Introduction
Melanoma is a malignancy that has the highest rate
of increase in incidence worldwide, with a high tendency for
metastasis (1-3). Among other subtypes of melanoma,
mucosal melanoma (MM) in the oral and maxillofacial regions is a
particularly aggressive malignancy with a poor prognosis (4). A variety of treatment strategies
have been utilized for improving upon the poor 5-year survival
rate, such as anti-programmed death-1/programmed death-ligand-1,
anti-cytotoxic T-cell lymphocyte-4 antibody immunotherapies
(5,6) and oncolytic viral therapy (7,8).
However, therapy for patients with MM presenting with distant
metastasis remains a major challenge in the clinical setting due to
the poorly understood pathological drivers.
Following metastasis, cancer cells acquire
advantageous alterations in their physiology that facilitate cell
proliferation, genome integrity maintenance and immune escape to
survive various stresses or nutrient deprivation (9). Previous in vivo models have
revealed that only 0.02% disseminated melanoma cells in the
circulation survive to form metastases (10). This is due to the fact that
disseminated cancer cells must overcome harsh environments,
including physical damage from hemodynamic shear forces,
immune-mediated killing, and various forms of cell death induced by
genetic dysregulation, including apoptosis, anoikis and ferroptosis
(11).
Ferroptosis is characterized as an reactive oxygen
species (ROS)-dependent form of regulated cell death that occurs
downstream of iron accumulation and excessive lipid
peroxidation-mediated membrane damage (12,13). Ferroptosis is initiated by
polyunsaturated fatty acid peroxidation and subsequent membrane
damage, of which malondialdehyde (MDA) is one of the by-products of
lipid peroxidation (14-16). To date, three pathways have been
demonstrated to induce ferroptosis. The glutathione peroxidase 4
(GPX4)-glutathione (GSH)-cysteine axis is the central and most
upstream node of the ferroptosis death cascade (17). In addition, there are the
ferroptosis suppressor protein 1 (FSP1)-ubiquinol-nicotinamide
adenine dinucleotide hydrogen/NADPH (18) and the GTP cyclohydrolase
I-tetrahydrobiopterin axes (19,20). Among these signaling axes, GSH is
considered to be the reducing substrate of GPX4, whereby the
intracellular GSH concentration is maintained by a complex
homeostatic mechanism (21).
Additionally, glutathione-disulfide reductase (GSR) is a
glutathione reductase that catalyzes the transformation of
glutathione disulfide (GSSG) to GSH. Previous studies have
demonstrated that therapy-resistant cancer cells are potentially
susceptible to regulation by the GPX4-/GSH-/cysteine axis,
indicating vulnerability to this form of cell death (22,23). By contrast, ferroptosis-resistant
cancer cells exhibit a greater viability by becoming dormant before
metastasizing when favorable conditions arise (24).
During metastatic progression, cancer cells can not
only remodel the tumor microenvironment to render it more suitable
for secondary tumorigenesis, but also improve their potential
plasticity (25). This can be
mediated by metabolic reprogramming and acquisition of the ability
to resist oxidative stress. Despite being generally known to be a
waste product of anaerobic metabolism, lactate has been previously
shown to be a major source of carbons for the tricarboxylic acid
cycle (TCA) in both normal and cancerous tissues, even in the
presence of glucose (26-28). Indeed, the range of lactate
detected in solid tumors has been found to be 10-40 mM (29). As an indicator of metabolic
reprogramming, lactate may serve as an energy source to meet the
cellular energy demands and activate the associated signaling
pathways (30). Monocarboxylate
transporters (MCTs) are members of a cytoplasmic membrane protein
family that rapidly exchange lactate to allow cells to use lactate
more efficiently (31), depending
on the lactate concen-tration and extracellular pH (28). MCTs have been reported to
contribute to cancer development through multiple mechanisms, such
as MCT1 and MCT4 (28,32,33). MCT1 and MCT4 are ubiquitously
expressed. In addition, MCT4 has the lowest affinity for lactate
among all members of MCTs and therefore primarily facilitates
lactate efflux from glycolytic cells (30,34). Aerobic glycolysis has been
suggested to regulate lactate production and NADH accumulation
(27,35). Accumulating clinical evidence has
indicated that high expression levels of MCT1 or MCT4 are
associated with poorer prognosis (36-38). Furthermore, increasing MCT1 or
MCT4 expression can promote colorectal, bladder, gastric,
esophageal and cervical cancer cell migration and metastasis
(28,33,39-42).
Cancer cell lines are extensively used to
investigate caner heterogeneity; however, cancer cells tend to
gradually lose heterogeneity during long-term cell culture
(43-45). Therefore, in the present study,
two novel MM cell lines were established to investigate whether
metabolic heterogeneity can protect metastatic MM cells from
ferroptosis to promote distal metastasis.
Materials and methods
Chemicals and reagents
The ferroptosis inhibitor, ferrostatin-1 (Fer-1, 10
mM, cat. no. HY-100579) and the ferroptosis inducer, Erastin (10
mM, cat. no. HY-15763), were obtained from MedChemExpress. The
apoptosis inhibitor, Z-VAD-FMK (1 mM, cat. no. S7023), the necrosis
inhibitor, necrostatin-1 (10 mM, cat. no. S8037), the MCT1
inhibitor, AZD3965 (10 mM, cat. no. S7339), and the
glucose-6-phosphate dehydrogenase (G6PD) inhibitor,
6-aminonicotinamide (6AN, 100 mM, cat. no. S9783), were purchased
form Selleck Chemicals. All reagents were dissolved in DMSO (cat.
no. D2650, MilliporeSigma) for in vitro experiments, the
treatment durations were 6 h, 12 h or 24 h, as indicated. Mice were
intraperitoneally with 6AN (10 mg/kg), AZD3965 (30 mg/kg) or Fer-1
(1 mg/kg) every 2 days, respectively. All reagents were dissolved
in corn oil (cat. no. C40543.36, Thermo Fisher Scientific, Inc.)
for the in vivo experiments. H2O2 (5
M, cat. no. C04045101; Nanjing Chemical Reagent Co., Ltd.) were
stored at 4°C and diluted with culture medium.
Healthy human serum (HHS)
Healthy human blood was obtained from healthy adult
donors. Donors provided written consent for using their blood, and
all personal information was kept confidential as required. The
experiment followed the protocol approved by the Ethics Committee
of the Stomatological Hospital of Sun Yat-sen University,
Guangzhou, China. The blood was centrifuged at 3,000 × g for 15 min
at 4°C, and the upper serum was carefully removed and filtered
through a 0.22 µm filter (MilliporeSigma) for further
analysis.
Human specimen and cell culture
Chinese oral mucosal melanoma (COMM)-1 and COMM-2
cell lines were established from human mucosal melanoma specimens
obtained from the Hospital of Stomatology, Sun Yat-sen University,
which was followed the protocol approved by the Ethics Committee of
the Stomatological Hospital of Sun Yat-sen University. The patients
provided written consent for the use of their tissue in research,
and all personal information was kept confidential as required. All
cells were cultured in Dulbecco's modified Eagle's medium/F12
(DF12, MilliporeSigma) supplemented with 10% fetal bovine serum
(FBS, Bioloical Industries). 293T cells and A375 cells were
purchased from the American Type Culture Collection (ATCC) and
cultured in Dulbecco's modified Eagle's medium (DMEM,
MilliporeSigma) supplemented with 10% FBS (Bioloical Industries).
All the cell lines were confirmed to be free of bacteria and
mycoplasma contamination and cultured at 37°C in humidified chamber
with 5% CO2. All the cells treated with the different
reagents including inhibitors, erastin or
H2O2 which were cultured in serum-free DF12
supplemented with 10 µg/ml human insulin, 5 µg/ml
human transferrin, 10 µg/ml heparin, 10 µg/ml
ascorbic acid, 5 µg/ml bovine serum albumin-oleic acid
(BSA-oleid), 10 µM 2-aminoethanol, 10 nM sodium selenite and
10 µM mercaptoethanol. The authentication of both cell lines
and tissues was confirmed by short tandem repeat (STR, Guangzhou
Ige Biotechnology, Ltd.).
Hematoxylin and eosin (H&E) staining,
immunohisto-chemistry (IHC) and immunofluorescence (IF)
analysis
The primary and secondary antibodies used in the
present study are presented in Table
SI. The tissues were fixed in 10% formalin for 24 h, and then
embedded in paraffin, and sliced into 4.5-µm-thick sections.
Following deparaffinization in xylene twice for 10 min each and
then rehydration in 100, 90, 80 and 70% ethanol and water for 5 min
each, the sections were stained with H&E or were processed for
IHC. For H&E, the sections were stained with hematoxylin (cat.
no. CTS-1090; MXB Biotechnologies) at room temperature for 10 min
and rinsed with tap water for 15 min. The sections were then
stained with eosin and dehydrated in ascending ethanol solutions
(70, 80, 90 and 100%). They were then rinsed in xylene and
cover-slipped with synthetic neutral resin (cat. no. G8590-100,
Beijing Solarbio Science & Technology Co., Ltd.). For IHC, the
sections were incubated in fresh 3% hydrogen peroxide/methanol
solution at room temperature for 10 min to quench endogenous
peroxidase activity. The sections were then incubated in citrate
buffer and heated at 100°C for 10 min for antigen retrieval. After
cooling, the tissue slides were washed with PBS and incubated with
5% goat serum (cat. no. AR0009; Wuhan Boster Biological Technology,
Ltd.) at room temperature to block non-specific binding sites and
incubated with primary antibody at 4°C overnight. After washing
with PBS, the sections were incubated with secondary antibody (cat.
no. KIT-5005, MaxVision™ HRP-Polymer anti-Rabbit IHC kit; MXB
Biotechnologies) at room temperature for 25 min. The sections were
rinsed and stained in diaminobenzidine (DAB, cat. no. DAB-0031 MXB
Biotechnologies), stained with hematoxylin at room temperature for
10 min and rinsed with tap water for 15 min. The sections were
examined under a microscope (Nikon Eclipse Ni-E fluorescence
microscope; Nikon Corporation).
For IF, following incubation with EdU incorporation
(500 mmol, Invitrogen; Thermo Fisher Scientific, Inc.) for 2 weeks
at 37°C in humidified chamber with 5% CO2, the cells
were seeded in 24-well plates and then treated with various
concentrations of sodium lactate for 48 h. Subsequently, the cells
were fixed with 4% paraformaldehyde for 30 min at room temperature.
After being permeabilized with 0.2% Triton X-100 in PBS for 30 min,
the cells dyed with a Click-labelling dye, including 1 M CuSO4
(Wako, Japan), 1 M ascorbic acid (FUJIFILM Wako Pure Chemical
Corporation), 10 µM FAM azide (Lumiprobe Corp.) for 1 h at
room temperature, as previously described (46). After washing with PBS, the cells
were blocked with 5% goat serum (cat. no. SP-KIT-B2, MXB
Biotechnologies) at 1 h for room temperature, the cells were then
stained with primary anti-body at 4°C overnight. After washing with
PBS, the secondary antibody was applied for 1 h in the dark at room
temperature. The samples were washed and incubated with DAPI
(1:1,000; Thermo Fisher Scientific, Inc.) for 5 min, washed and
mounted with fluorescence mounting medium (cat. no. S3023, Dako;
Agilent Technologies, Inc.). Images were acquired using a Nikon
Eclipse Ni-E fluorescence microscope. For each group, 100 pairs of
cells in division were captured and the number of asymmetric
division cells was calculated.
Flow cytometry (FAC S) analysis
The cells (1×106 cells/well) were
pre-seeded in 10 cm plates and the COMM-SUS cells were completely
removed from the whole cell population. The COMM-AD cells were
treated with PKH67 (cat. no. PKH47GL-1KT; MilliporeSigma) for 48 h
according to the instructions of the manufacturer. The COMM-AD and
COMM-SUS cells were then collected, and centrifuged at 140 × g for
4 min at room temperature, respectively. Stained samples were
acquired using a Beckman MoFlo Astrios EQs flow cytometer (Beckman
Coulter, Inc.). Data were analyzed using FlowJo V10 software
(FlowJo LLC).
Animal experiments
A total of 30 female BALB/c nude mice (4-6 weeks
old, weighing 16-18 g) were purchased from Guangdong Medical
Laboratory Animal Center (Guangdong, China). The mice were
maintained under specific pathogen-free conditions (12-h light/dark
cycle, temperature of 25°C and a relative humidity of 60%, with
free access to food and water) and randomly divided into the
indicated groups, including the PBS group, COMM-AD group, COMM-SUS
group, COMM-AD + Fer-1 group, COMM-SUS + AZD3965 group and COMM-SUS
+ 6AN group (n=5/group). All the animal experiments were conducted
in compliance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals after approval from the Animal
Ethics Committee of Sun Yat-sen University (approval no.
2020000828).
The cells with enhanced GFP (eGFP; 5×105
cells suspended in 100 µl PBS) were injected into the tail
vein of the mice. Mice were intraperitoneally injected with 6-AN
(10 mg/kg), AZD3965 (30 mg/kg) or Fer-1 (1 mg/kg) as indicated
above every 2 days, respectively. After 4 weeks, the mice were
anesthetized by an intraperitoneal injection of pentobarbital
sodium (40 mg/kg) and euthanized by cervical dislocation. The
organs were harvested and then fixed with 10% formalin for 24 h for
histological analysis.
Cell viability assay
Cell viability was determined using a
CellTiter-Gro® Luminescent Cell Viability Assay (cat.
no. G7571; Promega Corporation). The cells were collected at the
determined time points following treatment with the different
reagents and seeded into a 96-well black bottom plate. An equal
volume of reconstituted CellTiter-Glo reagent was added to 100
µl of cell suspension. The contents were incubated for 10
min at room temperature to stabilize the luminescent signal and
cell viability was detected by record luminescence using a BioTek
Synergy LX multimode reader (BioTek Instruments, Inc.). For trypan
blue staining, the cells were stained with 0.4% trypan blue (cat.
no. C0040; Beijing Solarbio Science & Technology Co., Ltd.) for
3 min at room temperature before counted. Unstained cells were
regarded as viable, and stained cells were regarded as dead. The
total cells number and the blue-positive cells number were counted
using a microscope (Nikon Eclipse Ti-U; Nikon Corporation). The
percentage of surviving cells was calculated using the formula:
(number of total cells-number of stained cells)/number of total
cells ×100.
NADH, NADPH, MDA and lactate
analysis
The intracellular NADH and NADPH levels were
determined using a NAD+/NADH Assay kit with WST-8 (cat.
no. S0175, Beyotime Institute of Biotechnology) and a
NADP+/NADPH Assay kit with WST-8 (cat. no. S0179;
Beyotime Institute of Biotechnology), respectively. The absorbance
values were measured at 450 nm by record luminescence using a
BioTek Synergy LX multimode reader (BioTek Instruments, Inc.). The
intracellular MDA expression and total protein were determined
using Lipid Peroxidative MDA Assay kit (cat. no. S0131S; Beyotime
Institute of Biotechnology) and a BCA Protein Assay kit (cat. no.
23227, Thermo Fisher Scientific, Inc.), respectively. MDA
expression was normalized to milligram protein. Cells
(5×105 cells/sample) were collected and cell lysis was
performed using a Lactate Assay kit-WST (λ=450 nm, cat. no. L256;
Dojindo Laboratories, Inc.). All the analyses were conducted
according to the instructions of the manufacturer.
Small interfering RNA (siRNA)
transfection
Synthetic siRNAs were purchased from Guangzhou
RiboBio Co., Ltd. and the detailed information for the siRNAs is
presented in Table SII. The
transfection of the duplex siRNAs (100 nM) was carried using
Lipofectamine 3000® (cat. no. L3000015; Thermo Fisher
Scientific, Inc.) according to the instructions of the
manufacturer. The transfection efficiency was determined at 72 h
post-transfection using reverse transcription-quantitative PCR, as
described below. The primer sequences used are presented in
Table SIII.
Confocal microscopy
The COMM-AD cells were gently digested into single
cells using 0.05% trypsin-EDTA, and the COMM-AD and COMM-SUS cells
were then transferred to 35 mm confocal dishes (cat. no. FCFC020;
Beyotime Institute of Biotechnology), respectively. The cells were
allowed to place on glass-bottom for 5 min, and confocal imaging
was performed using a Leica TCS SP8 confocal microscopy (Leica
Microsystems GmbH). The light at a 405 nm wavelength was used to
detect the autofluorescence of COMM-AD and COMM-SUS cells. Stacks
of images were acquired using Leica LAS X software (version 2.0;
Leica Microsystems GmbH), and the confocal microscope acquisition
settings were kept the same for all scans.
Vectors and cell transfection
The stable overexpression of eGFP in the COMM cells
was amplified from the Lenti-GFP-neo vector (Addgene, Inc.).
Lentivirus were amplified from 293T packaging cells with pSPAX2 and
pMD2G (Addgene, Inc.) helper plasmids. The virus-containing
supernatants were collected at 48 and 72 h following transfection.
The supernatants with 5% PEG8000 were centrifuged at 4,000 × g for
2 h at 4°C to concentrate the lentiviral particles, diluted in 200
µl PBS and then stored at -80°C. The COMM cells were then
transfected with the lentivirus (40 µl lentivirus in 2 ml
DMEM) for 8 h and then selected with 1 µg/ml puromycin (cat.
no. A1113803, Gibco; Thermo Fisher Scientific, Inc.) after 48 h to
stabilize eGFP expression.
RNA isolation and RT-qPCR
Total RNA was extracted using the EZ-press RNA
Purification kit (cat. no. B0004DP; EZBioscience). A total of 1
µg of RNA was used for cDNA synthesis using the First Strand
cDNA Synthesis kit ReverTra Ace (cat. no. FSQ-201, Toyobo Life
Science) at 37°C for 10 min, 50°C for 5 min and the reaction was
terminated by incubating at 95°C for 3 min. qPCR was performed
using a LightCycler® 480 SYBR-Green I Master (Roche
Diagnostics) according to the manufacturer's instructions. The PCR
system included 5 µl PCR mix, 0.2 µl upstream primer,
0.2 µl downstream primer, 2.6 µl RNase-free
ddH2O and 2 µl cDNA template. The thermocycling
conditions were applied at 95°C for 5 min, followed by 45 cycles of
95°C for 10 sec, 60°C for 20 sec and then at 72°C for 20 sec. mRNA
expression was normalized to hRPLP0 using the 2−ΔΔCq
method (47). The primer
sequences used for RT-qPCR are presented in Table SIII.
Statistical analysis
All the experiments were carried out at least three
times independently. The data are presented as the mean ± SD. Data
between two groups were compared using an unpaired Student's
t-test, and data among multiple groups were compared using one-way
analysis of variance (ANOVA) and two-way ANOVA followed by Tukey's
multiple comparisons test using GraphPad Prism Software, version 9
(GraphPad Software, Inc.). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Mucosal melanoma cells exhibit
heterogeneous phenotypes
In total, two low-passage COMM cell lines, namely
COMM-1 and COMM-2, were successfully established. H&E staining
and melan-A immunostaining confirmed the characteristics of
melanoma, whereas the COMM-1 and COMM-2 cells were verified using
an STR analysis, which compared to STR profiles of primary tumor
tissues (Figs. 1A and S1A and Table SIV). In addition,
transmission electron microscopy (performed as described in
supplementary Materials and methods; Data S1) revealed that these
two low-passage cell lines were rich in melanin granules (Fig. S1B).
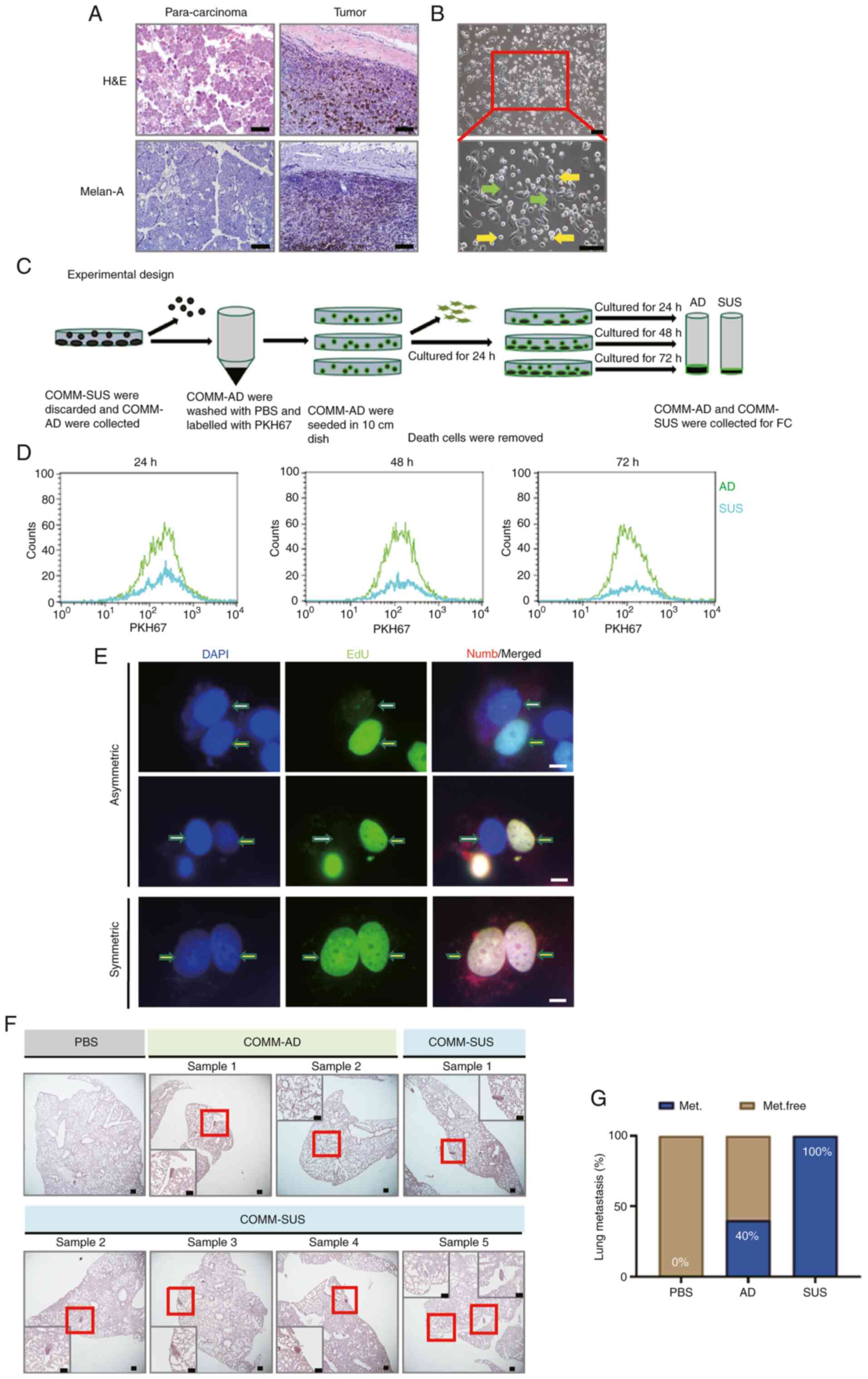 | Figure 1Malignant mucosal melanoma cells
exhibited a marked heterogeneous phenotype and biological
behaviors. (A) H&E and immunohistochemistry of COMM specimens.
Melan-A indicated COMM cells. Scale bar, 100.0 µm. (B) Two
subpopulations of COMM cells are indicated by the arrows; green
arrows indicate COMM-AD cells (abbreviated as AD), and yellow
arrows indicate COMM-SUS cells (abbreviated as SUS). (C) Schematic
diagram of the experimental design of flow cytometric analysis.
COMM-SUS cells were discarded and the whole adherent cell
population was labeled with PKH67, and the cells were then seeded
into culture plates for 24 h. After rinsing with the same medium,
the cells were further cultured for 24, 48 and 72 h, and then
analyzed by flow cytometry. (D) The results of flow cytometry of
PKH67-labeled cells. (E) Immunofluorescence of template DNA
co-segregate asymmetric and symmetric division. EdU (green)
staining indicated distribution of DNA. Positive EdU (yellow
arrows) indicated the DNA from parent cells. Negative EdU (white
arrows) indicated the newly synthesized DNA. DAPI (blue) staining
indicated nuclei. Numb (red) staining indicated the asymmetric
division of the cell membrane. Scale bar, 10.0 µm. (F) Lung
metastases were analyzed after tail intravenous injections of
COMM-AD and COMM-SUS cells with eGFP, respectively at 4 weeks.
Positive melan-A staining indicated COMM cells in lungs. Scale bar,
100.0 µm. (G) The frequency of developed lung metastasis in
mice, expressed as a percentage (Met, metastasis-positive;
Met.free, metastasis-free). n=5 mice per group. H&E,
hematoxylin and eosin; COMM, Chinese oral mucosal melanoma;
COMM-AD, cells with adhesive morphology; COMM-SUS, cells grown in
suspension. |
Compared with those of the malignant melanoma cell
line, A375, cultured long-term (data not shown), the COMM-1 and
COMM-2 cells exhibited highly heterogeneous phenotypes, where two
potential subpopulations were identified. In particular, one
exhibited a typical adherent monolayer morphology and were
designated 'COMM-AD' cells (Fig.
1B). By contrast, the other sub-group proliferated in
suspension and were designated as 'COMM-SUS' cells. The morphology
of the COMM-AD cells was spindle-shaped, whilst that of the
COMM-SUS cells exhibited a more rounded morphology (Fig. 1B).
COMM-SUS cells exhibit a greater
metastatic capacity
The COMM-AD and COMM-SUS cells exhibited a dynamic
conversion; the adherent cells became rounded and converted into
suspension cells (Video S1). The
COMM-SUS cells were completely removed from the whole cell
population, following which, the COMM-AD cells were labeled with
PKH67, a fluorescent reagent used for cell membrane staining, to
assess the association between the COMM-AD and COMM-SUS cells
(Fig. 1C). Cell viability was
then assessed using trypan blue staining prior to analysis using
flow cytometry. Flow cytometric analysis revealed that the majority
of the COMM-SUS cells remained viable (data not shown) and were
positive for PKH67 staining (Fig.
1D). A portion of COMM-AD cells transformed into COMM-SUS
cells, according to the observations of template DNA co-segregation
during asymmetric division (Fig.
1E). Numb immunostaining indicated that the cell membrane of
the COMM-AD cells was asymmetrically divided (Fig. 1E). These results suggested that
the COMM-AD cells divided asymmetrically into COMM-SUS cells and
maintained an interconversion state between the two
subpopulations.
Equivalent quantities of eGFP-labeled COMM-AD and
COMM-SUS cells (Fig. S1C) were
then injected into the tail vein of immunodeficient mice to analyze
their tumorigenic and metastatic potential. After 4 weeks, the mice
were euthanized before immunostaining for melan-A and GFP was
conducted in the lung tissues. Compared with those of the COMM-AD
cells (40% metastasis), the COMM-SUS cells were more capable of
establishing lesions in the lungs, with a 100% metastatic rate
(Figs. 1F and G, and S1D).
COMM-SUS cells survive in blood by
resisting ferroptosis
Based on the observations that the COMM-SUS cells
exhibited a greater capacity for metastasis, it was hypothesized
that the COMM-SUS cells resisted destruction in the blood
circulation during metastasis. The COMM-AD and COMM-SUS cells were
therefore cultured in HHS to evaluate their survival rates. The
COMM-SUS cells appeared to be more resilient, whilst the majority
of the COMM-AD cells died in HHS (Figs. 2A and B, and S2A). Subsequently, three types of cell
death inhibitors were used to investigate the mechanisms underlying
COMM-AD cell death in HHS. ZVAD-FMK, Fer-1 and necrostatin-1 were
used to treat the COMM-AD cells. The viability of the COMM-AD cells
treated with Fer-1 increased; however, ZVAD-FMK and necrostatin-1
were not able to significantly rescue cell survival (Figs. 2C and S2B). In addition, the COMM-AD cells
were treated with erastin; the majority of the COMM-AD cells died
following treatment with erastin, apart from those that were
pre-treated with Fer-1 (Fig. 2D).
In particular, the COMM-SUS cells survived, even in the presence of
erastin (Figs. 2E and S2C). Taken together, these results
provided evidence of the ability of the COMM-SUS cells to resist
ferroptosis. However, the COMM-AD cells presented a low metastatic
capacity due to ferroptotic cell death.
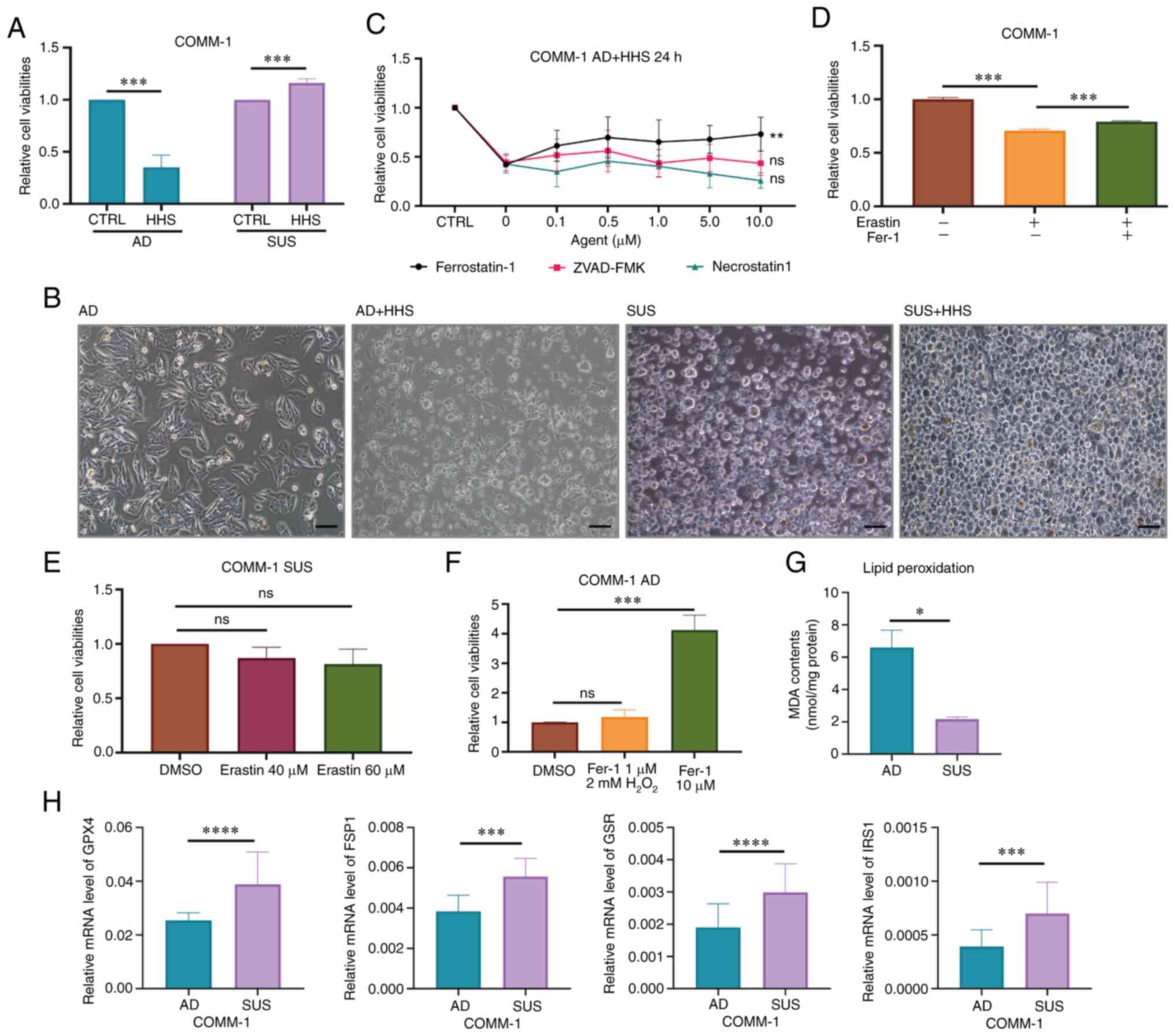 | Figure 2COMM-SUS cells survive in serum
plasma by gaining an ability of anti-ferroptosis. (A) The
viabilities of COMM-1 AD and COMM-1 SUS cells were measured after
culturing in HHS for 24 h. (B) The cell morphology of COMM-1 AD and
COMM-1 SUS cells following treatment with HHS for 24 h. (C) COMM-1
AD cells were treated with different inhibitors for 24 h. ZVAD-FMK
is the inhibitor of apoptosis, ferrostatin-1 is the inhibitor of
ferroptosis, and necrostatin-1 is the inhibitor of necrosis. (D)
COMM-1 AD cells were treated with the ferroptosis activator,
erastin, for 24 h with or without the pre-incubation of
ferrostatin-1 for 6 h. (E) COMM-1 SUS cells were treated with
erastin or DMSO (as the control) for 24 h. (F) COMM-1 AD cells were
treated with 2 mM H2O2 for 6 h following treatment with various
concentrations of ferrostatin-1 for 6 h. (G) The expression of MDA
in COMM-AD and COMM-SUS cells. (H) Detection of the expression of
GPX4, FSP1, GSR and IRS1 in COMM-AD and COMM-SUS cells using
reverse transcription-quantitative PCR. Significance was determined
using a Student's t-test or one-way ANONA or two-way ANOVA
(*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001; ns, not
significant, P>0.05). HHS, healthy human serum; MDA,
malondialdehyde; COMM, Chinese oral mucosal melanoma; COMM-AD,
cells with adhesive morphology; COMM-SUS, cells grown in
suspension. |
Due to the critical role of ROS in the hematogenous
spread of cancer cells, the capacity of resistance to oxidative
stress plays a crucial anti-ferroptotic role (48). In the present study, the viability
of the COMM-AD cells was found to be decreased following treatment
with H2O2 due its potent oxidant activity,
which was in turn rescued by treatment with 10 µM Fer-1
(Fig. 2F). The levels of MDA in
the COMM-AD and COMM-SUS cells were then measured. The MDA content
in the COMM-SUS cells was lower compared with that in the COMM-AD
cells (Fig. 2G). Furthermore,
RT-qPCR revealed that the COMM-SUS cells exhibited higher
expression levels of GPX4, FSP1, GSR and insulin receptor substrate
1 (IRS1) compared with those in the COMM-AD cells (Figs. 2H and S2D). These observations suggested that
the COMM-SUS cells survived in blood circulation by resisting
ferroptosis.
Lactate metabolism is reprogrammed to
promote NADH accumulation in COMM-SUS cells
Confocal microscopy images revealed a strong
autofluorescence in COMM-SUS cells (Fig. 3A). Subsequent flow cytometric
analysis also confirmed the high degree of autofluorescence at the
405-565 nm emission wavelengths following excitation with 405 nm
(Fig. 3B). According to the range
of emission wavelengths, it was suggested that the products
generating this autofluorescence were NADH and NADPH (400-510 nm),
lipofuscin (500-695 nm) and flavins (500-600 nm). The catalytic
reactions induced by FSP1 or GPX4 are associated with the flux of
NADH and NADPH. In addition, the anti-ferroptotic effects are also
associated with NADH and NADPH accumulation (49,50). Considering these factors of
emission wavelengths and oxidative stress resistance, it was
suggested that this was caused by NADH and NADPH accumulation in
COMM-SUS cells.
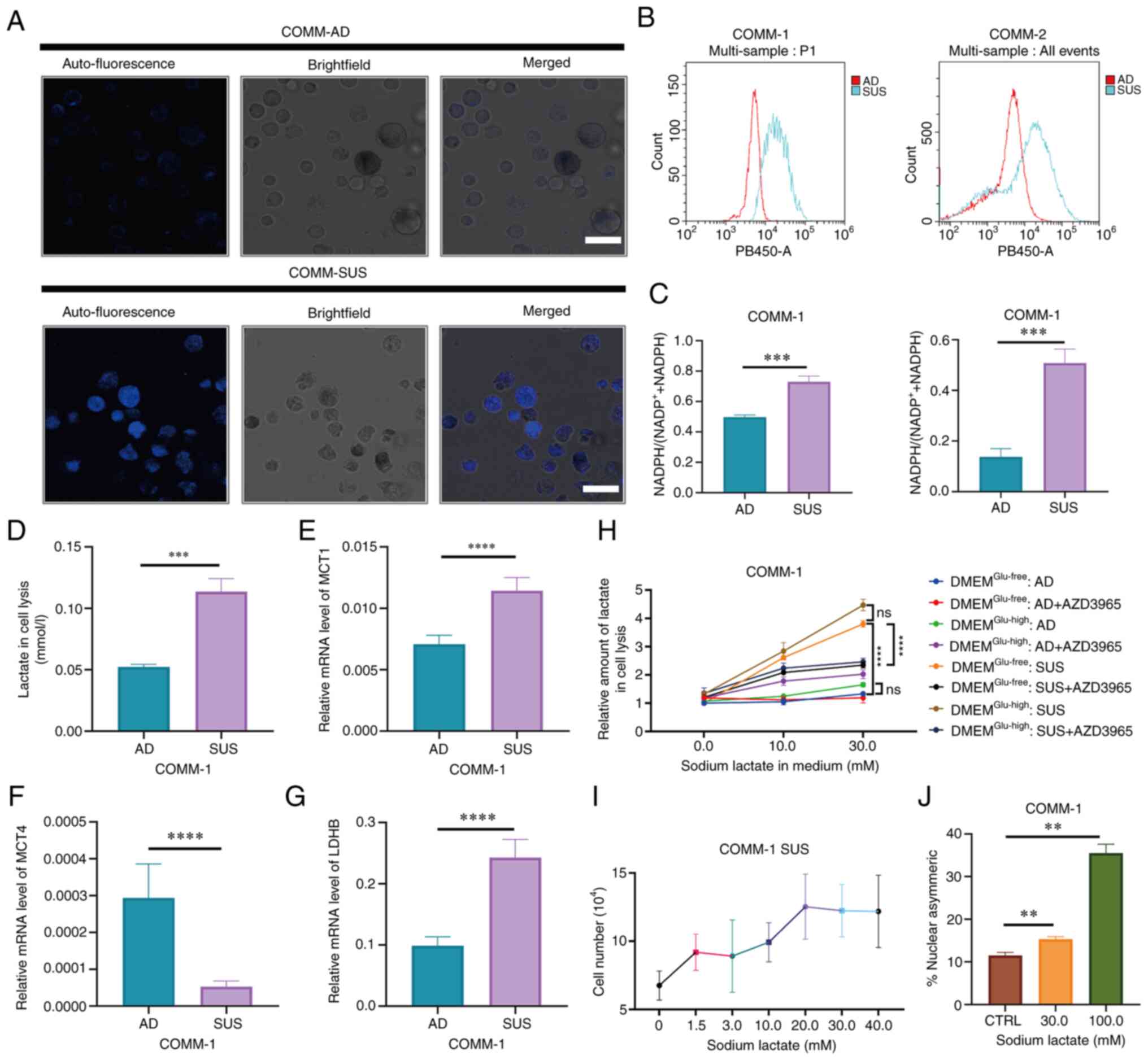 | Figure 3Lactate metabolism is reprogrammed in
COMM-SUS cells. (A) Autofluorescence in COMM-AD or COMM-SUS cells
was analyzed using a confocal microscope. COMM-AD cells were
enzymatically digested to obtain a single-cell suspension. Scale
bar, 10.0 µm. (B) Flow cytometric analysis of the
autofluorescence in COMM-AD and COMM-SUS cells; the excitation
wavelength was 405 nm. (C) Analyzing the amount of intracellular
NADH and NADPH in COMM-1 AD and COMM-1 SUS cells. (D) The total
lactate levels of COMM-1 AD and COMM-1 SUS cells were measured
using a Lactate Assay kit-WST. (E-G) Reverse
transcription-quantitative PCR analysis of MCT1, MCT4 and LDHB
expression in COMM-1 AD and COMM-1 SUS cells. (H) COMM-1 AD and
COMM-1 SUS cells were treated with or without AZD3965 for 6 h, and
cultured in glucose-free medium (DMEMGlu−free) or
glucose-high medium (DMEMGlu−high), which was
supplemented with 10 and 30 mM sodium lactate for 24 h. The amount
of intracellular lactate was measured. (I) The number of viable
COMM-1 SUS cells was determined in 72 h after treating with a
serial concentrations of sodium lactate. (J) Quantification of
immunofluorescence analysis for template DNA co-segregate
asymmetrically after the COMM-1 cells were treated with various
concentrations of sodium lactate. Significance was determined using
a Student's t-test or one-way ANONA or two-way ANOVA
(**P<0.01, ***P<0.001 and
****P<0.0001; ns, not significant, P>0.05). COMM,
Chinese oral mucosal melanoma; COMM-AD, cells with adhesive
morphology; COMM-SUS, cells grown in suspension; MCT,
monocarboxylate transporter; LDHB, lactate dehydrogenase B. |
The intracellular NADH and NADPH levels were
therefore assessed in the COMM cells. It was found that the
COMM-SUS cells accumulated higher levels of NADH and NADPH
(Figs. 3C and S2E). The lactate levels in the COMM-SUS
cells were also found to be significantly higher compared with
those in the COMM-AD cells (Figs.
3D and S2F). RT-qPCR also
revealed that the COMM-SUS cells expressed MCT1 and lactate
dehydrogenase (LDH)B at higher levels compared with those in the
COMM-AD cells, providing a potential explanation for the higher
degree of NADH accumulation observed in the COMM-SUS cells. In
addition, the lower lactate flux, but higher levels of MCT4
expression in COMM-AD cells suggested that the COMM-AD cells
preferentially used aerobic glycolysis, whereby lactate was
transported into the extracellular space by MCT1 and MCT4, which
was then consumed by COMM-SUS cells (Figs. 3E-G and S2G).
The cells were then treated with high glucose medium
or glucose-free medium supplemented with various concentrations of
sodium lactate according to the lactate range detected in solid
tumors to test the model lactate utilization in COMM-AD and
COMM-SUS cells. The intracellular lactate levels in COMM-AD cells
remained relatively stable even when the concentration of exogenous
sodium lactate increased. By contrast, the COMM-SUS cells exhibited
a significant increase in intracellular lactate levels. Compared
with the COMM-AD cells, the COMM-SUS cells took up lactate under
both high glucose and glucose-free conditions (Fig. 3H). The COMM-AD and COMM-SUS cells
were subsequently pre-treated with AZD3965 before being cultured in
high-glucose or glucose-free medium containing various sodium
lactate concentrations to assess the role of MCT1 in lactate
uptake. The intracellular lactate levels were significantly
increased in the COMM-AD cells treated with AZD3965 (Fig. 3H) in high-glucose medium. However,
the intracellular lactate levels were decreased in the COMM-SUS
cells after MCT1 was blocked (Fig.
3H). Collectively, these data suggest that the COMM-AD cells
mainly utilized glucose for energy, whereas the COMM-SUS cells
mainly utilized lactate as the energy source through MCT1.
A series of sodium lactate concentrations was added
to the cells to examine the effects of lactate on the
transformation of COMM-AD cells into COMM-SUS cells. The numbers of
COMM-SUS cells were observed to be increased following treatment
with increasing sodium lactate concentrations (Figs. 3I and S2H). The proportion of template DNA
co-segregation during asymmetric division was also confirmed by
calculating the 100 pairs of cells in division using
immunofluorescence staining (Figs.
3J and S2I) in each group,
suggesting that the COMM-AD cells asymmetrically divided to become
lactate-accumulating COMM-SUS cells.
COMM-SUS cells are resistant to oxidative
stress by accumulating NADPH through pentose phosphate pathway
(PPP) activation
Although the data thus far suggested that the
COMM-SUS cells principally utilized lactate as a metabolic
substrate and accumulated NADH, the mechanisms underlying this
phenomenon remain unknown. The classical pathways that are known to
produce NADPH in cells include the PPP, fatty acid synthesis
pathway (FASP) and folate metabolism pathway (FMP) (39). The key enzymes in these pathways
are G6PD, 6-phosphogluconate dehydrogenase (PGD), malic enzymes
(ME) 1, 2 and 3, and methylenetetrahydrofolate dehydrogenase
(MTHFD)2 (Fig. 4A). RT-qPCR
revealed higher expression levels of G6PD, GPD, ME2 and MTHFD2 in
the COMM-SUS cells compared with those in the COMM-AD cells
(Figs. 4B and S3A). However, ME1 and ME3 were not
detected in either the COMM-SUS or COMM-AD cells (data not
shown).
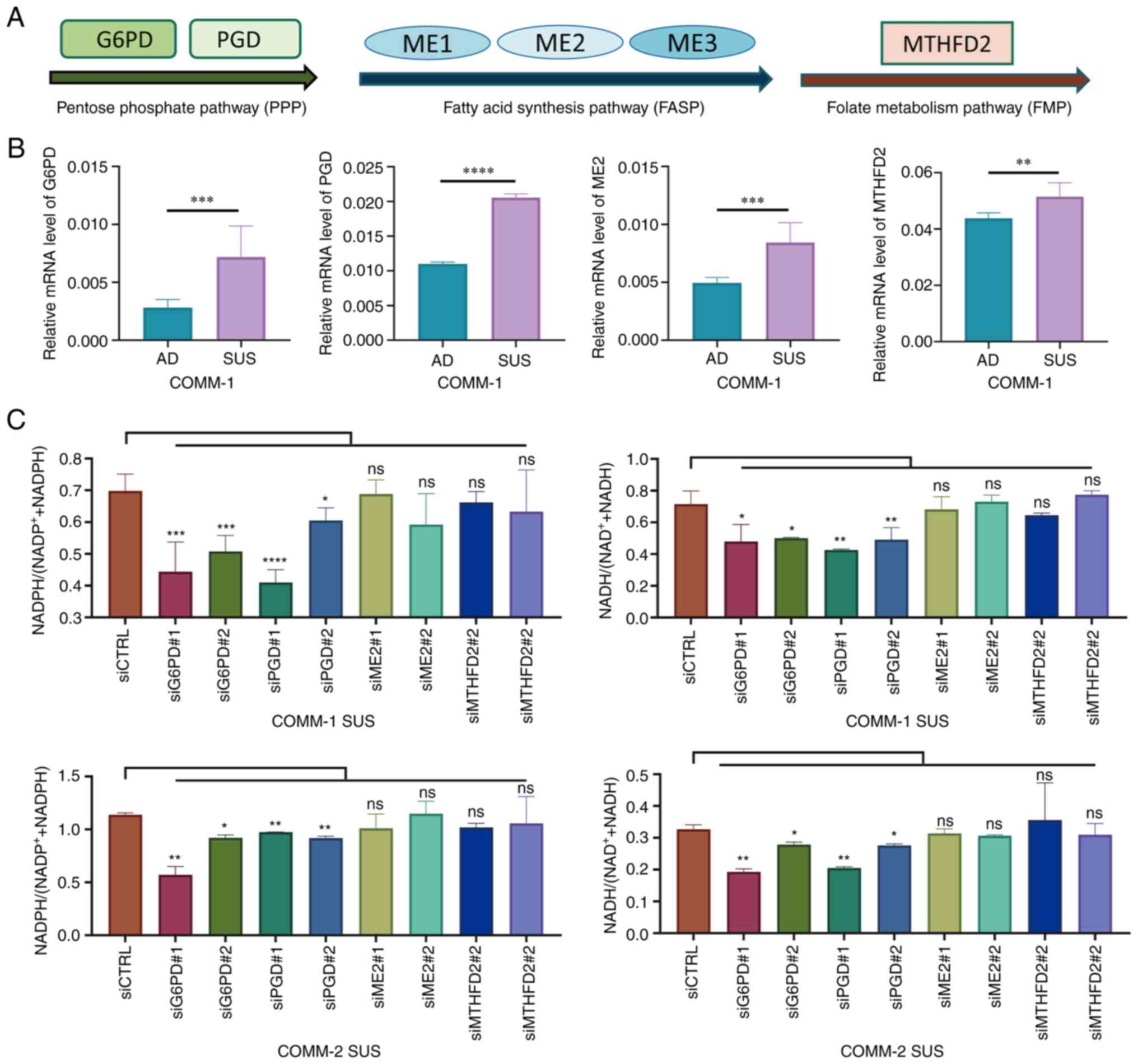 | Figure 4COMM-SUS cells resist oxidative
stress by NADPH accumulation via PPP. (A) Schematic diagram of the
main enzymes of the pentose phosphate pathway, fatty acid synthesis
pathway and folate metabolism pathway. The expression levels of the
enzymes were interfered with by corresponding siRNA. (B) The
expression of the main enzymes was measured in COMM-1 AD and COMM-1
SUS cells using reverse transcription-quantitative PCR. (C) The
amount of intracellular NADH and NADPH was evaluated after
suppressing enzyme expression. Significance was determined using a
Student's t-test or one-way ANONA (*P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001; ns, not significant, P>0.05). COMM,
Chinese oral mucosal melanoma; COMM-AD, cells with adhesive
morphology; COMM-SUS, cells grown in suspension; PGD,
6-phosphogluconate dehydrogenase; G6PD, glucose-6-phosphate
dehydrogenase. |
The COMM cells were subsequently transfected with
siRNAs individually targeting each of the detectable enzymes to
investigate which pathway was involved in NADPH production
(Fig. S3B). The levels of NADH
and NADPH were evaluated following the knockdown of enzyme
expression. The levels of NADH and NADPH in the COMM-SUS cells were
found to be significantly decreased when G6PD and PGD expression
was knocked down in both the COMM-1 and COMM-2 cell lines; however,
the levels of NADH and NADPH were not altered in the COMM-AD cells
(Figs. 4C and S3C). Collectively, these results
suggested that the PPP was activated in the COMM-SUS cells to
promote NADPH accumulation.
Lactate metabolism and PPP activation
inhibit ferroptosis in COMM-SUS cells
The COMM-SUS cells were pre-treated with AZD3965 and
cultured in HHS to investigate whether lactate metabolism is
associated with ferroptosis. The viability of the COMM-SUS cells
was found to be significantly decreased following AZD3965 treatment
(Fig. 5A). Consistent with the
status in HHS, the COMM-SUS cells exhibited resistance to oxidative
stress induced by H2O2, which was reversed by
AZD3965 treatment (Fig. 5B). In
addition to changes in cell viability, the intracellular NADH and
NADPH levels were decreased in the COMM-SUS cells following
treatment with AZD3965 (Fig. 5C and
D). After MCT1 was inhibited in the COMM-AD cells, lactate was
found to be accumulated in the intracytoplasmic areas, which
reduced pyruvate transformation into lactate and NADH accumulation.
Furthermore, the effects of lactate on the viability of COMM-AD
cells were then investigated. Lactate accumulation was deleterious
for the COMM-AD cells following treatment with AZD3965 to a certain
extent (Figs. 5E and F, and
S4A). Therefore, the COMM-AD
cells were unable to resist oxidative stress after culturing in HHS
even after increasing the NADH levels due to the downregulated
expression of GPX4 and FSP1.
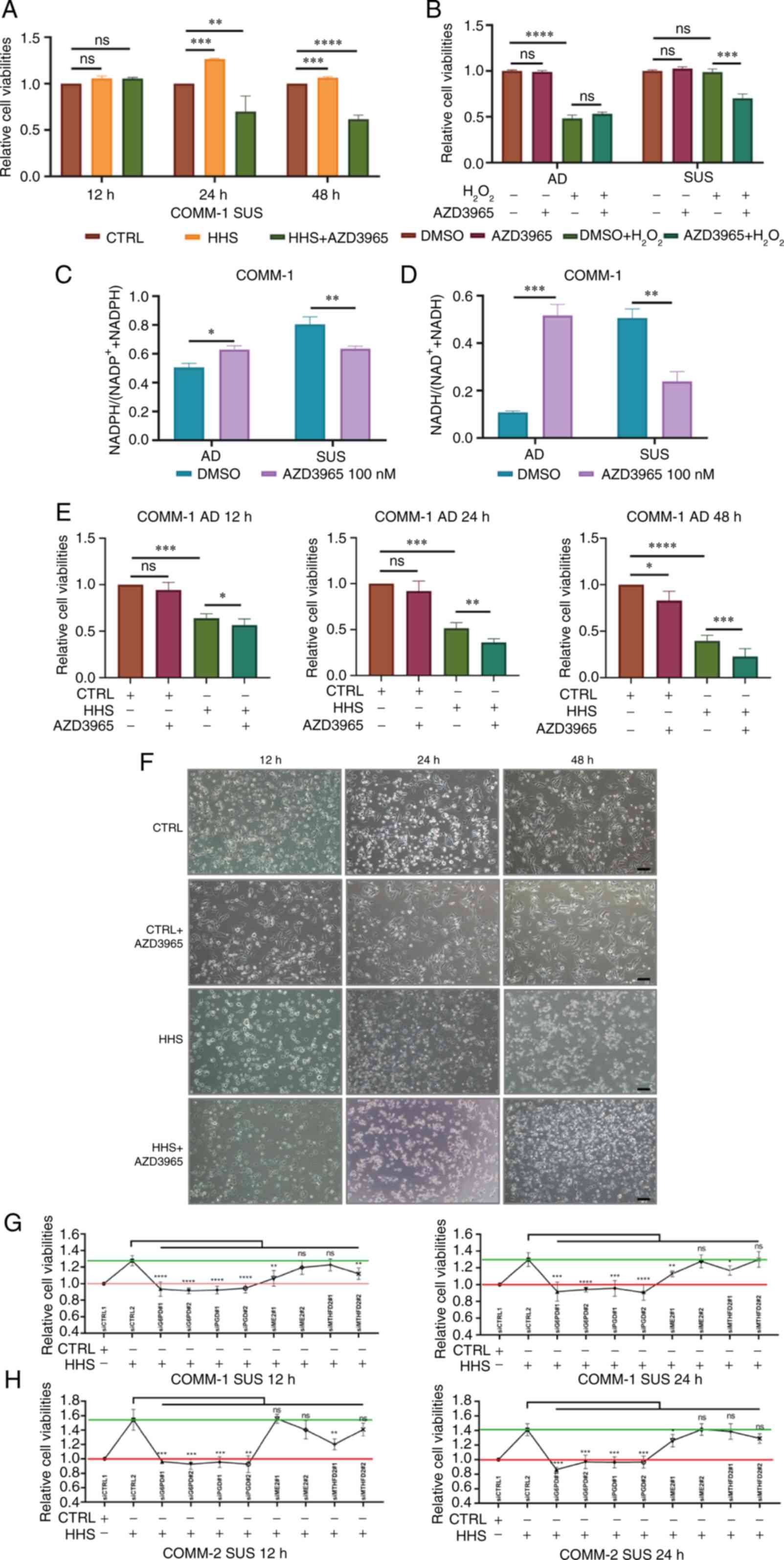 | Figure 5Lactate metabolism and pentose
phosphate pathway activation inhibit ferroptosis in COMM-SUS cells.
(A) COMM-1 SUS cells were treated with or without the MCT1
inhibitor, AZD3965, and then cultured in HHS for 12, 24 and 48 h.
Cell viabilities were analyzed. (B) Cell viabilities of COMM-1
cells were measured following treatment with or without AZD3965 for
6 h, and cultured with 2 mM H2O2 for 6 h. (C
and D) The amount of intracellular NADPH and NADH of COMM-1 cells
were measured after treating with 100 nM AZD3965 for 24 h. (E and
F) COMM-1 AD cells were treated with AZD3965, and then cultured in
HHS for 12, 24 and 48 h. Cell viabilities and morphologies were
analyzed. (G and H) Cell viabilities of COMM-1 SUS cells were
measured after siRNA transfection targeting PPP, FASP and FMP,
respectively. Significance was determined using one-way ANONA
(*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001; ns, not
significant, P>0.05). COMM, Chinese oral mucosal melanoma;
COMM-AD, cells with adhesive morphology; COMM-SUS, cells grown in
suspension; HHS, healthy human serum. |
The present study validated the anti-ferroptotic
efficacy of the PPP activation in COMM-SUS cells. The viability of
the COMM-SUS cells was decreased following the knockdown of
associated enzymes of the three pathways separately using siRNA in
HHS. Notably, cell viability was significantly decreased after PPP
was inhibited by siRNA targeting G6PD and PGD enzymes (Fig. 5G and H). These results suggested
that the PPP was activated in COMM-SUS cells to produce NADPH and
resist serum-induced death.
Inhibition of ferroptosis increases
COMM-AD cell metastasis, whilst the inhibition of MCT1 and the PPP
suppresses COMM-SUS cell metastasis
The COMM-AD and COMM-SUS cells were injected into
the tail vein of immunodeficient mice before Fer-1, AZD3965 and 6AN
were intraperitoneally injected every 2 days (Fig. 6A). 6AN is an anti-metabolite
analog of G6PD that inhibits the NADPH-producing PPP to render the
it more susceptible to oxidative stress. Consistent with the
results obtained in vitro, 80% of the mice in the COMM-AD
group treated with Fer-1 developed lung metastasis, suggesting that
ferrostatin-1 improved the anti-ferroptotic capacity of the COMM-AD
cells to promote metastasis. In addition, only 20% of the mice in
the COMM-SUS group treated with AZD3965 and 40% of the mice in the
COMM-SUS group treated with 6AN developed lung metastasis (Figs. 6B and C, and S4B). Based on these results, these
observations suggest that inhibition of lactate uptake and PPP
activity can significantly suppress the metastatic potential of
COMM-SUS cells.
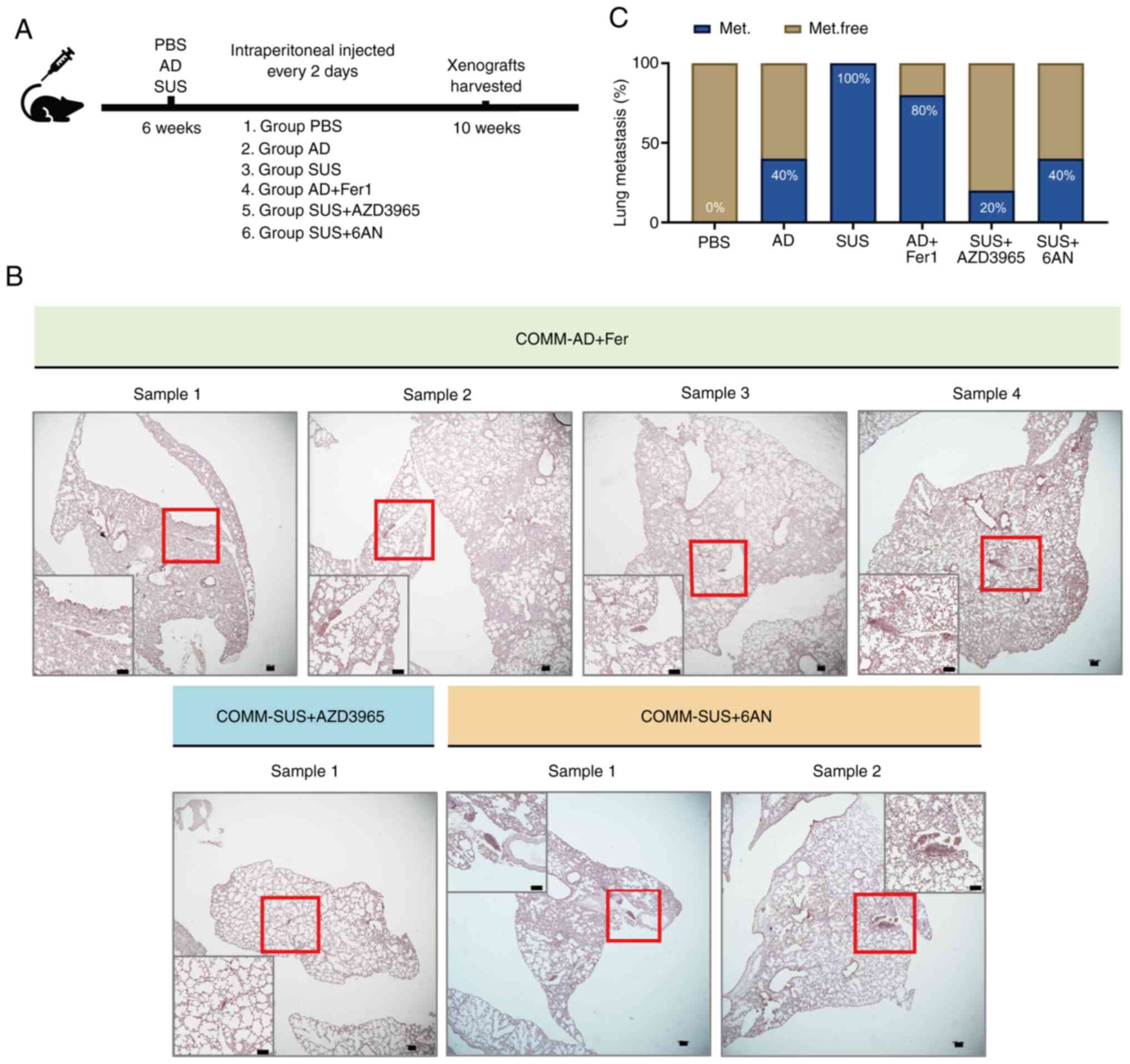 | Figure 6Inhibition of ferroptosis markedly
increases the metastatic capacity of COMM-AD cells, and the
inhibition of MCT1 and pentose phosphate pathway suppress the
metastatic capacity of COMM-SUS cells. (A) Schematic diagram of the
in vivo experimental design. COMM-AD and COMM-SUS cells were
tail intravenously injected to 6-week old immunodeficiency mice
respectively. Ferrostatin-1 (Fer-1), AZD3965 and 6AN were injected
intraperitoneally every 2 days for 4 weeks in corresponding group,
and the control group was injected with PBS. (B)
Immunohistochemical staining of Melan-A indicated COMM cells in
lung. Scale bar, 100.0 µm. (C) The frequency of developed
lung metastasis of mice shown in panel B and in Fig. 1F, expressed as a percentage; n=5
mice per group. 6AN, 6-aminonicotinamide; COMM, Chinese oral
mucosal melanoma; COMM-AD, cells with adhesive morphology;
COMM-SUS, cells grown in suspension; MCT, monocarboxylate
transporter. |
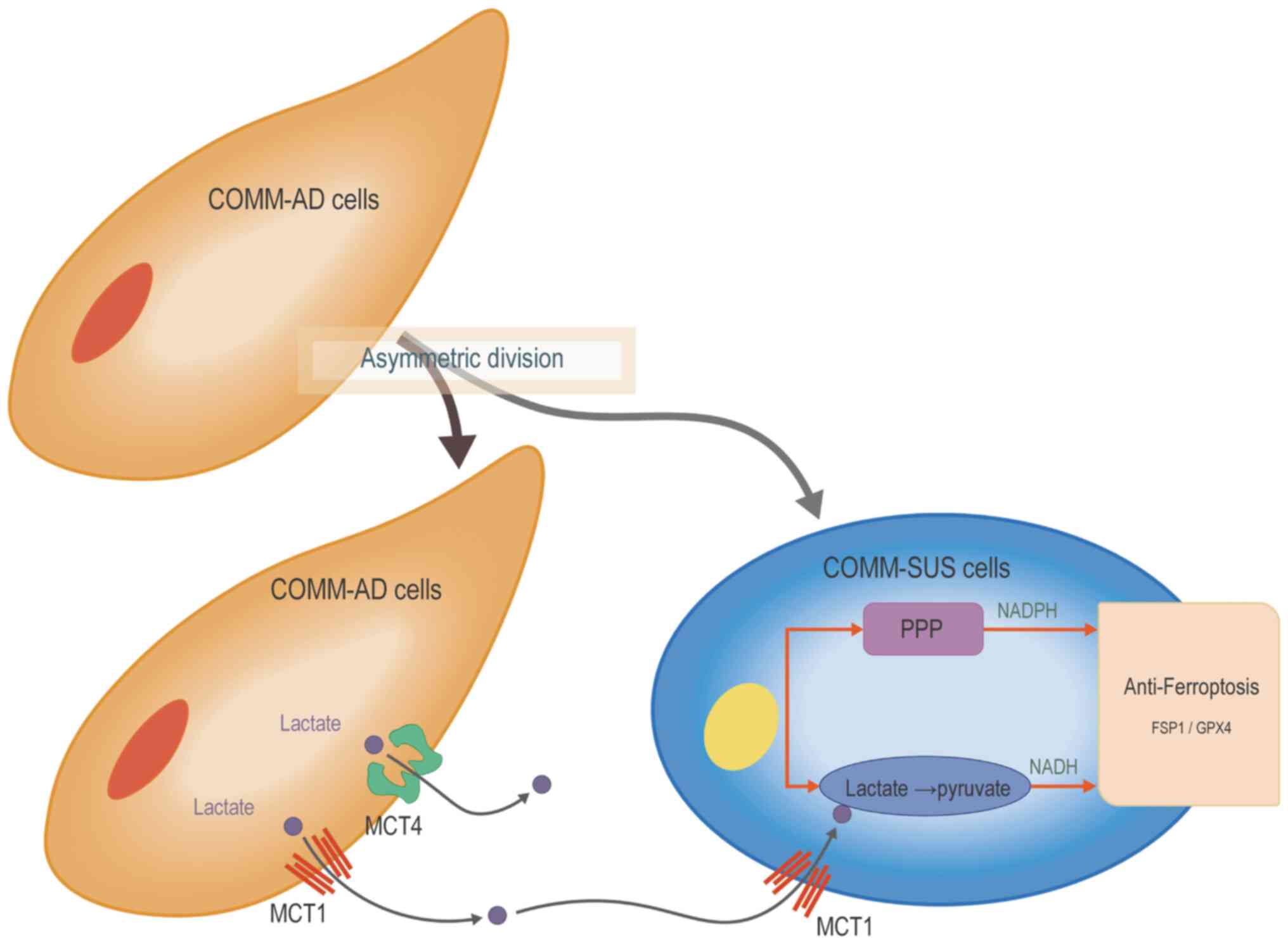
Collectively, these findings demonstrated that the
COMM cells exhibited heterogenous phenotypes, where the COMM-SUS
cells are able to inhibit ferroptosis and reprogram metabolism by
accumulating lactate and subsequently NADH to activate the PPP,
which in turn increases NADPH levels. Increased NADH and NADPH
accumulation then improves the ability of COMM-SUS cells to resist
oxidative stress and achieve distal metastases through hematogenous
spread (Fig. 7).
Discussion
To date, cancer heterogeneity represents an ongoing
challenge in clinical therapy (51). As novel technologies and reagents
continually emerge, primary cultured cells or low-passage cell
lines have been increasingly recognized for their advantages. This
is due to the fact that they have been shown to retain the original
heterogenous landscape, genetic and molecular profiles of their
corresponding tumors (51,52).
In addition, efforts have been made to characterize the biological
signatures and markers of different subpopulations within the same
cancer (53,54). Exploring the mechanism of tumor
heterogeneity may allow for the design of strategies to interfere
with the carcinogenic processes and ultimately improve cancer
management in the clinic. However, the majority of cancer cell
lines gradually lose their heterogeneity after long-term in
vitro culture (43). In the
present study, two MM cell lines with low-passage numbers were
established to explore the heterogeneity of MM.
Cancer initiation, propagation, therapy resistance
and relapse are potentially driven and maintained by asymmetric
cell division (55), which also
contributes to the heterogenic characteristics (56,57). In the present study, COMM-AD cells
presented with template DNA co-segregation and exhibited the
potential to asymmetrically divide into COMM-SUS cells, which
underwent metabolic reprogramming to use lactate as their primary
carbon source to enhance their own metastatic capabilities. It has
been suggested that different cell subsets within the same tumor
can divide metabolic tasks by exchanging intermediary metabolites,
such as lactate (58). The
findings of the present study suggested that the entire COMM cell
population can mimic the original tumor tissue. COMM-AD cells are
cancer cells in situ and are considered to be glycolytic
cancer cells that can produce and excrete lactate into the
extracellular microenvironment (27). By contrast, the COMM-SUS cells are
cancer cells that exhibit higher metastatic capacities to leave the
stroma and invade by taking up lactate as the main oxidative cell
type (30). This is consistent
with the hypothesis of 'metabolic symbiosis' (27,30,59). Glycolytic cells use glucose to
produce large quantities of lactate that are rapidly exported
through MCT4, where the oxidative cells located in perfused areas
use their MCT1 proteins to import the lactate (30). Once imported into the cytosol of
oxidative cancer cells, lactate is then oxidized into pyruvate by
LDH-1 (60). LDH typically exists
as a tetramer of the LDH-H protein encoded by the LDHB gene
and mediates the simultaneous reduction of NAD+ into
NADH (60). This pyruvate and
NADH then fuel the TCA cycle to facilitate ATP production. Based on
accumulating evidence, lactate is considered to be a major carbon
source for the TCA instead of being a waste product of anaerobic
metabolism in cancer cells (27,61). The present study demonstrated that
high levels of MCT1 expression were positively associated with
metastasis and shorter overall survival times in the clinic
(Fig. S4C). Based on this
finding, MCT1 may represent an ideal drug target for MM treatment.
The MCT1-specific inhibitor, AZD3965, has undergone a phase I
clinical trial in the UK for advanced solid tumors and lymphomas
(62,63). AZD3965 has been previously
reported to reduce tumor growth in breast cancer (64), small cell lung cancer (63), lymphoma (65) and colon carcinoma (66). In normal proliferating cells, 30%
of the intracellular NADPH has been found to be derived from the
PPP, whereas the other 30% is produced from glutaminolysis flux and
40% is derived from folate metabolism (67). However, cancer cells require
higher levels of NADPH for the acceleration of various
physiological processes, such as biomolecule synthesis and lipid
oxidation, especially redox hemostasis (68,69). Therefore, increased NADPH levels
are considered to be favorable for cancer metastasis (70). Metastatic melanoma cells tend to
have higher levels of NADPH generation to convert GSSG into GSH,
allowing them to withstand oxidative stress through the folate
pathway (14). NADPH has also
been shown to promote gastric cancer growth and metastasis by
upregulating the expression of ME1 (71). In the present study, in addition
to lactate uptake to increase NADH levels, metabolic reprogramming
in COMM-SUS cells also activated the PPP to accumulate additional
NADPH levels and confer resistance to oxidative stress.
Increased NADH and NADPH accumulation not only
functions as an antioxidant, but also increases autofluorescence
(72,73). High levels of autofluorescence
were confirmed in COMM-SUS cells in the present study. The
characteristic of autofluorescence holds promise as a potentially
powerful tool for detecting the first signs of metastasis during
clinical diagnosis (72).
Non-invasive diagnostic techniques for identifying malignant tumor
cells, monitoring distant metastasis and local recurrence of cancer
are improving. In particular, the use of inflammatory markers and
autofluorescence to diagnose cancer holds promise (30,72,74-77). In addition, red cell distribution
width (RDW) value may be applied as a novel inflammatory marker to
assess malignant thyroid nodules (74). Indeed, elevated RDW values have
been found in several cancer types, including esophageal cancer and
gastric cancer (78-81). Tumorigenesis is frequently
accompanied with alterations in the immune cell and cytokine
profile (82). The degree of
tumor immune infiltration altered in the tumor microenvironment,
whereas the cytokine secretion profile is altered in the
bloodstream (83,84). Previous studies have demonstrated
that inflammation is an important hallmark of tumorigenesis,
including head and neck squamous cell carcinoma and breast cancer
(82,85-87). Tumors at different stages of
development typically exhibit different characteristics of
cancer-associated inflammation, where the different inflammatory
environments will influence local immune responses (88). Therefore, the combination of
inflammatory markers and autofluorescence holds diagnostic value in
the clinical diagnosis of cancer.
Cancer cells are able to resist multitude of cancer
therapies due to cell plasticity, which include properties of
metabolic reprogramming, immune escape and suppression of cell
death (56,89-91). They chronically experience high
levels of oxidative stress, which may induce ferroptosis during
hematogenous metastasis (92). A
previous study revealed that the lymph nodes allows melanoma cells
to incorporate oleic acid and other antioxidants for protection
against ferroptosis (93). In the
present study, notable cellular heterogeneity was observed in the
mucosal MM cells tested. In particular, COMM-SUS cells exhibited
highly aggressive metastatic behavior following metabolic
reprogramming, resulting in the accumulation of NADH and NADPH and
protection against ferroptosis induced by oxidative stress from the
bloodstream. COMM-SUS cells were found to mainly depended on GPX4
and FSP1 for protection against ferroptosis. According to this
finding, GPX4 or FSP1 may represent potential drug targets for
suppressing MM cell metastasis. In addition, the metabolic
reprogramming event that was found in the COMM-SUS cells, where
increased quantities of lactate was taken up and then oxidized to
pyruvate, is most likely to be associated with ferroptosis
resistance. These metabolic activities resulting in or from
ferroptosis are inherently associated with energy metabolism.
Therefore, inhibiting metabolic reprogramming in aggressive
metastatic cancer cells also represents a possible drug target to
deny the high energetic needs of cancer cells.
In summary, the results from the present study
suggest that efficiently metastasizing melanoma cells are able to
reprogram their own metabolic pathways to increase the uptake
lactate to meet their energy demands. Mechanistically, this was
possibly mediated by the activation of the PPP to increase the
production of the antioxidants NADH and NADPH. In addition,
increases in the expression levels of GPX4 and FSP1 allowed the
cells to resist ferroptotic cell death induced by oxidative stress
to promote distant metastasis.
Heterogeneity is one of the important biological
characteristics of cancer, which results in the existence of a
diverse range of cell types and cell subpopulations with distant
physiological features within the same tumor. In addition,
different subpopulations of the same cancer type can utilize
different synergistic mechanisms to enhance tumorigenesis. The
dynamic conversion and interdependence of cancer subpopulations may
provide a perspective to deepen the understanding into tumor
characteristics. Cancer heterogeneity represents an ongoing
challenge in clinical settings. Exploring the mechanism of cancer
heterogeneity may allow research in methods of interfering with
dynamic cancer inter-cell interactions and ultimately improve
cancer management protocols in the clinic.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
YZ was involved in the conceptualization of the
study, as well as in project administration, funding acquisition,
and in the writing, reviewing and editing of the manuscript. HW was
involved in the conceptualization of the study, as well as in
project administration and funding acquisition. WL was involved in
the conceptualization of the study, as well as in the methodology,
visualization, and in the writing of the original draft. XL was
involved in the conceptualization of the study, as well as in the
methodology, and in the writing, reviewing and editing of the
manuscript. HY was involved in the study methodology and
visualization. LH, YT and SL were involved in the conceptualization
of the study. WH was involved in visualization. XL and HY confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The specimens obtained from Hospital of Stomatology
Sun Yatsen University followed the protocol approved by the Ethics
Committee of the Stomatological Hospital of Sun Yat-sen University,
Guangzhou, China. The patients provided written consent for the use
of their blood or tissue in research. All the animal experiments
were conducted in compliance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals after approval
from the Animal Ethics Committee of Sun Yatsen University (approval
no. 2020000828).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to Dr Junheng Zheng from
Sun Yat-sen university for his insightful advice and Mr Jiang Qian
from the Laboratory Animal Center, Sun Yat-sen University,
Guangzhou, China for his valuable support in performing the animal
experiments.
Funding
The present study was supported in part by a grant from the
National Natural Science Foundation of China (no. 31871413) and two
grants from the Programs of Guangdong Science and Technology (nos.
2017B020230002 and 2016B030231001).
References
|
1
|
Spencer KR and Mehnert JM: Mucosal
melanoma: Epidemiology, biology and treatment. Cancer Treat Res.
167:295–320. 2016. View Article : Google Scholar
|
|
2
|
Nassar KW and Tan AC: The mutational
landscape of mucosal melanoma. Semin Cancer Biol. 61:139–148. 2020.
View Article : Google Scholar :
|
|
3
|
Merkel EA and Gerami P: Malignant melanoma
of sun-protected sites: A review of clinical, histological, and
molecular features. Lab Invest. 97:630–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ascierto PA, Accorona R, Botti G, Farina
D, Fossati P, Gatta G, Gogas H, Lombardi D, Maroldi R, Nicolai P,
et al: Mucosal melanoma of the head and neck. Crit Rev Oncol
Hematol. 112:136–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis LE, Shalin SC and Tackett AJ:
Current state of melanoma diagnosis and treatment. Cancer Biol
Ther. 20:1366–1379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yde SS, Sjoegren P, Heje M and Stolle LB:
Mucosal melanoma: A literature review. Curr Oncol Rep. 20:282018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poh A: First oncolytic viral therapy for
melanoma. Cancer Discov. 6:62016. View Article : Google Scholar
|
|
8
|
Killock D: Skin cancer: T-VEC oncolytic
viral therapy shows promise in melanoma. Nat Rev Clin Oncol.
12:4382015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A, Kalluri R and Kshitiz: Systems biology of cancer
metastasis. Cell Syst. 9:109–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: The link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsoi J, Robert L, Paraiso K, Galvan C,
Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al:
Multi-stage differentiation defines melanoma subtypes with
differential vulnerability to drug-induced iron-dependent oxidative
stress. Cancer Cell. 33:890–904.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan Yanxiang, Guo J, et
al: Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu M, Zhang X, Zhang W, Chiou YS, Qian W,
Liu X, Zhang M, Yan H, Li S, Li T, et al: Cancer stem cell
regulated phenotypic plasticity protects metastasized cancer cells
from ferroptosis. Nat Commun. 13:13712022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohshima K and Morii E: Metabolic
reprogramming of cancer cells during tumor progression and
metastasis. Metabolites. 11:282021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Icard P, Shulman S, Farhat D, Steyaert JM,
Alifano M and Lincet H: How the Warburg effect supports
aggressiveness and drug resistance of cancer cells? Drug Resist
Updat. 38:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar :
|
|
29
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell
Metab. 30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
30
|
Pisarsky L, Bill R, Fagiani E, Dimeloe S,
Goosen RW, Hagmann J, Hess C and Christofori G: Targeting metabolic
symbiosis to overcome resistance to anti-angiogenic therapy. Cell
Rep. 15:1161–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
García-Cañaveras JC, Chen L and Rabinowitz
JD: The tumor metabolic microenvironment: Lessons from lactate.
Cancer Res. 79:3155–3162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tasdogan A, Faubert B, Ramesh V,
Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin
M, et al: Metabolic heterogeneity confers differences in melanoma
metastatic potential. Nature. 577:115–120. 2020. View Article : Google Scholar
|
|
33
|
Zhang G, Zhang Y, Dong D, Wang F, Ma X,
Guan F and Sun L: MCT1 regulates aggressive and metabolic
phenotypes in bladder cancer. J Cancer. 9:2492–2501. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Felmlee MA, Jones RS, Rodriguez-Cruz V,
Follman KE and Morris ME: Monocarboxylate transporters (SLC16):
Function, regulation, and role in health and disease. Pharmacol
Rev. 72:466–485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao HJ, Zhao MC, Zhang YJ, Zhou DS, Xu L,
Li GB, Chen MS and Liu J: Monocarboxylate transporter 4 predicts
poor prognosis in hepatocellular carcinoma and is associated with
cell proliferation and migration. J Cancer Res Clin Oncol.
141:1151–1162. 2015. View Article : Google Scholar
|
|
37
|
Wang Y, Li Y, Jiang L, Ren X, Cheng B and
Xia J: Prognostic value of glycolysis markers in head and neck
squamous cell carcinoma: A meta-analysis. Aging (Albany NY).
13:7284–7299. 2021. View Article : Google Scholar
|
|
38
|
Pertega-Gomes N, Felisbino S, Massie CE,
Vizcaino JR, Coelho R, Sandi C, Simoes-Sousa S, Jurmeister S,
Ramos-Montoya A, Asim M, et al: A glycolytic phenotype is
associated with prostate cancer progression and aggressiveness: A
role for monocarboxylate transporters as metabolic targets for
therapy. J Pathol. 236:517–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilde L, Roche M, Domingo-Vidal M, Tanson
K, Philp N, Curry J and Martinez-Outschoorn U: Metabolic coupling
and the reverse Warburg effect in cancer: Implications for novel
biomarker and anticancer agent development. Semin Oncol.
44:198–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huhta H, Helminen O, Palomäki S, Kauppila
JH, Saarnio J, Lehenkari PP and Karttunen TJ: Intratumoral lactate
metabolism in Barrett's esophagus and adenocarcinoma. Oncotarget.
8:228942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu Y and Zeng F: Expressions of GPR81,
MCT1 and MCT4 in squamous carcinoma and their clinical
significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 43:950–956.
2018.In Chinese. PubMed/NCBI
|
|
42
|
Nakayama Y, Torigoe T, Inoue Y, Minagawa
N, Izumi H, Kohno K and Yamaguchi K: Prognostic significance of
monocarboxylate transporter 4 expression in patients with
colorectal cancer. Exp Ther Med. 3:25–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hubert CG, Rivera M, Spangler LC, Wu Q,
Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE and Rich JN: A
three-dimensional organoid culture system derived from human
glioblastomas recapitulates the hypoxic gradients and cancer stem
cell heterogeneity of tumors found in vivo. Cancer Res.
76:2465–2477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Post Y, Puschhof J, Beumer J, Kerkkamp HM,
de Bakker MA, Slagboom J, de Barbanson B, Wevers NR, Spijkers XM,
Olivier T, et al: Snake venom gland organoids. Cell.
180:233–247.e21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boretto M, Maenhoudt N, Luo X, Hennes A,
Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I,
et al: Patient-derived organoids from endometrial disease capture
clinical heterogeneity and are amenable to drug screening. Nat Cell
Biol. 21:1041–1051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Haider N, Dutt P, van de Kooij B, Ho J,
Palomero L, Pujana MA, Yaffe M and Stambolic V: NEK10 tyrosine
phosphorylates p53 and controls its transcriptional activity.
Oncogene. 39:5252–5266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
48
|
Park E and Chung SW: ROS-mediated
autophagy increases intracellular iron levels and ferroptosis by
ferritin and transferrin receptor regulation. Cell Death Dis.
10:8222019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ding CC, Rose J, Sun T, Wu J, Chen PH, Lin
CC, Yang WH, Chen KY, Lee H, Xu E, et al: MESH1 is a cytosolic
NADPH phosphatase that regulates ferroptosis. Nat Metab. 2:270–277.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018. View Article : Google Scholar
|
|
52
|
Burrell RA, McGranahan N, Bartek J and
Swanton C: The causes and consequences of genetic heterogeneity in
cancer evolution. Nature. 501:338–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng H, Pomyen Y, Hernandez MO, Li C,
Livak F, Tang W, Dang H, Greten TF, Davis JL, Zhao Y, et al:
Single-cell analysis reveals cancer stem cell heterogeneity in
hepatocellular carcinoma. Hepatology. 68:127–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang DC and Wang X: Systems heterogeneity:
An integrative way to understand cancer heterogeneity. Semin Cell
Dev Biol. 64:1–4. 2017. View Article : Google Scholar
|
|
55
|
Hitomi M, Chumakova AP, Silver DJ, Knudsen
AM, Pontius WD, Murphy S, Anand N, Kristensen BW and Lathia JD:
Asymmetric cell division promotes therapeutic resistance in
glioblastoma stem cells. JCI Insight. 6:e1305102021. View Article : Google Scholar :
|
|
56
|
Marjanovic ND, Weinberg RA and Chaffer CL:
Cell plasticity and heterogeneity in cancer. Clin Chem. 59:168–179.
2013. View Article : Google Scholar
|
|
57
|
Gerlach C, Rohr JC, Perié L, van Rooij N,
van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB,
et al: Heterogeneous differentiation patterns of individual
CD8+ T cells. Science. 340:635–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bergers G and Fendt SM: The metabolism of
cancer cells during metastasis. Nat Rev Cancer. 21:162–180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu L, Chen X, Wang L and Chen S: The sweet
trap in tumors: Aerobic glycolysis and potential targets for
therapy. Oncotarget. 7:38908–38926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brisson L, Bański P, Sboarina M, Dethier
C, Danhier P, Fontenille MJ, Van Hée VF, Vazeille T, Tardy M,
Falces J, et al: Lactate dehydrogenase B controls lysosome activity
and autophagy in cancer. Cancer Cell. 30:418–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kennedy KM, Scarbrough PM, Ribeiro A,
Richardson R, Yuan H, Sonveaux P, Landon CD, Chi JT, Pizzo S,
Schroeder T and Dewhirst MW: Catabolism of exogenous lactate
reveals it as a legitimate metabolic substrate in breast cancer.
PLoS One. 8:e751542013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Silva A, Antunes B, Batista A,
Pinto-Ribeiro F, Baltazar F and Afonso J: In vivo anticancer
activity of AZD3965: A systematic review. Molecules. 27:1812021.
View Article : Google Scholar
|
|
63
|
Polański R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar
|
|
64
|
Benyahia Z, Blackman MC, Hamelin L,
Zampieri LX, Capeloa T, Bedin ML, Vazeille T, Schakman O and
Sonveaux P: In vitro and in vivo characterization of MCT1 inhibitor
AZD3965 confirms preclinical safety compatible with breast cancer
treatment. Cancers (Basel). 13. pp. 5692021, View Article : Google Scholar
|
|
65
|
Beloueche-Babari M, Wantuch S, Casals
Galobart T, Koniordou M, Parkes HG, Arunan V, Chung YL, Eykyn TR,
Smith PD and Leach MO: MCT1 inhibitor AZD3965 increases
mitochondrial metabolism, facilitating combination therapy and
noninvasive magnetic resonance spectroscopy. Cancer Res.
77:5913–5924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang G, Wang JJ, Yin PH, Xu K, Wang YZ,
Shi F, Gao J and Fu XL: New strategies for targeting glucose
metabolism-mediated acidosis for colorectal cancer therapy. J Cell
Physiol. 234:348–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fan J, Ye J, Kamphorst JJ, Shlomi T,
Thompson CB and Rabinowitz JD: Quantitative flux analysis reveals
folate-dependent NADPH production. Nature. 510:298–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lewis C A, Parker SJ, Fiske BP, McCloskey
D, Gui D Y, Green CR, Vokes NI, Feist AM, Vander Heiden MG and
Metallo CM: Tracing compartmentalized NADPH metabolism in the
cytosol and mitochondria of mammalian cells. Mol Cell. 55:253–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang X, Liu Z, Sun J, Song X, Bian M, Wang
F, Yan F and Yu Z: Inhibition of NADPH oxidase 4 attenuates
lymphangiogenesis and tumor metastasis in breast cancer. FASEB J.
35:e215312021.PubMed/NCBI
|
|
71
|
Lu YX, Ju HQ, Liu ZX, Chen DL, Wang Y,
Zhao Q, Wu QN, Zeng ZL, Qiu HB, Hu PS, et al: ME1 regulates NADPH
homeostasis to promote gastric cancer growth and metastasis. Cancer
Res. 78:1972–1985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tamošiūnas M, Plorina EV, Lange M, Derjabo
A, Kuzmina I, Bļizņuks D and Spigulis J: Autofluorescence imaging
for recurrence detection in skin cancer postoperative scars. J
Biophotonics. 13:e2019001622020. View Article : Google Scholar
|
|
73
|
Huang TT, Chen KC, Wong TY, Chen CY, Chen
WC, Chen YC, Chang MH, Wu DY, Huang TY, Nioka S, et al: Two-channel
auto-fluorescence analysis for oral cancer. J Biomed Opt. 24:1–10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gulali A, Mustafa S, Ibrahim K, Erkus E,
Ozer B, Kocak MZ, Yaman S, Keyif F, Altinordu R, Erkol H and Savli
H: Could red cell distribution width be a marker of thyroid cancer?
J Coll Physicians Surg Pak. 27:556–558. 2017.
|
|
75
|
Chamma E, Qiu J, Lindvere-Teene L,
Blackmore KM, Majeed S, Weersink R, Dickie CI, Griffin AM, Wunder
JS, Ferguson PC and DaCosta RS: Optically-tracked handheld
fluorescence imaging platform for monitoring skin response in the
management of soft tissue sarcoma. J Biomed Opt. 20:0760112015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Waaijer L, Filipe MD, Simons J, van der
Pol CC, de Boorder T, van Diest PJ and Witkamp AJ: Detection of
breast cancer precursor lesions by autofluorescence ductoscopy.
Breast Cancer. 28:119–129. 2021. View Article : Google Scholar
|
|
77
|
Borile G, Sandrin D, Filippi A, Anderson
KI and Romanato F: Label-free multiphoton microscopy: Much more
than fancy images. Int J Mol Sci. 22:26572021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Salvagno GL, Sanchis-Gomar F, Picanza A
and Lippi G: Red blood cell distribution width: A simple parameter
with multiple clinical applications. Crit Rev Clin Lab Sci.
52:86–105. 2015. View Article : Google Scholar
|
|
79
|
Ma W, Mao S, Bao M, Wu Y, Guo Y, Liu J,
Wang R, Li C, Zhang J, Zhang W and Yao X: Prognostic significance
of red cell distribution width in bladder cancer. Transl Androl
Urol. 9:295–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang PF, Song SY, Guo H, Wang TJ, Liu N
and Yan CX: Prognostic role of pretreatment red blood cell
distribution width in patients with cancer: A meta-analysis of 49
studies. J Cancer. 10:43052019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han F, Liu Y, Cheng S, Sun Z, Sheng C, Sun
X, Shang X, Tian W, Wang X, Li J, et al: Diagnosis and survival
values of neutrophil-lymphocyte ratio (NLR) and red blood cell
distribution width (RDW) in esophageal cancer. Clin Chim Acta.
488:150–158. 2019. View Article : Google Scholar
|
|
82
|
Ghosh M, Saha S, Bettke J, Nagar R,
Parrales A, Iwakuma T, van der Velden AWM and Martinez LA: Mutant
p53 suppresses innate immune signaling to promote tumorigenesis.
Cancer Cell. 39:494–508.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Watson MJ, Vignali PDA, Mullett SJ,
Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV,
Rittenhouse NL, DePeaux K, Whetstone RD, et al: Metabolic support
of tumour-infiltrating regulatory T cells by lactic acid. Nature.
591:645–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Karsten E, Breen E, McCracken SA, Clarke S
and Herbert BR: Red blood cells exposed to cancer cells in culture
have altered cytokine profiles and immune function. Sci Rep.
10:77272020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li ZX, Zheng ZQ, Wei ZH, Zhang LL, Li F,
Lin L, Liu RQ, Huang XD, Lv JW, Chen FP, et al: Comprehensive
characterization of the alternative splicing landscape in head and
neck squamous cell carcinoma reveals novel events associated with
tumorigenesis and the immune microenvironment. Theranostics.
9:7648–7665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Duan Q, Zhang H, Zheng J and Zhang L:
Turning cold into hot: Firing up the tumor microenvironment. Trends
Cancer. 6:605–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hou J, Karin M and Sun B: Targeting
cancer-promoting inflammation-have anti-inflammatory therapies come
of age? Nat Rev Clin Oncol. 18:261–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7:1932021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Boumahdi S and de Sauvage FJ: The great
escape: Tumour cell plasticity in resistance to targeted therapy.
Nat Rev Drug Discov. 19:39–56. 2020. View Article : Google Scholar
|
|
92
|
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL,
Cheng K, Sun RY, Zhou D, Han J and Wu Q: Tom20 senses
iron-activated ROS signaling to promote melanoma cell pyroptosis.
Cell Res. 28:1171–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ubellacker JM, Tasdogan A, Ramesh V, Shen
B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB,
Spitz DR, et al: Lymph protects metastasizing melanoma cells from
ferroptosis. Nature. 585:113–118. 2020. View Article : Google Scholar : PubMed/NCBI
|