Introduction
Cervical cancer is the fourth most common cancer in
the world among women (1).
Although the etiology of cervical cancer has not been fully
clarified, human papillomavirus has been identified as the main
causative agent of cervical cancer (2). However, smoking can also promote the
occurrence and development of cervical cancer because tobacco can
promote the epithelial-mesenchymal transition of cervical
epithelial cells (3). The cause
of the majority of the mortality cases from cervical cancer is
cancer metastasis (1-3). At present, surgery, radiotherapy and
chemotherapy remain to be the main treatment approaches for
cervical cancer and immunotherapy is also used (4,5).
However, these existing therapeutic approaches have limitations.
Radiotherapy and chemotherapy can cause serious side effects, such
as leucopenia and hemolysis (6).
Therefore, it is important to develop more effective and safer
therapeutics for the treatment of cervical cancer.
Previous studies have demonstrated that traditional
Chinese medicine may be a promising approach, since they appear to
have fewer side effects compared to chemically synthesized drugs
for the treatment of tumors (7-10).
Lycorine is an alkaloid that can be found in Lycoris bulbs and is
mainly used as an emetic in the clinic (7). Furthermore, lycorine has been
reported to inhibit the proliferation and migration of a variety of
tumors, such as colorectal cancer, breast cancer, gastric cancer
and liver cancer (8-11). It has also been previously
demonstrated that lycorine can inhibit the initiation and
progression of cervical cancer. However, its underlying mechanism
has not been determined (12-14).
In the present study, the effect of lycorine on
human cervical cancer cells was investigated and its possible
underlying mechanism of action was explored. It is hoped that the
present study will provide a novel approach for the treatment of
cervical cancer and reduce the disease burden of patients with
cervical cancer.
Materials and methods
Cell culture
HeLa cells were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone;
Cytiva) containing 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin. The cells
were cultured in a 1% humidified atmosphere of 5% CO2
and 95% air at 37°C.
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis
(GEPIA; http://gepia.cancer-pku.cn/) was used
to analyze the data from The Cancer Genome Atlas (TCGA; https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
to determine the mRNA expression levels and the effects of RBM10 on
the survival rates of patients with cervical cancer. The expression
of RBM10 in numerous types of cancer was analyzed using The Human
Protein Atlas (https://www.proteinatlas.org/).
Antibodies
Rabbit monoclonal phosphorylated (p)-Akt and rabbit
monoclonal Akt antibodies were purchased from Affinity Biosciences
(both 1:1,000; cat. no. af0016 and af0836, respectively). Rabbit
monoclonal E-cadherin and N-cadherin antibodies were purchased from
Boster Biological Technology (both 1:500; cat. no. BA0415 and
BA0673, respectively). Rabbit monoclonal cyclin D1 and polyclonal
β-catenin antibodies were purchased from Wuhan Servicebio
Technology Co., Ltd. (all 1:1,000; cat. no. gb111372 and gb11015,
respectively). A goat anti-rabbit IgG HRP-binding secondary
antibody was purchased from Wuhan Servicebio Technology Co., Ltd.
(1:1,000; cat. no. gb23303). Rabbit anti-GAPDH primary antibody was
obtained from Wuhan Servicebio Technology Co., Ltd. (1:500; cat.
no. gb11002).
Small interfering (si)RNA
transfection
The siRNAs for RBM10 (siRNA-RBM10-1 and
siRNA-RBM10-2) or the scrambled siRNA negative control (NC;
siRNA-NC) were synthesized via Guangzhou RiboBio Co., Ltd. siRNA
(20 nM) transfection into HeLa cells was performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 20 min. Cells were
harvested at 48 h after transfection for the subsequent
experiments. The siRNA sequences used are presented in Table SI.
Colony formation assay and crystal violet
staining
HeLa cells were seeded in 6-well dishes at a density
of 5×102 cells/dish and cultured at 37°C in DMEM with or
without 5 µg lycorine (cat. no. HY-N0288; MedChemExpress)
for 1 week. Subsequently, the cells were washed with PBS, fixed
with 4% paraformaldehyde for 30 min at room temperature and stained
with 0.1% crystal violet for 15 min at room temperature. The number
of colonies (>50 cells) formed was then quantified manually. For
quantitative crystal violet staining, the absorbance of each well
was measured at 595 nm using a multifunctional enzyme microplate
reader (iMark; Bio-Rad Laboratories, Inc.).
Wound healing assay
Cells from different treatment groups, with or
without 10 µM lycorine (5×105 cells/well) were
seeded into a six-well plate and DMEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS, respectively, allowed
to adhere to the bottom of the well. Cells were cultured to 80%
confluency at 37°C. Subsequently, a scratch wound was made using a
200 µl pipette tip and the wells were washed twice with PBS
to remove all floating cells. The cells in each well were then
exposed to serum-free DMEM/F12 with 3 µg lycorine for 24 and
48 h at 37°C. In the NC group, the same amount of PBS was added as
the control. Images were captured using an optical light microscope
(magnification, ×200). The distance of the scratch was measured
using ImageJ (version 1.53e, National Institutes of Health) at 0,
24 and 48 h following incubation and relative invasion distance was
compared with the NC group (without lycorine group). The
invasiveness is calculated by the following formula: Healing area
ratio=healing area/original area. Healing area was represented by
the difference between the initial scratch area and the area at the
indicated time points; whereas the original area was defined by
that originally made using the 200-µl pipette tip).
Transwell invasion assay
HeLa cells (2×104 cells/0.4 ml) were
seeded into Transwell inserts (pore size 8 µm) containing
Matrigel (Corning, Inc.) and the upper chamber was filled with DMEM
(containing 1% FBS) with 10 µM lycorine or with 10 µM
PBS. DMEM containing 10% FBS was placed in the lower chamber. The
cells were incubated at 37°C for 48 h. Subsequently, the cells were
fixed with 4% formaldehyde for 15 min at 37°C and stained with 0.1%
crystal violet in 0.01 M PBS for 15 min at room temperature. The
number of cells penetrating the membrane was quantified according
to a previously reported method (15). Images were captured using an
optical light microscope (magnification, ×200). The images of HeLa
cells that invaded through the Transwell membrane following
different treatments were analyzed using Image-Pro Plus 6.0
software (Media Cyberkinetics, Inc.).
Cytotoxicity assay
The Cell Counting Kit-8 (CCK-8; Thermo Fisher
Scientific, Inc.) assay was used to assess the cytotoxicity of HeLa
cells. In brief, cells were seeded into 96-well plates at a density
of 3×103 cells/well. In total, 10 µl CCK-8
reagent and lycorine (0, 2 or 4 µg) were then added to each
well and the cells were cultured for 12, 24 and 48 h at 37°C. In
the gene knockdown experiment, 5 µg lycorine was added into
each well and observed at 0, 24, 48 and 72 h. Cell cytotoxicity was
determined by assessing the optical density of each well at a
wavelength of 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.).
Flow cytometry analysis
HeLa cells (1×106 cells/well) were
pre-seeded into 10 cm plates. The HeLa cells were treated with 5
µg lycorine for 48 h at 37°C, according to the
manufacturer's instructions. The HeLa cells were then collected and
centrifuged at 140 × g for 4 min at room temperature. Annexin
V-FITC Apoptosis Detection Kit (MilliporeSigma) was used to
determine cell apoptosis at 37°C, according to the manufacturer's
protocols. Cells were fixed with cold 70% ethanol for 1 h before
being centrifuged (1,000 × g, 37°C and 5 min) and washed twice
using cold PBS. A total of 1×106 cells were then
incubated with Annexin V (1X) and propidium iodide (1X) for 30 min
in the dark at 37°C. Samples were analyzed using a Beckman MoFlo
Astrios EQs Flow Cytometer (Beckman Coulter, Inc.). Data were
analyzed using FlowJo V10 software (FlowJo LLC).
ELISA
ELISA was performed using a Human TNF-α Single Step
ELISA Kit (cat. no. ab181421; Abcam) according to the
manufacturer's instructions. The cell culture supernatant was
extracted and passed through a 0.45 µM filtering device
(MilliporeSigma). The 450 nm absorbance of the sample was assessed
using an ELISA reader and the standard curve.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted using the EZ-press RNA
Purification kit (cat. no. B0004DP; EZBioscience). A total of 1
µg RNA was used for complementary (c)DNA synthesis using the
First Strand cDNA Synthesis kit ReverTra Ace™ qPCR RT Master Mix
(cat. no. FSQ-201; Toyobo Life Science) at 37°C for 10 min and 50°C
for 5 min. The reaction was terminated by incubating the samples at
95°C for 3 min. qPCR was performed using the MonAmp SYBR Green qPCR
Mix (cat. no. RN04006M; Monad Biotech Co., Ltd.) with a RT
fluorescence quantitative PCR system (Light Cycler 96 SW 1.1; Roche
Diagnostics) according to the manufacturer's protocols. β-actin was
used as the internal reference gene. The PCR system included, 5
µl PCR mix, 0.2 µl upstream primer, 0.2 µl
downstream primer, 2.6 µl RNase-free double-distilled
H2O and 2 µl cDNA template. qPCR was performed
for the initial activation at 95°C for 20 sec, followed by 40
cycles at 95°C for 10 sec, 63°C for 30 sec, and 70°C for 30 sec.
mRNA expression levels were analyzed using the 2−ΔΔCq
method and were normalized using the ACTB gene (16). The primer sequences used are
presented in Table SII.
Xenograft mouse model of cervical
cancer
All animal experimental procedures were performed in
accordance with the guidelines provided by the National Institutes
of Health Guide for the Care and Use of Laboratory Animals
(17). The present study was
approved by the Ethics Committee of The School of Stomatology of
Shandong University. BALB/C nude mice (age, 4 weeks; weight, 30 g)
were used in the present study and were purchased from Beijing
Huafukang Biotechnology Co., Ltd. The mice were housed in
accordance with animal welfare regulations (17), under specific-pathogen-free
conditions at 25°C, 50% humidity and a 12-h light/dark cycle. The
animals also had free access to food and water. In total, 20 female
nude mice were randomly divided into the experimental group and
control group. HeLa cells (1×106 cells/100 µl
PBS) were injected into the groin of nude mice. At 1, 2 and 3
weeks, 100 µl lycorine or PBS was injected into the tail
vein of the nude mice once a week. The health and behavior of the
animals were monitored every 2 days. Tumor mass and volume (V) were
checked. The tumor was almost spherical with a radius of L and the
following formula was used: V=4/3πL3. The tumor diameter
was assessed every 5 days (on day 1, 6, 11, 16 and 21) and the
tumor volume was determined according to the formula. Subsequently,
21 days following cell injection, the mice were sacrificed via
cervical dislocation. The humane endpoints were as follows: A
marked reduction in food or water intake, labored breathing, the
inability to stand and no response to external stimuli. No abnormal
signs that signified the humane endpoints of the experiment were
observed in any of the mice during the experiment. When it was
confirmed that the experimental animals had no heartbeat or
breathing, the tumors were isolated and weighed (18).
Western blotting
Cells were collected and total protein was extracted
using RIPA lysis buffer (Beyotime Institute of Biotechnology).
Protein concentration was determined using the BCA method. Total
protein (10 µg/lane) was separated on a 10% gel using
SDS-PAGE and the separated proteins were transferred to a PVDF
membrane (MilliporeSigma). After blocking with 5% fat-free milk
powder in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h at
room temperature, the membrane was incubated with primary antibody
at 37°C for 45 min. After washing with TBST, the membrane was
sealed with 5% fat-free milk powder overnight at 4°C and incubated
with HRP-bound secondary antibody (1:1,000) in the dark for 1 h at
25°C. Finally, the protein bands were visualized using the Common
ECL chemiluminescence detection kit (cat. no. PK10001; ProteinTech
Group, Inc.) and were analyzed using Gel Pro Analyzer 4.0 software
(Media Cybernetics, Inc.). The Gel Pro Analyzer 4.0 software was
used to quantify the western blotting bands, assess the gray values
of the different bands, list the gray values obtained and determine
the protein expression levels using histograms (19).
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 8.0 software (GraphPad Software, Inc.). Two-tailed
unpaired Student's t-tests were used to analyze two groups. One-way
ANOVA was used to analyze multiple groups. The Tukey's post-hoc
test was used following ANOVA. All experiments are repeated at
least three times unless otherwise stated. All data are presented
as the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lycorine inhibits the proliferation and
migration of cervical cancer cells
To assess the effect of lycorine on the initiation
and progression of cervical cancer, the subsequent experiments were
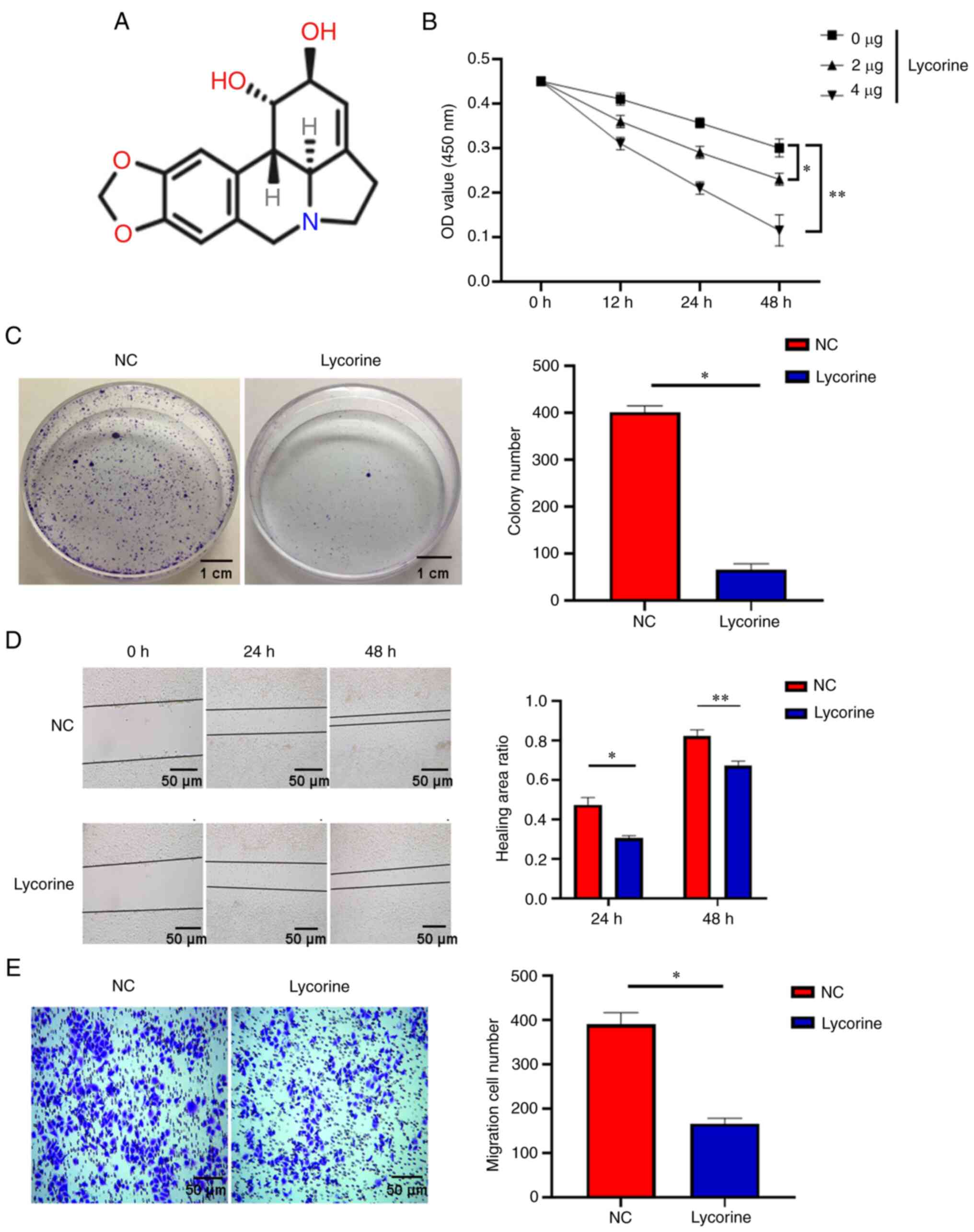
performed. The results of the CCK-8 assay demonstrated that
compared with NC group, lycorine (Fig. 1A) significantly inhibited the
proliferation of cervical cancer cells and this inhibition
significantly intensified in a dose- and time-dependent manner
(Fig. 1B). The subsequent plate
cloning experiments confirmed these results (Fig. 1C). Furthermore, to verify the
effect of lycorine on the migration of cervical cancer cells, the
wound healing assay was performed. The results demonstrated that at
24 and 48 h, the lycorine treatment significantly inhibited cell
migration compared with the NC. These results therefore indicated
that lycorine can potentially inhibit the migration of cervical
cancer cells (Fig. 1C). Moreover,
in the Transwell experiment, lycorine treatment significantly
reduced the number of cells migrating to the bottom of the insert
after 48 h compared with the NC, which suggested that lycorine can
potentially inhibit the invasion ability of cervical cancer cells
(Fig. 1D). These data therefore
indicated that lycorine can potentially inhibit the proliferation
and migration of cervical cancer cells.
Lycorine upregulates the expression of
RBM10 and promotes apoptosis
RBM10 is involved in a variety of inflammatory
processes and is closely associated with apoptosis (20-22). An imbalance of RBM10 expression is
linked to the initiation and progression of a variety of tumors
(23,24). Therefore, in the present study the
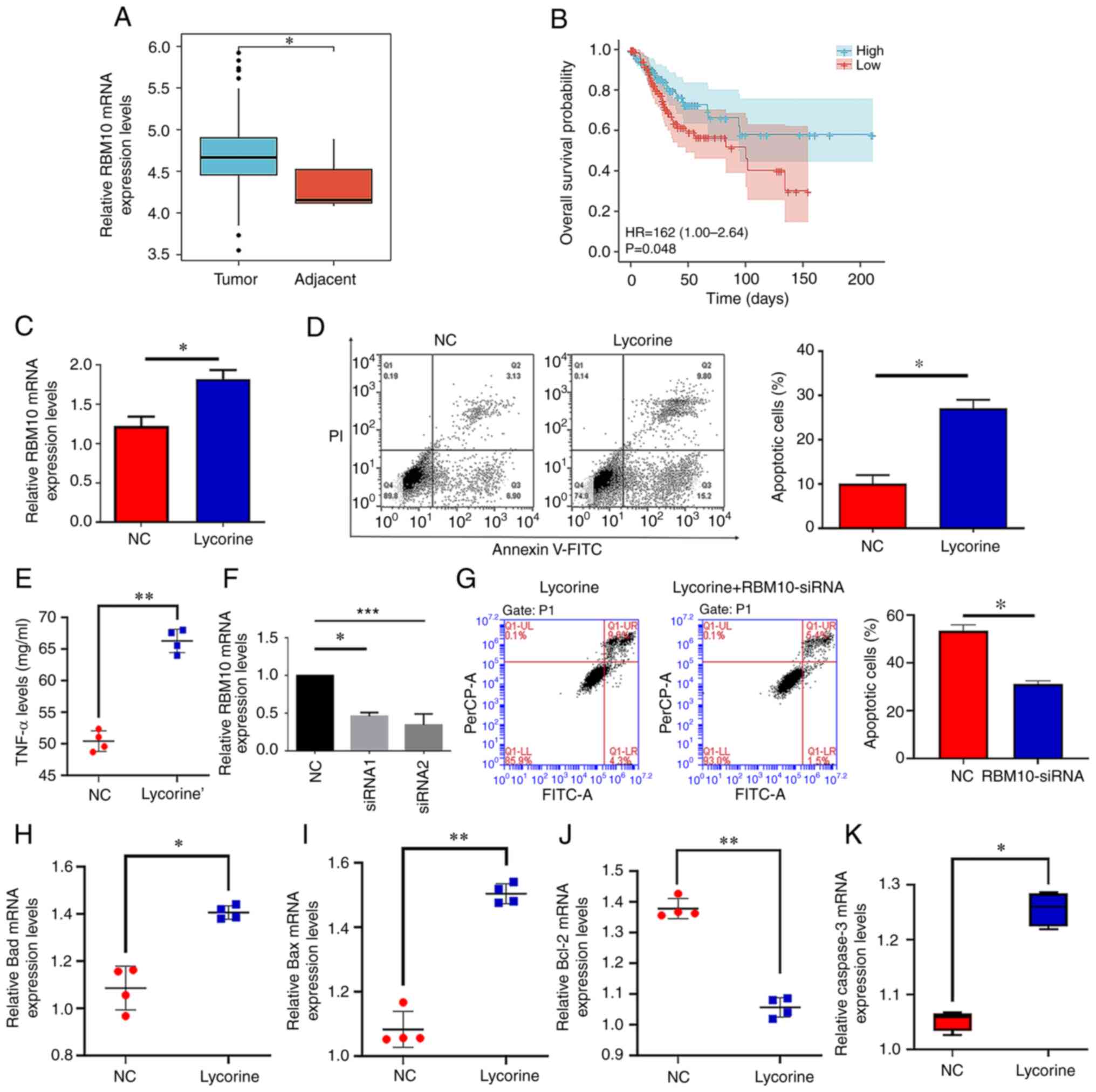
expression of RBM10 in tumor tissues was examined using the TCGA
database. The results demonstrated that the mRNA expression levels
of RBM10 in tumor tissues were significantly increased compared
with adjacent tissues (Fig. 2A).
Subsequently, it was demonstrated that patients with high RBM10
mRNA expression levels had significantly longer survival rates
compared with patients with low RBM10 expression (Fig. 2B). Therefore, low RBM10 expression
levels may be associated with a poor prognosis in patients with
cervical cancer. Furthermore, the mRNA expression levels of RBM10
significantly increased after the addition of lycorine compared
with those in the NC group (Fig.
2C). In the subsequent flow cytometry results, it was
demonstrated that the percentage of apoptotic cells significantly
increased following lycorine treatment compared with the NC
(Fig. 2D). As TNF-α is closely
associated with apoptosis (25),
the level of TNF-α in the two groups was investigated. The results
demonstrated that the expression level of TNF-α significantly
increased following lycorine treatment compared with the NC
(Fig. 2E). Moreover, when the
expression of RBM10 was knocked-down using siRNA, the results
demonstrated that siRNA significantly reduced the mRNA expression
levels of RBM10. (Fig. 2F), the
level of apoptosis was also significantly decreased compared with
the NC (Fig. 2G). Consistent with
the aforementioned results, the results demonstrated that the mRNA
expression levels of the proapoptotic factors Bax and Bad were
significantly increased (Fig. 2H and
I) and the Mrna expression levels of the antiapoptotic factor
Bcl-2 were significantly decreased (Fig. 2J) when cells were treated with
lycorine, compared with the NC. Furthermore, the mRNA expression
level of the apoptotic protein caspase-3 significantly increased
compared with the NC (Fig. 2K).
These data indicated that lycorine may potentially promote the
apoptosis of cervical cancer cells and that this mechanism may be
mediated by the upregulation of RBM10 expression.
Elevated expression of RBM10 reduces the
activation level of the AKT signaling pathway
RBM10 interacts with key proteins in a variety of
Akt signaling pathways (26),
which are involved in the initiation and progression of a variety
of malignancies (27,28). Moreover, the Akt signaling pathway
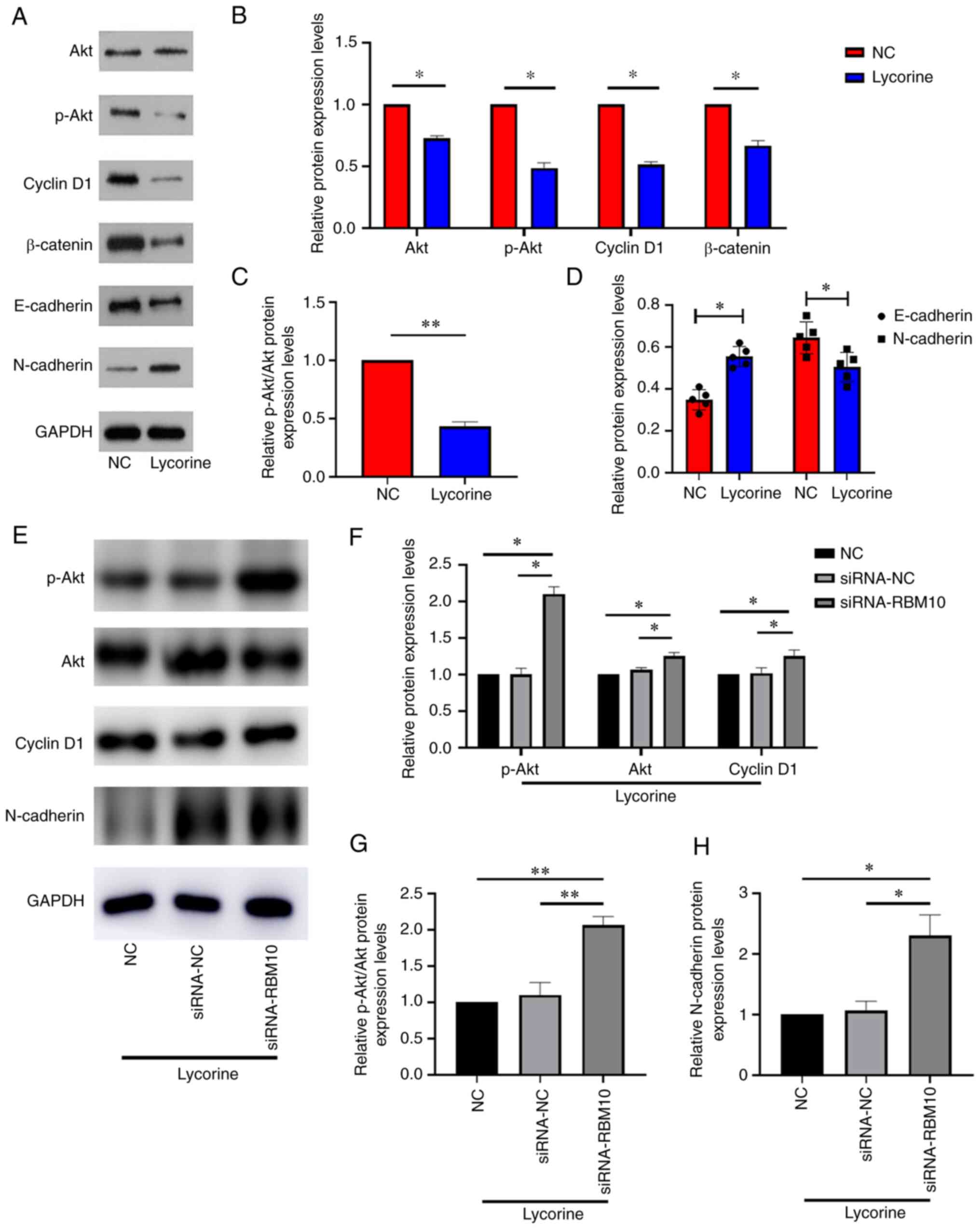
serves a key role in numerous types of tumor (29). As shown in Fig. 2C, lycorine treatment was able to
increase the expression of RBM10. To explore the relationship
between RBM10 and the Akt signaling pathway, western blotting was
performed to assess the activation level of the Akt signaling
pathway when RBM10 expression was increased via lycorine treatment.
The results demonstrated that the increased expression levels of
RBM10 resulted in changes in the protein expression levels of Akt
and p-Akt (Fig. 3A and B). The
ratio of p-Akt/Akt significantly decreased (Fig. 3C) following lycorine treatment
compared with the NC, which indicated that the phosphorylation of
Akt was potentially inhibited. To further test the hypothesis that
lycorine will reduce the activation level of the Akt signaling
pathway, other proteins of the Akt signaling pathway were
investigated, including cyclin D1 and β-catenin. The protein
expression levels of both proteins were significantly downregulated
via lycorine treatment compared with the NC. However, following
lycorine treatment, the expression of E-cadherin, a well-known
epithelial marker, was significantly increased and the expression
of N-cadherin was significantly decreased compared with the NC
(Fig. 3D). These results
therefore suggested that lycorine may potentially reverse the EMT
process via RBM10. To further support this conclusion, siRNA was
used to knockdown the expression of RBM10 and the activation level
of the Akt signaling pathway was assessed (Fig. 3E). It was demonstrated that the
decreased protein expression levels of RBM10 significantly improved
the decreased protein expression levels of Akt, p-Akt, cyclin D1
and β-catenin caused by lycorine compared with the NC and siRNA-NC
(Fig. 3F and G). Similarly,
RBM10-siRNA potentially improved the decreased expression of
E-cadherin (Fig. 3H), which was
opposite to the effect of lycorine. These results indicated that
lycorine may reduce the activation level of the Akt signaling
pathway, which is related to RBM10.
RBM10 knockdown can reduce the
therapeutic effect of lycorine
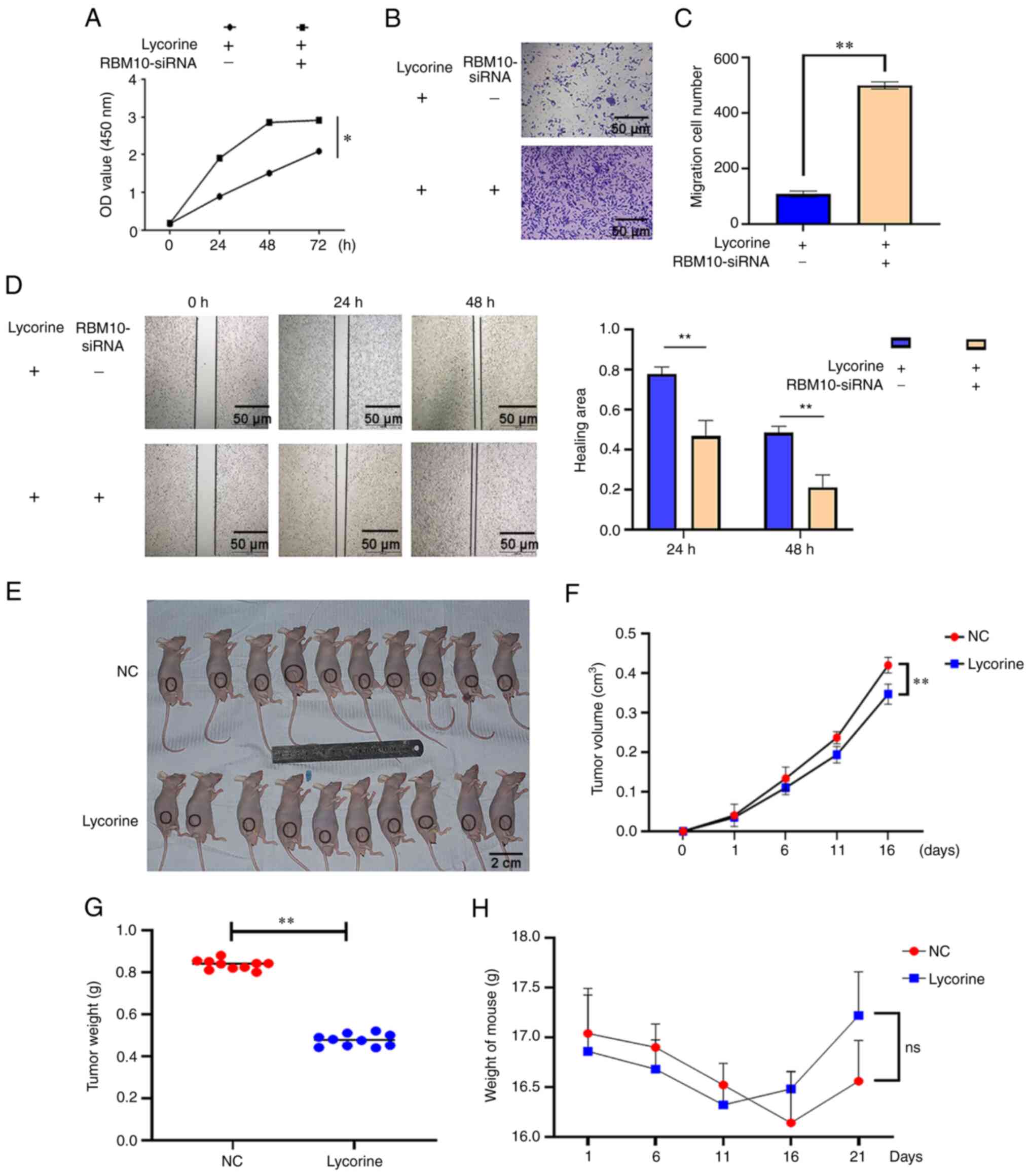
It was previous demonstrated in the present study
that lycorine inhibited the proliferation and migration of cervical
cancer cells and that RBM10 may be mediating this process. To
verify this hypothesis, RBM10 mRNA expression levels were
knocked-down using siRNA targeting RBM10. In the CCK-8, wound
healing and Transwell assays, the results demonstrated that a
reduction in RBM10 mRNA expression levels significantly increased
the proliferation and migration of cervical cancer cells compared
with the lycorine only group (Fig.
4A-D). Combined with the aforementioned experimental results,
these data indicated that lycorine may potentially inhibit the
proliferation and migration of cervical cancer cells, and that this
effect is mediated via apoptosis caused by the increased expression
of RBM10.
Lycorine can inhibit cervical cancer in
vivo
Subsequently, animal experiments were performed
(Fig. 4E). The results
demonstrated that the tumor volume and mass of the lycorine
treatment group were significantly smaller compared with the NC
group (Fig. 4F and G). The
largest tumor diameter observed was 5.31 mm and the largest tumor
weight observed was 0.87 g. To assess the safety of lycorine, the
weight changes of nude mice in the experimental group and the
control group were also assessed at various time points. The
results demonstrated that the weight of mice did not significantly
change regardless of treatment (Fig.
4H), which indicated that lycorine potentially had no obvious
toxic effect on nude mice. These results indicated that lycorine
may potentially inhibit cervical cancer in vivo and this
inhibition will not cause obvious damage to the health of
experimental animals.
Discussion
Cervical cancer is one of the most common malignant
tumors of the female reproductive system, as it is prone to lymph
node and blood-borne metastases (30). Therefore, even with systematic
treatment, the prognosis of patients is still poor (30). Developing more effective treatment
methods is an important issue in the field of cervical cancer
research. In the present study, the results demonstrated that
lycorine, a commonly used emetic, had an inhibitory effect on
cervical cancer cells. The present study reported that lycorine
significantly inhibited the proliferation and migration of human
cervical cancer cells in a time- and dose-dependent manner. The
therapeutic effect of lycorine was verified using in vivo
experiments. Lycorine was demonstrated to significantly reduce the
volume of the tumor in the experimental animals. Therefore, it can
be hypothesized that lycorine may act as a potential therapeutic
for the treatment of cervical cancer. Furthermore, the results
demonstrated that the therapeutic effect of lycorine was
potentially mediated via RBM10. When RBM10 expression was
knocked-down using siRNA, cervical cancer initiation and
progression were restored.
A loss of cell proliferation regulation is
considered to be an important mechanism for the initiation and
progression of malignant tumors, and the induction of apoptosis is
considered to be an effective factor to inhibit the growth of
malignant tumors (31,32). RBM10 is closely related to the
level of apoptosis (33). RBM10
is considered to promote apoptosis via the high expression of TNF-α
(33). In the present study, the
results demonstrated that the expression of RBM10 was significantly
decreased in tumor tissues, which may be related to the poor
prognosis of patients. It was further demonstrated that lycorine
can significantly induce high mRNA expression levels of RBM10 and
that the high expression of RBM10 can potentially significantly
elevate TNF-α levels and promote apoptosis. Bcl-2 family proteins
are key regulators of apoptosis. In the Bcl-2 family, Bcl-2 is an
antiapoptotic factor, whereas Bad and Bax are proapoptotic factors
(34). Moreover, it is well
established that caspase-3 serves a crucial role in apoptosis and
is considered to act as a marker that can indicate the level of
apoptosis (30). In the present
study, the flow cytometry results demonstrated that lycorine
significantly increased the level of apoptosis. To determine the
specific underlying mechanism, the mRNA expression levels of Bcl-2,
Bax, Bad and caspase-3 were assessed. It was demonstrated that
after lycorine treatment, the mRNA expression levels of the
antiapoptotic factor Bcl-2 significantly decreased, whereas the
mRNA expression levels of the proapoptotic factors Bax and Bad
significantly increased. These data indicated that lycorine
potentially promoted the apoptosis of human cervical cancer cells,
which was supported by the subsequent increase in caspase-3 mRNA
expression levels.
The regulation of intracellular signaling is closely
associated with the initiation and progression of malignant tumors
(35). The Akt signaling pathway
is considered to be closely linked to the initiation and
progression of cervical cancer (36). Akt signaling pathway upregulation
promotes the proliferation and migration of cervical cancer cells
(35,36). Furthermore, the loss of epithelial
characteristics is an important feature of tumor progression
(33). In the present study,
lycorine significantly downregulated the Akt signaling pathway.
Moreover, after knocking down RBM10 expression it was demonstrated
that the activation level of the Akt signaling pathway
significantly increased. It can therefore be hypothesized that the
lycorine-induced downregulation of the Akt signaling pathway is
potentially dependent on RBM10. Furthermore, RBM10 potentially
downregulated the Akt signaling pathway and reversed the EMT of
cervical cancer cells, which was demonstrated by the significantly
increased expression of the epithelial marker E-cadherin and the
significantly reduced expression of the mesenchymal marker
N-cadherin.
The present study also had certain limitations. For
example, the experiments were not repeated with other cell lines to
verify the conclusion. In the future, close attention will be given
to the research progress of treatment strategies for cervical
cancer and future research will continue to explore other effective
therapeutic approaches for the treatment of cervical cancer.
Moreover, MMPs are thought to be related to cancer migration
(28) and therefore MMPs should
be investigated in future studies.
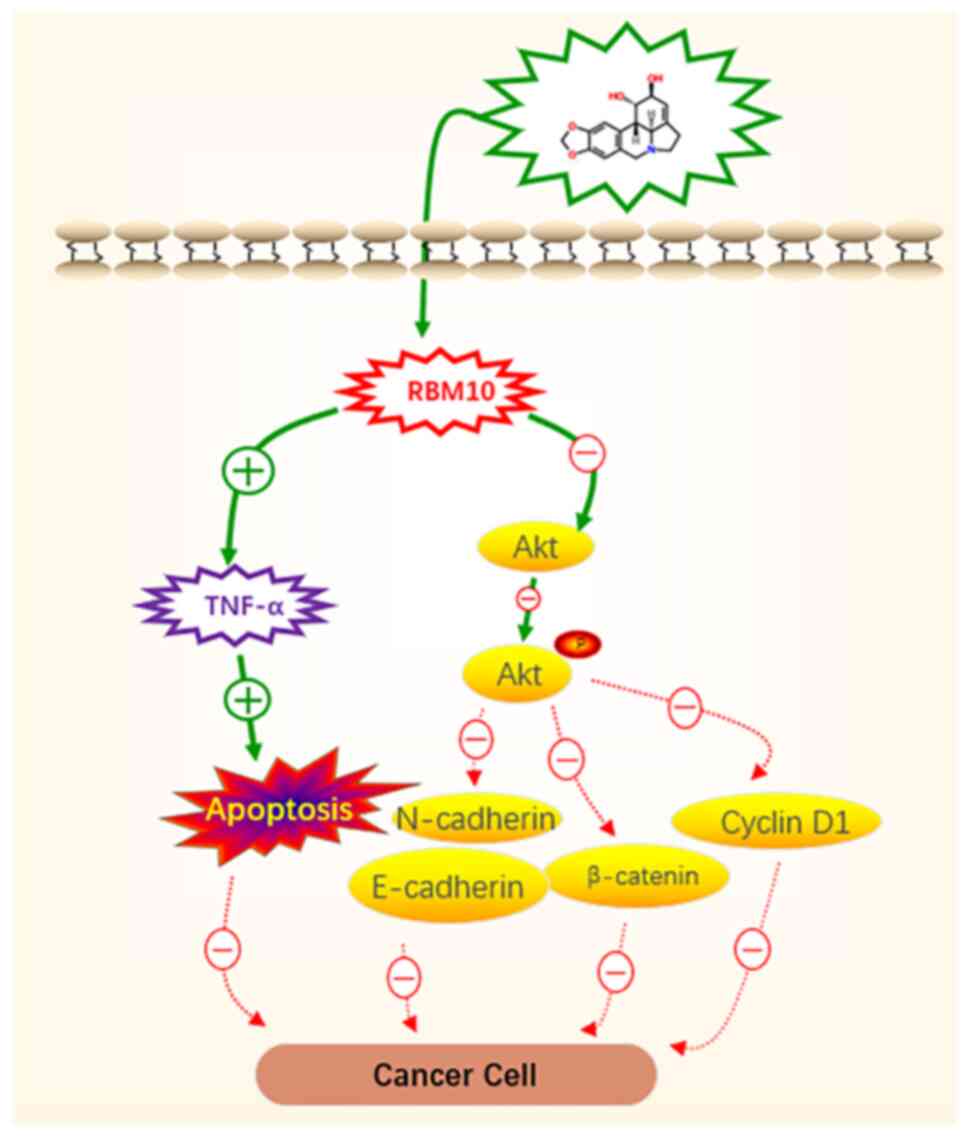
In conclusion, lycorine was demonstrated to
potentially inhibit the proliferation and migration of cervical
cancer cells. The results indicated that this inhibition was
potentially achieved via the induction of high RBM10 mRNA
expression levels and the promotion of apoptosis. Furthermore, the
data indicated that RBM10 potentially induces TNF-α, which is the
cause for the observed increase in apoptosis. Mechanistically, the
results indicated that the promotion of apoptosis and the
inhibition of the initiation and progression of cervical cancer by
RBM10 may potentially be accompanied by the downregulation of the
AKT signaling pathway (Fig. 5).
However, the mechanism of lycorine activity in cervical cancer
still remains to be elucidated. The study of the cell-cell
interaction between lycorine and cervical cancer cells may be of
great significance for the development of novel therapeutic
strategies for cervical cancer, which should be the focus of future
research.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, QZ and GL guided the project, analyzed the data
and wrote the manuscript. YL and XL conceived the technical details
and designed the experiments. ZL, QZ and XF performed the
experiments. QZ, GL and ZL confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Experiments were performed under a project license
(approval no. 20201002) granted by the Institutional Ethics Board
of the Stomatological Hospital of Shandong University (Jinan,
China), in compliance with Chinese national or institutional
guidelines for the care and use of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81402298), the Young Scholars
Program of the Shandong University, the opening project of the
Collaborative Innovation Center for classic and famous
prescriptions of traditional Chinese medicine in Shandong Province
entitled 'Functional mechanism of Chaihu Guizhi Ganjiang Decoction
in the treatment of Sjögren's syndrome' (grant no. 2019KFY10) and
the project 'Research on the functional mechanism of stem cell
exosomes in the treatment of Sjögren's syndrome' of Shandong Jiekai
Biotechnology Co., Ltd. (grant no. 1350022002).
References
|
1
|
Moga MA, Dima L, Balan A, Blidaru A,
Dimienescu OG, Podasca C and Toma S: Are bioactive molecules from
seaweeds a novel and challenging option for the prevention of HPV
infection and cervical cancer therapy?-A review. Int J Mol Sci.
22:6292021. View Article : Google Scholar
|
|
2
|
Davies-Oliveira JC, Smith MA, Grover S,
Canfell K and Crosbie EJ: Eliminating cervical cancer: Progress and
challenges for high-income countries. Clin Oncol (R Coll Radiol).
33:550–559. 2021. View Article : Google Scholar
|
|
3
|
Buskwofie A, David-West G and Clare CA: A
review of cervical cancer: Incidence and disparities. J Natl Med
Assoc. 112:229–232. 2020.PubMed/NCBI
|
|
4
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hill EK: Updates in cervical cancer
treatment. Clin Obstet Gynecol. 63:3–11. 2020. View Article : Google Scholar
|
|
6
|
Liontos M, Kyriazoglou A, Dimitriadis I,
Dimopoulos MA and Bamias A: Systemic therapy in cervical cancer: 30
Years in review. Crit Rev Oncol Hematol. 137:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Z, Yang P and Zhou Q: Multiple
biological functions and pharmacological effects of lycorine. Sci
China Chem. 56:1382–1391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai B, Zhang N, Han X, Li Q, Zhang M,
Chen X, Li G, Zhang R, Chen P, Wang W, et al: Molecular targets of
β-elemene, a herbal extract used in traditional Chinese medicine,
and its potential role in cancer therapy: A review. Biomed
Pharmacother. 114:1088122019. View Article : Google Scholar
|
|
10
|
Zhang P, Yuan X, Yu T, Huang H, Yang C,
Zhang L, Yang S, Luo X and Luo J: Lycorine inhibits cell
proliferation, migration and invasion, and primarily exerts in
vitro cytostatic effects in human colorectal cancer via activating
the ROS/p38 and PI3K/AKT signaling pathways. Oncol Rep. 45:192021.
View Article : Google Scholar
|
|
11
|
Shang H, Jang X, Shi L and Ma Y: Lycorine
inhibits cell proliferation and induced oxidative stress-mediated
apoptosis via regulation of the JAK/STAT3 signaling pathway in HT-3
cells. J Biochem Mol Toxicol. 35:e228822021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi J, Zhao H, Lian H, Ke L, Zhao L, Wang
C and Han Q: CD276 (B7H3) improve cancer stem cells formation in
cervical carcinoma cell lines. Transl Cancer Res. 10:65–72. 2021.
View Article : Google Scholar
|
|
13
|
Xu Y, Wu H, Huang C and Lu L: Clinical
value of traditional laparotomy, extensive vaginal hysterectomy,
and laparoscope-assisted vaginal hysterectomy in the treatment of
patients with cervical intraepithelial neoplasia III. Transl Cancer
Res. 10:2409–2415. 2021. View Article : Google Scholar
|
|
14
|
Wang W, Gao X, Zhu Y, Qi Y and Wang Y:
Diagnostic significance of a color Doppler ultrasound combined with
serum CXCL16 and E-cad in cervical cancer. Transl Cancer Res.
10:1492–1499. 2021. View Article : Google Scholar
|
|
15
|
Li Z, Wang S, Fang S, Li X, Li Y and Liu
G: Adipose-derived stem cells promote the proliferation, migration,
and invasion of oral squamous cell carcinoma cells by activating
the Wnt/planar cell polarity signaling pathway. Transl Cancer Res.
11:306–315. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition: Washington (DC): National Academies Press (US); 2011
|
|
18
|
Li Z, Fan X, Xu X, Zhou Q, Xing G and Liu
G: Adipose-derived stem cells postpone the progression of Sjögren's
syndrome by upregulating the Hippo signaling pathway. Exp Ther Med.
24:5872022. View Article : Google Scholar
|
|
19
|
Hu M, Tan J, Liu Z, Li L, Zhang H, Zhao D,
Li B, Gao X, Che N and Zhang T: Comprehensive comparative molecular
characterization of young and old lung cancer patients. Front
Oncol. 11:8068452022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung JH, Lee H, Zeng SX and Lu H: RBM10, a
new regulator of p53. Cells. 9:21072020. View Article : Google Scholar :
|
|
21
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Bacon ML, Tessier JJ, Rintala-Maki
ND, Tang V and Sutherland LC: RBM10 modulates apoptosis and
influences TNF-α gene expression. J Cell Death. 5:1–19. 2012.
View Article : Google Scholar
|
|
23
|
Jung JH, Lee H, Cao B, Liao P, Zeng SX and
Lu H: RNA-binding motif protein 10 induces apoptosis and suppresses
proliferation by activating p53. Oncogene. 39:1031–1040. 2020.
View Article : Google Scholar :
|
|
24
|
Han LP, Wang CP and Han SL: Overexpression
of RBM10 induces osteosarcoma cell apoptosis and inhibits cell
proliferation and migration. Med Sci (Paris). 34:81–86. 2018.
View Article : Google Scholar
|
|
25
|
Stratos I, Behrendt AK, Anselm C, Gonzalez
A, Mittlmeier T and Vollmar B: Inhibition of TNF-α restores muscle
force, inhibits inflammation, and reduces apoptosis of traumatized
skeletal muscles. Cells. 11:23972022. View Article : Google Scholar
|
|
26
|
Ji Y, Xie S, Jiang L, Liu L, Li L, Luo L,
Chen Y, Zhang J, Yu L, Zhang Y, et al: Increased cell apoptosis in
human lung adenocarcinoma and in vivo tumor growth inhibition by
RBM10, a tumor suppressor gene. Oncol Lett. 14:4663–4669. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Y, Di X, Zhang Q, Li R and Wang K:
RBM10 regulates tumor apoptosis, proliferation, and metastasis.
Front Oncol. 11:6039322021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Zhang P and Wang X: YBX1 promotes
tumor progression via the PI3K/AKT signaling pathway in laryngeal
squamous cell carcinoma. Transl Cancer Res. 10:4859–4869. 2021.
View Article : Google Scholar
|
|
29
|
Tateishi K, Nakamura T, Juratli TA,
Williams EA, Matsushita Y, Miyake S, Nishi M, Miller JJ, Tummala
SS, Fink AL, et al: PI3K/AKT/mTOR pathway alterations promote
malignant progression and xenograft formation in oligodendroglial
tumors. Clin Cancer Res. 25:4375–4387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng M, Zhou Q, Liu X, Wang C and Liu G:
CTHRC1 overexpression promotes cervical carcinoma progression by
activating the Wnt/PCP signaling pathway. Oncol Rep. 41:1531–1538.
2019.PubMed/NCBI
|
|
31
|
Castillo Ferrer C, Berthenet K and Ichim
G: Apoptosis-fueling the oncogenic fire. FEBS J. 288:4445–4463.
2021. View Article : Google Scholar
|
|
32
|
Obeng E: Apoptosis (programmed cell death)
and its signals-a review. Braz J Biol. 81:1133–1143. 2021.
View Article : Google Scholar
|
|
33
|
Inoue A: RBM10: Structure, functions, and
associated diseases. Gene. 783:1454632021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Y, Wang W, Xu X, Yang B, Yu X, Wu Y and
Wang J: Mettl3 inhibits the apoptosis and autophagy of chondrocytes
in inflammation through mediating Bcl2 stability via
Ythdf1-mediated m6A modification. Bone. 154:1161822022.
View Article : Google Scholar
|
|
35
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|