Introduction
Cancer is known as one of the underlying causes of
most public health threats and is considered the leading cause of
death after stroke and coronary heart disease on a global scale
(1). Based on reports from the
World Health Organisation (WHO), cancer is the first or second
leading cause of cancer-related deaths before the age of 70 years
in 112 countries. As reported by GLOBOCAN, there were approximately
19.3 million new cancer cases and 10.0 million cancer-related
deaths in 2020, and new cases are estimated to increase to 28.4
million in 2040 (1).
Cancer occurs mainly due to dynamic genetic
alterations, and the resultant genome deformities may promote the
uncontrolled growth of abnormal cells and spreading throughout the
body. It is well accepted that the generation of abnormal cells
accumulates mutations in essential genes, leading to cancer cell
formation. Accumulated mutations can further increase genomic
instability, including rearrangements and chromosomal changes
(2,3). Thus, genomic instability is the
main element and one of the underlying mechanisms in the initiation
and progression of human cancer (4).
The growing interest in the mechanistic
understanding of the specific factors in cancer development allows
researchers to investigate the role of the second human genome,
which is mitochondrial DNA (mtDNA). It is widely known that the
role of mitochondria is implicated in tumor promotion and
development. In the 1920s, Dr Otto Warburg observed that most
cancer cells have an altered metabolism characterized by high
glycolytic activity. This condition is referred to as ‘aerobic
glycolysis’ and is considered to be linked with a constant
diminished mitochondrial oxidative phosphorylation (OXPHOS)
(5). Since then, a considerable
amount of research has been conducted, which is devoted to the
dysfunction of mitochondrial respiration in cancer development
(6,7). Previous studies have revealed that
cancer cells exhibit a highly glycolytic phenotype and have the
capacity to utilize glucose at higher rates than their origin cells
(8,9). Moreover, it was observed that
cancer cell lines are incapable of adequately upregulating OXPHOS,
thus, increasing their dependency on glycolysis (10).
mtDNA is recognized to be highly unstable and liable
to damage compared to the nuclear genome, due to the persistent
exposure to oxidative attack coupled with deficient DNA repair
machinery (11). As a result,
mtDNA suffers from the formation of sequence alterations or
depletion of mtDNA content, consequently leading to various
disorders and cancer tumorigenesis. The levels of mtDNA copy number
(mtDNA-CN) may reflect the biogenesis and pathology of
mitochondria, which are responsible for the phenotypic changes in
malignant tumors. It implies that mtDNA-CN may serve as a potential
marker in a broad spectrum of cancers, including breast, lung,
colorectal, head and neck, and gastric cancers (10–14).
In the present review, the current knowledge
concerning the mtDNA-CN alterations found in human cancer were
investigated. Relevant literature was comprehensively searched
(between 2004 and 2022) using the online electronic databases:
PubMed, Web of Science, Scopus as well as other appropriate
resources. The key words searched for included ‘mtDNA-CN
alterations’ or ‘mtDNA content’ and ‘cancer’ or ‘neoplasm’. Of the
84 published articles, 76 were included and considered to meet the
inclusion criteria of the case-control studies that investigated
the association between mtDNA-CN alterations and various types of
human cancer. In this review, an update on the roles and mechanisms
of mtDNA-CN in tumorigenesis were outlined. In addition, available
evidence on the incidence of mtDNA content variations in a wide
range of human cancers were provided.
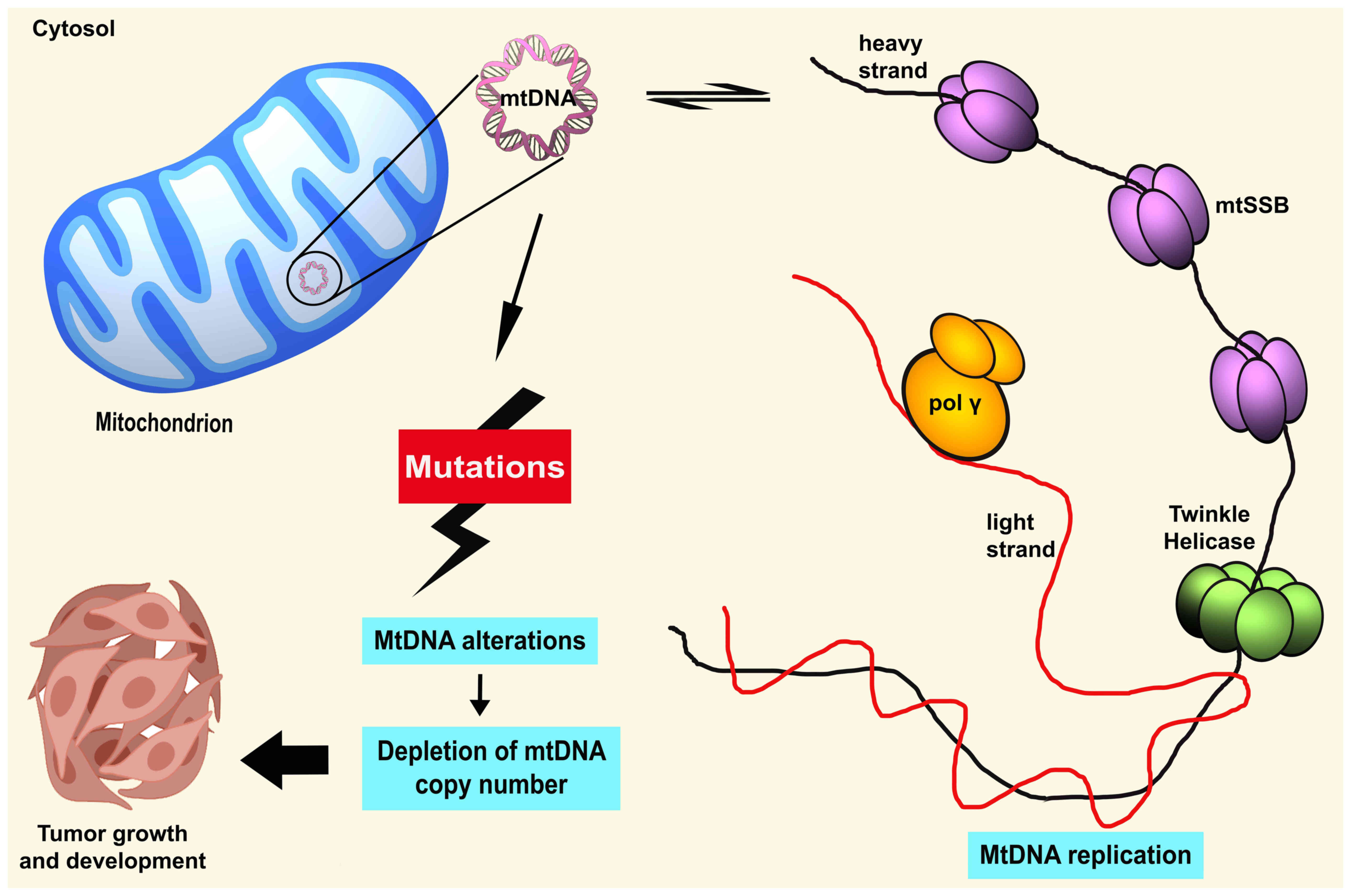
mtDNA
Mitochondria are eukaryotic organelles involved in a
broad spectrum of cellular processes, including the production of
ATP, cellular metabolism, reactive oxygen species (ROS) production,
activation of apoptosis, and calcium homeostasis. The generation of
mitochondrial energy is entirely dependent on five multi-subunit
protein complexes (complex I–V) of the OXPHOS system together with
the tricarboxylic acid (TCA) cycle (15). Protein complexes I, III, IV, and
V have contributions from both mtDNA and nuclear DNA (nDNA) genes,
but complex II is completely nuclear-encoded. Therefore, a proper
interaction between both the nucleus and mitochondria is needed to
achieve sufficient levels of a functional OXPHOS system.
Human mtDNA is maternally inherited due to the
higher number of mtDNA copies found in the egg of a female compared
to sperm which resides in the mitochondrial matrix (16). mtDNA is organized into nucleoids
that allocate across the mitochondrial networks (17). These nucleoids are composed of an
accumulation of mtDNA molecules and several elements of protein
factors, for instance, mitochondrial transcription factor A (TFAM),
mitochondrial single-stranded binding protein (mtSSB), polymerase γ
(pol γ), and helicase Twinkle (18). mtDNA is a close, double membrane
and circular form of 16,569 nucleotide pairs. mtDNA contains 37
genes, coding for two ribosomal RNAs, 22 transfer RNAs, and 13
elements consisting of seven subunits of complex I (ND 1 to 6 and
ND4L), one subunit of complex III (cytochrome b), three subunits of
complex IV (COX I, COX II and COX III), and two subunits of complex
V (ATPase 6 and ATPase 8) (18).
mtDNA contains two distinct strands based on the density
differences in caesium chloride gradient (19). The heavy strand (H-strand) has a
greater level of GC content and encodes for 12S and 16S rRNAs, 14
tRNAs, and 12 subunits of mitochondrial respiratory complexes. In
addition, the light strand (L-strand) encodes eight tRNAs and ND6
(18).
mtDNA carries only two non-coding regions and is
almost entirely composed by coding sequences (>90%), and their
genes do not present introns. The main non-coding region is termed
a displacement loop (D-loop) region covering 1,122 base pairs of
the genome (20). A D-loop has
all the essential materials for the transcription and replication
process: The origin of H-strand replication (OH), the promoters for
H- and L-strand transcription (HSP1, HSP2, and LSP), and three
conserved sequence blocks (CSB I, CSB II, and CSB III) (18). Therefore, D-loop variations may
affect the promoter sequences and consequent changes of protein
binding affinities of the inducers or modulators of mtDNA
transcription, which could induce replication error. On the other
hand, the origin of L-strand replication (OL) appears in the second
non-coding region with only 30 nucleotide pairs (18).
mtDNA-CN
Regulation of mtDNA-CN
Notably, the mtDNA-CN may represent the amount of
mitochondria in a cell. Almost hundreds or thousands of mtDNA
copies are available in each cell and vary considerably depending
on cell types and tissue origins (21). Of note, mtDNA-CN remains
consistent within each cell based on its energy requirement but may
markedly change during the aging process, cell differentiation,
hormone therapy, and exercise (13). Tissues with high energy demands,
such as the brain, and skeletal and cardiac muscles have more
mtDNA-CNs than other tissues, as for example, kidney and liver
cells (22,23). It has been determined that
mtDNA-CN in oocytes reaches up to 100 thousand copies, and recent
analyses have shown that there are ~4–6 thousand copies in the
heart and 0.5–2×103 copies in the lungs, liver, and kidneys
(24,25).
In general, each mitochondrial molecule per cell
shares similar mtDNA sequences referred to as homoplasmy. However,
due to the highly polymorphic nature of mtDNA, a mixture of
wild-type and mutant mtDNA coexist within the same cell, termed
heteroplasmy. This discrepancy could be attributed to numerous
somatic mtDNA mutations typically caused by oxidative stress, high
ROS exposure, replication errors, and aging (26). Heteroplasmy threshold occurs when
the frequency of heteroplasmy attains 60–90% of mutant mtDNA and
reflects the human mitochondrial pathology (6,27). Conceivably, the heterogeneity of
the mtDNA genome may act as an essential participant in
mitochondrial dysfunction, which is responsible for the development
of various complex diseases.
mtDNA replication
In 1972, Robberson et al studied mammalian mtDNA
replication by applying cesium chloride-purified mitochondrial DNA
and observed it under an electron microscope (28). With the combination of numerous
research studies, three models of mtDNA replication have been
proposed, including the asymmetric strand-displacement model,
strand-coupled bidirectional replication, and RNA incorporated
throughout the lagging strand (RITOLS) (20,29,30). Over the last 40 years, the
strand-displacement model has been the central reference in the
knowledge of mtDNA replication. Based on this model, the production
of a new leading strand (H strand) commences at the OH site within
the D-loop region. The synthesis proceeds continuously until 70%
(~11 kb) of the mtDNA genome circle, and when the OL origin of a
lagging strand (L strand) is exposed, the initiation of the lagging
strand occurs in the opposite direction (20).
Although several models of mtDNA replication have
been previously proposed, it is critical to highlight that the
understanding of the exact mechanism is still under debate and not
fully elucidated at present. However, it should be noted that the
mtDNA replication process occurs by a molecular machinery that is
different from the nuclear replisome. Nonetheless, nDNA is still
involved in the regulation of mtDNA-CN since all trans-acting
factors engaged in mtDNA replication are encoded by nDNA (31). Mitochondrial replication is
implemented by the onset of proteins which is initiated by
mitochondrial transcription factor A (TFAM) and aided by mtDNA pol
γ, hexameric Twinkle helicase, mtSSB, mitochondrial RNA polymerase
(POLRMT), transcription elongation factor (TEFM), transcription
factor B2 (TFB2M), exonuclease MGME1, DNA ligase III, and RNAse H1
(27).
Mitochondrial TFAM is a member of the high mobility
group (HMG) box domain family and is present at a ratio of 1
molecule per 16–17 base pairs of mtDNA (32). It is considered that TFAM is
sufficient to coat the mitochondrial genome, in addition to playing
a major role in the initiation of transcription and replication of
mtDNA. Furthermore, TFAM regulates protein binding at the
cis-regulatory region of the mitochondrial D-loop (31). It has been demonstrated that TFAM
plays a critical part in mtDNA-CN regulation and maintenance in
vivo (33,34). A previous study revealed that
loss of mtDNA was caused by mouse-TFAM deficiency, whereas the
elevated mtDNA-CN was driven by the overexpression of TFAM
(35). Moreover, the levels of
mtDNA molecules needed for the transcription and replication
process are dependent on TFAM concentrations available (36). Therefore, the expression level of
TFAM was hypothesized to be directly proportional to the level of
mtDNA molecules (35).
mtDNA pol γ is the sole DNA polymerase of
mitochondria responsible for replicating and repairing the mtDNA
genome (32). It has a high
proofreading ability to eradicate the misincorporation of DNA
bases. mtDNA pol γ also appears to function with Twinkle helicase
and mtSSB protein to perform, to some extent, complete mtDNA
replication. Korhonen et al discovered that the synthesis of ssDNA
molecules during replication is achieved with the cooperation of
mtDNA pol γ, Twinkle, and mtSSB (37). Twinkle is a 72-kDa monomer and is
greatly homologous to bacteriophage T7 gene 4 primase/helicase
(T7gp4) (38). A preliminary
study revealed that Twinkle has a 5′ to 3′ DNA helicase activity
and unwinds the dsDNA at the mtDNA replication fork (39). Milenkovic et al reported the
significance of Twinkle in nascent D-loop strand synthesis during
mtDNA replication (40).
However, the activity of both mtDNA pol γ and Twinkle is stimulated
by mtSSB (39). mtSSB was found
to be more abundant in human HeLa cells than mtDNA. mtSSB can
support the unwinding activity of Twinkle helicase and improve
mtDNA pol γ activity (41,42). The processivity of Twinkle
helicase increased when bound to mtSSB and could unwind the
duplexes longer than needed for strand separation (43). Furthermore, mtSSB binds to ssDNA
to preserve and maintain ssDNA in an active single-stranded state
(44). The regulation of mtSSB
has a possible mechanistic link with TFAM for D-loop stabilization
and mtDNA maintenance (41).
Mutations of regulatory factors
involved in mtDNA replication
It has been proven that the accumulation of somatic
mutations in critical regions, for instance, in conservative
sequences areas, replication sites, promoter, or transcription
factor binding sites, can lead to repression of mtDNA gene
expression and subsequently damage mitochondrial biosynthesis
(45) (Fig. 1). These alterations substantially
alter mitochondrial respiration capacity, other than stimulating a
higher rate of oxidative stress and apoptosis (45). mtDNA mutations frequently occur
within the hypervariable D-loop region, particularly in a
homopolycytidine stretch or near the replication origins of the
mtDNA H-strand (11). This
crucial region possesses a triple-stranded DNA structure, which is
severely liable to the persistent effect of oxidative insults,
thus, possibly increasing the rate of mutations. It was theorized
that aberration in the D-loop could alter the binding affinities of
several regulators of nuclear-encoded DNA on the regulatory site of
mtDNA (20). Thus, mutations in
this particular region may modify mtDNA transcription and
subsequently cause disturbances in mtDNA respiration and ROS
production (46). The damaged
mtDNA is removed by mitophagy in the cell, which causes the
reduction of mtDNA-CN (47).
Therefore, as suggested, the alterations in the D-loop region could
critically affect the regulation of mtDNA at the heteroplasmy level
and mtDNA-CN (48).
Available literature has revealed that somatic
mutations in the D-loop region are markedly associated with a
decreased level of mtDNA in hepatocellular carcinoma, invasive
breast cancer, and Ewing's sarcoma (49,50). Moreover, decreased mtDNA-CN was
correlated with tumors that harbored somatic mutations, which were
located close to replication origins of the H-strand or at the
homopolymeric C-stretch in the D-loop (50–52). Sequence mutations in the D-loop
region, specifically, in the polycytidine stretch caused by
continuous oxidative stress, could lead to slippage error and/or
mispairing during mtDNA replication or repair by mtDNA pol γ
(53). mtDNA pol γ has low
frameshift fidelity and is susceptible to mistakes, resulting in
decreased proofreading efficiency (54). Defects in mtDNA pol γ can arise
from increased oxidative damage, and in turn, this may induce
further mtDNA errors. Previously, published studies delineated that
transgenic mice with high-level proofreading-deficient pol γ
expression induce mitochondrial mutations in cardiac tissues
(55). However, the homozygous
knock-in mice carrying-expressed proofreading-deficient pol γ
developed a mutator phenotype with a higher rate of mutated and
deleted mtDNA (56). Thus, the
faulty mtDNA pol γ may reflect the biosynthesis of mtDNA, which
affects the regulation of mtDNA-CN.
A plethora of evidence has revealed the close
association of mtDNA pol γ and the amount of mtDNA-CN in human
cancers. Spelbrink et al identified that transient expression of
pol γ-myc mutants suppressed mtDNA polymerase activity and was
associated with depleted mtDNA in vivo (57). Furthermore, Singh et al revealed
that 63% of pol γ mutations in breast cancer tumors led to mtDNA
depletion (58). Data from a
previous study, indicated that mtDNA-CN was decreased in patients
with colorectal cancer, and exhibited somatic mutations in pol γ or
germline nucleotide mutations were found in the region encoding pol
γ polymerase domain (59).
Another previous study revealed that pol γ SNP genotype was
markedly associated with the increased level of mtDNA-CN and
improved survival among patients with hepatocellular carcinoma
(60). Indeed, aberrant mtDNA
pol γ is considered to contribute to repair system deficiency,
which leads to mtDNA-CN alterations.
The presence of Twinkle helicase is also critical in
mtDNA-CN, as the interactions of helicase-primase during mtDNA
replication play a part in controlling mtDNA levels in cells.
Theoretically, Twinkle has been suggested to be responsible for the
switching between abortive and full-length mtDNA replication during
the pre-termination events of triple-stranded D-loop structure
formation (38). This switching
thus determines the regulation of mtDNA levels in the cell. In a
preliminary study, Twinkle was identified as the causative gene of
autosomal dominant progressive external ophthalmoplegia (adPEO)
correlated with several mtDNA deletions (61). To date, >40 point mutations
have been detected in the TWNK gene encoding Twinkle, which is
associated with multiple diseases (38).
Previous research has touted that the role of
Twinkle helicase can be inferred from the regulation of the amount
of mtDNA. One study in 2004 reported that overexpression of the
Twinkle helicase in transgenic mouse lines led to an increase in
mtDNA-CN (62). Twinkle helicase
overexpression may also act on anti-helicase blocking, thus
permitting increased mtDNA-CN and successful mtDNA replication
(40). However, studies of
mutant Twinkle variants in vivo demonstrated that mutations of this
helicase lead to extreme mtDNA depletion and increased apoptosis in
flies (63,64). Therefore, this suggests that
Twinkle is the sole replicative helicase for mitochondrial
biogenesis.
In general, it appears that the knockout of these
regulatory elements in mtDNA replication could affect the mtDNA
genome, particularly within the non-coding region. Although any
defects or absence of these elements may perturb the regulation of
mtDNA copies, overexpression of a protein involved in this
machinery does not constantly increase mtDNA-CN. Moreover, mtDNA
depletion and secondary multiple deletions/duplications may be the
outcomes of the flaws in mtDNA machinery proteins with the
consequent mitochondrial dysregulation (38). Thus, common regulation of
mtDNA-CN may be limited, thus increasing the inability of
mitochondria to maintain normal function, subsequently establishing
a background liability of tumor development.
Effects of aberrant mtDNA-CN
It is well-recognized that regulation of mtDNA-CN is
significant to govern the cellular energy needs. mtDNA-CN was shown
to be markedly associated with the level of oxidative insults in
the mtDNA genome and involved in ROS production (up to 85%) to
modulate apoptosis or cell differentiation (65). Aberrant mtDNA-CN can lead to
elevation of mtDNA oxidative stress and disruption of mtDNA gene
expressions. Subsequently, this may affect overall mtDNA functions,
including the OXPHOS system, ROS production, signal transduction,
cell apoptosis, cell growth, and mitochondria-to-nucleus retrograde
signaling (66,67). Certainly, disturbances in the
OXPHOS system may result in a reduction of intracellular ATP
generation (6). Depleted mtDNA
causes a decrease in OXPHOS capacity, which triggers a high
compensatory increase in glycolysis to cover the total ATP
(7). Thus, the aberrant mtDNA-CN
can reduce the rate of mitochondrial biogenesis and normal cellular
function that eventually confer the emergence of tumors.
Previous findings have described the functional
significance of oxidative stress correlated with the mtDNA-CN.
Intrinsic oxidative stress induced by noxious ROS was revealed to
be closely associated to cell toxicity, DNA injury, copy number
changes, and malignant transformation in human cancer cells
(68). A previous study on
mtDNA-CN alterations conducted in human leukocytes revealed that
oxidative stress in blood circulation influences the level of
mtDNA-CN via the alterations of plasma antioxidants/oxidants and an
oxidative insult to DNA (69).
In addition, Al-Kafaji and Golbahar revealed the effects of the
glucose-induced oxidative stress and mtDNA-CN in human mesangial
cells, in an in vitro study. The results revealed a higher mtDNA-CN
in the mesangial cells, as a response following higher oxidative
stress stimulated by high glucose activity (70). In addition, mtDNA-CN and
oxidative stress are associated with environmental exposure to
tobacco, pollutants, smoke, drugs, xenobiotics, and radiation
(66).
It is also important to emphasize the functional
impact of mtDNA-CN alteration on nDNA instability. Notably,
aberrant mtDNA-CN could potentially interrupt mitochondrial
membrane potential, contributing to mitochondrial dysfunction
(71). As a result, the
initiation of retrograde signaling occurs and mitochondria
communicate their changing functional and metabolic state to the
nucleus. This response causes the impairment of the expression
profile of nuclear genes and modifies cell physiology and
morphology (71). In 1989,
Corral et al found that chemically-induced rat hepatomas and the
HT-1080 fibrosarcoma cell line exhibited increased copy numbers of
COI, COII, and COIII pseudogenes in nDNA compared with normal cells
(72). In addition, it was
demonstrated that mtDNA depletion in HeLa cells showed higher lipid
peroxidation and oxidative damage to nDNA (73).
Furthermore, it has been shown that aberrant
mtDNA-CN is associated with resistance to apoptosis and the
promotion of cancer (7).
mtDNA-depleted cells were demonstrated to activate the Akt pathway,
which could inhibit the apoptosis of cells (74). Furthermore, nuclear factor-κB/Rel
signaling, which is involved in apoptosis resistance and cancer
development, can be activated by depleted mtDNA content (75,76). By contrast, recent data revealed
that mtDNA-CN was increased in apoptotic tumor cell lines,
suggesting that increased mtDNA-CN acts as a defense mechanism of
tumor cells to prevent apoptosis (77). Decreased mtDNA-CN in tumor cells
increased ROS levels, the rate of apoptosis, and sensitivity
against chemotherapeutic drugs (77). This finding is in line with a
previous study that demonstrated that increased mtDNA-CN induces
survival and apoptosis resistance in colorectal cancer cells in
vivo and in vitro (78).
Previously, published studies have revealed
increased or decreased mtDNA-CN in some tumor entities. Several
studies have assessed the quantitative changes of mtDNA-CN in
peripheral blood specimens and the resected tumorous tissues
compared with non-tumorous tissues (65). Increased mtDNA-CN was observed in
breast, bladder, esophageal, head and neck squamous cell, kidney,
and liver cancers (except in lung carcinoma) compared with
non-tumorous tissues (79).
Moreover, decreased mtDNA-CN was revealed in kidney clear cell
carcinoma, hepatocellular carcinoma, and myeloproliferative tumors
(80). The association of
mtDNA-CN variation with the risk of cancer progression has also
been reported. A meta-analysis by Hu et al (66) discovered that increased mtDNA-CN
was markedly associated with the risk of lymphoma, melanoma and
breast cancer, but inversely associated with hepatic carcinoma.
This discrepant result of mtDNA-CN in different cancer types
implies that mtDNA-CN occurs in a cancer-specific manner, as a
consequence of different needs of energy metabolism in human cancer
tissues. Notably, knowledge of mtDNA-CN in tumorigenesis remains
elusive and requires elucidation.
Restoration of mtDNA-CN
The current understanding of mtDNA-CN regulation is
still unclear, although evidence of mtDNA replication and
maintenance has accumulated and improved over the years. Several
theories and models have been proposed to explain mtDNA-CN
regulation. It is hypothesized that all the binding proteins
involved in mtDNA replication, may be key factors in the
maintenance and regulation of mtDNA levels (79). Furthermore, a study performed by
Tang et al suggested that the regulation of mtDNA-CN could depend
on the availability of nucleotide pools (80). Subsequently, these authors
introduced another theory, which proposed that several replication
origins may affect the regulation of mtDNA-CN (81). Additionally, different levels of
mtDNA-CN (wild-type, duplicated, or deleted mtDNA) were shown to be
independent of mtDNA size, indicating that the copy numbers were
not controlled by the number of molecules or mtDNA genomes present
(81). In 2009, a threshold
model for mtDNA-CN control was proposed (31). The model suggested that
upregulation of mtDNA replication machinery could be the cause of a
lower mtDNA-CN threshold, whereas mtDNA degradation could be the
result of a higher mtDNA-CN threshold (31).
mtDNA-CN is important in early development and
differentiation, as mature cells need enough copies to meet their
specific demands (82).
Premature cells exhibit a high level of copy numbers during
oogenesis and achieve maximal levels in mature oocytes. Copy number
is subsequently decreased during the development of preimplantation
before gastrulation (83). A low
copy number in these undifferentiated cells indicates the mtDNA set
point, which allows these cells to acquire sufficient copies of
mtDNA to sustain their energy demands (18). Cells that utilize high ATP
consumption, including the heart, muscle, and brain cells contain
high mtDNA-CN, while cells that retain low copies such as
endothelial cells depend on glycolysis (84).
The mtDNA set point of a cell requires synchrony
between nDNA and mtDNA genomes to establish a cooperative process
of mtDNA differentiation and replication (84). However, cancer cells that contain
depleted mtDNA-CN have to re-establish their mtDNA set point
(85). It has been demonstrated
that levels of mtDNA-CN influence the frequency and progression of
GBM cells. The number and duration of tumor formation were
proportional to the mtDNA depletion level. Thus, mtDNA-depleted GBM
cells restored their mtDNA-CN for their tumor growth process
(84). In addition, mtDNA-CN can
be recovered by the mtDNA replication machinery of a cell and
mitochondrial horizontal gene transfer from adjacent cells
(85,86). The migration of mtDNA horizontal
gene transfer from normal cells to tumor cells with deficient mtDNA
results in the restoration of respiration, initiation of tumors,
and metastasis (87).
It is also plausible that mtDNA variations are
implicated in mtDNA-CN restoration. mtDNA with impaired respiration
function or carrying mutations deals with this damage by increasing
mtDNA-CN in accordance with the proportion of mtDNA-CN in the cell
(85). A preliminary study
reported that a high mtDNA-CN found in aging tissue cells acts as a
feedback mechanism to counterbalance the metabolic injury in
mitochondria carrying mutated mtDNA and a defective respiratory
chain (88). Additionally, a
previous study by Yeung et al revealed that GBM cells harbored the
accumulation of mtDNA variants in ND6 and ND4 genes, that encoded
the subunits of complex I of the respiratory chain, resulting in
impaired mtDNA function and consequently affecting mtDNA levels
(89). A more recent study
demonstrated a marked increase of mtDNA-CN in samples with
high-allele-frequency truncating mutations encoded by nDNA. The
authors suggested that the increased mtDNA-CN was necessary to
compensate for the damage induced by truncating mutations (90).
Assessment of mtDNA-CN
To date, numerous studies have determined the levels
of mtDNA-CN in tissues and peripheral blood samples. Notably,
peripheral blood has been proposed as a reliable source, due to the
easily obtainable and non-invasive approach in estimating mtDNA-CN
in human cancer. Moreover, mtDNA-CN alterations in peripheral blood
are proposed as a potential indicator, to study mitochondrial
function and aerobic metabolism and have been revealed to be
significantly correlated with the risk of cancer (91,92). However, since mitochondrial
numbers and mtDNA copies in a cell differ within tissue types, it
is crucial to use accurate methods in order to prevent any
compromised variables in assessing the copy numbers.
Several methods currently exist for the
quantification of mtDNA-CN (93–95). One of the most well-known methods
for assessing mtDNA-CN in human samples and model organisms is
quantitative real-time PCR (qPCR) which assesses the ratio of an
mtDNA gene to a reference gene (nuclear genome). Previous studies
have used several genes for mtDNA level assessment, including
MTATP8, MTND1, MTCOX1, 16S rRNA, and MTCYB for mtDNA, while ACTB,
B2M, HGB, and GAPDH have been used as nuclear genomes (10,14,66,96–98). Recently, droplet digital PCR
(ddPCR) was developed as a high-efficiency method to measure
mtDNA-CN without applying external standards (99,100). The ddPCR utilizes a similar
workflow as in qPCR technology, and a sample can be segmented into
thousands of oil emulsion droplets (99). The amplification occurs in
individual droplets to avoid bias in PCR efficacy and inhibitors
(100). However, the restricted
efficacy of ddPCR may affect quantification accuracy when measuring
a large copy number (100,101).
Other available methods to quantify mtDNA-CN include
genotyping microarray probe intensities, whole genome sequencing
(WGS), whole exome sequencing (WES), and DNA sequencing read counts
(102). It has been shown that
WGS data could provide a more sensitive and accurate assessment of
mtDNA levels compared with the qPCR method (101,102). The WGS data provide complete
genome sequencing reads including mtDNA and nDNA (101), and WGS serves as the only
current method which concurrently measures mtDNA-CN and mutations
(103).
It is conceivable that methods used in purifying DNA
are also critical in assessing mtDNA content. Methods utilized for
DNA extraction or isolation may affect the yield of mtDNA integrity
due to the circular and compact size of mtDNA (102,104). Any deviation from a specific
protocol may have a critical impact on the accuracy of mtDNA-CN
estimation. A number of studies with different extraction
techniques have been performed to establish a more accurate method
for measuring mtDNA-CN (93,95,105–108). The column kit isolation method
is often used to extract DNA fragments ≥50 kb and highly depends on
chaotropic salts and low pH to induce the DNA binding capacity.
This method potentially decreases total DNA, which may increase the
bias in the measurement of the DNA ratio (106). Previously, organic solvents
containing precipitated ethanol were demonstrated to be more
accurate compared to column-based methods (106). The organic solvent method is
time-consuming and mostly relies on technical skill, however, it
offers a high DNA yield (106).
Furthermore, a method with less usage of spin columns or without
spin columns may be able to diminish GC-dependent bias (109). Recently, it was revealed that
the lysis-based method has low variability and is more accurate
than other traditional methods. Cells are directly lysed without
any isolation or spin columns, ensuring minimal hands-on time in
addition to reducing biased mtDNA-CN estimation (102).
Involvement of mtDNA-CN in human
cancers
With the numerous types of cancers that have been
assessed for mtDNA content alterations, it is necessary to examine
the molecular mechanism of mtDNA-CN occurrence in carcinogenesis.
With this aim, an extensive search in Google Scholar, PubMed,
online databases, and published journal articles was performed, to
summarize the involvement of mtDNA-CN alterations in various types
of cancer (Table I).
 | Table I.Distribution of mtDNA copy number
levels in selected cancer types of different countries. |
Table I.
Distribution of mtDNA copy number
levels in selected cancer types of different countries.
| Type of cancer | No. of samples | Laboratory
methods | MthNA gene | Nuclear gene | MthNA levels | Country | (Refs.) |
|---|
| Breast | 302 | qPCR |
tRNALeu | B2M | Decreased | USA | (52) |
|
| 59 | qPCR | D˗loop | ACTB | Decreased | China | (51) |
|
| 102 | qPCR | MTATP8 | GADPH | Decreased | Switzerland | (108) |
|
| 183 | qPCR | MT˗ND1 | 18S RNA | Increased | Singapore | (113) |
|
| 103 | qPCR | MT˗ND1 | HGB | Increased | USA | (92) |
|
| 1000 | qPCR | MT˗ND1 | HGB | Increased | USA | (96) |
|
| 1,108 | qPCR | MT˗ND1 | ALB | Increased | UK | (112) |
|
| 506 | qPCR | mtDNA | B2M | Increased | China | (91) |
|
| 570 | qPCR | MT˗ND1 | ACTB | N/A | EPIC | (12) |
|
| 82 | qPCR |
tRNALeu | 18S rRNA | Decreased | Mexico | (111) |
|
| 60 | qPCR | MT˗ND1 | ACTB | Decreased | Taiwan | (107) |
|
| 60 | qPCR | MT˗ND1 | ACTB | Decreased | Taiwan | (110) |
| Colorectal | 60 | qPCR | MT˗ND1 | ACTB | Decreased | China | (121) |
|
| 444 | qPCR | MT˗ND1 | BRCA1 | Decreased | China | (122) |
|
| 736 | qPCR | MT˗ND2 | FASLG | Decreased | Canada | (120) |
|
| 74 | qPCR | D˗loop | B2M | Decreased | Netherlands | (123) |
|
| 422 | qPCR | MT˗ND1 | 18S rRNA | Increased | Singapore | (117) |
|
| 320 | qPCR | MT˗ND1 | HGB | Increased | China | (116) |
|
| 126 | qPCR | 16S rRNA | B2M | Increased | North India | (14) |
|
| 24 | qPCR | mtDNA | ACTB | Increased | China | (114) |
|
| 104 | qPCR | COXI | ACTB | Increased | China | (115) |
|
| 324 | qPCR | MT˗ND2 | AluYb8 | Decreased | USA | (119) |
| Gliomas | 28 | qPCR | MT˗ND2 | FALSG | Increased | Italy | (124) |
|
| 124 | qPCR | MT˗ND1 | ACTB | Increased | China | (122) |
|
| 336 | qPCR | MT˗ND1 | HGB | Increased | China | (127) |
|
| 390 | qPCR | MT˗ND1 | HGB | Increased | USA | (126) |
|
| 35 | qPCR | D˗loop &
COXII | ACTB | Decreased | Australia | (131) |
|
| 162 | qPCR | MT˗ND1 | RNase P | Decreased | India | (132) |
|
| 67 | qPCR | N/A | N/A | Increased | France | (128) |
|
| 414 | qPCR | MT˗ND1 | ACTB | Increased | China | (129) |
| Gastric | 76 | qPCR | mtDNA | ACTB | Decreased | China | (137) |
|
| 20 | PAGE | D˗loop | ACTB | Decreased | China | (133) |
|
| 31 | qPCR | MT˗ND1 | ACTB | Decreased | Taiwan | (134) |
|
| 109 | qPCR | COXI | ACTB | Increased | Korea | (67) |
|
| 103 | qPCR | MT˗ND1 | ACTB | Decreased | China | (135) |
|
| 984 | qPCR | MT˗ND1 | HGB | Increased | China | (16) |
|
| 162 | qPCR | mtDNA | HBB | Decreased | China | (136) |
|
| 109 | qPCR | COXI | HBB | Decreased | Korea | (98) |
| Prostate | 9 | qPCR | MT˗ND1 | HBB | Increased | USA | (138) |
|
| 196 | qPCR | MT˗ND1 | HGB | Decreased | USA | (139) |
|
| 102 | qPCR | MT˗ND1 | HBB | Increased | India | (140) |
|
| 793 | qPCR | MT˗ND1 | HBB | Increased | USA | (141) |
|
| 1,751 | qPCR | MT˗ND1 | HGB | Decreased | USA | (142) |
|
| 46 | qPCR | MT˗ND1 | B2M | Decreased | Australia | (143) |
|
| 317 | qPCR | MT˗ND1 | HGB | Decreased | USA | (144) |
| Esophageal | 20 | qPCR | mtDNA | B2M |
Increased/Decreased | USA | (145) |
|
| 42 | qPCR | N/A | N/A | Increased | China | (146) |
|
| 72 | qPCR | MT˗ND1 | 18S rRNA | Increased | Taiwan | (147) |
|
| 80 | qPCR | COXI | COXIV | Increased | Japan | (149) |
|
| 141 | qPCR | MT˗ND1 | ACTB | Increased | China | (148) |
| Lung | 29 | qPCR | MT˗ND1 | 18S rRNA | Decreased | Taiwan | (65) |
|
| 122 | qPCR | MT˗ND1 | HBB | Increased | China | (156) |
|
| 874 | qPCR | MT˗ND1 | HBB | N/A | China | (158) |
|
| 128 | qPCR | MT˗ND1 | 36B4 | Decreased | China | (155) |
|
| 227 | qPCR | MT˗ND1 | HBB | Increased | Finland | (157) |
|
| 37 | qPCR | MT˗HVI | HBB | Decreased | China | (13) |
| Renal cell | 37 | Southern blot | mtDNA | 18S rRNA | Decreased | Austria | (159) |
|
| 375 | qPCR | MT˗ND1 | HGB | Decreased | USA | (160) |
|
| 1,217 | qPCR | MT˗ND1 | HBB | Decreased | USA | (161) |
|
| 252 | qPCR | MT˗ND1 | HBB | Increased | USA | (163) |
|
| 5 | qPCR |
tRNALeu | 18S rRNA | Decreased | Taiwan | (162) |
|
| 57 | qPCR | MT˗ND1 | HBB | Increased | Egypt | (164) |
| Head and neck | 76 | qPCR | COXI & II | ACTB | Decreased | USA | (152) |
|
| 91 | qPCR | COXI | ACTB | Increased | USA | (151) |
|
| 75 | qPCR |
tRNALeu | 18S rRNA | Increased | Taiwan | (153) |
|
| 570 | qPCR | MT˗ND1 | HGB |
Increased/Decreased | China | (95) |
|
| 50 | qPCR | D˗loop | GADPH | Increased | India | (154) |
| Pancreatic | 43 | qPCR | MT˗ND1 | SLCO1B1 | Decreased | Poland | (172) |
|
| 406 | qPCR | MT˗ND1 | HBB | Increased | Taiwan | (171) |
|
| 476 | qPCR | MT˗ND1 | ALB | Decreased | EPIC | (173) |
| Endometrial | 20 | qPCR | MT˗ND1 | ACTB | Increased | Italy | (166) |
|
| 65 | qPCR | MT˗ND1 | HBB | Increased | China | (165) |
|
| 139 | qPCR | MT˗ND1 | HGB | Decreased | USA | (167) |
| Oral | 35 | qPCR | COXI & II | ACTB | Decreased | Japan | (170) |
|
| 124 | qPCR | D˗loop | GADPH | Decreased | India | (168) |
|
| 143 | qPCR | MT˗ND1 | HGB | Increased | USA | (169) |
| Ovarian | 42 | qPCR | MT˗ND1 | HBB | Increased | China | (174) |
|
|
|
|
|
SLCO2B1/SERPINA |
|
|
|
|
| 24 | qPCR | MT˗ND1/ND5 | 1 | Increased | Hungary | (175) |
| Melanoma | 136 | qPCR | MT˗ND1 | HBB | Increased | USA | (176) |
|
| 500 | qPCR | MT˗ND1 | HGB | Increased | USA | (177) |
| Laryngeal | 40 | qPCR | COXII | ACTB | Increased | China | (179) |
|
| 204 | qPCR | MT˗ND1 | ACTB | Increased | China | (178) |
Breast cancer
Several experimental approaches investigating
mtDNA-CN in patients with breast cancer have been aggressively
performed that demonstrated a varying spectrum of mtDNA
alterations. To date, most of the analyzed patients who suffered
from breast cancer exhibited a significant change in mtDNA-CN,
which was positively correlated with the risk of breast cancer.
The alterations of mtDNA-CN in breast cancer were
first reported in 2006, and the results revealed decreased mtDNA-CN
in 38 breast cancer samples compared with the paired non-tumorous
tissues (107). According to an
analysis of mtDNA-CN in 59 paired breast tumor tissues and
non-tumorous tissues in 2007, the mtDNA-CN level appeared to be
lower in tumor tissues, advanced age, and advanced tumor stage
(51). The authors also observed
that tumors harboring mutations that occurred in the D-loop region
had less mtDNA-CN. Consistent with this finding, the studies by Bai
et al and Jiang et al, also observed that somatic mutations that
occurred in that area may facilitate the reduction of the level of
mtDNA in breast tumorigenesis (52,91). Moreover, decreased mtDNA-CN was
found in 82% of invasive breast tissue samples compared with the
normal counterparts from a total of 102 tumor tissue samples
(108).
In 2010, Hsu et al investigated the depleted mtDNA
content in breast cancer in response to anthracycline treatment in
vivo and vitro (110). The
results revealed that decreased disease-free survival of patients
was associated with higher mtDNA content compared with patients
with decreased mtDNA content. These authors also demonstrated that
mtDNA-depleted breast cancer cells had greater sensitivity to
doxorubicin treatment and higher ROS production. These findings
indicate that the level of copies in mtDNA may serve as a valuable
biomarker in predicting response to anthracycline treatment in
breast cancer. A more recent analysis reported that mtDNA-CN was
markedly decreased in breast tumor tissue samples among 82 tumor
cases (111). The results also
revealed that mtDNA-CN was decreased in the sequences with three
deletions at A249del, A290del, and A291del or C16327T, while the
copy number was increased in sequences containing C16111T, G16319A,
or T16362C (111).
Notably, Shen et al examined the mtDNA-CN in
connection to certain endogenous antioxidants and oxidants, using
peripheral blood specimens (92). The study concluded that an
increase in the level of mtDNA content was associated with the
development of breast cancer. However, mtDNA-CN was inversely
associated with changes in antioxidant and oxidant status. In a
subsequent study, these authors also revealed that higher mtDNA
content was associated with a higher risk of breast cancer, in
addition to the presence of mitochondrial length heteroplasmy in
hypervariable I (HV1) and hypervariable II (HV2) regions (96). Thus, it was hypothesized that the
appearance of HV1 and HV2 length heteroplasmy may be involved in
the initiation and promotion of breast cancer.
Furthermore, a comprehensive study of mtDNA-CN in
peripheral blood cells by Lemnrau et al provided evidence that an
increased level in mtDNA-CN was shown to be associated with the
increased risk of breast cancer (112). This study also claimed that the
results revealed a stronger association with the risk of breast
cancer as mtDNA-CN was more accurately measured using prospectively
collected blood specimens and a large sample size. These data are
in line with a study by Thyagarajan et al which revealed that the
increase of mtDNA-CN in peripheral blood samples was associated
with a higher risk of breast cancer (113). In another study, the assessment
of leukocyte telomere length (LTL) and mtDNA-CN were investigated
in 570 breast tumor cases and 538 controls from the EPIC cohort,
using real-time qPCR analysis (12). The study, with collected samples
obtained 15 years apart, revealed a link between telomere length
and mtDNA-CN. Additionally, it was observed that longer LTL was
strongly associated with an increased risk of breast cancer, while
mtDNA content was not found to be associated with the risk of
breast cancer.
Colorectal cancer
mtDNA content was assessed in 24 patients with colon
cancer and 20 patients with rectal cancer by Feng et al (114). This study revealed a
significant increase in mtDNA-CN changes associated with tumor
stage, mainly in stages I and II, suggesting that mtDNA-CN is
involved in the early progression of colorectal cancer (114). Moreover, an analysis of mtDNA
4,977 bp deletion and mtDNA-CN was conducted in the same year, and
the results revealed that increased mtDNA-CN was associated with
the levels of the 4,977 bp deletion and with tumor stage (115). In a different study, Qu et al
demonstrated that higher mtDNA content was found in patients with
colorectal cancer than in the paired controls, and was shown to be
markedly associated with higher risk of colorectal cancer, similar
to a study by Kumar et al (14,116). However, Thyagarajan et al
observed a U-shaped association of mtDNA-CN and colorectal cancer
risk among Singaporean Chinese patients, indicating that patients
with increased and decreased mtDNA-CNs were at increased risk for
colorectal cancer (117).
A 2017 study by Tong et al investigated the effect
of D-loop demethylation on mtDNA content using five colorectal
cancer cell lines (118). In
this study, it was determined that a DNA hypomethylating agent
caused an increase of mtDNA-CN and alterations in the cell biology
of colorectal cancer (118).
Furthermore, Sun et al reported that increased mtDNA-CN was
correlated with the regulation of the survival and metastasis of
microsatellite-stable colorectal cancer cells (78). Recently, a study of mtDNA-CN
using blood specimens from 324 female patients and 658 paired
controls was performed by Yang et al. In this study, it was
demonstrated that mtDNA-CN was inversely associated with the risk
of colorectal cancer in a dose-dependent manner (119). In addition, previous research
identified the mtDNA-CN increase and reduction (39.6 and 60.4%) in
colorectal tissues compared with the non-cancerous rectum or colon
tissues, respectively (120).
This study also revealed that there was no statistically
significant association between the mtDNA-CN variable with the
overall survival and disease-free survival of the patients.
Several studies on colorectal cancer with reduced
mtDNA-CNs have also been published. As demonstrated by Cui et al,
mtDNA-CN was shown to be lower in colorectal cancer tissues than
its corresponding counterparts (121). Moreover, Huang et al also
observed lower mtDNA-CNs in blood specimens of female patients who
suffered from colorectal cancer than in those of controls (122). In addition, a subsequent study
revealed a significantly decreased mtDNA-CN in tumorous tissue than
in adjacent tissue (123). The
authors also revealed that the mtDNA-CN was markedly lower in
mutated BRAF and microsatellite instability (MSI) tumors but
increased in KRAS mutated tumors. These findings suggest that the
mtDNA-CN plays a significant role in tumorigenesis.
Gliomas
In 2013, Marucci et al observed the correlation
between mtDNA content with morphology and survival using an
immunohistochemical method in a group of patients with glioblastoma
(GBM) (124). The findings
revealed that 10 cases exhibited oncocytic changes, and nine of
these cases had markedly increased mtDNA content compared with
control tissues. In the same year, Dickinson et al determined
whether GBM cells can regulate mtDNA-CN and chromosomal gene
expression during differentiation compared with human neural stem
cells (hNSCs) (84). The results
revealed that GBM cells did not upregulate mtDNA-CN, respiratory
capacity, and the expression of nuclear-encoded mtDNA replication
factors during differentiation compared with hNSCs. It was also
determined that tumors originating from mtDNA-depleted GBM cells
retrieved mtDNA-CNs for tumor development, indicating that mtDNA
may play a crucial role in tumor progression (84).
An increased mtDNA content was also reported in a
case-control study of patients with glioma using peripheral blood
lymphocytes (PBLs) (125). This
study also revealed that increased mtDNA content in PBLs was
associated with the risk of glioma. This data is also in agreement
with a previous study by Shen et al that observed a higher mtDNA-CN
in glioma cases compared with control subjects and was
significantly associated with glioma risk (126). Chen et al reported that
increased mtDNA-CN was markedly correlated with a worse prognosis
in patients with glioma (127).
However, two separate studies demonstrated that increased mtDNA was
associated with an improved prognosis in patients with GBM
(128,129).
Conversely, a previous study revealed decreased
mtDNA-CN in temozolomide-resistant glioma cells (130). More recently, Shen et al
analyzed mtDNA alterations as a therapeutic method in pediatric
patients with high-grade gliomas. In this study, the reduction of
mtDNA-CN in glioma cases compared with normal brains, was reported
(131). Furthermore, Sravya et
al observed that decreased mtDNA-CN was associated with worse
prognosis and treatment resistance in GBM (132).
Gastric cancer
The incidence of mtDNA-CN in gastric cancer was
originally observed by Li et al in 2004 (133). In this study, a decreased
mtDNA-CN was observed in 20 cases of gastric cancer compared with
matched paracancerous tissues using the polyacrylamide gel
electrophoresis method (133).
Subsequently, Wu et al reported a significantly decreased mtDNA-CN
in 17 out of 31 cases of gastric cancer (134). In another study, Zhang et al
determined that most patients with gastric cancer had a low
mtDNA-CN compared with the non-tumorous gastric group (135). Moreover, it was found that
mtDNA content variation was involved in cancer-related deaths in
patients with advanced gastric cancer, as well as the risk of lymph
node metastasis.
A previous study revealed that leukocyte mtDNA-CN
was not associated with the risk of gastric cancer (136). Nevertheless, the authors noted
a possible early disease potential with a low level of mtDNA-CN in
gastric cancer. Furthermore, an analysis of D-loop demethylation in
association with mtDNA-CN was conducted in 76 gastric cancer and
the respective non-cancerous stomach tissues (137). The results revealed markedly
decreased mtDNA-CN in cancer tissues, specifically in advanced
stages, suggesting this mtDNA-CN decrease as a late molecular event
during gastric cancer development. However, mtDNA-CN was shown to
be increased in gastric cells following demethylation treatment.
Thus, it was inferred that demethylation in the D-loop region may
act as one of the factors that affect the relative mtDNA content
level in gastric cancer (137).
Conversely, Lee et al observed increased mtDNA
content in 64.2% of gastric cancer tissues compared with
non-tumorous tissues (67).
Another previous study also demonstrated increased mtDNA content in
gastric cancer compared with the control group and suggested that
mtDNA content and relative telomere length were the independent
factors for predicting the risk of gastric cancer (16). Additionally, Jung et al analyzed
the correlation between telomere length and mtDNA-CN. The authors
determined that telomere length was positively associated with
mtDNA-CN in gastric cancer tissues and corresponding non-tumorous
tissues (98).
Prostate cancer
A higher proportion of mtDNA-CNs in prostate cancer
has been reported in some previous studies. Based on research
conducted by Mizumachi et al, an increased mtDNA-CN (78%) was found
in prostate cancer tissue compared with adjacent normal tissues
(138). Similarly, a study by
Zhou et al revealed that patients who suffered from prostate cancer
exhibited a significantly increased mtDNA-CN compared with healthy
subjects, which was associated with a higher risk of prostate
cancer and advanced tumor stage (139). In 2017, Abhishek et al analyzed
the relationship between cadmium, zinc, and mtDNA-CN with the
clinopathological characteristics of patients with prostate cancer
(140). In this study, it was
revealed that higher mtDNA-CN, as well as zinc and cadmium
compounds were correlated with Gleason scores in prostate cancer
cases compared with normal controls (140). In another study, the authors
observed that a high mtDNA-CN was associated with and increased
risk of non-aggressive prostate cancer (141).
A decrease in mtDNA-CN was identified in the
peripheral blood of patients with prostate cancer (142). The study demonstrated that low
mtDNA-CN was correlated with aggressive prostate cancer, which
indicated poor progression-free survival among the patients
(142). In addition, a previous
study performed by Kalsbeek et al demonstrated a significantly
decreased mtDNA-CN in prostate tumor tissue compared with
non-tumorous tissue (143).
Recently, Xu et al revealed that patients with prostate cancer who
exhibited reduced mtDNA-CN were associated with a lower risk of
biochemical recurrence compared with those who harbored increased
mtDNA-CN (144).
Esophageal cancer
The first report of mtDNA-CN in esophageal cancer
was described by Tan et al (145). In this study, high and low
mtDNA-CNs were found in esophageal tumor tissue, and no correlation
was established between mtDNA-CN and mutations (145). The following year, a study
conducted by Liu et al examined mtDNA content in 42 tissue samples
and discovered an increased mtDNA-CN in esophageal cancer tissues
(146). In 2010, Lin et al
observed a gradual increase of mtDNA-CN among cigarette smokers and
wine drinkers in patients with esophageal squamous cell carcinoma
(147). In a previous study, Li
et al observed an elevated mtDNA-CN in patients with esophageal
squamous cell carcinoma compared with control subjects, and this
increase in mtDNA-CN was significantly associated with
cancer-associated mortality risk (148).
Relatively few studies, by contrast, have revealed
a low mtDNA-CN in esophageal tumor cases compared with controls. In
a previous study, Masuike et al identified a reduced mtDNA-CN
(56.0%) in resected tumorous samples compared with non-tumorous
specimens (149). Decreased
mtDNA-CN was shown to be correlated with the depth of tumor
invasion and tumor stage (149). In this study, it was also
revealed that patients with reduced mtDNA-CNs had a significantly
poor 5-year overall survival compared with patients with increased
mtDNA-CNs. Moreover, a study on mtDNA-CNs in esophageal
adenocarcinoma was carried out using peripheral blood leukocytes of
218 esophageal adenocarcinoma cases and the corresponding control
samples (150). The results
demonstrated a significantly decreased mtDNA-CN in tumor cases
compared with the controls, this decrease in mtDNA-CN was markedly
associated with a high risk of esophageal adenocarcinoma (150).
Head and neck cancer
mtDNA-CN alterations in head and neck cancer was
revealed in a study by Kim et al (151). In this study an evident
increase in mtDNA-CN was demonstrated from normal tissue to
malignant head and neck tumors. The study also identified that a
markedly elevated mtDNA-CN was associated with tumor grade. By
contrast, another study revealed a decreased mtDNA-CN in
postoperative salivary rinse samples of head and neck cancer
(152). Reduced mtDNA-CN was
associated with never-smoker status and in response to
post-treatment radiation therapy after the first surgery (152).
A case-control study of head and neck cancer of
Taiwanese patients showed a two-fold increase of mtDNA-CN, compared
with the control cohort (153).
It was also identified that patients with increased mtDNA-CN and
advanced cancer stage were associated with a higher mortality rate
(153). Similar outcomes were
also reported by Kumar et al, who analyzed the association of
mtDNA-CNs with smoke and smokeless tobacco, betel quid chewing, and
alcohol-using cell-free mtDNA samples (154). The findings revealed the
increased mtDNA-CN in patients with head and neck squamous cell
carcinoma with these observed habits compared with the healthy
control cohort. A different study that involved 570 head and neck
squamous cell carcinoma cases and 597 controls among the Chinese
population revealed an increase of mtDNA-CN in patients with head
and neck squamous cell carcinoma compared with the control group
(95). Moreover, the authors
also found a U-shaped association of mtDNA-CN and the risk of
cancer, which suggested the importance of mtDNA-CN in the
progression of head and neck cancer.
Lung cancer
A number of studies have investigated the
association between mtDNA-CN and lung cancer progression. In a
previous study, Lin et al found decreased mtDNA-CN and low levels
of oxidative mtDNA damage in lung tumor tissues after chemotherapy
(65). Moreover, another study
revealed that lower mtDNA-CN was associated with the late stage of
lung cancer, which harbored a poorer prognosis (155). In a separate study,
mitochondrial MSI (mtMSI) and mtDNA-CN were examined in 37 lung
carcinoma tissues and paired with non-cancerous tissue specimens
(13). This study demonstrated
that the mtDNA-CN in tumor tissue was lower than that in
non-cancerous tissue, and the mtDNA-CN in tumor tissue harboring
mtMSI was significantly reduced compared with the other lung
carcinoma specimens (13).
In 2009, Bonner et al determined that mtDNA-CN was
associated with a higher risk of lung cancer among older patients
(156). Furthermore, Hosgood et
al revealed that mtDNA-CN was markedly associated with age and
observed a dose-dependent association between mtDNA-CN and
increased lung cancer risk in heavy smokers (157). In a pooled analysis of three
study populations, there was no association between mtDNA-CN and
the risk of lung cancer (158).
Renal cell cancer
In 2004, Meierhofer et al analyzed mtDNA-CN changes
in 37 patients with renal cell cancer using Southern blot analysis.
The results of this study revealed a decreased mtDNA-CN in 92% of
renal carcinoma tissues compared with the non-cancerous tissues
(159). Previous studies also
showed a reduced mtDNA-CN in resected tissues of renal cell cancer
compared with normal tissues. These studies identified a
significant association between mtDNA-CN and the high risk of renal
cell cancer (160,161). Moreover, Xing et al determined
a high heritability of mtDNA content (65%) in this cancer,
suggesting a vital role of mtDNA-CN in renal cell cancer
development (160). A study by
Lin et al revealed that lower mtDNA-CN with the addition of reduced
mitochondrial enzyme activity may be involved in a drug-resistance
phenotype and the progression of renal cell cancer (162). However, mtDNA-CN was revealed
to be higher in peripheral blood samples of renal cell carcinoma
patients (163,164). These studies also revealed a
significant association between the increased levels of mtDNA-CN
and the increased risk of renal cell cancer (163,164).
Endometrial cancer
In a study by Wang et al mtDNA-CNs were examined in
65 endometrial cancer and 41 non-cancer cases. In this study, a
significant increase in mtDNA-CN in patients with endometrial
cancer compared with the non-cancer subjects, was reported. In
addition, it was discovered that patients who exhibited mtMSI at
nucleotide position 303 carried a markedly higher level of mtDNA-CN
(165). In addition, a previous
study revealed increased mtDNA-CN and citrate synthase activity in
endometrial cancer cases compared with those in the healthy group
(166).
By contrast, a cancer-based study which included
139 peripheral blood samples of patients with endometrial cancer
and 139 paired controls conducted in 2016 discovered decreasing
numbers of copies of mtDNA in the endometrial cancer cases. The
authors claimed that reduced mtDNA-CN had significant combined
effects with smoking status, obesity, hypertension, and diabetes
history in the increased risk of endometrial cancer (167).
Oral cancer
Studies of mtDNA-CNs in patients with oral cancer
have also been performed. In a study by Mondal et al, mtDNA-CN was
examined in 124 patients with oral cancer and 140 subjects as
controls (168). In this study,
mtDNA-CN was significant in tobacco-betel quid chewers than
tobacco-betel quid non chewers, while reduced mtDNA-CN was markedly
associated with higher tumor stage (168).
Conversely, a study in 2014 revealed that
individuals with elevated mtDNA-CNs were markedly associated with
an increased risk of oral cancer compared with individuals with a
decreased mtDNA-CN (169).
Furthermore, another previous study that involved real-time PCR and
immunohistochemistry revealed lower PGC-1α/TFAM expression and
mtDNA-CN in oral tumorous tissues compared with the corresponding
non-tumorous tissues. The authors suggest that the reduced
mitochondrial PGC-1α/TFAM pathway is involved in oral cancer
(170).
Pancreatic cancer
According to a study by Lynch et al, elevated
mtDNA-CN was found in patients with pancreatic cancer compared with
the controls (171). The
findings also revealed a significant association between increased
mtDNA-CN and the increased risk of pancreatic cancer (171). By contrast, another study
performed by Tuchalska-Czuroń et al demonstrated a markedly lower
mtDNA-CN in tumor tissues compared with corresponding normal
pancreatic tissues (172).
However, the study failed to identify a significant difference in
the overall survival of the patients, which indicated that mtDNA-CN
was not a prognostic indicator of pancreatic cancer (172). This result is in line with a
more recent study which revealed lower mtDNA-CN associated with
older age and high body mass index among EPIC patients (173).
Ovarian cancer
There are relatively few studies available on the
assessment of mtDNA-CN in human ovarian cancer. The earliest report
of mtDNA-CN in ovarian cancer was described by Wang et al in 2006
(174). In this study, an
increased mtDNA-CN was observed in tumorous tissues of ovarian
cancer compared with non-tumorous tissues. It was also observed
that the early stages of cancer harbored markedly higher mtDNA
copies compared with the late stages (174). In a relatively recent study, a
group of researchers performed mtDNA-CN assessment in blood and
plasma samples from 24 ovarian cancer patients and matched samples
(175). The results revealed
higher mtDNA copies in patients with advanced cancer stages than in
healthy subjects. In addition, the authors detected elevated levels
of mtDNA-CN in exosomes as well as in plasma, and peripheral blood
of patients with advanced-stage ovarian cancer, indicating that
mtDNA-CN varies depending on the needs of cell types and
physiological conditions (175).
Other cancers
Studies of the mtDNA-CN alterations have also been
documented previously in several other types of cancers. In a study
by Hyland et al the relationship between mtDNA-CN and the risk of
melanoma cancer in blood samples was investigated using
quantitative PCR. In this study, it was observed that increased
mtDNA-CN in melanoma cases were associated with CDKN2A mutations
(176). This data is consistent
with another study that demonstrated a higher proportion of mtDNA
copies in melanoma cases compared with controls, and that a high
number of mtDNA copies was significantly correlated with a higher
risk melanoma (177).
Furthermore, elevated mtDNA-CN has been reported in
laryngeal cancer cases (178,179). Guo et al determined mtDNA-CN in
40 resected tissue specimens of laryngeal cancer and matched blood
controls. The results showed increased mtDNA copies in tumor
tissues compared with the peripheral blood controls. Additionally,
patients that exhibited D-loop mutations carried a higher mtDNA-CN,
indicating significant effects of unstable mtDNA D-loop region with
mutational loads and polymorphisms in larynx tumorigenesis
(179). A separate study also
revealed a greater mtDNA-CN in paraffin-embedded tissues of
laryngeal cancer than in non-tumorous tissues (178). In addition, it was also
revealed that reduced mtDNA copies were markedly correlated with
smoking status, tumor invasion, and tumor stage (178).
Future approaches in determining mtDNA-CN
changes
mtDNA-CN has been proposed as a reliable biomarker
in predicting cancer prognosis. It is worth mentioning that control
groups, solid tumors, and other diseases can be distinguished by
the level of mtDNA-CN (100).
To date, there are still no standardized methods used for the
relative assessment of mtDNA-CNs in clinical settings.
Certainly, various quantification methods or
pre-analytical and analytical factors can influence final
estimation and produce varying data of mtDNA content among
laboratories (180). In this
review, the variations of mtDNA-CN were presented in tumorous
tissues compared with non-tumorous tissue specimens, or in body
fluids, including circulating blood cells, saliva, and cell-free
serum. Since tissues and organs are not easily accessible, body
fluids have been frequently used in numerous studies as indicators
in determining mtDNA content.
Notably, various DNA extraction methods produce
conflicting results, which lead to incorrect copy number
assessment. Extraction methods can influence the yield of total
mtDNA, and the failure to dilute genomic DNA appropriately due to
the varying genome sizes of the nuclear and mitochondrial genome
may affect the mtDNA-CN values (181). Therefore, new methods are
essential for measuring mtDNA integrity, since with the available
methods there is difficulty in differentiating between intact mtDNA
and damaged mtDNA fragments. Currently, mtDNA-CN can be readily
measured from cell lysates, which have less downstream utility and
are advantageous for small samples, except that they are restricted
only to cultured cells (102).
However, the first quantification of mtDNA-CN based on dried blot
spot samples has been developed, and has shown promising results
compared to conventional samples (182). The study demonstrated that the
average mtDNA-CN was markedly higher in dried blot spots than in
the whole blood specimens collected 5 to 10 years apart. A dried
blood spot sample may be a viable alternative as it is a more
stable, less costly, and less invasive method. It is easy to
transport and available for long-term storage at room temperature
(182). With all the features
mentioned, a dried blood spot sample is optimal for large-scale
studies of multiple research fields. However, future additional
research is still warranted in assessing mtDNA-CNs with the usage
of dried blood spot specimens.
Furthermore, the qPCR-based method serves as the
current gold standard in mtDNA-CN quantification and has been
extensively used in published findings. Generally, the use of both
targeted mtDNA and nDNA genes and external standards may vary among
laboratories (100). This may
lead to diverse data outcomes, and a challenge in standardizing
methods for clinical setup. To date, there is no gold standard for
reference genes as they are usually selected based on sample use
and diseases, with numerous analyses mostly using the repetitive
and altered regions (100).
Notably, the nuclear genome can duplicate >97% of the mtDNA
genome, giving rise to pseudogenes which are nuclear insertions of
mitochondrial origin (183).
The mtDNA genes used in the mtDNA-CN measurement may be capable of
co-amplifying the nuclear pseudogenes (181). In addition, mtDNA genome
duplication is inconsistent among individuals and diseases, hence,
it is difficult to recognize the regions in the mtDNA genome which
were not duplicated in the nuclear genome (100).
Presently, next-gene sequencing methods, including
WES and WGS quantification, provide great effectiveness and
comprehensive assessment of mtDNA changes. These methods may
provide better results of mtDNA-CN than the gold standard qPCR
method as they could detect changes in various samples, including
paraffin-embedded tissues, formalin-fixed specimens, and body
fluids (103). A recent study
optimized the detection of circulating cell-free mtDNA in patients
with cancer using a low-depth WGS approach, which revealed a
markedly decreased mtDNA-CN in plasma DNA samples compared with
fresh tumor tissues (184).
Plasma samples contain a very low abundance of mtDNA, and
therefore, the usage of qPCR is not applicable for mtDNA-CN
estimation in plasma samples due to its low quality (184). Thus, the advent of a
capture-based NGS method such as WGS is advantageous for mtDNA
content detection using challenging samples. As suggested by a
previous study, the NGS approach is anticipated to become a gold
standard in measuring mtDNA-CNs in the future (101).
Nonetheless, standardization of all parameters
involved in assessing mtDNA-CN such as sample preservation,
isolation methods, PCR inhibitors, and quantification methods is
significant to avoid bias and false assessment. While these
variables are normalized, reference values are crucial for each
measurement process, and research findings need to be compared
carefully (180). Thus, a
thorough appraisal of various approaches and factors involved in
mtDNA-CN assessment has yet to be completed (102).
Conclusion
In conclusion, emerging evidence suggests that
variations in mtDNA-CNs can contribute to the initiation and
progression of tumorigenesis. Despite the comprehensive findings of
mtDNA-CN alterations in various human cancers, there is still
limited understanding of the mechanisms of how cells modulate
mtDNA-CN. Although the relevant mechanism has yet to be clarified,
it is undeniable that mtDNA-CN is a very promising potential target
in cancer research. Therefore, more efforts are required to study
the undetermined mechanism of mtDNA-CN alterations in cancer
development.
Acknowledgements
Not applicable.
Funding
This present review was supported by the Ministry of Higher
Education Malaysia under the Fundamental Research Grant Scheme
(FRGS) with Project Code: FRGS/1/2021/SKK0/USM/02/2
(203.PPSP.6171310).
Availability of data and materials
Not applicable.
Authors' contributions
SMAR performed the literature search and wrote the
manuscript. AAMY contributed to conceptualization, designing,
drafting, and editing of the manuscript. SZNMK and SMAR designed
the figure and prepared the table. AAMY, AP, FA, ZI critically
reviewed and revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon JJ, Lu A and Moon C: Role of genomic
instability in human carcinogenesis. Exp Biol Med (Maywood).
244:227–240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pikor L, Thu K, Vucic E and Lam W: The
detection and implication of genome instability in cancer. Cancer
Metastasis Rev. 32:341–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yusoff AAM, Abdullah WSW, Khair SZNM and
Radzak SMA: A comprehensive overview of mitochondrial DNA 4977-bp
deletion in cancer studies. Oncol Rev. 13:4092019.PubMed/NCBI
|
|
5
|
Otto AM: Warburg effect(s)-a biographical
sketch of Otto Warburg and his impacts on tumor metabolism. Cancer
Metab. 4:52016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Modica-Napolitano JS, Kulawiec M and Singh
K: Mitochondria and human cancer. Curr Mol Med. 7:121–131. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen EI: Mitochondrial dysfunction and
cancer metastasis. J Bioenerg Biomembr. 44:619–622. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedersen PL: Bioenergetics of cancer
cells-a brief orientation to this minireview series. J Bioenerg
Biomembr. 29:301–302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rempel A, Mathupala SP, Griffin CA,
Hawkins AL and Pedersen PL: Glucose catabolism in cancer cells:
Amplification of the gene encoding type II hexokinase. Cancer Res.
56:2468–2471. 1996.PubMed/NCBI
|
|
10
|
Wu M, Neilson A, Swift AL, Moran R,
Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J,
et al: Multiparameter metabolic analysis reveals a close link
between attenuated mitochondrial bioenergetic function and enhanced
glycolysis dependency in human tumor cells. Am J Physiol Cell
Physiol. 292:125–C136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Radzak S, Khair Z, Ahmad F, Idris Z and
Yusoff A: Accumulation of mitochondrial DNA microsatellite
instability in Malaysian patients with primary central nervous
system tumors. Turk Neurosurg. 31:99–106. 2021.PubMed/NCBI
|
|
12
|
Campa D, Barrdahl M, Santoro A, Severi G,
Baglietto L, Omichessan H, Tumino R, Bueno-de-Mesquita HBA, Peeters
PH, Weiderpass E, et al: Mitochondrial DNA copy number variation,
leukocyte telomere length, and breast cancer risk in the European
prospective investigation into cancer and nutrition (EPIC) study.
Breast Cancer Res. 20:292018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai JG, Zhang ZY, Liu QX and Min JX:
Mitochondrial genome microsatellite instability and copy number
alteration in lung carcinomas. Asian Pacific J Cancer Prev.
14:2393–2399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar B, Bhat ZI, Bansal S, Saini S,
Naseem A, Wahabi K, Burman A, Kumar GT, Saluja SS and Rizvi MMA:
Association of mitochondrial copy number variation and T16189C
polymorphism with colorectal cancer in North Indian population.
Tumour Biol. 39:10104283177402962017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao RZ, Jiang S, Zhang L and Yu ZB:
Mitochondrial electron transport chain, ROS generation and
uncoupling (Review). Int J Mol Med. 44:3–15. 2019.PubMed/NCBI
|
|
16
|
Zhu X, Mao Y, Huang T, Yan C, Yu F, Du J,
Dai J, Ma H and Jin G: High mitochondrial DNA copy number was
associated with an increased gastric cancer risk in a Chinese
population. Mol Carcinog. 56:2593–2600. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilkerson R, Bravo L, Garcia I, Gaytan N,
Herrera A, Maldonado A and Quintanilla B: The mitochondrial
nucleoid: Integrating mitochondrial DNA into cellular homeostasis.
Cold Spring Harb Perspect Biol. 5:a0110802013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Facucho-Oliveira JM and St John JC: The
relationship between pluripotency and mitochondrial DNA
proliferation during early embryo development and embryonic stem
cell differentiation. Stem Cell Rev Rep. 5:140–158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montoya J, Christianson T, Levens D,
Rabinowitz M and Attardi G: Identification of initiation sites for
heavy-strand and light-strand transcription in human mitochondrial
DNA. Proc Natl Acad Sci USA. 79:7195–7199. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clayton DA: Transcription and replication
of mitochondrial DNA. Hum Reprod. 15 (Suppl 2):S11–S17. 2000.
View Article : Google Scholar
|
|
21
|
Turnbull HE, Lax NZ, Diodato D, Ansorge O
and Turnbull DM: The mitochondrial brain: From mitochondrial genome
to neurodegeneration. Biochim Biophys Acta. 1802:111–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chabi B, Mousson De Camaret B, Duborjal H,
Issartel JP and Stepien G: Quantification of mitochondrial DNA
deletion, depletion, and overreplication: Application to diagnosis.
Clin Chem. 49:1309–1317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castellani CA, Longchamps RJ, Sun J,
Guallar E and Arking DE: Thinking outside the nucleus:
Mitochondrial DNA copy number in health and disease. Mitochondrion.
53:214–223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Erchia AM, Atlante A, Gadaleta G, Pavesi
G, Chiara M, De Virgilio C, Manzari C, Mastropasqua F, Prazzoli GM,
Picardi E, et al: Tissue-specific mtDNA abundance from exome data
and its correlation with mitochondrial transcription, mass and
respiratory activity. Mitochondrion. 20:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Prosser R, Simonetti S, Sadlock J,
Jagiello G and Schon EA: Rearranged mitochondrial genomes are
present in human oocytes. Am J Hum Genet. 57:239–247.
1995.PubMed/NCBI
|
|
26
|
Yusoff AA, Ahmad F, Idris Z, Jaafar H and
Abdullah JM: Understanding mitochondrial DNA in brain
tumorigenesis. Mol Considerations Evol Surg Manag Issues Treat
Patients with a Brain Tumor. 3–28. 2015.
|
|
27
|
Fontana GA and Gahlon HL: Mechanisms of
replication and repair in mitochondrial DNA deletion formation.
Nucleic Acids Res. 48:11244–11258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robberson DL, Kasamatsu H and Vinograd J:
Replication of mitochondrial DNA. Circular replicative
intermediates in mouse L cells. Proc Natl Acad Sci USA. 69:737–741.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holt IJ, Lorimer HE and Jacobs HT: Coupled
leading-and lagging-strand synthesis of mammalian mitochondrial
DNA. Cell. 100:515–524. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yasukawa T, Reyes A, Cluett TJ, Yang MY,
Bowmaker M, Jacobs HT and Holt IJ: Replication of vertebrate
mitochondrial DNA entails transient ribonucleotide incorporation
throughout the lagging strand. EMBO J. 25:5358–5371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clay Montier LL, Deng JJ and Bai Y: Number
matters: Control of mammalian mitochondrial DNA copy number. J
Genet Genomics. 36:125–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Falkenberg M: Mitochondrial DNA
replication in mammalian cells: Overview of the pathway. Essays
Biochem. 62:287–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larsson NG, Wang J, Wilhelmsson H, Oldfors
A, Rustin P, Lewandoski M, Barsh GS and Clayton DA: Mitochondrial
transcription factor A is necessary for mtDNA maintenance and
embryogenesis in mice. Nat Genet. 18:231–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanki T, Ohgaki K, Gaspari M, Gustafsson
CM, Fukuoh A, Sasaki N, Hamasaki N and Kang D: Architectural role
of mitochondrial transcription factor A in maintenance of human
mitochondrial DNA. Mol Cell Biol. 24:9823–9834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ekstrand MI, Falkenberg M, Rantanen A,
Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM and Larsson
NG: Mitochondrial transcription factor A regulates mtDNA copy
number in mammals. Hum Mol Genet. 13:935–944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farge G, Mehmedovic M, Baclayon M, van den
Wildenberg SM, Roos WH, Gustafsson CM, Wuite GJ and Falkenberg M:
In vitro-reconstituted nucleoids can block mitochondrial DNA
replication and transcription. Cell Rep. 8:66–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korhonen JA, Pham XH, Pellegrini M and
Falkenberg M: Reconstitution of a minimal mtDNA replisome in vitro.
EMBO J. 23:2423–2429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peter B and Falkenberg M: Twinkle and
other human mitochondrial DNA helicases: Structure, function and
disease. Genes (Basel). 11:4082020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Korhonen JA, Gaspari M and Falkenberg M:
Twinkle has 5′ ->3′ DNA helicase activity and is specifically
stimulated by mitochondrial single-stranded DNA-binding protein. J
Biol Chem. 278:48627–48632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Milenkovic D, Matic S, kühl I, Ruzzenente
B, Freyer C, Jemt E, Park CB, Falkenberg M and Larsson NG: Twinkle
is an essential mitochondrial helicase required for synthesis of
nascent D-loop strands and complete mtDNA replication. Hum Mol
Genet. 22:1983–1993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takamatsu C, Umeda S, Ohsato T, Ohno T,
Abe Y, Fukuoh A, Shinagawa H, Hamasaki N and Kang D: Regulation of
mitochondrial D-loops by transcription factor A and single-stranded
DNA-binding protein. EMBO Rep. 3:451–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miralles Fusté J, Shi Y, Wanrooij S, Zhu
X, Jemt E, Persson Ö, Sabouri N, Gustafsson CM and Falkenberg M: In
vivo occupancy of mitochondrial single-stranded DNA binding protein
supports the strand displacement mode of DNA replication. PLoS
Genet. 10:e10048322014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Datta A and Brosh RM Jr: New insights into
DNA helicases as druggable targets for cancer therapy. Front Mol
Biosci. 5:592018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruhanen H, Borrie S, Szabadkai G,
Tyynismaa H, Jones AW, Kang D, Taanman JW and Yasukawa T:
Mitochondrial single-stranded DNA binding protein is required for
maintenance of mitochondrial DNA and 7S DNA but is not required for
mitochondrial nucleoid organisation. Biochim Biophys Acta.
1803:931–939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grzybowska-Szatkowska L and Slaska B:
Mitochondrial DNA and carcinogenesis (review). Mol Med Rep.
6:923–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reddy TV, Govatati S, Deenadayal M,
Sisinthy S and Bhanoori M: Impact of mitochondrial DNA copy number
and displacement loop alterations on polycystic ovary syndrome risk
in south Indian women. Mitochondrion. 44:35–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li HL, Slone J, Fei L and Huang T:
Mitochondrial DNA variants and common diseases: A mathematical
model for the diversity of age-related mtDNA mutations. Cells.
8:6082019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicholls TJ and Minczuk M: In D-loop: 40
years of mitochondrial 7S DNA. Exp Gerontol. 56:175–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee HC, Li SH, Lin JC, Wu CC, Yeh DC and
Wei YH: Somatic mutations in the D-loop and decrease in the copy
number of mitochondrial DNA in human hepatocellular carcinoma.
Mutat Res. 547:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu M, Wan Y and Zou Q: Decreased copy
number of mitochondrial DNA in Ewing's sarcoma. Clin Chim Acta.
411:679–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu M, Zhou Y, Shi Y, Ning L, Yang Y, Wei X
and Zhang N: Reduced mitochondrial DNA copy number is correlated
with tumor progression and prognosis in Chinese breast cancer
patients. IUBMB Life. 59:450–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bai R, Chang J, Yeh K, Lou MA, Lu JF, Tan
DJ, Liu H and Wong LJ: Mitochondrial DNA content varies with
pathological characteristics of breast cancer. J Oncol.
2011:4961892011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yusoff AAM, Radzak SMA and Khair SZNM:
Research highlights on contributions of mitochondrial DNA
microsatellite instability in solid cancers - an overview. Contemp
Oncol (Pozn). 26:8–26. 2022.PubMed/NCBI
|
|
54
|
Kazak L, Reyes A and Holt IJ: Minimizing
the damage : Repair pathways keep mitochondrial DNA intact. Nat Rev
Mol Cell Biol. 13:659–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang D, Mott JL, Chang SW, Denniger G,
Feng Z and Zassenhaus HP: Construction of transgenic mice with
tissue-specific acceleration of mitochondrial DNA mutagenesis.
Genomics. 69:151–161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trifunovic A, Wredenberg A, Falkenberg M,
Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors
A, Wibom R, et al: Premature ageing in mice expressing defective
mitochondrial DNA polymerase. Nature. 429:417–423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Spelbrink JN, Toivonen JM, Hakkaart GAJ,
Kurkela JM, Cooper HM, Lehtinen SK, Lecrenier N, Back JW, Speijer
D, Foury F and Jacobs HT: In vivo functional analysis of the human
mitochondrial DNA polymerase POLG expressed in cultured human
cells. J Biol Chem. 275:24818–24828. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Singh KK, Ayyasamy V, Owens KM, Koul MS
and Vujcic M: Mutations in mitochondrial DNA polymerase-gamma
promote breast tumorigenesis. J Hum Genet. 54:516–524. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Linkowska K, Jawien A, Marszałek A,
Skonieczna K and Grzybowski T: Searching for association of the CAG
repeat polymorphism in the mitochondrial DNA polymerase gamma gene
(POLG) with colorectal cancer. Acta Biochim Pol. 62:625–627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Long X, Wang X, Chen Y, Guo X, Zhou F, Fan
Y, Ge N, Guo M, Zhang Z and Dong G: Polymorphisms in POLG were
associated with the prognosis and mtDNA content in hepatocellular
carcinoma patients. Bull Cancer. 104:500–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Spelbrink JN, Li FY, Tiranti V, Nikali K,
Yuan QP, Tariq M, Wanrooij S, Garrido N, Comi G, Morandi L, et al:
Human mitochondrial DNA deletions associated with mutations in the
gene encoding Twinkle, a phage T7 gene 4-like protein localized in
mitochondria. Nat Genet. 28:223–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tyynismaa H, Sembongi H, Bokori-Brown M,
Granycome C, Ashley N, Poulton J, Jalanko A, Spelbrink JN, Holt IJ
and Suomalainen A: Twinkle helicase is essential for mtDNA
maintenance and regulates mtDNA copy number. Hum Mol Genet.
13:3219–3227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sanchez-Martinez A, Calleja M, Peralta S,
Matsushima Y, Hernandez-Sierra R, Whitworth AJ, Kaguni LS and
Garesse R: Modeling pathogenic mutations of human twinkle in
Drosophila suggests an apoptosis role in response to mitochondrial
defects. PLoS One. 7:e439542012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Matsushima Y and Kaguni LS: Differential
phenotypes of active site and human autosomal dominant progressive
external ophthalmoplegia mutations in Drosophila mitochondrial DNA
helicase expressed in Schneider cells. J Biol Chem. 282:9436–9444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin CS, Wang LS, Tsai CM and Wei YH: Low
copy number and low oxidative damage of mitochondrial DNA are
associated with tumor progression in lung cancer tissues after
neoadjuvant chemotherapy. Interact Cardiovasc Thorac Surg.
7:954–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hu L, Yao X and Shen Y: Altered
mitochondrial DNA copy number contributes to human cancer risk:
Evidence from an updated meta-analysis. Sci Rep. 6:358592016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lee H, Lee JH, Kim DC, Hwang I, Kang YN,
Gwon GJ, Choi IJ and Kim S: Is mitochondrial DNA copy number
associated with clinical characteristics and prognosis in gastric
cancer? Asian Pacific J Cancer Prev. 16:87–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Han Y and Chen JZ: Oxidative stress
induces mitochondrial DNA damage and cytotoxicity through
independent mechanisms in human cancer cells. Biomed Res Int.
2013:8250652013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK,
Ma YS and Wei YH: Oxidative stress-related alteration of the copy
number of mitochondrial DNA in human leukocytes. Free Radic Res.
37:1307–1317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Al-Kafaji G and Golbahar J: High
glucose-induced oxidative stress increases the copy number of
mitochondrial DNA in human mesangial cells. Biomed Res Int.
2013:7549462013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guha M and Avadhani NG: Mitochondrial
retrograde signaling at the crossroads of tumor bioenergetics,
genetics and epigenetics. Mitochondrion. 13:577–591. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Corral M, Paris B, Baffet G, Tichonicky L,
Guguen-Guillouzo C, Kruh J and Defer N: Increased level of the
mitochondrial ND5 transcript in chemically induced rat hepatomas.
Exp Cell Res. 184:158–166. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Delsite RL, Rasmussen LJ, Rasmussen AK,
Kalen A, Goswami PC and Singh KK: Mitochondrial impairment is
accompanied by impaired oxidative DNA repair in the nucleus.
Mutagenesis. 18:497–503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Suzuki S, Naito A, Asano T, Evans TT,
Reddy SAG and Higuchi M: Constitutive activation of AKT pathway
inhibits TNF-induced apoptosis in mitochondrial DNA-deficient human
myelogenous leukemia ML-1a. Cancer Lett. 268:31–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Biswas G, Anandatheerthavarada HK, Zaidi M
and Avadhani NG: Mitochondria to nucleus stress signaling: A
distinctive mechanism of NFkappaB/Rel activation through
calcineurin-mediated inactivation of IkappaBbeta. J Cell Biol.
161:507–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lu T and Stark GR: Cytokine overexpression
and constitutive NFkappaB in cancer. Cell Cycle. 3:1114–1117. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mei H, Sun S, Bai Y, Chen Y, Chai R and Li
H: Reduced mtDNA copy number increases the sensitivity of tumor
cells to chemotherapeutic drugs. Cell Death Dis. 6:e17102015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun X, Zhan L, Chen Y, Wang G, He L, Wang
Q, Zhou F, Yang F, Wu J, Wu Y, et al: Increased mtDNA copy number
promotes cancer progression by enhancing mitochondrial oxidative
phosphorylation in microsatellite-stable colorectal cancer. Signal
Transduct Target Ther. 3:82018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Moraes CT: What regulates mitochondrial
DNA copy number in animal cells? Trends Genet. 17:199–205. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tang Y, Schon EA, Wilichowski E,
Vazquez-Memije ME, Davidson E and King MP: Rearrangements of human
mitochondrial DNA (mtDNA): New insights into the regulation of
mtDNA copy number and gene expression. Mol Biol Cell. 11:1471–1485.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tang Y, Manfredi G, Hirano M and Schon EA:
Maintenance of human rearranged mitochondrial DNAs in long-term
cultured transmitochondrial cell lines. Mol Biol Cell.
11:2349–2358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kelly RD, Mahmud A, McKenzie M, Trounce IA
and St John JC: Mitochondrial DNA copy number is regulated in a
tissue specific manner by DNA methylation of the nuclear-encoded
DNA polymerase gamma A. Nucleic Acids Res. 40:10124–10138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lee W, Johnson J, Gough DJ, Donoghue J,
Cagnone GL, Vaghjiani V, Brown KA, Johns TG and St John JC:
Mitochondrial DNA copy number is regulated by DNA methylation and
demethylation of POLGA in stem and cancer cells and their
differentiated progeny. Cell Death Dis. 6:e16642015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dickinson A, Yeung KY, Donoghue J, Baker
MJ, Kelly RD, McKenzie M, Johns TG and St John JC: The regulation
of mitochondrial DNA copy number in glioblastoma cells. Cell Death
Differ. 20:1644–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun X and St John JC: Modulation of
mitochondrial DNA copy number in a model of glioblastoma induces
changes to DNA methylation and gene expression of the nuclear
genome in tumours. Epigenetics Chromatin. 11:532018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tan AS, Baty JW, Dong LF, Bezawork-Geleta
A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B,
et al: Mitochondrial genome acquisition restores respiratory
function and tumorigenic potential of cancer cells without
mitochondrial DNA. Cell Metab. 21:81–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Berridge MV, Crasso C and Neuzil J:
Mitochondrial genome transfer to tumor cells breaks the rules and
establishes a new precedent in cancer biology. Mol Cell Oncol.
5:e10239292018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee HC and Wei YH: Mitochondrial
biogenesis and mitochondrial DNA maintenance of mammalian cells
under oxidative stress. Int J Biochem Cell Biol. 37:822–834. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yeung KY, Dickinson A, Donoghue JF,
Polekhina G, White SJ, Grammatopoulos DK, McKenzie M, Johns TG and
St John JC: The identification of mitochondrial DNA variants in
glioblastoma multiforme. Acta Neuropathol Commun. 2:12014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yuan Y, Ju YS, Kim Y, Li J, Wang Y, Yoon
CJ, Yang Y, Martincorena I, Creighton CJ, Weinstein JN, et al:
Comprehensive molecular characterization of mitochondrial genomes
in human cancers. Nat Genet. 52:342–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jiang H, Zhao H, Xu H, Hu L, Wang W, Wei
Y, Wang Y, Peng X and Zhou F: Peripheral blood mitochondrial DNA
content, A10398G polymorphism, and risk of breast cancer in a Han
Chinese population. Cancer Sci. 105:639–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shen J, Platek M, Mahasneh A, Ambrosone CB
and Zhao H: Mitochondrial copy number and risk of breast cancer: A
pilot study. Mitochondrion. 10:62–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fazzini F, Schöpf B, Blatzer M, Coassin S,
Hicks AA, Kronenberg F and Fendt L: Plasmid-normalized
quantification of relative mitochondrial DNA copy number. Sci Rep.
8:153472018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Castellani CA, Longchamps RJ, Sumpter JA,
Newcomb CE, Lane JA, Grove ML, Bressler J, Brody JA, Floyd JS,
Bartz TM, et al: Mitochondrial DNA copy number can influence
mortality and cardiovascular disease via methylation of nuclear DNA
CpGs. Genome Med. 12:842020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang L, Lv H, Ji P, Zhu X, Yuan H, Jin G,
Dai J, Hu Z, Su Y and Ma H: Mitochondrial DNA copy number is
associated with risk of head and neck squamous cell carcinoma in
Chinese population. Cancer Med. 7:2776–2782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shen J, Wan J, Song R and Zhao H:
Peripheral blood mitochondrial DNA copy number, length heteroplasmy
and breast cancer risk: A replication study. Carcinogenesis.
36:1307–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xia P, An HX, Dang CX, Radpour R, Kohler
C, Fokas E, Engenhart-Cabillic R, Holzgreve W and Zhong XY:
Decreased mitochondrial DNA content in blood samples of patients
with stage I breast cancer. BMC Cancer. 9:4542009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jung SJ, Cho JH, Park WJ, Heo YR and Lee
JH: Telomere length is correlated with mitochondrial DNA copy
number in intestinal, but not diffuse, gastric cancer. Oncol Lett.
14:925–929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
O'Hara R, Tedone E, Ludlow A, Huang E,
Arosio B, Mari D and Shay JW: Quantitative mitochondrial DNA copy
number determination using droplet digital PCR with single-cell
resolution. Genome Res. 29:1878–1888. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Memon AA, Zöller B, Hedelius A, Wang X,
Stenman E, Sundquist J and Sundquist K: Quantification of
mitochondrial DNA copy number in suspected cancer patients by a
well optimized ddPCR method. Biomol Detect Quantif. 13:32–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Filograna R, Mennuni M, Alsina D and
Larsson NG: Mitochondrial DNA copy number in human disease: The
more the better? FEBS Lett. 595:976–1002. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Longchamps RJ, Castellani CA, Yang SY,
Newcomb CE, Sumpter JA, Lane J, Grove ML, Guallar E, Pankratz N,
Taylor KD, et al: Evaluation of mitochondrial DNA copy number
estimation techniques. PLoS One. 15:e02281662020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhou K, Mo Q, Guo S, Liu Y, Yin C, Ji X,
Guo X and Xing J: A novel next-generation sequencing-based approach
for concurrent detection of mitochondrial DNA copy number and
mutation. J Mol Diagn. 22:1408–1418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Picard M: Blood mitochondrial DNA copy
number: What are we counting? Mitochondrion. 60:1–11. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ware SA, Desai N, Lopez M, Leach D, Zhang
Y, Giordano L, Nouraie M, Picard M and Kaufman BA: An automated,
high-throughput methodology optimized for quantitative cell-free
mitochondrial and nuclear DNA isolation from plasma. J Biol Chem.
295:15677–15691. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo W, Jiang L, Bhasin S, Khan SM and
Swerdlow RH: DNA extraction procedures meaningfully influence
qPCR-based mtDNA copy number determination. Mitochondrion.
9:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tseng LM, Yin PH, Chi CW, Hsu CY, Wu CW,
Lee LM, Wei YH and Lee HC: Mitochondrial DNA mutations and
mitochondrial DNA depletion in breast cancer. Genes Chromosom
Cancer. 45:629–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fan AX, Radpour R, Haghighi MM, Kohler C,
Xia P, Hahn S, Holzgreve W and Zhong XY: Mitochondrial DNA content
in paired normal and cancerous breast tissue samples from patients
with breast cancer. J Cancer Res Clin Oncol. 135:983–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nacheva E, Mokretar K, Soenmez A, Pittman
AM, Grace C, Valli R, Ejaz A, Vattathil S, Maserati E, Houlden H,
et al: DNA isolation protocol effects on nuclear DNA analysis by
microarrays, droplet digital PCR, and whole genome sequencing, and
on mitochondrial DNA copy number estimation. PLoS One.
12:e01804672017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hsu CW, Yin PH, Lee HC, Chi CW and Tseng
LM: Mitochondrial DNA content as a potential marker to predict
response to anthracycline in breast cancer patients. Breast J.
16:264–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Domínguez-de-la-Cruz E, Muñoz ML,
Pérez-Muñoz A, García-Hernández N, Moctezuma-Meza C and
Hinojosa-Cruz JC: Reduced mitochondrial DNA copy number is
associated with the haplogroup, and some clinical features of
breast cancer in Mexican patients. Gene. 761:1450472020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lemnrau A, Brook MN, Fletcher O, Coulson
P, Tomczyk K, Jones M, Ashworth A, Swerdlow A, Orr N and
Garcia-Closas M: Mitochondrial DNA copy number in peripheral blood
cells and risk of developing breast cancer. Cancer Res.
75:2844–2850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Thyagarajan B, Wang R, Nelson H, Barcelo
H, Koh W and Yuan M: Mitochondrial DNA copy number is associated
with breast cancer risk. PLoS One. 8:e659682013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Feng S, Xiong L, Ji Z, Cheng W and Yang H:
Correlation between increased copy number of mitochondrial DNA and
clinicopathological stage in colorectal cancer. Oncol Lett.
2:899–903. 2011.PubMed/NCBI
|
|
115
|
Chen T, He J, Shen L, Fang H, Nie H, Jin
T, Wei X, Xin Y, Jiang Y, Li H, et al: The mitochondrial DNA
4,977-bp deletion and its implication in copy number alteration in
colorectal cancer. BMC Med Genet. 12:82011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qu F, Liu X, Zhou F, Yang H, Bao G, He X
and Xing J: Association between mitochondrial DNA content in
leukocytes and colorectal cancer risk: A case-control analysis.
Cancer. 117:3148–3155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Thyagarajan B, Wang R, Barcelo H, Koh WP
and Yuan JM: Mitochondrial copy number is associated with
colorectal cancer risk. Cancer Epidemiol Biomarkers Prev.
21:1574–1581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tong H, Zhang L, Gao J, Wen S, Zhou H and
Feng S: Methylation of mitochondrial DNA displacement loop region
regulates mitochondrial copy number in colorectal cancer. Mol Med
Rep. 16:5347–5353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang K, Li X, Forman MR, Monahan PO,
Graham BH, Joshi A, Song M, Hang D, Ogino S, Giovannucci EL, et al:
Pre-diagnostic leukocyte mitochondrial DNA copy number and
colorectal cancer risk. Carcinogenesis. 40:1462–1468. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mohideen AM, Dicks E, Parfrey P, Green R
and Savas S: Mitochondrial DNA polymorphisms, its copy number
change and outcome in colorectal cancer. BMC Res Notes. 8:2722015.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cui HH, Huang P, Wang ZJ, Zhang Y, Zhang
Z, Xu W, Wang X, Han Y and Guo X: Association of decreased
mitochondrial DNA content with the progression of colorectal
cancer. BMC Cancer. 13:1102013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Huang B, Gao YT, Shu XO, Wen W, Yang G, Li
G, Courtney R, Ji BT, Li HL, Purdue MP, et al: Association of
leukocyte mitochondrial DNA copy number with colorectal cancer
risk: Results from the Shanghai women's health study. Cancer
Epidemiol Biomarkers Prev. 23:2357–2365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
van Osch FH, Voets AM, Schouten LJ,
Gottschalk RW, Simons CC, van Engeland M, Lentjes MH, van den
Brandt PA, Smeets HJ and Weijenberg MP: Mitochondrial DNA copy
number in colorectal cancer: Between tissue comparisons,
clinicopathological characteristics and survival. Carcinogenesis.
36:1502–1510. 2015.PubMed/NCBI
|
|
124
|
Marucci G, Maresca A, Caporali L, Farnedi
A, Betts CM, Morandi L, de Biase D, Cerasoli S, Foschini MP, Bonora
E, et al: Oncocytic glioblastoma: A glioblastoma showing oncocytic
changes and increased mitochondrial DNA copy number. Hum Pathol.
44:1867–1876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang J, Li D, Qu F, Chen Y, Li G, Jiang
H, Huang X, Yang H and Xing J: Association of leukocyte
mitochondrial DNA content with glioma risk: Evidence from a Chinese
case-control study. BMC Cancer. 14:6802014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shen J, Song R, Lu Z and Zhao H:
Mitochondrial DNA copy number in whole blood and glioma risk: A
case control study. Mol Carcinog. 55:2089–2094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen Y, Zhang J, Huang X, Zhang J, Zhou X,
Hu J, Li G, He S and Xing J: High leukocyte mitochondrial DNA
content contributes to poor prognosis in glioma patients through
its immunosuppressive effect. Br J Cancer. 113:99–106. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dardaud LM, Bris C, Desquiret-Dumas V,
Boisselier B, Tabouret E, Mokhtari K, Figarella-Branger D, Rousseau
A and Procaccio V: High mitochondrial DNA copy number is associated
with longer survival in young patients with glioblastoma. Neuro
Oncol. 21:1084–1085. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhang Y, Qu Y, Gao K, Yang Q, Shi B, Hou P
and Ji M: High copy number of mitochondrial DNA (mtDNA) predicts
good prognosis in glioma patients. Am J Cancer Res. 5:1207–16.
2015.PubMed/NCBI
|
|
130
|
Oliva CR, Nozell SE, Diers A, McClugage SG
III, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie
GY, Landar A and Griguer CE: Acquisition of temozolomide
chemoresistance in gliomas leads to remodeling of mitochondrial
electron transport chain. J Biol Chem. 285:39759–39767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Shen H, Yu M, Tsoli M, Chang C, Joshi S,
Liu J, Ryall S, Chornenkyy Y, Siddaway R, Hawkins C and Ziegler DS:
Targeting reduced mitochondrial DNA quantity as a therapeutic
approach in pediatric high-grade gliomas. Neuro Oncol. 22:139–151.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sravya P, Nimbalkar VP, Kanuri NN, Sugur
H, Verma BK, Kundu P, Rao S, Uday Krishna AS, Somanna S, Kondaiah
P, et al: Low mitochondrial DNA copy number is associated with poor
prognosis and treatment resistance in glioblastoma. Mitochondrion.
55:154–163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li F, Wang X, Han C and Lin J: Decreased
mtDNA copy number of gastric cancer: A new tumor marker? Chin J
Clin Oncol. 1:250–255. 2004. View Article : Google Scholar
|
|
134
|
Wu CW, Yin PH, Hung WY, Li AF, Li SH, Chi
CW, Wei YH and Lee HC: Mitochondria DNA mutations and mitochondrial
DNA depletion in gastric cancer. Genes Chromosom Cancer. 44:19–28.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang G, Qu Y, Dang S, Yang Q, Shi B and
Hou P: Variable copy number of mitochondrial DNA (mtDNA) predicts
worse prognosis in advanced gastric cancer patients. Diagn Pathol.
8:1732013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Liao LM, Baccarelli A, Shu XO, Gao YT, Ji
BT, Yang G, Li HL, Hoxha M, Dioni L, Rothman N, et al:
Mitochondrial DNA copy number and risk of gastric cancer: A report
from the Shanghai women's health study. Cancer Epidemiol Biomarkers
Prev. 20:1944–1949. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wen SL, Zhang F and Feng S: Decreased copy
number of mitochondrial DNA: A potential diagnostic criterion for
gastric cancer. Oncol Lett. 6:1098–1102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mizumachi T, Muskhelishvili L, Naito A,
Furusawa J, Fan CY, Siegel ER, Kadlubar FF, Kumar U and Higuchi M:
Increased distributional variance of mitochondrial DNA content
associated with prostate cancer cells as compared with normal
prostate cells. Prostate. 68:408–417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhou W, Zhu M, Gui M, Huang L, Long Z,
Wang L, Chen H, Yin Y, Jiang X, Dai Y, et al: Peripheral blood
mitochondrial DNA copy number is associated with prostate cancer
risk and tumor burden. PLoS One. 9:e1094702014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Abhishek A, Singh V, Sinha RJ, Ansari NG,
Siddiqe MK, Verma M and Kumar M: To study the relationship between
cadmium, zinc and mtDNA copy number in North Indian patients
suffering from prostate cancer: A case control study. Afr J Urol.
23:126–132. 2017. View Article : Google Scholar
|
|
141
|
Moore A, Lan Q, Hofmann JN, Liu CS, Cheng
WL, Lin TT and Berndt SI: A prospective study of mitochondrial DNA
copy number and the risk of prostate cancer. Cancer Causes Control.
28:529–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tu H, Gu J, Meng QH, Kim J, Davis JW, He
Y, Wagar EA, Thompson TC, Logothetis CJ and Wu X: Mitochondrial DNA
copy number in peripheral blood leukocytes and the aggressiveness
of localized prostate cancer. Oncotarget. 6:41988–41996. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kalsbeek AM, Chan EK, Grogan J, Petersen
DC, Jaratlerdsiri W, Gupta R, Lyons RJ, Haynes AM, Horvath LG,
Kench JG, et al: Altered mitochondrial genome content signals worse
pathology and prognosis in prostate cancer. Prostate. 78:25–31.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu J, Chang WS, Tsai CW, Bau DT, Davis JW,
Thompson TC, Logothetis CJ and Gu J: Mitochondrial DNA copy number
in peripheral blood leukocytes is associated with biochemical
recurrence in prostate cancer patients in African Americans.
Carcinogenesis. 41:267–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Tan DJ, Chang J, Liu LL, Bai RK, Wang YF,
Yeh KT and Wong LJC: Significance of somatic mutations and content
alteration of mitochondrial DNA in esophageal cancer. BMC Cancer.
6:932006. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu Z, Zhao Z and Zhao Q: Change in the
mitochondrial DNA copy number and its significance in esophageal
squamous cell carcinoma. Chin J Clin Oncol. 34:7–9. 2007.
|
|
147
|
Lin CS, Chang SC, Wang LS, Chou TY, Hsu
WH, Wu YC and Wei YH: The role of mitochondrial DNA alterations in
esophageal squamous cell carcinomas. J Thorac Cardiovasc Surg.
139:189–197.e4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li H, Tian Z, Zhang Y, Yang Q, Shi B, Hou
P and Ji M: Increased copy number of mitochondrial DNA predicts
poor prognosis of esophageal squamous cell carcinoma. Oncol Lett.
15:1014–1020. 2018.PubMed/NCBI
|
|
149
|
Masuike Y, Tanaka K, Makino T, Yamasaki M,
Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, Mori M and Doki Y:
Esophageal squamous cell carcinoma with low mitochondrial copy
number has mesenchymal and stem-like characteristics, and
contributes to poor prognosis. PLoS One. 13:e01931592018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Xu E, Sun W, Gu J, Chow WH, Ajani JA and
Wu X: Association of mitochondrial DNA copy number in peripheral
blood leukocytes with risk of esophageal adenocarcinoma.
Carcinogenesis. 34:2521–2524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kim MM, Clinger JD, Masayesva BG, Ha PK,
Zahurak ML, Westra WH and Califano JA: Mitochondrial DNA quantity
increases with histopathologic grade in premalignant and malignant
head and neck lesions. Clin Cancer Res. 10:8512–8515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jiang WW, Rosenbaum E, Mambo E, Zahurak M,
Masayesva B, Carvalho AL, Zhou S, Westra WH, Alberg AJ, Sidransky
D, et al: Decreased mitochondrial DNA content in posttreatment
salivary rinses from head and neck cancer patients. Cancer Ther
Clin. 12:1564–1569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cheau-Feng Lin F, Jeng YC, Huang TY, Chi
CS, Chou MC and Chin-Shaw Tsai S: Mitochondrial DNA copy number is
associated with diagnosis and prognosis of head and neck cancer.
Biomarkers. 19:269–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kumar M, Srivastava S, Singh SA, Das AK,
Das GC, Dhar B, Ghosh SK and Mondal R: Cell-free mitochondrial DNA
copy number variation in head and neck squamous cell carcinoma: A
study of non-invasive biomarker from Northeast India. Tumor Biol.
Oct 26–2017.(Epub ahead of print). View Article : Google Scholar
|
|
155
|
Chen J, Zhang L, Yu X, Zhou H, Luo Y, Wang
W and Wang L: Clinical application of plasma mitochondrial DNA
content in patients with lung cancer. Oncol Lett. 16:7074–7081.
2018.PubMed/NCBI
|
|
156
|
Bonner MR, Shen M, Liu CS, DiVita M, He X
and Lan Q: Mitochondrial DNA content and lung cancer risk in Xuan
Wei, China. Lung Cancer. 63:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hosgood HD III, Liu CS, Rothman N,
Weinstein SJ, Bonner MR, Shen M, Lim U, Virtamo J, Cheng WL,
Albanes D and Lan Q: Mitochondrial DNA copy number and lung cancer
risk in a prospective cohort study. Carcinogenesis. 31:847–849.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kim C, Bassig BA, Seow WJ, Hu W, Purdue
MP, Shu XO, Huang WY, Liu CS, Cheng WL, Lin TT, et al: Pooled
analysis of mitochondrial DNA copy number and lung cancer risk in
three prospective studies. Cancer Epidemiol Biomarkers Prev.
23:2977–2980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Meierhofer D, Mayr JA, Foetschl U, Berger
A, Fink K, Schmeller N, Hacker GW, Hauser-Kronberger C, Kofler B
and Sperl W: Decrease of mitochondrial DNA content and energy
metabolism in renal cell carcinoma. Carcinogenesis. 25:1005–1010.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Xing J, Chen M, Wood CG, Lin J, Spitz MR,
Ma J, Amos CI, Shields PG, Benowitz NL, Gu J, et al: Mitochondrial
DNA content: Its genetic heritability and association with renal
cell carcinoma. J Natl Cancer Inst. 100:1104–1112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Purdue MP, Hofmann JN, Colt JS, Hoxha M,
Ruterbusch JJ, Davis FG, Rothman N, Wacholder S, Schwartz KL,
Baccarelli A and Chow WH: A case-control study of peripheral blood
mitochondrial DNA copy number and risk of renal cell carcinoma.
PLoS One. 7:e431492012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Lin CS, Lee HT, Lee MH, Pan SC, Ke CY,
Chiu AW and Wei YH: Role of mitochondrial DNA copy number
alteration in human renal cell carcinoma. Int J Mol Sci.
17:8142016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Hofmann JN, Hosgood HD III, Liu CS, Chow
WH, Shuch B, Cheng WL, Lin TT, Moore LE, Lan Q, Rothman N and
Purdue MP: A nested case-control study of leukocyte mitochondrial
DNA copy number and renal cell carcinoma in the prostate, lung,
colorectal and ovarian cancer screening trial. Carcinogenesis.
35:1028–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Elsayed ET, Hashad MM and Elgohary IE:
Mitochondrial DNA copy number variation as a potential predictor of
renal cell carcinoma. Int J Biol Markers. 32:e313–e318. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wang Y, Liu VW, Xue WC, Tsang PC, Cheung
AN and Ngan HY: The increase of mitochondrial DNA content in
endometrial adenocarcinoma cells: A quantitative study using
laser-captured microdissected tissues. Gynecol Oncol. 98:104–110.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Cormio A, Guerra F, Cormio G, Pesce V,
Fracasso F, Loizzi V, Resta L, Putignano G, Cantatore P, Selvaggi
LE and Gadaleta MN: Mitochondrial DNA content and mass increase in
progression from normal to hyperplastic to cancer endometrium. BMC
Res Notes. 5:2792012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Sun Y, Zhang L, Ho SS, Wu X and Gu J:
Lower mitochondrial DNA copy number in peripheral blood leukocytes
increases the risk of endometrial cancer. Mol Carcinog.
55:1111–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Mondal R, Ghosh SK, Choudhury JH, Seram A,
Sinha K, Hussain M, Laskar RS, Rabha B, Dey P, Ganguli S, et al:
Mitochondrial DNA copy number and risk of oral cancer: A report
from Northeast India. PLoS One. 8:e577712013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
He Y, Gong Y, Gu J, Lee JJ, Lippman SM and
Wu X: Increased leukocyte mitochondrial DNA copy number is
associated with oral premalignant lesions: an epidemiology study.
Carcinogenesis. 35:1760–1764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Takeda D, Hasegawa T, Ueha T, Sakakibara
A, Kawamoto T, Minamikawa T, Sakai Y and Komori T: Decreased
mitochondrial copy numbers in oral squamous cell carcinoma. Head
Neck. 36:1170–1175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Lynch SM, Weinstein SJ, Virtamo J, Lan Q,
Liu CS, Cheng WL, Rothman N, Albanes D and Stolzenberg-Solomon RZ:
Mitochondrial DNA copy number and pancreatic cancer in the
alpha-tocopherol beta-carotene cancer prevention study. Cancer Prev
Res (Phila). 4:1912–1919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Tuchalska-Czuroń J, Lenart J, Augustyniak
J and Durlik M: Is mitochondrial DNA copy number a good prognostic
marker in resectable pancreatic cancer? Pancreatology. 19:73–79.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Gentiluomo M, Katzke VA, Kaaks R,
Tjønneland A, Severi G, Perduca V, Boutron-Ruault MC, Weiderpass E,
Ferrari P, Johnson T, et al: Mitochondrial DNA copy-number
variation and pancreatic cancer risk in the prospective EPIC
cohort. Cancer Epidemiol Biomarkers Prev. 29:681–686. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wang Y, Liu VW, Xue WC, Cheung AN and Ngan
HY: Association of decreased mitochondrial DNA content with ovarian
cancer progression. Br J Cancer. 95:1087–1091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Keserű JS, Soltész B, Lukács J, Márton É,
Szilágyi-Bónizs M, Penyige A, Póka R and Nagy B: Detection of
cell-free, exosomal and whole blood mitochondrial DNA copy number
in plasma or whole blood of patients with serous epithelial ovarian
can. J Biotechnol. 298:76–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Hyland PL, Pfeiffer RM, Rotunno M, Hofmann
JN, Liu CS, Cheng WL, Yuenger J, Lan Q, Tucker MA, Goldstein AM and
Yang XR: Constitutive mitochondrial DNA copy number in peripheral
blood of melanoma families with and without CDKN2A mutations. J
Carcinog Mutagen. 2014 (Suppl 4):S0062014.
|
|
177
|
Shen J, Gopalakrishnan V, Lee JE, Fang S
and Zhao H: Mitochondrial DNA copy number in peripheral blood and
melanoma risk. PLoS One. 10:e01316492015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Dang S, Qu Y, Wei J, Shao Y, Yang Q, Ji M,
Shi B and Hou P: Low copy number of mitochondrial DNA (mtDNA)
predicts worse prognosis in early-stage laryngeal cancer patients.
Diagn Pathol. 9:282014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Guo W, Yang D, Xu H, Zhang Y, Huang J,
Yang Z, Chen X and Huang Z: Mutations in the D-loop region and
increased copy number of mitochondrial DNA in human laryngeal
squamous cell carcinoma. Mol Biol Rep. 40:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro
M, Moreno-Loshuertos R, Fernandez-Silva P, Enriquez JA and
Laclaustra M: Adjusting MtDNA quantification in whole blood for
peripheral blood platelet and leukocyte counts. PLoS One.
11:e01637702016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Malik AN and Czajka A: Is mitochondrial
DNA content a potential biomarker of mitochondrial dysfunction?
Mitochondrion. 13:481–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Anderson C, Fry RC, Hartwell H, Kleeberger
C, Sandler DP and Nichols HB: Measurement of mitochondrial DNA copy
number in dried blood spots: A pilot study. Mitochondrion.
56:35–39. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Parr RL, Dakubo GD, Thayer RE, McKenney K
and Birch-Machin MA: Mitochondrial DNA as a potential tool for
early cancer detection. Hum Genomics. 2:252–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Liu Y, Zhou K, Guo S, Wang Y, Ji X, Yuan
Q, Su L, Guo X, Gu X and Xing J: NGS-based accurate and efficient
detection of circulating cell-free mitochondrial DNA in cancer
patients. Mol Ther Nucleic Acids. 23:657–666. 2021. View Article : Google Scholar : PubMed/NCBI
|