Introduction
Epithelial cell adhesion molecule (EpCAM) is
expressed on the basolateral membrane of epithelial cells (1). EpCAM is a unique type I
transmembrane glycoprotein which possesses a different structure
and functions compared to other classical adhesion molecules,
including cadherins, selectins and integrins. EpCAM-mediated
homophilic and intercellular adhesion are essential for the
maintenance of the epithelial integrity (2). EpCAM also plays critical roles in
intercellular signaling. Following the cleavage of the EpCAM
intracellular domain, it functions as a transcriptional co-factor
with β-catenin and regulates the transcriptional targets involved
in cell proliferation, survival and stemness (3). Therefore, the overexpression of
EpCAM plays a key role in tumor development.
EpCAM is a critical marker for the isolation
circulating tumor cells (CTCs). CTCs provide critical prognostic
information as an indicator of micro-metastasis, and determine the
response to some cancer therapeutics (4). The US Food and Drug Administration
(FDA) confirmed the clinical importance of CTCs, and approved of
CellSearch®, a platform that can be used for the
isolation of EpCAM-positive, CD45-negative cells from whole blood
samples (5). The EpCAM-based
CellSearch® CTC test has been studied in several
clinical trials in lung (6),
breast (7) and prostate (8) cancers.
Therapeutic monoclonal antibodies (mAbs) are the
most crucial biological therapeutics for the treatment of various
tumors (9) and inflammatory
diseases, such as rheumatoid arthritis (10). EpCAM was the first identified
human tumor-associated antigen, and plays a critical role in tumor
development (11). Therefore,
EpCAM has been considered as a target of mAb therapies, including
Adecatumumab (12) and
Edrecolomab (13,14) in EpCAM-overexpressing breast,
prostate, gastrointestinal and colorectal cancers. A humanized
single chain Fv against EpCAM, fused to Pseudomonas exotoxin
A, Oportuzumab monatox, has been evaluated in bladder cancer, as
have been previously reviewed (15). A bispecific EpCAM/CD3-antibody,
Catumaxomab, functions as the trifunctional antibody. When
Catumaxomab recognizes EpCAM-high tumors, it also recruits T-cells
to tumors. This event promotes the formation of cytotoxic synapse
and T-cell-mediated cytotoxicity. Furthermore, Catumaxomab recruits
natural killer (NK) cells and macrophages through the Fc domain and
enhances antibody-dependent cellular cytotoxicity (ADCC) (16,17). Catumaxomab has demonstrated
promising outcomes in several clinical trials (18-20), and has been approved by the
European Union for the treatment of patients with malignant ascites
(21).
ADCC is mediated by NK cells upon the binding of the
FcγRIIIa to the Fc region of mAbs. The FcγRIIIa engagement can
stimulate NK cells, which attack and lyse target cells (9). However, this function is affected by
the N-linked glycosylation in the Fc region (22). In particular, a core fucose
deficiency on the Fc N-glycan has been revealed to enhance
the Fc binding to the FcγRIIIa on the effector cells (23). In a previous study on recombinant
mAb production using Chinese hamster ovary (CHO) cells, the Fc
N-glycans were determined as an heterogeneous biantennary
complex (22). Fucosyltransferase
8 (FUT8) is the only α1,6-fucosyltransferase transferring fucose
via an α1,6 linkage to the innermost N-acetylglucosamine on
N-glycans for core fucosylation. CHO cells subjected to FUT8
knockout have been revealed to produce completely defucosylated
recombinant antibodies. Furthermore, mAb produced by CHO cells
subjected to FUT8 knockout strongly binds to FcγRIIIa and potently
enhances ADCC activity compared to mAb produced by wild-type CHO.
Therefore, CHO cells subjected to FUT8 knockout may be an ideal
host cell for the production of completely defucosylated high-ADCC
mAb for therapeutic use (24).
An anti-EpCAM mAb, EpMab-37 (mouse IgG1,
kappa) has been previously established by the authors, using the
cell-based immunization and screening method (25). In the present study, a
defucosylated anti-EpCAM mAb (EpMab-37-mG2a-f) was
produced by using FUT8-deficient CHO cells to potentiate anti-tumor
activity and investigated the ability of EpMab-37-mG2a-f
to induce ADCC, complement-dependent cytotoxicity (CDC) and
antitumor activity in EpCAM-expressing cells.
Materials and methods
Cell lines and cell culture
CHO-K1 (ATCC CCL-61) and the human colorectal cancer
cell lines, Caco-2 (ATCC HTB-37), HCT116 (ATCC CCL-247), HT-29
(ATCC HTB-38), LS174T (ATCC CL-188), COLO201 (ATCC CCL-224), HCT-8
(ATCC CCL-244) and SW1116 (ATCC CCL-233), were purchased from the
ATCC. HCT-15 (TKG 0504), COLO205 (TKG 0457) and DLD-1 (TKG 0379)
were purchased from the Cell Resource Center for Biomedical
Research Institute of Development, Aging and Cancer at Tohoku
University. EpCAM cDNA plus a C-terminal PA tag (EpCAM-PA) was
subcloned into a pCAG-Ble vector (FUJIFILM Wako Pure Chemical
Corporation). CHO/EpCAM was established by transfecting the
pCAG/EpCAM-PA vector into CHO-K1 cells using the Neon Transfection
System (Thermo Fisher Scientific, Inc.). CHO-K1 cells
(1.5×106) were transfected with the pCAG/EpCAM-PA
vector. Positive cells for anti-EpCAM mAb (clone 9C4; cat. no.
324202; BioLegend, Inc.) were sorted using an SH800 cell sorter
(Sony Corporation) and selected as previously described (26,27). CHO-K1 and CHO/EpCAM cells were
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(Nacalai Tesque, Inc.), supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, 100 µg/ml streptomycin and 0.25 µg/ml
amphotericin B (Nacalai Tesque, Inc.). Caco-2 cells and Caco-2
cells in which EpCAM was knocked out (BINDS-16), which were
previously established (26),
were cultured in DMEM (Nacalai Tesque, Inc.), supplemented with 10%
FBS, 100 units/ml penicillin and 100 µg/ml streptomycin. The
COLO201, COLO205, SW1116 and DLD-1 cells were cultured in RPMI-1640
medium (Nacalai Tesque, Inc.), supplemented with 10% FBS, 100
units/ml penicillin and 100 µg/ml streptomycin (Nacalai
Tesque, Inc.). The HCT116, HT-29, LS174T and HCT-8 were cultured in
DMEM (Nacalai Tesque, Inc.), supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. All cell lines were
maintained at 37°C in a humidified atmosphere under 5%
CO2.
Animal experiments
All animal experiments were performed following
regulations and guidelines to minimize animal distress and
suffering in the laboratory by the Institutional Committee for
Experiments of the Institute of Microbial Chemistry (Numazu,
Japan). The animal study protocol was approved (approval no.
2022-024) by the Institutional Committee for Experiments of the
Institute of Microbial Chemistry (Numazu, Japan). BALB/c nude mice
(female, a total of 70 mice) were maintained on an 11-h light/13-h
dark cycle in a specific pathogen-free environment, across the
experimental period. Food and water were supplied ad
libitum. The weight of the mice was monitored twice per week
and their health was monitored three times per week. The loss of
original body weight was determined to a point >25% (28) and/or a maximum tumor size
>3,000 mm3 and/or significant changes in the
appearance of tumors as humane endpoints for euthanasia. Cervical
dislocation was used for euthanasia. Mouse death was confirmed by
respiratory arrest and rigor mortis.
Antibodies
Anti-EpCAM mAb, EpMab-37 was previously established
(25). To generate recombinant
EpMab-37, VH cDNA of EpMab-37 and CH of mouse
IgG2a was cloned into the pCAG-Ble vector. VL
cDNA of EpMab-37 and CL cDNA of mouse kappa light chain
were also subcloned into the pCAG-Neo vector (FUJIFILM Wako Pure
Chemical Corporation). The vector for the recombinant EpMab-37 was
transduced into BINDS-09 (FUT8-knockout ExpiCHO-S) cells using the
ExpiCHO Expression System (Thermo Fisher Scientific, Inc.), as
previously described (29-36).
EpMab-37-mG2a-f was purified using Ab-Capcher (ProteNova
Co., Ltd.). Mouse IgG (cat. no. 140-09511) and IgG2a
(cat. no. M7769) were purchased from FUJIFILM Wako Pure Chemical
Corporation and MilliporeSigma, respectively. 281-mG2a-f
[defucosylated anti-hamster podoplanin (PDPN) mAb, control mouse
IgG2a for ADCC reporter bioassay] was previously
described (37).
Flow cytometry
CHO-K1, CHO/EpCAM and Caco-2 cells were isolated
using 0.25% trypsin and 1 mM ethylenediamine tetraacetic acid
(EDTA; Nacalai Tesque, Inc.) treatment. The cells were treated with
EpMab-37-mG2a-f, or blocking buffer [0.1% bovine serum
albumin (BSA; Nacalai Tesque, Inc.) in phosphate-buffered saline
(PBS)] (control) for 30 min at 4°C. Subsequently, the cells were
incubated in Alexa Fluor 488-conjugated anti-mouse IgG (1:2,000;
cat. no. 4408; Cell Signaling Technology, Inc.) for 30 min at 4°C.
Fluorescence data were collected using the SA3800 Cell Analyzer and
analyzed using SA3800 software ver. 2.05 (Sony Corporation).
Determination of binding affinity
Serially diluted EpMab-37-mG2a-f
(0.006-100 µg/ml) was suspended with CHO/EpCAM and Caco-2
cells. The cells were further treated with Alexa Fluor
488-conjugated anti-mouse IgG (1:200). Fluorescence data were
collected using BD FACSLyric and analyzed using BD FACSuite
software version 1.3 (BD Biosciences). To determine the
dissociation constant (KD), GraphPad Prism 8 (the
fitting binding isotherms to built-in one-site binding models;
GraphPad Software, Inc.) was used.
ADCC of EpMab-37-mG2a-f
ADCC induction by EpMab-37-mG2a-f was
assayed as follows: Six female BALB/c nude mice (5 weeks old) were
purchased from Charles River Laboratories, Inc. The spleens were
aseptically removed and single-cell suspensions were obtained
through a sterile cell strainer (cat. no. 352360; BD Falcon).
Erythrocytes were removed with the treatment of ice-cold distilled
water. The splenocytes were resuspended in DMEM with 10% FBS; this
preparation was designated as effector cells. Target cells (CHO-K1,
CHO/EpCAM and Caco-2) were treated with 10 µg/ml Calcein AM
(Thermo Fisher Scientific, Inc.) (38). The target cells (2×104
cells) were plated in 96-well plates and mixed with effector cells
(effector-to-target ratio, 100:1), 100 µg/ml of
EpMab-37-mG2a-f or control mouse IgG2a.
Following incubation for 4.5 h at 37°C, the Calcein release into
the medium was measured with an excitation wavelength (485 nm) and
an emission wavelength (538 nm) using a microplate reader (Power
Scan HT; BioTek Instruments, Inc.).
The total percentage of cell lysis was determined as
follows: % lysis=(E-S)/(M-S) x100, where 'E' corresponds to the
fluorescence in the presence of both effector and target cells, 'S'
to the spontaneous fluorescence in the presence of only target
cells and 'M' to the maximum fluorescence by the treatment with a
lysis buffer [10 mM Tris-HCl (pH 7.4), 10 mM of EDTA and 0.5%
Triton X-100].
CDC of EpMab-37-mG2a-f
Target cells (CHO-K1, CHO/EpCAM and Caco-2) were
treated with 10 µg/ml Calcein AM (39). The target cells (2×104
cells) were mixed with rabbit complement (final dilution 1:10;
Low-Tox-M Rabbit Complement; Cedarlane Laboratories) and 100
µg/ml control mouse IgG2a or
EpMab-37-mG2a-f. Following incubation for 4.5 h at 37°C,
Calcein release into the medium was measured.
ADCC reporter bioassay
The ADCC reporter bioassay was performed using an
ADCC Reporter Bioassay kit (cat. no. G7018; Promega Corporation),
according to the manufacturer's instructions (40). Target cells (12,500 cells per
well) were inoculated into a 96-well white solid plate (cat. no.
655083; Greiner Bio-One). EpMab-37-mG2a-f, EpMab-37 and
281-mG2a-f were serially diluted and added to target
cells. Jurkat cells (a component of ADCC Reporter Bioassay kit)
stably expressing the human FcγRIIIa receptor, and a nuclear factor
of activated T-cells (NFAT) response element driving Firefly
luciferase, were used as effector cells. The engineered Jurkat
cells (75,000 cells in 25 µl) were then added and
co-cultured with antibody-treated target cells at 37°C for 6 h.
Luminescence using the Bio-Glo Luciferase Assay System was measured
with a GloMax luminometer (Promega Corporation).
Antitumor activity of
EpMab-37-mG2a-f in xenografts of CHO-K1 and
CHO/EpCAM
BALB/c nude mice (female, 32 mice) were purchased
from Charles River Laboratories, Inc. The CHO-K1 and CHO/EpCAM
cells (5×106 cells) suspended with BD Matrigel Matrix
Growth Factor Reduced (BD Biosciences) were inoculated into the
left flank of the mice subcutaneously. On day 6 after the
inoculation, 100 µg EpMab-37-mG2a-f (n=8) or
control mouse IgG (n=8) in 100 µl PBS were injected
intraperitoneally. Additional antibody injections were performed on
day 14. The dose was based on a biodistribution study of
mouse-derived anti-EpCAM mAb (MOC31) (41). The tumor volume was measured on
days 6, 11, 14, 18 and 20, and determined as previously described
(42). On day 18, ulcerations
began to appear in some tumors. As ulcerated tumors result in skin
breakdown and a decrease in tumor weight, it was decided to
terminate the experiment on day 20 prior to the diameter of the
ulcers exceeding 4 mm. The health and well-being of the animals did
not deteriorate during these 2 days. The xenograft tumors with or
without ulceration were carefully removed from the sacrificed mice,
and then weighed and photographed immediately.
Antitumor activity of
EpMab-37-mG2a-f in xenografts of Caco-2 and BINDS-16
cells
The Caco-2 and BINDS-16 cells (5×106
cells) resuspended with BD Matrigel Matrix Growth Factor Reduced
(BD Biosciences) were inoculated into the left flank of BALB/c nude
mice (female, 32 mice) subcutaneously. On day 6 after the
inoculation, 100 µg EpMab-37-mG2a-f (n=8) or
control mouse IgG (n=8) in 100 µl PBS were injected
intraperitoneally. Additional antibody injections were performed on
days 14 and 20. The tumor volume was measured on days 6, 11, 14,
18, 20, 25 and 27, and determined as previously described (42). The xenograft tumors were removed,
weighed and photographed as described above.
Statistical analyses
All data are expressed as the mean ± standard error
of the mean (SEM). In ADCC, CDC and tumor weight measurement,
unpaired Welch's t-test was conducted. ANOVA with Sidak's post hoc
test were utilized for tumor volume and mice weight. GraphPad Prism
8 (GraphPad Software, Inc.) was used for all calculations.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Flow cytometric analysis against
CHO/EpCAM cells using EpMab-37-mG2a-f
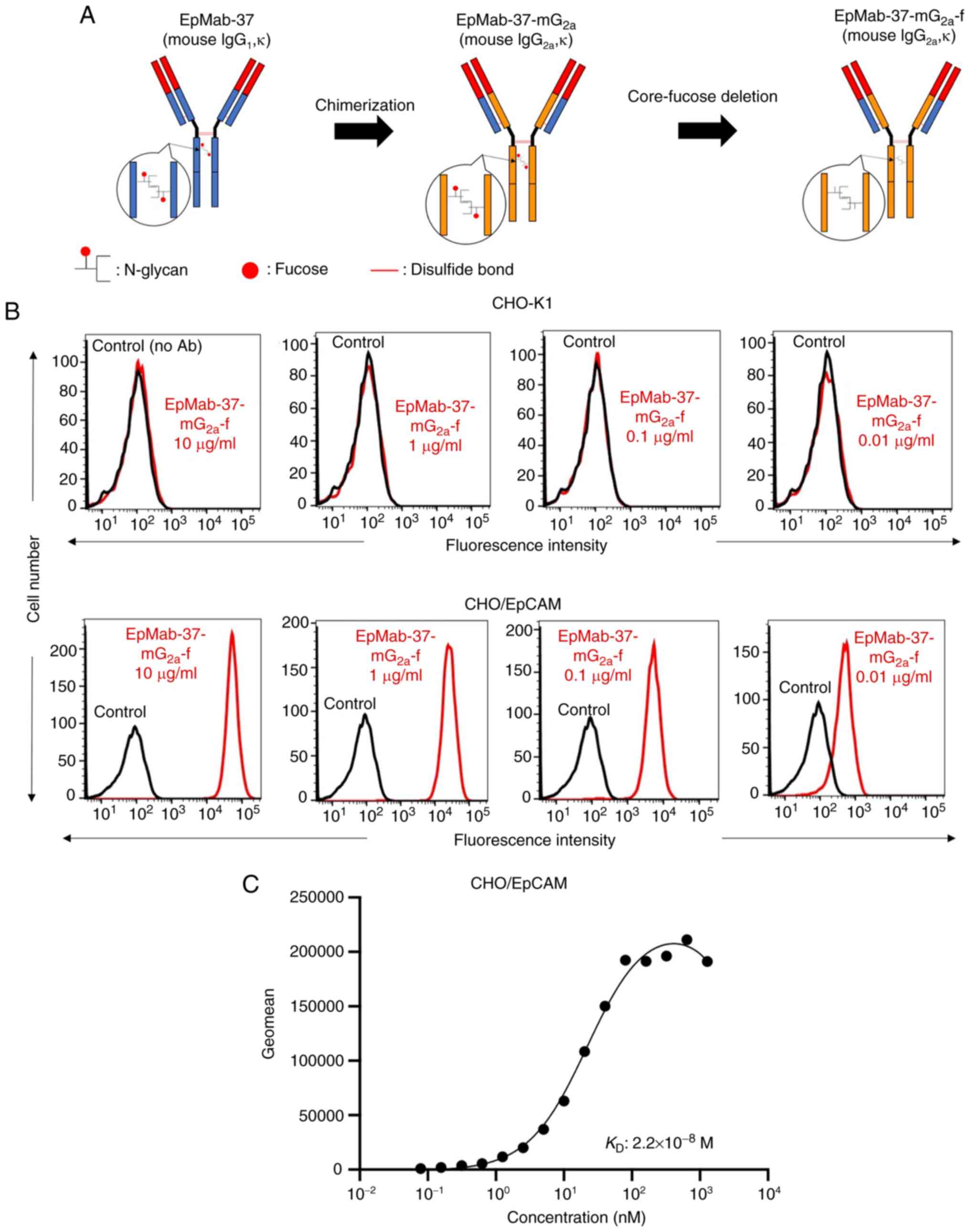
In a previous study by the authors, an anti-EpCAM
mAb (EpMab-37, mouse IgG1, kappa) was established, using
cancer-specific mAb (CasMab) technology (25). EpMab-37 was revealed to be
available for flow cytometry, western blotting and
immunohistochemistry (25). In
the present study, a defucosylated form of anti-EpCAM mAb
(EpMab-37-mG2a-f) was produced by combining
VH and VL of EpMab-37 with CH and
CL of mouse IgG2a, respectively (Fig. 1A). EpMab-37-mG2a-f
detected CHO/EpCAM and not parental CHO-K1 cells in a
concentration-dependent manner (Figs.
1B and S1A), indicating that
EpMab-37-mG2a-f reacted with EpCAM.
A kinetic analysis of the interactions of
EpMab-37-mG2a-f with CHO/EpCAM was performed using flow
cytometry. As demonstrated in Fig.
1C, the KD for the interaction of
EpMab-37-mG2a-f with CHO/EpCAM cells was
2.2×10−8 M, suggesting that EpMab-37-mG2a-f
may exhibit moderate affinity for CHO/EpCAM cells.
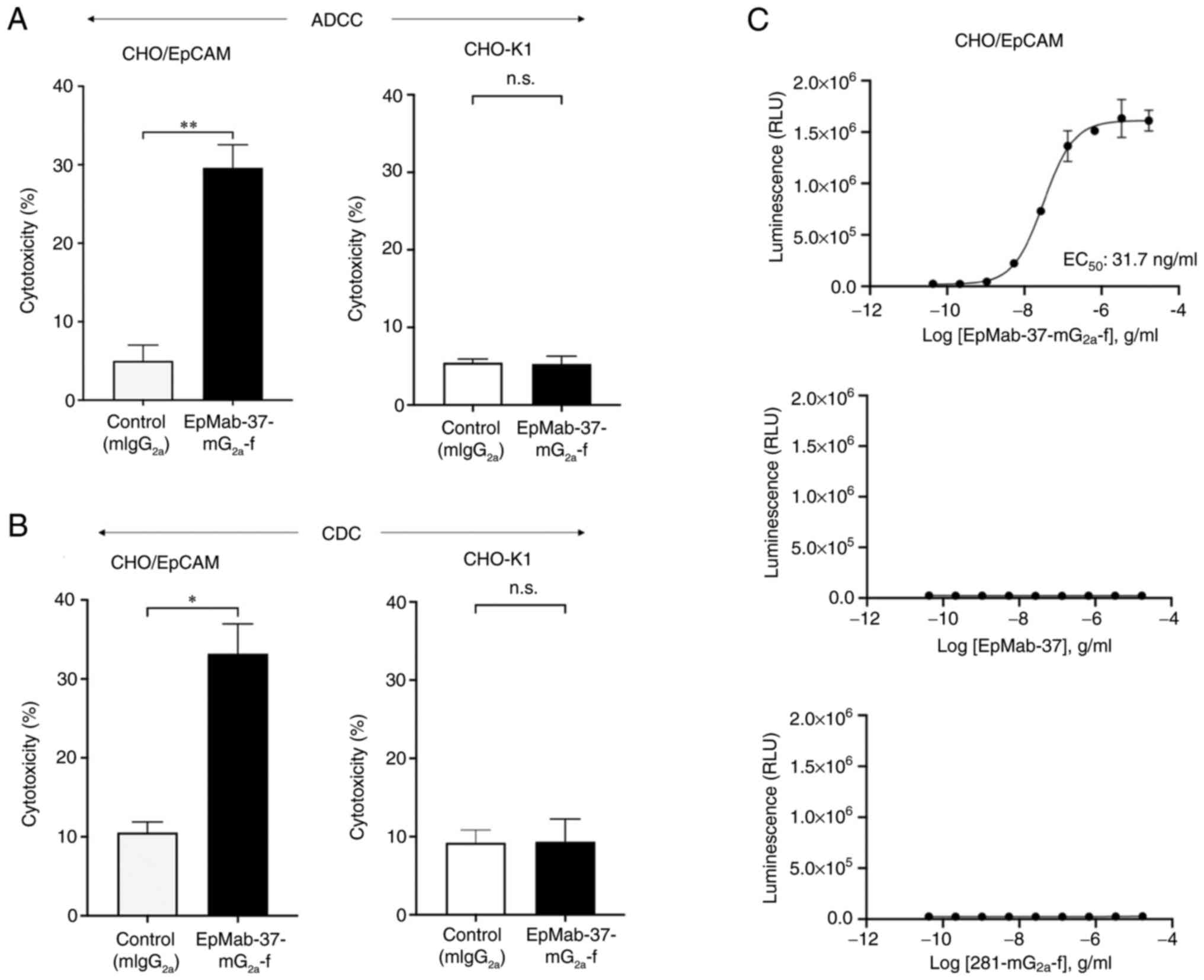
EpMab-37-mG2a-f-mediated A DCC
and CDC in CHO/EpCAM cells
The present study then investigated whether
EpMab-37-mG2a-f was capable of mediating ADCC against
CHO/EpCAM cells. EpMab-37-mG2a-f exhibited ADCC (29.6%
cytotoxicity) against CHO/EpCAM cells more effectively than the
control mouse IgG2a (5.0% cytotoxicity; P<0.01). No
notable difference was found between EpMab-37-mG2a-f and
control mouse IgG2a in ADCC levels against CHO-K1
(Fig. 2A).
Subsequently, it was examined whether
EpMab-37-mG2a-f could exert CDC against CHO/EpCAM cells.
As demonstrated in Fig. 2B,
EpMab-37-mG2a-f induced a higher degree of CDC (33.2%
cytotoxicity) in CHO/EpCAM cells compared with that induced by
control mouse IgG2a (10.5% cytotoxicity; P<0.05).
There was no marked difference between EpMab-37-mG2a-f
and control mouse IgG2a in the observed CDC levels
against CHO-K1 (Fig. 2B).
The ADCC reporter bioassay is a bioluminescent
reporter gene assay for the quantification of the biological
activity of the antibody via FcγRIIIa-mediated pathway activation
in an ADCC mechanism of action (40). In the present study, to compare
the ADCC pathway activation by EpMab-37-mG2a-f and
EpMab-37, the CHO/EpCAM cells were treated with serially diluted
mAbs, and then incubated with effector Jurkat cells, which express
the human FcγRIIIa receptor and an NFAT response element driving
Firefly luciferase. Furthermore, defucosylated anti-hamster PDPN
(mouse IgG2a Ab) (281-mG2a-f) was also used
as a control since defucosylated control mouse IgG2a was
unavailable. It was confirmed that 281-mG2a-f never
recognized the target cells. As demonstrated in Fig. 2C, EpMab-37-mG2a-f
activated the effector (EC50; 31.7 ng/ml) in a
concentration-dependent manner, whereas EpMab-37 and
281-mG2a-f did not. These results indicated that
EpMab-37-mG2a-f exhibits superior ADCC activity against
CHO/EpCAM cells compared with EpMab-37.
Antitumor effects of
EpMab-37-mG2a-f in the mouse xenografts of CHO/EpCAM
cells
In the CHO/EpCAM xenograft tumors,
EpMab-37-mG2a-f and control mouse IgG were injected into
mice intraperitoneally on days 6 and 14, following CHO/EpCAM cell
inoculation. On days 6, 11, 14, 18 and 20 following the
inoculation, the tumor volume was measured. The
EpMab-37-mG2a-f administration resulted in a significant
reduction in tumor volume on days 14 (P<0.05), 18 (P<0.01)
and 20 (P<0.01) compared with that of the control mouse IgG
(Fig. 3A). The
EpMab-37-mG2a-f administration resulted in a 50%
reduction in tumor volume compared with that of the control mouse
IgG on day 20.
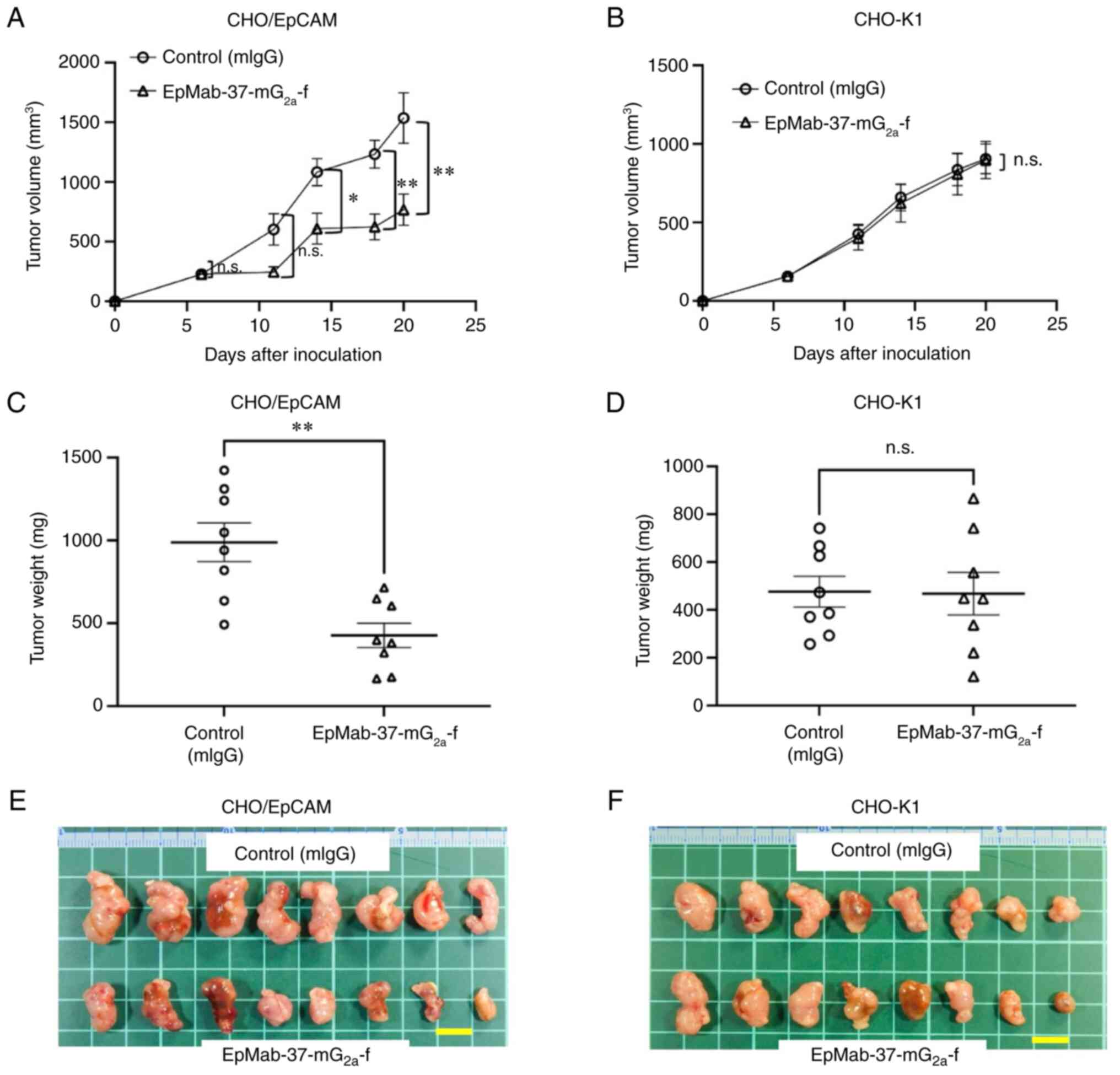 | Figure 3Antitumor activity of
EpMab-37-mG2a-f. (A and B) Measurement of tumor volume
in (A) CHO/EpCAM and (B) CHO-K1 xenograft models. CHO/EpCAM and
CHO-K1 cells (5×106 cells) were injected into mice
subcutaneously. On day 6, 100 µg EpMab-37-mG2a-f
or mIgG were injected into mice intraperitoneally. On day 14,
additional antibodies were injected. On days 6, 11, 14, 18 and 20
following the inoculation, the tumor volume was measured. Values
are presented as the mean ± SEM. *P<0.05 and
**P<0.01 (ANOVA and Sidak's multiple comparisons
test). (C and D) The weight of the excised (C) CHO/EpCAM and (D)
CHO-K1 xenografts was measured on day 20. Values are presented as
the mean ± SEM. **P<0.01 (Welch's t-test). (E and F)
The resected tumors appearance of (E) CHO/EpCAM and (F) CHO-K1
xenografts in the control mouse IgG and EpMab-37-mG2a-f
treated groups on day 20 (scale bar, 1 cm). n.s., not significant;
CHO, Chinese hamster ovary; EpCAM, epithelial cell adhesion
molecule; mIgG, mouse IgG. |
The weight of CHO/EpCAM tumors treated with
EpMab-37-mG2a-f was significantly lower than that of
tumors treated with control mouse IgG (57% reduction; P<0.01;
Fig. 3C). CHO/EpCAM tumors that
were resected from mice on day 20 are depicted in Fig. 3E.
In the CHO-K1 xenograft models,
EpMab-37-mG2a-f and control mouse IgG were
intraperitoneally injected into mice on days 6 and 14 following the
inoculation of CHO-K1 cells. On days 6, 11, 14, 18 and 20 after the
inoculation of cells, the tumor volume was measured. No marked
differences were observed between EpMab-37-mG2a-f and
control mouse IgG as regards CHO-K1 tumor volume (Fig. 3B) and weight (Fig. 3D). CHO-K1 tumors that were
resected from mice on day 20 are demonstrated in Fig. 3F.
The loss of body weight was not observed in the
CHO/EpCAM (Fig. 4A) and CHO-K1
(Fig. 4B) tumor-implanted mice.
The mice on day 20 are demonstrated in Fig. 4C and D.
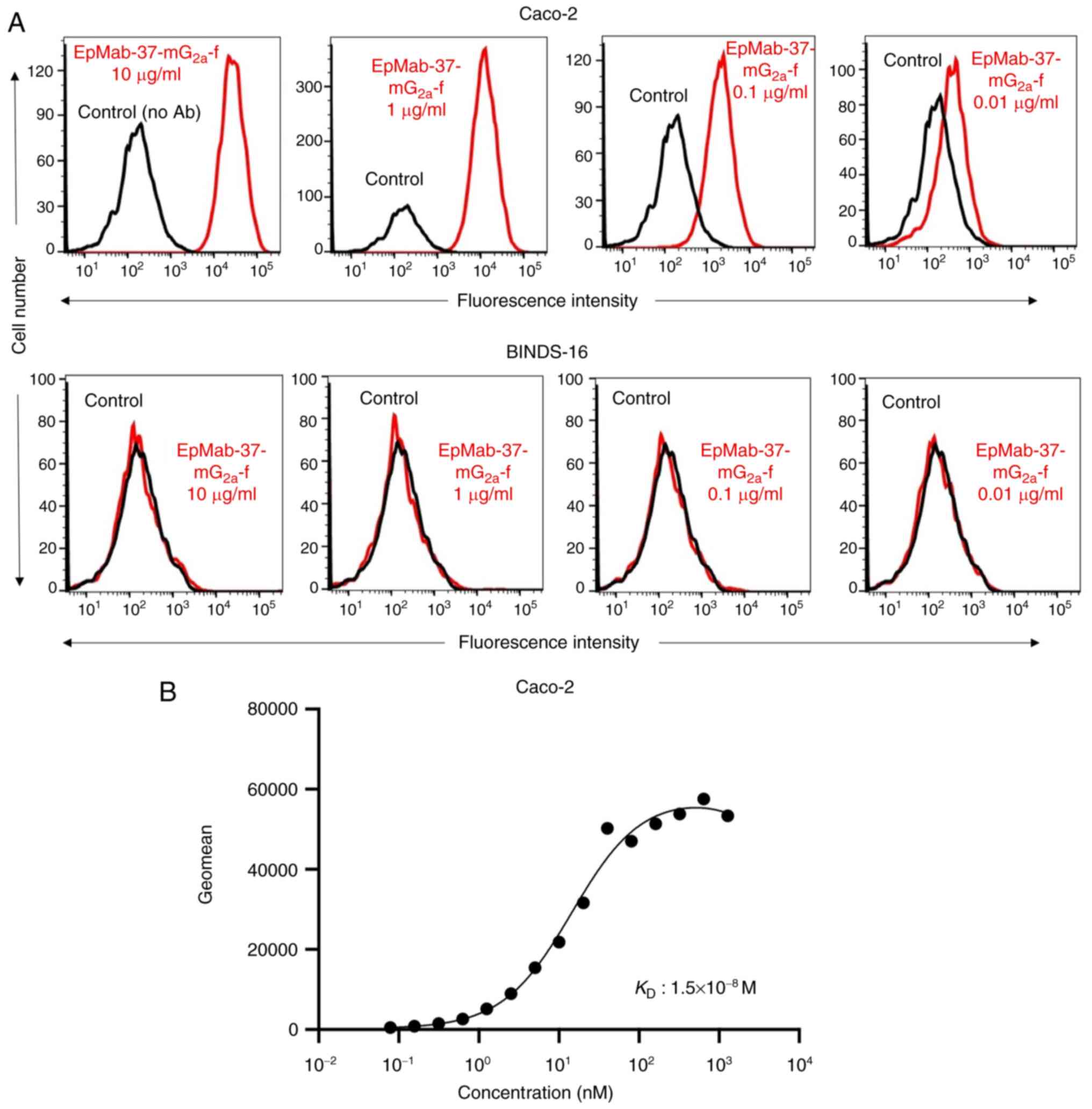
Flow cytometry of Caco-2 cells using
EpMab-37-mG2a-f
As demonstrated in Figs. 5A and S1B, EpMab-37-mG2a-f detected
Caco-2 cells in a concentration-dependent manner. By contrast,
EpMab-37 did not react with Caco-2 cells in which EpCAM was knocked
out (BINDS-16). The increased expression of EpCAM could also be
detected in other colorectal cancer cell lines tested (Fig. S2). A kinetic analysis of the
binding of EpMab-37-mG2a-f to Caco-2 cells was performed
using flow cytometry. The KD for the interaction
of EpMab-37-mG2a-f with Caco-2 cells was
1.5×10−8 M (Fig. 5B),
suggesting that EpMab-37-mG2a-f exhibits moderate
affinity for Caco-2 cells.
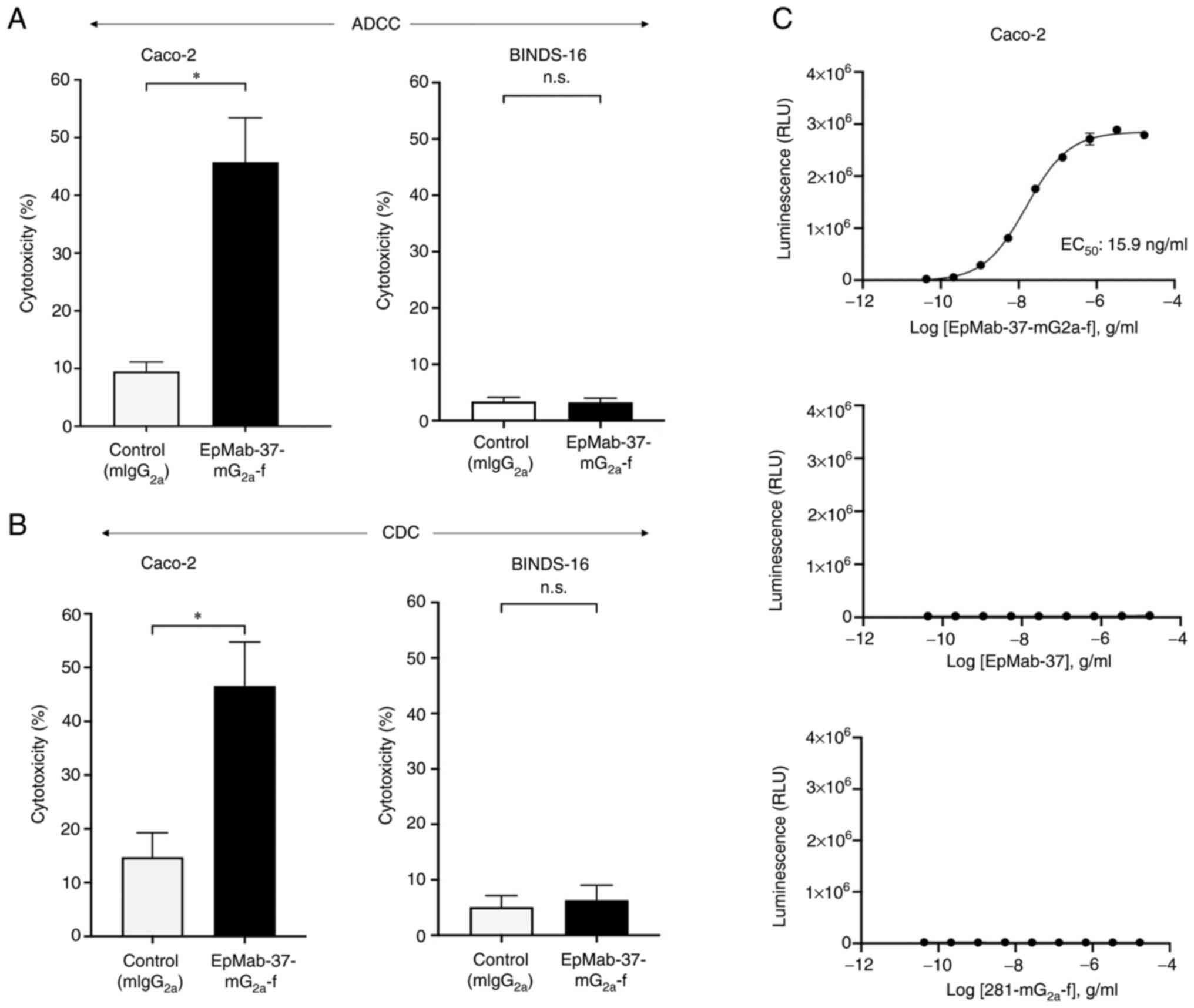
EpMab-37-mG2a-f-mediated ADCC
and CDC in Caco-2 and BINDS-16 cells
The present study then investigated whether
EpMab-37-mG2a-f is capable of mediating ADCC against
Caco-2 and BINDS-16 cells. As revealed in Fig. 6A, EpMab-37-mG2a-f
demonstrated ADCC (45.7% cytotoxicity) against Caco-2 cells, more
potently than the control mouse IgG2a (9.5%
cytotoxicity; P<0.05). It was then investigated whether
EpMab-37-mG2a-f exhibited CDC against Caco-2 cells.
EpMab-37-mG2a-f induced a higher degree of CDC (46.6%
cytotoxicity) in Caco-2 cells compared with that induced by control
mouse IgG2a (14.7% cytotoxicity; P<0.05) (Fig. 6B). By contrast, ADCC and CDC were
not induced by EpMab-37-mG2a-f in BINDS-16 cells
(Fig. 6A and B). The ADCC
reporter bioassay for Caco-2 cells also revealed that the only
EpMab-37-mG2a-f exhibited the ADCC effector activation
(EC50; 15.9 ng/ml) (Fig.
6C). These results demonstrated that EpMab-37-mG2a-f
exhibited potent ADCC and CDC against Caco-2 cells.
Antitumor effects of
EpMab-37-mG2a-f on Caco-2 and BINDS-16 xenografts
In the Caco-2 xenograft models,
EpMab-37-mG2a-f and control mouse IgG were
intraperitoneally injected on days 6, 14 and 20, following the
inoculation of Caco-2 cells. The tumor volume was measured on days
6, 11, 14, 18, 20, 25 and 27 after the injection. The
administration of EpMab-37-mG2a-f resulted in a
significant reduction in tumor growth on days 14 (P<0.05), 18
(P<0.01), 20 (P<0.01), 25 (P<0.01) and 27 (P<0.01) as
compared with the control mouse IgG (Fig. 7A). The administration of
EpMab-37-mG2a-f resulted in a 42% reduction in tumor
volume compared with the control mouse IgG on day 27. Tumors from
the EpMab-37-mG2a-f-treated mice weighed significantly
less than those from the control mouse IgG-treated mice (28%
reduction; P<0.01, Fig. 7C).
Tumors that were resected from mice on day 27 are demonstrated in
Fig. 7E. By contrast, the
antitumor effects of EpMab-37-mG2a-f on BINDS-16 were
not observed (Fig. 7B and D).
BINDS-16 tumors that were resected from mice on day 27 are
demonstrated in Fig. 7F.
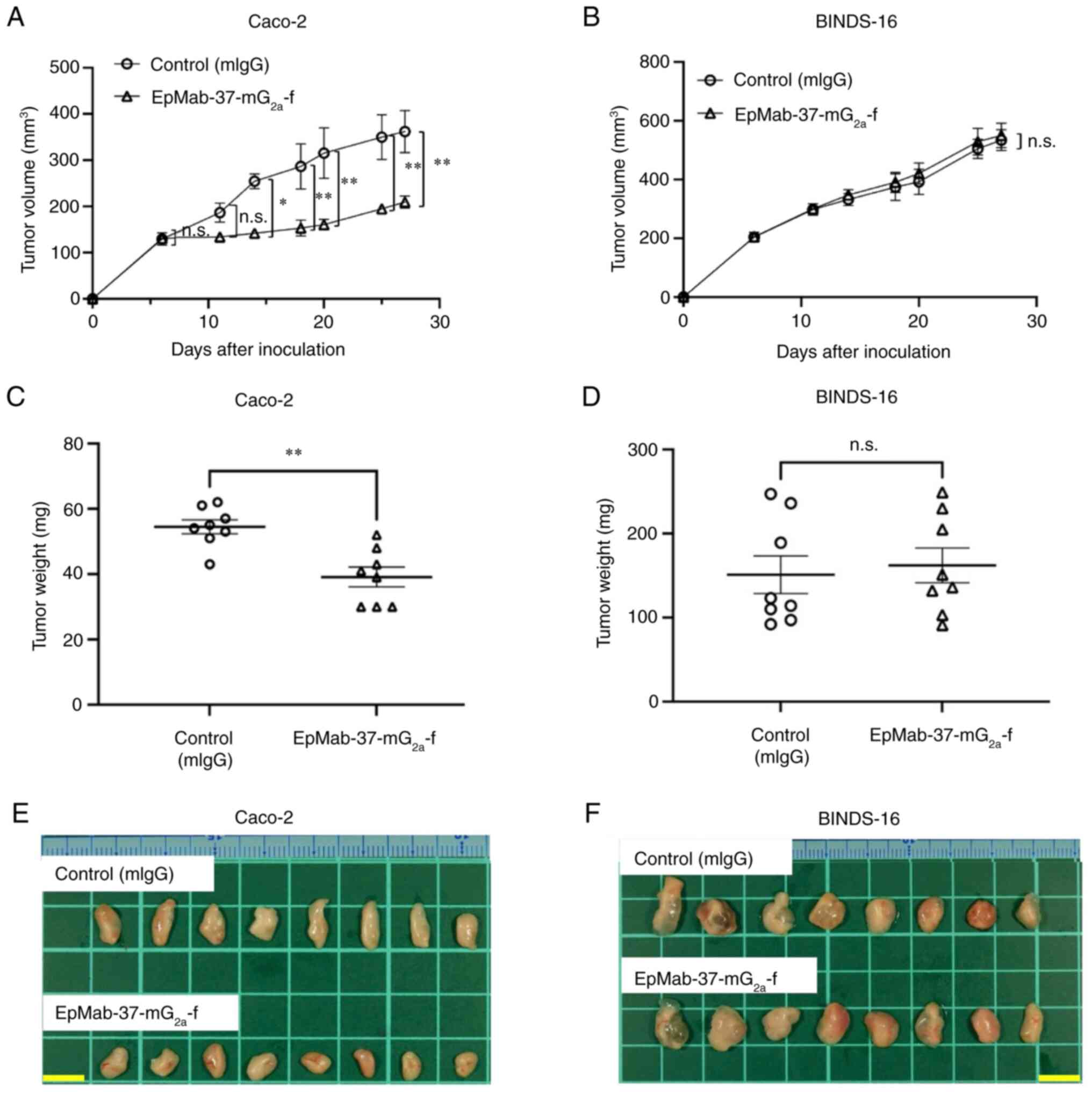 | Figure 7Antitumor activity of
EpMab-37-mG2a-f against Caco-2 and BINDS-16 xenografts.
(A and B) Measurement of tumor volume in (A) Caco-2 and (B)
BINDS-16 (Caco-2 cells in which EpCAM was knocked out)
xenograft-implanted mice. Caco-2 and BINDS-16 cells
(5×106 cells) were inoculated into mice subcutaneously.
On day 6, 100 µg EpMab-37-mG2a-f or mIgG were
injected into mice intraperitoneally. On days 14 and 20, additional
antibodies were injected. On days 6, 11, 14, 18, 20, 25 and 27
following the inoculation, the tumor volume was measured. Values
are presented as the mean ± SEM. *P<0.05 and
**P<0.01 (ANOVA and Sidak's multiple comparisons
test). (C and D) The weight of excised xenografts of (C) Caco-2 and
(D) BINDS-16 was measured on day 27. Values are presented as the
mean ± SEM. **P<0.01 (Welch's t-test). (E and F) The
resected tumors appearance of (E) Caco-2 and (F) BINDS-16
xenografts in the control mouse IgG and EpMab-37-mG2a-f
treated groups on day 27 (scale bar, 1 cm). n.s., not significant;
mIgG, mouse IgG; EpCAM, epithelial cell adhesion molecule. |
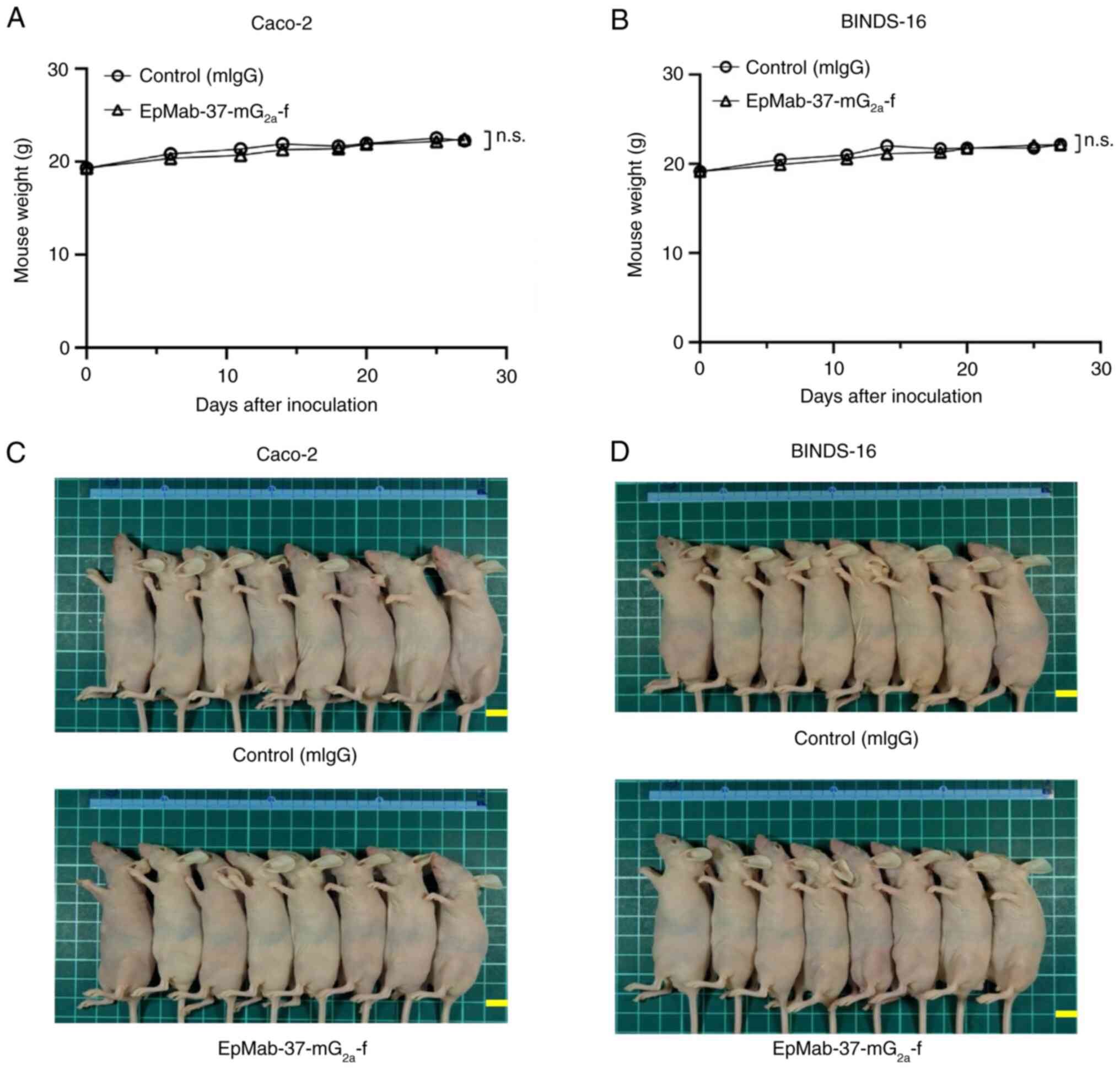
The loss of body weight was not observed in Caco-2
and BINDS-16 tumor-implanted mice (Fig. 8A and B). The mice on day 27 are
demonstrated in Fig. 8C and
D.
Discussion
In the present study, a mouse IgG1
subclass of EpMab-37 was converted into a mouse IgG2a,
and a defucosylated form (EpMab-37-mG2a-f) was produced
in order to enhance ADCC activity. In fact,
EpMab-37-mG2a-f exhibited a high ADCC activity in
vitro (Figs. 2 and 6), and exhibited potent antitumor
activity against CHO/EpCAM (Fig. 3A
and C) and Caco-2 xenografts (Fig. 7A and C). Notably,
EpMab-37-mG2a-f did not affect the growth of tumors
derived from Caco-2 cells in which EpCAM was knocked out (BINDS-16)
(Fig. 7B and D), indicating that
EpMab-37-mG2a-f can target EpCAM-positive tumors
selectively. Additionally, it was observed that a higher EpCAM
expression could be detected in HCT-15, COLO201 and COLO205 than
Caco-2 cells (Fig. S2). Further
studies are required to investigate the antitumor activity of
EpMab-37-mG2a-f against these cell lines.
The EpCAM N-terminal domain (residues 24-63)
contains the EGF-like domain, which is targeted by the vast
majority of anti-EpCAM mAbs, including HEA125 (used in
CellSearch®), 17-1A (Edrecolomab), C215 (used in
Catumaxomab) and MOC31 (used in Oportuzumab) probably due to its
high accessibility and antigenicity on plasma membrane (43,44). By contrast, the anti-EpCAM mAbs
targeting the thyroglobulin-like domain (residues 64-138) or the
extracellular C-terminal domain (residues 139-265; CD) are rare
(43). Among the clinically
tested mAbs, MT201 (Adecatumumab) was reported to recognize the
167-QKEIT-171 sequence in the EpCAM CD (45). The epitope mapping of EpMab-37 was
previously performed by the authors and revealed that EpCAM
(residues 144-164) are involved in its recognition (25). In crystal structure analysis, this
domain forms β-sheet and loop structures, and exposed on the
molecular surface when EpCAM forms cis-dimer or trans-tetramer
(43). Therefore, EpMab-37 has a
unique epitope, and is expected a different mode of action.
However, EpMab-37 never recognized EpCAM peptides including 144 to
163 amino acids, suggesting that EpMab-37 has the conformational
epitopes (25). To determine the
conformational epitopes, the epitope mapping strategy was
developed, including Arg, Ile, Glu, Asp and Leu (RIEDL)-insertion
for epitope mapping (REMAP) (46-49) and His-tag insertion for the
epitope mapping (HisMAP) method (50,51). The detailed determination of
EpMab-37 epitope could contribute the understanding of recognition
by EpMab-37.
It was observed that tumors derived from Caco-2
cells in which EpCAM was knocked out exhibited a larger weight and
volume compared to parental ones (Fig. 7). Huang et al (52) reviewed the effect of EpCAM
depletion in Caco-2 cells and Xenopus embryos. In
EpCAM-depleted Caco-2 cells, the elevated PKC activity resulted in
the downregulation of E-cadherin. A similar phenomenon was observed
in Xenopus embryos (53).
Huang et al (52)
mentioned that these events were 'two noteworthy exceptions' in the
regulation of Cadherins by EpCAM. E-cadherin downregulation
sometimes leads to epithelial-to-mesenchymal transition and an
increase in tumorigenicity (54).
Although the in vitro cell proliferation of parental and
EpCAM knocked out Caco-2 is similar in the experiments of the
present study, the aforementioned phenomenon may influence the
growth in vivo.
In the present study, mouse IgG2a and
281-mG2a-f were used as controls in ADCC/CDC and ADCC
reporter assays, respectively, which are mAbs that could recognize
unknown (mouse IgG2a) and known (281-mG2a-f)
targets. It was confirmed that they were not specific for the
target cells. Therefore, they are suitable as negative controls
in vitro for ADCC/CDC and ADCC reporter assays. However,
concerning the in vivo analysis, it could not be excluded
that they recognize unidentified target and exhibit the side
effects in mice. For the aforementioned reasons, normal mouse IgG
(a mixture of diverse antibodies) was used as the control in the
xenograft assays. In future xenograft studies, it will be also
necessary to test IgG, IgG2a and/or
281-mG2a-f and EpMab-37-mG2a-f.
EpCAM-overexpressing CTCs exhibit cancer stem cell
features (55). CTCs, which
express EpCAM, CD44, CD47 and MET, identify as a subset with
increased metastasis-initiating phenotype (56), suggesting that EpCAM plays a
crucial role in cancer stemness and metastasis. Recently, CTC
expansion methods, including two-dimensional (2D) long-term
expansion, 3D organoids/spheroids culture, and xenografts, have
been developed to evaluate the character of CTCs (57). Therefore, the biological
characteristic of affecting cell proliferation and invasion need to
be investigated as Adecatumumab, which has an epitope close to that
of EpMab-37, exerts weak anti-proliferative effects in the absence
of complement and immune effector cells (45). Moreover, it would be worthwhile to
investigate the effect of EpMab-37-mG2a-f on the CTC
expansion in vitro and metastasis in vivo.
Antibody-drug conjugate (ADC) exhibits cytotoxicity
through the contents released from endocytic receptor-bound
mAbs-drug conjugate (9).
Oportuzumab monatox (Vicinium, VB4-845) is an EpCAM-targeting scFv,
conjugated with Pseudomonas exotoxin A, an inhibitor of
translation through ADP-ribosylation of eukaryotic elongation
factor-2 (58). Vicinium has been
developed for the treatment of bacillus Calmette-Guérin
(BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC)
(15). Vicinium was approved by
the FDA as fast track designation and evaluated in the single-arm
Phase 3 VISTA study (NCT02449239) for the patients with high-grade
NMIBC previously treated with BCG. However, FDA did not approve
Vicinium for BCG-unresponsive NMIBC (59). An anti-dog PDPN mAb conjugated
anti-body with emtansine was developed as the contents (P38B-DM1)
(60-62) P38B-DM1 exhibited cytotoxicity to
dog PDPN expressed CHO-K1 cells, and demonstrated more a potent
anti-tumor effect than P38B in the xenograft model (63). Moreover, anti-trophoblast
cell-surface antigen 2 (TROP2) ADC (Sacituzumab govitecan-hziy) has
been approved by the FDA (64).
TROP2 is a paralogue of EpCAM (65). As Sacituzumab possesses the potent
internalization activity of TROP2 (66), the capacity of EpCAM
internalization by EpMab-37-mG2a-f needs to be further
investigated for the development of the ADC.
The applications of anti-EpCAM mAbs in the clinic
are still limited, since anti-EpCAM mAbs may generate side-effects
by affecting normal tissues. CasMabs targeting PDPN (67-70) and podocalyxin (71) have been previously developed by
the authors, which are currently applied to chimeric antigen
receptor T-cell therapy in mice models (72-74). It will be of great value to
establish cancer-specific anti-EpCAM mAbs using the CasMab method.
In the future, anti-EpCAM CasMab production may be applicable as a
basis for designing and optimizing potent immunotherapy modalities,
including ADCs and chimeric antigen receptor T-cell therapy.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
GL, TO, TT, MY, TN, TA and TY performed the
experiments. MKK, MK and YK designed the experiments. GL, TO, HS
and YK analyzed the data. GL, HS, and YK wrote the manuscript. All
authors have read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. HS and YK confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The animal study protocol was approved (approval
no. 2022-024) by the Institutional Committee for Experiments of the
Institute of Microbial Chemistry (Numazu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Saori Okuno and
Ms. Saori Handa (Department of Antibody Drug Development, Tohoku
University Graduate School of Medicine) for providing technical
assistance for the completion of the in vitro experiments,
and Mr. Shun-ichi Ohba and Ms. Akiko Harakawa [Institute of
Microbial Chemistry (BIKAKEN), Numazu, Microbial Chemistry Research
Foundation] for providing technical assistance in performing the
animal experiments.
Funding
The present study was supported in part by Japan Agency for
Medical Research and Development (AMED; grant nos. JP22ama121008,
JP21am0401013, JP22bm1004001, JP22ck0106730 and JP21am0101078).
Abbreviations:
|
EpCAM
|
epithelial cell adhesion molecule
|
|
mAb
|
monoclonal antibody
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
CDC
|
complement-dependent cytotoxicity
|
|
FDA
|
Food and Drug Administration
|
|
FcγR
|
Fcγ receptor
|
|
NK
|
natural killer
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
PBS
|
phosphate-buffered saline
|
|
KD
|
dissociation constant
|
References
|
1
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: More than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown TC, Sankpal NV and Gillanders WE:
Functional implications of the dynamic regulation of EpCAM during
epithelial-to-mesenchymal transition. Biomolecules. 11:9562021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eyvazi S, Farajnia S, Dastmalchi S,
Kanipour F, Zarredar H and Bandehpour M: Antibody based EpCAM
targeted therapy of cancer, review and update. Curr Cancer Drug
Targets. 18:857–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao J, Pohlmann PR, Isaacs C, Weinberg
BA, He AR, Schlegel R and Agarwal S: Circulating tumor cells:
Technologies and their clinical potential in cancer metastasis.
Biomedicines. 9:11112021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe M, Kenmotsu H, Ko R, Wakuda K,
Ono A, Imai H, Taira T, Naito T, Murakami H, Abe M, et al:
Isolation and molecular analysis of circulating tumor cells from
lung cancer patients using a microfluidic chip type cell sorter.
Cancer Sci. 109:2539–2548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lampignano R, Yang L, Neumann MHD, Franken
A, Fehm T, Niederacher D and Neubauer H: A novel workflow to enrich
and isolate patient-matched EpCAMhigh and
EpCAMlow/negative CTCs enables the comparative
characterization of the PIK3CA status in metastatic breast cancer.
Int J Mol Sci. 18:18852017. View Article : Google Scholar
|
|
8
|
Zapatero A, Gómez-Caamaño A, Cabeza
Rodriguez MÁ, Muinelo-Romay L, Martin de Vidales C, Abalo A, Calvo
Crespo P, Leon Mateos L, Olivier C and Vega Piris LV: Detection and
dynamics of circulating tumor cells in patients with high-risk
prostate cancer treated with radiotherapy and hormones: A
prospective phase II study. Radiat Oncol. 15:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsao LC, Force J and Hartman ZC:
Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer
Res. 81:4641–4651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McInnes IB and Gravallese EM:
Immune-mediated inflammatory disease therapeutics: Past, present
and future. Nat Rev Immunol. 21:680–686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herlyn M, Steplewski Z, Herlyn D and
Koprowski H: Colorectal carcinoma-specific antigen: Detection by
means of monoclonal antibodies. Proc Natl Acad Sci USA.
76:1438–1442. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baeuerle PA and Gires O: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sears HF, Atkinson B, Mattis J, Ernst C,
Herlyn D, Steplewski Z, Häyry P and Koprowski H: Phase-I clinical
trial of monoclonal antibody in treatment of gastrointestinal
tumours. Lancet. 1:762–765. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riethmüller G, Holz E, Schlimok G,
Schmiegel W, Raab R, Höffken K, Gruber R, Funke I, Pichlmaier H,
Hirche H, et al: Monoclonal antibody therapy for resected Dukes' C
colorectal cancer: Seven-year outcome of a multicenter randomized
trial. J Clin Oncol. 16:1788–1794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaplon H, Muralidharan M, Schneider Z and
Reichert JM: Antibodies to watch in 2020. MAbs. 12:17035312020.
View Article : Google Scholar :
|
|
16
|
Zhang X, Yang Y, Fan D and Xiong D: The
development of bispecific antibodies and their applications in
tumor immune escape. Exp Hematol Oncol. 6:122017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schönberger S, Kraft D, Nettersheim D,
Schorle H, Casati A, Craveiro RB, Mohseni MM, Calaminus G and
Dilloo D: Targeting EpCAM by a bispecific trifunctional antibody
exerts profound cytotoxic efficacy in germ cell tumor cell lines.
Cancers (Basel). 12:12792020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruf P, Kluge M, Jäger M, Burges A, Volovat
C, Heiss MM, Hess J, Wimberger P, Brandt B and Lindhofer H:
Pharmacokinetics, immunogenicity and bioactivity of the therapeutic
antibody catumaxomab intraperitoneally administered to cancer
patients. Br J Clin Pharmacol. 69:617–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mau-Sørensen M, Dittrich C, Dienstmann R,
Lassen U, Büchler W, Martinius H and Tabernero J: A phase I trial
of intravenous catumaxomab: A bispecific monoclonal antibody
targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother
Pharmacol. 75:1065–1073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knödler M, Körfer J, Kunzmann V, Trojan J,
Daum S, Schenk M, Kullmann F, Schroll S, Behringer D, Stahl M, et
al: Randomised phase II trial to investigate catumaxomab
(anti-EpCAM x anti-CD3) for treatment of peritoneal carcinomatosis
in patients with gastric cancer. Br J Cancer. 119:296–302. 2018.
View Article : Google Scholar
|
|
21
|
Linke R, Klein A and Seimetz D:
Catumaxomab: Clinical development and future directions. MAbs.
2:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira NA, Chan KF, Lin PC and Song Z:
The 'less-is-more' in therapeutic antibodies: Afucosylated
anti-cancer antibodies with enhanced antibody-dependent cellular
cytotoxicity. MAbs. 10:693–711. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shinkawa T, Nakamura K, Yamane N,
Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M,
Yamasaki M, et al: The absence of fucose but not the presence of
galactose or bisecting N-acetylglucosamine of human IgG1
complex-type oligosaccharides shows the critical role of enhancing
antibody-dependent cellular cytotoxicity. J Biol Chem.
278:3466–3473. 2003. View Article : Google Scholar
|
|
24
|
Yamane-Ohnuki N, Kinoshita S,
Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R,
Sakurada M, Uchida K, et al: Establishment of FUT8 knockout Chinese
hamster ovary cells: An ideal host cell line for producing
completely defucosylated antibodies with enhanced
antibody-dependent cellular cytotoxicity. Biotechnol Bioeng.
87:614–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li G, Suzuki H, Asano T, Tanaka T, Suzuki
H, Kaneko MK and Kato Y: Development of a novel anti-EpCAM
monoclonal anti-body for various applications. Antibodies (Basel).
11:412022. View Article : Google Scholar
|
|
26
|
Hosono H, Ohishi T, Takei J, Asano T,
Sayama Y, Kawada M, Kaneko MK and Kato Y: The anti-epithelial cell
adhesion molecule (EpCAM) monoclonal antibody EpMab-16 exerts
antitumor activity in a mouse model of colorectal adenocarcinoma.
Oncol Lett. 20:3832020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaneko MK, Ohishi T, Takei J, Sano M,
Nakamura T, Hosono H, Yanaka M, Asano T, Sayama Y, Harada H, et al:
Anti-EpCAM monoclonal antibody exerts antitumor activity against
oral squamous cell carcinomas. Oncol Rep. 44:2517–2526. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Queiroz AL, Dantas E, Ramsamooj S, Murthy
A, Ahmed M, Zunica ERM, Liang RJ, Murphy J, Holman CD, Bare CJ, et
al: Blocking ActRIIB and restoring appetite reverses cachexia and
improves survival in mice with lung cancer. Nat Commun.
13:46332022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takei J, Kaneko MK, Ohishi T, Hosono H,
Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al: A
defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts
antitumor effects in mouse xenograft models of oral squamous cell
carcinoma. Oncol Rep. 44:1949–1960. 2020.PubMed/NCBI
|
|
30
|
Takei J, Ohishi T, Kaneko MK, Harada H,
Kawada M and Kato Y: A defucosylated anti-PD-L1 monoclonal antibody
13-mG2a-f exerts antitumor effects in mouse xenograft
models of oral squamous cell carcinoma. Biochem Biophys Rep.
24:1008012020.
|
|
31
|
Li G, Ohishi T, Kaneko MK, Takei J, Mizuno
T, Kawada M, Saito M, Suzuki H and Kato Y: Defucosylated mouse-dog
chimeric anti-EGFR antibody exerts antitumor activities in mouse
xenograft models of canine tumors. Cells. 10:35992021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tateyama N, Nanamiya R, Ohishi T, Takei J,
Nakamura T, Yanaka M, Hosono H, Saito M, Asano T, Tanaka T, et al:
Defucosylated anti-epidermal growth factor receptor monoclonal
antibody 134-mG2a-f exerts antitumor activities in mouse
xenograft models of dog epidermal growth factor
receptor-overexpressed cells. Monoclon Antib Immunodiagn
Immunother. 40:177–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goto N, Suzuki H, Ohishi T, Harakawa A, Li
G, Saito M, Takei J, Tanaka T, Asano T, Sano M, et al: Antitumor
activities in mouse xenograft models of canine fibroblastic tumor
by defucosylated anti-epidermal growth factor receptor monoclonal
antibody. Monoclon Antib Immunodiagn Immunother. 41:67–73. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nanamiya R, Takei J, Ohishi T, Asano T,
Tanaka T, Sano M, Nakamura T, Yanaka M, Handa S, Tateyama N, et al:
Defucosylated anti-epidermal growth factor receptor monoclonal
antibody (134-mG2a-f) exerts antitumor activities in
mouse xenograft models of canine osteosarcoma. Monoclon Antib
Immunodiagn Immunother. 41:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki H, Ohishi T, Asano T, Tanaka T,
Saito M, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK and Kato Y:
Defucosylated mouse-dog chimeric anti-HER2 monoclonal antibody
exerts antitumor activities in mouse xenograft models of canine
tumors. Oncol Rep. 48:1542022. View Article : Google Scholar :
|
|
36
|
Tanaka T, Ohishi T, Saito M, Suzuki H,
Kaneko MK, Kawada M and Kato Y: Defucosylated anti-epidermal growth
factor receptor monoclonal antibody exerted antitumor activities in
mouse xenograft models of canine mammary gland tumor. Monoclon
Antib Immunodiagn Immunother. 41:142–149. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nanamiya R, Suzuki H, Takei J, Li G, Goto
N, Harada H, Saito M, Tanaka T, Asano T, Kaneko MK and Kato Y:
Development of monoclonal antibody 281-mG2a-f against
golden hamster podoplanin. Monoclon Antib Immunodiagn Immunother.
Apr 27–2022.Epub ahead of print.
|
|
38
|
Itai S, Ohishi T, Kaneko MK, Yamada S, Abe
S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y, et al:
Anti-podocalyxin antibody exerts antitumor effects via
antibody-dependent cellular cytotoxicity in mouse xenograft models
of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett 1. 9:2809–2816.
2020.
|
|
40
|
Garvin D, Stecha P, Gilden J, Wang J,
Grailer J, Hartnett J, Fan F, Cong M and Cheng ZJ: Determining ADCC
activity of antibody-based therapeutic molecules using two
bioluminescent reporter-based bioassays. Curr Protoc. 1:e2962021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kosterink JGW, McLaughlin PM, Lub-de Hooge
MN, Hendrikse HH, van Zanten J, van Garderen E, Harmsen MC and de
Leij LFMH: Biodistribution studies of epithelial cell adhesion
molecule (EpCAM)-directed monoclonal antibodies in the
EpCAM-transgenic mouse tumor model. J Immunol. 179:1362–1368. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato Y, Ohishi T, Takei J, Nakamura T,
Kawada M and Kaneko MK: An antihuman epidermal growth factor
receptor 2 monoclonal antibody (H2Mab-19) exerts
antitumor activity in glioblastoma xenograft models. Monoclon Antib
Immunodiagn Immunother. 39:135–139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pavšič M, Gunčar G, Djinović-Carugo K and
Lenarčič B: Crystal structure and its bearing towards an
understanding of key biological functions of EpCAM. Nat Commun.
5:47642014. View Article : Google Scholar
|
|
44
|
Gaber A, Lenarčič B and Pavšič M: Current
view on EpCAM structural biology. Cells. 9:13612020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Münz M, Murr A, Kvesic M, Rau D, Mangold
S, Pflanz S, Lumsden J, Volkland J, Fagerberg J, Riethmüller G, et
al: Side-by-side analysis of five clinically tested anti-EpCAM
monoclonal antibodies. Cancer Cell Int. 10:442010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asano T, Kaneko MK and Kato Y: Development
of a novel epitope mapping system: RIEDL insertion for epitope
mapping method. Monoclon Antib Immunodiagn Immunother. 40:162–167.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asano T, Kaneko MK, Takei J, Tateyama N
and Kato Y: Epitope mapping of the anti-CD44 monoclonal antibody
(C44Mab-46) using the REMAP method. Monoclon Antib
Immunodiagn Immunother. 40:156–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nanamiya R, Sano M, Asano T, Yanaka M,
Nakamura T, Saito M, Tanaka T, Hosono H, Tateyama N, Kaneko MK and
Kato Y: Epitope mapping of an anti-human epidermal growth factor
receptor monoclonal antibody (EMab-51) using the RIEDL insertion
for epitope mapping method. Monoclon Antib Immunodiagn Immunother.
40:149–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sano M, Kaneko MK, Aasano T and Kato Y:
Epitope mapping of an antihuman EGFR monoclonal antibody (EMab-134)
using the REMAP method. Monoclon Antib Immunodiagn Immunother.
40:191–195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asano T, Suzuki H, Kaneko MK and Kato Y:
Epitope mapping of rituximab using HisMAP method. Monoclon Antib
Immunodiagn Immunother. 41:8–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suzuki H, Asano T, Tanaka T, Kaneko MK and
Kato Y: Epitope mapping of the anti-CD20 monoclonal antibodies
(C20Mab-11 and 2H7) using HisMAP method. Monoclon Antib Immunodiagn
Immunother. 41:20–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang L, Yang Y, Yang F, Liu S, Zhu Z, Lei
Z and Guo J: Functions of EpCAM in physiological processes and
diseases (review). Int J Mol Med. 42:1771–1785. 2018.PubMed/NCBI
|
|
53
|
Maghzal N, Kayali HA, Rohani N, Kajava AV
and Fagotto F: EpCAM controls actomyosin contractility and cell
adhesion by direct inhibition of PKC. Dev Cell. 27:263–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Munz M, Baeuerle PA and Gires O: The
emerging role of EpCAM in cancer and stem cell signaling. Cancer
Res. 69:5627–5629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baccelli I, Schneeweiss A, Riethdorf S,
Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T,
Wallwiener M, et al: Identification of a population of blood
circulating tumor cells from breast cancer patients that initiates
metastasis in a xenograft assay. Nat Biotechnol. 31:539–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rupp B, Ball H, Wuchu F, Nagrath D and
Nagrath S: Circulating tumor cells in precision medicine:
Challenges and opportunities. Trends Pharmacol Sci. 43:378–391.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dieffenbach M and Pastan I: Mechanisms of
resistance to immunotoxins containing Pseudomonas exotoxin A in
cancer therapy. Biomolecules. 10:9792020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fragkoulis C, Glykas I, Bamias A,
Stathouros G, Papadopoulos G and Ntoumas K: Novel treatments in BCG
failure. Where do we stand today? Arch Esp Urol. 74:681–691.
2021.In English, Spanish. PubMed/NCBI
|
|
60
|
Kaneko MK, Honma R, Ogasawara S, Fujii Y,
Nakamura T, Saidoh N, Takagi M, Kagawa Y, Konnai S and Kato Y:
PMab-38 recognizes canine podoplanin of squamous cell carcinomas.
Monoclon Antib Immunodiagn Immunother. 35:263–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ito A, Ohta M, Kato Y, Inada S, Kato T,
Nakata S, Yatabe Y, Goto M, Kaneda N, Kurita K, et al: A real-time
near-infrared fluorescence imaging method for the detection of oral
cancers in mice using an indocyanine green-labeled podoplanin
antibody. Technol Cancer Res Treat. 17:15330338187679362018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kato Y, Ohishi T, Kawada M, Maekawa N,
Konnai S, Itai S, Yamada S and Kaneko MK: The mouse-canine chimeric
anti-dog podoplanin antibody P38B exerts antitumor activity in
mouse xenograft models. Biochem Biophys Rep. 17:23–26.
2018.PubMed/NCBI
|
|
63
|
Kato Y, Ito Y, Ohishi T, Kawada M,
Nakamura T, Sayama Y, Sano M, Asano T, Yanaka M, Okamoto S, et al:
Antibody-drug conjugates using mouse-canine chimeric anti-dog
podoplanin antibody exerts antitumor activity in a mouse xenograft
model. Monoclon Antib Immunodiagn Immunother. 39:37–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Engl J Med.
380:741–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pavšič M: Trop2 forms a stable dimer with
significant structural differences within the membrane-distal
region as compared to EpCAM. Int J Mol Sci. 22:106402021.
View Article : Google Scholar
|
|
66
|
Cardillo TM, Govindan SV, Sharkey RM,
Trisal P and Goldenberg DM: Humanized anti-Trop-2 IgG-SN-38
conjugate for effective treatment of diverse epithelial cancers:
Preclinical studies in human cancer xenograft models and monkeys.
Clin Cancer Res. 17:3157–3169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaneko MK, Nakamura T, Kunita A, Fukayama
M, Abe S, Nishioka Y, Yamada S, Yanaka M, Saidoh N, Yoshida K, et
al: ChLpMab-23: Cancer-specific human-mouse chimeric
anti-podoplanin antibody exhibits antitumor activity via
antibody-dependent cellular cytotoxicity. Monoclon Antib
Immunodiagn Immunother. 36:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kaneko MK, Yamada S, Nakamura T, Abe S,
Nishioka Y, Kunita A, Fukayama M, Fujii Y, Ogasawara S and Kato Y:
Antitumor activity of chLpMab-2, a human-mouse chimeric
cancer-specific antihuman podoplanin antibody, via
antibody-dependent cellular cytotoxicity. Cancer Med. 6:768–777.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Suzuki H, Kaneko MK and Kato Y: Roles of
podoplanin in malignant progression of tumor. Cells. 11:5752022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kaneko MK, Ohishi T, Kawada M and Kato Y:
A cancer-specific anti-podocalyxin monoclonal antibody
(60-mG2a-f) exerts antitumor effects in mouse xenograft
models of pancreatic carcinoma. Biochem Biophys Rep.
24:1008262020.
|
|
72
|
Ishikawa A, Waseda M, Ishii T, Kaneko MK,
Kato Y and Kaneko S: Improved anti-solid tumor response by
humanized anti-podoplanin chimeric antigen receptor transduced
human cytotoxic T cells in an animal model. Genes Cells.
27:549–558. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chalise L, Kato A, Ohno M, Maeda S,
Yamamichi A, Kuramitsu S, Shiina S, Takahashi H, Ozone S, Yamaguchi
J, et al: Efficacy of cancer-specific anti-podoplanin CAR-T cells
and oncolytic herpes virus G47Δ combination therapy against
glioblastoma. Mol Ther Oncolytics. 26:265–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shiina S, Ohno M, Ohka F, Kuramitsu S,
Yamamichi A, Kato A, Motomura K, Tanahashi K, Yamamoto T, Watanabe
R, et al: CAR T cells targeting podoplanin reduce orthotopic
glioblastomas in mouse brains. Cancer Immunol Res. 4:259–268. 2016.
View Article : Google Scholar : PubMed/NCBI
|