1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
general term for squamous epithelial malignancies originating in
the nasal cavity, oral cavity, pharynx and larynx, accounting for
>90% of head and neck cancers, with rapid progression, extensive
infiltration and a poor prognosis (1). The current treatment strategies for
HNSCC are mainly based on radiotherapy, chemotherapy and surgery
(2). However, patients with
advanced or metastatic HNSCC are highly susceptible to recurrence
and metastasis following surgery. These patients are usually
treated with cisplatin-based concurrent radiotherapy and targeted
drug-induced chemotherapy represented by cetuximab; the efficacy of
this treatment however, is hampered by the widespread development
of cisplatin resistance, thus rendering cisplatin-based
chemotherapeutic regimens ineffective. Previous studies have shown
that promoting ferroptosis in tumor cells is a more effective
method of reducing cisplatin resistance in cancer cells (3), and it can enhance the sensitivity of
tumor cells to radiotherapy and chemotherapeutic drugs (4).
Ferroptosis is a regulated form of cell death that
differs from the traditional cell death modality, depending on
lipid peroxidation, which is induced by iron ions and reactive
oxygen species (5). Initially,
researchers found that ferroptosis can cause multiple tissue damage
via oxidative stress pathways, which are associated with the
pathogenesis of several degenerative diseases, such as Alzheimer's
disease (6), ischemia-reperfusion
injury (7) and osteoarthritis
(8). Based on the ability of
ferroptosis to induce damage to multiple tissues, it has been
suggested that ferroptosis has immense therapeutic potential in the
treatment of cancer with active abnormal proliferation, and the
induction of ferroptosis in tumor cells may lead to the development
of novel treatment strategies (9).
Therefore, the present review summarizes the
mechanisms of action of ferroptosis and its regulators in HNSCC,
and also discusses the drugs regulated by ferroptosis in HNSCC, in
order to provide a theoretical basis for the further use of
ferroptosis in the treatment of HNSCC.
2. Data collection methods
Up to October 2022, the 'PubMed', 'Springer', 'Web
of Science' and 'CNKI' databases were searched for original
research articles and reviews on the progress of ferroptosis in
HNSCC. The terms for the search included the following:
Ferroptosis, ferroptosis and HNSCC, regulatory mechanism of
ferroptosis, iron metabolism, ferroptosis inducers, ferroptosis
inhibitors, ferroptosis and glutathione peroxidase 4 (GPX4),
ferroptosis and System Xc−, and ferroptosis and iron
(the detailed search strategies are presented in Table I).
 | Table ISummary of the search strategy used
in the present study. |
Table I
Summary of the search strategy used
in the present study.
| Items | Specification |
|---|
| Date of search | October 31,
2022 |
| Databases and other
sources searched | 'PubMed' 'Springer'
'Web of Science' 'CNKI' |
| Search terms
used | 'ferroptosis'
(title/abstract) |
| 'ferroptosis' and
'HNSCC' (title/abstract) |
| 'regulatory
mechanism of ferroptosis' (title/abstract) |
| 'iron metabolism'
(title/abstract) |
| 'ferroptosis
inducers' (title/abstract) |
| 'ferroptosis
inhibitors' (title/abstract) |
| 'ferroptosis' and
'GPX4' (title/abstract) |
| 'ferroptosis' and
'System Xc−' (title/abstract) |
| 'ferroptosis' and
'Iron' (title/abstract) |
| Timeframe | 2008-2022 |
| Inclusion and
exclusion criteria | Focus was placed on
original articles and reviews in the English language about the
regulatory factor of ferroptosis in head and neck squamous cell
carcinoma; articles that had no information about HNSCC and
ferroptosis were excluded |
3. The discovery and characteristics of
ferroptosis
Ferroptosis is a regulated form of cell death which
differs from apoptosis, necrosis and autophagy, which can be
inhibited by iron chelators and antioxidants (10), characterized by the excessive
accumulation of lipid peroxides and reactive oxygen species (ROS),
discovered by the Stockwell laboratory at Columbia University in
2012 (11,12). The study of ferroptosis has been
gradually applied to a variety of diseases, such as tissue
ischemia-reperfusion, neurological diseases, acute renal failure
and tumors (13).
Ferroptosis is caused by unrestricted lipid
peroxidation and plasma membrane rupture, the two main features of
which are iron ion accumulation and lipid peroxidation.
Morphologically, ferroptosis is manifested by cell membrane
fracture and effacement, mitochondrial atrophy, reduction or even
the disappearance of mitochondrial ridges, and increased cell
membrane density; biologically, it is manifested by the increased
generation of ROSs, iron ion aggregation, the activation of
mitogen-activated protein kinases (MAPKs), and the inhibition of
cysteine-glutamate reverse transporter by decreasing cystine uptake
and depleting glutathione (GSH); in immunologically, it is
manifested by the release of damage-associated molecular patterns,
which promote inflammatory responses (14).
4. Regulatory mechanisms of ferroptosis
It has been demonstrated that the sensitivity of
cells to ferroptosis is associated with multiple regulatory
mechanisms, mainly including three pathways: The regulation of iron
metabolism, the regulation of the system Xc−/GSH/GPX4
anti-oxidant system and the regulation of lipid peroxidation, which
can directly affect the sensitivity of cells to ferroptosis
(14) (Fig. 1).
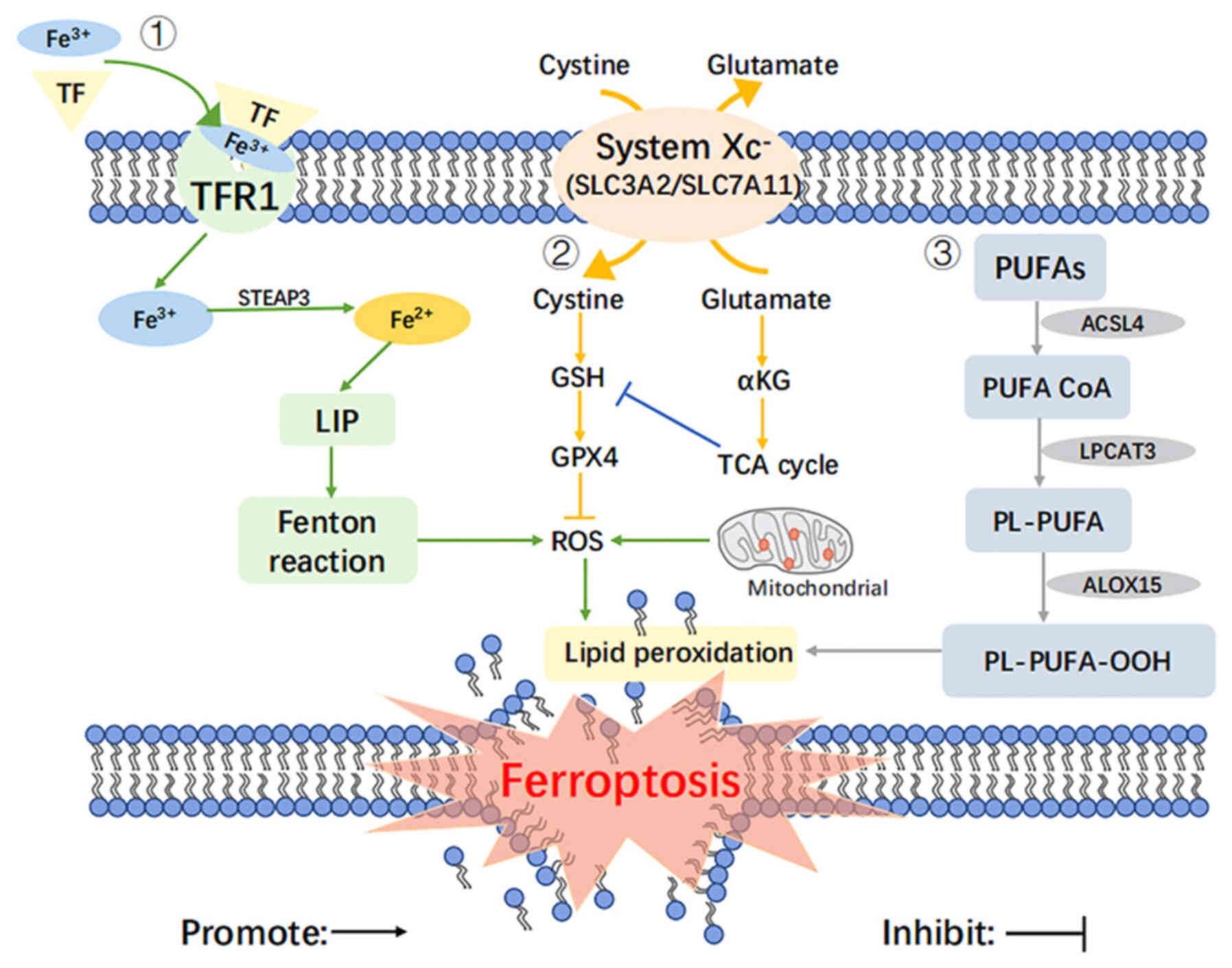 | Figure 1Regulatory mechanisms of ferroptosis.
The regulatory mechanisms of ferroptosis include the following: i)
The regulation of iron metabolism which involves TF, TFR1, LIP and
the Fenton reaction; ii) the regulation of the System Xc-/GSH/GPX4
antioxidant system which involves GSH, GPX4, ROS, α-KG and the TCA
cycle; iii) the regulation of lipid peroxidation which involves
PUFAs, ACSL4, PUFA CoA, LPCAT3, PL-PUFA, ALOX15, PL-PUFA-OOH. TF,
transferrin; TFR1, transferrin 1; LIP, labile iron pool; Fenton
reaction, Fe2+ +H2O2 →
Fe3++OH− + ·OH; GSH, glutathione; GPX4,
glutathione peroxidase 4; ROS, reactive oxygen species; α-KG,
α-ketoglutarate; TCA cycle, tricarboxylic acid cycle. PUFAs,
polyunsaturated fatty acids; ACSL4, long-chain acyl-CoA synthetase
4; PUFA CoA, polyunsaturated fatty acids acetyl CoA; LPCAT3,
lysophosphatidylcholine acyl-transferase 3; PL-PUFA, phospholipid
polyunsaturated fatty acids; ALOX15, arachidonate 15-lipoxygenase;
PL-PUFA-OOH, lipid hydrogen peroxide. |
Regulation of iron metabolism
Iron is an essential trace metal element with redox
activity in the human body; increased levels of iron and/or
iron-binding proteins and dysregulated iron metabolism lead to a
risk of carcinogenesis and promote tumor growth (15).
Iron in the body circulation is usually present and
is transported as Fe3+, stored in endosomes with
transferrin (TF) in the presence of TF receptor 1, and is reduced
to Fe2+ in the presence of iron oxidoreductase (STEAP3).
When Fe2+ is in excess, it can be transferred to the
cell plasma and eventually stored in ferritin to form labile iron
pool. When the buffering capacity of ferritin is exceeded, a large
amount of divalent iron ions (Fe2+) catalyze hydrogen
peroxide (H2O2), which generates toxic
hydroxyl radicals (·OH) and thus triggers cellular lipid
peroxidation (16). It has been
found that the concentration of H2O2 and iron
ions in a large number of tumor cells is generally low; thus, when
the disorder of iron metabolism causes an increase of free iron in
cells, iron catalyzes the production of ROS through the Fenton
reaction, and ROS further promotes lipid peroxidation and causes
lipid peroxide aggregation, which induces ferroptosis (17).
Cancer cells are more iron-dependent compared to
normal cells (18), and some
cancer cells exhibit iron ion aggregation. Thus, the regulation of
ferroptosis via iron homeostasis can effectively kill cancer cells
by increasing iron uptake, decreasing iron storage and limiting
iron efflux to promote ferroptosis; and by preventing ferroptosis
through iron chelators and antioxidants (19).
Cellular iron accumulation is one of the typical
markers of ferroptosis, and the detection of changes in iron ions
can indicate whether ferroptosis has occurred. The iron ion content
is positively associated with ferroptosis, and by using the
Fe2+ detection probe FerroOrange, flow cytometry or
confocal microscopy to detect intracellular iron content, the
orange fluorescence of FerroOrange is enhanced in cells in which
ferroptosis occurs (20).
Regulation of the System
Xc−/GSH/GPX4 antioxidant system
Ferroptosis can be induced by exogenous or
endogenous pathways. The exogenous pathway initiates ferroptosis
mainly by inhibiting the functional activity of System Xc-. System
Xc− is a critical intracellular antioxidant system
consisting of two subunits: Solute carrier family 7 member 11
(SLC7A11; xCT) and solute carrier family 3 member 2 (SLC3A2;
CD98hc). SLC7A11 is responsible for the major transport activities,
including cystine uptake and glutamate excretion; SLC3A2 functions
as a chaperone protein. Cystine is exchanged into the cell at a 1:1
ratio with glutamate and is rapidly reduced to cysteine, which is
involved in the synthesis of intracellular GSH and GPX4 (21).
The depletion of GSH, a key co-factor for the
function of GPX4, disrupts cellular redox homeostasis, leading to
the accumulation of ROS and ultimately inducing the onset of
ferroptosis. Erastin can reduce GSH synthesis by targeting the
inhibition of System Xc-, which reduces the cystine entering the
cell and ultimately induces the onset of ferroptosis (22).
The endogenous pathway then activates ferroptosis by
reducing the activity of GPX4. The basic function of GPX family
members is H2O2 reduction at the expense of
GSH, and GPX4 is the only enzyme that reduces cholesterol
hydroperoxide and esterifies oxidized fatty acids (23). GPX4 is also a key regulatory
component of the ferroptosis mechanism, and its reduced activity is
the hallmark event for the onset of ferroptosis (24).
Regulation of lipid peroxidation
Normal cells tend to maintain a dynamic redox
balance, whereas cancer cells are usually in an oxidative stress
state with higher metabolic levels than normal cells, accompanied
by ROS accumulation (25). The
production of ROS is associated with damage to proteins,
carbohydrates, lipids and nucleotides, which can eventually lead to
the development of malignancies. The primary indicator of
ferroptosis is the excessive accumulation of lipid peroxides and
ROS, which is currently detected in laboratory mainly by the
specific fluorescent probes, C11 BODIPY 581/591 and
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFH-DA)
(26).
In the process of ferroptosis, polyunsaturated fatty
acids (PUFAs) can be oxidized by intracellular ROS to produce
unstable lipid hydroperoxides and lipid peroxides, reaching the
lethal amounts and can lead to the destruction of bilayer lipid
structures, thus inducing ferroptosis (27). The level of intracellular PUFAs
determines the development of ferroptosis (28,29). The enzymatic reaction of lipid
peroxidation contains three key enzymes: Long-chain lipid acyl
coenzyme A synthesis 4 (ACSL4) (30), lysophosphatidylcholine
acyltransferase 3 (LPCAT3) (31)
and arachidonic acid 15-lipoxygenase (ALOX15). Among these, ACSL4
catalyzes the conversion of free PUFAs into polyunsaturated fatty
acids-acetyl coenzyme A (PUFA CoA), which is subsequently
esterified by LPCAT3 into phospholipid-type polyunsaturated fatty
acids (PL-PUFA), and finally ALOX15 participates in the
peroxidation process of membrane phospholipids to lipid peroxides
(32). In summary, disorders of
lipid metabolism are closely related to cellular ferroptosis, and
the accumulation of lipid oxides and ROS is necessary for
ferroptosis to occur.
5. Ferroptosis regulators in head and neck
squamous cell carcinoma
Cisplatin, one of the most widely used platinum
compounds, is the clinical first-line chemotherapeutic agent used
in the treatment of HNSCC (33).
Although ~80% of patients with HNSCC are initially sensitive to
this treatment, the resistance rate in subsequent treatment is as
high as 70% (34), which severely
diminishes the efficacy of cisplatin (35), and chemoresistance as a major
cause of treatment failure in HNSCC can no longer be
underestimated.
Recent research has identified some drugs that can
reverse chemoresistance in vitro; however, the majority of
these drugs have cytotoxic defects that limit their clinical
application, and thus, the search for a novel methods of
chemotherapy sensitization has become a hot topic and difficult
issue in HNSCC research (2).
Previous studies have reported that the resistance of HNSCC to
cisplatin can be reduced by inducing ferroptosis without affecting
normal tissues (36-38). This may provide a new strategy
with which to improve the post-operative survival of patients with
HNSCC. Thus, exploring ferroptosis regulators in HNSCC is critical
for improving the prognosis of patients with HNSCC.
Currently, in HNSCC, studies on ferroptosis have
focused on the System Xc−-GSH-GPX4 pathway, which
induces ferroptosis by directly or indirectly disrupting
intracellular redox homeostasis (39,40) (Table II and Fig. 2).
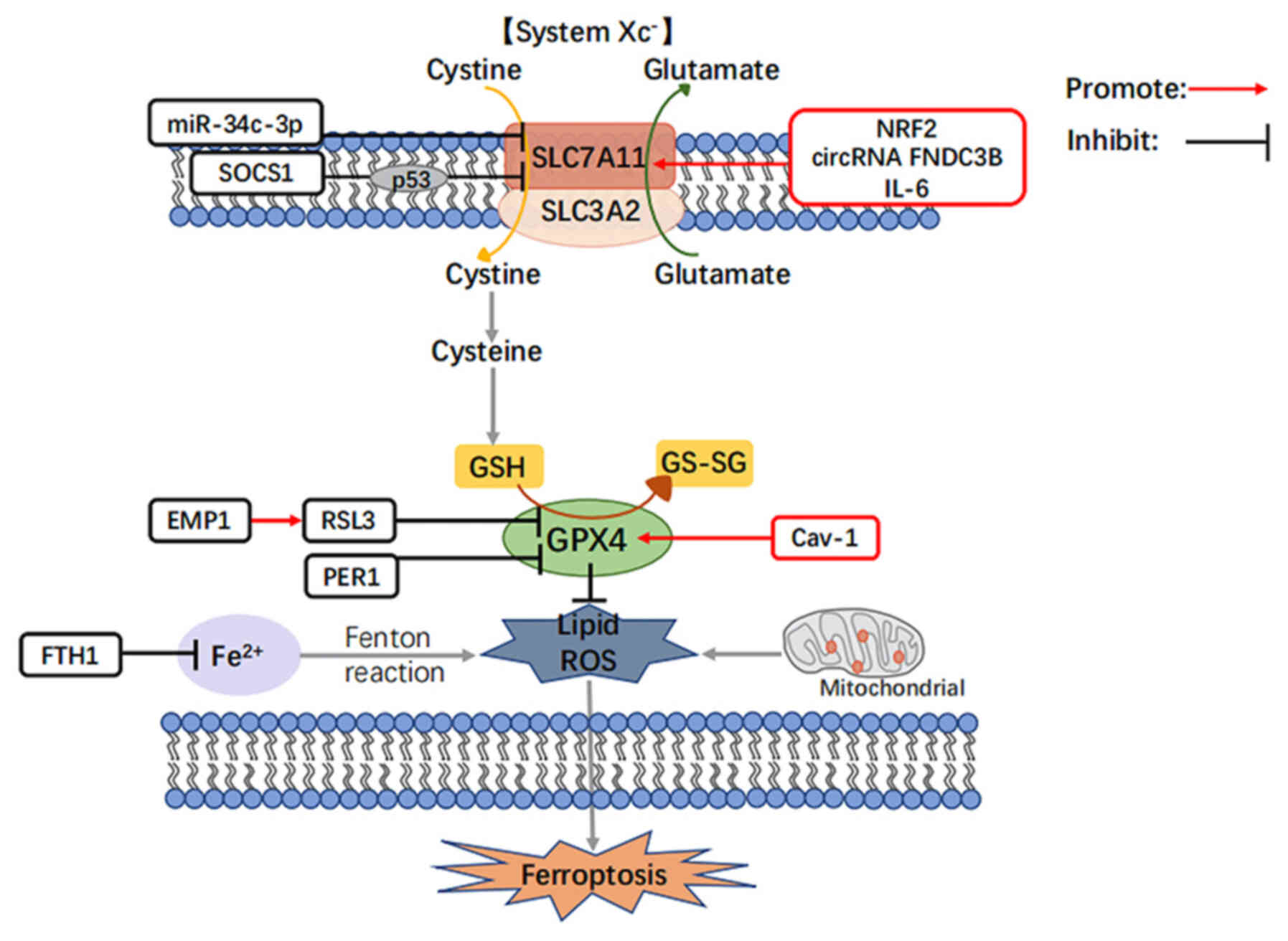 | Figure 2Roles and functions of the listed
regulators in the regulation of ferroptosis in head and neck
cancers. NRF2, nuclear factor erythroid 2-related factor 2; SOCS1,
suppressors of cytokine signaling 1; IL-6, interleukin-6; GPX4,
glutathione peroxidase 4; RSL3, an inhibitor of GPX4; EMP1,
epithelial membrane protein genes 1; PER1, circadian rhythm protein
1, period-1; Cav-1, caveolin-1; FTH1, ferritin heavy chain. |
 | Table IISummary of ferroptosis regulators in
HNSCC. |
Table II
Summary of ferroptosis regulators in
HNSCC.
| Mechanism | Compound | (Refs.) |
|---|
|
Activation/inhibition of ferroptosis
through regulation of SLC7A11 | miR-34c-3p | (58) |
| circRNA FNDC3B | (62) |
| NRF2 | (68) |
| SOCS1 | (75) |
| IL-6 | (78) |
|
Activation/inhibition of ferroptosis
through the regulation of GPX4 | RSL3 | (90) |
| EMP1 | (95) |
| PER1 | (105) |
| Cav-1 | (112) |
| Regulation of
ferroptosis through iron metabolic pathways | FTH1 | (75) |
Activation/inhibition of ferroptosis
through the regulation of SLC7A11
SLC7A11 expression has been found to be higher than
normal in a variety of cancer types (21,41,42) SLC7A11 can inhibit the occurrence
of ferroptosis, and its expression level is positively associated
with the clinical stage of patients with HNSCC (43); in human papillomavirus
(HPV)-positive patients with HNSCC, the expression level of SLC7A11
has been found to be lower than that HPV-negative patients, and
positive patients may be more responsive to radiotherapy and
chemotherapy (44). It is
hypothesized that the ferroptosis marker gene, SLC7A11, plays a
crucial role in the development of HNSCC, and targeting SLC7A11 may
selectively kill tumor cells while preserving normal cells
(45).
In HNSCC, multiple factors regulate the expression
of SLC7A11 and thus the occurrence of ferroptosis, and hotspot
factors currently studied include non-coding RNAs (ncRNAs), nuclear
factor erythroid 2-related factor 2 (NRF2), suppressor of cytokine
signaling 1 (SOCS1) and interleukin (IL)-6, whose regulatory
mechanisms are specified below.
ncRNAs
ncRNAs account for ~98% of the human transcribed
genome and are involved in a number of physiological and
pathological processes, including cancer, rendering them one of the
hotspots of current research (46). It has been found that ncRNAs,
particularly microRNAs (miRNAs/miRs) and circular RNAs (circRNAs),
are closely related to the biological process of ferroptosis, and
they can regulate ferroptosis in tumor cells through
transcriptional or post-transcriptional levels by mechanisms
involving glutamine catabolism, mitochondria-related proteins, iron
metabolism, glutathione metabolism, amino acid metabolism, lipid
peroxidation, cell cycle and the p53 signaling pathway (47). Therefore, ncRNAs and downstream
target genes associated with cellular ferroptosis may become novel
clinical markers and therapeutic targets for HNSCC.
a) miRNAs
It has been found that miRNAs can inhibit
ferroptosis in tumor cells by regulating the glutamine catabolic
pathway (48-50). miR-137 and miR-9 and other miRNAs
achieve the inhibition of ferroptosis in tumor cells by inhibiting
a link in the glutamine catabolic pathway, further revealing the
molecular mechanisms of the miRNA regulation of ferroptosis in
tumor cells, and promoting tumor cells by targeting related miRNAs
glutamine metabolism can improve the sensitivity of tumor cells to
ferroptosis (48,51). In addition, miRNAs can inhibit
tumor cell ferroptosis by regulating mitochondria-related proteins.
miRNA expression differences in tumor cells can affect
mitochondrial function and energy metabolism, which in turn can
achieve the regulation of tumor cell ferroptosis. Mitochondrial
transferrin mainly mediates the entry of Fe2+ into
mitochondria to maintain the coordinated intracellular distribution
and utilization of iron (52,53).
Previous studies have demonstrated that miRNAs are
key regulators of HNSCC carcinogenesis (54-56), and they have attracted widespread
attention in HNSCC. Shen et al (57) revealed that miR-34c-3p is closely
associated with the spread, migration, invasion and apoptosis of
HNSCC. As illustrated in Fig. 2,
miR-34c-3p inhibits the expression of SLC7A11 by binding to the 3′
non-coding region of SLC7A11, which inhibits the transport process
of System Xc−. The reduction of cysteine affects the
synthesis of cysteine and glutathione, which inhibits the
expression of GPX4 and ultimately induces lipid peroxidation and
ROS generation, leading to ferroptosis in HNSCC and inhibiting
HNSCC cell growth (58).
b) circRNAs
circRNA, which account for >90% of the human
transcriptome, but have minimal potential to encode proteins, are
involved in the regulation of ferroptosis in tumor cells by
adsorbing certain miRNAs, functioning as competing endogenous RNAs
to regulate the expression of relevant miRNA target genes and thus,
and their involvement in cancer ferroptosis (59). The potential regulatory mechanisms
include mitochondria-related proteins, iron metabolism, glutathione
metabolism and lipid peroxidation (60).
Some circRNAs have been reported to regulate head
and neck tumorigenesis (61). As
also illustrated in Fig. 2, Yang
et al (62) in HNSCC found
that circRNA FNDC3B further upregulated the expression level of
SLC7A11 by adsorbing miR-520d-5p, inhibited the occurrence of
ferroptosis, induced epithelial-mesenchymal transition, and
promoted HNSCC cell proliferation.
These studies have proven that ncRNA-based therapy
may be used for cancer treatment by regulating ferroptosis;
however, there is no mature technical means available for applying
ncRNA-mediated tumor therapy to clinical practice. The in-depth
study of the molecular mechanisms of the ferroptosis regulation of
tumor cells by ncRNAs may provide potential targets and novel
therapeutic strategies for cancer treatment. Thus far, circFNDC3B
and miR-34c-3p have only been studied in HNSCC.
NRF2
The redox state of the cell is determined by the
balance between the production of reactive substances and the
subsequent reduction of a series of antioxidant defense systems.
Excess reactive substances react to generate lipid peroxides, which
are associated with the progression of a number of diseases,
including cancer, diabetes, cardiovascular disease and liver
disease (63). The intracellular
antioxidant defense system is used to control the level of reactive
substances.
NRF2, the central transcriptional regulator in the
oxidative stress response, plays a key role in cellular antioxidant
responses, redox homeostasis and metabolic homeostasis, and is
involved in lipid peroxidation and free iron accumulation (64); numerous factors of the ferroptosis
cascade are target genes of NRF2 (65), indicating its critical role in
mediating the ferroptosis response. When oxidative stress occurs,
NRF2 activates and induces the expression of antioxidant genes to
eliminate ROS, and if excessive ROS generation causes peroxidation,
it initiates a cellular suicide program, in which ferroptosis is
the most critical. Furthermore, a number of antioxidants such as
GSH, heme oxygenase 1 and NAD(P)H quinone dehydrogenase 1 are
downstream genes of NRF2 (66).
Cancer and neurodegenerative diseases highly associated with
oxidative stress render NRF2 a crucial therapeutic target for these
diseases (64,67).
As illustrated in Fig.
2, in HNSCC, the high expression of NRF2 induces the high
expression of SLC7A11 by directly binding to the promoter region of
SLC7A11, which in turn induces System Xc− to transport
intracellular glutamate away and extracellular cystine in, and
elevated cystine leads to an increase in glutathione, which in turn
increases the expression of GPX4. In turn, the high expression of
GPX4 suppresses ROS and lipid peroxidation levels, ultimately
inhibiting the sensitivity of HNSCC cells to ferroptosis and
leading to increased patient resistance to radiation therapy
(68). Indeed, in other types of
cancer, such as colorectal (69),
lung (70), stomach (71) and ovarian cancers (72), NRF2 also functions as an oncogenic
transcription factor, and cancer cells frequently exhibit the
overexpression of NRF2, which plays a key role in counteracting
environmental or intracellular stress (21,73).
Therefore, controlling the progression of HNSCC by
modulating the NRF2/SLC7A11/ferroptosis pathway provides a
potential therapeutic target for enhancing the sensitivity of HNSCC
to radiotherapy, and exploring the combination of NRF2 inhibitors
with radiotherapy remains a direction for future research.
SOCS1
SOCS1 is considered as a negative feedback regulator
of cytokine signaling and is also involved in the formation of the
E3 ubiquitin ligase complex (74), which plays critical regulatory
roles in both cell growth and proliferation. As illustrated in
Fig. 2, in HNSCC, SOCS1 functions
as a 'ferroptosis activator' similar to miR-34c-3p by suppressing
the transcription of SLC7A11 and thus the uptake of cystine
(75).
IL-6
Smoking and betel nut chewing are two risk factors
for HNSCC, both of which upregulate the expression of the
pro-inflammatory cytokine, IL-6 (76), and oxidative stress caused by an
imbalance of oxidants and antioxidants is considered to underlie
the development of HNSCC after smoking (77). As also demonstrated in Fig. 2, Li et al found that IL-6
accumulation was associated with lipid peroxidation, which
inhibited HNSCC ferroptosis in vitro and in vivo
through the upregulation of SLC7A11 expression (78). In addition, IL-6 is secreted by
immune cells or tumor cells and can promote tumor cell
proliferation, epithelial-mesenchymal transition (EMT) through the
activation of signal transducer and transcriptional activator 3
pathways, thus promoting cancer progression and inducing
chemotherapy resistance, which is associated with a poor prognosis
of patients with HNSCC (76,79). Thus, IL-6 can function as an
inhibitor of ferroptosis that drives tumor progression, and it can
potentially be used as a tumor prevention and therapeutic
target.
Regulation of ferroptosis through the
GPX4 pathway
Compared with normal tissues, GPX4 expression levels
are significantly elevated in a variety of tumor tissues, such as
triple-negative breast (80),
gastric (81) and esophageal
(82) cancer, leading to the
hypothesis that GPX4 may be an oncogene. It has been shown to be a
key regulator of ferroptosis; the overexpression or knockdown of
GPX4 can affect the cell lethality of 12 ferroptosis inducers
(83). GPX4 can affect tumor stem
cell production and the EMT process by regulating ROS production
(84,85). Viswanathan et al (86) demonstrated that GPX4 inhibitors in
the majority of tumors can induce the ferroptosis of drug-resistant
cells and prevent tumor recurrence; the combination of the targeted
therapy of tumors with GPX4 inhibitors has provide new insight into
resolving the issue of drug resistance in cancer cells (87).
In HNSCC, GPX4 is strongly associated with the poor
prognosis of patients (88). A
previous study demonstrated that the knockdown of GPX4 decreased
cell viability, although the level of the caspase activity marker
was not increased, indicating that non-apoptotic cell death
occurred, which was reversed following pre-treatment with
ferrostatin-1, suggesting that cells die via the ferroptosis
pathway (89). Thus, in HNSCC,
GPX4 can function as an inhibitor of ferroptosis and its
downregulation induces tumor cell death; i.e., the modification of
GPX4 expression may be a novel therapeutic approach for HNSCC.
In HNSCC, various genes such as RAS-selective lethal
3 (RSL3), epithelial membrane protein 1 (EMP1), period circadian
regulator 1 (period 1; PER1) and caveolin-1 (Cav-1) regulate the
occurrence of ferroptosis by regulating GPX4 expression and their
specific regulatory mechanisms are described below.
RSL3
RSL3, an inhibitor of GPX4 that inhibits cysteine
and glutamate transporter proteins, is a ferroptosis activator. As
illustrated in Fig. 2, RSL3
functions as a 'ferroptosis activator' in HNSCC by downregulating
GPX4 expression, inducing an increase in ROS generation, and
catalyzing the lipid peroxidation of highly expressed unsaturated
fatty acids in the cell membrane (90).
EMP1
EMP1 is a member of the epithelial membrane protein
family, encoded by the peripheral myelin protein family, and is
involved in the proliferation, migration and differentiation of
tumor cells (91). It is mainly
expressed in the squamous epithelium and exhibits differential
expression levels in various normal tissues, such as esophagus,
stomach and gallbladder (92).
EMP1 plays a key role in cell adhesion, proliferation, apoptosis,
tumor formation and ferroptosis (93), and its decreased expression is
associated with the poor prognosis of patients with certain
malignancies and is an independent predictor of ferroptosis
(94).
As also illustrated in Fig. 2, Wang et al (95) reported that the overexpression of
EMP1 in HNSCC cells intensified the downregulation of GPX4 by the
ferroptosis activator, RSL3, increasing the levels of ROS and lipid
peroxidation in a time- and concentration-dependent manner,
ultimately inducing the development of ferroptosis. In addition,
EMP1 enhances the sensitivity of the tumor-targeting drug,
gefitinib, and can be used as a biomarker of tumor chemotherapeutic
resistance (96). Overall, EMP1
inhibits the malignant progression of HNSCC by activating
ferroptosis in HNSCC and provides new markers which may be used to
overcome the chemoresistance of tumors, contributing to the
development of future therapeutic agents for HNSCC.
PER1
Period genes are present in almost all types of
human tissues and organs and are involved in regulating a variety
of important physiological and biochemical processes in organisms,
and their abnormal expression is an important factor in the
development of many diseases, including cancer (97). PER1 is one of the core genes of
Period and is involved in regulating important physiological
processes, such as apoptosis, autophagy, DNA damage repair and
ferroptosis (98); its decreased
expression in a variety of cancers, including gastric cancer
(99), colon cancer (100), HNSCC (101) and non-small cell lung cancers
(102) predicts that PER1 may be
a key tumor suppressor gene (103).
In HNSCC, the decreased expression of PER1 is
significantly associated with the TNM stage and poor prognosis of
patients (104). PER1 negatively
correlates with the expression of GPX4, a key regulator of
ferroptosis, as shown in Fig. 2,
which combine to form the PER1/GPX4 negative feedback pathway,
thereby inducing the ferroptosis of cancer cells (105). In summary, the upregulation of
PER1 gene expression can inhibit the proliferation, invasion and
migration of HNSCC, which plays a similar role as RSL3 as a
'ferroptosis activator'; the in-depth study of this mechanism may
provide a novel therapeutic approach for the treatment of
HNSCC.
Cav-1
Caveolin is mainly composed of lipids and proteins,
among which, Cav-1 plays a major biological role (106), functioning as an oncogene in a
variety of tumors, including HNSCC, liver, colon, breast, kidney
and lung cancer (107-109). It plays a critical regulatory
role in substance transport, endothelial infiltration and
tumorigenesis (110). Deng et
al (111) demonstrated that
Cav-1 was also involved in the regulation of ferroptosis. As
demonstrated in Fig. 2, in HNSCC,
Cav-1 is highly expressed and inhibits ferroptosis by upregulating
GPX4 expression and suppressing ROS and lipid peroxidation. Lu
et al (112) found that
after knocking down Cav-1 in HNSCC, GPX4 expression was reduced,
ferroptosis was activated, and cell proliferation, invasion and
migration were all inhibited, suggesting that ferroptosis was
negatively regulated by Cav-1. Overall, in HNSCC, Cav-1 functions
as an inhibitor of ferroptosis and is likely to be a diagnostic
marker of HNSCC (112).
Regulation of ferroptosis through iron
metabolic pathways
Ferritin, the most critical form of iron storage in
humans, consists of two polypeptide chains: The ferritin heavy
chain (FTH1) and the light chain, which are essential for
maintaining iron homeostasis and preventing iron overload, and also
play a role in regulating the tumor microenvironment and immune
metabolism (113). FTH1 is the
major functional subunit of iron storage protein with iron oxidase
activity that effectively reduces Fe2+ toxicity
(114). Moreover, FTH1 is
closely associated with the development of HNSCC, as illustrated in
Fig. 2, where FTH1 expression
levels are upregulated in HNSCC compared to normal tissues
(75), which further inhibits the
Fenton reaction by downregulating the expression of ferrous ions,
thereby inhibiting cellular production of ROS and lipid peroxide
aggregation, and ultimately negatively regulating ferroptosis
(114). The further study of
iron metabolic pathways may aid in the better understanding of the
development of ferroptosis and may provide lead to the development
of novel cancer therapeutics.
6. Therapeutic modalities regulating
ferroptosis in head and neck squamous carcinoma
Drug treatment
Currently, drugs that have been experimentally
validated to modulate ferroptosis in HNSCC include dyclonine and
paclitaxel (PTX), and ferroptosis-based drug studies may alleviate
current resistance to radiotherapy and chemotherapy in HNSCC and
increase the sensitivity of the drugs.
Dyclonine, a covalent inhibitor of the cancer cell
resistance gene, ALDH3A1 (115),
plays a key role in inducing oxidative damage and cell death by
promoting the accumulation of lipid peroxides in GSH-resistant
cancer cells, and can reduce resistance to targeted therapy with
the SLC7A11 inhibitor, sulfasalazine (116).
PTX is a paclitaxel-like substance extracted from
plants that exerts anti-mitotic and apoptosis-inducing effects
(117); it also induces cellular
autophagy (118), downregulates
glutamine degradation-related genes (119), affects glutamine catabolism and
shifts metabolic reprogramming to oxidative phosphorylation, which
in turn regulates the onset of ferroptosis (90,120).
At this stage, the FDA has approved a number of
drugs for clinical use that inhibit the development of
epithelial-derived tumors, such as ovarian, endometrial and
squamous lung cancers through ferroptosis, including GPX4
inhibitors (altretamine), iron ion activators (lapatinib) and
SLC7A11 inhibitors (sorafenib) (121). However, although these drugs
have not yet been used in the clinical treatment of patients with
HNSCC, HNSCC also serves as a tumor of epithelial origin in which
these drugs are likely to play a similar role; thus, further
research is required into the use of these drugs in HNSCC.
Emerging therapies
Currently, the conventional treatment for HNSCC is
surgical resection with drug therapy; however, the, treatment
efficacy is poor. Photodynamic therapy (PDT) is one of the emerging
therapies for HNSCC. With the participation of photosensitizers and
the action of light, the body cells generate reactive oxygen
radicals through electron transfer and energy transfer, and
eventually interact with oxygen molecules. The generation of large
amounts of intracellular ROS leads to lipid peroxidation, causing
damage to the structure and function of cell and organelle
membranes (122,123).
The non-invasive nature of PDT allows organ function
to be preserved in patients with HNSCC. However, PDT is hampered by
hypoxia in the tumor microenvironment (TME) due to high
intracellular oxygen consumption and tumor vascular distortion.
Therefore, increasing oxygen production in the TME may be a new
approach to enhancing the efficacy of PDT. The study by Zhu et
al (124) in HNSCC found
that ferroptosis significantly enhanced the efficacy of PDT and
enhanced the anticancer effect through the generation of ROS and a
sustained supply of O2 through the Fenton reaction
(124). In other words,
ferroptosis offers new hope for overcoming PDT resistance
associated with hypoxia in the treatment of HNSCC.
7. Conclusions and future perspectives
HNSCC is the most common malignant tumors of the
head and neck, with a recurrence and metastasis rate >65% and a
5-year survival rate <50% (125). Although combined surgical
resection and radiotherapy have improved the survival rate of
patients with HNSCC to a certain extent over the past 20 years, in
the majority of patients, first-line drugs used for HNSCC, such as
platinum-based drugs, 5-fluorouracil, polyene paclitaxel and
cetuximab have minimal effects (2). Therefore, the effective treatment of
patients with HNSCC, and the improvement of the quality of life and
prognosis of patients are issues that still need to be addressed.
Several new therapeutic approaches have been developed for HNSCC,
among which PDT is one of the emerging therapies widely used in the
clinical treatment of HNSCC, and its treatment mechanism is
consistent with ferroptosis; the combination of ferroptosis and PDT
may lead to the development of a novel strategy for more effective
HNSCC treatment in the future (124).
Since its discovery in 2012, numerous studies have
demonstrated that ferroptosis plays a critical role as a novel cell
death modality in various diseases (126). Although a number of pathways
have been demonstrated to be involved in ferroptosis, to date, the
mechanisms of ferroptosis in vivo are mainly focused on
three pathways: Iron metabolism, the System Xc−/GSH/GPX4
antioxidant system and lipid metabolism. Among these, the pathway
involved in regulating HNSCC is mainly the System
Xc−/GSH/GPX4 pathway, which has been widely studied in
various types of cancer, such as liver cancer and non-small cell
lung cancer, and drugs targeting ferroptosis have been used in
clinical trials (127).
There are numerous factors in the System
Xc-/GSH/GPX4 pathway that can affect the occurrence of HNSCC, and
in the present review, the factors that regulate ferroptosis in
HNSCC have been described, based on existing studies. Among these,
NRF2 has been most comprehensively studied in ferroptosis and
HNSCC, and a large number of studies have shown that NRF2 exerts a
more pronounced inhibitory effect in HNSCC compared with other
influencing factors, and further clinical trials are required to
verify its role in regulating ferroptosis in HNSCC (128). Likewise, a number of researchers
have explored ncRNAs for factors that can affect HNSCC by
regulating ferroptosis; however, the research is not yet mature and
further investigations are warranted (129).
At this stage, the FDA has approved a number of
drugs for clinical use that inhibit the development of
epithelial-derived tumors, such as ovarian, endometrial and
squamous lung cancers by targeting ferroptosis (130,131). While these factors that target
ferroptosis have also been shown to be effective in in vitro
studies on HNSCC, all of these studies need to be explored in more
depth with regard to their effects on the development and
progression of HNSCC to demonstrate whether they can also be
applied to the clinical treatment of HNSCC. However, the emergence
of these targeted agents is certainly a glimmer of hope for the
current dilemma facing HNSCC.
Overall, ferroptosis studies help to address the
major issues of HNSCC and provide novel therapeutic targets and
strategies for the diagnosis and clinical treatment of HNSCC.
Availability of data and materials
Not applicable.
Authors' contributions
MY drafted the manuscript, and prepared the figures
and tables. RG and XC participated in the literature search and in
the analysis of the data to be included in the review. GS and FZ
edited and revised the manuscript. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 31772551).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roh JL, Kim EH, Jang HJ, Park JY and Shin
D: Induction of ferroptotic cell death for overcoming cisplatin
resistance of head and neck cancer. Cancer Lett. 381:96–103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Comish PB, Tang D and Kang R:
Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol.
9:6371622021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masaldan S, Belaidi AA, Ayton S and Bush
AI: Cellular senescence and iron dyshomeostasis in Alzheimer's
disease. Pharmaceuticals (Basel). 12:932019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan X, Li X, Yang X, Yan J, Shi P, Ba L,
Cao Y and Wang P: The neuroprotective effects of carvacrol on
ischemia/reperfusion-induced hippocampal neuronal impairment by
ferroptosis mitigation. Life Sci. 235:1167952019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv Z, Han J, Li J, Guo H, Fei Y, Sun Z,
Dong J, Wang M, Fan C, Li W, et al: Single cell RNA-seq analysis
identifies ferroptotic chondrocyte cluster and reveals TRPV1 as an
anti-ferroptotic target in osteoarthritis. EBioMedicine.
84:1042582022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stockwell BR, Jiang X and Gu W: Emerging
mechanisms and disease relevance of ferroptosis. Trends Cell Biol.
30:478–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang W and Ferrara N: Iron metabolism in
the tumor microenvironment: Contributions of innate immune cells.
Front Immunol. 11:6268122021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Z, Song J, Yung BC, Zhou Z, Wu A and
Chen X: Emerging strategies of cancer therapy based on ferroptosis.
Adv Mater. 30:e17040072018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YJ, Liu XY, Xing L, Wan X, Chang X and
Jiang HL: Fenton reaction-independent ferroptosis therapy via
glutathione and iron redox couple sequentially triggered lipid
peroxide generator. Biomaterials. 241:1199112020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manz DH, Blanchette NL, Paul BT, Torti FM
and Torti SV: Iron and cancer: Recent insights. Ann N Y Acad Sci.
1368:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galaris D, Barbouti A and Pantopoulos K:
Iron homeostasis and oxidative stress: An intimate relationship.
Biochim Biophys Acta Mol Cell Res. 1866:1185352019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Lin Y, Wang M, Yuan K, Wang Q, Mu
P, Du J, Yu Z, Yang S, Huang K, et al: Targeting ferroptosis
suppresses osteocyte glucolipotoxicity and alleviates diabetic
osteoporosis. Bone Res. 10:262022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar :
|
|
22
|
Tan Y, Huang Y, Mei R, Mao F, Yang D, Liu
J, Xu W, Qian H and Yan Y: HucMSC-derived exosomes delivered BECN1
induces ferroptosis of hepatic stellate cells via regulating the
xCT/GPX4 axis. Cell Death Dis. 13:3192022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maiorino M, Conrad M and Ursini F: GPx4,
lipid peroxidation, and cell death: Discoveries, rediscoveries, and
open issues. Antioxid Redox Signal. 29:61–74. 2018. View Article : Google Scholar
|
|
24
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar
|
|
25
|
Luo C, Sun J, Liu D, Sun B, Miao L,
Musetti S, Li J, Han X, Du Y, Li L, et al: Self-assembled redox
dual-responsive prodrug-nano-system formed by single
thioether-bridged paclitaxel-fatty acid conjugate for cancer
chemotherapy. Nano Lett. 16:5401–5408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: Novel targets for anticancer therapy.
J Cell Physiol. 231:2570–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar :
|
|
28
|
Lee JY, Kim WK, Bae KH, Lee SC and Lee EW:
Lipid metabolism and ferroptosis. Biology (Basel).
10:1842021.PubMed/NCBI
|
|
29
|
Lee H, Zandkarimi F, Zhang Y, Meena JK,
Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al:
Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat
Cell Biol. 22:225–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
31
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar
|
|
32
|
Gan B: ACSL4, PUFA, and ferroptosis: New
arsenal in anti-tumor immunity. Signal Transduct Target Ther.
7:1282022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hung CC, Chien CY, Chu PY, Wu YJ, Lin CS,
Huang CJ, Chan LP, Wang YY, Yuan SF, Hour TC and Chen JY:
Differential resistance to platinum-based drugs and 5-fluorouracil
in p22phox-overexpressing oral squamous cell carcinoma:
Implications of alternative treatment strategies. Head Neck.
39:1621–1630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kallunki T, Olsen OD and Jäättelä M:
Cancer-associated lysosomal changes: Friends or foes? Oncogene.
32:1995–2004. 2013. View Article : Google Scholar
|
|
35
|
Pan ST, Qin Y, Zhou ZW, He ZX, Zhang X,
Yang T, Yang YX, Wang D, Qiu JX and Zhou SF: Plumbagin induces G2/M
arrest, apoptosis, and autophagy via p38 MAPK- and
PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell
carcinoma cells. Drug Des Devel Ther. 9:1601–1626. 2015.PubMed/NCBI
|
|
36
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu W, Chen Y, Putluri N, Coarfa C,
Robertson MJ, Putluri V, Stossi F, Dubrulle J, Mancini MA, Pang JC,
et al: Acquisition of cisplatin resistance shifts head and neck
squamous cell carcinoma metabolism toward neutralization of
oxidative stress. Cancers (Basel). 12:16702020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han L, Li L and Wu G: Induction of
ferroptosis by carnosic acid-mediated inactivation of Nrf2/HO-1
potentiates cisplatin responsiveness in OSCC cells. Mol Cell
Probes. 64:1018212022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng H and Stockwell BR: Unsolved
mysteries: How does lipid peroxidation cause ferroptosis? PLoS
Biol. 16:e20062032018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu K, Li K, Lv J, Feng J, Chen J, Wu H,
Cheng F, Jiang W, Wang J, Pei H, et al: Suppression of the
SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant
lung adenocarcinoma. J Clin Invest. 130:1752–1766. 2020. View Article : Google Scholar :
|
|
42
|
Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Z, Zhang H, Lian M, Yue C, Dong G, Jin
Y, Li R, Wan H, Wang R, Wang Y, et al: SLC7A11, a component of
cysteine/glutamate transporter, is a novel biomarker for the
diagnosis and prognosis in laryngeal squamous cell carcinoma. Oncol
Rep. 38:3019–3029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hémon A, Louandre C, Lailler C, Godin C,
Bottelin M, Morel V, François C, Galmiche A and Saidak Z: SLC7A11
as a biomarker and therapeutic target in HPV-positive head and neck
squamous cell carcinoma. Biochem Biophys Res Commun. 533:1083–1087.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jyotsana N, Ta KT and DelGiorno KE: The
role of cystine/glutamate antiporter SLC7A11/xCT in the
pathophysiology of cancer. Front Oncol. 12:8584622022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Slaby O, Laga R and Sedlacek O:
Therapeutic targeting of non-coding RNAs in cancer. Biochem J.
474:4219–4251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie B and Guo Y: Molecular mechanism of
cell ferroptosis and research progress in regulation of ferroptosis
by noncoding RNAs in tumor cells. Cell Death Discov. 7:1012021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo M, Wu L, Zhang K, Wang H, Zhang T,
Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, et al: miR-137
regulates ferroptosis by targeting glutamine transporter SLC1A5 in
melanoma. Cell Death Differ. 25:1457–1472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang K, Wu L, Zhang P, Luo M, Du J, Gao
T, O'Connell D, Wang G, Wang H and Yang Y: miR-9 regulates
ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in
melanoma. Mol Carcinog. 57:1566–1576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Quirico L, Orso F, Cucinelli S, Paradzik
M, Natalini D, Centonze G, Dalmasso A, La Vecchia S, Coco M,
Audrito V, et al: miRNA-guided reprogramming of glucose and
glutamine metabolism and its impact on cell adhesion/migration
during solid tumor progression. Cell Mol Life Sci. 79:2162022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Wang B, Ren H and Chen W: miR-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun.
509:241–218. 2019. View Article : Google Scholar
|
|
52
|
Tomita K, Fukumoto M, Itoh K, Kuwahara Y,
Igarashi K, Nagasawa T, Suzuki M, Kurimasa A and Sato T: MiR-7-5p
is a key factor that controls radioresistance via intracellular
Fe2+ content in clinically relevant radioresistant cells. Biochem
Biophys Res Commun. 518:712–718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tomita K, Kuwahara Y, Takashi Y, Igarashi
K, Nagasawa T, Nabika H, Kurimasa A, Fukumoto M, Nishitani Y and
Sato T: Clinically relevant radioresistant cells exhibit resistance
to H2O2 by decreasing internal
H2O2 and lipid peroxidation. Tumour Biol.
40:10104283187992502018. View Article : Google Scholar
|
|
54
|
Amaral AJ, Andrade J, Foxall RB, Matoso P,
Matos AM, Soares RS, Rocha C, Ramos CG, Tendeiro R, Serra-Caetano
A, et al: miRNA profiling of human naive CD4 T cells links
miR-34c-5p to cell activation and HIV replication. EMBO J.
36:346–360. 2017. View Article : Google Scholar
|
|
55
|
Zhang B, Li Y, Hou D, Shi Q, Yang S and Li
Q: MicroRNA-375 inhibits growth and enhances radiosensitivity in
oral squamous cell carcinoma by targeting insulin like growth
factor 1 receptor. Cell Physiol Biochem. 42:2105–2117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang K, Jin J, Ma T and Zhai H: MiR-139-5p
inhibits the tumorigenesis and progression of oral squamous
carcinoma cells by targeting HOXA9. J Cell Mol Med. 21:3730–3740.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen Y, Sun C, Zhao B, Guo H, Li J, Xia Y,
Liu M, Piao S and Saiyin W: miR-34c-5p mediates the cellular
malignant behaviors of oral squamous cell carcinoma through
targeted binding of TRIM29. Ann Transl Med. 9:15372021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun K, Ren W, Li S, Zheng J, Huang Y, Zhi
K and Gao L: MiR-34c-3p upregulates erastin-induced ferroptosis to
inhibit proliferation in oral squamous cell carcinomas by targeting
SLC7A11. Pathol Res Pract. 231:1537782022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Balihodzic A, Prinz F, Dengler MA, Calin
GA, Jost PJ and Pichler M: Non-coding RNAs and ferroptosis:
Potential implications for cancer therapy. Cell Death Differ.
29:1094–1106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang X, Wang L, Li H, Zhang L, Zheng X
and Cheng W: Crosstalk between noncoding RNAs and ferroptosis: New
dawn for overcoming cancer progression. Cell Death Dis. 11:5802020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang J, Cao XH, Luan KF and Huang YD:
Circular RNA FNDC3B protects oral squamous cell carcinoma cells
from ferroptosis and contributes to the malignant progression by
regulating miR-520d-5p/SLC7A11 axis. Front Oncol. 11:6727242021.
View Article : Google Scholar
|
|
63
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song X and Long D: Nrf2 and ferroptosis: A
new research direction for neurodegenerative diseases. Front
Neurosci. 14:2672020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Q, Qu H, Chen Y, Luo X, Chen C, Xiao
B, Ding X, Zhao P, Lu Y, Chen AF and Yu Y: Atorvastatin induces
mitochondria-dependent ferroptosis via the modulation of
Nrf2-xCT/GPx4 axis. Front Cell Dev Biol. 10:8060812022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jelic MD, Mandic AD, Maricic SM and
Srdjenovic BU: Oxidative stress and its role in cancer. J Cancer
Res Ther. 17:22–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu
C and Li B: SLC7A11 regulated by NRF2 modulates esophageal squamous
cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl
Med. 19:3672021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X,
Jiang L and Ye L: Cetuximab promotes RSL3-induced ferroptosis by
suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant
colorectal cancer. Cell Death Dis. 12:10792021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sánchez-Ortega M, Carrera AC and Garrido
A: Role of NRF2 in lung cancer. Cells. 10:18792021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Farkhondeh T, Pourbagher-Shahri AM,
Azimi-Nezhad M, Forouzanfar F, Brockmueller A, Ashrafizadeh M,
Talebi M, Shakibaei M and Samarghandian S: Roles of Nrf2 in gastric
cancer: Targeting for therapeutic strategies. Molecules.
26:31572021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tossetta G, Fantone S, Montanari E,
Marzioni D and Goteri G: Role of NRF2 in ovarian cancer.
Antioxidants (Basel). 11:6632022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang XJ, Sun Z, Villeneuve NF, Zhang S,
Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, et al: Nrf2
enhances resistance of cancer cells to chemotherapeutic drugs, the
dark side of Nrf2. Carcinogenesis. 29:1235–1243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ying J, Qiu X, Lu Y and Zhang M: SOCS1 and
its potential clinical role in tumor. Pathol Oncol Res.
25:1295–1301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu ZW, Wen YH, Ma RQ, Chen L, Zeng XL, Wen
WP and Sun W: Ferroptosis driver SOCS1 and suppressor FTH1
independently correlate with M1 and M2 macrophage infiltration in
head and neck squamous cell carcinoma. Front Cell Dev Biol.
9:7277622021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee CH, Chang JS, Syu SH, Wong TS, Chan
JY, Tang YC, Yang ZP, Yang WC, Chen CT, Lu SC, et al: IL-1β
promotes malignant transformation and tumor aggressiveness in oral
cancer. J Cell Physiol. 230:875–884. 2015. View Article : Google Scholar
|
|
77
|
Zhao J, Dar HH, Deng Y, St Croix CM, Li Z,
Minami Y, Shrivastava IH, Tyurina YY, Etling E, Rosenbaum JC, et
al: PEBP1 acts as a rheostat between prosurvival autophagy and
ferroptotic death in asthmatic epithelial cells. Proc Natl Acad Sci
USA. 117:14376–14385. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li M, Jin S, Zhang Z, Ma H and Yang X:
Interleukin-6 facilitates tumor progression by inducing ferroptosis
resistance in head and neck squamous cell carcinoma. Cancer Lett.
527:28–40. 2022. View Article : Google Scholar
|
|
79
|
Jin S, Yang X, Li J, Yang W, Ma H and
Zhang Z: p53-targeted lincRNA-p21 acts as a tumor suppressor by
inhibiting JAK2/STAT3 signaling pathways in head and neck squamous
cell carcinoma. Mol Cancer. 18:382019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao
X, Wang M, Chen Y and Zhang Q: Identification of a small molecule
as inducer of ferroptosis and apoptosis through ubiquitination of
GPX4 in triple negative breast cancer cells. J Hematol Oncol.
14:192021. View Article : Google Scholar
|
|
81
|
Zhao L, Peng Y, He S, Li R, Wang Z, Huang
J, Lei X, Li G and Ma Q: Apatinib induced ferroptosis by lipid
peroxidation in gastric cancer. Gastric Cancer. 24:642–654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu CC, Li HH, Lin JH, Chiang MC, Hsu TW,
Li AF, Yen DH, Hsu HS and Hung SC: Esophageal cancer stem-like
cells resist ferroptosis-induced cell death by active Hsp27-GPX4
pathway. Biomolecules. 12:482021. View Article : Google Scholar
|
|
83
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chatterjee R and Chatterjee J: ROS and
oncogenesis with special reference to EMT and stemness. Eur J Cell
Biol. 99:1510732020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheng FZY, Wang X, Dou J and Wu Z:
Research progress on the role and mechanism of GPX4 in ferroptosis.
Mod Oncol. 29:1254–1258. 2021.
|
|
86
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole
D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL,
et al: Drug-tolerant persister cancer cells are vulnerable to GPX4
inhibition. Nature. 551:247–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee JR, Roh JL, Lee SM, Park Y, Cho KJ,
Choi SH, Nam SY and Kim SY: Overexpression of glutathione
peroxidase 1 predicts poor prognosis in oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 143:2257–2265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fukuda M, Ogasawara Y, Hayashi H, Okuyama
A, Shiono J, Inoue K and Sakashita H: Down-regulation of
glutathione peroxidase 4 in oral cancer inhibits tumor growth
through SREBP1 signaling. Anticancer Res. 41:1785–1792. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ye J, Jiang X, Dong Z, Hu S and Xiao M:
Low-concentration PTX and RSL3 inhibits tumor cell growth
synergistically by inducing ferroptosis in mutant p53
hypopharyngeal squamous carcinoma. Cancer Manag Res. 11:9783–9792.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ahmat Amin MKB, Shimizu A and Ogita H: The
pivotal roles of the epithelial membrane protein family in cancer
invasiveness and metastasis. Cancers (Basel). 11:16202019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu Y, Ding Y, Nie Y and Yang M: EMP1
promotes the proliferation and invasion of ovarian cancer cells
through activating the MAPK pathway. Onco Targets Ther.
13:2047–2055. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Durgan J, Tao G, Walters MS, Florey O,
Schmidt A, Arbelaez V, Rosen N, Crystal RG and Hall A: SOS1 and Ras
regulate epithelial tight junction formation in the human airway
through EMP1. EMBO Rep. 16:87–96. 2015. View Article : Google Scholar :
|
|
94
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: EMP1 regulates caspase-9 and VEGFC expression and suppresses
prostate cancer cell proliferation and invasion. Tumour Biol.
35:3455–3462. 2014. View Article : Google Scholar
|
|
95
|
Wang Y, Zhang L, Yao C, Ma Y and Liu Y:
Epithelial membrane protein 1 promotes sensitivity to RSL3-induced
ferroptosis and intensifies gefitinib resistance in head and neck
cancer. Oxid Med Cell Longev. 2022:47506712022.PubMed/NCBI
|
|
96
|
van Zandwijk N: Tolerability of gefitinib
in patients receiving treatment in everyday clinical practice. Br J
Cancer. 89(Suppl 2): S9–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu Y, Hao J, Yuan G, Wei M, Bu Y, Jin T
and Ma L: PER1 as a tumor suppressor attenuated in the malignant
phenotypes of breast cancer cells. Int J Gen Med. 14:7077–7087.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chakrabarti S and Michor F: Circadian
clock effects on cellular proliferation: Insights from theory and
experiments. Curr Opin Cell Biol. 67:17–26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhao H, Zeng ZL, Yang J, Jin Y, Qiu MZ, Hu
XY, Han J, Liu KY, Liao JW and Zou QF: Prognostic relevance of
Period1 (Per1) and Period2 (Per2) expression in human gastric
cancer. Int J Clin Exp Pathol. 7:619–630. 2014.PubMed/NCBI
|
|
100
|
Krugluger W, Brandstaetter A, Kállay E,
Schueller J, Krexner E, Kriwanek S, Bonner E and Cross HS:
Regulation of genes of the circadian clock in human colon cancer:
reduced period-1 and dihydropyrimidine dehydrogenase transcription
correlates in high-grade tumors. Cancer Res. 67:7917–7922. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hsu CM, Lin SF, Lu CT, Lin PM and Yang MY:
Altered expression of circadian clock genes in head and neck
squamous cell carcinoma. Tumour Biol. 33:149–155. 2012. View Article : Google Scholar
|
|
102
|
Liu B, Xu K, Jiang Y and Li X: Aberrant
expression of Per1, Per2 and Per3 and their prognostic relevance in
non-small cell lung cancer. Int J Clin Exp Pathol. 7:7863–7871.
2014.
|
|
103
|
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D
and Koeffler HP: The circadian gene per1 plays an important role in
cell growth and DNA damage control in human cancer cells. Mol Cell.
22:375–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang G, Yang Y, Tang H and Yang K: Loss of
the clock gene Per1 promotes oral squamous cell carcinoma
progression via the AKT/mTOR pathway. Cancer Sci. 111:1542–1554.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yang Y, Tang H, Zheng J and Yang K: The
PER1/HIF-1alpha negative feedback loop promotes ferroptosis and
inhibits tumor progression in oral squamous cell carcinoma. Transl
Oncol. 18:1013602022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Chen D and Che G: Value of caveolin-1 in
cancer progression and prognosis: Emphasis on cancer-associated
fibroblasts, human cancer cells and mechanism of caveolin-1
expression (Review). Oncol Lett. 8:1409–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang G, Goltsov AA, Ren C, Kurosaka S,
Edamura K, Logothetis R, DeMayo FJ, Troncoso P, Blando J,
DiGiovanni J and Thompson TC: Caveolin-1 upregulation contributes
to c-Myc-induced high-grade prostatic intraepithelial neoplasia and
prostate cancer. Mol Cancer Res. 10:218–229. 2012. View Article : Google Scholar
|
|
108
|
Sun J, Lu Y, Yu C, Xu T, Nie G, Miao B and
Zhang X: Involvement of the TGF-β1 pathway in caveolin-1-associated
regulation of head and neck tumor cell metastasis. Oncol Lett.
19:1298–1304. 2020.PubMed/NCBI
|
|
109
|
Nwosu ZC, Ebert MP, Dooley S and Meyer C:
Caveolin-1 in the regulation of cell metabolism: A cancer
perspective. Mol Cancer. 15:712016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Vered M, Lehtonen M, Hotakainen L, Pirilä
E, Teppo S, Nyberg P, Sormunen R, Zlotogorski-Hurvitz A, Salo T and
Dayan D: Caveolin-1 accumulation in the tongue cancer tumor
microenvironment is significantly associated with poor prognosis:
An in-vivo and in-vitro study. BMC Cancer. 15:252015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Deng G, Li Y, Ma S, Gao Z, Zeng T, Chen L,
Ye H, Yang M, Shi H, Yao X, et al: Caveolin-1 dictates ferroptosis
in the execution of acute immune-mediated hepatic damage by
attenuating nitrogen stress. Free Radic Biol Med. 148:151–161.
2020. View Article : Google Scholar
|
|
112
|
Lu T, Zhang Z, Pan X, Zhang J, Wang X,
Wang M, Li H, Yan M and Chen W: Caveolin-1 promotes cancer
progression via inhibiting ferroptosis in head and neck squamous
cell carcinoma. J Oral Pathol Med. 51:52–62. 2022. View Article : Google Scholar
|
|
113
|
Hu ZW, Chen L, Ma RQ, Wei FQ, Wen YH, Zeng
XL, Sun W and Wen WP: Comprehensive analysis of ferritin subunits
expression and positive correlations with tumor-associated
macrophages and T regulatory cells infiltration in most solid
tumors. Aging (Albany NY). 13:11491–11506. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Salatino A, Aversa I, Battaglia AM, Sacco
A, Di Vito A, Santamaria G, Chirillo R, Veltri P, Tradigo G, Di
Cello A, et al: H-ferritin affects cisplatin-induced cytotoxicity
in ovarian cancer cells through the modulation of ROS. Oxid Med
Cell Longev. 2019:34612512019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Black W, Chen Y, Matsumoto A, Thompson DC,
Lassen N, Pappa A and Vasiliou V: Molecular mechanisms of
ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal.
Free Radic Biol Med. 52:1937–1944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Okazaki S, Shintani S, Hirata Y, Suina K,
Semba T, Yamasaki J, Umene K, Ishikawa M, Saya H and Nagano O:
Synthetic lethality of the ALDH3A1 inhibitor dyclonine and xCT
inhibitors in glutathione deficiency-resistant cancer cells.
Oncotarget. 9:33832–33843. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yardley DA: Taxanes in the elderly patient
with metastatic breast cancer. Breast Cancer (Dove Med Press).
7:293–301. 2015.PubMed/NCBI
|
|
118
|
Choi YH and Yoo YH: Taxol-induced growth
arrest and apoptosis is associated with the upregulation of the Cdk
inhibitor, p21WAF1/CIP1, in human breast cancer cells. Oncol Rep.
28:2163–2169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lv C, Qu H, Zhu W, Xu K, Xu A, Jia B, Qing
Y, Li H, Wei HJ and Zhao HY: Low-dose paclitaxel inhibits tumor
cell growth by regulating glutaminolysis in colorectal carcinoma
cells. Front Pharmacol. 8:2442017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shen YA, Li WH, Chen PH, He CL, Chang YH
and Chuang CM: Intraperitoneal delivery of a novel
liposome-encapsulated paclitaxel redirects metabolic reprogramming
and effectively inhibits cancer stem cells in
Taxol(®)-resistant ovarian cancer. Am J Transl Res.
7:841–855. 2015.
|
|
121
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lin L, Song C, Wei Z, Zou H, Han S, Cao Z,
Zhang X, Zhang G, Ran J, Cai Y and Han W: Multifunctional
photodynamic/photothermal nano-agents for the treatment of oral
leukoplakia. J Nanobiotechnology. 20:1062022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gurudath S, Ganapathy K, D S, Pai A,
Ballal S and Ml A: Estimation of superoxide dismutase and
glutathione peroxidase in oral submucous fibrosis, oral leukoplakia
and oral cancer-a comparative study. Asian Pac J Cancer Prev.
13:4409–4412. 2012. View Article : Google Scholar
|
|
124
|
Zhu T, Shi L, Yu C, Dong Y, Qiu F, Shen L,
Qian Q, Zhou G and Zhu X: Ferroptosis promotes photodynamic
therapy: Supramolecular photosensitizer-inducer nanodrug for
enhanced cancer treatment. Theranostics. 9:3293–3307. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Liang F, Wang R, Du Q and Zhu S: An
epithelial-mesenchymal transition hallmark gene-based risk score
system in head and neck squamous-cell carcinoma. Int J Gen Med.
14:4219–4227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System Xc −/GSH/GPX4 axis: An
important antioxidant system for the ferroptosis in drug-resistant
solid tumor therapy. Front Pharmacol. 13:9102922022. View Article : Google Scholar
|
|
128
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tang Y, Li C, Zhang YJ and Wu ZH:
Ferroptosis-related long non-coding RNA signature predicts the
prognosis of head and neck squamous cell carcinoma. Int J Biol Sci.
17:702–711. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
















