1. Introduction
The recent coronavirus disease 2019 (COVID-19)
pandemic has prioritised multifaced international scientific effort
for adequate, state-of-the-art, health and medical care, worldwide
(1). The COVID-19 era has been a
very demanding and challenging period for all individuals,
including paediatric health professionals, newborns, children and
parents (2). Moreover, it has
highlighted the value of multi-disciplinary international
scientific cooperation and medical education, including online
learning using virtual, hybrid teaching modes (3). Paediatric viral infections continue
to represent an educational, clinical and research field with
increasing needs and future perspectives. The present review
describes the key messages addressed during the recent 8th Workshop
on Paediatric Virology, which was organised through the COVID-19
era by the Institute of Paediatric Virology (IPV; https://www.paediatricvirology.org) on October
20, 2022 (4). The workshop was
coordinated virtually by the Paediatric Virology Study Group
(PVSG); this is the third year that this workshop adopted a hybrid
format and did not require the physical presence of its
participants due to the COVID-19 pandemic (5,6).
The workshop focused on the following factors: i) New advances in
antiviral agents and vaccines against cytomegalovirus (CMV); ii)
hantavirus nephropathy in children; iii) human rhinovirus (HRV)
infections in children requiring paediatric intensive care; iv)
complications and management of human adenovirus (HAdV) infections;
v) challenges of post-COVID-19 syndrome (PCS) in children and
adolescents and vi) foetal magnetic resonance imaging (MRI) in
viral infections involving the central nervous system (CNS)
(Table I).
 | Table ITop key messages of the 8th Workshop
on Paediatric Virology. |
Table I
Top key messages of the 8th Workshop
on Paediatric Virology.
| Topic | Key messages |
|---|
| CMV | CMV is a common
infection in neonates and children and has a significant healthcare
burden. Several antivirals are available with more in development;
different doses for each medication have been suggested for
different clinical scenarios (e.g., congenital CMV infection,
immuno-compromised children, those with renal issues, prematurity
and various ages).
There are CMV vaccines in the pipeline although one getting to
licensure is several years away at least. |
| Hantavirus | Hantaviruses should
be considered as possible causes of interstitial nephritis with
decreased GFR in children even in areas with a low incidence of
this infection. In children with fever, thrombocytopenia and
haematuria or proteinuria, differential diagnosis should include
hantavirus induced nephropathy. |
| HRV | HRVs have been
recently associated with increased morbidity, hospitalisation rates
and PICU admissions; recent advances in NGS analyses have
highlighted the significant burden in PICU admissions due to SARI
caused by HRV as well as CNS disease and MODS.
As there is no antiviral-specific treatment for HRV, recognition of
signalling pathways involved and the profiling of patients
according to disease phenotypes may lead the way to future
treatment modalities in hyperinflammatory antiviral response. |
| HAdV | HAdVs are common
causes of self-limited febrile illnesses in young children;
however, in some cases, it may lead to disseminated disease, which
requires hospitalisation and intensive management with antivirals
and immunotherapy.
The most common adenovirus infection in healthy children involves
the respiratory system and eyes; outbreaks of epidemic
keratoconjunctivitis can occur in hospitals due to the rapid
transmission of HAdVs via the hands of health care workers,
contaminated instruments or eye drops.
Neonates and immunocompromised individuals are at a risk of
increased morbidity and mortality; in these groups antiviral
therapy with cidofovir, ganciclovir, ribavirin and vidarabine has
been used in case reports or uncontrolled series, leading to
inconclusive evidence for efficacy. |
| SARS-CoV-2 | PCS appears to be
an existing entity in the paediatric population; its prevalence is
as yet undetermined and can occur in SARS-CoV-2 positive children,
even if asymptomatic; the most frequent symptoms are headache,
fatigue-weakness, sleep disturbances, cognitive disorders and
myalgia-arthralgia.
The clarification of PCS diagnostic criteria and management may
help to improve the health of young patients and guide health
policy makers. |
| Foetal MRI and
viral infections | Congenital
infections represent a serious cause for foetal CNS injury with
serious sequelae in the neonate.
In the case that maternal viral infection is suspected, combining
prenatal ultrasound and foetal MRI may document the extent of
tissue damage and therefore contribute to treatment and
counselling. |
In the context of the workshop, Professor Anna
Kramvis, Professor of Virology at the University of the
Witwatersrand (Johannesburg, South Africa), was awarded (2022
Paediatric Virology Award) for her outstanding academic, research,
teaching and publishing contribution in hepatitis B virus research
in Africa (7-9). Dr Fergus Maher, Consultant in
Palliative Medicine at the Norfolk and Norwich University
Hospitals, NHS Foundation Trust (Norwich, UK) and Honorary
Associate Professor in Palliative Medicine at the Norwich Medical
School of the University of East Anglia (Norwich, UK), was also
awarded (2022 George N. Papanicolaou Humanitarian Award) due to his
state-of-the art clinical and academic contribution to palliative
medicine in the UK (10).
2. Developing antiviral agents and vaccines
against cytomegalovirus: New advances
CMV causes one of the most common infections
globally, with most individuals being infected at some point in
life (11). For most, it results
in a mild, self-resolving illness that leads to lifelong, latent
infection. However, for some high-risk individuals, such as those
who are immunocompromised, CMV infection can result in severe
disease and even mortality (12).
In addition, foetuses infected in utero can develop
congenital CMV infection with sequelae ranging from asymptomatic
infection through to multiorgan failure, severe neurodisability and
mortality (13). To date, several
antiviral medications are already available to treat CMV infection.
Ganciclovir, valganciclovir, foscarnet and cidofovir have been used
for a number of years (12,13). Aciclovir/valaciclovir, primarily
used to treat varicella-zoster virus (VZV) and herpes simplex virus
(HSV) infections, have activity against CMV infection and are used
to treat pregnant women to reduce the risk of transmission of CMV
to the foetus; the antivirals acyclovir/valaciclovir are less toxic
than other CMV antivirals (14).
Different doses for each medication have been suggested for
different clinical scenarios (e.g., congenital CMV infection,
immunocompromised children, those with renal issues, prematurity
and various ages) (12-14). Newer agents, such as
brincidofovir, letermovir and maribavir, have recently been
developed, although their use remains limited (15). Novel antivirals are in early-stage
development. These include classes of small molecules, such as
distinct acyclic nucleoside phosphonate analogues, non-nucleoside
inhibitors of DNA polymerase, monoclonal antibodies, and
host-targeted antivirals, such as sirtuins (16). The pipeline for CMV antivirals is
certainly very promising.
The search for a CMV vaccine has been ongoing for a
number of decades; however, new vaccine platforms, particularly
those developed during the COVID-19 pandemic have recently led to
significant advances. CMV vaccines are being designed to target two
key groups, namely women of child-bearing potential/pregnant women,
to try to reduce the burden of congenital CMV, and those who are
severely immunosuppressed, to prevent the complications of acute
and chronic CMV infection in this population. There are three main
vaccines in clinical development. The first, and currently most
promising, is a mRNA-based CMV vaccine, which uses the same
technology as that used in some of the COVID-19 vaccines. This
vaccine is being studied in several ongoing phase 1-3 clinical
trials in healthy adults and adolescents and patients, who have
received a haemopoietic stem-cell transplant to assess safety,
immunogenicity and efficacy potential [for further information
please visit the official website of the National Institutes of
Health (NIH), US National Library of Medicine, ClinicalTrials.gov: https://clinicaltrials.gov/ct2/results?cond=CMV&term=vaccine%2C+Moderna&cntry=&state=&city=&dist=&Search=Search].
Results from these studies are expected over the ensuing years. A
conditionally replication-defective CMV vaccine (17) was recently assessed in a phase 2
clinical trial in women of child-bearing potential (for further
information visit the official website of the NIH, U.S. National
Library of Medicine, ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03486834). It
was shown to be safe and immunogenic, and reduced the quantity and
duration of CMV shedding in those who received three doses of the
vaccine (18). However, neither
the three-dose nor the two-dose regimen demonstrated significant
efficacy against CMV infection (18). It is unclear whether the
development of this vaccine will continue. The third is a CMV
recombinant protein subunit vaccine consisting of a combination of
glycoprotein B and pentamer antigens, being evaluated in a phase 1
clinical trial in healthy adults (for further information visit the
official website of the NIH, US National Library of Medicine,
ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05089630?term=vaccine%2C+gsk&cond=CMV&draw=2&rank=3).
Although there is clearly a long road ahead, advances in vaccine
development consequent to the COVID-19 pandemic, suggest that the
prospect of a licensed CMV vaccine is becoming increasingly
promising.
3. Hantavirus nephropathy in children: Cases
in Europe
In children, hantaviruses can cause a wide spectrum
of clinical manifestations, which have recently been grouped into
two clinical syndromes: i) Haemorrhagic fever with renal syndrome
(HFRS), which is endemic in Europe; and ii) hantavirus
cardiopulmonary syndrome (19-22). Children can be infected following
exposure to aerosolized urine, droppings or saliva of infected
rodents or following exposure to dust from their nests; rodents are
the main hosts of hantaviruses. The virus is transmitted through
the exposure of injured skin or mucous membranes (eyes or nose) to
these materials; transmission from one human to another has rarely
been described; however, it is not impossible (19-22).
HFRS usually appears between the 7th and the 14th
day after contact with the virus through the infectious material,
although in some occasions, it may take up to 2 months to manifest.
Symptoms of the acute phase include intense headaches, back and
abdominal pain, fever, chills, nausea and blurred vision. Affected
patients may experience flushing of the face, inflammation or
redness of the eyes, or a rash. Low blood pressure, acute shock and
acute kidney injury can appear later on. Each virus is responsible
for different manifestations of the illness (23-25). The Hantaan and
Dobrava viruses are associated with severe disease, while
the Seoul, Saaremaa and Puumala virus can cause
milder symptoms. The recovery can vary from several days to months.
The key to the diagnosis of HFRS is the detection of hantavirus RNA
sequences in blood or tissue. The optimal strategy with which to
control the disease is by treating the basic symptoms and
supporting the patient with the management of fluids and
electrolytes; in the case that this is not applicable, dialysis may
be used. Intravenous ribavirin has been shown to decrease illness
and mortality associated with HFRS, if used in the very early
stages of the disease (26,27).
Cases in Europe
Dusek et al (28) referred to the first three children
with Puumala virus nephropathy diagnosis in the Czech
Republic. A boy and two girls were diagnosed with interstitial
nephritis. Their initial symptoms were similar to those of the
'common cold'. The children presented with mild fever and a high
erythrocyte sedimentation rate and C-reactive protein levels, and
low haemoglobin levels (28).
Kidney injury was verified by the presence of proteinuria, the high
serum creatinine levels and a renal biopsy. The serological tests
for Puumala virus antigen antibodies were positive. All
children reacted positively to the supportive therapy, which led to
a clinical improvement within 1 month (28). van der Werff ten Bosch et
al (29) described a young
girl from Belgium diagnosed with hantavirus infection; in that
case, the main symptoms and signs were high fever, proteinuria,
haematuria and eye lesions. No kidney injury or haematological
discrepancies were documented. The diagnosis was based on the
detection of hantavirus in the blood tests. No specialised therapy
was required and the child had a rapid recovery (29). Eboriadou et al (30) described a case of an 11-year-old
boy from Northeastern Greece with haemorrhagic fever with HFRS
caused by hantavirus infection. The child presented with a mild
fever with total lack of other symptoms. The creatinine and urea
levels progressively increased to 23 and 930 mg/l, respectively,
accompanied with oligoanuria, proteinuria and haematuria. The
glomerular filtration rate (GFR) was 47.3 ml/min. The IgG
Hantaan viral antibody titer was 1:2,048; no IgM antibody
titer was quantified. The symptomatic therapy with fluids and
electrolytes was in this case of crucial significance and concluded
to a complete renal recovery after 6 months of follow-up.
4. Human rhinovirus infections requiring
paediatric intensive care
HRV of the Enterovirus genus, belonging to
the family Picornaviridae, has been traditionally recognised
as the 'common cold' pathogen. However, the association with severe
acute respiratory infection (SARI) in children needs to be
highlighted as there is increasing evidence to indicate the
implication of HRV with increased rates of hospitalisation and
paediatric intensive care unit (PICU) admissions (31-33). Of note, a prospective cohort study
including >600 infants linked severe HRV-associated respiratory
tract illness with maternal atopic disease and HRV species, with
HRV group B being associated with longer hospital stay (34). Similarly, other authors have
highlighted the presence of comorbidities, such as bronchopulmonary
dysplasia, a very low birth weight and congenital heart disease, as
significant risk factors for SARI caused by HRV (35-37).
To date, an increasing number of studies have shown
the increased rate of HRV in PICU admissions due to SARI in
paediatric, as well as adult populations (31-39). In a recent retrospective study,
Smith and Wilson (33)
demonstrated that 1 in 3 children with HRV infection admitted to a
PICU had severe respiratory insufficiency, and 14% of these
children met the diagnostic criteria for paediatric acute
respiratory distress syndrome (ARDS). Similarly, Spaeder et
al (38) conducted a
multicentre cohort study, including 519 patients with HRV infection
admitted to a PICU, providing insightful morbidity and mortality
data. It is worth noting, however, that 12.5 and 6.4% of the
patients had viral and bacterial coinfection, respectively
(38). In the same study
(38), clinical presentation to
the PICU consisted of wheezing (36%), apnoea (3%), stridor or croup
(1.2%) and seizures (3%). In addition, 1 in 4 patients required
mechanical ventilation, 3% were diagnosed with multiple organ
dysfunction syndrome (MODS), 2% developed ARDS and 2 patients
underwent extracorporeal membrane oxygenation; the mortality rate
was 2%. Notably, HRV infection prevailed in PICU admissions among
other usual suspects, such as respiratory syncytial virus (RSV) and
influenza viruses (38). Finally,
Asner et al (32)
conducted a retrospective cohort study in 750 children randomly
selected from a cohort of >5,000 outpatients, exploring the
clinical severity of single positive HRV cases compared with other
viral infections. They concluded that children with HRV had a more
severe course of disease than those with RSV and influenza A and B,
and often had significant comorbidities, such as cardiorespiratory,
metabolic and immunocompromise that also leading to a relatively
higher mortality rate (4.7%) (32). Of note, during the past decade,
HRV C, a previously unrecognised genogroup of HRVs, has been
discovered and linked to severe respiratory illness associated with
PICU admission, mechanical ventilation support and prolonged
hospital stay in addition to its association with the exacerbation
of asthma (39-41).
Furthermore, HRV infection has been implicated in
critical illness with extrapulmonary complications, mainly CNS
involvement and multiple organ dysfunction (42). Liu et al (43) described the case of a 10-year-old
female patient without comorbidities infected with HRV, who
presented with seizures attributed to significant diffuse
encephaloedema, and subsequently developed septic shock requiring
intubation, inotropes support and continuous renal replacement
treatment. She had a prolonged hospital stay of 75 days and her
MODS was attributed to HRV A45 confirmed by metagenomic
next-generation sequencing (NGS) (43). Similar cases have been reported in
previously healthy children, whereas in a large analysis of
unexplained cases of encephalitis using metatransciptomic
sequencing, HRV was implicating in a considerable proportion of
them (44,45). Triantafilou et al (46) attempted to elucidate the mechanism
of host hyperinflammatory response to HRV via translational studies
in human cell lines. According to their experimental results, the
Toll-like receptors (TLR) 2, 7 and 8 and melanoma
differentiation-associated (MDA) protein 5 signalling pathways
mediated the recognition of the virus in non-immune cells, which
further triggered a synergistic pro-inflammatory cytokine release
and hyperinflammatory phenotype, components that may be targeted
for future treatment modalities (46).
5. Complications and management of human
adenovirus infections in children
First isolated in the 1950s, HAdVs are
double-stranded DNA viruses, which consist of a family
(Adenoviridae) of >60 serotypes, divided into seven
subgroups, A to G (47). Certain
serotypes are associated with distinct clinical manifestations,
reflecting preferential infection of the respiratory,
gastrointestinal and urinary tracts and conjunctiva; however, the
majority of infections are asymptomatic (47). HAdVs are among the most common
viruses isolated from young children with febrile illness.
Pharyngitis, exudative tonsillitis, otitis media, bronchiolitis and
pneumonia are common presentations of respiratory tract infections.
There is a high incidence of pulmonary sequelae following
adenoviral pneumonia in young children, including restrictive lung
disease, bronchiectasis and bronchiolitis obliterans (48). Extrapulmonary complications
include meningoencephalitis, hepatitis, myocarditis, nephritis,
neutropenia and disseminated intravenous coagulation (47).
Pharyngoconjunctival fever is a classic adenoviral
syndrome that consists of a benign conjunctivitis often accompanied
by a febrile pharyngitis and cervical adenitis (47,49). Outbreaks have been described,
particularly in summer camps and swimming pools. Epidemic
keratoconjunctivitis is a more severe disease characterized by
bilateral conjunctivitis with preauricular adenopathy, followed by
the development of painful corneal opacities. It may be transmitted
in hospitals via the hands of healthcare workers, contaminated
instruments or eye drops. As previously demonstrated, in a neonatal
intensive care unit, an outbreak of HAdV type 8 conjunctivitis
occurred in seven premature infants who had undergone
ophthalmological examination (49). In an American study by Bowles
et al (50), polymerase
chain reaction (PCR) testing identified HAdV as the most detected
virus in the myocardium of children and adults with acute
myocarditis and dilated cardiomyopathy.
It is unclear whether HAdV infection is a cause of
the recent 2022 outbreak of unexplained hepatitis in children. As
of July 8, 2022, 35 countries had reported to the World Health
Organization (WHO) probable cases of children with severe acute
hepatitis of unknown aetiology (51). To date, there is no established
link with COVID-19 or hepatitis viruses A, B, C, D and E. Although
HAdV serotype 41 was detected by PCR in 65% of the tested cases in
the UK and 44.6% in the USA (52), it remains uncertain whether the
virus is the causative agent, as HAdV does not typically cause
hepatitis in healthy children. Preprints from the UK suggest that
co-infection with HAdV-associated virus 2, a member of the
parvovirus family may play a role (53); however, additional studies are
required to confirm this. Management includes supportive therapy
and treatment of coagulopathy disorders and hepatic encephalopathy
(51).
HAdVs are rare causes of CNS disease, particularly
in immunocompetent children. The diseases spectrum is highly
variable, ranging from febrile seizures and mild aseptic meningitis
to severe, acute necrotizing encephalopathy, leading to death. In a
retrospective study by Schwartz et al (54) conducted at the Hospital for Sick
Children (Toronto, Canada), 5% of children with microbiologically
confirmed HAdV infection had neurologic symptoms with an
encephalitis incidence of 0.4%; alternatively, 1.9% of encephalitis
cases were attributed to HAdV. (54). In another paediatric study from
Taiwan by Huang et al (55), a similar rate (3.3%) of children
with culture-confirmed HAdV infections had symptoms or signs of
neurological dysfunction. Notably, in the study by Schwartz et
al (54), HAdV in
cerebrospinal fluid or brain tissue was only isolated in 15% of the
cases; the most prevalent recorded serotype was type 7, followed by
serotypes 3 and 2. Complex seizures, coagulopathy, younger age and
serotype 2 were associated with adverse outcomes.
Hemophagocytic lymphohistiocytosis (HLH) is a
life-threatening condition of immune dysregulation (56,57). It is characterized by a prolonged
fever, hepatosplenomegaly, cytopenia and abnormal laboratory tests,
including elevated levels of aspartate aminotransferase, alanine
transaminase, triglycerides, ferritin and serum soluble
interleukin-2 receptor. Young children often have a genetic
predisposition for primary or familiar HLH, while others suffer
from secondary HLH as a complication of infection, malignancy or
rheumatologic disease. In the literature, there are few reports on
HAdV-associated HLH in healthy children (56,57). Otto et al (56) described five cases of HAdV 7
associated HLH and HLH-like illness in children aged 2 months-16
years old during the 2018-2019 season. In another report (57), two neonates with disseminated HAdV
infection, developed ARDS and cardiovascular failure attributed to
virus-related HLH. No specific therapeutic protocols have been
established; however, intravenous immunoglobulin, dexamethasone,
cyclosporin A, etoposide and methotrexate have been used with
beneficial effects.
HAdVs can cause disease during the
post-transplantation period in patients who have received
haematopoietic stem cell or solid organ transplants (58). The spectrum of infection ranges
from asymptomatic shedding to fatal disseminated disease. Common
manifestations include pneumonia, hepatitis, haemorrhagic
cystitis/nephritis (accompanied by acute renal failure at ~90%),
colitis and encephalitis. In a retrospective study by Munoz et
al (58), 11 of 440 children
with HAdV infection had disseminated disease; among these, 54% (6
of 11) were immunocompromised. Mortality from disseminated disease
was 73%, overall (83% among immunocompromised and 60% among
immunocompetent hosts).
The majority of HAdV infections are self-limiting
and treatment is supportive. Patients with CNS involvement, severe
respiratory disease, severe keratitis and immunosuppression require
hospitalisation. Neonates and immunocompromised individuals are at
a risk of increased morbidity and mortality. In these groups,
antiviral therapy with cidofovir, ganciclovir, ribavirin and
vidarabine has been used in case reports or uncontrolled series;
however, the evidence of efficacy is inconclusive (59). Cidofovir appears more efficient
against HAdV than other antiviral drugs, although severe
nephrotoxicity is a major dose-limiting adverse event. In a
retrospective study on 45 patients with HAdV infection following
allogeneic haematopoietic stem cell transplantation, 69% were
successfully treated with cidofovir, even though 40% experienced
toxicity (60). Intravenous
immunoglobulin has also been used in conjunction with antivirals
(61). T-cell immunity is
critical for the recovery from HAdV infection following
haematopoietic stem cell transplantation. The rapid transfer of
donor-derived, virus-specific memory T-cells offers substantial
promise in controlling severe disease with low adverse effects in
those with intolerance or nonresponse to antivirals. In the study
by Leen et al (62), the
treatment of HAdV infections in 17 paediatric stem cell transplant
recipients with banked third-party virus-specific T-cells from
individuals with common human leukocyte antigen polymorphisms,
resulted in a complete or partial response in 78% of patients; 7
patients with complete response and 7 patients with partial
response.
6. Post-COVID-19 syndrome in children and
adolescents: Exploring current terminology, symptomatology and
management
Even though acute severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infection has proven to be a mild
disease for the majority of children, it has been recognized that
symptoms from various systems may persist, re-emerge or recur
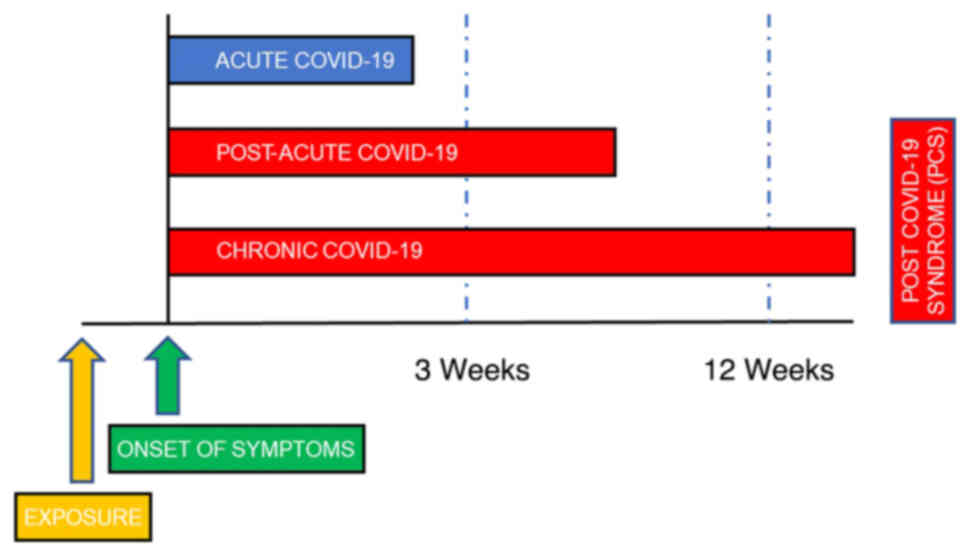
months following the acute phase, constituting the PCS or 'long
COVID' (Fig. 1). This syndrome,
which has been described primarily in adults, was first mentioned
in May 2020 on Twitter by patients themselves (adults), trying to
communicate inexplicable symptoms following acute SARS-CoV-2
infection, that made their everyday living difficult (63). In September 2020, WHO established
the codes U09 in the International System of Classification of
Diseases (ICD-10 and ICD-11) for this new condition (64) without mentioning the children.
Once again, in November, 2020, the media highlighted that children
may also be affected by PCS (65). Since then, there have been
numerous attempts made to define this new condition, mainly for the
adults, but lately also for the children, the most acceptable of
which are presented in Table
II.
 | Table IIDefinitions of the post-COVID-19
syndrome (PCS). |
Table II
Definitions of the post-COVID-19
syndrome (PCS).
| Scientific
corporation/institute/group | Definitions |
|---|
| WHO | In October 2021,
the WHO defined the post-COVID-19 syndrome as 'the condition that
occurs in people with a history of definite or probable SARS-CoV-2
infection, usually within 3 months of the onset of the COVID-19
disease, with symptoms that last at least 2 months and cannot be
attributed-explained by another diagnosis' (64). These symptoms may follow an
initial period of recovery or persist from the initial infection
and generally have an impact on the functions of daily life. This
definition does not include children. |
| CDC | In July 2021, the
CDC introduced post-COVID-19 as the condition where there is no
complete return to previous health status, where patients of all
ages experience new, recurrent, or persistent symptoms, at least 4
weeks after SARS-CoV-2 infection (90). Symptoms can recur after a period
of recovery from the initial infection and can occur regardless of
the severity of the acute infection. |
| NICE | In February 2022,
NICE defined long COVID-19 as persistent symptoms originating from
the infection (ongoing symptomatic COVID-19) and lasting for 4 up
to 12 weeks but also symptoms after the infection (post-COVID-19
syndrome) lasting ≥12 weeks (91). |
| NIHR | The NIHR has
proposed that post-COVID-19 syndrome consists of different
syndromes that include post-intensive care syndrome, post-viral
fatigue syndrome, long-term COVID-19 syndrome and chronic disease
that can result from organ damage due to of COVID-19, with patients
likely to suffer from more than one syndrome and some showing
different clusters of symptoms or patterns of symptoms (92). |
| CLoCk
Consortium | At the end of 2021,
the scientific group 'CLoCk Consortium' published a definition for
children and young people, corresponding to the WHO definition
(93). Post-COVID-19 condition
occurs in children and young people with a history of confirmed
SARS-CoV-2 infection, with one or more persistent physical
symptoms, for a minimum period of 12 weeks following the initial
diagnosis and cannot be explained by another diagnosis. Symptoms
must affect daily functioning, may persist or develop after
COVID-19, and may fluctuate or recur over time. |
To date, numerous terms have been used in order to
describe this syndrome, such as 'long-haulers', 'long COVID',
'post-acute sequalae of COVID-19 (PASC)', 'post-COVID', 'long term
effects of COVID', 'long-post-COVID' and 'chronic COVID' (66,67). Symptoms and signs from almost all
systems have been reported, including cardiovascular (chest pain,
palpitations, orthostatic intolerance),
neurological-neuropsychiatric (brain fog, concentration disorders,
sleep disorders, headaches, memory disorders, behavioural
disorders, irritability, smell/taste disorders, dizziness, night
sweats, anxiety, depression, convulsions), dermatological (rash,
chilblains), gastrointestinal (abdominal pain, epigastric pain,
diarrhoea, vomiting), musculoskeletal (myalgia, arthralgia),
respiratory (cough, dyspnoea, sore throat, nasal congestion),
haematological (coagulation disorders, haemorrhagic diathesis) and
endocrinological (hyperglycaemia) (68-70).
The reported prevalence varies widely, from <4 to
>66%, depending on the symptom and the methodology of the study
(68,71-73). The most frequently reported
symptoms are headache, fatigue-weakness, sleep disturbances,
cognitive disorders and myalgia-arthralgia (71). Lower prevalence rates (<2-3%)
are usually observed in children with no history of hospitalisation
and mild-to-moderate disease, whereas higher rates (>10%) are
recorded in children with more severe disease, with comorbidities,
who may had been hospitalised (72,74). Therefore, it is crucial for
studies to specify the level of severity of the initial disease. Of
note however, numerous children with a negative history of
infection also exhibit symptoms, such as anxiety, sleep
disturbances, headaches and a decline in mental functions,
attributed to the COVID-19 pandemic itself (75,76).
The clinical spectrum of PCS should be treated with
ample attention, whether originating from pathophysiological
alterations, or it is the result of increased stress and
aggravation of pre-existing psychological issues, which developed
due to the pandemic. The question that remains unanswered, is which
is the most appropriate management of those individuals who believe
they are suffering from it. The majority of studies focus on the
care of the adult population, and propose the provision of
supportive, holistic, individualized treatment of people with PCS,
initially at primary care level, but with immediate referral to
specialists whenever serious conditions emerge (77).
For the paediatric population, some advocate that
there should be a clinical assessment of children with a history of
SARS-CoV-2 infection, either confirmed or suspected, 4-12 weeks
after the primary infection (78,79). During the first visit, a clinical
examination should be performed and a detailed history should be
taken, aiming to detect symptoms, even of low intensity, that make
the daily lives of children difficult and may not have been
evaluated properly, either by the child, or by the caregiver
(68,73,78). Subsequently, there should be
re-evaluation visits, whenever symptom patterns change (79). Further management should be
undertaken on an individualized basis, consisting of laboratory
investigation (e.g., chest X-ray, blood biochemistry test,
respiratory tests, and other tests) or referral to an appropriate
specialty depending on the clinical manifestations. The management
of PCS means primarily listening to the patients, and validating
their experience (77). Special
care should be taken when assessing previously healthy adolescents,
who present with psychological issues, to not reinforce their
possibly false concept of being unwell (73,80). When the specialized
examination/investigation (pulmonological, neurological,
rheumatological, cardiological, allergological and psychiatric)
reveals specific conditions, the appropriate treatment (inhaled
bronchodilators, anti-inflammatories, β-blockers, antihistamines,
anxiolytics, etc.) should be administered (68,73,81).
Health care professionals should be trained and
informed about this new PCS condition. The long COVID-19 clinics,
which have been established by the majority of hospitals, play a
crucial role; however, it is critical to train primary health care
(PHC) professionals in this new entity, as they are going to face
the majority of such patients (73). In a Centers for Disease Control
and Prevention (CDC) publication by Kompaniyets et al
(70) on PCS syndrome in
children, who were 0-17 years of age, conditions that were more
likely to occur among children with a history of SARS-CoV-2
infection than without, included the following: Acute pulmonary
embolism, myocarditis and cardiomyopathy, acute renal failure and
type I and type II diabetes mellitus. These are very severe
conditions, which are not commonly found in paediatric patients;
thus, PHC workers should be aware of and prepared to combat these.
The majority of symptoms subside after 12 weeks from the infection
and the most valid criterion for recognizing improvement is the
testimony of the patient that they feel better. As more guidelines
are becoming available for the management of PCS, it is important
that these are followed by the health care workers involved
(68,73,78,81). Studying and understanding this new
entity is essential, as it will not only help improve the health of
young patients, but will also help health policy makers to
re-examine measures against the pandemic and re-evaluate the need
for different protection and management strategies against
SARS-CoV-2 infection in children.
7. Foetal magnetic resonance imaging and
viral infections: Indications and findings
Foetuses are susceptible to a wide variety of viral
infections, which can result in significant health issues
associated with morbidity and disabilities in the affected children
(82,83). CMV, HSV, human immunodeficiency
virus (HIV), enteroviruses, VZV, parvovirus B19 and Zika virus
account for transplacental foetal infection and most commonly
involve the CNS (83).
Neurosonography, including a foetal ultrasound brain scan, is the
primary technique for assessing the foetal brain, while foetal MRI
complements the ultrasound (82).
Maternal infections represent one of the main indications for
performing foetal CNS MRI, particularly if the ultrasound is
abnormal or equivocal (82,84). Although imaging is practically
unable to set the diagnosis of a viral infection in foetal life and
reveal the pathogens, it has the potential to accurately suggest
this scenario in foetal life, map the extent of involvement and
direct the investigation, the parental consultation and the
neonatal care accordingly. In fact, the developing brain is
particularly sensitive to neurotropic viruses and infections
acquired early in pregnancy, which may interfere with normal brain
development (83). Each pathogen
has a predilection for specific anatomical region/s. However, the
sequelae of an intrauterine infection reflect a combination of the
pathogens and the stage of foetal development at which the exposure
has occurred (85,86).
CMV appears to represent the most frequent and
critical maternal infection. The findings of microcephaly, gyration
anomalies and parenchymal lesions may provide a clue, although they
are usually non-specific to foetal CMV infection (84). As a neurotropic virus, CMV
haematogenously seeds the choroid plexus and replicates in the
ependymal, germinal matrix and capillary endothelium (83). It may severely affect the foetal
brain and cause permanent sequelae in almost 50% of symptomatic
neonates, but also in up to 25% of the asymptomatic infants, who
will develop sensorineural hearing loss usually by the age of 2
(83,86). Thus, foetal CNS MRI is indicated
in these pregnancies, even if the ultrasound is negative. If CMV
infection is acquired early in the second trimester, by
predilection of the germinal matrix cells, it appears to interfere
with normal neuro-glial migration and cause migrational and
cortical abnormalities, such as agyria/pachygyria, polymicrogyria,
schizencephaly, cerebellar hypoplasia and ventriculomegaly
(83,86). Capillary involvement may lead to
thrombosis and brain ischaemic lesions (83). This interesting association is
examined in the literature, where there is an attempt to associate
imaging findings with the timing of infection during pregnancy. In
late gestation infections, different kinds, usually less sever,
anomalies are noted, such as ventriculomegaly and myelin delay
(focal, patchy or confluent with posterior distribution but sparing
of the periventricular and subcortical white matter).
Periventricular cysts are more characteristic of CMV infection,
with variable locations, commonly in the anterior temporal lobes
where white matter abnormalities are also noted. The differential
diagnosis includes other cysts and parenchymal lesions. Parenchymal
calcifications of variable configuration may be seen throughout
pregnancy but they are not pathognomonic of CMV infection. These
are better appreciated on neonatal ultrasound or CT (83).
Intrauterine HSV infection is rare and may lead to
foetal hydrops, encephalomalacia, ventriculomegaly, microcephaly
and foetal demise (83). Foetal
HIV infection is globally decreasing due to preventative measures.
In HIV-infected foetuses, calcifications of the subcortical white
matter of the frontal lobes are typically seen and their severity
is associated with the viral load. Foetuses affected by Zika virus
at any time of the gestation present with a variety of severe brain
defects, including microcephaly, brain atrophy, ventriculomegaly,
cortical, callosal and posterior fossa abnormalities and ocular
anomalies. Congenital varicella infection, caused by in utero
transmission of VZV, causes variable imaging findings, which range
from lobar destruction, basal ganglia necrosis and cerebellar
hypoplasia to polymicrogyria and ventriculomegaly. Congenital
infections represent a serious cause for foetal CNS injury with
severe sequelae in the neonate (86). In suspicion of maternal viral
infection, combining prenatal ultrasound and foetal MRI may
document the extent of tissue damage and contribute to targeted
treatment and counselling (87,88).
8. Conclusions and future perspectives
The management and prevention of CMV infection
requires the administration of antiviral agents and the development
of effective vaccines approved by the international scientific
community. Hantaviruses should be considered as possible causes of
interstitial nephritis with decreased GFR in children. Recent
advances in NGS analyses have emphasized the contribution of
non-RSV, non-influenza viruses in the PICU disease burden. Among
these, HRV appears to be a common pathogen causing severe
respiratory disease requiring mechanical support, whereas CNS
involvement and hyperinflammatory response with multi-organ
involvement are being recognised as extrapulmonary HRV infection
complications in critically ill children. HAdVs can also lead to
disseminated disease, which requires hospitalisation and PICU
management with antivirals and immunotherapy. PCS has been proposed
as a new entity in the paediatric population; its prevalence is as
yet undetermined and can occur in SARS-CoV-2 positive children,
even if asymptomatic. The clarification of its definition and
management may help improve the health of young patients, and may
also guide health policy makers. Congenital infections represent a
severe cause of foetal CNS injury with severe sequelae in the
neonate. In the case that maternal viral infection is suspected,
combining prenatal ultrasound and foetal MRI may document the
extent of tissue damage and contribute to treatment and
counselling. The COVID-19 era requires continuous, intensive,
systematic, global scientific efforts in the clinic and in the
laboratory, focusing on the diagnosis, management and prevention of
all neonatal and paediatric viral infections.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (INM, SBD, CC, PK, AP, CK, TS, KM, HK,
GP, AK, AG, MT and DAS) contributed equally to the conception and
design of the study, wrote the original draft, edited and
critically revised the manuscript, and read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
The present study was published in the context of
the 8th Workshop on Paediatric Virology, which was organised
virtually on October 20, 2022, by the Institute of Paediatric
Virology (IPV; https://www.paediatricvirology.org) based on the
island of Euboea in Greece, under the auspices of the World Academy
of Sciences (WAS) and the support of the Department of Clinical
Virology of the University of Crete School of Medicine and the
First Department of Paediatrics of the University of Athens School
of Medicine. In the context of the same workshop three more
articles by Kramvis et al (7), Maher et al (10) and Mammas et al (89) were published in Biomedical
Reports, Medicine International and Experimental and Therapeutic
Medicine, respectively.
Funding
No funding was received.
References
|
1
|
Jansen M, Irving H, Gillam L, Sharwood E,
Preisz A, Basu S, Delaney C, McDougall R, Johnston C, Isaacs D and
Lister P: Ethical considerations for paediatrics during the
COVID-19 pandemic: A discussion paper from the Australian
Paediatric Clinical Ethics Collaboration. J Paediatr Child Health.
56:847–851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Child Health Group; Royal
College of Paediatrics & Child Health; Royal College of
Paediatrics & Child Hea: Impact of the COVID-19 pandemic on
global child health: Joint statement of the International Child
Health Group and the Royal College of Paediatrics and Child Health.
Arch Dis Child. 106:115–116. 2021. View Article : Google Scholar
|
|
3
|
Johnston R, Sen C and Baki Y: Virtual
paediatrics: What COVID-19 has taught us about online learning.
Arch Dis Child Educ Pract Ed. 108:125–129. 2023. View Article : Google Scholar
|
|
4
|
Mammas IN, Greenough A, Theodoridou M and
Spandidos DA: The foundation of the Institute of Paediatric
Virology on the island of Euboea, Greece (Review). Exp Ther Med.
20:3022020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mammas IN, Kramvis A, Papaevangelou V,
Doukas SG, Naya SD, Doukas PG, Melikoki V, Bouros D, Thiagarajan P,
Chrousos GP, et al: SARS-CoV-2 infection and children: Insights
from the 6th Workshop on Paediatric Virology (Review). World Acad
Sci J. 4:1–12. 2022. View Article : Google Scholar
|
|
6
|
Mammas IN, Liston M, Koletsi P, Vitoratou
DI, Koutsaftiki C, Papatheodoropoulou A, Kornarou H, Theodoridou M,
Kramvis A, Drysdale SB and Spandidos DA: Insights in paediatric
virology during the COVID-era (Review). Med Int (Lond).
2:172022.
|
|
7
|
Kramvis A, Mammas IN and Spandidos DA:
Exploring the optimal vaccination strategy against hepatitis B
virus in childhood (Review). Biomed Rep. 19:482023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kramvis A: The clinical implications of
hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol.
26:285–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kramvis A: Challenges for hepatitis B
virus cure in resource-limited settings in sub-Saharan Africa. Curr
Opin HIV AIDS. 15:185–192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maher F, Mammas IN and Spandidos DA: The
challenges and perspectives of palliative medicine: A webinar by
the Paediatric Virology Study Group. Med Int (Lond). 3:242023.
|
|
11
|
Fowler K, Mucha J, Neumann M, Lewandowski
W, Kaczanowska M, Grys M, Schmidt E, Natenshon A, Talarico C, Buck
PO and Diaz-Decaro J: A systematic literature review of the global
seroprevalence of cytomegalovirus: Possible implications for
treatment, screening, and vaccine development. BMC Public Health.
22:16592022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiskey L, Madigan T, Ristagno EH,
Razonable RR and Ferdjallah A: Prevention and management of human
cytomegalovirus in pediatric HSCT recipients: A review. Front
Pediatr. 10:10399382022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pesch MH, Kuboushek K, McKee MM, Thorne MC
and Weinberg JB: Congenital cytomegalovirus infection. BMJ.
373:n12122021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faure-Bardon V, Fourgeaud J, Stirnemann J,
Leruez-Ville M and Ville Y: Secondary prevention of congenital
cytomegalovirus infection with valacyclovir following maternal
primary infection in early pregnancy. Ultrasound Obstet Gynecol.
58:576–581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SJ, Wang SC and Chen YC: Challenges,
recent advances and perspectives in the treatment of human
cytomegalovirus infections. Trop Med Infect Dis. 7:4392022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Acosta E, Bowlin T, Brooks J, Chiang L,
Hussein I, Kimberlin D, Kauvar LM, Leavitt R, Prichard M and
Whitley R: Advances in the development of therapeutics for
cytomegalovirus infections. J Infect Dis. 221(Suppl 1): S32–S44.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Freed DC, Liu Y, Li F, Barrett DF,
Xiong W, Ye X, Adler SP, Rupp RE, Wang D, et al: A conditionally
replication-defective cytomegalovirus vaccine elicits potent and
diverse functional monoclonal antibodies in a phase I clinical
trial. NPJ Vaccines. 6:792021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Das R, Blazquez-Gamero D, Bernstein DI,
Gantt S, Bautista O, Beck K, BSN RN, Conlon A, Rosenbloom D, Wang
D, et al: 1048. Double-blind, randomized, placebo-controlled phase
2b multicenter trial of V160, a replication-defective human
cytomegalovirus (CMV) vaccine. Open Forum Infect Dis. 8(Suppl 1):
S615–S616. 2021. View Article : Google Scholar
|
|
19
|
Mustonen J, Huttunen NP,
Brummer-Korvenkontio M and Vaheri A: Clinical picture of
nephropathia epidemica in children. Acta Paediatr. 83:526–529.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peters CJ: Viral Hemorrhagic Fevers. Viral
Pathogenesis. Lippincott-Raven Publishers; New York, NY: pp.
779–794. 1997
|
|
21
|
Peters CJ, Simpson GL and Levy H: Spectrum
of hantavirus infection: Hemorrhagic fever with renal Syndrome and
hantavirus pulmonary Syndrome. Annu Rev Med. 50:531–545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linderholm M and Elgh F: Clinical
characteristics of hantavirus infections on the Eurasian continent.
Curr Top Microbiol Immunol. 256:135–151. 2001.PubMed/NCBI
|
|
23
|
Koskela S, Mäkelä S, Strandin T, Vaheri A,
Outinen T, Joutsi-Korhonen L, Pörsti I, Mustonen J and Laine O:
Coagulopathy in acute puumala hantavirus infection. Viruses.
13:15532021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Latus J, Schwab M, Tacconelli E, Pieper
FM, Wegener D, Rettenmaier B, Schwab A, Hoffmann L, Dippon J,
Müller S, et al: Acute kidney injury and tools for
risk-stratification in 456 patients with hantavirus-induced
nephropathia epidemica. Nephrol Dial Transplant. 30:245–251. 2015.
View Article : Google Scholar
|
|
25
|
Latus J, Kitterer D, Segerer S, Artunc F,
Alscher MD and Braun N: Severe thrombocytopenia in
hantavirus-induced nephropathia epidemica. Infection. 43:83–87.
2015. View Article : Google Scholar
|
|
26
|
Antoniades A, Grekas D, Rossi CA and LeDuc
JW: Isolation of a hantavirus from a severely ill patient with
hemorrhagic fever with renal syndrome in Greece. J Infect Dis.
156:1010–1013. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferres M and Vial P: Hantavirus infection
in children. Curr Opin Pediatr. 16:70–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dusek J, Pejcoch M, Kolsky A, Seeman T,
Nemec V, Stejskal J, Vondrak K and Janda J: Mild course of Puumala
nephropathy in children in an area with sporadic occurrence
Hantavirus infection. Pediatr Nephrol. 21:1889–1892. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Werff ten Bosch J, Heyman P,
Potters D, Peeters S, Cochez C and Piérard D: Hantavirus Puumala
infection as a cause of fever of unknown origin in a child. Acta
Paediatr. 93:1120–1122. 2004. View Article : Google Scholar
|
|
30
|
Eboriadou M, Kalevrosoglou I, Varlamis G,
Mitsiakos G, Papa A and Antoniadis A: Hantavirus nephropathy in a
child. Nephrol Dial Transplant. 14:1040–1041. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Papadopoulos NG, Moustaki M, Tsolia M,
Bossios A, Astra E, Prezerakou A, Gourgiotis D and Kafetzis D:
Association of rhinovirus infection with increased disease severity
in acute bronchiolitis. Am J Respir Crit Care Med. 165:1285–1289.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asner SA, Petrich A, Hamid JS, Mertz D,
Richardson SE and Smieja M: Clinical severity of
rhinovirus/enterovirus compared to other respiratory viruses in
children. Influenza Other Respir Viruses. 8:436–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith ME and Wilson PT: Human
rhinovirus/enterovirus in pediatric acute respiratory distress
Syndrome. J Pediatr Intensive Care. 9:81–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miller EK, Williams JV, Gebretsadik T,
Carroll KN, Dupont WD, Mohamed YA, Morin LL, Heil L, Minton PA,
Woodward K, et al: Host and viral factors associated with severity
of human rhinovirus-associated infant respiratory tract illness. J
Allergy Clin Immunol. 127:883–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Costa LF, Queiróz DA, Lopes da Silveira H,
Bernardino Neto M, de Paula NT, Oliveira TF, Tolardo AL and
Yokosawa J: Human rhinovirus and disease severity in children.
Pediatrics. 133:e312–e321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miller EK, Bugna J, Libster R, Shepherd
BE, Scalzo PM, Acosta PL, Hijano D, Reynoso N, Batalle JP, Coviello
S, et al: Human rhinoviruses in severe respiratory disease in very
low birth weight infants. Pediatrics. 129:e60–e67. 2012. View Article : Google Scholar :
|
|
37
|
Brand HK, de Groot R, Galama JM, Brouwer
ML, Teuwen K, Hermans PW, Melchers WJ and Warris A: Infection with
multiple viruses is not associated with increased disease severity
in children with bronchiolitis. Pediatr Pulmonol. 47:393–400. 2012.
View Article : Google Scholar
|
|
38
|
Spaeder MC, Custer JW, Miles AH, Ngo L,
Morin NP, Scafidi S, Bembea MM and Song X: A multicenter outcomes
analysis of children with severe rhino/enteroviral respiratory
infection. Pediatr Crit Care Med. 16:119–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Louie JK, Roy-Burman A, Guardia-Labar L,
Boston EJ, Kiang D, Padilla T, Yagi S, Messenger S, Petru AM,
Glaser CA and Schnurr DP: Rhinovirus associated with severe lower
respiratory tract infections in children. Pediatr Infect Dis J.
28:337–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Renwick N, Schweiger B, Kapoor V, Liu Z,
Villari J, Bullmann R, Miething R, Briese T and Lipkin WI: A
recently identified rhinovirus genotype is associated with severe
respiratory-tract infection in children in Germany. J Infect Dis.
196:1754–1760. 2007. View
Article : Google Scholar
|
|
41
|
Lee WM, Kiesner C, Pappas T, Lee I,
Grindle K, Jartti T, Jakiela B, Lemanske RF Jr, Shult PA and Gern
JE: A diverse group of previously unrecognized human rhinoviruses
are common causes of respiratory illnesses in infants. PLoS One.
2:e9662007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
To KK, Lau SK, Chan KH, Mok KY, Luk HK,
Yip CC, Ma YK, Sinn LH, Lam SH, Ngai CW, et al: Pulmonary and
extrapulmonary complications of human rhinovirus infection in
critically ill patients. J Clin Virol. 77:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Zhao H, Feng Z, Liu Y, Feng Q, Qian
S, Xu L, Gao H and Xie Z: A severe case of human rhinovirus A45
with central nervous system involvement and viral sepsis. Virol J.
19:722022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li CX, Burrell R, Dale RC, Kesson A, Blyth
CC, Clark JE, Crawford N, Jones CA, Britton PN and Holmes EC:
Diagnosis and analysis of unexplained cases of childhood
encephalitis in Australia using metatranscriptomic sequencing. J
Gen Virol. 1032022.
|
|
45
|
Hazama K, Shiihara T, Tsukagoshi H,
Matsushige T, Dowa Y and Watanabe M: Rhinovirus-associated acute
encephalitis/encephalopathy and cerebellitis. Brain Dev.
41:551–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Triantafilou K, Vakakis E, Richer EA,
Evans GL, Villiers JP and Triantafilou M: Human rhinovirus
recognition in non-immune cells is mediated by Toll-like receptors
and MDA-5, which trigger a synergetic pro-inflammatory immune
response. Virulence. 2:22–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Centers for Disease Control and Prevention
(CDC): Adenoviruses. CDC. Atlanta, GA: 2022, https://www.cdc.gov/adenovirus/hcp/index.html.
|
|
48
|
Edmond K, Scott S, Korczak V, Ward C,
Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I and
Campbell H: Long term sequelae from childhood pneumonia; systematic
review and meta-analysis. PLoS One. 7:e312392012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Birenbaum E, Linder N, Varsano N, Azar R,
Kuint J, Spierer A and Reichman B: Adenovirus type 8 conjunctivitis
outbreak in a neonatal intensive care unit. Arch Dis Child. 68(5
Spec No): 610–611. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bowles NE, Ni J, Kearney DL, Pauschinger
M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR and
Towbin JA: Detection of viruses in myocardial tissues by polymerase
chain reaction. Evidence of adenovirus as a common cause of
myocarditis in children and adults. J Am Coll Cardiol. 42:466–472.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
World Health Organization (WHO): Severe
acute hepatitis of unknown aetiology in children-Multi-country.
WHO; Geneva: 2022, https://www.who.int/emergen-cies/disease-outbreak-news/item/2022-DON400.
Accessed October 20, 2022
|
|
52
|
Cates J, Baker JM, Almendares O,
Kambhampati AK, Burke RM, Balachandran N, Burnett E, Potts CC,
Reagan-Steiner S, Kirking HL, et al: Interim analysis of acute
hepatitis of unknown etiology in children aged >10 years-United
States, October 2021-June 2022. MMWR Morb Mortal Wkly Rep.
71:852–858. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Morfopoulou S, Buddle S, Torres Montaguth
OE, Atkinson L, Guerra-Assunção JA, Storey N, Roy S, Lennon A, Lee
JCD, Williams R, et al: Genomic investigations of acute hepatitis
of unknown aetiology in children. View Article : Google Scholar : https://media.gosh.nhs.uk/documents/MEDRXIV-2022-277963v1-Breuer.pdf.
Accessed October 20, 2022
|
|
54
|
Schwartz KL, Richardson SE, MacGregor D,
Mahant S, Raghuram K and Bitnun A: Adenovirus-Associated central
nervous system disease in children. J Pediatr. 205:130–137. 2019.
View Article : Google Scholar
|
|
55
|
Huang YC, Huang SL, Chen SP, Huang YL,
Huang CG, Tsao KC and Lin TY: Adenovirus infection associated with
central nervous system dysfunction in children. J Clin Virol.
57:300–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Otto WR, Behrens EM, Teachey DT, Lamson
DM, Barrett DM, Bassiri H, Lambert MP, Mount S, Petrosa WL, Romberg
N, et al: Human adenovirus 7-associated hemophagocytic
lymphohistiocytosis-like Illness: Clinical and virological
characteristics in a cluster of five pediatric cases. Clin Infect
Dis. 73:e1532–e1538. 2021. View Article : Google Scholar :
|
|
57
|
Censoplano N, Gorga S, Waldeck K,
Stillwell T, Rabah-Hammad R and Flori H: Neonatal adenovirus
infection complicated by hemophagocytic lymphohistiocytosis
Syndrome. Pediatrics. 141(Suppl 5): S475–S480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Munoz FM, Piedra PA and Demmler GJ:
Disseminated adenovirus disease in immunocompromised and
immunocompetent children. Clin Infect Dis. 27:1194–1200. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Alcamo AM, Wolf MS, Alessi LJ, Chong HJ,
Green M, Williams JV and Simon DW: Successful use of cidofovir in
an immunocompetent child with severe adenoviral sepsis. Pediatrics.
145:e201916322020. View Article : Google Scholar
|
|
60
|
Ljungman P, Ribaud P, Eyrich M,
Matthes-Martin S, Einsele H, Bleakley M, Machaczka M, Bierings M,
Bosi A, Gratecos N, et al: Cidofovir for adenovirus infections
after allogeneic hematopoietic stem cell transplantation: A survey
by the Infectious Diseases Working Party of the European Group for
Blood and Marrow Transplantation. Bone Marrow Transplant.
31:481–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sofer A, Arger N and Vest M: Successful
Treatment of Adenovirus-Induced ARDS With Cidofovir and IVIG. Chest
Infect. 144:229A2013. View Article : Google Scholar
|
|
62
|
Leen AM, Bollard CM, Mendizabal AM, Shpall
EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P,
et al: Multicenter study of banked third-party virus-specific T
cells to treat severe viral infections after hematopoietic stem
cell transplantation. Blood. 121:5113–5123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Callard F and Perego E: How and why
patients made long COVID. Soc Sci Med. 268:1134262021. View Article : Google Scholar
|
|
64
|
Soriano JB, Murthy S, Marshall JC, Relan P
and Diaz JV; WHO Clinical Case Definition Working Group on
Post-COVID-19 condition: A clinical case definition of
post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis.
22:e102–e107. 2022. View Article : Google Scholar
|
|
65
|
Munblit D, Simpson F, Mabbitt J,
Dunn-Galvin A, Semple CO and Warner J: Legacy of COVID-19 infection
in children: Long-COVID will have a lifelong health/economic
impact. Arch Dis Child. 107:e22022. View Article : Google Scholar
|
|
66
|
Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del
Valle NC, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A and
Villapol S: Long COVID in children and adolescents: A systematic
review and meta-analyses. Sci Rep. 12:99502022. View Article : Google Scholar
|
|
67
|
Thallapureddy K, Thallapureddy K, Zerda E,
Suresh N, Kamat D, Rajasekaran K and Moreira A: Long-Term
complications of COVID-19 infection in adolescents and children.
Curr Pediatr Rep. 10:11–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fainardi V, Meoli A, Chiopris G, Motta M,
Skenderaj K, Grandinetti R, Bergomi A, Antodaro F, Zona S and
Esposito S: Long COVID in children and adolescents. Life (Basel).
12:2852022.PubMed/NCBI
|
|
69
|
Borch L, Holm M, Knudsen M,
Ellermann-Eriksen S and Hagstroem S: Long COVID symptoms and
duration in SARS-CoV-2 positive children-a nationwide cohort study.
Eur J Pediatr. 181:1597–1607. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kompaniyets L, Bull-Otterson L, Boehmer
TK, Baca S, Alvarez P, Hong K, Hsu J, Harris AM, Gundlapalli AV and
Saydah S: Post-COVID-19 symptoms and conditions among children and
adolescents-United States. March 1, 2020-January 31, 2022. MMWR
Morb Mortal Wkly Rep. 71:993–999. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zimmermann P, Pittet LF and Curtis N: How
common is Long COVID in children and adolescents? Pediatr Infect
Dis J. 40:e482–e487. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nittas V, Gao M, West EA, Ballouz T,
Menges D, Wulf Hanson S and Puhan MA: Long COVID through a public
health lens: An umbrella review. Public Health Rev. 43:16045012022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pavli A, Theodoridou M and Maltezou H:
Post-COVID Syndrome: Incidence, clinical spectrum and challenges
for Primary Healthcare Professionals. Arch Med Res. 52:575–581.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Osmanov IM, Spiridonova E, Bobkova P,
Gamirova A, Shikhaleva A, Andreeva M, Blyuss O, El-Taravi Y,
DunnGalvin A, Comberiati P, et al: Risk factors for post-COVID-19
condition in previously hospitalized children using the ISARIC
Global follow-up protocol: A prospective cohort study. Eur Respir
J. 59:2101342022. View Article : Google Scholar
|
|
75
|
Zimmermann P, Pittet LF and Curtis N: Long
covid in children and adolescents. BMJ. 376:o1432022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zimmermann P, Pittet LF and Curtis N: The
challenge of studying Long COVID: An updated review. Pediatr Infect
Dis J. 41:424–426. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Greenhalgh T, Sivan M, Delaney B, Evans R
and Milne R: Long covid-an update for primary care. BMJ.
378:e0721172022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Esposito S, Principi N, Azzari C,
Cardinale F, Di Mauro G, Galli L, Gattinara GC, Fainardi V, Guarino
A, Lancella L, et al: Italian intersociety consensus on management
of long covid in children. Ital J Pediatr. 48:422022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Goldman RD: Long COVID in children. Can
Fam Physician. 68:263–265. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cozzi G, Marchetti F and Barbi E:
Clinicians need to be careful that they do not confuse mental
health issues and long COVID in children and adolescents. Acta
Paediatr. 112:180–183. 2023. View Article : Google Scholar
|
|
81
|
Morrow AK, Malone LA, Kokorelis C,
Petracek LS, Eastin EF, Lobner KL, Neuendorff L and Rowe PC:
Long-term COVID 19 sequelae in adolescents: The overlap with
orthostatic intolerance and ME/CFS. Curr Pediatr Rep. 10:31–44.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Salomon LJ and Garel C: Magnetic resonance
imaging examination of the fetal brain. Ultrasound Obstet Gynecol.
30:1019–1032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Neuberger I, Garcia J, Meyers ML, Feygin
T, Bulas DI and Mirsky DM: Imaging of congenital central nervous
system infections. Pediatr Radiol. 48:513–523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rossi AC and Prefumo F: Additional value
of fetal magnetic resonance imaging in the prenatal diagnosis of
central nervous system anomalies: A systematic review of the
literature. Ultrasound Obstet Gynecol. 44:388–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lo CP and Chen CY: Neuroimaging of viral
infections in infants and young children. Neuroimaging Clin N Am.
18:119–132. viii2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Griffiths PD, Mooney C, Bradburn M and
Jarvis D: Should we perform in utero MRI on a fetus at increased
risk of a brain abnormality if ultrasonography is normal or shows
non-specific findings? Clin Radiol. 73:123–134. 2018. View Article : Google Scholar
|
|
87
|
Verstraelen H, Vanzieleghem B, Defoort P,
Vanhaesebrouck P and Temmerman M: Prenatal ultrasound and magnetic
imaging in fetal varicella syndrome: Correlation with pathology
findings. Prenat Diagn. 23:705–709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sanchez TR, Datlow MD and Nidecker AE:
Diffuse periventricular calcification and brain atrophy: A case of
neonatal central nervous system cytomegalovirus infection.
Neuroradiol J. 29:314–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mammas IN, Drysdale SB, Theodoridou M and
Spandidos DA: Exploring medical terminology inexpediencies:
Tripledemic vs. triple epidemic. Exp Ther Med. 26:3342023.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Centers for Disease Control and Prevention
(CDC): Post-COVID Conditions: Information for Healthcare Providers.
CDC; Atlanta, GA2022, https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
Accessed October 20, 2022
|
|
91
|
The National Institute for Health and Care
Excellence (NICE): COVID-19 rapid guideline: managing the long-term
effects of COVID-19. https://www.nice.org.uk/guidance/NG. pp. 188Accessed
October 20, 2022
|
|
92
|
National Institute for Health and Care
Research (NIHR): Living with Covid-19-Second Review. https://evidence.nihr.ac.uk/theme-dreview/living-with-covid19-second-review.
Accessed October 20, 2022
|
|
93
|
Stephenson T, Allin B, Nugawela MD, Rojas
N, Dalrymple E, Pinto Pereira S, Soni M, Knight M, Cheung EY,
Heyman I, et al: Long COVID (post-COVID-19 condition) in children:
A modified Delphi process. Arch Dis Child. 107:674–680. 2022.
View Article : Google Scholar : PubMed/NCBI
|