Inflammation and fibrosis are inseparable from
disease. Organ fibrosis is frequently induced by persistent chronic
inflammation triggered by several stimuli, although it may have
multiple causes (1). Type 2 T
helper (Th2) cells have strong profibrotic capacities and induce
M2-type expression of macrophages. Macrophages have essential
effects on inflammation and fibrosis via several pathways (2,3).
Chronic inflammation and epithelial-mesenchymal transition (EMT)
create a profibrotic environment that promotes the production of
collagen and smooth muscle α-actin (α-SMA) by infiltrating
hematopoietic cells and resident fibroblasts. Several interactions
among fibroblasts, fibrocytes and inflammatory cells also attenuate
or exacerbate fibrosis (4).
MicroRNAs (miRNAs) mediate several functions in
cells. They are pivotal regulators in cellular pathways and
numerous pathologies, although they do not code for proteins. With
RNA-polymerase II/III catalyst, miRNAs encoding genes are
transcribed into primary miRNAs. The primary miRNAs are cleaved
with Drosha (the first RNase III enzyme) into precursor miRNAs of
~60-70 nucleotides in the nucleus, and exportin-5 transports these
precursor miRNAs into the cytoplasm. Subsequently, the second RNase
III enzyme, Dicer, cleaves them into ~22-nucleotide double-stranded
miRNA molecules (5). The
RNA-induced silencing complex (RISC) incorporates the mature
miRNAs, in which they further perform their functions. Finally,
these miRNAs bind to the 3′-untranslated regions (3′UTRs) of the
target genes and degenerate or reduce mRNA expression (6). miRNAs mediate mRNA degradation
mainly by deadenylation, subsequent decapping and 5′-3′
exonucleolytic digestion (7).
In a physiological environment, the expression of
miR-146a is confined to immune cells, and it negatively inhibits
the innate and adaptive immune responses by regulating certain
adapters or transcription factors, including signal transducer and
activator of transcription 1 (STAT1) (8,9),
tumor necrosis factor receptor-associated factor 6 (TRAF6) and
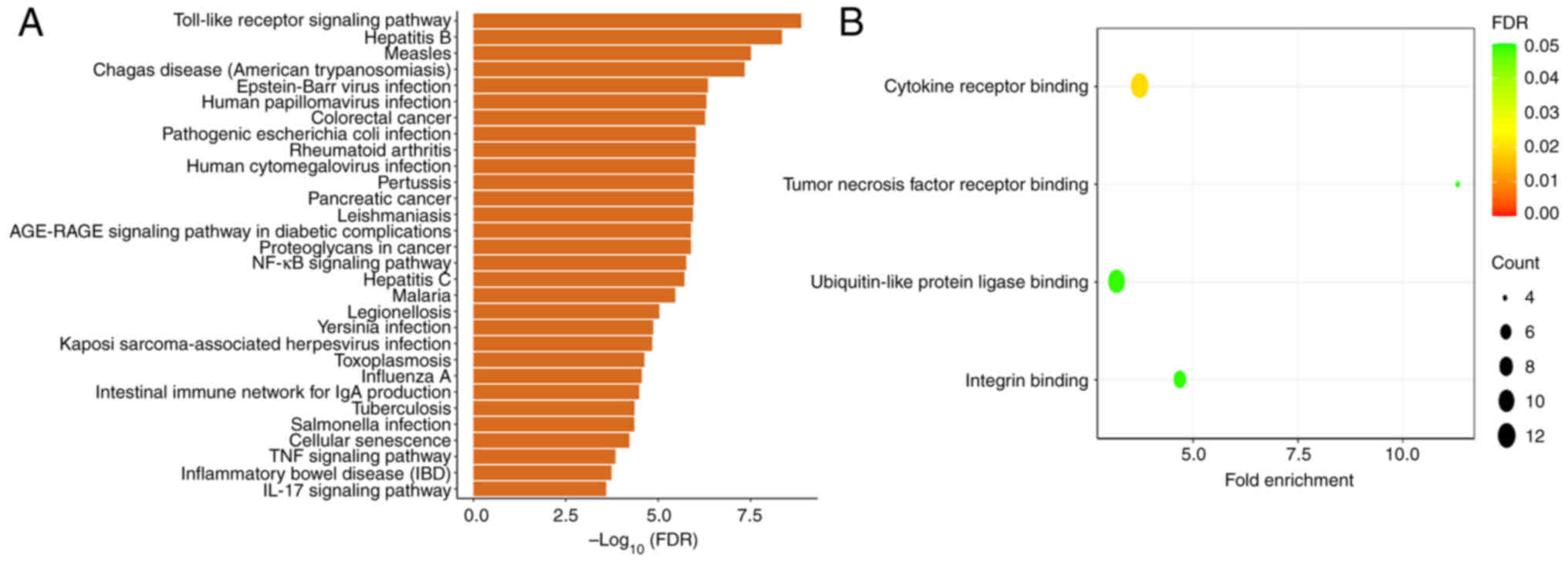
interleukin-1 receptor-associated kinase 1 (IRAK1) (10-13). Fig.
1 illustrates the biological functions and top 30 enriched
pathways of miR-146a-5p from the Gene Ontology and Kyoto
Encyclopedia of Genes and Genomes enrichment analysis [the results
were obtained from the CancerMIRNome database (http://bioinfo.jialab-ucr.org/CancerMIRNome/)]. The
nuclear factor-κB (NF-κB) signaling pathway and Toll-like receptor
(TLR) are related to inflammation. Binding cytokine receptor is
also an inflammation-related biological function. The present
review focused on describing the mechanisms and application
strategies of miRNA-146a in regulating inflammation and fibrosis at
molecular and cellular levels.
The miR-146 family (miR-146a and miR-146b) are
homologous, with only two nucleotides differing in their 3′ region.
Their coding genes are located on 10 (10q24.32) and chromosomes 5
(5q33.3) in humans (14). These
isoforms have similarities and differences in function and target
molecules. They have unique regulatory functions for the signaling
pathway (15,16). The miR-146a precursor contains a
5′ arm sequence (miR-146a-5p) and a 3′ arm sequence (miR-146a-3p).
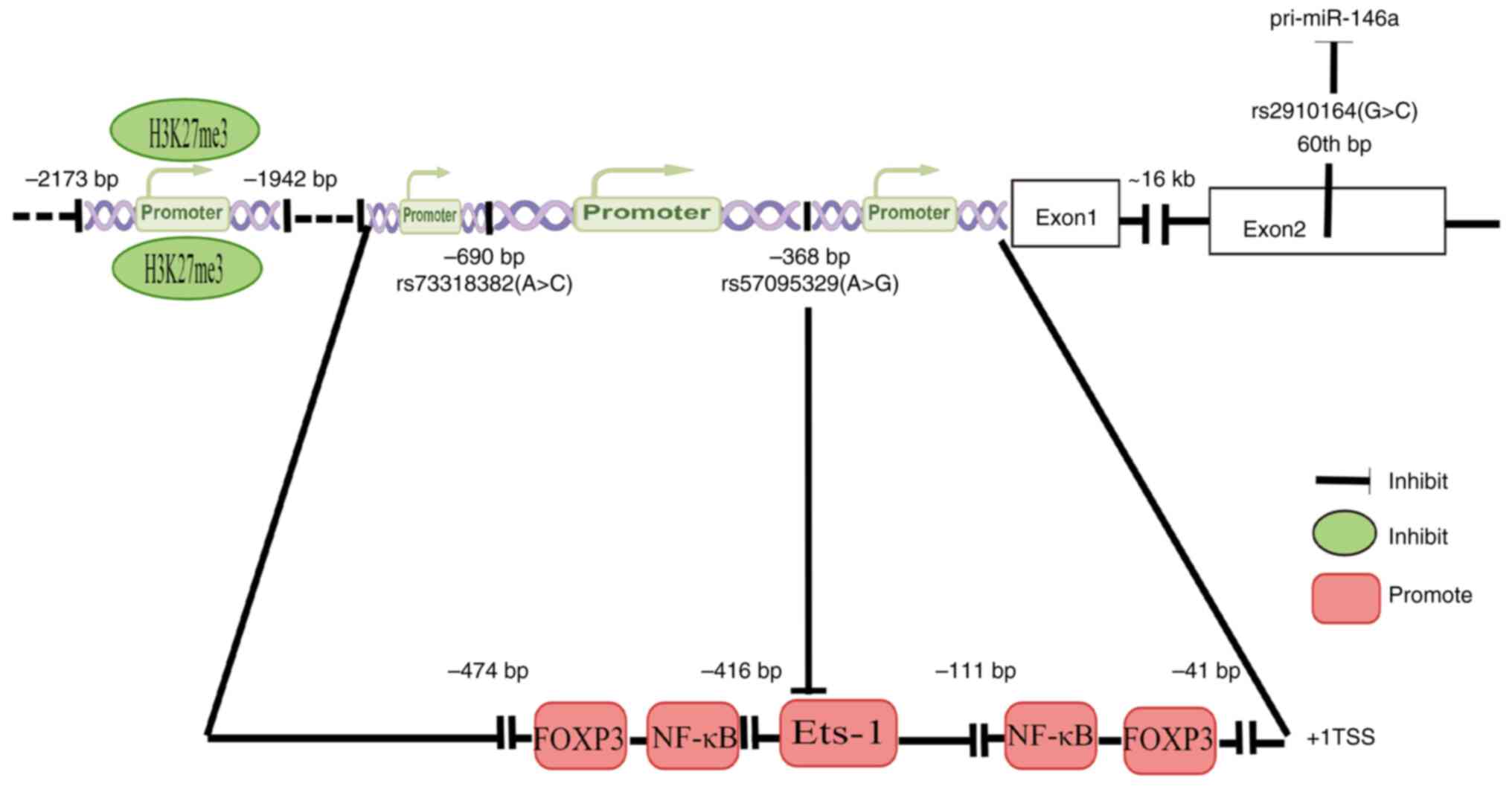
miR-146a is the main topic of the present review. The gene motif's
binding promoter of miR-146a reported in studies on inflammation or
fibrosis will be discussed. Liu et al (17) found two pairs of NF-κB binding
motifs (GGG ATT TCC and GGG ACT TTC C in humans) and forkhead box
protein 3 (FOXP3) binding motifs (GCC AAC A and GCA AAT A in
humans) in the miR-146a promoter. NF-κB is a pivotal factor in
inflammation and fibrosis, while FOXP3 is the transcription factor
of T-regulatory (Treg) cells and participants in immune responses.
IL-1 receptors (IL-1R)/TLRs stimuli, such as lipopolysaccharide
(LPS) and NF-κB signaling, lead to its expression. The expression
of miR-146a downregulates the activity of these pathways (14,15,18). In rats with acute spinal cord
injury, Ni et al (19)
found that tri-methylation recruitment at lysine 27 of histone H3
(H3K27me3) around the upstream region in the promoter of miR-146a
reduced miR-146 expression, suggesting that H3K27me3 participates
in the epigenetic regulation of miR-146a. By interfering with the
maturation process of miRNA, genetic variants in miRNA precursors
affect miRNA expression levels and thereby cause disease
susceptibility. These variants may occur in the miRNA promoter and
precursor regions. Single nucleotide polymorphisms (SNPs) are
essential. As a result of the rs2910164 polymorphism, the
pre-miR-146a's stem region is mismatched, which indirectly
influences the expression of mature miR-146a (20). G:U in rs2910164 changes to C:U,
causing mispairing in miR-146a's hairpin and decreasing the
efficiency of processing miRNA precursors into mature miRNA, thus
suppressing the expression of miR-146a (21). V-Ets oncogene homolog 1 (Ets-1) is
a member of the Ets family of transcription factors that activates
the miR-146a promoter and directly regulates miR-146a expression.
The inflammation risk-associated G allele of rs57095329 inhibits
the binding affinity for Ets-1 and leads to an inappropriate
compositional change, and is thus another genetic factor
suppressing miR-146a expression (22). These two SNPs were in a strong
linkage disequilibrium. Cui et al (23) found that the miRNA-146a rs57095329
A allele carried a lower risk and seizure frequency of
drug-resistant epilepsy (Fig.
2).
It is thought that downstream of these receptors are
similar signaling cascades, as TLRs and IL-1R both express
Toll/IL-1 receptor (TIR) domains (24). The current understanding of TLR
signaling is primarily based on studies focusing on downstream
IL-1R signaling. TLR2 is involved in peptidoglycan, lipoteichoic
acid (LTA) and bacterial lipoprotein metabolism. The stimuli of
TLR4 contain LPS, LTA and oxidized low-density lipoprotein
(25,26). TLRs transduce downstream signals
by mediating the myeloid differentiation factor 88 (MyD88) or TIR
domain-containing adapter molecule (TRIF)-related pathways. The
binding of IRAK4 and IRAK1/2 are induced to form the myddosome when
MyD88 recruits to the TIR domain of TLRs. TRAF6 further activates
downstream of MyD88 and triggers NF-κB signaling, inducing
pro-inflammatory responses. TRAF6 also induces endosomal TLR4,
which is related to TRIF and contributes to pro-inflammatory
responses (27). Activated
inhibitor κ kinase β induces the phosphorylation of inhibitor of
κBα (IκBα), leading to the ubiquitination and subsequent
degradation of IκBα, which promotes the release of NF-κB dimers
from the cytoplasm to the nucleus (28-30). The canonical NF κB pathway
regulates the production of pro inflammatory cytokines, growth
factors, chemokines, matrix metalloproteinase, anti apoptotic
proteins, pro inflammatory enzymes, intercellular adhesion molecule
1 and inhibitors of NFκ B signaling (such as IκBα) (31,32).
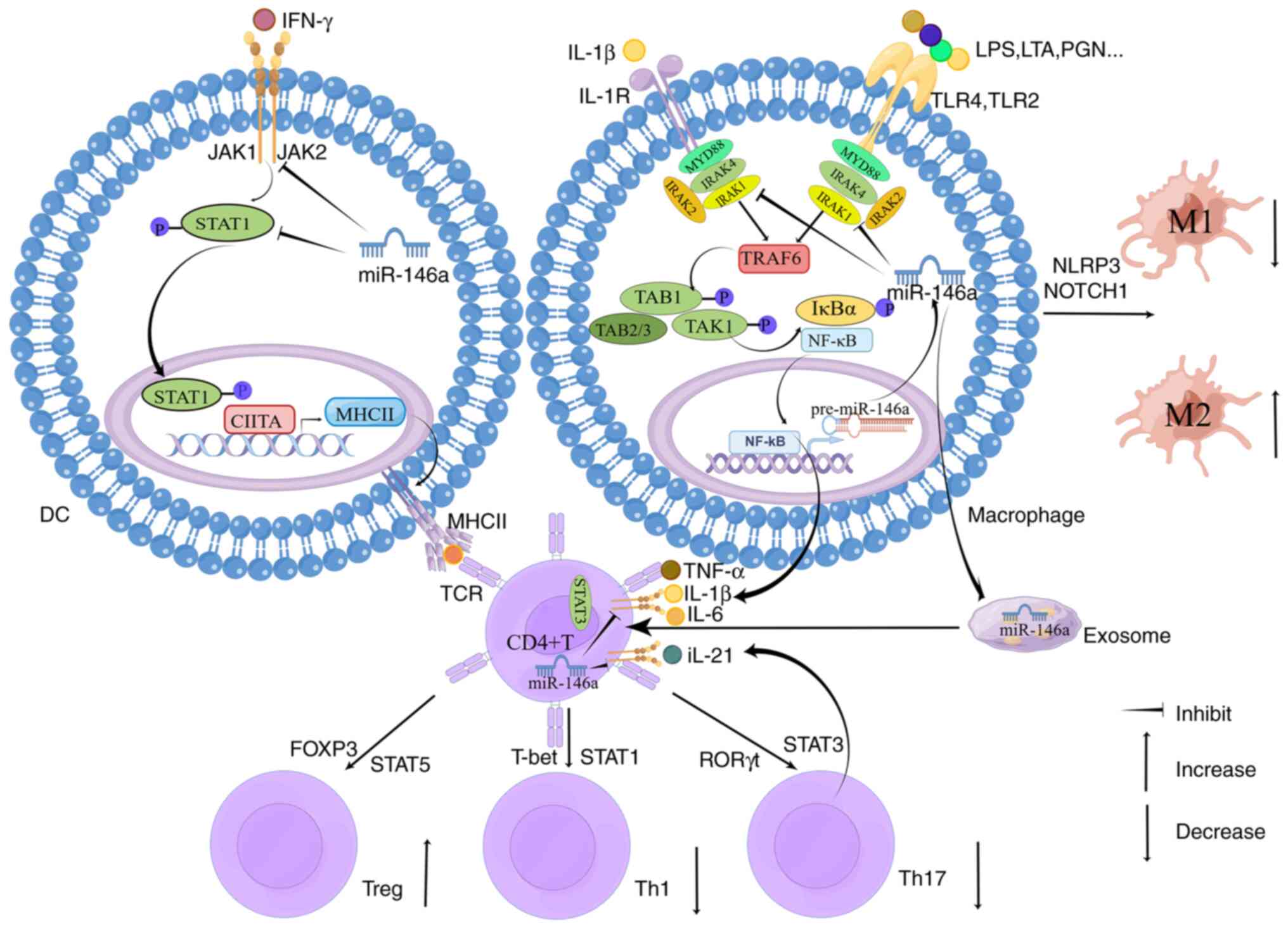
The IL-1R/TLRs-NF-κB signaling pathway functions
mainly by targeting IRAK1 and TRAF6. Numerous studies have reported
that miR-146a reduced gene expression of IRAK1 and TRAF6 (33-36). In a study of kidney
ischemia/reperfusion injury, Li et al (37) found that miR-146a downregulated
the expression of IRAK1 through binding to its 3′UTR and ultimately
inactivated NF-κB, inhibiting the infiltration of inflammatory
cells and protecting kidney functions. Another study reported that
miR-146a targeted the 3′UTR of TRAF6 and induced LPS-TLR4-NF-κB
pathway inhibition, reducing inflammatory mediators such as IL6 and
TNF-α (38). Zhang et al
(39) reported that the 3′UTR of
TRAF6 and IRAK1 had binding domains for miR-146a. The release of
inflammatory cytokines was decreased by the upregulation of
miR-146a, blunting IL-1R1/TLR4 (40). When TLR2 of THP-1 cells was
stimulated by bacterial lipoprotein, Quinn et al (41) found that overexpression of
miR-146a reduced TNF-α expression. When they used LPS to stimulate
TLR4 of the human hepatic stellate cell (HSC) line LX2, Chen et
al (42) found that
inflammatory responses and fibrogenesis were inhibited by the
overexpression of miR-146a. During inflammation, with the
overactivation of the NF-κB pathway, the expression and activity of
miR-146a in immune cells led to the downregulation of IRAK1 and
TRAF6 genes, finally decreasing the expression of NF-κB
transcription factors, particularly inflammatory cytokines. In
mice, acute and chronic inflammatory autoimmune responses were
overactive in miR-146a-deficient T cells (43). Certain studies reported worsening
inflammation in several diseases associated with deficiency of
miR-146a (44-46). miR-146a treatment of experimental
autoimmune anterior uveitis resulted in significant variations in
cytokine production. Pro-inflammatory cytokines were inhibited,
such as IL-1β, IL-6 and IFN-γ, while anti-inflammatory cytokines
such as IL-10 increased (47). Lv
et al (48) found that
overexpression of miR-146a inhibited TRAF6/NF-κB activation in
LPS-induced nucleus pulposus cells. Furthermore, when miR-146a was
deficient in injured mice, inflammation at the wound site
deteriorated due to dysregulation of pro-inflammatory cytokines,
IRAK1 and TRAF6 (49). These
findings suggest that miR-146a attenuates inflammatory responses by
targeting the upstream IRAK1 and TRAF6 of NF-κB, the center of the
IL-1R/TLRs signaling pathway.
STAT3 is involved in retinal endothelial
inflammation in type 1 diabetes and high glucose-induced
endoplasmic reticulum stress (52). Elevated STAT3 phosphorylation led
to increased production of inflammatory cytokines from macrophages
(53). Ocular JAK/STAT3 signaling
is activated by IL-6 (54-56),
while miR-146a inhibits IL-6. Ye and Steinle (57) reported that miR-146a inhibited
inflammation and apoptosis as a potential molecular treatment in
the diabetic retina by participating in inhibiting the IL-6-related
JAK/STAT3 pathway. Furthermore, miR-146a was reported to inhibit
the pro-inflammatory function of STAT3 by targeting
homeodomain-interacting protein kinases 3 (58). Downregulation of miR-146a
increased the levels of phosphorylated STAT3 protein (59), consistent with the result that
STAT3 had significantly higher activity in miR-146a-deficient mice
(60). A western blot study by
Sun et al (61) also
demonstrated that miRNA-146a-5p inhibited the activation of STAT3.
miR-146a is also involved in inflammation-related Treg/Th17
differentiation by targeting STAT1/STAT3, which will be introduced
in the next section.
To be consistent regarding Th1 and Th2
differentiation paradigms for T cells, macrophage differentiation
involved in the production of various phenotypes was divided into
canonical M1 polarization and alternative M2 polarization. M1
macrophages are predominantly pro-inflammatory (62,63). The enhanced expressions of
cell-surface activation factors, such as major histocompatibility
complex class II (MHCII), CD80 and CD86, and the production of
pro-inflammatory factors are the main characteristics involved in
antigen presentation functions for M1 activation. M2 macrophages
have numerous functions, including inhibiting inflammation and
promoting tissue repair (64-67). Peng et al (68) demonstrated miR-146a-induced M2
polarization through TLR4/NF-κB to attenuate inflammation and
repair wounds in mice with diabetic ulcers. miR-146a has also been
found to be involved in inhibiting M1 macrophage polarization
(69,70). Huang et al (70) demonstrated that the overexpression
of miR-146a in M1 macrophages significantly reduced the expression
of pro-inflammatory cytokines; on the contrary, miR-146a inhibition
promoted the polarization of M1 macrophages. miR-146a inhibits M1
pro-inflammatory responses and miR-146a-deficient diabetic mice
exhibited increased M1 and weakened M2 responses, aggravating renal
injury (45). Overexpression of
miR-146a improved LPS-induced inflammatory responses in macrophages
of older mice (71). Macrophage
maturation was downregulated by miR-146a through decreased
expression of CD80 and CD86, attenuating inflammation (72). miR-146a inhibited M1 polarization
and induced M2 polarization by regulating NLRP3 (73) and NOTCH1 (74). The inflammatory inhibition of
miR-146a by regulating macrophage polarization was also identified
in other studies. By sponging miR-146a, long non-coding RNAs
(lncRNAs) such as lncRNA CHRF and lncRNA HCG18 induced M1
polarization and inflammatory responses (75,76).
DC maturation is characterized by the upregulation
of the surface antigens CD80 and CD86 (83). It has been reported that miR-146a
mimics contributed to immature DC, inducing Treg differentiation
and reducing the expression of CD80 and CD86 in the DC surface via
the Notch-1 signal pathway. By contrast, miR-146a inhibition had
the opposite effect (84).
miR-146a appears to be involved in DC tolerogenic properties based
on these findings. A study reported that miR-146a overexpression
inhibited DC maturation and antigen presentation (85). Through targeting the JAK/STAT1
signaling pathway, miR-146a was indicated to downregulate the
expression of MHCII and activation of DCs and decrease the
production of inflammatory factors (86). These findings are consistent with
another study (87). miR-146a
expression in human plasmacytoid DCs reduced their ability to
mediate the proliferation of CD4+ T-cell proliferation by
downregulating costimulatory molecules and MHCII on its surface
(88). Park et al
(89) demonstrated that miR-146a
overexpression in mature DCs decreased cytokine production and
enhanced DC apoptosis by targeting IRAK1 and TRAF6. These studies
indicate that upregulation of miR-146a suppresses the activation,
maturation and antigen presentation function of DCs via several
signaling pathways, and miR-146a may mediate Treg differentiation
via DCs.
Approximately 45-50% of deaths may be ascribed to
fibrosis in developed countries (1,93).
Excessive extracellular matrix (ECM) production is a feature of
fibrosis and excessive matrix shrinkage in various organs (94). Fibrosis is primarily caused by
chronic inflammation mediated by several stimuli (1). In fibrosis models, epithelial cells
trans-differentiate into myofibroblasts via EMT activated by
chronic inflammation (95).
Myofibroblasts produce ECM in the progression of physiological
tissue repair and organ fibrosis. Myofibroblasts are derived from
endothelial cells, epithelial cells, fixed fibroblasts, smooth
muscle cells, pericytes, bone marrow-derived progenitor cell lines
and bone marrow-derived fibroblasts (96). The deposition of connective tissue
components leads to increased stiffness in the affected tissue and
impairs the diffusion of oxygen and nutrients, further inducing
sustained myofibroblast activation and cell damage (97-99). Epigenetic modifications induced by
profibrotic environments shield myofibroblasts from external
stimuli, forming a malignant expansion loop (100,101). Due to the profibrotic effect of
excessive fibrosis, TGF-β and other signaling pathways trigger
further fibrosis, culminating in organ failure and dysfunction
(102).
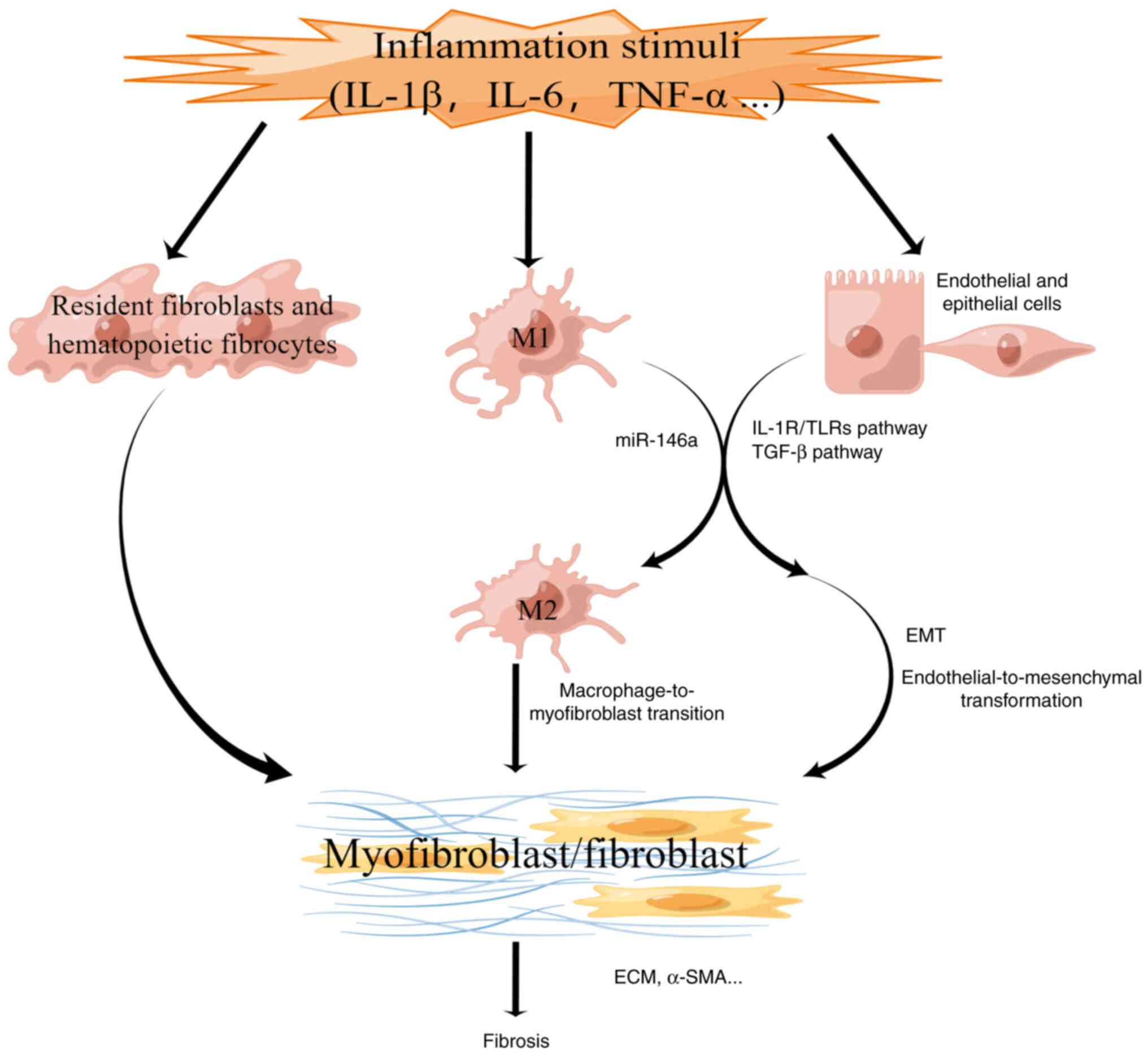
These studies indicate that miR-146a regulates the
fibrosis process inside cells through signaling pathways, which are
classified into two types in the present review (Fig. 4). In addition, it is also
necessary to explore the functions of miR-146a at the level of
fibrosis-related cells in the future.
The TGF-β signaling pathway regulates adaptive and
innate immunity, fibrosis and inflammation (115). TGF-β switching accelerated the
development or progression of fibrosis in chronic inflammatory
autoimmune conditions (116,117). The activation of fibrotic
factors produced by inflammatory and epithelial cells was observed
during the progression of chronic autoimmune diseases. Organ
fibrosis is characterized by sustained fibroblast proliferation and
inflammation (118). TGF-β1
treatment of HSCs led to elevated expression of α-SMA and Col-1 and
decreased expression of miR-146a-5p (119). Overexpression of miR-146a
reduced the levels of α-SMA and Col-1 in TGF-β-treated HSCs
(110).
The primary signaling pathway mediated by TGF-β
involves the phosphorylation of Smad2/3 protein to form
receptor-mediated Smad/co-regulated Smad4 complex (120). This Smad complex accumulates in
the nucleus and triggers the transcription of target genes (e.g.,
SNAIL) by activating transcription factors (121) and mediating signaling events
associated with EMT activation. SNAIL is involved in the
downregulation of E-cadherin and claudins, upregulating vimentin
(VIM) and fibronectin (122).
The induction of EMT markers and SNAIL activation led to
upregulation of the ECM markers Col-1 and VIM and the suppression
of the epithelial marker E-cadherin. Several studies indicated that
Smad4 is the target gene of miR-146a (119,123,124). After miR-146a was transferred,
the expression of α-SMA, VIM and Col-1 in injured mice was
significantly reduced; however, after simultaneous treatment with
Smad4 and miR-146a, the expression of these fibrosis markers was
significantly enhanced, suggesting that miR-146 ameliorates
skeletal muscle fibrosis by downregulating Smad4 (111). The inflammatory
microenvironment, including IL-17, IL-22, IL-6 and other
pro-inflammatory cytokines, may induce EMT through TGF-β/Smad or
non-Smad signaling pathways (125). miR-146a may be crucial in the
process of EMT-related fibrosis mediated by TGF-β.
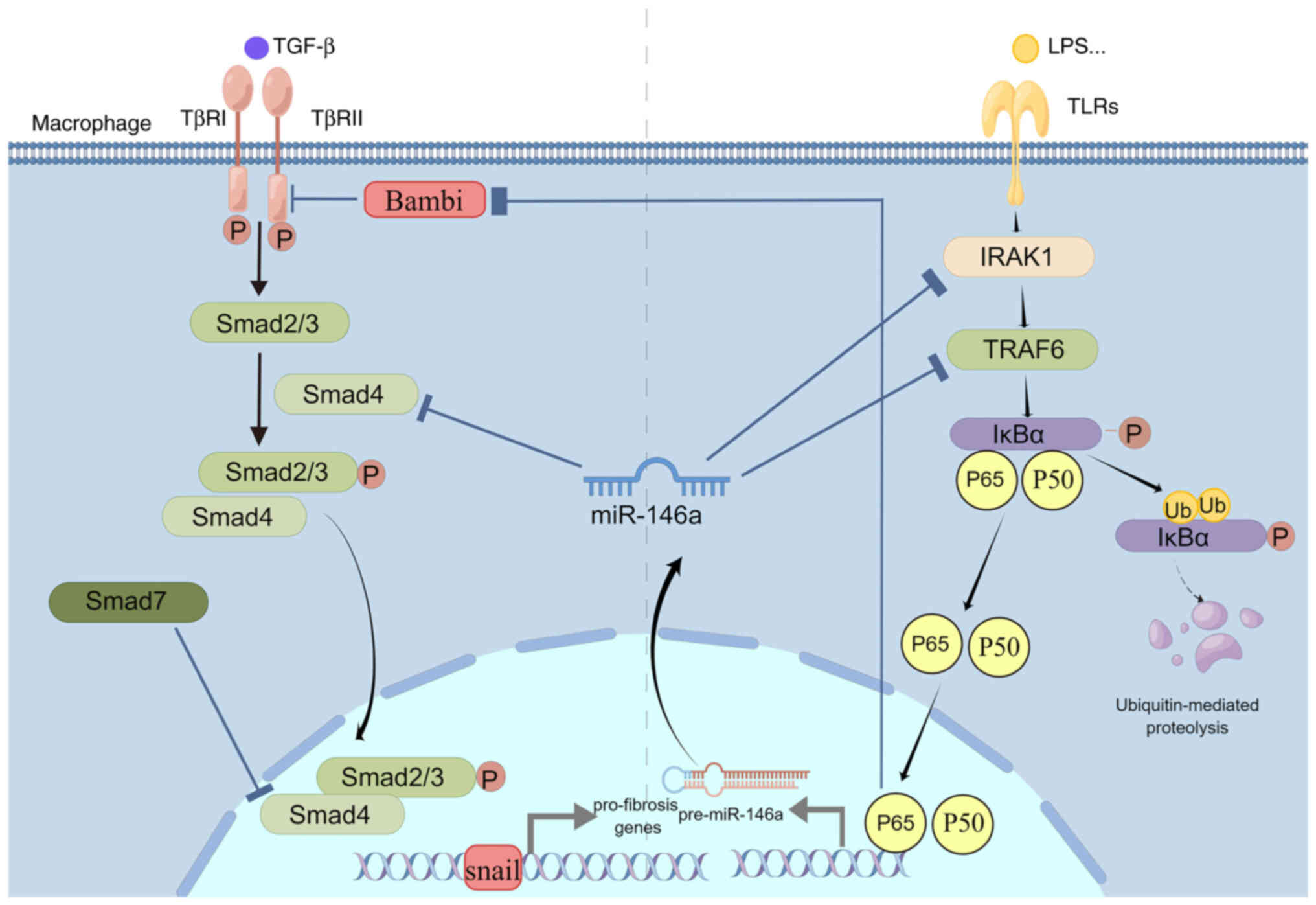
In quiescent HSCs, TGF-β1 signaling was suppressed
by Bambi, an endogenous decoy receptor for TGF-β (126). In addition to serving as bait,
Bambi directly interfered with TGF-β1 signaling by targeting Smad7
(127). Jiang et al
(128) found that Enhancer of
zeste homolog 2 (EZH2) inhibition led to transcriptional block of
the TGF-β1 pathway, the cell cycle pathway and numerous ECM
components in primary HSCs. EZH2 inhibition reduced the recruitment
of H3K27me3 to target genes encoding Bambi and increased Bambi
expression. Ni et al (19)
observed that LPS upregulated H3K27me3 and EZH2 inhibitors
increased miR-146a expression by regulating H3K27me3 methylation.
This finding suggests that suppressing the recruitment of H3K27me3
around the promoter may increase the expression of miR-146a and
Bambi. Under profibrotic stimulation, Bambi expression was
downregulated, activating the TGF-β1 pathway of hematopoietic stem
cells and activating hematopoietic stem cells (129). LPS is an essential factor in the
downregulation of Bambi during cirrhosis progression (130). Bambi is a functional inhibitor
of the TGF-β receptor that is downregulated by NF-κB. With the
progression of cirrhosis, bacterial translocation and LPS levels
increased (131). LPS binds to
TLR4 and recruits TRAF6, IRAK1 and TGF-β-activated kinase 1,
inducing phosphorylation, ubiquitination and degradation of IκBα.
Subsequently, NF-κB dissociates from IκBα and enters the nucleus,
inducing downregulation of Bambi, which elevates the response of
HSCs to TGF-β1 stimulation (132,133). A gene expression array of total
RNA from miR-146a-overexpressed HSC-2 cells and control cells
indicated that Bambi mRNA was upregulated in clones overexpressing
miR-146a-5p (134). Zou et
al (107) found that Bambi
protein was highly expressed after mi-146a-5p overexpression in
TGF-β1-stimulated cells pretreated by LPS.
During fibrosis, miR-146a suppresses the activation
of NF-κB by targeting its upstream genes IRAK1 and TRAF6. The
expression of Bambi then increases due to the inactivated NF-κB and
induces the inhibition of the TGF-β/SAMD signaling pathway. While
miR-146a regulates fibrosis, H3K27me3-mediated epigenetic
regulation has a considerable role. The effect of miR-146a on
fibrosis is summarized in Table
I. These findings indicate that miR-146a regulates EMT and
fibrotic factors such as α-SMA, COL-1 and COL-4 to exert its
antifibrotic functions via these signaling pathways (Fig. 5).
Macrophages derived from monocytes produce numerous
factors, which modulate fibrosis and tissue repair. TGF-β derived
from macrophages induces fibroblast migration and promotes
fibroblast growth, activation and collagen synthesis (146,147). Due to the essential role of
macrophages in regulating fibrosis, researchers adopted several
approaches to transfer, reduce and restrain macrophage migration
into tissues (148,149). Bhatt et al (45) found that the expression of M1
polarization antigens was enhanced, while the expression of M2
polarization antigens was inhibited in miR-146a-deficient
macrophages, inducing the infiltration of macrophages and kidney
fibrosis. Another study reported that delivery of miR-146a using
polyethyleneimine nanoparticles inhibited macrophage infiltration
and renal fibrosis in vivo (105). Another study found that miR-146a
attenuated fibrosis in hepatic schistosomiasis by regulating
macrophage differentiation into M2 cells (51). The differentiation of M1
macrophages to M2 macrophages reduced the stimulation of
inflammation to fibrosis and promoted matrix remodeling and injury
healing (3). There is substantial
opportunity for studies on miR-146a regulating macrophages in
fibrosis.
miRNAs are crucial regulators of gene expression,
suggesting that they have the potential to serve as biomarkers for
diagnosis and prediction of prognosis, as well as treatment targets
for diseases (150).
Due to its significant relationship with
inflammation and fibrosis, numerous scientists suggest that
miR-146a may be essential for diagnosing related diseases.
Shumnalieva et al (151)
found that miR-146a was overexpressed in peripheral blood compared
with a healthy control group. miR-146a was also downregulated in
cases with lupus nephritis compared to negative controls, and based
on its expression levels, it was possible to identify lupus
nephritis and assess its activity (152). Abou-Zeid et al (153) found that miR-146a levels were
elevated in cases with rheumatoid arthritis and may act as a
potentially effective marker of disease activity. Li et al
(154) reported that miR-146a
regulated the inflammatory response during Helicobacter
pylori infection via regulating the NF-κB pathway. miR-146a and
IL-17A expression were positively correlated in H.
pylori-infected human gastric mucosa (154). As inflammation has a crucial
role in the pathogenesis of acute coronary syndrome, serum exome
miR-146a levels were significantly higher in patients than in
normal coronary arteries (155).
Yang et al (156)
reported that miR-146a inhibited the accumulation of low-density
lipoprotein cholesterol and inflammatory responses by targeting
TLR-4 signaling. They also demonstrated that expression levels of
miR-146a increased initially and then decreased in atherosclerotic
endothelial cells (156). In
most fibrosis models, miR-146a expression was downregulated, while
certain studies indicated that expression may be upregulated in the
early stages of fibrosis (142).
These findings suggest that increased miR-146a expression may be a
protective factor in early disease stages.
The primary challenge for delivering miRNAs is their
limited ability to penetrate cell membranes due to their negative
charge and rapid degradation in vivo. Effective
internalization of therapeutic agents into target cells without
cytotoxic or immunogenic effects is critical for obtaining the
desired therapeutic effect, particularly when the aim is to target
unwanted inflammation. To date, several substances have been used
to develop delivery systems for miR-146a and its mimics, including
viral vectors such as adenovirus (162) and nano-carriers consisting of
polyethylene glycol-poly (lactic acid) nanoparticles (163), cerium oxide nanoparticles
(164) and polyethyleneimine
nanoparticles (105). To promote
absorption, the nanoparticles are frequently modified with lectins
or penetrating peptides (165)
or doped with chitosan (166).
Although viral vectors possess good stability and transfection
efficiency, they may induce adverse inflammatory responses.
Therefore, nano-carriers appear to be the primary focus. Studies
indicated anti-fibrotic effects after delivering extracellular
vesicles containing miR-146a (37,143,167,168). Studies on the delivery of
miR-146a to treat inflammation and fibrosis are summarized in
Table II. Their treatment
effects appear to be dose-dependent.
The present study focused on the functions of
miR-146a in inflammation and fibrosis. The former may involve the
IL-1R/TLRs-NF-κB axis and the JAK-STAT signaling pathway. By
regulating microglial M1 and M2 polarization, miR-146a demonstrated
critical roles in inflammation and fibrosis. The effects of
miR-146a on fibrosis development were discussed in the present
study. However, substantial knowledge gaps remain. There was
frequent disagreement among the results obtained by different
studies, possibly due to preanalytical factors such as acquiring
samples, storage and small sample sizes (179). For instance, studies reported
that they controlled inflammatory responses via the inhibition of
miR-146a expression (173,180), while for inflammation therapy,
miR-146a mimics were delivered in vivo to upregulate
miR-146a levels (176). These
principles appear mutually contradictory. In addition, M1
macrophages have a pro-inflammatory effect, while M2 tends to
confine inflammation and remodel the matrix or promote fibrosis
when inflammation cannot be resolved. Therefore, miR-146a
inhibiting M1 polarization of macrophages and promoting M2
polarization may lead to increased fibrosis, while simultaneously
serving anti-inflammatory functions, which appears contradictory. A
reasonable explanation is that miR-146a promotes a proportional
balance between them (51). It
should be noted that off-target effects complicate the therapeutic
journey, as a single miRNA possesses various targets controlled by
cellular substances and environments.
Furthermore, exogenous miRNAs may alter the balance
of miRISC-endogenous miRNAs by interacting with miRISC
unnecessarily. Treatments based on oligonucleotides, such as miRNA
mimics and miRNA inhibitors, should be specific to certain cell
types. Synthetic double-stranded miRNAs, which regulate the
expression of endogenous miRNA, must be constituted in a manner
that allows them to be freely adsorbed by cells, as well as keeping
their effectiveness for the intended time (181). A system that is able to deliver
the exogenous miRNAs in a standardized and efficient manner should
be established. Furthermore, it is necessary to determine mRNA
target-specific activity based on a profound comprehension of
cellular complexity.
A substantial body of evidence demonstrated that
various miRNAs, particularly miR-146a, are essential regulators of
inflammation and fibrosis, focusing on the potential of miR-146a as
a biomarker and target with a clinical application value. A deeper
understanding of the miR-146a-related signaling pathway and its
functions will facilitate the early diagnosis and treatment of
disease.
Not applicable.
ZFL and GFS made all contributions to the design
and conception for the study; RJZ performed the literature search;
ZFL and GFS wrote and revised the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Natural Science Foundation of
Ningbo Municipality (grant nos. 202003N4269 and 2019C50069), the
grants of basic public welfare projects in Zhejiang province (grant
no. LGF19H020004), Zhejiang Province Medical and Health Project
(grant nos. 2017ZD026 and 2020KY273), Ningbo Health Branding
Subject Fund (grant no. PPXK2018-01) and Ningbo 'Technology
Innovation 2025' Major Special Project (grant no. 2022Z150).
|
1
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
2
|
Mack M: Inflammation and fibrosis. Matrix
Biol. 68-69:106–121. 2018. View Article : Google Scholar
|
|
3
|
Tang PM, Nikolic-Paterson DJ and Lan HY:
Macrophages: Versatile players in renal inflammation and fibrosis.
Nat Rev Nephrol. 15:144–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleaveland KR, Moore BB and Kim KK:
Paracrine functions of fibrocytes to promote lung fibrosis. Expert
Rev Respir Med. 8:163–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gregory RI, Chendrimada TP, Cooch N and
Shiekhattar R: Human RISC couples microRNA biogenesis and
posttranscriptional gene silencing. Cell. 123:631–640. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011. View Article : Google Scholar
|
|
7
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu LF, Boldin MP, Chaudhry A, Lin LL,
Taganov KD, Hanada T, Yoshimura A, Baltimore D and Rudensky AY:
Function of miR-146a in controlling Treg cell-mediated regulation
of Th1 responses. Cell. 142:914–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Zhang X, Ju Y, Zhao B, Yan X, Hu
J, Shi L, Yang L, Ma Z, Chen L, et al: MicroRNA-146a feedback
suppresses T cell immune function by targeting Stat1 in patients
with chronic hepatitis B. J Immunol. 191:293–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boldin MP, Taganov KD, Rao DS, Yang L,
Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J,
et al: miR-146a is a significant brake on autoimmunity,
myeloproliferation, and cancer in mice. J Exp Med. 208:1189–1201.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao JL, Rao DS, Boldin MP, Taganov KD,
O'Connell RM and Baltimore D: NF-kappaB dysregulation in
microRNA-146a-deficient mice drives the development of myeloid
malignancies. Proc Natl Acad Sci USA. 108:9184–9189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho BC, Yu IS, Lu LF, Rudensky A, Chen HY,
Tsai CW, Chang YL, Wu CT, Chang LY, Shih SR, et al: Inhibition of
miR-146a prevents enterovirus-induced death by restoring the
production of type I interferon. Nat Commun. 5:33442014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alexander M, Hu R, Runtsch MC, Kagele DA,
Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM and
O'Connell RM: Exosome-delivered microRNAs modulate the inflammatory
response to endotoxin. Nat Commun. 6:73212015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paterson MR and Kriegel AJ: MiR-146a/b: A
family with shared seeds and different roots. Physiol Genomics.
49:243–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curtale G, Mirolo M, Renzi TA, Rossato M,
Bazzoni F and Locati M: Negative regulation of Toll-like receptor 4
signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA.
110:11499–11504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kutty RK, Nagineni CN, Samuel W,
Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK,
Hooks JJ and Redmond TM: Differential regulation of microRNA-146a
and microRNA-146b-5p in human retinal pigment epithelial cells by
interleukin-1β, tumor necrosis factor-α, and interferon-γ. Mol Vis.
19:737–750. 2013.
|
|
17
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni S, Yang B, Xia L and Zhang H: EZH2
mediates miR-146a-5p/HIF-1 α to alleviate inflammation and
glycolysis after acute spinal cord injury. Mediators Inflamm.
2021:55915822021. View Article : Google Scholar
|
|
20
|
Damodaran M, Paul SFD and Venkatesan V:
Genetic polymorphisms in miR-146a, miR-196a2 and miR-125a genes and
its association in prostate cancer. Pathol Oncol Res. 26:193–200.
2020. View Article : Google Scholar
|
|
21
|
Chae YS, Kim JG, Lee SJ, Kang BW, Lee YJ,
Park JY, Jeon HS, Park JS and Choi GS: A miR-146a polymorphism
(rs2910164) predicts risk of and survival from colorectal cancer.
Anticancer Res. 33:3233–3239. 2013.PubMed/NCBI
|
|
22
|
Luo X, Yang W, Ye DQ, Cui H, Zhang Y,
Hirankarn N, Qian X, Tang Y, Lau YL, de Vries N, et al: A
functional variant in microRNA-146a promoter modulates its
expression and confers disease risk for systemic lupus
erythematosus. PLoS Genet. 7:e10021282011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui L, Tao H, Wang Y, Liu Z, Xu Z, Zhou H,
Cai Y, Yao L, Chen B, Liang W, et al: A functional polymorphism of
the microRNA-146a gene is associated with susceptibility to
drug-resistant epilepsy and seizures frequency. Seizure. 27:60–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang JY and Lee JO: Structural biology of
the Toll-like receptor family. Annu Rev Biochem. 80:917–941. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rowe DC, McGettrick AF, Latz E, Monks BG,
Gay NJ, Yamamoto M, Akira S, O'Neill LA, Fitzgerald KA and
Golenbock DT: The myristoylation of TRIF-related adaptor molecule
is essential for Toll-like receptor 4 signal transduction. Proc
Natl Acad Sci USA. 103:6299–6304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanimura N, Saitoh S, Matsumoto F,
Akashi-Takamura S and Miyake K: Roles for LPS-dependent interaction
and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys
Res Commun. 368:94–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Nardo D: Toll-like receptors:
Activation, signalling and transcriptional modulation. Cytokine.
74:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LF and Greene WC: Shaping the nuclear
action of NF-kappaB. Nat Rev Mol Cell Biol. 5:392–401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoffmann A, Natoli G and Ghosh G:
Transcriptional regulation via the NF-kappaB signaling module.
Oncogene. 25:6706–6716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo JL, Kamata H and Karin M:
IKK/NF-kappaB signaling: Balancing life and death-a new approach to
cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
type I IFN production in macrophages by targeting TRAF6, IRAK1, and
IRAK2. J Immunol. 183:2150–2158. 2006. View Article : Google Scholar
|
|
34
|
He L, Wang Z, Zhou R, Xiong W, Yang Y,
Song N and Qian J: Dexmedetomidine exerts cardioprotective effect
through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the
NF-κB pathway. Biomed Pharmacother. 133:1109932021. View Article : Google Scholar
|
|
35
|
Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li
F, Han J, Sun H, Ouyang Q, Hua S, et al: Human umbilical cord
mesenchymal stem cell-derived exosomal miR-146a-5p reduces
microglial-mediated neuroinflammation via suppression of the
IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging (Albany
NY). 13:3060–3079. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hou J, Deng Q, Deng X, Zhong W, Liu S and
Zhong Z: MicroRNA-146a-5p alleviates lipopolysaccharide-induced
NLRP3 inflammasome injury and pro-inflammatory cytokine production
via the regulation of TRAF6 and IRAK1 in human umbilical vein
endothelial cells (HUVECs). Ann Transl Med. 9:14332021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su
Q, Huang H, Wei Y, Gao Y, et al: Human urine-derived stem cells
protect against renal ischemia/reperfusion injury in a rat model
via exosomal miR-146a-5p which targets IRAK1. Theranostics.
10:9561–9578. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XP, Luoreng ZM, Zan LS, Li F and Li
N: Bovine miR-146a regulates inflammatory cytokines of bovine
mammary epithelial cells via targeting the TRAF6 gene. J Dairy Sci.
100:7648–7658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Guo Y, Xu X, Tang T, Sun L, Wang
H, Zhou W, Fang L, Li Q and Xie P: miR-146a promotes Borna disease
virus 1 replication through IRAK1/TRAF6/NF-κB signaling pathway.
Virus Res. 271:1976712019. View Article : Google Scholar
|
|
40
|
Iori V, Iyer AM, Ravizza T, Beltrame L,
Paracchini L, Marchini S, Cerovic M, Hill C, Ferrari M, Zucchetti
M, et al: Blockade of the IL-1R1/TLR4 pathway mediates
disease-modification therapeutic effects in a model of acquired
epilepsy. Neurobiol Dis. 99:12–23. 2017. View Article : Google Scholar
|
|
41
|
Quinn EM, Wang JH, O'Callaghan G and
Redmond HP: MicroRNA-146a is upregulated by and negatively
regulates TLR2 signaling. PLoS One. 8:e622322013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Wu Z, Yuan B, Dong Y, Zhang L and
Zeng Z: MicroRNA-146a-5p attenuates irradiation-induced and
LPS-induced hepatic stellate cell activation and hepatocyte
apoptosis through inhibition of TLR4 pathway. Cell Death Dis.
9:222018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang L, Boldin MP, Yu Y, Liu CS, Ea CK,
Ramakrishnan P, Taganov KD, Zhao JL and Baltimore D: miR-146a
controls the resolution of T cell responses in mice. J Exp Med.
209:1655–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lochhead RB, Ma Y, Zachary JF, Baltimore
D, Zhao JL, Weis JH, O'Connell RM and Weis JJ: MicroRNA-146a
provides feedback regulation of lyme arthritis but not carditis
during infection with Borrelia burgdorferi. PLoS Pathog.
10:e10042122014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhatt K, Lanting LL, Jia Y, Yadav S, Reddy
MA, Magilnick N, Boldin M and Natarajan R: Anti-inflammatory role
of MicroRNA-146a in the pathogenesis of diabetic nephropathy. J Am
Soc Nephrol. 27:2277–2288. 2016. View Article : Google Scholar :
|
|
46
|
Ammari M, Presumey J, Ponsolles C,
Roussignol G, Roubert C, Escriou V, Toupet K, Mausset-Bonnefont AL,
Cren M, Robin M, et al: Delivery of miR-146a to Ly6Chigh
monocytes inhibits pathogenic bone erosion in inflammatory
arthritis. Theranostics. 8:5972–5985. 2018. View Article : Google Scholar
|
|
47
|
Hsu YR, Chang SW, Lin YC and Yang CH:
MicroRNA-146a alleviates experimental autoimmune anterior uveitis
in the eyes of lewis rats. Mediators Inflamm. 2017:96013492017.
View Article : Google Scholar
|
|
48
|
Lv F, Huang Y, Lv W, Yang L, Li F, Fan J
and Sun J: MicroRNA-146a ameliorates inflammation via TRAF6/NF-κB
pathway in intervertebral disc cells. Med Sci Monit. 23:659–664.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bi X, Zhou L, Liu Y, Gu J and Mi QS:
MicroRNA-146a deficiency delays wound healing in normal and
diabetic mice. Adv Wound Care (New Rochelle). 11:19–27. 2022.
View Article : Google Scholar
|
|
50
|
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y,
Huang X, Zhou H, de Vries N, Tak PP, et al: MicroRNA-146A
contributes to abnormal activation of the type I interferon pathway
in human lupus by targeting the key signaling proteins. Arthritis
Rheum. 60:1065–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He X, Tang R, Sun Y, Wang YG, Zhen KY,
Zhang DM and Pan WQ: MicroR-146 blocks the activation of M1
macrophage by targeting signal transducer and activator of
transcription 1 in hepatic schistosomiasis. EBioMedicine.
13:339–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen Y, Wang JJ, Li J, Hosoya KI, Ratan R,
Townes T and Zhang SX: Activating transcription factor 4 mediates
hyperglycaemia-induced endothelial inflammation and retinal
vascular leakage through activation of STAT3 in a mouse model of
type 1 diabetes. Diabetologia. 55:2533–2545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shirai T, Nazarewicz RR, Wallis BB, Yanes
RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC,
Assimes TL, et al: The glycolytic enzyme PKM2 bridges metabolic and
inflammatory dysfunction in coronary artery disease. J Exp Med.
213:337–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Elsaeidi F, Bemben MA, Zhao XF and Goldman
D: Jak/Stat signaling stimulates zebrafish optic nerve regeneration
and overcomes the inhibitory actions of Socs3 and Sfpq. J Neurosci.
34:2632–2644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fasler-Kan E, Barteneva NS, Ketterer S,
Wunderlich K, Reschner A, Nurzhanova A, Flammer J, Huwyler J and
Meyer P: Human cytokines activate JAK-STAT signaling pathway in
porcine ocular tissue. Xenotransplantation. 20:469–480. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Samardzija M, Wenzel A, Aufenberg S,
Thiersch M, Remé C and Grimm C: Differential role of Jak-STAT
signaling in retinal degenerations. FASEB J. 20:2411–2413. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ye EA and Steinle JJ: miR-146a suppresses
STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in
primary human retinal microvascular endothelial cells in high
glucose conditions. Vision Res. 139:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo H, Zhang Y, Liao Z, Zhan W, Wang Y,
Peng Y, Yang M, Ma X, Yin G and Ye L: MiR-146a upregulates FOXP3
and suppresses inflammation by targeting HIPK3/STAT3 in allergic
conjunctivitis. Ann Transl Med. 10:3442022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li T, Li M, Xu C, Xu X, Ding J, Cheng L
and Ou R: miR-146a regulates the function of Th17 cell
differentiation to modulate cervical cancer cell growth and
apoptosis through NF-κB signaling by targeting TRAF6. Oncol Rep.
41:2897–2908. 2019.PubMed/NCBI
|
|
60
|
Ferrer-Marín F, Arroyo AB, Bellosillo B,
Cuenca EJ, Zamora L, Hernández-Rivas JM, Hernández-Boluda JC,
Fernandez-Rodriguez C, Luño E, García Hernandez C, et al: miR-146a
rs2431697 identifies myeloproliferative neoplasm patients with
higher secondary myelofibrosis progression risk. Leukemia.
34:2648–2659. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun W, Ma J, Zhao H, Xiao C, Zhong H, Ling
H, Xie Z, Tian Q, Chen H, Zhang T, et al: Resolvin D1 suppresses
pannus formation via decreasing connective tissue growth factor
caused by upregulation of miRNA-146a-5p in rheumatoid arthritis.
Arthritis Res Ther. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dai X, Mao C, Lan X, Chen H, Li M, Bai J,
Deng J, Liang Q, Zhang J, Zhong X, et al: Acute Penicillium
marneffei infection stimulates host M1/M2a macrophages polarization
in BALB/C mice. BMC Microbiol. 17:1772017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Khan J, Sharma PK and Mukhopadhaya A:
Vibrio cholerae porin OmpU mediates M1-polarization of
macrophages/monocytes via TLR1/TLR2 activation. Immunobiology.
220:1199–1209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vinuesa E, Hotter G, Jung M,
Herrero-Fresneda I, Torras J and Sola A: Macrophage involvement in
the kidney repair phase after ischaemia/reperfusion injury. J
Pathol. 214:104–113. 2008. View Article : Google Scholar
|
|
65
|
Huen SC and Cantley LG:
Macrophage-mediated injury and repair after ischemic kidney injury.
Pediatr Nephrol. 30:199–209. 2015. View Article : Google Scholar
|
|
66
|
Lee S, Huen S, Nishio H, Nishio S, Lee HK,
Choi BS, Ruhrberg C and Cantley LG: Distinct macrophage phenotypes
contribute to kidney injury and repair. J Am Soc Nephrol.
22:317–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Alikhan MA, Jones CV, Williams TM,
Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG,
Deane JA, et al: Colony-stimulating factor-1 promotes kidney growth
and repair via alteration of macrophage responses. Am J Pathol.
179:1243–1256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Peng X, He F, Mao Y, Lin Y, Fang J, Chen
Y, Sun Z, Zhuo Y and Jiang J: miR-146a promotes M2 macrophage
polarization and accelerates diabetic wound healing by inhibiting
the TLR4/NF-κB axis. J Mol Endocrinol. 69:315–327. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu XS, Fan B, Szalad A, Jia L, Wang L,
Wang X, Pan W, Zhang L, Zhang R, Hu J, et al: MicroRNA-146a mimics
reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes.
66:3111–3121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang C, Liu XJ, QunZhou, Xie J, Ma TT,
Meng XM and Li J: MiR-146a modulates macrophage polarization by
inhibiting Notch1 pathway in RAW264.7 macrophages. Int
Immunopharmacol. 32:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiang M, Xiang Y, Wang D, Gao J, Liu D,
Liu Y, Liu S and Zheng D: Dysregulated expression of miR-146a
contributes to age-related dysfunction of macrophages. Aging Cell.
11:29–40. 2012. View Article : Google Scholar
|
|
72
|
Li Z, Wang S, Zhao W, Sun Z, Yan H and Zhu
J: Oxidized low-density lipoprotein upregulates microRNA-146a via
JNK and NF-κB signaling. Mol Med Rep. 13:1709–1716. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang Y, Huang G, Xu Q, Zhao G, Jiang J, Li
Y and Guo Z: miR-146a-5p attenuates allergic airway inflammation by
inhibiting the NLRP3 inflammasome activation in macrophages. Int
Arch Allergy Immunol. 183:919–930. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen X, Su C, Wei Q, Sun H, Xie J and Nong
G: Exosomes derived from human umbilical cord mesenchymal stem
cells alleviate diffuse alveolar hemorrhage associated with
systemic lupus erythematosus in mice by promoting M2 macrophage
polarization via the microRNA-146a-5p/NOTCH1 axis. Immunol Invest.
51:1975–1993. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Luo S, Ding X, Zhao S, Mou T, Li R and Cao
X: Long non-coding RNA CHRF accelerates LPS-induced acute lung
injury through microRNA-146a/Notch1 axis. Ann Transl Med.
9:12992021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ren W, Xi G, Li X, Zhao L, Yang K, Fan X,
Gao L, Xu H and Guo J: Long non-coding RNA HCG18 promotes M1
macrophage polarization through regulating the miR-146a/TRAF6 axis,
facilitating the progression of diabetic peripheral neuropathy. Mol
Cell Biochem. 476:471–482. 2021. View Article : Google Scholar
|
|
77
|
Cobb BS, Hertweck A, Smith J, O'Connor E,
Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG and
Merkenschlager M: A role for Dicer in immune regulation. J Exp Med.
203:2519–2527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Smigielska-Czepiel K, van den Berg A,
Jellema P, van der Lei RJ, Bijzet J, Kluiver J, Boots AM, Brouwer E
and Kroesen BJ: Comprehensive analysis of miRNA expression in
T-cell subsets of rheumatoid arthritis patients reveals defined
signatures of naive and memory Tregs. Genes Immun. 15:115–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Y, Yang Y, Guo J, Cui L, Yang L, Li
Y, Mou Y, Jia C, Zhang L and Song X: miR-146a enhances regulatory
T-cell differentiation and function in allergic rhinitis by
targeting STAT5b. Allergy. 77:550–558. 2022. View Article : Google Scholar
|
|
80
|
Li B, Wang X, Choi IY, Wang YC, Liu S,
Pham AT, Moon H, Smith DJ, Rao DS, Boldin MP and Yang L: miR-146a
modulates autoreactive Th17 cell differentiation and regulates
organ-specific autoimmunity. J Clin Invest. 127:3702–3716. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Yang L, Wang L, Yang Y and Wang Y:
Forkhead box p3 controls progression of oral lichen planus by
regulating microRNA-146a. J Cell Biochem. 119:8862–8871. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang J, Zhai X, Guo J, Li Y, Yang Y, Wang
L, Yang L and Liu F: Long non-coding RNA DQ786243 modulates the
induction and function of CD4+ Treg cells through
Foxp3-miR-146a-NF-κB axis: Implications for alleviating oral lichen
planus. Int Immunopharmacol. 75:1057612019. View Article : Google Scholar
|
|
83
|
Schmidt SV, Nino-Castro AC and Schultze
JL: Regulatory dendritic cells: There is more than just immune
activation. Front Immunol. 3:2742012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tang H, Lai Y, Zheng J, Chen K, Jiang H
and Xu G: MiR-146a promotes tolerogenic properties of dendritic
cells and through targeting notch1 signaling. Immunol Invest.
49:555–570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Du J, Wang J, Tan G, Cai Z, Zhang L, Tang
B and Wang Z: Aberrant elevated microRNA-146a in dendritic cells
(DC) induced by human pancreatic cancer cell line
BxPC-3-conditioned medium inhibits DC maturation and activation.
Med Oncol. 29:2814–2823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Stickel N, Hanke K, Marschner D, Prinz G,
Köhler M, Melchinger W, Pfeifer D, Schmitt-Graeff A, Brummer T,
Heine A, et al: MicroRNA-146a reduces MHC-II expression via
targeting JAK/STAT signaling in dendritic cells after stem cell
transplantation. Leukemia. 31:2732–2741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jurkin J, Schichl YM, Koeffel R, Bauer T,
Richter S, Konradi S, Gesslbauer B and Strobl H: miR-146a is
differentially expressed by myeloid dendritic cell subsets and
desensitizes cells to TLR2-dependent activation. J Immunol.
184:4955–4965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Karrich JJ, Jachimowski LC, Libouban M,
Iyer A, Brandwijk K, Taanman-Kueter EW, Nagasawa M, de Jong EC,
Uittenbogaart CH and Blom B: MicroRNA-146a regulates survival and
maturation of human plasmacytoid dendritic cells. Blood.
122:3001–3009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar :
|
|
90
|
Xu D, Han Q, Hou Z, Zhang C and Zhang J:
miR-146a negatively regulates NK cell functions via STAT1
signaling. Cell Mol Immunol. 14:712–720. 2017. View Article : Google Scholar :
|
|
91
|
Pesce S, Squillario M, Greppi M, Loiacono
F, Moretta L, Moretta A, Sivori S, Castagnola P, Barla A, Candiani
S and Marcenaro E: New miRNA signature heralds human NK cell
subsets at different maturation steps: Involvement of miR-146a-5p
in the regulation of KIR expression. Front Immunol. 9:23602018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang H, Zhang Y, Wu X, Wang Y, Cui H, Li
X, Zhang J, Tun N, Peng Y and Yu J: Regulation of human natural
killer cell IFN-γ production by MicroRNA-146a via targeting the
NF-κB signaling pathway. Front Immunol. 9:2932018. View Article : Google Scholar
|
|
93
|
Friedman SL, Sheppard D, Duffield JS and
Violette S: Therapy for fibrotic diseases: Nearing the starting
line. Sci Transl Med. 5:167sr12013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rosenbloom J, Mendoza FA and Jimenez SA:
Strategies for anti-fibrotic therapies. Biochim Biophys Acta.
1832:1088–1103. 2013. View Article : Google Scholar
|
|
95
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
McAnulty RJ: Fibroblasts and
myofibroblasts: Their source, function and role in disease. Int J
Biochem Cell Biol. 39:666–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beyer C, Schett G, Gay S, Distler O and
Distler JHW: Hypoxia. Hypoxia in the pathogenesis of systemic
sclerosis. Arthritis Res Ther. 11:2202009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lokmic Z, Musyoka J, Hewitson TD and Darby
IA: Hypoxia and hypoxia signaling in tissue repair and fibrosis.
Int Rev Cell Mol Biol. 296:139–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Santos A and Lagares D: Matrix stiffness:
The conductor of organ fibrosis. Curr Rheumatol Rep. 20:22018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Parker MW, Rossi D, Peterson M, Smith K,
Sikström K, White ES, Connett JE, Henke CA, Larsson O and Bitterman
PB: Fibrotic extracellular matrix activates a profibrotic positive
feedback loop. J Clin Invest. 124:1622–1635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Watson CJ, Collier P, Tea I, Neary R,
Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann
A, et al: Hypoxia-induced epigenetic modifications are associated
with cardiac tissue fibrosis and the development of a
myofibroblast-like phenotype. Hum Mol Genet. 23:2176–2188. 2014.
View Article : Google Scholar
|
|
102
|
Kanzler S, Lohse AW, Keil A, Henninger J,
Dienes HP, Schirmacher P, Rose-John S, zum Büschenfelde KH and
Blessing M: TGF-beta1 in liver fibrosis: An inducible transgenic
mouse model to study liver fibrogenesis. Am J Physiol.
276:G1059–G1068. 1999.
|
|
103
|
Zhu H, Li Y, Qu S, Luo H, Zhou Y, Wang Y,
Zhao H, You Y, Xiao X and Zuo X: MicroRNA expression abnormalities
in limited cutaneous scleroderma and diffuse cutaneous scleroderma.
J Clin Immunol. 32:514–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jia C, Xiong M, Wang P, Cui J, Du X, Yang
Q, Wang W, Chen Y and Zhang T: Notoginsenoside R1 attenuates
atherosclerotic lesions in ApoE deficient mouse model. PLoS One.
9:e998492014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Morishita Y, Imai T, Yoshizawa H, Watanabe
M, Ishibashi K, Muto S and Nagata D: Delivery of microRNA-146a with
polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int
J Nanomedicine. 10:3475–3488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu Z, Lu CL, Cui LP, Hu YL, Yu Q, Jiang
Y, Ma T, Jiao DK, Wang D and Jia CY: MicroRNA-146a modulates
TGF-β1-induced phenotypic differentiation in human dermal
fibroblasts by targeting SMAD4. Arch Dermatol Res. 304:195–202.
2012. View Article : Google Scholar
|
|
107
|
Zou Y, Cai Y, Lu D, Zhou Y, Yao Q and
Zhang S: MicroRNA-146a-5p attenuates liver fibrosis by suppressing
profibrogenic effects of TGFβ1 and lipopolysaccharide. Cell Signal.
39:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Skhirtladze C, Distler O, Dees C,
Akhmetshina A, Busch N, Venalis P, Zwerina J, Spriewald B,
Pileckyte M, Schett G and Distler JH: Src kinases in systemic
sclerosis: Central roles in fibroblast activation and in skin
fibrosis. Arthritis Rheum. 58:1475–1484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hu M, Che P, Han X, Cai GQ, Liu G, Antony
V, Luckhardt T, Siegal GP, Zhou Y, Liu RM, et al: Therapeutic
targeting of SRC kinase in myofibroblast differentiation and
pulmonary fibrosis. J Pharmacol Exp Ther. 351:87–95. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yuan BY, Chen YH, Wu ZF, Zhuang Y, Chen
GW, Zhang L, Zhang HG, Cheng JC, Lin Q and Zeng ZC:
MicroRNA-146a-5p attenuates fibrosis-related molecules in
irradiated and TGF-beta1-treated human hepatic stellate cells by
regulating PTPRA-SRC signaling. Radiat Res. 192:621–629. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sun Y, Li Y, Wang H, Li H, Liu S, Chen J
and Ying H: miR-146a-5p acts as a negative regulator of TGF-β
signaling in skeletal muscle after acute contusion. Acta Biochim
Biophys Sin (Shanghai). 49:628–634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu W, Ma C, Li HY, Chen L, Yuan SS and Li
KJ: MicroRNA-146a downregulates the production of hyaluronic acid
and collagen I in Graves' ophthalmopathy orbital fibroblasts. Exp
Ther Med. 20:382020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Amrouche L, Desbuissons G, Rabant M,
Sauvaget V, Nguyen C, Benon A, Barre P, Rabaté C, Lebreton X,
Gallazzini M, et al: MicroRNA-146a in human and experimental
ischemic AKI: CXCL8-dependent mechanism of action. J Am Soc
Nephrol. 28:479–493. 2017. View Article : Google Scholar :
|
|
114
|
Xiao Y, Qiao W, Wang X, Sun L and Ren W:
MiR-146a mediates TLR-4 signaling pathway to affect myocardial
fibrosis in rat constrictive pericarditis model. J Thorac Dis.
13:935–945. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yoshimura A, Wakabayashi Y and Mori T:
Cellular and molecular basis for the regulation of inflammation by
TGF-beta. J Biochem. 147:781–792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-β1 signaling pathway as an attractive
target in the fibrosis pathogenesis of Sjögren's syndrome.
Mediators Inflamm. 2018:19659352018. View Article : Google Scholar
|
|
117
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
He Y, Huang C, Sun X, Long XR, Lv XW and
Li J: MicroRNA-146a modulates TGF-beta1-induced hepatic stellate
cell proliferation by targeting SMAD4. Cell Signal. 24:1923–1930.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wrighton KH, Lin X and Feng XH:
Phospho-control of TGF-beta superfamily signaling. Cell Res.
19:8–20. 2009. View Article : Google Scholar
|
|
121
|
Hill CS: Transcriptional control by the
SMADs. Cold Spring Harb Perspect Biol. 8:a0220792016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang Q, Cai R, Tang G, Zhang W and Pang
W: MiR-146a-5p targeting SMAD4 and TRAF6 inhibits adipogenensis
through TGF-β and AKT/mTORC1 signal pathways in porcine
intra-muscular preadipocytes. J Anim Sci Biotechnol. 12:122021.
View Article : Google Scholar
|
|
124
|
Milano G, Biemmi V, Lazzarini E, Balbi C,
Ciullo A, Bolis S, Ameri P, Di Silvestre D, Mauri P, Barile L and
Vassalli G: Intravenous administration of cardiac progenitor
cell-derived exosomes protects against
doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc Res.
116:383–392. 2020.
|
|
125
|
Sisto M, Ribatti D and Lisi S: Organ
fibrosis and autoimmunity: The role of inflammation in
TGFβ-dependent EMT. Biomolecules. 11:3102021. View Article : Google Scholar
|
|
126
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning
Y and Chen YG: Human BAMBI cooperates with Smad7 to inhibit
transforming growth factor-beta signaling. J Biol Chem.
284:30097–30104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jiang Y, Xiang C, Zhong F, Zhang Y, Wang
L, Zhao Y, Wang J, Ding C, Jin L, He F and Wang H: Histone H3K27
methyltransferase EZH2 and demethylase JMJD3 regulate hepatic
stellate cells activation and liver fibrosis. Theranostics.
11:361–378. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu C, Chen X, Yang L, Kisseleva T,
Brenner DA and Seki E: Transcriptional repression of the
transforming growth factor β (TGF-β) pseudoreceptor BMP and activin
membrane-bound inhibitor (BAMBI) by nuclear factor κB (NF-κB) p50
enhances TGF-β signaling in hepatic stellate cells. J Biol Chem.
289:7082–7091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wiest R, Lawson M and Geuking M:
Pathological bacterial translocation in liver cirrhosis. J Hepatol.
60:197–209. 2014. View Article : Google Scholar
|
|
132
|
Pradere JP, Troeger JS, Dapito DH, Mencin
AA and Schwabe RF: Toll-like receptor 4 and hepatic fibrogenesis.
Semin Liver Dis. 30:232–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Maubach G, Lim MCC, Chen J, Yang H and
Zhuo L: miRNA studies in in vitro and in vivo activated hepatic
stellate cells. World J Gastroenterol. 17:2748–2773. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chen Y, Zeng Z, Shen X, Wu Z, Dong Y and
Cheng JC: MicroRNA-146a-5p negatively regulates pro-inflammatory
cytokine secretion and cell activation in lipopolysaccharide
stimulated human hepatic stellate cells through inhibition of
Toll-like receptor 4 signaling pathways. Int J Mol Sci.
17:10762016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xiao L, Gu Y, Ren G, Chen L, Liu L, Wang X
and Gao L: miRNA-146a mimic inhibits NOX4/P38 signalling to
ameliorate mouse myocardial ischaemia reperfusion (I/R) injury.
Oxid Med Cell Longev. 2021:63662542021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Li J, Jiang ZZ, Li YY, Tang WT, Yin J and
Long XP: LncRNA CHRF promotes TGF-β1 induced EMT in alveolar
epithelial cells by inhibiting miR-146a up-regulating L1CAM
expression. Exp Lung Res. 47:198–209. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Feng B, Chen S, Gordon AD and Chakrabarti
S: miR-146a mediates inflammatory changes and fibrosis in the heart
in diabetes. J Mol Cell Cardiol. 105:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chen Y, Yuan B, Chen G, Zhang L, Zhuang Y,
Niu H and Zeng Z: Circular RNA RSF1 promotes inflammatory and
fibrotic phenotypes of irradiated hepatic stellate cell by
modulating miR-146a-5p. J Cell Physiol. 235:8270–8282. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Du J, Niu X, Wang Y, Kong L, Wang R, Zhang
Y, Zhao S and Nan Y: MiR-146a-5p suppresses activation and
proliferation of hepatic stellate cells in nonalcoholic fibrosing
steatohepatitis through directly targeting Wnt1 and Wnt5a. Sci Rep.
5:161632015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang H, Wen H and Huang Y: MicroRNA-146a
attenuates isoproterenol-induced cardiac fibrosis by inhibiting
FGF2. Exp Ther Med. 24:5062022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Editorial Office: Erratum to MiR-146a
mediates TLR-4 signaling pathway to affect myocardial fibrosis in
rat constrictive pericarditis model. J Thorac Dis. 13:4623–4624.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ma C, Qi X, Wei YF, Li Z, Zhang HL, Li H,
Yu FL, Pu YN, Huang YC and Ren YX: Amelioration of ligamentum
flavum hypertrophy using umbilical cord mesenchymal stromal
cell-derived extracellular vesicles. Bioact Mater. 19:139–154.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Saferding V, Puchner A, Goncalves-Alves E,
Hofmann M, Bonelli M, Brunner JS, Sahin E, Niederreiter B, Hayer S,
Kiener HP, et al: MicroRNA-146a governs fibroblast activation and
joint pathology in arthritis. J Autoimmun. 82:74–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jang SY, Park SJ, Chae MK, Lee JH, Lee EJ
and Yoon JS: Role of microRNA-146a in regulation of fibrosis in
orbital fibroblasts from patients with Graves' orbitopathy. Br J
Ophthalmol. 102:407–414. 2018. View Article : Google Scholar
|
|
146
|
Acharya PS, Majumdar S, Jacob M, Hayden J,
Mrass P, Weninger W, Assoian RK and Puré E: Fibroblast migration is
mediated by CD44-dependent TGF beta activation. J Cell Sci.
121:1393–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Clark RA, McCoy GA, Folkvord JM and
McPherson JM: TGF-beta 1 stimulates cultured human fibroblasts to
proliferate and produce tissue-like fibroplasia: A fibronectin
matrix-dependent event. J Cell Physiol. 170:69–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Saha B, Kodys K and Szabo G: Hepatitis C
virus-induced monocyte differentiation into polarized M2
macrophages promotes stellate cell activation via TGF-β. Cell Mol
Gastroenterol Hepatol. 2:302–316.e8. 2016. View Article : Google Scholar
|
|
149
|
Tang PM, Zhou S, Li CJ, Liao J, Xiao J,
Wang QM, Lian GY, Li J, Huang XR, To KF, et al: The proto-oncogene
tyrosine protein kinase Src is essential for
macrophage-myofibroblast transition during renal scarring. Kidney
Int. 93:173–187. 2018. View Article : Google Scholar
|
|
150
|
Long H, Wang X, Chen Y, Wang L, Zhao M and
Lu Q: Dysregulation of microRNAs in autoimmune diseases:
Pathogenesis, biomarkers and potential therapeutic targets. Cancer
Lett. 428:90–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shumnalieva R, Kachakova D,
Shoumnalieva-Ivanova V, Miteva P, Kaneva R and Monov S: Whole
peripheral blood miR-146a and miR-155 expression levels in systemic
lupus erythematosus patients. Acta Reumatol Port. 43:217–225.
2018.PubMed/NCBI
|
|
152
|
Zhu Y, Xue Z and Di L: Regulation of
MiR-146a and TRAF6 in the diagnose of lupus nephritis. Med Sci
Monit. 23:2550–2557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Abou-Zeid A, Saad M and Soliman E:
MicroRNA 146a expression in rheumatoid arthritis: Association with
tumor necrosis factor-alpha and disease activity. Genet Test Mol
Biomarkers. 15:807–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li N, Wang J, Yu W, Dong K, You F, Si B,
Tang B, Zhang Y, Wang T and Qiao B: MicroRNA-146a inhibits the
inflammatory responses induced by interleukin-17A during the
infection of Helicobacter pylori. Mol Med Rep. 19:1388–1395.
2019.
|
|
155
|
Li LJ, Gu YJ, Wang LQ, Wan W, Wang HW,
Yang XN, Ma LL, Yang LH and Meng ZH: Serum exosomal microRNA-146a
as a novel diagnostic biomarker for acute coronary syndrome. J
Thorac Dis. 13:3105–3114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu
J, Sun Z and Shen WF: MiR-146a inhibits oxidized low-density
lipoprotein-induced lipid accumulation and inflammatory response
via targeting toll-like receptor 4. FEBS Lett. 585:854–860. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wu W, Xuan Y, Ge Y, Mu S, Hu C and Fan R:
Plasma miR-146a and miR-365 expression and inflammatory factors in
patients with osteoarthritis. Malays J Pathol. 43:311–317.
2021.PubMed/NCBI
|
|
158
|
Ghotloo S, Motedayyen H, Amani D, Saffari
M and Sattari M: Assessment of microRNA-146a in generalized
aggressive periodontitis and its association with disease severity.
J Periodontal Res. 54:27–32. 2019. View Article : Google Scholar
|
|
159
|
Sabbatinelli J, Giuliani A, Matacchione G,
Latini S, Laprovitera N, Pomponio G, Ferrarini A, Svegliati Baroni
S, Pavani M, Moretti M, et al: Decreased serum levels of the
inflammaging marker miR-146a are associated with clinical
non-response to tocilizumab in COVID-19 patients. Mech Ageing Dev.
193:1114132021. View Article : Google Scholar
|
|
160
|
Cai P, Mu Y, Olveda RM, Ross AG, Olveda DU
and McManus DP: Serum exosomal miRNAs for grading hepatic fibrosis
due to schistosomiasis. Int J Mol Sci. 21:35602020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cai P, Mu Y, Olveda RM, Ross AG, Olveda DU
and McManus DP: Circulating miRNAs as footprints for liver fibrosis
grading in schistosomiasis. EBioMedicine. 37:334–343. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen J, Chen T, Zhou J, Zhao X, Sheng Q
and Lv Z: MiR-146a-5p mimic inhibits NLRP3 inflammasome downstream
inflammatory factors and CLIC4 in neonatal necrotizing
enterocolitis. Front Cell Dev Biol. 8:5941432021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang Y, Zhang S and Benoit DSW: Degradable
poly(ethylene glycol) (PEG)-based hydrogels for spatiotemporal
control of siRNA/nanoparticle delivery. J Control Release.
287:58–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Niemiec SM, Hilton SA, Wallbank A,
Azeltine M, Louiselle AE, Elajaili H, Allawzi A, Xu J, Mattson C,
Dewberry LC, et al: Cerium oxide nanoparticle delivery of
microRNA-146a for local treatment of acute lung injury.
Nanomedicine. 34:1023882021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Chen B, Yoo K, Xu W, Pan R, Han XX and
Chen P: Characterization and evaluation of a peptide-based siRNA
delivery system in vitro. Drug Deliv Transl Res. 7:507–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Su Y, Sun B, Gao X, Liu S, Hao R and Han
B: Chitosan hydrogel doped with PEG-PLA nanoparticles for the local
delivery of miRNA-146a to treat allergic rhinitis. Pharmaceutics.
12:9072020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chiabotto G, Ceccotti E, Tapparo M,
Camussi G and Bruno S: Human liver stem cell-derived extracellular
vesicles target hepatic stellate cells and attenuate their
pro-fibrotic phenotype. Front Cell Dev Biol. 9:7774622021.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liang YC, Wu YP, Li XD, Chen SH, Ye XJ,
Xue XY and Xu N: TNF-α-induced exosomal miR-146a mediates
mesenchymal stem cell-dependent suppression of urethral stricture.
J Cell Physiol. 234:23243–23255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Shafei S, Khanmohammadi M, Ghanbari H,
Nooshabadi VT, Tafti SHA, Rabbani S, Kasaiyan M, Basiri M and
Tavoosidana G: Effectiveness of exosome mediated miR-126 and
miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res.
390:71–92. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Carreras-Badosa G, Maslovskaja J,
Periyasamy K, Urgard E, Padari K, Vaher H, Tserel L, Gestin M,
Kisand K, Arukuusk P, et al: NickFect type of cell-penetrating
peptides present enhanced efficiency for microRNA-146a delivery
into dendritic cells and during skin inflammation. Biomaterials.
262:1203162020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wang WX, Prajapati P, Vekaria HJ, Spry M,
Cloud AL, Sullivan PG and Springer JE: Temporal changes in
inflammatory mitochondria-enriched microRNAs following traumatic
brain injury and effects of miR-146a nanoparticle delivery. Neural
Regen Res. 16:514–522. 2021. View Article : Google Scholar :
|
|
172
|
Bobba CM, Fei Q, Shukla V, Lee H, Patel P,
Putman RK, Spitzer C, Tsai M, Wewers MD, Lee RJ, et al:
Nanoparticle delivery of microRNA-146a regulates
mechanotransduction in lung macrophages and mitigates injury during
mechanical ventilation. Nat Commun. 12:2892021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fouad MR, Salama RM, Zaki HF and El-Sahar
AE: Vildagliptin attenuates acetic acid-induced colitis in rats via
targeting PI3K/Akt/NFκB, Nrf2 and CREB signaling pathways and the
expression of lncRNA IFNG-AS1 and miR-146a. Int Immunopharmacol.
92:1073542021. View Article : Google Scholar
|
|
174
|
Pan Y, Wang J, Xue Y, Zhao J, Li D, Zhang
S, Li K, Hou Y and Fan H: GSKJ4 protects mice against early sepsis
via reducing proinflammatory factors and up-regulating MiR-146a.
Front Immunol. 9:22722018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sun W, Ma M and Yu H and Yu H: Inhibition
of lncRNA X inactivate-specific transcript ameliorates inflammatory
pain by suppressing satellite glial cell activation and
inflammation by acting as a sponge of miR-146a to inhibit
Nav 1.7. J Cell Biochem. 119:9888–9898. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhou Z, Zhu Y, Gao G and Zhang Y: Long
noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate
LPS-induced WI-38 cell apoptosis and inflammation in acute
pneumonia. Life Sci. 228:189–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zhu D, Hu B, Zhou Y, Sun X, Chen J, Chen
L, Ji Z, Zhu J and Duan Y: microRNA-146a is involved in
rSjP40-inhibited activation of LX-2 cells by targeting Smad4
expression. J Cell Biochem. 119:9249–9253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Madhavan D, Cuk K, Burwinkel B and Yang R:
Cancer diagnosis and prognosis decoded by blood-based circulating
microRNA signatures. Front Genet. 4:1162013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Li Y, Zhang S, Zhang C and Wang M: LncRNA
MEG3 inhibits the inflammatory response of ankylosing spondylitis
by targeting miR-146a. Mol Cell Biochem. 466:17–24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Stenvang J, Petri A, Lindow M, Obad S and
Kauppinen S: Inhibition of microRNA function by antimiR
oligonucleotides. Silence. 3:12012. View Article : Google Scholar : PubMed/NCBI
|