Introduction
Ulcerative colitis (UC), one of the two major types
of inflammatory bowel disease (IBD), is widespread globally and
poses notable health risks and economical burdens to human society
(1). It was estimated that
ulcerative colitis affected ~5 million individuals globally, with
its incidence increasing globally (2). Patients with UC generally present
with symptoms such as abdominal pain, bloody stool, weight loss and
enterorrhea (3). Although the
etiology of UC has yet to be fully elucidated yet, there are
intrinsic connections between the pathogenesis and progression of
UC and environment factors, intestinal barrier dysfunction and
immune system disorder (4,5).
Existing pharmaceutical strategies for treatment of UC have
principally focused on controlling the aggravated host immune
response in a broad or selective manner. Administration of
biological agents, salicylates and immunomodulators results in a
limited remission rate and serious side effects, including
potential liver and kidney damage, in clinical practice (6). Therefore, it is imperative to
identify novel strategies to intervene with UC and seek therapeutic
agents with superior therapeutic efficacy.
Numerous natural products have therapeutic potential
against UC and side effects are comparatively low (7,8).
Curculigoside (CUR), a phenolic glycoside component of Curculigo
orchioides Gaertn, has been reported to alleviate the symptoms
of UC (9). The aim of the present
study was to elucidate the mechanisms underlying the anti-oxidative
stress and anti-UC effects caused by CUR.
Oxidative stress is associated with a number of
diseases including UC (10).
Oxidative stress results from persistent inflammation, in which
high levels of reactive oxygen species (ROS) are produced from a
variety of cell types (11). When
the production of ROS surpasses the rate at which bowel tissue can
eliminate them, ROS residues bind to specific receptors or
signaling molecules associated with inflammation or cell death,
including NF-κB and caspase-dependent pathways, therefore worsening
inflammation (12). This, in
turn, induces apoptosis of the intestinal epithelium, causing bowel
damage. Development of new means of preventing the bowel from being
'attacked' by ROS, ROS-activated cells and inflammatory cytokines
secreted by activated cells during progression of UC may offer
novel therapeutic strategies.
Nrf-2 is a promising target that may fulfill such an
aim (13). As a crucial
transcription factor, Nrf-2 maintains homeostasis at both the
tissue and cellular levels by upregulating antioxidant genes
(14). Attenuating the production
of ROS, with the subsequent decrease in pro-inflammatory cytokine
secretion in IBD, has been found to result from activation of Nrf-2
signaling (15). In addition, the
role of autophagy in tissue protection and damage prevention is
increasingly recognized and activation of autophagy has been
developed as a strategy for treatment of various types of disease,
such as cancer, polycystic ovary syndrome and osteoarthritis, among
others (16-19). However, the underlying mechanism
and pathway by which autophagy is activated have yet to be fully
elucidated. The present study aimed to explore whether CUR induces
autophagy to protect the intestine/colon.
Kelch-like ECH-associated protein 1 (Keap1) is the
predominant suppressor protein of Nrf-2. The inhibition of Keap1
activity decreases the rate of degradation of Nrf-2 by the
ubiquitin proteasome system, resulting in accretion of newly
synthesized Nrf-2 and its translocation into the nucleus, where it
triggers transcription of a series of antioxidant and
cytoprotective genes, leading to the activation of cellular defense
systems (20). However, the
development of UC is associated with downregulation of Keap1/Nrf-2
signaling along with downstream target genes. For example, in the
presence of ROS or oxidative stress, the Keap1-Nrf-2 complex
sequesters inactivated Nrf-2 molecules in the cytoplasm, which are
translocated to the nucleus (21). Subsequently, expression of
antioxidant genes is regulated through binding of the complex to
antioxidant response elements. This signal transduction process
suggests that the Keap1/Nrf-2 pathway is a potential target for UC
treatment (22), activation of
which may prevent the detrimental effects caused by of ROS on
intestinal epithelium.
The present study aimed to investigate the
protective function, as well as the underlying mechanisms, of CUR
on UC.
Materials and methods
Chemicals and reagents
CUR (purity, ≥98%) was purchased from
MedChemExpress. Fluorescein (FITC)-conjugated Affinipure donkey
anti-goat IgG (cat. no. SA 079103-719) was purchased from
MilliporeSigma. Zonula occludens-1 (ZO-1), occludin, Nrf-2,
microtubule-associated protein 1A/1B-light chain 3 (LC3), P62 and
Beclin 1 antibodies were purchased from Cell Signaling Technology,
Inc. Dextran sulfate sodium (DSS) was purchased from MP
Biomedicals, LLC.
Experimental animals
A total of 30 male C57BL/6 mice (6 weeks, 20±2 g)
and 5 male Nrf-2−/− (knockout) mice (6 weeks,
20±2 g) were purchased from Labbio Biotechnology Co., Ltd. and
housed at Nanjing University of Chinese Medicine. The mice were
kept under conditions of 23±1°C temperature, relative humidity 50
to 60%, and a light-dark cycle of 12 h and had ad libitum access to
water and food. The animal experiments were approved by the Ethics
Committee of Nanjing University of Chinese Medicine (approval no.
2022042501). Following 1 week acclimatization, the mice were
randomly divided into the following groups (n=6 per group):
Control, model (3% DSS), low- and high-dose CUR and positive
control. Mice in the model group were fed 3% DSS for 8 days.
C57BL/6 and Nrf-2−/− mice in the low- and
high-dose CUR treatment groups were administered CUR
intragastrically at doses of 10 and 20 mg/kg/day, respectively, for
8 days. The positive control group was administered oral dose of
260 mg/kg/d of 5-aminosalicylic acid (5-ASA, MedChemExpress) for 8
days. The control group of mice intragastrically received an
equivalent volume of normal saline. On day 9, mice were
anaesthetized by intraperitoneal injection of sodium pentobarbital
(50 mg/kg) and executed sacrificed by cervical dislocation.
Measurement of disease activity index
(DAI)
The DAI score was calculated as previously described
(23). In summary, the following
parameters were employed: Body weight loss (0 points, none; 1,
<5%; 2, 5-10%; 3, 11-20% and 4, >20%), hematochezia (0
points, no bleeding; 2, slight bleeding and 4, noticeable bleeding)
and diarrhea (0 points, normal stool; 2, loose stool and 4, watery
diarrhea). The DAI score, which ranges from 0 to 12, was derived by
combining the values of these three indicators.
Histopathological analysis
The colon tissue of mice was fixed using 4%
paraformaldehyde solution at room temperature for 24 h and
subsequently embedded in paraffin. The sections (5 μm) were
stained with hematoxylin for 5 min and eosin for 2 min (H&E) at
room temperature. Finally, histopathological changes in the colon
were observed under a fluorescence microscope (Olympus,
magnification, ×4).
Immunofluorescence analysis
The colon tissue or cells was fixed in 4%
paraformaldehyde at room temperature for 24 h. Subsequently, it was
embedded in paraffin and cut into 5 μm sections. The
paraffin-embedded sections were subjected to dewaxing process,
involving xylene, anhydrous ethanol, 90% ethanol, 75% ethanol, and
50% ethanol. Subsequently, they were immersed in an antigen
retrieval solution and a membrane permeabilization solution (cat.
C1035, Solarbio Life Sciences). Finally, the sections were sealed
using a neutral resin (cat. no. G8590, Solarbio Life Sciences).
Slides were incubated with 5% bovine serum albumin (BSA, Dalian
Meilun Biotechnology Co., Ltd.) at 37°C for 1 h and subsequently
with primary antibodies targeting ZO-1 (1:200, #13663, Cell
Signaling Technology) overnight at 4°C. Diluted secondary
antibodies goat anti-rabbit IgG, HRP (H+L; 1:500, cat. no. A11036,
Thermo Fisher Scientific) were then added to the slides for 1 h at
room temperature. Add ProLong® Gold Antifade Reagent
with DAPI (#8961, Cell Signaling Technology) or Hoechst (#62249,
Thermo Fisher Scientific), and incubate at room temperature in a
light-protected environment for 10 min. After staining, the slides
were observed under an inverted fluorescence microscope (Olympus,
magnification, ×4).
Permeability assay in vivo
To assess colonic permeability, FITC-dextran was
employed. C57BL/6 mice received intragastric administration of
FITC-dextran at a dose of 60 mg/100 g, and blood samples were
collected 4 h later. The blood was left to stand in the dark for 2
h, followed by centrifugation at 1,000 g for 15 min under 4°C.
Serum samples were collected and the concentration of FITC-dextran
in the serum was detected at 530 nm with an excitation wavelength
of 485 nm using a microplate reader.
Western blotting
Cells and tissue lysate were prepared in RIPA
(Dalian Meilun Biotechnology Co., Ltd.) buffer supplemented with a
proteinase inhibitor. Nuclear and cytosolic proteins were isolated
following the manufacturer's instructions, utilizing the nuclear
and cytosolic protein extraction kit provided by Thermo Fisher
Scientific. Protein concentrations were determined using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology). Proteins
samples (30 μg/lane) were separated by SDS-PAGE (12.5%) and
electroblotted onto PVDF membranes. After blocking the membranes
with 5% BSA at room temperature for 1 h, membranes were
subsequently incubated with primary antibody ZO-1 (1:1,000, #13663,
Cell Signaling Technology, Inc.), Occludin (1:1,000, #91131, Cell
Signaling Technology), GAPDH (1:1,000, #5174, Cell Signaling
Technology), Nrf2 (1:1,000, #12721, Cell Signaling Technology),
Lamin B1 (1:1,000, #13435, Cell Signaling Technology) at 4°C
overnight. The membranes were then washed with TBST buffer (1%
Tween-20 used in TBST) three times and incubated for 1 h with
secondary antibody HRP Goat Anti-Rabbit IgG (1:5,000, 9300014001,
ABclonal Technology) at room temperature. Samples were washed again
with TBST three times, after signals were detected using an
Enhanced Chemiluminescence kit (WBKLS0100, Millipore) and ImageJ
Software (version no. v1.8.0, National Institutes of Health).
Myeloperoxidase (MPO) assay
The colon tissue was homogenized for 1 min in
ice-cold PBS. Subsequently, homogenates were centrifuged at 10,000
× g for 15 min at 4°C. The supernatant was collected and examined
for MPO activity using the MPO ELISA kit (ab155458, Abcam)
according to the manufacturer's directions.
Measurement of ROS
Flow cytometric analysis was performed for
measurement of ROS levels. Cells were collected, washed with PBS
and subsequently incubated with DCFDA for 30 min at 37°C in
darkness. After washing twice with PBS, FITC fluorescence was
analyzed by flow cytometry (Beckman CytoFlex LX) and FlowJo V.10
software (Beijing Tianyu Rongzhi Software Co.).
Measurement of colonic oxidative
stress
The contents of glutathione (GSH; cat. no.
E-EL-0026c, Elabscience Biotechnology, Inc.), malonaldehyde (MDA,
cat. no. E-EL-0060c, Elabscience Biotechnology, Inc.), superoxide
dismutase (SOD, SBJ-M0412-48T, GoldenRain/sbj) and catalase (CAT,
E-BC-K031-M, Elabscience Biotechnology, Inc.) were measured in
colonic tissue and cell suspensions according to the manufacturer's
instructions in the commercial kit.
Determination of cytokine
concentration
The concentrations of TNF-α and IL-6 and -1β in
mouse colonic tissue homogenates were detected using the respective
ELISA kits (cat. nos. E-EL-M3063, E-MSEL-M0001, E-EL-M0037c,
Elabscience Biotechnology, Inc.), following the manufacturer's
instructions.
Cell culture and treatment
Caco2 human epithelial intestinal adenocarcinoma
cell line was obtained from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences. Cells were cultured in a
5% CO2, 37°C incubator in DMEM (Dalian Meilun
Biotechnology Co., Ltd.) containing 10% fetal bovine serum (Gibco),
100 μg/ml streptomycin and 100 U/ml penicillin. For the
H2O2-induced oxidative stress experiment,
Caco2 cells were pre-treated with CUR (0, 30, 40 μM) for 20
h at 37°C incubator, followed by treatment with 100 μM
H2O2 for 4 h to induce cellular oxidative
stress.
Measurement of transepithelial electrical
resistance (TEER)
TEER of the Caco-2 cell monolayer was evaluated
using the EVOM volt-ohm meter (World Precision Instruments) as
previously described (24).
Briefly, Caco2 cells (1×105 cells/ml) were cultured at
37°C in 12-well Transwell filters with a 0.4 μm pore size
for 21 days. Cells were exposed to 20 ng/ml TNF-α for 24 h at 37°C.
TEER value was determined using the EVOM volt-ohm meter.
Luciferase gene reporter assay
pGL-ARE-reporter (5′-CGA GGA TAT TCT AGC TTG GAA ATG
ACA TTG CTA ATG GTG ACA AAG CAA CTT TTA GCT TGG AAA TGA CAT TGC TAA
TGG TGA CA A AGC A AC TCA AGA TCTG-3′) and pCWV-renilla luciferase
vector (5′-CTA GCA AAA TAG GCT GTC CC-3′) were purchased from
Genecopoeia Company. 293 cells (Cell Bank, Chinese Academy of
Sciences) (1×104) were seeded into 96-well plates for 12
h at 37°C. Subsequently, plasmid was transfected using
Lipofectamine™ 3000 reagent (Thermo Fisher Scientific, Inc.). At 24
h post-transfection, the cells were treated with 10-40 μM
CUR for 24 h. The luminescence was quantified and normalized using
Nano-Glo Dual-Luciferase Reporter Assay (Promega Corporation). The
luciferase activity was assessed with a Double-Luciferase Reporter
Assay kit, purchased from Promega Biotech Co., Ltd., using the
Dual-Light Chemiluminescent Reporter Gene Assay System, which was
normalized to firefly luciferase activity.
Cellular thermal shift assay (CETSA)
Cells were treated with DMSO (3 μl) and CUR
(100 μM) for 1 h at 37°C and subsequently subjected to a
temperature gradient (43, 46, 49, 52°C) for 3 min for each step.
After heat treatment, cells were lysed (PBS supplemented with
protease inhibitor (1:100, cat. no. 87785, Thermo Fisher
Scientific, Inc.) and the soluble fraction was separated by
centrifugation at 16,000 × g for 10 min at 4°C. The lysate was
analyzed by western blotting to detect the Keap1 as aforementioned.
Protein levels were quantified and the resulting melting curves
were used to determine the interaction between the target and the
compound in living cells.
Small interfering (si)RNA knockdown
siNrf2 (5′-GAC ATG GAT TTG ATT GAC ATA CT-3′) and
scrambled siRNA negative control (siNC; 5′-CAC TTG AAT CCG ACG GAT
TT G CA-3′) were purchased from Shanghai GenePharma Co., Ltd. Caco2
cells were transfected with siNrf-2 or siNC (100 nM) for 24 h at
37°C using Lipofectamine™ 2000 transfection reagent (Thermo Fisher
Scientific). On the following day, cells were exposed to the
subsequent treatments. Following CUR treatment, protein expression
of the siRNA target was detected by western blotting, as
aforementioned.
Crypt isolation and organoid culture
Crypt isolation was performed as previously descried
(25). In brief, crypts were
obtained from the murine small intestine by incubation for 30 min
at 4°C in Dulbecco's Phosphate-Buffered Saline (D-PBS) containing
3.3 mM EDTA (Dalian Meilun Biotechnology Co., Ltd.). Isolated
crypts were embedded in Matrigel, placed in 48-well culture plate
and cultured in IntestCult™ OGM Mouse Basal Medium (Stemcell
Technologies, Inc.) at 37°C for 7 days.
Molecular docking assay
The 3D structure of Keap1-Kelch protein complex (ID
no. 3WN7) was retrieved from the Research Collaboratory for
Structural Bioinformatics Protein Data Bank database (https://www.rcsb.org/). Autodock Tools version 4.2.6
(https://autodock.scripps.edu/) was used
to preprocess initial protein structure, which involved removing
water molecules, adding hydrogen atoms and exporting the structure
to a pdbqt file for docking. The 2D structure of CUR was obtained
from the PubChem database (pubchem.ncbi.nlm.nih.gov/) and converted into a Mol2
file using OpenBabel 2.3.1 (http://openbabel.org/wiki/Main_Page) software.
Subsequently, hydrogen atoms were added, the charge was
self-distributed and torsional construction detection was performed
on the CUR structure. Docking was performed using Autodock Tools
and the results were optimized using the PyMOL Molecular Graphics
System software 2.4 (pymol.org/2/).
Microscale thermophoresis (MST)
assay
CUR was diluted (1×10−9,
4×10−9, 7×10−9, 10−8,
4×10−8, 7×10−8, 10−7,
4×10−7, 7×10−7, 10−6,
4×10−6, 7×10−6, 1×10−5,
4×10−5 M) using standard buffer (Tris/HCl, pH 7.4) and
combined with the recombinant Keap1 protein solution (Thermo Fisher
Scientific) in a 1:1 ratio. Following incubation at room
temperature for 15 min, all samples were loaded into standard glass
capillaries and analyzed using MST Monolith NT.115 instrument
(NanoTemper Technologies GmbH) at 25°C with a laser and LED power
set at 40%. The Nanotemper Analysis software (NanoTemper
Technologies GmbH; version no.PR.48) was employed to calculate the
dissociation constant (Kd) values.
Statistical analysis
Data are presented as the mean ± SD (n=6).
Statistical analysis was performed using GraphPad Prism software
9.0 (Dotmatics). One-way ANOVA was used to analyze data followed by
student-Newman-Keuls (three groups) or Tukey's multiple comparisons
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CUR alleviates DSS-induced colitis in
mice
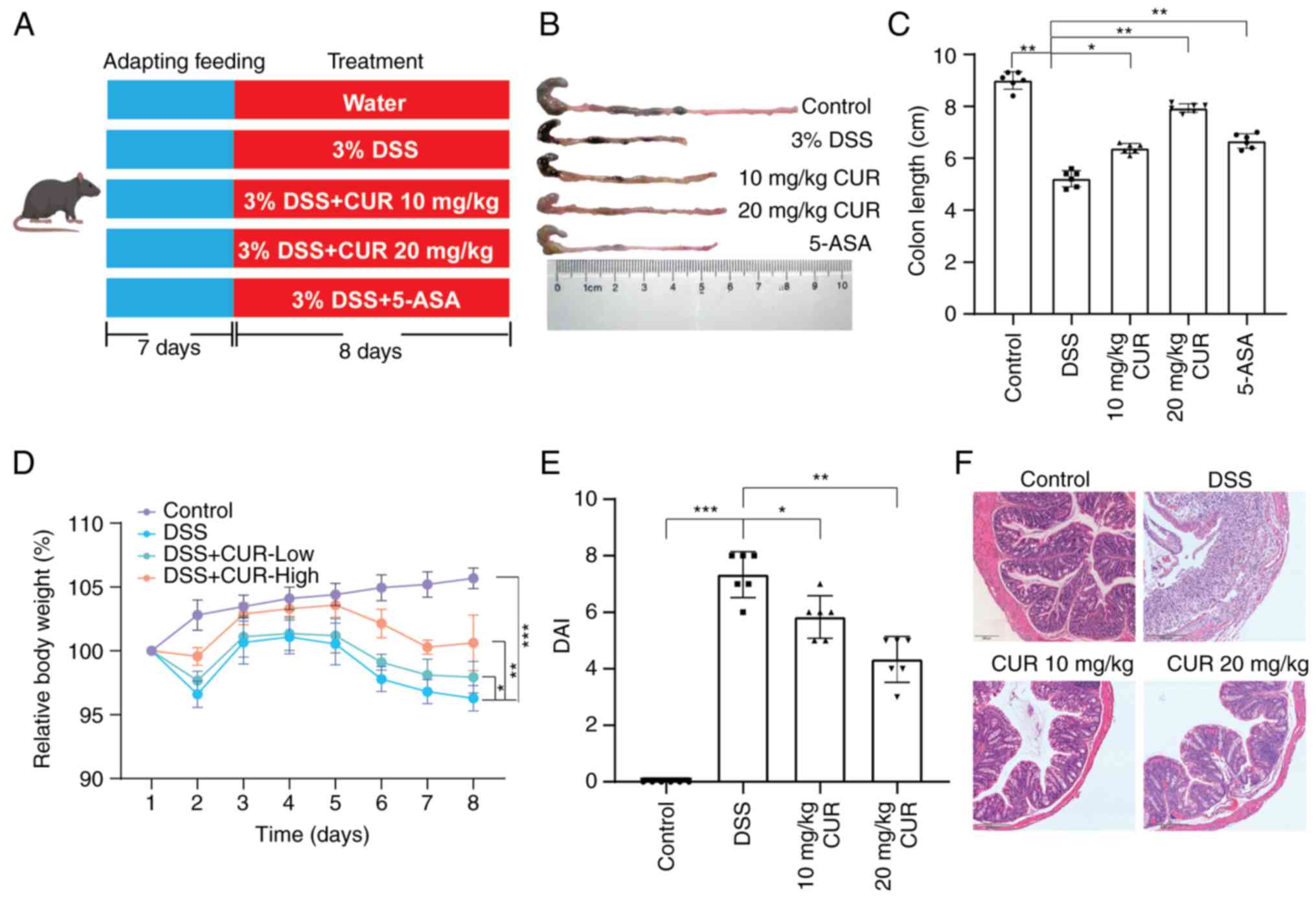
The effects of CUR on attenuating UC were evaluated
using a model of DSS-induced mouse colitis (Fig. 1A). Compared with the control
group, DSS-induced mice had significantly shortened colons,
although these symptoms were attenuated following administration of
CUR and 5-ASA (Fig. 1B and C).
The body weight of mice in the DSS group was significantly
decreased on day 4 compared with the control group. However, in the
CUR treatment and 5-ASA groups, weight loss was reversed on day 6
(Fig. 1D). Additionally, CUR and
5-ASA treatment resulted in attenuation of DSS-induced colonic
tissue lesions, with DAI scores significantly lower compared with
those in the DSS group (Fig. 1E).
Furthermore, the regression of symptoms of colitis, including
tissue damage and the inflammatory infiltration of immune cells,
was observed on the basis of histological staining following CUR
and 5-ASA treatment (Fig. 1F).
These data demonstrated the therapeutic effects of CUR and 5-ASA on
DSS-induced colitis.
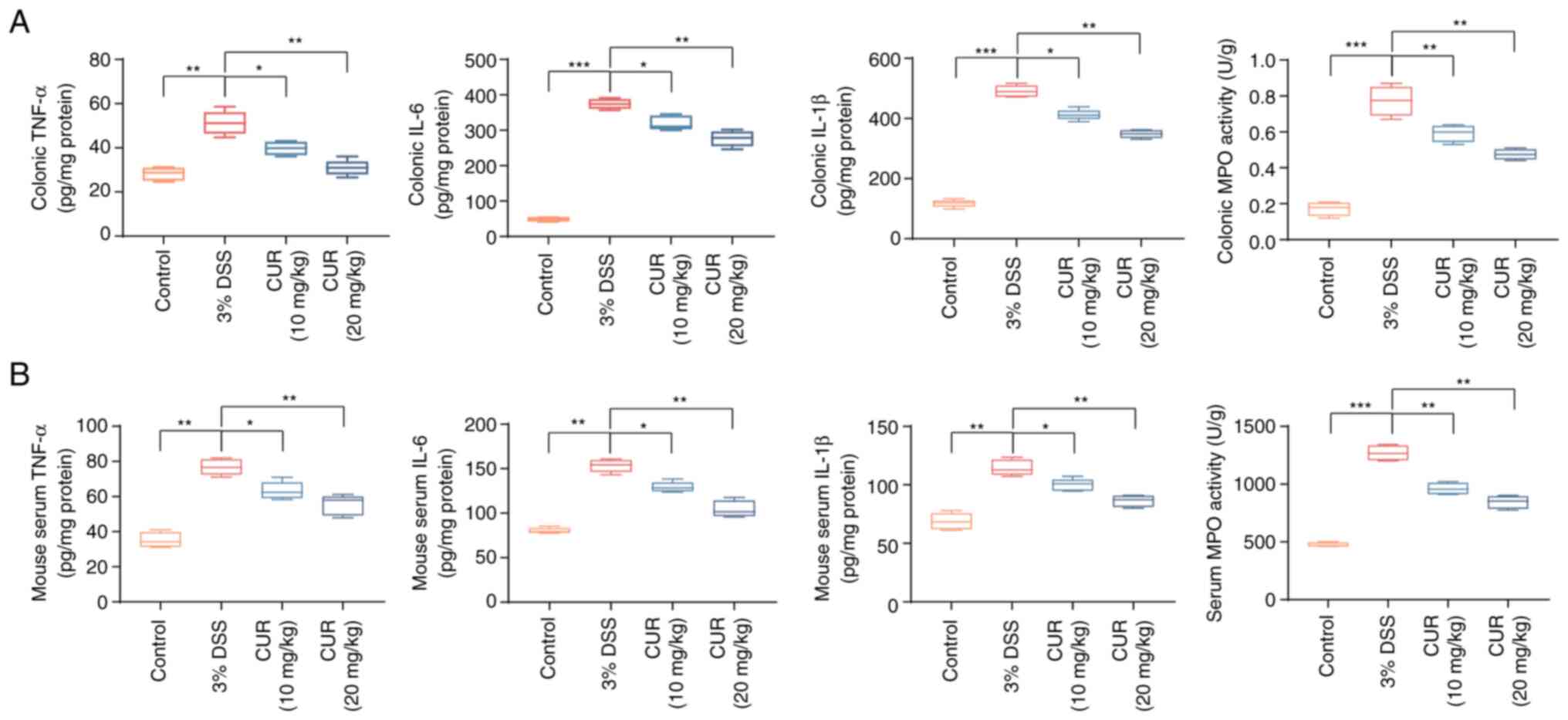
Effect of CUR on inflammation of colonic
tissue
Given the importance of inflammation in the
pathogenesis of UC (26), the
effect of CUR on inflammatory cytokine expression (TNF-α, IL-6 and
IL-1β) in colons and serum of mice with DSS-induced colitis was
explored (Fig. 2A and B).
Although secretion levels of TNF-α, IL-6 and IL-1β were
significantly increased in the DSS group, CUR intervention
significantly reversed this trend and expression levels of these
cytokines returned to normal. MPO activity is a key marker of
neutrophil infiltration into colon tissue (27) and was therefore used to measure
the influence of CUR on the infiltration of neutrophils. MPO
activity in both the colonic tissue and serum from the DSS group
was significantly higher compared with that in the control group.
Following CUR intervention, however, changes in MPO activity were
reversed. In conclusion, taken together, these results suggested
that CUR could effectively decrease the inflammatory response in
DSS-induced mice.
Effect of CUR on intestinal epithelial
barrier damage in vivo and in vitro
The tight junction (TJ) is a key structure
consisting of multiple protein complexes responsible for
maintaining the barrier function of the intestine (28). To determine the role of CUR in the
integrity of the intestinal epithelial barrier, the levels of
TJ-associated proteins ZO-1 and occluding were detected using
western blot and immunofluorescence assay. A significant decrease
in TJ-associated proteins was observed in mouse colon tissue in the
DSS group, although expression levels of these two proteins were
restored in the CUR groups, indicating that CUR was able to restore
the integrity of the intestinal mucosal barrier (Fig. 3A and B). Intragastric
administration of FITC-dextran was used to detect intestinal
permeability (Fig. 3C). CUR
intervention was able to substantially prevent the increase in
serum FITC-dextran in DSS mice.
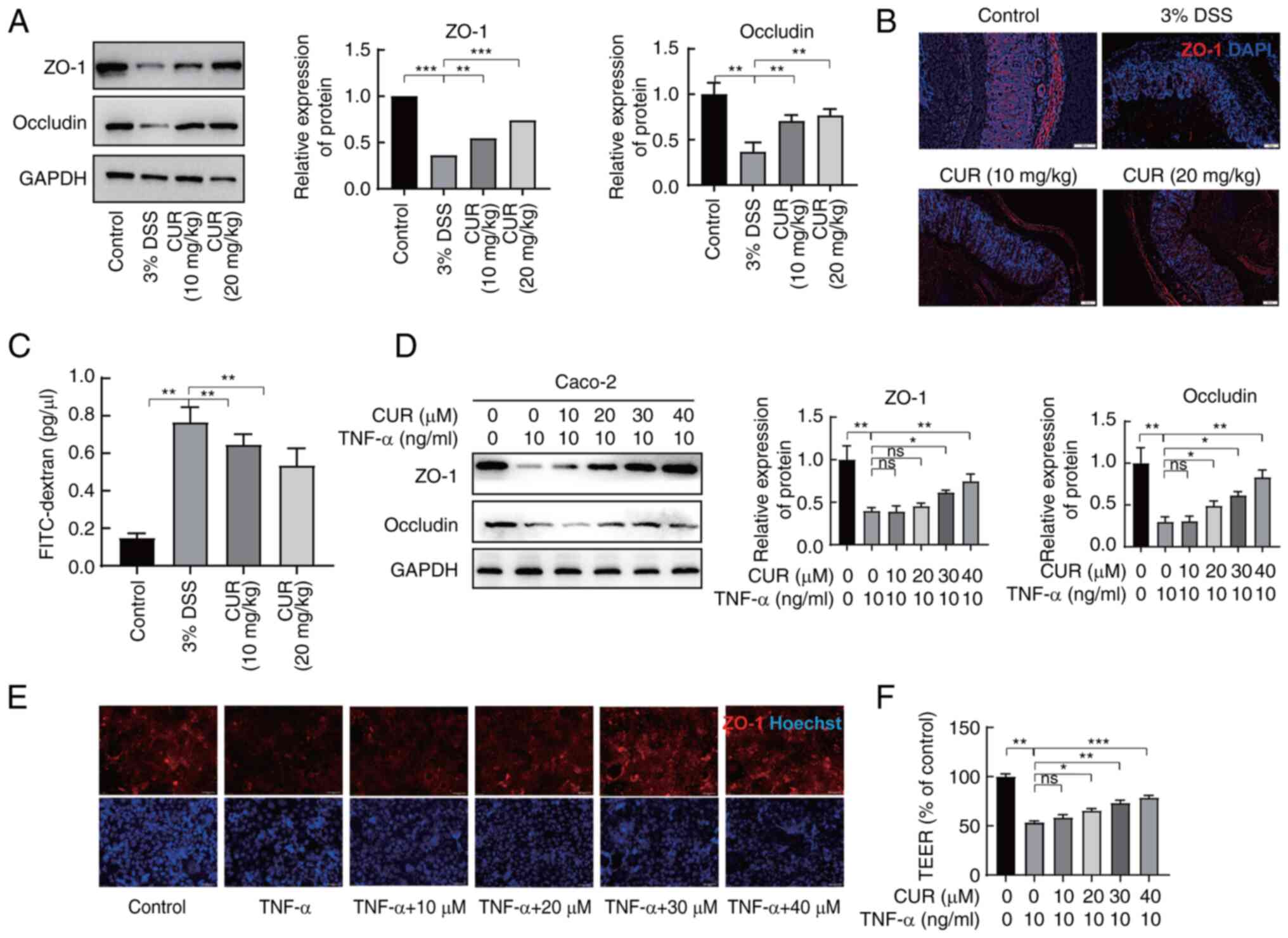 | Figure 3CUR maintains intestinal epithelial
barrier integrity in vivo and in vitro. Expression of
ZO-1 and occludin in colon tissue as determined by (A) western blot
and (B) immunofluorescence assay. Scale bar, 100 μm. (C)
Mice were gavaged with FITC-dextran for 4 h, then serum was
collected and FITC concentrations of each sample was detected. (D)
Caco2 cells were treated with CUR (10, 20, 30, 40 μM) for 4
h followed by TNF-α (100 ng/ml) for 24 h. The protein levels of
ZO-1 and occludin were assessed by western blot. (E) Expression of
ZO-1 in Caco2 cells was evaluated by immunofluorescence assay.
Scale bar, 50 μm. (F) Changes in the TEER value across Caco2
monolayers over time. Cells were treated with CUR prior to TNF-α.
The results are representative of 6 independent experiments and
expressed as the mean ± SD. *P<0.05,
**P<0.01, ***P<0.001. CUR,
curculigoside; ZO-1, Zonula occluden-1; TEER, transepithelial
electrical resistance; DSS, dextran sulfate sodium. |
To explore the effect of CUR on the properties of
the TJ, expression levels of TJ marker proteins were detected and a
cell barrier integrity test was performed on the TNF-α-treated
Caco2 cells (Fig. 3D and E).
TNF-α serves a key role in the inflammatory cascade response that
induces chronic intestinal inflammation in IBD (29). Notable levels of TNF-α were
secreted by mice following DSS administration (Fig. 2A). TEER was used to assess the
barrier function of Caco2 cells (Fig.
3F). CUR led to a significant mitigation of the decrease in
TEER induced by TNF-α. These results suggested that CUR could
potentially rescue damage of intestinal barrier function in UC.
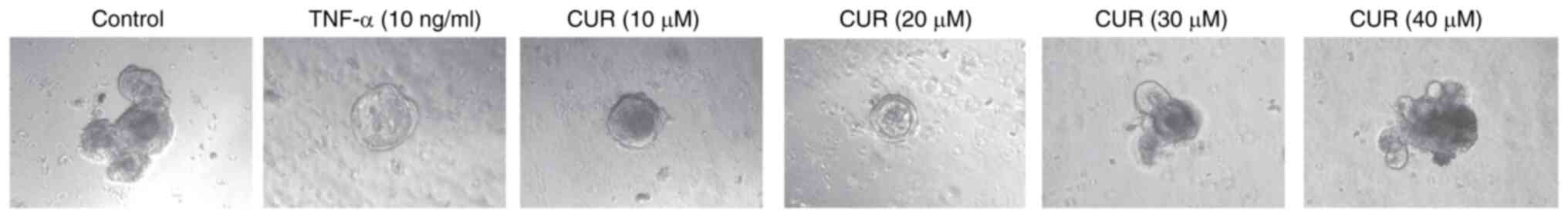
Effect of CUR on intestinal organoids
following TNF-α damage
To explore the effects of CUR intervention on
DSS-induced colitis, co-culture system consisting of intestinal
organoids and CUR was constructed. The decrease in surface area and
morphological protection of intestinal organoids induced by TNF-α
were promoted following addition of CUR (Fig. 4). These results suggested that CUR
promoted intestinal organoid growth.
Effect of CUR on oxidative stress in vivo
and in vitro
The severity of UC depends on degree of antioxidant
defense deficiency in the intestinal mucosa, which counteracts
oxidative stress in the colon that contributes to inflammatory
diseases (30). DSS reduced the
activity of CAT, GSH, and SOD, while increasing the activity of
MAD; however, CUR effectively reversed these trends (Fig. 5A).
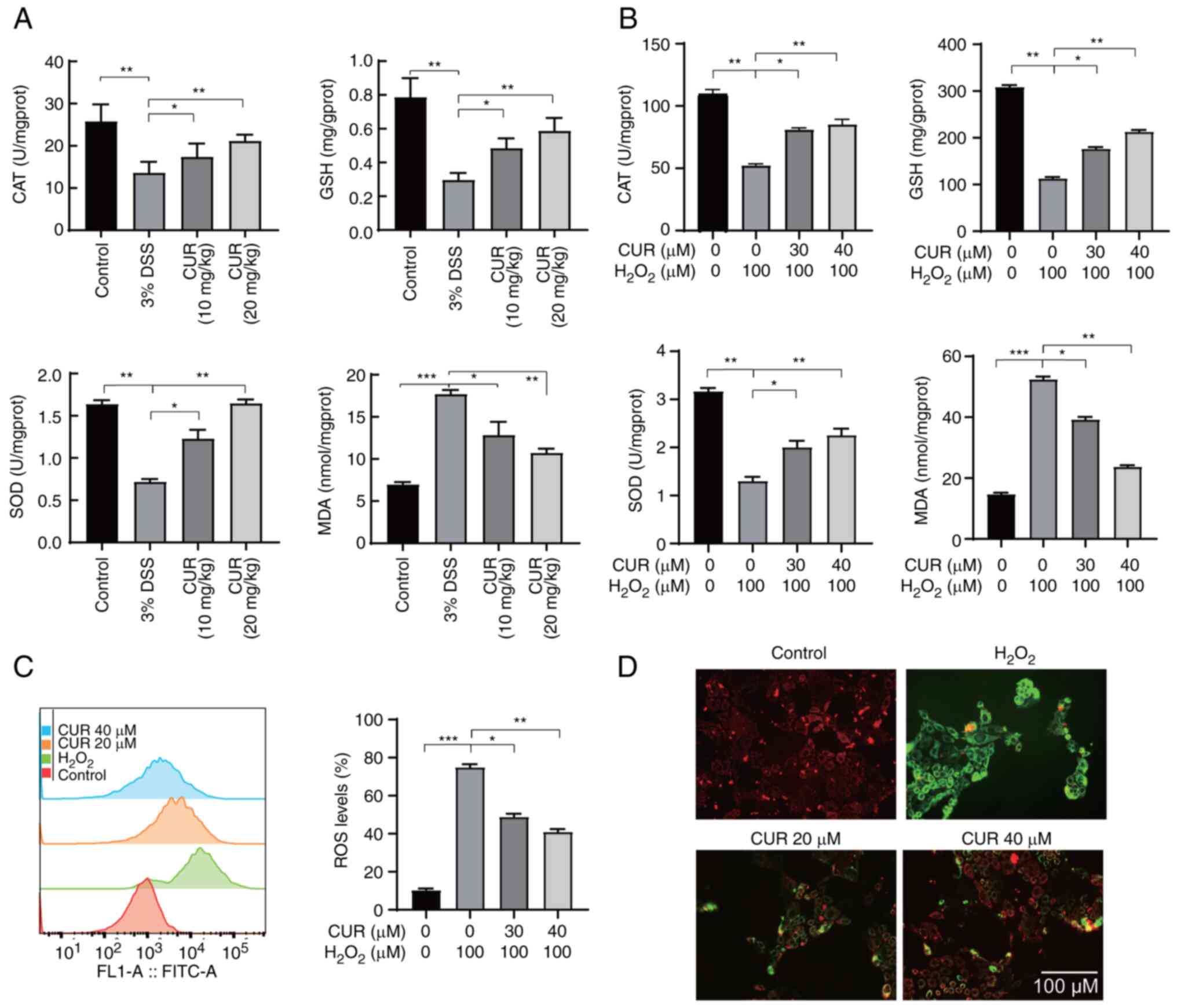 | Figure 5CUR inhibits oxidative stress in
vivo and in vitro. (A) CAT, GSH, SOD and MDA in (A)
mouse colon and (B) cells were detected by corresponding kits. (C)
Caco2 cell ROS levels were measured by DCFHDA ROS assay kit. (D)
Mitochondrial membrane potential was detected using JC-1 probe.
Scale bar, 100 μm. The results are representative of 6
independent experiments and expressed as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001. CUR, curculigoside; CAT, catalase; GSH,
glutathione; SOD, superoxide dismutase; MDA, malondialdehyde; ROS,
reactive oxygen species; DSS, dextran sulfate sodium; prot,
protein. |
Hydrogen peroxide (H2O2) was
used to induce oxidative stress in the Caco-2 cells to examine the
effect and underlying mechanism of CUR intervention on oxidative
stress. Significant decreases in levels of CAT, GSH and SOD were
observed after treating Caco2 cells with 100 μM
H2O2 for 4 h. This trend, however, was
reversed following CUR treatment (Fig. 5B). The levels of MDA and
intracellular ROS were increased following
H2O2 stimulation, although their levels were
significantly decreased in Caco-2 cells that had been pretreated
with CUR for 12 h, indicating the antioxidant activity of CUR
(Fig. 5C). Furthermore, a JC-1
probe was utilized to evaluate the damage caused by matrix
metalloproteinases (MMPs). H2O2 decreased the
level of red fluorescence (JC-1 aggregate), whereas that of green
fluorescence (JC-1 monomer) increased. However, CUR intervention
led to a promotion of the red fluorescence, and a reduction in
green fluorescence (Fig. 5D).
CUR ameliorates oxidative stress in DSS
mice and H2O2-treated Caco-2 cells by
activating Nrf-2
Nrf-2 is a key transcription mediator involved in
oxidative stress (31).
Expression of Nrf-2 in mouse colonic tissue was measured using
immunofluorescence and western blot assay. CUR intervention led to
a significant increase in Nrf-2 (Fig.
6A and B).
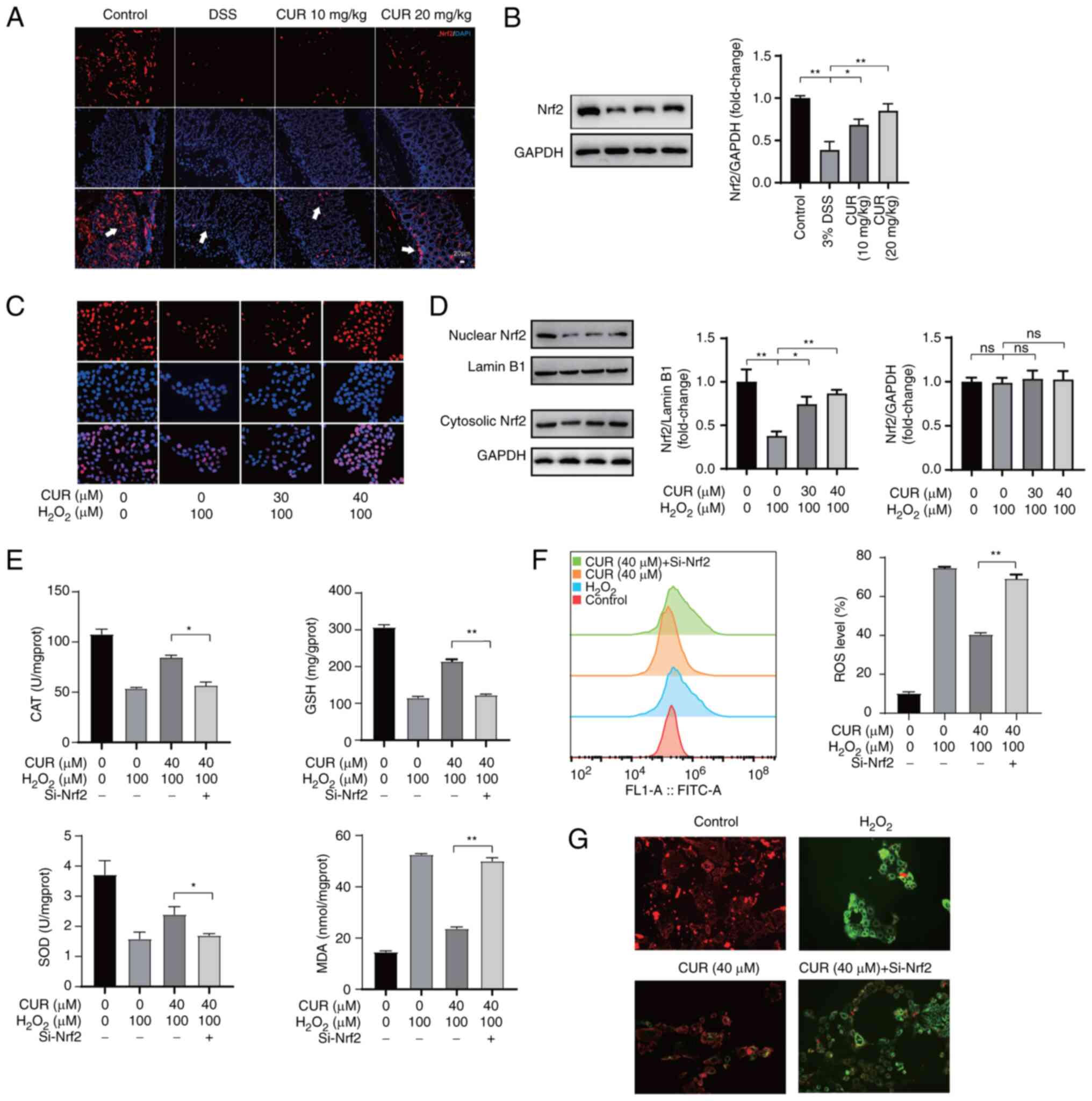 | Figure 6CUR relieves oxidative stress in
DSS-administrated mice and H2O2-treated
Caco-2 cells by activating Nrf-2. (A) Nrf-2 (arrow) distribution in
mouse colon was measured using immunofluorescence. (The arrowhead
represents Nrf-2 expression). Scale bar, 20 μm. (B) Western
blot was used to determine expression of Nrf-2. (C) Nrf-2
distribution in cells was measured using immunofluorescence. Scale
bar, 100 μm. (D) Expression of Nrf-2 in cytosolic and
nuclear extract was determined by western blot. Lamin B1 and GAPDH
were used as nuclear and cytoplasmic markers, respectively (E)
Following Nrf2 siRNA transfection, Caco2 cells were treated with
CUR for 24 h followed by H2O2 stimulation for
4 h. CAT, GSH, SOD and MDA were detected by corresponding kits. (F)
ROS was evaluated by spectrofluorometer. (G) Mitochondrial membrane
potential was determined by JC-1 probe. Scale bar, 100 μm.
The results are representative of 6 independent experiments and
expressed as the mean ± SD. *P<0.05,
**P<0.01. CUR, curculigoside; DSS, dextran sulfate
sodium; si, small interfering; CAT, catalase; GSH, glutathione;
SOD, superoxide dismutase; MDA, malondialdehyde; prot, protein. |
Immunofluorescence assay was employed to detect the
intracellular distribution of Nrf-2 in Caco-2 cells treated with
control vehicle or a combination of H2O2 and
CUR. Nrf2 was evenly localized in the cytoplasm of
H2O2-administered Caco-2 cells, whereas the
intensity of fluorescence was markedly increased in nuclei of cells
treated with CUR (Fig. 6C). The
effect of CUR on distribution of Nrf-2 in Caco-2 cells was further
investigated using western blot assay. Following treating the
Caco-2 cells with H2O2 for 4 h, the nuclear
Nrf-2 levels were decreased, whereas pretreatment with CUR led to
enhanced expression of nuclear Nrf-2 (Fig. 6D).
To determine whether CUR exerts an anti-oxidative
stress effect via Nrf-2, the expression of Nrf-2 was knocked down
by siRNA (Fig. S1). The contents
of GSH, CAT and SOD in the H2O2 + CUR +
siNrf-2 group were markedly decreased compared with those of the
H2O2 + CUR group (Fig. 6E). In addition, compared with the
H2O2 + CUR group, MDA and accumulation of ROS
were found to be markedly increased in the
H2O2 + CUR + siNrf-2 group (Fig. 6F). Consistently, the
H2O2 + CUR + siNrf=2 group showed higher
levels of MMP damage compared with the H2O2 +
CUR group (Fig. 6G).
Collectively, these data suggested that Nrf-2 promoted the
influence of CUR on H2O2-induced oxidative
stress injury.
CUR activates autophagy to protect cells
via Nrf-2
Through the removal of N-acetyl-p-benzoquinone imine
protein adducts or damaged mitochondria, the activation of
autophagy protects tissues or organs from being damaged by
oxidative stress. The present study investigated whether the
presence of CUR could activate autophagy for intestine protection.
The levels of autophagy markers, including LC3I, P62 and beclin-1,
were detected. Under oxidative stress conditions (100 μM
H2O2), LC3I was upregulated in a
dose-dependent manner following administration of CUR, whereas that
of the reference protein, LC3II, remained unchanged (Fig. 7A). The levels of positive
regulator of autophagy beclin-1 followed a similar trend. By
contrast, negative regulator of autophagy P62 was downregulated as
the dose of CUR increased. Furthermore, under oxidative stress
conditions, immunofluorescence staining of LC3 confirmed the
dose-dependent increase in expression with changes in dose of
administered CUR. Collectively, these data demonstrated that CUR
activated autophagy in a dose-dependent manner, as demonstrated by
the upregulation of the positive regulators of autophagy, along
with downregulation of negative regulator of autophagy.
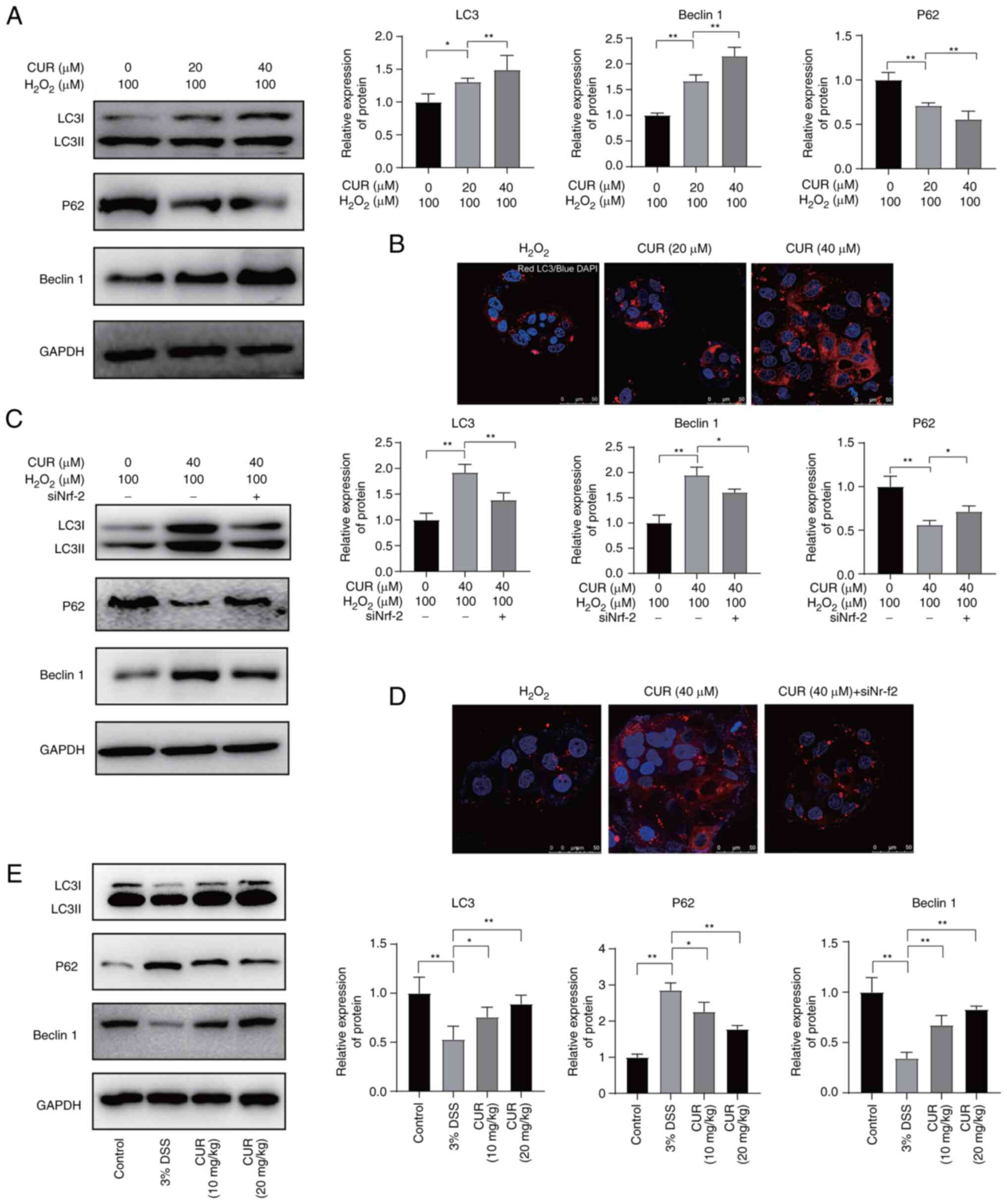 | Figure 7CUR regulates autophagy by activating
Nrf-2. (A) Caco2 cells were treated with H2O2
or CUR + H2O2. Western blot was used to
measure the protein expression of LC3I/II, P62 and Beclin 1. (B)
Immunofluorescence images of LC3I/II. LC3I/II was marked using CY3
red immunofluorescence and nuclei were labeled using Hoechst. Scale
bar, 50 μm. (C) After Nrf2 siRNA transfection, Caco2 cells
were treated with CUR for 24 h followed by
H2O2 stimulation for 4 h. Western blot was
used to measure protein expression of LC3I/II, P62 and Beclin 1.
(D) Immunofluorescence images of LC3I/II. Scale bar, 50 μm.
(E) Protein expression of LC3I/II, P62 and Beclin 1 in mouse
colonic tissue was determined by western blot. The results are
representative of 6 independent experiments and expressed as the
mean ± SD. *P<0.05, **P<0.01. CUR,
curculigoside; si, small interfering; DSS, dextran sulfate
sodium |
To identify through which pathway CUR activated
autophagy of cells, Nrf-2 was silenced by siRNA, which was
hypothesized to be the key protein to be targeted by CUR.
Upregulation of the positive regulators of autophagy LC3 and P62,
as well as downregulation of the negative regulator, beclin-1, were
observed when Nrf-2 was silenced (Fig. 7C), which indicated that autophagy
was initiated by CUR through Nrf-2 signaling under oxidative
stress. Immunofluorescence further confirmed this (Fig. 7D).
Subsequently, in vivo experiments were
performed on in mice with UC. The establishment of UC induced
downregulation of the autophagy-positive regulators LC3 and
beclin-1 and upregulation of the negative regulator P62, suggesting
that autophagy was suppressed (Fig.
7E). By contrast, CUR was found to reverse this trend in a
dose-dependent manner (Fig. 7E),
suggesting that it induced autophagy, thereby protecting intestine
tissue from damage caused by oxidative stress.
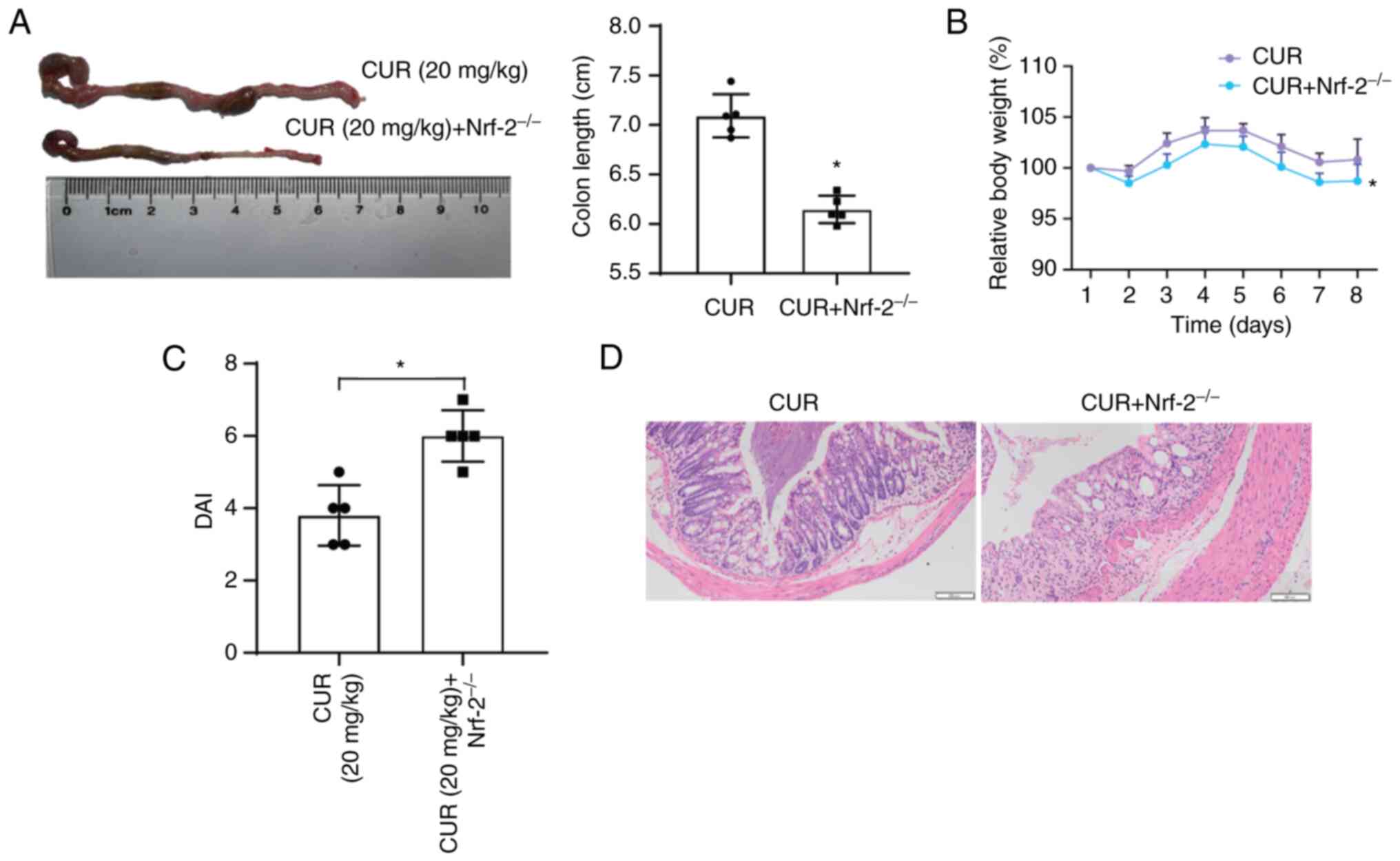
Mitigation of UC by CUR is mediated by
Nrf-2 signaling
To test the hypothesis that mitigation of UC by CUR
is mediated by Nrf-2 signaling, Nrf-2−/−
knockout was induced in C57BL/J mice and the effects on the
amelioration of UC by CUR were explored. The severity of mouse
colon damage worsened significantly with progression of UC when
Nrf-2 was knockout, even in the presence of CUR. (Fig. 8A), along with a marked reduction
in body weight (Fig. 8B). DAI
score and HE staining of the colon indicated that Nrf-2-knockout
mice experienced more severe damage during UC compared with the
normal group (Fig. 8C and D).
Collectively, these data suggested that the CUR protective effects
on the mouse colon were mediated through Nrf-2 signaling.
CUR interferes with the interaction
between Keap1 and Nrf-2 by binding to Keap1 protein
The aforementioned results demonstrated that CUR
activated Nrf-2; therefore, the present study investigated whether
CUR interacts with Keap1, thereby resulting in activation of Nrf-2
signaling. In the present study, CUR interacted with the target
protein Keap1-Kelch. The hydroxyphenyl group of CUR formed hydrogen
bonds with Val561 and Thr560 of Keap1, whereas the glucopyranoside
group established hydrogen bonded with Val418 and Ile559.
Additionally, the dimethoxybenzyl and hydroxyphenyl groups of CUR
participated in π-alkyl interactions with Val467 and Cys513 of
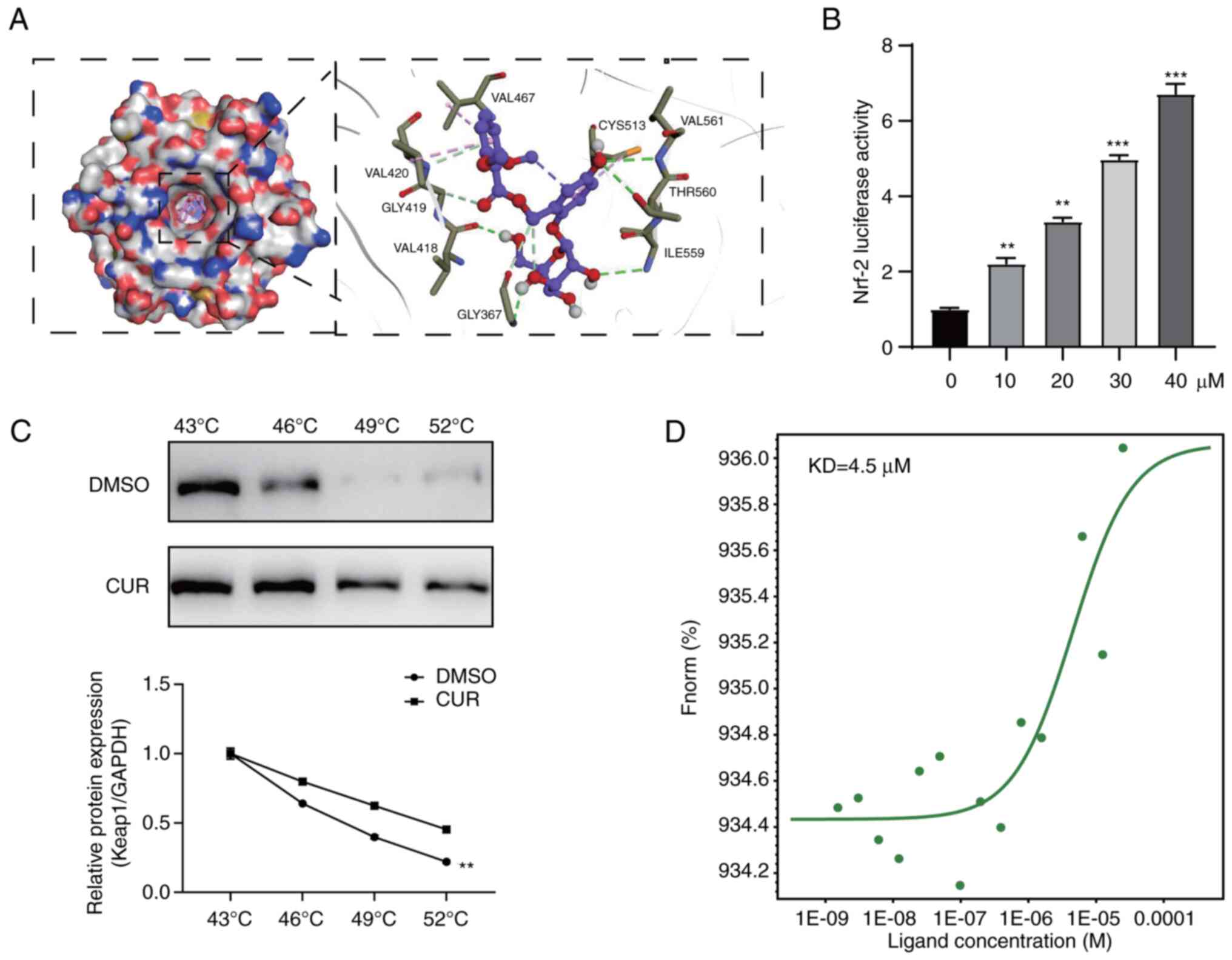
Keap1, respectively (Fig. 9A).
The binding energy of CUR with Keap1-Kelch-8.901 kcal/mol,
indicating a stable interaction between the ligand and protein
(Fig. 9A). In addition,
luciferase reporter gene assay demonstrated that CUR activated the
Nrf2, which is involved in activation of downstream antioxidant
enzymes (Fig. 9B). CETSA was
performed, which enables detection of the binding of small
molecules to target proteins. This analysis demonstrated that the
thermal stability of CUR was promoted in living Caco2 cells in the
temperature range 43-52°C (Fig.
9C). Finally, the direct interaction between CUR and Keap1 was
investigated using MST. Upon calibrating the binding curve using
the reference compound (Fig. 9D),
Kd of CUR was determined to be 4.5 μM,
suggesting a strong interaction between CUR and Keap1. Taken
together, this revealed that CUR directly bound to Keap1 to induce
Nrf-2 activation.
Discussion
UC, one of the two primary types of IBD (the other
being Crohn's disease), has been recognized as a major concern for
health, resulting in major social-economic burdens for human
society (32). Although
conventional treatment strategies that involve suppressing
inflammatory responses via administration of antibiotics are
available, the limited cure rate and serious side effects
associated with these therapies make the development of novel and
potent alternative treatment strategies for UC an urgent need.
The present study investigated the effects of CUR in
terms of relieving the symptoms of chronic colitis in a mouse UC
model. Consistent with these findings, the inflammation of mice
with chronic colitis was alleviated through administering CUR, as
demonstrated by downregulated expression of a number of
inflammatory cytokines, including TNF-α, IL-6 and IL-1β, decreased
neutrophil infiltration and downregulated MPO activity.
Furthermore, CUR helped to maintain the barrier functions of the
intestinal epithelium. In addition, in vitro organoid and
Caco2 cell experiments demonstrated the capabilities of CUR in
terms of rescuing cells from oxidative stress. CUR could promote
the autophagy of cells under conditions of oxidative stress through
Nrf-2 signaling and autophagy promoted the ability of CUR to
protect the colon tissue from damage by oxidative stress.
Mechanistic studies demonstrated that activation of Nrf-2 served a
pivotal role in inhibition of UC by CUR. This hypothesis was
confirmed using an Nrf-2 knockout mouse and interaction of CUR with
protein Keap1/Kelch. 5-ASA, also known as mesalazine is a
first-line drug for treatment of inflammatory bowel diseases such
as UC, with a high effective rate of induction and maintenance of
remission. It has antioxidant activity and decreases tissue damage.
5-ASA is important for prevention of T cell activation and
proliferation. It negatively regulates the cyclooxygenase and
lipoxygenase pathways and decreases production of prostaglandins
and leukotrienes (33). Therefore
5-ASA was used as a positive drug for comparison.
As a natural phenolic glycoside compound, CUR has
been shown to inhibit the progression of various types of diseases
(34,35); furthermore, it promotes tissue
regeneration, including osteogenesis, and modulates immune
responses (36). In the present
study, the therapeutic effect of CUR on UC via modulating
interaction of Keap1/Nrf-2 in the Nrf-2 signaling pathway was
demonstrated. The results were consistent with those of a previous
study in terms of the protective effects of CUR on UC (9), although the aforementioned study
focused on the protective effects of CUR on ferroptosis in UC.
To the best of our knowledge, the present study is
the first to demonstrate the role of Nrf-2 and Keap1 on the
therapeutic effects of CUR on UC. Keap1 is the primary negative
modulator of Nrf-2, which regulates the steady-state levels of
Nrf-2 under diverse intracellular redox states (37). The role of Nrf-2 activation in
inflammation suppression has been investigated in a variety of
diseases. For example, Huang et al (38) demonstrated the role of Nrf-2
activation in the attenuation of lung injury and resultant
oxidative stress. Wu et al (39) reported that the interaction of
Keap1/Nrf2 with the natural product acacetin alleviates myocardial
ischemia/reperfusion injury. Other examples include the therapeutic
effects of sleep deprivation-induced neuroinflammation (40), cardiovascular injury (41) and different types of cancer
(42,43). These reports demonstrated the
effects of Nrf-2 activation on treatment of diseases associated
with inflammation.
In addition, ROS elimination or depletion stimulates
Nrf-2 activation; this has been investigated in the treatment of
the aforementioned diseases (44). In the present study, DSS led to a
notable decrease in the activities of CAT, GSH and SOD, indicating
the loss of the antioxidant enzyme defense system; CUR, however,
effectively reversed these trends. In addition, compared with the
H2O2 + CUR group, MDA and ROS accumulation
were markedly increased in the H2O2 + CUR +
siNrf-2 group.
To protect the body from peroxidative damage, cells
form a complex antioxidant enzyme defense system, primarily
comprising SOD, CAT and GSH, in which SOD converts toxic superoxide
anion into H2O2 and CAT converts
H2O2 into water. CAT is also able to regulate
levels of H2O2, in addition to acting as a
protective agent of hemoglobin and other sulfhydryl proteins
(45). Reduced GSH is oxidized to
glutathione disulfide (GSSG) and the ratio of GSH to GSSG in cells
provides a measure of cellular oxidative stress (46). In the present study,
downregulation of levels of SOD, CAT and GSH suggested that the
activity of the antioxidant enzyme system induced by bowel
inflammation was suppressed, whereas this trend was reversed upon
administering CUR, indicating recovery of the activity of the
antioxidant enzyme defense system. In addition, MAD is a lipid
peroxidation metabolite commonly used as a marker to measure lipid
peroxidation metabolism in vivo, serving as an indicator to
assess the generation of free radicals and damage they cause to the
structure of the membrane lipid bilayer (47). In the present study,
downregulation of MAD following CUR administration demonstrated
elimination of oxidative stress mediated by CUR that occurred
during inflammation. These data highlighted the therapeutic effect
of CUR in terms of eliminating ROS, suggesting its potential
clinical utility. Future studies should investigate the protective
effects of CUR on local tissue and organs in other types of disease
involving increased oxidative stress either locally or
systematically, regardless of whether oxidative stress acts as the
upstream regulator or downstream effector.
In conclusion, CUR is potentially a potent
therapeutic compound for UC that is able to activate Nrf-2
signaling, as demonstrated both in an animal model and in in
vitro cell and organoid models. The molecular docking
simulation demonstrated that CUR targeted the interaction of Keap1
and Nrf-2. Taken together, these data confirmed the therapeutic
effects of CUR on UC and the underlying mechanism. These findings
may facilitate the clinical application of CUR in UC therapy.
Supplementary Data
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ designed and performed the experiments, analyzed
data and edited the manuscript. DL performed the experiments and
wrote and edited the manuscript. FL designed the experiments and
analyzed data. HH designed and performed the experiments and
analyzed data. PZ and XD designed the experiments. JJ performed the
experiments and wrote and edited the manuscript. All authors have
read and approved the final manuscript. LJ and JJ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Ethics approval for animal experiments was received
from the Experimental Animal Ethics Committee of Nanjing University
Of Chinese Medicine (approval no. 2022053209).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CUR
|
curculigoside
|
|
UC
|
ulcerative colitis
|
|
IBD
|
inflammatory bowel disease
|
|
DSS
|
dextran sulfate sodium
|
|
ROS
|
reactive oxygen species
|
|
Keap1
|
Kelch-like ECH-associated protein
1
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
TJ
|
tight junction
|
|
MST
|
microscale thermophoresis
|
Acknowledgments
Not applicable.
Funding
The present study was supported by Jiangsu Traditional Chinese
Medicine Science and Technology Development Project (grant no.
MS2021058); Natural Science Foundation of Nanjing University of
Chinese Medicine (grant no. XZR2020062); Suzhou Municipal Science
and Technology Bureau Supporting Project (grant no. SKY2022072);
Changshu Municipal Science and Technology Bureau Supporting Project
(grant nos. CS202233 and CS202030) and Open Project of Zhenjiang
Traditional Chinese Medicine Spleen and Stomach Diseases Clinical
Medicine Research Center (grant no. SSPW2022-KF08).
References
|
1
|
Thorsteinsdottir S, Gudjonsson T, Nielsen
OH, Vainer B and Seidelin JB: Pathogenesis and biomarkers of
carcinogenesis in ulcerative colitis. Nat Rev Gastroenterol
Hepatol. 8:395–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le Berre C, Honap S and Peyrin-Biroulet L:
Ulcerative colitis. Lancet. 402:571–584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aarestrup J, Jess T, Kobylecki CJ,
Nordestgaard BG and Allin KH: Cardiovascular risk profile among
patients with inflammatory bowel disease: A population-based study
of more than 100 000 individuals. J Crohns Colitis. 13:319–323.
2019. View Article : Google Scholar
|
|
4
|
Larabi A, Barnich N and Nguyen HTT: New
insights into the interplay between autophagy, gut microbiota and
inflammatory responses in IBD. Autophagy. 16:38–51. 2020.
View Article : Google Scholar :
|
|
5
|
Nikolaus S and Schreiber S: Diagnostics of
inflammatory bowel disease. Gastroenterology. 133:1670–1689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Gao W, Cai X, Xu J, Zou D, Li Z,
Hu B and Zheng Y: Nanozyme-mediated catalytic nanotherapy for
inflammatory bowel disease. Theranostics. 9:2843–2855. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Fu Y, Xu B, Liu C, Wang Q, Luo S,
Nong F, Wang X, Huang S, Chen J, et al: Wogonoside alleviates
colitis by improving intestinal epithelial barrier function via the
MLCK/pMLC2 pathway. Phytomedicine. 68:1531792020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong Y, Liu W, Xiong Y, Li Y, Wan Q, Zhou
W, Zhao H, Xiao Q and Liu D: Astragaloside IV alleviates ulcerative
colitis by regulating the balance of Th17/Treg cells.
Phytomedicine. 104:1542872022. View Article : Google Scholar
|
|
9
|
Wang S, Liu W, Wang J and Bai X:
Curculigoside inhibits ferroptosis in ulcerative colitis through
the induction of GPX4. Life sci. 259:1183562020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elmaksoud HAA, Motawea MH, Desoky AA,
Elharrif MG and Ibrahimi A: Hydroxytyrosol alleviate intestinal
inflammation, oxidative stress and apoptosis resulted in ulcerative
colitis. Biomed Pharmacother. 142:1120732021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roessner A, Kuester D, Malfertheiner P and
Schneider-Stock R: Oxidative stress in ulcerative
colitis-associated carcinogenesis. Pathol Res Pract. 204:511–524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colombo BB, Fattori V, Guazelli CFS,
Zaninelli TH, Carvalho TT, Ferraz CR, Bussmann AJC, Ruiz-Miyazawa
KW, Baracat MM, Casagrande R and Verri WA Jr: Vinpocetine
ameliorates acetic acid-induced colitis by inhibiting NF-κB
activation in mice. Inflammation. 41:1276–1289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torrente L and DeNicola GM: Targeting NRF2
and its downstream processes: Opportunities and challenges. Annu
Rev Pharmacol Toxicol. 62:279–300. 2022. View Article : Google Scholar
|
|
14
|
Liu S, Pi J and Zhang Q: Signal
amplification in the KEAP1-NRF2-ARE antioxidant response pathway.
Redox Biol. 54:1023892022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauer C, Duewell P, Mayer C, Lehr HA,
Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E and Schnurr M:
Colitis induced in mice with dextran sulfate sodium (DSS) is
mediated by the NLRP3 inflammasome. Gut. 59:1192–1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai X, Hua S, Deng J, Du Z, Zhang D, Liu
Z, Khan NU, Zhou M and Chen Z: Astaxanthin activated the Nrf2/HO-1
pathway to enhance autophagy and inhibit ferroptosis, ameliorating
acetaminophen-induced liver injury. ACS Appl Mater Interfaces.
14:42887–42903. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao K, Shi Q, Liu Y and Wang C: Enhanced
autophagy and NFE2L2/NRF2 pathway activation in SPOP
mutation-driven prostate cancer. Autophagy. 18:2013–2015. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Bio.
24:560–575. 2023. View Article : Google Scholar
|
|
19
|
Kumariya S, Ubba V, Jha RK and Gayen JR:
Autophagy in ovary and polycystic ovary syndrome: Role, dispute and
future perspective. Autophagy. 17:2706–2733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Z, Yi W, Tang J, Sun Y, Huang J, Lan
T, Dai X, Xu S, Jin ZG and Wu X: Urolithin A protects against
acetaminophen-induced liver injury in mice via sustained activation
of Nrf2. Int J Biol Sci. 18:2146–2162. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Negrette-Guzmán M, Huerta-Yepez S, Tapia E
and Pedraza-Chaverri J: Modulation of mitochondrial functions by
the indirect antioxidant sulforaphane: A seemingly contradictory
dual role and an integrative hypothesis. Free Radical Bio Med.
65:1078–1089. 2013. View Article : Google Scholar
|
|
22
|
Piotrowska M, Swierczynski M, Fichna J and
Piechota-Polanczyk A: The Nrf2 in the pathophysiology of the
intestine: Molecular mechanisms and therapeutic implications for
inflammatory bowel diseases. Pharmacol Res. 163:1052432021.
View Article : Google Scholar
|
|
23
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corpetti C, Del Re A, Seguella L, Palenca
I, Rurgo S, De Conno B, Pesce M, Sarnelli G and Esposito G:
Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced
cytotoxicity and inflammation through a PPARγ-dependent
TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line.
Phytother Res. 35:6893–6903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geng H, Bu HF, Liu F, Wu L, Pfeifer K,
Chou PM, Wang X, Sun J, Lu L, Pandey A, et al: In inflamed
intestinal tissues and epithelial cells, interleukin 22 signaling
increases expression of H19 long noncoding RNA, which promotes
mucosal regeneration. Gastroenterology. 155:144–155. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Podolsky DK: Inflammatory bowel disease.
New Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Jha R, Li A, Liu H, Zhang Z, Zhang
C, Zhai Q and Zhang J: Probiotics (Lactobacillus plantarum HNU082)
supplementation relieves ulcerative colitis by affecting intestinal
barrier functions, immunity-related gene expression, gut
microbiota, and metabolic pathways in mice. Microbiol Spectr.
10:e1651222022. View Article : Google Scholar
|
|
28
|
Foerster EG, Mukherjee T, Cabral-Fernandes
L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls
the intestinal epithelial barrier. Autophagy. 18:86–103. 2022.
View Article : Google Scholar :
|
|
29
|
Michielan A and D'Incà R: Intestinal
permeability in inflammatory bowel disease: Pathogenesis, clinical
evaluation, and therapy of leaky gut. Mediat Inflamm.
2015:6281572015. View Article : Google Scholar
|
|
30
|
Perico L, Morigi M, Rota C, Breno M, Mele
C, Noris M, Introna M, Capelli C, Longaretti L, Rottoli D, et al:
Human mesenchymal stromal cells transplanted into mice stimulate
renal tubular cells and enhance mitochondrial function. Nat Commun.
8:9832017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Z, Hu B, Zang F, Wang J, Zhang X and
Chen H: Nrf2 drives oxidative stress-induced autophagy in nucleus
pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect
intervertebral disc from degeneration. Cell Death Dis. 10:5102019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghafouri-Fard S, Eghtedarian R and Taheri
M: The crucial role of non-coding RNAs in the pathophysiology of
inflammatory bowel disease. Biomed Pharmacother. 129:1105072020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le Berre C, Roda G, Nedeljkovic PM, Danese
S and Peyrin-Biroulet L: Modern use of 5-aminosalicylic acid
compounds for ulcerative colitis. Expert Opin Biol Ther.
20:363–378. 2020. View Article : Google Scholar
|
|
34
|
Guo H, Zheng L, Guo Y, Han L, Yu J and Lai
F: Curculigoside represses the proliferation and metastasis of
osteosarcoma via the JAK/STAT and NF-κB signaling pathways. Biol
Pharm Bull. 45:1466–1475. 2022. View Article : Google Scholar
|
|
35
|
Han J, Wan M, Ma Z, Hu C and Yi H:
Prediction of targets of curculigoside A in osteoporosis and
rheumatoid arthritis using network pharmacology and experimental
verification. Drug Des Devel Ther. 14:5235–5250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen Q, Zeng D, Zhou Y, Xia L, Zhao Y,
Qiao G, Xu L, Liu Y, Zhu Z and Jiang X: Curculigoside promotes
osteogenic differentiation of bone marrow stromal cells from
ovariectomized rats. J Pharm Pharmacol. 65:1005–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamamoto M, Kensler TW and Motohashi H:
The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for
maintaining redox homeostasis. Physiol Rev. 98:1169–1203. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang CY, Deng JS, Huang WC, Jiang WP and
Huang GJ: Attenuation of lipopolysaccharide-induced acute lung
injury by hispolon in mice, through regulating the
TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing
oxidative stress-mediated ER stress-induced apoptosis and
autophagy. Nutrients. 12:17422020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu C, Chen RL, Wang Y, Wu WY and Li G:
Acacetin alleviates myocardial ischaemia/reperfusion injury by
inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1
pathway. Pharm Biol. 60:553–561. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue R, Wan Y, Sun X, Zhang X, Gao W and Wu
W: Nicotinic mitigation of neuroinflammation and oxidative stress
after chronic sleep deprivation. Front Immunol. 10:25462019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zou L, Liang B, Gao Y, Ye T, Li M, Zhang
Y, Lu Q, Hu X, Li H, Yuan Y and Xing D: Nicotinic acid riboside
regulates Nrf-2/P62-related oxidative stress and autophagy to
attenuate doxorubicin-induced cardiomyocyte injury. Biomed Res Int.
2022:62933292022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nair N and Gongora E: Oxidative stress and
cardiovascular aging: Interaction between NRF-2 and ADMA. Curr
Cardiol Rev. 13:183–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nazmeen A, Chen G and Maiti S: Dependence
between estrogen sulfotransferase (SULT1E1) and nuclear
transcription factor Nrf-2 regulations via oxidative stress in
breast cancer. Mol Biol Rep. 47:4691–4698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu J, Chu T, Yu T, Li N, Wang C, Li C,
Zhang Y, Meng H and Nie G: Design of diselenide-bridged hyaluronic
acid nano-antioxidant for efficient ROS scavenging to relieve
colitis. Acs Nano. 16:13037–13048. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moniruzzaman M, Ghosal I, Das D and
Chakraborty SB: Melatonin ameliorates
H2O2-induced oxidative stress through
modulation of Erk/Akt/NFkB pathway. Biol Res. 51:172018. View Article : Google Scholar
|
|
46
|
Giustarini D, Dalle-Donne I, Milzani A,
Fanti P and Rossi R: Analysis of GSH and GSSG after derivatization
with N-ethylmaleimide. Nat Protoc. 8:1660–1669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan SC, Rajendran R, Bhattamisra SK,
Krishnappa P, Davamani F, Chitra E, Ambu S, Furman B and Candasamy
M: Effect of madecassoside in reducing oxidative stress and blood
glucose in streptozotocin-nicotinamide-induced diabetes in rats. J
Pharm Pharmacol. 75:1034–1045. 2023. View Article : Google Scholar : PubMed/NCBI
|