1. Introduction
Advanced glycation end-products (AGEs) are highly
heterogeneous group of chemical species formed through
non-enzymatic reactions of glucose or other carbohydrates with
proteins and other biomolecules (1). AGEs are formed due to condensation
between the carbonyl group of a reducing sugar and free amine group
of proteins, lipids or nucleic acids with the irreversible
formation of end-products (2).
Depending on the molecules involved in glycation, AGEs have been
classified into three groups as follows: i) Glycated proteins
[e.g., glycated hemoglobin (HbA1c), ApoB100, crystallin, etc.]; ii)
low molecular weight AGEs [pyrraline, carboxyethyl lysine (CEL),
carboxymethyl lysine (CML), pentosidine, imidazole]; iii) AGEs
formed by modification with a particular glycating agent [glucose
(AGE-1), glyceraldehyde (AGE-2), glycolaldehyde (AGE-3),
methylglyoxal (AGE-4), glyoxal (AGE-5), 3-deoxyglucosone (AGE-6)
and acetaldehyde (AA-AGE)] (3).
AGEs have also been classified as fluorescent (pentosidine,
methylglyoxal-lysine dimer) and non-fluorescent (CML, CEL and
pyrraline) (1).
AGEs are formed both endogenously and exogenously
through a number of mechanisms (Fig.
1). One of the mechanisms of AGE formation termed the Maillard
reaction involves a series of non-enzymatic reactions with the
formation of a Schiff base and its subsequent rearrangement into a
more stable Amadori product. AGEs are also formed through the
interaction of reactive carbonyl species, including glyoxal or
methylglyoxal with protein amino acid residues (4). At the same time, multiple other
mechanisms may also contribute to the formation of AGEs (5,6).
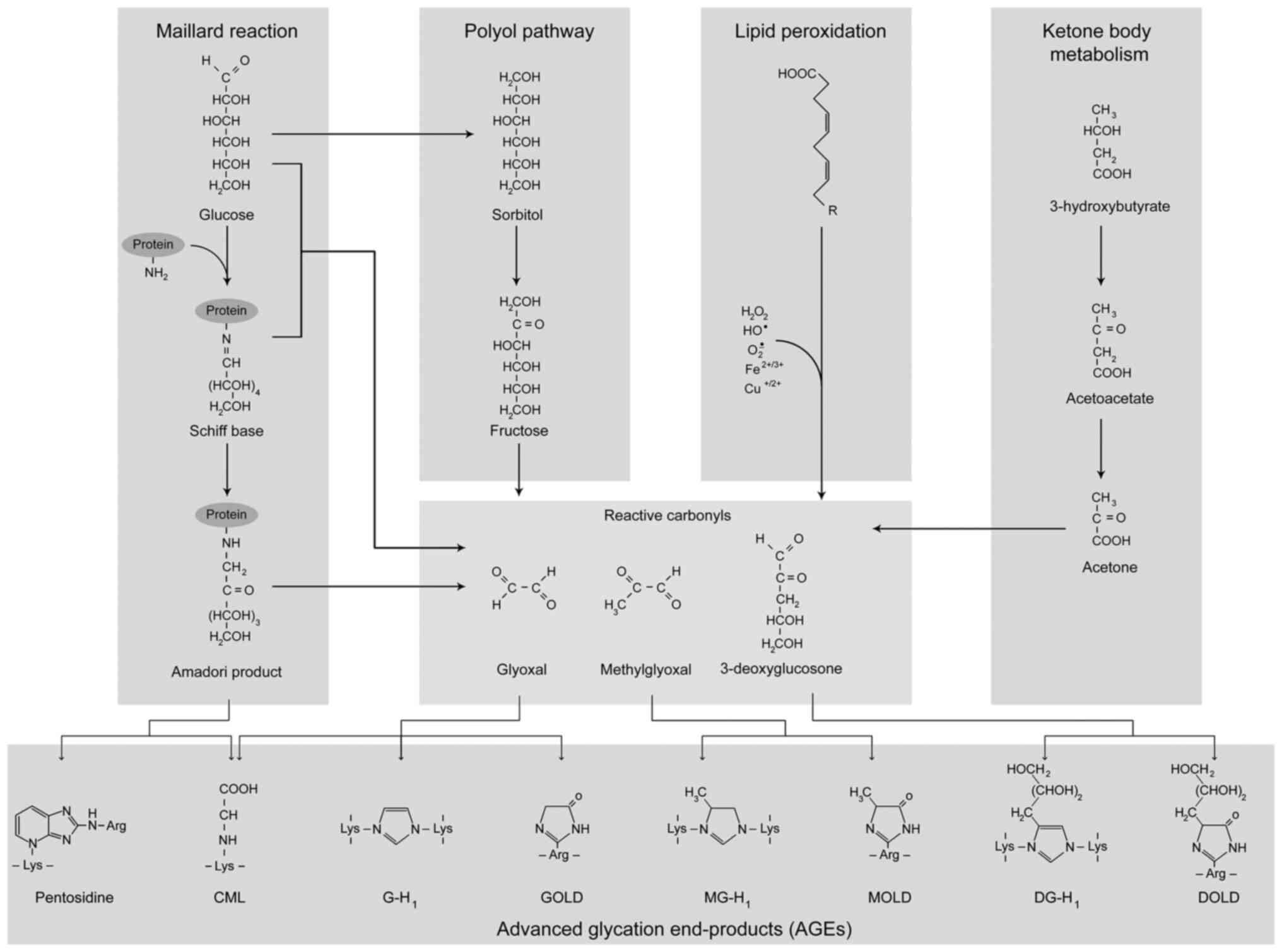 | Figure 1Common pathways of AGE formation
in vivo. The first step of the Maillard reaction includes a
reaction between the carbonyl group of a reducing sugar with a free
protein amino group with the formation of a Schiff base that is
transformed to a more stable Amadori product through a series of
rearrangements. Following a series of rearrangements, oxidation and
dehydration reactions, Amadori products are transformed into AGEs,
such as CML and pentosidine. Another mechanism of AGE formation
involves the generation of reactive carbonyls, glyoxal,
methylglyoxal and 3-deoxyglucosone that are considered AGE
precursors. Specifically, the interaction of glyoxal, methylglyoxal
and 3-deoxyglucosone with protein molecules yields CML, GOLD and
G-H1, MOLD and MG-H1, as well as DOLD and 3DG-H1, respectively. In
addition, other sources of reactive carbonyls may include polyol
pathway, lipid peroxidation and ketone body metabolism. AGE,
advanced glycation end-product; CML, carboxymethyllysine; GOLD,
glyoxal-lysine dimer; G-H1, glyoxal-derived hydroimidazolone; MOLD,
methylglyoxal-derived di-lysine imidazolium crosslink; MG-H1,
methylglyoxal-derived hydroimidazolone; DOLD, desoxyglucosone
lysine dimer; 3DG-H1, 3-deoxyglucosone-derived hydroimidazolone
1. |
Endogenously formed AGEs are generated at high
amounts in diabetes mellitus due to insulin resistance and
persistent hyperglycemia (7).
AGEs impart toxic effects to cells through the induction of
oxidative stress, endoplasmic reticulum stress, mitochondrial
dysfunction, apoptosis and inflammation dysregulation (8,9).
Excessive AGE formation along with its toxicity in diabetes
mellitus is considered a potential mechanism linking diabetes with
other metabolic disorders (10).
In addition to diabetes and metabolic syndrome (11), AGEs have been shown to be involved
in the pathogenesis of a variety of other diseases, including
neurodegeneration (12), cancer
(13), osteoporosis (14), infertility (15), chronic kidney disease (16) and aging (17,18). Correspondingly, the results of a
recent meta-analysis demonstrated a significant association between
circulating AGEs and their soluble receptor levels and both
all-cause and cardiovascular mortality (19).
Dietary AGE intake also significantly contributes to
AGE accumulation and toxicity (20). Western diets which are based on
highly processed and heat-treated foods are known to contain high
levels of AGEs (21). Given these
associations, AGEs are considered a potential link between the
modern diet and adverse health outcomes (22).
It has been proposed that the modulation of the gut
microbiota significantly contributes to the effect of AGEs on human
health (23), and mediates the
differences observed between the effects of low and high molecular
mass glycation products in the organism (24). However, the existing data are
inconsistent and the potential contribution of the gut microbiota
in the modulation of AGE-induced toxicity and glycation stress, as
well as the health effects of AGE accumulation are unclear.
Therefore, the aim of the present review was to summarize and
discuss the potential interactive effects between the gut
microbiota and AGE accumulation and toxicity in the host, as well
as to reveal the potential mediatory effects of the gut microbiota
on AGE-related health effects.
2. Bacterial AGE metabolism
The existing data demonstrate that the gut
microbiota may be considered as a source of AGEs. Specifically, it
has been demonstrated that Escherichia coli (E. coli)
cultures release AGEs during growth (25). Such an effect may be mediated by
the bacterial secretion of methylglyoxal (MGO), which is considered
as a reactive carbonyl species and an AGE precursor (4). High MGO-producing activity has been
demonstrated for Proteus spp. (26), E. coli (27), Pediococcus acidilactici and
other bacteria (28).
MGO is formed in bacterial cells as a product of
multiple metabolic processes (29), although its overaccumulation with
a subsequent increase in AGE formation has been shown to exert
toxic effects (30) and limit
bacterial growth (31).
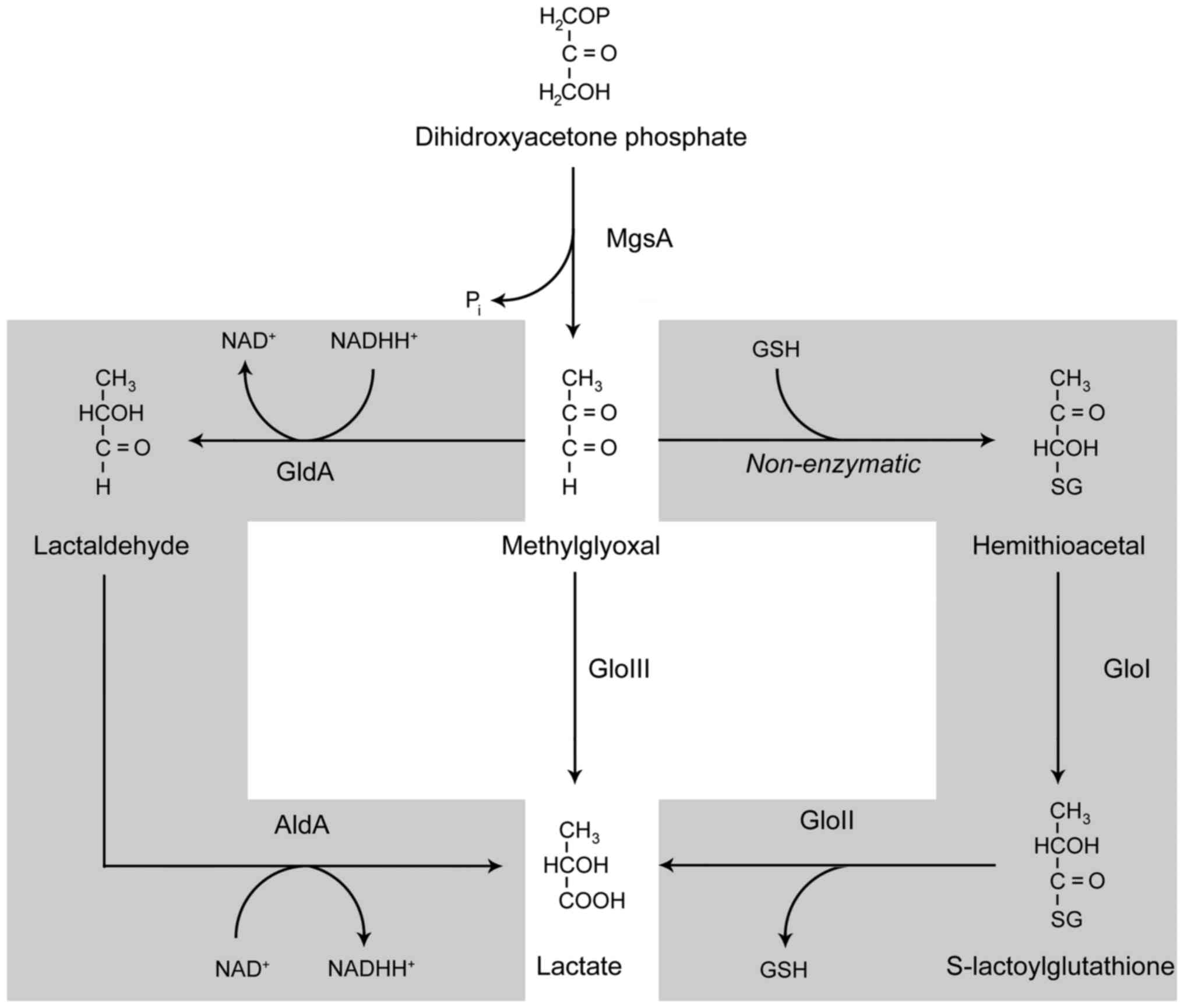
The main source of MGO is glucose catabolism,
including both enzymatic and non-enzymatic reactions. The key
mechanism of MGO synthesis is the transformation of
dihydroxyacetone phosphate catalyzed by methylglyoxal synthase
(MgsA). In turn, non-enzymatic MGO formation may result from the
fragmentation of triosephosphates via phosphoenediolate
intermediate (29).
The toxicity of MGO for bacterial cells is
associated with its high reactivity and modification of nucleic
acids and proteins, resulting in AGE formation (30). Specifically, it has been
demonstrated that MGO exposure is toxic to both Gram-positive
(Bacillus subtilis and Staphylococcus aureus) and
Gram-negative (Pseudomonas aeruginosa and E. coli)
bacteria, inhibiting their growth and inducing structural
alterations in bacterial fimbriae and flagella (31).
To overcome the toxic effects of increasing MGO
concentrations and subsequent cell death (32), MGO decomposition is strictly
regulated by a number of mechanisms involving glyoxalases and
NAD-dependent enzymes (Fig. 2).
Glyoxalases I and II catalyze the glutathione (GSH)-dependent
conversion of MGO to D-lactate through the formation of
S-D-lactoylglutathione, whereas glyoxalase III catalyzes this
conversion without consuming GSH. The NAD-dependent enzymes,
glycerol dehydrogenase (GldA) and aldehyde dehydrogenase (AldA),
catalyze the transformation of MGO to D-lactate through
D-lactaldehyde (30). The
resulting D-lactate may be subsequently transformed to pyruvate or
excreted (33).
In addition to MGO, which is considered a precursor
of AGEs, existing data demonstrate that gut bacteria can metabolize
other AGEs. Specifically, it has been demonstrated that the human
gut microbiota is able to degrade Maillard reaction products with a
substantial reduction at 24 h observed for fructosyllysine (100%)
> carboxymethyllysine (41%) > pyrraline (20%), but not
maltosine (34). A recent study
by Bui et al (35)
demonstrated that the gut microbiota is capable of anaerobic
carboxymethyllysine degradation to carboxymethylated biogenic
amines and 11 carboxylic acids with Oscillibacter and
Cloacibacillus evryensis being the potential responsible
taxa. It has been demonstrated that adult fecal microbiota and
particularly Intestinimonas spp. can convert
Nε-fructosyllysine to butyrate, whereas such a property in bacteria
isolated from 3-4-month-old infants was dependent on the type of
feeding. Specifically, the microbiota of breast-fed infants was
unable to degrade Nε-fructosyllysine, whereas that of formula-fed
infants possessed a Nε-fructosyllysine-converting ability due to
the presence of this AGE in infant formulas following thermal
exposure, thus being indicative of the adaptation of microbiota
metabolism to dietary compounds (36). In addition, E. coli has
been shown to metabolize CML with the formation of three
metabolites, N-carboxymethylcadaverine,
N-carboxymethylaminopentanoic acid and the N-carboxymet
hyl-Δ1-piperideinium ion, although the particular end-product may
be strain-specific (37).
It has also been demonstrated that bacterial
metabolites can modulate AGE toxicity. The existing data
demonstrate that gut microbiota-derived metabolite trimethylamine
N-oxide (TMAO) can increase the production of AGEs in the aorta,
thus promoting arterial stiffening (38), and underlying the role of TMAO and
AGEs in the progression of cardiovascular and chronic kidney
diseases (39).
3. The impact of dietary AGE exposure on gut
microbiota characteristics and host metabolism
Diet has a significant impact on the biodiversity
and metabolism of the gut microbiota (40). Correspondingly, the results of a
recent systematic review demonstrated that the characteristics of
dietary protein, including protein glycation can modulate gut
biodiversity, although significant inconsistencies still exist
(41).
Adverse effects of dietary AGEs on the
gut microbiota
Consistent with the overall understanding of AGEs as
perilous molecules, several studies have demonstrated that the
dietary intake of AGEs induces the dysfunction of the gut
microbiota along with unfavorable effects in the host organism.
Specifically, in a human study, it was demonstrated that the
increased exposure to glycated BSA significantly affected the
colonic microbiota sampled from the feces of both healthy subjects
and patients with ulcerative colitis, with a profound decrease in
beneficial bacteria (eubacteria and bifidobacteria) and an
increased abundance of more hazardous phyla (clostridia,
bacteroides, sulfate-reducing bacteria) (42). The detailed study by Seiquer et
al (43) revealed the
significant association between AGEs intake and gut microbiota
composition both in humans and rats. Specifically, the relative
abundance of lactobacteria was inversely associated with dietary
hydroxymethylfurfural and carboxymethyl-lysine, whereas the
relative numbers of bifidobacterial were inversely associated with
the intake of Amadori compounds in adolescents. Similarly, in rats,
the dietary intake of the Amadori compounds, hydroxymethylfurfural
and carboxymethyl-lysine, was negatively associated with both total
bacteria and lactobacteria (43).
In a laboratory study, it was demonstrated that
AGE-rich food significantly aggravated alterations in gut
microbiota biodiversity in a time-dependent manner, being more
profound at 18 weeks of exposure, as compared to 6 and 12 weeks.
Furthermore, it was shown that the high-AGE group ws characterized
by a significant decrease in the abundance of Ruminococcaceae,
Lachnospiraceae, Alloprevotella, Mollicutes,
Christensenellaceae, Treponema, Prevotellaceae,
Sphaerochaeta, Elusimicrobium, Butyrivibrio
and Anaeroplasma, whereas that of Oscillibacter,
Allobaculum, Anaerotruncus, Barnesiella,
Fusicatenibacter and Veillonellaceae was elevated (44). Feeding rats with a heat-treated
diet rich in AGEs resulted in a significant increase in the
relative abundance of Parabacteroides, Alloprevotella,
Helicobacter, Ruminococcaceae_UCG-014, and unclassified
Rhodospirillaceae, whereas populations of Desulfovibrio,
Rikenellaceae, Lachnospiraceae and Alistipes, were
significantly reduced, being associated with perturbations of
microbial metabolites and certain metabolic pathways, including
impaired carbohydrate and amino acid metabolism (45). Correspondingly, in another study,
the administration of an AGE-rich diet, obtained by heating the
standard chow, to C57BL/6 mice resulted in a significant increase
in circulating and tissue AGEs levels, systemic inflammation, and
the alteration of the gut microbiota mainly characterized by the
increased abundance of Clostridium_sensu_stricto_1,
Turicibacter and Parasutterella, and in particular,
Dubosiella at the genera level, as well as the increased
abundance of Clostridiaceae_1, Erysipelotrichaceae and
Burkholderiaceae families (46).
Dietary AGE-induced effects on gut microbiota have
also been linked to several diseases. Specifically, a previous
study demonstrated that feeding C57BL/6 mice with an AGE-rich diet
significantly increased systemic AGE (CML) levels, protein
glycosylation, receptor for AGEs (RAGE) expression in the ileum and
submandibular glands, as well as complex alterations in gut
microbiota composition. AGE intake significantly increased
Lawsonia, Parabacteroides and Ruminococcus abundance,
whereas the relative numbers of Lactobacillus, Prevotella,
Anaerostipes and Candidatus Arthromitus were
significantly reduced, altogether being associated with impaired
insulin signal transduction (47). It has been also demonstrated that
feeding rats with casein glycated with methylglyoxal
5-hydro-5-methylimidazolone significantly affects the intestinal
microbiota, which may at least in part be responsible for a
reduction in systemic gastric inhibitory polypeptide and
glucagon-like peptide-1 levels, consistent with an altered
incretin-insulin axis, as well as with the overproduction of the
pro-inflammatory cytokines, interleukin (IL)-1β and IL-17 and
plasminogen activator inhibitor-1 (48).
Potentially beneficial effects of dietary
AGEs on the gut microbiota
In contrast to the aforementioned findings, a number
of studies have demonstrated that administration of dietary AGEs
can afford beneficial effects, both to the gut microbiota and host
metabolism. It is notable that the majority of results
demonstrating the positive influence of AGEs on gut microbiota were
attributable to administration of glycated fish proteins.
Specifically, the glycation of dietary grass carp myofibrillar
proteins resulting in increased furosine levels in glycoconjugates
significantly increased gut microbiota biodiversity and butyrate
production that was positively associated with the relative
abundance of Mitsuokella, Lachnospiraceae_UCG-004,
Sutterella, Salinimicrobium, Fodinibius and
Nitriliruptor, being inversely associated with that of
Enterococcus, Dorea, Escherichia-Shigella and
Phascolarctobacterium, thus being indicative of the
potential beneficial effects of protein glycation on gut health
(49). Correspondingly, another
study demonstrated that the administration of glycated fish protein
to rats significantly increased the relative abundance of
Allobaculum, Akkermansia, Turicibacter and
Lactobacillus animalis, and reduced that of
Escherichia-Shigella, Fusobacterium and
Erysipelatoclostridium in the caecum, altogether being
associated with the increased production of butyrate from
fructoselysine (50). Similar
findings were obtained in another study, where the administration
of fish peptide glycated with galactooligosaccharide resulted in
increased abundance of the Veillonellaceae, Prevotellaceae and
Coriobacteriaceae families, and increased the abundance of the
genera, Anaerovibrio, Collinsella,
Prevotella_9, as well as reduced Alloprevotella,
Holdemanella, Escherichia-Schigella and
Streptococcus when compared to the control rats (51). Increasing fish protein glycation
through heating for 24 or 48 h with its subsequent in vitro
fermentation in a model of human distal colon significantly
increased the relative abundance of Lactococcus in parallel
with a decrease in Bacteroides. Moreover, the exposure of
gut bacteria to glycated fish protein heated for 48 h resulted in a
greater abundance of Holdemania, Streptococcus,
Enterococcus and Lactobacillus, as well as a
reduction of Parabacteroides when compared to a less
glycated protein (24 h of heating). These changes, and particularly
a decrease in Bacteroides, Dialister and
Parabacteroides, were associated with reduced ammonia and
indole production (52). The
intake of glycated fish protein in high-fat fed rats also decreased
relative abundance of Ruminiclostridium and
Desulfovibrio, as well as dose-dependent effects, including
increased Ruminococcus and Roseburia abundance in low
glycated protein diet and decreased Helicobacter and
Lachnospiraceae upon high-dose glycated protein intake. Moreover,
the observed AGE-induced modulation of gut microbiota composition
was associated with a significant reduction of systemic
proinflammatory cytokine (IL-1β and IL-6) levels and lipid profile
improvement, thus indicative of the potential beneficial effects on
gut and metabolism (53).
It has been also demonstrated that the glycation of
the milk proteins, β-lactoglobulin and casein, significantly
increased fermentability of the proteins by Lactobacillus
and Bifidobacterium thus promoting their growth, although
the effect was more profound at the initial steps of Maillard
reaction (54).
β-lactoglobulin-galactose conjugate was also shown to promote
Clostridium coccoides-Eubacterium rectal group
growth, as well as increase bacterial acetate production (55). Notably, the modification of
β-lactoglobulin by glycation and ultrasonication has been shown to
reduce milk protein allergenicity, which may be mediated by lower
digestibility, modification of allergenic epitopes on the protein
molecule, as well as modulation of gut microbiota composition
(56).
The administration of glycated whey proteins to aged
male non-obese diabetic mice with autoimmune prostatitis
significantly increased mice survival, reduced prostatic
inflammation, as well as an increased abundance of
Allobaculum, Anaerostipes, Bacteroides,
Parabacteroides and Prevotella and reduced abundance
Adlercreutzia and Roseburia, whereas the population
of Bacteroides acidifaciens significantly correlated with
the observed effects, indicative of the role of gut microbiota
modulation in protective effects of glycated whey proteins
(57). Similarly, a beneficial
effect on the gut microbiota was demonstrated for glycated pea
protein which increased Bacteroides,
Lactobacillus/Enterococcus and Bifidobacterium growth
as well as short chain fatty acids (SCFAs), acetate, propionate,
lactate, and butyrate production (58).
The dietary restriction of AGEs was shown to reduce
systemic CML and MG levels, as well as affect the gut microbiota by
increasing the relative abundance of Alistipes indistinctus,
Clostridium citroniae, Clostridium hathewayi, and
Ruminococcus gauvreauii, in parallel with a decrease in
Prevotella copri and Bifidobacterium animalis in
peritoneal dialysis patients (59). Concomitantly, in another study, a
reduction in dietary AGEs intake did not have a significant effect
on the most abundant gut bacteria in healthy obese subjects. As
compared to the group with a high AGE intake, the low dietary AGE
group was characterized by a greater abundance of
Tyzzerella, Family_XII_UCG-001 and Christensenellaceae_R-7
Group, as well as a lower abundance of Negativibacillus,
Oscillibacter and Anaerostipes (60).
An insight into the distinct effects of
various AGEs on the gut microbiota
As clearly detailed in previous studies, the effects
of dietary AGEs on the gut microbiota vary significantly, depending
on their characteristics, including both the dose, source and
chemical properties. The study by Cao et al (61) proposed that the observed
inconsistencies in the reported effects of AGE intake on the gut
microbiota and overall health were dependent on the dose of the
glycated protein in the diet. The intake of low levels of glycated
fish proteins for 15 weeks in mice resulted not only in an
increased abundance of the butyrate-producing bacteria,
Lachnospiraceae and Allobaculum, but also increased
intestinal tight junction protein expression (occludin and Zonula
occludens-1), reduced pro-inflammatory cytokine production (IL-1β
and IL-6) and improved insulin sensitivity. By contrast, inverse
effects were observed upon exposure to high-dose glycated fish
protein in parallel with a reduction in Bifidobacterium and
Lactobacillus abundance (61). It has been posited that free AGEs,
such as carboxymethyllysine have detrimental effects on the
composition and functions of the gut microbiota, whereas bound AGEs
have a more beneficial effect, although certain detrimental effects
may be also observed (62). It
was hypothesized that the positive effects of glycated proteins,
namely fish proteins on the gut microbiota are due to the use of
such proteins as a slow fermentable protein source or carbonyl
donors providing additional energy to gut microbiota (46). In addition, free and protein-bound
AGEs in the diet have distinct effects on the gut microbiota due to
differences in digestibility (63). In addition to the AGE species, the
molecular weight of the ligand has a significant impact on
digestibility. Specifically, in a previous study, in a model of
glyoxal-glycated casein digests, CML was degraded predominantly in
the low molecular weight fraction (38.7%) followed by medium
(21.7%) and high molecular weight fractions (9.6%) which may be
mediated by the lower activity of proteases (64).
4. Involvement of AGE-gut microbiota
interplay in disease pathogenesis
Type 2 diabetes mellitus
The understanding of the pathogenesis of diabetes
mellitus sheds light onto the toxicological effects of AGEs and
their role in metabolic diseases (10). Although the dysfunction of the gut
microbiota has been known to play a significant role in
diabetogenesis (65), the
potential interplay between gut microbiota dysfunction and
excessive protein glycation in diabetes has been studied only
recently. Wu et al (66)
demonstrated that dietary AGE exposure significantly affected the
gut microbiota, with an irreversible increase in
Bacteroidetes populations and a decrease in
Firmicutes abundance at the phylum level, whereas at the
genera level, a high-AGE diet stimulated Helicobacter,
Bacteroides, Rikenella, Alistipes,
Bifidobacterium, Candidatus Saccharimonas,
Faecalibaculum, Clostridiales, Erysipelatoclostridium
and Intestinimonas, and decreased unidentified
Lachnospiraceae, Roseburia, Oscillibacter,
Anaerotruncus, Blautia, Mucispirillum,
Angelakisella, Lachnoclostridium, Lachnospira,
Ruminiclostridium, Acetatifactor, and
Desulfovibrio. These perturbations were shown to contribute
to diabetes pathogenesis through the modulation of glyceraldehyde
and pyruvate production with the subsequent aggravation of insulin
resistance and other alterations in carbohydrate and lipid
metabolism, as well as inflammation due to higher systemic
lipopolysaccharide (LPS) levels (66). A comparative analysis demonstrated
that despite a significant increase in circulating AGEs and the
induction of insulin resistance in mice exposed to both an AGE-rich
diet or purified methylglyoxal-bovine serum protein (exogenous
AGE), profound alterations of intestinal permeability and
microbiota structure were observed only upon exogenous AGE intake.
A high AGE intake was shown to reduce the abundance of
Bacteroidales_S24-7, Bacteroidaceae, Porphyromonadaceae,
Odoribacteraceae, Lachnospiraceae, Rikenellaceae, and
Erysipelotrichaceae in parallel with an increase in
Desulfovibrionaceae abundance (67). It was proposed that a decrease in
butyrate production by butyrate-producing bacteria may promote the
impairment of the intestinal epithelial barrier and induce
inflammation, thus contributing to systemic insulin resistance
(67). The observed increase in
Desulfovibrio abundance generally corresponds to the early
observed positive association between these bacteria with blood
glucose indices (68) and
Parkinson's disease (69),
although the results of the Guangdong Gut Microbiome Project
demonstrated that the abundance of Desulfovibrio may be
inversely associated with body mass index and triglyceride levels
(70). Moreover, earlier findings
in diabetic db/db mice exposed to high levels of dietary AGEs
demonstrated in a significant increase in gut permeability, as well
as an elevation of the Firmicutes-to-Bacteroidetes
ratio, altogether being associated with kidney damage and
albuminuria, whereas the administration of resistant starch
ameliorated these effects (71).
It is also notable that the formation of Maillard reaction products
with subsequent protein aggregation in bacterial species shares
certain similarity to that observed in Parkinson's and Alzheimer's
disease (72). Therefore,
preliminary data demonstrate that AGE-induced alterations in the
gut microbiota can contribute to the aggravation of insulin
resistance through a number of mechanisms, including the impairment
of the intestinal epithelial barrier and subsequent increase in
circulating LPS levels.
Ageing-associated diseases
Recent studies have demonstrated that alterations in
the gut microbiota, as well as increased levels of AGEs are
associated with aging, contributing to the development of
age-related diseases (73,74).
Age-related changes in the gut microbiota characterized by a
reduced Firmicutes-to-Bacteroidetes ratio at the
phylum level, as well as by the increased abundance of
Turibacter, Alloprevotella, Parasutterella,
Bifidobacterium, Macellibacteroides, Alistipes
sensu stricto 1, Peptostreptococcaceae incertae sedis and
Parabacteroides, and the lower abundance of Pantoea,
Anoxybacillus, Lachnospiraceae incertae sedis,
Cutrobacterium and Acetatifactor at the genera level,
were shown to contribute to the accumulation of
N6-carboxymethyllysine in microglia and subsequent
oxidative stress and mitochondrial dysfunction by increasing
intestinal permeability, whereas germ-free mice brain microglia
were characterized by lower oxidative stress and mitochondrial
damage (75). In corroboration,
the translocation of fecal microbiota from aged to young rats
impaired cognition, induced synaptic dysfunction, along with
oxidative stress and inflammation, which may be at least partially
mediated by an increased AGE production and RAGE expression
(76). Correspondingly, the
antibiotic treatment of 5xFAD mice, a model of Alzheimer's disease
that is known to be age-related, resulted in a significant decrease
in intestinal Lactobacillaceae abundance, being also associated
with reduced hippocampal plaque formation, antidiabetic effect and
decreased RAGE expression (77).
Taken together, even these limited data demonstrate that
age-related alterations of the gut microbiota may contribute to AGE
accumulation, particularly in brain tissues, indicating the gut
microbiota-AGE interplay in age-related neurodegeneration.
Other diseases
A recent study in ethanol-fed mice demonstrated an
increased abundance of Bacteroidetes and a decrease in
Firmicutes numbers, which was associated with an elevation
in AGE and RAGE levels in colonic tissues, and considered a
potential mechanism of ethanol-related colorectal cancer
pathogenesis (78).
5. Effects of gut microbiota modulation on
AGE metabolism and toxicity
Probiotics
Several studies have demonstrated that the
modulation of the gut microbiota using probiotics is also
associated with reduced AGE accumulation and toxicity, thus also
supporting the role of the gut microbiota in AGE toxicity.
Specifically, in a previous study, in a model of Alzheimer's
disease, the modulation of the gut microbiota through the
administration of SLAB51 probiotic significantly reduced brain AGE
accumulation and tau phosphorylation, and also improved insulin
signaling through the Akt/AMPK pathway (79). In another laboratory in
vivo study, the administration of probiotic Lactobacillus
paraplantarum BGCG11 significantly reduced AGE accumulation in
parallel with inhibiting hyperglycemia, oxidative stress, DNA
damage, liver and kidney fibrosis in rats with
streptozotocin-induced diabetes (80). In another study, the
administration of the commercial probiotic, Protexin®,
in Cd-exposed rats significantly reduced the serum MGO levels, as
well as decreased tissue Cd accumulation and Cd-induced oxidative
stress (81). Lactococcus
lactis KF140 supplementation has also been shown to reduce
serum CML levels and hepatic CML accumulation that may be at least
partially mediated by activity of bacteria-derived β-galactosidase
(82).
In addition to probiotics, it has been demonstrated
that treatment with prebiotics may also modulate AGE accumulation
and toxicity. Specifically, the administration of the prebiotic,
resistant dextrin, has been shown to significantly reduce
carboxymethyl lysine, soluble RAGE, as well as several other
cardiometabolic risk factors in adult women (83).
At the same time, additional studies, including
clinical trials are required to address the impact of microbiota
modulation by probiotics and prebiotics on AGE toxicity and RAGE
signaling, as well as the clinical validity of these
interventions.
Phytochemicals
Polyphenols have also been shown to have a
significant beneficial effect on gut microbiota and glycative
stress, and these effects appear partially interrelated (84). Specifically, the administration of
Physalis alkekengi L. fruit polysaccharide to AGE-fed mice
was shown to modulate gut microbiota by increasing the abundance of
Rikenallaceae, Alistipes, Nocardiaceae, Rhodococus,
Bacilli, Lactobacillaceae, Bacteroidaceae and
Burkholderiaceae, improving the
Bacteroidetes/Firmicutes ratio, as well as decreasing
LPS production. Treatment with Physalis alkekengi
polysaccharide also improved the bacterial production of SCFAs,
namely acetic and propionic acids, which may at least partially
mediate the treatment-induced reduction of insulin resistance
(85). It has also been
demonstrated that the administration of quercetin to AGE-fed mice
significantly ameliorated cognitive dysfunction through the
reduction of tau phosphorylation, cathepsin B and
neuroinflammation, as well as increased gut microbiota biodiversity
and reduced the abundance of Verrucomicrobia phylum, and
Blautia and Anaerotruncus genera (86). In addition, it has been proposed
that an increase in Lactobacteria and particularly
Bifidobacteria by Geranium dielsianum extract may at
least partially mediate antiglycative effect of the extract
(87). These data demonstrate
that the protective effects of phytochemicals against AGE toxicity
are mediated by its influence on the composition of the gut
microbiota.
6. Role of lipopolysaccharide in the
interplay between microbiota and AGE toxicity
LPS, also known as endotoxin, is a cell wall
component of Gram-negative bacteria. In the human gut microbiota,
Bacteroidetes, and to a lesser extent, Proteobacteria
phyla, are considered as key sources of LPS production (88). LPS mediates a substantial part of
the effects of altered microbiota on the host, including the
regulation of systemic inflammation (89).
Several studies have demonstrated the significant
effect of LPS on cellular production and the accumulation of AGEs
or their precursors. Specifically, a previous study demonstrated
that the stimulation of RAW264.7 murine macrophages with LPS
resulted in a significant increase in intracellular methylglyoxal
generation upon high-glucose conditions in parallel with HIF-1
downregulation and pyroptosis (90). A similar effect was observed in
LPS-stimulated J774A.1 macrophages and N11 microglia (91). Correspondingly, long-term LPS
treatment was shown to increase aortal AGE accumulation (92). In turn, in rat aortic smooth
muscle cells, MGO treatment was shown to inhibit LPS-stimulated
inducible nitric oxide synthase expression by inhibiting Akt
phosphorylation that may be involved in diabetic vascular
dysfunction (93). These findings
generally corroborate recent data obtained by Kitaura et al
(94), demonstrating that AGEs
may reduce LPS uptake by RAW264.7 macrophages, which may be at
least partially mediated by RAGE activation, resulting in altered
immune response in diabetes (94).
LPS is a potent pro-inflammatory agent that induces
an inflammatory response through a number of mechanisms. It has
been shown that AGEs potentiate the pro-inflammatory effects of LPS
on gingival fibroblasts under high-glucose conditions, as evidenced
by an elevated IL-8 secretion (95). The potentiation of the
pro-inflammatory effects of LPS and AGEs may be mediated by the
activation of mitogen-activated protein kinases and NF-κB
activation in endothelial cells (96).
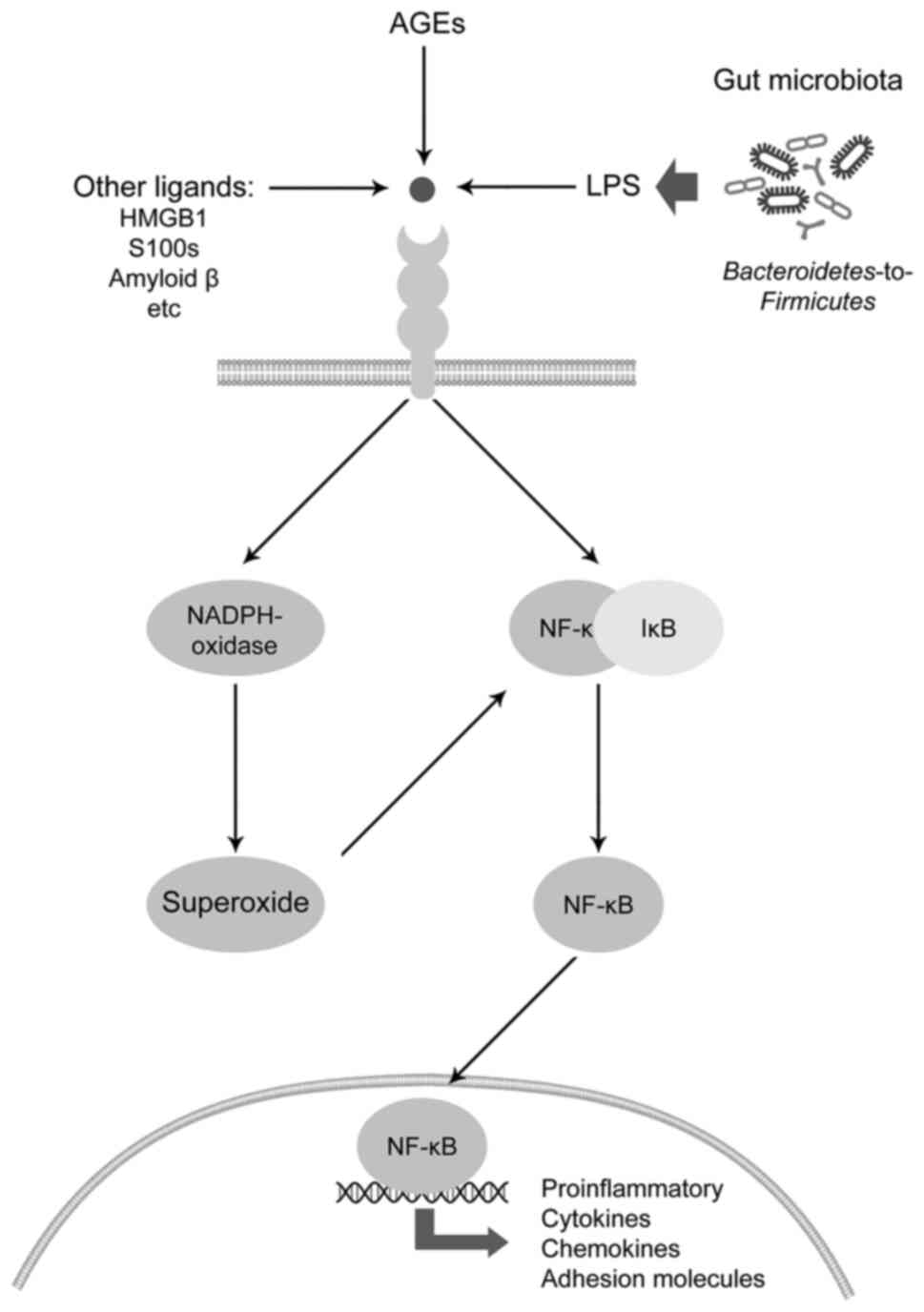
One of the mechanisms underlying the
pro-inflammatory effects of LPS and its impact on NF-κB signaling
is the modulation of RAGE signaling (97) (Fig.
3). The activation of RAGE signaling has been shown to mediate
certain effects of glucotoxicity, as well as the toxic effects of
environmental factors (98,99).
Given the role of AGEs as ligands for RAGE, the
modulation of RAGE signal transduction by LPS may also be
considered as one of the aspects of microbiota-AGE interplay.
Specifically, in human umbilical vein endothelial cells, LPS
treatment has been shown to increase both RAGE expression and NF-κB
activation, as well as p65 nuclear translocation, whereas anti-RAGE
antibody ameliorated the LPS-induced NF-κB activation and
subsequent endothelial barrier dysfunction, thus being indicative
of the role of RAGE in the LPS-induced inflammatory reaction
(100). Anti-RAGE antibody has
also been shown to reduce LPS-induced acute lung injury in a
neonatal rat model (101).
Concomitantly, tje inhibition of NF-κB signaling significantly
decreases LPS-induced RAGE expression in alveolar type I epithelial
cells, whereas RAGE knockdown inhibits both basal and LPS-induced
NF-κB activation (102).
A previous study demonstrated that LPS may directly
bind RAGE with a subsequent NF-κB-dependent inflammatory response
in a murine model of septic shock, whereas the injection of soluble
RAGE significantly reduced LPS-induced proinflammatory cytokine
expression and tissue damage (103). It has been proposed that the
ratio between cell surface RAGE and soluble RAGE (sRAGE) may
significantly mediate inflammatory response to bacterial molecules
(103). Additional research has
demonstrated that the direct interaction between LPS, high mobility
group box 1 (HMGB1) and AGEs results in the formation of a triplet
complex and subsequent increase in HMGB1 mobility, altogether
leading to increased TNF-α mRNA expression in RAW264.7 macrophages
through Toll-like receptor 4 (TLR4) and RAGE activation (104).
Another study demonstrated that the inhibition of
RAGE signaling thwarted the LPS-induced upregulation of HMGB1 and
IL-6 expression through a NF-κB-mediated mechanism (105). Accordingly, the inhibition of
RAGE activation has been considered as one of the mechanisms
underlying the protective effects of certain agents including
β-caryophyllene and perindopril against LPS-induced liver injury
(106) and amyloidogenesis
(107), respectively. In
agreement with this, the phytochemicals, icariin and icaritin, have
been found to significantly reduce LPS-induced hippocampal
neuroinflammation through the downregulation of HMGB1-RAGE
signaling (108). Concomitantly,
preconditioning with HMGB1 has been shown to induce LPS tolerance
in a RAGE-dependent manner (109).
In addition to the role of RAGE signaling in
LPS-induced inflammation, this mechanism may also trigger the
adverse effects of LPS on the cytoskeleton and tight junction
proteins. Specifically, RAGE signaling has been shown to be
involved in LPS-induced cytoskeletal alterations in mouse pulmonary
microvascular endothelial cells, as demonstrated in RAGE-knockout
pulmonary microvascular endothelial cells, which did not develop
F-actin rearrangement and stress fiber formation upon LPS
stimulation (110). In another
study, RAGE-deficient mice were also found to be resistant to
LPS-induced leukocyte infiltration and proinflammatory cytokine
secretion, as well as alteration of lung Zonula occludens-1,
sodium-potassium ATPase (Na, K-ATPase), and epithelial sodium
channel expression. Moreover, in patients with infection-induced
acute respiratory distress syndrome, bronchial alveolar lavage
fluid sRAGE levels were increased, being associated with
pro-inflammatory cytokine levels and pulmonary vascular
permeability (111). In line
with these observations, it was previously demonstrated that the
inhibition of RAGE signaling not only reduced pro-inflammatory
cytokine expression in a model of LPS-induced acute lung injury,
but also prevented the downregulation of claudin-2 and occludin
expression (112). While
discussing the role of AGE signaling and LPS in the alteration of
cell contacts, it is important to note that glycated caseinate
hydrolysate has been shown to possess significantly lower
barrier-protective effects in LPS-exposed intestinal IEC-6 cells as
compared to unmodified caseinate hydrolysate (113).
The activation of LPS-RAGE signaling has also been
shown to mediate carcinogenesis. Specifically, the upregulation of
HMGB1/RAGE signaling has been found to be responsible for
LPS-induced inflammation and the subsequent malignant
transformation of normal cervical epithelial cells (114). Moreover, a previous study
demonstrated that the breast tumor microbiota was enriched with
Gram-negative bacteria producing LPS. In vitro LPS treatment
was shown to upregulate S100A7 expression in breast cancer cells,
resulting in the upregulation of RAGE expression along with a
reduced TLR4 expression that may contribute to tumor growth
progression (115).
At the same time, it has been proposed that RAGE
signaling may mediate the inflammatory response to bacteria through
reactions to other bacterial molecules than LPS (116).
Taken together, these findings demonstrate that
AGEs can modulate the pro-inflammatory effects of bacterial LPS,
that is normally released by gut microbiota, whereas the
pro-inflammatory signals of LPS are mediated by RAGE activation,
which is also activated by AGEs.
7. Conclusions and future perspectives
The existing data demonstrate a bilateral
association between gut microbiota and the effects of AGEs. Such an
association may be summarized by the following aspects: i) Dietary
AGEs may have a significant impact on the richness and diversity of
the gut microbiota; ii) the gut microbiota may metabolize dietary
AGEs; iii) the composition of the gut microbiota is tightly
associated with AGE accumulation in the host organism; iv) certain
effects of AGE accumulation in the organism may be mediated by the
modulation of the gut microbiota; v) the alteration of the gut
microbiota may mediate the development of comorbidities associated
with ageing and diabetes; vi) LPS may be considered as the molecule
mediating the association between the gut microbiota and AGEs, and
particularly, RAGE signaling; vii) dietary interventions aimed at
the improvement of the gut microbiota may exert protective effects
against AGEs toxicity. Given a mutual interaction between AGE
toxicity and dysbiosis, it can be hypothesized that exposure to
dietary AGEs may induce gut dysbiosis that further promotes AGE
production, thus composing a vicious circle involved in disease
pathogenesis. This vicious circle may be involved in the
development of opportunistic infections and systemic inflammation
in diabetic patients characterized by high levels of endogenous
AGE. Therefore, the modulation of the gut microbiota with
probiotics or other nutrients may be considered as a potential
protective strategy against AGE-induced glycative stress and
systemic inflammation. However, further studies on the molecular
aspects of the interaction between gut microbiota and AGE
metabolism and toxicity are required.
Availability of data and materials
Not applicable.
Authors' contributions
MA and AAT were involved in the conceptualization
of the study. MA, AVS, VAG, OLK, AS, JBTR, IPZ, AT and AAT were
involved in the investigation of the literature and in the curation
of data for inclusion in the review. VAG, OLK, IPZ and AAT were
involved in the writing and preparation of the original draft. MA,
AVS, AS, JBTR, DAS and AT were involved in the writing, reviewing
and editing of the manuscript. AAT was involved in visualization.
MA, AVS, AS, JBTR, DAS and AT supervised the study. AAT was
involved in funding acquisition. All authors have read and agreed
to the published version of the manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was performed with the financial support of
the Russian Science Foundation (project No. 20-16-00078).
References
|
1
|
Perrone A, Giovino A, Benny J and
Martinelli F: Advanced glycation end products (AGEs): Biochemistry,
signaling, analytical methods, and epigenetic effects. Oxid Med
Cell Longev. 2020:38181962020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Twarda-Clapa A, Olczak A, Białkowska AM
and Koziołkiewicz M: Advanced glycation end-products (AGEs):
Formation, chemistry, classification, receptors, and diseases
related to AGEs. Cells. 11:13122022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuzan A: Toxicity of advanced glycation
end products (Review). Biomed Rep. 14:462021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aaseth J, Skalny AV, Roos PM, Alexander J,
Aschner M and Tinkov AA: Copper, iron, selenium and lipo-glycemic
dysmetabolism in Alzheimer's disease. Int J Mol Sci. 22:94612021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luevano-Contreras C, Garay-Sevilla ME and
Chapman-Novakofski K: Role of dietary advanced glycation end
products in diabetes mellitus. J Evid Based Complementary Altern
Med. 18:50–66. 2013. View Article : Google Scholar
|
|
6
|
Song Q, Liu J, Dong L, Wang X and Zhang X:
Novel advances in inhibiting advanced glycation end product
formation using natural compounds. Biomed Pharmacother.
140:1117502021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vlassara H and Uribarri J: Advanced
glycation end products (AGE) and diabetes: Cause, effect, or both?
Curr Diab Rep. 14:4532014. View Article : Google Scholar :
|
|
8
|
Uribarri J, del Castillo MD, de la Maza
MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH,
Markowicz Bastos DH, Medrano A, Menini T, et al: Dietary advanced
glycation end products and their role in health and disease. Adv
Nutr. 6:461–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sruthi CR and Raghu KG: Advanced glycation
end products and their adverse effects: The role of autophagy. J
Biochem Mol Toxicol. 35:e227102021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruiz HH, Ramasamy R and Schmidt AM:
Advanced glycation end products: Building on the concept of the
'common soil' in metabolic disease. Endocrinology. 161:bqz0062020.
View Article : Google Scholar
|
|
11
|
Margina D, Gradinaru D, Manda G, Neagoe I
and Ilie M: Membranar effects exerted in vitro by
polyphenols-quercetin, epigallocatechin gallate and curcumin-on
HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem
Toxicol. 61:86–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chrysanthou M, Miro Estruch I, Rietjens
IMCM, Wichers HJ and Hoppenbrouwers T: In vitro methodologies to
study the role of advanced glycation end products (AGEs) in
neurodegeneration. Nutrients. 14:3632022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan H, Khan MS and Ahmad S: The in vivo
and in vitro approaches for establishing a link between advanced
glycation end products and lung cancer. J Cell Biochem.
119:9099–9109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge W, Jie J, Yao J, Li W, Cheng Y and Lu
W: Advanced glycation end products promote osteoporosis by inducing
ferroptosis in osteoblasts. Mol Med Rep. 25:1402022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu JL, Cai YQ, Long SL, Chen Z and Mo ZC:
The role of advanced glycation end products in human infertility.
Life Sci. 255:1178302020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bettiga A, Fiorio F, Di Marco F, Trevisani
F, Romani A, Porrini E, Salonia A, Montorsi F and Vago R: The
modern western diet rich in advanced glycation end-products (AGEs):
An overview of its impact on obesity and early progression of renal
pathology. Nutrients. 11:17482019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moskalev A, Stambler I and Caruso C:
Innate and adaptive immunity in aging and longevity: The foundation
of resilience. Aging Dis. 11:1363–1373. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borsa C, Gradinaru D, Margina D, Prada G
and Peña C: Receptor of advanced glycation end products and
cardiovascular risk in elderly with type 2 diabetes mellitus. J
Biol Res. 90:81–86. 2017.
|
|
19
|
Sharifi-Zahabi E, Sharafabad FH,
Abdollahzad H, Malekahmadi M and Rad NB: Circulating advanced
glycation end products and their soluble receptors in relation to
all-cause and cardiovascular mortality: A systematic review and
meta-analysis of prospective observational studies. Adv Nutr.
12:2157–2171. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nowotny K, Schröter D, Schreiner M and
Grune T: Dietary advanced glycation end products and their
relevance for human health. Ageing Res Rev. 47:55–66. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inan-Eroglu E, Ayaz A and Buyuktuncer Z:
Formation of advanced glycation endproducts in foods during cooking
process and underlying mechanisms: A comprehensive review of
experimental studies. Nutr Res Rev. 33:77–89. 2020. View Article : Google Scholar
|
|
22
|
Gill V, Kumar V, Singh K, Kumar A and Kim
JJ: Advanced glycation end products (AGEs) may be a striking link
between modern diet and health. Biomolecules. 9:8882019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JA, Wu CH and Yen GC: Perspective of
advanced glycation end products on human health. J Agric Food Chem.
66:2065–2070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Dongen KCW, Kappetein L, Miro Estruch
I, Belzer C, Beekmann K and Rietjens IMCM: Differences in kinetics
and dynamics of endogenous versus exogenous advanced glycation end
products (AGEs) and their precursors. Food Chem Toxicol.
164:1129872022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srebreva LN, Stoynev GA and Ivanov IG:
Evidence for excretion of glycation agents from E. coli cells
during growth. Biotechnol Biotechnol Equip. 23:1068–1071. 2009.
View Article : Google Scholar
|
|
26
|
Baskaran S, Rajan DP and Balasubramanian
KA: Formation of methylglyoxal by bacteria isolated from human
faeces. J Med Microbiol. 28:211–215. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao C, Dong H, Zhang Y and Li Y:
Discovery of potential genes contributing to the biosynthesis of
short-chain fatty acids and lactate in gut microbiota from
systematic investigation in E. coli. NPJ Biofilms Microbiomes.
5:192019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Popkov VA, Zharikova AA, Demchenko EA,
Andrianova NV, Zorov DB and Plotnikov EY: Gut microbiota as a
source of uremic toxins. Int J Mol Sci. 23:4832022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chakraborty S, Karmakar K and Chakravortty
D: Cells producing their own nemesis: Understanding methylglyoxal
metabolism. IUBMB Life. 66:667–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee C and Park C: Bacterial responses to
glyoxal and methylglyoxal: reactive electrophilic species. Int J
Mol Sci. 18:1692017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabie E, Serem JC, Oberholzer HM, Gaspar
AR and Bester MJ: How methylglyoxal kills bacteria: An
ultrastructural study. Ultrastruct Pathol. 40:107–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Booth IR, Ferguson GP, Miller S, Li C,
Gunasekera B and Kinghorn S: Bacterial production of methylglyoxal:
A survival strategy or death by misadventure? Biochem Soc Trans.
31:1406–1408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferguson GP, Tötemeyer S, MacLean MJ and
Booth IR: Methylglyoxal production in bacteria: Suicide or
survival? Arch Microbiol. 170:209–218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hellwig M, Bunzel D, Huch M, Franz CMAP,
Kulling SE and Henle T: Stability of individual maillard reaction
products in the presence of the human colonic microbiota. J Agric
Food Chem. 63:6723–6730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bui TPN, Troise AD, Fogliano V and de Vos
WM: Anaerobic degradation of N-ε-carboxymethyllysine, a major
glycation end-product, by human intestinal bacteria. J Agric Food
Chem. 67:6594–6602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bui TPN, Troise AD, Nijsse B, Roviello GN,
Fogliano V and de Vos WM: Intestinimonas-like bacteria are
important butyrate producers that utilize Nε-fructosyllysine and
lysine in formula-fed infants and adults. J Funct Foods.
70:1039742020. View Article : Google Scholar
|
|
37
|
Hellwig M, Auerbach C, Müller N, Samuel P,
Kammann S, Beer F, Gunzer F and Henle T: Metabolization of the
advanced glycation end product N-ε-carboxymethyllysine (CML) by
different probiotic E. coli Strains. J Agric Food Chem.
67:1963–1972. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brunt VE, Casso AG, Gioscia-Ryan RA,
Sapinsley ZJ, Ziemba BP, Clayton ZS, Bazzoni AE, VanDongen NS,
Richey JJ, Hutton DA, et al: Gut microbiome-derived metabolite
trimethylamine N-oxide induces aortic stiffening and increases
systolic blood pressure with aging in mice and humans.
Hypertension. 78:499–511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taguchi K, Fukami K, Elias BC and Brooks
CR: Dysbiosis-related advanced glycation endproducts and
trimethylamine N-oxide in chronic kidney disease. Toxins (Basel).
13:3612021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scott KP, Gratz SW, Sheridan PO, Flint HJ
and Duncan SH: The influence of diet on the gut microbiota.
Pharmacol Res. 69:52–60. 2013. View Article : Google Scholar
|
|
41
|
Wu S, Bhat ZF, Gounder RS, Mohamed Ahmed
IA, Al-Juhaimi FY, Ding Y and Bekhit AEA: Effect of dietary protein
and processing on gut microbiota-A systematic review. Nutrients.
14:4532022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mills DJS, Tuohy KM, Booth J, Buck M,
Crabbe MJC, Gibson GR and Ames JM: Dietary glycated protein
modulates the colonic microbiota towards a more detrimental
composition in ulcerative colitis patients and non-ulcerative
colitis subjects. J Appl Microbiol. 105:706–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seiquer I, Rubio LA, Peinado MJ,
Delgado-Andrade C and Navarro MP: Maillard reaction products
modulate gut microbiota composition in adolescents. Mol Nutr Food
Res. 58:1552–1560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qu W, Yuan X, Zhao J, Zhang Y, Hu J, Wang
J and Li J: Dietary advanced glycation end products modify gut
microbial composition and partially increase colon permeability in
rats. Mol Nutr Food Res. 61:2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qu W, Nie C, Zhao J, Ou X, Zhang Y, Yang
S, Bai X, Wang Y, Wang J and Li J: Microbiome-metabolomics analysis
of the impacts of long-term dietary advanced-glycation-end-product
consumption on C57BL/6 mouse fecal microbiota and metabo-lites. J
Agric Food Chem. 66:8864–8875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Dongen KCW, Linkens AMA, Wetzels SMW,
Wouters K, Vanmierlo T, van de Waarenburg MPH, Scheijen JLJM, de
Vos WM, Belzer C and Schalkwijk CG: Dietary advanced glycation
endproducts (AGEs) increase their concentration in plasma and
tissues, result in inflammation and modulate gut microbial
composition in mice; evidence for reversibility. Food Res Int.
147:1105472021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mastrocola R, Collotta D, Gaudioso G, Le
Berre M, Cento AS, Ferreira Alves G, Chiazza F, Verta R, Bertocchi
I, Manig F, et al: Effects of exogenous dietary advanced glycation
end products on the cross-talk mechanisms linking microbiota to
metabolic inflammation. Nutrients. 12:24972020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gaudioso G, Collotta D, Chiazza F,
Mastrocola R, Cento A, Fava F, Aragno M, Collino M and Tuohy K:
Advanced glycation end products (AGEs) in metabolic disease:
Linking diet, inflammation and microbiota. Proc Nutr Soc.
79:E3682020. View Article : Google Scholar
|
|
49
|
Han K, Yao Y, Dong S, Jin S, Xiao H, Wu H
and Zeng M: Chemical characterization of the glycated myofibrillar
proteins from grass carp (Ctenopharyngodon idella) and their
impacts on the human gut microbiota in vitro fermentation. Food
Funct. 8:1184–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han K, Jin W, Mao Z, Dong S, Zhang Q, Yang
Y, Chen B, Wu H and Zeng M: Microbiome and butyrate production are
altered in the gut of rats fed a glycated fish protein diet. J
Funct Foods. 47:423–433. 2018. View Article : Google Scholar
|
|
51
|
Jin W, Han K, Dong S, Yang Y, Mao Z, Su M
and Zeng M: Modifications in gut microbiota and fermentation
metabolites in the hindgut of rats after the consumption of
galactooligosaccharide glycated with a fish peptide. Food Funct.
9:2853–2864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Wu H, Dong S, Jin W, Han K, Ren Y
and Zeng M: Glycation of fish protein impacts its fermentation
metabolites and gut microbiota during in vitro human colonic
fermentation. Food Res Int. 113:189–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mao Z, Ren Y, Zhang Q, Dong S, Han K, Feng
G, Wu H and Zhao Y: Glycated fish protein supplementation modulated
gut microbiota composition and reduced inflammation but increased
accumulation of advanced glycation end products in high-fat diet
fed rats. Food Funct. 10:3439–3451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Corzo-Martínez M, Ávila M, Moreno FJ,
Requena T and Villamiel M: Effect of milk protein glycation and
gastrointestinal digestion on the growth of bifidobacteria and
lactic acid bacteria. Int J Food Microbiol. 153:420–427. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Corzo-Martínez M, Hernandez-Hernandez O,
Villamiel M, Rastall RA and Moreno FJ: In vitro bifidogenic effect
of Maillard-type milk protein-galactose conjugates on the human
intestinal microbiota. Int Dairy J. 31:127–131. 2013. View Article : Google Scholar
|
|
56
|
Shao YH, Zhang Y, Zhang L, Liu J and Tu
ZC: Mechanism of reduction in allergenicity and altered human
intestinal microbiota of digested β-lactoglobulin modified by
ultrasonic pretreatment combined with glycation. J Agric Food Chem.
69:14004–14012. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen Y, Guo KM, Nagy T and Guo TL: Chronic
oral exposure to glycated whey proteins increases survival of aged
male NOD mice with autoimmune prostatitis by regulating the gut
microbiome and anti-inflammatory responses. Food Funct. 11:153–162.
2020. View Article : Google Scholar :
|
|
58
|
Świątecka D, Narbad A, Ridgway KP and
Kostyra H: The study on the impact of glycated pea proteins on
human intestinal bacteria. Int J Food Microbiol. 145:267–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yacoub R, Nugent M, Cai W, Nadkarni GN,
Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil
SA, et al: Advanced glycation end products dietary restriction
effects on bacterial gut microbiota in peritoneal dialysis
patients; a randomized open label controlled trial. PLoS One.
12:e01847892017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Linkens AMA, van Best N, Niessen PM,
Wijckmans NEG, de Goei EEC, Scheijen JLJM, van Dongen MCJM, van
Gool CCJAW, de Vos WM, Houben AJHM, et al: A 4-week diet low or
high in advanced glycation endproducts has limited impact on gut
microbial composition in abdominally obese individuals: The
deAGEing trial. Int J Mol Sci. 23:53282022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cao C, Tang M, Zhao N, Dong S and Wu H:
Effects of fish protein with glycation extent on gut microbiota and
colonic barrier function in mice fed a high-fat diet. J Funct
Foods. 85:1046362021. View Article : Google Scholar
|
|
62
|
Yuan X, Nie C, Liu H, Ma Q, Peng B, Zhang
M, Chen Z and Li J: Comparison of metabolic fate, target organs,
and microbiota interactions of free and bound dietary advanced
glycation end products. Crit Rev Food Sci Nutr. Oct 26–2021.Epub
ahead of print. View Article : Google Scholar
|
|
63
|
Zhao D, Sheng B, Wu Y, Li H, Xu D, Nian Y,
Mao S, Li C, Xu X and Zhou G: Comparison of free and bound advanced
glycation end products in food: A review on the possible influence
on human health. J Agric Food Chem. 67:14007–14018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu D, Li L, Zhang X, Yao H, Yang M, Gai Z,
Li B and Zhao D: Degradation of peptide-bound maillard reaction
products in gastrointestinal digests of glyoxal-glycated casein by
human colonic microbiota. J Agric Food Chem. 67:12094–12104. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gurung M, Li Z, You H, Rodrigues R, Jump
DB, Morgun A and Shulzhenko N: Role of gut microbiota in type 2
diabetes pathophysiology. EBioMedicine. 51:1025902020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu Y, Zong M, Wu H, He D, Li L, Zhang X,
Zhao D and Li B: Dietary advanced glycation end-products affects
the progression of early diabetes by intervening in carbohydrate
and lipid metabolism. Mol Nutr Food Res. 66:e22000462022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Cai W, Yu J, Liu H, He S, Zhu L
and Xu J: Dietary advanced glycation end products shift the gut
microbiota composition and induce insulin resistance in mice.
Diabetes Metab Syndr Obes. 15:427–437. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Su L, Hong Z, Zhou T, Jian Y, Xu M, Zhang
X, Zhu X and Wang J: Health improvements of type 2 diabetic
patients through diet and diet plus fecal microbiota
transplantation. Sci Rep. 12:11522022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Murros KE, Huynh VA, Takala TM and Saris
PEJ: Desulfovibrio bacteria are associated with Parkinson's
disease. Front. Cell Infect Microbiol. 11:6526172021. View Article : Google Scholar
|
|
70
|
Chen YR, Jing QL, Chen FL, Zheng H, Chen
LD and Yang ZC: Desulfovibrio is not always associated with adverse
health effects in the guangdong gut microbiome project. PeerJ.
9:e120332021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Snelson M, Tan SM, Sourris K,
Thallas-Bonke V, Ziemann M, El-Osta S, Cooper M and Coughlan M:
SAT-301 resistant starch ameliorates advanced glycation
endproduct-induced gut dysbiosis and albuminuria in a mouse model
of type 2 diabetes. Kidney Int Rep. 4(Suppl): S1342019. View Article : Google Scholar
|
|
72
|
Reddy VP, Aryal P and Darkwah EK: Advanced
glycation end products in health and disease. Microorganisms.
10:18482022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Semba RD, Nicklett EJ and Ferrucci L: Does
accumulation of advanced glycation end products contribute to the
aging phenotype? J Gerontol A Biol Sci Med Sci. 65:963–975. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Maynard C and Weinkove D: The gut
microbiota and ageing. Subcell Biochem. 90:351–371. 2018.
View Article : Google Scholar
|
|
75
|
Mossad O, Batut B, Yilmaz B, Dokalis N,
Mezö C, Nent E, Nabavi LS, Mayer M, Maron FJM, Buescher JM, et al:
Gut microbiota drives age-related oxidative stress and
mitochondrial damage in microglia via the metabolite
N6-carboxymethyllysine. Nat Neurosci. 25:295–305. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li Y, Ning L, Yin Y, Wang R, Zhang Z, Hao
L, Wang B, Zhao X, Yang X, Yin L, et al: Age-related shifts in gut
microbiota contribute to cognitive decline in aged rats. Aging
(Albany NY). 12:7801–7817. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guilherme MDS, Nguyen VTT, Reinhardt C and
Endres K: Impact of gut microbiome manipulation in 5xFAD mice on
Alzheimer's disease-like pathology. Microorganisms. 9:8152021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ohira H, Tsuruya A, Oikawa D, Nakagawa W,
Mamoto R, Hattori M, Waki T, Takahashi S, Fujioka Y and Nakayama T:
Alteration of oxidative-stress and related marker levels in mouse
colonic tissues and fecal microbiota structures with chronic
ethanol administration: Implications for the pathogenesis of
ethanol-related colorectal cancer. PLoS One. 16:e02465802021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bonfili L, Cecarini V, Gogoi O, Berardi S,
Scarpona S, Angeletti M, Rossi G and Eleuteri AM: Gut microbiota
manipulation through probiotics oral administration restores
glucose homeostasis in a mouse model of Alzheimer's disease.
Neurobiol Aging. 87:35–43. 2020. View Article : Google Scholar
|
|
80
|
Mihailović M, Živković M, Jovanović JA,
Tolinački M, Sinadinović M, Rajić J, Uskoković A, Dinić S, Grdović
N, Golić N and Vidaković M: Oral administration of probiotic
Lactobacillus paraplantarum BGCG11 attenuates diabetes-induced
liver and kidney damage in rats. J Funct Foods. 38:427–437. 2017.
View Article : Google Scholar
|
|
81
|
Al-Enazi AMM, Virk P, Hindi A, Awad MA,
Elobeid M and Qindeel R: Protective effect of probiotic bacteria
and its nanoformulation against cadmium-induced oxidative stress in
male Wistar rat. J King Saud Univ Sci. 32:3045–3051. 2020.
View Article : Google Scholar
|
|
82
|
Park HY, Lee HB, Lee SY, Oh MJ, Ha SK, Do
E, Lee HHL, Hur J, Lee KW, Nam MH, et al: Lactococcus lactis KF140
reduces dietary absorption of Nε-(Carboxymethyl)lysine
in rats and humans via β-galactosidase activity. Front Nutr.
9:9162622022. View Article : Google Scholar
|
|
83
|
Farhangi MA, Dehghan P and Namazi N:
Prebiotic supple-mentation modulates advanced glycation
end-products (AGEs), soluble receptor for AGEs (sRAGE), and
cardiometabolic risk factors through improving metabolic
endotoxemia: A randomized-controlled clinical trial. Eur J Nutr.
59:3009–3021. 2020. View Article : Google Scholar
|
|
84
|
Li Y, Peng Y, Shen Y, Zhang Y, Liu L and
Yang X: Dietary polyphenols: Regulate the advanced glycation end
products-RAGE axis and the microbiota-gut-brain axis to prevent
neurodegenerative diseases. Crit Rev Food Sci Nutr. May
19–2022.Epub ahead of print.
|
|
85
|
Wu Y, Dong L, Song Y, Wu Y, Zhang Y and
Wang S: Preventive effects of polysaccharides from Physalis
alkekengi L. on dietary advanced glycation end product-induced
insulin resistance in mice associated with the modulation of gut
microbiota. Int J Biol Macromol. 204:204–214. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang S, Zhou H, Wang G, Zhong XH, Shen QL,
Zhang XJ, Li RY, Chen LH, Zhang YH and Wan Z: Quercetin is
protective against short-term dietary advanced glycation end
products intake induced cognitive dysfunction in aged ICR mice. J
Food Biochem. 44:e131642020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yonei Y, Ikeda T, Ogawa H, Yagi N, Takabe
W, Ito M and Morii H: Anti-glycation and improvement microbiota by
Geranium dielsianum extract: Relation to health problems in
athletes. Glycative Stress Res. 6:31–38. 2019.
|
|
88
|
Di Lorenzo F, De Castro C, Silipo A and
Molinaro A: Lipopolysaccharide structures of Gram-negative
populations in the gut microbiota and effects on host interactions.
FEMS Microbiol Rev. 43:257–272. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mohr AE, Crawford M, Jasbi P, Fessler S
and Sweazea KL: Lipopolysaccharide and the gut microbiota:
Considering structural variation. FEBS Lett. 596:849–875. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Aki T, Funakoshi T, Noritake K, Unuma K
and Uemura K: Extracellular glucose is crucially involved in the
fate decision of LPS-stimulated RAW264.7 murine macrophage cells.
Sci Rep. 10:105812020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dhananjayan K, Gunawardena D, Hearn N,
Sonntag T, Moran C, Gyengesi E, Srikanth V and Münch G: Activation
of macrophages and microglia by interferon-γ and lipopolysaccharide
increases methylglyoxal production: A new mechanism in the
development of vascular complications and cognitive decline in type
2 diabetes mellitus? J Alzheimers Dis. 59:467–479. 2017. View Article : Google Scholar
|
|
92
|
Ko YH, Tsai MS, Lee PH, Liang JT and Chang
KC: Methylprednisolone stiffens aortas in
lipopolysaccharide-induced chronic inflammation in rats. PLoS One.
8:e696362013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shamsaldeen YA, Alsugoor MH, Lione LA and
Benham CD: Dysfunction in nitric oxide synthesis in streptozotocin
treated rat aorta and role of methylglyoxal. Eur J Pharmacol.
842:321–328. 2019. View Article : Google Scholar
|
|
94
|
Kitaura A, Nishinaka T, Hamasaki S,
Hatipoglu OF, Wake H, Nishibori M, Mori S, Nakao S and Takahashi H:
Advanced glycation end-products reduce lipopolysaccharide uptake by
macrophages. PLoS One. 16:e02459572021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chiu HC, Fu MM, Yang TS, Fu E, Chiang CY,
Tu HP, Chin YT, Lin FG and Shih KC: Effect of high glucose,
Porphyromonas gingivalis lipopolysaccharide and advanced glycation
end-products on production of interleukin-6/-8 by gingival
fibroblasts. J Periodontal Res. 52:268–276. 2017. View Article : Google Scholar
|
|
96
|
Liu J, Zhao S, Tang J, Li Z, Zhong T, Liu
Y, Chen D, Zhao M, Li Y, Gong X, et al: Advanced glycation end
products and lipopolysaccharide synergistically stimulate
proinflammatory cytokine/chemokine production in endothelial cells
via activation of both mitogen-activated protein kinases and
nuclear factor-kappaB. FEBS J. 276:4598–4606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yamamoto Y and Yamamoto H: Interaction of
receptor for advanced glycation end products with advanced
oxidation protein products induces podocyte injury. Kidney Int.
82:733–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Pinkas A, Cunha Martins A Jr and Aschner
M: C. elegans-an emerging model to study metal-induced RAGE-related
pathologies. Int J Environ Res Public Health. 15:14072018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lawes M, Pinkas A, Frohlich BA, Iroegbu
JD, Ijomone OM and Aschner M: Metal-induced neurotoxicity in a
RAGE-expressing C. elegans model. Neurotoxicology. 80:71–75. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang L, Wu J, Guo X, Huang X and Huang Q:
RAGE plays a role in LPS-induced NF-κB activation and endothelial
hyperpermeability. Sensors (Basel). 17:7222017. View Article : Google Scholar
|
|
101
|
Li Y, Wu R, Tian Y, Yu M, Tang Y, Cheng H
and Tian Z: RAGE/NF-κB signaling mediates lipopolysaccharide
induced acute lung injury in neonate rat model. Int J Clin Exp Med.
8:13371–13376. 2015.
|
|
102
|
Li Y, Wu R, Zhao S, Cheng H, Ji P, Yu M
and Tian Z: RAGE/NF-κB pathway mediates lipopolysaccharide-induced
inflammation in alveolar type I epithelial cells isolated from
neonate rats. Inflammation. 37:1623–1629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yamamoto Y, Harashima A, Saito H,
Tsuneyama K, Munesue S, Motoyoshi S, Han D, Watanabe T, Asano M,
Takasawa S, et al: Septic shock is associated with receptor for
advanced glycation end products ligation of LPS. J Immunol.
186:3248–3257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Watanabe M, Toyomura T, Tomiyama M, Wake
H, Liu K, Teshigawara K, Takahashi H, Nishibori M and Mori S:
Advanced glycation end products (AGEs) synergistically potentiated
the proinflammatory action of lipopolysaccharide (LPS) and high
mobility group box-1 (HMGB1) through their direct interactions. Mol
Biol Rep. 47:7153–7159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang J, Xiong T, Zhang Z, Tan Y and Guo
L: Inhibition of the receptor for advanced glycation inhibits
lipopolysaccharide-mediated high mobility group protein B1 and
Interleukin-6 synthesis in human gingival fibroblasts through the
NF-κB signaling pathway. Arch Oral Biol. 105:81–87. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cho HI, Hong JM, Choi JW, Choi HS, Hwan
Kwak J, Lee DU, Kook Lee S and Lee SM: β-Caryophyllene alleviates
D-galactosamine and lipopolysaccharide-induced hepatic injury
through suppression of the TLR4 and RAGE signaling pathways. Eur J
Pharmacol. 764:613–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Goel R, Bhat SA, Hanif K, Nath C and
Shukla R: Perindopril attenuates lipopolysaccharide-induced
amyloidogenesis and memory impairment by suppression of oxidative
stress and RAGE activation. ACS Chem Neurosci. 7:206–217. 2016.
View Article : Google Scholar
|
|
108
|
Liu L, Zhao Z, Lu L, Liu J, Sun J, Wu X
and Dong J: Icariin and icaritin ameliorated hippocampus
neuroinflammation via inhibiting HMGB1-related pro-inflammatory
signals in lipopolysaccharide-induced inflammation model in C57BL/6
J mice. Int Immunopharmacol. 68:95–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Aneja RK, Tsung A, Sjodin H, Gefter JV,
Delude RL, Billiar TR and Fink MP: Preconditioning with high
mobility group box 1 (HMGB1) induces lipopolysaccharide (LPS)
tolerance. J Leukoc Biol. 84:1326–1334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou XY, Zhang WJ, Huang QB and Guo XH:
Role of RAGE in lipopolysaccharide-induced cytoskeletal changes in
mouse pulmonary microvascular endothelial cells. Nan Fang Yi Ke Da
Xue Xue Bao. 35:6–11. 2015.In Chinese. PubMed/NCBI
|
|
111
|
Wang H, Wang T, Yuan Z, Cao Y, Zhou Y, He
J, Shen Y, Zeng N, Dai L, Wen F and Chen L: Role of receptor for
advanced glycation end products in regulating lung fluid balance in
lipopolysaccharide-induced acute lung injury and infection-related
acute respiratory distress syndrome. Shock. 50:472–482. 2018.
View Article : Google Scholar
|
|
112
|
Li J, Wang K, Huang B, Li R, Wang X, Zhang
H, Tang H and Chen X: The receptor for advanced glycation end
products mediates dysfunction of airway epithelial barrier in a
lipopolysaccharides-induced murine acute lung injury model. Int
Immunopharmacol. 93:1074192021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Shi J, Fu Y, Zhao XH and Lametsch R:
Glycation sites and bioactivity of lactose-glycated caseinate
hydrolysate in lipopolysaccharide-injured IEC-6 cells. J Dairy Sci.
104:1351–1363. 2021. View Article : Google Scholar
|
|
114
|
You L, Cui H, Zhao F, Sun H, Zhong H, Zhou
G and Chen X: Inhibition of HMGB1/RAGE axis suppressed the
lipopolysaccharide (LPS)-induced vicious transformation of cervical
epithelial cells. Bioengineered. 12:4995–5003. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wilkie T, Verma AK, Zhao H, Charan M,
Ahirwar DK, Kant S, Pancholi V, Mishra S and Ganju RK:
Lipopolysaccharide from the commensal microbiota of the breast
enhances cancer growth: Role of S100A7 and TLR4. Mol Oncol.
16:1508–1522. 2022. View Article : Google Scholar :
|
|
116
|
Ramsgaard L, Englert JM, Manni ML,
Milutinovic PS, Gefter J, Tobolewski J, Crum L, Coudriet GM,
Piganelli J, Zamora R, et al: Lack of the receptor for advanced
glycation end-products attenuates E. coli pneumonia in mice. PLoS
One. 6:e201322011. View Article : Google Scholar : PubMed/NCBI
|