Introduction
Breast cancer is the leading cause of cancer death
for women worldwide. In addition, the incidence rates of breast
cancer have markedly increased over the past few years. The
incident cases of BC increased from 876,990 in 1990 to 2,002,350 in
2019, and the estimated annual percentage change for incidence
increased by an average of 0.33% per year worldwide (1). The cure rate is high when the
cancer is diagnosed early and treated comprehensively with surgical
resection, radiation and chemotherapy. However, an increase in
mortality has been reported due to treatment failure caused by
cancer recurrence after an apparent cure or metastasis to distant
sites from the original tumor. Furthermore, 25-30% of all patients
with breast cancer experience disease recurrence, which often leads
to mortality (2). Expression of
receptors such as estrogen receptor (ER), progesterone receptor
(PR) and HER2 is vital for the evaluation of treatments.
Triple-negative breast cancer (TNBC) is characterized by the
absence of ER, PR and HER2, and has the worst prognosis among
aggressive breast cancer subtypes due to the lack of treatment
targets (3). General anticancer
therapy and radiation therapy are used after surgery for patients
with TNBC if there is no metastasis when TNBC is diagnosed.
However, if treatment resistance is acquired, anticancer drugs and
radiation do not exhibit therapeutic efficacy, ultimately
increasing tumor recurrence and metastasis (4,5).
Due to the acquisition of therapy resistance, patients with TNBC
have a short survival period and a mortality rate of 40% within 5
years from diagnosis (6).
Therefore, innovative treatment strategies for patients with TNBC
with acquired treatment resistance are urgently needed.
In our previous study, radiotherapy-resistant
(RT-R)-TNBC cells (RT-R-MDA-MB-231 cells) were established and
their characteristics compared with MDA-MB-231 cells were clarified
(7). RT-R-MDA-MB-231 cells
exhibited increased migration, adhesion to endothelial cells (ECs),
and invasion through ECs-Matrigel-coated membranes by upregulating
adhesion molecules (such as intercellular adhesion molecule 1 and
vascular cell adhesion molecule 1) and epithelial-mesenchymal
transition (EMT)-associated proteins (such as MMP-9, Snail-1 and
β-catenin) (7). Furthermore,
RT-R-MDA-MB-231 cells may contribute to tumor progression by
enhancing premetastatic niche formation through the
hypoxia-inducible factor 1α (HIF-1α)-lysyl oxidase axis (8). ATP-binding cassette (ABC)
transporters, the largest family of transmembrane proteins, are
divided into seven subfamilies (ABCA-ABCG). The role of ABC
transporters in multidrug resistance is well recognized because
they are responsible for ATP-dependent export of various
xenobiotics, including drugs (9,10). However, the drug
efflux-independent roles of ABC transporters in cancer biology are
not well known. ABC transporters are involved in the export of
lipids and metabolic products across plasma and intracellular
membranes (11). These
substances can act as signaling substances for cells and
surrounding other cancer cells. For example, ABCC4 can export
prostaglandins; ABCC1 can export leukotriene C4,
sphingosine-1-phosphate and lysophosphatidyl inositol; ABCB5 can
export IL1β; and ABCG2 can export androgens (12). ABCB1/2/5, ABCC1/2/3/4/5/6/10 and
ABCG2 are known to be directly related to resistance to
chemotherapy by mediating the efflux of chemotherapy agents
(13,14). However, ABCG5 and ABC subfamily G
member 8 (ABCG8) are known to be partially involved in the outflow
of sterols and there is little research on their function in cancer
by mediating sterol efflux (15,16). Therefore, the present study aimed
to identify the differences in gene expression levels of ABC
transporters between RT-R-MDA-MB-231 cells and MDA-MB-231 cells.
The present study also aimed to investigate the role of the ABC
transporter subtype that shows the most significant change between
these two cell lines.
Materials and methods
Cell culture and treatment
The MCF10A breast epithelial cell line was obtained
from American Type Culture Collection, and the MCF7 (non-TNBC), and
MDA-MB-231 and MDA-MB-453 (TNBC) human breast cancer cell lines
were obtained from the Korean Cell Line Bank; Korean Cell Line
Research Foundation. RT-R breast cancer cells (RT-R-MCF7 and
RT-R-MDA-MB-231 cells) were generated by repeatedly applying 2 Gy
X-ray irradiation until a final dose of 50 Gy (2 Gy; 25 times) was
achieved, as described previously (7). MCF10A cells were cultured in
DMEM/F-12 (cat. no. 11320-033; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 5% horse serum (cat. no. 16050122; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100 g/ml
streptomycin, 0.5 g/ml hydrocortisone, 10 g/ml insulin, 20 ng/ml
epidermal growth factor, 2 mM L-glutamine and 0.5 μg/ml
amphotericin B. All cancer cell lines were cultured in RPMI 1640
medium (cat. no. SH30027.01; Cytiva) supplemented with 10% fetal
bovine serum (cat. no. 160000-044; Gibco; Thermo Fisher Scientific,
Inc.), 1% penicillin and streptomycin (cat. no. SV30010; Cytiva).
The cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2. The RT-R breast cancer cells were
used through five passages. MCF10A, MCF7, RT-R-MCF7, MDA-MB-231,
RT-R-MDA-MB-231 and MDA-MB-453 cells were used for the detection of
ABC transporter subtypes (ABCA9, ABCB4, ABCG5 and ABCG8) by western
blot analysis. In other assays, MDA-MB-231 and RT-R-MDA-MB-231
cells were used. RT-R-MDA-MB-231 and MDA-MB-231 cells were treated
with digoxin (cat. no. D6003; Supelco; Merck KGaA; dissolved in
DMSO) at a dose of 400 nM for 24 h at 37°C or cholesterol (C4951;
Sigma-Aldrich; Merck KGaA; dissolved in sterile water) at various
concentrations (0, 10 and 20 μM) for 24 h at 37°C for the
indicated experiments.
Cell viability assay
MDA-MB-231 and RT-R-MDA-MB-231 cells were seeded at
a density of 104 cells/well in 24-well plates. The cells
were treated with different concentrations (0.01, 0.05, 0.1, 0.5
and 1 μM) of doxorubicin (DOXO; cat. no. D1515;
Sigma-Aldrich; Merck KGaA; dissolved in sterile water) or
paclitaxel (PTX; cat. no. T7402; Sigma-Aldrich; Merck KGaA;
dissolved in DMSO) for 24-72 h at 37°C. Cell viability was assessed
using the D-Plus™ CCK cell viability assay kit (cat. no. CCK-3000;
Donginbiotech Co., Ltd.). After treatment, 20 μl CCK-8
solution was added to 200 μl serum-free medium in each well,
and the cells were incubated for 30 min at 37°C in the dark. The
absorbance at 450 nm was measured using a microplate reader
(Molecular Devices VersaMax; Molecular Devices, LLC).
Gene expression array analysis
Gene expression profiling was performed using the
QuantiSeq 3′ mRNA-Seq Service (ebiogen, Inc.). Briefly, total RNA
was extracted from MDA-MB-231 and RT-R-MDA-MB-231 cells using
TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The quality of the RNA was evaluated using the
Agilent 2100 Bioanalyzer System (Agilent Technologies, Inc.).
Libraries were prepared from the total RNA (500 ng) using the
Lexogen Quant-Seq 96 prep kit (cat. no. 015.2X96; Lexogen GmbH)
according to the provided instructions. The library concentration
was measured using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc.) and a Qubit™ Flex Fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc.), and the library concentration per
sample was ~4 nM, which was diluted to a concentration of 20 pM.
The final library concentration was adjusted to 1.5 pM after
quantitative PCR-based quantification (StepOnePlusTM;
Applied Biosystems; Thermo Fisher Scientific, Inc.) before
sequencing. Reverse transcription was performed using an oligo-dT
primer containing an Illumina-compatible sequence. The reaction
conditions were 85°C for 3 min (primer hybridization) and 42°C for
15 min (cDNA synthesis). The Lexogen Quant-Seq 96 prep kit (cat.
no. 015.2X96; Lexogen GmbH) contained reverse transcriptase and
master mix, including buffer, dNTPs and oligo-dT primer. The RNA
template was then degraded, and second strand synthesis was
initiated using a random primer with an Illumina-compatible linker
sequence at its 5′ end by incubation at 98°C for 1 min and 25°C for
30 min. Subsequently, the double-stranded cDNA library was
amplified as follows: Initial denaturation at 98°C for 30 sec, 12
cycles of 98°C for 10 sec, 65°C for 20 sec and 72°C for 30 sec, and
a final extension at 72°C for 1 min. This procedure was also
performed using the Lexogen Quant-Seq 96 prep kit. Purified
libraries were sequenced using an Illumina NextSeq500 system with
Single-End 75 bp (cat. no. 20024906; Illumina, Inc.). The raw
sequencing data underwent quality control checks using FastQC
(version 0.11.5; https://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
Data mining was carried out using ExDEGA (v5.2.1; ebiogen, Inc.).
The corresponding datasets have been submitted to a public database
(Gene Expression Omnibus; www.ncbi.nlm.nih.gov/geo). ABC transporters were
selected from the dataset (GSE287883) and analyzed further.
Gene silencing with small interfering
(si)RNA
MDA-MB-231 cells and RT-R-MDA-MB-231 cells were
transfected with 100 nM negative control siRNA (siCTRL; cat. no.
SN-1003; Bioneer Corporation), ABCG8 siRNA (siABCG8; cat. no.
64241-1; Bioneer Corporation) and LDL receptor (LDLR)-related
protein 6 (LRP6) siRNA (siLRP6; cat. nos. 4040-1; Bioneer
Corporation) using Lipofectamine® 3000 (cat. no.
L3000015; Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The cells were incubated in
complete medium containing transfection reagent for 24 h at 36°C,
and the medium was then replaced with fresh medium and cells were
used for experiments after 24 h of incubation. The siRNA sequences
were as follows: siCTRL forward, 5′-CCUACGCCACCAAUUUCGU-3′ and
reverse, 5′-ACGAAAUUGGUGGCGUAGG-3′; siABCG8 forward,
5′-CGUCAGAUUUCCAACGACU-3′ and reverse, 5′-AGUCGUUGGAAAUCUGACG-3′;
siLRP6 1 forward, 5′-CUGCUUUGGAUUUUGAUGU-3′ and reverse,
5′-ACAUCAAAAUCCAAAGCAG-3′; aiLRP6 2 forward,
5′-UGAGAACACCUAUUCUACA-3′ and reverse, 5′-UGUAGAGGUGUUCUCA-3′; and
siLRP6 3 forward, 5′-UGUUGACCAGUUAUCAGUA-3′ and reverse,
5′-UACUGAUAACUGGUCAACA-3′. The silencing efficiency was determined
by western blot analysis.
Western blot analysis
MDA-MB-231 cells and RT-R-MDA-MB-231 cells were
harvested, and lysed in RIPA buffer containing 50 mM Tris-HCl (pH
7.5), 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate and
protease inhibitors. The samples were centrifuged at 16,000 × g for
20 min at 4°C and the supernatants were collected. The protein
concentration was determined using the Bradford method. Equal
amounts of protein (20-50 μg/lane) were subjected to 8-10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto Hybond-P+ polyvinylidene fluoride membranes
(Amersham; Cytiva). The membranes were blocked with 5% non-fat milk
in Tris-buffered saline containing 0.05% Tween-20 for 1 h at room
temperature, and then incubated with the following primary
antibodies overnight at 4°C: Anti-ABCA9 (cat. no. PA5-75606;
1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.), anti-ABCB4
(cat. no. PA5-78692; 1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc.), anti-ABCG5 (cat. no. bs-5013R; 1:1,000; BIOSS), anti-ABCG8
(cat. no. ab223056; 1:1,000; Abcam), anti-GAPDH (cat. no.
MA5-15738; 1:5,000; Invitrogen; Thermo Fisher Scientific, Inc.),
anti-Cyclin D1 (cat. no. ab16663; 1:1,000; Abcam), anti-Cyclin B1
(cat. no. ab32053; 1:1,000; Abcam), anti-OCT4 (cat. no. ab18976;
1:1,000; Abcam), anti-β-catenin (cat. no. sc-7963; 1:1,000; Santa
Cruz Biotechnology, Inc.), anti-AKT (cat. no. sc-8312; 1:1,000;
Santa Cruz Biotechnology, Inc.), anti-GSK3β (cat. no. sc-9166;
1:1,000; Santa Cruz Biotechnology, Inc.), anti-p-GSK3β (cat. no.
sc-11358; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-p-AKT
(cat. no. 9271; 1:1,000; Cell Signaling Technology, Inc.),
anti-fatty acid synthase (FASN; cat. no. 3180; 1:1,000; Cell
Signaling Technology, Inc.), anti-STAT3 (cat. no. 4904; 1:1,000;
Cell Signaling Technology, Inc.), anti-p-STAT3 (cat. no. 9131;
1:1,000; Cell Signaling Technology, Inc.), anti-N-cadherin (cat.
no. 4061; 1:1,000; Cell Signaling Technology, Inc.), anti-HIF-1α
(cat. no. ab179483; 1:1,000; Abcam), anti-inducible degrader of LDL
(IDOL; cat. no. ab74562; 1:1,000; Abcam), anti-LDLR (cat. no.
ab52818; 1:1,000; Abcam), anti-LRP6 (cat. no. 3395; 1:1,000; Cell
Signaling Technology, Inc.), anti-phosphorylated-(p-)LRP6 (cat. no.
2568; 1:1,000; Cell Signaling Technology, Inc.), anti-liver X
receptor (LXR) α/β (cat. no. sc-377260; 1:1,000; Santa Cruz
Biotechnology, Inc.), anti-Polo-like kinase 1 (PLK1; cat. no. 4513;
1:1,000; Cell Signaling Technology, Inc.) and anti-sterol
regulatory element binding protein 1 (SREBP1; cat. no. sc-13551;
1:1,000; Santa Cruz Biotechnology, Inc.) (precursor form 125 kDa,
mature form 68 kDa). The expression of the proteins was detected
using horseradish peroxidase-conjugated secondary antibodies for 1
h at room temperature, including anti-rabbit IgG (cat. no. 12-348;
1:5,000; Sigma-Aldrich; Merck KGaA) and anti-mouse IgG (cat. no.
A90-116P; 1:5,000; BETHYL; Fortis Life Sciences, LLC), and western
ECL substrates (cat. no. 170-5061; Bio-Rad Laboratories, Inc.) for
western blotting detection. The protein levels were normalized to
β-actin (cat. no. MA5-15739; 1:5,000; Thermo Fisher Scientific,
Inc.). The density of protein was assessed using the ChemiDoc™ XRS+
system (Bio-Rad Laboratories, Inc.), and relative protein levels
were semi-quantified using ImageJ software (version 1.53e; National
Institutes of Health).
Total cholesterol quantification
For quantification of intracellular total
cholesterol, 1×106 cells (MDA-MB-231 and RT-R-MDA-MB-231
cells) were collected in 100 μl PBS (Ph 7.2-7.4). Cells were
frozen for a few seconds using liquid nitrogen, and thawed at room
temperature, which was repeated five times to release intracellular
components. Subsequently, the supernatant was collected after
centrifuging for 20 min at 4°C at 400-800 × g. For quantification
of total cholesterol in the cell media, cell culture media were
collected into aseptic tubes and centrifuged for 1 min at 4°C at
400-800 × g, and the supernatant was transferred into new tubes.
Total cholesterol was quantified using the Human Total Cholesterol
ELISA Kit (cat. no. ab287836; Abcam) according to the
manufacturer's instructions. The absorbance at 450 nm was measured
with a microplate reader (Molecular Devices VersaMax; Molecular
Devices, LLC).
Cell cycle analysis
MDA-MB-231 or RT-R-MDA-MB-231 cells, which were
transfected with siCTRL or siABCG8, were fixed with 75% ethanol at
4°C overnight, and stained with PI solution (50 μg/ml PI;
cat. no. 537059; Sigma-Aldrich; Merck KGaA; 0.7 μg/ml RNase
A; cat. no. R6513; Sigma-Aldrich; Merck KGaA) at room temperature
for 30 min in the dark. DNA content was analyzed using flow
cytometry (FACSverse; BD Biosciences) and the data were analyzed
using FlowJo software (version 10; Tree Star, Inc.).
Statistical analysis
All data were statistically analyzed using GraphPad
Prism 8 software (Dotmatics). Statistical comparisons were
performed using an unpaired Student's t-test for two-group
comparisons or one-way ANOVA followed by Tukey's post hoc test for
comparisons of three or more groups. The results of the cell
viability assay were compared using two-way ANOVA followed by
Sidak's post hoc test. The data are presented as the mean ± SD of
three to five independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
ABCG8 expression is increased in TNBC
cells compared with in non-TNBC cells, and is significantly
increased in RT-R-TNBC cells compared with in TNBC cells
Gene expression array analysis of MDA-MB-231 and
RT-R-MDA-MB-231 cells revealed that ABCA9, ABCB4 and ABCG8 were
upregulated by >2-fold in RT-R-MDA-MB-231 cells (RT-R-TNBC)
compared with in MDA-MB-231 cells (TNBC) (Fig. 1A). The protein levels of ABCA9,
ABCB4 and ABCG8 in MCF10A (normal epithelial cells), MCF7
(non-TNBC), RT-R-MCF-7, MDA-MB-231 (TNBC) and RT-R-MDA-MB-231 cells
were subsequently determined by western blot analysis. Because
ABCG5 can form a heterodimer with ABCG8, and this heterodimer of
ABCG5/G8 serves as a major sterol transporter in liver and
intestinal cholesterol efflux (16), the expression levels of ABCG5
were also detected. The results showed that ABCG5 and ABCG8 protein
levels were higher in MDA-MB-231 cells than in MCF7 cells.
Furthermore, the expression levels of ABCG8, which were upregulated
in MDA-MB-231 cells, were significantly increased in
RT-R-MDA-MB-231 cells (Fig. 1B and
F), although the increase of ABCG8 expression at the protein
level in RT-R-MDA-MB-231 cells (1.6-fold compared with MDA-MB-231
cells) was slightly lower than that at the RNA level (~2.88-fold
compared with MDA-MB-231 cells), which may be due to methodological
differences. In addition, it was confirmed that ABCG8 protein
expression was increased in other TNBC cell lines, such as
MDA-MB-453 cells compared with in MCF7 cells, a non-TNBC cell line
(Fig. 1G and H). However, ABCG5
protein expression did not significantly differ between MDA-MB-231
and RT-R-MDA-MB-231 cells. Therefore, in subsequent experiments,
the role of ABCG8 in cancer progression was examined in
RT-R-MDA-MB-231 cells.
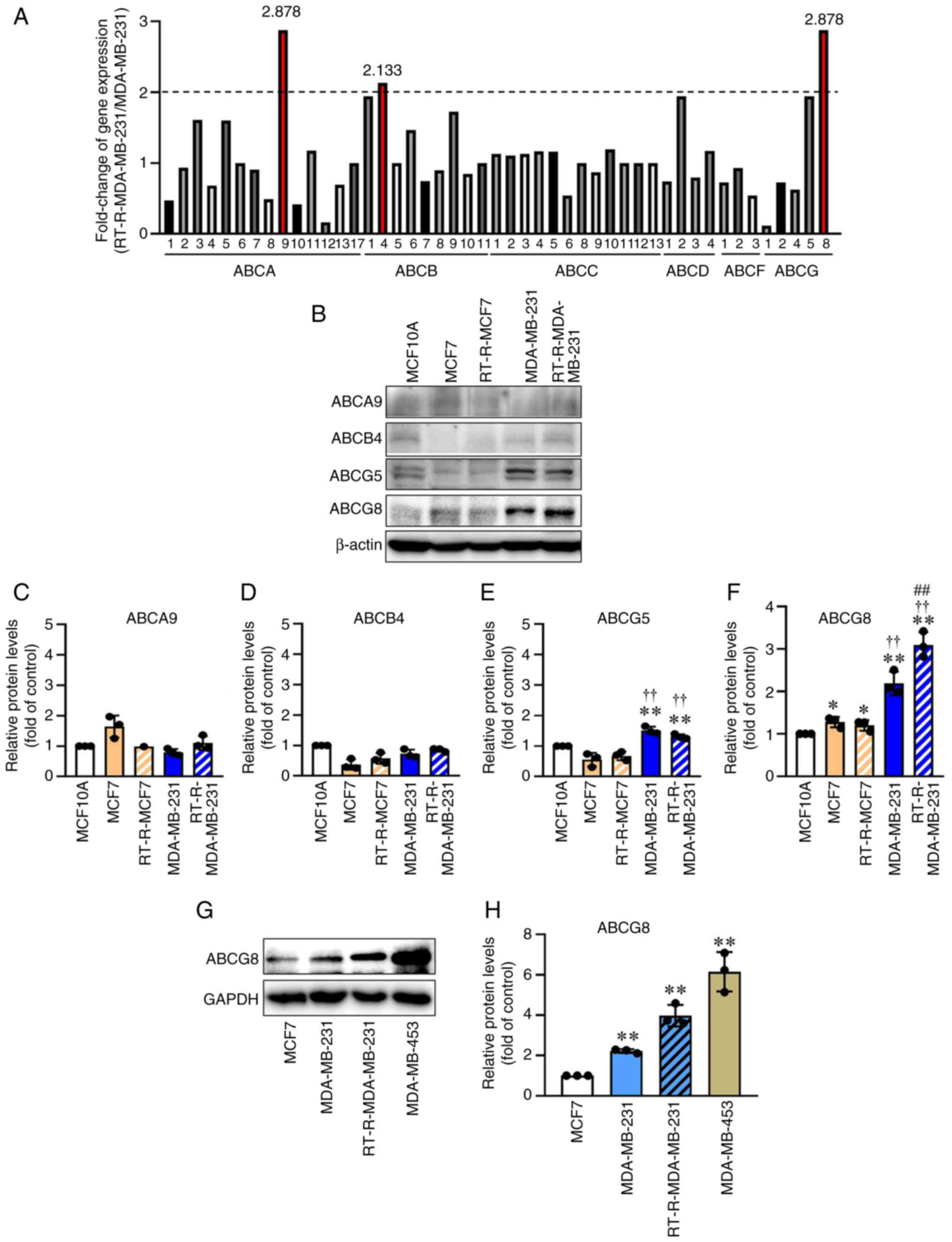 | Figure 1ABCG8 expression is increased in TNBC
cells compared with that in non-TNBC cells, with this increase
being more pronounced in RT-R-TNBC cells. (A) Differential
expression of ABC transporters in RT-R-MDA-MB-231 and MDA-MB-231
cells was determined by gene expression array analysis. ABC
transporter isotypes that were upregulated >2-fold in
RT-R-MDA-MB-231 cells compared with MDA-MB-231 cells are shown in
red. (B) ABCA9, ABCB4, ABCG5 and ABCG8 were selected, and the
expression levels of these proteins were confirmed in MCF10A normal
epithelial cells, MCF7 and RT-R-MCF7 non-TNBC cells, and MDA-MB-231
and RT-R-MDA-MB-231 TNBC cells by western blot analysis (n=3). The
levels of (C) ABCA9, (D) ABCB4, (E) ABCG5 and (F) ABCG8 were
semi-quantified and normalized to β-actin. Data are presented as
the mean ± SD of three independent experiments.
*P<0.05, **P<0.01 compared with MCF10A
cells; ††P<0.01 compared with MCF7 cells;
##P<0.01 compared with MDA-MB-231 cells. (G) Protein
levels of ABCG8 were compared between non-TNBC (MCF7) and TNBC
(MDA-MB-231, MDA-MB-453 and RT-R-MDA-MB-231) cells by western blot
analysis. (H) ABCG8 protein levels were compared among TNBC cell
lines, including MDA-MB-231, RT-R-MDA-MB-231 and MDA-MB-453 cells,
and non-TNBC cells such as MCF7 cells. Data are presented as the
mean ± SD of three independent experiments. **P<0.01
compared with MCF7 cells. ABC, ATP-binding cassette; ABCG8, ABC
subfamily G member 8; RT-R, radiotherapy-resistant; TNBC,
triple-negative breast cancer. |
ABCG8 knockdown enhances
chemotherapy-mediated cytotoxicity in TNBC and RT-R-TNBC cells
ABC transporters are known to mediate chemotherapy
resistance by pumping out chemotherapeutic drugs (14,17). Therefore, the present study
examined the effect of ABCG8 knockdown on chemotherapy-mediated
cytotoxicity in TNBC and RT-R-TNBC cells. After confirming the
effectiveness of ABCG8 knockdown using siABCG8 by western blot
analysis (Fig. 2A and B), the
effect of ABCG8 knockdown on the viability of MDA-MB-231 and
RT-R-MDA-MB-231 cells treated with Doxo or PTX (0.01, 0.05, 0.1,
0.5 and 1 μM) for 24-72 h was determined (Table SI). As shown in Table SI and Fig. 2C and D, Doxo and PTX reduced the
viability of MDA-MB-231 and RT-R-MDA-MB-231 cells in a
dose-dependent manner over 24-72 h, and the reduction in cell
viability caused by Doxo or PTX was even greater when cells were
transfected with siABCG8. Specifically, for 1 μM Doxo
treatment, knockdown of ABCG8 significantly decreased cell
viability from 86, 67 and 51% to 65, 57 and 43% at 24, 48 and 72 h,
respectively, in MDA-MB-231 cells, and from 96, 67 and 46% to 72,
61 and 36% at 24, 48 and 72 h, respectively, in RT-R-MDA-MB-231
cells (Fig. 2C). For 1 μM
PTX, siABCG8 transfection led to a significant reduction from 60 to
46% at 72 h in MDA-MB-231 cells, and from 70 and 67% to 54 and 43%
at 48 and 72 h in RT-R-MDA-MB-231 cells (Fig. 2D). Notably, both MDA-MB-231 and
RT-R-MDA-MB-231 cells transfected with siABCG8 exhibited
substantial reductions in viability after 48 and 72 h of incubation
(Fig. 2E). These results
indicated that the presence of ABCG8 affected cell viability, and
thus, ABCG8 may have additional functions beyond its role as a drug
pump.
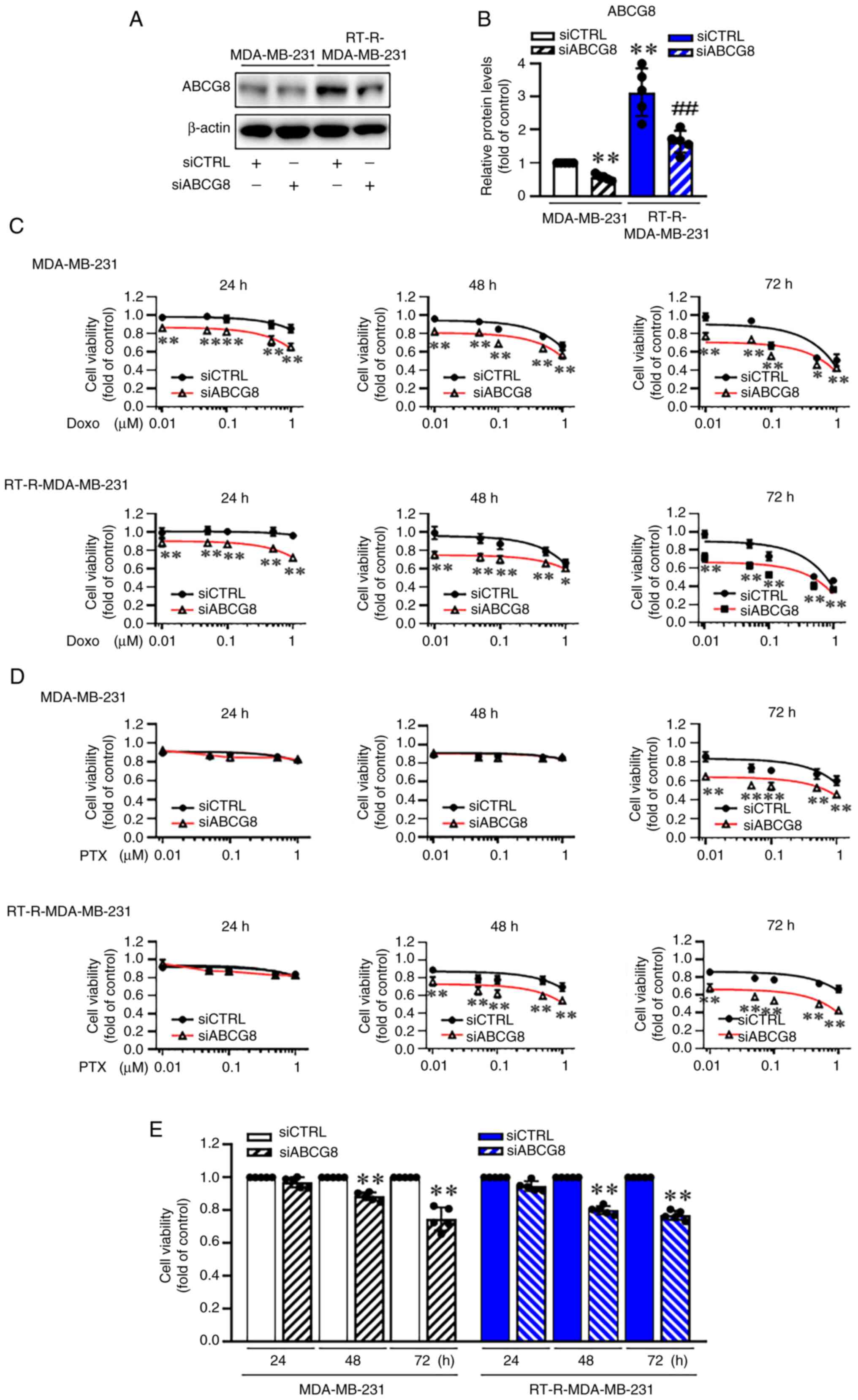 | Figure 2siABCG8 transfection enhances
chemotherapy (Doxo and PTX)-mediated cytotoxicity in
RT-R-MDA-MB-231 and MDA-MB-231 cells, with siABCG8 itself
decreasing cell viability. (A) Cells were transfected with siCTRL
or siABCG8 (100 nM) for 24 h and the transfection efficiency of
siABCG8 was confirmed by western blot analysis. (B) ABCG8
expression was semi-quantified and normalized to β-actin. Data are
presented as the mean ± SD of five independent experiments.
**P<0.01 compared with siCTRL-transfected MDA-MB-231
cells; ##P<0.01 compared with siCTRL-transfected
RT-R-MDA-MB-231 cells. Cells transfected with siCTRL or siABCG8
were treated with different concentrations (0.01, 0.05, 0.1, 0.5
and 1 μM) of (C) Doxo or (D) PTX for 24-72 h. Cell viability
was assessed using a CCK-8 cell viability assay kit. Data are
presented as the mean ± SD of five independent experiments.
*P<0.05; **P<0.01 compared between the
siCTRL group and the siABCG8 group. (E) Cells transfected with
siCTRL or siABCG8 were cultured in fresh media for 24-72 h. Cell
viability was determined using the CCK-8 cell viability assay kit.
Data are presented as the mean ± SD of five independent
experiments. **P<0.01 compared with the siCTRL group
for each time point. ABCG8, ATP-binding cassette subfamily G member
8; CCK-8, Cell Counting Kit-8; CTRL, control; Doxo, doxorubicin;
PTX, paclitaxel; RT-R, radiotherapy-resistant; si, small
interfering RNA. |
ABCG8 knockdown is associated with
accumulation of intracellular cholesterol but reduces the
expression levels of β-catenin and EMT proteins
The ABCG5/ABCG8 protein is a major sterol
transporter, and tumor cells need lipids for their rapid
proliferation (18). The present
study examined the cholesterol production in RT-R-TNBC and TNBC
cells. Both intracellular and medium cholesterol levels were
significantly higher in the RT-R-MDA-MB-231 group compared with
those in the MDA-MB-231 group (Fig.
3A). Subsequently, to investigate the role of ABCG8 in the
cholesterol efflux from the cytosol into the media, total
cholesterol levels in the cell lysates and culture media were
measured. The level of total cholesterol in the lysate of
RT-R-MDA-MB-231 cells was higher compared with that in MDA-MB-231
cells, and this increase was further enhanced by siABCG8
transfection. By contrast, the increased cholesterol levels in the
culture media from RT-R-MDA-MB-231 cells, compared with those from
MDA-MB-213 cells, were significantly decreased by siABCG8
transfection (Fig. 3B). These
findings suggested that siABCG8 transfection decreased cholesterol
efflux from the cytosol into the media. To investigate whether
ABCG8 may be involved in regulating EMT-related proteins by
expelling cholesterol, the levels of EMT-related proteins,
β-catenin, N-cadherin and OCT4, were measured in MDA-MB-231 and
RT-R-MDA-MB-231 cells transfected with siABCG8. As shown in
Fig. 3C and D, β-catenin,
N-cadherin and OCT4 were more highly expressed in RT-R-MDA-MB-213
cells than in MDA-MB-231 cells. However, their expression levels
were significantly reduced by siABCG8 transfection. These results
suggested that cholesterol pumped out of cells by ABCG8, rather
than cholesterol inside cells, may affect the induction of
EMT-related proteins. However, the levels of induced p-AKT, p-STAT3
and p-GSK3β were not influenced by siABCG8 transfection (Fig. 3E and F), suggesting that
ABCG8-mediated cholesterol efflux did not affect the induction of
EMT-related proteins through the p-AKT, p-STAT3 or p-GSK3β
pathways.
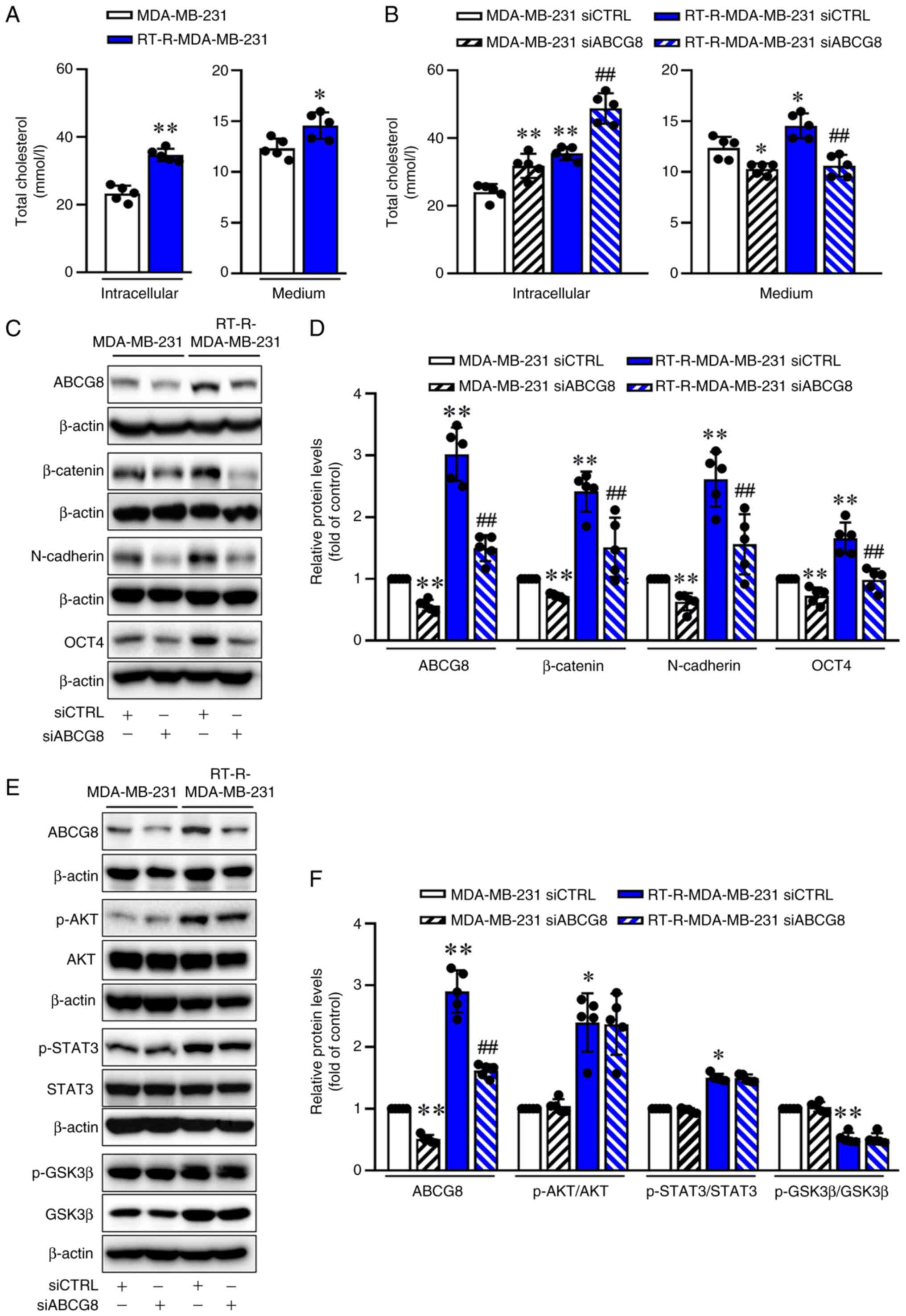 | Figure 3siABCG8 transfection leads to
intracellular cholesterol accumulation and reduces
epithelial-mesenchymal transition signaling. (A) Total cholesterol
levels were quantified in cell lysates and culture media from
MDA-MB-231 and RT-R-MDA-MB-231 cells using the Human Total
Cholesterol ELISA Kit. Data are presented as the mean ± SD of five
independent experiments. *P<0.05;
**P<0.01 compared with the MDA-MB-231 cells. (B)
Cells were transfected with siCTRL or siABCG8 (100 nM) for 24 h.
Total cholesterol levels in both cell lysates and culture media
were then quantified using a Human Total Cholesterol ELISA Kit.
Data are presented as the mean ± SD of five independent
experiments. *P<0.05; **P<0.01 compared
with the siCTRL-transfected MDA-MB-231 cells;
##P<0.01 compared with the siCTRL-transfected
RT-R-MDA-MB-231 cells. Protein levels of (C) ABCG8, β-catenin,
N-cadherin and OCT4, or (E) ABCG8, p-AKT/AKT, p-STAT3/STAT3 and
p-GSK3β/GSK3β were determined by western blot analysis. (D) ABCG8,
β-catenin, N-cadherin and OCT4, or (F) ABCG8, p-AKT/AKT,
p-STAT3/STAT3 and p-GSK3β/GSK3β protein levels were semi-quantified
and normalized to β-actin. Data are presented as the mean ± SD of
five independent experiments. *P<0.05;
**P<0.01 compared with the siCTRL-transfected
MDA-MB-231 cells; ##P<0.01 compared with the
siCTRL-transfected RT-R-MDA-MB-231 cells. ABCG8, ATP-binding
cassette subfamily G member 8; CTRL, control; p-, phosphorylated;
RT-R, radiotherapy-resistant; si, small interfering RNA. |
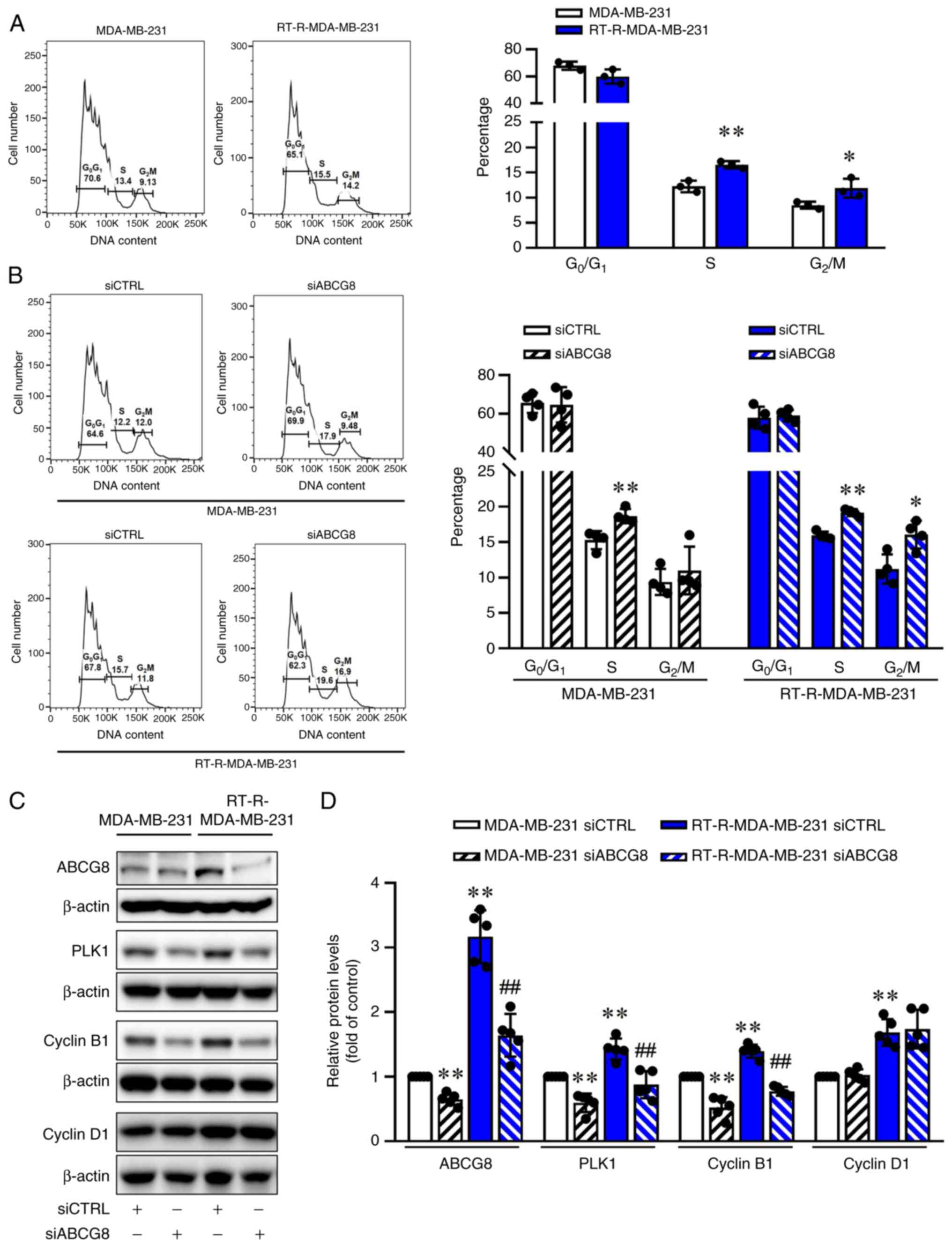
ABCG8 knockdown induces cell cycle arrest
at the G2/M phase in RT-R-MDA-MB-231 cells by inhibiting
PLK1 and Cyclin B1
Dysregulation of the cell cycle is a hallmark of
cancer that enables limitless cell division (19). The Wnt/β-catenin pathway can
modulate the cell cycle. Conversely, the cell cycle also influences
the Wnt/β-catenin pathway (20).
Therefore, the present study subsequently determined the role of
ABCG8 in the cell cycle. Flow cytometry data showed that there were
a higher proportion of RT-R-MDA-MB-231 cells in the S and
G2/M phases compared with MDA-MB-231 cells (Fig. 4A). Furthermore, when ABCG8 was
knocked down, a 20% increase in cells in the S phase and a 17%
increase in cells in the G2/M phase in MDA-MB-231 cells,
and a 20% increase in cells in the S phase and a 40% increase in
cells in the G2/M phase in RT-R-MDA-MB-231 cells were
observed (Fig. 4B). This result
suggested that ABCG8 knockdown affected the G2/M phase.
However, the changes observed in the G2/M phase
resulting from ABCG8 knockdown were not significant in the
MDA-MB-231 cells. The increase in the G2/M cell
population due to ABCG8 knockdown was more pronounced in
RT-R-MDA-MB-231 cells than in MDA-MB-231 cells. PLKs serve an
essential role as key mitotic kinases and cell cycle regulators,
and they also serve key roles in proliferation and cell growth
(21). Among the PLKs, PLK1 has
been shown to be upregulated in various types of cancer and to be
associated with poor patient prognosis (22). PLK1 is a central actor during
mitosis (23), which promotes
entry into mitosis in the cell cycle by activating Cyclin B1
(24). Furthermore, β-catenin
can regulate transcriptional induction of cell cycle regulators
such as PLKs, particularly PLK1 (25,26). Therefore, the present study
subsequently determined the effect of ABCG8 knockdown on PLK1 and
Cyclin B1 expression. RT-R-MDA-MB-231 cells exhibited significantly
increased protein expression levels of PLK1 and Cyclin B1 compared
with those in MDA-MB-231 cells, which was suppressed by siABCG8
transfection. However, Cyclin D1 protein expression was
significantly increased in RT-R-MDA-MB-231 cells, but was not
affected by aiABCG8 transfection (Fig. 4C and D). These results indicated
that ABCG8 knockdown led to downregulation of PLK1 and Cyclin B1,
which resulted in cell cycle arrest in the G2/M phase by
inhibiting cells from entering mitosis.
LRP6, the Wnt co-receptor, is activated
more in RT-R-TNBC cells than in TNBC cells, and is involved in the
induction of β-catenin, PLK1 and Cyclin B1
It has been reported that LDLR mediates the entry of
cholesterol into cells (27),
while LRP6, the Wnt co-receptor, activates the Wnt/β-catenin
pathway upon cholesterol binding, resulting in the proliferation of
cancer cells (28,29). If cholesterol imported into cells
by LDLR activates Wnt/β-catenin signaling, increased intracellular
cholesterol levels observed in siABCG8-transfected cells are
expected to increase β-catenin. However, siABCG8-transfected cells
exhibited increased intracellular cholesterol levels but reduced
β-catenin (Fig. 3B-D).
Therefore, the possibility that increased intracellular cholesterol
via LDLR-mediated endocytosis could activate Wnt/β-catenin
signaling in cells was excluded. Instead, the present study
investigated whether the cholesterol-binding receptor LRP6, a Wnt
co-receptor, could activate β-catenin and the associated
EMT-related gene expression. RT-R-MDA-MB-231 cells exhibited
increased levels of p-LRP6 compared with those in MDA-MB-231 cells
(Fig. 5A and B). Knockdown of
ABCG8 using siRNA decreased both LRP6 expression and LRP6
phosphorylation (Fig. 5C and D).
Additionally, silencing of LRP6 resulted in reduced levels of
β-catenin, PLK1 and Cyclin B1 compared with the siCTRL-transfected
group (Fig. 5E-G). Furthermore,
extracellular cholesterol treatment (10 and 20 μM; 24 h) did
not significantly affect LRP6 phosphorylation and expression, and
β-catenin signaling in RT-R-MDA-MB-231 cells at 10 μM, but
did at 20 μM (Fig. S1A and
B). When cholesterol was administered to MDA-MB-231 cells,
which secreted less cholesterol than RT-R-TNBC cells, LRP6
phosphorylation and β-catenin induction were more prominent
compared with those in RT-R-MDA-MB-231 cells from 10 μM
(Fig. S1C and D). These
findings indicated that increased LRP6 activation in
RT-R-MDA-MB-231 cells was dependent on ABCG8 expression, and was
associated with the induction of β-catenin, PLK1 and Cyclin B1
expression, suggesting that extracellular cholesterol exported by
ABCG8 may affect LRP6 to activate the Wnt/β-catenin pathway.
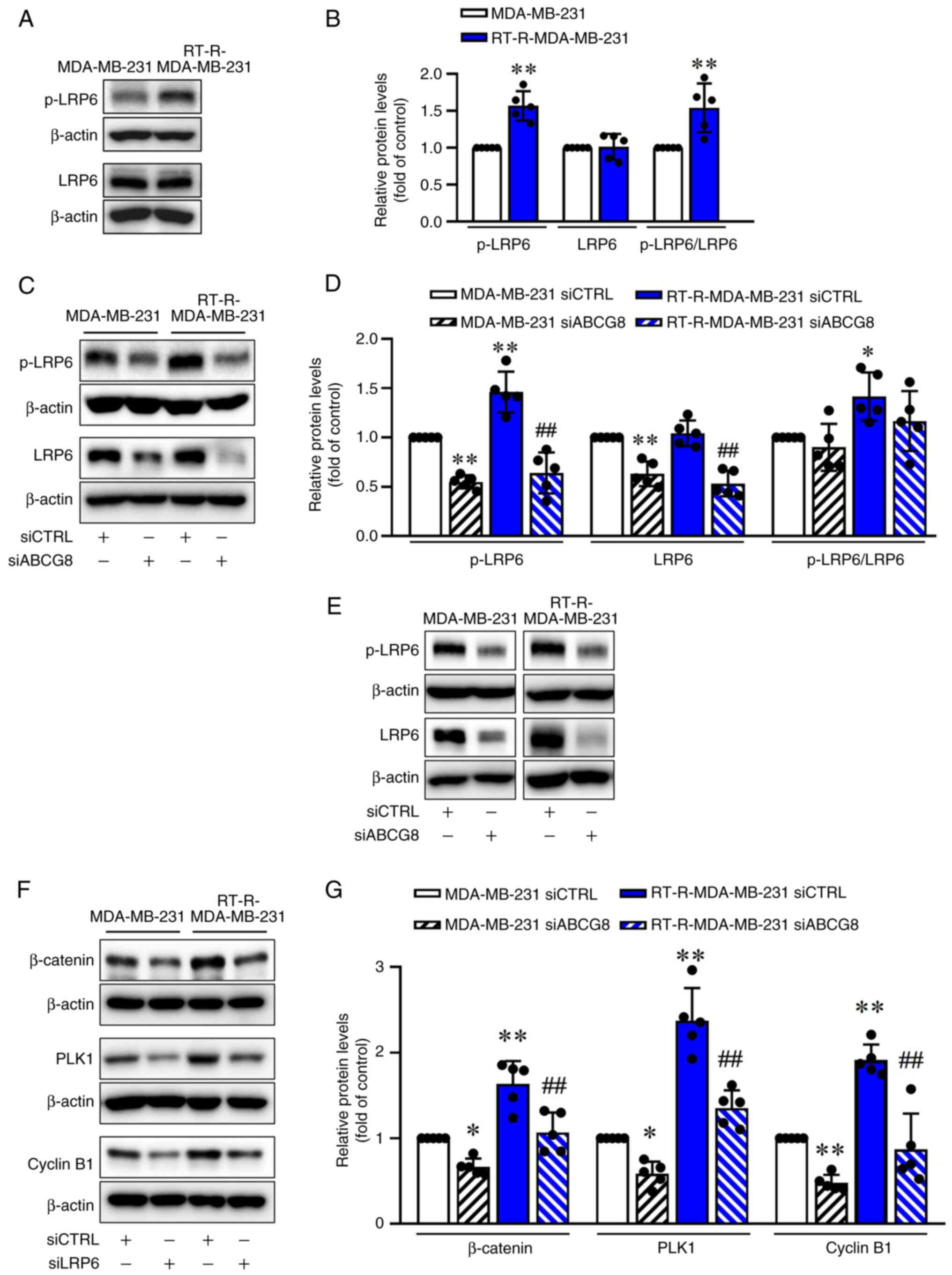 | Figure 5LRP6 is activated in RT-R-MDA-MB-231
cells, with its activation mediating cholesterol-dependent
activation of the Wnt/β-catenin pathway. (A) Levels of LRP6 and
p-LRP6 in MDA-MB-231 and RT-R-MDA-MB-231 cells were determined
using western blot analysis. (B) Levels of LRP6 and p-LRP6 were
semi-quantified and normalized to β-actin. Data are presented as
the mean ± SD of five independent experiments.
**P<0.01 compared with MDA-MB-231 cells. (C) Protein
levels of LRP6 and p-LRP6 in cells transfected with siCTRL or
siABCG8 were determined by western blot analysis. (D) Levels of
LRP6, p-LRP6 and p-LRP6/LRP6 were semi-quantified and normalized to
β-actin. Data are presented as the mean ± SD of five independent
experiments. *P<0.05, **P<0.01 compared
with the siCTRL-transfected MDA-MB-231 cells;
##P<0.01 compared with the siCTRL-transfected
RT-R-MDA-MB-231 cells. (E) Cells were transfected with siCTRL or
siLRP6 (100 nM) for 24 h and the transfection efficiency of siLRP6
was determined by western blot analysis. (F) Cells were transfected
with siCTRL and siLRP6 for 24 h. Levels of β-catenin, PLK1 and
Cyclin B1 were then determined by western blotting. (G) Levels of
proteins were semi-quantified and normalized to β-actin. Data are
presented as the mean ± SD of five independent experiments.
*P<0.05; **P<0.01 compared with the
siCTRL-transfected MDA-MB-231 cells; ##P<0.01
compared with the siCTRL-transfected RT-R-MDA-MB-231 cells. ABCG8,
ATP-binding cassette subfamily G member 8; CTRL, control; LRP6,
low-density lipoprotein receptor-related protein 6; p-,
phosphorylated; PLK1, Polo-like kinase 1; RT-R,
radiotherapy-resistant; si, small interfering RNA. |
RT-R-MDA-MB-231 cells exhibit increased
SREBP1 (mature) and FASN expression and reduced LXRα/β and IDOL
expression compared with MDA-MB-231 cells
SREBPs are well-known key transcription factors that
control the expression of genes important for the uptake and
synthesis of cholesterol, fatty acids and phospholipids (30,31), and it has been reported that FASN
and cholesterol synthesis are linked under specific conditions
(32,33). In particular, the
SREBP1/FASN/cholesterol axis facilitates radioresistance in
colorectal cancer (34);
therefore, the present study investigated the SREBP1/FASN signaling
pathways. RT-R-MDA-MB-231 cells exhibited a significant increase in
the mature form of SREBP1 and FASN compared with MDA-MB-231 cells
(Fig. 6A and B). Consistent with
a report that hypoxia induces changes in lipid metabolism (35), RT-R-MDA-MB-231 cells exhibited
increased HIF-1α levels (Fig. 6A and
B), and treatment with digoxin, a HIF-1α inhibitor (at 400 nM
for 24 h), led to a marked reduction in SREBP1 (mature form), and
FASN protein expression in RT-R-MDA-MB-231 cells (Fig. 6C and D). However, ABCG8
expression was not affected by digoxin (Fig. 6C and D). In addition,
RT-R-MDA-MB-231 cells exhibited increased levels of LDLR and
decreased levels of LXRα/β and IDOL compared with those in
MDA-MB-231 cells (Fig. 6E and
F). These results suggested that RT-R-TNBC increased
intracellular cholesterol levels by inducing synthesis of
cholesterol and by dysregulating feedback mechanisms such as the
LXRα/β and IDOL feedback mechanisms to reduce intracellular
cholesterol by decreasing LDLR expression.
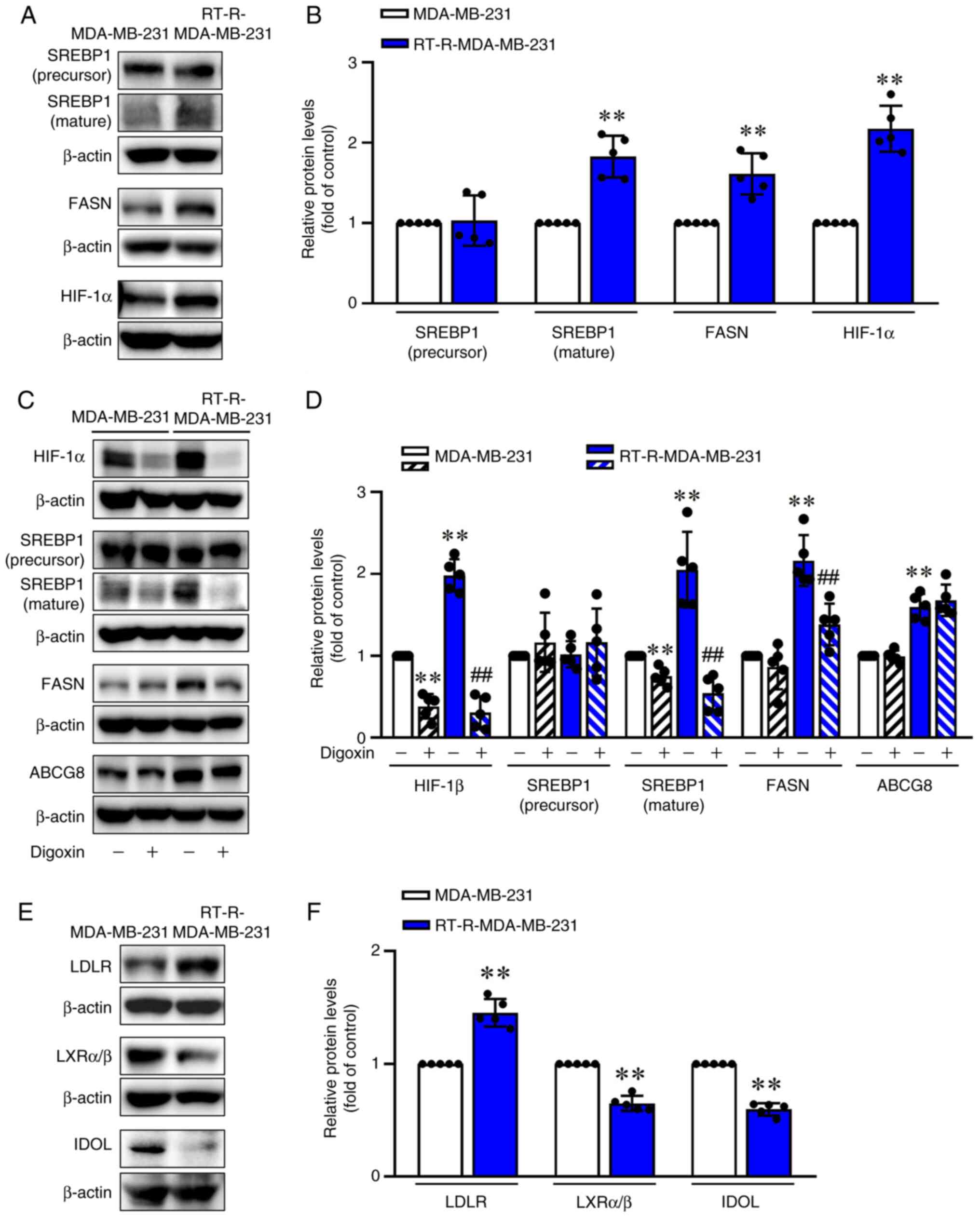 | Figure 6RT-R-MDA-MB-231 cells exhibit
increased SREBP1 (mature) and FASN expression compared with
MDA-MB-231 cells. (A) Protein levels of SREBP1 (precursor and
mature form), FASN and HIF-1α in MDA-MB-231 and RT-R-MDA-MB-231
cells were analyzed by western blotting. (B) Levels of proteins
were semi-quantified and normalized to β-actin. Data are presented
as the mean ± SD of five independent experiments.
**P<0.01 compared with MDA-MB-231 cells. (C) Cells
were treated with 400 nM digoxin, an inhibitor of HIF-1α, for 24 h,
and protein levels of HIF-1α, SREBP1, FASN and ABCG8 were then
analyzed by western blotting. (D) Levels of proteins were
semi-quantified and normalized to β-actin. Data are presented as
the mean ± SD of five independent experiments.
**P<0.01 compared with the untreated MDA-MB-231
cells; ##P<0.01 compared with the untreated
RT-R-MDA-MB-231 cells. (E) LDLR, LXRα/β and IDOL protein levels in
MDA-MB-231 and RT-R-MDA-MB-231 cells were analyzed by western
blotting. (F) Levels of proteins were semi-quantified and
normalized to β-actin. Data are presented as the mean ± SD of five
independent experiments. **P<0.01 compared with
MDA-MB-231 cells. ABCG8, ATP-binding cassette subfamily G member 8;
FASN, fatty acid synthase; HIF-1α, hypoxia-inducible factor 1α;
IDOL, inducible degrader of LDL; LDLR, LDL receptor; LDL,
low-density lipoprotein; LXRα/β, liver X receptor α/β; RT-R,
radiotherapy-resistant; SREBP1, sterol regulatory element binding
protein 1. |
Discussion
ABC transporters are ATP-dependent transporters
composed of 48 genes. They are subdivided into seven subfamilies
(ABCA-ABCG) (36), and transport
xenobiotics, metabolites and signaling molecules across
intracellular and extracellular membranes (11). ABC transporters are well known
for mediating multidrug resistance in cancer, which can lead to
chemotherapy failure (37).
Studies have suggested the important roles of ABC transporters in
cancer beyond their established function as a multidrug efflux pump
(11); they have been reported
to influence tumor cells in various cancer types by regulating
their malignant potential, including tumor cell proliferation,
differentiation, migration and invasion (38,39). However, there is still a lack of
understanding of how ABC transporters affect cancer
progression.
In the present study, RT-R-MDA-MB-231 cells
exhibited a significant increase in ABCG8 expression compared with
MDA-MB-231 cells. Furthermore, knockdown of ABCG8 not only
increased Doxo- and PTX-mediated cytotoxicity, but also
significantly reduced cell viability on its own after 48 and 72 h
of incubation, suggesting the additional function of ABCG8 beyond
its role as a drug pump. Since ABCG8 is involved in sterol efflux
(16), the present study
investigated the levels of cholesterol inside cells and in the
surrounding medium. The levels of cholesterol in cells and
surrounding medium were significantly higher in the RT-R-MDA-MB-231
group than in the MDA-MB-231 group. Additionally, when ABCG8 was
knocked down, there was a further increase in intracellular
cholesterol levels but a reduction in medium cholesterol levels.
This result demonstrated that RT-R-MDA-MB-231 cells exhibited
higher levels of cholesterol, and that ABCG8 in RT-R-MDA-MB-231
cells increases cholesterol efflux.
Tumor cells have an increased need for lipids to
grow and proliferate quickly. Lipid synthesis and uptake are
elevated in various types of cancer, including breast cancer, and
represent a novel characteristic of human cancer (18,40). Cholesterol is a major component
of cell membranes; it controls cellular processes such as cell
proliferation, differentiation, survival and apoptosis, and is also
considered a risk factor and prognostic factor in cancer (41). Studies have shown that
cholesterol serves a crucial role as a signaling molecule,
regulating several pathways such as the PI3K, Hedgehog and
Wnt/β-catenin pathways (42,43). Therefore, the present study
investigated whether cholesterol exported by ABCG8 could function
as a signaling molecule and whether it might be involved in cancer
progression. The present results demonstrated that the expression
levels of EMT-related proteins (β-catenin, N-cadherin and OCT4)
were significantly higher in RT-R-MDA-MB-231 cells than in
MDA-MB-231 cells. However, these increases were reduced by siABCG8
transfection. EMT-related proteins are known to be regulated by the
PI3K/AKT signaling pathway in cancer (44,45). Additionally, the STAT3 pathway is
a key regulator of breast cancer metastasis, promoting cancer
progression, drug resistance, inhibition of apoptosis and EMT
(46,47). However, although intracellular
cholesterol levels were increased by siABCG8 transfection, the
increased levels of p-AKT and p-STAT3 in RT-R-MDA-MB-231 cells were
not affected by siABCG8 transfection. Therefore, this result
suggested that cholesterol exported by ABCG8 affected the induction
of EMT-related proteins but not via the p-AKT or p-STAT3
pathways.
It has been reported that cholesterol may serve a
role in cancer biology by activating the Wnt pathway through
specific interactions with Dishevelled and LRP6 (48). LRP6 is a crucial Wnt co-receptor
in the Wnt/β-catenin signaling pathway. LRP6 expression is
frequently upregulated in human breast cancer, and LRP6 is more
frequently upregulated in TNBC (49). LRP6 promotes TNBC cell migration
and invasion by interacting with frizzled (a transmembrane receptor
family) and by activating the Wnt/β-catenin signaling pathway
(50,51) involved in various physiological
processes, such as proliferation, apoptosis, migration, invasion
and homeostasis. Emerging reports have suggested that abnormal
activation of the Wnt/β-catenin signaling pathway can promote
cancer stem cell progression and cancer metastasis (52,53), and patients with TNBC with
upregulated Wnt signaling often have a poor prognosis (54). Based on the significance of LRP6
in binding cholesterol and activating the Wnt/β-catenin pathway,
which ultimately contributes to the maintenance and proliferation
of cancer cells, the present study investigated the expression
levels of LRP6 in RT-R-MDA-MB-231 cells, and revealed that the
levels of p-LRP6 were increased in RT-R-MDA-MB-231 cells compared
with MDA-MB-231 cells. siLRP6 transfection reduced the expression
levels of β-catenin, PLK1 and Cyclin B1. Based on these results, it
was hypothesized that increased cholesterol in RT-R-MDA-MB-231
cells may be exported by ABCG8 and that the elevated cholesterol
could subsequently influence LRP6/Wnt/β-catenin signaling to
activate the expression of β-catenin and other EMT-related genes.
ABCG8 protein levels were higher in TNBC cells such as MDA-MB-231
and MDA-MB-453 cells than in MCF7 cells, a non-TNBC cell line.
Furthermore, siABCG8 transfection also affected cholesterol efflux
and the LRP6/Wnt/β-catenin signaling pathway in MDA-MB-231 cells.
Therefore, ABCG8 may mediate cholesterol efflux and activate
LRP6/Wnt/β-catenin signaling in TNBC. The present study emphasized
that ABCG8 expression, cholesterol efflux from cytosol to media,
and activation of the LRP6/Wnt/β-catenin signaling were enhanced in
RT-R-MDA-MB-231 cells compared with MDA-MB-231 cells. In addition,
knockdown of ABCG8 using siRNA reduced the levels of p-LRP6/LRP6,
β-catenin and EMT-related proteins without reducing p-GSKβ or GSKβ.
This result indicated that the levels of β-catenin may be
controlled by factors other than GSKβ in the Wnt signaling
pathway.
PLKs serve an essential role as key mitotic kinases
and cell cycle regulators, and they also serve a role in
proliferation and cell growth (21). PLKs are upregulated in various
types of cancer, including ovarian cancer (22), breast cancer (55) and colorectal cancer (56), and promote entry into mitosis in
the cell cycle by activating Cyclin B1 (23). Additionally, Wnt
signaling-mediated β-catenin can activate cJUN, which results in
increased expression levels of PLK1 (26). c-Jun is known as a
transcriptional target and an interacting partner of β-catenin
(57) and is also considered a
possible transcriptional factor regulating PLK1 expression
(58). Kim et al
(26) demonstrated that
TGFβ-activated PLK1 can phosphorylate β-catenin at Ser311, leading
to increased stability, and promotes its nuclear translocation.
Consequently, this process leads to elevated c-JUN expression,
which, as part of the AP-1 complex, upregulates PLK1 expression,
forming a positive feedback loop that facilitates EMT in metastatic
non-small cell lung cancer. In the present study, ABCG8 knockdown
significantly reduced the levels of PLK1 and Cyclin B1 in
RT-R-MDA-MB-231 cells, resulting in cell cycle arrest in the
G2/M phase. LRP6 siRNA transfection also reduced
β-catenin, PLK1 and Cyclin B1 levels. These results suggested that
cholesterol exported by ABCG8 could activate the LRP6/Wnt/β-catenin
signaling pathway, which in turn could induce the expression of
PLK1 and Cyclin B1, ultimately leading to cell cycle
progression.
SREBP is a crucial transcription factor that
controls the expression of enzymes involved in lipid metabolism and
synthesis (30), and its
expression is increased in various types of cancer, including
colorectal cancer (59), breast
cancer (60) and hepatocellular
carcinoma (61). HIF-1α is a
transcription factor that is induced under hypoxia, which is
involved in tumor progression, including metastasis, drug
resistance and cell cycle progression. HIF-1α is related to poor
prognosis in various types of cancer (62-64), including colon cancer, prostate
cancer and breast cancer (65).
Under hypoxic conditions, tumor cells can activate HIF-1α.
Activated HIF-1α then upregulates SREBP1 and activates FASN, which
can increase hypoxia-induced chemotherapy resistance and promote
tumor cell survival (66,67).
Our previous study revealed that increased HIF-1α expression in
RT-R-MDA-MB-231 cells contributed to tumor progression (8). In the present study, the expression
levels of the mature form of SREBP1 and FASN were significantly
increased in RT-R-MDA-MB-231 cells compared with in MDA-MB-231
cells, and increased SREBP1 and FASN levels in RT-R-MDA-MB-231
cells were significantly reduced by treatment with digoxin, an
inhibitor of HIF-1α. These results suggested that the cholesterol
levels in RT-R-MDA-MB-231 cells were increased via HIF-1α-mediated
SREBP1-FASN induction. However, ABCG8 induction in RT-R-MDA-MB-231
cells was not HIF-1α-dependent, as digoxin, a HIF-1α inhibitor,
failed to inhibit ABCG8 induction in RT-R-MDA-MB-231 cells. Further
investigation is needed to understand the mechanism by which
RT-R-MDA-MB-231 cells induce ABCG8. In normal cells, a high level
of cholesterol triggers regulatory mechanisms that control the
synthesis and uptake of cholesterol, leading to downregulation of
SREBP and degradation of β-hydroxy β-methylglutaryl-CoA reductase.
In addition, LXRα/β and IDOL are increased to reduce intracellular
cholesterol by decreasing LDLR expression (68). However, cancer cells may be able
to evade these feedback mechanisms in a cholesterol-rich
environment. The present results showed that the expression of
LXRα/β and IDOL, a feedback mechanism for the downregulation of
intracellular cholesterol levels, was reduced in RT-R-MDA-MB-231
cells, resulting in increased LDLR expression. Dysregulation of
feedback mechanisms as well as induction of cholesterol synthesis
might maintain a high level of cholesterol within cells. The
cholesterol was exported by ABCG8, which was upregulated in
RT-R-MDA-MB-231 cells. The exported cholesterol in turn activated
LRP6, the Wnt co-receptor, leading to the mediation of
Wnt/β-catenin signaling. This activation induced the expression of
PLK1, Cyclin B1 and EMT-related proteins, ultimately contributing
to cancer progression (Fig.
7).
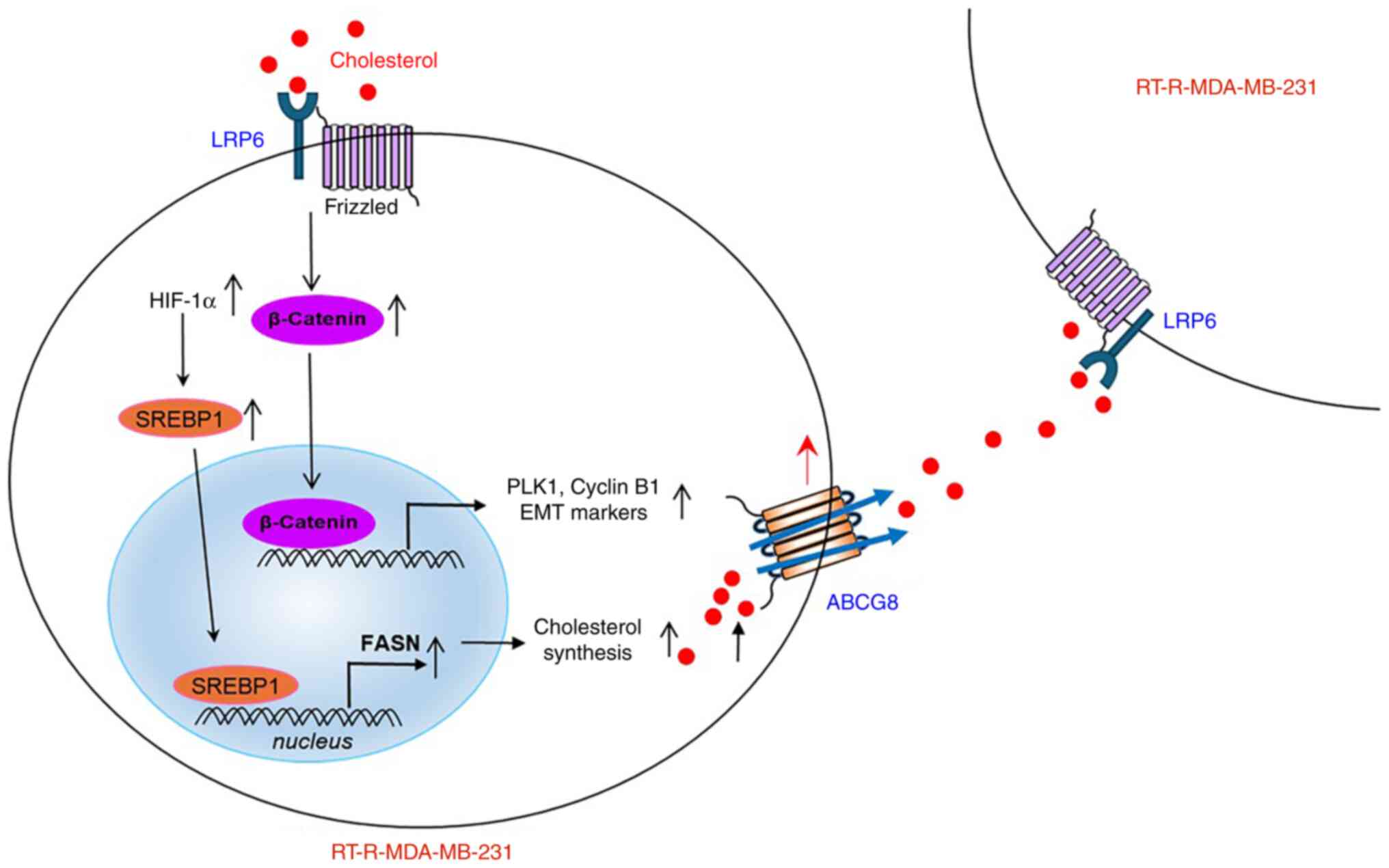 | Figure 7A proposed mechanism suggests that
ABCG8 upregulation in RT-R-MDA-MB-231 cells mediates cholesterol
efflux, leading to increased cancer cell progression through the
LRP6/Wnt/β-catenin signaling pathway in RT-R-MDA-MB-231 cells.
Induced HIF-1α in RT-R-MDA-MB-231 cells increases SREBP1 (mature
form) and FASN, resulting in the production of cholesterol.
Produced cholesterol is exported by ABCG8 to activate Wnt/β-catenin
signaling through LRP6 receptors. Activated β-catenin can induce
the expression of target genes such as PLK1 and Cyclin B1,
ultimately leading to increased proliferation of cancer cells and
tumor growth. ABCG8, ATP-binding cassette subfamily G member 8;
EMT, epithelial-mesenchymal transition; FASN, fatty acid synthase;
HIF-1α, hypoxia-inducible factor 1α; LRP6, low-density lipoprotein
receptor-related protein 6; PLK1, Polo-like kinase 1; RT-R,
radiotherapy-resistant; SREBP1, sterol regulatory element binding
protein 1. |
The increased cholesterol levels in highly
metastatic TNBC that has acquired radiotherapy resistance may
affect the maintenance of cell morphology because cholesterol is a
component of cell membranes and vital for cell membrane integrity
(41,69). However, to the best of our
knowledge, the present study was the first to suggest the role of
ABCG8 and that the cholesterol exported by ABCG8, not inside the
cells, affects cancer progression through the LRP6/Wnt/β-catenin
signaling pathway in RT-R-TNBC cells. The present results
demonstrated that intracellular and medium cholesterol levels were
significantly higher in the RT-R-MDA-MB-231 group compared with in
the MDA-MB-231 group, and LRP6 was highly phosphorylated and
β-catenin signaling was upregulated at baseline without any
external stimulation in RT-R-MDA-MB-231 cells. This may explain why
the effect of externally administered cholesterol was minimal in
RT-R-MDA-MB-231 cells. Therefore, it may be hypothesized that high
levels of intracellular cholesterol exported by ABCG8 may serve an
important role in cancer progression by maintaining high levels of
pericellular cholesterol and continuously stimulating cells. This
needs to be further investigated. In conclusion, targeting the
LRP6-Wnt/β-catenin signaling pathway may provide a potential
therapeutic strategy for the treatment of RT-R-TNBC.
Supplementary Data
Availability of data and materials
The sequencing data generated in the present study
may be found in the Gene Expression Omnibus database under
accession number GSE287883 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE287883.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
YSK performed the experiments, analyzed the data and
wrote the manuscript. JYW, HJ, NBN, YW and VN conducted the
experiments and analyzed the data. SPY and SWP interpreted the
results, developed the methodology and revised the manuscript. HJK
conceived and designed the study, directed the project, and revised
the manuscript. YSK, HJ and HJK confirmed the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
CTRL
|
control
|
|
EC
|
endothelial cell
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ER
|
estrogen receptor
|
|
FASN
|
fatty acid synthase
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
IDOL
|
inducible degrader of LDL
|
|
LDL
|
low-density lipoprotein
|
|
LDLR
|
LDL receptor
|
|
LRP6
|
LDLR-related protein 6
|
|
LXRα/β
|
liver X receptor α/β
|
|
PLK1
|
Polo-like kinase 1
|
|
PR
|
progesterone receptor
|
|
RT-R
|
radiotherapy-resistant
|
|
siRNA
|
small interfering RNA
|
|
SREBP1
|
sterol regulatory element binding
protein 1
|
|
TNBC
|
triple-negative breast cancer
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea, funded
by the Ministry of Education (grant nos. 2021R1A2B5B01001446 and
RS-2024-003397886138211653 0101), and by the Development Fund
Foundation, Gyeongsang National University, 2024.
References
|
1
|
Xu Y, Gong M, Wang Y, Yang Y, Liu S and
Zeng Q: Global trends and forecasts of breast cancer incidence and
deaths. Sci Data. 10:3342023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Courtney D, Davey MG, Moloney BM, Barry
MK, Sweeney K, McLaughlin RP, Malone CM, Lowery AJ and Kerin MJL:
Breast cancer recurrence: Factors impacting occurrence and
survival. Ir J Med Sci. 191:2501–2510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Ruijter TC, Veeck J, de Hoon JP, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:1831922011. View Article : Google Scholar
|
|
4
|
Bai X, Ni J, Beretov J, Graham P and Li Y:
Triple-negative breast cancer therapeutic resistance: Where is the
Achilles' heel? Cancer Lett. 28(497): 100–111. 2021. View Article : Google Scholar
|
|
5
|
Zhang C, Wang S, Israel HP, Yan SX,
Horowitz DP, Crockford S, Gidea-Addeo D, Chao KSC, Kalinsky K and
Connolly EP: Higher locoregional recurrence rate for
triple-negative breast cancer following neoadjuvant chemotherapy,
surgery and radiotherapy. Springer Plus. 4:3862015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dent R, Trudeau M, Pritchard KI, Wedad MP,
Harriet KH, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ko YS, Jin H, Lee JS, Park SW, Chang KC,
Kang KM, Jeong BK and Kim HJ: Radioresistant breast cancer cells
exhibit increased resistance to chemotherapy and enhanced invasive
properties due to cancer stem cells. Oncol Rep. 40:3752–3762.
2018.PubMed/NCBI
|
|
8
|
Ko YS, Rugira T, Jin H, Joo YN and Kim HJ:
Radiotherapy-resistant breast cancer cells enhance tumor
progression by enhancing premetastatic niche formation through the
HIF-1α-LOX axis. Int J Mol Sci. 21:80272020. View Article : Google Scholar
|
|
9
|
Modi A, Roy D, Sharma S, Vishnoi JR,
Pareek P, Elhence P, Sharma P and Purohit P: ABC transporters in
breast cancer: Their roles in multidrug resistance and beyond. J
Drug Target. 9:927–947. 2022. View Article : Google Scholar
|
|
10
|
Beretta GL, Cassinelli G, Pennate M, Zuco
V and Gatti L: Overcoming ABC transporter-mediated multidrug
resistance: The dual role of tyrosine kinase inhibitors as
multitargeting agents. Eur J Med Chem. 142:271–289. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher JI, Henderson MJ and Norris MD:
ABC transporters in cancer: More than just drug efflux pumps. Nat
Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Begicevic RR and Falasca M: ABC
transporters in cancer stem cells: Beyond chemoresistance. Int J
Mol Sci. 18:23622017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen ZS and Tiwari AK: Multidrug
resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic
diseases. FEBS J. 278:3266–3245. 2011. View Article : Google Scholar
|
|
14
|
Sun YL, Patel A, Kumar P and Chen ZS: Role
of ABC transporters in cancer chemotherapy. Chin J Cancer.
31:51–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sabeva NS, Liu J and Graf GA: The
ABCG5ABCG8 sterol transporter and phytosterols: Implications for
cardiometabolic disease. Curr Opin Endocrinol Diabetes Obes.
16:172–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Mitsche MA, Lütjohann D, Cohen JC,
Xie XS and Hobbs HH: Relative roles of ABCG5/ABCG8 in liver and
intestine. J Lipid Res. 56:319–330. 2015. View Article : Google Scholar :
|
|
17
|
Xiao H, Zheng Y, Ma L, Tian L and Sun Q:
Clinically-relevant ABC transporter for anti-Cancer drug
resistance. Front Pharmacol. 19(12): 6484072021. View Article : Google Scholar
|
|
18
|
Cheng X, Li J and Guo D: SCAP/SREBPs are
central players in lipid metabolism and novel metabolic targets in
cancer therapy. Curr Top Med Chem. 18:484–493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thu KL, Soria-Bretones I, Mak TW and
Cescona DW: Targeting the cell cycle in breast cancer: Towards the
next phase. Cell Cycle. 17:1871–1885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lecarpentier Y, Schussler O, Hébert JL and
Vallée A: Multiple targets of the canonical WNT/β-Catenin signaling
in cancers. Front Oncol. 9:12482019. View Article : Google Scholar
|
|
21
|
Lee SY, Jang C and Lee KA: Polo-like
kinases (plks), a key regulator of cell cycle and new potential
target for cancer therapy. Dev Reprod. 18:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kressin M, Fietz D, Becker S and
Strebhardt K: Modelling the functions of Polo-Like Kinases in mice
and their applications as cancer targets with a special focus on
ovarian cancer. Cells. 10:11762021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solc P, Kitajima TS, Yoshida S, Brzakova
A, Kaido M, Baran V, Mayer A, Samalova P, Motlik J and Ellenber J:
Multiple requirements of PLK1 during mouse oocyte maturation. PLoS
One. 10:e01167832015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S, Sharma G, Chakraborty C, Sharma
AR and Kim JB: Regulatory functional territory of PLK-1 and their
substrates beyond mitosis. Oncotarget. 8:37942–37962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah K and Kazi J:
Phosphorylation-dependent regulation Wnt/beta-catenin signaling.
Front oncol. 12:8587822022. View Article : Google Scholar
|
|
26
|
Kim DE, Shin SB, Kim CH, Kim YB, Oh HJ and
Yim HS: PLK1-mediated phosphrylatioh of β-catenin enhances its
stability and transcriptional activity for extracellular matrix
remodeling in metastaic NSCLC. Theranostics. 13:1198–1216. 2023.
View Article : Google Scholar :
|
|
27
|
Brown MS and Goldstein JL: A
receptor-mediated pathway for cholesterol homeostasis. Science.
232:34–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alrefaei AF and Abu-Elmagd M: LRP6
receptor plays essential functions in development and human
diseases. Genes (Basel). 13:1202022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raisch J, Côté-Biron A and Rivard N: A
role for the WNT Co-receptor LRP6 in pathogenesis and therapy of
epithelial cancers. Cancers (Basel). 11:11622019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bengoechea-Alonso MT and Ericsson J: SREBP
in signal transduction: Cholesterol metabolism and beyond. Curr
Opin Cell Biol. 19:215–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eid W, Dauner K, Courtney KC, Gagnon A,
Parks RJ, Sorisky A and Zha X: mTORC1 activates SREBP-2 by
suppressing cholesterol trafficking to lysosomes in mammalian
cells. Proc Natl Acad Sci USA. 114:7999–8004. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carroll RG, Zasłona Z, Galvan-Pena S,
Koppe EL, Sevin DC, Angiari S, Triantafilou M, Triantafilou K,
Modis LK and O'Neill LA: An unexpected link between fatty acid
synthase and cholesterol synthesis in proinflammatory macrophage
activation. J Biol Chem. 293:5509–5521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin Y, Chen Z, Dong J, Wang B, Fan S, Yang
X and Cui M: SEBP1/FASN/cholesterol axis facilitates
radioresistance in colorectal cancer. FEBS Open Bio. 11:1343–1352.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mylonis I, Simos G and Paraskeva E:
Hypoxia-inducible factors and the regulation of lipid metabolism.
Cells. 8:2142019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dean M: The genetics of ATP-binding
cassette transporters. Methods Enzymol. 400:409–429. 2005.
View Article : Google Scholar
|
|
37
|
Fletcher JI, Williams RT, Henderson MJ,
Norris MD and Haber M: ABC transporters as mediators of drug
resistance and contributors to cancer cell biology. Drug Resist
Updat. 26:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Copsel S, Garcia C, Diez F, Vermeulem M,
Baldi A, Bianciotti LG, Russel FGM, Shayo C and Davio C: Multidrug
resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels
and controls human leukemia cell proliferation and differentiation.
J Biol Chem. 286:6979–6988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Henderson MJ, Haber M, Porro A, Munoz MA,
Iraci N, Xue C, Murray J, Flemming CL, Smith J and Fletcher JI:
ABCC multidrug transporters in childhood neuroblastoma: Clinical
and biological effects independent of cytotoxic drug efflux. J Natl
Cancer Inst. 103:1236–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Beloribi-Djefaflia S, Vasseur S and
Guillaumond F: Lipid metabolic reprogramming in cancer cells.
Oncogenesis. 5:e1892016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan A, Jia Z, Qiao C, Wang M and Ding X:
Cholesterol metabolism in drug-resistant cancer. Int J Oncol.
57:1103–1115. 2020.
|
|
42
|
Sheng R, Chen Y, Gee HY, Stec E, Melowic
HR, Blatner NR, Tun MP, Kim YJ, Källberg M and Fujiwara TK:
Cholesterol modulates cell signaling and protein networking by
specifically interacting with PDZ domain-containing scaffold
proteins. Nat Commun. 3:12492012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Halimi H and Farjadian S: Cholesterol: An
important actor on the cancer immune scene. Front Immunol.
13:10575462022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song JW: Targeting Epithelial-mesenchymal
transition pathway in hepatocellular carcinoma. Clin Mol Hepatol.
26:484–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maharati A and Moghbeli M: PI3K/AKT
signaling pathway as a critical regulator of Epithelial-mesenchymal
transition in colorectal tumor cells. Cell Commun Signal.
21:2012023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manore SG, Doheny DL, Wong GL and Lo HW:
IL-6/JAK/STAT3 signaling in breast cancer metastasis: Biology and
Treatment. Front Oncol. 12:8660142022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang G, Hou S, Li S, Wang Y and Cui W:
Role of STAT3 in cancer cell Epithelial-mesenchymal transition. Int
J Oncol. 64:482024. View Article : Google Scholar
|
|
48
|
Sheng R, Kim HJ, Lee HY, Xin Y, Chen Y,
Tian Y, Cui Y, Choi JC, Doh JS, Han JK and Cho WH: Cholesterol
selectively activates canonical Wnt signalling over Non-canonical
Wnt signaling. Nat Commun. 5:43932014. View Article : Google Scholar
|
|
49
|
Liu CC, Prior J, Piwnica-Worms D and Bu G:
LRP6 overexpression defines a class of breast cancer subtype and is
a target for therapy. Proc Natl Acad Sci USA. 107:5136–5141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang L, Wu X, Wang Y, Zhang KW, Ju Y, Yuan
YC, Deng X, Chen L, Kim CCH and Lau S: FZD7 has a critical role in
cell proliferation in triple negative breast cancer. Oncogene.
30:4437–4446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma J, Lu W, Chen D, Xu B and Li Y: Role of
Wnt Co-receptor LRP6 in triple negative breast cancer cell
migration and invasion. J Cell Biochem. 118:2968–2976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020. View Article : Google Scholar
|
|
53
|
Paskeh MDA, Mirzaei S, Ashrafizadeh M,
Zarrabi A and Sethi G: Wnt/β-Catenin signaling as a driver of
hepatocellular carcinoma progression: An emphasis on molecular
pathways. J Hepatocell Carcinoma. 8:1415–1444. 2021. View Article : Google Scholar
|
|
54
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Weichert W: Polo-like kinase isoforms in
breast cancer: Expression patterns and prognostic implications.
Virchows Arch. 446:442–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Takahashi T: Polo-like kinase 1 (PLK1) is
overexpressed in primary colorectal cancers. Cancer Sci.
94:148–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mann B, Gelos M, Siedow A, Hanski ML,
Gratchev A and Ilyas M: Target genes of beta-catenin-T
cell-factor/lymphoid-enhancer-factor signaling in human colorectal
carcinomas. Proc Natl Acad Sci USA. 96:1603–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martin BT and Strebhardt K: Polo-like
kinase 1: Target and regulator of transcriptional control. Cell
Cycle. 5:2881–2085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gao Y, Nan X, Shi X, Mu X, Liu B and Zhu
H: SREBP1 promotes the invasion of colorectal cancer accompanied
upregulation of MMP7 expression and NF-Kappa b pathway activation.
BMC Cancer. 19:6852019. View Article : Google Scholar
|
|
60
|
Zhu Z, Zhao X, Zhao L, Yang H, Liu L and
Li J: P54(nrb)/NONO regulates lipid metabolism and breast cancer
growth through SREBP-1A. Oncogene. 35:1399–1410. 2006. View Article : Google Scholar
|
|
61
|
Li C, Yang W, Zhang J, Zheng X, Yao Y and
Tu K: SREBP-1 has a prognostic role and contributes to invasion and
metastasis in human hepatocellular carcinoma. Int J Mol Sci.
15:7124–7138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yong L, Tang S, Yu H, Zhang H, Zhang Y,
Wan Y and Cai F: The role of hypoxia-inducible factor-1 alpha in
multidrug-resistant breast cancer. Front Oncol. 12:9649342022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jun JC, Rathore A, Younas H, Gilkes D and
Polotsky YV: Hypoxia-inducible factors and cancer. Curr Sleep Med
Rep. 3:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
65
|
Generali D, Berruti A, Brizzi MP, Campo L,
Bonardi S and Wigfield S: Hypoxia-inducible factor-1alpha
expression predicts a poor response to primary chemoendocrine
therapy and disease-free survival in primary human breast cancer.
Clin Cancer Res. 12:4562–4568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ezzeddini R, Taghikhani M, Somi MH, Samadi
N and Rasaee MJ: Clinical importance of FASN in relation to HIF-1α
and SREBP-1c in gastric adenocarcinoma. Life Sci. 224:169–176.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Furuta E, Pai SK, Zhan R, Bandyopadhyay S,
Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T and Miura K: Fatty
Acid Synthase gene is up-regulated by hypoxia via activation of Akt
and Sterol Regulatory Element Binding Protein-1. Cancer Res.
68:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang L, Reue K, Fong LG, Young SG and
Tontonoz P: Feedback regulation of cholesterol uptake by the
LXR-IDOL-LDLR axis. Arterioscler Thromb Vasc Biol. 32:2541–2546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yoon HJ, Jillian L, Shaw JL, Haigis MC and
Greka A: Lipid metabolism in sickness and in health: Emerging
regulators of lipotoxicity. Mol Cell. 81:3708–3730. 2021.
View Article : Google Scholar : PubMed/NCBI
|