Introduction
Acute pancreatitis (AP) is a common exocrine
inflammatory disease of the pancreas that can cause severe
abdominal pain, multiple organ dysfunction, pancreatic necrosis and
persistent organ failure. AP is associated with a mortality rate of
1-5% (1). However, the mortality
rate of patients with severe AP is between 15 and 35% (2). Ulinastatin has been shown to
attenuate lipopolysaccharide (LPS)-induced associated lung injury
(ALI) by suppressing the activation of the Toll-like receptor
4/NF-κB pathway, associated with neutrophil, macrophage and
myeloperoxidase activities (3).
One of the potential mechanisms of ulinastatin is through the
inflammatory response, whose main signaling pathways are TNF-α, and
the high mobility group box 1-mediated inflammatory amplification
of NF-κB and IL-6-mediated JAK2/STAT3 signaling pathway in AP-ALI
(4). Melatonin has been
demonstrated to exert a protective effect on AP-ALI in a rat model,
possibly mediated via the upregulation of IL-22 and Th22 levels to
improve the innate immunity of tissue cells and promote lung tissue
regeneration (5). At present, the
therapeutic efficacy of AP-ALI needs to be improved, and immune
cell-related therapy may be a beneficial approach.
Adropin is a peptide hormone encoded by the energy
homeostasis-associated (Enho) gene, composed of 76 amino
acids. Adropin is mainly expressed in the liver, brain, heart,
kidneys, pancreas, coronary artery, umbilical vein and other
tissues, with the highest expression observed in the brain. Adropin
can participate in the negative regulation of the immune system and
play an anti-inflammatory role in atherosclerosis, fatty
inflammation, fatty liver, non-alcoholic hepatitis, pulmonary
vasculitis (6) and multiple
sclerosis (7), where the level of
adropin in multiple sclerosis is significantly reduced. Notably,
adropin therapy has been shown to effectively reduce the monolayer
migration of macrophages induced by monocyte chemotactic protein-1
regulation (8). A previous study
demonstrated that the relative number of Tregs in patients with
fatty pancreas and type 2 diabetes was positively associated with
adropin (9). Another study also
demonstrated and adropin knockout (Adro-KO) mice presented with
myeloperoxidase (MPO)-anti-neutrophil cytoplasm
autoantibody-associated alveolar bleeding and abnormal Treg cell
function (10).
Therefore, it was hypothesized that adropin may play
a role in the immune regulation of AP-ALI. Combined with the
aforementioned experimental findings, it was hypothesized that
adropin may exert a protective effect against AP-ALI mediated by
the regulation of macrophage phenotypes. The present study aimed to
explore the protective effects of adropin on AP-ALI and its
possible mechanisms of action using a combination of clinical
samples and animal models.
Materials and methods
Clinical samples
A total of 23 serum samples were collected from
patients with AP and healthy controls between November, 2021 and
January, 2022 at The First Affiliated Hospital of Fujian Medical
University, Fuzhou, China. The mean age of the patients in the
AP-ALI and control groups was 49.9 and 45.0 years, respectively,
and the proportion of female patients was 60 and 46.2%,
respectively. All patients were confirmed by lipase, amylase and
imaging examinations. All patients provided informed consent for
excess specimens to be used for research purposes and all protocols
employed in the study were approved by the Medical Ethics Committee
of The First Affiliated Hospital of Fujian Medical University
[MTCA, ECFAH of FMU(2015) 084-1].
Animal model
The Adro-KO mice used in the present study were
developed by Shanghai Southern Model Biotechnology Co., Ltd, with a
genetic background of C57BL/6J. Non-homologous recombination repair
induced reading frame shift mutations were introduced using
CRISPR/Cas9 to generate the loss of Enho gene function
(9) (Fig. S1). A total of 30 male KO mice,
weighing 20-25 g and 8 weeks old, were used in the present
study.
In addition, 48 wild-type male mice weighing 20-25 g
and aged 8 weeks were purchased from the Wu's Animal Laboratory
Center (http://www.wssydw.com/m/index.asp) and housed in a
clean feeding environment. All the mice were kept at 24-26%
temperature and 50-60% humidity, and a 12/12 h light/dark cycle,
and were provided with free access to food and water. All animal
experiments complied with the National Institutes of Health
guidelines for the care and used of laboratory animals, and the
protocol was approved by the Ethics Committee of Fujian Medical
University (IACUC FJMU 2022-0428).
The mouse model of AP-ALI induced by the use of 10%
L-arginine (L-Arg) was established according to a previously
published protocol (11).
Briefly, the male mice were intraperitoneally injected with L-Arg
at 4 g/kg/time at an interval of 1 h. The animals were sacrificed
at 48 h following the intraperitoneal injection of L-Arg (cat. no.
A8220, Beijing Solarbio Science & Technology Co., Ltd.). The
animals were anesthetized using isofluorane and euthanized by
exsanguination through the inferior vena cava; the induction and
maintenance concentrations were 3 and 1%, respectively. Following
sacrifice, pain response, cardiac and respiratory arrest were
observed to confirm euthanasia. Serum samples from the inferior
vena cava were collected and pancreatic tissue samples were
harvested. Pancreatic and lung tissues were stored at −80°C for
protein analysis and the remaining samples were fixed in 10%
formalin for pathological examination.
The mice were randomly assigned to one of the
following groups: i) The WT + NS group (n=12), where the mice
received an intraperitoneal injection of normal saline; ii) the WT
+ L-Arg group (n=12), where the mice were intraperitoneally
injected with 10% L-Arg (4 mg/kg); iii) the Adro-KO + NS group
(n=12), where Adro-KO transgenic mice were intraperitoneally
injected with normal saline; iv) the Adro-KO + L-Arg group (n=12),
where the Adro-KO transgenic mice received an intraperitoneal
injection of 10% L-Arg (4 mg/kg); v) the Adro-KO + L-Arg +
Adro(34−76) group (n=12), where the Adro-KO transgenic
mice received an intraperitoneal injection of 10% L-Arg (4 mg/kg)
plus adropin(34−76) [cat. no. 126418, GL Biochem
(Shanghai) Ltd. 450 nmol/kg via intraperitoneal injection] five
times (Fig. S2) (12,13).
Hematoxylin and eosin (H&E) staining
and Masson's staining
The tissue samples were paraffin-sectioned with 4%
polyformaldehyde for 24 h, and 4-µm-thick tissue slices were
prepared, which were dipped in hematoxylin solution for 5-10 min,
followed by placement in eosin solution for 0.5-2 min (cat. no.
G1076, Wuhan Servicebio Technology Co., Ltd.) at room temperature.
The sections were placed in 95% ethanol for de-hydration and placed
in xylene and sealed with neutral gum. Images was obtained using a
light microscope (Leica Microsystems GmbH). The pancreatic tissue
sections were scored for the severity of pancreatitis based on
edema, inflammation, vacuolization and necrosis. The individual
scores were then added to obtain the total pathological score for
the pancreatic tissue. As previously mentioned, the scales of
interstitial and alveolar edema, interstitial and alveolar
leukocyte infiltration and fibrosis were used to assess the extent
of lung injury (14).
The tissue samples were paraffin-sectioned with 4%
polyformaldehyde for 24 h and 4-µm-thick tissue slices were
prepared. Masson's reagent (cat. no. G1006, Wuhan Servicebio
Technology Co., Ltd.) was used as per the manufacturer's
instructions at room temperature. Images was obtained using a light
microscope (Leica Microsystems GmbH). Masson's staining was
subsequently quantified by determining the collagen fiber
area/non-collagen fiber area.
Immunohistochemistry
The paraffin-embedded sections were de-waxed, soaked
in recovery buffer containing EDTA antigen (Wuhan Servicebio
Technology Co., Ltd.), and incubated with the primary antibodies at
4°C overnight. The sections were then incubated in HRP-secondary
antibody (1:200; cat. no. G1213, Wuhan Servicebio Technology Co.,
Ltd.) at room temperature for 2 h. Following incubation with DAB
(cat. no. G1212, Wuhan Servicebio Technology Co., Ltd.) and
hematoxylin (cat. no. G1004, Wuhan Servicebio Technology Co.,
Ltd.), the sections were sealed with neutral resin (cat. no.
WG10004160, Wuhan Servicebio Technology Co., Ltd.). Images was
obtained using a light microscope (Leica Microsystems GmbH). The
primary antibodies used were the following: MPO (1:200, cat. no.
YT5351, ImmunoWay), CD68 (1:200, cat. no. ab283654, Abcam),
caspase-3 (1:200, cat. no. A0214, ABclonal), poly(ADP-ribose)
polymerase 1 (PARP1; 1:200, cat. no. A19596, ABclonal) and TGF-β
(1:200, cat. no. bs-0103R, BIOSS).
Immunofluorescence
The paraffin-embedded sections were completely
dewaxed with xylene, anhydrous ethanol, 90% ethanol, 75% ethanol
and 50% ethanol, and immersed in antigen repair solution, membrane
breaking solution (cat. no. G1204, Wuhan Servicebio Technology Co.,
Ltd.), and, the sections were sealed with neutral resin (cat. no.
WG10004160, Wuhan Servicebio Technology Co., Ltd.). The sections
were incubated in primary antibody at 4°C overnight, followed by
incubation with goat anti-rat FITC (1:200, cat. no. BA1108, Boster
Bio) and goat anti-rabbit Alexa Fluor 594 (1:200, cat. no. ASP1365,
Abcepta) antibodies at room temperature for 2 h, respectively.
Following incubation with DAPI (2 µg/ml; cat. no. G1012,
Wuhan Servicebio Technology Co., Ltd.) at room temperature for 5
min, the sections were sealed with anti-fluorescence quenching
reagent (cat. no. G1401, Wuhan Servicebio Technology Co., Ltd.).
Visual fluorescence signals were obtained using a fluorescence
microscope (Olympus Corporation). The primary antibodies used were
the following: adropin (1:200; cat. no. PA5-72781, Thermo Fisher
Scientific, Inc.), CD68 (1:200, cat. no. ab53444, Abcam), inducible
nitric oxide synthase (iNOS; 1:200, cat. no. ab3523, Abcam) and
CD206 (1:200, cat. no. ab64693, Abcam).
TUNEL fluorescence staining
The paraffin-embedded sections were completely
dewaxed with xylene, anhydrous ethanol, 90% ethanol, 75% ethanol
and 50% ethanol, and incubated in protease K (cat. no. K1133A,
ApexBio) at room temperature for 20 min. After the sections were
slightly dried, they were incubated in equilibration buffer (cat.
no. K1133, ApexBio) at room temperature for 20 min. An appropriate
amount of TUNEL staining solution (TDT enzyme, dUTP, buffer mixed
at a ratio of 1:5:50, cat. no. K1133, ApexBio) was added to cover
the tissues. The sections were incubated in an incubator at 37°C
for 60 min. After PBS washes were applied, DAPI was applied for 10
min at room temperature. The sections were subsequently sealed with
anti-fluorescence quenching sealing reagent, and photographed under
a fluorescence microscope (Olympus Corporation).
Enzyme-linked immunosorbent assay
(ELISA)
The serum samples were prepared for the ELISA of
human adropin protein according to the manufacturer's instructions
(Jiangsu Enzymatic Co., Ltd.; Lot: MM-50924H2). A standard curve
was generated and the absorbance values were detected at 450 nm
using a Synergy 2 Multi-Mode microplate reader (BioTek Instruments,
Inc.).
Western blot analysis
The tissue proteins extracts were harvested from
fresh pancreatic tissue using RIPA lysis buffer with protease and
phosphatase inhibitors (cat. nos. G2002, G2006 and G2007, Wuhan
Servicebio Technology Co., Ltd.). The protein concentration was
quantified using the BCA method. Total proteins (30 µg) were
separated by 10% SDS-PAGE and transferred to PVDF membranes. The
PVDF membranes were blocked at room temperature for 20 min using a
rapid sealing solution. The primary antibodies were used at 4°C
overnight, and the antibody included Adropin (1:1,000, cat. no.
PA5-72781, Thermo Fisher Scientific, Inc.); GADPH (1:10,000; cat.
no. AC001, ABclonal); peroxisome proliferator-activated receptor γ
(PPARγ; 1:1,000, cat. no. bsm-52220R, BIOSS); p-PPARγ Ser112
(1:1,000, cat. no. bs-3737R, BIOSS); p-PPARγ Ser273 (1:1,000, cat.
no. bs-2875R, BIOSS), caspase-3 (1:1,000, cat. no. YC0006,
ImmunoWay Biotechnology Company) and PARP1 (1:1,000, cat. no.
A0942, ABclonal). Visualization reagent (PL101, Shenzhen SunView
technology Co., Ltd.) was used, and all blotted bands were analyzed
using ImageJ 1.48 software (National Institutes of Health), and the
intensity values were normalized to GADPH.
Reverse transcription-quantitative PCR
(RT-qPCR)
Lung tissues were isolated using RNA isolater Total
RNA Extraction Reagent (cat. no. RC112, Vazyme). The concentration
of total RNA in the samples was determined using a
spectrophotometer (Multiskan Go lDrop, Thermo Fisher Scientific,
Inc). cDNA was synthesized from 2,000 ng total RNA using the
SweScript RT I First Strand cDNA Synthesis kit (cat. no. G3331,
Wuhan Servicebio Technology Co., Ltd.). The cDNA was used for
quantitative PCR (qPCR) using the 2X SYBR-Green qPCR Master Mix
(cat. no. G3326, Wuhan Servicebio Technology Co., Ltd.) and the
threshold cycle (CT) was determined using the ABI QuantStudio 5
(Thermo Fisher Scientific, Inc.). The PCR thermocycling: (95°C 30
sec) ×1 cycle; (95°C 15 sec + 60°C 10 sec + 72°C 30 sec) ×40
cycles. Relative gene expression was calculated based on
normalization to β-actin. The primers and sequences used are
presented in Table SI).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and GraphPad prism5.0 software (GraphPad Software, Inc.) was
used for statistical analyses. An unpaired Student's t-test was
utilized for comparisons between two groups. ANOVA was used to
analyze multiple sets of data, and the Student-Newman-Keuls
analysis was used to make pair-to-pair comparisons (three groups),
and Tukey's multiple comparisons test was used to make pair-to-pair
comparisons (four groups). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
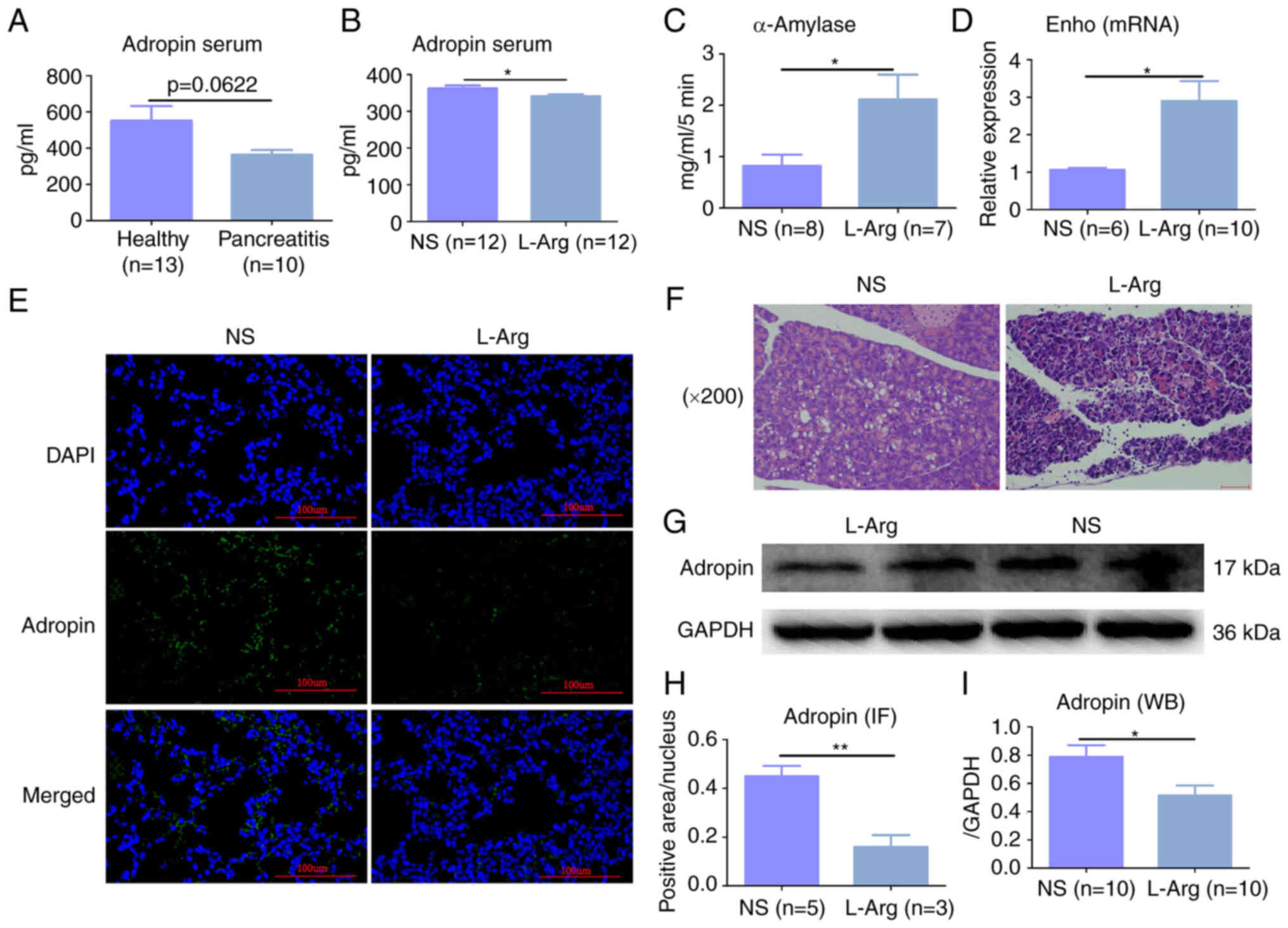
Adropin expression is lower in AP
Clinical samples were collected from patients with
AP and a mouse model of AP-ALI was constructed to explore the
expression of adropin in the AP model. ELISA revealed that the
average expression levels of adropin in the serum of patients with
AP was lower than that of the healthy controls (551.8l vs. 362.8
pg/ml); however, there was no significant difference between the
groups (P=0.0622, Fig. 1A).
Moreover, the expression of serum adropin in the WT + L-Arg mouse
group was significantly lower than in the WT + NS mouse group
(P<0.05, Fig. 1B). In
addition, serum α-amylase expression in the WT + L-Arg group was
higher than that in the WT + NS group (P<0.05, Fig. 1C). H&E staining revealed
evident intralobular and interlobular edema, diffuse acinar cell
necrosis of the pancreas, inflammatory cell infiltration around the
necrotic area, with the isolation of some acinar cells, nuclear
contraction and notable inflammatory changes in the pancreatic
tissues (Fig. 1F). RT-qPCR
revealed that Enho mRNA expression in the WT + L-Arg group
was higher than that in the WT + NS group (P<0.05, Fig. 1D), demonstrating that the
expression of adropin in the WT + L-Arg group was lower than that
in the WT + NS group; this was also shown by both
immunofluorescence and western blot analysis (P<0.05, Fig. 1E, G, H and I).
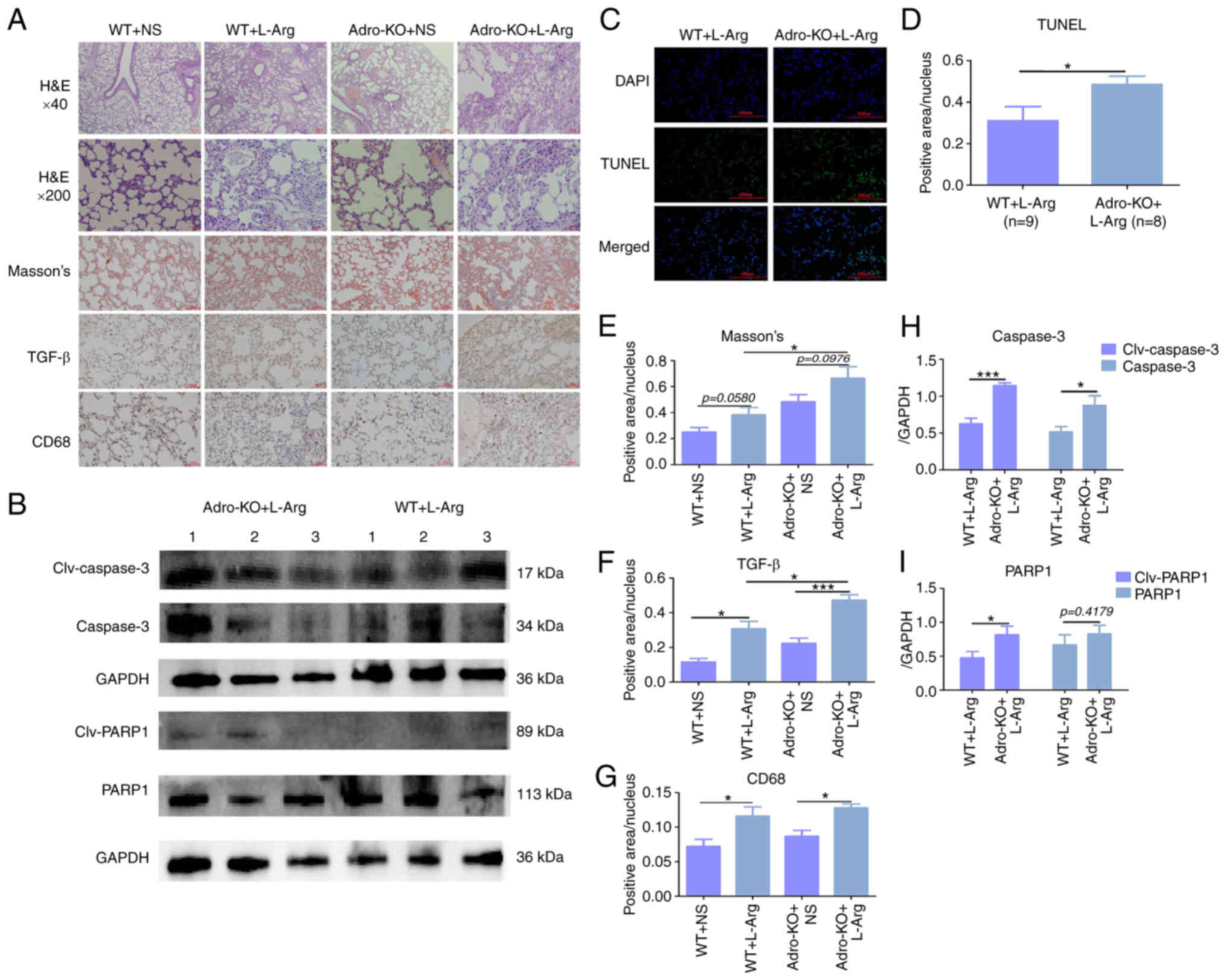
Animal model of Adro-KO and L-Arg-induced
AP exhibits severe AP-ALI
An Adro-KO mouse model and a model of AP were
constructed, and the degree of lung injury was evaluated using
immunohistochemistry and western blot analysis. Masson's staining
revealed that the level of fibrosis in the Adro-KO + L-Arg group
was higher than that in the WT + L-Arg group (P<0.05, Fig. 2A and E). Immunohistochemistry
revealed that the positive intensity of TGF-β in the Adro-KO +
L-Arg group was higher than that in the Adro-KO + NS and WT + L-Arg
groups, while the WT + L-Arg group demonstrated higher levels of
TGF-β than the WT + NS group (P<0.05, Fig. 2A and F). The positive intensity of
CD68 in the Adro-KO + L-Arg group was higher than that in the
Adro-KO + NS group, while the intensity of CD68 in the WT + L-Arg
group was higher than that in the WT + NS group (P<0.05,
Fig. 2A and G). Western blot
analysis demonstrated that the protein expression of caspase-3 and
PARP1 in the lung tissues collected from the mice in the Adro-KO +
L-Arg group was higher than that in the WT + L-Arg group
(P<0.05, Fig. 2B, H and I).
TUNEL staining similarly revealed that the level of apoptosis in
the lung tissues from the mice in the Adro-KO + L-Arg group was
higher than that in the WT + L-Arg group (P<0.05, Fig. 2C and D).
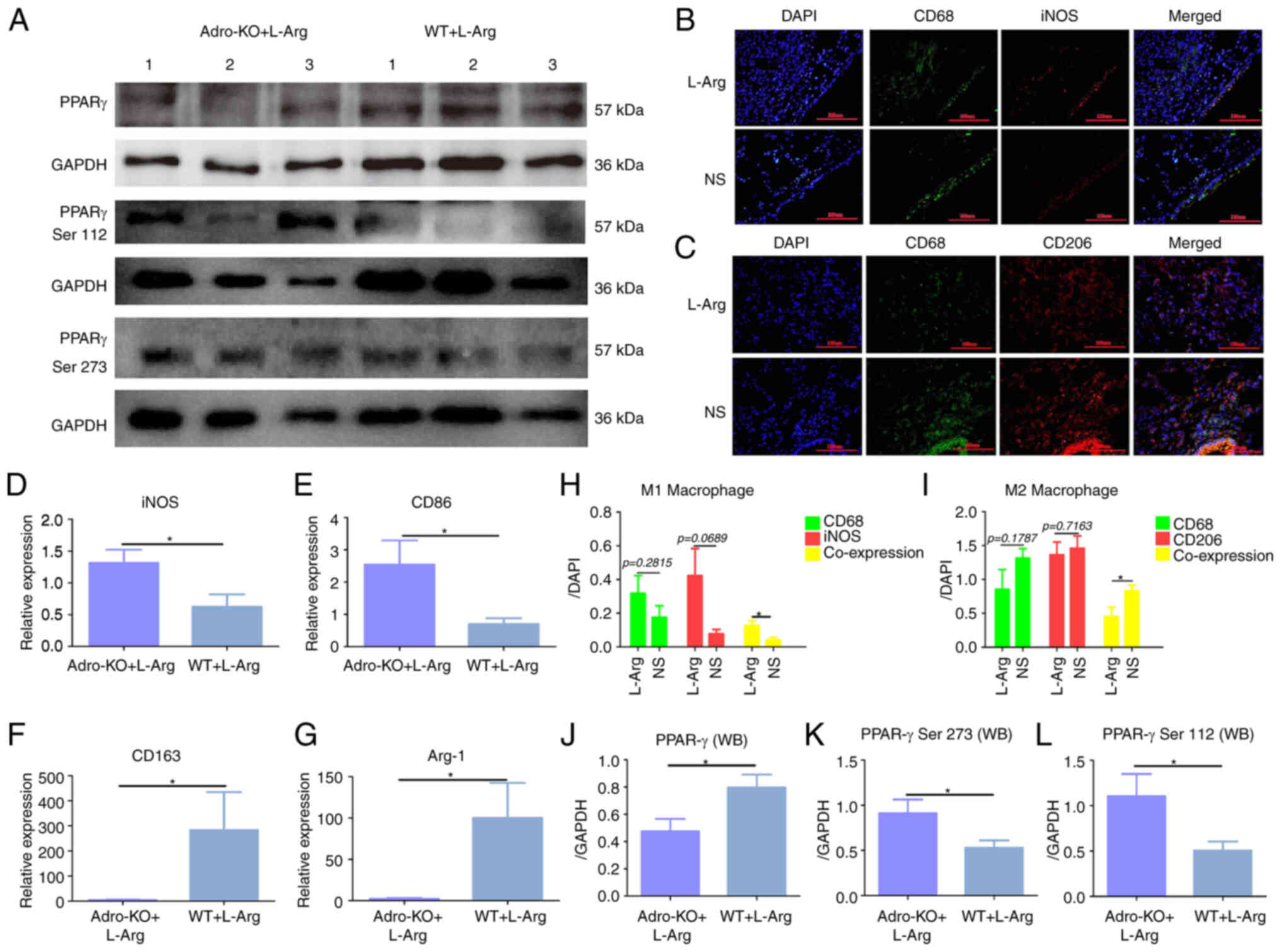
Adro-KO and L-Arg lead to excessive M1
macrophage polarization
In addition, using the established models of Adro-KO
AP, the phosphorylation of PPARγ and the polarization of
macrophages were evaluated using immunofluorescence and western
blot analysis. Western blot analysis revealed that the protein
expression of PPARγ in the lung tissues of mice from the Adro-KO +
L-Arg group was lower than that in the WT + L-Arg group (P<0.05,
Fig. 3A and J). The
phosphorylation levels of PPARγ Ser112 and PPARγ Ser273 in the
lungs of mice in the Adro-KO + L-Arg group were higher than those
in the WT + L-Arg group (P<0.05, Fig. 3A, K and L). The results also
indicated that the co-expression of iNOS and CD68 in the lung
tissues from the WT + L-Arg group was higher than that in the WT +
NS group (P<0.05, Fig. 3B and
H). The co-expression of CD206 and CD68 in the lung tissues
from the WT + L-Arg group was lower than that in the WT + NS group
(P<0.05, Fig. 3C and I). In
addition, the results demonstrated that the mRNA expression of iNOS
and CD86 in the lung tissue from the Adro-KO + L-Arg group was
higher than that in the WT + L-Arg group (P<0.05, Fig. 3D and E). It was also found that
the mRNA levels of CD163 and arginase 1 (Arg-1) in the lung tissues
from the Adro-KO + L-Arg group were lower than those in the WT +
L-Arg group (P<0.05, Fig. 3F and
G).
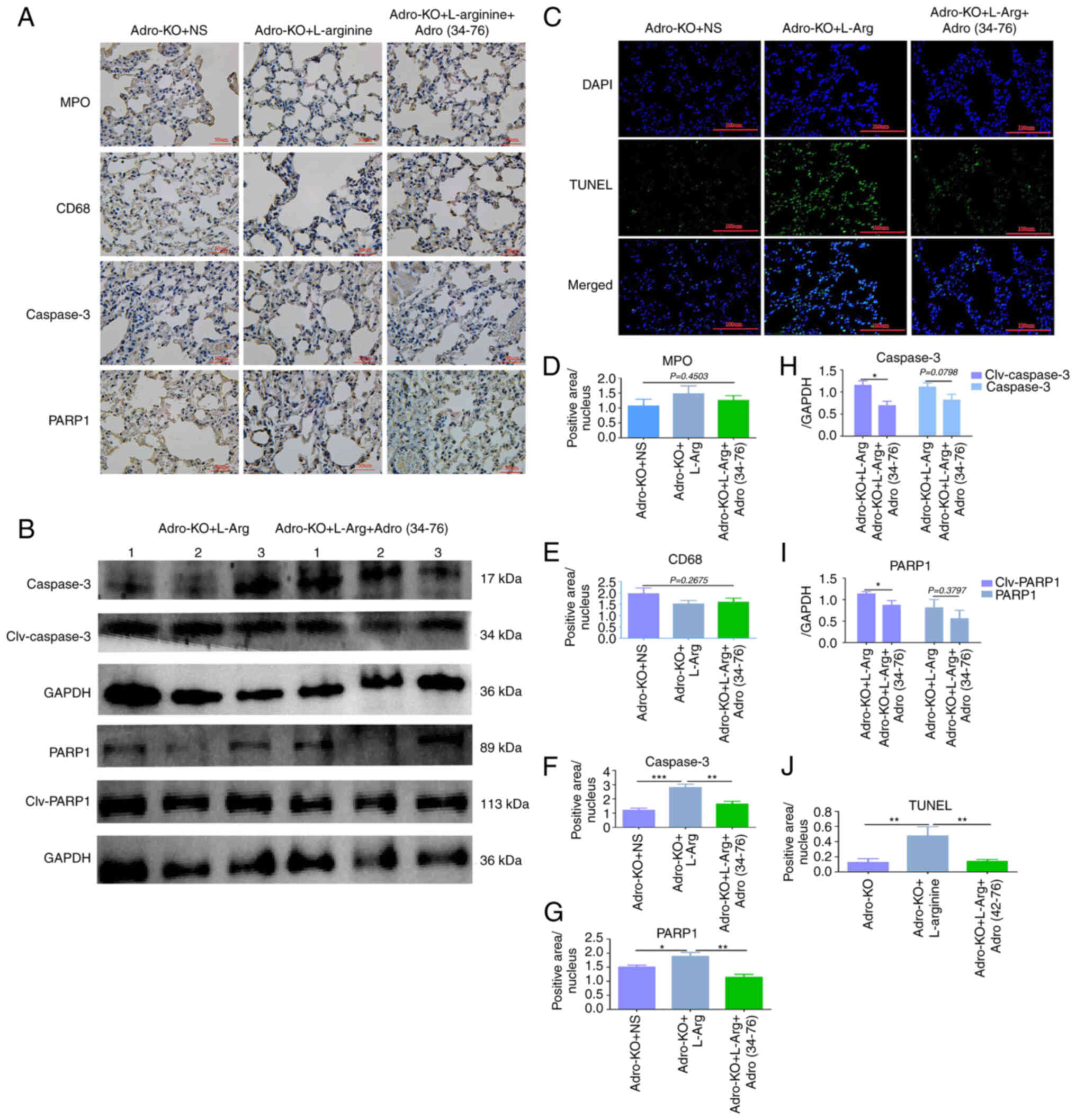
Adropin exogenous supplement attenuates
AP-ALI
Rescue experiments were then performed using
exogenous adropin and the protective effect of adropin was examined
in animal models of AP. Immunohistochemistry revealed that the
positive intensity of PARP1 and caspase-3 in lung tissues from the
Adro-KO + L-Arg + Adro(34−76) group was lower than that
in the Adro-KO + L-Arg group (P<0.05, Fig. 4A, F and G). There were no
significant differences in the protein expression of MPO and CD68
in the lung tissues between the Adro-KO +
L-Arg+Adro(34−76) and Adro-KO + L-Arg groups (P<0.05,
Fig. 4A, D and E). The results
also demonstrated that the protein expression levels of cleaved
caspase-3 and cleaved PARP1 in the lung tissues from the Adro-KO +
L-Arg + Adro(34−76) group were lower than those in the
Adro-KO + L-Arg group (P<0.05, Fig. 4B, H and I). Of note, the protein
expression levels of caspase-3 and PARP1 in the lung tissues from
the Adro-KO + L-Arg + Adro(34−76) and Adro-KO + L-Arg
groups exhibited no significant differences (Fig. 4B, H and I). These results also
indicated that the intensity of TUNEL staining in the lung tissue
obtained from the Adro-KO + L-Arg + Adro(34−76) group
was lower than that in the Adro-KO + L-Arg group (P<0.05,
Fig. 4C and J).
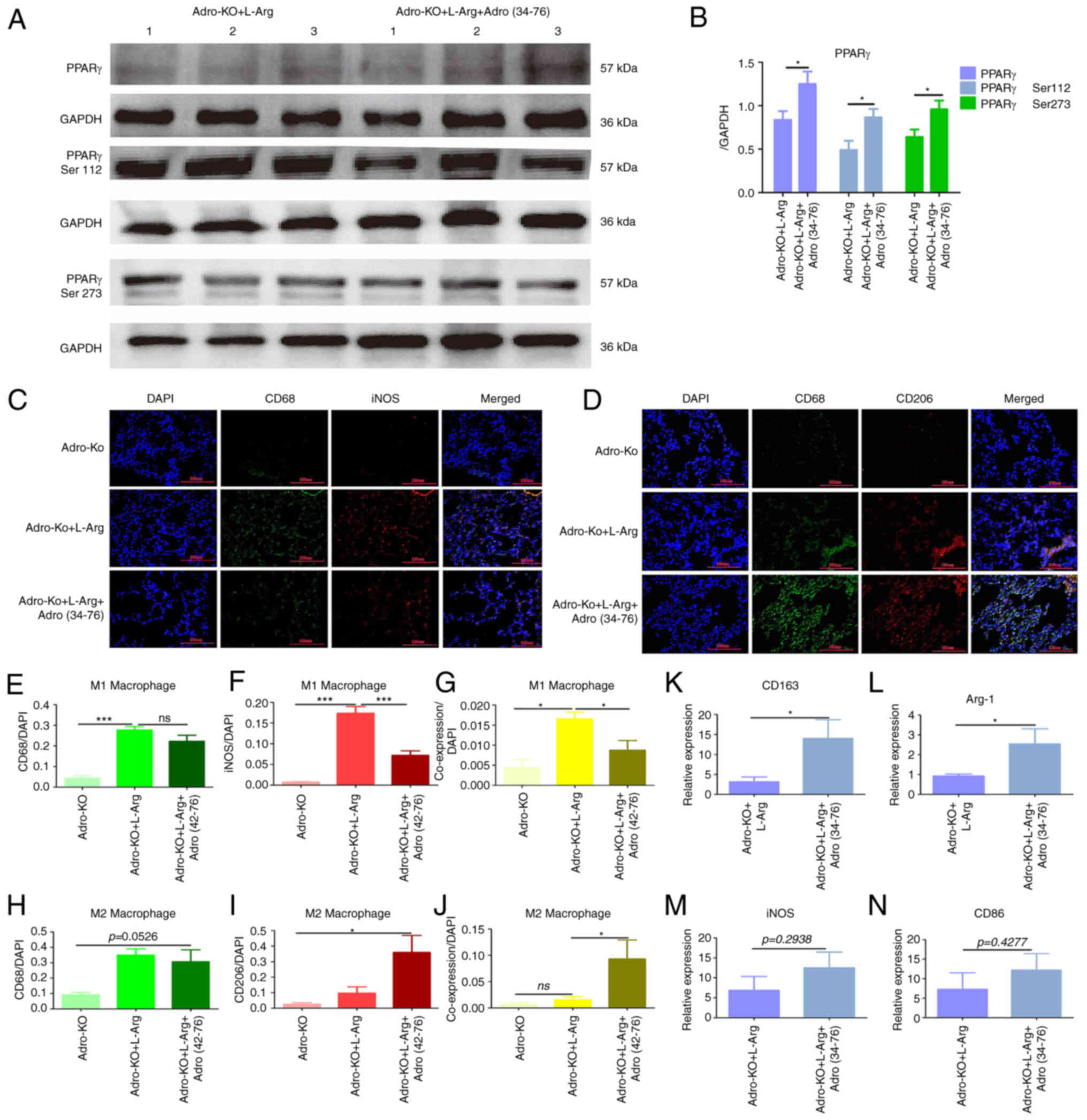
Adropin exogenous supplement induceS M2
macrophage polarization
Rescue experiments were then performed using
exogenous adropin to further explore the effects of exogenous
adropin on PPARγ phosphorylation and macrophage polarization.
Western blot analysis revealed that the protein expression levels
of PPARγ, PPARγ Ser112 and PPARγ Ser273 in the Adro-KO + L-Arg +
Adro(34−76) group were higher than those in the Adro-KO
+ L-Arg group (P<0.05, Fig. 5A and
B). Immunofluorescence revealed that the co-expression of iNOS
and CD68 in the lung tissues from the Adro-KO + L-Arg +
Adro(34−76) group was lower than that in the Adro-KO +
L-Arg group (P<0.05, Fig. 5C and
E-G). Furthermore, the results indicated that the co-expression
of CD206 and CD68 in the lung tissues from the Adro-KO + L-Arg +
Adro(34−76) group was higher than that in the Adro-KO +
L-Arg group (P<0.05, Fig. 5D and
H-J). In addition, RT-qPCR demonstrated that the mRNA
expression of CD163 and Arg-1 in the Adro-KO + L-Arg +
Adro(34−76) group was higher than that in the Adro-KO +
L-Arg group (P<0.05, Fig. 5K and
L). Comparatively, the mRNA expression of iNOS and CD86 in the
Adro-KO + L-Arg + Adro(34−76) group and the Adro-KO + L
-Arg group exhibited no significant difference (P>0.05, Fig. 5M and N).
Discussion
The results of the present study suggested that
adropin may play a crucial role in the progression of AP-ALI. In
contrast to the decreased protein expression of adropin in lung and
serum in AP, the increased mRNA expression of Enho was found
in lung tissue in AP, suggesting that the low expression of adropin
may be related to increased degradation. An animal model of Adro-KO
AP was established, observing that the level of apoptosis in lung
tissue in the Adro-KO group was significantly higher than that in
the NS group. It was also found that the expression of PPAR-γ in
Adro-KO group was downregulated and that M1 macrophage polarization
was increased. Based on these findings, it was hypothesized that
the decreased expression of adropin could exacerbate AP-ALI by
affecting the function of PPAR-γ protein and the polarization of
pulmonary macrophages. To further examine this hypothesis, adropin
rescue experiments were conducted, discovering that adropin
exogenous supplementation resulted in a lower level of lung
apoptosis in the Adro-KO + L-Arg + Adro(34−76) group
compared with the Adro-KO + L-Arg group. At the same time,
adropin(34−76) was evaluated in an adropin-KO model,
demonstrating an increased expression of PPAR-γ, a decrease in M1
macrophage polarization and an increased M2 macrophage
polarization. Rescue experiments also supported the aforementioned
hypothesis. In summary, the results preliminarily demonstrated that
adropin regulated AP-ALI by affecting the protein function of
PPAR-γ and the polarization of lung macrophages.
The results of the present study demonstrated that
adropin expression in lung tissue and serum in AP-ALI models ws
decreased, supporting the likelihood of its involvement in the
progression of AP-ALI. Adropin can regulate renal function and
inflammatory responses, exerting a protective effect on the
progression of systemic inflammation and renal failure (15). Adropin has been shown to inhibit
inflammation by reducing the levels of pro-inflammatory cytokines
and IL-6 in tissues (16).
Additionally, adropin has been found to improve non-alcoholic
steatohepatitis by inhibiting the activation of the NLRP3
inflammasome (17). Plasma
adropin concentrations have been shown to be negatively associated
with plasma biomarkers of systemic inflammation, suggesting that
adropin may play an anti-inflammatory role in obstructive sleep
apnea (18). Combined with the
findings of the present study and those reported above, it was
hypothesized that the decreased expression of adropin in lung and
serum may exacerbate immune disorders and inflammatory damage in
AP-ALI models.
The present study found an increased number of
CD68-positive macrophages in the lungs of mice with AP-ALI. At the
same time, it was found that in the Adro-KO + L-Arg group, the mRNA
expression levels of CD206 and Arg-1 were decreased, while the mRNA
expression levels of iNOS and CD86 were increased. Moreover, the
results of immunofluorescence staining demonstrated that the
expression of CD206 in the Adro-KO + L-Arg group decreased, while
the expression of iNOS increased, suggesting that the absence of
adropin was associated with macrophage M1 polarization. The
expression of PPARγ was downregulated, and the phosphorylation of
Ser112 and Ser273 was increased. The results of the present study
are consistent with those reported previously in the literature.
For example, adropin has been shown to regulate macrophage
polarization by regulating the expression of PPAR-γ, a gene related
to fatty acid metabolism (6).
Adropin has also been found to transfer the phenotype to the
anti-inflammatory M2 rather than pro-inflammatory M1 during
monocyte differentiation into macrophages through the upregulation
of PPAR-γ (19). The long-term
administration of adropin in Apoe−/− mice has been shown
to reduce the development of aortic atherosclerotic lesions,
mononuclear/macrophage infiltration and smooth muscle cell content
in plaque (20). The upregulation
of PPAR-γ expression and induced PPAR-γ dephosphorylation at the
Ser112 site plays an anti-inflammatory role (21). The inhibition of the
phosphorylation of PPAR-γ at the Ser112 site in macrophages
activates PPAR-γ, inducing the expression of ATP-binding box
transporter A1 and producing anti-atherosclerotic effects (22). It was hypothesized that Adro-KO
would lead to an increased phosphorylation of PPARγ at Ser112 and
Ser273 sites, and inhibit PPARγ function, resulting in an increased
M1 type of macrophages in lung tissue.
The results of the present study revealed that the
proportion of M1 macrophages increased and lung tissue damage
increased in the Adro-KO + L-Arg model. Moreover, it was found that
exogenous adropin improved M2 macrophage polarization and reduced
lung inflammatory damage through rescue experiments. The results of
pathological molecular experiments revealed that the intensity of
TGF-β and Masson's staining in the Adro-KO + L-Arg group was higher
than that in the WT + L-Arg group. The results of western blot
analysis demonstrated that the expression of cleaved caspse-3 and
cleaed PARP1 was decreased in the Adro-KO + L-Arg +
Adro(34−76) group, indicating that adropin exerted an
anti-apoptotic effect on AP-ALI. It has been found that alveolar
macrophage death plays a crucial role in the progression of
pneumonia by affecting other immune cell populations in the lungs
(23). A recent study
demonstrated that macrophages were key regulatory factors in the
pathogenesis of acute lung injury/acute respiratory distress
syndrome. As such, regulating macrophage polarization may improve
the prognosis of acute lung injury (24). Macrophages are involved in the
development and progression of acute lung injury through the
secretion of inflammatory cytokines/chemokines and the activation
of transcription factors in the pathogenesis of inflammatory lung
diseases (25). The activation of
PPAR-γ in alveolar macrophages demonstrates a protective effect
against LPS-induced acute lung injury in mice (26). Macrophages play a critical role in
the regulation of lung inflammatory lung injury. Combined with our
result and the literature above (6,26).
Adropin may reduce inflammatory injury by regulating the
polarization of lung macrophages, identifying a potential
therapeutic target for lung injury in acute pancreatitis.
One limitation of the present study is the lack of
high-throughput protein sequencing to construct a relatively
complete adropin-related signaling pathway. The authors hope to
perfrom such an investigation in the future. The present study may
have also benefited from flow cytometry for the serum
neutralization of lung macrophages. Finally, the dose selection in
the rescue experiment was based on reference literature instead of
a concentration gradient. Due to the lack of an
adropin(34−76) gradient, it could not be ruled out
whether some results were attributable to an insufficient dose or
functional limitations of adropin(34−76). In the future,
further studies are required to explore the immune regulatory
effects of adropin on acute pancreatitis. The key questions
remaining to be answered include: i) Whether adropin regulates
macrophage polarization and the molecular mechanisms in AP-ALI; ii)
the regulatory effects of adropin expression in the occurrence and
development of AP-ALI.
In conclusion, the present study demonstrates that
the decreased expression of adropin in lung tissue and serum may
aggravate immune disorders and inflammatory injury in AP. The
knockout of adropin resulted in the increased expression of PPARγ
at Ser112 and Ser273, inhibiting PPARγ function and resulting in
increased M1 macrophage in lung tissue. Exogenous adropin
alleviated inflammatory damage by regulating the polarization of
lung macrophages. This finding may be leveraged to improve
AP-associated pneumonia and may markedly improve the prognosis of
patients with AP.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and SW planned the study. FD and FG conceived and
designed the study. FD and GL performed the collection of the
samples. FD and ZZ performed the immunohistochemistry experiment.
GL and YH performed the analyses of expression levels. FD and GL
confirm the authenticity of all the raw data. All the authors
carefully reviewed the manuscript and all authors have read and
approved the final version.
Ethics approval and consent to
participate
All patients provided informed consent for excess
specimens to be used for research purposes and all protocols
employed in the study were approved by the Medical Ethics Committee
of The First Affiliated Hospital of Fujian Medical University
[MTCA, ECFAH of FMU(2015) 084-1]. All animal experiments complied
with the National Institutes of Health guidelines for the care and
used of laboratory animals, and the protocol was approved by the
Ethics Committee of Fujian Medical University (IACUC FJMU
2022-0428).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by grants from the Sailing
Foundation of Fujian Medical University (no. 2022QH2033) and the
Natural Science Foundation of Fujian Province (no. 2020J01961).
References
|
1
|
Petrov MS and Yadav D: Global epidemiology
and holistic prevention of pancreatitis. Nat Rev Gastroenterol
Hepatol. 16:175–184. 2019. View Article : Google Scholar :
|
|
2
|
Liu W, Du JJ, Li ZH, Zhang XY and Zuo HD:
Liver injury associated with acute pancreatitis: The current status
of clinical evaluation and involved mechanisms. World J Clin Cases.
9:10418–10429. 2021. View Article : Google Scholar
|
|
3
|
Cao C, Yin C, Shou S, Wang J, Yu L, Li X
and Chai Y: Ulinastatin protects against LPS-induced acute lung
injury by attenuating TLR4/NF-κB pathway activation and reducing
inflammatory mediators. Shock. 50:595–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu D, Wen L, Wang Z, Hai Y, Yang D, Zhang
Y, Bai M, Song B and Wang Y: The Mechanism of lung and intestinal
injury in acute pancreatitis: A review. Front Med (Lausanne).
9:9040782022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huai JP, Sun XC, Chen MJ, Jin Y, Ye XH, Wu
JS and Huang ZM: Melatonin attenuates acute pancreatitis-associated
lung injury in rats by modulating interleukin 22. World J
Gastroenterol. 18:5122–5128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Chen Q, Lin X, Chen M and Liu Q:
A review of adropin as the medium of dialogue between energy
regulation and immune regulation. Oxid Med Cell Longev.
2020:39478062020.PubMed/NCBI
|
|
7
|
Algul S and Ozcelik O: Evaluating the
energy regulatory hormones of nesfatin-1, irisin, adropin and
preptin in multiple sclerosis. Mult Scler Relat Disord.
68:1042212022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dodd WS, Patel D, Lucke-Wold B, Hosaka K,
Chalouhi N and Hoh BL: Adropin decreases endothelial monolayer
permeability after cell-free hemoglobin exposure and reduces
MCP-1-induced macrophage transmigration. Biochem Biophys Res
Commun. 582:105–110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Zeng K, Liu QC, Guo Z, Zhang S,
Chen XR, Lin JH, Wen JP, Zhao CF, Lin XH and Gao F: Adropin
deficiency worsens HFD-induced metabolic defects. Cell Death Dis.
8:e30082017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao F, Fang J, Chen F, Wang C, Chen S,
Zhang S, Lv X, Zhang J, He Q, Weng S, et al: Enho mutations causing
low adropin: A possible pathomechanism of MPO-ANCA associated lung
injury. EBioMedicine. 9:324–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen J, Wan R, Hu G, Wang F, Shen J and
Wang X: Involvement of thrombopoietin in acinar cell necrosis in
L-arginine-induced acute pancreatitis in mice. Cytokine.
60:294–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Ghoshal S, Zhang L, Stevens JR,
McCommis KS, Finck BN, Lopaschuk GD and Butler AA: The peptide
hormone adropin regulates signal transduction pathways controlling
hepatic glucose metabolism in a mouse model of diet-induced
obesity. J Biol Chem. 294:13366–13377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao S, McMillan RP, Zhu Q, Lopaschuk GD,
Hulver MW and Butler AA: Therapeutic effects of adropin on glucose
tolerance and substrate utilization in diet-induced obese mice with
insulin resistance. Mol Metab. 4:310–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mikawa K, Nishina K, Takao Y and Obara H:
ONO-1714, a nitric oxide synthase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
97:1751–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Memi G and Yazgan B: Adropin and spexin
hormones regulate the systemic inflammation in adenine-induced
chronic kidney failure in rat. Chin J Physiol. 64:194–201. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali II, D'Souza C, Singh J and Adeghate E:
Adropin's role in energy homeostasis and metabolic disorders. Int J
Mol Sci. 23:83182022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Liu L, Wei Y, Fang C, Liu S, Zhou
F, Li Y, Zhao G, Guo Z, Luo Y and Li L: Exercise suppresses NLRP3
inflammasome activation in mice with diet-induced NASH: A plausible
role of adropin. Lab Invest. 101:369–380. 2021. View Article : Google Scholar
|
|
18
|
Bozic J, Borovac JA, Galic T, Kurir TT,
Supe-Domic D and Dogas Z: Adropin and inflammation biomarker levels
in male patients with obstructive sleep apnea: A link with glucose
metabolism and sleep parameters. J Clin Sleep Med. 14:1109–1118.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato K, Yamashita T, Shirai R, Shibata K,
Okano T, Yamaguchi M, Mori Y, Hirano T and Watanabe T: Adropin
contributes to anti-atherosclerosis by suppressing
monocyte-endothelial cell adhesion and smooth muscle cell
proliferation. Int J Mol Sci. 19:12932018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L and Xie W: LncRNA HDAC11-AS1
suppresses atherosclerosis by inhibiting HDAC11-mediated adropin
histone deacetylation. J Cardiovasc Transl Res. 15:1256–1269. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu T, Gao M, Yang P, Liu D, Wang D, Song
F, Zhang X and Liu Y: Insulin promotes macrophage phenotype
transition through PI3K/Akt and PPAR-γ signaling during diabetic
wound healing. J Cell Physiol. 234:4217–4231. 2019. View Article : Google Scholar
|
|
22
|
Ishii N, Matsumura T, Kinoshita H, Fukuda
K, Motoshima H, Senokuchi T, Nakao S, Tsutsumi A, Kim-Mitsuyama S,
Kawada T, et al: Nifedipine induces peroxisome
proliferator-activated receptor-gamma activation in macrophages and
suppresses the progression of atherosclerosis in apolipoprotein
E-deficient mice. Arterioscler Thromb Vasc Biol. 30:1598–1605.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan EKY and Fan J: Regulation of alveolar
macrophage death in acute lung inflammation. Respir Res. 19:502018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Tang J, Shuai W, Meng J, Feng J
and Han Z: Macrophage polarization and its role in the pathogenesis
of acute lung injury/acute respiratory distress syndrome. Inflamm
Res. 69:883–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JW, Chun W, Lee HJ, Min JH, Kim SM,
Seo JY, Ahn KS and Oh SR: The role of macrophages in the
development of acute and chronic inflammatory lung diseases. Cells.
10:8972021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Y, Sun Z and Guo X: Citral inhibits
lipopolysaccharide-induced acute lung injury by activating PPAR-γ.
Eur J Pharmacol. 747:45–51. 2015. View Article : Google Scholar
|