The tendon is composed of longitudinally arranged
collagen fibers and a scattered distribution of spindle-shaped
tendon cells. Its primary function is to transmit the force
generated by contraction of the muscles and to drive the movement
of the bones (1). It is
hypothesized that when the tendon is overused for a long time,
bears a large load or is stretched repeatedly, many pathological
changes will occur in the tendon including cell and extracellular
matrix (ECM) lesions, increased proteoglycan and damage to the
collagen structure (2), which
leads to tendon injury. In addition, other factors may also lead to
tendon injury, including age, incorrectly performed exercise,
previous injury, weight and medication (3). In recent years (4), injured athletes have accounted for
the majority of injured people. Numerous athletes suffer from
chronic tendinopathy due to overwork, especially those who play
basketball, football and volleyball, as well as those who perform
in the high jump. The injured areas include the Achilles and
patellar tendon, rotator cuff and the tendons around the elbow
joint, all of which result in inconvenience to the lives of those
affected.
In recent years, studies have shown that MSCs are a
promising treatment method. Many clinical trials have demonstrated
that MSCs have good therapeutic effects (7-9).
Firstly, MSCs differentiate into targeted cell types, and under
specific induction conditions in vivo or in vitro,
they can differentiate into tendon cells to stimulate tendon tissue
regeneration. Secondly, they have a paracrine effect and can
secrete cytokines and growth factors into neighboring cells,
thereby promoting vascularization and cell proliferation in damaged
tissue and helping to repair the damaged area (10). MSCs also have immunomodulatory
properties and decrease the inflammatory response of damaged tissue
(11). Additionally, MSCs have a
wide range of sources; they can be isolated and prepared from bone
marrow, adipose tissue, placenta, umbilical cord and other tissues.
Overall, MSCs have numerous advantages over other conservative
treatments such as NSAIDs, low level lasers, including strong
multiplication capability, safety, economy and efficiency. The
present review summarized the mechanisms, progress, and challenges
of MSCs in the treatment of tendon injury based on published
literature and provides support for future clinical practice and
research.
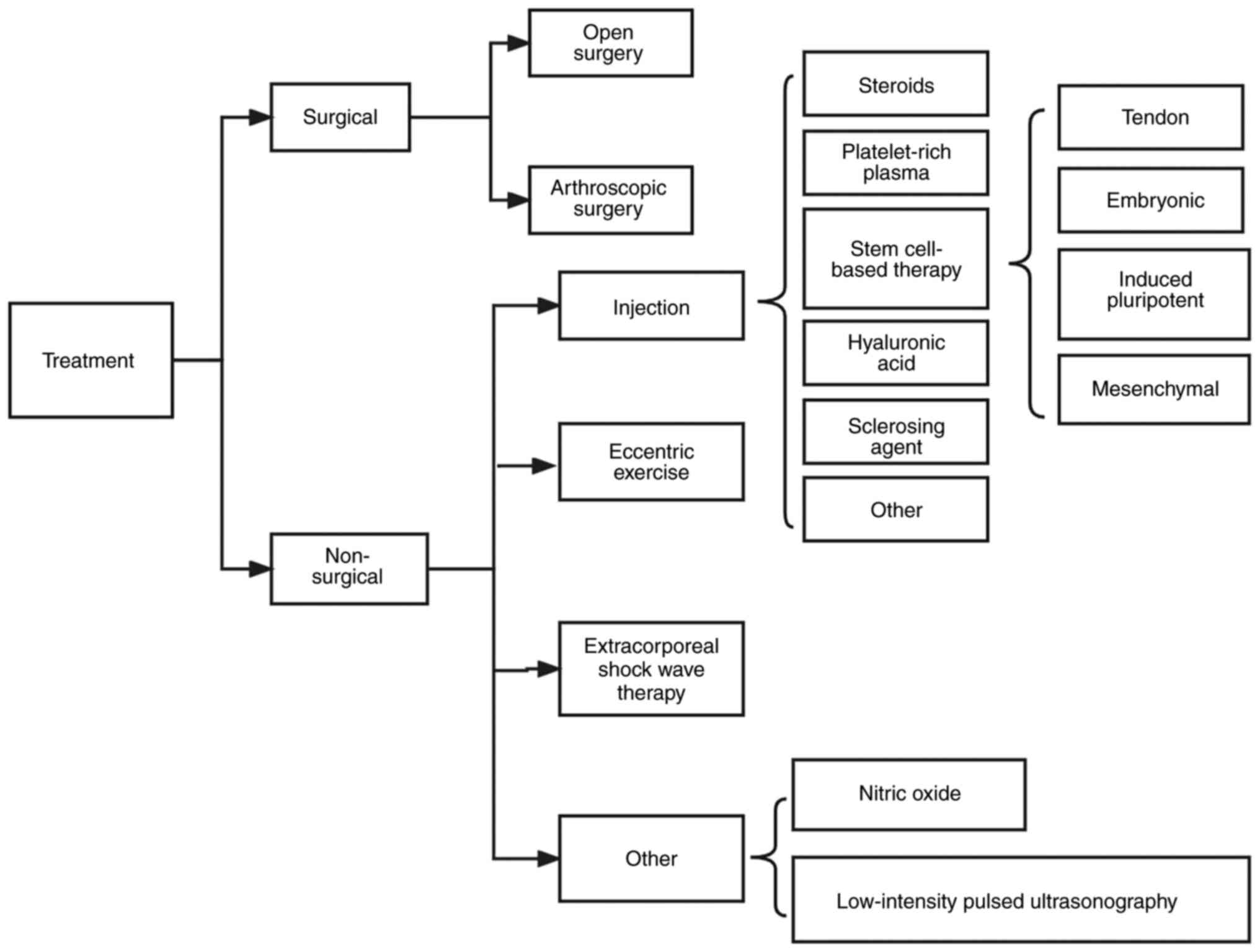
Cell-based tissue regeneration therapies are
attractive and well-explored therapeutic approaches, especially in
the application of tendon repair (12,13). The most discussed cell-based
therapies include MSCs (from sources such as bone marrow, adipose,
umbilical cord) and tendon, embryonic and induced pluripotent SCs
(iPSCs) (14-17). Tables I and II summarize the properties of MSCs and
other cellular therapies in tendon repair and their
characteristics.
MSCs are widely available, relatively simple to
obtain and can be injected directly or following processing,
purification and amplification. Several studies have shown that
MSCs in the tendon are actively involved in the tendon repair
process (15,18). They migrate to the injury site
following tendon injury and secrete growth factors and other
soluble cytokines that induce cell proliferation and regulate
signaling, in addition to enhancing the tendon-forming properties
of tendon stem/progenitor cells (TSPCs), thereby promoting tendon
repair (19). Studies have showed
that the efficiency of differentiation of MSCs into tendon cells
could be better improved by injecting growth factors such as bone
morphogenetic protein-12 (BMP-12), growth/differentiation factor-5
(GDF-5) and TGF-β compared to treatment with MSCs alone (20,21). There are numerous studies on the
use of exogenous MSCs of different sources in tendon repair
following injury through intravenous or topical wound injection,
bioengineered scaffolds and gels (22-24). For example, Smith et al
(25) injected bone marrow MSCs
(BMSCs) into racehorses with tendon injury; after 6 months of
treatment, the tendons of racehorses showed enhanced biomechanics,
morphology and normalization of extracellular matrix (ECM)
component of the tendon (25). In
particular, adipose-derived MSCs (ADSCs) show advantages over other
sources of BMSC in terms of decreased donor morbidity and avoiding
ethical concerns. Therefore, it has a high value in the treatment
of tendon injuries (26).
The tendon also contains a small population of
resident cells that maintain homeostasis of tendon growth and
repair (27). Similar to other
SCs, these TSPCs have the capacity to undergo self-renewal and
multidirectional differentiation. Numerous studies have exploited
this feature to promote the self-proliferation of TSPCs and induce
differentiation to tendon cells by injecting growth factors such as
TGF-β and basic fibroblast growth factor (bFGF) in vivo or
creating hypoxic states in vitro. Subsequently, TSPCs
upregulate IL-10 and TIMP-3 through the JNK/STAT signaling pathway,
thus playing a regulatory role in inflammation and tendon
remodeling (16,28). However, alterations in the tendon
microenvironment following injury may lead to misdifferentiation of
TSPCs to chondrocytes, osteoblasts and adipocytes, resulting in
failure of tendon healing (29).
There are many triggers that lead to misdifferentiation, including
age-associated cellular aging, mechanical stretch stimulation
>8% and some inflammatory factors such as prostaglandin E2
(PGE2) (30,31). Understanding the factors that
induce TSPC (mis)differentiation may facilitate use of TSPCs in the
treatment of tendon injuries.
Embryonic SCs (ESCs) are isolated from early embryos
(before protointestinal embryonic stage) that have properties of
unlimited proliferation, self-renewal and multidirectional
differentiation (32). ESCs can
be induced to differentiate into almost all cell types, both in
vitro and in vivo. Therefore, they have potential in
regenerative medicine (17). It
has been shown that human ESCs can be induced to differentiate into
tendon-like cells by the addition of exogenous BMP-12, GDF-7 and
BMP-13 (33). It has also been
shown that tendon injury sites treated with ESCs recover better and
collagen fibers can be restored to a more normal linear fiber
pattern compared to other cellular therapies (33). However, there are concerns
regarding the use of ESCs. First, ESC isolation destroys the
embryo, which may be considered a violation of bioethics. Despite
the potential use of ESCs in both basic research and clinical
applications, research on ESCs and their applications is prohibited
in some countries, such as the United States and some European
countries where religious organizations are prevalent (14). ESCs can theoretically be induced
into various types of somatic cells for tissue regeneration.
Therefore, there is a risk of teratoma formation following
application of ESCs for treatment (34). Teratomas consist of three
embryonic germ layers, which are due to residual undifferentiated
cells in the transplanted population. Therefore, it is necessary to
remove residual undifferentiated stem cells from ESCs before
application (35). Similar to
ESCs, iPSCs) can be prepared from the patient's own somatic cells,
thus avoiding immune rejection (36). Compared with ESCs, iPSCs can be
obtained from more convenient sources, such as fibroblasts and
hepatocytes, and do not involve ethical concerns. In horses, the
application of iPSCs promotes tendon tissue regeneration and
significantly decreases the frequency of re-injury (37). However, since iPSCs have the
ability of multidirectional differentiation, there is also a risk
of teratoma formation (38). In
addition, the time and cost required to prepare iPSCs may prevent
them from becoming a therapeutic option.
Tendons are dense tissues that connect muscle and
bones. Their unique composition and structure give them appropriate
mechanical properties (39).
Therefore, understanding of the association between the
composition, structure and mechanical properties of normal tendons
can help to prevent tendon injury and select the most appropriate
method for treatment.
In terms of ultrastructure, tendons are hierarchical
structures with a regular arrangement of collagen fibers (40). This hierarchical structure
provides ideal load-bearing and tensile force transmission
properties (41). The smallest
structural unit of the tendon is the fibril, with a diameter of
20-500 nm, consisting of rod-shaped collagen molecules (42). Electron microscopy in the absence
of load shows fibrils become 'crimped'. This is thought to be due
to a non-linear change in the strain-stress curve caused by small
tensile forces at low strains. Studies have shown that this change
can be effective for cushioning and shock absorption in tendons
(43,44). The fibrils are cross-linked to
form a stable structure, referred to as a collagen fiber. Multiple
collagen fibers reassemble to form the tendon fascicle, which is
the largest structural unit of the tendon. The tendon fascicle is a
tubular-like structure 150-500 µm in diameter, aligned
parallel to the long axis of the tendon. Each fascicle is
surrounded by connective tissue called the endotenon, thus forming
a complete tendon structural unit (45). The tendon is covered with a layer
of connective tissue attached to the endotenon called the epitenon.
The epitenon effectively reduces friction between the tendon and
adjacent tissue (46). In
addition, there are nerves and blood and lymphatic vessels on the
endotenon and epitenon, which serve a key role in development of
the tendon. A layer of loose connective tissue, called paratenon,
surrounds the tendon away from the joint area. During joint
movement, the paratenon facilitates the smooth gliding of the
tendon and completion of the movement (47). Normal tendons consist of collagen,
water, proteoglycans, glycoproteins, cells and other components
(48). Type I collagen is the
primary component of tendons and is also the main element in
connective tissue responsible for transmitting force (49). Type III collagen is less abundant
in normal tendons (50). Due to
its rapid cross-linking properties, type III collagen increases
rapidly following tendon injury, allowing for rapid repair in the
injured area and tendon healing (51). In addition to containing high
amounts of type I and type III collagen, collagen fibers expressed
at lower levels, including types V, VI, XI, XII and XIV, serve key
roles in regulating the biological properties of fibers in terms of
diameter, number and density (52-54). Tendons also contain a large amount
of water; studies have shown that the higher the water content, the
lower the stiffness of tendons (55,56).
In addition to collagen, non-collagenous
glycoproteins, proteoglycans, elastin, and other components of the
ECM also play important roles in the growth and development of
tendons (57). Non-collagenous
glycoproteins mediate signaling between TSPCs and muscle, thereby
promoting maturation of the tendon-muscle junction and maintaining
tendon stability (58).
Proteoglycan is an important component of extracellular matrix
(59); it mainly regulates the
diameter of linear and lateral fibers during the later stage of
tendon development and cooperates with growth factors to regulate
cell proliferation, thus promoting collagen production (60,61). Glycoproteins are part of the ECM;
cartilage oligomeric matrix protein (COMP) is the most abundant
glycoprotein in tendons. The amount of COMP is positively
associated with tensile stress and stiffness (62). Tenascin-C is glycoprotein
expressed at low levels that is primarily present in locations
where the tendon is subjected to higher load (63). Tenascin-C maintains mechanical
properties of the ECM by interacting with collagen fibers (64). Different numbers of elastic fibers
are found between tendon fascicles and around tenocytes; multiple
elastic fibers surround groups of tenocytes and travel
longitudinally along tendon, whereas fibers present between
fascicles form a loose mesh-like organization oriented in the
transverse direction (65); these
play important roles in maintaining tendon strength and conferring
elasticity to tissue such as ligaments, aorta and skin (66). Thus, elastin fibers may contribute
to recovery of fibers to their original form after stretching
(46).
Tendon cells are primarily divided into two
categories. Tendon cells present between collagen fibers, known as
tenocytes, are the main cells in the tendon tissue. These produce
ECM components such as collagen, fibronectin and proteoglycans, and
therefore serve an important role in maintaining tendon homeostasis
(67). Furthermore, tenocytes
increase expression levels of the junctional and stress fiber
components when exposed to tensile load. Thus, in tendinopathy,
loading with an appropriate tensile load promotes recovery of
mechanical properties of the damaged tendon (68). Another group of cells located in
the interfascicular matrix (IFM) is called interfascicular cells
(69). Interfascicular cells are
round in shape and are more densely distributed compared with
tenocytes. They are also more metabolically active because of
faster protein turnover in the IFM (47). Marr showed that CD146+ cells are
interfascicular cells (70).
Following injury to a rat Achilles tendon, CD146+ cells migrate
toward the injury on days 4 and 7 and to fill the wound on day 21
after the injury (70). Studies
have demonstrated that CD146+ cells promote cell proliferation
through mTORC2 signaling and thus serve a role in tendon repair
(71,72). CD146+ cells also bind Laminin α4
and may play a role in the resolution of inflammation but the exact
mechanism of action is currently unknown (73). The presence of interfascicular
cells may be key for maintaining tendon function but their exact
mechanism of action requires further study. In addition to the
aforementioned cells, other cells are present in tendons, such as
chondrocytes, synovial cells and tendon SCs (46). Among them, the recently identified
tendon SCs have good ability to maintain homeostasis of tendons and
promote repair of tendinopathy (74). Tendon SCs have the ability to
self-renew and differentiate into tendon cells (75). Their differentiation into tendon
cells is promoted at low levels of mechanical stretch (4%) and
produces collagen, thereby promoting remodeling of the tendon ECM.
However, at high mechanical stretch (8%), tendon SCs are induced to
differentiate into non-tendon cells, such as adipocytes,
chondrocytes and osteocytes, resulting in histopathological
features of lipid deposition, proteoglycan accumulation and
calcification in the tendon, thus leading to the development of
tendinopathy (76).
Because of the unique layered structure and
composition, tendons have characteristic biomechanical properties
such as high mechanical strength and viscoelasticity (46). The unique mechanical properties of
tendons are reflected in a stress-strain curve composed of four
regions. In the initial area when the tendon is stretched <2%,
the curled fiber is straightened. When the degree of stretch is
2-4%, it is referred to as the linear area. As the tendon is
stretched, the stiffness of fibers increases rapidly and they
distort in a linear manner. The slope of this region is called the
Young's modulus of the tendon and is used to express the stiffness
of the tendon. When a tendon is stretched >4%, microscopic tears
occur in the fibers. Tendons undergo significant tissue damage when
subjected to >8% strain and continued stretching leads to tendon
rupture (46). Tendons have
several other mechanical properties, including non-linearity,
viscoelasticity, and heterogeneity (77). Viscoelasticity may result from the
interaction between collagen, water and proteoglycan (78). Viscoelasticity is important for
load transfer in tendons. Tendons are more prone to deformation at
low strain rates and are less effective in transferring loads. At
high strain rates, tendons are less prone to deformation with
higher stiffness and are more effective at transmitting larger
loads (79). Thus, the unique
mechanical properties of tendons are key to their function of
carrying and transmitting loads. The high levels and regular
arrangement of collagen create high tensile strength required to
provide efficient load transfer. However, it is not clear how small
changes in the structure and composition of the tendon lead to
changes in mechanical properties.
MSCs are considered to be 'medicinal cell factories'
that are capable of secreting a range of bioactive molecules,
either in the form of soluble biofactors or through MSC-exosomes
(Exos), which have functions in immunomodulation, anti-apoptotic
activity, and promoting synthesis of ECM components, such as
collagen (80). MSCs have been
used in regenerative medicine since their discovery in the late
1960s (80). MSCs are isolated
from a number of tissues, including adipose, muscle, tendon,
synovial sac, dental pulp, skin, lung, placenta and umbilical cord
(11). Furthermore, MSCs
self-renew and differentiate to produce specialized cell types such
as chondrocytes, muscle cells, and skin cells (82). MSCs derived from BM and adipose
are most frequently used in the treatments of tendon injury as they
exhibit self-renewal, multidirectional differentiation, and
paracrine functions. And they can also lead to tendon healing and
functional improvement through minimally invasive treatment
approaches (83). The effect of
MSCs from different sources in tendon healing is summarized in
Table III.
In recent years, more attention has turned to ADSCs
because they are easier to isolate compared to other sources of
MSCs. And because BMSCs need to be collected using a trocar to
drill through the iliac crest, while ADSCs can be collected using
only minimally invasive liposuction techniques, this is easier and
less painful for the patient. In addition, over time, the donor
site providing BMSCs is prone to pain and stiffness, whereas ADSCs
have a much lower incidence (15,88,89). Specifically, adipose tissue is
easily aspirated from abdominal subcutaneous adipose; collected
tissue is passed through specific systems including forming,
granulating, cutting, purification, centrifugation, nitrification,
absorption of antimicrobial or antitoxin arrangements, cleansing,
partition, and lyophilization to obtain ADSCs (90,91). Furthermore, ADSCs can be obtained
from autologous or allogeneic sources, but ADSCs isolated from
autologous adipose tissue are the best candidates for the treatment
of tendon injuries because they do not induce immune rejection
after application in injured tendons (92). Studies have detected transcription
factors associated with hypoxia, such as hypoxia-inducible factor-1
(HIF-1), in models of ruptured Achilles tendon that show ectopic
ossification; therefore, hypoxia may be associated with formation
of cartilage in tendons (93,94). Adding ADSCs in the early stage of
tendon healing can reverse or prevent hypoxia by inhibiting
inflammation and promoting formation of new blood vessels to
inhibit occurrence of heterotopic ossification (95). Consequently, ADSCs have advantages
over BMSCs in the treatment of tendon injury. In addition, compared
with MSCs from other sources, ADSCs enhance tenogenic properties of
tendon resident cells, increase the ratio of collagen I/III and
promote repair of ECM (15).
These characteristics make ADSCs promising in tendon healing.
MSCs secrete biologically active soluble factors
(cytokines, growth factors, chemokines, MSC-Exos) to accelerate
healing of tendons (96,97). MSC-Exos are extracellular vesicles
(EVs) containing complex RNAs and proteins that target cells via
endocytosis, membrane fusion or receptor-ligand interactions and
are key paracrine factors for MSCs. MSC-Exos serve important roles
in immune regulation, apoptosis and tissue regeneration in numerous
types of disease such as osteoarthritis (98). In recent years, there have been
numerous studies on the use of MSC-Exos in tendon repair (99-101). MSC-Exos serve roles in tendon
repair by regulating biological factors and activating signaling
pathways (102). For example,
MSC-Exos inhibit the inflammatory response, primarily by promoting
AMPK signaling (103). The
angiogenesis phase, which is important for tendon healing, also
involves MSC-Exos, which secrete growth factors such as vascular
endothelial growth factor (VEGF), which is associated with
angiogenesis (104). In
addition, MSC-Exos play a key role in subsequent cell proliferation
and collagen synthesis. MSC-Exos promote proliferation and
differentiation of tenocytes by activating the SMAD signaling
pathway and increasing the ratio of type I/III collagen genes,
thereby promoting type I collagen synthesis (105,106). Other growth factors secreted by
MSCs that serve important roles in tendon repair include
insulin-like growth factor-I, TGF-β, platelet-derived growth factor
(PDGF) and bFGF. These growth factors promote tendon repair by
participating in intercellular messaging, as well as signaling
pathways during the three phases of tendon healing: inflammation,
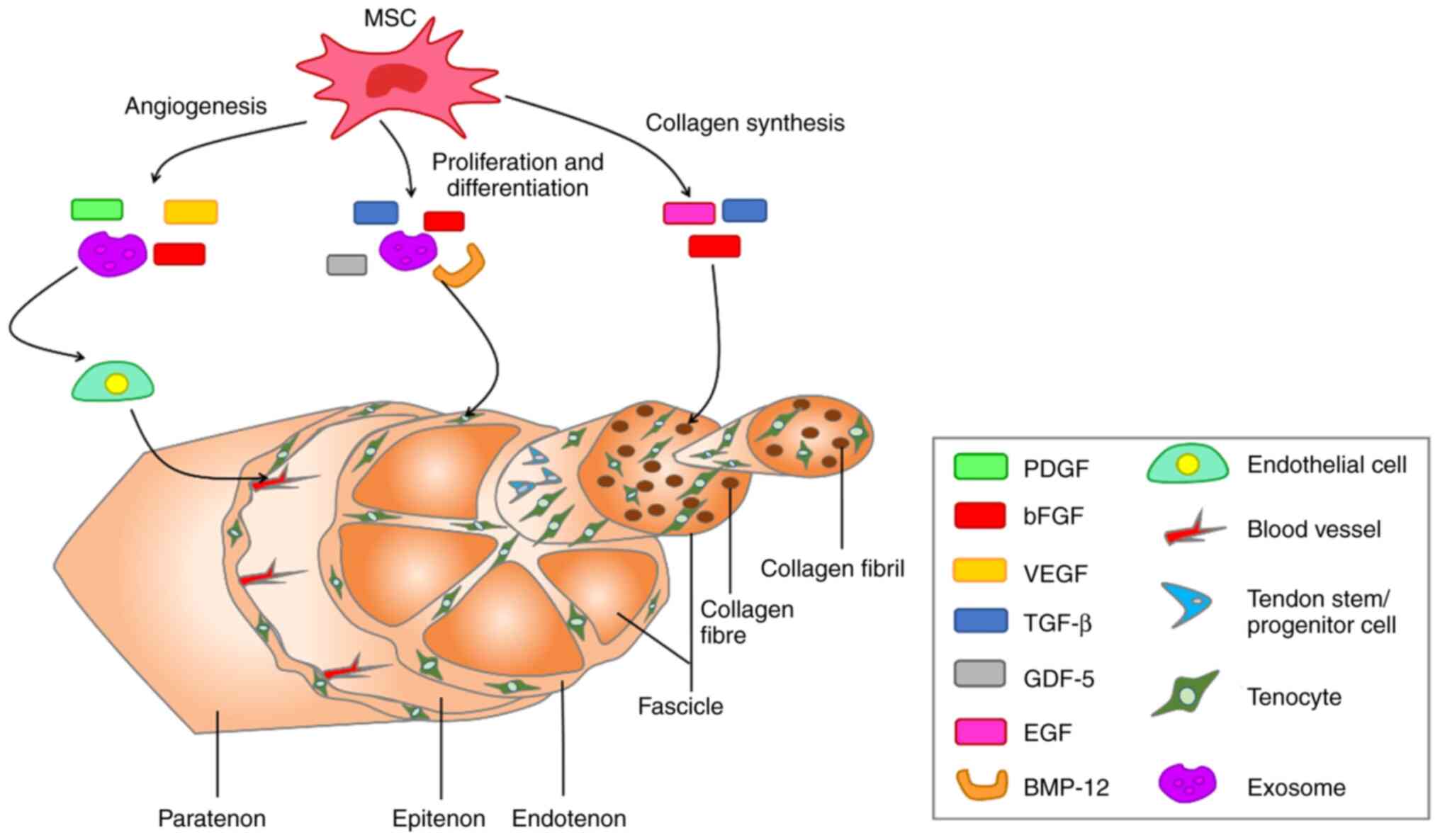
proliferation, and remodeling (10,107). The biologically active soluble
factors secreted by MSCs and their effects on the molecular
structure of the tendon are presented in Fig. 3. In addition, mechanical
stimulation induces MSCs to differentiate into tenocytes, thereby
accelerating the repair of tendon tissue (108,109). For example, in Zhu's (110) experiments, a mechanical
stretching force of 10% was applied to MSCs and stimulated for 6 h
with 30 cycles per minute. However, in Kasper's (111) experiments, he applied a
mechanical load of 10 kPa to MSCs at a frequency of 1 Hz for 72 h
of stimulation.
After tendon injury, the injury site may show signs
of pain, exudation, redness and dysfunction (112). Although studies have shown that
tendon injury is a degenerative condition caused by excessive use
of tendons and does not involve inflammatory cells, there is
mounting evidence that inflammatory factors serve an important role
following tendon injury (113-115). Tendon healing involves three
phase: Inflammatory, proliferative and remodeling phase (116). The inflammatory phase removes
necrotic cells and creates a temporary ECM to prepare for
proliferation and differentiation of new tenocytes in the
subsequent repair process. The immune system begins to recruit
immune cells, such as macrophages and mast cells. It also starts to
secrete cytokines such as IL-1β, IL-4 thus stimulating cell
proliferation and tissue remodeling. These inflammatory responses
are directed by type I (pro-inflammatory) and type II
(anti-inflammatory) immune regimens (117). S100A9, an alarmin that can form
calprotectin (CP) heterodimers with S100A8, is mainly produced by
keratinocytes and innate immune cells. In the type I immune
response, S100A8 and S100A9 serve as alarm elements that are
released into the extracellular environment by necrotic cells or
activated immune cells (118).
This leads to enhanced recruitment of immune cells [T helper (Th)1
T, neutrophils, M1-type macrophages] and promotes release of
pro-inflammatory factors such as tumor necrosis factor-α (TNF-α),
IFN-γ, IL-1β and inducible nitric oxide synthase (iNOS) from tendon
cells (119). Subsequently,
downstream inflammatory signaling pathways such as NF-κB and NLRP3
are activated, regulating inflammatory gene expression and
transcription (120,121). The presence of pro-inflammatory
factors breaks down ECM and promotes new ECM deposition (122). To prevent the excessive
pro-inflammatory type I immune response, the body activates the
type II immune anti-inflammatory response by secreting IL-4 or
IL-33 from damaged cells (123).
The release of IL-33 triggers downstream responses from
macrophages, T regulatory cells (Tregs) and other intrinsic immune
cells (124). Tregs can produce
IL-10, which acts as a key anti-inflammatory factor to resolve
inflammation caused by the type I immune response. IL-4 also
promotes conversion of naive CD4 T cells and macrophages to Th2
cells and M2-type macrophages, thus exerting anti-inflammatory
effects (125).
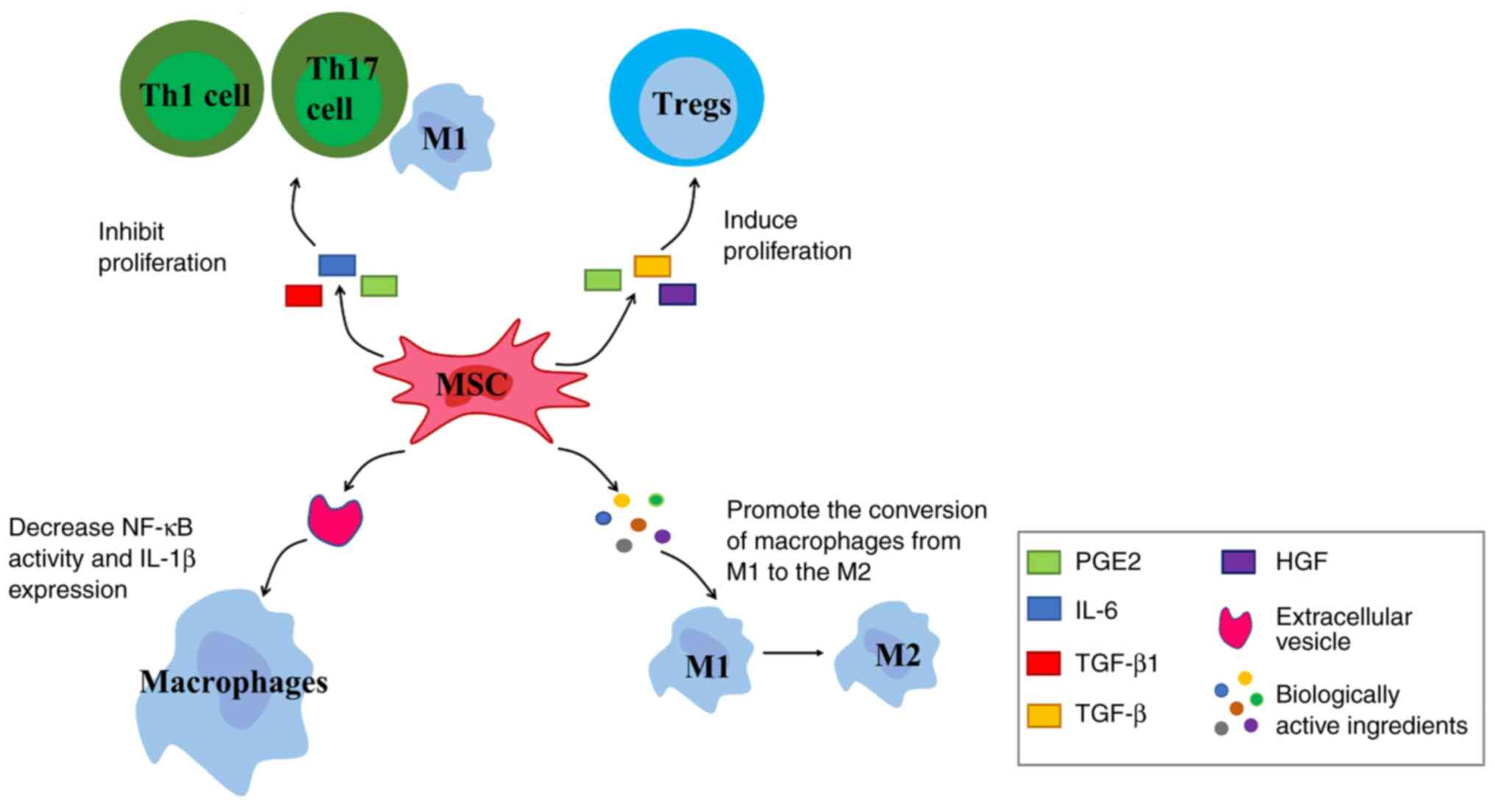
Chemokine receptors are expressed by MSCs; MSCs
detect inflammation signals through chemokine receptors and migrate
to them. Under stimulation of pro-inflammatory factors, MSCs exert
immunomodulatory effects by secreting immunomodulatory mediators
such as chemokines, cytokines and growth factors (Fig. 4). For example, TGF-β1, IL-6 and
PGE2 inhibit proliferation of Th1 and Th17 cells, M1-type
macrophages and other pro-inflammatory cells (126). MSCs also express and secrete
soluble factors such as PGE2, TGF-β and hepatocyte growth factor.
These factors induce proliferation of Tregs, thereby controlling
inflammation (127).
EVs secreted by MSCs (MSC-EVs) play an important
role in the anti-inflammatory response. The inflammatory response
at the injury site stimulates MSCs to secrete EVs. MSC-EVs target
macrophages and decrease NF-κB activity and IL-1β expression,
thereby decreasing inflammation in the early stage of tendon repair
(128). IL-1 is a key
inflammatory cytokine (129). It
plays an important role in degrading ECM, inhibiting tendon cell
marker expression and inducing pain. Following tendon injury,
inflammatory factors such as IL-1 and TNF-α are released by
inflammatory cells such as neutrophils and macrophages during the
exogenous healing phase (130).
IL-1β downregulates gene expression of early growth response gene 1
and type I and III collagen while upregulating expression of MMP1,
3, 8, and 13 (131). MMPs
mediate catabolism of collagen, leading to sustained tissue
degradation (132). In addition,
IL-1β downregulates expression of tendon cell markers scleraxis
(SCX) and tenomodulin (TNMD), which leads to a decrease in ultimate
tensile strength and elastic modulus of the repaired tendon
(133). In damaged tissues, PGE2
acts to promote vasodilation and elicit a pain hypersensitivity
response. IL-1β accelerates the conversion from PGH2 to PGE2 and
causes an increase in pain by enhancing expression of prostaglandin
E synthase (134). This suggests
that IL-1 serves a key role in the development of the inflammatory
response. After IL-1 and TNF-α are released, they bind to toll-like
receptor 4 (TLR4) on the cell membrane (135). The polymerization of TLR4
enables signal transduction into cells; there is a toll/IL-1
receptor region in the cell membrane of TLR4 that binds to the
carboxy terminus of myeloid differentiation primary response gene
88 (MyD88) (136). The
amino-terminal death domain of MyD88 binds to the amino-terminal
death domain of IL-1 receptor-associated kinase (IRAK). This
promotes the phosphorylation of IRAK and acquisition of free IRAK1,
2 and 4, which in turn activates TNF-α receptor-associated factor 6
(TRAF-6). TRAF-6 binds to NF-κB kinase and phosphorylates the β
subunit (IKKβ), thereby activating the IκB kinase (IKK) complex
(137). IKK induces IκB
phosphorylation at residues Ser32 and Ser36 of IκBα and residues
Ser19 and Ser23 of IκBβ via the 26S proteasome (138). IκB is an inhibitory protein of
NF-κB, which binds to NF-κB dimer to inhibit its activity. IκB is
degraded, which results in entry of p50-p65 complex into the
nucleus to initiate expression of downstream genes regulated by
NF-κB (139,140). NF-κB serves as a powerful
pro-inflammatory signaling pathway that drives the production of
pro-inflammatory cytokines, including IL-1, IL-6, C-C motif
chemokine ligand 2 and TNF-α. These inflammatory factors reactivate
NF-κB activity so there is often a persistent inflammatory response
during tendon healing (141).
The persistent inflammatory environment has a negative impact on
tendon healing and leads to tissue adhesion during the collagen
remodeling phase (142).
MSCs-Exos can downregulate phosphorylated (p-)P65 by secreting
microRNA (miR)-23a-3p (143).
While NF-κB is a typical heterodimer, its common structure is a
complex composed of proteins P65 and P50; MSCs-Exos directly
inhibits NF-κB activity by downregulating activity of p-P65
(144). In addition, Shen et
al (128) showed that ADSC
EVs inhibit NF-κB activity in an indirect manner by inhibiting
IL-1β secretion via macrophages, thereby blocking IL-1β-induced
activation of the NF-κB signaling pathway.
Tendons have a specific structure with lower
metabolic demands compared with other tissue and therefore have
lower cellular and vascular content (149). However, tendons are surrounded
by a rich network of blood vessel. Degenerative lesions of tendons
tend to occur in areas with decreased vascularity and decreased
blood supply can lead to hypoxia (150). Hypoxia is one of the most common
environmental stresses experienced by cells and serves an important
role in the early stages of tendinopathy (151). Following tendon injury,
decreased tissue perfusion and increased energy demands lead to a
lack of oxygen and nutrients in local tissue, which in turn creates
a hypoxic environment (152). In
tenocytes, hypoxia induces expression of key cytokines and
pro-inflammatory molecules, including platelet-derived growth
factor, IL-6, IL-8 and platelet-activating factor, which may
disrupt the balance of ECM repair (153). HIF-1 is a heterodimer composed
of subunits HIF-1α and HIF-1β. HIF-1α is ubiquitous in cells and
plays an important role in intracellular hypoxia response (154). During hypoxia, the activity of
hydroxylase such as Prolyl Hydroxylase (PHD) and factor-inhibiting
hydroxylase, is inhibited, which activates the NF-κB signaling
pathway (155). At the same
time, the activated NF-κB pathway leads to upregulation of HIF mRNA
levels, which further promotes the activation of signaling. HIF-1
can induce expression of NF-κB target genes, including
cyclooxygenase-2, TNF-α, IL-6 and macrophage phagophageal
inflammatory protein-2, which leads to the continued development of
inflammation (155). Thus,
decreased vascularity in injured tendons and a hypoxic state induce
an inflammatory response in tendon cells and lead to decreased
synthesis of collagen matrix. Moreover, the recovery process takes
longer in less vascularized tissue, with a greater likelihood of
re-injury occurring before full recovery (156). Therefore, it is key to promote
neovascularization in tendon repair.
MSCs secrete VEGF to enhance the proliferation and
differentiation of vascular endothelial cells, thereby directly
promoting angiogenesis (157-159). Studies have identified numerous
cytokines that promote blood vessel formation, such as VEGF, PDGF
and bFGF; VEGF has been proven to act on vascular endothelial cells
(160,161). Angiogenesis is caused by the
degradation of the basement membrane by MMPs. In the early stages
of tendon healing, high levels of VEGF are secreted after injection
of MSCs and its receptors are highly expressed (162). VEGF stimulates the expression of
MMPs, accelerates degradation of the basement membrane and ECM
components and initiates migration of endothelial cells.
Subsequently, it responds to locally produced factors such as PDGF
and bFGF, promoting development of capillary structure and forming
an anastomosis with other blood vessels (163,164). VEGF may be considered the most
effect mitogen promoting vascular growth. After VEGF secreted by
MSCs binds to receptors VEGFR 2 and vascular endothelial growth
factor receptor 1 (Flt-1) on endothelial cells with high affinity,
it directly stimulates proliferation of vascular endothelial cells,
inducing their migration and leading to formation of new blood
vessels. At the same time, it can increase the permeability of
capillaries (165). The ability
to drive angiogenesis is significantly enhanced after culturing
MSCs under hypoxic conditions (166,167). This is because hypoxic
conditions inhibit cellular senescence, increase cell proliferation
and enhance the differentiation potential of MSCs. Thus, the
biological activity of MSCs is significantly increased in hypoxic
environments, while hypoxia activates the angiogenic pathway by
regulating the paracrine function of MSCs, leading to enhanced
secretion of growth factors, including VEGF and IL-6 (168,169).
Studies have also shown that MSC-Exos deliver
biologically active molecules, including microRNAs (miRs), to
endothelial cells and mediate angiogenesis (129,170). miR-30b serves a key role in
MSC-mediated angiogenesis. Delta-like protein 4 (DLL4), an miR-30
family target, is responsible for regulating blood vessel growth
and branching during angiogenesis (171). In addition, miR-125a is a key
factor in promoting angiogenesis. In the early stages of tendon
healing, MSC-Exos promote secretion of MMP2 and miR-125a, which
targets endothelial cells, thereby increasing endothelial cell
migration and leading to increased angiogenesis, thereby
accelerating tendon healing (172). MSCs promote endothelial cell
proliferation via paracrine cytokines and MSC-Exos to increase
blood vessel formation, which is necessary in tendon healing. The
formation of new blood vessels ensures that sufficient nutrients,
such as oxygen and growth factors, are provided to the injured area
(150,173).
Tenocytes (also known as tendon fibroblasts) are the
primary cells in tendons. They produce ECM components such as
collagen, fibronectin and proteoglycans; thus, tenocytes play a
crucial role in maintaining the stability of the ECM (67). During the proliferative phase of
tendon healing, tenocytes gradually move to the injury site and
proliferate, while production of collagen and glycoprotein
increases to promote tissue regeneration (77). However, the proliferation and
remodeling phases of tendon healing are usually slow due to low
levels of tenocytes, as well as the relatively poor blood supply
due to the low vascularity in the tendon, resulting in a mismatch
between the rates of production of ECM components and tendon
healing, which eventually leads to incomplete recovery of
mechanical properties (174).
Studies have identified another cell population in tendons, TSPCs,
which account for 1-4% of the total number of cells within the
tendon (85,175). TSPCs differentiate into
tenocytes as well as chondrogenic, osteogenic and adipogenic
lineages following induction in vitro and may then form
tendon, cartilage, bone and tendon-bone junction tissues in animal
models (176,177). Injection of TSPCs into a rat
model of Achilles tendon injury shows strong healing ability
(178). TSPCs are primarily
responsible for the rapid replenishment of tenocytes after tendon
injury to maintain numbers of tenocytes (179). However, the limited number of
TSPCs isolated from tendon tissue requires expansion in
vitro; this process may lead to genetic drift, which negatively
affects proliferation and differentiation into tenocytes (180). Therefore, during in vitro
amplification of TSPCs, the construction of a medium suitable for
the amplification and survival of TSPCs is crucial for tendon
regeneration.
Therapeutic approaches have focused on expansion of
endogenous TSPCs and tenocytes by MSCs, as well as secretion of
growth factors that induce TSPC differentiation to promote tendon
regeneration (181). MSCs
promote activation of protein kinase B (Akt) and extracellular
signal-regulated kinase (ERK)-1/2, which are involved in tenocyte
proliferation and migration via MEK/ERK1/2 and PI3K/Akt signaling
(182). In addition, MSCs-Exos
can promote activation of SMAD2/3 and SMAD1/5/9 signaling pathways,
which significantly increases expression of TNMD, type I collagen
and SCX protein (183). SCX and
Mohawk are the major tendon cell-specific transcription factors
that support matrix generation, tenocyte proliferation and
differentiation. Moreover, they inhibit the differentiation of
non-tendinous lineages including osteogenesis, chondrogenesis, and
adipogenesis, thus promoting proliferation and differentiation of
TSPCs to tenocytes (184). Thus,
MSCs promote tendon healing in indirect and direct manners. A
number of growth factors promote differentiation of co-cultured
MSCs into tenocytes, including connective tissue growth factor
(CTGF), TGF-β, GDF-7 and GDF-5 (20,21,185). Among them, CTGF, a member of the
CCN protein family, has satisfactory effects on tendon repair when
co-cultured with ADSCs; differentiation of ADSCs to tenocytes is
induced by CTGF. CTGF significantly enhances the mRNA and protein
expression of SCX and TNMD in a time- and dose-dependent manner
(186). The most effective dose
and treatment duration of CTGF is 100 ng/ml for 14 days (187). On the other hand, CTGF can
induce the self-proliferation of ADSCs. This may be mediated by the
FAK/ERK1/2 signaling pathway, which is the typical pathway for cell
division and proliferation (188). Research (187) has shown that treatment of ADSCs
with CTGF promotes proliferation in a dose-dependent manner on days
5 and 7. CTGF induces ERK1/2 phosphorylation in 5 and FAK
phosphorylation in 15 min, both of which can last for 120 min. DNA
methylation is induced via the FAK/ERK1/2 signaling pathway,
increasing chromatin condensation and nuclear stiffness, thereby
promoting cell migration (189).
Therefore, combining CTGF with ADSCs can effectively increase
tendon healing and provide a molecular and cytological basis for
better application of tissue engineering methods to promote tendon
healing. Another growth factor, BMP-12, induces differentiation of
MSCs to tenocytes; this process is mainly mediated through the
SMAD1/5/8 signaling pathway (190). However, the induction of
differentiation of MSCs by transgenic BMP-12 is currently
controversial: Although this has been shown to be effective in
animal experiments, there are difficulties in clinical application
of transgenic cells in humans, including possibility of side
effects and ethical issues (191). In addition to the aforementioned
growth factors, epidermal growth factor (EGF), platelet-derived
growth factor-BB and TGF-β3 can also effectively increase
expression of tendonogenic genes such as SRY-box containing gene 9
and TNMD when co-cultured with MSCs (192).
Although BMSCs are frequently used in the treatment
of tendon repair and express a number of tendon-related markers,
including SCX, TNMD, proteoglycan and type I and III collagen, it
is hypothesized that levels of these markers are lower in BMSCs
than in TSPCs (85). Therefore,
co-culture of TSPCs with BMSCs may be a good option for the
treatment of tendon injuries. In vitro experiment (193) have shown that bi-directional
crosstalk between TSPCs and BMSCs upregulates tendon-associated
genes (including SCX and TNMD) and tendon ECM markers (such as type
I collagen, decorin and tenascin) and promotes ECM deposition.
Thus, co-culture serves a role in inducing cell differentiation to
tenocytes (193). Furthermore,
adding mechanical stimuli to the surface of the medium can affect
cell density, cellular arrangement and ECM deposition. For example,
this results in a significantly higher cross-sectional cell density
and a 2.5-fold increase in cell alignment (194,195). Cells can be cultured on
micropatterned silicone substrates and subjected to cyclic
stretching to simulate the in vivo biomechanical environment
during tendon healing (195).
When cells in medium are exposed to intermittent cyclic strain,
cell differentiation to tenocytes is induced (196). When the tensile strength is
increased to 4 and 8%, MSCs cultured in vitro exhibit a
spindle-like shape and produce type I collagen (197). In summary, studying the effects
of growth factors or TSPCs in combination with MSCs and mechanical
stimulation may provide novel options for differentiation of MSCs
to tenocytes (198).
In normal tendons, the levels of type I collagen in
the ECM are high, accounting for 95%, and type III collagen is
expressed at lower levels (49,85). Type III collagen is weaker than
type I collagen bearing mechanical loads and type I collagen plays
a crucial role in the tensile strength of the tendon (199). The activity of MMPs is key in
the remodeling phase of tendon repair. MMPs are collagen hydrolases
that break down damaged collagen (200,201). During the collagen remodeling
phase, MMP13 and MMP3 are highly expressed and their increased
expression degrades type I and III collagen and proteoglycans in
ECM. During the early stages of tendon healing, VEGF and its
receptors are highly expressed, which stimulates the expression of
MMPs (202). ADSC-Exos
significantly inhibit the expression of MMP9/13 genes and
indirectly increase the ratio of type I/III collagen, thus
promoting collagen synthesis and tendon healing (203). In addition, BMSCs cultured under
hypoxic conditions exhibit high expression of type I/III collagen
α1, decorin and TNMD in the early stages of tendon repair (168). Decorin regulates the diameter of
collagen fibers and works in combination with growth factors to
regulate cell proliferation, thereby promoting collagen production
(61). TNMD is a specific marker
of tendon maturation. TNMD promotes proliferation, migration and
tendon differentiation of TSPCs and prevents scar formation during
early stage of tendon healing; TNMD also regulates levels of type I
collagen and promotes collagen remodeling (101). Therefore, BMSCs are an effective
therapeutic method to promote tendon tissue regeneration.
Similarly, ADSC-Exos increase expression of TNMD, type I collagen
and SCX in TSPCs by activating SMAD2/3 and SMAD1/5/9 signaling
pathways, thereby promoting TSPC proliferation, migration and
tendon differentiation (99).
SMAD2/3 and SMAD1/5/9 are typical SMAD signaling pathways and SMAD3
is also a key transcription factor for type I collagen synthesis
(204). SMAD3 activates TGF-β
signaling pathway via TGF-β type I and II transmembrane receptors
(205); SMAD2 and SMAD3
dissociate from the receptor after phosphorylation, form a complex
with SMAD4 and translocate to the nucleus (206). SMAD3 is involved in
transcription of genes associated with cell proliferation,
inflammatory response and ECM formation. Therefore, TGF-β signaling
is involved in regulating collagen formation, MMPs activity and
tissue remodeling during tendon healing via the transcription
factor SMAD3 (207).
Studies have shown that MSCs injected following
tendon injury secrete growth factors, including TGF-β, bFGF and EGF
(208,209). These factors accelerate ECM
deposition and remodeling at the injured site and start collagen
synthesis and maturation (210,211). TGF-β promotes collagen
production, which increases the strength of the repaired tendon;
however, when TGF-β is overexpressed, the overproduction of
disordered collagen may lead to the formation of adhesions at the
tendon (212). bFGF promotes
cell mitosis, increases proliferation of fibroblasts and secretes
type I and III collagen (213).
There is a unique pattern of collagen production during tendon
repair. Type III collagen increases significantly in the early
stages of tendon healing, providing a 'quick fix' for the damaged
site. At 6-8 weeks after injury, the tissue replaces type III
collagen with type I collagen and restores its linear structure,
resulting in increased collagen fiber crosslinking and tendine-like
tissue formation (113,173). In co-culture experiments with
ADSCs and tenocytes, ratio of ADSCs to tenocytes of 3:1 increases
proliferation of tenocytes; ADSCs also differentiate into tendon
cells and expression of tenascin-C and SCX increases (214). Tenascin-C regulates the number
of collagen fibers as well as their growth direction and is key for
maintaining the mechanical stability of the ECM (64). Likewise, SCX serves an important
role in tenocyte differentiation as well as tendon development. In
SCX-knockout mice, tendon development is notably disrupted
(215). Certain genes, including
type I collagen α1 and TNMD, are potential direct target genes of
SCX in tenocytes but how SCX regulates tenocyte differentiation is
unknown (216). ADSCs increase
proliferation rate and gene expression in tenocytes, thereby
enhancing the function of tenocytes, accelerating the turnover of
ECM and increasing the proportion of normal collagen structure in
tendons. The strength of the tendon is quickly restored, thereby
inhibiting further degeneration of the tendon (217).
Tendon injury is common in orthopedics. After
tendon injury, the tendon shows a local inflammatory response,
hypocellularity, lack of collagen and blood vessels and increase
levels of proteoglycans (218).
The injured tendon exhibits discontinuous and disorganized tendon
fibers. Since tendon is a tissue with low cellular content and poor
blood supply, the tendon has a limited ability to heal (219,220). The process of tendon healing is
divided into three overlapping phases: Inflammatory, proliferative
and remodeling phase. During the remodeling phase, scars often form
(116). Scars have different
biomechanical properties compared with natural tendons, including
decreased strength, increased stiffness and greater tendency to
form adhesions. Reconstructed tendon tissue exhibits poorer
biochemical and mechanical properties compared with uninjured
tendon (221). This leads to
dysfunction in the limb and makes the tendon more prone to
re-rupture (11). With the
development of SC research, MSCs have attracted attention (222). MSCs have high proliferation and
self-renewal capacity (223).
MSCs differentiate into target cells and directly promote tissue
regeneration; MSCs also secrete biological factors and EVs, thus
indirectly affecting tissue repair (220). MSCs can be obtained from
numerous types of tissue and MSCs from different sources show
different characteristics, indicating potential advantages and
disadvantages of each type of MSC for specific clinical
applications (224). Among
sources, the most commonly used are BM and adipose tissue. ADSCs
are more readily available compared with BM, yield more abundant
MSCs after isolation and also decrease donor site morbidity
(225). This is because BMSCs
need to be collected using a trocar to drill through the iliac
crest, while ADSCs can be collected using only minimally invasive
liposuction techniques, this is easier and less painful for the
patient. In addition, over time, the donor site providing BMSCs is
prone to pain and stiffness, whereas ADSCs have a much lower
incidence (88). In addition to
application in tendon injury, MSCs can also be used to treat
fractures, osteoarthritis and other disease (226). MSCs have shown satisfactory
results in tissue engineering and regenerative medicine, not only
in promoting tissue regeneration, but also in restoring the tissue
to its original biomechanical function to the greatest extent
possible (227).
The roles and mechanisms of MSCs may involve
promoting angiogenesis, cell proliferation and differentiation and
collagen formation and decreasing inflammation during tendon repair
(129,228). MSCs participate in localized
anti-inflammatory response in the early stage following tendon
damage. Anti-inflammatory factors and MSCs-EVs are hypothesized to
be intercellular messengers in immune regulation (229). They interact with various types
of immune cell, including T and B lymphocytes, natural killer
cells, macrophages, neutrophils and monocytes (230). MSCs promote angiogenesis mainly
by releasing VEGF and Exos (157). During the remodeling and
collagen production phase, in vitro experiment indicated
MSCs enhance their ability to differentiate into tenocytes by
co-culture with growth factors and TSPCs, thus promoting tenocyte
proliferation and differentiation, as well as collagen fiber
production and ECM remodeling (198,217).
The present review summarized the functions and
mechanisms of MSCs in tendon repair but there are still some issues
with the clinical application of MSCs that need to be addressed. To
the best of our knowledge is no consensus on practical
considerations regarding the source, dose, administration technique
and timing of MSC usage (231).
Although the commonly used sources of MSCs are adipose and BM
tissue, there are still debates on the applications of both; for
example, whether ectopic bone can develop after application of
BMSCs for treatment and whether the application of ADSCs involves
ethical issues. MSCs isolated from young living sources survive
longer, secrete more Exos and have a broader differentiation
capacity than MSCs isolated from older tissue (232). Therefore, MSCs isolated from
embryonic sources may be promising therapeutic tools for tendon
repair and regeneration (233).
The current mode of administration is direct injection of MSCs at
the site of injury, but a study (234) has shown that intravenous MSCs
can promote better interaction with the immune system and initiate
systemic anti-inflammatory effects. However, due to small sample,
more research is needed on intravenous MSC administration (235). Although study have shown that
MSCs show satisfactory therapeutic effects when co-cultured with
growth factors or TSPCs, few studies have compared the effects of
culture conditions (236,237).
Therefore, randomized controlled trials are required (238). In addition, current treatments
to promote tendon repair lack standardization so treatment results
may differ. Therefore, future studies should investigate the
effects of clinical treatment with MSCs alone to develop
standardized treatment modalities, which may lead to more uniform
outcomes.
In conclusion, MSCs are a promising cell therapy to
promote tendon healing and understanding of the functions and
mechanisms of MSCs in tendon healing can help improve its
efficiency. However, further studies are required to maximize the
therapeutic value of MSCs.
Not applicable.
LJ, JL, YC, KL, LL, XW, TL and SL performed study
conception and design. KL, LL and XW wrote the manuscript. LJ
performed the literature review. LJ, TL and SL edited the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Mr Robert
Constantine, Everett Adult Learning Center, Everett, Massachusetts,
United States for language editing the manuscript.
The present study was supported by 2022 Sichuan Provincial
Science and Technology Plan Project (grant no. 22ZDYF3799), Luzhou
Science and Technology Program Project (grant no. 2020-SYF-31),
Luzhou Municipal Government-Southwest Medical University Joint
Project (grant no. 2021LZXNYD-J10), Sichuan Science and Technology
Program Project (grant no. 2022NSFSC0688) and Southwest Medical
University Applied Basic Fundamental Research Project (grant no.
2021ZKMS050).
|
1
|
Andarawis-Puri N, Flatow EL and Soslowsky
LJ: Tendon basic science: Development, repair, regeneration, and
healing. J Orthop Res. 33:780–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cook JL and Purdam C: Is compressive load
a factor in the development of tendinopathy? Br J Sports Med.
46:163–168. 2012. View Article : Google Scholar
|
|
3
|
Beatty NR, Félix I, Hettler J, Moley PJ
and Wyss JF: Rehabilitation and prevention of proximal hamstring
tendinopathy. Curr Sports Med Rep. 16:162–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silbernagel KG, Hanlon S and Sprague A:
Current Clinical concepts: Conservative management of achilles
tendinopathy. J Athl Train. 55:438–447. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Figueroa D, Figueroa F and Calvo R:
Patellar tendinopathy: Diagnosis and treatment. J Am Acad Orthop
Surg. 24:e184–e192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun SW, Kim W, Lee SY, Lim CY, Kim K, Kim
JG, Park CH, Hong SH, Yoo HJ and Chung SG: A randomized controlled
trial of stem cell injection for tendon tear. Sci Rep. 12:8182022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Z, Jin M, He H, Dong J, Li J, Nie J,
Wang Z, Xu J and Wu F: Mesenchymal stem cells and macrophages and
their interactions in tendon-bone healing. J Orthop Translat.
39:63–73. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malekpour K, Hazrati A, Zahar M, Markov A,
Zekiy AO, Navashenaq JG, Roshangar L and Ahmadi M: The potential
use of mesenchymal stem cells and their derived exosomes for
orthopedic diseases treatment. Stem Cell Rev Rep. 18:933–951. 2022.
View Article : Google Scholar
|
|
9
|
Xu Y, Zhang WX, Wang LN, Ming YQ, Li YL
and Ni GX: Stem cell therapies in tendon-bone healing. World J Stem
Cells. 13:753–775. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8:7842019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Torres-Torrillas M, Rubio M, Damia E,
Cuervo B, Del Romero A, Peláez P, Chicharro D, Miguel L and Sopena
JJ: Adipose-derived mesenchymal stem cells: A promising tool in the
treatment of musculoskeletal diseases. Int J Mol Sci. 20:31052019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Docheva D, Müller SA, Majewski M and Evans
CH: Biologics for tendon repair. Adv Drug Deliv Rev. 84:222–239.
2015. View Article : Google Scholar :
|
|
13
|
Mirghaderi SP, Valizadeh Z, Shadman K,
Lafosse T, Oryadi-Zanjani L, Yekaninejad MS and Nabian MH: Cell
therapy efficacy and safety in treating tendon disorders: A
systemic review of clinical studies. J Exp Orthop. 9:852022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lui PP: Stem cell technology for tendon
regeneration: Current status, challenges, and future research
directions. Stem Cells Cloning. 8:163–174. 2015.PubMed/NCBI
|
|
15
|
Migliorini F, Tingart M and Maffulli N:
Progress with stem cell therapies for tendon tissue regeneration.
Expert Opin Biol Ther. 20:1373–1379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo D, Li H, Liu Y, Yu X, Zhang X, Chu W,
She Y, Wang D and Chen G: Fibroblast growth factor-2 promotes the
function of tendon-derived stem cells in Achilles tendon
restoration in an Achilles tendon injury rat model. Biochem Biophys
Res Commun. 521:91–97. 2020. View Article : Google Scholar
|
|
17
|
Cohen S, Leshansky L, Zussman E, Burman M,
Srouji S, Livne E, Abramov N and Itskovitz-Eldor J: Repair of
full-thickness tendon injury using connective tissue progenitors
efficiently derived from human embryonic stem cells and fetal
tissues. Tissue Eng Part A. 16:3119–3137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yea JH, Kim Y and Jo CH: Comparison of
mesenchymal stem cells from bone marrow, umbilical cord blood, and
umbilical cord tissue in regeneration of a full-thickness tendon
defect in vitro and in vivo. Biochem Biophys Rep.
34:1014862023.PubMed/NCBI
|
|
19
|
Baberg F, Geyh S, Waldera-Lupa D,
Stefanski A, Zilkens C, Haas R, Schroeder T and Stühler K:
Secretome analysis of human bone marrow derived mesenchymal stromal
cells. Biochim Biophys Acta Proteins Proteom. 1867:434–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomopoulos S, Das R, Sakiyama-Elbert S,
Silva MJ, Charlton N and Gelberman RH: bFGF and PDGF-BB for tendon
repair: Controlled release and biologic activity by tendon
fibroblasts in vitro. Ann Biomed Eng. 38:225–234. 2010. View Article : Google Scholar :
|
|
21
|
Gelberman RH, Linderman SW, Jayaram R,
Dikina AD, Sakiyama-Elbert S, Alsberg E, Thomopoulos S and Shen H:
Combined administration of ASCs and BMP-12 promotes an M2
macrophage phenotype and enhances tendon healing. Clin Orthop Relat
Res. 475:2318–2331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yea JH, Bae TS, Kim BJ, Cho YW and Jo CH:
Regeneration of the rotator cuff tendon-to-bone interface using
umbilical cord-derived mesenchymal stem cells and gradient
extracellular matrix scaffolds from adipose tissue in a rat model.
Acta Biomater. 114:104–116. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim WL, Chowdhury SR, Ng MH and Law JX:
Physicochemical properties and biocompatibility of electrospun
polycaprolactone/gelatin nanofibers. Int J Environ Res Public
Health. 18:47642021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ciardulli MC, Marino L, Lovecchio J,
Giordano E, Forsyth NR, Selleri C, Maffulli N and Porta GD: Tendon
and cytokine marker expression by human bone marrow mesenchymal
stem cells in a hyaluronate/poly-lactic-co-glycolic acid
(PLGA)/fibrin three-dimensional (3D) scaffold. Cells. 9:12682020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith RK, Werling NJ, Dakin SG, Alam R,
Goodship AE and Dudhia J: Beneficial effects of autologous bone
marrow-derived mesenchymal stem cells in naturally occurring
tendinopathy. PLoS One. 8:e756972013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manning CN, Martel C, Sakiyama-Elbert SE,
Silva MJ, Shah S, Gelberman RH and Thomopoulos S: Adipose-derived
mesenchymal stromal cells modulate tendon fibroblast responses to
macrophage-induced inflammation in vitro. Stem Cell Res Ther.
6:742015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan Q, Lui PP and Lee YW: In vivo identity
of tendon stem cells and the roles of stem cells in tendon healing.
Stem Cells Dev. 22:3128–3140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P, Xu Y, Gan Y, Song L, Zhang C, Wang L
and Zhou Q: Role of the ERK1/2 signaling pathway in osteogenesis of
rat tendon-derived stem cells in normoxic and hypoxic cultures. Int
J Med Sci. 13:629–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rui YF, Lui PP, Chan LS, Chan KM, Fu SC
and Li G: Does erroneous differentiation of tendon-derived stem
cells contribute to the pathogenesis of calcifying tendinopathy?
Chin Med J (Engl). 124:606–610. 2011.PubMed/NCBI
|
|
30
|
Nie D, Zhou Y, Wang W, Zhang J and Wang
JHC: Mechanical overloading induced-activation of mTOR signaling in
tendon stem/progenitor cells contributes to tendinopathy
development. Front Cell Dev Biol. 9:6878562021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J and Wang JH: Prostaglandin E2
(PGE2) exerts biphasic effects on human tendon stem cells. PLoS
One. 9:e877062014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bajada S, Mazakova I, Richardson JB and
Ashammakhi N: Updates on stem cells and their applications in
regenerative medicine. J Tissue Eng Regen Med. 2:169–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dale TP, Mazher S, Webb WR, Zhou J,
Maffulli N, Chen GQ, El Haj AJ and Forsyth NR: Tenogenic
differentiation of human embryonic stem cells. Tissue Eng Part A.
24:361–368. 2018. View Article : Google Scholar
|
|
34
|
Blum B and Benvenisty N: The
tumorigenicity of human embryonic stem cells. Adv Cancer Res.
100:133–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wyles SP, Yamada S, Oommen S, Maleszewski
JJ, Beraldi R, Martinez-Fernandez A, Terzic A and Nelson TJ:
Inhibition of DNA topoisomerase II selectively reduces the threat
of tumorigenicity following induced pluripotent stem cell-based
myocardial therapy. Stem Cells Dev. 23:2274–2282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aguiar C, Theoret C, Smith O, Segura M,
Lemire P and Smith LC: Immune potential of allogeneic equine
induced pluripotent stem cells. Equine Vet J. 47:708–714. 2015.
View Article : Google Scholar
|
|
37
|
Bavin EP, Smith O, Baird AEG, Smith LC and
Guest DJ: Equine induced pluripotent stem cells have a reduced
tendon differentiation capacity compared to embryonic stem cells.
Front Vet Sci. 2:552015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Polo JM, Liu S, Figueroa ME, Kulalert W,
Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al:
Cell type of origin influences the molecular and functional
properties of mouse induced pluripotent stem cells. Nat Biotechnol.
28:848–855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nourissat G, Berenbaum F and Duprez D:
Tendon injury: From biology to tendon repair. Nat Rev Rheumatol.
11:223–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang JH: Mechanobiology of tendon. J
Biomech. 39:1563–1582. 2006. View Article : Google Scholar
|
|
41
|
Whittaker P and Canham PB: Demonstration
of quantitative fabric analysis of tendon collagen using
two-dimensional polarized light microscopy. Matrix. 11:56–62. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Canty EG and Kadler KE: Procollagen
trafficking, processing and fibrillogenesis. J Cell Sci.
118:1341–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herchenhan A, Kalson NS, Holmes DF, Hill
P, Kadler KE and Margetts L: Tenocyte contraction induces crimp
formation in tendon-like tissue. Biomech Model Mechanobiol.
11:449–459. 2012. View Article : Google Scholar
|
|
44
|
Stammers M, Niewczas IS, Segonds-Pichon A
and Clark J: Mechanical stretching changes crosslinking and
glycation levels in the collagen of mouse tail tendon. J Biol Chem.
295:10572–10580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thorpe CT, Udeze CP, Birch HL, Clegg PD
and Screen HR: Specialization of tendon mechanical properties
results from interfascicular differences. J R Soc Interface.
9:3108–3117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang JHC, Guo Q and Li B: Tendon
biomechanics and mechanobiology-a minireview of basic concepts and
recent advancements. J Hand Ther. 25:133–141. 2012. View Article : Google Scholar
|
|
47
|
Thorpe CT and Screen HR: Tendon structure
and composition. Adv Exp Med Biol. 920:3–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang G, Young BB, Ezura Y, Favata M,
Soslowsky LJ, Chakravarti S and Birk DE: Development of tendon
structure and function: Regulation of collagen fibrillogenesis. J
Musculoskelet Neuronal Interact. 5:5–21. 2005.PubMed/NCBI
|
|
49
|
Screen HRC, Berk DE, Kadler KE, Ramirez F
and Young MF: Tendon functional extracellular matrix. J Orthop Res.
33:793–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Riechert K, Labs K, Lindenhayn K and Sinha
P: Semiquantitative analysis of types I and III collagen from
tendons and ligaments in a rabbit model. J Orthop Sci. 6:68–74.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu SH, Yang RS, al-Shaikh R and Lane JM:
Collagen in tendon, ligament, and bone healing. A current review.
Clin Orthop Relat Res. 265–278. 1995.PubMed/NCBI
|
|
52
|
Birk DE and Mayne R: Localization of
collagen types I, III and V during tendon development. Changes in
collagen types I and III are correlated with changes in fibril
diameter. Eur J Cell Biol. 72:352–361. 1997.PubMed/NCBI
|
|
53
|
Wenstrup RJ, Smith SM, Florer JB, Zhang G,
Beason DP, Seegmiller RE, Soslowsky LJ and Birk DE: Regulation of
collagen fibril nucleation and initial fibril assembly involves
coordinate interactions with collagens V and XI in developing
tendon. J Biol Chem. 286:20455–20465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Izu Y, Ansorge HL, Zhang G, Soslowsky LJ,
Bonaldo P, Chu ML and Birk DE: Dysfunctional tendon collagen
fibrillogenesis in collagen VI null mice. Matrix Biol. 30:53–61.
2011. View Article : Google Scholar
|
|
55
|
Taye N, Karoulias SZ and Hubmacher D: The
'other' 15-40%: The role of non-collagenous extracellular matrix
proteins and minor collagens in tendon. J Orthop Res. 38:23–35.
2020. View Article : Google Scholar
|
|
56
|
Birch HL: Tendon matrix composition and
turnover in relation to functional requirements. Int J Exp Pathol.
88:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ristaniemi A, Regmi D, Mondal D,
Torniainen J, Tanska P, Stenroth L, Finnilä MAJ, Töyräs J and
Korhonen RK: Structure, composition and fibril-reinforced
poroviscoelastic properties of bovine knee ligaments and patellar
tendon. J R Soc Interface. 18:202007372021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schwartz AG, Lipner JH, Pasteris JD, Genin
GM and Thomopoulos S: Muscle loading is necessary for the formation
of a functional tendon enthesis. Bone. 55:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thorpe CT, Birch HL, Clegg PD and Screen
HRC: The role of the non-collagenous matrix in tendon function. Int
J Exp Pathol. 94:248–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen S and Birk DE: The regulatory roles
of small leucine-rich proteoglycans in extracellular matrix
assembly. FEBS J. 280:2120–2137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Halper J: Proteoglycans and diseases of
soft tissues. Adv Exp Med Biol. 802:49–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Smith RKW, Gerard M, Dowling B, Dart AJ,
Birch HL and Goodship AE: Correlation of cartilage oligomeric
matrix protein (COMP) levels in equine tendon with mechanical
properties: A proposed role for COMP in determining
function-specific mechanical characteristics of locomotor tendons.
Equine Vet J Suppl. 241–244. 2002.PubMed/NCBI
|
|
63
|
Järvinen TAH, Józsa L, Kannus P, Järvinen
TL, Hurme T, Kvist M, Pelto-Huikko M, Kalimo H and Järvinen M:
Mechanical loading regulates the expression of tenascin-C in the
myotendinous junction and tendon but does not induce de novo
synthesis in the skeletal muscle. J Cell Sci. 116:857–866. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pajala A, Melkko J, Leppilahti J, Ohtonen
P, Soini Y and Risteli J: Tenascin-C and type I and III collagen
expression in total Achilles tendon rupture. An immunohistochemical
study. Histol Histopathol. 24:1207–1211. 2009.PubMed/NCBI
|
|
65
|
Grant TM, Thompson MS, Urban J and Yu J:
Elastic fibres are broadly distributed in tendon and highly
localized around tenocytes. J Anat. 222:573–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thakkar D, Grant TM, Hakimi O and Carr AJ:
Distribution and expression of type VI collagen and elastic fibers
in human rotator cuff tendon tears. Connect Tissue Res. 55:397–402.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang J and Wang JHC: Characterization of
differential properties of rabbit tendon stem cells and tenocytes.
BMC Musculoskelet Disord. 11:102010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ralphs JR, Waggett AD and Benjamin M:
Actin stress fibres and cell-cell adhesion molecules in tendons:
Organisation in vivo and response to mechanical loading of tendon
cells in vitro. Matrix Biol. 21:67–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang S, Ju W and Chen X, Zhao Y, Feng L,
Yin Z and Chen X: Hierarchical ultrastructure: An overview of what
is known about tendons and future perspective for tendon
engineering. Bioact Mater. 8:124–139. 2022. View Article : Google Scholar
|
|
70
|
Marr N, Meeson R, Kelly EF, Fang Y,
Peffers MJ, Pitsillides AA, Dudhia J and Thorpe CT: CD146
Delineates an interfascicular cell sub-population in tendon that is
recruited during injury through its ligand laminin-α4. Int J Mol
Sci. 22:97292021. View Article : Google Scholar
|
|
71
|
Xu W, Hua H, Chiu YH, Li G, Zhi H, Yu Z,
Ren F, Luo Y and Cui W: CD146 regulates growth factor-induced
mTORC2 activity independent of the PI3K and mTORC1 pathways. Cell
Rep. 29:1311–1322.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Grol MW, Haelterman NA, Lim J, Munivez EM,
Archer M, Hudson DM, Tufa SF, Keene DR, Lei K, Park D, et al:
Tendon and motor phenotypes in the Crtap−/− mouse model
of recessive osteogenesis imperfecta. Elife. 10:e634882021.
View Article : Google Scholar
|
|
73
|
Flanagan K, Fitzgerald K, Baker J,
Regnstrom K, Gardai S, Bard F, Mocci S, Seto P, You M, Larochelle
C, et al: Laminin-411 is a vascular ligand for MCAM and facilitates
TH17 cell entry into the CNS. PLoS One. 7:e404432012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wei B and Lu J: Characterization of
tendon-derived stem cells and rescue tendon injury. Stem Cell Rev
Rep. 17:1534–1551. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou Z, Akinbiyi T, Xu L, Ramcharan M,
Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL
and Sun HB: Tendon-derived stem/progenitor cell aging: Defective
self-renewal and altered fate. Aging Cell. 9:911–915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang J and Wang JHC: Mechanobiological
response of tendon stem cells: Implications of tendon homeostasis
and pathogenesis of tendinopathy. J Orthop Res. 28:639–643. 2010.
View Article : Google Scholar
|
|
77
|
Voleti PB, Buckley MR and Soslowsky LJ:
Tendon healing: repair and regeneration. Annu Rev Biomed Eng.
14:47–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Elliott DM, Robinson PS, Gimbel JA, Sarver
JJ, Abboud JA, Iozzo RV and Soslowsky LJ: Effect of altered matrix
proteins on quasilinear viscoelastic properties in transgenic mouse
tail tendons. Ann Biomed Eng. 31:599–605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Winnicki K, Ochała-Kłos A, Rutowicz B,
Pękala PA and Tomaszewski KA: Functional anatomy, histology and
biomechanics of the human Achilles tendon-A comprehensive review.
Ann Anat. 229:1514612020. View Article : Google Scholar
|
|
80
|
Zhang M, Liu H, Cui Q, Han P, Yang S, Shi
M, Zhang T, Zhang Z and Li Z: Tendon stem cell-derived exosomes
regulate inflammation and promote the high-quality healing of
injured tendon. Stem Cell Res Ther. 11:4022020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Margiana R, Markov A, Zekiy AO, Hamza MU,
Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar
HH, et al: Clinical application of mesenchymal stem cell in
regenerative medicine: A narrative review. Stem Cell Res Ther.
13:3662022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chanda D, Kumar S and Ponnazhagan S:
Therapeutic potential of adult bone marrow-derived mesenchymal stem
cells in diseases of the skeleton. J Cell Biochem. 111:249–257.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
McDougall RA, Canapp SO and Canapp DA:
Ultrasonographic findings in 41 dogs treated with bone marrow
aspirate concentrate and platelet-rich plasma for a supraspinatus
tendinopathy: A retrospective study. Front Vet Sci. 5:982018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ruzzini L, Longo UG, Rizzello G and Denaro
V: Stem cells and tendinopathy: State of the art from the basic
science to clinic application. Muscles Ligaments Tendons J.
2:235–238. 2012.
|
|
85
|
Bi Y, Ehirchiou D, Kilts TM, Inkson CA,
Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al:
Identification of tendon stem/progenitor cells and the role of the
extracellular matrix in their niche. Nat Med. 13:1219–1227. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chong AKS, Ang AD, Goh JCH, Hui JHP, Lim
AYT, Lee EH and Lim BH: Bone marrow-derived mesenchymal stem cells
influence early tendon-healing in a rabbit achilles tendon model. J
Bone Joint Surg Am. 89:74–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Harris MT, Butler DL, Boivin GP, Florer
JB, Schantz EJ and Wenstrup RJ: Mesenchymal stem cells used for
rabbit tendon repair can form ectopic bone and express alkaline
phosphatase activity in constructs. J Orthop Res. 22:998–1003.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Arnaud-Franco Á, Lara-Arias J,
Marino-Martínez IA, Cienfuegos-Jiménez O, Barbosa-Quintana Á and
Peña-Martínez VM: Effect of adipose-derived mesenchymal stem cells
(ADMSCs) application in achilles-tendon injury in an animal model.
Curr Issues Mol Biol. 44:5827–5838. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen
P, Ma K and Zhou C: Comparative analysis of mesenchymal stem cells
from bone marrow, cartilage, and adipose tissue. Stem Cells Dev.
17:761–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chu DT, Nguyen Thi Phuong T, Tien NLB,
Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH and Thi Nga V:
Adipose tissue stem cells for therapy: An update on the progress of
isolation, culture, storage, and clinical application. J Clin Med.
8:9172019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gentile P, Calabrese C, De Angelis B,
Pizzicannella J, Kothari A and Garcovich S: Impact of the different
preparation methods to obtain human adipose-derived stromal
vascular fraction cells (AD-SVFs) and human adipose-derived
mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus
mechanical centrifugation. Int J Mol Sci. 20:54712019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Itro A, Trotta MC, Miranda R, Paoletta M,
De Cicco A, Lepre CC, Tarantino U, D'Amico M, Toro G and Schiavone
Panni A: Why use adipose-derived mesenchymal stem cells in
tendinopathic patients: A systematic review. Pharmaceutics.
14:11512022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin L, Shen Q, Xue T and Yu CL:
Heterotopic ossification induced by Achilles tenotomy via
endochondral bone formation: Expression of bone and cartilage
related genes. Bone. 46:425–431. 2010. View Article : Google Scholar
|
|
94
|
Schipani E: Posttranslational
modifications of collagens as targets of hypoxia and Hif-1alpha in
endochondral bone development. Ann N Y Acad Sci. 1192:317–321.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kokubu S, Inaki R, Hoshi K and Hikita A:
Adipose-derived stem cells improve tendon repair and prevent
ectopic ossification in tendinopathy by inhibiting inflammation and
inducing neovascularization in the early stage of tendon healing.
Regen Ther. 14:103–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lee SY, Kwon B, Lee K, Son YH and Chung
SG: Therapeutic mechanisms of human adipose-derived mesenchymal
stem cells in a rat tendon injury model. Am J Sports Med.
45:1429–1439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Naji A, Eitoku M, Favier B, Deschaseaux F,
Rouas-Freiss N and Suganuma N: Biological functions of mesenchymal
stem cells and clinical implications. Cell Mol Life Sci.
76:3323–3348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fu G, Lu L, Pan Z, Fan A and Yin F:
Adipose-derived stem cell exosomes facilitate rotator cuff repair
by mediating tendon-derived stem cells. Regen Med. 16:359–372.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu H, Zhang M, Shi M, Zhang T, Lu W, Yang
S, Cui Q and Li Z: Adipose-derived mesenchymal stromal cell-derived
exosomes promote tendon healing by activating both SMAD1/5/9 and
SMAD2/3. Stem Cell Res Ther. 12:3382021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shi Y, Kang X, Wang Y, Bian X, He G, Zhou
M and Tang K: Exosomes derived from bone marrow stromal cells
(BMSCs) enhance tendon-bone healing by regulating macrophage
polarization. Med Sci Monit. 26:e9233282020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu XD, Kang L, Tian J, Wu Y, Huang Y, Liu
J, Wang H, Qiu G and Wu Z: Exosomes derived from magnetically
actuated bone mesenchymal stem cells promote tendon-bone healing
through the miR-21-5p/SMAD7 pathway. Mater Today Bio.
15:1003192022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Heo JS, Choi Y and Kim HO: Adipose-derived
mesenchymal stem cells promote M2 macrophage phenotype through
exosomes. Stem Cells Int. 2019:79217602019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ragni E, Papait A, Perucca Orfei C, Silini
AR, Colombini A, Viganò M, Libonati F, Parolini O and de Girolamo
L: Amniotic membrane-mesenchymal stromal cells secreted factors and
extracellular vesicle-miRNAs: Anti-inflammatory and regenerative
features for musculoskeletal tissues. Stem Cells Transl Med.
10:1044–1062. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hong P, Yang H, Wu Y, Li K and Tang Z: The
functions and clinical application potential of exosomes derived
from adipose mesenchymal stem cells: A comprehensive review. Stem
Cell Res Ther. 10:2422019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen SH, Chen ZY, Lin YH, Chen SH, Chou
PY, Kao HK and Lin FH: Extracellular vesicles of adipose-derived
stem cells promote the healing of traumatized achilles tendons. Int
J Mol Sci. 22:123732021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Racchetti G and Meldolesi J: Extracellular
vesicles of mesenchymal stem cells: Therapeutic properties
discovered with extraordinary success. Biomedicines. 9:6672021.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tetta C, Consiglio AL, Bruno S, Tetta E,
Gatti E, Dobreva M, Cremonesi F and Camussi G: The role of
microvesicles derived from mesenchymal stem cells in tissue
regeneration; a dream for tendon repair? Muscles Ligaments Tendons
J. 2:212–221. 2012.
|
|
108
|
Rodas G, Soler-Rich R, Rius-Tarruella J,
Alomar X, Balius R, Orozco L, Masci L and Maffulli N: Effect of
autologous expanded bone marrow mesenchymal stem cells or
leukocyte-poor platelet-rich plasma in chronic patellar
tendinopathy (with gap > 3 mm): Preliminary outcomes after 6
months of a double-blind, randomized, prospective study. Am J
Sports Med. 49:1492–1504. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Caplan AI: Review: Mesenchymal stem cells:
Cell-based reconstructive therapy in orthopedics. Tissue Eng.
11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhu Z, Gan X, Fan H and Yu H: Mechanical
stretch endows mesenchymal stem cells stronger angiogenic and
anti-apoptotic capacities via NFκB activation. Biochem Biophys Res
Commun. 468:601–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kasper G, Dankert N, Tuischer J, Hoeft M,
Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M,
Matziolis G and Duda GN: Mesenchymal stem cells regulate
angiogenesis according to their mechanical environment. Stem Cells.
25:903–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Longo UG, Garau G, Denaro V and Maffulli
N: Surgical management of tendinopathy of biceps femoris tendon in
athletes. Disabil Rehabil. 30:1602–1607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Millar NL, Murrell GAC and McInnes IB:
Inflammatory mechanisms in tendinopathy-towards translation. Nat
Rev Rheumatol. 13:110–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Dakin SG, Newton J, Martinez FO, Hedley R,
Gwilym S, Jones N, Reid HAB, Wood S, Wells G, Appleton L, et al:
Chronic inflammation is a feature of Achilles tendinopathy and
rupture. Br J Sports Med. 52:359–367. 2018. View Article : Google Scholar
|
|
115
|
Frich LH, Fernandes LR, Schrøder HD,
Hejbøl EK, Nielsen PV, Jørgensen PH, Stensballe A and Lambertsen
KL: The inflammatory response of the supraspinatus muscle in
rotator cuff tear conditions. J Shoulder Elbow Surg. 30:e261–e275.
2021. View Article : Google Scholar
|
|
116
|
Yang G, Rothrauff BB and Tuan RS: Tendon
and ligament regeneration and repair: Clinical relevance and
developmental paradigm. Birth Defects Res C Embryo Today.
99:203–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Arvind V and Huang AH: Reparative and
maladaptive inflammation in tendon healing. Front Bioeng
Biotechnol. 9:7190472021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Crowe LAN, McLean M, Kitson SM, Melchor
EG, Patommel K, Cao HM, Reilly JH, Leach WJ, Rooney BP, Spencer SJ,
et al: S100A8 & S100A9: Alarmin mediated inflammation in
tendinopathy. Sci Rep. 9:14632019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Marsolais D, Côté CH and Frenette J:
Neutrophils and macrophages accumulate sequentially following
Achilles tendon injury. J Orthop Res. 19:1203–1209. 2001.
View Article : Google Scholar
|
|
120
|
Abraham AC, Shah SA, Golman M, Song L, Li
X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM and
Thomopoulos S: Targeting the NF-κB signaling pathway in chronic
tendon disease. Sci Transl Med. 11:eaav43192019. View Article : Google Scholar
|
|
121
|
Thankam FG, Dilisio MF, Dietz NE and
Agrawal DK: TREM-1, HMGB1 and RAGE in the shoulder tendon: Dual
mechanisms for inflammation based on the coincidence of
glenohumeral arthritis. PLoS One. 11:e01654922016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Voloshin I, Gelinas J, Maloney MD, O'Keefe
RJ, Bigliani LU and Blaine TA: Proinflammatory cytokines and
metalloproteases are expressed in the subacromial bursa in patients
with rotator cuff disease. Arthroscopy. 21:1076.e1–1076.e9. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Eming SA, Wynn TA and Martin P:
Inflammation and metabolism in tissue repair and regeneration.
Science. 356:1026–1030. 2017. View Article : Google Scholar
|
|
124
|
Jin R, Xu J, Gao Q, Mao X, Yin J, Lu K,
Guo Y, Zhang M and Cheng R: IL-33-induced neutrophil extracellular
traps degrade fibronectin in a murine model of bronchopulmonary
dysplasia. Cell Death Discov. 6:332020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gause WC, Wynn TA and Allen JE: Type 2
immunity and wound healing: Evolutionary refinement of adaptive
immunity by helminths. Nat Rev Immunol. 13:607–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shi Y and Wang Y, Li Q, Liu K, Hou J, Shao
C and Wang Y: Immunoregulatory mechanisms of mesenchymal stem and
stromal cells in inflammatory diseases. Nat Rev Nephrol.
14:493–507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Selmani Z, Naji A, Zidi I, Favier B,
Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et
al: Human leukocyte antigen-G5 secretion by human mesenchymal stem
cells is required to suppress T lymphocyte and natural killer
function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem
Cells. 26:212–222. 2008. View Article : Google Scholar
|
|
128
|
Shen H, Yoneda S, Abu-Amer Y, Guilak F and
Gelberman RH: Stem cell-derived extracellular vesicles attenuate
the early inflammatory response after tendon injury and repair. J
Orthop Res. 38:117–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Huang Y, He B, Wang L, Yuan B, Shu H,
Zhang F and Sun L: Bone marrow mesenchymal stem cell-derived
exosomes promote rotator cuff tendon-bone healing by promoting
angiogenesis and regulating M1 macrophages in rats. Stem Cell Res
Ther. 11:4962020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Challoumas D, Biddle M and Millar NL:
Recent advances in tendinopathy. Fac Rev. 9:162020. View Article : Google Scholar
|
|
131
|
Zhang K, Asai S, Yu B and Enomoto-Iwamoto
M: IL-1β irreversibly inhibits tenogenic differentiation and alters
metabolism in injured tendon-derived progenitor cells in vitro.
Biochem Biophys Res Commun. 463:667–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tohyama H, Yasuda K, Uchida H and
Nishihira J: The responses of extrinsic fibroblasts infiltrating
the devitalised patellar tendon to IL-1beta are different from
those of normal tendon fibroblasts. J Bone Joint Surg Br.
89:1261–1267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Guerquin MJ, Charvet B, Nourissat G, Havis
E, Ronsin O, Bonnin MA, Ruggiu M, Olivera-Martinez I, Robert N, Lu
Y, et al: Transcription factor EGR1 directs tendon differentiation
and promotes tendon repair. J Clin Invest. 123:3564–3576. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang G, Im HJ and Wang JHC: Repetitive
mechanical stretching modulates IL-1beta induced COX-2, MMP-1
expression, and PGE2 production in human patellar tendon
fibroblasts. Gene. 363:166–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
136
|
Gohda J, Matsumura T and Inoue JI: Cutting
edge: TNFR-associated factor (TRAF) 6 is essential for
MyD88-dependent pathway but not toll/IL-1 receptor
domain-containing adaptor-inducing IFN-beta (TRIF)-dependent
pathway in TLR signaling. J Immunol. 173:2913–2917. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Striz I, Brabcova E, Kolesar L and
Sekerkova A: Cytokine networking of innate immunity cells: A
potential target of therapy. Clin Sci (Lond). 126:593–612. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schottelius AJ, Mayo MW, Sartor RB and
Baldwin AS Jr: Interleukin-10 signaling blocks inhibitor of kappaB
kinase activity and nuclear factor kappaB DNA binding. J Biol Chem.
274:31868–31874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: Key elements of proinflammatory
signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
141
|
Xie F, Teng L, Xu J, Lu J, Zhang C, Yang
L, Ma X and Zhao M: Interleukin-10 modified bone marrow mesenchymal
stem cells prevent hypertrophic scar formation by inhibiting
inflammation. Pharmazie. 75:571–575. 2020.PubMed/NCBI
|
|
142
|
Best KT, Nichols AEC, Knapp E, Hammert WC,
Ketonis C, Jonason JH, Awad HA and Loiselle AE: NF-κB activation
persists into the remodeling phase of tendon healing and promotes
myofibroblast survival. Sci Signal. 13:eabb72092020. View Article : Google Scholar
|
|
143
|
Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B
and Chen L: BMSC-derived exosomes promote tendon-bone healing after
anterior cruciate ligament reconstruction by regulating M1/M2
macrophage polarization in rats. Stem Cell Res Ther. 13:2952022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Gumina S, Natalizi S, Melaragni F,
Leopizzi M, Carbone S, Postacchini F, Milani A and Della Rocca C:
The possible role of the transcription factor nuclear factor-κB on
evolution of rotator cuff tear and on mechanisms of cuff tendon
healing. J Shoulder Elbow Surg. 22:673–680. 2013. View Article : Google Scholar
|
|
145
|
Kirkham AM, Bailey AJM, Tieu A, Maganti
HB, Montroy J, Shorr R, Campbell TM, Fergusson DA, Lalu MM,
Elmoazzen H and Allan DS: MSC-derived extracellular vesicles in
preclinical animal models of bone injury: A systematic review and
meta-analysis. Stem Cell Rev Rep. 18:1054–1066. 2022. View Article : Google Scholar
|
|
146
|
Tardito S, Martinelli G, Soldano S,
Paolino S, Pacini G, Patane M, Alessandri E, Smith V and Cutolo M:
Macrophage M1/M2 polarization and rheumatoid arthritis: A
systematic review. Autoimmun Rev. 18:1023972019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chamberlain CS, Clements AEB, Kink JA,
Choi U, Baer GS, Halanski MA, Hematti P and Vanderby R:
Extracellular vesicle-educated macrophages promote early achilles
tendon healing. Stem Cells. 37:652–662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Andia I, Sanchez M and Maffulli N: Tendon
healing and platelet-rich plasma therapies. Expert Opin Biol Ther.
10:1415–1426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Durgam S and Stewart M: Cellular and
molecular factors influencing tendon repair. Tissue Eng Part B Rev.
23:307–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Pufe T, Petersen W, Tillmann B and
Mentlein R: The angiogenic peptide vascular endothelial growth
factor is expressed in foetal and ruptured tendons. Virchows Arch.
439:579–585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kannus P: Etiology and pathophysiology of
chronic tendon disorders in sports. Scand J Med Sci Sports.
7:78–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Taylor CT: Interdependent roles for
hypoxia inducible factor and nuclear factor-kappaB in hypoxic
inflammation. J Physiol. 586:4055–4059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Berse B, Hunt JA, Diegel RJ, Morganelli P,
Yeo K, Brown F and Fava RA: Hypoxia augments cytokine (transforming
growth factor-beta (TGF-beta) and IL-1)-induced vascular
endothelial growth factor secretion by human synovial fibroblasts.
Clin Exp Immunol. 115:176–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Semenza GL: Hydroxylation of HIF-1: Oxygen
sensing at the molecular level. Physiology (Bethesda). 19:176–182.
2004.PubMed/NCBI
|
|
155
|
Cummins EP and Taylor CT:
Hypoxia-responsive transcription factors. Pflugers Arch.
450:363–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wezenbeek E, Willems T, Mahieu N, De
Muynck M, Vanden Bossche L, Steyaert A, De Clercq D and Witvrouw E:
The role of the vascular and structural response to activity in the
development of achilles tendinopathy: A prospective study. Am J
Sports Med. 46:947–954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kaigler D, Krebsbach PH, Polverini PJ and
Mooney DJ: Role of vascular endothelial growth factor in bone
marrow stromal cell modulation of endothelial cells. Tissue Eng.
9:95–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Furumatsu T, Shen ZN, Kawai A, Nishida K,
Manabe H, Oohashi T, Inoue H and Ninomiya Y: Vascular endothelial
growth factor principally acts as the main angiogenic factor in the
early stage of human osteoblastogenesis. J Biochem. 133:633–639.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Al-Khaldi A, Eliopoulos N, Martineau D,
Lejeune L, Lachapelle K and Galipeau J: Postnatal bone marrow
stromal cells elicit a potent VEGF-dependent neoangiogenic response
in vivo. Gene Ther. 10:621–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Rauniyar K, Jha SK and Jeltsch M: Biology
of vascular endothelial growth factor C in the morphogenesis of
lymphatic vessels. Front Bioeng Biotechnol. 6:72018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Boyer MI, Watson JT, Lou J, Manske PR,
Gelberman RH and Cai SR: Quantitative variation in vascular
endothelial growth factor mRNA expression during early flexor
tendon healing: An investigation in a canine model. J Orthop Res.
19:869–872. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Conway EM, Collen D and Carmeliet P:
Molecular mechanisms of blood vessel growth. Cardiovasc Res.
49:507–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Karamysheva AF: Mechanisms of
angiogenesis. Biochemistry (Mosc). 73:751–762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ferrara N: Molecular and biological
properties of vascular endothelial growth factor. J Mol Med (Berl).
77:527–543. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y,
Duan D, Hu Z, Chen P and Lu M: Extracellular vesicles derived from
hypoxia-preconditioned olfactory mucosa mesenchymal stem cells
enhance angiogenesis via miR-612. J Nanobiotechnology. 19:3802021.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Hou J, Zhong T, Guo T, Miao C, Zhou C,
Long H, Wu H, Zheng S, Wang L and Wang T: Apelin promotes
mesenchymal stem cells survival and vascularization under
hypoxic-ischemic condition in vitro involving the upregulation of
vascular endothelial growth factor. Exp Mol Pathol. 102:203–209.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chen G, Zhang W, Zhang K, Wang S, Gao Y,
Gu J, He L, Li W, Zhang C, Zhang W, et al: Hypoxia-induced
mesenchymal stem cells exhibit stronger tenogenic differentiation
capacities and promote patellar tendon repair in rabbits. Stem
Cells Int. 2020:88226092020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Haque N, Rahman MT, Abu Kasim NH and
Alabsi AM: Hypoxic culture conditions as a solution for mesenchymal
stem cell based regenerative therapy. ScientificWorldJournal.
2013:6329722013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing
X and Liu L: miRNA-126-3p carried by human umbilical cord
mesenchymal stem cell enhances endothelial function through
exosome-mediated mechanisms in vitro and attenuates vein graft
neointimal formation in vivo. Stem Cell Res Ther. 11:4642020.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul
C, Millard RW, Xiao DS, Ashraf M and Xu M: Mesenchymal stem cells
release exosomes that transfer miRNAs to endothelial cells and
promote angiogenesis. Oncotarget. 8:45200–45212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Liang X, Zhang L, Wang S, Han Q and Zhao
RC: Exosomes secreted by mesenchymal stem cells promote endothelial
cell angiogenesis by transferring miR-125a. J Cell Sci.
129:2182–2189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wu F, Nerlich M and Docheva D: Tendon
injuries: Basic science and new repair proposals. EFORT Open Rev.
2:332–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Thomopoulos S, Parks WC, Rifkin DB and
Derwin KA: Mechanisms of tendon injury and repair. J Orthop Res.
33:832–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Walia B and Huang AH: Tendon stem
progenitor cells: Understanding the biology to inform therapeutic
strategies for tendon repair. J Orthop Res. 37:1270–1280. 2019.
View Article : Google Scholar :
|
|
176
|
Lovati AB, Corradetti B, Lange Consiglio
A, Recordati C, Bonacina E, Bizzaro D and Cremonesi F:
Characterization and differentiation of equine tendon-derived
progenitor cells. J Biol Regul Homeost Agents. 25(2 Suppl):
S75–S84. 2011.PubMed/NCBI
|
|
177
|
Qin S, Wang W, Liu Z, Hua X, Fu S, Dong F,
Li A, Liu Z, Wang P, Dai L, et al: Fibrochondrogenic
differentiation potential of tendon-derived stem/progenitor cells
from human patellar tendon. J Orthop Translat. 22:101–108. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Al-Ani MKH, Xu K, Sun Y, Pan L, Xu Z and
Yang L: Study of bone marrow mesenchymal and tendon-derived stem
cells transplantation on the regenerating effect of achilles tendon
ruptures in rats. Stem Cells Int. 2015:9841462015. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Leong DJ and Sun HB: Mesenchymal stem
cells in tendon repair and regeneration: Basic understanding and
translational challenges. Ann N Y Acad Sci. 1383:88–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Vermeulen S, Vasilevich A, Tsiapalis D,
Roumans N, Vroemen P, Beijer NRM, Dede Eren A, Zeugolis D and de
Boer J: Identification of topographical architectures supporting
the phenotype of rat tenocytes. Acta Biomater. 83:277–290. 2019.
View Article : Google Scholar
|
|
181
|
Lee CH, Cook JL, Mendelson A, Moioli EK,
Yao H and Mao JJ: Regeneration of the articular surface of the
rabbit synovial joint by cell homing: A proof of concept study.
Lancet. 376:440–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Seuntjens E, Umans L, Zwijsen A,
Sampaolesi M, Verfaillie CM and Huylebroeck D: Transforming growth
factor type beta and Smad family signaling in stem cell function.
Cytokine Growth Factor Rev. 20:449–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong
X, Zhang Z, Hu J, Yin Z, Heng BC, et al: Mohawk promotes the
tenogenesis of mesenchymal stem cells through activation of the
TGFβ signaling pathway. Stem Cells. 33:443–455. 2015. View Article : Google Scholar
|
|
185
|
Kim HM, Galatz LM, Das R, Havlioglu N,
Rothermich SY and Thomopoulos S: The role of transforming growth
factor beta isoforms in tendon-to-bone healing. Connect Tissue Res.
52:87–98. 2011. View Article : Google Scholar
|
|
186
|
Lee CH, Shah B, Moioli EK and Mao JJ: CTGF
directs fibroblast differentiation from human mesenchymal
stem/stromal cells and defines connective tissue healing in a
rodent injury model. J Clin Invest. 125:39922015. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Li X, Pongkitwitoon S, Lu H, Lee C,
Gelberman R and Thomopoulos S: CTGF induces tenogenic
differentiation and proliferation of adipose-derived stromal cells.
J Orthop Res. 37:574–582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Lee CH, Lee FY, Tarafder S, Kao K, Jun Y,
Yang G and Mao JJ: Harnessing endogenous stem/progenitor cells for
tendon regeneration. J Clin Invest. 125:2690–2701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhang B, Luo Q, Chen Z, Shi Y, Ju Y, Yang
L and Song G: Increased nuclear stiffness via FAK-ERK1/2 signaling
is necessary for synthetic mechano-growth factor E peptide-induced
tenocyte migration. Sci Rep. 6:188092016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Shen H, Gelberman RH, Silva MJ,
Sakiyama-Elbert SE and Thomopoulos S: BMP12 induces tenogenic
differentiation of adipose-derived stromal cells. PLoS One.
8:e776132013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Violini S, Ramelli P, Pisani LF, Gorni C
and Mariani P: Horse bone marrow mesenchymal stem cells express
embryo stem cell markers and show the ability for tenogenic
differentiation by in vitro exposure to BMP-12. BMC Cell Biol.
10:292009. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Gonçalves AI, Rodrigues MT, Lee SJ, Atala
A, Yoo JJ, Reis RL and Gomes ME: Understanding the role of growth
factors in modulating stem cell tenogenesis. PLoS One.
8:e837342013. View Article : Google Scholar
|
|
193
|
Wu T, Liu Y, Wang B, Sun Y, Xu J, Yuk-Wai
LW, Xu L, Zhang J and Li G: The use of cocultured mesenchymal stem
cells with tendon-derived stem cells as a better cell source for
tendon repair. Tissue Eng Part A. 22:1229–1240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Kuo CK, Marturano JE and Tuan RS: Novel
strategies in tendon and ligament tissue engineering: Advanced
biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil
Ther Technol. 2:202010.PubMed/NCBI
|
|
195
|
Park SA, Kim IA, Lee YJ and Shin JW, Kim
CR, Kim JK, Yang YI and Shin JW: Biological responses of ligament
fibroblasts and gene expression profiling on micropatterned
silicone substrates subjected to mechanical stimuli. J Biosci
Bioeng. 102:402–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Riboh J, Chong AK, Pham H, Longaker M,
Jacobs C and Chang J: Optimization of flexor tendon tissue
engineering with a cyclic strain bioreactor. J Hand Surg Am.
33:1388–1396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Yang G, Crawford RC and Wang JH:
Proliferation and collagen production of human patellar tendon
fibroblasts in response to cyclic uniaxial stretching in serum-free
conditions. J Biomech. 37:1543–1550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Costa-Almeida R, Calejo I and Gomes ME:
Mesenchymal stem cells empowering tendon regenerative therapies.
Int J Mol Sci. 20:30022019. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Crovace A, Lacitignola L, Rossi G and
Francioso E: Histological and immunohistochemical evaluation of
autologous cultured bone marrow mesenchymal stem cells and bone
marrow mononucleated cells in collagenase-induced tendinitis of
equine superficial digital flexor tendon. Vet Med Int.
2010:2509782010. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Bramono DS, Richmond JC, Weitzel PP,
Kaplan DL and Altman GH: Matrix metalloproteinases and their
clinical applications in orthopaedics. Clin Orthop Relat Res.
272–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Oshiro W, Lou J, Xing X, Tu Y and Manske
PR: Flexor tendon healing in the rat: A histologic and gene
expression study. J Hand Surg Am. 28:814–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Gruber R, Kandler B, Holzmann P,
Vögele-Kadletz M, Losert U, Fischer MB and Watzek G: Bone marrow
stromal cells can provide a local environment that favors migration
and formation of tubular structures of endothelial cells. Tissue
Eng. 11:896–903. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zhang X, Cai Z, Wu M, Huangfu X, Li J and
Liu X: Adipose stem cell-derived exosomes recover impaired matrix
metabolism of torn human rotator cuff tendons by maintaining tissue
homeostasis. Am J Sports Med. 49:899–908. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Katzel EB, Wolenski M, Loiselle AE, Basile
P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC and
O'Keefe RJ: Impact of Smad3 loss of function on scarring and
adhesion formation during tendon healing. J Orthop Res. 29:684–693.
2011. View Article : Google Scholar
|
|
205
|
Brown KA, Pietenpol JA and Moses HL: A
tale of two proteins: Differential roles and regulation of Smad2
and Smad3 in TGF-beta signaling. J Cell Biochem. 101:9–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Li F, Zeng B, Chai Y, Cai P, Fan C and
Cheng T: The linker region of Smad2 mediates TGF-beta-dependent
ERK2-induced collagen synthesis. Biochem Biophys Res Commun.
386:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Moustakas A, Pardali K, Gaal A and Heldin
CH: Mechanisms of TGF-beta signaling in regulation of cell growth
and differentiation. Immunol Lett. 82:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Han Y, Yang J, Fang J, Zhou Y, Candi E,
Wang J, Hua D, Shao C and Shi Y: The secretion profile of
mesenchymal stem cells and potential applications in treating human
diseases. Signal Transduct Target Ther. 7:922022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Almalki SG: Adipose-derived mesenchymal
stem cells and wound healing: Potential clinical applications in
wound repair. Saudi Med J. 43:1075–1086. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Chen X, Song XH, Yin Z, Zou XH, Wang LL,
Hu H, Cao T, Zheng M and Ouyang HW: Stepwise differentiation of
human embryonic stem cells promotes tendon regeneration by
secreting fetal tendon matrix and differentiation factors. Stem
Cells. 27:1276–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Chen X, Zou XH, Yin GL and Ouyang HW:
Tendon tissue engineering with mesenchymal stem cells and
biografts: An option for large tendon defects? Front Biosci (Schol
Ed). 1:23–32. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
212
|
Wang XT, Liu PY and Tang JB: Tendon
healing in vitro: Modification of tenocytes with exogenous vascular
endothelial growth factor gene increases expression of transforming
growth factor beta but minimally affects expression of collagen
genes. J Hand Surg Am. 30:222–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wang QW, Chen ZL and Piao YJ: Mesenchymal
stem cells differentiate into tenocytes by bone morphogenetic
protein (BMP) 12 gene transfer. J Biosci Bioeng. 100:418–422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Kraus A, Woon C, Raghavan S, Megerle K,
Pham H and Chang J: Co-culture of human adipose-derived stem cells
with tenocytes increases proliferation and induces differentiation
into a tenogenic lineage. Plast Reconstr Surg. 132:754e–766e. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Sugimoto Y, Takimoto A, Akiyama H, Kist R,
Scherer G, Nakamura T, Hiraki Y and Shukunami C: Scx+/Sox9+
progenitors contribute to the establishment of the junction between
cartilage and tendon/ligament. Development. 140:2280–2288. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Liu H, Xu J, Lan Y, Lim HW and Jiang R:
The scleraxis transcription factor directly regulates multiple
distinct molecular and cellular processes during early tendon cell
differentiation. Front Cell Dev Biol. 9:6543972021. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Oshita T, Tobita M, Tajima S and Mizuno H:
Adipose-derived stem cells improve collagenase-induced tendinopathy
in a rat model. Am J Sports Med. 44:1983–1989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Aicale R, Tarantino D and Maffulli N:
Overuse injuries in sport: A comprehensive overview. J Orthop Surg
Res. 13:3092018. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Steinmann S, Pfeifer CG, Brochhausen C and
Docheva D: Spectrum of tendon pathologies: Triggers, trails and
end-state. Int J Mol Sci. 21:8442020. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Canapp SO Jr, Canapp DA, Ibrahim V, Carr
BJ, Cox C and Barrett JG: The use of adipose-derived progenitor
cells and platelet-rich plasma combination for the treatment of
supraspinatus tendinopathy in 55 dogs: A retrospective study. Front
Vet Sci. 3:612016. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Bruns J, Kampen J, Kahrs J and Plitz W:
Achilles tendon rupture: Experimental results on spontaneous repair
in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 8:364–369.
2000. View Article : Google Scholar
|
|
222
|
Im GI and Kim TK: Stem cells for the
regeneration of tendon and ligament: A perspective. Int J Stem
Cells. 13:335–341. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Arnhold S, Elashry MI, Klymiuk MC and
Wenisch S: Biological macromolecules and mesenchymal stem cells:
Basic research for regenerative therapies in veterinary medicine.
Int J Biol Macromol. 123:889–899. 2019. View Article : Google Scholar
|
|
224
|
Harman RM, Patel RS, Fan JC, Park JE,
Rosenberg BR and Van de Walle GR: Single-cell RNA sequencing of
equine mesenchymal stromal cells from primary donor-matched tissue
sources reveals functional heterogeneity in immune modulation and
cell motility. Stem Cell Res Ther. 11:5242020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Gonçalves AI, Gershovich PM, Rodrigues MT,
Reis RL and Gomes ME: Human adipose tissue-derived tenomodulin
positive subpopulation of stem cells: A promising source of tendon
progenitor cells. J Tissue Eng Regen Med. 12:762–774. 2018.
View Article : Google Scholar
|
|
226
|
Dias IE, Cardoso DF, Soares CS, Barros LC,
Viegas CA, Carvalho PP and Dias IR: Clinical application of
mesenchymal stem cells therapy in musculoskeletal injuries in
dogs-a review of the scientific literature. Open Vet J. 11:188–202.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Loebel C and Burdick JA: Engineering stem
and stromal cell therapies for musculoskeletal tissue repair. Cell
Stem Cell. 22:325–339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Lui PPY: Mesenchymal stem cell-derived
extracellular vesicles for the promotion of tendon repair-an update
of literature. Stem Cell Rev Rep. 17:379–389. 2021. View Article : Google Scholar
|
|
229
|
Brennan MÁ, Layrolle P and Mooney DJ:
Biomaterials functionalized with MSC secreted extracellular
vesicles and soluble factors for tissue regeneration. Adv Funct
Mater. 30:19091252020. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Peroni JF and Borjesson DL:
Anti-inflammatory and immunomodulatory activities of stem cells.
Vet Clin North Am Equine Pract. 27:351–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Shojaee A and Parham A: Strategies of
tenogenic differentiation of equine stem cells for tendon repair:
Current status and challenges. Stem Cell Res Ther. 10:1812019.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Gugjoo MB, Pal Amar, Makhdoomi DM and
Sharma GT: Equine mesenchymal stem cells: Properties, sources,
characterization, and potential therapeutic applications. J Equine
Vet Sci. 72:16–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Iacono E, Pascucci L, Rossi B, Bazzucchi
C, Lanci A, Ceccoli M and Merlo B: Ultrastructural characteristics
and immune profile of equine MSCs from fetal adnexa. Reproduction.
154:509–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Ikehara S: A novel BMT technique for
treatment of various currently intractable diseases. Best Pract Res
Clin Haematol. 24:477–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
De Becker A and Riet IV: Homing and
migration of mesenchymal stromal cells: How to improve the efficacy
of cell therapy? World J Stem Cells. 8:73–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Zhao T, Qi Y, Xiao S, Ran J, Wang J,
Ghamor-Amegavi EP, Zhou X, Li H, He T, Gou Z, et al: Integration of
mesenchymal stem cell sheet and bFGF-loaded fibrin gel in knitted
PLGA scaffolds favorable for tendon repair. J Mater Chem B.
7:2201–2211. 2019. View Article : Google Scholar
|
|
237
|
Supokawej A, Korchunjit W and Wongtawan T:
The combination of BMP12 and KY02111 enhances tendon
differentiation in bone marrow-derived equine mesenchymal stromal
cells (BM-eMSCs). J Equine Sci. 33:19–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Huang TF, Yew TL, Chiang ER, Ma HL, Hsu
CY, Hsu SH, Hsu YT and Hung SC: Mesenchymal stem cells from a
hypoxic culture improve and engraft Achilles tendon repair. Am J
Sports Med. 41:1117–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Han P, Cui Q, Yang S, Wang H, Gao P and Li
Z: Tumor necrosis factor-α and transforming growth factor-β1
facilitate differentiation and proliferation of tendon-derived stem
cells in vitro. Biotechnol Lett. 39:711–719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Ouyang HW, Goh JCH and Lee EH: Use of bone
marrow stromal cells for tendon graft-to-bone healing: Histological
and immunohistochemical studies in a rabbit model. Am J Sports Med.
32:321–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Yao Z, Li J, Xiong H, Cui H, Ning J, Wang
S, Ouyang X, Qian Y and Fan C: MicroRNA engineered umbilical cord
stem cell-derived exosomes direct tendon regeneration by mTOR
signaling. J Nanobiotechnology. 19:1692021. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Shen H, Kormpakis I, Havlioglu N,
Linderman SW, Sakiyama-Elbert SE, Erickson IE, Zarembinski T, Silva
MJ, Gelberman RH and Thomopoulos S: The effect of mesenchymal
stromal cell sheets on the inflammatory stage of flexor tendon
healing. Stem Cell Res Ther. 7:1442016. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Li J, Yao Z, Xiong H, Cui H, Wang X, Zheng
W, Qian Y and Fan C: Extracellular vesicles from
hydroxycamptothecin primed umbilical cord stem cells enhance
anti-adhesion potential for treatment of tendon injury. Stem Cell
Res Ther. 11:5002020. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Geburek F, Mundle K, Conrad S, Hellige M,
Walliser U, van Schie HT, van Weeren R, Skutella T and Stadler PM:
Tracking of autologous adipose tissue-derived mesenchymal stromal
cells with in vivo magnetic resonance imaging and histology after
intralesional treatment of artificial equine tendon lesions-a pilot
study. Stem Cell Res Ther. 7:212016. View Article : Google Scholar
|
|
245
|
Yea JH, Park JK, Kim IJ, Sym G, Bae TS and
Jo CH: Regeneration of a full-thickness defect of rotator cuff
tendon with freshly thawed umbilical cord-derived mesenchymal stem
cells in a rat model. Stem Cell Res Ther. 11:3872020. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Xue Y, Kim HJ, Lee J, Liu Y, Hoffman T,
Chen Y, Zhou X, Sun W, Zhang S, Cho HJ, et al: Co-electrospun silk
fibroin and gelatin methacryloyl sheet seeded with mesenchymal stem
cells for tendon regeneration. Small. 18:e21077142022. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Uyar İ, Altuntaş Z, Fındık S, Yıldırım
MEC, Yarar S, Aktan M and Avcı A: The effects of a combination
treatment with mesenchymal stem cell and platelet-rich plasma on
tendon healing: An experimental study. Turk J Med Sci. 52:237–247.
2022.PubMed/NCBI
|
|
248
|
Kang K, Geng Q, Cui L, Wu L, Zhang L, Li
T, Zhang Q and Gao S: Upregulation of Runt related transcription
factor 1 (RUNX1) contributes to tendon-bone healing after anterior
cruciate ligament reconstruction using bone mesenchymal stem cells.
J Orthop Surg Res. 17:2662022. View Article : Google Scholar : PubMed/NCBI
|