Introduction
Stroke, a prevalent cerebrovascular disease, is
characterized by high incidence, recurrence, morbidity and
mortality rates, ranking as the second leading cause of disability
and death globally (1). It
primarily encompasses two major categories: Ischemic stroke and
hemorrhagic stroke, with ischemic stroke being more prevalent,
accounting for ~60-80% of all stroke cases (2). Ischemic stroke, also known as
cerebral infarction, is a clinical syndrome that arises from
cerebral ischemia and hypoxia due to stenosis or occlusion of
cerebral vessels, leading to tissue damage or necrosis and
subsequent rapid onset of corresponding neurological deficits. In
the pursuit of therapeutic strategies, it has gradually become
apparent that non-neuronal cells within the central nervous system
(CNS), particularly astrocytes, play a pivotal role in the
pathophysiological processes of ischemic stroke.
As one of the most abundant and versatile cell types
in the CNS, astrocytes not only provide essential nutritional
support, structural scaffolding, and physical protection to
neurons, but also contribute to the formation of the blood-brain
barrier (BBB), regulate potassium ion concentration in the neuronal
extracellular environment, and maintain the stability of the
neuronal milieu. Furthermore, astrocytes play critical roles in
neurotransmitter metabolism, immune responses, pathological
processes and tissue repair, underscoring their indispensable
function in safeguarding the health and functionality of the
nervous system (3). Metabolic
reprogramming refers to the mechanism by which cells alter their
metabolic patterns to facilitate growth, proliferation and
adaptation to environmental changes in response to energy and
biosynthetic demands. As a crucial strategy for cells to cope with
external environmental alterations, metabolic reprogramming
encompasses, but is not limited to, the regulation of multiple
metabolic pathways such as glycolysis, oxidative phosphorylation,
fatty acid metabolism and amino acid metabolism (4). During ischemic stroke, astrocytes
swiftly respond by initiating a series of intricate metabolic
reprogramming processes to adapt to the extreme ischemic and
hypoxic conditions. However, the specific roles and underlying
mechanisms of astrocytes in ischemic stroke remain to be further
systematically investigated.
Therefore, the current understanding of the roles
and mechanisms of astrocyte metabolic reprogramming in ischemic
stroke have been summarized, delving into their molecular
mechanisms, functional alterations and interactions with other cell
types. By consolidating existing literature, it is aimed to uncover
the central role of astrocyte metabolic reprogramming in the
pathophysiological processes of ischemic stroke, thereby providing
a theoretical basis for the development of novel therapeutic
strategies.
The physiological function of astrocyte
The CNS is composed of a large number of
non-neuronal cells called glia, with astrocytes being the primary
type of glial cells that are ubiquitous throughout the entire CNS
and interact with a variety of cells.
Formation and maintenance of the BBB
The BBB, situated between the blood and brain
tissues, serves as a highly selective permeable interface that
safeguards the brain from harmful substances. It is primarily
composed of brain microvascular endothelial cells, astrocytes,
pericytes and the basal lamina, playing a crucial role in
maintaining normal CNS function and internal environmental
homeostasis (5). Structurally,
the BBB can be divided into three layers: The inner layer,
consisting of brain microvascular endothelial cells held together
by tight and adherens junctions, serves as the core component that
restricts the passage of blood-borne substances into the brain. The
middle layer is composed of the basal lamina formed by the
extracellular matrix and pericytes embedded within it. The outer
layer comprises astrocyte foot processes adhering to the
microvascular basal lamina (6).
Astrocytes, with their unique morphology and
functions, play a pivotal role in the formation and maintenance of
BBB integrity, being essential cells that contribute to the
physiological homeostasis of the CNS (7). Once the BBB is formed, mature
astrocytes regulate and maintain the barrier. These cells extend
numerous processes from their cell bodies, which tightly envelop
cerebral capillaries, providing support and separation for neuronal
cells. This intimate contact not only reinforces the structural
stability of the cerebral capillary walls but also participates in
the formation of the basal lamina through the secretion of matrix
proteins by the astrocytes, further enhancing the barrier function
of the BBB (8). Additionally,
astrocytes are crucial for water and potassium homeostasis in the
CNS, as they control the exchange of water and certain solutes
through abundant expression of aquaporin-4 (9).
Ion transport and neuromodulation
Astrocytes are the primary cell type responsible for
regulating brain homeostasis, encompassing the maintenance of ionic
homeostasis and neuronal regulation. Potassium and sodium ions are
crucial for neuronal electrical activity, and the stability of
their concentration is essential for maintaining the normal
function of neurons. Potassium ions predominantly reside within
cells, whereas a large inward electrochemical gradient of sodium
ions serves to generate action potentials. Upon neuronal
excitation, a significant concentration of potassium ions is
released into the extracellular space, leading to a local increase
in potassium ion concentration. Astrocytes, through the expression
of inward rectifier potassium channels such as Kir4.1, can rapidly
uptake these excess potassium ions and distribute them to adjacent
astrocytes via gap junctions, thereby maintaining a stable
potassium ion concentration around neurons. This spatial buffering
of potassium ions helps prevent neuronal hyper-excitation and
damage (10,11). Sodium ions play a pivotal role in
regulating the intracellular pH of astrocytes. While the activation
of the sodium-potassium ATPase in response to elevated
extracellular potassium levels may decrease intracellular sodium
ions, the co-transport of neurotransmitters such as glutamate with
sodium ions leads to an increase in sodium ions within astrocytes,
thereby modulating downstream metabolic pathways (12).
Astrocytes also play a vital role in neuromodulation
within the CNS. They are capable of sensing and responding to
neurotransmitters and other signaling molecules released by
neurons, and influence neuronal excitability and synaptic
plasticity through the release of their own neuroactive substances
(13). Furthermore, astrocytes
form intricate network structures through gap junctions among
themselves and with neurons, enabling them to synchronize their
activities and transmit information. This signal transduction and
neuromodulation function contribute to maintaining the homeostasis
and adaptability of the nervous system (14).
Nutrition and support
Neurotrophic factors are proteins that play a
crucial role in the development, survival and apoptosis of neurons.
Under normal physiological conditions, astrocytes can release
various neurotrophic factors, such as brain-derived neurotrophic
factor and nerve growth factor. These neurotrophic factors are
essential for the growth, development, survival and functional
maintenance of neurons. They facilitate the growth of neuronal
axons and dendrites, enhance synaptic connections between neurons,
and thereby increase the complexity and functionality of neural
networks.
Relationship between astrocyte and ischemic
stroke
In response to the damage of CNS, neurodegenerative
diseases, or other infectious diseases, the genes, morphology, and
functions of astrocytes undergo rapid changes, and these reactions
are collectively referred to as astrocyte reactivity (15). In recent years, with the
intensifying exploration into the intricate functional diversity of
astrocytes, researchers have uncovered their ability to
differentiate into distinct functional phenotypes under various
pathological conditions, notably the A1 and A2 subtypes. After the
onset of stroke, astrocytes are activated and release
pro-inflammatory or anti-inflammatory factors, leading to a complex
neuroinflammatory cascade reaction, which has a dual effect on
neuroinflammation after ischemic stroke (16). These subtypes exhibit profoundly
different mechanisms of action during the pathological process of
ischemic stroke, significantly impacting disease progression,
outcome and neural repair.
Through genetic profiling of reactive astrocytes in
mouse models of ischemic stroke and neuroinflammation, Zamanian
et al (17) demonstrated
that reactive astrocytes can be induced by different types of
insults to adopt either an A1 or A2 phenotype. Specifically,
ischemic injury triggers the emergence of so-called trophic A2
astrocytes, whereas inflammatory insults induce the more neurotoxic
A1 astrocytes (17). Liddelow
et al (18) further
elucidated that ischemia and neuroinflammation can prompt M1
microglia to secrete cytokines, which in turn induce astrocytes to
transform into the neurotoxic A1 phenotype. These A1 astrocytes
typically display reduced normal functions and significantly
upregulate numerous genes previously shown to be detrimental to
synapses. Additionally, A1 astrocytes may secrete neurotoxins,
contributing to the death of neurons and oligodendrocytes (18). By contrast, A2 astrocytes appear
to upregulate neurotrophic factors or anti-inflammatory genes that
promote neuronal survival and growth, and support repair functions,
such as TGF-β, which can promote synaptogenesis and exhibit
neuroprotective effects against cerebral ischemic injury (19). To ascertain the relative roles of
A1/A2 astrocytes in CNS diseases, intervention with the critical
intracellular and extracellular signaling pathways that drive the
polarization of astrocytes into either beneficial or detrimental
phenotypes is necessary. Research strongly indicates that the
Stat3-mediated signaling pathway may be crucial for A2 astrocytes
to proliferate and support neuronal regeneration in acute injury
models, while the NF-κB signaling pathway may underlie the
induction of A1 astrocytes (20,21). While the simplistic A1/A2
dichotomy does not fully capture the broad spectrum of astrocyte
phenotypes, it provides a valuable framework for understanding
astrocyte reactivity in various CNS diseases.
Glial fibrillary acidic protein (GFAP) is a type of
intermediate filament protein specifically expressed in astrocytes
within the central and peripheral nervous systems. It plays a
crucial role in maintaining the morphology, structure and function
of astrocytes, while also participating in their reactive and
proliferative processes. In the event of ischemic stroke, oxygen
levels in the brain tissue decrease, leading to damage to
surrounding cellular tissues. Consequently, astrocytes become
activated and release GFAP, which subsequently enters the
bloodstream through the compromised BBB. In patients with acute
ischemic stroke, serum GFAP levels exhibit a moderate positive
correlation with the degree of neurological deficit and brain
injury as assessed by the National Institutes of Health Stroke
Scale (NIHSS) score. Elevated serum GFAP levels are associated with
higher NIHSS scores, indicating more severe neurological deficits
(22). Furthermore, high serum
GFAP levels serve as a marker for poor neurological prognosis in
patients with ischemic stroke, enabling the prediction of
neurological outcomes one-year post-stroke (23). The overview of astrocyte related
to ischemic stroke is demonstrated in Table I (16,17,24-33).
 | Table IOverview of astrocyte related to
ischemic stroke. |
Table I
Overview of astrocyte related to
ischemic stroke.
| First author/s
year | Object of
study | Cohort
description | Sample type | Outcome
characteristics | Microbiota analysis
approach | (Refs.) |
|---|
| Zamanian et
al, 2012 | Mice from the
transgenic mouse line | Transient ischemia
was induced by occluding the MCAO | Brain tissues | Reactive astrocyte
phenotype strongly depended on the type of inducing injury | Affymetrix GeneChip
arrays | (17) |
| Song et al,
2020 | SD rats | Rats subjected to
MCAO/R | Brain tissues | Increase the
glutamate disposal by astrocytes and protecte neurons from
excitotoxicity in response to I/R insults | Decrease the
oxidative stress mediated by SDH | (24) |
| Shi et al,
2021 | MEGF10-flox founder
mice | The surgery of
MCAO | Brain tissues | Reactive
microgliosis and astrogliosis hinder brain repair by engulfing
synapses | Immunostaining,
fluorescence in situ hybridization, western blotting,
quantitative PCR, Golgi-Cox staining | (25) |
| Williamson et
al, 2021 | C57BL/6 mice | Photothrombotic
infarcts were created in forelimb motor cortex | Vasculature | Reactive astrocytes
are crucial for vascular repair and remodeling after ischemic
stroke in mice | In vivo
two-photon imaging | (26) |
| Ma et al,
2022 | C57BL/6 mice | tMCAO model | Brain tissues | Significant
differences in the expression of genes and metabolic pathways in
the astrocytes at 12 and 24 h after ischemic stroke | Single-cell
sequencing | (27) |
| Denecke et
al, 2022 | Cell | Rat astrocytes were
cultured in rat astrocyte basal medium containing growth serum | Cells | When subjected to
hypoxia, and nutrient starvation, astrocytes within the penumbral
region generated in the microdevice exhibited long-lasting,
significantly altered signaling capacity | polystyrene-based
microfluidic device | (28) |
| Wang et al,
2023 | C57BL/6 mice | reversible right
MCAO surgery | Brain tissues | Astrocyte-derived
KLF4 has a critical role in regulating the activation of A1/A2
reactive astrocytes following AIS | Immunofluorescent
staining, antibodies, RNA extraction, reverse-transcription,
western blot analysis and quantitative PCR | (16) |
| Lochhead et
al, 2023 | SD rats | MCAO surgery | Brain tissues | Astrocyte
activation may contribute to or be in response to neurodegeneration
following MCAO | Confocal microscopy
strategy, TTC Staining, Laser Doppler Blood Flow,
immunofluorescence staining | (29) |
| Zhou et al,
2024 | C57BL/6J mice | Transient focal
cerebral ischemia was induced by right MCAO | Brain tissues | Astrocytic LRP1
regulated the transfer of healthy mitochondria from astrocytes into
neurons and protected against brain ischemia-reperfusion
injury | Extracellular
mitochondria collection | (30) |
| Scott et al,
2024 | C57/Bl6J mice | Stroke-injured or
uninjured controls | Cells | The acquisition of
different astrocytic states after stroke that are influenced by
time and proximity to the stroke lesion site | tDISCO alongside
two high-throughput platforms for spatial (Visium) and single-cell
transcriptomics (10X Chromium) | (31) |
| Wang et al,
2024 | C57BL/6J mice | The barrel cortex
ischemic stroke | Brain tissues | A2 astrocytes and
A1 astrocytes are enriched in the acute and chronic phases of
ischemic stroke respectively | Flow cytometry,
Total RNA extraction, RNA-Seq, quantitative PCR, Vibrissae-Evoked
Forelimb-Placing Test, Immunofluorescence | (32) |
| Bormann et
al, 2024 | Wistar rats and
mice | Cerebral ischemia
was induced by intraluminal occlusion of the MCAO | Brain tissues | Astrocytes belong
to one of the most transcriptionally perturbed populations and
exhibit infarct and subtype specific molecular features. | Single nucleus RNA
sequencing, immunofluorescence | (33) |
The role of astrocyte in ischemic
stroke
A close connection between astrocytes and the
pathogenesis of CNS diseases has been recently suggested (34). Alzheimer's disease (AD) is the
most prevalent progressive neurodegenerative disorder,
characterized clinically by comprehensive dementia manifestations
such as memory impairment, aphasia, apraxia and agnosia. It
constitutes the primary cause of dementia in the elderly. In AD,
astrocytes undergo sustained activation and release significant
levels of inflammatory cytokines and neurotoxic substances. This
process triggers or exacerbates the hyperphosphorylation pathology
of tau protein and amyloid-β protein, thereby facilitating the
formation of amyloid plaques and neurofibrillary tangles, as well
as contributing to neuronal death and the decline of cognitive
function (35-37). Furthermore, the distinct
pathological phenotypes they develop may exert crucial influences
on the progression of AD (38).
Parkinson's disease (PD), the second most common neurodegenerative
disorder, is primarily characterized by heterogeneous classic motor
features associated with Lewy bodies and the loss of dopaminergic
neurons in the substantia nigra. Additionally, it exhibits
clinically significant non-motor features. Research has
demonstrated that patients with PD exhibit abnormal proliferation
and increased senescence of astrocytes in the brain, which
participate in inflammatory responses within the substantia nigra,
thereby promoting disease progression (39-41). Multiple sclerosis (MS) is a
neurological autoimmune disorder primarily characterized by
inflammatory demyelination in the white matter of the CNS,
ultimately leading to progressive physical disability in patients.
Astrocytes play a pivotal role in MS by modulating immune responses
and inflammatory reactions, thereby influencing the severity and
progression rate of the disease (42-44). Furthermore, ischemic stroke, as
another prevalent disorder of the CNS, is also intimately
associated with abnormal functions of astrocytes.
Dual roles of glial scar formation
In the pathological process of ischemic stroke,
astrocytes exhibit diverse roles in the CNS, with a notable
response being the formation of glial scars. During the acute phase
of the disease, this process aids in sealing the injury site,
remodeling tissues, and controlling local immune responses.
However, during the recovery phase, it may restrict the recovery of
neurological function.
At the onset of the disease, damaged cells produce
and release cytokines, triggering reactive proliferation of
astrocytes that migrate towards the injury area to form glial scars
(45). The formation of glial
scars helps limit the spread of inflammatory reactions, reduces
further tissue damage, and provides a physical barrier around the
injured area to prevent the infiltration of harmful substances,
thus maintaining CNS homeostasis and creating conditions for
subsequent repair processes. However, during neural recovery,
excessive formation and persistence of glial scars become physical
barriers that hinder neuronal synaptic reconnection. Glial scars
are primarily composed of proliferated astrocytes and the
extracellular matrix proteins they secrete, forming a dense barrier
that severely impedes neuronal regeneration and axonal extension.
Additionally, glial scars may further inhibit the neural repair
process by releasing inhibitory factors, leading to limited
restoration of neurological function (46).
Mediation of inflammatory response and
oxidative stress
Astrocytes actively participate in the regulation of
inflammatory responses and oxidative stress in ischemic stroke.
Inflammatory response, while being one of the primary causes of CNS
injury, can exacerbate brain tissue damage when excessive.
Following ischemic stroke, astrocytes are activated into reactive
astrocytes, releasing a surge of inflammatory factors and inducing
the production of neurotoxic mediators. This inflammatory response
leads to the disruption of the BBB and the upregulation of cellular
adhesion molecules, increasing the permeability of the BBB and
allowing immune cells to infiltrate into brain tissues. This fact
further triggers inflammatory reactions such as cerebral edema,
ultimately resulting in neuronal cell death (47). Concurrently, astrocytes can also
release anti-inflammatory neuroprotective factors, attracting
immune cells, clearing necrotic tissues, and promoting tissue
repair, thereby inhibiting inflammation (48).
During ischemic stroke, the severe inflammatory
response caused by ischemia and hypoxia in brain tissue induces the
production of reactive oxygen species (ROS) and other oxidative
products in large concentrations, resulting in the accumulation of
numerous free radicals in the body. This, in turn, damages cell
structures and human tissues, triggering an oxidative stress
response. As the primary glial cell type in the CNS, astrocytes
rapidly respond to this stress state by upregulating the expression
and activity of antioxidant enzymes to combat excessive ROS
accumulation and mitigate damage to neurons and their own cells.
Glutathione (GSH) is a crucial antioxidant and free radical
scavenger that participates in redox reactions in the body. It
binds to peroxides and free radicals, reducing ROS toxicity and
serving as a key factor in preventing the aggravation of ischemic
injury in brain tissue. Studies have shown that astrocytes are rich
in GSH and enzymes related to GSH metabolism. By enhancing the
synthesis and release of antioxidants such as GSH and storing
nitric oxide in the cytoplasm, astrocytes can reduce oxidative
stress toxicity, alleviate oxidative stress damage to neurons, and
thereby promote neuronal survival (49-51).
Antagonizing neurotoxicity and promoting
neurological recovery
Glutamate is the primary excitatory neurotransmitter
in the CNS, responsible for virtually all excitatory synaptic
activities in the brain. This crucial amino acid circulates
extensively between neurons and astrocytes, known as the
glutamate-glutamine cycle. Extracellular glutamate is taken up by
nearby astrocytes, converted into glutamine, which then migrates to
the extracellular environment, enters presynaptic neurons, and is
reconverted into glutamate (52). In the early stage of ischemic
stroke, the brain tissue suffers from ischemia and hypoxia, leading
to reduced ATP production, gradual energy depletion, and impaired
ion pump function, which results in presynaptic calcium overload.
The subsequent accumulation of glutamate in the synapses activates
post-synaptic glutamate receptors, causing post-synaptic calcium
overload, initiating profound neurotoxicity, and ultimately
triggering neuronal death (53).
Astrocytes can effectively remove glutamate from the synaptic cleft
by expressing high-affinity glutamate transporters, maintaining its
homeostatic levels and preventing excessive neuronal excitation and
damage (54). Synaptic
regeneration after neural injury is a crucial process that enhances
neural plasticity, and astrocytes contribute to this process in
multiple ways. The close morphological relationship between
astrocytic membranes and pre- and post-synaptic neurons, along with
the widespread expression of neurotransmitter receptors in
astrocytes, forms the astrocytic tripartite synapse. This structure
prevents the diffusion of neurotransmitters from the synapse and
serves as an important structural unit in the nervous system
(55). Besides, astrocytes
facilitate the release of various growth factors and neurotrophins.
These factors are vital during ischemia for neuronal survival and
growth, angiogenesis, synaptic regeneration, and the restoration of
other neurological functions (56).
Transformation into neurons
Astrocytes are the most widely distributed cells in
the CNS, and they can rapidly activate and proliferate under
pathological conditions, making them the ideal target cell type for
induction into neuronal cells (57). Reactive astrocytes can transform
into neurons under specific circumstances (58). Although neural stem cells (NSCs)
are the primary source of endogenous neural repair, their migratory
capacity is limited. When the site of injury is far from the region
where NSCs reside, reactive astrocytes can be activated through
neurogenic programs, acquiring certain stem cell properties, and
then re-differentiating into neurons to promote local neuronal
regeneration. In addition, astrocytes also aid in the migration of
differentiated NSCs (59,60).
Astrocyte metabolic reprogramming in
ischemic stroke
In recent years, with the deepening of research into
neurological diseases, the metabolic flexibility and functional
diversity of astrocytes have garnered increasing attention, and
notable advancements have been made in the study of metabolic
reprogramming of astrocytes. Research has revealed that mutations
in the intellectual disability-associated gene SNX27 can induce
metabolic reprogramming of astrocytes, subsequently impairing
cognitive functions. Specifically, SNX27 mutations lead to a
decreased ability of astrocytes to uptake glucose via GLUT1,
resulting in reduced lactate production and a transition from
homeostatic astrocytes to reactive astrocytes. This transformation
impacts the energy supply to neighboring neurons (61). Additionally, astrocytes possess
the capacity to modulate the pathogenicity of glioblastoma by
reprogramming the immune characteristics of the tumor
microenvironment and supporting the non-oncogenic metabolic
dependence of glioblastoma on cholesterol (62).
Astrocytes form functional networks through gap
junctions, enabling intercellular communication with other cells
via various mechanisms, playing a crucial role in maintaining
homeostasis within the CNS (63). The unique cellular structure and
quantitative characteristics of astrocytes confer upon them a high
degree of metabolic plasticity, allowing them to adjust their
metabolic pathways in response to environmental changes and
physiological demands. They not only harness glucose for energy
production through glycolysis and aerobic oxidative phosphorylation
but also acquire energy and synthesize essential biomolecules
through fatty acid and amino acid metabolism pathways (64). However, during ischemic stroke,
local cerebral blood flow is reduced or interrupted due to cerebral
vascular occlusion, leading to insufficient oxygen supply and a
precipitous decline in energy supply. In this emergent situation,
astrocytes promptly respond by initiating metabolic reprogramming
to adapt to the energy crisis.
Astrocyte glucose metabolism in ischemic
stroke
Glucose serves as the primary energy source for the
brain, and its metabolism directly impacts brain function,
particularly the interplay between astrocytes and neurons (65). Under normal physiological
conditions, astrocytes efficiently uptake glucose via glucose
transporter proteins. Once inside the cell, glucose is initially
phosphorylated to glucose-6-phosphate (G6P), marking the crucial
initiating step in glucose metabolism. G6P then bifurcates into two
primary metabolic routes: glycolysis and the pentose phosphate
pathway (PPP). Glycolysis, one of the major branches of glucose
metabolism, involves the gradual degradation of G6P into pyruvate,
generating small amounts of ATP and NADH along the way. In aerobic
conditions, the majority of pyruvate enters mitochondria where it
undergoes complete oxidation to carbon dioxide and water through
the tricarboxylic acid (TCA) cycle, releasing substantial energy
for ATP synthesis. This process is known as aerobic oxidative
phosphorylation (66).
Furthermore, astrocytes also retain the capacity to convert a
portion of pyruvate into lactate, which can be released
extracellularly to regulate pH balance and contribute to energy
supply. The PPP, as a shunt from glycolysis, diverges at G6P and is
catalyzed by G6P dehydrogenase, yielding NADPH and 5-phosphoribose,
among other products. NADPH, an essential cytosolic reductive
equivalent, effectively scavenges ROS, thereby safeguarding cells
from oxidative stress damage. Consequently, PPP activity is often
regarded as an important indicator of cellular antioxidant defense.
Notably, under hypoxic conditions, astrocytes upregulate glucose
flux towards the PPP to provide additional NADPH for GSH synthesis,
thereby protecting neurons from oxidative insult (67). During ischemic stroke, localized
hypoxia in brain tissue ensues due to reduced blood flow,
concurrently diminishing glucose and oxygen supply. In response to
this emergency, astrocytes reprogram their glucose metabolic
pathways by enhancing glucose uptake, accelerating glycolysis, and
increasing lactate release. These concerted changes collectively
enhance the tolerance of astrocytes to ischemic and hypoxic
environments (68) (Fig. 1).
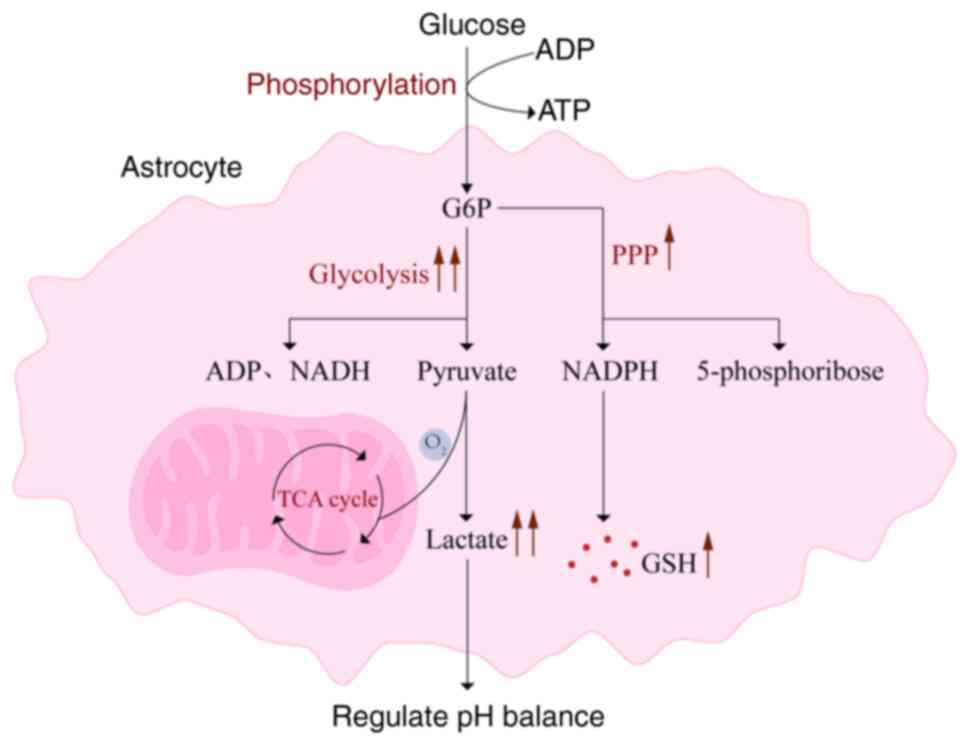 | Figure 1Astrocyte glucose metabolism in
ischemic stroke. Under normal physiological conditions, astrocytes
efficiently uptake glucose via glucose transporter proteins. Upon
entering the cell, glucose is initially phosphorylated to G6P.
Subsequently, G6P diverges into two primary metabolic pathways:
glycolysis and the pentose phosphate pathway. In glycolysis, G6P is
gradually degraded into pyruvate. Under aerobic conditions, the
majority of pyruvate enters the mitochondria and undergoes complete
oxidation to carbon dioxide and water through the TCA cycle,
releasing substantial energy for ATP synthesis. Meanwhile, a
portion of pyruvate is converted into lactate, which is released
extracellularly. In the PPP, G6P is catalyzed by G6PDH to yield
NADPH and 5-phosphoribose, among other products. During ischemic
stroke, astrocytes reprogram their glucose metabolic pathways by
enhancing glucose uptake, accelerating glycolysis, and increasing
lactate release. G6P, glucose-6-phosphate; TCA, tricarboxylic acid;
G6PDH, G6P dehydrogenase; PPP, pentose phosphate pathway. |
Astrocytes enhance their glucose uptake capacity by
upregulating the expression of glucose transporter proteins, with
GLUT1, a 45 kDa subtype, being primarily responsible for basal
glucose transport in astrocytes (69). GLUT1 is a transmembrane protein
widely distributed in vascular endothelial cells of the BBB and
various brain tissue cells. It exhibits high specificity and
affinity, enabling rapid transfer of glucose from the bloodstream
into cells, providing essential energy sources (70). During ischemia, astrocytes
increase the quantity and activity of GLUT1 to ensure enhanced
glucose influx into the cells, meeting the urgent metabolic demands
(71). By enhancing glucose
uptake efficiency, astrocytes sustain a certain level of energy,
enabling them to continue supporting neuronal function and
regulating the intracranial environment. Under hypoxic conditions
resulting from ischemic stroke, the aerobic oxidative
phosphorylation pathway is severely inhibited, leading to a drastic
reduction in ATP production. In response to this energetic crisis,
most eukaryotic cells reprogram their primary metabolic route,
shifting from mitochondrial respiration to glycolysis, in order to
sustain ATP levels. This hypoxia-induced metabolic reprogramming is
crucial for meeting cellular energy demands during acute hypoxic
stress (72). Glycolysis, a
glucose metabolic pathway that operates even under anaerobic or
hypoxic conditions, albeit generating fewer ATP molecules compared
with aerobic oxidative phosphorylation, operates at a faster rate
and is independent of oxygen. By enhancing the proportion of
aerobic glycolysis, astrocytes can rapidly generate sufficient ATP
to maintain fundamental cellular functions, which is pivotal in
preserving cellular stability and preventing further damage.
However, this process also leads to a corresponding elevation in
lactate levels, which promotes the formation of Kla protein,
significantly exacerbating brain injury in ischemic stroke
(73). Excessive accumulation of
lactate also causes a significant decrease in cellular pH, exposing
brain tissue to acidosis-induced damage (74). To alleviate this crisis,
astrocytes not only adapt to their own metabolic changes but also
release lactate into the extracellular space through the lactate
shuttle mechanism. This lactate is then taken up by neurons,
serving as an alternative energy source under hypoxic conditions.
Through gluconeogenesis, it can be reconverted into glucose or
enter the TCA cycle for oxidative phosphorylation to generate ATP,
thereby effectively protecting neurons from further damage. This
process is known as the astrocyte-neuron lactate shuttle (75). Not only does this process provide
crucial energy support for neurons, but it also promotes the
recycling of glucose and lactate within the brain, enhancing
overall metabolic efficiency.
Astrocyte fatty acid metabolism in
ischemic stroke
As the preferred energy substrate for the brain,
when glucose supply decreases, fatty acid oxidation and the
resulting ketone bodies (KBs) become the primary alternative energy
sources, assisting in maintaining the stability of neuronal
synaptic function and structure (76). Astrocytes serve as the primary
site of fatty acid oxidation in the brain (77). Fatty acids first enter astrocytes
through specific transporters and are then stored as lipid droplets
(LDs) in the endoplasmic reticulum. When needed, these LDs are
converted into fatty acyl-CoA and transported to mitochondria to
participate in β-oxidation, ultimately generating acetyl-CoA, which
enters the TCA cycle and other pathways to produce energy.
Furthermore, fatty acids are transported to support myelin
formation or regeneration. Additionally, astrocytes are the primary
source of KBs production in the brain (78).
Under normal physiological conditions, fatty acid
oxidation constitutes a pivotal pathway for astrocytes to derive
energy. However, during ischemic stroke, the brain tissue rapidly
becomes ischemic and hypoxic, necessitating a steep rise in
neuronal energy demands. In response to this energy crisis,
astrocytes swiftly engage their distinctive metabolic adaptation
mechanisms. They upregulate the expression of fatty acid
transporter proteins, such as fatty acid-binding proteins, to
augment their capacity for fatty acid uptake and concurrently
activate lipid synthesis pathways to augment intracellular fatty
acid storage. While this process may be somewhat hindered by the
deficient energy supply, astrocytes persist in maintaining their
metabolic functions (79).
Nevertheless, excessive expression of fatty acid transporter
proteins in ischemic stroke is not uniformly beneficial. In certain
scenarios, it may lead to lipid droplet accumulation within
astrocytes, further impeding normal cellular physiology. LDs,
fundamental components of astrocytic lipid metabolism, modulate
fatty acid storage and hydrolysis in eukaryotes (80). Their primary purpose is to
provide fuel for fatty acid oxidation, offering an alternative
energy-generating route for astrocytes and other tissues during
energy source depletion, ensuring an adequate fatty acid reserve
for cell survival and neuronal metabolism (81). At the onset of ischemia and
hypoxia, astrocytes intensify fatty acid oxidation and breakdown to
generate more ATP, crucial for maintaining cellular survival and
fulfilling neuronal energy demands, thereby conferring
neuroprotection. Consequently, metabolites associated with fatty
acid catabolism may elevate initially (82). Yet, over time, mitochondrial
dysfunction and impairment of oxidative phosphorylation may hinder
effective fatty acid entry into mitochondria, suppressing fatty
acid oxidation (83) (Fig. 2). Additionally, reduced activity
of vital enzymes such as carnitine palmitoyl-transferase 1A
(CPT1A), a crucial regulator for long-chain fatty acid entry into
mitochondria for oxidation, further decelerates fatty acid
oxidation rates. The decreased CPT1A activity directly contributes
to reduced fatty acid oxidation, instigating fatty acid metabolic
disturbances and exacerbating the energy supply crisis (84).
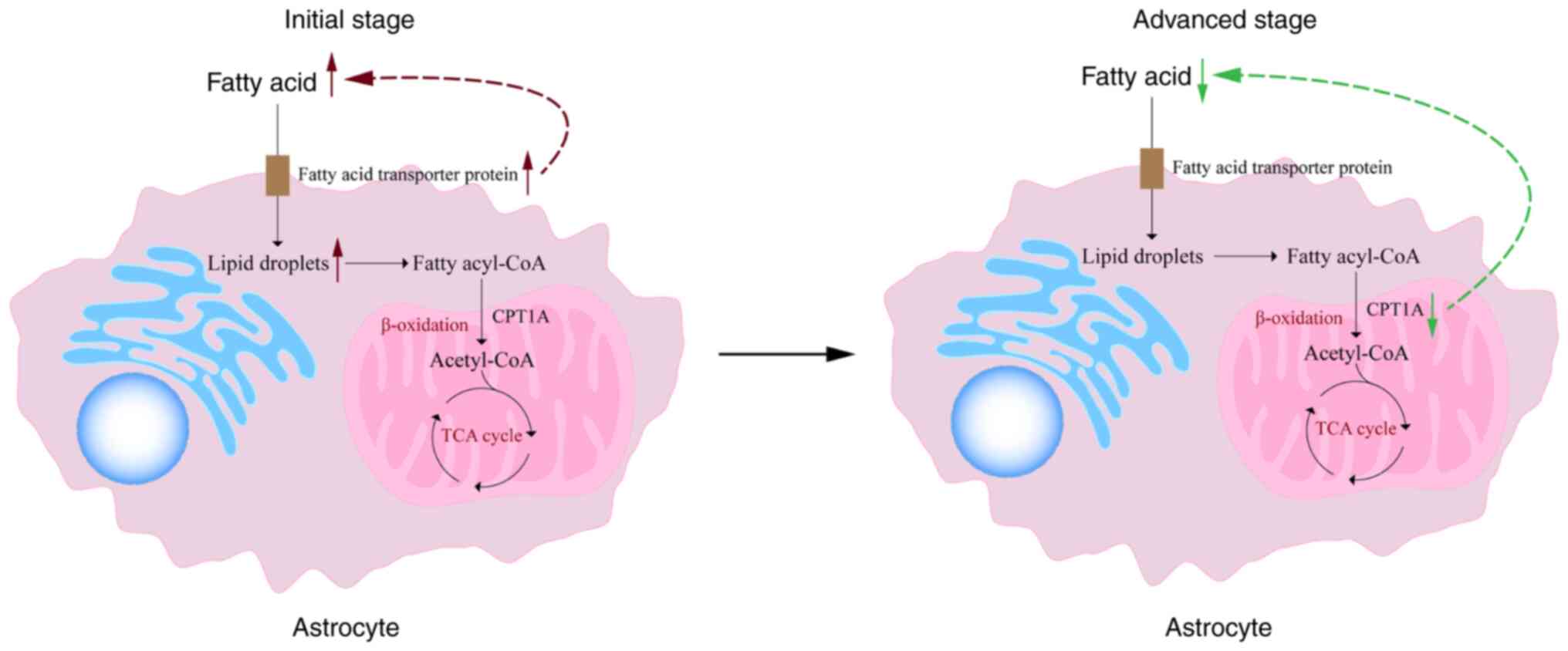 | Figure 2Astrocyte fatty acid metabolism in
ischemic stroke. Fatty acids initially enter astrocytes through
specific transporter proteins and are subsequently stored as LDs in
the endoplasmic reticulum. These LDs are converted into fatty
acyl-CoA when needed and transported to mitochondria to participate
in β-oxidation, ultimately generating acetyl-CoA, which enters the
TCA cycle and other pathways to produce energy. However, following
ischemic stroke, astrocytes upregulate the expression of fatty acid
transporter proteins, leading to the accumulation of LDs within
astrocytes. Over time, mitochondrial dysfunction and impairment of
the oxidative phosphorylation pathway may prevent fatty acids from
effectively entering mitochondria, thereby inhibiting the
progression of fatty acid oxidation. LDs, lipid droplets; TCA,
tricarboxylic acid; CPT1A, carnitine palmitoyl-transferase 1A. |
Astrocyte amino acid metabolism in
ischemic stroke
In parallel with energy metabolic pathways, amino
acid metabolism within astrocytes plays a pivotal role in
regulating brain function. As fundamental constituents of proteins,
amino acids not only participate in protein synthesis and
degradation but also modulate neuronal excitability, synaptic
plasticity, and the synthesis and release of neurotransmitters
through their transport and metabolic processes.
Glutamate serves as the primary excitatory
neurotransmitter in the CNS, with neurons and astrocytes being the
primary players in its metabolism. The exchange of glutamate,
γ-aminobutyric acid (GABA), and glutamine between these two cell
types, known as the glutamate/GABA-glutamine cycle, is crucial for
maintaining CNS homeostasis (85). During cerebral ischemia and
hypoxia, neuronal damage leads to the release of excess glutamate
into the synaptic cleft, where it acts as an excitatory
neurotransmitter binding to post-synaptic receptors. Under these
conditions, the expression of excitatory glutamate transporters
such as GLT-1 and GLAST-1 is significantly downregulated, resulting
in reduced clearance of glutamate from the synaptic cleft (86). The subsequent accumulation of
glutamate excessively stimulates post-synaptic neurons, leading to
excitotoxicity. A portion of the unbound glutamate is sequestered
by astrocytes through high-affinity glutamate transporters and
converted to glutamine within the cells, thereby reducing
extracellular glutamate concentrations and protecting neurons from
excitotoxic damage to a certain extent. In some instances, this
unbound glutamate may also be retrogradely transported back to
neurons via glutamate transporters, participating in new rounds of
neurotransmission (87).
Additionally, astrocytes have been shown to release small amounts
of glutamate to surrounding neurons, contributing to the
synchronization of action potential firing and regulating neural
excitability transmission (88).
Concurrently, GABA, synthesized by neurons, is released as an
inhibitory neurotransmitter to dampen post-synaptic neuronal
excitability. Imbalances between excitatory and inhibitory
neurotransmitter signaling contribute to the onset of
excitotoxicity. After release, GABA can also be taken up by
astrocytes, where it is converted to succinic semialdehyde by GABA
transaminase and enters the TCA cycle for energy metabolism
(89). Moreover, astrocytes can
synthesize and release GABA into the extracellular space,
fine-tuning local neural networks (90). However, extensive uptake of
glutamate and GABA by astrocytes depletes the corresponding
neurotransmitter pools in neurons. To counteract this, astrocytes
convert ingested glutamate into glutamine and shuttle it back to
neurons via the glutamine-glutamate cycle for reuse, which is
essential for replenishing glutamate and GABA at neuronal terminals
(91) (Fig. 3).
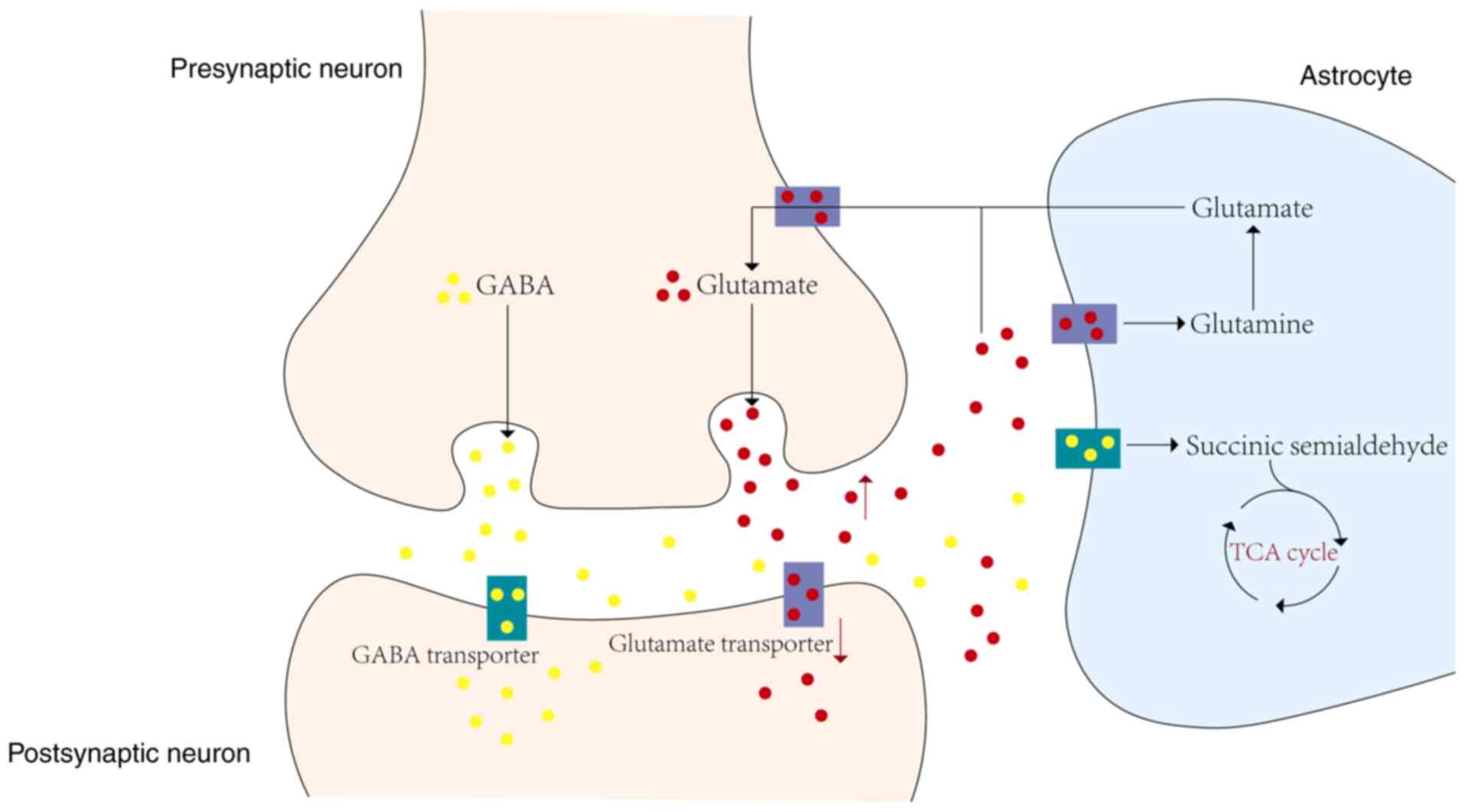 | Figure 3Astrocyte amino acid metabolism in
ischemic stroke. When the brain is in a state of ischemia and
hypoxia, a substantial level of glutamate is released into the
synaptic cleft, resulting in a significant downregulation of
excitatory glutamate transporters expression. This leads to a
reduction in the efficiency of glutamate clearance from the
synaptic cleft. Among the glutamate released, some that is not
bound by post-synaptic neurons is received by astrocytes via
high-affinity glutamate transporters and converted into glutamine
within the cells. In certain circumstances, this unbound glutamate
may also be directly retrogradely transported back to neurons via
glutamate transporters, participating in a new round of
neurotransmission. Simultaneously, GABA, synthesized by neurons, is
released as an inhibitory neurotransmitter to inhibit the
excitability of post-synaptic neurons. The imbalance between the
release of excitatory and inhibitory neurotransmitter signals leads
to the occurrence of excitotoxicity. Released GABA can also be
taken up by astrocytes and converted into succinate semialdehyde
via GABA transaminase within the astrocytes, entering the TCA cycle
for energy metabolism. However, the extensive uptake of glutamate
and GABA by astrocytes depletes the corresponding neurotransmitter
pools in neurons. To counteract this, astrocytes convert the
received glutamate into glutamine and transport it back to neurons
through the glutamine-glutamate cycle for reuse, which is crucial
for replenishing glutamate and GABA at neuronal terminals. GABA,
γ-aminobutyric acid; TCA, tricarboxylic acid. |
In the pathological process of ischemic stroke,
metabolic reprogramming of astrocytes plays a pivotal role. From
the basal metabolism, glucose metabolism, fatty acid metabolism, to
amino acid metabolism of astrocytes, all aspects exhibit adaptive
changes to ischemic and hypoxic environments. Such metabolic
reprogramming not only enhances the survival capacity of astrocytes
but also provides robust support for neuronal and brain tissue
repair by modulating various aspects including antioxidation,
energy supply, inflammatory response and neuroprotection.
Consequently, a profound understanding of the mechanisms underlying
metabolic reprogramming of astrocytes in ischemic stroke holds
significant importance for the development of novel therapeutic
strategies.
Regulatory factors of astrocyte metabolic
reprogramming in ischemic stroke
Hypoxia-inducible factors (HIFs) constitute pivotal
transcription factors that are responsive to intracellular oxygen
concentration fluctuations. Under hypoxic conditions, HIFs exert
transcriptional regulatory activity, governing the expression of an
array of metabolism-related genes, thereby facilitating cellular
and systemic adaptation to hypoxic environments. They also emerge
as crucial regulators in metabolic reprogramming of astrocytes. By
modulating the expression of an extensive network of target genes,
HIFs orchestrate reprogramming of astrocyte pathways associated
with glucose metabolism, fatty acid metabolism and amino acid
metabolism, markedly enhancing astrocytic resilience against
ischemic and hypoxic insults. In ischemic stroke, the ensuing
inflammatory response and cellular damage induced by ischemia and
hypoxia activate various signaling cascades. Numerous of these
pathways are intricately linked to the reactivity of astrocytes,
with the Jak-STAT3 signaling pathway playing a pivotal role in
coordinating the proliferation of reactive astrocytes by modulating
the production of cytokines and chemokines. This pathway serves as
a central regulatory hub for numerous molecular and functional
alterations in reactive astrocytes (92). A previous study revealed a marked
upregulation of the transcription factor STAT3 in reactive
astrocytes following ischemic stroke (93). STAT3 has been demonstrated to
control phenotypic changes and metabolic reprogramming of
astrocytes during the inflammatory phase of ischemic stroke,
upregulating lactate-directed glycolysis while impeding
mitochondrial function, thus serving as a key modulator of
astrocyte phenotype, metabolic shift and associated neurotoxicity.
Moreover, in reactive astrocytes, elevated expression of activated
pSTAT3 correlates with larger infarct sizes and reduced synaptic
densities in the peri-infarct zone (94). Beyond cell proliferation, STAT3
also regulates GFAP expression in reactive astrocytes, as the GFAP
promoter harbors STAT3 consensus binding sites essential for GFAP
induction. Consequently, the STAT3 signaling pathway emerges as a
promising pharmacological target for modifying reactive astrocytes
in the context of ischemic stroke (95).
Glycophagy, as a selective autophagy process for
glycogen, is emerging as a pivotal metabolic pathway for the
delivery of glycolytic fuel substrates, playing a crucial role in
maintaining cellular metabolic homeostasis in peripheral tissues
(96). It regulates cellular
redox homeostasis, glutamine utilization, fatty acid oxidation in
skeletal muscle, and lipid droplet formation in adipocytes
(97,98). Guo et al (99) discovered during reperfusion in
ischemic stroke patients and mice that dysfunction in glycophagy in
astrocytes is induced by the downregulation of GABA A
receptor-associated protein-like 1, with the activation of the
PI3K-Akt pathway also involved. Following ischemic stroke, as key
enzymes in glycolysis in astrocytes are inhibited, glucose sourced
from glycophagy enters the PPP more significantly than it does
glycolysis and the TCA cycle. Restoring glycophagy in astrocytes
can not only enhance their resistance to ischemic and hypoxic
environments but also aid in the survival of nearby neurons when
the entire brain is under energy stress (99).
Therapeutic approaches targeting astrocyte
metabolic reprogramming in ischemic stroke
Currently, the frontline treatments for ischemic
stroke encompass intravenous administration of recombinant tissue
plasminogen activator (rt-PA) and mechanical thrombectomy (100). Nevertheless, due to their
narrow therapeutic windows, the options for treating ischemic
stroke remain limited. As such, the pursuit of novel candidate
drugs for ischemic stroke management poses an urgent challenge. In
the context of ischemic stroke, astrocytes undergo significant
metabolic reprogramming, transitioning from a quiescent state to a
reactive state. Thus, the therapeutic strategies targeting
metabolic reprogramming of astrocytes in ischemic stroke focus on
modulating their metabolic pathways and functional states, with the
aim of inhibiting excessive astrocyte activation or regulating the
ratio of A1/A2 phenotypes, thereby maximizing neuroprotective
effects while minimizing neurotoxicity, ultimately facilitating
repair and regeneration following brain injury.
In the early stages of ischemic stroke, due to blood
flow interruption, brain tissue experiences hypoxia, prompting
astrocytes to shift towards anaerobic glycolysis for rapid ATP
production. However, this metabolic pathway is prone to lactate
accumulation, leading to acidosis. Following ischemic reperfusion,
astrocyte glycogen mobilization augments the production of NADPH
and GSH via the PPP, resulting in a reduction in ROS levels. This,
in turn, triggers the inhibition of the NF-κB signaling pathway and
the activation of the STAT3 signaling pathway, ultimately leading
to the suppression of A1 astrocytes and the enhancement of A2
astrocytes (101). HIF-1
enhances the intrinsic tolerance of brain tissue to ischemia by
augmenting glucose uptake in astrocytes, facilitating glycogen
synthesis, and initiating a metabolic shift towards glycolytic
energy production (68).
Similarly, AMPK exerts a specific influence on glycolysis during
ischemic preconditioning in brain tissue (102). These pathways present promising
therapeutic targets for addressing reperfusion injury in ischemic
stroke. Under ischemic and hypoxic stimuli, astrocytes release free
fatty acids from LDs into mitochondria, causing mitochondrial
damage, reducing metabolic support from astrocytes, and thereby
exacerbating neuronal injury (103). Cao et al (104) revealed that n-3 polyunsaturated
fatty acids can decrease the cerebral infarction volume and improve
neurological function in mice with transient middle cerebral artery
occlusion. Additionally, it reduced the polarization of A1
astrocytes induced by ischemic stroke both in vivo and in
vitro (104). In addition,
the interaction between glutamate and astrocytes jointly influences
the pathological process of ischemic stroke. In ischemic stroke,
glutamate, acting as a neurotransmitter, experiences an abnormal
elevation in its levels, exerting neurotoxic effects on neurons.
Conversely, astrocytes are responsible for modulating the uptake
and metabolism of glutamate, thereby mitigating its deleterious
impact on neurons. However, to date, no literature has confirmed
the existence of drugs that target this mechanism to affect
astrocyte phenotype changes during ischemic stroke.
As a crucial therapeutic target in ischemic stroke,
promoting the differentiation of astrocytes towards the A2
phenotype may offer novel neuroprotective strategies with profound
therapeutic implications. Research has demonstrated that the
overexpression of the chemokine-like signaling protein
prokineticin-2 and its small molecule agonist IS20 in primary
astrocytes or in the brains of mice in vivo can induce the
A2 astrocyte phenotype. This leads to the upregulation of key
protective genes and A2 reactive markers, while simultaneously
reducing inflammatory cytokines (105). The TGF-β secreted by M2
macrophages potentially elicits the differentiation of astrocytes
towards the A2 phenotype through the activation of the PI3K/Akt
signaling cascade (106).
Furthermore, cytokines such as IL-4, IL-10 and IL-1β can induce the
differentiation of astrocytes towards the A2 phenotype in
vitro, further elucidating their protective and repair
functions under inflammatory responses (107,108). In summary, therapeutic
approaches aimed at restoring tissue damage and metabolic balance
in the brain through the modulation of astrocyte metabolic
reprogramming have emerged as promising targets for the treatment
of ischemic stroke. However, this area remains relatively
under-explored, and there is a pressing need for future research
endeavors to delve deeper into this domain. The relevant
therapeutic approaches targeting astrocyte metabolic reprogramming
in ischemic stroke are demonstrated in Table II (16,73,104,109-116).
 | Table IITherapeutic approaches targeting
astrocyte metabolic reprogramming in ischemic stroke. |
Table II
Therapeutic approaches targeting
astrocyte metabolic reprogramming in ischemic stroke.
| First author/s,
year | Strategy | Regulation on
astrocytes polarization and metabolism | (Refs.) |
|---|
| Liu et al,
2020 | Cottonseed oil | Reduce A1 phenotype
neurotoxic astrocyte activation by inhibiting TLR4/NF-κB pathway,
which may affect glucose metabolism | (109) |
| Zhang et al,
2024 | AD16 | Reduce A1
polarization of astrocytes by downregulating NF-κB and JAK2/STAT3
pathways, which may affect glucose metabolism | (110) |
| Zou et al,
2024 | Scutellarin | Inhibit the
activation of astrocytes to A1 type by inhibiting the activation of
NF-κB pathway, which may affect glucose metabolism | (111) |
| Zhou et al,
2022 | Tetrahedral
framework nucleic acid | Facilitate the
astrocytic polarization from the proinflammatory A1 phenotype to
the neuroprotective A2 phenotype by downregulating the TLRs/NF-κB
pathway, which may affect glucose metabolism | (112) |
| Liu et al,
2024 | Genistein-3′-sodium
sulfonate | Enhance astrocyte
polarization to A2 phenotype by inactivating TLR4/NF-κB pathway,
which may affect glucose metabolism | (113) |
| Chen et al,
2024 | Edaravone
Dexborneol | Suppress activation
of neurotoxic A1 astrocytes by inhibiting NF-κB pathway, which may
affect glucose metabolism | (114) |
| Wang et al,
2023 | Kruppel-like
transcription factor-4 | Inhibit the
activation of A1 astrocyte and promote A2 astrocyte polarization by
modulating expressions of NF-κB pathway, which may affect glucose
metabolism | (16) |
| Li et al,
2024 | Buyang Huanwu
Decoction | Suppress astrocytes
A1 polarization and promote astrocyte A2 polarization by increasing
expression of phosphorylated AMPK and inhibiting NF-κB pathway,
which may affect glucose metabolism | (115) |
| Xia et al,
2024 | Gypenosides | Inhibit astrocytes'
polarization into the A1 type, by inhibiting STAT-3/HIF1-α and
TLR-4/NF-κB/HIF1-α pathways, which may affect glucose
metabolism | (116) |
| Xiong et al,
2024 | Oxamate | Inhibit the
activation of A1 astrocyte by inhibiting the production of lactate,
blocking the shuttle of lactate to neurons, and inhibiting the
formation of Kla protein, which may affect glucose metabolism | (73) |
| Cao et al,
2021 | N-3 polyunsaturated
fatty acids | Reduce the A1
astrocyte polarization both in vivo and in vitro by
attenuating mitochondrial oxidative stress and increasing the
mitophagy of astrocytes, which may affect fatty acid
metabolism | (104) |
Conclusions and perspectives
In conclusion, ischemic stroke, a prevalent and
devastating condition, poses significant challenges in terms of
disability and mortality globally. The current pharmacological
treatment, intravenous thrombolysis with rt-PA, while effective,
has notable limitations, necessitating the exploration of novel
therapeutic approaches. Astrocytes, as crucial and versatile cells
in the CNS, play multifaceted roles in supporting neuronal
function, maintaining the BBB, and regulating ion concentrations.
Importantly, recent research has highlighted the involvement of
astrocytes in neurotransmitter metabolism, immune response and
tissue repair, with their metabolic characteristics influencing the
progression and outcome of ischemic stroke. This understanding
underscores the potential of targeting astrocyte metabolic
reprogramming as a therapeutic strategy for ischemic stroke. By
summarizing the current knowledge on astrocyte metabolic
reprogramming mechanisms, regulatory factors and pathways, as well
as strategies to promote polarization balance, the present review
provides insights into the promising potential of astrocyte
immunometabolism-targeted therapies.
However, there is still a long way to go in the
study of metabolic reprogramming of astrocytes in ischemic stroke.
Given the intricate involvement of multiple molecular and signaling
pathways, the specific molecular mechanisms and regulatory networks
underlying astrocyte metabolic reprogramming in ischemic stroke
have yet to be fully elucidated. Therefore, future studies should
delve deeper into the precise molecular mechanisms of astrocyte
metabolic reprogramming, particularly its dynamic evolution across
different stages of ischemic stroke, the interplay and regulatory
relationships among various metabolic pathways, and the intricate
molecular mechanisms of its complex interactions with neurons. A
profound understanding of the molecular mechanisms and regulatory
networks of astrocyte metabolic reprogramming holds significant
clinical implications for developing novel therapeutic strategies
for ischemic stroke and identifying potential drug targets.
Currently, research on astrocytes in ischemic stroke primarily
focuses on cellular levels and animal models, and the applicability
and translatability of these findings in humans remain to be
validated. Future endeavors should concentrate on drug targets
capable of modulating astrocyte metabolic pathways and functional
states, emphasizing human-level exploration and validation. This
includes investigating astrocyte function and metabolic changes
using clinical samples, conducting relevant clinical trials, and
leveraging structural biology, medicinal chemistry and pharmacology
to design and optimize targeted therapies against these identified
targets. Ultimately, these efforts aim to assess the efficacy and
safety of astrocyte-targeted therapies.
Availability of data and materials
Not applicable.
Authors' contributions
LW and TYM conceived and designed the study. WXC
wrote the first draft of the manuscript. RH, RM and YXX
participated in editing of specific paragraphs and figures. TYM
supervised the study and critically revised the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 8237142870).
References
|
1
|
Saini V, Guada L and Yavagal DR: Global
epidemiology of stroke and access to acute ischemic stroke
interventions. Neurology. 97(20 Suppl 2): S6–S16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2019 Stroke Collaborators: Global,
regional, and national burden of stroke and its risk factors,
1990-2019: A systematic analysis for the Global Burden of Disease
Study 2019. Lancet Neurol. 20:795–820. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han RT, Kim RD, Molofsky AV and Liddelow
SA: Astrocyte-immune cell interactions in physiology and pathology.
Immunity. 54:211–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiellini G: Metabolic reprogramming in
health and disease. Int J Mol Sci. 21:27682020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Candelario-Jalil E, Dijkhuizen RM and
Magnus T: Neuroinflammation, stroke, blood-brain barrier
dysfunction, and imaging modalities. Stroke. 53:1473–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu D, Chen Q, Chen X, Han F, Chen Z and
Wang Y: The blood-brain barrier: Structure, regulation, and drug
delivery. Signal Transduct Target Ther. 8:2172023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verkhratsky A and Nedergaard M: Physiology
of astroglia. Physiol Rev. 98:239–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacoste B, Prat A, Freitas-Andrade M and
Gu C: The blood-brain barrier: Composition, properties, and roles
in brain health. Cold Spring Harb Perspect Biol. a0414222024.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vandebroek A and Yasui M: Regulation of
AQP4 in the central nervous system. Int J Mol Sci. 21:16032020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hertz L and Chen Y: Importance of
astrocytes for potassium ion (K+) homeostasis in brain
and glial effects of K+ and its transporters on
learning. Neurosci Biobehav Rev. 71:484–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seifert G, Henneberger C and Steinhäuser
C: Diversity of astrocyte potassium channels: An update. Brain Res
Bull. 136:26–36. 2018. View Article : Google Scholar
|
|
12
|
Felix L, Delekate A, Petzold GC and Rose
CR: Sodium fluctuations in astroglia and their potential impact on
astrocyte function. Front Physiol. 11:8712020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allen NJ and Eroglu C: Cell biology of
astrocyte-synapse interactions. Neuron. 96:697–708. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oliveira JF and Araque A: Astrocyte
regulation of neural circuit activity and network states. Glia.
70:1455–1466. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hara M, Kobayakawa K, Ohkawa Y, Kumamaru
H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima
Y and Okada S: Interaction of reactive astrocytes with type I
collagen induces astrocytic scar formation through the
integrin-N-cadherin pathway after spinal cord injury. Nat Med.
23:818–828. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C and Li L: The critical role of KLF4
in regulating the activation of A1/A2 reactive astrocytes following
ischemic stroke. J Neuroinflammation. 20:442023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou
L, Giffard RG and Barres BA: Genomic analysis of reactive
astrogliosis. J Neurosci. 32:6391–6410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liddelow SA, Guttenplan KA, Clarke LE,
Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS,
Peterson TC, et al: Neurotoxic reactive astrocytes are induced by
activated microglia. Nature. 541:481–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel MR and Weaver AM: Astrocyte-derived
small extracellular vesicles promote synapse formation via
fibulin-2-mediated TGF-β signaling. Cell Rep. 34:1088292021.
View Article : Google Scholar
|
|
20
|
Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea
TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ and Sofroniew MV:
Astrocyte scar formation aids central nervous system axon
regeneration. Nature. 532:195–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lian H, Yang L, Cole A, Sun L, Chiang AC,
Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky
JL, et al: NFκB-activated astroglial release of complement C3
compromises neuronal morphology and function associated with
Alzheimer's disease. Neuron. 85:101–115. 2015. View Article : Google Scholar
|
|
22
|
Amalia L: Glial fibrillary acidic protein
(GFAP): Neuroinflammation biomarker in acute ischemic stroke. J
Inflamm Res. 14:7501–7506. 2021. View Article : Google Scholar
|
|
23
|
Liu G and Geng J: Glial fibrillary acidic
protein as a prognostic marker of acute ischemic stroke. Hum Exp
Toxicol. 37:1048–1053. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song X, Gong Z and Liu K, Kou J, Liu B and
Liu K: Baicalin combats glutamate excitotoxicity via protecting
glutamine synthetase from ROS-induced 20S proteasomal degradation.
Redox Biol. 34:1015592020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi X, Luo L, Wang J, Shen H, Li Y,
Mamtilahun M, Liu C, Shi R, Lee JH, Tian H, et al: Stroke
subtype-dependent synapse elimination by reactive gliosis in mice.
Nat Commun. 12:69432021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williamson MR, Fuertes CJA, Dunn AK, Drew
MR and Jones TA: Reactive astrocytes facilitate vascular repair and
remodeling after stroke. Cell Rep. 35:1090482021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma H, Zhou Y, Li Z, Zhu L, Li H, Zhang G,
Wang J, Gong H, Xu D, Hua W, et al: Single-cell RNA-sequencing
analyses revealed heterogeneity and dynamic changes of metabolic
pathways in astrocytes at the acute phase of ischemic stroke. Oxid
Med Cell Longev. 2022:18177212022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Denecke KM, McBain CA, Hermes BG, Teertam
SK, Farooqui M, Virumbrales-Muñoz M, Panackal J, Beebe DJ, Famakin
B and Ayuso JM: Microfluidic model to evaluate astrocyte activation
in penumbral region following ischemic stroke. Cells. 11:23562022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lochhead JJ, Williams EI, Reddell ES, Dorn
E, Ronaldson PT and Davis TP: High resolution multiplex confocal
imaging of the neurovascular unit in health and experimental
ischemic stroke. Cells. 12:6452023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Zhang L, Peng J, Zhang X, Zhang F,
Wu Y, Huang A, Du F, Liao Y, He Y, et al: Astrocytic LRP1 enables
mitochondria transfer to neurons and mitigates brain ischemic
stroke by suppressing ARF1 lactylation. Cell Metab.
36:2054–2068.e14. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scott EY, Safarian N, Casasbuenas DL,
Dryden M, Tockovska T, Ali S, Peng J, Daniele E, Nie Xin Lim I,
Bang KWA, et al: Integrating single-cell and spatially resolved
transcriptomic strategies to survey the astrocyte response to
stroke in male mice. Nat Commun. 15:15842024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Pan Y, Zhang C, Zhao Y, Wang H, Ma
H, Sun J, Zhang S, Yao J, Xie D and Zhang Y: Transcriptome analysis
reveals dynamic microglial-induced A1 astrocyte reactivity via
C3/C3aR/NF-κB signaling after ischemic stroke. Mol Neurobiol.
61:10246–10270. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bormann D, Knoflach M, Poreba E, Riedl CJ,
Testa G, Orset C, Levilly A, Cottereau A, Jauk P, Hametner S, et
al: Single-nucleus RNA sequencing reveals glial cell type-specific
responses to ischemic stroke in male rodents. Nat Commun.
15:62322024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Endo F, Kasai A, Soto JS, Yu X, Qu Z,
Hashimoto H, Gradinaru V, Kawaguchi R and Khakh BS: Molecular basis
of astrocyte diversity and morphology across the CNS in health and
disease. Science. 378:eadc90202022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Preman P, Alfonso-Triguero M, Alberdi E,
Verkhratsky A and Arranz AM: Astrocytes in Alzheimer's disease:
Pathological significance and molecular pathways. Cells.
10:5402021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Price BR, Johnson LA and Norris CM:
Reactive astrocytes: The nexus of pathological and clinical
hallmarks of Alzheimer's disease. Ageing Res Rev. 68:1013352021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai Z, Wan CQ and Liu Z: Astrocyte and
Alzheimer's disease. J Neurol. 264:2068–2074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim J, Yoo ID, Lim J and Moon JS:
Pathological phenotypes of astrocytes in Alzheimer's disease. Exp
Mol Med. 56:95–99. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Booth HDE, Hirst WD and Wade-Martins R:
The role of astrocyte dysfunction in Parkinson's disease
pathogenesis. Trends Neurosci. 40:358–370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang T, Sun Y and Dettmer U: Astrocytes in
Parkinson's disease: From role to possible intervention. Cells.
12:23362023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cohen J and Torres C: Astrocyte
senescence: Evidence and significance. Aging Cell. 18:e129372019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ponath G, Park C and Pitt D: The role of
astrocytes in multiple sclerosis. Front Immunol. 9:2172018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yi W, Schlüter D and Wang X: Astrocytes in
multiple sclerosis and experimental autoimmune encephalomyelitis:
Star-shaped cells illuminating the darkness of CNS autoimmunity.
Brain Behav Immun. 80:10–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aharoni R, Eilam R and Arnon R: Astrocytes
in multiple sclerosis-essential constituents with diverse
multifaceted functions. Int J Mol Sci. 22:59042021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu S, Lu J, Shao A, Zhang JH and Zhang J:
Glial cells: Role of the immune response in ischemic stroke. Front
Immunol. 11:2942020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Z and Chopp M: Astrocytes, therapeutic
targets for neuroprotection and neurorestoration in ischemic
stroke. Prog Neurobiol. 144:103–120. 2016. View Article : Google Scholar :
|
|
47
|
Alsbrook DL, Di Napoli M, Bhatia K, Biller
J, Andalib S, Hinduja A, Rodrigues R, Rodriguez M, Sabbagh SY,
Selim M, et al: Neuroinflammation in acute ischemic and hemorrhagic
stroke. Curr Neurol Neurosci Rep. 23:407–431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu W, Chen Z and Wen J: Flavonoids and
ischemic stroke-induced neuroinflammation: Focus on the glial
cells. Biomed Pharmacother. 170:1158472024. View Article : Google Scholar
|
|
49
|
Shen XY, Gao ZK, Han Y, Yuan M, Guo YS and
Bi X: Activation and role of astrocytes in ischemic stroke. Front
Cell Neurosci. 15:7559552021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu G, Wang X, Chen L, Lenahan C, Fu Z,
Fang Y and Yu W: Crosstalk between the oxidative stress and glia
cells after stroke: From mechanism to therapies. Front Immunol.
13:8524162022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Qin C, Huang J, Tang X, Liu C,
Huang K, Xu J, Guo G, Tong A and Zhou L: The role of astrocytes in
oxidative stress of central nervous system: A mixed blessing. Cell
Prolif. 53:e127812020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hertz L and Rothman DL: Glucose, lactate,
β-hydroxybutyrate, acetate, GABA, and succinate as substrates for
synthesis of glutamate and GABA in the glutamine-glutamate/GABA
cycle. Adv Neurobiol. 13:9–42. 2016. View Article : Google Scholar
|
|
53
|
Wang F, Xie X, Xing X and Sun X:
Excitatory synaptic transmission in ischemic stroke: A new outlet
for classical neuroprotective strategies. Int J Mol Sci.
23:93812022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Andersen JV, Markussen KH, Jakobsen E,
Schousboe A, Waagepetersen HS, Rosenberg PA and Aldana BI:
Glutamate metabolism and recycling at the excitatory synapse in
health and neurodegeneration. Neuropharmacology. 196:1087192021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Perea G, Navarrete M and Araque A:
Tripartite synapses: Astrocytes process and control synaptic
information. Trends Neurosci. 32:421–431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Patabendige A, Singh A, Jenkins S, Sen J
and Chen R: Astrocyte activation in neurovascular damage and repair
following ischaemic stroke. Int J Mol Sci. 22:42802021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu X, Nagai J and Khakh BS: Improved tools
to study astrocytes. Nat Rev Neurosci. 21:121–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mollinari C, Zhao J, Lupacchini L, Garaci
E, Merlo D and Pei G: Transdifferentiation: A new promise for
neurodegenerative diseases. Cell Death Dis. 9:8302018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Popa-Wagner A, Hermann D and Gresita A:
Genetic conversion of proliferative astroglia into neurons after
cerebral ischemia: A new therapeutic tool for the aged brain?
Geroscience. 41:363–368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jiang MQ, Yu SP, Wei ZZ, Zhong W, Cao W,
Gu X, Wu A, McCrary MR, Berglund K and Wei L: Conversion of
reactive astrocytes to induced neurons enhances neuronal repair and
functional recovery after ischemic stroke. Front Aging Neurosci.
13:6128562021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang H, Zheng Q, Guo T, Zhang S, Zheng S,
Wang R, Deng Q, Yang G, Zhang S, Tang L, et al: Metabolic
reprogramming in astrocytes results in neuronal dysfunction in
intellectual disability. Mol Psychiatry. 29:1569–1582. 2024.
View Article : Google Scholar
|
|
62
|
Perelroizen R, Philosof B, Budick-Harmelin
N, Chernobylsky T, Ron A, Katzir R, Shimon D, Tessler A, Adir O,
Gaoni-Yogev A, et al: Astrocyte immunometabolic regulation of the
tumour microenvironment drives glioblastoma pathogenicity. Brain.
145:3288–3307. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Giaume C, Naus CC, Sáez JC and Leybaert L:
Glial connexins and pannexins in the healthy and diseased brain.
Physiol Rev. 101:93–145. 2021. View Article : Google Scholar
|
|
64
|
Zhang YM, Qi YB, Gao YN, Chen WG, Zhou T,
Zang Y and Li J: Astrocyte metabolism and signaling pathways in the
CNS. Front Neurosci. 17:12174512023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Falkowska A, Gutowska I, Goschorska M,
Nowacki P, Chlubek D and Baranowska-Bosiacka I: Energy Metabolism
of the brain, including the cooperation between astrocytes and
neurons, especially in the context of glycogen metabolism. Int J
Mol Sci. 16:25959–25981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chandel NS: Glycolysis. Cold Spring Harb
Perspect Biol. 13:a0405352021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Takahashi S: Neuroprotective function of
high glycolytic activity in astrocytes: Common roles in stroke and
neurodegenerative diseases. Int J Mol Sci. 22:65682021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Madai S, Kilic P, Schmidt RM, Bas-Orth C,
Korff T, Büttner M, Klinke G, Poschet G, Marti HH and Kunze R:
Activation of the hypoxia-inducible factor pathway protects against
acute ischemic stroke by reprogramming central carbon metabolism.
Theranostics. 14:2856–2880. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Koepsell H: Glucose transporters in brain
in health and disease. Pflugers Arch. 472:1299–1343. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Veys K, Fan Z, Ghobrial M, Bouché A,
García-Caballero M, Vriens K, Conchinha NV, Seuwen A, Schlegel F,
Gorski T, et al: Role of the GLUT1 glucose transporter in postnatal
CNS angiogenesis and blood-brain barrier integrity. Circ Res.
127:466–482. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Alquisiras-Burgos I and Aguilera P:
Involvement of glucose transporter overexpression in the protection
or damage after ischemic stroke. Neural Regen Res. 17:783–784.
2022. View Article : Google Scholar
|
|
72
|
Kierans SJ and Taylor CT: Regulation of
glycolysis by the hypoxia-inducible factor (HIF): Implications for
cellular physiology. J Physiol. 599:23–37. 2021. View Article : Google Scholar
|
|
73
|
Xiong XY, Pan XR, Luo XX, Wang YF, Zhang
XX, Yang SH, Zhong ZQ, Liu C, Chen Q, Wang PF, et al:
Astrocyte-derived lactate aggravates brain injury of ischemic
stroke in mice by promoting the formation of protein lactylation.
Theranostics. 14:4297–4317. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
M Tóth O, Menyhárt Á, Frank R, Hantosi D,
Farkas E and Bari F: Tissue acidosis associated with ischemic
stroke to guide neuroprotective drug delivery. Biology (Basel).
9:4602020.PubMed/NCBI
|
|
75
|
Bonvento G and Bolaños JP:
Astrocyte-neuron metabolic cooperation shapes brain activity. Cell
Metab. 33:1546–1564. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Romano A, Koczwara JB, Gallelli CA,
Vergara D, Micioni Di Bonaventura MV, Gaetani S and Giudetti AM:
Fats for thoughts: An update on brain fatty acid metabolism. Int J
Biochem Cell Biol. 84:40–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ebert D, Haller RG and Walton ME: Energy
contribution of octanoate to intact rat brain metabolism measured
by 13C nuclear magnetic resonance spectroscopy. J Neurosci.
23:5928–5935. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Le Foll C and Levin BE: Fatty acid-induced
astrocyte ketone production and the control of food intake. Am J
Physiol Regul Integr Comp Physiol. 310:R1186–R1192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo Q, Kawahata I, Cheng A, Jia W, Wang H
and Fukunaga K: Fatty acid-binding proteins: Their roles in
ischemic stroke and potential as drug targets. Int J Mol Sci.
23:96482022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mallick K, Paul S and Banerjee S and
Banerjee S: Lipid droplets and neurodegeneration. Neuroscience.
549:13–23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lee JA, Hall B, Allsop J, Alqarni R and
Allen SP: Lipid metabolism in astrocytic structure and function.
Semin Cell Dev Biol. 112:123–136. 2021. View Article : Google Scholar
|
|
82
|
Wang X, Zhang L, Sun W, Pei LL, Tian M,
Liang J, Liu X, Zhang R, Fang H, Wu J, et al: Changes of
metabolites in acute ischemic stroke and its subtypes. Front
Neurosci. 14:5809292021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sayre NL, Sifuentes M, Holstein D, Cheng
SY, Zhu X and Lechleiter JD: Stimulation of astrocyte fatty acid
oxidation by thyroid hormone is protective against ischemic
stroke-induced damage. J Cereb Blood Flow Metab. 37:514–527. 2017.
View Article : Google Scholar :
|
|
84
|
Du W, Zhang L, Brett-Morris A, Aguila B,
Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, et
al: HIF drives lipid deposition and cancer in ccRCC via repression
of fatty acid metabolism. Nat Commun. 8:17692017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Andersen JV, Schousboe A and Verkhratsky
A: Astrocyte energy and neurotransmitter metabolism in Alzheimer's
disease: Integration of the glutamate/GABA-glutamine cycle. Prog
Neurobiol. 217:1023312022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hsu PC, Lan YJ, Chen CC, Lee LY, Chen WP,
Wang YC and Lee YH: Erinacine A attenuates glutamate transporter 1
downregulation and protects against ischemic brain injury. Life
Sci. 306:1208332022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shen Z, Xiang M, Chen C, Ding F, Wang Y,
Shang C, Xin L, Zhang Y and Cui X: Glutamate excitotoxicity:
Potential therapeutic target for ischemic stroke. Biomed
Pharmacother. 151:1131252022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mahmoud S, Gharagozloo M, Simard C and
Gris D: Astrocytes maintain glutamate homeostasis in the CNS by
controlling the balance between glutamate uptake and release.
Cells. 8:1842019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Andersen JV, Jakobsen E, Westi EW, Lie
MEK, Voss CM, Aldana BI, Schousboe A, Wellendorph P, Bak LK,
Pinborg LH and Waagepetersen HS: Extensive astrocyte metabolism of
γ-aminobutyric acid (GABA) sustains glutamine synthesis in the
mammalian cerebral cortex. Glia. 68:2601–2612. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kwak H, Koh W, Kim S, Song K, Shin JI, Lee
JM, Lee EH, Bae JY, Ha GE, Oh JE, et al: Astrocytes control sensory
acuity via tonic inhibition in the thalamus. Neuron.
108:691–706.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Qureshi T, Bjørkmo M, Nordengen K,
Gundersen V, Utheim TP, Watne LO, Storm-Mathisen J, Hassel B and
Chaudhry FA: Slc38a1 conveys astroglia-derived glutamine into
GABAergic interneurons for neurotransmitter GABA synthesis. Cells.
9:16862020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ceyzériat K, Abjean L, Carrillo-de Sauvage
MA, Ben Haim L and Escartin C: The complex STATes of astrocyte
reactivity: How are they controlled by the JAK-STAT3 pathway?
Neuroscience. 330:205–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rakers C, Schleif M, Blank N, Matušková H,
Ulas T, Händler K, Torres SV, Schumacher T, Tai K, Schultze JL, et
al: Stroke target identification guided by astrocyte transcriptome
analysis. Glia. 67:619–633. 2019. View Article : Google Scholar
|
|
94
|
Borbor M, Yin D, Brockmeier U, Wang C,
Doeckel M, Pillath-Eilers M, Kaltwasser B, Hermann DM and Dzyubenko
E: Neurotoxicity of ischemic astrocytes involves STAT3-mediated
metabolic switching and depends on glycogen usage. Glia.
71:1553–1569. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wu M, Wang L, Li F, Hu R, Ma J, Zhang K
and Cheng X: Resveratrol downregulates STAT3 expression and
astrocyte activation in primary astrocyte cultures of rat.
Neurochem Res. 45:455–464. 2020. View Article : Google Scholar
|
|
96
|
Koutsifeli P, Varma U, Daniels LJ,
Annandale M, Li X, Neale JPH, Hayes S, Weeks KL, James S, Delbridge
LMD and Mellor KM: Glycogen-autophagy: Molecular machinery and
cellular mechanisms of glycophagy. J Biol Chem. 298:1020932022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Heden TD, Chow LS, Hughey CC and Mashek
DG: Regulation and role of glycophagy in skeletal muscle energy
metabolism. Autophagy. 18:1078–1089. 2022. View Article : Google Scholar :
|
|
98
|
Mayeuf-Louchart A, Lancel S, Sebti Y,
Pourcet B, Loyens A, Delhaye S, Duhem C, Beauchamp J, Ferri L,
Thorel Q, et al: Glycogen dynamics drives lipid droplet biogenesis
during brown adipocyte differentiation. Cell Rep. 29:1410–1418.e6.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Guo H, Li Y, Wang S, Yang Y, Xu T, Zhao J,
Wang J, Zuo W, Wang P, Zhao G, et al: Dysfunction of astrocytic
glycophagy exacerbates reperfusion injury in ischemic stroke. Redox
Biol. 74:1032342024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: Guidelines for the early management of patients
with acute ischemic stroke: 2019 Update to the 2018 guidelines for
the early management of acute ischemic stroke: A guideline for
healthcare professionals from the american heart
association/american stroke association. Stroke. 50:e344–e418.
2019. View Article : Google Scholar
|
|
101
|
Guo H, Fan Z, Wang S, Ma L, Wang J, Yu D,
Zhang Z, Wu L, Peng Z, Liu W, et al: Astrocytic A1/A2 paradigm
participates in glycogen mobilization mediated neuroprotection on
reperfusion injury after ischemic stroke. J Neuroinflammation.
18:2302021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Geng J, Zhang Y, Li S, Li S, Wang J, Wang
H, Aa J and Wang G: Metabolomic profiling reveals that
reprogramming of cerebral glucose metabolism is involved in
ischemic preconditioning-induced neuroprotection in a rodent model
of ischemic stroke. J Proteome Res. 18:57–68. 2019.
|
|
103
|
Huang XX, Li L, Jiang RH, Yu JB, Sun YQ,
Shan J, Yang J, Ji J, Cheng SQ, Dong YF, et al: Lipidomic analysis
identifies long-chain acylcarnitine as a target for ischemic
stroke. J Adv Res. 61:133–149. 2024. View Article : Google Scholar :
|
|
104
|
Cao J, Dong L, Luo J, Zeng F, Hong Z, Liu
Y, Zhao Y, Xia Z, Zuo D, Xu L and Tao T: Supplemental N-3
polyunsaturated fatty acids limit A1-specific astrocyte
polarization via attenuating mitochondrial dysfunction in ischemic
stroke in mice. Oxid Med Cell Longev. 2021:55247052021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Neal M, Luo J, Harischandra DS, Gordon R,
Sarkar S, Jin H, Anantharam V, Désaubry L and Kanthasamy A and
Kanthasamy A: Prokineticin-2 promotes chemotaxis and alternative A2
reactivity of astrocytes. Glia. 66:2137–2157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pang QM, Zhang Q, Wu XC, Yang RL, Fu SP,
Fan ZH, Liu J, Yu LM, Peng JC and Zhang T: Mechanism of M2
macrophages modulating astrocyte polarization through the
TGF-β/PI3K/Akt pathway. Immunol Lett. 259:1–8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chistyakov DV, Gavrish GE, Goriainov SV,
Chistyakov VV, Astakhova AA, Azbukina NV and Sergeeva MG: Oxylipin
profiles as functional characteristics of acute inflammatory
responses in astrocytes pre-treated with IL-4, IL-10, or LPS. Int J
Mol Sci. 21:17802020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shiow LR, Favrais G, Schirmer L, Schang
AL, Cipriani S, Andres C, Wright JN, Nobuta H, Fleiss B, Gressens P
and Rowitch DH: Reactive astrocyte COX2-PGE2 production inhibits
oligodendrocyte maturation in neonatal white matter injury. Glia.
65:2024–2037. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao
J, Fu Q, Liu Y, Li H, Lou J, et al: Cottonseed oil alleviates
ischemic stroke injury by inhibiting the inflammatory activation of
microglia and astrocyte. J Neuroinflammation. 17:2702020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang L, Zhao G, Luo Z, Yu Z, Liu G, Su G,
Tang X, Yuan Z, Huang C, Sun HS, et al: AD16 attenuates
neuroinflammation induced by cerebral ischemia through
down-regulating astrocytes A1 polarization. Biomed Pharmacother.
178:1172092024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zou Y, Pei J, Wan C, Liu S, Hu B, Li Z and
Tang Z: Mechanism of scutellarin inhibition of astrocyte activation
to type A1 after ischemic stroke. J Stroke Cerebrovasc Dis.
33:1075342024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhou M, Zhang T, Zhang X, Zhang M, Gao S,
Zhang T, Li S, Cai X, Li J and Lin Y: Effect of tetrahedral
framework nucleic acids on neurological recovery via ameliorating
apoptosis and regulating the activation and polarization of
astrocytes in ischemic stroke. ACS Appl Mater Interfaces.
14:37478–37492. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu R, Yu Y, Ge Q, Feng R, Zhong G, Luo L,
Han Z, Wang T, Huang C, Xue J and Huang Z: Genistein-3′-sodium
sulfonate promotes brain functional rehabilitation in ischemic
stroke rats by regulating astrocytes polarization through NF-κB
signaling pathway. Chem Biol Interact. 400:1111592024. View Article : Google Scholar
|
|
114
|
Chen Z, Li T, Tang HB, Lu ZW, Chen ZY,
Zhao ZH, Yang XL, Zhao LL, Dang MJ, Li Y, et al: Edaravone
Dexborneol provides neuroprotective effect by inhibiting neurotoxic
activation of astrocytes through inhibiting NF-κB signaling in
cortical ischemia. Brain Res Bull. 218:1110972024. View Article : Google Scholar
|
|
115
|
Li MC, Li MZ, Lin ZY, Zhuang YM, Wang HY,
Jia JT, Lu Y, Wang ZJ, Zou HY and Zhao H: Buyang Huanwu Decoction
promotes neurovascular remodeling by modulating astrocyte and
microglia polarization in ischemic stroke rats. J Ethnopharmacol.
323:1176202024. View Article : Google Scholar
|
|
116
|
Xia X, Chen J, Ren H, Zhou C, Zhang Q,
Cheng H and Wang X: Gypenoside pretreatment alleviates the cerebral
ischemia injury via inhibiting the microglia-mediated
neuroinflammation. Mol Neurobiol. 61:1140–1156. 2024. View Article : Google Scholar
|

















