1. Introduction
Metabolic syndrome is a combination of symptoms,
including abdominal obesity, atherogenic dyslipidemia,
hypertension, insulin resistance characterized by hyperglycemia,
proinflammatory state and prothrombotic state [National Cholesterol
Education Programs Adult Treatment Panel III report (ATP III)]
(1). The comorbidities associated
with the metabolic syndrome include cardiovascular disease, type 2
diabetes and other diseases including polycystic ovary syndrome,
fatty liver, asthma, cholesterol gallstones, sleep disturbances and
several cancers (1-3).
Abdominal obesity is characterized by a waist
circumference of >102 cm in men and >88 cm in women (1). Obesity is often caused by excessive
food intake and a lack of physical activity, resulting in an energy
imbalance, where energy intake is greater than energy expenditure,
leading to an elevated body mass index (BMI >30
kg/m2) and increased body fat mass (4,5).
It has been linked to increased proinflammatory states due to
chronic adipose tissue inflammation, leading to the development of
insulin resistance and type 2 diabetes (6). Atherogenic dyslipidemia is
manifested by increased plasma triglyceride levels, a high
concentration of plasma low-density lipoprotein (LDL)-cholesterol
(LDL-C) and low level of high-density lipoprotein (HDL) cholesterol
(HDL-C), increasing the risk of cardiovascular disease (1). Hypertension is caused by a high
blood pressure where systolic blood pressure of is >130 mm Hg
and diastolic >85 mm Hg (1).
Insulin resistance occurs when fasting blood glucose level is ≥110
mg/dl (1). The World Health
Organization criteria for metabolic syndrome is the presence of
insulin resistance accompanied with any two of the other symptoms
of metabolic syndrome including increased waist/hip ratio and
increased urinary albumin excretion rate (7).
Currently, there are several pharmacological drugs
available for obesity treatment, including orlistat (Xenical) and
sibutramine; however, these drugs have several undesirable side
effects including mood changes, and gastrointestinal or
cardiovascular complications (8).
Plant-derived natural compounds have been revealed to demonstrate
positive effects on obesity, diabetes, renal and cardiovascular
disease (9). Thus, natural
products from plants have been suggested as a better alternative
for treating obesity and cardiometabolic syndrome (10).
α-mangostin is a xanthone by chemical structure
(Fig. 1), and one of the
significant phytochemical constituents in the tropical fruit
Garcinia mangostana (11-13). It has also been found in other
Garcinia (G.) species, including G. dulcis
(14), G. staudtii
(15) G. merguensis
(16) and G. cowa
(17), and in the perennial
tropical trees Cratoxylum cochinchinense (18), Cratoxylum arborescens
(19), Cratoxylum formosum
(20) and Pentadesma
butyracea (21). In G.
mangostana, α-mangostin is mainly found in the fruit pericarp
(12,22) which has been traditionally used to
treat several health conditions, including abdominal pain,
diarrhea, dysentery, wound infections, suppuration, and chronic
ulcers (23).
Previous reports have demonstrated that α-mangostin
exerts numerous health-promoting effects including anti-obesity
(24), antidiabetic (25), antioxidant (26), anti-inflammatory (27), antiallergic (28), anticancer (29), neuroprotective (30), hepatoprotective (31), cardioprotective (32), antimicrobial (33) and antifungal (34) properties. Although previous
reviews have summarized the health properties of α-mangostin
(30,35-40), limited information is available on
its molecular mechanisms in cardiometabolic disease. Hence, the
present review aimed to elucidate the potential molecular effects
of α-mangostin on metabolic syndrome parameters, observed in
biological models of cardiometabolic syndrome and other related
models.
2. Anti-obesity effects of α-mangostin
The anti-obesity effects of α-mangostin and
α-mangostin-rich materials have been extensively studied. Various
doses of purified α-mangostin compound have been revealed to reduce
body weight in animal models (mice and rats) even when treated with
G. mangostana fruit pericarp/rind/peel or flesh (12,41-46).
Kim et al (25) suggested that the treatment of high
fat-fed C57BL/6 mice with 50 mg/kg of α-mangostin per day reduced,
their body weight, cholesterol levels, serum triglycerides, and
increased adiponectin levels, also noting that the treatment
reduced epididymal adipose tissue size and reduced the crown like
structures in adipocytes. Choi et al (24) also stated that an α-mangostin dose
of 50 mg/kg per day in C57BL/6 mice reduced body weight, total
cholesterol (TC), LDL-C and free fatty acids in mice fed a high-fat
diet. Furthermore, they found that α-mangostin increased the
expression of hepatic peroxisome proliferator-activated receptor γ
(PPARγ), sirtuin (SIRT) 1, AMP-activated protein kinase (AMPK) and
retinoid-X-receptor alpha, suggesting that the anti-obesity and
hepatoprotective effects of α-mangostin are mediated via the
SIRT1-AMPK and PPARγ pathways in mice with obesity induced by a
high fat diet. Li et al (42) treated aged mice with an
α-mangostin dose of 25 and 50 mg/kg per day and observed a
reduction in body weight, epididymal and inguinal white adipose
tissue, serum concentrations of triglycerides, LDL-C and TC. The
treatment increased phosphorylated (p-) protein kinase B (p-AKT)
expression in epididymal white adipose tissue (42).
The hallmark of dyslipidemia in obesity includes
increased triglycerides and free fatty acids, low HDL-C or slightly
increased LDL-C (47). John et
al (12) demonstrated that
the administration of an α-mangostin dose of 168 mg/kg per day from
G. mangostana rind to rats on a high fat/carbohydrate diet
decreased their body weight gain and visceral fat accumulation
accompanied by reduced adipocyte size and plasma triglycerides. In
a monosodium glutamate, high-calorie diet-induced male Wistar rat
model, Abuzaid et al (41)
noted that the administration of 200 and 500 mg/kg G.
mangostana extract per day, equivalent to a dose of 60 and 150
mg/kg α-mangostin per day, respectively, caused a reduction in body
weight gain, which was associated with a reduction in fatty acid
synthase activity in adipose tissue and serum. In the study by
Mohamed et al (46), the
treatment of Balb/c mice with high-fat non-alcoholic fatty liver
disease (NAFLD) and non-alcoholic steatohepatitis (NASH) with 50
mg/kg α-mangostin per day also reduced body weight gain and free
fatty acid levels.
Chae et al (45) examined the effect of the 50 and
200 mg/kg dose of G. mangostana extract per day, equivalent
to a dose of ~12.5 mg/kg and 50 mg/kg α-mangostin per day,
respectively, in C57BL/6 mice fed a high-fat diet. The treatment
decreased body weight and visceral fat (epididymal, inguinal and
mesenteric), although not retroperitoneal fat. The treatment also
improved lipid metabolism by reducing triglycerides, LDL-C, TC at
both concentrations. Protein expression analysis revealed that
α-mangostin activated the AMPK and SIRT-1 pathways, aiding in body
weight reduction. In the 200 mg/kg per day group, the PPARγ levels
decreased, suggesting a reduction of lipogenesis in adipocyte
differentiation and an increase in carnitine palmitoyl transferase
1a (CPT1a), which in turn promotes fatty oxidation (45). In the study by Tsai et al
(43) rats fed a high fat-diet
were treated with 25 mg/day of mangosteen pericarp extract for 11
weeks. A decrease in body weight gain, plasma free fatty acids and
hepatic triglyceride accumulation was observed. In another study,
Sprague-Dawley rats fed a high-fat diet were treated with dried
G. mangostana flesh doses of 200, 400, 600 mg/kg per day and
exhibited a reduced body weight, food intake, plasma cholesterol
and TC levels (44). However, in
that study, the amount of α-mangostin was not quantified (44).
In vitro studies using α-mangostin have
demonstrated similar conclusions as in vivo studies. The
treatment of breast cancer cell lines with α-mangostin (1-4
µM) led to the inhibition of fatty acid synthase (FAS)
expression and activity (29). In
another study, 3T3-L1 pre-adipocytes treated with α-mangostin
exhibited a concentration-dependent reduction in intracellular fat
accumulation up to 44.4% relative to methylisobutylxanthine,
dexamethasone, insulin (MDI)-treated control cells at a 50
µM concentration. PPARγ expression and pre-adipocyte
differentiation were suppressed by α-mangostin (48). Additionally, the use of
α-mangostin resulted in leptin production increase (48) and exerted potent inhibitory
effects against pancreatic lipase (49).
The treatment with G. mangostana pericarp,
rich in α-mangostin, demonstrated similar effects across studies.
John et al (12), Li et
al (42) and Chae et
al (45) observed reduced
white adipose tissue deposition, TC, free fatty acids,
triglyceride, and visceral fat accumulation. Li et al
(42) and Chae et al
(45) additionally observed
reduced LDL-C with pericarp treatment. The treatment has also been
previously reported to increase hepatic AMPK and SIRT1 (24) and reduce PPARγ expression
(45). AMPK activity maintains
cellular energy storage by activating catabolic pathways that
generate ATP, primarily by increasing oxidative metabolism and
mitochondrial biogenesis, while 'switching off' anabolic pathways
that utilize ATP (50).
In principle, the reduction of body weight by
α-mangostin is associated with a decrease in adipose tissue size
and accumulation, decreased fatty acid synthase activity, decreased
intracellular fat accumulation, increased adiponectin expression,
increased fatty acid oxidation via an increased CPT1a expression,
the increased activation of the SIRT1-AMPK, and reduced PPARγ
pathways (Fig. 2 and Table I). This may suggest that the main
methods of action in obesity reduction by α-mangostin occur through
fatty acid metabolism and adipose tissue biology as the major depot
for fatty acids, requiring further elucidation.
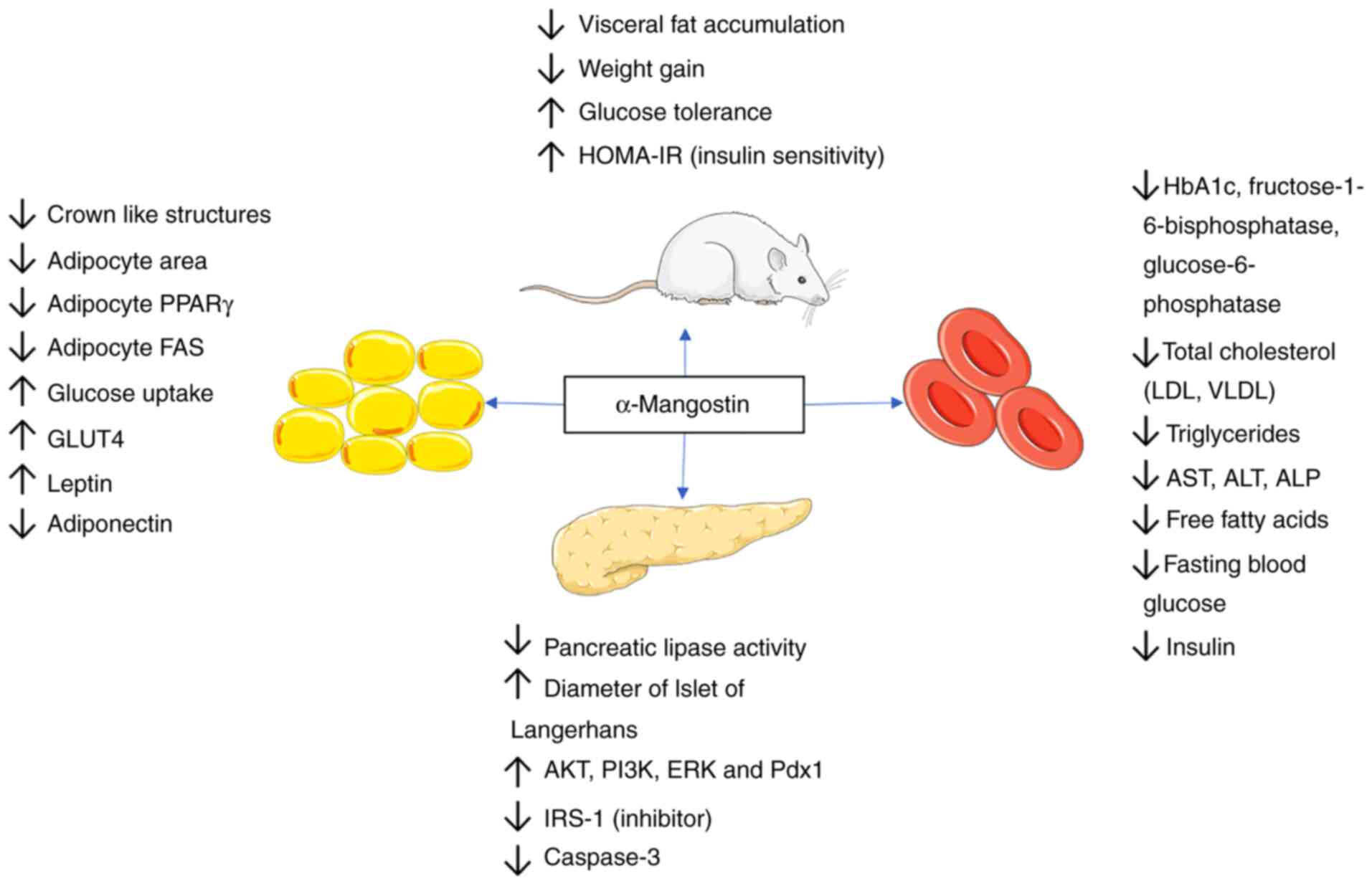 | Figure 2Anti-obesity and antidiabetic effects
of α-mangostin. The anti-obesity effects of α-mangostin are
mediated via the modulation of adipose tissue biology, reduction in
visceral fat accumulation and inhibition of fatty acid synthase.
Its antidiabetic effects are mediated through an improvement in
insulin sensitivity and glucose tolerance, increased pancreatic
lipase activity, increased glucose transporter activity, the
increased stimulation of insulin receptor and the increased
phosphorylation of the PI3K, AKT and ERK signaling cascades. PPARγ,
peroxisome proliferator-activated receptor γ; GLUT4, glucose
transporter 4; HOMA-IR, homeostatic model assessment for insulin
resistance; Pdx1, pancreatic and duodenal homeobox 1; LDL,
low-density lipoprotein; VLDL, very low-density lipoprotein; AST,
aspartate aminotransferase; ALT, alanine aminotransferase; ALP,
alkaline phosphatase. |
 | Table IAnti-obesity effects of
a-mangostin. |
Table I
Anti-obesity effects of
a-mangostin.
| Authors | Source of
α-mangostin | Model: in
vitro/in vivo | Dosage and duration
of treatment | Mechanisms of
action of anti-obesity effects | (Refs.) |
|---|
| John et
al | G.
mangostana rind | Wistar rats (High
carbohydrate, high fat) | 168 mg/kg per day 8
weeks | • ↓Weight
gain
• ↓Visceral fat accumulation
• ↓Adipocyte area
• ↓Plasma triglyceride
• ↓FFA | (12) |
| Taher et
al | G.
malaccencis | 3T3-L1
preadipocytes | 10, 25, and 50
µM of α-mangostin 2 days | • ↓Intracellular
fat accumulation
• ↓PPARγ expression | (48) |
| Chae et
al | G.
mangostana | In vitro pancreatic
lipase assay model | IC50=5.0
µM | • ↓Pancreatic
lipase activity | (49) |
| Abuzaid et
al | G.
mangostana pericarp | Wistar rats | 200 mg/kg body
weight per day 500 mg/kg body weight per day (~60 mg/kg per day and
150 mg/kg α-mangostin per day) 9 weeks | • ↓Body
weight
• ↓Fatty acid synthase (adipose tissue/serum) | (41) |
| Chang et
al | Mangosteen
concentrate drink | Sprague-Dawley
rats | 13 mg/day 6
weeks | • ↓Fasting plasma
triglyceride
• ↓Total cholesterol
• ↓Hepatic CAT | (107) |
| Tsai et
al | Mangosteen pericarp
extract | Sprague-Dawley
rats; rat primary hepatocytes | 25 mg/day-rat 11
weeks 10-30 µM-rat hepatocytes 24 h | • ↓Plasma
FFA
• ↓Weight gain | (43) |
| Muhamad Adyab et
al | G.
mangostana flesh | Sprague-Dawley
rats | 200-600 mg/kg
(α-mangostin concentration not detailed) 7 weeks | • ↓Weight
gain
• ↓Plasma LDL-C
• ↓Total cholesterol | (44) |
| Chae et
al | G.
mangostana peel | Male C57BL/6
mice | 50 and 200 mg/kg
per day (~12.5 and 50 mg/kg of α-mangostin per day) 45 days | • ↓Weight
gain
• ↓AST and ALT
• ↓LDL cholesterol
• ↓Total cholesterol
• ↓Triglyceride
• ↓FFA, ↓glucose
• ↓Visceral fat accumulation
• ↓PPARγ
• ↑Hepatic SIRT1 and AMPK | (45) |
| Mohamed et
al | G.
mangostana extract | Balb/c mice | Group III-50 mg/kg
of α-mangostin per day 16 weeks | • ↓Weight
gain
• ↓FFA | (46) |
| Kim et
al | Purified
α-mangostin | C57BL/6 mice
RAW264.7 macrophages Mesenteric adipose tissue culture | 50 mg/kg per day 12
weeks 25 µM/ml | • ↓Body
weight
• ↓Cholesterol
• ↓Serum triglyceride
• ↑Adiponectin
• ↓Serum ALT
• ↓Crown-like structures (adipocytes)
• ↓Epididymal adipose tissue size | (25) |
| Li et
al | Purified
α-mangostin | MCF-7, estrogen
receptor-positive cells, MDA-MB-231, estrogen receptor-negative
cells | 1, 2, 3, 4
µM 24 h | • ↓FAS
expression
• ↓Intracellular FAS activity | (29) |
| Li et
al | Purified
α-mangostin | Mouse derived
RAW264.7 macrophage 3T3-L1 preadipocytes Male C57BL/6J | 10 mg/kg per day
(inflammation mice) 5 days 25 and 50 mg/kg per day 8 weeks (aged
mice) | • ↓Weight, indexes
eWAT and iWAT
• ↑Insulin sensitivity (HOMA-IR), p-AKT level
• ↓Total cholesterol, triglyceride, LDL-cholesterol
• ↑HDL-C | (42) |
| Choi et
al | Purified
α-mangostin | Male CB57L/6
mice | 50 mg/kg per day 6
weeks | • ↓Weight
gain
• ↓FFA
• ↓Total cholesterol
• ↓LDL-C
• ↑Hepatic PPARγ,
SIRT1, AMPK and RXRα | (24) |
3. Antidiabetic effects of α-mangostin
Diabetes is characterized by insulin resistance and
hyperglycemia, and is associated with hyperlipidemia. The main
hallmarks of diabetes include insulin resistance and pancreatic
β-cell dysfunctions (51). In
animal studies, treating high fat-fed mice with a dose of 50 mg/kg
α-mangostin per day has been reported to improve glucose tolerance,
increase insulin sensitivity as evaluated by the homeostatic model
assessment for insulin resistance (HOMA-IR), increase adiponectin
levels and increase the phosphorylation levels of insulin receptor
substrate 1 (IRS-1) and AKT in both liver and adipose tissues
(25). This indicates that
α-mangostin affects insulin signaling in both tissues.
By using rat pancreatic INS-1 cells, Lee et
al (52) revealed that the
use of α-mangostin at a concentration between 1-10 µM
increased insulin secretion in a concentration-dependent manner in
cells grown under high glucose conditions (16.7 mM glucose) without
inducing cytotoxicity, which was maintained for 48 h. Additionally,
it was further demonstrated that high glucose levels reduced the
phosphorylated or active insulin receptor (p-IR), phosphoinositide
3-kinases (PI3K), p-AKT, protein kinase R-like endoplasmic
reticulum kinase and pancreatic and duodenal homeobox 1 (Pdx1)
protein levels, but increased the expression of IRS-1
phosphorylated at Ser 1101 (p-IRS-1Ser1101) in INS-1 cells
(52).
Reduced p-IR levels indicate a response to the
increased serine phosphorylation of IRS-1 proteins (53). The phosphorylation of IRS proteins
on serine residues negatively regulates IRS signaling (54,55). IRS-1 acting downstream of pIR,
recruits p85, a regulatory subunit of PI3K, and activates the
PI3K/AKT pathway (56). This
pathway activates PDK kinases, which phosphorylate AKT and permit
its translocation to the nucleus, activating genes involved in
glucose intake and blocking FOXO genes, inducing gluconeogenesis
(57). High glucose levels
essentially shut down insulin signaling by altering IR
phosphorylation. This shutdown mechanism is attributed to the
increased proteasome degradation of IRS proteins, the failure to
recruit p85 to IRS proteins, thereby not activating the PI3K/AKT
signaling pathway (54).
Treatment with a 5 µM concentration of
α-mangostin, has been reported to protect against these effects, by
reducing the inhibitory pIRS-1Ser1101, and restoring IR signaling
as evidenced by increased PI3K, AKT and Pdx1 proteins (52). The loss of Pdx1 has been revealed
to be associated with a decrease in b cell mass. It should be noted
that hyperinsulinemia lowers the expression of IRS1 family of
proteins in both cultured cells and mouse tissues, which has been
linked to insulin resistance in animal models. The mechanism has
been largely attributed to increased degradation (by
ubiquitination) and decreased synthesis of IRS proteins (54,55).
Streptozotocin (STZ) is commonly used to induce type
1 diabetes in animal models, which induces oxidative stress in
pancreatic β-cells, resulting in the increased activation of p38
MAPKs, JNK proteins and cleaved caspase-3 protease which causes
hyperglycemia, the increased generation of reactive oxygen species
(ROS), osmotic stress, proinflammatory cytokine secretion and
apoptosis (52,58). However, co-treatment with
α-mangostin (5 µM) has been found to reduce caspase-3
protease levels, reduce the apoptosis of the cells, and increase
p-PI3K-AKT levels, demonstrating the protective effect of this
compound in the presence of STZ (52). The antiapoptotic properties of
α-mangostin could be attributed to its anti-oxidant properties, as
described in a following section. The effects of α-mangostin in
reducing pro-apoptotic proteins levels, particularly caspase-3
levels have been also observed in a previous study in human
umbilical vein endothelial cultured cells (59), where α-mangostin led to an
increase in Bcl-2, an antiapoptotic protein, and a reduction in
Bax, a pro-apoptotic protein, in a concentration-dependent manner
(59).
Hyperglycemia also induces ROS generation via a
series of complex processes involving diacylglycerol, protein
kinase C and NADPH-oxidase (60).
ROS production eventually leads to tissue damage, which decreases
insulin production as pancreatic β-cells are damaged over time,
leading to hyperglycemia. These effects are attenuated by
α-mangostin, which protects the pancreatic β-cells from damage and
restores damaged pancreatic cells to allow for optimal insulin
release (59,61,62).
In another in vivo study, histological
samples of mice with STZ-induced diabetes treated with α-mangostin
exhibited a restored diameter of islets of Langerhans (61), thereby improving insulin
secretion. Jariyapongskul et al (63), who studied the effects of
α-mangostin on hyperglycemia induced ocular hypoperfusion retinal
leakage in rats, revealed that α-mangostin treatment significantly
improved ocular flow and reduced leakage in rats, by reducing
malondialdehyde (MDA) levels and lipid peroxidation in the retina.
Hyperglycemic tissues have been shown to exhibit increased levels
of MDA and advanced glycation end-products (AGEs). MDA is an end
product of lipid peroxidation prevalent in hyperglycemia due to
increased free radicals (63).
AGEs are involved in the pathogenesis of diabetes-related
complications, including cardiomyopathy, retinopathy and
nephropathy (64). At a 25
µM concentration, α-mangostin was found to reduce the
production of AGEs (65). In
another study, the treatment of mice with STZ-induced diabetes with
α-mangostin resulted in decreased glycated hemoglobin levels and
reduced key gluconeogenic enzymes fructose-1-6-bisphosphatase and
glucose-6-phosphatase lowering glucose production (66).
A pilot study previously investigated the effects of
mangosteen supplement (400 mg, once per day) in obese female
patients with insulin resistance (67). In that study, Watanabe et
al (67) revealed that the
treatment significantly improved insulin sensitivity (HOMA-IR)
along with a reduction in insulin levels, improved HDL-C and
lowered high-sensitivity C-reactive protein levels.
In general, treatment with α-mangostin or a diet
rich in α-mangostin in various obese and diabetic rat models, In
vitro studies and human studies has revealed that the compound
can improve the markers of diabetes by lowering fasting blood
glucose concentration, lowering insulinemia, increasing glucose
tolerance and increasing insulin sensitivity, as measured by
HOMA-IR, and increasing glucose uptake. The treatment with
α-mangostin promotes glucose uptake by increasing the expression of
glucose transporters in tissues, including glucose transporter
(GLUT)4 in adipose tissues, adipocytes, cardiac tissues, and
skeletal muscles and GLUT2 in hepatic tissues (25,68). Furthermore, α-mangostin may
stimulate insulin release in the pancreatic, liver and adipose
tissues by activating IRs and increasing the phosphorylation of
PI3K, AKT and ERK signaling cascades (25,52). These observations could explain
the increased glucose uptake and plasma glucose level reduction in
cells or animals treated with α-mangostin. A summary of the
mechanism of α-mangostin is presented in Fig. 2 and Table II.
 | Table IIAntidiabetic effects of
a-mangostin. |
Table II
Antidiabetic effects of
a-mangostin.
| Authors | Source of
α-mangostin | Model In
vitro/in vivo | Dosage and
duration | Mechanisms of
action of anti-diabetic effects | (Refs.) |
|---|
| Kim et
al | Purified
α-mangostin | C57BL/6 mice
RAW264.7 macrophages Mesenteric adiposetissue culture | 50 mg/kg per day 12
weeks 25 µM/ml 24 h | • ↑Gucose
tolerance
• ↑HOMA-IR (insulin sensitivity)
• ↑p-AKT
• ↑p-IRS-1
• ↑GLUT4 (adipose)
• ↑GLUT2 (liver) | (25) |
| Taher et
al | G.
malaccencis | 3T3-L1
preadipocytes | 10, 25 and 50
µM of α-mangostin 2 days | • ↑Glucose uptake
(1, 25 µM only)
• ↑GLUT4 (adipocyte)
• ↑Leptin | (48) |
| Jiang et
al | Purified
α-mangostin | C57BL/KsJ diabetic
(db/db) mice Primary aortic endothelial cells | 10 mg/kg/d, i.p.;
mice 12 weeks 15 µM α-mangostin; cell culture 24 and 48
h | • ↓Fasting blood
glucose
• ↓Insulin
• ↓Ceramide and aSMase signaling and accumulation | (79) |
| John et
al | G.
mangostana rind | Wistar rats | 168 mg/kg per day 8
weeks | • ↑Glucose
tolerance | (12) |
| Lazarus et
al | α-mangostin
compound | Wistar rats | 100, 200 mg/kg per
day 8 weeks | • ↑Insulin
sensitivity (HOMA-IR)
• ↑GLUT4 (skeletal muscle) expression
• ↓STZ-induced weight loss | (108) |
| Ratwita et
al | α-mangostin
compound | Wistar rats
adipocytes (WAT) | 5, 10, 20 mg/kg day
21 days 3.125 mM; 6.25 and 25 mM (cell culture)/48 h | • ↑Glucose
tolerance
• ↑GLUT4 (adipocytes)
• ↑GLUT4 (cardiac) | (68) |
| Luo and Lei | α-mangostin | Human umbilical
vein endothelial cells | 5, 10, and 15
µM of α-mangostin Effects noted at 15 µM 24 h | • ↓Glucose induced
cell apoptosis
• ↓Pro-apoptotic proteins
• ↑Anti-apoptotic proteins | (59) |
| Soetikno et
al | α-mangostin | Male Wistar
rats | 100 and 200 mg/kg,
8 weeks | • ↑Insulin
sensitivity
• ↓Lipid profiles (LDH)
• ↓Fasting blood glucose | (98) |
| Jariyapongskul
et al | Purified, extracted
α-mangostin | Male Sprague-Dawley
rats | 200 mg/kg 8
weeks | • ↑Insulin
sensitivity
• ↓Fasting blood glucose
• ↓Blood cholesterol and triglycerides
• ↓TNF-α
• Re-establishes ocular blood flow and reduces retinal blood
leakage | (63) |
| Husen et
al | α-mangostin | Male BALB/C
mice | 2, 4 and 8 mg/kg
per day 14 days | • ↓Fasting blood
glucose
• ↓Blood cholesterol
• ↑Diameter of Islet of Langerhans | (61) |
| Lee et
al | α-mangostin
extracted and purified | Rat insulinoma,
INS-1 cells (store and secrete insulin) | 1, 2.5 and 5
µM 1 h | • ↑Insulin
secretion after glucose stimulation
• ↑Active insulin receptor
• ↑AKT, PI3K, ERK and Pdx1
• ↓IRS-1 (inhibitor)
• ↑Protection of INS-1 cells in presence of damaging
chemicals
• ↓Pro-apoptotic caspase 3 | (52) |
| Kumar et
al | α-mangostin
compound |
Streptozotozin-induced diabetes in Wistar
rat | 25, 50 and 100
mg/kg 56 days Toxicity test up to 1,250 mg/kg; 48 h | • ↓Blood
glucose
• ↓Glycated hemoglobin, fructose-1-6-bisphosphatase,
glucose-6-phosphatase
• ↓Total cholesterol (LDL, VLDL)
• ↓Triglycerides
• ↓AST, ALT, ALP
• ↓Structural renal and hepatic damage
• ↓IL-6, CRP, TNF-α concentrations | (66) |
| Usman et
al | α-mangostin | Male Sprague-Dawley
rats | Determined
IC50 | • ↑Potential to
lengthen α-mangostin release
• ↓Hyperglycemia | (158) |
| Watanabe et
al | G.
mangostana extract | Obese female
patients with insulin resistance | 400 mg/day
26-week | • ↓Insulin
levels
• ↓HOMA-IR
• ↓HsCRP
• ↑HDL-C | (67) |
4. Anti-steatotic and hepatoprotective
effects of α-mangostin
The use of α-mangostin and products rich in
α-mangostin from G. mangostana peel have been extensively
studied in both cell culture and rodent models of hepatic diseases.
G. mangostana peel has been revealed to decrease hepatic fat
vacuole accumulation (12) and
reduce hepatic triglyceride accumulation (43). The infusion of G.
mangostana peel decreases hepatic structural damage induced by
hydrogen peroxide (H2O2) (69). The ability of G. mangostana
peel to improve liver morphology has also been reported by Hassan
et al (70), John et
al (12), Yan et al
(71), and Fu et al
(72) in various hepatic disease
models.
The improvement in hepatic structure has been
associated with improved liver function tests indicated by reduced
levels of aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) (70,72,73). The reduction in liver fibrosis has
also been observed following treatment with G. mangostana
peel (12,73). Mangosteen peel extract (doses of
250 and 500 mg/kg per day) administration in a
thioacetamide-induced hepatoxicity rat model, prevented the
development of liver changes, decreased fibrosis through reduced
expression of α smooth muscle actin (α-SMA) and transforming growth
factor β1 (TGF-β1) genes (73).
Acute and chronic liver injury activate TGF-β1 from the
extracellular matrix, activating hepatic stellate cells to
transdifferentiate into myofibroblasts expressing a large amount of
α-SMA (74). Additionally,
treatment with G. mangostana peel also increases hepatic
PPARγ, AMPK and SIRT1 activation, which are linked to its
anti-obesity effect (45).
In an acute acetaminophen-induced liver injury study
by Yan et al (71),
α-mangostin from G. mangostana peel presented with
hepatoprotective benefits by increasing antioxidant markers,
glutathione (GSH) and MDA, and reducing inflammatory cytokines,
including tumor necrosis factor α (TNF-α) and interleukin (IL)-1β.
α-mangostin also inhibited the expression of autophagy-related
microtubule-associated protein light chain 3 (LC3) and
Bcl-2/adenovirus E1B protein-interacting protein 3. Western blot
analysis further indicated that α-mangostin partially hindered the
activation of apoptotic signaling pathways by increasing Bcl-2
expression, concurrently reducing Bax and cleaved caspase 3
proteins. α-mangostin also increased the expression of p62,
phosphorylated mammalian target of rapamycin (mTOR), phosphorylated
AKT and reduced LC3 II/LC3 I ratio in autophagy signaling pathways
in mouse liver. This indicates that the effect of α-mangostin on
this model may be related to alteration in the AKT/mTOR pathway
(71).
NAFLD is caused by multiple factors, including
hepatic oxidative stress, lipotoxicity, and mitochondrial
dysfunction. Obesity is among the risk factors for NAFLD alongside
type 2 diabetes mellitus and hyperlipidemia. In NAFLD and NASH
models of high fat diet-induced liver disease, treatment with an
α-mangostin concentration of 50 mg/kg per day was shown to reduce
the liver weight coefficient, AST and ALT levels, reduce hepatic
fibrosis and reduce plasma cholesterol, triglyceride and LDL-C
cholesterol levels. The treatment also improved hepatic structure
and function, and increased glycogen storage, as shown by PAS
staining (46). Additionally,
α-mangostin reduced the levels of caspase-3, a marker of apoptosis,
increased autophagy process and reduced CD68-positive macrophages,
and reduced sequestosome-1 (SQSTM1)/p62, LC3 and α-SMA expression
levels (46).
The accretion of lipids in non-adipose tissues,
including the liver, promotes free fatty acid accumulation in the
hepatocytes and increases apoptosis through production of ROS,
lysosomal pathway and death receptor mediated pathway (75). In high-fat diet models, the
significant increase in SQSTM1/P62 and LC3 expression in the obese
group signifies marked autophagy suppression (46,76), possibly suggesting that a lack of
lysosomal activity may affect autophagic processes and may cause
SQSTM1/P62 and LC3 accumulation (77). Yang et al (76) revealed that hepatic autophagy was
suppressed in dietary and genetic obesity models, due to the
decreased expression of key autophagy molecules such as Atg7.
Hepatic autophagy activation has been reported to promote fatty
acid β-oxidation; thus, autophagy suppression has been suggested to
reduce fatty acid α-oxidation in both in vivo and in
vitro models (78). The
reduced expression of SQSTM1/P62 and LC3 in α-mangostin groups may
occur due to the upregulation of autophagy by inducing
pre-autophagosomal structure expression levels and reducing
SQSTM1/p62 and LC3 within hepatocytes (46,47). This is coupled with the
downregulation of the apoptosis process by improved cellular
antioxidant and antioxidant enzymatic capacity and reduced lipid
peroxidation, due to reduced oxidative stress (43).
The treatment of rats with 25 mg/kg body weight
mangosteen extract a day has been reported to increase hepatic
antioxidant enzyme activities and reduce ROS in rat liver tissue
(43). Treated rats and mice have
demonstrated reduced plasma free fatty acid and hepatic
thiobarbituric acid reactive substances levels, while antioxidant
enzymes and the activities of NADH-cytochrome c reductase, and
succinate-cytochrome c reductase (SCCR) were increased (43,79). In vitro research also
demonstrated showed that α-mangostin also increased membrane
potential, cellular oxygen rate, decreased total ROS and
mitochondrial ROS levels, and reduced calcium and cytochrome c
release from the mitochondria, which reduced caspase-9 and -3
activities linked to the apoptotic processes (43).
There are numerous reports on the hepatoprotective
effects of α-mangostin as an individual compound. Kim et al
(25) reported the reduction of
hepatic lipid droplet, tissue weight, hepatic triglyceride in obese
mice treated with α-mangostin. They further reported that the
changes were associated with reduced expression of sterol
regulatory element-binding transcription factor 1, sterol
regulatory element-binding transcription factor (SREBP)-2,
SREBP-1c, lipoprotein lipase (LPL) and stearoyl-CoA desaturase-1
(SCD1) (25). Li et al
(42) also examined the effects
of α-mangostin on aged mice and noted that the treatment reduced
liver injury, AST and ALT levels, and reduced the expression of
microRNA (miRNA/miR)-155 from epididymal white adipose tissue and
macrophage/monocyte-like (RAW264.7) cells and bone marrow-derived
macrophages. miRNA-155 is a crucial mediator in liver steatosis and
fibrosis and its expression is increased during inflammatory
responses in macrophages (42).
α-mangostin also reduced hepatic steatosis by reducing hepatic
triglyceride and fat accumulation and reducing AST and ALT
(24).
Following the administration of α-mangostin at a
dose of 5 mg/kg per day in a thioacetamide induced hepatic fibrosis
rat model, it was noted that the expression of TGF-β1, α-SMA,
tissue inhibitor of metalloproteinases 1 (TIMP-1) was downregulated
(31). In another study by
Rahmaniah et al (80),
α-mangostin was observed to decrease the ratio of pSmad/Smad and
pAKT/AKT in TGF-β-induced liver fibrosis model using human hepatic
stellate (LX-2) cells. They also noted that this treatment reduced
the expression of antigen Ki-67, collagen type I alpha 1 chain
(COL1A1), TIMP-1, plasminogen activator inhibitor-1, α-SMA and
phosphorylated Smad3 (p-Smad3) (80).
In another study, the levels of the hepatic enzymes,
fatty acid transporter and β-hydroxy β-methylglutaryl-CoA (HMG-CoA)
synthase, were significantly suppressed in apolipoprotein E
(Apoe)-deficient mice treated with α-mangostin. However, HMG-CoA
reductase levels increased, and this may be attributed to
compensatory mechanisms triggered by the decrease in HMG-CoA
synthase. Histologically, the treatment also reduced hepatic lipid
accumulation and fibrosis and could be linked to the reduction of
TC due to HMG-CoA synthase inhibition and a reduction of fatty acid
transporter gene expression (81).
In a STZ diabetic mouse model, α-mangostin treatment
(doses of 25, 50 and 100 mg/kg per day) increased plasma insulin
levels, increased superoxide dismutase (SOD), catalase (CAT) and
GSH and reduced TC, triglycerides, LDL-C, very low-density
lipoprotein cholesterol (VLDL-C), AST, ALT, alkaline phosphatase
(ALP) and lipid peroxidation. The treatment also improved hepatic
damage induced by streptozotocin (66).
Another study reported that α-mangostin (10 and 20
µM) inhibited acetaldehyde-induced hepatic stellate (LX-2)
cell proliferation through the downregulation of Ki-67; and
activation through the reduced expression of α-SMA (82). The treatment also reduced the
hepatic stellate cell (HSC) migration markers: Matrix
metallopeptidase (MMP)-2 and -9 as well as expression and
concentration of TGF-β1. The phosphorylation of ERK1/2 and the
expression levels of the fibrogenic markers, COL1A1, TIMP-1 and
TIMP-3, were also reduced. In addition, α-mangostin upregulated the
expression of the antioxidant defenses manganese superoxide
dismutase and glutathione peroxidase (GPx), and reduced
intracellular ROS levels (82).
Overall, α-mangostin reduced acetaldehyde-induced HSC proliferation
and activation via the TGF-β and ERK 1/2 pathways (82).
A sophisticated transcriptomic study conducted by
Chae et al (83), revealed
several novel pathways in lipid and cholesterol metabolism when
HepG2 and Huh7 cells were treated with α-mangostin (10 and 20
µM) for 24 h. The compound decreased the expression levels
of several cholesterol biosynthetic genes, including SQLE, HMGCR,
LSS and DHCR7, and controlled the specific cholesterol
trafficking-associated genes, ABCA1, SOAT1 and PCSK9. α-mangostin
also reduced SREBP2 expression, indicating that SREBP2 is an
essential transcriptional factor in lipid or cholesterol
metabolism, as observed by the decreased amount of SREBP2-SCAP
complex. When exogenous cholesterol was added, α-mangostin reduced
SREBP2 expression and the synthesis of PCSK9 which could increase
cholesterol uptake in cells and provide a feasible explanation of
the cholesterol-reducing properties of α-mangostin (83). Overall, α-mangostin treatment in
HepG2 cells, controlled cholesterol homeostasis through a reduction
in the expression of SREBP2 and its downstream target genes in
cholesterol synthesis (SQLE and IDI1) and cholesterol trafficking
(ABCA1 and PCSK9) (83).
α-mangostin also downregulated the expression levels of FADS1,
FADS2 and ACAT2 involved in the lipid metabolic pathway.
Overall, the molecular effects of α-mangostin in
hepatic tissue are multifaceted and complex, reflecting the key
functions of the liver in metabolism. The improvement in hepatic
structure is associated with decreased collagen deposition and
fibrosis observed by several researchers associated with the
modulation of genes or proteins involved in fibrosis, including
TGF-β1, smad3, TIMP-3, TIMP-1, PAI1, COL1A1, miRNA-155-5p and
α-SMA. α-mangostin also prevents the apoptosis of hepatic tissues,
as demonstrated by the decrease in cleaved caspase-3 and 9-activity
levels in liver cells, regulating hepatic lipid and carbohydrate
homeostasis, reducing fat vacuoles or steatosis, improving liver
function, and preventing hepatic inflammation and oxidative stress,
as well as upregulating hepatic autophagy. The molecular mechanisms
of α-mangostin in hepatoprotective effects are summarized in
Fig. 3 and Table III.
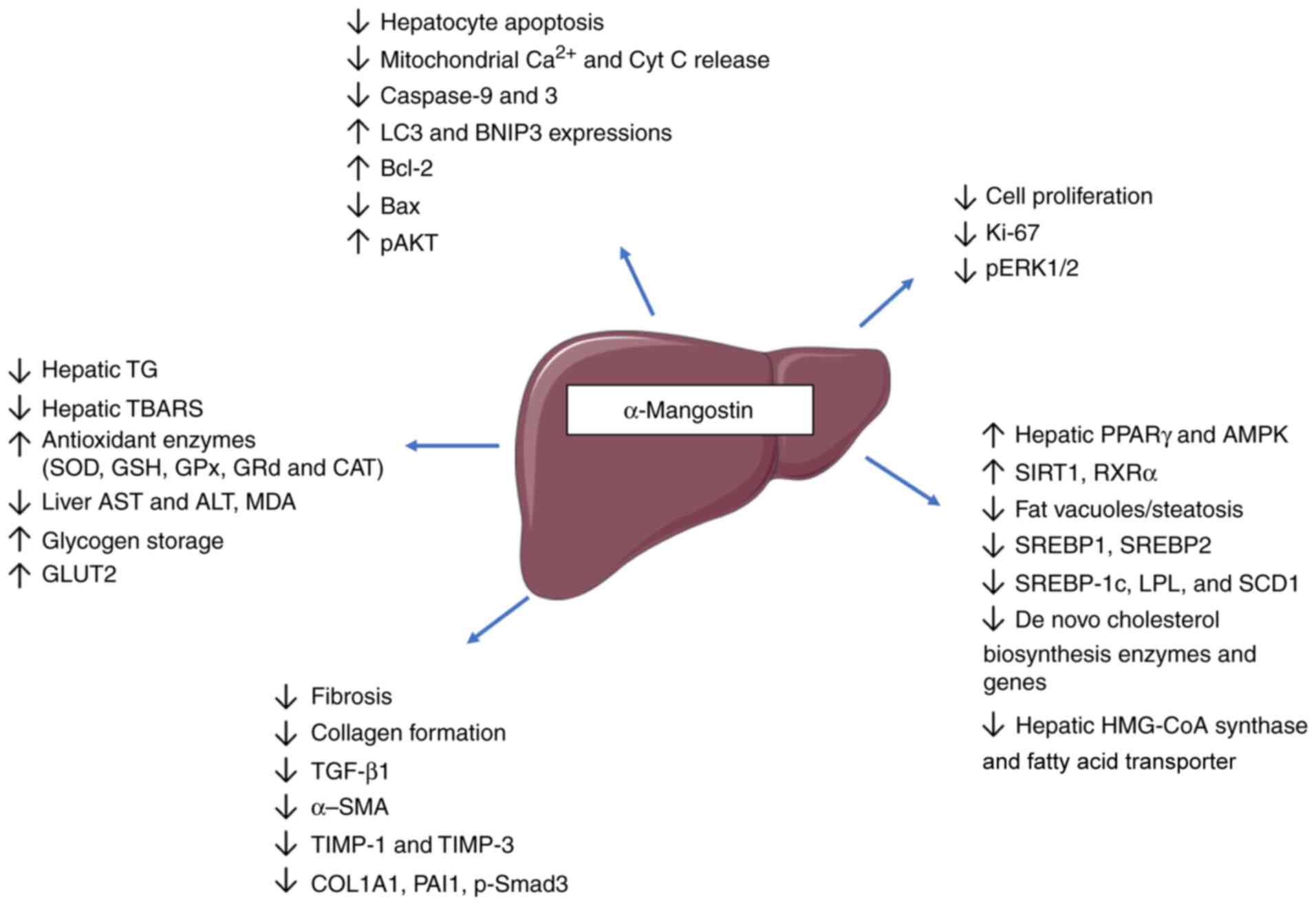 | Figure 3Anti-steatotic and hepatoprotective
effects of α-mangostin. The improvement in hepatic structure and
function by α-mangostin is mediated through decreased collagen
deposition and fibrosis, affecting related genes/proteins (TGF-β1,
Smad3, TIMP-3, TIMP-1, PAI1, COL1A1, miRNA-155-5p and α-SMA).
α-mangostin also prevents the apoptosis of hepatic tissues,
regulates hepatic lipid and carbohydrate homeostasis via AMPK,
PPARγ, SIRT1 and RXRα, reduces steatosis, improves liver function,
prevents inflammation and oxidative stress and upregulates hepatic
autophagy. TIMP, tissue inhibitor of metalloproteinases; PAI1,
plasminogen activator inhibitor-1; COL1A1, collagen type I alpha 1
chain; α-SMA, α-smooth muscle actin; TG, triglyceride; TBARS,
thiobarbituric acid reactive substances; SOD, superoxide dismutase;
GSH, glutathione; GPx, glutathione peroxidase; GRd, glutathione
reductase; CAT, catalase; AST, aspartate aminotransferase; ALT,
alanine aminotransferase; MDA, malondialdehyde; GLUT, glucose
transporter; PPARγ, peroxisome proliferator-activated receptor γ;
SIRT1, sirtuin 1; RXRα, retinoid-X-receptor α; SREBP, sterol
regulatory element-binding transcription factor; LPL, lipoprotein
lipase; SCD1, stearoyl-CoA desaturase-1; HMG-CoA, β-hydroxy
β-methylglutaryl-CoA. |
 | Table IIIAnti-steatotic and hepatoprotective
effects of a-mangostin. |
Table III
Anti-steatotic and hepatoprotective
effects of a-mangostin.
| Authors | Source of
α-mangostin | Model
in-vitro/in vivo | Dosage and
duration | Mechanisms of
action of hepatoprotective effects | (Refs.) |
|---|
| Tsai et
al | Mangosteen pericarp
extract | Sprague-Dawley; Rat
primary hepatocytes | 25 mg/day; rat 11
weeks 10-30 µM; rat hepatocytes 24 h | • ↓Hepatic
TG
• ↓Hepatic TBARS
• ↑Antioxidant enzymes (SOD, GSH, GPx, GRd and
CAT)
• ↑NCCR and SCCR activities in liver tissue Cell
study
• ↑OCR
• ↓tROS and mitoROS
• ↓Mitochondrial Ca2+
and cytochrome c release
• ↓Caspase-9 and -3 | (43) |
| Chae et
al | G.
mangostana peel | Male C57BL/6
mice | 200 mg/kg per day
45 days | • ↑Hepatic
PPARγ, AMPK, SIRT1 | (45) |
| John et
al | G.
mangostana rind | Wistar rats | 168 mg/kg per day 8
weeks | • ↓Hepatic
fat vacuoles and inflammatory cells
• ↓Collagen formation | (12) |
| Mohamed et
al | G.
mangostana extract | Balb/c mice | Group III, 50 mg/kg
of α-mangostin per day for 16 weeks Group IV, 50 mg/kg of
α-mangostin per day for the last 2 weeks | • ↓Liver
weight coefficient
• ↓Liver AST and ALT
• ↓Plasma cholesterol, triglycerides and LDL-C,
↑HDL-C
• ↑Hepatic structure and function
• ↓Hepatic fibrosis
• ↑Glycogen storage
• ↑Autophagy process
• ↓Hepatocyte apoptosis (caspase 3)
• ↓CD68-positive macrophages
• ↓p62 expression
• ↓LC3 expression
• ↓α-SMA expression | (46) |
| Muhamad Adyab et
al | G.
mangostana flesh | Sprague Dawley
rats | 200-600 mg/kg (α
mangostin concentration not detailed) 7 weeks | • ↓Hepatic
lipid accumulation | (44) |
| Rusman et
al | G.
mangostana peel infusion | Wistar rats | 0.25-2% 1
month | • ↓Hepatic
structural damage induced by H2O2 | (69) |
| Fu et
al | G.
mangostana fruit rind |
lipopolysaccharide/d-galactosamine
(LPS/D-GalN)-induced acuteliver failure mice model | 12.5, 25 mg/kg 7
days | • ↑Liver
morphology
• ↓Hepatic MDA, ALT and AST | (72) |
| Abood et
al | G.
mangostana peel | Sprague-Dawley
rats | 250 mg/kg per day
and 500 mg/kg per day 8 weeks | • ↓Liver
index
• ↓Hepatocyte proliferation (PCNA staining)
• ↓Hepatic fibrosis
• ↓α-SMA, TGF-β1
• ↓Serum bilirubin
• ↓Total protein, albumin and liver enzymes (ALP, ALT and
AST) | (73) |
| Hassan et
al | G.
mangostana fruit rind | Wistar rats | 500 mg/kg per day
for 30 days after irradiation | • ↑Liver
function test
• ↑Liver morphology | (70) |
| Yan et
al | Purified
α-mangostin | ICR mice exposed to
acetaminophen, acute liver injury model | 100 and 200 mg/kg 7
days | • ↑Liver
morphology
• ↓Expression of LC3, BNIP3
• ↑expression Bcl-2
• ↓Bax and cleaved caspase 3
• ↑p-mTOR, ↑ p-AKT
• ↓LC3 II/LC3 I ratio in autophagy signaling pathways in
mouse liver
• ↓Degradation of p62/SQSTM1 protein | (71) |
| Rodniem et
al | Purified
α-mangostin |
Thioacetamide-induced hepatic fibrosis rat
model | 5 mg/kg (twice a
week) 8 weeks | • ↓TGF-β1,
α-SMA, TIMP-1 | (31) |
| Rahmaniah et
al | Purified
α-mangostin | Human hepatic
stellate cells, LX-2 | (5 or 10 µM)
24 h | • ↓TGF-β
concentration
• ↓Ki-67 and p-Akt expression
• ↓Expression of COL1A1, TIMP1, PAI1, α-SMA
• ↓p-Smad3 as fibrogenic markers | (80) |
| Lestari et
al | Purified
α-mangostin | Acetaldehyde
induced human hepatic stellate cells (HSC), LX-2 | (10 µM) 24
h | •
↓proliferation and migration of HSC
• ↓Ki-67
• ↓pERK1/2
• ↓TGF-β
• ↓COL1A1
• ↓TIMP1 and TIMP3
• ↑Expression MnSOD and GPx
• ↓α-SMA
• ↓ROS | (82) |
| Kim et
al | Purified
α-mangostin | C57BL/6 mice
RAW264.7 macrophages Mesenteric adipose tissue culture | 50 mg/kg per day 12
weeks 25 µM/ml 24 h | • ↓Lipid
droplets
• ↓Liver tissue weight
• ↓Liver triglyceride
• ↓SREBP1, SREBP2
• ↓Expression of hepatic SREBP-1c, LPL and SCD1
• ↑Liver functions (AST and ALT) | (25) |
| Li et
al | Purified
α-mangostin | Mouse derived
RAW264.7 macrophage 3T3-L1 preadipocytes Male C57BL/6J | 25 and 50 mg/kg per
day 8 weeks (old mice) | •
↓MicroRNA-155-5p from macrophages, eWAT and serum
• ↓AST, ALT
• ↑p-AKT
• ↓Liver injury | (42) |
| Choi et
al | Purified
α-mangostin | Male CB57L/6
mice | 50 mg/kg per day 6
weeks | • ↓Hepatic
TG
• ↓Hepatic fat accumulation
• ↓Serum AST and ALT | (24) |
| Chae et
al | Purified
α-mangostin from G. mangostana | HepG2 cell lines
Hur7 cells | 0.8, 1, 10, 20
µM 24 h | • ↓FDFT1,
SQLE, LSS, CYP51A1, MSMO1, HSD17B7 and DHCR7 (de novo
cholesterol biosynthesis)
• ↓PCSK9, SQLE, HMGCR and LSS (enzyme-encoding metabolic
genes)
• ↓DHCR7, FDFT1, FDPS, HMGCR, IDI1, PCSK9, SQLE and SREBP2
(cholesterol biosynthesis)
• ↓SCAP-SREBP2 complexes formation in endoplasmic reticulum
and Golgi
• ↑Cholesterol and LDL-C uptake
• ↓FADS1, FADS2 and ACAT2 expression | (83) |
| Shibata et
al | G.
mangostana extract rich in α-mangostin | Male
Apoe−/− mice | 0, 0.3, 0.4% of
α-mangostin 17 weeks | • ↓Body
weight
• ↓Hepatic HMG-CoA synthase and fatty acid
transporter
• ↓Hepatic steatosis
• ↑Serum lipoprotein lipase | (81) |
| Ibrahim et
al | α-mangostin
(Cratoxylum arborescens) | ICR female and male
mice Human Normal hepatic cells (WRL-68) | 100, 500 and 1,000
mg/kg body weight IC50, 65 mg/ml | • No
toxicity | (19) |
5. Cardioprotective and anti-atherogenic
effects of α-mangostin
Garcinia mangostana pericarp extract has been
previously suggested to counteract the effects of
NG-nitro-L-arginine methyl ester in a hypertensive rat model
(84). The administration of
G. mangostana extract at a concentration of 200 mg/kg per
day partially attenuated the effects of the drug by reducing
hypertension, arterial wall thickness, and cardiovascular
remodeling. The treatment also attenuated oxidative stress activity
induced by the drug by decreasing plasma MDA, increasing plasma
nitric oxide (NO) metabolites and reducing
p47phoxNADPH oxidase subunit and inducible
nitric oxide synthase (iNOS) protein expression in aortic tissues
(84). The addition of G.
mangostana rind in powder to the diet of obese rats (daily
intake equivalent to α-mangostin concentration of 168 mg/kg per
day) resulted in improved cardiovascular structures by reducing
fibrosis and collagen deposition. Cardiac structure improvement was
accompanied by a reduction in cardiac stiffness, as measured by
Langendorff's isolated heart contraction assessment. The treatment
also improved aortic endothelial tissue activity, while lowering
blood pressure (12).
Pre-treatment with α-mangostin (200 mg/kg per body
weight per day) for 8 days in Wistar rats significantly reduced
cardiac TNF-α and cyclooxygenase (COX)-2 expression induced by
Isoproterenol (ISO). α-mangostin also reduced lysosomal hydrolases
in both serum and cardiac tissues, preserved myocardial membrane
integrity through restoring membrane-bound phosphatases function,
and reduced ISO-induced oxidative stress and cellular damage
(32). In another study,
pre-treatment with α-mangostin (200 mg/kg/day) for 8 days in Wistar
rats decreased the functional abnormalities and mitochondrial
function disturbance induced by ISO (72). Treatment with α-mangostin improved
cardiac endothelial NOS (eNOS) expression and NO concentration. The
administration of α-mangostin also increased cardiac mitochondrial
cytochrome c, c1, b and aa3 levels, and improved NADH
dehydrogenase and cytochrome c oxidase activity. The reduction of
lipid peroxides in the treatment group was associated with enhanced
antioxidant enzyme activity. The findings have suggested that
α-mangostin may present with cardioprotective effects in myocardial
cells by upregulating oxidative mitochondrial enzymes (85).
In an Apoe-deficient mouse model, α-mangostin
(0.3 and 0.4%) reduced TC, triglycerides (0.4%) and VLDL-C
(81). The treatment also reduced
the risk of atherosclerosis by decreasing the deposition of
cholesterol in the aortic arch, aortic hiatus and renal artery
bifurcation area, and improved hepatic lipid droplets. The level of
lipoprotein lipase enzyme was significantly increased in the
0.4%-concentration group; however, no changes were observed in the
serum level of the proatherogenic indicator, soluble lectin-like
oxidized LDL receptor-1. Macrophages analysis revealed that the
expression of Cd163 gene (M2 macrophage marker) was
increased, whereas CD68 levels (pan-macrophage marker) and Nos1 (M1
macrophage marker) were not significantly altered. The M2
macrophage populations were more frequent in atherosclerotic
lesions exposed with 0.4% treatment. Inflammatory cytokine levels
(IFN-γ, TNF-α and IL-1β) exhibited a decreasing trend, while the
IL-13 (M2 polarizing cytokine) was increased (81). The accumulation of M1 is a
hallmark for atherosclerosis progression, however, the M2 type is
predominant in atherosclerosis recession. Plausibly, α-mangostin
has been suggested to induce the microenvironment for M2
polarization observed in the treatment group and subsequently
reduce the development of atherosclerotic lesions (81).
A previous study using human umbilical vein
endothelial cells (HUVECs) grown in a high glucose environment
treated with α-mangostin has revealed the treatment decreased high
glucose-induced ROS formation and high glucose-induced apoptosis.
Further analysis then revealed that α-mangostin reduced apoptosis
through the suppression of JNK and p38-MAPK pathway via the
inhibition of JNK and p38-MAPK phosphorylation (86). Another study using HUVECs grown in
high glucose (60 mM) significantly reduced cellular viability and
increased reactive oxygen species and cellular senescence through
the reduction of senescence-associated β-galactosidase activity
(87). A high glucose environment
also elevated p53, acetyl-p53 and p21 protein levels and IL-6
secretions; however, it reduced SIRT1 and total AMPK protein
levels. Of note, α-mangostin (1.25 µM) reversed the toxic
effects of high glucose in HUVECs by reducing apoptosis, ROS, IL-6
secretion, p53 expression while increasing SIRT1 expression. These
results demonstrated that in high-glucose conditions, α-mangostin
has demonstrated beneficial effects in endothelial cells,
suggesting protective effects on the vasculature, and
anti-senescence effects, most likely due to its antioxidant
activity through the SIRT1 pathway. Thus, α-mangostin may act as a
natural agent to protect against high glucose-induced vascular
damage in diabetic patients (87).
In another study, the use of α-mangostin to treat
CoCl2-induced hypoxic injury in H9C2 cardiomyocytes
demonstrated increased cell viability in the treatment group, with
the concentration of 0.06 mM being the most effective concentration
(88). Additionally, the
treatment also reduced ROS and MDA, while increasing SOD levels.
α-mangostin treatment reduced the number of apoptotic cells treated
with CoCl2. RT-qPCR analysis further revealed that the
treatment also increased expression of Bcl-2, while reducing gene
expression of Bax, caspase-3 and-9, involved in apoptosis. This
finding was in accordance with the reduction of protein levels of
Bax, caspase-3 and caspase-9, and revealed that α-mangostin exerted
cardioprotective effects by reducing apoptotic genes and oxidative
stress (88).
Hyperglycemia affects the vascular system, leading
to vascular complications, which are the leading causes of
mortality among individuals with diabetes. The damage to the
endothelial stems from an aberrant accumulation of ceramide
(59), occurs when NO production
is impaired (79). NO is a
natural molecule produced by endothelial cells that controls the
vascular tone in a paracrine manner (89). Hyperglycemia activates the acid
sphingomyelinase (aSMase)/ceramide pathway. The activation of this
pathway leads to ROS generation, inhibiting NO production in
endothelial cells (90) and
affecting the vascular tone. Ceramide accumulation and its
metabolites exert damaging effects on insulin sensitivity,
pancreatic β-cell function, vascular reactivity and mitochondrial
metabolism (91).
In a previous study, diabetic mice treated with
α-mangostin (10 mg/kg) for 12 weeks presented with limited aSMase
activity and ceramide deposition in the aortas and partially
improved vascular dysfunction (79). The endothelial vascular
dysfunction was also improved through eNOS/NO pathway as
α-mangostin increased the expression of phosphorylated eNOS in
diabetic mouse aortas. In isolated aortas, α-mangostin was also
reported to prevent the activation of aSMase/ceramide pathway
induced by high glucose. Following a testing of the compound in
high glucose environment endothelial cell culture, α-mangostin
reversed the upregulation of aSMase/ceramide pathway. That study
suggested that α-mangostin may improve endothelial dysfunction, by
affecting the aSMase/ceramide pathway (79). α-mangostin could exert this effect
as it has been revealed to be a competitive inhibitor of the aSMase
enzyme (92). The restoration of
the vascular function resulted from increased NO generation and
eNOS phosphorylation. α-mangostin has also been proposed to reduce
endothelial vasoconstrictor, endothelin-1 (ET-1) (63). ET-1 is overexpressed due to
hyperglycemia-induced oxidative stress and the generation of free
radicals, and enhances vascular resistance (93,94).
In a previous study using a rat model of
doxorubicin-induced cardiotoxicity, treatment with α-mangostin (100
and 200 mg/kg) was suggested to improve electrocardiograph
recordings, heart/body weight ratio and histological structures,
increase systolic blood pressure, decrease MDA levels, improved the
GSH level and normalize creatine kinase-MB (CK-MB) levels and
lactate dehydrogenase (LDH) compared to doxorubicin-treated (DOX)
rats (95). CK-MB detection in
serum is a sensitive indicator in the early stages of cardiac
damage, whereas LDH levels will increase when further cardiac
damage occurs (96,97). As previously demonstrated,
α-mangostin (100 mg/kg) reduced the ratio of Bax/Bcl-2 as compared
to DOX in heart tissues. It also decreased apoptotic protein
caspase-9 and-3 levels, and myocardial IL-1β and TNF-α expression
levels (95). Similarly, Soetikno
et al (98) revealed that
treatment with α-mangostin (100 and 200 mg) decreased CK-MB, LDH,
blood pressure and cardiac proinflammatory cytokine levels (TNFα,
MCP-1, IL-6 and IL-1β), by inhibiting the infiltration of cardiac
tissue with immune cells, and decreasing cardiac hypertrophy and
fibrosis in rats with STZ-induced diabetes.
In a prospective cohort study, patients with
high-risk Framingham Score treated with 2,520 mg of G.
mangostana extract daily for 90 days, exhibited reduced MDA
levels and increased SOD levels, as compared to the placebo group
(99). The excessive formation of
ROS can trigger the production of MDA, as a result of lipid
peroxidation, endogenous antioxidants combined with exogenous
antioxidant compounds could counteract MDA formation. The
anti-atherogenic effects observed in that study could most possibly
be attributed to the antioxidant properties of xanthones from G.
mangostana, resulting in the inhibition of LDL oxidization and
MDA formation (99).
In an animal model fed a high-cholesterol diet, the
administration of 400 and 800 mg/kg ethanolic extract of mangosteen
pericarp, rich in α- and γ-mangostin, significantly counteracted
the effects of the high-cholesterol diet by reducing
H2O2 plasma concentration, increasing CAT
activity and inhibited the formation of foam cells in the aorta
(100). The reduction of plasma
H2O2 could possibly be attributed to the
increased conversion of this compound into oxygen and water.
However, it could also be potentiated by the antioxidant activities
of phytocompounds in the pericarp extract (100). Another study using an animal
model similar to the aforementioned one demonstrated that daily
treatment with a 400 and 800 mg/kg body weight dose of ethanolic
extract of mangosteen pericarp, reduced NF-κB and iNOS levels,
while maintaining eNOS activity in treated rats (101).
Furthermore, Wistar rats fed a high-fat diet and
treated with 200, 400 and 800 mg/kg body weight of ethanolic
extract for 8 weeks exhibited a decreased thickness of aortic
perivascular adipose tissue and reduced thickening of tunica
intima-media compared to control high fat group (102). Smooth muscle vascular cell
adhesion protein 1 expression was significantly decreased in
treated rats, according to the evaluation by double-staining
immunofluorescence. Additionally, HDL-C levels were increased and
LDL-C levels were reduced in treated rats, along with the reduction
of TG and TC, particularly in the 400 and 800 mg/kg groups
(102).
In summary, α-mangostin exhibits cardioprotective
activities through various mechanisms, including: i) Blood
pressure, arterial wall thickness and cardiovascular remodeling
reduction; ii) improvement of cardiovascular structures by reducing
fibrosis and collagen deposition and cardiac stiffness; iii)
reduction of lysosomal hydrolases in both serum and cardiac
tissues; iv) preservation of myocardial membrane integrity by
restoring membrane-bound phosphatases function; v) restoration of
mitochondrial functions; vi) reduction of atherosclerosis risk by
increasing M2 macrophage populations in atherosclerotic lesions;
vii) reduction of cardiac and endothelial cell apoptosis through
pathways including suppression of JNK and p38-MAPK pathway; viii)
reduction of endothelial cell senescence through activation of
SIRT1; ix) reduction of aortic aSMase and ceramide deposition; x)
improvement of cardiac and aortic eNOS expression and NO
concentration and reduction of iNOS and NFκB expressions while
maintaining eNOS expression; xi) reduction of CK-MB and lactate
dehydrogenase; xii) reduction of aortic perivascular adipose tissue
and tunica intima-media thickening; and xiii) reduction of
inflammation and oxidative stress. The molecular mechanisms of the
cardioprotective and anti-atherogenic effects of α-mangostin are
summarized in Fig. 4 and Table IV.
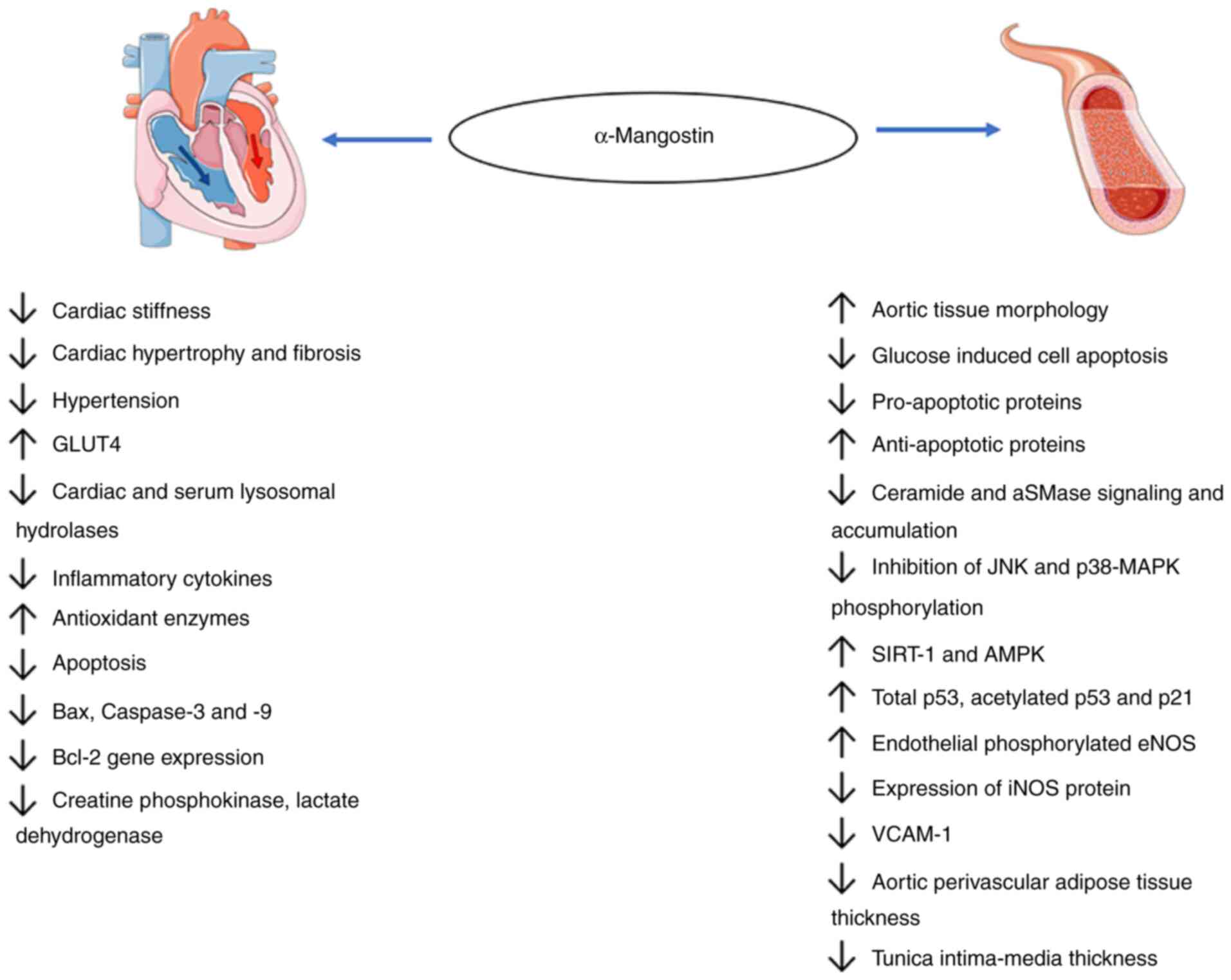 | Figure 4Cardioprotective and anti-atherogenic
effects of α-mangostin. α-mangostin protects the heart and blood
vessels against several stressors, including reactive oxygen
species, drug-induced stressors, lipids, aSMase activity,
hyperglycemia and various potentially harmful signaling pathways.
It lowers the levels of inflammatory cytokines and proapoptotic
proteins, such as Bax and caspase-3 and-9, leading to tissue death,
lactate accumulation and aSMase/ceramide signaling. α-mangostin
increases the levels of anti-apoptotic proteins (p53) and
antioxidant enzymes. It restores the heart and blood vessel
morphology by reducing creatine kinase-MB and lactate
dehydrogenase, reducing aortic perivascular adipose tissue
deposition and reducing VCAM-1 expression, tunica intima-media
thickening. aSMase, acid sphingomyelinase; VCAM-1, vascular cell
adhesion molecule 1; GLUT, glucose transporter; SIRT1, sirtuin
1. |
 | Table IVCardioprotective and anti-atherogenic
effects of a-mangostin. |
Table IV
Cardioprotective and anti-atherogenic
effects of a-mangostin.
| Authors | Source of
α-mangostin | Model
in-vitro/in-vivo | Dosage and
duration | Mechanism of action
on cardioprotective effects | (Refs.) |
|---|
| Sampath and
Vijayaragavan | Purified
α-mangostin | Isoproterenol
induced-myocardial necrosis Wistar rats | 200 mg/kg/body
weight 8 days | • ↓TNF-α and
COX-2
• ↓Activities of membrane-bound phosphatases
• ↓Cardiac and serum lysosomal hydrolases | (32) |
| Sampath and
Kannan | Purified
α-mangostin | Isoproterenol
induced-myocardial necrosis; Wistar rats | 200 mg/kg/body
weight (pre-treatment) 8 days | • ↑Cytochrome
c, c1, aa3 and b levels
• ↑NADH dehydrogenase and cytochrome c oxidase
activities
• ↑Antioxidant enzymes (GSH, GPx, GST, SOD, CAT)
• ↓Lipid peroxides
• ↑Cardiac eNOS | (85) |
| Jittiporn et
al | α-mangostin
extracted from G. mangostana peel | Human umbilical
vein endothelial cells | 10-100 nM 72 h | • ↓ROS, ↓
apoptosis
• ↓Inhibition of JNK and p38-MAPK phosphorylation | (86) |
| Fang et
al | Purified
α-mangostin | CoCl2-induced
apoptotic damage H9C2 cardiomyoblasts | 0.012, 0.06, 0.3,
0.6 or 1.2 mM 24 h | • ↓ROS,
MDA
• ↑SOD
• ↓Apoptosis
• ↓Bax, caspase-9 and caspase-3 gene expression and
protein
• ↓Bcl-2 gene expression | (88) |
| Tousian et
al | Purified
α-mangostin | Human umbilical
vein endothelial cells | 1.25 µM
(non-toxic xconcentration) 6 days | • ↑Total p53,
acetylated p53 and p21
• ↓SA-β-GAL
• ↑SIRT-1 and AMPK | (87) |
| Jiang et
al | Purified
α-mangostin | Primary aortic
endothelial cells C57BL/KsJ; diabetic (db/db) mice | 10 mg/kg/day, i.p.;
mice 12 weeks 15 µM α-mangostin; cell culture 24 and 48
h | • ↓Serum aSMase and
ceramide
• ↓Aortic aSMase and ceramide
• ↑Endothelial cell NO production
• ↑Endothelial phosphorylated eNOS | (79) |
| Eisvand et
al | Purified
α-mangostin | Doxorubicin-induced
cardiotoxicity rat model Heart cells MC7 cells | 50, 100, 200 mg/kg
per day 19 days | • ↓MDA, caspase-3
and -9
• ↓Inflammatory markers
• ↑Heart weight
• ↓Creatine phosphokinase, lactate dehydrogenase
• ↓IL-1β and TNF-α | (95) |
| Soetikno et
al | Purified
α-mangostin | Wistar rat | 100, 200 mg/kg per
day 8 weeks | • ↓CK-MB,
LDH
• ↓Prevent weight loss in diabetic rats
• ↓Blood pressure
• ↓AST and ALT
• ↓Total cholesterol and triglyceride
• ↓Cardiac pro-inflammatory levels (TNFα, MCP-1, IL-6,
IL-1β)
• ↓Cardiac hypertrophy and fibrosis | (98) |
| Shibata et
al | G.
mangostana extract rich in α-mangostin | Male
Apoe−/− mice | 0%, 0.3%, 0.4% of
α-mangostin; 17 weeks | • ↑Aortic tissue
morphology
• ↓Total cholesterol (VLDL)
• ↓Triglyceride
• ↑Serum lipoprotein lipase
• ↑CD163
• ↑IL-13
• ↑M2 polarization
• ↓IFN-γ, TNF-α and IL-1β | (81) |
| John et
al | G.
mangostana rind rich in α-mangostin | Wistar rats (high
carbohydrate, high fat) | 168 mg/kg per day 8
weeks | • ↓Systolic blood
pressure
• ↓Cardiac stiffness
• ↓Cardiac hypertrophy and fibrosis
• ↑Endothelial tissue activity | (12) |
| Boonprom et
al | G.
mangostana pericarp extract | Sprague-Dawley rats
(L-Name induced hypertension) | 200 mg/kg per day
(extract) concentration (extract) concentration of α-mangostin; not
detailed; 5 weeks | • ↓Hypertension and
cardiovascular remodeling
• ↓Oxidative stress (MDA) and inflammation (TNF-α)
• ↓Expression of p47phoxNADPH oxidase
subunit
• ↓Expression of iNOS protein in aortic tissues
• ↓Arterial wall thickness
• ↑Plasma NO metabolites | (84) |
| Ismail et
al | G. mangstana
Pericarp extract | Patients with
high-risk Framingham C score | 2,520 mg/day
(extract) oncentration of α-mangostin; not detailed; 90 days | • ↑Plasma
SOD
• ↓Plasma MDA
• ↓Atherosclerosis risk | (99) |
| Adiputro et
al | G.
mangostana Ethanolic pericarp extract | Wistar rats
(High-chole-sterol diet) | 200, 400, 800 mg/kg
per day (containing 0.064% α-mangostin and 6.144% of γ-mangostin)
(Treatment duration not stated) | • ↓Plasma
H2O2
• ↑Plasma CAT
• ↓Foam cells | (100) |
| Wihastuti et
al | G.
mangostana Ethanolic pericarp extract | Wistar rats
(High-chole-sterol diet) | 200, 400, 800 mg/kg
per day 3 months | • ↓NF-κB
• ↓iNOS
• Maintain eNOS | (101) |
| Wihastuti et
al | G.
mangostana Ethanolic pericarp extract | Wistar rats
(High-chole-sterol diet) | 200, 400, 800 mg/kg
per day 2 months | • ↓Aortic
perivascular adipose tissue thickness
• ↓Tunica intima-media thickness
• ↓VCAM-1 expression | (102) |
6. Antioxidant effects of α-mangostin
Antioxidants are of physiological importance as
they reduce ROS generation, and are linked to tissue damage, aging
and chronic inflammation (103).
The human body has an antioxidant system involving SOD, GPx and
CAT. These enzymes function together with SOD, converting the
superoxide anion (O2-) to H2O2,
which GPx and CAT convert in turn to water. Another antioxidant
protein is GSH, that reduces ROS accumulation (104). According to a previous study,
α-mangostin was reported to exert protective effects against
oxidative stress by modulating the production of SOD, GPx, GSH and
CAT, via the nuclear factor-erythroid 2-related factor 2 (Nrf2)
transcription factor which targets genes involved in antioxidant,
detoxification, metabolism and inflammatory pathways (104).
Fang et al (105) used a mouse light damage model to
induce retinal death via the production of
H2O2. H2O2 produces
oxidative stress, acting as ROS and activates caspase 3, leading to
apoptotic reactions. Treatment with α-mangostin (30 mg/kg)
increased Nrf2 translocation to the nucleus following light
exposure to, inducing the expression of antioxidant genes, reducing
cleaved caspase-3 expression and retinal damage. The interaction of
α-mangostin with Nrf2 is a common mechanism, inducing antioxidant
expression, leading to resistance to oxidant stress. In the same
mouse experiment, treatment with α-mangostin restored the levels of
SOD, GPx and GSH. In the same study, retinal pigment epithelia 19
(ARPE-19) cells exposed to H2O2 were used to
induce cytotoxicity. Pre-treatment with α-mangostin led to a
reduced apoptosis in a dose-dependent manner (4-12 µM), with
an apoptotic rate of 5.85% observed at 12 µM. α-mangostin
reduced ROS production, as observed by DCF fluorescence in flow
cytometry. A similar effect was observed in cultured cells in terms
of restoring levels of SOD, GPx, GSH, and increasing heme oxygenase
1 (HO-1) expression, revealing the robustness of this molecule
(105).
In a previous study by Fu et al (72) in a mouse model of
lipopolysaccharide (LPS)/d-galactosamine (D-GalN)-induced acute
liver failure, α-mangostin also interacted with Nrf2. In that
study, treatment with α-mangostin resulted in an increased
expression of Nrf2, heme oxygenase 1 (HO-1) hepatic GSH, SOD and
CAT with a reduction in hepatic MDA levels (72). This suggests that α-mangostin
either positively interacts with Nrf2 or negatively with Kelch-like
ECH-associated protein 1 (KEAP1). KEAP1 is a negative regulator of
Nrf2, as it ubiquitinates Nrf2, targeting it for degradation
(106). Further research has
revealed that α-mangostin stimulation induces the dissociation of
KEAP1 from Nrf2 in the cytosol and in vivo, supporting the
notion that α-mangostin could dissociate Nrf2 from KEAP1,
permitting Nrf2 protein accumulation and nuclear translocation
(105).
α-mangostin also has anti-oxidant activity by
inhibiting the aSMase enzyme (79). A high-fat/carbohydrate diet
decreases SOD and GPx levels, favors the accumulation of
glucose-induced ROS, and results in increased levels of the
pro-inflammatory markers, TNF-α and IL-6 (44,79). Increased ROS generation aggravates
the system by activating the aSMase/ceramide pathway, leading to
ceramide-induced cell death. By using primary endothelial cells and
a db/db diabetic mice, Jiang et al (79) revealed that α-mangostin reversed
the high glucose-induced ROS production and aSMase/ceramide pathway
activation by inhibiting the aSMase enzyme, as α-mangostin is a
competitive inhibitor of the aSMase enzyme according to another
study (92). This results in an
upregulation of eNOS/NO pathway in aortas from diabetic mice,
reducing ROS levels and restoring their structure and function
(79). Muhamad Adyab et al
(44), using an obese rat model,
demonstrated that rats fed a high-fat/carbohydrate diet
supplemented with a 200-600 mg/kg dose of mangosteen flesh extract
improved SOD and GPx levels (44).
α-mangostin can counteract the effects of
lactate-induced ROS production via MDA generation, and liver damage
due to ionizing radiation and thioacetamide. In a previous study by
Chang et al (107), rats
subjected to high rates of exhaustive exercise accumulated high
levels of lactate in both liver and muscle tissues, which was
rapidly cleared in rats given α-mangostin. Levels of MDA also
diminished in both tissues (5-fold in liver and 10-fold in muscle)
and levels of CAT and GPx increased in both tissues. Thioacetamide-
and radiation-induced liver damage, as measured by ALT, AST and ALP
liver biomarkers, has been also reported to be reversed in
α-mangostin-treated mice (70,73). Hassan et al (70) previously exposed rats to ionizing
radiation, in order to mimic the situations in which the liver is
damaged due to radiotherapy treatment or accidental exposure.
Radiation caused alterations in liver protein homeostasis and
increased plasma liver markers like ALT, AST and ALP, indicating
liver damage. SOD and CAT levels were significantly reduced after
radiation; however, their levels were restored by treatment with
α-mangostin at the 500 mg/kg equivalent dose per day. MDA and NO
levels in the radiated liver doubled compared to the control;
however, this was reduced to normal levels by α-mangostin
treatment.
Abood et al (73) examined the effects of in
thioacetamide-induced liver cirrhosis in rats. Thioacetamide
increased liver markers, AST, ALP and ALT, increased MDA levels,
and reduced SOD and CAT enzymes, while α-mangostin (250 and 500
mg/kg), significantly reduced the effects of thioacetamide
treatment and conserved the liver, heart and kidney from
thioacetamide damage. In a previously reported experiment by
Lazarus et al (108), SOD
levels increased significantly in the heart with an α-mangostin
dose of 100 mg/kg. However, a significant improvement was observed
with 200 mg/kg treatment in the kidneys. GSH also increased in both
tissues, and α-mangostin reversed MDA levels in the liver, kidneys,
and heart tissues. Further study is still required, in order to
finalize the ideal concentration at which beneficial effects are
seen.
Harliansyah et al (109) also reported on the potential
tissue-specific potency of α-mangostin. By using HepG2 and WRL-68
cells, it was observed that when they were exposed to ROS
stimulating chemicals, α-mangostin reduced ROS levels in both cell
lines at comparable levels in a concentration-dependent manner
(5-1,000 µg/ml). However, when assessing MDA levels,
α-mangostin led to a more significant MDA reduction in the HepG2
cancer line, possibly due to the increased oxidative stress in
comparison with the WRL-68 cell line. Notably, when compared to
WRL-68, a normal human hepatic cell line, HepG2 cell line presented
with a more notable reduction of MDA (109). It is possible that HepG2
presented with more reduced MDA because of the effects of both ROS
that stabilizes Nrf2, and oncogenic signaling via KRAS and BRAF
that has been revealed to induce Nrf2 stabilization (110), leading to enhanced production of
antioxidant proteins. They also analyzed protein carbonyl levels,
evaluating the amount of ROS oxidized protein, and observed that
α-mangostin-treated cells demonstrated decreased ROS levels. The
effect was more intense in the WRL-68 cell line, indicating that
this cell line was more responsive, highlighting the potential
anticancer properties of α-mangostin.
Mitochondria are essential organelles involved in
energetic homeostasis and the production of reactive oxygen
species. In a previous study, treatment of proximal tubule Lilly
laboratory culture porcine kidney (LLC-PK1) cells with
Cis-dichlorodiammineplatinum II (CDDP)-induced damage with
α-mangostin (4 µM) demonstrated that the compound preserved
mitochondrial function and mass (111). α-mangostin inhibited the
CDDP-induced decrease in cell respiratory states, in the maximum
capacity of the electron transfer system and the respiration
associated with oxidative phosphorylation protein, preventing
changes in mitochondrial bioenergetics alterations. It also
prevented mitochondrial mass reduction and fragmentation through
the preservation of the mitofusin 2 fusion marker, reducing
induction of autophagy by CDDP (111), and revealing that α-mangostin
can modulate the ROS production at the organelle level.
In another study, in a model of sodium
iodate-induced ROS-dependent toxicity using ARPE-19 cells,
α-mangostin (3.75, 7.5 and 15 µM) prevented cell death,
although not at the 20 µM dose. α-mangostin also prevented
mitochondrial damage as revealed by JC-1 staining, reduced
intracellular ROS levels and the extracellular
H2O2 concentration, increased CAT and GSH
levels, and decreased SOD levels (112). This treatment also prevented
cell apoptosis through the regulation of apoptosis-related
proteins. α-mangostin treatment protected ARPE cells against sodium
iodate-induced oxidative damage by reducing SIRT-3 expression,
mediated by the PI3K/AKT/PGC-1α signaling pathway. Treatment with
α-mangostin in this mouse model revealed that it could prevent
retinal degradation and apoptosis induced by sodium iodate
(112). The proposed mechanism
was that α-mangostin modulated the SIRT-3 pathway (113). SIRT-3, a member of the sirtuin
family, is a mitochondrial enzyme that modulates deacetylation and
acetylation of mitochondrial enzymes and is known to prevent ROS
and the development of cancerous cells or apoptosis (114). As previously explained,
α-mangostin treatment reduced caspase-3 protease levels, reduced
cell apoptosis, and increased p-PI3K-AKT levels, demonstrating the
protective effects of this compound in the presence of STZ
(52,58) and high glucose (59).
In summary, the antioxidant effects of α-mangostin
are exerted primarily through the stabilization of cytoplasmic
Nrf2, the increase in heme oxygenase 1 (HO-1) expression, the
modulation of the aSMase/ceramide pathway, kinase signaling
pathways (p38 MAPKs and JNK kinases) and the acetylation activity
of SIRT-3 enzymes. The mechanisms through which α-mangostin affects
these enzymes remain unknown. However, a recent study investigating
α-mangostin and α-glucosidase suggested, that in the presence of
α-mangostin, α-glucosidase has a more α-helical secondary
structure, making it more compact and decreasing its catalytic
activity (65). Acting via these
mechanisms, antioxidant enzymes including SOD, GPx, CAT, GSH are
increased and oxidative stress markers including MDA and ROS are
reduced. There is also a mechanism controlling the apoptotic
pathways, protecting cells from ROS induced caspase 3 cell damage.
α-mangostin also protects the mitochondria via the preservation of
mitochondrial respiratory processes, leading to reduced ROS
production and improvement in cell homeostasis. The antioxidant
effects of α-mangostin are summarized in Fig. 5 and Table V.
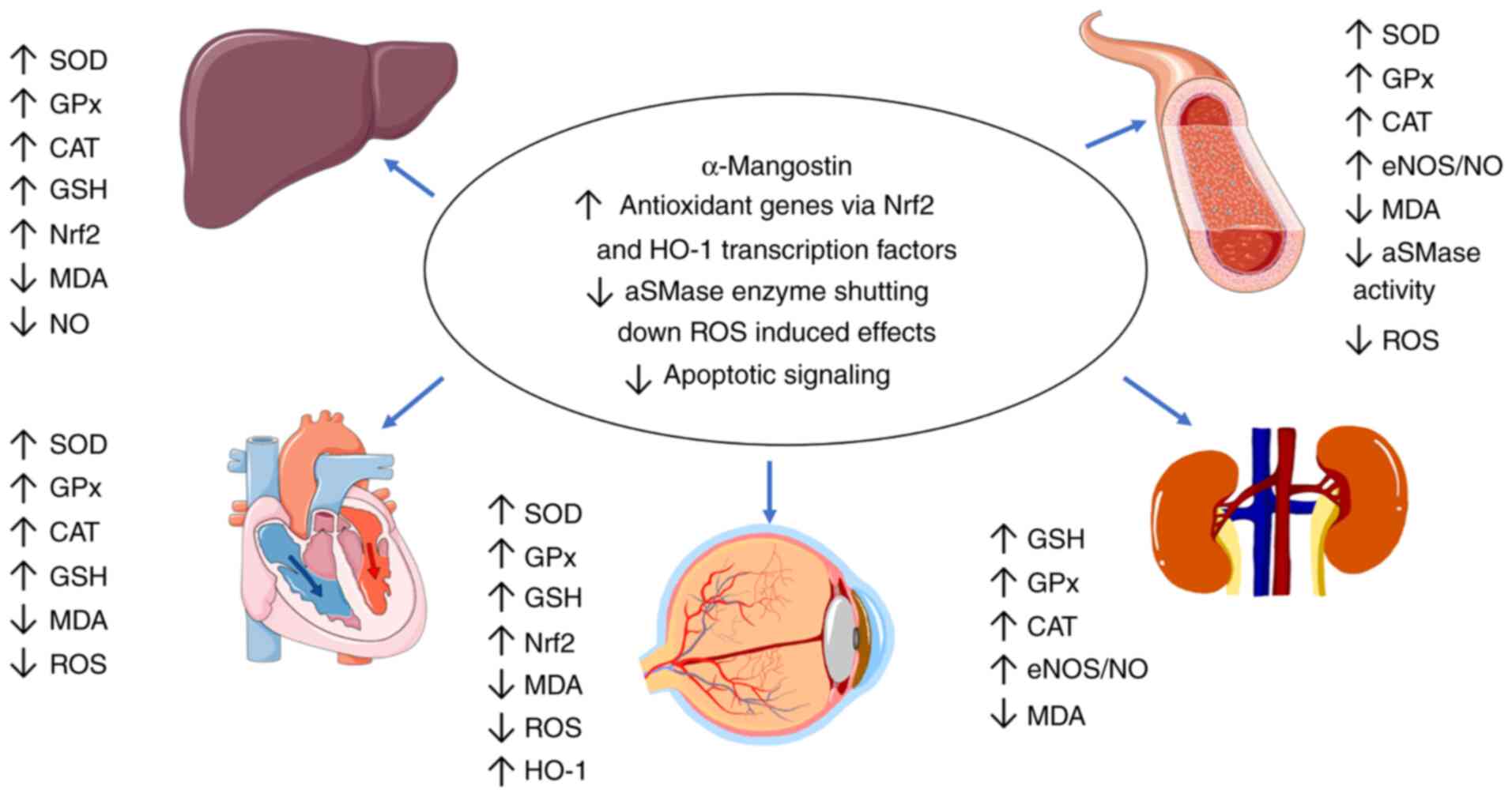 | Figure 5Antioxidant mechanisms of
α-mangostin. α-mangostin exhibits antioxidant activity via three
main mechanisms as demonstrated in the present review. It
stabilizes Nrf2, which is a transcription factor that leads to the
production of antioxidant proteins. It inhibits aSMase activity and
modulates various signaling pathways. These actions in turn
increase production of SOD, GPx, CAT and GSH in various tissues,
leading to a decrease in ROS and MDA levels. aSMase, acid
sphingomyelinase; ROS, reactive oxygen species; SOD, superoxide
dismutase; GSH, glutathione; GPx, glutathione peroxidase; CAT,
catalase; MDA, malondialdehyde; NO, nitric oxide; Nrf2, nuclear
factor-erythroid 2-related factor 2; HO-1, heme oxygenase 1; eNOS,
endothelial nitrix oxide synthase. |
 | Table VAntioxidant effects of
a-mangostin. |
Table V
Antioxidant effects of
a-mangostin.
| Authors | Source of
α-mangostin | Model In
vitro/in vivo | Dosage and
duration | Mechanisms of
action of antioxidant effects | (Refs.) |
|---|
| Chang et
al | Mangosteen
concentrate drink | Sprague-Dawley
rats | 13 mg/day 6
weeks | • ↓Hepatic
MDA
• ↓Hepatic and muscular GPx
• ↓Hepatic and muscular SOD
• ↓Hepatic and muscular CAT | (107) |
| Jiang et
al | Purified
α-mangostin | C57BL/KsJ; diabetic
(db/db) mice primary aortic endothelial cells | 10 mg/kg/day; i.p.;
mice 12 weeks 15 µM α-mangostin; cell culture 24 and 48
h | • ↓Hepatic
TBARS
• ↑Hepatic antioxidant enzymes (GSH, GPx, GRd, SOD, CAT)
• ↓Primary hepatocytes (TBARS, tROS, mitoROS, cytocrhome
c)
• ↑Primary hepatocytes mitochondrial function (NCCR mito complex I
and III) and SCCR (mito complex II and III)
• ↑Primary hepatocytes mitochondrial oxygen consumption rate | (79) |
| Abood et
al | G.
mangostana peel extract | Sprague-Dawley
rats | 250 and 500 mg/kg
per day (α-mangostin concentration not detailed) 8 weeks | • ↓Hepatic
MDA
• ↑Hepatic SOD and CAT | (73) |
| Hassan et
al | G.
mangostana fruit rind | Wistar rats | 500 mg/kg per day
for 30 days after irradiation | • ↓Hepatic MDA, NO,
SOD and CAT | (70) |
| Yan et
al | G.
mangostana fruit hull | ICR mice induced by
acetaminophen, acute liver injury model | 100 and 200 mg/kg 7
days | • ↑Serum AST,
GSH
• ↓Serum MDA | (71) |
| Fu et
al | G.
mangostana fruit rind |
Lipopolysaccharide/d-galactosamine
(LPS/D-GalN)- induced acute liver failure mouse model | 12.5, 25 mg/kg 7
days | • ↓Hepatic
MDA
• ↑Hepatic GSH, SOD, CAT
• ↑Expression of Nrf2 and HO-1 | (72) |
| Fang et
al | α-mangostin
powder | Hydrogen peroxide
(H2O2)-stressed RPE cells, human retinal
pigment epithelial cell line, light-damaged mice model | 10 and 30 mg/kg;
mice; 7 days 10 µM; cell culture 24 h | • ↓MDA
• ↑SOD, GPx, Gsh
• ↑Expression Nrf2 and HO-1
• ↑Expression PKC-δ
• ↓Expression of MAPK, ERK1/2, JNK, P38 | (105) |
| Harliansyah et
al | α-mangostin
powder | HepG2 Cells and
WRL-68 cells | 5-1,000
µg/ml 24 h | • ↓MDA
• ↓Protein carbonyl
• ↓ROS | (109) |
| Lazarus et
al | α-mangostin
compound | Wistar rats | 100, 200 mg/kg per
day 8 weeks | • ↓MDA (hepatic,
heart, kidney)
• ↑SOD, GSH | (108) |
| Tousian et
al | Purified
α-mangostin | Human umbilical
vein endothelial cells | 1.25 µM 6
days | • ↓ROS | (87) |
| Reyes- Fermín et
al | Purified
α-mangostin | CDDP-induced damage
in proximal tubule Lilly laboratory culture porcine kidney
(LLC-PK1) cells | 4 µM 24
h | • ↓CDDP-induced
cell death
• ↓Respiratory states alterations
• ↑MFN2 fusion marker
• ↓Mitochondrial mass reduction and fragmentation
• ↓Mitochondrial biogenesis alterations and induction of
mitophagy. | (111) |
| Chuang et
al | Purified
α-mangostin |
NaIO3-induced reactive oxygen
species (ROS)- dependent toxicity in ARPE-19 cells BABL/c mice | 3.75, 7.5, and 15
µM 24 h 20 mg/kg Administered before injection of
NaIO3 | • ↑Cell viability
and intracellular antioxidant enzymes
• ↓Apoptosis
• ↓Bax, cleaved PARP-1, cleaved caspase-3 expression
• ↑Bcl-2 protein
• ↓Intracellular ROS and extracellular
H2O2
• ↓CAT
• ↓PI3K-AKT-PGC-1α-STRT-3 signaling
• ↑Retinal structure and thickness | (112) |
| Muhamad Adyab et
al | G.
mangostana flesh | Sprague-Dawley
rats | 200-600 mg/kg (No
α-mangostin concentration) 7 weeks | • ↑Plasma
GPx
• ↑Antioxidant capacity | (44) |
7. Anti-inflammatory effects of
α-mangostin
The increased expression of pro-inflammatory
cytokines has been reported in obesity (115). This has been linked to the
changes in adipose tissue biology in the excess nutrient
environment of obesity, by undergoing hypertrophy and hyperplasia
(116). Adipocyte hypertrophy
reduces blood supply to adipocytes and promotes hypoxia (117). Hypoxia leads to necrosis and
macrophage migration into adipose tissues, enhancing the production
of proinflammatory chemokines, including TNF-α and IL-6 resulting
in systemic inflammation (118,119). Chronic low-grade inflammation
has been associated with the development of insulin resistance and
type 2 diabetes in obese subjects (6). Excluding obesity, various factors
have been suggested to induce inflammation, including pathogens,
damaged cells and toxic compounds (120). The anti-inflammatory effects of
α-mangostin have been extensively investigated and reviewed,
underlining the importance of this compound as an anti-inflammatory
agent.
In a previous study, in a mouse model fed a
high-fat diet, treatment with-mangostin (50 mg/kg per day) reduced
macrophage infiltration in white adipose tissue as tested using
F4/80 macrophage marker. Obese mice treated with α-mangostin also
presented with reduced levels of M1 macrophage marker, CD11c,
diminished collagen staining, and increased levels of the M2
macrophage marker, CD206 (25).
α-mangostin-treatment in obese mice reduced macrophage genes F4/80
and CD11c, in both the white adipose tissue and liver tissue.
α-mangostin-treatment also reduced proinflammatory genes MCP-1 and
IL-6 in white tissue and reduced TNFα, MCP-1 and C-C chemokine
receptor type 2 (CCR2) in the liver. The levels of the
anti-inflammatory genes, IL-10 and M2, and the macrophage marker,
CD206, increased (25). Kim et
al (25) also demonstrated
that α-mangostin reversed hepatic steatosis, delayed the movement
of macrophages in tissues, and reduced the expression of
proinflammatory cytokines (TNFα, MCP-1, CCR2 and IL-6).
In another study, in mice exposed to LPS, the serum
concentrations of IL-6, TNFα, and MCP1 were reduced in the
α-mangostin group, and their expression was downregulated in
epididymal white adipose tissue (eWAT) (42). Gene expression analysis on eWAT
revealed a reduced expression of the chemokines, MCP-1, macrophage
inflammatory protein-1α (Mip-1α) and the CXCL10, CCL11, CX3CL1 and
CCL5. Pre-treatment with α-mangostin also reduced the gene
expression in eWAT of macrophage-specific markers, F4/80 and Cd68.
This reduced expression indicates a decreased macrophage content in
eWAT. Moreover, the M1 macrophage marker, Cd11c, was suppressed;
however, the levels of M2 macrophage markers, Cd206 and Arginase-1,
were increased in treated mice. Additionally, α-mangostin reduced
iNOS expression, increased SIRT3 expression, reduced the activation
of the MAPK and NF-κB pathways in eWAT, and reduced Iκ activation
in macrophages. These findings were consistent with those obtained
with RAW264.7 macrophages and demonstrated that α-mangostin reduced
inflammation by suppressing MAPK and NF-κB activation, while
promoting SIRT3 expression (42).
Similarly, in the study by Li et al (42) in aged mice, it was also revealed
that α-mangostin alleviated aging-related adipose tissue
inflammation by significantly reducing the adipocyte size and the
amount of F4/80+ macrophages in eWAT in aged mice. A
Transwell chemotaxis assay revealed that the pre-treatment of
RAW264.7 macrophages with α-mangostin reduced macrophage migration
towards the adipocyte cellular matrix, revealing that α-mangostin
promoted a shift towards anti-inflammatory macrophage polarization.
In comparison to LPS-exposed mice, similar effects of α-mangostin
in aged mice were also observed, with the reduced expression of the
chemokines, Mcp-1, Mip-1α, Cx3cl1 and Ccl5, and reduced adipose
tissue inflammation through the inhibition of iNOS, TNF-α, IL-1β
and COX-2 expression levels, while increasing SIRT3 expression.
Furthermore, α-mangostin protected aged mice from liver injury by
inhibiting macrophage release of miR-155-5p (42).
Using the LPS-induced inflammation IEC-6 cell line
model, Yin et al (121)
demonstrated that the expression levels of the NLRP3 inflammasome
and caspase-1, proteins that initiate inflammation and trigger the
release of the proinflammatory cytokines, IL-1β and IL-18, were
significantly reduced following the administration of α-mangostin,
as indicated by RT-qPCR, western blotting and immunohistochemistry.
Whole-genome transcriptomic analysis in the IEC-6 lines revealed
that α-mangostin upregulated 175 genes and downregulated 324 genes.
Gene Ontology (GO) analysis suggested that two groups of
differentially expressed genes affected by either LPS or
α-mangostin are mainly linked with inflammation and oxidative
stress. The Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
revealed that pre-treatment with α-mangostin affected the TNF,
JAK-STAT, p53 and MAPK signaling pathway cytokine-cytokine receptor
interaction (121). α-mangostin
also normalized the intestinal villus morphology of mitochondria
and nucleus and improved the swelling of the villi in an
LPS-induced inflammatory rat model. The tips of the intestinal
villi were relatively intact, the damage to the lamina propria was
reduced, congestion was clearly improved and bleeding was
significantly reduced (121). In
addition, pre-treatment with α-mangostin (2.5, 5 and 10 µM)
in LPS-stimulated IEC-6 cells strongly decreased the
phosphorylation of NF-κB subunits IB and p65, as well as their
upstream proteins, transforming growth factor β-activated kinase 1
and Iκκ. This notably inhibited the degradation of NF-κB inhibitor
IB, according to Zou et al (122).
COX-2 expression increases when there is an
inflammatory reaction and results in prostaglandin 2 (PGE-2) and NO
production via NF-κB, which localizes to the nucleus and promotes
the expression of inflammatory genes (123). Previous research has indicated
that α-mangostin can inhibit the NF-κB signaling pathway. RAW 264.7
cells treated with LPS culminated in inflammatory cytokine level
increase, including PGE-2, TNF-α, IL-6 and increased NO production
and iNOS. A high NO concentration is toxic and proinflammatory
(124), and TNF-α is a potent
inflammatory cytokine. In a previous study, α-mangostin reversed
such effects in a concentration-dependent manner by reducing the
translocation of NF-κB into the nucleus compared to LPS only
treated cells (123). Using a
mouse peritonitis model, Mohan et al (123) also demonstrated that α-mangostin
inhibited the infiltration of mononuclear immune cells and
neutrophils in inflamed tissue in a dose-dependent manner.
Inhibiting the infiltration of immune cells into tissues is
particularly important as immune cells, such as neutrophils,
release proinflammatory cytokines. A decreased tissue infiltration
is associated with the reduced presence of TNF-α and IL-1β
(123). Widowati et al
(125) reported that the
concentrations of inflammatory mediators (COX-2, IL-6 and IL-1β)
and NO in treated LPS-exposed RAW 264.7 cells were reduced by
α-mangostin and γ-mangostin. An in silico analysis of
α-mangostin demonstrated that it preferentially binds to COX-2
rather than COX-1 (123). This
is important as it shows that α-mangostin could acts as an
anti-inflammatory agent via COX-2, suggesting that it can be a
potential alternative lead compound, since current
anti-inflammatory drugs target COX-1, a physiologically important
enzyme with adverse effects when blocked.
Franceschelli et al (126) examined the effects of
α-mangostin on LPS-treated U937 cells; LPS is an inducer of
inflammation. α-mangostin (10 µM) inhibited LPS-induced NO
production by 40%. Furthermore, α-mangostin markedly increased
SIRT-1 expression, blocking the p65 acetylation, suggesting that
the anti-inflammatory action of α-mangostin involves NF-κB pathway
inhibition. This is supported by the reduced expression of iNOS and
COX-2. An experiment using EX-527, an inhibitor of SIRT1, confirmed
that α-mangostin inhibits proinflammatory NF-κB signaling through
SIRT1 induction. α-mangostin also reduced the expression of IL-1β
and TNFα but increased the anti-inflammatory IL-10. Similarly, the
effect of α-mangostin on LPS-induced human monocytes demonstrated
similar anti-inflammatory action through the modulation of
SIRT-1/NF-κB signaling (126).
Additionally, according to Sugiyanto et al
(127), the treatment with
α-mangostin (5, 10 and 20 µM) reduced the expression of both
TNF-α and IFN-γ in human peripheral blood mononuclear cells (PBMCs)
in a concentration-dependent manner (P=0.01) following both a 24-
and a 48-h post-infection. In another previous in vivo
study, similar findings were reported on the role of α-mangostin in
inhibiting the NF-κB signaling pathway (128). Yin et al (128) revealed that the ability of rat
PBMCs, obtained from rats with collagen-induced arthritis treated
with mangostin, to promote the production of cytokines (TNF-α and
IL-1β) was lost.
Moreover, Tarasuk et al (129) demonstrated that various
concentrations (10, 15 and 20 µM) of α-mangostin
significantly reduced cytokine/chemokine transcription in Dengue
virus (DENV-2)-infected cells. The percentage reduction of
cytokines (IL-6 and TNF-α), and chemokine macrophage inflammatory
protein 1β (MIP-1β), regulated upon activation, normally
T-expressed, and presumably secreted (RANTES) and interferon
gamma-induced protein 10 (IP-10) transcription was 94, 95, 92, 87
and 95%, respectively, as measured using RT-qPCR. At 48 h after
treatment, α-mangostin considerably reduced TNF-α, MIP-1, and
RANTES transcription, whereas IL-6 and IP-10 transcription was
reduced, although not significantly, in comparison to the untreated
control. Following 72 h, the effects on all parameters were
diminished. Yongpitakwattana et al (130) observed that the inhibitory
effects of α-mangostin (25 µM) on the expression levels of
the genes, TNF-α, CCL4, CCL5, CXCL10 and IFN-α, gene involved in
the vascular leakage and immunopathogenesis of DENV2-infected
immature monocyte-derived dendritic cells were significantly
reduced. However, it was noted that the levels of the
anti-inflammatory cytokine, IL-10, were also reduced in that study,
contrary to other studies reporting an increase in the levels of
this cytokine following α-mangostin treatment (20,126).
In an arthritic animal model, Herrera-Aco et
al (131) revealed that both
doses of α-mangostin (10 and 40 mg/kg) significantly reduced the
production of the chemokines, LIX/CXCL5, IP-10/CXCL10, MIG/CXCL9,
RANTES/CCL5, IL-6 and IL-33. Furthermore, at a dose of 40 mg/kg,
α-mangostin reduced the development of anti-collagen II IgG2a
antibodies in DBA/1J mice in which arthritis was induced by
collagen. This could indicate that one of the mechanisms through
which α-mangostin exerts anti-inflammatory effects in mice with
collage-induced arthritis is by altering the humoral response,
which is reflected in decreased autoantibody synthesis and, most
likely, immune complex development (131). In rats with adjuvant-induced
arthritis, Zuo et al (132) found that α-mangostin reduced paw
swelling, inflammatory cell infiltration and TNF-α and IL-1β
release in joint serum. Additionally, Zuo et al (132) demonstrated that treatment with
α-mangostin at 10 µg/ml suppressed the expression and
phosphorylation of key proteins implicated in NF-κB pathway, and
inhibited the nuclear translocation of p65 using human
fibroblast-like synoviocytes/rheumatoid arthritis cells.
In rat chondrocytes treated with IL-1β, Pan et
al (27) discovered that
α-mangostin suppressed the expression of MMP-13 and ADAMTs-5, and
promoted the expression of SOX-9 (27). They also observed that α-mangostin
inhibited the expression of pro-apoptotic proteins, including Bax,
cytochrome c and caspase-3, while increasing the anti-apoptotic
protein, Bcl-2. In the same model, the treatment of α-mangostin
also reduced NO and PGE2 production, and reduced the expression of
iNOS, COX-2, MMP-3, MMP-9 and MMP-13, and also attenuated the
degradation of collagen II and aggrecan (27,133). Moreover, α-mangostin has been
demonstrated to inhibit the NF-κB signaling pathway by suppressing
IL-1β-induced p65 nuclear translocation (27,133). In vivo, α-mangostin has
been also observed to suppress the development of osteoarthritis in
rat models underlined by decreased cartilage degeneration, most
likely due to the downregulation of inflammation through the NF-κB
pathway (27). Xu et al
(134) observed similar findings
on the reduction of the expression of IL-6 and TNF-α serum proteins
and the downregulation of the NF-κB pathway in the paw tissue of
male Wistar rats with adjuvant-induced arthritis treated with
α-mangostin.
Under diabetic conditions, increased lipolysis
leads to the increased formation of unsaturated fats. In turn,
unsaturated fats activate immune cells to produce inflammatory
proteins. One protein included in this category is IL-1β, which
deactivates the insulin response in tissues and organs, including
the liver, muscle and adipose tissues (135), leading to insulin resistance and
type 2 diabetes symptoms (136).
Soetikno et al (98)
demonstrated that α-mangostin specifically reduced IL-1β levels in
a dose-dependent manner, as well as the levels of other
proinflammatory proteins, including MCP-1, IL-6 and TNF-α using
rats with STZ-induced diabetes fed a high-fat diet. Jariyapongskul
et al (63) revealed that
treating type 2 diabetic rats with α-mangostin treatment reduced
the expression levels of AGE, receptor for AGEs, TNF-α and VEGF to
63.2, 40.9, 27.8, 65.6 and 22.3%, respectively, implying a possible
mechanism by which α-mangostin suppresses inflammation and
proinflammatory cytokine production.
A study investigating the anti-inflammatory effects
of α-mangostin in neuroinflammation demonstrated that this compound
decreased brain endothelial cell activation induced by peripheral
LPS administration (137). The
study by Nava Catorce et al (137) demonstated that α-mangostin
inhibited the generation of the proinflammatory cytokines, IL-6,
COX-2 and 18-kDa translocator protein (TSPO), in the brains of
LPS-treated mice. The decline in COX-2 levels observed in that
study was attributable to a decrease in IL-6 levels, which binds to
its receptor on brain endothelial cells. Furthermore,
immunofluorescence labelling revealed a decrease in TSPO-positive
cells across the entire brain in mice supplied with α-mangostin
(137). These findings suggest
that α-mangostin could be further evaluated as an adjuvant therapy
for the prevention or treatment of neurodegenerative diseases in
pre-clinical models.
An interesting observation was made by Yang et
al (138), revealing a novel
anti-inflammatory mechanism of α-mangostin, involving cholinergic
anti-inflammatory pathway (CAP) control, which markedly lowered
IL-1β and TNF-α levels in the serum of rats with LPS-induced acute
lung injury (ALI). These findings may support the hypothesis that
the activation of the CAP through raising peripheral acetylcholine,
upregulating α7nAchR expression, which leads to NF-κB inhibition,
is involved in the therapeutic effects of α-mangostin on ALI.
In mice using a tape-stripping model,
Tatiya-Aphiradee et al (139) revealed that formulations of
G. mangostana pericarp ethanolic extract (GME) and
α-mangostin (equivalent to -mangostin in 10% GME) decreased TNF-α,
IL-6, IL-1β and TLR-2 gene mRNA expression levels in
methicillin-resistant Staphylococcus aureus-induced superficial
skin infection. Lim et al (140) reported that α-mangostin
inhibited the production of IL-6, IL-8 and PGE2 in P.
gingivalis KCOM 2804-immortalized human gingival fibroblast
cells.
Based on the study by Fu et al (72), pre-treatment with α-mangostin
(12.5 and 25 mg/kg) significantly decreased LPS/D-GalN-induced
liver inflammation in mice. Furthermore, α-mangostin inhibited the
LPS/D-GalN-induced upregulation of Toll-like receptor 4 (TLR4), and
simultaneously phosphorylated NF-κB p65 and IκB, indicating that
α-mangostin inhibited cytokine production by inhibiting
TLR4-mediated NF-κB activation. In an acute acetaminophen-induced
liver injury study, α-mangostin from G. mangostana peels
reduced the levels of inflammatory cytokines, including TNF-α and
IL-1β (71).
In a placebo-controlled clinical trial involving 60
healthy adults (30 females and 30 males), Xie et al
(141) demonstrated
significantly decreased C-reactive protein (CRP) levels from 2.9
mg/l on day 1 to 1.6 mg/l on 30 days following the consumption of
the mangosteen product. The considerable reduction in CRP indicated
that the daily consumption of the mangosteen product may help
individuals reduce their inflammatory condition, although the
amount of α-mangostin taken was not stated in that study. CRP
reduction was also observed, along with the reduced infiltration of
inflammatory cells in liver and heart tissues of obese rats treated
with G. mangostana pericarp rich in α-mangostin (12). In the study by Hassan et al
(70), α-mangostin reduced IL-6
and TNF-α levels in liver tissues, reduced CRP, and inhibited
NF-κB/TGF-β1 signaling in Wistar rats exposed to γ-irradiation.
In summary, α-mangostin possesses exceptional
anti-inflammatory properties, as demonstrated by numerous studies,
as described above in this section. This compound exerts its
anti-inflammatory effects by modulating the TNF-α, JAK-STAT,
SIRT1/NF-κB, TLR4/NF-κB and NF-κB/TGF-β1 pathways, suppressing MAPK
activation, increasing macrophage polarization to anti-inflammatory
M2, reducing proinflammatory cytokine level (IL-6, MCP-1, TNF-α,
IL-1β, IL-18, IFN-γ and COX-2), NLRP3 inflammasome and chemokine
expression levels (MIP-1α, MIP-1β, CXCL10, CCL11, CX3CL1, CCL5,
RANTES, IP-10), increasing SIRT3 and SIRT2 expression, reducing
iNOS expression, and NO and PGE2 production, as well as the
expression of TLR-2 and TLR-4 genes, and increasing
anti-inflammatory cytokine expression levels (IL-10). The molecular
mechanisms of the anti-inflammatory effects of α-mangostin are
summarized in Fig. 6 and Table VI. Collectively, these findings
demonstrate that α-mangostin has great potential for use as an
anti-inflammatory agent.
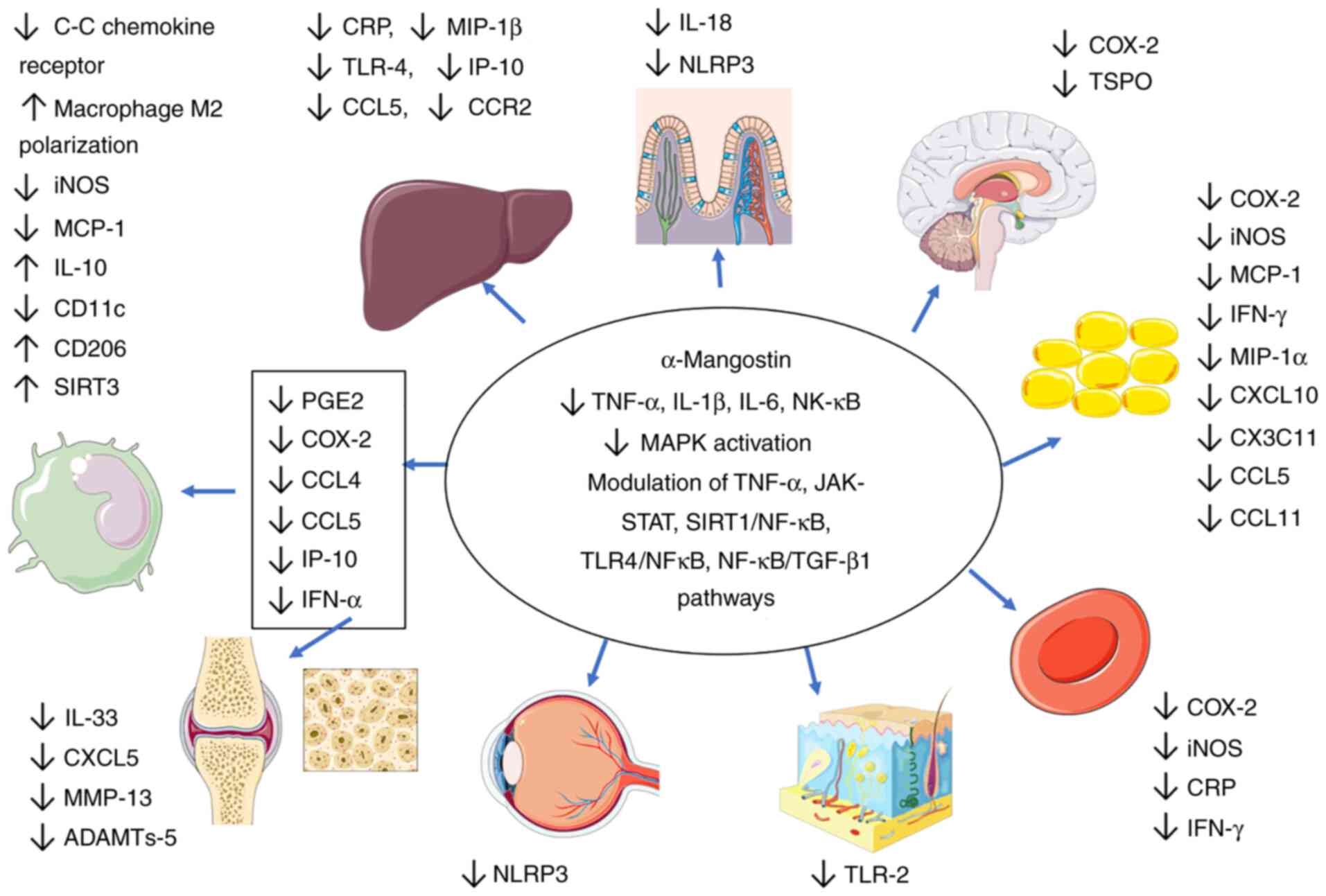 | Figure 6Anti-inflammatory effects of
α-mangostin. Prolonged inflammatory responses lead to tissue
damage. α-mangostin exerts anti-inflammatory effects and the
effects are observed throughout the body. It modulates signaling
pathways (JAK-STAT, TGF-β1, TLR4 and SIRT1 pathways) that terminate
with NF-κB translocating into the nucleus. NF-κB activates
inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, which are
released mainly through white blood cells. α-mangostin also reduces
COX-2 signaling, suppresses MAPK activation, increases macrophage
polarization to anti-inflammatory M2, reduces proinflammatory
cytokine levels, reduces NLRP3 inflammasome levels, reduces
chemokine expression (MIP-1α, MIP-1β, CXCL10, CCL11, CX3CL1, CCL5,
RANTES, IP-10), increases SIRT3 and SIRT2 expression, reduces iNOS
expression, reduces NO and PGE2 production, reduces the expression
of TLR-2 and TLR-4 genes and increases anti-inflammatory cytokine
levels (IL-10). TLR, Toll-like receptor; SIRT1, sirtuin 1; COX-2,
cyclooxygenase 2; MIP, macrophage inflammatory protein; RANTES,
regulated on activation, normal t cell expressed and secreted;
IP-10, interferon gamma-induced protein 10; NO, nitric oxide; PGE2,
prostaglandin 2; iNOS, intracellular nitric oxide synthase; CRP,
C-reactive protein; TSPO, translocator protein. |
 | Table VIAnti-inflammatory effects of
a-mangostin. |
Table VI
Anti-inflammatory effects of
a-mangostin.
| Authors | Source of
α-mangostin | Model In
vitro/in vivo | Dosage and
duration | Mechanisms of
action of anti-inflammatory effects | (Refs.) |
|---|
| Kim et
al | Purified
α-mangostin | C57BL/6 mice
RAW264.7 macrophages Mesenteric adipose tissue culture | 50 mg/kg per day 12
weeks 25 µM/ml 24 h | • ↓Pro-inflammatory
cytokine levels (TNF-α, IL-6, MCP1, IL-1β, CCR2)
• ↑Anti-inflammatory cytokines (IL-10)
• ↓C-C chemokine receptor (reduces infiltration of immune cells in
tissues)
• ↓M1 macrophage marker CD11c
• ↑M2 macrophage marker CD206 | (25) |
| Li et
al | Purified
α-mangostin | Mouse derived
RAW264.7 macrophage 3T3-L1 preadipocytes Male C57BL/6J (LPS-induced
acute inflammation and aged mice) | RAW264.7
macrophages; 1 h 10 mg/kg per day (inflammation mice) 5 days 25 and
50 mg/kg per day 8 weeks (aged mice) | • LPS
group
• ↓Il-6, TNF-α and MCP-1-serum and eWAT
• ↓Chemokines in eWAT-MCP-1, Mip-1α and the Cxcl10, Ccl11, Cx3cl1,
and Ccl5
• ↓Gene expression of F4/80 and Cd68 which are macrophage-specific
markers
• ↓Cd11c
• ↑Cd206 and Arg-1
• ↓iNOS and IKK and ↑ SIRT3
• ↑MAPK
• ↓NF-κB activation-adipose Aged mice
• ↓The adipocyte size and the amount of F4/80+
macrophages in eWAT
• ↓Expression of F4/80 and Cd68 in eWAT
• ↓Cd11c, ↑ Cd206
• ↓Mcp-1, Mip-1α, Cx3cl1, and Ccl5
• ↓Reduced macrophage migration towards adipocytes
• ↓MicroRNA-155-5p from macrophages, eWAT and serum and LPS
stimulated RAW264.7 macrophages and bone marrow derived macrophages
(BMDM) | (42) |
| Yin et
al | Purified
α-mangostin | LPS-induced
inflammation; intestinal epithelial cells, IEC-6 cell line
(CRL21592) Sprague-Dawley rats (LPS-induced enteritis model) | 10 µM; 1 h
50 mg/kg 5 days (intragastric) | • α-mangostin
regulates 475 genes mainly related to inflammation and oxidative
stress (151 genes up-regulated, 324 genes downregulated) in IEC-6
sample
• ↓Reduced intestinal villus congestion and hemorrhage Preserve
epithelial nuclei and mitochondrial morphology
• ↓Expression of inflammatory genes in LPS induced cells (IL-18 and
IL-1β)
• ↓Production of NLRP3 inflammasomes
• ↓Caspase 1 | (121) |
| Zou et
al | Purified
α-mangostin | IEC-6 cells | 2.5, 5, and 10
µM 1-h pre-treatment + 24 h LPS exposure | • ↓Production of
inflammatory factors
• ↓Activation of TAK1–NF-κB signaling pathway-related proteins, and
the entry of p65 into the nucleus | (122) |
| Mohan et
al | Pericarp of
Garcinia mangostana | RAW 264.7 cells
Male ICR mice | 24 h 1 to 25 mg/kg
60 min or 30 min before carrageenan injection | • ↓Production of
PGE2, nitric oxide, iNOS protein expression.
• ↓TNF-α and IL-6 Inhibit the translocation of NFkB and suppressing
the COX-2 enzymes
• ↓Total leukocyte migration
• ↓TNFα and IL-1β in the peritoneal fluids | (123) |
| Widowati et
al | Purified
α-mangostin Garcinia mangostana peel extract (GMPE) | RAW264.7 cells | α-mangostin: 75,
50, 25 µM 24 h with LPS GMPE: 20, 10, 5 µg/ml 24 h
with LPS | • ↓COX-2, IL-6,
IL-1β, and NO production | (125) |
| Franceschelli et
al | Purified
α-mangostin | LPS treated human
myeloid leukemia cell (U937 cell line) Human peripheral blood
monocytes | Garcinia
mangostana extract (50, 100, 500 ng/ml, 1, 5, 10, 50, 100, 500,
1 mg/ml) for 24 h (1, 5, 10, 50, and 100 mM) for 24 h | • ↓NF-κB subunit
p65 acetylation and pro-inflammatory gene products as COX-2,
iNOS
• ↑SIRT1 activation
• ↓NO production, IL-1β, TNFα
• ↑Anti-inflammatory IL-10 | (126) |
| Sugiyanto et
al | Purified
α-mangostin from G. mangostana | Human peripheral
blood mononuclear cells | 5, 10, and 20
µM 24- and 48-h post infection | • ↓TNF-α and IFN-γ
cytokines expression at 24- and 48-h post infection. | (127) |
| Yin et
al | Purified
α-mangostin | Collagen induced-
arthritic rats Human and rat peripheral blood mononuclear
cells | 40 mg/kg 45 days
2.5, 5, and 10 µg/ml (AChE activity) 2 h | • ↓Secretion of
TNF-α and IL-1β
• ↓PBMCs potential to stimulate NF-κB activation and
proinflammatory cytokine production | (128) |
| YP et
al | Purified
α-mangostin | Monocytes of
healthy individuals infected with dengue virus | 25 µM 24
h | • ↓TNF-α, CCL4,
CCL5, CXCL10, IL-6, IL1β, IL10, and IFN-α transcription | (130) |
| Tarasuk et
al | Purified
α-mangostin | Dengue virus (DENV)
infected HepG2 cells | 20 µM 24,
48, and 72 h | • ↓IL-6 and TNF-α
cytokines transcription
• ↓RANTES, MIP-1β, and IP-10 cytokines transcription | (129) |
| Herrera- Aco et
al | Pericarp of
Garcinia mangostana | DBA/1J mice | 10 and 40 mg/kg per
day 33 days | • Affect the
humoral response
• ↓PGE2 in joints
• Block the production of pleiotropic cytokine IL-6
• ↓ IL-33
• ↓LIX/CXCL5
• ↓IP-10/CXCL10
• ↓RANTES/CCL5 | (131) |
| Zuo et
al | Purified
α-mangostin | HFLS-RA cells Male
Sprague- Dawley rats | 6, 8, 10, 12 and 14
µg/ml 24 h 40 mg/kg per day 35 days | • ↓Inflammatory
cells infiltration and secretion of TNF-α and IL-1β
• ↓NF-κB induced by αMN by reducing the expression of p-p65 and
VEGF | (132) |
| Pan et
al | Purified
α-mangostin | Rat chondrocytes
Male Sprague- Dawley rats | 0, 3, 6, and 12
µM 24 h 10 mg/kg Every two days for 8 weeks | • ↓Expression of
MMP-13 and ADAMTs-5
• ↑Expression of SOX-9 in rat chondrocytes stimulated with
interleukin-1β (IL-1β)
• ↓Expression of pro-apoptotic proteins including Bax, Cyto-c, and
C-caspase3
• ↑Expression of the anti-apoptotic protein Bcl-2
• ↓IL-1β-induced activation of the NF-kB signaling pathway. | (27) |
| Pan et
al | Purified
α-mangostin | Rat chondrocytes
Male Sprague- Dawley rats | 0, 1.25, 2.5, and
5.0 µg/ml 24 h 10 mg/kg Every two days for 8 weeks | • ↓Production of NO
and PGE2
• ↓Expression of INOS, COX-2, MMP-3, MMP-9, and MMP-13
• ↓Phosphorylation of the NF-κB signaling pathway
• ↓IL-1β-induced p65 nuclear translocation
• ↓Cartilage degeneration | (133) |
| Xu et
al | Purified
α-mangostin | Male Wistar rats
(paw tissue) | 100 mg/kg 21
days | • ↓TNF-α, IL-6 and
NF-κB mRNA expression | (134) |
| Soetikno et
al | Purified
α-mangostin | Wistar rat | 100, 200 mg/kg per
day 8 weeks | • ↓Cardiac
pro-inflammatory levels (TNFα, MCP-1, IL-6, IL-1β) | (98) |
| Jariyapong- skul
et al | Pericarp of
Garcinia mangostana | Male Sprague-
Dawley rats | 200 mg/kg per day 8
weeks | • ↓MDA, AGE, RAGE,
TNF-α, and VEGF | (63) |
| Nava Catorce et
al | Pericarp of
Garcinia mangostana | Young (2-month-old)
female C57BL/6J mice | 40 mg/kg 14
days | • ↓Brain levels of
proinflammatory cytokine of cyclooxygenase-2 (COX-2)
• ↓Brain levels of proinflammatory cytokinetranslocator protein
(TSPO)
• ↓IL-6 | (137) |
| Yang et
al | Purified
α-mangostin | Acute lung injury
model male Sprague-Dawley rats | 40 mg/kg 3
days | • ↓Nucleus
translocation of p65 subunit, and secretion of TNF-α and IL-1β | (138) |
| Tatiya- Aphiradee
et al | Pericarp of
Garcinia mangostana Purified α-mangostin |
Methicillin-resistant Staphylococcus
aureus-induced superficial skin infection in mice (tape stripping
model) | 10% GME, 1.32% α-MG
in 10% ethanol (equivalent to α-MG in 10% GME) 10 days | • ↓TNF-α, IL-6,
IL-1β, and TLR-2 genes. | (139) |
| Fu et
al | Purified
α-mangostin |
Lipopolysaccharide/d-galactosamine
(LPS/D-GalN)- induced acute liver failure mice model | 12.5, 25 mg/kg 7
days | • ↓TLR-4
expression, p-NF-κB p65 and p-IκB activation
• ↓Expression of TNF-α, IL-6, IL-1β | (72) |
| Yan et
al | G.
mangostana fruit hull | ICR mice induced by
acetaminophen, acute liver injury model | 100 and 200 mg/kg 7
days | • ↓TNFα and
IL-1β | (71) |
| Lim et
al | Pericarp of
Garcinia mangostana | Immortalized human
gingival fibroblasts (hTERT-hNOF) cells | 1 µg/ml 24
h | • ↓Expression
levels of IL-6, IL-8, and PGE2 | (140) |
| Xie et
al | Mangosteen juice
and mangosteen extract | Healthy adults | 245 ml 30 days | • ↓CRP protein
level by 46% | (141) |
| John et
al | G.
mangostana rind | Wistar rat
(obese) | 168 mg/kg per day 8
weeks | • ↓CRP
proteins
• ↓Inflammatory cells (liver, heart) | (12) |
| Hassan et
al | G.
mangostana fruit rind | Wistar rats
(irradiation model) | 500 mg/kg per day
for 30 days after irradiation | • ↓TNF-α, CRP and
IL-6
• ↓Transcription of NF-κB/TGF-β1 signaling pathways | (70) |
8. Toxicity and bioavailability of
α-mangostin
It has been reported that the consumption of a
semi-purified diet with 845 mg/kg α-mangostin does not result in
adverse effect in mice (142).
The treatment of ICR mice with a 1,000 mg/kg α-mangostin dose did
not lead to any detrimental effects on the mice (19). In another study, toxicity tests
using α-mangostin at up to 1,250 mg/kg body weight did not
significantly affect mice with STZ-induced diabetes (66). In an acute toxicity study on the
effects of α-mangostin in rats, there were no toxic symptoms or
mortality observed with treatment at up to 2,000 mg/kg administered
orally at up to 48 h post-administration (143). However, in another study, the
intraperitoneal administration of α-mangostin for 72 h caused
mortality with the median lethal dose of 150 mg/kg, indicating that
the route of administration could affect the bioavailability of
this compound (144). In that
study, the mortality observed could be most likely attributed to
key organ damage, including liver, stomach, spleen, kidney, lung,
heart and brain tissues, as demonstrated in the tissue distribution
study following the intravenous administration of α-mangostin
(144). Another study on the
effects of the addition of α-mangostin in E3 medium on zebrafish
(Danio rerio) embryos for 72 h, revealed that it could
induce mortality and abnormal development with an LC50
value of 5.75 µmol/l (145). That study also demonstrated that
α-mangostin was potentially teratogenic and affected the embryonic
ROS balance and erythropoiesis (145). Due to the variabilities among
species, it is worth noting that the effects of α-mangostin may
differ between species, suggesting that further studies are
required to analyze the toxicity of this compound in human
subjects.
As a xanthone, α-mangostin is a hydrophobic
molecule affecting its solubility in aqueous environments, and is
also known to have high permeation (146). The low solubility affects the
bioavailability of the compound thus affecting final dose (147). A number of factors affect the
solubility of compounds, including the solvent, particle size,
molecular structure and nature of compound (148). In addition, α-mangostin and
other cytotoxic drugs have several limitations that influence their
effectiveness, including a first pass metabolism reaction, efflux
reactions induced by intercellular transporters, a non-specific
target site and fast drug release (149).
The bioavailability of α-mangostin has been
previously studied, demonstrating different responses when pure
α-mangostin compound and mangosteen extract rich in α-mangostin
were administered to mice. Previously, pharmacokinetic analysis of
the intravenous administration of α-mangostin or mangosteen extract
revealed that the area under curve (AUC) of the compound mean
arterial plasma concentration was higher in the extract group
compared to α-mangostin group (144). This corresponds to the higher
total body clearance of α-mangostin provided as an individual
compound compared to extract formulation, suggesting that
α-mangostin was mainly removed via a non-renal route, also reported
by other studies (150,151). Previously, it has been reported
that α-mangostin is metabolized through the hepatic microsomal
cytochrome p450 (CYP) 1A2 and/or glucuronide/sulfate conjugates
(151), therefore suggesting
that other constituents or metabolites in mangosteen extract could
interfere with glucuronide and/or sulfate conjugation of
α-mangostin (144).
When orally administered, the peak plasma
concentration (Cmax) of α-mangostin was higher in the extract group
(0.0865 µg/ml) compared to α-mangostin group (0.0408
µg/ml); however, the time required to reach Cmax was shorter
in the α-mangostin group (15 min) compared to the extract group (60
min) (144). The tissue
distributions in the groups orally administered α-mangostin
exhibited a higher concentration present in tissues (liver,
stomach, small intestine, mesentery, spleen, kidney, muscle, lung
and heart) at 45 min in the group treated with mangostin extract
compared to the group treated with the pure compound (144). Notably, the concentration of
α-mangostin was higher in the fat tissues of the pure compound
group administered the agent either orally or intravenously,
demonstrating the lipophilic property of this compound and its
potential in causing prolonged biological effects on this tissue.
In vitro metabolism analyses using tissue homogenates
demonstrated that α-mangostin was rapidly metabolized in the liver
and small intestine which may also explain its in vivo
metabolism. The oral administration of extract and pure compound
both improved the bioavailability of α-mangostin due to decreased
hepatic metabolism; however, it is still limited by increased
intestinal metabolism (144).
Rigorous clinical trials using this product will
further explain its potential toxicity and prove its credibility as
a drug candidate for various conditions, including cardiometabolic
diseases in human. For instance, a clinical study using
xanthone-rich mangosteen juice in human volunteers reported
increased antioxidant capacity measured as oxygen radical
absorbance capacity. It was hypothesized that other constituents in
the extract could have had synergistic effects with the xanthone
(152). In another study
investigating the efficacy and safety of herbal medicines it was
revealed that they were safe and had advantages over common
medication: The comparison between the turmeric extract and
paracetamol against knee pain, revealed the efficacy and safety of
the extract, which also reduced the levels of CRP and TNF-α more
effectively than paracetamol after 6 weeks (153).
9. Future perspectives
The application of α-mangostin, particularly in
cardiometabolic diseases, has been steadily investigated over the
years. Investigations have been performed using in vitro
cellular models, in vivo animal models and human volunteer
intervention, with the majority of studies demonstrating promising
results. Although numerous studies have assessed the effects of
G. mangostana products rich in α-mangostin, the interest of
the impact of this single compound is increasing, as reflected in
the literature. Although several studies have reported the in
vitro digestion products, the exact metabolites of this
compound in biological in vivo models remain to be further
elucidated (154-156). The methods with which to
increase the bioavailability of this compound also warrant further
attention, due to the low solubility of α-mangostin in aqueous
solution. The pharmaceutical industry has been trying to discover
means with which to increase solubility by manipulating the
different factors affecting solubility. Increasing solubility would
help achieve therapeutic plasma level concentrations, as this
therapeutic can be absorbed in the gastrointestinal tract. The
nanotechnology approach has demonstrated improved success, as it
reduces molecule size, increases the surface area, interaction with
the solvent, and solubility and bioavailability (157). In a previous study,
α-mangostin-loaded polymeric nanosponges made from hyper
crosslinked ethyl cellulose polymers, were administered to rats at
a predetermined minimum IC50. When compared to rats
administered pure α-mangostin, the loaded nanoparticles slowly
released α-mangostin over time, requiring lower doses and
prolonging the antidiabetic response in the rats (158), suggesting that nanotechnology
could enhance the delivery of α-mangostin.
Nanoparticle technology also permits specific
targeting of therapeutics. The study by Sodalee et al
(159) using emulsion
nanoparticles (nanoemulsion) revealed that this method increased
the solubility and dissolution rates of α-mangostin. Nanoemulsion
encourages self-microemulsion (160). Sodalee et al (159) observed greater distribution in
lymphatic organs and increased digestive tract absorption.
Increased bioavailability has the potential for clinical drug
efficacy (159). There are
numerous effects of α-mangostin in biological models on various
diseases and studies have reported its molecular docking mechanisms
(161,162); however, comprehensive reports on
other receptors are still required.
In addition, as obesity forms the main comorbidity
in metabolic syndrome involving the excessive accumulation of
adipose tissue, it is of utmost importance to further explore the
mechanisms of action of α-mangostin in adipose tissue. The
upregulation of uncoupling protein 1 (UCP-1) in white adipose
tissue promotes thermogenesis, energy metabolism and
differentiation into a beige or brown phenotype (163). Phytochemicals, including
quercetin and resveratrol have been demonstrated to enhance UCP-1
expression, implying increased white adipose tissue browning
through the activation of the AMPK/PPARγ (164) and AMPKα1 pathways (165), respectively. This is one among
numerous mechanisms through which α-mangostin could affect adipose
tissue biology, including but not limited to, its effects on the
secretion of adipocytokines, which are not yet widely reported. It
is also unclear whether the actions of α-mangostin can be
potentiated, synergized or antagonized by other phytochemical
compounds or synthetic drugs; thus, further investigations are
warranted in this matter. As the present review only aimed to
discuss the potential mechanisms of α-mangostin in modulating the
comorbidities of metabolic syndrome, the interrelations of the
biological and molecular effects discussed herein with other
diseases including, but not limited to, cancer, infection and
neurodegenerative diseases, were not discussed and thus deserve
further exploration.
10. Conclusions
α-mangostin, as a medicinal compound, has gained
increasing attention, since the research community gradually delves
into naturally-sourced bioactive compounds. The present review
discussed the metabolic and molecular mechanisms through which
α-mangostin functions to exert positive effects on metabolic
syndrome parameters, including anti-obesity, antidiabetic,
hepatoprotective and anti-steatotic, cardioprotective and
anti-atherogenic, antioxidant and anti-inflammatory effects,
without any severe adverse effects. Novel drug delivery systems are
promising approaches in overcoming the low α-mangostin solubility
and increasing the targeted delivery of α-mangostin to specific
organs. When this system is optimized, dosage studies on humans can
be thoroughly conducted. Importantly, rigorous clinical trials
using products rich in α-mangostin may further demonstrate its
potential toxicity and prove its credibility as a drug candidate
for various conditions, including cardiometabolic diseases.
Availability of data and materials
Not applicable.
Authors' contributions
ODJ designed the overall manuscript. ODJ, ATM and
RMG participated in the writing process, reviewed the literature
and shared the writing drafts. ODJ and NS performed the final
review of the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The chemical structure of α-mangostin was drawn
using ChemSketch Freeware by ACD/Labs. Images were designed using
Microsoft PowerPoint and Servier Medical Art (smart.servier.com).
Funding
No funding was received
References
|
1
|
Grundy SM, Brewer HB Jr, Cleeman JI, Smith
SC Jr and Lenfant C; American Heart Association: National Heart,
Lung, and Blood Institute: Definition of metabolic syndrome: Report
of the national heart, lung, and blood institute/American heart
association conference on scientific issues related to definition.
Circulation. 109:433–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pite H, Aguiar L, Morello J, Monteiro EC,
Alves AC, Bourbon M and Morais-Almeida M: Metabolic dysfunction and
asthma: Current perspectives. J Asthma Allergy. 13:237–247. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Neill S and O'Driscoll L: Metabolic
syndrome: A closer look at the growing epidemic and its associated
pathologies. Obes Rev. 16:1–12. 2015. View Article : Google Scholar
|
|
4
|
ASMBS Clinical Issues Committee: Bariatric
surgery in class I obesity (body mass index 30-35
kg/m2). Surg Obes Relat Dis. 9:e1–e10. 2013. View Article : Google Scholar
|
|
5
|
Hill JO, Wyatt HR and Peters JC: Energy
balance and obesity. Circulation. 126:126–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zatterale F, Longo M, Naderi J, Raciti GA,
Desiderio A, Miele C and Beguinot F: Chronic adipose tissue
inflammation linking obesity to insulin resistance and type 2
diabetes. Front Physiol. 10:16072020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zafar U, Khaliq S, Ahmad HU, Manzoor S and
Lone KP: Metabolic syndrome: An update on diagnostic criteria,
pathogenesis, and genetic links. Hormones (Athens). 17:299–313.
2018. View Article : Google Scholar
|
|
8
|
Halpern A, Pepe RB, Monegaglia AP, Beyruti
M, de Melo ME and Mancini MC: Efficacy and tolerability of the
association of sibutramine and orlistat for six months in
overweight and obese patients. J Obes. 2010:6025372010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Francini-Pesenti F, Spinella P and Calò
LA: Potential role of phytochemicals in metabolic syndrome
prevention and therapy. Diabetes Metab Syndr Obes. 12:1987–2002.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu QY, Wang YT and Lin LG: New insights
into the anti-obesity activity of xanthones from Garcinia
mangostana. Food Funct. 6:383–393. 2015. View Article : Google Scholar
|
|
11
|
John OD, Brown L and Panchal SK: Garcinia
fruits: Their potential to combat metabolic syndrome.
Nutraceuticals and Natural Product Derivatives: Disease Prevention
& Drug Discovery. Ullah MF and Ahmad A: John Wiley & Sons,
Inc; Hoboken, NJ: pp. 39–80. 2019
|
|
12
|
John OD, Mouatt P, Panchal SK and Brown L:
Rind from purple mangosteen (Garcinia mangostana) attenuates
diet-induced physiological and metabolic changes in obese rats.
Nutrients. 13:3192021. View Article : Google Scholar :
|
|
13
|
Ee GC, Daud S, Taufiq-Yap YH, Ismail NH
and Rahmani M: Xanthones from Garcinia mangostana (Guttiferae). Nat
Prod Res. 20:1067–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khamthong N and Hutadilok-Towatana N:
Phytoconstituents and biological activities of Garcinia dulcis
(Clusiaceae): A review. Nat Prod Commun. 12:453–460.
2017.PubMed/NCBI
|
|
15
|
Ngoupayo J, Tabopda TK and Ali MS:
Antimicrobial and immunomodulatory properties of prenylated
xanthones from twigs of Garcinia staudtii. Bioorg Med Chem.
17:5688–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kijjoa A, Gonzalez MJ, Pinto MM,
Nascimento MS, Campos N, Mondranondra IO, Silva AM, Eaton G and
Herz W: Cytotoxicity of prenylated xanthones and other constituents
from the wood of Garcinia merguensis. Planta Med. 74:864–866. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fatma Sri W, Daud Ahmad Israf A, Nordin Hj
L and Dachriyanus: Anti-inflammatory activity of isolated compounds
from the stem bark of Garcinia cowa Roxb. Pharmacogn J. 9:55–57.
2017.
|
|
18
|
Phuwapraisirisan P, Udomchotphruet S,
Surapinit S and Tip-Pyang S: Antioxidant xanthones from Cratoxylum
cochinchinense. Nat Prod Res. 20:1332–1337. 2006. View Article : Google Scholar
|
|
19
|
Ibrahim MY, Hashim NM, Mohan S, Abdulla
MA, Abdelwahab SI, Arbab IA, Yahayu M, Ali LZ and Ishag OE:
α-Mangostin from Cratoxylum arborescens: An in vitro and in vivo
toxicological evaluation. Arab J Chem. 8:129–137. 2015. View Article : Google Scholar
|
|
20
|
Thaweboon S, Thaweboon B, Nisalak P and
Kaypetch R: Inhibitory effect of Cratoxylum formosum gum on candida
glabrata and its α-mangostin content. MATEC Web Conf. 65:030042016.
View Article : Google Scholar
|
|
21
|
Lenta BN, Kamdem LM, Ngouela S, Tantangmo
F, Devkota KP, Boyom FF, Rosenthal PJ and Tsamo E: Antiplasmodial
constituents from the fruit pericarp of Pentadesma butyracea.
Planta Med. 77:377–379. 2011. View Article : Google Scholar
|
|
22
|
Ghasemzadeh A, Jaafar HZE, Baghdadi A and
Tayebi-Meigooni A: Alpha-mangostin-rich extracts from mangosteen
pericarp: Optimization of green extraction protocol and evaluation
of biological activity. Molecules. 23:18522018. View Article : Google Scholar :
|
|
23
|
Pedraza-Chaverri J, Cárdenas-Rodríguez N,
Orozco-Ibarra M and Pérez-Rojas JM: Medicinal properties of
mangosteen (Garcinia mangostana). Food Chem Toxicol. 46:3227–3239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi YH, Bae JK, Chae HS, Kim YM, Sreymom
Y, Han L, Jang HY and Chin YW: α-Mangostin regulates hepatic
steatosis and obesity through SirT1-AMPK and PPARγ pathways in
high-fat diet-induced obese mice. J Agric Food Chem. 63:8399–8406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HM, Kim YM, Huh JH, Lee ES, Kwon MH,
Lee BR, Ko HJ and Chung CH: α-Mangostin ameliorates hepatic
steatosis and insulin resistance by inhibition C-C chemokine
receptor 2. PLoS One. 12:e01792042017. View Article : Google Scholar
|
|
26
|
Vien LC, Chinnappan S and Mogana R:
Antioxidant activity of Garcinia mangostana L and alpha mangostin:
A review. Res J Pharm Technol. 14:4466–4470. 2021. View Article : Google Scholar
|
|
27
|
Pan T, Chen R, Wu D, Cai N, Shi X, Li B
and Pan J: Alpha-mangostin suppresses interleukin-1β-induced
apoptosis in rat chondrocytes by inhibiting the NF-κB signaling
pathway and delays the progression of osteoarthritis in a rat
model. Int Immunopharmacol. 52:156–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chae HS, Oh SR, Lee HK, Joo SH and Chin
YW: Mangosteen xanthones, α-and γ-mangostins, inhibit allergic
mediators in bone marrow-derived mast cell. Food Chem. 134:397–400.
2012. View Article : Google Scholar
|
|
29
|
Li P, Tian W and Ma X: Alpha-mangostin
inhibits intracellular fatty acid synthase and induces apoptosis in
breast cancer cells. Mol Cancer. 13:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang A, Liu C, Wu J, Kou X and Shen R: A
review on α-mangostin as a potential multi-target-directed ligand
for Alzheimer's disease. Eur J Pharmacol. 897:1739502021.
View Article : Google Scholar
|
|
31
|
Rodniem S, Tiyao V, Nilbu-Nga C, Poonkhum
R, Pongmayteegul S and Pradidarcheep W: Protective effect of
alpha-mangostin on thioacetamide-induced liver fibrosis in rats as
revealed by morpho-functional analysis. Histol Histopathol.
34:419–430. 2019.
|
|
32
|
Sampath PD and Vijayaragavan K:
Ameliorative prospective of alpha-mangostin, a xanthone derivative
from Garcinia mangostana against beta-adrenergic
cathecola-mine-induced myocardial toxicity and anomalous cardiac
TNF-α and COX-2 expressions in rats. Exp Toxicol Pathol.
60:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sivaranjani M, Prakash M, Gowrishankar S,
Rathna J, Pandian SK and Ravi AV: In vitro activity of
alpha-mangostin in killing and eradicating Staphylococcus
epidermidis RP62A biofilms. Appl Microbiol Biotechnol.
101:3349–3359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaomongkolgit R, Jamdee K and Chaisomboon
N: Antifungal activity of alpha-mangostin against Candida albicans.
J Oral Sci. 51:401–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang KJ, Gu QL, Yang K, Ming XJ and Wang
JX: Anticarcinogenic effects of α-mangostin: A review. Planta Med.
83:188–202. 2017.
|
|
36
|
Chen G, Li Y, Wang W and Deng L:
Bioactivity and pharmacological properties of α-mangostin from the
mangosteen fruit: A review. Expert Opin Ther Pat. 28:415–427. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ibrahim MY, Hashim NM, Mariod AA, Mohan S,
Abdulla MA, Abdelwahab SI and Arbab IA: α-Mangostin from Garcinia
mangostana Linn: An updated review of its pharmacological
properties. Arab J Chem. 9:317–329. 2016. View Article : Google Scholar
|
|
38
|
Jindarat S: Xanthones from mangosteen
(Garcinia mangostana): Multi-targeting pharmacological properties.
J Med Assoc Thai. 97(Suppl 2): S196–S201. 2014.PubMed/NCBI
|
|
39
|
Tousian Shandiz H, Razavi BM and
Hosseinzadeh H: Review of Garcinia mangostana and its xanthones in
metabolic syndrome and related complications. Phytother Res.
31:1173–1182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang MH, Zhang KJ, Gu QL, Bi XL and Wang
JX: Pharmacology of mangostins and their derivatives: A
comprehensive review. Chin J Nat Med. 15:81–93. 2017.PubMed/NCBI
|
|
41
|
Abuzaid AS, Sukandar E, Kurniati NF and
Adnyana IK: Preventive effect on obesity of mangosteen (Garcinia
mangostana L.) pericarp ethanolic extract by reduction of fatty
acid synthase level in monosodium glutamate and high-calorie
diet-induced male wistar rats. Asian J Pharm Clin Res. 9:257–260.
2016.
|
|
42
|
Li D, Liu Q, Lu X, Li Z, Wang C, Leung CH,
Wang Y, Peng C and Lin L: α-Mangostin remodels visceral adipose
tissue inflammation to ameliorate age-related metabolic disorders
in mice. Aging (Albany NY). 11:11084–11110. 2019. View Article : Google Scholar
|
|
43
|
Tsai SY, Chung PC, Owaga EE, Tsai IJ, Wang
PY, Tsai JI, Yeh TS and Hsieh RH: Alpha-mangostin from mangosteen
(Garcinia mangostana Linn.) pericarp extract reduces high fat-diet
induced hepatic steatosis in rats by regulating mitochondria
function and apoptosis. Nutr Metab (Lond). 13:882016. View Article : Google Scholar
|
|
44
|
Muhamad Adyab NS, Rahmat A, Abdul Kadir
NAA, Jaafar H, Shukri R and Ramli NS: Mangosteen (Garcinia
mangostana) flesh supplementation attenuates biochemical and
morphological changes in the liver and kidney of high fat
diet-induced obese rats. BMC Complement Altern Med. 19:3442019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chae HS, Kim YM, Bae JK, Sorchhann S, Yim
S, Han L, Paik JH, Choi YH and Chin YW: Mangosteen extract
attenuates the metabolic disorders of high-fat-fed mice by
activating AMPK. J Med Food. 19:148–154. 2016. View Article : Google Scholar
|
|
46
|
Mohamed SM, Mohammed DS, Abd Elhaliem NG,
Elbadry MI and Abu-Dief EE: Mangosteen can improve steatohepatitis
through modulating inflammatory and autophagy/apoptosis cell
injury: An animal model study. Cytol Genet. 55:480–490. 2021.
View Article : Google Scholar
|
|
47
|
Klop B, Elte JWF and Cabezas MC:
Dyslipidemia in obesity: Mechanisms and potential targets.
Nutrients. 5:1218–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taher M, Mohamed Amiroudine MZ, Tengku
Zakaria TM, Susanti D, Ichwan SJ, Kaderi MA, Ahmed QU and Zakaria
ZA: α-Mangostin improves glucose uptake and inhibits adipocytes
differentiation in 3T3-L1 cells via PPARγ, GLUT4, and leptin
expressions. Evid Based Complementary Altern Med. 2015:7402382015.
View Article : Google Scholar
|
|
49
|
Chae HS, Kim EY, Han L, Kim NR, Lam B,
Paik JH, Yoon KD, Choi YH and Chin YW: Xanthones with pancreatic
lipase inhibitory activity from the pericarps of Garcinia
mangostana L.(Guttiferae). Eur J Lipid Sci Technol. 118:1416–1421.
2016. View Article : Google Scholar
|
|
50
|
Cantó C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+ metabolism
and SIRT1 activity. Nature. 458:1056–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fu Z, R Gilbert E and Liu D: Regulation of
insulin synthesis and secretion and pancreatic Beta-cell
dysfunction in diabetes. Curr Diabetes Rev. 9:25–53. 2013.
View Article : Google Scholar
|
|
52
|
Lee D, Kim YM, Jung K, Chin YW and Kang
KS: Alpha-mangostin improves insulin secretion and protects INS-1
cells from streptozotocin-induced damage. Int J Mol Sci.
19:14842018. View Article : Google Scholar :
|
|
53
|
Langlais P, Yi Z, Finlayson J, Luo M,
Mapes R, De Filippis E, Meyer C, Plummer E, Tongchinsub P, Mattern
M and Mandarino LJ: Global IRS-1 phosphorylation analysis in
insulin resistance. Diabetologia. 54:2878–2889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
De Meyts P: The insulin receptor and its
signal transduction network. Endotext [Internet]. MDText.com, Inc. South Dartmouth, MA: 2016
|
|
55
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signalling pathways: Insights into insulin
action. Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Okada S, Crosson S, Mori M, Saltiel AR and
Pessin JE: Insulin action, post-receptor mechanisms. Encyclopedia
of Endocrine Diseases. Martini L: Elsevier; pp. 14–22. 2004,
View Article : Google Scholar
|
|
57
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Y, Mei H, Shan W, Shi L, Chang X,
Zhu Y, Chen F and Han X: Lentinan protects pancreatic β cells from
STZ-induced damage. J Cell Mol Med. 20:1803–1812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Luo Y and Lei M: α-Mangostin protects
against high-glucose induced apoptosis of human umbilical vein
endothelial cells. Biosci Rep. 37:BSR201707792017. View Article : Google Scholar
|
|
60
|
Volpe CMO, Villar-Delfino PH, Dos Anjos
PMF and Nogueira-Machado JA: Cellular death, reactive oxygen
species (ROS) and diabetic complications. Cell Death Dis.
9:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Husen SA, Salamun, Ansori ANM, Joko R,
Susilo K, Hayaza S and Winarni D: The effect of alpha-mangostin in
glucose level, cholesterol level and diameter of the islets of
langerhans of STZ-induced diabetic mice. In: Proceedings of the 2nd
International Conference Postgraduate School (ICPS 2018); Science
and Technology Publications, Lda; pp. 561–566. 2018
|
|
62
|
Kurniawati M, Mahdi C and Aulanni'am A:
The effect of juice mangosteen rind (Garcinia mangostana L.) to
blood sugar levels and histological of pancreatic rats with the
induction of streptozotocin. J Pure App Chem Res. 3:1–6. 2014.
View Article : Google Scholar
|
|
63
|
Jariyapongskul A, Areebambud C, Suksamrarn
S and Mekseepralard CJBri: Alpha-mangostin attenuation of
hyperglycemia-induced ocular hypoperfusion and blood retinal
barrier leakage in the early stage of type 2 diabetes rats. Biomed
Res Int. 2015:7858262015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Paul S, Ali A and Katare R: Molecular
complexities underlying the vascular complications of diabetes
mellitus-a comprehensive review. J Diabetes Complications.
34:1076132020. View Article : Google Scholar
|
|
65
|
Djeujo FM, Francesconi V, Gonella M,
Ragazzi E, Tonelli M and Froldi G: Anti-α-glucosidase and
antiglycation activities of α-mangostin and new xanthenone
derivatives: Enzymatic kinetics and mechanistic insights through in
vitro studies. Molecules. 27:5472022. View Article : Google Scholar
|
|
66
|
Kumar V, Bhatt PC, Kaithwas G, Rashid M,
Al-abbasi F, Khan J, Anwar F and Verma A: α-Mangostin mediated
pharmacological modulation of hepatic carbohydrate metabolism in
diabetes induced Wistar rat. Beni-Suef Univ J Basic Appl Sci.
5:255–276. 2016.
|
|
67
|
Watanabe M, Gangitano E, Francomano D,
Addessi E, Toscano R, Costantini D, Tuccinardi D, Mariani S,
Basciani S, Spera G, et al: Mangosteen extract shows a potent
insulin sensitizing effect in obese female patients: A prospective
randomized controlled pilot study. Nutrients. 10:5862018.
View Article : Google Scholar :
|
|
68
|
Ratwita W, Sukandar EY, Adnyana IK and
Kurniati NF: Alpha mangostin and xanthone from Mangosteen (Garcinia
mangostana L.) role on glucose tolerance and glucose transporter-4
in diabetes mellitus. Int J Pharmacogn Phytochem Res. 9:1206–1212.
2017.
|
|
69
|
Rusman JRA, Sundari SA, Nuriliani A and
Saragih HT: Ameliorative effect of Mangosteen (Garcinia mangostana
L.) peel infusion on the histopathological structures of the liver
and kidney of rats (Rattus norvegicus Berkenhout, 1769) after
H2O2 induction. Vet World. 14:1579–1587.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hassan AA, Moustafa EM, EL-Khashab IH and
Mansour SZ: Mangosteen hinders gamma radiation-mediated oxidative
stress and liver injury by down-regulating TNF-α/NF-κB and
pro-fibrotic factor TGF-β1 inducing inflammatory signaling. Dose
Response. 19:155932582110251902021. View Article : Google Scholar
|
|
71
|
Yan XT, Sun YS, Ren S, Zhao LC, Liu WC,
Chen C, Wang Z and Li W: Dietary α-mangostin provides protective
effects against acetaminophen-induced hepatotoxicity in mice via
Akt/mTOR-mediated inhibition of autophagy and apoptosis. Int J Mol
Sci. 19:13352018. View Article : Google Scholar
|
|
72
|
Fu T, Li H, Zhao Y, Cai E, Zhu H, Li P and
Liu J: Hepatoprotective effect of α-mangostin against
lipopolysaccharide/d-galactosamine-induced acute liver failure in
mice. Biomed Pharmacother. 106:896–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Abood WN, Bradosty SW, Shaikh FK, Salehen
N, Farghadani R, Agha FS, Al-Medhtiy MH, Kamil TDA, Agha S, Abdulla
MA, et al: Garcinia mangostana peel extracts exhibit
hepatoprotective activity against thioacetamide-induced liver
cirrhosis in rats. J Funct Foods. 74:1042002020. View Article : Google Scholar
|
|
74
|
Dewidar B, Meyer C, Dooley S and
Meindl-Beinker N: TGF-β in hepatic stellate cell activation and
liver fibrogenesis-updated 2019. Cells. 8:14192019. View Article : Google Scholar
|
|
75
|
Seki S, Kitada T, Yamada T, Sakaguchi H,
Nakatani K and Wakasa K: In situ detection of lipid peroxidation
and oxidative DNA damage in non-alcoholic fatty liver diseases. J
Hepatol. 37:56–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang L, Li P, Fu S, Calay ES and
Hotamisligil GS: Defective hepatic autophagy in obesity promotes ER
stress and causes insulin resistance. Cell Metab. 11:467–478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Inami Y, Yamashina S, Izumi K, Ueno T,
Tanida I, Ikejima K and Watanabe S: Hepatic steatosis inhibits
autophagic proteolysis via impairment of autophagosomal
acidification and cathepsin expression. Biochem Biophys Res Commun.
412:618–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sinha RA, You SH, Zhou J, Siddique MM, Bay
BH, Zhu X, Privalsky ML, Cheng SY, Stevens RD, Summers SA, et al:
Thyroid hormone stimulates hepatic lipid catabolism via activation
of autophagy. J Clin Invest. 122:2428–2438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jiang M, Huang S, Duan W, Liu Q and Lei M:
Alpha-mangostin improves endothelial dysfunction in db/db mice
through inhibition of aSMase/ceramide pathway. J Cell Mol Med.
25:3601–3609. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rahmaniah R, Yuyuntia Y, Soetikno V,
Arozal W, Antarianto RD and Louisa M: Alpha mangostin inhibits
hepatic stellate cells activation through TGF-β/smad and AKT
signaling pathways: An in vitro study in LX2. Drug Res (Stuttg).
8:153–158. 2018.
|
|
81
|
Shibata MA, Harada-Shiba M, Shibata E,
Tosa H, Matoba Y, Hamaoka H, Iinuma M and Kondo Y: Crude
α-mangostin suppresses the development of atherosclerotic lesions
in apoe-deficient mice by a possible M2 macrophage-mediated
mechanism. Int J Mol Sci. 20:17222019. View Article : Google Scholar
|
|
82
|
Lestari N, Louisa M, Soetikno V, Suwana
AG, Ramadhan PA, Akmal T and Arozal W: Alpha mangostin inhibits the
proliferation and activation of acetaldehyde induced hepatic
stellate cells through TGF-β and ERK 1/2 pathways. J Toxicol.
2018:53604962018. View Article : Google Scholar
|
|
83
|
Chae HS, Kim HJ, Ko HJ, Lee CH, Choi YH
and Chin YW: Transcriptome analysis illuminates a hub role of
SREBP2 in cholesterol metabolism by α-mangostin. ACS Omega.
5:31126–31136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Boonprom P, Boonla O, Chayaburakul K,
Welbat JU, Pannangpetch P, Kukongviriyapan U, Kukongviriyapan V,
Pakdeechote P and Prachaney P: Garcinia mangostana pericarp extract
protects against oxidative stress and cardiovascular remodeling via
suppression of p47phox and iNOS in nitric oxide
deficient rats. Ann Anat. 212:27–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sampath PD and Kannan V: Mitigation of
mitochondrial dysfunction and regulation of eNOS expression during
experimental myocardial necrosis by alpha-mangostin, a xanthonic
derivative from Garcinia mangostana. Drug Chem Toxicol. 32:344–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jittiporn K, Moongkarndi P, Samer J and
Suvitayavat W: Protective effect of α-mangostin on high glucose
induced endothelial cell apoptosis. Walailak J Sci Technol.
15:579–587. 2018. View Article : Google Scholar
|
|
87
|
Tousian H, Razavi BM and Hosseinzadeh H:
Alpha-mangostin decreased cellular senescence in human umbilical
vein endothelial cells. Daru. 28:45–55. 2020. View Article : Google Scholar :
|
|
88
|
Fang Z, Luo W and Luo Y: Protective effect
of α-mangostin against CoCl2-induced apoptosis by suppressing
oxidative stress in H9C2 rat cardiomyoblasts. Mol Med Rep.
17:6697–6704. 2018.PubMed/NCBI
|
|
89
|
Chen LG, Yang LL and Wang CC:
Anti-inflammatory activity of mangostins from Garcinia mangostana.
Food Chem Toxicol. 46:688–693. 2008. View Article : Google Scholar
|
|
90
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chaurasia B and Summers SA:
Ceramides-lipotoxic inducers of metabolic disorders. Trends
Endocrinol Metab. 26:538–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Okudaira C, Ikeda Y, Kondo S, Furuya S,
Hirabayashi Y, Koyano T, Saito Y and Umezawa K: Inhibition of
acidic sphingomyelinase by xanthone compounds isolated from
Garcinia speciosa. J Enzyme Inhib. 15:129–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Idris-Khodja N, Ouerd S, Mian MOR,
Gornitsky J, Barhoumi T, Paradis P and Schiffrin EL: Endothelin-1
overexpression exaggerates diabetes-induced endothelial dysfunction
by altering oxidative stress. Am J Hypertens. 29:1245–1251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Schneider JG, Tilly N, Hierl T, Sommer U,
Hamann A, Dugi K, Leidig-Bruckner G and Kasperk C: Elevated plasma
endothelin-1 levels in diabetes mellitus. Am J Hypertens.
15:967–972. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Eisvand F, Imenshahidi M, Ghasemzadeh
Rahbardar M, Tabatabaei Yazdi SA, Rameshrad M, Razavi BM and
Hosseinzadeh H: Cardioprotective effects of alpha-mangostin on
doxorubicin-induced cardiotoxicity in rats. Phytother Res.
36:506–524. 2022. View Article : Google Scholar
|
|
96
|
Wu Y, Pan N, An Y, Xu M, Tan L and Zhang
L: Diagnostic and prognostic biomarkers for myocardial infarction.
Front Cardiovasc Med. 7:6172772021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Aydin S, Ugur K, Aydin S, Sahin İ and
Yardim M: Biomarkers in acute myocardial infarction: Current
perspectives. Vasc Health Risk Manag. 15:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Soetikno V, Murwantara A, Andini P,
Charlie F, Lazarus G, Louisa M and Arozal W: Alpha-mangostin
improves cardiac hypertrophy and fibrosis and associated
biochemical parameters in high-fat/high-glucose diet and low-dose
streptozotocin injection-induced type 2 diabetic rats. J Exp
Pharmacol. 12:27–38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ismail AMZ, Sargowo D, Tjahjono CT, Widito
S, Rizal A and Rahimah AF: The role of Garcinia mangostana pericarp
extract as antioxidant to inhibit atherosclerosis process in high
risk framingham score patient; original article. Heart Sci J.
2:25–29. 2021. View Article : Google Scholar
|
|
100
|
Adiputro DL, Khotimah H, Widodo MA,
Romdoni R and Sargowo D: Effects of ethanolic extracts of Garcinia
mangostana fruit pericap on oxidant-antioxidant status and foam
cells in atherosclerotic rats. Oxid Antioxid Med Sci. 2:61–64.
2013. View Article : Google Scholar
|
|
101
|
Wihastuti TA, Widodo MA, Heriansyah T and
Sari NAK: Study of the inhibition effect of ethanolic extract of
mangosteen pericarp on atherogenesis in hypercholesterolemic rat.
Asian Pac J Trop Dis. 5:830–834. 2015. View Article : Google Scholar
|
|
102
|
Wihastuti TA, Aini FN, Tjahjono CT and
Heriansyah T: Dietary ethanolic extract of mangosteen pericarp
reduces VCAM-1, perivascular adipose tissue and aortic intimal
medial thickness in hypercholesterolemic rat model. Open Access
Maced J Med Sci. 7:3158–3163. 2019. View Article : Google Scholar
|
|
103
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
104
|
He F, Antonucci L and Karin M: NRF2 as a
regulator of cell metabolism and inflammation in cancer.
Carcinogenesis. 41:405–416. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Fang Y, Su T, Qiu X, Mao P, Xu Y, Hu Z,
Zhang Y, Zheng X, Xie P and Liu Q: Protective effect of
alpha-mangostin against oxidative stress induced-retinal cell
death. Sci Rep. 6:210182016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Taguchi K and Yamamoto M: The KEAP1-NRF2
system in cancer. Front Oncol. 7:852017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chang CC, Chen CW, Owaga E, Lee WT, Liu TN
and Hsieh RH: Mangosteen concentrate drink supplementation promotes
antioxidant status and lactate clearance in rats after exercise.
Nutrients. 12:14472020. View Article : Google Scholar :
|
|
108
|
Lazarus G, Alexander S, Kusuma GO, Wijaya
K and Soetikno V: Antioxidative activities of alpha-mangostin in
high-fat/high-glucose diet and streptozotocin-induced
insulin-resistant rodents. J Appl Pharm Sci. 10:035–039. 2020.
|
|
109
|
Harliansyah H, Rahmah NA and Kuslestari K:
α-Mangosteen as An Oxidative Inhibitor in Hepatocellular Carcinoma.
Indones J Cancer Chemoprevention. 12:106–113. 2021. View Article : Google Scholar
|
|
110
|
Sanghvi VR, Leibold J, Mina M, Mohan P,
Berishaj M, Li Z, Miele MM, Lailler N, Zhao C, de Stanchina E, et
al: The oncogenic action of NRF2 depends on de-glycation by
fructosamine-3-kinase. Cell. 178:807–819.e21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Reyes-Fermín LM, Avila-Rojas SH,
Aparicio-Trejo OE, Tapia E, Rivero I and Pedraza-Chaverri J: The
protective effect of alpha-mangostin against cisplatin-induced cell
death in LLC-PK1 cells is associated to mitochondrial function
preservation. Antioxidants (Basel). 8:1332019. View Article : Google Scholar
|
|
112
|
Chuang CJ, Wang M, Yeh JH, Chen TC, Tsou
SC, Lee YJ, Chang YY and Lin HW: The protective effects of
α-mangostin attenuate sodium iodate-induced cytotoxicity and
oxidative injury via mediating SIRT-3 inactivation via the
PI3K/AKT/PGC-1α pathway. Antioxidants (Basel). 10:18702021.
View Article : Google Scholar
|
|
113
|
Ruankham W, Suwanjang W, Phopin K,
Songtawee N, Prachayasittikul V and Prachayasittikul S: Modulatory
effects of alpha-mangostin mediated by SIRT1/3-FOXO3a pathway in
oxidative stress-induced neuronal cells. Front Nutr. 8:7144632022.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang J, Xiang H, Liu J, Chen Y, He RR and
Liu B: Mitochondrial sirtuin 3: New emerging biological function
and therapeutic target. Theranostics. 10:8315–8342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Halberg N, Wernstedt-Asterholm I and
Scherer PE: The adipocyte as an endocrine cell. Endocrinol Metab
Clin North Am. 37:753–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cinti S, Mitchell G, Barbatelli G, Murano
I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS and Obin MS:
Adipocyte death defines macrophage localization and function in
adipose tissue of obese mice and humans. J Lipid Res. 46:2347–2355.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Trayhurn P and Wood IS: Adipokines:
Inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017. View Article : Google Scholar
|
|
121
|
Yin P, Zou W, Li J, Jin N, Gao Q and Liu
F: Using high-throughput sequencing to explore the
anti-inflammatory effects of α-mangostin. Sci Rep. 9:156262019.
View Article : Google Scholar
|
|
122
|
Zou W, Yin P, Shi Y, Jin N, Gao Q, Li J
and Liu F: A novel biological role of α-mangostin via TAK1-NF-κB
pathway against inflammatory. Inflammation. 42:103–112. 2019.
View Article : Google Scholar
|
|
123
|
Mohan S, Syam S, Abdelwahab SI and
Thangavel N: An anti-inflammatory molecular mechanism of action of
α-mangostin, the major xanthone from the pericarp of Garcinia
mangostana: an in silico, in vitro and in vivo approach. Food
Funct. 9:3860–3871. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dong BM, Abano JB and Egan TM: Nitric
oxide ventilation of rat lungs from non-heart-beating donors
improves posttransplant function. Am J Transplant. 9:2707–2715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Widowati W, Darsono L, Suherman J, Fauziah
N, Maesaroh M and Erawijantari PP: Anti-inflammatory effect of
mangosteen (Garcinia mangostana L.) peel extract and its compounds
in LPS-induced RAW264.7 cells. Nat Prod Sci. 22:147–153. 2016.
View Article : Google Scholar
|
|
126
|
Franceschelli S, Pesce M, Ferrone A,
Patruno A, Pasqualone L, Carlucci G, Ferrone V, Carlucci M, de
Lutiis MA, Grilli A, et al: A novel biological role of α-mangostin
in modulating inflammatory response through the activation of
SIRT-1 signaling pathway. J Cell Physiol. 231:2439–2451. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sugiyanto Z, Yohan B, Hadisaputro S,
Dharmana E, Suharti C, Winarto, Djamiatun K, Rahmi FL and Sasmono
RT: Inhibitory effect of alpha-mangostin to dengue virus
replication and cytokines expression in human peripheral blood
mononuclear cells. Nat Prod Bioprospect. 9:345–349. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yin Q, Wu YJ, Pan S, Wang DD, Tao MQ, Pei
WY and Zuo J: Activation of cholinergic anti-inflammatory pathway
in peripheral immune cells involved in therapeutic actions of
α-mangostin on collagen-induced arthritis in rats. Drug Des Devel
Ther. 14:1983–1993. 2020. View Article : Google Scholar :
|
|
129
|
Tarasuk M, Songprakhon P, Chimma P,
Sratongno P, Na-Bangchang K and Yenchitsomanus PT: Alpha-mangostin
inhibits both dengue virus production and cytokine/chemokine
expression. Virus Res. 240:180–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yongpitakwattana P, Morchang A, Panya A,
Sawasdee N and Yenchitsomanus PT: Alpha-mangostin inhibits dengue
virus production and pro-inflammatory cytokine/chemokine expression
in dendritic cells. Arch Virol. 166:1623–1632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Herrera-Aco DR, Medina-Campos ON,
Pedraza-Chaverri J, Sciutto-Conde E, Rosas-Salgado G and
Fragoso-González G: Alpha-mangostin: Anti-inflammatory and
antioxidant effects on established collagen-induced arthritis in
DBA/1J mice. Food Chem Toxicol. 124:300–315. 2019. View Article : Google Scholar
|
|
132
|
Zuo J, Yin Q, Wang YW, Li Y, Lu LM, Xiao
ZG, Wang GD and Luan JJ: Inhibition of NF-κB pathway in
fibroblast-like synoviocytes by α-mangostin implicated in
protective effects on joints in rats suffering from
adjuvant-induced arthritis. Int Immunopharmacol. 56:78–89. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Pan T, Wu D, Cai N, Chen R, Shi X, Li B
and Pan J: Alpha-mangostin protects rat articular chondrocytes
against IL-1β-induced inflammation and slows the progression of
osteoarthritis in a rat model. Int Immunopharmacol. 52:34–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xu Y, Zhou H and Cai L: Alpha-mangostin
attenuates oxidative stress and inflammation in adjuvant-induced
arthritic rats. Trop J Pharm Res. 16:2611–2616. 2017. View Article : Google Scholar
|
|
135
|
Wen H, Gris D, Lei Y, Jha S, Zhang L,
Huang MT, Brickey WJ and Ting JP: Fatty acid-induced NLRP3-ASC
inflammasome activation interferes with insulin signaling. Nat
Immunol. 12:408–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Duan Y, Zeng L, Zheng C, Song B, Li F,
Kong X and Xu K: Inflammatory links between high fat diets and
diseases. Front Immunol. 9:26492018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Nava Catorce M, Acero G, Pedraza-Chaverri
J, Fragoso G, Govezensky T and Gevorkian G: Alpha-mangostin
attenuates brain inflammation induced by peripheral
lipopolysaccharide administration in C57BL/6J mice. J Neuroimmunol.
297:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yang Z, Yin Q, Olatunji OJ, Li Y, Pan S,
Wang DD and Zuo J: Activation of cholinergic anti-inflammatory
pathway involved in therapeutic actions of α-mangostin on
lipopolysaccharide-induced acute lung injury in rats. Int J
Immunopathol Pharmacol. 34:20587384209549412020. View Article : Google Scholar
|
|
139
|
Tatiya-Aphiradee N, Chatuphonprasert W and
Jarukamjorn K: Anti-inflammatory effect of Garcinia mangostana
Linn. pericarp extract in methicillin-resistant Staphylococcus
aureus-induced superficial skin infection in mice. Biomed
Pharmacother. 111:705–713. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lim YK, Yoo SY, Jang YY, Lee BC, Lee DS
and Kook JK: Anti-inflammatory and in vitro bone formation effects
of Garcinia mangostana L. and propolis extracts. Food Sci
Biotechnol. 29:539–548. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Xie Z, Sintara M, Chang T and Ou B: Daily
consumption of a mangosteen-based drink improves in vivo
antioxidant and anti-inflammatory biomarkers in healthy adults: A
randomized, double-blind, placebo-controlled clinical trial. Food
Sci Nutr. 3:342–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chitchumroonchokchai C, Thomas-Ahner JM,
Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD
and Failla ML: Anti-tumorigenicity of dietary α-mangostin in an
HT-29 colon cell xenograft model and the tissue distribution of
xanthones and their phase II metabolites. Mol Nutr Food Res.
57:203–211. 2013. View Article : Google Scholar
|
|
143
|
Nelli GB, K AS and Kilari EK: Antidiabetic
effect of α-mangostin and its protective role in sexual dysfunction
of streptozotocin induced diabetic male rats. Syst Biol Reprod Med.
59:319–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Choi YH, Han SY, Kim YJ, Kim YM and Chin
YW: Absorption, tissue distribution, tissue metabolism and safety
of α-mangostin in mangosteen extract using mouse models. Food Chem
Toxicol. 66:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kittipaspallop W, Taepavarapruk P,
Chanchao C and Pimtong W: Acute toxicity and teratogenicity of
α-mangostin in zebrafish embryos. Exp Biol Med (Maywood).
243:1212–1219. 2018. View Article : Google Scholar
|
|
146
|
Fajeriyati N, Muchtaridi M and Sopyan I:
Methods For improving alpha-mangostin solubility: A review. Int J
Appl Pharm. 13:47–54. 2021. View Article : Google Scholar
|
|
147
|
Aisha AF, Ismail Z, Abu-Salah KM and Majid
AM: Solid dispersions of α-mangostin improve its aqueous solubility
through self-assembly of nanomicelles. J Pharm Sci. 101:815–825.
2012. View Article : Google Scholar
|
|
148
|
Savjani KT, Gajjar AK and Savjani JK: Drug
solubility: Importance and enhancement techniques. ISRN Pharm.
2012:1957272012.PubMed/NCBI
|
|
149
|
Wathoni N, Rusdin A, Motoyama K, Joni IM,
Lesmana R and Muchtaridi M: Nanoparticle drug delivery systems for
α-mangostin. Nanotechnol Sci Appl. 13:23–36. 2020. View Article : Google Scholar :
|
|
150
|
Li L, Han AR, Kinghorn AD, Frye RF,
Derendorf H and Butterweck V: Pharmacokinetic properties of pure
xanthones in comparison to a mangosteen fruit extract in rats.
Plant Med. 79:646–653. 2013. View Article : Google Scholar
|
|
151
|
Foti RS, Pearson JT, Rock DA, Wahlstrom JL
and Wienkers LC: In vitro inhibition of multiple cytochrome P450
isoforms by xanthone derivatives from mangosteen extract. Drug
Metab Dispos. 37:1848–1855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kondo M, Zhang L, Ji H, Kou Y and Ou B:
Bioavailability and antioxidant effects of a xanthone-rich
mangosteen (Garcinia mangostana) product in humans. J Agric Food
Chem. 57:8788–8792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Singhal S, Hasan N, Nirmal K, Chawla R,
Chawla S, Kalra BS and Dhal A: Bioavailable turmeric extract for
knee osteoarthritis: A randomized, non-inferiority trial versus
paracetamol. Trials. 22:1052021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bumrungpert A, Kalpravidh RW, Suksamrarn
S, Chaivisuthangkura A, Chitchumroonchokchai C and Failla ML:
Bioaccessibility, biotransformation, and transport of
alpha-mangostin from Garcinia mangostana (mangosteen) using
simulated digestion and Caco-2 human intestinal cells. Mol Nutr
Food Res. 53(Suppl 1): S54–S61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chitchumroonchokchai C, Riedl KM,
Suksumrarn S, Clinton SK, Kinghorn AD and Failla ML: Xanthones in
mangosteen juice are absorbed and partially conjugated by healthy
adults. J Nutr. 142:675–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Rukthong P, Sereesongsang N, Kulsirirat T,
Boonnak N and Sathirakul K: In vitro investigation of metabolic
fate of α-mangostin and gartanin via skin permeation by LC-MS/MS
and in silico evaluation of the metabolites by ADMET
predictor™. BMC Complement Med Ther. 20:3592020.
View Article : Google Scholar
|
|
157
|
Thassu D, Pathak Y and Deleers M:
Nanoparticulate drug-delivery systems: An overview Nanoparticulate
drug delivery systems. CRC Press; Boca Raton, FL: pp. 1–31.
2007
|
|
158
|
Usman F, Shah HS, Zaib S, Manee S,
Mudassir J, Khan A, Batiha GE, Abualnaja KM, Alhashmialameer D and
Khan I: Fabrication and biological assessment of antidiabetic
α-mangostin loaded nanosponges: In vitro, in vivo, and in silico
studies. Molecules. 26:66332021. View Article : Google Scholar
|
|
159
|
Sodalee K, Sapsuphan P, Wongsirikul R and
Puttipipatkhachorn S: Preparation and evaluation of alpha-mangostin
solid self-emulsifying drug delivery system. Asian J Pharm Sci.
11:225–226. 2016. View Article : Google Scholar
|
|
160
|
Xu WK, Jiang H, Yang K, Wang YQ, Zhang Q
and Zuo J: Development and in vivo evaluation of self-microemulsion
as delivery system for α-mangostin. Kaohsiung J Med Sci.
33:116–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Mahmudah R, Adnyana IK and Sukandar EY:
Molecular docking studies of α-mangostin, γ-mangostin, and xanthone
on peroxisome proliferator-activated receptor gamma diphenyl
peptidase-4 enzyme, and aldose reductase enzyme as an antidiabetic
drug candidate. J Adv Pharm Technol Res. 12:196–208. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Sunitha J, Mahendra J, Mahendra L and
Devaraj N: Molecular docking studies of a-mangostin with oral
cancer targets ARRB1, FLNA, CALM3 and HTT. Bioinformation.
16:625–630. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Herz CT and Kiefer FW: Adipose tissue
browning in mice and humans. J Endocrinol. 241:R97–R109. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Choi H, Kim CS and Yu R: Quercetin
upregulates uncoupling protein 1 in white/brown adipose tissues
through sympathetic stimulation. J Obes Metab Syndr. 27:102–109.
2018. View Article : Google Scholar
|
|
165
|
Wang S, Liang X, Yang Q, Fu X, Zhu M,
Rodgers BD, Jiang Q, Dodson MV and Du M: Resveratrol enhances brown
adipocyte formation and function by activating AMP-activated
protein kinase (AMPK) α1 in mice fed high-fat diet. Mol Nutr Food
Res. 61: View Article : Google Scholar : 2017.
|




















