LINC00520, which targets miR-27b-3p,
regulates the expression of oncostatin M receptor to promote the
development of acute kidney injuries through the PI3K/AKT signaling
pathway (131). The lncRNA
np_5318 promotes renal I/R injuries through the TGF-β/Smad
signaling pathway (132). The
lncRNA XLOC_032768 protects renal tubular epithelial cells against
apoptosis in renal I/R injuries by regulating FNDC3B/TGF-β1
signaling pathway (133).
Additionally, a previous clinical study demonstrated that changes
in the concentration of circulating lncRNAs in the plasma of
patients with acute kidney injury were predictive of cohort
mortality (134). This also
reveals the practical application of lncRNAs in clinical ischemic
injuries.
6. Role of lncRNAs in lung ischemic
injury
Lung I/R is a common type of ischemic injury, as
well as a common post-operative complication of lung
transplantation with high morbidity and mortality rates. The
elucidation of the mechanisms of pulmonary ischemic injury is of
utmost significance for the clinical diagnosis and treatment of the
condition. However, only a few studies to date have analyzed the
role of lncRNAs in pulmonary ischemic injury, at least to the best
of our knowledge.
In line with its effects on the brain, heart and
kidneys, it is also assumed that MALAT1 plays a crucial role in
ischemic lung injuries. In 2019, Wei et al (12) explored the role of MALAT1 in
inflammatory injuries following lung transplantation I/R (LTIR).
The expression of MALAT1 was significantly increased in the lung
tissues of rats with LTIR. The expression of IL-8 was the highest
among all examined inflammatory factors and was significantly
higher in the serum and in bronchoalveolar lavage fluid of rats
with LTIR compared with the control animals. The knockdown of
MALAT1 significantly reduced the expression of IL-8 (mRNA and
protein) and suppressed the apoptosis of pulmonary epithelial
cells. In fact, MALAT1 was directly bound to p300 and activated
Il-8 transcription. The silencing of MALAT1 alleviated inflammatory
injury in rats with LTIR by suppressing IL-8 expression, and
inhibiting neutrophil activation and infiltration (8).
Primary graft dysfunction (PGD) is an acute lung
injury caused by I/R injury, which is the main cause of early
morbidity and mortality following transplantation (135). A previous study found that
lncRNA X-inactive-specific transcript (XIST) upregulated IL-12A
expression by binding to miR-21, thus inducing the formation of
neutrophil extracellular traps (NETs) and accelerated PGD following
lung transplantation (136). In
that study, miR-21 expression was significantly reduced in patients
with PGD following lung transplantation compared with non-PGD
patients, while its expression level was inversely associated with
the PGD grade. Moreover, the level of the pro-inflammatory
cytokines, IL-6, CXCL10, CCL2, and the chemokine, IL-8, in patients
with PGD was higher than that in patients without PGD. An elevated
expression of XIST was also detected in the alveolar lavage fluid
from patients with PGD (136).
During acute immune responses, neutrophils migrate to the site of
inflammation and decompose their nuclear inclusions to form NETs,
which are a possible risk factor for PGD. XIST was found to
negatively correlate with miR-21, but to positively correlate with
IL-12A. Silencing XIST also downregulated the expression of IL-12A
by upregulating miR-21, inhibiting the formation of NETs, and
ultimately reducing PGD following lung transplantation (136).
7. Overview of lncRNAs in ischemic
injuries
A large number of in vitro and in vivo
experiments have demonstrated that multiple lncRNAs are involved in
the ischemic injury to different organs, among which MALAT1, H19,
MEG3 and TUG1 are the most commonly described lncRNAs. Some lncRNAs
exhibit similar expression patterns in ischemic cell and animal
models and in the plasma of ischemic patients (Table SI), while the
knockdown/overexpression of lncRNAs in animal models recapitulates
the pathology of ischemic patients, suggesting that lncRNAs may
contribute to the formation and progression of ischemic injury in
patients. However, the mechanisms and associated signaling pathways
of the majority of lncRNAs in ischemic injuries remain unclear and
the effect of some lncRNAs remains controversial (126,127).
Based on review of the entire literature, it can be
concluded that MALAT1 plays a critical role not only in cerebral
ischemic injury, but also in the ischemic injury of the heart,
kidneys and lungs. Why is the lncRNA MALAT1 of such vital
importance to ischemic injury? MALAT1 is a highly conserved nuclear
retained lncRNA and plays a role in the transcriptional and
post-transcriptional regulation of gene expression in an
environment-dependent manner (137,138). Furthermore, MALAT1 is involved
in a variety of physiological processes, including alternative
splicing, epigenetic modification of gene expression, synapse
formation and myogenesis (138). In recent years, it has been
found that an abnormal MALAT1 expression is closely related to
cancer. MALAT1 regulates cancer progression by interacting with
molecules, such as proteins, RNA and DNA to alter different
signaling pathways (23). In
addition to lung cancer, recent studies have shown that MALAT1 is
involved in other types of cancer, such as breast, pancreatic,
prostate cancer, glioma and leukemia (139). MALAT1 is therefore considered
as a potential biomarker for the diagnosis and prediction of
cancer, as well as a therapeutic target for specific tumors.
The present review article demonstrates that MALAT1
plays a key role in ischemic injury, and is involved in the heart,
brain, lungs and kidneys (Fig.
1). Similarly, studies have shown that in most cases, MALAT1
expression or overexpression can aggravate ischemic injury in these
organs. Moreover, MALAT1 is involved in the regulation of ischemic
injury mainly through the miRNA-mediated regulation of inflammatory
factors, apoptosis, autophagy and other pathways. MALAT1 promotes
the development of stroke by regulating MDM2 and subsequently
activating the p53 signaling pathway. Further study of the
MALAT1/MDM2/p53 signaling pathway may provide a more effective
clinical treatment strategy for patients with ischemic stroke
(Fig. 1). Accordingly, MALAT1
may be a novel target for the treatment of cerebral ischemic
stroke. In heart ischemic injury, MALAT1 can function as a ceRNA
for miR-20b-5p to upregulate expression of Beclin1 and enhance
autophagy-mediated cardiomyocyte injury (109). MALAT1 can also sponge miR-133
and miRNA-320 to enhance inflammation in ischemia-reperfusion
injury and apoptosis in acute myocardial infarction (110,111). The Wnt/β-catenin signal pathway
has been shown to exert a protective effect in cerebral, hepatic
and myocardial I/R injury (140-142). The knockdown of MALAT1
attenuates I-R induced myocardial infarction and downregulates
β-catenin expression (112).
The mechanisms underlying the regulation of β-catenin expression by
MALAT1 require further investigation. MALTA1 also exacerbates I/R
injury following lung transplantation by upregulating IL-8
expression (12). However,
MALAT1 has no effect or, in fact, the opposite effect on renal I/R
injury (111,127). The underlying mechanisms of the
functional difference of MALAT1 between organs warrant further
investigation.
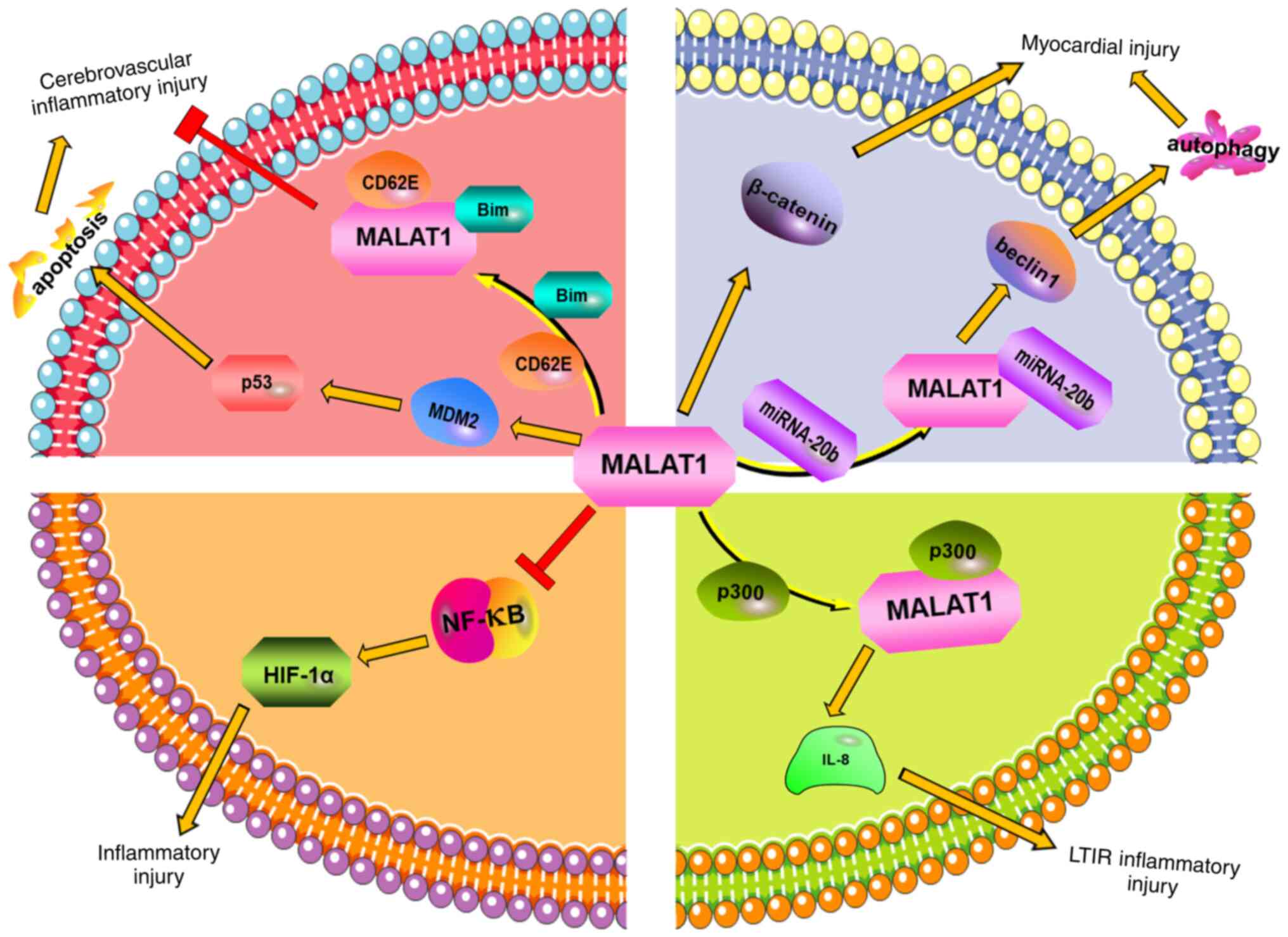 | Figure 1In cerebral ischemic injury, MALAT1
directly binds to Bim and CD62E, thereby reducing the expression of
Bim and the level of pro-inflammatory cytokines following ischemic
injury and reducing inflammatory injury. MALAT1 can promote
apoptosis through the MDM2/p53 pathway, and then aggravate the
inflammatory injury of cerebrovascular endothelium. In ischemic
heart injury, MALAT1 can directly promote β-catenin expression. It
can also promote the expression of Beclin1 by binding with
miRNA-20, enhance autophagy, and finally further aggravating
myocardial injury. In renal ischemic injury, the knockdown of
MALAT1 can activate NF-κB, increase the expression of HIF-1α, and
thereby reduce the inflammatory injury caused by renal
ischemia/reperfusion injuries. In ischemic lung injury, MALAT1 can
directly bind to p300, then upregulate IL-8, and finally promote
the inflammatory damage caused by lung transplantation
ischemia-reperfusion. MALAT1, metastatic-associated lung
adenocarcinoma transcript 1; MDM2, murine double minute 2. |
The function of lncRNAs in ischemic injury appears
to be complex. An single lncRNA may have multiple miRNA targets and
is associated with different signaling pathways. For example, in
brain ischemic injury, lncRNA TUG1 can sponge miR-9, miR-29b-1-5p,
miR-145, miR-410, miR-410-3p and miR-493-3p, can regulate
expression of downstream targeting genes, markedly induce apoptosis
and inflammation, and aggravate brain ischemic injury (Fig. 2) (44,84,143-145); in heart ischemic injury, lncRNA
TUG1 can bind to miR-9, miR-132-3p, miR-142-3p and miR-340,
subsequently regulating the expression of targeting genes,
enhancing oxidative damage, autophagy and apoptosis, and promoting
heart ischemic injury (Fig. 2)
(104-106,146); in kidney ischemic injury, the
reported effects of lncRNA TUG1 on ischemic injury are not
consistent. A previous study demonstrated that lncRNA TUG1 sponged
miR-494-3p and disinhibited E-cadherin, reducing I/R induced acute
kidney injury (147). However,
another study reported that lncRNA TUG1 interacted with miR-449b-5p
and induced apoptosis and inflammation, aggravating I/R induced
kidney injury (Fig. 2) (130). It is interesting that lncRNA
TUG1 sponges the same miRNA (miR-9), but that the downstream target
is different (FOXO3 in the brain and RLF5 in the heart) (Fig. 2). The complex multiple targets
and associated signaling pathways of lncRNA renders the development
of treatments more challenging.
 | Figure 2Individual lncRNAs have multiple
miRNA targets and involve different signaling pathways. In brain
ischemic injury, for example, lncRNA TUG1 can bind miR-9,
upregulate FOXO3 expression and increase neuronal apoptosis in mice
with middle cerebral artery occlusion (143); lncRNA TUG1 binds miR-29b-1-5p,
activates the NF-κB/IL-β signaling pathway, and induces
inflammatory damage in rats with spinal cord I/R injury (144); lncRNA TUG1 can directly
interact with miR-145 and function as a competing endogenous RNA of
miR-145, regulate AQP4 expression and induce cell damage in
cerebral I/R injury (84);
lncRNA TUG1 binds miR-410, regulates FOXO3 expression and induces
apoptosis and inflammation in cerebral I/R injury (145); lncRNA TUG1 binds miR-410-3p and
miR-493-3p, activates the JNK and p38 MAPK signaling pathways, and
induces inflammation and oxidative damage in cerebral I/R injury
(44). In heart ischemic injury,
lncRNA TUG1 binds miR-9, regulates KLF5 expression and induces
apoptosis in myocardial I/R injury (106); lncRNA TUG1 activates HDAC3 by
sponging miR-132-3p, stimulates intracellular ROS accumulation and
aggravates myocardial ischemic injury (104); lncRNA TUG1 binds miR-142-3p,
regulates expression of HMGB1 and Rac1, and induces the apoptosis
and autophagy of ischemic/hypoxia cardiomyocytes (104); lncRNA TUG1 binds miR-340,
regulates HDAC4 expression, mediates β-catenin/GLUT1 and induces
apoptosis in myocardial I/R injury (146). In kidney ischemic injury,
lncRNA TUG1 interacts with miR-449b-5p, and then regulates
expression of HMGB1 and MMP2, inducing apoptosis and inflammation
in I/R injury (130). However,
lncRNA TUG1 binds miR-494-3p, regulates E-cadherin expression and
inhibits apoptosis, alleviating I/R induced acute kidney injury
(147). lncRNA, long non-coding
RNA; TUG1, taurine up-regulated gene 1; I/R, ischemia/reperfusion;
KLF5, Kruppel like factor 5; HMGB1, high mobility group box 1;
HDAC3, histone deacetylase 3. |
8. Conclusion and future perspectives
Ischemic injuries cause severe trauma to the body
and are associated with high morbidity and mortality rates.
Ischemic stroke, myocardial infarction and renal transplantation
failures have attracted extensive clinical attention. Numerous
studies (cited in the present review) have demonstrated that
lncRNAs play an essential role in ischemic injuries and that their
interaction with miRNA is the main mode of action. The
downregulation of the majority of lncRNAs reduces inflammation,
apoptosis, autophagy, or other cell damage, thus alleviating
ischemic injuries. Since the plethora of data are derived from
in vitro and pre-clinical studies, further investigations
are urgently required to develop novel therapeutic strategies for
patients with ischemic injury.
Supplementary Data
Availability of data and materials
Not applicable.
Authors' contributions
LS and BY contributed to the conception of the
study. YC, JL, QL, KH and NJ analyzed the data from the literature
for inclusion in the review and prepared the manuscript. JR and XS
revised the manuscript. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Outstanding
Youth Project of the Hunan Provincial Department of Education (no.
19B508), the Jiangxi Provincial Natural Science Foundation (no.
20202BABL206061), the Cultivation Scientific Research Fund for the
Junior Teachers of Medicine in NanChang University (PY201826), and
the Lotus Scholarship Program of Hunan Province (2019-23).
References
|
1
|
Akbari G: Role of zinc supplementation on
ischemia/reperfusion injury in various organs. Biol Trace Elem Res.
196:1–9. 2020. View Article : Google Scholar
|
|
2
|
Akella A, Bhattarai S and Dharap A: Long
noncoding RNAs in the pathophysiology of ischemic stroke.
Neuromolecular Med. 21:474–483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haddad G, Kölling M, Wegmann UA, Dettling
A, Seeger H, Schmitt R, Soerensen-Zender I, Haller H, Kistler AD,
Dueck A, et al: Renal AAV2-mediated overexpression of long
non-coding RNA H19 attenuates ischemic acute kidney injury through
sponging of microRNA-30a-5p. J Am Soc Nephrol. 32:323–341. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frangogiannis NG: Pathophysiology of
myocardial infarction. Compr Physiol. 5:1841–1875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L and Wang H: Long non-coding RNA in
CNS injuries: A new target for therapeutic intervention. Mol Ther
Nucleic Acids. 17:754–766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar :
|
|
7
|
Das A, Samidurai A and Salloum FN:
Deciphering non-coding RNAs in cardiovascular health and disease.
Front Cardiovasc Med. 5:732018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Chen H and Fan Y: Systematic
analysis of the expression profile of non-coding RNAs involved in
ischemia/reperfusion-induced acute kidney injury in mice using RNA
sequencing. Oncotarget. 8:100196–100215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Hao M, Yang B, Shi T, Zhang Y, Feng
J and Chen J: Long non-coding RNAs expression profile and
functional analysis of acute ischemic stroke. Medicine (Baltimore).
99:e229642020. View Article : Google Scholar
|
|
10
|
Wang Y, Pan WY, Ge JS, Wang XD, Chen W,
Luo X and Wang YL: A review of the relationship between long
noncoding RNA and post-stroke injury repair. J Int Med Res.
47:4619–4624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su M, Hu X, Lin J, Zhang L, Sun W, Zhang
J, Tian Y and Qiu W: Identification of candidate genes involved in
renal ischemia/reperfusion injury. DNA Cell Biol. 38:256–262. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei L, Li J, Han Z, Chen Z and Zhang Q:
Silencing of lncRNA MALAT1 prevents inflammatory injury after lung
transplant ischemia-reperfusion by downregulation of IL-8 via p300.
Mol Ther Nucleic Acids. 18:285–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali T and Grote P: Beyond the
RNA-dependent function of LncRNA genes. Elife. 9:e605832020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uszczynska-Ratajczak B, Lagarde J,
Frankish A, Guigó R and Johnson R: Towards a complete map of the
human long non-coding RNA transcriptome. Nat Rev Genet. 19:535–548.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang S, Zhang L, Guo J, Niu Y, Wu Y, Li H,
Zhao L, Li X, Teng X, Sun X, et al: NONCODEV5: A comprehensive
annotation database for long non-coding RNAs. Nucleic Acids Res.
46(D1): D308–D314. 2018. View Article : Google Scholar :
|
|
16
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar
|
|
17
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Statello L, Guo CJ, Chen LL and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar
|
|
19
|
Charles Richard JL and Eichhorn PJA:
Platforms for investigating LncRNA functions. SLAS Technol.
23:493–506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SW, Liu Z and Shi ZS: Non-coding RNA
in acute ischemic stroke: Mechanisms, biomarkers and therapeutic
targets. Cell Transplant. 27:1763–1777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Chen M, Cao RY, Li Q and Zhu F:
The role of circular RNAs in cerebral ischemic diseases: Ischemic
stroke and cerebral ischemia/reperfusion injury. Adv Exp Med Biol.
1087:309–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li ZX, Zhu QN, Zhang HB, Hu Y, Wang G and
Zhu YS: MALAT1: A potential biomarker in cancer. Cancer Manag Res.
10:6757–6768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Yan G, Zhang J and Yu L: Knockdown
of long noncoding RNA (lncRNA) metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1) inhibits proliferation,
migration, and invasion and promotes apoptosis by targeting miR-124
in retinoblastoma. Oncol Res. 26:581–591. 2018. View Article : Google Scholar
|
|
24
|
Lelli A, Nolan KA, Santambrogio S,
Gonçalves AF, Schönenberger MJ, Guinot A, Frew IJ, Marti HH,
Hoogewijs D and Wenger RH: Induction of long noncoding RNA MALAT1
in hypoxic mice. Hypoxia (Auckl). 3:45–52. 2015.
|
|
25
|
Zhang X, Tang X, Liu K, Hamblin MH and Yin
KJ: Long noncoding RNA Malat1 regulates cerebrovascular pathologies
in ischemic stroke. J Neurosci. 37:1797–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang T, Wang H, Li Q, Fu J, Huang J and
Zhao Y: MALAT1 activates the P53 signaling pathway by regulating
MDM2 to promote ischemic stroke. Cell Physiol Biochem.
50:2216–2228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo D, Ma J, Yan L, Li T, Li Z, Han X and
Shui S: Down-regulation of lncrna MALAT1 attenuates neuronal cell
death through suppressing beclin1-dependent autophagy by regulating
Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem.
43:182–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Zheng X, Jin J, Zheng L, Guan T,
Huo Y, Xie S, Wu Y and Chen W: LncRNA MALAT1 silencing protects
against cerebral ischemia-reperfusion injury through miR-145 to
regulate AQP4. J Biomed Sci. 27:402020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao DW, Liu MM, Duan R, Tao YF, Zhou JS,
Fang WR, Zhu JR, Niu L and Sun JG: The lncRNA Malat1 functions as a
ceRNA to contribute to berberine-mediated inhibition of HMGB1 by
sponging miR-181c-5p in poststroke inflammation. Acta Pharmacol
Sin. 41:22–33. 2020. View Article : Google Scholar :
|
|
30
|
Zhang L, Yang H, Li WJ and Liu YH: LncRNA
MALAT1 promotes OGD-induced apoptosis of brain microvascular
endothelial cells by sponging miR-126 to repress PI3K/Akt signaling
pathway. Neurochem Res. 45:2091–2099. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruan W, Li J, Xu Y, Wang Y, Zhao F, Yang
X, Jiang H, Zhang L, Saavedra JM, Shi L and Pang T: MALAT1
Up-regulator polydatin protects brain microvascular integrity and
ameliorates stroke through C/EBPβ/MALAT1/CREB/PGC-1α/PPARγ pathway.
Cell Mol Neurobiol. 39:265–286. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Li J and Tang N: Long noncoding RNA
Malat1 is a potent autophagy inducer protecting brain microvascular
endothelial cells against oxygen-glucose
deprivation/reoxygenation-induced injury by sponging miR-26b and
upregulating ULK2 expression. Neuroscience. 354:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Rugeebah A, Alanazi M and Parine NR:
MEG3: An oncogenic long non-coding RNA in different cancers. Pathol
Oncol Res. 25:859–874. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
You D and You H: Repression of long
non-coding RNA MEG3 restores nerve growth and alleviates
neurological impairment after cerebral ischemia-reperfusion injury
in a rat model. Biomed Pharmacother. 111:1447–1457. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang J, Wang Q, Li JQ, Guo T and Yu D:
Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion
injury through increasing pyroptosis by targeting miR-485/AIM2
axis. Exp Neurol. 325:1131392020. View Article : Google Scholar
|
|
36
|
Han L, Dong Z, Liu N, Xie F and Wang N:
Maternally expressed gene 3 (MEG3) enhances PC12 cell hypoxia
injury by targeting MiR-147. Cell Physiol Biochem. 43:2457–2469.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng D and Liang H: Silencing MEG3
protects PC12 cells from hypoxic injury by targeting miR-21. Artif
Cells Nanomed Biotechnol. 48:610–619. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou XM, Liu J, Wang Y and Zhang MH:
Silencing of long noncoding RNA MEG3 enhances cerebral protection
of dexmedetomidine against hypoxic-ischemic brain damage in
neonatal mice by binding to miR-129-5p. J Cell Biochem: Nov.
28:2018.Epub ahead of print.
|
|
39
|
Zhong L, Liu P, Fan J and Luo Y: Long
non-coding RNA H19: Physiological functions and involvements in
central nervous system disorders. Neurochem Int. 148:1050722021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu H, Wang L, Chen J, Shen H and Chen Z:
Mechanisms underlying abnormal expression of lncRNA H19 in neonatal
hypoxic-ischemic encephalopathy. Am J Perinatol. Oct 27–2020.Epub
ahead of print.
|
|
41
|
Fang H, Li HF, Pan Q, Yang M, Zhang FX,
Wang RR, Wang QY and Zhang JP: Long noncoding RNA H19
overexpression protects against hypoxic-ischemic brain damage by
inhibiting miR-107 and up-regulating vascular endothelial growth
factor. Am J Pathol. 191:503–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu S, Zheng J, Du Z and Wu G: Knock down
of lncRNA H19 promotes axon sprouting and functional recovery after
cerebral ischemic stroke. Brain Res. 1732:1466812020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao Z, Qiu Y, Lin Y, Medina R, Zhuang S,
Rosenblum JS, Cui J, Li Z, Zhang X and Guo L: Blocking lncRNA
H19-miR-19a-Id2 axis attenuates hypoxia/ischemia induced neuronal
injury. Aging (Albany NY). 11:3585–3600. 2019. View Article : Google Scholar
|
|
44
|
Du J, Li W and Wang B: Long non-coding RNA
TUG1 aggravates cerebral ischemia and reperfusion injury by
sponging miR-493-3p/miR-410-3p. Open Med (Wars). 16:919–930. 2021.
View Article : Google Scholar
|
|
45
|
Yin M, Chen WP, Yin XP, Tu JL, Hu N and Li
ZY: LncRNA TUG1 demethylated by TET2 promotes NLRP3 expression,
contributes to cerebral ischemia/reperfusion inflammatory injury.
ASN Neuro. 13:175909142110032472021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cai J, Shangguan S, Li G, Cai Y, Chen Y,
Ma G, Miao Z, Liu L and Deng Y: Knockdown of lncRNA Gm11974 protect
against cerebral ischemic reperfusion through miR-766-3p/NR3C2
axis. Artif Cells Nanomed Biotechnol. 47:3847–3853. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gai HY, Wu C, Zhang Y and Wang D: Long
non-coding RNA CHRF modulates the progression of cerebral
ischemia/reperfusion injury via miR-126/SOX6 signaling pathway.
Biochem Biophys Res Commun. 514:550–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li EY, Zhao PJ, Jian J, Yin BQ, Sun ZY, Xu
CX, Tang YC and Wu H: LncRNA MIAT overexpression reduced neuron
apoptosis in a neonatal rat model of hypoxic-ischemic injury
through miR-211/GDNF. Cell Cycle. 18:156–166. 2019. View Article : Google Scholar :
|
|
49
|
Jing H, Liu L, Jia Y, Yao H and Ma F:
Overexpression of the long non-coding RNA Oprm1 alleviates
apoptosis from cerebral ischemia-reperfusion injury through the
Oprm1/miR-155/GATA3 axis. Artif Cells Nanomed Biotechnol.
47:2431–2439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yao P, Li YL, Chen Y, Shen W, Wu KY and Xu
WH: Overexpression of long non-coding RNA Rian attenuates cell
apoptosis from cerebral ischemia-reperfusion injury via
Rian/miR-144-3p/GATA3 signaling. Gene. 737:1444112020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu S, Yu M, He X, Wen L, Bu Z and Feng J:
KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7
pathway in cerebral ischemic stroke. Aging Cell. 18:e129402019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tan X, Guo W, Peng Z, Gu C, Xiang P, Tu Y,
Fei H, Liu X, Lu Y, Li M, et al: LncRNA-Malat1 down-regulates
miR-211-5p expression to promote neuronal damage from cerebral
ischemia reperfusion injury. Biochem Pharmacol. 192:1146942021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meng S, Wang B and Li W: LncRNA MALAT1
improves cerebral ischemia-reperfusion injury and cognitive
dysfunction by regulating miR-142-3p/SIRT1 axis. Int J Neurosci.
1:192021.Epub ahead of print.
|
|
54
|
Zhang G, Wang Q, Su D and Xie Y: Long
non-coding RNAMALAT1 knockdown alleviates cerebral
ischemia/reperfusion injury of rats through regulating the
miR-375/PDE4D axis. Front Neurol. 11:5787652020. View Article : Google Scholar
|
|
55
|
Jia Y, Yi L, Li Q, Liu T and Yang S:
LncRNA MALAT1 aggravates oxygen-glucose
deprivation/reoxygenation-induced neuronal endoplasmic reticulum
stress and apoptosis via the miR-195a-5p/HMGA1 axis. Biol Res.
54:82021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hu Y, Ye C, Cheng S and Chen J: Propofol
downregulates lncRNA MALAT1 to alleviate cerebral
ischemia-reperfusion injury. Inflammation. 44:2580–2591. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi YL, Wang Q and Wei JC: Influence of
lncRNA-MALAT1 on neuronal apoptosis in rats with cerebral
infarction through regulating the ERK/MAPK signaling pathway. Eur
Rev Med Pharmacol Sci. 23:8039–8048. 2019.PubMed/NCBI
|
|
58
|
Wang LQ and Zhou HJ: LncRNA MALAT1
promotes high glucose-induced inflammatory response of microglial
cells via provoking MyD88/IRAK1/TRAF6 signaling. Sci Rep.
8:83462018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jin J, Wang H, Zheng X, Xie S, Zheng L and
Zhan R: Inhibition of LncRNA MALAT1 attenuates cerebral ischemic
reperfusion injury via regulating AQP4 expression. Eur Neurol.
83:581–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang L, Wang L, Wang J and Liu P: Long
non-coding RNA Gm11974 aggravates oxygen-glucose
deprivation-induced injury via miR-122-5p/SEMA3A axis in ischaemic
stroke. Metab Brain Dis. 36:2059–2069. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo HC, Yi TZ, Huang FG, Wei Y, Luo XP and
Luo QS: Role of long noncoding RNA MEG3/miR-378/GRB2 axis in
neuronal autophagy and neurological functional impairment in
ischemic stroke. J Biol Chem. 295:14125–14139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li TH, Sun HW, Song LJ, Yang B, Zhang P,
Yan DM, Liu XZ and Luo YR: Long non-coding RNA MEG3 regulates
autophagy after cerebral ischemia/reperfusion injury. Neural Regen
Res. 17:824–831. 2022. View Article : Google Scholar
|
|
63
|
Zhang F, Wang Z, Sun B, Huang Y, Chen C,
Hu J, Li L, Xia P and Ye Z: Propofol rescued astrocytes from
LPS-induced inflammatory response via blocking LncRNA-MEG3/NF-κB
axis. Curr Neurovasc Res. Mar 16–2022.Epub ahead of print.
View Article : Google Scholar
|
|
64
|
Chen C, Huang Y, Xia P, Zhang F, Li L,
Wang E, Guo Q and Ye Z: Long noncoding RNA Meg3 mediates
ferroptosis induced by oxygen and glucose deprivation combined with
hyperglycemia in rat brain microvascular endothelial cells, through
modulating the p53/GPX4 axis. Eur J Histochem. 65:32242021.
View Article : Google Scholar :
|
|
65
|
Li H, Tang C and Wang D: LncRNA H19
promotes inflammatory response induced by cerebral
ischemia-reperfusion injury through regulating the miR-138-5p-p65
axis. Biochem Cell Biol. 98:525–536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu J, Wang C, Meng F and Xu P: Long
non-coding RNA H19 inhibition ameliorates oxygen-glucose
deprivation-induced cell apoptosis and inflammatory cytokine
expression by regulating the microRNA-29b/SIRT1/PGC-1α axis. Mol
Med Rep. 23:1312021. View Article : Google Scholar
|
|
67
|
Huang Y, Deng L, Zeng L, Bao S, Ye K, Li
C, Hou X, Yao Y, Li D and Xiong Z: Silencing of H19 alleviates
oxygen-glucose deprivation/reoxygenation-triggered injury through
the regulation of the miR-1306-5p/BCL2L13 axis. Metab Brain Dis.
36:2461–2472. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gao N, Tang H, Gao L, Tu GL, Luo H and Xia
Y: LncRNA H19 aggravates cerebral ischemia/reperfusion injury by
functioning as a ceRNA for miR-19a-3p to target PTEN. Neuroscience.
437:117–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang J, Cao B, Han D, Sun M and Feng J:
Long non-coding RNA H19 induces cerebral ischemia reperfusion
injury via activation of autophagy. Aging Dis. 8:71–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen H and Li X: LncRNA ROR is involved in
cerebral hypoxia/reoxygenation-induced injury in PC12 cells via
regulating miR-135a-5p/ROCK1/2. Am J Transl Res. 11:6145–6158.
2019.PubMed/NCBI
|
|
71
|
Zhou Q, An Y and Tang Y: Long noncoding
RNA-regulator of reprogramming alleviates hypoxia-induced cerebral
injury in mice model and human via modulating apoptosis complexes.
J Integr Neurosci. 18:431–437. 2019. View Article : Google Scholar
|
|
72
|
Yin WL, Yin WG, Huang BS and Wu LX: LncRNA
SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral
ischemia/reperfusion injury through activating AMPK signaling
pathway. Neurosci Lett. 690:188–195. 2019. View Article : Google Scholar
|
|
73
|
Deng Y, Chen D, Wang L, Gao F, Jin B, Lv
H, Zhang G, Sun X, Liu L, Mo D, et al: Silencing of long noncoding
RNA nespas aggravates microglial cell death and neuroinflammation
in ischemic stroke. Stroke. 50:1850–1858. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yao X, Yao R, Huang F and Yi J: LncRNA
SNHG12 as a potent autophagy inducer exerts neuroprotective effects
against cerebral ischemia/reperfusion injury. Biochem Biophys Res
Commun. 514:490–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu Y, Huang Y, Cai J, Zhang D, Liu S and
Pang B: LncRNA SNHG12 improves cerebral ischemic-reperfusion injury
by activating SIRT1/FOXO3a pathway through I nhibition of autophagy
and oxidative stress. Curr Neurovasc Res. 17:394–401. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p/ROCK1.
Cancer Gene Ther. 26:234–247. 2019. View Article : Google Scholar
|
|
77
|
Deng Z, Ou H, Ren F, Guan Y, Huan Y, Cai H
and Sun B: LncRNA SNHG14 promotes OGD/R-induced neuron injury by
inducing excessive mitophagy via miR-182-5p/BINP3 axis in HT22
mouse hippocampal neuronal cells. Biol Res. 53:382020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bu X, Zhao Y, Chang M and Ge X:
Downregulation of lncRNA SNHG14 alleviates neurons injury by
modulating the miR-181c-5p/BMF axis in ischemic stroke. Brain Res
Bull. 174:379–388. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang G, Li T, Chang X and Xing J: Long
noncoding RNA SNHG14-promotes ischemic brain injury via regulating
miR-199b/AQP4 axis. Neurochem Res. 46:1280–1290. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Deng W, Fan C, Shen R, Wu Y, Du R and Teng
J: Long noncoding MIAT acting as a ceRNA to sponge microRNA-204-5p
to participate in cerebral microvascular endothelial cell injury
after cerebral ischemia through regulating HMGB1. J Cell Physiol.
235:4571–4586. 2020. View Article : Google Scholar
|
|
81
|
Guo X, Wang Y, Zheng D, Cheng X and Sun Y:
LncRNA-MIAT promotes neural cell autophagy and apoptosis in
ischemic stroke by up-regulating REDD1. Brain Res. 1763:1474362021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang H, Liao S, Li H, Chen Y and Yu J:
Long non-coding RNA TUG1 sponges Mir-145a-5p to regulate microglial
polarization after oxygen-glucose deprivation. Front Mol Neurosci.
12:2152019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xiang P, Hu J, Wang H, Luo Y, Gu C, Tan X,
Tu Y, Guo W, Chen L, Gao L, et al: miR-204-5p is sponged by TUG1 to
aggravate neuron damage induced by focal cerebral ischemia and
reperfusion injury through upregulating COX2. Cell Death Discov.
8:892022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shan W, Chen W, Zhao X, Pei A, Chen M, Yu
Y, Zheng Y and Zhu S: Long noncoding RNA TUG1 contributes to
cerebral ischaemia/reperfusion injury by sponging mir-145 to
up-regulate AQP4 expression. J Cell Mol Med. 24:250–259. 2020.
View Article : Google Scholar
|
|
85
|
Li L, Zhang Q, Wang Y, Yin S, Chi S, Han F
and Wang W: Knockdown of lncRNA TUG1 attenuates cerebral
ischemia/reperfusion injury through regulating miR-142-3p.
Biofactors. 47:819–827. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qiao P, Yan H and Wang J: EGb761 protects
brain microvascular endothelial cells against oxygen-glucose
deprivation-induced injury through lncRNA Rmst/miR-150 axis.
Neurochem Res. 45:2398–2408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hou XX and Cheng H: Long non-coding RNA
RMST silencing protects against middle cerebral artery occlusion
(MCAO)-induced ischemic stroke. Biochem Biophys Res Commun.
495:2602–2608. 2018. View Article : Google Scholar
|
|
88
|
Wu Z, Wu P, Zuo X, Yu N, Qin Y, Xu Q, He
S, Cen B, Liao W and Ji A: LncRNA-N1LR enhances neuroprotection
against ischemic stroke probably by inhibiting p53 phosphorylation.
Mol Neurobiol. 54:7670–7685. 2017. View Article : Google Scholar
|
|
89
|
Lejay A, Fang F, John R, Van JA, Barr M,
Thaveau F, Chakfe N, Geny B and Scholey JW: Ischemia reperfusion
injury, ischemic conditioning and diabetes mellitus. J Mol Cell
Cardiol. 91:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lncRNA AK139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cai B, Ma W, Ding F, Zhang L, Huang Q,
Wang X, Hua B, Xu J, Li J, Bi C, et al: The long noncoding RNA
CAREL controls cardiac regeneration. J Am Coll Cardiol. 72:534–550.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao J, Chen F, Ma W and Zhang P:
Suppression of long noncoding RNA NEAT1 attenuates hypoxia-induced
cardiomyocytes injury by targeting miR-378a-3p. Gene.
731:1443242020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rui PF, Wang JH and Xu J: Long non-coding
NEAT1 weakens the protective role of sevoflurane on myocardial
ischemia/reperfusion injury by mediating the microRNA-140/RhoA
axis. J Biol Regul Homeost Agents. 35:933–944. 2021.PubMed/NCBI
|
|
94
|
Wei Q, Zhou HY, Shi XD, Cao HY and Qin L:
Long noncoding RNA NEAT1 promotes myocardiocyte apoptosis and
suppresses proliferation through regulation of miR-129-5p. J
Cardiovasc Pharmacol. 74:535–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ruan Z, Wang S, Yu W and Deng F: LncRNA
NEAT1 aggravates diabetic myocardial ischemia-reperfusion injury
through regulating PINK1 by targeting miR-27b. Int J Cardiol.
286:1362019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang SM, Liu GQ, Xian HB, Si JL, Qi SX and
Yu YP: LncRNA NEAT1 alleviates sepsis-induced myocardial injury by
regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol
Sci. 23:4898–4907. 2019.PubMed/NCBI
|
|
97
|
Gidlöf O, Bader K, Celik S, Grossi M,
Nakagawa S, Hirose T, Metzler B, Olde B and Erlinge D: Inhibition
of the long non-coding RNA NEAT1-protects cardiomyocytes from
hypoxia in vitro via decreased pri-miRNA processing. Cell Death
Dis. 11:6772020. View Article : Google Scholar
|
|
98
|
Zhang BF, Chen J and Jiang H: LncRNA H19
ameliorates myocardial ischemia-reperfusion injury by targeting
miR-22-3P. Int J Cardiol. 278:2242019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang BF, Jiang H, Chen J, Hu Q, Yang S,
Liu XP and Liu G: LncRNA H19 ameliorates myocardial
infarction-induced myocardial injury and maladaptive cardiac
remodelling by regulating KDM3A. J Cell Mol Med. 24:1099–1115.
2020. View Article : Google Scholar
|
|
100
|
Luo H, Wang J, Liu D, Zang S, Ma N, Zhao
L, Zhang L, Zhang X and Qiao C: The lncRNA H19/miR-675 axis
regulates myocardial ischemic and reperfusion injury by targeting
PPARα. Mol Immunol. 105:46–54. 2019. View Article : Google Scholar
|
|
101
|
Zhang X, Cheng L, Xu L, Zhang Y, Yang Y,
Fu Q, Mi W and Li H: The lncRNA, H19 mediates the protective effect
of hypoxia postconditioning against hypoxia-reoxygenation injury to
senescent cardiomyocytes by targeting microRNA-29b-3p. Shock.
52:249–256. 2019. View Article : Google Scholar
|
|
102
|
Choong OK, Chen CY, Zhang J, Lin JH, Lin
PJ, Ruan SC, Kamp TJ and Hsieh PCH: Hypoxia-induced H19/YB-1
cascade modulates cardiac remodeling after infarction.
Theranostics. 9:6550–6567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen C, Liu M, Tang Y, Sun H, Lin X, Liang
P and Jiang B: LncRNA H19 is involved in myocardial ischemic
preconditioning via increasing the stability of nucleolin protein.
J Cell Physiol. 235:5985–5994. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Su Q, Liu Y, Lv XW, Dai RX, Yang XH and
Kong BH: LncRNA TUG1 mediates ischemic myocardial injury by
targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol.
318:H332–H344. 2020. View Article : Google Scholar
|
|
105
|
Su Q, Liu Y, Lv XW, Ye ZL, Sun YH, Kong BH
and Qin ZB: Inhibition of lncRNA TUG1 upregulates miR-142-3p to
ameliorate myocardial injury during ischemia and reperfusion via
targeting HMGB1- and Rac1-induced autophagy. J Mol Cell Cardiol.
133:12–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang D, Yu J, Liu HB, Yan XQ, Hu J, Yu Y,
Guo J, Yuan Y and Du ZM: The long non-coding RNA TUG1-miR-9a-5p
axis contributes to ischemic injuries by promoting cardiomyocyte
apoptosis via targeting KLF5. Cell Death Dis. 10:9082019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Song T, Wang P and Xin L: LncRNA TUG1
Contributes to hypoxia-induced myocardial cell injury through
downregulating miR-29a-3p in AC16 cells. J Cardiovasc Pharmacol.
76:533–539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cai X, Wang S, Hong L, Yu S, Li B, Zeng H,
Yang X, Zhang P and Shao L: Long noncoding RNA taurine-upregulated
gene 1 knockdown protects cardiomyocytes against
hypoxia/reoxygenation-induced injury through regulating
miR-532-5p/Sox8 axis. J Cardiovasc Pharmacol. 76:556–563. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang S, Yao T, Deng F, Yu W, Song Y, Chen
J and Ruan Z: LncRNA MALAT1 promotes oxygen-glucose deprivation and
reoxygenation induced cardiomyocytes injury through sponging
miR-20b to enhance beclin1-mediated autophagy. Cardiovasc Drugs
Ther. 33:675–686. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yu SY, Dong B, Tang L and Zhou SH: LncRNA
MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in
ischemia-reperfusion injured heart. Int J Cardiol. 254:502018.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tian H, Wu M, Zhou P, Huang C, Ye C and
Wang L: The long non-coding RNA MALAT1 is increased in renal
ischemia-reperfusion injury and inhibits hypoxia-induced
inflammation. Ren Fail. 40:527–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xu XZ, Luo B, Xiao Y and Zheng WQ: Effects
of lncRNA MALAT1-mediated β-catenin signaling pathway on myocardial
cell apoptosis in rats with myocardial ischemia/reperfusion injury.
Eur Rev Med Pharmacol Sci. 23:9557–9565. 2019.PubMed/NCBI
|
|
113
|
Shu L, Zhang W, Huang C, Huang G, Su G and
Xu J: lncRNA ANRIL protects H9c2 cells against hypoxia-induced
injury through targeting the miR-7-5p/SIRT1 axis. J Cell Physiol.
235:1175–1183. 2020. View Article : Google Scholar
|
|
114
|
Li L, Zhang M, Chen W, Wang R, Ye Z, Wang
Y, Li X and Cai C: LncRNA-HOTAIR inhibition aggravates oxidative
stress-induced H9c2 cells injury through suppression of MMP2 by
miR-125. Acta Biochim Biophys Sin (Shanghai). 50:996–1006. 2018.
View Article : Google Scholar
|
|
115
|
Du J, Yang ST, Liu J, Zhang KX and Leng
JY: Silence of LncRNA GAS5 protects cardiomyocytes H9c2 against
hypoxic injury via sponging miR-142-5p. Mol Cells. 42:397–405.
2019.PubMed/NCBI
|
|
116
|
Liu CY, Zhang YH, Li RB, Zhou LY, An T,
Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, et al: LncRNA CAIF
inhibits autophagy and attenuates myocardial infarction by blocking
p53-mediated myocardin transcription. Nat Commun. 9:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li Z, Zhang Y, Ding N, Zhao Y, Ye Z, Shen
L, Yi H and Zhu Y: Inhibition of lncRNA XIST improves myocardial
I/R injury by targeting miR-133a through inhibition of autophagy
and regulation of SOCS2. Mol Ther Nucleic Acids. 18:764–773. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li Y, Li J, Zhang P, Jiang X, Pan Z, Zheng
W and Lin H: LncRNA-LET relieves hypoxia-induced injury in H9c2
cells through regulation of miR-138. J Cell Biochem. 121:259–268.
2020. View Article : Google Scholar
|
|
119
|
Li T, Tian H, Li J, Zuo A, Chen J, Xu D,
Guo Y and Gao H: Overexpression of lncRNA Gm2691 attenuates
apoptosis and inflammatory response after myocardial infarction
through PI3K/Akt signaling pathway. IUBMB Life. 71:1561–1570. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen L, Zhang D, Yu L and Dong H:
Targeting MIAT reduces apoptosis of cardiomyocytes after
ischemia/reperfusion injury. Bioengineered. 10:121–132. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kong F, Jin J, Lv X, Han Y, Liang X, Gao Y
and Duan X: RETRACTED: Long noncoding RNA RMRP upregulation
aggravates myocardial ischemia-reperfusion injury by sponging
miR-206 to target ATG3 expression. Biomed Pharmacother.
109:716–725. 2019. View Article : Google Scholar
|
|
122
|
Ong SB, Katwadi K, Kwek XY, Ismail NI,
Chinda K, Ong SG and Hausenloy DJ: Non-coding RNAs as therapeutic
targets for preventing myocardial ischemia-reperfusion injury.
Expert Opin Ther Targets. 22:247–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Basile DP, Donohoe D, Roethe K and Osborn
JL: Renal ischemic injury results in permanent damage to
peritubular capillaries and influences long-term function. Am J
Physiol Renal Physiol. 281:F887–F899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Tao Q, Tianyu W, Jiangqiao Z, Zhongbao C,
Xiaoxiong M, Long Z and Jilin Z: Expression analysis of long
non-coding RNAs in a renal ischemia-reperfusion injury model. Acta
Cir Bras. 34:e2019004032019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Liu F, Yang Y, Liu T, Deng J, Zhang H, Luo
D and Lou YL: Analysis of differentially expressed long noncoding
RNA in renal ischemia-reperfusion injury. Kidney Blood Press Res.
45:686–701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kölling M, Genschel C, Kaucsar T, Hübner
A, Rong S, Schmitt R, Sörensen-Zender I, Haddad G, Kistler A,
Seeger H, et al: Hypoxia-induced long non-coding RNA Malat1 is
dispensable for renal ischemia/reperfusion-injury. Sci Rep.
8:34382018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Puthanveetil P, Gutschner T and Lorenzen
J: MALAT1: A therapeutic candidate for a broad spectrum of vascular
and cardiorenal complications. Hypertens Res. 43:372–379. 2020.
View Article : Google Scholar
|
|
128
|
Jiang X, Li D, Shen W, Shen X and Liu Y:
LncRNA NEAT1 promotes hypoxia-induced renal tubular epithelial
apoptosis through downregulating miR-27a-3p. J Cell Biochem.
120:16273–16282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Geng X, Song N, Zhao S, Xu J, Liu Y, Fang
Y, Liang M, Xu X and Ding X: LncRNA GAS5 promotes apoptosis as a
competing endogenous RNA for miR-21 via thrombospondin 1 in
ischemic AKI. Cell Death Discov. 6:192020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xu Y, Niu Y, Li H and Pan G:
Downregulation of lncRNA TUG1 attenuates inflammation and apoptosis
of renal tubular epithelial cell induced by ischemia-reperfusion by
sponging miR-449b-5p via targeting HMGB1 and MMP2. Inflammation.
43:1362–1374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tian X, Ji Y, Liang Y, Zhang J, Guan L and
Wang C: LINC00520 targeting miR-27b-3p regulates OSMR expression
level to promote acute kidney injury development through the
PI3K/AKT signaling pathway. J Cell Physiol. 234:14221–14233. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lu J, Miao J and Sun J: LncRNA np_5318
promotes renal ischemia-reperfusion injury through the TGF-β/Smad
signaling pathway. Exp Ther Med. 19:2833–2840. 2020.PubMed/NCBI
|
|
133
|
Zhou X, Li Y, Wu C, Yu W and Cheng F:
Novel lncRNA XLOC_032768 protects against renal tubular epithelial
cells apoptosis in renal ischemia-reperfusion injury by regulating
FNDC3B/TGF-β1. Ren Fail. 42:994–1003. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lorenzen JM, Schauerte C, Kielstein JT,
Hübner A, Martino F, Fiedler J, Gupta SK, Faulhaber-Walter R,
Kumarswamy R, Hafer C, et al: Circulating long noncoding RNATapSaki
is a predictor of mortality in critically ill patients with acute
kidney injury. Clin Chem. 61:191–201. 2015. View Article : Google Scholar
|
|
135
|
Shah RJ and Diamond JM: Primary graft
dysfunction (PGD) following lung transplantation. Semin Respir Crit
Care Med. 39:148–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li J, Wei L, Han Z, Chen Z and Zhang Q:
Long non-coding RNA X-inactive specific transcript silencing
ameliorates primary graft dysfunction following lung
transplantation through microRNA-21-dependent mechanism.
EBioMedicine. 52:1026002020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Arun G, Aggarwal D and Spector DL: MALAT1
long non-coding RNA: Functional implications. Noncoding RNA.
6:222020.
|
|
138
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun Y and Ma L: New insights into long
non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel).
11:2162019. View Article : Google Scholar
|
|
140
|
Li P, Zhang Y and Liu H: The role of
Wnt/β-catenin pathway in the protection process by dexmedetomidine
against cerebral ischemia/reperfusion injury in rats. Life Sci.
236:1169212019. View Article : Google Scholar
|
|
141
|
Lehwald N, Tao GZ, Jang KY, Sorkin M,
Knoefel WT and Sylvester KG: Wnt-β-catenin signaling protects
against hepatic ischemia and reperfusion injury in mice.
Gastroenterology. 141:707–718. 718.e1–e5. 2011. View Article : Google Scholar
|
|
142
|
Ban Q, Qiao L, Xia H, Xie B, Liu J, Ma Y,
Zhang L, Zhang M, Liu LG, Jiao W, et al: β-catenin regulates
myocardial ischemia/reperfusion injury following heterotopic heart
transplantation in mice by modulating PTEN pathways. Am J Transl
Res. 12:4757–4771. 2020.
|
|
143
|
Xiong ZJ, Zhang Q, Wang DX and Hu L:
Overexpression of TUG1 promotes neuronal death after cerebral
infarction by regulating microRNA-9. Eur Rev Med Pharmacol Sci.
22:7393–7400. 2018.PubMed/NCBI
|
|
144
|
Jia H, Li Z, Chang Y, Fang B, Zhou Y and
Ma H: Downregulation of long noncoding RNA TUG1 attenuates
MTDH-mediated inflammatory damage via targeting miR-29b1-5p after
spinal cord ischemia reperfusion. J Neuropathol Exp Neurol.
80:254–264. 2021. View Article : Google Scholar
|
|
145
|
He Z, Zhao Y, Zhu Y, Wang W, Liu X and Lu
F: Interfering TUG1 attenuates cerebrovascular endothelial
apoptosis and inflammatory injury after cerebral
ischemia/reperfusion via TUG1/miR-410/FOXO3 ceRNA axis. Neurotox
Res. 40:1–13. 2022. View Article : Google Scholar
|
|
146
|
Wu X, Liu Y, Mo S, Wei W, Ye Z and Su Q:
LncRNA TUG1 competitively binds to miR-340 to accelerate myocardial
ischemia-reperfusion injury. FASEB J. 35:e211632021.
|
|
147
|
Chen L, Xu JY and Tan HB: LncRNA TUG1
regulates the development of ischemia-reperfusion mediated acute
kidney injury through miR-494-3p/E-cadherin axis. J Inflamm (Lond).
18:122021. View Article : Google Scholar
|
















