Introduction
The incidence of malignant tumors has been
increasing annually and has become a common issue faced by mankind.
Traditional therapies such as surgery, conventional chemotherapy
and radiation therapy have been playing a leading role in the
treatment of cancer. However, the effects of these therapies are
limited, particularly in the advanced stages of disease. Thus, it
is difficult for patients to benefit from these treatments in the
long term, and they cannot fundamentally solve or change their
quality of life or survival. Therefore, researchers are
increasingly focusing on the field of immunotherapy.
Immunotherapy has provided novel opportunities and
hope for the treatment of tumors, in which immune checkpoint
inhibitors, therapeutic antibodies, cytokine-immunomodulators,
tumor vaccines and over-the-counter cellular therapies have
markedly improved the prognosis of patients with tumors, and some
patients with advanced-stage tumors have achieved long-term
survival (1–3).
Cytokines are an integral part of the tumor
microenvironment (TME) and those released in response to infection,
inflammation and immunity can inhibit or promote tumor development.
Thus, cytokines play a vital role in tumor pathogenesis. Numerous
types of recombinant protein products are currently available,
among which, antibody-cytokine conjugates are a class of cytokine
drugs with immense value in clinical applications (4–6).
With the continuous application of antibody drugs in more
therapeutic fields, the structures of these agents and their
therapeutic mechanisms have become increasing complex and diverse.
Among these agents, nanobodies have attracted ample attention due
to their small size and unique molecular structure, rendering them
suitable for a few applications, such as disease diagnosis and
treatment (7–11). The present review summarizes the
application of nanobody-related immunocytokines,
agonists/inhibitors, engager cytokines and cytokine receptors in
tumor immunotherapy and immunoimaging.
Cytokines and cytokine-based
immunodrugs
Cytokines are a class of small proteins with a wide
range of biological activities. These molecules are synthesized and
secreted by immune cells [such as monocytes, macrophages, T-cells,
B-cells and natural killer (NK) cells] and some non-immune cells
(such as endothelial cells, epidermal cells and fibroblasts) under
stimulation, and play a critical role in cell signaling (12,13). Cytokines are classified as
interleukins (ILs), interferon (IFNs), tumor necrosis factor
superfamily, colony-stimulating factors, chemokines and growth
factors. They act through cell surface receptors and are
particularly important in the immune system, regulating the balance
between humoral and cell-based immune responses, as well as the
maturation, growth, and responsiveness of specific cell
populations. The effect of individual cytokines on immunity depends
on the local cytokine concentration, the pattern of their receptor
expression and the integration of multiple signaling pathways in
the immune response cells (14–16). As molecular messengers, cytokines
facilitate communication between immune cells, which perform
regulatory and effector functions in a number of diseases.
Consequently, cytokines and their receptors are increasingly being
used in immunotherapy, particularly in host immune responses to
inflammation (16), infection
(17–21), tumors (4–6)
and trauma (22–24).
As immunomodulators, numerous cytokines have been
used in the form of recombinant, synthetic and natural agents for
activation and suppression immunotherapy, including ILs (IL-2, IL-7
and IL-12) (25–30), chemokines (CCL3, CCL26 and CXCL9)
(31–33) and other cytokines [IFN,
granulocyte colony-stimulating factor (G-CSF)] (34–36).
During immunotherapy, cytokines directly stimulate
immune effector cells and stromal cells at tumor sites to enhance
immune effector cytotoxicity. Studies using animal tumor models
have demonstrated that cytokines have broad antitumor activities,
and many have been used in cancer therapy (4,6,37,38). Several cytokine drugs have been
approved by the Food and Drug Administration (FDA), such as
high-dose IL-2 for the treatment of melanoma and renal cell
carcinoma in 1992 (39,40) and IFN-α for the adjuvant treatment
of stage III melanoma (41).
Several other cytokines, such as GM-CSF, IL-7, IL-12, IL-15 and
IL-18, are also being investigated in trials with different
clinical research statuses (NCT02978222, NCT04833504, NCT02451748,
NCT01055522, NCT01189383, NCT05297084, NCT05307068) (39–44).
In activation immunotherapy, G-CSF (35,36) is used to stimulate peripheral
blood stem cells to produce lymphocytes that are later co-cultured
with tumor antigens in vitro and infused back into the
patient. Combined with stimulating cytokines to enhance immunity,
the tumor cells carrying the same antigens are destroyed to achieve
the therapeutic effect. IL-7 and IL-2 can be used to restore the
immune system in immunocompromised patients, and this approach is
already being evaluated in trials with different clinical research
statuses (NCT01339000, NCT03308786) (45,46). By contrast, the focus of
suppressive immunotherapy is on reducing normal immune responses to
prevent rejection in cell or organ transplantation (47,48) or suppressing abnormal immune
responses in autoimmune diseases (49,50).
The potent immunomodulatory effects of cytokines,
such as IL-2, IL-7, IL-15, IL-21 and IL-12, and IFNs have been used
with some success in humans (51). However, the development of
cytokine therapeutics in clinical practice is hindered by multiple
issues, mainly polymorphisms in cytokine immunomodulation, the
short half-life and toxicity due to the activation of off-target
cells (52–55). These toxicities occur due to the
uptake of large doses of free pro-inflammatory cytokines by
surrounding tissues before reaching their intended destination and
the lack of effective concentrations within the tumor. With a
better understanding of the structural principles and functional
signaling of cytokine-receptor interactions, artificial
modifications of cytokines through methods, such as protein
engineering have facilitated the generation of effective drugs
(56). Engineered cytokines,
including immunocytokines, cytokine agonists/inhibitors and engager
cytokines with antibodies have been developed. Antibodies of
engineered cytokines are directed mainly against tumor markers and
the cytokines currently fused with antibodies include IL-2
(57), IL-10 (58), TNF-α (59), IL-12 (60,61), IL-15 (62), IL-21 (63) and IL-4 (64). This approach has exhibited great
promise as a useful platform for the development of effective
antitumor therapeutics. However, traditional antibodies have
disadvantages, such as a long expression cycle, high costs, poor
stability and cumbersome genetic engineering, thus limiting their
application as cytokine drugs.
Since nanobodies are simple in structure, stable,
soluble, easy to express and have a low immunogenicity, they have
become the focus of research in the development of immunotherapy,
widely used in various fields such as research, diagnosis,
detection and drug development (65–70).
Nanobodies and their advantages in
immunotherapy
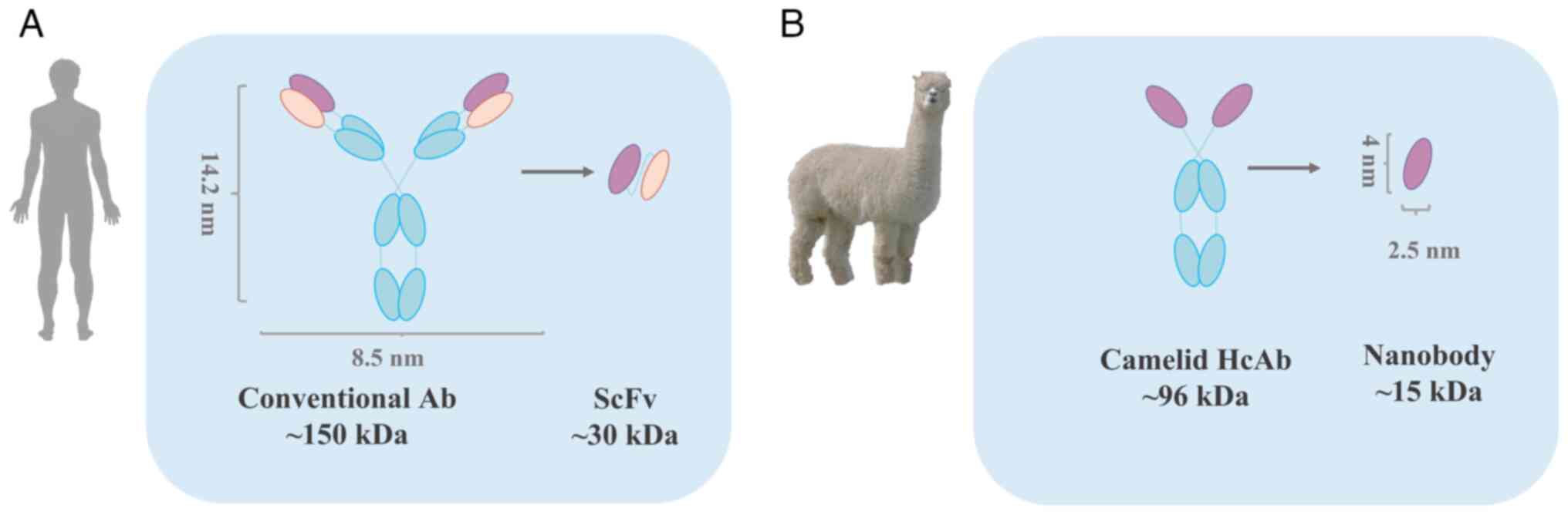
Nanobodies were first reported by the Belgian
scientist, Hamers-Casterman et al (71) in 1993; they, as well as others
found that some antibodies in the blood of camelids (camels,
alpacas and their relatives) existed as heavy chain antibodies with
missing light chains (71,72).
These naturally occurring light chain-deficient antibodies, also
known as single-domain heavy chain antibodies, are found in Asian
camels (Camelus bactrianus) and African dromedaries
(Camelus dromedarius), as well as alpacas (Vicugna
pacos), large alpacas (Lama glama) and llamas (Lama
guanicoe) (71,72). These antibodies contain only two
heavy chain variable regions (VHH) and two heavy chain CH2 and CH3
regions (Fig. 1). The VHH region
retains intact antigen-binding capacity and is the smallest intact
antigen-binding fragment, known as single-domain antibodies
(73). VHH crystals are 2.5×4 nm
in size, with a molecular weight of only 15 kDa.
Compared with traditional antibody fragments, such
as the fragment of antigen binding (Fab) and single chain antibody
fragment (scFv), nanobodies have significant advantages, such as
weak immunogenicity, low production costs, good water solubility
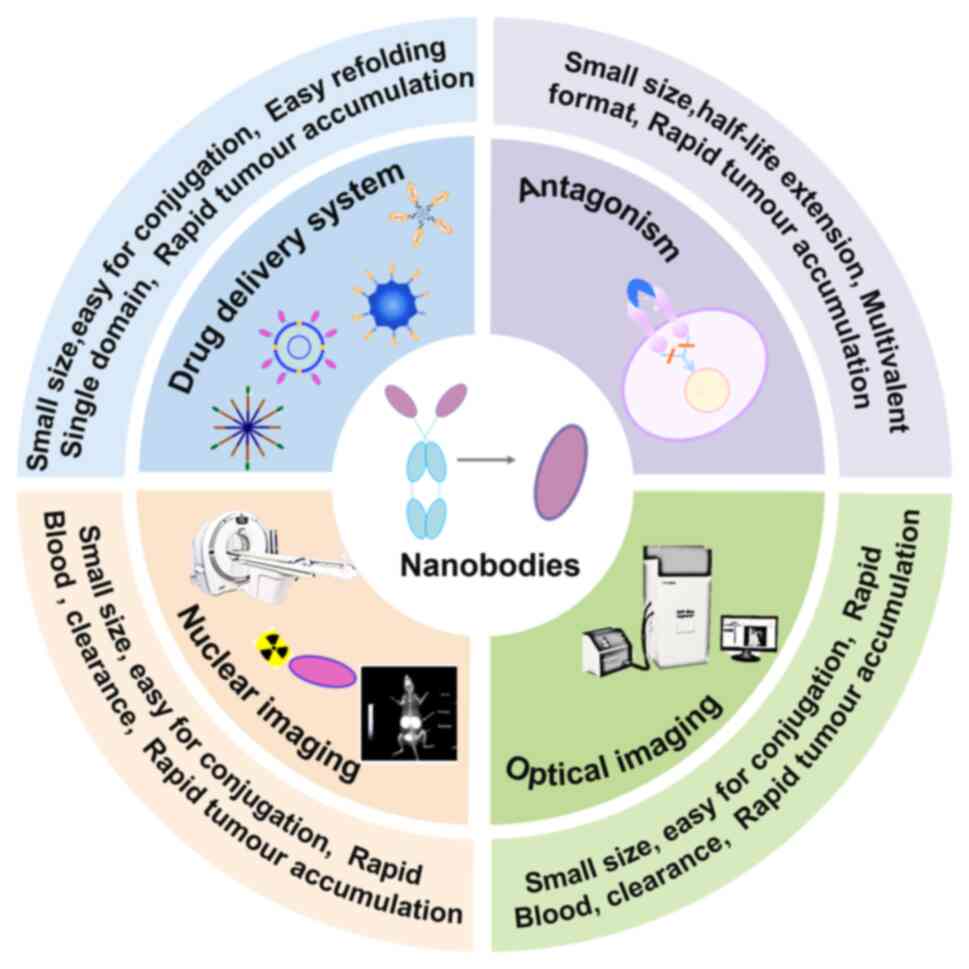
and tissue permeability, as well as good stability (Table I). It is these excellent
properties that have led to the widespread application of
nanobodies in biotechnology (74–77). They have been widely used in
multiple medical fields, such as infection and immunity (78–80), and oncology (81–83) and exhibit immense potential for
new drug discovery (Fig. 2). A
comparison of the properties of nanobodies and conventional
antibodies is presented in Table
I.
 | Table I.Comparison of the properties of
nanobodies and conventional antibodies. |
Table I.
Comparison of the properties of
nanobodies and conventional antibodies.
|
Characteristics | Nanobody | Conventional
antibody | (Refs.) |
|---|
| Stability | High stability and
high temperature resistance | Low stability,
easily inactivated, temperature sensitive | (65–67) |
| Immunogenicity | Low | High | (68) |
| Tissue
penetration | High, penetrates
the blood-brain barrier | Low | (66,68) |
| Half-life
period | Short, fast serum
clearance | Long half-life | (68,69) |
| Expression
systems | Mammalian cell
expression system, yeast expression system, E. coli
expression system, low-cost, high water solubility of expressed
antibodies | Mammalian cell
expression system, high cost and long expression period | (68–70) |
The present review summarizes the application of
nanobodies in immunocytokines, cytokine agonists/inhibitors,
engager cytokines and cytokine receptors.
Application of nanobodies in
cytokine-mediated immunotherapy and immunoimaging
Immunocytokines (nanobody-cytokine
fusion proteins)
High concentrations of cytokines in the TME markedly
enhance the effectiveness of tumor immunotherapy. Since cytokines
lack targeting capacity, methods designed to direct cytokines to
disease sites are critical for improving the therapeutic
effectiveness of cytokine drugs. Antibodies or peptides against
disease site-specific biomarkers may be ideal ‘vehicles’ for
targeted cytokine delivery (84).
Antibody-cytokine fusion proteins targeting tumor biomarkers have
been shown to significantly increase the selective accumulation of
corresponding cytokines at sites of tissue remodeling in many mouse
models. These fusion proteins exploit the tumor-targeting ability
of antibodies to specifically direct cytokines to tumor sites,
where they can stimulate a more desirable antitumor immune response
while avoiding the systemic toxicity that limits the potential
efficacy of current cytokine doses (85–89). Such antibody-cytokine fusion
proteins are referred to as immunocytokines (47,90), which have recently been
redeveloped and described as a next-generation cytokine product. A
summary of immunocytokine-based nanobodies currently under
investigation is presented in Table
II.
 | Table II.Summary of immunocytokine-based
nanobodies. |
Table II.
Summary of immunocytokine-based
nanobodies.
|
Immunocytokines | Cytokines | Fusion
nanobody | Function | (Refs.) |
|---|
| CEA-IL15 | IL-15 | Anti-CEA
nanobody | Antitumor | (92) |
| Ia1-TNFα | TNF-α | Anti-EGFR nanobody
(Ia1) | Antitumor | (100) |
| IL-2
immunocytokine | IL-2 | Nanobody of
fibronectin EIIIB domain | Antitumor | (89) |
IL-2-based immunocytokines
As a water-soluble cytokine, IL-2 was the first IL
to be discovered and cloned. In the 1980s and 1990s, the immune
enhancing properties of IL-2 made it a promising immune-enhancing
drug for the pharmaceutical market. Lutz et al (91) engineered IL-2 immunocytokines
fused to nanobodies that specifically targeted the fibronectin
EIIIB structural domain of tumors with nanomolar to picomolar
affinity. This high affinity allows for the specific targeting of
the immunocytokine to the tumor, where IL-2 exerts an antitumor
effect in the TME. Moreover, the intra-tumoral administration
improves the survival rates of tumor-bearing mice, indicating that
this is a promising route for the targeted delivery of small
molecules and cytokines.
IL-15-based immunocytokines
IL-15 belongs to the same cytokine family as IL-2.
However, IL-15 has no immunosuppressive function and may exert a
more potent antitumor effect than IL-2 (62,92). High doses of IL-15 are required to
achieve biological function, but this carries the risk of toxicity
(93). Therefore, the extension
and enhancement of the therapeutic activity of IL-15 has been
explored (93,94). Such approaches include targeting
IL-15 to the TME to promote the specific chemotaxis of immune cells
in the TME, thereby enhancing the antitumor function of IL-15.
Liu et al (95) constructed an anti-CEA-IL15
structure by fusing anti-CEA nanobody-Fc and IL15Rα-IL15. This
fusion protein recognizes CEA-positive tumor cells, while
possessing potent cytokine activity that activates and mobilizes
the immune system against cancer cells. The anti-CEA-IL15 fusion
protein promotes immune cell proliferation in vitro and
targets the TME, where it exerts potent antitumor activity in a
xenograft model in vivo. These data support the further
development of the anti-CEA-IL15 immunocytokine for cancer
immunotherapy, and highlight the potential application of this
strategy to other cytokines and tumor-targeting molecules to
enhance the antitumor efficacy.
TNF-α-based immunocytokines
TNF-α belongs to the TNF superfamily of cytokines
(96–98) and is a multifunctional molecule
that regulates biological processes, including cell proliferation
(98), differentiation (98,99), apoptosis (98,99) and anti-tumor activity (100–103). TNF-α represents a key link in
the cell signaling pathway and has thus become the target of
numerous drugs. TNF-α-targeting inhibitors (TNF inhibitors) are
mainly monoclonal antibodies, including infliximab (Remicade),
adalimumab (Humira), certolizumab (Cimzia) and golimumab (Simponi),
or the TNF receptor-antibody fusion protein etanercept (Enbrel).
Clinically, therapeutic antibodies against TNF-α have been used
successfully in the treatment of rheumatoid arthritis (RA) and
Crohn's disease, and studies have shown positive efficacy in
psoriasis and ankylosing spondylitis (101). However, the systemic
administration of TNF-α inhibitors can lead to severe adverse
effects, such as shock and organ failure, which greatly limits its
clinical application (101,102).
Osaki et al (103) constructed an antibody-cytokine
fusion protein (Ia1-TNF-α) consisting of a single-domain antibody
(also called nanobody) Ia1 and a TNF-α domain. Ia1 targets the
epidermal growth factor receptor (EGFR), which is overexpressed in
epithelial tumors, while TNF-α has antitumor activity. By using the
E. coli expression system, Ia1-TNF-α was produced in a
soluble form, which exists mainly in a trimeric form that is
consistent with the multimeric state of TNF-α. Flow cytometric
analysis revealed the specific binding of Ia1-TNF-α to
EGFR-expressing tumor cells, with higher binding activity than
monovalent Ia1, indicating that the fusion protein binds
multivalently to tumor cells. Taken together, these results suggest
that the fusion of TNFα and single-domain antibodies Ia1 may be a
cost-effective method to produce antitumor therapeutics (103).
Cytokine agonists/inhibitors
In diseases or defense states in which large amounts
of cytokines are expressed, excessive levels of cytokine can cause
systemic or local toxic responses, such as cytokine storms, and
therefore inhibitors are required to limit their activity and
tightly control cytokine levels (104–107). Antibodies obtained by immunizing
animals with cytokines or their receptors as immunogens, exert
poent inhibitory effects on cytokines and can be used to treat
diseases caused by excess cytokines. In addition to inhibitors,
agonists targeting certain cytokine receptors to mobilize agonistic
activity can be used in tumor immunotherapy. If nanobodies
targeting different cytokine receptors or different epitopes of the
same cytokine receptor are designed as bispecific antibodies, their
agonistic/inhibitor activity can also be enhanced by cross-linking.
Bispecific nanobodies targeting two receptors concurrently were
designed as antibody agonists for diverse purposes, such as
nanobodies targeting IL-2 receptor and IL-15 receptor for tumor
immunotherapy, IFN receptors for COVID-19 treatment, IL-2 receptor
and IL-10 receptor for the activation of NK cells and T-cells
(108).
Details of the cytokine agonists/inhibitors in the
form of nanobodies currently under investigation are summarized in
Table III.
 | Table III.Summary of cytokine
agonists/inhibitors in the form of nanobodies. |
Table III.
Summary of cytokine
agonists/inhibitors in the form of nanobodies.
| Nanobody-cytokine
agonists/inhibitors | Cytokine | Function | Clinical trials:
Stage and status | (Refs.) |
|---|
| Monovalent and
multivalent anti-hIL-23 | IL-23 | Treating
inflammatory diseases | Preclinical | (112) |
| Nanobodies |
|
|
|
|
| Anti-IL13
nanobody | IL-13 | Inhibition of IL-13
function | Preclinical | (113) |
| Anti-IL-17A
nanobody | IL-17A | Neutralization
nanobody | Preclinical | (116) |
| M1095 | IL-17A and
IL-17F | Block IL-17A/
IL-17F | Clinical Phase
IIb | (117,118) |
| BI-655088 | Anti-CX3CR1 | CX3CR1
biotherapeutic antagonist, inhibition of atherosclerosis | Clinical Phase
Ib | (119) |
| V-L-R-H | TNF-α | Inhibit tumor cells
migration and proliferation | Preclinical | (120) |
| TNF-α nanobody
(NT-3) | TNF-α | Block TNF
signaling | Preclinical | (121) |
| VHH-Zag | TNF-α | Prolonged VHH
half-life and improved Pharmacokinetics | Preclinical | (122) |
| Ozoralizumab
(ATN-103) | TNF-α | Treatment of
patients with rheumatoid arthritis | Clinical Phase
IIIb | (123) |
| Lactic acid
bacteria secreting anti-murine TNF nanobodies | TNF-α | Treatment of
chronic colitis | Preclinical | (124) |
IL-23 specific nanobody
IL-23 is a heterodimer consisting of two subunits,
p40 and p19. In isolation, p19 has no biological activity, but
combines with p40 to form biologically active IL-23 (109), which plays a role in innate and
adaptive immunity and is a key cytokine that promotes inflammatory
responses in various target organs (110,111). Desmyter et al (112) prepared monovalent anti-human
IL23 nanobodies comprising the nanobody 37D5, which blocks p19, and
the nanobody 22E11, which blocks p40. The same research group also
constructed multivalent IL23-specific blocking nanobodies by fusing
the monovalent nanobodies 37D5, 22E11, 124C4 and the serum albumin
antibody Alb1, thus providing improved hIL23 neutralization ability
compared with the monovalent nanobodies. With an extended half-life
that prolongs in vivo exposure, this multivalent nanobody
construct represents an excellent drug candidate for the treatment
of inflammatory diseases (112).
IL-13 specific nanobody
IL-13 is involved in allergies, inflammation and
fibrosis in response to a number of different cell types, such as
mast cells, B-cells and fibroblasts. The inhibition of IL-13
function has exhibited great potential in preclinical studies in
animal models (113). However,
targeting IL-13 with conventional monoclonal antibodies alone has
failed to improve the therapeutic efficacy (113). Nanobodies targeting IL-13 were
prepared by Gevenois et al (113) for combination therapy or for
novel strategies, such as local (pulmonary) administration. They
designed a multimeric structure resulting in a 36-fold increase in
affinity and a 300-fold increase in biological activity, while
maintaining high specificity for IL-13 (113). This approach therefore provides
new insight for the development of cytokine nanobody drugs.
IL-17A specific nanobody
As a member of the IL-17 family, IL-17A plays a
crucial role in host defense, autoimmune disease pathogenesis and
tumorigenesis, particularly in promoting the inflammatory
autoimmunity in diseases, such as psoriasis, psoriatic arthritis
and ankylosing spondylitis (114,115).
To alleviate the immune system disturbance caused by
cytokine overproduction, known as hypercytokinemia, Yao et
al (116) constructed a
novel immunosorbent containing anti-IL-17A nanobodies to remove
IL-17A in the blood. Such nanobody-loaded immunosorbents represent
a simple and cost-effective platform technology for the removal of
single or even multiple cytokines from plasma.
M1095 (117)
(also known as ALX-0761, Sonelokimab) is a trivalent bispecific
nanobody targeting IL-17A and IL-17F. It consists of three
single-domain antibodies comprising the anti-IL-17A domain,
anti-IL-17F domain and anti-human serum albumin domain, which are
coupled to enhance targeting and prolong the serum half-life.
Papp et al (117) and Svecova et al (118) conducted a clinical study of
M1095 in patients with plaque psoriasis and evaluated the safety of
M1095 in multiple dose increments in terms of susceptibility,
tolerability and immunogenicity. Their results demonstrated a
significant clinical benefit of M1095 at doses ≤120 mg for the
treatment of moderate-to-severe plaque psoriasis, with rapid onset
of action, durable improvement and an acceptable safety profile.
Therefore, these data indicate the potential value of 17A/F
nanobodies for clinical application.
C-X3-C motif chemokine receptor 1
(CX3CR1) specific nanobody
CX3CR1 is a multifunctional inflammatory chemokine,
with diverse biological effects in the immune system and the TME.
BI-655088, a nanobody targeting the CX3CR1 protein, is currently in
phase I clinical studies (NCT02696616) for the treatment of chronic
kidney disease. Low et al (119) reported that when added to
standard-of-care treatments including statins, BI-655088 reduced
inflammation in atherosclerotic plaques, which may contribute to
the significant reduction in atherothrombotic events in patients
with existing cardiovascular disease.
TNF-α specific nanobody
The biological functions of TNF-α are diverse and
its action mechanisms are complex. In addition to its anti-tumor
effects, TNF-α plays a pathological role in a variety of autoimmune
diseases, in which pro- and anti-inflammatory cytokines modulate
the function of the immune system. Anti-TNF-α therapy has been used
with varying degrees of success in arthritis, psoriasis,
inflammatory bowel disease, psoriasis, Crohn's disease and
non-infectious uveitis (102).
In addition to tumor therapy, TNF-α nanobodies are also used for
in vivo molecular imaging. However, certain side-effects
involve rashes, transaminitis, anemia have also limited the
clinical use of TNF-α inhibitors (102). Researchers at home and abroad
have been exploring the mechanisms underlying the therapeutic
effects of TNF-α and its inhibitors in order to ‘target and
pinpoint’ more effective treatment strategies.
Ji et al (120) and Nie et al (121) developed an anti-TNF-α nanobody
and experimentally demonstrated that the anti-TNF-α nanobody
inhibited proliferation and promoted the apoptosis of tumor cells.
However, since nanobodies are rapidly cleared from the circulation,
novel strategies are required to extend their half-life for
therapeutic use. Morais et al (122) innovatively designed novel
nanobodies that couple a bacterial albumin binding structural
domain from protein Zag and anti-human serum albumin antibody
(123) to the TNF nanobodies,
this fusion protein enhances the in vivo performance of TNF
nanobodies and reduces blood clearance, renal retention and
excretion.
Vandenbroucke et al (124) innovatively established lactic
acid bacteria (Lactococcus lactis) that secrete anti-murine
TNF (mTNF)-α nanobodies, which were shown to neutralize mTNF in
vitro. Furthermore, the daily oral administration of the
engineered Lactococcus lactis facilitated the local delivery
of the anti-mTNF nanobodies to the colon and significantly reduced
inflammation in model mice with dextran sodium sulfate-induced
chronic colitis.
Engager cytokines
Bispecific immunoconjugates are designed with two
arms, one targeting tumor antigens, while the other targets T-cells
or NK cells. For example, bispecific T-cell engagers (BiTEs) are an
engineered molecule that targets CD3 on T-cells via one arm and
cancer cells through the other arm (80–83). Bispecific antibodies that bind
CD3-activated T-cells have been successful in treating hematologic
tumors, such as blinatumomab targeting CD3/CD19 (125), primarily by bridging T-cells to
CD19-expressing tumor cells, while activating T-cells to release
associated cytokines to kill tumor cells. In the context of
bridging the activation of NK cells to kill tumor cells, an
increasing number of studies are now adding cytokines, such as
IL-15 and IL-2, to enhance the efficacy of these platforms. For
example, IL-15 has been incorporated into the development of
bispecific killer cell engagers (BiKEs) to promote the survival and
expansion of NK cells (126–128). Details of the engager cytokines
in the form of nanobodies currently under investigation are
summarized in Table IV.
 | Table IV.Summary of nanobody-engager
cytokines. |
Table IV.
Summary of nanobody-engager
cytokines.
| Nanobody-engager
cytokines | Cytokine-related
factors | Nanobody-related
factors | Function | (Refs.) |
|---|
| CD16-EGFR
bispecific VHH | EGFR | Anti-CD16 nanobody
C21 | Tumor
immunotherapy | (129) |
| CAM1615HER2 | IL-15 | Anti-CD16
nanobody | Tumor
immunotherapy | (130) |
Toffoli et al (129) prepared a novel bispecific
single-domain antibody (VHH) that binds to CD16 (FcRgammaIII) on NK
cells and EGFR on epithelial-derived tumor cells. This bispecific
VHH triggered CD16- and EGFR-dependent activation of NK cells and
subsequent tumor cells lysis, independent of the tumor KRAS
mutation status. The enhanced activation of NK cells by bispecific
VHH was also observed when NK cells from patients with colorectal
cancer (CRC) were co-cultured with EGFR-expressing tumor cells.
Finally, higher levels of cytotoxicity against patient-derived
metastatic CRC cells were found in the presence of bispecific VHH
and autologous peripheral blood monocytes cells or allogeneic
CD16-expressing NK cells. Thus, the antitumor activity of CD16-EGFR
bispecific VHHs warrants further exploration.
Vallera et al (130) prepared the human epidermal
growth factor receptor 2 (HER2) tri-specific killer cell engagers
(TriKEs) CAM1615HER2, consisting of a camelid VHH antibody fragment
recognizing CD16, a single chain variable fragment (scFv)
recognizing HER2, and cross-linked human IL-15. This
triple-specific killer attractor (TriKETM) has been shown to induce
NK cell proliferation in vitro and exhibits strong cytotoxic
activity against acute myelogenous leukemia (AML) cell lines and
patient-derived AML cells.
Nanobody targeting
cytokine-receptors
Cytokines generally exert their biological effects
by binding to the corresponding cytokine receptors on the cell
surface. This interaction initiates complex intracellular molecular
changes that ultimately regulate cellular gene transcription.
Cytokine receptors are capable of mediating a remarkable array of
biological responses. Details of the nanobody-related
cytokine-receptors currently under investigation are summarized in
Table V.
 | Table V.Summary of nanobody-related
cytokine-receptors. |
Table V.
Summary of nanobody-related
cytokine-receptors.
| Cytokine receptor
nanobody | Target | Function | (Refs.) |
|---|
| ALX-0061 | IL-6R | IL-6 signal
blockade | (133) |
| VHH26 nanobody | G-CSF receptor
(G-CSF-R) | Blockade of
downstream G-CSF-R signaling | (138,139) |
| VEGFR2-specific
nanobody (3VGR19) | Vascular
endothelial growth factor receptor-2 (VEGFR2) | VEGFR2 signaling
blockade | (141) |
| V21-DOS47 | Anti-VEGFR2
nanobody (V21) | VEGFR site blockade
with cytotoxicity | (142) |
| Second-generation
CAR-T cell based on a VEGFR2-nanobody (anti-VEGFR2 CARs) | VEGFR2
nanobody | T cell
immunotherapy for solid tumors | (143) |
| Anti-EGFR
nanobody | EGFR | EGFR signal
blockade | (146) |
| 7D12, EgA1and
9G8 | EGFR | EGFR signal
blockade | (147) |
| 7D12-IR | EGFR
nanobody-7D12 | Rapid preclinical
optical imaging | (148) |
| 7D12-800CW | EGFR
nanobody-7D12 | Head and neck
cancer surveillance | (149) |
| (99m) Tc-D10 | EGFR
nanobody-D10 | Specific and high
contrast in vivo visualization of small human tumors
overexpressing EGFR | (152) |
| OA-cb6-(99m)
Tc | EGFR nanobody-
OA-cb6 | Tumor imaging in
cancers with high EGFR expression | (153) |
| Tc radiolabeled EG2
(mTc-sdAb EG2) | EGFR nanobody
EG2 | EGFR detection for
monitoring tumor cells | (154) |
| Multivalent
antibodies EG2-RHCC and EG2-COMP | EGFR nanobody
EG2 | EGFR detection for
monitoring tumor cells | (157) |
| 68Ga labeled 7D12
[(68)Ga-nanobody] | EGFR nanobody
7D12 | Tumor detection and
imaging | (155) |
|
DTPA-IRDye700DX-7D12 | EGFR
nanobody-7D12 | Nuclear imaging and
targeted photodynamic therapy of EGFR-positive tumors | (156) |
Il-6 specific nanobody
IL-6 is a key pro-inflammatory cytokine that plays a
crucial role in systemic inflammation and joint destruction in
patients with RA (131). The
biological activity of IL-6 is mediated through a hexameric
signaling complex consisting of two molecules each of IL-6, IL-6R
and glycoprotein (107,132). Unlike other cytokines, IL-6
exerts biological functions by binding to membrane-bound receptors
(mIL-6R; classical signaling) or soluble receptors (sIL-6R; trans
signaling) (132). Van Roy et
al (133) developed
Nanobody® ALX-0061 consisting of one domain that targets
IL-6R and a second domain designed to improve the pharmacokinetic
properties by binding to human serum albumin. In cynomolgus
monkeys, ALX-0061 exhibited the dose-dependent and complete
inhibition of hIL-6-induced inflammatory parameters including
plasma C-reactive protein, fibrinogen and platelet levels. It also
exhibited a longer plasma half-life of 6.6 days, with albumin
binding yielding the expected half-life extension technique
(133). ALX-0061 is currently in
clinical development with promising results obtained in a phase
I/II trial in RA (NCT02518620). A number of preclinical
pharmacological properties of ALX-0061 support its development as a
clinical agent in RA.
G-CSF specific nanobody
G-CSF induces the proliferation and differentiation
of hematopoietic precursor cells, and the activation of mature
neutrophils (134). G-CSF is
overexpressed in certain malignancies and the blockade of its
binding to the receptor markedly reduces tumor growth, tumor
vascularization and metastasis (135). In addition, targeting the G-CSF
receptor has been shown to exhibit therapeutic efficacy in
conditions such as RA (136), as
well as progressive neurodegenerative diseases (137).
A nanobody known as VHH26 developed by Bakherad
et al (138,139) specifically binds to the G-CSF-R
on the surface of NFS60 cells and effectively blocks the downstream
signaling pathway of G-CSF-R in a dose-dependent manner. Based on
this, Bakherad et al (138,139) modified the G-CSF-R targeting
nanobody (VHH1) to increase its affinity for G-CSF-R by redesigning
the complementary determining region 3 domain of the VHH1 nanobody
to mimic the interaction of G-CSF with its receptor. The newly
engineered nanobodies exhibited dose-dependent binding to the
G-CSF-R on the surface of NFS60 cells, as well as higher biological
activity compared to the parental nanobodies. These newly developed
nanobodies may be beneficial for tumor imaging and therapy and lay
the foundation for the development of other engineered nanobodies.
However, further studies are required to better characterize these
nanobodies and evaluate their interaction with G-CSF-R both in
vitro and in vivo.
Vascular endothelial growth factor
receptor (VEGFR) specific nanobody
VEGFR2 is a critical tumor-associated receptor that
is responsible for >85% of cancer-related deaths in solid tumors
that require angiogenesis to promote their growth and metastasis.
Targeting VEGFR2, which is overexpressed on tumor blood vessels,
has emerged as a promising strategy for antiangiogenic therapy
(140). Behdani et al
(141) described the
identification of the VEGFR2-specific nanobody, 3VGR19, isolated
from dromedary camels immunized with cell lines expressing high
levels of VEGFR2. Flow cytometric analysis revealed that 3VGR19
specifically bound VEGFR2 on the cell surface of 293KDR and HUVECs,
and effectively inhibited the formation of capillary-like
structures (141). These data
demonstrate the potential of nanobodies to block VEGFR2 signaling
and provide a basis for the development of novel cancer
therapies.
In addition to blocking VEGFR2 as receptors,
nanobodies are also combined with other proteins to increase their
destructive effects on tumor cells. Tian et al (142) prepared the fusion protein
V21-DOS47, consisting of a VEGFR2-specific nanobody (V21) and
urease, which converts endogenous urea to toxic ammonia. Thus, the
V21-DOS47 immunocomplex not only blocks the VEGFR site, but also
targets cytotoxic activity to the tumor. To improve the stability
of the immunoconjugate, the research group generated two versions
of the V21 antibody (V21H1 and V21H4) by adding several amino acid
residues at the C-terminus to modulate the activity of the V21
antibody. In addition, different chemical cross-linkers were used
to conjugate to urease. The heterofunctional cross-linker
succinimidyl-[(N-maleimidopropionamido)-diethyleneglycol] ester was
used to couple V21H1 to urease, whereas a homofunctional
cross-linker 1,8-bis (male imide) diethylene glycol was used to
conjugate V21H4. Bioactivity analysis revealed that V21H4-DOS47 was
expressed at high levels, was easy to purify and produce, and
retained higher binding activity than V21H1-DOS47 (142). This strategy for amino acid
regulation of nanobodies and modification of complex cross-linker
agents has highlighted new avenues for the development of cytokine
nanobody-related drugs.
Taheri et al (143) developed second-generation CAR
T-cells based on a nanobody (VHH) that targets VEGFR2-expressing
tumor cells. This CAR T-cell was developed by linking the
anti-VEGFR2 VHH to the signaling domains of CD28 and CD3 zeta.
Following co-culture with VEGFR2-expressing cells, CAR T-cells
exhibited a 41 and 48% expression of the activation markers, CD69
and CD25, and produced 470 and 360 pg/ml of IL-2 and IFN-γ,
respectively. The CAR T-cells exhibited 30% cytotoxic activity at
an effector-to-target ratio of 9:1. Thus, anti-VEGFR2 CARs are
implicated as candidates for T-cell immunotherapy for solid
tumors.
EGFR-specific nanobody for
immunotherapy
EGFR, which plays a crucial role in cell growth,
reproduction and differentiation, has long been recognized as a
promising target for cancer diagnostic and therapeutic
applications. EGFR tyrosine kinase-mediated signaling is closely
related to tumor development, and the inhibition of this receptor
activity can effectively suppress tumors (144). Anti-EGFR monoclonal antibodies
block EGFR downstream signaling pathways, thereby inhibiting
uncontrolled cell proliferation (145). Monoclonal antibodies and other
large antibodies spread slowly in tumors, limiting their efficacy.
To develop low molecular weight probes against EGFR and other tumor
cell receptors, Gottlin et al (146) constructed a VHH phage library by
immunizing llamas with the extracellular domain of EGFR, as well as
the oncogenic mutant receptor EGFRvIII and tumor cell line
extracts. The EGFR-specific nanobodies identified by screening were
found to be cross-specific with existing anti-EGFR monoclonal
antibodies, with a high affinity in the nM range (146).
Schmitz et al (147) described the X-ray crystal
structures of three inhibitory nanobodies (7D12, EgA1 and 9G8)
binding to the extracellular domain of EGFR. All three nanobodies
inhibited EGFR activation, although via different mechanisms. 7D12
sterically blocked ligand binding to EGFR in a cetuximab-like
manner, while EgA1 and 9G8 were shown to bind to epitopes near the
EGFR domain II/III junction, thereby preventing the conformational
changes of the receptor required for high-affinity ligand binding
and dimerization. This epitope contacts the convex VHH paratope,
but not with the flat paratope of the monoclonal antibody (147). Understanding the binding and
inhibition modes of these VHH domains will aid their development in
tumor imaging and/or cancer therapy.
To improve the performance for rapid preclinical
optical imaging and visualization, Oliveira et al (148) developed a nanobody-based
anti-EGFR probe consisting of the anti-EGFR nanobody 7D12 and
cetuximab attached to the near-infrared fluorophore IRDye800CW.
7D12-IR allowed the visualization of tumors up to 30 min
post-injection, whereas no signal above background was observed at
the tumor site when cetuximab-IR was used, indicating that this
nanobody-based anti-EGFR probe has excellent performance for rapid
preclinical optical imaging and is expected to be applied as an
auxiliary diagnostic tool in humans in the future (148).
Subsequently, van Driel et al (149) used a similar 7D12-800CW probe
for head and neck cancer surveillance. Lymph node metastases in the
neck were clearly detected following the injection of 75 µg
7D12-800CW (149). Applying this
approach in clinical practice may thus improve the survival rate of
radical surgical resection.
To block EGF-mediated EGFR activation van Lith
et al (150) prepared a
conjugate of a cell-penetrating peptide and 7D12 that specifically
binds and internalizes EGFR cells. The VHH-CPP conjugate exhibited
a combination of activities implemented through the application of
a very powerful new design principle that blocks receptor
activation by removing the receptor from the cell surface. To
further apply 7D12 to antitumor therapy, van Lith et al
(151) functionalized VHH 7D12
with the photosensitizer PS (IRDye700DX) to develop
photoimmunotherapy (PIT). The use of low molecular weight camelid
single-domain antibodies (VHHs, nanobodies) in PIT is preferred
over full-size antibodies due to improved tumor penetration
(151). Both VHH([PS]) and
VHH([PS])-CPP conjugates specifically induced the death of cancer
cells expressing high EGFR under near-infrared light, including
tumor cell lines highly expressing EGFR and cells highly expressing
EGFR extracted from the ascites of patients with high-grade
plasmacytotic ovarian cancer. However, the VHH([PS]) were
significantly more effective compared to internalized
VHH([PS])-CPP, suggesting that cell surface binding is necessary
for optimal therapeutic activity.
EGFR-specific nanobody for
immunoimaging
Given that EGFR is overexpressed in several types
of human epithelial cancers, the non-invasive molecular imaging of
this receptor using specific monoclonal antibodies against EGFR as
probes has important implications for the diagnosis of certain
tumors. However, the long half-life of monoclonal antibodies in
blood has prompted the development of smaller probes. Nanobodies
are the smallest intact antigen-binding fragments (15 kDa), and the
variable domains of heavy chain antibodies (VHHs) are valuable
reagents for tumor diagnosis and therapy when combined with
diagnostic probes and therapeutic compounds. For example, EGFR
nanobodies labeled with (99m) Tc (152–154) or Ga (155) were used in positron emission
tomography (PET) to image EGFR expression in mouse tumors. In
addition, EGFR nanobodies labeled with photosensitizers such as
IRDye 700DX (152,156) and IRDye 800CW (149,150) were used for near-infrared (NIR)
fluorescence imaging studies in tumor-bearing mice.
Krüwel et al (152) used the (99m) Tc-labeled EGFR
Nanobody D10 [(99m) Tc-D10] for the visual detection of small tumor
lesions <100 mm. EGFR-overexpressing small human tumors were
visualized specifically and with high contrast in vivo by
preclinical multi-pinhole SPECT following the intravenous injection
of (99m) Tc-D10 (153).
Piramoon et al (153) synthesized and labeled the
anti-EGFR nanobody, OA-cb6, with (99m) Tc(CO)3(+) and evaluated its
specific targeting properties in the A431 human epidermal carcinoma
cells. In their study, stable radiolabeled nanobodies were obtained
with a high yield and radiochemical purity. Biodistribution
analysis in nude mice revealed a good tumor-to-muscle ratio and the
location of the tumor was visible 4 h after the injection of the
radiolabeled nanobodies. Thus, the OA-cb6-(99m) Tc radiolabeled
nanobody is a promising radiolabeled biomolecule for tumor imaging
in cancers with a high EGFR expression (153).
Li et al (154) used a tricarbonyl kit to Tc
radiolabel EG2, a nanobody targeting the EGFR, and single-photon
emission computer imaging revealed that A431 tumor images were
clearly visible as early as 1 h after the injection of (99m)
Tc-sdAb EG2. Biodistribution analyses demonstrated that (99m)
Tc-sdAb EG2 uptake by A431 tumors was blocked by ~51% 3 h following
an overdose injection. This indicated that sdAb EG2 effectively
targets EGFR and highlights its potential as a molecular probe for
EGFR detection (154). To
prepare more sensitive tracers, Li's group (157) fused the nanobody targeting the
EGFR with a right-handed helical coil (RHCC) and human cartilage
oligomeric matrix protein (COMP) to form multivalent antibodies
EG2-COMP. SPECT imaging showed that A431 expressing high EGFR
levels was clearly visible 6 h after injection of (99m)
Tc-EG2-COMP. Therefore, EG2-COMP shows promise for clinical
application in the real-time monitoring of tumor cells.
For PET imaging, Vosjan et al (155) used a novel bifunctional iron
chelator for 68Ga labeling of the anti-EGFR nanobody 7D12. The 68
Ga nanobody was successfully prepared and exhibited high tumor
uptake in nude mice with HoA431 ×enograft tumors that were clearly
visible in PET imaging studies. Thus, the 68 Ga nanobody conjugate
represents a tool with clinical application for tumor detection and
imaging.
Using chelating agents and photosensitizers, Renard
et al (156) achieved
dual labeling of EGFR nanobodies for nuclear imaging and the
targeted photodynamic therapy of EGFR-Positive tumors. The
site-specific binding of the chelator DTPA and photosensitizer
IRDye700DX to the anti-EGFR nanobody 7D12 were optimized for
nuclear imaging and photodynamic therapy applications. Using a
dichlorotetrazine-conjugated platform, 7D12 was site-specifically
equipped with the bimodal probe DTPA-tetrazine-IRDye700DX. The
[111In] In-DTPA-IRDye700DX-7D12 was shown to bind specifically to
A431 cells and efficiently induce their destruction under light
exposure both in vitro and in vivo. In addition,
SPECT and fluorescence imaging confirmed that dTPA-IRDye700DX-7D12
binds to A431 ×enografts cells in vivo. The
dichlorotetrazolium platform provides a feasible approach for
site-specific dual labeling of nanobody 7D12, preserving its
affinity and therapeutic efficacy. Moreover, the flexibility of
this method facilitates modification of the properties of the probe
for other combinations of diagnostic and therapeutic compounds
(156).
Conclusions and future perspectives
Cytokines function as molecular messengers that
allow communication between immune system cells to mediate
regulatory and effector functions in a number of diseases.
Therefore, cytokines and their receptors have important value in
immunotherapy, and cytokines were the first tumor immunotherapy
drug approved by the US FDA.
However, cytokine therapy is prone to various
side-effects and has a narrow therapeutic window, which represents
a challenge to the use of natural cytokines as clinical candidates.
A growing number of research teams are developing new cytokine
drugs, such as ‘immunocytokines’, cytokine agonists/inhibitors and
engager cytokines. Compared with traditional antibodies, nanobodies
are structurally different and exhibit different properties, and
have broad application prospects in basic medical research and
disease treatment. The introduction of nanobodies into the
preparation of cytokine drugs has shown promise as an antitumor
therapy.
Among the nanobody-related ‘immunocytokine’ drugs,
nanobodies target tumor biomarkers by targeting the CEA, EGFR, the
fibronectin EIIIB structural domain of tumors, while cytokines such
as IL-15, TNF-α and IL-2 are introduced locally into the tumors
simultaneously to achieve antitumor effects. Nanobody-engager
cytokines, mainly in the form of BiKEs, are designed to activate
the killing capacity of NK cells or deliver IL-15 in tandem to
activate NK cells for efficient antitumor activity. Compared with
cytokine agonists, greater focus has been placed on
nanobodies-based inhibitors of cytokines, such as IL-23, IL-17,
IL-13 and TNF-α, to neutralize and block inhibitory signals for the
purpose of treating disease. Nanobodies to EGFR are used to block
EGFR downstream signaling and also to generate probes as
non-invasive molecular imaging for diagnostic imaging of
tumors.
The main targets of the current nanobody probes
include tumor membrane antigens and targets in the TME, including,
but not limited to EGFR, HER, VEGF, VEGFR, lymphocyte activation
gene-3 and programmed cell death ligand 1. Due to their small
molecular weight and short circulation time, nanobody probes are
rapidly metabolized by the kidneys; thus, the significant uptake of
high affinity nanobody probes at the tumor site can be achieved
within a short period of time. Nanobodies are therefore novel
targeting molecules for the construction of molecular imaging
probes, which, when labeled with radionuclides, have the advantage
of obtaining high quality images in a short period of time,
providing a comprehensive assessment of the disease and guiding
individualized and precise treatment. However, persistent high
uptake in the kidneys and short circulation times in vivo
have hindered the use of nanobody probes in oncology. To promote
the clinical application of such probes for target-specific
diagnosis and therapy, the pharmacokinetics of nanobody drugs and
probes require further optimization to achieve improved target
affinity, reduced renal uptake and an extended circulation time
in vivo, thereby mitigating drug-induced adverse effects.
and safeguarding patient safety.
Nanobody-related cytokine drugs will soon become a
research focus in accurate tumor diagnosis and targeting therapy.
The following issues are worth paying attention to: First, the
selection of cytokines is the key to immunocytokine therapy. The
same cytokine may have differential effects on different tumor
types, or on different patients with the same tumor. Therefore,
cytokines for antitumor treatment should be selected according to
the characteristics of tumor type, tumor immune microenvironment
and cytokine function. Second, the selection of nanobody targets is
also a key point in cytokine-related immunotherapy. In addition to
tumor antigens, anti-angiogenic factors, immune checkpoints, and
TME factors are good choices for the preparation of ‘immunocytokine
drugs’. Third, the optimization and modification of cytokine drug
structure and expression system are required to improve the quality
of nanobody-related cytokine drugs. Fourth, a wider cytokine
spectrum is still required. An increasing number of previously
unidentified cytokines or new functions of cytokines are being
discovered, which will lead to the development and application of
unexpected cytokine drugs. Finally, for the successful application
of emerging nanobody-based cytokine drugs in immunodiagnosis and
therapy, a multidisciplinary team of researchers, clinicians and
regulators needs to be established to construct an overall
framework for clinical translation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Scientific Foundation of China (grant no. 82003250), the
Liuzhou Science and Technology Program (grant no. 2020PAAA0605) and
the Guangxi Young and Middle-aged Teachers' Basic Research Ability
Enhancement Project (grant no. 2020KY08029).
Availability of data and materials
Not applicable.
Authors' contributions
XZ, JW, YT, CC, ST, SZ and QQ wrote the original
draft of the manuscript. HH and SD designed and revised the
manuscript. All authors contributed to the article and have read
and approved the final version. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Santoni M, Rizzo A, Mollica V, Matrana MR,
Rosellini M, Faloppi L, Marchetti A, Battelli N and Massari F: The
impact of gender on The efficacy of immune checkpoint inhibitors in
cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol.
170:1035962022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santoni M, Rizzo A, Kucharz J, Mollica V,
Rosellini M, Marchetti A, Tassinari E, Monteiro FSM, Soares A,
Molina-Cerrillo J, et al: Complete remissions following
immunotherapy or immuno-oncology combinations in cancer patients:
The MOUSEION-03 meta-analysis. Cancer Immunol Immunother.
72:1365–1379. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rizzo A, Cusmai A, Giovannelli F,
Acquafredda S, Rinaldi L, Misino A, Montagna ES, Ungaro V, Lorusso
M and Palmiotti G: Impact of Proton Pump Inhibitors and
Histamine-2-Receptor Antagonists on Non-Small Cell Lung Cancer
Immunotherapy: A Systematic Review and Meta-Analysis. Cancers
(Basel). 14:14042022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gout DY, Groen LS and van Egmond M: The
present and future of immunocytokines for cancer treatment. Cell
Mol Life Sci. 79:5092022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mortara L, Balza E, Bruno A, Poggi A,
Orecchia P and Carnemolla B: Anti-cancer Therapies Employing IL-2
cytokine tumor targeting: Contribution of innate, adaptive and
immunosuppressive cells in the anti-tumor efficacy. Front Immunol.
9:29052018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JS, Jun SY and Kim YS: Critical issues
in the development of immunotoxins for anticancer therapy. J Pharm
Sci. 109:104–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muyldermans S: Applications of Nanobodies.
Annu Rev Anim Biosci. 9:401–421. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jovčevska I and Muyldermans S: The
therapeutic potential of nanobodies. BioDrugs. 34:11–26. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verhaar ER, Woodham AW and Ploegh HL:
Nanobodies in cancer. Semin Immunol. 52:1014252021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muyldermans S: A guide to: Generation and
design of nanobodies. FEBS J. 288:2084–2102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naidoo DB and Chuturgoon AA: Nanobodies
enhancing cancer visualization, diagnosis and therapeutics. Int J
Mol Sci. 22:97782021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conlon KC, Miljkovic MD and Waldmann TA:
Cytokines in the treatment of cancer. J Interferon Cytokine Res.
39:6–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waldmann TA: Cytokines in cancer
immunotherapy. Cold Spring Harb Perspect Biol. 10:a0284722018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong C: Cytokine regulation and function
in T cells. Annu Rev Immunol. 39:51–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang W and O'Garra A: IL-10 Family
Cytokines IL-10 and IL-22: From basic science to clinical
translation. Immunity. 50:871–891. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A, Dinarello CA, Molgora M and
Garlanda C: Interleukin-1 and related cytokines in the regulation
of inflammation and immunity. Immunity. 50:b778–795. 2019.
View Article : Google Scholar
|
|
17
|
Krayem I and Lipoldová M: Role of host
genetics and cytokines in Leishmania infection. Cytokine.
147:1552442021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fajgenbaum DC and June CH: Cytokine Storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ragab D, Salah Eldin H, Taeimah M, Khattab
R and Salem R: The COVID-19 Cytokine Storm; What we know so far.
Front Immunol. 11:14462020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Huang Z, Huang M and Zeng J:
Predicting severe enterovirus 71-infected hand, foot, and mouth
disease: Cytokines and chemokines. Mediators Inflamm.
2020:92732412020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long DL, Song HL and Qu PP: Cytokines
profiles in cervical mucosa in patients with cervical high-risk
human papillomavirus infection. J Infect Dev Ctries. 15:719–725.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji X, Yue H, Li G and Sang N: Maternal
smoking-induced lung injuries in dams and offspring via
inflammatory cytokines. Environ Int. 156:1066182021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumari M, Mathur P, Aggarwal R, Madan K,
Sagar S, Gupta A, Khurana S, Sreenivas V and Kumar S: Changes in
extracellular cytokines in predicting disease severity and final
clinical outcome of patients with blunt chest trauma.
Immunobiology. 226:1520872021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller ES, Loftus TJ, Kannan KB, Plazas
JM, Efron PA and Mohr AM: Systemic Regulation of Bone Marrow
Stromal Cytokines After Severe Trauma. J Surg Res. 243:220–228.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Z, Ren Z, Yang K, Liu Z, Cao S, Deng
S, Xu L, Liang Y, Guo J, Bian Y, et al: Author Correction: A
next-generation tumor-targeting IL-2 preferentially promotes
tumor-infiltrating CD8+ T-cell response and effective tumor
control. Nat Commun. 11:17162020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chandran E, Meininger L, Karzai F and
Madan RA: Signaling new therapeutic opportunities: Cytokines in
prostate cancer. Expert Opin Biol Ther. 22:1233–1243. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cirella A, Luri-Rey C, Di Trani CA,
Teijeira A, Olivera I, Bolaños E, Castañón E, Palencia B, Brocco D,
Fernández-Sendin M, et al: Novel strategies exploiting
interleukin-12 in cancer immunotherapy. Pharmacol Ther.
239:1081892022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shum T, Omer B, Tashiro H, Kruse RL,
Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R, et al:
Constitutive signaling from an engineered IL7 receptor promotes
durable tumor elimination by tumor-redirected T cells. Cancer
Discov. 7:1238–1247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
interleukin-12 for cancer immunotherapy. Front Immunol.
11:5755972020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hicks KC, Chariou PL, Ozawa Y, Minnar CM,
Knudson KM, Meyer TJ, Bian J, Cam M, Schlom J and Gameiro SR:
Tumour-targeted interleukin-12 and entinostat combination therapy
improves cancer survival by reprogramming the tumour immune cell
landscape. Nat Commun. 12:51512021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokunaga R, Zhang W, Naseem M, Puccini A,
Berger MD, Soni S, McSkane M, Baba H and Lenz HJ: CXCL9, CXCL10,
CXCL11/CXCR3 axis for immune activation-A target for novel cancer
therapy. Cancer Treat Rev. 63:40–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Humblin E and Kamphorst AO: CXCR3-CXCL9:
It's all in the tumor. Immunity. 50:1347–1349. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karin N: Chemokines and cancer: New immune
checkpoints for cancer therapy. Curr Opin Immunol. 51:140–145.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ivashkiv LB: IFNγ: Signalling, epigenetics
and roles in immunity, metabolism, disease and cancer
immunotherapy. Nat Rev Immunol. 8:545–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rizzo A: Use of granulocyte
colony-stimulating factor for adult cancer patients: Current issues
and future directions. Future Oncol. 17:3411–3413. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu L, Liu Y, Yan X, Zhou C and Xiong X:
The role of granulocyte colony stimulating factor in breast cancer
development: A review. Mol Med Rep. 21:2019–2029. 2020.PubMed/NCBI
|
|
37
|
MaruYama T, Chen W and Shibata H: TGF-β
and cancer immunotherapy. Biol Pharm Bull. 45:155–161. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Märkl F, Huynh D, Endres S and Kobold S:
Utilizing chemokines in cancer immunotherapy. Trends Cancer.
8:670–682. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu EJ, Cao X, Moon B, Bae J, Sun Z, Liu Z
and Fu YX: A cytokine receptor-masked IL2 prodrug selectively
activates tumor-infiltrating lymphocytes for potent antitumor
therapy. Nat Commun. 12:27682021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hernandez R, Põder J, LaPorte KM and Malek
TR: Engineering IL-2 for immunotherapy of autoimmunity and cancer.
Nat Rev Immunol. 22:614–628. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mirlekar B and Pylayeva-Gupta Y: IL-12
family cytokines in cancer and immunotherapy. Cancers (Basel).
13:1672021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiu Y, Su M, Liu L, Tang Y, Pan Y and Sun
J: Clinical application of cytokines in cancer immunotherapy. Drug
Des Devel Ther. 15:2269–2287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Runbeck E, Crescioli S, Karagiannis SN and
Papa S: Utilizing immunocytokines for cancer therapy. Antibodies
(Basel). 10:102021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coppola C, Hopkins B, Huhn S, Du Z, Huang
Z and Kelly WJ: Investigation of the Impact from IL-2, IL-7, and
IL-15 on the growth and signaling of activated CD4+ T Cells. Int J
Mol Sci. 21:78142020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JH, Lee KJ and Lee SW: Cancer
immunotherapy with T-cell targeting cytokines: IL-2 and IL-7. BMB
Rep. 54:21–30. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park JH, Waickman AT, Reynolds J, Castro M
and Molina-París C: IL7 receptor signaling in T cells: A
mathematical modeling perspective. Wiley Interdiscip Rev Syst Biol
Med. 11:e14472019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pol JG, Caudana P, Paillet J, Piaggio E
and Kroemer G: Effects of interleukin-2 in immunostimulation and
immunosuppression. J Exp Med. 217:e201912472020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang T, Li J, Li R, Yang C, Zhang W, Qiu
Y, Yang C and Rong R: Correlation between MDSC and immune tolerance
in transplantation: Cytokines, pathways and cell-cell interaction.
Curr Gene Ther. 19:81–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kucuksezer UC, Ozdemir C, Cevhertas L,
Ogulur I, Akdis M and Akdis CA: Mechanisms of allergen-specific
immunotherapy and allergen tolerance. Allergol Int. 69:549–560.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Propper DJ and Balkwill FR: Harnessing
cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol.
19:237–253. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spangler JB, Moraga I, Mendoza JL and
Garcia KC: Insights into cytokine-receptor interactions from
cytokine engineering. Annu Rev Immunol. 33:139–167. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bentebibel SE and Diab A: Cytokines in the
treatment of melanoma. Curr Oncol Rep. 23:832021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo J, Liang Y, Xue D, Shen J, Cai Y, Zhu
J, Fu YX and Peng H: Tumor-conditional IL-15 pro-cytokine
reactivates anti-tumor immunity with limited toxicity. Cell Res.
31:1190–1198. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Briukhovetska D, Dörr J, Endres S, Libby
P, Dinarello CA and Kobold S: Interleukins in cancer: From biology
to therapy. Nat Rev Cancer. 21:481–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zheng X, Wu Y, Bi J, Huang Y, Cheng Y, Li
Y, Wu Y, Cao G and Tian Z: The use of supercytokines,
immunocytokines, engager cytokines, and other synthetic cytokines
in immunotherapy. Cell Mol Immunol. 19:192–209. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Weiss T, Puca E, Silginer M, Hemmerle T,
Pazahr S, Bink A, Weller M, Neri D and Roth P: Immunocytokines are
a promising immunotherapeutic approach against glioblastoma. Sci
Transl Med. 12:eabb23112020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qiao J, Liu Z, Dong C, Luan Y, Zhang A,
Moore C, Fu K, Peng J, Wang Y, Ren Z, et al: Targeting Tumors with
IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis.
Cancer Cell. 35:901–915.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Papadia F, Basso V, Patuzzo R, Maurichi A,
Di Florio A, Zardi L, Ventura E, González-Iglesias R, Lovato V,
Giovannoni L, et al: Isolated limb perfusion with the
tumor-targeting human monoclonal antibody-cytokine fusion protein
L19-TNF plus melphalan and mild hyperthermia in patients with
locally advanced extremity melanoma. J Surg Oncol. 107:173–179.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Morillon YM II, Su Z, Schlom J and Greiner
JW: Temporal changes within the (bladder) tumor microenvironment
that accompany the therapeutic effects of the immunocytokine
NHS-IL12. J Immunother Cancer. 7:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Halin C, Gafner V, Villani ME, Borsi L,
Berndt A, Kosmehl H, Zardi L and Neri D: Synergistic therapeutic
effects of a tumor targeting antibody fragment, fused to
interleukin 12 and to tumor necrosis factor alpha. Cancer Res.
63:3202–3210. 2003.PubMed/NCBI
|
|
62
|
Knudson KM, Hicks KC, Ozawa Y, Schlom J
and Gameiro SR: Functional and mechanistic advantage of the use of
a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer.
8:e0004932020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Deng S, Sun Z, Qiao J, Liang Y, Liu L,
Dong C, Shen A, Wang Y, Tang H, Fu YX and Peng H: Targeting tumors
with IL-21 reshapes the tumor microenvironment by proliferating
PD-1intTim-3-CD8+ T cells. JCI Insight. 5:e1320002020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hemmerle T, Doll F and Neri D:
Antibody-based delivery of IL4 to the neovasculature cures mice
with arthritis. Proc Natl Acad Sci USA. 111:12008–12012. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li T, Cai H, Yao H, Zhou B, Zhang N, van
Vlissingen MF, Kuiken T, Han W, GeurtsvanKessel CH, Gong Y, et al:
A synthetic nanobody targeting RBD protects hamsters from
SARS-CoV-2 infection. Nat Commun. 12:46352021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mir MA, Mehraj U, Sheikh BA and Hamdani
SS: Nanobodies: The ‘Magic Bullets’ in therapeutics, drug delivery
and diagnostics. Hum Antibodies. 28:29–51. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wicke N, Bedford MR and Howarth M:
Gastrobodies are engineered antibody mimetics resilient to pepsin
and hydrochloric acid. Commun Biol. 4:9602021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kang W, Ding C, Zheng D, Ma X, Yi L, Tong
X, Wu C, Xue C, Yu Y and Zhou Q: Nanobody conjugates for targeted
cancer therapy and imaging. Technol Cancer Res Treat.
20:153303382110101172021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yu S, Li Z, Li J, Zhao S, Wu S, Liu H, Bi
X, Li D, Dong J, Duan S and Hammock BD: Generation of dual
functional nanobody-nanoluciferase fusion and its potential in
bioluminescence enzyme immunoassay for trace glypican-3 in serum.
Sens Actuators B Chem. 336:1297172021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Marco A: Recombinant expression of
nanobodies and nanobody-derived immunoreagents. Protein Expr Purif.
172:1056452020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hamers-Casterman C, Atarhouch T,
Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N and
Hamers R: Naturally occurring antibodies devoid of light chains.
Nature. 363:446–448. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Eggers M, Rühl F, Haag F and Koch-Nolte F:
Nanobodies as probes to investigate purinergic signaling. Biochem
Pharmacol. 187:1143942021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Salema V and Fernández LÁ: Escherichia
coli surface display for the selection of nanobodies. Microb
Biotechnol. 10:1468–1484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu B and Yang D: Easily established and
multifunctional synthetic nanobody libraries as research tools. Int
J Mol Sci. 23:14822022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Verkhivker G: Structural and computational
studies of the SARS-CoV-2 spike protein binding mechanisms with
nanobodies: From structure and dynamics to avidity-driven nanobody
engineering. Int J Mol Sci. 23:29282022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Manoutcharian K, Perez-Garmendia R and
Gevorkian G: Recombinant antibody fragments for neurodegenerative
diseases. Curr Neuropharmacol. 15:779–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu M, Li L, Jin D and Liu Y: Nanobody-A
versatile tool for cancer diagnosis and therapeutics. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 13:e16972021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mei Y, Chen Y, Sivaccumar JP, An Z, Xia N
and Luo W: Research progress and applications of nanobody in human
infectious diseases. Front Pharmacol. 13:9639782022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Koenig PA, Das H, Liu H, Kümmerer BM, Gohr
FN, Jenster LM, Schiffelers LDJ, Tesfamariam YM, Uchima M, Wuerth
JD, et al: Structure-guided multivalent nanobodies block SARS-CoV-2
infection and suppress mutational escape. Science.
371:eabe62302021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yu S, Xiong G, Zhao S, Tang Y, Tang H,
Wang K, Liu H, Lan K, Bi X and Duan S: Nanobodies targeting immune
checkpoint molecules for tumor immunotherapy and immunoimaging
(Review). Int J Mol Med. 47:444–454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lecocq Q, De Vlaeminck Y, Hanssens H,
D'Huyvetter M, Raes G, Goyvaerts C, Keyaerts M, Devoogdt N and
Breckpot K: Theranostics in immuno-oncology using nanobody
derivatives. Theranostics. 9:7772–7791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sun S, Ding Z, Yang X, Zhao X, Zhao M, Gao
L, Chen Q, Xie S, Liu A, Yin S, et al: Nanobody: A small antibody
with big implications for tumor therapeutic strategy. Int J
Nanomedicine. 16:2337–2356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gurbatri CR, Lia I, Vincent R, Coker C,
Castro S, Treuting PM, Hinchliffe TE, Arpaia N and Danino T:
Engineered probiotics for local tumor delivery of checkpoint
blockade nanobodies. Sci Transl Med. 12:eaax08762020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Di Nitto C, Neri D, Weiss T, Weller M and
De Luca R: Design and characterization of novel antibody-cytokine
fusion proteins based on interleukin-21. Antibodies (Basel).
11:192022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hutmacher C and Neri D: Antibody-cytokine
fusion proteins: Biopharmaceuticals with immunomodulatory
properties for cancer therapy. Adv Drug Deliv Rev. 141:67–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Murer P and Neri D: Antibody-cytokine
fusion proteins: A novel class of biopharmaceuticals for the
therapy of cancer and of chronic inflammation. N Biotechnol.
52:42–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Valedkarimi Z, Nasiri H, Aghebati-Maleki L
and Majidi J: Antibody-cytokine fusion proteins for improving
efficacy and safety of cancer therapy. Biomed Pharmacother.
95:731–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Neri D: Antibody-Cytokine Fusions:
Versatile products for the modulation of anticancer immunity.
Cancer Immunol Res. 7:348–354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ziffels B, Stringhini M, Probst P, Fugmann
T, Sturm T and Neri D: Antibody-Based delivery of cytokine payloads
to carbonic anhydrase IX leads to cancer cures in immunocompetent
tumor-bearing mice. Mol Cancer Ther. 18:1544–1554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Corbellari R, Nadal L, Villa A, Neri D and
De Luca R: The immunocytokine L19-TNF eradicates sarcomas in
combination with chemotherapy agents or with immune check-point
inhibitors. Anticancer Drugs. 31:799–805. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lutz EA, Jailkhani N, Momin N, Huang Y,
Sheen A, Kang BH, Wittrup KD and Hynes RO: Intratumoral
nanobody-IL-2 fusions that bind the tumor extracellular matrix
suppress solid tumor growth in mice. PNAS Nexus. 1:pgac2442022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rhode PR, Egan JO, Xu W, Hong H, Webb GM,
Chen X, Liu B, Zhu X, Wen J, You L, et al: Comparison of the
superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics
in animal models. Cancer Immunol Res. 4:49–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu H, Buhtoiarov IN, Guo H and Cheung NV:
A novel multimeric IL15/IL15Rα-Fc complex to enhance cancer
immunotherapy. Oncoimmunology. 10:18935002021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Corbellari R, Stringhini M, Mock J, Ongaro
T, Villa A, Neri D and De Luca R: A novel Antibody-IL15 fusion
protein selectively localizes to tumors, synergizes with TNF-based
immunocytokine, and inhibits Metastasis. Mol Cancer Ther.
20:859–871. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu Y, Wang Y, Xing J, Li Y, Liu J and
Wang Z: A novel multifunctional anti-CEA-IL15 molecule displays
potent antitumor activities. Drug Des Devel Ther. 12:2645–2654.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zelová H and Hošek J: TNF-α signalling and
inflammation: Interactions between old acquaintances. Inflamm Res.
62:641–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Vanamee ÉS and Faustman DL: Structural
principles of tumor necrosis factor superfamily signaling. Sci
Signal. 11:eaao49102018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mitoma H, Horiuchi T, Tsukamoto H and Ueda
N: Molecular mechanisms of action of anti-TNF-α agents-Comparison
among therapeutic TNF-α antagonists. Cytokine. 101:56–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang Y, Li X, Chihara T, Dong H and
Kagami H: Effect of TNF-α and IL-6 on compact bone-derived cells.
Tissue Eng Regen Med. 18:441–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tsimberidou AM and Giles FJ: TNF-alpha
targeted therapeutic approaches in patients with hematologic
malignancies. Expert Rev Anticancer Ther. 2:277–286. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kemanetzoglou E and Andreadou E: CNS
Demyelination with TNF-α Blockers. Curr Neurol Neurosci Rep.
17:362017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jang DI, Lee AH, Shin HY, Song HR, Park
JH, Kang TB, Lee SR and Yang SH: The role of tumor necrosis factor
alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in
therapeutics. Int J Mol Sci. 22:27192021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Osaki T, Nakanishi T, Aoki M, Omizu T,
Nishiura D and Kitamura M: Soluble expression in escherichia coli
of a single-domain antibody-tumor necrosis factor α fusion protein
specific for epidermal growth factor receptor. Monoclon Antib
Immunodiagn Immunother. 37:20–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tang Y, Sun J, Pan H, Yao F, Yuan Y, Zeng
M, Ye G, Yang G, Zheng B, Fan J, et al: Aberrant cytokine
expression in COVID-19 patients: Associations between cytokines and
disease severity. Cytokine. 143:1555232021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Saxton RA, Glassman CR and Garcia KC:
Emerging principles of cytokine pharmacology and therapeutics. Nat
Rev Drug Discov. 21:21–37. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oppenheim JJ: The future of the cytokine
discipline. Cold Spring Harb Perspect Biol. 10:a0284982018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kaur S, Bansal Y, Kumar R and Bansal G: A
panoramic review of IL-6: Structure, pathophysiological roles and
inhibitors. Bioorg Med Chem. 28:1153272020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yen M, Ren J, Liu Q, Glassman CR, Sheahan
TP, Picton LK, Moreira FR, Rustagi A, Jude KM, Zhao X, et al:
Facile discovery of surrogate cytokine agonists. Cell.
185:1414–1430.e19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Schinocca C, Rizzo C, Fasano S, Grasso G,
La Barbera L, Ciccia F and Guggino G: Role of the IL-23/IL-17
pathway in rheumatic diseases: An overview. Front Immunol.
12:6378292021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Neurath MF: IL-23 in inflammatory bowel
diseases and colon cancer. Cytokine Growth Factor Rev. 45:1–8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Huang J, Wang L, Yu C, Fu Z, Liu C, Zhang
H, Wang K, Guo X and Wang J: Characterization of a reliable
cell-based reporter gene assay for measuring bioactivities of
therapeutic anti-interleukin-23 monoclonal antibodies. Int
Immunopharmacol. 85:1066472020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Desmyter A, Spinelli S, Boutton C,
Saunders M, Blachetot C, de Haard H, Denecker G, Van Roy M,
Cambillau C and Rommelaere H: Neutralization of human interleukin
23 by multivalent nanobodies explained by the structure of
cytokine-nanobody complex. Front Immunol. 8:8842017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gevenois PJY, De Pauw P, Schoonooghe S,
Delporte C, Sebti T, Amighi K, Muyldermans S and Wauthoz N:
Development of neutralizing multimeric nanobody constructs directed
against IL-13: From immunization to lead optimization. J Immunol.
207:2608–2620. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
von Stebut E, Boehncke WH, Ghoreschi K,
Gori T, Kaya Z, Thaci D and Schäffler A: IL-17A in psoriasis and
beyond: Cardiovascular and metabolic implications. Front Immunol.
10:30962020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Brevi A, Cogrossi LL, Grazia G,
Masciovecchio D, Impellizzieri D, Lacanfora L, Grioni M and Bellone
M: Much more than IL-17A: Cytokines of the IL-17 family between
microbiota and cancer. Front Immunol. 11:5654702020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yao G, Huang C, Ji F, Ren J, Zang B and
Jia L: Nanobody-loaded immunosorbent for highly-specific removal of
interleukin-17A from blood. J Chromatogr A. 1654:4624782021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Papp KA, Weinberg MA, Morris A and Reich
K: IL17A/F nanobody sonelokimab in patients with plaque psoriasis:
A multicentre, randomised, placebo-controlled, phase 2b study.
Lancet. 397:1564–1575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Svecova D, Lubell MW, Casset-Semanaz F,
Mackenzie H, Grenningloh R and Krueger JG: A randomized,
double-blind, placebo-controlled phase 1 study of multiple
ascending doses of subcutaneous M1095, an anti-interleukin 17A/F
nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol.
81:196–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Low S, Wu H, Jerath K, Tibolla A, Fogal B,
Conrad R, MacDougall M, Kerr S, Berger V, Dave R, et al: VHH
antibody targeting the chemokine receptor CX3CR1 inhibits
progression of atherosclerosis. MAbs. 12:17093222020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ji X, Han T, Kang N, Huang S and Liu Y:
Preparation of RGD4C fused anti-TNFα nanobody and inhibitory
activity on triple-negative breast cancer in vivo. Life Sci.
260:1182742020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nie J, Ma X, Hu F, Miao H, Feng X, Zhang
P, Han MH, You F, Yang Y, Zhang W and Zheng W: Designing and
constructing a phage display synthesized single domain antibodies
library based on camel VHHs frame for screening and identifying
humanized TNF-α-specific nanobody. Biomed Pharmacother.
137:1113282021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Morais M, Cantante C, Gano L, Santos I,
Lourenço S, Santos C, Fontes C, Aires da Silva F, Gonçalves J and
Correia JD: Biodistribution of a (67)Ga-labeled anti-TNF VHH
single-domain antibody containing a bacterial albumin-binding
domain (Zag). Nucl Med Biol. 41 (Suppl):e44–e48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ishiwatari-Ogata C, Kyuuma M, Ogata H,
Yamakawa M, Iwata K, Ochi M, Hori M, Miyata N and Fujii Y:
Ozoralizumab, a Humanized Anti-TNFα NANOBODY® compound,
exhibits efficacy not only at the onset of arthritis in a human TNF
transgenic mouse but also during secondary failure of
administration of an Anti-TNFα IgG. Front Immunol. 13:8530082022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Vandenbroucke K, de Haard H, Beirnaert E,
Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler
L, Remaut E, et al: Orally administered L: Lactis secreting an
anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal
Immunol. 3:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Moazzami R, Mirzahosein H, Nematollahi L,
Barkhordari F, Raigani M, Hajari Taheri F, Mahboudi F and Davami F:
Woodchuck hepatitis virus post-transcriptional regulation element
(WPRE) Promotes Anti-CD19 BiTE Expression in Expi293 Cells. Iran
Biomed J. 25:275–283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sarhan D, Brandt L, Felices M, Guldevall
K, Lenvik T, Hinderlie P, Curtsinger J, Warlick E, Spellman SR,
Blazar BR, et al: 161533 TriKE stimulates NK-cell function to
overcome myeloid-derived suppressor cells in MDS. Blood Adv.
2:1459–1469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yun HD, Felices M, Vallera DA, Hinderlie
P, Cooley S, Arock M, Gotlib J, Ustun C and Miller JS: Trispecific
killer engager CD16×IL15×CD33 potently induces NK cell activation
and cytotoxicity against neoplastic mast cells. Blood Adv.
2:1580–1584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Vallera DA, Felices M, McElmurry R,
McCullar V, Zhou X, Schmohl JU, Zhang B, Lenvik AJ,
Panoskaltsis-Mortari A, Verneris MR, et al: IL15 trispecific killer
engagers (TriKE) make natural killer cells specific to CD33+
targets while also inducing persistence, in vivo expansion, and
enhanced function. Clin Cancer Res. 22:3440–3450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Toffoli EC, Sheikhi A, Lameris R, King LA,
van Vliet A, Walcheck B, Verheul HMW, Spanholtz J, Tuynman J, de
Gruijl TD and van der Vliet HJ: Enhancement of NK Cell antitumor
effector functions using a bispecific single domain antibody
targeting CD16 and the epidermal growth factor receptor. Cancers
(Basel). 13:54462021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Vallera DA, Oh F, Kodal B, Hinderlie P,
Geller MA, Miller JS and Felices M: A HER2 Tri-Specific NK cell
engager mediates efficient targeting of human ovarian cancer.
Cancers (Basel). 13:39942021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Narazaki M, Tanaka T and Kishimoto T: The
role and therapeutic targeting of IL-6 in rheumatoid arthritis.
Expert Rev Clin Immunol. 13:535–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Baran P, Hansen S, Waetzig GH, Akbarzadeh
M, Lamertz L, Huber HJ, Ahmadian MR, Moll JM and Scheller J: The
balance of interleukin (IL)-6, IL-6·soluble IL-6 receptor (sIL-6R),
and IL-6·sIL-6R·sgp130 complexes allows simultaneous classic and
trans-signaling. J Biol Chem. 293:6762–6775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Van Roy M, Ververken C, Beirnaert E,
Hoefman S, Kolkman J, Vierboom M, Breedveld E, 't Hart B, Poelmans
S, Bontinck L, et al: The preclinical pharmacology of the high
affinity anti-IL-6R Nanobody® ALX-0061 supports its
clinical development in rheumatoid arthritis. Arthritis Res Ther.
17:1352015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chang YJ, Zhao XY and Huang XJ:
Granulocyte colony-stimulating factor-primed unmanipulated
haploidentical blood and marrow transplantation. Front Immunol.
10:25162019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Karagiannidis I, Salataj E, Said Abu Egal
E and Beswick EJ: G-CSF in tumors: Aggressiveness, tumor
microenvironment and immune cell regulation. Cytokine.
142:1554792021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Christensen AD, Haase C, Cook AD and
Hamilton JA: Granulocyte colony-stimulating factor (G-CSF) plays an
important role in immune complex-mediated arthritis. Eur J Immunol.
46:1235–1245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tsai ST, Chu SC, Liu SH, Pang CY, Hou TW,
Lin SZ and Chen SY: Neuroprotection of granulocyte
colony-stimulating factor for early stage Parkinson's disease. Cell
Transplant. 26:409–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bakherad H, Farahmand M, Setayesh N and
Ebrahim-Habibi A: Engineering an anti-granulocyte colony
stimulating factor receptor nanobody for improved affinity. Life
Sci. 257:1180522020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bakherad H, Gargari SLM, Sepehrizadeh Z,
Aghamollaei H, Taheri RA, Torshabi M, Yazdi MT, Ebrahimizadeh W and
Setayesh N: Identification and in vitro characterization of novel
nanobodies against human granulocyte colony-stimulating factor
receptor to provide inhibition of G-CSF function. Biomed
Pharmacother. 93:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cheng K, Liu CF and Rao GW:
Anti-angiogenic Agents: A review on vascular endothelial growth
factor receptor-2 (VEGFR-2) inhibitors. Curr Med Chem.
28:2540–2564. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Behdani M, Zeinali S, Khanahmad H,
Karimipour M, Asadzadeh N, Azadmanesh K, Khabiri A, Schoonooghe S,
Habibi Anbouhi M, Hassanzadeh-Ghassabeh G and Muyldermans S:
Generation and characterization of a functional Nanobody against
the vascular endothelial growth factor receptor-2; angiogenesis
cell receptor. Mol Immunol. 50:35–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tian B, Wong WY, Uger MD, Wisniewski P and
Chao H: Development and characterization of a camelid single domain
antibody-urease conjugate that targets vascular endothelial growth
factor receptor 2. Front Immunol. 8:9562017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Hajari Taheri F, Hassani M, Sharifzadeh Z,
Behdani M, Arashkia A and Abolhassani M: T cell engineered with a
novel nanobody-based chimeric antigen receptor against VEGFR2 as a
candidate for tumor immunotherapy. IUBMB Life. 71:1259–1267. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Rajaram P, Chandra P, Ticku S, Pallavi BK,
Rudresh KB and Mansabdar P: Epidermal growth factor receptor: Role
in human cancer. Indian J Dent Res. 28:687–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liang R, Yang L and Zhu X: Nimotuzumab, an
Anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal
carcinoma. Cancer Control. 28:10732748219893012021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Gottlin EB, Xiangrong Guan, Pegram C,
Cannedy A, Campa MJ and Patz EF Jr: Isolation of novel
EGFR-specific VHH domains. J Biomol Screen. 14:77–85. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Schmitz KR, Bagchi A, Roovers RC, van
Bergen en Henegouwen PM and Ferguson KM: Structural evaluation of
EGFR inhibition mechanisms for nanobodies/VHH domains. Structure.
21:1214–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Oliveira S, van Dongen GA, Stigter-van
Walsum M, Roovers RC, Stam JC, Mali W, van Diest PJ and van Bergen
en Henegouwen PM: Rapid visualization of human tumor xenografts
through optical imaging with a near-infrared fluorescent
anti-epidermal growth factor receptor nanobody. Mol Imaging.
11:33–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
van Driel PB, van der Vorst JR, Verbeek
FP, Oliveira S, Snoeks TJ, Keereweer S, Chan B, Boonstra MC,
Frangioni JV, van Bergen en Henegouwen PM, et al: Intraoperative
fluorescence delineation of head and neck cancer with a fluorescent
anti-epidermal growth factor receptor nanobody. Int J Cancer.
134:2663–2673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
van Lith SAM, van den Brand D, Wallbrecher
R, van Duijnhoven SMJ, Brock R and Leenders WPJ: A conjugate of an
anti-epidermal growth factor receptor (EGFR) VHH and a
cell-penetrating peptide drives receptor internalization and blocks
EGFR activation. Chembiochem. 18:2390–2394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
van Lith SAM, van den Brand D, Wallbrecher
R, Wübbeke L, van Duijnhoven SMJ, Mäkinen PI, Hoogstad-van Evert
JS, Massuger L, Ylä-Herttuala S, Brock R and Leenders WPJ: The
effect of subcellular localization on the efficiency of
EGFR-targeted VHH photosensitizer conjugates. Eur J Pharm Biopharm.
124:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Krüwel T, Nevoltris D, Bode J, Dullin C,
Baty D, Chames P and Alves F: In vivo detection of small tumour
lesions by multi-pinhole SPECT applying a (99m)Tc-labelled nanobody
targeting the Epidermal Growth Factor Receptor. Sci Rep.
6:218342016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Piramoon M, Hosseinimehr SJ, Omidfar K,
Noaparast Z and Abedi SM: 99m Tc-anti-epidermal growth
factor receptor nanobody for tumor imaging. Chem Biol Drug Des.
89:498–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li C, Wen B, Wang L, Feng H, Xia X, Ding
Z, Gao B, Zhang Y and Lan X: 99mTc-labeled single-domain antibody
EG2 in targeting epidermal growth factor receptor: An in vitro and
mouse model in-vivo study. Nucl Med Commun. 36:452–460. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Vosjan MJ, Perk LR, Roovers RC, Visser GW,
Stigter-van Walsum M, van Bergen En Henegouwen PM and van Dongen
GA: Facile labelling of an anti-epidermal growth factor receptor
Nanobody with 68Ga via a novel bifunctional desferal chelate for
immuno-PET. Eur J Nucl Med Mol Imaging. 38:753–763. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Renard E, Collado Camps E, Canovas C, Kip
A, Gotthardt M, Rijpkema M, Denat F, Goncalves V and van Lith SAM:
Site-Specific Dual-Labeling of a VHH with a chelator and a
photosensitizer for nuclear imaging and targeted photodynamic
therapy of EGFR-Positive tumors. Cancers (Basel). 13:4282021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Li C, Feng H, Xia X, Wang L, Gao B, Zhang
Y and Lan X: (99m) Tc-labeled tetramer and pentamer of
single-domain antibody for targeting epidermal growth factor
receptor in xenografted tumors. J Labelled Comp Radiopharm.
59:305–312. 2016. View Article : Google Scholar : PubMed/NCBI
|