1. Introduction
Hydrogen peroxide-inducible clone 5 (Hic-5) was
isolated from a transforming growth factor-β (TGF-β) or hydrogen
peroxide-inducible gene clone by ablative hybridization in 1994 by
Shibanuma et al (1).
Subsequently, after studying Hic-5 at the molecular and cellular
level, it was found that Hic-5 is a homolog of Paxillin and is
currently considered a member of the Paxillin protein family
(2). The Paxillin family includes
Paxillin, Leupaxin and Hic-5, and they function as molecular
adapters that deliver signals when the cellular adhesion
environment changes (2).
Hic-5 has a high degree of homology with Paxillin
and its intracellular localization is similar (Table I) (2-19).
It is primarily restricted to focal adhesion sites, and sites of
adhesion with the extracellular matrix (ECM) via integrins
(2). In contrast to the abnormal
development of extra-embryonic tissues, and the segmentation of the
heart and body in mouse fetuses following the knockout of Paxillin
in mice (20), no effects on
homeostasis and development were observed in Hic-5 knockout mice
(13). This indicates that Hic-5
is not required for development, and suggests that Paxillin and
Hic-5 have different physiological functions. Hic-5 expression has
been detected in smooth muscle cells (SMCs) of various tissues
(21), and a higher level of
expression has been observed in the lungs and spleen (1). Hic-5 expression has been assessed
and shown to vary in several cell lines (2). It is highly expressed in mesenchymal
cell lines, such as fibroblasts, whilst it exhibits lower levels of
expression in epithelial cell lines (2). The fact that Hic-5 expression can be
detected in different tissues and cells also implies that it plays
a central role in the pathophysiology of different organs.
 | Table ICharacterization and expression of
Paxillin and Hic-5. |
Table I
Characterization and expression of
Paxillin and Hic-5.
| Protein | Molecular weight
(kDa) | Chromosome | Domain
structure | Expressing tissues
or cells | Functions | Related
disease | (Refs.) |
|---|
| Hic-5 | 55 | 16p11 (human) 7
(mouse) | Four LD motifs Four
LIM domains | Widespread high
level of expression in SMCs, platelets, and fibroblasts, etc. | Regulation of the
expression of multiple genes through transcriptional regulation.
Interacts with multiple structural and signaling molecules | Vascular injury,
fibrosis, cancer, etc. | (2-14) |
| Paxillin | 68 | 12q24.2 (human) 5
(mouse) | Five LD motifs Four
LIM domains | Universally
expressed | Embryogenesis,
involved in the adhesion and metastasis of tumor cells, regulates
signaling pathways, etc. | Cancer,
inflammation, etc. | (15-19) |
However, the physiological and pathological roles of
Hic-5 have not yet been systematically clarified. Therefore, the
present review summarized the expression of Hic-5 in different
organs and its role in various diseases.
2. Structure and function of Hic-5
Hic-5 has a molecular weight of 55 kDa and consists
of 444 amino acids; its gene is located in chromosome 16p11 in
humans and in chromosome 7 mice (3). A long intron can be found in the
genetic structure of Hic-5 between the N-terminal and C-terminal
structural domains (22). Hic-5
consists of four Leu- and Asp-rich LD domains in the N-terminal
region; LD1 is missing in one isoform; and there are four LiM
domains with two zinc fingers in the C-terminal region (2). Hic-5 also includes multiple
phosphorylation sites; tyrosine phosphorylation can occur in
response to stimuli, such as osmotic pressure, serum factors,
lysophosphatidic acid (LPA) and endothelin (8,23),
which further regulate signaling associated with lamellar
pseudopodia formation to influence cell motility (24). The structural domains involved in
protein interactions include the LD and LiM structural domains, and
these allow Hic-5 to function as a junction molecule and thus
facilitate protein-protein interactions. It can also serve as a
docking site for signaling proteins, such as vinculin and focal
adhesion kinase (FAK) (25,26). Additionally, Hic-5 can function as
a linker molecule in the integrin substrate complex and can
modulate integrin signaling through interactions with binding
molecules (2). Under normal
culture conditions, the majority of cellular behaviors are almost
unaffected by Hic-5 expression (2). When healthy adhesion maintenance is
compromised under conditions of stress that affect focal adhesion
sites, Hic-5 may inhibit adhesion and excessive changes in
cytoskeletal structure by antagonizing Paxillin (21,27).
There is a nuclear export signal (NES) that overlaps
with the amino-terminal LD structural domain, and this allows Hic-5
to enter the nucleus from the cytoplasm via an oxidation-sensitive
NES (28). Hic-5 is transferred
from a focal adhesion site to actin stress fibers in the presence
of organic stress, thereby regulating cell contractility (21). In various pathophysiological
processes, Hic-5 functions primarily through two different
pathways. First, it can affect the transcriptional levels of
several nuclear receptors (29),
including the glucocorticoid receptor (GR) (4,30),
the androgen receptor (AR) (31)
and the progesterone receptor (13). Hic-5 may affect the genomic
occupancy of GR, as well as the assembly of transcriptional
complexes and may thus function as a glucocorticoid regulatory
switch for several genes (30).
After entering the nucleus, Hic-5 can regulate the expression of
several genes through transcriptional regulation (4-6).
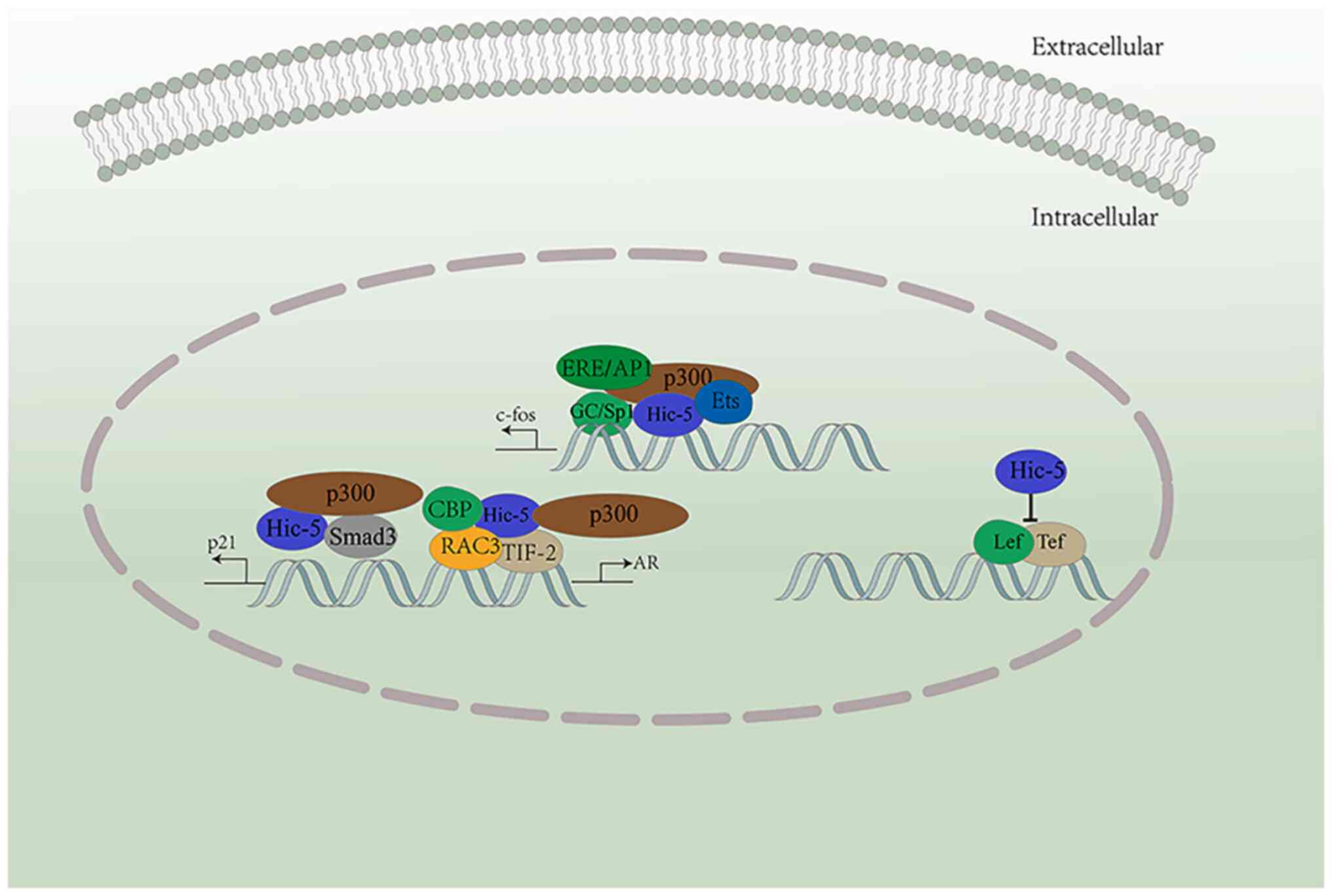
For example, it can repress Lef/Tcf-driven transcription. Multiple
sequences upstream of c-fos (such as GC/Sp1 and Ets, amongst
others) can activate Hic-5 (Fig.
1) (5). The P21 promoter
region can also respond to Hic-5 in the nucleus, which can bind to
Sp1, Smad3, and p300 to create a transcriptional complex (6). Hic-5 also interacts with
glucocorticoid response promoters (such as TIF-2 and p300, among
others) and coactivators, and thus functions as a steroid receptor
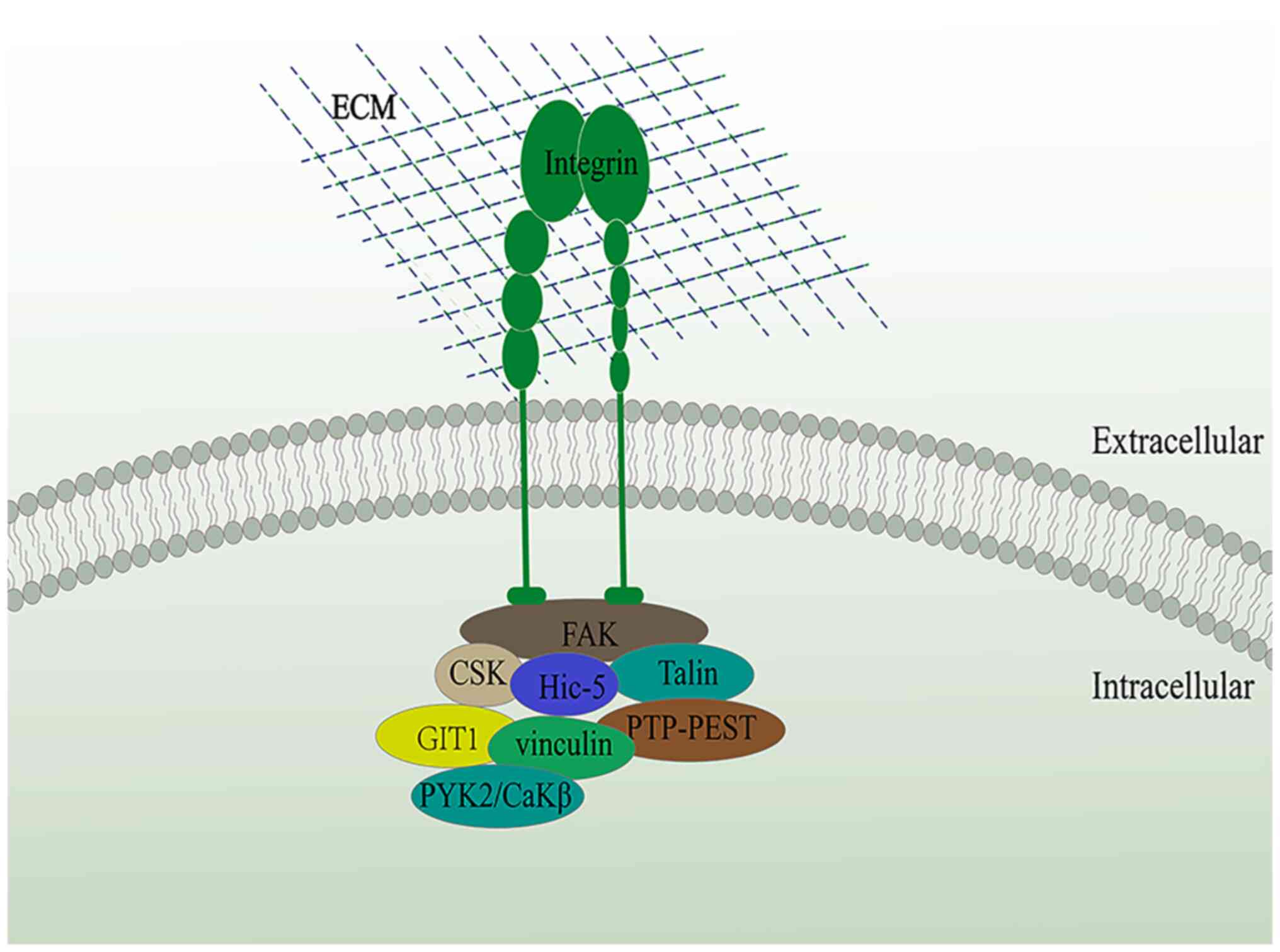
coactivator (6,32-34). In addition, Hic-5 acts in
conjunction with various structural and signaling molecules (such
as FAK, vinculin and PtP-PEST, amongst others) to form a scaffold
for integrin signaling (Fig. 2)
(8-10,14). Hic-5 has been shown to be
essential to the adhesion formation of the three-dimensional (3D)
structure of the ECM (35).
Moreover, when Hic-5 expression is low, fibroblasts exhibit higher
migratory capacity, and when its expression is high, the migratory
capacity of fibroblasts is reduced and the contractile capacity is
increased, increasing the contraction and matrix remodeling of the
ECM (36). This allows Hic-5 to
function as a key factor in various types of fibrotic diseases
(7,11,37).
3. Hic-5 and the regulation of signaling
pathways
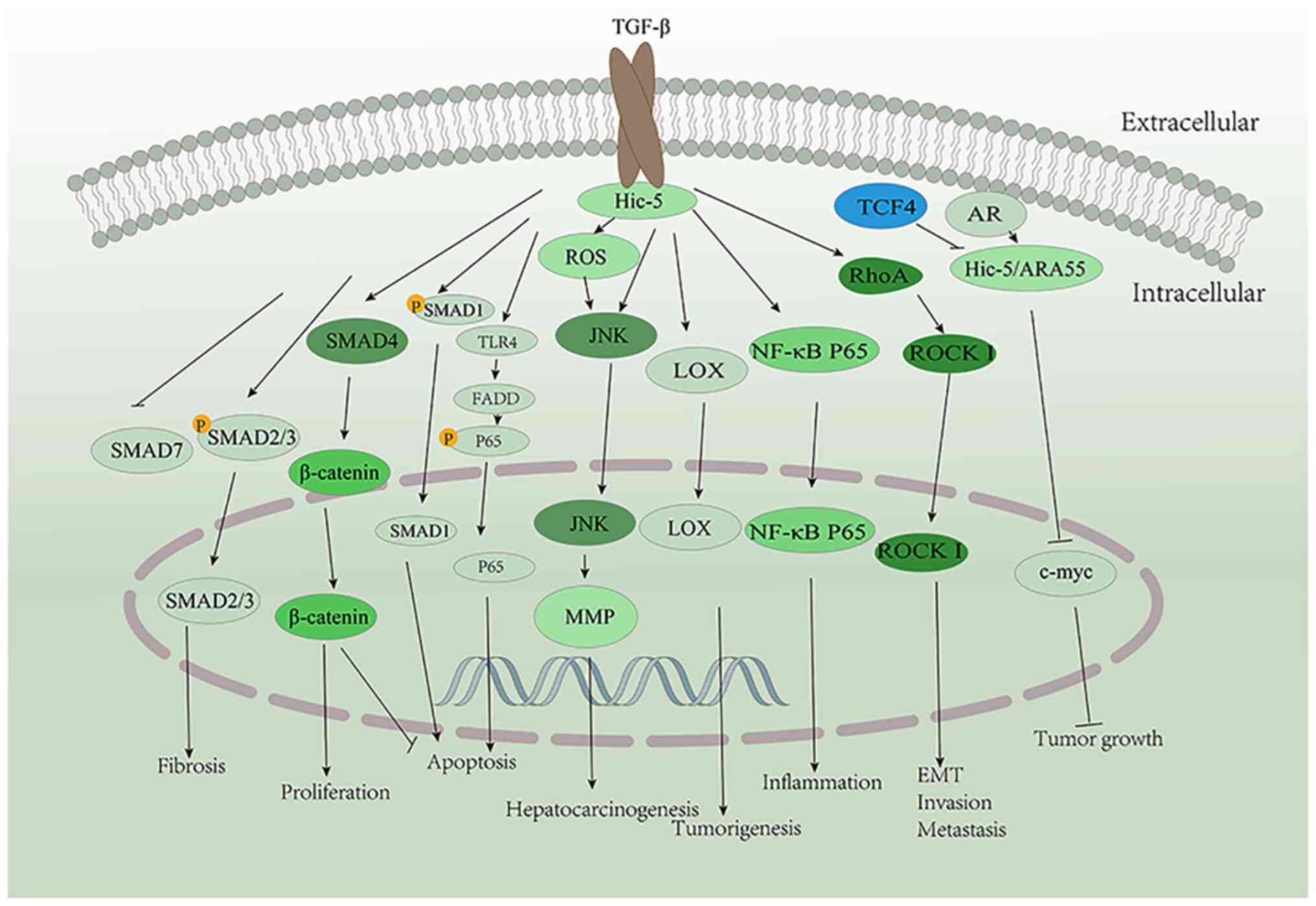
A main mechanism through which Hic-5 exerts its
effects is via the activation of various signaling pathways
(Fig. 3). It can induce the
formation of abdominal aortic aneurysm (AAA) by promoting the
activity of the JNK pathway (38). The TGF-β/Smad pathway is a crucial
factor in fibrotic diseases (39,40), and TGF-β can promote Hic-5
expression (1). Previous studies
have also demonstrated that Hic-5 can promote the development of
liver fibrosis and pancreatic fibrosis via the activation of
Smad2/Smad3 and inhibition of Smad7 (7,41).
It also inhibits the apoptosis of prostate cancer cells by binding
and interacting with Smad1 (42).
Hic-5 inhibits β-catenin function upon interaction with Smad4,
thereby promoting the proliferation and inhibiting apoptosis of
osteosarcoma (OS) cells (43).
Hic-5 can also promote inflammation by activating the activity of
the NF-κB pathway (44) and can
promote cell apoptosis through the Toll-like receptor 4
(TLR4)/Fas-associated protein with death domain (FADD) signaling
pathway (45). Hic-5 can promote
hepatocarcinogenesis by activating the reactive oxygen species
(ROS)/JNK pathway (46), while in
a variety of tumors, Hic-5 can induce epithelial-mesenchymal
transition (EMT) by activating RhoA/ROCK I signaling, thus
promoting invasion and metastasis (12,47,48). Hic-5 may play a critical role in
other signaling pathways; however, current research is limited, and
further research is required to elucidate the roles of Hic-5 in
other signaling pathways.
4. Role of Hic-5 in the cardiovascular
system
A high expression of Hic-5 has been detected in both
vascular and visceral SMCs in mice and humans (49); under physiological conditions,
Hic-5 expression has been shown to play a very limited role in
intravascular homeostasis (13).
Moreover, it has been demonstrated Hic-5−/− mice do not
exhibit any notable abnormalities compared with the wild-type mice
in terms of arterial structure and function (3). However, other researchers have found
a reduced endothelial cell (EC) expansion in capillaries and an
impaired structural organization of cells on basement membrane
extracts following the knockdown of Hic-5 (50). Hic-5 has also been found to be
expressed in mouse ECs, which are involved in both EC spreading and
migration (3). In ECs, the
knockdown of Hic-5 has been found to reduce EC sprouting and lumen
formation, whereas the sprouting deficiency is restored upon the
re-expression of Hic-5. Hic-5 can interact with membrane type-1
matrix metalloproteinase (MT1-MMPs) under the stimulation of
pro-angiogenic factors (51).
During EC sprouting, the expression of MT1-MMPs and Hic-5 complexes
increases, and this promotes the formation of MT1-MMPs and FAK
complexes, thus playing a role in EC matrix proteolysis and cell
motility (51). All types of
vessels contain ECs and vascular SMCs (VSMCs); this suggests that
Hic-5 functions as an essential factor in all types of vascular
diseases.
Hic-5 and vascular injury
A previous study on Hic-5 found that it carried out
a regulatory function in the activation of integrin αIIbβ3 and
platelet aggregation in mice (52). However, a subsequent study found
that the knockdown of Hic-5 did not affect hemostasis and
experimental thrombosis (53),
possibly as the function affected by Hic-5 absence was compensated
for by Paxillin and Leupaxin, which are also expressed in mouse
platelets (54).
In a previous study, in a rat model of wire-mediated
femoral artery injury, the expression of Hic-5 was significantly
downregulated after 4 days, followed by a gradual recovery to
normal levels after 2 weeks, suggesting that Hic-5 expression is
downregulated during the acute phase of vascular injury (13). By contrast, in Hic-5−/−
mice, a significant reduction in the arterial mesangial area with
vascular injury was observed along with the accelerated formation
of neointima and promotion of chronic apoptosis of VSMCs (13). Similarly, in another study, in the
mouse SM SV30 cell line, the expression of Hic-5 was notably
increased after differentiation above undifferentiated levels, and
furthermore, in a rat model of acute vascular injury following the
local delivery of Hic-5 using an adenoviral vector, neointima
formation was inhibited, primarily by reducing the migration of
vascular SM cells (55). However,
further investigations are required to explore the specific
underlying mechanisms.
Hic-5 and atherosclerosis
The adhesion of monocytes and ECs is a key factor in
the development of atherosclerotic plaques (56,57). The APOE−/− model is
currently the most well-recognized and widely used model for
studying atherosclerosis. Arita-Okubo et al (58) extracted the aortas of
APOE−/− and LDLR−/− mice and found that Hic-5
deficiency inhibited the adhesion of THP-1 monocytes to the aorta
typically stimulated with TNF-α or oxidized low-density
lipoprotein. Electron microscopic analysis of aortas extracted from
APOE−/− mice revealed that Hic-5 deletion significantly
inhibited TNF-α-induced microvilli-like structures. Further
experiments revealed that the knockdown of Hic-5 significantly
inhibited atherosclerotic changes in mice. The same reduction in
the number of surface microvillus-like structures was found in
human umbilical vein ECs (HUVECs) in which Hic-5 was knocked down,
while the number of surface microvillus-like structures was
significantly increased in HUVECs after overexpression of Hic-5
(58). These findings indicate
that Hic-5 in ECs not only promotes the formation of
microvillous-like structures, but also promotes the recruitment of
monocytes, which ultimately leads to atherosclerosis (58). However, the exact role of Hic-5
has not yet been determined, thus the mechanisms through which
Hic-5 affects atherosclerosis warrant further investigations in
future studies.
Hic-5 and AAA
In a previous study in which models of AAA were
constructed by the administration of angiotensin II in
APOE−/− and APOE−/− Hic-5−/− mice,
it was found that Hic-5−/− mice exhibited a
significantly lower degree of AAA and had smaller maximum aortic
diameters (38). Abdominal artery
rupture and mass hemorrhaging were observed in the
APOE−/− mice, but not in the APOE−/−
Hic-5−/− mice, indicating that the knockdown of Hic-5
prevented AAA formation and rupture (38). Further mechanistic analyses
revealed that Hic-5 promoted the activation of the JNK pathway,
subsequently increasing MMP expression and promoting the
development of AAAs (38).
Hic-5 and hypertrophic heart disease
Hic-5 has also been shown to be expressed in
neonatal rat ventricular myocytes (NRVMs) (59). In NRVMs, epinephrine induced the
expression of Hic-5, and Hic-5 overexpression significantly
increased the number of cells with organized cytoskeleton (59). Mechanistically, Hic-5 exerted its
effects via regulation of the ERK1/2 signaling pathway (59). Increased levels of Hic-5 were
found in a mouse model of hypertrophic heart disease established
using thoracic aortic constriction (59). However, the elevated of Hic-5 in
the model alone did not suggest that it played a role in the
development of hypertrophic heart disease; thus, future
investigations are required to determine its role in hypertrophic
heart disease.
5. Role of Hic-5 in the digestive
system
Hic-5 and the liver
Hic-5 and liver fibrosis
Previous studies have demonstrated that Hic-5 is not
expressed in quiescent hepatic stellate cells (HSCs), although its
expression is significantly increased following HSC activation
(7,60). The significant upregulation of
HIC-5 expression has also been found in liver tissues from patients
with fibrosis, or in mice subjected to bile duct ligation or mice
with CCl4-induced mouse liver fibrosis, as well as in activated
HSCs (7). It has been revealed
that HSCs from Hic-5−/− mice exhibit a significantly
reduced activation when cultured (7). Additionally, Hic-5 has been found to
promote fibrosis by activating the TGF-β/Smad2 pathway, while
inhibiting Smad7 (7).
Hic-5 and hepatic ischemia-reperfusion
injury (HIRI)
In a previous study, Hic-5 expression was shown to
be increased in models of HIRI, and the expression of inflammatory
chemokines was found to be decreased in a Hic-5−/− HIRI
mouse model, while the extent of liver injury was found to be
reduced compared with wild-type mice (45). Further experiments revealed that
Hic-5 activated the NF-κB signaling pathway to enhance the
inflammatory response, and activated the TLR4/FADD pathway to
increase hepatocyte apoptosis, which ultimately led to the
exacerbation of the lesions in mice (45). This suggests that Hic-5 targeted
therapy may be beneficial for liver transplantation-induced HIRI.
Similarly, Hic-5 may play a role in ischemia-reperfusion injury in
other organs (such as the lungs); however, further studies are
required to confirm this.
Hic-5 and chronic pancreatitis (CP)
Similar to liver fibrosis, pancreatic fibrosis is
also primarily caused by the activation of pancreatic stellate
cells (PSCs) (61). A high
expression of Hic-5 was likewise found in pancreatic tissues of
patients with CP and was primarily expressed in activated PSCs
(41). In a previous study, by
culturing pancreatic primary PSCs, it was found that the knockdown
of HIC-5 inhibited PSC activation. The same study subsequently
found a significant attenuation of pancreatic fibrosis in
Hic-5−/− mice in a model of caerulein-induced CP,
suggesting that Hic-5 can promote the development of CP (41). By culturing primary PSCs, Hic-5
was shown to increase the activation of PSCs by increasing the
phosphorylation of Smad2, ultimately promoting the development of
CP (41). Another study also
found that the knockdown of Hic-5 inhibited NF-κB/p65 expression
(44). This also suggested that
Hic-5 can be used as a diagnostic marker and a potential
therapeutic target in CP.
6. Hic-5 and the urinary system
Hic-5 and the kidneys
In rat kidneys, Hic-5 expression has been detected
in mesenchymal cells, mesangial cells and ECs (62). This suggests that Hic-5 may be
involved in the development of glomerulosclerosis (11). Another study also demonstrated
that the increased expression of Hic-5 in glomerular mesangial
cells was not dependent on the TGF-β-induced pro-sclerotic
phenotype (63); however, that
study only illustrated the increased expression of Hic-5 in
glomerulosclerosis, which does not yet indicate a role in the
development of glomerulosclerosis, and therefore further studies
are required to determine this.
Hic-5 in the prostate
Previous research detected Hic-5 expression in the
normal prostate mesenchyme and exhibited a lesser reduction in the
tumor-associated mesenchyme (31). Hic-5 expression was subsequently
found in both the normal and tumor prostate epithelium, as well as
in prostate cancer cells and prostate cancer tissues (42,64,65). Hic-5 regulates the transcription
of AR (31). When steroid ligands
are absent, Hic-5 can interact with nuclear receptor co-blockers to
inhibit transcription (34,66). In prostate myofibroblasts, Hic-5
rapidly translocates to the nucleus in response to the action of
androgens, consistent with the increased phosphorylation of
adherent spot kinase. Hic-5 acts as a co-regulator and AR can lead
to androgen-induced transcriptional enhancement through interaction
with the coactivator, Hic-5/ARA55, thereby affecting androgenic
effects on growth, cell adhesion, motility and invasion regulation
(67). It was similarly found
that the mRNA levels of Hic-5/ARA55 in normal or benign prostate
hypertrophy tissues were reduced in hormone-independent prostate
cancer tissues (12,64); in normal prostate tissues, it was
primarily expressed in the interstitium (31,68), and in prostate cancer tissues it
was also expressed in the interstitium. Hic-5/ARA55 was also
observed in the cytoplasm and focal adhesion sites of the prostate
mesenchymal cell line, WPMY-1 (31). Hic-5/ARA55 activates AR activity
by interacting with the endogenous androgen response promoter
(31). CYP24A1 encodes the 1,25D3
metabolizing enzyme 24-hydroxylase (69). Hic-5 is a coactivator of the
vitamin D3 receptor (VDR) and can limit the negative feedback
circuit of VDR activity by participating in VDR-mediated
transcription of CYP24A1 (69).
7. Hic-5 and tumors
Tumor cells exhibit two interchangeable patterns of
cell movement, mesenchymal or amoeboid; during invasion, tumor
cells can migrate to different 3D microenvironments through these
two switchable motility modes, and this allows them to invade the
tumor stroma and circulatory system (35,70). Gulvady et al (71) found that the expression levels of
Hic-5 varied greatly by analyzing the expression of Paxillin and
Hic-5 in a variety of tumor cells, while the levels of Paxillin
were relatively stable. It was also found that cancer cell lines
with low Hic-5:Paxillin ratios lacked the ability to efficiently
convert to a mesenchymal phenotype, while cell lines with a high
Hic-5:Paxillin ratios were able to adequately switch from an
amoeboidal to a mesenchymal phenotype. In a variety of tumors,
Hic-5 has been shown to induce EMT by activating RhoA/ROCK I
signaling, thus promoting invasion and metastasis (12,47,48), which highlights its potential as a
biomarker for metastasis in several tumors and as a therapeutic
target.
Hic-5 and hepatocellular carcinoma
(HCC)
Hic-5 is also involved in HCC progression. The
upregulation of proline-rich tyrosine kinase 2 (Pyk2) is expressed
in HCC liver tissues and is associated with a worse prognosis
(72). Other studies have
demonstrated that Pyk2 upregulates the activation and localization
of Hic-5 (73). In another study,
Wu et al (46) found that
Hic-5 expression was upregulated in HCC cell lines that originated
from high motility cancers, but not in HCC cells originated from
low motility cancers when they analyzed liver tissues from patients
with HCC, suggesting that its overexpression was closely associated
with the metastasis of HCC. This suggests that Hic-5 may be used as
a biomarker for metastasis in with HCC. The same authors also found
that the knockdown of Hic-5 in high motility-derived HCC cells
significantly reduced the invasive ability of HCC cells (46). Further analyses revealed that
Hic-5 affected HCC progression through the ROS/JNK pathway
(46). However, that study was
limited to assessing the progression of HCC in vitro, and
further studies are thus required to confirm its role in
vivo and to elucidate the specific underlying mechanisms.
Hic-5 and pancreatic cancer (PC)
In a previous study, the upregulated expression of
Hic-5 was detected in PC, and the knockdown of Hic-5 revealed that
PC cell (PCC) proliferation was suppressed and apoptosis was
increased, while PCC invasion and migration were reduced (74). The same study also found that
patients with a high expression of Hic-5 in PC had lower survival
rates (74). However, there are
fewer studies investigating the role of Hic-5 in PC; thus, further
cellular and animal models are required to examine the mechanisms
through which Hic-5 specifically affects PC and to identify novel
strategies for the management of PC.
Hic-5 and esophageal cancer
The tumor microenvironment plays an essential role
in tumor development and metastasis (75); cancer-associated fibroblasts
(CAFs) are an important component of the tumor microenvironment
(76) that release cytokines into
the ECM, thereby promoting circulating tumor metastasis (77,78). In a previous study, the high
expression of Hic-5 was detected in the CAFs of esophageal squamous
cell carcinoma (ESCC) (79). That
study further demonstrated that the invasion and migration of cells
was inhibited following the knockdown of Hic-5 in KYSE150/TE1
esophageal cancer cells co-cultured with CAFs (79). RNA-seq revealed that Hic-5 in CAFs
may promote tumor progression by regulating cytokines (such as
CCL2) and altering the ECM (79).
The same study also found an association with esophageal cancer
lymph node metastasis through survival analysis (79). This suggests that the levels of
Hic-5 may be used as a marker of lymph node metastasis in ESCC. The
effect of Hic-5 on the biological behavior of esophageal cancer
cells has only been demonstrated in vitro; thus, in
vivo models are warranted to explore the role of Hic-5 and its
specific mechanisms of action in esophageal cancer.
Hic-5 and colon cancer
PPARγ is a major regulator of adipocyte
differentiation (80). Hic-5 has
been shown to bind to and activate PPARγ, and both Hic-5 and PPARγ
are expressed in normal and malignant intestines (33). In a mouse colon cancer model, the
expression of both PPARγ and Hic-5 has been found to be notably
decreased (33). Hic-5 has also
been found to enhance PPARγ-mediated epithelial gene induction in
colon cancer cells (33).
The high expression of Hic-5 was also previously
detected in CAFs of tumor tissues from patients with colon cancer
(81). The addition of CAF
supernatant to normal fibroblasts induced the expression of Hic-5
(81). Following the
establishment of a mouse colon tumor model by azoxymethane
induction, no tumors were found in Hic-5−/− mice
compared with wild-type mice, where tumors were found in 55% of
mice after 20 weeks; proliferative polyps were found in only one
location after 24 weeks, and advanced adenocarcinomas was only
observed in wild-type mice (81).
Further analyses revealed that HIC-5 induced lysyl oxidase (LOX)
expression following nucleation, and LOX promoted the cross-linking
of collagen fibers and thus increased the stiffness of ECM, which
eventually promoted tumor progression (81). This suggests that Hic-5 may serve
as a therapeutic target for the management of colon cancer.
Hic-5 and prostate cancer
Previous research has shown that Hic-5 can interact
with Smad1 to inhibit the apoptosis of prostate cancer cells
(42). LNCaP is a prostate
epithelial cell line that does not express Hic-5. Hic-5
overexpression using lentiviral transfection and subsequent
administration in mice has been shown to result in the inhibition
of tumor growth (64). Further
mechanistic research has revealed that Hic-5/ARA55 can inhibit
c-Myc expression in an androgen-dependent manner (65). The expression of c-Myc is
increased when deprived of androgens, and Hic-5/ARA55 also inhibits
c-Myc expression by suppressing it in a TCF4-dependent and
non-dependent manner (64).
Hic-5 could interact with 1,25D3 to inhibit prostate
cancer cell proliferation. Following androgen deprivation, Hic-5
induces different responses in prostate tumors to 1,25D3,
particularly during androgen deprivation therapy (69). Hic-5 can play a differential role
in the adjuvant treatment of VDR activity in prostate cancer. This
suggests that patients with prostate cancer with a downregulated
expression of Hic-5 may benefit more from treatment with VDR
ligands (69).
Hic-5 and breast cancer
Gulvady et al (71) found that the expression of Hic-5
in breast cancer cells was upregulated. They further constructed a
mouse polyoma middle T-antigen breast cancer model and found that
CAFs in Hic-5−/− mice could not effectively form
fibrillar adhesion in both 2D and 3D (82). Using bioinformatics analysis, it
was found that the high expression of Hic-5 in patients with breast
cancer was negatively associated with the distant metastasis-free
survival (DMFS) of patients (83).
Hic-5 and ovarian cancer
The high expression of Hic-5 was previously found in
advanced ovarian cancer, and EMT independent of TGFβ-1 was induced
by the overexpression of Hic-5 in the ovarian cancer cell line,
A2780 (which is typically morphologically epithelial), accompanied
with enhanced cell proliferation and migratory/invasive ability, as
well as increased resistance to chemotherapeutic agents (48). In addition, in epithelial ovarian
cancer cells, the knockdown of Hic-5 inhibited its proliferation,
migration/invasion and induced mesenchymal-epithelial transition,
and it was further shown that it may promote EMT in ovarian cancer
via the regulation of the RhoA/ROCK signaling pathway (48). However, further in vivo
studies are required to investigate the role of Hic-5 in ovarian
cancer.
Hic-5 and osteosarcoma (OS)
The high expression of Hic-5 was previously detected
in both OS tissues and cells, and a higher percentage of low-grade
OS tissues exhibited an upregulated expression of Hic-5 (46.7%),
while its expression was reduced in the majority of high-grade OS
patient tissues (53.3%) (43).
The knockdown of Hic-5 suppressed the proliferation of OS cells,
whereas it promoted apoptosis. Additionally, it was found that
Hic-5 expression in exosomes was similarly reduced following the
knockdown of Hic-5, and it was further found that Hic-5 interacted
with Smad4 through the exosomal pathway to promote the activation
of β-catenin, thus promoting the proliferation of OS cells
(43).
Hic-5 and melanoma
Hic-5 expression was previously detected in both
human melanoma cells and murine B16-F1 cells (84). The knockdown of Hic-5 in murine
melanoma B16-F1 cells was shown to result in reduced cell
proliferation and migration, and significantly inhibited tumor
growth and lung metastasis in further subcutaneous tumorigenesis
assays in mice (84). It was also
found an increase in RhoA activity upon the knockdown of Hic-5,
accompanied by an altered amoeboid-like phenotype, leading to a
loss of cell plasticity, which may also be responsible for its
effect on cell motility (84).
This suggests that Hic-5 may influence melanoma development via
modulation of the RhoA/ROCK signaling pathway.
8. Hic-5 and other diseases
Hic-5 and Alzheimer's disease (AD)
In a previous study using a model AD, an increased
expression of Hic-5 was detected in pyramidal neurons in the CA1
region of the hippo-campus (85).
It is possible that Hic-5-mediated intracellular signaling in the
ECM is involved in the pathogenesis of AD. However, to the best of
our knowledge, no further studies have been performed to date to
confirm whether Hic-5 is involved in AD development, and thus
further research is required to determine this.
Hic-5 and osteoarthritis
A mouse model of osteoarthritis of the knee was
previously established by surgical induction, and a significantly
lower degree of cartilage degradation was observed in
Hic-5−/− mice than in wild-type mice after 8 weeks
(86). Furthermore, by extracting
primary chondrocyte cultures, it was found that Hic-5 promoted the
development of osteoarthritis primarily by promoting the expression
of inflammatory cytokines, or mechanical load-induced MMP13, and a
disintegrin and metalloproteinase with thrombospondin type 1 motif
5 (86).
Hic-5 and skin
Hic-5 modulates androgen sensitivity in hair
follicle papillae (87), while in
scar-forming myofibroblasts, autocrine TGF-β upregulates Hic-5
expression, further downregulating the autocrine loop of Hic-5 and
collagen synthesis (88). The
overexpression of Hic-5 in keloid-derived fibroblasts was
previously found to increase collagen expression in keloid scars
(89). Similarly, Hic-5 was
confirmed to be expressed in keloid specimens (8/15) using
immunohistochemistry, and was not observed in normal tissues; its
expression was shown to be primarily localized in the nucleus where
it stimulated Smad2/3 expression via other pathways not dependent
on Smad7 thus promoting scar formation (89). Hic-5 expression was found to be
increased in patients with systemic sclerosis (SSc) and SSc dermal
fibroblasts. In addition, in SSc fibroblasts, the knockdown of
Hic-5 reduced the production of type I collagen by >50%. This
suggests that Hic-5 can promote the formation of fibrosis in SSc,
indicating that it may be a therapeutic target for the management
of SSc fibrosis (90). However,
that study had a small clinical sample; thus, further clinical
samples are warranted to confirm these findings and the underlying
molecular mechanism need to be assessed.
Hic-5 and the eyes
Aqueous humor (AH) homeostasis is critical for
maintaining normal intraocular pressure (91). By contrast, increased obstruction
or resistance to the atrial fluid flow through the trabecular
meshwork (TM) causes increased intraocular pressure and is a major
risk element for primary intraocular pressure elevation (92). In a previous study, in human TM
(HTM) cells, Hic-5 expression was limited to the focal adhesion of
HTM cells, as well as throughout the TM and AH efflux pathways of
the Schlemm's canal; the administration of recombinant Hic-5 was
found to result in the redistribution of actin stress fibers, focal
adhesion and αv integrins in the focal adhesion, along with an
increase in a-SMA, and increased collagen-1 expression, whereas the
knockdown of Hic-5 reversed these effects. In the presence of
dexamethasone, Hic-5 in TM increased resistance to AH efflux via
the trabecular pathway, which suggests that Hic-5 plays a critical
role in regulating eye AH outflow through the TM (93).
9. Perspectives of Hic-5 in clinical
applications
TGF-β plays a key role in the progression of
various diseases. Hic-5 expression can be induced by TGF-β, plays a
key role in various diseases (Table
II) (7,11,13,31,38,41-46,48,55,58-60, 64,71,74,79,81,83-86,90,94-122) and the negative effects of its
knockdown are limited and not severe; thus, targeting Hic-5 may be
a suitable approach for the management of several diseases. Hic-5
expression was previously found to be significantly elevated in
liver fibrosis, pancreatic fibrosis, HIRI, glomerulosclerosis and
SSc, while disease pathology was reduced following the knockdown of
Hic-5 (7,11,41,44,45,88,90), which also suggests its potential
use as a biomarker for the diagnosis of fibrotic diseases and as a
potential therapeutic target. The role of Hic-5 in other fibrotic
diseases likely promotes fibrosis via the activation of the
TGF-β/Smad pathway.
 | Table IIRole of Hic-5 and TGF-β in different
diseases. |
Table II
Role of Hic-5 and TGF-β in different
diseases.
| Disease | Hic-5 | TGF-β | (Refs.) |
|---|
| Vascular
injury | • Expression was
downregulated during the acute phase of vascular injury.
Hic-5−/− mice exhibited a significant reduction in the
arterial mesangial area as well as accelerated neonatal intima
formation and increased levels of chronic apoptosis of vascular
SMCs. | • Increased
expression after vascular injury.
• Promotes the proliferation of neoplastic endothelium. | (13,55,94,95) |
|
Atherosclerosis | • Plays an
essential role in the formation of microvillous structures of
endothelial cells.
• Promotes atherosclerosis by recruiting monocytes and interacting
with monocytes. | • Decreased TGF-β
levels in patients with advanced atherosclerosis.
• Promotes the development of atherosclerosis. | (58,95,96) |
| Abdominal aortic
aneurysm | • Activates the JNK
pathway to enhance MMP expression in VSMCs and promote AAA
formation. | • Decrease aneurysm
formation and progression.
• TGF-β1 can decrease pseudoaneurysm formation and
progression. | (38,96,97) |
| Hypertrophic heart
disease | • Overexpression of
Hic-5 increases the number of cells in the cytoskeletal
tissue. | • Not reported | (59) |
| Liver fibrosis | • Expression is
upregulated in patients with liver fibrosis and in mouse
models.
• Exerts its effect by activating the TGF-β/Smad2 pathway while
suppressing Smad7. | • Activation of
hepatic stellate cells through the
• TGF-β/Smad signaling pathway, thereby promoting liver
fibrosis | (7,60,98,99) |
| Hepatic
ischemia-reperfusion injury (HIRI) | • Highly expressed
in HIRI models.
• Promotes hepatocyte apoptosis through the TLR4-FADD pathway and
inflammation through the NF-κB pathway. | • Serum TGF-β1
levels are upregulated in the HIRI mouse model. | (45,100) |
| Hepatocellular
carcinoma (HCC) | • Highly expressed
in HCC cells originating from cells with a high degree of
motility.
• Promotes HCC development through the ROS-JNK signaling
pathway. | • Limiting
hepatocyte proliferation under normal conditions, but enabling
chemically induced HCC in the heterozygous state.
• Promotes HCC progression and immune escape. | (46,99,101-103) |
| Chronic
pancreatitis | • Highly expressed
in patients with CP.
• Exerts its effect by activating the TGF-β/Smad2 signaling pathway
and NF-κB signaling pathway. | • Highly expressed
in a mouse model of CP.
• Promotes CP progression. | (41,44,104) |
| Pancreatic
cancer | • Highly expressed
in pancreatic cancer.
• Promotes the proliferation of PCCs, reduces apoptosis and
promotes the invasion and migration of PCCs. | • Increased
expression in patients with pancreatic ductal adenocarcinoma
(PDAC).
• Inhibition of TGF-β receptor inhibits tumor growth and
metastasis. | (74,99,105) |
| Esophageal
cancer | • Highly expressed
in ESCC.
• Promotes invasion and migration of esophageal squamous carcinoma
cells. Could be used as a marker of lymph node metastasis in
ESCC. | • Associated with
the overall survival time in esophageal cancer. | (79,99,106) |
| Colorectal
cancer | • Highly expressed
in the CAFs of patients with CRC.
• Promotes the expression of LOX, promotes the cross-linking of
collagen fibers, and thus increases the stiffness of the ECM,
ultimately promoting tumor progression. | • Highly expressed
in CRC.
• Inhibition of TGF-β signaling inhibits CRC metastasis | (81,99,107,108) |
|
Glomerulosclerosis | • Increased
expression in a rat model of glomerulosclerosis.
• Kidney fibrosis can be reduced by targeting TGF-β downstream
molecules. | • Highly expressed
in various kidney diseases associated with fibrosis. | (11,109,110) |
| Prostate
cancer | • Expressed in
tumor prostate epithelium, AR-deficient prostate cancer cells, and
in tissue from untreated stage IV prostate cancer.
• Interacts with Smad1 to inhibit apoptosis. | • Inhibits
proliferation in early stages of prostate cancer, promotes
proliferation and metastasis in advanced stages. | (31,42,64, 111-113) |
| Breast cancer | • Increased
expression in breast cancer cells.
• Negatively associated with the DMFS of breast cancer
patients. | • TGF-β1 activated
cancer-associated fibroblasts to promote breast cancer invasion,
metastasis and EMT | (71,83,114) |
| Ovarian cancer | • Highly expressed
in ovarian cancer.
• Promotes EMT in ovarian cancer via RhoA/ROCK signaling. | • Induces
metastasis or EMT in advanced ovarian cancer | (48,115) |
| Osteosarcoma | • Highly expressed
in the osteosarcoma.
• Activates β-catenin by interacting with smad4 to promote
osteosarcoma cell proliferation and inhibit osteosarcoma
apoptosis. | • Serum levels are
increased in patients with osteosarcoma. | (43,116,117) |
| Melanoma | • Expressed in
human melanoma cells.
• Promotes proliferation and migration. | • Promotes invasion
and metastasis.
• Promotes the invasion and metastasis of melanoma cells. | (84,118) |
| Alzheimer's
disease | • Increased
expression in a rat model of AD | • Serum levels are
increased in patients with AD.
• Reduce amyloid β-protein (Aβ) pathology of brain
parenchyma.
• May be a useful biological marker for patients with AD. | (85,119,120) |
| Osteoarthritis | • Promotes the
development of osteoarthritis by increasing the expression of MMP13
and ADAMTS5 | • Serum levels are
increased in patients with osteoarthritis.
• Promotes osteoarthritis progression. | (86,121) |
| Systemic
sclerosis | • Increased
expression in systemic sclerosis skin. | • Elevated serum
levels in patients with systemic sclerosis. | (90,122) |
In tumors, the high expression of Hic-5 was found
to promote the transformation of tumor cells with an amoeboid
phenotype to a mesenchymal phenotype and promote EMT through the
RhoA/ROCK pathway, thus promoting tumor cell plasticity and
invasiveness (12,47,48), which also suggests that Hic-5 may
be used as a biomarker for tumor metastasis. Related studies have
demonstrated that Hic-5 expression is upregulated in HCC with a low
migratory capacity and is also associated with lymph node
metastasis in patients with esophageal cancer (46,79). Therefore, it may be used as a
biomarker for the detection of metastases in patients with HCC, and
lymph node metastases in patients with esophageal cancer. It is
also negatively associated with the survival of with PC and the
DMFS of patients with breast cancer and may serve as a biomarker
for predicting a poor prognosis of patients with PC and breast
cancer. The knockdown of Hic-5 in HCC cells, PC cells, esophageal
cancer cells, ovarian cancer, melanoma and cholangiocarcinoma cells
led to the inhibition of tumor cell invasion and migration
(43,46,48,74,79,84,123), and the knockdown of Hic-5 in
HuCCT1 cholangiocarcinoma cells was also found to inhibit
cholangiocarcinoma migration in a concentration-dependent manner
(123). This suggests that it
may be possible to inhibit tumor metastasis in these tumors by
targeting Hic-5. However, the effects of the knockdown of Hic-5
were only verified at the cellular level in these tumors, and
further in vivo experiments are thus required to confirm
these results. In addition, the knockdown of Hic-5 was previously
found to suppress tumorigenesis in mouse models of colon and breast
cancer (81,82). In patients with PC, those with a
downregulated HIC-5 expression were found to benefit more from VDR
ligand therapy (69).
Furthermore, the majority of research has focused on the effects of
Hic-5 on tumor metastasis, and thus further studies are required to
explore the effects of Hic-5 on tumorigenesis.
In future studies, drugs targeting Hic-5 need to be
investigated, which may provide novel options for the treatment of
tumors. Moreover, Hic-5 promotes CAF protofiber adhesion by direct
interaction with Tensin1, and the interaction is through a
phosphorylation-dependent mechanism (83). Therefore, Hic-5 plays a key role
in CAFs, and future research on drugs targeting the tumor
microenvironment using Hic-5 as a target may provide novel avenues
for the management and treatment of several types of cancer.
10. Conclusions
As an important component of focal adhesion sites,
Hic-5 plays an essential role in cell proliferation, migration,
differentiation and apoptosis. It also plays a key role in
transcriptional regulation, as well as in the regulation of
signaling pathways, and in the suppression of its functions can
prevent the development of several diseases. Thus, Hic-5 may serve
as a diagnostic biomarker, as well as a therapeutic target for a
wide range of diseases, including tumors. Several studies have
confirmed the expression of Hic-5 in various organs and its
pathological roles in different systems; however, the exact
physiological and pathological roles of Hic-5 in several other
organs remain unclear and additional studies are required to
explore its functions. The present review summarized the role of
Hic-5 in a variety of organs and their associated diseases with the
aim of providing an up-to-date background on the current body of
literature from which future studies may be designed.
Availability of data and materials
Not applicable.
Authors' contributions
SY and ZT drafted the manuscript and contributed
equally. XY, LiZhang, YZ, LZheng and HW participated in the
literature search and analysis of the data to be included in the
review. ZY, JA and HJ were involved in the design of the study and
assisted in the preparation of the figures and tables. GW and BT
edited and revised the manuscript. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81960507, 82073087
and 82160112), and the Collaborative Innovation Center of Chinese
Ministry of Education (2020-39), the Science and Technology Bureau
fund of Zunyi City [grant no. ZUN SHI KE HE HZ ZI (2019)93-Hao] and
the Science and Technology Plan Project of Guizhou Province [grant
nos. QIAN KE HE JI CHU-ZK (2021) YI BAN451 and QIAN KE HE LH ZI
(2017)7095 HAO].
References
|
1
|
Shibanuma M, Mashimo J, Kuroki T and Nose
K: Characterization of the TGF beta 1-inducible hic-5 gene that
encodes a putative novel zinc finger protein and its possible
involvement in cellular senescence. J Biol Chem. 269:26767–26774.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibanuma M, Mori K and Nose K: HIC-5: A
mobile molecular scaffold regulating the anchorage dependence of
cell growth. Int J Cell Biol. 2012:4261382012. View Article : Google Scholar
|
|
3
|
Kim-Kaneyama JR, Lei XF, Arita S, Miyauchi
A, Miyazaki T and Miyazaki A: Hydrogen peroxide-inducible clone 5
(Hic-5) as a potential therapeutic target for vascular and other
disorders. J Atheroscler Thromb. 19:601–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L, Guerrero J, Hong H, DeFranco DB
and Stallcup MR: Interaction of the tau2 transcriptional activation
domain of glucocorticoid receptor with a novel steroid receptor
coactivator, Hic-5, which localizes to both focal adhesions and the
nuclear matrix. Mol Biol Cell. 11:2007–2018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim-Kaneyama JR, Shibanuma M and Nose K:
Transcriptional activation of the c-fos gene by a LIM protein,
Hic-5. Biochem Biophys Res Commun. 299:360–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibanuma M, Kim-Kaneyama JR, Sato S and
Nose K: A LIM protein, Hic-5, functions as a potential coactivator
for Sp1. J Cell Biochem. 91:633–645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei XF, Fu W, Kim-Kaneyama JR, Omoto T,
Miyazaki T, Li B and Miyazaki A: Hic-5 deficiency attenuates the
activation of hepatic stellate cells and liver fibrosis through
upregulation of Smad7 in mice. J Hepatol. 64:110–117. 2016.
View Article : Google Scholar
|
|
8
|
Matsuya M, Sasaki H, Aoto H, Mitaka T,
Nagura K, Ohba T, Ishino M, Takahashi S, Suzuki R and Sasaki T:
Cell adhesion kinase beta forms a complex with a new member, Hic-5,
of proteins localized at focal adhesions. J Biol Chem.
273:1003–1014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishiya N, Shirai T, Suzuki W and Nose K:
Hic-5 interacts with GIT1 with a different binding mode from
paxillin. J Biochem. 132:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita H, Kamiguchi K, Cho D, Shibanuma M,
Morimoto C and Tachibana K: Interaction of Hic-5, A
senescence-related protein, with focal adhesion kinase. J Biol
Chem. 273:26516–26521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hornigold N, Craven RA, Keen JN, Johnson
T, Banks RE and Mooney AF: Upregulation of Hic-5 in
glomerulosclerosis and its regulation of mesangial cell apoptosis.
Kidney Int. 77:329–338. 2010. View Article : Google Scholar
|
|
12
|
Mestayer C, Blanchère M, Jaubert F, Dufour
B and Mowszowicz I: Expression of androgen receptor coactivators in
normal and cancer prostate tissues and cultured cell lines.
Prostate. 56:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim-Kaneyama JR, Takeda N, Sasai A,
Miyazaki A, Sata M, Hirabayashi T, Shibanuma M, Yamada G and Nose
K: Hic-5 deficiency enhances mechanosensitive apoptosis and
modulates vascular remodeling. J Mol Cell Cardiol. 50:77–86. 2011.
View Article : Google Scholar
|
|
14
|
Nishiya N, Iwabuchi Y, Shibanuma M, Côté
JF, Tremblay ML and Nose K: Hic-5, a paxillin homologue, binds to
the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3
domain. J Biol Chem. 274:9847–9853. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
López-Colomé AM, Lee-Rivera I,
Benavides-Hidalgo R and López E: Paxillin: A crossroad in
pathological cell migration. J Hematol Oncol. 10:502017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma X and Hammes SR: Paxillin actions in
the nucleus. Steroids. 133:87–92. 2018. View Article : Google Scholar :
|
|
17
|
Xu W, Alpha KM, Zehrbach NM and Turner CE:
Paxillin promotes breast tumor collective cell invasion through
maintenance of adherens junction integrity. Mol Biol Cell.
33:ar142022. View Article : Google Scholar :
|
|
18
|
Tanaka N, Minemura C, Asai S, Kikkawa N,
Kinoshita T, Oshima S, Koma A, Kasamatsu A, Hanazawa T, Uzawa K and
Seki N: Identification of miR-199-5p and miR-199-3p target genes:
Paxillin facilities cancer cell aggressiveness in head and neck
squamous cell carcinoma. Genes (Basel). 12:19102021. View Article : Google Scholar
|
|
19
|
Ripamonti M, Wehrle-Haller B and de Curtis
I: Paxillin: A hub for mechano-transduction from the β3
integrin-talin-kindlin axis. Front Cell Dev Biol. 10:8520162022.
View Article : Google Scholar
|
|
20
|
Hagel M, George EL, Kim A, Tamimi R, Opitz
SL, Turner CE, Imamoto A and Thomas SM: The adaptor protein
paxillin is essential for normal development in the mouse and is a
critical transducer of fibronectin signaling. Mol Cell Biol.
22:901–915. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim-Kaneyama JR, Suzuki W, Ichikawa K,
Ohki T, Kohno Y, Sata M, Nose K and Shibanuma M: Uni-axial
stretching regulates intracellular localization of Hic-5 expressed
in smooth-muscle cells in vivo. J Cell Sci. 118:937–949. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mashimo J, Shibanuma M, Satoh H, Chida K
and Nose K: Genomic structure and chromosomal mapping of the mouse
hic-5 gene that encodes a focal adhesion protein. Gene. 249:99–103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panetti TS, Hannah DF, Avraamides C,
Gaughan JP, Marcinkiewicz C, Huttenlocher A and Mosher DF:
Extracellular matrix molecules regulate endothelial cell migration
stimulated by lysophosphatidic acid. J Thromb Haemost. 2:1645–1656.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hetey SE, Lalonde DP and Turner CE:
Tyrosine-phosphorylated Hic-5 inhibits epidermal growth
factor-induced lamellipodia formation. Exp Cell Res. 311:147–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tumbarello DA, Brown MC and Turner CE: The
paxillin LD motifs. FEBS Lett. 513:114–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown MC and Turner CE: Paxillin: Adapting
to change. Physiol Rev. 84:1315–1339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishiya N, Tachibana K, Shibanuma M,
Mashimo JI and Nose K: Hic-5-reduced cell spreading on fibronectin:
Competitive effects between paxillin and Hic-5 through interaction
with focal adhesion kinase. Mol Cell Biol. 21:5332–5345. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibanuma M, Kim-Kaneyama JR, Ishino K,
Sakamoto N, Hishiki T, Yamaguchi K, Mori K, Mashimo J and Nose K:
Hic-5 communicates between focal adhesions and the nucleus through
oxidant-sensitive nuclear export signal. Mol Biol Cell.
14:1158–1171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heitzer MD and DeFranco DB: Hic-5, an
adaptor-like nuclear receptor coactivator. Nucl Recept Signal.
4:e0192006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chodankar R, Wu DY, Schiller BJ, Yamamoto
KR and Stallcup MR: Hic-5 is a transcription coregulator that acts
before and/or after glucocorticoid receptor genome occupancy in a
gene-selective manner. Proc Natl Acad Sci USA. 111:4007–4012. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heitzer MD and DeFranco DB: Hic-5/ARA55, a
LIM domain-containing nuclear receptor coactivator expressed in
prostate stromal cells. Cancer Res. 66:7326–7333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghogomu SM, van Venrooy S, Ritthaler M,
Wedlich D and Gradl D: HIC-5 is a novel repressor of lymphoid
enhancer factor/T-cell factor-driven transcription. J Biol Chem.
281:1755–1764. 2006. View Article : Google Scholar
|
|
33
|
Drori S, Girnun GD, Tou L, Szwaya JD,
Mueller E, Xia K, Shivdasani RA and Spiegelman BM: Hic-5 regulates
an epithelial program mediated by PPARgamma. Genes Dev. 19:362–375.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heitzer MD and DeFranco DB: Mechanism of
action of Hic-5/androgen receptor activator 55, a LIM
domain-containing nuclear receptor coactivator. Mol Endocrinol.
20:56–64. 2006. View Article : Google Scholar
|
|
35
|
Deakin NO and Turner CE: Distinct roles
for paxillin and Hic-5 in regulating breast cancer cell morphology,
invasion, and metastasis. Mol Biol Cell. 22:327–341. 2011.
View Article : Google Scholar :
|
|
36
|
Vohnoutka RB, Gulvady AC, Goreczny G,
Alpha K, Handelman SK, Sexton JZ and Turner CE: The focal adhesion
scaffold protein Hic-5 regulates vimentin organization in
fibroblasts. Mol Biol Cell. 30:3037–3056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paul J, Singh AK, Kathania M, Elviche TL,
Zeng M, Basrur V, Theiss AL and Venuprasad K: IL-17-driven
intestinal fibrosis is inhibited by Itch-mediated ubiquitination of
HIC-5. Mucosal Immunol. 11:427–436. 2018. View Article : Google Scholar
|
|
38
|
Lei XF, Kim-Kaneyama JR, Arita-Okubo S,
Offermanns S, Itabe H, Miyazaki T and Miyazaki A: Identification of
Hic-5 as a novel scaffold for the MKK4/p54 JNK pathway in the
development of abdominal aortic aneurysms. J Am Heart Assoc.
3:e0007472014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frangogiannis N: Transforming growth
factor-β in tissue fibrosis. J Exp Med. 217:e201901032020.
View Article : Google Scholar
|
|
40
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Mol Cancer. 21:1042022. View Article : Google Scholar
|
|
41
|
Gao L, Lei XF, Miyauchi A, Noguchi M,
Omoto T, Haraguchi S, Miyazaki T, Miyazaki A and Kim-Kaneyama JR:
Hic-5 is required for activation of pancreatic stellate cells and
development of pancreatic fibrosis in chronic pancreatitis. Sci
Rep. 10:191052020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shola DT, Wang H, Wahdan-Alaswad R and
Danielpour D: Hic-5 controls BMP4 responses in prostate cancer
cells through inter-acting with Smads 1,5 and 8. Oncogene.
31:2480–2490. 2012. View Article : Google Scholar
|
|
43
|
Sha L, Ma D and Chen C: Exosome-mediated
Hic-5 regulates proliferation and apoptosis of osteosarcoma via
Wnt/β-catenin signal pathway. Aging (Albany NY). 12:23598–23608.
2020. View Article : Google Scholar
|
|
44
|
Chen H, Tan P, Qian B, Du Y, Wang A, Shi
H, Huang Z, Huang S, Liang T and Fu W: Hic-5 deficiency protects
cerulein-induced chronic pancreatitis via down-regulation of the
NF-κB (p65)/IL-6 signalling pathway. J Cell Mol Med. 24:1488–1503.
2020. View Article : Google Scholar
|
|
45
|
Gao L, Qian B, Chen H, Wang A, Li Q, Li J,
Tan P, Xia X, Du Y and Fu W: Hic-5 deficiency attenuates hepatic
ischemia reperfusion injury through TLR4/NF-κB signaling pathways.
Life Sci. 249:1175172020. View Article : Google Scholar
|
|
46
|
Wu JR, Hu CT, You RI, Pan SM, Cheng CC,
Lee MC, Wu CC, Chang YJ, Lin SC, Chen CS, et al: Hydrogen peroxide
inducible clone-5 mediates reactive oxygen species signaling for
hepatocellular carcinoma progression. Oncotarget. 6:32526–32544.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tumbarello DA and Turner CE: Hic-5
contributes to epithelial-mesenchymal transformation through a
RhoA/ROCK-dependent pathway. J Cell Physiol. 211:736–747. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sheta R, Wang ZQ, Bachvarova M, Plante M,
Gregoire J, Renaud MC, Sebastianelli A, Gobeil S, Morin C,
Macdonald E, et al: Hic-5 regulates epithelial to mesenchymal
transition in ovarian cancer cells in a TGFβ1-independent manner.
Oncotarget. 8:82506–82530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yuminamochi T, Yatomi Y, Osada M, Ohmori
T, Ishii Y, Nakazawa K, Hosogaya S and Ozaki Y: Expression of the
LIM proteins paxillin and Hic-5 in human tissues. J Histochem
Cytochem. 51:513–521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Komorowsky C, Samarin J, Rehm M, Guidolin
D and Goppelt-Struebe M: Hic-5 as a regulator of endothelial cell
morphology and connective tissue growth factor gene expression. J
Mol Med (Berl). 88:623–631. 2010. View Article : Google Scholar
|
|
51
|
Dave JM, Abbey CA, Duran CL, Seo H,
Johnson GA and Bayless KJ: Hic-5 mediates the initiation of
endothelial sprouting by regulating a key surface
metalloproteinase. J Cell Sci. 129:743–756. 2016.PubMed/NCBI
|
|
52
|
Kim-Kaneyama JR, Miyauchi A, Lei XF, Arita
S, Mino T, Takeda N, Kou K, Eto K, Yoshida T, Miyazaki T, et al:
Identification of Hic-5 as a novel regulatory factor for integrin
αIIbβ3 activation and platelet aggregation in mice. J Thromb
Haemost. 10:1867–1874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Popp M, Thielmann I, Nieswandt B and
Stegner D: Normal platelet integrin function in mice lacking
hydrogen peroxide-induced clone-5 (Hic-5). PLoS One.
10:e01334292015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gao J, Huang M, Lai J, Mao K, Sun P, Cao
Z, Hu Y, Zhang Y, Schulte ML, Jin C, et al: Kindlin supports
platelet integrin αIIbβ3 activation by interacting with paxillin. J
Cell Sci. 130:3764–3775. 2017.PubMed/NCBI
|
|
55
|
Kim-Kaneyama JR, Wachi N, Sata M, Enomoto
S, Fukabori K, Koh K, Shibanuma M and Nose K: Hic-5, an adaptor
protein expressed in vascular smooth muscle cells, modulates the
arterial response to injury in vivo. Biochem Biophys Res Commun.
376:682–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vergallo R and Crea F: Atherosclerotic
plaque healing. N Engl J Med. 383:846–857. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Almeida SO and Budoff M: Effect of statins
on atherosclerotic plaque. Trends Cardiovasc Med. 29:451–455. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Arita-Okubo S, Kim-Kaneyama JR, Lei XF, Fu
WG, Ohnishi K, Takeya M, Miyauchi A, Honda H, Itabe H, Miyazaki T
and Miyazaki A: Role of Hic-5 in the formation of microvilli-like
structures and the monocyte-endothelial interaction that
accelerates atherosclerosis. Cardiovasc Res. 105:361–371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yund EE, Hill JA and Keller RS: Hic-5 is
required for fetal gene expression and cytoskeletal organization of
neonatal cardiac myocytes. J Mol Cell Cardiol. 47:520–527. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ji J, Yu F, Ji Q, Li Z, Wang K, Zhang J,
Lu J, Chen L, E Q, Zeng Y and Ji Y: Comparative proteomic analysis
of rat hepatic stellate cell activation: A comprehensive view and
suppressed immune response. Hepatology. 56:332–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vege SS and Chari ST: Chronic
pancreatitis. N Engl J Med. 386:869–878. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jamba A, Kondo S, Urushihara M, Nagai T,
Kim-Kaneyama JR, Miyazaki A and Kagami S: Hydrogen
peroxide-inducible clone-5 regulates mesangial cell proliferation
in proliferative glomerulonephritis in mice. PLoS One.
10:e01227732015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hornigold N and Mooney A: Extracellular
matrix-induced Hic-5 expression in glomerular mesangial cells leads
to a prosclerotic phenotype independent of TGF-β. FASEB J.
29:4956–4967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li X, Martinez-Ferrer M, Botta V,
Uwamariya C, Banerjee J and Bhowmick NA: Epithelial Hic-5/ARA55
expression contributes to prostate tumorigenesis and castrate
responsiveness. Oncogene. 30:167–177. 2011. View Article : Google Scholar :
|
|
65
|
Cárdenas S, Colombero C, Panelo L,
Dakarapu R, Falck JR, Costas MA and Nowicki S: GPR75 receptor
mediates 20-HETE-signaling and metastatic features of
androgen-insensitive prostate cancer cells. Biochim Biophys Acta
Mol Cell Biol Lipids. 1865:1585732020. View Article : Google Scholar :
|
|
66
|
Lee BH and Stallcup MR: Different
chromatin and DNA sequence characteristics define glucocorticoid
receptor binding sites that are blocked or not blocked by
coregulator Hic-5. PLoS One. 13:e01969652018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Leach DA, Need EF, Trotta AP, Grubisha MJ,
DeFranco DB and Buchanan G: Hic-5 influences genomic and
non-genomic actions of the androgen receptor in prostate
myofibroblasts. Mol Cell Endocrinol. 384:185–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li P, Yu X, Ge K, Melamed J, Roeder RG and
Wang Z: Heterogeneous expression and functions of androgen receptor
co-factors in primary prostate cancer. Am J Pathol. 161:1467–1474.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Solomon JD, Heitzer MD, Liu TT, Beumer JH,
Parise RA, Normolle DP, Leach DA, Buchanan G and DeFranco DB: VDR
activity is differentially affected by Hic-5 in prostate cancer and
stromal cells. Mol Cancer Res. 12:1166–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng C, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gulvady AC, Dubois F, Deakin NO, Goreczny
GJ and Turner CE: Hic-5 expression is a major indicator of cancer
cell morphology, migration, and plasticity in three-dimensional
matrices. Mol Biol Cell. 29:1704–1717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shen T and Guo Q: Role of Pyk2 in human
cancers. Med Sci Monit. 24:8172–8182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sun CK, Ng KT, Lim ZX, Cheng Q, Lo CM,
Poon RT, Man K, Wong N and Fan ST: Proline-rich tyrosine kinase 2
(Pyk2) promotes cell motility of hepatocellular carcinoma through
induction of epithelial to mesenchymal transition. PLoS One.
6:e188782011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Qian B, Wei L, Yang Z, He Q, Chen H, Wang
A, Yang D, Li Q, Li J, Zheng S and Fu W: Hic-5 in pancreatic
stellate cells affects proliferation, apoptosis, migration,
invasion of pancreatic cancer cells and postoperative survival time
of pancreatic cancer. Biomed Pharmacother. 121:1093552020.
View Article : Google Scholar
|
|
75
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Biffi G and Tuveson DA: Diversity and
biology of cancer-associated fibroblasts. Physiol Rev. 101:147–176.
2021. View Article : Google Scholar :
|
|
77
|
Sun X, He X, Zhang Y, Hosaka K, Andersson
P, Wu J, Wu J, Jing X, Du Q, Hui X, et al: Inflammatory
cell-derived CXCL3 promotes pancreatic cancer metastasis through a
novel myofibroblast-hijacked cancer escape mechanism. Gut.
71:129–147. 2022. View Article : Google Scholar
|
|
78
|
Zhang M, Liu ZZ, Aoshima K, Cai WL, Sun H,
Xu T, Zhang Y, An Y, Chen JF, Chan LH, et al: CECR2 drives breast
cancer metastasis by promoting NF-κB signaling and
macrophage-mediated immune suppression. Sci Transl Med.
14:eabf54732022. View Article : Google Scholar
|
|
79
|
Du X, Xu Q, Pan D, Xu D, Niu B, Hong W,
Zhang R, Li X and Chen S: HIC-5 in cancer-associated fibroblasts
contributes to esophageal squamous cell carcinoma progression. Cell
Death Dis. 10:8732019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hernandez-Quiles M, Broekema MF and
Kalkhoven E: PPARgamma in metabolism, immunity, and cancer: Unified
and diverse mechanisms of action. Front Endocrinol (Lausanne).
12:6241122021. View Article : Google Scholar
|
|
81
|
Omoto T, Kim-Kaneyama JR, Lei XF, Orimo A,
Ohnishi K, Yoshihara K, Miyauchi A, Li S, Gao L, Umemoto T, et al:
The impact of stromal Hic-5 on the tumorigenesis of colorectal
cancer through lysyl oxidase induction and stromal remodeling.
Oncogene. 37:1205–1219. 2018. View Article : Google Scholar
|
|
82
|
Goreczny GJ, Ouderkirk-Pecone JL, Olson
EC, Krendel M and Turner CE: Hic-5 remodeling of the stromal matrix
promotes breast tumor progression. Oncogene. 36:2693–2703. 2017.
View Article : Google Scholar :
|
|
83
|
Goreczny GJ, Forsythe IJ and Turner CE:
Hic-5 regulates fibrillar adhesion formation to control tumor
extracellular matrix remodeling through interaction with tensin1.
Oncogene. 37:1699–1713. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Noguchi F, Inui S, Nakajima T and Itami S:
Hic-5 affects proliferation, migration and invasion of B16 murine
melanoma cells. Pigment Cell Melanoma Res. 25:773–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Caltagarone J, Hamilton RL, Murdoch G,
Jing Z, DeFranco DB and Bowser R: Paxillin and hydrogen
peroxide-inducible clone 5 expression and distribution in control
and Alzheimer disease hippocampi. J Neuropathol Exp Neurol.
69:356–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Miyauchi A, Kim-Kaneyama JR, Lei XF, Chang
SH, Saito T, Haraguchi S, Miyazaki T and Miyazaki A: Alleviation of
murine osteoarthritis by deletion of the focal adhesion
mechanosensitive adapter, Hic-5. Sci Rep. 9:157702019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Inui S, Fukuzato Y, Nakajima T, Kurata S
and Itami S: Androgen receptor co-activator Hic-5/ARA55 as a
molecular regulator of androgen sensitivity in dermal papilla cells
of human hair follicles. J Invest Dermatol. 127:2302–2306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dabiri G, Tumbarello DA, Turner CE and Van
de Water L: Hic-5 promotes the hypertrophic scar myofibroblast
phenotype by regulating the TGF-beta1 autocrine loop. J Invest
Dermatol. 128:2518–2525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Inui S, Shono F, Noguchi F, Nakajima T,
Hosokawa K and Itami S: In vitro and in vivo evidence of pathogenic
roles of Hic-5/ARA55 in keloids through Smad pathway and
profibrotic transcription. J Dermatol Sci. 58:152–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Piera-Velazquez S, Fertala J,
Huaman-Vargas G, Louneva N and Jiménez SA: Increased expression of
the transforming growth factor β-inducible gene HIC-5 in systemic
sclerosis skin and fibroblasts: A novel antifibrotic therapeutic
target. Rheumatology (Oxford). 59:3092–3098. 2020. View Article : Google Scholar
|
|
91
|
Reina-Torres E, De Ieso ML, Pasquale LR,
Madekurozwa M, van Batenburg-Sherwood J, Overby DR and Stamer WD:
The vital role for nitric oxide in intraocular pressure
homeostasis. Prog Retin Eye Res. 83:1009222021. View Article : Google Scholar :
|
|
92
|
Nair KS, Srivastava C, Brown RV, Koli S,
Choquet H, Kang HS, Kuo YM, Grimm SA, Sutherland C, Badea A, et al:
GLIS1 regulates trabecular meshwork function and intraocular
pressure and is associated with glaucoma in humans. Nat Commun.
12:48772021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Pattabiraman PP and Rao PV: Hic-5
regulates actin cytoskeletal reorganization and expression of
fibrogenic markers and myocilin in trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 56:5656–5669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
You Q, Duan L, Wang F, Du X and Xiao M:
Characterization of the inhibition of vein graft intimal
hyperplasia by a biodegradable vascular stent. Cell Biochem
Biophys. 59:99–107. 2011. View Article : Google Scholar
|
|
95
|
Low EL, Baker AH and Bradshaw AC: TGFβ,
smooth muscle cells and coronary artery disease: A review. Cell
Signal. 53:90–101. 2019. View Article : Google Scholar :
|
|
96
|
Bai H, Lee JS, Hu H, Wang T, Isaji T, Liu
S, Guo J, Liu H, Wolf K, Ono S, et al: Transforming growth
factor-β1 inhibits pseudoaneurysm formation after aortic patch
angioplasty. Arterioscler Thromb Vasc Biol. 38:195–205. 2018.
View Article : Google Scholar
|
|
97
|
Goumans MJ and Ten Dijke P: TGF-β
signaling in control of cardiovascular function. Cold Spring Harb
Perspect Biol. 10:a0222102018. View Article : Google Scholar
|
|
98
|
Boers W, Aarrass S, Linthorst C, Pinzani
M, Elferink RO and Bosma P: Transcriptional profiling reveals novel
markers of liver fibrogenesis: Gremlin and insulin-like growth
factor-binding proteins. J Biol Chem. 281:16289–16295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gough NR, Xiang X and Mishra L: TGF-β
signaling in liver, pancreas, and gastrointestinal diseases and
cancer. Gastroenterology. 161:434–452.e15. 2021. View Article : Google Scholar
|
|
100
|
Nogueira MA, Coelho AM, Sampietre SN,
Patzina RA, Pinheiro da Silva F, D'Albuquerque LA and Machado MC:
Beneficial effects of adenosine triphosphate-sensitive K+ channel
opener on liver ischemia/reperfusion injury. World J Gastroenterol.
20:15319–15326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan
J, Li R, Zhao Q, Wei L, Yang T and Lu J: TGF-β regulates
hepatocellular carcinoma progression by inducing Treg cell
polarization. Cell Physiol Biochem. 35:1623–1632. 2015. View Article : Google Scholar
|
|
103
|
Caja L, Dituri F, Mancarella S,
Caballero-Diaz D, Moustakas A, Giannelli G and Fabregat I: TGF-β
and the tissue microenvironment: Relevance in fibrosis and cancer.
Int J Mol Sci. 19:12942018. View Article : Google Scholar
|
|
104
|
Bansod S, Doijad N and Godugu C: Berberine
attenuates severity of chronic pancreatitis and fibrosis via
AMPK-mediated inhibition of TGF-β1/Smad signaling and M2
polarization. Toxicol Appl Pharmacol. 403:1151622020. View Article : Google Scholar
|
|
105
|
Gore J, Imasuen-Williams IE, Conteh AM,
Craven KE, Cheng M and Korc M: Combined targeting of TGF-β, EGFR
and HER2 suppresses lymphangiogenesis and metastasis in a
pancreatic cancer model. Cancer Lett. 379:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Song W, Dai WJ, Zhang MH, Wang H and Yang
XZ: Comprehensive analysis of the expression of TGF-β signaling
regulators and prognosis in human esophageal cancer. Comput Math
Methods Med. 2021:18122272021. View Article : Google Scholar
|
|
107
|
Calon A, Lonardo E, Berenguer-Llergo A,
Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M,
Palomo-Ponce S, Tauriello DV, Byrom D, et al: Stromal gene
expression defines poor-prognosis subtypes in colorectal cancer.
Nat Genet. 47:320–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tauriello DVF, Palomo-Ponce S, Stork D,
Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M,
Ibiza S, Cañellas A, Hernando-Momblona X, et al: TGFβ drives immune
evasion in genetically reconstituted colon cancer metastasis.
Nature. 554:538–543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Meng XM: Inflammatory mediators and renal
fibrosis. Adv Exp Med Biol. 1165:381–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8:1232020. View Article : Google Scholar
|
|
111
|
Wang H, Song K, Krebs TL, Yang J and
Danielpour D: Smad7 is inactivated through a direct physical
interaction with the LIM protein Hic-5/ARA55. Oncogene.
27:6791–6805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Thompson-Elliott B, Johnson R and Khan SA:
Alterations in TGFβ signaling during prostate cancer progression.
Am J Clin Exp Urol. 9:318–328. 2021.
|
|
113
|
Mirzaei S, Paskeh MDA, Saghari Y, Zarrabi
A, Hamblin MR, Entezari M, Hashemi M, Aref AR, Hushmandi K, Kumar
AP, et al: Transforming growth factor-beta (TGF-β) in prostate
cancer: A dual function mediator? Int J Biol Macromol. 206:435–452.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang M, Fu M, Wang J, Xia C, Zhang H,
Xiong Y, He J, Liu J, Liu B, Pan S and Liu F: TGF-β1-activated
cancer-associated fibroblasts promote breast cancer invasion,
metastasis and epithelial-mesenchymal transition by autophagy or
overexpression of FAP-α. Biochem Pharmacol. 188:1145272021.
View Article : Google Scholar
|
|
115
|
Roane BM, Arend RC and Birrer MJ: Review:
Targeting the transforming growth factor-beta pathway in ovarian
cancer. Cancers (Basel). 11:6682019. View Article : Google Scholar
|
|
116
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xu S, Yang S, Sun G, Huang W and Zhang Y:
Transforming growth factor-beta polymorphisms and serum level in
the development of osteosarcoma. DNA Cell Biol. 33:802–806. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bu MT, Chandrasekhar P, Ding L and Hugo W:
The roles of TGF-β and VEGF pathways in the suppression of
antitumor immunity in melanoma and other solid tumors. Pharmacol
Ther. 240:1082112022.Epub ahead of print. View Article : Google Scholar
|
|
119
|
Zheng C, Zhou XW and Wang JZ: The dual
roles of cytokines in Alzheimer's disease: Update on interleukins,
TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 5:72016. View Article : Google Scholar
|
|
120
|
Park JK, Lee KJ, Kim JY and Kim H: The
association of blood-based inflammatory factors IL-1β, TGF-β and
CRP with cognitive function in Alzheimer's disease and mild
cognitive impairment. Psychiatry Investig. 18:11–18. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
van der Kraan PM: The changing role of
TGFβ in healthy, ageing and osteoarthritic joints. Nat Rev
Rheumatol. 13:155–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kuźnik-Trocha K, Winsz-Szczotka K,
Komosińska-Vassev K, Jura-Półtorak A, Kotulska-Kucharz A, Kucharz
EJ, Kotyla P and Olczyk K: Plasma glycosaminoglycan profiles in
systemic sclerosis: Associations with MMP-3, MMP-10, TIMP-1,
TIMP-2, and TGF-beta. Biomed Res Int. 2020:64165142020. View Article : Google Scholar
|
|
123
|
Wu WS, Ling CH, Lee MC, Cheng CC, Chen RF,
Lin CF, You RI and Chen YC: Targeting Src-Hic-5 signal cascade for
preventing migration of cholangiocarcinoma cell HuCCT1.
Biomedicines. 10:10222022. View Article : Google Scholar : PubMed/NCBI
|