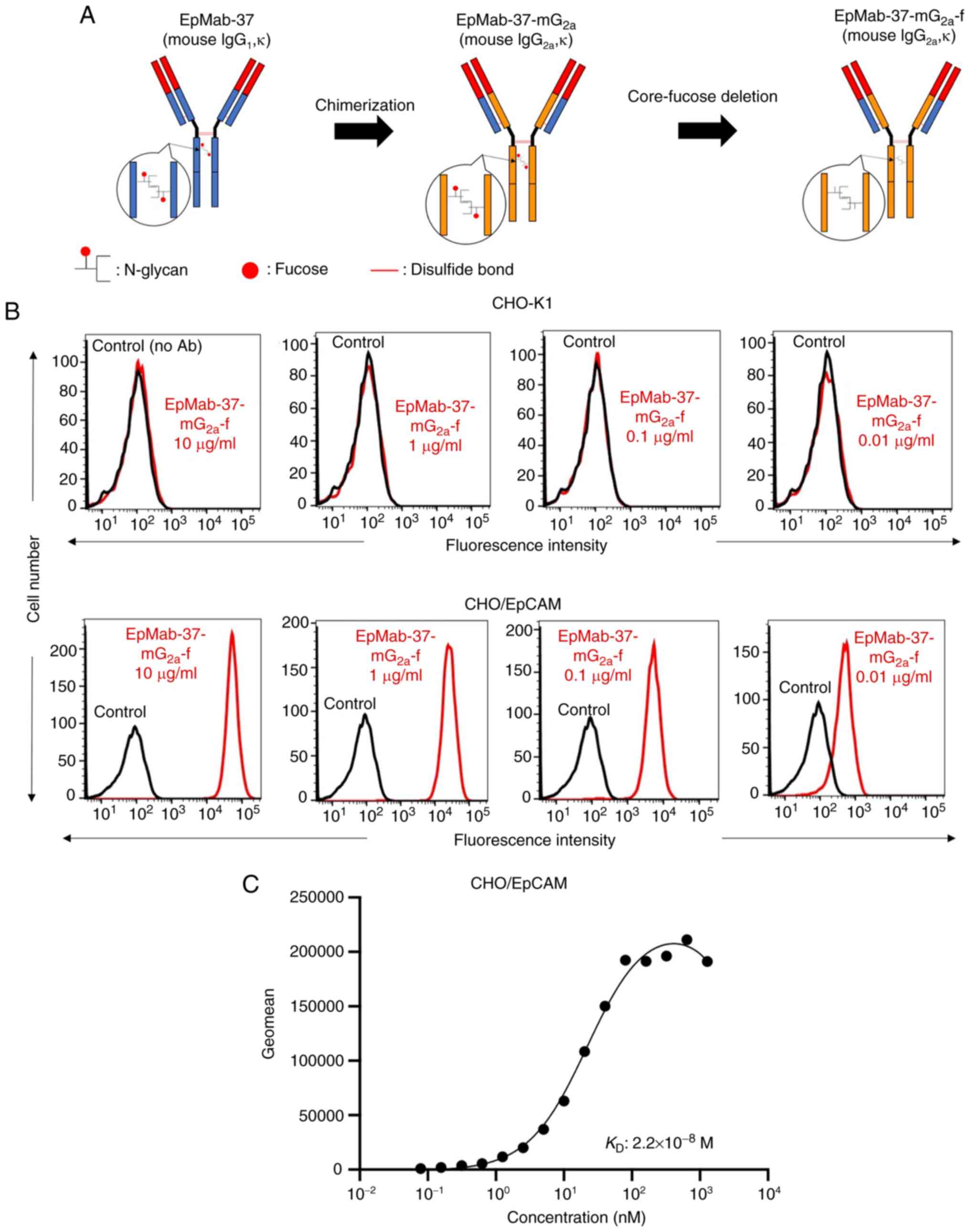
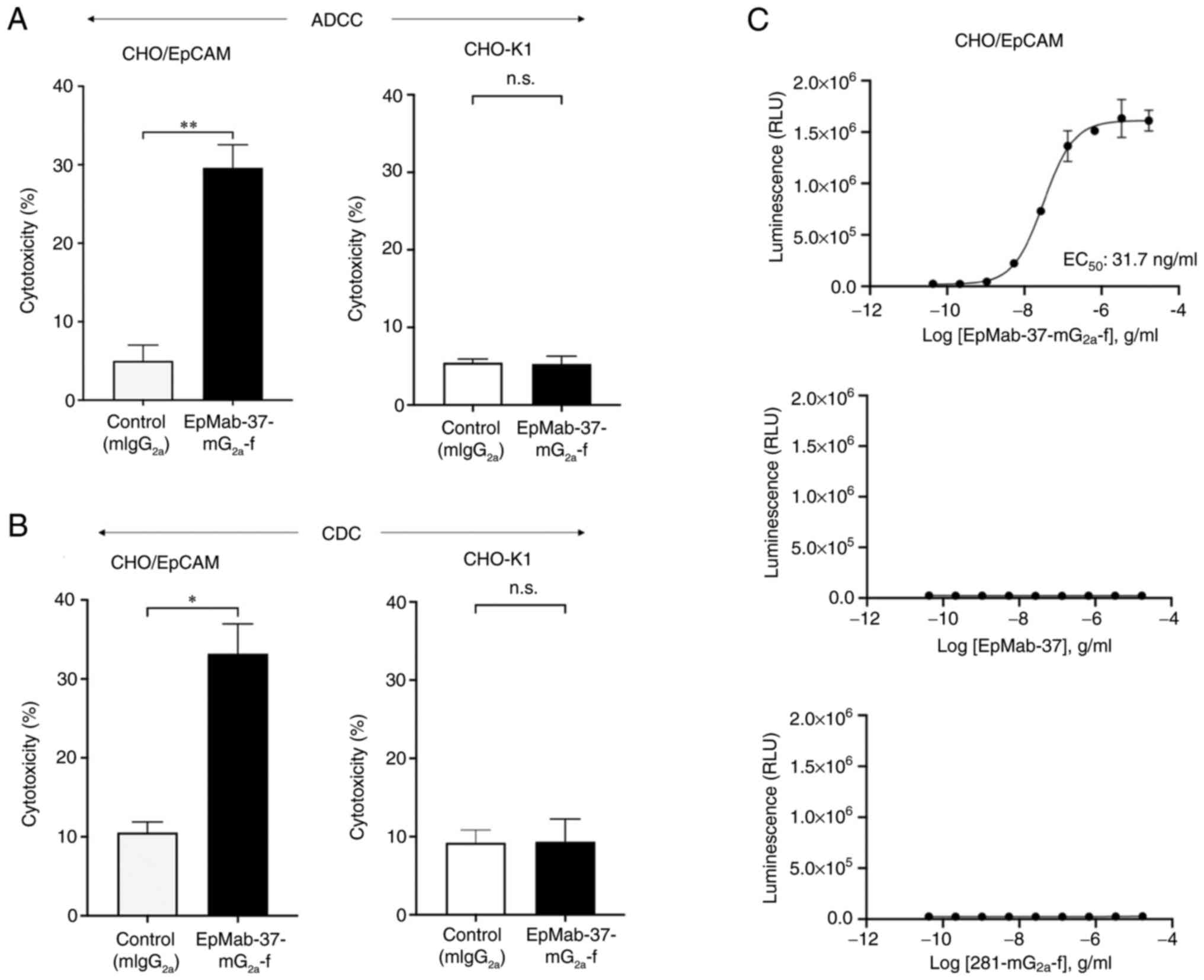
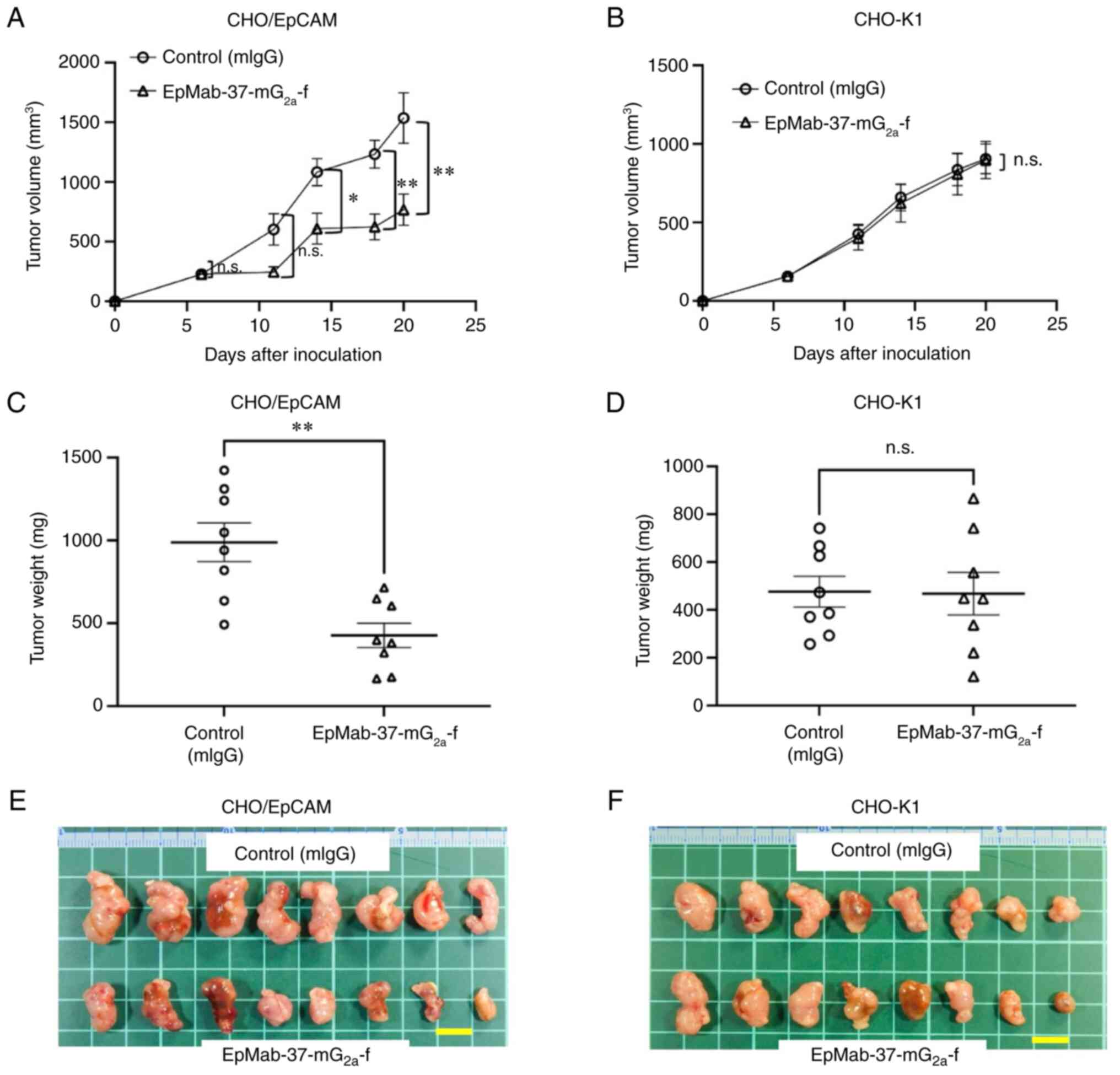
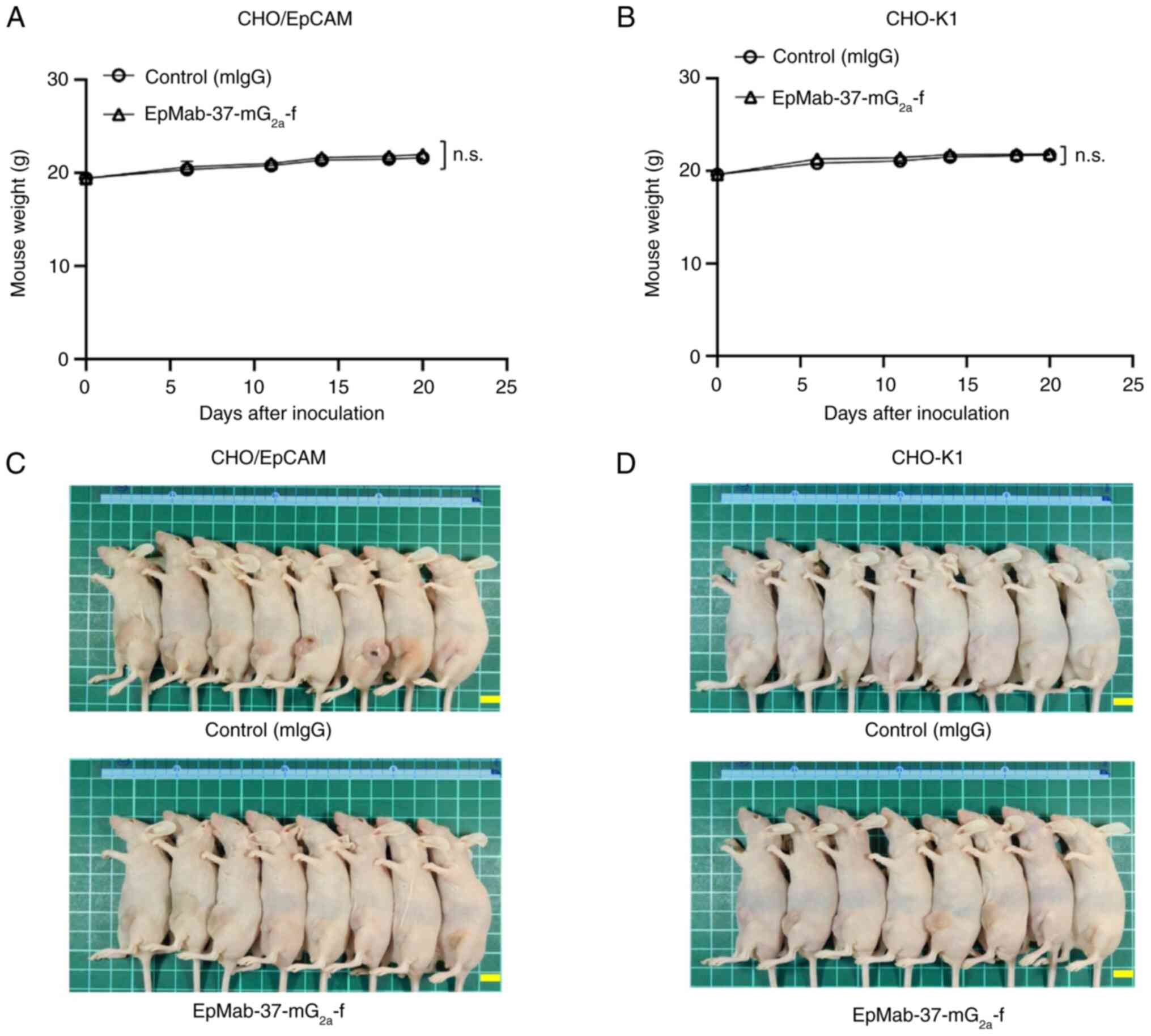
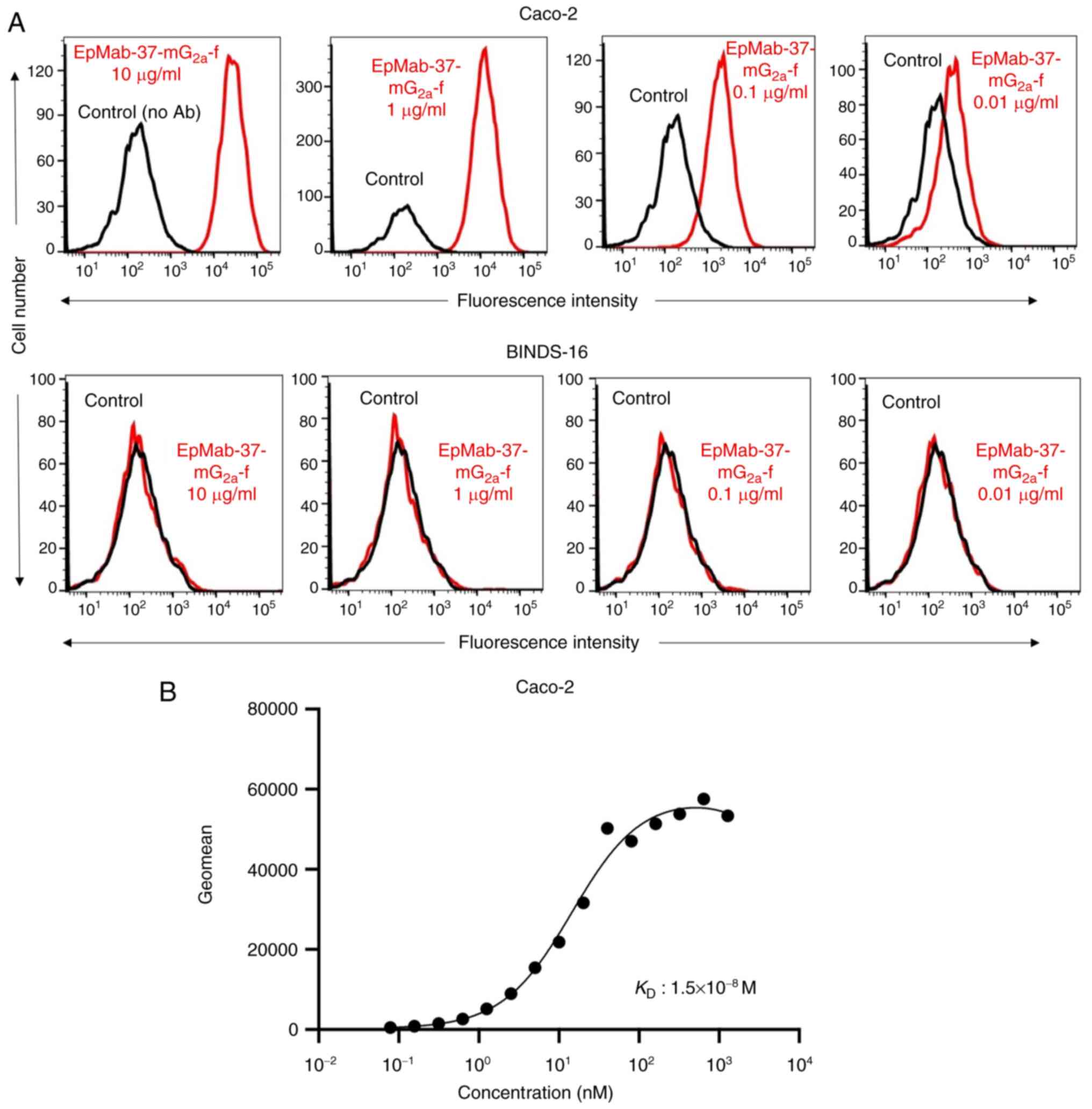
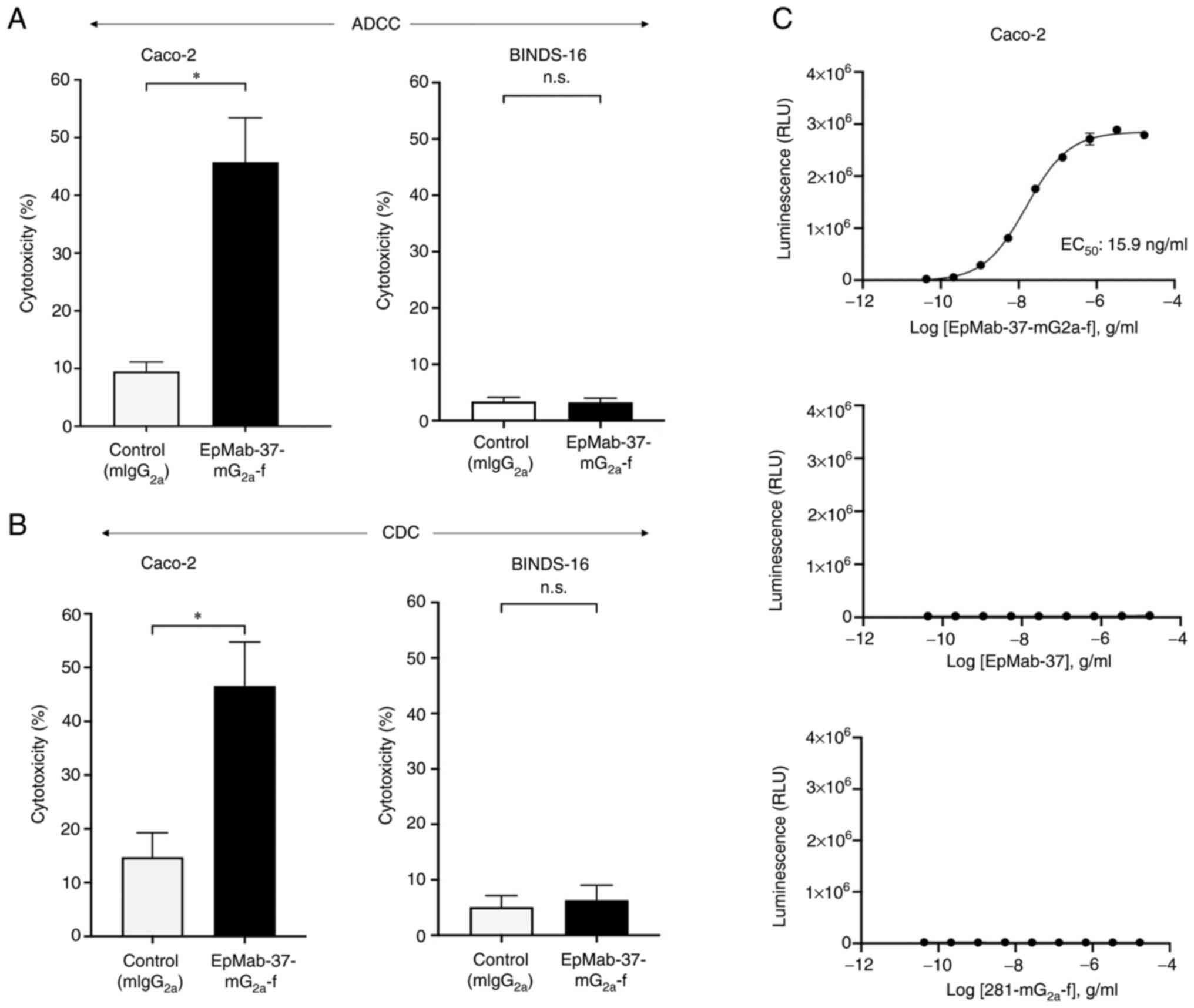
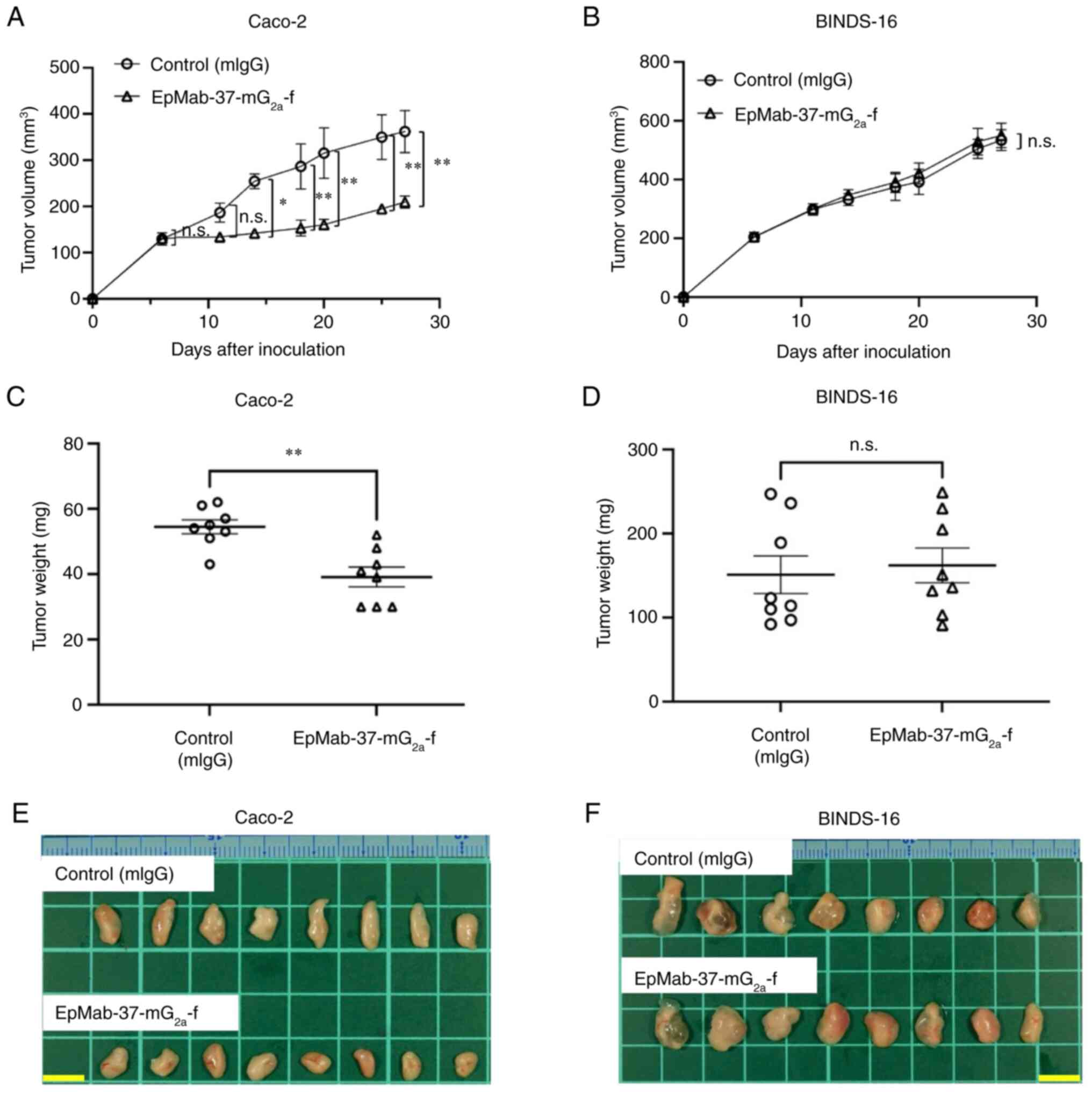
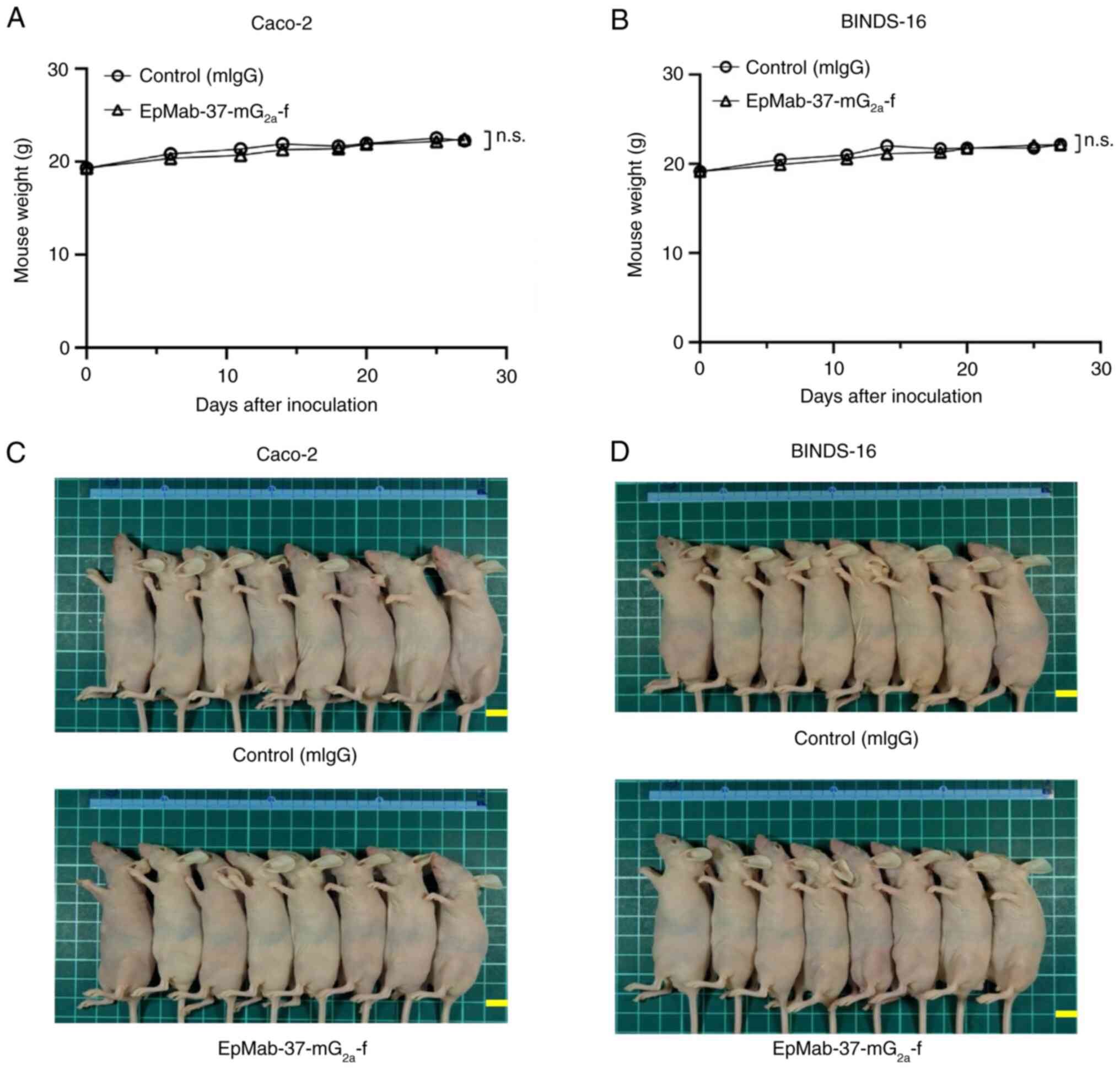
|
1
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: More than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown TC, Sankpal NV and Gillanders WE:
Functional implications of the dynamic regulation of EpCAM during
epithelial-to-mesenchymal transition. Biomolecules. 11:9562021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eyvazi S, Farajnia S, Dastmalchi S,
Kanipour F, Zarredar H and Bandehpour M: Antibody based EpCAM
targeted therapy of cancer, review and update. Curr Cancer Drug
Targets. 18:857–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao J, Pohlmann PR, Isaacs C, Weinberg
BA, He AR, Schlegel R and Agarwal S: Circulating tumor cells:
Technologies and their clinical potential in cancer metastasis.
Biomedicines. 9:11112021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe M, Kenmotsu H, Ko R, Wakuda K,
Ono A, Imai H, Taira T, Naito T, Murakami H, Abe M, et al:
Isolation and molecular analysis of circulating tumor cells from
lung cancer patients using a microfluidic chip type cell sorter.
Cancer Sci. 109:2539–2548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lampignano R, Yang L, Neumann MHD, Franken
A, Fehm T, Niederacher D and Neubauer H: A novel workflow to enrich
and isolate patient-matched EpCAMhigh and
EpCAMlow/negative CTCs enables the comparative
characterization of the PIK3CA status in metastatic breast cancer.
Int J Mol Sci. 18:18852017. View Article : Google Scholar
|
|
8
|
Zapatero A, Gómez-Caamaño A, Cabeza
Rodriguez MÁ, Muinelo-Romay L, Martin de Vidales C, Abalo A, Calvo
Crespo P, Leon Mateos L, Olivier C and Vega Piris LV: Detection and
dynamics of circulating tumor cells in patients with high-risk
prostate cancer treated with radiotherapy and hormones: A
prospective phase II study. Radiat Oncol. 15:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsao LC, Force J and Hartman ZC:
Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer
Res. 81:4641–4651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McInnes IB and Gravallese EM:
Immune-mediated inflammatory disease therapeutics: Past, present
and future. Nat Rev Immunol. 21:680–686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herlyn M, Steplewski Z, Herlyn D and
Koprowski H: Colorectal carcinoma-specific antigen: Detection by
means of monoclonal antibodies. Proc Natl Acad Sci USA.
76:1438–1442. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baeuerle PA and Gires O: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sears HF, Atkinson B, Mattis J, Ernst C,
Herlyn D, Steplewski Z, Häyry P and Koprowski H: Phase-I clinical
trial of monoclonal antibody in treatment of gastrointestinal
tumours. Lancet. 1:762–765. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riethmüller G, Holz E, Schlimok G,
Schmiegel W, Raab R, Höffken K, Gruber R, Funke I, Pichlmaier H,
Hirche H, et al: Monoclonal antibody therapy for resected Dukes' C
colorectal cancer: Seven-year outcome of a multicenter randomized
trial. J Clin Oncol. 16:1788–1794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaplon H, Muralidharan M, Schneider Z and
Reichert JM: Antibodies to watch in 2020. MAbs. 12:17035312020.
View Article : Google Scholar :
|
|
16
|
Zhang X, Yang Y, Fan D and Xiong D: The
development of bispecific antibodies and their applications in
tumor immune escape. Exp Hematol Oncol. 6:122017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schönberger S, Kraft D, Nettersheim D,
Schorle H, Casati A, Craveiro RB, Mohseni MM, Calaminus G and
Dilloo D: Targeting EpCAM by a bispecific trifunctional antibody
exerts profound cytotoxic efficacy in germ cell tumor cell lines.
Cancers (Basel). 12:12792020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruf P, Kluge M, Jäger M, Burges A, Volovat
C, Heiss MM, Hess J, Wimberger P, Brandt B and Lindhofer H:
Pharmacokinetics, immunogenicity and bioactivity of the therapeutic
antibody catumaxomab intraperitoneally administered to cancer
patients. Br J Clin Pharmacol. 69:617–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mau-Sørensen M, Dittrich C, Dienstmann R,
Lassen U, Büchler W, Martinius H and Tabernero J: A phase I trial
of intravenous catumaxomab: A bispecific monoclonal antibody
targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother
Pharmacol. 75:1065–1073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knödler M, Körfer J, Kunzmann V, Trojan J,
Daum S, Schenk M, Kullmann F, Schroll S, Behringer D, Stahl M, et
al: Randomised phase II trial to investigate catumaxomab
(anti-EpCAM x anti-CD3) for treatment of peritoneal carcinomatosis
in patients with gastric cancer. Br J Cancer. 119:296–302. 2018.
View Article : Google Scholar
|
|
21
|
Linke R, Klein A and Seimetz D:
Catumaxomab: Clinical development and future directions. MAbs.
2:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira NA, Chan KF, Lin PC and Song Z:
The 'less-is-more' in therapeutic antibodies: Afucosylated
anti-cancer antibodies with enhanced antibody-dependent cellular
cytotoxicity. MAbs. 10:693–711. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shinkawa T, Nakamura K, Yamane N,
Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M,
Yamasaki M, et al: The absence of fucose but not the presence of
galactose or bisecting N-acetylglucosamine of human IgG1
complex-type oligosaccharides shows the critical role of enhancing
antibody-dependent cellular cytotoxicity. J Biol Chem.
278:3466–3473. 2003. View Article : Google Scholar
|
|
24
|
Yamane-Ohnuki N, Kinoshita S,
Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R,
Sakurada M, Uchida K, et al: Establishment of FUT8 knockout Chinese
hamster ovary cells: An ideal host cell line for producing
completely defucosylated antibodies with enhanced
antibody-dependent cellular cytotoxicity. Biotechnol Bioeng.
87:614–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li G, Suzuki H, Asano T, Tanaka T, Suzuki
H, Kaneko MK and Kato Y: Development of a novel anti-EpCAM
monoclonal anti-body for various applications. Antibodies (Basel).
11:412022. View Article : Google Scholar
|
|
26
|
Hosono H, Ohishi T, Takei J, Asano T,
Sayama Y, Kawada M, Kaneko MK and Kato Y: The anti-epithelial cell
adhesion molecule (EpCAM) monoclonal antibody EpMab-16 exerts
antitumor activity in a mouse model of colorectal adenocarcinoma.
Oncol Lett. 20:3832020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaneko MK, Ohishi T, Takei J, Sano M,
Nakamura T, Hosono H, Yanaka M, Asano T, Sayama Y, Harada H, et al:
Anti-EpCAM monoclonal antibody exerts antitumor activity against
oral squamous cell carcinomas. Oncol Rep. 44:2517–2526. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Queiroz AL, Dantas E, Ramsamooj S, Murthy
A, Ahmed M, Zunica ERM, Liang RJ, Murphy J, Holman CD, Bare CJ, et
al: Blocking ActRIIB and restoring appetite reverses cachexia and
improves survival in mice with lung cancer. Nat Commun.
13:46332022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takei J, Kaneko MK, Ohishi T, Hosono H,
Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al: A
defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts
antitumor effects in mouse xenograft models of oral squamous cell
carcinoma. Oncol Rep. 44:1949–1960. 2020.PubMed/NCBI
|
|
30
|
Takei J, Ohishi T, Kaneko MK, Harada H,
Kawada M and Kato Y: A defucosylated anti-PD-L1 monoclonal antibody
13-mG2a-f exerts antitumor effects in mouse xenograft
models of oral squamous cell carcinoma. Biochem Biophys Rep.
24:1008012020.
|
|
31
|
Li G, Ohishi T, Kaneko MK, Takei J, Mizuno
T, Kawada M, Saito M, Suzuki H and Kato Y: Defucosylated mouse-dog
chimeric anti-EGFR antibody exerts antitumor activities in mouse
xenograft models of canine tumors. Cells. 10:35992021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tateyama N, Nanamiya R, Ohishi T, Takei J,
Nakamura T, Yanaka M, Hosono H, Saito M, Asano T, Tanaka T, et al:
Defucosylated anti-epidermal growth factor receptor monoclonal
antibody 134-mG2a-f exerts antitumor activities in mouse
xenograft models of dog epidermal growth factor
receptor-overexpressed cells. Monoclon Antib Immunodiagn
Immunother. 40:177–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goto N, Suzuki H, Ohishi T, Harakawa A, Li
G, Saito M, Takei J, Tanaka T, Asano T, Sano M, et al: Antitumor
activities in mouse xenograft models of canine fibroblastic tumor
by defucosylated anti-epidermal growth factor receptor monoclonal
antibody. Monoclon Antib Immunodiagn Immunother. 41:67–73. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nanamiya R, Takei J, Ohishi T, Asano T,
Tanaka T, Sano M, Nakamura T, Yanaka M, Handa S, Tateyama N, et al:
Defucosylated anti-epidermal growth factor receptor monoclonal
antibody (134-mG2a-f) exerts antitumor activities in
mouse xenograft models of canine osteosarcoma. Monoclon Antib
Immunodiagn Immunother. 41:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki H, Ohishi T, Asano T, Tanaka T,
Saito M, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK and Kato Y:
Defucosylated mouse-dog chimeric anti-HER2 monoclonal antibody
exerts antitumor activities in mouse xenograft models of canine
tumors. Oncol Rep. 48:1542022. View Article : Google Scholar :
|
|
36
|
Tanaka T, Ohishi T, Saito M, Suzuki H,
Kaneko MK, Kawada M and Kato Y: Defucosylated anti-epidermal growth
factor receptor monoclonal antibody exerted antitumor activities in
mouse xenograft models of canine mammary gland tumor. Monoclon
Antib Immunodiagn Immunother. 41:142–149. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nanamiya R, Suzuki H, Takei J, Li G, Goto
N, Harada H, Saito M, Tanaka T, Asano T, Kaneko MK and Kato Y:
Development of monoclonal antibody 281-mG2a-f against
golden hamster podoplanin. Monoclon Antib Immunodiagn Immunother.
Apr 27–2022.Epub ahead of print.
|
|
38
|
Itai S, Ohishi T, Kaneko MK, Yamada S, Abe
S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y, et al:
Anti-podocalyxin antibody exerts antitumor effects via
antibody-dependent cellular cytotoxicity in mouse xenograft models
of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett 1. 9:2809–2816.
2020.
|
|
40
|
Garvin D, Stecha P, Gilden J, Wang J,
Grailer J, Hartnett J, Fan F, Cong M and Cheng ZJ: Determining ADCC
activity of antibody-based therapeutic molecules using two
bioluminescent reporter-based bioassays. Curr Protoc. 1:e2962021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kosterink JGW, McLaughlin PM, Lub-de Hooge
MN, Hendrikse HH, van Zanten J, van Garderen E, Harmsen MC and de
Leij LFMH: Biodistribution studies of epithelial cell adhesion
molecule (EpCAM)-directed monoclonal antibodies in the
EpCAM-transgenic mouse tumor model. J Immunol. 179:1362–1368. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato Y, Ohishi T, Takei J, Nakamura T,
Kawada M and Kaneko MK: An antihuman epidermal growth factor
receptor 2 monoclonal antibody (H2Mab-19) exerts
antitumor activity in glioblastoma xenograft models. Monoclon Antib
Immunodiagn Immunother. 39:135–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pavšič M, Gunčar G, Djinović-Carugo K and
Lenarčič B: Crystal structure and its bearing towards an
understanding of key biological functions of EpCAM. Nat Commun.
5:47642014. View Article : Google Scholar
|
|
44
|
Gaber A, Lenarčič B and Pavšič M: Current
view on EpCAM structural biology. Cells. 9:13612020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Münz M, Murr A, Kvesic M, Rau D, Mangold
S, Pflanz S, Lumsden J, Volkland J, Fagerberg J, Riethmüller G, et
al: Side-by-side analysis of five clinically tested anti-EpCAM
monoclonal antibodies. Cancer Cell Int. 10:442010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asano T, Kaneko MK and Kato Y: Development
of a novel epitope mapping system: RIEDL insertion for epitope
mapping method. Monoclon Antib Immunodiagn Immunother. 40:162–167.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asano T, Kaneko MK, Takei J, Tateyama N
and Kato Y: Epitope mapping of the anti-CD44 monoclonal antibody
(C44Mab-46) using the REMAP method. Monoclon Antib
Immunodiagn Immunother. 40:156–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nanamiya R, Sano M, Asano T, Yanaka M,
Nakamura T, Saito M, Tanaka T, Hosono H, Tateyama N, Kaneko MK and
Kato Y: Epitope mapping of an anti-human epidermal growth factor
receptor monoclonal antibody (EMab-51) using the RIEDL insertion
for epitope mapping method. Monoclon Antib Immunodiagn Immunother.
40:149–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sano M, Kaneko MK, Aasano T and Kato Y:
Epitope mapping of an antihuman EGFR monoclonal antibody (EMab-134)
using the REMAP method. Monoclon Antib Immunodiagn Immunother.
40:191–195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asano T, Suzuki H, Kaneko MK and Kato Y:
Epitope mapping of rituximab using HisMAP method. Monoclon Antib
Immunodiagn Immunother. 41:8–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suzuki H, Asano T, Tanaka T, Kaneko MK and
Kato Y: Epitope mapping of the anti-CD20 monoclonal antibodies
(C20Mab-11 and 2H7) using HisMAP method. Monoclon Antib Immunodiagn
Immunother. 41:20–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang L, Yang Y, Yang F, Liu S, Zhu Z, Lei
Z and Guo J: Functions of EpCAM in physiological processes and
diseases (review). Int J Mol Med. 42:1771–1785. 2018.PubMed/NCBI
|
|
53
|
Maghzal N, Kayali HA, Rohani N, Kajava AV
and Fagotto F: EpCAM controls actomyosin contractility and cell
adhesion by direct inhibition of PKC. Dev Cell. 27:263–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Munz M, Baeuerle PA and Gires O: The
emerging role of EpCAM in cancer and stem cell signaling. Cancer
Res. 69:5627–5629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baccelli I, Schneeweiss A, Riethdorf S,
Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T,
Wallwiener M, et al: Identification of a population of blood
circulating tumor cells from breast cancer patients that initiates
metastasis in a xenograft assay. Nat Biotechnol. 31:539–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rupp B, Ball H, Wuchu F, Nagrath D and
Nagrath S: Circulating tumor cells in precision medicine:
Challenges and opportunities. Trends Pharmacol Sci. 43:378–391.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dieffenbach M and Pastan I: Mechanisms of
resistance to immunotoxins containing Pseudomonas exotoxin A in
cancer therapy. Biomolecules. 10:9792020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fragkoulis C, Glykas I, Bamias A,
Stathouros G, Papadopoulos G and Ntoumas K: Novel treatments in BCG
failure. Where do we stand today? Arch Esp Urol. 74:681–691.
2021.In English, Spanish. PubMed/NCBI
|
|
60
|
Kaneko MK, Honma R, Ogasawara S, Fujii Y,
Nakamura T, Saidoh N, Takagi M, Kagawa Y, Konnai S and Kato Y:
PMab-38 recognizes canine podoplanin of squamous cell carcinomas.
Monoclon Antib Immunodiagn Immunother. 35:263–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ito A, Ohta M, Kato Y, Inada S, Kato T,
Nakata S, Yatabe Y, Goto M, Kaneda N, Kurita K, et al: A real-time
near-infrared fluorescence imaging method for the detection of oral
cancers in mice using an indocyanine green-labeled podoplanin
antibody. Technol Cancer Res Treat. 17:15330338187679362018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kato Y, Ohishi T, Kawada M, Maekawa N,
Konnai S, Itai S, Yamada S and Kaneko MK: The mouse-canine chimeric
anti-dog podoplanin antibody P38B exerts antitumor activity in
mouse xenograft models. Biochem Biophys Rep. 17:23–26.
2018.PubMed/NCBI
|
|
63
|
Kato Y, Ito Y, Ohishi T, Kawada M,
Nakamura T, Sayama Y, Sano M, Asano T, Yanaka M, Okamoto S, et al:
Antibody-drug conjugates using mouse-canine chimeric anti-dog
podoplanin antibody exerts antitumor activity in a mouse xenograft
model. Monoclon Antib Immunodiagn Immunother. 39:37–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Engl J Med.
380:741–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pavšič M: Trop2 forms a stable dimer with
significant structural differences within the membrane-distal
region as compared to EpCAM. Int J Mol Sci. 22:106402021.
View Article : Google Scholar
|
|
66
|
Cardillo TM, Govindan SV, Sharkey RM,
Trisal P and Goldenberg DM: Humanized anti-Trop-2 IgG-SN-38
conjugate for effective treatment of diverse epithelial cancers:
Preclinical studies in human cancer xenograft models and monkeys.
Clin Cancer Res. 17:3157–3169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaneko MK, Nakamura T, Kunita A, Fukayama
M, Abe S, Nishioka Y, Yamada S, Yanaka M, Saidoh N, Yoshida K, et
al: ChLpMab-23: Cancer-specific human-mouse chimeric
anti-podoplanin antibody exhibits antitumor activity via
antibody-dependent cellular cytotoxicity. Monoclon Antib
Immunodiagn Immunother. 36:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kaneko MK, Yamada S, Nakamura T, Abe S,
Nishioka Y, Kunita A, Fukayama M, Fujii Y, Ogasawara S and Kato Y:
Antitumor activity of chLpMab-2, a human-mouse chimeric
cancer-specific antihuman podoplanin antibody, via
antibody-dependent cellular cytotoxicity. Cancer Med. 6:768–777.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Suzuki H, Kaneko MK and Kato Y: Roles of
podoplanin in malignant progression of tumor. Cells. 11:5752022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kaneko MK, Ohishi T, Kawada M and Kato Y:
A cancer-specific anti-podocalyxin monoclonal antibody
(60-mG2a-f) exerts antitumor effects in mouse xenograft
models of pancreatic carcinoma. Biochem Biophys Rep.
24:1008262020.
|
|
72
|
Ishikawa A, Waseda M, Ishii T, Kaneko MK,
Kato Y and Kaneko S: Improved anti-solid tumor response by
humanized anti-podoplanin chimeric antigen receptor transduced
human cytotoxic T cells in an animal model. Genes Cells.
27:549–558. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chalise L, Kato A, Ohno M, Maeda S,
Yamamichi A, Kuramitsu S, Shiina S, Takahashi H, Ozone S, Yamaguchi
J, et al: Efficacy of cancer-specific anti-podoplanin CAR-T cells
and oncolytic herpes virus G47Δ combination therapy against
glioblastoma. Mol Ther Oncolytics. 26:265–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shiina S, Ohno M, Ohka F, Kuramitsu S,
Yamamichi A, Kato A, Motomura K, Tanahashi K, Yamamoto T, Watanabe
R, et al: CAR T cells targeting podoplanin reduce orthotopic
glioblastomas in mouse brains. Cancer Immunol Res. 4:259–268. 2016.
View Article : Google Scholar : PubMed/NCBI
|