Introduction
Autoimmune hepatitis (AIH) is a type of chronic
progressive liver disease and has recently exhibited a global
increasing trend in incidence (1,2).
Immune-mediated injury to hepatocytes is the major
pathophysiological feature of AIH, which is pivotal in directing
liver injury progression due to the resultant liver inflammation,
leading to fibrosis (3). To date,
the precise etiology of AIH remains largely unknown; thus, the
treatment of AIH poses a great challenge for physicians (4). Clinically, the current therapeutic
options available for AIH mainly consist of various
immunosuppressive agents (4),
whereas the therapeutic efficacy of immunosuppressive agents is
limited. Patients with AIH are unable to obtain long-term
histological remission only by receiving immunosuppressive
treatment (5). Furthermore, some
patients with AIH still respond insufficiently to immunosuppressive
agents, presenting with a prolonged course and repeated onset
(1). Additionally, the adverse
side-effects of immunosuppressive agents appear inevitable
(6). Therefore, the
identification of novel effective drugs which can be used to
prevent the progression of AIH is of utmost importance.
Chinese herbal medicines are considered penitential
therapeutic agents for AIH and have unique advantages in the
treatment of hepatic fibrosis (7). Sophoricoside (SOP) is an isoflavone
glycoside extracted from the dried fruit of Sophora japonica
L., which belongs to the traditional Chinese herb (8). Previous studies using animal
experiments have revealed that SOP exerts therapeutic effects on
non-alcoholic fatty liver disease (NAFLD), allergic asthma,
dermatitis and lipopolysaccharide (LPS)-induced lung injury
(9-12). The pharmacological properties of
SOP reported thus far include the modulation of lipogenesis,
anticancer, immune regulation and antioxidant effects (9-12).
However, the pharmacological effects and regulatory mechanisms of
SOP have not yet been explored in AIH or in autoimmune
diseases.
In the present study, a mouse model of AIH was
established to evaluate the effects of SOP on AIH. A mouse model of
concanavalin A (ConA)-induced hepatitis was also used, as this
model is commonly used in the study of AIH (13). However, the liver injury induced
by ConA developed rapidly and could not fully mimic the chronic
progression of AIH in the human body. Moreover, the acute liver
injury induced by ConA exhibited a self-healing tendency, which
usually disappeared after 48 h. Furthermore, the specific
autoantibodies against liver, tissue fibrosis and the
characteristic features of hepatic pathology could not be detected
in ConA-induced AIH (13).
Cytochrome P450 2D6 (CYP2D6) is a recognized human autoantigen in
type-2 AIH and the adenovirus expressing human CYP2D6 was first
used in an attempt to establish a mouse model of chronic AIH by
Holdener et al (14).
Based on previous research, an improved chronic CYP2D6-AIH mouse
model was established by repeated injections of the human CYP2D6
expression plasmid targeting the liver (15). This model of CYP2D6-induced AIH
can well resemble the chronic pathological process of AIH in
vivo, and chronic inflammation, liver fibrosis and
autoantibodies against the liver, characteristic of hepatic
pathology can all be detected in this model mouse (15). Herein, the pharmacological effects
and regulatory mechanisms of SOP in AIH were explored in the
improved CYP2D6-AIH mouse model in combination with the
ConA-induced acute immune-mediated liver injury model.
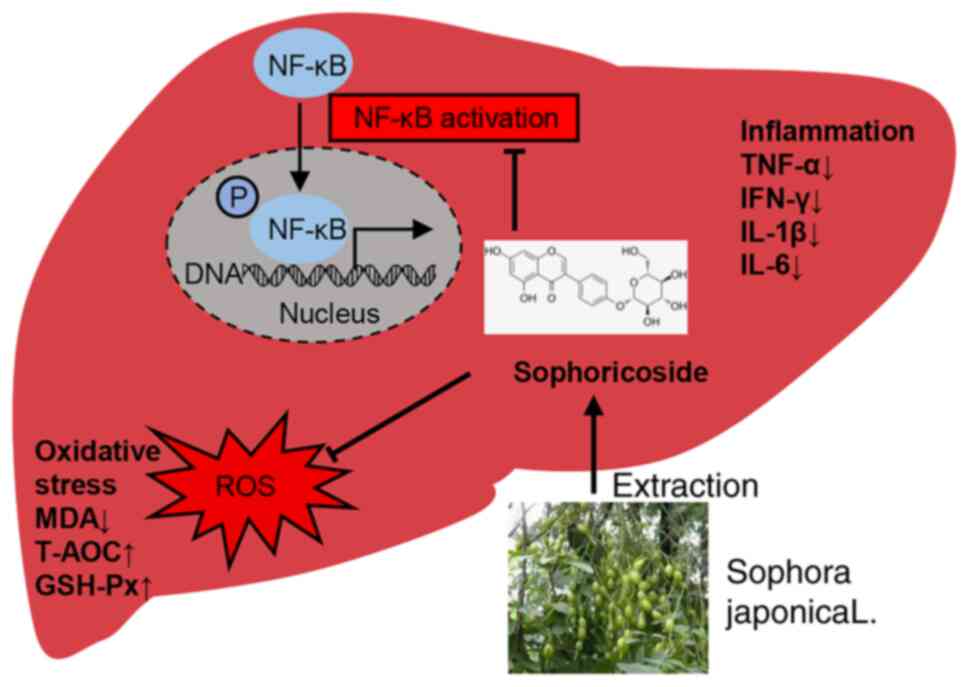
The present study demonstrates that SOP exerts
therapeutic effects against autoimmune-mediated hepatic damage in
mice. It is demonstrated that mechanistically, SOP attenuates liver
inflammation and fibrosis via the inhibition of oxidative stress
and NF-κB signaling pathway activation in hepatocytes.
Materials and methods
Animals and experimental protocol
Specific pathogen-free C57BL/6 male mice (n=48, 6-8
weeks old, weighing 20-25 g) were purchased from GemPharmatech Co.,
Ltd. The mice were housed in the specific pathogen-free environment
at 24±2°C with an alternating 12 h light/dark cycle and allowed
free access to food and water at the Laboratory Animal Center of
Tongji Hospital of Tongji Medical College. All experimental
protocols were conducted following the Chinese National Guidelines
for ethical review of animal welfare (GB/T 35892-2018) and approved
by the Ethics Committee of Animal Experiments of Tongji Hospital,
Tongji Medical College, Huazhong University of Science and
Technology (approval no. TJH-202104021).
Establishment of moue model of AIH and
drug administration
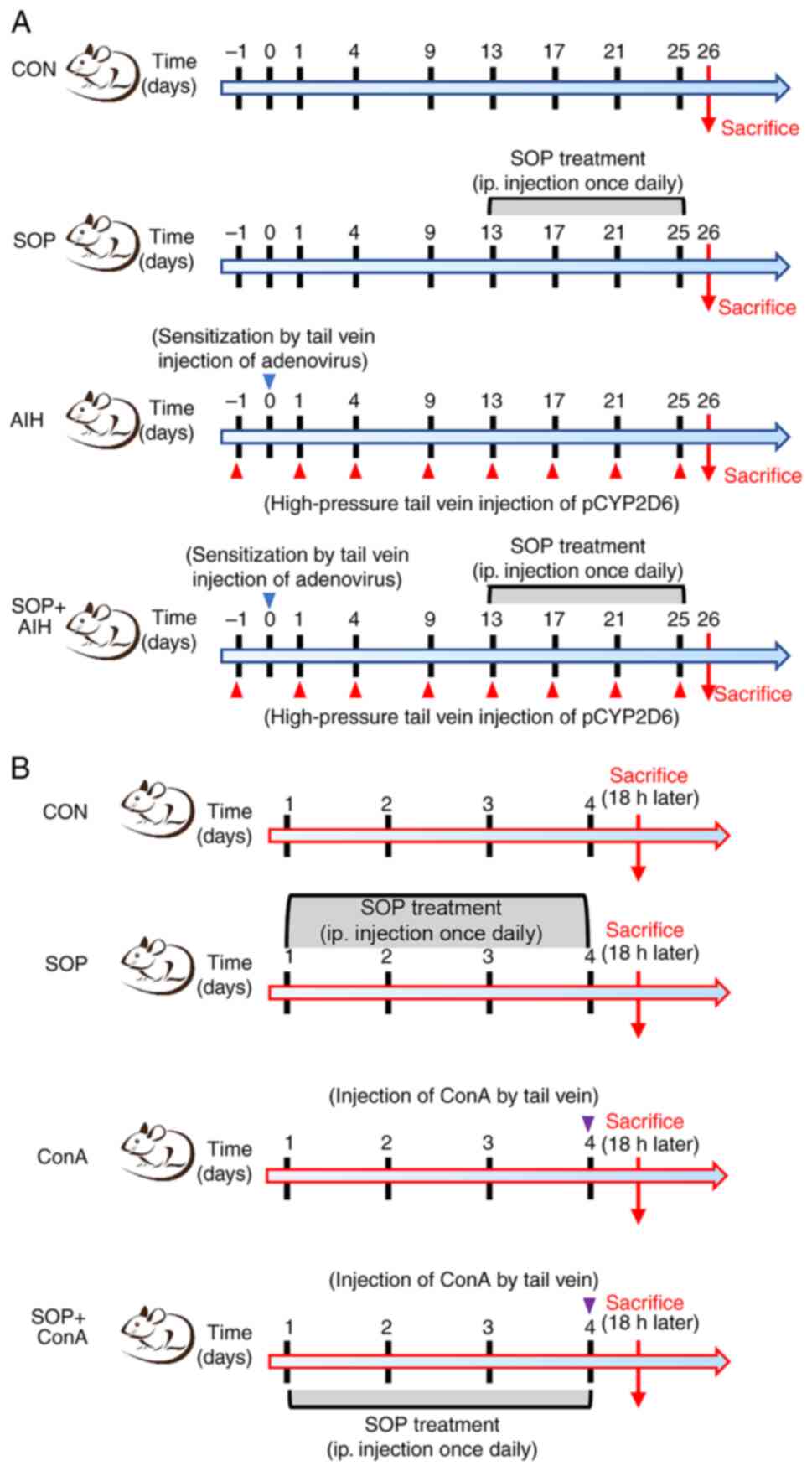
The mouse model of chronic CYP2D6-AIH was
established by an adenovirus infection first and followed by a
repeated tail vein injection of the CYP2D6 overexpression plasmid
(pCYP2D6, 60 µg per injection) on days 1, 4, 9, 13, 17, 21,
25, as previously described (15). SOP (cat. no. HY-N0423;
C21H20O10, purity >99%) was
purchased from MedChemExpress. For research on the improved chronic
CYP2D6-AIH mouse model, the mice were randomly divided into four
groups as follows: The CON group (n=6), SOP group (n=6), AIH group
(n=6) and SOP + AIH group (n=6). SOP was administered by
intraperitoneal injection once daily (30 mg/kg) from the 13th day.
The detailed experimental protocol for each group is illustrated in
Fig. 1A.
For research using the mouse model of ConA-induced
acute hepatitis, the mice were randomly divided into the normal CON
group (n=6), SOP group (n=6), ConA group (n=6) and SOP + ConA group
(n=6). The mice were injected with a single dose of ConA (15 mg/kg,
cat. no. L7647, MilliporeSigma) via the tail vein to induce acute
autoimmune-mediated liver injury, as previously described (16). The mice were treated with SOP 3
days prior to the ConA injection. The treatment scheme of each
group is presented in Fig.
1B.
At the endpoint of the experiment, the mice were
anesthetized with pentobarbital sodium (70 mg/kg, administered
intraperitoneally) for the harvest of blood from the orbital sinus
and were sacrificed subsequently by cervical vertebra dislocation.
Immediately following sacrifice, liver specimens were collected.
Part of the livers were fixed in 4% paraformaldehyde solution and
the remaining parts were preserved at −80°C.
Network pharmacology
The two-dimensional structure of SOP was obtained
from the PubChem database. The potential target genes of SOP were
retrieved from online databases, including traditional Chinese
medicine (TCM) systems pharmacology (TCMSP) (https://tcmsp-e.com/tcmsp.php) and PharmMapper
(http://lilab-ecust.cn/pharmmapper/index.html), and the
duplicates of two databases were removed. To collect the common
targets of SOP on AIH, 'autoimmune hepatitis' was used as the key
word for searching in the GeneCards database. The intersection
between SOP targets and AIH-related genes was then screened out
using an online Venn diagram tool (http://www.ehbio.com/test/venn/).
Protein-protein interaction (PPI) target
network
The intersection targets between SOP targets and
AIH-related genes were input into the STRING database (http://string-db.org/) to construct a PPI network.
Cytoscape software 3.6.0 (http://www.cytoscape.org) was used to visualize the
network and underwent the calculation of topological parameters.
The top 10 common targets were selected according to the degree and
above-average betweenness centrality (17).
Enrichment analysis
To predict the underlying regulatory mechanisms of
SOP in AIH, the Gene Ontology (GO) biological functional enrichment
and Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling
pathway enrichment analysis was performed using the DAVID database
(https://david.ncifcrf.gov/tools.jsp).
The GO biological functional enrichment analysis consists of
biological processes (GO_BP), cellular components (GO_CC) and
molecular function (GO_MF) analysis. The enrichment analysis of
target genes in Transcription Factor Targets was performed using
the Metascape database (http://metascape.org).
Cells, cell culture and drug
intervention
The AML12 cell lines were kept in the Institute of
Liver and Gastrointestinal Diseases (Tongji Hospital, Huazhong
University of Science and Technology). The AML12 cells were
cultured in DMEM/F12 medium with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 1%
insulin-transferrin-selenium solution [cat. no. 60708ES10, Yeasen
Biotechnology (Shanghai) Co., Ltd.] and 40 ng/ml dexamethasone
(cat. no. HY-14648, MedChemExpress). The cells were incubated at
37°C in 5% CO2, as previously described (18). After the AML12 cells were cultured
until they adhered to the wells, LPS (cat. no. L4391,
MilliporeSigma) was added at a concentration of 1,000 ng/ml for 24
h. To explore the in vitro effects of SOP, the AML12 cells
were treated with SOP at 50 and 100 µM for 24 h prior to LPS
stimulation.
Biochemistry measurements
The venous blood of mice was collected and
centrifuged (3,000 × g, 10 min, at 4°C). The supernatants were
submitted to the Clinical Laboratory of Tongji Hospital (Wuhan,
China) to measure the level of transaminase including alanine
aminotransferase (ALT) and aspartate aminotransferase (AST).
For the detection of malondialdehyde (MDA), total
antioxidant capacity (T-AOC) and glutathione peroxidase (GSH-Px)
levels in mouse liver tissue and AML12 cells, the lysates sample
were first collected by repeated ultrasonic spallation, and was
measured using the malondialdehyde assay kit (cat. no. A003-1-2,
Nanjing Jiancheng Taihao Biotechnology Co., Ltd.), total
antioxidant capacity assay kit (cat. no. A015-2-1, Nanjing
Jiancheng Taihao Biotechnology Co., Ltd.) and Glutathione
Peroxidase assay kit (cat. no. A006-2-1, Nanjing Jiancheng Taihao
Biotechnology Co., Ltd.), respectively, according to the
manufacturer's instructions.
Histopathological analysis and
immunohistochemistry
The livers were carefully isolated from the mice,
fixed in a 4% paraformaldehyde solution (at least 24 h at 25°C),
and subsequently embedded in paraffin, and cut to yield
3-µm-thick sections. The sliced sections were stained with
hematoxylin and eosin (H&E solution; 4 min at 25°C) and Sirius
red (20 min at 25°C) to evaluate liver inflammation and fibrosis,
respectively. The stains and fixative used, and technical support
for H&E and Sirius staining were supplied by the Hubei Bios
Biological Technology Co., Ltd. Images of H&E and Sirius
staining were obtained using an inverted-phase contrast microscope
(Olympus Corporation).
For immunohistochemistry, following dewaxing in
xylene, rehydration in ethanol and antigen retrieval, the sliced
sections were incubated with the 8-hydroxy-2-deoxyguanosine
(8-OHDG; 1:100; cat. no sc-393871, Santa Cruz Biotechnology, Inc.)
or phosphorylated (p-)p65 NF-κB (1:100; cat. no. 13346, Cell
Signaling Technology, Inc.) primary antibodies overnight at 4°C.
The following day, the sliced sections were incubated with
horseradish peroxidase-conjugated polyclonal goat anti-rabbit
secondary antibodies (1:200; cat. no. AS014, ABclonal) for 1 h at
room temperature. Finally, the sections were visualized with DAB
and hematoxylin (Hubei Bios Biological Technology Co., Ltd.) using
inverted phase contrast microscope (Olympus Corporation), as
previously described (19).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the liver tissue or
AML12 cells using the MolPure TRleasy Plus Total RNA kit [cat. no.
19211ES60, Yeasen Biotechnology (Shanghai) Co., Ltd.].
Complementary DNA (cDNA) was synthesized using HiScript II Q RT
SuperMix for qPCR (cat. no. R222-01, Nanjing Vazyme Biotech Co.,
Ltd.). The quantification of target mRNA was performed using ChamQ
Universal SYBR qPCR Master Mix (cat. no. Q711-02, Nanjing Vazyme
Biotech Co., Ltd.) on an ABI StepOne Real-Time PCR system (Thermo
Fisher Scientific, Inc.). qPCR was performed under the following
conditions: Initial denaturation at 95°C for 3 min, followed by 45
cycles at 95°C for 5 sec and 60°C for 30 sec, and a final step at
95°C for 5 sec and 60°C for 1 min. The expression levels of mRNAs
were normalized to GAPDH expression, and the 2−ΔΔCq
method was used to calculate the expression levels (19,20). All primers were synthesized by
Beijing Tsingke Biological Technology, Co. Ltd., and are listed in
Table SI.
Protein extraction and western blot
analysis
Liver tissue or AML12 cells were collected and
placed in RIPA Lysis Buffer (cat. no. 2002, Wuhan Servicebio
Technology Co., Ltd.) containing a cocktail of protease inhibitors,
homogenized on ice, and centrifugated at 12,000 × g, 4°C for 15
min. The supernatant was collected and the protein concentration
was measured using a BCA kit (cat. no. 2026-200T, Wuhan Servicebio
Technology Co., Ltd.). A total of 40 µg protein per well was
electrophoresed on 10% SDS polyacrylamide gel. The protein bands
were then transferred onto PVDF membranes (MilliporeSigma). The
PVDF membranes were then blocked with 5% non-fat milk for 1 h at
room temperature and incubated with the primary antibodies
overnight at 4°C. The following antibodies were used: p-p65 NF-κB
(1:1,000; cat. no. 13346; Cell Signaling Technology, Inc.), p65
NF-κB (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.),
β-actin (1:5,000; cat. no. 66009-1-Ig; Proteintech Group, Inc.),
TNF-α (1:1,000; cat. no. 60291-1-Ig; Proteintech Group, Inc.),
IFN-γ (1:1,000; cat. no. 15365-1-AP; Proteintech Group, Inc.),
IL-1β (1:1,000; cat. no. 16806-1-AP; Proteintech), IL-6 (1:1,000;
cat. no. A0286; ABclonal), followed by incubation with goat
anti-mouse IgG (H+L)-HRP (1:2,000; cat. no. SA00001-1; Proteintech
Group, Inc.) or goat anti-rabbit IgG (H+L)-HRP (1:2,000; cat. no.
SA00001-2; Proteintech Group, Inc.) for 1 h at room temperature.
The blots referred to the expression of the antibody-linked protein
was visualized by enhanced chemiluminescence using an ECL assay kit
(cat. no. E412-01, Nanjing Vazyme Biotech Co., Ltd.). Image J
software (version 1.50i; National Institutes of Health) was used to
quantify the protein bands intensity.
Cell viability assay
The AML12 cells were uniformly seeded in 96-well
plates at a density of 5,000 cells per well. Following treatment
with various concentrations of SOP (50, 100 and 200 µM) for
48 h, the viability of the cells in each group was detected using
the Cell Counting Kit (CCK-8) assay [10 µl CCK-8 per well,
cat. no. 30203ES60, Yeasen Biotechnology (Shanghai) Co., Ltd.]. The
cell viability was reflected by the absorbance value at 450 nm, and
was measured using a microplate reader (BioTek Instruments,
Inc.).
Measurement of reactive oxygen species
(ROS) levels
The AML12 cells were uniformly seeded in six-well
plates and incubated with 10 µM H2DCFDA (cat. no.
HY-D0940, MedChemExpress) for 30 min at 37°C, and the fluorescence
intensity of the cells subjected to the different treatments was
then evaluated using a fluorescence microscope (Olympus
Corporation).
Immunofluorescence staining assay
The cells were sequentially fixed in 4%
paraformaldehyde solution, permeabilized in 0.3% Triton-X, blocked
by 10% goat serum, and incubated with p65 NF-κB antibody (1:100;
cat. no. 8242; Cell Signaling Technology, Inc.) at 4°C overnight.
The following day, the cells were incubated with CY3-conjugated
goat anti-rabbit IgG (1:200; cat. no. BA1032, Boster Bio) for 1 h
at room temperature. Images were recorded using an inverted
fluorescence phase contrast microscope (Olympus Corporation).
Statistical analysis
The resulting data are presented as the mean ±
standard error of the mean (SEM). Statistical analysis was
performed using GraphPad Prism software version 6.0 (GraphPad
Software, Inc.). The differences between groups were compared using
the non-parametric one-way analysis of variance (ANOVA) followed by
Tukey's post hoc tests. A value of P<0.05 was considered to
indicate a statistically significant difference. All experiments
were repeated at least three times.
Results
Key targets of SOP in AIH
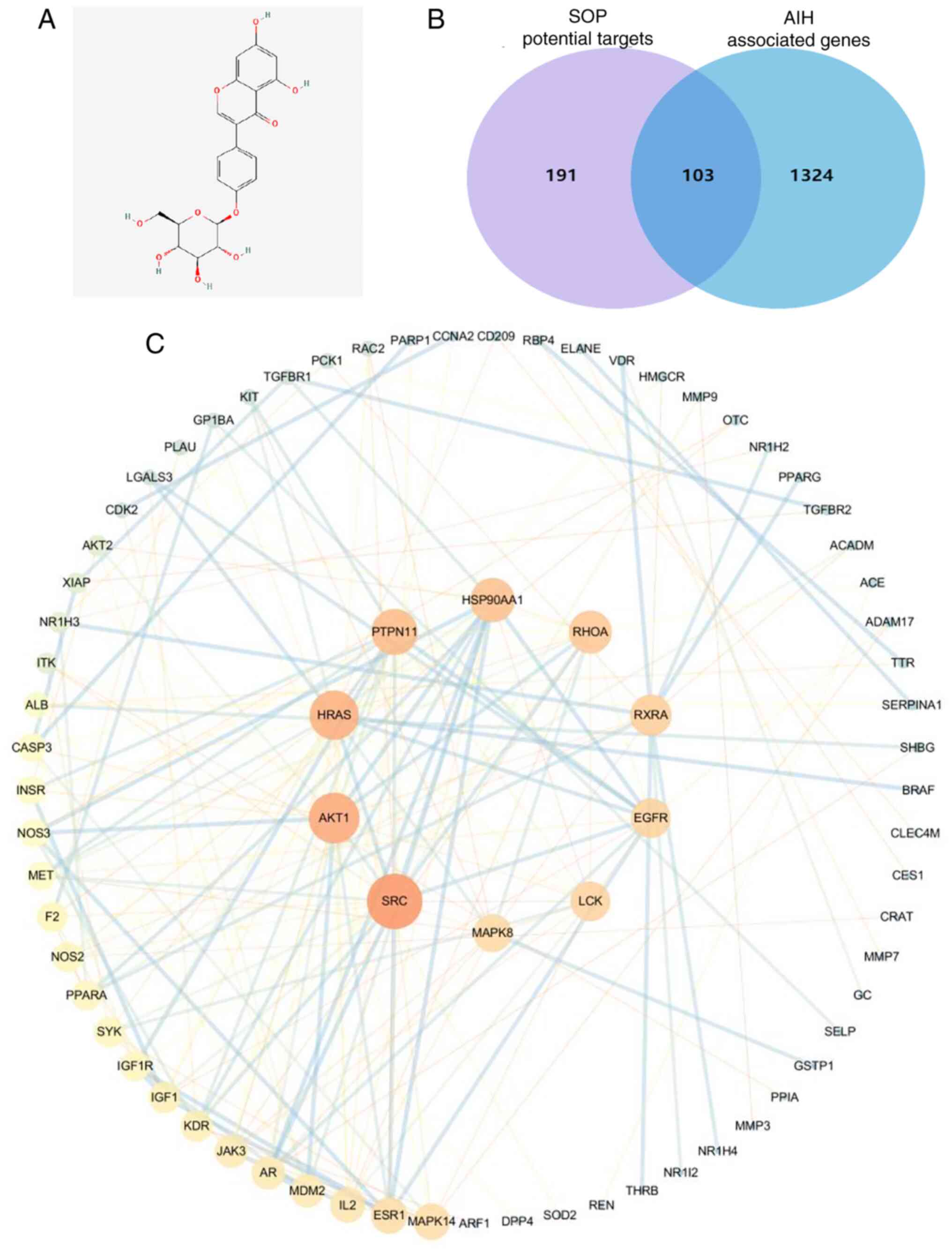
The two-dimensional structure of SOP is presented in
Fig. 2A. To identify the
potential target genes of SOP, network pharmacological analysis was
performed using the TCMSP database. After removing the duplicates,
a total of 294 target genes of SOP were screened out. Moreover,
through the GeneCards database, 1,427 AIH-related genes were
obtained by using the quartile relevance score as the cut-off. The
SOP targets were then intersected with the AIH-related targets.
Finally, a total of 103 commonly matched targets were obtained
(Fig. 2B). To explore the
association among these targets, a PPI network was established
using the STRING database, and the topological parameters,
including the closeness centrality, between centrality and degree
centrality were calculated (Fig.
2C). The top 10 key targets of SOP against AIH were selected
for subsequent analyses (Fig. 2C
and Table SII).
Potential mechanisms underlying the
effects of SOP on AIH
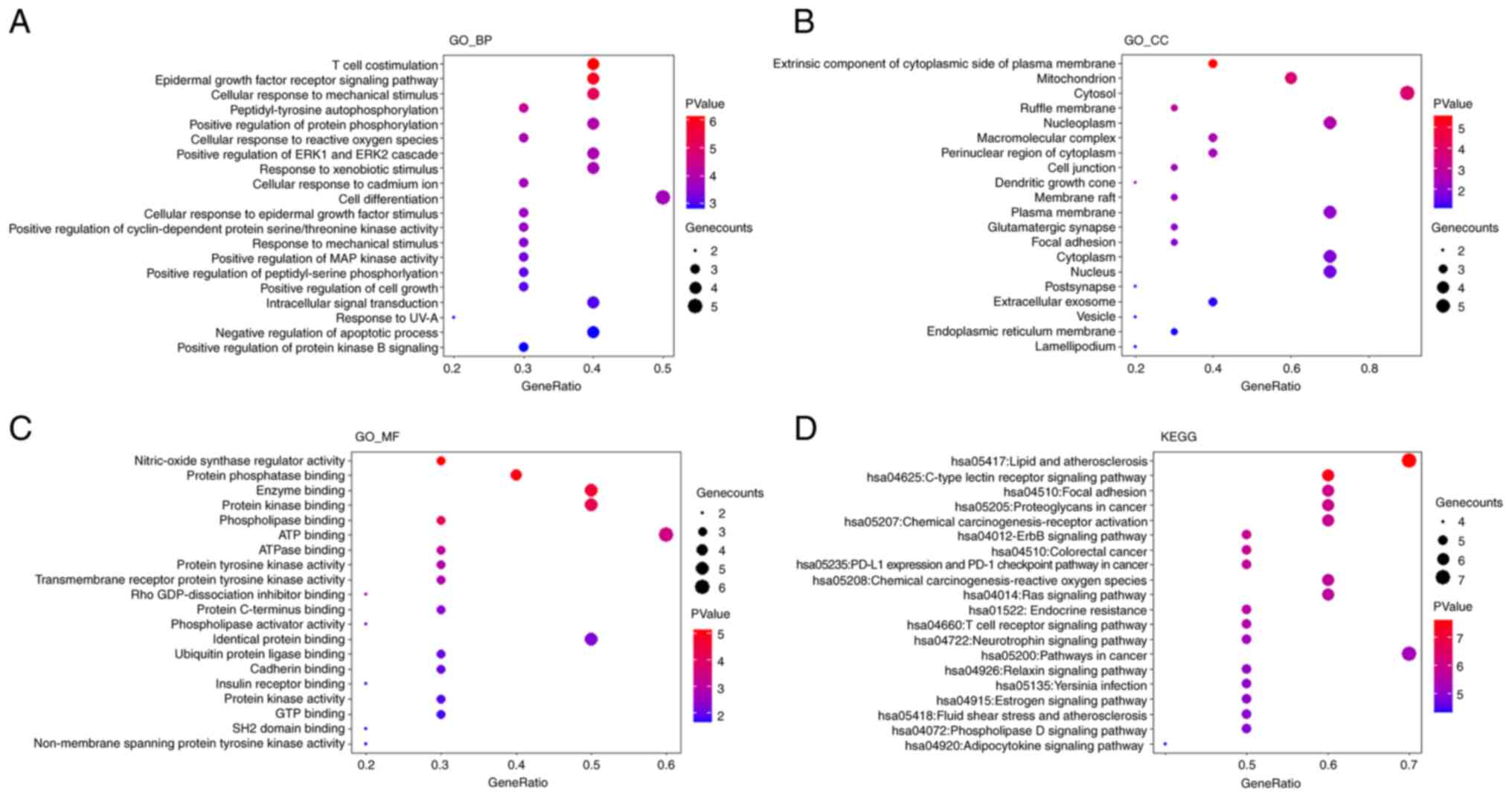
To explore the underlying regulatory mechanisms of
SOP in AIH, the selected top 10 common target genes were subjected
to GO and KEGG enrichment analyses using the DAVID database. Based
on the significance level, the top 20 significantly enriched terms
were selected and are exhibited in Fig. 3A-D, respectively. By taking a
comprehensive view of the enriched terms, it was noted that the
enriched terms associated with the metabolism of ROS were highly
ranked in all categories of enrichment analysis. In the GO_BP
enrichment analysis, the results revealed that SOP was involved in
the regulation of cellular response to ROS (Fig. 3A). The mitochondrion, which was
the primary source of ROS in cells, was the top two cellular
components influenced by SOP (Fig.
3B). The results of GO_MF analysis revealed that SOP was
related to a series of reactions related to ROS production,
including nitric-oxide synthase regulator activity, ATP binding and
ATPase binding (Fig. 3C).
Moreover, the results of KEGG enrichment analysis suggested that
SOP participated in the chemical carcinogenesis by affecting ROS
(Fig. 3D). In general, the data
from GO and KEGG enrichment analyses predicted that the effects of
SOP on AIH were mainly exerted by regulating oxidative stress.
SOP treatment attenuates
autoimmune-mediated liver injury in an improved chronic AIH mouse
model
To further confirm the effects of SOP treatment on
AIH, an improved autoimmune-mediated hepatitis model was adopted,
namely the CYP2D6-AIH mouse model. SOP was administered daily at a
dose of 30 mg/kg (intraperitoneally). At the endpoint of the
experiment, compared with the CON group, mice from the AIH group
manifested evident pathological changes of AIH, including an
elevation in the levels of serum liver enzymes, enhanced
inflammatory cytokine production, interface hepatitis and tissue
fibrosis (Fig. 4A-D). After
almost 2 weeks of SOP treatment, the elevation in the levels of
serum ALT/AST in AIH mice decreased significantly (Fig. 4A). The results of H&E staining
revealed that in the mice with AIH treated with SOP, the
histological damage was attenuated in contrast to that of the mice
from the AIH group (Fig. 4B).
Consistent with the results of histological analysis, the enhanced
production of inflammatory cytokines in the mice with AIH was
significantly suppressed by SOP administration (Fig. 4C and D). Moreover, the mice from
the SOP + AIH group exhibited markedly attenuated liver fibrosis
compared to the AIH group mice (Fig.
4E).
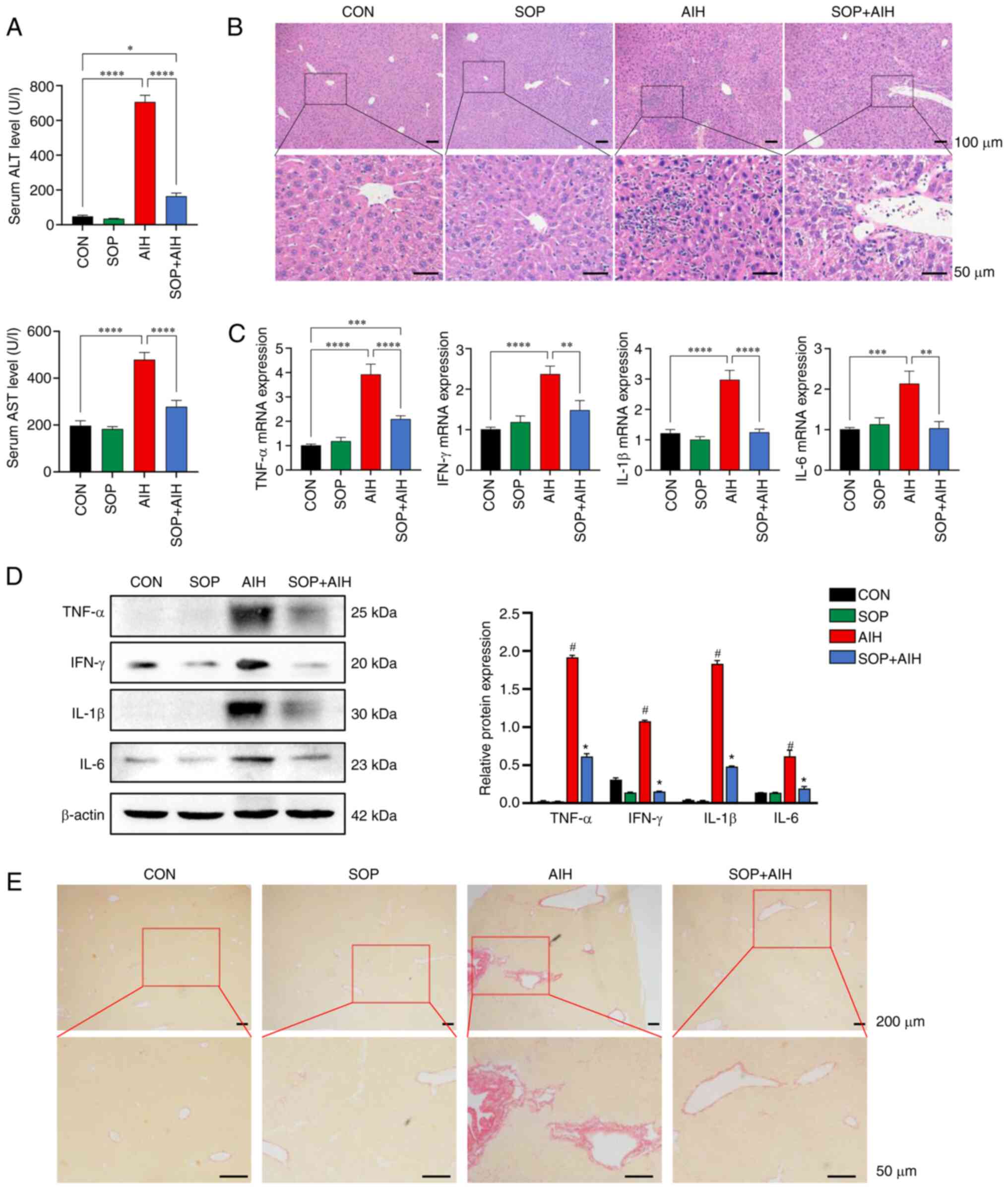 | Figure 4SOP treatment attenuates hepatic
inflammation and fibrosis in an improved mouse model of chronic
AIH. (A) Serum levels of ALT (top panel) and AST (bottom panel) in
mice. (B) Hematoxylin and eosin staining of liver tissues (upper
panels: Magnification ×100; scale bars, 100 µm; bottom
panels: Magnification, ×400; scale bars, 50 µm). The
relative expression levels of inflammatory cytokines in the liver
were analyzed using (C) reverse transcription PCR and (D) western
blot analysis. (E) Sirius red staining of mouse liver tissues.
(upper panels: Magnification, ×40; scale bars, 200 µm;
bottom panels: Magnification, ×200; scale bars, 50 µm). The
data represent the mean ± SEM, n=6 per group. (A-C)
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. (D)
#P<0.05, between the AIH and CON group;
*P<0.05 between the SOP + AIH and AIH group. SOP,
sophoricoside; AIH, autoimmune hepatitis; CON, control; ALT,
alanine aminotransferase; AST, aspartate aminotransferase. |
SOP treatment suppresses hepatocyte
oxidative stress in mice with AIH and in LPS-stimulated AML12
cells
As mentioned above, bioinformatics analysis
predicted that the major biological process in the pathogenesis of
AIH targeted by SOP was oxidative stress, which is characterized by
excessive ROS production. Considering that the distressed
hepatocytes are the key source of ROS in the liver (21), several indices reflecting the
degree of oxidative stress in hepatocytes were evaluated.
Hepatocyte injury in the mouse liver induced by ROS in the present
study was detected by examining the level of 8-OHDG, which is
commonly used as a marker of oxidative damage. According to the
data, following SOP treatment, the elevation in the levels of
hepatic MDA in mice with chronic AIH was significantly decreased
(Fig. 5A), and the overall
decreased hepatic antioxidant ability in mice with AIH measured by
T-AOC and GSH-Px exhibited a considerable enhancement (Fig. 5B and C). The oxidative damage to
mouse liver cells induced by ROS was further assessed by
immunohistochemical staining with 8-OHDG. The results revealed that
compared with the mice from the CON group, oxidative damage to
hepatocytes was enhanced in mice from the AIH group, while mice
from the SOP + AIH group exhibited alleviated hepatic oxidative
damage compared with the AIH group (Fig. 5D). The results of in vitro
experiments revealed that treatment with SOP significantly reversed
the excessive increase in MDA levels in AML12 cells induced by LPS
(Fig. 5E), and upregulated the
low levels of T-AOC and GSH-Px in a concentration-dependent manner
(Fig. 5F and G). Moreover, it was
observed that the level of ROS in AML12 cells extensively increased
following LPS stimulation and decreased in cells pre-treated with
SOP (Fig. 5H). These results
indicated that SOP treatment exerted hepatoprotective effects by
eliminating ROS in hepatocytes and promoting the antioxidant
defense mechanisms.
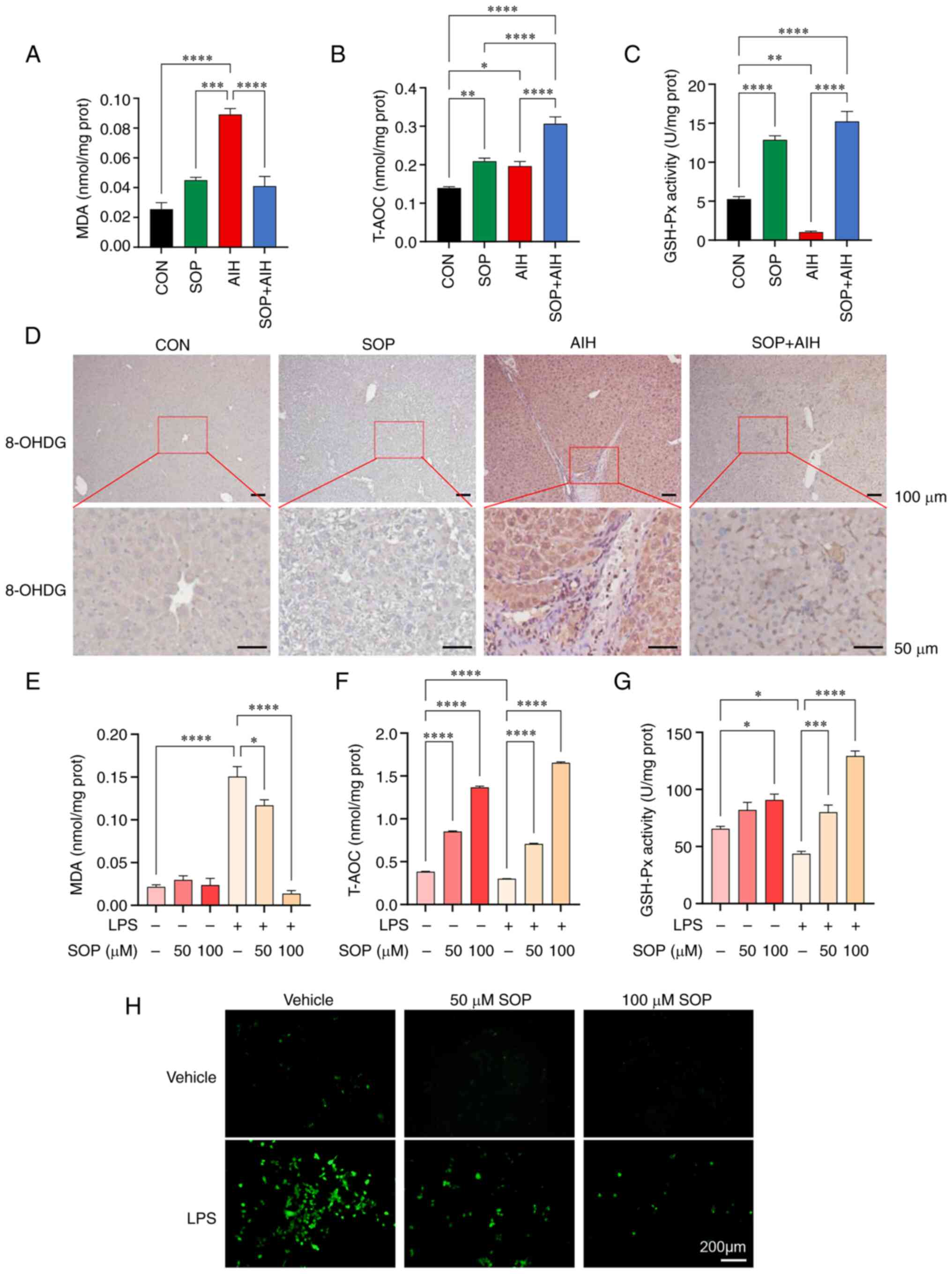 | Figure 5SOP treatment inhibits hepatocellular
oxidative stress in a mouse model of chronic AIH and in
LPS-stimulated AML12 cells. (A) Lysates of mouse liver tissue were
collected for the detection of (A) MDA, (B) T-AOC, and (C) GSH-Px.
(D) Representative images of immunostaining for the ROS marker,
8-OHDG, in the liver. AML12 cells were treated with SOP at 50 and
100 µM with or without LPS (1,000 ng/ml) for 24 h. Cell
lysates were collected for the detection of (E) MDA, (F) T-AOC, and
(G) GSH-Px. (H) Fluorescence microscopic images of ROS level in
AML12 cells treated with SOP at 50 and 100 µM with or
without LPS (1,000 ng/ml) for 24 h. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. SOP, sophoricoside; AIH, autoimmune
hepatitis; CON, control; LPS, lipopolysaccharide; 8-OHDG,
8-hydroxy-2-deoxyguanosine; MDA, malondialdehyde; T-AOC, total
antioxidant capacity; GSH-Px, glutathione peroxidase. |
SOP treatment suppresses the activation
of the NF-κB signaling pathway in mice with AIH and in
LPS-stimulated AML12 cells
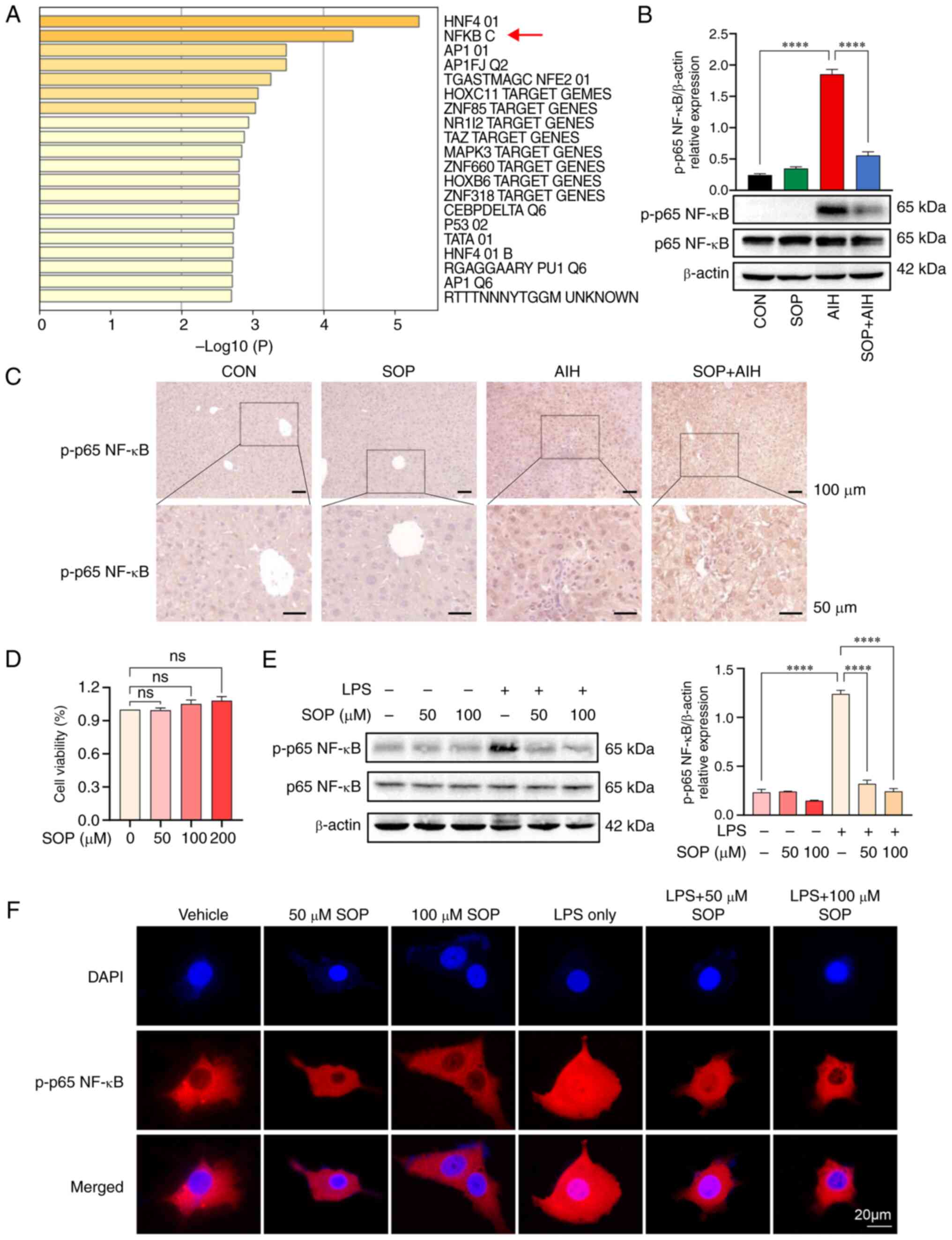
Through enrichment analysis of the key target genes
in Transcription Factor Targets, it was identified that the targets
of SOP were primarily regulated by the gene set NF-κB (GO: M12240)
(Fig. 6A). According to the in
vivo experimental data, the activation of the NF-κB signaling
pathway in the liver was enhanced in mice with AIH. The results of
western blot analysis indicated that the mice with AIH treated with
SOP exhibited a decreased activation of the NF-κB signaling pathway
compared to the mice in the AIH group (Fig. 6B). Immunohistochemical staining
further confirmed that the increased protein expression of p-p65
NF-κB in the liver cells of mice with AIH was decreased by SOP
treatment (Fig. 6C). In addition,
the effects of SOP treatment on the NF-κB signaling pathway were
further investigated in vitro. Firstly, the potential
cytotoxicity of SOP on AML12 cells was evaluated by CCK-8 assay
after the AML12 cells were incubated with SOP for 48 h. The results
indicated no cellular toxicity of SOP against the AML12 cells at
concentrations ranging from 50 to 200 µM (Fig. 6D). Cell lysates were collected and
the content of p-p65/p65 NF-κB protein was detected using western
blot analysis. It was found that the relative protein expression of
p-p65 NF-κB was upregulated in the LPS-stimulated AML12 cells and
was subsequently suppressed by SOP treatment (Fig. 6E). Simultaneously, the results of
immunofluorescence staining revealed that the LPS-induced nuclear
translocation of p65 NF-κB in the AML12 cells was significantly
blocked by SOP treatment (Fig.
6F). These data suggested that SOP treatment blocked the
activation of the NF-κB signaling pathway in hepatocytes.
SOP treatment inhibits oxidative stress
and NF-κB activation in mice with ConA-induced hepatitis
To further examine the protective effects of SOP
against autoimmune-mediated liver injury, the ConA-induced acute
AIH mouse model was additionally adopted to assess the grade of
oxidative stress and NF-κB activation in the mouse liver before and
after SOP treatment. According to the results obtained, the mice
from the ConA group presented with elevated serum levels of
ALT/AST, increased inflammatory cytokine expression levels and
interface hepatitis. Compared with the ConA group mice, the SOP +
ConA group mice exhibited significantly lower serum levels of
ALT/AST and inflammatory cytokine expression levels, as well as an
alleviated pathophysiological damage in the liver (Fig. 7A-D). Furthermore, the results of
western blot analysis revealed that the ConA-induced enhancement of
p-p65 NF-κB protein expression in the mouse liver was significantly
suppressed by SOP administration (Fig. 7E). Additionally, mice with
ConA-induced liver injury exhibited increased levels of MDA, and
decreased T-AOC and GSH-Px levels; these effects were partially
restored in mice treated with SOP (Fig. 7F).
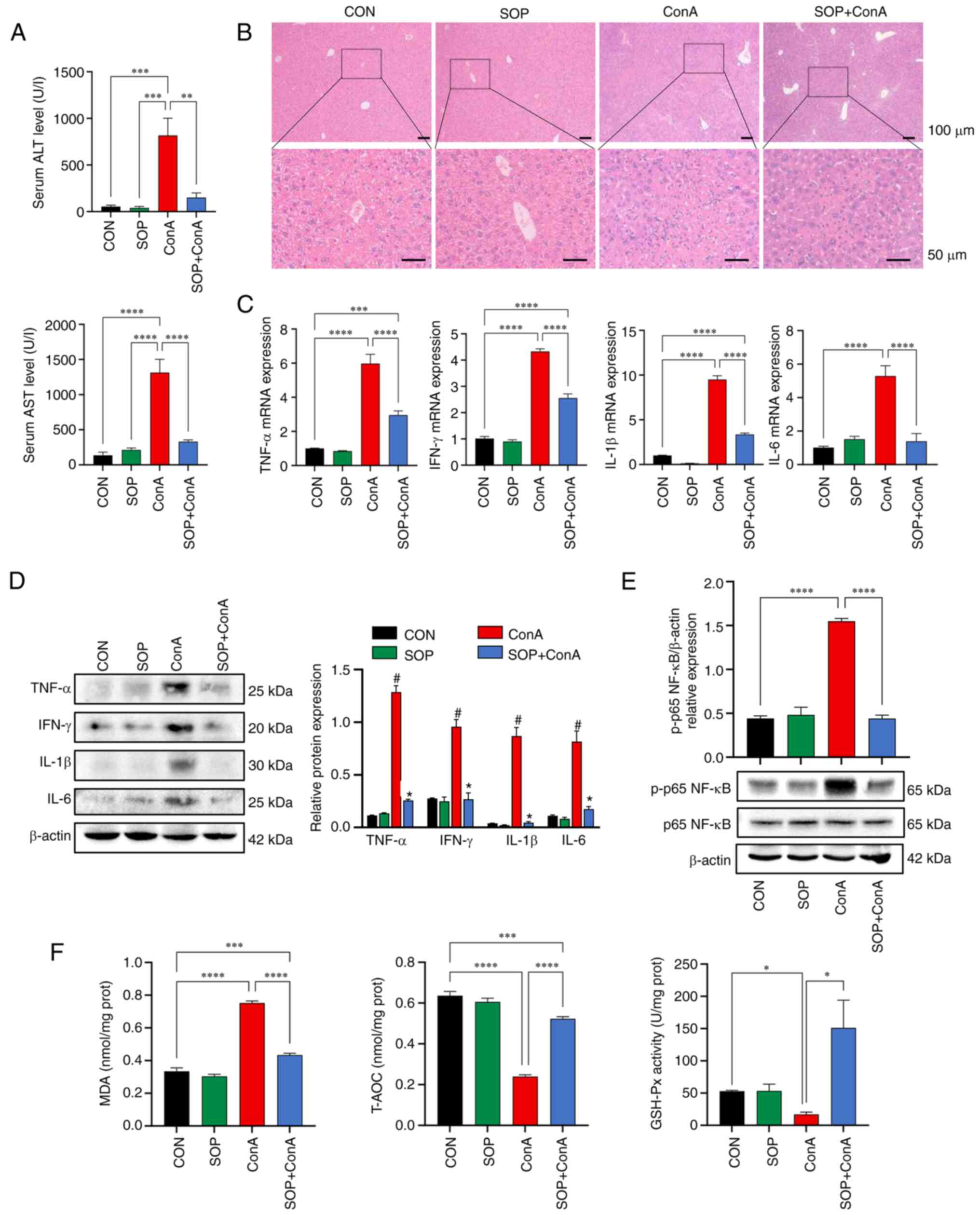 | Figure 7The ConA-induced hepatitis was
alleviated in SOP-treated mice accompanied by reduced oxidative
stress and NF-κB activation in the liver. (A) Quantification of
serum ALT and AST levels in mice. (B) The histology of mouse
hepatic tissue was assessed using hematoxylin and eosin staining.
(C) The mRNA expression levels of TNF-α, IFN-γ, IL-1β and IL-6 in
mouse liver tissues were measured using reverse
transcription-quantitative PCR. (D) The protein expression levels
of TNF-α, IFN-γ, IL-1β and IL-6 in mouse liver tissues were
detected using western blot analysis. (E) Western blot analysis of
NF-κB activation in liver tissue. (F) Lysates of mouse liver tissue
were collected for the detection of MDA, T-AOC and GSH-Px.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. (D)
#P<0.05, between the ConA and CON group;
*P<0.05, between the SOP + ConA and ConA group. ConA,
concanavalin-A; SOP, sophoricoside; AIH, autoimmune hepatitis; CON,
control; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; MDA, malondialdehyde; T-AOC, total antioxidant
capacity; GSH-Px, glutathione peroxidase. |
Discussion
AIH is characterized by autoimmune-mediated
inflammatory damage to the liver with a chronic course (22). Due to the complex etiology of AIH,
there are currently no effective drugs available with which to
reduce hepatic fibrosis and achieve histological remission in
patients with AIH (22).
Therefore, the exploration of novel drugs for AIH is still
essential. TCM includes a diverse selection of agents which can be
used for human healthcare. SOP is a bioactive component of the
Chinese herbal medicine, Sophora japonica L. (8). In the present study, it was found
that SOP attenuated chronic and acute autoimmune-mediated liver
injury by suppressing oxidative stress and the NF-κB signaling
pathway activation in liver cells.
The pathophysiological mechanisms of AIH are
complex. There is evidence to indicate that a number of factors,
such as immune disorder, oxidative stress, the dysregulation of
immune-related signaling pathways and intestinal dysbiosis, can
contribute to the development of AIH and destroy hepatocytes
(23-25). In light of the complex factors
involved in the pathogenesis of AIH, herein, network pharmacology
was performed to predict which of these pathophysiological
mechanisms in AIH are regulated by SOP. Network pharmacology is a
platform utilized to predict the molecular targets and regulatory
mechanisms, as well as components of TCM for disease (26). In a comprehensive view of the
network pharmacological results, it was noted that the modulation
of oxidative stress may be a key mechanism associated with the
benefits of SOP in AIH. Based on the top 20 significantly enriched
terms, it was found that cellular response to ROS was respectively
the second and the ninth significant term in GO_BP and KEGG
enrichment analysis. Following the GO_CC enrichment analysis, the
second significant term was mitochondrion, which is the major
source of cellular ROS (27). The
GO_MF analysis also revealed that SOP mainly influenced the process
of cellular respiration, including nitric-oxide synthase regulatory
activity, ATP binding and ATPase binding (28). Therefore, it was anticipated that
SOP mainly exerted effects on AIH by regulating oxidative
stress.
The studies by Kim and Lee (9,10),
Zhang et al (11), Wu
et al (12), Gao et
al (29) and Li and Lu
(30) have confirmed that SOP
treatment can exert therapeutic effects in a number of diseases,
such as NAFLD, dermatitis, allergic asthma, LPS-induced lung
injury, cardiac hypertrophy, fructose-induced liver injury
(9-12,29,30). The results of the present study
indicated that SOP treatment reduce the serum ALT/AST level and
suppressed the expression of pro-inflammatory cytokines, including
TNF-α, IFN-γ, IL-1β and IL-6 in the mouse model of AIH. Moreover,
the histological findings revealed that hepatic inflammation and
tissue fibrosis in chronic autoimmune-mediated hepatitis could be
prevented by SOP treatment. The normalization of serum transaminase
is considered a key marker of full biochemical remission in
patients with AIH (31). In
addition, high levels of TNF-α, IFN-γ, IL-1β and IL-6 are the major
pathogenetic inflammatory cytokines in AIH (22,32). The extension of hepatic fibrosis
may proceed to cirrhosis and liver failure, leading to the
deterioration of the clinical outcomes of patients (31). Taken together, these data suggest
that SOP treatment can improve chronic autoimmune-mediated liver
injury.
Oxidative stress is characterized by the excessive
production of ROS and has been implicated in the pathogenesis of
AIH (33-35). The hepatocyte is the major cell
population within the liver that generates ROS under conditions of
stress (21). It has been
suggested that patients with AIH tend to present an increased level
of hepatic ROS (24). The
redundant ROS induced by oxidative stress attacks the cellular
membrane and disrupts mitochondrial function, resulting in the
death of hepatocytes and in tissue fibrosis (36-38). Moreover, ROS may promote hepatic
fibrosis by stimulating hepatic stellate cells (39,40). It has been reported that SOP can
exert antioxidant pharmacological effects in the treatment of NAFLD
and LPS-induced acute lung injury (11,12). Consistently, the results of the
in vivo and in vitro experiments in the present study
indicated that treatment with SOP reduced the MDA levels and ROS,
and upregulated T-AOC and GSH-Px in hepatocytes subjected to
immune-mediated injury. MDA is one of the peroxidation products of
polyunsaturated fatty acids, which is the most commonly used marker
of oxidant stress (41). T-AOC
and GSH-Px are indexes that reflect the anti-oxidant capability of
tissue (42). Therefore, these
results further validate the prediction from network pharmacology
that SOP treatment can protect against AIH through the inhibition
of oxidative stress, accompanied by the decreased production and
enhanced elimination of ROS in hepatocytes.
On the other hand, the present study suggested that
SOP also attenuated autoimmune-mediated liver injury via the
inhibition of the NF-κB signaling pathway in hepatocytes. The
enrichment analysis in Transcription Factor Targets indicated that
the majority of genes targeted by SOP were significantly regulated
by the transcription factor, NF-κB. The experimental data supported
this prediction, demonstrating that treatment with SOP reduced the
enhanced nuclear translocation of NF-κB in LPS-stimulated AML12
cells and suppressed the activation of p65 NF-κB protein in the
livers of mice with AIH. It has been demonstrated that the
activation of the NF-κB signaling pathway is vital in the
progression of inflammatory liver disease (43). Patients with AIH often present
with increased levels of NF-κB-induced inflammatory cytokines, such
as TNF-α, IFNγ, IL-1β and IL-6 (30). The blockade of the NF-κB-mediated
inflammatory signaling pathway can alleviate liver inflammation
(44). The data presented herein
demonstrated that SOP treatment inhibited the activation of the
NF-κB signaling pathway and reduced inflammatory cytokine levels in
mice with AIH.
In order to further validate the effects of SOP
treatment on AIH, the present study adopted a mouse model of
ConA-induced hepatitis. The ConA model is a canonical animal model
for AIH research, which is mediated by T-cells and characterized by
acute autoimmune-mediated liver injury resembling AIH (45). The results revealed that the
hepatic damage induced by ConA was ameliorated by SOP treatment.
Mice in the ConA model treated with SOP exhibited decreased levels
of oxidative stress and NF-κB activation in the liver. However, a
limitation of the present study was that the role of the NF-κB
signaling pathway during SOP treatment of AIH was not further
verified by the overexpression of p-p65 NF-κB protein in mice with
AIH treated with SOP. In addition, whether the NF-κB signaling
pathway interacted with oxidative stress in the pathogenesis of AIH
was not identified in the present study.
In conclusion, the present study demonstrates that
SOP may have a potential therapeutic effect on AIH. Treatment with
SOP can attenuate liver inflammation and prevent hepatic fibrosis
progression in mice with chronic or acute autoimmune-mediated liver
injury via the inhibition of oxidative stress and NF-κB signaling
pathway activation in hepatocytes (Fig. 8).
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and YL designed the study and wrote the
manuscript. YC, LW, JX and SW performed all the experiments
cooperatively. ZP and MY collected and analyzed the experimental
data. FX and HW interpreted the experimental results and critically
reviewed the manuscript. ML and DT conceived the study and
supervised the study. All authors have read and approved the final
manuscript. ML and DT confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All experiments involving animals were conducted
following the Chinese National Guidelines for ethical review of
animal welfare (GB/T 35892-2018) and approved by the Ethics
Committee of Animal Experiments of Tongji Hospital, Tongji Medical
College, Huazhong University of Science and Technology (approval
no. TJH-202104021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
SOP
|
sophoricoside
|
|
AIH
|
autoimmune hepatitis
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
ConA
|
concanavalin-A
|
|
LPS
|
lipopolysaccharide
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
ROS
|
reactive oxygen species
|
|
pCYP2D6
|
plasmid CYP2D6
|
|
8-OHDG
|
8-hydroxy-2-deoxyguanosine
|
|
MDA
|
malondialdehyde
|
|
T-AOC
|
total antioxidant capacity
|
|
GSH-Px
|
glutathione peroxidase
|
|
SEM
|
standard error of the mean
|
|
ANOVA
|
one-way analysis of variance
|
|
TCM
|
traditional Chinese medicine
|
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (nos. 81974071 and
82270558).
References
|
1
|
Mieli-Vergani G, Vergani D, Czaja AJ,
Manns MP, Krawitt EL, Vierling JM, Lohse AW and Montano-Loza AJ:
Autoimmune hepatitis. Nat Rev Dis Primers. 4:180172018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Floreani A, Restrepo-Jiménez P, Secchi MF,
De Martin S, Leung PSC, Krawitt E, Bowlus CL, Gershwin ME and Anaya
JM: Etiopathogenesis of autoimmune hepatitis. J Autoimmun.
95:133–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sirbe C, Simu G, Szabo I, Grama A and Pop
TL: Pathogenesis of autoimmune hepatitis-cellular and molecular
mechanisms. Int J Mol Sci. 22:135782021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manns MP, Lohse AW and Vergani D:
Autoimmune hepatitis-update 2015. J Hepatol. 62(Suppl 1):
S100–S111. 2015. View Article : Google Scholar
|
|
5
|
Expert Panel on Gastrointestinal Imaging;
Hindman NM, Arif-Tiwari H, Kamel IR, Al-Refaie WB, Bartel TB, Cash
BD, Chernyak V, Goldstein A, Grajo JR, et al: ACR appropriateness
criteria® jaundice. J Am Coll Radiol. 16:S126–S140.
2019. View Article : Google Scholar
|
|
6
|
Mack CL, Adams D, Assis DN, Kerkar N,
Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH and Czaja AJ:
Diagnosis and management of autoimmune hepatitis in adults and
children: 2019 Practice guidance and guidelines from the American
association for the study of liver diseases. Hepatology.
72:671–722. 2020. View Article : Google Scholar
|
|
7
|
Li H: Advances in anti hepatic fibrotic
therapy with traditional Chinese medicine herbal formula. J
Ethnopharmacol. 251:1124422020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang L, Ren Y, Cao L, Sun Y, Sun Q, Sheng
N, Yuan L, Zhi X and Zhang L: Simultaneous determination and
pharmacokinetic study of six flavonoids from fructus sophorae
extract in rat plasma by LC-MS/MS. J Chromatogr B Analyt Technol
Biomed Life Sci. 904:59–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim BH and Lee S: Sophoricoside from
styphnolobium japonicum improves experimental atopic dermatitis in
mice. Phytomedicine. 82:1534632021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim BH and Lee S: Sophoricoside from
Sophora japonica ameliorates allergic asthma by preventing mast
cell activation and CD4+ T cell differentiation in
ovalbumin-induced mice. Biomed Pharmacother. 133:1110292021.
View Article : Google Scholar
|
|
11
|
Zhang Y, Li F, Jiang X, Jiang X, Wang Y,
Zhang H, Zhang L, Fan S, Xin L, Yang B, et al: Sophoricoside is a
selective LXRβ antagonist with potent therapeutic effects on
hepatic steatosis of mice. Phytother Res. 34:3168–3179. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YX, Zeng S, Wan BB, Wang YY, Sun HX,
Liu G, Gao ZQ, Chen D, Chen YQ, Lu MD and Pang QF: Sophoricoside
attenuates lipopolysaccharide-induced acute lung injury by
activating the AMPK/Nrf2 signaling axis. Int Immunopharmacol.
90:1071872021. View Article : Google Scholar
|
|
13
|
Hao J, Sun W and Xu H: Pathogenesis of
concanavalin A induced autoimmune hepatitis in mice. Int
Immunopharmacol. 102:1084112022. View Article : Google Scholar
|
|
14
|
Holdener M, Hintermann E, Bayer M, Rhode
A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter
J, Manns MP, et al: Breaking tolerance to the natural human liver
autoantigen cytochrome P450 2D6 by virus infection. J Exp Med.
205:1409–1422. 2008. View Article : Google Scholar :
|
|
15
|
Wang H, Yan W, Feng Z, Gao Y, Zhang L,
Feng X and Tian D: Plasma proteomic analysis of autoimmune
hepatitis in an improved AIH mouse model. J Transl Med. 18:32020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Huang Z, Lei Y, Han X, Tian D,
Gong J and Liu M: Celastrol alleviates autoimmune hepatitis through
the PI3K/AKT signaling pathway based on network pharmacology and
experiments. Front Pharmacol. 13:8163502022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei Y, Wang S, Liu J, Yan W, Han P and
Tian D: Identification of MCM family as potential therapeutic and
prognostic targets for hepatocellular carcinoma based on
bioinformatics and experiments. Life Sci. 272:1192272021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Li X, Zhang W, Liu W, Lv Z, Gui R,
Li M, Li Y, Sun X, Liu P, et al: ETNPPL impairs autophagy through
regulation of the ARG2-ROS signaling axis, contributing to palmitic
acid-induced hepatic insulin resistance. Free Radic Biol Med.
199:126–140. 2023. View Article : Google Scholar
|
|
19
|
Lei Y, Han P, Chen Y, Wang H, Wang S, Wang
M, Liu J, Yan W, Tian D and Liu M: Protein arginine
methyltransferase 3 promotes glycolysis and hepatocellular
carcinoma growth by enhancing arginine methylation of lactate
dehydrogenase A. Clin Transl Med. 12:e6862022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Crosas-Molist E and Fabregat I: Role of
NADPH oxidases in the redox biology of liver fibrosis. Redox Biol.
6:106–111. 2015. View Article : Google Scholar
|
|
22
|
Webb GJ, Hirschfield GM, Krawitt EL and
Gershwin ME: Cellular and molecular mechanisms of autoimmune
hepatitis. Annu Rev Pathol. 13:247–292. 2018. View Article : Google Scholar
|
|
23
|
Longhi MS, Mieli-Vergani G and Vergani D:
Regulatory T cells in autoimmune hepatitis: An updated overview. J
Autoimmun. 119:1026192021. View Article : Google Scholar
|
|
24
|
Kaffe ET, Rigopoulou EI, Koukoulis GK,
Dalekos GN and Moulas AN: Oxidative stress and antioxidant status
in patients with autoimmune liver diseases. Redox Rep. 20:33–41.
2015. View Article : Google Scholar
|
|
25
|
Wei Y, Li Y, Yan L, Sun C, Miao Q, Wang Q,
Xiao X, Lian M, Li B, Chen Y, et al: Alterations of gut microbiome
in autoimmune hepatitis. Gut. 69:569–577. 2020. View Article : Google Scholar
|
|
26
|
Wen Y, Han C, Liu T, Wang R, Cai W, Yang
J, Liang G, Yao L, Shi N, Fu X, et al: Chaiqin chengqi decoction
alleviates severity of acute pancreatitis via inhibition of TLR4
and NLRP3 inflammasome: Identification of bioactive ingredients via
pharmacological sub-network analysis and experimental validation.
Phytomedicine. 79:1533282020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oyewole AO and Birch-Machin MA:
Mitochondria-targeted antioxidants. FASEB J. 29:4766–4771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maynard AG and Kanarek N: NADH ties
one-carbon metabolism to cellular respiration. Cell Metab.
31:660–662. 2020. View Article : Google Scholar
|
|
29
|
Gao M, Hu F, Hu M, Hu Y, Shi H, Zhao GJ,
Jian C, Ji YX, Zhang XJ, She ZG, et al: Sophoricoside ameliorates
cardiac hypertrophy by activating AMPK/mTORC1-mediated autophagy.
Biosci Rep. 40:BSR202006612020. View Article : Google Scholar :
|
|
30
|
Li W and Lu Y: Hepatoprotective effects of
sophoricoside against fructose-induced liver injury via regulating
lipid metabolism, oxidation, and inflammation in mice. J Food Sci.
83:552–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
European Association for the Study of the
Liver: EASL clinical practice guidelines: Autoimmune hepatitis. J
Hepatol. 63:971–1004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seki E and Schwabe RF: Hepatic
inflammation and fibrosis: Functional links and key pathways.
Hepatology. 61:1066–1079. 2015. View Article : Google Scholar
|
|
33
|
Sanz-Cameno P, Medina J, Garcia-Buey L,
García-Sánchez A, Borque MJ, Martín-Vílchez S, Gamallo C, Jones EA
and Moreno-Otero R: Enhanced intrahepatic inducible nitric oxide
synthase expression and nitrotyrosine accumulation in primary
biliary cirrhosis and autoimmune hepatitis. J Hepatol. 37:723–729.
2002. View Article : Google Scholar
|
|
34
|
Pemberton PW, Aboutwerat A, Smith A,
Burrows PC, McMahon RF and Warnes TW: Oxidant stress in type I
autoimmune hepatitis: The link between necroinflammation and
fibrogenesis? Biochim Biophys Acta. 1689:182–189. 2004. View Article : Google Scholar
|
|
35
|
Beyazit Y, Kocak E, Tanoglu A and Kekilli
M: Oxidative stress might play a role in low serum vitamin D
associated liver fibrosis among patients with autoimmune hepatitis.
Dig Dis Sci. 60:1106–1108. 2015. View Article : Google Scholar
|
|
36
|
Richter K, Konzack A, Pihlajaniemi T,
Heljasvaara R and Kietzmann T: Redox-fibrosis: Impact of TGFβ1 on
ROS generators, mediators and functional consequences. Redox Biol.
6:344–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh R and Czaja MJ: Regulation of
hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol.
22(Suppl 1): S45–S48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Czaja AJ: Nature and implications of
oxidative and nitrosative stresses in autoimmune hepatitis. Dig Dis
Sci. 61:2784–2803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui W, Matsuno K, Iwata K, Ibi M,
Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ and
Yabe-Nishimura C: NOX1/nicotinamide adenine dinucleotide phosphate,
reduced form (NADPH) oxidase promotes proliferation of stellate
cells and aggravates liver fibrosis induced by bile duct ligation.
Hepatology. 54:949–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hernández-Gea V, Hilscher M, Rozenfeld R,
Lim MP, Nieto N, Werner S, Devi LA and Friedman SL: Endoplasmic
reticulum stress induces fibrogenic activity in hepatic stellate
cells through autophagy. J Hepatol. 59:98–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar
|
|
42
|
Dadheech G, Mishra S, Gautam S and Sharma
P: Evaluation of antioxidant deficit in schizophrenia. Indian J
Psychiatry. 50:16–20. 2008. View Article : Google Scholar
|
|
43
|
He G and Karin M: NF-κB and STAT3-key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar
|
|
44
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
45
|
Liberal R, de Boer YS and Heneghan MA:
Established and novel therapeutic options for autoimmune hepatitis.
Lancet Gastroenterol Hepatol. 6:315–326. 2021. View Article : Google Scholar : PubMed/NCBI
|