Introduction
Ovarian cancer (OC) is a major health concern among
women worldwide, ranking as the seventh most commonly diagnosed
cancer and the eighth leading cause of cancer-associated death
(1,2). By the end of 2023, it is predicted
that 19,710 new OC cases will be diagnosed and 13,270 OC-related
deaths will occur in the United States (3). OC is considered the most lethal
gynecological cancer due to its asymptomatic early stages and the
inadequate screening methods for detecting precancerous lesions,
often resulting in a late-stage diagnoses (at stage III/IV). In
addition to traditional surgery, the treatment options for OC
include platinum-based combined chemotherapy and radiotherapy
(4). Most patients are treated
with surgery first, followed by platinum-based combined
chemotherapy (5). However, it has
been reported that both chemotherapy and radiotherapy have serious
side effects (6), and tumor
recurrence arises as a result of treatment resistance (7). Therefore, identifying novel
therapeutic strategies is crucial to treat OC.
Traditional Chinese medicine (TCM) has a history of
2,500 years and has been used to treat diseases since ancient times
(8). Paeoniae Radix Rubra (PRR,
also known as Chishao) is a type of Chinese herbal medicine, which
is the dried root of Paeonia lactiflora Pall. PRR is often
used in TCM formulations to clinically treat cancer (9,10),
such as the Guizhi Fuling Formulation (11) for the treatment of bladder cancer,
OC and breast cancer. Paeoniflorin has been identified as one of
the main components in PRR with antitumor activity (12). A water-soluble monoterpene
glycoside known as paeoniflorin has been reported to have a good
antitumor function in various types of cancer, such as liver cancer
(13), gastric cancer (14), breast cancer (15), lung cancer (16) and bladder cancer (12), and the molecular mechanisms
involved include the nuclear factor κB, STAT3 and p53/14-3-3
signaling pathways (17).
Paeoniflorigenone (PFG), another monoterpene
compound found in Paeonia species, has been shown to possess
a range of bioactive properties. Previous research has shown that
PFG is a depolarizing neuromuscular blocking agent in frogs and
mice, being similar to succinylcholine (18). PFG can also improve blood
circulation through anticoagulant and antiplatelet activities
(19). In addition, it has been
reported that PFG induces Jurkat cells, HeLa cells and HL-60 tumor
cell lines to undergo apoptosis, possibly through the activation of
caspase-3 (20). Additionally,
PFG can suppress the growth of cancerous HeLa cells through
triggering S and G2/M cell cycle arrest and inhibiting
mitosis (21). Nevertheless, to
the best of our knowledge, the mechanism underlying the antitumor
activity of PFG in OC has not been reported.
Over 90% of cases of epithelial OC (EOC), including
platinum-resistant tumors, exhibit upregulation of the type I
transmembrane glycoprotein mucin 1 (MUC1), which is highly
glycosylated (22,23). Following O-glycosylation and
N-glycosylation, MUC1 matures into a mucin that is capable of
performing its function (24).
Glycosylation is thought to be responsible for 50-90% of the total
weight of MUC1, and MUC1 can range in weight from 250 to 500 kDa,
depending on the quantity of tandem repeats and the level of
glycosylation (25). Notably,
MUC1 can serve as a target and prognostic biomarker for
immunotherapy (26).
Overexpression of MUC1 has been shown to promote the invasive
growth and metastasis of tumor cells (27,28). Furthermore, MUC1 functions as an
anti-adhesion factor in cancer, and may destroy cell-to-cell and
cell-to-matrix adhesion, releasing cells from tumor nests and
causing micro-metastasis (29,30). Additionally, MUC1 induces
epithelial-mesenchymal transition (EMT) at the post-transcriptional
level by altering the expression of microRNAs that regulate the
expression of EMT-related genes (31,32). As a result, tumor-associated MUC1
represents a prospective therapeutic target for EOC.
The present study aimed to scrutinize the inhibitory
effect of PFG on SKOV3 and A2780 OC cells, and to investigate the
role of MUC1 in the anti-OC effects of PFG by knocking down and
overexpressing MUC1.The present study also explored the association
of MUC1 expression with the pharmaceutical effectiveness of PFG,
potentially providing a new approach for developing MUC1-targeted
drugs.
Materials and methods
Cell culture
China Center for Type Culture Collection supplied
the human OC cell lines SKOV3, A2780 and HEY-T30. SKOV3 cells were
grown in McCoy's 5A medium (Cienry Biotechnology, Inc.), whereas
A2780 and HEY-T30 cells were grown in RPMI 1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). Both media were supplemented with
10% (v/v) fetal bovine serum (FBS; Invigentech, Inc.), 100 U/ml
penicillin and 100 μg/ml streptomycin (Beyotime Institute of
Biotechnology). The cells were grown at 37°C in an atmosphere
containing 5% CO2 with saturated humidity.
Reagents and antibodies
PFG (cat. no. B31203; molecular weight: 318.326;
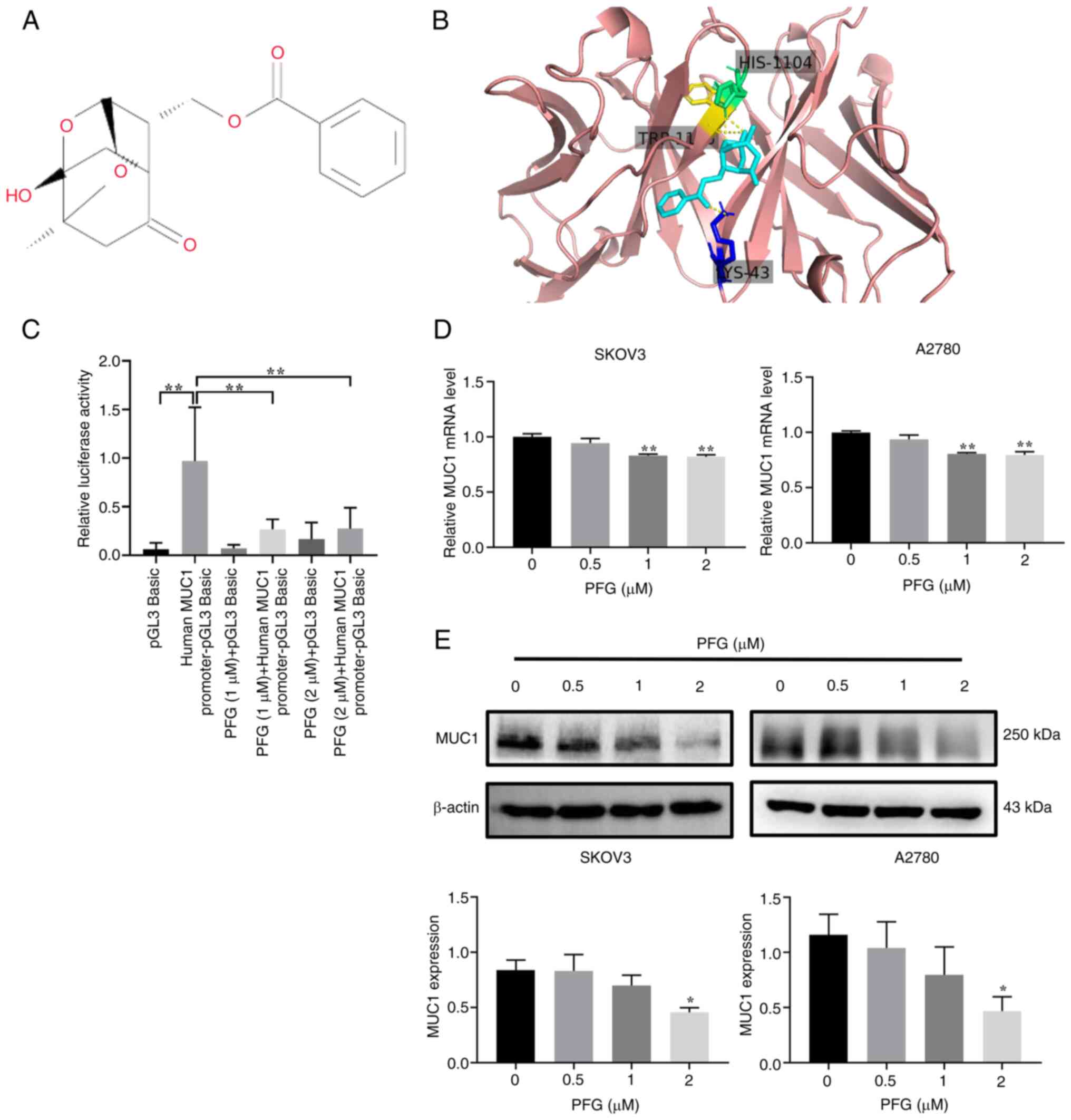
purity ≥98%; lot no. D15GB171320; Fig. 1A) was purchased from Shanghai
Yuanye Bio-Technology Co., Ltd. PFG was mixed with dimethyl
sulfoxide and stored at -80°C. To reduce the effects of serum and
other chemicals in the culture medium on pharmacological action,
PFG was diluted in cell culture medium containing 2% FBS and
applied in all experiments. Docetaxel (cat. no. HY-B0011; purity
99.42%) was purchased from MedChemExpress, and was used to treat
SKOV3 and A2780 cells for 24 h at 37°C at a dose of 80 nM. The
Wnt/β-catenin pathway inhibitor XAV939 (cat. no. S1180; molecular
weight: 312.31; purity 99.95%) was purchased from Selleck
Chemicals, which was used to treat SKOV3 and A2780 cells for 24 h
at 37°C at a dose of 10 μM. The MUC1 (cat. no. 4538S),
N-cadherin (cat. no. 13116S), Vimentin (cat. no. 5741S), Snail
(cat. no. 3879S), β-catenin (cat. no. 8480S) and cyclin D1 (cat.
no. 55506S) antibodies were purchased from Cell Signaling
Technology, Inc. Antibodies against MMP2 (cat. no. ab92536), MMP9
(cat. no. ab76003) and CDK2 (cat. no. ab32147) were acquired from
Abcam. Antibodies against c-Myc (cat. no. sc-40), cyclin A (cat.
no. sc-239) and cyclin E (cat. no. sc-247) were obtained from Santa
Cruz Biotechnology, Inc. The β-actin antibody (cat. no. AC026) was
obtained from ABclonal, Inc. HRP-conjugated goat anti-mouse IgG
H&L (cat. no. BK-M050) and anti-rabbit IgG H&L (cat. no.
BK-R050) antibodies were purchased from BIOKER Biotechnology, Inc.
For western blotting, antibodies were used at a 1:1,000
dilution.
Molecular docking
The Research Collaboratory for Structural
Bioinformatics Protein Data Bank database (https://www.rcsb.org/) and the PubChem database
(https://pubchem.ncbi.nlm.nih.gov/)
were utilized to find the three-dimensional structures of the MUC1
protein and molecular ligand structures, respectively. The Dockthor
tool (https://docthor.lncc.br/v2/) was used to
process proteins and ligands, including removing water molecules,
performing hydrogenation and calculating protein charges. The final
docking conformation should have the strongest affinity. According
to this principle, the conformation was selected and visualized
using Pymol 2.3 software (33).
Cell counting kit-8 (CCK-8) assay
Cell proliferation and the drug half maximal
inhibitory concentration (IC50) were examined by CCK-8
assay (Biosharp Life Sciences). SKOV3 and A2780 cells were
inoculated in 96-well plates at a density of 4×103
cells/well. The cells were incubated overnight with
5%CO2 at 37°C in 5% CO2, and were then
treated with various doses of PFG (0, 0.5, 1, 2, 5, 10, 20 and 40
μM) for 24, 48 and 72 h at 37°C. As recommended, 10
μl CCK-8 reagent was then added per well and incubated for 1
h at 37°C in 5% CO2. A New Epoch™ 2 Epoch Microplate
Spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure the absorbance at 450 nm.
Colony formation assay
SKOV3 and A2780 cells were exposed to different
concentrations of PFG (0.5, 1 and 2 μM) for 24 h at 37°C in
5% CO2, then grown in McCoy's 5A or RPMI 1640 media
supplemented with 10% FBS for 2 weeks, after being plated at a
density of 1,000 cells/well in 6-well plates. Subsequently, 4%
paraformaldehyde was employed to fix the colonies for 20 min and
they were stained with 0.1% crystal violet for 30 min at room
temperature. An optical microscope was used to count colonies
containing >50 cells.
EdU assay
Using an EdU assay kit (Beyotime Institute of
Biotechnology), the cell proliferation capacity was detected. At a
density of 4×105 cells/well, cells were plated into
6-well plates and exposed to different concentrations of PFG (0.5,
1 and 2 μM) for 24 h at 37°C in 5% CO2. After
SKOV3 and A2780 cells underwent a 2 h incubation in 50 μM
EdU buffer at 37°C in 5% CO2, they were fixed for 30 min
in 4% paraformaldehyde, and then permeabilized for 20 min in 0.1%
Triton X-100. After the culture was infused with click reaction
solution, prepared according to the manufacturer's instructions,
and then incubated in the dark for 30 min at room temperature, the
nuclei were stained with 1X Hoechst 33342 at room temperature in
the dark for 10 min. A fluorescence microscope was used to
visualize the cells.
Cell cycle and apoptosis assay
To analyze cell apoptosis, the Annexin-V FITC/PI
Apoptosis Analysis Kit (Beyotime Institute of Biotechnology) was
used. Several concentrations of PFG (0.5, 1 and 2 μM) were
used to pretreat SKOV3 and A2780 cells for 24 h at 37°C in 5%
CO2. According to the manufacturer's instructions, prior
to staining with 5 μl Annexin-V/FITC and 10 μl PI at
room temperature in the dark for 15 min, centrifuged cells (1,000 ×
g for 5 min at 25°C) were rinsed in precooled PBS. In addition, as
a positive control, cells were treated with 80 nM docetaxel for 24
h at 37°C in 5% CO2. To assess cell cycle progression,
the Cell Cycle Analysis Kit (Shanghai Yuanye Bio-Technology Co.,
Ltd.) was performed. The same cells were assessed as for the
apoptosis experiment. According to the manufacturer's instructions,
centrifuged cells (1,000 × g for 5 min at 25°C) were rinsed gently
in precooled PBS before being stained with 500 μl 50
μg/ml PI staining reagent (containing 200 μg/ml
RNase) at room temperature in the dark for 15 min. The test was
repeated three times for each sample. Finally, FlowJo-V10 software
(FlowJo, LLC) was used to investigate the apoptosis and cell cycle
data, which were collected using Beckman CytoFlex (Beckman Coulter,
Inc.). The apoptotic rate was calculated using the formula:
Apoptotic rate=Q1-LR + Q1-UR. LR refers to early apoptotic rate and
UR to late apoptotic rate.
DNA fragmentation analysis
DNA fragmentation, which is a hallmark of apoptosis,
was studied using the One-Step TUNEL Apoptosis Detection Kit (FITC)
(MedChemExpress). SKOV3 and A2780 cells (2×104/well)
were seeded in 24-well plates and treated with PFG (0.5, 1 and 2
μM) or docetaxel (80 nM) at 37°C in 5% CO2 for 24
h. Cells were fixed with 10% neutral buffered formalin at room
temperature for 20 min, and stained according to the manufacturer's
protocol. Slides were then cover-slipped with antifade mounting
medium with DAPI (Vector Laboratories, Inc.). Images were acquired
using a fluorescence microscope, and the fluorescence intensity was
statistically analyzed by ImageJ v1.48 software (National
Institutes of Health). The experiment was repeated three times.
Wound-healing assay
OC cells (3×106 cells/well) were plated
on 6-well plates and allowed to adhere to the plates for 24 h. To
maintain a healthy cell growth state, when the fusion degree of the
cell monolayer reached >80%, a straight line was scratched into
the monolayer using a 200-μl pipette tip. After three washes
with PBS, PFG (0.5, 1 and 2 μM) with 2% FBS was added to
each plate at 37°C in 5% CO2 for 24 h. The distance that
cells had migrated was photographed under a light microscope at 0,
12 and 24 h for the subsequent calculation. Taking 24 h as an
example, migration was assessed as follows: Migration rate
(%)=(At=0 h)-(At=24 h)/(At=0 h). (At=0 h) refers to the area of the
wound measured immediately after scratching; (At=24 h) refers to
the area of the wound measured 24 h after the scratch was
performed.
Migration and invasion assays
For the migration and invasion assays, PFG was
applied to SKOV3 and A2780 at 0.5, 1 and 2 μM at 37°C in 5%
CO2 for 24 h. Subsequently, the upper chamber of a
Transwell system (pore size, 8 μm; Corning, Inc.) was seeded
with 2×104 cells resuspended in 100 μl serum-free
medium, and the bottom of the chamber contained the cell culture
medium with 10% FBS. After the cells had migrated for 24 h at 37°C
in 5% CO2, the chamber was removed, the unmigrated cells
were scraped off, and the migrated cells were fixed with 4%
paraformaldehyde for 20 min at room temperature and stained with
0.01% crystal violet (Beijing Solarbio Science & Technology
Co., Ltd.) for 15 min at room temperature. Using a light microscope
(×200 magnification; Olympus Corporation) cell images were captured
and cell counts from five random fields were measured. For the
invasion assay, the upper chamber of the insert was coated with 100
μl growth factor-reduced Matrigel (Corning, Inc.) overnight
at 37°C.
Establishment of stable cell lines with
MUC1 knockdown and overexpression
To infect the SKOV3 and A2780 cell lines, Shanghai
GeneChem Co., Ltd. provided both the lentivirus-based short hairpin
(sh) RNA vector GV493 (hU6 -MCS-CBh-gcGFP-IRES-puromycin) and the
lentivirus-based overexpression RNA vector GV721 (CMV
enhancer-MCS-3FLAG-EF1a-firefly_ Luciferase-SV40-puromycin).
According to the manufacturer's instructions, the recombined
GV493/GV721 lentiviral vector plasmid or the negative control
lentiviral vector plasmid, and pHelper 1.0 and pHelper 2.0 plasmids
(Shanghai Genechem Co., Ltd.) were co-transfected into 293T cells
(American Type Culture Collection) using HitransG enhanced
infection solution (Shanghai Genechem Co., Ltd.) at 37°C in 5%
CO2 for 6 h. Subsequently, the culture supernatant was
removed and replaced with fresh high-glucose DMEM supplemented with
10% FBS and the culture supernatants were collected at 48 h
post-transfection. Following centrifugation at 4,000 × g for 10 min
at 4°C to remove cell debris, the supernatant was filtered through
0.45-μm filters. The concentrated viral supernatant was
aliquoted and maintained at -80°C prior to use. The lentivirus was
then diluted with serum-free McCoy's 5A or 1640 medium and used to
infect SKOV3 and A2780 cells at a multiplicity of infection of 10.
After 8 h at 37°C in 5% CO2, the medium was refreshed.
Subsequently, at 72 h post-infection, puromycin (2 μg/ml)
was employed to screen out the stable knockdown and overexpression
cell lines at 37°C in 5% CO2 for 48 h, and then 1
μg/ml puromycin was used for maintenance. Finally, the SKOV3
and A2780 cells were harvested for further analysis. The following
shRNA sequences were used: sh-MUC1, 5'-CCG GGA TAC CTA CCA TCC TAT
P-3'; sh-Control (non-targeting negative control viral vector),
5'-TTCTCCGAACGTGTCACGT-3'.
Promoter-reporter assays
According to the manufacturer's protocol, the pGL3
Basic human MUC1 promoter (HIBio Biotechnology Co., Ltd.) was
transfected into human OC SKOV3 cells using
Lipofectamine® 3000 Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). As an internal control, a pRL-TK
plasmid carrying the Renilla luciferase gene (HIBio
Biotechnology Co., Ltd.) was co-transfected with pGL3 Basic human
MUC1 promoter in SKOV3 cells. Briefly, 24-well plates were used for
transfection; the plasmid was transfected into cells at 37°C in 5%
CO2 for 8 h, then the medium was replaced with fresh
culture medium for 24 h. Subsequently, PFG (1 and 2 μM) was
added for 24 h at 37°C in 5% CO2. Utilizing the
Dual-luciferase Reporter Assay Kit (Promega Corporation), according
to the guidelines provided by the manufacturer, cells transfected
with vector were added to perform luciferase assays in triplicate
in 96-well plates after puromycin resistance screening. GraphPad
Prism 8 (Dotmatics) was used to evaluate and plot the exported
data.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), RNA was extracted from the cells. The
Nanodrop 2000 system (Thermo Fisher Scientific, Inc.) was employed
to measure the quality and quantity of RNA. Subsequently, 1
μg total RNA was used to generate cDNA using the PrimeScript
RT reagent kit (Takara Bio, Inc.) according to the manufacturer's
protocol. qPCR was conducted on a CFX96 Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.) using the SYBR Premix Ex Taq™
kit (Takara Bio, Inc.). The thermocycling conditions were as
follows: An initial denaturation step at 95°C for 10 sec, followed
by 40 cycles, (denaturation at 95°C for 10 sec, annealing at 64°C
for 25 sec), and a final extension step at 55°C for 1 min and 95°C
for 10 sec. GAPDH was used as an internal control. To obtain the
relative gene expression levels, the 2-ΔΔCq method
(34) was used. The following
primer sequences were used: MUC1, forward 5'-ATA CCT ACC ATC CTA
TGA G CG A-3', reverse 5'-CTG CTG GGT TTG TGT AAG AGA-3'; and
GAPDH, forward 5'-GGT GGT CTC CTC TGA CTT CAA CA-3' and reverse
5'-GTT GCT GTA GCC AAA TTC GTT GT-3'.
Western blotting
Cells were lysed in RIPA buffer with a proteinase
inhibitor cocktail (Beyotime Institute of Biotechnology), and the
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.) was used to
quantify the amount of protein in the extracts. Proteins (20
μg) were separated by SDS-PAGE on 8% gels and were then
transferred to polyvinylidene fluoride membranes (MilliporeSigma).
To prevent nonspecific binding, the membranes were blocked for 2 h
with 5% skim milk (Bio-Rad Laboratories, Inc.) diluted in
Tris-buffered saline. Prior to being incubated with HRP-conjugated
secondary antibodies at room temperature for 2 h, membranes were
pre-incubated with a specific primary antibody for an overnight
period at 4°C. An ECL Western Blotting Detection kit (Biosharp Life
Sciences) was utilized for detecting protein signals. After
standardization to β-actin, ImageJ v1.48 software (National
Institutes of Health) was used to measure the optical density of
the blot.
Statistical analysis
GraphPad Prism version 8 software was used to
investigate statistical significance via a Student's unpaired
t-test, one-way ANOVA or two-way ANOVA with a Bonferroni multiple
comparisons test (if the variance is homogeneous) or Games-Howell
multiple comparison test (if the variance is uneven). All studies
were conducted at least in triplicate, and all data are presented
as the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference.
Results
PFG inhibits the expression of MUC1 in OC
cells
The binding mode of PFG to MUC1 was predicted by
molecular docking, and it was revealed that PFG formed three
hydrogen bonds with HIS-1104, LYS-43 and TRP-1105 amino acid
residues (Fig. 1B). These results
indicated that MUC1 is a potential target protein of PFG. Next, the
effects of PFG on the promoter activity of MUC1 were assessed using
a dual-luciferase assay. Compared with the human MUC1 promoter-pGL3
Basic group, the PFG (1 and 2 μM) groups showed a
significant decrease in relative fluorescence activity, indicating
that PFG treatment affected the binding of transcription factors to
the MUC1 promoter in SKOV3 cells (Fig. 1C). Subsequently, quantification of
MUC1 expression in SKOV3 and A2780 cells was performed using
RT-qPCR and western blotting. The results suggested that PFG
inhibited MUC1 expression and exhibited a concentration-dependent
effect on the downregulation of MUC1 expression at concentrations
of 0.5, 1 and 2 μM (Fig. 1D
and E). According to these findings, PFG may inhibit MUC1
expression by decreasing MUC1 promoter activity in OC cells.
PFG suppresses the proliferation of OC
cells
To understand the anticancer effect of PFG on OC
cells, SKOV3 and A2780 cells were treated with PFG (0.5-40
μM) for 24, 48 and 72 h, and cell viability was detected by
CCK-8 assay. According to the results, compared with in the control
group, PFG suppressed the viability of SKOV3 and A2780 cells, and
the inhibition was dose-dependent (Fig. 2A). The IC50 value at 24
h for SKOV3 cells was 10.79 μM, and the IC50
value at 24 h for A2780 cells was 3.845 μM. To rule out the
potential bias in subsequent experiments caused by the cytotoxicity
of high doses of PFG, 0.5, 1 and 2 μM concentrations were
selected for subsequent experiments.
 | Figure 2PFG suppresses the proliferation of
ovarian cancer cells. (A) PFG was applied to SKOV3 and A2780 cells
at concentrations of 0, 0.5, 1, 2, 5, 10, 20 and 40 μM. Cell
viability was evaluated using the Cell Counting Kit-8 assay at 24,
48 and 72 h. (B) EdU assay measured the proliferation of SKOV3 and
A2780 cells after 24 h of PFG treatment. Cells stained with red
fluorescence indicated those that were in a proliferative state
(magnification, ×200). (C) Colony formation assay was performed to
assess the clonogenic potential of PFG-treated SKOV3 and A2780
cells for 24 h. Data are presented as the mean ± SEM (n=3). One-way
ANOVA was used to establish statistical significance.
*P<0.05, **P<0.01 vs. control group.
PFG, paeoniflorigenone. |
The EdU assay also indicated that PFG treatment
inhibited the proliferation of both cell lines (Fig. 2B). Moreover, the colony formation
assay revealed that PFG significantly inhibited the clonogenic
ability of both cell lines (Fig.
2C).
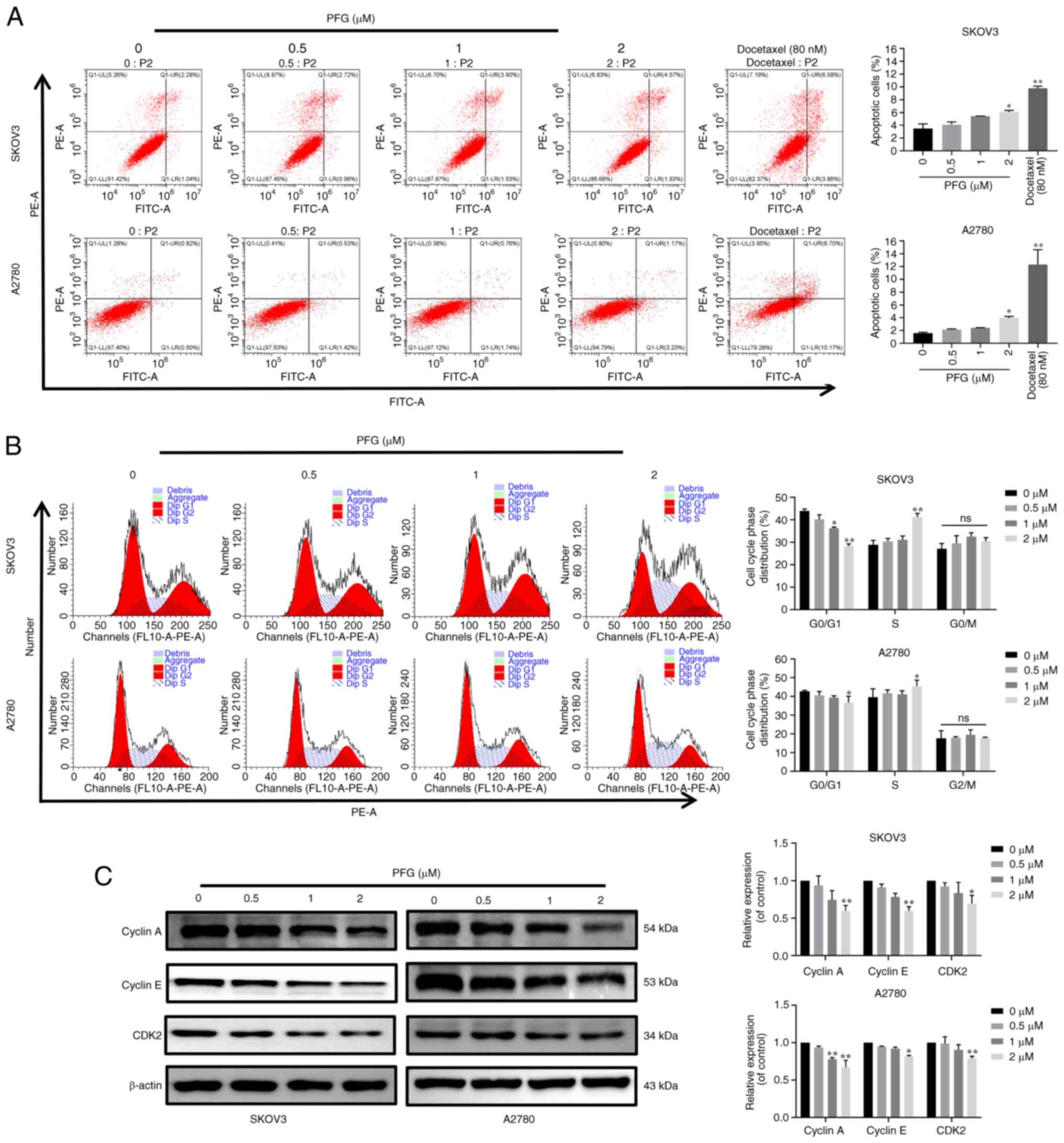
To further assess the mechanism underlying the
anti-proliferative effects of PFG, flow cytometry was performed to
detect its effects on the apoptosis and cell cycle progression of
the two cell lines. The results showed that in SKOV3 and A2780
cells, the apoptotic rate was significantly different in the PFG (2
μM) and docetaxel (80 nM) treatment groups compared with
that in the control group (Fig.
3A), TUNEL detection indicated the same trend change in SKOV3
and A2780 cells (Fig. S1).
However, the maximum apoptotic rate detected by the two
experimental methods was <10%, which indicates that the effect
of PFG on the apoptosis of SKOV3 and A2780 cells may have no
biological significance. Cell cycle experiments demonstrated that
PFG treatment could block the cell cycle in S phase, resulting in
shortening of the G0/G1 phase and prolonging
the S phase (Fig. 3B), with no
significant change in the proportion of cells in G2
phase. To explore the molecular mechanism underlying PFG-induced
S-phase blockade, the expression levels of specific proteins
associated with S-phase regulation were examined by western
blotting. Notably, PFG treatment had a marked dose-dependent
inhibitory effect on the protein expression levels of cyclin A,
cyclin E and CDK2 (Fig. 3C).
These results suggested that PFG may induce S-phase cell cycle
arrest in OC cell lines through controlling the levels of S-phase
cell cycle regulatory proteins.
PFG suppresses cell migration, invasion
and EMT in OC
OC frequently exhibits distant metastasis, and
patients with metastatic OC exhibit a low survival rate when
compared with primary OC (35).
In order to determine if PFG has anti-metastatic effects, the
migration of SKOV3 and A2780 cells was assessed using a
wound-healing assay. Compared with in the control group, PFG
significantly inhibited the migration of both cell lines in a
dose-dependent manner (Fig. 4A).
Furthermore, the effects of PFG on migration and invasion were
assessed using the Transwell assay, and it was revealed that PFG
markedly inhibited the migration and invasion of both cell lines in
a dose-dependent manner (Fig.
4B).
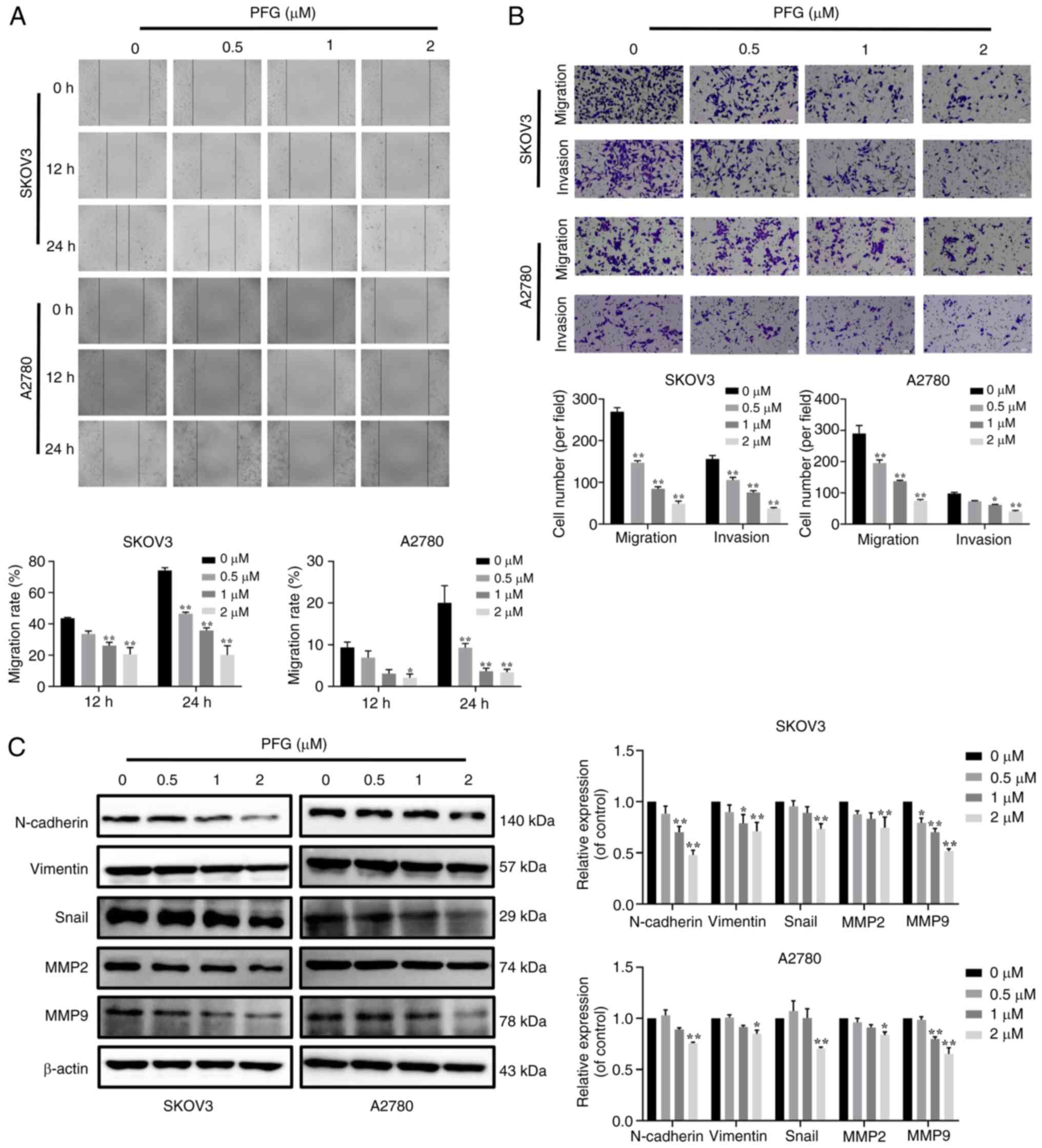 | Figure 4Functions of PFG on the migration,
invasion and EMT of OC cells. (A) Cell migration was examined using
the wound-healing assay and was observed under a light microscopy
at 0, 12 and 24 h (magnification, ×200); cell migration rate
calculation using wound healing area. (B) Inhibitory effect of PFG
on the ability of OC cells to invade and migrate, as identified
using the Transwell assay (magnification, ×200). (C) Inhibitory
effect of PFG on EMT-related proteins (N-cadherin, Vimentin and
Snail) and cell invasion-related proteins (MMP2 and MMP9) in OC
cells was tested using a western blotting. Three independent
repetitions of the experiment were performed. Data are presented as
the mean ± SEM (n=3). One-way ANOVA was applied to establish
statistical significance. *P<0.05,
**P<0.01 vs. control group. EMT,
epithelial-mesenchymal transition; OC, ovarian cancer; PFG,
paeoniflorigenone. |
Due to the importance of the EMT in the distant
metastases of OC, identifying a new therapy that can target EMT is
critical. To the best of our knowledge, the specific mechanism
underlying the effects of PFG on the EMT of OC cells has not been
reported. Therefore, after 24 h of PFG treatment, the expression
levels of EMT-related proteins in the two OC cell lines were
examined via western blot analysis. It was revealed that PFG could
significantly inhibit the expression levels of MMP2 and MMP9, and
downregulate the expression levels of mesenchymal markers, such as
N-cadherin, Vimentin and Snail (Fig.
4C). These results indicated that PFG could block the
activation of EMT in OC cells. Overall, it may be concluded that
PFG suppresses OC cell migration and invasion via blocking EMT.
MUC1 promotes the proliferation of OC
cells
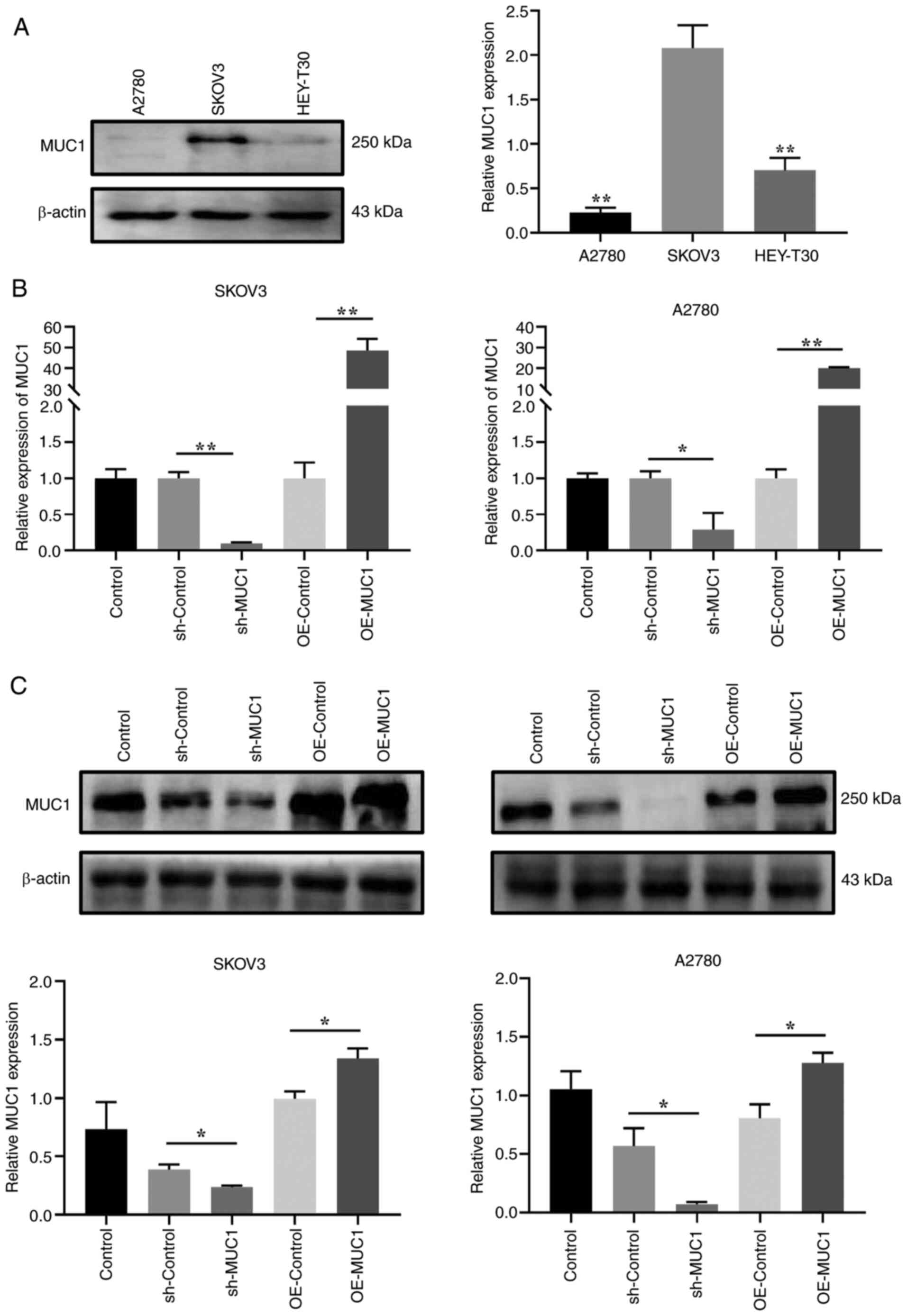
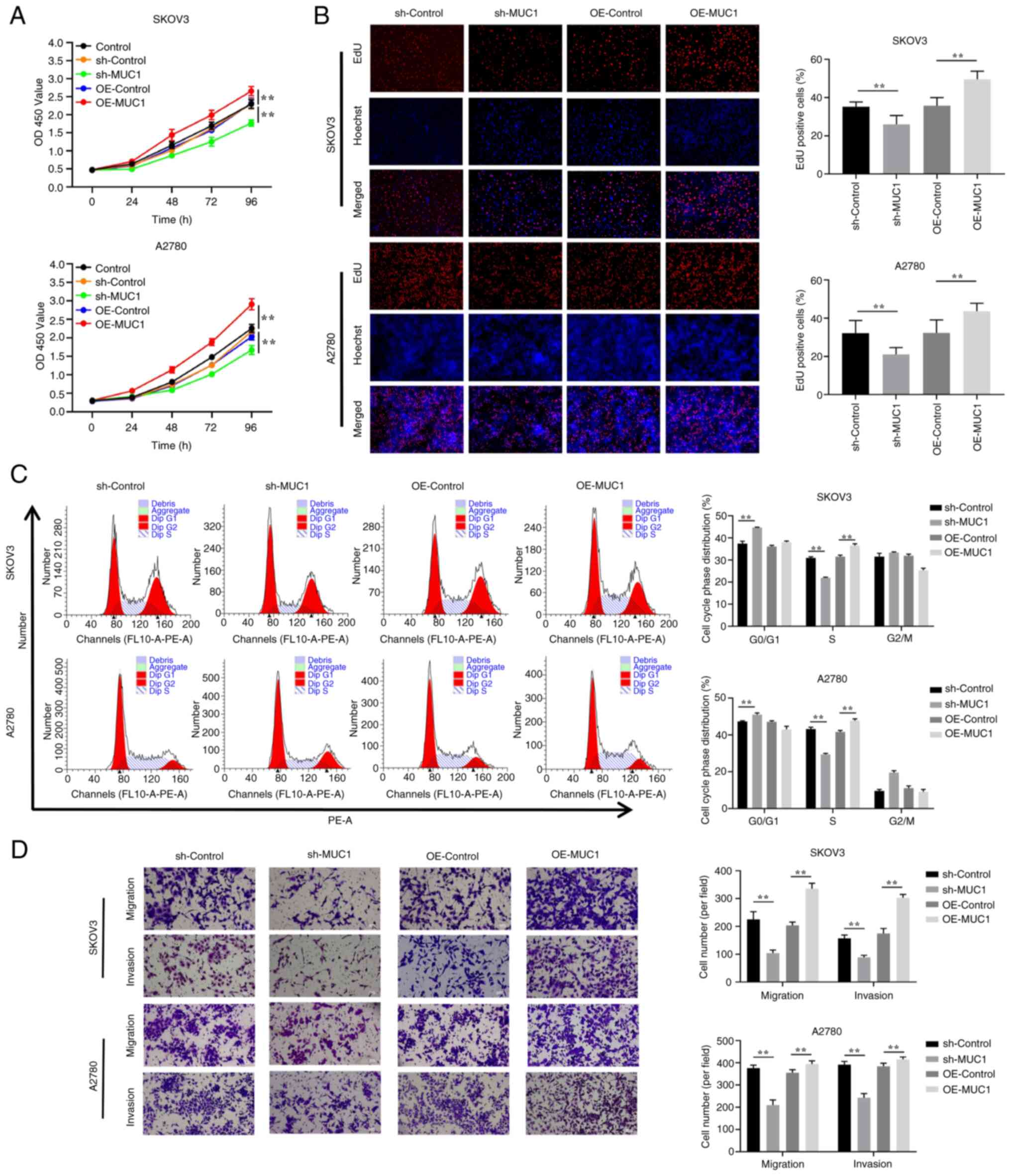
To investigate the effects of MUC1 on OC cells, MUC1
protein expression levels were compared in three OC cell lines, and
SKOV3 cells with the highest relative expression levels and A2780
cells with the lowest relative expression levels were selected for
the construction of cells with stable MUC1 knockdown and
overexpression for further mechanistic studies (Fig. 5A). Accordingly, RT-qPCR and
western blot experiments verified the knockdown and overexpression
of MUC1 (Fig. 5B and C). Compared
with in the sh-Control cells, the results demonstrated that MUC1
knockdown had a significant effect on inhibiting the proliferation
of OC cells (Fig. 6A and B),
inducing cell cycle arrest at G0/G1 phase
(Fig. 6C), and reducing cell
migration and invasion (Fig. 6D).
By contrast, compared with in the overexpression control
(OE-control) cells, MUC1 overexpression promoted OC cell
proliferation, accelerated cell cycle progression, and increased
cell migration and invasion. These results implied that MUC1 is
essential for OC cell proliferation and metastasis.
PFG targets MUC1 to limit OC cell
migration and invasion
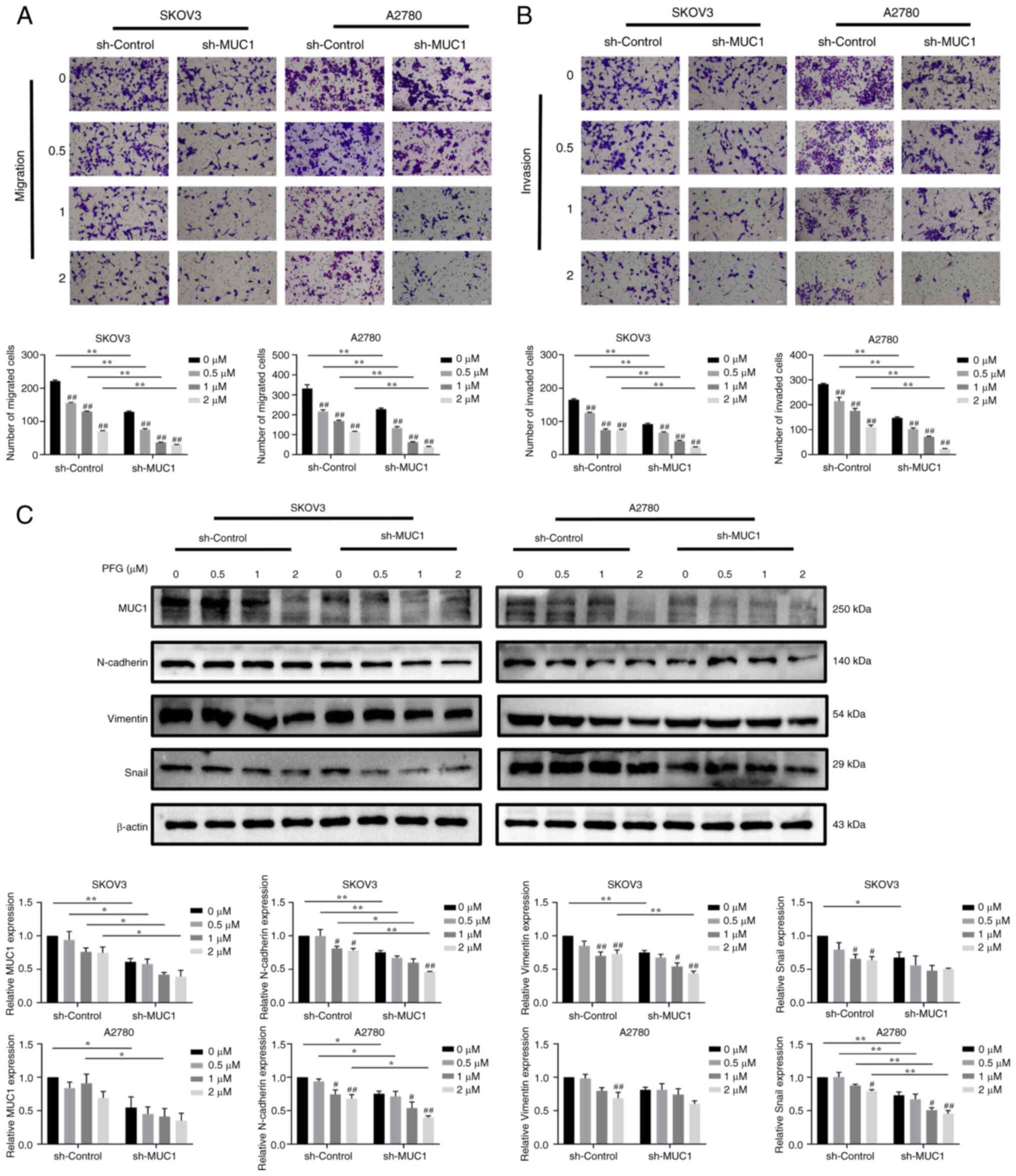
To clarify whether PFG-induced antitumor effects
were mediated by MUC1, the changes in migration and invasion were
detected in cells with MUC1 knockdown and overexpression after
treatment with various concentrations of PFG. The migration and
invasion rate of sh-MUC1 cells was more affected by PFG than that
of sh-control cells (Fig. 7A and
B). Compared with the sh-control cells, in sh-MUC1 cells, the
expression levels of MUC1 were significantly decreased and the
inhibitory effect of PFG on EMT-related proteins (including
N-cadherin, Vimentin and Snail) was enhanced (Fig. 7C).
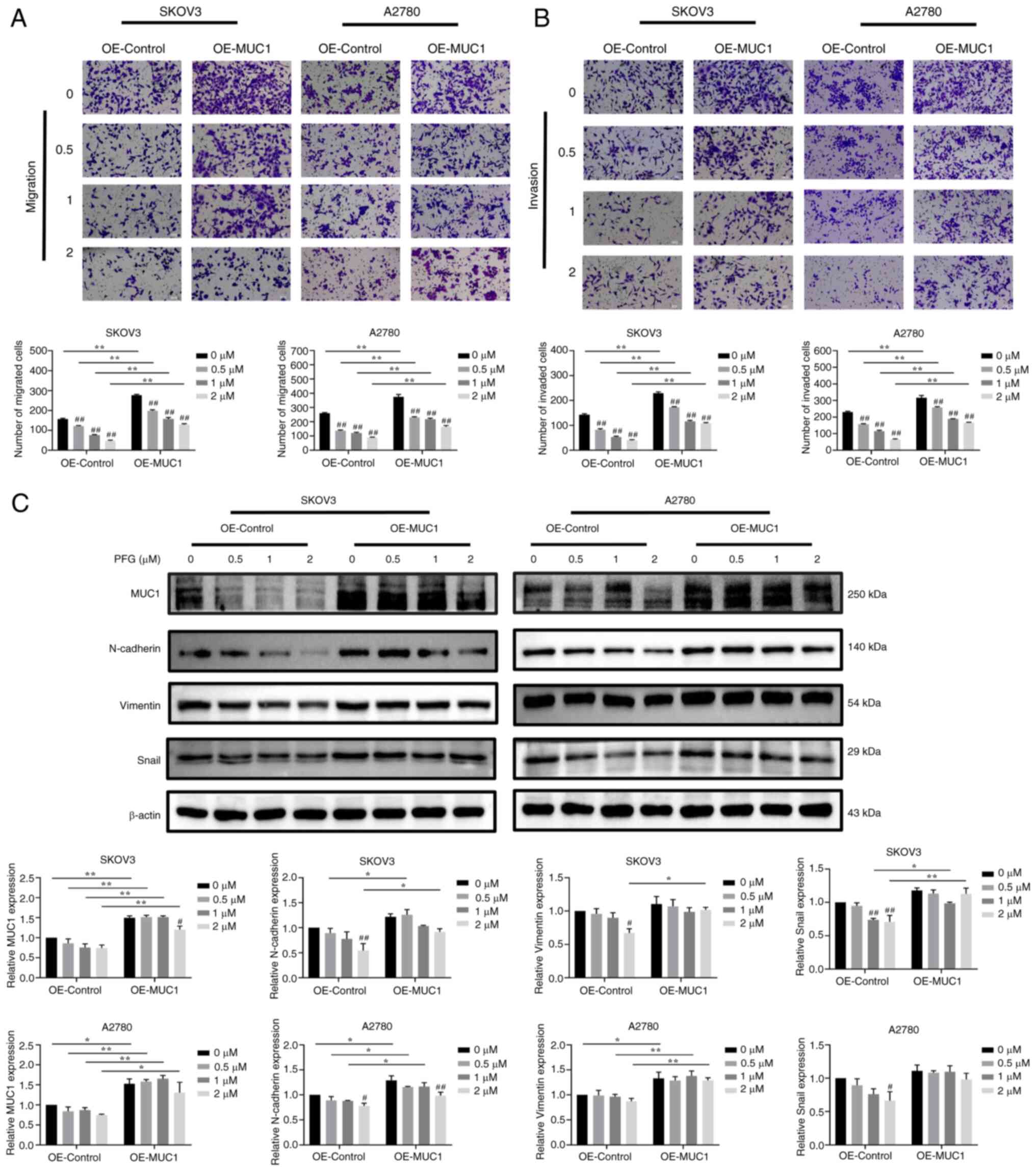
By contrast, MUC1 overexpression restored and
enhanced the migratory and invasive ability of SKOV3 and A2780
cells, while also activating EMT. Compared with the OE-control
cells, the inhibitory effect of different concentrations of PFG
supplementation on cell migration and invasion was reduced in
MUC1-overexpressing cells (Fig. 8A
and B). Compared with the OE-control cells, the expression
levels of MUC1 were significantly increased in OE-MUC1 cells, and
MUC1 overexpression partially reversed the PFG-induced decrease in
N-cadherin, Vimentin and Snail protein expression levels. (Fig. 8C). These results indicated that
MUC1 may be the dominant target protein through which PFG inhibits
SKOV3 and A2780 cells migration, invasion and EMT.
PFG blocks the Wnt/β-catenin signaling
pathway by inhibiting MUC1
The Wnt/β-catenin pathway is closely related to the
migration and invasion of tumor cells (36). To evaluate the role of the
Wnt/β-catenin pathway in the anti-OC effect of PFG, the
Wnt/β-catenin pathway inhibitor XAV939 was used. Western blot
analysis revealed that PFG and AXV939 exhibited similar inhibitory
effects on SKOV3 and A2780 cells. The combination of PFG and XAV939
further suppressed the expression levels of β-catenin in SKOV3
cells, as well as the downstream target protein MMP9 in SKOV3 and
A2780 cells (Fig. S2A). The
results of the wound-healing assay showed that combining PFG and
XAV939 markedly improved the inhibitory effect of PFG on OC cell
migration (Fig. S2B). These
results suggested that the Wnt/β-catenin pathway may have an
important role in the inhibition of OC by PFG.
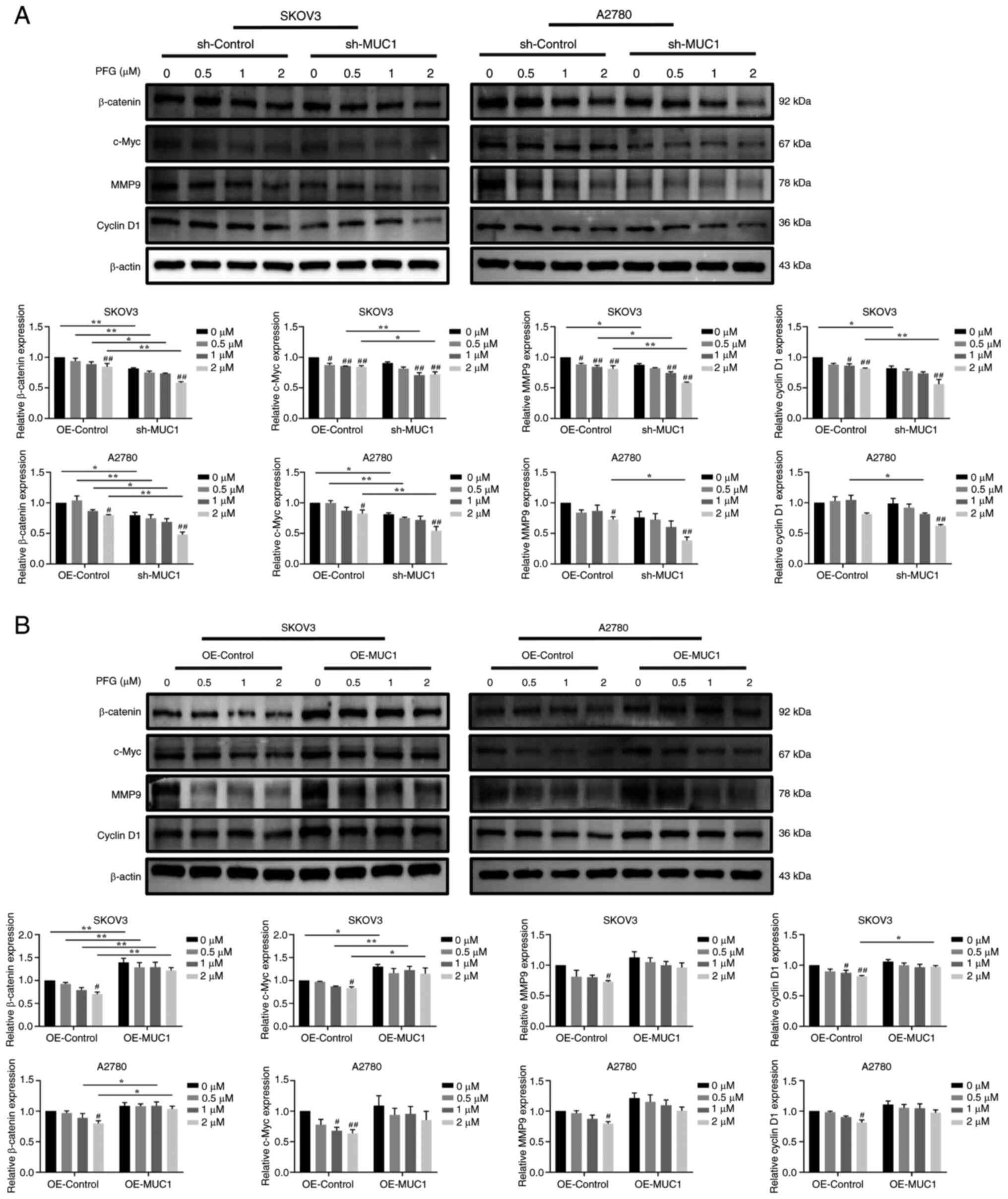
To elucidate whether PFG regulates the Wnt/β-catenin
pathway and cancer motility via inhibiting the expression of MUC1,
the protein expression levels of Wnt/β-catenin-related proteins and
their downstream target proteins, which are linked to tumor cell
metastasis and invasion were assessed. PFG (2 μM) treatment
significantly reduced the protein expression levels of β-catenin,
c-Myc, cyclin D1 and MMP9 in the sh-control cells and OE-control
cells, when compared with the control cells (0 μM PFG),
indicating that PFG inhibited Wnt/β-catenin pathway signaling in
both OC cell lines. In addition, compared with sh-control cells,
the silencing of MUC1 accelerated the decrease in β-catenin, c-Myc,
MMP9 and cyclin D1 protein expression levels induced by PFG in
SKOV3 and A2780 cells (Fig.
9A).
By contrast, compared with in the OE-control group,
overexpression of MUC1 partially reversed the decrease in β-catenin
protein expression levels in SKOV3 and A2780 cells induced by PFG,
as well as c-Myc and cyclin D1 protein expression levels in SKOV3
cells (Fig. 9B). In summary, it
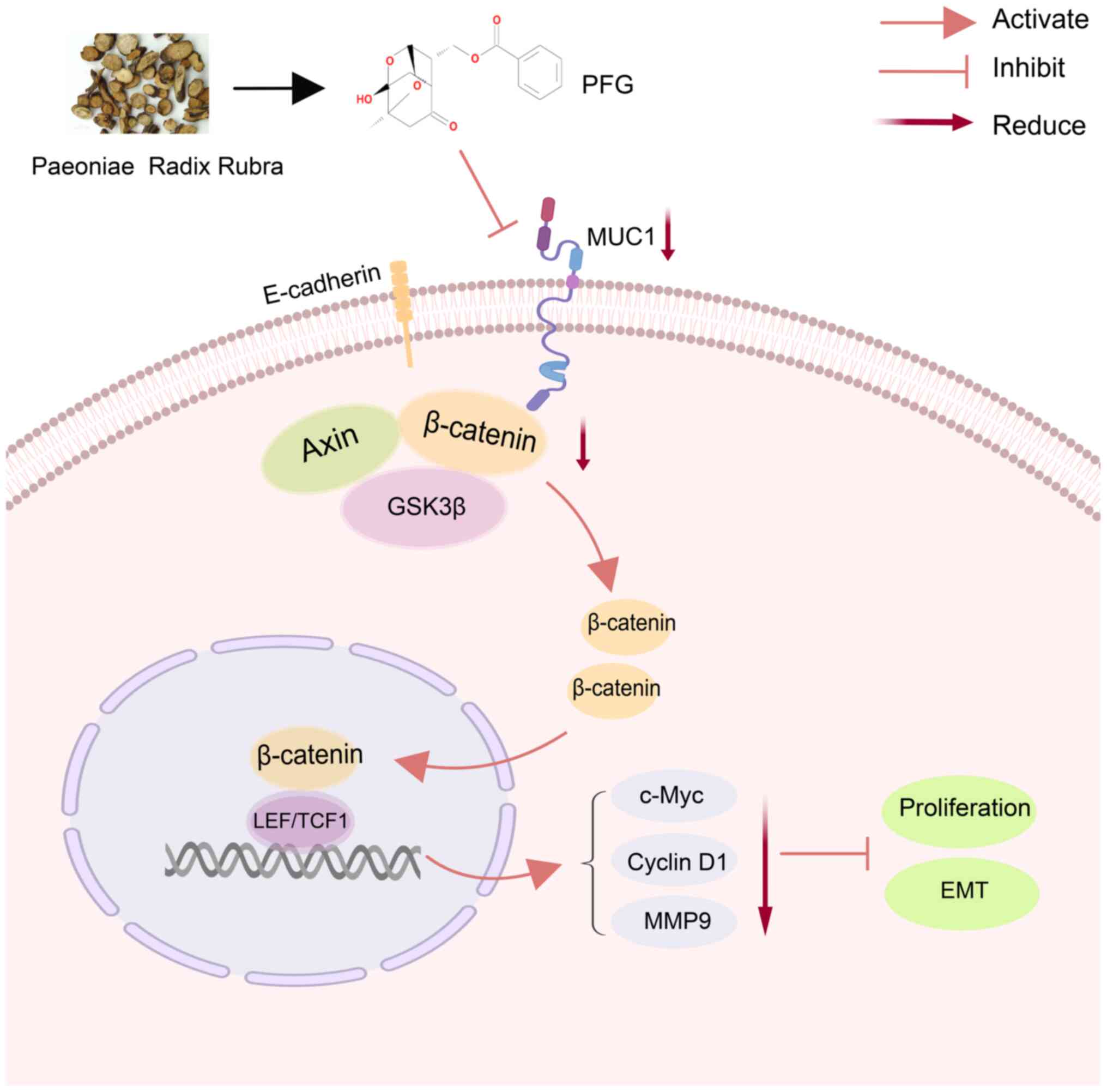
may be suggested that PFG can prevent the EMT process by blocking
the Wnt/β-catenin signaling pathway through inhibiting MUC1 in OC
cells (Fig. 10).
Discussion
The present study indicated that PFG can inhibit the
proliferation of OC cells, induce cell cycle arrest in the S phase,
and suppress cell migration and invasion. Moreover, PFG inhibited
the promoter activity of MUC1, lowered its protein expression, and
suppressed the production of crucial proteins in the Wnt/β-catenin
signaling pathway.
PFG has been shown in several studies to reduce the
proliferative capacity of tumor cells, and is cytotoxic and
selectively induces apoptosis in cancer cell lines (20,21); notably, the present study
confirmed these effects in two OC cell lines. The latest research
shows that PFG exerts anticancer effects by inducing apoptotic cell
death and blocking metastatic processes via the inactivation of
PI3K/AKT/mTOR/p70S6K signaling (37). In the present study, although PFG
significantly affected the apoptotic rate of OC cells, the maximum
apoptotic rate was <10% in both flow cytometry and TUNEL
experiments, which is not biologically significant, suggesting that
when the survival rate of SKOV3 and A2780 cells is >80%,
induction of apoptosis is not the primary mechanism by which PFG
inhibits OC cells. Subsequently, it was revealed that PFG
significantly inhibited the expression levels of the cell
cycle-related proteins cyclin A, cyclin E and CDK2, leading to cell
cycle arrest in S phase. This could be one of the key reasons why
PFG inhibits the proliferation of OC cells. Furthermore, MUC1
knockdown caused cell cycle arrest in the
G0/G1 phase, which differed from the direct
action of PFG on OC cells. PFG has previously been shown to lower
the expression of cyclin D1, CDK4 and CDK6 in tumor cells, which
may affect progression of the G1 phase (37); however, another study demonstrated
that PFG can promote S-phase arrest in HeLa cells (21). These findings indicated that the
regulatory effects of PFG on cells is multifaceted. Although the
present study demonstrated that PFG can directly bind to MUC1, it
is also plausible that PFG interacts with other molecules at the
same time, influencing cell cycle-related protein expression. The
results of the present study showed that PFG only significantly
affected S phase in SKOV3 and A2780 cells.
Cancer cell expansion, motility, invasion and EMT
are associated with MUC1. In the present study, knockdown and
overexpression of MUC1 in OC cells confirmed its crucial function
in OC progression, which is consistent with previous research
(38-40). Research on MUC1 has increased as a
result of its promise as an OC therapeutic target. For example, it
has been suggested that patients with EOC may have a higher chance
of overall survival if IgG antibodies to MUC1 are induced (41). Furthermore, the anti-MUC1
monoclonal antibody C595 alone, and in combination with docetaxel,
greatly improves the efficiency of OC cell death and induces
apoptosis (42,43). Moreover, the new vaccine BN-CV301,
which encodes the tumor-associated antigens MUC1 and CEA, induces
T-cell responses to these antigens, resulting in high levels of
immunogenicity that may have therapeutic benefits either alone or
in combination with anti-PD-1/L1 drugs (44-46). These results offer an experimental
foundation for ongoing preclinical assessment of MUC1 targeting.
GO-203, a cell-penetrating peptide-based MUC1-cytoplasmic tail (CT)
inhibitor, is still in phase I clinical trials for the treatment of
breast cancer (25). However,
there are currently few targeted drugs that target MUC1, and PFG, a
monomer compound of traditional Chinese medicine, is expected to be
a good candidate inhibitor of MUC1. The present study discovered
that the TCM compound PFG directly inhibited MUC1 expression, and
that knocking down MUC1 via external intervention further enhanced
its efficacy. By contrast, MUC1 overexpression antagonized the
antitumor activity of PFG. As a result, it may be hypothesized that
MUC1 is a critical target for PFG to exert its anticancer effects.
More investigation is warranted to determine which region of PFG
primarily binds to MUC1 and affects its function.
A complex between β-catenin and T cell-specific
transcription factor that drives transcriptional activity is formed
as a result of the Wnt/β-catenin pathway. Notably, the onset,
development, progression and worsening of numerous malignancies are
linked to elevated transcriptional activity of this pathway
(47-49). Recent research has demonstrated
that cancer cell migration can be inhibited and the Wnt/β-catenin
signaling pathway can be blocked by MUC1 silencing (50). Furthermore, interfering with MUC1
via small interfering RNA can decrease the interaction between
MUC1-CT and β-catenin, inhibiting β-catenin nuclear translocation
and target gene expression during carcinogenesis (51,52).
During the present study, it was revealed that the
Wnt/β-catenin pathway is critical in the inhibition of OC by PFG.
Furthermore, PFG and the pathway inhibitor XAV939 had a similar
inhibitory effect on OC cell migration, and their combination
considerably increased the inhibitory effects of PFG. Moreover, it
was revealed that the expression levels of key Wnt/β-catenin
pathway proteins, including β-catenin, and critical downstream
genes, such as c-Myc, MMP9 and cyclin D1, were significantly
altered in two OC cell lines with MUC1 knockdown and
overexpression. Additionally, it was observed that PFG treatment
inhibited this pathway more effectively in cells with MUC1
knockdown and less effectively in MUC1-overexpressing cells. Taken
together, it may be hypothesized that PFG can prevent OC cell
migration and invasion by decreasing MUC1 expression and blocking
the Wnt/β-catenin signaling pathway. PFG could be used as an
effective inhibitor targeting MUC1, providing a new experimental
basis for the treatment of OC with TCM.
The present study investigated the antitumor
activity of PFG against OC. The binding relationship between PFG
and its target MUC1 was identified, and further experiments were
performed to assess how MUC1 expression affects PFG efficacy. The
detailed mechanistic investigation revealed that PFG may exert
anti-migratory, -invasive and -EMT effects on OC by preventing MUC1
from being expressed and thus suppressing the Wnt/β-catenin
signaling pathway. These results indicated that a possible
therapeutic option for OC is MUC1, and PFG could be used to treat
OC as a MUC1 inhibitor. However, there are several limitations to
the present study that require further exploration: i) Only the
inhibitory effect of PFG on EOC cells was investigated, suggesting
that other types of OC may involve different mechanisms; ii)
further extensive investigations are essential to explore the
inhibitory mechanism of PFG on MUC1; and iii) additional in
vivo experiments are needed to support the findings of this
study. iv) In addition, the specific mechanism of PFG-induced
S-phase arrest of OC cells needs to be further explored.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FT and YX designed the study, revised the manuscript
and finalized the article. QL performed experiments and wrote the
manuscript. QL and LJ collected and analyzed the data. YZ and FS
performed the statistical analysis. XT, WL, JL and XJ contributed
to data analysis and interpretation. QL and LJ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Abbreviations:
|
OC
|
ovarian cancer
|
|
PFG
|
paeoniflorigenone
|
|
MUC1
|
mucin 1
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TCM
|
traditional Chinese medicine
|
|
PRR
|
Paeoniae Radix Rubra
|
|
FBS
|
fetal bovine serum
|
|
CCK-8
|
Cell Counting Kit-8
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82074391 and 82204875), the
Zhejiang Provincial Natural Science Foundation of China (grant nos.
LY21H270004 and LQ23H270012), the Youth Project of Guangdong Basic
and Applied Basic Regional Joint Fund (grant no. 2020A1515110281),
and the Graduate Research Foundation of Zhejiang Chinese Medical
University (grant no. 2021YKJ19).
References
|
1
|
Coburn SB, Bray F, Sherman ME and Trabert
B: International patterns and trends in ovarian cancer incidence,
overall and by histologic subtype. Int J Cancer. 140:2451–2460.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo N, Peiretti M, Parma G, Lapresa M,
Mancari R, Carinelli S, Sessa C and Castiglione M; ESMO Guidelines
Working Group: Newly diagnosed and relapsed epithelial ovarian
carcinoma: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21(Suppl 5): v23–v30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christie EL and Bowtell DDL: Acquired
chemotherapy resistance in ovarian cancer. Ann Oncol. 28(suppl_8):
viii13–viii15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar
|
|
9
|
Sun Z, Su YH and Yue XQ: Professor ling
Changquan's experience in treating primary liver cancer: An
analysis of herbal medication. Zhong Xi Yi Jie He Xue Bao.
6:1221–1225. 2008.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian AP, Yin YK, Yu L, Yang BY, Li N, Li
JY, Bian ZM, Hu SY, Weng CX and Feng L: Low-Frequency sonophoresis
of chinese medicine formula improves efficacy of malignant pleural
effusion treatment. Chin J Integr Med. 26:263–269. 2020. View Article : Google Scholar
|
|
11
|
Gao J, Yang J, Lu Z, Dong X and Xu Y: The
Multiple pharmacologic functions and mechanisms of action of guizhi
fuling formulation. Evid Based Complement Alternat Med.
2022:68134212022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Ren Y, Lou ZG, Wan X, Weng GB and
Cen D: Paeoniflorin inhibits the growth of bladder carcinoma via
deactivation of STAT3. Acta Pharm. 68:211–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Zang L, Zhao J, Wang Z and Li L:
Paeoniflorin inhibits cell viability and invasion of liver cancer
cells via inhibition of Skp2. Oncol Lett. 19:3165–3172.
2020.PubMed/NCBI
|
|
14
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Wang Q, Li X, Luo G, Shen M, Shi
J, Wang X and Tang L: Paeoniflorin Sensitizes breast cancer cells
to tamoxifen by Downregulating microRNA-15b via the
FOXO1/CCND1/β-Catenin axis. Drug Des Devel Ther. 15:245–257. 2021.
View Article : Google Scholar :
|
|
16
|
Gao J, Song L, Xia H, Peng L and Wen Z:
6'-O-galloylpaeoniflorin regulates proliferation and metastasis of
non-small cell lung cancer through AMPK/miR-299-5p/ATF2 axis.
Respir Res. 21:392020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XZ, Xia L, Zhang XY, Chen Q, Li X,
Mou Y, Wang T and Zhang YN: The multifaceted mechanisms of
Paeoniflorin in the treatment of tumors: State-of-the-Art. Biomed
Pharmacother. 149:1128002022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura M, Kimura I, Nojima H, Takahashi K,
Hayashi T, Shimizu M and Morita N: Blocking effects of a new
component, paeoniflorigenone, in paeony root on neuromuscular
junctions of frogs and mice. Jpn J Pharmacol. 35:61–66. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koo YK, Kim JM, Koo JY, Kang SS, Bae K,
Kim YS, Chung JH and Yun-Choi HS: Platelet anti-aggregatory and
blood anti-coagulant effects of compounds isolated from Paeonia
lactiflora and Paeonia suffruticosa. Pharmazie. 65:624–628.
2010.PubMed/NCBI
|
|
20
|
Huang Y, Ohno O, Suenaga K and Miyamoto K:
Apoptosis-inducing activity and antiproliferative effect of
Paeoniflorigenone from moutan cortex. Biosci Biotechnol Biochem.
81:1106–1113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Y, Ohno O and Miyamoto K: PFG acted
as an inducer of premature senescence in TIG-1 normal diploid
fibroblast and an inhibitor of mitosis in the HeLa cells. Biosci
Biotechnol Biochem. 83:986–995. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu XF, Yang E, Li J and Xing PX: MUC1
cytoplasmic tail: A potential therapeutic target for ovarian
carcinoma. Expert Rev Anticancer Ther. 6:1261–1271. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Elssen CH, Frings PW, Bot FJ, Van de
Vijver KK, Huls MB, Meek B, Hupperets P, Germeraad WT and Bos GM:
Expression of aberrantly glycosylated Mucin-1 in ovarian cancer.
Histopathology. 57:597–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gendler SJ: MUC1, the renaissance
molecule. J Mammary Gland Biol Neoplasia. 6:339–353. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Zhang Z, Zhang S, Zhu P, Ko JK and
Yung KK: MUC1: Structure, function, and clinic application in
epithelial cancers. Int J Mol Sci. 22:65672021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mommers EC, Leonhart AM, von
Mensdorff-Pouilly S, Schol DJ, Hilgers J, Meijer CJ, Baak JP and
van Diest PJ: Aberrant expression of MUC1 mucin in ductal
hyperplasia and ductal carcinoma In situ of the breast. Int J
Cancer. 84:466–469. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gaemers IC, Vos HL, Volders HH, van der
Valk SW and Hilkens J: A stat-responsive element in the promoter of
the episialin/MUC1 gene is involved in its overexpression in
carcinoma cells. J Biol Chem. 276:6191–6199. 2001. View Article : Google Scholar
|
|
29
|
Wesseling J, van der Valk SW, Vos HL,
Sonnenberg A and Hilkens J: Episialin (MUC1) overexpression
inhibits integrin-mediated cell adhesion to extracellular matrix
components. J Cell Biol. 129:255–265. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng H, Ghazizadeh M, Konishi H and Araki
T: Expression of MUC1 and MUC2 mucin gene products in human ovarian
carcinomas. Jpn J Clin Oncol. 32:525–529. 2002. View Article : Google Scholar
|
|
31
|
Mohr AM, Bailey JM, Lewallen ME, Liu X,
Radhakrishnan P, Yu F, Tapprich W and Hollingsworth MA: MUC1
regulates expression of multiple microRNAs involved in pancreatic
tumor progression, including the miR-200c/141 cluster. PLoS One.
8:e733062013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajabi H, Alam M, Takahashi H, Kharbanda
A, Guha M, Ahmad R and Kufe D: MUC1-C oncoprotein activates the
ZEB1/miR-200c regulatory loop and epithelial-mesenchymal
transition. Oncogene. 33:1680–1689. 2014. View Article : Google Scholar
|
|
33
|
Seeliger D and de Groot BL: Ligand docking
and binding site analysis with PyMOL and Autodock/Vina. J Comput
Aided Mol Des. 24:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Gardner AB, Charo LM, Mann AK, Kapp DS,
Eskander RN and Chan JK: Ovarian, uterine, and cervical cancer
patients with distant metastases at diagnosis: Most common
locations and outcomes. Clin Exp Metastasis. 37:107–113. 2020.
View Article : Google Scholar
|
|
36
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park KR, Lee H, Kim SH and Yun HM:
Paeoniflorigenone regulates apoptosis, autophagy, and necroptosis
to induce anti-cancer bioactivities in human head and neck squamous
cell carcinomas. J Ethnopharmacol. 288:1150002022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins
AC and Li Y: Expression of MUC1 in primary and metastatic human
epithelial ovarian cancer and its therapeutic significance. Gynecol
Oncol. 105:695–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng J, Wang L, Chen H, Li L, Ma Y, Ni J
and Li Y: The role of tumour-associated MUC1 in epithelial ovarian
cancer metastasis and progression. Cancer Metastasis Rev.
32:535–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Q, Song J, Wang S and He N: MUC1
regulates AKT signaling pathway by upregulating EGFR expression in
ovarian cancer cells. Pathol Res Pract. 224:1535092021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oei AL, Moreno M, Verheijen RH, Sweep FC,
Thomas CM, Massuger LF and von Mensdorff-Pouilly S: Induction of
IgG antibodies to MUC1 and survival in patients with epithelial
ovarian cancer. Int J Cancer. 123:1848–1853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Chen H, Liu F, Madigan MC, Power
CA, Hao J, Patterson KI, Pourgholami MH, O'Brien PM, Perkins AC and
Li Y: Monoclonal antibody targeting MUC1 and increasing sensitivity
to docetaxel as a novel strategy in treating human epithelial
ovarian cancer. Cancer Lett. 300:122–133. 2011. View Article : Google Scholar
|
|
43
|
Wang L, Chen H, Pourgholami MH, Beretov J,
Hao J, Chao H, Perkins AC, Kearsley JH and Li Y: Anti-MUC1
monoclonal antibody (C595) and docetaxel markedly reduce tumor
burden and ascites, and prolong survival in an in vivo ovarian
cancer model. PLoS One. 6:e244052011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mohebtash M, Tsang KY, Madan RA, Huen NY,
Poole DJ, Jochems C, Jones J, Ferrara T, Heery CR, Arlen PM, et al:
A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in
patients with metastatic breast and ovarian cancer. Clin Cancer
Res. 17:7164–7173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morse MA, Niedzwiecki D, Marshall JL,
Garrett C, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S,
Hobeika AC, et al: A randomized phase II study of immunization with
dendritic cells modified with poxvectors encoding CEA and MUC1
compared with the same poxvectors plus GM-CSF for resected
metastatic colorectal cancer. Ann Surg. 258:879–886. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gatti-Mays ME, Strauss J, Donahue RN,
Palena C, Del Rivero J, Redman JM, Madan RA, Marté JL, Cordes LM,
Lamping E, et al: A phase I Dose-escalation trial of BN-CV301, a
recombinant poxviral vaccine targeting MUC1 and CEA with
costimulatory molecules. Clin Cancer Res. 25:4933–4944. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tsuchiya K, Kiyoshi M, Hashii N, Fujita M,
Kurohara T, Ishii-Watabe A, Fukuhara K, Misawa T and Demizu Y:
Development of a penetratin-conjugated stapled peptide that
inhibits Wnt/β-catenin signaling. Bioorg Med Chem. 73:1170212022.
View Article : Google Scholar
|
|
48
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020. View Article : Google Scholar
|
|
49
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar
|
|
50
|
Song F, Chen FY, Wu SY, Hu B, Liang XL,
Yang HQ, Cheng JW, Wang PX, Guo W, Zhou J, et al: Mucin 1 promotes
tumor progression through activating WNT/β-catenin signaling
pathway in intrahepatic cholangiocarcinoma. J Cancer. 12:6937–6947.
2021. View Article : Google Scholar :
|
|
51
|
Liu X, Caffrey TC, Steele MM, Mohr A,
Singh PK, Radhakrishnan P, Kelly DL, Wen Y and Hollingsworth MA:
MUC1 regulates cyclin D1 gene expression through p120 catenin and
β-catenin. Oncogenesis. 3:e1072014. View Article : Google Scholar
|
|
52
|
Wang Z, Sun J, Hu X and Huang S:
Interference of mucin 1 inhibits progression of colon carcinoma by
repression of Wnt/β-catenin signaling. DNA Cell Biol. 33:162–170.
2014. View Article : Google Scholar : PubMed/NCBI
|