Introduction
Osteoarthritis (OA) is a chronic degenerative
arthropathy hallmarked by cartilage degeneration, inflammatory
response, space narrowing and osteophyte formation (1). OA progression can lead to articular
synovitis, meniscus rupture, varus deformity and even disability
(2). The increased incidence of
OA poses an enormous burden on global health and economic
development. An imbalance between articular cartilage metabolic
effects is the main cause of progressive OA. Moreover, inflammation
is a key risk regulator of OA development. Artificial joint
replacement is almost the sole intervention therapy for
advanced-stage OA (3). Although
non-steroidal anti-inflammatory drugs are clinically prescribed to
relieve OA, they have limited efficacy and cause an increased
clinical risk of gastrointestinal peptic ulcers and cardiovascular
diseases (4-8). Therefore, the development of safe
and effective clinical drugs to ameliorate osteoarthritis is
urgently required.
Studies have demonstrated the presence of metabolic
disorders in patients with osteoarthritis, such as the low
expression of collagen type II (COL II) and aggrecan, and the high
expression of matrix metalloproteinases (MMPs) (9,10).
In addition, previous studies have indicated that the progression
of OA is accompanied by hollow cartilage lacunae and
hypocellularity, indicating the occurrence of chondrocyte
senescence and death, demonstrating their roles in the development
of OA (11,12). As OA progresses, cell death is
complex as it involves apoptosis, senescence, oxidative stress,
chondroptosis, necrosis and autophagy (11,12). Accordingly, autophagy activation,
antioxidation, antisenescence and decreased apoptosis serve as
therapeutic strategies against the progression of OA.
Previous studies have confirmed the crucial role of
the activation of the nuclear factor-κB (NF-κB) pathway in the
interleukin (IL)-1β-induced inflammatory response and OA
progression (4,13,14). An IL-1β stimulus induces the
phosphorylation of Akt, which further phosphorylates IκBα,
displaying the nuclear localization signal on the NF-κB complex.
Consequently, the stimulation and translocation of the NF-κB p65
subunit (p65) into the nucleus triggers downstream gene
transcription. Therefore, Akt phosphorylation triggers the
downstream phosphorylation of p65 and IκBα. NF-κB is a vital
signalling substance for the phosphoinositide-3-kinase (PI3K)/Akt
pathway (4,15,16), which comprises several
serine/threonine protein kinases. Thus, the classic PI3K/Akt/NF-κB
pathway contributes to the progression of OA and is considered a
primary target for the treatment of OA.
Mitogen-activated protein kinases (MAPKs) are
serine/threonine kinases, such as p38, extracellular
signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK).
It has been demonstrated that the MAPK signalling pathway, which
transmits inflammatory cellular signals to facilitate inflammation,
markedly contributes to articular cartilage degradation (17). Moreover, MAPK phosphorylation is
linked to the expression level of MMPs, which cause the
deterioration of aggrecan and COL II. Furthermore, the inhibition
of MAPKs downregulates the IL-1β-induced activity of cyclooxygenase
(COX)2 and prostaglandin E2 in chondrocytes (17).
Rutaecarpine (RUT), a novel drug of the class 'COX2
inhibitors' and an indolopyridoquinazoline alkaloid isolated from
Evodia rutaecarpa (18),
has been shown to exert inhibitory effects against inflammation.
RUT has been shown to inhibit Kelch-like ECH-associated protein
1-nuclear factor erythroid 2-related factor 2 (Nrf2) binding to
trigger Nrf2 and attenuate dextran sulfate sodium-induced colitis
(19). Furthermore, RUT has been
found to inhibit prostaglandin synthesis in macrophages (20) and mitigate inflammation in RAW
264.7 macrophages by reducing PI3K/Akt/NF-κB and MAPK signal
transduction (21). The potential
value of RUT in the synovitis of rheumatoid arthritis has also been
reported (22). Moreover, it has
been shown to effectively inhibit the apoptosis and production of
inflammatory cytokines in neuronal injury by regulating the signal
transduction of the Nrf2/heme oxygenase-1 and ERK1/2 pathways
(23). In addition, a previous
study demonstrated that RUT effectively inhibited
osteoclastogenesis by impairing macrophage colony stimulating
factor and receptor activator of nuclear factor κ-B
ligand-stimulated signalling pathways (24). Thus, RUT has exhibited various
pharmacological effects, such as anti-inflammatory, anti-apoptotic
and antioxidant effects. However, whether RUT can alleviate murine
osteoarthritis remains unexplored. Hence, the present study aimed
to examine the curative effects of RUT on IL-1β-activated murine
chondrocytes.
Materials and methods
Chemicals, reagents and antibodies
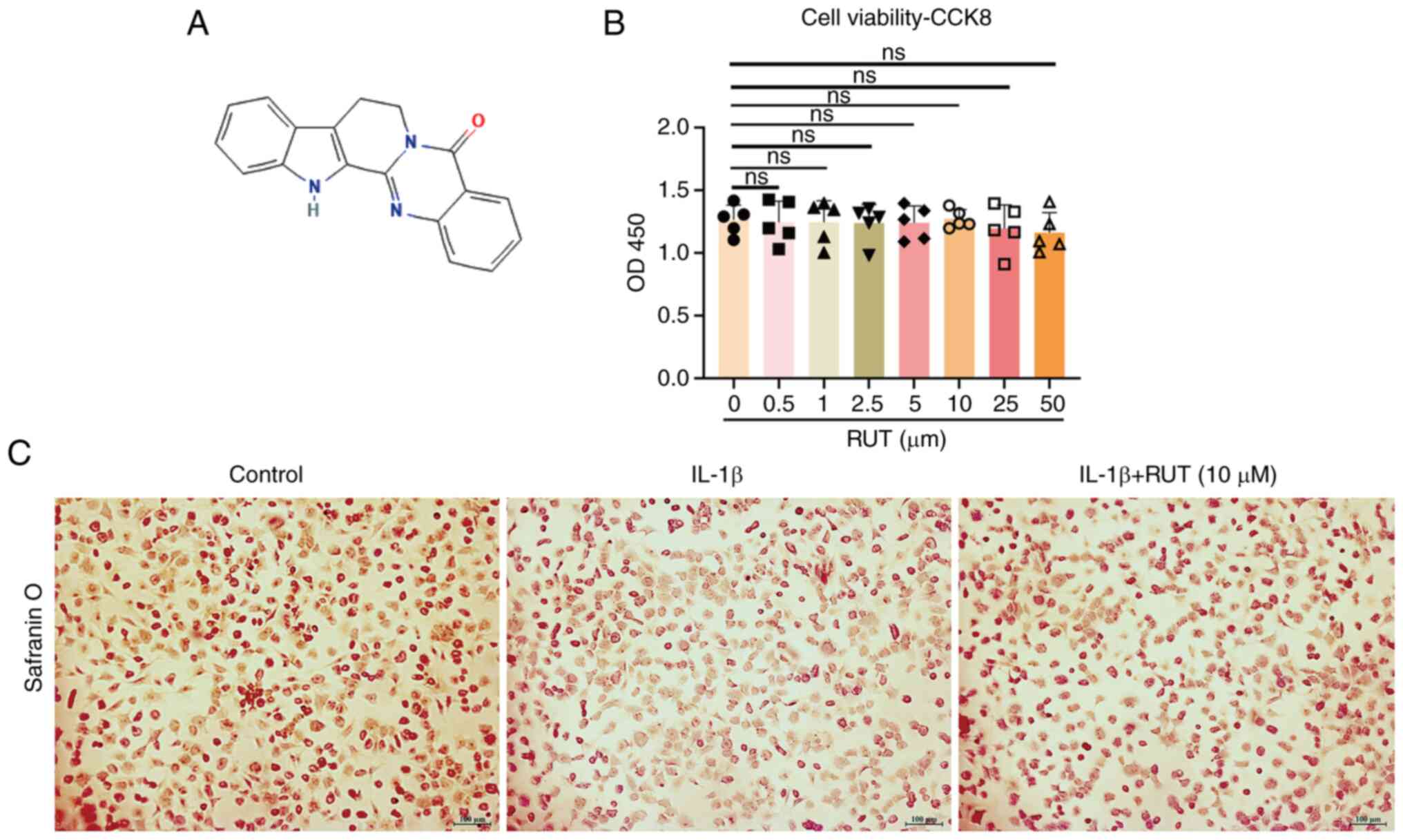
RUT (CAS 84-26-4) was purchased from Shandong
Topscience Biotech Co., Ltd. and its molecular structure is
illustrated in Fig. 1A.
Recombinant mouse IL-1β cytokine was obtained from R&D Systems,
Inc. (401-ML-010) and Safranin O solution was obtained from Beijing
Solarbio Science & Technology Co., Ltd. (cat. no. G1067).
Corresponding primary antibodies against COL II (cat. no.
28459-1-AP), SOX9 (cat. no. 67439-1-Ig), aggrecan (cat. no.
13880-1-AP) and MMP13 (cat. no. 18165-1-AP) were acquired from
Proteintech Group, Inc. and used at a 1:1,000 dilution. GAPDH
monoclonal antibody (cat. no. 60004-1-Ig, Proteintech Group, Inc.)
was used at a 1:50,000 dilution. Wuhan Boster Biological
Technology, Ltd. provided the primary antibodies against MMP3 (cat.
no. BM4074, 1:1,000), trypsin, as well as the reagents, collagenase
type II and phosphate-buffered saline (PBS), HRP-AffiniPure goat
anti-rabbit IgG (1:10,000; cat. no. BM3894), HRP-AffiniPure goat
anti-mouse IgG (1:10,000; cat. no. BM3895) and CY3-conjugated
AffiniPure goat anti-rabbit IgG (1:200; cat. no. BA1032) for
western blot analysis and immunofluorescence analyses. Cell
Signalling Technology, Inc. provided the corresponding primary
antibodies against COX2 (cat. no. 12282), BAX (cat. no. 14796),
BCL2 (cat. no. 3498), autophagy-related 5 (ATG5, cat. no. 12994)
and microtubule-associated protein light chain 3 (LC3I/II, cat. no.
12741), and constituents of the MAPK and PI3K/Akt/NF-κB pathways:
p38 (cat. no. 8690), phosphorylated (p-)p38 (cat. no. 4511), ERK1/2
(cat. no. 4695), p-ERK (#4370), JNK (cat. no. 9258), p-JNK (cat.
no. 9255), PI3K (cat. no. 4257), p-PI3K (cat. no. 4228), AKT (cat.
no. 4691), p-AKT (cat. no. 4060), p65 (cat. no. 8242) and p-p65
(cat. no. 3033); all these antibodies were used at a 1:1,000
dilution. A primary antibody against p21 (cat. no. ab188224,
1:1,000) was acquired from Abcam. p16 (INK4A) antibody (cat. no.
A0262, 1:750) was purchased from ABclonal Biotech Co., Ltd. Rabbit
antibodies against TNF-α (cat. no. 48136) and IL-6 (cat. no. 32064)
were acquired from Signalway Antibody and used at a 1:1,000
dilution. The Annexin V-FITC/PI Apoptosis Detection kit was
acquired from Vazyme Biotech Co., Ltd. The senescence
β-galactosidase staining kit was purchased from the Beyotime
Institute of Biotechnology. LC3 autophagy double-labelled
adenovirus (cat. no. HBAD-1007) was purchased from HanBio Inc. In
addition, Gibco; Thermo Fisher Scientific, Inc. provided foetal
bovine serum (FBS) and Dulbecco's modified Eagle's medium F12
(DMEM/F12).
Chondrocyte extraction and culture
As previously described, chondrocytes were obtained
from the knee joints of sacrificed C57BL/6J mice (5 days old) by
sequential enzymatic digestion (25). The total knee cartilage was
extracted and placed in PBS. The synovium and other attachments
were stripped thoroughly from the cartilage surface. Subsequently,
the separated cartilage was crumbled into miniature patches and
immersed in 0.25% trypsin for digestion in a cell incubator for 30
min 37°C. The sediment was transferred and regurgitated with 0.2%
collagenase II for 6 h in a hybridization oven at 37°C after being
centrifuged at 362 × g for 5 min 37°C. The chondrocyte sediment was
then isolated from suspension by centrifugation at 362 × g for 5
min 37°C and cultured in a medium including 10% FBS, 1%
penicillin/streptomycin and DMEM/F12 culture medium in a cell
incubator. Matured chondrocytes were collected for subsequent in
vitro evaluation.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) (cat. no. AR1199,
Wuhan Boster Biological Technology, Ltd.) was employed to detect
the cytotoxic action of a graded concentration of RUT on mouse
chondrocytes that were seeded and cultured for a day using a
96-well plate at a density of 5,000 to 10,000 cells per well.
Following cell treatment using a concentration gradient of RUT (0,
1, 2.5, 5 and 10 μM) for 1 day, 100 μl of the mixture
(containing 10 μl CCK-8 reagent) were added and the
chondrocytes were incubated for 1 h at 37°C in the dark.
Subsequently, a microplate reader (Bio-Rad Laboratories, Inc.) was
used to detect the cell absorbance at 450 nm wavelength.
Safranin O staining of cells
Safranin O staining was used to relatively quantify
the proteoglycan content. The chondrocytes seeded into six-well
plates were treated with IL-1β (5 ng/ml) (9,25)
for 1 day with/without RUT (5 or 10 μM) when the cells
reached 80% confluency. PBS was used to wash the cells three times,
and the cells were then stabilized using 4% paraformaldehyde (PFA)
at room temperature for 30 min prior to incubation with Safranin O
reagent for 2 h at 37°C, followed by dye removal and washing with
PBS. A light microscope was used to capture the brightness of
Safranin O/fast green staining (EVOS FL auto, Life Technologies;
Thermo Fisher Scientifc, Inc.).
Western blot analysis
The chondrocytes seeded in six-well plates were
placed on ice, and the culture medium was discarded, followed by
washing with PBS three times. The cell lysate was prepared
following the thorough drying of the plates using filter paper. The
cells were lysed with lysis buffer comprised
radioimmunoprecipitation assay (RIPA) lysis buffer, phosphatase and
protease inhibitors at a ratio of 100:1:1 (Wuhan Boster Biological
Technology, Ltd.). Following cell lysis on ice for 20 min, the
cells mixed with lysate were scraped off using cell wipers and
collected. The mixture was fully crushed using an ultrasonic
crusher (Sonicator Q125, Qsonica, LLC.) and centrifuged at 16,099 ×
g for 30 min at 4°C. Following centrifugation, the protein
concentration in the supernatant was measured using BCA assay
(Wuhan Boster Biological Technology, Ltd.). The supernatants were
mixed with loading buffer (4:1), refrigerated for 5 min and
denatured at 95°C for 10 min, leading to the final protein samples.
These were then subjected to sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (8.0-12.5%) and transferred onto a
polyvinylidene difluoride membrane. Subsequently, 5% bovine serum
albumin (Wuhan Boster Biological Technology, Ltd.) was then
employed to denature the membrane sites for 1 h at 25°C. The
membranes were then incubated with specific primary antibodies at
4°C overnight, rinsed three times using Tris-buffered saline with
0.1% Tween®-20 for 15 min, incubated at room temperature
with specific secondary antibodies for 1 h, and then washed three
times for 15 min each time with the same saline and buffer mixture.
Finally, the target protein bands were exposed using enhanced
chemiluminescence (Abbkine), saved using Image LabTM
Software v.4.0 (Bio-Rad Laboratories, Inc.) and quantified using
ImageJ software v.1.8.0 (National Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to evaluate the expression
levels of inflammatory cytokines in the RUT-treated chondrocytes.
The chondrocytes were plated into a six-well plate and cultured
with medium containing IL-1β (5 ng/ml) for 1 day with/without RUT
(5 or 10 μM). The extraction of total RNA was performed
using the Total RNA kit I (Omega Bio-tek) according to the
manufacturer's instructions. Complementary DNA (cDNA) synthesis was
executed using a Rever Tra Ace qPCR RT kit (cat. no. FSQ-101,
Toyobo Life Science), and subsequently amplified using a RT-qPCR
kit [cat. no. 13117ES, Yeasen Biotechnology (Shanghai) Co., Ltd.]
on a Bio Rad Q5 instrument (Bio-Rad Laboratories, Inc.) under the
following conditions: 5 min at 95°C, and thereafter 40 cycles of 10
sec at 95°C and 30 sec at 60°C. The relative levels of target gene
expression were normalized to the internal reference gene, GAPDH.
The results were quantified using the relative 2-ΔΔCq
method (26). The sequences of
the primers used for RT-qPCR are presented in Table I.
 | Table IPrimer sequences used in RT-qPCR. |
Table I
Primer sequences used in RT-qPCR.
| Gene | Sequence |
|---|
| GAPDH | F:
5′-CCCAGCTTAGGTTCATCAGG-3′ |
| R:
5′-ATCTCCACTTTGCCACTGC-3′ |
| IL-6 | F:
5′-CCTTCCTACCCCAAT TTCCAAT-3′ |
| R:
5′-GCCACTCCTTCTGTGACTCCAG-3′ |
| TNF-α | F:
5′-ATGTCTCAGCCTCTTCTC-3′ |
| R:
5′-GCCATTTGGGAACTTCTC-3′ |
| Integrin αV | F:
5′-AATAAGATCTGCCCGTTGCC-3′ |
| R:
5′-GTAGAAGCTCCACCTGGAAG-3′ |
| Integrin β3 | F: 5′-TGATG
GGCACTGTCACATTG-3′ |
| R:
5′-TTCTGGTAAAGGCTGACGAC-3′ |
Immunofluorescence staining
Confocal dishes were prepared for COL II and p65
staining, and the chondrocytes were evenly seeded at
2×104 per dish and cultured for 24 h. These cells were
then stimulated with IL-1β (5 ng/ml) for 24 h with/without RUT (10
μM). Subsequently, 4% PFA was used to fix the plates for 30
min at 25°C, followed by rinsing with 0.2% Triton X-100 for 15 min.
The unbound sites on the plates were then blocked using 1% bovine
serum albumin for 30 min at normal temperature. Subsequently,
primary antibodies against COL II (1:200; cat. no. 28459-1-AP,
Proteintech Group, Inc.) and p65 (1:400; cat. no. 8242, Cell
Signaling Technology, Inc.) were used to incubate the cells at 4°C
overnight. Following a wash with PBS, the chondrocytes were
incubated with Cy3-conjugated Affinipure goat anti-rabbit secondary
antibody (1:200; cat. no. BA1032, Wuhan Boster Biological
Technology, Ltd.) for 1 h at room temperature in the dark. The
chondrocyte nuclei were then subjected to staining using
4′,6-diamidino-2-phenylindole (cat. no. AR1176, Wuhan Boster
Biological Technology, Ltd.) for 10 min at room temperature in the
dark. A confocal microscope (Nikon Corporation) was used for
screening and obtaining images.
Senescence β-galactosidase staining
β-galactosidase activity was evaluated using a
senescence β-galactosidase staining kit. The chondrocytes seeded
into six-well plates were treated for 1 day with IL-1β (5 ng/ml)
with/without RUT (10 μM). The plate was then triple-washed
with PBS, stabilized in 4% PFA for 15 min at room temperature,
incubated with the mixture staining fluid at 37°C overnight without
CO2, and finally observed under a light microscope (EVOS
FL auto, Life Technologies; Thermo Fisher Scientific, Inc.). The
density of the senescent cells was measured using ImageJ software
v.1.8.0 (National Institutes of Health) as the cell count (cells
with blue spots).
Apoptosis evaluation
Flow cytometric analysis was utilized to calculate
the chondrocyte apoptotic rates. The chondrocytes were seeded in
six-well plates and cultured in an integrated medium containing 10%
FBS, 1% penicillin/streptomycin and DMEM/F12 culture medium (Thermo
Fisher Scientific, Inc.). When the cells reached 80% confluency,
they were treated with IL-1β (5 ng/ml), and treated with or without
RUT (1, 2.5, 5 and 10 μM) for 1 day, digested using trypsin,
rinsed using PBS and resuspended in 100 μl binding buffer
for 5 min. Subsequently, 5 μl FITC Annexin V and 10
μl propidium iodide from the Annexin V-FITC/PI apoptosis kit
were combined with the suspension without light at room temperature
for 5-10 min. Subsequently, 400 μl binding buffer were
added, and finally, a flow cytometer was used to detect the
apoptotic cells (Moflo XDP, Beckman Coulter) and FlowJo v10
software (FlowJo LLC) was used to analyse the apoptotic cells.
mRFP-GFP-LC3 adenovirus infection and
confocal microscopy
LC3 protein was overexpressed using mRFP-GFP-LC3
autophagy double-labelled adenovirus (HanBio Inc.) and labelled
with red and green fluorescence to observe the autophagic flux. The
chondrocytes were seeded in confocal dishes and incubated in
complete medium consisting of 10% FBS, 1% penicillin/streptomycin
and DMEM/F12 culture medium (Thermo Fisher Scientific, Inc.). When
the cells reached approximately 50% confluency, adenoviral vectors
(HanBio Inc.) were used to infect them at a formerly defined
multiplicity of infection (MOI) of 20 for 1 day (9). IL-1β (5 ng/ml) was then used to
treat the cells with/without RUT (10 μM) for 24 h.
Subsequently, the autophagic flux was recorded using a confocal
microscope (Nikon Corporation). Due to acid sensitivity, the green
fluorescence was quenched following autolysosome formation, and
only red fluorescence could be detected. The number of green and
red nodes was recorded to evaluate the intensity of autophagy and
the number of autophagosomes, respectively.
Bioinformatics analysis of potential
mechanisms of RUT intervention in OA
Predicted RUT target genes were acquired from the
PharmMapper database and then input into the Database for
Annotation, Visualization, and Integrated Discovery (DAVID) to
obtain RUT-related Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathways. Simultaneously, OA-related pathways were screened from
the miRWalk2.0 database (http://mirwalk.umm.uni-heidelberg.de/). The KEGG
pathways of RUT-target genes were intersected with the OA-related
pathways to obtain common pathways by Venn Diagram (Venny2.1).
RNA-seq data were obtained from the Gene Expression Omnibus (GEO)
database (accession no. GSE210476) (27), and the R package ggplot2 was used
for quantitative analysis of gene-level expression. A molecular
docking analysis was performed using Autodock Vina on RUT
(C18H13N3O) and integrin αVβ3 (PDB: 6MSL). PyMOL (https://pymol.org/2/) was used to visualize the
docking of molecules.
siRNA
Specific integrin αV (ItgαV) and β3 (Itgβ3) siRNAs
(Table II) were synthesized by
Guangzhou Ribobio Co., Ltd. with two repeats of each siRNA to knock
down integrins αV and β3. The chondrocytes seeded in six-well
plates were transfected with negative control or siRNA against
integrins αV and β3 (50 nM) using Lipofectamine 3000®
reagent (Thermo Fisher Scientific, Inc.) and Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C for 24 h. The knockdown
efficiency of each repeat was examined using RT-qPCR as described
above. Subsequently, the repeat with the highest effectivity of
ItgαV and Itgβ3 siRNA was used to transfect the chondrocytes for 24
h to confirm the amelioration of osteoarthritis by RUT in the
presence or absence of integrin αVβ3.
 | Table IIsiRNA sequences used for siRNA
assays. |
Table II
siRNA sequences used for siRNA
assays.
| siRNA | Sequence |
|---|
| ItgαV siRNA#1 |
GAGGATCTCTTCAACTCTA |
| ItgαV siRNA#1 |
ACCCGTTGTCACTGTAAAT |
| ItgαV siRNA#1 |
GCAAGAAAGAGAACAGCTT |
| Itgβ3 siRNA#1 |
CCGTGAATTGTACCTACAA |
| Itgβ3 siRNA#1 |
GCTGATGACTGAGAAACTA |
| Itgβ3 siRNA#1 |
CCATGACCGGAAGGAATTT |
| Negative control
siRNA |
TTCTCCGAACGTGTCACGT |
Histological staining and
immunohistochemical analysis
RUT was intra-articularly injected at a dose of 25
mg/kg per week following the establishment of a destabilization of
the medial meniscus (DMM) model using mice, as described below.
After 2 months, all mice were sacrificed to obtain their right knee
joints. The soft tissue of the joints was thoroughly removed, and
the joints were immersed in 4% PFA (Wuhan Boster Biological
Technology, Ltd.) at room temperature for 1 day, decalcified in 10%
ethylenediaminetetraacetic acid for 30 days and embedded in
paraffin. In addition, 5-μm-thick sections were sliced for
tissue staining and immunohistological evaluation. Haematoxylin and
eosin (H&E), Safranin O/fast green, and toluidine blue (Beijing
Solarbio Science & Technology Co., Ltd.) were used for tissue
section staining following the corresponding protocols [staining at
room temperature; haematoxylin (5 min) eosin (1 min), Safranin (2
min), fast green (5 min), toluidine blue (30 min)]. Images were
obtained using an Olympus BX63 microscope (Olympus Corporation).
The degree of mouse cartilage damage was determined according to
the Osteoarthritis Research Society International (OARSI)
guidelines (28). Following
deparaffinization and blocking with 5% bovine serum albumin at 37°C
for 1 h, the sections were incubated overnight at 4°C using primary
antibodies against the anabolic factor, aggrecan (1:200; cat. no.
13880-1-AP), and the catabolic factor, MMP13 (1:100; cat. no.
18165-1-AP) (both from Proteintech Group, Inc.). HRP-AffiniPure
goat anti-rabbit IgG (1:500; cat. no. BM3894, Wuhan Boster
Biological Technology, Ltd.) was then incubated with the sections
at 37°C for 1 h, followed by development and observation under an
Olympus BX63 microscope (Olympus Corporation).
Animal experiments
The present study was conducted as per the approval
and regulations of the Ethics and Animal Research Committee of
Huazhong University of Science and Technology [(2021) IACUC no.
2908]. A total of 60 C57BL/6J mice (6-8 weeks old, male) were
supplied by the Experimental Animal Centre of Tongji Hospital and
fed with normal chow and water in an SPF animal laboratory at 25°C
with 12:12 light/dark cycle. The mice were randomly divided into
three groups (the control, OA and OA + RUT groups). Following
inhalation-induced anaesthesia with 2% isoflurane and maintenance
with 1.5% isoflurane, the model of DMM was constructed in the OA
and OA + RUT groups by cutting at the joint cavity and transecting
the anterior medial meniscus-tibial ligament to immobilize the
medial meniscus, as previously described (4,9,10,15). The mice in the control group
underwent joint cavity opening and only anterior fat pad excision.
A 1 week after the surgery, the articular cavity of the mice in the
OA + RUT group was injected with a 10-μl solution of RUT at
25 mg/kg per week for 8 weeks. Simultaneously, the mice in the
control and OA group were administered 10 μl saline
solution. Following the completion of the intra-articular injection
course, the mice were sacrificed by cervical dislocation following
deep anaesthesia with 5% isoflurane, and the right knee joints were
obtained and subjected to further evaluation. The three-dimensional
reconstruction and the evaluation of bone volume fraction (BV/TV),
trabecular separation (TB.Sp) and trabecular number (TB.N) were all
performed using the micro-computed tomography system
(μ-CT50, SCANCO Medical AG).
Statistical analysis
The experimental data analysed using GraphPad Prism
v.8.4.0 (Dotmatics). The outcomes are presented as the mean ± SD.
The Student's t-test was employed to compare two groups to
highlight their variations. One-way analysis of variance (ANOVA)
with Tukey's post hoc test were employed to compare two or more
groups. A P-value <0.05 was considered to indicate a
statistically significant difference. All analyses were repeated
independently at least three times.
Results
Cell viability and identification of
mouse chondrocytes
The viability of the chondrocytes treated with
various concentrations of RUT (0, 1, 2.5, 5 and 10 μM) for
24 h was detected using a CCK-8 kit. As shown in Fig. 1B, RUT did not exert any
significant cytotoxic effects on the chondrocytes; therefore, the
1, 2.5, 5 and 10 μM concentrations of RUT were chosen for
use in further experimentations. Furthermore, Safranin O staining
was conducted to observe chondrocyte morphology. A mild loss of
staining was observed in the IL-1β-stimulated chondrocytes that was
reversed by RUT treatment in a concentration-dependent manner. The
proteoglycan content revealed that RUT administration promoted
anabolism in chondrocytes (Fig.
1C).
RUT attenuates the IL-1β-induced
expression of inflammatory mediators and catabolic factors and
reverses the IL-1β-induced degeneration of anabolic factors in
mouse chondrocytes
COX2, IL-6, TNF-α and IL-1β are major inflammatory
cytokines and their levels are enhanced in IL-1β-stimulated
chondrocytes (4,17). To determine the anti-inflammatory
effects of RUT, chondrocytes that reached 80% confluency were
treated with various concentrations of RUT with/without IL-1β (5
ng/ml) for 1 day, and the cell lysate was subjected to western blot
analysis and RT-qPCR. As shown in Fig. 2A and C-E, a marked elevation in
the expression levels of the aforementioned proteins was observed
in the chondrocytes stimulated by IL-1β, and the increasing pattern
was averted in a concentration-dependent manner by RUT.
Additionally, the present study evaluated the mRNA expression
levels of inflammatory cytokines in chondrocytes with or without
RUT treatment when IL-1β (5 ng/ml) was used for 24 h. As
illustrated in Fig. 2D and E, RUT
significantly suppressed the mRNA expression of IL-6 and TNF-α
induced by IL-1β. Moreover, as COL II, aggrecan and SOX9 are highly
significant anabolic enzymes with crucial roles in maintaining
extracellular matrix (ECM) cartilage homeostasis, the expression of
these proteins was determined to analyse the anabolism level in
chondrocytes. The results revealed an attenuation in COL II,
aggrecan and SOX9 production by IL-1β, and the suppressive trend
was mitigated by RUT in a concentration-dependent manner (Fig. 2B and F). Furthermore, the
expression levels of catabolic enzymes were significantly augmented
in the IL-1β-stimulated chondrocytes, indicating the disruption of
ECM homeostasis. The expression of the key chondrocyte catabolic
indicators, MMP3 and MMP13, was examined to verify the beneficial
effects of RUT on IL-1β-stimulated chondrocytes from mice with OA.
As shown in Fig. 2B and G, IL-1β
abnormally upregulated MMP-3 and MMP-13 expression. The
IL-1β-stimulated chondrocytes treated with RUT exhibited a
concentration-dependent decrease in catabolic enzyme expression. In
addition, the immunofluorescence analysis of COL II revealed
similar changes following the treatment of IL-1β-stimulated
chondrocytes with/without RUT (Fig.
2H).
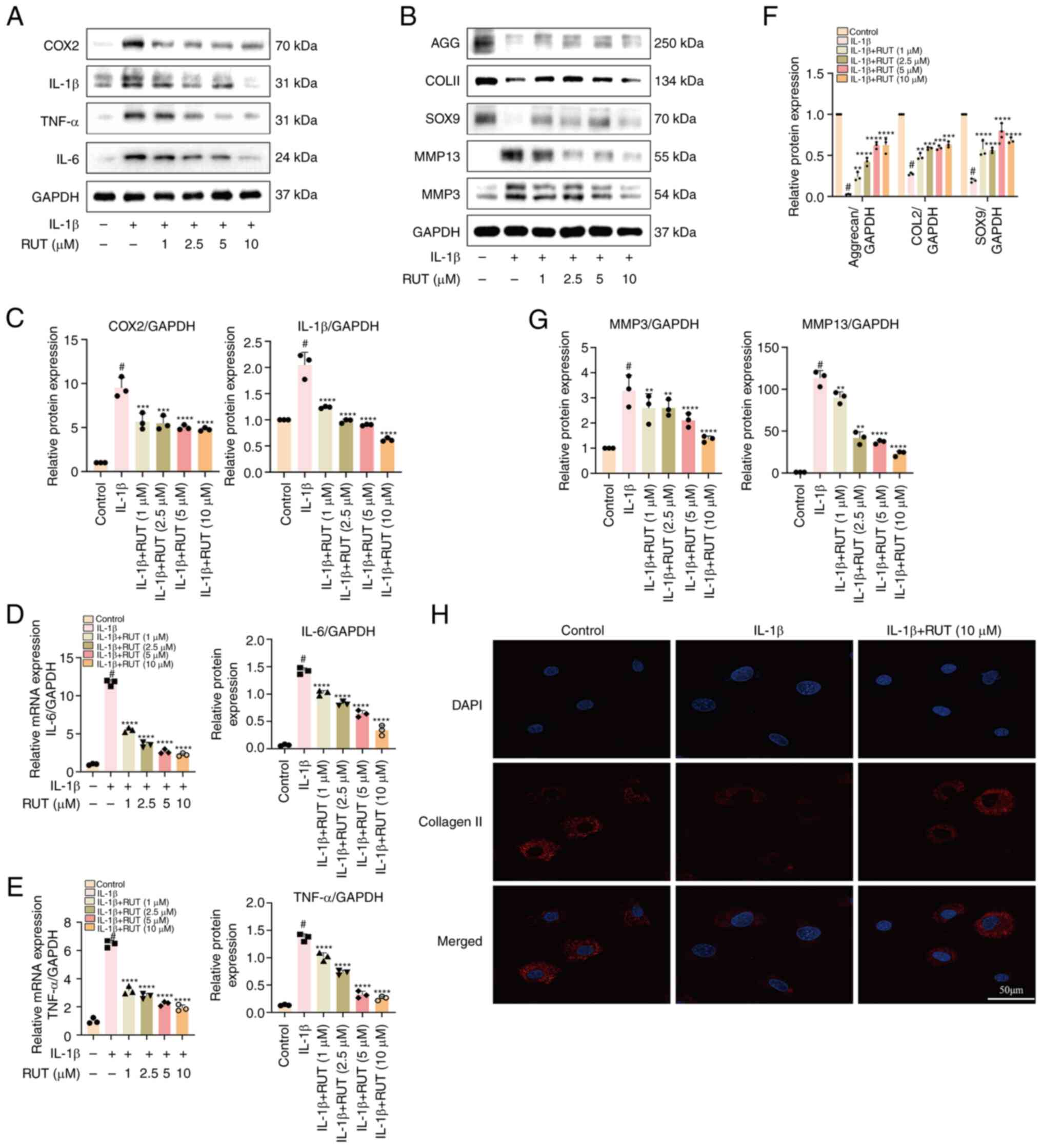 | Figure 2Protective effects of RUT against
IL-1β-induced inflammation and extracellular matrix degradation in
mouse chondrocytes. The cells were exposed to IL-1β (5 ng/ml)
with/without RUT (1, 2.5, 5 and 10 μM) for 24 h. (A and B)
The protein expression levels of MMP3, MMP13, IL-1β, COX2, IL-6,
TNF-α, SOX9, COL II and aggrecan were determined using western blot
analysis. (C-G) Quantification analysis of the results of western
blotting. (D and E) Relative mRNA expression levels of IL-6 and
TNF-α in chondrocytes stimulated with IL-1β (5 ng/ml) and treated
with or without RUT (1, 2.5, 5 and 10 μM) for 24 h. The
values are presented as the mean ± SD of three independent
experiments. #P<0.05 vs. control group;
**P<0.01, ***P<0.001 and
****P<0.0001 vs. IL-1β group. (H) The protein
expression of COL II was detected using immunofluorescence
following treatment of the cells with IL-1β (5 ng/ml) with/without
RUT (10 μM) for 24 h. IL-1β, interleukin 1 β; RUT,
rutaecarpine; MMP, matrix metalloproteinase; COX2, cyclooxygenase
2; SOX9, SRY-box transcription factor 9; COL II, collagen type
II. |
RUT attenuates IL-1β-induced senescence
and apoptosis, and impairs chondrocyte autophagy
Chondrocyte senescence occurs with OA progression,
with inflammation as the primary process regulator (11). The activity of β-galactosidase, a
critical enzyme, regulates cell senescence (9). Herein, the expression of
β-galactosidase markedly increased in the IL-1β-stimulated
chondrocytes compared to the normal chondrocytes for 24 h, and
treatment with RUT (10 μM) effectively mitigated
β-galactosidase activity (Fig. 3A and
B). Consistent with this phenomenon of enhanced expression with
IL-1β stimuli and its reversal in the presence of RUT, a similar
effect was observed in the activity of senescence marker proteins,
including p16 (INK4A) and p21 in chondrocytes (Fig. 3C and D). Additionally, the
expression of BCL2 (an anti-apoptotic protein) and BAX (a
pro-apoptotic protein) was determined using western blot analysis.
Consistent with the findings of a previous study (1), stimulation with IL-1β for 24 h
significantly augmented BAX activity and reduced BCL2 expression in
the chondrocytes (Fig. 3G and H).
However, these effects were effectively reversed in a
concentration-dependent manner by RUT.
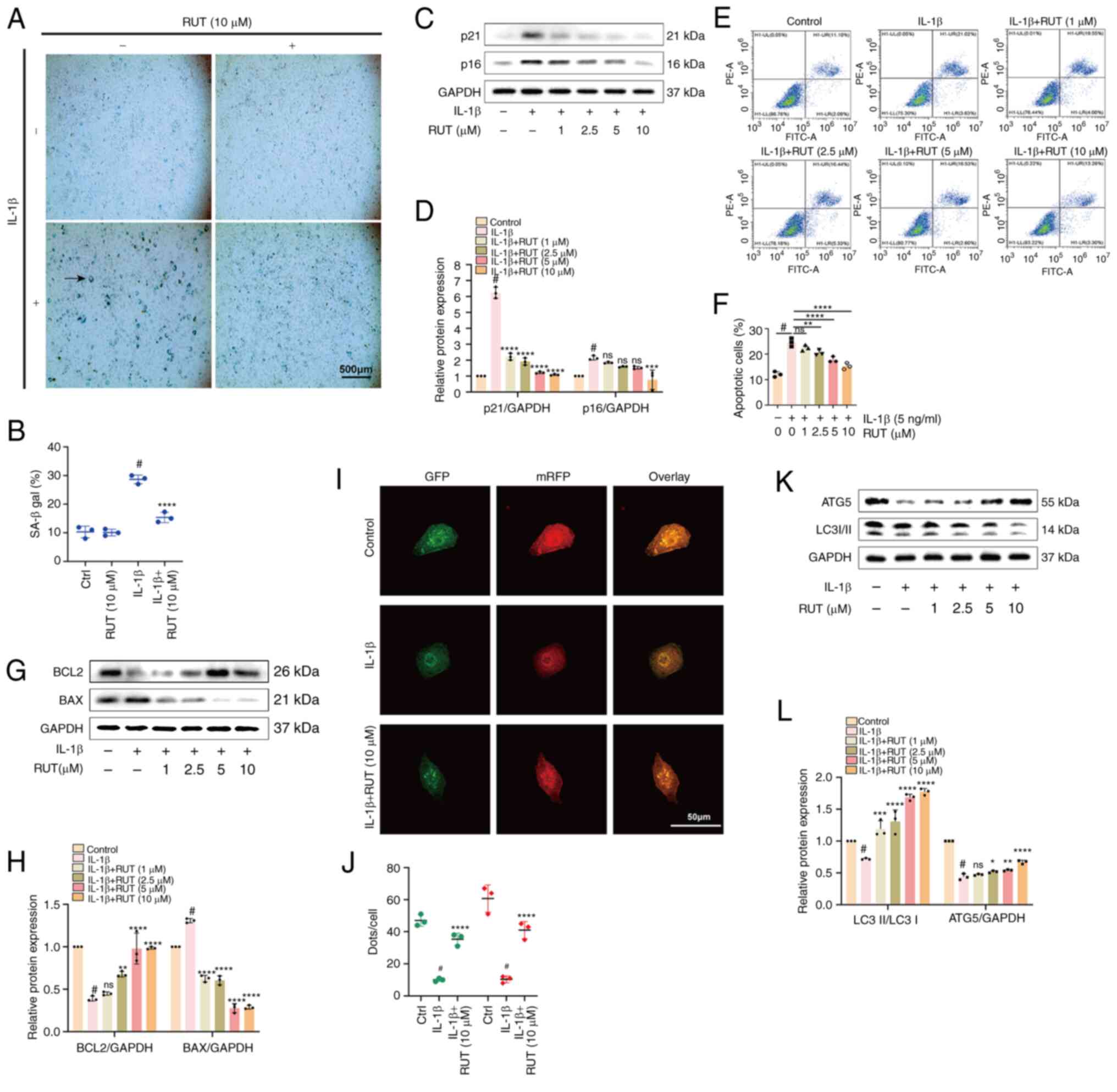 | Figure 3Effects of RUT on IL-1β-induced
chondrocyte senescence, apoptosis and autophagy. The cells were
stimulated with 5 ng/ml IL-1β in the presence or absence of RUT (10
μM) for 24 h. (A) β-galactosidase staining of chondrocytes.
Scale bar, 500 μm. (B) Quantification analysis of
β-galactosidase activity. (C and D) Results of western blot
analysis of the senescence marker proteins, p21 and p16 (INK4A),
and corresponding quantification analysis. (E and F) Apoptotic
chondrocytes and corresponding apoptosis rates. (G and H) Levels of
the apoptosis-related proteins, BAX and BCL2. (I) Yellow puncta
represent autophagosomes, and red puncta represent autolysosomes in
the merged images. (J) Quantification analysis of dots/cells. (K
and L) The outcomes of western blot analysis and quantification
analysis of proteins linked to autophagy (ATG5 and LC3I/II). The
values are presentd as the mean ± SD of three independent
experiments. #P<0.05 vs. control group;
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. IL-1β
group; ns, no significant difference (P>0.05). IL-1β,
interleukin 1 β; RUT, rutaecarpine; ATG5, autophagy-related 5; LC3,
microtubule-associated protein light chain 3. |
Furthermore, the results of flow cytometric analysis
(Fig. 3E and F) demonstrated that
the rate of apoptosis increased in chondrocytes stimulated with
IL-1β for 1 day and decreased following treatment with RUT in a
concentration-dependent manner. These data collectively
demonstrated the potential anti-apoptotic effects of RUT on
IL-1β-stimulated chondrocytes. Furthermore, the expression of
autophagy-related proteins and the autophagic flux were determined
to verify whether the ameliorating effects of RUT on mouse OA
involved autophagy. The results of western blot analysis revealed
that the inhibition of proteins linked to autophagy, including
LC3I/II and ATG5, under IL-1β stimulation (Fig. 3K and L) was reversed by RUT in a
concentration-dependent manner. Moreover, treatment with RUT (10
μM) enhanced the number of autophagosomes and autolysosomes
in contrast to the group stimulated with IL-1β for 24 h (Fig. 3I and G). Thus, RUT may have a
potential ameliorating effect on impaired autophagy in
chondrocytes.
Bioinformatics analysis of the potential
therapeutic mechanisms of RUT
Based on the PharmMapper database, the potential
target genes of RUT were identified using bioinformatic methods.
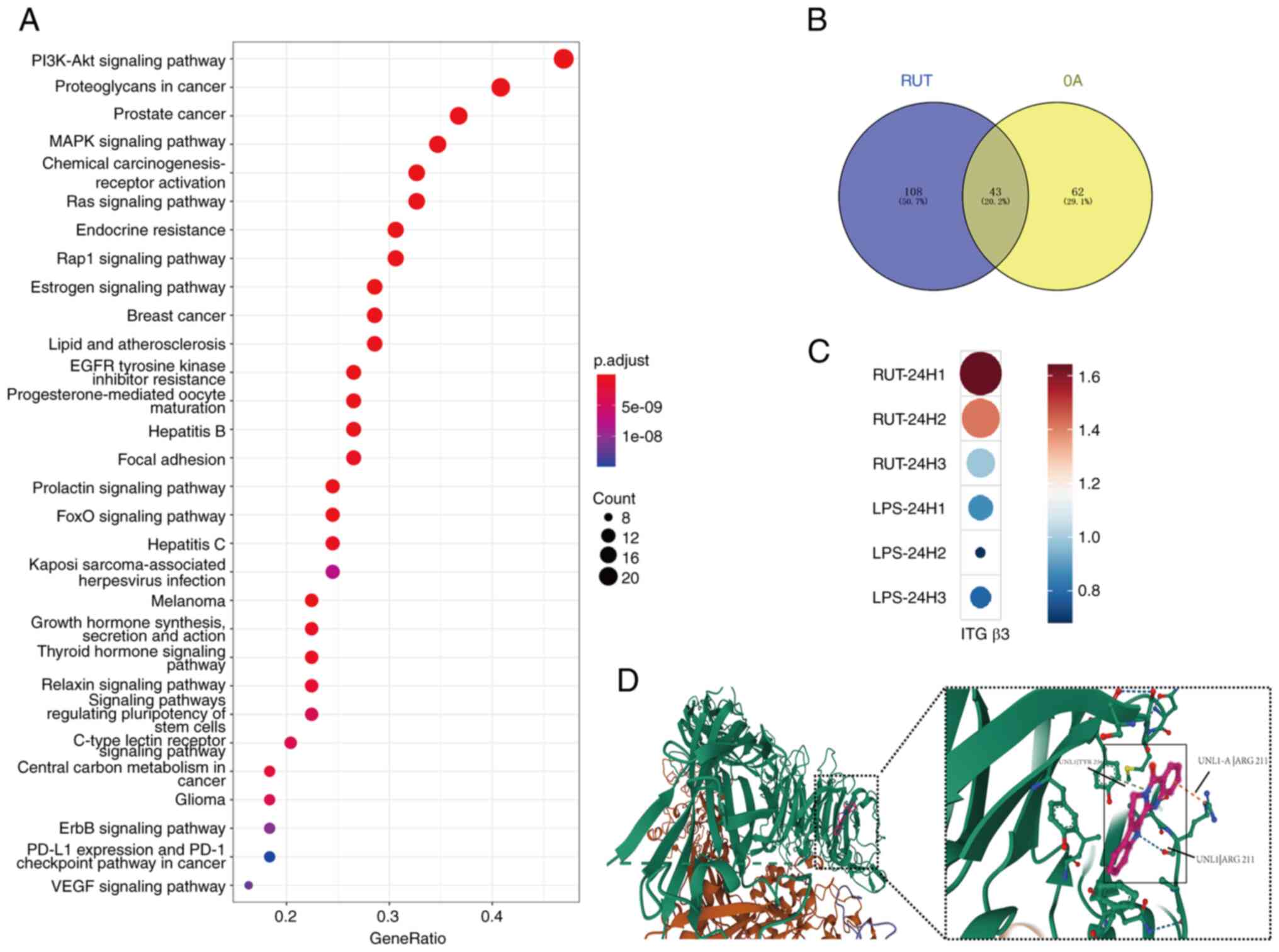
KEGG pathway enrichment analysis was used to enrich 151 pathways to
analyse RUT target genes. The PI3K/Akt, proteoglycans in cancer,
prostate cancer and MAPK signalling pathways constituted the top
four pathways with the greatest number of genes (Fig. 4A). Simultaneously, 43 common
pathways were obtained via the intersection of OA-related pathways
and KEGG pathways of RUT target genes (Fig. 4B), which may potentially underlie
the therapeutic mechanisms of RUT. The data obtained from RNA
sequencing (GSE210476) were analysed in primary human macrophages
treated with or without lipopolysaccharide (LPS; 100 ng/ml) and RUT
(10 μM) (27). The gene
expression of integrin β3 markedly increased in the RUT-treated
macrophages compared to the LPS-stimulated groups for 24 h
(Fig. 4C). An intervention with
RUT increased the expression of integrin β3. Hence, integrin β3
might be a potential target gene of RUT. With the assistance of
AutoDock Vina, a ligand docking analysis was conducted to further
investigate the possible interaction modes between RUT and integrin
β3. A docking analysis revealed that the RUT protein formed three
hydrogen bond interactions with the amino acids in the integrin β3
protein, which had a strong association (Fig. 4D).
RUT mitigates the activation of the
PI3K/Akt/NF-κB and MAPK pathways in mouse chondrocytes stimulated
with IL-1β
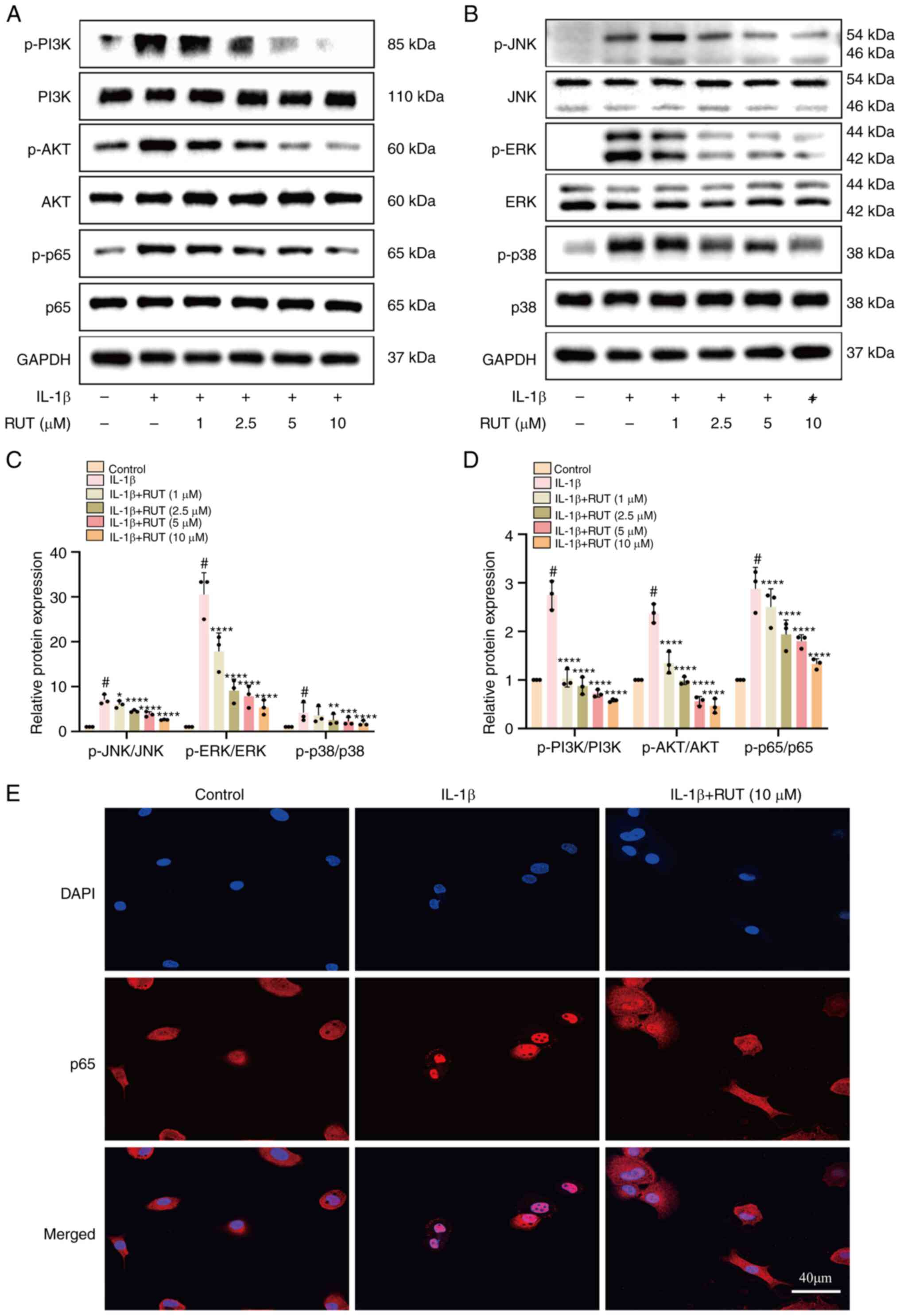
According to the prediction results of
bioinformatics analysis, the PI3K/AKT and MAPK pathways were
regarded as two potential mechanisms of the effects of RUT on OA
occurrence and progression, as they are closely associated with
inflammation, metabolism and autophagy (18). The chondrocytes were treated with
RUT in a concentration gradient for 1 day and then cultured
with/without IL-1β (5 ng/ml) for 30 min. The expression levels of
the related proteins in the two aforementioned pathways were
determined using western blot analysis. RUT significantly blocked
the phosphorylation levels of the related proteins, which indicated
that RUT suppressed the activation of the two pathways in a
concentration-dependent manner (Fig.
5A-D). Consistent with the results of western blot analysis,
immunofluorescence assay demonstrated that RUT interrupted the
nuclear translocation of p65 in IL-1β-stimulated chondrocytes
(Fig. 5E). These data suggested
that RUT alleviated mouse OA by suppressing the stimulation of two
signalling pathways.
Alleviating effects of RUT on
IL-1β-stimulated chondrocytes are attenuated following the
knockdown of integrin αVβ3
The decreasing trend in integrin αV/β3 (ItgαV/β3)
levels induced by IL-1β was reversed following treatment of the
chondrocyte with RUT, and the knockdown efficiency of the three
repeats of siRNA was validated using RT-qPCR (Fig. 6A and B). The most effective
combination was selected: ItgαV siRNA#1 and Itgβ3 siRNA#1 to knock
down ItgαV/β3. The chondrocytes were treated only using 5 ng/ml
IL-1β; IL-1β with 10 μM RUT; or IL-1β, 10 μM RUT and
ItgαV/β3 siRNA. The activity levels of inflammatory, anabolic and
catabolic markers were detected using western blot analysis. The
results revealed that RUT alleviated OA by enhancing cartilage
anabolism, and inhibiting cartilage catabolism and inflammatory
markers. This effect was reversed by the knockdown of ItgαV/β3
(Fig. 6C-E and H-M).
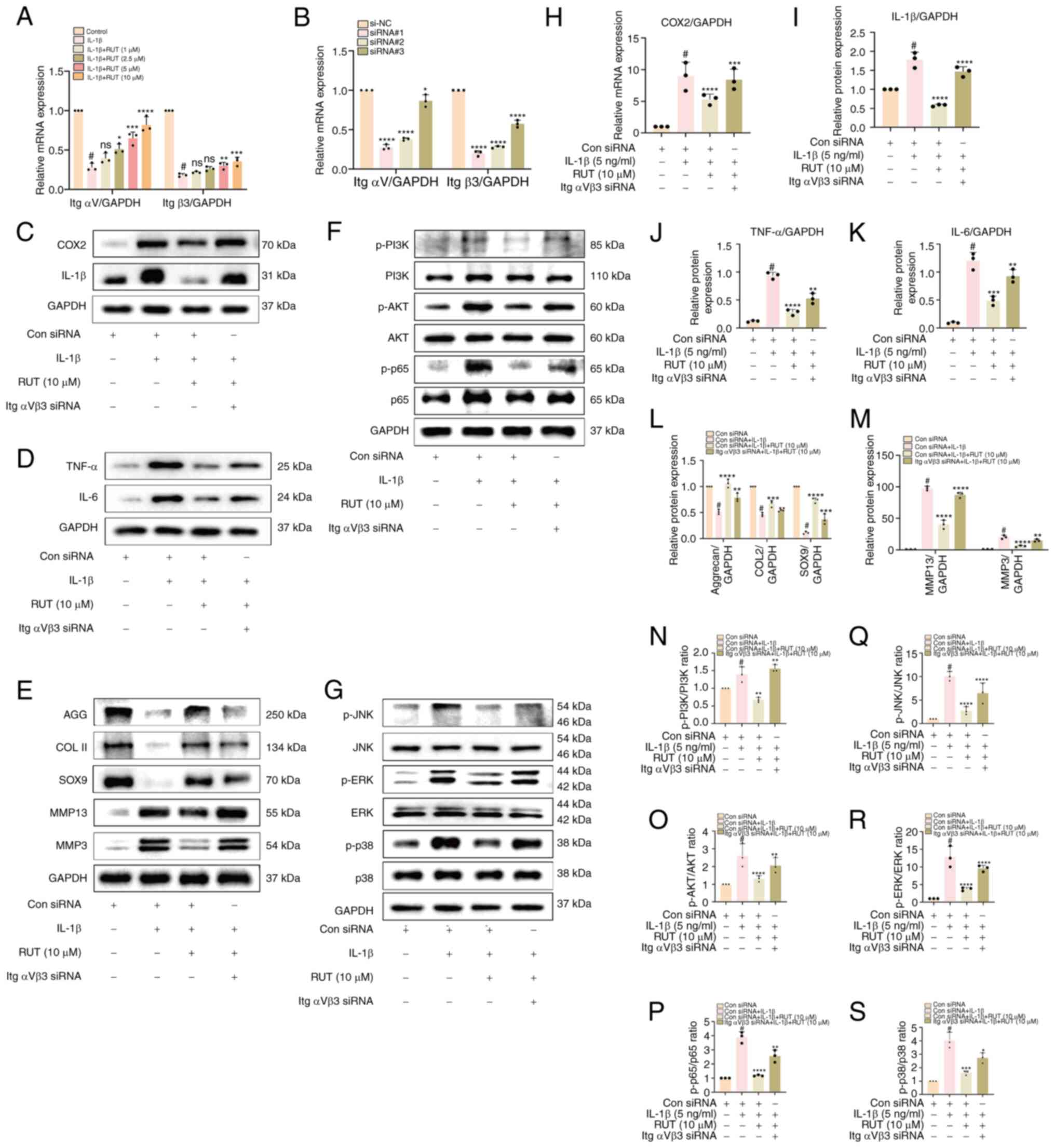 | Figure 6The beneficial effects of RUT on
IL-1β-stimulated chondrocytes were attenuated following integrin
αVβ3 knockdown. (A and B) Relative mRNA levels of ItgαV and Itgβ3
in chondrocytes exposed to 5 ng/ml IL-1β with/without RUT (1, 2.5,
5 and 10 μM) for 24 h. The knockdown efficiency of ItgαV and
Itgβ3 siRNA transfection was verified using RT-qPCR. (C-E and H-M)
The expression levels of inflammation and extracellular matrix
degradation-related proteins were determined using western blot
analysis; the outcomes of the chondrocytes were quantified in the
presence/absence of 5 ng/ml IL-1β, RUT (10 μM) and ItgαVβ3
for 24 h. (F and G, and N-S) Western blot analysis and
quantification analysis were used to measure the levels of
PI3K/Akt/NF-κB and MAPK-related signalling proteins in IL-1β (5
ng/ml)-stimulated chondrocytes for 30 min following transfection
with/without ItgαVβ3 siRNA and RUT (10 μM) for 24 h. Data
are presented as the mean ± SD of three independent experiments.
#P<0.05 vs. control group; *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. IL-1β group; ns, no significant
difference (P>0.05). IL-1β, interleukin 1 β; RUT, rutaecarpine;
MMP, matrix metalloproteinase; COX2, cyclooxygenase 2; SOX9,
SRY-box transcription factor 9; PI3K, phosphoinositide-3-kinase;
COL II, collagen type II; AGG, aggrecan. |
3RUT attenuates the destruction of
cartilage in vivo in a mouse model of OA
As shown in Fig.
7A, the superficial articular cartilage exhibited more notable
abrasion and proteoglycan depletion in the DMM group than in the
control group, and the worsening situation was reversed by RUT. A
relatively smoother cartilaginous surface and richer proteoglycan
were observed in the RUT-treated group than in the untreated DMM
group. Furthermore, the calculated OARSI score revealed that RUT
administration attenuated the severity of OA (Fig. 7B). Based on the findings of the
in vitro analysis, the activity of aggrecan and MMP13
exhibited a similar change in the DMM + RUT group. RUT intervention
reduced MMP13 synthesis and facilitated aggrecan production
(Fig. 7C and D).
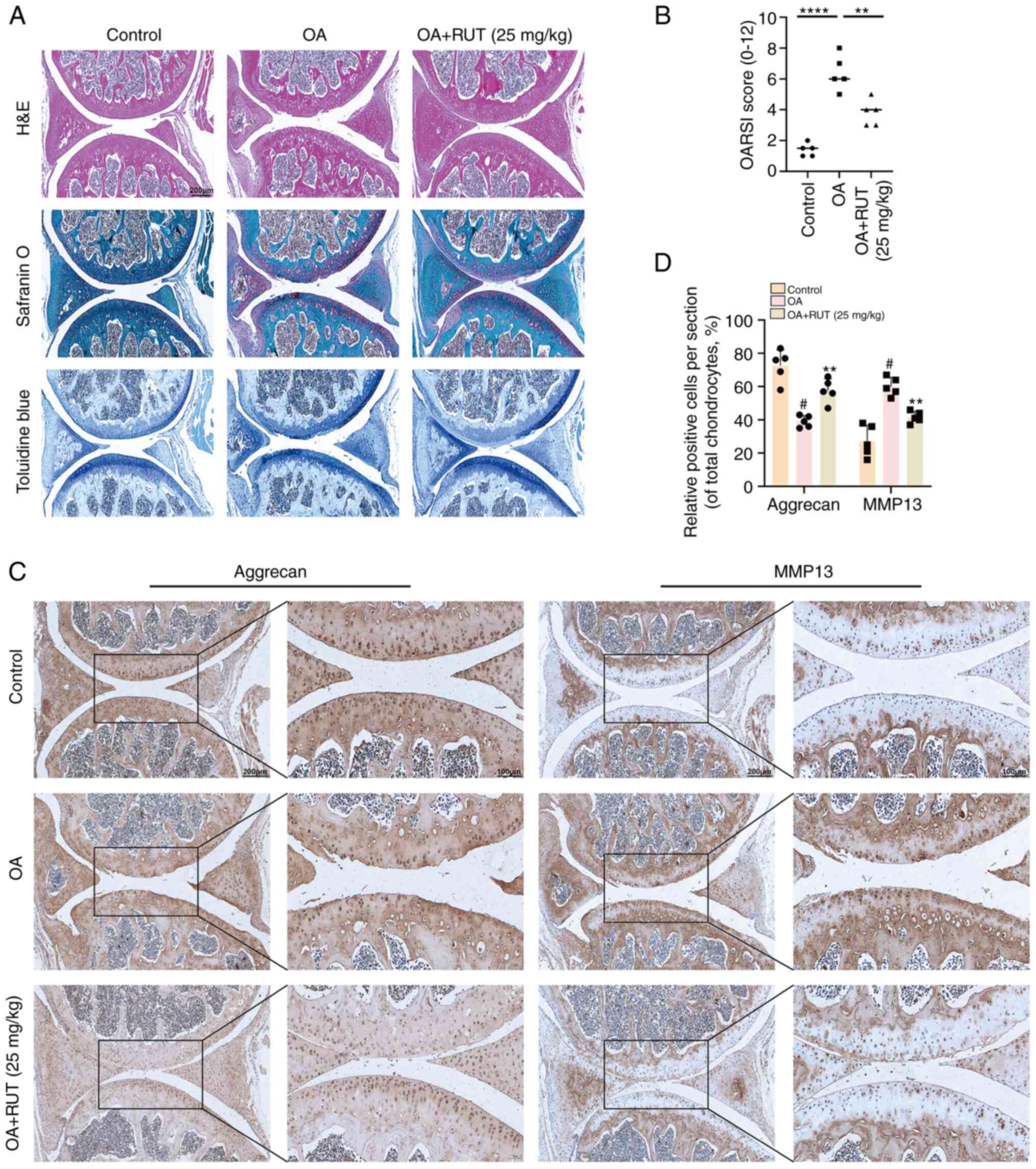 | Figure 7RUT attenuates cartilage destruction
in vivo in the mouse model of OA. (A) H&E, Safranin
O/fast green, toluidine blue staining, and (B) OARSI scores of the
mouse knee joints from the control, OA and OA + RUT groups at 8
weeks following the corresponding treatment (scale bars, 100
μm). (C) Immunohistochemical staining and (D) quantification
analysis of the expression of aggrecan and MMP13 were conducted
among the three groups (scale bars, 50 and 100 μm). Data are
presented as the mean ± SD (n=5). #P<0.05 vs. control
group; **P<0.01 and ****P<0.0001 vs. OA
group. RUT, rutaecarpine; OA, osteoarthritis; OARSI, Osteoarthritis
Research Society International; H&E, haematoxylin and eosin;
MMP, matrix metalloproteinase. |
RUT attenuates subchondral bone
remodelling in vivo in the mouse model of OA
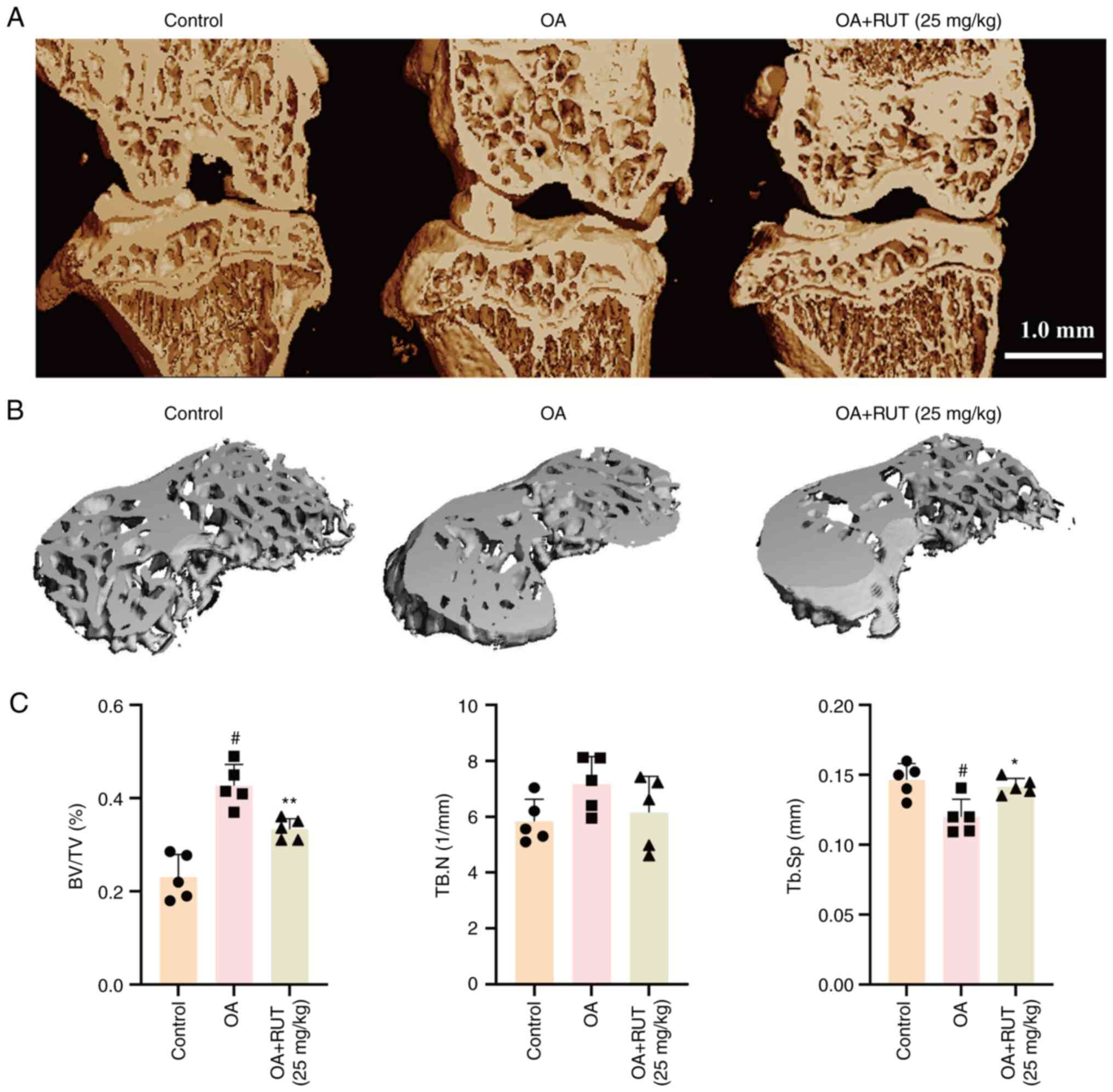
Furthermore, microcomputed tomography was used to
examine the effects of RUT on subchondral bone remodelling in a
mouse model of OA. The 3D CT images of the right knee from the mice
in the control, OA, and OA + RUT groups are presented in Fig. 8A and B. The mice in the OA group
exhibited a more significant BV/TV than the control group mice,
while a noticeable decrease in TB.Sp was observed and was reversed
following treatment with RUT. No statistically significant
difference was observed in TB.N between the OA and control groups
(Fig. 8C). These findings suggest
that RUT had a promising ameliorating effect on subchondral bone
remodelling in mice with OA.
Discussion
Previous research has identified the medicinal
significance of RUT (19,21,22); however, its role in chondrocytes
remains undetermined. In the present study, inflammatory responses
were indeed observed in IL-1β-stimulated chondrocytes. However,
treatment of the chondrocytes with RUT led to the downregulation of
COX2, IL-1β, IL-6 and TNF-α. Additionally, RUT markedly decreased
the generation of catabolic enzymes (MMP3 and MMP13) and
upregulated the expression of anabolic enzymes (COL II, aggrecan
and SOX9). The results of immunofluorescence staining confirmed the
ameliorating effect, similar to the findings of western blot
analysis. These results indicated that RUT promoted
anti-inflammation, anti-catabolism and pro-anabolism in
chondrocytes. Furthermore, cartilage preservation effects, such as
anti-apoptosis, anti-senescence and autophagy repair were also
verified in the chondrocytes treated with RUT.
Furthermore, the mutual suppression of the
activation of the PI3K/AKT/NF-κB and MAPK signalling pathways by
RUT was also observed. Previous research has confirmed that the
rapid activation of PI3K/AKT contributes to the activation of NF-κB
(29) and subsequently enhances
the expression of COX2. Furthermore, the production of catabolic
factors is significantly augmented in OA (30). In the present study, the
phosphorylation level of PI3K/Akt/NF-κB in mouse chondrocytes
induced by IL-1β was reversed in a concentration-dependent manner
by RUT. Moreover, the results of the immunofluorescence staining of
p65 confirmed that pre-treatment with RUT impeded the nuclear
translocation of NF-κB heterodimers. The aforementioned results
strongly indicate that the anti-inflammatory effects of RUT on
chondrocytes may be executed by suppressing the activation of the
PI3K/Akt/NF-κB signalling pathway. Moreover, the present study
revealed that RUT pre-treatment inhibited the IL-1β-mediated
phosphorylation of p38, ERK1/2 and JNK in chondrocytes, which
suggested that the MAPK signalling pathway may be targeted by RUT
for its anti-inflammatory effects.
Integrin αVβ3 is a cell adhesion receptor vital to
mediating cellular interaction with the ECM. Previous studies have
demonstrated that integrin αVβ3 is expressed in normal adult
articular chondrocytes and exerts chondroprotective effects by
mitigating the levels of inflammatory factors in IL-1β-stimulated
chondrocytes (31,32). Another study suggested that
blocking integrin αVβ3 destroyed osteocyte morphology, contributing
to inhibition in spread area and process retraction (33). However, a previous study reported
the opposite result and stated that integrin αVβ3 was highly active
in OA-affected chondrocytes, and its stimulation contributed to OA
progression (34). By contrast, a
previous study further revealed that the activation of integrin
αVβ3 decreased chondrocyte apoptosis (35). Recently, it was shown that
integrin αVβ3 expression was decreased in IL-1β-stimulated
chondrocytes, and knocking it down aggravated mouse OA through the
stimulation of the two pathways (36). This supported the role of integrin
αVβ3 as an upstream target of the aforementioned pathways in OA
treatment (36). Another study
suggested that integrin αVβ3 has a positive influence on
WNT1-inducible-signaling pathway protein 1, protecting rat
chondrocytes from senescence and apoptosis (37). In the present study, it was found
that there was a positive association between integrin β3 and RUT
via RNA-seq data analysis of primary human macrophages treated with
or without LPS (100 ng/ml) and RUT (10 μM) from a previous
study (27). However, one of the
limitations of the present study is that RNA-seq analysis was not
performed on chondrocytes treated with IL-1β (5 ng/ml) with/without
RUT (10 μM). Therefore, it can only be hypothesized that
there may be an association between integrin β3 and RUT in
chondrocytes stimulated with IL-1β, and that integrin αVβ3 may be a
potential target of RUT in the treatment of OA. Consequently,
chondrocytes were transfected with ItgαV and Itgβ3 siRNA to
determine the ameliorating effect of RUT on OA in the presence or
absence of integrin αVβ3. The findings demonstrated that the
knockdown of integrin αVβ3 significantly inhibited the
anti-inflammatory, anticatabolic and pro-anabolic effects of RUT on
IL-1β-stimulated chondrocytes. Additionally, the inhibition of the
PI3K/Akt/NF-κB and MAPK pathways was mitigated following the
knockdown of integrin αVβ3. All these outcomes indicated that
integrin αVβ3 may be a pivotal factor in the inhibition of OA by
RUT.
In the present study, the mouse model of OA
exhibited signs of osteophyte hyperplasia, knee joint cartilage
abrasion, joint space narrowing and other pathological changes. The
histological examination of the mouse knees revealed that RUT
treatment decreased articular cartilage impairment in the mouse
with OA. Moreover, the results of microcomputed tomography
demonstrated that RUT contributed to the remodelling of subchondral
bone. Since the protective effects of RUT on OA remained, the
present study provides exclusive data confirming the effect of RUT
on reducing articular cartilage deterioration in mouse models of
OA.
In summary, RUT was found to exert anti-inflammatory
and anticatabolic functions in IL-1β-stimulated chondrocytes by
suppressing the activation of the PI3K/Akt/NF-κB and MAPK
signalling pathways through integrin αVβ3. Collectively, these
results emphasize the potential of RUT as a reliable drug for the
treatment of OA. However, the findings of the present study need to
be expanded upon in the future. The present study was limited to
investigating the effects of RUT on OA and did not examine the
systemic effects on the body. Intra-articular administration has a
number of advantages, including increased local bioavailability,
reduced adverse events and reduced costs (38). However, the present study did not
investigate the drug retention time and drug metabolism pattern of
RUT in the joint cavity. Additionally, due to its potential as an
anti-inflammatory agent, it is crucial to understand how RUT
promotes cartilage regeneration by mediating OA-related signalling
pathways and potential targets of action. Additionally, further
research is required to determine whether RUT can improve the
progression of OA by acting on other OA-related cells, such as
immune cells, following injection into the joint cavity. Studies
such as transcriptome sequencing and metabolome sequencing may
enable the further understanding of the mechanisms involved in the
effects of RUT on OA in the future.
In conclusion, to the best of our knowledge, the
present study is the first to reveal the in vivo and in
vitro ameliorating effects of RUT on OA-affected cartilage.
These beneficial effects are mediated through anti-inflammatory
effects, the suppression of cartilage degradation and the
suppression of the PI3K/AKT/NF-κB and MAPK signalling pathways in
mice. The cartilage protective effects of RUT on OA may be
regulated by integrin αVβ3. RUT or integrin αVβ3 may thus great
potential to serve as a drug or molecular targets in the treatment
of OA.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (JW, ML, XYuan, XYu, AC, MS, HK and PC)
were responsible for the reliability of the data sources and the
data collation process and analysis operations. The present study
was conceptualized and designed by AC, MS, HK and PC. JW, ML, XYuan
and XYu performed the experiments. All authors (JW, ML, XYuan, XYu,
AC, MS, HK and PC) were involved in data analysis and
interpretation. JW and ML participated in the drafting and in the
critical revision of the important intellectual content of the
article and made significant contributions to the completion of the
final version of this article. JW, ML, XYuan and XYu revised the
manuscript. JW and ML confirm the authenticity of all the raw data.
All authors had no reservations and have read and approved the
publication of the final version.
Ethics approval and participation
consent
The present study was conducted as per the approval
and regulations of the Ethics and Animal Research Committee of
Huazhong University of Science and Technology [(2021) IACUC no.
2908].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
RUT
|
rutaecarpine
|
|
OA
|
osteoarthritis
|
|
COX2
|
cyclooxygenase-2
|
|
IL-1β
|
interleukin-1β
|
|
MMP3
|
matrix metalloproteinases
|
|
SOX9
|
SRY-box transcription factor 9
|
|
CCK-8
|
Cell Counting Kit-8
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K/AKT/NF-κB
|
phosphoinositide-3-kinase/Akt/nuclear
factor-κB
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
H&E
|
haematoxylin and eosin
|
|
OARSI
|
Osteoarthritis Research Society
International
|
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (no. 81672168) and the Natural
Science Foundation of Hubei Province of China (no. 2020CFB216).
References
|
1
|
Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B
and Wang SN: Aucubin protects chondrocytes against IL-1β-induced
apoptosis in vitro and inhibits osteoarthritis in mice model. Drug
Des Devel Ther. 13:3529–3538. 2019. View Article : Google Scholar :
|
|
2
|
Cui J, Shibata Y, Zhu T, Zhou J and Zhang
J: Osteocytes in bone aging: Advances, challenges, and future
perspectives. Ageing Res Rev. 77:1016082022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar
|
|
4
|
Wu Y, Wang Z, Fu X, Lin Z and Yu K:
Geraniol-mediated osteoarthritis improvement by down-regulating
PI3K/Akt/NF-κB and MAPK signals: In vivo and in vitro studies. Int
Immunopharmacol. 86:1067132020. View Article : Google Scholar
|
|
5
|
Rannou F, Pelletier JP and
Martel-Pelletier J: Efficacy and safety of topical NSAIDs in the
management of osteoarthritis: Evidence from real-life setting
trials and surveys. Semin Arthritis Rheum. 45:S18–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schurman DJ and Smith RL: Osteoarthritis:
Current treatment and future prospects for surgical, medical, and
biologic intervention. Clin Orthop Relat Res. (427 Suppl):
S183–S189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang
SB and Kim JH: Disease-modifying therapeutic strategies in
osteoarthritis: Current status and future directions. Exp Mol Med.
53:1689–1696. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou K, Wong J, Abdullah N, Chen X, Smith
T, Doherty M and Zhang W: Examination of overall treatment effect
and the proportion attributable to contextual effect in
osteoarthritis: Meta-analysis of randomised controlled trials. Ann
Rheum Dis. 75:1964–1970. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni B, Pei W, Qu Y, Zhang R, Chu X, Wang Y,
Huang X and You H: MCC950, the NLRP3 inhibitor, protects against
cartilage degradation in a mouse model of osteoarthritis. Oxid Med
Cell Longev. 2021:41390482021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Li J, Xiang X, Zhou B, Zhao C,
Wei Q, Sun Y, Chen J, Lai B, Luo Z and Li A: Tert-butylhydroquinone
attenuates osteoarthritis by protecting chondrocytes and inhibiting
macrophage polarization. Bone Joint Res. 10:704–713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou S, Shi J, Wen H, Xie W, Han X and Li
H: A chondroprotective effect of moracin on IL-1β-induced primary
rat chondrocytes and an osteoarthritis rat model through Nrf2/HO-1
and NF-κB axes. Food Funct. 11:7935–7945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Wu Z, He Y, Chen Z, Xu K, Yu W, Fang
W, Ma C, Moqbel SAA, Ran J, et al: MFN2 contributes to metabolic
disorders and inflammation in the aging of rat chondrocytes and
osteoarthritis. Osteoarthritis Cartilage. 28:1079–1091. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Y, Tang S, Nie X, Zhou Z, Ruan G, Han
W, Zhu Z and Ding C: Decreased miR-214-3p activates NF-κB pathway
and aggravates osteoarthritis progression. EBioMedicine.
65:1032832021. View Article : Google Scholar
|
|
14
|
Chang SH, Mori D, Kobayashi H, Mori Y,
Nakamoto H, Okada K, Taniguchi Y, Sugita S, Yano F, Chung UI, et
al: Excessive mechanical loading promotes osteoarthritis through
the gremlin-1-NF-κB pathway. Nat Commun. 10:14422019. View Article : Google Scholar
|
|
15
|
Zhang C, Shao Z, Hu X, Chen Z, Li B, Jiang
R, Bsoul N, Chen J, Xu C and Gao W: Inhibition of
PI3K/Akt/NF-kappaB signaling by Aloin for ameliorating the
progression of osteoarthritis: In vitro and in vivo studies. Int
Immunopharmacol. 89:1070792020. View Article : Google Scholar
|
|
16
|
Huang X, Xi Y, Mao Z, Chu X, Zhang R, Ma
X, Ni B, Cheng H and You H: Vanillic acid attenuates cartilage
degeneration by regulating the MAPK and PI3K/AKT/NF-κB pathways.
Eur J Pharmacol. 859:1724812019. View Article : Google Scholar
|
|
17
|
Nieminen R, Korhonen R, Moilanen T, Clark
AR and Moilanen E: Aurothiomalate inhibits cyclooxygenase 2, matrix
metalloproteinase 3, and interleukin-6 expression in chondrocytes
by increasing MAPK phosphatase 1 expression and decreasing p38
phosphorylation: MAPK phosphatase 1 as a novel target for
antirheumatic drugs. Arthritis Rheum. 62:1650–1659. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon TC, Murakami M, Kudo I, Son KH, Kim
HP, Kang SS and Chang HW: A new class of COX-2 inhibitor,
rutaecarpine from Evodia rutaecarpa. Inflamm Res. 48:621–625. 1999.
View Article : Google Scholar
|
|
19
|
Zhang Y, Yan T, Sun D, Xie C, Wang T, Liu
X, Wang J, Wang Q, Luo Y, Wang P, et al: Rutaecarpine inhibits
KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran
sulfate sodium-induced colitis. Free Radic Biol Med. 148:33–41.
2020. View Article : Google Scholar :
|
|
20
|
Woo HG, Lee CH, Noh MS, Lee JJ, Jung YS,
Baik EJ, Moon CH and Lee SH: Rutaecarpine, a quinazolinocarboline
alkaloid, inhibits prostaglandin production in RAW264.7
macrophages. Planta Med. 67:505–509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jayakumar T, Lin KC, Chang CC, Hsia CW,
Manubolu M, Huang WC, Sheu JR and Hsia CH: Targeting MAPK/NF-κB
Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on
Src/FAK-Mediated Macrophage Migration. Int J Mol Sci. 23:2021.
View Article : Google Scholar
|
|
22
|
Guo B, Zhao C, Zhang C, Xiao Y, Yan G, Liu
L and Pan H: Elucidation of the anti-inflammatory mechanism of Er
Miao San by integrative approach of network pharmacology and
experimental verification. Pharmacol Res. 175:1060002022.
View Article : Google Scholar
|
|
23
|
Han M, Hu L and Chen Y: Rutaecarpine may
improve neuronal injury, inhibits apoptosis, inflammation and
oxidative stress by regulating the expression of ERK1/2 and
Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion
injury. Drug Des Devel Ther. 13:2923–2931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukuma Y, Sakai E, Komaki S, Nishishita K,
Okamoto K and Tsukuba T: Rutaecarpine attenuates osteoclastogenesis
by impairing macrophage colony stimulating factor and receptor
activator of nuclear factor κ-B ligand-stimulated signalling
pathways. Clin Exp Pharmacol Physiol. 45:863–865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing S, Wan J, Wang T, He Z, Ding Q, Sheng
G, Wang S, Zhao H, Zhu Z, Wu H and Li W: Flavokawain A alleviates
the progression of mouse osteoarthritis: An in vitro and in vivo
study. Front Bioeng Biotechnol. 10:10717762022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
John SP, Singh A, Sun J, Pierre MJ,
Alsalih L and Lipsey C: Small-molecule screening identifies Syk
kinase inhibition and rutaecarpine as modulators of macrophage
training and SARS-CoV-2 infection. Cell Rep. 41:1114412022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAlindon T: Osteoarthritis research
society international (OARSI) Classification and Guidelines. HSS J.
8:66–67. 2012. View Article : Google Scholar :
|
|
29
|
Sun K, Luo J, Jing X, Xiang W, Guo J, Yao
X, Liang S, Guo F and Xu T: Hyperoside ameliorates the progression
of osteoarthritis: An in vitro and in vivo study. Phytomedicine.
80:1533872021. View Article : Google Scholar
|
|
30
|
Lepetsos P, Papavassiliou KA and
Papavassiliou AG: Redox and NF-κB signaling in osteoarthritis. Free
Radic Biol Med. 132:90–100. 2019. View Article : Google Scholar
|
|
31
|
Maki K, Nava MM, Villeneuve C, Chang M,
Furukawa KS, Ushida T and Wickstrom SA: Hydrostatic pressure
prevents chondrocyte differentiation through heterochromatin
remodeling. J Cell Sci. 134:jcs2476432021. View Article : Google Scholar :
|
|
32
|
Attur MG, Dave MN, Clancy RM, Patel IR,
Abramson SB and Amin AR: Functional genomic analysis in
arthritis-affected cartilage: Yin-yang regulation of inflammatory
mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J
Immunol. 164:2684–2691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haugh MG, Vaughan TJ and Mcnamara LM: The
role of integrin α(V)β(3) in Osteocyte mechanotransduction. J Mech
Behav Biomed Mater. 42:67–75. 2015. View Article : Google Scholar
|
|
34
|
Wang Q, Onuma K, Liu C, Wong H, Bloom MS,
Elliott EE, Cao RR, Hu N, Lingampalli N, Sharpe O, et al:
Dysregulated integrin alphaVbeta3 and CD47 signaling promotes joint
inflammation, cartilage breakdown, and progression of
osteoarthritis. JCI Insight. 4:e1286162019. View Article : Google Scholar
|
|
35
|
Wang Z, Boyko T, Tran MC, LaRussa M,
Bhatia N, Rashidi V, Longaker MT and Yang GP: DEL1 protects against
chondrocyte apoptosis through integrin binding. J Surg Res.
231:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu R, Yu X, Liang S, Cheng P, Wang Z, He
ZY, Lv ZT, Wan JL, Mo H, Zhu WT and Chen AM: Physalin A Inhibits
MAPK and NF-κB signal transduction through integrin αVβ3 and exerts
chondroprotective effect. Front Pharmacol. 12:7619222021.
View Article : Google Scholar
|
|
37
|
Cheng C, Tian J, Zhang F, Deng Z, Tu M, Li
L, Yang H, Xiao K, Guo W, Yang RQ, et al: WISP1 protects against
chondrocyte senescence and apoptosis by regulating αvβ3 and
PI3K/Akt pathway in osteoarthritis. DNA Cel Biol. 40:629–637. 2021.
View Article : Google Scholar
|
|
38
|
Emami A, Tepper J, Short B, Yaksh TL,
Bendele AM, Ramani T, Cisternas AF, Chang GH and Mellon RD:
Toxicology evaluation of drugs administered via uncommon routes:
Intranasal, intraocular, intrathecal/intraspinal, and
intra-articular. Int J Toxicol. 37:4–27. 2018. View Article : Google Scholar
|