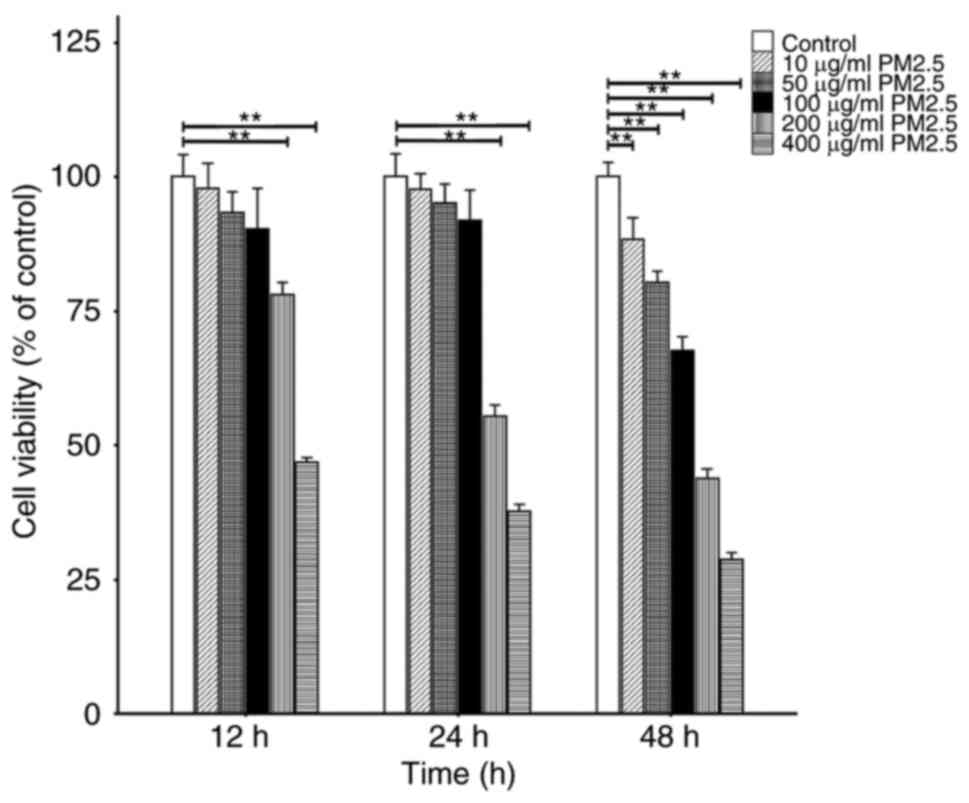
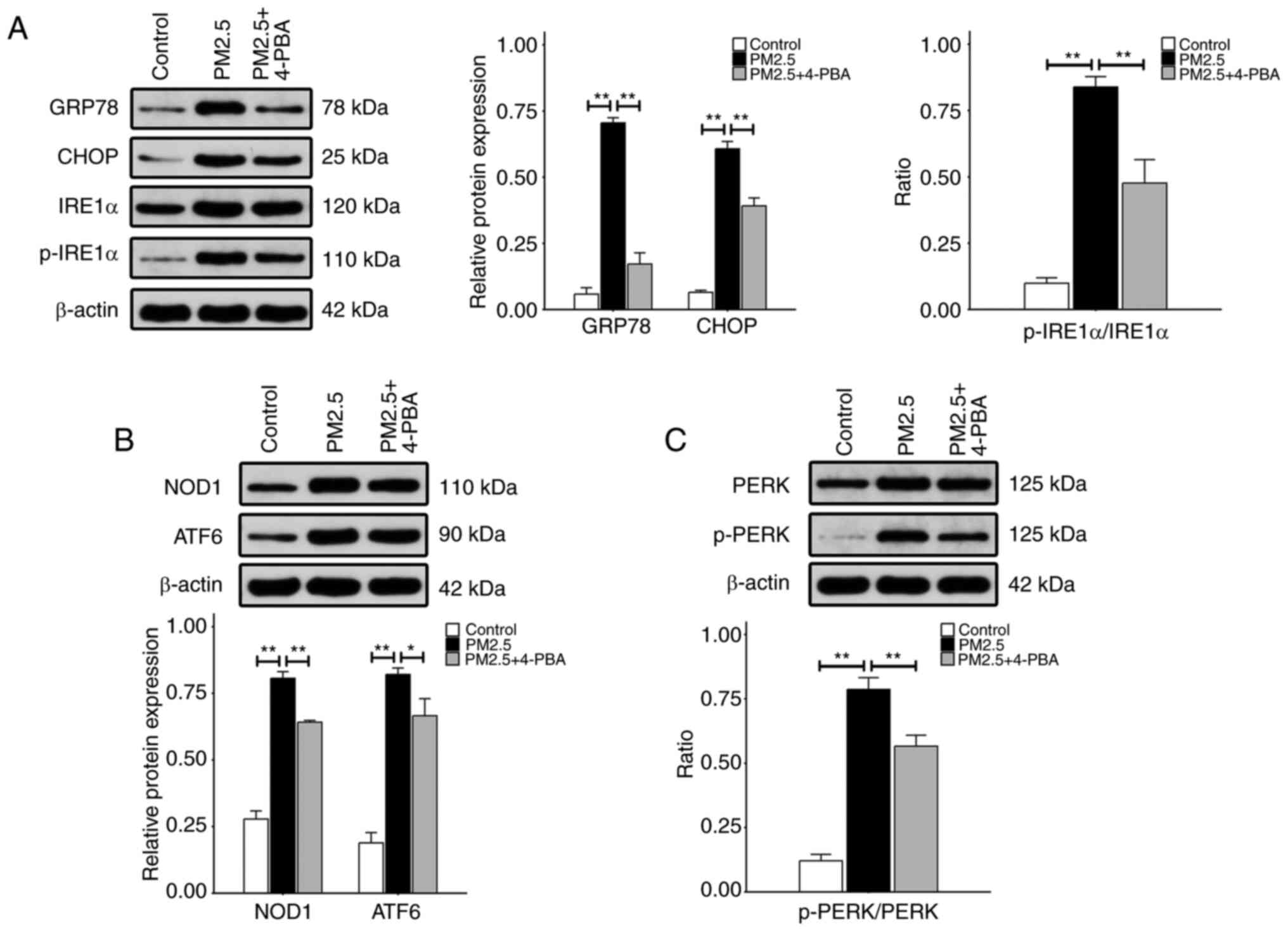
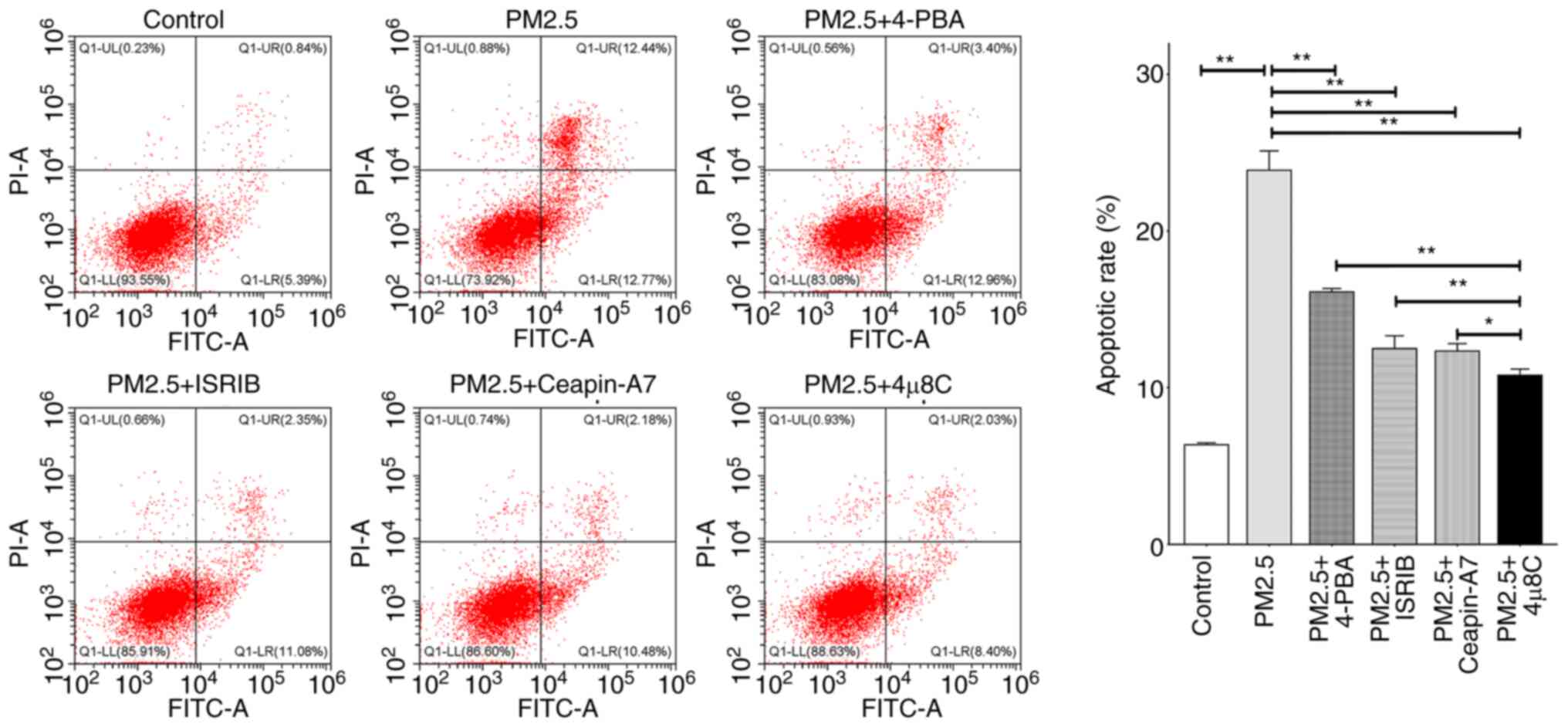
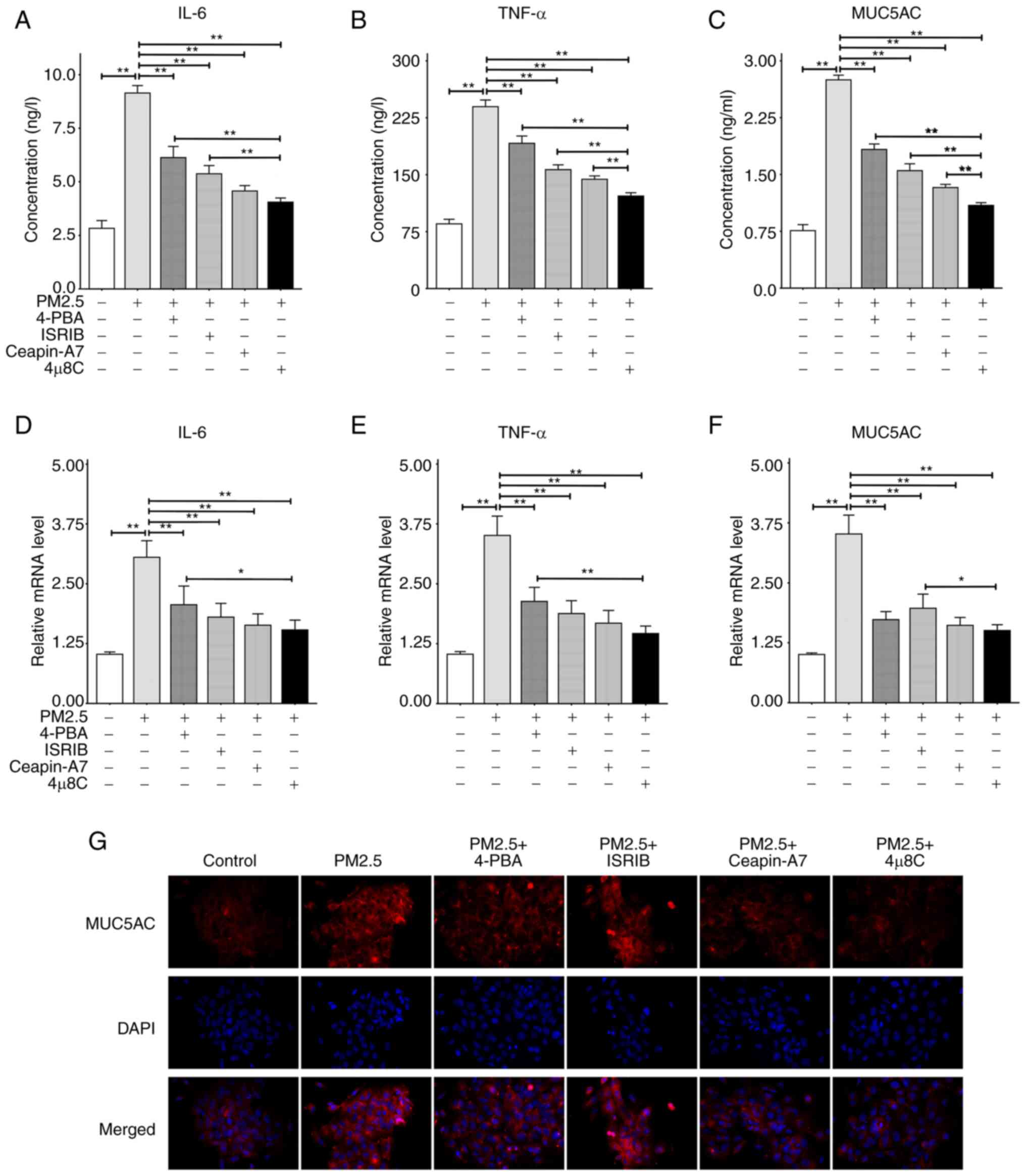
|
1
|
Gleason JA, Bielory L and Fagliano JA:
Associations between ozone, PM2.5, and four pollen types on
emergency department pediatric asthma events during the warm season
in New Jersey: A case-crossover study. Environ Res. 132:421–429.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montoya-Estrada A, Torres-Ramos YD,
Flores-Pliego A, Ramirez-Venegas A, Ceballos-Reyes GM,
Guzman-Grenfell AM and Hicks JJ: Urban PM2.5 activates GAPDH and
induces RBC damage in COPD patients. Front Biosci (Schol Ed).
5:638–649. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davel AP, Lemos M, Pastro LM, Pedro SC, de
André PA, Hebeda C, Farsky SH, Saldiva PH and Rossoni LV:
Endothelial dysfunction in the pulmonary artery induced by
concentrated fine particulate matter exposure is associated with
local but not systemic inflammation. Toxicology. 295:39–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller MR, Shaw CA and Langrish JP: From
particles to patients: Oxidative stress and the cardiovascular
effects of air pollution. Future Cardiol. 8:577–602. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown DM, Donaldson K, Borm PJ, Schins RP,
Dehnhardt M, Gilmour P, Jimenez LA and Stone V: Calcium and
ROS-mediated activation of transcription factors and TNF-alpha
cytokine gene expression in macrophages exposed to ultrafine
particles. Am J Physiol Lung Cell Mol Physiol. 286:L344–L353. 2004.
View Article : Google Scholar
|
|
6
|
Liu K, Hua S and Song L: PM2.5 exposure
and asthma development: The key role of oxidative stress. Oxid Med
Cell Longev. 2022:36188062022.PubMed/NCBI
|
|
7
|
Kim MH, Bae CH, Choi YS, Na HG, Song SY
and Kim YD: Endoplasmic reticulum stress induces MUC5AC and MUC5B
expression in human nasal airway epithelial cells. Clin Exp
Otorhinolaryngol. 12:181–189. 2019. View Article : Google Scholar :
|
|
8
|
Park SH, Gong JH, Choi YJ, Kang MK, Kim YH
and Kang YH: Kaempferol inhibits endoplasmic reticulum
stress-associated mucus hypersecretion in airway epithelial cells
and ovalbumin-sensitized mice. PLos One. 10:e1435262015. View Article : Google Scholar
|
|
9
|
Martino MB, Jones L, Brighton B, Ehre C,
Abdulah L, Davis CW, Ron D, O'Neal WK and Ribeiro CMP: The ER
stress transducer IRE1β is required for airway epithelial mucin
production. Mucosal Immunol. 6:639–654. 2013. View Article : Google Scholar
|
|
10
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Bio. 13:89–102. 2012. View
Article : Google Scholar
|
|
12
|
Keestra-Gounder AM, Byndloss MX, Seyffert
N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham
OH, Tiffany CR, et al: NOD1 and NOD2 signalling links ER stress
with inflammation. Nature. 532:394–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Li Q, Li L, Zeng M, Zhou X and Cheng
Z: Endoplasmic reticulum stress/XBP1 promotes airway mucin
secretion under the influence of neutrophil elastase. Int J Mol
Med. 47:812021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai Y, Wang Y, Lu S, Deng X, Niu X, Guo Z,
Qian R, Zhou M and Peng X: Autophagy attenuates particulate matter
2.5-induced damage in HaCaT cells. Ann Transl Med. 9:9782021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iordache C and Duszyk M: Sodium
4-phenylbutyrate upregulates ENaC and sodium absorption in T84
cells. Exp Cell Res. 313:305–311. 2007. View Article : Google Scholar
|
|
16
|
Yan C, Zhang L, Lu B, Lyu D, Chen H, Song
F, Wang X, Chen Z, Fu Q and Yao K: Trans, trans-2,4-decadienal
(tt-DDE), a composition of cooking oil fumes, induces oxidative
stress and endoplasmic reticulum stress in human corneal epithelial
cells. Toxicol In Vitro. 68:1049332020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kubra K, Akhter MS, Saini Y, Kousoulas KG
and Barabutis N: Activating transcription factor 6 protects against
endothelial barrier dysfunction. Cell Signal. 99:1104322022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinto AR, Américo MF, Terenzi H and
Silveira DB: Inhibiting IRE-1 RNase signaling decreases HIV-1
Tat-induced inflammatory M1 state in microglial cells. Biochim
Biophys Acta Gen Subj. 1866:1302192022. View Article : Google Scholar
|
|
19
|
Porcherie A, Cunha P, Trotereau A, Roussel
P, Gilbert FB, Rainard P and Germon P: Repertoire of Escherichia
coli agonists sensed by innate immunity receptors of the bovine
udder and mammary epithelial cells. Vet Res. 43:142012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scherbakov AM, Vorontsova SK, Khamidullina
AI, Mrdjanovic J, Andreeva OE, Bogdanov FB, Salnikova DI, Jurisic
V, Zavarzin IV and Shirinian VZ: Novel pentacyclic derivatives and
benzylidenes of the progesterone series cause anti-estrogenic and
antiproliferative effects and induce apoptosis in breast cancer
cells. Invest New Drug. 41:142–152. 2023. View Article : Google Scholar
|
|
21
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomic. 17:663–674. 2020.
View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Bio. 21:421–438. 2020. View Article : Google Scholar
|
|
25
|
Cui X, Zhang Y, Lu Y and Xiang M: ROS and
endoplasmic reticulum stress in pulmonary disease. Front Pharmacol.
13:8792042022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Yao N, Fu X, Wei L, Ding M, Pang
Y, Liu D, Ren Y and Guo M: Butylphthalide ameliorates airway
inflammation and mucus hypersecretion via NF-κB in a murine asthma
model. Int Immunopharmacol. 76:1058732019. View Article : Google Scholar
|
|
27
|
Ma B, Athari SS, Nasab EM and Zhao L:
PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors attenuate
pathological mechanisms of allergic asthma. Inflammation.
44:1895–1907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008. View Article : Google Scholar
|
|
30
|
Shi Y, Ji Y, Sun H, Hui F, Hu J, Wu Y,
Fang J, Lin H, Wang J, Duan H and Lanza M: Nanoscale
characterization of PM2.5 airborne pollutants reveals high
adhesiveness and aggregation capability of soot particles. Sci Rep.
5:112322015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marshall J: PM 2.5. Proc Natl Acad Sci
USA. 110:87562013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao SS, Luo KL and Shi L: Endoplasmic
reticulum stress interacts with inflammation in human diseases. J
Cell Physiol. 231:288–294. 2016. View Article : Google Scholar
|
|
33
|
Hasnain SZ, Lourie R, Das I, Chen AC and
McGuckin MA: The interplay between endoplasmic reticulum stress and
inflammation. Immunol Cell Biol. 90:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei J, Rahman S, Ayaub EA, Dickhout JG and
Ask K: Protein misfolding and endoplasmic reticulum stress in
chronic lung disease. Chest. 143:1098–1105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molagoda IMN, Kavinda MHD, Choi YH, Lee H,
Kang CH, Lee MH, Lee CM and Kim GY: Fisetin protects HaCaT human
keratinocytes from fine particulate matter (PM2.5)-induced
oxidative stress and apoptosis by inhibiting the endoplasmic
reticulum stress response. Antioxidants (Basel). 10:14922021.
View Article : Google Scholar
|
|
37
|
Kim SR, Kim HJ, Kim DI, Lee KB, Park HJ,
Jeong JS, Cho SH and Lee YC: Blockade of interplay between IL-17A
and endoplasmic reticulum stress attenuates LPS-induced lung
injury. Theranostics. 5:1343–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroeder BW, Verhaeghe C, Park S,
Nguyenvu LT, Huang X, Zhen G and Erle DJ: AGR2 is induced in asthma
and promotes allergen-induced mucin overproduction. Am J Respir
Cell Mol Biol. 47:178–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Yang X, Li Y, Wang X, Zhang Y, Dai
X, Niu B, Wu J, Yuan X, Xiong A, et al: Lyn kinase represses mucus
hypersecretion by regulating IL-13-induced endoplasmic reticulum
stress in asthma. EBioMedicine. 15:137–149. 2017. View Article : Google Scholar :
|
|
40
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar
|
|
41
|
Mijošek V, Lasitschka F, Warth A, Zabeck
H, Dalpke AH and Weitnauer M: Endoplasmic reticulum stress is a
danger signal promoting innate inflammatory responses in bronchial
epithelial cells. J Innate Immun. 8:464–478. 2016. View Article : Google Scholar
|
|
42
|
Delmotte P and Sieck GC: Interaction
between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress),
mitochondrial signaling and Ca(2+) regulation in airway smooth
muscle (ASM). Can J Physiol Pharmacol. 93:97–110. 2015. View Article : Google Scholar
|
|
43
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling - from basic mechanisms to clinical applications. FEBS
J. 286:241–278. 2019. View Article : Google Scholar
|
|
44
|
Laing S, Wang G, Briazova T, Zhang C, Wang
A, Zheng Z, Gow A, Chen AF, Rajagopalan S, Chen LC, et al: Airborne
particulate matter selectively activates endoplasmic reticulum
stress response in the lung and liver tissues. Am J Physiol Cell
Ph. 299:C736–C749. 2010. View Article : Google Scholar
|
|
45
|
Urano F, Bertolotti A and Ron D: IRE1 and
efferent signaling from the endoplasmic reticulum. J Cell Sci.
21:3697–3702. 2000. View Article : Google Scholar
|
|
46
|
Duan Q, Zhou Y and Yang D: Endoplasmic
reticulum stress in airway hyperresponsiveness. Biomed
Pharmacother. 149:1129042022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duvigneau JC, Luís A, Gorman AM, Samali A,
Kaltenecker D, Moriggl R and Kozlov AV: Crosstalk between
inflammatory mediators and endoplasmic reticulum stress in liver
diseases. Cytokine. 124:1545772019. View Article : Google Scholar
|
|
48
|
Lei Y, Wang S, Ren B, Wang J, Chen J, Lu
J, Zhan S, Fu Y, Huang L and Tan J: CHOP favors endoplasmic
reticulum stress-induced apoptosis in hepatocellular carcinoma
cells via inhibition of autophagy. PLoS One. 12:e1836802017.
View Article : Google Scholar
|
|
49
|
Kropski JA and Blackwell TS: Endoplasmic
reticulum stress in the pathogenesis of fibrotic disease. J Clin
Invest. 128:64–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Siddesha JM, Nakada EM, Mihavics BR,
Hoffman SM, Rattu GK, Chamberlain N, Cahoon JM, Lahue KG, Daphtary
N, Aliyeva M, et al: Effect of a chemical chaperone,
tauroursodeoxycholic acid, on HDM-induced allergic airway disease.
Am J Physiol Lung Cell Mol Physiol. 310:L1243–L1259. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aghapour M, Ubags ND, Bruder D, Hiemstra
PS, Sidhaye V, Rezaee F and Heijink IH: Role of air pollutants in
airway epithelial barrier dysfunction in asthma and COPD. Eur
Respir Rev. 31:2101122022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gon Y and Hashimoto S: Role of airway
epithelial barrier dysfunction in pathogenesis of asthma. Allergol
Int. 67:12–17. 2018. View Article : Google Scholar
|
|
53
|
Bradley KL, Stokes CA, Marciniak SJ,
Parker LC and Condliffe AM: Role of unfolded proteins in lung
disease. Thorax. 76:92–99. 2021. View Article : Google Scholar
|
|
54
|
Byndloss MX, Keestra-Gounder AM, Bäumler
AJ and Tsolis RM: NOD1 and NOD2: New functions linking endoplasmic
reticulum stress and inflammation. DNA Cell Biol. 35:311–313. 2016.
View Article : Google Scholar : PubMed/NCBI
|