There is a delicate balance between immune tolerance
and responsiveness against foreign assault. If the balanceis
shifted towardstolerance it may underpin the development of
pathological conditions, such as cancer. However, if the immune
system is overly responsive, this may induce autoimmune diseases
and allergic disorders (1,2). Hyper responsiveness of the immune
system is responsible for different allergic conditions in atopic
individuals (3). Studies have
estimated that >25% of the population in developed countries
suffers from immunoglobulin (IgE)-mediated allergies or ‘type I
hypersensitivity’ (4–6). Allergen-specific immunotherapy (AIT),
also known as ‘allergy vaccination’ or ‘desensitization’, is a
treatment that fine-tunes the defense system of the body to become
tolerant to a specific allergen over a period of time (7,8). AIT
is accompanied by several potential drawbacks, such as local and
systemic immune reactions during AIT administration, and variable
patient outcome (9,10). Despite the risk and differential
response among individuals, AIT is still the most effective
approach and the only therapeutic approach that is specific for the
treatment of allergy. AIT reduces the activation and proliferation
of lymphocytes in response to allergenic stimulus and further
enhances the immune tolerance mechanisms towards specific allergens
(11). Principally, during AIT,
the allergen is administered to the host via different routes at
increasing concentrations to achieve an effective immunotherapy
(12). Theprocessis divided into
twophases: The ‘build up phase'and the ‘maintenance phase’
(13).
During the first phase, or ‘build up phase’ of
immunotherapy, an increasing dose of the therapeutic formulation is
administered to the host 2 or 3 times in a week, which enhances the
allergen tolerance over time (14). The length of this phase varies
based on the frequency of injections and effectiveness of the
therapeutic formulation but generally ranges between 3 and 6 months
(14,15). After achieving an effective dose in
the ‘buildup phase’, the second phase of AIT is started as a
‘maintenance phase’ (15). The gap
between therapeutic injections throughout the maintenance phase
varies and may range from 2–4 weeks to 2–5 years (15,16).
This desensitization process increases the threshold dose of the
allergen to cause allergic reactivity (13). Several studies have provided
evidence that administration of a suitable allergen for
immunotherapy not only provides protection against its own
allergenicity but also reduces the probability of developing
sensitization against other allergens (17,18).
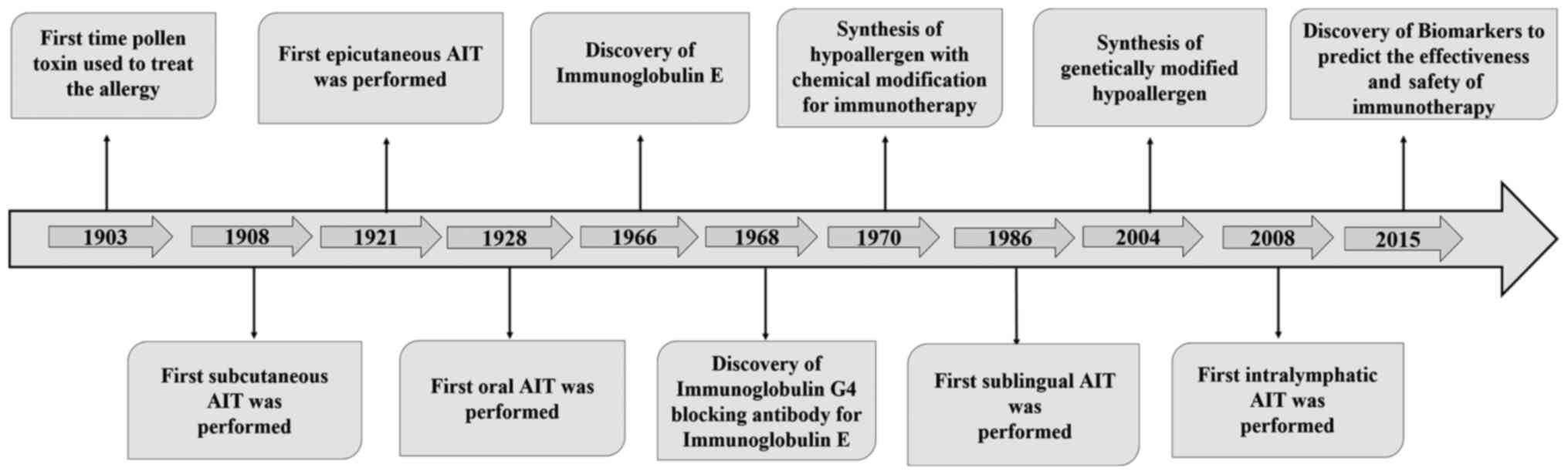
The first specific immunotherapy for grass pollen-induced hay fever
was introduced in 1903 (19).
However, over time, the treatment options with different routes for
AIT expanded the scope of immunotherapy to other allergic diseases
(20–24). The identification of IgE and a
blocking antibody of IgE, IgG4, provided a leap forward in the
field of AIT (25,26). With further advances in
technologies in later years, such as the synthesis of chemically
modified and genetically engineered allergens with low allergenic
activity and their use for AIT, the scope of modifying the
allergens for an improved clinical outcome broadened (27,28).
In addition, novel biomarkers have been identified that could be
useful for monitoring the effectiveness and predicting the safety
of immunotherapy (29). The
practices of allergen immunotherapy have been knowingly or
unknowingly used for several decades. Fig. 1 provides a roadmap of findings
associated with AIT (19–30). The present review attempts to
provide a comprehensive overview of the history and diverse
clinical applications of AIT, and to explore developments in the
scientific understanding of therapy along with future
perspectives.
Allergens are a complex mixture of allergenic and
non-allergenic ingredients comprising single or multitudinous
combinations of proteins, carbohydrates, lipids and glycoproteins
(31). The allergenic ingredients
that are responsible for induction of allergy could potentially
also be used for the diagnosis and specific immunotherapy of the
same allergy (32). Usually, the
therapeutic formulations are directly prepared from a natural
source of allergen, which contains allergenic as well as
non-allergenic components (31,32).
The concentration of an allergen for inducing effective
immunotherapy depends on several bio-variable factors, such as the
ratio of allergic and non-allergic ingredients, their quantity,
processes used for their isolation and purification, and genetic
makeup of the affected atopic individual (33). Crude allergenic extract is
extensively used for immunotherapy; however, four major problems
are often witnessed during the course of this method: i) Allergen
extracts comprise a variety of allergenic as well as non-allergenic
proteins, other macromolecules and toxic ingredients, which is
often difficult to standardize involving high batch-to-batch
variability; ii) development of specificities against newer
proteins; iii) unpredictable clinical response due to systemic
administration of intact allergens through extracts; and iv)
effective therapeutic doses are often difficult to achieve due to a
lack of standardized extracts (33–35).
Therefore, the standardization of allergen extracts from their
natural source is necessary to diminish their allergenic potential
and to ensure their consistent composition and potency for AIT
(36). At present, various
injectable and non-injectable, Food and Drug Administration
(FDA)-approved allergen extract-based therapeutic formulations are
used for the diagnosis and treatment of different allergic diseases
(37–39). However, the majority of the
FDA-approved allergen extract-based therapeutic formulations are
non-injectable. For example, to treat allergic rhinitis and
conjunctivitis, the FDA-approved GRASTEK (timothy grass pollen
allergen extract), ODACTRA (house dust mites allergen extract),
RAGWITEK (short ragweed pollen allergen extract) and ORALAIR (sweet
vernal, orchard, perennial rye, Timothy and Kentucky bluegrass
mixed pollens allergen extract) are available as tablets for
sublingual AIT (37,40–42).
Similarly, PALFORZIA (peanut allergen powder) is also an
FDA-approved allergen extract-based therapeutic formulation that is
used for the treatment of peanut allergy through oral immunotherapy
(OIT) (38,43). In addition, numerous other
injectable and non-injectable allergen extract-based therapeutic
formulations are being investigated in different phases of clinical
trials (39,44,45).
Table I provides an overview of
allergen extracts approved by the FDA or undergoing clinical
trials.
At present, novel ways are being developed to
introduce chemical modifications in the allergenic extracts
intending to lower their allergenic potential without affecting the
immunogenicity, and such modified extracts are termed as
‘allergoids’ (27,46,47).
To enhance the efficacy of the immunotherapeutic approaches and
decrease the allergenic properties of a given protein, different
chemical, structural and recombinational modifications can be
introduced in the allergen (34).
The generation of hypoallergenic hybrid molecules through
conjugation of allergens to adjuvant substances activating innate
immune cells, such as CpG oligonucleotides, carbohydrate-based
particles, or nanosized therapeutic formulations are examples of
these modifications (48–50). These alterations are primarily
intended to modify the IgE-specific epitopes present on allergens,
while keeping the T cell epitopes intact (51). Methods of chemical modifications to
prepare hypo allergens are advantageous over others as they can be
applied on different homogenous as well as heterogeneous types of
allergens. For example, coupling of allergens with polyethylene
glycol, glutaraldehyde and formaldehyde has been illustrated to
modify the IgE epitopes of allergens (34,51).
Similarly, treatment of allergens with maleic acid anhydride
generates recognition sites for different scavenger receptors in
allergens, thereby facilitating their intake by phagocytic cells,
and immunotherapy with these hypo allergens has been observed to
induce the type 1 helper cell (Th1) dominated immune response
(52,53). Similarly, conjugation of allergens
with synthetic oligo-deoxy nucleotides carrying immune-stimulatory
CpG sequences from bacterial DNA can mask IgE-specific epitopes on
allergens and could potentially block the cross-linking between
allergens and IgE bound to high affinity IgE receptor (FcεRI)
present on the surface of mast cells and basophils (54). In order to further refine this
strategy, further investigations that compare the relative
efficiency of the chemical modifications and determine their
potential synergistic or additive effects are required.
Recombinant DNA technology has also been used for
targeted alteration in allergenic proteins by means of mutations,
deletions, fusion, site-directed mutagenesis and hybridization
(32). Recombination of the genes
of allergens requires knowledge of their sequences and positions of
amino acids, as well as their three-dimensional conformation, which
is helpful for targeted modification (34,55).
Using this approach, hypo allergens for the timothy grass Phil p 5b
and American Cockroach Per a 1 allergen have been successfully
prepared by deleting the IgE epitopes present in the corresponding
gene segments, and the consequent hypo allergens were observed to
have reduced IgE binding properties, reduced histamine-releasing
activity and reduced skin reactivity (56,57).
After modification, allergens may exhibit low allergenicity but
also carry the risk of generating new epitopes that may induce
allergic reactions (48).
Therefore, it is important to subject the newly synthesized hypo
allergens to pertinent in vitro and in vivo
evaluation tests before approving them for therapeutic
applications.
Allergy is fundamentally an undesirable hyperactive
immune response to allergens, which occurs due to a breach of
peripheral tolerance and dysregulation of immune homeostasis
mediated by cellular and molecular factors, such as TLR4 or TLR8,
regulatory T cells (Tregs), T cell, immunoglobulin and mucin and
allergen-specific MHC class II tetramer+ cells (58,59).
Forkhead box P3 (FoxP3)-positive Tregs are pivotal for generating
tolerance against self-antigens and harmless non-self-antigens
(60,61). Usually, Tregs present at the
mucosal surfaces suppress the immune cells involved in the
mediation of allergic responses, such as type 2 CD4+
helper cells, mast cells and eosinophils (62,63).
Distinct approaches have been used in various AIT studies; however,
there is a profound overlap in the mechanism underlying these AITs
and their allergen-specific tolerogenic features (64,65).
The major differences among various approaches primarily involve
the role of antigen presenting cells (APCs) associated with
differentroutes of immunotherapy, memory cell or Treg responses,
characteristic immunoglobulins produced, and interaction with other
immune cell types present at the interface niche, where the primary
encounter of the tolerogenic protein occurs with the host (12,66,67).
Notably, the APC phenotype present at the host-environment
interface servesan important role in peripheral insensitivity or
immunogenicity to innocuous antigens (68,69).
For example, dendritic cells (DCs) are specialized
antigen-presenting cells, which initiate and sustain allergic
inflammation, or support tolerance induction (70,71).
After being triggered by an antigen, immature DCs polarize into
either dendritic cells-1, dendritic cells-2 (DC2s), dendritic
cells-17 or regulatory dendritic cells (DCregs), which induce the
differentiation of naïve T cells into Th1, type 2 helper (Th2) or T
helper 17 cell (Th17), or Tregs, respectively (29,71,72).
Compared with immature DCs, DCregs or tolerogenic DCs represent an
intermediate stage of DC maturation characterized by higher
expression levels of class II major histocompatibility complex
(MHC) and co-stimulatory molecules, but often lack the capacity of
proinflammatory cytokine secretion (73,74).
The DCs involved in AIT are primarily myeloid DCs (mDCs) and
Langerhans cells (LCs), which are also characterized by expression
of FcεRI on their surface (75,76).
mDCs secrete IL-12, which tilts the Th1/Th2 balance towards Th1
responses, whereas LCs promote the development of T helper 3
regulatory cells via production of IL-10 and TGF-β, thereby
attenuating the Th2 immune responses (71,75).
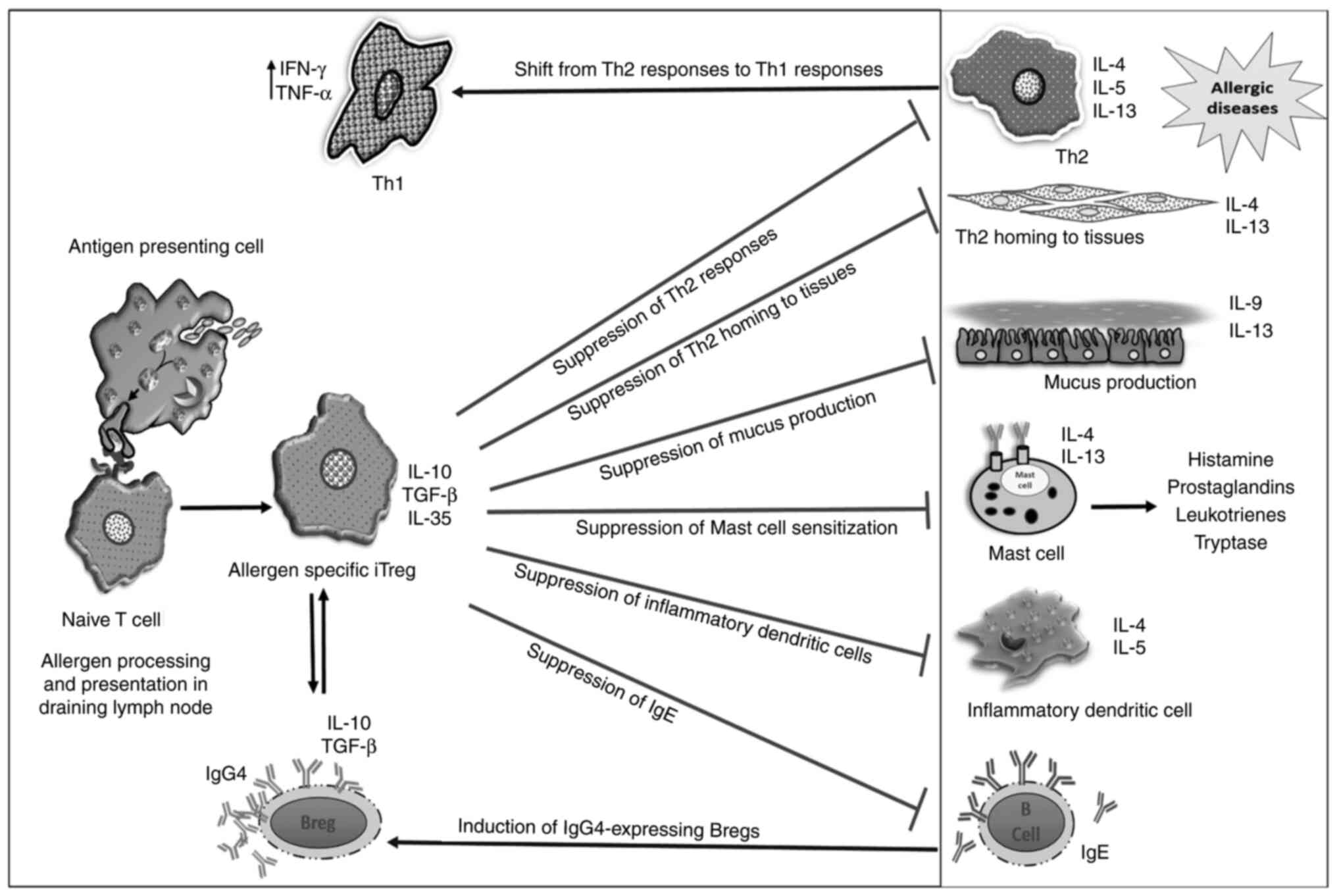
The induction of allergen-specific Tregs is the central factor in
all types of AITs; however, how the allergen-specific effector T
cells or naïve T cells transform into allergen-specific Tregs is
not well understood. It would appear that the myriad of signals
present in the microenvironment of immature DCs after the
phagocytosis of allergens orchestrates the development of the
Th2-mediated allergic response or tolerogenic response against
allergens spearheaded by Tregs (76,77).
It has been demonstrated in several models that coincident exposure
of pathogens or endotoxin with allergen may lead to onset of
IgE-mediated allergic responses (78,79).
However, in the absence of pathogenic signals during AIT, the
immature DC sunder go tolerogenic interaction with T cells of the
lymph node (80). This promotes
the development of IL-10, TGF-β and IL-35-secreting Tregs, thereby
inducing allergen-specific peripheral tolerance (81–83).
These suppressive cytokines (IL-10 and TGF-β) are
known to inhibit the differentiation, proliferation and activation
of effector T cells, and further bring about desensitization of
mast cells and basophils (84).
IL-10 acts by decreasing the production of allergen-specific IgE,
while increasing the levels of immunoglobulin G4 (IgG4) and
immunoglobulin G2a (IgG2a) secretion from B cells (85). In addition, TGF-β is also involved
in the induction of allergen-specific tolerance during AIT
(86). Tregs are the major source
of TGF-β, which affects T cell proliferation and differentiation,
and inhibits Th2 differentiation by suppressing GATA binding
protein 3 (GATA-3) expression and IL-4-mediatedSTAT6 activity
(87–89).
Apart from the suppressive Tregs and DCregs, a
population of IL-10-secreting suppressor B cells has also been
identified, and this is known as regulatory B cells (Bregs)
(90,91). The primary function of Bregs is to
support immunological tolerance and inhibit unwanted inflammation
(92). IL-10-secreting Bregs serve
an important role in the tolerance induction during AITs (93). IL-10 is a key suppressive cytokine
associated with Bregs; however, TGF-β and IL-35 have also been
identified as Breg-associated suppressor molecules (94,95).
Different subsets of Bregs have been described in humans and a
defective development and function of Bregs may result in various
chronic inflammatory diseases, such as collagen-induced arthritis
and chronic hepatitis B virus infection (96,97).
Although IL-10 secretion is common to all Bregs, they are further
grouped into different subsets based on their differential
functions (98). The
immature/transitional Bregs
(CD19+CD24hiCD38hi) suppress
effector T cells but enhance Treg function (99). Similarly, another sub-population of
Bregs, known as memory B Cells/B10 Bregs
(CD19+CD24hiCD27+), enhance and
stabilize the expression of FoxP3 in Tregs (100,101). Another subset of Bregs
(CD25highCD71highCD73low) prevents
peripheral tolerance by producing IL-10 and blocking antibody IgG4
(93,102). Notably, it has been reported that
the relative percentage of CD19+
CD24hiCD27+ Bregs was decreased in patients
with allergic rhinitis, whereas an increase in the percentage of
CD19+CD24hiCD38hi Bregs was
observed in comparison with healthy individuals; however, the
significance of this finding is unclear (103). It has been noted that Bregs serve
a critical role in effective AIT. After AIT, the percentage of
IL-10 and IgG4-secreting Bregs increases, which suppresses the
allergen-specific CD4+ T-cell proliferation and further
ameliorates the allergic airway inflammation via FoxP3-positive T
regulatory cells (93,104). In another study conducted on bee
venom antigen allergic patients subjected to AIT, an enhanced
percentage of IL-10 and IgG4-secreting
CD25hiCD71hiCD73low Bregs, which
potently suppress allergen-specific CD4+ T-cell
proliferation and produce increased amounts of IgG4, was found
(93). Notably, a 10-100-fold
increase in serum allergen-specific IgG4 isotype has been observed
for AITs (85). IgG4 functions as
a blocking antibody for IgE and considerably reduces the binding of
IgE to its receptor present on the surface of mast cells and
basophils (105). This process
prevents mast cell degranulation, which in turn downregulates the
activity of eosinophils and neutrophils (106). IgG4 antibodies also inhibit the
proliferative response of T-cell clones by blocking IgE-facilitated
allergen binding to B cells and thereby inhibiting the presentation
of allergenic peptides by B-cells to allergen-specific T-cell
clones (93). Additionally, an
increase in IgG2 a in the serum shifts the Th1/Th2 immune response
towards a Th1-dominated immune response (107). Despite early generation of Tregs
following AIT, it may still take years to effectuate a marked
reduction in IgE levels in the allergic individuals (108). An analysis of the mechanisms of
AIT has been summarized in Fig. 2,
and a comparison of various AITs is presented in Table II.
The most important factor, which contributes to the
duration of AIT, is the route of administration and this has a
marked influence on the clinical outcome of immunotherapy (12). There a large variations in the
immune niche present at various external tissue interfaces
associated with different AITS, which serve a major role in fine
orchestration of immune responses (12,109,110). The routes of administration of
AIT can be categorized into subcutaneous immunotherapy (SCIT),
sublingual immunotherapy (SLIT), OIT, intralymphatic immunotherapy
(ILIT) and epicutaneous immunotherapy (EPIT).
Historically, SCIT has been the first form of
immunotherapy, where in a small amount of allergen extract is
administered by injectioninto the subcutaneous layer of skin and
this is commonly called an‘allergy shot’ (111). It was used for the first time
approximately a century ago by Noon (20,112) in 1911 as a useful measure to
tackle hay fever symptoms (20,112). Until the discovery of IgE in
1965, SCIT was used without having a proper understanding of the
primary allergic mediators and the regulatory mechanisms targeted
by immunotherapy (20,113). However, in a number of cases, the
therapy proved effective in reducing the symptoms of allergic
diseases for a prolonged period (114). Previous studies have demonstrated
that improvements can be observed within 3 months after initiation
of therapy, and those benefits may be long lasting with decreases
in seasonal symptoms and use of anti-allergic medications, which
further persisted for at least 2 years even after discontinuation
of immunotherapy (115,116). A case study of apatient with
atopic keratoconjunctivitis revealed that SCIT was fairly
successful in controlling the allergic symptoms and disease
exacerbation (117). The
therapeutic benefits of SCIT are attributed to different types of
regulatory mechanisms. In a study comparing the efficacy of SLIT
vs. SCIT against grass allergy, it was demonstrated that SCIT could
induce comparatively high levels of IgE blocking antibody IgG4, by
suppressing Th2 cytokine production more efficiently (114,118). The subcutaneous administration of
allergens activates IL-10-secreting DCs, which further induces
IL-10 or TGF-β secretion from Tregs, thereby establishing the
homeostatic balance between Th1 and Th2 cytokines. IL-10 secreted
by Tregs induces B cells to secrete allergen-specific IgE-blocking
antibodies, such as IgG4 andimmunoglobulin A, which further trap
allergens before their binding to receptor-bound IgE (119–121). The enhanced IL-10 also leads to
induction of specific non-reactivity of allergen-specific T cells
during the later phase of therapy either by inducing clonal anergy
in allergen-specific effector T cells or by generating
immunosuppressive Tregs (114,122). Principally, in SCIT, the target
APCs are DCs of the non-vasculature part of the skin, i.e.,
subcutaneous administration of allergens modulates the distribution
of various subpopulations of DCs and their ability to produce
different proinflammatory cytokines, such as IFN-α and IL-6
(71,123). In addition, SCIT alters the
distribution of type 2 innate lymphoide cells (ILC2s), which have
been recognized to servean important role in the initiation and
establishment of allergic responses through production of thetype 2
cytokines IL-5 and IL-13 (124).
ILC2s also empower DCs to potentiate memory T helper 2 cells, and
thus may enforce the recall immune response against allergens
(125).
Despite its potential efficacy, SCIT has been found
to trigger adverse allergic reactions, including rashes at the site
of injection, swelling, itchiness, breathlessness and even
anaphylaxis leading to death, numerous times (126). Furthermore, it takes a long time
(>50 injections in 3–5 years) to achieve the effective
therapeutic dose for sustained allergen tolerance (114).
Unlike during SCIT, where allergy shots are
administered through injection, during SLIT, a minute amount of
allergen extract is kept under the tongue of the patient, held for
2 min and then swallowed, thus avoiding the irritation of injection
(127–129). However, the doses administered
during SLIT are restricted by the available concentration of the
allergen extract and the volume of liquid that can be held under
the tongue (129,130). During the initial 4–6 months of
SLIT, the allergen extract with a low allergenic potential is
administered to the patients at gradually increasing doses,
followed by a constant maintenance dose administered daily for up
to 3 years (115). Compared with
SCIT, where subcutaneous DCs are important, LCs are central to
tolerance development during SLIT (71,131). The LCs prominently express high
affinity IgE receptor (FcεR1), MHC class I and II, and other
co-stimulatory and co-inhibitory receptors on their surface, which
makes them suitable for receptor-mediated IgE-dependent allergen
uptake and subsequent presentation to T cells (132). This triggers the transformation
of naive T-cells into Tregs implicated in allergen-specific immune
tolerance (133,134). In addition, cross-linking of
allergen-bound IgE with FcεRI on oral LCs results in the production
of IL-10, which facilitates inflammation resolution (135). Notably, during the early phase of
SLIT, IL-10 is also contributed by allergen-specific
IL-10-producing Tregs, thus establishing a concordance between the
innate and adaptive arms of immunity for induction of a tolerogenic
microenvironment (136,137). In a clinical study, it was
observed that 12 months of SLIT against house dust mite allergy was
advantageous in inducing allergen tolerance (127). Several clinical trials have also
illustrated the clinical efficacy of single allergen tablets (grass
and ragweed) and extract solution (ragweed) at the primary level
(37,42). After getting clinical approval from
the FDA, SLIT has been commercialized in several parts of the world
(127,128,131).
Compared with SCIT, SLIT delivers a more
satisfactory clinical outcome in children and adults as
demonstrated by its efficacy to prevent reoccurrence of allergic
symptoms for a longer period (118). According to World Allergy
Organization, SLIT is the most innocuous immunotherapy that is used
as an alternative to injection-based immunotherapy (46,138). Importantly, considering the
relative safety of SLIT in clinical trials over the years and
standardization of modalities with several allergen extracts, such
as ragweed and grass pollen, certain SLIT tablets have also been
permitted to be taken at home without medical supervision (138). At present, this is the only form
of immunotherapy that provides this flexibility to allergic
patients. The local reactions during SLIT are mild and resolve
themselves without requiring any allergen dose adjustment or
adjunct medication (129). In an
observational safety study evaluating the safety of SLIT, only 11
out of 65 children subjected to SLIT were reported to exhibit
adverse reactions, and even the observed reactions were not severe
enough to necessitate modification or discontinuation of therapy
(129,139). At present, there has been no
report of mortality associated with SLIT (140).
In OIT, an allergen extract is taken either in
encapsulated form or administrated with an aqueous solution
(145). The swallowed allergen
extract is adsorbed by the gut mucosal membrane and phagocytosed by
APCs in the gastrointestinal tract, which further stimulates gut
mucosal Tregs (146,147). OIT has been observed to provide
symptomatic relief in allergic asthma through induction of blocking
antibody (IgG4) with concurrent reduction in serum IgE levels
(148). In a study monitoring the
sustained clinical efficacy of OIT, wherein 24 volunteers were
subjected to 5 years of peanut OIT, half of the volunteers
developed the capability to tolerate 5 g in a double-blind,
placebo-controlled food challenge and could successfully
incorporate peanut into their diet (149). In spite of several reports
demonstrating the therapeutic efficacy of OIT, there is also a
considerable risk of serious allergic reactions and anaphylaxis
involved with OIT, which has restricted the over-the-counter sale
of OIT allergy shots (150–152).
EPIT for the treatment of allergies was first
introduced in early 1917. The procedure of EPIT requires
administration of an allergen to the epicutaneous layer of the
skin, where large numbers of professional antigen presenting LCs
facilitate the trafficking of allergens into the lymph nodes
(153). EPIT is a strategy
gradually evolving for the treatment of different allergic
conditions and particularly food allergy, considering the potential
risk associated with OIT (154).
In the context of food allergy, another important phenomenon which
requires particular mention is ‘gut homing’, which is migration of
T and B cells from primary lymphoid organs to the inflamed and
non-inflamed regions of the intestine (155). Food allergy is characterized by a
disturbed gastrointestinal immune environment and gut homing, which
hampers the movement of tolerogenic Tregs into gastrointestinal
immunological tissues. This compromises the body's ability to cope
with local and systemic immune responses induced by the oral
administration of harmless antigens, such as food proteins
(156). EPIT can regenerate
gut-homing via selective expansion of unique TGF-β+
Tregs, which impart protection against anaphylactic reactions
(157,158). Furthermore, EPIT provides a
naturally safer alternative to other AITs, because the allergen
delivered through the epicutaneous layer of the skin reaches the
systemic circulation in minute amounts compared with other routes
of administration (159). The
epicutaneous Viaskin® Patch- (EV patch) system has been
developed for EPIT, which enhances the allergen delivery across
intact skin (160). Therapeutic
formulation or allergen extract can be directly applied on the
groove of the EV patch, which facilitates allergen exposure to APCs
in the superficial layers of the skin (161). Repeated application of the EV
patch in mice for 8 weeks resulted in desensitization with no
significant increase in histamine after oral challenge with
allergen (160). The advancements
indicate that the epidermal layer of the skin with a
non-vasculature system could be exploited as amoresuitable route
for immunotherapy with fewer side effects.
ILIT is characterized by the delivery of allergen
directly into lymph nodes, which generates immune tolerance earlier
compared with other AITs (162).
ILIT came into consideration when it was observed that only a
minute amount of allergen was channelized into the lymph node when
administered through other routes (163). In this regard, intralymphatic
administration of allergens is associated with a marked enhancement
in the effectiveness of allergy vaccination, even with a low dose
of allergen (164,165). In a murine model, it has been
demonstrated that ILIT induces higher levels of serum cytokines,
such as IL-10, IL-4 and IL-2, compared with SCIT (166). In congruence, ILIT also
encourages the switching of Th2-dependent hyperactive allergic
responses to Th1 phenotype, which boosts the production of IgG2a
and IgG4, albeit with a markedly lower dose of allergen compared
with SCIT (167). In an open
trial study to determine the safety and efficacy of ILIT, 6
patients were subjected to intralymphatic inguinal injections of
either birch or grass-pollen extract, and all patients stayed
healthy and reported symptomatic relief from allergy alongside
decreased medicinal requirement (24). In another study comparing the
therapeutic outcome of SCIT vs. ILIT in patients with pollen
allergy, ILIT was observed to be more efficacious in reducing the
frequency of rescue medication and provided improved symptomatic
relief with reduced skin-prick sensitivity in patients (168). Furthermore, allergen tolerance is
induced markedly faster in the ILIT, as early as by 4 months,
compared with other AITs, which take 2–5 years (169,170).
Since the fundamental process of immunotherapy
involves challenging the sensitized patients with increasing doses
of allergen, there is always an acute possibility of undesirable
minor to severe allergic reactions. Furthermore, during the ‘build
up’ or ‘escalation’ phase of immunotherapy, local and systemic
reactions are often witnessed with increasing doses, which impedes
the procedural efforts to achieve a therapeutically active
‘maintenance’ dose (15).
Furthermore, due to huge variations in the sensitivity of different
patients to an allergen, the therapeutic formulation applicable for
one atopic individual may not be promising for others (10). Another confounding factor is the
variation in the composition of allergenic extracts available for
immunotherapy from different manufacturers, which may arise due to
differences in allergen sources and allergen extract preparation
protocols (171). Allergen
extracts prepared from divergent natural sources may get
contaminated with pathogens and allergens from other sources, which
yields undesirable immunogenicity and even new IgE-mediated
allergies (46). One of the causes
underlying the limited success and variable outcome of
immunotherapeutic approaches so far is the absence of standardized
procedures and regulatory guidelines for preparation of allergen
extracts and their characterization (10,172). Another major bottleneck hindering
the progress of AIT is the lack of appropriate biomarkers that can
predict the efficacy of AIT (29).
The present review summarizes a consensus on the
AIT guidelines followed by regulators on a global scale, which are
fundamentally based on the factors influencing the therapeutic
efficacy of AIT (Table III).
These guidelines are largely based on meta-analyses, which include
reports published over the past two decades, and primarily aim to
ascertain the efficacy and safety of AIT (10,126,173–177). However, at present, the drug and
vaccine safety monitoring system for AIT is poorly organized and is
only based on the voluntary reporting of side effects and efficacy.
In this regard, there is a clear need to institutionalize dedicated
monitoring systems of allergen immunotherapy outcomes and to
further streamline uniform regulatory guidelines on the modalities
of AIT.
At present, no surrogate biomarkers that can
predict the effectiveness of AIT have been identified (178). Decreased levels of
allergen-specific IgE and increased levels of serum IgG4 have been
acknowledged as biomarkers to predict the clinical efficacy of AIT
(179,180). Increased numbers of IL-10 and
TGF-β-producing Tregs during and after immunotherapy are also
crucial emerging biomarkers to assess the clinical response of AIT
(29,181). In addition, AIT induces other
molecular markers in DC2s, such as CD141, GATA-3 and
receptor-interacting serine/threonine kinase 4, as well as in
DCregs, such as complement C1q chain receptor variant IIIA of IgG
Fc, which can also be used as a potential biomarker to predict the
efficacy of AIT (182–184). However, neither of these
biomarkers is appropriate to precisely predict the prognosis and
clinical efficacy of immunotherapy in all AIT-receiving patients.
Therefore, it is further required to refine the understanding of
the specific mechanistic involvement of these biomarkers in the
successful progression of AIT. This will help in monitoring the
progress of therapy and integrating appropriate solutions, which
will improve the clinical outcome.
Another growing concern is the problem of
‘polysensitization’, which is a sensitivity of atopic individuals
to two or more allergens, and this condition is referred to as
‘polyallergy’ after clinical confirmation (185). According to estimates, 60–80% of
allergic patients are polysensitized (186). An increasing prevalence of
polyallergy has been documented with age, which necessitates the
development of immunotherapeutic approaches that can take care of
more than one allergen simultaneously (186,187). However, an additive preparation
of mixture of allergens for simultaneous AIT may not yield the
desirable outcome, as one allergen may affect the stability and
optimal dose of the other allergen, thus affecting its
immunotherapeutic potential, efficacy and even safety (188–190). In this regard, the European
Medicines Agency has suggested that AIT should not be performed
with a mixture of two non-homologous allergens, and should be
performed separately for seasonal or perineal allergens (10). This creates the problem and
annoyance of enduring separate immunotherapeutic procedures by
patients for addressing multiple allergen sensitivities on an
individual basis (190). The
polyallergic condition in patients may arise due to
‘cross-reactivity’ or ‘co-sensitization’ (186). Cross-reactivity is defined as IgE
reactivity against structurally related proteins when the sequence
homology is often >70%, whereas co-sensitization may involve
multiple IgE sensitizations against structurally unrelated allergen
groups (191). It is essential to
understand the nature of polyallergy with respect to
‘cross-reactivity’ or ‘co-sensitization’ to design a safe and
effective AIT (192). With the
advancement in science and technology, it is now possible to
isolate the pure allergic components from their natural source for
refined diagnosis and treatment of allergies. Component-resolved
diagnosis (CRD) utilizes purified native or recombinant allergens
to detect IgE sensitivity against individual allergen molecules and
has assumed increasing importance in clinical investigation of
IgE-mediated allergies (193).
The CRD technique quantifies serum specific IgE against individual
allergenic proteins or even allergenic peptides present in natural
sources, rather than quantifying IgE against the whole natural
extract (194). At present, CRD
diagnosis is used in laboratory practices as single plex and
multiplex arrays and offers a promising technology that could
replace conventional serum specific IgE assays in the near future
(195). One of the major
advantages of CRD is that it can discriminate true allergens from
the cross-reactive allergen molecules and polyallergy of other
related allergens (196).
However, CRD analysis utilizes intact proteins or random peptides
in its present form, which makes the data interpretation complex
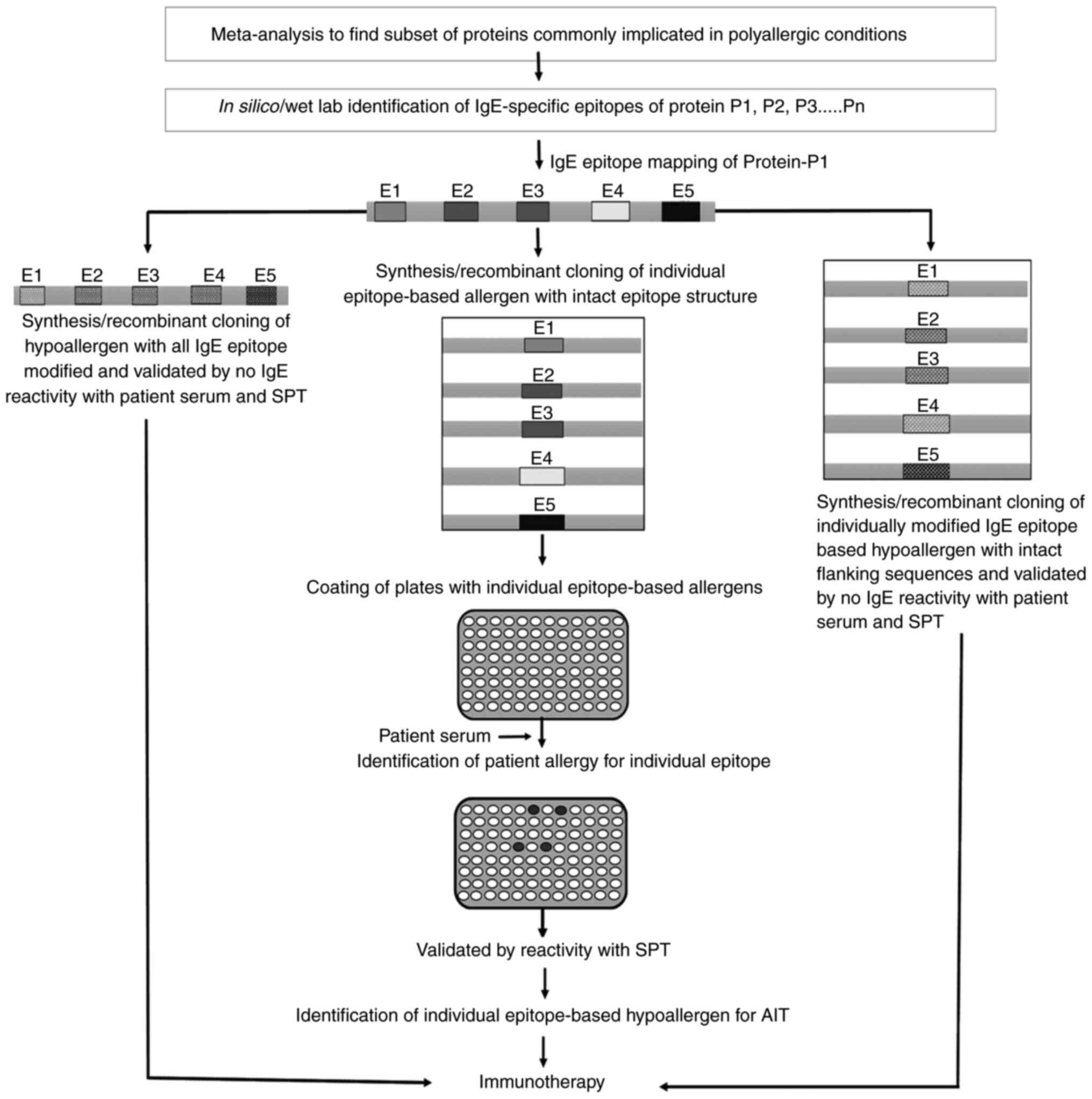
and ambiguous (192,196). A more refined CRD approach could
entail the use of individual IgE epitope-based recombinant
fragments present in a protein, rather than using the whole
allergenic protein components (192). In silico analysis in
conjunction with wet lab validation allows determination of
specific IgE epitopes present in an allergen, which can be further
employed for predicting epitope specific IgE reactivity of patient
serum (197). The present review
describes a strategy for developing allergy arrays with potential
application for AIT in patients with polyallergy (Fig. 3).
The strategy offers a simple and robust tool with a
high resolution for predicting IgE cross-reactivity or
co-sensitization from single or multiple allergenic sources. After
a thorough characterization of the IgE sensitivity profile of a
patient, the same IgE epitope-based recombinant fragments can also
be used for generating hypoallergens intended for use in AIT
(198,199). The hypoallergen could be prepared
by modifying the IgE specific epitopes of the particular allergen
either by coupling them with chemical modifiers or by altering the
coding sequence of the allergy-responsive component of the allergen
using recombinant DNA techniques (46,200,201). A combination of these
hypoallergenic epitope-based proteins may be employed for AIT
through a single dosing regimen plan (202). However, before the onset of AIT,
it should be ensured by a skin prick test that the serum of the
patient shows IgE reactivity towards the allergens but not the
hypoallergens (202).
There is a need for devising strategies aiming at
improved predictability of AIT, minimization of side effects,
annoyance of injection, irritability, fatal outcomes and a shorter
immunotherapeutic duration along with sustenance of life-long
tolerance for the allergen. Several combinatorial therapies, which
involve administration of allergen extracts with immunomodulatory
or suppressive cytokines, such as TGF-β, IL-35 and IL-10, have
yielded encouraging results; however, these approaches may markedly
escalate the cost of immunotherapy (203,204). Different endogenous specialized
proresolving lipid mediators (SPMs) have also shown promise as
therapeutic agents in the resolution of allergic inflammation.
Results from several experimental systems indicate that SPMs,
including lipoxins, resolvins, maresins and protectins, are
multi-pronged and potent regulators of inflammation and stimulate
resolution (205,206). For example, a combination of
resolvin D1 (RvD1) and 17-hydroxydocosahexaenoic acid has been
demonstrated to inhibit IgE production by human B cells and it also
suppresses the differentiation of naïve B cells into IgE-secreting
cells by specifically blocking epsilon germline transcript
(207). Furthermore, other
studies have also investigated the roles of SPMs in murine models
of allergic airway inflammation and have revealed their protective
role in allergic asthma (208–210). RvD1 is also known to reduce the
allergic airway inflammation by targeting eosinophils and
proinflammatory mediators involved in the Th2 signaling pathway,
while resolvin E1regulates the development of Th17 cells and IL-23
production (205). Similarly,
exogenous administration of maresin1 (MaR1) during the allergen
challenge phase attenuates allergen-triggered inflammation by
decreasing the multiple allergy-associated parameters, such as
numbers of eosinophils, allergen-specific IgE levels and type 2
cytokines in bronchoalveolar lavage fluid (BALF), and increasing
TGF-β levels (211). The
MaR1-mediated increase in BALF TGF-β triggers Tregs to limit type 2
innate lymphoid cell activation, and thus, promotes resolution of
lung inflammation. In addition, MaR1 promotes lung catabasis for
allergic asthma by suppressing ILC2-derived IL-5 and IL-13, while
stimulating the expression of amphiregulin (211). Furthermore, amphiregulin itself
contributes to a constitutive, low-level release of bio-active
TGF-β within tissues, leading to continuous tissue regeneration and
to an immunosuppressive environment, which may keep
inflammation-prone tissues in the homeostatic state (212). Considering the multi-pronged
beneficial actions of SPMs, they are important potential candidates
for combinatorial AITs.
Furthermore, as aforementioned, the lack of
appropriate biomarkers indicating successful progression of AIT is
a major bottleneck affecting the clinical outcome of allergy
immunotherapy. Clinical investigations examining the expression
levels and biosynthesis of SPMs in relation to efficacious AIT may
help to identify prospective biomarkers. In a model of allergic
lung inflammation, MaR1 production declined upon allergen challenge
but increased with resolution of allergic inflammation (211). This finding suggests that levels
of MaR1 in tissues before and after allergen-specific immunotherapy
may be tested as a biomarker of successful immunotherapy.
The knowledge gathered in the past decades has
helped in developing an improved understanding of the mutual
interaction between immune cell types presents in diverse
immunological niches, thereby propelling the evolution of different
AIT routes of administration and improved therapeutic formulations.
As a noteworthy breakthrough, the over-the-counter sale of certain
AIT formulations is also now possible, the self-administration of
which does not require any special hospital supervision. However,
there is still much to be done to address the issues of
standardizing AIT formulations, the risk of frequent adverse
reactions, the maintenance of tolerance to allergens, the reduction
in the duration of AIT, and other concerns, such as polyallergy.
Developing immune tolerance against allergens is the primary aim of
AIT but the current understanding of the precise mechanism
underlying the induction of allergen-specific Tregs is still in its
infancy. An improved scientific understanding of key events guiding
antigen-specific tolerance would pave the way for the advent of
novel non-invasive technologies targeting induction of
allergen-specific Tregs for an improved prognosis of AIT and
complete cure of allergies.
Not applicable.
Funding: No funding was received.
Not applicable.
AT conceptualized the review article and was
thoroughly involved in the critical review of the manuscript for
important intellectual content. SY carried out the literature
survey and prepared the review. SS and PM drafted the tables and
figures. Data authentication is not applicable. All authors
participated in the design and revision of the manuscript. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhang P and Lu Q: Genetic and epigenetic
influences on the loss of tolerance in autoimmunity. Cell Mol
Immunol. 15:575–585. 2018. View Article : Google Scholar
|
|
2
|
Tulic MK, Hodder M, Forsberg A, McCarthy
S, Richman T, D'Vaz N, van den Biggelaar AH, Thornton CA and
Prescott SL: Differences in innate immune function between allergic
and nonallergic children: New insights into immune ontogeny. J
Allergy Clin Immunol. 127:470–478.e1. 2011. View Article : Google Scholar
|
|
3
|
Rajan TV: The Gell-Coombs classification
of hypersensitivity reactions: A re-interpretation. Trends Immunol.
24:376–379. 2003. View Article : Google Scholar
|
|
4
|
Passali D, Cingi C, Staffa P, Passali F,
Muluk NB and Bellussi ML: The International study of the allergic
rhinitis survey: Outcomes from 4 geographical regions. Asia Pac
Allergy. 8:e72018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meltzer EO: Allergic rhinitis: Burden of
illness, quality of life, comorbidities, and control. Immunol
Allergy Clin North Am. 36:235–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pritchard DI, Falcone FH and Mitchell PD:
The evolution of IgE-mediated type I hypersensitivity and its
immunological value. Allergy. 76:1024–1040. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooke RA, Barnard JH, Hebald S and Stull
A: Serological evidence of immunity with coexisting sensitization
in a type of human allergy (Hay Fever). J Exp Med. 62:733–750.
1935. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Worm M, Lee HH, Kleine-Tebbe J, Hafner RP,
Laidler P, Healey D, Buhot C, Verhoef A, Maillère B, Kay AB and
Larché M: Development and preliminary clinical evaluation of a
peptide immunotherapy vaccine for cat allergy. J Allergy Clin
Immunol. 127:89–97, 97.e1-14. 2011. View Article : Google Scholar
|
|
9
|
Bonini S: Regulatory aspects of
allergen-specific immunotherapy: Europe sets the scene for a global
approach. World Allergy Organ J. 5:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaur SN: Guidelines for allergen
immunotherapy in India: 2017-An update. Indian J Allergy Asthma
Immunol. 31:1–2. 2017. View Article : Google Scholar
|
|
11
|
Von Baehr V, Hermes A, Von Baehr R, Scherf
HP, Volk HD, Fischer von Weikersthal-Drachenberg KJ and Woroniecki
S: Allergoid-specific T-cell reaction as a measure of the
immunological response to specific immunotherapy (SIT) with a
Th1-adjuvanted allergy vaccine. J Investig Allergol Clin Immunol.
15:234–241. 2005.
|
|
12
|
Hochfelder JL and Ponda P: Allergen
immunotherapy: Routes, safety, efficacy, and mode of action.
Immunotargets Ther. 2:61–71. 2013.PubMed/NCBI
|
|
13
|
Chaoul N, Albanesi M, Giliberti L, Rossi
MP, Nettis E, Di Bona D, Caiaffa MF and Macchia L:
Maintenance-phase subcutaneous immunotherapy with house dust mites
induces cyclic immunologic effects. Int Arch Allergy Immunol.
179:37–42. 2019. View Article : Google Scholar
|
|
14
|
Choi JS, Ryu HR, Yoon CH, Kim JH, Baek JO,
Roh JY and Lee JR: Treatment of patients with refractory atopic
dermatitis sensitized to house dust mites by using sublingual
allergen immunotherapy. Ann Dermatol. 27:82–86. 2015. View Article : Google Scholar
|
|
15
|
Cox L, Nelson H, Lockey R, Calabria C,
Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI,
Blessing-Moore J, et al: Allergen immunotherapy: A practice
parameter third update. J Allergy Clin Immunol. 127 (1
Suppl):S1–S55. 2011. View Article : Google Scholar
|
|
16
|
Feuille E and Nowak-Wegrzyn A:
Allergen-specific immunotherapies for food allergy. Allergy Asthma
Immunol Res. 10:189–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobsen L, Niggemann B, Dreborg S,
Ferdousi HA, Halken S, Høst A, Koivikko A, Norberg LA, Valovirta E,
Wahn U, et al: Specific immunotherapy has long-term preventive
effect of seasonal and perennial asthma: 10-year follow-up on the
PAT study. Allergy. 62:943–948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pajno GB, Barberio G, De Luca F, Morabito
L and Parmiani S: Prevention of new sensitizations in asthmatic
children monosensitized to house dust mite by specific
immunotherapy. A six-year follow-up study. Clin Exp Allergy.
31:1392–1397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
No authors listed. The specific treatment
of hay fever. JAMA. 41:1081903.
|
|
20
|
Noon L: Prophylactic inoculation against
hay fever. Lancet. 177:1572–1573. 1911. View Article : Google Scholar
|
|
21
|
Black JH: The oral administration of
pollen: A clinical report. J Lab Clin Med. 13:709–713. 1928.
|
|
22
|
Mackenzie GM and Baldwin LB: Local
desensitization in hypersensitive individuals and its bearing on
the prevention of hay-fever. Arch Intern Med (Chic). 28:722–732.
1921. View Article : Google Scholar
|
|
23
|
Scadding GK and Brostoff J: Low dose
sublingual therapy in patients with allergic rhinitis due to house
dust mite. Clin Allergy. 16:483–491. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senti G, Prinz Vavricka BM, Erdmann I,
Diaz MI, Markus R, McCormack SJ, Simard JJ, Wüthrich B, Crameri R,
Graf N, et al: Intralymphatic allergen administration renders
specific immunotherapy faster and safer: A randomized controlled
trial. Proc Natl Acad Sci USA. 105:17908–17912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stanworth DR: The discovery of IgE.
Allergy. 48:67–71. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lichtenstein LM, Holtzman NA and Burnett
LS: A quantitative in vitro study of the chromatographic
distribution and immunoglobulin characteristics of human blocking
antibody. J Immunol. 101:317–324. 1968.
|
|
27
|
Marsh DG, Lichtenstein LM and Campbell DH:
Studies on ‘allergoids’ prepared from naturally occurring
allergens: I. Assay of allergenicity and antigenicity of
formalinized rye group I component. Immunology. 18:705–722.
1970.PubMed/NCBI
|
|
28
|
Niederberger V, Horak F, Vrtala S,
Spitzauer S, Krauth MT, Valent P, Reisinger J, Pelzmann M, Hayek B,
Kronqvist M, et al: Vaccination with genetically engineered
allergens prevents progression of allergic disease. Proc Natl Acad
Sci USA. 101 (Suppl 2):S14677–S14682. 2004. View Article : Google Scholar
|
|
29
|
Kouser L, Kappen J, Walton RP and Shamji
MH: Update on biomarkers to monitor clinical efficacy response
during and post treatment in allergen immunotherapy. Curr Treat
Options Allergy. 4:43–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bachmann MF and Kündig TM:
Allergen-specific immunotherapy: Is it vaccination against toxins
after all? Allergy. 72:13–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esch RE: Allergen source materials and
quality control of allergenic extracts. Methods. 13:2–13. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jutel M, Jaeger L, Suck R, Meyer H, Fiebig
H and Cromwell O: Allergen-specific immunotherapy with recombinant
grass pollen allergens. J Allergy Clin Immunol. 116:608–613. 2005.
View Article : Google Scholar
|
|
33
|
Grier TJ: How's my dosing? A one-step,
math-free guide for comparing your clinic's maintenance
immunotherapy doses to current practice parameter recommendations.
Ann Allergy Asthma Immunol. 108:201–205. 2012. View Article : Google Scholar
|
|
34
|
Ferreira F, Briza P, Infuhr D, Schmidt G,
Wallner M, Wopfner N, Thalhamer J and Achatz G: Modified
recombinant allergens for safer immunotherapy. Inflamm Allergy Drug
Targets. 5:5–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jaye T: Allergy immunotherapy update. Curr
Allergy Clin Immunol. 32:91–94. 2019.
|
|
36
|
Spiric J, Reuter A and Rabin R: Mass
spectrometry to complement standardization of house dust mite and
other complex allergenic extracts. Clin Exp Allergy. 47:604–617.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Köberlein J and Mösges R:
Oralair(®): A causal treatment for grass pollen-induced
allergic rhinoconjunctivitis. Immunotherapy. 5:13–21. 2013.
View Article : Google Scholar
|
|
38
|
Dougherty JA, Wagner JD and Stanton MC:
Peanut allergen powder-dnfp: A novel oral immunotherapy to mitigate
peanut allergy. Ann Pharmacother. 55:344–353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuidmeer-Jongejan L, Huber H, Swoboda I,
Rigby N, Versteeg SA, Jensen BM, Quaak S, Akkerdaas JH, Blom L,
Asturias J, et al: Development of a hypoallergenic recombinant
parvalbumin for first-in-man subcutaneous immunotherapy of fish
allergy. Int Arch Allergy Immunol. 166:41–51. 2015. View Article : Google Scholar
|
|
40
|
Rizvi AY and Panchal AS: Timothy grass
pollen allergen extract (Grastek) for allergic rhinitis. Am Fam
Physician. 92:1096–1097. 2015.PubMed/NCBI
|
|
41
|
Cho SW, Han DH, Kim JW, Kim DY and Rhee
CS: House dust mite sublingual immunotherapy in allergic rhinitis.
Immunotherapy. 10:567–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nelson HS: Ragweed allergy immunotherapy
tablet MK-3641 (Ragwitek®) for the treatment of allergic
rhinitis. Expert Rev Clin Immunol. 14:1003–1011. 2018. View Article : Google Scholar
|
|
43
|
Erlich D: Peanut allergen powder
(Palforzia) for peanut allergy. Am Fam Physician. 105:20–21.
2022.PubMed/NCBI
|
|
44
|
Thompson CP, Silvers S and Shapiro MA:
Intralymphatic immunotherapy for mountain cedar pollinosis: A
randomized, double-blind, placebo-controlled trial. Ann Allergy
Asthma Immunol. 125:311–318.e2. 2020. View Article : Google Scholar
|
|
45
|
Senti G, von Moos S, Tay F, Graf N,
Sonderegger T, Johansen P and Kündig TM: Epicutaneous
allergen-specific immunotherapy ameliorates grass pollen-induced
rhinoconjunctivitis: A double-blind, placebo-controlled dose
escalation study. J Allergy Clin Immunol. 129:128–135. 2012.
View Article : Google Scholar
|
|
46
|
Gaur SN: Allergoid preparations for
allergen immunotherapy: A brief overview. Indian J Allergy Asthma
Immunol. 32:1–3. 2018. View Article : Google Scholar
|
|
47
|
Carnes J, Gallego MT, Moya R and Iraola V:
Allergoids for allergy treatment. Recent Pat Inflamm Allergy Drug
Discov. 12:110–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Focke-Tejkl M and Valenta R: Safety of
engineered allergen-specific immunotherapy vaccines. Curr Opin
Allergy Clin Immunol. 12:555–563. 2012. View Article : Google Scholar
|
|
49
|
Senti G, Johansen P, Haug S, Bull C,
Gottschaller C, Müller P, Pfister T, Maurer P, Bachmann MF, Graf N
and Kündig TM: Use of A-type CpG oligodeoxynucleotides as an
adjuvant in allergen-specific immunotherapy in humans: A phase
I/IIa clinical trial. Clin Exp Allergy. 39:562–570. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Satitsuksanoa P, Globinska A, Jansen K,
van de Veen W and Akdis M: Modified allergens for immunotherapy.
Curr Allergy Asthma Rep. 18:92018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Akdis CA and Blaser K: Regulation of
specific immune responses by chemical and structural modifications
of allergens. Int Arch Allergy Immunol. 121:261–269. 2000.
View Article : Google Scholar
|
|
52
|
Singh N, Bhatia S, Abraham R, Basu SK,
George A, Bal V and Rath S: Modulation of T cell cytokine profiles
and peptide-MHC complex availability in vivo by delivery to
scavenger receptors via antigen maleylation. J Immunol.
160:4869–4880. 1998.
|
|
53
|
Bhatia S, Mukhopadhyay S, Jarman E, Hall
G, George A, Basu SK, Rath S, Lamb JR and Bal V: Scavenger
receptor-specific allergen delivery elicits IFN-gamma-dominated
immunity and directs established TH2-dominated responses to a
nonallergic phenotype. J Allergy Clin Immunol. 109:321–328. 2002.
View Article : Google Scholar
|
|
54
|
Tighe H, Takabayashi K, Schwartz D,
Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ Jr, Spiegelberg
HL and Raz E: Conjugation of protein to immunostimulatory DNA
results in a rapid, long-lasting and potent induction of
cell-mediated and humoral immunity. Eur J Immunol. 30:1939–1947.
2000. View Article : Google Scholar
|
|
55
|
Takai T, Mori A, Yuuki T, Okudaira H and
Okumura Y: Non-anaphylactic combination of partially deleted
fragments of the major house dust mite allergen Der f 2 for
allergen-specific immunotherapy. Mol Immunol. 36:1055–1065. 1999.
View Article : Google Scholar
|
|
56
|
Swoboda I, de Weerd N, Bhalla PL,
Niederberger V, Sperr WR, Valent P, Kahlert H, Fiebig H, Ebner C,
Spitzauer S, et al: Hypoallergenic forms of the ryegrass pollen
allergen Lol p 5 as candidates for immunotherapy. Int Arch Allergy
Immunol. 124:380–382. 2001. View Article : Google Scholar
|
|
57
|
Wu CH, Lee MF, Yang JS and Tseng CY:
IgE-binding epitopes of the American cockroach Per a 1 allergen.
Mol Immunol. 39:459–464. 2002. View Article : Google Scholar
|
|
58
|
Kücüksezer UC, Palomares O, Rückert B,
Jartti T, Puhakka T, Nandy A, Gemicioğlu B, Fahrner HB, Jung A,
Deniz G, et al: Triggering of specific Toll-like receptors and
proinflammatory cytokines breaks allergen-specific T-cell tolerance
in human tonsils and peripheral blood. J Allergy Clin Immunol.
131:875–885. 2013. View Article : Google Scholar
|
|
59
|
Li L and Boussiotis V: Control and
regulation of peripheral tolerance in allergic inflammatory
disease: Therapeutic consequences. Chem Immunol Allergy.
94:178–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Akbari O, Freeman GJ, Meyer EH, Greenfield
EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH and Umetsu DT:
Antigen-specific regulatory T cells develop via the
ICOS-ICOS-ligand pathway and inhibit allergen-induced airway
hyperreactivity. Nat Med. 8:1024–1032. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Legoux FP, Lim JB, Cauley AW, Dikiy S,
Ertelt J, Mariani TJ, Sparwasser T, Way SS and Moon JJ: CD4+ T cell
tolerance to tissue-restricted self antigens is mediated by
antigen-specific regulatory T cells rather than deletion. Immunity.
43:896–908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Soroosh P, Doherty TA, Duan W, Mehta AK,
Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH and
Croft M: Lung-resident tissue macrophages generate Foxp3+
regulatory T cells and promote airway tolerance. J Exp Med.
210:775–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun CM, Hall JA, Blank RB, Bouladoux N,
Oukka M, Mora JR and Belkaid Y: Small intestine lamina propria
dendritic cells promote de novo generation of Foxp3 T reg cells via
retinoic acid. J Exp Med. 204:1775–1785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Akdis CA and Akdis M: Advances in allergen
immunotherapy: Aiming for complete tolerance to allergens. Sci
Transl Med. 7:280ps2862015. View Article : Google Scholar
|
|
65
|
Smarr CB, Bryce PJ and Miller SD:
Antigen-specific tolerance in immunotherapy of Th2-associated
allergic diseases. Crit Rev Immunol. 33:389–414. 2013. View Article : Google Scholar
|
|
66
|
Akkoc T, Akdis M and Akdis CA: Update in
the mechanisms of allergen-specific immunotheraphy. Allergy Asthma
Immunol Res. 3:11–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Larché M, Akdis CA and Valenta R:
Immunological mechanisms of allergen-specific immunotherapy. Nat
Rev Immunol. 6:761–771. 2006. View Article : Google Scholar
|
|
68
|
Hughes CE, Benson RA, Bedaj M and Maffia
P: Antigen-presenting cells and antigen presentation in tertiary
lymphoid organs. Front Immunol. 7:4812016. View Article : Google Scholar
|
|
69
|
Janeway Jr CA, Travers P, Walport M and
Shlomchik MJ: Principles of innate and adaptive immunity.
Immunobiology: The Immune System in Health and Disease. 5th
edition. Garland Science; New York: pp. 13–25. 2001
|
|
70
|
Kappen JH, Durham SR, Veen HI and Shamji
MH: Applications and mechanisms of immunotherapy in allergic
rhinitis and asthma. Ther Adv Respir Dis. 11:73–86. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lawrence MG, Steinke JW and Borish L:
Basic science for the clinician: Mechanisms of sublingual and
subcutaneous immunotherapy. Ann Allergy Asthma Immunol.
117:138–142. 2016. View Article : Google Scholar
|
|
72
|
Chang K, Song JY and Lim DS: Tolerogenic
dendritic cell-based immunotherapy. Oncotarget. 8:90630–90631.
2017. View Article : Google Scholar
|
|
73
|
Choo EH, Lee JH, Park EH, Park HE, Jung
NC, Kim TH, Koh YS, Kim E, Seung KB, Park C, et al: Infarcted
myocardium-primed dendritic cells improve remodeling and cardiac
function after myocardial infarction by modulating the regulatory T
cell and macrophage polarization. Circulation. 135:1444–1457. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lee JH, Kim TH, Park HE, Lee EG, Jung NC,
Song JY, Seo HG, Seung KB, Chang K and Lim DS: Myosin-primed
tolerogenic dendritic cells ameliorate experimental autoimmune
myocarditis. Cardiovasc Res. 101:203–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Allam JP, Würtzen PA, Reinartz M, Winter
J, Vrtala S, Chen KW, Valenta R, Wenghoefer M, Appel T, Gros E, et
al: Phl p 5 resorption in human oral mucosa leads to dose-dependent
and time-dependent allergen binding by oral mucosal Langerhans
cells, attenuates their maturation, and enhances their migratory
and TGF-beta1 and IL-10-producing properties. J Allergy Clin
Immunol. 126:638–645.e1. 2010. View Article : Google Scholar
|
|
76
|
Mascarell L, Lombardi V, Louise A,
Saint-Lu N, Chabre H, Moussu H, Betbeder D, Balazuc AM, Van
Overtvelt L and Moingeon P: Oral dendritic cells mediate
antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T
cells. J Allergy Clin Immunol. 122:603–609.e5. 2008. View Article : Google Scholar
|
|
77
|
Morianos I and Semitekolou M: Dendritic
cells: Critical regulators of allergic asthma. Int J Mol Sci.
21:79302020. View Article : Google Scholar
|
|
78
|
Reuter S, Lemmermann NAW, Maxeiner J,
Podlech J, Beckert H, Freitag K, Teschner D, Ries F, Taube C, Buhl
R, et al: Coincident airway exposure to low-potency allergen and
cytomegalovirus sensitizes for allergic airway disease by viral
activation of migratory dendritic cells. PLoS Pathog.
15:e10075952019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zakeri A and Russo M: Dual role of
Toll-like Receptors in human and experimental asthma models. Front
Immunol. 9:10272018. View Article : Google Scholar
|
|
80
|
Manicassamy S and Pulendran B: Dendritic
cell control of tolerogenic responses. Immunol Rev. 241:206–227.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ray A, Khare A, Krishnamoorthy N, Qi Z and
Ray P: Regulatory T cells in many flavors control asthma. Mucosal
Immunol. 3:216–229. 2010. View Article : Google Scholar
|
|
82
|
Akdis CA and Akdis M: Mechanisms of immune
tolerance to allergens: Role of IL-10 and Tregs. J Clin Invest.
124:4678–4680. 2014. View Article : Google Scholar
|
|
83
|
Shamji MH, Layhadi JA, Achkova D, Kouser
L, Perera-Webb A, Couto-Francisco NC, Parkin RV, Matsuoka T,
Scadding G, Ashton-Rickardt PG and Durham SR: Role of IL-35 in
sublingual allergen immunotherapy. J Allergy Clin Immunol.
143:1131–1142.e4. 2019. View Article : Google Scholar
|
|
84
|
Fujita H, Soyka MB, Akdis M and Akdis CA:
Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy.
2:22012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jeannin P, Lecoanet S, Delneste Y, Gauchat
JF and Bonnefoy JY: IgE versus IgG4 production can be
differentially regulated by IL-10. J Immunol. 160:3555–3561.
1998.
|
|
86
|
Jutel M, Akdis M, Budak F,
Aebischer-Casaulta C, Wrzyszcz M, Blaser K and Akdis CA: IL-10 and
TGF-beta cooperate in the regulatory T cell response to mucosal
allergens in normal immunity and specific immunotherapy. Eur J
Immunol. 33:1205–1214. 2003. View Article : Google Scholar
|
|
87
|
Quakyi IA and Ahlers JD: Assessing CD4+
helper T-lymphocyte responses by lymphoproliferation. Methods Mol
Med. 72:369–383. 2002.PubMed/NCBI
|
|
88
|
Gorelik L, Fields PE and Flavell RA:
Cutting edge: TGF-beta inhibits Th type 2 development through
inhibition of GATA-3 expression. J Immunol. 165:4773–4777. 2000.
View Article : Google Scholar
|
|
89
|
Heath VL, Murphy EE, Crain C, Tomlinson MG
and O'Garra A: TGF-beta1 down-regulates Th2 development and results
in decreased IL-4-induced STAT6 activation and GATA-3 expression.
Eur J Immunol. 30:2639–2649. 2000. View Article : Google Scholar
|
|
90
|
Nakamura T and Ushigome H: Myeloid-derived
suppressor cells as a regulator of immunity in organ
transplantation. Int J Mol Sci. 19:23572018. View Article : Google Scholar
|
|
91
|
Chekol Abebe E, Asmamaw Dejenie T, Mengie
Ayele T, Dagnew Baye N, Agegnehu Teshome A and Tilahun Muche Z: The
role of regulatory B cells in health and diseases: A systemic
review. J Inflamm Res. 14:75–84. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Katz SI, Parker D and Turk JL: B-cell
suppression of delayed hypersensitivity reactions. Nature.
251:550–551. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
van de Veen W, Stanic B, Yaman G,
Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA and Akdis M:
IgG4 production is confined to human IL-10-producing regulatory B
cells that suppress antigen-specific immune responses. J Allergy
Clin Immunol. 131:1204–1212. 2013. View Article : Google Scholar
|
|
94
|
Lee KM, Stott RT, Zhao G, SooHoo J, Xiong
W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, et al:
TGF-β-producing regulatory B cells induce regulatory T cells and
promote transplantation tolerance. Eur J Immunol. 44:1728–1736.
2014. View Article : Google Scholar
|
|
95
|
Shen P, Roch T, Lampropoulou V, O'Connor
RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C,
et al: IL-35-producing B cells are critical regulators of immunity
during autoimmune and infectious diseases. Nature. 507:366–370.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Carter NA, Rosser EC and Mauri C: IL-10
produced by B cells is crucial for the suppression of Th17/Th1
responses, induction of Tr1 cells and reduction of collagen-induced
arthritis. Arthritis Res Ther. 14:R322012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Das A, Ellis G, Pallant C, Lopes AR,
Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, et al:
IL-10-producing regulatory B cells in the pathogenesis of chronic
hepatitis B virus infection. J Immunol. 189:3925–3935. 2012.
View Article : Google Scholar
|
|
98
|
Mauri C and Menon M: The expanding family
of regulatory B cells. Int Immunol. 27:479–486. 2015. View Article : Google Scholar
|
|
99
|
Zaimoku Y, Patel BA, Kajigaya S, Feng X,
Alemu L, Quinones Raffo D, Groarke EM and Young NS: Deficit of
circulating CD19+ CD24hi CD38hi
regulatory B cells in severe aplastic anaemia. Br J Haematol.
190:610–617. 2020. View Article : Google Scholar
|
|
100
|
Blair PA, Noreña LY, Flores-Borja F,
Rawlings DJ, Isenberg DA, Ehrenstein MR and Mauri C: CD19+
CD24hiCD38hi B cells exhibit regulatory capacity in healthy
individuals but are functionally impaired in systemic lupus
erythematosus patients. Immunity. 32:129–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Khoder A, Sarvaria A, Alsuliman A, Chew C,
Sekine T, Cooper N, Mielke S, de Lavallade H, Muftuoglu M,
Fernandez Curbelo I, et al: Regulatory B cells are enriched within
the IgM memory and transitional subsets in healthy donors but are
deficient in chronic GVHD. Blood. 124:2034–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kim AS, Doherty TA, Karta MR, Das S, Baum
R, Rosenthal P, Beppu A, Miller M, Kurten R and Broide DH:
Regulatory B cells and T follicular helper cells are reduced in
allergic rhinitis. J Allergy Clin Immunol. 138:1192–1195.e5. 2016.
View Article : Google Scholar
|
|
103
|
Luo J, Guo H, Liu Z, Peng T, Hu X, Han M,
Yang X, Zhou X and Li H: Analysis of peripheral B cell subsets in
patients with allergic rhinitis. Allergy Asthma Immunol Res.
10:236–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Amu S, Saunders SP, Kronenberg M, Mangan
NE, Atzberger A and Fallon PG: Regulatory B cells prevent and
reverse allergic airway inflammation via FoxP3-positive T
regulatory cells in a murine model. J Allergy Clin Immunol.
125:1114–1124.e8. 2010. View Article : Google Scholar
|
|
105
|
Kanagaratham C, El Ansari YS, Lewis OL and
Oettgen HC: IgE and IgG antibodies as regulators of mast cell and
basophil functions in food allergy. Front Immunol. 11:6030502020.
View Article : Google Scholar
|
|
106
|
Santos AF, James LK, Bahnson HT, Shamji
MH, Couto-Francisco NC, Islam S, Houghton S, Clark AT, Stephens A,
Turcanu V, et al: IgG4 inhibits peanut-induced basophil and mast
cell activation in peanut-tolerant children sensitized to peanut
major allergens. J Allergy Clin Immunol. 135:1249–1256. 2015.
View Article : Google Scholar
|
|
107
|
James LK, Shamji MH, Walker SM, Wilson DR,
Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ and Durham
SR: Long-term tolerance after allergen immunotherapy is accompanied
by selective persistence of blocking antibodies. J Allergy Clin
Immunol. 127:509–516.e1-e5. 2011. View Article : Google Scholar
|
|
108
|
Hassan G, Kant S, Prakash V, Verma AK,
Saheer S, Singh A, Singh A, Jena NN and Wani NA: Allergen
immunotherapy: Basic concepts. Indian J Allergy Asthma Immunol.
27:9–18. 2013. View Article : Google Scholar
|
|
109
|
Aricigil M, Muluk NB, Sakarya EU, Sakalar
EG, Senturk M, Reisacher WR and Cingi C: New routes of allergen
immunotherapy. Am J Rhinol Allergy. 30:193–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Di Bona D, Plaia A, Leto-Barone MS, La
Piana S and Di Lorenzo G: Efficacy of subcutaneous and sublingual
immunotherapy with grass allergens for seasonal allergic rhinitis:
A meta-analysis-based comparison. J Allergy Clin Immunol.
130:1097–1107.e2. 2012. View Article : Google Scholar
|
|
111
|
Cox L, Calderon M and Pfaar O:
Subcutaneous allergen immunotherapy for allergic disease: Examining
efficacy, safety and cost-effectiveness of current and novel
formulations. Immunotherapy. 4:601–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Krishna MT and Huissoon AP: Clinical
immunology review series: An approach to desensitization. Clin Exp
Immunol. 163:131–146. 2011. View Article : Google Scholar
|
|
113
|
Bergmann KC and Ring J: History of
Allergy. Vol 100. Karger Medical and Scientific Publishers;
Switzerland: 2014
|
|
114
|
Hesse L, Brouwer U, Petersen AH, Gras R,
Bosman L, Brimnes J, Oude Elberink JNG, van Oosterhout AJM and
Nawijn MC: Subcutaneous immunotherapy suppresses Th2 inflammation
and induces neutralizing antibodies, but sublingual immunotherapy
suppresses airway hyperresponsiveness in grass pollen mouse models
for allergic asthma. Clin Exp Allergy. 48:1035–1049. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jacobsen L, Wahn U and Bilo MB:
Allergen-specific immunotherapy provides immediate, long-term and
preventive clinical effects in children and adults: The effects of
immunotherapy can be categorised by level of benefit -the centenary
of allergen specific subcutaneous immunotherapy. Clin Transl
Allergy. 2:82012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Scadding GW, Calderon MA, Shamji MH, Eifan
AO, Penagos M, Dumitru F, Sever ML, Bahnson HT, Lawson K, Harris
KM, et al: Effect of 2 years of treatment with sublingual grass
pollen immunotherapy on nasal response to allergen challenge at 3
years among patients with moderate to severe seasonal allergic
rhinitis: The GRASS randomized clinical trial. JAMA. 317:615–625.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jongkhajornpong P and Laisuan W:
Successful subcutaneous allergen-specific immunotherapy in
refractory atopic keratoconjunctivitis: A case report. Case Rep
Ophthalmol. 8:562–567. 2017. View Article : Google Scholar
|
|
118
|
Aasbjerg K, Backer V, Lund G, Holm J,
Nielsen NC, Holse M, Wagtmann VR and Würtzen PA: Immunological
comparison of allergen immunotherapy tablet treatment and
subcutaneous immunotherapy against grass allergy. Clin Exp Allergy.
44:417–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Schülke S: Induction of Interleukin-10
producing dendritic cells as a tool to suppress allergen-specific T
helper 2 responses. Front Immunol. 9:4552018. View Article : Google Scholar
|
|
120
|
Bellinghausen I, König B, Böttcher I, Knop
J and Saloga J: Inhibition of human allergic T-helper type 2 immune
responses by induced regulatory T cells requires the combination of
interleukin-10-treated dendritic cells and transforming growth
factor-beta for their induction. Clin Exp Allergy. 36:1546–1555.
2006. View Article : Google Scholar
|
|
121
|
Taylor A, Verhagen J, Blaser K, Akdis M
and Akdis CA: Mechanisms of immune suppression by interleukin-10
and transforming growth factor-beta: The role of T regulatory
cells. Immunology. 117:433–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Maggi E: T-cell responses induced by
allergen-specific immunotherapy. Clin Exp Immunol. 161:10–18. 2010.
View Article : Google Scholar
|
|
123
|
Sousa L, Martín-Sierra C, Pereira C,
Loureiro G, Tavares B, Pedreiro S, Martinho A and Paiva A:
Subcutaneous immunotherapy induces alterations in monocytes and
dendritic cells homeostasis in allergic rhinitis patients. Allergy
Asthma Clin Immunol. 14:452018. View Article : Google Scholar
|
|
124
|
Lao-Araya M, Steveling E, Scadding GW,
Durham SR and Shamji MH: Seasonal increases in peripheral innate
lymphoid type 2 cells are inhibited by subcutaneous grass pollen
immunotherapy. J Allergy Clin Immunol. 134:1193–1195. e42014.
View Article : Google Scholar
|
|
125
|
Halim TY, Hwang YY, Scanlon ST, Zaghouani
H, Garbi N, Fallon PG and McKenzie AN: Group 2 innate lymphoid
cells license dendritic cells to potentiate memory TH2 cell
responses. Nat Immunol. 17:57–64. 2016. View Article : Google Scholar
|
|
126
|
Ring J, Beyer K, Biedermann T, Bircher A,
Duda D, Fischer J, Friedrichs F, Fuchs T, Gieler U, Jakob T, et al:
Guideline for acute therapy and management of anaphylaxis: S2
Guideline of the German society for allergology and clinical
immunology (DGAKI), the association of German allergologists
(AeDA), the society of pediatric allergy and environmental medicine
(GPA), the German academy of allergology and environmental medicine
(DAAU), the German professional association of pediatricians
(BVKJ), the Austrian society for allergology and immunology (ÖGAI),
the Swiss society for allergy and immunology (SGAI), the German
society of anaesthesiology and intensive care medicine (DGAI), the
German society of pharmacology (DGP), the German society for
psychosomatic medicine (DGPM), the German working group of
anaphylaxis training and education (AGATE) and the patient
organization German allergy and asthma association (DAAB). Allergo
J Int. 23:96–112. 2014. View Article : Google Scholar
|
|
127
|
Okamoto Y, Fujieda S, Okano M, Yoshida Y,
Kakudo S and Masuyama K: House dust mite sublingual tablet is
effective and safe in patients with allergic rhinitis. Allergy.
72:435–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lim JH, Kim JY, Han DH, Lee CH, Hong SN,
Wee JH, Park SK and Rhee CS: Sublingual immunotherapy (SLIT) for
house dust mites does not prevent new allergen sensitization and
bronchial hyper-responsiveness in allergic rhinitis children. PLoS
One. 12:e01822952017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Frati F, Scurati S, Puccinelli P, David M,
Hilaire C, Capecce M, Marcucci F and Incorvaia C: Development of a
sublingual allergy vaccine for grass pollinosis. Drug Des Devel
Ther. 4:99–105. 2010.PubMed/NCBI
|
|
130
|
Calderon MA, Penagos M, Sheikh A, Canonica
GW and Durham SR: Sublingual immunotherapy for allergic
conjunctivitis: Cochrane systematic review and meta-analysis. Clin
Exp Allergy. 41:1263–1272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Allam JP, Stojanovski G, Friedrichs N,
Peng W, Bieber T, Wenzel J and Novak N: Distribution of Langerhans
cells and mast cells within the human oral mucosa: New application
sites of allergens in sublingual immunotherapy? Allergy.
63:720–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Schulten V, Tripple V, Aasbjerg K, Backer
V, Lund G, Würtzen PA, Sette A and Peters B: Distinct modulation of
allergic T cell responses by subcutaneous vs. sublingual
allergen-specific immunotherapy. Clin Exp Allergy. 46:439–448.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Larche M: Immune mechanisms of sublingual
immunotherapy: Are oral Langerhans cells the masters of tolerance?
J Allergy Clin Immunol. 126:646–647. 2010. View Article : Google Scholar
|
|
134
|
Allam JP, Novak N, Fuchs C, Asen S, Bergé
S, Appel T, Geiger E, Kochan JP and Bieber T: Characterization of
dendritic cells from human oral mucosa: a new Langerhans' cell type
with high constitutive FcepsilonRI expression. J Allergy Clin
Immunol. 112:141–148. 2003. View Article : Google Scholar
|
|
135
|
Moingeon P, Lombardi V, Baron-Bodo V and
Mascarell L: Enhancing allergen-presentation platforms for
sublingual immunotherapy. J Allergy Clin Immunol Pract. 5:23–31.
2017. View Article : Google Scholar
|
|
136
|
Francis JN, Till SJ and Durham SR:
Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy.
J Allergy Clin Immunol. 111:1255–1261. 2003. View Article : Google Scholar
|
|
137
|
Bohle B, Kinaciyan T, Gerstmayr M,
Radakovics A, Jahn-Schmid B and Ebner C: Sublingual immunotherapy
induces IL-10-producing T regulatory cells, allergen-specific
T-cell tolerance, and immune deviation. J Allergy Clin Immunol.
120:707–713. 2007. View Article : Google Scholar
|
|
138
|
Canonica GW, Cox L, Pawankar R,
Baena-Cagnani CE, Blaiss M, Bonini S, Bousquet J, Calderón M,
Compalati E, Durham SR, et al: Sublingual immunotherapy: World
Allergy Organization position paper 2013 update. World Allergy
Organ J. 7:62014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Fiocchi A, Pajno G, La Grutta S, Pezzuto
F, Incorvaia C, Sensi L, Marcucci F and Frati F: Safety of
sublingual-swallow immunotherapy in children aged 3 to 7 years. Ann
Allergy Asthma Immunol. 95:254–258. 2005. View Article : Google Scholar
|
|
140
|
Saporta D: Sublingual immunotherapy: A
useful tool for the allergist in private practice. Biomed Res Int.
2016:93238042016. View Article : Google Scholar
|
|
141
|
Feuille E and Nowak-Węgrzyn A: Oral
immunotherapy for food allergies. Ann Nutr Metab. 68 (Suppl
1):S19–S31. 2016. View Article : Google Scholar
|
|
142
|
Wang YT, Liu HC, Chen HC, Lee YC, Tsai TC,
Chen HL, Fan HC and Chen CM: Oral immunotherapy with the ingestion
of house dust mite extract in a murine model of allergic asthma.
Allergy Asthma Clin Immunol. 14:432018. View Article : Google Scholar
|
|
143
|
Khoriaty E and Umetsu DT: Oral
immunotherapy for food allergy: Towards a new horizon. Allergy
Asthma Immunol Res. 5:3–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Pandiyan P, Bhaskaran N, Zou M, Schneider
E, Jayaraman S and Huehn J: Microbiome dependent regulation of
Tregs and Th17 cells in Mucosa. Front Immunol.
10:4262019. View Article : Google Scholar
|
|
145
|
Faria AM and Weiner HL: Oral tolerance:
Mechanisms and therapeutic applications. Adv Immunol. 73:153–264.
1999. View Article : Google Scholar
|
|
146
|
Smaldini PL, Orsini Delgado ML, Fossati CA
and Docena GH: Orally-induced intestinal CD4+ CD25+ FoxP3+ Treg
controlled undesired responses towards oral antigens and
effectively dampened food allergic reactions. PLoS One.
10:e01411162015. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mizrahi M and Ilan Y: The gut mucosa as a
site for induction of regulatory T-cells. Curr Pharm Des.
15:1191–1202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Vickery BP, Lin J, Kulis M, Fu Z, Steele
PH, Jones SM, Scurlock AM, Gimenez G, Bardina L, Sampson HA and
Burks AW: Peanut oral immunotherapy modifies IgE and IgG4 responses
to major peanut allergens. J Allergy Clin Immunol.
131:128–134.e1-3. 2013. View Article : Google Scholar
|
|
149
|
Vickery BP, Scurlock AM, Kulis M, Steele
PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S,
Christie L, et al: Sustained unresponsiveness to peanut in subjects
who have completed peanut oral immunotherapy. J Allergy Clin
Immunol. 133:468–475. 2014. View Article : Google Scholar
|
|
150
|
Sampson HA: Peanut oral immunotherapy: Is
it ready for clinical practice? J Allergy Clin Immunol Pract.
1:15–21. 2013. View Article : Google Scholar
|
|
151
|
Chu DK, Wood RA, French S, Fiocchi A,
Jordana M, Waserman S, Brożek JL and Schünemann HJ: Oral
immunotherapy for peanut allergy (PACE): A systematic review and
meta-analysis of efficacy and safety. Lancet. 393:2222–2232. 2019.
View Article : Google Scholar
|
|
152
|
Anagnostou A: Weighing the benefits and
risks of oral immunotherapy in clinical practice. Allergy Asthma
Proc. 42:118–123. 2021. View Article : Google Scholar
|
|
153
|
Dioszeghy V, Mondoulet L, Laoubi L, Dhelft
V, Plaquet C, Bouzereau A, Dupont C and Sampson H: Antigen uptake
by langerhans cells is required for the induction of regulatory T
cells and the acquisition of tolerance during epicutaneous
immunotherapy in OVA-sensitized mice. Front Immunol. 9:19512018.
View Article : Google Scholar
|
|
154
|
Senti G, von Moos S and Kündig TM:
Epicutaneous immunotherapy for aeroallergen and food allergy. Curr
Treat Options Allergy. 1:68–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Gorfu G, Rivera-Nieves J and Ley K: Role
of beta7 integrins in intestinal lymphocyte homing and retention.
Curr Mol Med. 9:836–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yu LC: Intestinal epithelial barrier
dysfunction in food hypersensitivity. J Allergy (Cairo).
2012:5960812012.PubMed/NCBI
|
|
157
|
Tordesillas L, Mondoulet L, Blazquez AB,
Benhamou PH, Sampson HA and Berin MC: Epicutaneous immunotherapy
induces gastrointestinal LAP+ regulatory T cells and
prevents food-induced anaphylaxis. J Allergy Clin Immunol.
139:189–201.e4. 2017. View Article : Google Scholar
|
|
158
|
Plunkett CH and Nagler CR: The influence
of the microbiome on allergic sensitization to food. J Immunol.
198:581–589. 2017. View Article : Google Scholar
|
|
159
|
Mondoulet L, Dioszeghy V, Ligouis M,
Dhelft V, Dupont C and Benhamou PH: Epicutaneous immunotherapy on
intact skin using a new delivery system in a murine model of
allergy. Clin Exp Allergy. 40:659–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Mondoulet L, Dioszeghy V, Puteaux E,
Ligouis M, Dhelft V, Letourneur F, Dupont C and Benhamou PH: Intact
skin and not stripped skin is crucial for the safety and efficacy
of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl
Allergy. 2:222012. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Dupont C, Kalach N, Soulaines P,
Legoué-Morillon S, Piloquet H and Benhamou PH: Cow's milk
epicutaneous immunotherapy in children: A pilot trial of safety,
acceptability, and impact on allergic reactivity. J Allergy Clin
Immunol. 125:1165–1167. 2010. View Article : Google Scholar
|
|
162
|
Senti G, Johansen P and Kundig TM:
Intralymphatic immunotherapy. Curr Opin Allergy Clin Immunol.
9:537–543. 2009. View Article : Google Scholar
|
|
163
|
Hylander T, Latif L, Petersson-Westin U
and Cardell LO: Intralymphatic allergen-specific immunotherapy: An
effective and safe alternative treatment route for pollen-induced
allergic rhinitis. J Allergy Clin Immunol. 131:412–420. 2013.
View Article : Google Scholar
|
|
164
|
Kim ST, Park SH, Lee SM and Lee SP:
Allergen-specific intralymphatic immunotherapy in human and animal
studies. Asia Pac Allergy. 7:131–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Senti G, Freiburghaus AU,
Larenas-Linnemann D, Hoffmann HJ, Patterson AM, Klimek L, Di Bona
D, Pfaar O, Ahlbeck L, Akdis M, et al: Intralymphatic
immunotherapy: Update and unmet needs. Int Arch Allergy Immunol.
178:141–149. 2019. View Article : Google Scholar
|
|
166
|
Martínez-Gómez JM, Johansen P, Erdmann I,
Senti G, Crameri R and Kündig TM: Intralymphatic injections as a
new administration route for allergen-specific immunotherapy. Int
Arch Allergy Immunol. 150:59–65. 2009. View Article : Google Scholar
|
|
167
|
Freiberger SN, Zehnder M, Gafvelin G,
Gronlund H, Kundig TM and Johansen P: IgG4 but no IgG1 antibody
production after intralymphatic immunotherapy with recombinant
MAT-Feld1 in human. Allergy. 71:1366–1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Konradsen JR, Grundström J, Hellkvist L,
Tran TAT, Andersson N, Gafvelin G, Kiewiet MBG, Hamsten C, Tang J,
Parkin RV, et al: Intralymphatic immunotherapy in pollen-allergic
young adults with rhinoconjunctivitis and mild asthma: A randomized
trial. J Allergy Clin Immunol. 145:1005–1007.e7. 2020. View Article : Google Scholar
|
|
169
|
Rajakulendran M, Tham EH, Soh JY and Van
Bever HP: Novel strategies in immunotherapy for allergic diseases.
Asia Pac Allergy. 8:e142018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Fischer N, Rostaher A and Favrot C:
Intralymphatic immunotherapy: An effective and safe alternative
route for canine atopic dermatitis. Schweiz Arch Tierheilkd.
158:646–652. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Nelson MR and Cox L: Allergen
immunotherapy extract preparation manual. Practice Management
Resource Guide. 2014 edition. American Academy of Allergy, Asthma
& Immunology; pp. 1–30. 2014
|
|
172
|
Nony E, Martelet A, Jain K and Moingeon P:
Allergen extracts for immunotherapy: To mix or not to mix? Expert
Rev Clin Pharmacol. 9:401–408. 2016. View Article : Google Scholar
|
|
173
|
Helyeh S, David L and Gary S: Advances in
the management of food allergy in children. Curr Pediatr Rev.
14:150–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Pajno GB, Fernandez-Rivas M, Arasi S,
Roberts G, Akdis CA, Alvaro-Lozano M, Beyer K, Bindslev-Jensen C,
Burks W, Ebisawa M, et al: EAACI Guidelines on allergen
immunotherapy: IgE-mediated food allergy. Allergy. 73:799–815.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Pfaar O, Alvaro M, Cardona V, Hamelmann E,
Mosges R and Kleine-Tebbe J: Clinical trials in allergen
immunotherapy: Current concepts and future needs. Allergy.
73:1775–1783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner
C, Eng P, Friedrichs F, Fuchs T, Hamelmann E, Hartwig-Bade D, et
al: Guideline on allergen-specific immunotherapy in IgE-mediated
allergic diseases: S2k guideline of the German society for
allergology and clinical immunology (DGAKI), the society for
pediatric allergy and environmental medicine (GPA), the medical
association of German allergologists (AeDA), the Austrian society
for allergy and immunology (OGAI), the Swiss Society for Allergy
and Immunology (SGAI), the German Society of Dermatology (DDG), the
German Society of Oto- Rhino-Laryngology, Head and Neck Surgery
(DGHNO-KHC), the German society of pediatrics and adolescent
medicine (DGKJ), the society for pediatric pneumology (GPP), the
German respiratory society (DGP), the German association of ENT
surgeons (BV-HNO), the professional federation of paediatricians
and youth doctors (BVKJ), the federal association of pulmonologists
(BDP) and the German dermatologists association (BVDD). Allergo J
Int. 23:282–319. 2014. View Article : Google Scholar
|
|
177
|
Halken S, Larenas-Linnemann D, Roberts G,
Calderón MA, Angier E, Pfaar O, Ryan D, Agache I, Ansotegui IJ,
Arasi S, et al: EAACI guidelines on allergen immunotherapy:
prevention of allergy. Pediatr Allergy Immunol. 28:728–745. 2017.
View Article : Google Scholar
|
|
178
|
Globinska A, Boonpiyathad T, Satitsuksanoa
P, Kleuskens M, van de Veen W, Sokolowska M and Akdis M: Mechanisms
of allergen-specific immunotherapy: Diverse mechanisms of immune
tolerance to allergens. Ann Allergy Asthma Immunol. 121:306–312.
2018. View Article : Google Scholar
|
|
179
|
Chen J, Zhou Y, Wang Y, Zheng Y, Lai X,
Westermann-Clark E, Cho SH and Kong W: Specific immunoglobulin E
and immunoglobulin G4 toward major allergens of house-dust mite
during allergen-specific immunotherapy. Am J Rhinol Allergy.
31:156–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ciprandi G, Tosca MA and Silvestri M: The
practical role of serum allergen-specific IgE as potential
biomarker for predicting responder to allergen immunotherapy.
Expert Rev Clin Immunol. 10:321–324. 2014. View Article : Google Scholar
|
|
181
|
Palomares O, Martin-Fontecha M, Lauener R,
Traidl-Hoffmann C, Cavkaytar O, Akdis M and Akdis CA: Regulatory T
cells and immune regulation of allergic diseases: Roles of IL-10
and TGF-beta. Genes Immun. 15:511–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Licari A, Castagnoli R, Brambilla I, Tosca
MA, De Filippo M, Marseglia G and Ciprandi G: Biomarkers of
immunotherapy response in patients with allergic rhinitis. Expert
Rev Clin Immunol. 14:657–663. 2018. View Article : Google Scholar
|
|
183
|
Gueguen C, Bouley J, Moussu H, Luce S,
Duchateau M, Chamot-Rooke J, Pallardy M, Lombardi V, Nony E,
Baron-Bodo V, et al: Changes in markers associated with dendritic
cells driving the differentiation of either TH2 cells or regulatory
T cells correlate with clinical benefit during allergen
immunotherapy. J Allergy Clin Immunol. 137:545–558. 2016.
View Article : Google Scholar
|
|
184
|
Caruso M, Cibella F, Emma R, Campagna D,
Tringali G, Amaradio MD and Polosa R: Basophil biomarkers as useful
predictors for sublingual immunotherapy in allergic rhinitis. Int
Immunopharmacol. 60:50–58. 2018. View Article : Google Scholar
|
|
185
|
Wise SK, Lin SY, Toskala E, Orlandi RR,
Akdis CA, Alt JA, Azar A, Baroody FM, Bachert C, Canonica GW, et
al: International consensus statement on allergy and rhinology:
Allergic rhinitis. Int Forum Allergy Rhinol. 8:108–352. 2018.
View Article : Google Scholar
|
|
186
|
Demoly P, Passalacqua G, Pfaar O, Sastre J
and Wahn U: Management of the polyallergic patient with allergy
immunotherapy: A practice-based approach. Allergy Asthma Clin
Immunol. 12:22016. View Article : Google Scholar
|
|
187
|
Ciprandi G, Alesina R, Ariano R, Aurnia P,
Borrelli P, Cadario G, Capristo A, Carosso A, Casino G, Castiglioni
G, et al: Characteristics of patients with allergic
polysensitization: The POLISMAIL study. Eur Ann Allergy Clin
Immunol. 40:77–83. 2008.
|
|
188
|
Shamji MH and Durham SR: Mechanisms of
allergen immunotherapy for inhaled allergens and predictive
biomarkers. J Allergy Clin Immunol. 140:1485–1498. 2017. View Article : Google Scholar
|
|
189
|
Daigle BJ and Rekkerth DJ: Practical
recommendations for mixing allergy immunotherapy extracts. Allergy
Rhinol (Providence). 6:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Bahceciler NN, Galip N and Cobanoglu N:
Multiallergen-specific immunotherapy in polysensitized patients:
Where are we? Immunotherapy. 5:183–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Chruszcz M, Kapingidza AB, Dolamore C and
Kowal K: A robust method for the estimation and visualization of
IgE cross-reactivity likelihood between allergens belonging to the
same protein family. PLoS One. 13:e02082762018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Dodig S and Čepelak I: The potential of
component-resolved diagnosis in laboratory diagnostics of allergy.
Biochem Med (Zagreb). 28:0205012018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Treudler R and Simon JC: Overview of
component resolved diagnostics. Curr Allergy Asthma Rep.
13:110–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Ebo DG: Component-resolved allergy
diagnosis: A new era? Verh K Acad Geneeskd Belg. 73:163–179.
2011.PubMed/NCBI
|
|
195
|
Valenta R, Campana R, Marth K and van Hage
M: Allergen-specific immunotherapy: From therapeutic vaccines to
prophylactic approaches. J Intern Med. 272:144–157. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Luengo O and Cardona V: Component resolved
diagnosis: When should it be used? Clin Transl Allergy. 4:282014.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Alessandri C, Ferrara R, Bernardi ML,
Zennaro D, Tuppo L, Giangrieco I, Tamburrini M, Mari A and
Ciardiello MA: Diagnosing allergic sensitizations in the third
millennium: Why clinicians should know allergen molecule
structures. Clin Transl Allergy. 7:212017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Marth K, Focke-Tejkl M, Lupinek C, Valenta
R and Niederberger V: Allergen peptides, recombinant allergens and
hypoallergens for allergen-specific immunotherapy. Curr Treat
Options Allergy. 1:91–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Curin M, Garib V and Valenta R: Single
recombinant and purified major allergens and peptides: How they are
made and how they change allergy diagnosis and treatment. Ann
Allergy Asthma Immunol. 119:201–209. 2017. View Article : Google Scholar
|
|
200
|
Gaur SN: Future modalities in allergen
immunotherapy: A brief overview. Indian J Allergy Asthma Immunol.
32:43–46. 2018. View Article : Google Scholar
|
|
201
|
Vrtala S, Focke-Tejkl M, Swoboda I, Kraft
D and Valenta R: Strategies for converting allergens into
hypoallergenic vaccine candidates. Methods. 32:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Valenta R, Campana R, Focke-Tejkl M and
Niederberger V: Vaccine development for allergen-specific
immunotherapy based on recombinant allergens and synthetic allergen
peptides: Lessons from the past and novel mechanisms of action for
the future. J Allergy Clin Immunol. 137:351–357. 2016. View Article : Google Scholar
|
|
203
|
Larsen JN and Dreborg S: Standardization
of allergen extracts. Methods Mol Med. 138:133–145. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Akdis CA, Blesken T, Akdis M, Wuthrich B
and Blaser K: Role of interleukin 10 in specific immunotherapy. J
Clin Invest. 102:98–106. 1998. View Article : Google Scholar
|
|
205
|
Levy BD: Resolvin D1 and Resolvin E1
promote the resolution of allergic airway inflammation via shared
and distinct molecular counter-regulatory pathways. Front Immunol.
3:3902012. View Article : Google Scholar
|
|
206
|
Lotfi R, Rezaiemanesh A, Mortazavi SH,
Karaji AG and Salari F: Immunoresolvents in asthma and allergic
diseases: Review and update. J Cell Physiol. 234:8579–8596. 2019.
View Article : Google Scholar
|
|
207
|
Kim N, Ramon S, Thatcher TH, Woeller CF,
Sime PJ and Phipps RP: Specialized proresolving mediators (SPMs)
inhibit human B-cell IgE production. Eur J Immunol. 46:81–91. 2016.
View Article : Google Scholar
|
|
208
|
Karra L, Haworth O, Priluck R, Levy BD and
Levi-Schaffer F: Lipoxin B4 promotes the resolution of
allergic inflammation in the upper and lower airways of mice.
Mucosal Immunol. 8:852–862. 2015. View Article : Google Scholar
|
|
209
|
Flesher RP, Herbert C and Kumar RK:
Resolvin E1 promotes resolution of inflammation in a mouse model of
an acute exacerbation of allergic asthma. Clin Sci (Lond).
126:805–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Levy BD, Kohli P, Gotlinger K, Haworth O,
Hong S, Kazani S, Israel E, Haley KJ and Serhan CN: Protectin D1 is
generated in asthma and dampens airway inflammation and
hyperresponsiveness. J Immunol. 178:496–502. 2007. View Article : Google Scholar
|
|
211
|
Krishnamoorthy N, Burkett PR, Dalli J,
Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK,
Serhan CN and Levy BD: Cutting edge: Maresin-1 engages regulatory T
cells to limit type 2 innate lymphoid cell activation and promote
resolution of lung inflammation. J Immunol. 194:863–867. 2015.
View Article : Google Scholar
|
|
212
|
Zaiss DM, Minutti CM and Knipper JA:
Immune- and non-immune-mediated roles of regulatory T-cells during
wound healing. Immunology. 157:190–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Di Bona D, Plaia A, Scafidi V, Leto-Barone
MS and Di Lorenzo G: Efficacy of sublingual immunotherapy with
grass allergens for seasonal allergic rhinitis: A systematic review
and meta-analysis. J Allergy Clin Immunol. 126:558–566. 2010.
View Article : Google Scholar
|
|
214
|
Harrison OJ and Powrie FM: Regulatory T
cells and immune tolerance in the intestine. Cold Spring Harb
Perspect Biol. 5:a0183412013. View Article : Google Scholar
|
|
215
|
de Chaisemartin L: Lymphocyte Homing and
Trafficking. Encyclopedia of Inflammatory Diseases. Parnham M:
Birkhäuser; Basel: pp. 1–8. 2013, View Article : Google Scholar
|
|
216
|
De Calisto J, Villablanca EJ, Wang S, Bono
MR, Rosemblatt M and Mora JR: T-cell homing to the gut mucosa:
General concepts and methodological considerations. Methods Mol
Biol. 757:411–434. 2012. View Article : Google Scholar
|
|
217
|
Weinberg EG: The WAO white book on allergy
2011–2012. Curr Allergy Clin Immunol. 24:156–157. 2011.
|
|
218
|
Ansotegui IJ, Melioli G, Canonica GW,
Caraballo L, Villa E, Ebisawa M, Passalacqua G, Savi E, Ebo D,
Gómez RM, et al: IgE allergy diagnostics and other relevant tests
in allergy, a World Allergy Organization position paper. World
Allergy Organ J. 13:1000802020. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Pitsios C, Demoly P, Bilò MB, Gerth van
Wijk R, Pfaar O, Sturm GJ, Rodriguez del Rio P, Tsoumani M, Gawlik
R, Paraskevopoulos G, et al: Clinical contraindications to allergen
immunotherapy: An EAACI position paper. Allergy. 70:897–909. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Wollenberg A, Barbarot S, Bieber T,
Christen-Zaech S, Deleuran M, Fink-Wagner A, Gieler U, Girolomoni
G, Lau S, Muraro A, et al: Consensus-based European guidelines for
treatment of atopic eczema (atopic dermatitis) in adults and
children: Part II. J Eur Acad Dermatol Venereol. 32:850–878. 2018.
View Article : Google Scholar
|
|
221
|
Lommatzsch M: Current asthma treatment in
light of new asthma guidelines. Dtsch Med Wochenschr. 143:806–810.
2018.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Wollenberg A, Barbarot S, Bieber T,
Christen-Zaech S, Deleuran M, Fink-Wagner A, Gieler U, Girolomoni
G, Lau S, Muraro A, et al: Consensus-based European guidelines for
treatment of atopic eczema (atopic dermatitis) in adults and
children: Part I. J Eur Acad Dermatol Venereol. 32:657–682. 2018.
View Article : Google Scholar
|