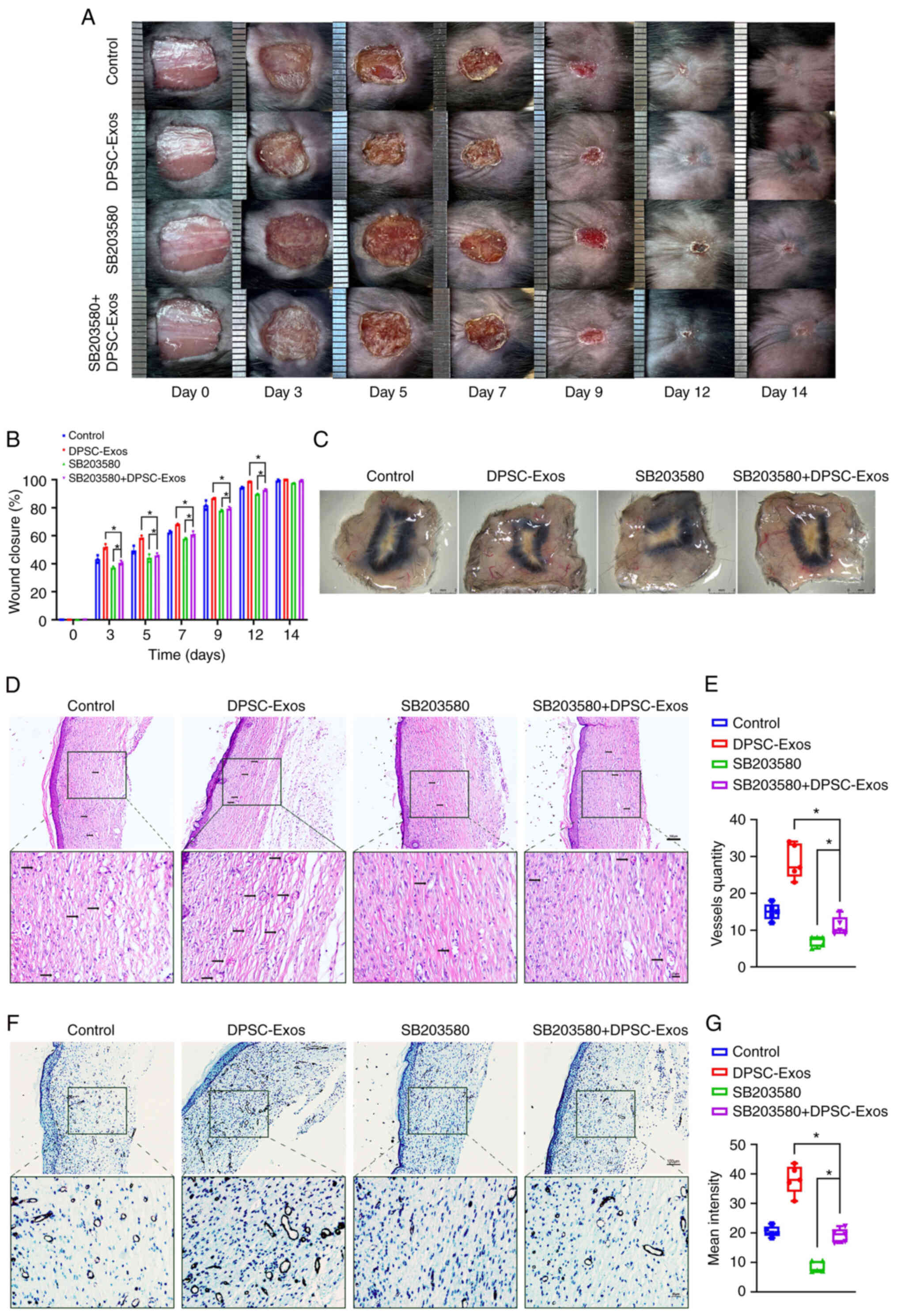
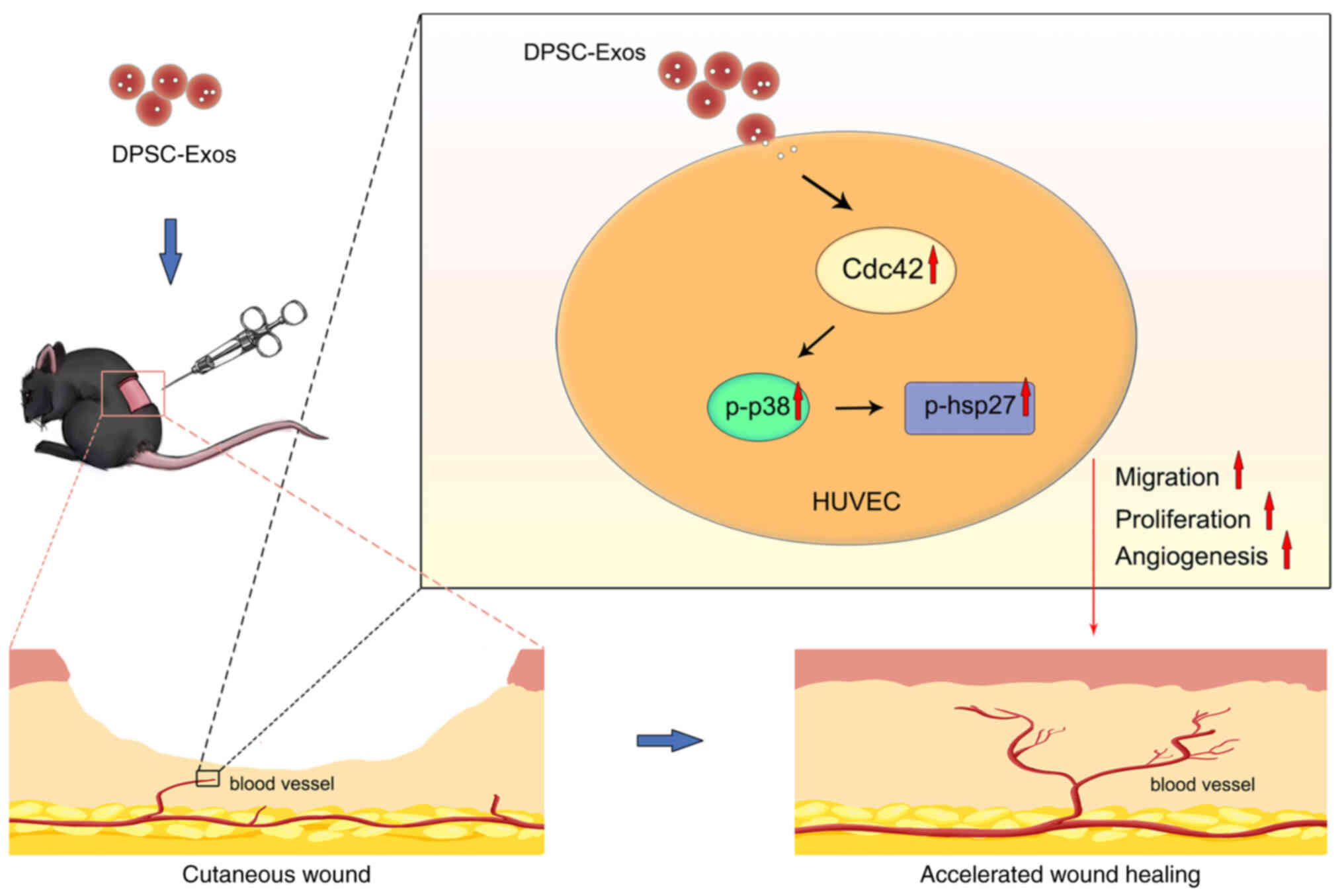
|
1
|
Strecker-McGraw MK, Jones TR and Baer DG:
Soft tissue wounds and principles of healing. Emerg Med Clin North
Am. 25:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues M, Kosaric N, Bonham CA and
Gurtner GC: Wound healing: A cellular perspective. Physiol Rev.
99:665–706. 2019. View Article : Google Scholar :
|
|
3
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Zhang YP and Kirsner RS:
Angiogenesis in wound repair: Angiogenic growth factors and the
extracellular matrix. Microsc Res Tech. 60:107–114. 2003.
View Article : Google Scholar
|
|
5
|
Ding J, Wang X, Chen B, Zhang J and Xu J:
Exosomes derived from human bone marrow mesenchymal stem cells
stimulated by deferoxamine accelerate cutaneous wound healing by
promoting angiogenesis. Biomed Res Int. 2019:97427652019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Xie X, Lian W, Shi R, Han S, Zhang
H, Lu L and Li M: Exosomes from adipose-derived stem cells
overexpressing Nrf2 accelerate cutaneous wound healing by promoting
vascularization in a diabetic foot ulcer rat model. Exp Mol Med.
50:1–14. 2018.
|
|
7
|
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo
J, Li HM, Zhang WS, Chen CY and Xie H: Exosomes from human
umbilical cord blood accelerate cutaneous wound healing through
miR-21-3p-mediated promotion of angiogenesis and fibroblast
function. Theranostics. 8:169–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang B, Chen Y and Shi J: Exosome
biochemistry and advanced nanotechnology for next-generation
theranostic platforms. Adv Mater. 31:e18028962019. View Article : Google Scholar
|
|
10
|
Gonzalez-King H, García NA, Ontoria-Oviedo
I, Ciria M, Montero JA and Sepúlveda P: Hypoxia inducible factor-1α
potentiates jagged 1-mediated angiogenesis by mesenchymal stem
cell-derived exosomes. Stem Cells. 35:1747–1759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Yan Z, Yang F, Huang Y, Yu Y, Zhou
L, Sun Z, Cui D and Yan Y: Exosomes derived from human umbilical
cord mesenchymal stem cells accelerate cutaneous wound healing by
enhancing angiogenesis through delivering angiopoietin-2. Stem Cell
Rev Rep. 17:305–317. 2021. View Article : Google Scholar
|
|
12
|
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu
Y, Luo J, Liu YW, Yin H, Huang J, et al: Exosomal DMBT1 from human
urine-derived stem cells facilitates diabetic wound repair by
promoting angiogenesis. Theranostics. 8:1607–1623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsutsui TW: Dental pulp stem cells:
Advances to applications. Stem Cells Cloning. 13:33–42.
2020.PubMed/NCBI
|
|
14
|
Yoon JK, Kang ML, Park JH, Lee KM, Shin
YM, Lee JW, Kim HO and Sung HJ: Direct control of stem cell
behavior using biomaterials and genetic factors. Stem Cells Int.
2018:86429892018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BC, Bae H, Kwon IK, Lee EJ, Park JH,
Khademhosseini A and Hwang YS: Osteoblastic/cementoblastic and
neural differentiation of dental stem cells and their applications
to tissue engineering and regenerative medicine. Tissue Eng Part B
Rev. 18:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Botelho J, Cavacas MA, Machado V and
Mendes JJ: Dental stem cells: Recent progresses in tissue
engineering and regenerative medicine. Ann Med. 49:644–651. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martínez-Sarrà E, Montori S, Gil-Recio C,
Núñez-Toldrà R, Costamagna D, Rotini A, Atari M, Luttun A and
Sampaolesi M: Human dental pulp pluripotent-like stem cells promote
wound healing and muscle regeneration. Stem Cell Res Ther.
8:1752017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mead B, Logan A, Berry M, Leadbeater W and
Scheven BA: Concise review: Dental pulp stem cells: A novel cell
therapy for retinal and central nervous system repair. Stem Cells.
35:61–67. 2017. View Article : Google Scholar
|
|
19
|
Huang CC, Narayanan R, Alapati S and
Ravindran S: Exosomes as biomimetic tools for stem cell
differentiation: Applications in dental pulp tissue regeneration.
Biomaterials. 111:103–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jarmalavičiūtė A, Tunaitis V, Pivoraitė U,
Venalis A and Pivoriūnas A: Exosomes from dental pulp stem cells
rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced
apoptosis. Cytotherapy. 17:932–939. 2015. View Article : Google Scholar
|
|
21
|
Swanson WB, Zhang Z, Xiu K, Gong T, Eberle
M, Wang Z and Ma PX: Scaffolds with controlled release of
pro-mineralization exosomes to promote craniofacial bone healing
without cell transplantation. Acta Biomater. 118:215–232. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Zhong Y, Kong Y, Chen Y, Feng J and
Zheng J: Lineage-specific exosomes promote the odontogenic
differentiation of human dental pulp stem cells (DPSCs) through
TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell
Res Ther. 10:1702019. View Article : Google Scholar
|
|
23
|
Izar B, Tirosh I, Stover EH, Wakiro I,
Cuoco MS, Alter I, Rodman C, Leeson R, Su MJ, Shah P, et al: A
single-cell landscape of high-grade serous ovarian cancer. Nat Med.
26:1271–1279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rochereau N, Roblin X, Michaud E, Gayet R,
Chanut B, Jospin F, Corthésy B and Paul S: NOD2 deficiency
increases retrograde transport of secretory IgA complexes in
Crohn's disease. Nat Commun. 12:2612021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fornabaio G, Barnhill RL, Lugassy C,
Bentolila LA, Cassoux N, Roman-Roman S, Alsafadi S and Bene FD:
Angiotropism and extravascular migratory metastasis in cutaneous
and uveal melanoma progression in a zebrafish model. Sci Rep.
8:104482018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa
C, Remold-O'Donnell E, Zhao BQ, Cifuni SM and Wagner DD:
Inflammation induces hemorrhage in thrombocytopenia. Blood.
111:4958–4964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marcinkiewicz AL, Lieknina I, Kotelovica
S, Yang X, Kraiczy P, Pal U, Lin YP and Tars K: Eliminating factor
H-Binding activity of borrelia burgdorferi CspZ combined with
virus-like particle conjugation enhances its efficacy as a lyme
disease vaccine. Front Immunol. 9:1812018. View Article : Google Scholar :
|
|
28
|
Albelda SM, Muller WA, Buck CA and Newman
PJ: Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A
novel vascular cell-cell adhesion molecule. J Cell Biol.
114:1059–1068. 2018. View Article : Google Scholar
|
|
29
|
Ju Lee H, Bartsch D, Xiao C, Guerrero S,
Ahuja G, Schindler C, Moresco JJ, Yates JR III, Gebauer F, Bazzi H,
et al: A post-transcriptional program coordinated by CSDE1 prevents
intrinsic neural differentiation of human embryonic stem cells. Nat
Commun. 8:14562017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng H, Castillo-Cabrera J, Manser M, Lu
B, Yang Z, Strande V, Begue D, Zamponi R, Qiu S, Sigoillot F, et
al: Genome-wide CRISPR screening reveals genetic modifiers of
mutant EGFR dependence in human NSCLC. Elife. 19:e502232019.
View Article : Google Scholar
|
|
31
|
Kumar S, Jiang MS, Adams JL and Lee JC:
Pyridinylimidazole compound SB 203580 inhibits the activity but not
the activation of p38 mitogen-activated protein kinase. Biochem
Biophys Res Commun. 263:825–831. 2017. View Article : Google Scholar
|
|
32
|
Tu GW, Ju MJ, Zheng YJ, Hao GW, Ma GG, Hou
JY, Zhang XP, Luo Z and Lu LM: CXCL16/CXCR6 is involved in
LPS-induced acute lung injury via P38 signalling. J Cell Mol Med.
23:5380–5389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu
Q, Li J, Huang H, Cai T, Ji H, et al: MAPK-Mediated YAP activation
controls mechanical-tension-induced pulmonary alveolar
regeneration. Cell Rep. 16:1810–1819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koch S and Claesson-Welsh L: Signal
transduction by vascular endothelial growth factor receptors. Cold
Spring Harb Perspect Med. 2:a0065022012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurogane Y, Miyata M, Kubo Y, Nagamatsu Y,
Kundu RK, Uemura A, Ishida T, Quertermous T, Hirata KI and Rikitake
Y: FGD5 mediates proangiogenic action of vascular endothelial
growth factor in human vascular endothelial cells. Arterioscler
Thromb Vasc Biol. 32:988–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rousseau S, Houle F, Landry J and Huot J:
p38 MAP kinase activation by vascular endothelial growth factor
mediates actin reorganization and cell migration in human
endothelial cells. Oncogene. 15:2169–2177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Armstrong SC, Delacey M and Ganote CE:
Phosphorylation state of hsp27 and p38 MAPK during preconditioning
and protein phosphatase inhibitor protection of rabbit
cardiomyocytes. J Mol Cell Cardiol. 31:555–567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans IM, Britton G and Zachary IC:
Vascular endothelial growth factor induces heat shock protein (HSP)
27 serine 82 phosphorylation and endothelial tubulogenesis via
protein kinase D and independent of p38 kinase. Cell Signal.
20:1375–1384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou T, Yang Z, Chen Y, Chen Y, Huang Z,
You B, Peng Y and Chen J: Estrogen accelerates cutaneous wound
healing by promoting proliferation of epidermal keratinocytes via
Erk/Akt signaling pathway. Cell Physiol Biochem. 38:959–968. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie L, Guan Z, Zhang M, Lyu S, Thuaksuban
N, Kamolmattayakul S and Nuntanaranont T: Exosomal circLPAR1
promoted osteogenic differentiation of homotypic dental pulp stem
cells by competitively binding to hsa-miR-31. Biomed Res Int.
2020:63193952020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Venugopal CKS, Rai KS, Pinnelli VB, Kutty
BM and Dhanushkodi A: Neuroprotection by human dental pulp
mesenchymal stem cells: From billions to nano. Curr Gene Ther.
18:307–323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pivoraitė U, Jarmalavičiūtė A, Tunaitis V,
Ramanauskaitė G, Vaitkuvienė A, Kašėta V, Biziulevičienė G, Venalis
A and Pivoriūnas A: Exosomes from human dental pulp stem cells
suppress carrageenan-induced acute inflammation in mice.
Inflammation. 38:1933–1941. 2015. View Article : Google Scholar
|
|
43
|
Han Y, Ren J, Bai Y, Pei X and Han Y:
Exosomes from hypoxia-treated human adipose-derived mesenchymal
stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem
Cell Biol. 109:59–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bakhtyar N, Jeschke MG, Herer E,
Sheikholeslam M and Amini-Nik S: Exosomes from acellular Wharton's
jelly of the human umbilical cord promotes skin wound healing. Stem
Cell Res Ther. 9:1932018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tonnesen MG, Feng X and Clark RA:
Angiogenesis in wound healing. J Investig Dermatol Symp Proc.
5:40–46. 2000. View Article : Google Scholar
|
|
46
|
Ribeiro MF, Zhu H, Millard RW and Fan GC:
Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes.
2:54–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu P, Zhang B, Shi H, Qian H and Xu W:
MSC-exosome: A novel cell-free therapy for cutaneous regeneration.
Cytotherapy. 20:291–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu M, Liu W, Li J, Lu J, Lu H, Jia W and
Liu F: Exosomes derived from atorvastatin-pretreated MSC accelerate
diabetic wound repair by enhancing angiogenesis via AKT/eNOS
pathway. Stem Cell Res Ther. 11:3502020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Uppal H, Demaria M, Desprez PY,
Campisi J and Kapahi P: Simvastatin suppresses breast cancer cell
proliferation induced by senescent cells. Sci Rep. 5:178952015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu J, He X, Corbett SA, Lowry SF, Graham
AM, Fässler R and Li S: Integrins are required for the
differentiation of visceral endoderm. J Cell Sci. 122:233–242.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lamalice L, Houle F, Jourdan G and Huot J:
Phosphorylation of tyrosine 1214 on VEGFR2 is required for
VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene.
23:434–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lamalice L, Houle F and Huot J:
Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment
of Nck and activation of Fyn leading to SAPK2/p38 activation and
endothelial cell migration in response to VEGF. J Biol Chem.
281:34009–34020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feng PC, Ke XF, Kuang HL, Pan LL, Ye Q and
Wu JB: BMP2 secretion from hepatocellular carcinoma cell HepG2
enhances angiogenesis and tumor growth in endothelial cells via
activation of the MAPK/p38 signaling pathway. Stem Cell Res Ther.
10:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Correa AR, Berbel AC, Papa MP, Morais AT,
Peçanha LM and Arruda LB: Dengue virus directly stimulates
polyclonal B cell activation. PLoS One. 10:e01433912015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Morrison VL, James MJ, Grzes K, Cook P,
Glass DG, Savinko T, Lek HS, Gawden-Bone C, Watts C, Millington OR,
et al: Loss of beta2-integrin-mediated cytoskeletal linkage
reprogrammes dendritic cells to a mature migratory phenotype. Nat
Commun. 5:53592014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maji S, Chaudhary P, Akopova I, Nguyen PM,
Hare RJ, Gryczynski I and Vishwanatha JK: Exosomal annexin II
promotes angiogenesis and breast cancer metastasis. Mol Cancer Res.
15:93–105. 2017. View Article : Google Scholar
|
|
57
|
Liang B, Liang JM, Ding JN, Xu J, Xu JG
and Chai YM: Dimethyloxaloylglycine-stimulated human bone marrow
mesenchymal stem cell-derived exosomes enhance bone regeneration
through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell
Res Ther. 10:3352019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou X, Yan T, Huang C, Xu Z, Wang L,
Jiang E, Wang H, Chen Y, Liu K, Shao Z and Shang Z: Melanoma
cell-secreted exosomal miR-155-5p induce proangiogenic switch of
cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling
pathway. J Exp Clin Cancer Res. 37:2422018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xue C, Shen Y, Li X, Li B, Zhao S, Gu J,
Chen Y, Ma B, Wei J, Han Q and Zhao RC: Exosomes derived from
hypoxia-treated human adipose mesenchymal stem cells enhance
angiogenesis through the PKA signaling pathway. Stem Cells Dev.
27:456–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park S, Guo Y, Negre J, Preto J, Smithers
CC, Azad AK, Overduin M, Murray AG and Eitzen G: Fgd5 is a
rac1-specific Rho GEF that is selectively inhibited by
aurintricarboxylic acid. Small GTPases. 12:147–160. 2021.
View Article : Google Scholar :
|
|
61
|
Heldin J, O'Callagha n P, Vera RH, Fuchs
PF, Gerwins P and Kreuger J: FGD5 sustains vascular endothelial
growth factor A (VEGFA) signaling through inhibition of
proteasome-mediated VEGF receptor 2 degradation. Cell Signal.
40:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|