Introduction
Heart failure (HF) is a complex clinical syndrome
that is induced by the functional impairment of ventricular
systolic or diastolic functions (1). Worldwide, >1 million patients are
hospitalized annually due to HF (2), and ~40% of these patients are
re-hospitalized or succumb to the condition within ~1 year
following diagnosis (3). The
pathophysiological mechanisms of HF involve a variety of factors,
including cardiomyocyte apoptosis, oxidative stress, calcium
overload, inflammation and mitochondrial dysfunction. Notably,
cardiomyocyte apoptosis remains the main cause of HF (4). Increased cardiomyocyte apoptosis is
observed in both patients with end-stage HF and in experimental
rats with HF (5). As HF exerts a
negative effect on morbidity and mortality, successful treatment
options are required to improve patient survival rates and quality
of life (6).
Previous research has indicated that elevation of
angiotensin II (Ang II) levels is present in patients with HF,
which highlights that Ang II stimulation is associated with HF
(7). The results of a previous
study demonstrated that rats treated with Ang II exhibited
decreased cardiac systolic and diastolic function, accompanied by
notable cardiomyocyte apoptosis in myocardial tissue (8). Therefore, Ang II is often used for
the establishment of models of HF (9). Cardiac apoptosis is involved in the
decline of cardiac function induced by Ang II (10). An increase in the levels of Ang II
may trigger the activation of the PI3K/Akt signaling pathway to
induce apoptosis (11). Thus, in
the present study, Ang II was used to mimic cardiomyocyte apoptosis
in a model of HF.
Compound Kushen injection (CKI) is a type of
traditional Chinese medicine (TCM) that is extracted from Kushen
(Radix Sophorae Flavescentis) and Tufuling (Rhizoma
Smilacis Chinae) (12,13).
The active compounds of CKI consist of matrine, oxymatrine,
sophocarpine, sophoridine and kurarinone. The results of a previous
study demonstrated numerous pharmacological properties of CKI,
including antitumor, analgesic, immunity-enhancing and
anti-inflammatory activities (14). CKI is extensively used in the
treatment of numerous malignancies, including breast, lung and
liver cancer (15). CKI is often
used alone, or in combination with chemotherapy or radiotherapy
(16). Notably, the results of a
previous study demonstrated that CKI regulated cell proliferation
via tge downregulation of the PI3K/Akt/mTOR pathway (17). The PI3K family is involved in a
number of signaling pathways, and it regulates cell proliferation,
differentiation, survival and apoptosis (18). The PI3K/Akt pathway is the main
signaling pathway involved in cardiomyocyte apoptosis (19). An increased expression of PI3K
leads to cardiomyocyte apoptosis and ultimately results in impaired
ventricular systolic and diastolic function. The inhibition of the
PI3K/Akt pathway may exert protective effects against HF (20). Thus, it was hypothesized that CKI
may attenuate cardiomyocyte apoptosis and exert protective effects
on heart function via the inhibition of the PI3K/Akt pathway.
Matrine and oxymatrine are the major active
components of CKI. The cardioprotective effects of matrine and
oxymatrine have been previously reported (21,22). Matrine can decrease myocardial
stiffness and ameliorate myocardial compliance, and contributes to
improving cardiac function (23).
Cardiovascular injury is attenuated by matrine through the
regulation of the PI3K/Akt pathway (24). Matrine ameliorates apoptosis in
Ang II-mediated HF via the regulation of Bcl-2/Bax expression and
caspase-3 activation (25).
Additionally, previous research has reported that oxymatrine
pre-treatment protects cardiomyocytes from cell damage, cell
apoptosis and oxidative stress induced by hypoxia/reoxygenation by
modulating the PI3K/Akt pathway (26).
As CKI is a combination of matrine, oxymatrine,
sophocarpine, sophoridine and kurarinone, it remains unclear as to
whether CKI protects against HF by attenuating PI3K/Akt-induced
apoptosis. The present study thus aimed to evaluate these
mechanisms through the establishment of an Ang II-mediated HF model
in vivo and in vitro.
Materials and methods
Materials, reagents and antibodies
Human Ang II (24738) and phosphate-buffered saline
(PBS; 600221) was obtained from Cayman Chemical Company. CKI with a
total alkaloid concentration of 26.5 mg/ml in a 5-ml ampoule was
obtained from Shanxi Zhendong Pharmaceutical Co. Ltd. Commercial
enzyme-linked immunosorbent assay (ELISA) kits for cardiac troponin
I (cTn I; E08421m) and N-terminal (NT)-pro hormone B-type
natriuretic peptide (NT-proBNP; E05153m) were purchased from
Cusabio Technology, LLC. The Epon812 resin (02334),
dodecenylsuccinic anhydride (00563), dimethylaminomethyl phenol
(17806), methyl nadic anhydride (00886), uranyl acetate (21447) and
lead citrate (25350) were purchased from Polysciences, Inc. The
TUNEL In Situ Cell Death Detection kit was purchased from
the Beyotime Institute of Biotechnology. The
4′,6′-diamidino-2-phenylindole (DAPI) was purchased from AAT
Bioquest, Inc. The 1X binding buffer, Annexin V-PE, 7-AAD and
propidium iodide (PI) were purchased from BD Biosciences.
Trypsin-EDTA solution, DMEM, penicillin and streptomycin were
obtained from Gibco; Thermo Fisher Scientific, Inc. Fetal bovine
serum (FBS) was purchased from Shanghai ExCell Biology, Inc. The
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Laboratories, Inc. The BCA protein quantification kit was purchased
from Vazyme Biotech Co., Ltd. The BioTrace NT nitrocellulose
membrane (66487) was purchased from Pall Life Sciences. Skim milk
(abs9175) was obtained from Absin Bioscience Inc. The
chemiluminescence detection kit (BL520A) was obtained from Biosharp
Life Sciences. Primary antibodies against total Akt (t-Akt) (cat.
no. GB111114), Bax (cat. no. GB11690) and Bcl-2 (cat. no. GB112382)
were purchased from Wuhan Servicebio Technology Co., Ltd.
Phosphorylated Akt (p-Akt) (cat. no. 66444-1-lg) and GAPDH (cat.
no. BC004109) anti-bodies were purchased from Proteintech Group,
Inc. Primary antibodies against cleaved caspase-3 (cat. no. 9661)
and cleaved caspase-9 (cat. no. 9507) were purchased from Cell
Signaling Technology, Inc. Primary antibodies against cytochrome
c (cyto c) (cat. no. Ab-AF0146), p27 (cat. no.
Ab-AF6324) and cyclin D1 (cat. no. Ab-AF0931) were purchased from
Affinity Biosciences. Goat anti-rabbit IgG (cat. no. A23920) and
goat anti-mouse IgG (cat. no. A23710) were obtained from Abbkine
Scientific Co., Ltd. Primary antibodies against PI3K (cat. no.
ab191606), goat anti-mouse IgG H&L (Alexa Fluor®647,
cat. no. ab150115) and goat anti-rabbit IgG H&L (Alexa
Fluor®647, cat. no. ab150077) were provided by Abcam.
LY294002 (cat. no. 440202) and RIPA buffer (cat. no. 42029053) was
acquired from MilliporeSigma.
Osmotic minipumps were purchased from Durect
Corporation (Alzet Model 2004). The M-mode echocardiograph imaging
system was purchased from FUJIFILM VisualSonics (Vevo 2100 system).
A transmission electron microscope was purchased from Hitachi, Ltd.
TUNEL analysis software was obtained from Image Pro-Plus software
(Media Cybernetics, Inc.). An Olympus Fluoview FV1000 microscope
was purchased from Olympus Corporation. A microplate reader was
obtained from PerkinElmer, Inc. A FC-500 type flow cytometer and
EXPO 32 ADC software (version no. 1.2) were obtained from Beckman
Coulter, Inc. Multicycle AV software was purchased from Phoenix
Pharmaceuticals, Inc. (version no. 275).
Animals
All animal procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (27), and all animal experiments were
approved by the Institutional Animal Care and Use Committee of The
First Hospital of Hebei Medical University, Shijiazhuang, China
(ethics approval no. 20220634; date of approval: June 10,
2022).
Male C57BL/6 mice (age, 6 weeks; weight, 20-22 g)
were purchased from Skbex Biotechnology, housed in cages with a
12/12-h light/dark cycle and provided with free access to water and
food at 24°C and a relative humidity of 50-70%. The mice were
randomly divided into the following four groups: i) The control
group (CON group, n=10); ii) the CKI group (n=10); iii) the Ang II
group (Ang II group, n=10); and iv) the Ang II plus CKI group (AC
group, n=10).
All mice were anesthetized with an intraperitoneal
injection of pentobarbital sodium (40 mg/kg) prior to surgery.
Subsequently, the mice in the Ang II and AC groups were treated
with Ang II (2 µg/kg/min) through osmotic minipumps for 3
weeks, as previously described (28). The minipump was subcutaneously
implanted into the back of each mouse for 21 days. In addition,
mice in the CON and CKI groups were subcutaneously implanted with
osmotic minipumps containing 200 µl PBS.
Zhao et al (29) reported that CKI treatment was
maximally effective at concentrations of ≥25 mg/kg/day. Thus, in
the present study, CKI was administered at 25 mg/kg/day. The mice
were intraperitoneally injected with CKI once a day for 3 weeks in
the CKI and AC groups, and mice in the CON and Ang II groups were
injected with an equal volume of PBS at the same time intervals. In
the present study, each experiment was carried out three times.
Hemodynamics and echocardiography
The mice were anesthetized with an intraperitoneal
injection of pentobarbital sodium (40 mg/kg) following 3 weeks of
treatment. Subsequently, M-mode echocardiography was carried out
using a 30-MHz probe. Changes in left ventricular end-systolic
diameter (LVDS), left ventricular end-diastolic diameter (LVDD),
left ventricular ejection fraction (LVEF) and left ventricular
fraction shortening (LVFS) were determined.
ELISA
Following M-mode echocardiography, when the mice
were still anesthetized with pentobarbital sodium, a glass
capillary was used to collect blood from the inner canthus of the
mice. The blood samples were centrifuged at 1,100 × g for 5 min at
room temperature, the separated sera were used to determine the
serum concentrations of cTn I and NT-proBNP. The concentrations
were measured using commercial ELISA kits, as aforementioned. The
absorbance was recorded at 450 nm using a Multiskan®
96-well plate reader.
Morphometric analysis
At the end of the monitoring period, 40 mice were
sacrificed via spinal cord dislocation. The heart tissues were
removed, and five samples from each group were used for
morphometric analyses, flow cytometry (FCM) and western blot (WB)
analysis. The left ventricle of the heart was removed for
transmission electron microscopy (TEM). For TEM, the tissues were
prefixed with 2.5% glutaraldehyde in 0.1 M PBS pH 7.2 for 24 h at
4°C. The samples were then washed twice with 0.1 M PBS and
post-fixed for 1 h with 1% osmium tetroxide in 0.1 M PBS at 4°C.
The samples were respectively dehydrated according to the gradient
of 50, 70, 90 and 100% dehydrated acetone and the dehydration was
performed for 10 min with each concentration of dehydrated acetone
three times. The dehydrated samples were then treated at 37°C for
24 h in a mixture of epoxy resin and pure acetone (1:1), followed
by 24 h of embedding at 60°C in a mixture of Epon812 resin,
dodecenylsuccinic anhydride, dimethylaminomethyl phenol and methyl
nadic anhydride (26:6:1:19). The tissues were then cut into
ultrathin slices (70 nm), followed by double staining with uranyl
acetate (30 min) and lead citrate (10 min) at room temperature. The
observation of ultrastructural alterations in the myocardial
sections was performed using a transmission electron
microscope.
FCM assay
Left ventricular tissue was removed as described
above. The tissues were washed with ice-cold PBS and cut into
sections of 1-mm3. Subsequently, an enzyme solution
(0.1% trypsin and 0.02% EDTA) was added, and the samples were
maintained in a 37°C water bath or thermostat shaker for digestion
for 20-60 min. Digestion was terminated following the addition of
serum-containing medium. Cell suspensions were collected in batches
and filtered through a 100-mesh filter, followed by centrifugation
at 2,000 × g for 5 min at 4°C. Subsequently, the supernatant was
discarded. The cells were washed with ice-cold PBS and centrifuged
at 2,000 × g for 5 min at 4°C, and l-ml single-cell suspension was
collected from each group. The cells were washed with ice-cold PBS
and suspended in 100 µl 1X binding buffer. Subsequently, 5
µl Annexin V-PE were added, and the mixture was placed on
ice for 15 min in the dark. A total of 400 µl 1X binding
buffer and 5 µl 7-AAD were subsequently added. Following
incubation on ice for 15 min in the dark at room temperature, the
cells were washed once with ice-cold PBS, resuspended in 1 ml PBS
and analyzed using an FC-500 flow cytometer. The apoptotic rate was
measured using EXPO 32 ADC software (version no. 1.2).
TUNEL assay
The remaining five heart tissues from each group
were fixed in 4% paraformaldehyde (in PBS, pH 7.4) for 30 min at
room temperature, embedded in paraffin and cut into 5-mm-thick
serial sections for TUNEL staining to evaluate cardiomyocyte
apoptosis. TUNEL detection solution was added and sections were
incubated the dark at 37°C for 2 h. Nuclear staining was conducted
using 1 µg/ml DAPI in PBS for 15 min at room temperature.
The stained samples were analyzed using an Olympus Fluoview FV1000
microscope, and cells from 10 visual fields were randomly selected
to determine the apoptotic index. The In Situ Cell Death
Detection kit was used to analyze cardiomyocyte apoptosis. The
digitally captured images of immunofluorescence in five randomly
selected fields from each sample were analyzed using analysis
software. The percentage of apoptotic cells was measured and
recorded as the apoptotic index (AI%).
Cell culture and cell viability
assay
The H9C2 cell line (cat. no. 1101RAT-PUMC000219) was
purchased from the China Infrastructure of Cell Line Resources
(Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences). The cells were cultured in DMEM supplemented with 10%
FBS, penicillin (100 U/ml) and streptomycin (100 mg/ml) in a 37°C
humidified atmosphere containing 5% CO2 and 95%
O2. Ang II (1 µmol/l) was used to mimic HF in
vitro, as previously described (10). For all the in vitro
experiments performed in the present study, CKI was used at a
dilution of a final concentration of 2 mg/ml total alkaloids
(30). LY294002 (25
µmol/l) was used as a PI3K/Akt pathway inhibitor, as
previously reported (31). The
H9C2 cells (3×104 cells/ml) were seeded in a 96-well
plate and subsequently divided into four groups for incubation for
48 h at 37°C as follows: The CON group was treated with medium (50
µl), the Ang II group was treated with Ang II (50
µl), the AC group was treated with Ang II (50 µl) +
CKI (50 µl), and the AL group was treated with Ang II (50
µl) and the PI3K/Akt pathway inhibitor, LY294002 (50
µl).
CCK-8 assay was used to detect cell viability. A
total of 10 µl of the CCK-8 solution was added to each well
and incubated for 2 h at 37°C, and the optical density was measured
at 450 nm using a microplate reader.
Cell cycle distribution analysis
The effect of CKI on cell cycle distribution was
analyzed using FCM with PI staining. Briefly, a total of l ml of
single-cell suspension was collected from each group following the
aforementioned treatments. The H9C2 cells were washed with ice-cold
PBS and fixed with 70% alcohol at 4°C for 24 h. Following washing
twice with ice-cold PBS, the cells were suspended in 100 µl
PBS, and 1 ml PI was added to the suspension for staining at 4°C
for 30 min, prior to cell cycle detection using an FC-500 type flow
cytometer. Data were analyzed using Multicycle AV software.
WB analysis
Protein was extracted from left ventricular tissue
or H9C2 cells for WB analysis. The tissues or cells were lysed
using RIPA buffer (50 mM Tris-HCl; pH 7.4; 150 mM NaCl; 1% Triton
X-100; 1% sodium deoxycholate). Protein concentrations was
determined by BCA assay. Equal amounts of protein (20 µg)
were separated with 6-15% SDS-PAGE gel and transferred onto a
BioTrace NT nitrocellulose membrane and incubated with 5% skimmed
milk for 1 h at room temperature. Subsequently, the membrane was
incubated with the primary antibodies overnight at 4°C. The
reaction was then performed using a secondary antibody matching the
primary antibody for 1 h at room temperature. The primary
antibodies employed for WB were against PI3K (1:1,000), p-Akt
(1:5,000), t-Akt (1:1,000), Bax (1:1,000), Bcl-2 (1:1,000), cyto
c (1:500), cleaved caspase-9 (1:1,000), cleaved caspase-3
(1:1,000), p27 (1:500), cyclin D1 (1:500) and GAPDH (1:20,000).
Secondary antibodies were all diluted at 1:2,000. The target
proteins in the membranes were visualized using an enhanced
chemiluminescence detection kit with an Odyssey Imaging System
(Odyssey; version no. 3.0). Protein bands were quantified using
ImageJ (National Institutes of Health) software (version 1.8.0) for
densitometric analysis and normalized to GAPDH.
Statistical analysis
All data are presented as the mean ± standard error
of the mean (M ± SEM). The results were analyzed using GraphPad
Prism (version 8.0; GraphPad Software, Inc.). Depending on the
design of the experiment, the data were analyzed using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
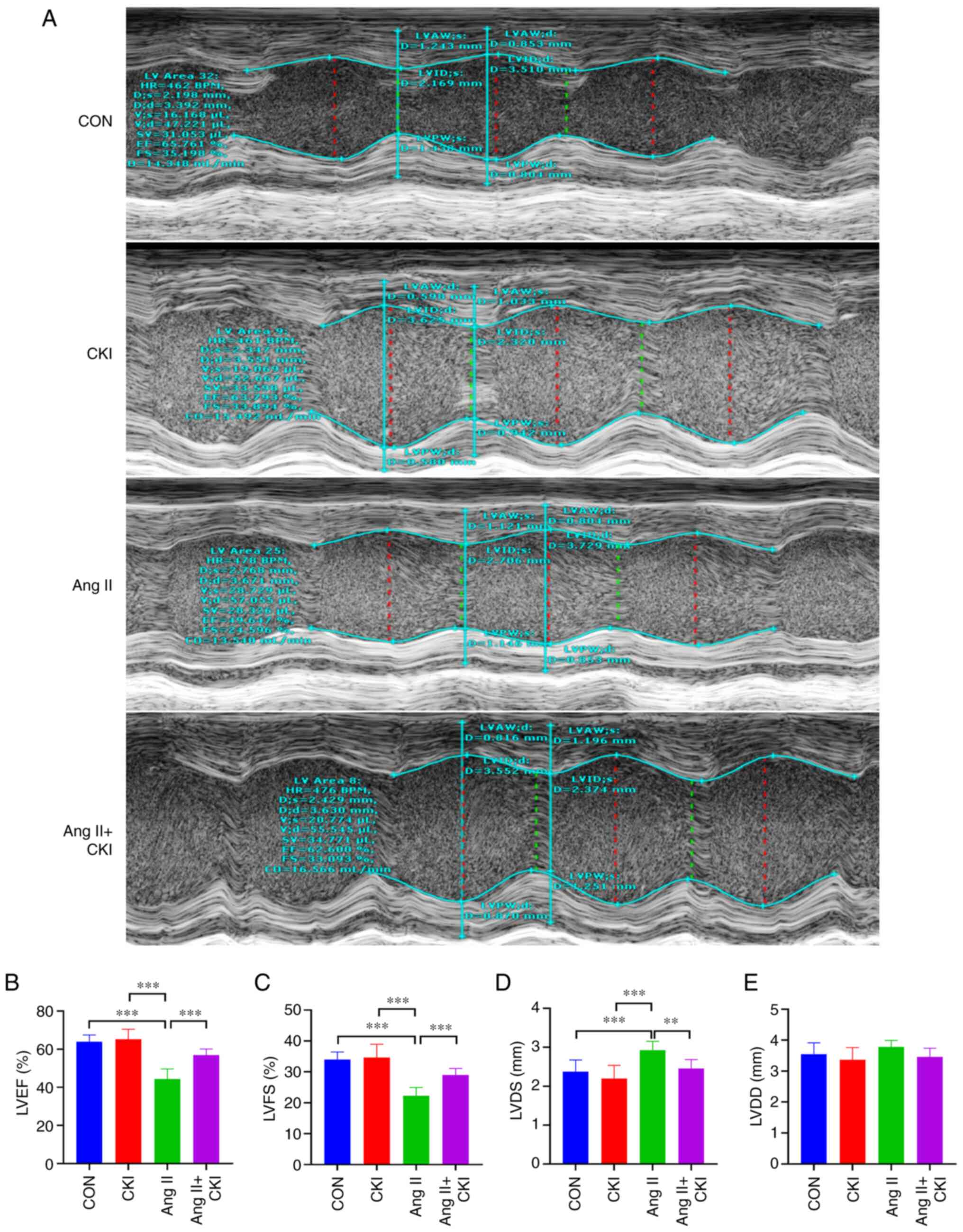
CKI improves cardiac compliance
Heart functions were evaluated using Doppler M-mode
ultrasonography, as illustrated in Fig. 1A. The results demonstrated that
the LVEF values were notably higher in the AC group than in the Ang
II group (P<0.001; n=5; Fig.
1B). Similar trends for LVFS were demonstrated among all
different groups (Fig. 1C). A
higher LVFS was detected in the AC group (P<0.001) than in the
Ang II group. Co-treatment with CKI decreased the LVDS values,
compared with those of the Ang II group (P<0.01; Fig. 1D). However, there was no
significant difference in LVDD between the two groups (Fig. 1E). These results indicated that
the Ang II-treated mice exhibited a lower ejection force; however,
co-treatment with CKI significantly improved the systolic function
of the ventricle. Thus, CKI attenuated Ang II-mediated HF in
mice.
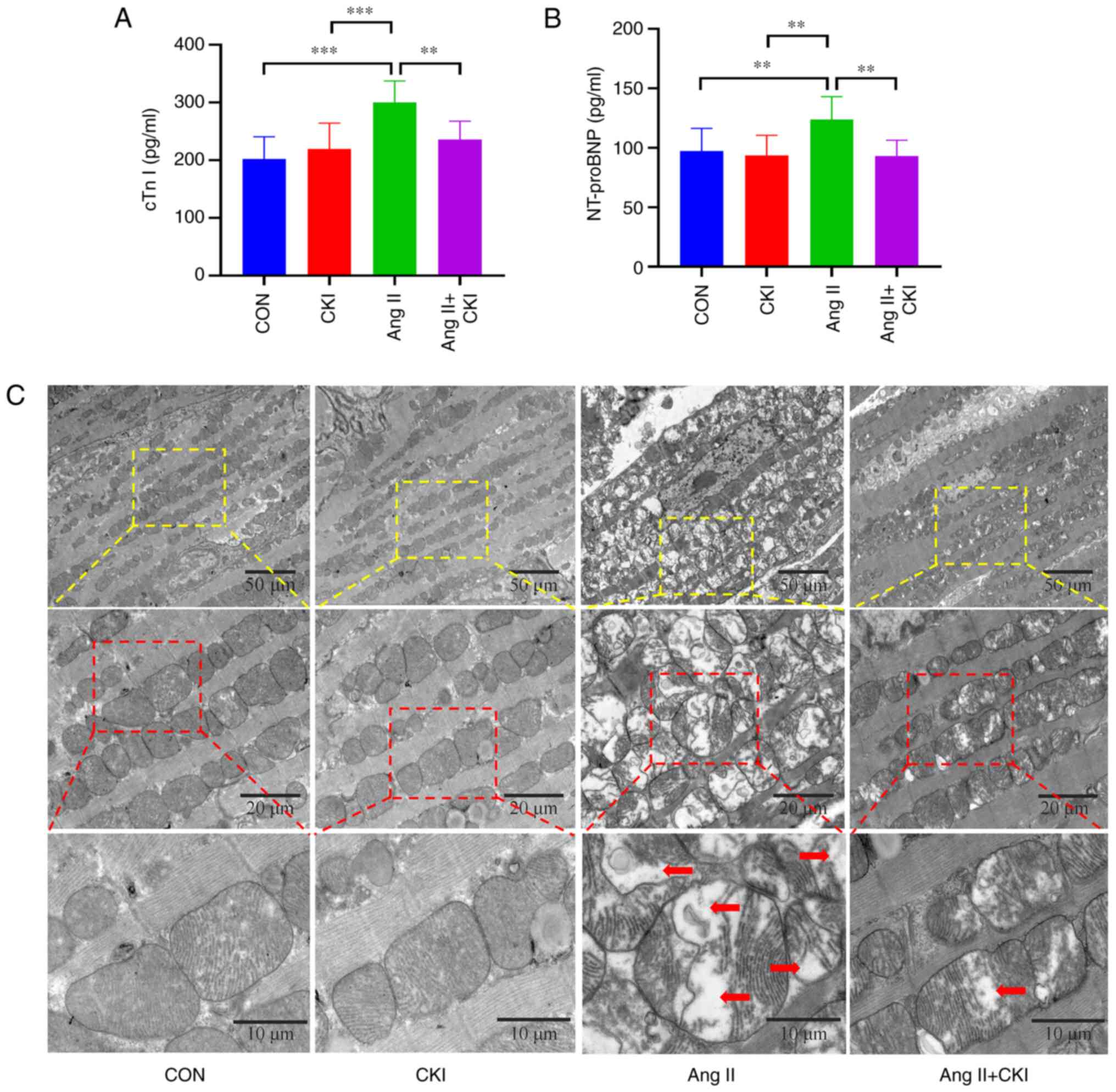
CKI preserves myocardial injury
biomarkers
The results presented in Fig. 2A and B confirmed that CKI
significantly decreased the levels of myocardial injury biomarkers.
The levels of cTn I were markedly increased in the Ang II group,
compared with the control group (P<0.001; Fig. 2A). Co-treatment with CKI
significantly reduced the levels of cTn I, compared with those of
the Ang II group (P<0.01). The results also revealed that the
mice exhibited lower NT-proBNP plasma levels in the AC group than
in the Ang II group (P<0.01; Fig.
2B). The NT-proBNP levels were significantly reduced in the AC
group, compared with those of the Ang II group (P<0.01). These
results indicated that CKI reversed Ang II-induced myocardial
damage.
CKI regulates myocardial structural
damage
The ultrastructure of the myocardium in the left
ventricle was observed using TEM (Fig. 2C). TEM images of the healthy
control mice and CKI-treated mice demonstrated evenly scattered
mitochondria throughout the myocardium, and these displayed a
regular shape, with compact cristae, an intact membrane and orderly
arrayed myocardial filaments with vivid Z lines. The Ang II-treated
group exhibited cardiomyocyte edema, as well as multiple forms of
damage to the cardiomyocyte membrane. The results of the present
study also demonstrated irregularly arranged and severely ruptured
myocardial filaments in the Ang II-treated group. In addition, the
mitochondria exhibited diffuse swelling and differing sizes, with
numerous vacuoles. Moreover, the cristae were ruptured, lysed and
vacuolized. The mice co-treated with CKI exhibited heart
mitochondria with no swelling, slightly damaged cristae, healthy
myofibrils and orderly arranged myocardial filaments with vivid Z
lines. The cardiomyocyte hypertrophy, edema and mitochondrial
damage rates were markedly higher in the Ang II group than in the
AC group.
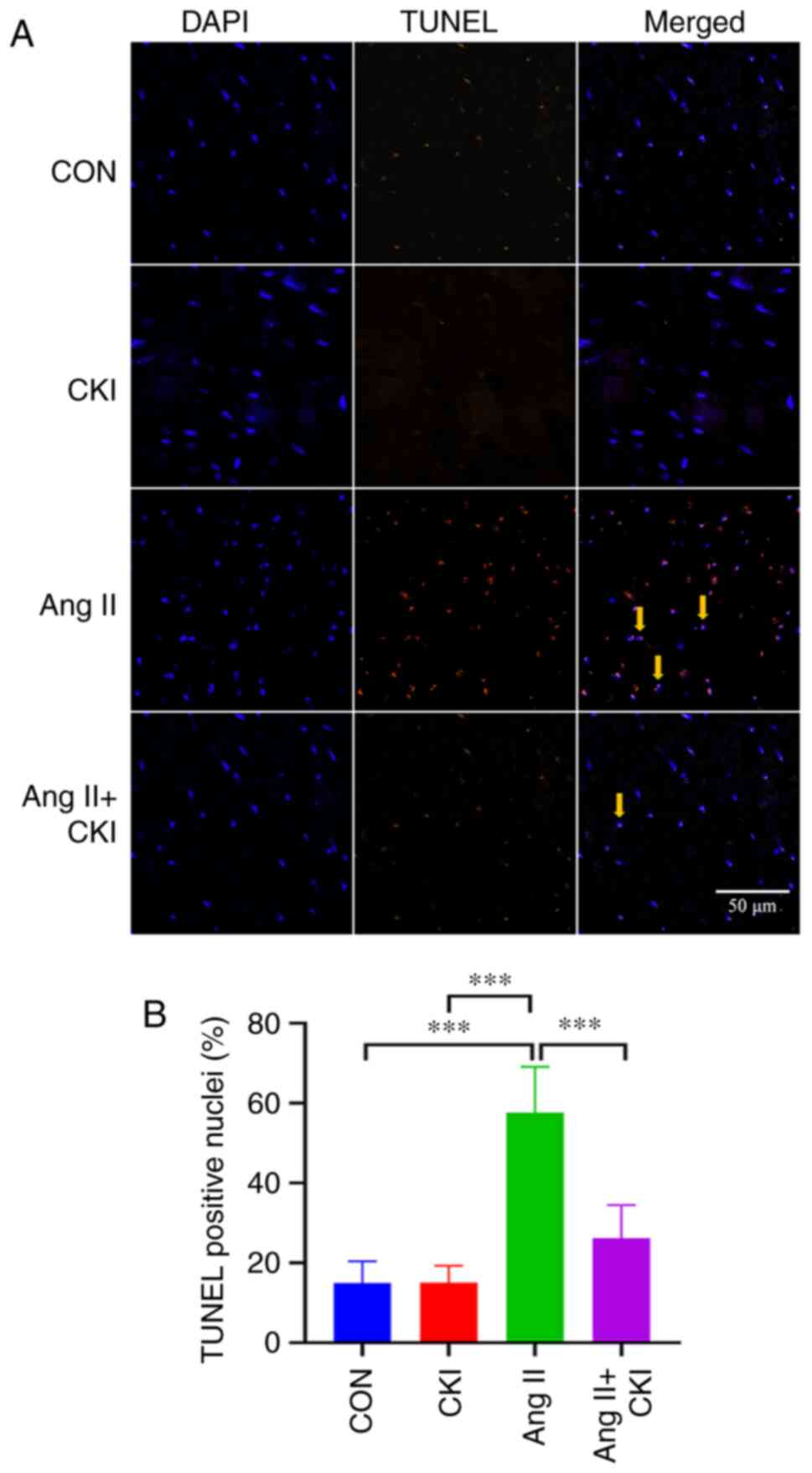
CKI reduces apoptosis in heart tissue in
vivo
The results of TUNEL assay demonstrated that
continuous Ang II treatment significantly increased the number of
TUNEL-positive cells (Fig. 3).
Co-treatment with CKI decreased the number of TUNEL-positive cells
in the AC group, and Ang II increased the apoptotic rate, compared
with that in the AC group (P<0.001; Fig. 3). No notable trend was observed
for the incidence of apoptosis in the CON or CKI groups, compared
with the Ang II group (P<0.001; Fig. 3). Thus results of TUNEL assay thus
revealed that CKI markedly reduced the apoptosis in the heart
tissue.
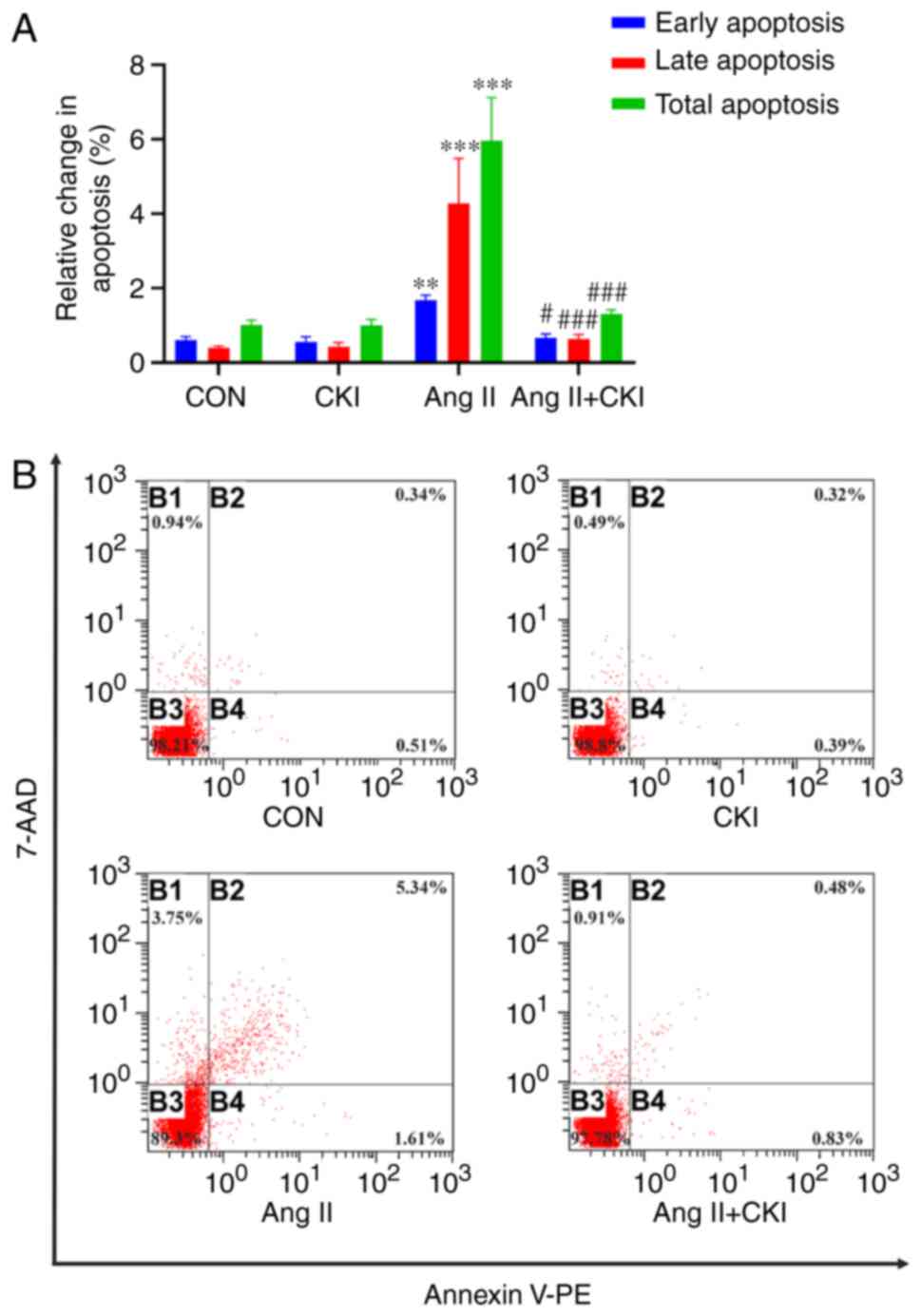
FCM assay with Annexin V-PE/7-AAD double staining
indicated that CKI inhibited cardiomyocyte apoptosis in the left
ventricle (Fig. 4A). In the Ang
II group, the population at the upper-right quadrant (Annexin
V-PE+/7-AAD+), indicative of late apoptotic
cells, was notably increased (4.284±1.071%), compared with that in
the CON group (0.4±0.045%; P<0.001; Fig. 4B). In addition, the population at
the lower-right quadrant (Annexin
V-PE+/7-AAD−), indicative of early apoptotic
cells, was significantly increased in the Ang II group
(1.682±0.122%), compared with that in the CON group (0.61±0.082%;
P<0.01, Fig. 4B). However,
following co-treatment with CKI and Ang II, the population at the
upper-right quadrant (0.64±0.122%) and lower-right quadrant
(0.672±0.092%) was markedly decreased in the AC group, compared
with the Ang II group. The total apoptotic rate, including early
and late apoptosis, also decreased in the AC group (1.312±0.1%),
compared with that of the Ang II group (5.966±1.038%; P<0.001;
Fig. 4A). These findings revealed
that CKI inhibited Ang II-mediated cardiomyocyte apoptosis.
The results of WB analysis (Fig. 5) demonstrated that Ang II notably
increased the expression levels of PI3K, Bax, cyto c,
cleaved caspase-9, cleaved caspase-9/total caspase-9, cleaved
caspase-3 and cleaved caspase-3/total caspase 3 in the left
ventricular tissue (Fig. 5B, G, H, I,
K, L and N). In addition, Ang II notably decreased the
expression levels of p-Akt, p-Akt/t-Akt and Bcl-2 (Fig. 5C, E and F). By contrast, CKI
markedly increased the expression levels of p-Akt, p-Akt/t-Akt and
Bcl-2 (Fig. 5C, E and F), and
decreased the expression levels of PI3K, Bax, cyto c,
cleaved caspase-9, cleaved caspase-9/total caspase-9, cleaved
caspase-3 and cleaved caspase-3/total caspase-3 in the AC group
(Fig. 5B, G, H, I, K, L and N).
No significant differences were observed in the expression levels
of t-Akt, total caspase-9 and total caspase-3 among the four groups
(Fig. 5D, J and M).
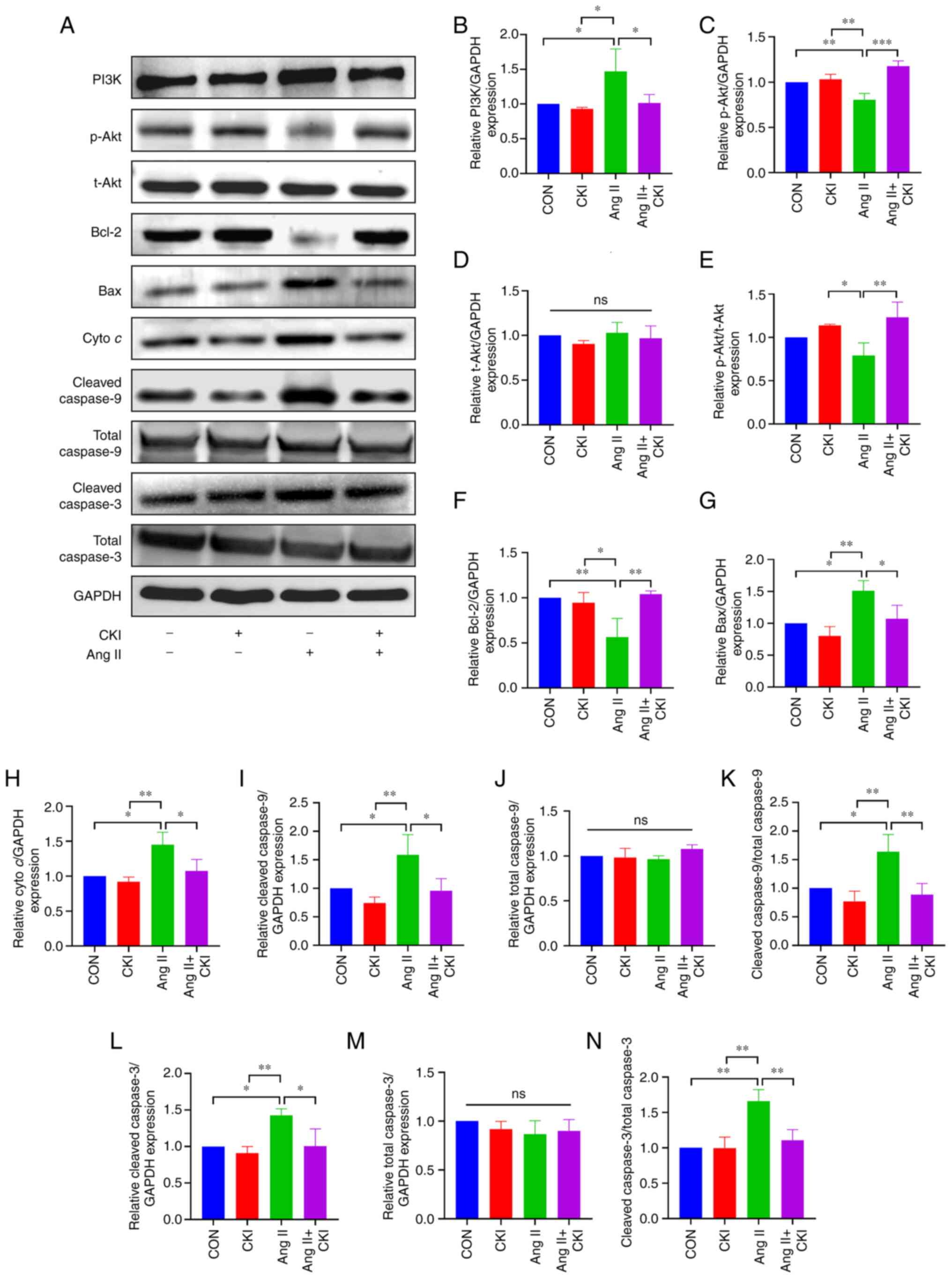 | Figure 5Expression of apoptosis-related
proteins. (A) The results of western blot analysis for each
protein. (B-N) The expression of representative proteins. (B) PI3K
expression, (C) p-Akt expression, (D) t-Akt expression, (E)
p-Akt/t-Akt ratio, (F) Bcl-2 expression, (G) Bax expression, (H)
cyto c expression, (I) cleaved caspase-9 expression, (J) total
caspase-9 expression, (K) cleaved caspase-9/total caspase-9, (L)
cleaved caspase-3 expression, (M) total caspase-3 expression and
(N) cleaved caspase-3/total caspase-3 in the different groups. Data
are presented as the mean ± SEM. One-way ANOVA and post-hoc Tukey's
multiple comparisons test were used to determine statistically
significant differences (n=3 mice per group).
*P<0.05, **P<0.01 and
***P<0.001. ns, not significant; CON, control; CKI,
compound Kushen injection; Ang II, angiotensin II; cyto c,
cytochrome c. |
CKI reduces the apoptosis of myocardial
cells in vitro
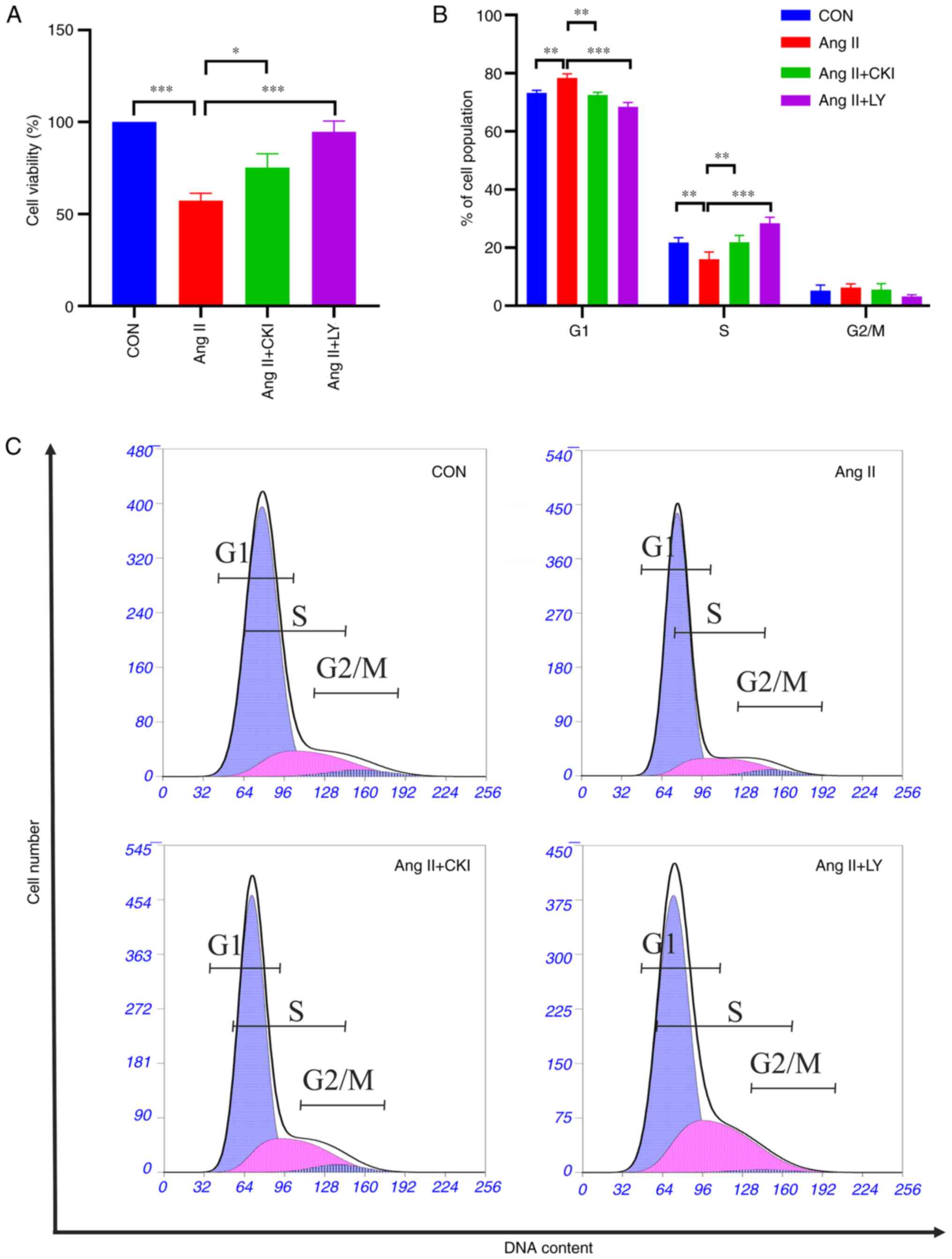
The effects of CKI on the viability of the H9C2
cells were investigated using a CCK-8 assay. The results
demonstrated that Ang II treatment significantly decreased H9C2
cell viability (P<0.001; Fig.
6A), and this decrease was reversed following treatment with
CKI (P<0.05) and the PI3K/Akt pathway inhibitor, LY294002
(P<0.001) (Fig. 6A).
To examine whether CKI affects the cell cycle in
myocardial cells, cell cycle distribution was investigated
following treatment with CKI and LY294002, for 48 h. As shown in
Fig. 6B and C, Ang II increased
cell cycle arrest at the G1 phase (P<0.01 vs. CON
group) and decreased cell cycle arrest at the S phase in the H9C2
cells (P<0.01 vs. CON group). Following CKI treatment, the
percentage of cells in the G1 phase decreased (P<0.01
vs. Ang II group), and cell cycle arrest at the G1 phase
decreased. In addition, the percentage of cells at the S phase
increased (P<0.01 vs. Ang II group). Similar trends were
observed among the different phases in the AL group.
The results of WB analysis (Fig. 7) demonstrated that CKI
significantly decreased the expression levels of PI3K, Bax and p27
in H9C2 cells (Fig. 7G, H and I).
Moreover, the expression levels of p-Akt, Bcl-2 and cyclin D1, were
significantly higher in the AC and AL groups, compared with the Ang
II group (Fig. 7B, E and F). A
high p-Akt/t-Akt ratio was detected in the AC and AL groups
(Fig. 7D). On the whole, these
results demonstrated that CKI significantly reduced the expression
of pro-apoptotic proteins in the H9C2 cells, and a similar trend
was observed following treatment with the PI3K/Akt pathway
inhibitor, LY294002.
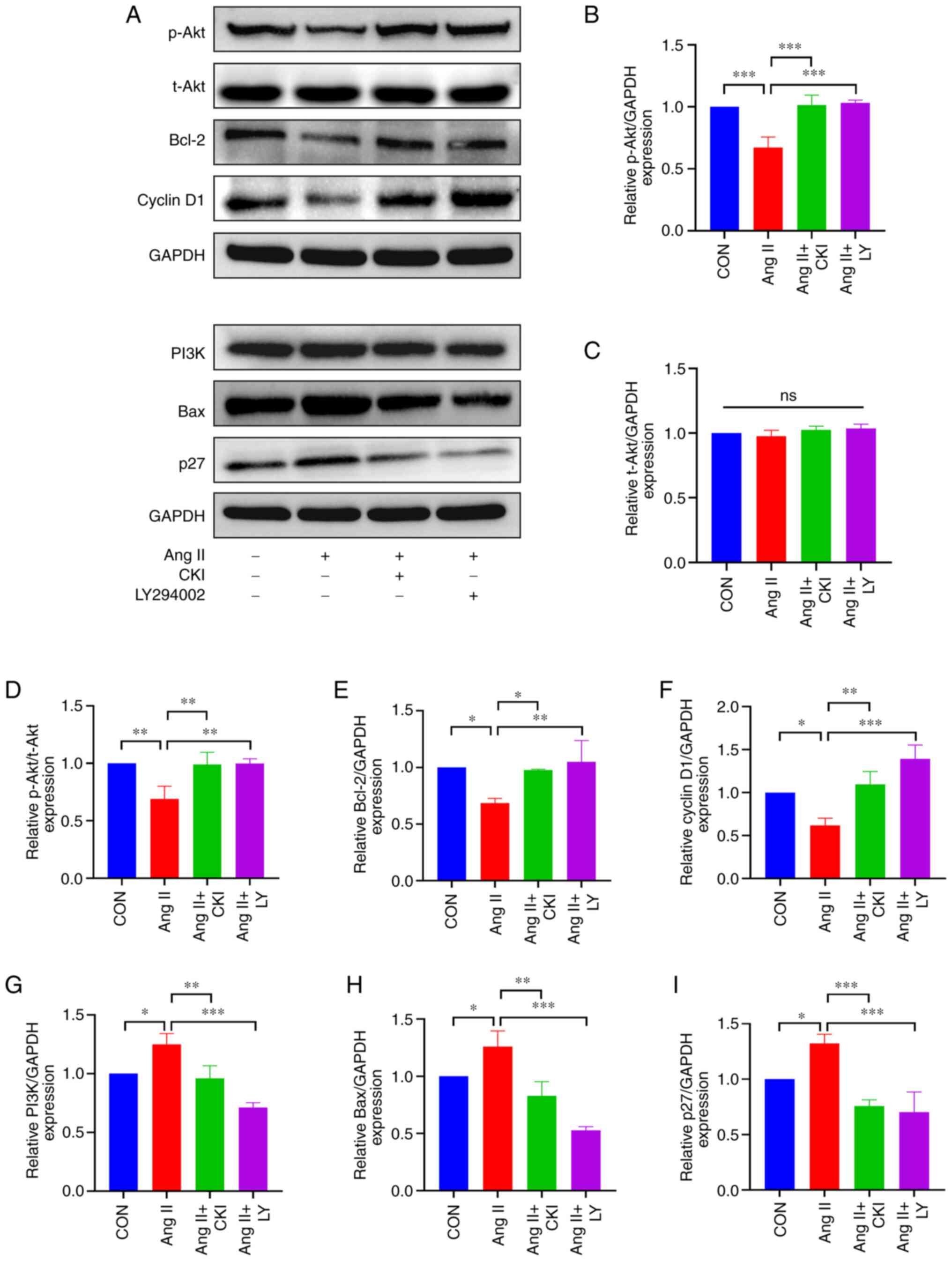 | Figure 7Expression of apoptosis-related
proteins in vitro. (A) The results of western blot analysis
for each protein. (B-I) The expression of representative proteins.
(B) p-Akt expression, (C) t-Akt expression, (D) p-Akt/t-Akt ratio,
(E) Bcl-2 expression, (F) cyclin D1 expression, (G) PI3K
expression, (H) Bax expression, and (I) p27 expression in the
different groups. Data are presented as the mean ± SEM. One-way
ANOVA and post-hoc Tukey's multiple comparisons test were used to
determine statistically significant differences (n=3 per group).
*P<0.05, **P<0.01 and
***P<0.001. CON, control; CKI, compound Kushen
injection; Ang II, angiotensin II; LY, LY294002 (an inhibitor of
the PI3K/Akt pathway). |
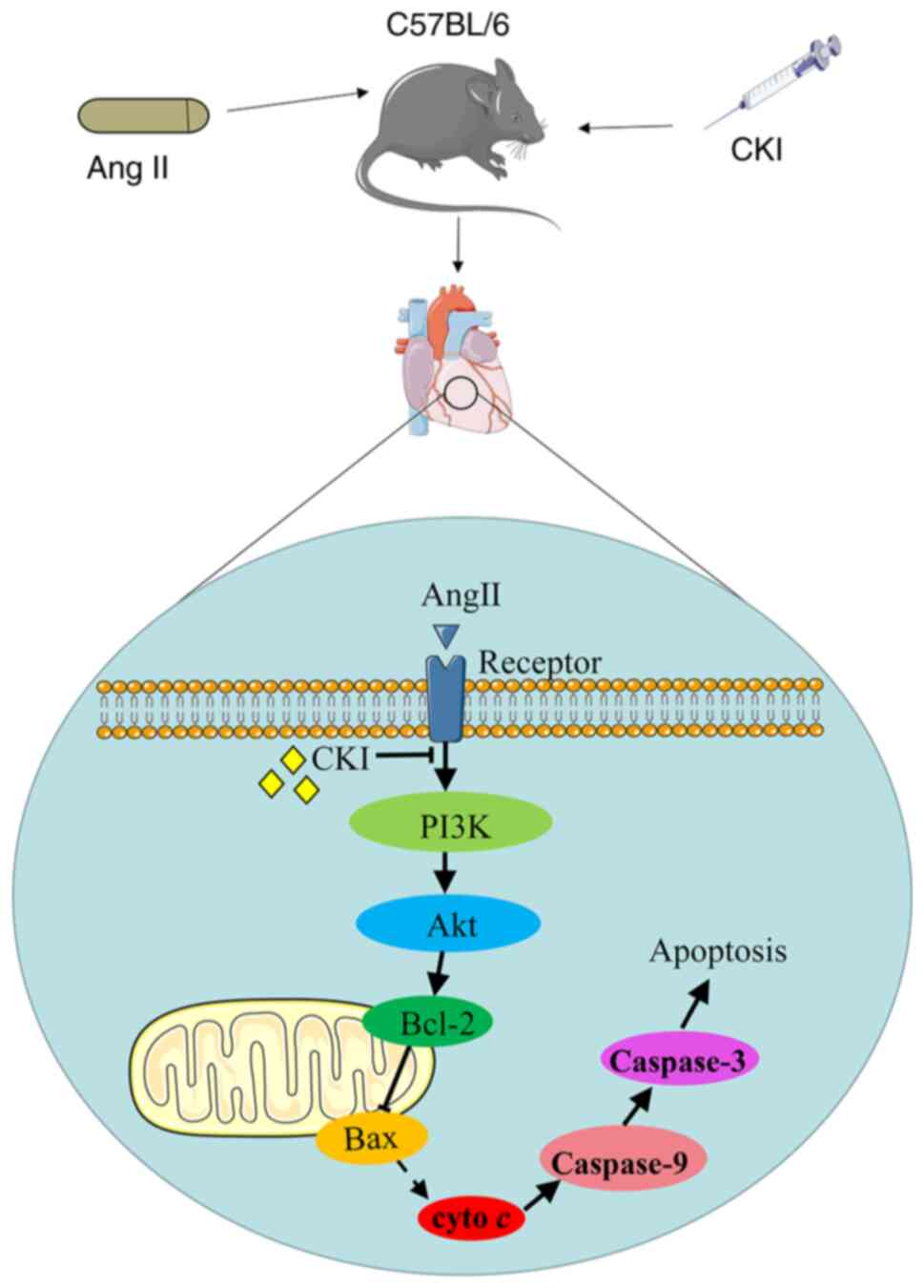
Discussion
HF is the final stage of numerous cardiac diseases.
Inhibiting myocardial apoptosis may prevent cardiac remodeling and
protect cardiac functions. To the best of our knowledge, the
present study is the first to explore the association between the
cardioprotective effects of CKI and the PI3K/Akt apoptotic pathway.
CKI may promote healthy cardiac function, inhibit heart structure
remodeling and decrease cardiomyocyte apoptosis in vivo and
in vitro. These effects were closely associated with the
inactivation of pro-apoptotic proteins, such as PI3K, Bax, cyto
c, cleaved caspase-9 and cleaved caspase-3, and the
activation of apoptosis inhibitors, such as p-Akt and Bcl-2 in the
PI3K/Akt pathway (Fig. 8). The
results of the present study indicated that CKI may exert
protective effects against HF.
Left ventricular enlargement and cardiac function
decline may be early signs of HF. Notably, these factors may be
associated with myocardial cell apoptosis, ventricular hypertrophy
and cardiac structural remodeling. In the present study, the
results of the M-mode ultrasonography demonstrated that the
ventricular systolic and diastolic function were protected by CKI.
cTnI is the gold standard biomarker for the detection of cardiac
injury and myocardial cell necrosis (32). In addition, NT-proBNP is also used
as a key biomarker to detect HF (33). In the present study, CKI
significantly decreased cTnI and NT-proBNP. These results
demonstrated that CKI protected the myocardium from damage.
The main pathological feature of HF is a decrease in
cardiomyocytes caused by apoptosis, necrosis or myocardial fibrosis
(34). The mitochondria are not
only the main site of intracellular reactive oxygen species, but
also the first target of myocardial function injury (35). Ang II promotes cardiomyocyte
apoptosis, which is a main mechanism leading to HF (7). TEM images obtained in the present
study demonstrated that Ang II elicited diffuse mitochondrial
swelling, ruptured cristae and ruptured myocardial filaments. In
addition, CKI treatment reduced mitochondrial apoptosis and
promoted a regular structure. As the main site of myocardial cell
death and survival, the mitochondria are of particular importance
in the occurrence of HF. CKI may promote healthy cardiac function
through reducing mitochondrial and cardiomyocyte apoptosis.
Apoptosis, as a type of programmed cell death,
exerts a major effect on cell injury (36). In the present study, the apoptotic
rates were analyzed using TUNEL and FCM assays. The results of
TUNEL assay indicated that the Ang II group exhibited a markedly
increased apoptotic rate. Following treatment with CKI, the number
of apoptotic cardiomyocytes significantly decreased. In addition,
the results of FCM assay demonstrated that CKI decreased the
population of early and late apoptotic cells. The results of the
TUNEL and FCM assays both confirmed that CKI reduced the apoptotic
rate of cardiomyocytes.
To further determine whether the effects of CKI on
HF are due to the inhibition of the PI3K/Akt pathway, the
expression levels of representative signal proteins were determined
using WB analysis. The PI3K/Akt apoptotic pathway is a key
signaling pathway in HF (37). It
plays a pro-apoptotic role by regulating the concentration of
calcium, mitochondrial membrane stability and apoptotic gene
expression (38). During
myocardial injury, the PI3K/Akt signaling pathway is activated.
Herein, CKI decreased the transcriptional activities of PI3K and
Bax, and increased the expression of p-Akt and Bcl-2. As an
apoptosis suppressor gene, Bcl-2 alleviates mitochondrial damage
(39). An increased Bcl-2
expression inhibits apoptosis, which increases the stability of the
mitochondrial membrane. The inhibition of the pro-apoptotic
protein, Bax, may reduce mitochondrial damage (40). Cyto c is the main molecule
released by mitochondria to promote cell apoptosis, which forms
apoptosomes with Apoptotic protease activating factor-1 (APAF-1),
ATP and caspase-9 precursor molecules in the cytoplasm (41). Apoptosomes trigger the activation
of caspase-9 and increase cleaved caspase-9 expression, which
activates caspase-3. Caspase-3 is a critical apoptosis-executing
enzyme in the caspase family that plays a key role in various cell
apoptotic pathways (42). When
caspase-3 is activated, apoptosis occurs. The results of the
present study demonstrated that Ang II activated the PI3K/Akt
pathway and increased cleaved caspase-3 expression in myocardial
cells. On the other hand, CKI inhibited the PI3K/Akt pathway and
decreased cleaved caspase-3 expression. The persistence of
cardiomyocyte apoptosis leads to a progressive cell loss in the
myocardium, ventricle dilation and gradual cardiac remodeling,
eventually leading to HF. In the present study, CKI alleviated
myocyte injury and reduced cardiomyocyte apoptosis, thereby
inhibiting HF. Collectively, these results indicate that the
effects of CKI may be associated with the inactivation of
pro-apoptotic mediators, such as PI3K, Bax, cyto c, cleaved
caspase-9 and cleaved caspase-3, and the activation of apoptosis
inhibitors, such as p-Akt and Bcl-2, in heart tissues.
To further determine the effects and potential
underlying mechanisms, H9C2 cells were used to simulate an in
vitro model of HF. In addition, the cells were treated with the
PI3K/Akt pathway inhibitor, LY294002. The results of CCK-8 assay
demonstrated that CKI and LY294002 increased H9C2 cell viability,
compared with the Ang II group. The cell cycle is a repeating
series of events that occur in a cell, leading to cell division and
DNA replication to produce two daughter cells (43). The cell cycle is divided into the
interphase phase and metaphase phase (M). The cell interphase can
be further divided into three phases, namely, the G1
phase (period of time before mitosis and DNA replication), the S
phase (period of DNA synthesis) and the G2 phase (period
between the S and M phase). Cells undergoing a temporary pause in
cell division are in the G0 phase. The disruption of the
cell cycle inhibits cell growth and activates the process of
apoptosis (44). The regulation
of the cell cycle is usually dependent on G1 phase
arrest. The proliferation index (PI) is calculated using the
following formula (45): PI=(S +
G2/M)/(G0/G1 + S +
G2/M) ×100%. As shown in Fig. 6C, the cells were accumulated in
the G1 phase in the Ang II group, while the percentage
of cells in the S phase decreased. By contrast, the opposite
results were observed following treatment with CKI and LY294002.
Thus, the PI of the CKI and LY294002 group was higher than that in
the Ang II group. These results indicate that CKI may promote
myocardial cell proliferation and inhibit myocardial cell
apoptosis.
The PI3K/Akt pathway plays a crucial role in
regulating the cell cycle and cell apoptosis (18). Cell cycle progression is
controlled by the cyclin-CDK complex and CDK inhibitor proteins. In
the G1/S checkpoint, cyclin D1 forms a complex with CDK4
and therefore inhibits pRb via phosphorylation, resulting in the
release of E2F to promote progression through the G1
phase (46). On the other hand,
the activity of the CDK4-cyclin D1 complex is negatively controlled
by CDK inhibitor proteins, such as p27. In the present study, the
results of WB analysis indicated that Ang II-induced G1
arrest may be attributed to the decreased expression of cyclin D1
and the increased expression of p27. Treatment with CKI increased
the expression of cyclin D1 and decreased that of p27, similar to
the effects of LY294002. Notably, the results of the in
vitro analysis suggested that CKI inhibited the apoptosis of
H9C2 cells, similar to the effects of the PI3K/Akt pathway
inhibitor, LY294002.
CKI is a type of TCM formulation. The active
compounds of CKI consist of matrine, oxymatrine, sophocarpine,
sophoridine, sophorine and kurarinone. The cardioprotective
function of CKI may be associated with the multiple components. As
aforementioned, matrine has been shown to exhibit a number of
therapeutic effects on the cardiovascular system. Matrine has been
shown to improve the function of HF in mice by resisting the
inflammatory response and oxidative stress, and decreasing
cardiomyocyte apoptosis (47).
Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad
pathway in experimental diabetic cardiomyopathy (48). Furthermore, oxymatrine
pre-treatment has been shown to protect cardiomyocytes from
hypoxia/reoxygenation injury by modulating the PI3K/Akt pathway
(26). Oxymatrine can attenuate
HF by improving cardiac function and this amelioration is
associated with the upregulation of sarcoplasmic/endoplasmic
reticulum Ca2+ ATPase 2a and 6,7-dihydropteridine
reductase (49). A series of
previous studies demonstrated that sophocarpine exerted a number of
protective effects on the heart, vessels and other tissues.
Sophocarpine has been shown to reduce the myocardial infarct size
and improve cardiac function follwing ischemia-reperfusion in rats
via NF-κB inactivation (50).
Sophocarpine potentially exerts antifibrotic effects on the heart
by modulating the balance between pro-inflammatory cytokine
expression and the collagen content level, as well as MMP
expression via the NF-κB signaling pathway (51). In summary, different components
protect cardiac functions via different pathways. In the present
study, it was demonstrated that CKI, as a compound preparation,
exerted cardioprotective effects by inhibiting the PI3K/Akt
pathway.
The present study has certain limitations which
should be mentioned. It remains unknown as to whether the
cardiovascular protective effects of CKI are dependent on matrine
or other compounds, and which compound or compounds are responsible
for the cardioprotective effects. In addition, the present study
only briefly referred to certain some previous studies (21-26,47-51) on matrine, oxymatrine and
sophocarpine. There may be other components with beneficial
cardioprotective effects. On the other hand, in the present study,
it was indicated that CKI inhibited the PI3K/Akt pathway. This
pathway may contribute to the effects of CKI, but may not be the
primary target. To further demonstrate that the suppression of this
pathway is the main mechanism of action of CKI, additional studies
are warranted using transgenic animals or using the combination
treatment of CKI and a PI3K/Akt pathway agonist. Due to the limited
sample size, the authors were unable to adequately assess the most
effective compound or pathway. Further research is thus required to
verify which compound or pathway is primarily responsible for the
cardioprotective effects. Moreover, although the results of the
present study verified that CKI inhibited apoptosis, the percentage
of TUNEL-positive cells observed following TUNEL staining was
inconsistent with the apoptotic rate of cardiomyocytes determined
by FCM. These results may differ due to different experimental
techniques. Additional external validations and evaluations are
required to verify the apoptotic rate.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that CKI attenuates Ang
II-mediated HF in vivo and in vitro, and this
amelioration is associated with the inhibition of the PI3K/Akt
pathway. CKI markedly decreased the expression of pro-apoptotic
proteins, including PI3K, Bax, cyto c, cleaved caspase-9 and
cleaved caspase-3, while it significantly increased the expression
of p-Akt and Bcl-2. In addition, CKI reduced cardiomyocyte
apoptosis, decreased myocardial damage, prevented heart structure
remodeling and promoted healthy cardiac function. Therefore, CKI
may exhibit potential as a novel treatment option for promoting
healthy cardiac function.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and DL conceived and conducted the experiments.
WW wrote the manuscript. LY and LC established the animal models.
MM established the cell models. YL and YY performed the data
curation. MW and GL supervised the experimental process and
performed the statistical analysis. MZ and YA designed the research
and provided funding acquisition. All authors confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were carried out in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health, and all animal experiments
were approved by the Institutional Animal Care and Use Committee of
The First Hospital of Hebei Medical University, Shijiazhuang, China
(ethics approval no. 20220634).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Hebei Key Laboratory of
Heart and Metabolism (grant no. SZX2021003), the China Hebei
International Joint Research Center for Structural Heart Disease,
the S&T Program of Hebei (grant nos. 203777117D and 19277757D),
and the Natural Science Foundation of Hebei Province (grant nos.
H2021206399 and H2020206420).
Abbreviations:
|
HF
|
heart failure
|
|
Ang II
|
angiotensin II
|
|
CKI
|
compound Kushen injection
|
|
TCM
|
traditional Chinese medicine
|
|
PBS
|
phosphate-buffered saline
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
DAPI
|
4',6'-diamidino-2-phenylindole
|
|
PI
|
propidium iodide
|
|
FBS
|
fetal bovine serum
|
|
CCK-8
|
Cell Counting Kit-8
|
|
t-Akt
|
total Akt
|
|
p-Akt
|
phosphorylated Akt
|
|
cyto c
|
cytochrome c
|
|
LVDS
|
left ventricular end-systolic
diameter
|
|
LVDD
|
left ventricular end-diastolic
diameter
|
|
LVFS
|
left ventricular fraction
shortening
|
|
LVEF
|
left ventricular ejection
fraction
|
|
cTn I
|
cardiac troponin I
|
|
FCM
|
flow cytometry
|
|
WB analysis
|
western blot analysis
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Pang H, Han B, Yu T and Zong Z: Effect of
apelin on the cardiac hemodynamics in hypertensive rats with heart
failure. Int J Mol Med. 34:756–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American heart association. Circulation.
131:e29–e322. 2015.
|
|
3
|
Sinphitukkul K, Manotham K and Eiam-Ong S
and Eiam-Ong S: Aldosterone nongenomically induces angiotensin II
receptor dimerization in rat kidney: Role of mineralocorticoid
receptor and NADPH oxidase. Arch Med Sci. 15:1589–1598. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao G, Chen W, Yan M, Liu J, Luo H, Wang C
and Yang P: Rapamycin regulates the balance between cardiomyocyte
apoptosis and autophagy in chronic heart failure by inhibiting mTOR
signaling. Int J Mol Med. 45:195–209. 2020.
|
|
5
|
Kang PM and Izumo S: Apoptosis and heart
failure: A critical review of the literature. Circ Res.
86:1107–1113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan GX, Ji LH, Xia WB, Cheng L and Zhang
YG: Bioinformatics identification of potential candidate blood
indicators for doxorubicin-induced heart failure. Exp Ther Med.
16:2534–2544. 2018.PubMed/NCBI
|
|
7
|
Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y,
Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y,
et al: Angiotensin II type 2 receptor is upregulated in human heart
with interstitial fibrosis, and cardiac fibroblasts are the major
cell type for its expression. Circ Res. 83:1035–1046. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurado Acosta A, Rysä J, Szabo Z, Moilanen
AM, Serpi R and Ruskoaho H: Phosphorylation of GATA4 at serine 105
is required for left ventricular remodelling process in angiotensin
II-induced hypertension in rats. Basic Clin Pharmacol. 127:178–195.
2020. View Article : Google Scholar
|
|
9
|
Fukami K, Ueda S, Yamagishi S, Kato S,
Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, et
al: AGEs activate mesangial TGF-beta-Smad signaling via an
angiotensin II type I receptor interaction. Kidney Int.
66:2137–2147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Meng H, Wang Q, Shao M, Lu W, Chen
X, Jiang Y, Li C, Wang Y and Tu P: Baoyuan decoction ameliorates
apoptosis via AT1-CARP signaling pathway in H9C2 cells and heart
failure post-acute myocardial infarction rats. J Ethnopharmacol.
252:1125362020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan Y, Abe I, Nakagawa M, Ishii Y, Kira
S, Miyoshi M, Oniki T, Kondo H, Teshima Y, Yufu K, et al: A
traditional herbal medicine rikkunshito prevents angiotensin
II-Induced atrial fibrosis and fibrillation. J Cardiol. 76:626–635.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, You RL, Qin WJ, Hai LN, Fang MJ,
Huang GH, Kang RX, Li MH, Qiao YF, Li JW and Li AP: Anti-tumor
activities of active ingredients in compound Kushen injection. Acta
Pharmacol Sin. 36:676–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang HY, Hu HY, Rong H and Zhao XW:
Effects of compound Kushen injection on pathology and angiogenesis
of tumor tissues. Oncol Lett. 17:2278–2282. 2019.PubMed/NCBI
|
|
14
|
Xu W, Lin H, Zhang Y, Chen X, Hua B, Hou
W, Qi X, Pei Y, Zhu X, Zhao Z and Yang L: Compound Kushen injection
suppresses human breast cancer stem-like cells by down-regulating
the canonical Wnt/β-catenin pathway. J Exp Clin Canc Res.
30:1032011. View Article : Google Scholar
|
|
15
|
Lai BY, Chu AJ, Yu BW, Jia LY, Fan YY, Liu
JP and Pei XH: Clinical effectiveness and safety of Chinese herbal
medicine compound Kushen injection as an add-on treatment for
breast cancer: A systematic review and meta-analysis. Evid Based
Complement Alternat Med. 2022:81184082022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Z, Liao H and Ju Y: Effect of
compound Kushen injection on T-cell subgroups and natural killer
cells in patients with locally advanced non-small-cell lung cancer
treated with concomitant radiochemotherapy. J Tradit Chin Med.
36:14–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Qu Z, Yao H, Sun L, Harata-Lee Y,
Cui J, Aung TN, Liu X, You R, Wang W, et al: An effective drug
sensitizing agent increases gefitinib treatment by down regulating
PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell
lung cancer. Biomed Pharmacother. 118:1091692019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong YX,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu MH, Zhang Y, He J, Tan TP, Wu SJ, Guo
DM, He H, Peng J, Tang ZH and Jiang ZS: Hydrogen sulfide protects
H9c2 cardiac cells against doxorubicin-induced cytotoxicity through
the PI3K/Akt/FoxO3a pathway. Int J Mol Med. 37:1661–1668. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravingerová T, Matejíková J, Neckář J,
Andelová E and Kolář F: Differential role of PI3K/Akt pathway in
the infarct size limitation and antiarrhythmic protection in the
rat heart. Mol Cell Biochem. 297:111–120. 2007. View Article : Google Scholar
|
|
21
|
Huang J and Xu H: Matrine: Bioactivities
and structural modifications. Curr Top Med Chem. 16:3365–3378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao TT, Wang YY, Zhang Y, Bai CH and Shen
XC: Similar to spironolactone, oxymatrine is protective in
aldosterone-induced cardiomyocyte injury via inhibition of calpain
and apoptosis-inducing factor signaling. PLoS One. 9:e888562014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou YH, Shan H, Qiao G, Sui X, Lu Y and
Yang B: Inotropic effects and mechanisms of matrine, a main
alkaloid from Sophora flavescens AIT. Biol Pharm Bull.
31:2057–2062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Guo S, Gao XB, Liu A, Jiang W,
Chen X, Yang P, Liu LN, Shi L and Zhang Y: Matrine attenuates
high-fat diet-induced in vivo and ox-LDL-induced in vitro vascular
injury by regulating the PKCα/eNOS and PI3K/Akt/eNOS pathways. J
Cell Mol Med. 23:2731–2743. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Wang B, Zhou C and Bi Y: Matrine
induces apoptosis in angiotensin II-stimulated hyperplasia of
cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3
activation. Basic Clin Pharmacol Toxicol. 101:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Qin X, Wang Z, Li Y, Chen F, Chen
R, Li C, Zhang W and Zhang M: Oxymatrine pretreatment protects H9c2
cardiomyocytes from hypoxia/reoxygenation injury by modulating the
PI3K/Akt pathway. Exp Ther Med. 21:5562021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Research Council of The National
Academies: Guide for the care and use of laboratory animals. Eighth
edition. The National Academies Press; Washington, DC: 2011
|
|
28
|
Liu D, Zhan Y, Ono K, Yin Y, Wang L, Wei
M, Ji L, Liu M, Liu G, Zhou X and Zheng M: Pharmacological
activation of estrogenic receptor G protein-coupled receptor 30
attenuates angiotensin II-induced atrial fibrosis in ovariectomized
mice by modulating TGF-β1/smad pathway. Mol Biol Rep. 49:6341–6355.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Z, Fan H, Higgins T, Qi J, Haines D,
Trivett A, Oppenheim JJ, Wei H, Li J, Lin H and Howard OM: Fufang
Kushen injection inhibits sarcoma growth and tumor-induced
hyperalgesia via TRPV1 signaling pathways. Cancer Lett.
355:232–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qu Z, Cui J, Harata-Lee Y, Aung TN, Feng
Q, Raison JM, Kortschak RD and Adelson DL: Identification of
candidate anti-cancer molecular mechanisms of compound Kushen
injection using functional genomics. Oncotarget. 7:660032016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin
Q, Zhu J, Mai L, Shan Z, Yu X, et al: Panax notoginseng saponins
inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway
in cardiomyocytes. J Ethnopharmacol. 137:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Omland T, de Lemos JA, Sabatine MS,
Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh
BJ, Rouleau JL, et al: A sensitive cardiac troponin T assay in
stable coronary artery disease. N Engl J Med. 361:2538–2547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vergaro G, Gentile F, Meems LMG, Aimo A,
Januzzi JL Jr, Richards AM, Lam CSP, Latini R, Staszewsky L, Anand
IS, et al: NT-proBNP for risk prediction in heart failure:
Identification of optimal cutoffs across body mass index
categories. JACC Heart Fail. 9:653–663. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tham YK, Bernardo BC, Ooi JYY, Weeks KL
and McMullen JR: Pathophysiology of cardiac hypertrophy and heart
failure: Signaling pathways and novel therapeutic targets. Arch
Toxicol. 89:1401–1438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goldenthal MJ: Mitochondrial involvement
in myocyte death and heart failure. Heart Fail Rev. 21:137–155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI
|
|
37
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Z, Zhang Y, Lin L and Zhou J:
Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes
subjected to myocardial ischemia-reperfusion injury via
upregulation of the PI3K/Akt pathway. Mol Med Rep. 18:1560–1570.
2018.PubMed/NCBI
|
|
39
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Er E, Oliver L, Cartron PF, Juin P, Manon
S and Vallette FM: Mitochondria as the target of the pro-apoptotic
protein Bax. Biochim Biophys Acta. 1757:1301–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Riedl SJ and Salvesen GS: The apoptosome:
Signalling platform of cell death. Nat Rev Mol Cell Bio. 8:405–413.
2007. View Article : Google Scholar
|
|
42
|
Jeong SY, Han MH, Jin CY, Kim GY, Choi BT,
Nam TJ, Kim SK and Choi YH: Apoptosis induction of human leukemia
cells by Streptomyces sp. SY-103 metabolites through activation of
caspase-3 and inactivation of Akt. Int J Mol Med. 25:31–40.
2010.
|
|
43
|
Qin J, Tao D, Duan R, Leng Y, Shen M, Zhou
H, Feng Y, Gao C, Yu Y, Li QQ, et al: Cytokinetic analysis of cell
cycle and sub-phases in MOLT-4 cells by cyclin E + A/DNA
multiparameter flow cytometry. Oncol Rep. 9:1041–1045.
2002.PubMed/NCBI
|
|
44
|
Meikrantz W and Schlegel R: Apoptosis and
the cell cycle. J Cell Biochem. 58:160–174. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Y, Chen Y, Wang J and Liu L: Effects
of ATP-binding cassette transporter G2 in extracellular vesicles on
drug resistance of laryngeal cancer cells in in vivo and in vitro.
Oncol Lett. 21:3642021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lukas J, Bartkova J, Rohde M, Strauss M
and Bartek J: Cyclin D1 is dispensable for G1 control in
retinoblastoma gene-deficient cells independently of cdk4 activity.
Mol Cell Biol. 15:2600–2611. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Lian Q, Li X, Zhou X, Gao H and Cui
T: Matrine alleviates heart failure in rats by resisting
inflammatory response and oxidative stress and reducing cardiac
myocyte apoptosis. Lat Am J Pharm. 38:2170–2176. 2019.
|
|
48
|
Zhang Y, Cui L, Guan G, Wang J, Qiu C,
Yang T, Guo Y and Liu Z: Matrine suppresses cardiac fibrosis by
inhibiting the TGF-β/Smad pathway in experimental diabetic
cardiomyopathy. Mol Med Rep. 17:1775–1781. 2018.
|
|
49
|
Hu ST, Tang Y, Shen YF, Ao HH, Bai J, Wang
YL and Yang YJ: Protective effect of oxymatrine on chronic rat
heart failure. J Physiol Sci. 61:363–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li C, Gao Y, Tian J, Shen J, Xing Y and
Liu Z: Sophocarpine administration preserves myocardial function
from ischemia-reperfusion in rats via NF-κB inactivation. J
Ethnopharmacol. 135:620–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Li L, Chu H, Sun X and Ge Z: Oral
sophocarpine protects rat heart against pressure overload-induced
cardiac fibrosis. Pharm Biol. 52:1045–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|