To systemically study the association between
pituitary hormones and female-related tumors, a PubMed search was
performed using the key terms 'growth hormone', 'prolactin',
'luteinizing hormone', 'follicle-stimulating hormone' or 'female',
including articles from 2017 to 2021, with some exceptions from the
last 10 years. The search strategy included research articles and
reviews. In total, 158 studies were included in the present review.
The pituitary gland is the most important endocrine gland in the
human body, controlling the secretion of key hormones involved in
metabolism, growth, development, and reproduction. Six main
hormones are secreted by adenohypophyseal cells: growth hormone
(GH), prolactin (PRL), thyroid-stimulating hormone (TSH),
gonadotropin [luteinizing hormone (LH) and follicle-stimulating
hormone (FSH)], and adrenocorticotropic hormone. In the present
review, the association between the GH, gonadotropin-related
hormones, and PRL, and the occurrence of associated diseases was
summarized, with a focus on tumor development.
The regulation of hypothalamic GH secretion is
mediated by the growth hormone-releasing hormone (GHRH),
somatostatin (SST), and ghrelin (1). GHRH promotes GH release by
interacting with the growth hormone-releasing hormone receptor
(GHRHR) in the somatotroph cells of the adenohypophysis through the
cyclic adenosine monophosphate (cAMP)-mediated signal transduction
pathway (2). SST inhibits GH
secretion by binding to SST receptors (2,3).
In addition, ghrelin, a hormone-releasing peptide, promotes GH
secretion by activating the growth hormone secretagogue receptor
(GHS-R) in the hypothalamus and pituitary (3). Growth hormone receptors (GHRs) are
widely distributed through the body, including in bones, muscles,
heart, brain, kidney, and fat cells (4). GH secretion may vary with age, sex,
and nutritional status, peaking at a young age due to increased
estrogen or testosterone concentrations (4-6).
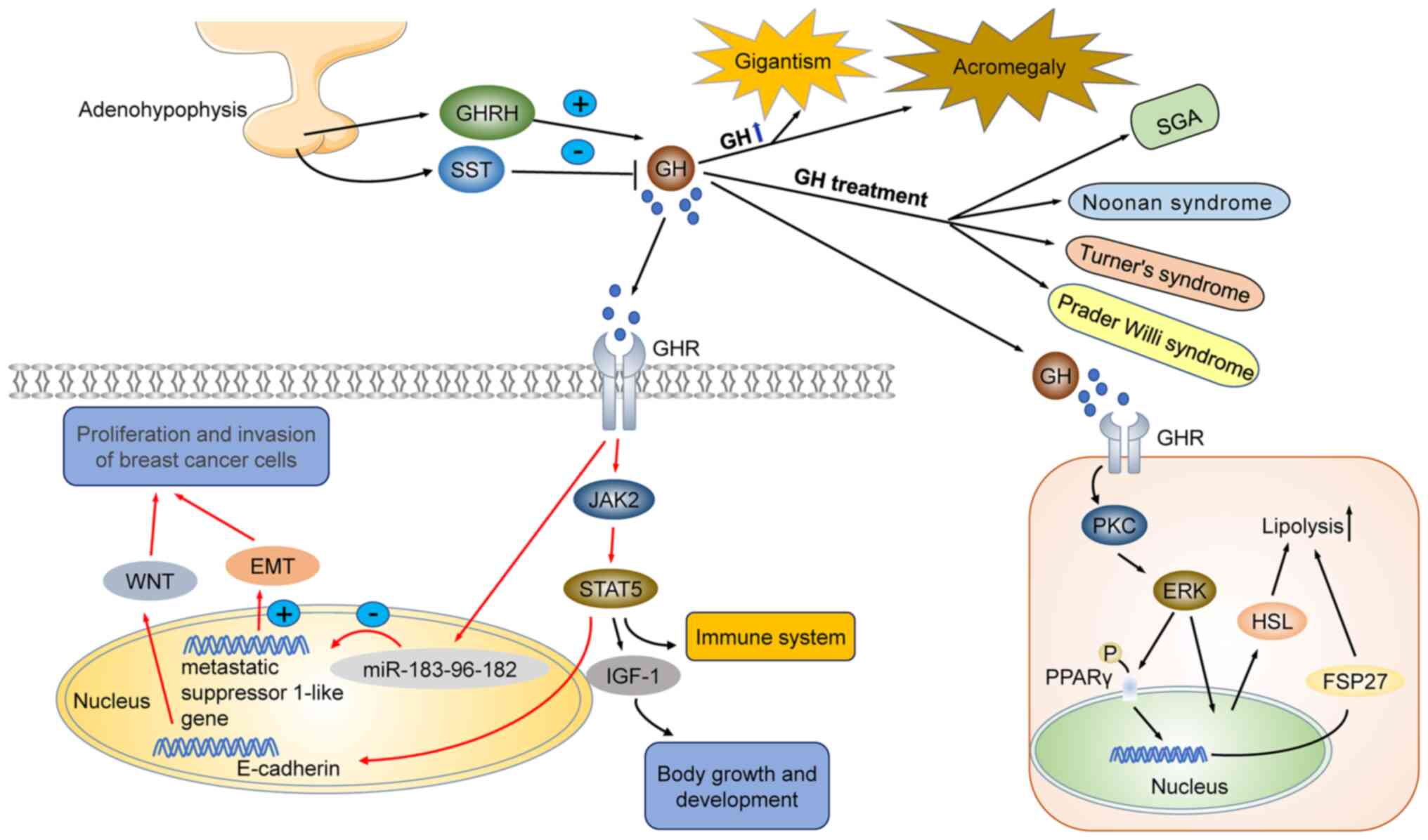
Effects of GH on growth and development. GH
misregulation is linked to several diseases. Growth hormone
deficiency (GHD) incidence is highest in children. It has been
reported that children with GHD after birth are often characterized
by slow growth and short stature (7,8).
Being a positive modulator of growth, GH is used to treat diseases
characterized by short statures, such as short stature homeobox
(SHOX) deficiency (linked to SHOX gene mutations), Turner syndrome,
Prader-Willi syndrome, Noonan syndrome, and small for gestational
age (SGA) (9-13). GH regulates human growth by
modulating the metabolism of carbohydrates, proteins and lipids
(14). Moreover, adults with GHD
experience increased total and visceral body fat, low bone and
muscle mass, reduced muscle strength, impaired anaerobic
performance, poor cardiovascular status, and poor quality of life
(15,16). Previous research has shown that GH
acts directly in chondrocytes to promote differentiation and
chondrocyte proliferation, by stimulating peripheral tissues via
upregulation of the insulin-like growth factor-1 (IGF-1),
especially in the liver (17). In
adults, excessive growth hormone secretion and increased IGF-1
concentration may lead to acromegaly. In children, excessive
secretion of GH and IGF-1 leads to gigantism (18,19). Therefore, accurate regulation of
GH is crucial to modulate human growth and development at all
ages.
Some evidence shows a close association between the
neuroendocrine and the immune systems. In vitro and in
vivo studies have demonstrated that GH is involved in immune
response, by regulating the proliferation and activity of immune
cells, namely the proliferation of leukocyte subsets with
expression of GHRs (20).
GH-activated protein kinase C (PKC) and ERK-activated
hormone-sensitive lipase (HSL) regulate lipolysis and improve sleep
efficiency (21,22). Furthermore, it is likely that GH
exerts important roles in ovulation and fertility of women
(23). Polycystic ovary syndrome
(PCOS) is the most common female endocrine disorder, affecting
5-10% of women at reproductive age. Oxidative stress (OS) plays a
central role in the pathophysiology of PCOS and other diseases. GH
reduces OS-induced apoptosis by activating the PI3K/Akt signaling
pathway, which may provide a theoretical basis for PCOS treatment
(24). Furthermore, GH
administration has been associated with successful in vitro
fertilization (IVF). Exogenous GH administration alleviates
mitochondrial dysfunction and improves oocyte quality in women
above 40, and in patients with poor ovarian response. Studies have
shown that GH treatment during ovarian stimulation in young women
who have failed IVF may increase IVF success rates by improving
oocyte quality (25).
The incidence of malignant tumors, including breast
cancer, is major public health concern in Chinese women. A
significant portion of cancers are hormone-dependent, and GH plays
key roles in the proliferation and invasion of breast cancer cells
(26). Human GH (hGH) promotes
body growth via activation of distinct biological processes,
including IGF-1 synthesis and secretion in the liver, and
activation of JAK2/STAT5 and BRAF/MEK/ERK signaling pathways. GH
triggers cell proliferation in breast cancer cells by increasing
JAK2 and STAT5 gene expression. Conversely, silencing of GHRs
blocks JAK2/STAT5 and BRAF/MEK/ERK signaling pathways, thereby
inhibiting the growth of breast cancer cells (27,28). Clinicopathological studies of
cases with breast cancer revealed that hGH expression was
positively correlated with the presence of metastases, high
clinical stage and HER2 positive lymph nodes. It has also been
reported that elevated GH levels may induce malignant
transformation of mammary epithelial cells, promote proliferation
and metastasis via activation of epithelial-mesenchymal transition
(EMT), through miR-183-96-182 cluster activation. During EMT,
epithelial cells reduce their polarity and intercellular contacts,
therefore acquiring migration and motility capacities, enabling
them to invade adjacent tissues and distant tissues. EMT is
triggered after cells receive microenvironmental signals and it is
activated during the pathogenesis of cancer (29-31).
GH promotes the proliferation and invasion of breast
cancer cells by promoting EMT. GH-mediated EMT in breast cancer may
occur via two distinct mechanisms: i) GH may directly promote EMT,
or ii) GH may promote the EMT of breast cancer cells by inducing
microRNA clustering and inhibiting the expression of breast cancer
metastasis suppressor 1-like (BRMS1L) (32,33). In addition, GH may promote the
development of breast cancer cells by downregulating connexin
E-cadherin expression and inducing the WNT signaling pathway
(32,34). Abnormal secretion of GH is
additionally associated with several human diseases. Previous
research has revealed that hGH expression is increased in
hepatocellular carcinoma (HCC) and is associated with poor survival
outcomes in HCC patients. hGH secreted by HCC promotes invasion and
cancer stem cell-like properties by inhibiting the expression of
the tight junction component CLAUDIN-1. Thus, inhibiting hGH and
hGHR signaling pathways may be a potential therapeutic approach to
limit HCC progression and recurrence (35). The specific molecular mechanism is
presented in Fig. 1.
Excessive GH secretion is often surgically treated,
followed by post-operative adjuvant drug therapy, which may include
GHR antagonists, dopamine agonists (DAs), and SST receptor ligands.
Pegvisomant is a recombinant GHR antagonist, similar to hGH, that
binds and blocks GHR activity (36). Pegvisomant treatment reestablishes
normal levels of IGF-1 in patients with acromegaly and relieves
symptoms related to excess GH (37). Cabergoline (CAB) is a dopamine
agonist that can be used in combination with pegvisomant. CAB is
the first-line treatment for hyperprolactinemia, being additionally
used to treat acromegaly (38,39). SST is a cyclic-released hormone
that functions as an inhibitory peptide. SST receptor ligand drugs,
such as octreotide, lanreotide, and pasireotide, are used in the
clinic to treat diseases caused by the excessive release of GH.
Octreotide and lanreotide are specific for the SST receptor
somatostatin receptor 2 (SSTR2), a key regulator of GH secretion
(40,41). Acromegaly is characterized by
hypersecretion of GH and elevated levels of IGF-1. The reported
leading cause of acromegaly is the presence of a GH-secreting
pituitary neuroendocrine tumor (PitNET) or GH plus PRL-secreting
mixed adenoma. Combination therapy including SST and dopamine
inhibit GH and IGF-1 secretion by binding to SSTR2 and dopamine D2
receptor (D2R), thereby inhibiting the acral lesions caused by
GH-secreting PitNETs (41-43).
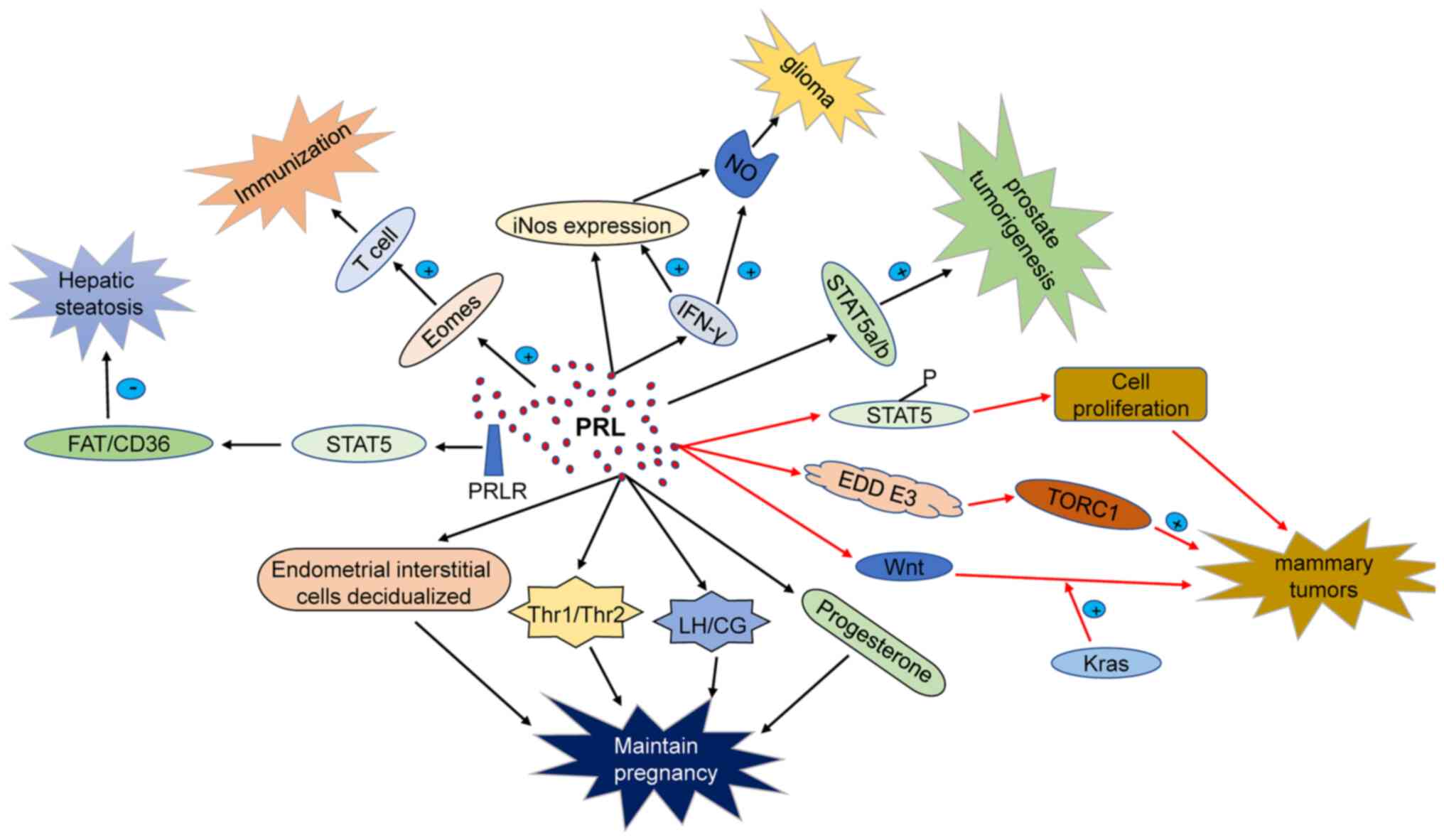
PRL is a versatile hormone and serves a wide variety
of physiological functions apart from lactation. PRL lactotrophs in
the pituitary are released in a circadian pattern depending on the
physiological state. PRL content in the human body varies slightly
with sex; with women presenting higher levels than men during
puberty (44,45). PRL further participates in immune
regulation, by binding to PRL receptors (PRLRs) in the surface of
monocytes, lymphocytes, and thymic epithelial cells (46,47). PRL secretion is controlled by
hypothalamic PRL release factors and PRL release-inhibiting
hormones, which include TSH-releasing hormone, vasoactive
intestinal peptide, serotonin, PRL release-inhibiting hormone
dopamine class material, growth hormone-releasing inhibitory
hormone (GHIH), gamma-aminobutyric acid (GABA), and thyroid
hormone, among others (48). In
addition, PRL regulates luteal function via modulation of LH
receptors in the ovaries (49).
PRL belongs to the family of growth factors that
regulate cellular and humoral immunity. During pregnancy, PRL is
key for intrauterine homeostasis and normal embryo development. PRL
levels in women who have suffered from miscarriage are
significantly lower than in women with healthy pregnancies.
Moreover, low levels of PRL are associated with low weight at birth
and premature birth (50).
PRL is synthesized in the endometrium during embryo
implantation, providing a suitable microenvironment for blastocyst
implantation through PRLR, to improve the endometrial receptivity.
Precise regulation of PRL concentration is key to promote growth
and adhesion of the endometrial cells which are essential for
embryo implantation and development (51). The space-temporal regulation of
PRLR expression suggests that PRL regulates several functions
related to mitosis and differentiation of ovarian tissue. PRL
production is positively correlated with the degree of the
decidualization of endometrial stromal cells (48). PRL secreted by decidua during
pregnancy increases after embryo implantation, reaching the peak
around 20-25 weeks of pregnancy (52). PRLR expression has further been
detected in the placental trophoblast, and amniotic epithelial
cells (53). Thus, PRL should be
adequately added as hormone replacement therapy in premature
infants (54).
In recent years, the association between human T
helper cells (Th1/Th2) in serum, lymphocytes, decidua, and
spontaneous abortion has attracted much attention. It is maintained
that Th1 cells inhibit embryo development. In contrast, Th2 cells
play key roles in maintaining a normal pregnancy. PRL regulates the
Th1/Th2 balance, particularly acting on Th1 cells. In addition, PRL
decreases natural killer (NK) cells and Th1 activity. PRL and PRLR
are abundantly expressed in uterine decidual tissue in early
pregnancy (55-57). PRL further stimulates the
expression of LH/placental secretory chorionic gonadotropin
receptor (CG receptor) in the corpus luteum after fertilization.
Moreover, PRLR, coordinates with FSH and LH, thereby regulating
cAMP and progesterone synthesis, both of which are essential during
pregnancy (58-60). Based on these studies, PRL may
regulate the local microenvironment of uterine decidua that
controls the synthesis and secretion levels of hormones and factors
by autocrine and paracrine pathways. Endogenous or exogenous
factors blocking PRL synthesis and secretion may affect the
expression of LH/CG receptors in the pregnant corpus luteum of the
ovary, which may result in miscarriage. In conclusion, PRL levels
during early pregnancy are essential to normal embryo development,
acting via luteal maintenance, immune function regulation, and
decidual development.
PRL is an essential growth factor with direct
influence on the division and proliferation of somatic cells.
Particularly, PRL plays a key role in regulating breast development
and terminal differentiation of breast epithelial cells.
Epidemiological research has revealed that elevated serum PRL
levels are associated with increased risk of breast cancer. PRL and
PRLR overexpression is observed in several tumors, including breast
cancer, and is associated with increased cell proliferation,
reduced apoptosis, and a shorter cell cycle (61).
PRL is involved in several biological processes that
include immune response, neuroprotection, and metabolic regulation.
PRL participates in immune regulation by interacting with PRLR on
the surface of immune cells such as leukocytes, macrophages,
lymphocytes, among others (46).
PRL misregulation is associated with increased risk of multiple
sclerosis and rheumatoid arthritis by regulating the number of
circulating lymphocytes (57).
PRL has been reported to exert neuroprotective functions. For
instance, in a rat glutamate (Glu) injury model, PRL maintained the
function of neuronal mitochondria by promoting intracellular
calcium release and NF-κB. PRL further regulates nerve regeneration
of non-transferrin bound iron (NTBI) by preventing calcium overload
(73,74). Finally, PRL maintains
physiological homeostasis through the regulation of the level of
insulin, liver-related enzyme activity, and intestinal
Ca2+ absorption (75-77).
Excessive secretion of PRL may lead to
hyperprolactinemia, which is frequent in hermaphroditism. DAs are
used as first-line treatment. Most commonly used drugs are CAB,
bromocriptine (BRC), and pergolide (PER) (78-80), all of which act on dopamine
receptors in the lactating cell membrane of the pituitary gland to
reduce serum levels of PRL (81),
thereby relieving the clinical symptoms of patients, and recovering
the menstruation and fertility of women. Antipsychotics also
increase the level of PRL. Among them, amisulpride, risperidone,
and paliperidone significantly increase the levels of PRL, even at
low doses (82). PRL dysfunction
inhibits the male reproductive system and reduces semen quality
(83). The antipsychotic drug
aripiprazole (ARI) decreases serum PRL levels in children and
adolescents (84). With the
increasing research on the molecular and cellular biology of the
pituitary, different target genes and gene transfer methods are
under study as therapeutic candidates against PitNET. In the
future, gene therapy will likely focus on the treatment of invasive
PitNET and residual drug resistance diseases with local invasion
following surgery (85). Gene
therapy will undoubtedly enlighten the future clinical treatment of
infections caused by abnormal PRL secretion.
Gonadotropin-releasing hormone (GnRH) is a
heterodimeric glycoprotein composed of an α subunit and a β subunit
(86-88). Gonadotropin, a member of the
glycoprotein hormone family, consists of FSH and LH. There are
several subtypes of FSH generated by post-translational
modifications, all of which affect FSH half-life through the change
of carbohydrate pattern (86).
FSH participates in follicular development and regulates follicular
secretion, maturation, and atresia by modulating granulosa cells.
Late follicular stages may induce LH receptor (LHR) synthesis by
granulosa cells, thus supporting LHR interaction with LH, resulting
in the production of a luteinizing follicle (87). In women, LH stimulates the
secretion of estrogen by theca cells, promotes ovulation, and
maintains the physiological role of luteinization. In addition,
inhibition of the hypothalamus is performed by inhibin A to
regulate the menstrual cycle of women. GnRH is a therapeutic drug
that stimulates the release of inhibin A, enhances the activity of
LH, selectively inhibits the synthesis and secretion of FSH, and
promotes the selection of dominant follicles (DF), thereby
regulating reproductive function (89-92). Duan et al described four
luteinizing hormone-choriogonadotropin receptor (LHCGR) structures
using cryo-electron microscopy (cryo-EM): Two of the structures
were the wild-type receptor in the inactive or in the active
states; and the other two structures were the constitutively active
mutated receptor. Research has revealed a unique mechanism of
receptor activation, providing a rationale for drug discovery in
endocrine-related diseases (93,94).
A new action factor, the neuropeptide kisspeptin,
which acts upstream of GnRH, has attracted the attention of the
scientific community in recent years (95). Other neuropeptides (gonadotropin
inhibitory hormone/radiofrequency amide-related peptide and other
members of the radiofrequency amide peptide superfamily), and
various non-peptide neurotransmitters (glial fibrillary acidic
protein, dopamine, neuron-derived neurotrophic factor, and
serotonin), also function as regulators of GnRH secretion and
synthesis, as well as of LH and FSH secretion (95-98). Kisspeptin/neurokinin B/dynorphin
(KNDy) neuron is a GnRH pulse generator that maintains gonadotropin
levels and follicular development (99). Nuclear receptors, such as the
steroid generation factor 1 and liver receptor homolog 1 (LRH-1),
regulate progesterone receptors to control FSH and LH. These are
also critical regulators of steroid generation, cell proliferation
and migration, and cytoskeleton remodeling (100). Concurrently, makorin ring-finger
protein 3 (MKRN3) inhibits the reproductive axis through its role
in kisspeptin-expressing neurons involved in a MKRN3-guided
ubiquitination mediation mechanism (101,102). A previous study revealed that a
high-fat diet (HFD) intake by parents may also be a risk factor for
prostate hyperplasia and cancer at advanced ages, due to a negative
impact on the reproductive system of male offspring (102,103). Thus, it is advised that
epidemiological and clinical research on semen quality must include
male offspring of overweight and/or obese parents (103,104).
Gonadotropin plays a key role in reproduction, and
the evaluation of FSH and LH is an index of ovarian reserve. There
are several recombinant preparations used to treat gonadotropin
deficiency and gonadal dysfunction. Abnormal FSH may lead to
abnormal ovarian function, such as follicular development disorder
and abnormal follicular atresia (105,106). The combination of urinary
follicle-stimulating hormone (uFSH) and recombinant
follicle-stimulating hormone (rFSH) improves ovarian stimulation
(107). In addition, GnRH
upregulates the anti-Mullerian hormone (AMH), which leads to
ovulation dysfunction and androgen elevation by causing abnormal
follicular growth and abnormal secretion of granulosa cells
(108). Furthermore, GnRH
affects bone mineral density in postmenopausal women by regulating
LH and FSH levels (109).
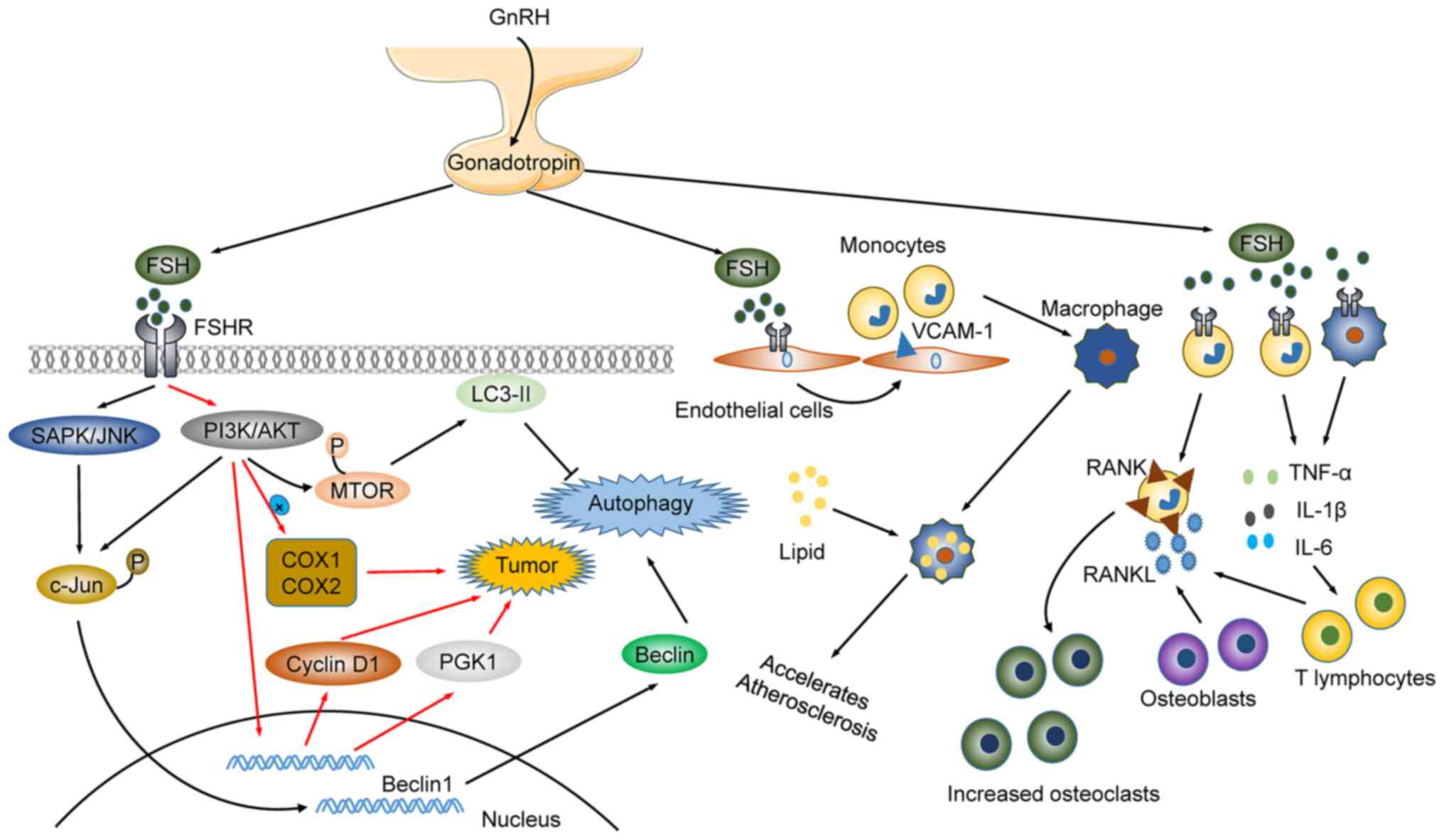
Postmenopausal osteoporosis is a risk factor for bone fracture. It
was initially proposed that estrogen decay was responsible for the
onset of postmenopausal osteoporosis. However, recent studies have
shown that FSH, which increases along with estrogen decreasing,
acts on FSH receptor (FSHR) in osteoclasts. FSH promotes osteoclast
formation and accelerates bone loss by enhancing the expression of
receptor activator of nuclear factor-κB (RANK). This suggests that
FSH, along with estrogen, may be linked to the risk of osteoporosis
in postmenopausal women (110,111). In addition, LH and FSH are
distinctly regulated. For example, LH and FSH reduce
nicotine-induced oocyte autophagy in different manners. LH reduces
nicotine-induced autophagy by restoring the phosphorylation of
adenosine 5′-monophosphate-activated protein kinase α-1. By
contrast, FSH reduces autophagy by downregulating the
phosphorylation of forkhead box O1 (FoxO1) and light chain 3
(LC3)-II (112,113). Paradoxically, FSH upregulates
Beclin1 through the PI3K/JNK/c-Jun pathway to accelerate the
degradation of lipid droplets in mammalian follicular granulosa
cells, thus enhancing autophagy. FSH affects follicular
development, and participates in the regulation of lipid metabolism
(such as cholesterol and fat), regulates bone density, and is
associated with the onset of cardiovascular diseases, and the
occurrence of breast cancer (112). FSH activates liver cholesterol
biosynthesis and decreases serum cholesterol through the
Gi2α/β-inhibin-2/Akt pathway during menopause (114). Elderly postmenopausal women with
higher FSH levels have higher bone marrow obesity rates, lower bone
density, fat, and lean meat mass than elderly postmenopausal women
with lower FSH levels (115,116). It is also suggested that FSH
regulates bone formation and regeneration, and that its
dysregulation enhances joint inflammation in rheumatoid arthritis.
Mechanistically, FSH triggers pathogenesis through the regulation
of activin A or inhibin B in the respiratory system (Fig. 3) (117,118).
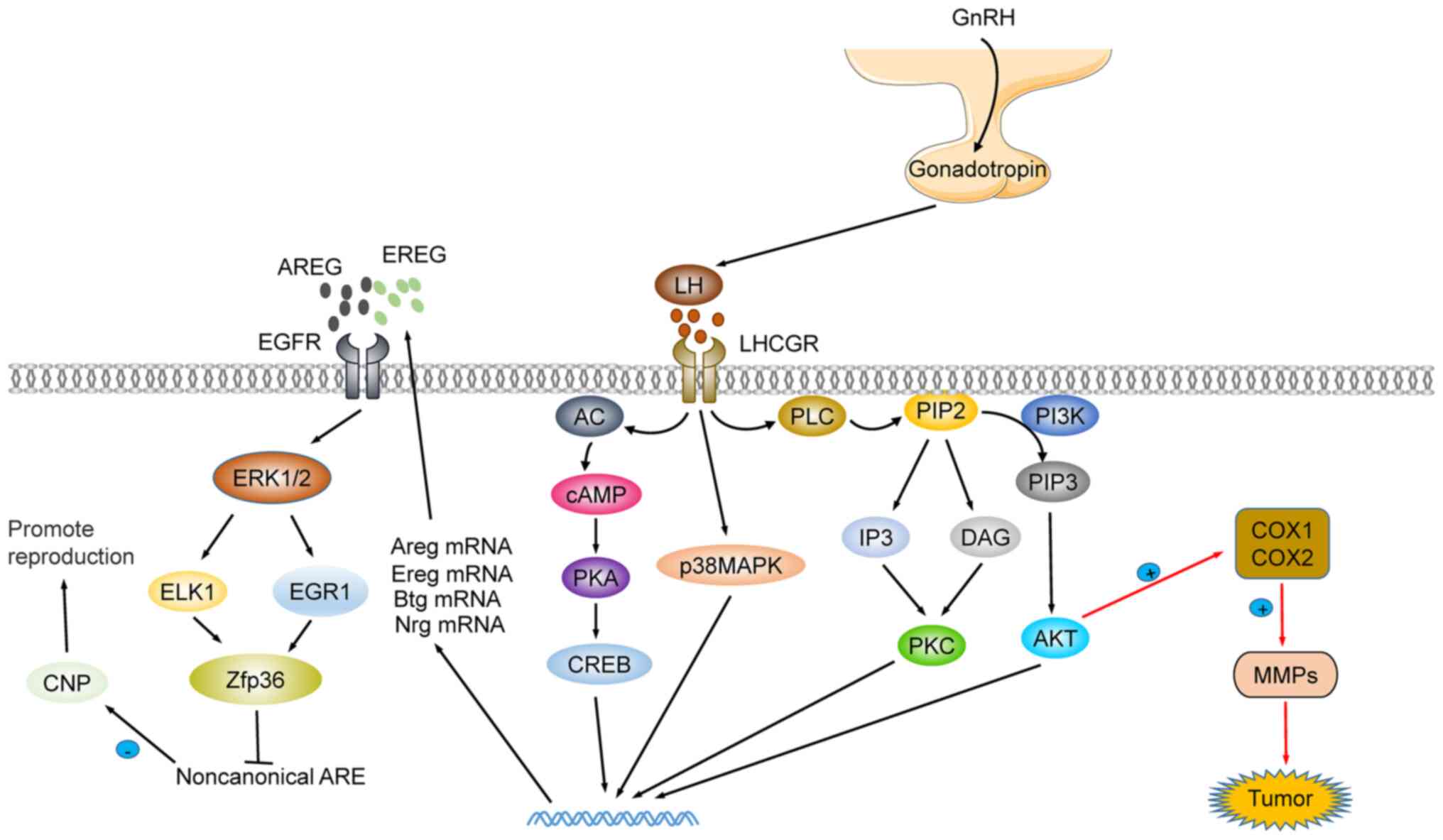
At present, research on LH function is mainly
focused on the maturation and development of follicles and the
recovery of the hematopoietic system (119,120). LH coordinates the process of
oocyte meiosis via the activation of zinc finger protein 36 (ZFP36)
expression in an EGFR-ERK1/2 dependent pathway (86,121). Moreover, LH participates in the
homeostasis of hematopoietic stem cells (HSC). The lack of LHCGR
has been revealed to accelerate the development of acute myeloid
leukemia in mice (119). The LH
blocker leuprorelin improves hematopoietic recovery after
radiotherapy or chemotherapy, by protecting LHR-expressing HSCs
(122,123). Additionally, LH is an
inflammation modulator. During follicular maturation and
development, the first responders to the LH wave are granulosa and
theca cells, which produce steroids, prostaglandins, chemokines,
and cytokines. These mediators activate ovarian cells and resident
immune cells in the ovary, and further attract additional immune
cells to the ovaries. These jointly regulate the proteolytic
pathway to recombine the follicular matrix, destroy the basal layer
of granulosa cells and promote the invasion of vascular endothelial
cells (124,125). In mice, it was revealed that LH
reduced the expression of the apoptosis-promoting protein Tap63,
inhibited cisplatin-induced oocyte apoptosis, and protected the
reproductive function of female mice following treatment (126). In addition, Li et al
reported that p62 depletion in the pituitary impairs LH synthesis
through mitochondrial OXPHOS signal transduction and leads to
female infertility. This provided a new theoretical basis for
studying female reproductive dysfunction in gonadotropic cells: The
GnRH-p62-oxphos (ndufa2)-Ca2+/ATP-LH pathway (127). Notably, LH has been described as
a therapeutic candidate in treating Alzheimer's disease (AD) and
recovering from cognitive impairment. The increase in LH has been
associated with increased risk of cognitive impairment and AD.
Cognitive improvement after LH level reestablishment, and remission
of AD symptoms after treatment with specific LHCGR blockers, have
been observed (128,129). The precise molecular mechanism
is revealed in Fig. 4.
In recent years, the expression of GnRH and its
receptor have been found in a variety of gynecological tumors,
including uterine fibroids, endometrial cancer, ovarian cancer, and
breast cancer. Ovarian cancer is a common reproductive tumor in
women. The most frequent subtype is epithelioid ovarian cancer
(EOC), which can be further divided in serous ovarian cancer (SOC),
endometrioid carcinoma, clear cell carcinoma (CCOC), mucinous
carcinoma and other less diagnosed types (130,131).
Previous studies have shown that genetic, hormonal,
and reproductive factors affect the incidence of ovarian cancer,
but its etiology and pathogenesis remain unclear. The
hypothalamus-pituitary ovary axis is an important neuroendocrine
system that plays a crucial role in regulating the female
reproductive system. Gonadotropin, as the key hub of this axis,
plays an important role in ovarian cancer incidence (132). Tumor cell metabolic
reprogramming and microenvironmental changes have been linked to
the development of ovarian tumors (133-135). In this context, FSH and LH
increase the synthesis of cyclooxygenase (COX)1 and COX2 through
the PI3K/Akt signaling pathway and the production of prostaglandin
E2 (PGE2), which itself is linked to disease progression
(hypertension, diabetes, and dyslipidemia) (136-138). PEG2 promotes the progression of
ovarian cancer by increasing the production of matrix
metalloproteinase (MMP)2 and MMP9 (139,140). In addition, FSH promotes ovarian
cancer progression by affecting the glycolysis process. For
instance, FSH promotes glycolysis in ovarian cancer cells and the
decrease of pH in the microenvironment by promoting the expression
of pyruvate kinase M2 (PKM2) and phosphoglycerate kinase 1 (PGK1)
(141,142). Therefore, gonadotropin may be
involved in the progression of ovarian cancer by modulating tumor
cell metabolism.
FSH affects the expression of progranulin (PGRN) in
CCOC, through activation of the PI3K/Akt pathway via FSHR, thereby
supporting the development of ovarian cancer. Nevertheless, FSH
regulates different molecular mechanisms via PKC modulation in
different subtypes of ovarian cancer including SOC (143). FSH increases the expression of
Gankyrin through the PI3K/Akt pathway, and promotes the
proliferation of ovarian cancer cells by regulating cyclin D1
(144). In addition, the
specific expression of FSH in the reproductive system supports the
hypothesis that FSH may be a candidate therapeutic target in
ovarian cancer (145-147). LH also regulates the PI3K/Akt
pathway by upregulating vascular endothelial growth factor (VEGF)
and Slit2 (148). VEGF can be
used as a gonadotropin to promote the progression of advanced
ovarian cancer (149). In
conclusion, gonadotropin is closely related to the occurrence and
development of ovarian cancer, and may provide insights for
targeted treatment.
The treatment of gonadotropin-associated disorders
is performed by gonadotropin-releasing hormone agonists (GnRH-a),
gonadotropin-releasing hormone antagonists (GnRH ant), estrogen
receptor modulators, aromatase inhibitors, and hormone analogs.
GnRH-a promotes the release of gonadotropin. However, the long-term
use of the GnRH-a leuprorelin results in decreased bone mineral
density (150,151). The GnRH ant, cetrorelix inhibits
the synthesis and secretion of gonadotropin by competing with GnRH
receptor on the surface of pituitary cells to block GnRH secretion
(152-154). In addition, clomiphene citrate,
an estrogen receptor regulator, increases the secretion of GnRH in
the hypothalamus by inhibiting the negative feedback regulation of
estrogen. In addition to treating female ovulation dysfunction,
clomiphene citrate also improves the levels of testosterone and
GnRH in men (155-157). Aromatase inhibitors, which are
estrogen receptor regulators, promote the release of GnRH by
modulating estrogen levels. Furthermore, gonadotropin drugs are
used to replace endogenous hormones. For example, human chorionic
gonadotropin (hCG) is used as a LH substitute to induce ovulation
(158). Hormone administration
is often more effective than oral administration, however at the
cost of the occurrence of side effects, such as increasing the risk
of multiple births and ovarian hyperstimulation syndrome
(OHSS).
In recent years, major progress in our knowledge
concerning the mechanisms of action of the three pituitary hormones
(GH, gonadotropin-related hormones, and PRL) and their roles in
human health and diseases has advanced significantly, particularly
their involvement in growth and development. GH promotes body
growth and development, and its abnormal secretion is associated
with a variety of diseases. GH is involved in breast cancer through
dysregulation of the JAK2/STAT5 pathway and promotion of EMT. PRL
is involved in distinct biological processes: It promotes milk
secretion and gonad development, and is essential for metabolism,
immunity, and fetal growth and development. In addition, PRL
misregulation is linked to human diseases including breast and
liver diseases, as well as autoimmune and endocrine diseases.
PRL-based therapy is used to manage pain, particularly migraines in
women. Moreover, PRL-based treatments are applied in the context of
different cancers, including, but not limited to, pancreatic ductal
carcinoma, glioma, breast cancer, uterine cancer, and prostate
cancer. Dysregulation of LH and FSH hormones is associated with
diseases of reproductive development in both men and women. In this
context, an accurate FSH-LH balance is critical to seminiferous
tubular development in early sexual maturation. The increase of FSH
has been observed in primary amenorrhea, congenital ovarian
hypoplasia, and primary hyper reproductive function. During the
menstrual cycle, an increase in FSH is observed during the fertile
period. Elevated FSH levels in the follicular phase are a marker of
decreased ovarian function, which in turn leads to secondary
decreases in ovarian estrogen and progesterone secretion. LH
induces testosterone synthesis, thus supporting the production of
mature sperm. Therefore, reduced LH levels are associated with a
decrease in fertility, and ultimately with infertility.
The exact role of pituitary hormones in metabolic
homeostasis remains to be fully elucidated, with recently described
functions expanding further than the standard roles of these
hormones. GH plays a unique biological role in various stages of
human development through GHRs which are expressed in different
organs. Lack of GH in childhood leads to slow growth and
development, and to dwarfism in extreme cases. In adults, lack of
GH leads to increased fat content and a high risk of cardiovascular
disease. Moreover, GH impacts fertilization by affecting egg
quality, and alleviates PCOS by reducing OS. An excessive
gonadotropin level in adults is an indicator of premature ovarian
failure, whereas its excessive levels confer a higher risk for bone
loss and cognitive impairment during the perimenopausal period.
Similarly, PRL functions are timely regulated. PRL promotes
lactation in women, and its accurate regulation is fundamental for
the development of healthy pregnancies. Thus, data indicate that
these hormones play indispensable roles in different growth stages
of the human body.
Overall, hormone regulation of the pituitary
function is extremely complex and integrates multiple regulatory
levels, ranging from subcellular to extracellular processes.
Hormonal recombination agents are more effective than oral drugs.
Although hormonal recombination agents are used to treat
developmental abnormalities and reproductive disorders, the
therapeutic effects come at the expense of several side effects,
such as the increased risk of multiple births, in cases where
gonadotropins are used to treat infertility. The use of hormonal
therapy, using single or multiple agents requires further research,
in order to limit the currently observed side effects of such
treatment approaches. The present review integrated recent research
results, aiming to provide new guidelines for future treatment
strategies for clinical management and further drug
development.
Not applicable.
ZW performed the data collection. HQ performed the
data curation. HW wrote the manuscript. WT and SQ prepared the
figures. JD and PW revised the manuscript. Data authentication is
not applicable. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was financially supported by the National
Natural Science Foundation of China (grant nos. 81602327 and
81500798) and the Funds for Zhishan Young Scholars (Southeast
University; grant no. 2242021R41070).
|
1
|
Birzniece V and Ho KKY: Mechanisms in
endocrinology: Paracrine and endocrine control of the growth
hormone axis by estrogen. Eur J Endocrinol. 184:R269–R278. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caputo M, Pigni S, Agosti E, Daffara T,
Ferrero A, Filigheddu N and Prodam F: Regulation of GH and GH
signaling by nutrients. Cells. 10:13762021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donato J Jr, Wasinski F, Furigo IC,
Metzger M and Frazão R: Central regulation of metabolism by growth
hormone. Cells. 10:1292021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Z, Huang L, Waters MJ and Chen C:
Insulin and growth hormone balance: Implications for obesity.
Trends Endocrinol Metab. 31:642–654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roelfsema F, Yang RJ, Bowers CY and
Veldhuis JD: Modulating effects of progesterone on spontaneous
nocturnal and ghrelin-induced GH secretion in postmenopausal women.
J Clin Endocrinol Metab. 104:2385–2394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cuny T, Graillon T, Defilles C, Datta R,
Zhang S, Figarella-Branger D, Dufour H, Mougel G and Brue T:
Characterization of the ability of a, second-generation SST-DA
chimeric molecule, TBR-065, to suppress GH secretion from human
GH-secreting adenoma cells. Pituitary. 24:351–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boguszewski MCS, Carlsson M, Lindberg A,
Dahlgren J, Aydin F, Camacho-Hübner C and Hokken-Koelega ACS:
Near-adult height after growth hormone treatment in children born
prematurely-data from KIGS. J Clin Endocrinol Metab.
105:dgaa2032020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hjelholt AJ, Charidemou E, Griffin JL,
Pedersen SB, Gudiksen A, Pilegaard H, Jessen N, Møller N and
Jørgensen JOL: Insulin resistance induced by growth hormone is
linked to lipolysis and associated with suppressed pyruvate
dehydrogenase activity in skeletal muscle: A 2x2 factorial,
randomised, crossover study in human individuals. Diabetologia.
63:2641–2653. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Binder G: Short stature due to SHOX
deficiency: Genotype, phenotype, and therapy. Horm Res Paediatr.
75:81–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donato B and Ferreira MJ: Cardiovascular
risk in turner syndrome. Rev Port Cardiol (Engl Ed). 37:607–621.
2018.In English, Portuguese. View Article : Google Scholar
|
|
11
|
Muscogiuri G, Formoso G, Pugliese G,
Ruggeri RM, Scarano E and Colao A: RESTARE: Prader-Willi syndrome:
An uptodate on endocrine and metabolic complications. Rev Endocr
Metab Disord. 20:239–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts AE, Allanson JE, Tartaglia M and
Gelb BD: Noonan syndrome. Lancet. 381:333–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verkauskiene R, Petraitiene I and
Albertsson Wikland K: Puberty in children born small for
gestational age. Horm Res Paediatr. 80:69–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guevara-Aguirre J, Guevara A, Palacios I,
Pérez M, Prócel P and Terán E: GH and GHR signaling in human
disease. Growth Horm IGF Res. 38:34–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boguszewski CL: Individual sensitivity to
growth hormone replacement in adults. Rev Endocr Metab Disord.
22:117–14. 2021. View Article : Google Scholar
|
|
16
|
Gasco V, Caputo M, Lanfranco F, Ghigo E
and Grottoli S: Management of GH treatment in adult GH deficiency.
Best Pract Res Clin Endocrinol Metab. 31:13–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tritos NA and Klibanski A: Effects of
growth hormone on bone. Prog Mol Biol Transl Sci. 138:193–211.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beckers A, Petrossians P, Hanson J and
Daly AF: The causes and consequences of pituitary gigantism. Nat
Rev Endocrinol. 14:705–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domene HM and Fierro-Carrión G: Genetic
disorders of GH action pathway. Growth Horm IGF Res. 38:19–23.
2018. View Article : Google Scholar
|
|
20
|
Villares R, Criado G, Juarranz Y,
Lopez-Santalla M, Garcia-Cuesta EM, Rodriguez-Frade JM, Leceta J,
Lucas P, Pablos JL, Martínez-A C, et al: Inhibitory role of growth
hormone in the induction and progression phases of collagen-induced
arthritis. Front Immunol. 9:11652018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kopchick JJ, Berryman DE, Puri V, Lee KY
and Jorgensen JOL: The effects of growth hormone on adipose tissue:
Old observations, new mechanisms. Nat Rev Endocrinol. 16:135–146.
2020. View Article : Google Scholar :
|
|
22
|
Shukur HH, Hussain-Alkhateeb L, Farholt S,
Nørregaard O, Jørgensen AP and Hoybye C: Effects of growth hormone
treatment on sleep-related parameters in adults with Prader-Willi
syndrome. J Clin Endocrinol Metab. 106:e3634–e3643. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devesa J and Caicedo D: The role of growth
hormone on ovarian functioning and ovarian angiogenesis. Front
Endocrinol (Lausanne). 10:4502019. View Article : Google Scholar
|
|
24
|
Gong Y, Luo S, Fan P, Zhu H, Li Y and
Huang W: Growth hormone activates PI3K/Akt signaling and inhibits
ROS accumulation and apoptosis in granulosa cells of patients with
polycystic ovary syndrome. Reprod Biol Endocrinol. 18:1212020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tesarik J, Galán-Lázaro M, Conde-López C,
Chiara-Rapisarda AM and Mendoza-Tesarik R: The effect of GH
administration on oocyte and zygote quality in young women with
repeated implantation failure after IVF. Front Endocrinol
(Lausanne). 11:5195722020. View Article : Google Scholar
|
|
26
|
Subramani R, Nandy SB, Pedroza DA and
Lakshmanaswamy R: Role of growth hormone in breast cancer.
Endocrinology. 158:1543–1555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coker-Gurkan A, Celik M, Ugur M, Arisan
ED, Obakan-Yerlikaya P, Durdu ZB and Palavan-Unsal N: Curcumin
inhibits autocrine growth hormone-mediated invasion and metastasis
by targeting NF-κB signaling and polyamine metabolism in breast
cancer cells. Amino Acids. 50:1045–1069. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu X, Li Y, Xu G and Fu C: Growth hormone
receptor promotes breast cancer progression via the BRAF/MEK/ERK
signaling pathway. FEBS Open Bio. 10:1013–1020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang N, Ng AS, Cai S, Li Q, Yang L and
Kerr D: Novel therapeutic strategies: Targeting
epithelial-mesenchymal transition in colorectal cancer. Lancet
Oncol. 22:e358–e368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lambert AW and Weinberg RA: Linking EMT
programmes to normal and neoplastic epithelial stem cells. Nat Rev
Cancer. 21:325–338. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baskari S, Govatati S, Madhuri V,
Nallabelli N, K PM, Naik S, Poornachandar, Balka S, Tamanam RR and
Devi VR: Influence of autocrine growth hormone on NF-κB activation
leading to epithelial-mesenchymal transition of mammary carcinoma.
Tumour Biol. 39:10104283177191212017. View Article : Google Scholar
|
|
33
|
Chesnokova V and Melmed S: Growth hormone
in the tumor microenvironment. Arch Endocrinol Metab. 63:568–575.
2019. View Article : Google Scholar
|
|
34
|
Brittain AL, Basu R, Qian Y and Kopchick
JJ: Growth hormone and the epithelial-to-mesenchymal transition. J
Clin Endocrinol Metab. 102:3662–3673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen YJ, You ML, Chong QY, Pandey V,
Zhuang QS, Liu DX, Ma L, Zhu T and Lobie PE: Autocrine human growth
hormone promotes invasive and cancer stem cell-like behavior of
hepatocellular carcinoma cells by STAT3 dependent inhibition of
CLAUDIN-1 expression. Int J Mol Sci. 18:12742017. View Article : Google Scholar :
|
|
36
|
Neggers SJ, Muhammad A and van der Lely
AJ: Pegvisomant treatment in acromegaly. Neuroendocrinology.
103:59–65. 2016. View Article : Google Scholar
|
|
37
|
Tritos NA and Biller BM: Pegvisomant: A
growth hormone receptor antagonist used in the treatment of
acromegaly. Pituitary. 20:129–135. 2017. View Article : Google Scholar
|
|
38
|
Kuhn E and Chanson P: Cabergoline in
acromegaly. Pituitary. 20:121–128. 2017. View Article : Google Scholar
|
|
39
|
Chanson P: Medical treatment of acromegaly
with dopamine agonists or somatostatin analogs. Neuroendocrinology.
103:50–58. 2016. View Article : Google Scholar
|
|
40
|
Maffezzoni F, Formenti AM, Mazziotti G,
Frara S and Giustina A: Current and future medical treatments for
patients with acromegaly. Expert Opin Pharmacother. 17:1631–1642.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang JW, Li Y, Mao ZG, Hu B, Jiang XB,
Song BB, Wang X, Zhu YH and Wang HJ: Clinical applications of
somatostatin analogs for growth hormone-secreting pituitary
adenomas. Patient Prefer Adherence. 8:43–51. 2014.PubMed/NCBI
|
|
42
|
Colao A, Grasso LFS, Giustina A, Melmed S,
Chanson P, Pereira AM and Pivonello R: Acromegaly. Nat Rev Dis
Primers. 5:202019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Valea A, Ghervan C, Carsote M, Morar A,
Iacob I, Tomesc F, Pop DD and Georgescu C: Effects of combination
therapy: Somatostatin analogues and dopamine agonists on GH and
IGF1 levels in acromegaly. Clujul Med. 88:310–313. 2015.
|
|
44
|
Augustine RA, Ladyman SR, Bouwer GT,
Alyousif Y, Sapsford TJ, Scott V, Kokay IC, Grattan DR and Brown
CH: Prolactin regulation of oxytocin neurone activity in pregnancy
and lactation. J Physiol. 595:3591–3605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Z, Luo J, Zhang C, Ma Y, Sun S, Zhang
T and Loor JJ: Mechanism of prolactin inhibition of miR-135b via
methylation in goat mammary epithelial cells. J Cell Physiol.
233:651–662. 2018. View Article : Google Scholar
|
|
46
|
Borba VV, Zandman-Goddard G and Shoenfeld
Y: Prolactin and autoimmunity. Front Immunol. 9:732018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
García-Rizo C, Vázquez-Bourgon J, Labad J,
Ortiz García de la Foz V, Gómez-Revuelta M, Juncal Ruiz M and
Crespo-Facorro B: Prolactin, metabolic and immune parameters in
naïve subjects with a first episode of psychosis. Prog
Neuropsychopharmacol Biol Psychiatry. 110:1103322021. View Article : Google Scholar
|
|
48
|
Bernard V, Young J and Binart N:
Prolactin-a pleiotropic factor in health and disease. Nat Rev
Endocrinol. 15:356–365. 2019. View Article : Google Scholar
|
|
49
|
Bernard V, Young J, Chanson P and Binart
N: New insights in prolactin: Pathological implications. Nat Rev
Endocrinol. 11:265–275. 2015. View Article : Google Scholar
|
|
50
|
Moghbeli M: Genetics of recurrent
pregnancy loss among Iranian population. Mol Genet Genomic Med.
7:e8912019. View Article : Google Scholar :
|
|
51
|
Kavarthapu R and Dufau ML: Essential role
of endogenous prolactin and CDK7 in estrogen-induced upregulation
of the prolactin receptor in breast cancer cells. Oncotarget.
8:27353–27363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ezoe K, Miki T, Ohata K, Fujiwara N,
Yabuuchi A, Kobayashi T and Kato K: Prolactin receptor expression
and its role in trophoblast outgrowth in human embryos. Reprod
Biomed Online. 42:699–707. 2021. View Article : Google Scholar
|
|
53
|
Mestre Citrinovitz AC, Langer L,
Strowitzki T and Germeyer A: Resveratrol enhances decidualization
of human endometrial stromal cells. Reproduction. 159:453–463.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Napso T, Yong HEJ, Lopez-Tello J and
Sferruzzi-Perri AN: The role of placental hormones in mediating
maternal adaptations to support pregnancy and lactation. Front
Physiol. 9:10912018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kalu E, Bhaskaran S, Thum MY, Vishwanatha
R, Croucher C, Sherriff E, Ford B and Bansal AS: Serial estimation
of Th1:th2 cytokines profile in women undergoing in-vitro
fertilization-embryo transfer. Am J Reprod Immunol. 59:206–211.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Soh MC and Moretto M: The use of biologics
for autoimmune rheumatic diseases in fertility and pregnancy.
Obstet Med. 13:5–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Borba VV, Zandman-Goddard G and Shoenfeld
Y: Prolactin and autoimmunity: The hormone as an inflammatory
cytokine. Best Pract Res Clin Endocrinol Metab. 33:1013242019.
View Article : Google Scholar
|
|
58
|
Proietto S, Cortasa SA, Corso MC, Inserra
PIF, Charif SE, Schmidt AR, Di Giorgio NP, Lux-Lantos V, Vitullo
AD, Dorfman VB and Halperin J: Prolactin is a strong candidate for
the regulation of luteal steroidogenesis in vizcachas (Lagostomus
maximus). Int J Endocrinol. 2018:19106722018. View Article : Google Scholar :
|
|
59
|
Trott JF, Schennink A, Petrie WK, Manjarin
R, VanKlompenberg MK and Hovey RC: Triennial lactation symposium:
Prolactin: The multifaceted potentiator of mammary growth and
function. J Anim Sci. 90:1674–1686. 2012. View Article : Google Scholar
|
|
60
|
Chen Y, Moutal A, Navratilova E,
Kopruszinski C, Yue X, Ikegami M, Chow M, Kanazawa I, Bellampalli
SS, Xie J, et al: The prolactin receptor long isoform regulates
nociceptor sensitization and opioid-induced hyperalgesia
selectively in females. Sci Transl Med. 12:eaay75502020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Anderson MG, Zhang Q, Rodriguez LE,
Hecquet CM, Donawho CK, Ansell PJ, Ansell PJ and Reilly EB:
ABBV-176, a PRLR antibody drug conjugate with a potent DNA-damaging
PBD cytotoxin and enhanced activity with PARP inhibition. BMC
Cancer. 21:6812021. View Article : Google Scholar
|
|
62
|
Li D, San M, Zhang J, Yang A, Xie W, Chen
Y, Lu X, Zhang Y, Zhao M, Feng X and Zheng Y: Oxytocin receptor
induces mammary tumorigenesis through prolactin/p-STAT5 pathway.
Cell Death Dis. 12:5882021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Borcherding DC, Hugo ER, Fox SR, Jacobson
EM, Hunt BG, Merino EJ and Ben-Jonathan N: Suppression of breast
cancer by small molecules that block the prolactin receptor.
Cancers (Basel). 13:26622021. View Article : Google Scholar
|
|
64
|
O'Leary KA, Rugowski DE, Shea MP, Sullivan
R, Moser AR and Schuler LA: Prolactin synergizes with canonical Wnt
signals to drive development of ER+ mammary tumors via activation
of the Notch pathway. Cancer Lett. 503:231–239. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Campbell KM, O'Leary KA, Rugowski DE,
Mulligan WA, Barnell EK, Skidmore ZL, Krysiak K, Griffith M,
Schuler LA and Griffith OL: A spontaneous aggressive ERα+ mammary
tumor model is driven by Kras activation. Cell Rep.
28:1526–1537.e4. 2019. View Article : Google Scholar
|
|
66
|
MacDonald TM, Thomas LN, Daze E, Marignani
P, Barnes PJ and Too CK: Prolactin-inducible EDD E3 ubiquitin
ligase promotes TORC1 signalling, anti-apoptotic protein
expression, and drug resistance in breast cancer cells. Am J Cancer
Res. 9:1484–1503. 2019.PubMed/NCBI
|
|
67
|
Chen X, Wu D, Zheng Y, Liu X and Wang J:
Preparation of a growth hormone receptor/prolactin receptor
bispecific antibody antagonist which exhibited anti-cancer
activity. Front Pharmacol. 11:5984232020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dandawate P, Kaushik G, Ghosh C, Standing
D, Ali Sayed AA, Choudhury S, Subramaniam D, Manzardo A, Banerjee
T, Santra S, et al: Diphenylbutylpiperidine antipsychotic drugs
inhibit prolactin receptor signaling to reduce growth of pancreatic
ductal adenocarcinoma in mice. Gastroenterology. 158:1433–1449.e27.
2020. View Article : Google Scholar
|
|
69
|
Ramirez-Hernandez G, Adan-Castro E,
Diaz-Lezama N, Ruiz-Herrera X, Martinez de la Escalera G, Macotela
Y and Clapp C: Global deletion of the prolactin receptor aggravates
streptozotocin-induced diabetes in mice. Front Endocrinol
(Lausanne). 12:6196962021. View Article : Google Scholar
|
|
70
|
Wen Y, Wang Y, Chelariu-Raicu A, Stur E,
Liu Y, Corvigno S, Bartsch F, Redfern L, Zand B, Kang Y, et al:
Blockade of the short form of prolactin receptor induces
FOXO3a/EIF-4EBP1-mediated cell death in uterine cancer. Mol Cancer
Ther. 19:1943–1954. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Asad AS, Nicola Candia AJ, Gonzalez N,
Zuccato CF, Seilicovich A and Candolfi M: The role of the prolactin
receptor pathway in the pathogenesis of glioblastoma: What do we
know so far? Expert Opin Ther Targets. 24:1121–1133. 2020.
View Article : Google Scholar
|
|
72
|
Boutillon F, Pigat N, Sala LS, Reyes-Gomez
E, Moriggl R, Guidotti JE and Goffin V: STAT5a/b deficiency delays,
but does not prevent, prolactin-driven prostate tumorigenesis in
mice. Cancers (Basel). 11:9292019. View Article : Google Scholar
|
|
73
|
ivero-Segura NA, Flores-Soto E, García de
la Cadena S, Coronado-Mares I, Gomez-Verjan JC, Ferreira DG,
Cabrera-Reyes EA, Lopes LV, Massieu L and Cerbón M:
Prolactin-induced neuroprotection against glutamate excitotoxicity
is mediated by the reduction of [Ca2+]i overload and NF-κB
activation. PLoS One. 12:e01769102017. View Article : Google Scholar
|
|
74
|
Yousefvand S, Hadjzadeh MA, Vafaee F and
Dolatshad H: The protective effects of prolactin on brain injury.
Life Sci. 263:1185472020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lopez-Vicchi F, De Winne C, Brie B,
Sorianello E, Ladyman SR and Becu-Villalobos D: Metabolic functions
of prolactin: Physiological and pathological aspects. J
Neuroendocrinol. 32:e128882020. View Article : Google Scholar
|
|
76
|
Charoenphandhu N and Krishnamra N:
Prolactin is an important regulator of intestinal calcium
transport. Can J Physiol Pharmacol. 85:569–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ponce AJ, Galván-Salas T, Lerma-Alvarado
RM, Ruiz-Herrera X, Hernández-Cortés T, Valencia-Jiménez R,
Cárdenas-Rodríguez LE, Martínez de la Escalera G, Clapp C and
Macotela Y: Low prolactin levels are associated with visceral
adipocyte hypertrophy and insulin resistance in humans. Endocrine.
67:331–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tatum RC, McGowan CM and Ireland JL:
Efficacy of pergolide for the management of equine pituitary pars
intermedia dysfunction: A systematic review. Vet J. 266:1055622020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Harris K, Murphy KE, Horn D, MacGilivray J
and Yudin MH: Safety of cabergoline for postpartum lactation
inhibition or suppression: A systematic review. J Obstet Gynaecol
Can. 42:308–315.e20. 2020. View Article : Google Scholar
|
|
80
|
Krysiak R and Okopień B: Sexual
functioning in hyperprolactinemic patients treated with cabergoline
or bromocriptine. Am J Ther. 26:e433–e440. 2019. View Article : Google Scholar
|
|
81
|
Khalil G, Khan FA, Jamal QM, Saleem A,
Masroor H and Abbas K: Change in insulin sensitivity and lipid
profile after dopamine agonist therapy in patients with
prolactinoma. Cureus. 13:e178242021.PubMed/NCBI
|
|
82
|
Peuskens J, Pani L, Detraux J and De Hert
M: The effects of novel and newly approved antipsychotics on serum
prolactin levels: A comprehensive review. CNS Drugs. 28:421–453.
2014.PubMed/NCBI
|
|
83
|
Drobnis EZ and Nangia AK: Psychotropics
and male reproduction. Adv Exp Med Biol. 1034:63–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Safer DJ, Calarge CA and Safer AM:
Prolactin serum concentrations during aripiprazole treatment in
youth. J Child Adolesc Psychopharmacol. 23:282–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Davis JR and McNeilly AS: Is pituitary
gene therapy realistic? Clin Endocrinol (Oxf). 55:427–433. 2001.
View Article : Google Scholar
|
|
86
|
Filatov M, Khramova Y, Parshina E, Bagaeva
T and Semenova M: Influence of gonadotropins on ovarian follicle
growth and development in vivo and in vitro. Zygote. 25:235–243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ulloa-Aguirre A and Lira-Albarran S:
Clinical applications of gonadotropins in the male. Prog Mol Biol
Transl Sci. 143:121–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Das N and Kumar TR: Molecular regulation
of follicle-stimulating hormone synthesis, secretion and action. J
Mol Endocrinol. 60:R131–R155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Casarini L, Santi D, Brigante G and Simoni
M: Two hormones for one receptor: Evolution, biochemistry, actions,
and pathophysiology of LH and hCG. Endocr Rev. 39:549–592. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Troppmann B, Kleinau G, Krause G and
Gromoll J: Structural and functional plasticity of the luteinizing
hormone/choriogonadotrophin receptor. Hum Reprod Update.
19:583–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Son WY, Das M, Shalom-Paz E and Holzer H:
Mechanisms of follicle selection and development. Minerva Ginecol.
63:89–102. 2011.PubMed/NCBI
|
|
92
|
Themmen APN and Huhtaniemi IT: Mutations
of gonadotropins and gonadotropin receptors: Elucidating the
physiology and pathophysiology of pituitary-gonadal function.
Endocr Rev. 21:551–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Duan J, Xu P, Cheng X, Mao C, Croll T, He
X, Shi J, Luan X, Yin W, You E, et al: Structures of full-length
glycoprotein hormone receptor signalling complexes. Nature.
598:688–692. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jiang X, Dias JA and He X: Structural
biology of glycoprotein hormones and their receptors: Insights to
signaling. Mol Cell Endocrinol. 382:424–451. 2014. View Article : Google Scholar
|
|
95
|
Abbara A, Clarke SA and Dhillo WS:
Clinical potential of kisspeptin in reproductive health. Trends Mol
Med. 27:807–823. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Skorupskaite K and Anderson RA:
Hypothalamic neurokinin signalling and its application in
reproductive medicine. Pharmacol Ther. 230:1079602022. View Article : Google Scholar
|
|
97
|
Messina A, Pulli K, Santini S, Acierno J,
Känsäkoski J, Cassatella D, Xu C, Casoni F, Malone SA, Ternier G,
et al: Neuron-derived neurotrophic factor is mutated in congenital
hypogonadotropic hypogonadism. Am J Hum Genet. 106:58–70. 2020.
View Article : Google Scholar :
|
|
98
|
Vanacker C, Defazio RA, Sykes CM and
Moenter SM: A role for glial fibrillary acidic protein
(GFAP)-expressing cells in the regulation of gonadotropin-releasing
hormone (GnRH) but not arcuate kisspeptin neuron output in male
mice. Elife. 10:e682052021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Uenoyama Y, Nagae M, Tsuchida H, Inoue N
and Tsukamura H: Role of KNDy neurons expressing kisspeptin,
neurokinin B, and dynorphin A as a GnRH pulse generator controlling
mammalian reproduction. Front Endocrinol (Lausanne). 12:7246322021.
View Article : Google Scholar
|
|
100
|
Hughes CHK and Murphy BD: Nuclear
receptors: Key regulators of somatic cell functions in the
ovulatory process. Mol Aspects Med. 78:1009372021. View Article : Google Scholar
|
|
101
|
Abreu AP, Toro CA, Song YB, Navarro VM,
Bosch MA, Eren A, Liang JN, Carroll RS, Latronico AC, Rønnekleiv
OK, et al: MKRN3 inhibits the reproductive axis through actions in
kisspeptin-expressing neurons. J Clin Invest. 130:4486–4500.
2020.PubMed/NCBI
|
|
102
|
Li M, Chen Y, Liao B, Tang J, Zhong J and
Lan D: The role of kisspeptin and MKRN3 in the diagnosis of central
precocious puberty in girls. Endocr Connect. 10:1147–1154. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sertorio MN, Estadella D, Ribeiro DA and
Pisani LP: Could parental high-fat intake program the reproductive
health of male offspring? A review. Crit Rev Food Sci Nutr. 1–8.
2021.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Santoro N, Schauer IE, Kuhn K, Fought AJ,
Babcock-Gilbert S and Bradford AP: Gonadotropin response to insulin
and lipid infusion reproduces the reprometabolic syndrome of
obesity in eumenorrheic lean women: A randomized crossover trial.
Fertil Steril. 116:566–574. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hsueh AJ and He J: Gonadotropins and their
receptors: Coevolution, genetic variants, receptor imaging, and
functional antagonists. Biol Reprod. 99:3–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Chu YL, Xu YR, Yang WX and Sun Y: The role
of FSH and TGF-β superfamily in follicle atresia. Aging (Albany
NY). 10:305–321. 2018. View Article : Google Scholar
|
|
107
|
Smitz J, Wolfenson C, Chappel S and Ruman
J: Follicle-stimulating hormone: A review of form and function in
the treatment of infertility. Reprod Sci. 23:706–716. 2016.
View Article : Google Scholar
|
|
108
|
di Clemente N, Racine C, Pierre A and
Taieb J: Anti-Müllerian hormone in female reproduction. Endocr Rev.
42:753–782. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mills EG, Yang L, Nielsen MF, Kassem M,
Dhillo WS and Comninos AN: The relationship between bone and
reproductive hormones beyond estrogens and androgens. Endocr Rev.
42:691–719. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhu D, Li X, Macrae VE, Simoncini T and Fu
X: Extragonadal effects of follicle-stimulating hormone on
osteoporosis and cardiovascular disease in women during menopausal
transition. Trends Endocrinol Metab. 29:571–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chin KY: The relationship between
follicle-stimulating hormone and bone health: Alternative
explanation for bone loss beyond oestrogen? Int J Med Sci.
15:1373–1383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu WX, Zhang YJ, Wang YF, Klinger FG, Tan
SJ, Farini D, De Felici M, Shen W and Cheng SF: Protective
mechanism of luteinizing hormone and follicle-stimulating hormone
against nicotine-induced damage of mouse early folliculogenesis.
Front Cell Dev Biol. 9:7233882021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kumariya S, Ubba V, Jha RK and Gayen JR:
Autophagy in ovary and polycystic ovary syndrome: Role, dispute and
future perspective. Autophagy. 17:2706–2733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Guo Y, Zhao M, Bo T, Ma S, Yuan Z, Chen W,
He Z, Hou X, Liu J, Zhang Z, et al: Blocking FSH inhibits hepatic
cholesterol biosynthesis and reduces serum cholesterol. Cell Res.
29:151–166. 2019. View Article : Google Scholar :
|
|
115
|
Veldhuis-Vlug AG, Woods GN, Sigurdsson S,
Ewing SK, Le PT, Hue TF, Vittinghoff E, Xu K, Gudnason V,
Sigurdsson G, et al: Serum FSH is associated with BMD, bone marrow
adiposity, and body composition in the AGES-Reykjavik study of
older adults. J Clin Endocrinol Metab. 106:e1156–e1169. 2021.
View Article : Google Scholar :
|
|
116
|
Wu KC, Ewing SK, Li X, Sigurðsson S,
Guðnason V, Kado DM, Hue TF, Woods GN, Veldhuis-Vlug AG,
Vittinghoff E, et al: FSH level and changes in bone mass and body
composition in older women and men. J Clin Endocrinol Metab.
106:2876–2889. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bloise E, Ciarmela P, Dela Cruz C, Luisi
S, Petraglia F and Reis FM: Activin A in mammalian physiology.
Physiol Rev. 99:739–780. 2019. View Article : Google Scholar
|
|
118
|
Bernard DJ, Smith CL and Brûlé E: A tale
of two proteins: Betaglycan, IGSF1, and the continuing search for
the inhibin B receptor. Trends Endocrinol Metab. 31:37–45. 2020.
View Article : Google Scholar
|
|
119
|
Peng YJ, Yu H, Hao X, Dong W, Yin X, Lin
M, Zheng J and Zhou BO: Luteinizing hormone signaling restricts
hematopoietic stem cell expansion during puberty. EMBO J.
37:e989842018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Del Castillo LM, Buigues A, Rossi V,
Soriano MJ, Martinez J, De Felici M, Lamsira HK, Di Rella F,
Klinger FG, Pellicer A and Herraiz S: The cyto-protective effects
of LH on ovarian reserve and female fertility during exposure to
gonadotoxic alkylating agents in an adult mouse model. Hum Reprod.
36:2514–2528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xi G, An L, Wang W, Hao J, Yang Q, Ma L,
Lu J, Wang Y, Wang W, Zhao W, et al: The mRNA-destabilizing protein
tristetraprolin targets 'meiosis arrester' Nppc mRNA in mammalian
preovulatory follicles. Proc Natl Acad Sci USA.
118:e20183451182021. View Article : Google Scholar
|
|
122
|
Dalle IA, Paranal R, Zarka J, Paul S,
Sasaki K, Li W, Ning J, Short NJ, Ohanian M, Cortes JE, et al:
Impact of luteinizing hormone suppression on hematopoietic recovery
after intensive chemotherapy in patients with leukemia.
Haematologica. 106:1097–1105. 2021.
|
|
123
|
Elias HK and Van den Brink MRM: New option
for improving hematological recovery: Suppression of luteinizing
hormone. Haematologica. 106:929–931. 2021.
|
|
124
|
Navarro VM: Metabolic regulation of
kisspeptin-the link between energy balance and reproduction. Nat
Rev Endocrinol. 16:407–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Duffy DM, Ko C, Jo M, Brannstrom M and
Curry TE: Ovulation: Parallels with inflammatory processes. Endocr
Rev. 40:369–416. 2019. View Article : Google Scholar :
|
|
126
|
Rossi V, Lispi M, Longobardi S, Mattei M,
Di Rella F, Salustri A, De Felici M and Klinger FG: LH prevents
cisplatin-induced apoptosis in oocytes and preserves female
fertility in mouse. Cell Death Differ. 24:72–82. 2017. View Article : Google Scholar :
|
|
127
|
Li X, Zhou L, Peng G, Liao M, Zhang L, Hu
H, Long L, Tang X, Qu H, Shao J, et al: Pituitary P62 deficiency
leads to female infertility by impairing luteinizing hormone
production. Exp Mol Med. 53:1238–1249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Blair JA, Bhatta S, McGee H and Casadesus
G: Luteinizing hormone: Evidence for direct action in the CNS. Horm
Behav. 76:57–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Burnham VL and Thornton JE: Luteinizing
hormone as a key player in the cognitive decline of Alzheimer's
disease. Horm Behav. 76:48–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Natanzon Y, Goode EL and Cunningham JM:
Epigenetics in ovarian cancer. Semin Cancer Biol. 51:160–169. 2018.
View Article : Google Scholar :
|
|
131
|
Kossaï M, Leary A, Scoazec JY and Genestie
C: Ovarian cancer: A heterogeneous disease. Pathobiology. 85:41–49.
2018. View Article : Google Scholar
|
|
132
|
Cheung J, Lokman NA, Abraham RD,
Macpherson AM, Lee E, Grutzner F, Ghinea N, Oehler MK and
Ricciardelli C: Reduced gonadotrophin receptor expression is
associated with a more aggressive ovarian cancer phenotype. Int J
Mol Sci. 22:712020. View Article : Google Scholar
|
|
133
|
Wang Z and Dong C: Gluconeogenesis in
cancer: Function and regulation of PEPCK, FBPase, and G6Pase.
Trends Cancer. 5:30–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
MacLean DM and Jayaraman V: Acid-sensing
ion channels are tuned to follow high-frequency stimuli. J Physiol.
594:2629–2645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu J, Leng T, Jing L, Jiang N, Chen D, Hu
Y, Xiong ZG and Zha XM: Two di-leucine motifs regulate trafficking
and function of mouse ASIC2a. Mol Brain. 9:92016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Dong HW, Wang K, Chang XX, Jin FF, Wang Q,
Jiang XF, Liu JR, Wu YH and Yang C: Beta-ionone-inhibited
proliferation of breast cancer cells by inhibited COX-2 activity.
Arch Toxicol. 93:2993–3003. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.
Cancer Sci. 107:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Davenport JR, Cai Q, Ness RM, Milne G,
Zhao Z, Smalley WE, Zheng W and Shrubsole MJ: Evaluation of
pro-inflammatory markers plasma C-reactive protein and urinary
prostaglandin-E2 metabolite in colorectal adenoma risk. Mol
Carcinog. 55:1251–1261. 2016. View Article : Google Scholar :
|
|
139
|
Feng D, Zhao T, Yan K, Liang H, Liang J,
Zhou Y, Zhao W and Ling B: Gonadotropins promote human ovarian
cancer cell migration and invasion via a cyclooxygenase 2-dependent
pathway. Oncol Rep. 38:1091–1098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lau MT, Wong AS and Leung PC:
Gonadotropins induce tumor cell migration and invasion by
increasing cyclooxygenases expression and prostaglandin E(2)
production in human ovarian cancer cells. Endocrinology.
151:2985–2993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li S, Ji X, Wang R and Miao Y:
Follicle-stimulating hormone promoted pyruvate kinase isozyme type
M2-induced glycolysis and proliferation of ovarian cancer cells.
Arch Gynecol Obstet. 299:1443–1451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang J, Zhang J, Wei Y, Li Q and Wang Q:
ACTL6A regulates follicle-stimulating hormone-driven glycolysis in
ovarian cancer cells via PGK1. Cell Death Dis. 10:8112019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Perez-Juarez CE, Arechavaleta-Velasco F,
Mendez C and Díaz-Cueto L: Progranulin expression induced by
follicle-stimulating hormone in ovarian cancer cell lines depends
on the histological subtype. Med Oncol. 37:592020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen J, Bai M, Ning C, Xie B, Zhang J,
Liao H, Xiong J, Tao X, Yan D, Xi X, et al: Gankyrin facilitates
follicle-stimulating hormone-driven ovarian cancer cell
proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway.
Oncogene. 35:2506–2517. 2016. View Article : Google Scholar
|
|
145
|
Zhang M, Zhang M, Wang J, Cai Q, Zhao R,
Yu Y, Tai H, Zhang X and Xu C: Retro-inverso follicle-stimulating
hormone peptide-mediated polyethylenimine complexes for targeted
ovarian cancer gene therapy. Drug Deliv. 25:995–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang MX, Hong SS, Cai QQ, Zhang M, Chen
J, Zhang XY and Xu CJ: Transcriptional control of the MUC16
promoter facilitates follicle-stimulating hormone
peptide-conjugated shRNA nanoparticle-mediated inhibition of
ovarian carcinoma in vivo. Drug Deliv. 25:797–806. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jiang N, Wu J, Leng T, Yang T, Zhou Y,
Jiang Q, Wang B, Hu Y, Ji YH, Simon RP, et al: Region specific
contribution of ASIC2 to acidosis-and ischemia-induced neuronal
injury. J Cereb Blood Flow Metab. 37:528–540. 2017. View Article : Google Scholar :
|
|
148
|
Liao H, Zhou Q, Gu Y, Duan T and Feng Y:
Luteinizing hormone facilitates angiogenesis in ovarian epithelial
tumor cells and metformin inhibits the effect through the mTOR
signaling pathway. Oncol Rep. 27:1873–1888. 2012.PubMed/NCBI
|
|
149
|
Garrido MP, Bruneau N, Vega M, Selman A,
Tapia JC and Romero C: Follicle-stimulating hormone promotes nerve
growth factor and vascular endothelial growth factor expression in
epithelial ovarian cells. Histol Histopathol. 35:961–971.
2020.PubMed/NCBI
|
|
150
|
Zhang J, Sun YF, Xu YM, Shi BJ, Han Y, Luo
ZY, Zhao ZM, Hao GM and Gao BL: Effect of endometrium thickness on
clinical outcomes in luteal phase short-acting GnRH-a long protocol
and GnRH-Ant protocol. Front Endocrinol (Lausanne). 12:5787832021.
View Article : Google Scholar
|
|
151
|
Sauerbrun-Cutler MT and Alvero R: Short-
and long-term impact of gonadotropin-releasing hormone analogue
treatment on bone loss and fracture. Fertil Steril. 112:799–803.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Tepekoy F, Uysal F, Acar N, Ustunel I and
Akkoyunlu G: The effect of GnRH antagonist cetrorelix on Wnt
signaling members in pubertal and adult mouse ovaries. Histochem
Cell Biol. 152:423–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Doroszko M, Chrusciel M, Stelmaszewska J,
Slezak T, Anisimowicz S, Plöckinger U, Quinkler M, Bonomi M,
Wolczynski S, Huhtaniemi I, et al: GnRH antagonist treatment of
malignant adrenocortical tumors. Endocr Relat Cancer. 26:103–117.
2019. View Article : Google Scholar
|
|
154
|
Xu H, Zhao S, Gao X, Wu X, Xia L, Zhang D,
Li J, Zhang A and Xu B: GnRH antagonist protocol with cessation of
cetrorelix on trigger day improves embryological outcomes for
patients with sufficient ovarian reserve. Front Endocrinol
(Lausanne). 12:7588962021. View Article : Google Scholar
|
|
155
|
Practice Committee of the American Society
for Reproductive Medicine. Electronic address: asrm@asrm.org:
Practice Committee of the American Society for Reproductive
Medicine: Evidence-based treatments for couples with unexplained
infertility: A guideline. Fertil Steril. 113:305–322. 2020.
View Article : Google Scholar
|
|
156
|
Krzastek SC, Sharma D, Abdullah N, Sultan
M, Machen GL, Wenzel JL, Ells A, Chen X, Kavoussi M, Costabile RA,
et al: Long-term safety and efficacy of clomiphene citrate for the
treatment of hypogonadism. J Urol. 202:1029–1035. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Miller GD, Moore C, Nair V, Hill B,
Willick SE, Rogol AD and Eichner D:
Hypothalamic-pituitary-testicular axis effects and urinary
detection following clomiphene administration in males. J Clin
Endocrinol Metab. 104:906–914. 2019. View Article : Google Scholar
|
|
158
|
Kirshenbaum M, Haas J, Nahum R, Aizer A,
Yinon Y and Orvieto R: The effect of ovarian stimulation on
endothelial function-A prospective cohort study using peripheral
artery tonometry. J Clin Endocrinol Metab. 105:dgaa6812020.
View Article : Google Scholar : PubMed/NCBI
|