The cardiovascular system consists of the heart and
its blood vessels. Cardiovascular disease (CVD) is a general term
that describes a type of disease that affects any of these
components. The majority of the anatomical structures that are
related to the cardiovascular system display a common tissue
organization, with endothelial cells (ECs), smooth muscle cells
(SMCs) and myocardial cells being three of the major cell types at
the cellular level. In this context, several types of CVD exist,
affecting only the blood vessels or the heart, but most commonly,
both of these, either simultaneously as result of a common cause or
as a sequalae arising, for example, from one condition that leads
to other diseases within the group (e.g., coronary vessel disease
that leads to ischemic cardiac disease or arrhythmias) (1).
Endometriosis is a common, benign,
estrogen-dependent gynecological disease, associated with chronic
pelvic pain and subfertility, defined by the presence of ectopic
endometrial glands and stroma outside of the uterine cavity on
other organs (2,3). It affects 10 to 15% of women of
reproductive age, exhibiting varying symptoms such as severe pelvic
pain, irregular menstrual bleeding, heavy menstrual pain, urinary
tract and gastrointestinal symptoms and pain during intercourse,
and can significantly affect the quality of life of patients
(4-6). Notably, endometriosis possesses
numerous features that are reminiscent of a benign neoplastic
process, which has the potential for malignant transformation.
Genetic factors contribute to the development of this condition, in
combination with environmental ones, such as toxins and pollution
agents (3,7). Although the exact molecular and
pathophysiological pathways leading to endometriosis remain
unclear, various hypotheses have been suggested thus far, and all
cases are not able to be explained by one theory alone (8). There is strong evidence to suggest
the contribution of not only hormonal aspects, but also of multiple
immune-mediated processes related to chronic inflammation,
increased oxidative stress and an atherogenic lipid profile
(9,10).
Women with endometriosis, which is a heterogeneous
condition, are at a high risk of developing several other chronic
diseases, including cancer (11)
and various autoimmune diseases, such as systemic lupus
erythematosus (SLE), multiple sclerosis, rheumatoid arthritis (RA),
Crohn's disease, scleroderma, ulcerative colitis, autoimmune
thyroid disorder, Sjögren's syndrome, coeliac disease and
ankylosing spondylitis (12-14). Of note, previous findings have
suggested that endometriosis can increase the susceptibility for
cardiovascular disorders in these women, considering that both
conditions share pathogenic mechanisms based on hormonal
deviations, as well as aberrant immunology and genetic profiles
(15-17). Moreover, studies have reported a
similar potential association between endometriosis and
atherosclerotic CVD, underlying similar pathogenic mechanisms
including coronary microvascular dysfunction, endothelial
dysfunction and atherogenic lipid profile (18,19). There is an extended amount of
literature focusing on the increased risk that women with
endometriosis have for developing myocardial infarction, ischemic
heart disease, hypertension, atherosclerosis requiring bypass and
angioplasty or stenting procedures (16,20-24). However, CVD in women with
endometriosis remains underdiagnosed and, therefore, detailed
studies are required to fully understand the clinical relevance, as
well as the underlying pathophysiological mechanisms of the
interactions between these conditions.
The present review discusses and summarizes the
observed increased risk of CVD among women with endometriosis from
the genetic point of view, focusing on the potential underlying
shared genetic factors that warrant further study. Understanding
these associations in depth requires mechanistic and functional
genomics research in an effort to determine the causal shared risk
factors.
Recent advances in human genetics, mainly due to the
rapid development of genome-wide association studies (GWAS),
polygenic risk score and next-generation sequencing (NGS)
technologies, have revealed thousands of genetic associations
between common DNA sequence variants [e.g., single nucleotide
polymorphisms (SNPs)] and complex human diseases or traits, thus
leading to a marked increase in the number of risk alleles
identified in patients with complex diseases. CVDs are the leading
public health problem worldwide (25). Among these, atherosclerosis of the
coronary circulation along with its complications of acute coronary
syndromes, and ischemic heart failure are the principal entities.
Genetic studies have led to a more in-depth knowledge of the
pathophysiological processes in coronary artery disease (CAD) and
the identification of novel treatment targets (e.g., in lipid
metabolism).
Currently, known CAD genes that target the vessel
wall and the process of atherosclerosis independently of the genes
involved in the regulation of risk factors, can be classified as
genes affecting plaque progression and platelet function, although
they are not limited to this classification. Genes involved in the
pathophysiological process include genes affecting the vascular
remodeling (VSMCs), the proliferation/mitosis of angiogenic cells,
transcriptional regulation, inflammation, angiogenesis and nitric
oxide signaling (35).
Endometriosis is a complex disease and genetic,
epigenetic and environmental influences interact with each other,
thus leading to the disease phenotype. Apart from the aberrant
immunological responses, angiogenesis processes and biochemical
alterations leading to the growth of endometrial tissue outside the
uterus, which impair fertility, the disease has a strong genetic
component, as firstly shown by monozygotic twin-based and family
studies performed (36,37). Considering that the association
between genetic polymorphisms and clinical disease has long been
recognized, 'candidate gene' studies have greatly assisted
investigators in identifying causal genetic variants underlying
endometriosis over the past decades, taking into account hundreds
of genes and SNPs (38,39), while GWAS have succeeded in
identifying common genetic variants of moderate effects for various
complex diseases (40-44). Currently, the number of novel
endometriosis-associated loci is increased due to the developments
in NGS techniques, which result in the detection of common or rarer
variants conferring a high risk of disease. However, apart from the
progress made in the identification and confirmation of novel SNPs
associated with endometriosis, many studies have obtained
controversial results, mainly due to the enrolment of small
populations and the unclear definition of race and/or ethnicity
(38). Thus, some of these
endometriosis-associated loci were confirmed in other populations
and/or replication studies, while further new loci were also
identified through meta-analyses, as presented in a comprehensive
analysis by Sapkota et al (45). In a meta-analysis conducted by
Rahmioglu et al (39),
involving four GWAS and four replication datasets, a genome-wide
level association of SNPs in six out of nine genetic loci was
detected for rs12700667 on 7p15.2, rs7521902 near Wingless and
Int-1 (Wnt) family member 4 (WNT4), rs10859871 near vezatin
adherens junctions transmembrane protein (VEZT) gene,
rs1537377 near CDKN2B antisense RNA 1 (CDKN2B-AS1),
rs7739264 near inhibitor of DNA binding 4 (ID4) gene and
rs13394619 in growth regulating estrogen receptor binding 1
(GREB1) gene with all these loci; however, VEZT SNP
exhibited a stronger association with stage III/IV of
endometriosis. A further meta-analysis by Sapkota et al
(44) identified five novel loci
significantly (at a genome-wide level) associated with the risk of
developing endometriosis, with these genes being involved in sex
steroid hormone pathways [fibronectin 1, coiled-coil domain
containing 170, estrogen receptor 1 (ESR1), spectrin repeat
containing nuclear envelope protein 1 and follicle stimulating
hormone subunit beta]. Since then, in the largest GWAS and
replication meta-analysis of endometriosis that was performed to
date, 42 loci (31 novel) were identified. According to that study,
these loci explained 5.5% of disease variance with roles in
progesterone resistance, cell cycle regulation, oncogenesis and
ovarian tissue enhancers (46).
Of note, whole exome sequencing (WES) that was used for the first
time in family studies allowed for the detection of two genes, UDP
glucuronosyltransferase family 2 member B28 (UGT2B28) and
ubiquitin specific peptidase 17 like family member 2
(USP17L2), which are novel endometriosis-associated genes,
by investigating a three-generation family from Crete, Greece
(47).
Several well-conducted basic science studies on
atherosclerosis and CVD over the past years have undoubtedly
established the concept that the deregulation of the immune system
plays a major role in its pathophysiology (48-51). The translational confirmation of
this concept was provided by the Canakinumab Anti-inflammatory
Thrombosis Outcome Study (CANTOS study) (52,53) and studies using colchicine in
patients with CAD (54,55). These pioneer studies proved that
independently of the control of dyslipidemia as a traditional risk
factor, targeting inflammatory cytokines reduced cardiovascular
complications in high-risk subjects. Data from these studies
definitively established the role of inflammation in CVD and
several additive experimental evidence [summarized in (56)], is now supported by genetic data.
Of particular importance, genetic data from GWAS assigned to the
inflammation pathways associated with CAD, do not contain the
expected and easily predictable molecules, such as interleukin
(IL)-1β (57) or components of
the NOD-like receptor protein 3 inflammasome (58) or classic pro-atherogenic
chemokines [e.g., chemokine (C-X-C motif) ligand (CXCL)1] (59). Instead of usual suspects, a member
of the CXCL family, CXCL12 and IL-6 receptor were
identified as CAD genetic risk loci (26,60). As is known, CXCL12 is the ligand
for C-X-C motif chemokine receptor 4. Signaling pathways via this
receptor play a complex role (61); however, generally, in ECs, it
appears to have pro-atherosclerotic properties (62).
Immune and inflammatory cells are not the only
players in the inflammation 'game' of atherosclerosis. Although it
is well known that subsets of ECs in different tissues and organs
have functions, such as alloimmunity, immune cell recruitment,
immune tolerance and vascular inflammation (63-65), additive immune functions, typical
of immune cells, have been recognized. Co-stimulation and
co-inhibition, (66), the
induction of apoptosis in other cells (67) and roles as
semi-professional-antigen-presenting cells, have all been assigned
to ECs.
Of utmost importance, within the past 20 years, new
data have confirmed that atherosclerosis is an inflammatory and
immune-mediated disease, reducing the rank of traditional risk
factors. There is accumulating evidence to suggest that ~40% of
individuals with confirmed CVD were never exposed to smoking or
diagnosed with diabetes mellitus. On the other hand, it is well
established that atherogenesis is accelerated by autoimmune
diseases, such as SLE, antiphospholipid syndrome, RA and vasculitis
(68). In this context, it is not
surprising that a marked improvement in the understanding of the
mechanisms regulating the engagement and activation of various
immune cells in atherosclerosis and the production of several
chemo- and cytokines that regulate these recruitment and process,
may serve as a common molecular mechanism explaining the
co-occurrence of atherosclerosis in several other diseases with
strong inflammatory and immune components, independently of the
male sex, older age or other classic risk factors such as
endometriosis, known to affect young women without traditional risk
factors for CVD (as exactly is the point in SLE).
Taking the aforementioned data into account,
endometriosis and CVD may be considered as inflammatory diseases.
Inflammatory cytokines, chemokines and growth factors that are
involved in the generation of localized inflammation have been
found in atherosclerotic plaque and the peritoneal fluid of female
patients with endometriosis (10). For example, the deregulated
production of interferon-γ has been reported as an underlying
mechanism between endometriosis and atherosclerosis, indicating
that female patients with endometriosis are at an increased risk of
developing microvascular dysfunction and atherosclerosis (69).
Detailed studies have been conducted in an attempt
to elucidate the influence of inflammation in endometriosis, thus
shedding light on the pathogenetic mechanisms leading to this
disorder. The association between inflammation and endometriosis
was initially suggested in infertile women, characterized by an
extended intra-peritoneal inflammation (70). Endometriosis, highlighted by
systemic inflammation in affected organs throughout the body, is
caused by a variety of inflammatory factors, such as cytokines,
prostaglandins, macrophages, and tumor necrosis factor (TNF).
Inflammation is part of the non-specific immune response, playing a
key role in the pathophysiology of the disease, by altering the
function of immune cells and increasing the levels of
pro-inflammatory mediators in the peritoneal cavity, endometrium
and blood. In particular, this marked elevation refers to
pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), while JNK in
eutopic endometrial cells from women with endometriosis upregulates
the expression of inflammatory cytokines (71). Furthermore, levels of various
inflammatory factors, such as intracellular adhesion molecule 1
(ICAM-1), C-reactive protein, IL-1, IL-6, TNF-α and vascular
endothelial growth factor (VEGF) have been found to be elevated in
the peritoneal fluid and peripheral blood of women with
endometriosis (72). In addition,
endometriosis has been shown to be accompanied by alterations in
the levels and function of inflammatory products, including
macrophage migration inhibitory factor, C-C chemokine monocyte
chemoattractant protein-1, serum amyloid A and chemokine (C-C
motif) receptor (CCR)1 (73).
Various studies have significantly associated
endometriosis with angiogenesis, lymphangiogenesis and
neurogenesis, which may be mediated by the activation of
inflammatory cells, thus leading to the ectopic growth of
endometrial tissue. Angiogenesis is significantly involved in
endometriosis in general. A continuum vascular development results
in the development of endometriosis, through the induction of
angiogenesis and lymphangiogenesis, while it is well known that
endometriotic lesions have lymphangiogenic properties (80,81). Angiogenesis and neurogenesis are
activated coordinately, with angiogenesis allowing the maintenance
of lesions, supplying them with functional blood vessels, thus
forming a dense vascularization (82). Taking into account that pelvic
pain represents a usual manifestation of endometriosis, the
endometriosis-associated increase in neurogenesis is likely to be a
consequence of the observed proximity between mast cells and nerve
fibers and, therefore, mast cells may contribute to pain (83). In this framework, it has been
suggested that neurogenesis contributes to the growth of nerve
fibers, the subsequent peripheral neuroinflammation and the
generation of chronic pain (84).
Notably, the identification of novel molecular factors associated
with angiogenesis and lymphangiogenesis may facilitate the
development of novel therapeutic strategies for this disorder.
Moreover, in a recent study focusing on the measurement and
validation of circulating proteins in predominantly adolescents and
young adult women with endometriosis, a significant enrichment and
increased activation of proteins that are related to angiogenesis
and cell migration pathway was observed (85).
Although in adults the majority of ECs remain
quiescent, postnatal angiogenesis can take place under both
physiological and pathological stimuli, including reproduction,
inflammation, tissue regeneration and tumor growth. In line with
this phenomenon, pathological angiogenesis has been implicated in
the pathogenesis of numerous diseases, such as cancer,
atherosclerosis, ischemic heart disease, hypertension and vascular
retinopathies. The mechanisms of angiogenesis are numerous, with
angiogenic factors and their corresponding signaling pathways
playing a key role in blood vessel growth and morphogenesis
(86). In the cascade of
angiogenesis and vascular diseases, important functions for ncRNAs
have been assigned. ncRNAs can be classified as microRNAs (miRNAs
or miRs), circular RNAs, other small RNAs (including tRNAs, 5S and
5.8S ribosomal RNA, small nuclear RNAs and piRNAs) and lncRNAs.
Currently, although the functions and mechanisms of miRs in
angiogenesis and vascular diseases are well established (87,88), the perspective role of lncRNAs
remains unknown. What definitely be concluded about the functional
mechanisms of lncRNAs regarding their expression, regulation and
roles in angiogenesis and vascular disease, is that central
mechanisms predicted from shared genetic background between
endometriosis and CVD, are cross-linked in several molecular
pathways that are significantly modified by lncRNAs, thus
emphasizing the rationale for a detailed description of these
mechanisms [reviews on lncRNAs in CVD and therapeutic angiogenesis
have been previously provided (89,90)].
The standard sequence of pathogenic events involved
in the progression of atherosclerosis has been described, including
endothelial dysfunction and endothelial activation as initial
events, followed by monocyte/macrophage adhesion, activation and
migration, lipid deposition, extracellular matrix synthesis, SMC
migration and proliferation and finally, plaque neovascularization
(48,91). Specific environments in
atherosclerotic areas (relative anoxia, inflammation, oxidative
stress) induce angiogenic factors that primarily promote sprouting
angiogenesis from pre-existing vasa vasorum (92-97). Neovascularization acts by
supplying nutrients and O2 to the local
microenvironment; however, the incomplete maturation and the
fragility of neocapillaries promote intra-plaque hemorrhages that
may lead to plaque instability and rupture (98). Indeed, the presence of
neocapillaries in atherosclerotic plaques play a role in their
progression and complications (93), with the adventitial delivery of
VEGF promoting neoangiogenesis and intimal hyperplasia (99), whereas inhibitors of angiogenesis
attenuate plaque growth (100).
It appears reasonable that the vascular wall where
resident, recruited and circulating cells and lipids interact for
the initial formation and proceeding plaque progression, along with
increasing evidence of the involvement of immune cells,
inflammation [for a detailed overview please see (101), and for an overview of platelets
please see (102)] in
atherosclerosis, has been subjected to detailed investigations.
VSMCs have been already described to influence atherosclerosis via
vascular tone and via local processes, including inflammation
andvascular remodeling, leading either to the stabilization or
destabilization of the plaque. Significant genetic overlap exists
between these two functions. The nitric oxide signaling pathway
induces vasodilatation via the second messenger cyclic guanosine
monophosphate (103) and
simultaneously inhibits the migration of VSMCs (104), which itself is a hallmark of
athero-progression (105).
Another genetic locus affecting VSMC biology is the 9p21 locus
(33,34), which will be described in the
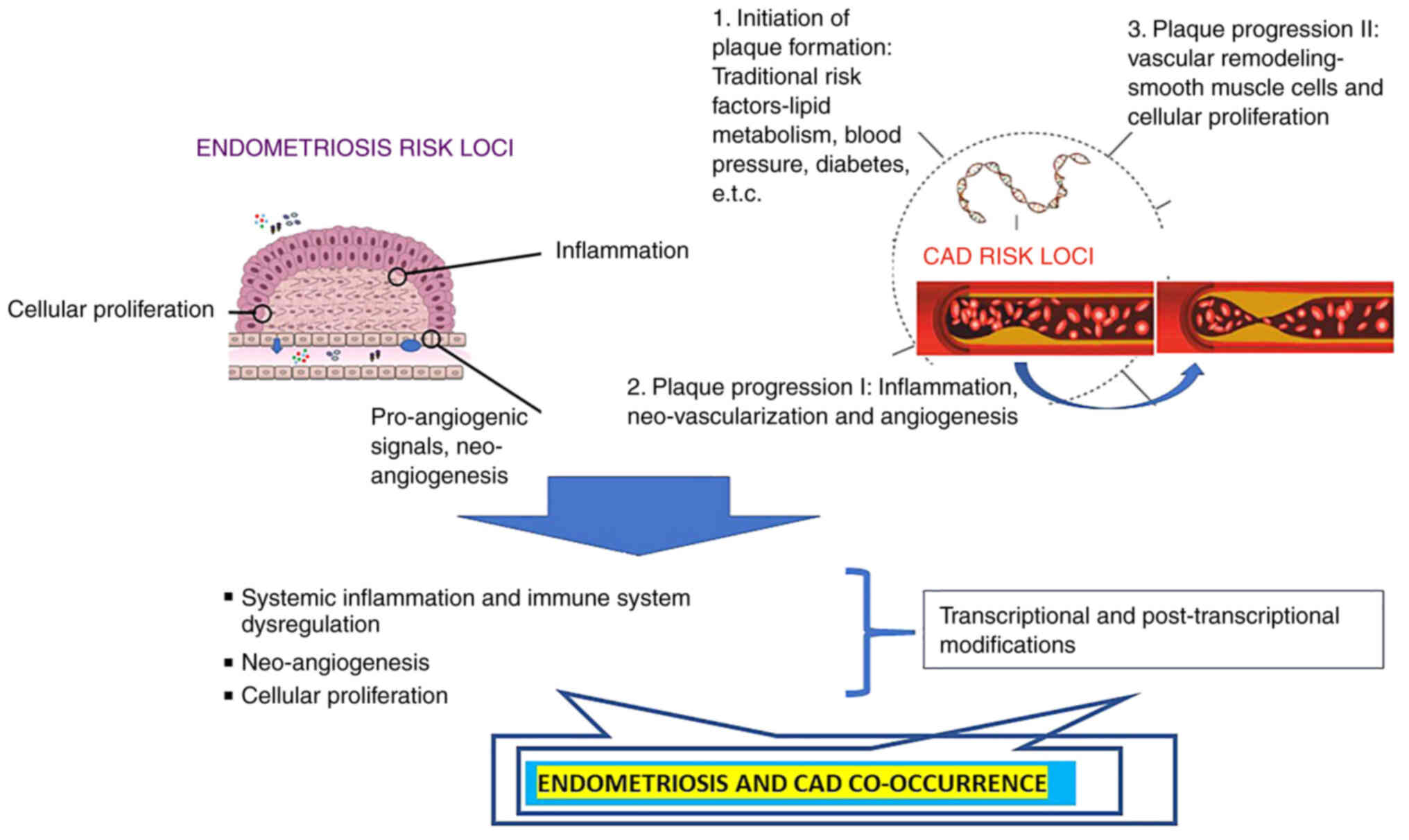
following sections of the present review. A schematic diagram of
the shared pathogenesis of CVDs and endometriosis is presented in
Fig. 1.
Previous findings have suggested an association
between endometriosis and an increased risk of developing CVD in
various populations (15,17-23), with this observation posing the
interesting question regarding the potential role of a shared
genetic background on the co-occurrence of endometriosis and CVD.
Considering that various genes involved in inflammation are
significantly associated with both endometriosis and CVD, it
appears reasonable that shared genetic components between these
diseases exist. Indeed, recent findings have linked endometriosis
to several pathological mechanisms, ranging from systemic
inflammation and enhanced oxidative stress to endothelial
dysfunction (106). Given the
fact that CVD is also a systemic and multifactorial condition, it
may be intriguing to further explore the shared mechanisms
underlying endometriosis and CVD, by highlighting key clinical
evidence that links endometriosis with adverse cardiovascular
events. Considering that the aforementioned mechanisms of systemic
inflammation and endothelial dysfunction are considered the key
mechanisms in the development of atherosclerosis/CVD and that both
endometriosis and CVD are characterized by a strong genetic
component, as described above, the present review presents current
knowledge regarding the potential shared genetic background of
these diseases by performing an extensive search of the current
literature.
With a closer look at these common genetic findings,
one can easily conclude that they can be grouped into three major
gene groups, representing inflammation and immunity, angiogenesis
and cell proliferation and, finally, the group of ncRNAs (either
miRs or lncRNAs) that influence several biological pathways by
transcriptional and post-transcriptional regulation, cross-linking
all the above pathways in one way or another (9,147-150). As it is widely acknowledged that
endometriosis is a multifactorial condition involving hormonal,
pro-inflammatory, pro-angiogenic, immunological and genetic
processes that have been previously extensively reviewed (8,9,106), in the following section, the
present review describes in further detail the influence of this
common genetic background in the context of possible risk
prediction for both diseases and putative implications in
therapeutic management, which is the ultimate task in every genetic
analysis.
Cytokines modify immune responses and contribute to
the maintenance of the balance between pro-inflammatory and
anti-inflammatory stimuli in the process of CVD. The IL-6
gene, located on chromosome 7p21, encodes a multifunctional
pro-inflammatory cytokine and represents a physiological link
between the endocrine and the immune systems. An association of the
IL-6 promoter region rs1800796 (-634C/G) SNP with the
increased susceptibility to both CAD and endometriosis has been
reported (114,115). This SNP influences IL-6
transcription rates in vitro and basal IL-6 levels
in vivo (159), while
elevated levels of IL-6 were assessed in both peritoneal fluid and
serum of women with endometriosis (130). Similarly, Lu et al
(115) reported that the G
allele of this SNP may influence the expression of the IL-6
gene, given that bioinformatics analysis revealed that it is
located in a potential transcription factor binding site. It was
suggested that this SNP predisposes to CAD, considering that
IL-6 is expressed at relatively high levels in human
atherosclerotic plaques (160).
IL-10, produced by Th2 cells and macrophages, is an
immunomodulatory, anti-inflammatory cytokine, involved in the
ongoing coronary inflammation and may inhibit the activation and
function of T-cells, macrophages and monocytes (161). The human IL-10 gene maps
to chromosome 1q31-32. It has been demonstrated that IL-10
promoter polymorphisms are associated with the production of
anti-CA II antibody in patients with endometriosis (162). Previous research on women with
endometriosis has demonstrated that allele C of rs1800871 SNP is
associated with a 2-fold reduced risk of developing the disease
compared with the common TT genotype, while the C allele is
associated with higher levels of IL-10 compared with the T allele
in women suffering from this condition (163). Furthermore, a significant
increase in the IL-10 serum level in patients with CVD has been
previously reported (164).
Estrogen exerts its effects on tissues upon binding
(with high affinity) to and activating estrogen receptors (ERs).
ERs are steroid nuclear ligands that are involved in the regulation
of the transcription of the estrogen-responsive genes (113). Apart from the initial thoughts
that estrogens are hormones involved in female reproduction, it has
been found that they are also involved in lipid homeostasis,
vascular tissue repair, insulin signaling and CVD (174,175). The eESR1 gene, considered
the primary receptor for estrogen, is located on chromosome 6q25.1
and consists of eight exons separated by seven introns. The
rs9340799 SNP, which is an XbaI restriction site
polymorphism involving an A-to-G transition in intron-1, has been
found in previous studies to increase the risk of developing both
CVD and endometriosis (112,113). It has been suggested that the A
to G transition of rs9340799 may alter transcription, thus exerting
its effect on the gene expression levels as well as ER-related
molecular mechanisms (176). In
particular, a decreased number of ERs may result in less effective
estrogen signaling and, as a consequence, may indirectly influence
molecular signaling mechanisms that are related to risk factors for
CVD (113). Moreover, numerous
studies have shown an association between G allele of rs9340799 SNP
and at least one risk factor for CVD, while the GG genotype was
reported to increase the risk of developing endometriosis 4-fold
(177,178).
The ABO blood group is determined by the presence
of A and B antigens on the surface of the red blood cells with the
frequency of the common ABO phenotypes varying among different
populations, probably due to evolutionary selection (183). ABO blood groups have been shown
to be associated with various disease phenotypes, including
cardiometabolic diseases (184).
rs507666 at ABO/9q34.2, located within intron 1, has been
previously shown to be associated with CVD and, recently, with
endometriosis as well (124,141). Various studies have shown that
the ABO glycotransferase may result in the development of
atherosclerotic CVD by a broader mechanism than simply modulating
thrombosis (141). The study by
Pare et al (185)
demonstrated that rs507666, which represents a perfect tag for ABO
blood group A1, was associated with decreased levels of circulating
soluble ICAM-1 (sICAM-1) when compared to the O allele. Moreover,
in a previous meta-analysis, it was found that heterozygotes, as
well homozygotes for the minor allele of rs507666 had lower plasma
levels of sICAM-1, soluble P-selectin (sP-selectin) and soluble
E-selectin (sE-selectin) (186).
Since the first GWAS on CAD, the chr9p21 risk locus
has emerged as a top signal in GWAS of atherosclerotic CVD and was
shown to increase susceptibility to CAD in carriers of certain
alleles of SNPs located within this locus (26,187). This locus codes for an antisense
RNA (CDKN2B-AS1 or ANRIL), which is located close to the
CDKN2A-CDKN2B gene cluster (188). Both the CDKN2A and
CDKN2B genes have been reported to be significantly
associated with an increased risk of CAD (26). Thus, rs10757272 of CDKN2B,
as well as the rs10965235 of ANRIL were found to be
associated with CAD (189),
while rs1333049 of ANRIL has been found to be associated
with myocardial infarction (128,131,190). Notably, all the aforementioned
SNPs have been associated with endometriosis as well (129,191). It has been reported that the
majority of genetic variants associated with CAD are located within
intronic and flanking sequences of ANRIL, which is involved
in the regulation of both CDKN2A and CDKN2B genes
(189). This locus mediates its
risk at the vessel wall, given that the repression of these genes
causes SMC proliferation, which appears at the coronary artery wall
during the initial stages of atherosclerosis (192). In this framework, it has also
been reported that the genotype of individuals determines the
production of atherogenic (linear) over anti-atherogenic
ANRIL RNA species (circular), thus pointing to the
functional role of ANRIL regulation in CAD (131). However, the molecular mechanisms
through which the genotype of this locus controls the ratio of
linear and circular ANRIL are currently unclear (131).
Based on the aforementioned data, a clear
identification of a link between endometriosis and lifelong CVD
would necessitate a radical shift in public health management.
Although endometriosis remains an enigmatic disease and little is
understood about the main causes of the disease, it has been
associated with various diseases, including CVD, thus suggesting
that women with endometriosis may represent a high-risk group for
CVD. Of note, Taskin et al (225) pointed out that endometriosis
should be considered as a risk factor for CVD, thus requiring
specific counseling and prevention. Key questions that arise from
these studies refer to whether gynecologists have to recommend
women with endometriosis for a cardiology assessment, as well as
the possibility of these findings leading to substantial changes in
treatment options. However, it should not be under-recognized that
other known risk factors for CVD in women, such as diabetes, LDL-C
levels, obesity and hypertension, may have a larger combined effect
on the risk of CVD compared to endometriosis alone. Evidently, an
in-depth understanding of the association between these conditions
may enrich the existing knowledge and may provide new insight into
the chronic consequences of endometriosis. Furthermore, given the
high prevalence of endometriosis, the development of preventive and
early detection guidelines for CVD for women with endometriosis may
prove beneficial for public health (226).
Advances in cardiovascular genetics prompted the
American Heart Association (AHA) to publish a very recent
Scientific Statement for the incorporation of polygenic risk scores
to the management of five cardiometabolic diseases (coronary artery
disease, hypercholesterolemia, type 2 diabetes, atrial
fibrillation, and venous thromboembolic disease) (227). This report summarizes the dogma
for the CVD as a multifactorial and complex one. Despite
advancements being made, risk prediction remains imprecise with
persistently high rates of incident CVD. Currently, such scores are
often used in research, one field being the study the
inter-relationship between the risk of CAD and other phenotypes. In
an attempt to unravel the mechanisms underlying the risk
association between endometriosis and CVD, various possible
explanations can be suggested. Thus, it can be hypothesized that
endometriosis may cause chronic inflammation, considering that
inflammation is the precursor to a number of different disease
pathologies, including CVD. To this end, various markers of
endothelial function (including common carotid intima-media
thickness) can be useful for an evaluation of a preclinical and
subclinical risk of atherosclerosis in women with endometriosis,
not only in the peritoneal cavity, but also at a systemic level
(10). Notably, a higher risk of
CHD has been observed among women who have had a
hysterectomy/oophorectomy than in those who have not undergone this
surgical procedure and, as a consequence, this fact has to be taken
into account before a decision for this invasive treatment
(15). The data presented herein
provide evidence of various genetic factors that are shared between
endometriosis and CVD, thus demonstrating apparent genetic links
between these conditions. Noteworthy, it is beneficial for
clinicians to be aware of the possibility of a co-occurrence of
these diseases in order to provide suitable medication to women
with endometriosis. Given the existing link between endometriosis
and early menopause (228),
hormone replacement therapy during perimenopause has shown that
estrogen therapy is cardioprotective (229). However, although estrogens have
been found to be generally cardioprotective in women with early
atherogenesis, it has been reported that it is potentially harmful
in women with established atherosclerosis (230). In addition, the common treatment
of endometriosis by the inhibition of the production of
prostaglandins (using drugs such as ibuprofen or naproxen) in an
attempt to significantly reduce the symptoms of disease should be
avoided, considering that these can lead to an increased
cardiovascular risk as an undesirable secondary effect (231).
Future research should attempt to fully unravel the
shared molecular pathways underpinning the association between
endometriosis and CVD, thus allowing physicians to develop and
customize novel therapeutic interventions based on an individual's
molecular and clinical profiles.
Not applicable.
VMV, MIZ, EE and GNG designed the present study and
drafted the manuscript. GNG, MIZ, DV, LP, DAS and DC searched the
literature. DC, EE, DV, DC, LP, MIZ and VMV analyzed and organized
the data to be included in the review. DAS, DV, LP and DC
critically revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Organization WH: Global status report on
noncommunicable diseases 2014. World Health Organization; 2014
|
|
2
|
Berkley KJ, Rapkin AJ and Papka RE: The
pains of endometriosis. Science. 308:1587–1589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Symons LK, Miller JE, Kay VR, Marks RM,
Liblik K, Koti M and Tayade C: The immunopathophysiology of
endometriosis. Trends Mol Med. 24:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpson JL, Elias S, Malinak LR and
Buttram VC Jr: Heritable aspects of endometriosis. I. Genetic
studies. Am J Obstet Gynecol. 137:327–331. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maroun P, Cooper MJ, Reid GD and Keirse
MJ: Relevance of gastrointestinal symptoms in endometriosis. Aust N
Z J Obstet Gynaecol. 49:411–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Graaff AA, D'Hooghe TM, Dunselman GA,
Dirksen CD and Hummelshoj L; WERF EndoCost Consortium and Simoens
S: The significant effect of endometriosis on physical, mental and
social wellbeing: Results from an international cross-sectional
survey. Human Reprod. 28:2677–2685. 2013. View Article : Google Scholar
|
|
7
|
Vassilopoulou L, Matalliotakis M, Zervou
MI, Matalliotaki C, Krithinakis K, Matalliotakis I, Spandidos DA
and Goulielmos GN: Defining the genetic profile of endometriosis.
Exp Ther Med. 17:3267–3281. 2019.PubMed/NCBI
|
|
8
|
Vercellini P, Vigano P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014. View Article : Google Scholar
|
|
9
|
Matalliotakis M, Zervou MI, Eliopoulos E,
Matalliotaki C, Rahmioglu N, Kalogiannidis I, Zondervan K,
Spandidos DA, Matalliotakis I and Goulielmos GN: The role of IL16
gene polymorphisms in endometriosis. Int J Mol Med. 41:1469–1476.
2018.PubMed/NCBI
|
|
10
|
Santanam N, Song M, Rong R, Murphy AA and
Parthasarathy S: Atherosclerosis, oxidation and endometriosis. Free
Radical Res. 36:1315–1321. 2002. View Article : Google Scholar
|
|
11
|
Kvaskoff M, Mu F, Terry KL, Harris HR,
Poole EM, Farland L and Missmer SA: Endometriosis: A high-risk
population for major chronic diseases? Hum Reprod Update.
21:500–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigesi N, Kvaskoff M, Kirtley S, Feng Q,
Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT and Becker
CM: The association between endometriosis and autoimmune diseases:
A systematic review and meta-analysis. Hum Reprod Update.
25:486–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zervou MI, Vlachakis D, Papageorgiou L,
Eliopoulos E and Goulielmos GN: Increased risk of rheumatoid
arthritis in patients with endometriosis: Genetic aspects.
Rheumatology (Oxford). 61:4252–4262. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin Z, Low HY, Chen BS, Huang KS, Zhang Y,
Wang YH, Ye Z and Wei JC: Risk of ankylosing spondylitis in
patients with endometriosis: A population-based retrospective
cohort study. Front Immunol. 13:8779422022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn SH, Khalaj K, Young SL, Lessey BA,
Koti M and Tayade C: Immune-inflammation gene signatures in
endometriosis patients. Fertility Sterility. 106:1420–1431.e7.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hai-Feng T, Wei W, Yuan-Yuan Y, Jun Z,
Su-Ping G and Hui-Ming L: Association between polymorphisms in
IL-16 genes and coronary heart disease risk. Pakistan J Med Sci.
29:1033–1037. 2013.
|
|
17
|
Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia
A, Khan MA and Roohi N: Association of Inflammatory
Markers/Cytokines with cardiovascular risk manifestation in
patients with endometriosis. Mediators Inflamm. 2021:34255602021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinugasa S, Shinohara K and Wakatsuki A:
Increased asymmetric dimethylarginine and enhanced inflammation are
associated with impaired vascular reactivity in women with
endometriosis. Atherosclerosis. 219:784–788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC,
Poli-Neto OB, Ferriani RA and Vieira CS: Unfavorable lipid profile
in women with endometriosis. Fertil Steril. 93:2433–2436. 2010.
View Article : Google Scholar
|
|
20
|
Mu F, Rich-Edwards J, Rimm EB, Spiegelman
D and Missmer SA: Endometriosis and risk of coronary heart disease.
Circ Cardiovasc Qual Outcomes. 9:257–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akinjero A, Adegbala O and Akinyemiju T:
Abstract P320: Is Co-occurring endometriosis among women with
myocardial infarction associated with worse In-hospital outcomes?
Findings from the nationwide inpatient sample. Circulation.
135:AP320. 2017. View Article : Google Scholar
|
|
22
|
Chiang HJ, Lan KC, Yang YH, Chiang JY,
Kung FT, Huang FJ, Lin YJ, Su YT and Sung PH: Risk of major adverse
cardiovascular and cerebrovascular events in Taiwanese women with
endometriosis. J Formos Med Assoc. 120:327–336. 2021. View Article : Google Scholar
|
|
23
|
Okoth K, Wang J, Zemedikun D, Thomas GN,
Nirantharakumar K and Adderley NJ: Risk of cardiovascular outcomes
among women with endometriosis in the United Kingdom: A
retrospective matched cohort study. BJOG. 128:1598–1609. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marchandot B, Curtiaud A, Matsushita K,
Trimaille A, Host A, Faller E, Garbin O, Akladios C, Jesel L and
Morel O: Endometriosis and cardiovascular disease. Eur Heart J
Open. 2:oeac0012022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart disease and stroke statistics-2019
Update: A report from the american heart association. Circulation.
139:e56–e528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samani NJ, Erdmann J, Hall AS,
Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund
P, Wichmann HE, et al: Genomewide association analysis of coronary
artery disease. N Engl J Med. 357:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McPherson R, Pertsemlidis A, Kavaslar N,
Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA,
Tybjaerg-Hansen A, Folsom AR, et al: A common allele on chromosome
9 associated with coronary heart disease. Science. 316:1488–1491.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helgadottir A, Thorleifsson G, Manolescu
A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A,
Sigurdsson A, Baker A, Palsson A, et al: A common variant on
chromosome 9p21 affects the risk of myocardial infarction. Science.
316:1491–1493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wellcome Trust Case Control Consortium:
Genome-wide association study of 14,000 cases of seven common
diseases and 3,000 shared controls. Nature. 447:661–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clarke R, Peden JF, Hopewell JC, Kyriakou
T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, et
al: Genetic variants associated with Lp(a) lipoprotein level and
coronary disease. N Engl J Med. 361:2518–2528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Visel A, Zhu Y, May D, Afzal V, Gong E,
Attanasio C, Blow MJ, Cohen JC, Rubin EM and Pennacchio LA:
Targeted deletion of the 9p21 non-coding coronary artery disease
risk interval in mice. Nature. 464:409–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holdt LM and Teupser D: Recent studies of
the human chromosome 9p21 locus, which is associated with
atherosclerosis in human populations. Arterioscler Thromb Vasc
Biol. 32:196–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holdt LM, Stahringer A, Sass K, Kulak NA,
Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G,
et al: Circular non-coding RNA ANRIL modulates ribosomal RNA
maturation and atherosclerosis in humans. Nat Commun. 7:124292016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lo Sardo V, Chubukov P, Ferguson W, Kumar
A, Teng EL, Duran M, Zhang L, Cost G, Engler AJ, Urnov F, et al:
Unveiling the role of the most impactful cardiovascular risk locus
through haplotype editing. Cell. 175:1796–1810.20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kessler T and Schunkert H: Coronary artery
disease genetics enlightened by genome-wide association studies.
JACC Basic Transl Sci. 6:610–623. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stortoni P, Cecati M, Giannubilo SR,
Sartini D, Turi A, Emanuelli M and Tranquilli AL: Placental
thrombomodulin expression in recurrent miscarriage. Reprod Biol
Endocrinol. 8:12010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saha R, Pettersson HJ, Svedberg P,
Olovsson M, Bergqvist A, Marions L, Tornvall P and Kuja-Halkola R:
Heritability of endometriosis. Fertil Steril. 104:947–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falconer H, D'Hooghe T and Fried G:
Endometriosis and genetic polymorphisms. Obstet Gynecol Surv.
62:616–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rahmioglu N, Montgomery GW and Zondervan
KT: Genetics of endometriosis. Women's Health. 11:577–586. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nyholt DR, Low SK, Anderson CA, Painter
JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin
NG, et al: Genome-wide association meta-analysis identifies new
endometriosis risk loci. Nat Genet. 44:1355–1359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahmioglu N, Nyholt DR, Morris AP, Missmer
SA, Montgomery GW and Zondervan KT: Genetic variants underlying
risk of endometriosis: Insights from meta-analysis of eight
genome-wide association and replication datasets. Hum Reprod
Update. 20:702–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uimari O, Rahmioglu N, Nyholt DR, Vincent
K, Missmer SA, Becker C, Morris AP, Montgomery GW and Zondervan KT:
Genome-wide genetic analyses highlight mitogen-activated protein
kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum
Reprod. 32:780–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Watanabe K, Taskesen E, van Bochoven A and
Posthuma D: Functional mapping and annotation of genetic
associations with FUMA. Nat Commun. 8:18262017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sapkota Y, Steinthorsdottir V, Morris AP,
Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards
TL, Jones S, et al: Meta-analysis identifies five novel loci
associated with endometriosis highlighting key genes involved in
hormone metabolism. Nat Commun. 8:155392017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sapkota Y, Fassbender A, Bowdler L, Fung
JN, Peterse D, O D, Montgomery GW, Nyholt DR and D'Hooghe TM:
Independent replication and meta-analysis for endometriosis risk
loci. Twin Res Hum Genet. 18:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rahmioglu N, Mortlock S, Ghiasi M, Møller
PL, Stefansdottir L, Galarneau G, Turman C, Danning R, Law MH,
Sapkota Y, et al: The genetic basis of endometriosis and
comorbidity with other pain and inflammatory conditions. Nat Genet.
In press.
|
|
47
|
Albertsen HM, Matalliotaki C,
Matalliotakis M, Zervou MI, Matalliotakis I, Spandidos DA, Chettier
R, Ward K and Goulielmos GN: Whole exome sequencing identifies
hemizygous deletions in the UGT2B28 and USP17L2 genes in a
threegeneration family with endometriosis. Mol Med Rep.
19:1716–1720. 2019.PubMed/NCBI
|
|
48
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moore KJ and Tabas I: Macrophages in the
pathogenesis of atherosclerosis. Cell. 145:341–355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Randolph GJ: The fate of monocytes in
atherosclerosis. J Thromb Haemost. 7(Suppl 1): S28–S30. 2009.
View Article : Google Scholar
|
|
51
|
Swirski FK and Nahrendorf M: Leukocyte
behavior in atherosclerosis, myocardial infarction, and heart
failure. Science. 339:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ridker PM: Residual inflammatory risk:
Addressing the obverse side of the atherosclerosis prevention coin.
Eur Heart J. 37:1720–1722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tardif JC, Kouz S, Waters DD, Bertrand OF,
Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al:
Efficacy and safety of low-dose colchicine after myocardial
infarction. N Engl J Med. 381:2497–2505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nidorf SM, Fiolet ATL, Mosterd A,
Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA,
Lenderink T, et al: Colchicine in patients with chronic coronary
disease. N Engl J Med. 383:1838–1847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schloss MJ, Swirski FK and Nahrendorf M:
Modifiable cardiovascular risk, hematopoiesis, and innate immunity.
Circ Res. 126:1242–1259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vromman A, Ruvkun V, Shvartz E,
Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, Folco E, Gram H,
Nahrendorf M, Swirski FK, et al: Stage-dependent differential
effects of interleukin-1 isoforms on experimental atherosclerosis.
Eur Heart J. 40:2482–2491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Grebe A, Hoss F and Latz E: NLRP3
inflammasome and the IL-1 pathway in atherosclerosis. Circ Res.
122:1722–1740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zernecke A, Shagdarsuren E and Weber C:
Chemokines in atherosclerosis: An update. Arterioscler Thromb Vasc
Biol. 28:1897–1908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schunkert H, Konig IR, Kathiresan S,
Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M,
Gieger C, et al: Large-scale association analysis identifies 13 new
susceptibility loci for coronary artery disease. Nat Genet.
43:333–338. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Doring Y, Noels H, van der Vorst EPC,
Neideck C, Egea V, Drechsler M, Mandl M, Pawig L, Jansen Y,
Schröder K, et al: Vascular CXCR4 limits atherosclerosis by
maintaining arterial integrity: Evidence from mouse and human
studies. Circulation. 136:388–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Doring Y, van der Vorst EPC, Duchene J,
Jansen Y, Gencer S, Bidzhekov K, Atzler D, Santovito D, Rader DJ,
Saleheen D and Weber C: CXCL12 derived from endothelial cells
promotes atherosclerosis to drive coronary artery disease.
Circulation. 139:1338–1340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lohse AW, Knolle PA, Bilo K, Uhrig A,
Waldmann C, Ibe M, Schmitt E, Gerken G, Meyer Zum and Büschenfelde
KH: Antigen-presenting function and B7 expression of murine
sinusoidal endothelial cells and Kupffer cells. Gastroenterology.
110:1175–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wedgwood JF, Hatam L and Bonagura VR:
Effect of interferon-gamma and tumor necrosis factor on the
expression of class I and class II major histocompatibility
molecules by cultured human umbilical vein endothelial cells. Cell
Immunol. 111:1–9. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Carman CV and Martinelli R: T
Lymphocyte-endothelial interactions: Emerging understanding of
trafficking and antigen-specific immunity. Front Immunol.
6:6032015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Motz GT, Santoro SP, Wang LP, Garrabrant
T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F and Coukos
G: Tumor endothelium FasL establishes a selective immune barrier
promoting tolerance in tumors. Nat Med. 20:607–615. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Full LE, Ruisanchez C and Monaco C: The
inextricable link between atherosclerosis and prototypical
inflammatory diseases rheumatoid arthritis and systemic lupus
erythematosus. Arthritis Res Ther. 11:2172009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Glavind MT, Forman A, Arendt LH, Nielsen K
and Henriksen TB: Endometriosis and pregnancy complications: A
Danish cohort study. Fertil Steril. 107:160–166. 2017. View Article : Google Scholar
|
|
70
|
Haney AF, Jenkins S and Weinberg JB: The
stimulus responsible for the peritoneal fluid inflammation observed
in infertile women with endometriosis. Fertil Steril. 56:408–413.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Uz YH, Murk W, Bozkurt I, Kizilay G, Arici
A and Kayisli UA: Increased c-Jun N-terminal kinase activation in
human endometriotic endothelial cells. Histochem Cell Biol.
135:83–91. 2011. View Article : Google Scholar
|
|
72
|
Bedaiwy MA and Falcone T: Peritoneal fluid
environment in endometriosis. Clinicopathological implications.
Minerva Ginecol. 55:333–345. 2003.PubMed/NCBI
|
|
73
|
Agic A, Xu H, Finas D, Banz C, Diedrich K
and Hornung D: Is endometriosis associated with systemic
subclinical inflammation? Gynecol Obstet Invest. 62:139–147. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Monsanto SP, Edwards AK, Zhou J,
Nagarkatti P, Nagarkatti M, Young SL, Lessey BA and Tayade C:
Surgical removal of endometriotic lesions alters local and systemic
proinflammatory cytokines in endometriosis patients. Fertil Steril.
105:968–977.e965. 2016. View Article : Google Scholar :
|
|
75
|
Graziottin A, Skaper SD and Fusco M: Mast
cells in chronic inflammation, pelvic pain and depression in women.
Gynecol Endocrinol. 30:472–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Indraccolo U and Barbieri F: Effect of
palmitoylethanolamide-polydatin combination on chronic pelvic pain
associated with endometriosis: Preliminary observations. Eur J
Obstet Gynecol Reprod Biol. 150:76–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Grandi G, Mueller MD, Papadia A, Kocbek V,
Bersinger NA, Petraglia F, Cagnacci A and McKinnon B: Inflammation
influences steroid hormone receptors targeted by progestins in
endometrial stromal cells from women with endometriosis. J Reprod
Immunol. 117:30–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garcia-Gomez E, Vazquez-Martinez ER,
Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I and Cerbon M:
Regulation of Inflammation Pathways and Inflammasome by Sex Steroid
Hormones in Endometriosis. Front Endocrinol. 10:9352019. View Article : Google Scholar
|
|
79
|
Bao C, Wang H and Fang H: Genomic evidence
supports the recognition of endometriosis as an inflammatory
systemic disease and reveals disease-specific therapeutic
potentials of targeting neutrophil degranulation. Front Immunol.
13:7584402022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Malhotra N, Karmakar D, Tripathi V, Luthra
K and Kumar S: Correlation of angiogenic cytokines-leptin and IL-8
in stage, type and presentation of endometriosis. Gynecol
Endocrinol. 28:224–227. 2012. View Article : Google Scholar
|
|
81
|
Kobayashi H, Higashiura Y, Shigetomi H and
Kajihara H: Pathogenesis of endometriosis: The role of initial
infection and subsequent sterile inflammation (Review). Mol Med
Rep. 9:9–15. 2014. View Article : Google Scholar
|
|
82
|
Shifren JL, Tseng JF, Zaloudek CJ, Ryan
IP, Meng YG, Ferrara N, Jaffe RB and Taylor RN: Ovarian steroid
regulation of vascular endothelial growth factor in the human
endometrium: implications for angiogenesis during the menstrual
cycle and in the pathogenesis of endometriosis. J Clin Endocrinol
Metab. 81:3112–3118. 1996.PubMed/NCBI
|
|
83
|
Kirchhoff D, Kaulfuss S, Fuhrmann U,
Maurer M and Zollner TM: Mast cells in endometriosis: Guilty or
innocent bystanders? Expert Opin Ther Targets. 16:237–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu J, Xie H, Yao S and Liang Y: Macrophage
and nerve interaction in endometriosis. J Neuroinflammation.
14:532017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sasamoto N, Ngo L, Vitonis AF, Dillon ST,
Missmer SA, Libermann TA and Terry KL: Circulating proteomic
profiles associated with endometriosis in adolescents and young
adults. Hum Reprod. 37:2042–2053. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ahmed Z and Bicknell R: Angiogenic
signalling pathways. Methods Mol Biol. 467:3–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Landskroner-Eiger S, Moneke I and Sessa
WC: miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect
Med. 3:a0066432013. View Article : Google Scholar
|
|
88
|
Wang S and Olson EN: AngiomiRs-key
regulators of angiogenesis. Curr Opin Genet Dev. 19:205–211. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar :
|
|
91
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Michel JB, Thaunat O, Houard X, Meilhac O,
Caligiuri G and Nicoletti A: Topological determinants and
consequences of adventitial responses to arterial wall injury.
Arterioscler Thromb Vasc Biol. 27:1259–1268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Moreno PR, Purushothaman KR, Sirol M, Levy
AP and Fuster V: Neovascularization in human atherosclerosis.
Circulation. 113:2245–2252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Conway EM, Collen D and Carmeliet P:
Molecular mechanisms of blood vessel growth. Cardiovasc Res.
49:507–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Simons M: Angiogenesis: Where do we stand
now? Circulation. 111:1556–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tanaka K, Nagata D, Hirata Y, Tabata Y,
Nagai R and Sata M: Augmented angiogenesis in adventitia promotes
growth of atherosclerotic plaque in apolipoprotein E-deficient
mice. Atherosclerosis. 215:366–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Michel JB, Martin-Ventura JL, Nicoletti A
and Ho-Tin-Noe B: Pathology of human plaque vulnerability:
Mechanisms and consequences of intraplaque haemorrhages.
Atherosclerosis. 234:311–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bhardwaj S, Roy H, Heikura T and
Yla-Herttuala S: VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced
intimal hyperplasia in carotid arteries. Eur J Clin Invest.
35:669–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Moulton KS, Heller E, Konerding MA, Flynn
E, Palinski W and Folkman J: Angiogenesis inhibitors endostatin or
TNP-470 reduce intimal neovascularization and plaque growth in
apolipoprotein E-deficient mice. Circulation. 99:1726–1732. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Swirski FK and Nahrendorf M:
Cardioimmunology: The immune system in cardiac homeostasis and
disease. Nat Rev Immunolo. 18:733–744. 2018. View Article : Google Scholar
|
|
102
|
Dang TA, Schunkert H and Kessler T: cGMP
signaling in cardiovascular diseases: Linking genotype and
phenotype. J Cardiovasc Pharmacol. 75:516–525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feil R and Kemp-Harper B: cGMP signalling:
From bench to bedside. Conference on cGMP generators, effectors and
therapeutic implications. EMBO Rep. 7:149–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dubey RK, Jackson EK and Luscher TF:
Nitric oxide inhibits angiotensin II-induced migration of rat
aortic smooth muscle cell. Role of cyclic-nucleotides and
angiotensin1 receptors. J Clin Invest. 96:141–149. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bianco B, André GM, Vilarino FL, Peluso C,
Mafra FA, Christofolini DM and Barbosa CP: The possible role of
genetic variants in autoimmune-related genes in the development of
endometriosis. Hum Immunol. 73:306–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu D, Song J, Ji X, Liu Z, Cong M and Hu
B: Association of genetic polymorphisms on VEGFA and VEGFR2 with
risk of coronary heart disease. Medicine. 95:e34132016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma H, Marti-Gutierrez N, Park SW, Wu J,
Hayama T, Darby H, Van Dyken C, Li Y, Koski A, Liang D, Ma, et al:
reply. Nature. 560:E10–E23. 2018. View Article : Google Scholar
|
|
111
|
Shimizu Y, Arima K, Noguchi Y, Yamanashi
H, Kawashiri SY, Nobusue K, Nonaka F, Aoyagi K, Nagata Y and Maeda
T: Vascular endothelial growth factor (VEGF) polymorphism rs3025039
and atherosclerosis among older with hypertension. Sci Rep.
12:55642022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Eldafira E, Prasasty VD, Abinawanto A,
Syahfirdi L and Pujianto DA: Polymorphisms of estrogen receptor-α
and estrogen receptor-β genes and its expression in endometriosis.
Turk J Pharm Sci. 18:91–95. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Casazza K, Page GP and Fernandez JR: The
association between the rs2234693 and rs9340799 estrogen receptor
alpha gene polymorphisms and risk factors for cardiovascular
disease: A review. Biol Res Nurs. 12:84–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mao T, Zong LL, Wang YF, Zhao X, Fu YG,
Zeng J and Rao XQ: Association of the IL-6 gene 634C/G polymorphism
with susceptibility to endometriosis. Zhonghua Yi Xue Yi Chuan Xue
Za Zhi. 28:555–558. 2011.In Chinese. PubMed/NCBI
|
|
115
|
Lu S, Wang Y, Wang Y, Hu J, Di W, Liu S,
Zeng X, Yu G, Wang Y and Wang Z: The IL-6 rs1800795 and rs1800796
polymorphisms are associated with coronary artery disease risk. J
Cell Mol Med. 24:6191–6207. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kumari R and Kumar S, Ahmad MK, Singh R,
Kant Kumar S, Pradhan A, Chandra S and Kumar S: Promoter variants
of TNF-α rs1800629 and IL-10 rs1800871 are independently associated
with the susceptibility of coronary artery disease in north Indian.
Cytokine. 110:131–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Juo SH, Wu R, Lin CS, Wu MT, Lee JN and
Tsai EM: A functional promoter polymorphism in interleukin-10 gene
influences susceptibility to endometriosis. Fertil Steril.
92:1228–1233. 2009. View Article : Google Scholar
|
|
118
|
Zheng J, Chen T and Lin H: IL-10, IL-18
gene polymorphisms might influence predisposition to coronary
artery disease in east asians: A meta-analysis. Immunol Invest.
50:37–46. 2021. View Article : Google Scholar
|
|
119
|
Chen Y, Huang H, Liu S, Pan LA, Zhou B,
Zhang L and Zeng Z: IL-16 rs11556218 gene polymorphism is
associated with coronary artery disease in the Chinese Han
population. Clin Biochem. 44:1041–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Luo Z, Lu Z, Muhammad I, Chen Y, Chen Q,
Zhang J and Song Y: Associations of the MTHFR rs1801133
polymorphism with coronary artery disease and lipid levels: A
systematic review and updated meta-analysis. Lipids Health Dis.
17:1912018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Szczepanska M, Mostowska A, Wirstlein P,
Lianeri M, Marianowski P, Skrzypczak J and Jagodziński PP:
Polymorphic variants of folate and choline metabolism genes and the
risk of endometriosis-associated infertility. Eur J Obstet Gynecol
Reprod Biol. 157:67–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rodriguez-Rodriguez L, Lopez-Mejias R,
Garcia-Bermudez M, Gonzalez-Juanatey C, Gonzalez-Gay MA and Martin
J: Genetic markers of cardiovascular disease in rheumatoid
arthritis. Mediators Inflamm. 2012:5748172012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sundqvist J, Falconer H, Seddighzadeh M,
Vodolazkaia A, Fassbender A, Kyama C, Bokor A, Stephansson O,
Padyukov L, Gemzell-Danielsson K and D'Hooghe TM: Endometriosis and
autoimmune disease: Association of susceptibility to
moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil
Steril. 95:437–440. 2011. View Article : Google Scholar
|
|
124
|
Nilufer R, Karina B, Paraskevi C, Rebecca
D, Genevieve G, Ayush G, Stuar M, Sally M, Yadav S, Andrew SJ, et
al: Large-scale genome-wide association meta-analysis of
endometriosis reveals 13 novel loci and genetically-associated
comorbidity with other pain conditions. bio Rxiv. 406967. 2018,
View Article : Google Scholar
|
|
125
|
Zhang H, Mooney CJ and Reilly MP: ABO
blood groups and cardiovascular diseases. Int J Vasc Med.
2012:6419172012.PubMed/NCBI
|
|
126
|
Omrani-Nava V, Hedayatizadeh-Omran A,
Alizadeh-Navaei R, Mokhberi V, Jalalian R, Janbabaei G, Amjadi O,
Rahmatpour G and Mozaffari A: TP53 single nucleotide polymorphism
(rs1042522) in Iranian patients with coronary artery disease.
Biomed Rep. 9:259–265. 2018.PubMed/NCBI
|
|
127
|
Li J, Chen Y, Mo Z and Li L: TP53 Arg72Pro
polymorphism (rs1042522) and risk of endometriosis among Asian and
Caucasian populations. Eur J Obstet Gynecol Reprod Biol. 189:73–78.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Shakhtshneider E, Orlov P, Semaev S,
Ivanoshchuk D, Malyutina S, Gafarov V, Ragino Y and Voevoda M:
Analysis of polymorphism rs1333049 (Located at 9P21.3) in the white
population of western siberia and associations with clinical and
biochemical markers. Biomolecules. 9:2902019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lalami I, Abo C, Borghese B, Chapron C and
Vaiman D: Genomics of endometriosis: From genome wide association
studies to exome sequencing. Int J Mol Sci. 22:72972021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kang YJ, Jeung IC, Park A, Park YJ, Jung
H, Kim TD, Lee HG, Choi I and Yoon SR: An increased level of IL-6
suppresses NK cell activity in peritoneal fluid of patients with
endometriosis via regulation of SHP-2 expression. Hum Reprod.
29:2176–2189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Holdt LM and Teupser D: Long noncoding RNA
ANRIL: Lnc-ing genetic variation at the chromosome 9p21 locus to
molecular mechanisms of atherosclerosis. Front Cardiovasc Med.
5:1452018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Matoo S, Fallah MS, Daneshpour MS, Mousavi
R, Sedaghati Khayat B, Hasanzad M and Azizi F: Increased risk of
CHD in the presence of rs7865618 (A allele): Tehran lipid and
glucose study. Arch Iran Med. 20:153–157. 2017.PubMed/NCBI
|
|
133
|
Manjula G, Pranavchand R, Kumuda I, Reddy
BS and Reddy BM: The SNP rs7865618 of 9p213 locus emerges as the
most promising marker of coronary artery disease in the southern
Indian population. Sci Rep. 10:215112020. View Article : Google Scholar
|
|
134
|
Yang Y, Shi X, Du Z, Zhou G and Zhang X:
Associations between genetic variations in microRNA and myocardial
infarction susceptibility: A meta-analysis and systematic review.
Herz. 47:524–535. 2022. View Article : Google Scholar
|
|
135
|
Farsimadan M, Ismail Haje M, Khudhur
Mawlood C, Arabipour I, Emamvirdizadeh A, Takamoli S, Masumi M and
Vaziri H: MicroRNA variants in endometriosis and its severity. Br J
Biomed Sci. 78:206–210. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Cai MY, Cheng J, Zhou MY, Liang LL, Lian
SM, Xie XS, Xu S, Liu X and Xiong XD: The associationbetween
pre-miR-27a rs 895819 polymorphism and myocardial infarction risk
in a Chinese Han population. Lipids Health Dis. 17:72018.
View Article : Google Scholar
|
|
137
|
Jaafar SO, Jaffar JO, Ibrahim SA and
Jarjees KK: MicroRNA Variants miR-27a rs895819 and miR-423
rs6505162, but not miR-124-1 rs531564, are Linked to endometriosis
and its severity. Br J Biomed Sci. 79:102072022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang Z, Li H, Zhao Z, Gao B, Meng L and
Feng X: miR-146b level and variants is associated with
endometriosis related macrophages phenotype and plays a pivotal
role in the endometriotic pain symptom. Taiwan J Obstet Gynecol.
58:401–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhao Y, Ponnusamy M, Zhang L, Zhang Y, Liu
C, Yu W, Wang K and Li P: The role of miR-214 in cardiovascular
diseases. Eur J Pharmacol. 816:138–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jones Buie JN, Goodwin AJ, Cook JA,
Halushka PV and Fan H: The role of miRNAs in cardiovascular disease
risk factors. Atherosclerosis. 254:271–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
CARDIoGRAMplusC4D Consortium; Deloukas P,
Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR,
Ingelsson E, Saleheen D, Erdmann J, et al: Large-scale association
analysis identifies new risk loci for coronary artery disease. Nat
Genet. 45:25–33. 2013. View Article : Google Scholar
|
|
142
|
Ghafouri-Fard S, Shoorei H and Taheri M:
Role of Non-coding RNAs in the pathogenesis of endometriosis. Front
Oncol. 10:13702020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Besseling J, Hovingh GK, Huijgen R,
Kastelein JJP and Hutten BA: Statins in familial
hypercholesterolemia: Consequences for coronary artery disease and
All-cause mortality. J Am Coll Cardiol. 68:252–260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Jha CK, Mir R, Elfaki I, Khullar N, Rehman
S, Javid J, Banu S and Chahal SMS: Potential impact of MicroRNA-423
gene variability in coronary artery disease. Endocr Metab Immune
Disord Drug Targets. 19:67–74. 2019. View Article : Google Scholar
|
|
145
|
Chang CY, Lai MT, Chen Y, Yang CW, Chang
HW, Lu CC, Chen CM, Chan C, Chung C, Tseng CC, et al: Up-regulation
of ribosome biogenesis by MIR196A2 genetic variation promotes
endometriosis development and progression. Oncotarget.
7:76713–76725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fragoso JM, Ramirez-Bello J, Martinez-Rios
MA, Peña-Duque MA, Posadas-Sánchez R, Delgadillo-Rodríguez H,
Jiménez-Morales M, Posadas-Romero C and Vargas-Alarcón G: miR-196a2
(rs11614913) polymorphism is associated with coronary artery
disease, but not with in-stent coronary restenosis. Inflamm Res.
68:215–221. 2019. View Article : Google Scholar
|
|
147
|
Davignon J: Beneficial cardiovascular
pleiotropic effects of statins. Circulation. 109(23 Suppl 1):
III39–II43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Leuschner F, Rauch PJ, Ueno T, Gorbatov R,
Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al:
Rapid monocyte kinetics in acute myocardial infarction are
sustained by extramedullary monocytopoiesis. J Exp Med.
209:123–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hoyer FF, Naxerova K, Schloss MJ, Hulsmans
M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, et
al: Tissue-specific macrophage responses to remote injury impact
the outcome of subsequent local immune challenge. Immunity.
51:899–914 e897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhao N and Zhang J: Role of alternative
splicing of VEGF-A in the development of atherosclerosis. Aging.
10:2695–2708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhao W, Li Y, Zhao J and Kang S: A
functional promoter polymorphism in interleukin 12B gene is
associated with an increased risk of ovarian endometriosis. Gene.
666:27–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Buroker NE: SNP (rs1570360) in
transcriptional factor binding sites of the VEGFA promoter is
associated with hypertensive nephropathy and diabetic retinopathy.
Austin J Endocrinol Diabetes. 2:1–5. 2015.
|
|
154
|
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS and
Koh GY: Vascular endothelial growth factor expression of
intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion
molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B
activation in endothelial cells. J Biol Chem. 276:7614–7620. 2001.
View Article : Google Scholar
|
|
155
|
McCarron SL, Edwards S, Evans PR, Gibbs R,
Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA and Howell
WM: Influence of cytokine gene polymorphisms on the development of
prostate cancer. Cancer Res. 62:3369–3372. 2002.PubMed/NCBI
|
|
156
|
Howell WM, Bateman AC, Turner SJ, Collins
A and Theaker JM: Influence of vascular endothelial growth factor
single nucleotide polymorphisms on tumour development in cutaneous
malignant melanoma. Genes Immunity. 3:229–232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Vigano P, Parazzini F, Somigliana E and
Vercellini P: Endometriosis: Epidemiology and aetiological factors.
Best Pract Res Clin Obstet Gynaecol. 18:177–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang W, Xu A and Xu H: The roles of
vascular endothelial growth factor gene polymorphisms in congenital
heart diseases: A meta-analysis. Growth Factors. 36:232–238. 2018.
View Article : Google Scholar
|
|
159
|
Wieser F, Fabjani G, Tempfer C,
Schneeberger C, Sator M, Huber J and Wenzl R: Analysis of an
interleukin-6 gene promoter polymorphism in women with
endometriosis by pyrosequencing. J Soc Gynecol Investig. 10:32–36.
2003.PubMed/NCBI
|
|
160
|
Schieffer B, Schieffer E, Hilfiker-Kleiner
D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W
and Drexler H: Expression of angiotensin II and interleukin 6 in
human coronary atherosclerotic plaques: Potential implications for
inflammation and plaque instability. Circulation. 101:1372–1378.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Moore KW, de Waal Malefyt R, Coffman RL
and O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kitawaki J, Obayashi H, Ohta M, Kado N,
Ishihara H, Koshiba H, Kusuki I, Tsukamoto K, Hasegawa G, Nakamura
N, et al: Genetic contribution of the interleukin-10 promoter
polymorphism in endometriosis susceptibility. Am J Reprod Immunol.
47:12–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang X, Hei P, Deng L and Lin J:
Interleukin-10 gene promoter polymorphisms and their protein
production in peritoneal fluid in patients with endometriosis. Mol
Hum Reprod. 13:135–140. 2007. View Article : Google Scholar
|
|
164
|
Tabrez S, Ali M, Jabir NR, Firoz CK,
Ashraf GM, Hindawi S, Damanhouri GA and Nabil Alama M: A putative
association of interleukin-10 promoter polymorphisms with
cardiovascular disease. IUBMB Life. 69:522–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Center DM, Kornfeld H and Cruikshank WW:
Interleukin-16. Int J Biochem Cell Biol. 29:1231–1234. 1997.
View Article : Google Scholar
|
|
166
|
Mathy NL, Scheuer W, Lanzendörfer M,
Honold K, Ambrosius D, Norley S, Kurth R, et al: Interleukin-16
stimulates the expression and production of pro-inflammatory
cytokines by human monocytes. Immunology. 100:63–69. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Cheong YC, Shelton JB, Laird SM, Richmond
M, Kudesia G, Li TC and Ledger WL: IL-1, IL-6 and TNF-alpha
concentrations in the peritoneal fluid of women with pelvic
adhesions. Hum Reprod. 17:69–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Watkins D and Rosenblatt DS: Inherited
disorders of folate and cobalamin transport and Metabolism. The
Online Metabolic and Molecular Bases of Inherited Disease. Valle
DL, Antonarakis S, Ballabio A, Beaudet AL and Mitchell GA:
McGraw-Hill Education; New York, NY: 2019
|
|
169
|
Lian Z, Lv FF, Yu J and Wang JW: The
anti-inflammatory effect of microRNA-383-3p interacting with IL1R2
against homocysteine-induced endothelial injury in rat coronary
arteries. J Cell Biochem. 119:6684–6694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Cyster JG: Leukocyte migration: Scent of
the T zone. Curr Biol. 10:R30–R33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li G, Zhao J, Li B, Ma J, Zhao Q, Wang X,
Lv Z, Li K, Du Z, Ma X and Liu J: Associations between CCL21 gene
polymorphisms and susceptibility to rheumatoid arthritis: A
meta-analysis. Rheumatol Int. 37:1673–1681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Damas JK, Smith C, Øie E, Fevang B,
Halvorsen B, Waehre T, Boullier A, Breland U, Yndestad A,
Ovchinnikova O, et al: Enhanced expression of the homeostatic
chemokines CCL19 and CCL21 in clinical and experimental
atherosclerosis: Possible pathogenic role in plaque
destabilization. Arterioscler Thromb Vasc Biol. 27:614–620. 2007.
View Article : Google Scholar
|
|
173
|
Nakayama T, Kitaya K, Okubo T, Kuroboshi
H, Daikoku N, Fushiki S and Honjo H: Fluctuation of 6Ckine
expression in human endometrium during the menstrual cycle. Fertil
Steril. 80:1461–1465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Nilsson S and Gustafsson JA: Biological
role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol.
37:1–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Howard BV, Criqui MH, Curb JD, Rodabough
R, Safford MM, Santoro N, Wilson AC and Wylie-Rosett J: Risk factor
clustering in the insulin resistance syndrome and its relationship
to cardiovascular disease in postmenopausal white, black, hispanic,
and Asian/Pacific Islander women. Metabolism. 52:362–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Huang Q, Wang TH, Lu WS, Mu PW, Yang YF,
Liang WW, Li CX and Lin GP: Estrogen receptor alpha gene
polymorphism associated with type 2 diabetes mellitus and the serum
lipid concentration in Chinese women in Guangzhou. Chin Med J
(Engl). 119:1794–1801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lawlor DA, Timpson N, Ebrahim S, Day IN
and Smith GD: The association of oestrogen receptor
alpha-haplotypes with cardiovascular risk factors in the British
Women's Heart and Health Study. Eur Heart J. 27:1597–1604. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Almeida S and Hutz MH: Genetic variation
of estrogen metabolism and the risks of cardiovascular disease.
Curr Opin Investig Drugs. 8:814–820. 2007.PubMed/NCBI
|
|
179
|
Naccarati A, Polakova V, Pardini B,
Vodickova L, Hemminki K, Kumar R and Vodicka P: Mutations and
polymorphisms in TP53 gene-an overview on the role in colorectal
cancer. Mutagenesis. 27:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bennett BJ, Davis RC, Civelek M, Orozco L,
Wu J, Qi H, Pan C, Packard RR, Eskin E, Yan M, et al: Genetic
Architecture of atherosclerosis in mice: A systems genetics
analysis of common inbred strains. PLoS Genet. 11:e10057112015.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Frank AK, Leu JI, Zhou Y, Devarajan K,
Nedelko T, Klein-Szanto A, Hollstein M and Murphy ME: The codon 72
polymorphism of p53 regulates interaction with NF-{kappa}B and
transactivation of genes involved in immunity and inflammation. Mol
Cell Biol. 31:1201–1213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Dumont C, Corsoni-Tadrzak A, Ruf S, de
Boer J, Williams A, Turner M, Kioussis D and Tybulewicz VL: Rac
GTPases play critical roles in early T-cell development. Blood.
113:3990–3998. 2009. View Article : Google Scholar :
|
|
183
|
Storry JR and Olsson ML: The ABO blood
group system revisited: A review and update. Immunohematology.
25:48–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Reilly MP, Li M, He J, Ferguson JF,
Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson
JR, et al: Identification of ADAMTS7 as a novel locus for coronary
atherosclerosis and association of ABO with myocardial infarction
in the presence of coronary atherosclerosis: Two genome-wide
association studies. Lancet. 377:383–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Pare G, Ridker PM, Rose L, Barbalic M,
Dupuis J, Dehghan A, Bis JC, Benjamin EJ, Shiffman D, Parker AN and
Chasman DI: Genome-wide association analysis of soluble ICAM-1
concentration reveals novel associations at the NFKBIK, PNPLA3,
RELA, and SH2B3 loci. PLoS Genet. 7:e10013742011. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kiechl S, Pare G, Barbalic M, Qi L, Dupuis
J, Dehghan A, Bis JC, Laxton RC, Xiao Q, Bonora E, et al:
Association of variation at the ABO locus with circulating levels
of soluble intercellular adhesion molecule-1, soluble P-selectin,
and soluble E-selectin: A meta-analysis. Circ Cardiovasc Genet.
4:681–686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Roberts R and Stewart AF: 9p21 and the
genetic revolution for coronary artery disease. Clin Chem.
58:104–112. 2012. View Article : Google Scholar
|
|
188
|
Broadbent HM, Peden JF, Lorkowski S, Goel
A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G,
et al: Susceptibility to coronary artery disease and diabetes is
encoded by distinct, tightly linked SNPs in the ANRIL locus on
chromosome 9p. Hum Mol Genet. 17:806–814. 2008. View Article : Google Scholar
|
|
189
|
Cunnington MS, Santibanez Koref M, Mayosi
BM, Burn J and Keavney B: Chromosome 9p21 SNPs associated with
multiple disease phenotypes correlate with ANRIL expression. PLoS
Genet. 6:e10008992010. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kalpana B, Murthy DK and Balakrishna N:
9p213 coronary artery disease risk locus and interferon alpha 21:
Association study in an Asian Indian population. Indian Heart J.
71:476–480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ,
Hwang K and Chae SJ: Association of CDKN2B-AS and WNT4 genetic
polymorphisms in Korean patients with endometriosis. Fertil Steril.
102:1393–1397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Motterle A, Pu X, Wood H, Xiao Q, Gor S,
Ng FL, Chan K, Cross F, Shohreh B, Poston RN, et al: Functional
analyses of coronary artery disease associated variation on
chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet.
21:4021–4029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Boettger T, Beetz N, Kostin S, Schneider
J, Krüger M, Hein L and Braun T: Acquisition of the contractile
phenotype by murine arterial smooth muscle cells depends on the
Mir143/145 gene cluster. J Clin Invest. 119:2634–2647. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Elia L, Quintavalle M, Zhang J, Contu R,
Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J,
et al: The knockout of miR-143 and -145 alters smooth muscle cell
maintenance and vascular homeostasis in mice: Correlates with human
disease. Cell Death Differ. 16:1590–1598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Elia L, Kunderfranco P, Carullo P,
Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R,
Condorelli G and Quintavalle M: UHRF1 epigenetically orchestrates
smooth muscle cell plasticity in arterial disease. J Clin Invest.
128:2473–2486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Xin M, Small EM, Sutherland LB, Qi X,
McAnally J, Plato CF, Richardson JA, Bassel-Duby R and Olson EN:
MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and
responsiveness of smooth muscle cells to injury. Genes Dev.
23:2166–2178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Quintavalle M, Elia L, Condorelli G and
Courtneidge SA: MicroRNA control of podosome formation in vascular
smooth muscle cells in vivo and in vitro. J Cell Biol. 189:13–22.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Climent M, Quintavalle M, Miragoli M, Chen
J, Condorelli G and Elia L: TGFβ Triggers miR-143/145 transfer from
smooth muscle cells to endothelial cells, thereby modulating vessel
stabilization. Circ Res. 116:1753–1764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Faccini J, Ruidavets JB, Cordelier P,
Martins F, Maoret JJ, Bongard V, Ferrières J, Roncalli J, Elbaz M
and Vindis C: Circulating miR-155, miR-145 and let-7c as diagnostic
biomarkers of the coronary artery disease. Sci Rep. 7:429162017.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Hall IF, Climent M, Viviani Anselmi C,
Papa L, Tragante V, Lambroia L, Farina FM, Kleber ME, März W,
Biguori C, et al: rs41291957 controls miR-143 and miR-145
expression and impacts coronary artery disease risk. EMBO Mol Med.
13:e140602021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Nimi-Hoveidi E, Kohan L and Hashemi SS:
Association of miR-143 rs41291957 and rs4705342 genetic variants
with endometriosis risk in infertile women. Feyz J Kashan Univ Med
Sci. 20:441–446. 2016.
|
|
203
|
Bashti O, Noruzinia M, Garshasbi M and
Abtahi M: miR-31 and miR-145 as potential non-invasive regulatory
biomarkers in patients with endometriosis. Cell J. 20:84–89.
2018.PubMed/NCBI
|
|
204
|
Ounzain S, Micheletti R, Arnan C,
Plaisance I, Cecchi D, Schroen B, Reverter F, Alexanian M, Gonzales
C, Ng SY, et al: CARMEN, a human super enhancer-associated long
noncoding RNA controlling cardiac specification, differentiation
and homeostasis. J Mol Cell Cardiol. 89:98–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Plaisance I, Perruchoud S,
Fernandez-Tenorio M, Gonzales C, Ounzain S, Ruchat P, Nemir M,
Niggli E and Pedrazzini T: Cardiomyocyte lineage specification in
adult human cardiac precursor cells via modulation of
enhancer-associated long noncoding RNA expression. JACC Basic
Transl Sci. 1:472–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu
Q, Deitch EA, Huo Y, Delphin ES and Zhang C: MicroRNA-145, a novel
smooth muscle cell phenotypic marker and modulator, controls
vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Wang X, Hu G and Zhou J: Repression of
versican expression by microRNA-143. J Biol Chem. 285:23241–23250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Dong K, Shen J, He X, Hu G, Wang L, Osman
I, Bunting KM, Dixon-Melvin R, Zheng Z, Xin H, et al: CARMN is an
evolutionarily conserved smooth muscle cell-specific LncRNA that
maintains contractile phenotype by binding myocardin. Circulation.
144:1856–1875. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Li M, Wang J, Fang Y, Gong S, Li M, Wu M,
Lai X, Zeng G, Wang Y, Yang K and Huang X: microRNA-146a promotes
mycobacterial survival in macrophages through suppressing nitric
oxide production. Sci Rep. 6:233512016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Sidorkiewicz M, Grek M, Jozwiak B, Krol A
and Piekarska A: The impact of chronic hepatitis C infection on
cholesterol metabolism in PBMCs is associated with microRNA-146a
expression. Eur J Clin Microbiol Infect Dis. 36:697–702. 2017.
View Article : Google Scholar
|
|
211
|
Takahashi Y, Satoh M, Minami Y, Tabuchi T,
Itoh T and Nakamura M: Expression of miR-146a/b is associated with
the Toll-like receptor 4 signal in coronary artery disease: Effect
of renin-angiotensin system blockade and statins on miRNA-146a/b
and Toll-like receptor 4 levels. Clin Sci. 119:395–405. 2010.
View Article : Google Scholar
|
|
212
|
Gangwar RS, Rajagopalan S, Natarajan R and
Deiuliis JA: Noncoding RNAs in cardiovascular disease: Pathological
relevance and emerging role as biomarkers and therapeutics. Am J
Hypertens. 31:150–165. 2018. View Article : Google Scholar :
|
|
213
|
Zhou L, Zhao X, Han Y, Lu Y, Shang Y, Liu
C, Li T, Jin Z, Fan D and Wu K: Regulation of UHRF1 by miR-146a/b
modulates gastric cancer invasion and metastasis. FASEB J.
27:4929–4939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Ramirez-Moya J, Wert-Lamas L and
Santisteban P: MicroRNA-146b promotes PI3K/AKT pathway
hyperactivation and thyroid cancer progression by targeting PTEN.
Oncogene. 37:3369–3383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Zhu Y, Wu G, Yan W, Zhan H and Sun P:
miR-146b-5p regulates cell growth, invasion, and metabolism by
targeting PDHB in colorectal cancer. Am J Cancer Res. 7:1136–1150.
2017.PubMed/NCBI
|
|
216
|
Jin H, Zhang H, Ma T, Lan H, Feng S, Zhu H
and Ji Y: Resveratrol protects murine chondrogenic ATDC5 cells
against LPS-induced inflammatory injury through Up-regulating
MiR-146b. Cell Physiol Biochem. 47:972–980. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Gao S, Zhao Z, Wu R, Wu L, Tian X and
Zhang Z: MiR-146b inhibits autophagy in prostate cancer by
targeting the PTEN/Akt/mTOR signaling pathway. Aging. 10:2113–2121.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Curtale G, Mirolo M, Renzi TA, Rossato M,
Bazzoni F and Locati M: Negative regulation of Toll-like receptor 4
signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA.
110:11499–11504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: The role of miR-126 in embryonic angiogenesis, adult vascular
homeostasis, and vascular repair and its alterations in
atherosclerotic disease. J Mol Cell Cardiol. 97:47–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Urbich C, Kaluza D, Frömel T, Knau A,
Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel JN,
Hergenreider E, et al: MicroRNA-27a/b controls endothelial cell
repulsion and angiogenesis by targeting semaphorin 6A. Blood.
119:1607–1616. 2012. View Article : Google Scholar
|
|
221
|
Su X, Hu Y, Li Y, Cao JL, Wang XQ, Ma X
and Xia HF: The polymorphism of rs6505162 in the MIR423 coding
region and recurrent pregnancy loss. Reproduction. 150:65–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Medford HM and Marsh SA: The role of
O-GlcNAc transferase in regulating the gene transcription of
developing and failing hearts. Future Cardiol. 10:801–812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Luo P, He T, Jiang R and Li G:
MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in
cardiomyocytes. Mol Med Rep. 12:1163–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Amin MMJ, Trevelyan CJ and Turner NA:
MicroRNA-214 in health and disease. Cells. 10:32742021. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Taskin O, Rikhraj K, Tan J, Sedlak T, Rowe
TC and Bedaiwy MA: Link between Endometriosis, Atherosclerotic
Cardiovascular Disease, and the Health of Women Midlife. J Minim
Invasive Gynecol. 26:781–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Missmer SA: Commentary:
Endometriosis-epidemiologic considerations for a potentially
'high-risk' population. Int J Epidemiol. 38:1154–1155. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
O'Sullivan JW, Raghavan S, Marquez-Luna C,
Luzum JA, Damrauer SM, Ashley EA, O'Donnell CJ, Willer CJ and
Natarajan P; American Heart Association Council on Genomic and
Precision Medicine; et al Polygenic risk scores for cardiovascular
disease: A scientific statement from the American Heart
Association. Circulation. 146. pp. e93–e118. 2022
|
|
228
|
Bulun SE, Monsavais D, Pavone ME, Dyson M,
Xue Q, Attar E, Tokunaga H and Su EJ: Role of estrogen receptor-β
in endometriosis. Semin Reprod Med. 30:39–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Stevenson JC: Long-term benefits and risks
of HRT (Section 11): Cardiovascular disease. Post Reprod Health.
22:80–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Newson L: Menopause and cardiovascular
disease. Post Reprod Health. 24:44–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J
and Taylor RN: Pathogenesis of endometriosis: Interaction between
Endocrine and inflammatory pathways. Best Pract Res Clin Obstet
Gynaecol. 50:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|