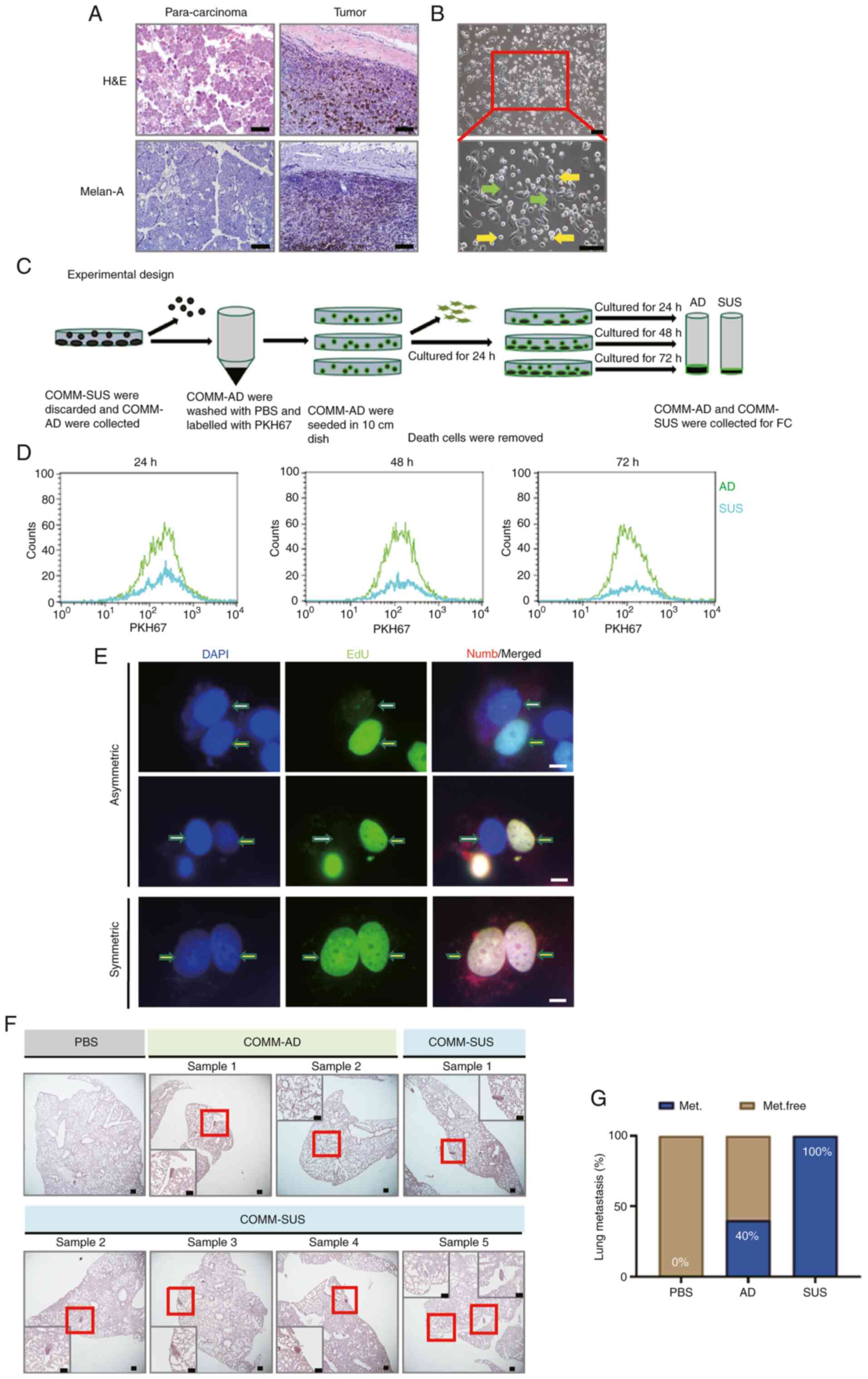
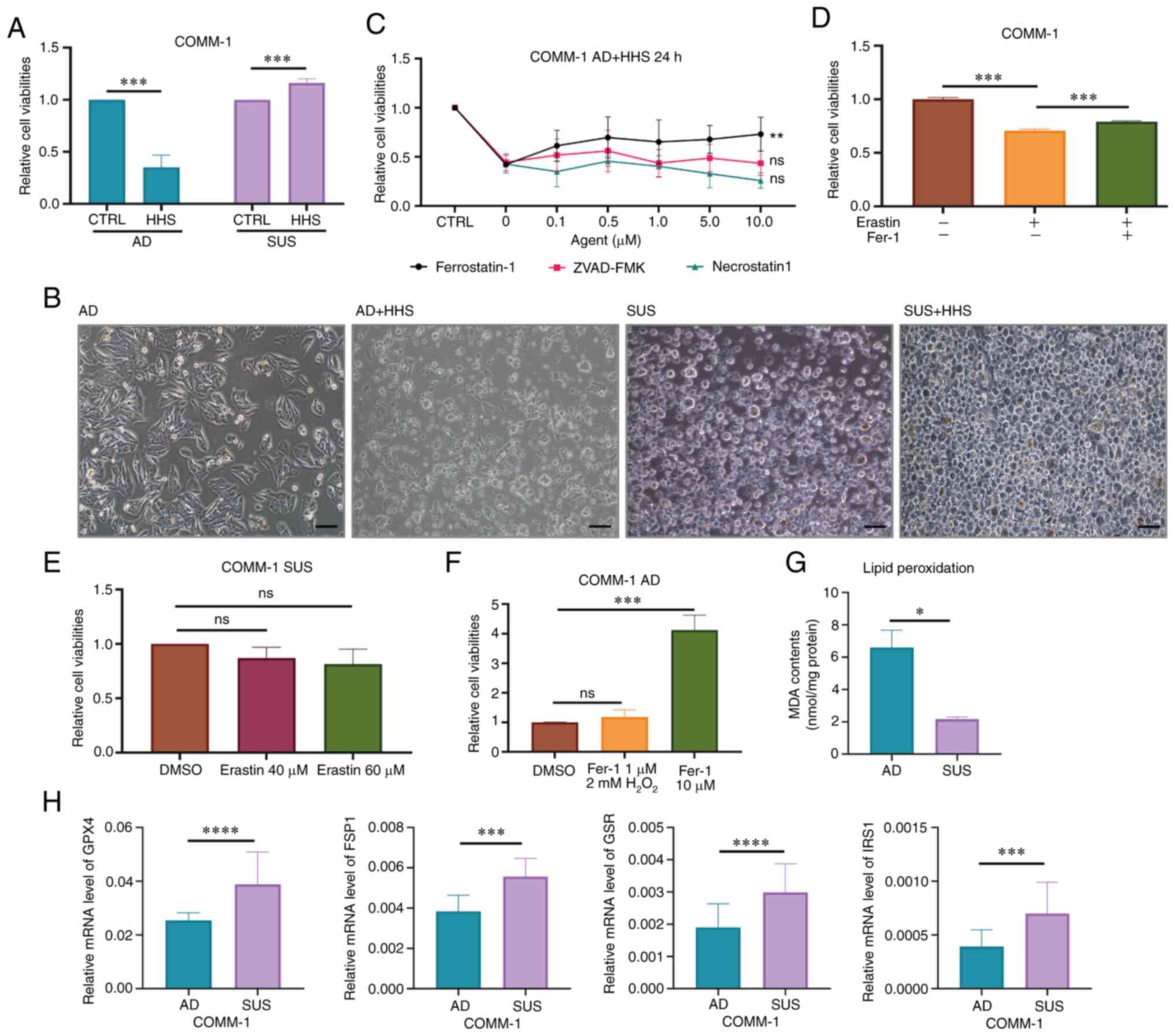
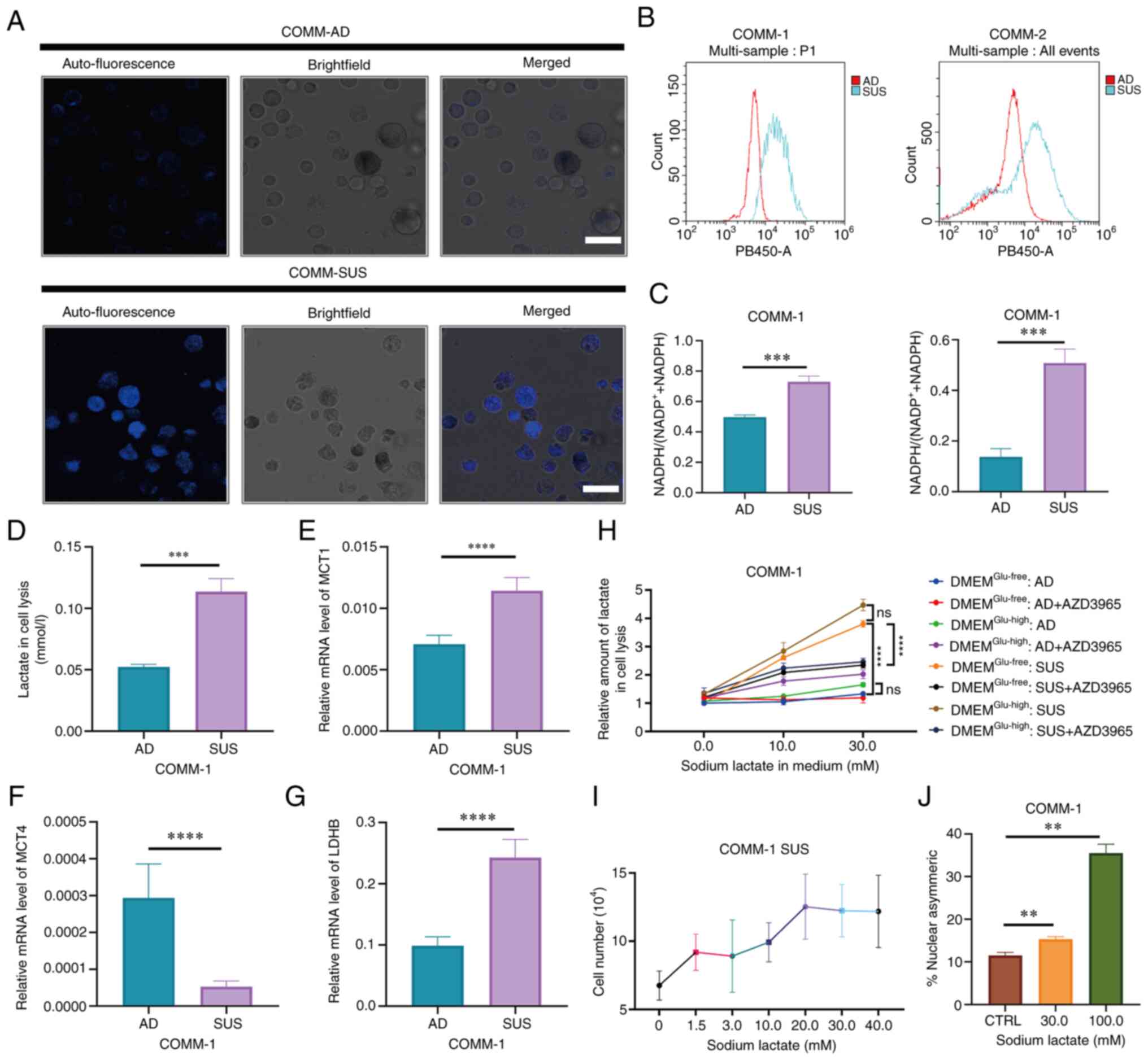
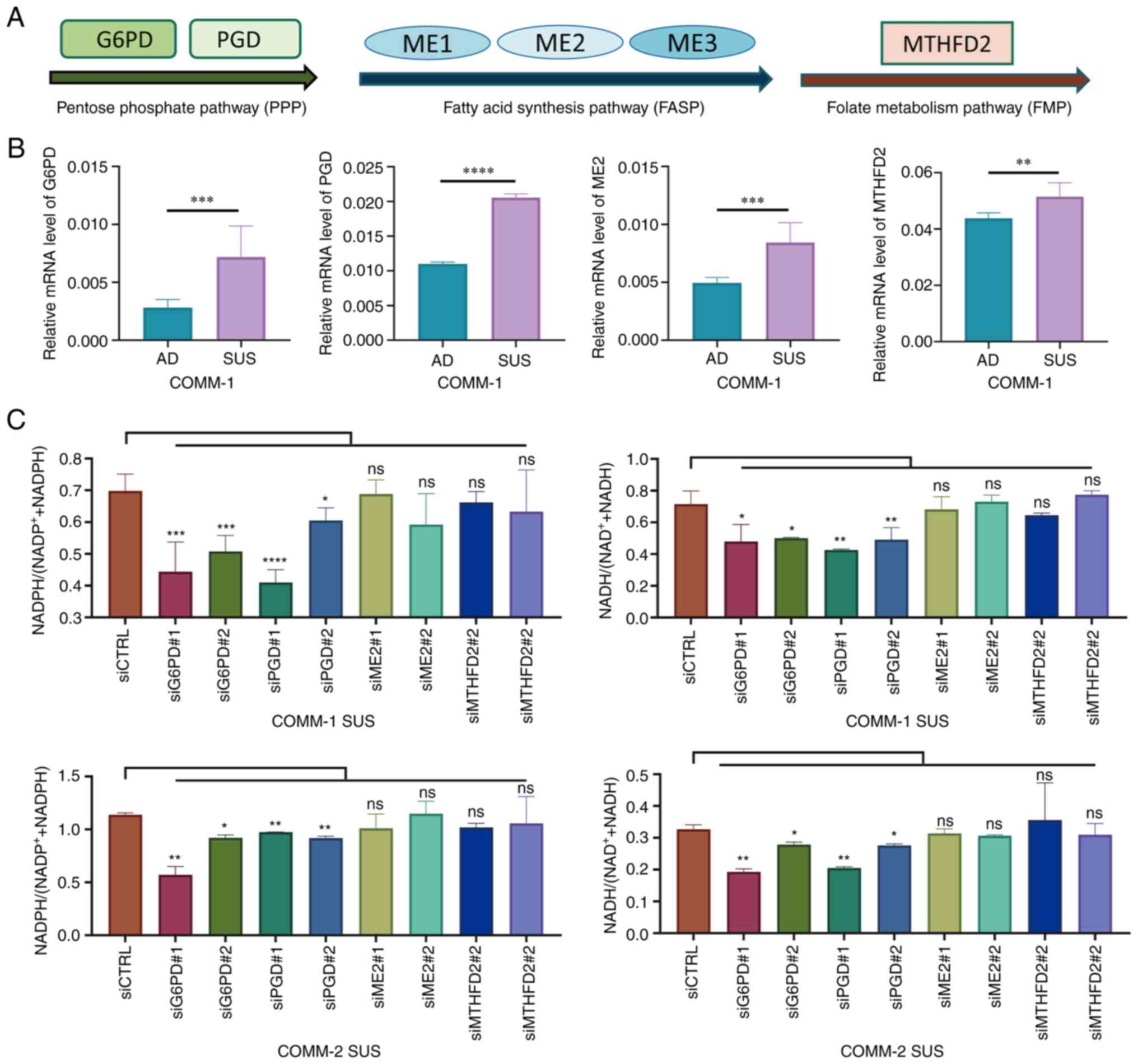
|
1
|
Spencer KR and Mehnert JM: Mucosal
melanoma: Epidemiology, biology and treatment. Cancer Treat Res.
167:295–320. 2016. View Article : Google Scholar
|
|
2
|
Nassar KW and Tan AC: The mutational
landscape of mucosal melanoma. Semin Cancer Biol. 61:139–148. 2020.
View Article : Google Scholar :
|
|
3
|
Merkel EA and Gerami P: Malignant melanoma
of sun-protected sites: A review of clinical, histological, and
molecular features. Lab Invest. 97:630–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ascierto PA, Accorona R, Botti G, Farina
D, Fossati P, Gatta G, Gogas H, Lombardi D, Maroldi R, Nicolai P,
et al: Mucosal melanoma of the head and neck. Crit Rev Oncol
Hematol. 112:136–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis LE, Shalin SC and Tackett AJ:
Current state of melanoma diagnosis and treatment. Cancer Biol
Ther. 20:1366–1379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yde SS, Sjoegren P, Heje M and Stolle LB:
Mucosal melanoma: A literature review. Curr Oncol Rep. 20:282018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poh A: First oncolytic viral therapy for
melanoma. Cancer Discov. 6:62016. View Article : Google Scholar
|
|
8
|
Killock D: Skin cancer: T-VEC oncolytic
viral therapy shows promise in melanoma. Nat Rev Clin Oncol.
12:4382015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A, Kalluri R and Kshitiz: Systems biology of cancer
metastasis. Cell Syst. 9:109–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: The link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsoi J, Robert L, Paraiso K, Galvan C,
Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al:
Multi-stage differentiation defines melanoma subtypes with
differential vulnerability to drug-induced iron-dependent oxidative
stress. Cancer Cell. 33:890–904.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan Yanxiang, Guo J, et
al: Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu M, Zhang X, Zhang W, Chiou YS, Qian W,
Liu X, Zhang M, Yan H, Li S, Li T, et al: Cancer stem cell
regulated phenotypic plasticity protects metastasized cancer cells
from ferroptosis. Nat Commun. 13:13712022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohshima K and Morii E: Metabolic
reprogramming of cancer cells during tumor progression and
metastasis. Metabolites. 11:282021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Icard P, Shulman S, Farhat D, Steyaert JM,
Alifano M and Lincet H: How the Warburg effect supports
aggressiveness and drug resistance of cancer cells? Drug Resist
Updat. 38:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar :
|
|
29
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell
Metab. 30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
30
|
Pisarsky L, Bill R, Fagiani E, Dimeloe S,
Goosen RW, Hagmann J, Hess C and Christofori G: Targeting metabolic
symbiosis to overcome resistance to anti-angiogenic therapy. Cell
Rep. 15:1161–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
García-Cañaveras JC, Chen L and Rabinowitz
JD: The tumor metabolic microenvironment: Lessons from lactate.
Cancer Res. 79:3155–3162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tasdogan A, Faubert B, Ramesh V,
Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin
M, et al: Metabolic heterogeneity confers differences in melanoma
metastatic potential. Nature. 577:115–120. 2020. View Article : Google Scholar
|
|
33
|
Zhang G, Zhang Y, Dong D, Wang F, Ma X,
Guan F and Sun L: MCT1 regulates aggressive and metabolic
phenotypes in bladder cancer. J Cancer. 9:2492–2501. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Felmlee MA, Jones RS, Rodriguez-Cruz V,
Follman KE and Morris ME: Monocarboxylate transporters (SLC16):
Function, regulation, and role in health and disease. Pharmacol
Rev. 72:466–485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao HJ, Zhao MC, Zhang YJ, Zhou DS, Xu L,
Li GB, Chen MS and Liu J: Monocarboxylate transporter 4 predicts
poor prognosis in hepatocellular carcinoma and is associated with
cell proliferation and migration. J Cancer Res Clin Oncol.
141:1151–1162. 2015. View Article : Google Scholar
|
|
37
|
Wang Y, Li Y, Jiang L, Ren X, Cheng B and
Xia J: Prognostic value of glycolysis markers in head and neck
squamous cell carcinoma: A meta-analysis. Aging (Albany NY).
13:7284–7299. 2021. View Article : Google Scholar
|
|
38
|
Pertega-Gomes N, Felisbino S, Massie CE,
Vizcaino JR, Coelho R, Sandi C, Simoes-Sousa S, Jurmeister S,
Ramos-Montoya A, Asim M, et al: A glycolytic phenotype is
associated with prostate cancer progression and aggressiveness: A
role for monocarboxylate transporters as metabolic targets for
therapy. J Pathol. 236:517–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilde L, Roche M, Domingo-Vidal M, Tanson
K, Philp N, Curry J and Martinez-Outschoorn U: Metabolic coupling
and the reverse Warburg effect in cancer: Implications for novel
biomarker and anticancer agent development. Semin Oncol.
44:198–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huhta H, Helminen O, Palomäki S, Kauppila
JH, Saarnio J, Lehenkari PP and Karttunen TJ: Intratumoral lactate
metabolism in Barrett's esophagus and adenocarcinoma. Oncotarget.
8:228942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu Y and Zeng F: Expressions of GPR81,
MCT1 and MCT4 in squamous carcinoma and their clinical
significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 43:950–956.
2018.In Chinese. PubMed/NCBI
|
|
42
|
Nakayama Y, Torigoe T, Inoue Y, Minagawa
N, Izumi H, Kohno K and Yamaguchi K: Prognostic significance of
monocarboxylate transporter 4 expression in patients with
colorectal cancer. Exp Ther Med. 3:25–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hubert CG, Rivera M, Spangler LC, Wu Q,
Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE and Rich JN: A
three-dimensional organoid culture system derived from human
glioblastomas recapitulates the hypoxic gradients and cancer stem
cell heterogeneity of tumors found in vivo. Cancer Res.
76:2465–2477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Post Y, Puschhof J, Beumer J, Kerkkamp HM,
de Bakker MA, Slagboom J, de Barbanson B, Wevers NR, Spijkers XM,
Olivier T, et al: Snake venom gland organoids. Cell.
180:233–247.e21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boretto M, Maenhoudt N, Luo X, Hennes A,
Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I,
et al: Patient-derived organoids from endometrial disease capture
clinical heterogeneity and are amenable to drug screening. Nat Cell
Biol. 21:1041–1051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Haider N, Dutt P, van de Kooij B, Ho J,
Palomero L, Pujana MA, Yaffe M and Stambolic V: NEK10 tyrosine
phosphorylates p53 and controls its transcriptional activity.
Oncogene. 39:5252–5266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
48
|
Park E and Chung SW: ROS-mediated
autophagy increases intracellular iron levels and ferroptosis by
ferritin and transferrin receptor regulation. Cell Death Dis.
10:8222019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ding CC, Rose J, Sun T, Wu J, Chen PH, Lin
CC, Yang WH, Chen KY, Lee H, Xu E, et al: MESH1 is a cytosolic
NADPH phosphatase that regulates ferroptosis. Nat Metab. 2:270–277.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018. View Article : Google Scholar
|
|
52
|
Burrell RA, McGranahan N, Bartek J and
Swanton C: The causes and consequences of genetic heterogeneity in
cancer evolution. Nature. 501:338–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng H, Pomyen Y, Hernandez MO, Li C,
Livak F, Tang W, Dang H, Greten TF, Davis JL, Zhao Y, et al:
Single-cell analysis reveals cancer stem cell heterogeneity in
hepatocellular carcinoma. Hepatology. 68:127–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang DC and Wang X: Systems heterogeneity:
An integrative way to understand cancer heterogeneity. Semin Cell
Dev Biol. 64:1–4. 2017. View Article : Google Scholar
|
|
55
|
Hitomi M, Chumakova AP, Silver DJ, Knudsen
AM, Pontius WD, Murphy S, Anand N, Kristensen BW and Lathia JD:
Asymmetric cell division promotes therapeutic resistance in
glioblastoma stem cells. JCI Insight. 6:e1305102021. View Article : Google Scholar :
|
|
56
|
Marjanovic ND, Weinberg RA and Chaffer CL:
Cell plasticity and heterogeneity in cancer. Clin Chem. 59:168–179.
2013. View Article : Google Scholar
|
|
57
|
Gerlach C, Rohr JC, Perié L, van Rooij N,
van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB,
et al: Heterogeneous differentiation patterns of individual
CD8+ T cells. Science. 340:635–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bergers G and Fendt SM: The metabolism of
cancer cells during metastasis. Nat Rev Cancer. 21:162–180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu L, Chen X, Wang L and Chen S: The sweet
trap in tumors: Aerobic glycolysis and potential targets for
therapy. Oncotarget. 7:38908–38926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brisson L, Bański P, Sboarina M, Dethier
C, Danhier P, Fontenille MJ, Van Hée VF, Vazeille T, Tardy M,
Falces J, et al: Lactate dehydrogenase B controls lysosome activity
and autophagy in cancer. Cancer Cell. 30:418–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kennedy KM, Scarbrough PM, Ribeiro A,
Richardson R, Yuan H, Sonveaux P, Landon CD, Chi JT, Pizzo S,
Schroeder T and Dewhirst MW: Catabolism of exogenous lactate
reveals it as a legitimate metabolic substrate in breast cancer.
PLoS One. 8:e751542013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Silva A, Antunes B, Batista A,
Pinto-Ribeiro F, Baltazar F and Afonso J: In vivo anticancer
activity of AZD3965: A systematic review. Molecules. 27:1812021.
View Article : Google Scholar
|
|
63
|
Polański R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar
|
|
64
|
Benyahia Z, Blackman MC, Hamelin L,
Zampieri LX, Capeloa T, Bedin ML, Vazeille T, Schakman O and
Sonveaux P: In vitro and in vivo characterization of MCT1 inhibitor
AZD3965 confirms preclinical safety compatible with breast cancer
treatment. Cancers (Basel). 13. pp. 5692021, View Article : Google Scholar
|
|
65
|
Beloueche-Babari M, Wantuch S, Casals
Galobart T, Koniordou M, Parkes HG, Arunan V, Chung YL, Eykyn TR,
Smith PD and Leach MO: MCT1 inhibitor AZD3965 increases
mitochondrial metabolism, facilitating combination therapy and
noninvasive magnetic resonance spectroscopy. Cancer Res.
77:5913–5924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang G, Wang JJ, Yin PH, Xu K, Wang YZ,
Shi F, Gao J and Fu XL: New strategies for targeting glucose
metabolism-mediated acidosis for colorectal cancer therapy. J Cell
Physiol. 234:348–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fan J, Ye J, Kamphorst JJ, Shlomi T,
Thompson CB and Rabinowitz JD: Quantitative flux analysis reveals
folate-dependent NADPH production. Nature. 510:298–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lewis C A, Parker SJ, Fiske BP, McCloskey
D, Gui D Y, Green CR, Vokes NI, Feist AM, Vander Heiden MG and
Metallo CM: Tracing compartmentalized NADPH metabolism in the
cytosol and mitochondria of mammalian cells. Mol Cell. 55:253–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang X, Liu Z, Sun J, Song X, Bian M, Wang
F, Yan F and Yu Z: Inhibition of NADPH oxidase 4 attenuates
lymphangiogenesis and tumor metastasis in breast cancer. FASEB J.
35:e215312021.PubMed/NCBI
|
|
71
|
Lu YX, Ju HQ, Liu ZX, Chen DL, Wang Y,
Zhao Q, Wu QN, Zeng ZL, Qiu HB, Hu PS, et al: ME1 regulates NADPH
homeostasis to promote gastric cancer growth and metastasis. Cancer
Res. 78:1972–1985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tamošiūnas M, Plorina EV, Lange M, Derjabo
A, Kuzmina I, Bļizņuks D and Spigulis J: Autofluorescence imaging
for recurrence detection in skin cancer postoperative scars. J
Biophotonics. 13:e2019001622020. View Article : Google Scholar
|
|
73
|
Huang TT, Chen KC, Wong TY, Chen CY, Chen
WC, Chen YC, Chang MH, Wu DY, Huang TY, Nioka S, et al: Two-channel
auto-fluorescence analysis for oral cancer. J Biomed Opt. 24:1–10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gulali A, Mustafa S, Ibrahim K, Erkus E,
Ozer B, Kocak MZ, Yaman S, Keyif F, Altinordu R, Erkol H and Savli
H: Could red cell distribution width be a marker of thyroid cancer?
J Coll Physicians Surg Pak. 27:556–558. 2017.
|
|
75
|
Chamma E, Qiu J, Lindvere-Teene L,
Blackmore KM, Majeed S, Weersink R, Dickie CI, Griffin AM, Wunder
JS, Ferguson PC and DaCosta RS: Optically-tracked handheld
fluorescence imaging platform for monitoring skin response in the
management of soft tissue sarcoma. J Biomed Opt. 20:0760112015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Waaijer L, Filipe MD, Simons J, van der
Pol CC, de Boorder T, van Diest PJ and Witkamp AJ: Detection of
breast cancer precursor lesions by autofluorescence ductoscopy.
Breast Cancer. 28:119–129. 2021. View Article : Google Scholar
|
|
77
|
Borile G, Sandrin D, Filippi A, Anderson
KI and Romanato F: Label-free multiphoton microscopy: Much more
than fancy images. Int J Mol Sci. 22:26572021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Salvagno GL, Sanchis-Gomar F, Picanza A
and Lippi G: Red blood cell distribution width: A simple parameter
with multiple clinical applications. Crit Rev Clin Lab Sci.
52:86–105. 2015. View Article : Google Scholar
|
|
79
|
Ma W, Mao S, Bao M, Wu Y, Guo Y, Liu J,
Wang R, Li C, Zhang J, Zhang W and Yao X: Prognostic significance
of red cell distribution width in bladder cancer. Transl Androl
Urol. 9:295–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang PF, Song SY, Guo H, Wang TJ, Liu N
and Yan CX: Prognostic role of pretreatment red blood cell
distribution width in patients with cancer: A meta-analysis of 49
studies. J Cancer. 10:43052019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han F, Liu Y, Cheng S, Sun Z, Sheng C, Sun
X, Shang X, Tian W, Wang X, Li J, et al: Diagnosis and survival
values of neutrophil-lymphocyte ratio (NLR) and red blood cell
distribution width (RDW) in esophageal cancer. Clin Chim Acta.
488:150–158. 2019. View Article : Google Scholar
|
|
82
|
Ghosh M, Saha S, Bettke J, Nagar R,
Parrales A, Iwakuma T, van der Velden AWM and Martinez LA: Mutant
p53 suppresses innate immune signaling to promote tumorigenesis.
Cancer Cell. 39:494–508.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Watson MJ, Vignali PDA, Mullett SJ,
Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV,
Rittenhouse NL, DePeaux K, Whetstone RD, et al: Metabolic support
of tumour-infiltrating regulatory T cells by lactic acid. Nature.
591:645–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Karsten E, Breen E, McCracken SA, Clarke S
and Herbert BR: Red blood cells exposed to cancer cells in culture
have altered cytokine profiles and immune function. Sci Rep.
10:77272020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li ZX, Zheng ZQ, Wei ZH, Zhang LL, Li F,
Lin L, Liu RQ, Huang XD, Lv JW, Chen FP, et al: Comprehensive
characterization of the alternative splicing landscape in head and
neck squamous cell carcinoma reveals novel events associated with
tumorigenesis and the immune microenvironment. Theranostics.
9:7648–7665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Duan Q, Zhang H, Zheng J and Zhang L:
Turning cold into hot: Firing up the tumor microenvironment. Trends
Cancer. 6:605–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hou J, Karin M and Sun B: Targeting
cancer-promoting inflammation-have anti-inflammatory therapies come
of age? Nat Rev Clin Oncol. 18:261–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7:1932021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Boumahdi S and de Sauvage FJ: The great
escape: Tumour cell plasticity in resistance to targeted therapy.
Nat Rev Drug Discov. 19:39–56. 2020. View Article : Google Scholar
|
|
92
|
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL,
Cheng K, Sun RY, Zhou D, Han J and Wu Q: Tom20 senses
iron-activated ROS signaling to promote melanoma cell pyroptosis.
Cell Res. 28:1171–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ubellacker JM, Tasdogan A, Ramesh V, Shen
B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB,
Spitz DR, et al: Lymph protects metastasizing melanoma cells from
ferroptosis. Nature. 585:113–118. 2020. View Article : Google Scholar : PubMed/NCBI
|