Introduction
Ultraviolet radiation (UVR) poses the greatest
threat to the skin as external component. UVR in composed of three
wavelength ranges: UVA (320-400 nm), UVB (290-320 nm) and UVC
(200-290 nm) (1). Different UVR
wavelengths cause skin damage through various mechanisms. UVA
exhibits a strong ability to penetrate the skin, reaching the
epidermis, dermis and even subcutaneous tissue. On the other hand,
the penetrative ability of UVB is weaker, mainly damaging the
epidermis and superficial dermis (2-4).
However, as the energy of UVR decreases with increasing wavelength,
UVB has a stronger detrimental impact on the epidermis compared to
UVA (5). Additionally, UVB
radiation indirectly stimulates higher levels of epidermal melanin,
which serves to protect the skin against DNA damage (6,7).
Repetitive exposure to UVB increases the levels of
cellular reactive oxygen species (ROS), which can promote
carcinogenesis, cell senescence and other skin pathologies
(8-10). Excessive levels of ROS upregulate
the production of matrix metalloproteinases (MMPs), which can
degrade extracellular matrix components and inhibit collagen
synthesis, causing skin relaxation, wrinkles and erythema (11). The skin possesses various
protective mechanisms against UVB-induced oxidative damage,
including antioxidants such as catalase (CAT), superoxide dismutase
(SOD), glutathione peroxidase (GPX) and reduced glutathione (GSH)
(12). However, when ROS levels
surpass the capacity of these antioxidant defenses, excessive ROS
accumulation can disrupt the balance of the oxidation/antioxidant
system, leading to DNA damage and cell cycle arrest (13). Given the crucial role of ROS in
photoaging, reducing ROS accumulation presents a potential approach
for safeguarding the skin against photo-damage.
UVB-mediated DNA damage activates multiple cell
signaling pathways related to cell growth, apoptosis, senescence,
DNA damage repair, connective tissue degradation and inflammation
(14,15). Histone H2AX plays a central role
in several repair mechanisms (16,17) and its phosphorylation is
associated with DNA double-strand breaks (DSBs). The phosphorylated
form of H2AX (Ser139), known as γH2AX, leads to changes
in chromatin structure and facilitates the recruitment of DNA
repair factors, including p53-binding protein 1, Nijmegen breakage
syndrome 1, breast cancer type 1 susceptibility protein, radiation
sensitive 52 (RAD52) and mediator of DNA damage checkpoint 1
(18).
Exosomes, which are nanoscale extracellular
vesicles, are secreted by all living cells (19). They transfer functional cargo,
such as bioactive proteins, messenger ribonucleic acids (mRNAs) and
microRNAs, which can mediate cell responses and regulate some
biological processes in target cells (20). For example, exosomes derived from
human induced pluripotent stem cells (iPSCs) have been reported to
significantly reduce the expression levels of MMP-1/3 and
senescence-associated β-galactosidase (SA-β-Gal), while
upregulating the expression of collagen type I in human dermal
fibroblasts (hDFs) (21).
Furthermore, Choi et al (22) found that exosomes derived from
adipose-derived stem cells (ADSCs) effectively suppressed the
overexpression of MMPs, and enhanced the expression of collagen
type I and elastin (22).
hDFs are the predominant cell type in the dermis and
are responsible for regulating the extracellular matrix (ECM),
collagen production and wound healing (23). These cells play critical roles in
preventing skin aging, and maintaining the normal structure and
functions of the skin (24),
rendering them essential for skin regeneration and repair. Previous
research has demonstrated that hDFs constitute a useful cell line
for studying particular aspects of skin aging as they are easily
grown in culture and respond to various age-inducing stimuli
(25). Additionally, the
foreskin is considered a valuable tissue source because it contains
immunotherapeutic molecules (26). However, the anti-photoaging
activities of exosomes derived from human neonatal foreskin
fibroblast cells have not yet been reported, at least to the best
of our knowledge. The present study examined the protective effects
of exosomes derived from BJ-5ta cells (BJ-5ta Exo) on UVB-induced
photoaging. The data demonstrate that BJ-5ta Exo can protect the
skin against UVB-induced photoaging.
Materials and methods
Isolation and characterization of
exosomes
Human hTERT-immortalized foreskin fibroblast
(BJ-5ta) cells were purchased from ATCC (CRL-4001) and maintained
in a 4:1 mixture of DMEM and Medium 199 (WelGENE Inc.),
supplemented with 10% fetal bovine serum (FBS; HyClone; Cytiva) and
1% penicillin/streptomycin at 37°C in a humidified incubator with
5% CO2. To isolate the exosomes, the cells were
incubated in serum-free DMEM. The conditioned medium was then
collected and centrifuged at 300 × g for 10 min and 2,000 × g for
20 min at 4°C, followed by filtration to remove the cells and
cellular debris using 0.22-μm filters. The clarified
supernatant was collected and concentrated using a sterile-membrane
T-series cassette (Pall Life Sciences) with tangential flow
filtration using a membrane with a molecular weight cut-off (MWCO)
of 100 kDa (Sartorius AG). The mixture was then centrifuged at
100,000 × g for 3 h at 4°C (Fig.
S1). The morphology of the BJ-5ta Exo was imaged using a
field-emission scanning electron microscope (FE-SEM; Sigma HD, Carl
Zeiss Meditec AG). The size distribution was determined by
nanoparticle tracking analysis (NTA) using ZetaView (Particle
Metrix). In total, two positive exosome markers, CD63 and ALIX,
were used, while Calnexin was used as the negative protein
marker.
UVB irradiation
Prior to UVB irradiation, the cells were treated
with BJ-5ta Exo at a concentration of 104 particles/ml
for 6 h in a serum-free 4:1 mixture of DMEM and Medium 199 (WelGENE
Inc.). UVB irradiation was performed at 30 mJ/cm2 for
<1 min in a thin layer of phosphate-buffered saline (PBS) using
a UVB-emitting system from Biospectra (Vilber Lourmat Sto).
Following irradiation, the cells were incubated in serum-free
medium, and both the cells and cell supernatants were used for
further analysis.
Measuring cell viability
The cells were seeded and cultured in 96-well plates
(Corning, Inc.) until they reached a confluency of 90%. The cells
were treated with BJ-5ta Exo (0, 103, 104,
105, 106 and 107 particles/ml) for
24 h or exposed to various doses of UVB radiation (20, 30, and 40
mJ/cm2), followed by incubation at 37°C with 5%
CO2 for 24 h. In addition, the cells were treated with
BJ-5ta Exo (104 particles/ml) for 6 h before being
exposed to UVB radiation (30 mJ/cm2) and then incubated
at 37°C with 5% CO2 for 24 h. Cell viability was
assessed using a WST-8 assay kit (QuantiMax™, Biomax). The
absorbance was measured at 450 nm using a microplate
spectrophotometer (SpectraMax 340; Moleular Devices, Inc.).
Measurement of intracellular ROS
levels
The intracellular production of ROS was measured
using the Cellular ROS Detection Assay kit (cat. no. ab113851,
Abcam). The cells were pre-treated with various concentrations of
BJ-5ta Exo for 6 h, washed with 1X assay buffer (Abcam), and
incubated in 20 μM in 2,7-dichlorofluorescin diacetate
(DCFDA) solution (Abcam) (100 μl) for 45 min at 37°C with 5%
CO2 in the dark. Subsequently, the cells were exposed to
UVB radiation (30 mJ/cm2) and incubated in complete
medium containing 10% fetal bovine serum (FBS), but lacking phenol
red (WelGENE Inc.) for 2 h. The samples were observed using a
fluorescence microscope (DMi8, Leica Microsystems GmbH), and
fluorescence readings were obtained using a spectrophotometer
(SpectraMax 340; Molecular Devices, Inc.) at wavelengths of 485 and
535 nm.
SA-β-Gal staining
SA-β-gal staining was performed according to the
instructions provided with the SA-β-gal Staining kit (cat. no.
9860, Cell Signaling Technology, Inc.). After washing with PBS (pH
6.0), the cells were fixed in 4% paraformaldehyde (4% PFA) for 10
min at room temperature (RT) and stained at 37°C for overnight in a
dry incubator without CO2. The SA-β-gal-positive cells
were observed using an optical microscope (DMi8, Leica Microsystems
GmbH), and the count was determined by examining 400 cells per dish
using the Image Pro Plus (IPP) 6.0 software (Media Cybernetics,
Inc.). The proportions of cells exhibiting SA-β-gal activity are
presented as percentages of the total cells count in each dish.
Cell cycle assay
The BJ-5ta fibroblasts were pre-treated with BJ-5ta
Exo at a concentration of 104 particles/ml for 1 h and
then irradiated with UVB at a dose of 30 mJ/cm2.
Following 24 h of incubation at 37°C with 5% CO2, the
cells were fixed with cold ethanol (70%) and then treated with
propidium iodide (PI; MilliporeSigma) and RNase A (Thermo Fisher
Scientific, Inc.). The cell cycle was assessed using a BD FAC
Symphony A1 flow cytometer (BD Biosciences) and analyzed using
FlowJo software v10.
Apoptosis analysis
An Annexin V-FITC apoptosis detection kit (V13241,
Invitrogen; Thermo Fisher Scientific, Inc.) was used to determine
the number of apoptotic cells. The cells were pre-treated with
BJ-5ta Exo at concentrations of 104 particles/ml for 1 h
and then irradiated with UVB (30 mJ/cm2). Following
exposure, the cells were incubated for 24 h at 37°C with 5%
CO2, and then collected and centrifuged at 252 × g for
10 min at RT, washed three times with cold PBS and resuspended in
100 μl binding buffer solution. Finally, the cells were
incubated with Annexin V-FITC (5 μl) and PI (5 μl) at
room temperature for 15 min in the dark. The fluorescence of the
cells was immediately assessed using a flow cytometer (BD
Biosciences). In the FACS diagram, the early apoptotic cells and
the late apoptotic cells are respectively represented in the lower
right quadrant and upper right quadrant. The total apoptotic rates
were calculated using the following formula: Total apoptosis rate
(%)=early apoptosis rate + late apoptosis rate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Gallic acid (GA), a phenolic antioxidant found in
numerous types of plants, a positive control was used due to its
antioxidant activity (27). The
BJ-5ta fibroblasts were pre-treated with BJ-5ta Exo or GA at a
concentration of 104 particles/ml for 6 h and then
irradiated with UVB at a dose of 30 mJ/cm2. Following 1
h of incubation at 37°C with 5% CO2, total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Single-strand cDNA synthesis was performed using
reverse transcription using PrimeScript TM RT Master Mix (Takara
Bio, Inc.). The resulting cDNA was subjected to qPCR on a CFX96
thermocycler (Bio-Rad Laboratories, Inc.) using qPCR PreMIX
SYBR-Green (Enzynomics). The following thermal cycling conditions
were used: PCR initial activation step for 15 min at 95°C;
three-step cycling: Denaturation for 10 sec at 95°C, annealing for
15 sec at 60°C, elongation for 30 sec at 72°C; for 45 cycles. Gene
expression levels were calculated as a cycle threshold (Ct) value
using the 2−ΔΔCq quantification method and normalized to
that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (28). The primers used for qPCR are
listed in Table I.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Primer sequence (5′
to 3′) |
|---|
| Human SOD1 | F:
CGACAGAAGGAAAGTAATG |
| R:
TGGATAGAGGATTAAAGTGAGG |
| Human SOD2 | F:
GCCCTGGAACCTCACATCAA |
| R:
GGTACTTCTCCTCGGTGACGTT |
| Human CAT | F:
CGTGCTGAATGAGGAACAGA |
| R:
AGTCAGGGTGGACCTCAGTG |
| Human GPX | F:
CAACCAGTTTGGGCATCAG |
| R:
TTCACCTCGCACTTCTCG |
| Human GAPDH | F:
TGGAAATCCCATCACCATCTTC |
| R:
CGCCCCACTTGATTTTGG |
Western blot analysis
Whole protein lysates were extracted from BJ-5ta
cells using RIPA buffer (Thermo Fisher Scientific, Inc.), and the
protein content was quantified using Bradford reagent
(MilliporeSigma). Equal amounts of protein (10 μg) were
separated on a 10% SDS-PAGE gel and transferred to nitrocellulose
membranes (Cytiva, Amersham, United States), which were then
blocked in 5% skim milk in Tris-buffered saline containing 0.1%
Tween-20 (TBS-T) for 2 h at RT and probed overnight at 4°C with
primary antibodies listed in Table
II. The membranes were then incubated with HRP-conjugated
anti-mouse (1:5,000, PI-2000-1, Vector Laboratories, Inc.) or
anti-rabbit (1:5,000,PI-1000-1, Vector Laboratories, Inc.)
secondary antibodies at room temperature for 1 h. Immunodetection
was performed using an Amersham ECL kit (GE Healthcare; Cytiva)
according to the manufacturer's protocol. The protein bands were
visualized using a ChemiDoc™ MP Imaging System (Bio-Rad
Laboratories, Inc.) and analyzed using ImageJ software V1.8.0
(National Institutes of Health).
 | Table IIAntibodies used for western blot
analysis. |
Table II
Antibodies used for western blot
analysis.
| Antibodies | Catalogue
number | Company | Dilution |
|---|
| Anti-γH2AX | MA1-2022 | Thermo Fisher
Scientific, Inc. | 1:5,000 |
| Anti-iNOS | PA1-038 | Thermo Fisher
Scientific, Inc. | 1:1,000 |
| Anti-MMP1 | PA5-27210 | Thermo Fisher
Scientific, Inc. | 1:5,000 |
| Anti-p53 | ab131442 | Abcam | 1:1,000 |
| Anti-p16 | ab81278 | Abcam | 1:1,000 |
| Anti-collagen type
I | ab21965 | Abcam | 1:1,000 |
| Anti-RAD51 | ab176458 | Abcam | 1:5,000 |
| ALIX | ab275377 | Abcam | 1:1,000 |
| CD63 | ab134045 | Abcam | 1:1,000 |
| CALNEXIN | Ab22595 | Abcam | 1:1,000 |
| Anti-p21 | 2947s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-β-actin | 3700s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-cleaved
PARP | 5625s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-p-NF-κB
(Ser536) | 3033s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-NF-κB | 8242s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-COX-2 | 12282s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-p-p38
(Thr180/Tyr182) | 4511s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-p38 | 9212s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-p-JNK
(Thr183/Tyr185) | 9251s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-JNK | 9252s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-p-ERK
(Thr202/Tyr204) | 9101s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-ERK | 9102s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-p-c-Jun
(Ser73) | 9164s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-c-Jun | 9165s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-p-c-Fos
(Ser32) | 5348s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-c-Fos | 2250s | Cell Signaling
Technology, Inc. | 1:5,000 |
| Anti-Smad7 | 365846s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-Smad2 | 3108s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-p-Smad3
(Ser423/425) | 9520s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-Smad3 | 9250s | Cell Signaling
Technology, Inc. | 1:1,000 |
| Anti-p-Smad2
(Ser465/467) | 12570-1-ap | Proteintech Group,
Inc. | 1:1,000 |
| Anti-TGFβ-1 | 21898-1-ap | Proteintech Group,
Inc. | 1:5,000 |
| Anti-Nrf2. | PA5-27882 | Thermo Fisher
Scientific, Inc. | 1:1,000 |
| Anti-Ki67 | MA5-14520 | Thermo Fisher
Scientific, Inc. | 1:500 |
| Anti-procollagen
type I | ABT-257 | Merck | 1:1,000 |
| Anti-elastin | 58756s | Cell Signaling
Technology, Inc. | 1:250 |
Immunocytochemistry (ICC)
The cells were fixed with 4% PFA for 30 min, washed
with PBS, blocked with 3% bovine serum albumin (BSA) and 0.2%
Triton X-100 in PBS at RT for 1 h, and incubated overnight at 4°C
with primary antibodies listed in Table II. After washing with PBS, the
cells were incubated with anti-rabbit IgG-FITC secondary antibodies
(1:3,000, ab6717, Abcam) for 1 h at RT in the dark. The cell nuclei
were counterstained with 4°C counterstained
4′,6-diamidino-2-phenylindole (DAPI; cat. no. AR-6501-01,
ImmunoBioScience Corp.) at RT for 30 min, and the stained cells
were observed using a confocal microscope (LSM 880, Zeiss AG).
Experimental animals and UVB
irradiation
Female SKH-1 hairless mice (7 weeks old, 17-22 g)
were purchased from Saeron Bio, Inc. The mice were acclimatized for
1 week under the following conditions: A temperature of 23±2°C,
55±10% humidity, and a 12-h-light/12-h-dark cycle. All animal
experiments were conducted in accordance with the principles of
laboratory animal care at the National Institutes of Health and
with the approval of the Ethics Committee for Laboratory Animals at
Chung-Ang University (IACUC no. A2022053). The mice were randomly
assigned to four groups (n=6 per group) as follows: Group 1, normal
control; group 2, UVB only; group 3, UVB + BJ-5ta Exo
106 (particles/ml); and group 4, UVB + BJ-5ta Exo
108 (particles/ml). The mice were exposed to UVB
irradiation using a BIO-SPECTRA (Vilber Lourmat Sta), three times a
week for 8 weeks. Initially, the dose was set at 100
mJ/cm2 for 2 weeks, and this was then increased to 150
mJ/cm2 for 4 weeks. In addition, the mice were
irradiated with UVB at 150 or 200 mJ/cm2 for 2 weeks.
Immediately after UVB irradiation, 100 μl BJ-5ta Exo were
injected subcutaneously into the backs of the mice, three times a
week. The normal mice were subcutaneously injected with saline
three times a week. The wrinkles on the backs of the mice were
photographed using a DSLR camera (Canon EOS 700D) and PRIMOS CR
(SnTLab).
Anesthesia/euthanasia
The mice were euthanized by CO2
asphyxiation. The mice were placed in a new cage, and immediately
euthanized by the displacement of air with 100% CO2 (30%
chamber volume/min), within 5 min and decapitated for tissue
collection (29,30).
Morphological analysis, histological
observation and immunohistochemistry (IHC)
Morphological changes in the dorsal skin were
observed using a DSLR camera (Canon EOS 700D) and PRIMOS CR (SnT
Lab Co., Ltd.). at 12 weeks. Mouse skin roughness (RA) was analyzed
using PRIMOS CR software version 5.8E (SnT Lab Co., Ltd.). Skin
biopsies were fixed in 10% formalin for 24 h. Paraffin-embedded
3-μm-thick sections were cut, mounted on
POLYSINE® Slides (Thermo Fisher Scientific, Inc.),
dewaxed in xylene, and then dehydrated in an ethanol series.
Hematoxylin and eosin (H&E) staining was performed to examine
the histological features and skin thickness. Masson's trichome
(MT) staining and Verhoeff-van Gieson (VVG) staining were performed
to evaluate collagen and elastin fibers, respectively. H&E
staining was performed as follows: Hematoxylin solution was applied
for 5 min, followed by eosin solution (Muto Pure Chemical Co.,
Ltd.) for 2 min, both at RT. Additionally, Masson's trichrome
staining was conducted using the following steps: The mordant
solution for 25 min, 0.75% orange G solution (Muto Pure Chemical
Co., Ltd.) for 1 min, Masson's B stain solution for 25 min, 2.5%
phosphotungstic acid solution for 20 min, and aniline blue (Muto
Pure Chemical Co., Ltd.) for 8 min, all at RT. For VVG staining,
the procedure included an initial incubation in Verhoeff's solution
for 1 h, followed by washing with tap water. Subsequently, samples
were exposed to 2% FeCl3 (MilliporeSigma) for 5 min, followed by
treatment with 5% sodium thiosulfate (MilliporeSigma). For
counterstaining, slides were stained with VVG's solution for 5 min.
The samples subsequently underwent dehydration using 95% alcohol,
followed by two changes of 100% alcohol. For IHC, the sliced
sections were subjected to antigen retrieval with Tris-EDTA at 4°C
for 15 min, incubated with BLOXALL blocking Solution (Vector
Laboratories, Inc.) at RT for 30 min, and then incubated overnight
at 4°C with the primary antibodies listed in Table II, including procollagen type I,
collagen type I, MMP-1, elastin and filaggrin antibodies. The
slides were then incubated with HRP using the ImmPRESS®
Excel Amplified Polymer Staining kit (Vector Laboratories, Inc.).
The staining was developed using the 3,3′-diaminobenzidine (DAB)
peroxidase substrate kit (Vector Laboratories, Inc.). To identify
nuclei, the slides were counterstained with hematoxylin at RT for
<1 min. The stained slides were photographed using a slide
scanner (Pannoramic MIDI; 3DHISTECH Ltd.), observed using Case
Viewer software (V 2.7; 3DHISTECH Ltd.), and analyzed using ImageJ
software (V1.8.0; National Institutes of Health).
Assessment of skin hydration
The hydration of the dorsal skin was assessed using
a Corneometer® CM 825 (Courage + Khazaka Electronic
GmbH). The amount of transepidermal water loss (TEWL) was measured
using a Tewameter® TM 300 (Courage + Khazaka Electronic
GmbH) after 8 weeks.
Reconstructed human skin model
analysis
The reconstructed human skin model
Neoderm®-ED was purchased from Tego Science, Inc.
Neoderm®-ED was removed from medium-containing agar and
transferred onto 12-well plates for equilibration at 37°C (5%
CO2) for 1 day. Under treatment with BJ-5ta Exo,
Neoderm®-ED was irradiated with UVB (128
mJ/cm2), and the tissue samples were incubated at 37°C
with 5% CO2 for 48 h.
ELISA of the reconstructed human skin
model
The quantitative measurement of procollagen-type I
and MMP-1 production from reconstructed human skin
(Neoderm®-ED) in the supernatant was conducted using a
procollagen type I ELISA kit (cat. no. MK101, Takara Bio, Inc.) and
a Human MMP-1 (Sandwich ELISA) ELISA kit (cat. no. ab215083,
Abcam), following the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ducted using a
procollagen type I ELISA kit independent experiments. Data analyses
were performed using unpaired one-way analysis of variance (ANOVA)
followed by the Bonferroni post hoc test. Statistical analysis was
performed using GraphPad Prism 7.0 software (GraphPad Software
Inc.). All experiments were repeated at least three times.
Results
Treatment with BJ-5ta Exo attenuates the
UVB-irradiation-induced inhibition of cell viability
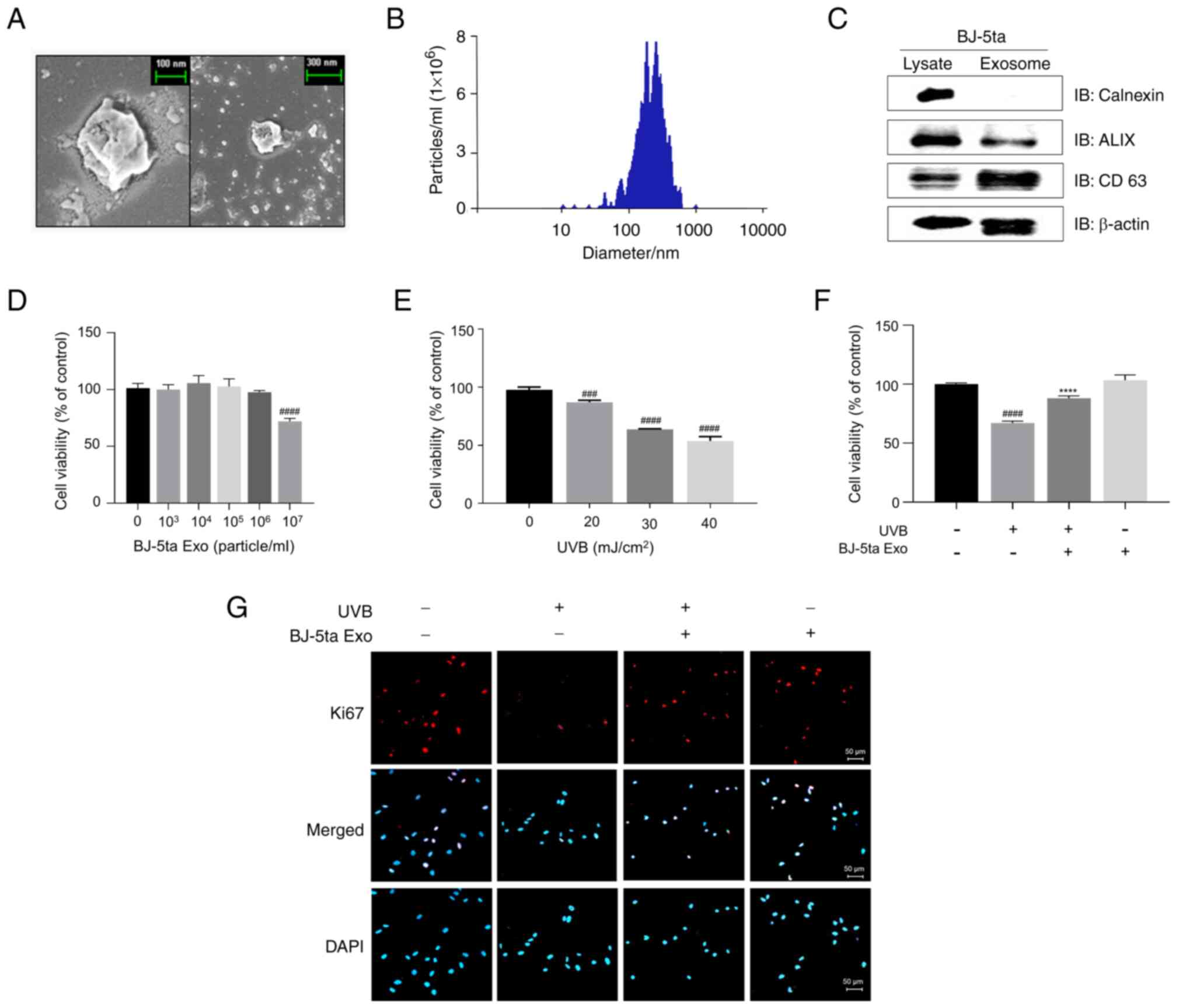
BJ-5ta-Exo exhibited a spherical morphology
(Fig. 1A). The number and size
of the total particles were quantified using NTA as follows: The
mean size of the exosomes was 215.4±116.1 nm and the number was
2.33×1010±2.22×109/ml (Fig. 1B). The two positive markers for
the exosomes (ALIX and CD63) were abundant in the BJ-5ta-Exo, while
the negative protein marker, calnexin, was absent in the BJ-5ta Exo
(Fig. 1C). The BJ-5ta cells were
treated with BJ-5ta Exo (103 to 107
particles/ml) for 24 h, and cell viability was then measured using
WST-8 assay. BJ-5ta Exo at doses of up to 106
particles/ml was not toxic to the BJ-5ta cells (Fig. 1D). UVB irradiation inhibited cell
viability by 36 and 46% at doses of 30-40 mJ/cm2,
respectively (Fig. 1E). However,
BJ-5ta Exo (104 particles/ml) prevented the inhibition
of cell viability induced by UVB (Fig. 1F). In addition, UVB significantly
decreased the percentage of cells expressing Ki67. Conversely,
BJ-5ta Exo reversed this trend (Fig.
1G).
BJ-5ta-Exo treatment reduces UV-induced
oxidative stress
UVB-mediated ROS generation can trigger genes
related to skin photoaging and lead to oxidative stress. UVB
significantly increased intracellular ROS levels compared to the
control group, while BJ-5ta Exo attenuated ROS generation induced
by UVB (Fig. 2A and B). UVB
irradiation induced a decrease in the mRNA levels of antioxidant
enzymes (SOD-1, SOD-2, CAT and GPX); however, BJ-5ta
Exo prevented the downregulation of these genes (Fig. 2C). Furthermore, UVB irradiation
resulted in the downregulation of NF-E2-related factor 2 (Nrf2),
which is responsible for inducing the transcription of antioxidant
and cytoprotective genes. However, BJ-5ta Exo attenuated this
decrease in Nrf2 expression (Fig.
2D). In addition, it was confirmed that BJ-5ta Exo inhibited
the UVB-mediated decrease of Nrf2 in the nucleus (Fig. 2E). These results thus suggested
that BJ-5ta Exo suppressed UVB-induced oxidative stress by
increasing the expression levels of Nrf2.
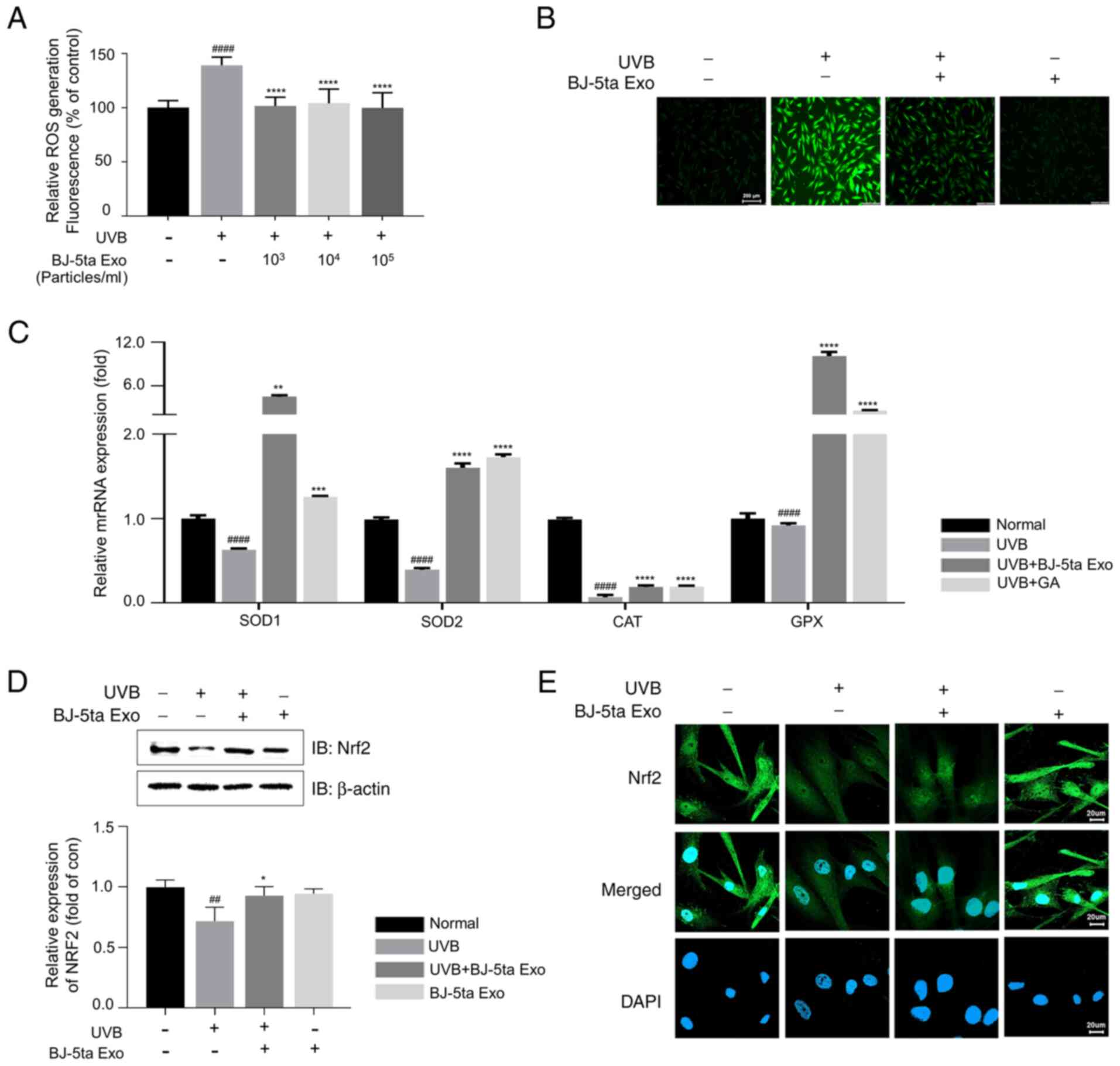 | Figure 2Effects of BJ-5ta Exo on
intercellular ROS levels. (A) UVB-induced intracellular ROS
generation was attenuated by BJ-5ta Exo
(103,104 and 105 particles/ml) in
BJ-5ta cells. (B) Representative intracellular ROS images of cells
pre-treated with BJ-5ta Exo (104 particles/ml) for 6 h,
followed by UVB (30 mJ/cm2) irradiation. Scale bar, 200
μm. (C) The mRNA expression levels of Nrf2 downstream
antioxidant enzymes (SOD-1 or 2, CAT and GPX). (D) Total Nrf2
protein levels. (E) Nrf2 localization was determined using
immunocytochemistry with an anti-Nrf2 antibody (green fluorescence)
and DAPI staining (blue fluorescence). Scale bar, 20 μm.
BJ-5ta cells were pre-treated with BJ-5ta Exo (104
particles/ml) for 6 h, followed by exposure to UVB (30
mJ/cm2). Following 3 h of incubation (C-E), the cells
were analyzed using RT-qPCR, western blot analysis, or
immunocytochemistry. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001, compared
with UVB-irradiated cells; ##P<0.01,
####P<0.0001, compared with normal cells. BJ-5ta Exo,
exosomes derived from BJ-5ta cells; UVB, ultraviolet B; ROS,
reactive oxygen species; SOD, superoxide dismutase; CAT, catalase;
GPX, glutathione peroxidase; Nrf2, nuclear factor erythroid
2-related factor 2. |
BJ-5ta Exo reduce UVB-induced DNA damage,
p53/p21 pathway activation and senescence
UVB exposure caused an increase in γH2AX expression,
while BJ-5ta Exo suppressed this increase (Fig. 3A). Using ICC staining (Fig. 3B), it was found that BJ-5ta Exo
inhibited the UVB-induced γH2AX foci and nuclear accumulation.
BJ-5ta Exo also prevented the UVB-induced upregulation of RAD51 and
cleaved PARP-1 (Fig. 3C). In
addition, the apoptotic cells were analyzed using Annexin V and PI
solution followed by flow cytometric analysis. UVB irradiation
increased total apoptosis by 20.9% compared to the control group.
However, BJ-5ta Exo significantly reduced apoptosis, resulting in
an 11.39% inhibition compared to the UVB-alone group (Fig. 3D). Cell cycle analysis also
revealed that UVB irradiation decreased the cell populations in the
G0/G1 phase (from 82.73±1.38 to 62.30±5.31%). However, BJ-5ta Exo
partially prevented the G2-phase cell cycle alteration compared
with the UVB group (from 16.90±4.20 to 9.32±0.41) (Fig. 3E). Subsequently, it was confirmed
that BJ-5ta Exo efficiently reversed the UVB-mediated increase in
p53 and p21 expression (Fig.
3F). The number of cells expressing SA-β-Gal and the expression
levels of p16 were also significantly increased after UVB
irradiation compared to the control cells; however, these effects
were attenuated by BJ-5ta Exo (Fig.
3G and H).
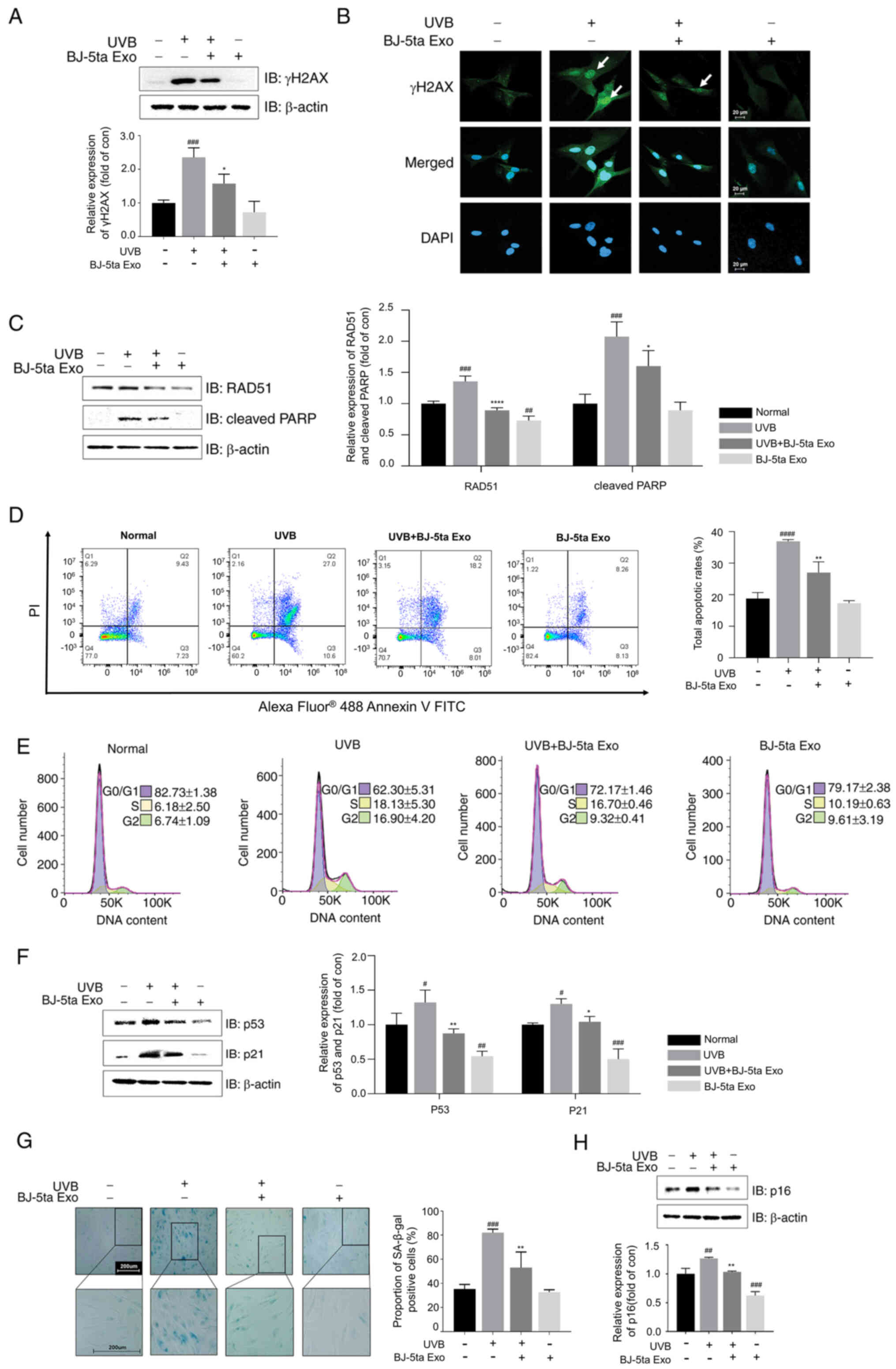 | Figure 3Effects of BJ-5ta Exo on UVB-induced
DNA damage, the p53/p21 pathway and senescence in BJ-5ta cells. (A)
Cell lysates were analyzed using western blot analysis with
antibodies against γH2AX. (B) The expression of γH2AX was
determined using immunocytochemistry with an anti-γH2AX antibody
(green fluorescence) and DAPI (blue fluorescence). Scale bar, 20
μm. Protein levels of (C) RAD51 and cleaved PARP were
analyzed using western blot analysis. (D) Apoptosis and (E) cell
cycle distribution was analyzed using flow cytometry. (F) The
levels of p53, and its downstream effector p21, were assessed using
western blot analysis. (G) SA-β-Gal-positive cells were observed in
BJ-5ta cells pre-treated with and without BJ-5ta Exo
(104 particles/ml) and exposed to UVB (30
mJ/cm2). Scale bar, 200 μm. (H) The protein
levels of p16, a marker of cellular senescence, were determined
using western blot analysis. Cells were pre-treated with and
without BJ-5ta Exo (104 particles/ml) for 6 h and then
exposed to UVB radiation (30 mJ/cm2). Following 24 h of
incubation, the cells were analyzed using western blot analysis or
immunocytochemistry. In western blot analysis, the protein levels
were quantified and presented relative to the β-actin levels. The
results are expressed as the mean ± standard deviation.
*P<0.05, **P<0.01 and
****P<0.0001, compared with UVB-irradiated cells;
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001, compared
with normal cells. BJ-5ta Exo, exosomes derived from BJ-5ta cells;
UVB, ultraviolet B; SA-β-Gal, senescence-associated
β-galactosidase. |
BJ-5ta Exo alleviate UVB-induced
inflammation
UVB increased COX-2 and iNOS expression (4.0-fold
and 1.4-fold, respectively, compared to the control group), while
BJ-5ta Exo significantly reduced the COX-2 and iNOS levels by
0.3-fold compared to the UVB-only group (Fig. 4A). The levels of p-NF-kB
(Ser536) were found to be elevated by UVB, although this
effect was reversed by BJ-5ta Exo (Fig. 4B). Furthermore, ICC staining
confirmed that BJ-5ta Exo significantly inhibited the UVB-induced
translocation of NF-κB to the nucleus (Fig. 4C).
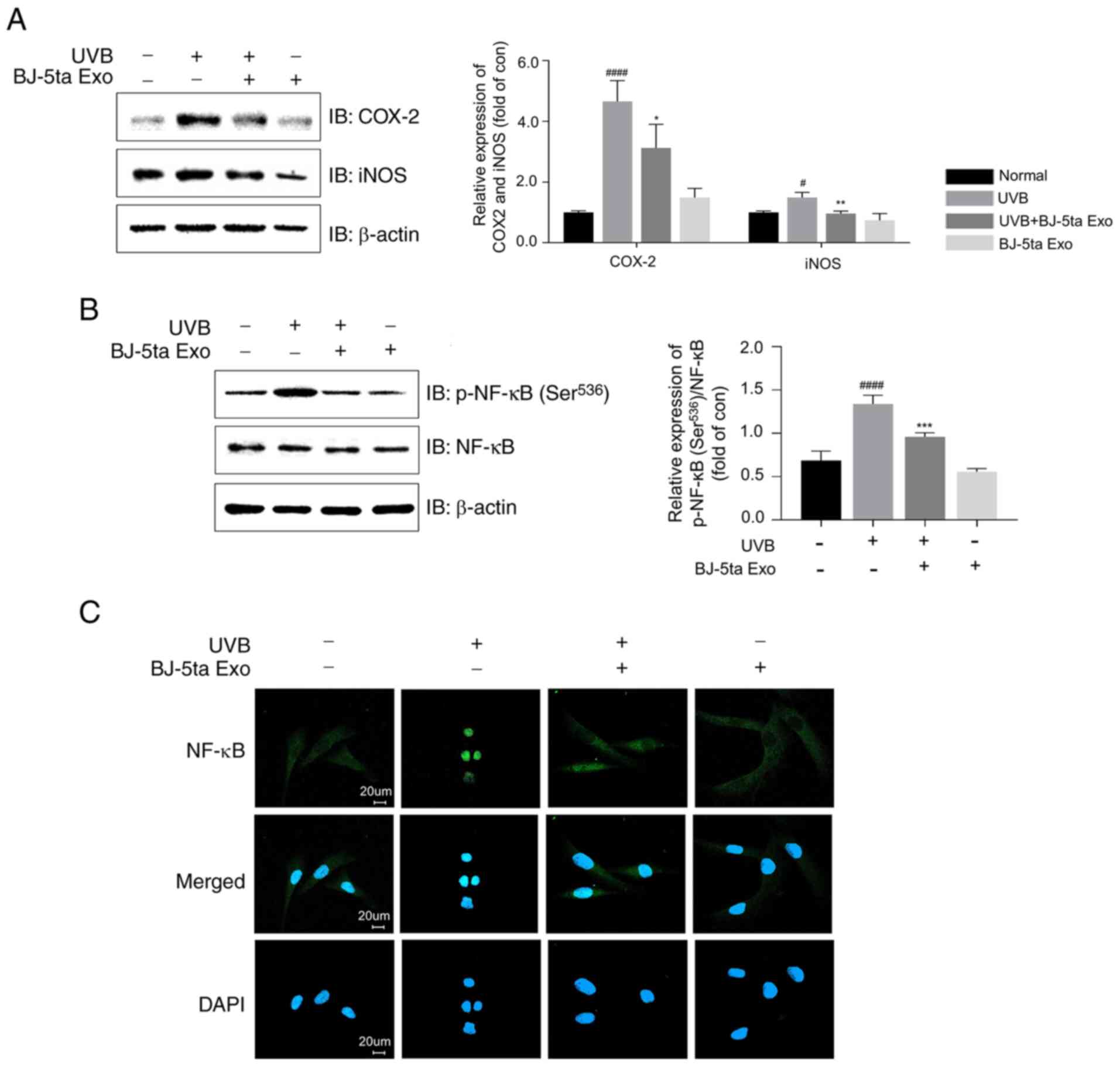 | Figure 4Inhibition of UVB-induced expression
of p-NF-κB, iNOS and COX-2 by BJ-5ta Exo. BJ-5ta cells were treated
with and without BJ-5ta-Exo for 6 h and then irradiated with UVB
(30 mJ/cm2). After 24 h, cell lysates were examined
using western blot analysis for the protein levels of (A) COX-2 and
iNOS, and (B) p-NF-κB (Ser536). (C) NF-κB localization
was determined using immunocytochemistry with an anti-NF-κB
antibody (green fluorescence) and DAPI (blue fluorescence). The
levels of the phosphorylated proteins were normalized to β-actin or
total NF-κB proteins. The results are expressed as the mean ±
standard error. P<0.05, **P<0.01,
***P<0.001 compared with UVB-irradiated cells;
##P<0.01, ####P<0.0001 compared with
normal cells. BJ-5ta Exo, exosomes derived from BJ-5ta cells; UVB,
ultraviolet B; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxynase 2. |
BJ-5ta Exo reverse the UVB-induced
increase in MMP-1 and the decrease in collagen type I expression
through the MAPK/AP-1/TGF-β1/Smad signaling pathway
BJ-5ta Exo suppressed the UVB-induced decrease in
collagen type I levels, while increasing MMP-1 expression (Fig. 5A). The TGF-β1 signaling pathway
plays a key role in the regulation of ECM biosynthesis, including
procollagen (31). As shown in
Fig. 5B, UVB significantly
decreased TGF-β1 expression, as well as the phosphorylation levels
of Smad2 (Ser465/467) and Smad3 (Ser423/425),
which were reduced by 0.2-fold, while the level of Smad7 increased
by 1.3-fold compared to the control group. However, BJ-5ta Exo
inhibited the UVB-induced suppression of the TGF-β1/Smad pathway.
The UVB-induced production of MMPs can be mediated by protein
kinase cascades, such as MAPK and AP-1 (32). BJ-5ta Exo prevented the
UVB-mediated increase in the levels of p-p38
(Thr180/Tyr182), p-JNK
(Thr183/Tyr185) and
p-ERK(Thr202/Tyr204) (Fig. 5C). In addition, BJ-5ta Exo
suppressed the UVB-induced phosphorylation of AP-1 subunits (c-Fos
on Ser32 and c-Jun on Ser73) (Fig. 5D).
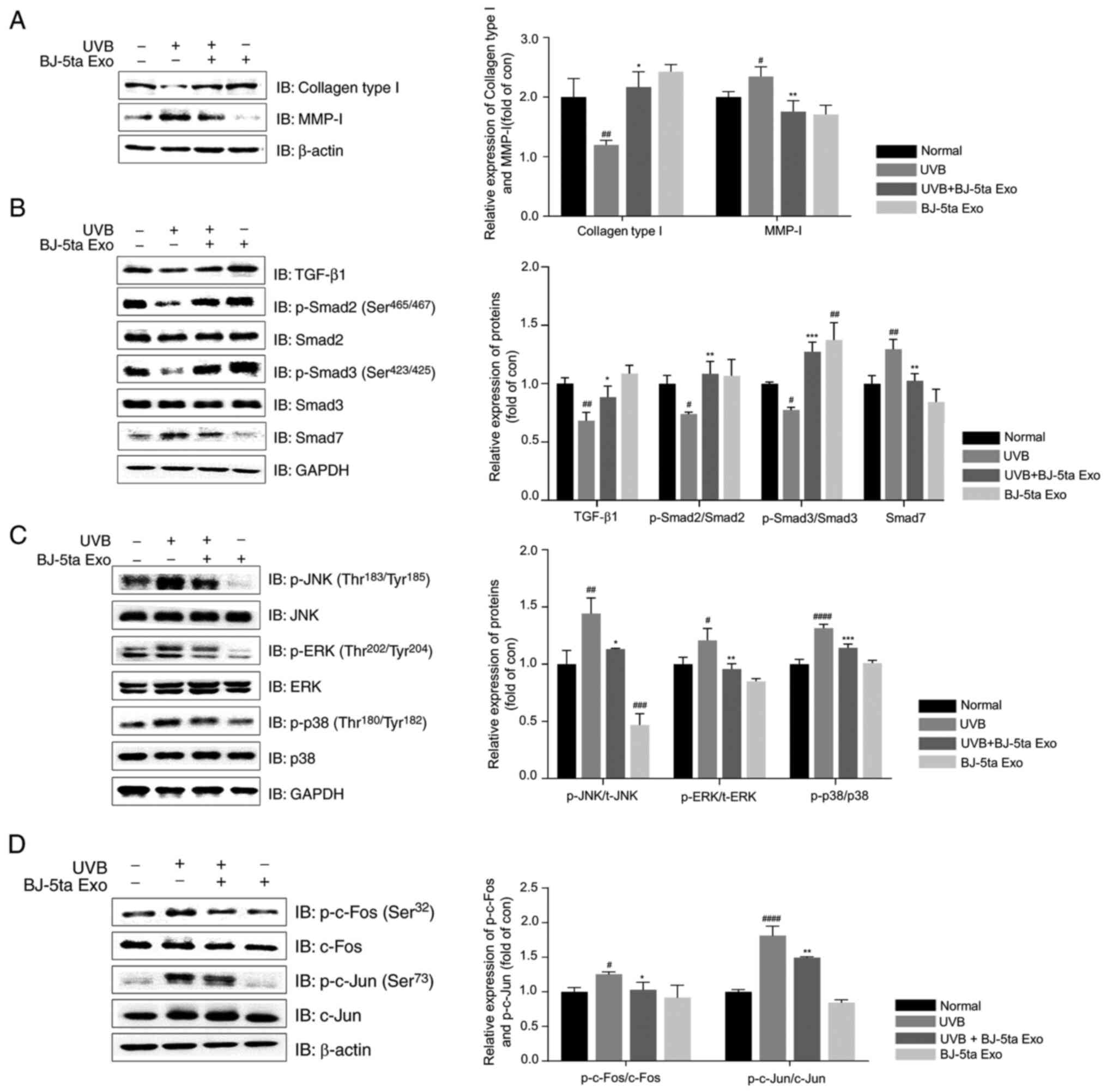 | Figure 5Effects of BJ-5ta Exo on the levels
of collagen type I, MMP-I, TGF-β1/Smads and MAPK/AP-1
phosphorylation in UVB-irradiated BJ-5ta fibroblasts. Protein
levels of (A) collagen type I and MMP-1; (B) TGF-β1, p-Smad2
(Ser465/467), p-Smad3 (Ser423/425) and Smad7;
(C) p-JNK (Thr183/Tyr185), p-ERK
(Thr202/Tyr204), and p-p38
(Thr180/Tyr182); (D) p-c-Fos
(Ser32) and p-c-Jun (Ser73) were examined
using western blot analysis. Cells were pre-treated with and
without BJ-5ta Exo (104 particles/ml) for 6 h and then
exposed to UVB radiation (30 mJ/cm2). Following (A) 24 h
or (B-D) 30 min of incubation, the cells were examined using
western blot analysis. The levels of the phosphorylated proteins
were normalized to β-actin or total proteins. The results are
expressed as the mean ± standard error. *P<0.05,
**P<0.01, ***P<0.001 compared with
UVB-irradiated cells; #P<0.05,
##P<0.01, ###P<0.001 and
####P<0.0001 compared with normal cells. BJ-5ta Exo,
exosomes derived from BJ-5ta cells; UVB, ultraviolet B; MMP, matrix
metalloproteinase. |
BJ-5ta-Exo reduce UVB-induced wrinkle
formation by suppressing collagen degradation and decreasing MMP-1
expression
To examine whether BJ-5ta Exo reduce wrinkle
formation caused by UVB irradiation in SKH-1 hairless mice, BJ-5ta
Exo were subcutaneously injected into the dorsal skin of each group
of mice for 8 weeks following irradiation with UVB (Fig. S2A). Ultimately, there was no
significant difference in the body weights of the mice treated with
various concentrations of BJ-5ta Exo alongside UVB exposure,
indicating the safety of BJ-5ta Exo for the mice (Fig. S2B). A visual assessment
indicated that the administration of BJ-5ta Exo reduced the
UVB-induced wrinkle formation on the back skins of the mice
compared to the saline-treated UVB-irradiated mice (Fig. 6A). To quantitatively measure the
roughness, relevant parameters in the images were analyzed using
PRIMOS CR software version 5.8E, an optical three-dimensional skin
measurement system. The roughness values in the UVB-saline group
were significantly higher (1.4-fold) compared with those in the
control group. By contrast, the mice treated with BJ-5ta Exo at
concentrations of 1×106 particles/ml and
1×108 particles/ml exhibited a reduction in total
wrinkle area of 0.3- and 0.4-fold, respectively, compared to the
saline-treated group (Fig. 6E).
BJ-5ta Exo also restored skin hydration and inhibited moisture
evaporation compared to the UVB-saline group (Fig. 6F and G). As shown in Fig. 6H and I-K, the saline-treated
UVB-irradiated mice had a thicker epidermal surface and a marked
decrease in collagen (blue) and elastin (black) fibers in the
dermis compared to the control mice, while the treated mice
exhibited a significant increase in the abundance and density of
collagen and elastin fibers in the dermis, and a significant
decrease in the thickness of the epidermal layer. In addition,
using IHC staining, it was observed that the expression levels of
procollagen type I, collagen type I and elastin were restored by
1.4, 1.3, and 1.3-fold, respectively, in the BJ-5ta Exo
106-treated group, and by 1.5, 1.3, and 1.3-fold,
respectively, in the BJ-5ta Exo 108-treated group,
compared to the UVB-saline group. By contrast, MMP-1 expression
decreased by 0.3-fold in the BJ-5ta Exo 106-treated
group and 0.4-fold in the BJ-5ta Exo 108-treated group
(Fig. 6L and M). These results
suggest that BJ-5ta Exo can ameliorate skin photodamage by
increasing ECM components.
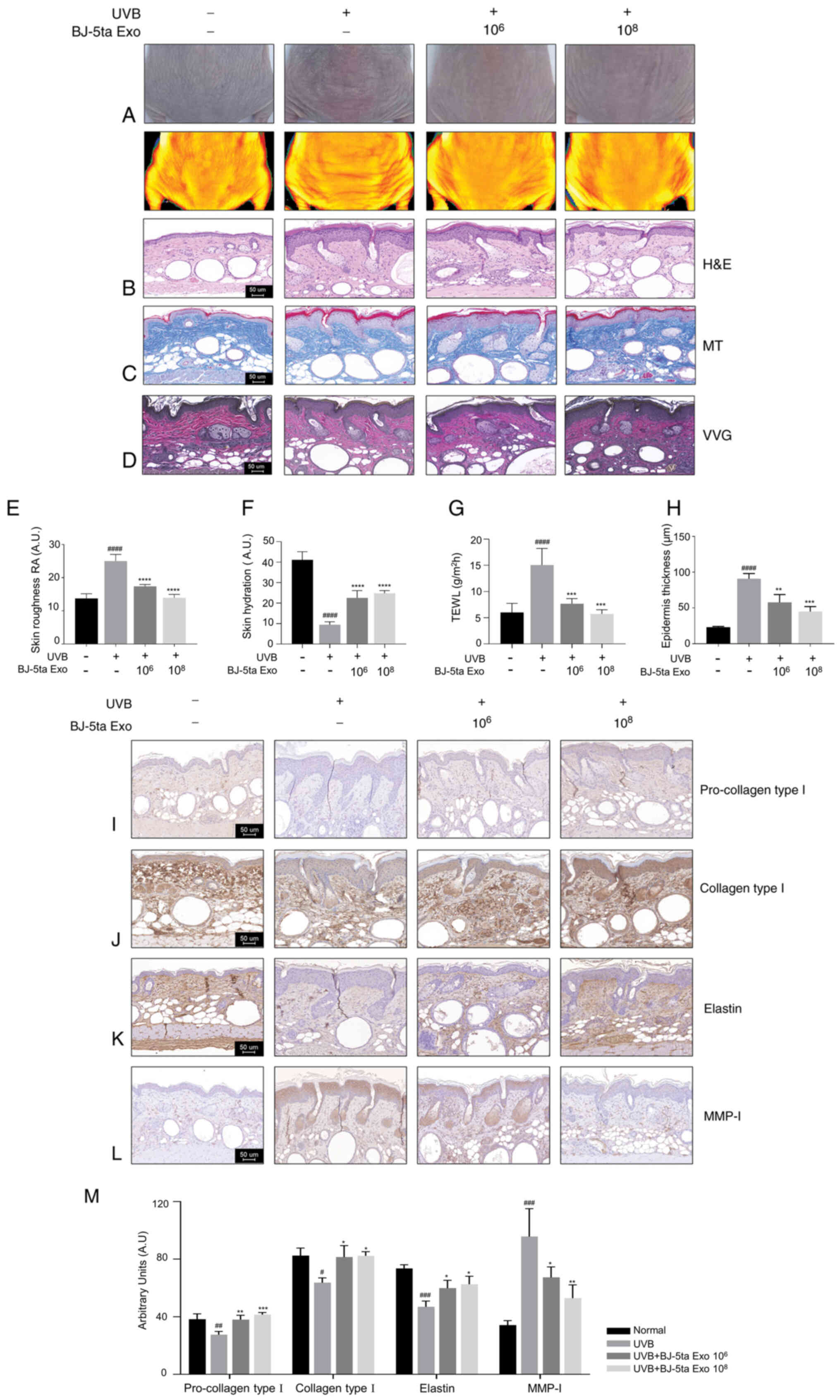 | Figure 6Subcutaneous injection of BJ-5ta Exo
attenuates UVB-induced wrinkle formation in skin of mice. (A) To
evaluate the changes in dorsal wrinkle formation, images were
obtained using a DSLR and PRIMOS CR. (B) H&E staining. (C) MT
staining. (D) VVG staining. (E) The quantification of wrinkles
(roughness) revealed that the depth of the wrinkles was reduced by
BJ-5ta Exo. RA, average roughness. (F) Skin hydration was evaluated
using a Corneometer. (G) TEWL was evaluated using a Tewameter
TM300. (H) Epidermal thickness was quantified using ImageJ
software. (I) Procollagen type I, (J) collagen type I, (K) elastin,
and (L) MMP-1 were analyzed using immunohistochemistry. Scale bar,
50 μm. (M) Immunohistochemistry arbitrary units (a.u.) of
procollagen type I, collagen type I, MMP-1 and elastin in SKH-1
mice. The results are expressed as the mean ± standard error.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001, compared
with UVB irradiation; #P<0.05,
##P<0.01, ###P<0.001 and
####P<0.0001, compared with the control. BJ-5ta Exo,
exosomes derived from BJ-5ta cells; UVB, ultraviolet B; H&E,
hematoxylin and eosin; MT, Masson's trichome; VVG, Verhoeff-van
Gieson; TEWL, transepidermal water loss. |
BJ-5ta Exo inhibit UVB-induced MMP-1
expression and collagen reduction in a reconstructed human skin
model
To further investigate the effects of BJ-5ta Exo on
UVB-induced photodamage in human skin, the Neoderm-ED reconstructed
human skin model was employed. The thickness of the epidermis
increased as a result of UVB exposure. However, BJ-5ta Exo
effectively reduced the increased epidermal thickness caused by UVB
(Fig. 7A and E). UVB radiation
causes an abnormal reduction in procollagen type I and elastin, as
well as the production of MMP-1. Using IHC staining for collagen
type I, elastin and MMP-1, it was confirmed that BJ-5ta Exo
inhibited the UVB-induced downregulation of collagen type I and
elastin, while also suppressing the UVB-induced increase in MMP-1
(Fig. 7B-D). In addition, ELISA
assay used to quantify procollagen type I and MMP-1. Compared to
the UVB-alone group, the groups treated with BJ-5ta Exo at
concentrations of 106 and 108 particles/ml
exhibited an elevated expression of procollagen type I, with an
increased rate of 34.53 and 35.30%, respectively. Additionally,
they exhibited a decrease in MMP-1 levels by 6.85 and 20.14%,
respectively. (Fig. 7F and G).
These observations indicated that BJ-5ta Exo effectively restored
the tissue damage induced by UVB in the layers of the reconstructed
human skin model.
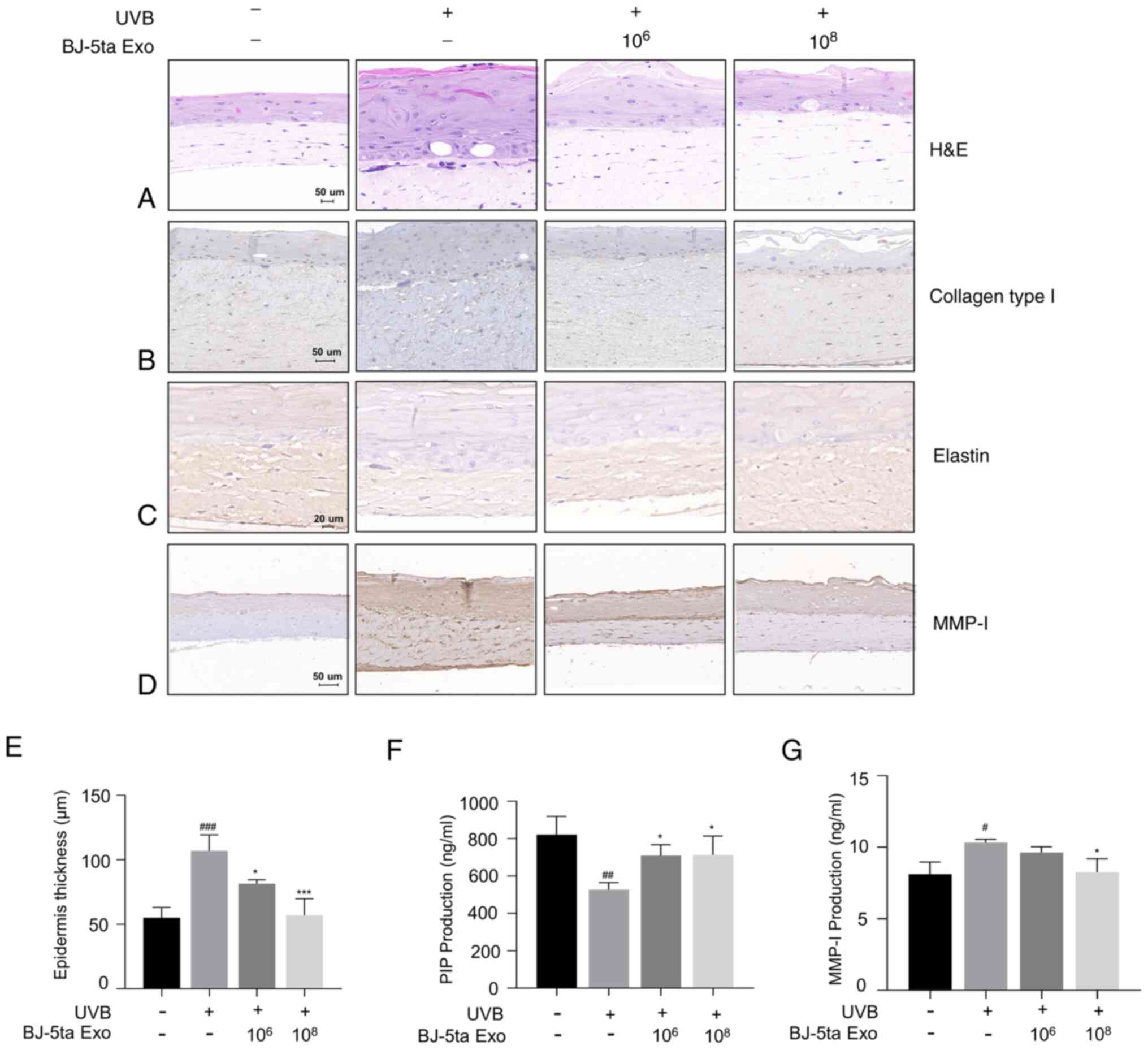 | Figure 7Protective effects of BJ-5ta Exo on
UVB-induced photoaging was evaluated using a reconstructed human
skin model, Neoderm®-ED. The impact of BJ-5ta Exo on
UVB-induced skin tissue damage was assessed. Following UVB
irradiation, the skin tissues were subjected to staining with (A)
H&E, (B) collagen type I, (C) elastin, and (D) MMP-1. (E)
Epidermal thickness was quantified using ImageJ software. (F) PIP
and (G) MMP-1 expression levels were analyzed using ELISA. The
reconstructed human skin model was exposed to UVB (128
mJ/cm2), and the supernatants were collected after 48 h.
BJ-5ta Exo, exosomes derived from BJ-5ta cells; UVB, ultraviolet B;
H&E, hematoxylin and eosin; MMP, matrix metalloproteinase; PIP,
procollagen type I C peptide. *P<0.05 and
***P<0.001, compared with UVB irradiation;
#P<0.05, ##P<0.01 and
###P<0.001, compared with the control. |
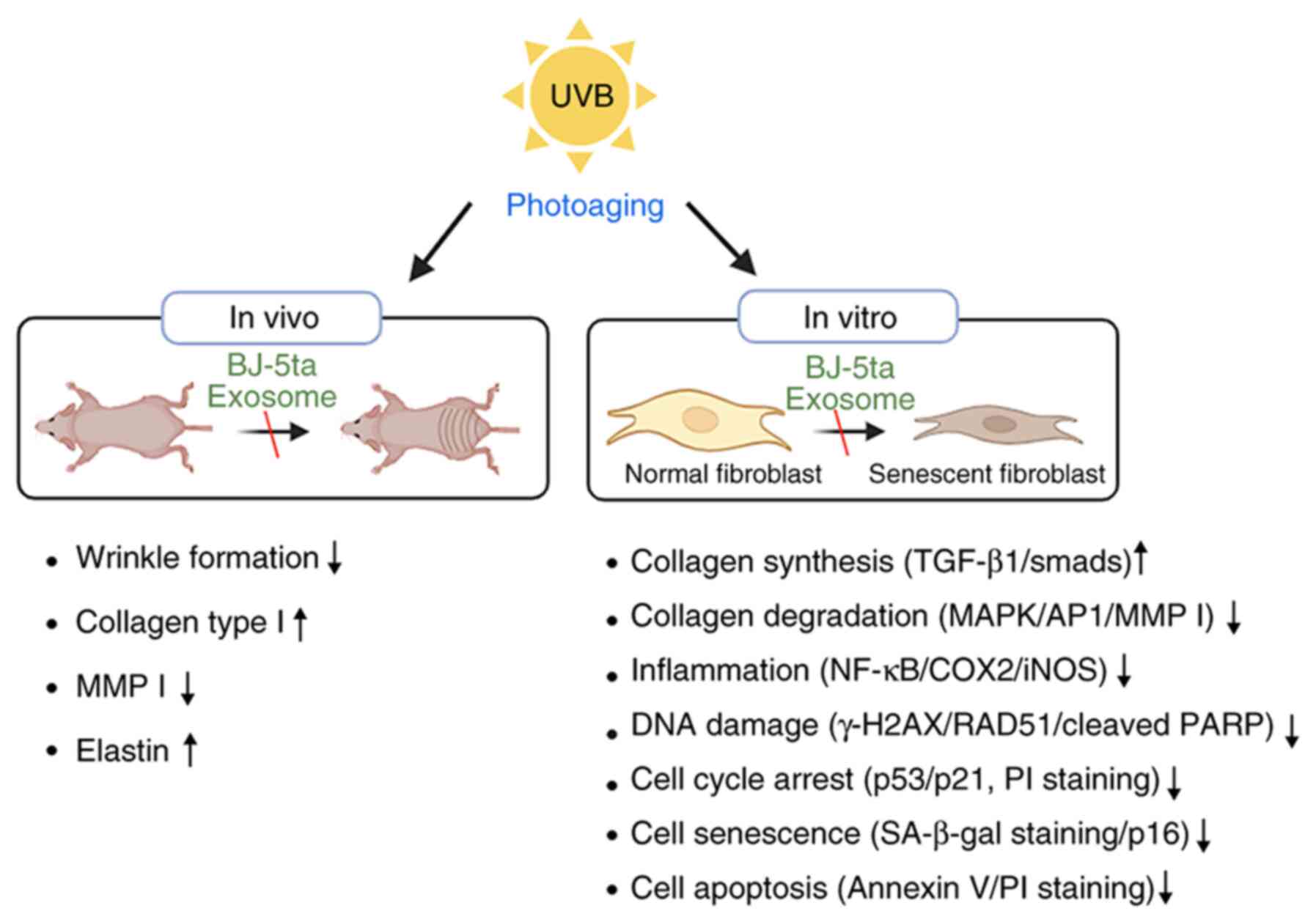
Working model of BJ-5ta exosome with
anti-photoaging effects
BJ-5ta Exo treatment contributes to the removal of
oxidative stress by inhibiting ROS generation and preventing the
decrease in the expression levels of SOD1 and SOD2, GPX and
catalase. It also provides protection against UVB radiation by
activating the DNA repair system through the regulation of both
γH2AX and RAD51. BJ-5ta Exo prevent UVB-induced collagen
degradation by activating the TGF-β1/Smads pathway and inhibiting
the MAPK/AP-1 pathway. Furthermore, BJ-5ta Exo suppress UVB-induced
cellular senescence by inhibiting the expression of SA-β-gal and
p16. In a UVB-induced photoaging model, it was confirmed that
BJ-5ta-Exo treatment decreased the level of TEWL, wrinkle formation
and MMP-1 expression. On the other hand, BJ-5ta Exo increased the
levels of collagen type-I and elastin in the dorsal skin (Fig. 8).
Discussion
Recently, there has been extensive research on the
effects of exosomes on various skin defects. Exosomes have several
key benefits, including high stability, non-immune rejection and
the ability to directly stimulate target cells (20,33). Exosomes derived from ADSCs or
mesenchymal stem cells play crucial roles in aging, atopic
dermatitis and wound healing (34,35). Moreover, recent studies have
found that exosomes derived from autologous hDFs can more
effectively promote cutaneous wound healing (36,37), indicating that exosomes from hDFs
may be potential materials for protecting and repairing skin
damage.
In addition to the effects mentioned above, there
have been reports on the photoprotective effects of exosomes
against UVB damage. Gao et al (38) investigated the role of ADSCs-Exo
in hDFs exposed to UVB radiation. They found that ADSCs-Exo
treatment attenuated UVB-induced photoaging in hDFs (38). In addition, Ellistasari et
al (39) reported that
exosomes derived from human umbilical vein endothelial cells
(HUVEC-Exo) ameliorated UVB-induced photoaging in skin fibroblasts.
These findings indicate that exosomes are effective in preventing
UVB-mediated dermal photoaging in in vitro models. The
present study further investigated the effects of BJ-5ta-Exo on a
UVB-irradiated photoaging model using human neonatal foreskin
fibroblast BJ-5ta, SKH1 hairless mice and a reconstructed human
skin model, as well as its mechanism of action.
The skin barrier, mainly composed of the epidermis,
serves as a physical barrier against pathogens, irritants and UV
radiation. It also prevents water and solute loss, while
maintaining homeostasis (40).
Exposure to UVB radiation can impair the function of the epidermis
barrier, leading to sunburn, skin dryness and an increased
thickness of the epidermis (41). The present study found that
BJ-5ta-Exo prevented the loss of skin hydration, the increase in
TEWL and epidermal hyperplasia (Fig.
6F-H). Furthermore, it was confirmed that the levels of
filaggrin, which plays a key role in the skin barrier (42), increased in the dorsal skin of
mice treated with BJ-5ta-Exo prior to UVB radiation (Fig. S3). Collectively, these results
suggest that BJ-5ta-Exo has the ability to maintain a healthy skin
barrier against UVB radiation.
As shown in Fig.
2C, BJ-5ta Exo increased the expression of CAT, SOD-1, SOD-2
and GPX, indicating that BJ-5ta-Exo can alleviate UVB-induced
oxidative stress by stimulating the expression antioxidant-related
genes. The Nrf2 transcription factor is a key regulator of cellular
oxidative stress (43). Under
UVR exposure, Nrf2 is typically translocated from the cytoplasm to
the nucleus, where it activates various target genes, including
heme oxygenase-1, NAD(P)H quinone dehydrogenase 1,
glutamate-cysteine ligase and glutathione synthetase, which help
mitigate cellular oxidative stress (44). However, if UVR increases
extremely, the balance of defense systems such as Nrf2 be disrupted
(13). As shown in Fig. 2D and E, BJ-5ta Exo suppressed
UVB-mediated Nrf2 downregulation, which suggests that BJ-5ta Exo
attenuated UVB-induced oxidative stress by increasing Nrf2
activity.
RAD51 plays a central role in eukaryotic homologous
recombination (HR), where it identifies and invades homologous DNA
sequences to facilitate the accurate and timely repair of the DNA
(45). The phosphorylated form
of histone H2AX (γH2AX) is widely regarded as the most sensitive
indicator of DSB formation (46). BJ-5ta Exo attenuated the
induction of γH2AX by UVB irradiation. Mechanistically, BJ-5ta Exo
functioned by downregulating RAD51, which in turn promoted the
repair of damaged DNA (Fig. 3A and
B).
The increased ROS production induced by UVB may
result in the activation of p53 and p21, which are hallmarks of
cellular senescence associated with cell cycle arrest and decreased
cell proliferation (47). As
shown in Fig. 3F, BJ-5ta Exo
alleviated the UVB-induced increase in p53 and p21. The protein
c-PARP serves as a marker for cells undergoing apoptosis (48). BJ-5ta Exo also inhibited the
UVB-induced increase in cleaved PARP levels. In addition, it was
confirmed that BJ-5ta-Exo reduced cell cycle arrest and apoptosis
(Fig. 3D and E), indicating that
BJ-5ta Exo promote DNA repair, resulting in a decrease in cellular
apoptosis and prompting cell cycle arrest.
Collagen and elastin networks comprise the majority
of the ECM in the skin. Collagen constitutes ~70% of the dermal
layer and provides tensile stiffness and strength, whereas the
biopolymer elastin, in the form of elastic fibers, provides
compliance and supports stress during multiaxial deformation
(49). UVB not only hinders
collagen synthesis and promotes its breakdown, but also boosts the
degradation of elastin in fibroblasts (50). It was observed that BJ-5ta-Exo
attenuated UVB-mediated wrinkle formation (Fig. 6A and E). Consistent with these
findings, BJ-5ta Exo suppressed the UVB-mediated decrease in
procollagen type I, collagen type I and elastin. Additionally,
BJ-5ta Exo downregulated the UVB-induced increase in MMP-1 levels
(Fig. 7D and G). These results
strongly support the effectiveness of BJ-5ta Exo against
UVB-induced photoaging. Indeed, exosomes contain proteins, lipids
and RNAs that are specific to their cell origin and could deliver
cargo to both nearby and distant cells. As a result, the
investigation of exosome cargo contents may provide offer
opportunities for disease detection and treatment. Therefore, the
authors aim to analyze protein cargo using liquid
chromatography-mass spectrometry in future studies.
In conclusion, the findings of the present study
indicate that BJ-5ta Exo have the potential to serve as a
preventive and therapeutic option for improving
photoaging-associated skin diseases.
Supplementary Data
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AYP was involved in the study methodology,
investigation, data curation, formal analysis, and in the writing
of the manuscript. JOL was involved in the writing, reviewing and
editing of the manuscript, as well as in data investigation and
data curation, and in funding acquisition. YNJ, YJK, SYK, and JML
were involved in data investigation and data curation. KHY was
involved in the conceptualization of the study, in data
investigation, and in the reviewing and editing of the manuscript.
BJK was involved in the conceptualization and methodology of the
study, in data investigation, and in project administration. All
authors have read and approved the final manuscript. JOL and NYJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the principles of laboratory animal care at the National
Institutes of Health and with the approval of the Ethics Committee
for Laboratory Animals at Chung-Ang University (IACUC no.
A2022053).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Chung-Ang University
Research Scholarship Grants in 2023.
References
|
1
|
Kageyama H and Waditee-Sirisattha R:
Antioxidative, anti-inflammatory, and anti-aging properties of
mycosporine-like amino acids: Molecular and cellular mechanisms in
the protection of skin-aging. Marine Drugs. 17:2222019.
|
|
2
|
Agar NS, Halliday GM, Barnetson RS,
Ananthaswamy HN, Wheeler M and Jones AM: The basal layer in human
squamous tumors harbors more UVA than UVB fingerprint mutations: A
role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA.
101:4954–4959. 2004.
|
|
3
|
Battie C, Jitsukawa S, Bernerd F, Del Bino
S, Marionnet C and Verschoore M: New insights in photoaging, UVA
induced damage and skin types. Exp Dermatol. 23:7–12. 2014.
|
|
4
|
Ansary TM, Hossain MR, Kamiya K, Komine M
and Ohtsuki M: Inflammatory molecules associated with ultraviolet
radiation-mediated skin aging. Int J Mol Sci. 22:39742021.
|
|
5
|
Elmets CA and Athar M: Milestones in
photocarcinogenesis. J Invest Dermatol. 133:E13–E17. 2013.
|
|
6
|
D'Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013.
|
|
7
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008.
|
|
8
|
Fuller B: Role of PGE-2 and other
inflammatory mediators in skin aging and their inhibition by
topical natural anti-inflammatories. Cosmetics-Basel. 6:2019.
|
|
9
|
de Jager TL, Cockrell AE and Du Plessis
SS: Ultraviolet light induced generation of reactive oxygen
species. Adv Exp Med Biol. 996:15–23. 2017.
|
|
10
|
Amaro-Ortiz A, Yan B and D'Orazio JA:
Ultraviolet Radiation, Aging and the Skin: Prevention of damage by
Topical cAMP manipulation. Molecules. 19:6202–6219. 2014.
|
|
11
|
Kammeyer A and Luiten RM: Oxidation events
and skin aging. Ageing Res Rev. 21:16–29. 2015.
|
|
12
|
Tyrrell RM: Modulation of gene expression
by the oxidative stress generated in human skin cells by UVA
radiation and the restoration of redox homeostasis. Photoch
Photobio Sci. 11:135–147. 2012.
|
|
13
|
Thiele JJ, Traber MG and Packer L:
Depletion of human stratum corneum vitamin E: An early and
sensitive in vivo marker of UV induced photo-oxidation. J Invest
Dermatol. 110:756–761. 1998.
|
|
14
|
Cinat D, Coppes RP and Barazzuol L: DNA
Damage-induced inflammatory microenvironment and adult stem cell
response. Front Cell Dev Biol. 9:7291362021.
|
|
15
|
Tanveer MA, Rashid H and Tasduq SA:
Molecular basis of skin photoaging and therapeutic interventions by
plant-derived natural product ingredients: A comprehensive review.
Heliyon. 9:e135802023.
|
|
16
|
Halicka HD, Huang X, Traganos F, King MA,
Dai W and Darzynkiewicz Z: Histone H2AX phosphorylation after cell
irradiation with UV-B-Relationship to cell cycle phase and
induction of apoptosis. Cell Cycle. 4:339–345. 2005.
|
|
17
|
Revet I, Feeney L, Bruguera S, Segalés J,
Díaz I, Galindo-Cardiel IJ, Martínez E, Darwich L, Fang Y,
Maldonado J, et al: Functional relevance of the histone gammaH2Ax
in the response to DNA damaging agents. Proc Natl Acad Sci USA.
108:8663–8667. 2011.
|
|
18
|
Georgoulis A, Vorgias CE, Chrousos GP and
Rogakou EP: Genome instability and gammaH2AX. Int J Mol Sci.
18:19792017.
|
|
19
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells-Basel.
8:7272019.
|
|
20
|
Zhang Y, Liu YF, Liu HY and Tang WH:
Exosomes: Biogenesis, biologic function and clinical potential.
Cell Biosci. 9:192019.
|
|
21
|
Oh M, Lee J, Kim YJ, Rhee WJ and Park JH:
Exosomes derived from human induced pluripotent stem cells
ameliorate the aging of skin fibroblasts. Int J Mol Sci.
19:17152018.
|
|
22
|
Choi JS, Cho WL, Choi YJ, Kim JD, Park HA,
Kim SY, Park JH, Jo DG and Cho YW: Functional recovery in
photo-damaged human dermal fibroblasts by human adipose-derived
stem cell extracellular vesicles. J Extracell Vesicles.
8:15658852019.
|
|
23
|
Tracy LE, Minasian RA and Caterson EJ:
Extracellular matrix and dermal fibroblast function in the healing
wound. Adv Wound Care. 5:119–136. 2016.
|
|
24
|
Shin JW, Kwon SH, Choi JY, Na JI, Huh CH,
Choi HR and Park KC: Molecular mechanisms of dermal aging and
antiaging approaches. Int J Mol Sci. 20:21262019.
|
|
25
|
Greussing R, Hackl M, Charoentong P, Pauck
A, Monteforte R, Cavinato M, Hofer E, Scheideler M, Neuhaus M,
Micutkova L, et al: Identification of microRNA-mRNA functional
interactions in UVB-induced senescence of human diploid
fibroblasts. BMC Genomics. 14:2242013.
|
|
26
|
Najar M, Crompot E, van Grunsven LA, Dolle
L and Lagneaux L: Foreskin-derived mesenchymal stromal cells with
aldehyde dehydrogenase activity: Isolation and gene profiling. BMC
Cell Biol. 19:42018.
|
|
27
|
Badhani B, Sharma N and Kakkar R: Gallic
acid: A versatile antioxidant with promising therapeutic and
industrial applications. Rsc Adv. 5:27540–27557. 2015.
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
29
|
Ko MJ, Mulia GE and van Rijn RM: Commonly
used Anesthesia/Euthanasia methods for brain collection
differentially impact MAPK activity in male and female C57BL/6
Mice. Front Cell Neurosci. 13:962019.
|
|
30
|
Boivin GP, Hickman DL, Creamer-Hente MA,
Pritchett-Corning KR and Bratcher NA: Review of CO2 as a Euthanasia
agent for laboratory rats and mice. J Am Assoc Lab Anim Sci.
56:491–499. 2017.
|
|
31
|
Kim YI, Kim KS, Ahn HJ, Kang IH and Shin
MK: Reduced matrix metalloproteinase and collagen transcription
mediated by the TGF-β/Smad pathway in passaged normal human dermal
fibroblasts. J Cosmet Dermatol. 19:1211–1218. 2020.
|
|
32
|
Oh JH, Joo YH, Karadeniz F, Ko J and Kong
CS: Syringaresinol inhibits UVA-induced MMP-1 expression by
suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human
dermal fibroblasts. Int J Mol Sci. 21:39812020.
|
|
33
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020.
|
|
34
|
Shafei S, Khanmohammadi M, Heidari R,
Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M,
Sanikhani NS, Absalan M and Tavoosidana G: Exosome loaded alginate
hydrogel promotes tissue regeneration in full-thickness skin
wounds: An in vivo study. J Biomed Mater Res A. 108:545–556.
2020.
|
|
35
|
Wang C, Wang M, Xu T, Zhang X, Lin C, Gao
W, Xu H, Lei B and Mao C: Engineering Bioactive Self-Healing
antibacterial exosomes hydrogel for promoting chronic diabetic
wound healing and complete skin regeneration. Theranostics.
9:65–76. 2019.
|
|
36
|
Fafian-Labora JA, Rodriguez-Navarro JA and
O'Loghlen A: Small extracellular vesicles have GST activity and
ameliorate senescence-related tissue damage. Cell Metab.
32:71–86.e5. 2020.
|
|
37
|
Han X, Wu P, Li L, Sahal HM, Ji C, Zhang
J, Wang Y, Wang Q, Qian H, Shi H and Xu W: Exosomes derived from
autologous dermal fibroblasts promote diabetic cutaneous wound
healing through the Akt/β-catenin pathway. Cell Cycle. 20:616–629.
2021.
|
|
38
|
Gao W, Wang X, Si Y, Pang J, Liu H, Li S,
Ding Q and Wang Y: Exosome derived from ADSCs attenuates
ultraviolet B-mediated photoaging in human dermal fibroblasts.
Photochem Photobiol. 97:795–804. 2021.
|
|
39
|
Ellistasari EY, Kariosentono H, Purwanto
B, Wasita B, Riswiyant RCA, Pamungkasari EP and Soetrisno S:
Exosomes derived from secretome human umbilical vein endothelial
cells (Exo-HUVEC) Ameliorate the Photo-Aging of Skin Fibroblast.
Clin Cosmet Inv Derm. 15:1583–1591. 2022.
|
|
40
|
Tanaka Y, Uchi H and Furue M: Antioxidant
cinnamaldehyde attenuates UVB-induced photoaging. J Dermatol Sci.
96:151–158. 2019.
|
|
41
|
Wang L, Yang K, Jing R, Zhao W, Guo K, Hu
Z, Liu G, Xu N, Zhao J, Lin L and Gao S: Protective effect of
Saussurea involucrata polysaccharide against skin dryness induced
by ultraviolet radiation. Front Pharmacol. 14:10895372023.
|
|
42
|
Sandilands A, Sutherland C, Irvine AD and
McLean WH: Filaggrin in the frontline: Role in skin barrier
function and disease. J Cell Sci. 122:1285–1294. 2009.
|
|
43
|
Caricchio R, McPhie L and Cohen PL:
Ultraviolet B radiation-induced cell death: Critical role of
ultraviolet dose in inflammation and lupus autoantigen
redistribution. J Immunol. 171:5778–5786. 2003.
|
|
44
|
Zhang M, An C, Gao Y, Leak RK, Chen J and
Zhang F: Emerging roles of Nrf2 and phase II antioxidant enzymes in
neuroprotection. Prog Neurobiol. 100:30–47. 2013.
|
|
45
|
Angelis KJ, Zaveska Drabkova L, Vagnerova
R and Hola M: RAD51 and RAD51B play diverse roles in the repair of
DNA double strand breaks in physcomitrium patens. Genes (Basel).
14:3052023.
|
|
46
|
Deng M, Xu Y, Yu Z, Wang X, Cai Y, Zheng
H, Li W and Zhang W: Protective effect of fat extract on
UVB-Induced photoaging in vitro and in vivo. Oxid Med Cell Longev.
2019:61469422019.
|
|
47
|
Granados-Lopez AJ, Manzanares-Acuña E,
López-Hernández Y, Castañeda-Delgado JE, Fraire-Soto I,
Reyes-Estrada CA, Gutiérrez-Hernández R and López JA: UVB Inhibits
proliferation, cell cycle and induces apoptosis via p53, E2F1 and
microtubules system in cervical cancer cell lines. Int J Mol Sci.
22:51972021.
|
|
48
|
Tewari M, Quan LT, Orourke K, Desnoyers S,
Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995.
|
|
49
|
Henninger HB, Ellis BJ, Scott SA and Weiss
JA: Contributions of elastic fibers, collagen, and extracellular
matrix to the multiaxial mechanics of ligament. J Mech Behav
Biomed. 99:118–126. 2019.
|
|
50
|
Varani J, Spearman D, Perone P, Fligiel
SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ and Voorhees JJ:
Inhibition of type I procollagen synthesis by damaged collagen in
photoaged skin and by collagenase-degraded collagen in vitro. Am J
Pathol. 158:931–942. 2001.
|