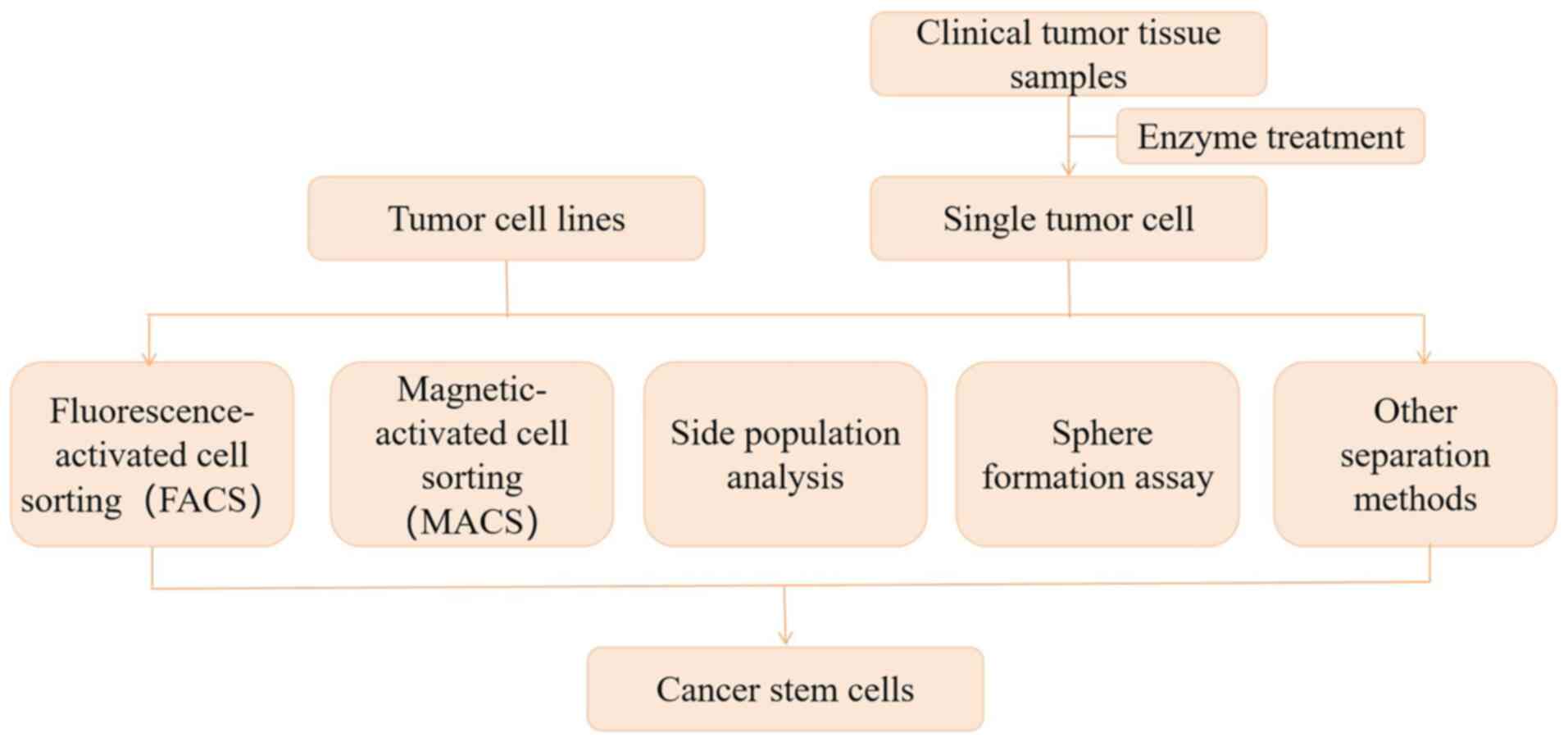
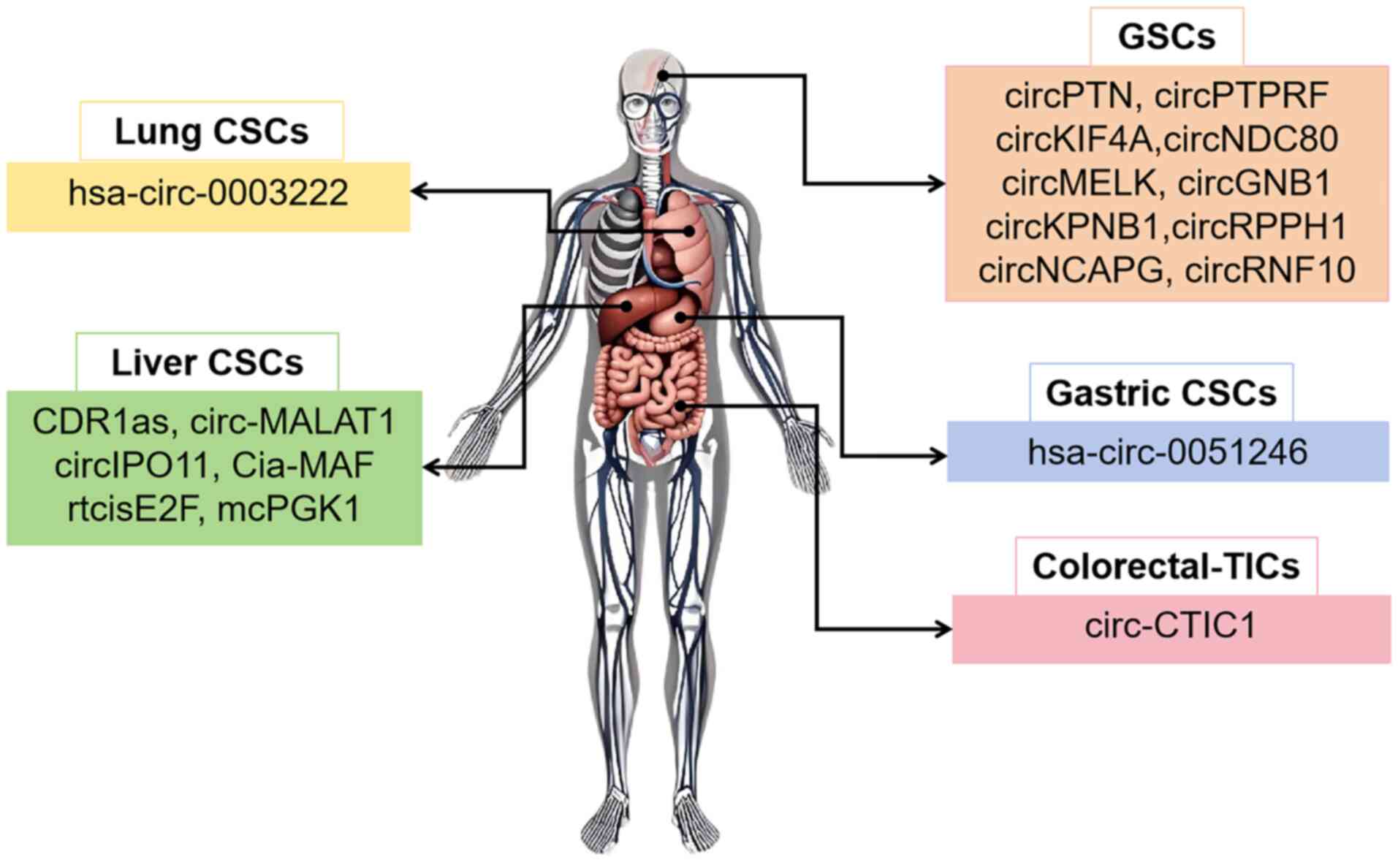
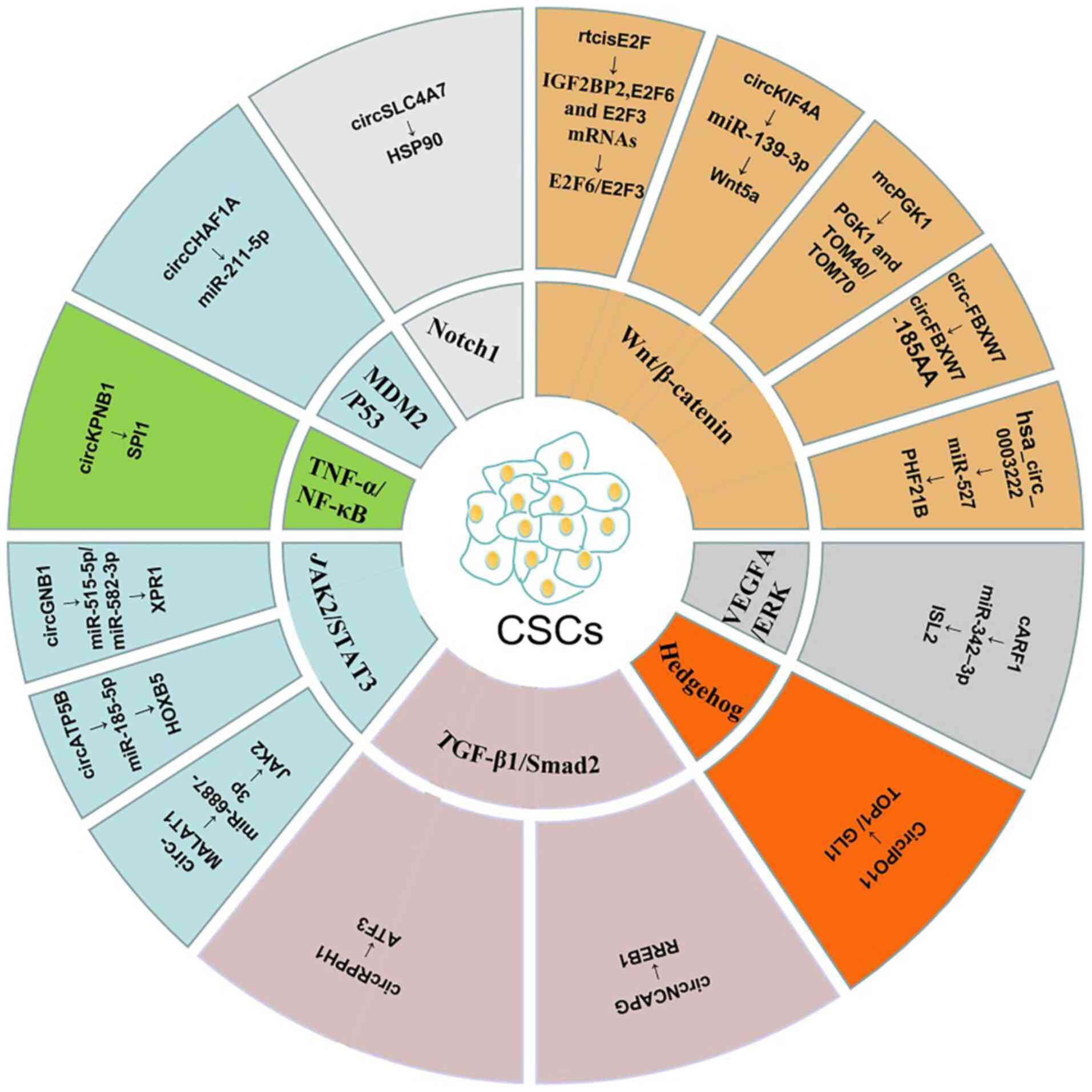
|
1
|
Sanger HL, Klotz G, Riesner D, Gross HJ and Kleinschmidt AK: Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu MT and Coca-Prados M: Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al: Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 19:141–157. 2013. View Article : Google Scholar :
|
|
5
|
Kelly S, Greenman C, Cook PR and Papantonis A: Exon skipping is correlated with exon circularization. J Mol Biol. 427:2414–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt CA, Giusto JD, Bao A, Hopper AK and Matera AG: Molecular determinants of metazoan tricRNA biogenesis. Nucleic Acids Res. 47:6452–6465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang Y, Li X, Wu Z, Yang D, Zhou Y, et al: Circular RNAs in cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol Cancer. 18:902019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeck WR and Sharpless NE: Detecting and characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki H and Tsukahara T: A view of pre-mRNA splicing from RNase R resistant RNAs. Int J Mol Sci. 15:9331–9342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glažar P, Papavasileiou P and Rajewsky N: circBase: A database for circular RNAs. RNA. 20:1666–1670. 2014. View Article : Google Scholar
|
|
12
|
Xia S, Feng J, Lei L, Hu J, Xia L, Wang J, Xiang Y, Liu L, Zhong S, Han L and He C: Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief Bioinform. 18:984–992. 2017.
|
|
13
|
Guo JU, Agarwal V, Guo H and Bartel DP: Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salzman J, Chen RE, Olsen MN, Wang PL and Brown PO: Cell-type specific features of circular RNA expression. PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Wu J, Wu P and Li Y: Emerging roles of circular RNAs in stem cells. Genes Dis. 10:1920–1936. 2023. View Article : Google Scholar :
|
|
16
|
Reya T, Morrison SJ, Clarke MF and Weissman IL: Stem cells, cancer, and cancer stem cells. Nature. 414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and Dick JE: A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takebe N, Harris PJ, Warren RQ and Ivy SP: Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
19
|
Pattabiraman DR and Weinberg RA: Tackling the cancer stem cells-what challenges do they pose? Nat Rev Drug Discov. 13:497–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Espinoza I and Miele L: Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 341:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cojoc M, Mäbert K, Muders MH and Dubrovska A: A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin Cancer Biol. 31:16–27. 2015. View Article : Google Scholar
|
|
23
|
Huntly BJ and Gilliland DG: Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 5:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaffer CL and Weinberg RA: A perspective on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steeg PS: Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 12:895–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao Q, Zhao Y, Renner A, Niess H, Seeliger H, Jauch KW and Bruns CJ: Cancer stem cells in pancreatic cancer. Cancers (Basel). 2:1629–1641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velasco-Velazquez MA, Popov VM, Lisanti MP and Pestell RG: The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol. 179:2–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verma P, Shukla N, Kumari S, Ansari MS, Gautam NK and Patel GK: Cancer stem cell in prostate cancer progression, metastasis and therapy resistance. Biochim Biophys Acta Rev Cancer. 1878:1888872023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mei X, Chen YS, Chen FR, Xi SY and Chen ZP: Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 19:1109–1118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, et al: CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 55:807–820. 2012. View Article : Google Scholar
|
|
31
|
Bussolati B, Bruno S, Grange C, Ferrando U and Camussi G: Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 22:3696–3705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raha D, Wilson TR, Peng J, Peterson D, Yue P, Evangelista M, Wilson C, Merchant M and Settleman J: The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 74:3579–3590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng E, Mitra A, Tripathi K, Finan MA, Scalici J, McClellan S, Madeira da Silva L, Reed E, Shevde LA, Palle K and Rocconi RP: ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS One. 9:e1071422014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, et al: Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 7:279–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Svensson A, Engervall P, Söderstrom T and Hansson M: PBSC harvests individually optimized by using pre-collection CD34(+) values and on-line flow cytometric analysis of the mononuclear cell enrichment. Cytotherapy. 1:165–174. 1999. View Article : Google Scholar
|
|
37
|
Miltenyi S, Müller W, Weichel W and Radbruch A: High gradient magnetic cell separation with MACS. Cytometry. 11:231–238. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonnet D and Dick JE: Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greve B, Kelsch R, Spaniol K, Eich HT and Götte M: Flow cytometry in cancer stem cell analysis and separation. Cytometry A. 81:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L and Borlak J: Advances in liver cancer stem cell isolation and their characterization. Stem Cell Rev Rep. 17:1215–1238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang DG, Jiang AG, Lu HY, Zhang LX and Gao XY: Isolation, cultivation and identification of human lung adenocarcinoma stem cells. Oncol Lett. 9:47–54. 2015. View Article : Google Scholar
|
|
42
|
Goodell MA, Brose K, Paradis G, Conner AS and Mulligan RC: Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boesch M, Zeimet AG, Reimer D, Schmidt S, Gastl G, Parson W, Spoeck F, Hatina J, Wolf D and Sopper S: The side population of ovarian cancer cells defines a heterogeneous compartment exhibiting stem cell characteristics. Oncotarget. 5:7027–7039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng L, Wu JB and Yi FM: Isolation and phenotypic characterization of cancer stem-like side population cells in colon cancer. Mol Med Rep. 12:3531–3536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li R, Wu X, Wei H and Tian S: Characterization of side population cells isolated from the gastric cancer cell line SGC-7901. Oncol Lett. 5:877–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ho MM, Ng AV, Lam S and Hung JY: Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ai J, Tan G, Li W, Liu H, Li T, Zhang G, Zhou Z and Gan Y: Exosomes loaded with circPARD3 promotes EBV-miR-BART4-induced stemness and cisplatin resistance in nasopharyngeal carcinoma side population cells through the miR-579-3p/SIRT1/SSRP1 axis. Cell Biol Toxicol. 39:537–556. 2023. View Article : Google Scholar
|
|
48
|
Nimmakayala RK, Leon F, Rachagani S, Rauth S, Nallasamy P, Marimuthu S, Shailendra GK, Chhonker YS, Chugh S, Chirravuri R, et al: Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma. Oncogene. 40:215–231. 2021. View Article : Google Scholar
|
|
49
|
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lobo NA, Shimono Y, Qian D and Clarke MF: The biology of cancer stem cells. Annu Rev Cell Dev Biol. 23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al: Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 8:323–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Waldron NN, Kaufman DS, Oh S, Inde Z, Hexum MK, Ohlfest JR and Vallera DA: Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther. 10:1829–1838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Son MJ, Ryu JS, Kim JY, Kwon Y, Chung KS, Mun SJ and Cho YS: Upregulation of mitochondrial NAD+ levels impairs the clonogenicity of SSEA1+ glioblastoma tumor-initiating cells. Exp Mol Med. 49:e3442017. View Article : Google Scholar
|
|
54
|
Xu J, Zhang G, Hu J, Li H, Zhao J, Zong S, Guo Z, Jiang Y and Jing Z: UPF1/circRPPH1/ATF3 feedback loop promotes the malignant phenotype and stemness of GSCs. Cell Death Dis. 13:6452022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen L, Kong R, Wu C, Wang S, Liu Z and Liu S, Li S, Chen T, Mao C and Liu S: Circ-MALAT1 functions as both an mRNA Translation brake and a microRNA sponge to promote self-renewal of hepatocellular cancer stem cells. Adv Sci (Weinh). 7:19009492020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L and Ambudkar SV: Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 102:1637–1652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma L, Lai D, Liu T, Cheng W and Guo L: Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin (Shanghai). 42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nicot C: RNA-seq reveals novel CircRNAs involved in breast cancer progression and patient therapy response. Mol Cancer. 19:762020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu Y, Liu X, Cheng W, Wei YQ, Peng Y and Li W: RNA-Seq profiling of circular RNA in human lung adenocarcinoma and squamous cell carcinoma. Mol Cancer. 18:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Szabo L and Salzman J: Detecting circular RNAs: Bioinformatic and experimental challenges. Nat Rev Genet. 17:679–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li S, Teng S, Xu J, Su G, Zhang Y, Zhao J, Zhang S, Wang H, Qin W, Lu ZJ, et al: Microarray is an efficient tool for circRNA profiling. Brief Bioinform. 20:1420–1433. 2019. View Article : Google Scholar
|
|
62
|
Shi Y and Shang J: Circular RNA Expression Profiling by Microarray-A technical and practical perspective. Biomolecules. 13:6792023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu W, Zhao F and Zhang J: circAtlas 3.0: A gateway to 3 million curated vertebrate circular RNAs based on a standardized nomenclature scheme. Nucleic Acids Res. 52:D52–D60. 2024. View Article : Google Scholar
|
|
64
|
Dong R, Ma XK, Li GW and Yang L: CIRCpedia v2: An updated database for comprehensive circular RNA annotation and expression comparison. Genomics Proteomics Bioinformatics. 16:226–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, et al: The landscape of circular RNA in cancer. Cell. 176:869–881.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen X, Han P, Zhou T, Guo X, Song X and Li Y: circRNADb: A comprehensive database for human circular RNAs with protein-coding annotations. Sci Rep. 6:349852016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
John B, Enright AJ, Aravin A, Tuschl T, Sander C and Marks DS: Human MicroRNA targets. PLoS Biol. 2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar :
|
|
70
|
Xia S, Feng J, Chen K, Ma Y, Gong J, Cai F, Jin Y, Gao Y, Xia L, Chang H, et al: CSCD: A database for cancer-specific circular RNAs. Nucleic Acids Res. 46:D925–D929. 2018. View Article : Google Scholar :
|
|
71
|
Vromman M, Vandesompele J and Volders PJ: Closing the circle: Current state and perspectives of circular RNA databases. Brief Bioinform. 22:288–297. 2021. View Article : Google Scholar :
|
|
72
|
Gao Y, Wang J and Zhao F: CIRI: An efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 16:42015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ma XK, Wang MR, Liu CX, Dong R, Carmichael GG, Chen LL and Yang L: CIRCexplorer3: A CLEAR pipeline for direct comparison of circular and linear RNA expression. Genomics Proteomics Bioinformatics. 17:511–521. 2019. View Article : Google Scholar
|
|
74
|
Wang K, Singh D, Zeng Z, Coleman SJ, Huang Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, et al: MapSplice: Accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 38:e1782010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang J, Chen S, Yang J and Zhao F: Accurate quantification of circular RNAs identifies extensive circular isoform switching events. Nat Commun. 11:902020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chuang TJ, Wu CS, Chen CY, Hung LY, Chiang TW and Yang MY: NCLscan: Accurate identification of non-co-linear transcripts (fusion, trans-splicing and circular RNA) with a good balance between sensitivity and precision. Nucleic Acids Res. 44:e292016. View Article : Google Scholar :
|
|
77
|
Cheng J, Metge F and Dieterich C: Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics. 32:1094–1096. 2016. View Article : Google Scholar
|
|
78
|
Hansen TB, Venø MT, Damgaard CK and Kjems J: Comparison of circular RNA prediction tools. Nucleic Acids Res. 44:e582016. View Article : Google Scholar :
|
|
79
|
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin XM, Yang S, Zhao Q, Wu T, Li ZX, et al: Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 9:3526–3540. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al: EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yan N, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al: Circular RNA profile indicates circular RNA VRK1 is negatively related with breast cancer stem cells. Oncotarget. 8:95704–95718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rengganaten V, Huang CJ, Tsai PH, Wang ML, Yang YP, Lan YT, Fang WL, Soo S, Ong HT, Cheong SK, et al: Mapping a circular RNA-microRNA-mRNA-signaling regulatory axis that modulates stemness properties of cancer stem cell populations in colorectal cancer spheroid cells. Int J Mol Sci. 21:78642020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sohn EJ: Differentially expression and function of circular RNAs in ovarian cancer stem cells. J Ovarian Res. 15:972022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tao T, Yuan S, Liu J, Shi D, Peng M, Li C and Wu S: Cancer stem cell-specific expression profiles reveal emerging bladder cancer biomarkers and identify circRNA_103809 as an important regulator in bladder cancer. Aging (Albany NY). 12:3354–3370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function as efficient microRNA sponges. Nature. 495:384–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P and Yang BB: Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zeng Y, Du WW, Wu Y, Yang Z, Awan FM, Li X, Yang W, Zhang C, Yang Q, Yee A, et al: A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 7:3842–3855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen Q, Wang H, Li Z, Li F, Liang L, Zou Y, Shen H, Li J, Xia Y, Cheng Z, et al: Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol. 76:135–147. 2022. View Article : Google Scholar
|
|
89
|
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang L, Long H, Zheng Q, Bo X, Xiao X and Li B: Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol Cancer. 18:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wen SY, Qadir J and Yang BB: Circular RNA translation: Novel protein isoforms and clinical significance. Trends Mol Med. 28:405–420. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lapointe S, Perry A and Butowski NA: Primary brain tumours in adults. Lancet. 392:432–446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G and Elicci G: CD133 is essential for glioblastoma stem cell maintenance. Stem Cells. 31:857–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang J, Cai H, Sun L, Zhan P, Chen M, Zhang F, Ran Y and Wan J: LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 37:2252018. View Article : Google Scholar
|
|
95
|
Sun J, Li B, Shu C, Ma Q and Wang J: Functions and clinical significance of circular RNAs in glioma. Mol Cancer. 19:342020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen J, Chen T, Zhu Y, Li Y, Zhang Y, Wang Y, Li X, Xie X, Wang J, Huang M, et al: circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res. 38:3982019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhou J, Wang C, Liu Y, Cui D, Wang Z, Jiang Y and Gao L: Circular RNA circPTPRF promotes the progression of GBM via sponging miR-1208 to up-regulate YY1. Cancer Cell Int. 22:3592022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Luo K, Liu A, Wu H, Liu Q, Dai J, Liu Y and Wang Z: CircKIF4A promotes glioma growth and temozolomide resistance by accelerating glycolysis. Cell Death Dis. 13:7402022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huo LW, Wang YF, Bai XB, Zheng HL and Wang MD: circKIF4A promotes tumorogenesis of glioma by targeting miR-139-3p to activate Wnt5a signaling. Mol Med. 26:292020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jiang Y, Wang Z, Ying C, Hu J, Zeng T and Gao L: FMR1/circCHAF1A/miR-211-5p/HOXC8 feedback loop regulates proliferation and tumorigenesis via MDM2-dependent p53 signaling in GSCs. Oncogene. 40:4094–4110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhao J, Jiang Y, Zhang H, Zhou J, Chen L, Li H, Xu J, Zhang G and Jing Z: The SRSF1/circATP5B/miR-185-5p/HOXB5 feedback loop regulates the proliferation of glioma stem cells via the IL6-mediated JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res. 40:1342021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Y, Wang B, Zhou F, Lv K, Xu X and Cao W: CircNDC80 promotes glioblastoma multiforme tumorigenesis via the miR-139-5p/ECE1 pathway. J Transl Med. 21:222023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hou D, Wang Z, Li H, Liu J, Liu Y, Jiang Y and Lou M: Circular RNA circASPM promotes the progression of glioblastoma by acting as a competing endogenous RNA to regulate miR-130b-3p/E2F1 axis. J Cancer. 13:1664–1678. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jiang Y, Zhao J, Liu Y, Hu J, Gao L, Wang H and Cui D: CircKPNB1 mediates a positive feedback loop and promotes the malignant phenotypes of GSCs via TNF-α/NF-kappaB signaling. Cell Death Dis. 13:6972022. View Article : Google Scholar
|
|
105
|
Xu G, Qu J, Zhang M and Wang Q: C-Fos-activated circRPPH1 contributes to glioma stemness. Clin Transl Oncol. 25:1277–1286. 2023. View Article : Google Scholar
|
|
106
|
Li H, Jiang Y, Hu J, Xu J, Chen L, Zhang G, Zhao J, Zong S, Guo Z, Li X, et al: The U2AF65/circNCAPG/RREB1 feedback loop promotes malignant phenotypes of glioma stem cells through activating the TGF-beta pathway. Cell Death Dis. 14:232023. View Article : Google Scholar
|
|
107
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi L: Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ahir BK, Engelhard HH and Lakka SS: Tumor development and angiogenesis in adult brain tumor: Glioblastoma. Mol Neurobiol. 57:2461–2478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tischer E, Gospodarowicz D, Mitchell R, Silva M, Schilling J, Lau K, Crisp T, Fiddes JC and Abraham JA: Vascular endothelial growth factor: A new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 165:1198–206. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kurihara T, Westenskow PD and Friedlander M: Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv Exp Med Biol. 801:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Segawa H, Miyashita T, Hirate Y, Higashijima S, Chino N, Uyemura K, Kikuchi Y and Okamoto H: Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish. Neuron. 30:423–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jiang Y, Zhou J, Zhao J, Zhang H, Li L, Li H, Chen L, Hu J, Zheng W and Jing Z: The U2AF2/circRNA ARF1/miR-342-3p/ISL2 feedback loop regulates angiogenesis in glioma stem cells. J Exp Clin Cancer Res. 39:1822020. View Article : Google Scholar
|
|
113
|
Jiang X, Stockwell BR and Conrad M: Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jiang Y, Zhao J, Li R, Liu Y, Zhou L, Wang C, Lv C, Gao L and Cui D: CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J Exp Clin Cancer Res. 41:3072022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang C, Zhang M, Liu Y, Cui D, Gao L and Jiang Y: CircRNF10 triggers a positive feedback loop to facilitate progression of glioblastoma via redeploying the ferroptosis defense in GSCs. J Exp Clin Cancer Res. 42:2422023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Marquardt JU, Andersen JB and Thorgeirsson SS: Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 15:653–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sun JH, Luo Q, Liu LL and Song GB: Liver cancer stem cell markers: Progression and therapeutic implications. World J Gastroenterol. 22:3547–3557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Weinberg AG and Finegold MJ: Primary hepatic tumors of childhood. Hum Pathol. 14:512–537. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fiegel HC, Glüer S, Roth B, Rischewski J, von Schweinitz D, Ure B, Lambrecht W and Kluth D: Stem-like cells in human hepatoblastoma. J Histochem Cytochem. 52:1495–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen J, Yang J, Fei X, Wang X and Wang K: CircRNA ciRS-7: A novel oncogene in multiple cancers. Int J Biol Sci. 17:379–389. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen L, Shi J, Wu Y, Qiu R, Zeng L, Lou L, Su J, Liao M and Deng X: CircRNA CDR1as promotes hepatoblastoma proliferation and stemness by acting as a miR-7-5p sponge to upregulate KLF4 expression. Aging (Albany NY). 12:19233–19253. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Forner A, Reig M and Bruix J: Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, Zhang P, Xiong Z, He C, Huang Z, et al: ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 11:422–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jiang X, Xing L, Chen Y, Qin R, Song S, Lu Y, Xie S, Wang L, Pu H, Gui X, et al: CircMEG3 inhibits telomerase activity by reducing Cbf5 in human liver cancer stem cells. Mol Ther Nucleic Acids. 23:310–323. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ventham NT, Kennedy NA, Nimmo ER and Satsangi J: Beyond gene discovery in inflammatory bowel disease: The emerging role of epigenetics. Gastroenterology. 145:293–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G, Li L, Dai N, Si J, Tao Q, et al: Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 53:843–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Niewiadomski P, Niedziółka SM, Markiewicz Ł, Uśpieński T, Baran B and Chojnowska K: Gli proteins: Regulation in development and cancer. Cells. 8:1472019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gu Y, Wang Y, He L, Zhang J, Zhu X, Liu N, Wang J, Lu T, He L, Tian Y and Fan Z: Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol Cancer. 20:132021. View Article : Google Scholar
|
|
129
|
Chen Z, Lu T, Huang L, Wang Z, Yan Z, Guan Y, Hu W, Fan Z and Zhu P: Circular RNA cia-MAF drives self-renewal and metastasis of liver tumor-initiating cells via transcription factor MAFF. J Clin Invest. 131:e1480202021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Vidal AF: Read-through circular RNAs reveal the plasticity of RNA processing mechanisms in human cells. RNA Biol. 17:1823–1826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen Z, Huang L, Wang K, Zhang L, Zhong X, Yan Z, Liu B and Zhu P: rtcisE2F promotes the self-renewal and metastasis of liver tumor-initiating cells via N6-methyladenosine-dependent E2F3/E2F6 mRNA stability. Sci China Life Sci. 65:1840–1854. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liu X, Wang X, Li J, Hu S, Deng Y, Yin H, Bao X, Zhang QC, Wang G, Wang B, et al: Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci China Life Sci. 63:1429–1449. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu X, Yang Y and Shan G: Identification and detection of mecciRNAs. Methods. 196:147–152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen Z, He Q, Lu T, Wu J, Shi G, He L, Zong H, Liu B and Zhu P: mcPGK1-dependent mitochondrial import of PGK1 promotes metabolic reprogramming and self-renewal of liver TICs. Nat Commun. 14:11212023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Siegel RL, Miller KD, Wagle NS and Jemal A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A and Siegel RL: Breast cancer statistics, 2022. CA Cancer J Clin. 72:524–541. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang L, Chen W, Liu S and Chen C: Targeting breast cancer stem cells. Int J Biol Sci. 19:552–570. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG: Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li W, Yang X, Shi C and Zhou Z: Hsa_circ_002178 promotes the growth and migration of breast cancer cells and maintains cancer Stem-like cell properties through regulating miR-1258/KDM7A Axis. Cell Transplant. 29:9636897209601742020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chen W, Cen S, Zhou X, Yang T, Wu K, Zou L, Luo J, Li C, Lv D and Mao X: Circular RNA CircNOLC1, upregulated by NF-KappaB, promotes the progression of prostate cancer via miR-647/PAQR4 axis. Front Cell Dev Biol. 8:6247642020. View Article : Google Scholar
|
|
142
|
Chen S, Wu W, Li QH, Xie BM, Shen F, Du YP, Zong ZH, Wang LL, Wei XQ and Zhao Y: Circ-NOLC1 promotes epithelial ovarian cancer tumorigenesis and progression by binding ESRP1 and modulating CDK1 and RhoA expression. Cell Death Discov. 7:222021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Liu YP, Heng JY, Zhao XY and Li EY: The inhibition of circular RNA circNOLC1 by propofol/STAT3 attenuates breast cancer stem cells function via miR-365a-3p/STAT3 signaling. J Transl Med. 19:4672021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z and Yang BB: Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017. View Article : Google Scholar :
|
|
145
|
Chen D, Zeng S, Qiu H, Yang M, Lin X, Lv X, Li P, Weng S, Kou S, Luo K, et al: Circ-FOXO3 inhibits triple-negative breast cancer growth and metastasis via regulating WHSC1-H3K36me2-Zeb2 axis. Cell Signal. 117:1110792024. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kamalabadi-Farahani M, Atashi A and Eslami MM: Downregulation of circ-Foxo3 in breast cancer stem-like cells. BMC Res Notes. 16:1322023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, et al: Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 16:45–55. 2010. View Article : Google Scholar
|
|
148
|
Kamalabadi-Farahani M, Karimi R and Atashi A: High percentage of Cancer Stem cells in metastatic locations: Upregulation of cicBIRC6 in highly metastatic breast Cancer Subline. Mol Biol Rep. 50:1303–1309. 2023. View Article : Google Scholar
|
|
149
|
Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De Maria R: Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar
|
|
150
|
Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al: Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA and Katz RL: Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chen YW, Du QR, He YJ, Chen WS, Jiang WY, Gui Q, Xu CC, Wang W and Cheng HY: Circ_0044516 regulates miR-136/MAT2A pathway to facilitate lung cancer development. J Immunol Res. 2021:55108692021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li C, Zhang J, Yang X, Hu C, Chu T, Zhong R, Shen Y, Hu F, Pan F, Xu J, et al: hsa_circ_0003222 accelerates stemness and progression of non-small cell lung cancer by sponging miR-527. Cell Death Dis. 12:8072021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
MacDonagh L, Gallagher MF, Ffrench B, Gasch C, Breen E, Gray SG, Nicholson S, Leonard N, Ryan R, Young V, et al: Targeting the cancer stem cell marker, aldehyde dehydrogenase 1, to circumvent cisplatin resistance in NSCLC. Oncotarget. 8:72544–72563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
MacDonagh L, Gray SG, Breen E, Cuffe S, Finn SP, O'Byrne KJ and Barr MP: BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 428:117–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li K, Peng ZY, Wang R, Li X, Du N, Liu DP, Zhang J, Zhang YF, Ma L, Sun Y, et al: Enhancement of TKI sensitivity in lung adenocarcinoma through m6A-dependent translational repression of Wnt signaling by circ-FBXW7. Mol Cancer. 22:1032023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang L, Liu X, Ren Y, Zhang J, Chen J, Zhou W, Guo W, Wang X, Chen H, Li M, et al: Cisplatin-enriching cancer stem cells confer multidrug resistance in non-small cell lung cancer via enhancing TRIB1/HDAC activity. Cell Death Dis. 8:e27462017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhao Y, Zheng R, Chen J and Ning D: CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes to cisplatin resistance in non-small cell lung cancer (NSCLC). Cancer Cell Int. 20:2892020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Beck B and Blanpain C: Unravelling cancer stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xu G, Shen J, Ou Yang X, Sasahara M and Su X: Cancer stem cells: The 'heartbeat' of gastric cancer. J Gastroenterol. 48:781–797. 2013. View Article : Google Scholar
|
|
162
|
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC: Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang C, Li C, He F, Cai Y and Yang H: Identification of CD44+CD24+ gastric cancer stem cells. J Cancer Res Clin Oncol. 137:1679–1686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Xing Y, Chen H, Guo Z and Zhou X: Circular RNA circ0007360 attenuates gastric cancer progression by altering the miR-762/IRF7 Axis. Front Cell Dev Biol. 10:7890732022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Chen QY, Xu KX, Huang XB, Fan DH, Chen YJ, Li YF, Huang Q, Liu ZY, Zheng HL, Huang ZN, et al: Circ-0075305 hinders gastric cancer stem cells by indirectly disrupting TCF4-beta-catenin complex and downregulation of SOX9. Commun Biol. 7:5452024. View Article : Google Scholar
|
|
166
|
Xia Y, Lv J, Jiang T, Li B, Li Y, He Z, Xuan Z, Sun G, Wang S, Li Z, et al: CircFAM73A promotes the cancer stem cell-like properties of gastric cancer through the miR-490-3p/HMGA2 positive feedback loop and HNRNPK-mediated β-catenin stabilization. J Exp Clin Cancer Res. 40:1032021. View Article : Google Scholar
|
|
167
|
Deng M, Xu Y, Yao Y, Wang Y, Yan Q, Cheng M and Liu Y: Circular RNA hsa_circ_0051246 acts as a microRNA-375 sponge to promote the progression of gastric cancer stem cells via YAP1. PeerJ. 11:e165232023. View Article : Google Scholar
|
|
168
|
Hui Y, Wenguang Y, Wei S, Haoran W, Shanglei N and Ju L: circSLC4A7 accelerates stemness and progression of gastric cancer by interacting with HSP90 to activate NOTCH1 signaling pathway. Cell Death Dis. 14:4522023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Biller LH and Schrag D: Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA. 325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y and Chen Q: CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li Z: CD133: A stem cell biomarker and beyond. Exp Hematol Oncol. 2:172013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T and Sato T: Visualization and targeting of LGR5+ human colon cancer stem cells. Nature. 545:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Leng Z, Xia Q, Chen J, Li Y, Xu J, Zhao E, Zheng H, Ai W and Dong J: Lgr5+CD44+EpCAM+ strictly defines cancer stem cells in human colorectal cancer. Cell Physiol Biochem. 46:860–872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zahran AM, Rayan A, Fakhry H, Attia AM, Ashmawy AM, Soliman A, Elkady A and Hetta HF: Pretreatment detection of circulating and tissue CD133+ CD44+ cancer stem cells as a prognostic factor affecting the outcomes in Egyptian patients with colorectal cancer. Cancer Manag Res. 11:1237–1248. 2019. View Article : Google Scholar :
|
|
175
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 18:42019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ma X, Wang C, Chen J, Wei D, Yu F and Sun J: circAGFG1 sponges miR-28-5p to promote non-small-cell lung cancer progression through modulating HIF-1α level. Open Med (Wars). 16:703–717. 2021. View Article : Google Scholar
|
|
177
|
Luo J, Zhong H, Guo M, Xiao P, Cao R, Zhao M and Jing Y: CircAGFG1 promotes ovarian cancer progression through the miR-409-3 p/ZEB1 axis. Technol Cancer Res Treat. 23:153303382412524232024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li T, Xing G, Lu L, Kong X and Guo J: CircAGFG1 promotes osteosarcoma progression and stemness by competing with miR-302a-3p to upregulate the expression of LATS2. Evid Based Complement Alternat Med. 2022:63707662022.PubMed/NCBI
|
|
179
|
Zhang L, Dong X, Yan B, Yu W and Shan L: CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 11:5422020. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sun J, Liu J, Zhu Q, Xu F, Kang L and Shi X: Hsa_circ_0001806 Acts as a ceRNA to facilitate the stemness of colorectal cancer cells by increasing COL1A1. Onco Targets Ther. 13:6315–6327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Rengganaten V, Huang CJ, Wang ML, Chien Y, Tsai PH, Lan YT, Ong HT, Chiou SH and Choo KB: Circular RNA ZNF800 (hsa_circ_0082096) regulates cancer stem cell properties and tumor growth in colorectal cancer. BMC Cancer. 23:10882023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chen Z, He L, Zhao L, Zhang G, Wang Z, Zhu P and Liu B: circREEP3 drives colorectal cancer progression via activation of FKBP10 transcription and restriction of antitumor immunity. Adv Sci (Weinh). 9:e21051602022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Meyer KD and Jaffrey SR: Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol. 33:319–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhang L, Hou C, Chen C, Guo Y, Yuan W, Yin D, Liu J and Sun Z: The role of N6-methyladenosine (m6A) modification in the regulation of circRNAs. Mol Cancer. 19:1052020. View Article : Google Scholar
|
|
185
|
Zeng W, Zhu JF, Guo J, Huang GJ, Ai LS, Zeng Y and Liao WJ: m6A-modified circFNDC3B inhibits colorectal cancer stemness and metastasis via RNF41-dependent ASB6 degradation. Cell Death Dis. 13:10082022. View Article : Google Scholar
|
|
186
|
Zhan W, Liao X, Wang Y, Li L, Li J, Chen Z, Tian T and He J: circCTIC1 promotes the self-renewal of colon TICs through BPTF-dependent c-Myc expression. Carcinogenesis. 40:560–568. 2019. View Article : Google Scholar
|
|
187
|
Chen Z, Wu J, Liu B, Zhang G, Wang Z, Zhang L, Wang K, Fan Z and Zhu P: Identification of cis-HOX-HOXC10 axis as a therapeutic target for colorectal tumor-initiating cells without APC mutations. Cell Rep. 36:1094312021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Chen Q, Yin Q, Mao Y, Zhang Z, Wu S, Cheng Z, Chen X, Xu H, Jin S, Jiang H and Yang C: Hsa_circ_0068307 mediates bladder cancer stem cell-like properties via miR-147/c-Myc axis regulation. Cancer Cell Int. 20:1512020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Fan L, Yang J, Shen C, Wu Z and Hu H: Circ_0030586 inhibits cell proliferation and stemness in bladder cancer by inactivating the ERK signaling via miR-665/NR4A3 axis. Acta Histochem. 123:1517452021. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Tian Y, Gao P, Dai D, Chen L, Chu X and Mei X: Circular RNA circSETD3 hampers cell growth, migration, and stem cell properties in bladder cancer through sponging miR-641 to upregulate PTEN. Cell Cycle. 20:1589–1602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Gu C, Zhou N, Wang Z, Li G, Kou Y, Yu S, Feng Y, Chen L, Yang J and Tian F: circGprc5a promoted bladder oncogenesis and metastasis through Gprc5a-Targeting peptide. Mol Ther Nucleic Acids. 13:633–641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Yang J, Yang L, Li S and Hu N: HGF/c-Met promote renal carcinoma cancer stem cells enrichment through upregulation of Cir-CCDC66. Technol Cancer Res Treat. 19:15330338199011142020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang Y, Yang Z, Gu J, Zhang Y, Wang X, Teng Z, Wang D, Gao L, Li W, Yeh S and Han Z: Estrogen receptor beta increases clear cell renal cell carcinoma stem cell phenotype via altering the circPHACTR4/miR-34b-5p/c-Myc signaling. FASEB J. 36:e221632022.PubMed/NCBI
|
|
195
|
Lin G, Fei Y and Zhang Y: Zhang, Hsa-circ_0003420 induces apoptosis in acute myeloid leukemia stem cells and impairs stem cell properties. Immunopharmacol Immunotoxicol. 43:622–631. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Shi P, Li Y and Guo Q: Circular RNA circPIP5K1A contributes to cancer stemness of osteosarcoma by miR-515-5p/YAP axis. J Transl Med. 19:4642021. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Schulenburg A, Blatt K, Cerny-Reiterer S, Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC and Valent P: Cancer stem cells in basic science and in translational oncology: Can we translate into clinical application? J Hematol Oncol. 8:162015. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 8:806–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Nassar D and Blanpain C: Cancer stem cells: Basic concepts and therapeutic implications. Annu Rev Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhou F, Wang B, Wang H, Hu L, Zhang J, Yu T, Xu X, Tian W, Zhao C, Zhu H and Liu N: circMELK promotes glioblastoma multiforme cell tumorigenesis through the miR-593/EphB2 axis. Mol Ther Nucleic Acids. 25:25–36. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Hu J, Zhang G, Wang Y, Xu K, Chen L, Luo G, Xu J, Li H, Pei D, Zhao X, et al: CircGNB1 facilitates the malignant phenotype of GSCs by regulating miR-515-5p/miR-582-3p-XPR1 axis. Cancer Cell Int. 23:1322023. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhang D, Yang L, Liu X, Gao J, Liu T, Yan Q and Yang X: Hypoxia modulates stem cell properties and induces EMT through N-glycosylation of EpCAM in breast cancer cells. J Cell Physiol. 235:3626–3633. 2020. View Article : Google Scholar
|
|
203
|
Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J Clin Pathol. 64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ and Visvader JE: Generation of a functional mammary gland from a single stem cell. Nature. 439:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N and Liu N: CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 32:544–553. 2013. View Article : Google Scholar
|
|
206
|
Fillmore CM and Kuperwasser C: Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA and Weinberg RA: A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 16:1105–1117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Nishikawa S, Nishikawa S, Konno M, Hamabe A, Hasegawa S, Kano Y, Ohta K, Fukusumi T, Sakai D, Kudo T, et al: Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int J Oncol. 42:1437–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Lau WM, Teng E, Chong HS, Lopez KA, Tay AY, Salto-Tellez M, Shabbir A, So JB and Chan SL: CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 74:2630–2641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Zhu Y, Yu J, Wang S, Lu R, Wu J and Jiang B: Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep. 32:2437–2444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Fujikuni N, Yamamoto H, Tanabe K, Naito Y, Sakamoto N, Tanaka Y, Yanagihara K, Oue N, Yasui W and Ohdan H: Hypoxia-mediated CD24 expression is correlated with gastric cancer aggressiveness by promoting cell migration and invasion. Cancer Sci. 105:1411–1420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Xue Z, Yan H, Li J, Liang S, Cai X, Chen X, Wu Q, Gao L, Wu K, Nie Y and Fan D: Identification of cancer stem cells in vincristine preconditioned SGC7901 gastric cancer cell line. J Cell Biochem. 113:302–312. 2012. View Article : Google Scholar
|
|
213
|
Wenqi D, Li W, Shanshan C, Bei C, Yafei Z, Feihu B, Jie L and Daiming F: EpCAM is overexpressed in gastric cancer and its downregulation suppresses proliferation of gastric cancer. J Cancer Res Clin Oncol. 135:1277–1285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Wang C, Gao Y, Liang W, Lu Y, Zhang K, Wu D, Zhuang Z, Li K, Qiao Z, Xi H and Chen L: Rspondin-1 contributes to the progression and stemness of gastric cancer by LGR5. Biochem Biophys Res Commun. 627:91–96. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Zhang SS, Huang ZW, Li LX, Fu JJ and Xiao B: Identification of CD200+ colorectal cancer stem cells and their gene expression profile. Oncol Rep. 36:2252–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Tseng JY, Yang CY, Yang SH, Lin JK, Lin CH and Jiang JK: Circulating CD133(+)/ESA(+) cells in colorectal cancer patients. J Surg Res. 199:362–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Ren F, Sheng WQ and Du X: CD133: A cancer stem cells marker, is used in colorectal cancers. World J Gastroenterol. 19:2603–2611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al: Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Zhou F, Mu YD, Liang J, Liu ZX, Zhou D, Ning WL, Li YZ, Ding D and Zhang JF: Aldehyde dehydrogenase 1: A specific cancer stem cell marker for human colorectal carcinoma. Mol Med Rep. 11:3894–3899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Peng H, Ye T, Deng L, Yang X, Li Q, Tong J and Guo J: Activin and hepatocyte growth factor promotes colorectal cancer stemness and metastasis through FOXM1/SOX2/CXCR4 signaling. Gut Liver. 18:476–488. 2024. View Article : Google Scholar :
|
|
221
|
Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, Wucherpfennig K, Michor F, Clevers H and Shivdasani RA: Ascl2-Dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell. 26:377–390.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Seyfrid M, Maich WT, Shaikh VM, Tatari N, Upreti D, Piyasena D, Subapanditha M, Savage N, McKenna D, Mikolajewicz N, et al: CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J Immunother Cancer. 10:e0032892022. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wang A, Qu L and Wang L: At the crossroads of cancer stem cells and targeted therapy resistance. Cancer Lett. 385:87–96. 2017. View Article : Google Scholar
|
|
224
|
Schulte A, Günther HS, Phillips HS, Kemming D, Martens T, Kharbanda S, Soriano RH, Modrusan Z, Zapf S, Westphal M and Lamszus K: A distinct subset of glioma cell lines with stem cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia. 59:590–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Lu Y, Wang W and Tan S: EHD1 promotes the cancer stem cell (CSC)-like traits of glioma cells via interacting with CD44 and suppressing CD44 degradation. Environ Toxicol. 37:2259–2268. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Gopinath S, Malla R, Alapati K, Gorantla B, Gujrati M, Dinh DH and Rao JS: Cathepsin B and uPAR regulate self-renewal of glioma-initiating cells through GLI-regulated Sox2 and Bmi1 expression. Carcinogenesis. 34:550–559. 2013. View Article : Google Scholar :
|
|
227
|
Rasper M, Schäfer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C and Schlegel J: Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro Oncol. 12:1024–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Mani SK, Zhang H, Diab A, Pascuzzi PE, Lefrançois L, Fares N, Bancel B, Merle P and Andrisani O: EpCAM-regulated intramembrane proteolysis induces a cancer stem cell-like gene signature in hepatitis B virus-infected hepatocytes. J Hepatol. 65:888–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Akbari S, Kunter I, Azbazdar Y, Ozhan G, Atabey N, Firtina Karagonlar Z and Erdal E: LGR5/R-Spo1/Wnt3a axis promotes stemness and aggressive phenotype in hepatoblast-like hepatocellular carcinoma cell lines. Cell Signal. 82:1099722021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Li Y, Wang R, Xiong S, Wang X, Zhao Z, Bai S, Wang Y, Zhao Y and Cheng B: Cancer-associated fibroblasts promote the stemness of CD24+ liver cells via paracrine signaling. J Mol Med (Berl). 97:243–255. 2019. View Article : Google Scholar
|
|
231
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Wang R, Li Y, Tsung A, Huang H, Du Q, Yang M, Deng M, Xiong S, Wang X, Zhang L, et al: iNOS promotes CD24+CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc Natl Acad Sci USA. 115:E10127–E10136. 2018.
|
|
233
|
Zhang K, Che S, Pan C, Su Z, Zheng S, Yang S, Zhang H, Li W, Wang W and Liu J: The SHH/Gli axis regulates CD90-mediated liver cancer stem cell function by activating the IL6/JAK2 pathway. J Cell Mol Med. 22:3679–3690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and Li J: Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cance. 126:2067–2078. 2010. View Article : Google Scholar
|
|
235
|
Cao HZ, Liu XF, Yang WT, Chen Q and Zheng PS: LGR5 promotes cancer stem cell traits and chemoresistance in cervical cancer. Cell Death Dis. 8:e30392017. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Leung CON, Deng W, Ye TM, Ngan HYS, Tsao SW, Cheung ANY, Ziru N, Yuen DCK, Pang RTK and Yeung WSB: MicroRNA-135a-induced formation of CD133+ subpopulation with cancer stem cell properties in cervical cancer. Carcinogenesis. 41:1592–1604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Zhang J, Chen X, Bian L, Wang Y and Liu H: CD44+/CD24+-expressing cervical cancer cells and radioresistant cervical cancer cells exhibit cancer stem cell characteristics. Gynecol Obstet Invest. 84:174–182. 2019. View Article : Google Scholar
|
|
238
|
Liu SY and Zheng PS: High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget. 4:2462–2475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Zhou T, Liu J, Xie Y, Yuan S, Guo Y, Bai W, Zhao K, Jiang W, Wang H, Wang H, et al: ESE3/EHF, a promising target of rosiglitazone, suppresses pancreatic cancer stemness by downregulating CXCR4. Gut. 71:357–371. 2022. View Article : Google Scholar
|
|
240
|
Amsterdam A, Raanan C, Schreiber L, Polin N and Givol D: LGR5 and Nanog identify stem cell signature of pancreas beta cells which initiate pancreatic cancer. Biochem Biophys Res Commun. 433:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Lin L, Jou D, Wang Y, Ma H, Liu T, Fuchs J, Li PK, Lü J, Li C and Lin J: STAT3 as a potential therapeutic target in ALDH+ and CD44+/CD24+ stem cell-like pancreatic cancer cells. Int J Oncol. 49:2265–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Shi J, Lu P, Shen W, He R, Yang MW, Fang Y, Sun YW, Niu N and Xue J: CD90 highly expressed population harbors a stemness signature and creates an immunosuppressive niche in pancreatic cancer. Cancer Lett. 453:158–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Sasaki N, Ishii T, Kamimura R, Kajiwara M, Machimoto T, Nakatsuji N, Suemori H, Ikai I, Yasuchika K and Uemoto S: Α-fetoprotein-producing pancreatic cancer cells possess cancer stem cell characteristics. Cancer Lett. 308:152–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
van der Horst G, Bos L and van der Pluijm G: Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 10:995–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Verma A, Kapoor R and Mittal RD: Cluster of Differentiation 44 (CD44) gene variants: A putative cancer stem cell marker in risk prediction of bladder cancer in north indian population. Indian J Clin Biochem. 32:74–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA and Jiang F: Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 19:327–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Ooki A, VandenBussche CJ, Kates M, Hahn NM, Matoso A, McConkey DJ, Bivalacqua TJ and Hoque MO: CD24 regulates cancer stem cell (CSC)-like traits and a panel of CSC-related molecules serves as a non-invasive urinary biomarker for the detection of bladder cancer. Br J Cancer. 119:961–970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Gao MQ, Choi YP, Kang S, Youn JH and Cho NH: CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 29:2672–2680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, et al: Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Kryczek I, Liu S, Roh M, Vatan L, Szeliga W, Wei S, Banerjee M, Mao Y, Kotarski J, Wicha MS, et al: Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 130:29–39. 2012. View Article : Google Scholar
|
|
251
|
Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L and Rocconi RP: CD44+/CD24-ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 29:939–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Tachezy M, Zander H, Wolters-Eisfeld G, Müller J, Wicklein D, Gebauer F, Izbicki JR and Bockhorn M: Activated leukocyte cell adhesion molecule (CD166): An 'inert' cancer stem cell marker for non-small cell lung cancer? Stem Cells. 32:1429–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Wang J, Shao F, Yang Y, Wang W, Yang X, Li R, Cheng H, Sun S, Feng X, Gao Y, et al: A non-metabolic function of hexokinase 2 in small cell lung cancer: Promotes cancer cell stemness by increasing USP11-mediated CD133 stability. Cancer Commun (Lond). 42:1008–1027. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Nian WQ, Chen FL, Ao XJ and Chen ZT: CXCR4 positive cells from Lewis lung carcinoma cell line have cancer metastatic stem cell characteristics. Mol Cell Biochem. 355:241–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Qiu X, Wang Z, Li Y, Miao Y, Ren Y and Luan Y: Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 323:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Heo SK, Noh EK, Ju LJ, Sung JY, Jeong YK, Cheon J, Koh SJ, Min YJ, Choi Y and Jo JC: CD45dimCD34+CD38-CD133+ cells have the potential as leukemic stem cells in acute myeloid leukemia. BMC Cancer. 20:2852020. View Article : Google Scholar
|
|
259
|
Riether C, Schürch CM, Bührer ED, Hinterbrandner M, Huguenin AL, Hoepner S, Zlobec I, Pabst T, Radpour R and Ochsenbein AF: CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. 214:359–380. 2017. View Article : Google Scholar :
|
|
260
|
Aoki T, Shiba N, Tsujimoto S, Yamato G, Hara Y, Kato S, Yoshida K, Ogawa S, Hayashi Y, Iwamoto S, et al: High IL2RA/CD25 expression is a prognostic stem cell biomarker for pediatric acute myeloid leukemia without a core-binding factor. Pediatr Blood Cancer. 71:e308032024. View Article : Google Scholar
|
|
261
|
Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al: The interleukin-3 receptor α chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 14:1777–1784. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y and Akashi K: TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 7:708–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Zhang Y, Zhou SY, Yan HZ, Xu DD, Chen HX, Wang XY, Wang X, Liu YT, Zhang L, Wang S, et al: miR-203 inhibits proliferation and self-renewal of leukemia stem cells by targeting survivin and Bmi-1. Sci Rep. 6:199952016. View Article : Google Scholar : PubMed/NCBI
|