1. Introduction
Macrophages can differentiate into distinct
phenotypes in response to various stimuli. The two distinct
polarized states of macrophages are M1-type and M2-type, each
displaying unique functional characteristics. M1-type macrophages,
activated by lipopolysaccharides (LPS) and interferon-gamma
(IFN-γ), play an active role in promoting the formation and rupture
of unstable atherosclerotic plaques, pathogen resistance and tumor
control mainly through innate and adaptive immune responses
(1,2). These macrophages upregulate
inflammatory genes such as nuclear transcription factor-κB (NF-κB)
signal expression, leading to proinflammatory responses and the
release of cytokines like IL-1, IL-1β and TNF-α (3-6).
By contrast, M2-type macrophages induced by IL-4 or IL-13 express
high levels of the mannose receptor (MR, also known as CD206), IL-1
receptor (IL-IR), and C-C motif chemokine ligand 17 (CCL17). They
also secrete pro-fibrosis factors and exhibit high arginase-1
(ARG-1) activity. The distinct characteristics of M2-type
macrophages contribute to their involvement in various
physiological and pathological processes, including pathogen and
parasite clearance, anti-inflammatory reactions, wound healing,
tissue remodeling and immune regulation (1,7-10). The plasticity of macrophages in
the microenvironment underscores the critical role of their
polarization state in determining their function in different
diseases. Consequently, the regulation of macrophage polarization
represents a significant therapeutic target for macrophage-based
treatments across a range of diseases.
The polarization state of macrophages is intricately
linked to alterations in various metabolic processes, including
glycolysis, the pentose phosphate pathway (PPP), oxidative
phosphorylation (OXPHOS), lipid metabolism (synthesis and oxidation
of fatty acids), amino acid metabolism, the tricarboxylic acid
(TCA) cycle and iron metabolism. M1-type macrophages rely on
glycolysis, PPP, heightened fatty acid synthesis (FAS), and iron
retention to generate nitric oxide (NO) and sustain an inflammatory
response. Nevertheless, their TCA cycle and OXPHOS are
dysfunctional. By contrast, M2-type macrophages predominantly
fulfill an anti-inflammatory role by enhancing fatty acid oxidation
(FAO), OXPHOS and glutamine metabolism, while decreasing PPP
activity (11,12).
Targeting macrophage in metabolic regulation has
emerged as a pivotal strategy for addressing metabolic disorders.
Advanced nanomaterials offer unique advantages in targeting
macrophage metabolism for disease treatment, including independence
from the microenvironment, enhanced immune activity and minimal
side effects. These nanomaterials can be composed of organic
materials (lipids, peptides, glycosylated compounds, hyaluronic
acid, or nucleic acids), as well as metals, inorganic materials
(iron oxides and gold), or combinations of these materials. The
utilization of nanomaterials to regulate metabolic abnormalities,
such as glycolysis, lipid metabolism, iron metabolism and glutamine
metabolism, has emerged as a promising therapeutic strategy
(13-16). For instance, polymer composites
have been designed to modulate macrophage glycolysis signals,
reverse the immunosuppressive phenotype of tumor-associated
macrophages (TAMs), and regulate the immunosuppressive tumor
microenvironment (TME) in the past few years (17). This approach introduces an
innovative strategy for targeting macrophage metabolism using
nanomaterials to treat diseases.
The present review provided a comprehensive overview
of state-of-the-art nanomaterials targeting macrophage polarization
for the treatment of metabolic diseases. More specifically, the
intrinsic connection between macrophage metabolism and polarization
was initially discussed. Subsequently, emphasis was given to
describing how advanced nanomaterials are utilized to mitigate the
progression of the disease including cancer and atherosclerosis by
regulating macrophage metabolism.
2. Macrophage metabolism
Typically, M1-type macrophages exhibit elevated
glycolysis, PPP activity, FAS and iron storage. Conversely, M2-type
macrophages predominantly rely on OXPHOS, glutamine metabolism and
fatty acid oxidation (FAO) (as shown in Table I). It is essential to target
macrophage metabolism for regulating macrophages in the
microenvironment of the lesion. In the following sections, a
detailed review of the metabolic processes associated with
macrophages will be provided.
 | Table IClassical metabolism pathway in
M1-type and M2-type macrophage. |
Table I
Classical metabolism pathway in
M1-type and M2-type macrophage.
| M1-type
macrophages | M2-type
macrophages |
|---|
| Glycolysis | Enhanced
glycolysis | / |
| PPP | | |
| OXPHOS | Inhabited
OXPHOS | Increased
OXPHOS |
| Amino acid
metabolism | Express iNOS to NO
from arginine; Glutamine metabolism promote the synthesis of
succinic acid; | Hydrolyzed arginine
to ornithine and urea by arginase; Glutamine metabolism drive
M2-polarization; |
| TCA cycle | Rupture after
citrate and succinate | Entire TCA
cycle |
| Iron
metabolism | High levels of
ferritin and iron deposition | Decrease in
intracellular iron level |
Glycolysis
Glycolysis is a vital metabolic pathway in
macrophages, responsible for converting glucose into lactic acid
and generating a limited quantity of ATP through anaerobic
metabolism. Glycolysis is indispensable for glucose metabolism and
breakdown in macrophages, and the enhancement of glycolysis results
in M1-type polarization. Following bacterial infection or
activation by LPS, macrophages elevate glucose uptake, displaying
augmented aerobic glycolysis. Within the cytoplasm, glucose
undergoes enzymatic processing, ultimately resulting in the
generation of pyruvate and ATP (18).
Pyruvate can be further converted to lactic acid by
lactate dehydrogenase (LDH), or enter the mitochondria to
participate in TCA cycle (19).
In the TCA cycle, pyruvate is transformed into citrate, which is
subsequently transported to the cytoplasm to generate acetyl-CoA, a
precursor molecule that enhances the expression of genes encoding
inflammatory molecules through histone acetylation (20). The metabolic transition from
OXPHOS to glycolysis is evident in classically activated M1-type
macrophages, promoting lactic acid synthesis and the secretion of
inflammatory mediators, thereby contributing to the inflammatory
response (21,22).
Lactic acid, a byproduct of glycolysis, has the
potential to enhance pyruvate kinase activity and facilitate the
polarization of macrophages toward a reparative phenotype (23). Elevated lactic acid
concentrations have the capacity to hinder glycolysis in immune
cells, decrease the extracellular acidification rate, and augment
the oxygen consumption rate. Lactic acid may additionally impede
the activation of YAP and NF-κB in the inflammatory process through
GPR81-mediated signaling, thus inhibiting the pro-inflammatory
response of LPS-stimulated macrophages (24).
The flux of glycolysis is regulated by a range of
enzymes, including glycolytic enzyme and pyruvate kinase
(PKM1/PKM2). Hypoxia-inducible factor 1α (HIF-1α) is a metabolic
regulator that, when overexpressed, increases the expression of
glycolysis-related genes. It upregulates glucose uptake by
stimulating the expression of glucose transporters (such as GLUT1),
as well as genes involved in glycolysis, such as hexokinase 2, PK
and LDHA. Ultimately, HIF-1α mediates M1 type polarization of
macrophages (25,26).
PPP
The PPP is a metabolic pathway that plays a critical
role in nucleotide synthesis and the generation of NADPH
(nicotinamide adenine dinucleotide phosphate). NADPH functions as a
crucial cofactor in diverse cellular processes, encompassing lipid
biosynthesis and the production of NO and reactive oxygen species
(ROS). Activated by LPS and IFN-γ, the PPP is upregulated, leading
to increased synthesis of NADPH in macrophages. The enhanced PPP
activity in activated macrophages supports their heightened
phagocytic function. NADPH derived from the PPP is particularly
essential for cholesterol metabolism and FAS, these pathways are
crucial for macrophage function. Additionally, the increased
synthesis of NADPH through the PPP contributes to the expansion of
the Golgi apparatus and endoplasmic reticulum in macrophages. This
expansion facilitates the secreted production of inflammatory
cytokines, further promoting the immune response (27).
OXPHOS
OXPHOS plays a critical role in inflammation
resolution, and its reduction in M1-type macrophages contributes to
the accumulation of TCA cycle intermediates such as citrate,
succinate, fumarate and malate. Toll-like receptor 4 (TLR4) is
involved in OXPHOS regulation with the PI3K/Akt axis playing a
crucial role in this process. On the other hand, in M2-type
macrophages, OXPHOS is upregulated to support ATP production, which
is essential during the tissue repair phase and serves as the main
functional pathway.
Lipid metabolism
Lipid metabolism is a pivotal aspect of macrophage
function, integral to the modulation of inflammatory responses and
phagocytosis (28). In
classically activated macrophages (M1), stimulation by LPS enhances
de novo lipogenesis (DNL) by converting the cytosolic pool
of glucose-derived citrate into acetyl-CoA, a key component of FAS
(29). The increased
biosynthesis of fatty acids in these macrophages results in the
esterification of fatty acids into triglycerides, serving as a
storage form of lipids (30).
Consequently, LPS-activated macrophages increase glucose
utilization to support DNL and triglyceride synthesis, which is
crucial for maintaining the connection between the actin
cytoskeletal network and the plasma membrane, promoting enhanced
macrophage phagocytosis (30).
In non-inflammatory-activated macrophages (M2), the upregulation of
FAMIN protein expression establishes a connection between DNL and
FAO, intensifying the flow of oxidative metabolism in macrophages
following IL-4 activation (31,32).
FAO
Fatty acid metabolism like FAS and FAO serves
distinct roles in M1-type and M2-type macrophages, respectively
(33,34). FAS is intricately linked to the
pro-inflammatory function of macrophages, whereas M2-type
macrophages rely more on FAO for energy acquisition (34,35). In M1-type macrophages, when the
TCA cycle is disrupted, citrate accumulates and is transported from
the mitochondria to the cytoplasm, where it is converted to
acetyl-CoA through the ATP citrate lyase (ACLY). This sequence of
events stimulates the de novo synthesis of fatty acids
(36). The de novo
synthesis pathway of fatty acids is interconnected with glucose and
lipid metabolism, further supporting M1-type macrophage
polarization (37-40).
Saturated fatty acids (SFAs) have been demonstrated
to induce NF-κB activation and the expression of inflammatory
markers in macrophages through the activation of TLR signaling by
SFA metabolites. This process results in macrophage inflammation,
with the NLRP3 inflammasome orchestrating the activation of
caspase-1 and the release of pro-inflammatory factors (IL-1β and
IL-18) (41). Mitochondrial
uncoupling protein-2 has been found to upregulate FASN-dependent
lipid synthesis and positively regulate NLRP3 inflammasome-mediated
caspase-1 activation in macrophages (42,43). On the other hand, M2-type
macrophages exhibit an increased reliance on FAO (32). FAO generates acetyl-CoA, NADH and
FADH2, which are utilized in the TCA cycle to produce abundant ATP
(44,45). In macrophages treated with LPS,
there is a notable reduction in the ability to oxidize fatty acids
to CO2 due to decreased expression of proteins such as
CPT1α and CPT1β, which are responsible for facilitating the entry
of fatty acids into the mitochondria for oxidation. The FAO pathway
diminishes NLRP3 activation causing the suppression of the
inflammatory response in macrophages (46,47).
Cholesterol metabolism
Cholesterol is a critical lipid in macrophages,
essential for maintaining the integrity, fluidity and functionality
of the macrophage membrane. Precise regulation of intracellular
cholesterol is pivotal for the effective execution of a range of
macrophage functions. Macrophages can synthesize cholesterol, and
it can also be directly imported through the internalization of
lipoproteins (48). Excessive
cholesterol accumulation in macrophages can result in profound
cellular dysfunction and the activation of inflammatory pathways,
leading to IL-1β-mediated inflammation (49). The expression of cholesterol
biosynthesis is regulated by the transcription factor sterol
regulatory element-binding protein 2 (SREBP2). Cholesterol
25-hydroxylase (CH25H) is particularly important in inflammation
and macrophage biology and responsible for producing
25-hydroxycholesterol (25HC), which is considered an interferon
regulatory gene (50).
During the inflammatory response to viruses and
certain microorganisms, changes in cholesterol homeostasis observed
in macrophages are often caused by upregulation of CH25H mediated
by interferon and the subsequent increase in 25HC production
(51). Interferon signaling and
pattern recognition receptors (PRRs) that induce IFN responses,
such as TLR3-TRIF signaling, downregulate cholesterol biosynthesis
and promote cholesterol storage in the form of cholesterol esters
(50,52,53). The synthesis of cholesterol in
macrophages is not directly linked to alterations in the overall
level of intracellular cholesterol. Interferon-stimulated
macrophages, instead of altering cholesterol biosynthesis, respond
to interferon through other mechanisms, including increased
cholesterol uptake or reduced cholesterol efflux (50,54).
Conversely, MyD88-dependent PRRs, such as TLR-2,
TLR-7 and TLR-9, result in increased cholesterol biosynthesis and
overall cholesterol content in macrophages (50). Macrophages preferentially acquire
cholesterol through receptor-mediated endocytosis of
cholesterol-rich lipoproteins. The transport of cholesterol out of
macrophages relies on transport proteins ABCA1 and ABCG1.
Stimulation of macrophages with LPS can decrease the activity of
ABCA1 and ABCG1, impairing cholesterol efflux and leading to
intracellular cholesterol accumulation. Excessive cholesterol
accumulation in macrophages can promote inflammatory responses. In
mice lacking ABCA1 and ABCG1, macrophages exhibit enhanced
cholesterol levels, and the immune response is heightened when
stimulated by TLRs. This is due to the accumulation of cholesterol
potentially causing the formation of cholesterol crystals, which
can activate macrophage inflammasomes or act as phagocytic targets,
triggering inflammatory responses (55,56).
Amino acid metabolism
The availability of amino acids is crucial for
maintaining proper immune cell function during the immune response.
Insufficient amino acids supply can lead to deficiencies in immune
cell activity. Macrophages, in particular, depend on amino acid
catabolism to support immune activation and swiftly adjust to
fluctuating nutrient sources (55). Amino acid metabolism plays a
significant role in regulating various macrophage response
pathways, such as mTOR signaling and NO production. Moreover, amino
acid metabolites possess immunomodulatory properties that influence
macrophage response. For instance, in LPS + IFN-γ-stimulated
macrophages, glutamine is essential for LPS-induced IL-1β
secretion, while arginine is metabolized by inducible NO synthase
(iNOS) to produce NO, which acts as both an antimicrobial agent and
a signaling molecule involved in vasodilation, angiogenesis and
insulin secretion. Macrophages can polarize into an
anti-inflammatory M2 phenotype, leading to significant alterations
in amino acid metabolism, including arginine and proline
metabolism, alanine, aspartic, and glutamic acid metabolism,
cysteine and methionine metabolism, and taurine metabolism
(56). By contrast, M1-type
macrophages rely on glutamine metabolism (57,58). In the following section, the
present review will delve into how arginine metabolism and
glutamine metabolism processes govern macrophage function.
Arginine metabolism
Arginine metabolism indeed serves as a prominent
example of macrophage amino acid metabolism, with ARG-1 being a
classical marker of the M2 phenotype. The metabolic fate of
arginine regulates the polarization of M1-type and M2-type
macrophages. In macrophages stimulated by LPS + IFN-γ, there is an
overexpression of iNOS, which results in citrulline production.
Arginine succinate synthase 1 converts citrulline into arginine
succinate, which is rapidly broken down to regenerate arginine and
sustain NO production. This conversion of arginine to citrulline
and NO through iNOS promotes the loss of mitochondrial complex at
the later stage of M1 polarization (59). On the other hand,
anti-inflammatory M2 macrophages consistently express ARG-1, which
is involved in arginine catabolism. During this process, ornithine
is produced, which can control cell growth and promote tissue
repair when converted to polyamines by ornithine decarboxylase
(60,61). These metabolic pathways highlight
the dynamic regulation of arginine metabolism in macrophage
polarization. The balance between the production of NO and the
metabolism of arginine plays a crucial role in determining the M1
or M2 phenotype of macrophages.
Glutamine metabolism
Different pathways of glutamine metabolism
contribute to the polarization of macrophages into distinct
phenotypes upon activation. Glutamine metabolism in M1-type
macrophages promotes the synthesis of succinic acid by entering the
TCA cycle. Simultaneously, glutamine metabolism also drives M2
polarization (62,63). The degradation of glutamine
generates α-ketoglutaric acid (αKG), which plays a critical role in
OXPHOS and FAO processes in M2-type macrophages. αKG can also
facilitate macrophage epigenetic reprogramming, thereby promoting
the M2 phenotype (64). The
production of α-KG from glutamine decomposition is influenced by
the SENP-Sirt3 signaling pathway, which deacetylates GLUD1,
increasing its activity in glutamine decomposition and promoting
αKG production (65). This
mechanism further modulates M2 polarization by controlling the
αKG/succinic acid ratio. A high ratio favors M2 phenotype, while a
low ratio strengthens the pro-inflammatory phenotype in classically
activated (M1) macrophages. Consequently, αKG contributes to
endotoxin tolerance following M1 activation (64).
Additionally, glutamine metabolism contributes to
the UDP-GlcNAc synthesis pathway, which experiences upregulation in
M2-type macrophages. Glutamine serves as a substrate for glutamine
synthetase (GS), which is highly expressed in M2 macrophages. GS
induces the synthesis of intracellular glutamate and ammonia,
leading to the production of glutamine. The ablation of GS in TAMs
results in reduced expression of M2-type markers, such as ARG1 and
CD206 (66,67). These findings elucidate the
intricate interplay between metabolic and epigenetic reprogramming
through glutamine metabolism in customizing the immune response of
macrophages.
TCA cycle
The TCA cycle, commonly referred to as the Krebs
cycle, serves as the central pathway for the oxidation of
carbohydrates, fatty acids and amino acids. It plays a crucial role
in cellular anabolism (such as gluconeogenesis and lipid synthesis)
and catabolism (including glycolysis) (68). Research has revealed that the TCA
cycle is disrupted at various nodes in M1-type macrophages,
resulting in the accumulation of specific metabolites such as
citrate, itaconic acid and succinate (69,70). By contrast, M2-type macrophages
demonstrate an intact TCA cycle, accompanied by increased OXPHOS
and ATP levels (71).
The production and transformation of citrate are
intricately linked to both mitochondrial and cytoplasmic
metabolism. Citrate is generated via the condensation of
oxaloacetic acid and acetyl coenzyme A in the TCA cycle. In
macrophages activated by LPS, the expression of the mitochondrial
citrate carrier (CIC/SLC25a1) mRNA and protein significantly
increases. Citrate output supports FAS, which is essential for the
production of prostaglandin E2 (PGE2), as well as the reduction of
NADP+ to NADPH (72,73).
Itaconic acid, generated by the upregulation of
aconitic acid decarboxylase 1 in classically activated macrophages,
acts as a key regulator of macrophage function. Itaconic acid exits
the TCA cycle and has demonstrated the ability to diminish the
production of proinflammatory mediators in LPS-treated macrophages.
Additionally, it contributes to maintaining the stability of the
anti-inflammatory transcription factor nuclear factor erythroid
2-related factor 2 (NRF2).
Succinate, another metabolite, accumulates during
macrophage activation and exhibits proinflammatory properties.
LPS-induced macrophage activation leads to intracellular buildup of
succinate and the activation of the γ-aminobutyric acid shunt
pathway. In response to inflammatory signals, macrophages express
GPR91, a receptor for succinate. Succinate triggers GPR91-mediated
signaling, maintaining a proinflammatory phenotype, and
facilitating the production of IL-1β. GPR91 is expressed in diverse
cell types and responds to extracellular succinate (74,75). This process has been implicated
in diseases such as diabetic retinopathy (76), diabetic nephropathy (77), hypertension (78) and atherothrombotic thrombosis
(79).
Iron metabolism
Iron plays a crucial role in the development,
differentiation and function of macrophages. Macrophages are
essential for maintaining systemic iron homeostasis. The
characteristics of M1 and M2 macrophages are closely linked to
their iron status. M1-type macrophages have high levels of ferritin
and are prone to iron accumulation, while M2-type macrophages can
metabolize and export iron, leading to a decrease in intracellular
iron levels. Macrophages acquire iron directly through transporters
and receptors such as LDL-related receptor 1, transferrin receptor
1 and the hemoglobin-haptoglobin receptor (CD163). Intracellular
iron homeostasis in macrophages is regulated post-transcriptionally
by the iron regulatory protein (IRP)/iron-responsive element (IRE)
system. Iron regulation in macrophages is intricately connected to
immune function, with intracellular iron levels directly impacting
macrophage polarization (80-82). The mechanisms through which iron
mediates macrophage polarization involve modulation of
intracellular signaling pathways such as NF-κB, MAPK and ROS
generation (83).
Iron storage in macrophages is primarily achieved
through ferritin binding. In M1-type macrophages, iron overload
leads to abundant iron storage due to higher expression of hepcidin
(Hamp) and ferritin heavy (FTH)/ferritin light (FTL), and lower
expression of hepcidin-ferroportin (FPN) and IRP1/2 (84). Iron-overloaded macrophages are
often accompanied by increased expression of various M1-type
cytokines, including IL-1β, TNFα and IL-6, as well as decreased
levels of the M2-type marker TGM2 (85). This iron accumulation in
macrophages is associated with increased glycolytic metabolism and
p53 acetylation (86,87). Additionally, iron-overloaded
macrophages manifest increased ROS production (87) and lipid peroxidation through the
Fenton reaction, leading to iron-induced cell death (88). Specific cytokines released by
macrophages can either induce or inhibit iron-induced cell death
through diverse mechanisms. For instance, TNF-α upregulates enzymes
such as ACSL3 and ACSL57, which participate in acyl coenzyme A
synthesis, fostering lipid accumulation in macrophages and
establishing conditions for iron-induced cell death. IL-1β, a
typical inflammatory cytokine, enhances the expression of
phosphorylated c-Jun N-terminal kinase and its substrates (c-Jun
and b-Jun), leading to FPN degradation and iron-induced cell death
in macrophages (89,90). Conversely, macrophages deficient
in FPN show increased iron accumulation and elevated expression of
inflammatory cytokines (91).
Both TNF-α and IL-1β have proinflammatory functions and play
important roles in promoting iron-induced cell death in
macrophages. iNOS, a hallmark of M1-type macrophages, has been
found to induce lipid peroxidation in macrophages, which has a
protective function against iron-induced cell death (92,93). Alterations in iron metabolism in
macrophages are closely associated with macrophage polarization,
production of inflammatory factors, lipid processing, angiogenesis
and iron sequestration, all of which impact the progression of
various diseases including cancer and atherosclerosis.
3. Nanomaterials regulate macrophage
metabolism to treat diseases
Nanomaterials have emerged as promising therapeutic
agents for addressing metabolic disorders, owing to their
distinctive size and physicochemical characteristics. Their
expansive specific surface area, high bioavailability, targeting
capabilities and adjustable release rates make them valuable tools
for regulating metabolic abnormalities in diseases. This article
provides a comprehensive review of the current status and prospects
of using nanomaterials to target the abnormal metabolism of
macrophages for the treatment of cancer and atherosclerosis
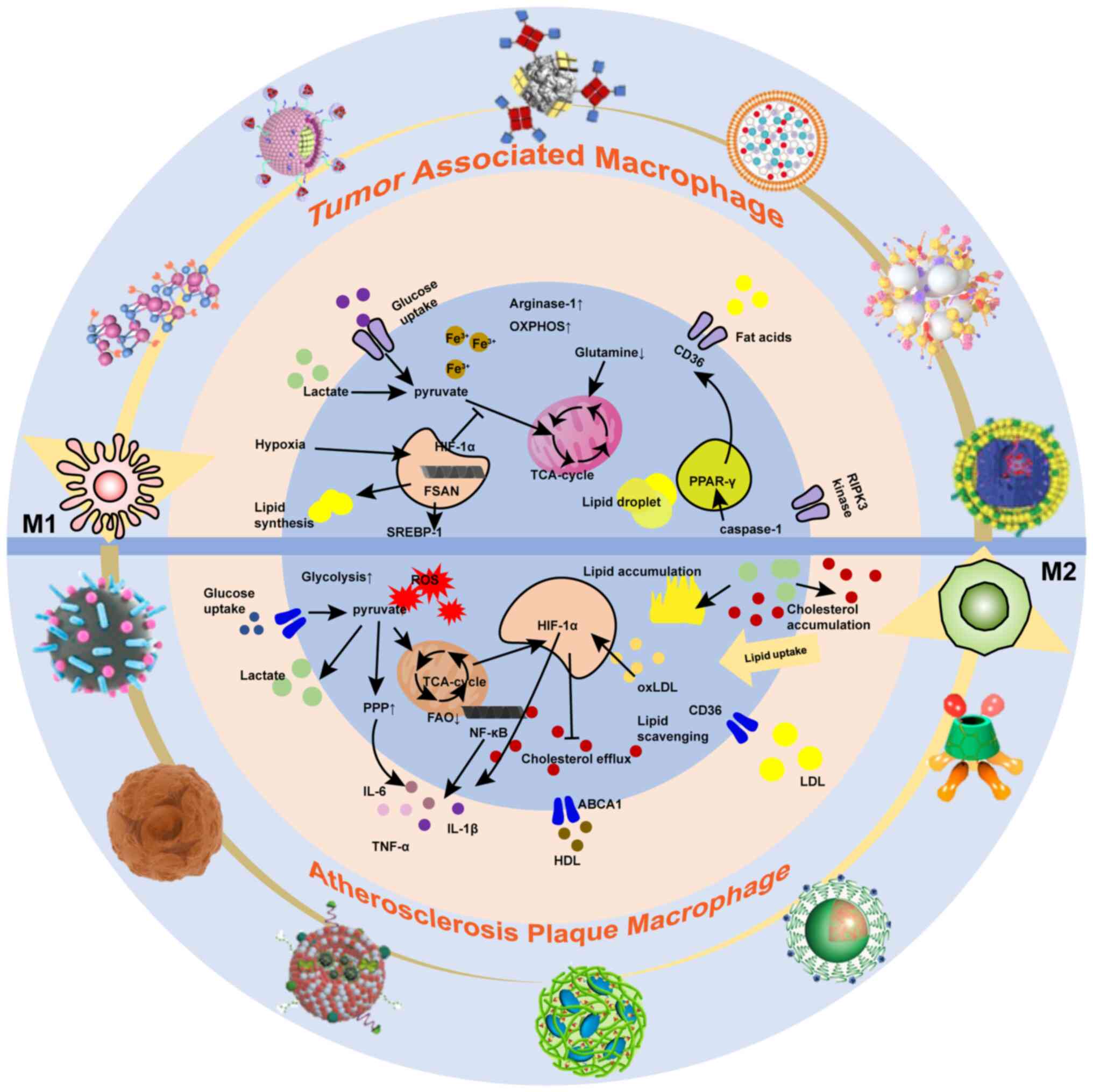
(Fig. 1).
Cancer
Tumors are intricate multicellular systems, and
among the constituents of the TME, TAMs assume a pivotal role. TAMs
exhibit heightened metabolic activity in pathways such as
glycolysis, FAS, FAO, as well as altered glutamate metabolism and
aberrant iron uptake. These metabolic alterations contribute to the
tumor-promoting functions of TAMs. In this section, the unique
metabolic pathways observed in TAMs were discussed and the
utilization of advanced nanomaterials to modulate these pathways
was examined.
TAMs, being the predominant immune cell population
within tumors, have an immunosuppressive role during tumor
progression. Cancer cells fulfill their energy demands for rapid
proliferation by consuming large amounts of glucose and relying on
glycolysis. Consequently, TAMs must shift to alternative metabolic
pathways such as OXPHOS and FAO to meet their energy requirements
and maintain an immunosuppressive phenotype in the
glucose-deficient TME (94).
Reprogramming TAMs using nanodrugs to enhance glycolysis or inhibit
OXPHOS and FAO in the TME holds promise for mitigating tumor
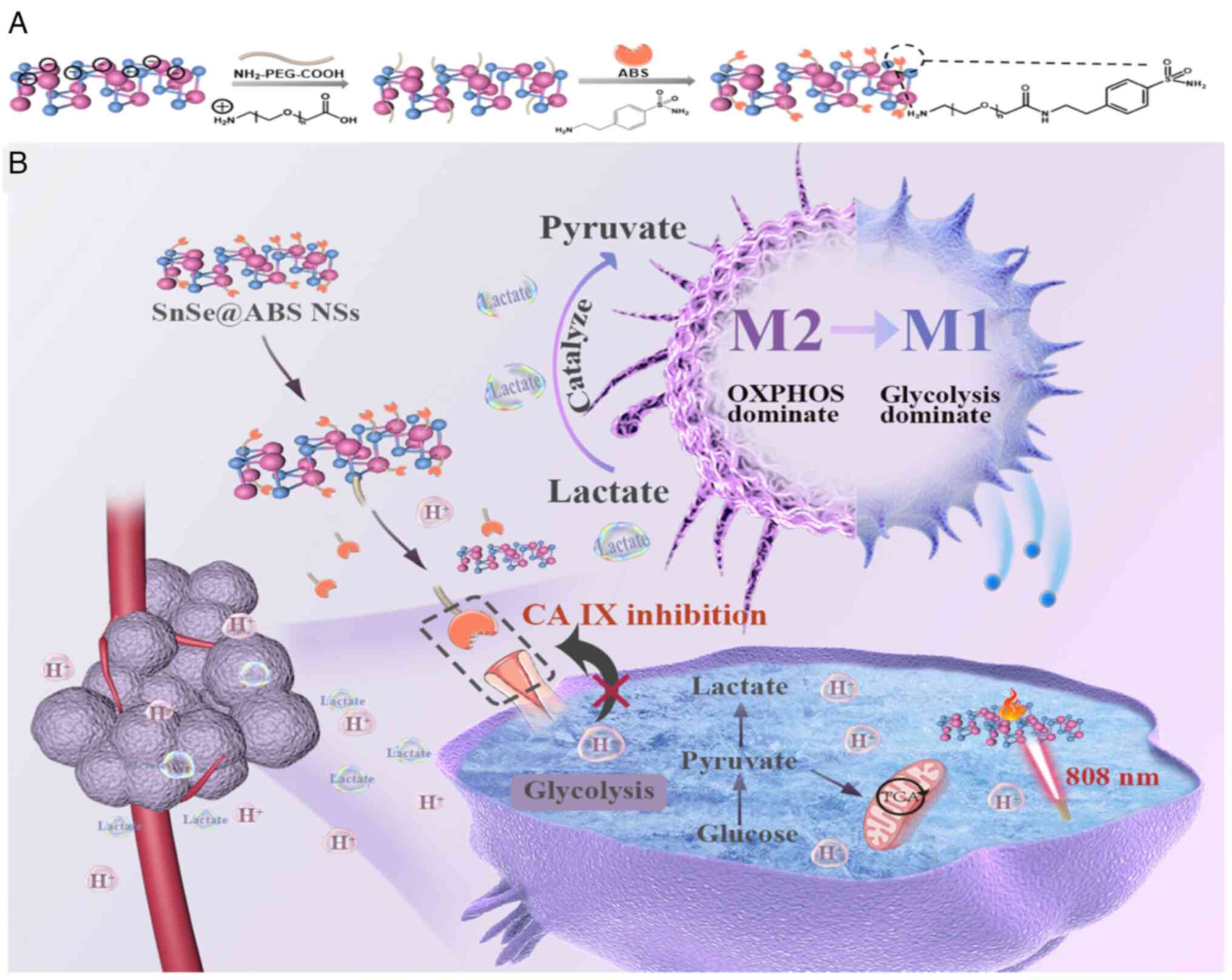
development. For instance, Jiabao et al (95) employed LDH mimicking SnSe
nanosheets equipped with carbonic anhydrase IX inhibitors to shift
TAM metabolism from mitochondrial OXPHOS to glycolysis. This
approach activated TAMs into M1-like macrophages and enhanced the
efficacy of TAM-based antitumor immunotherapy (Fig. 2).
Previous studies have revealed that TAMs exhibit
higher glucose uptake and elevated levels of glycolytic metabolism
(96-98), though the specific mechanisms
underlying these observations require further investigation. The
downstream metabolite of TAM glycolysis is lactate. M1-like TAMs
are predominantly found in the normoxic tumor regions, while
M2-like TAMs are concentrated in the hypoxic regions. Hypoxia,
characterized by low oxygen levels, enhances the tumor-promoting
activities of TAMs. The transcription factor HIF-1α plays a crucial
role in regulating glycolysis under hypoxic conditions. Within
TAMs, HIF-1α activates pyruvate dehydrogenase kinase 1, leading to
the inactivation of pyruvate dehydrogenase (PDH) and preventing
pyruvate from entering the TCA cycle, resulting in lactic acid
accumulation. TAMs predominantly rely on glycolysis as their
primary metabolic pathway, which distinguishes them from the
traditional M2-type macrophages. In M2-type macrophages, pyruvate
is converted to lactic acid by LDHA. The excessive accumulation of
lactic acid promotes cancer cell proliferation and favors the
polarization of macrophages towards an immunosuppressive M2
phenotype in the TME (99-104). Sustained lactic acid release in
malignant tumors is associated with cancer progression, and lactic
acid polarizes macrophages towards M2-like phenotypes by regulating
the acetylation level of macrophage histones, thereby promoting TAM
polarization (105-108). Lactate secreted by breast
cancer cells has been found to increase ROS levels in macrophages
via NRF2, inducing M2-type macrophage polarization, VEGF
expression, and promoting epithelial-mesenchymal transition in
cancer cells (109). Increased
availability of lactic acid in the TME facilitates the catabolism
of arginine by ARG-1 and ARG-2 in macrophages, inhibiting the
secretion of anticancer substances such as NO and citrulline
(110). This results in the
production of tumor-supporting cytokines such as ornithine and
polyamines by TAMs (111).
Consequently, preventing lactic acid efflux from cancer cells has
emerged as an important strategy to regulate the immunosuppressive
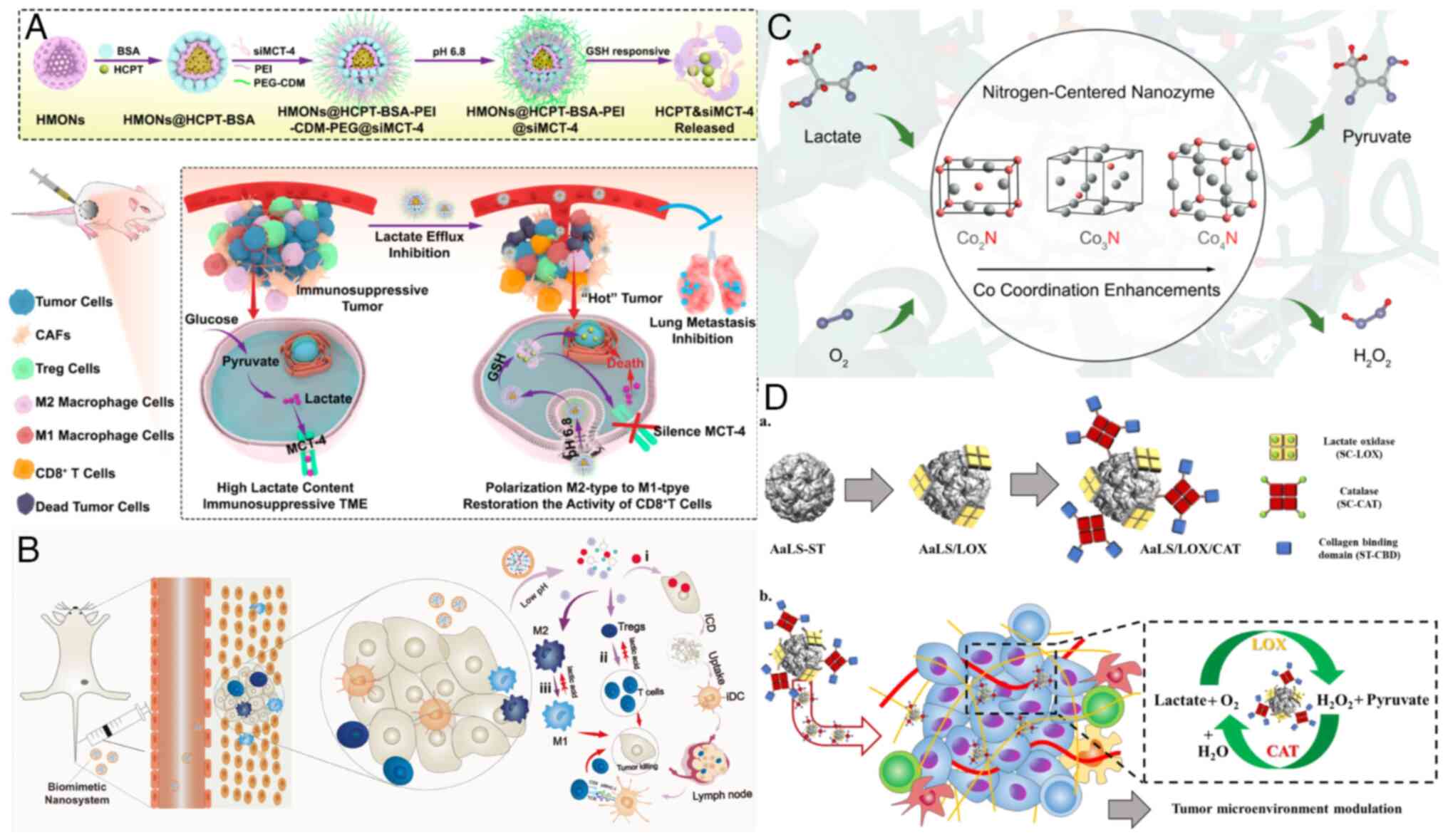
TME. Li et al (112)
developed a nano-cascade platform that responds to the weakly
acidic TME and high glutathione (GSH) levels in tumor cells. This
platform enables the sustained release of hydroxycamptothecin and
siMCT-4, inhibiting intracellular lactate efflux. By combining
chemotherapy with lactate efflux modulation, this nanoplatform
effectively reshapes the immunosuppressive TME, repolarizes TAMs
from the M2 phenotype to the M1 phenotype and significantly
inhibits tumor growth (Fig. 3A).
Currently, advanced nanomaterials primarily target the consumption
of lactic acid accumulated in the TME, assuming that this lactic
acid originates from tumor cell secretion (112-115) (Fig. 3). Nonetheless, the exploration of
other metabolic pathways in TAMs, such as amino acid and iron
metabolism, remains a promising direction for the development of
new nanomaterials in the comprehensive study of macrophage
metabolism within the TME.
As aforementioned, reprogramming the metabolic
processes of TAMs has emerged as a promising approach to modulate
their tumor-promoting function. One of the metabolic pathways
closely related to TAM polarization is OXPHOS. Inhibition of the
OXPHOS pathway has been explored as a strategy to promote the
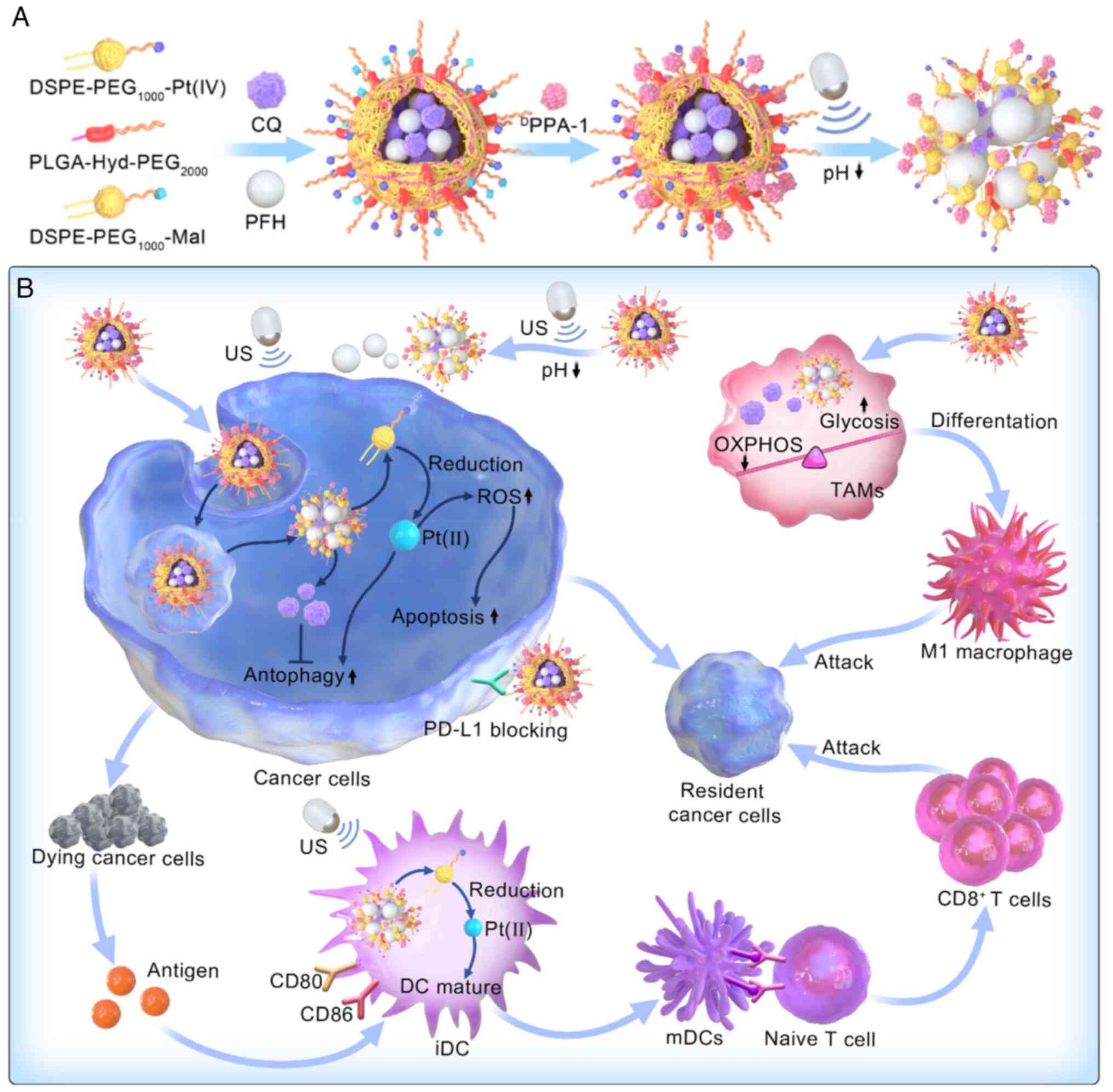
transition of M2-type TAMs to the M1 phenotype. Yang et al
(116) developed
nano-ultrasound contrast agents (Pt(IV)/CQ/PFH NPs-DPPA-1) that
could reprogram the metabolic processes of TAMs, enhancing
glycolysis and reducing OXPHOS. This reprogramming increased the
proportion of pro-inflammatory macrophages and enabled combined
chemical and immunotherapy using Pt (IV) and anti-PD-L1 peptide
(DPPA-1) (Fig. 4).
In addition to enhancing glycolysis and inhibiting
OXPHOS, the development of FAO inhibitors to induce phenotypic
transformation of macrophages and inhibit tumor development is also
a promising avenue of research. TAMs utilize FAO as an energy
source through the scavenger receptor CD36, mediating lipid uptake
and catabolism (117). In the
TME, activation of the RIPK3 kinase in cancer cells promotes lipid
accumulation in TAMs by activating PPAR-γ via caspase 1 (118,119). Macrophages with increased lipid
content and endoplasmic reticulum (ER) stress promote tumor
development through the combination of β-Glucoceramide with
receptors on TAMs, triggering stress responses in the ER tubular
organelles (120). Hypoxia, a
characteristic feature of solid tumors, activates HIF, which
upregulates SREBP1 (121,122). Activated FASN, the main
transcriptional regulator of FASN genes, promotes de novo
lipid synthesis under hypoxia stress, leading to the accumulation
of lipids stored as lipid droplets, which support biofilm
formation, energy production and protein modification (123,124). Accumulated lipid droplets in
macrophages can induce TAM polarization toward the M2 phenotype by
regulating the catabolism of unsaturated fatty acids in
mitochondrial respiration (117). Targeting lipid synthesis is a
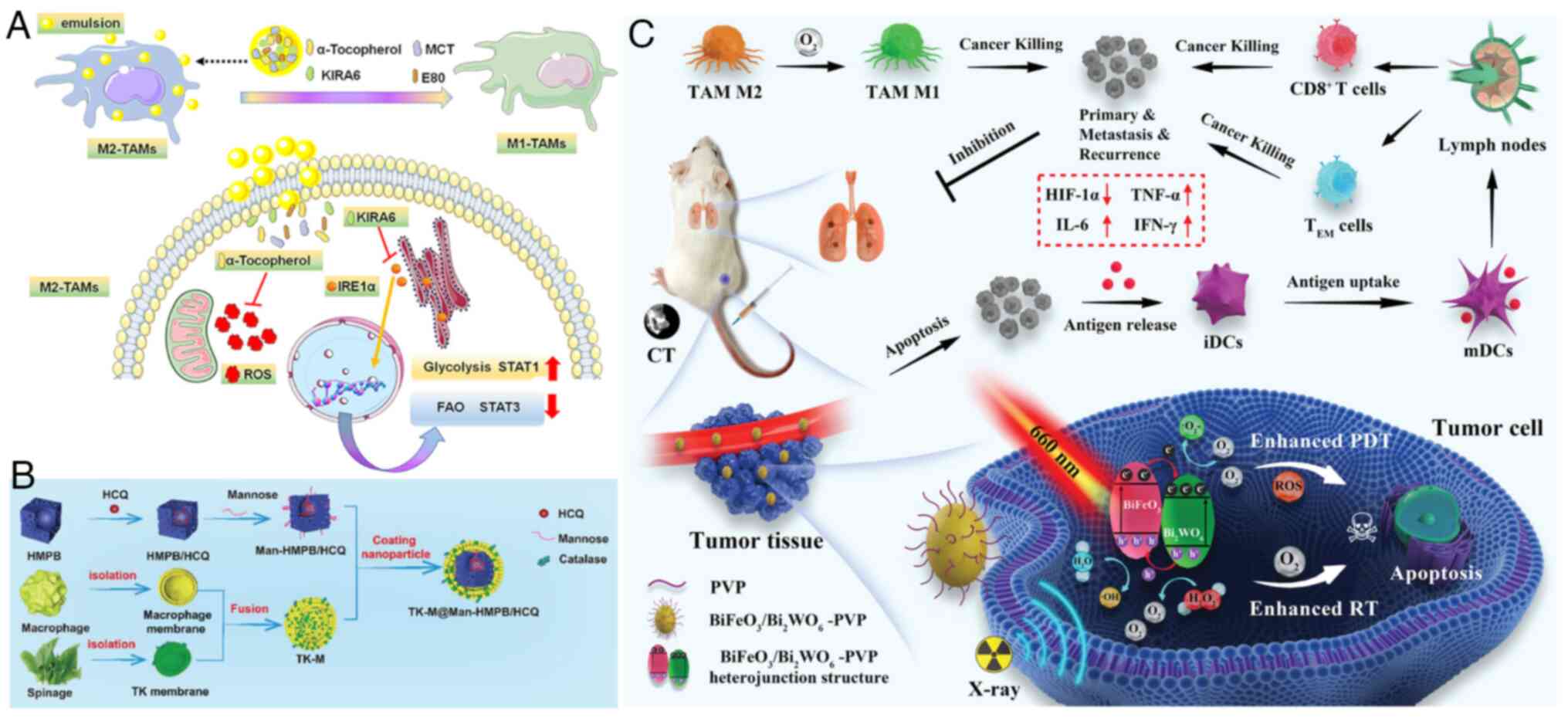
promising strategy for tumor treatment (125). Jiang et al (126) developed a nano-emulsion
containing α-tocopherol, encapsulating the IRE1-XBP1 pathway
inhibitor KIRA6, to inhibit ER stress and oxidative stress. This
dual inhibitory effect reprogrammed M2-type TAMs by increasing
glycolysis and inhibiting FAO, thereby delaying tumor growth
(Fig. 5A). Hou et al
(127) constructed a hollow
mesoporous Prussian blue (HMPB) nano-system with mannose
modification and hydroxychloroquine (HCQ) adsorption. They combined
macrophages and thylakoid (TK) membranes on the surface of the
nanoparticles, resulting in TK-M@Man-HMPB/HCQ, which effectively
alleviated TAM polarization induced by the hypoxic microenvironment
and promoted cytotoxic T lymphocyte (CTL) infiltration, leading to
significant inhibition of cancer growth (Fig. 5B). Yang et al (128) developed a novel poly
(vinylpyrrolidone) (PVP)-modified
BiFeO3/Bi2WO6 (BFO/BWO) with a
p-n-type heterojunction that catabolizes H2O2
to produce O2, thereby alleviating tumor hypoxia and
enhancing the sensitivity of photodynamic therapy (PDT) and
radiotherapy (RT). The PVP-modified BFO/BWO nanoparticles also
reduced the expression of HIF-1α and promoted the polarization of
TAMs toward the antitumor M1 phenotype (Fig. 5C).
Iron metabolism is another important aspect of TAM
function, as M2-type TAMs are key players in iron uptake,
metabolism, storage and export. TAMs provide iron to promote tumor
growth through multiple transport routes, making targeting TAM iron
delivery systems a potential strategy to enhance the anti-tumor
immune response (129).
Macrophages regulate intracellular iron levels by modulating
hepcidin/iron transporters. When this balance is disrupted, excess
iron is exported, resulting in tissue iron overload and
iron-induced cell death. Inducing iron-mediated cell death in TAMs
can inhibit tumor development by transforming or sacrificing them.
Zhang et al (130)
developed a biomimetic magnetosome using
Fe3O4 magnetic nanoclusters as the core,
bearing PD-1 antibodies on the membrane surface and loaded with a
TGF-β inhibitor. This biomimetic magnetosome induced TAM
polarization from M2 to M1, resulting in increased release of Fe
ions and subsequent hydrogen peroxide production, which induced
iron-induced cell death in tumor cells. Iron overload stress can
also modulate TAM signaling activation and metabolic function,
offering a promising antitumor therapeutic approach. Gu et
al (131) developed
iron-based metal-organic framework nanoparticles equipped with an
iron death inducer. These nanoparticles synergistically enhanced
TAM mitochondrial glycolysis, promoted macrophage M1 activation and
increased the secretion of antitumor cytokines, thereby
strengthening the tumoricidal activity of macrophages (Fig. 6).
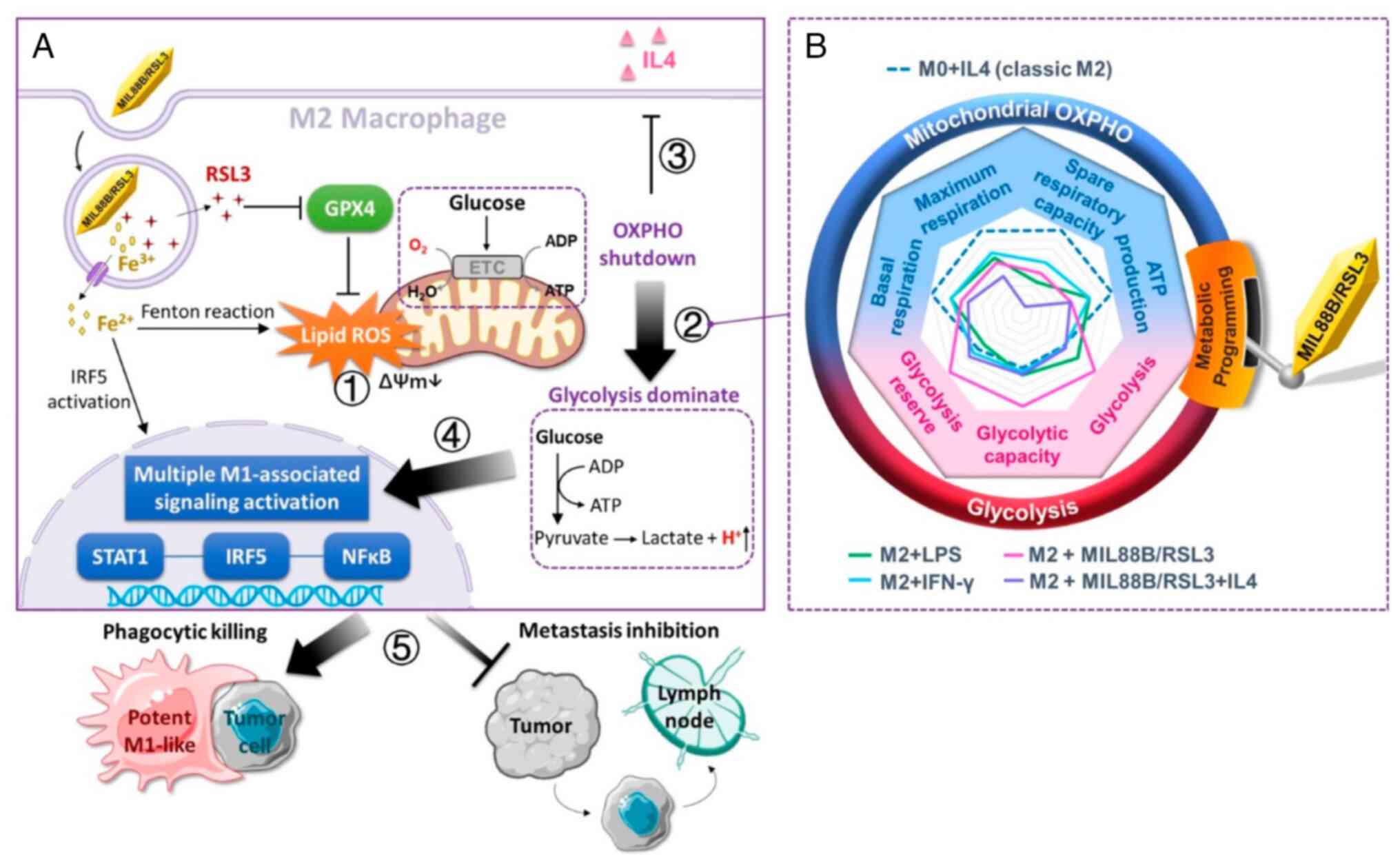 | Figure 6MIL88B/RSL3-Induced metabolic
programming and tumoricidal macrophage polarization. (A) When M2
macrophages are treated with MIL88/RSL3, the iron species and RSL3
synergizes to induce ferroptosis-associated lipid peroxidation,
which disrupts mitochondrial activity ①, embodied in the loss of
membrane potential. The shutdown of OXPHOS forces macrophage
metabolism to shift to glycolysis for ATP ② and effectively
counteracts the stimulation of M2 anti-inflammatory cytokine ③.
This MIL88/RSL3 drives potent M1 polarization via activation of
multiple M1-associated nuclear transcriptional factors ④.
Collectively, MIL88/RSL3 significantly promotes M1 population in
breast cancer tumors and elicits excellent tumoricidal activities ⑤
including phagocytic killing and metastasis inhibition. (B)
MIL88B/RSL3 shifted M2 macrophages from OXPHOS to glycolytic
metabolism, dramatically elevating the level of glycolysis,
glycolytic capacity and glycolysis reserve, and lowering
mitochondrial basal respiration, maximum respiration, spare
respiratory capacity and ATP production [Reprinted with permission
from Ref. (131); Copyright
(2021) American Chemical Society]. OXPHOS, oxidative
phosphorylation. |
In addition to the previously discussed
abnormalities in glycolysis, lipid metabolism and iron metabolism,
M2-TAMs significantly contribute to the immunosuppressive TME
through their amino acid metabolism. Amino acids play a crucial
role in the survival of TAMs, with glutamine being a key amino acid
for cancer cells and immunosuppressive TAMs (132,133). In vitro experiments have
demonstrated that glutamine ligase promotes the polarization of
TAMs towards the M2 phenotype by catalyzing the conversion of
glutamate to glutamine. Inhibiting the uptake of glutamine by TAMs
can repolarize them towards the M1 phenotype, enhancing their
antitumor function. An effective approach for tumor immunotherapy
involves targeting macrophages through nanomaterial delivery and
regulating their glutamine metabolism. Certain studies have
employed small molecule inhibitors of glutamine metabolism, such as
the prodrug of small molecule-6-diazo-5-oxo-L-demethylleucine, to
target glutamine metabolism and increase the population of
inflammatory TAMs, thereby inhibiting tumor growth (134).
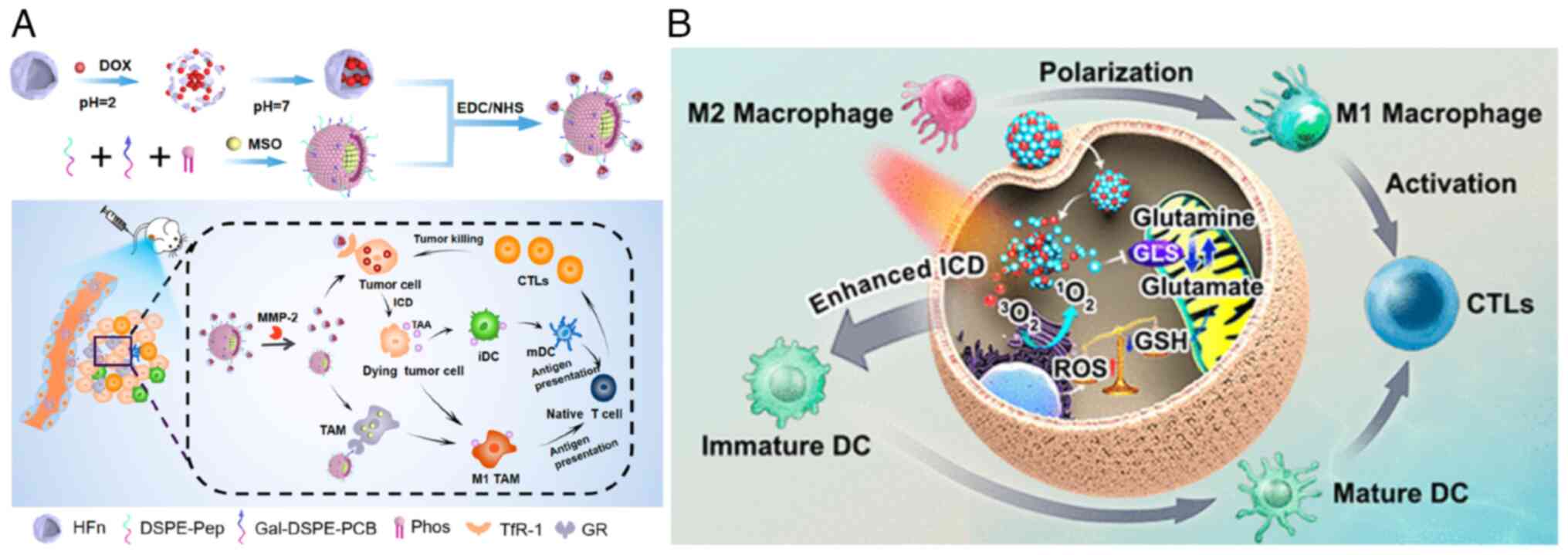
Du et al (135) conducted a study involving an
endogenous stimulus-responsive nano-delivery system (DOX@
HFn-MSO@PGZL). This innovative system incorporated L-methionine
sulfoxideimine (MSO) to disrupt the glutamate metabolism of TAMs in
tumor-bearing mice. This disruption promoted the formation of the
M1 phenotype, stimulated M1-TAMs to restore their antigen
presentation function, and synergistically interacted with mature
dendritic cells to enhance antigen presentation efficiency.
Consequently, this activation of tumor-killing T cells resulted in
a potent antitumor effect (Fig.
7A). Furthermore, the use of glutamine enhances the
pro-inflammatory response induced by FAO and optimizes the NAD/NADH
ratio of glutamine-producing lactic acid, contributing to antitumor
immunity. CD40, strongly expressed by macrophages and other
antigen-presenting cells, has been targeted for the phenotypic
re-education of TAM in tumor-bearing mice (136-138). Activation of CD40-mediated
signaling with agonistic anti-CD40 monoclonal antibodies has been
shown to promote macrophage glutamine and fatty acid metabolism,
thereby facilitating epigenetic reprogramming of pro-inflammatory
genes and antitumor phenotypes in ACLY-dependent macrophages
(139).
The immunotherapeutic effect of PDT can be
attenuated by tumor defense mechanisms associated with glutamine
metabolism. To overcome this, Mai et al (140) developed a carrier-free
immunotherapy enhancer, C6SN, with a dual synergistic effect. This
enhancer combines the self-assembly of glutamine inhibitor compound
9 (C968) and the photosensitizer Chlorin e6 (68) to block glutamine metabolism in
macrophages and polarize TAM towards the M1 phenotype.
Consequently, it further recruits and activates CTL while
remodeling the immunosuppressive TME (Fig. 7B).
In summary, the metabolic processes of TAMs drive
their immunosuppressive functions, including increased glucose and
lipid uptake, as well as glutamine and glutamic acid accumulation
during tumor growth. These insights highlight the potential of
designing nanodrugs specifically tailored to target
macrophage-specific metabolic changes, offering a promising avenue
to enhance anti-tumor immunotherapy.
Atherosclerosis
Macrophages within atherosclerotic plaques often
exhibit metabolic abnormalities, including dysregulated glycolysis,
PPP, iron overload, excessive intracellular lipid accumulation and
reduced cholesterol efflux. This section delves into the
atherosclerotic microenvironment and the concomitant metabolic
aberrations. Additionally, nanomaterials that target these abnormal
metabolic processes, ranging from the modulation of macrophage
cholesterol efflux and lipid accumulation to the inhibition of
macrophage foam cell formation and plaque injury, were briefly
discussed.
Firstly, a concise overview of the microenvironment
within the atherosclerotic plaque and the metabolic characteristics
of macrophages dwelling within it was provided. During the early
stage of atherosclerosis, monocytes are attracted to the arterial
wall through chemokine-receptor interactions and the secretion of
intercellular adhesion molecule-1 and vascular adhesion molecule-1
by endothelial cells (141).
Subsequently, these monocytes differentiate into macrophages,
acquiring either pro-inflammatory or anti-inflammatory phenotypes
under the influence of the local microenvironment (142). Pro-inflammatory macrophages are
predominantly found in early plaques and exhibit a heightened
affinity for oxidized low-density lipoprotein (oxLDL) via scavenger
receptor CD36, as opposed to the LDL receptor (143,144). This leads to the accumulation
of lipids within macrophages, resulting in the formation of foam
cells characterized by excessive cholesterol and triglyceride
storage in cytoplasmic lipid droplets. Cholesterol accumulation
within macrophages triggers Toll-like receptor signaling,
NF-kB-mediated NLRP3 inflammasome activation and the promotion of
macrophage inflammation, thereby exacerbating the chronic
inflammatory state associated with atherosclerosis (145). Moreover, untreated cholesterol
overload induces macrophage toxicity and apoptosis while impairing
their capacity to migrate and remove plaques. High-density
lipoprotein (HDL) levels in the bloodstream are inversely
correlated with the risk of atherosclerosis, primarily through the
process of reverse cholesterol transport, which facilitates the
transport of excess cholesterol from surrounding cells and tissues
back to the liver while regulating inflammation to prevent lipid
accumulation (146). However,
in the inflammatory environment of atherosclerosis, increased
macrophage myeloperoxidase (MPO) activity leads to HDL oxidation,
causing partial loss of its functionality. Furthermore,
MPO-mediated oxidation of the cholesterol transport receptor ABCA1
impairs cholesterol excretion by macrophages. Therefore, it is
crucial to target macrophages within the plaque environment,
modulate their lipid metabolism, and regulate cholesterol influx
and efflux for the progression of atherosclerosis (147).
Given that the inability to efflux cholesterol from
macrophages within atherosclerotic plaques is a major contributor
to disease progression, numerous therapeutic strategies focus on
restoring cholesterol efflux capacity. Consequently, various
nanomaterials have been designed to target diseased macrophages and
plaques by addressing the underlying metabolic abnormalities and
metabolites in this environment. In the subsequent section, the
review shall focus on the nanomaterials designed to facilitate
cholesterol efflux from macrophages, mitigate inflammation in
atherosclerosis, and ameliorate the disease.
In the atherosclerotic plaque environment, the
abnormal accumulation of cholesterol in macrophages triggers local
inflammation by promoting the production of ROS and the secretion
of inflammatory cytokines and chemokines, including TNF-α, IL-1β,
IL-6, IL-8 and TGF-β. These inflammatory responses further attract
immune cells to the site (148,149). Given the unique characteristics
of the atherosclerotic plaque environment, which is characterized
by high levels of ROS, environmentally responsive nanomaterials
have been developed to modulate the abnormal lipid metabolism in
macrophages.
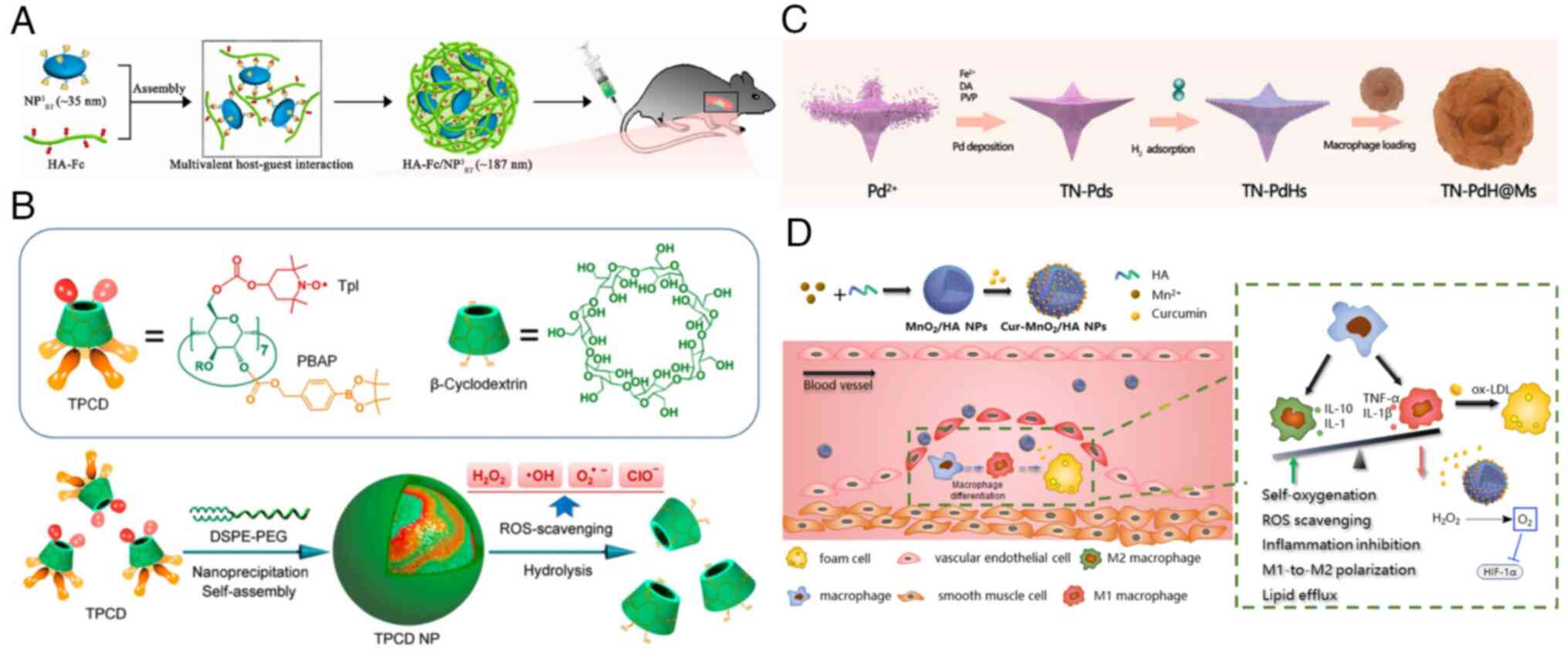
For instance, He et al (150) reported the development of a
nano-module called HA-Fc/NP3ST, comprising disc-shaped high-density
lipoprotein, hyaluronic acid-ferrocene conjugate anchored by
β-cyclodextrin. This nanomaterial exhibits ROS responsiveness and
undergoes size reduction. In atherosclerotic mice, it demonstrates
a potent therapeutic effect by releasing HDL in response to
excessive ROS. This deepens plaque penetration and targets
cholesterol efflux from macrophages, leading to a significant
reduction in plaque area and lipid deposition (Fig. 8A).
Wang et al (151) prepared nanoparticles by
covalently coupling superoxide dismutase simulator tempol and
pinacol phenylborate, which are hydrogen peroxide elimination
compounds, to cyclic polysaccharide β-cyclodextrin. This
nanomaterial effectively inhibits the internalization of oxLDL,
thus preventing the formation of foam cells in macrophages and
vascular smooth muscle cells. By significantly reducing ROS-induced
inflammation and apoptosis in macrophages, it stabilizes
atherosclerotic plaques and reduces the necrotic core (Fig. 8B).
Another innovative approach was introduced by Hu
et al (152), who
developed a unique quadruped needle-like PDH nano-enzyme that is
loaded into macrophages and specifically targets arterial plaques.
This nanomaterial demonstrates ROS scavenging activity,
anti-inflammatory properties and the activation of autophagy. It
yields highly favorable outcomes in the management and treatment of
atherosclerosis by concurrently addressing multiple facets of the
disease (Fig. 8C).
Additionally, Sun et al (153) have developed a nano-drug based
on MnO2, which is used to reprogram macrophages and
target atherosclerosis. The incorporation of curcumin (Cur) with
antioxidant and anti-inflammatory properties into MnO2
enables the polarization of M1 macrophages into the M2 phenotype.
MnO2 also inhibit HIF-1α and restores the lipid efflux
function of macrophages, thereby suppressing foam cell formation
and removing lipids and ROS from cells. This nanomaterial exhibits
a robust anti-atherosclerotic effect (Fig. 8D).
The use of photosensitizers engulfed by cells in
combination with near-infrared light irradiation can generate ROS
or heat, which regulates the cholesterol efflux capacity of
macrophages in the plaque and affects necrosis, apoptosis and
autophagy of foam cells in atherosclerotic plaques, thereby slowing
down the progression of atherosclerosis. For instance, upconversion
fluorescent nanoparticles encapsulating chloroproteins e6
(UCNPs-Ce6) were used to mediate PDT, enhancing the cholesterol
efflux ability and inducing autophagy in THP-1 macrophage-derived
foam cells (154). In another
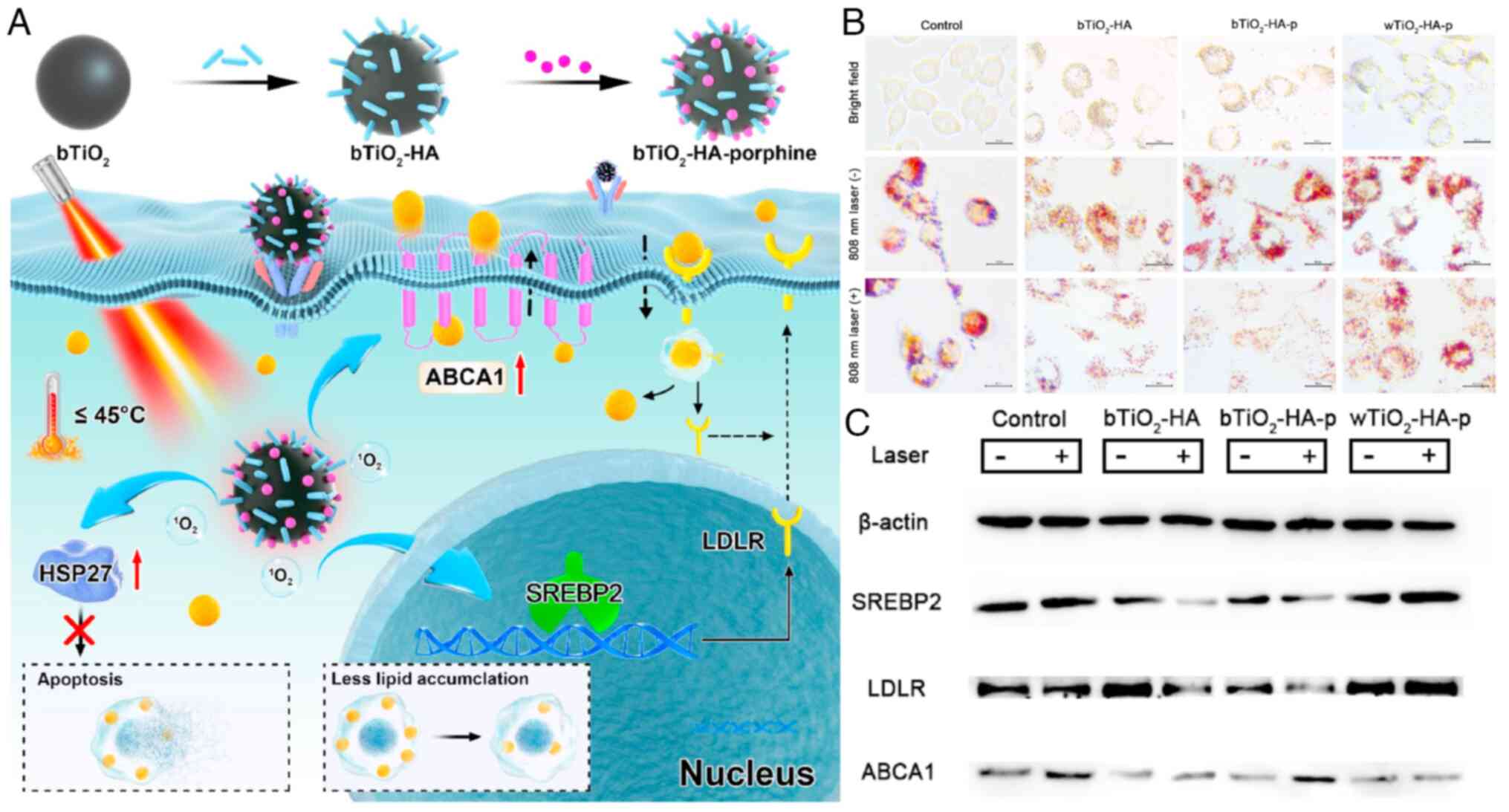
study, Dai et al (155)
designed a nanoprobe that combines photothermal therapy and PDT by
loading hyaluronic acid and porphyrin onto black TiO2.
This nanomaterial effectively targets macrophage foam cells in
atherosclerotic plaques without inducing extensive apoptosis and
necrosis that could damage the plaque. Through the SREBP2/LDLR
pathway, it reduces cholesterol production and excess cholesterol
uptake in cells while initiating ABCA1-mediated cholesterol efflux,
thus inhibiting lipid accumulation in foam cells (Fig. 9).
Apart from the surge of ROS, another characteristic
of atherosclerotic lesion environment is acidic pH. The acidic
microenvironment in atherosclerotic plaques is primarily caused by
the accumulation of lactic acid secreted by macrophages due to
enhanced glycolysis. The underlying mechanisms were described in
detail.
During the development of atherosclerotic plaques,
oxygen consumption in the vascular wall increases, but the narrow
vascular lumen leads to insufficient oxygen supply, resulting in
tissue hypoxia in the plaque lesions. This hypoxic condition
stabilizes the HIF-1α transcription factor in macrophages,
activating the glycolysis pathway to generate energy. Therefore,
the accumulation of lactic acid within plaques is a byproduct of
macrophage glycolysis. Increased glucose uptake by macrophages and
enhanced glycolytic metabolism in atherosclerotic vascular walls
contribute to increased inflammatory burden and plaque progression.
The mechanism involves increased glucose uptake and glycolytic
flux, which generate mitochondrial ROS, promoting the
phosphorylation of PKM2 and transcription factor STAT3. This leads
to the production of inflammatory cytokines such as TNF-α, IL-6 and
IL-1β. A significant portion of macrophages in atherosclerotic
plaques originate from the proliferation of resident macrophages
within the plaques. Increased activity of PPP is crucial for the
synthesis of proteins in inflammatory macrophages and the amino
acids required for RNA and DNA synthesis. Glycolysis provides fuel
for the PPP, and its activation leads to the removal of electrons
and production of ROS through mitochondrial and phagosome NADPH
oxidase. ROS contribute to oxidative stress in atherosclerotic
plaques by oxidizing proteins and fatty acids.
Excessive lactic acid plays a role in promoting
angiogenesis and vascular calcification within atherosclerotic
plaques. Moreover, accumulated lactic acid can increase cholesterol
accumulation inside and outside cells through various mechanisms,
including inducing extracellular acidification, enhancing
lipoprotein retention and modification, and reducing apolipoprotein
E secretion. Therefore, inhibiting the process of glycolysis,
preventing lactic acid accumulation and promoting lactic acid
consumption will be effective ways to alleviate atherosclerosis.
However, there are limited nanomaterials that target these
processes. Moreover, corresponding nanomaterials with acidic pH
responsiveness have emerged as an effective platform for the
on-demand release of anti-atherosclerotic drugs in the inflammatory
microenvironment. These pH-responsive nano-materials have been
designed for controlled drug release and imaging diagnostic
applications in atherosclerosis. Drugs such as metformin,
pioglitazone and statins have shown efficacy in regulating
macrophages and mitigating the progression of atherosclerosis.
Nonetheless, using pure drugs lacks targeting specificity.
Therefore, numerous studies have utilized the acidic environment to
design nano-drug delivery systems with pH-responsive properties,
specifically targeting diseased macrophages for the treatment of
atherosclerosis.
For instance, Zhang et al (156) employed poly-β-cyclodextrin as a
cholesterol crystal solubilizer and benzimidazole-grafted dextran
sulfate as a pH-sensitive switch to form a supramolecular
nano-assembly. This system enhanced cholesterol efflux and promoted
the regression of atherosclerosis (Fig. 10A). You et al (157) reported a hybrid nanomaterial
composed of a mixed membrane-coated graphene oxide quantum dot
loaded with atorvastatin. This hybrid membrane included hyaluronic
acid. This formulation effectively suppressed the inflammatory
state of diseased macrophages, reduced lipid influx, enhanced
autophagy to promote cholesterol efflux, and significantly
inhibited plaque development (Fig.
10B). Li et al (158) designed pH-responsive and
integrin-targeted nanoparticles derived from cyclodextrin and
microRNA-33 (anti-miR33) antisense oligonucleotide for the precise
treatment of atherosclerosis. This approach significantly promoted
reverse cholesterol transport, alleviated atherosclerosis in mice,
and markedly reduced vulnerable plaque (Fig. 10C).
Advanced atherosclerosis is characterized by the
presence of extensive necrotic cores, increased apoptosis, and the
accumulation of oxLDL, all of which promote the transformation of
macrophages into foam cells. This process contributes to plaque
instability and poor prognosis. In the late stage of
atherosclerosis, macrophage function becomes impaired, hindering
the clearance of necrotic cells and resulting in the outflow of
lipid-laden necrotic cells and infiltration of inflammatory cells.
Therefore, the induction of macrophage autophagy to rectify
irregular lipid metabolism has emerged as a pivotal therapeutic
strategy for alleviating arterial congestion.
A recent study harnessed optimized
mannose-functionalized dendritic polymer nanoparticles to
simultaneously deliver scavenger receptor-A siRNA (to reduce LDL
uptake) and liver X receptor ligand (to stimulate cholesterol
outflow). This approach effectively reduced the cholesterol content
of macrophages, promoted the regression of atherosclerotic plaques
and facilitated plaque stabilization (159) (Fig. 11A). Wu et al (160) developed a carrier-free
nanomotor driven by NO based on the reaction between trehalose (one
of the mTOR-independent autophagy inducers), L-arginine and
phosphatidylserine. This nanomotor precisely targeted macrophages
in atherosclerotic plaques, regulated their polarization to the M2
phenotype, promoted lipid excretion and facilitated the
reconstruction of the endothelial barrier, enabling multifaceted
treatment of atherosclerosis (Fig.
11B).
In addition to abnormalities in lipid metabolism
and glycolysis, amino acid metabolism and iron metabolism also play
important roles in atherosclerosis. The immunomodulator ARG1,
crucial in macrophages, regulates atherosclerotic plaque
progression by inhibiting NO-mediated cytotoxicity through
L-arginine consumption, primarily in anti-inflammatory macrophages
within the plaques. This modulation decelerates plaque progression,
promotes the formation of fibrous caps and increases plaque
stability. NO and ARG1 are commonly used as indicators to assess
changes in macrophage phenotype. Glutamine, another extensively
studied amino acid in atherosclerotic macrophages, has been found
to stabilize inflammation. Anti-inflammatory macrophages exhibit
increased uptake of glutamine. Iron overload in macrophages within
atherosclerosis contributes to their transformation into foam
cells, exacerbates glycolysis and macrophage inflammation, and
worsens the severity of the disease.
Although advanced nanomaterial development has
primarily focused on addressing lipid metabolism, inhibiting foam
cell formation and reducing the expression of inflammatory factors,
there is a pressing need to explore the potential of nanomaterials
in addressing iron metabolism and amino acid metabolism. These
areas hold great promise as therapeutic targets in the
comprehensive study of macrophage metabolism within the
atherosclerotic plaque environment, thus warranting the development
of new nanomaterials.
4. Conclusion
In the present review, the typical metabolic
pathways of macrophages were comprehensively outlined, highlighting
their inherent relationship with macrophage polarization and
functional activation. Furthermore, the aberrant metabolic
processes in macrophages associated with cancer and atherosclerosis
were illustrated. Additionally, an overview of recent advancements
in nanomaterials aimed at reprogramming macrophage metabolism,
contributing to disease progression inhibition, was provided. At
present, in the context of immunosuppressive TAMs, nanomaterials
are primarily engineered to restrain glucose and lipid uptake,
while also curbing the accumulation of glutamine and glutamate. In
the case of atherosclerosis, nanomaterials are specially formulated
to enhance cholesterol efflux and inhibit lipid accumulation,
mitigating the formation of macrophage foam cells and plaque
damage.
Nevertheless, there remain pressing issues within
the realm of nanomaterials aimed at regulating the aberrant
metabolism of macrophages in cancer and atherosclerosis. Firstly,
although metabolic pathways such as glycolysis, OXPHOS, FAO and
amino acid metabolism have received extensive scrutiny in the
context of tumors and atherosclerosis, certain abnormal metabolic
processes, such as those associated with iron and glutamine
metabolism in atherosclerosis, remain inadequately understood. This
knowledge gap holds paramount importance for the development of
precise nanomaterials. Currently, these unelucidated abnormal
metabolic processes lack corresponding nanomaterial interventions
and thus demand further investigation.
Secondly, the prolonged biosafety of nanomaterials
designed to target the abnormal metabolism of macrophages in cancer
and atherosclerosis is a significant concern within this
discipline. Consequently, the ongoing development of
next-generation nanomaterials, including those based on proteins
and DNA, is imperative.
In conclusion, the advent of advanced nanomaterials
has expedited the advancement of therapeutic approaches targeting
abnormal macrophage metabolism in cancer and atherosclerosis. It is
evident that by harnessing the capabilities of nanomaterials and
furthering the understanding of macrophage metabolism, the path for
innovative immunotherapies and personalized treatments can be
paved, effectively modulating macrophage function in a range of
diseases.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
MMX and YC contributed equally to the conception of
the review, wrote the original draft, edited and critically revised
the manuscript. SYW, XLC, YYC, SJT, AQY and WWC contributed equally
to data curation. LXW approved the final version of the manuscript,
and agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 62227803, 62288102 and 62235008),
the Natural Science Foundation of Jiangsu-Major Project (grant no.
BK20212012), τηε Natural Science Foundation of Jiangsu (grant no.
BK20220387), the Belt and Road Innovation Cooperation Project of
Jiangsu (grant no. BZ2022011) and the Natural Science Research
Start up Foundation of Recruiting Talents of Nanjing University of
Posts and Telecommunications (grant no. NY221146).
References
|
1
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray PJ and Wynn TAJ: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Juhas U, Ryba-Stanisławowska M, Szargiej P
and Myśliwska J: Different pathways of macrophage activation and
polarization. Postepy Hig Med Dosw (Online). 69:496–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T and He C: Pro-inflammatory
cytokines: The link between obesity and osteoarthritis. Cytokine
Growth Factor Rev. 44:38–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ploeger DT, Hosper NA, Schipper M, Koerts
JA, de Rond S and Bank RA: Cell plasticity in wound healing:
paracrine factors of M1/M2 polarized macrophages influence the
phenotypical state of dermal fibroblasts. Cell Commun Signal.
11:292013. View Article : Google Scholar
|
|
8
|
Tu Z, Chen M, Wang M, Shao Z, Jiang X,
Wang K, Yao Z, Yang S, Zhang X, Gao W, et al: Engineering bioactive
M2 macrophage-polarized anti-inflammatory, antioxidant, and
antibacterial scaffolds for rapid angiogenesis and diabetic wound
repair. Adv Funct Mater. 31:21009242021. View Article : Google Scholar
|
|
9
|
Yin C, Zhao Q, Li W, Zhao Z, Wang J, Deng
T, Zhang P, Shen K, Li Z and Zhang Y: Biomimetic anti-inflammatory
nano-capsule serves as a cytokine blocker and M2 polarization
inducer for bone tissue repair. Acta Biomater. 102:416–426. 2020.
View Article : Google Scholar
|
|
10
|
Kim J: Regulation of immune cell functions
by metabolic reprogramming. J Immunol Res. 2018:86054712018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Chen F, Tang Y, Wang J, Chen X, Li
X and Zhang X: Regulation of macrophage polarization and functional
status by modulating hydroxyapatite ceramic micro/nano-topography.
Mater Des. 213:1103022022. View Article : Google Scholar
|
|
12
|
O'Neill LAJ and Hardie DG: Metabolism of
inflammation limited by AMPK and pseudo-starvation. Nature.
493:346–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu B, Gao Y, Sun F, Shi M and Huang Y:
Lipid metabolism regulation based on nanotechnology for enhancement
of tumor immunity. Front Pharmacol. 13:8404402022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin L, Chen H, Zhao R, Zhu M and Nie G:
Nanomedicine targets iron metabolism for cancer therapy. Cancer
Sci. 113:828–837. 2022. View Article : Google Scholar :
|
|
15
|
Lin X, Xiao Z, Chen T, Liang SH and Guo H:
Glucose metabolism on tumor plasticity diagnosis, and treatment.
Front Oncol. 10:3172020. View Article : Google Scholar
|
|
16
|
Prasad CP, Gogia A and Batra AJC:
Essential role of aerobic glycolysis in epithelial-to-mesenchymal
transition during carcinogenesis. Clin Transl Oncol. 24:1844–1855.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang B and Shi J: Chemistry of advanced
nanomedicines in cancer cell metabolism regulation. Adv Sci
(Weinh). 7:20013882020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garedew A, Henderson SO and Moncada S:
Activated macrophages utilize glycolytic ATP to maintain
mitochondrial membrane potential and prevent apoptotic cell death.
Cell Death Differ. 17:1540–1550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galván-Peña S and O'Neill LAJ: Metabolic
reprograming in macrophage polarization. Front Immunol.
5:4202014.PubMed/NCBI
|
|
20
|
Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai
G, Liu R, Gao H, Tao B, Li W, et al: Macrophage-associated PGK1
phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol
Cell. 71:201–215.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bailey JD, Diotallevi M, Nicol T, McNeill
E, Shaw A, Chuaiphichai S, Hale A, Starr A, Nandi M, Stylianou E,
et al: Nitric oxide modulates metabolic remodeling in inflammatory
macrophages through TCA cycle regulation and itaconate
accumulation. Cell Rep. 28:218–230.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Na YR, Je S and Seok SH: Metabolic
features of macrophages in inflammatory diseases and cancer. Cancer
Lett. 413:46–58. 2018. View Article : Google Scholar
|
|
23
|
Wang J, Yang P, Yu T, Gao M, Liu D, Zhang
J, Lu C, Chen X, Zhang X and Liu Y: Lactylation of PKM2 suppresses
inflammatory metabolic adaptation in pro-inflammatory macrophages.
Int J Biol Sci. 18:6210–6225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T,
Williams DL and Li C: Lactate suppresses macrophage
pro-inflammatory response to LPS stimulation by inhibition of YAP
and NF-κB activation via GPR81-mediated signaling. Front Immunol.
11:5879132020. View Article : Google Scholar
|
|
25
|
Wang F, Zhang S, Vuckovic I, Jeon R,
Lerman A, Folmes CD, Dzeja PP and Herrmann J: Glycolytic
stimulation is not a requirement for M2 macrophage differentiation.
Cell Metab. 28:463–475.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang T, Liu H, Lian G, Zhang SY, Wang X
and Jiang C: HIF1α-induced glycolysis metabolism is essential to
the activation of inflammatory macrophages. Mediators Inflamm.
2017:90293272017. View Article : Google Scholar
|
|
27
|
Zhihua Y, Yulin T, Yibo W, Wei D, Yin C,
Jiahao X, Runqiu J and Xuezhong X: Hypoxia decreases macrophage
glycolysis and M1 percentage by targeting microRNA-30c and mTOR in
human gastric cancer. Cancer Sci. 110:2368–2377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Everts B, Amiel E, Huang SCC, Smith AM,
Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt
GJ, et al: TLR-driven early glycolytic reprogramming via the
kinases TBK1-IKKε supports the anabolic demands of dendritic cell
activation. Nat Immunol. 15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Im SS, Yousef L, Blaschitz C, Liu JZ,
Edwards RA, Young SG, Raffatellu M and Osborne TF: Linking lipid
metabolism to the innate immune response in macrophages through
sterol regulatory element binding protein-1a. Cell Metab.
13:540–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gordon S: Phagocytosis: An immunobiologic
process. Immunity. 44:463–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cader MZ, Boroviak K, Zhang Q, Assadi G,
Kempster SL, Sewell GW, Saveljeva S, Ashcroft JW, Clare S,
Mukhopadhyay S, et al: C13orf31 (FAMIN) is a central regulator of
immunometabolic function. Nat Immunol. 17:1046–1056. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nomura M, Liu J, Rovira II,
Gonzalez-Hurtado E, Lee J, Wolfgang MJ and Finkel T: Fatty acid
oxidation in macrophage polarization. Nat Immunol. 17:216–217.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schönfeld P and Wojtczak L: Short- and
medium-chain fatty acids in energy metabolism: The cellular
perspective. J Lipid Res. 57:943–954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coniglio S, Shumskaya M and Vassiliou E:
Unsaturated fatty acids and their immunomodulatory properties.
Biology (Basel). 12:2792023.PubMed/NCBI
|
|
35
|
Deng Y, Li W, Zhang Y, Li J, He F, Dong K,
Hong Z, Luo R and Pei X: α-Linolenic acid inhibits RANKL-induced
osteoclastogenesis in vitro and prevents inflammation in vivo.
Foods. 12:6822023. View Article : Google Scholar
|
|
36
|
Laval T, Chaumont L and Demangel C: Not
too fat to fight: The emerging role of macrophage fatty acid
metabolism in immunity to Mycobacterium tuberculosis. Immunol Rev.
301:84–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki M, Takaishi S, Nagasaki M, Onozawa
Y, Iino I, Maeda H, Komai T and Oda T: Medium-chain fatty
acid-sensing receptor, GPR84, is a proinflammatory receptor. J Biol
Chem. 288:10684–10691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Wu X, Simonavicius N, Tian H and
Ling L: Medium-chain fatty acids as ligands for orphan G
protein-coupled receptor GPR84. J Biol Chem. 281:34457–34464. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hidalgo MA, Carretta MD and Burgos RA:
Long chain fatty acids as modulators of immune cells function:
Contribution of FFA1 and FFA4 receptors. Front Physiol.
12:6683302021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Forsman H, Dahlgren C, Mårtensson J,
Björkman L and Sundqvist M: Function and regulation of GPR84 in
human neutrophils. Br J Pharmacol. Mar 4–2023.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Danielski LG, Giustina AD, Bonfante S,
Barichello T and Petronilho F: The NLRP3 inflammasome and its role
in sepsis development. Inflammation. 43:24–31. 2020. View Article : Google Scholar
|
|
42
|
Kuhajda FP: Fatty-acid synthase and human
cancer: New perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon JS, Lee S, Park MA, Siempos II,
Haslip M, Lee PJ, Yun M, Kim CK, Howrylak J, Ryter SW, et al:
UCP2-induced fatty acid synthase promotes NLRP3 inflammasome
activation during sepsis. J Clin Invest. 125:665–680. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Namgaladze D and Brüne B: Fatty acid
oxidation is dispensable for human macrophage IL-4-induced
polarization. Biochim Biophys Acta. 1841:1329–1335. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu L, Zhao Q, Yang T, Ding W and Zhao Y:
Cellular metabolism and macrophage functional polarization. Int Rev
Immunol. 34:82–100. 2015. View Article : Google Scholar
|
|
46
|
Hohensinner PJ, Lenz M, Haider P, Mayer J,
Richter M, Kaun C, Goederle L, Brekalo M, Salzmann M, Sharma S, et
al: Pharmacological inhibition of fatty acid oxidation reduces
atherosclerosis progression by suppression of macrophage NLRP3
inflammasome activation. Biochem Pharmacol. 190:1146342021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sola-García A, Cáliz-Molina MÁ, Espadas I,
Petr M, Panadero-Morón C, González-Morán D, Martín-Vázquez ME,
Narbona-Pérez ÁJ, López-Noriega L, Martínez-Corrales G, et al:
Metabolic reprogramming by Acly inhibition using SB-204990 alters
glucoregulation and modulates molecular mechanisms associated with
aging. Commun Biol. 6:2502023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo J, Yang H and Song BL: Mechanisms and
regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol.
21:225–245. 2020. View Article : Google Scholar
|
|
49
|
Guo H, Callaway JB and Ting JP:
Inflammasomes: Mechanism of action, role in disease, and
therapeutics. Nat Med. 21:677–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou QD, Chi X, Lee MS, Hsieh WY,
Mkrtchyan JJ, Feng AC, He C, York AG, Bui VL, Kronenberger EB, et
al: Interferon-mediated reprogramming of membrane cholesterol to
evade bacterial toxins. Nat Immunol. 21:746–755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao J, Chen J, Li M, Chen M and Sun C:
Multifaceted functions of CH25H and 25HC to modulate the lipid
metabolism, immune responses, and broadly antiviral activities.
Viruses. 12:7272020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Platanias LC: Mechanisms of type-I- and
type-II-interferon-mediated signalling. Nat Rev Immunol. 5:375–386.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hsieh WY, Zhou QD, York AG, Williams KJ,
Scumpia PO, Kronenberger EB, Hoi XP, Su B, Chi X, Bui VL, et al:
Toll-like receptors induce signal-specific reprogramming of the
macrophage lipidome. Cell Metab. 32:128–143.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
York AG, Williams KJ, Argus JP, Zhou QD,
Brar G, Vergnes L, Gray EE, Zhen A, Wu NC, Yamada DH, et al:
Limiting cholesterol biosynthetic flux spontaneously engages type I
IFN signaling. Cell. 163:1716–1729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kieler M, Hofmann M and Schabbauer G: More
than just protein building blocks: How amino acids and related
metabolic pathways fuel macrophage polarization. FEBS J.
288:3694–3714. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yuan P, Hu X and Zhou Q: The
nanomaterial-induced bystander effects reprogrammed macrophage
immune function and metabolic profile. Nanotoxicology.
14:1137–1155. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Puchalska P, Huang X, Martin SE, Han X,
Patti GJ and Crawford PA: Isotope tracing untargeted metabolomics
reveals macrophage polarization-state-specific metabolic
coordination across intracellular compartments. Science. 9:298–313.
2018.
|
|
58
|
O'Neill LA, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qualls JE, Subramanian C, Rafi W, Smith
AM, Balouzian L, DeFreitas AA, Shirey KA, Reutterer B, Kernbauer E,
Stockinger S, et al: Sustained generation of nitric oxide and
control of mycobacterial infection requires argininosuccinate
synthase 1. Cell Host Microbe. 12:313–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yue Y, Huang W, Liang J, Guo J, Ji J, Yao
Y, Zheng M, Cai Z, Lu L and Wang J: IL4I1 is a novel regulator of
M2 macrophage polarization that can inhibit T cell activation via
L-tryptophan and arginine depletion and IL-10 production. PLoS One.
10:e01429792015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Opitz CA, Litzenburger UM, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang SCC, Smith AM, Everts B, Colonna M,
Pearce EL, Schilling JD and Pearce EJ: Metabolic reprogramming
mediated by the mTORC2-IRF4 signaling axis is essential for
macrophage alternative activation. Immunity. 45:817–830. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Covarrubias AJ, Aksoylar HI, Yu J, Snyder
NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V,
Polynne-Stapornkul T, et al: Akt-mTORC1 signaling regulates Acly to
integrate metabolic input to control of macrophage activation.
Elife. 5:e116122016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu PS, Wang H, Li X, Chao T, Teav T,
Christen S, Di Conza G, Cheng WC, Chou CH, Vavakova M, et al:
α-ketoglutarate orchestrates macrophage activation through
metabolic and epigenetic reprogramming. Nat Immunol. 18:985–994.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou W, Hu G, He J, Wang T, Zuo Y, Cao Y,
Zheng Q, Tu J, Ma J, Cai R, et al: SENP1-Sirt3 signaling promotes
α-ketoglutarate production during M2 macrophage polarization. Cell
Rep. 39:1106602022. View Article : Google Scholar
|
|
66
|
Palmieri EM, Menga A, Martín-Pérez R,
Quinto A, Riera-Domingo C, De Tullio G, Hooper DC, Lamers WH,
Ghesquière B, McVicar DW, et al: Pharmacologic or genetic targeting
of glutamine synthetase skews macrophages toward an M1-like
phenotype and inhibits tumor metastasis. Cell Rep. 20:1654–1666.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mazzone M, Menga A and Castegna A:
Metabolism and TAM functions-it takes two to tango. FEBS J.
285:700–716. 2018. View Article : Google Scholar
|
|
68
|
Ryan DG and O'Neill LAJ: Krebs cycle
reborn in macrophage immunometabolism. Annu Rev Immunol.
38:289–313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
McGettrick AF and O'Neill LAJ: How
metabolism generates signals during innate immunity and
inflammation. J Biol Chem. 288:22893–22898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
O'Neill LAJ: A broken krebs cycle in
macrophages. Immunity. 42:393–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jha AK, Huang SCC, Sergushichev A,
Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart
KM, Ashall J, Everts B, et al: Network integration of parallel
metabolic and transcriptional data reveals metabolic modules that
regulate macrophage polarization. Immunity. 42:419–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Infantino V, Pierri CL and Iacobazzi V:
Metabolic routes in inflammation: The citrate pathway and its
potential as therapeutic target. Curr Med Chem. 26:7104–7116. 2019.
View Article : Google Scholar
|
|
73
|
Infantino V, Iacobazzi V, Palmieri F and
Menga A: ATP-citrate lyase is essential for macrophage inflammatory
response. Biochem Biophys Res Commun. 440:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tannahill G, Curtis A, Adamik J,
Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ,
Kelly B, Foley NH, et al: Succinate is an inflammatory signal that
induces IL-1β through HIF-1α. Nature. 496:238–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
He W, Miao FJ, Lin DC, Schwandner RT, Wang
Z, Gao J, Chen JL, Tian H and Ling L: Citric acid cycle
intermediates as ligands for orphan G-protein-coupled receptors.
Nature. 429:188–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Toma I, Kang JJ, Sipos A, Vargas S, Bansal
E, Hanner F, Meer E and Peti-Peterdi J: Succinate receptor GPR91
provides a direct link between high glucose levels and renin
release in murine and rabbit kidney. J Clin Invest. 118:2526–2534.
2008.PubMed/NCBI
|
|
77
|
Peti-Peterdi J, Kang JJ and Toma I:
Activation of the renal renin-angiotensin system in diabetes-new
concepts. Nephrol Dial Transplant. 23:3047–3049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sadagopan N, Li W, Roberds SL, Major T,
Preston GM, Yu Y and Tones MA: Circulating succinate is elevated in
rodent models of hypertension and metabolic disease. Am J
Hypertens. 20:1209–1215. 2007.PubMed/NCBI
|
|
79
|
Macaulay IC, Tijssen MR, Thijssen-Timmer
DC, Gusnanto A, Steward M, Burns P, Langford CF, Ellis PD,
Dudbridge F, Zwaginga JJ, et al: Comparative gene expression
profiling of in vitro differentiated megakaryocytes and
erythroblasts identifies novel activatory and inhibitory platelet
membrane proteins. Blood. 109:3260–3269. 2007. View Article : Google Scholar
|
|
80
|
Wu JY, Huang TW, Hsieh YT, Wang YF, Yen
CC, Lee GL, Yeh CC, Peng YJ, Kuo YY, Wen HT, et al: Cancer-derived
succinate promotes macrophage polarization and cancer metastasis
via succinate receptor. Mol Cell. 77:213–227.e5. 2020. View Article : Google Scholar
|
|
81
|
Wunderer F, Traeger L, Sigurslid HH,
Meybohm P, Bloch DB and Malhotra R: The role of hepcidin and iron
homeostasis in atherosclerosis. Pharmacol Res. 153:1046642020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xia Y, Li Y, Wu X, Zhang Q, Chen S, Ma X
and Yu M: Ironing out the details: How iron orchestrates macrophage
polarization. Front Immunol. 12:6695662021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liang G, Sakamoto A, Cornelissen A, Hong
CC and Finn AV: Ironing-out the role of hepcidin in
atherosclerosis. Arterioscler Thromb Vasc Biol. 39:303–305. 2019.
View Article : Google Scholar
|
|
84
|
Marques L, Negre-Salvayre A, Costa L and
Canonne-Hergaux F: Iron gene expression profile in atherogenic Mox
macrophages. Biochim Biophys Acta. 1862:1137–1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Handa P, Thomas S, Morgan-Stevenson V,
Maliken BD, Gochanour E, Boukhar S, Yeh MM and Kowdley KV: Iron
alters macrophage polarization status and leads to steatohepatitis
and fibrogenesis. J Leukoc Biol. 105:1015–1026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hu X, Cai X, Ma R, Fu W, Zhang C and Du X:
Iron-load exacerbates the severity of atherosclerosis via inducing
inflammation and enhancing the glycolysis in macrophages. J Cell
Physiol. 234:18792–18800. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX,
You Y, Gong JP and Liu ZJ: Iron overloaded polarizes macrophage to
proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer
Med. 7:4012–4022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang CY and Babitt JL: Hepcidin regulation
in the anemia of inflammation. Curr Opin Hematol. 23:189–197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kanamori Y, Murakami M, Matsui T and
Funaba M: JNK facilitates IL-1β-induced hepcidin transcription via
JunB activation. Cytokine. 111:295–302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kanamori Y, Murakami M, Sugiyama M,
Hashimoto O, Matsui T and Funaba M: Hepcidin and IL-1β. Vitam Horm.
110:143–156. 2019. View Article : Google Scholar
|
|
91
|
Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao
Y, Wu Q, Zhang Y, Yu Y, Ning B, et al: Ferroportin1 deficiency in
mouse macrophages impairs iron homeostasis and inflammatory
responses. Blood. 118:1912–1922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jiang L, Zheng H, Lyu Q, Hayashi S, Sato
K, Sekido Y, Nakamura K, Tanaka H, Ishikawa K, Kajiyama H, et al:
Lysosomal nitric oxide determines transition from autophagy to
ferroptosis after exposure to plasma-activated Ringer's lactate.
Redox Biol. 43:1019892021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Krümmel B, Plötz T, Jörns A, Lenzen S and
Mehmeti I: The central role of glutathione peroxidase 4 in the
regulation of ferroptosis and its implications for pro-inflammatory
cytokine-mediated beta-cell death. Biochim Biophys Acta Mol Basis
Dis. 1867:1661142021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
de Goede KE, Driessen AJM and Van den
Bossche J: Metabolic cancer-macrophage crosstalk in the tumor
microenvironment. Biology (Basel). 9:3802020.PubMed/NCBI
|
|
95
|
Ling J, Chang Y, Yuan Z, Chen Q, He L and
Chen T: Designing lactate dehydrogenase-mimicking SnSe nanosheets
to reprogram tumor-associated macrophages for potentiation of
photothermal immunotherapy. ACS Appl Mater Interfaces.
14:27651–27665. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE,
Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, et al: Tumor-associated
macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer
Res. 79:795–806. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lin Y, Xu J and Lan H: Tumor-associated
macrophages in tumor metastasis: Biological roles and clinical
therapeutic applications. J Hematol Oncol. 12:762019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu D, Chang C, Lu N, Wang X, Lu Q, Ren X,
Ren P, Zhao D, Wang L, Zhu Y, et al: Comprehensive proteomics
analysis reveals metabolic reprogramming of tumor-associated
macrophages stimulated by the tumor microenvironment. J Proteome
Res. 16:288–297. 2017. View Article : Google Scholar
|
|
99
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Goswami KK, Banerjee S, Bose A and Baral
R: Lactic acid in alternative polarization and function of
macrophages in tumor microenvironment. Hum Immunoll. 83:409–417.
2022. View Article : Google Scholar
|
|
101
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chiu DKC, Xu IMJ, Lai RKH, Tse AP, Wei LL,
Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al: Hypoxia induces
myeloid-derived suppressor cell recruitment to hepatocellular
carcinoma through chemokine (C-C motif) ligand 26. Hepatology.
64:797–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xu Y, Lu J, Tang Y, Xie W, Zhang H, Wang
B, Zhang S, Hou W, Zou C, Jiang P and Zhang W: PINK1 deficiency in
gastric cancer compromises mitophagy, promotes the Warburg effect,
and facilitates M2 polarization of macrophages. Cancer Lett.
529:19–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Locatelli SL, Careddu G, Serio S, Consonni
FM, Maeda A, Viswanadha S, Vakkalanka S, Castagna L, Santoro A,
Allavena P, et al: Targeting cancer cells and tumor
microenvironment in preclinical and clinical models of hodgkin
lymphoma using the dual PI3Kδ/γ inhibitor RP6530. Clin Cancer Res.
25:1098–1112. 2019. View Article : Google Scholar
|
|
106
|
Ohashi T, Aoki M, Tomita H, Akazawa T,
Sato K, Kuze B, Mizuta K, Hara A, Nagaoka H, Inoue N and Ito Y:
M2-like macrophage polarization in high lactic acid-producing head
and neck cancer. Cancer Sci. 108:1128–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kumar V: Targeting macrophage
immunometabolism: Dawn in the darkness of sepsis. Int
Immunopharmacol. 58:173–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kanmani P and Kim H: Protective effects of
lactic acid bacteria against TLR4 induced inflammatory response in
hepatoma HepG2 cells through modulation of toll-like receptor
negative regulators of mitogen-activated protein kinase and NF-κB
signaling. Front Immunol. 9:15372018. View Article : Google Scholar
|
|
109
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Carmona-Fontaine C, Deforet M, Akkari L,
Thompson CB, Joyce JA and Xavier JB: Metabolic origins of spatial
organization in the tumor microenvironment. Proc Natl Acad Sci USA.
114:2934–2939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li K, Lin C, He Y, Lu L, Xu K, Tao B, Xia
Z, Zeng R, Mao Y, Luo Z and Cai K: Engineering of
cascade-responsive nanoplatform to inhibit lactate efflux for
enhanced tumor chemo-immunotherapy. ACS Nano. 14:14164–14180. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Choi H, Yeo M, Kang Y, Kim HJ, Park SG,
Jang E, Park SH, Kim E and Kang S: Lactate
oxidase/catalase-displaying nanoparticles efficiently consume
lactate in the tumor microenvironment to effectively suppress tumor
growth. J Nanobiotechnology. 21:52023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang H, Wu C, Tong X and Chen S: A
biomimetic metal-organic framework nanosystem modulates
immunosuppressive tumor microenvironment metabolism to amplify
immunotherapy. J Control Release. 353:727–737. 2023. View Article : Google Scholar
|
|
115
|
Zhao S, Li H, Liu R, Tao N, Deng L, Xu Q,
Hou J, Sheng J, Zheng J, Wang L, et al: Nitrogen-centered lactate
oxidase nanozyme for tumor lactate modulation and microenvironment
remodeling. J Am Chem Soc. 145:10322–10332. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang X, Zhao M, Wu Z, Chen C, Zhang Y,
Wang L, Guo Q, Wang Q, Liang S, Hu S, et al: Nano-ultrasonic
contrast agent for chemoimmunotherapy of breast cancer by immune
metabolism reprogramming and tumor autophagy. ACS Nano.
16:3417–3431. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wu H, Han Y, Rodriguez Sillke Y, Deng H,
Siddiqui S, Treese C, Schmidt F, Friedrich M, Keye J, Wan J, et al:
Lipid droplet-dependent fatty acid metabolism controls the immune
suppressive phenotype of tumor-associated macrophages. EMBO Mol
Med. 11:e106982019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wu L, Zhang X, Zheng L, Zhao H, Yan G,
Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al: RIPK3 orchestrates
fatty acid metabolism in tumor-associated macrophages and
hepatocarcinogenesis. Cancer Immunol Res. 8:710–721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen
B, Wang Q, Zhao X, Chen J, Cheng N, et al: Caspase-1 cleaves PPARγ
for potentiating the pro-tumor action of TAMs. Nat Commun.
8:7662017. View Article : Google Scholar
|
|
120
|
Di Conza G, Tsai CH, Gallart-Ayala H, Yu
YR, Franco F, Zaffalon L, Xie X, Li X, Xiao Z, Raines LN, et al:
Tumor-induced reshuffling of lipid composition on the endoplasmic
reticulum membrane sustains macrophage survival and pro-tumorigenic
activity. Nat Immunol. 22:1403–1415. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bidault G, Virtue S, Petkevicius K, Jolin
HE, Dugourd A, Guénantin AC, Leggat J, Mahler-Araujo B, Lam BYH, Ma
MK, et al: SREBP1-induced fatty acid synthesis depletes macrophages
antioxidant defences to promote their alternative activation. Nat
Metab. 3:1150–1162. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhao Q, Lin X and Wang G: Targeting
SREBP-1-mediated lipogenesis as potential strategies for cancer.
Front Oncol. 12:9523712022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang T, Guo Z, Huo X, Gong Y, Li C, Huang
J, Wang Y, Feng H, Ma X, Jiang C, et al: Dysregulated lipid
metabolism blunts the sensitivity of cancer cells to EZH2
inhibitor. EBioMedicine. 77:1038722022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen M and Huang J: The expanded role of
fatty acid metabolism in cancer: New aspects and targets. Precis
Clin Med. 2:183–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xiang W, Shi R, Kang X, Zhang X, Chen P,
Zhang L, Hou A, Wang R, Zhao Y, Zhao K, et al: Monoacylglycerol
lipase regulates cannabinoid receptor 2-dependent macrophage
activation and cancer progression. Nat Commun. 9:25742018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jiang M, Li X, Zhang J, Lu Y, Shi Y, Zhu
C, Liu Y, Qin B, Luo Z, Du Y, et al: Dual inhibition of endoplasmic
reticulum stress and oxidation stress manipulates the polarization
of macrophages under hypoxia to sensitize immunotherapy. ACS Nano.
15:14522–14534. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hou L, Gong X, Yang J, Zhang H, Yang W and
Chen X: Hybrid-membrane-decorated prussian blue for effective
cancer immunotherapy via tumor-associated macrophages polarization
and hypoxia relief. Adv Mater. 34:22003892022. View Article : Google Scholar
|
|
128
|
Yang Z, Luo Y, Yu H, Liang K, Wang M, Wang
Q, Yin B and Chen H: Reshaping the tumor immune microenvironment
based on a light-activated nanoplatform for efficient cancer
therapy. Adv Mater. 34:21089082022. View Article : Google Scholar
|
|
129
|
Costa da Silva M, Breckwoldt MO, Vinchi F,
Correia MP, Stojanovic A, Thielmann CM, Meister M, Muley T, Warth
A, Platten M, et al: Iron induces anti-tumor activity in
tumor-associated macrophages. Front Immunol. 8:14792017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv
Y, Bao W, Gao X, Wei W, Pu K and Xie HY: Engineering magnetosomes
for ferroptosis/immunomodulation synergism in cancer. ACS Nano.
13:5662–5673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gu Z, Liu T, Liu C, Yang Y, Tang J, Song
H, Wang Y, Yang Y and Yu C: Ferroptosis-strengthened metabolic and
inflammatory regulation of tumor-associated macrophages provokes
potent tumoricidal activities. Nano Lett. 21:6471–6479. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Altman BJ, Stine ZE and Dang CV: From
Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev
Cancer. 16:619–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhu Y, Zhang S, Sun J, Wang T, Liu Q, Wu
G, Qian Y, Yang W, Wang Y and Wang W: Cigarette smoke promotes oral
leukoplakia via regulating glutamine metabolism and M2 polarization
of macrophage. Int J Oral Sci. 13:252021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Oh MH, Sun IH, Zhao L, Leone RD, Sun IM,
Xu W, Collins SL, Tam AJ, Blosser RL, Patel CH, et al: Targeting
glutamine metabolism enhances tumor-specific immunity by modulating
suppressive myeloid cells. J Clin Invest. 130:3865–3884. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Du B, Jiao Q, Bai Y, Yu M, Pang M, Zhao M,
Ma H and Yao H: Glutamine metabolism-regulated nanoparticles to
enhance chemoimmunotherapy by increasing antigen presentation
efficiency. ACS Appl Mater Interfaces. 14:8753–8765. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hoves S, Ooi CH, Wolter C, Sade H,
Bissinger S, Schmittnaegel M, Ast O, Giusti AM, Wartha K, Runza V,
et al: Rapid activation of tumor-associated macrophages boosts
preexisting tumor immunity. J Exp Med. 215:859–876. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kashyap AS, Schmittnaegel M, Rigamonti N,
Pais-Ferreira D, Mueller P, Buchi M, Ooi CH, Kreuzaler M,
Hirschmann P, Guichard A, et al: Optimized antiangiogenic
reprogramming of the tumor microenvironment potentiates CD40
immunotherapy. Proc Natl Acad Sci USA. 117:541–551. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Beatty GL, Chiorean EG, Fishman MP,
Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL,
et al: CD40 agonists alter tumor stroma and show efficacy against
pancreatic carcinoma in mice and humans. Science. 331:1612–1616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu PS, Chen YT, Li X, Hsueh PC, Tzeng SF,
Chen H, Shi PZ, Xie X, Parik S, Planque M, et al: CD40 signal
rewires fatty acid and glutamine metabolism for stimulating
macrophage anti-tumorigenic functions. Nat Immunol. 24:452–462.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Mai Z, Zhong J, Zhang J, Chen G, Tang Y,
Ma W, Li G, Feng Z, Li F, Liang XJ, et al: Carrier-free
immunotherapeutic nano-booster with dual synergistic effects based
on glutaminase inhibition combined with photodynamic therapy. ACS
Nano. 17:1583–1596. 2023. View Article : Google Scholar
|
|
141
|
Tabas I and Bornfeldt KE: Intracellular
and intercellular aspects of macrophage immunometabolism in
atherosclerosis. Circ Res. 126:1209–1227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhu X, Owen JS, Wilson MD, Li H, Griffiths
GL, Thomas MJ, Hiltbold EM, Fessler MB and Parks JS: Macrophage
ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to
lipid rafts by reduction of lipid raft cholesterol. J Lipid Res.
51:3196–3206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Stewart CR, Stuart LM, Wilkinson K, van
Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA,
et al: CD36 ligands promote sterile inflammation through assembly
of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol.
11:155–161. 2010. View Article : Google Scholar :
|
|
144
|
Miller YI, Viriyakosol S, Worrall DS,
Boullier A, Butler S and Witztum JL: Toll-like receptor 4-dependent
and -independent cytokine secretion induced by minimally oxidized
low-density lipoprotein in macrophages. Arterioscler Thromb Vasc
Biol. 25:1213–1219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 464:1357–1361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen J, Su Y, Pi S, Hu B and Mao L: The
dual role of low-density lipoprotein receptor-related protein 1 in
atherosclerosis. Front Cardiovasc Med. 8:6823892021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tomas L, Edsfeldt A, Mollet IG, Perisic
Matic L, Prehn C, Adamski J, Paulsson-Berne G, Hedin U, Nilsson J,
Bengtsson E, et al: Altered metabolism distinguishes high-risk from
stable carotid atherosclerotic plaques. Eur Heart J. 39:2301–2310.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mügge A: The role of reactive oxygen
species in atherosclerosis. Z Kardiol. 87:851–864. 1998.
|
|
149
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta J: Oxidative stress in atherosclerosis. Curr Atheroscler Rep.
19:422017. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
He J, Zhang W, Zhou X, Xu F, Zou J, Zhang
Q, Zhao Y, He H, Yang H and Liu J: Reactive oxygen species
(ROS)-responsive size-reducible nanoassemblies for deeper
atherosclerotic plaque penetration and enhanced macrophage-targeted
drug delivery. Bioact Mater. 19:115–126. 2022.PubMed/NCBI
|
|
151
|
Wang Y, Li L, Zhao W, Dou Y, An H, Tao H,
Xu X, Jia Y, Lu S, Zhang J and Hu H: Targeted therapy of
atherosclerosis by a broad-spectrum reactive oxygen species
scavenging nanoparticle with intrinsic anti-inflammatory activity.
ACS Nano. 12:8943–8960. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hu R, Dai C, Dong C, Ding L, Huang H, Chen
Y and Zhang B: Living macrophage-delivered tetrapod PdH nanoenzyme
for targeted atherosclerosis management by ROS scavenging, hydrogen
anti-inflammation, and autophagy activation. ACS Nano.
16:15959–15976. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Sun W, Xu Y, Yao Y, Yue J, Wu Z, Li H,
Shen G, Liao Y, Wang H and Zhou W: Self-oxygenation mesoporous
MnO2 nanoparticles with ultra-high drug loading capacity
for targeted arteriosclerosis therapy. J Nanobiotechnology.
20:882022. View Article : Google Scholar
|
|
154
|
Han XB, Li HX, Jiang YQ, Wang H, Li XS,
Kou JY, Zheng YH, Liu ZN, Li H, Li J, et al: Upconversion
nanoparticle-mediated photodynamic therapy induces autophagy and
cholesterol efflux of macrophage-derived foam cells via ROS
generation. Cell Death Dis. 8:e28642017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dai T, He W, Tu S, Han J, Yuan B, Yao C,
Ren W and Wu A: Black TiO2 nanoprobe-mediated mild
phototherapy reduces intracellular lipid levels in atherosclerotic
foam cells via cholesterol regulation pathways instead of
apoptosis. Bioact Mater. 17:18–28. 2022.PubMed/NCBI
|
|
156
|
Zhang Y, Gong F, Wu Y, Hou S, Xue L, Su Z
and Zhang C: Poly-β-cyclodextrin supramolecular nanoassembly with a
pH-sensitive switch removing lysosomal cholesterol crystals for
antiatherosclerosis. Nano Lett. 21:9736–9745. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
You P, Mayier A, Zhou H, Yang A, Fan J, Ma
S, Liu B and Jiang Y: Targeting and promoting atherosclerosis
regression using hybrid membrane coated nanomaterials via
alleviated inflammation and enhanced autophagy. Appl Mater Today.
26:1013862022. View Article : Google Scholar
|
|
158
|
Li C, Dou Y, Chen Y, Qi Y, Li L, Han S,
Jin T, Guo J, Chen J and Zhang J: Site-specific microRNA-33
antagonism by pH-responsive nanotherapies for treatment of
atherosclerosis via regulating cholesterol efflux and adaptive
immunity. Adv Funct Mater. 30:20021312020. View Article : Google Scholar
|
|
159
|
He H, Wang J, Yannie PJ, Korzun WJ, Yang H
and Ghosh S: Nanoparticle-based 'two-pronged' approach to regress
atherosclerosis by simultaneous modulation of cholesterol influx
and efflux. Biomaterials. 260:1203332020. View Article : Google Scholar
|
|
160
|
Wu Z, Zhou M, Tang X, Zeng J, Li Y, Sun Y,
Huang J, Chen L, Wan M and Mao C: Carrier-free trehalose-based
nanomotors targeting macrophages in inflammatory plaque for
treatment of atherosclerosis. ACS Nano. 16:3808–3820. 2022.
View Article : Google Scholar : PubMed/NCBI
|