Introduction
Diabetic kidney disease (DKD) is a major
complication of diabetes and the prominent cause of chronic kidney
disease and end-stage kidney disease (ESKD). It has been reported
that DKD develops in 30% of individuals with type 1 diabetes
mellitus (DM) and in 20-50% of patients with type 2 DM (T2DM)
(1). The prevalence of DKD has
been increasing worldwide, which parallels the great rise in the
prevalence of diabetes, hypertension, obesity and aging (2,3).
DKD causes a tremendous global disease burden and markedly upsurges
the risk of renal failure and cardiovascular diseases (4). It was reported that the number of
deaths caused by DKD was increased by 94% between 1990 and 2012
(5). Currently, the application
of the renin-angiotensin-aldosterone system (RAS) inhibitor and
multidisciplinary treatments, such as control for blood glucose,
blood pressure and lipid levels, have been shown to be effective in
treating DKD (6). In addition,
sodium-dependent glucose transporters 2 (SGLT2) inhibitors have
been shown to be a novel treatment option for DKD (7,8).
It has been reported that 5.27% (116/2,202) of patients with DKD
continue to develop ESKD even if they are treated with RAS and
SGLT2 inhibitors (6). Therefore,
there is an urgent need to identify novel therapeutic treatment
agents for this disease.
Autophagy is a highly conserved intracellular
catabolic process that extensively degrades a number of damaged
proteins and organelles to preserve cell homeostasis under various
stress conditions through the lysosomal pathway (9). It has been reported that autophagy
can regulate systemic blood glucose and lipid metabolism in
mammals. Moreover, higher levels of basal autophagy are responsible
for podocyte homeostasis (10),
and activation of autophagy enhances the adaptive response in
podocytes. In addition, autophagy is also reported to be
responsible for podocyte differentiation, contributing to the
development of the mouse kidney (11). Therefore, targeting autophagy may
be used as a potential novel therapeutic treatment for DKD
(12).
Recently, an increasing body of evidence suggests
that senolytic drugs, such as the combination of dasatinib and
quercetin (DQ) present beneficial effects on various age-related
disorders, such as Alzheimer's disease (AD) (13) and intervertebral disc
degeneration (14). The
potential mechanisms include removal of senescent cells, reduction
of inflammation and modulation of the gut microbiome (15,16). Dasatinib is a second-generation
dual-specificity tyrosine kinase inhibitor that is permitted for
the treatment of chronic myeloid leukemia (17). Quercetin is a flavonoid and a
natural substance with a phenolic structure, which is abundant in
several fruits, vegetables, leaves, seeds and grains (18). Quercetin possesses antioxidant,
anti-inflammatory and anticancer activities. However, a limited
number of studies have explored the effects of DQ on DKD (19,20). A recent study showed that single
3-day oral course of DQ selectively decreases the number of
senescent cells in patients with DKD (19). In addition, another study
revealed that DQ alleviated insulin resistance, proteinuria and
podocyte dysfunction in high fat diet-induced or genetically obese
mice by targeting senescent cells (20). The effects of DQ on DKD and its
specific pathways and molecular mechanisms require further
exploration.
Although autophagy and senescence share a number of
characteristics and are closely related, they are not necessarily
interdependent responses. It has not yet been established whether
the effects of DQ on DKD are mediated by targeting autophagy.
Therefore, in the present study, in vivo and in vitro
experiments were performed to verify these effects and clarify the
associated mechanism. The present study may provide a novel option
for the treatment of DKD.
Materials and methods
Animals and treatment
A total of 8-week-old male diabetic db/db mice
(n=16) and age-matched wild-type non-diabetic db/m mice (n=8) were
purchased from Charles River Laboratories, Inc. The animals were
raised in a specific pathogen free room at 22±2°C with a relative
humidity of 40-60% and a 12-h light-12-h dark cycle. The mice were
provided free access to standard pellet food and fresh water. The
animals were randomly divided into three groups (n=8 in each group)
as follows: db/m, db/db and db/db + DQ. The diabetic model was
successfully induced if the fasting blood glucose was ≥16.7 mM. The
mice in the db/m and db/db groups were administered PBS, while the
mice in the db/db + DQ group were treated with senolytic cocktail
dasatinib (5 mg/kg, LC Laboratories) and quercetin (50 mg/kg,
Sigma-Aldrich; Merck KGaA) by gavage for 20 weeks (Fig. 1A). The health and behavior of the
mice was monitored once a day and the body weight of the mice was
measured weekly from week 10 to 20. The duration of the experiment
was 20 weeks. Mice were anesthetized with isoflurane inhalation
(4.0% induction and 2% maintenance) (21) and euthanized by cervical
dislocation following anesthesia. Mouse death was verified by the
cessation of respiratory movements. A total of 24 mice were used
and euthanized in the experiment, and no death occurred before the
end of the experiment. Animal death was confirmed if respiratory
and cardiac arrest, and pupil dilation were observed for ≥10 min.
All animal welfare considerations were taken, including efforts to
minimize suffering and distress, use of analgesics or anesthetics,
or special housing conditions. The animal experiments were carried
out according to the Guide for the Care and Use of Laboratory
Animals of the National Research Council and approved by the Ethics
Committee of The First Affiliated Hospital of China Medical
University [approval no. (2019) 136].
Blood and urine tests
Blood glucose was determined using the
Accu-Chek® Aviva glucometer (Roche Diabetes Care
Limited), and the levels of urine albumin-creatinine ratio (ACR)
were assessed using the immunoturbidimetric assay with Tina-quant
Albumin Gen.2 (ALBT2; Roche Diagnostics) at the indicated time
(week 10, 12, 16 and 20). Serum creatinine (Scr) was examined using
the compensated Jaffé method in a Roche/Integra 400 Analyzer (Roche
Diagnostics) and blood urea nitrogen (BUN) was assessed by a urea
assay kit (BioAssay System) at week 20.
Immunohistochemical (IHC) staining
Kidneys were fixed with 10% neutral buffered
formalin for 24-48 h at room temperature, embedded in paraffin and
cut into 4-μm-thick sections. After deparaffinization and
rehydration in ethanol baths of 100, 90 and 60%, respectively, for
5 min each, the sections underwent antigen retrieval using
microwave heating at high heat (>100°C) for 8 min, medium and
low heat (50-60°C) for 20 min, and cooled at room temperature for 1
h. The sections were washed with PBS for 5 min ×3 times at room
temperature. Subsequently, the sections were blocked with 5% normal
goat serum in PBS at 37°C for 30 min and then incubated with a
primary antibody against either fibronectin (FN; cat. no. ab2413;
Abcam) or nephrin (cat. no. ab216341; Abcam) overnight for 12 h at
4°C, followed by incubation with secondary antibodies against
biotin-conjugated goat anti-rabbit IgG H&L (1:500; cat. no.
ab207995) for 1 h at room temperature. Following staining with
diaminobenzidine at room temperature for 2-5 min and
counterstaining with hematoxylin at room temperature for 3 min, the
sections were visualized using a light microscope (BX53, Olympus
Corporation) at a magnification of ×400.
Histological staining
The histopathological changes of the kidney tissues
were assessed by periodic acid-Schiff (PAS) and Masson's trichrome
staining. Briefly, tissues were fixed with 10% buffered formalin
for 24 h at room temperature, embedded in paraffin and cut into
4-μm-think sections. Subsequently, the sections were stained
with either PAS solution for 15-20 min at room temperature (ScyTek
Laboratories, Inc.) according to the manufacturer's guidelines as
previously described (22). For
Masson's trichrome staining, the sections were stained in Weigert's
iron hematoxylin staining for 3 min at room temperature, rinsed
under running water and differentiated with 1% hydrochloric acid
alcohol. Then, the sections were stained in Biebrich scarlet-acid
fuchsin solution for 5-10 min at room temperature, washed in
distilled water and differentiated in
phosphomolybdic-phosphotungestic acid solution for 1-3 min at room
temperature. Thereafter, the sections were transferred to aniline
blue solution for 3-6 min at room temperature and differentiated in
1% acetic acid solution. After gradient dehydration with ethanol,
the sections were stained with eosin solution for 5 min at room
temperature. The images were captured using a light microscope
(BX53, Olympus Corporation) at a magnification of ×200 or ×400. PAS
was performed to assess the tubular injury. The tubular damage
score was evaluated based on a semiquantitative scale of
0-5+ (23) as
follows: i) 0, no lesion; ii) 1+, ≤10%; iii)
2+, 11-25%; iv) 3+, 26-45%; v) 4+,
46-75%; and vi) 5+, ≥76%.
Immunofluorescence (IF) staining
The frozen sections (3 μm) of the kidney
tissues were fixed with 4% paraformaldehyde for 15 min at room
temperature. Podocytes cultured on coverslips were fixed with cold
methanol/acetone for 10 min at room temperature. Following blocking
with 10% donkey serum for 60 min at room temperature, the slides
were immunostained with primary antibodies against FN (cat. no.
ab2413; Abcam), podocin (cat. no. SAB4200810; Sigma-Aldrich; Merck
KGaA), synaptopodin (synap; cat. no. SAB3500585; Sigma-Aldrich;
Merck KGaA), LC3 (cat. no. ab192890; Abcam), p62 (cat. no.
MA5-27800; Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C;
subsequently, they were incubated with a secondary antibodies
against Alexa Fluor 647-conjugated donkey anti-rabbit IgG H&L
antibody (1:700; cat. no. ab150075; Abcam), Alexa Fluor
647-conjugated goat anti-mouse IgG H&L antibody (1:700; cat.
no. ab150115; Abcam), Alexa Fluor® 488-conjugated goat
anti-mouse IgG H&L antibody (1:500; cat. no. ab150113; Abcam)
for 2 h at 37°C. The counterstaining of the cell nuclei was
performed using 4′,6-diamidino-2-phenylindole (Sigma-Aldrich; Merck
KGaA) for 10 min at room temperature. The images were obtained
using a confocal microscope at a magnification of ×400.
Western blotting
Total protein was extracted from both kidney tissues
and mouse podocytes using RIPA buffer (Cell Signaling Technology,
Inc.) and quantified by the bicinchoninic acid protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). The samples (30
μg) were subjected to 8-15% SDS-PAGE and the proteins were
transferred to polyvinylidene fluoride membranes (Pierce; Thermo
Fisher Scientific, Inc.). Subsequently, the membranes were blocked
with bovine serum albumin (BSA; cat. no. A7030; Sigma Aldrich;
Merck KGaA) at 37°C for 1 h, and incubated with the following
primary antibodies overnight for 12 h at 4°C: FN (1:500; cat. no.
ab2413; Abcam), type I collagen (Col I; 1:500; cat. no. ab260043;
Abcam), nephrin (1:500; cat. no. sc-376522; Santa Cruz
Biotechnology, Inc.), podocin (1:500; cat. no. ab181143; Abcam),
synap (1:500; cat. no. SAB3500585; Sigma-Aldrich; Merck KGaA), LC3
(1:500; cat. no. ab192890; Abcam), p62 (1:500; cat. no. ab109012;
Abcam) and Notch intracellular domain (NICD; 1:500; cat. no.
ab52627; Abcam). β-actin was used as a reference. Following
washing, the membranes were incubated with secondary antibodies
against HRP-conjugated goat anti-rabbit IgG H&L antibody
(1:5,000; cat. no. ab6721; Abcam) or HRP-conjugated goat anti-mouse
IgG H&L antibody (1:5,000; cat. no. ab205719; Abcam) for 2 h at
room temperature. The protein bands were imaged with Millipore
Immobilon Western Chemiluminescent HRP Substrate (MilliporeSigma).
The images were finally quantified by ImageJ software (version
1.51j8; National Institutes of Health).
Cell culture and treatment
Conditionally immortalized mouse podocytes were
kindly provided by Dr Perter Mundel (Mount Sinai School of
Medicine) and cultured in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), 100 U/ml penicillin and 100 μg/ml streptomycin
at 37°C and 5 % CO2 (24). Conditionally immortalized cells
refer the cell lines with unlimited proliferation capacity and
resistance to apoptosis, which are created by transduction of
specific cellular or viral immortalizing genes. All experiments
were performed on passage 10-14 podocytes. The podocytes were
treated with 5.5 mmol/l glucose corresponding to a normal glucose
(NG) group or with 30 mmol/l glucose corresponding to a
high-glucose (HG) group. In addition, DQ (250 nM dasatinib + 50
μm quercetin) (25) and
3-methyladenine (3-MA; 1 mg/ml, Sigma-Aldrich; Merck KGaA)
(26) were treated with the
cells under HG conditions.
Cell transfection
Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for cell transfection according
to the manufacturer's instructions. Briefly, podocytes
(1×104 cells/well) were plated in 96-well plates. When
the cells reached 70-80% confluency, the podocytes were transfected
with lentivirus-mediated NICD overexpression plasmid (Genepharm,
Inc.) at 37°C for 6 h using the transfection reagent. The empty
vector was used as a control for overexpression vector. The
overexpression plasmid backbone was amplified and cloned by Gibson
assembly. The concentration of nucleic acid was 500 ng/ml. The 3rd
generation of lentiviral packaging system was used and DNA was
transfected into 293T cells (American Type Culture Collection) at
37°C for 48 h. The ratio used for the lentivirus, packaging and
envelope plasmids was 4:3:1. The collection of lentiviral particles
was 20 transduction unit with a multiplicity of infection of 20.
The quantity of lentiviral plasmid used for transfection was 5
μg. The duration of transfection into the cells of interest
was 16 h. Subsequent experiments were performed 48 h after
transfection.
Transmission electron microscopy
(TEM)
TEM was performed to examine ultrastructural changes
in podocytes as previously described (27). Briefly, podocytes were fixed with
2.5% glutaraldehyde solution (cat. no. P1126; Beijing Solarbio
Science & Technology Co., Ltd.) in PBS overnight at 4°C for 12
h. Subsequently, the ultrathin sections (40-50 nm) were treated
with 1% osmium tetroxide (Epon812; SPI), washed, and dehydrated in
graded ethanols at room temperature (50, 70, 90, 95 and 100%) for 8
min each, embedded in Spurr resin for 3-4 h at 60°C, and
polymerized for 48 h at 60°C. Thereafter, the ultrathin sections
were stained in 5% uranyl acetate for 30 min at room temperature
followed by 0.1% lead citrate for 10 min at room temperature.
Finally, the samples were observed using TEM (LL7650, Hitachi,
Ltd.) at a magnification of ×4,000.
Statistical analysis
The data are expressed as mean ± standard deviation.
Comparisons between two groups were performed using unpaired
Student's t-test, and those between ≥3 groups were performed with
one-way analysis of variance and Tukey's post hoc test using SPSS
(version 20.0; IBM Corp.). P≤ 0.05 was considered to indicate a
statistically significant difference.
Results
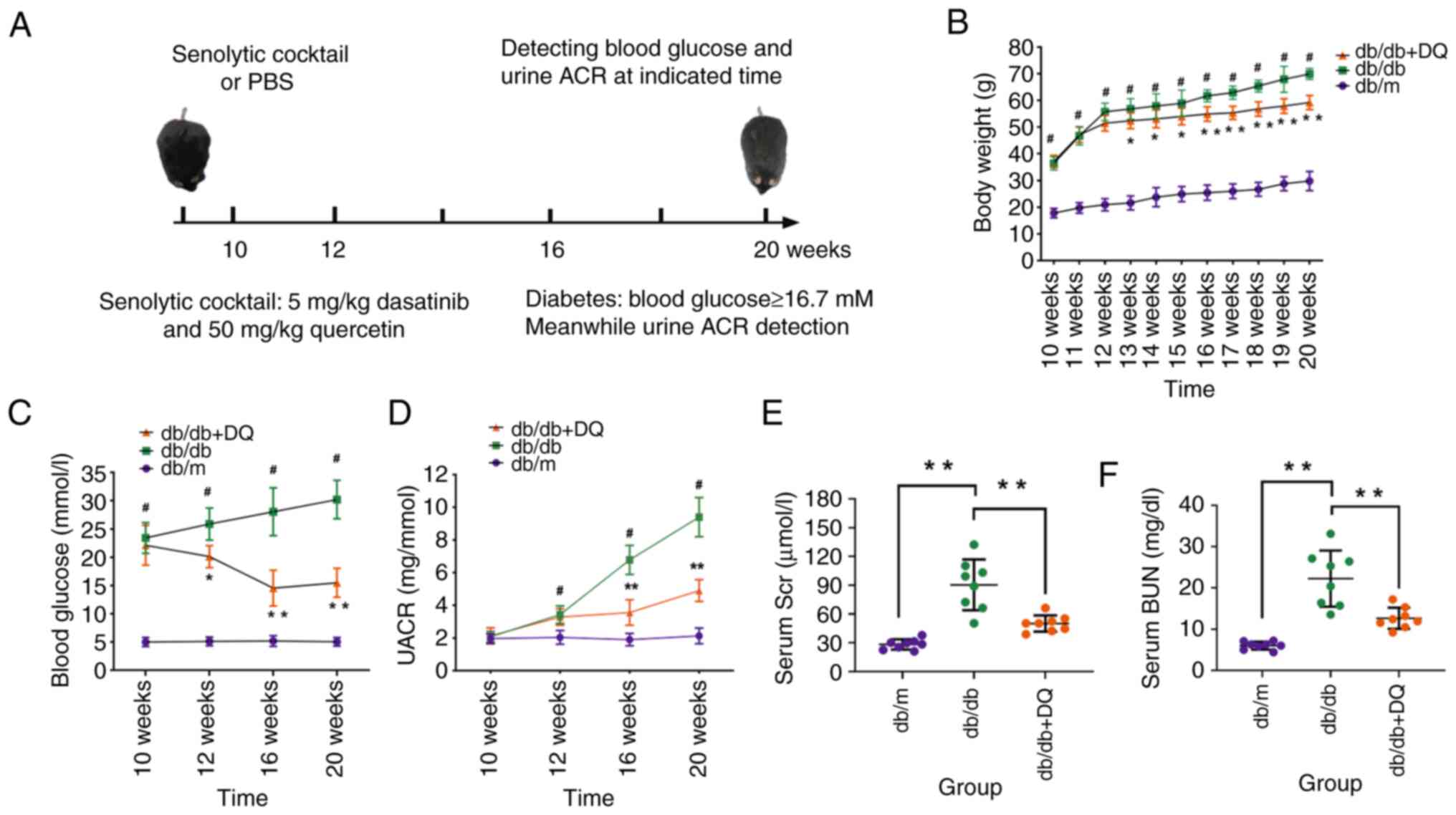
DQ improves renal function in diabetic
mice
The body weight of the mice in each group was
measured weekly. The results indicated that the body weight was
increased as the age of mice increased in each group. The body
weight was significantly higher in the db/db group than that noted
in the db/m group at each week of age (P<0.05); the body weight
was decreased in the db/db + DQ group compared with that of the
db/db group following 3 weeks of intervention (P<0.05 or
P<0.01). This change was more apparent following 6 weeks of
intervention (Fig. 1B). The
blood glucose levels were detected at different time points in each
group, and it was found that they were higher in the db/db group
than those in the db/m group at each time point (P<0.05); the
blood glucose levels were reduced in the db/db DQ group following 2
weeks of intervention compared with those of the db/db group
(Fig. 1C). The most significant
glucose-lowering effect was noted following 6 weeks of intervention
(Fig. 1C). In addition, it was
observed that the levels of urine ACR were significantly increased
in the db/db group compared with those of the db/m group at week
12, 16 and 20 (P<0.05), while the levels of urine ACR were
significantly decreased in the db/db + DQ group compared with those
of the db/db group at week 16 and 20 (P<0.01; Fig. 1D). Scr and BUN were measured at
week 20 of age, and it was found that their levels were
significantly downregulated in the db/db DQ group compared with
those in the db/db group, indicating that DQ significantly improved
the renal function of db/db mice (Fig. 1E and F).
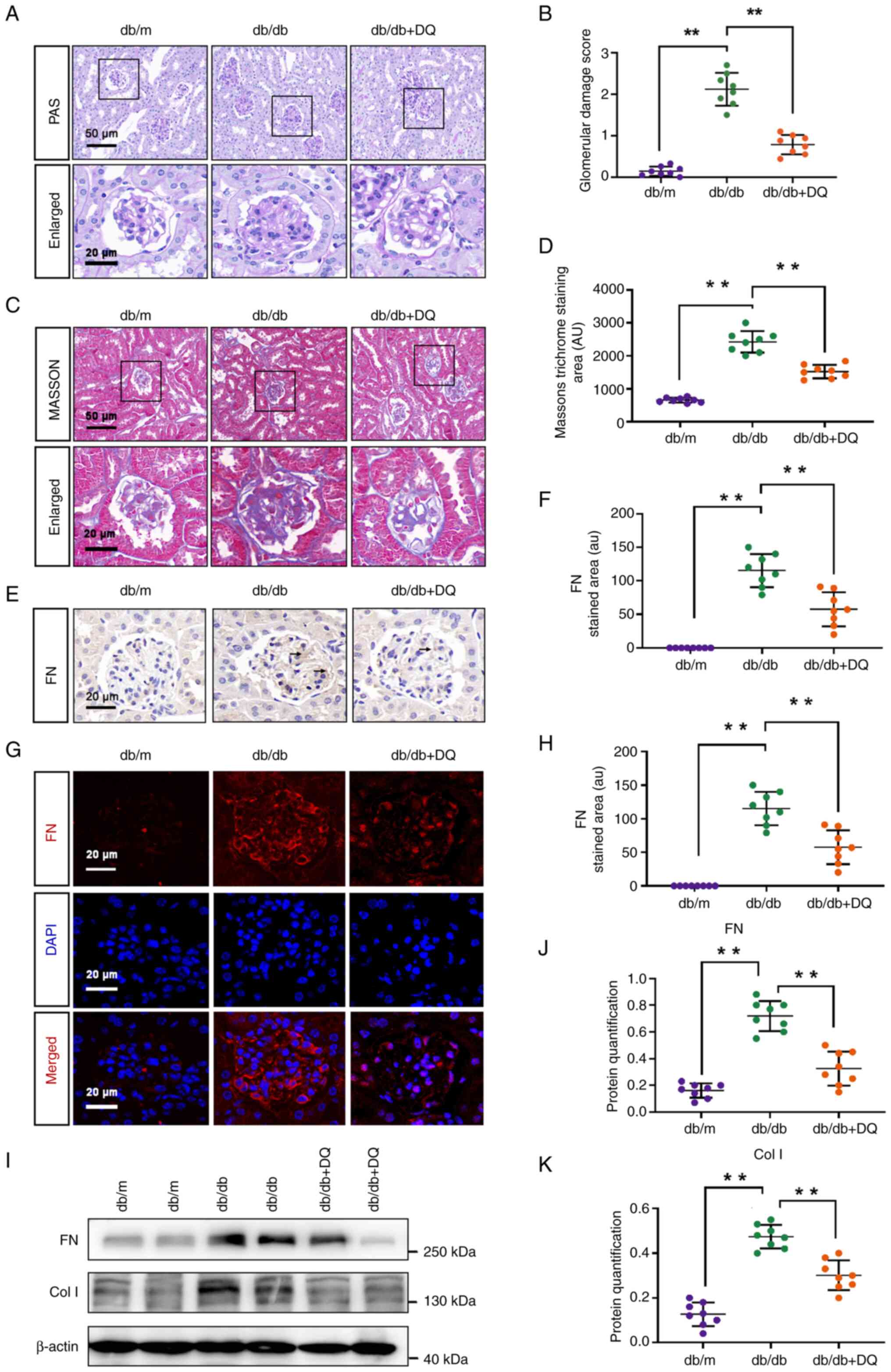
DQ improves pathological changes in
diabetic mouse kidneys
Kidney tissues were collected from mice at week 20
and stained with PAS and Masson's stains to assess the degree of
pathological changes in the kidneys of the mice from each group.
The results indicated that glomerular basement membrane thickening
and mesangial matrix hyperplasia were observed in the kidney
tissues of the db/db mice. The glomerular damage score and renal
fibrosis levels were significantly increased compared with the db/m
group (P<0.01). Treatment with DQ for 10 weeks significantly
improved these histopathological changes, including reduction of
glomerular damage score (P<0.01) and renal fibrosis levels
(P<0.01; Fig. 2A-D). IHC and
IF analyses revealed that the expression levels of FN in the
glomeruli of db/db mice were significantly increased compared with
those of the db/m mice (P<0.01) and the expression levels of FN
could be significantly reduced with DQ treatment for 10 weeks
(P<0.01; Fig. 2E-H),
respectively. The expression levels of the extracellular matrix
(ECM) proteins FN and Col I in the kidney tissues of the groups
were detected by western blotting. The protein expression levels of
FN and Col I were significantly increased in the db/db renal
tissues compared with those in the db/m group (P<0.01). DQ
administration significantly reduced the protein expression levels
of FN and Col I in the db/db + DQ group (P<0.01; Fig. 2I-K).
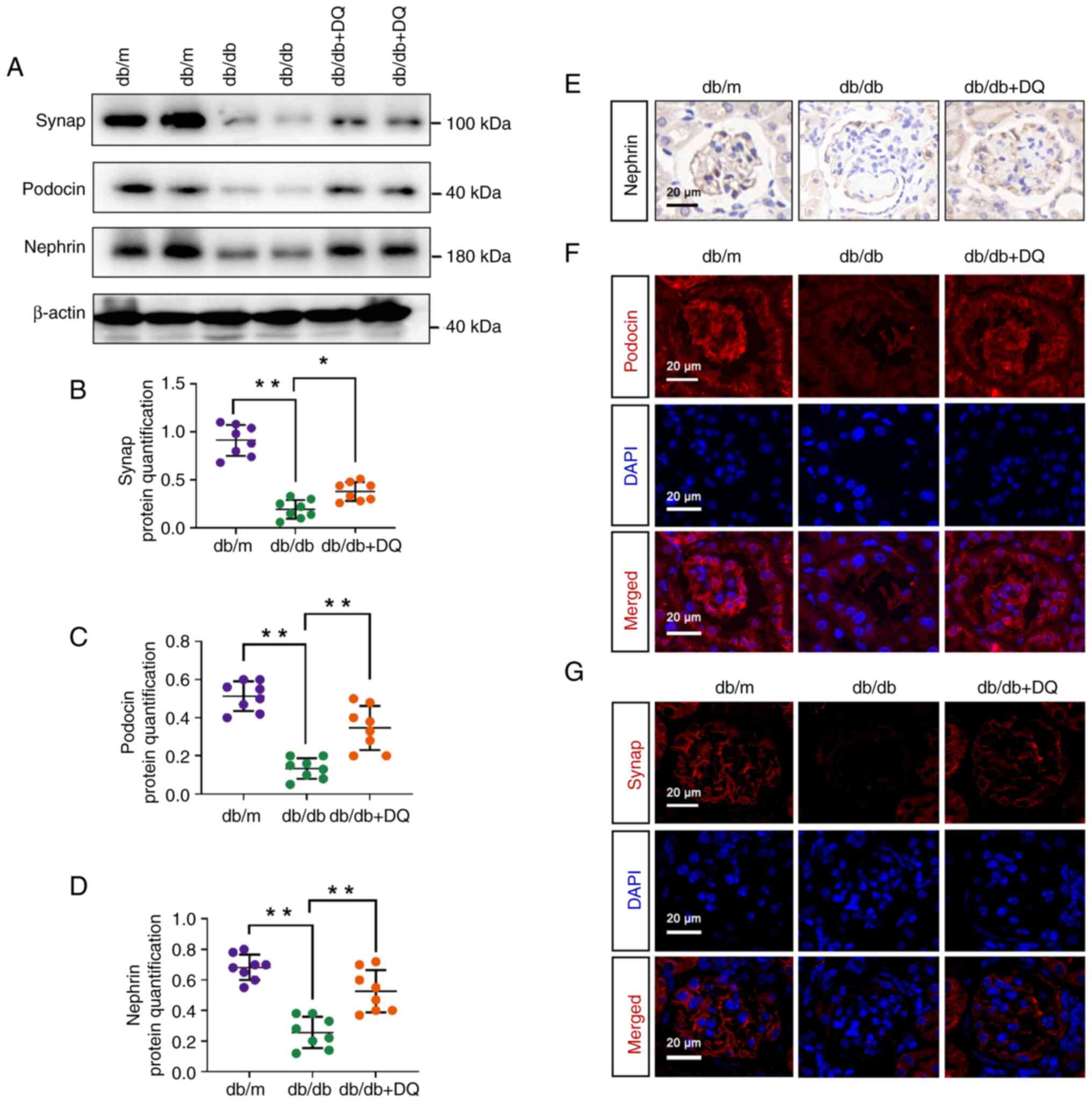
DQ alleviates podocyte dedifferentiation
in diabetic mice
Western blotting was used to detect the expression
of podocyte differentiation proteins in mice. The results
demonstrated that the expression levels of synap, podocin and
nephrin were significantly downregulated in db/db mice compared
with those in the db/m mice (P<0.01), whereas they were
significantly upregulated following DQ intervention in the db/db +
DQ group compared with those in the db/db group (P<0.05 or 0.01;
Fig. 3A-D). IHC staining was
used to detect the localization and expression of nephrin and its
expression trend was consistent with the western blotting results
(Fig. 3E). IF was used to detect
the expression levels of podocin and synap; results demonstrated
that both the fluorescence intensity of podocin and synap were
apparently decreased in db/db mice and increased following DQ
intervention (Fig. 3F and G).
The aforementioned results suggested that DQ protected against
diabetic podocyte injury by alleviating podocyte
dedifferentiation.
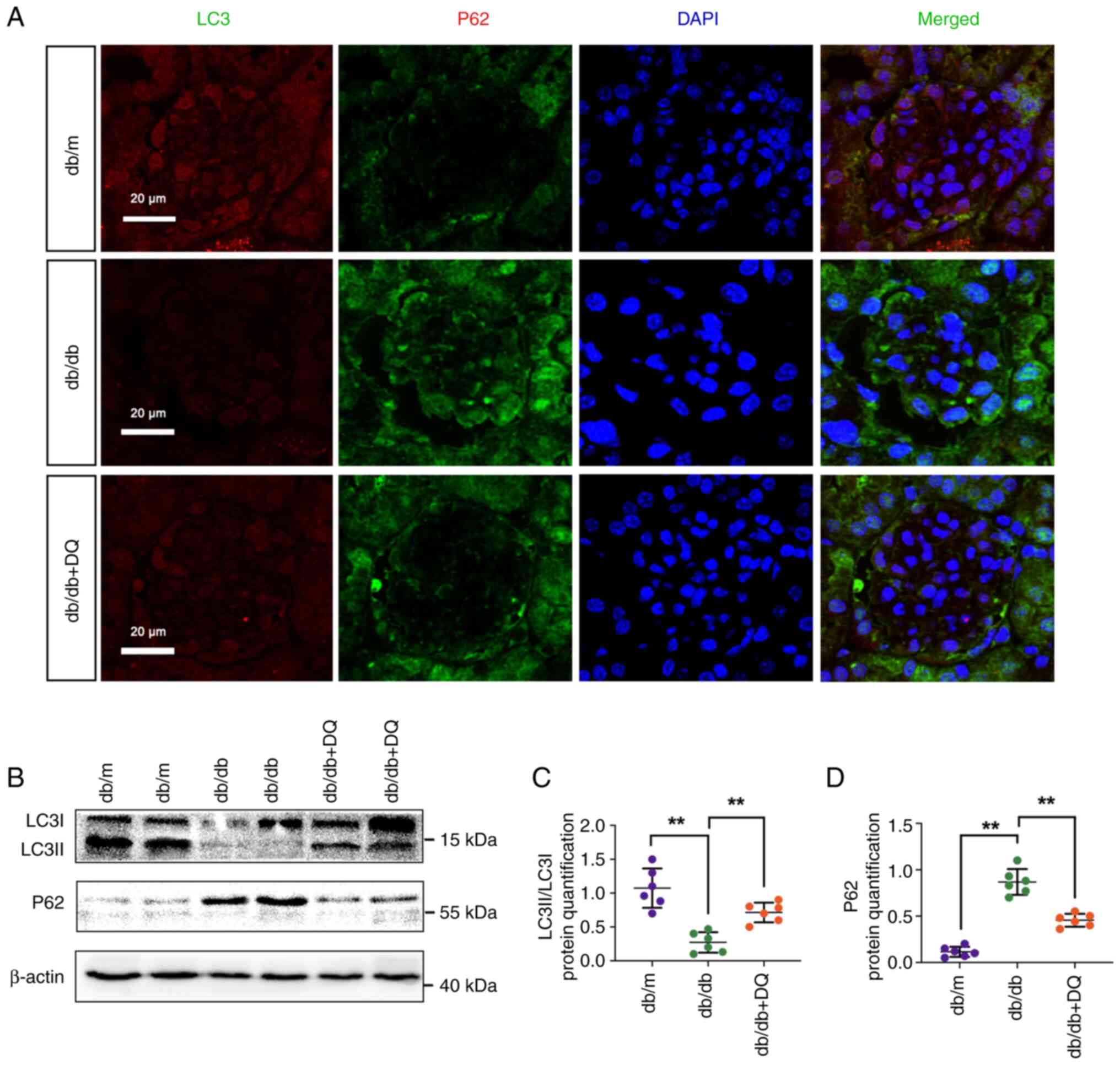
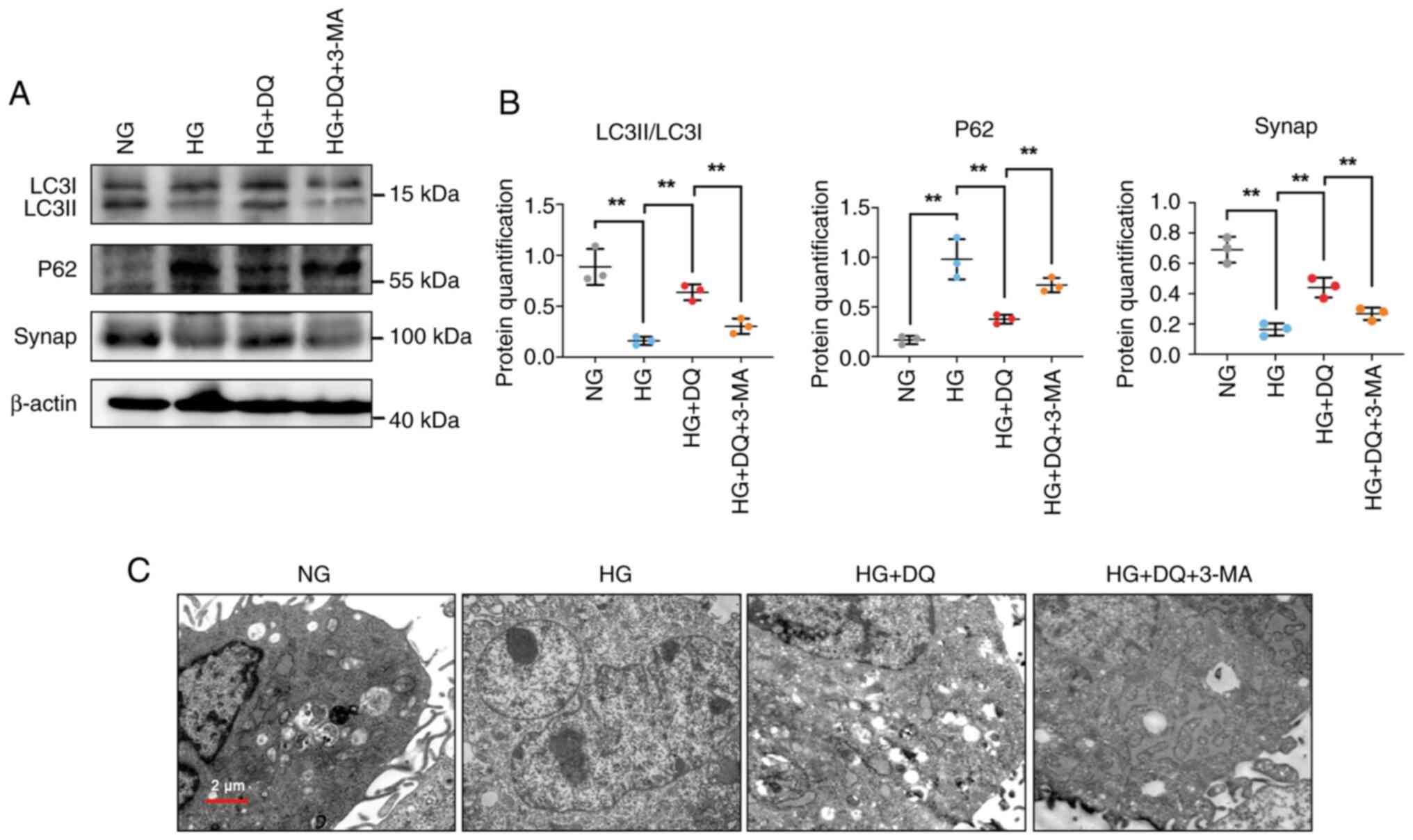
DQ activates autophagy in diabetic
mice
Podocyte autophagy dysfunction plays an important
role in diabetic podocyte differentiation. Therefore, the levels of
autophagy were examined in the renal tissues of each group. IF
staining was performed to detect the localization and expression
levels of LC3 and p62. The results indicated that the fluorescence
intensity of LC3 was weakened, whereas the fluorescence intensity
of p62 was markedly increased in db/db mice. However, the
fluorescence intensity of LC3 and p62 was noticeably decreased
following the intervention of DQ (Fig. 4A). Western blotting was further
performed to detect the expression levels of autophagy-related
proteins. The results revealed that the expression levels of
LC3II/LC3I were significantly decreased and the expression levels
of the p62 protein were significantly increased in db/db mice
(P<0.01). This effect could be reversed following DQ
intervention (P<0.01; Fig.
4B-D). The aforementioned results suggested that DQ
intervention could significantly promote autophagy in diabetic
mice.
DQ protects against diabetic podocyte
injury by activation of autophagy to alleviate podocyte
dedifferentiation
To further verify whether DQ exerted protective
effects on podocytes through autophagy, the autophagy inhibitor
3-MA was added to mouse podocytes by establishment of the cell
hyperglycemia model. As shown in Fig. 5A and B, it was observed that the
expression levels of LC3II/LC3I were significantly decreased,
whereas the expression levels of p62 were significantly increased
in podocytes cultured under HG conditions; however, they were
significantly reversed by administration of DQ (P<0.01).
Moreover, the expression levels of the podocyte differentiation
protein synap were significantly decreased under HG conditions,
whereas its levels were significantly overturned by treatment with
DQ (P<0.01). Furthermore, the number of autophagic vesicles was
measured by TEM. The results displayed that DQ intervention could
apparently upregulate the number of autophagic vesicles (Fig. 5C). However, the aforementioned
results were further reversed by addition of 3-MA, suggesting that
3-MA inhibited the protective effect of DQ on podocytes. These
results implied that DQ exerted protective effects on diabetic
podocyte injury by activation of autophagy to alleviate podocyte
dedifferentiation.
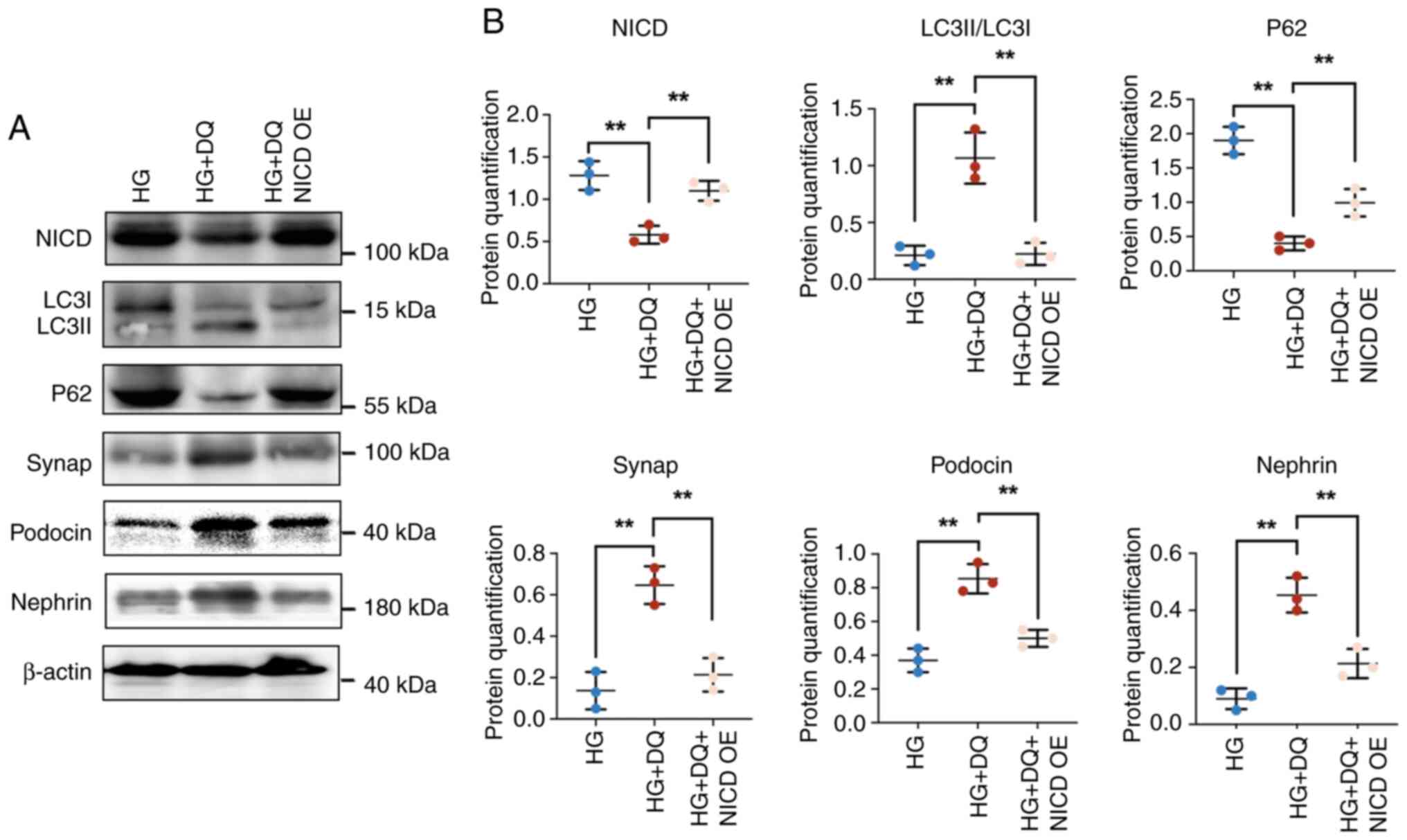
DQ protects against diabetic podocyte
injury through activation of autophagy to alleviate podocyte
dedifferentiation via the Notch pathway
The transfection efficiency of NICD was shown in
Fig. S1. The results showed
that NICD was markedly upregulated by transfection with NICD
overexpression plasmid, indicating high transfection efficiency.
Further, the effects of NICD overexpression on expression of
autophagy-related proteins and differentiation proteins were
explored. The results indicated that DQ intervention caused a
significant downregulation of the expression of NICD and p62
(P<0.01) and a significant upregulation of the expression of
LC3II/LC3I, synap, podocin and nephrin (P<0.01). Addition of a
NICD overexpression plasmid significantly reversed the
aforementioned results (P<0.01; Fig. 6A and B). These data suggested
that DQ exerted protective effects on diabetic podocyte injury by
activating autophagy to promote podocyte differentiation via the
Notch pathway.
Discussion
In recent years, DKD has become a life-threatening
disease that affects human life. Despite tremendous advances in the
development of renal protective medicine, the management of DKD
remains a challenge for clinicians and researchers (28). Therefore, it is essential to
understand the potential pathological mechanism of DKD to develop
innovative therapeutic approaches. The senolytic combination of DQ
has been reported to be effective in DKD (19,20). Nevertheless, the potential
mechanism requires further exploration.
To explore the effects of DQ on DKD, a DKD animal
model was established using db/db mice. The mice were administered
5 mg/kg D and 50 mg/kg Q by gavage for 20 weeks. The doses of D and
Q used were reported in previous studies (25,29-31). Subsequently, the levels of
several indices of DKD were evaluated, and the results indicated
that DKD could significantly reduce body weight, blood glucose,
ACR, Scr and BUN levels. This demonstrated that DQ alleviated renal
function in diabetic mice. The histopathological changes noted in
mice were investigated following DQ intervention. The results
indicated that treatment with DQ for 10 weeks significantly reduced
glomerular damage score and renal fibrosis levels. These results
implied that DQ exerted protective effects on kidney function of
diabetic mice. The present study is in line with a previous study
(20). The potential molecular
mechanism of DQ was further assessed. The disturbance in glomerular
ECM is involved in the progression of DKD (32). FN and Col I are notable
ECM-related proteins found in glomerular basement membranes. The
increase in FN and Col I levels contributes to basement membrane
thickening (33). The data
demonstrated that DQ could decrease the expression levels of FN and
Col I, indicating reduction of ECM deposition by DQ treatment.
It has been acknowledged that podocyte injury and
dedifferentiation are essential for the loss of selective
permeability at the glomerular barrier in DKD (34). Podocyte loss is an important
disorder contributing to the development of DKD (35). Podocytes are terminally
differentiated cells and the loss of podocytes is an irreversible
event that results in a deteriorated function of the glomerular
filtration barrier (36).
Therefore, improvement of podocyte dedifferentiation and survival
may be beneficial for the treatment of DKD. Synap is a specific
synaptic junction protein that is exclusively found in the podocyte
foot (37). Loss of synap is a
hallmark of podocyte differentiation (38). Nephrin is a key transmembrane
protein in the slit diaphragm complex contributing to the survival
of podocytes (39). Podocin is
an integral membrane protein that is found in the glomerular slit
diaphragm, which interacts with nephrin and anchors it into the
lipid raft (40). In addition,
podocin has been reported to be a promising marker for early
detection of DKD in T2DM (41).
Therefore, the expression levels of the three proteins were
examined by western blotting, IHC and IF. The results revealed that
DQ could significantly upregulate the expression levels of synap,
nephrin and podocin, suggesting that it could alleviate podocyte
dedifferentiation.
Targeting or reducing senescent cells has been
reported to be one potential mechanism for the effects of the
senolytics DQ on DKD. For example, Hickson et al (19) suggested that DQ decreased
senescent cells in patients with DKD. Palmer et al (20) indicated that DQ could largely
alleviate insulin resistance, proteinuria and renal podocyte
dysfunction in DKD by targeting senescent cells. Senescence and
autophagy are two distinct cellular responses to stress, which are
involved in acute kidney injury and renal repair (42). Although they are distinct, they
have various common characteristics and are closely interconnected.
In addition, it has been reported that interfering with autophagy
suppresses senescence (43).
Therefore, it was hypothesized that the effects of DQ on DKD may
also be mediated via regulation of autophagy. Previous studies
suggested that profound autophagy dysregulation was responsible for
both glomerular and tubulointerstitial pathologies in DKD (44,45). Autophagy is activated when kidney
cells are under stress conditions, such as hypoxia and oxidative
stress, and plays a key role in cell survival (46). However, autophagy was suppressed
in proximal tubules (47) and
podocytes (48) of
streptozotocin-induced diabetic animals. Activation of autophagy
may be a therapeutic target for DKD (44,49,50). To confirm this hypothesis, the
levels of the autophagy-related proteins were measured including
LC3 and p62 following treatment with DQ. It is noteworthy that the
results of the present study demonstrated that DQ could upregulate
the expression levels of LC3II/LC3I and downregulate the expression
levels of p62, indicating the activation of autophagy by DQ. To
further confirm these results, in vitro experiments were
performed in mouse podocytes. The podocytes were administered with
the autophagy inhibitor 3-MA under HG conditions. Subsequently, the
expression levels of autophagy-related proteins were measured
again. The results demonstrated that 3-MA could reverse the effects
of DQ on the expression of autophagy-related proteins, which
suggested that DQ protected against diabetic podocyte injury by
activation of autophagy. In consideration of the function of
podocyte dedifferentiation and deregulated autophagy in the
development of DKD, it was further hypothesized that a potential
link may be present between podocyte differentiation and autophagy
that contributes to the protection of DQ on DKD. To address this
hypothesis, the levels of podocyte differentiation-related proteins
were assessed following administration with 3-MA under HG
conditions. It is noteworthy that 3-MA could reduce the ability of
podocyte differentiation by downregulation of synap levels. The
data demonstrated that DQ could protect against DKD by activation
of autophagy to alleviate podocyte dedifferentiation. The present
study is similar with a previous study, in which the authors
demonstrated that autophagy contributed to normal kidney
development and podocyte differentiation (11). The present study is the first to
demonstrate a link between autophagy and podocyte differentiation
in DKD.
It has been reported that the Notch pathway plays a
significant role in podocyte injury in DKD (51) and is also responsible for the
regulation of autophagy-related proteins (52). Niranjan et al (53) demonstrated that activation of
Notch in mature podocytes of diabetic mice and humans leads to the
development of glomerular disease. Moreover, ectopic expression of
NICD in genetically engineered mouse podocytes results in severe
glomerular abnormalities resembling focal segmental
glomerulosclerosis in humans. Walsh et al (54) also reported an increased
expression of the Notch signaling pathway-related proteins in the
tubulointerstitial section of patients with diabetic nephropathy.
Selective activation of Notch in adult podocytes is accompanied by
apoptosis and activation of Notch is able to prompt podocyte foot
process regression and depletion, leading to albuminuria and
glomerulosclerosis (54).
Abnormal expression of NICD in Notch transgenic mice induced severe
podocyte foot process effacement and decreased expression of
specific podocyte markers including nephrin and podocin (55). In the present study, the levels
of NICD were overexpressed and the protein levels of
autophagy-related proteins and specific podocyte markers were
assessed. Similarly, the present study demonstrated that
overexpression of NICD significantly decreased the levels of
autophagy and the expression levels of synap, nephrin and podocin,
indicating that the protective effects of DQ on DKD were mediated
by the enhancement of autophagy to reduce podocyte differentiation
through the Notch pathway.
However, it seems that the relationship between
autophagy and apoptosis should also be discussed to a certain
extent. It has been reported that DQ can effectively reduce
apoptosis, which contributes its protective effects (56). It is noteworthy that autophagy
and apoptosis are not mutually exclusive. The two pathways share
several of the same regulatory signals, and each pathway can
regulate and alter the activity of the other, thereby having
different effects on the fate of the stressed cells. The functional
cross-talk between autophagy and apoptosis is complicated and
usually manifests in three situations (57-59). Firstly, autophagy antagonizes
apoptosis by promoting cell survival and removing damaged
organelles that act as oxidative stressors, by breaking down
cellular molecules to provide energy and nutrient sources, or by
degrading unfolded protein aggregates to limit endoplasmic
reticulum stress. Secondly, autophagy acts upstream of apoptosis,
allowing apoptotic signaling to be transmitted, or participating in
certain ATP-dependent morphological changes in the final stage of
apoptosis, such as phosphatidylserine exposure, membrane cleavage
and apoptotic body formation. Thirdly, autophagy and apoptosis can
cooperate in parallel, or autophagy can assist apoptosis and
promote cell death. Whether DQ enhances autophagy through
regulation of apoptosis in DKD requires further exploration.
In conclusion, the present study demonstrated that
the combination DQ protected against DKD by activating autophagy to
alleviate podocyte dedifferentiation via the Notch pathway.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY designed the present study. XZ, CZ, LL and LX
contributed to material preparation and data collection. XZ and LX
analyzed the data. XZ and LX wrote the manuscript and LY polished
it. LY and XZ confirmed the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The animal experiments were carried out according to
the National Research Council's Guide for the Care and Use of
Laboratory Animals and approved by the Ethics Committee of The
First Affiliated Hospital of China Medical University [approval no.
(2019) 136].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82070763).
References
|
1
|
Reutens AT: Epidemiology of diabetic
kidney disease. Med Clin North Am. 97:1–18. 2013.
|
|
2
|
Hoogeveen EK: The epidemiology of diabetic
kidney disease. Kidney Dial. 2:433–442. 2022.
|
|
3
|
Lv JC and Zhang LX: Prevalence and disease
burden of chronic kidney disease. Adv Exp Med Biol. 1165:3–15.
2019.
|
|
4
|
Tuttle KR, Agarwal R, Alpers CE, Bakris
GL, Brosius FC, Kolkhof P and Uribarri J: Molecular mechanisms and
therapeutic targets for diabetic kidney disease. Kidney Int.
102:248–260. 2022.
|
|
5
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
|
|
6
|
Yamazaki T, Mimura I, Tanaka T and Nangaku
M: Treatment of diabetic kidney disease: Current and future.
Diabetes Metab J. 45:11–26. 2021.
|
|
7
|
Zou H, Zhou B and Xu G: SGLT2 inhibitors:
A novel choice for the combination therapy in diabetic kidney
disease. Cardiovasc Diabetol. 16:652017.
|
|
8
|
Perkovic V, Jardine MJ, Neal B, Bompoint
S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull
S, et al: Canagliflozin and renal outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 380:2295–2306. 2019.
|
|
9
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014.
|
|
10
|
Hartleben B, Gödel M, Meyer-Schwesinger C,
Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ,
Lindenmeyer MT, et al: Autophagy influences glomerular disease
susceptibility and maintains podocyte homeostasis in aging mice. J
Clin Invest. 120:1084–1096. 2010.
|
|
11
|
Zhang C, Li W, Wen J and Yang Z: Autophagy
is involved in mouse kidney development and podocyte
differentiation regulated by Notch signalling. J Cell Mol Med.
21:1315–1328. 2017.
|
|
12
|
Yasuda-Yamahara M, Kume S, Tagawa A,
Maegawa H and Uzu T: Emerging role of podocyte autophagy in the
progression of diabetic nephropathy. Autophagy. 11:2385–2386.
2015.
|
|
13
|
Gonzales MM, Garbarino VR, Marques Zilli
E, Petersen RC, Kirkland JL, Tchkonia T, Musi N, Seshadri S, Craft
S and Orr ME: Senolytic therapy to modulate the progression of
Alzheimer's disease (SToMP-AD): A pilot clinical trial. J Prev
Alzheimers Dis. 9:22–29. 2022.
|
|
14
|
Novais EJ, Tran VA, Johnston SN, Darris
KR, Roupas AJ, Sessions GA, Shapiro IM, Diekman BO and Risbud MV:
Long-term treatment with senolytic drugs dasatinib and quercetin
ameliorates age-dependent intervertebral disc degeneration in mice.
Nat Commun. 12:52132021.
|
|
15
|
Krzystyniak A, Wesierska M, Petrazzo G,
Gadecka A, Dudkowska M, Bielak-Zmijewska A, Mosieniak G, Figiel I,
Wlodarczyk J and Sikora E: Combination of dasatinib and quercetin
improves cognitive abilities in aged male Wistar rats, alleviates
inflammation and changes hippocampal synaptic plasticity and
histone H3 methylation profile. Aging (Albany NY). 14:572–595.
2022.
|
|
16
|
Saccon TD, Nagpal R, Yadav H, Cavalcante
MB, Nunes ADC, Schneider A, Gesing A, Hughes B, Yousefzadeh M,
Tchkonia T, et al: Senolytic combination of dasatinib and quercetin
alleviates intestinal senescence and inflammation and modulates the
gut microbiome in aged mice. J Gerontol A Biol Sci Med Sci.
76:1895–1905. 2021.
|
|
17
|
Levêque D, Becker G, Bilger K and
Natarajan-Amé S: Clinical pharmacokinetics and pharmacodynamics of
dasatinib. Clin Pharmacokinet. 59:849–856. 2020.
|
|
18
|
Banerjee S, Sarkar R, Mukherjee A, Miyoshi
SI, Kitahara K, Halder P, Koley H and Chawla-Sarkar M: Quercetin, a
flavonoid, combats rotavirus infection by deactivating
rotavirus-induced pro-survival NF-κB pathway. Front Microbiol.
13:9517162022.
|
|
19
|
Hickson LJ, Langhi Prata LGP, Bobart SA,
Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q,
Jordan KL, et al: Senolytics decrease senescent cells in humans:
Preliminary report from a clinical trial of dasatinib plus
quercetin in individuals with diabetic kidney disease.
EBioMedicine. 47:446–456. 2019.
|
|
20
|
Palmer AK, Xu M, Zhu Y, Pirtskhalava T,
Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E,
Casaclang-Verzosa G, et al: Targeting senescent cells alleviates
obesity-induced metabolic dysfunction. Aging Cell.
18:e129502019.
|
|
21
|
Kumari R, Bettermann K, Willing L, Sinha K
and Simpson IA: The role of neutrophils in mediating stroke injury
in the diabetic db/db mouse brain following hypoxia-ischemia.
Neurochem Int. 139:1047902020.
|
|
22
|
You YK, Huang XR, Chen HY, Lyu XF, Liu HF
and Lan HY: C-reactive protein promotes diabetic kidney disease in
db/db mice via the CD32b-Smad3-mTOR signaling pathway. Sci Rep.
6:267402016.
|
|
23
|
You YK, Wu WF, Huang XR, Li HD, Ren YP,
Zeng JC, Chen H and Lan HY: Deletion of Smad3 protects against
C-reactive protein-induced renal fibrosis and inflammation in
obstructive nephropathy. Int J Biol Sci. 17:3911–3922. 2021.
|
|
24
|
Xu L, Fan Q, Wang X, Li L, Lu X, Yue Y,
Cao X, Liu J, Zhao X and Wang L: Ursolic acid improves podocyte
injury caused by high glucose. Nephrol Dial Transplant.
32:1285–1293. 2017.
|
|
25
|
Dungan CM, Murach KA, Zdunek CJ, Tang ZJ,
Nolt GL, Brightwell CR, Hettinger Z, Englund DA, Liu Z, Fry CS, et
al: Deletion of SA β-Gal+ cells using senolytics improves muscle
regeneration in old mice. Aging Cell. 21:e135282022.
|
|
26
|
Kong ZL, Che K, Hu JX, Chen Y, Wang YY,
Wang X, Lü WS, Wang YG and Chi JW: Orientin protects podocytes from
high glucose induced apoptosis through mitophagy. Chem Biodivers.
17:e19006472020.
|
|
27
|
Zhu X, Zhang C, Shi M, Li H, Jiang X and
Wang L: IL-6/STAT3-mediated autophagy participates in the
development of age-related glomerulosclerosis. J Biochem Mol
Toxicol. 35:e226982021.
|
|
28
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: global dimension and perspectives. Lancet. 382:260–272.
2013.
|
|
29
|
Huang Y, Wang B, Hassounah F, Price SR,
Klein J, Mohamed TMA, Wang Y, Park J, Cai H, Zhang X and Wang XH:
The impact of senescence on muscle wasting in chronic kidney
disease. J Cachexia Sarcopenia Muscle. 14:126–141. 2023.
|
|
30
|
Li C, Shen Y, Huang L, Liu C and Wang J:
Senolytic therapy ameliorates renal fibrosis postacute kidney
injury by alleviating renal senescence. FASEB J. 35:e212292021.
|
|
31
|
Cavalcante MB, Saccon TD, Nunes ADC,
Kirkland JL, Tchkonia T, Schneider A and Masternak MM: Dasatinib
plus quercetin prevents uterine age-related dysfunction and
fibrosis in mice. Aging (Albany NY). 12:2711–2722. 2020.
|
|
32
|
Adeva-Andany MM and Carneiro-Freire N:
Biochemical composition of the glomerular extracellular matrix in
patients with diabetic kidney disease. World J Diabetes.
13:498–520. 2022.
|
|
33
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012.
|
|
34
|
Canney AL, Cohen RV, Elliott JA, M Aboud
C, Martin WP, Docherty NG and le Roux CW: Improvements in diabetic
albuminuria and podocyte differentiation following Roux-en-Y
gastric bypass surgery. Diab Vasc Dis Res.
17:14791641198790392020.
|
|
35
|
Weil EJ, Lemley KV, Mason CC, Yee B, Jones
LI, Blouch K, Lovato T, Richardson M, Myers BD and Nelson RG:
Podocyte detachment and reduced glomerular capillary endothelial
fenestration promote kidney disease in type 2 diabetic nephropathy.
Kidney Int. 82:1010–1017. 2012.
|
|
36
|
Nagata M: Podocyte injury and its
consequences. Kidney Int. 89:1221–1230. 2016.
|
|
37
|
Mundel P, Heid HW, Mundel TM, Krüger M,
Reiser J and Kriz W: Synaptopodin: An actin-associated protein in
telencephalic dendrites and renal podocytes. J Cell Biol.
139:193–204. 1997.
|
|
38
|
Harvey SJ, Jarad G, Cunningham J, Goldberg
S, Schermer B, Harfe BD, McManus MT, Benzing T and Miner JH:
Podocyte-specific deletion of dicer alters cytoskeletal dynamics
and causes glomerular disease. J Am Soc Nephrol. 19:2150–2158.
2008.
|
|
39
|
Li X, Chuang PY, D'Agati VD, Dai Y, Yacoub
R, Fu J, Xu J, Taku O, Premsrirut PK, Holzman LB and He JC: Nephrin
preserves podocyte viability and glomerular structure and function
in adult kidneys. J Am Soc Nephrol. 26:2361–2377. 2015.
|
|
40
|
Nishibori Y, Liu L, Hosoyamada M, Endou H,
Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw
D, et al: Disease-causing missense mutations in NPHS2 gene alter
normal nephrin trafficking to the plasma membrane. Kidney Int.
66:1755–1765. 2004.
|
|
41
|
ElShaarawy A, Behairy MA, Bawady SA,
Abdelsattar HA and Shadad E: Urinary podocin level as a predictor
of diabetic kidney disease. J Nephropathol. 8:e262019.
|
|
42
|
Baisantry A, Bhayana S, Wrede C, Hegermann
J, Haller H, Melk A and Schmitt R: The impact of autophagy on the
development of senescence in primary tubular epithelial cells. Cell
Cycle. 15:2973–2979. 2016.
|
|
43
|
Gewirtz DA: Autophagy and senescence: A
partnership in search of definition. Autophagy. 9:808–812.
2013.
|
|
44
|
Gonzalez CD, Carro Negueruela MP, Nicora
Santamarina C, Resnik R and Vaccaro MI: Autophagy dysregulation in
diabetic kidney disease: From pathophysiology to pharmacological
interventions. Cells. 10:24972021.
|
|
45
|
Yang D, Livingston MJ, Liu Z, Dong G,
Zhang M, Chen JK and Dong Z: Autophagy in diabetic kidney disease:
Regulation, pathological role and therapeutic potential. Cell Mol
Life Sci. 75:669–688. 2018.
|
|
46
|
Huber TB, Edelstein CL, Hartleben B, Inoki
K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et
al: Emerging role of autophagy in kidney function, diseases and
aging. Autophagy. 8:1009–1031. 2012.
|
|
47
|
Barbosa Júnior Ade A, Zhou H,
Hültenschmidt D, Totovic V, Jurilj N and Pfeifer U: Inhibition of
cellular autophagy in proximal tubular cells of the kidney in
streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B
Cell Pathol Incl Mol Pathol. 61:359–366. 1992.
|
|
48
|
Vallon V, Rose M, Gerasimova M, Satriano
J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC and Rieg T:
Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia
and glomerular hyperfiltration but not kidney growth or injury in
diabetes mellitus. Am J Physiol Renal Physiol. 304:F156–F167.
2013.
|
|
49
|
Matboli M, Eissa S, Ibrahim D, Hegazy MGA,
Imam SS and Habib EK: Caffeic acid attenuates diabetic kidney
disease via modulation of autophagy in a high-fat
diet/streptozotocin-induced diabetic rat. Sci Rep. 7:22632017.
|
|
50
|
Ren H, Shao Y, Wu C, Ma X, Lv C and Wang
Q: Metformin alleviates oxidative stress and enhances autophagy in
diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell
Endocrinol. 500:1106282020.
|
|
51
|
Zheng D, Tao M, Liang X, Li Y, Jin J and
He Q: p66Shc regulates podocyte autophagy in high glucose
environment through the Notch-PTEN-PI3K/Akt/mTOR pathway. Histol
Histopathol. 35:405–415. 2020.
|
|
52
|
Yoshida G, Kawabata T, Takamatsu H, Saita
S, Nakamura S, Nishikawa K, Fujiwara M, Enokidani Y, Yamamuro T,
Tabata K, et al: Degradation of the NOTCH intracellular domain by
elevated autophagy in osteoblasts promotes osteoblast
differentiation and alleviates osteoporosis. Autophagy.
18:2323–2332. 2022.
|
|
53
|
Niranjan T, Bielesz B, Gruenwald A, Ponda
MP, Kopp JB, Thomas DB and Susztak K: The Notch pathway in
podocytes plays a role in the development of glomerular disease.
Nat Med. 14:290–298. 2008.
|
|
54
|
Walsh DW, Roxburgh SA, McGettigan P,
Berthier CC, Higgins DG, Kretzler M, Cohen CD, Mezzano S, Brazil DP
and Martin F: Co-regulation of Gremlin and Notch signalling in
diabetic nephropathy. Biochim Biophys Acta. 1782:10–21. 2008.
|
|
55
|
Waters AM, Wu MYJ, Onay T, Scutaru J, Liu
J, Lobe CG, Quaggin SE and Piscione TD: Ectopic notch activation in
developing podocytes causes glomerulosclerosis. J Am Soc Nephrol.
19:1139–1157. 2008.
|
|
56
|
Wang C, Kang Y, Liu P, Liu W, Chen W,
Hayashi T, Mizuno K, Hattori S, Fujisaki H and Ikejima T: Combined
use of dasatinib and quercetin alleviates overtraining-induced
deficits in learning and memory through eliminating senescent cells
and reducing apoptotic cells in rat hippocampus. Behav Brain Res.
440:1142602023.
|
|
57
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752.
2007.
|
|
58
|
Scarlatti F, Granata R, Meijer AJ and
Codogno P: Does autophagy have a license to kill mammalian cells?
Cell Death Differ. 16:12–20. 2009.
|
|
59
|
Rubinstein AD and Kimchi A: Life in the
balance-a mechanistic view of the crosstalk between autophagy and
apoptosis. J Cell Sci. 125:5259–5268. 2012.
|