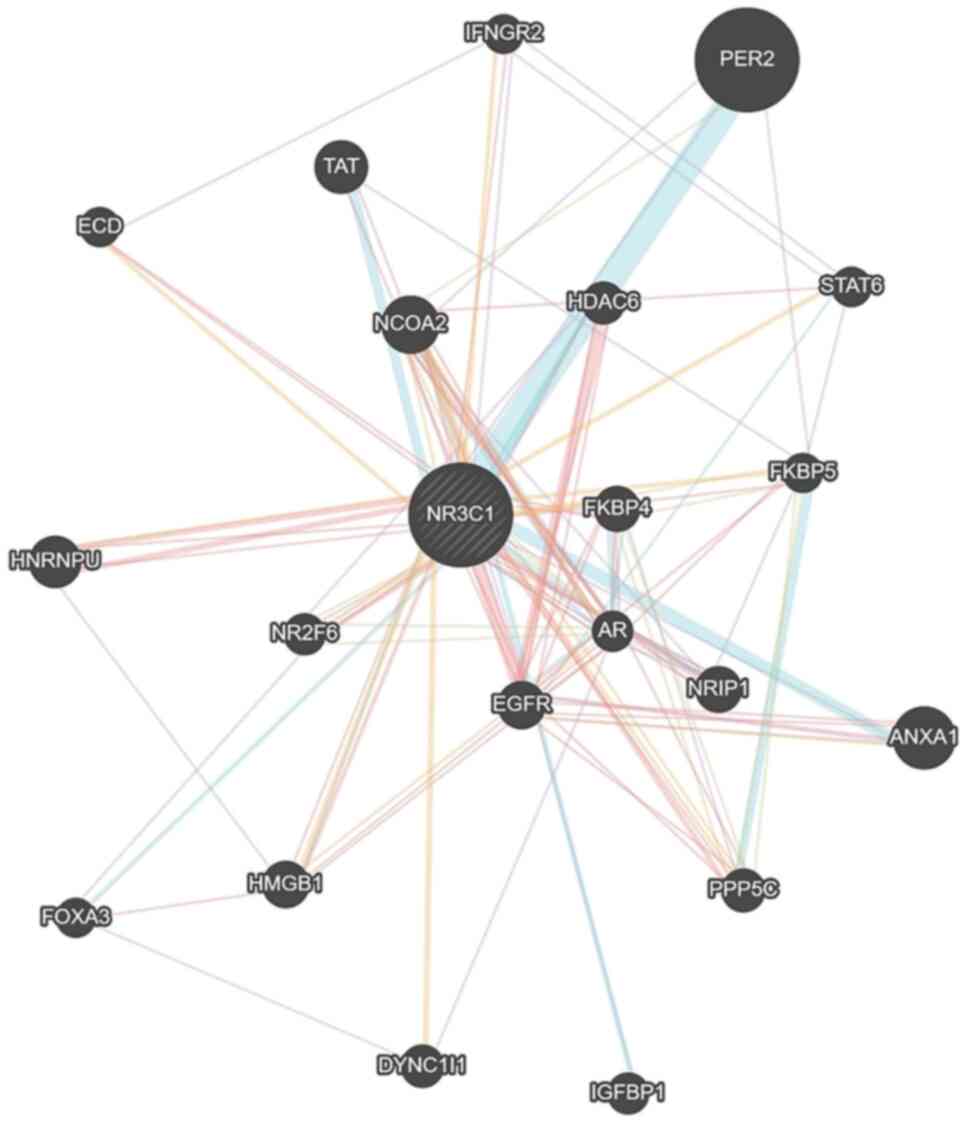
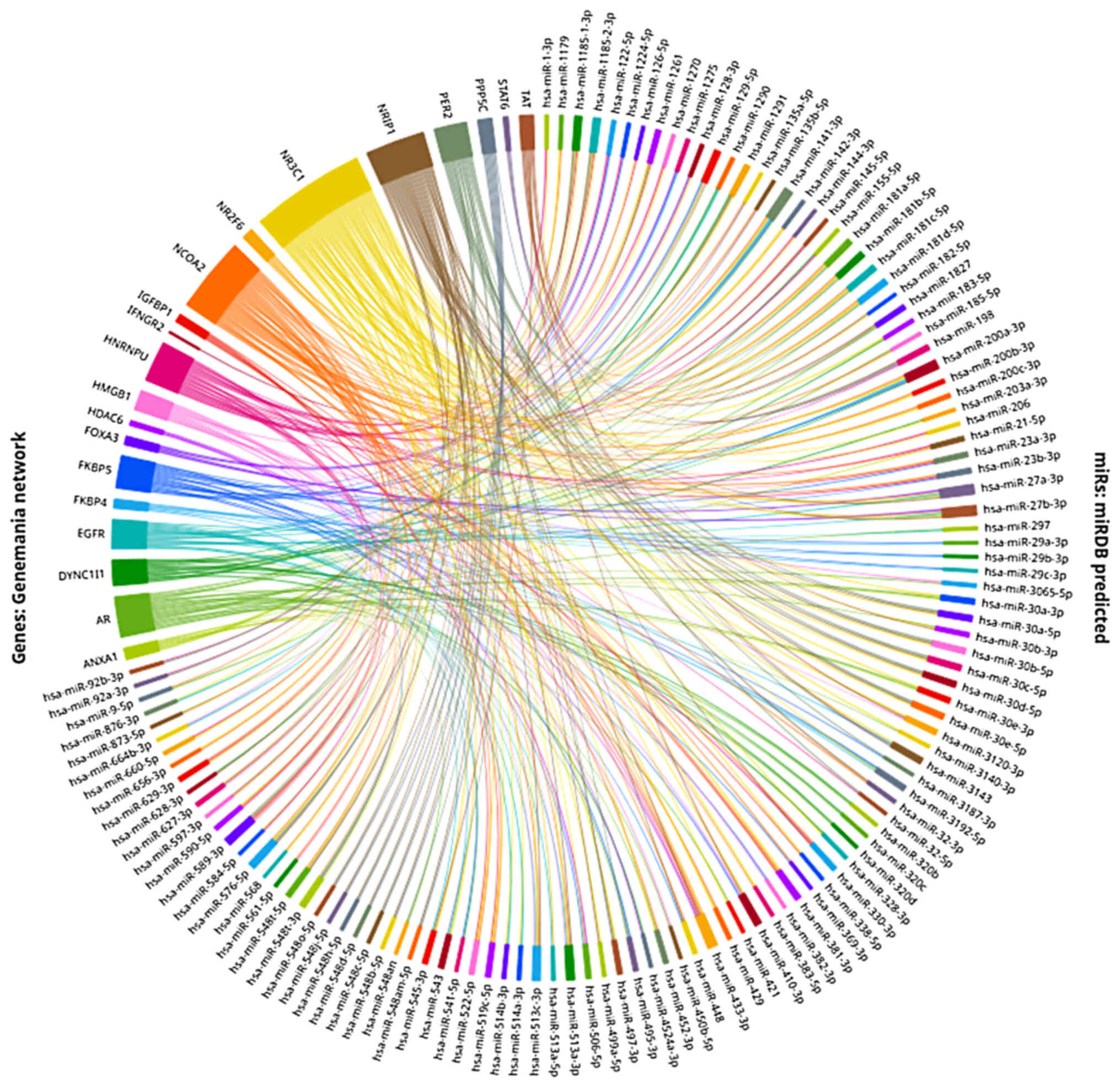
|
1
|
Chrousos GP: Stress and disorders of the
stress system. Nat Rev Endocrinol. 5:374–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsigos C, Kyrou I, Kassi E and Chrousos
GP: Stress: Endocrine Physiology and Pathophysiology. Endotext.
Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan
K, Grossman A, Hershman JM, Hofland J, Kaltsas G, et al: MDText.com, Inc. Copyright© 20002021, MDText.com, Inc. South Dartmouth, MA: 2000
|
|
3
|
Charmandari E, Kino T, Souvatzoglou E and
Chrousos GP: Pediatric stress: Hormonal mediators and human
development. Horm Res. 59:161–179. 2003.PubMed/NCBI
|
|
4
|
Yaribeygi H, Panahi Y, Sahraei H, Johnston
TP and Sahebkar A: The impact of stress on body function: A review.
EXCLI J. 16:1057–1072. 2017.PubMed/NCBI
|
|
5
|
Russell G and Lightman S: The human stress
response. Nat Re Endocrinol. 15:525–534. 2019. View Article : Google Scholar
|
|
6
|
Smith SM and Vale WW: The role of the
hypothalamicpituitary-adrenal axis in neuroendocrine responses to
stress. Dialogues Clin Neurosci. 8:383–395. 2006. View Article : Google Scholar
|
|
7
|
Nicolaides NC, Charmandari E, Kino T and
Chrousos GP: Stress-related and circadian secretion and target
tissue actions of glucocorticoids: Impact on Health. Front
Endocrinol (Lausanne). 8:70. 2017. View Article : Google Scholar
|
|
8
|
Dunlavey CJ: Introduction to the
Hypothalamic-pituitary-adrenal axis: Healthy and dysregulated
stress responses, developmental stress and neurodegeneration. J
Undergrad Neurosci Educ. 16:R59–R60. 2018.PubMed/NCBI
|
|
9
|
DeMorrow S: Role of the
Hypothalamic-pituitary-adrenal axis in health and disease. Int J
Mol Sci. 19:9862018. View Article : Google Scholar
|
|
10
|
Chrousos GP: Stressors, stress, and
neuroendocrine integration of the adaptive response. The 1997 hans
selye memorial lecture. Ann N Y Acad Sci. 851:311–335. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evanson NK, Tasker JG, Hill MN, Hillard CJ
and Herman JP: Fast feedback inhibition of the HPA axis by
glucocorticoids is mediated by endocannabinoid signaling.
Endocrinology. 151:4811–4819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Gou X, Jiang T and Ouyang J: The
effects of microRNAs on glucocorticoid responsiveness. J Cancer Res
Clin Oncoly. 143:1005–1011. 2017. View Article : Google Scholar
|
|
13
|
Kawa MP, Sobuś A, Litwińska Z,
Osowicz-Korolonek L, Cymbaluk-Płoska A, Stecewicz I, Zagrodnik E,
Romanowska H, Walczak M, Syrenicz A and Machaliński B: Expression
of selected angiogenesis-related small microRNAs in patients with
abnormally increased secretion of glucocorticoids. Endokryno Pol.
70:489–495. 2019. View Article : Google Scholar
|
|
14
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Douma LG, Solocinski K, Masten SH, Barral
DH, Barilovits SJ, Jeffers LA, Alder KD, Patel R, Wingo CS, Brown
KD, et al: EDN1-AS, a novel long non-coding rna regulating
endothelin-1 in human proximal tubule cells. Front Physiol.
11:2092020. View Article : Google Scholar :
|
|
16
|
Silverman MN and Sternberg EM:
Glucocorticoid regulation of inflammation and its functional
correlates: From HPA axis to glucocorticoid receptor dysfunction.
Ann N Y Acad Sci. 1261:55–63. 2012. View Article : Google Scholar
|
|
17
|
Timmermans S, Souffriau J and Libert C: A
general introduction to glucocorticoid biology. Front Immunol.
10:1545. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flynn BP: Glucocorticoid ultradian
rhythms. Curr Opin Endocrine Metabolic Res. 25:1003622022.
View Article : Google Scholar
|
|
19
|
Kalafatakis K, Russell GM, Ferguson SG,
Grabski M, Harmer CJ, Munafò MR, Marchant N, Wilson A, Brooks JC,
Thakrar J, et al: Glucocorticoid ultradian rhythmicity
differentially regulates mood and resting state networks in the
human brain: A randomised controlled clinical trial.
Psychoneuroendocrinology. 124:1050962021. View Article : Google Scholar :
|
|
20
|
Dickmeis T: Glucocorticoids and the
circadian clock. J Ndocrinol. 200:3–22. 2009. View Article : Google Scholar
|
|
21
|
Sevilla LM and Pérez P: Roles of the
Glucocorticoid and mineralocorticoid receptors in skin
pathophysiology. Int J Mol Sci. 19:19062018. View Article : Google Scholar :
|
|
22
|
Sarabdjitsingh RA, Meijer OC and de Kloet
ER: Specificity of glucocorticoid receptor primary antibodies for
analysis of receptor localization patterns in cultured cells and
rat hippocampus. Brain Res. 1331:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Desmet SJ and De Bosscher K:
Glucocorticoid receptors: Finding the middle ground. J Clin Invest.
127:1136–1145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicolaides NC, Skyrla E, Vlachakis D,
Psarra AM, Moutsatsou P, Sertedaki A, Kossida S and Charmandari E:
Functional characterization of the hGRαT556I causing Chrousos
syndrome. Eur J Clin Invest. 46:42–49. 2016. View Article : Google Scholar
|
|
25
|
Paragliola RM, Papi G, Pontecorvi A and
Corsello SM: Treatment with synthetic glucocorticoids and the
hypothalamus-pituitary-Adrenal Axis. Int J Mol Sci. 18:22012017.
View Article : Google Scholar :
|
|
26
|
Mazaira GI, Zgajnar NR, Lotufo CM,
Daneri-Becerra C, Sivils JC, Soto OB, Cox MB and Galigniana MD: The
nuclear receptor field: A historical overview and future
challenges. Nucl Receptor Res. 5:1013202018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porter BA, Ortiz MA, Bratslavsky G and
Kotula L: Structure and function of the nuclear receptor
superfamily and current targeted therapies of prostate cancer.
Cancers (Basel). 11:18522019. View Article : Google Scholar
|
|
28
|
Weikum ER, Okafor CD, D'Agostino EH,
Colucci JK and Ortlund EA: Structural analysis of the
glucocorticoid receptor ligand-binding domain in complex with
triamcinolone acetonide and a fragment of the atypical coregulator,
small heterodimer partner. Mol Pharmacol. 92:12–21. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan CK and Wahli W: A trilogy of
glucocorticoid receptor actions. Proc Natl Acad Sci USA.
113:1115–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nicolaides NC, Galata Z, Kino T, Chrousos
GP and Charmandari E: The human glucocorticoid receptor: Molecular
basis of biologic function. Steroids. 75:1–12. 2010. View Article : Google Scholar :
|
|
31
|
Kaziales A, Barkovits K, Marcus K and
Richter K: Glucocorticoid receptor complexes form cooperatively
with the Hsp90 co-chaperones Pp5 and FKBPs. Sci Rep. 10:10733.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baker JD, Ozsan I, Rodriguez Ospina S,
Gulick D and Blair LJ: Hsp90 heterocomplexes regulate steroid
hormone receptors: From stress response to psychiatric disease. Int
J Mol Sci. 20:792018. View Article : Google Scholar
|
|
33
|
Louw A: GR Dimerization and the Impact of
GR Dimerization on GR protein stability and half-life. Front
Immunol. 10:1693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson S, Hapgood JP and Louw A:
Glucocorticoid receptor concentration and the ability to dimerize
influence nuclear translocation and distribution. Steroids.
78:182–194. 2013. View Article : Google Scholar
|
|
35
|
Frego L and Davidson W: Conformational
changes of the glucocorticoid receptor ligand binding domain
induced by ligand and cofactor binding, and the location of
cofactor binding sites determined by hydrogen/deuterium exchange
mass spectrometry. Protein Sci. 15:722–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vandevyver S, Dejager L and Libert C: On
the trail of the glucocorticoid receptor: Into the nucleus and
back. Traffic. 13:364–374. 2012. View Article : Google Scholar
|
|
37
|
Hudson WH, Youn C and Ortlund EA: The
structural basis of direct glucocorticoid-mediated transrepression.
Nat Struct Mol Biol. 20:53–58. 2013. View Article : Google Scholar :
|
|
38
|
Groeneweg FL, van Royen ME, Fenz S, Keizer
VI, Geverts B, Prins J, de Kloet ER, Houtsmuller AB, Schmidt TS and
Schaaf MJ: Quantitation of glucocorticoid receptor DNA-binding
dynamics by single-molecule microscopy and FRAP. PLoS One.
9:e905322014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quatrini L and Ugolini S: New insights
into the cell- and tissue-specificity of glucocorticoid actions.
Cell Mol Immunol. 18:269–278. 2021. View Article : Google Scholar
|
|
40
|
Petta I, Dejager L, Ballegeer M, Lievens
S, Tavernier J, De Bosscher K and Libert C: The Interactome of the
glucocorticoid receptor and its influence on the actions of
glucocorticoids in combatting inflammatory and infectious diseases.
Microbiol Mol Biol Rev. 80:495–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
42
|
Rao NA, McCalman MT, Moulos P, Francoijs
KJ, Chatziioannou A, Kolisis FN, Alexis MN, Mitsiou DJ and
Stunnenberg HG: Coactivation of GR and NFKB alters the repertoire
of their binding sites and target genes. Genome Res. 21:1404–1416.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shimba A and Ikuta K: Control of immunity
by glucocorticoids in health and disease. Semin Immunopathol.
42:669–680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilson KS, Tucker CS, Al-Dujaili EA,
Holmes MC, Hadoke PW, Kenyon CJ and Denvir MA: Early-life
glucocorticoids programme behaviour and metabolism in adulthood in
zebrafish. J Endocrinol. 230:125–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rog-Zielinska EA, Craig MA, Manning JR,
Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes
MC, Hardie DG, et al: Glucocorticoids promote structural and
functional maturation of foetal cardiomyocytes: A role for PGC-1α.
Cell Death Differ. 22:1106–1116. 2015. View Article : Google Scholar
|
|
47
|
Whirledge S and DeFranco DB:
Glucocorticoid signaling in health and disease: Insights from
tissue-specific GR knockout mice. Endocrinology. 159:46–64. 2018.
View Article : Google Scholar :
|
|
48
|
Meszaros K and Patocs A: Glucocorticoids
influencing Wnt/β-catenin pathway; multiple sites, heterogeneous
effects. Molecules. 25:14892020. View Article : Google Scholar
|
|
49
|
Steptoe A and Kivimäki M: Stress and
cardiovascular disease. Nat Rev Cardiol. 9:360–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duma D, Collins JB, Chou JW and Cidlowski
JA: Sexually dimorphic actions of glucocorticoids provide a link to
inflammatory diseases with gender differences in prevalence. Sci
Signal. 3:ra742010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goodwin JE, Zhang J and Geller DS: A
critical role for vascular smooth muscle in acute
glucocorticoid-induced hypertension. J Am Soc Nephrol.
19:1291–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Goodwin JE, Feng Y, Velazquez H and Sessa
WC: Endothelial glucocorticoid receptor is required for protection
against sepsis. Proc Natl Acad Sci USA. 110:306–311. 2013.
View Article : Google Scholar :
|
|
53
|
Akalestou E, Genser L and Rutter GA:
Glucocorticoid metabolism in obesity and following weight loss.
Front Endocrinol (Lausanne). 11:592020. View Article : Google Scholar
|
|
54
|
Kuo T, McQueen A, Chen TC and Wang JC:
Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med
Biol. 872:99–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ferris HA and Kahn CR: New mechanisms of
glucocorticoid-induced insulin resistance: Make no bones about it.
J Clin Invest. 122:3854–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vegiopoulos A and Herzig S:
Glucocorticoids, metabolism and metabolic diseases. Mol Cell
Endocrinol. 275:43–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Madalena KM and Lerch JK: The effect of
glucocorticoid and glucocorticoid receptor interactions on brain,
spinal cord, and glial cell plasticity. Neural Plast.
2017:86409702017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen H, Lombès M and Le Menuet D:
Glucocorticoid receptor represses brain-derived neurotrophic factor
expression in neuron-like cells. Mol Brain. 10:122017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Myers B, McKlveen JM and Herman JP:
Glucocorticoid actions on synapses, circuits, and behavior:
Implications for the energetics of stress. Front Neuroendocrinol.
35:180–196. 2014. View Article : Google Scholar
|
|
60
|
Joëls M: Corticosteroids and the brain. J
Endocrinol. 238:R121–R130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fietta P and Fietta P: Glucocorticoids and
brain functions. Riv Biol. 100:403–418. 2007.
|
|
62
|
McEwen BS and Akil H: Revisiting the
stress concept: Implications for affective disorders. J Neurosci.
40:12–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Smoller JW and Finn CT: Family, twin, and
adoption studies of bipolar disorder. Am J Med Genet C Semin Med
Genet. 123C:48–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Geschwind DH and Flint J: Genetics and
genomics of psychiatric disease. Science. 349:1489–1494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Akil H, Gordon J, Hen R, Javitch J,
Mayberg H, McEwen B, Meaney MJ and Nestler EJ: Treatment resistant
depression: A multi-scale, systems biology approach. Neurosci
Biobehav Rev. 84:272–288. 2018. View Article : Google Scholar
|
|
66
|
Wray NR, Ripke S, Mattheisen M,
Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM,
Andlauer TMF, et al: Genome-wide association analyses identify 44
risk variants and refine the genetic architecture of major
depression. Nat Genet. 50:668–681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Aurbach EL, Inui EG, Turner CA, Hagenauer
MH, Prater KE, Li JZ, Absher D, Shah N, Blandino P Jr, Bunney WE,
et al: Fibroblast growth factor 9 is a novel modulator of negative
affect. Proc Natl Acad Sci USA. 112:11953–11958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Salmaso N, Stevens HE, McNeill J, ElSayed
M, Ren Q, Maragnoli ME, Schwartz ML, Tomasi S, Sapolsky RM, Duman R
and Vaccarino FM: Fibroblast growth factor 2 modulates hypothalamic
pituitary axis activity and anxiety behavior through glucocorticoid
receptors. Biol Psychiatry. 80:479–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chaudhury S, Aurbach EL, Sharma V,
Blandino P Jr, Turner CA, Watson SJ and Akil H: FGF2 is a target
and a trigger of epigenetic mechanisms associated with differences
in emotionality: Partnership with H3K9me3. Proc Natl Acad Sci USA.
111:11834–11839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tyrka AR, Parade SH, Eslinger NM, Marsit
CJ, Lesseur C, Armstrong DA, Philip NS, Josefson B and Seifer R:
Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor
gene promoter and exposure to adversity in preschool-aged children.
Dev Psychopathol. 27:577–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sinclair D, Fillman SG, Webster MJ and
Weickert CS: Dysregulation of glucocorticoid receptor co-factors
FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness.
Sci Rep. 3:35392013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sinclair D, Tsai SY, Woon HG and Weickert
CS: Abnormal glucocorticoid receptor mRNA and protein isoform
expression in the prefrontal cortex in psychiatric illness.
Neuropsychopharmacology. 36:2698–2709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sinclair D, Webster MJ, Fullerton JM and
Weickert CS: Glucocorticoid receptor mRNA and protein isoform
alterations in the orbitofrontal cortex in schizophrenia and
bipolar disorder. BMC Psychiatry. 12:842012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39:D152–D157. 2011. View Article : Google Scholar :
|
|
76
|
Griffiths-Jones S: miRBase: MicroRNA
Sequences and Annotation. Curr Protoc Bioinformatics. Chapter 12:
Unit 12.9.1-10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: Tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar :
|
|
78
|
Griffiths-Jones S: miRBase: The microRNA
sequence database. Methods Mol Biol. 342:129–138. 2006.PubMed/NCBI
|
|
79
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: MicroRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar :
|
|
80
|
Griffiths-Jones S: The microRNA Registry.
Nucleic Acids Res. 32:D109–D111. 2004. View Article : Google Scholar :
|
|
81
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47:D155–D162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hollins SL and Cairns MJ: MicroRNA: Small
RNA mediators of the brains genomic response to environmental
stress. Prog Neurobiol. 143:61–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
de Kloet ER, Fitzsimons CP, Datson NA,
Meijer OC and Vreugdenhil E: Glucocorticoid signaling and
stress-related limbic susceptibility pathway: About receptors,
transcription machinery and microRNA. Brain Res. 1293:129–141.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moisiadis VG and Matthews SG:
Glucocorticoids and fetal programming part 2: Mechanisms. Nat Rev
Endocrinol. 10:403–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pufall MA: Glucocorticoids and cancer. Adv
Exp Med Biol. 872:315–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar :
|
|
88
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar :
|
|
89
|
Liu W and Wang X: Prediction of functional
microRNA targets by integrative modeling of microRNA binding and
target expression data. Genome Biol. 20:182019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ledderose C, Möhnle P, Limbeck E, Schütz
S, Weis F, Rink J, Briegel J and Kreth S: Corticosteroid resistance
in sepsis is influenced by microRNA-124-induced downregulation of
glucocorticoid receptor-α. Crit Care Med. 40:2745–2753. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang SS, Mu RH, Li CF, Dong SQ, Geng D,
Liu Q and Yi LT: microRNA-124 targets glucocorticoid receptor and
is involved in depression-like behaviors. Prog Neuropsychopharmacol
Biol Psychiatry. 79:417–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Roy B, Dunbar M, Shelton RC and Dwivedi Y:
Identification of MicroRNA-124-3p as a putative epigenetic
signature of major depressive disorder. Neuropsychopharmacology.
42:864–875. 2017. View Article : Google Scholar :
|
|
93
|
Dwivedi Y: microRNA-124: A putative
therapeutic target and biomarker for major depression. Expert Opin
Ther Targets. 21:653–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vreugdenhil E, Verissimo CS, Mariman R,
Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T,
Meijer OC, de Kloet ER and Fitzsimons CP: MicroRNA 18 and 124a
down-regulate the glucocorticoid receptor: Implications for
glucocorticoid responsiveness in the brain. Endocrinology.
150:2220–2228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Uchida S, Nishida A, Hara K, Kamemoto T,
Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen
BS and Watanabe Y: Characterization of the vulnerability to
repeated stress in Fischer 344 rats: Possible involvement of
microRNA-mediated down-regulation of the glucocorticoid receptor.
Eur J Neurosci. 27:2250–2261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Vallès A, Martens GJ, De Weerd P, Poelmans
G and Aschrafi A: MicroRNA-137 regulates a glucocorticoid
receptor-dependent signalling network: Implications for the
etiology of schizophrenia. J Psychiatry Neurosc. 39:312–320. 2014.
View Article : Google Scholar
|
|
97
|
Li S, Ma H, Yuan X, Zhou X, Wan Y and Chen
S: MicroRNA-382-5p targets nuclear receptor subfamily 3 group C
member 1 to regulate depressive-like behaviors induced by chronic
unpredictable mild stress in rats. Neuropsychiatr Dis Treat.
16:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kim J, Jeong D, Nam J, Aung TN, Gim JA,
Park KU and Kim SW: MicroRNA-124 regulates glucocorticoid
sensitivity by targeting phosphodiesterase 4B in diffuse large B
cell lymphoma. Gene. 558:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liang YN, Tang YL, Ke ZY, Chen YQ, Luo XQ,
Zhang H and Huang LB: MiR-124 contributes to glucocorticoid
resistance in acute lymphoblastic leukemia by promoting
proliferation, inhibiting apoptosis and targeting the
glucocorticoid receptor. J Steroid Biochem Mol Biol. 172:62–68.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang
H, Hong M, Jiang T, Jiang Q, Lu J, et al: An oncogenic role of
miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by
targeting glucocorticoid receptor-alpha and cAMP/PKA pathways.
Leukemia. 26:769–777. 2012. View Article : Google Scholar
|
|
101
|
Riester A, Issler O, Spyroglou A, Rodrig
SH, Chen A and Beuschlein F: ACTH-dependent regulation of microRNA
as endogenous modulators of glucocorticoid receptor expression in
the adrenal gland. Endocrinology. 153:212–222. 2012. View Article : Google Scholar
|
|
102
|
Tessel MA, Benham AL, Krett NL, Rosen ST
and Gunaratne PH: Role for microRNAs in regulating glucocorticoid
response and resistance in multiple myeloma. Horm Cancer.
2:182–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sionov RV: MicroRNAs and
glucocorticoid-induced apoptosis in lymphoid malignancies. ISRN
Hematol. 2013:3482122013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Vigorito E, Kohlhaas S, Lu D and Leyland
R: miR-155: An ancient regulator of the immune system. Immunol Rev.
253:146–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Elmesmari A, Fraser AR, Wood C, Gilchrist
D, Vaughan D, Stewart L, McSharry C, McInnes IB and
Kurowska-Stolarska M: MicroRNA-155 regulates monocyte chemokine and
chemokine receptor expression in Rheumatoid Arthritis. Rheumatology
(Oxford). 55:2056–2065. 2016. View Article : Google Scholar
|
|
106
|
Kurowska-Stolarska M, Alivernini S,
Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna
M, Fraser AR, Stolarski B, et al: MicroRNA-155 as a proinflammatory
regulator in clinical and experimental arthritis. Proc Natl Acad
Sci USA. 108:11193–11198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang ZH, Liang YB, Tang H, Chen ZB, Li ZY,
Hu XC and Ma ZF: Dexamethasone down-regulates the expression of
microRNA-155 in the livers of septic mice. PLoS One. 8:e805472013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zheng Y, Xiong S, Jiang P, Liu R, Liu X,
Qian J, Zheng X and Chu Y: Glucocorticoids inhibit
lipopolysaccharide-mediated inflammatory response by downregulating
microRNA-155: A novel anti-inflammation mechanism. Free Radic Biol
Med. 52:1307–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Curtale G, Renzi TA, Drufuca L, Rubino M
and Locati M: Glucocorticoids downregulate TLR4 signaling activity
via its direct targeting by miR-511-5p. Eur J Immunol.
47:2080–2089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Puimège L, Van Hauwermeiren F, Steeland S,
Van Ryckeghem S, Vandewalle J, Lodens S, Dejager L, Vandevyver S,
Staelens J, Timmermans S, et al: Glucocorticoid-induced
microRNA-511 protects against TNF by down-regulating TNFR1. EMBO
Mol Med. 7:1004–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Clayton SA, Jones SW, Kurowska-Stolarska M
and Clark AR: The role of microRNAs in glucocorticoid action. J
Biol Chemistry. 293:1865–1874. 2018. View Article : Google Scholar
|
|
112
|
Davis TE, Kis-Toth K, Szanto A and Tsokos
GC: Glucocorticoids suppress T cell function by up-regulating
microRNA-98. Arthritis Rheum. 65:1882–1890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhu QY, Liu Q, Chen JX, Lan K and Ge BX:
MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation
of MAPKs in macrophages. J Immunol. 185:7435–7442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mogilyansky E and Rigoutsos I: The
miR-17/92 cluster: A comprehensive update on its genomics,
genetics, functions and increasingly important and numerous roles
in health and disease. Cell Death Differ. 20:1603–1614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Molitor is JK, McColl KS and Distelhorst
CW: Glucocorticoid-mediated repression of the oncogenic microRNA
cluster miR-17~92 contributes to the induction of Bim and
initiation of apoptosis. Mol Endocrinol. 25:409–420. 2011.
View Article : Google Scholar
|
|
116
|
Harada M, Pokrovskaja-Tamm K, Söderhäll S,
Heyman M, Grander D and Corcoran M: Involvement of miR17 pathway in
glucocorticoid-induced cell death in pediatric acute lymphoblastic
leukemia. Leuk Lymphoma. 53:2041–2050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Palagani A, Op de Beeck K, Naulaerts S,
Diddens J, Sekhar Chirumamilla C, Van Camp G, Laukens K, Heyninck
K, Gerlo S, Mestdagh P, et al: Ectopic microRNA-150-5p
transcription sensitizes glucocorticoid therapy response in MM1S
multiple myeloma cells but fails to overcome hormone therapy
resistance in MM1R cells. PLoS One. 9:e1138422014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhao JJ, Chu ZB, Hu Y, Lin J, Wang Z,
Jiang M, Chen M, Wang X, Kang Y, Zhou Y, et al: Targeting the
miR-221-222/PUMA/BAK/BAX pathway abrogates dexamethasone resistance
in multiple myeloma. Cancer Res. 75:4384–4397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Murray MY, Rushworth SA, Zaitseva L,
Bowles KM and Macewan DJ: Attenuation of dexamethasone-induced cell
death in multiple myeloma is mediated by miR-125b expression. Cell
Cycle. 12:2144–2153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kotani A, Ha D, Hsieh J, Rao PK, Schotte
D, den Boer ML, Armstrong SA and Lodish HF: miR-128b is a potent
glucocorticoid sensitizer in MLL-AF4 acute lymphocytic leukemia
cells and exerts cooperative effects with miR-221. Blood.
114:4169–4178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cruz-Topete D, Oakley RH, Xu X and
Cidlowski JA: Glucocorticoid receptor signaling is critical for
microRNA Gender-specific regulation of gene expression in the adult
mouse heart. FASEB J. 31:687.4. 2017.
|
|
122
|
Jung SH, Wang Y, Kim T, Tarr A, Reader B,
Powell N and Sheridan JF: Molecular mechanisms of repeated social
defeat-induced glucocorticoid resistance: Role of microRNA. Brain
Behav Immun. 44:195–206. 2015. View Article : Google Scholar
|
|
123
|
Ko JY, Chuang PC, Ke HJ, Chen YS, Sun YC
and Wang FS: MicroRNA-29a mitigates glucocorticoid induction of
bone loss and fatty marrow by rescuing Runx2 acetylation. Bone.
81:80–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Schroeder M, Jakovcevski M, Polacheck T,
Drori Y, Luoni A, Röh S, Zaugg J, Ben-Dor S, Albrecht C and Chen A:
Placental miR-340 mediates vulnerability to activity based anorexia
in mice. Nat Commun. 9:15962018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Fu Q, Liu CJ, Zhang X, Zhai ZS, Wang YZ,
Hu MX, Xu XL, Zhang HW and Qin T: Glucocorticoid receptor regulates
expression of microRNA-22 and downstream signaling pathway in
apoptosis of pancreatic acinar cells. World J Gastroenterol.
24:5120–5130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Buschmann D, González R, Kirchner B,
Mazzone C, Pfaffl MW, Schelling G, Steinlein O and Reithmair M:
Glucocorticoid receptor overexpression slightly shifts microRNA
expression patterns in triple-negative breast cancer. Int J Oncol.
52:1765–1776. 2018.PubMed/NCBI
|
|
127
|
Tejos-Bravo M, Oakley RH, Whirledge SD,
Corrales WA, Silva JP, García-Rojo G, Toledo J, Sanchez W,
Román-Albasini L, Aliaga E, et al: Deletion of hippocampal
Glucocorticoid receptors unveils sex-biased microRNA expression and
neuronal morphology alterations in mice. Neurobiol Stress.
14:1003062021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zheng D, Sabbagh JJ, Blair LJ, Darling AL,
Wen X and Dickey CA: MicroRNA-511 binds to FKBP5 mRNA, which
encodes a chaperone protein, and regulates neuronal
differentiation. J Biol Chemistry. 291:17897–17906. 2016.
View Article : Google Scholar
|
|
129
|
Pelleymounter LL, Moon I, Johnson JA,
Laederach A, Halvorsen M, Eckloff B, Abo R and Rossetti S: A novel
application of pattern recognition for accurate SNP and indel
discovery from high-throughput data: Targeted resequencing of the
glucocorticoid receptor co-chaperone FKBP5 in a Caucasian
population. Mol Genet Metab. 104:457–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Clemson CM, McNeil JA, Willard HF and
Lawrence JB: XIST RNA paints the inactive X chromosome at
interphase: Evidence for a novel RNA involved in nuclear/chromosome
structure. J Cell Biol. 132:259–275. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Swiezewski S, Liu F, Magusin A and Dean C:
Cold-induced silencing by long antisense transcripts of an
arabidopsis polycomb target. Nature. 462:799–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Houseley J, Rubbi L, Grunstein M,
Tollervey D and Vogelauer M: A ncRNA modulates histone modification
and mRNA induction in the yeast GAL gene cluster. Mol Cell.
32:685–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Reeves MB, Davies AA, McSharry BP,
Wilkinson GW and Sinclair JH: Complex I binding by a virally
encoded RNA regulates mitochondria-induced cell death. Science.
316:1345–1348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Clark MB and Mattick JS: Long noncoding
RNAs in cell biology. Semin Cell Dev Biol. 22:366–376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Martianov I, Ramadass A, Serra Barros A,
Chow N and Akoulitchev A: Repression of the human dihydrofolate
reductase gene by a non-coding interfering transcript. Nature.
445:666–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Tripathi V, Ellis JD, Shen Z, Song DY, Pan
Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al: The
nuclear-retained noncoding RNA MALAT1 regulates alternative
splicing by modulating SR splicing factor phosphorylation. Mol
Cell. 39:925–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mourtada-Maarabouni M, Hedge VL, Kirkham
L, Farzaneh F and Williams GT: Growth arrest in human T-cells is
controlled by the non-coding RNA growth-arrest-specific transcript
5 (GAS5). J Cell Sci. 121:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Place RF and Noonan EJ: Non-coding RNAs
turn up the heat: An emerging layer of novel regulators in the
mammalian heat shock response. Cell Stress Chaperones. 19:159–172.
2014. View Article : Google Scholar :
|
|
142
|
Jarroux J, Morillon A and Pinskaya M:
History, discovery, and classification of lncRNAs. Adv Exp Med
Biol. 1008:1–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ma L, Bajic VB and Zhang Z: On the
classification of long non-coding RNAs. RNA Biol. 10:925–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mayama T, Marr AK and Kino T: Differential
expression of glucocorticoid receptor noncoding RNA repressor Gas5
in autoimmune and inflammatory diseases. Horm Metab Res.
48:550–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lucafò M, Di Silvestre A, Romano M, Avian
A, Antonelli R, Martelossi S, Naviglio S, Tommasini A, Stocco G,
Ventura A, Decorti G and De Iudicibus S: Role of the long
non-coding RNA growth Arrest-Specific 5 in glucocorticoid response
in children with inflammatory bowel disease. Basic Clin Pharmacol
Toxicol. 122:87–93. 2018. View Article : Google Scholar
|
|
146
|
Gharesouran J, Taheri M, Sayad A,
Ghafouri-Fard S, Mazdeh M and Omrani MD: The growth arrest-specific
Transcript 5 (GAS5) and nuclear receptor Subfamily 3 Group C Member
1 (NR3C1): Novel Markers involved in multiple sclerosis. Int J Mol
Cell Med. 7:102–110. 2018.PubMed/NCBI
|
|
147
|
Esguerra JLS, Ofori JK, Nagao M, Shuto Y,
Karagiannopoulos A, Fadista J, Sugihara H, Groop L and Eliasson L:
Glucocorticoid induces human beta cell dysfunction by involving
riborepressor GAS5. LincRNA Mol Metab. 32:160–167. 2020. View Article : Google Scholar
|
|
148
|
Ketab FNG, Gharesouran J, Ghafouri-Fard S,
Dastar S, Mazraeh SA, Hosseinzadeh H, Moradi M, Javadlar M,
Hiradfar A, Rezamand A, et al: Dual biomarkers long non-coding RNA
GAS5 and its target, NR3C1, contribute to acute myeloid leukemia.
Exp Mol Pathol. 114:1043992020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Pulido T, Adzerikho I, Channick RN,
Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO,
Mehta S, et al: Macitentan and morbidity and mortality in pulmonary
arterial hypertension. N Engl J Med. 369:809–818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Speed JS and Pollock DM: Endothelin,
kidney disease, and hypertension. Hypertension. 61:1142–1145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Holm SJ, Sánchez F, Carlén LM, Mallbris L,
Ståhle M and O'Brien KP: HLA-Cw*0602 associates more strongly to
psoriasis in the Swedish population than variants of the novel
6p213 gene PSORS1C3. Acta Derm Venereol. 85:2–8. 2005. View Article : Google Scholar
|
|
152
|
Robinson PC, Leo PJ, Pointon JJ, Harris J,
Cremin K, Bradbury LA; Wellcome Trust Case Control Consortium;
Australasian Osteoporosis Genetics Consortium (AOGC); Stebbings S,
Harrison AA, et al: The genetic associations of acute anterior
uveitis and their overlap with the genetics of ankylosing
spondylitis. Genes Immun. 17:46–51. 2016. View Article : Google Scholar
|
|
153
|
Murphy TM, Crawford B, Dempster EL, Hannon
E, Burrage J, Turecki G, Kaminsky Z and Mill J: Methylomic
profiling of cortex samples from completed suicide cases implicates
a role for PSORS1C3 in major depression and suicide. Transl
Psychiatry. 7:e9892017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Mirzadeh Azad F, Malakootian M and Mowla
SJ: lncRNA PSORS1C3 is regulated by glucocorticoids and fine-tunes
OCT4 expression in non-pluripotent cells. Sci Rep. 9:83702019.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Redfern AD, Colley SM, Beveridge DJ, Ikeda
N, Epis MR, Li X, Foulds CE, Stuart LM, Barker A, Russell VJ, et
al: RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and
Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad
Sci USA. 110:6536–6541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Hatchell EC, Colley SM, Beveridge DJ, Epis
MR, Stuart LM, Giles KM, Redfern AD, Miles LE, Barker A, MacDonald
LM, et al: SLIRP, a small SRA binding protein, is a nuclear
receptor corepressor. Mol Cell. 22:657–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Yang L, Lin C, Jin C, Yang JC, Tanasa B,
Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al:
lncRNA-dependent mechanisms of androgen-receptor-regulated gene
activation programs. Nature. 500:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Vitellius G, Trabado S, Bouligand J,
Delemer B and Lombès M: Pathophysiology of Glucocorticoid
Signaling. Ann Endocrinol (Paris). 79:98–106. 2018. View Article : Google Scholar
|
|
159
|
Nicolaides NC, Geer EB, Vlachakis D,
Roberts ML, Psarra AM, Moutsatsou P, Sertedaki A, Kossida S and
Charmandari E: A novel mutation of the hGR gene causing Chrousos
syndrome. Eur J Clin Invest. 45:782–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Volden PA and Conzen SD: The influence of
glucocorticoid signaling on tumor progression. Brain Behav Immun.
30(Suppl): S26–S31. 2013. View Article : Google Scholar
|
|
161
|
Oakley RH and Cidlowski JA: Glucocorticoid
signaling in the heart: A cardiomyocyte perspective. J Steroid
Biochem Mol Biol. 153:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Oakley RH and Cidlowski JA: The biology of
the glucocorticoid receptor: New signaling mechanisms in health and
disease. J Allergy Clin Immunol. 132:1033–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zabirowicz ES and Gan TJ: 34-Pharmacology
of Postoperative Nausea and Vomiting. Pharmacology and Physiology
for Anesthesia (Second Edition). Hemmings HC and Egan TD: Elsevier;
Philadelphia: pp. 671–692. 2019, View Article : Google Scholar
|
|
164
|
Flor M: R interface to D3 chord diagrams.
Chorddiag. https://github.com/mattflor/chorddiag. Accessed
February 18, 2022.
|